Abstract
Prior to the second half of the 20th century, most clinical decision-making was based on expert opinion. By the 1960s, experience in actuarial and research cohort studies had provided strong evidence that blood pressure was an important risk factor for cardiovascular disease. The landmark 1967 and 1970 Veterans Administration Cooperative Study trials confirmed the value of antihypertensive drug therapy in preventing stroke, myocardial infarction, and heart failure in adults with a high level of diastolic blood pressure. They also provided an impetus to develop the first blood pressure-related clinical practice guideline in 1977. In subsequent years, more structured and comprehensive blood pressure guidelines have evolved to become a major resource in clinical and public health practice. Despite some limitations, these guidelines provide useful evidence-based guidance for diagnosis and management of high blood pressure. The core advice in most of the current comprehensive blood pressure guidelines is more similar than different. Modeling studies suggest that better adherence to guideline recommendations would result in a lower average blood pressure and substantial improvement in public health.
Brief summary
Clinical practice guidelines provide recommendations based on structured review of the available evidence. They are especially useful in areas like high blood pressure that are common, costly, well informed by high-quality research, and associated with wide variations in practice patterns. Guidance in the principal blood pressure guidelines tends to be more similar than different. Greater implementation of their recommendations would not only substantially improve prevention and control of hypertension but markedly improve public health.
Evidence in Medicine
Evidence has always been a central tenet of clinical decision-making. Historical examples of seminal advances based on prior scientific evidence include the use of cowpox inoculation to prevent smallpox, administration of citrus fruits to prevent scurvy and demonstration that infectious diseases resulted from specific pathogens. For many years, however, clinical decisions were primarily based on expert opinion rather than on stronger scientific principles. As a result, treatments such as blood-letting persisted for more than 2,500 years before evidence demonstrated that it was, at best, of no value. During the early 20th century, William Welch and some other leading figures in North American medicine were strong proponents of the premise that medical practice and education should be based on scientific principles rather than expert opinion and apprenticeship. However, the type of evidence available to make clinical judgements remained limited until the second half of the 20th century. Egas Moniz is recognized for his pioneering introduction of cerebral angiography but received the Nobel Prize in Medicine in 1949 for his “discovery of the therapeutic value of leucotomy in certain psychoses.” Based on his original experience, Moniz concluded that “Prefrontal leucotomy is a simple operation, always safe.” It was only later that the serious adverse consequences of lobectomy became apparent and prefrontal lobotomy was replaced by the introduction of effective psychotropic drugs1. During the first half of the 20th century radiation therapy was used to treat enlargement of the thymus in infants and to manage other benign conditions in children2. It was considered to be a safe and effective treatment until evidence eventually emerged documenting a strong cancer relationship, especially in the thyroid gland3.
Measurement of Blood Pressure
Palpation of the pulse was practiced by the early Egyptians and Hales description of blood pressure (BP) measurement in his 1733 “Haemostaticks: Volume II of the Statical Essays” attracted considerable interest and recognition by the major scientific societies of his era4. However, accurate measurement of BP in humans was not possible until the introduction of the mercury manometer by Poisuille in 18284 During the mid to latter part of the nineteenth century there was intense interest in development of sphygmanometry devices for measurement of BP. However, when Osler’s first edition of The Principles and Practice of Medicine was published in 1892 there was no mention of high BP because there was still no practical method of BP measurement in clinical practice5. It was not until Riva-Rocci introduced cuff-based sphygmomanometry in 1896 that estimation of BP became feasible in clinical practice6. In 1901, Cushing introduced sphygmomanometry to U.S. physicians as a simple tool for obliteration of the radial pulse and measurement of systolic BP (SBP)7. Janeway and Crile were early supporters of the scientific utility of sphygmomanometry and its superiority over the traditional use of palpation to estimate the force of blood flow in the radial artery7. Much like today, however, there was great variation in measurement methods and little attention to quality control. In 1905, Korotkov described the auscultatory method of BP measurement and this technique was disseminated remarkedly rapidly8.
Blood Pressure as a Risk Factor
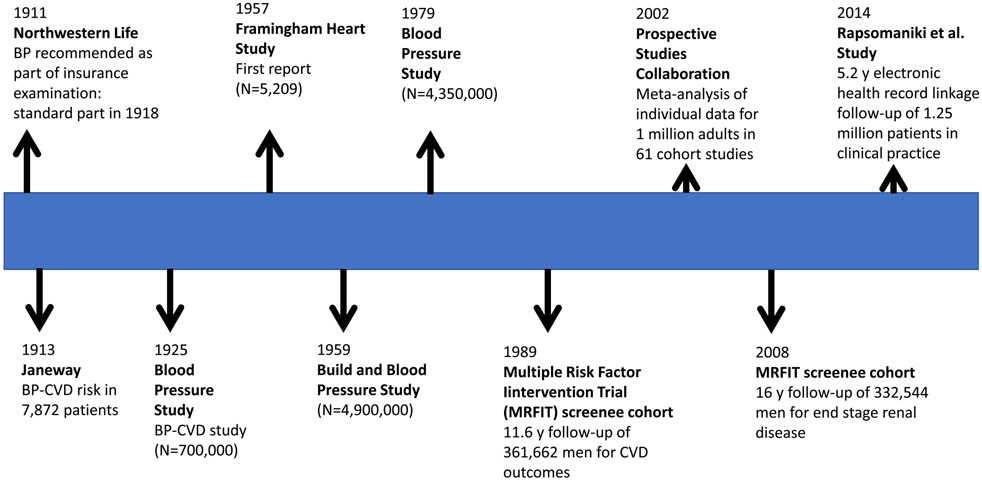
Figure 1 provides a timeline for some of the most important studies that have informed our knowledge of BP-related cardiovascular disease (CVD) and all-cause mortality. Actuaries and life insurance company medical directors were among the first to recognize the value of BP in estimating future risk. By 1905 there was considerable discussion regarding the value of the sphygmomanometer and BP measurements. In 1911, John Fisher, M.D., Medical Director, Northwestern Mutual Life Insurance Company, wrote “No practitioner of medicine should be without a sphygmomanometer. He has in this instrument a most valuable aid in diagnosis. The sphygmomanometer is indispensable in life-insurance examinations, and the time is not far distant when all progressive life-insurance companies will require its use in all examinations of applicants for life insurance”9. In 1912, Osler published his initial clinical observations that only some patients with high BP had signs of cardio-vascular disease10. Only one year later, Janeway reported clinical observations in 7,872 patients that suggested a strong qualitative relationship between high BP and risk of cardiovascular disease (CVD) risk11. In 1918, BP measurement became a standard part of insurance examinations for all companies12.
Figure 1:
Timeline for selected advances in knowledge related to risks of high blood pressure.
Beginning in the 1920s, large actuarial studies conducted by the insurance industry began to provide quantitative documentation of a strong association between level of BP and subsequent risk of CVD events, with a report published in 1925 being based on a study of 707,000 insured persons13. Although the data were imperfect, higher than average levels of both SBP and diastolic BP (DBP) were associated with increased mortality whereas the reverse was true for lower than average levels of SBP and DBP. Unfortunately, these findings were ignored by many opinion leaders in the medical community. With the exception of malignant hypertension, high BP was generally thought to be relatively benign and a physiological consequence of aging14,15. As a result, the term “benign essential hypertension” was in common use well into the second half of the 20th century.
In 1957, the Framingham Heart Study (N=5,209) reported a threefold higher incidence of atherosclerotic heart disease in 98 adults with a baseline SBP/DBP ≥160/95 mm Hg compared to 310 with a baseline SBP/DBP <140/90 mm Hg16. The 1959 Build and Blood Pressure Study pooled data from 26 insurance companes to provide actuarial experience for 4,900,000 persons who were insured between 1934 and 195917. This study identified a robust quantitative relationship between BP and mortality, both in men and women, with even small increments in SBP and DBP being associated with higher mortality. Cause of death analyses identified a strong relationship between level of BP and diseases of the heart and circulation. Important subsequent risk investigations have included the 1979 Blood Pressure Study in which 4,350,000 insured men and women were followed for periods in excess of 20 years18, the MRFIT cohort of 361,662 35-57 year old men screened for trial participation whose baseline BP were linked to CVD mortality and end stage renal disease experience19, the Prospective Studies Collaboration study of BP as a risk factor in their meta-analysis of individual data for one million adults in 61 prospective studies20 and the Rapsomaniki et al. electronic health record study of 1.25 million patients in clinical practice21. Collectively, these reports have substantially enhanced and refined our knowledge of the BP-CVD relationship but there was already little doubt as to the importance of BP as a risk factor for CVD by the 1960s. The 1979 Blood Pressure Study18 and many subsequent cohort study reports, especially the large MRFIT cohort investigation19,22, provided convincing evidence that SBP was more important than DBP as a predictor of risk, especially in older adults. Despite this, a misconception which seems to have originated in 192623, led to a widely held belief that high SBP reflected a strong heart whereas high DBP resulted from raised peripheral resistance and should be the focus of clinical attention. This type of thinking influenced the design of antihypertensive drug treatment trials well into the 1980s.
Treatment of High Blood Pressure
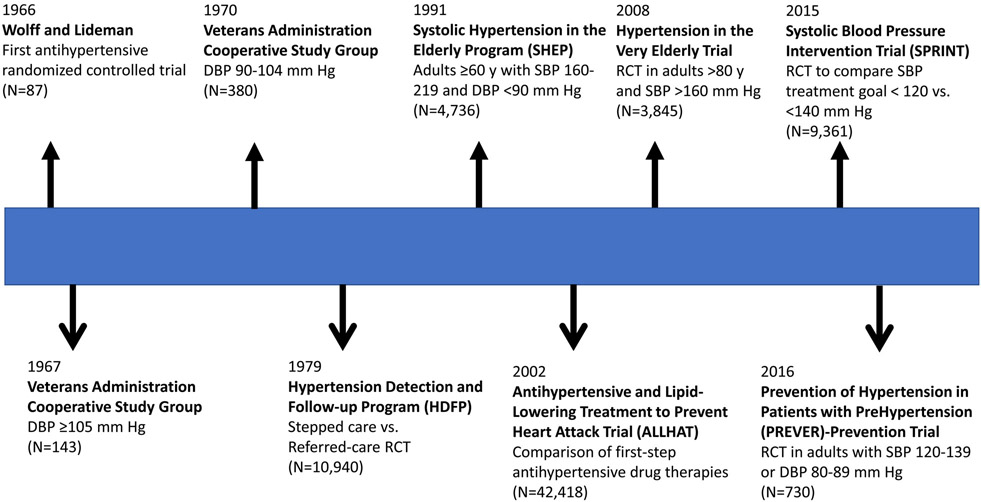
The era of effective antihypertensive drug therapy can be traced to the introduction of diuretics during the latter part of the 1950s. In the early 1960s, several historically controlled trials of antihypertensive drug therapy provided compelling evidence for benefit in adults with malignant hypertension or very high levels of BP. These studies were superseded by randomized controlled trials (RCTs), starting in the late 1960s. Figure 2 provides a timeline for some of the most important RCTs that have documented the value of antihypertensive drug therapy in adults with high BP. The first RCT of antihypertensive drug therapy was reported in 196624 but it was quickly followed by two landmark Veterans Administration Cooperative Study Group multicenter trials that provided convincing evidence for treatment benefits in adults with a baseline DBP ≥105 mm Hg in 196725 and in those with a baseline DBP 90-104 mm Hg in 197026. Subsequently, larger trials provided evidence of benefit for stepped-care compared to referred-care (usual) antihypertensive drug therapy27, and this treatment approach was widely recommended for many years. Until the late 1980s, RCTs were largely based on treatment of diastolic hypertension and DBP goals. Around this time, several RCTs were designed to test the value of antihypertensive drug therapy in older adults with high SBPs28-30. The Systolic Hypertension in the Elderly Program (SHEP) trial was the first to prove the value of treating well defined isolated systolic hypertension in adults ≥60 years28. Later trials conducted in Europe and China, also confirmed the value of drug treatment in older adults with a combination of high SBPs and low DBPs31,32. The 2008 Hypertension in the Very Elderly Trial (HYVET) established the value of BP lowering even in those 80 years of age or older33. More recently, the Systolic Blood Pressure Intervention Trial (SPRINT) demonstrated benefit with more intensive therapy to mm <120 Hg compared to a goal of 140 mm Hg34. The benefit seemed to apply to all prespecified subgroups, including those ≥75 years at baseline35. In addition to the primary CVD composite outcome benefit, more intensive therapy significantly reduced mild cognitive impairment and the combination of mild cognitive impairment and dementia36,37. In the best designed comparison of first-step antihypertensive drug therapy, the Antihypertensive and Lipid-Lowering to Treat Heart Attack Trial (ALLHAT) investigators demonstrated the superiority of a long-acting diuretic (chlorthalidone) compared to an α-receptor blocker (doxazosin)38. First-step therapy with chlorthalidone, the calcium channel blocker amlodipine, or the angiotensin converting enzyme inhibitor lisinopril resulted in a similar incidence of the primary coronary heart disease outcome and all-cause mortality but decompensated heart failure was less common with chlorthalidone, especially compared to amlodipine39. Meta-analyses of the many trials of antihypertensive drug therapy RCTs provide the scientific underpinning for pharmacological treatment of hypertension in adults40-43. All of the trials included in these meta-analyses were conducted in adults at high risk for CVD. The effectiveness of antihypertensive drug therapy for prevention of CVD events has not been proven in those at lower risk for CVD44 but low-dose pharmacotherapy has been demonstrated to be effective in preventing hypertension45-47 and reducing left ventricular mass in adults with high normal BP47.
Figure 2:
Timeline for selected advances in knowledge related to antihypertensive drug treatment of high blood pressure
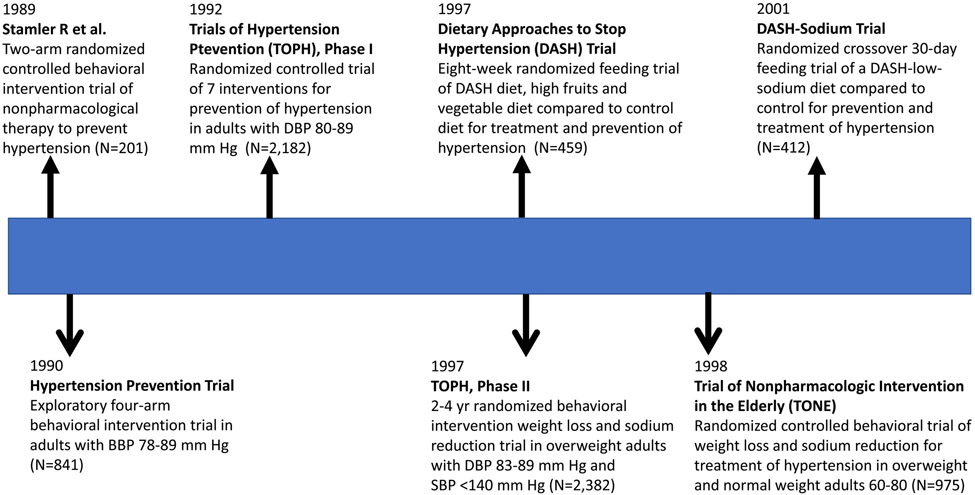
Figure 3 provides a temporal sequence for some of the more important RCTs that have confirmed the value of nonpharmacological therapy for prevention and treatment of high BP. In 1989, Stamler et al. demonstrated in 201 adults that a “nutritional-hygienic” behavioral intervention targeting weigh, dietary sodium, physical activity and alcohol consumption was effective for prevention of hypertension48. The following year, many of the same investigators reported a larger (N=841) 4-arm trial that suggested weight loss and sodium reduction as the most effective interventions for prevention of hypertension49. In a larger test of seven behavioral and supplement interventions in 2,182 adults, Phase I of The Trials of Hypertension Prevention (TOHP) identified weight loss and sodium reduction as the two most effective approaches for prevention of hypertension50. TOHP, Phase II confirmed the effectiveness of weight loss and sodium reduction during 3-4 years of follow-up in 2,182 overweight adults but also demonstrated the difficulty of maintaining behavioral intervention effects during long-term follow-up51. A three-arm eight-week feeding study demonstrated the value of the Dietary Approaches to Stop Hypertension (DASH) diet (high in fruits and vegetables and in low-fat dairy products) for BP lowering in 459 adults with and without hypertension52. In a subsequent crossover RCT conducted in 412 adults with and without hypertension, sodium reduction in combination with either the DASH or control diet was shown to effective in lowering BP, with the greatest reduction in BP being noted in those assigned to the DASH and the lowest level of sodium intake (1,500 mg/day)53. The Trial of Nonpharmacologic Interventions in the Elderly (TONE) demonstrated the value of weight loss and sodium reduction, especially when combined, for BP lowering in 975 adults 60-80 years with hypertension already “controlled” on a single antihypertensive medication (mean SBP = 127.5 in the usual care group) and the capacity to maintain an acceptable level of BP following withdrawal of their BP lowering medication, particularly in those who maintained their behavioral intervention54. Long-term post-trial follow-up of the TOHP I and II participants has suggested sodium reduction is effective in preventing CVD and mortality fram all-causes in addition to lowering BP55-57.
Figure 3:
Timeline for selected advances in knowledge related to nonpharmacological prevention and treatment of high blood pressure
Blood Pressure Clinical Practice Guidelines
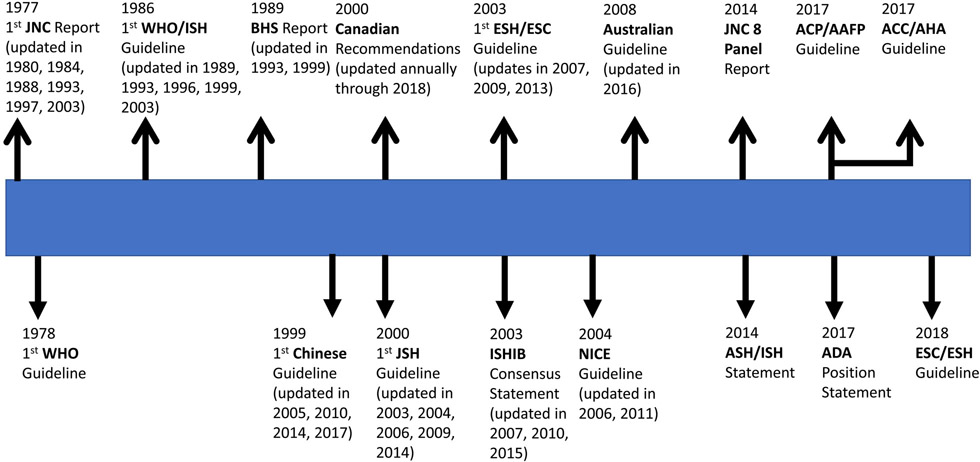
Publication of Framingham Heart Study BP-related CVD risk results and demonstration of the benefits of antihypertensive drug treatment in the two Veterans Administration Cooperative Study Group trials provided the impetus for creation of a National High Blood Pressure Education Program (NHBPEP) at the National Heart, Lung, and Blood Institute (NHLBI) in 1972. Under the auspices of the NHBPEP, a task force was created to identify the prevalence of high BP, determine who would be expected to benefit from antihypertensive therapy, and recommend appropriate therapeutic regimens for BP lowering. The task force issued a report in 1973 and this was followed by a more thorough Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure report (JNC 1) in 197758. The six-page JNC 1 report was a consensus-based document that provided advice on diagnosis of high BP in adults and recommended stepped-care antihypertensive drug therapy in those with a DBP ≥105 mm Hg. The report did not address non-drug interventions and staging of BP was based entirely on DBP. Three JNC updates were published during the 1980s59-61, reflecting the rapid expansion of biomedical research findings detailing BP-related CVD risk and benefits of antihypertensive drug treatment. The 1980 JNC 2 Report recommended antihypertensive drug therapy for adults with a DBP ≥104 mm Hg and possibly for adults with a DBP 90-104 mm Hg who had target organ damage or other evidence of increased CVD risk59. The 1984 JNC 3 Report was the first to include SBP in addition to DBP for diagnosis of hypertension and the first to to emphasize the role of nonpharmacological therapy for treatment of “mild” hypertension (DBP 90-94 mm Hg) and as an adjunct to drug therapy in adults with more severe hypertension. However, initial drug treatment recommendations were restricted to adults with a DBP ≥95 mm Hg, with individualization of decisions in those with a DBP 90-94 mm Hg following nonpharmacological therapy as well as those with isolated systolic hypertension. The goal of treatment was a DBP goal <90 mm Hg due to lack of RCT evidence for a SBP goal60,61. The 1988 JNC 4 Report provided slightly modified treatment recommendations that included stepped-care antihypertensive drug therapy for all adults with an average DBP >94 mm Hg, for those with a DBP of 90-94 mm Hg “despite vigorous attempts with nonpharmacologic approaches”, and for those with isolated systolic hypertension (SBP >160 mm Hg and DBP <90 mm Hg) as well as a treatment goal (SBP/DBP <140/90 mm Hg) that included SBP for the first time61.
In 1989, the U.S. Congress created the Agency for Health Care Policy and Research (AHCPR), now known as the Agency for Healthcare Research and Quality (AHRQ), to focus on outcomes and effectiveness research. AHCPR contracted with the Institute of Medicine (IOM) to provide advice on development and formulation of clinical practice guidelines. In 1990, an IOM Committee to Advise the Public Health Service on Clinical Practice Guidelines issued what would be the first of several reports on the topic, Clinical Practice Guidelines: Directions for a New Program62. The most recent in the series, Clinical Practice Guidelines We can Trust, was published in 201163. Based on the IOM recommendations, progressively more structured and detailed JNC reports were published in 199364, 199765 and 200366. The 1993 JNC 5 expanded the recommendation for nonpharmacological therapy to include prevention of hypertension64, a concept that was published in greater detail in a NHLBI Working Group Report67. Although a SBP <140 mm Hg and DBP <90 mm Hg continued to be identified as the target during drug therapy, JNC 5 recommended consideration of “further reduction to levels of 130/85 mm Hg”, especially in older persons. The 1997 JNC 6 recommended that treatment decisions be influenced by CVD risk stratification, specifically general reliance on “vigorous lifestyle modification” for adults with no CVD risk factors or target organ damage (Group A), consideration of initial combined lifestyle modification and antihypertensive drug therapy for those with at least one CVD risk factor but no diabetes, target organ damage or clinical CVD (Group B), and prompt drug and lifestyle modification not only in adults with hypertension but in those with “high normal BP” (SBP 130-139 mm Hg or DBP 80-89 mm Hg) who manifested target organ damage, clinical CVD or diabetes (Group C)65. In addition, lower BP targets were recommended during treatment of adults with diabetes (SBP/DBP <130/85 mm Hg) and in those with renal disease (SBP/DBP of 125/75 mm Hg for those with heavy proteinuria and 130/85 mm Hg for those with less proteinuria). The final JNC Report (JNC 7), published in 2003, emphasized the importance of SBP in older adults, encouraged use of two or more antihypertensive drugs in most adults with hypertension, consideration of initial therapy with two or more drugs for those with a SBP/DBP >20/10 mm Hg above goal, use of thiazide-type diuretics for first-step drug therapy, and a general SBP/DBP goal of <140/90 mm Hg but <130/80 in those with diabetes or chronic kidney disease66. In March 2008, NHLBI appointed a panel to develop a JNC 8 Report. In 2013, prior to publication of the panel’s report, the NHLBI transferred responsibility for development of CVD prevention clinical practice guidelines (CPGs) to the American College of Cardiology (ACC) and American Heart Association (AHA)68,69. The members appointed to the JNC 8 panel elected to publish a guideline limited to three antihypertensive drug therapy questions in 2014, without endorsement from NHLBI or any professional society70. The most controversial of their recommendations was to employ a SBP treatment threshold of ≥150 mm Hg and a SBP treatment goal of <150 mm Hg during antihypertensive drug therapy in adults ≥60 years. Five members of the panel published a minority view that supported the JNC 7 recommendation for an SBP treatment threshold of 140 mm Hg and BP goal of <140 mm Hg during drug therapy in adults ≥60 years71. During the same year (2014), the ACC, AHA and nine partner professional societies with an interest in BP appointed a 21-member writing committee to develope a comprehensive BP CPG, which was subsequently released in 201772. The writing committee emphasized the importance of accurate BP measurements, averaging of readings within and between visits, use of out-of-office measurements to confirm high office readings and to recognize white coat and masked hypertension. In addition, the committee recommended a new BP classification system that characterized hypertension as an average of correctly measured SBPs ≥130 mm Hg or DBPs ≥80 mm Hg and also recommended quantification of atherosclerotic CVD (ASCVD) risk using the ACC/AHA pooled cohorts equation73 for guidance of treatment decisions. Emphasis was placed on SBP but DBP recommendations were also provided, with the DBP goals being based on expert opinion. Antihypertensive drug therapy, in addition to nonpharmacological therapy, was recommended for the approximately 30% of US adults with stage 1 hypertension (SBP 130-139 mm Hg or DBP 80-89 mm Hg) with a 10-year risk of ASCVD ≥10% and all those with a SBP ≥140 mm Hg or DBP ≥90 mm Hg. A general SBP/DBP treatment goal of <130/80 mm Hg was recommended, with the caveat that the focus should be restricted to SBP (<80 mm Hg) in non-institutionalized ambulatory community-dwelling adults ≥65 years. A variety of other US-based BP guidelines have been published by professional societies such as the International Society of Hypertension in Blacks74-77, the American Society of Hypertension (in conjunction with the International Society of Hypertension)78, the American College of Physicians (ACP)/American Academy of Family Physicians (AAFP), confined to pharmacologic treatment in adults ≥60 years79, and the American Diabetes Association80. In a quantitative analysis, identical antihypertensive treatment recommendations are advocated for a high percentage of US adults with diabetes in the 2017 ACC/AHA CPG and 2017 American Diabetes Association Position Statement81. For example, concordant management recommendations were made for 95.7% of those with a BP above goal during treatment. Likewise, the BP treatment goals in the 2017 ACC/AHA, and the most recent major BP CPGs in Europe, Canada and Australia have all been considerably lower than in previous CPGs. The most aberrant of the current US guidelines is the ACP/AAFP CPG, which provides recommendations for antihypertensive drug therapy in adults ≥60 years79. In contrast to the ACC/AHA CPG focus on functionality and recommendation to employ similar SBP goals (<130 mm Hg) in older compared to younger adults for non-institutionalized community-dwelling patients who tolerate their treatment, the ACP/AAFP guideline recommends initiating treatment when SBP >150 mm Hg and a SBP treatment goal of <150 mm Hg, with consideration of <140 in high risk patients. No guidance is provided regarding BP measurement methods for the ACP/AAFP treatment decisions. As indicated below, the other major English language CPG publications are more in keeping with the 2017 ACC/AHA recommendations than those suggested by the ACP/AAFP.
Outside the U.S., the World Health Organization (WHO) issued a technical report with recommendations for detection and management of hypertension in 197882, followed by a collaboration with the International Society of Hypertension that resulted in progressively more comprehensive CPGs in 198683, 198984 and 1993. A WHO Expert Committee Report was released in 199685, followed by a WHO/ISH statement on management of hypertension in 1999 and a statement that addressed the role of CVD risk for treatment decisions in 200386. The British Hypertension Society published hypertension CPGs in 198987, 199388 and 199989, followed by hypertension recommendations for England and Wales by the National Institute for Health and Clinical Excellence (NICE) in 200490, 200691 and 201192. The European Society of Hypertension (ESH) and European Society of Cardiology (ESC) collaborated to produce their first comprehensive BP CPG in 200393, which was subsequently updated in 200794, 200995 and 201396. In 2018, the same two professional societies published an ESC/ESH hypertension CPG97. Despite some important differences, especially in classification of hypertension, the 2017 ACC/AHA and 2018 ESC/ESH have many common recommendations. These include lower than previous thresholds for initiation of therapy and lower BP targets during drug therapy, as well as a focus on functionality rather than chronological age during treatment in older adults98. The Canadian Hypertension Education Program was established in 1999 as a public-private partnership to develop and implement evidence-based CPGs for diagnosis and management of hypertension in Canadian primary care practices99. Starting with a hypertension CPG in 2000100,101, the recommendations have been comprehensively updated on an annual basis until the present102. The first (1999) hypertension CPG in China was published in 2000103. In subsequent years, a variety of hypertension CPGs have been released by different organizations, with some of the more important being published in 2005104, 2011105, 2015106 and 2017107. The Japanese Society of Hypertension produced its first hypertension CPG in 2001108, followed by updates in 2005109, 2009110, and 2014111, with an update expected in 2019. The Brazilian Society of Hypertension has published seven hypertension CPGs in 1991112, 1994113, 1998114, 2004115, 2007116, 2010117, and 2016118. In Australia, a comprehensive hypertension CPG was published in 2008119 (updated in 2010) and more recently replaced by a CPG published in 2016120. In a manner similar to the ACC/AHA, ESC/ESH and Canadian BP CPGs, the 2016 Australian document recommends a much lower SBP treatment target (<120 mm Hg if tolerated) than in the earlier Australian CPGs. Comprehensive hypertension CPGs have also been developed on a continuing basis by many other professional societies121 and countries122,123.
Strengths and Challenges of Blood Pressure Clinical Practice Guidelines
In most countries, BP-related CPGs are now an established part of clinical and public health practice. CPGs are especially well suited to areas like high BP that are common, result in substantial cost and use of heath resources, demonstrate variation in practice patterns, and have high-quality evidence to support recommendations. One of the challenges in a “mature” area like high BP is that many guidelines with variable rigor and quality are developed. To avoid the potential for a conflict of interest, membership of the 2017 ACC/AHA BP CPG writing committee was restricted to individuals devoid of any relationship with pharmaceutical companies or device manufacturers that have an interest in BP-related products. The review process was used to allow knowledgeable opinion leaders who were ineligible for committee membership to provide input. Although desirable, this approach is not feasible in all countries and may be less critical in the current era when many of the antihypertensive drugs used in clinical practice are generic124. Currently, conflicting recommendations is a more serious issue. It is a natural consequence of CPGs generated by multiple writing committees because they may well interpret the same data in different ways72,79 and choice of CVD risk instruments as well as treatment recommendations are highly influenced by the country/region of focus. Even when the core recommendations are very similar81,98, there is a tendency to look for differences125,126 and suggest that even the experts cannot agree. This is unfortunate because it can lead to confusion and uncertainty among clinicians and the general public. This inevitably results in therapeutic inertia, despite the inadequacy of hypertension control rates in high income countries like the US127 and Canada128, and a dismal rate of hypertension control worldwide129. CPG treatment recommendations are heavily influenced by the results of RCTs and systematic reviews based on meta-analysis of clinical trials. RCTs provide the best evidence for treatment recommendations but interpretation of their results is constrained by study participant selection and the knowledge that landmark trials have been based on efficacy rather than effectiveness studies. Although many BP CPGs are developed, only a small number are based on a comprehensive, rigorous and independent review of the evidence. The 2017 ACC/AHA guideline72, 2018 ESC/ESH guideline97, annually updated Hypertension Canada series102, and 2016 National Heart Foundation of Australia guideline120 are good examples of rigorous, comprehensive BP CPGs that have been developed by independent writing committees.
Many of the most careful BP CPGs have been designed to meet the needs of practitioners in specific countries or regions. Generalizing recommendations in these guidelines to other populations has the advantage that such reports tend to be based on thoughtful interpretation of the available evidence. Advice in some areas, such as BP measurement, has almost universal application. However, other areas such as choice of CVD risk-prediction instruments, antihypertensive treatment decisions and BP goals are highly dependent on availability of valid instruments for measurement of CVD risk, economic conditions, existing systems of health care delivery, accessibility and affordability of antihypertensive medications, and the extent to which high BP, especially severe hypertension, is already being controlled. Most high BP and its complications are now centered in low- and middle-income countries where CVD risk estimating tools are imperfect130, few RCTs have been conducted131, systems of care are often imperfect, and control rates are extremely low even for levels of BP at which combined nonpharmacological and antihypertensive drug therapy are recommended in almost all BP CPGs129,132. In contrast to this dismal situation, control rates have improved progressively in high-income countries129. In the US general population, antihypertensive drug treatment and control to a SBP/DBP <140/90 mm Hg has been slightly in excess of 50 % in recent years127. However, when a focused approach is applied to management of high BP in RCTs34,133 and in systems of care134,135 substantially better control rates have been achieved. In a recent report from the Kaiser Permanente System in Northern California control to a SBP/DBP <140/90 mm Hg was reported in 90% of members receiving drug treatment for hypertension. Such reports from US systems of care have been based on Healthcare Effectiveness Data and Information Set (HEDIS) or similar requirements and are likely subject to some overestimation bias. However, there is little doubt that the level of BP control has improved progressively in settings where there has been a commitment to a comprehensive plan aimed at improved treatment and control. Both the 2017 ACC/AHA and 2018 ESC/ESH CPGs placed considerable emphasis on approaches to improving treatment and control of high BP. Strategies recommended in the ACC/AHA CPG included improved adherence to prescribed therapy, promotion of lifestyle modification, team-based care, active use of electronic health record systems, telehealth initiatives, performance and quality improvement measures, and to a lesser extent financial incentives72. Many of the same strategies recommended to improve hypertension treatment and control in the ACC/AHA document were also identified in a subsequent systematic review and meta-analysis136. RCTs have repeatedly identified team-based care as having a major effect on control rates136,137.
Recognizing the limitations of BP CPGs, they provide useful structed guidance for the clinical and public health communities. Implementation of CPG recommendations is challenging and involves several steps including dissemination of knowledge, commitment on the part of providers and policy-decision makers, and funding agencies to successfully implement all or some of the recommendations. Many clinician survey reports suggest insufficient familiarity with the content of BP CPGs138-140 but these inferences are based on studies with serious methodologic limitations, including low response rates and use of selected practitioner samples. Surveys that have employed better methods and have achieved a relatively high response rate provide more convincing evidence that patients have limited awareness of important knowledge elements in BP CPGs. Whatever the knowledge and attitudes of providers and patients, control rates127,129 support the need for better dissemination and implementation of BP CPGs. Modeling studies suggest this would result in a lower average level of BP and substantial improvement in the health of the public141-143. Fortunately, several large-scale initiatives are being implemented to improve detection and management of hypertension and CVD. In the US, these include the Million Hearts Initiative144,145 sponsored by the Centers for Disease Control and Prevention (CDC) and the the Centers for Medicare and Medicaid Services (CMS) and Target: BP146 sponsored by the American Heart Association and American Medical Association. Globally, major initiatives include the WHO sponsored Global Hearts Initiative147 and the Resolve to Save Lives Cardiovascular Health Initiative148,149 sponsored by private philanthropy. Hopefully, these and other initiatives will result in much improved treatment and control of high BP.
Conclusions
Clinical practice guidelines have evolved to become an important resource for advice on diagnosis and management of high blood pressure. Most of the major blood pressure guidelines provide recommendations that are more similar than different. Modeling studies suggest that better adherence to the advice in blood pressure guidelines would result in a lower average level of blood pressure and a substantial improvement in health status.
Figure 4:
Timeline for selected major blood pressure clinical practice guidelines. JNC = Joint National Committee. WHO = World Health Organization. ISH = International Society of Hypertension. BHS = British Hypertension Society. JSH = Japanese Society of Hypertension. ESH = European Society of Hypertension. ESC = European Society of Cardiology. ISHIB = International Society of Hypertension in Blacks. NICE = National Institute for Care and Excellence. ASH = American Society of Hypertension. ISH = International Society of Hypertension. ACP = American College of Physicians. AAFP = American Academy of Family Physicians. ADA = American Diabetes Association. ACC = American College of Cardiology. AHA = American Heart Association.
Acknowledgments
Funding sources: Centers for Biomedical Research Excellence grant support from the National Institute of General Medical Sciences (P20GM109036).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Swayze VW 2nd. Frontal leukotomy and related psychosurgical procedures in the era before antipsychotics (1935-1954): a historical overview. Am J Psychiatry. 1995;152(4): 505–515. [DOI] [PubMed] [Google Scholar]
- 2.Dewing SB. Radiotherapy of benign disease. Springfield, Illinois: Charles C Thomas; 1965. [Google Scholar]
- 3.Ron E, Lubin JH, Shore RE, et al. Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiat Res. 1995;141(3):259–277. [PubMed] [Google Scholar]
- 4.Cartwright FF. A short history of blood pressure measurement. Proc Roy Soc Med. 1997;70:793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osler W The principles and practice of medicine. New York: D Appleton and Company; 1892. [Google Scholar]
- 6.Riva-Rocci S Un nuovo sfigmomanometro Gazz Medi Torino. 1896;50:981–996. [Google Scholar]
- 7.Crenner CW. Introduction of the blood pressure cuff into U.S. medical practice: technology and skilled practice. Ann Intern Med. 1998;128(6):488–493. [DOI] [PubMed] [Google Scholar]
- 8.Korotkoff NC. To the question of methods of determining the blood pressure. Rep Imp Military Acad. 1905;11:365–367. [Google Scholar]
- 9.Fisher JW. The diagnostic value of the sphygmomanometer in examinations for life insurance. JAMA. 1914;63:1752–1754. [Google Scholar]
- 10.Osler W An Address ON HIGH BLOOD PRESSURE: ITS ASSOCIATIONS, ADVANTAGES, AND DISADVANTAGES: Delivered at the Glasgow Southern Medical Society. Br Med J. 1912;2(2705):1173–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janeway T A clinical study of hypertensive cardiovascular disease. Arch Intern Med. 1913;12:755–798. [Google Scholar]
- 12.Brown AE. The Association of Life Insurance Medical Directors of America 100 years of progress. J Insur Med. 1989;21(3):156–163. [Google Scholar]
- 13.Dwight EW, Rogers OH, Gage H et al. Report of The Joint Committee on Mortality of the Association of Life Insurance Medical Directors and The Actuarial Society of America. Blood pressure study of 1925.: New York: Acruarial society of America and Association of Life Insurance Medical Directors; New York, 1925. [Google Scholar]
- 14.Hay J A British Medical Association Lecture on THE SIGNIFICANCE OF A RAISED BLOOD PRESSURE. Br Med J. 1931;2(3679):43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White PD. Heart Disease. New York: Mac Millan Co.; 1937. [Google Scholar]
- 16.Dawber TR, Moore FE, Mann GV. Coronary heart disease in the Framingham study. Am J Public Health Nations Health. 1957;47(4 Pt 2):4–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Build and Blood Pressure Study, 1959. Vol 1 and 2 Chicago: Society of Actuaries; 1959. [Google Scholar]
- 18.Blood Pressure Study, 1979. Chicago: Society of Actuaries and Association of Life Insurance Medical Directors of America; 1980. [Google Scholar]
- 19.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J. End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA. 1997;277(16):1293–1298. [PubMed] [Google Scholar]
- 20.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies C. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349): 1903–1913. [DOI] [PubMed] [Google Scholar]
- 21.Rapsomaniki E, Timmis A, George J, et al. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet. 2014;383(9932):1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stamler J, Stamler R, Neaton JD. Blood pressure, systolic and diastolic, and cardiovascular risks. US population data. Arch Intern Med. 1993;153(5):598–615. [DOI] [PubMed] [Google Scholar]
- 23.Rolleston H A lecture on high blood pressure from the clinical aspect. The Lancet. 1926;208(5389): 1203–1207. [Google Scholar]
- 24.Wolff FW, Lindeman RD. Effects of treatment in hypertension. Results of a controlled study. J Chronic Dis. 1966;19(3):227–240. [DOI] [PubMed] [Google Scholar]
- 25.Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA. 1967;202(11):1028–1034. [PubMed] [Google Scholar]
- 26.Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA. 1970;213(7):1143–1152. [PubMed] [Google Scholar]
- 27.Five-year findings of the hypertension detection and follow-up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. Hypertension Detection and Follow-up Program Cooperative Group. JAMA. 1979;242(23):2562–2571. [PubMed] [Google Scholar]
- 28.Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991;265(24):3255–3264. [PubMed] [Google Scholar]
- 29.Dahlof B, Lindholm LH, Hansson L, Schersten B, Ekbom T, Wester PO. Morbidity and mortality in the Swedish Trial in Old Patients with Hypertension (STOP-Hypertension). Lancet. 1991;338(8778):1281–1285. [DOI] [PubMed] [Google Scholar]
- 30.Medical Research Council trial of treatment of hypertension in older adults: principal results. MRC Working Party. BMJ. 1992;304(6824):405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Staessen JA, Thijisq L, Fagard R, et al. Effects of immediate versus delayed antihypertensive therapy on outcome in the Systolic Hypertension in Europe Trial. J Hypertens. 2004;22(4):847–857. [DOI] [PubMed] [Google Scholar]
- 32.Liu L, Wang JG, Gong L, Liu G, Staessen JA. Comparison of active treatment and placebo in older Chinese patients with isolated systolic hypertension. Systolic Hypertension in China (Syst-China) Collaborative Group. J Hypertens. 1998;16(12 Pt 1):1823–1829. [DOI] [PubMed] [Google Scholar]
- 33.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358(18):1887–1898. [DOI] [PubMed] [Google Scholar]
- 34.SPRINT Research Group. Wright JT Jr., Williamson JD, Whelton PK, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373(22):2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged >/=75 Years: A Randomized Clinical Trial. JAMA. 2016;315(24):2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yaffe K Prevention of Cognitive Impairment With Intensive Systolic Blood Pressure Control. JAMA. 2019;321:548–549. [DOI] [PubMed] [Google Scholar]
- 37.The SPRINT MIND Investigators for the SPRINT Research Group. Intensive versus Standard Blood Pressure Control, Mild Cognitive Impairment, and Dementia: A Randomized Clinical Trial. JAMA. 2019;321:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antihypertensive, Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research G. Diuretic versus alpha-blocker as first-step antihypertensive therapy: final results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Hypertension. 2003;42(3):239–246. [DOI] [PubMed] [Google Scholar]
- 39.The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981–2997. [DOI] [PubMed] [Google Scholar]
- 40.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ. 2009;338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 3. Effects in patients at different levels of cardiovascular risk--overview and meta-analyses of randomized trials. J Hypertens. 2014;32(12):2305–2314. [DOI] [PubMed] [Google Scholar]
- 42.Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957–967. [DOI] [PubMed] [Google Scholar]
- 43.Bundy JD, Li C, Stuchlik P, et al. Systolic Blood Pressure Reduction and Risk of Cardiovascular Disease and Mortality: A Systematic Review and Network Meta-analysis. JAMA Cardiol. 2017;2(7):775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lonn EM, Bosch J, Lopez-Jaramillo P, et al. Blood-Pressure Lowering in Intermediate-Risk Persons without Cardiovascular Disease. N Engl J Med. 2016;374(21):2009–2020. [DOI] [PubMed] [Google Scholar]
- 45.Julius S, Nesbitt SD, Egan BM, et al. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med. 2006;354(16):1685–1697. [DOI] [PubMed] [Google Scholar]
- 46.Luders S, Schrader J, Berger J, et al. The PHARAO study: prevention of hypertension with the angiotensin-converting enzyme inhibitor ramipril in patients with high-normal blood pressure: a prospective, randomized, controlled prevention trial of the German Hypertension League. J Hypertens. 2008;26(7):1487–1496. [DOI] [PubMed] [Google Scholar]
- 47.Fuchs SC, Poli-de-Figueiredo CE, Figueiredo Neto JA, et al. Effectiveness of Chlorthalidone Plus Amiloride for the Prevention of Hypertension: The PREVER-Prevention Randomized Clinical Trial. J Am Heart Assoc. 2016;5(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stamler R, Stamler J, Gosch FC, et al. Primary prevention of hypertension by nutritional-hygienic means. Final report of a randomized, controlled trial. JAMA. 1989;262(13): 1801–1807. [PubMed] [Google Scholar]
- 49.The Hypertension Prevention Trial: three-year effects of dietary changes on blood pressure. Hypertension Prevention Trial Research Group. Arch Intern Med. 1990;150(1):153–162. [PubMed] [Google Scholar]
- 50.The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels. Results of the Trials of Hypertension Prevention, Phase I. JAMA. 1992;267(9):1213–1220. [DOI] [PubMed] [Google Scholar]
- 51.Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The Trials of Hypertension Prevention, phase II. The Trials of Hypertension Prevention Collaborative Research Group. Arch Intern Med. 1997;157(6):657–667. [PubMed] [Google Scholar]
- 52.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336(16):1117–1124. [DOI] [PubMed] [Google Scholar]
- 53.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344(1):3–10. [DOI] [PubMed] [Google Scholar]
- 54.Whelton PK, Appel LJ, Espeland MA, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA. 1998;279(11):839–846. [DOI] [PubMed] [Google Scholar]
- 55.Cook NR, Cutler JA, Obarzanek E, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ. 2007;334(7599):885–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cook NR, Appel LJ, Whelton PK. Lower levels of sodium intake and reduced cardiovascular risk. Circulation. 2014;129(9):981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cook NR, Appel LJ, Whelton PK. Sodium Intake and All-Cause Mortality Over 20 Years in the Trials of Hypertension Prevention. J Am Coll Cardiol. 2016;68(15):1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. A cooperative study. JAMA. 1977;237(3):255–261. [PubMed] [Google Scholar]
- 59.The 1980 report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1980;140(10):1280–1285. [PubMed] [Google Scholar]
- 60.The 1984 Report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1984;144(5):1045–1057. [PubMed] [Google Scholar]
- 61.The 1988 report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure. Arch Intern Med. 1988;148(5):1023–1038. [PubMed] [Google Scholar]
- 62.Institute of Medicine (US) Committee to Advise the Public Health Service on Clinical Practice Guidelines. Field MJ and Lohr KN eds. Clinical practice guidelines: Directions for a new program. . Washington, DC: IOM (Institute of Medicine);1990. [PubMed] [Google Scholar]
- 63.Committee on Standards for Developing Trustworthy Clinical Practice Guidelines. Board on Health Care Services Graham R, Mancher M, Wolman DM, Greenfield S, and Steinberg E, eds. Clinical practice guidelines we can trust. Washington, D.C: Institute of Medicine of the National Academies of Science. The National Academies Press;2011. [PubMed] [Google Scholar]
- 64.The fifth report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC V). Arch Intern Med. 1993; 153(2): 154–183. [PubMed] [Google Scholar]
- 65.The sixth report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157(21):2413–2446. [DOI] [PubMed] [Google Scholar]
- 66.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. [DOI] [PubMed] [Google Scholar]
- 67.National High Blood Pressure Education Program Working Group report on primary prevention of hypertension. Arch Intern Med. 1993;153(2):186–208. [PubMed] [Google Scholar]
- 68.Gibbons GH, Shurin SB, Mensah GA, Lauer MS. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. J Am Coll Cardiol. 2013;62(15):1396–1398. [DOI] [PubMed] [Google Scholar]
- 69.Gibbons GH, Harold JG, Jessup M, Robertson RM, Oetgen WJ. The next steps in developing clinical practice guidelines for prevention. J Am Coll Cardiol. 2013;62(15):1399–1400. [DOI] [PubMed] [Google Scholar]
- 70.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–520. [DOI] [PubMed] [Google Scholar]
- 71.Wright JT Jr., Fine LJ, Lackland DT, Ogedegbe G, Dennison Himmelfarb CR Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med. 2014;160(7):499–503. [DOI] [PubMed] [Google Scholar]
- 72.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):1269–1324. [DOI] [PubMed] [Google Scholar]
- 73.Goff DC Jr., Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Douglas JG, Bakris GL, Epstein M, et al. Management of high blood pressure in African Americans: consensus statement of the Hypertension in African Americans Working Group of the International Society on Hypertension in Blacks. Arch Intern Med. 2003;163(5):525–541. [DOI] [PubMed] [Google Scholar]
- 75.Williams RA, Flack JM, Gavin JR 3rd, Schneider WR, Hennekens CH Guidelines for management of high-risk African Americans with multiple cardiovascular risk factors: recommendations of an expert consensus panel. Ethn Dis. 2007;17(2):214–220. [PubMed] [Google Scholar]
- 76.Flack JM, Sica DA, Bakris G, et al. Management of high blood pressure in Blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension. 2010;56(5):780–800. [DOI] [PubMed] [Google Scholar]
- 77.Egan BM, Bland VJ, Brown AL, et al. Hypertension in african americans aged 60 to 79 years: statement from the international society of hypertension in blacks. J Clin Hypertens (Greenwich). 2015;17(4):252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich). 2014;16(1):14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qaseem A, Wilt TJ, Rich R, et al. Pharmacologic Treatment of Hypertension in Adults Aged 60 Years or Older to Higher Versus Lower Blood Pressure Targets: A Clinical Practice Guideline From the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med. 2017;166(6):430–437. [DOI] [PubMed] [Google Scholar]
- 80.de Boer IH, Bangalore S, Benetos A, et al. Diabetes and Hypertension: A Position Statement by the American Diabetes Association. Diabetes Care. 2017;40(9): 1273–1284. [DOI] [PubMed] [Google Scholar]
- 81.Muntner P, Whelton PK, Woodward M, Carey RM. A Comparison of the 2017 American College of Cardiology/American Heart Association Blood Pressure Guideline and the 2017 American Diabetes Association Diabetes and Hypertension Position Statement for U.S. Adults With Diabetes. Diabetes Care. 2018; 41(11):2322–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.WHO Expert Committee on Arterial Hypertension & World Health Organization. (1978) Arterial hypertension : report of a WHO expert committeeArterial hypertension. Geneva: World Health Organization;1978. [Google Scholar]
- 83.1986 guidelines for the treatment of mild hypertension: memorandum from a WHO/ISH meeting. J Hypertens. 1986;4(3):383–386. [DOI] [PubMed] [Google Scholar]
- 84.1989 guidelines for the management of mild hypertension: memorandum from a WHO/ISH meeting. Bull World Health Organ. 1989;67(5):493–498. [PMC free article] [PubMed] [Google Scholar]
- 85.Hypertension control. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1996;862:1–83. [PubMed] [Google Scholar]
- 86.Whitworth JA, World Health Organization ISoHWG. 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21(11):1983–1992. [DOI] [PubMed] [Google Scholar]
- 87.Swales JD, Ramsay LE, Coope JR et al. Treating mild hypertension. Report of the British Hypertension Society working party. BMJ. 1989;298(6675):694–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sever P, Beevers G, Bulpitt C, et al. Management guidelines in essential hypertension: report of the second working party of the British Hypertension Society. BMJ. 1993;306(6883):983–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ramsay LE, Williams B, Johnston GD, et al. British Hypertension Society guidelines for hypertension management 1999: summary. BMJ. 1999;319(7210):630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clinical Guideline 18. Hypertension - management of hypertension in adults in primary care. London: National Institute for Health and Care Excellence (NICE);2004. [Google Scholar]
- 91.Hypertension:management of hypertension in adults in primary care. Clinical guideline 34. London: National Institute for Health and Care Excellence (NICE) 2006. [Google Scholar]
- 92.Krause T, Lovibond K, Caulfield M, McCormack T, Williams B, Guideline Development G. Management of hypertension: summary of NICE guidance. BMJ. 2011;343:d4891. [DOI] [PubMed] [Google Scholar]
- 93.European Society of Hypertension-European Society of Cardiology Guidelines C. 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21(6):1011–1053. [DOI] [PubMed] [Google Scholar]
- 94.Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2007;28(12):1462–1536. [DOI] [PubMed] [Google Scholar]
- 95.Mancia G, Laurent S, Agabiti-Rosei E, et al. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens. 2009;27(11):2121–2158. [DOI] [PubMed] [Google Scholar]
- 96.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(28):2159–2219. [DOI] [PubMed] [Google Scholar]
- 97.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. [DOI] [PubMed] [Google Scholar]
- 98.Whelton PK, Williams B. The 2018 European Society of Cardiology/European Society of Hypertension and 2017 American College of Cardiology/American Heart Association Blood Pressure Guidelines: More Similar Than Different. JAMA. 2018;320(17):1749–1750. [DOI] [PubMed] [Google Scholar]
- 99.Zarnke KB, Campbell NR, McAlister FA, Levine M, Canadian Hypertension Recommendations Working G. A novel process for updating recommendations for managing hypertension: rationale and methods. Can J Cardiol. 2000;16(9):1094–1102. [PubMed] [Google Scholar]
- 100.McAlister FA, Levine M, Zarnke KB, et al. The 2000 Canadian recommendations for the management of hypertension: Part one--therapy. Can J Cardiol. 2001;17(5):543–559. [PubMed] [Google Scholar]
- 101.Zarnke KB, Levine M, McAlister FA, et al. The 2000 Canadian recommendations for the management of hypertension: part two--diagnosis and assessment of people with high blood pressure. Can J Cardiol. 2001;17(12):1249–1263. [PubMed] [Google Scholar]
- 102.Nerenberg KA, Zarnke KB, Leung AA, et al. Hypertension Canada's 2018 Guidelines for Diagnosis, Risk Assessment, Prevention, and Treatment of Hypertension in Adults and Children. Can J Cardiol. 2018;34(5):506–525. [DOI] [PubMed] [Google Scholar]
- 103.Liu LS and the Drafting Committee of the 1999 China Guidelines for Prevention and Treatment of Hypertension. 1999 China Guidelines for Prevention and Treatment of Hypertension. Chin J Hypertens. 2000;1:94–102 and 103-112. [Google Scholar]
- 104.Liu LS and Drafting Committee of China Guidelines for Prevention and Traeatment of Hypertension. China Guidelines for Prevention and Treatment of Hypertension. China J Hpertens. 2005;Suppl 134:2–41. [Google Scholar]
- 105.Liu LS and Drafting Committee of the 2010 Guidelines for Prevention and Treatment of Hypertension. 2010 Guidelines for Prevention and Treatment of Hypertension. Chin J Cardiol. 2011;39:579–615. [Google Scholar]
- 106.Guide to Basic Management of Hypertension in China. China Hypertension Grassroots Managenrnt Guide (2014 Revision). China J Hypertens. 2015;23:24–43. [Google Scholar]
- 107.Grassroots Hypertension Management Expert Committee. 2017 Gudelines for the Prevention and Treatment of Hypertension. China Circ J. 2017;32:1041–1048 [Google Scholar]
- 108.Japanese Society of Hypertension Guidelines Subcommittee for the Management of H. Guidelines for the management of hypertension for general practitioners. Hypertens Res. 2001;24(6):613–634. [DOI] [PubMed] [Google Scholar]
- 109.Saruta T The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2004). Nihon Rinsho. 2005;63(6):952–958. [PubMed] [Google Scholar]
- 110.Ogihara T, Kikuchi K, Matsuoka H, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2009). Hypertens Res. 2009;32(1):3–107. [PubMed] [Google Scholar]
- 111.Shimamoto K, Ando K, Fujita T, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2014). Hypertens Res. 2014;37(4):253–390. [DOI] [PubMed] [Google Scholar]
- 112.Consenso Brazileiro de Hipertensao Arterial. Arq Bras Cardiol. 1991;56 Suppl A:A1–16. [PubMed] [Google Scholar]
- 113.Consenso Brazileiro de Hipertensao Arterial. Arq Bras Cardiol. 1994;63(4):333–347. [PubMed] [Google Scholar]
- 114.Consenco Brasileiro de Hipertensao Arterial. Rev Bras Clin Terap. 1998;24:231–272. [Google Scholar]
- 115.Mion D, Machado CA, Gomes MAM et al. Brazilian guidelines in arterial hypertension. DArq Bras Cardiol. 2004;82 Suppl 4:7–22. [PubMed] [Google Scholar]
- 116.Mion D, Kohlmann O, Machado CA. Brazilian Guidelines in Arterial Hypertension. Arq Bras Cardiol. 2007;89(3):e24–79. [PubMed] [Google Scholar]
- 117.Sociedade Brasileira de Cardiologia, Sociedade Brasileira de Hipertensao, Sociedade Brasileira de Nefrologia. Brazilian Guidelines on Hypertension. Arq Bras Cardiol. 2010;95(1 Suppl):1–51. [PubMed] [Google Scholar]
- 118.Malachias MV, Souza WK, Plavnik FL et al. 7th Brazilian Guideline of Arterial Hypertension. Arq Bras Cardiol. 2016;107:1–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.National Heart Foundation of Australia. Guide to management of hypertension - 2008. Melbourne: National Heart Foundation of Australia, 2008. [Google Scholar]
- 120.National Heart Foundation of Australia. Guideline for the diagnosis and management of hypertension in adults- 2016. Melbourne: National Heart Foundation of Australia, 2016. [Google Scholar]
- 121.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int Suppl. 2012;2:337–414. [Google Scholar]
- 122.Chiang CE, Wang TD, Lin TH, et al. The 2017 Focused Update of the Guidelines of the Taiwan Society of Cardiology (TSOC) and the Taiwan Hypertension Society (THS) for the Management of Hypertension. Acta Cardiol Sin. 2017;33(3):213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Association of Physicians of India. Indian guidelines on hypertension. J Assoc Physicians India. 2013;61(2 Suppl):6–36. [PubMed] [Google Scholar]
- 124.Cummings DM, Letter AJ, Howard G, et al. Generic medications and blood pressure control in diabetic hypertensive subjects: results from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Diabetes Care. 2013;36(3):591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.de Boer IH, Bakris G, Cannon CP. Individualizing Blood Pressure Targets for People With Diabetes and Hypertension: Comparing the ADA and the ACC/AHA Recommendations. JAMA. 2018;319(13): 1319–1320. [DOI] [PubMed] [Google Scholar]
- 126.Messerli FH, Bangalore S. The Blood Pressure Landscape: Schism Among Guidelines, Confusion Among Physicians, and Anxiety Among Patients. J Am Coll Cardiol. 2018;72(11):1313–1316. [DOI] [PubMed] [Google Scholar]
- 127.Whelton PK. The elusiveness of population-wide high blood pressure control. Annu Rev Public Health. 2015;36:109–130. [DOI] [PubMed] [Google Scholar]
- 128.McAlister FA, Wilkins K, Joffres M, et al. Changes in the rates of awareness, treatment and control of hypertension in Canada over the past two decades. CMAJ. 2011;183(9): 1007–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mills KT, Bundy JD, Kelly TN, et al. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation. 2016;134(6):441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Whelton PK and Colantonio LD. Cardiovascular disease risk estimation in China. Ann Intern Med. 2019. doi: 10.7326/M18-3301. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Salem K KA. Hypertension in low and middle-income countries: challenges, gaps and limited resources specific strategies. World J Hypertens. 2017;7:19–23. [Google Scholar]
- 132.Chow CK, Teo KK, Rangarajan S, et al. Prevalence, awareness, treatment, and control of hypertension in rural and urban communities in high-, middle-, and low-income countries. JAMA. 2013;310(9):959–968. [DOI] [PubMed] [Google Scholar]
- 133.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981–2997. [DOI] [PubMed] [Google Scholar]
- 134.Fletcher RD, Amdur RL, Kolodner R, et al. Blood pressure control among US veterans: a large multiyear analysis of blood pressure data from the Veterans Administration health data repository. Circulation. 2012;125(20):2462–2468. [DOI] [PubMed] [Google Scholar]
- 135.Jaffe MG, Lee GA, Young JD, Sidney S, Go AS. Improved blood pressure control associated with a large-scale hypertension program. JAMA. 2013;310(7):699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mills KT, Obst KM, Shen W, et al. Comparative Effectiveness of Implementation Strategies for Blood Pressure Control in Hypertensive Patients: A Systematic Review and Meta-analysis. Ann Intern Med. 2018;168(2): 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Carter BL, Rogers M, Daly J, Zheng S, James PA. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med. 2009;169(19):1748–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cuspidi C, Michev I, Meani S, et al. Awareness of hypertension guidelines in primary care: results of a regionwide survey in Italy. J Hum Hypertens. 2003;17(8):541–547. [DOI] [PubMed] [Google Scholar]
- 139.Hagemeister J, Schneider CA, Barabas S, et al. Hypertension guidelines and their limitations--the impact of physicians' compliance as evaluated by guideline awareness. J Hypertens. 2001;19(11): 2079–2086. [DOI] [PubMed] [Google Scholar]
- 140.Hyman DJ, Pavlik VN. Self-reported hypertension treatment practices among primary care physicians: blood pressure thresholds, drug choices, and the role of guidelines and evidence-based medicine. Arch Intern Med. 2000;160(15):2281–2286. [DOI] [PubMed] [Google Scholar]
- 141.Bundy JD, Mills KT, Chen J, Li C, Greenland P, He J. Estimating the Association of the 2017 and 2014 Hypertension Guidelines With Cardiovascular Events and Deaths in US Adults: An Analysis of National Data. JAMA Cardiol. 2018;3(7):572–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Colantonio LD, Booth JN 3rd, Bress AP, et al. 2017 ACC/AHA Blood Pressure Treatment Guideline Recommendations and Cardiovascular Risk. J Am Coll Cardiol. 2018;72(11): 1187–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bress AP CL, Cooper R, Kramer H, Booth JN, Odden MC, Bibbins-Domingo K, Shimbo D, Whelton PK, Levitan EB, Howard G, Bellows BK, Kleindorfer D, Safford MM, Muntner P, Moran AE. Potential cardiovascular disease events prevented with adoption of the 2017 American College of Cardiology/American Heart Association blood pressure guideline. Circulation. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Frieden TR, Berwick DM. The "Million Hearts" initiative--preventing heart attacks and strokes. N Engl J Med. 2011;365(13):e27. [DOI] [PubMed] [Google Scholar]
- 145.Wright JS, Wall HK, Briss PA, Schooley M. Million hearts--where population health and clinical practice intersect. Circ Cardiovasc Qual Outcomes. 2012;5(4):589–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.American Heart Association and American Medical Association. Target: BP. 2019; https://targetbp.org/.
- 147.World Health Organization. Global Hearts Initiative, working together to promote cardiovascular health. 2019; https://www.who.int/cardiovascular_diseases/global-hearts/en/.
- 148.Frieden TR, Bloomberg MR. Saving an additional 100 million lives. Lancet. 2018;391(10121):709–712. [DOI] [PubMed] [Google Scholar]
- 149.Jaffe MG, Frieden TR, Campbell NRC, et al. Recommended treatment protocols to improve management of hypertension globally: A statement by Resolve to Save Lives and the World Hypertension League (WHL). J Clin Hypertens (Greenwich). 2018;20(5):829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]