Abstract
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews (Issue 1, 2010) on 'Vitamin D for the treatment of chronic painful conditions in adults'.
Vitamin D is produced in the skin after exposure to sunlight and can be obtained through food. Vitamin D deficiency has been linked with a range of conditions, including chronic pain. Observational and circumstantial evidence suggests that there may be a role for vitamin D deficiency in the aetiology of chronic painful conditions.
Objectives
To assess the efficacy and safety of vitamin D supplementation in chronic painful conditions when tested against placebo or against active comparators.
Search methods
For this update, we searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and EMBASE to February 2015. This was supplemented by searching the reference lists of retrieved articles, reviews in the field, and online trial registries.
Selection criteria
We included studies if they were randomised double‐blind trials of vitamin D supplementation compared with placebo or with active comparators for the treatment of chronic painful conditions in adults.
Data collection and analysis
Two review authors independently selected the studies for inclusion, assessed methodological quality, and extracted data. We did not undertake pooled analysis due to the heterogeneity of the data. Primary outcomes of interest were pain responder outcomes, and secondary outcomes were treatment group average pain outcomes and adverse events.
Main results
We included six new studies (517 participants) in this review update, bringing the total of included studies to 10 (811 participants). The studies were heterogeneous with regard to study quality, the chronic painful conditions that were investigated, the dose of vitamin D given, co‐interventions, and the outcome measures reported. Only two studies reported responder pain outcomes; the other studies reported treatment group average outcomes only. Overall, there was no consistent pattern that vitamin D treatment was associated with greater efficacy than placebo in any chronic painful condition (low quality evidence). Adverse events and withdrawals were comparatively infrequent, with no consistent difference between vitamin D and placebo (good quality evidence).
Authors' conclusions
The evidence addressing the use of vitamin D for chronic pain now contains more than twice as many studies and participants than were included in the original version of this review. Based on this evidence, a large beneficial effect of vitamin D across different chronic painful conditions is unlikely. Whether vitamin D can have beneficial effects in specific chronic painful conditions needs further investigation.
Plain language summary
Vitamin D for the treatment of chronic painful conditions in adults
Chronic pain is pain of moderate or severe intensity lasting three months or more. It can have a variety of causes, but most comes from musculoskeletal conditions such as arthritis, or pain in muscles. Chronic pain usually affects older people more than younger people. Chronic pain is disabling, and has a large negative impact on quality of life.
Vitamin D has a variety of roles in the body. It is made in the skin through the action of sunlight and can also be obtained from food. A lack of vitamin D has been implicated in a number of conditions, including chronic pain. Additionally, associations of such diverse types of pain as headache, abdominal pain, knee pain, and back pain with season of the year and latitude provide indirect support for a role for vitamin D. The possibility of a link between low levels of vitamin D and chronic pain has attracted interest because ‐ if it were true ‐ vitamin D would be an inexpensive and relatively safe treatment.
We searched scientific databases for studies comparing vitamin D supplementation with placebo (a dummy treatment) or active medicines for the treatment of chronic painful conditions in adults. The evidence is current to February 2015.
There is a small amount of evidence supporting this link but it is not of high quality and may not be reliable. This update of a review sought high quality evidence from randomised controlled trials (studies where participants are randomly allocated to receive one of several treatments) on vitamin D for chronic painful conditions.
We found no consistent pattern that vitamin D treatment was better than placebo for any chronic painful condition, but the studies had methodological shortcomings (low quality evidence).
More research is needed to determine if vitamin D is a useful pain treatment in any particular chronic painful condition. That research should examine whether any effect is restricted to people who are vitamin D deficient. It should also examine how much vitamin D is required, and for how long, before beneficial effects occur.
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews (Issue 1, 2010) on 'Vitamin D for the treatment of chronic painful conditions in adults'.
Vitamin D (calciferol) is a fat‐soluble vitamin that is synthesised from a precursor in the skin after exposure to ultraviolet B (UVB) radiation from the sun, and it is also obtained from dietary sources (eg oily fish, vitamin D‐fortified milk and breakfast cereals, or dietary supplements). Vitamin D can be of plant origin (vitamin D2 or ergocalciferol) or of animal origin (vitamin D3 or cholecalciferol). Vitamin D from the skin and diet is metabolised in the liver to 25‐hydroxyvitamin D, and further metabolised in the kidneys to its active form, 1,25‐dihydroxyvitamin D. Vitamin D exerts its effects by modulating gene expression after binding to a nuclear vitamin D receptor. Most tissues in the human body express vitamin D receptors (Holick 2007), supporting the concept of a wide‐ranging importance of vitamin D.
Vitamin D deficiency can arise from a number of causes including insufficient UVB exposure, decreased bioavailability (eg malabsorption disorders), or diseases that affect the metabolism of vitamin D, such as liver and kidney disease. Some medications can also cause vitamin D deficiency. These include anticonvulsants, glucocorticoids, and antiretroviral drugs (Holick 2007).
Lack of vitamin D is known to be implicated in diseases of the musculoskeletal system, where its deficiency is classically a cause of rickets (in children) and osteomalacia (in adults). Insufficient vitamin D has been implicated in a range of diseases involving other body systems, including metabolic, autoimmune, psychiatric, respiratory, and cardiovascular disorders; cancers (especially colon, prostate, and breast cancer); and chronic pain (Gröber 2013; Holick 2007). It has been suggested that there is widespread vitamin D deficiency or insufficiency in many populations and that sensible sun exposure and vitamin D supplementation may be called for (Gröber 2013; Holick 2007). Indeed, one meta‐analysis found a reduced all‐cause mortality with vitamin D supplementation (Autier 2007). One more recent Cochrane review found that, overall, vitamin D slightly decreased mortality, but further analyses suggested that the benefit in terms of vitamin D supplementation decreasing mortality was more limited; there may be benefit with vitamin D3 supplementation for elderly people (Bjelakovic 2014).
The Institute of Medicine recommends a dietary intake of 600 international units (IU) of vitamin D (800 IU for people older than 70 years) (IOM 2011), other publications recommend higher intakes, at least without adequate sun exposure (Gröber 2013; Holick 2007). One IU corresponds to 25 ng of vitamin D.
The precise definition of vitamin D deficiency is a matter of debate. It has been argued that serum 25‐hydroxyvitamin D levels below 20 ng/mL signify pronounced deficiency and that levels between 21 and 29 ng/mL signify moderate deficiency, or insufficiency (Gröber 2013; Hossein‐nezhad 2013). It has further been suggested that a 25‐hydroxyvitamin D level between 40 and 60 ng/mL is ideal and that toxicity is expected only at levels greater than 150 ng/mL (Gröber 2013). For the purpose of this review, we have regarded levels of vitamin D below 20 ng/mL as evidence of deficiency, levels between 20 and 29 ng/mL as evidence of insufficiency, and levels of 30 ng/mL or greater as evidence of sufficiency.
Description of the condition
Chronic pain is generally described as pain experienced on most days for at least three months. Chronic pain in its various forms is very common, one of the most common health problems of all: one recent review of prevalence studies estimated the average prevalence of non‐cancer chronic pain in adults at about 20% (Moore 2014), with some individual studies claiming substantially higher prevalence rates. The prevalence of benign chronic back pain alone has been estimated to be between 2% and 40%, depending on the population studied (Verhaak 1998). The prevalence of arthritis was estimated at almost 16% in one large multinational study (Alonso 2004). Fibromyalgia is another chronic pain disorder that affects 1% to 6% of the population (Bannwarth 2009; Mas 2008; McQuay 2007; Vincent 2013). Furthermore, acute pain can become chronic. For example, one systematic review showed that chronic pain after surgery was surprisingly common, up to 47% after thoracic surgery (Perkins 2000). Not surprisingly, chronic pain conditions can have a profound effect on functioning and quality of life (Alonso 2004; Mas 2008; Moore 2014), with considerable economic and social impact. Chronic pain is a common cause of sickness absence from work (Moore 2014; Saastamoinen 2009).
Low levels of 25‐hydroxyvitamin D have been linked with a higher incidence of chronic pain (Atherton 2009; Benson 2006; Lotfi 2007). Additionally, associations of such diverse types of pain as headache, abdominal pain, knee pain, and back pain with season of the year and latitude may suggest that 25‐hydroxyvitamin D levels are important in this context (Mitsikostas 1996; Saps 2008; Zeng 2004). Therefore, it seems possible that vitamin D deficiency may be involved in the aetiology of chronic pain.
Description of the intervention
Vitamin D supplementation can be given orally or parenterally. A number of different preparations and dosing regimens of vitamin D treatment have been employed in clinical practice. This review considered all vitamin D preparations and dosing regimens when used in the treatment of chronic pain. Treatment with vitamin D holds great appeal because it is inexpensive; has relatively few, usually mild, adverse effects; and there is a positive public perception of vitamin supplementation that could result in high rates of adherence. Excessive vitamin D intake can result in hypercalcaemia, but this is rare.
How the intervention might work
Vitamin D supplementation increases 25‐hydroxyvitamin D blood levels (25‐hydroxyvitamin D is the form of the vitamin usually measured and recommended to be measured (Holick 2011), with assays normally detecting vitamin D2 and vitamin D3) and can, therefore, potentially correct the effects of vitamin D deficiency (Gröber 2013; Holick 2007). The details of how this might work (molecular mechanism, time to effect, extent of reversibility of pain potentially associated with vitamin D deficiency) are unclear at present.
Why it is important to do this review
At the time that the original version of this Cochrane review was written, no systematic review of vitamin D supplementation in chronic pain had been published in the Cochrane Database of Systematic Reviews. A systematic review of vitamin D treatment for chronic pain was necessary to answer the questions of whether chronic pain could be helped by vitamin D supplementation and, indirectly, whether low levels of 25‐hydroxyvitamin D in people with chronic pain were causal or coincidental.
Our initial review on this topic was published in "Pain". This was based on a MEDLINE (PubMed) search and used evidence from a variety of study architectures (Straube 2009). We felt it was important to develop this further by using a more extensive search strategy and, at the same time, more stringent inclusion criteria to focus on the high quality evidence that is most relevant to clinical practice for publication in The Cochrane Library. The original version of this Cochrane review was published in 2010 (Straube 2010). A number of studies investigating vitamin D for chronic painful conditions have been published since that time, so that an update of our Cochrane review was called for.
Objectives
To assess the efficacy and safety of vitamin D supplementation in chronic painful conditions when tested against placebo or against active comparators.
Methods
Criteria for considering studies for this review
Types of studies
We included studies if they were randomised, double‐blind, controlled trials of vitamin D supplementation compared with placebo or with active interventions in the treatment of chronic painful conditions. Randomisation and double‐blinding are known to minimise bias and were therefore deemed essential study features (Moore 2006; Moore 2010). Parallel‐group design and cross‐over studies were both acceptable.
We excluded the following types of report.
Review articles, case series, case reports, and clinical observations.
Studies of experimentally induced pain.
Studies where pain relief (or pain intensity with baseline level) was not measured or not patient‐reported.
Types of participants
Studies of adults (generally over 15 years of age) with all types of chronic pain conditions were eligible for inclusion. To qualify for inclusion, all study participants needed to have a chronic painful condition. Studies in conditions that were painful in some people but could be asymptomatic in other people (eg osteoporosis) did not qualify for inclusion if they did not explicitly state that all study participants had chronic pain.
Types of interventions
We compared vitamin D supplementation (oral or parenteral) with placebo or active comparator, with no restriction regarding type, dose, and frequency of vitamin D treatment. We included studies that investigated combined treatment with vitamin D and other concurrent interventions only if the other interventions were also given to the comparator group, because we wanted to isolate the treatment effect due to vitamin D.
Types of outcome measures
We collected data on participant characteristics (age, sex, and condition to be treated) and on baseline and end‐of‐trial 25‐hydroxyvitamin D levels where they were reported.
Primary outcomes
The primary outcome was patient‐reported clinically significant pain relief. We used the following hierarchy of outcome measures, in order of preference.
Number of participants with at least 50% pain relief.
Number of participants reporting a global impression of change of "much" or "very much" improvement.
Number of participants with undefined (any) improvement in pain.
Secondary outcomes
Other patient‐rated pain outcomes (typically reported as group mean values).
Numbers of participants with adverse events: any adverse event, any serious adverse event.
Numbers of withdrawals: all‐cause, lack of efficacy, and adverse event withdrawals.
Quality of life (any scale).
Search methods for identification of studies
We identified studies primarily by searching electronic databases, with no language restriction.
Electronic searches
For the original version of this review, the following electronic databases were searched, as far back as they would allow, without specified start dates: Cochrane Central Register of Controlled Trials (CENTRAL; Issue 3, 2009), MEDLINE (to September 2009), EMBASE (to September 2009), and the Oxford Pain Relief Database (Jadad 1996a).
For this update of the review, the trials search co‐ordinator of the Cochrane Pain, Palliative and Supportive Care group and the review authors conducted searches in the databases listed below.
CENTRAL (via CRSO, 2009 to 2 February 2015).
MEDLINE (via Ovid, September 2009 to 2 February 2015).
EMBASE (via Ovid, September 2009 to 2 February 2015).
See Appendix 1 for the CENTRAL search strategy, Appendix 2 for the MEDLINE search strategy, and Appendix 3 for the EMBASE search strategy.
We also searched MEDLINE (via PubMed) in December 2014 using a number of search terms to identify chronic diseases in combination with terms to identify vitamin D treatment, and we searched using the "related citations" feature in PubMed for each of the included studies.
The Oxford Pain Relief Database is no longer being updated and was not searched again for this update.
Searching other resources
We searched additional studies for this update from the reference lists of the retrieved articles and reviews in the field. We searched two online trial registries for trials that have not yet been published or are still ongoing: ClinicalTrial.gov (clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trial Registry Platform (ICTRP) (apps.who.int/trialsearch/). These trial registries were searched on 3 December 2014. We did not contact manufacturers of vitamin D preparations since vitamin D does not have an indication in chronic pain.
Data collection and analysis
Review authors were not blinded to the study authors' names and institutions, journal of publication, or study results at any stage of the review. Two review authors independently selected the studies identified for this review update for inclusion, assessed methodological study quality, and extracted data. We resolved disagreements through discussion.
Selection of studies
We assessed titles and abstracts of publications identified by the searches on screen to eliminate studies that obviously did not satisfy the inclusion criteria. We obtained full publications of the remaining studies to determine inclusion in the review.
Data extraction and management
Two review authors independently extracted data using a standard form.
Assessment of risk of bias in included studies
We used the Oxford Quality Scale score as the basis for inclusion, limiting inclusion to studies that were randomised and double‐blind as a minimum (Jadad 1996b).
We also assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011); we assessed the following for each study.
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, eg random number table; computer random number generator); unclear risk of bias (method used to generate sequence was not clearly stated). We excluded studies using a non‐random process that were therefore at high risk of bias (eg odd or even date of birth; hospital or clinic record number).
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions before assignment determines whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (eg telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method was not clearly stated). We excluded studies that did not conceal allocation and were therefore at high risk of bias (eg open list).
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study stated that it was blinded and described the method used to achieve blinding, eg identical tubes containing gel, or identical plasters; matched in appearance and smell); unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how blinding was achieved). We excluded studies that were not double‐blind and therefore at high risk of bias.
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk of bias (less than 10% of participants did not complete the study or used 'baseline observation carried forward' analysis, or both); unclear risk of bias (used 'last observation carried forward' analysis and 10% of participants or more did not complete the study); or high risk of bias (used 'completer' analysis and 10% of participants or more did not complete the study).
Size (checking for possible biases confounded by small size). Small studies overestimate treatment effects, probably due to methodological weaknesses (Dechartres 2013; Nüesch 2010). We assessed studies as at low risk of bias if they had at least 200 participants in each treatment arm, at unclear risk if they had 50 to 199 participants, and at high risk if they had fewer than 50 participants.
Measures of treatment effect
We planned to estimate treatment effect for dichotomous outcomes by calculating risk ratio (RR) to establish statistical difference, and numbers needed to treat to benefit (NNT) and pooled percentages as absolute measures of benefit or harm (Cook 1995; Morris 1995). We did not plan to pool continuous outcome data because response to treatment does not usually follow a normal (Gaussian) distribution.
However, because of clinical and methodological heterogeneity between the studies, we did not undertake any pooled analysis.
Unit of analysis issues
Randomisation was to the individual participant.
Dealing with missing data
Analysis was conducted according to the intention‐to‐treat (ITT) principle. Missing data and imputation methods were incorporated in the risk of bias assessment.
Assessment of heterogeneity
We planned to examine the heterogeneity of studies with regard to intervention effects visually using L'Abbé plots (L'Abbé 1987). However, we performed no pooled analysis.
Assessment of reporting biases
We did not formally assess reporting biases.
Data synthesis
We planned to pool data where the same or comparable measures were used. We did not carry out any data synthesis because of substantial clinical and methodological heterogeneity in the included studies.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses for different painful conditions, but we performed no pooled analysis.
Sensitivity analysis
We planned sensitivity analyses for different treatment regimens and for participants with different baseline levels of 25‐hydroxyvitamin D (the effects of vitamin D treatment might differ in people who are deficient or insufficient in 25‐hydroxyvitamin D versus people who have sufficient levels), but we performed no pooled analysis.
Results
Description of studies
Results of the search
The electronic database searches in CENTRAL (Appendix 1), MEDLINE (Appendix 2), and EMBASE (Appendix 3) conducted for this update identified 792 hits after de‐duplication, of which 769 were excluded based on titles and abstracts. We examined 23 articles fully, and included four studies (McAlindon 2013; Sanghi 2013a; Schreuder 2012; Wepner 2014). Other searches identified four studies to be examined as full texts, of which we included two (Hansen 2014; Salesi 2012) (Figure 1).
1.
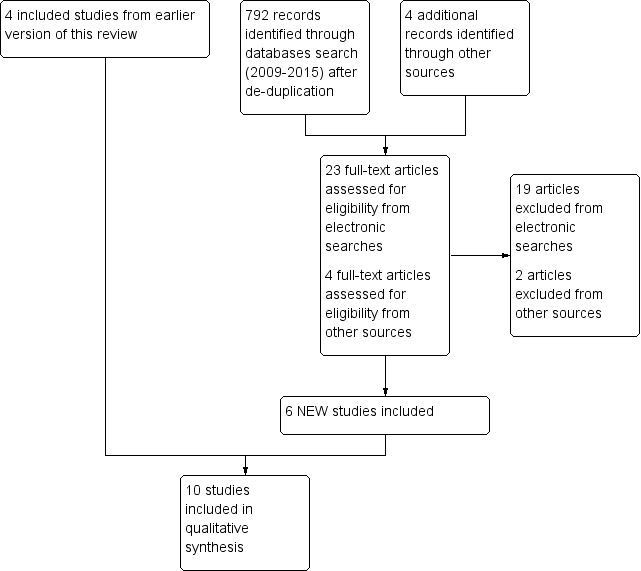
Study flow diagram. Six NEW studies
In addition, we found 12 studies ongoing (ACTRN12610000322033; Cao 2012; CTRI/2014/06/004658; EUCTR 2009‐015835‐34‐IT; EUCTR 2014‐000047‐33‐NL; ISRCTN94818153; NCT00279461; NCT01023490; NCT01351805; NCT01426347; NCT02002000; NCT02243800). Details are in the Characteristics of ongoing studies table. We were unable to determine exactly how many participants were enrolled in these studies.
Included studies
For this update, we included six new studies with 517 participants (Hansen 2014; McAlindon 2013; Salesi 2012; Sanghi 2013a; Schreuder 2012; Wepner 2014), and four studies with 294 participants from the earlier review (Brohult 1973; Di Munno 1989; Warner 2008; Yamauchi 1989).
The 10 included studies assessed people with rheumatoid arthritis (Brohult 1973; Hansen 2014; Salesi 2012; Yamauchi 1989), knee osteoarthritis (McAlindon 2013; Sanghi 2013a), polymyalgia rheumatica (Di Munno 1989), 'non‐specific' musculoskeletal pain (Schreuder 2012), 'diffuse' musculoskeletal pain (Warner 2008), and fibromyalgia (Wepner 2014). The mean age ranged from 42 to 68 years, and most studies included more women than men (range of women 41% to 100%).
Vitamin D preparation, dose, and duration varied considerably between the studies. Yamauchi 1989 used 1‐hydroxycholecalciferol, Di Munno 1989 and Salesi 2012 used 25‐hydroxyvitamin D, the other studies used calciferol preparations. Wepner 2014 gave some people in the active treatment group as little as 1200 IU of calciferol daily for 25 weeks, Brohult 1973 gave 100,000 IU of calciferol daily for one year. McAlindon 2013 used dose adjustments to keep vitamin D levels in a target range and treated over two years. Schreuder 2012 gave a single dose of 150,000 IU vitamin D.
Not all studies reported concentrations of vitamin D or metabolites in blood at the start and end of the treatment period. For those studies that did, results for placebo and treatment are in Appendix 4, and in Figure 2 and Figure 3. This shows that most studies demonstrated major change with treatment but not placebo, but only two had initial serum 25‐hydroxyvitamin D levels below 20 ng/mL signifying pronounced deficiency (Schreuder 2012; Warner 2008), one had initial levels of about 40 ng/mL (Salesi 2012), and that others had levels in the insufficiency range (Hansen 2014; McAlindon 2013; Wepner 2014). Weighted mean levels with placebo rose by 3 ng/mL and with active treatment by 14 ng/mL.
2.
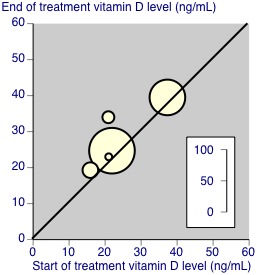
Vitamin D concentrations at start and end of treatment with placebo. The size of the symbols is proportional to the number of participants (inset scale).
3.
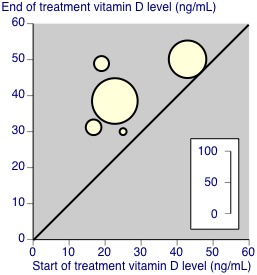
Vitamin D concentrations at start and end of treatment with vitamin D supplementation (active treatment). The size of the symbols is proportional to the number of participants (inset scale).
Full details are in the Characteristics of included studies table.
Excluded studies
We excluded 21 studies from records identified in searching for this update; 11 studies were excluded in the original review (Abou‐Raya 2014; Arden 2006; Arden 2014; Björkman 2008; Chlebowski 2013; de Nijs 2006; Ding 2013; Dottori 1982; Felson 2013; Gendelman 2014; Grove 1981; Kessenich 2010; Knutsen 2014; Kragstrup 2011; Krocker 2008; Lyritis 1994; Mascitelli 2012; McAlindon 2010; Osunkwo 2012a; Osunkwo 2012b; Osunkwo 2012c; Ringe 2000; Ringe 2004; Ringe 2005; Ringe 2007; Sakalli 2012; Sanghi 2011; Sanghi 2012; Sanghi 2013b; Sanghi 2014; Wicherts 2011; Wynn 2013). Exclusions were largely because studies were reported as short conference abstracts rather than full publications, were not double‐blind randomised controlled trials, or because it was not clear that all participants had a chronic painful condition. Full details are in the Characteristics of excluded studies table.
Risk of bias in included studies
Five studies scored the maximum of 5/5 on the Oxford Quality Scale (McAlindon 2013; Sanghi 2013a; Schreuder 2012; Warner 2008; Wepner 2014), one scored 4/5 (Hansen 2014), three scored 3/5 (Brohult 1973; Salesi 2012; Yamauchi 1989), and one scored 2/5 (Di Munno 1989). We have reported individual scores in the Characteristics of included studies table. Points were lost mainly due to failure to describe methods of randomisation and blinding adequately.
We assessed the risk of bias in included studies using the 'Risk of bias' tool, and have summarised the findings below and in Figure 4. Details for each study are in the Characteristics of included studies table.
4.
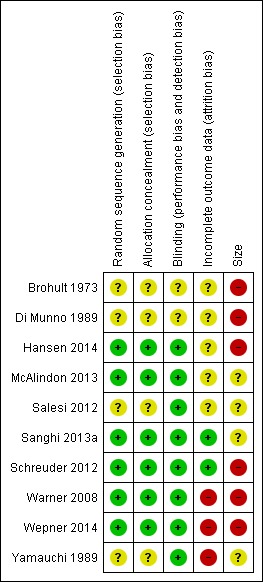
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
All the studies stated that they were randomised, but four did not provide adequate descriptions of the methods used to generate the random sequence or conceal the random allocation (Brohult 1973; Di Munno 1989; Salesi 2012; Yamauchi 1989).
Blinding
All the studies blinded participants and study personal, but two did not provide adequate descriptions of the methods used to blind the interventions (Brohult 1973; Di Munno 1989).
Incomplete outcome data
Warner 2008, Wepner 2014, and Yamauchi 1989 were at high risk of bias due to incomplete outcome data. The other studies were at unclear or low risk.
Selective reporting
While there was no clear evidence of selective reporting, only six of the 10 studies reported on vitamin D levels at the beginning and end of the trial (Hansen 2014; McAlindon 2013; Salesi 2012; Sanghi 2013a; Warner 2008; Wepner 2014). The others did not report either time point (Brohult 1973; Di Munno 1989; Yamauchi 1989), or only baseline vitamin D levels (Schreuder 2012).
Other potential sources of bias
None of the included studies enrolled 200 or more participants per treatment arm, which we consider is the minimum required to give confidence in the results. Six studies enrolled fewer than 50 participants in each treatment arm and we judged them to be at high risk of bias (Brohult 1973; Di Munno 1989; Hansen 2014; Schreuder 2012; Warner 2008; Wepner 2014). The remaining four studies enrolled between 50 and 199 participants per treatment arm and we judged them to be at unclear risk of bias (McAlindon 2013; Salesi 2012; Sanghi 2013a; Yamauchi 1989).
Effects of interventions
Because the studies were heterogeneous with regard to the included participants, interventions (amount and schedule of administration of vitamin D, co‐interventions), duration, and outcomes reported, we did not undertake a pooled analysis. Below, we discussed the studies by type of outcomes (efficacy or safety) and by the conditions treated.
Efficacy outcomes
Appendix 4 summarises the efficacy outcomes in individual studies.
Rheumatoid arthritis
Four studies investigated participants with rheumatoid arthritis (Brohult 1973; Hansen 2014; Salesi 2012; Yamauchi 1989).
In a trial of calciferol 100,000 IU daily conducted over 12 months, Brohult 1973 showed that the consumption of analgesics and anti‐inflammatory drugs decreased significantly in the calciferol group while there was no change in the placebo group (no direct between groups comparison was reported). Hansen 2014 investigated ergocalciferal 50,000 IU plus calcium initially three times weekly and then twice monthly for a total duration of 12 months, finding a worse outcome with vitamin D than with placebo (just calcium) in one pain comparison; the Patient Assessment of Global Health was also worse with vitamin D. Salesi 2012 gave participants vitamin D 50,000 IU weekly plus methotrexate or methotrexate alone for a duration of 12 weeks. There was no difference between the groups in visual analogue scale pain scores. None of these three studies investigated our preferred responder pain outcomes. One trial of alfacalcidol (1‐hydroxycholecalciferol) over 16 weeks found that similar proportions of participants reported "very much" or "much" improvement in the Patient Global Impression of Change in the two treatment groups (alfacalcidol 1 to 2 μg) and in the placebo group (Yamauchi 1989).
Overall, the studies in rheumatoid arthritis did not show a consistent direction of effect of vitamin D. Hansen 2014 and Salesi 2012 reported baseline and end‐of‐trial vitamin D levels, finding greater increases in vitamin D levels with vitamin D than placebo, but the increases were small to moderate on average. Only in Hansen 2014 did participants have vitamin D levels in the insufficiency range at the start of the trial and reach sufficiency (30 ng/mL) with vitamin D treatment. In Salesi 2012, the participants were already vitamin D sufficient at the start of the treatment.
Knee osteoarthritis
Two studies investigated vitamin D for knee osteoarthritis (McAlindon 2013; Sanghi 2013a).
McAlindon 2013 initially administered cholecalciferol 2000 IU daily with later dose adjustments, or placebo. The active treatment moved participants from vitamin D insufficiency at the beginning of the trial well into the sufficiency range at trial end, after two years. Pain responder outcomes were not reported. Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain and function score changes were not significantly different between the groups. Sanghi 2013a administered vitamin D 60,000 IU daily for 10 days followed by the same dose given once a month, or placebo, for 12 months. This moved participants in the active treatment group from vitamin D deficiency at trial beginning to sufficiency at trial end. Although Sanghi 2013a did not report any responder pain outcomes, the comparison of average visual analogue pain scale changes revealed a significant difference in favour of the vitamin D treatment. WOMAC pain score changes and total score changes also indicated superiority of vitamin D over placebo in that study.
Other painful conditions
One trial of 25‐hydroxyvitamin D (35 μg daily for 25 days a month) for nine months in people with polymyalgia rheumatica found that subjective pain on movement decreased in both vitamin D and placebo groups over time, with little difference between the groups (no statistics for between‐group comparisons reported) (Di Munno 1989).
Schreuder 2012 investigated non‐specific musculoskeletal pain in non‐Western immigrants who were vitamin D deficient at the onset of the study, and used a one‐time treatment of vitamin D 150,000 IU or placebo. After six weeks, the combined outcome of "much less pain" and "less pain" revealed a significant difference in favour of vitamin D. End‐of‐trial vitamin D levels were not reported.
One study of ergocalciferol 50,000 IU for the treatment of diffuse musculoskeletal pain over three months investigated pain with a visual analogue scale (finding no significant difference between vitamin D and placebo groups) and with a functional pain score (with an outcome that slightly favoured placebo in one comparison) (Warner 2008) (Appendix 4). In Warner 2008, participants were, on average, vitamin D deficient at trial onset and at the lower end of the sufficiency range at trial end in the active treatment group. Although vitamin D levels also increased in the placebo group, people in the placebo group on average remained in the deficiency range at trial end.
Wepner 2014 treated participants with fibromyalgia with cholecalciferol 1200 to 2400 IU daily (dose depending on vitamin D levels) or placebo. Both active and placebo groups were on the borderline of the vitamin D deficiency and insufficiency ranges (around 20 ng/mL), and both groups were in the sufficiency range after 25 weeks of treatment. Although responder pain outcomes were not reported, some other pain and quality of life outcomes suggested benefit with vitamin D.
Safety outcomes
Appendix 5 summarises the adverse event and withdrawal outcomes in the individual studies.
Overall, adverse events were infrequent and, where reported, the numbers of participants with any adverse event or with any serious adverse events did not seem to differ systematically between vitamin D and placebo groups. Salesi 2012 found 7/60 participants had adverse events with methotrexate plus vitamin D versus 3/57 with methotrexate alone.
All‐cause withdrawals were reported in all but two studies, with no consistent difference between vitamin D and placebo. Lack of efficacy withdrawals and adverse event withdrawals, when reported, also did not show a consistent difference between active treatment and placebo.
Wepner 2014 reported that one person receiving vitamin D had mild hypercalcaemia (2.71 mmol/L); the study medication was interrupted and the serum calcium level returned to the normal range. Brohult 1973 reported that after 10 months of treatment, one participant in the calciferol group had a serum calcium level (3.5 mmol/L) that exceeded the upper limit of the normal range, and that two participants in the calciferol group reported polydipsia and increased frequency of urination on one occasion (these can be symptoms of hypercalcaemia, but they are not specific for it). None of the other trials reported cases with hypercalcaemia as an adverse event.
Discussion
Summary of main results
This updated review found no convincing beneficial effect of vitamin D supplementation on chronic pain. The review has more information than a previous version of the review that reached similar conclusions.
This result has to be interpreted with caution. A major concern must be whether the studies were sufficiently sensitive to detect a difference with treatment. Only one study demonstrated unequivocal vitamin D deficiency before treatment, with 25‐hydroxyvitamin D levels below 10 ng/mL (Schreuder 2012). Other studies had initial values generally of 20 ng/mL or higher. The effects of vitamin D supplementation on 25‐hydroxyvitamin D levels was modest; although the five studies reporting end‐of‐study levels reported average 25‐hydroxyvitamin D levels above 30 ng/mL, two were borderline, and the weighted mean increase of 14 ng/mL was only a 50% increase on the initial level of 28 ng/mL. Some trial participants were vitamin D sufficient at the onset of the trial (Salesi 2012), thus, a vitamin D effect might be difficult to demonstrate in any case. Therefore, there would appear to be major questions as to whether the studies had designs able to sensitively demonstrate effects of vitamin D on pain.
Another reason why results have to be interpreted with caution is that responder outcomes of clinically useful benefit were reported only infrequently (Dworkin 2008; Moore 2010). Relying on group mean values for pain intensity or pain relief is problematic because the underlying distributions of individual data are often skewed, and using average data from such skewed distributions can produce misleading results (McQuay 1996; Moore 2013). This is why dichotomous responder analysis has been suggested to produce more useful results in clinical practice both in the acute (Moore 1998), and chronic (Moore 2008), pain settings.
With these caveats in mind, we found that there was no consistent effect of vitamin D on pain in rheumatoid arthritis. In knee osteoarthritis, one (smaller and shorter) study reported significantly better outcomes with vitamin D (Sanghi 2013a), while another (larger and longer) study found no difference between the groups (McAlindon 2013). With other painful conditions there is some suggestion of benefit in fibromyalgia (Warner 2008), and non‐specific musculoskeletal pain in non‐Western immigrants (Schreuder 2012), while there was no evidence of superiority of vitamin D over placebo in one study on polymyalgia rheumatica (Di Munno 1989). One study on diffuse musculoskeletal pain even reported benefit with placebo in one outcome (Warner 2008). Overall, there is no consistent pattern that vitamin D treatment was associated with greater efficacy than placebo in chronic painful conditions. Adverse events and withdrawals were comparatively infrequent, also with no consistent difference between vitamin D and placebo.
Overall completeness and applicability of evidence
A number of potentially eligible studies had methodological limitations and had to be excluded from this review. Other potentially eligible studies were identified by searching trial registries but were not (yet) published and did not have data available for analysis. The 10 studies included in this review were conducted at different times; in different locations; treating a number of painful conditions in participants with vitamin D levels in the deficiency, insufficiency, and sufficiency ranges with different vitamin D doses and schedules of administration over different periods of time; and assessing different outcomes. This heterogeneity meant that no meaningful pooled analysis could be undertaken.
Because of these limitations and the infrequent use of the responder pain outcomes now thought to be most informative of clinical benefit, the evidence has to be judged to be incomplete. Based on the included studies, there is no clearly demonstrable benefit associated with vitamin D in chronic pain in adults. It can be argued that if substantial benefit was present across different chronic painful conditions, it would have become apparent; however, this was not the case.
Quality of the evidence
Though the included studies were largely assessed to be at low or unclear risk of bias, the relative paucity of trials and participants in high quality studies, together with the inadequacy of outcome measures used, implies that the evidence base regarding the use of vitamin D in chronic painful conditions is weak.
Potential biases in the review process
Because of adherence to quality criteria and an extensive search strategy, biases in the review process were minimised.
Agreements and disagreements with other studies or reviews
This review agrees with a previous review on the subject in that there is little high quality evidence for an effect of vitamin D in chronic pain, despite the existence of a number of studies of lower methodological quality (not double‐blind randomised controlled trials) that suggest an effect (Straube 2009). As we have previously stated, there is a 'beguiling attraction' in a link between low 25‐hydroxyvitamin D levels and chronic pain (Straube 2009). If effective, vitamin D would be a simple and inexpensive treatment with limited adverse effects. However, good evidence for this effectiveness is lacking.
There are a number of examples where early observational studies and treatment studies of lower methodological quality appear to demonstrate effects that are not subsequently confirmed by high quality trials. For example, the use of hyperbaric oxygen in multiple sclerosis appeared beneficial based on observational and non‐randomised studies, but subsequent randomised controlled trails found no benefit (Bennett 2001). Likewise, for the use of transcutaneous electrical nerve stimulation for postoperative pain, non‐randomised trials were largely positive while randomised trials were largely negative (Carroll 1996). Our previous review on vitamin D for chronic pain came to a similar conclusion when comparing high and low quality treatment studies (Straube 2009). This present review, updated to include 10 studies, relied on a wider search and more strict inclusion criteria than our previous review (Straube 2009), but also did not identify consistent evidence of an effect of vitamin D.
Authors' conclusions
Implications for practice.
For people with chronic pain
The evidence base did not indicate that vitamin D supplementation for chronic painful conditions in adults is beneficial in terms of bringing about pain relief, though the evidence is insufficient to reach a definitive conclusion for clinical practice.
For clinicians
The evidence base did not indicate that vitamin D supplementation for chronic painful conditions in adults is beneficial in terms of bringing about pain relief. Frank vitamin D deficiency or insufficiency may dictate vitamin D treatment, but pain intensity reduction should not be expected.
For policy makers
There is no evidence that vitamin D supplementation reduces pain in people with chronic pain.
For funders
Treating chronic pain with vitamin D is unlikely to have any benefits, or be cost‐effective.
Implications for research.
General
Even though this Cochrane review update now includes more than twice as many studies and participants as the original review (Straube 2010), there is still a need for more work in this area. A number of studies that have been identified by searching online trial registries seem to be ongoing, so that there is the possibility of having more data available to assess the effect of vitamin D in chronic pain in the future. Even though the present evidence does not suggest a beneficial effect, such an effect of vitamin D on chronic pain is theoretically possible, even in the absence of a clearly identifiable mode of action, because of the wide range of effects of vitamin D (Holick 2007), and the many molecular and neural mechanisms involved in the pathogenesis of various chronic pain conditions. While there is little evidence from double‐blind randomised controlled trials, other study types do suggest that there may be a link (Straube 2009). Because vitamin D is inexpensive and relatively safe, a use in chronic pain could be advocated even if the benefit was of modest size.
Design
Large, double‐blind, randomised controlled trials in a variety of chronic pain conditions conducted over long enough periods of time with multiple assessments to capture short, medium, and long term effects are called for. To provide the highest quality evidence, these trials need to be stratified by baseline 25‐hydroxyvitamin D levels, with defined treatments, with clinically relevant pain outcomes (such as the primary outcomes that this review looked for), and ideally with outcomes analysed by post‐treatment 25‐hydroxyvitamin D level. Because it is unclear whether vitamin D has an effect at all, trials need to include a placebo group; and because it is unclear which dose of vitamin D ‐ if any ‐ is effective, different treatment regimens need to be compared. Finally, trials need to report on adverse events and give details of withdrawals and drop‐outs (all‐cause, lack of efficacy, and adverse event withdrawals), as is now established standard in the pain field.
Measurement (endpoints)
Standard pain outcomes should be used. The ideal outcome is people achieving at least 50% pain intensity reduction, or being in a final pain state of no worse than mild pain.
What's new
| Date | Event | Description |
|---|---|---|
| 18 February 2020 | Amended | Clarification added to Declarations of interest. |
| 22 March 2018 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 2, 2009 Review first published: Issue 1, 2010
| Date | Event | Description |
|---|---|---|
| 30 April 2015 | Review declared as stable | This review will be assessed for further updating in 2018. |
| 16 February 2015 | New citation required but conclusions have not changed | New studies did not change the overall conclusion that there is a lack of good evidence supporting the use of vitamin D for chronic pain. Issues around study design are highlighted. |
| 2 February 2015 | New search has been performed | New searches identified six new studies (517 participants) for inclusion and 21 studies for exclusion. |
| 12 December 2014 | Amended | Work on the update has started. Searches have been completed. |
| 27 June 2012 | Amended | Contact details updated. |
| 25 April 2012 | Review declared as stable | The authors will update this review in 2014 or sooner if evidence comes to light to alter the current conclusions. |
| 24 September 2010 | Amended | Contact details updated. |
Notes
A restricted search in March 2018 did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. There is ongoing relevant work and therefore the review will be re‐assessed for updating in two years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions
Acknowledgements
The Oxford Pain Relief Trust, the NHS Cochrane Collaboration Programme Grant Scheme, and the NIHR Biomedical Research Centre Programme provided support for the earlier review.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group. Disclaimer: the views and opinions expressed herein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS), or the Department of Health.
The review authors are grateful to Joanne Abbott for conducting searches. We thank Henry J McQuay for contributing to the previous version of this review. We thank Mari Imamura, who had translated a paper from Japanese for the previous version of this review (Yamauchi 1989).
Appendices
Appendix 1. CENTRAL (CRSO) search strategy
MESH DESCRIPTOR vitamin D EXPLODE ALL TREES
(vitamin D):TI,AB,KY
(vitamin D2):TI,AB,KY
(vitamin D3):TI,AB,KY
(1‐alpha hydroxyvitamin D3):TI,AB,KY
(1‐alpha hydroxy‐vitamin D3):TI,AB,KY
(1‐alpha hydroxycalciferol):TI,AB,KY
1‐alpha‐hydroxy‐calciferol:TI,AB,KY
(1,25 dihydroxyvitamin D3):TI,AB,KY
(1,25‐dihydroxy‐vitamin D3):TI,AB,KY
(1,25 dihydroxycholecalciferol):TI,AB,KY
1,25‐dihydroxycholecalciferol:TI,AB,KY
25‐hydroxycholecalciferol:TI,AB,KY
(25 hydroxycholecalciferol):TI,AB,KY
(25 hydroxyvitamin D):TI,AB,KY
(25‐hydroxy‐vitamin D):TI,AB,KY
alfacalcidol:TI,AB,KY
calcidiol:TI,AB,KY
calcitriol:TI,AB,KY
calcifediol:TI,AB,KY
calciferol:TI,AB,KY
ergocalciferol:TI,AB,KY
cholecalciferol:TI,AB,KY
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23
pain*:TI,AB,KY
MESH DESCRIPTOR pain EXPLODE ALL TREES
#25 OR #26
#24 AND #27
2009 TO 2015:YR
#28 AND #29
Appendix 2. MEDLINE (OVID) search strategy
exp Vitamin D/
vitamin D.tw.
vitamin D2.tw.
vitamin D3.tw.
1‐alpha hydroxyvitamin D3.tw.
1‐alpha‐hydroxy‐vitamin D3.tw.
1‐alpha hydroxycalciferol.tw.
1‐alpha‐hydroxy‐calciferol.tw.
1,25 dihydroxyvitamin D3.tw.
1,25‐dihydroxy‐vitamin D3.tw.
1,25 dihydroxycholecalciferol.tw.
1,25‐dihydroxycholecalciferol.tw.
25‐hydroxycholecalciferol.tw.
25 hydroxycholecalciferol.tw.
25 hydroxyvitamin D.tw.
25‐hydroxy‐vitamin D.tw.
alfacalcidol.tw.
calcidiol.tw.
calcitriol.tw.
calcifediol.tw.
calciferol.tw.
ergocalciferol.tw.
cholecalciferol.tw.
or/1‐23
pain*.tw.
exp pain/
25 or 26
24 and 27
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
29 or 30 or 31 or 32 or 33 or 34 or 35 or 36
exp animals/ not humans.sh.
37 not 38
28 and 39
(200909*or 200910* or 200911* or 200912* or 2010* or 2011* or 2012* or 2013* or 2014* or 2015*).ed.
40 and 41
Appendix 3. EMBASE (OVID) search strategy
exp Vitamin D/
vitamin D.tw.
vitamin D2.tw.
vitamin D3.tw.
1‐alpha hydroxyvitamin D3.tw.
1‐alpha‐hydroxy‐vitamin D3.tw.
1‐alpha hydroxycalciferol.tw.
1‐alpha‐hydroxy‐calciferol.tw.
1,25 dihydroxyvitamin D3.tw.
1,25‐dihydroxy‐vitamin D3.tw.
1,25 dihydroxycholecalciferol.tw.
1,25‐dihydroxycholecalciferol.tw.
25‐hydroxycholecalciferol.tw.
25 hydroxycholecalciferol.tw.
25 hydroxyvitamin D.tw.
25‐hydroxy‐vitamin D.tw.
alfacalcidol.tw.
calcidiol.tw.
calcitriol.tw.
calcifediol.tw.
calciferol.tw.
ergocalciferol.tw.
cholecalciferol.tw.
or/1‐23
pain*.tw.
exp pain/
25 or 26
24 and 27
random$.tw.
factorial$.tw.
crossover$.tw.
cross over$.tw.
cross‐over$.tw.
placebo$.tw.
(doubl$ adj blind$).tw.
(singl$ adj blind$).tw.
assign$.tw.
allocat$.tw.
volunteer$.tw.
Crossover Procedure/
double‐blind procedure.tw.
Randomized Controlled Trial/
Single Blind Procedure/
or/29‐43
(animal/ or nonhuman/) not human/
44 not 45
28 and 46
(200909*or 200910* or 200911* or 200912* or 2010* or 2011* or 2012* or 2013* or 2014* or 2015*).dd.
47 and 48
limit 49 to embase
Appendix 4. Summary of outcomes in individual studies: efficacy
| Study ID | Condition | Intervention |
Baseline 25‐hydroyx‐ vitamin D (ng/mL) (± SD) |
End‐of‐trial 25‐hydroxy‐ vitamin D (ng/mL) (± SD) |
≥ 50% pain relief | PGIC much or very much improved | Any pain improvement |
Other pain outcomes (95% CI) |
QoL Improvement (95% CI) |
| Rheumatoid arthritis | |||||||||
| Brohult 1973 | Rheumatoid arthritis | (1) Calciferol 100,000 IU/day, n = 24 (2) Placebo, n = 5 | No data | No data | No data | No data | No data | Consumption of "analgesics and antiinflammatory medicines" decreased significantly in calciferol group (P value < 0.01), no change in control group (no direct between groups comparison reported) | No data |
| Hansen 2014 | Rheumatoid arthritis | (1) Ergocalciferol 50,000 IU, 3 times/week for 4 weeks, then twice monthly for 11 months, plus calcium 500 mg 3 times daily, n = 11 (2) Placebo plus calcium 500 mg 3 times daily, n = 11 |
(1) 25 ± 24 (2) 21 ± 9 |
(1) 30 ± 11 (2) 23 ± 11 |
No data | No data | No data | Change in pain (scale 0 to 10) (1) 2.9 (95% CI 1.8 to 4.1) to 3.9 (95% CI 2.6 to 5.2) (2) 2.9 (95% CI 1.8 to 4.1) to 2.4 (95% CI 1.1 to 3.7) Significant difference (P value = 0.03) for end‐of‐trial values between groups; worse pain in vitamin D group |
Change in: Health Assessment Questionnaire (scale 0 to 3; 3 is worst functional status) (1) 0.61 (95% CI 0.35 to 0.87) to 0.68 (95% CI 0.39 to 0.96) (2) 0.61 (95% CI 0.35 to 0.87) to 0.44 (95% CI 0.15 to 0.73) No significant difference at end of trial Patient Assessment of Global Health (scale 0 to 5; 5 is poor health) (1) 2.4 (95% CI 2.0 to 2.8) to 3.2 (95% CI 2.6 to 3.7) (2) 2.4 (95% CI 2.0 to 2.8) to 2.2 (95% CI 1.7 to 2.7) Significant difference for end‐of‐trial values; worse with vitamin D Also significant decline in physical function domain of SF‐36 in vitamin D‐treated participants |
| Salesi 2012 | Rheumatoid arthritis | (1) Methotrexate (7.5‐20 mg/week) plus vitamin D 50,000 IU/week, n = 60 (2) Methotrexate (7.5‐20 mg/week plus placebo, n = 57 |
(1) 43 ± 11 (2) 37 ± 13 |
(1) 50 ± 9.0 (2) 40 ± 12 |
No data | No data | No data | Change in visual analogue scale (0 to 100): (1) 63 ± 18 to 46 ± 20 (2) 61 ± 22 to 39 ± 20 Significant improvements in both groups, no significant difference between groups at either time point |
No data |
| Yamauchi 1989 | Rheumatoid arthritis | (1) Alfacalcidol 1 μg/day, n = 59 (2) Alfacalcidol 2 μg/day, n = 55 (3) Placebo, n = 57 |
No data | No data | No data | (1) 8/59 (2) 7/55 (3) 8/57 |
No data | No data | No data |
| Knee osteoarthritis | |||||||||
| McAlindon 2013 | Knee osteoarthritis | (1) Cholecalciferol 2000 IU daily, initially, with adjustments at 4, 8, and 12 months for a target vitamin D level of 36‐100 ng/mL, n = 73 (2) Placebo, n = 73 |
(1) 23 ± 11 (2) 22 ± 8.3 |
(1) 39 (2) 25 |
No data | No data | No data | WOMAC knee pain score (range 0 to 20) change baseline to trial end (1) ‐2.3 (95% CI ‐3.2 to ‐1.4) (2) ‐1.5 (95% CI ‐2.3 to ‐0.6) No significant difference between groups |
WOMAC function score change (1) ‐7.0 (95% CI ‐9.8 to ‐4.2) (2) ‐3.8 (95% CI ‐6.0 to ‐1.7) No significant difference between groups |
| Sanghi 2013a | Knee osteoarthritis | (1) Vitamin D 60,000 IU/day for 10 days, followed by 60,000 IU once a month for 12 months, n = 53 (2) Placebo, n = 53 |
(1) 15 ± 3.0 (2) 14 ± 3.0 |
(1) 33 (2) 15 |
No data | No data | No data | Visual analogue pain scale change (1) ‐0.26 (95% CI ‐2.8 to ‐1.4) (2) 0.13 (95% CI ‐0.03 to 0.29) Significant difference (P value = 0.02) WOMAC pain score change (1) ‐0.55 (95% CI ‐0.07 to 1.02) (2) 1.2 (95% CI 0.82 to 1.2) Significant difference (P value < 0.001) |
WOMAC total score change (1) ‐2.1 (95% CI ‐2.8 to ‐1.4) (2) 1.4 (95% CI 0.95 to 1.86) Significant difference (P value < 0.001) |
| Other | |||||||||
| Di Munno 1989 | Polymyalgia rheumatica | (1) 25‐Hydroxyvitamin D 35 μg/day for 25 days/month, n = 12
(2) Placebo, n = 12 All had calcium 500 mg and 6‐methylprednisolone |
No data | No data | No data | No data | No data | Subjective pain on movement (scale 0 to 4): significantly decreased in both groups over time, no great difference between groups (no statistics for between groups comparison reported) | No data |
| Schreuder 2012 | Nonspecific musculoskeletal pain | (1) Vitamin D3 150,000 IU once, n = 44 (2) Placebo, n = 40 |
(1) 7.9 ± 4.3 (2) 7.9 ± 3.5 |
No data | "much less pain" (5‐point scale) (1) 2/44 (2) 1/40 |
No data | "much less pain" or "less pain" (5‐point scale) (1) 15/44 (2) 7/40 Significant difference (P value = 0.04) |
Visual analogue scale for pain: no significant improvement with vitamin D | No data |
| Warner 2008 | Diffuse musculoskeletal pain | (1) Ergocalciferol 50,000 IU/week, n = 25 (2) Placebo, n = 25 | (1) 17 ± 2.9 (2) 16 ± 3.6 | (1) 31 ± 6.2 (2) 19 ± 6.5 (P value < 0.001) | No data | No data | No data | Visual analogue scale: no significant difference between groups; functional pain score: slightly favours placebo (P value = 0.05) | No data |
| Wepner 2014 | Fibromyalgia | (1) Cholecalciferol 1200‐2400 IU daily, depending on vitamin D level, n = 24 (2) Placebo, n = 18 |
(1) 19 ± 5.9 (2) 21 ± 6.3 |
(1) 49 ± 11 (2) 34 ± 12 |
No data | No data | No data | Visual analogue scale score change week 1 to week 25 (1) 69 ± 12 to 53 ± 29 (2) 62 ± 20 to 65 ± 16 A 2 groups x 4 time points variance analysis showed a significant group effect (P value = 0.025) |
SF‐36 scores showed no significant time or groups effect in the physical and metal health summaries. A group‐specific significance was seen in the physical functioning subscale: the treatment group improved (P value = 0.014); the placebo group remained unchanged (P value = 0.480) FIQ scores, week 1 to week 25: (1) 43 ± 12 to 44 ± 16 (2) 42 ± 17 to 48 ± 12 No group‐specific effects seen overall (P value = 0.615); FIQ question on morning fatigue: a significantly better outcome was observed for the intervention group (P value = 0.007) |
| CI: confidence interval; FIQ: Fibromyalgia Impact Questionnaire; n = number; PGIC: Patient Global Impression of Change; QoL: quality of life; SD: standard deviation; SF‐36: Short Form (36) Health Survey; WOMAC: Western Ontario and McMaster Universities Arthritis Index. Yamauchi 1989 included 191 participants in total (65 in the placebo, 64 in the alfacalcidol 1 μg, and 62 in the alfacalcidol 2 μg). Of these, 20 (8, 5, and 7 in the previously mentioned groups) were excluded after randomisation due to violation of inclusion criteria or concurrent drugs. These 20 participants who were excluded from the original study due to protocol violation were not included in our intention‐to‐treat analysis. | |||||||||
Appendix 5. Summary of outcomes in individual studies: adverse events and withdrawals
| Study ID | Condition | Intervention | Any AE | Serious AE | All‐cause withdrawal | LoE withdrawal | AE withdrawal |
| Rheumatoid arthritis | |||||||
| Brohult 1973 | Rheumatoid arthritis | (1) Calciferol 100,000 IU/day, n = 24 (2) Placebo, n = 25 | No data | No data | (1) 4/24 (2) 6/25 | Worsening of disease: (1) 3/24 (2) 6/25 | Mortality: (1) 1/24 (PE) (2) 0/25 |
| Hansen 2014 | Rheumatoid arthritis | (1) Ergocalciferol 50,000 IU , 3 times/week for 4 weeks, then twice monthly for 11 months, plus 500 mg calcium 3 times daily, n = 11 (2) Placebo plus 500 mg calcium 3 times daily, n = 11 |
No data | No data | No data | No data | No data |
| Salesi 2012 | Rheumatoid arthritis | (1) Methotrexate 7.5‐20 mg/week plus vitamin D 50,000 IU/week, n = 60 (2) Methotrexate (7.5‐20 mg/week) plus placebo, n = 57 |
(1) 7/60 (2) 3/57 |
Due to intervention: (1) 0/60 (2) 0/57 Also 1 death due to heart disease in (1) |
(1) 10/60 (2) 9/57 |
(1) 0/60 (2) 0/57 |
(1) 1/60 (2) 1/57 |
| Yamauchi 1989 | Rheumatoid arthritis | (1) Alfacalcidol 1 μg/day, n = 59 (2) Alfacalcidol 2 μg/day, n = 55 (3) Placebo, n = 57 |
(1) 1/59 (2) 2/55 (3) 3/57 |
(1) 1/59 (2) 0/55 (3) 0/57 |
(1) 14/59 (2) 9/55 (3) 8/57 |
(1) 1/59 (2) 0/55 (3) 0/57 |
(1) 1/59 (2) 2/55 (3) 3/57 |
| Knee osteoarthritis | |||||||
| McAlindon 2013 | Knee osteoarthritis | (1) Cholecalciferol 2000 IU daily, initially, with adjustments at 4, 8, and 12 months for a target vitamin D level of 36‐100 ng/mL, n = 73 (2) Placebo, n = 73 |
(1) 47/73 (2) 46/73 |
(1) 16/73 (2) 16/73 |
(1) 9/73 (2) 13/73 |
1) 0/73 (2) 0/73 (2 with unclear ("other") reason for drop‐out) |
1) 0/73 (2) 0/73 (2 with unclear ("other") reason for drop‐out) |
| Sanghi 2013a | Knee osteoarthritis | (1) Vitamin D 60,000 IU/day for 10 days, followed by 60,000 IU once per month for 12 months, n = 53 (2) Placebo, n = 53 |
No data | No data | (1) 1/53 (2) 2/53 |
(1) 0/53 (2) 0/53 |
(1) 0/53 (2) 0/53 |
| Other | |||||||
| Di Munno 1989 | Polymyalgia rheumatica | (1) 25‐Hydroxyvitamin D 35 μg/day for 25 days/month, n = 12
(2) Placebo, n = 12 All had calcium 500 mg and 6‐methylprednisolone |
(1) 0/12 (2) 0/12 | (1) 0/12 (2) 0/12 | No data | No data | No data |
| Schreuder 2012 | Non‐specific musculoskeletal pain | (1) Vitamin D3 150,000 IU once, n = 44 (2) Placebo, n = 40 |
"did not find any adverse effects of vitamin D supplementation" | Presumably none | (1) 1/44 (2) 4/40 |
(1) 0/44 (2) 0/40 |
(1) 0/44 (2) 0/40 |
| Warner 2008 | Diffuse musculoskeletal pain | (1) Ergocalciferol 50,000 IU ergocalciferol/week, n = 25 (2) Placebo, n = 25 | No data | No data | (1) 3/25 (2) 5/25 | No data | (1) 0/25 (2) 0/25 |
| Wepner 2014 | Fibromyalgia | (1) Cholecalciferol 1200‐2400 IU daily, depending on vitamin D level, n = 24 (2) Placebo, n = 18 |
No data | No data | (1) 9/24 (2) 3/18 |
No data | No data |
| AE: adverse event; LoE: lack of efficacy; n: number; PE: pulmonary embolus. Yamauchi 1989 reported that there were 2 participants with adverse events in the placebo groups. However, because there were 3 adverse event withdrawals in that group the number of participants with adverse events is given as 3 in the above table. | |||||||
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brohult 1973.
| Methods | Randomised, double‐blind, placebo‐controlled study. Duration: 12 months | |
| Participants | Participants (N = 49) with rheumatoid arthritis of at least 2 years' duration (average duration of disease 7 years). Mean age 52 years (range 18‐69 years), 68% women | |
| Interventions | Calciferol 100,000 IU/day (no more details given) (n = 24) Placebo (n = 25) |
|
| Outcomes | Consumption of analgesics and antiinflammatory drugs, all‐cause withdrawals, lack of efficacy withdrawals (worsening of disease), adverse event withdrawals (death) | |
| Notes | Oxford Quality Score: R1, D1, W1 = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated to be randomised. 1 of the authors chose whether participants received placebo or active treatment, but it is not stated by which method |
| Allocation concealment (selection bias) | Unclear risk | 1 of the authors chose whether participants received placebo or active treatment (though it is not stated how) and was, therefore, possibly aware of the allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Stated to be double‐blind. 1 of the authors was blinded but the other was possibly aware of treatment allocation. However, the authors arrived at their assessments independently and the other author's opinion (not aware of allocation or blood calcium data) was adopted in case of disagreement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10/49 participants dropped out; last observation carried forward was used |
| Size | High risk | < 50 participants in each treatment arm |
Di Munno 1989.
| Methods | Randomised, double‐blind, placebo‐controlled study. Duration: 9 months | |
| Participants | Participants (N = 24) with polymyalgia rheumatica of recent onset, receiving 6‐metholprednisolone. Mean age 67.9 years (range 51‐82 years), 63% women | |
| Interventions | 25‐hydroxyvitamin D3 35 μg/day for 25 days/month (n = 12) Placebo (n = 12) All participants received calcium 500 mg/day |
|
| Outcomes | Pain on movement, any adverse events, and serious adverse events | |
| Notes | Oxford Quality Score: R1, D1, W0 = 2/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated to be randomised. Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Stated to be double‐blind. Method of blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐outs and imputation method not stated |
| Size | High risk | < 50 participants in each treatment arm |
Hansen 2014.
| Methods | Randomised, double‐blind, placebo‐controlled study. Duration: 12 months | |
| Participants | Participants (N = 22) with rheumatoid arthritis. Vitamin D: 36% women, mean (± SD) age 63 ± 12 years. Placebo: 55% women, mean (± SD) age 53 ± 11 years | |
| Interventions | Ergocalciferol 50,000 IU 3 times/week for 4 weeks, then 50,000 IU twice monthly for 11 months (n = 11) Placebo (n = 11) All participants received calcium 500 mg 3 times daily throughout the study and were asked to apply sunscreen between May and September |
|
| Outcomes | 11‐point pain scale, Health Assessment Questionnaire, Patient Assessment of Global Health, and baseline and end‐of‐trial vitamin D levels | |
| Notes | Oxford Quality Score: R2, D2, W0 = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Randomisation done by research pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Matching placebo" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐outs and imputation method not stated |
| Size | High risk | < 50 participants in each treatment arm |
McAlindon 2013.
| Methods | Randomised, double‐blind, placebo‐controlled study. Duration: 2 years | |
| Participants | Participants (N = 146) with symptomatic knee osteoarthritis. 61% women, mean (± SD) age 62.4 ± 8.5 years | |
| Interventions | Cholecalciferol 2000 IU daily initially, with subsequent dose adjustments in 2000 IU increments at 4, 8, and 12 months to keep 25‐hydroxyvitamin D levels between 36 and 100 ng/mL (n = 73) Placebo (n = 73) |
|
| Outcomes | Western Ontario and McMaster Universities Arthritis Index, any adverse events, serious adverse events, all‐cause withdrawals, and baseline and end‐of‐trial vitamin D levels | |
| Notes | Oxford Quality Score: R2, D2, W1 = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation system, 1 : 1 assignments in permuted blocks of 6, randomisation list generated by study statistician |
| Allocation concealment (selection bias) | Low risk | Randomisation list provided by study statistician to research pharmacy, list was concealed from the investigative team. "Labeling and dispensing of study pills was performed by the research pharmacy" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical placebo, dummy dose changes in placebo group. "Monitoring of laboratory test results was performed by the pathology department staff, independently of the clinical team" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 22/146 participants dropped out; multiple imputation used |
| Size | Unclear risk | > 50 but < 200 participants in each treatment arm |
Salesi 2012.
| Methods | Randomised, double‐blind, placebo‐controlled study. Duration: 12 weeks | |
| Participants | Participants (N = 117) with rheumatoid arthritis and on treatment with methotrexate, 98 completers (91% women). Median methotrexate dose at baseline 10 mg/week. Vitamin D: mean (± SD) age 49.9 ± 13 years. Placebo: mean (± SD) age 50 ± 12.7 years | |
| Interventions | 25‐hydroxyvitamin D 50,000 IU/week (plus methotrexate, n = 60) Placebo (plus methotrexate, n = 57) |
|
| Outcomes | Visual analogue pain scale, any adverse events, serious adverse events, all‐cause withdrawals, lack of efficacy withdrawals, adverse event withdrawals, and baseline and end‐of‐trial vitamin D levels | |
| Notes | Oxford Quality Score: R1, D1, W1 = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated to be randomised. Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Matching placebo" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 19 of 117 participants dropped out; imputation method not stated |
| Size | Unclear risk | > 50 but < 200 participants in each treatment arm |
Sanghi 2013a.
| Methods | Randomised, double‐blind, placebo‐controlled study. Duration: 12 months | |
| Participants | Participants (N = 106) with knee osteoarthritis and vitamin D insufficiency (≤ 50 nmol/L); 103 completers. Vitamin D: 69% women, mean (± SD) age 53.2 ± 9.6 years; placebo: 59% women, mean (± SD) age 53.0 ± 7.4 years | |
| Interventions | Cholecalciferol 60,000 IU/day for 10 days, then 60,000 IU once a month for 12 months (n = 53) Placebo (n = 53) |
|
| Outcomes | Visual analogue pain scale, Western Ontario and McMaster Universities Arthritis Index, all‐cause withdrawals, and baseline and end‐of‐trial vitamin D levels | |
| Notes | Oxford Quality Score: R2, D2, W1 = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random allocation sequence |
| Allocation concealment (selection bias) | Low risk | Random allocation sequence generated by study statistician. Stated that the allocation process was done in a completely concealed manner and that investigator, participants, and staff were unaware of the sequence |
| Blinding (performance bias and detection bias) All outcomes | Low risk | States that the investigator, participants, and pharmacist dispensing the interventions were all blinded to group assignment. Vitamin D and placebo given as capsules |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/106 participants dropped out |
| Size | Unclear risk | > 50 but < 200 participants in each treatment arm |
Schreuder 2012.
| Methods | Randomised, double‐blind, placebo‐controlled study. Total duration: 12 weeks. Semi‐cross‐over at week 6 (main outcome assessed then) | |
| Participants | Non‐Western immigrants (N = 84) presenting to general practitioner with non‐specific musculoskeletal pain and with 25‐hydroxyvitamin D levels < 50 nmol/L. 76% women, mean (± SD) age 41.9 ± 10.4 years | |
| Interventions | Vitamin D3 150,000 IU as a single dose (n = 44) Placebo (n = 40) (Re‐randomisation at week 6 with all placebo participants receiving vitamin D, and vitamin D participants receiving either placebo or a second dose of vitamin D) |
|
| Outcomes | 5‐point pain scale, visual analogue pain scale, any adverse events, all‐cause withdrawals, and baseline vitamin D level | |
| Notes | Oxford Quality Score: R2, D2, W1 = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomisation list" |
| Allocation concealment (selection bias) | Low risk | Pharmacists unaware of treatment allocation dispensed vitamin D or placebo |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo had same appearance and taste as active treatment. Participants, general practitioners, and interviewers were blinded during the whole trial period |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/84 participants dropped out |
| Size | High risk | < 50 participants in each treatment arm |
Warner 2008.
| Methods | Randomised, double‐blind, placebo‐controlled study. Duration: 3 months | |
| Participants | Participants (N = 50) with diffuse musculoskeletal pain and 25‐hydroxyvitamin D levels between 9 and 20 ng/mL. Mean (± SD) age 56.2 ± 9.3 years, 100% women | |
| Interventions | Ergocalciferol 50,000 IU/week (n = 25) Placebo (n = 25) |
|
| Outcomes | Visual analogue pain scale, functional pain score, all‐cause withdrawals, adverse event withdrawals, and baseline and end‐of‐trial 25‐hydroxyvitamin D levels | |
| Notes | Oxford Quality Score: R2, D2, W1 = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Vitamin D and placebo capsules (identical appearance) dispensed in identical prescription containers by hospital pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Colour matched, identical appearing capsules of vitamin D and placebo |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 8/50 participants dropped out; completer analysis was used |
| Size | High risk | < 50 participants in each treatment arm |
Wepner 2014.
| Methods | Randomised, double‐blind, placebo‐controlled study. Duration: 25 weeks of treatment, follow‐up examination at week 49 | |
| Participants | Participants (N = 42) with fibromyalgia and serum calcifediol levels < 32 ng/L; 30 completers (90% women): mean (± SD) age 48.4 ± 5.3 years, range 35‐55 years | |
| Interventions | Cholecalciferol (in triglyceride solution) 1200 or 2400 IU daily, depending on vitamin D level Placebo: triglyceride solution |
|
| Outcomes | Visual analogue pain scale, Short Form (36) Health Survey (SF‐36), Fibromyalgia Impact Questionnaire, all‐cause withdrawals, and baseline and end‐of‐trial 25‐hydroxyvitamin D levels | |
| Notes | Oxford Quality Score: R2, D2, W1 = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator |
| Allocation concealment (selection bias) | Low risk | Random allocation done by statistician who was not involved in testing or treatment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Cholecalciferol in triglyceride solution as active, triglyceride solution alone as placebo. Mock dose changes in placebo group |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12/42 participants dropped out, completer analysis |
| Size | High risk | < 50 participants in each treatment arm |
Yamauchi 1989.
| Methods | Randomised, double‐blind, placebo‐controlled study. Duration: 16 weeks | |
| Participants | Participants (N = 171) with rheumatoid arthritis. Data for 140 participants (excluding 31 drop‐outs): mean age not reported; age groups: < 29 years (n = 3), 30‐39 years (n = 12), 40‐49 years (n = 19), 50‐59 years (n = 51), 60‐69 years (n = 32), > 70 years (n = 23); 83% women | |
| Interventions | Alfacalcidol (1‐hydroxycholecalciferol) 1.0 μg/day (n = 59) Alfacalcidol 2.0 μg/day (n = 55) Placebo (n = 57) |
|
| Outcomes | Patient Global Impression of Change, any adverse events, serious adverse events, all‐cause withdrawals, adverse event withdrawals, and lack of efficacy withdrawals | |
| Notes | Oxford Quality Score: R1, D1, W1 = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated by 'controller'; no further details given |
| Allocation concealment (selection bias) | Unclear risk | Administered by (presumably independent) 'controller' |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical placebo; verified by 'controller' |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 31/171 participants dropped out; completer analysis was used |
| Size | Unclear risk | > 50 but < 200 participants in each treatment arm |
IU: international unit; N: total number in study population, n: number in a treatment arm; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abou‐Raya 2014 | Abstract only |
| Arden 2006 | Not all participants had chronic pain. Did not report on outcomes of vitamin D therapy |
| Arden 2014 | Abstract only |
| Björkman 2008 | Pain not always self rated ("self‐ or nurse‐reported"). No other outcomes of interest |
| Chlebowski 2013 | Included women who did not have pain |
| de Nijs 2006 | Participants were not required to have chronic pain |
| Ding 2013 | Letter to the editor, not a trial |
| Dottori 1982 | Not explicitly described as double‐blind |
| Felson 2013 | Commentary, not a trial |
| Gendelman 2014 | Abstract only |
| Grove 1981 | Fluoride and calcium administered concurrently with calciferol but not given to comparator group |
| Kessenich 2010 | Case report |
| Knutsen 2014 | Not all participants had chronic pain |
| Kragstrup 2011 | Editorial, not a trial |
| Krocker 2008 | All participants received vitamin D. Described as "pseudorandomised". Not described as double‐blind |
| Lyritis 1994 | Not clear that all participants had chronic pain |
| Mascitelli 2012 | Letter to the editor, not a trial |
| McAlindon 2010 | Abstract only |
| Osunkwo 2012a | Abstract only |
| Osunkwo 2012b | Abstract only |
| Osunkwo 2012c | Trial in children and adolescents |
| Ringe 2000 | Not clear that all participants had chronic pain. Not described as double‐blind |
| Ringe 2004 | Not clear that all participants had chronic pain. Not described as double‐blind |
| Ringe 2005 | Not clear that all participants had chronic pain. Not described as double‐blind. Reports on same results as Ringe 2004 |
| Ringe 2007 | Not clear that all participants had chronic pain. Not described as double‐blind |
| Sakalli 2012 | Not clear that all participants had chronic pain |
| Sanghi 2011 | Abstract only |
| Sanghi 2012 | Abstract only |
| Sanghi 2013b | Abstract only |
| Sanghi 2014 | Abstract only |
| Wicherts 2011 | Not limited to participants with chronic pain |
| Wynn 2013 | Review, not a trial |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12610000322033.
| Trial name or title | The Role of Vitamin D in the Treatment of Nerve Pain in Diabetes |
| Methods | Randomised, placebo‐controlled study, parallel groups. Duration: 6 months |
| Participants | Participants with type 2 diabetes and with painful neuropathy with a serum 25‐hydroxyvitamin D level < 20 ng/mL Estimated enrolment: N = 60 |
| Interventions | Intramuscular vitamin D injection (300,000 IU) at baseline Placebo injection (normal saline) at baseline |
| Outcomes | Pain scores visual analogue scale and McGill short form pain questionnaire, SF‐36 |
| Starting date | 1 April 2010 |
| Contact information | Paul Lee, Department of Endocrinology, Garvan Institute of Medical Research, 384 Victoria Street, Darlinghurst, New South Wales 2010, Australia |
| Notes |
Cao 2012.
| Trial name or title | Does Vitamin D Supplementation Prevent Progression of Knee Osteoarthritis? A Randomised Controlled Trial |
| Methods | Randomised, double‐blind, placebo‐controlled study, parallel group. Duration: 2 years |
| Participants | Men and women with symptomatic knee osteoarthritis for at least 6 months with a pain visual analogue scale score of at least 20 mm; serum vitamin D level of > 5 ng/mL and < 24 ng/mL Estimated enrolment: N = 400 |
| Interventions | Cholecalciferol 50,000 IU tablets given once monthly for 2 years Placebo tablets |
| Outcomes | Knee pain, assessed with Western Ontario and McMaster Universities Arthritis Index (WOMAC) |
| Starting date | 1 August 2010 |
| Contact information | Changhai Ding, Menzies Research Institute, 17 Liverpool St, Hobart 7000, Tasmania; Department of Epidemiology & Preventive Medicine, 99 Commercial Rd, Melbourne 3004, Victoria, Australia |
| Notes |
CTRI/2014/06/004658.
| Trial name or title | Effect of Vitamin D Supplementation on Progression of Osteoarthritis Knee |
| Methods | Randomised, double‐blind, parallel group study. Duration: 2 years |
| Participants | Men and women diagnosed with knee osteoarthritis with serum 25‐hydroxyvitamin D level ≤ 20 ng/mL |
| Interventions | Cholecalciferol 60,000 IU for 10 days continuously, then 60,000 IU once a month for 2 years Placebo |
| Outcomes | Knee pain, quality of life |
| Starting date | 22 July 2014 |
| Contact information | R N Srivastava, Dept. of Orthopaedic Surgery, King George's Medical University, Lucknow, Uttar Pradesh, 226003, India |
| Notes |
EUCTR 2009‐015835‐34‐IT.
| Trial name or title | Vitamin D Effect on T Lymphocytes and Osteoclastogenesis in Rheumatoid Arthritis |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel group study |
| Participants | Women with rheumatoid arthritis |
| Interventions | Cholecalciferol solution (300,000 IU) Placebo solution |
| Outcomes | |
| Starting date | 9 April 2010 |
| Contact information | |
| Notes |
EUCTR 2014‐000047‐33‐NL.
| Trial name or title | The Effect of Intensive Muscle Strength Training and Vitamin D Supplements in Persons with Knee Osteoarthritis |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel group study. Duration: 12 months |
| Participants | Participants with knee osteoarthritis and vitamin D deficiency defined as 25‐hydroxyvitamin D levels > 6 ng/mL and < 20 ng/mL (in winter) or < 28 ng/mL (in summer) |
| Interventions | Cholecalciferol (1200 IU) Placebo |
| Outcomes | Knee pain, global perceived effect |
| Starting date | 20 March 2014 |
| Contact information | Trial co‐ordinator, Dr. Jan van Breemenstraat, 1056AB, Amsterdam, The Netherlands |
| Notes |
ISRCTN94818153.
| Trial name or title | A Randomised, Double‐Blind, Placebo‐Controlled Trial of Vitamin D Supplementation in the Management of Symptomatic Knee Osteoarthritis (the VIDEO study) |
| Methods | Randomised, double‐blind, placebo‐controlled study. Duration: 36 months |
| Participants | Men and women with primary knee osteoarthritis Estimated enrolment: N = 800 |
| Interventions | Cholecalciferol 800 IU/day Placebo |
| Outcomes | Pain, quality of life |
| Starting date | 1 February 2004 |
| Contact information | Richard Keen, Metabolic Unit, Royal National Orthopaedic Hospital Stanmore, Brockley Hill, Stanmore, HA7 4LP, UK |
| Notes | Trial status: completed (end date: 31 August 2011) |
NCT00279461.
| Trial name or title | Vitamin D Deficiency Causes Immune Dysfunction and Enables or Perpetuates the Development of Rheumatoid Arthritis |
| Methods | Randomised, double‐blind, parallel group, placebo‐controlled study. Duration: 6 months |
| Participants | Men and women with rheumatoid arthritis and a 25‐hydroxyvitamin D level < 30 ng/mL Estimated enrolment: N = 50 |
| Interventions | Cholecalciferol 2000 IU capsule daily for 6 months Placebo capsule |
| Outcomes | American College of Rheumatology response criterion of 20% improvement (ACR20) |
| Starting date | May 2009 |
| Contact information | Ernesto N Levy, erlevy@iupui.edu |
| Notes |
NCT01023490.
| Trial name or title | Vitamin D Treatment to Patients Suffering from Chronic Pain and Vitamin D Hypovitaminosis |
| Methods | Randomised, double‐blind, parallel group, placebo‐controlled study. Duration: 1 year |
| Participants | Men and women with chronic pain and hypovitaminosis D Estimated enrolment: N = 12 |
| Interventions | Vitamin D (34,500 IU/week) Placebo |
| Outcomes | Pain intensity |
| Starting date | January 2010 |
| Contact information | |
| Notes | Trial status: completed October 2012. Other study ID: TASMC‐09‐SB‐0494‐CTIL |
NCT01351805.
| Trial name or title | Vitamin D and Fish Oil for Autoimmune Disease, Inflammation and Knee Pain |
| Methods | Randomised, double‐blind, placebo‐controlled study |
| Participants | The parent trial of this study, VITamin D and OmegA‐3 TriaL (VITAL; NCT 01169259), is a randomised controlled trial in 25,876 US men and women investigating the effects of daily dietary supplementation with cholecalciferol or omega‐3 fatty acids This study (NCT01351805) is conducted among VITAL participants. The study will examine whether vitamin D or fish oil have effects on autoimmune disease incidence, biomarkers of systemic inflammation, and chronic knee pain. It involves a randomly selected subcohort of 2000 participants analysed for changes in biomarkers of systemic inflammation. Approximately 2000 participants with chronic knee pain at baseline are to be followed regarding their knee pain as a subcohort |
| Interventions | Marine omega‐3 fatty acids (eicosapentaenoic acid (EPA) 465 mg and docosahexaenoic acid (DHA) 375 mg) Cholecalciferol 2000 IU/day Placebo Marine omega‐3 fatty acids (EPA 465 mg and DHA 375 mg) plus cholecalciferol 2000 IU/day |
| Outcomes | Severity of knee pain |
| Starting date | January 2011 |
| Contact information | |
| Notes | Study ongoing, but not recruiting participants |
NCT01426347.
| Trial name or title | Treatment of Vitamin D Deficiency in Patients with Rheumatoid Arthritis |
| Methods | Randomised, double‐blind, parallel group, placebo‐controlled study. Duration: 6 months |
| Participants | Men and women with rheumatoid arthritis Estimated enrolment, N = 140 |
| Interventions | Ergocalciferol 50,000 IU/week for 16 weeks Placebo |
| Outcomes | AIMS‐SF (probably refers to: Arthritis Impact Measurement Scale‐Short Form) |
| Starting date | January 2009 |
| Contact information | |
| Notes |
NCT02002000.
| Trial name or title | Chronic Pain and Vitamin D (DOVID) |
| Methods | Randomised, double‐blind, parallel group, placebo‐controlled study. Duration: 3 months |
| Participants | Men and women with chronic, diffuse musculoskeletal pain with 25‐hydroxyvitamin D deficiency < 20.8 ng/mL Estimated enrolment: N = 100 |
| Interventions | Cholecalciferol, 3 doses: 200,000 IU at baseline, 200,000 IU on day 15, and 200,000 IU on day 30 Placebo |
| Outcomes | Brief Pain Inventory, SF‐36 |
| Starting date | December 2013 |
| Contact information | Anne‐Marie Schott, anne‐marie.schott‐pettelaz@chu‐lyon.fr Julie Haesebaert, julie.haesebaert@chu‐lyon.fr |
| Notes |
NCT02243800.
| Trial name or title | Study of the Efficacy and Safety of Cholecalciferol Supplementation on the Activity of Rheumatoid Arthritis in Patients with Vitamin D Deficiency |
| Methods | Randomised, double‐blind, parallel group, placebo‐controlled study. Duration: 24 weeks |
| Participants | Men and women with rheumatoid arthritis with a serum 25‐hydroxyvitamin D < 30 ng/mL Estimated enrolment: N = 164 |
| Interventions | Cholecalciferol Placebo |
| Outcomes | Visual analogue score pain, quality of life (SF‐36 and others) |
| Starting date | November 2011 |
| Contact information | Patrick Lacarin, placarin@chu‐clermontferrand.fr |
| Notes |
N: total number in study population; SF‐36: Short Form (36) Health Survey
Differences between protocol and review
After the search and examination of the retrieved studies it was decided, at the stage of the original version of this review, to include an additional secondary outcome ("other patient‐rated pain outcomes"). For the 2015 update, the risk of bias assessment has been extended to bring it into line with current standards.
Contributions of authors
For this update, CS and SS assessed the studies identified by the searches, extracted data and performed the quality scoring.
SS has drafted the review update manuscript.
All review authors read and approved the final manuscript.
SS will be responsible for further updates.
Sources of support
Internal sources
-
Oxford Pain Research Trust, UK.
General institutional support
External sources
-
The National Institute for Health Research (NIHR), UK.
NIHR Cochrane Programme Grant: 13/89/29 ‐ Addressing the unmet need of chronic pain: providing the evidence for treatments of pain
Declarations of interest
SS has no conflicts relating to this review or any similar product.
SD has no conflicts relating to this review or any similar product.
CS has no conflicts relating to this review or any similar product.
RAM has no conflicts relating to this review or any similar product.
For transparency, SS, SD, and RAM have received research support from charities, government, and industry sources at various times, but none relate to this review. SD and RAM are funded by the NIHR for work on a series of reviews informing the unmet need of chronic pain and providing the evidence for treatments of pain.
This review was identified in a 2019 audit as not meeting the current definition of the Cochrane Commercial Sponsorship policy. At the time of its publication it was compliant with the interpretation of the existing policy. As with all reviews, new and updated, at update this review will be revised according to 2020 policy update.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Brohult 1973 {published data only}
- Brohult J, Jonson B. Effects of large doses of calciferol on patients with rheumatoid arthritis. Scandinavian Journal of Rheumatology 1973;2(4):173‐6. [DOI] [PubMed] [Google Scholar]
Di Munno 1989 {published data only}
- Munno O, Beghe F, Favini P, Giuseppe P, Pontrandolfo A, Occhipinti G, et al. Prevention of glucocorticoid‐induced osteopenia: effect of oral 25‐hydroxyvitamin D and calcium. Clinical Rheumatology 1989;8(2):202‐7. [DOI] [PubMed] [Google Scholar]
Hansen 2014 {published data only}
- Hansen KE, Bartels CM, Gangnon RE, Jones AN, Gogineni J. An evaluation of high‐dose vitamin D for rheumatoid arthritis. Journal of Clinical Rheumatology 2014;20(2):112‐4. [DOI: 10.1097/RHU.0000000000000072] [DOI] [PMC free article] [PubMed] [Google Scholar]
McAlindon 2013 {published data only}
- McAlindon T, LaValley M, Schneider E, Nuite M, Lee JY, Price LL, et al. Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis: a randomized controlled trial. JAMA 2013;309(2):155‐62. [DOI: 10.1001/jama.2012.164487] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salesi 2012 {published data only}
- Salesi M, Farajzadegan Z. Efficacy of vitamin D in patients with active rheumatoid arthritis receiving methotrexate therapy. Rheumatology International 2012;32(7):2129‐33. [DOI: 10.1007/s00296-011-1944-5] [DOI] [PubMed] [Google Scholar]
Sanghi 2013a {published data only}
- Sanghi D, Mishra A, Sharma AC, Singh A, Natu SM, Agarwal S, et al. Does vitamin D improve osteoarthritis of the knee: a randomized controlled pilot trial. Clinical Orthopaedics and Related Research 2013;471(11):3556‐62. [DOI: 10.1007/s11999-013-3201-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schreuder 2012 {published data only}
- Schreuder F, Bernsen RM, Wouden JC. Vitamin D supplementation for nonspecific musculoskeletal pain in non‐Western immigrants: a randomized controlled trial. Annals of Family Medicine 2012;10(6):547‐55. [DOI: 10.1370/afm.1402] [DOI] [PMC free article] [PubMed] [Google Scholar]
Warner 2008 {published data only}
- Warner AE, Arnspiger SA. Diffuse musculoskeletal pain is not associated with low vitamin D levels or improved by treatment with vitamin D. Journal of Clinical Rheumatology: Practical Reports on Rheumatic & Musculoskeletal Diseases 2008;14(1):12‐6. [DOI: 10.1097/RHU.0b013c31816356a9] [DOI] [PubMed] [Google Scholar]
Wepner 2014 {published data only}
- Wepner F, Scheuer R, Schuetz‐Wieser B, Machacek P, Pieler‐Bruha E, Cross HS, et al. Effects of vitamin D on patients with fibromyalgia syndrome: a randomized placebo‐controlled trial. Pain 2014;155(2):261‐8. [DOI: 10.1016/j.pain.2013.10.002] [DOI] [PubMed] [Google Scholar]
Yamauchi 1989 {published data only}
- Yamauchi Y, Tsunematsu T, Konda S, Hoshino T, Itokawa Y, Hoshizaki H. A double blind trial of alfacalcidol on patients with rheumatoid arthritis (RA). Ryumachi. [Rheumatism] 1989;29(1):11‐24. [PubMed] [Google Scholar]
References to studies excluded from this review
Abou‐Raya 2014 {published data only}
- Abou‐Raya S, Abou‐Raya A, Helmii M. Efficacy of vitamin D supplementation in the treatment of fibromyalgia: randomized controlled trial. Annals of the Rheumatic Diseases 2014;73:295. [DOI: 10.1136/annrheumdis-2014-eular.2767] [DOI] [Google Scholar]
Arden 2006 {published data only}
- Arden NK, Crozier S, Smith H, Anderson F, Edwards C, Raphael H, et al. Knee pain, knee osteoarthritis, and the risk of fracture. Arthritis and Rheumatism 2006;55(4):610‐5. [DOI: 10.1002/art.22088] [DOI] [PubMed] [Google Scholar]
Arden 2014 {published data only}
- Arden NK, Cro S, Dore C, Cooper C, O'Neil T, Birrell F, et al. Effect of vitamin D supplementation on radiographic knee osteoarthritis. Osteoarthritis and Cartilage 2014;22:S375. [DOI: 10.1016/j.joca.2014.02.699] [DOI] [PMC free article] [PubMed] [Google Scholar]
Björkman 2008 {published data only}
- Björkman M, Sorva A, Tilvis R. Vitamin D supplementation has no major effect on pain or pain behavior in bedridden geriatric patients with advanced dementia. Aging Clinical and Experimental Research 2008;20(4):316‐21. [DOI] [PubMed] [Google Scholar]
Chlebowski 2013 {published data only}
- Chlebowski RT, Pettinger M, Johnson KC, Wallace R, Womack C, Mossavar‐Rahmani Y, et al. Calcium plus vitamin D supplementation and joint symptoms in postmenopausal women in the women's health initiative randomized trial. Journal of the Academy of Nutrition and Dietetics 2013;113(10):1302‐10. [DOI: 10.1016/j.jand.2013.06.007] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Nijs 2006 {published data only}
- Nijs RN, Jacobs JW, Lems WF, Laan RF, Algra A, Huisman AM, et al. STOP Investigators. Alendronate or alfacalcidol in glucocorticoid‐induced osteoporosis. New England Journal of Medicine 2006;355(7):675‐84. [CTG: NCT00138983] [DOI] [PubMed] [Google Scholar]
Ding 2013 {published data only}
- Ding C, Cicuttini F, Jones G. Vitamin D supplementation in patients with osteoarthritis. JAMA 2013;309(15):1583. [DOI: 10.1001/jama.2013.3775] [DOI] [PubMed] [Google Scholar]
Dottori 1982 {published data only}
- Dottori L, D'Ottavio D, Brundisini B. Calcifediol and calcitonin in the therapy of rheumatoid arthritis. A short‐term controlled study [Calcifediolo e calcitonina nella terapia dell'artrite reumatoide. Studio controllato a breve termine]. Minerva Medica 1982;73(43):3033‐40. [PubMed] [Google Scholar]
Felson 2013 {published data only}
- Felson DT. CORR insights: does vitamin D improve osteoarthritis of the knee: a randomized controlled pilot trial. Clinical Orthopaedics and Related Research 2013;471(11):3563‐4. [DOI: 10.1007/s11999-013-3269-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gendelman 2014 {published data only}
- Gendelman O, Itzhaki D, Amital H. A randomized double‐blind placebo‐controlled study adding high dose vitamin D to analgesic regimens in patients with musculoskeletal pain. Annals of the Rheumatic Diseases 2014:1090. [DOI: 10.1136/annrheumdis-2014-eular.2555] [DOI] [PubMed] [Google Scholar]
Grove 1981 {published data only}
- Grove O, Halver B. Relief of osteoporotic backache with fluoride, calcium, and calciferol. Acta Medica Scandinavica 1981;209(6):469‐71. [DOI] [PubMed] [Google Scholar]
Kessenich 2010 {published data only}
- Kessenich CR. Vitamin D deficiency and leg pain in the elderly. Nurse Practitioner 2010;35(3):12‐3. [DOI: 10.1097/01.NPR.0000368901.92828.17] [DOI] [PubMed] [Google Scholar]
Knutsen 2014 {published data only}
- Knutsen KV, Madar AA, Brekke M, Meyer HE, Natvig B, Mdala I, et al. Effect of vitamin D on musculoskeletal pain and headache: A randomized, double‐blind, placebo‐controlled trial among adult ethnic minorities in Norway. Pain 2014;155(12):2591‐8. [DOI: 10.1016/j.pain.2014.09.024] [DOI] [PubMed] [Google Scholar]
Kragstrup 2011 {published data only}
- Kragstrup TW. Vitamin D supplementation for patients with chronic pain. Scandinavian Journal of Primary Health Care 2011;29(1):4‐5. [DOI: 10.3109/02813432.2010.530738] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krocker 2008 {published data only}
- Krocker D, Ullrich H, Buttgereit F, Perka C. Influence of adjuvant pain medication on quality of life in the treatment of postmenopausal osteoporosis [Einfluss der adjuvanten Schmerztherapie in der Behandlung der postmenopausalen Osteoporose auf die Lebensqualität]. Der Orthopäde 2008;37(5):435‐9. [DOI: 10.1007/s00132-008-1259-8] [DOI] [PubMed] [Google Scholar]
Lyritis 1994 {published data only}
- Lyritis GP, Androulakis C, Magiasis B, Charalambaki Z, Tsakalakos N. Effect of nandrolone decanoate and 1‐alpha‐hydroxy‐calciferol on patients with vertebral osteoporotic collapse. A double‐blind clinical trial. Bone and Mineral 1994;27(3):209‐17. [DOI] [PubMed] [Google Scholar]
Mascitelli 2012 {published data only}
- Mascitelli L, Goldstein MR, Grant WB. Hypovitaminosis D and pain in cystic fibrosis. Pain Medicine 2012;13(5):735‐6. [DOI: 10.1111/j.1526-4637.2012.01372.x] [DOI] [PubMed] [Google Scholar]
McAlindon 2010 {published data only}
- McAlindon TE, Dawson‐Hughes B, Driban J, LaValley M, Lee JY, Lo GH, et al. Clinical trial of vitamin D to reduce pain and structural progression of knee osteoarthritis (OA). Arthritis and Rheumatism 2010;62(Suppl 10):706. [DOI: 10.1002/art.28474] [DOI] [Google Scholar]
Osunkwo 2012a {published data only}
- Osunkwo I, Ziegler T, Alvarez J, George J, Cherry K, Rhodes J, et al. A randomized double blind, placebo controlled study of vitamin D to ameliorate sickle cell chronic pain. Journal of Pain 2012;13(4):S73. [DOI: 10.1016/j.jpain.2012.01.302] [DOI] [Google Scholar]
Osunkwo 2012b {published data only}
- Osunkwo I, Ziegler T, Cherry K, Alvarez J, Ghosh S, Rhodes J, et al. Results of a randomized double blind placebo controlled trial of vitamin D to ameliorate sickle cell chronic pain. American Journal of Hematology 2012;87(7):E56‐7. [Google Scholar]
Osunkwo 2012c {published data only}
- Osunkwo I, Ziegler TR, Alvarez J, McCracken C, Cherry K, Osunkwo CE, et al. High dose vitamin D therapy for chronic pain in children and adolescents with sickle cell disease: results of a randomized double blind pilot study. British Journal of Haematology 2012;159(2):211‐5. [DOI: 10.1111/bjh.12019.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ringe 2000 {published data only}
- Ringe JD, Cöster A, Meng T, Schacht E, Umbach R. Therapy of glucocorticoid‐induced osteoporosis with alfacalcidol/calcium and vitamin D/calcium [Therapie der Glucocorticoid‐induzierten Osteoporose mit Alfacalcidol/Kalzium und Vitamin D/Kalzium]. Zeitschrift für Rheumatologie 2000;59(3):176‐82. [DOI] [PubMed] [Google Scholar]
Ringe 2004 {published data only}
- Ringe JD, Dorst A, Faber H, Schacht E, Rahlfs VW. Superiority of alfacalcidol over plain vitamin D in the treatment of glucocorticoid‐induced osteoporosis. Rheumatology International 2004;24(2):63‐70. [DOI: 10.1007/s00296-003-0361-9] [DOI] [PubMed] [Google Scholar]
Ringe 2005 {published data only}
- Ringe JD, Faber H, Fahramand P, Schacht E. Alfacalcidol versus plain vitamin D in the treatment of glucocorticoid/inflammation‐induced osteoporosis. Journal of Rheumatology Supplement 2005;76:33‐40. [PubMed] [Google Scholar]
Ringe 2007 {published data only}
- Ringe JD, Farahmand P, Schacht E, Rozehnal A. Superiority of a combined treatment of Alendronate and Alfacalcidol compared to the combination of Alendronate and plain vitamin D or Alfacalcidol alone in established postmenopausal or male osteoporosis (AAC‐Trial). Rheumatology International 2007;27(5):425‐34. [DOI: 10.1007/s00296-006-0288-z] [DOI] [PubMed] [Google Scholar]
Sakalli 2012 {published data only}
- Sakalli H, Arslan D, Yucel AE. The effect of oral and parenteral vitamin D supplementation in the elderly: a prospective, double‐blinded, randomized, placebo‐controlled study. Rheumatology International 2012;32(8):2279‐83. [DOI: 10.1007/s00296-011-1943-6] [DOI] [PubMed] [Google Scholar]
Sanghi 2011 {published data only}
- Sanghi D, Srivastava RN, Agarwal S, Natu SM, Singh A, Avasthi S. Role of vitamin D in osteoarthritis knee: a six month double blind, randomized, placebo control trial. Osteoarthritis and Cartilage 2011;19(S1):S36. [Google Scholar]
Sanghi 2012 {published data only}
- Sanghi D, Srivastava RN. Role of vitamin D in osteoarthritis knee: a six month double blind, randomized, placebo control trial. Annals of the Rheumatic Disease 2012;71(Suppl 3):416. [Google Scholar]
Sanghi 2013b {published data only}
- Sanghi D, Srivastava RN, Mishra A, Natu S, Mishra R, Agarwal S. Role of vitamin D in osteoarthritis knee: a six month double blind, randomized, placebo control trial. Osteoarthritis and Cartilage 2013;21:S28‐9. [Google Scholar]
Sanghi 2014 {published data only}
- Sanghi D, Srivastava RN, Raj S, Mishra A, Natu SM, Agarwal S. Vitamin D receptor gene polymorphisms modulate the clinico‐radiological response to vitamin D supplementation in knee osteoarthritis. Osteoarthritis and Cartilage 2014;22:S235‐6. [Google Scholar]
Wicherts 2011 {published data only}
- Wicherts IS, Boeke AJ, Meer IM, Schoor NM, Knol DL, Lips P. Sunlight exposure or vitamin D supplementation for vitamin D‐deficient non‐western immigrants: a randomized clinical trial. Osteoporosis International 2011;22(3):873‐82. [DOI: 10.1007/s00198-010-1343-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wynn 2013 {published data only}
- Wynn RL. Vitamin D deficiency and nonspecific musculoskeletal pain. General Dentistry 2013;61(3):10‐3. [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12610000322033 {published data only}
- ACTRN12610000322033. Vitamin D as an analgesic for neuropathic pain in type 2 diabetes. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=335359 (accessed 2 March 2015).
Cao 2012 {published data only}
- Cao Y, Jones G, Cicuttini F, Winzenberg T, Wluka A, Sharman J, et al. Vitamin D supplementation in the management of knee osteoarthritis: study protocol for a randomized controlled trial. Trials 2012;13:131. [CTG: NCT01176344; DOI: 10.1186/1745-6215-13-131; ACTRN12610000495022] [DOI] [PMC free article] [PubMed] [Google Scholar]
CTRI/2014/06/004658 {published data only}
- CTRI/2014/06/004658. Effect of vitamin D on progression of primary osteoarthritis knee ‐ a double blind randomized control trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2014/06/004658 (accessed 2 March 2015).
EUCTR 2009‐015835‐34‐IT {published data only}
- EUCTR2009‐015835‐34‐IT. Vitamin D effect on T lymphocytes and osteoclastogenesis in rheumatoid arthritis. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2009‐015835‐34‐IT (accessed 2 March 2015).
EUCTR 2014‐000047‐33‐NL {published data only}
- EUCTR 2014‐000047‐33/NL. The effect of high‐resistance muscle strength training and vitamin D supplementation in persons with knee osteoarthritis ‐ vitD‐EX. www.clinicaltrialsregister.eu/ctr‐search/trial/2014‐000047‐33/NL (accessed 2 March 2015).
ISRCTN94818153 {published data only}
- ISRCTN94818153. A randomised, double‐blind, placebo‐controlled trial of vitamin D supplementation in the management of symptomatic knee osteoarthritis (the VIDEO study). isrctn.com/ISRCTN94818153 (accessed 2 March 2015).
NCT00279461 {published data only}
- NCT00279461. Vitamin D deficiency causes immune dysfunction and enables or perpetuates the development of rheumatoid arthritis. clinicaltrials.gov/ct2/show/NCT00279461?term=NCT00279461&rank=1 (accessed 2 March 2015).
NCT01023490 {published data only}
- Vitamin D treatment to patients suffering from chronic pain and vitamin D hypovitaminosis. clinicaltrials.gov/ct2/show/NCT01023490?term=NCT01023490&rank=1 (accessed 2 March 2015).
NCT01351805 {published data only}
- NCT01351805. Vitamin D and fish oil for autoimmune disease, inflammation and knee pain. clinicaltrials.gov/ct2/show/NCT01351805?term=NCT01351805&rank=1 (accessed 2 March 2015).
NCT01426347 {published data only}
- NCT01426347. Treatment of vitamin D deficiency in patients with rheumatoid arthritis. clinicaltrials.gov/ct2/show/NCT01426347?term=NCT01426347&rank=1 (accessed 2 March 2015).
NCT02002000 {published data only}
- NCT02002000. Chronic pain and vitamin D. clinicaltrials.gov/ct2/show/NCT02002000?term=NCT02002000&rank=1 (accessed 2 March 2015).
NCT02243800 {published data only}
- NCT02243800. Study of the efficacy and safety of cholecalciferol supplementation on the activity of rheumatoid arthritis in patients with vitamin D deficiency (SCORPION). clinicaltrials.gov/ct2/show/NCT02243800?term=NCT02243800&rank=1 (accessed 2 March 1915).
Additional references
Alonso 2004
- Alonso J, Ferrer M, Gandek B, Ware JE Jr, Aaronson NK, Mosconi P, et al. Health‐related quality of life associated with chronic conditions in eight countries: results from the International Quality of Life Assessment (IQOLA) Project. Quality of Life Research 2004;13(2):283‐98. [DOI] [PubMed] [Google Scholar]
Atherton 2009
- Atherton K, Berry DJ, Parsons T, Macfarlane GJ, Power C, Hypponen E. Vitamin D and chronic widespread pain in a white middle‐aged British population: evidence from a cross‐sectional population survey. Annals of the Rheumatic Diseases 2009;68(6):817‐22. [DOI: 10.1136/ard.2008.090456] [DOI] [PubMed] [Google Scholar]
Autier 2007
- Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta‐analysis of randomized controlled trials. Archives of Internal Medicine 2007;167:1730‐7. [DOI] [PubMed] [Google Scholar]
Bannwarth 2009
- Bannwarth B, Blotman F, Roué‐Le Lay K, Caubère JP, André E, Taïeb C. Fibromyalgia syndrome in the general population of France: a prevalence study. Joint Bone Spine 2009;76(2):184‐7. [DOI] [PubMed] [Google Scholar]
Bennett 2001
- Bennett M, Heard R. Treatment of multiple sclerosis with hyperbaric oxygen therapy. Undersea and Hyperbaric Medicine 2001;28(3):117‐22. [PubMed] [Google Scholar]
Benson 2006
- Benson J, Wilson A, Stocks N, Moulding N. Muscle pain as an indicator of vitamin D deficiency in an urban Australian Aboriginal population. Medical Journal of Australia 2006;185:76‐7. [DOI] [PubMed] [Google Scholar]
Bjelakovic 2014
- Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Wetterslev J, Simonetti RG, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD007470.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carroll 1996
- Carroll D, Tramèr M, McQuay H, Nye B, Moore A. Randomization is important in studies with pain outcomes: systematic review of transcutaneous electrical nerve stimulation in acute postoperative pain. British Journal of Anaesthesia 1996;77(6):798‐803. [DOI] [PubMed] [Google Scholar]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310:452‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dechartres 2013
- Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta‐epidemiological study. BMJ 2013;346:f2304. [DOI: 10.1136/bmj.f2304] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dworkin 2008
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105‐21. [DOI: 10.1016/j.jpain.2007.09.005] [DOI] [PubMed] [Google Scholar]
Gröber 2013
- Gröber U, Spitz J, Reichrath J, Kisters K, Holick MF. Vitamin D: update 2013: from rickets prophylaxis to general preventive healthcare. Dermato‐Endocrinology 2013;5(3):331‐47. [DOI: 10.4161/derm.26738] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holick 2007
- Holick MF. Vitamin D deficiency. New England Journal of Medicine 2007;357:266‐81. [DOI] [PubMed] [Google Scholar]
Holick 2011
- Holick MF, Binkley NC, Bischoff‐Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 2011;96(7):1911‐30. [DOI: 10.1210/jc.2011-0385.] [DOI] [PubMed] [Google Scholar]
Hossein‐nezhad 2013
- Hossein‐nezhad A, Holick MF. Vitamin D for health: a global perspective. Mayo Clinic Proceedings 2013;88(7):720‐55. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
IOM 2011
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press, 2011. [PubMed] [Google Scholar]
Jadad 1996a
- Jadad AR, Carroll D, Moore A, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. Pain 1996;66(2‐3):239‐46. [DOI: 10.1016/0304-3959(96)03033-3] [DOI] [PubMed] [Google Scholar]
Jadad 1996b
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI: 10.1016/0197-2456(95)00134-4] [DOI] [PubMed] [Google Scholar]
L'Abbé 1987
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐33. [DOI] [PubMed] [Google Scholar]
Lotfi 2007
- Lotfi A, Abdel‐Nasser AM, Hamdy A, Omran AA, El‐Rehany MA. Hypovitaminosis D in female patients with chronic low back pain. Clinical Rheumatology 2007;26:1895‐901. [DOI: 10.1007/s10067-007-0603-4] [DOI] [PubMed] [Google Scholar]
Mas 2008
- Mas AJ, Carmona L, Valverde M, Ribas B, EPISER Study Group. Prevalence and impact of fibromyalgia on function and quality of life in individuals from the general population: results from a nationwide study in Spain. Clinical and Experimental Rheumatology 2008;26(4):519‐26. [PubMed] [Google Scholar]
McQuay 1996
- McQuay H, Carroll D, Moore A. Variation in the placebo effect in randomised controlled trials of analgesics: all is as blind as it seems. Pain 1996;64(2):331‐5. [DOI: 10.1016/0304-3959(95)00116-6] [DOI] [PubMed] [Google Scholar]
McQuay 2007
- McQuay HJ, Smith LA, Moore RA. Chronic pain. In: Stevens A, Raferty J, Mant J, Simpson S editor(s). Health Care Needs Assessment. Oxford: Radcliffe Publishing Ltd, 2007:519‐600. [ISBN: 978‐1‐84619‐063‐6] [Google Scholar]
Mitsikostas 1996
- Mitsikostas DD, Tsaklakidou D, Athanasiadis N, Thomas A. The prevalence of headache in Greece: correlations to latitude and climatological factors. Headache 1996;36:168‐73. [DOI] [PubMed] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything‐large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. [DOI: 10.1016/S0304-3959(98)00140-7] [DOI] [PubMed] [Google Scholar]
Moore 2006
- Moore A, McQuay H. Bandolier's Little Book of Making Sense of the Medical Evidence. Oxford: Oxford University Press, 2006. [ISBN: 0‐19‐856604‐2] [Google Scholar]
Moore 2008
- Moore RA, Moore OA, Derry S, McQuay HJ. Numbers needed to treat calculated from responder rates give a better indication of efficacy in osteoarthritis trials than mean pain scores. Arthritis Research and Therapy 2008;10(2):R39. [DOI: 10.1186/ar2394] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2010
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. ACTINPAIN Writing Group of the IASP Special Interest Group on Systematic Reviews in Pain Relief, Cochrane Pain, Palliative and Supportive Care Systematic Review Group Editors. "Evidence" in chronic pain ‐ establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. [DOI: 10.1016/j.pain.2010.05.011] [DOI] [PubMed] [Google Scholar]
Moore 2013
- Moore RA, Straube S, Aldington D. Pain measures and cut‐offs ‐ 'no worse than mild pain' as a simple, universal outcome. Anaesthesia 2013;68(4):400‐12. [DOI: 10.1111/anae.12148] [DOI] [PubMed] [Google Scholar]
Moore 2014
- Moore AR, Derry S, Taylor RS, Straube S, Phillips CJ. The costs and consequences of adequately managed chronic non‐cancer pain and chronic neuropathic pain. Pain Practice 2014;14(1):79‐94. [DOI: 10.1111/papr.12050.] [DOI] [PubMed] [Google Scholar]
Morris 1995
- Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratios and standardised ratios and rates. In: Gardner MJ, Altman DG editor(s). Statistics with Confidence ‐ Confidence Intervals and Statistical Guidelines. London: British Medical Journal, 1995:50‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nüesch 2010
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta‐analyses of osteoarthritis trials: meta‐epidemiological study. BMJ 2010;341:c3515. [DOI: 10.1136/bmj.c3515] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perkins 2000
- Perkins FM, Kehlet H. Chronic pain as an outcome of surgery: a review of predictive factors. Anesthesiology 2000;93:1123‐33. [DOI] [PubMed] [Google Scholar]
Saastamoinen 2009
- Saastamoinen P, Laaksonen M, Lahelma E, Leino‐Arjas P. The effect of pain on sickness absence among middle‐aged municipal employees. Journal of Occupational and Environmental Medicine 2009;66(2):131‐6. [DOI: 10.1136/oem.2008.040733] [DOI] [PubMed] [Google Scholar]
Saps 2008
- Saps M, Blank C, Khan S, Seshadri R, Marshall B, Bass L, et al. Seasonal variation in the presentation of abdominal pain. Journal of Pediatric Gastroenterology and Nutrition 2008;46:279‐84. [DOI: 10.1097/MPG.0b013e3181559bd3] [DOI] [PubMed] [Google Scholar]
Verhaak 1998
- Verhaak PF, Kerssens JJ, Dekker J, Sorbi MJ, Bensing JM. Prevalence of chronic benign pain disorder among adults: a review of the literature. Pain 1998;77(3):231‐9. [DOI: 10.1016/S0304-3959(98)00117-1] [DOI] [PubMed] [Google Scholar]
Vincent 2013
- Vincent A, Lahr BD, Wolfe F, Clauw DJ, Whipple MO, Oh TH, et al. Prevalence of fibromyalgia: a population‐based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology Project. Arthritis Care and Research 2013;65(5):786‐92. [DOI: 10.1002/acr.21896.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeng 2004
- Zeng QY, Chen R, Xiao ZY, Huang SB, Liu Y, Xu JC, et al. Low prevalence of knee and back pain in southeast China; the Shantou COPCORD study. Journal of Rheumatology 2004;31:2439‐43. [PubMed] [Google Scholar]
References to other published versions of this review
Straube 2009
- Straube S, Moore RA, Derry S, McQuay HJ. Vitamin D and chronic pain. Pain 2009;141(1‐2):10‐3. [DOI: 10.1016/j.pain.2008.11.010] [DOI] [PubMed] [Google Scholar]
Straube 2010
- Straube S, Derry S, Moore RA, McQuay HJ. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007771.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


