Abstract
Background
Pancreatic cancer (PC) is a highly lethal disease with few effective treatment options. Over the past few decades, many anti‐cancer therapies have been tested in the locally advanced and metastatic setting, with mixed results. This review attempts to synthesise all the randomised data available to help better inform patient and clinician decision‐making when dealing with this difficult disease.
Objectives
To assess the effect of chemotherapy, radiotherapy or both for first‐line treatment of advanced pancreatic cancer. Our primary outcome was overall survival, while secondary outcomes include progression‐free survival, grade 3/4 adverse events, therapy response and quality of life.
Search methods
We searched for published and unpublished studies in CENTRAL (searched 14 June 2017), Embase (1980 to 14 June 2017), MEDLINE (1946 to 14 June 2017) and CANCERLIT (1999 to 2002) databases. We also handsearched all relevant conference abstracts published up until 14 June 2017.
Selection criteria
All randomised studies assessing overall survival outcomes in patients with advanced pancreatic ductal adenocarcinoma. Chemotherapy and radiotherapy, alone or in combination, were the eligible treatments.
Data collection and analysis
Two review authors independently analysed studies, and a third settled any disputes. We extracted data on overall survival (OS), progression‐free survival (PFS), response rates, adverse events (AEs) and quality of life (QoL), and we assessed risk of bias for each study.
Main results
We included 42 studies addressing chemotherapy in 9463 patients with advanced pancreatic cancer. We did not identify any eligible studies on radiotherapy.
We did not find any benefit for chemotherapy over best supportive care. However, two identified studies did not have sufficient data to be included in the analysis, and many of the chemotherapy regimens studied were outdated.
Compared to gemcitabine alone, participants receiving 5FU had worse OS (HR 1.69, 95% CI 1.26 to 2.27, moderate‐quality evidence), PFS (HR 1.47, 95% CI 1.12 to 1.92) and QoL. On the other hand, two studies showed FOLFIRINOX was better than gemcitabine for OS (HR 0.51 95% CI 0.43 to 0.60, moderate‐quality evidence), PFS (HR 0.46, 95% CI 0.38 to 0.57) and response rates (RR 3.38, 95% CI 2.01 to 5.65), but it increased the rate of side effects. The studies evaluating CO‐101, ZD9331 and exatecan did not show benefit or harm when compared with gemcitabine alone.
Giving gemcitabine at a fixed dose rate improved OS (HR 0.79, 95% CI 0.66 to 0.94, high‐quality evidence) but increased the rate of side effects when compared with bolus dosing.
When comparing gemcitabine combinations to gemcitabine alone, gemcitabine plus platinum improved PFS (HR 0.80, 95% CI 0.68 to 0.95) and response rates (RR 1.48, 95% CI 1.11 to 1.98) but not OS (HR 0.94, 95% CI 0.81 to 1.08, low‐quality evidence). The rate of side effects increased. Gemcitabine plus fluoropyrimidine improved OS (HR 0.88, 95% CI 0.81 to 0.95), PFS (HR 0.79, 95% CI 0.72 to 0.87) and response rates (RR 1.78, 95% CI 1.29 to 2.47, high‐quality evidence), but it also increased side effects. Gemcitabine plus topoisomerase inhibitor did not improve survival outcomes but did increase toxicity. One study demonstrated that gemcitabine plus nab‐paclitaxel improved OS (HR 0.72, 95% CI 0.62 to 0.84, high‐quality evidence), PFS (HR 0.69, 95% CI 0.58 to 0.82) and response rates (RR 3.29, 95% CI 2.24 to 4.84) but increased side effects. Gemcitabine‐containing multi‐drug combinations (GEMOXEL or cisplatin/epirubicin/5FU/gemcitabine) improved OS (HR 0.55, 95% CI 0.39 to 0.79, low‐quality evidence), PFS (HR 0.43, 95% CI 0.30 to 0.62) and QOL.
We did not find any survival advantages when comparing 5FU combinations to 5FU alone.
Authors' conclusions
Combination chemotherapy has recently overtaken the long‐standing gemcitabine as the standard of care. FOLFIRINOX and gemcitabine plus nab‐paclitaxel are highly efficacious, but our analysis shows that other combination regimens also offer a benefit. Selection of the most appropriate chemotherapy for individual patients still remains difficult, with clinicopathological stratification remaining elusive. Biomarker development is essential to help rationalise treatment selection for patients.
Plain language summary
The effects of anti‐cancer therapies on advanced pancreatic cancer
Review question
This review aimed to answer the question, which therapies are the most effective for advanced pancreatic cancer?
Background
Pancreatic cancer (PC) is a serious, often fatal disease, and many people are not diagnosed until they have advanced tumours that cannot be removed with surgery. Symptoms include abdominal pain, weight loss, and yellowing of the skin and eyes. Up until recently, gemcitabine was the standard drug for treating advanced pancreatic cancer, but this gave people only a modest benefit.
Study characteristics
We looked for all studies in people with pancreatic cancer that could not be operated on (locally advanced) or that had already spread beyond the pancreas (metastatic). We found 42 clinical studies involving 9463 participants who were receiving their first therapy for PC. Our search is current to June 2017.
The studies compared one therapy against either best supportive care (symptom management only) or another type of therapy. Studies had to evaluate overall survival (or time to death). The study could be testing either chemotherapy (drugs that kill or slow the growth of cancer cells) or radiotherapy (X‐ray treatment). We collected data on survival, tumour response rate, side effects and quality of life. The results of clinical studies addressing targeted/biological therapies, immunotherapies, second‐line therapies and local treatments for locally advanced disease will be reported in a separate Cochrane Review.
Key results
This review has shown that in advanced disease, combination chemotherapy with FOLFIRINOX (5‐fluorouracil, irinotecan, oxaliplatin combination); GEMOXEL (gemcitabine, oxaliplatin and capecitabine); cisplatin/epirubicin/5FU/gemcitabine; gemcitabine plus nab‐paclitaxel; and gemcitabine plus a fluoropyrimidine agent, provide a survival advantage over gemcitabine alone. These combinations do increase side effects. Gemcitabine given slowly using a fixed rate of infusion may be more effective than giving it in the standard way, which is quickly over 30 minutes.
Quality of the evidence
The quality of the evidence varied greatly amongst comparisons. The highest quality evidence was for gemcitabine versus fixed dose rate gemcitabine and some of the gemcitabine combinations (fluoropyrimidine, topoisomerase, and taxane). We judged the studies for quality using factors like how well they were conducted, how well they reported results and whether they used a placebo.
Summary of findings
Background
Recently published global cancer statistics show that pancreatic cancer (PC) accounted for 184,400 deaths worldwide in 2012, with the highest incidence in men in high‐income countries at 8.6 cases per 100,000 (Torre 2015). In Australia, although PC is relatively uncommon (incidence of 11 per 100,000), it is highly lethal, representing the fourth leading cause of death from cancer (Tracey 2010). The US National Cancer Institute has reported a five‐year survival of 21.5% for those with localised disease (www.cancer.gov); however, a review of the Finnish Cancer Registry showed five‐year survival of only 4.3% for those with localised disease and an overall five‐year survival of 0.2% (Carpelan 2005).
PC is a notoriously insidious cancer, commonly presenting with vague, non‐specific symptoms that classically consist of the triad of epigastric abdominal pain, weight loss and jaundice (Howard 1977; Warshaw 1992), which gradually worsen over time. Physical examination is often normal, with the commonest sign of an enlarged liver present in fewer than half of patients (Von Hoff 2005). Thus, most patients have advanced disease when they are diagnosed.
Approximately 10% of early stage pancreatic carcinomas are amenable to curative surgery (Siegel 2013). However, the risk of relapse after surgical resection is still quite high, with only 10% of patients surviving for five years (Conlon 1996; Shahrudin 1997). Although studies have reported a benefit for chemotherapy in advanced disease (Burris 1997; Heinemann 2008; Conroy 2011; Von Hoff 2013), the role of second and subsequent lines of chemotherapy remains controversial (Nagrial 2015). The benefits of radiotherapy, either alone or in combination, as a palliative treatment for advanced or relapsed disease, is uncertain (Sultana 2007). Hammel 2013 tested contemporary chemotherapy and radiotherapy techniques but did not demonstrate a survival benefit in locally advanced disease. Biological therapies are emerging in the treatment of pancreatic cancer and but have yet to find their place in routine clinical practice (Castellanos 2011).
There are other published meta‐analyses that look at various aspects covered by this review. Li 2014 analysed eight studies that assessed randomised data using gemcitabine and fluoropyrimidine agents, finding a benefit using gemcitabine plus fluoropyrimidine. Petrelli 2014 analysed 29 studies that assessed gemcitabine monotherapy versus chemotherapy combinations, finding improved outcomes with the chemotherapy combinations. Two studies have used a Bayesian network meta‐analysis to perform direct and indirect comparisons of chemotherapy combinations (Chan 2014; Gresham 2014). Chan 2014 concluded that FOLFIRINOX was likely to be the most efficacious regimen in the advanced stage. Two meta‐analyses have assessed chemotherapy plus radiotherapy (Bernstein 2014; Chen 2013), both finding a small benefit to adding chemotherapy to radiation; however, neither included the recent study conducted by Hammel 2013.
Anti‐cancer therapies in the metastatic setting ideally aim to improve people's quality and length of life, with tolerable side effects. This review will analyse both the anti‐cancer effects and the adverse effects of treatments in patients with pancreatic cancer.
Description of the condition
Pancreatic ductal adenocarcinoma (PDAC) is a cancer arising from the ducts in the pancreas gland. It can be localised to the pancreas (local disease), locally advanced (still confined to the area around the pancreas but possibly involving lymph glands or other immediately adjacent structures) or metastatic (with cancer spread to distant areas).
This review includes studies in patients with locally advanced (not amenable to local therapies) or metastatic PC, formally defined as follows (Callery 2009).
-
Locally advanced or unresectable, defined by:
greater than 180° of superior mesenteric vein encasement, any coeliac abutment;
unreconstructable superior mesenteric vein or portal occlusion;
aortic invasion or encasement;
nodal involvement beyond the field of resection.
Metastatic, defined by distant sites of disease.
Description of the intervention
Chemotherapy
Chemotherapy encompasses all cytotoxic or antineoplastic drug treatments, intravenous or oral, which work by killing or slowing the growth of cancer cells. Although the schedules differ between therapies, most are given on a four‐weekly basis (one cycle) for up to six cycles.
Radiotherapy
Radiation therapy uses X‐rays to destroy or injure cancer cells so they cannot multiply (Queensland Cancer Fund 2012). It is given in a number of different ways.
External beam radiotherapy: delivered over a number of sessions (fractions) utilising an external radiotherapy source emitting X‐rays, gamma rays, electrons or heavy particles.
Stereotactic body radiation therapy: a highly conformal (targeted) technique for delivering external beam radiotherapy in a single fraction (stereotactic radiosurgery) or a number of fractions (stereotactic radiotherapy).
Brachytherapy: internal radiotherapy utilising a radioactive source placed into or adjacent to the pancreas and administered in a single fraction or number of fractions, given alone or in combination with external beam radiotherapy.
Intraoperative radiotherapy: administration of external source radiotherapy or brachytherapy at the time of surgery, given alone or in combination with external beam radiotherapy.
Best supportive care
Best supportive care in advanced disease is defined as anything other than chemotherapy. It may include symptom control by radiotherapy (not to the primary site), palliative surgery, biliary stent insertion, analgesia, blood transfusion, and psychological or social support.
How the intervention might work
The primary goal for all treatments for locally advanced or metastatic pancreatic cancer is to palliate symptoms and improve overall survival (see Appendix 1, 'Glossary of terms'). In general, chemotherapy and radiotherapy can potentially kill cancer cells in the body and reduce the severity of the disease. This can in turn, reduce symptoms and increase survival times. In the advanced setting, chemotherapy and radiotherapy do not offer a cure. Best supportive care is usually administered alongside chemotherapy and radiotherapy, but it can be the sole treatment given to some patients. All anti‐cancer therapies can cause side effects, which commonly include fatigue, nausea, vomiting, low blood counts (haemoglobin, white cells and platelets) and diarrhoea. Radiotherapy can cause local pain, skin rash, fatigue, nausea and vomiting.
Why it is important to do this review
Given the poor prognosis of PC, evidence‐based clinical decision‐making is paramount in guiding patients through treatments. Performing a meta‐analysis of studies will ensure that clinicians and patients have a reference to inform their clinical choices.
The meta‐analysis published previously in Yip 2009 has been criticised for not using hazard ratios to assess survival (Sultana 2007). This update will use hazard ratios and also assess quality of life.
PC is a notoriously difficult cancer in which to perform clinical studies, and much controversy exists. Although there is evidence in the first line setting that supports the use of FOLFIRINOX (Conroy 2011), gemcitabine plus erlotinib (Moore 2007), gemcitabine plus fluoropyrimidine (Cunningham 2009), or nab‐paclitaxel (Von Hoff 2013), questions remain with regard to toxicity, cost and survival benefits. There is conflicting evidence on the place for and schedule of chemoradiation as well as debate about the optimum drug and dose (Kim 2007; Philip 2011).
Previous meta‐analyses have had narrow search criteria (Chan 2014; Li 2014; Petrelli 2014), or they have used only phase III randomised data (Gresham 2014). Here, we have attempted to synthesise and organise all available randomised data concerning patients having treatment for advanced pancreas cancer in order to help inform clinical decision‐making and guide further research in this area.
Objectives
To assess the effect of chemotherapy, radiotherapy or both for first‐line treatment of advanced pancreatic cancer. Our primary outcome was overall survival, while secondary outcomes include progression‐free survival, grade 3/4 adverse events, therapy response and quality of life.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled studies, both published and unpublished, comparing one of the intervention types versus placebo, another intervention type or best supportive care.
Types of participants
People with a diagnosis of pancreatic adenocarcinoma established by either histological or cytological findings (investigations on body tissue or cells). Studies enrolling people with advanced, unresectable or recurrent disease were eligible for inclusion.
Types of interventions
Any type of chemotherapy, radiotherapy or combination of chemotherapy plus radiotherapy versus placebo, no treatment, best supportive care or another chemotherapy and/or radiotherapy treatment regimen.
Best supportive care in advanced disease may include symptom control by radiotherapy (not to the primary site), palliative surgery, biliary stent insertion, analgesia, blood transfusion and psychological or social support.
We looked for interventions falling into the following comparisons.
Any chemotherapy treatment versus placebo, no treatment or best supportive care.
Any chemotherapy treatment versus any other chemotherapy treatment.
Any radiotherapy treatment versus placebo, no treatment or best supportive care.
Any radiotherapy treatment versus any other radiotherapy treatment.
Any combination of radiotherapy and chemotherapy versus placebo, no treatment or best supportive care.
Any combination of radiotherapy and chemotherapy versus any other combination of radiotherapy and chemotherapy.
After searching was complete, the studies were organised into four specific comparisons.
Anti‐cancer therapy versus best supportive care
Various types of chemotherapy versus gemcitabine
Gemcitabine combinations versus gemcitabine alone
Fluoropyrimidine combinations versus fluoropyrimidines alone
Types of outcome measures
Primary outcomes
Overall survival (OS) ‐ survival until death from any cause
Secondary outcomes
Progression‐free survival (PFS) ‐ time to progression of disease on a given therapy. This is usually detected by an increase of the size or number of cancer lesions seen on a computer tomography scan (CT) using the Response Evaluation Criteria in Solid Tumours (RECIST) criteria (Nishino 2010).
Quality of life (QoL), measured with a validated instrument, such as the European Organisation for Research and Treatment of Cancer (EORTC) quality of life questionnaire for cancer patients (QLQ‐C30) (eortc.be/qol/).
Response rates ‐ this relates to the shrinkage of a cancer in response to therapy and is usually measured on CT scans, with cancer shrinkage defined according to the RECIST criteria (Nishino 2010).
Grade 3/4 adverse events ‐ adverse events are defined by the National Cancer Institute (cancer.gov) as an unfavourable and unintended sign or symptom associated with a medical treatment. Severity is graded. Grade 3 is classed as a severe or medically significant event but not immediately life threatening. Hospitalisation is indicated, and the effects limit the patients' ability to self care. Grade 4 is classed as a life‐threatening event requiring urgent attention.
Search methods for identification of studies
The authors completed searches to identify all relevant published and unpublished randomised controlled studies. Articles published in any language were eligible for inclusion.
We searched the following electronic databases.
Cochrane Central Register of Controlled Trials (CENTRAL; 2017; Issue 6), which includes the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group Trials Register, in the Cochrane Library (searched 14 June 2017); Appendix 2.
MEDLINE (1946 to 14 June 2017); Appendix 3.
EMBASE (1980 to 14 June 2017); Appendix 4.
CANCERLIT (1999 to 2002). We did not undertake subsequent searches in CANCERLIT, as the database merged with MEDLINE in 2002.
To identify randomised controlled studies, we applied phases one, two and three of the Cochrane highly sensitive search strategy, as described in the Cochrane Handbook for Reviews of Interventions (Higgins 2011).
Electronic searches
We handsearched reference lists from studies and review articles from the electronic searching to identify further relevant studies. We also handsearched published abstracts from the following conference proceedings.
American Gastroenterological Association (AGA) (1994 to 2014).
American Society of Clinical Oncology (ASCO) (1996 to 2016).
American Association of Cancer Research (AACR) (1957 to 2014).
American Pancreatic Association (APA) (2001 to 2014).
Digestive Disease Week (DDW) (1994 to 2014).
European Cancer Conference (ECCO) (1997, 1999, 2001, 2003, 2005, 2007, 2009, 2011, 2013).
European Society of Medical Oncology (ESMO) (1998, 2000, 2002, 2004, 2006, 2008, 2010, 2012, 2014).
Joint ECCO/ESMO meeting (2009, 2010, 2011, 2013).
European Pancreatic Club (EPC) (2000 to 2014).
Gastrointestinal Cancers Symposium (2007 to 2015).
United European Gastroenterology Week (UEGF) (1960 to 2014).
We searched the following information resources.
National Cancer Institute Physician Data Query.
UK Co‐ordinating Committee on Cancer Research.
We also searched the following study registers.
Australian and New Zealand Clinical Trials Registry.
National Research Register.
Medical Research Council.
Clinicaltrials.gov.
Current Controlled Trials.
Trialscentral.
Center Watch.
Searching other resources
We searched the Internet using the Google search engine. In addition, we contacted members of the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group and other experts in the field and ask them to provide details of outstanding clinical studies and any relevant unpublished materials that were known to them.
Data collection and analysis
Selection of studies
We scanned titles of studies from the electronic search, removing duplicates. Two independent review authors (VC and AN) then considered the titles and abstracts to exclude clearly ineligible studies. We retrieved the full text of all remaining records, and two review authors (VC and AN) independently assessed them against inclusion criteria for the review, resolving disagreements with adjudication by a third review author (DY) according to the process outlined in Chapter 7.2.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We documented reasons for excluding studies according to Higgins 2011.
Data extraction and management
Two independent review authors (VC and AN) extracted data, recording the inclusion/exclusion criteria, number of participants and treatment arms for each study. For survival outcomes, we recorded hazard ratios (HRs) for OS and PFS from the published data where possible. If not reported, then we extracted time‐to‐event data and derived the HRs using the methods described in Tierney 2007. We also extracted median survival times. For response rates and adverse events (AEs), we recorded the number of people who had experienced an event of interest and the total number of people evaluated for that event to determine the risk ratio (RR). We extracted details on QoL in a descriptive fashion as published.
Assessment of risk of bias in included studies
Two review authors used the Cochrane 'Risk of bias' tool to independently assess risk of bias in the studies, with a a third independent review author settling disputes (Higgins 2011).
We summarised the results in a 'Risk of bias summary' graph. We interpreted the results of meta‐analyses in light of the findings of the risk of bias assessments.
Measures of treatment effect
For survival data, we used the HR with 95% confidence intervals (CI) and median survival times. For dichotomous data (response rates and grade 3/4 AEs), we used the risk ratio (RR) with a 95% CI. We report quality of life in a descriptive, tabulated fashion.
Unit of analysis issues
For studies that compared more than one treatment arm with a control arm in the same meta‐analysis, we divided the number of participants in the control group by the number of treatment arms. There were no other unit of analysis issues.
Dealing with missing data
When we could not extract data from the text, or when statistics were missing, we attempted to contact the authors of the original article to obtain the necessary information.
Assessment of heterogeneity
We assessed heterogeneity by visual inspection of the forest plots and statistically with the Chi² test for homogeneity and the I² statistic for inconsistency.
Assessment of reporting biases
Had we included comparisons with more than 10 included studies, we would have constructed funnel plots to assess reporting bias.
Data synthesis
We used the generic inverse variance method for all meta‐analyses according to the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Due to the heterogeneity of the interventions and comparators, we used a random‐effects model in all instances. We performed all analyses using Review Manager 5 (RevMan 5) software (RevMan 2014), following an intention‐to‐treat principle when data permitted.
Subgroup analysis and investigation of heterogeneity
We did not perform any subgroup analyses.
Sensitivity analysis
We planned to perform sensitivity analyses by excluding studies at high risk of bias from the meta‐analysis, but due to the small number of studies in the various comparisons, we were unable to do so.
Summary of findings table
We created four summary of findings tables describing the primary outcome measure of OS for participants. We included a narrative summary of the toxicity and QoL data in the comments section of the table. We calculated the median 12‐month survival rate for the control arm to calculate the assumed risk for each comparison. We used the percentage of people alive at 12 months if it was available, otherwise we extracted the data from the Kaplan‐Meier curves. We then applied the summary HR to this rate to give an anticipated effect on the rate of death with the intervention versus the comparator, expressed as number of events per 1000 people. We used the 6‐month survival rate if all control arm participants had died by 12 months.
We used the GRADE approach to assess the quality of the body of evidence for the outcome OS as described by the GRADE Working Group and in the GRADE Handbook (Guyatt 2011; Schünemann 2013).
Results
Description of studies
Results of the search
Figure 1 presents the study flow chart. We identified 1304 studies through electronic searches and an additional 80 studies through handsearching. After removing duplicates and studies that were clearly not eligible for inclusion, we assessed 215 full‐text articles. Of these, we excluded 155, including 49 that did not meet the inclusion criteria for the review, and 106 that will be reported in a separate Cochrane Review.
1.

1 Study flow diagram.
Included studies
The original published protocol had wide inclusion criteria. Due to the large number of studies identified, we decided to split the review. Therefore, we will report studies addressing biological agents, immunotherapy, second‐line therapies and local therapies for locally advanced disease separately. This report focuses on studies of either chemotherapy or radiotherapy in the advanced setting only.
We included sixty studies assessing the effects on chemotherapy in advanced PC (Characteristics of included studies). We did not identify any studies that addressed radiotherapy in the advanced setting. Of the included studies, we were able to include 42 with data on 9463 participants in a meta‐analysis.
We categorised these studies into five main categories.
Any anti‐cancer treatment versus best supportive care (6 studies: Andren‐Sandberg 1983; Frey 1981; Glimelius 1996; Huguier 2001,Takada 1998; Xinopoulos 2008).
Various types of chemotherapy versus gemcitabine (8 studies: Burris 1997; Cheverton 2004; Conroy 2011; Poplin 2009; Poplin 2013; Singhal 2014; Smith 2003; Tempero 2003).
Gemcitabine combination versus gemcitabine alone (7 studies addressing platinum plus gemcitabine: Colucci 2002; Colucci 2010; Heinemann 2006; Li 2004; Louvet 2005; Viret 2004; Wang 2002; 10 studies addressing fluoropyrimidine plus gemcitabine: Berlin 2002; Cunningham 2009; Di Costanzo 2005; Herrmann 2007; Lee 2017; Ohkawa 2004; Ozaka 2012; Riess 2005; Scheithauer 2003; Ueno 2013; 3 studies addressing topoisomerase inhibitors plus gemcitabine: Abou‐Alfa 2006; Rocha Lima 2004; Stathopoulos 2006; 1 study addressing taxane plus gemcitabine: Von Hoff 2013; 2 studies addressing multi‐drug combinations including gemcitabine: Petrioli 2015; Reni 2005; and 4 studies of other agents combined with gemcitabine: Gansauge 2002; Meng 2012; Oettle 2005; Ueno 2013 – EPA study).
Fluoropyrimidine‐based studies (4 studies: Ducreux 2004; Kovach 1974; Maisey 2002; Moertel 1979).
Single studies addressing unique treatment comparisons (13 studies: Afchain 2009; Boeck 2008; Bukowski 1983; Corrie 2017; Hirao 2011; Kelsen 1991; Kulke 2009; Levi 2004; Lohr 2012; Lutz 2005; Moertel 1977; Reni 2012; Topham 1991).
1 Anti‐cancer therapy versus best supportive care
Six studies compared a type of anticancer therapy with best supportive care (BSC). Andren‐Sandberg 1983 (N = 47) compared 5FU/CCNU plus vincristine (n = 25) versus BSC (n = 22). Frey 1981 included 152 participants with unresectable PC and assessed 5‐fluorouracil (5FU) plus chloroethylcyclohexylnitrosurea (CCNU). Glimelius 1996 studied people with advanced PC or biliary tract cancer; of the 53 participants with PC, 29 were given 5FU/LV, with or without etoposide, and 24 received BSC. Huguier 2001 included 45 participants with unresectable PC; the treatment arm was cisplatin plus 5FU plus leucovorin (LV). Takada 1998 included 83 people with unresectable PC; the treatment arm was 5FU plus doxorubicin plus mitomycin C (MMC). Xinopoulos 2008 included 49 people with locally advanced PC; the treatment arm was gemcitabine.
2 Various types of chemotherapy versus gemcitabine
Eight studies compared various types of chemotherapy versus gemcitabine.
2.1 5FU versus gemcitabine
There was one study in this group involving 126 people with symptomatic advanced PC; 63 were given 5FU and 63 gemcitabine chemotherapy (Burris 1997).
2.2 FOLFIRINOX versus gemcitabine
Conroy 2011 tested FOLFIRINOX in 342 people, and Singhal 2014 in 310 people, with metastatic PC.
2.3 CO‐101 versus gemcitabine
One study in 367 participants with metastatic PC compared CO‐101 (lipid conjugate form of gemcitabine) versus gemcitabine (Poplin 2013).
2.4 ZD9331 versus gemcitabine
One study addressed this comparison (Smith 2003), including 55 participants with locally advanced (LA) or metastatic PC. The treatment arm was ZD9331 (thymidylate synthase inhibitor).
2.5 Fixed‐dose rate gemcitabine versus standard infusional gemcitabine
Two studies were available for analysis: Poplin 2009 and Tempero 2003. Both had slightly different schedules: Poplin 2009 involved 824 participants with LA or metastatic PC and compared gemcitabine at 1000 mg/m² given over 30 min weekly for 7 out of 8 weeks then 3 out of 4 weeks versus gemcitabine given at 1500 mg/m² over 150 min 3 out of 4 weeks. Tempero 2003 involved 92 people with LA or metastatic PC and compared a dose‐dense regimen of gemcitabine 2200 mg/m² weekly, 3 out of 4 weeks versus gemcitabine 1500 mg/m² given at 10 mg/m²/min, weekly, 3 out of 4 weeks.
2.6 Exatecan (DX‐8951f) versus gemcitabine
One study addressed this comparison (Cheverton 2004), including 339 chemotherapy‐naive participants with LA or metastatic PC. The treatment arm was exatecan (a hexacyclic, water‐soluble, topoisomerase‐1 inhibitor).
3 Gemcitabine combination studies
3.1 Gemcitabine plus a platinum agent versus gemcitabine alone
Seven studies compared gemcitabine plus a platinum agent versus gemcitabine alone (Colucci 2002; Colucci 2010; Heinemann 2006; Li 2004; Louvet 2005; Viret 2004; Wang 2002). Louvet 2005 used oxaliplatin, while the rest used cisplatin. All studies had gemcitabine alone as the control arm and gemcitabine plus a platinum agent in the treatment arm. Colucci 2002 (N = 107), Colucci 2010 (N = 400), Heinemann 2006 (N = 195). Li 2004 (N = 46) and Louvet 2005 (N = 326) all included people with LA or metastatic PC, while Viret 2004 (N = 83) and Wang 2002 (N = 42) included participants with stage III/IV PC.
3.2 Gemcitabine plus fluoropyrimidine versus gemcitabine alone
Ten studies compared gemcitabine plus fluoropyrimidine versus gemcitabine alone (Berlin 2002; Cunningham 2009; Di Costanzo 2005; Herrmann 2007; Lee 2017; Ohkawa 2004; Ozaka 2012; Riess 2005; Scheithauer 2003; Ueno 2013).
Two studies assessed infusional 5FU in 567 participants with with LA/metastatic PC (Di Costanzo 2005; Riess 2005), and one study tested bolus 5FU in 322 participants with unresectable PC (Berlin 2002).
Four studies used capecitabine in: 533 people with LA/metastatic PC (Cunningham 2009), 319 people with inoperable/metastatic PC (Herrmann 2007), 214 people with LA/metastatic PC (Lee 2017), and 83 people with metastatic PC (Scheithauer 2003).
Two studies used oral tegafur (S1) in LA/metastatic PC: Ozaka 2012 included 112 participants and Ueno 2013 832. Ueno 2013 was a multi‐armed study that compared gemcitabine versus S1 versus gemcitabine plus S1.
One study assessed tegafur‐uracil (UFT) in 19 participants (Ohkawa 2004).
3.3 Gemcitabine plus toposiomerase inhibitor versus gemcitabine alone
Three studies compared gemcitabine plus a toposiomerase inhibitor versus gemcitabine alone in participants with LA or metastatic PC (Abou‐Alfa 2006; Rocha Lima 2004; Stathopoulos 2006). Rocha Lima 2004 (N = 360) and Stathopoulos 2006 (N = 130) tested irinotecan, and Abou‐Alfa 2006 (N = 349) used exatecan.
3.4 Gemcitabine plus taxane versus gemcitabine alone
Only one study, in 861 participants with metastatic PC, was suitable for analysis (Von Hoff 2013).
3.5 Gemcitabine plus other combinations of chemotherapy versus gemcitabine alone
Two studies assessed gemcitabine plus other combinations of chemotherapy: Petrioli 2015 included 67 people with metastatic PC and combined oxaliplatin plus capecitabine plus gemcitabine (GEMOXEL). Reni 2005 assessed 99 people with LA/metastatic PC and used a combination cisplatin‐epirubicin‐5FU‐gemcitabine.
3.6 Gemcitabine in combination with other agents versus gemcitabine alone
Four studies examined different agents in combination with gemcitabine: Gansauge 2002 looked at 90 participants with unresectable PC and used Ukrain (herbal medicine), Meng 2012 assessed 76 people with unresectable PC and used huachansu (Chinese herbal medicine), Oettle 2005 included 565 people with LA/metastatic PC and used pemetrexed, and Ueno 2013 – EPA study included 66 people with advanced PC and used eicosapentaenoic acid supplement (EPA).
4 Fluoropyrimidine combinations versus fluoropyrimidine alone
Four studies compared fluoropyrimidine combinations versus fluoropyrimidine alone (Ducreux 2004; Kovach 1974; Maisey 2002; Moertel 1979). Ducreux 2004 was a three‐armed study in 63 participants with LA or metastatic PC, and Kovach 1974 included 82 participants with unresectable PC and compared 5FU versus bis‐chloroethylnitrosurea (BCNU) alone versus 5FU plus BCNU. Maisey 2002 analysed 209 participants with LA or metastatic PC and compared 5FU versus 5FU plus mitomycin C (MMC). Moertel 1979 involved 176 people with metastatic PC and used streptozocin in the treatment arm. We were unable to include Cullinan 1985 and Cullinan 1990 in the meta‐analysis, as they were multi‐armed studies in which the control arm could not be split.
5 Single studies addressing unique treatment comparisons
Many studies addressed unique comparisons, so we could not group them with other studies.
Boeck 2008 studied capecitabine plus oxaliplatin (n = 61) versus capecitabine plus gemcitabine (n = 64) versus modified gemcitabine plus oxaliplatin (n = 63).
Kulke 2009 was a multi‐armed study comparing fixed dose rate gemcitabine (n = 64) versus infusional gemcitabine plus cisplatin (n = 66) versus infusional gemcitabine plus docetaxel (n = 65) versus infusional gemcitabine plus irinotecan (n = 60).
Afchain 2009 compared standard gemcitabine plus oxaliplatin (n = 20) versus a simplified gemcitabine plus oxaliplatin protocol (n = 37).
Bukowski 1983 compared mitomycin C plus 5FU (MF) (n = 73) versus streptozocin plus MMC plus 5FU (SMF) (n = 72).
Hirao 2011 looked at gemcitabine given on a three‐week schedule (n = 45) versus gemcitabine given on a four‐week schedule (n = 45).
Kelsen 1991 compared streptozocin plus MMC plus 5FU (SMF) (n = 42) versus cisplatin plus ara‐C plus caffeine (CAC) (n = 40).
Levi 2004 studied 5FU given either as a constant or chronomodulated infusion, with (n = 52) versus without (n = 55) cisplatin.
Lutz 2005 compared gemcitabine plus docetaxel (n = 49) versus cisplatin plus docetaxel (n = 47).
Moertel 1977 looked at streptozocin plus 5FU (n = 40) versus streptozocin plus cyclophosphamide (n = 48).
Reni 2012 compared capecitabine plus cisplatin plus gemcitabine plus docetaxel (PDXG) (n = 53) versus capecitabine plus cisplatin plus gemcitabine plus epirubicin (PEXG) (n = 48).
Finally, Topham 1991 looked at epirubicin (n = 32) versus 5FU plus epirubicin plus MMC (n = 30).
Excluded studies
We excluded 155 studies. Other Cochrane Reviews will cover the 53 studies addressing biological agents, the 11 assessing immunotherapies, the 25 looking at local therapies in locally advanced disease and the 17 focusing on second‐line therapies. We excluded the remaining 49 studies for the following reasons.
Five studies did not mandate histological confirmation in the study protocol (Abdel Wahab 1999; Johnson 2001; Mallinson 1980; Nakai 2012; Palmer 1994).
Two studies included some participants who did not have advanced stage PC (Andersen 1981; Lygidakis 1995).
Fifteen studies did not provide sufficient data (Baker 1976; Cohen 2010; GITSG 1985; Kim 2011; Oberic 2011; Queisser 1979; Ramanathan 2011; Sakata 1992; Senzer 2006; Shapiro 2005; Sultana 2009; Sun 2011; Tagliaferri 2013; Trouilloud 2012; Van Cutsem 2013).
Nine studies included people with non‐PDAC histologies (Ducreux 2002; GITSG 1988; Lokich 1979; Mizuno 2013; Moertel 1981; Oster 1986; Schein 1978; Sudo 2014; Takada 1994).
Five were cross‐over studies (Berglund 2010; Dahan 2010; Heinemann 2013 (GUT); Horton 1981; Javle 2011).
Five had a non‐randomised study design (Bukowski 1993; Gong 2007; Mitry 2006; Yongxiang 2001; Zemskov 2000).
Three studies published only interim results (GITSG 1979; Topham 1993; Tuinmann 2008).
Survival was not an endpoint in three studies (Ardalan 1988; Meyer 2008; Schmitz‐Winnenthal 2013).
Risk of bias in included studies
Figure 2 and Figure 3 summarise the risk of bias of all included studies. Many studies did not publish sufficient details to make a judgement on selection bias. Of those that did, all were judged to be at a low risk of bias because they used centralised randomisation techniques. Only one study was double‐blind and placebo controlled (Meng 2012), and we judged it to be at low risk for performance bias. We assessed the remainder of the studies to be at a high risk of bias. We considered studies that used OS as the primary endpoint to be at a low risk for detection bias (Abou‐Alfa 2006; Berlin 2002; Cheverton 2004; Colucci 2010; Conroy 2011; Cullinan 1985; Cullinan 1990; Cunningham 2009; Frey 1981; Gansauge 2002; Glimelius 1996; Heinemann 2006; Herrmann 2007; Huguier 2001; Kulke 2009; Lee 2017; Levi 2004; Li 2004; Lohr 2012; Louvet 2005; Oettle 2005; Poplin 2009; Poplin 2013; Riess 2005; Rocha Lima 2004; Singhal 2014; Smith 2003; Stathopoulos 2006; Takada 1998; Tempero 2003; Ueno 2013; Von Hoff 2013; Xinopoulos 2008). If tumour assessments were needed to assess the primary outcome (e.g. RR or PFS), we assigned a low risk of bias only if an independent reviewer or by a blinded radiologist conducted the assessments (Ducreux 2004; Reni 2005; Reni 2012; Scheithauer 2003). We judged all other studies to be at a high risk of bias. We deemed studies that reported the intention‐to‐treat population (all participants randomised on the study regardless if they received any treatment or not) to be at a low risk of attrition bias, while we considered studies that did not report all randomised patients to be at a high risk of bias (Bukowski 1983; Cullinan 1985; Ducreux 2004; Kelsen 1991; Louvet 2005; Moertel 1977; Ozaka 2012). We detected selective reporting bias in only two studies (Bukowski 1983; Moertel 1979), the former because only the participants with measurable disease were reported in detail and the latter because the toxicity data were not comprehensively reported.
2.
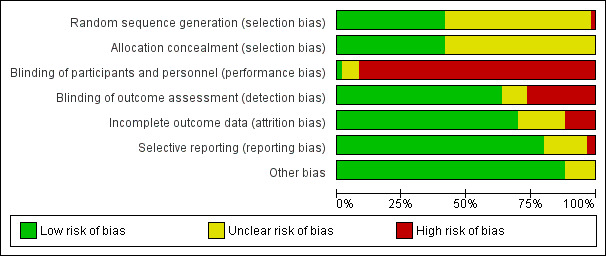
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
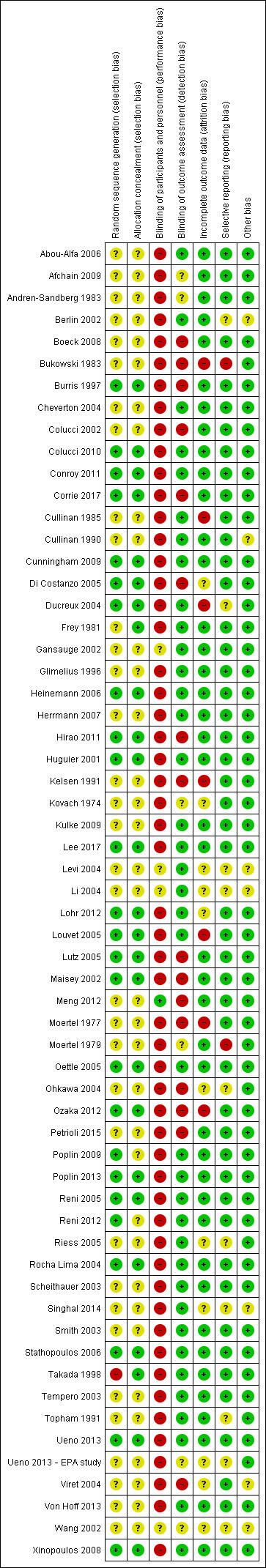
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We describe details of the risk of bias of the included studies in the Effects of interventions section.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Anti‐cancer therapy versus best supportive care for advanced pancreatic cancer.
| Anti‐cancer therapy versus best supportive care for advanced pancreatic cancer | |||||||
| Person or population: advanced pancreatic cancer Setting: first‐line therapy Intervention: anti‐cancer therapy Comparison: best supportive care | |||||||
| Outcomes | Anticipated risk of death* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | Toxicity and QoL | |
| Risk with best supportive care | Risk with anti‐cancer therapy | ||||||
| Overall survival | Study population | HR 1.08 (0.88 to 1.33) | 298 (4 RCTs) | ⊕⊕⊕⊝ Moderatea | — | The analysis showed that toxicity data were inconsistently reported. Most studies reporting this outcome noted that gastrointestinal adverse events were the most frequent, occurring in between 15% to 31%. 1 study noted haematological toxicity was present in 81.5% of people. 2 out of the 3 studies that analysed QoL demonstrated a benefit with anti‐cancer therapy. 1 study showed no difference between the 2 groups. | |
| 707 per 1000 | 734 per 1000 (660 to 804) | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; RCT: randomised controlled trial. | |||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
aConfidence interval include both benefit and harm; optimal information size not met.
Summary of findings 2. Various types of chemotherapy versus gemcitabine for advanced pancreatic cancer.
| Various types of chemotherapy versus gemcitabine for advanced pancreatic cancer | |||||||
| Person or population: advanced pancreatic cancer Setting: first‐line therapy Intervention: various types of chemotherapy Comparison: gemcitabine | |||||||
| Outcomes | Anticipated risk of death* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | Toxicity and QoL | |
| Risk with gemcitabine | Risk with various types of chemotherapy | ||||||
| Overall survival ‐ 5FU | Study population | HR 1.69 (1.26 to 2.27) | 126 (1 RCT) | ⊕⊕⊕⊝ Moderatea | Only 1 study | More toxicity was seen in the gemcitabine arm. Clinical benefit was improved in the gemcitabine arm | |
| 825 per 1000 | 948 per 1000 (889 to 981) | ||||||
| Overall survival ‐ FOLFIRINOX | Study population | HR 0.51 (0.43 to 0.60) | 652 (2 RCTs) | ⊕⊕⊕⊝ Moderateb | — | More toxicity was seen in the FOLFIRINOX arm. Longer time to degradation of QoL in FOLFIRINOX arm | |
| 794 per 1000 | 554 per 1000 (494 to 613) | ||||||
| Overall survival ‐ Fixed dose rate gemcitabine | Study population | HR 0.79 (0.66 to 0.94) | 644 (2 RCTs) | ⊕⊕⊕⊕ High | — | More toxicity in the fixed‐dose rate arm. QoL was not tested | |
| 880 per 1000 | 812 per 1000 (753 to 863) | ||||||
| Overall survival ‐ CO‐101 | Study population | HR 1.07 (0.86 to 1.34) | 367 (1 RCT) | ⊕⊕⊕⊝ Moderatec | Only 1 study | Toxicity was similar in both arms, QoL was not tested | |
| 854 per 1000 | 872 per 1000 (809 to 924) | ||||||
| Overall survival ‐ ZD9331 | Study population | HR 0.86 (0.42 to 1.76) | 55 (1 RCT) | ⊕⊕⊕⊝ Moderatea,c | Only 1 study | Toxicity was similar in both arms, QoL was not tested | |
| 560 per 1000 | 506 per 1000 (292 to 764) | ||||||
| Overall survival ‐ Exatecan | Study population | HR 1.27 (0.96 to 1.68) | 339 (1 RCT) | ⊕⊕⊕⊝ Moderatec | Only 1 study | Toxicity was similar in both arms, QoL was superior in the gemcitabine arm | |
| 776 per 1000 | 851 per 1000 (763 to 919) | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; RCT: randomised controlled trial. | |||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
aSmall sample size; optimal information size not met. bModerate statistical heterogeneity. cConfidence interval includes both benefit and harm.
Summary of findings 3. Gemcitabine combinations versus gemcitabine alone for advanced pancreatic cancer.
| Gemcitabine combinations versus gemcitabine alone for advanced pancreatic cancer | |||||||
| Person or population: advanced pancreatic cancer Setting: first‐line therapy Intervention: gemcitabine combinations Comparison: gemcitabine alone | |||||||
| Outcomes | Anticipated risk of death* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | Toxicity and QoL | |
| Risk with gemcitabine alone | Risk with gemcitabine combinations | ||||||
| Overall survival ‐ Gemcitabine plus platinum agent | Study population | HR 0.94 (0.81 to 1.08) | 1140 (6 RCTs) | ⊕⊕⊝⊝ Lowa,b | — | More toxicity in the combination arm with no differences shown in QoL | |
| 705 per 1000 | 683 per 1000 (628 to 733) | ||||||
| Overall survival ‐ Gemcitabine plus fluoropyrimidine | Study population | HR 0.89 (0.81 to 0.97) | 2718 (10 RCTs) | ⊕⊕⊕⊕ High | — | More toxicity in the combination arm. 2 studies showed no difference in QoL, 2 studies showed an improved QoL in the combination arm | |
| 690 per 1000 | 648 per 1000 (613 to 679) | ||||||
| Overall survival ‐ Gemcitabine plus topoisomerase inhibitor | Study population | HR 1.01 (0.87 to 1.16) | 839 (3 RCTs) | ⊕⊕⊕⊕ High | — | More toxicity in the combination arm. In 1 study, QoL was not different between the 2 arms | |
| 800 per 1000 | 803 per 1000 (753 to 845) | ||||||
| Overall survival ‐ Gemcitabine plus taxane | Study population | HR 0.72 (0.62 to 0.84) | 861 (1 RCT) | ⊕⊕⊕⊕ High | 1 study only | More toxicity in the combination arm. QoL not measured | |
| 779 per 1000 | 663 per 1000 (608 to 719) | ||||||
| Overall survival ‐ Gemcitabine plus other combinations of chemotherapy | Study population | HR 0.55 (0.39 to 0.79) | 166 (2 RCTs) | ⊕⊕⊝⊝ Lowc,d,e | — | Toxicity measured in 1 study and was not different. QoL was shown to be improved in the combination arms in both studies | |
| 850 per 1000 | 648 per 1000 (523 to 777) | ||||||
| Overall survival ‐ Gemcitabine plus other agent(s) | Study population | HR 0.79 (0.56 to 1.10) | 767 (4 RCTs) | ⊕⊕⊝⊝ Lowb,f | There was an increase in anaemia in the combination arm. 2 studies measured QoL and it was similar in both treatment arms | ||
| 825 per 1000 | 748 per 1000 (624 to 853) | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; RCT: randomised controlled trial. | |||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
aTwo studies were in abstract form and could not have full assessment completed. bConfidence interval includes both benefit and harm. cOne study did not publish sufficient details to make a full assessment. dThere was moderate statistical heterogeneity. eOptimal information size not met. fHigh statistical heterogeneity which is likely due to the difference in agents used in the treatment arms.
Summary of findings 4. Fluoropyrimidine combinations versus fluoropyrimidine alone for advanced pancreatic cancer.
| Fluoropyrimidine combinations versus fluoropyrimidine alone for advanced pancreatic cancer | ||||||
| Person or population: advanced pancreatic cancer Setting: first line therapy Intervention: fluoropyrimidine combinations Comparison: fluoropyrimidine alone | ||||||
| Outcomes | Anticipated risk of death* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Toxicity and QoL | |
| Risk with fluoropyrimidine alone | Risk with fluoropyrimidine combinations | |||||
| Overall survival | Study population | HR 0.84 (0.61 to 1.15) | 491 (4 RCTs) | ⊕⊕⊝⊝ Lowa,b | Toxicity was not different between the 2 treatment arms. QoL was measured in 1 study and showed an improvement in the combination arm | |
| 838 per 1000 | 783 per 1000 (671 to 877) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aHigh statistical heterogeneity. bConfidence interval includes both benefit and harm.
1 Anti‐cancer therapy versus best supportive care (BSC)
Six studies addressed any anti‐cancer therapy versus best supportive care (Andren‐Sandberg 1983; Frey 1981; Glimelius 1996; Huguier 2001,Takada 1998; Xinopoulos 2008). The main potential source of bias in these studies came from their non‐blinded design; however, we did not feel this significantly affected the results for overall survival (Figure 2; Figure 3). In three studies the risk of selection bias was unclear due to insufficient reporting (Andren‐Sandberg 1983; Glimelius 1996; Xinopoulos 2008).
Four of the six studies provided data in sufficient detail to derive hazard ratios (HR) for OS, with 298 people analysed. Pooled data of four studies in 298 people showed an HR of 1.08 (95% CI 0.88 to 1.33; Analysis 1.1). There was no statistical heterogeneity between studies (I² = 0%). Median survival ranged from 3.0 to 8.6 months in the anti‐cancer therapy group and 2.5 to 7.0 months in the BSC group. The difference in median survival times ranged from 0.9 months in favour of BSC to 3.5 months in favour of anticancer therapy (Table 5).
1.1. Analysis.
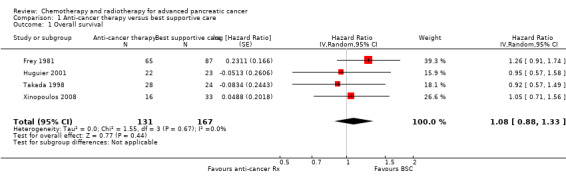
Comparison 1 Anti‐cancer therapy versus best supportive care, Outcome 1 Overall survival.
1. Median survival times and quality of life results of anti‐cancer therapy versus best supportive care.
| Study | Anti‐cancer therapy details | Median survival:anti‐cancer therapy vs best supportive care (months) | Quality of life |
| Andren‐Sandberg 1983 | 5FU + CCNU | 5 vs 4 | No difference in Karnofsky performance status (KPS) score |
| Frey 1981 | 5FU + CCNU | 3.0 vs 3.9 | Not addressed |
| Glimelius 1996 | 5FU + LV | 6.0 vs 2.5 | EORTC QLQ‐C30 results favoured the anti‐cancer therapy (NB: high rate of dropouts in the later time points) |
| Huguier 2001 | 5FU + LV + cisplatin | 8.6 vs 7.0 | Not addressed |
| Takada 1998 | 5FU + doxorubicin + MMC | 4.9 vs 5.0 | Not addressed |
| Xinopoulos 2008 | Gemcitabine | 5.25 vs 5.5 | Superior QoL (EORTC QLQ‐C30) in the gemcitabine group during the 1st month (P = 0.028), no difference from the 2nd to the 4th month; in the 5th and 6th month superior QoL in the BSC group (P = 0.010 and < 0.001) |
5FU: 5‐Fluorouracil; CCNU: chloroethylcyclohexylnitrosurea; EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer quality of life questionnaire for cancer patients; LV: leucovorin; MMC: 5FU+doxorubicin + mitomycin C
Three studies reported quality of life (Table 5). Andren‐Sandberg 1983 did not find a difference in Karnofsky performance status (KPS) score. In Glimelius 1996, the EORTC QLQ‐C30 results favoured the treatment group; however, there was a high rate of dropouts in the later time points. The third study (Xinopoulos 2008) demonstrated a superior QoL (EORTC QLQ‐C30) in the gemcitabine group during the first month (P = 0.028), but there was no difference in months two to four, and the BSC group had a superior QoL in months five (P = 0.010) and six (P = 0.0003).
Trials either did not study or did not adequately report PFS and response rates, with the exception of Takada 1998. This study reported complete or partial response in one person in the anti‐cancer therapy group versus none in the BSC group.
With respect to adverse effects or toxicity in the anti‐cancer therapy group, Frey 1981 reported that 31% of participants experienced at least one toxicity, with the most common being gastrointestinal. Huguier 2001 reported that the most common toxicities were haematological and gastrointestinal (each seen in 15% of people). Takada 1998 showed that the commonest grade 3/4 adverse events (AEs) were anorexia, which occurred in in 15/28 participants and nausea/vomiting, in 5/24 participants. Haematological toxicities were the most common in Xinopoulos 2008, with leucopenia occurring in 81.5% of participants.
2 Various types of chemotherapy versus gemcitabine
Eight studies compared various types of chemotherapy versus gemcitabine (Burris 1997; Cheverton 2004; Conroy 2011; Poplin 2009; Poplin 2013; Singhal 2014; Smith 2003; Tempero 2003), analysing a total of 1844 participants in six treatment subgroups. Due to the heterogeneity of the investigational agents, we did not pool the results. Five studies provided PFS data (Burris 1997; Conroy 2011; Poplin 2009; Singhal 2014; Smith 2003). The main potential source of bias in these studies came from the non‐blinded study design. We were unable to comprehensively assess selection bias in some studies (Cheverton 2004; Singhal 2014; Smith 2003; Tempero 2003), and there was a high risk of detection bias noted in Burris 1997,Poplin 2013 and Smith 2003; however, we did not consider that it significantly affected results for overall survival.
2.1 5FU versus gemcitabine
Burris 1997 (N = 126) was the only study to compare 5FU with gemcitabine, showing an HR for OS of 1.69 (95% CI 1.26 to 2.27, P < 0.001; Analysis 2.1). The difference in median survival was 1.3 months in favour of gemcitabine (Table 6). The analysis of PFS showed an HR of 1.47 (95% CI 1.12 to 1.92, P = 0.005; Analysis 2.2). There were better outcomes for both OS and PFS with gemcitabine, and this group also showed more treatment response (0 in the 5FU arm versus 3 in the gemcitabine arm; risk ratio (RR) 0.14, 95% CI 0.01 to 2.71, P = 0.19). On the other hand, the gemcitabine arm showed a higher risk of most types of grade 3/4 toxicity: anaemia (0 in the 5FU arm versus 6 events in the gemcitabine arm: RR 0.08, 95% CI 0.0 to 1.34, P = 0.08; Analysis 2.5); neutropenia (3 events versus 16 events: RR 0.19, 95% CI 0.06 to 0.61, P = 0.006; Analysis 2.6); thrombocytopenia (1 event versus 6 events: RR 0.17, 95% CI 0.02 to 1.34, P = 0.09; Analysis 2.7); and nausea (3 events versus 8 events: RR 0.38, 95% CI 0.10 to 1.35, P = 0.13; Analysis 2.8). Diarrhoea was the exception (3 events in the 5FU arm versus 1 event in the gemcitabine arm: RR 3.00, 95% CI 0.32 to 28.07, P = 0.34; Analysis 2.9). Clinical benefit was superior in the gemcitabine arm compared with the 5FU arm, with a higher clinical benefit response (23.8% versus 4.8%), shorter median time to clinical benefit response (3 weeks versus 7 weeks) and longer duration of clinical benefit response (18 weeks versus 13 weeks) (Table 6).
2.1. Analysis.
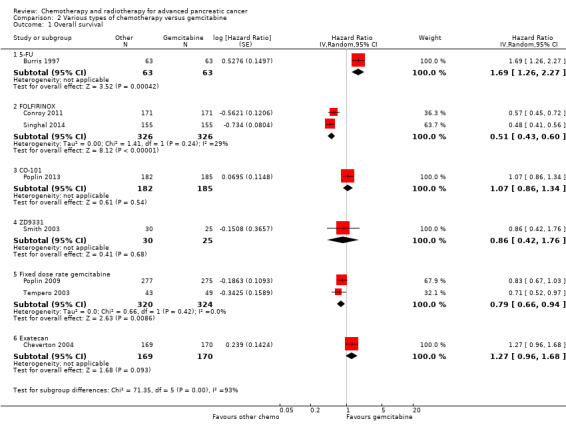
Comparison 2 Various types of chemotherapy versus gemcitabine, Outcome 1 Overall survival.
2. Median survival times and quality of life results of various types of chemotherapy versus gemcitabine.
| Study | Type of other chemotherapy | Median survival:other chemotherapy vs gemcitabine (months) | Quality of life |
| Burris 1997 | 5FU | 4.4 vs 5.7 | Improved clinical benefit 4.8% vs 23.8%. Median time to benefit 7 vs 3 weeks. Duration of benefit 18 vs 13 weeks |
| Conroy 2011 | FOLFIRINOX | 11.1 vs 6.8 | QLQ‐C30: decrease in Global Health Status and QoL scale at 3 months 17% vs 31%; at 6 months 31% vs 66% Median time to definitive deterioration: not reached vs 5.7 months |
| Singhal 2014 | FOLFIRINOX | 10.8 vs 7.4 | Definitive degradation of QoL at six months: 29% vs 59% |
| Poplin 2013 | CO‐101 | 5.2 vs 6.0 | Not addressed |
| Smith 2003 | ZD‐9331 | 5.0 vs 3.6 | Not addressed |
| Poplin 2009 | Fixed dose rate gemcitabine 1500 mg/m² over 150 min | 6.2 vs 4.9 | Not addressed |
| Tempero 2003 | Fixed dose rate gemcitabine 1500 mg/m² at 10 mg/m²/min | 8.0 vs 5.0 | Not addressed |
| Cheverton 2004 | Exatecan (DX‐8951f) | 5.0 vs 6.6 | Time to worsening of clinical benefit was longer in the gemcitabine group. Pain (3.7 vs 7.9 months; P = 0.0493), KPS (3.4 vs 4.6 months; P = 0.0111) and weight (2.3 vs 3.8 months; P = 0.0203). QoL measured with QLQ‐C3 and QLQ‐PAN26 were similar in the 2 groups |
5FU: 5‐Fluorouracil; FOLFIRINOX: 5‐fluorouracil + irinotecan + oxaliplatin; QoL: quality of life; QLQ‐C30 and QLQ‐PAN26: general and pancreatic cancer specific QoL questionnaire.
2.2. Analysis.

Comparison 2 Various types of chemotherapy versus gemcitabine, Outcome 2 Progression‐free survival.
2.5. Analysis.
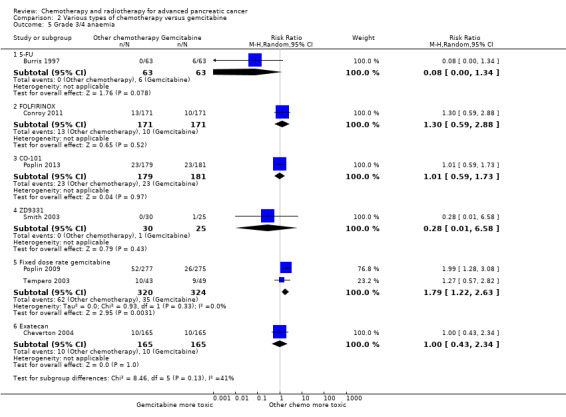
Comparison 2 Various types of chemotherapy versus gemcitabine, Outcome 5 Grade 3/4 anaemia.
2.6. Analysis.

Comparison 2 Various types of chemotherapy versus gemcitabine, Outcome 6 Grade 3/4 neutropenia.
2.7. Analysis.

Comparison 2 Various types of chemotherapy versus gemcitabine, Outcome 7 Grade 3/4 thrombocytopenia.
2.8. Analysis.
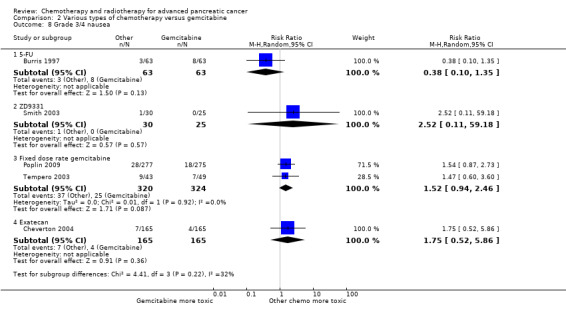
Comparison 2 Various types of chemotherapy versus gemcitabine, Outcome 8 Grade 3/4 nausea.
2.9. Analysis.

Comparison 2 Various types of chemotherapy versus gemcitabine, Outcome 9 Grade 3/4 diarrhoea.
2.2 FOLFIRINOX versus gemcitabine
Two studies in 652 people assessed the effects of FOLFIRINOX versus gemcitabine (Conroy 2011; Singhal 2014). The FOLFIRINOX group generally outperformed gemcitabine, showing improved OS (HR 0.51, 95% CI 0.43 to 0.60, P < 0.001; I² = 29%; Analysis 2.1), longer median survival (4.3 months versus 3.4 months; Table 6), longer PFS (HR 0.46, 95% CI 0.38 to 0.57, N = 652, P < 0.001; I² = 0%; Analysis 2.2), longer time to degradation of QoL (HR 0.46, 95% CI 0.35 to 0.61, P < 0.001; I² = 0%; Analysis 2.3; Table 6), and more treatment responses (54 responses versus 16 responses: RR 3.38, 95% CI 2.01 to 5.65, P < 0.001; Analysis 2.4). On the other hand, FOLFIRINOX also showed more grade 3/4 haematological toxicity for: anaemia (13 events versus 10 events: RR 1.30, 95% CI 0.59 to 2.88, P = 0.52; Analysis 2.5), neutropenia (75 events versus 35 events: RR 2.14, 95% CI 1.52 to 3.01, P < 0.001: Analysis 2.6), and thrombocytopenia (15 events versus 6 events: RR 2.50, 95% CI 0.99 to 6.29, P = 0.05; Analysis 2.7).
2.3. Analysis.

Comparison 2 Various types of chemotherapy versus gemcitabine, Outcome 3 Degradation of QoL at 6 months.
2.4. Analysis.
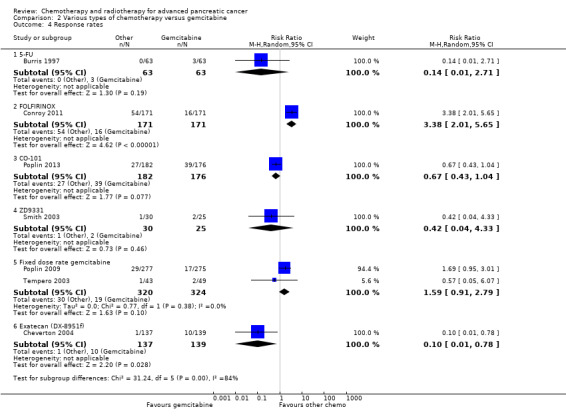
Comparison 2 Various types of chemotherapy versus gemcitabine, Outcome 4 Response rates.
2.3 CO‐101 versus gemcitabine
Poplin 2013 tested CO‐101 in 367 people. Outcomes were not different for participants in either arm. The HR for OS was 1.07 (95% CI 0.86 to 1.34, P = 0.68; Analysis 2.1). Median survival was similar in both groups, 5.2 months for CO‐101 and 6.0 months for gemcitabine (Table 6). The trial did not report PFS. The RR for response was 0.67 (95% CI 0.43 to 1.04, P = 0.08; Analysis 2.4). We could neither prove nor rule out differences in various types of grade 3/4 toxicity (Analysis 2.5; Analysis 2.6; Analysis 2.7).
2.4 ZD9331 versus gemcitabine
Smith 2003 compared ZD9331 versus gemcitabine in 55 people. There was no difference in survival for participants in either arm. The HR for OS was 0.86 (95% CI 0.42 to 1.76, P = 0.68; Analysis 2.1) and for PFS, it was 0.78 (95% CI 0.46 to 1.32, P = 0.36; Analysis 2.2). Median survival was 5.0 months and 3.6 months, respectively (Table 6). The RR for response was 0.42 (95% CI 0.04 to 4.33, P = 0.46, Analysis 2.4). We could neither prove nor rule out differences in various types of grade 3/4 toxicity (Analysis 2.5; Analysis 2.6; Analysis 2.7; Analysis 2.8; Analysis 2.9).
2.5 Fixed dose rate gemcitabine (FDR‐gem) versus standard infusional gemcitabine
Two studies assessed the effects of FDR‐gem in 644 people (Poplin 2009; Tempero 2003). OS was improved in the FDR‐gem group (HR 0.79, 95% CI 0.66 to 0.94, P = 0.009, I² = 0%; Analysis 2.1). In the two studies, median survival was 1.3 months and 3.0 months longer in the FDR‐gem group (Table 6). Only Poplin 2009 (N = 552) reported PFS, finding no significant difference between groups (HR 0.88, 95% CI 0.77 to 1.01, P = 0.06, Analysis 2.2). There were more responses seen in the FDR‐gem group (30 responses versus 19 responses), but this was not significant (RR 1.59, 95% CI 0.91 to 2.79, P = 0.10; Analysis 2.4). Analyses also showed more grade 3/4 toxicity in the FDR‐gem group: anaemia (62 events versus 35 events: RR 1.79, 95% CI 1.22 to 2.63, P = 0.003; Analysis 2.5), neutropenia (183 events versus 100 events: RR 1.85, 95% CI 1.53 to 2.23, P < 0.001; Analysis 2.6), thrombocytopenia (107 events versus 39 events: RR 2.77, 95% CI 1.99 to 3.86, P < 0.001; Analysis 2.7), and nausea (37 events versus 25 events: RR 1.52, 95% CI 0.94 to 2.46, P = 0.09; Analysis 2.8). Diarrhoea was the exception (5 events versus 12 events: RR 0.44, 95% CI 0.16 to 1.23, P = 0.12; Analysis 2.9).
2.6 Exatecan (DX‐8951f) versus gemcitabine
Cheverton 2004 demonstrated that exatecan had an inferior effect on OS compared with gemcitabine (HR 1.27, 95% CI 0.96 to 1.68, P = 0.093). Median survival in the two respective groups was 5 months versus 6.6 months; 6‐month survival rates were 44.1% versus 51.1%; and 12‐month survival rates, 17.9% versus 22.1%. There were insufficient data to include this study in the PFS analysis; however, median PFS was 2.8 months versus 4.4 months. Response rates were available in 276 people (1 response versus 10 responses: RR 0.10, 95% CI 0.01 to 0.78, P = 0.03; Analysis 2.4). Toxicity data were available in 330 people and showed that both agents performed similarly for grade 3/4 anaemia (10 events versus 10 events: RR 1.00, 95% CI 0.43 to 2.34, P = 1.00; Analysis 2.5), neutropenia (32 events versus 32 events: RR 1.00, 95% CI 0.64 to 1.55, P = 1.00; Analysis 2.6), thrombocytopenia (12 events versus 16 events: RR 0.75, 95% CI 0.37 to 1.54, P = 0.43; Analysis 2.7) and nausea (7 events versus 4 events: RR 1.75, 95% CI 0.52 to 5.86, P = 0.36; Analysis 2.8). QoL analysis showed that time to worsening of clinical benefit was longer in the gemcitabine arm, with 3.7 months to worsening of pain in the exatecan group versus 7.9 months in the gemcitabine group (P = 0.049). The gemcitabine group also showed a longer time to worsening KPS (3.4 months versus 4.6 months; P = 0.011) and to weight loss (2.3 months versus 3.8 months; P = 0.020). Global and pancreas‐specific QoL questionnaires failed to elicit significant differences between the two groups. (Table 6).
3 Gemcitabine combination studies
We identified six subgroups in this comparison, and we pooled results in the subgroups only and not overall.
3.1 Gemcitabine plus a platinum agent versus gemcitabine alone
The HR for OS based on six studies in 1140 participants showed no difference between the treatment groups, 0.94 (95% CI 0.81 to 1.08, P = 0.38; Analysis 3.1). There was some statistical heterogeneity (I² = 15%). Four studies in 1015 participants reported PFS and showed some improvement in the gemcitabine + platinum group, giving an HR of 0.80 (95% CI 0.68 to 0.95, P = 0.01; Analysis 3.2). There was high statistical heterogeneity (I² = 46%). The median survival times are listed in Table 7.
3.1. Analysis.
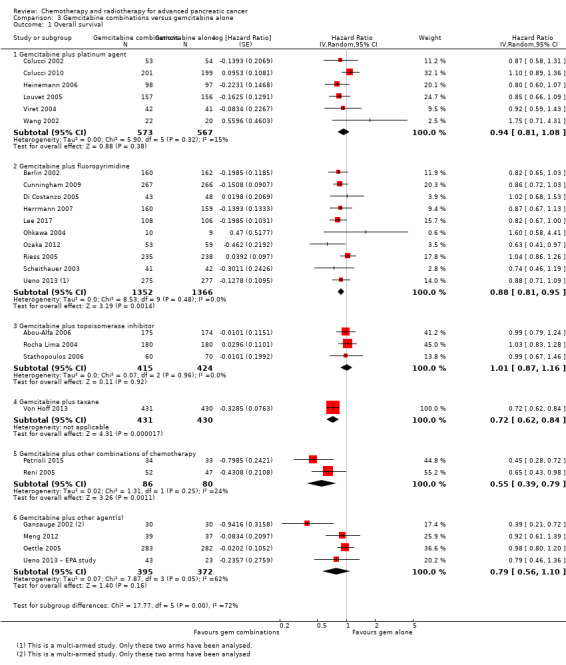
Comparison 3 Gemcitabine combinations versus gemcitabine alone, Outcome 1 Overall survival.
3.2. Analysis.

Comparison 3 Gemcitabine combinations versus gemcitabine alone, Outcome 2 Progression‐free survival.
3. Median survival times and quality of life results of gemcitabine combinations versus gemcitabine alone.
| Study | Gemcitabine combination details | Median survival:gemcitabine combination vs gemcitabine alone (months) | Quality of life |
| Platinum combinations | |||
| Colucci 2002 | Gemcitabine + cisplatin | 7.5 vs 5.0 | Not addressed |
| Colucci 2010 | Gemcitabine + cisplatin | 7.2 vs 8.3 | The mean difference from baseline in global QoL (EORTC C30) was not significantly different between the 2 groups: 0.09 (gemcitabine/cisplatin) vs 6.20 (gemcitabine), P = 0.07 |
| Heinemann 2006 | Gemcitabine + cisplatin | 7.5 vs 6.0 | No difference was detected in the 2 groups with either the Spitzer index or the pain intensity score |
| Li 2004 | Gemcitabine + cisplatin | 5.6 vs 4.6 | Clinical benefit (pain control, performance status, body weight gain) 29% vs 36% (P > 0.05); Quality adjusted life months 3.8 vs 5.6 (P < 0.001) |
| Louvet 2005 | Gemcitabine + oxaliplatin | 9.0 vs 7.1 | Not addressed |
| Viret 2004 | Gemcitabine + cisplatin | 8.0 vs 6.7 | Q‐TWiST results did not differ significantly between the 2 arms (EORTC C30) |
| Wang 2002 | Gemcitabine + cisplatin | 7.2 vs 9.1 | Not addressed |
| Fluoropyrimidine combinations | |||
| Berlin 2002 | Gemcitabine + 5FU (weekly) | 6.7 vs 5.4 | Not addressed |
| Cunningham 2009 | Gemcitabine + capecitabine | 7.1 vs 6.2 | 89% of people completed QoL questionnaires (EORTC QLQ‐C30 + ESPAC). No differences seen at baseline between the 2 groups and no differences across treatment groups at 3 or 6 months |
| Di Costanzo 2005 | Gemcitabine + daily 5FU | 7.5 vs 7.75 | No differences were seen between the 2 groups in mean disturbed days after cycle 1 or 2 or mean of days a person would like to cancel treatment in cycle 1 or 2 |
| Herrmann 2007 | Gemcitabine + capecitabine | 8.4 vs 7.2 | CBR seen in 29% of people in combination arm and 20% of people in gemcitabine arm. Median duration of response 9.5 and 6.5 weeks, respectively (P < 0.02). No differences in QoL as measured by LASA |
| Lee 2017 | Gemcitabine + capecitabine | 10.3 vs 7.5 | Not addressed |
| Ohkawa 2004 | Gemcitabine + UFT | Not stated | Not addressed |
| Ozaka 2012 | Gemcitabine + S1 | 13.7 vs 8.0 | Not addressed |
| Riess 2005 | Gemcitabine + 5FU (24 hour infusion) + FA | Not stated | Not addressed |
| Scheithauer 2003 | Gemcitabine + capecitabine | 9.5 vs 8.2 | The gemcitabine + capecitabine arm had an improvement in pain (35.5 vs 20%), KPS (41.9 vs 27%), but not weight (9.7 vs 17%) |
| Ueno 2013 | Gemcitabine + S1 | 10.1 vs 8.8 | The gemcitabine + S1 group showed an improvement in QALYs 0.525 vs 0.401, P < 0.001 |
| Topoisomerase combinations | |||
| Abou‐Alfa 2006 | Gemcitabine + exatecan | 6.2 vs 6.7 | Not addressed |
| Rocha Lima 2004 | Gemcitabine + irinotecan | 6.3 vs 6.5 | FACT‐Hep questionnaires were completed by 80% of people in irinotecan/gemcitabine group and 73% of the gemcitabine group during the first 30 weeks of the study. There were no differences between the 2 groups. |
| Stathopoulos 2006 | Gemcitabine + irinotecan | 6.4 vs 6.5 | Not addressed |
| Taxane combinations | |||
| Von Hoff 2013 | Gemcitabine + nab‐paclitaxel | 8.5 vs 6.7 | Not addressed |
| Other combination chemotherapy including gemcitabine | |||
| Petrioli 2015 | Gemcitabine + oxaliplatin + capecitabine (GEMOXEL) | 11.9 vs 7.1 | The global QoL score was higher in the combination chemotherapy group at 2 months (61 vs 56) and 4 months (72 vs 66) |
| Reni 2005 | Cisplatin/epirubicin/gemcitabine/5FU (PEFG) | Not stated | The EORTC‐QLQ Pan 26 questionnaire was done but the sample size was insufficient to obtain adequate statistical power to reliably detect differences between groups for multiple comparisons. People in PEFG group 20% to 44% more likely to have improvement in emotional functioning, overall quality of life, cognitive measures, pain, fatigue, indigestion, dyspnoea, appetite loss and flatulence. However, people in gemcitabine group had better scores for sexual function and body image |
| Other agents in combination with gemcitabine | |||
| Gansauge 2002 | Gemcitabine + Ukrain | 10.4 vs 5.2 | Not addressed |
| Meng 2012 | Gemcitabine + huachansu | 5.2 vs 5.3 | No significant differences were seen between the treatment groups with either the FACT‐G or MDASI assessments |
| Oettle 2005 | Gemcitabine + pemetrexed | 6.2 vs 6.3 | People in the gemcitabine group had better financial difficulties score, better physical functioning score and better cognitive functioning score. People in the gemcitabine/pemetrexed group had better pain scores. Performance status improvements was seen in 11.4% of gemcitabine/pemetrexed group and 9.4% of gemcitabine group. Weight gain was seen in 10.2% of gemcitabine/pemetrexed group and 5.7% of gemcitabine group |
| Ueno 2013 – EPA study | Gemcitabine + EPA | 8.2 vs 9.7 | Not addressed |
5FU: fluorouracil; CBR: clinical benefit response; ESPAC: European Study Group for Pancreatic Cancer; EORTC: European Organisation for Research and Treatment of Cancer; FACT‐G: Functional Assessment of Cancer Therapy; FA: folinic acid; KPS: Karnofsky performance status; LASA: linear‐analog self‐assessment indicators; MDASI: MD Anderson Symptom Inventory; QALY: quality‐adjusted life year; QLQ‐C30: quality of life questionnaire for cancer patients; QoL: quality of life; Q‐TWiST: quality‐adjusted time without symptoms or toxicity.
All studies (N = 1186) reported response rates favouring the combined treatment arm (100 responses versus 67 responses: RR 1.48, 95% CI 1.11 to 1.98, P = 0.007, I² = 0%; Analysis 3.3). Data from all studies (N = 1156) contributed to meta‐analyses for grade 3/4 anaemia (62 events in the gemcitabine plus platinum group versus 45 events in the gemcitabine alone group: RR 1.41, 95% CI 0.87 to 2.31, P = 0.17; Analysis 3.4) and neutropenia (122 events versus 97 events: RR 1.34, 95% CI 0.90 to 1.97, P = 0.14; Analysis 3.5), with similar rates between groups. For other adverse events, data in 1110 participants from six studies showed more grade 3/4 AEs in the combination group: thrombocytopenia (78 events versus 35 events: RR 1.96, 95% CI 1.00 to 3.84, P = 0.05; Analysis 3.6) and nausea (52 events versus 22 events: RR 2.28, 95% CI 1.40 to 3.71, P = 0.001; Analysis 3.7), although for diarrhoea, we could not rule out the possibility that these results were due to chance (23 events versus 14 events: RR 1.48, 95% CI 0.62 to 3.53, P = 0.38; Analysis 3.8).
3.3. Analysis.

Comparison 3 Gemcitabine combinations versus gemcitabine alone, Outcome 3 Response rates.
3.4. Analysis.

Comparison 3 Gemcitabine combinations versus gemcitabine alone, Outcome 4 Grade 3/4 anaemia.
3.5. Analysis.

Comparison 3 Gemcitabine combinations versus gemcitabine alone, Outcome 5 Grade 3/4 neutropenia.
3.6. Analysis.
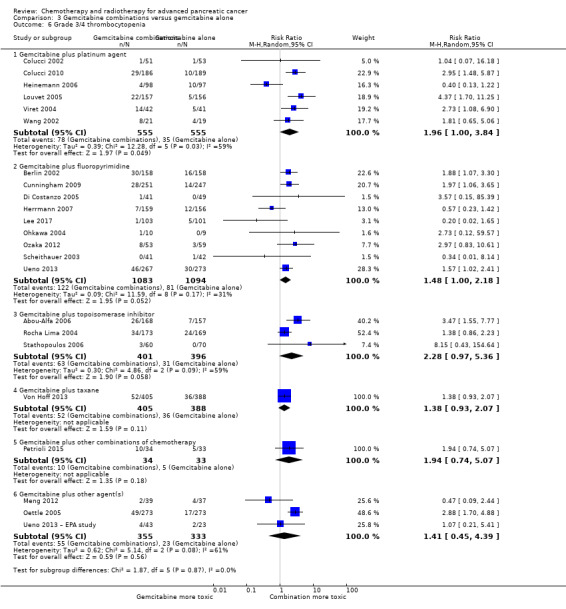
Comparison 3 Gemcitabine combinations versus gemcitabine alone, Outcome 6 Grade 3/4 thrombocytopenia.
3.7. Analysis.
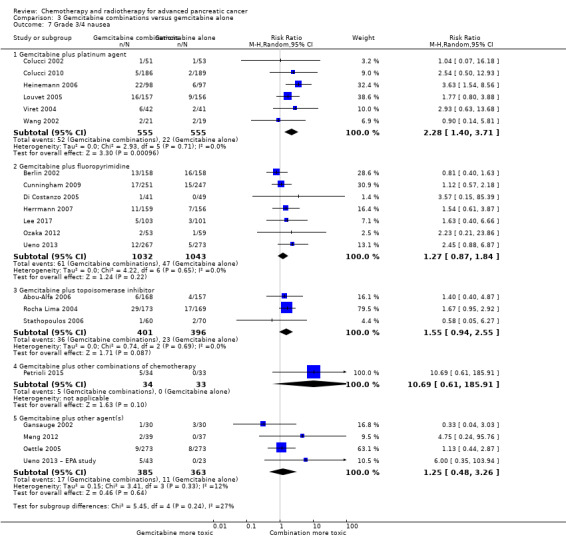
Comparison 3 Gemcitabine combinations versus gemcitabine alone, Outcome 7 Grade 3/4 nausea.
3.8. Analysis.
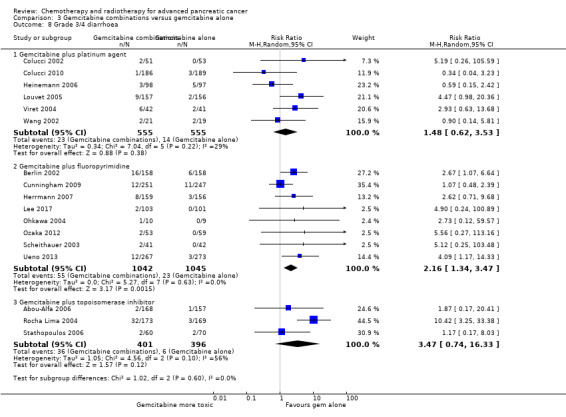
Comparison 3 Gemcitabine combinations versus gemcitabine alone, Outcome 8 Grade 3/4 diarrhoea.
Four studies reported QoL data. Colucci 2010 measured QoL using the EORTC QLQ C30 questionnaires in multiple areas. Scores were from a scale of 0‐100. The mean difference (MD) between baseline scores and scores after 4 weeks of treatment were measured. The study did not find a significant MD in global QoL scores between those taking gemcitabine alone (MD 6.20) versus gemcitabine plus platinum (MD 0.09), P = 0.07. Heinemann 2006 found no difference between the treatment groups in either the Spitzer index or pain intensity score, nor did Viret 2004 find any difference in the EORTC‐QLQ C30 results between treatment groups. Li 2004 reported finding no difference in clinical benefit but better quality of life outcomes in the gemcitabine alone arm (3.8 months versus 5.6 months in QoL‐adjusted life months gained P < 0.001; Table 7).
In the one study that we could not include in the meta‐analysis (Li 2004), there were no differences between the control and treatment groups for OS (4.6 months versus 5.6 months) or PFS (2.8 months versus 2.8 months; Table 7).
The main source of bias identified in these studies was their non‐blinded study design. There was a high risk of attrition bias in Louvet 2005 and insufficient details in Viret 2004 and Wang 2002 reports to make a comprehensive assessment of risk of bias.
3.2 Gemcitabine plus fluoropyrimidine versus gemcitabine alone
Ten studies reported OS in 2718 participants. A benefit for adding fluoropyrimidine to gemcitabine was detected (HR 0.88, 95% CI 0.81 to 0.95, P = 0.001; Analysis 3.1), with no statistical heterogeneity (I² = 0%). Eight studies reported PFS in 2608 participants and abenefit for the combination arm was also shown (HR 0.79, 95% CI 0.72 to 0.87, P < 0.001). There was moderate statistical heterogeneity with an I² of 34% (Analysis 3.2). The median survival times ranged from 5.4 months to 8.8 months in the gemcitabine alone group and from 6.7 months to 13.7 months in the combination group (Table 7). Ueno 2013 was a multi‐armed study that compared gemcitabine alone versus S1 alone versus gemcitabine plus S1. The analysis in this review includes only the gemcitabine alone and gemcitabine plus S1 arms.
Nine studies reported response rates in 2176 participants. Responses were more common in the combination group (228 responses in the combination group versus 124 responses in the gemcitabine alone group), RR 1.78 (95% CI 1.29 to 2.47, P < 0.001; Analysis 3.3), with high statistical heterogeneity (I² = 52%). Eight studies reported grade 3/4 AEs in 2158 participants in the combination group versus the gemcitabine alone group, with the combination treatment group tending to experience more AEs: anaemia (97 events versus 89 events: RR 1.11, 95% CI 0.84 to 1.45, P = 0.47; Analysis 3.4), neutropenia (353 events versus 234 events: RR 1.53, 95% CI 1.34 to 1.74, P < 0.001; Analysis 3.5), thrombocytopenia (122 events versus 81 events: RR 1.48, 95% CI 1.00 to 2.18, P = 0.05; Analysis 3.6), nausea (61 events versus 47 events: RR 1.27, 95% CI 0.87 to 1.84, P = 0.22; Analysis 3.7), and diarrhoea (55 events versus 23 events: RR 2.16, 95% CI 1.34 to 3.47, P = 0.002; Analysis 3.8).
Five studies recorded QoL data. Cunningham 2009 used the Memorial pain assessment card, EORTC QLQ C30 and ESPAC QoL questionnaires. Di Costanzo 2005 recorded mean disturbed days and the mean days the person would like to cancel treatment. Herrmann 2007 used a linear‐analogue self‐assessment (LASA) indicators for clinical benefit response (CBR). Scheithauer 2003 recorded a combination of pain, KPS and weight, and Ueno 2013 recorded quality adjusted life years (QALYs). Cunningham 2009 did not find any significant differences in QoL between treatment groups. Likewise, Di Costanzo 2005 did not show any differences in QoL outcomes. Herrmann 2007 did not show a difference in either CBR or QoL (measured by LASA); however, in those people who did have a CBR, the duration was longer in the combination arm (9.5 weeks versus 6.5 weeks, P < 0.02). Scheithauer 2003 demonstrated an improvement in pain response and KPS but not weight gain in the combination arm, and Ueno 2013 showed a statistically significant improvement in QALYs in the combination group: 0.401 versus 0.525, P < 0.001 (Table 7).
The main source of bias identified in this comparison was due to the non‐blinded study design. The risk of selection bias was unclear in Berlin 2002; Herrmann 2007; Ohkawa 2004; Riess 2005 and Scheithauer 2003, but we did not consider that this significantly affected the results.
3.3 Gemcitabine plus topoisomerase inhibitor versus gemcitabine alone
Three studies reported OS data in 839 participants, giving an HR of 1.01 (95% CI 0.87 to 1.16, P = 0.92; Analysis 3.1), indicating no difference between groups. There was no heterogeneity (I² = 0%). Two studies reported similar PFS in 709 participants (HR 0.91, 95% CI 0.78 to 1.07, P = 0.26, I² = 0%; Analysis 3.2). The median survival times were very similar between the two groups (Table 7). All studies reported response rates, with data on 729 participants (49 responses in the combined treatment group versus 22 responses in the gemcitabine alone group: RR 1.50, (95% CI 0.92 to 2.46, P = 0.11, I² = 0%; Analysis 3.3). The combination arms were shown to be more toxic with data for grade 3/4 AEs in 797 participants: anaemia (41 events versus 37 events: RR 1.09, 95% CI 0.72 to 1.66, P = 0.68; Analysis 3.4), neutropenia (132 events versus 88 events: RR 1.54, 95% CI 1.04 to 2.30, P = 0.03; Analysis 3.5), thrombocytopenia (63 events versus 31 events: RR 2.28, 95% CI 0.97 to 5.36, P = 0.06; Analysis 3.6), nausea (36 events versus 23 events: RR 1.55, 95% CI 0.94 to 2.55, P = 0.09; Analysis 3.7) and diarrhoea (36 events versus 6 events: RR 3.47, 95% CI 0.74 to 16.33, P = 0.12; Analysis 3.8).
Rocha Lima 2004 was the only study to record QoL data (FACT‐Hep questionnaire) and reported no significant differences between the two groups (Table 7).
The main source of bias identified in this comparison was due to the non‐blinded study design, but we did not consider that this affected the results.
3.4 Gemcitabine plus taxane versus gemcitabine alone
Von Hoff 2013 was the only study in this group, and trialists analysed all 861 participants for OS, PFS and response rate. A benefit in survival outcomes was demonstrated in the combination arm. For OS, the HR was 0.72 (95% CI 0.62 to 0.84; P < 0.001; Analysis 3.1), and for PFS, HR was 0.69 (95% CI 0.58 to 0.82; P < 0.001; Analysis 3.2). The median survival time was 8.5 months in the combination group versus 6.7 months in the gemcitabine control (Table 7). There was a higher response rate in the combination arm (99 responses versus 30 responses: RR 3.29, 95% CI 2.24 to 4.84, P < 0.001; Analysis 3.3). Data on grade 3/4 AEs were available for 793 participants and overall, toxicity was more common in the combination arm: anaemia (53 events versus 48 events: RR 1.06, 95% CI 0.73 to 1.52, P = 0.76; Analysis 3.4), neutropenia (153 events versus 103 events: RR 1.42, 95% CI 1.16 to 1.75, P < 0.001; Analysis 3.5), thrombocytopenia (52 events versus 36 events: RR 1.38, 95% CI 0.93 to 2.07, P = 0.11; Analysis 3.6), neuropathy (70 events versus 3 events: RR 22.35, 95% CI 7.10 to 70.40, P < 0.001; Analysis 3.9) and fatigue (70 events versus 27 events: RR 2.48, 95% CI 1.63 to 3.79, P < 0.001; Analysis 3.10). The studies did not report on QoL.
3.9. Analysis.

Comparison 3 Gemcitabine combinations versus gemcitabine alone, Outcome 9 Grade 3/4 neuropathy.
3.10. Analysis.
Comparison 3 Gemcitabine combinations versus gemcitabine alone, Outcome 10 Grade 3/4 fatigue.
Corrie 2017 was a unique study that we could not include in this analysis, addressing nab‐paclitaxel plus gemcitabine versus the same agents given in a sequential dosing schedule. Here the standard arm had similar results to the nab‐paclitaxel plus gemcitabine arm of Von Hoff 2013, with a median survival of 7.9 months, median PFS of 4.0 months and response rate of 33%.
Likewise, we could not include Lohr 2012 in the analysis as it was a multi‐armed study. It showed that overall survival for the gemcitabine alone arm was 6.8 months, compared to 8.1 months in combination with liposomal paclitaxel 11 mg/m², 8.7 months in combination with liposomal paclitaxel 22 mg/m² and 9.3 months in combination with liposomal paclitaxel 44 mg/m². When comparing each combination arm with gemcitabine alone the HRs all crossed the line of null effect: for concomitant doses of 11 mg/m²: HR 0.93 (95% CI 0.60 to 1.43); for 22 mg/m²: HR 0.69 (95% CI 0.44 to 1.07); and for 44 mg/m²: HR 0.66 (95% CI 0.43 to 1.03). PFS in the gemcitabine alone group was 2.7 months compared with each of the combination arms: 4.1 months, 4.6 months and 4.4 months (11 mg/m², 22 mg/m² and 44 mg/m², respectively). When comparing each experimental arm with gemcitabine alone for PFS, the HRs were 0.84 (95% CI 0.44 to 1.28), 0.58 (95% CI 0.38 to 0.90) and 0.74 (95% CI 0.49 to 1.13), respectively. The number of responses were similar in all groups (14%, 14%, 14% and 16%, respectively). Neutropenia and fatigue were the commonest AEs and occurred at similar rates across the four groups. The trials did not report QoL. Toxicity was more common in the combination arm with a dose dependent increase in thrombocytopenia, chills and pyrexia.
Although there were insufficient details to make an assessment of selection bias, overall we assessed the study as being at low risk of bias, the main source being due to the non‐blinded study design, which we considered to not affect the results.
3.5 Gemcitabine plus other combinations of chemotherapy versus gemcitabine alone
Two studies reported OS data on 166 participants which showed improved survival in the combination group (HR 0.55, 95% CI 0.39 to 0.79, P = 0.001; Analysis 3.1). There was some statistical heterogeneity (I² = 24%). Both studies reported PFS and again showed a benefit to the combination arm, with an HR of 0.43 (95% CI 0.30 to 0.62, P < 0.001, I² = 17%; Analysis 3.2). Median survival times were only available for Petrioli 2015, who reported that the combined treatment group survived for a median of 11.9 months versus 7.1 months in the gemcitabine alone group (Table 7). Only Petrioli 2015 reported response rates in 67 participants (12 responses versus 6 responses: RR 1.94, 95% CI 0.83 to 4.56, P = 0.13; Analysis 3.3). The same study reported grade 3/4 AEs. Although AEs were more common in the combination arm, the small number of events makes it difficult to assess the real difference between the arms: anaemia (6 events versus 3 events: RR 1.94, 95% CI 0.53 to 7.13, P = 0.32; Analysis 3.4), neutropenia (8 events versus 4 events: RR 1.94, 95% CI 0.65 to 5.83, P = 0.24; Analysis 3.5), thrombocytopenia (10 events versus 5 events: RR 1.94, 95% CI 0.74 to 5.07, P = 0.11; Analysis 3.6) and nausea (5 events versus 0 events: RR 10.69, 95% CI 0.61 to 185.91, P = 0.10; Analysis 3.7).
Both studies reported QoL data. Petrioli 2015 used the EORTC QLQ C30 and McGill Melzack questionnaires, and Reni 2005 used the EORTC‐QLQ Pan 26 questionnaire. Petrioli 2015 showed that global QoL was improved in the combined treatment group at two and four months. Reni 2005 stated that the sample size was insufficient to obtain statistical power to detect differences between the control and treatment groups. However, the treatment group had better average emotional functioning, overall QoL, cognitive measures, pain, fatigue, indigestion, dyspnoea, appetite loss and flatulence, while sexual function and body image were better in the control group (Table 7).
Petrioli 2015 did not publish enough data to make a full assessment of selection bias and had a high risk of performance and detection bias. Reni 2005 was a non‐blinded study but otherwise had a low risk of bias.
3.6 Gemcitabine plus other agent(s) versus gemcitabine alone
Four studies assessed OS in 767 participants, with no differences in survival detected (HR 0.79, 95% CI 0.56 to 1.10, P = 0.16; I² = 62%; Analysis 3.1). Only Meng 2012 reported PFS data in 76 people, with no differences seen, HR 1.05 (95% CI 0.68 to 1.62, P = 0.83; Analysis 3.2). Median survival times in the gemcitabine group ranged from 5.2 months to 9.7 months and in the combination group from 5.2 months to 10.4 months (Table 7). Three studies reported response rates in 691 participants (61 responses versus 22 responses: with RR 3.66, 95% CI 1.04 to 12.82, P = 0.04; Gansauge 2002; Meng 2012; Oettle 2005; Analysis 3.3). Three studies reported haematological toxicity data for grade 3/4 events in 688 participants revealing more anaemia in the combination arm (Meng 2012; Oettle 2005; Ueno 2013 – EPA study): anaemia (49 events versus 12 events: RR 3.58, 95% CI 1.93 to 6.62, P < 0.001; Analysis 3.4), neutropenia (140 events versus 45 events: RR 2.02, 95% CI 0.88 to 4.66, P = 0.10; Analysis 3.5), and thrombocytopenia (55 events versus 23 events: RR 1.41, 95% CI 0.45 to 4.39, P = 0.56; Analysis 3.6). Four studies reported on nausea in 748 participants (17 events versus 11 events: RR 1.25, 95% CI 0.48 to 3.26, P = 0.64; Analysis 3.7).
Two studies reported on QoL: Meng 2012 used the FACT‐G and MD Anderson Symptom Inventory questionnaires, and Oettle 2005 used the EORTC QLQ‐C30 questionnaire. Meng 2012 did not find a difference in either of the scales used (FACT‐G and MD Anderson Symptom Inventory questionnaire) at eight weeks. Oettle 2005 showed that people in the gemcitabine alone group had lower financial difficulties and better physical and cognitive functioning, but the combination arm had lower pain scores. There was no clear trend in QoL scores between the treatment groups, however (Table 7).
There was an unclear risk of selection bias in Gansauge 2002 and Meng 2012 due to insufficient details being published. Ueno 2013 – EPA study did not provide enough details to perform a comprehensive assessment.
4 Fluoropyrimidine combinations versus fluoropyrimidine alone
Four studies reported OS in 491 participants receiving either fluoropyrimidine combinations or fluoropyrimidine alone with no differences in survival detected (HR 0.84, 95% CI 0.61 to 1.15, P = 0.27; Analysis 4.1). There was high statistical heterogeneity with an I² of 66%. Ducreux 2004, which studied 5FU with or without oxaliplatin, showed a large benefit in the treatment group in contrast to the other three studies, which did not show much benefit with the combination arms. Only two studies reported PFS in 255 participants, and there were no differences (HR 0.52, 95% CI 0.19 to 1.38, P = 0.19; Analysis 4.2), again, with large statistical heterogeneity (I² = 89%). Median survival times ranged from 3.7 months to 6.5 months in the combination group and from 3.4 months to 5.25 months in the 5FU group (Table 8). All four studies reported response rates, but there were no differences between arms (32 responses versus 24 responses: RR 1.18, 95% CI 0.52 to 2.68, P = 0.10; I² = 52%; Analysis 4.3). Two studies (N = 255) reported rates of grade 3/4 anaemia, neutropenia, thrombocytopenia, nausea, and diarrhoea (Ducreux 2004; Maisey 2002). There were no significant differences between groups in: anaemia (8 events versus 11 events: RR 0.48, 95% CI 0.06 to 3.62, P = 0.16; Analysis 4.4); neutropenia (7 events versus 0 events: RR 5.70, 95% CI 0.73 to 44.46, P = 0.10; Analysis 4.5); thrombocytopenia (5 events versus 3 events: RR 1.40, 95% CI 0.34 to 5.80, P = 0.65; Analysis 4.6); nausea (7 events versus 5 events, RR 1.06, 95% CI 0.32 to 3.53, P = 0.93; Analysis 4.8); or diarrhoea (6 events versus 6 events: RR 0.92, 95% CI 0.31 to 2.78, P = 0.89; Analysis 4.9). Maisey 2002 reported similar rates of grade 3/4 fatigue in both arms (26 events versus 30 events: RR 0.91, 95% CI 0.58 to 1.43, P = 0.68; Analysis 4.7).
4.1. Analysis.

Comparison 4 Fluoropyrimidine combinations versus fluoropyrimidine alone, Outcome 1 Overall survival.
4.2. Analysis.
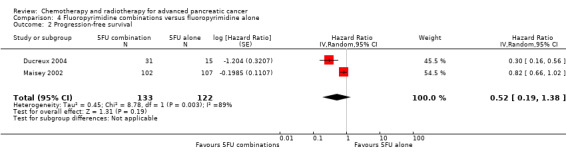
Comparison 4 Fluoropyrimidine combinations versus fluoropyrimidine alone, Outcome 2 Progression‐free survival.
4. Median survival times and quality of life results for fluoropyrimidine combinations versus fluoropyrimidine alone.
| Study | Fluoropyrimidine combination details | Median survival:fluoropyrimidine combination vs fluoropyrimidine alone (months) | Quality of life |
| Ducreux 2004 | 5FU + oxaliplatin | 3.7 vs 3.4 | Not addressed |
| Kovach 1974 | 5FU + BCNU | Not stated | Not addressed |
| Maisey 2002 | 5FU + MMC | 6.5 vs 5.1 | EORTC‐QLQ C30 showed that at 24 weeks, global QoL was superior in the combination arm compared to baseline (P = 0.035), and the pain score was also improved (P = 0.048). There was less dyspnoea at 12 weeks in the combination arm when compared to baseline (P = 0.033). |
| Moertel 1979 | 5FU + streptozocin | 4.5 vs 5.25 | Not addressed |
5FU: fluorouracil; BCNU: bis‐chloroethylnitrosourea (carmustine); EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer quality of life questionnaire for cancer patients; MMC: 5FU+doxorubicin + mitomycin C; QoL: quality of life.
4.3. Analysis.
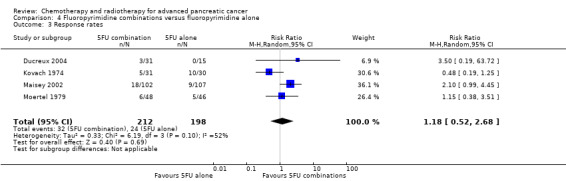
Comparison 4 Fluoropyrimidine combinations versus fluoropyrimidine alone, Outcome 3 Response rates.
4.4. Analysis.

Comparison 4 Fluoropyrimidine combinations versus fluoropyrimidine alone, Outcome 4 Grade 3/4 anaemia.
4.5. Analysis.
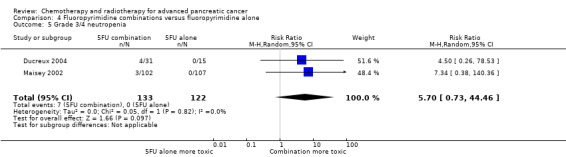
Comparison 4 Fluoropyrimidine combinations versus fluoropyrimidine alone, Outcome 5 Grade 3/4 neutropenia.
4.6. Analysis.

Comparison 4 Fluoropyrimidine combinations versus fluoropyrimidine alone, Outcome 6 Grade 3/4 thrombocytopenia.
4.8. Analysis.

Comparison 4 Fluoropyrimidine combinations versus fluoropyrimidine alone, Outcome 8 Grade 3/4 nausea.
4.9. Analysis.
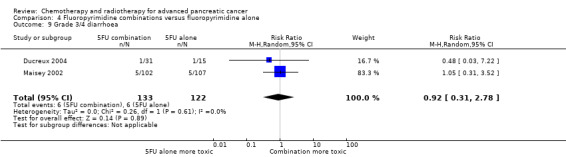
Comparison 4 Fluoropyrimidine combinations versus fluoropyrimidine alone, Outcome 9 Grade 3/4 diarrhoea.
4.7. Analysis.

Comparison 4 Fluoropyrimidine combinations versus fluoropyrimidine alone, Outcome 7 Grade 3/4 fatigue.
One study recorded QoL data (Maisey 2002), using the EORTC‐QLQ C30 questionnaire, which did not demonstrate a difference between the two groups at baseline, 12 weeks or 24 weeks (Table 8).
The main source of bias was in the non‐blinded study design. We assessed both Ducreux 2004 and Kovach 1974 as being at high risk of attrition bias, and this may have affected the results.
5 Single studies addressing unique treatment comparisons
Ten studies addressed unique comparisons that could not be categorised under the above‐mentioned comparisons (Table 9).
5. Results of studies addressing unique treatment comparisons.
| Study | Treatment arms/no. of participants | Survival outcomes | Response rates | Adverse events | Quality of life |
| Multi‐armed studies | |||||
| Boeck 2008 | Capecitabine/oxaliplatin (n = 61) versus capecitabine/gemcitabine (n = 64) versus modified gemcitabine/oxaliplatin (n = 63) | OS: 8.1 vs 9.0 v 6.9 months PFS 4.2 vs 5.7 v 3.9 months |
PR 13% vs 25% vs 13% SbD: 36% vs 39% vs 43% |
Haematological AEs more common in the gemcitabine containing arms | Not studied |
| Cullinan 1985 | 5FU (n = 50) versus 5FU/doxorubicin (n = 44) versus 5FU/doxorubicin/mitomycin C (n = 50) | Median survival of 22 weeks in all treatment groups | 30% vs 30% vs 7.7% | Haematological AEs more common in the 5FU and 5FU/doxorubicin arm, however the subgroup with PC were not reported separately. | Not studied |
| Cullinan 1990 | 5FU (n = 64) versus 5FU/cyclophosphamide/methotrexate 'Mallinson Regimen' (n = 61) versus 5FU/doxorubicin/cisplatin 'FAP' (n = 59) | OS: 3.5 vs 4.5 vs 3.5 months respectively PFS: 2.5 vs 2.5 vs 2.5 months |
7% vs 21% vs 15% | More AEs reported in the combination arms compared with 5FU alone | Not studied |
| Kulke 2009 | Gemcitabine (fixed dose rate) (n = 64) versus infusional gemcitabine + cisplatin (n = 66) versus infusional gemcitabine + docetaxel (n = 65) versus infusional gemcitabine + irinotecan (n = 60) | OS: 6.4 vs 6.7 vs 6.4 vs 7.1 months, respectively. Time to progression: 3.3 vs 4.5 vs 4.1 vs 4.0 months | 14 vs 12.5 vs 12 vs 14% | Neutropenia and fatigue most common AE and same in all groups | Not studied |
| Other studies | |||||
| Afchain 2009 | Gemcitabine/oxaliplatin (n = 20) vs simplified gemcitabine/oxaliplatin (n = 37) | OS: 3.2 vs 7.6 months PFS: 2.5 vs 4.0 months |
PR: 10% vs 27% SbD: 45% vs 43% |
Peripheral neuropathy more common in the simplified GemOx arm | Not studied |
| Bukowski 1983 | Mitomycin C/5FU (MF) (n = 73) vs Streptozocin/mitomycin C/5FU (SMF) (n = 72) | OS: 17 vs 18 weeks | PR: 8% v 34% | More gastrointestinal and renal toxicity in the SMF arm | Not studied |
| Corrie 2017 | Standard nab‐paclitaxel and gemcitabine (n = 75) vs sequential nab‐paclitaxel and gemcitabine (n = 71) | OS: 7.9 vs 10.1 months (HR 0.88) PFS: 4.0 vs 5.8 months (HR 0.66) |
PR: 33% vs 50% SbD: 28% vs 42% |
Neutropenia more common in the sequential arm | QoL score dropped by −12.1 points at 24 weeks in the standard arm vs −2.1 in the sequential arm |
| Hirao 2011 | Gemcitabine 3‐week schedule (n = 45) vs gemcitabine 4‐week schedule (n = 45) | OS: 250 vs 206 days PFS: 114 vs 112 days |
17.1% vs 14.2% | Thrombocytopenia more common in the 4‐week schedule | Not studied |
| Kelsen 1991 | Streptozocin/mitomycin C/5FU (SMF) (n = 42) vs cisplatin/ara‐C/caffeine (CAC) (n = 40) | OS: 10 vs 5 months | 10% vs 6% | Nausea and vomiting more common in CAC arm. | Not studied |
| Levi 2004 | 5FU constant infusion vs 5FU constant infusion/cisplatin versus 5FU chronomodulated infusion vs 5FU chronomodulated infusion/cisplatin (no cisplatin n = 55, with cisplatin n = 52) | OS: 5.4 vs 8.3 months (no cis vs cis) OS: 6.1 vs 6.7 months (continuous vs chronomodulated) PFS: 2.1 vs 3.2 months |
Not reported | Cisplatin increased rates of haematological AEs. Chronomodulated regimen increased rates of mucositis | Not studied |
| Lutz 2005 | Gemcitabine + docetaxel (n = 49) vs cisplatin + docetaxel (n = 47) | OS: 7.0 vs 7.5 months PFS: 3.9 vs 2.8 months |
19.4% vs 23.5% | Febile neutropenia more common in the cisplatin/docetaxel arm | Not studied |
| Moertel 1977 | Streptozocin + 5FU (n = 40) vs streptozocin + cyclophosphamide (n = 48) | OS: 13 vs 9 weeks | CR: 3 vs 6 PR: 2 vs 0 SbD: 9 vs 9 |
Haematological AEs more common in the cyclophosphamide arm | Not studied |
| Reni 2012 | Capecitabine + cisplatin + gemcitabine + docetaxel (PDXG) (n = 53) vs capecitabine + cisplatin + gemcitabine + epirubicin (PEXG) (n = 52) | OS: 10.7 vs 11 months PFS: 7.4 vs 7.6 months |
CR: 2 vs 4% PR: 58 vs 33% |
Neutropenia more common in the PEXG arm | Not studied |
| Topham 1991 | Epirubicin (n = 32) vs 5FU + epirubicin + mitomycin C (n = 30) | 1 year survival rates 15.4 vs 23.2% | 8% vs 11% | AEs were similar in both arms | Not studied |
5FU: fluorouracil; AE: adverse event; CR: complete response; OS: overall survival; PC: pancreatic cancer; PR: partial response; SbD: stable disease.
Boeck 2008 showed that capecitabine plus gemcitabine had superior median survival (9.0 months) and response rate (25%) compared with 8.1 months/13% in the capecitabine/oxaliplatin group and 6.9 months/13% in the gemcitabine/oxaliplatin group. Haematological AEs were more common in the gemcitabine‐containing regimens.
Kulke 2009 showed a similar OS in all four treatment groups, ranging from 6.4 months to 7.1 months and response rates of 12% to 14%. AEs were similar across treatment arms, with neutropenia and fatigue being the most common.
Afchain 2009 found that a simplified gemcitabine/oxaliplatin regimen was superior to a standard gemcitabine/oxaliplatin regimen with an OS of 7.6 months versus 3.2 months and response rate of 27% versus 10%. Peripheral neuropathy was more common in the simplified arm, however.
Bukowski 1983 did not demonstrate a difference in OS for streptozocin/MMC/5FU (SMF) versus MMC/5FU (18 weeks versus 17 weeks); however, there was an increase in response rate of 34% versus 8%. There was more gastrointestinal and renal toxicity in the SMF arm.
Hirao 2011 showed a slight increase in OS for the three‐week schedule of gemcitabine versus the four‐week schedule (250 days versus 206 days), but there was a similar response rate (17.1% versus 14.2%). Thrombocytopenia was more common in the four‐week schedule.
Kelsen 1991 found that the SMF arm had a longer OS than the cisplatin/ara‐C/caffeine arm (10 months versus 5 months), but a similar response rate (10% versus 6%). Nausea and vomiting were more common in the caffeine‐containing arm.
Levi 2004 showed that adding cisplatin to 5FU increased OS (8.3 months versus 5.4 months), but there was no difference between the continuous versus the chronomodulated arms (6.1 months versus 6.7 months). Cisplatin increased the rates of haematological AEs, and the chronomodulated regimen increased rates of mucositis.
Lutz 2005 did not demonstrate any striking differences between gemcitabine/docetaxel and cisplatin/docetaxel (OS 7.0 months versus 7.5 months); however, febrile neutropenia was more common in the cisplatin containing arm.
Moertel 1977 showed a slightly increased OS in the streptozocin/5FU arm compared with streptozocin/cyclophosphamide (13 weeks versus 9 weeks), with the cyclophosphamide arm experiencing more haematological AEs.
Reni 2012 showed a similar OS between capecitabine/cisplatin/gemcitabine/docetaxel (PDXG) and capecitabine/cisplatin/gemcitabine/epirubicin (PEXG) (10.7 months versus 11 months); however, there was a higher partial response rate in the PDXG group (58% versus 33%). The PEXG arm had more neutropenia.
Topham 1991 found a slightly higher one‐year survival rate in the 5FU/epirubicin/MMC arm compared with epirubicin alone (23.2% versus 15.4%), and the AEs were similar in both arms.
Discussion
Summary of main results
1 Anti‐cancer therapy versus best supportive care
We could neither prove nor rule out a survival benefit for anti‐cancer therapy versus BSC alone (moderate‐quality evidence due to imprecision; Table 1). This is in contrast to the previous version of this review, which found a benefit in the odds for death at both 6 months (OR 0.37, 95% CI 0.25 to 0.57, P < 0.001) and 12 months (OR 0.46, 95% CI 0.25 to 0.84, P = 0.01). Due to the new protocol used in this study, we excluded two studies that had featured in the previous review because they included people without histological confirmation (Mallinson 1980; Palmer 1994); this is the likely cause of these discrepant results. The differences in median survival were modest and ranged from 0.9 months in favour of BSC to 3.5 months in favour of anti‐cancer therapy (Table 5).
There is evidence for improved QoL with the use of anti‐cancer therapy in one study (Glimelius 1996), with Xinopoulos 2008 showing an early benefit that was not sustained after month 5.
Readers should interpret these results with caution, as the included studies span over 30 years, and Xinopoulos 2008 was the only study to use contemporary chemotherapy regimens. As it is unlikely that further studies will be conducted using BSC as the control arm, additional randomised data showing the effects of contemporary chemotherapy over BSC in the first‐line setting may never be generated.
2 Various types of chemotherapy versus gemcitabine
The one study addressing gemcitabine versus 5FU chemotherapy, Burris 1997, showed inferior outcomes for OS (HR 1.69; P = 0.004), PFS (HR 1.47; P = 0.005) and QoL with the 5FU arm. Table 2 shows a rating of moderate‐quality evidence due to only one small study being available for analysis. These results demonstrate that using gemcitabine reduces the risk of death by 41% and progression by 32% compared with 5FU therapy. The absolute improvement in OS is modest at just over one month. Gemcitabine may result in more grade 3/4 AEs. There is an improvement in QoL (clinical benefit response).
The analysis of two studies comparing FOLFIRINOX versus gemcitabine demonstrated an improvement in OS (HR 0.51; P < 0.001), PFS (HR 0.46; P < 0.001) and response rate (RR 3.38; P < 0.001) but also significantly more neutropenia and thrombocytopenia (Conroy 2011; Singhal 2014). There was improved QoL. Table 2 demonstrates the moderate quality of evidence rating based on inconsistency. These results suggest that FOLFIRINOX reduces the risk of death by 49%, reduces the risk of progression by 54% and triples the rate of response compared with gemcitabine. The absolute survival gains are still modest, with OS in the gemcitabine alone arm ranging from 6.8 months to 7.4 months and in the FOLFIRINOX arms between 10.8 months to 11.1 months.
The two studies that assessed the effects of giving gemcitabine at a fixed dose rate showed an improvement in OS (HR 0.79; P = 0.009) but also more haematological toxicity (Poplin 2009; Tempero 2003). Granted, the 'standard' gemcitabine arms differed between the two studies, but the study using a more intense control arm (gemcitabine 2200 mg/m² weekly) still found superiority in the FDR‐gem arm. Table 2 details a high quality of evidence rating. This analysis suggests that using FDR‐gem reduces the risk of death by 21%; however, the absolute survival gains are again small, with OS in the standard infusional gemcitabine arm ranging from 4.9 months to 5.0 months and in the FDR‐gem arm from 6.2 months to 8.0 months.
The studies comparing exatecan, CO‐101 and ZD9331 to gemcitabine did not show a survival benefit (Cheverton 2004; Poplin 2013; Smith 2003). None of these studies showed a difference in toxicity and in exatecan, analyses showed QoL to be superior in the gemcitabine arm. We rated each comparison as having moderate‐quality evidence due to imprecision (Table 2).
3 Gemcitabine combination studies
3.1 Gemcitabine plus a platinum agent versus gemcitabine alone
The analysis of seven studies has shown that the combination of gemcitabine with a platinum agent did not significantly improve OS (HR 0.94; P = 0.38) but may improve PFS (HR 0.80; P = 0.01) (Colucci 2002; Colucci 2010; Heinemann 2006; Louvet 2005; Viret 2004; Wang 2002). This equates to a reduction in the risk of progression of 20%. Table 3 shows that the quality of evidence in this analysis was low, due to two studies being in abstract form and not publishing sufficient data to make a full assessment, along with imprecision. These results are in keeping with the findings of the previous review, which found a benefit in 6‐month mortality (OR 0.59, P = 0.001) but not 12‐month mortality. We were not able to include all the studies from the previous review (Li 2004 did not publish sufficient data); however, we included two additional studies (Colucci 2010; Viret 2004). The addition of platinum improved response rates but increased thrombocytopenia and nausea. There were no significant differences found in QoL between the control and treatment arms in the people tested. This suggests that while adding platinum increases side effects, this does not translate into a worse QoL. The median survival times were similar in the two groups (Table 7).
3.2 Gemcitabine plus fluoropyrimidine versus gemcitabine alone
The analysis of 10 studies shows that adding a fluoropyrimidine agent can improve OS (HR 0.88; P = 0.001), PFS (0.79; P < 0.001) and response rate (RR 1.78; P < 0.001), but at the cost of increased rates of neutropenia and diarrhoea (Berlin 2002; Cunningham 2009; Di Costanzo 2005; Herrmann 2007; Lee 2017; Ohkawa 2004; Ozaka 2012; Riess 2005; Scheithauer 2003; Ueno 2013). Table 3 show that the quality of evidence is high. This shows that the addition of 5FU reduces the risk of death by 12%, reduces the risk of progression by 21% and nearly doubles the rate of response, but it also increases toxicity. Two studies did not report any differences in QoL with the addition of a fluoropyrimidine agent; however, two studies did report an improvement, with Scheithauer 2003 showing less pain and Ueno 2013 showing an improvement in QALYs. The previous version of this review did not find significant benefits for adding fluoropyrimidine to gemcitabine; however, that version analysed only 5 studies, compared to the 10 studied here. Because this analysis included both intravenous and oral fluoropyrimidine agents, these results must be interpreted with caution. Moreover, two studies used S1 (Ozaka 2012; Ueno 2013), and one study used UFT (Ohkawa 2004), agents that have not been well studied in non‐Asian populations. The absolute improvement in OS is small, ranging from 5.4 months to 8.8 months in the gemcitabine alone arm and 6.7 months to 13.7 months in the combination arm (Table 7).
3.3 Gemcitabine plus topoisomerase inhibitor versus gemcitabine alone
The analysis of three studies shows that the addition of a topoisomerase inhibitor to gemcitabine does not significantly improve OS (HR 1.01; P = 0.92) or PFS (HR 0.91; P = 0.26) (Abou‐Alfa 2006; Rocha Lima 2004; Stathopoulos 2006). Response rates were also not significantly improved (RR 1.50; P = 0.11); however, neutropenia did. Only one study measured QoL and failed to find any differences between the two groups. The median survival times were similar in the two groups (Table 7).
We assessed the quality of evidence as high (Table 3).
3.4 Gemcitabine plus taxane versus gemcitabine alone
Our search yielded only one study that we could analyse in this category (Von Hoff 2013), and it found that adding nab‐paclitaxel to gemcitabine significantly improved OS (HR 0.72; P < 0.001), PFS (HR 0.69; P < 0.001) and response rates (RR 3.29; P < 0.001). Table 3 show that the quality of evidence is high; however, there is only one study. This demonstrates that the addition of nab‐paclitaxel to gemcitabine reduces the risk of death by 28%, reduces the risk of progression by 31% and more than triples the rate of response. There is an increased risk of neutropenia, neuropathy and fatigue, and QoL was not measured. Although there is only one study in this analysis, there was also another study, Corrie 2017, which we could not include; it used gemcitabine plus nab‐paclitaxel as the control group and published similar OS, PFS and response data.
3.5 Gemcitabine plus other combinations of chemotherapy versus gemcitabine alone
The two studies analysed showed that combining gemcitabine with multiple other agents improves OS (HR 0.55; P = 0.001) and PFS (HR 0.43; P < 0.001) (Petrioli 2015; Reni 2005). Only one study reported response rates, which were not different between groups. Likewise, one study reported similar incidence of AEs. QoL was improved in both studies. Table 3 shows the low rating for quality of evidence due to one study not publishing enough data to make a full assessment and because of inconsistency. Given that only one study reported response rates and grade 3/4 AEs, the numbers of events in these analyses are small, and the conclusions that we can draw here are limited. This analysis suggests that the use of combination therapies containing gemcitabine may reduce the risk of death by 45% and reduce the risk of progression by 57%; however, we cannot make any assessment regarding the rates of side effects. There may be an improvement in QoL. Just one study reported median survival times, showing OS in the gemcitabine arm to be 7.1 months compared with 11.9 months in the combination arm (Table 7).
Multi‐drug combinations including gemcitabine may be effective in improving survival outcomes, and given the positive results of the Conroy 2011 study, which uses FOLFIRINOX, the findings add weight to the argument that intensive chemotherapy has a place in the treatment of PC.
3.6 Gemcitabine plus other agent(s) versus gemcitabine alone
This group contains studies that did not fall into any of the other pooled analyses. The four studies analysed here are heterogenous in terms of the agents used (Gansauge 2002; Meng 2012; Oettle 2005; Ueno 2013 – EPA study). The analysis shows that OS is not significantly different in the combination arm. Three studies show improved response rates but also increased anaemia. There was high statistical heterogeneity seen in both survival analyses, which is likely to be accounted for by the varied agents used. QoL was not significantly different in the two studies that reported this outcome. Median survival times were longer in the Gansauge 2002 study but otherwise very similar (Table 7).
These data need to be interpreted with caution, as the studies used a wide range of agents. The results for Ukrain in Gansauge 2002 are highly provocative and may warrant further study in larger numbers, supported by a meta‐analysis across different cancer types (Ernst 2005). We assessed the quality of evidence as low due to imprecision and inconsistency (Table 3).
4 Fluoropyrimidine combinations versus fluoropyrimidine alone
This analysis showed that pooling data from studies that added an agent to 5FU did not result in a significant benefit in OS (HR 0.84; P = 0.27) or PFS (0.52; P = 0.19) compared to 5FU alone (Ducreux 2004; Kovach 1974; Maisey 2002; Moertel 1979). However, in these two analyses, there was high statistical heterogeneity (I² = 66% and 89%, respectively), likely due to the range of agents tested. Three studies used fairly outdated chemotherapies (BCNU, MMC and streptozocin), whereas one study used oxaliplatin (Ducreux 2004). This study accounts for most of the heterogeneity seen, as it found a statistically significant benefit in both OS and PFS in contrast to the other studies. Response rates were not significantly improved (RR 1.18; P = 0.69), again with high statistical heterogeneity that was mainly due to the Kovach 1974 study, testing BCNU and reporting higher responses in the 5FU alone group. Grade 3/4 AEs were not significantly different between the two groups. Only Maisey 2002 assessed QoL, demonstrating an improvement in dyspnoea.
The conclusions that we can draw from this analysis are limited. It seems that from the results of the Ducreux 2004 study, oxaliplatin plus 5FU is an active combination compared with 5FU alone and does not measurably increase side effects.
The quality of evidence was assessed as low due to imprecision and statistical heterogeneity (Table 4).
Overall completeness and applicability of evidence
To our knowledge, this review contains a complete review of all the available evidence up until the censor date. We have made every attempt to conduct the analysis in a clinically relevant way in order to fulfil the objective of assisting patients and clinicians in decision‐making.
Quality of the evidence
Two review authors independently assessed the risk of bias of the individual studies using the GRADE criteria, and we tabulate this information in Figure 2, Figure 3 and the 'Summary of findings' tables. Only four subgroup comparisons were of high quality, whereas the remainder of the comparisons provided moderate‐ or low‐quality evidence. This was mainly due to inconsistency and small sample sizes. Given PC is a rare condition which is commonly seen in the elderly, recruiting to clinical studies is incredibly difficult. In addition, recent large scale sequencing studies have revealed the marked genetic heterogeneity in PC, which is likely to contribute to the inconsistent effects seen between studies (Bailey 2016). This should guide future studies and encourage stratified study design.
Potential biases in the review process
In order to reduce the potential biases in the review process, two separate review authors independently evaluated studies and extracted data, resolving disputes with adjudication by a third review author. We did not identify any other potential biases.
Agreements and disagreements with other studies or reviews
Unlike the previous version of this review (Yip 2009), we were unable to replicate the benefit seen for anti‐cancer therapy versus best supportive care alone. As discussed in the main text, this was mainly due to the fact we were unable to include all the previously analysed studies due to lack of available time‐to‐event data.
We have added to the scope and results of the previous review by widening the inclusion criteria and have been able to provide wider recommendations.
Authors' conclusions
Implications for practice.
Currently there is no way of rationally selecting the 'best' chemotherapy regimen for people with pancreatic cancer. For decades, gemcitabine has been the gold standard; however, there are now several more efficacious options that treating clinicians can consider. The treatment choice must be tailored to the person, taking into account the their performance status and the side effect profiles of the chemotherapy agents. The results of this analysis shows that in advanced pancreas cancer:
based on one study, gemcitabine is superior to 5FU alone, reducing the risk of death and progression and improving QoL;
compared to gemcitabine alone, multi‐drug combinations improve survival outcomes and response rates in PC. FOLFIRINOX, GEMOXEL and gemcitabine/cisplatin/epirubicin and 5FU are active regimens. These data suggest that in people who are fit, multi‐drug regimens may be appropriate, but the potential for increased toxicity must be taken into account;
gemcitabine given using a fixed dose rate schedule improves overall survival but increases toxicity compared with standard dosing;
gemcitabine plus platinum‐based chemotherapy does not improve OS but does improve PFS and response rates;
gemcitabine plus fluoropyrimidine‐based chemotherapy improves survival and response rates, albeit by a small amount;
based on one study, gemcitabine plus taxane improves survival outcomes and response rates but increases toxicity.
Implications for research.
The results of this analysis suggest that using multi‐drug regimens for advanced PC has the potential to improve outcomes. This must be weighed against the increase in toxicity. Currently, there are no effective biomarkers to predict in whom an aggressive approach is warranted, and this should be an area of further research. In addition, this analysis shows that there are many different chemotherapies which are beneficial in this disease, but currently there is no way of rationally selecting the 'best' chemotherapy regimen. Biomarker development has the potential to stratify people early in their disease course, inform clinical study design and avoid exposing people to ineffective chemotherapy.
History
Protocol first published: Issue 6, 2013 Review first published: Issue 3, 2018
| Date | Event | Description |
|---|---|---|
| 30 October 2008 | Amended | Converted to new review format. |
Acknowledgements
The authors would like to thank the Cochrane Upper Gastrointestinal and Pancreatic Diseases Editorial Team, in particular Karin Dearness, Sarah Rhodes and Racquel Simpson.
We would also like to acknowledge the two librarians who provided assistance in sourcing many of the papers in this analysis: Ms Cheng Sui (Garvan Institute of Medical Research and the University of NSW) and Mr Glenn Forbes (University of NSW).
We would like to thank Prof Val Gebski from the Clinical Trials Centre, Sydney, for his assistance with statistics.
The following investigators kindly responded to requests for further information on their studies: N Maisey, A Jacobs, , U Pelzer, S Ohkawa.
Special thanks to Dr Kazu Kikuchi, Dr Niantao Deng and Dr David Herrmann for assistance with translating articles into English.
Appendices
Appendix 1. Glossary of terms
Adenocarcinoma: cancer arising from glandular tissue Analgesia: medication used to relieve pain Anti‐neoplastic: stopping or preventing the growth and spread of cancerous cells Antibody: a protein produced to neutralise another protein. In the case of cancer treatment, these proteins block particular cancer pathways Aortic: the large artery that originates in the heart and supplies the body with blood Biliary: related to the structures that carry bile (a substance which is produced by the liver and responsible for helping the digestion of fats) Cobalt source: radioisotope from which radiation is emitted Coeliac abutment: when tumour touches but does not invade the coeliac vessels, the blood supply around the pancreas Complete response: when a tumour is no longer seen on imaging in response to treatment Cytotoxic: chemicals or drugs capable of killing cells Dyspnoea: difficulty breathing Epigastric: the top, middle part of the abdomen, the area around the stomach Flatulence: gas Insomnia: difficulty sleeping Jaundice: the yellowing of the skin, whites of of the eyes and mucous membranes due to high levels of bilirubin Lethal: capable of causing death Mesenteric vein: one of the two veins responsible for draining the intestines Neutropenia: low white cell count. Can pre‐dispose patients to getting serious infections Nodal: related to lymph nodes Palliative: treatment with the intention of improving symptoms, not cure Partial response: when a tumour shrinks on imaging in response to treatment Placebo: sham or fake treatment Portal occlusion: the blockage of the portal vein, a large vein in the abdomen Resection: surgical removal Stable disease: when tumour growth stabilises in response to treatment (does not change in size between scans) Stent: a small tube used to relieve blockages Thrombocytopenia: low platelet count. Can pre‐dispose patients to serious bleeding Thromboembolic: blood clots in the calf or lung veins Toxicities: side effects
Appendix 2. CENTRAL search strategy
exp Pancreas/
(carcin$ or cancer$ or neoplas$ or tumour$ or tumor$ or growth$ or adenocarcin$ or malig$).mp.
1 and 2
Carcinoma, Pancreatic Ductal/
Pancreatic Neoplasms/
or/3‐5
Antineoplastic Protocols/
chemotherap*.tw.
Radiotherapy/
chemoradiotherap*.tw.
chemo‐radiotherap*.tw.
radiochemotherap*.tw.
radio‐chemotherap*.tw.
Biological Therapy/
Immunotherapy, Adoptive/
exp Immunotherapy, Active/
cetuximab.tw.
erlotinib.tw.
bevacuzimab.tw.
panitumumab.tw.
trastuzumab.tw.
Protein‐Tyrosine Kinases/ai [Antagonists & Inhibitors]
tyrosine kinase inhibitor*.tw.
interleukins.tw.
exp Interleukins/
Cancer Vaccines/
Antibodies, Monoclonal/
exp Interferons/
Molecular Targeted Therapy/
or/7‐29
6 and 30
Appendix 3. MEDLINE search strategy
exp Pancreas/
(carcin$ or cancer$ or neoplas$ or tumour$ or tumor$ or growth$ or adenocarcin$ or malig$).mp.
1 and 2
Carcinoma, Pancreatic Ductal/
Pancreatic Neoplasms/
or/3‐5
Antineoplastic Protocols/
chemotherap*.tw.
Radiotherapy/
exp Chemoradiotherapy/
chemoradiotherap*.tw.
chemo‐radiotherap*.tw.
radiochemotherap*.tw.
radio‐chemotherap*.tw.
Biological Therapy/
Immunotherapy, Adoptive/
exp Immunotherapy, Active/
cetuximab.tw.
erlotinib.tw.
bevacuzimab.tw.
panitumumab.tw.
trastuzumab.tw.
Protein‐Tyrosine Kinases/ai [Antagonists & Inhibitors]
tyrosine kinase inhibitor*.tw.
interleukins.tw.
exp Interleukins/
Cancer Vaccines/
*Antibodies, Monoclonal/
exp Interferons/
Molecular Targeted Therapy/
or/7‐30
6 and 31
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
clinical trials as topic.sh.
randomly.ab.
trial.ti.
or/33‐39
exp animals/ not humans.sh.
40 not 41
32 and 42
Appendix 4. EMBASE search strategy
exp Pancreas/
(carcin$ or cancer$ or neoplas$ or tumour$ or tumor$ or growth$ or adenocarcin$ or malig$).mp.
1 and 2
Carcinoma, Pancreatic Ductal/
Pancreatic Neoplasms/
or/3‐5
Cancer chemotherapy/
Cancer radiotherapy/
exp Chemoradiotherapy/
chemoradiotherap*.tw.
chemo‐radiotherap*.tw.
radiochemotherap*.tw.
radio‐chemotherap*.tw.
Biological Therapy/
exp Immunotherapy, Active/
vaccine/ or cancer vaccine/ or tumor cell vaccine/ or tumor vaccine/
active immunization/
antineoplastic agent/
cetuximab/
erlotinib/
bevacizumab/
panitumumab/
trastuzumab/
protein tyrosine kinase inhibitor/
interleukin derivative/
cancer vaccine/
monoclonal antibody/
exp interferon/
immunotherapy/ or adoptive immunotherapy/ or cancer immunization/
molecularly targeted therapy/
or/7‐30
6 and 31
random:.tw. or placebo:.mp. or double‐blind:.tw.
32 and 33
Data and analyses
Comparison 1. Anti‐cancer therapy versus best supportive care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 4 | 298 | Hazard Ratio (Random, 95% CI) | 1.08 [0.88, 1.33] |
Comparison 2. Various types of chemotherapy versus gemcitabine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 8 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 5‐FU | 1 | 126 | Hazard Ratio (Random, 95% CI) | 1.69 [1.26, 2.27] |
| 1.2 FOLFIRINOX | 2 | 652 | Hazard Ratio (Random, 95% CI) | 0.51 [0.43, 0.60] |
| 1.3 CO‐101 | 1 | 367 | Hazard Ratio (Random, 95% CI) | 1.07 [0.86, 1.34] |
| 1.4 ZD9331 | 1 | 55 | Hazard Ratio (Random, 95% CI) | 0.86 [0.42, 1.76] |
| 1.5 Fixed dose rate gemcitabine | 2 | 644 | Hazard Ratio (Random, 95% CI) | 0.79 [0.66, 0.94] |
| 1.6 Exatecan | 1 | 339 | Hazard Ratio (Random, 95% CI) | 1.27 [0.96, 1.68] |
| 2 Progression‐free survival | 5 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 5‐FU | 1 | 126 | Hazard Ratio (Random, 95% CI) | 1.47 [1.12, 1.92] |
| 2.2 FOLFIRINOX | 2 | 652 | Hazard Ratio (Random, 95% CI) | 0.46 [0.38, 0.57] |
| 2.3 ZD9331 | 1 | 55 | Hazard Ratio (Random, 95% CI) | 0.78 [0.46, 1.32] |
| 2.4 Fixed dose rate gemcitabine | 1 | 552 | Hazard Ratio (Random, 95% CI) | 0.88 [0.77, 1.01] |
| 3 Degradation of QoL at 6 months | 2 | Hazard Ratio (Random, 95% CI) | 0.46 [0.35, 0.61] | |
| 4 Response rates | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 5‐FU | 1 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.71] |
| 4.2 FOLFIRINOX | 1 | 342 | Risk Ratio (M‐H, Random, 95% CI) | 3.38 [2.01, 5.65] |
| 4.3 CO‐101 | 1 | 358 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.43, 1.04] |
| 4.4 ZD9331 | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.04, 4.33] |
| 4.5 Fixed dose rate gemcitabine | 2 | 644 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.91, 2.79] |
| 4.6 Exatecan (DX‐8951f) | 1 | 276 | Risk Ratio (M‐H, Random, 95% CI) | 0.10 [0.01, 0.78] |
| 5 Grade 3/4 anaemia | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 5‐FU | 1 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.08 [0.00, 1.34] |
| 5.2 FOLFIRINOX | 1 | 342 | Risk Ratio (M‐H, Random, 95% CI) | 1.3 [0.59, 2.88] |
| 5.3 CO‐101 | 1 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.59, 1.73] |
| 5.4 ZD9331 | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.01, 6.58] |
| 5.5 Fixed dose rate gemcitabine | 2 | 644 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [1.22, 2.63] |
| 5.6 Exatecan | 1 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.43, 2.34] |
| 6 Grade 3/4 neutropenia | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 5‐FU | 1 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.06, 0.61] |
| 6.2 FOLFIRINOX | 1 | 342 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [1.52, 3.01] |
| 6.3 CO‐101 | 1 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.83, 2.07] |
| 6.4 ZD9331 | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 4.17 [0.52, 33.37] |
| 6.5 Fixed dose rate gemcitabine | 2 | 644 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [1.53, 2.23] |
| 6.6 Exatecan | 1 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.64, 1.55] |
| 7 Grade 3/4 thrombocytopenia | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 5‐FU | 1 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.02, 1.34] |
| 7.2 FOLFIRINOX | 1 | 342 | Risk Ratio (M‐H, Random, 95% CI) | 2.5 [0.99, 6.29] |
| 7.3 CO‐101 | 1 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.51, 2.34] |
| 7.4 ZD9331 | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 3.33 [0.40, 27.94] |
| 7.5 Fixed dose rate gemcitabine | 2 | 644 | Risk Ratio (M‐H, Random, 95% CI) | 2.77 [1.99, 3.86] |
| 7.6 Exatecan | 1 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.37, 1.54] |
| 8 Grade 3/4 nausea | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 5‐FU | 1 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.10, 1.35] |
| 8.2 ZD9331 | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 2.52 [0.11, 59.18] |
| 8.3 Fixed dose rate gemcitabine | 2 | 644 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.94, 2.46] |
| 8.4 Exatecan | 1 | 330 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.52, 5.86] |
| 9 Grade 3/4 diarrhoea | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 5‐FU | 1 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.32, 28.07] |
| 9.2 ZD9331 | 1 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.01, 6.58] |
| 9.3 Fixed dose rate gemcitabine | 2 | 644 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.16, 1.23] |
Comparison 3. Gemcitabine combinations versus gemcitabine alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 26 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Gemcitabine plus platinum agent | 6 | 1140 | Hazard Ratio (Random, 95% CI) | 0.94 [0.81, 1.08] |
| 1.2 Gemcitabine plus fluoropyrimidine | 10 | 2718 | Hazard Ratio (Random, 95% CI) | 0.88 [0.81, 0.95] |
| 1.3 Gemcitabine plus topoisomerase inhibitor | 3 | 839 | Hazard Ratio (Random, 95% CI) | 1.01 [0.87, 1.16] |
| 1.4 Gemcitabine plus taxane | 1 | 861 | Hazard Ratio (Random, 95% CI) | 0.72 [0.62, 0.84] |
| 1.5 Gemcitabine plus other combinations of chemotherapy | 2 | 166 | Hazard Ratio (Random, 95% CI) | 0.55 [0.39, 0.79] |
| 1.6 Gemcitabine plus other agent(s) | 4 | 767 | Hazard Ratio (Random, 95% CI) | 0.79 [0.56, 1.10] |
| 2 Progression‐free survival | 18 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 Gemcitabine plus platinum agent | 4 | 1015 | Hazard Ratio (Random, 95% CI) | 0.80 [0.68, 0.95] |
| 2.2 Gemcitabine plus fluoropyrimidine | 8 | 2608 | Hazard Ratio (Random, 95% CI) | 0.79 [0.72, 0.87] |
| 2.3 Gemcitabine plus topoisomerase inhibitor | 2 | 709 | Hazard Ratio (Random, 95% CI) | 0.91 [0.78, 1.07] |
| 2.4 Gemcitabine plus taxane | 1 | 861 | Hazard Ratio (Random, 95% CI) | 0.69 [0.58, 0.82] |
| 2.5 Gemcitabine plus other combinations of chemotherapy | 2 | 166 | Hazard Ratio (Random, 95% CI) | 0.43 [0.30, 0.62] |
| 2.6 Gemcitabine plus other agent(s) | 1 | 76 | Hazard Ratio (Random, 95% CI) | 1.05 [0.68, 1.62] |
| 3 Response rates | 24 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Gemcitabine plus platinum agent | 7 | 1186 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.11, 1.98] |
| 3.2 Gemcitabine plus fluoropyrimidine | 9 | 2176 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [1.29, 2.47] |
| 3.3 Gemcitabine plus topoisomerase inhibitor | 3 | 729 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.92, 2.46] |
| 3.4 Gemcitabine plus taxane | 1 | 861 | Risk Ratio (M‐H, Random, 95% CI) | 3.29 [2.24, 4.84] |
| 3.5 Gemcitabane plus other combinations of chemotherapy | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [0.83, 4.56] |
| 3.6 Gemcitabine plus other agent(s) | 3 | 691 | Risk Ratio (M‐H, Random, 95% CI) | 3.66 [1.04, 12.82] |
| 4 Grade 3/4 anaemia | 23 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Gemcitabine plus platinum agent | 7 | 1156 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.87, 2.31] |
| 4.2 Gemcitabine plus fluoropyrimidine | 8 | 2158 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.84, 1.45] |
| 4.3 Gemcitabine plus topoisomerase inhibitor | 3 | 797 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.72, 1.66] |
| 4.4 Gemcitabine plus taxane | 1 | 793 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.73, 1.52] |
| 4.5 Gemcitabine plus other combinations of chemotherapy | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [0.53, 7.13] |
| 4.6 Gemcitabine plus other agent(s) | 3 | 688 | Risk Ratio (M‐H, Random, 95% CI) | 3.58 [1.93, 6.62] |
| 5 Grade 3/4 neutropenia | 23 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Gemcitabine plus platinum agent | 6 | 961 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.90, 1.97] |
| 5.2 Gemcitabine plus fluoropyrimidine | 9 | 2177 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [1.34, 1.74] |
| 5.3 Gemcitabine plus topoisomerase inhibitor | 3 | 797 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [1.04, 2.30] |
| 5.4 Gemcitabine plus taxane | 1 | 793 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.16, 1.75] |
| 5.5 Gemcitabine plus other combinations of chemotherapy | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [0.65, 5.83] |
| 5.6 Gemcitabine plus other agent(s) | 3 | 688 | Risk Ratio (M‐H, Random, 95% CI) | 2.02 [0.88, 4.66] |
| 6 Grade 3/4 thrombocytopenia | 23 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Gemcitabine plus platinum agent | 6 | 1110 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [1.00, 3.84] |
| 6.2 Gemcitabine plus fluoropyrimidine | 9 | 2177 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.00, 2.18] |
| 6.3 Gemcitabine plus topoisomerase inhibitor | 3 | 797 | Risk Ratio (M‐H, Random, 95% CI) | 2.28 [0.97, 5.36] |
| 6.4 Gemcitabine plus taxane | 1 | 793 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.93, 2.07] |
| 6.5 Gemcitabine plus other combinations of chemotherapy | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [0.74, 5.07] |
| 6.6 Gemcitabine plus other agent(s) | 3 | 688 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.45, 4.39] |
| 7 Grade 3/4 nausea | 21 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Gemcitabine plus platinum agent | 6 | 1110 | Risk Ratio (M‐H, Random, 95% CI) | 2.28 [1.40, 3.71] |
| 7.2 Gemcitabine plus fluoropyrimidine | 7 | 2075 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.87, 1.84] |
| 7.3 Gemcitabine plus topoisomerase inhibitor | 3 | 797 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.94, 2.55] |
| 7.4 Gemcitabine plus other combinations of chemotherapy | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 10.69 [0.61, 185.91] |
| 7.5 Gemcitabine plus other agent(s) | 4 | 748 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.48, 3.26] |
| 8 Grade 3/4 diarrhoea | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Gemcitabine plus platinum agent | 6 | 1110 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.62, 3.53] |
| 8.2 Gemcitabine plus fluoropyrimidine | 8 | 2087 | Risk Ratio (M‐H, Random, 95% CI) | 2.16 [1.34, 3.47] |
| 8.3 Gemcitabine plus topoisomerase inhibitor | 3 | 797 | Risk Ratio (M‐H, Random, 95% CI) | 3.47 [0.74, 16.33] |
| 9 Grade 3/4 neuropathy | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Gemcitabine plus taxane | 1 | 793 | Risk Ratio (M‐H, Random, 95% CI) | 22.35 [7.10, 70.40] |
| 10 Grade 3/4 fatigue | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 Gemcitabine plus taxane | 1 | 793 | Risk Ratio (M‐H, Random, 95% CI) | 2.48 [1.63, 3.79] |
Comparison 4. Fluoropyrimidine combinations versus fluoropyrimidine alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 4 | 491 | Hazard Ratio (Random, 95% CI) | 0.84 [0.61, 1.15] |
| 2 Progression‐free survival | 2 | 255 | Hazard Ratio (Random, 95% CI) | 0.52 [0.19, 1.38] |
| 3 Response rates | 4 | 410 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.52, 2.68] |
| 4 Grade 3/4 anaemia | 2 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.06, 3.62] |
| 5 Grade 3/4 neutropenia | 2 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 5.70 [0.73, 44.46] |
| 6 Grade 3/4 thrombocytopenia | 2 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.34, 5.80] |
| 7 Grade 3/4 fatigue | 1 | 209 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.58, 1.43] |
| 8 Grade 3/4 nausea | 2 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.32, 3.53] |
| 9 Grade 3/4 diarrhoea | 2 | 255 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.31, 2.78] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abou‐Alfa 2006.
| Methods | Randomised phase III trial | |
| Participants | Study was conducted in North America in 349 participants with locally advanced/metastatic pancreatic adenocarcinoma. The mean age in the gemcitabine + exatecan group was 63 years, and the mean age in gemcitabine group 62.3 years. Previous radiotherapy for locally advanced disease was allowed. 174 received gemcitabine. 175 received gemcitabine + exatecan. | |
| Interventions | Gemcitabine: 1000 mg/m² 7/8 weeks, then 3/4 weeks Gemcitabine + exatecan: gemcitabine 1000 mg/m² and exatecan 2 mg/m² days 1 and 8 every 3 weeks |
|
| Outcomes | Overall survival Time to progression Safety Quality of life Response rate Progression‐free survival |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Overall survival primary endpoint |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in the survival analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods are reported in the results |
| Other bias | Low risk | No indication of other bias |
Afchain 2009.
| Methods | Randomised phase II study | |
| Participants | Study was conducted in France. 57 participants with metastatic adenocarcinoma without prior chemotherapy or radiotherapy. 20 participants received gemcitabine/oxaliplatin and 37 participants received simplified gemcitabine/oxaliplatin. Mean age was 66.6 years in the gemcitabine/oxaliplatin group and 64.9 years in the simplified gemcitabine/oxaliplatin group. | |
| Interventions | Gemcitabine/oxaliplatin: gemcitabine 1000 mg/m² on day 1, oxaliplatin 100 mg/m² day 2, every 2 weeks Simplified regimen: gemcitabine 1000 mg/m² day 1, oxaliplatin 100 mg/m² day 1, every 2 weeks |
|
| Outcomes | Progression‐free survival Overall survival Response rate |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient details published |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods are reported in the results |
| Other bias | Low risk | No indication of other bias |
Andren‐Sandberg 1983.
| Methods | Randomised study | |
| Participants | Study was conducted in Sweden. 47 participants with inoperable pancreatic cancer less than 71 years old. 22 received best supportive care and 25 received 5FU + CCNU + vincristine. The mean age was 58 years in the treatment group and 60 years in the best supportive care group. | |
| Interventions | 5FU: 500 mg orally days 2‐5 CCNU: 40 mg/m² orally days 2 + 3 Vincristine: 1 mg/m² day 1 Given every 6 weeks |
|
| Outcomes | Survival Quality of life |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient details published |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in the survival analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods are reported in the results |
| Other bias | Low risk | No indication of other bias |
Berlin 2002.
| Methods | Randomised phase III trial | |
| Participants | Study was conducted in North America. 322 participants with unresectable pancreatic ductal adenocarcinoma. Were allowed to have received adjuvant gemcitabine if completed > 6 months prior. Were allowed to have received radiotherapy if completed more than 4 weeks prior. 162 received gemcitabine. 160 received gemcitabine + 5‐fluorouracil (5FU). The median age in the gemcitabine + 5FU group was 65.8 years and 64.3 years in the gemcitabine group. | |
| Interventions | Gemcitabine 1000 mg/m² 3/4 weeks Gemcitabine + 5FU: gemcitabine as above + 5FU 600 mg/m²/week bolus given 3/4 weeks |
|
| Outcomes | Overall survival Time to progression Response rate |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in survival analysis |
| Selective reporting (reporting bias) | Unclear risk | Insufficient details published |
| Other bias | Unclear risk | No indication of other bias |
Boeck 2008.
| Methods | Randomised phase II trial | |
| Participants | Study was conducted in Germany. 190 participants with advanced pancreatic cancer. 61 received capecitabine + oxaliplatin (CapOx), 64 received capecitabine + gemcitabine (CapGem) and 63 received gemcitabine + oxaliplatin (mGemOx). The median age was 62 years (CapOx), 63 years (CapGem) and 63 years (mGemOx) in the treatment groups. | |
| Interventions | CapOx: capecitabine 1000 mg/m² orally twice daily, days 1‐14 every 3 weeks + oxaliplatin 130 mg/m² day 1 CapGem: capecitabine 825 mg/m² orally twice daily, days 1‐14 every 3 weeks + gemcitabine 1000 mg/m² days 1 + 8 mGemOx: gemcitabine 1000 mg/m² days 1 + 8 + oxaliplatin 130 mg/m² day 8 |
|
| Outcomes | Progression‐free survival after 3 months Overall survival Overall response rate Clinical benefit response Ca19.9 response |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | High risk for primary endpoint (PFS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat population reported for survival analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods are reported in the results |
| Other bias | Low risk | No indication of other bias |
Bukowski 1983.
| Methods | Randomised study | |
| Participants | Study was conducted in North America. 145 participants with inoperable pancreatic adenocarcinoma with no previous chemotherapy or radiotherapy. 73 were given mitomycin C + 5FU (MF), 72 were given streptozocin, mitomycin C and 5FU (SMF). The median age for participants in the SMF arm were 59 years and 59.5 years for those with measurable and non‐measurable disease respectively. In the MF arm the median age was 60 years and 62 years for those with measurable and non‐measurable disease respectively. | |
| Interventions | MF 'good risk' ‐ mitomycin C 20 mg/m² on day 1 + 5FU 1000 mg/m² days 1‐4 and 29‐32 every 56 days MF 'poor risk' ‐ mitomycin C 15 mg/m² on day 1 with the same 5FU regimen above SMF 'good risk' ‐ streptozocin 400 mg/m² days 1‐4 and 29‐32, mitomycin C 15 mg/m² on day 1 and 5FU 1000 mg/m² days 1‐4 and 29‐32, every 56 days SMF 'poor risk' ‐ mitomycin given at 10 mg/m² day 1, with streptozocin and 5FU given as above |
|
| Outcomes | Overall survival Performance‐free survival Response rate |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Primary outcome measure not stated, no intention‐to‐treat analysis |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only participants with measurable disease were included in survival analysis |
| Selective reporting (reporting bias) | High risk | Participants with non‐measurable disease not comprehensively reported |
| Other bias | Low risk | No indication of other bias |
Burris 1997.
| Methods | Randomised trial | |
| Participants | Study was conducted in the United States and Canada. 126 participants with advanced, symptomatic pancreas cancer with stabilised pain. 63 received 5‐fluorouracil (5FU). 63 received gemcitabine. Median age in the 5FU arm was 61 years and 62 years in the gemcitabine arm. | |
| Interventions | 5FU 600 mg/m² weekly Gemcitabine 1000 mg/m² 7/8 weeks then 3/4 weeks |
|
| Outcomes | Clinical benefit Response rate Overall survival Progression‐free survival Safety |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization of patients with stabilized pain to treatment with either gemcitabine or 5‐FU occurred immediately before starting study drug treatment and was performed at a central location" |
| Allocation concealment (selection bias) | Low risk | "Randomization of patients with stabilized pain to treatment with either gemcitabine or 5‐FU occurred immediately before starting study drug treatment and was performed at a central location" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Treatment was single blind. The study drug was not blinded to the investigator, because a rash was a potential side effect of treatment with both 5‐FU and gemcitabine" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | High risk for the primary endpoint (clinical benefit) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled participants included in survival analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods are reported in the results |
| Other bias | Low risk | No indications of other bias |
Cheverton 2004.
| Methods | Randomised phase III study | |
| Participants | Study was conducted in Europe. 339 participants with locally advanced or metastatic pancreatic cancer and no prior chemotherapy. 170 received gemcitabine. 169 received exatecan. Of these 330 (165 vs 165) received treatment. Median age was not published. | |
| Interventions | Gemcitabine 1000 mg/m² given 3/4 then 7/8 weeks Exatecan 0.5 mg/m² daily for 5 days every 3 weeks |
|
| Outcomes | Overall survival Response rates Time to tumour progression Quality of life |
|
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published to make an assessment |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published to make an assessment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled participants included in survival analysis (intention‐to‐treat population reported). |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods are reported in the results |
| Other bias | Low risk | No indications of other bias |
Colucci 2002.
| Methods | Randomised phase II trial | |
| Participants | Study was conducted in Italy. 107 participants with locally advanced, metastatic pancreatic ductal adenocarcinoma with measurable disease and no prior therapy. 54 received gemcitabine. 53 received gemcitabine + cisplatin. The median age in the gemcitabine + cisplatin arm was 60 years, and it was 63 years in the gemcitabine alone arm. | |
| Interventions | Gemcitabine 1000 mg/m² weekly × 7, then 2 weeks rest. Then 3/4 weeks Gemcitabine + cisplatin: gemcitabine as above. Cisplatin 25 mg/m² days 1, 8, 15, 29, 36, 42 then 2 weeks rest. Then gemcitabine and cisplatin days 1, 8, 15 every 4 weeks |
|
| Outcomes | Overall response rate Time to progression (assessed at week 7 and then every 2 cycles of treatment) Overall survival |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Time to progression was the primary endpoint |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis was reported for the primary endpoint |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods are reported in the results |
| Other bias | Low risk | No indication of other bias |
Colucci 2010.
| Methods | Randomised phase III trial | |
| Participants | Study conducted in Italy. 400 participants with unresectable or metastatic pancreatic ductal adenocarcinoma with no prior chemotherapy. 199 received gemcitabine. 201 received gemcitabine + cisplatin. The median age of participants was 63 years. | |
| Interventions | Gemcitabine: 1000 mg/m² 7/8 weeks then 3/4 weeks Gemcitabine + cisplatin: gemcitabine as above. Cisplatin 25 mg/m² days 1, 8, 15, 29, 36, 42 then 1 week rest. Then cisplatin 25 mg/m² days 1, 8, 15 every 4 weeks |
|
| Outcomes | Overall survival Progression‐free survival Overall response rate Toxicity Clinical benefit Quality of life |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to standard arm or experimental arm in a 1:1 ratio. Telephone random assignment was performed centrally (Clinical Trials Unit, National Cancer Institute, Napoli, Italy), by a computer‐driven minimization procedure" |
| Allocation concealment (selection bias) | Low risk | "Patients were randomly assigned to standard arm or experimental arm in a 1:1 ratio. Telephone random assignment was performed centrally (Clinical Trials Unit, National Cancer Institute, Napoli, Italy), by a computer‐driven minimization procedure" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods are reported in the results |
| Other bias | Low risk | No indication of other bias |
Conroy 2011.
| Methods | Randomised phase II/III study | |
| Participants | Study was conducted in France. 342 participants with measurable, metastatic pancreatic ductal adenocarcinoma and no previous chemotherapy. 171 received gemcitabine. 171 received 5‐fluorouracil (5FU) + oxaliplatin + irinotecan (FOLFIRINOX). The median age was 61 in both treatment groups. | |
| Interventions | Gemcitabine 1000 mg/m² 7/8 weeks then 3/4 weeks FOLFIRINOX: 5FU bolus 400 mg/m², 5FU CI 2400 mg/m² over 46 hours + leucovorin 400 mg/m² + oxaliplatin 85 mg/m² + irinotecan 180 mg/m² every 2 weeks |
|
| Outcomes | Overall survival Progression‐free survival Response rate Safety Quality of life |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed centrally in a 1:1 ratio with stratification according to center, performance status (0 vs. 1), and primary tumor localization" |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed centrally in a 1:1 ratio with stratification according to center, performance status (0 vs. 1), and primary tumor localization" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint and other endpoints. "Independent review of CT scans was performed" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All analyses were performed on an intention‐to‐treat‐basis" |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods are reported in the results |
| Other bias | Low risk | No indications of other bias |
Corrie 2017.
| Methods | Randomised phase II study | |
| Participants | Study was conducted in the UK. 146 participants with metastatic pancreatic adenocarcinoma with no prior treatment. 75 received standard concomitant nab‐paclitaxel and gemcitabine and 71 received sequential administration of nab‐paclitaxel and gemcitabine. Median age was 66 years. | |
| Interventions | Standard regimen: nab‐paclitaxel 125 mg/m² and gemcitabine 1000 mg/m² given immediately after each other on days 1, 8, 15 of a 4 week cycle Sequential regimen: nab‐paclitaxel 125 mg/m² on days 1, 8, 15 and gemcitabine 1000 mg/m² on days 2, 9, 16 of a 4‐week cycle |
|
| Outcomes | Progression‐free survival Safety Response rate Overall survival Quality of life |
|
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Web based randomisation system with stratified block randomisation |
| Allocation concealment (selection bias) | Low risk | No evidence of selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | High for the primary endpoint (PFS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in the survival analyses |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods are reported in the results |
| Other bias | Low risk | None found |
Cullinan 1985.
| Methods | Randomised trial | |
| Participants | Study was conducted in North America. 305 participants with unresectable or metastatic pancreatic or gastric adenocarcinoma. Of the participants with pancreatic cancer, 50 received 5‐fluorouracil (5FU), 44 received 5FU + doxorubicin (FA). 50 received 5FU + doxorubicin + mitomycin C (FAM). The majority of participants in the study were between 50 to 69 years old. | |
| Interventions | 5FU: 500 mg/m² days 1‐5 week 1, 4 and 8, then every 5 weeks FA: 5FU 400 mg/m² days 1‐4 + doxorubicin 40 mg/m² day 1 week 1, 4 and 8, then every 5 weeks FAM: 5FU 600 mg/m² days 1, 8, 29, 36 + doxorubicin 30 mg/m² days 1 and 29 + mitomycin C 10 mg/m² day 1, every 8 weeks |
|
| Outcomes | Overall survival Progression‐free survival Response rate Toxicity Symptom control |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10 participants excluded from survival analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods are reported in the results |
| Other bias | Low risk | No indication of other bias |
Cullinan 1990.
| Methods | Randomised phase III trial | |
| Participants | Study was conducted in North America. 187 participants with measurable, metastatic ductal or undifferentiated pancreatic cancer with no prior chemotherapy. 64 received 5‐fluorouracil (5FU). 61 received 5FU + cyclophosphamide + methotrexate + vincristine (Mallison regimen). 59 received 5FU + doxorubicin + cisplatin (FAP). Median age for the 5FU, Mallinson and FAP arm was 60, 62 and 62 years respectively. | |
| Interventions | 5FU: 500 mg/m²/day for 5 days every 5 weeks Mallinson: 5FU 270 mg/m²/day days 1‐5 + cyclophosphamide 160 mg/m² days 1 + 5 + methotrexate 11 mg/m² days 1 + 4 + vincristine 0.7 mg/m² days 2 + 5 then maintenance with 5FU 350 mg/m² days 1‐5 + mitomycin C 3.5 mg/m² days 1‐5 every 5 weeks FAP: 5FU 300 mg/m²/day days 1‐5 + doxorubicin 40 mg/m² day 1 + cisplatin 60 mg/m² day 1, every 5 weeks |
|
| Outcomes | Overall survival | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for the primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Survival analysis conducted on all enrolled patients |
| Selective reporting (reporting bias) | Low risk | Only OS listed specifically as an endpoint in the methods, this is reported on all patients |
| Other bias | Unclear risk | No indication of other bias |
Cunningham 2009.
| Methods | Randomised phase III trial | |
| Participants | Study was conducted in the UK. 533 participants with locally advanced/metastatic pancreatic ductal adenocarcinoma. 266 received gemcitabine. 267 received gemcitabine + capecitabine. Median age of participants was 62 years. | |
| Interventions | Gemcitabine: 1000 mg/m² 7/8 weeks then 3/4 weeks Gemcitabine + capecitabine: gemcitabine 1000 mg/m² 3/4 weeks + capecitabine 830 mg/m² twice daily orally for 3 weeks then 1 week rest |
|
| Outcomes | Overall survival Progression‐free survival Overall response rate Toxicity Quality of life Pain |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to each treatment arm on a 1:1 basis according to a computer‐generated variable‐size blocked randomization method. Randomization was stratified by performance status (0, 1 versus 2) and extent of disease" |
| Allocation concealment (selection bias) | Low risk | "Patients were randomly assigned to each treatment arm on a 1:1 basis according to a computer‐generated variable‐size blocked randomization method. Randomization was stratified by performance status (0, 1 versus 2) and extent of disease" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat population reported |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of other bias |
Di Costanzo 2005.
| Methods | Randomised phase II trial | |
| Participants | Study was conducted in Italy. 94 participants with locally advanced/metastatic pancreatic ductal adenocarcinoma with measurable disease. 48 received gemcitabine. 43 received gemcitabine + 5‐fluorouracil (5FU). Median age of participants was 63 years | |
| Interventions | Gemcitabine: 1000 mg/m² weekly for 7 weeks then 2 weeks rest, then 3/4 weeks Gemcitabine + 5FU: Gemcitabine as above. 5FU 200 mg/m²/day for 6 weeks then 2 weeks rest, then 3/4 weeks |
|
| Outcomes | Response rate Overall survival Safety Quality of life |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were centrally randomised by the central office of the Italian Oncology Group for Clinical Research (GOIRC) to receive: GEM alone (arm A) or in combination with CI 5‐FU (arm B)" |
| Allocation concealment (selection bias) | Low risk | "Patients were centrally randomised by the central office of the Italian Oncology Group for Clinical Research (GOIRC) to receive: GEM alone (arm A) or in combination with CI 5‐FU (arm B)" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | High risk for the primary outcome (response rate) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only patients "evaluable for response" were assessed for the primary endpoint |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of other bias |
Ducreux 2004.
| Methods | Randomised phase II study | |
| Participants | Study was conducted in France. 63 participants with locally advanced/metastatic pancreatic adenocarcinoma and measurable disease. Were allowed to have had previous 5FU and radiotherapy if more than 3 months prior to randomisation. 17 received oxaliplatin, 31 received 5FU + oxaliplatin and 15 received 5FU alone. The mean age was 57 years. | |
| Interventions | Oxaliplatin: 130 mg/m² every 3 weeks 5FU/ oxaliplatin: 5FU 1000 mg/m²/day, days 1‐4 and oxaliplatin as above 5FU alone given as above |
|
| Outcomes | Response rate | |
| Notes | The oxaliplatin alone arm was not included in the meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible patients in this open‐label study were stratified by center and disease stage (locally advanced versus metastatic) and centralized block randomization was used to assign patients to one of three arms" |
| Allocation concealment (selection bias) | Low risk | "Eligible patients in this open‐label study were stratified by center and disease stage (locally advanced versus metastatic) and centralized block randomization was used to assign patients to one of three arms" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | External radiologist used to assess tumour response |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 56 participants were evaluable for response, 4 withdrew prior to first assessment and 2 participants had baseline assessments which were old or missing |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of other bias |
Frey 1981.
| Methods | Randomised study | |
| Participants | Study was conducted in North America. 152 male participants with unresectable cancer of the pancreas. 65 received 5‐fluorouracil (5FU) and CCNU; 87 received best supportive care. The majority of participants were between the age of 50 and 59 years. | |
| Interventions | 5FU 9 mg/kg days 1‐5 + CCNU 70 mg/m² day 1, every 6 weeks | |
| Outcomes | Overall survival Toxicity |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details regarding the method of randomisation provided |
| Allocation concealment (selection bias) | Low risk | "Assignment of patients to treated or control groups in this multi‐institutional trial was made by means of sealed, sequentially numbered envelopes distributed by a statistician from the Follow‐up Agency, National Academy of Sciences‐National Research Council." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled participants included in survival analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indications of other bias |
Gansauge 2002.
| Methods | Randomised phase II trial | |
| Participants | Study was conducted in Germany. 90 participants with unresectable pancreatic adenocarcinoma. 30 received gemcitabine. 30 received Ukrain (NSC‐31570). 30 received Ukrain + gemcitabine. The mean age for the gemcitabine, Ukrain and Gemcitabine + Ukrain groups were 63.8, 60.6 and 58.2 respectively. | |
| Interventions | Gemcitabine: 1000 mg/m² 7/8 weeks then 34 weeks Ukrain: 20 mg weekly for 7 weeks, 1 week rest then 3/4 weeks up to 12 cycles Gemcitabine + Ukrain: as above |
|
| Outcomes | Overall survival | |
| Notes | This was a multi‐armed study. Event rates were not available for the 3 arms. Only the gemcitabine and gemcitabine + Ukrain arms analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient details published |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in the survival analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of other bias |
Glimelius 1996.
| Methods | Randomised study | |
| Participants | Study was conducted in Sweden. 90 participants with non‐curable pancreatic or biliary tract cancer. 53 had pancreatic cancer. 29 received 5FU/LV +/‐ etoposide. 24 received best supportive care. Median age for the chemotherapy and best supportive care arms were 65 and 64 respectively. | |
| Interventions | If participant was > 60 years, then 5FU/LV: 5FU 500 mg/m² + LV 60 mg/m² on days 1 + 2 every 14 days If participant was < 60 years old, then 5FU/LV: 5FU 500 mg/m² + LV 60 mg/m² + etoposide 120 mg/m² |
|
| Outcomes | Overall survival Quality of life Objective responses Toxicity |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention to treat analysis reported |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of other bias |
Heinemann 2006.
| Methods | Randomised phase III trial | |
| Participants | Study was conducted in Germany. 195 participants with locally advanced/metastatic pancreatic adenocarcinoma with measurable disease. Previous radiotherapy was allowed if not on the target lesion. 97 received gemcitabine. 98 received gemcitabine + cisplatin. The median age of the gemcitabine + cisplatin and gemcitabine alone groups was 64 and 66 years respectively. | |
| Interventions | Gemcitabine: 1000 mg/m² day 1, 8, 15 every 4 weeks Gemcitabine + cisplatin: gemcitabine as above. Cisplatin 50 mg/m² days 1, 8, 15 every 4 weeks |
|
| Outcomes | Overall survival Progression‐free survival Response rate Safety Quality of life |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Central random assignment was performed before the start of treatment, and patients were assigned to one of the treatment arms." |
| Allocation concealment (selection bias) | Low risk | "Central random assignment was performed before the start of treatment, and patients were assigned to one of the treatment arms." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "The primary outcome measure was OS, which was determined for all randomly assigned patients from the date of random assignment to the date of death or last contact." |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of other bias |
Herrmann 2007.
| Methods | Randomised phase III trial | |
| Participants | Study conducted in eight European countries. 319 participants with inoperable/metastatic pancreatic ductal adenocarcinoma. 159 received gemcitabine. 160 received gemcitabine + capecitabine. The median age was not stated. | |
| Interventions | Gemcitabine: 1000 mg/m² 7/8 weeks then 3/4 weeks Capecitabine: 650 mg/m² twice daily orally, days 1‐14 every 3 weeks |
|
| Outcomes | Overall survival Progression‐free survival Overall response rate Safety Quality of life |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Likely to be low risk but actual method of randomisation/allocation not stated in publication |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for the primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Intent‐to‐treat analysis was applied to the analysis of all end points" |
| Selective reporting (reporting bias) | Low risk | No indication of reporting bias |
| Other bias | Low risk | No indication of other bias |
Hirao 2011.
| Methods | Randomised phase II trial | |
| Participants | Study was conducted in Japan. 90 participants with unresectable, metastatic pancreatic ductal adenocarcinoma with no prior therapy. 45 received gemcitabine on a 3 week schedule. 45 received gemcitabine on a 4‐week schedule. The median age in the 4 week and 3 week schedule was 67 years and 66 years, respectively. | |
| Interventions | 3 weeks: gemcitabine 1000 mg/m² days 1 and 8 4 weeks: gemcitabine 1000 mg/m² days 1, 8 and 15 |
|
| Outcomes | Compliance rate Overall survival Progression‐free survival Toxicity Response rate |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed centrally, and the random‐allocation sequence had been generated previously by a statistician using a computer‐generated random code" |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed centrally, and the random‐allocation sequence had been generated previously by a statistician using a computer‐generated random code" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | High risk for primary endpoint (compliance rate) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat population included in analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of reporting bias |
Huguier 2001.
| Methods | Randomised study | |
| Participants | Study was conducted in France. 45 participants with unresectable pancreatic ductal adenocarcinoma. 22 received 5‐fluorouracil (5FU) + leucovorin (LV) + cisplatin; 23 received best supportive care. The median age of participants was 63.4 years. | |
| Interventions | 5FU: 375 mg/m²/day days 1‐5 LV 200 mg/m²/day days 1‐5 Cisplatin 15 mg/m²/day days 1‐5 |
|
| Outcomes | Overall survival Side effects |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Assignment of patients to chemotherapy or control group used a centralised random permuted block technique" |
| Allocation concealment (selection bias) | Low risk | "Assignment of patients to chemotherapy or control group used a centralised random permuted block technique" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled participants included in the survival analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indications of other bias |
Kelsen 1991.
| Methods | Phase III randomised study | |
| Participants | Study was conducted in North America. 82 participants with advanced pancreatic adenocarcinoma and no prior therapy. 42 received streptozocin, mitomycin C and 5FU (SMF). 50 received cisplatin, cytosine arabinoside and caffeine (CAC). The median age of participants was 59 years. | |
| Interventions | SMF: streptozocin 1 g/m² days 1, 8, 29 and 36 + mitomycin C 10 mg/m² day 1 + 5FU 600 mg/m² days 1, 8 and 36 every 8 weeks CAC: cisplatin 100 mg/m² day 1 + cytosine arabinoside 2 g/m² two doses day 1 + caffeine 400 mg/m² subcutaneous 2 doses day 1 |
|
| Outcomes | Response | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | High risk for primary outcome (response rate) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not all participants were assessed for the primary outcome measure |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of other bias |
Kovach 1974.
| Methods | Randomised study | |
| Participants | Study was conducted in North America. 82 with unresectable pancreatic adenocarcinoma and measurable disease. 31 received 5FU, 21 received 1,3‐bis‐(2‐chloroethyl)‐1‐nitrosurea (BCNU)and 30 received 5FU + BCNU | |
| Interventions | 5FU: 13.5 mg/kg/day for 5 days every 5 weeks BCNU: 50 mg/m²/day for 5 days every 8 weeks 5FU + carmustine: 5FU 10 mg/kg/day for 5 days and BCNU 40 mg/m²/day for 5 days every 8 weeks |
|
| Outcomes | Not stated | |
| Notes | This is a multi‐armed study. Event rates for each arm were not available. Only the 5FU and 5FU + carmustine arms were analysed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Primary outcome measure unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if all participants were included in survival analysis |
| Selective reporting (reporting bias) | Low risk | No indication of reporting bias |
| Other bias | Low risk | No indication of other bias |
Kulke 2009.
| Methods | Randomised phase II trial | |
| Participants | Study was conducted in North America. 245 participants with metastatic pancreatic adenocarcinoma. Adjuvant 5‐fluorouracil was permitted with completed > 2 weeks prior. 62 received gemcitabine + cisplatin. 58 received gemcitabine. 65 received gemcitabine + docetaxel. 60 received gemcitabine + irinotecan. Median age of participants was 60.5 years. | |
| Interventions | Gemcitabine + cisplatin: gemcitabine 1000 mg/m² weekly × 3, cisplatin 50 mg/m² days 1 and 15 every 4 weeks. Gemcitabine: 1500 mg/m² at 10 mg/m²/min, weekly × 3, every 4 weeks Gemcitabine + docetaxel: gemcitabine 1000 mg/m² weekly × 3, docetaxel 40 mg/m² days 1 + 8, every 4 weeks Gemcitbine + irinotecan: gemcitabine 1000 mg/m² days 1 and 8, irinotecan 100 mg/m² days 1 and 8 every 3 weeks |
|
| Outcomes | Overall survival Response rate Time to progression Toxicity |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published. "Patients were randomly assigned to receive one of the following four regimens: gemcitabine/cisplatin (arm A), fixed dose rate gemcitabine (arm B), gemcitabine/docetaxel (arm C), or gemcitabine/irinotecan (arm D)" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published. "Patients were randomly assigned to receive one of the following four regimens: gemcitabine/cisplatin (arm A), fixed dose rate gemcitabine (arm B), gemcitabine/docetaxel (arm C), or gemcitabine/irinotecan (arm D)" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in the outcome analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of other bias |
Lee 2017.
| Methods | Phase III trial | |
| Participants | Study was conducted in Korea. 214 treatment naive participants with locally advanced or metastatic pancreatic adenocarcinoma with ECOG 0‐2. 108 participants received gemcitabine + capecitabine and 106 participants received gemcitabine alone. Median age was 54 years years. | |
| Interventions | Gemcitabine 1000 mg/m² 3/4 weeks Capecitabine 1600 mg/m² daily for 3/4 weeks |
|
| Outcomes | Overall survival Progression‐free survival Overall response rate Disease control rate Toxicity |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We randomly assigned eligible patients to each treatment arm on a 1:1 basis according to a computer‐generated variable‐size blocked randomization method." |
| Allocation concealment (selection bias) | Low risk | "We randomly assigned eligible patients to each treatment arm on a 1:1 basis according to a computer‐generated variable‐size blocked randomization method."s |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients included in the survival analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | None found |
Levi 2004.
| Methods | Randomised study | |
| Participants | Study was conducted in Europe. 107 participants with advanced pancreatic cancer. Factorial design randomised participants to either 5FU given at a constant rate infusion or chronomodulated infusion, with or without cisplatin. Median age of participants was 63 years. | |
| Interventions | 5FU: 5 g/m² (cycle 1) or 6 g/m² (cycle 2) or 6.5 g/m² (cycle 3) either at a constant rate infusion or chronomodulated (given between 10 pm and 10 am) Cisplatin: 100 mg/m² once per cycle |
|
| Outcomes | OS | |
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient details published |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient details published |
| Selective reporting (reporting bias) | Unclear risk | Insufficient details published |
| Other bias | Unclear risk | Insufficient details published |
Li 2004.
| Methods | Randomised study | |
| Participants | Study was conducted in China. 46 participants with metastatic pancreatic adenocarcinoma. 25 received gemcitabine. 21 received gemcitabine + cisplatin | |
| Interventions | Gemcitabine: 1000 mg/m² IV 3/4 weeks Cisplatin: 25 mg/m²/week, 3/4 weeks |
|
| Outcomes | Overall survival | |
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient details published |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient details published |
| Selective reporting (reporting bias) | Unclear risk | Insufficient details published |
| Other bias | Unclear risk | Insufficient details published |
Lohr 2012.
| Methods | Randomised phase II trial | |
| Participants | Study was conducted in Europe. 200 participants with locally advanced/unresectable or metastatic pancreatic adenocarcinoma. 50 received gemcitabine. 50 received liposomal‐paclitaxel (ET) 11 mg/m². 50 received ET 22 mg/m². 50 received ET 44 mg/m². The median age for the gemcitabine alone, ET 11mg/m2, ET 22mg/m2 and ET 44mg/m2 group was 59.5, 63, 61 and 62.5 years, respectively. | |
| Interventions | Gemcitabine: 1000 mg/m² weekly × 7 ET: Dose given twice weekly × 14 |
|
| Outcomes | Overall survival Progression‐free survival Response rate Quality of life Adverse events |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were centrally randomized" |
| Allocation concealment (selection bias) | Low risk | "Patients were centrally randomized" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | PFS for intention‐to‐treat population not reported ‐ only the "modified intention to treat" population |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of other bias |
Louvet 2005.
| Methods | Randomised phase III trial | |
| Participants | Study was conducted in France. 326 participants with unresectable/metastatic pancreatic ductal adenocarcinoma with measurable disease. 156 received gemcitabine. 157 received gemcitabine + oxaliplatin. The median age was 60.1 years and 61.3 years in the gemcitabine and gemcitabine + oxaliplatin groups respectively. | |
| Interventions | Gemcitabine 1000 mg/m² 7/8 weeks then 3/4 weeks Gemcitabine + oxaliplatin: gemcitabine 1000 mg/m² day 1 + oxaliplatin 100 mg/m² day 2, every 2 weeks |
|
| Outcomes | Overall survival Clinical benefit Progression‐free survival Safety |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed centrally, and the minimization method was used to balance treatment allocation according to center, stage of disease (locally advanced v metastatic), and PS (0 or 1 v 2)." |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed centrally, and the minimization method was used to balance treatment allocation according to center, stage of disease (locally advanced v metastatic), and PS (0 or 1 v 2)." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 'per protocol' participants analysed |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of other bias |
Lutz 2005.
| Methods | Randomised phase II trial | |
| Participants | Study was conducted in Europe. 96 participants with metastatic or locally advanced pancreatic ductal adenocarcinoma with no previous treatment. 49 received gemcitabine + docetaxel. 47 received cisplatin + docetaxel. The median age of participants was 58 years and 59 years in the gemcitabine + docetaxel and cisplatin and docetaxel groups respectively. | |
| Interventions | Gemcitabine + docetaxel: gemcitabine 800 mg/m² days 1 and 8 + docetaxel 85 mg/m² day 8 every 3 weeks. Cisplatin + docetaxel: cisplatin 75 mg/m² day 1 every 3 weeks |
|
| Outcomes | Tumour response Rates of febrile neutropenia Duration of response Progression‐free survival Overall survival |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were centrally randomly assigned at the EORTC Data Center, Brussels, Belgium, and stratified using the minimization technique according to institution, performance status (0 v 1), and extent of disease (metastatic v locoregionally advanced)" |
| Allocation concealment (selection bias) | Low risk | "Patients were centrally randomly assigned at the EORTC Data Center, Brussels, Belgium, and stratified using the minimization technique according to institution, performance status (0 v 1), and extent of disease (metastatic v locoregionally advanced)" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | High risk for primary endpoint (tumour response) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat population included in analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of other bias |
Maisey 2002.
| Methods | Randomised phase III trial | |
| Participants | Study was conducted in the UK. 209 participants with locally advanced/metastatic pancreatic ductal adenocarcinoma. 107 received 5‐fluorouracil (5FU). 102 received 5FU + mitomycin C (MMC). The median age of participants was 62 years and 61 years in the 5FU and 5FU + MMC groups respectively. | |
| Interventions | 5FU 300 mg/m²/day via protracted venous infusion (PVI) for 12 weeks. If no progression, another 12 weeks 5FU + MMC: 5FU 300 mg/m²/day + MMC 10 mg/m² every 6 weeks × 4 cycles |
|
| Outcomes | Response rate Survival Toxicity QoL |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...patients were randomly assigned to treatment with PVI 5‐FU or PVI 5‐FU/MMC on a 1:1 basis according to a computer generated randomization code. The patients were randomized centrally in blocks of six and stratified by center" |
| Allocation concealment (selection bias) | Low risk | "...patients were randomly assigned to treatment with PVI 5‐FU or PVI 5‐FU/MMC on a 1:1 basis according to a computer generated randomization code. The patients were randomized centrally in blocks of six and stratified by center" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | High risk for primary endpoint (response rate) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in the survival analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of other bias |
Meng 2012.
| Methods | Randomised, placebo controlled, phase II trial | |
| Participants | Study was conducted in China. 76 participants with unresectable pancreatic adenocarcinoma with measurable disease. 37 received gemcitabine + placebo. 39 received gemcitabine + huachansu. The median age of participants was 60.9 years. | |
| Interventions | Gemcitabine + placebo: 1000 mg/m² 3/4 weeks + saline Gemcitabine + huachansu: gemcitabine as above, huachansu 20 mL/m² 5 days per week, 3 weeks on, 1 week off |
|
| Outcomes | 4 month progression‐free survival Overall survival Overall response rate Time to progression Toxicity |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published ‐ "Patients were randomised using a Bayesian algorithm" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published ‐ "Patients were randomised using a Bayesian algorithm" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | High risk for primary endpoint (PFS at 4 months) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in the survival analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of other bias |
Moertel 1977.
| Methods | Randomised trial | |
| Participants | Study was conducted in North America. 88 participants with unresectable pancreatic ductal adenocarcinoma with measurable disease. 40 received streptozocin + 5‐fluorouracil (5FU). 48 received streptozocin and cyclophosphamide. Most participants were aged between 50 to 59 years old. | |
| Interventions | Streptozocin 500 mg/m² days 1‐5 5FU 400 mg/m² days 1‐5 every 6 weeks Cyclophosphamide 1000 mg/m² days 1 and 21 every 6 weeks |
|
| Outcomes | Not stated | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants who died early or who were unable to continue their assigned treatment were declared to have progressive disease |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 74 participants included in survival analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of other bias |
Moertel 1979.
| Methods | Randomised trial | |
| Participants | Study conducted in North American. 176 participants with metastatic pancreatic ductal adenocarcinoma. 89 received 5‐fluorouracil (5FU). 87 received 5FU + streptozocin. Details on the age of participants not stated. | |
| Interventions | 5FU 450 mg/m² days 1‐5 every 5 weeks 5FU + streptozocin: 5FU 400 mg/m² days 1‐5 + streptozocin 400 mg/m² days 1‐5 every 5 weeks Participants were also randomised +/‐ spironolactone 50 mg 3 times per day |
|
| Outcomes | Survival | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient details published |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in the survival analysis |
| Selective reporting (reporting bias) | High risk | Toxicity data scarcely reported |
| Other bias | Low risk | No indication of other bias |
Oettle 2005.
| Methods | Randomised phase III trial | |
| Participants | Study was conducted in Germany. 565 participants with locally advanced/metastatic pancreatic adenocarcinoma with measurable disease. Radiotherapy permitted if completed > 4 weeks prior. 282 received gemcitabine. 283 received gemcitabine + pemetrexed. The median age of participants was 63 years. | |
| Interventions | Gemcitabine (G): 1000 mg/m² 3/4 weeks Gemcitabine + pemetrexed (PG): gemcitabine 1250 mg/m² days 1 and 8, pemetrexed 500 mg/m² day 8, every 21 days |
|
| Outcomes | Overall survival Progression‐free survival Time to treatment failure Response rate Quality of life |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible patients were randomly assigned using a centralized, automated randomization procedure to either the PG arm or the G arm" |
| Allocation concealment (selection bias) | Low risk | "Eligible patients were randomly assigned using a centralized, automated randomization procedure to either the PG arm or the G arm" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in the survival analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of other bias |
Ohkawa 2004.
| Methods | Randomised trial | |
| Participants | Study was conducted in Japan. 19 participants with advanced pancreatic ductal adenocarcinoma with no previous treatment. 9 received gemcitabine. 10 received gemcitabine + tegafur‐uracil (UFT). The median age of participants for the gemcitabine alone and gemcitabine + UFT groups was 58.4 years and 60.5 years respectively. | |
| Interventions | Gemcitabine: 1000 mg/m² 3/4 weeks. UFT 300 mg/day continuous |
|
| Outcomes | Response rate Survival time Time to progression |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | High risk for primary endpoint (response rate) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient details published |
| Selective reporting (reporting bias) | Unclear risk | Insufficient details published |
| Other bias | Low risk | No indication of other bias |
Ozaka 2012.
| Methods | Randomised phase II trial | |
| Participants | Study was conducted in Japan. 112 participants with locally advanced/metastatic pancreatic ductal adenocarcinoma with measurable disease. 59 received gemcitabine. 53 received gemcitabine + S1. The median age of participants was 64 years. | |
| Interventions | Gemcitabine: 1000 mg/m² 3/4 weeks Gemcitabine + S1: gemcitabine 1000 mg/m² day 1 and 8, S1 80 mg/m² twice daily orally, days 1‐14 every 3 weeks |
|
| Outcomes | Response rate Toxicity Clinical benefit rate Progression‐free survival Overall survival |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random assignment was performed centrally by a web‐based assistant system (Xexible license assisted data server, JACCRO, Tokyo), using a computer‐driven minimization procedure" |
| Allocation concealment (selection bias) | Low risk | "Random assignment was performed centrally by a web‐based assistant system (Xexible license assisted data server, JACCRO, Tokyo), using a computer‐driven minimization procedure" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | High risk for primary endpoint (response rate) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat population not reported for PFS |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of other bias |
Petrioli 2015.
| Methods | Randomised phase II study | |
| Participants | Study was conducted in Italy. 67 participants with metastatic, histologically proven pancreatic cancer and ECOG ≤ 2 and no prior chemotherapy. 33 given gemcitabine alone and 34 given gemcitabine + oxaliplatin + capecitabine (GEMOXEL). The median age of participants in the GEMOXEL and gemcitabine groups was 69 years and 67 years, respectively. | |
| Interventions | Gemcitabine: 1000 mg/m² for 7/8 weeks then days 1, 8 and 15 every 28 days GEMOXEL: gemcitabine 1000 mg/m² days 1, 8, 15 and 22. Oxaliplatin 100 mg/m² day 2, capectiabine 1500 mg/m²/day in 2 divided doses, days 1‐14 every 21 days |
|
| Outcomes | Disease control rate in per protocol population Safety Progression‐free survival Quality of life Overall survival |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | High risk for primary endpoint (disease control rate) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in the survival analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of other bias |
Poplin 2009.
| Methods | Randomised phase III trial | |
| Participants | Study was conducted in North America. 824 participants with locally advanced/metastatic pancreatic ductal adenocarcinoma with measurable disease. Were allowed to have had adjuvant radiotherapy if completed more than 4 weeks prior. 275 received standard gemcitabine. 277 received fixed dose rate gemcitabine (FDR). 272 received gemcitabine + oxaliplatin. The median age was 64 years, 61 years and 63 years for the gemcitabine, FDR and gemcitabine + oxaliplatin groups, respectively. | |
| Interventions | Gemcitabine: 1000 mg/m² over 30 min 7/8 weeks then 3/4 weeks FDR: gemcitabine 1500 mg/m² given over 150 min infusion day 1, 8, 15 every 4 weeks Gemcitabine + oxaliplatin: gemcitabine 1000 mg/m² over 100 min day 1 + oxaliplatin 100 mg/m² day 2, every 2 weeks |
|
| Outcomes | Overall survival Response rate Progression‐free survival Symptoms |
|
| Notes | Gemcitabine + oxaliplatin arm has not been analysed as the gemcitabine dose schedule is not standard. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to treatment using a dynamic balancing algorithm that stratified for performance status, 0 to 1 and versus 2, and for locally advanced versus metastatic disease" |
| Allocation concealment (selection bias) | Unclear risk | "Patients were randomly assigned to treatment using a dynamic balancing algorithm that stratified for performance status, 0 to 1 and versus 2, and for locally advanced versus metastatic disease" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat population included in survival analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of other bias |
Poplin 2013.
| Methods | Randomised, multicentre, phase II trial | |
| Participants | This was an international study. 367 participants with metastatic pancreatic ductal adenocarcinoma. 185 received gemcitabine. 182 received lipid‐drug conjugate of gemcitabine (CO‐101). The median age of participants in the low hENT1 group was 62 years, and was 61 years in the high hENT group. | |
| Interventions | Gemcitabine 100 mg/m² 7/8 weeks then 3/4 weeks CO‐101 120 mg/m² 3/4 weeks |
|
| Outcomes | Overall survival in low hENT1 participants Overall survival Progression‐free survival Response rate |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Treatment on the Low hENT1 in Adenocarcinoma of the Pancreas (LEAP) study was randomly assigned (1:1 to gemcitabine or CO‐101; Fig 1), and treatment allocation was stratified for Eastern Cooperative Oncology Group (ECOG) performances status (PS; 0 v 1) and geographic location (North America v South America v Australia v Eastern Europe v Western Europe)." |
| Allocation concealment (selection bias) | Low risk | "Treatment on the Low hENT1 in Adenocarcinoma of the Pancreas (LEAP) study was randomly assigned (1:1 to gemcitabine or CO‐101; Fig 1), and treatment allocation was stratified for Eastern Cooperative Oncology Group (ECOG) performances status (PS; 0 v 1) and geographic location (North America v South America v Australia v Eastern Europe v Western Europe)." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in the analyses |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indications of other bias |
Reni 2005.
| Methods | Randomised phase III trial | |
| Participants | Study was conducted in Italy. 99 participants with locally advanced/metastatic pancreatic adenocarcinoma with measurable disease. 47 received gemcitabine. 52 received cisplatin, epirubicin, gemcitabine and 5‐fluorouracil (5FU) (PEGF). The median age of participants was 62 years and 59 years in the PEGF and gemcitabine groups respectively. | |
| Interventions | Gemcitabine: 1000 mg/m² 7/8 weeks then 3/4 weeks PEGF: cisplatin 40 mg/m² day 1, epirubicin 40 mg/m² day 1, gemcitabine 600 mg/m² days 1 and 8, 5FU 200 mg/m²/day every 28 days |
|
| Outcomes | Progression‐free survival Overall survival Response rate Safety Quality of life |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisationwas done by a secretary at a central location by a phone call. The random‐allocation sequence had been generated previously by a statistician (LG) by use of a computer‐generated random code." |
| Allocation concealment (selection bias) | Low risk | "Randomisationwas done by a secretary at a central location by a phone call. The random‐allocation sequence had been generated previously by a statistician (LG) by use of a computer‐generated random code." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | PFS was primary outcome, but radiologist evaluating progression was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in the survival analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of other bias |
Reni 2012.
| Methods | Randomised phase II trial | |
| Participants | Study was conducted in Italy. 105 participants with unresectable/metastatic pancreatic ductal adenocarcinoma. 53 participants had capecitabine, cisplatin, gemcitabine and docetaxel (PDXG). 52 participants had capecitabine, epirubicin, cisplatin, gemcitabine (PEXG). The median age of participants was 61 years and 59 years in the PDXG and PEXG arms, respectively. | |
| Interventions | PDXG: capecitabine 1250 mg/m² days 1‐28, cisplatin 30 mg/m² days 1 and 15, gemcitabine 800 mg/m² days 1 and 15, docetaxel 25 mg/m² days 1 and 15 every 4 weeks PEXG: capecitabine as above, cisplatin as above, gemcitabine as above, epirubicin 30 mg/m² days 1 and 15 every 4 weeks |
|
| Outcomes | Progression‐free survival at 6 months Overall survival Toxicity Response rate |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients fulfilling all inclusion criteria were registered by the attending physician at an independent Contract Research Organization (CRO) that performed randomization on a 1:1 basis to either arm A or B. Patients were stratified according to stage of disease (III vs. IV)" |
| Allocation concealment (selection bias) | Unclear risk | "Patients fulfilling all inclusion criteria were registered by the attending physician at an independent Contract Research Organization (CRO) that performed randomization on a 1:1 basis to either arm A or B. Patients were stratified according to stage of disease (III vs. IV)" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | PFS primary outcome but radiologists blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat population included in survival analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of other bias |
Riess 2005.
| Methods | Randomised phase III trial | |
| Participants | 473 participants with locally advanced/metastatic pancreatic ductal adenocarcinoma with no prior therapy. 238 received gemcitabine. 235 received gemcitabine + 5‐fluorouracil (5FU) + folinic acid (FA) | |
| Interventions | Gemcitabine: 1000 mg/m² 7/8 weeks, then 3/4 weeks Gemcitabine + 5FU + FA: gemcitabine 1000 mg/m² + 5FU 750 mg/m² as a 24 hour infusion + FA 200 mg/m² days 1, 8, 15 every 6 weeks |
|
| Outcomes | Overall survival Time to progression Toxicity |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient details published |
| Selective reporting (reporting bias) | Unclear risk | Insufficient details published |
| Other bias | Low risk | No indication of other bias |
Rocha Lima 2004.
| Methods | Randomised phase III trial | |
| Participants | Study was conducted in North America. 360 participants with locally advanced/metastatic pancreatic adenocarcinoma with measurable disease. Adjuvant radiotherapy and 5‐fluorouracil (5FU) were permitted. 180 received gemcitabine. 180 received gemcitabine + irinotecan. The median age of participants was 63.2 years and 60.2 years in the gemcitabine + irinotecan and the gemcitabine alone group, respectively. | |
| Interventions | Gemcitabine: 1000 mg/m² 7/8 weeks then 3/4 weeks Gemcitabine + irinotecan: gemcitabine 1000 mg/m² and irinotecan 100 mg/m² days 1 and 8, every 21 days |
|
| Outcomes | Overall survival Quality of life |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were centrally randomly assigned and stratified by ECOG performance status (0, 1, or 2), extent of disease (locally advanced or metastatic), and previous radiotherapy for pancreatic cancer (yes or no)." |
| Allocation concealment (selection bias) | Low risk | "Patients were centrally randomly assigned and stratified by ECOG performance status (0, 1, or 2), extent of disease (locally advanced or metastatic), and previous radiotherapy for pancreatic cancer (yes or no)." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the survival analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of other bias |
Scheithauer 2003.
| Methods | Randomised phase II trial | |
| Participants | Study was conducted in Austria. 83 participants with metastatic pancreatic ductal adenocarcinoma. Adjuvant 5‐fluorouracil (5FU) and radiotherapy (RT) was permitted if completed > 6 months prior to randomisation. 42 received gemcitabine. 41 received gemcitabine + capecitabine. The median age of participants was 66 years and 64 years in the gemcitabine alone and the gemcitabine + capecitabine groups respectively. | |
| Interventions | Gemcitabine: 2200 mg/m² day 1, every 2 weeks Gemcitabine + capecitabine: gemcitabine as above, capecitabine 2500 mg/m²/day orally days 1‐7 every 2 weeks |
|
| Outcomes | Progression‐free survival Overall survival Response rate |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published. "Patients were then assigned to one treatment regimen by the central office located at the University in Vienna" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published. "Patients were then assigned to one treatment regimen by the central office located at the University in Vienna" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | PFS primary endpoint but independently reviewed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in the survival analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of other bias |
Singhal 2014.
| Methods | Phase III randomised trial | |
| Participants | 310 participants with metastatic PC. Half received FOLFIRINOX and half received gemcitabine. Details on age of participants not published. | |
| Interventions | FOLFIRINOX: Oxaliplatin 85 mg/m² + irinotecan 180 mg/m² + LV 400 mg/m² + 5FU 400 mg/m² bolus + 5FU 2400 mg/m² as 46 hours continuous infusion every 2 weeks Gemcitabine: 1000 mg/m² days 1, 8, 15 every 28 days |
|
| Outcomes | Overall survival Progression‐free survival Response rate Quality of life Adverse events |
|
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient details published |
| Selective reporting (reporting bias) | Unclear risk | Insufficient details published |
| Other bias | Unclear risk | Insufficient details published |
Smith 2003.
| Methods | Randomised phase II/III study | |
| Participants | Study was conducted in the UK. 55 participants with locally advanced or metastatic pancreatic adenocarcinoma with no prior treatment. 30 received ZD9331, 25 received gemcitabine alone. The median age of participants was 59.8 years and 60.8 years in the ZD9331 and gemcitabine arms respectively. | |
| Interventions | ZD9331: 130 mg/m² days 1, 8 every 21 days Gemcitabine: 1000 mg/m² weekly, 7/8 weeks, then 3/4 weeks |
|
| Outcomes | Tumour response Clinical benefit response PFS OS |
|
| Notes | Study supported by Astra Zeneca Ltd | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published ‐ "Patients were then randomised to receive either ZD9331 or gemcitabine and were stratified by centre..." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published ‐ "Patients were then randomised to receive either ZD9331 or gemcitabine and were stratified by centre..." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat population included in survival analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of other bias |
Stathopoulos 2006.
| Methods | Randomised phase III trial | |
| Participants | Study was conducted in Greece. 130 participants with locally advanced/metastatic pancreatic adenocarcinoma and no prior therapy. 70 received gemcitabine. 60 received gemcitabine + irinotecan. The median age of participants was 64 years. | |
| Interventions | Gemcitabine: 900 mg/m², 3/4 weeks Gemcitabine + irinotecan: gemcitabine 900 mg/m² days 1 and 8, irinotecan 300 mg/m² day 8, every 3 weeks |
|
| Outcomes | Overall survival Response rate Progression‐free survival Toxicity |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were centrally randomised by computer at a one‐to‐one ratio to receive either monotherapy (arm G) with gemcitabine" |
| Allocation concealment (selection bias) | Low risk | "Patients were centrally randomised by computer at a one‐to‐one ratio to receive either monotherapy (arm G) with gemcitabine" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed for primary outcome |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of other bias |
Takada 1998.
| Methods | Randomised study | |
| Participants | Study was conducted in Japan. 83 participants with unresectable pancreatic adenocarcinoma or biliary tract carcinoma, aged < 75 years. Of the participants with pancreatic cancer, 28 received 5‐fluorouracil (5FU), doxorubicin and mitomycin C (MMC), and 24 received palliative surgery. The median age in the chemotherapy arm was 62.8 years and was 61.5 years in the palliative surgery arm. | |
| Interventions | 5FU 200 mg/m² Doxorubicin 15 mg/m² MMC 5 mg/m² given weekly × 4, then 1 week break. 2 cycles given |
|
| Outcomes | Overall survival Response rates Performance status Adverse events |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Patients were assigned at random to the therapy group or the control group using the envelope method in each facility..." |
| Allocation concealment (selection bias) | Low risk | "Registration procedures were conducted by telephoning the Study Group Office when the envelope was opened" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enrolled participants included in survival analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indications of other bias |
Tempero 2003.
| Methods | Randomised phase II trial | |
| Participants | Study was conducted in North America. 92 participants with locally advanced/metastatic pancreatic ductal adenocarcinoma. 49 received dose‐intense gemcitabine. 43 received fixed dose rate infusion gemcitabine (FDR). The median age of participants was 62 years. | |
| Interventions | Dose‐intense gemcitabine: 2200 mg/m² IV over 30 min given days 1, 8, 15 every 28 days FDR: 1500 mg/m² given at 10 mg/m²/min given days 1, 8, 15 every 28 days. |
|
| Outcomes | Time to treatment failure Time to progression Median survival Response rate Toxicity |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published ‐ "Patients were randomly assigned to the following two treatment arms" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed for the primary endpoint |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indications of other bias |
Topham 1991.
| Methods | Randomised trial | |
| Participants | Study was conducted in the UK. 62 participants with locally advanced or metastatic pancreatic adenocarcinoma. 32 were given epirubicin alone, 30 were given 5FU + epirubicin + mitomycin C (FEM). No details on the median ages of participants was published. | |
| Interventions | Epirubicin: 100 mg IV every 4 weeks FEM: 5FU 1g IV days 1 and 28, epirubicin 600 mg IV days 1 + 8, mitomycin C 10 mg day 1 every 8 weeks |
|
| Outcomes | Not stated | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Insufficient details published. Unclear what the primary endpoint was. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat population included in survival analysis |
| Selective reporting (reporting bias) | Unclear risk | Endpoints not clearly stated in the methods. |
| Other bias | Low risk | No indication of other bias |
Ueno 2013.
| Methods | Randomised phase III trial | |
| Participants | Study was conducted in Japan. 832 participants with locally advanced/metastatic pancreatic ductal adenocarcinoma, ECOG 0‐1. 277 received gemcitabine. 280 received S1. 275 received gemcitabine + S1. Half the patients were under 65 years old and half were 65 years old or more. | |
| Interventions | Gemcitabine: 1000 mg/m² 3/4 weeks S1: orally, twice daily. Body surface area (BSA) < 1.25 m², 80 mg/day; BSA 1.25 m² to 1.5 m², 100 mg/day; BSA > 1.5 m², 120 mg/day. Days 1‐28, every 42 days. Gemcitabine + S1: gemcitabine 1000 mg/m² days 1 and 8, S1 (dosing as above), days 1‐14 every 21 days |
|
| Outcomes | Overall survival Progression‐free survival Overall response rate Safety Quality of life |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random assignment was performed centrally with stratification by extent of disease (locally advanced disease v metastatic disease) and institution using the minimization method" |
| Allocation concealment (selection bias) | Low risk | "Random assignment was performed centrally with stratification by extent of disease (locally advanced disease v metastatic disease) and institution using the minimization method" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the survival analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of other bias |
Ueno 2013 – EPA study.
| Methods | Randomised phase II trial | |
| Participants | Study was conducted in Japan. 66 participants with advanced pancreatic adenocarcinoma. 23 received gemcitabine. 43 received gemcitabine + EPA enriched oral supplement. Median ages of participants were not published | |
| Interventions | Gemcitabine: 1000 mg/m² 3/4 weeks Gemcitabine + EPA: gemcitabine as above. EPA 1 tablet orally, daily continuous |
|
| Outcomes | 1‐year survival | |
| Notes | Abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient details published |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient details published |
| Selective reporting (reporting bias) | Unclear risk | Insufficient details published |
| Other bias | Low risk | No indication of other bias |
Viret 2004.
| Methods | Randomised phase II trial | |
| Participants | Study was conducted in France. 83 participants with stage III/IV pancreatic ductal adenocarcinoma. 41 received gemcitabine. 42 received gemcitabine + cisplatin. Median age was 63 years and 61.5 years in the gemcitabine alone and the gemcitabine + cisplatin arms respectively. | |
| Interventions | Gemcitabine 1000 mg/m² 7/8 weeks then 3/4 weeks Gemcitabine + cisplatin: gemcitabine 1000 mg/m² weekly × 3, cisplatin 75 mg/m² day 15 every 4 weeks |
|
| Outcomes | Time to treatment failure Toxicity Overall survival Reponse rate Quality of life |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | High risk for primary endpoint (time to treatment failure) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient details published |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Unclear risk | Insufficient details published |
Von Hoff 2013.
| Methods | Randomised phase III trial | |
| Participants | This was an international study. 861 participants with metastatic pancreatic ductal adenocarcinoma with measurable disease and no prior therapy. 43 received gemcitabine. 431 received gemcitabine + nab‐paclitaxel. The median age of participants was 63 years. | |
| Interventions | Gemcitabine: 1000 mg/m² 7/8 weeks then 3/4 weeks Gemcitabine + nab‐paclitaxel: nab‐paclitaxel 125 mg/m² followed by gemcitabine 1000 mg/m² days 1, 8, 15 every 4 weeks |
|
| Outcomes | Overall survival Progression‐free survival Response rate |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Likely to be low, but insufficient details published‐ "In this international, multicenter, open‐label, randomized, phase 3 study, we randomly assigned eligible patients, in a 1:1 ratio" |
| Allocation concealment (selection bias) | Unclear risk | Likely to be low, but insufficient details published ‐ "In this international, multicenter, open‐label, randomized, phase 3 study, we randomly assigned eligible patients, in a 1:1 ratio" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in the survival analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indication of other bias |
Wang 2002.
| Methods | Randomised phase III trial | |
| Participants | 42 participants with measurable or evaluable stage III/IV pancreatic cancer. 20 received gemcitabine. 22 participants with gemcitabine + cisplatin | |
| Interventions | Gemcitabine 1000 mg/m² 7/8 weeks then 3/4 weeks Gemcitabine + cisplatin: gemcitabine 1000 mg/m² 3/4 weeks + cisplatin 60 mg/m² day 15 every 3 every 4 weeks |
|
| Outcomes | Clinical benefit Duration of clinical benefit Duration of response Time to progression Survival Toxicity |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient details published |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details published |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient details published |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient details published |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient details published |
| Selective reporting (reporting bias) | Unclear risk | Insufficient details published |
| Other bias | Unclear risk | Insufficient details published |
Xinopoulos 2008.
| Methods | Randomised study | |
| Participants | Study was conducted in Greece. 49 participants with locally advanced PC with normal liver function tests after biliary stent insertion. 33 received no further treatment after stent insertion, 16 received gemcitabine. Median age of participants not published. | |
| Interventions | Gemcitabine 1000 mg/m² weekly × 3, then 1 week off | |
| Outcomes | Overall survival Quality of life Requirement for 2nd stent insertion |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients' allocation into the 2 arms was based on a sequence of random binary numbers (i.e.111100111010...) that was developed in a computer based program" |
| Allocation concealment (selection bias) | Low risk | "Patients' allocation into the 2 arms was based on a sequence of random binary numbers (i.e.111100111010...) that was developed in a computer based program" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Low risk for primary endpoint (OS) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in the survival analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results |
| Other bias | Low risk | No indications of other bias |
5FU: 5‐fluorouracil; IV: intravenous; OS: overall survival; PFS: progression‐free survival: QoL: quality of life.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdel Wahab 1999 | May include participants who did not have histological confirmation of their tumour. An attempt to contact authors was made |
| Aigner 1998 | Participants with locally advanced pancreatic cancer (addressed in another Cochrane Review) |
| Alberts 2005 | Biological agent (addressed in another Cochrane Review) |
| Andersen 1981 | Not all participants had advanced pancreatic cancer. |
| Ardalan 1988 | Survival was not an endpoint. |
| Astsaturov 2011 | Second.line treatment (addressed in another Cochrane Review). |
| Baker 1976 | The survival data of the subgroup of participants with pancreatic cancer are not published separately. |
| Benavides 2014 | Biological agent (addressed in another Cochrane Review) |
| Benson 2014 | Biological agent (addressed in another Cochrane Review) |
| Benson 2017 | Biological agent ‐ addressed in another review |
| Berglund 2010 | Cross‐over study. |
| Bramhall 2001 | Biological agent (addressed in another Cochrane Review) |
| Bramhall 2002 | Biological agent (addressed in another Cochrane Review) |
| Buanes 2009 | Immunotherapy (addressed in another Cochrane Review) |
| Bukowski 1993 | Non‐randomised study |
| Burtness 2016 | Biological agent (addressed in another Cochrane Review) |
| Cantore 2004 | Second‐line treatment (addressed in another Cochrane Review) |
| Cascinu 2008 | Biological agent (addressed in another Cochrane Review) |
| Cascinu 2013 | Biological agent (addressed in another Cochrane Review) |
| Catenacci 2013 | Biological agent (addressed in another Cochrane Review) |
| Chai 2013 | Immunotherapy (addressed in another Cochrane Review) |
| Chauffert 2008 | Participants with locally advanced pancreatic cancer (addressed in another Cochrane Review) |
| Chen 2006 | Biological agent (addressed in another Cochrane Review) |
| Chung 2004 | Participants with locally advanced pancreatic cancer (addressed in another Cochrane Review) |
| Chung 2015 | Second‐line treatment (addressed in another Cochrane Review) |
| Ciuleanu 2009 | Second‐line treatment (addressed in another Cochrane Review) |
| Cohen 2005 | Participants with locally advanced pancreatic cancer (addressed in another Cochrane Review) |
| Cohen 2010 | Insufficient data published |
| Dahan 2010 | Cross‐over study |
| Dalgleish 2015 | Immunotherapy (addressed in another Cochrane Review) |
| Deplanque 2015 | Biological agent (addressed in another Cochrane Review) |
| Ducreux 2002 | Contains ampullary cancers |
| Duffy 2015 | Second‐line study ‐ addressed in another review (ongoing study) |
| El‐Khoueiry 2012 | Biological agent (addressed in another Cochrane Review) |
| Evans 2014 | Participants with locally advanced pancreatic cancer (addressed in another Cochrane Review) |
| Friess 2006 | Biological agent (addressed in another Cochrane Review) |
| Fuchs 2015 | Biological agent (addressed in another Cochrane Review) |
| Fukutomi 2015 | Participants with locally advanced pancreatic cancer (addressed in another Cochrane Review) |
| Gill 2014 | Second‐line treatment (addressed in another Cochrane Review) |
| Gilliam 2012 | Immunotherapy (addressed in another Cochrane Review) |
| GISTG 1985 (radiotherapy) | Locally advanced study (addressed in another Cochrane Review) |
| GITSG 1979 | Preliminary results only. Included acinar and undifferentiated pathologies |
| GITSG 1985 | Insufficient data published |
| GITSG 1988 | Participants with acinar pathology included |
| Gong 2007 | Non‐randomised study |
| Gonçalves 2012 | Biological agent (addressed in another Cochrane Review) |
| Haas 2015 | Biological agent ‐ addressed in another review (ongoing study) |
| Hammel 2013 | Participants with locally advanced pancreatic cancer (addressed in another Cochrane Review) |
| Han 2006 | Participants with locally advanced pancreatic cancer (addressed in another Cochrane Review) |
| Hazel 1981 | Participants with locally advanced pancreatic cancer (addressed in another Cochrane Review) |
| Heinemann 2013 | Biological agent (addressed in another Cochrane Review) |
| Heinemann 2013 (GUT) | Cross‐over study |
| Herman 2013 | Participants with locally advanced pancreatic cancer (addressed in another Cochrane Review) |
| Hingorani 2015 | Biological agent ‐ addressed in another review (ongoing study) |
| Horton 1981 | Cross‐over study |
| Hurwitz 2015 | Second‐line treatment (addressed in another Cochrane Review) |
| Hurwitz 2015 (JANUS 1) | Biological agent ‐ addressed in another review (ongoing study) |
| Infante 2013 | Biological agent (addressed in another Cochrane Review) |
| Ioka 2009 | Participants with locally advanced pancreatic cancer (addressed in another Cochrane Review) |
| Ioka 2013 | Second‐line treatment (addressed in another Cochrane Review) |
| Jacobs 2004 | Second‐line treatment (addressed in another Cochrane Review) |
| Javle 2011 | Cross‐over study |
| Johnson 2001 | Not all participants had a histological diagnosis |
| Kim 2011 | Insufficient data published |
| Kindler 2008 | Biological agent (addressed in another Cochrane Review) |
| Kindler 2010 | Biological agent (addressed in another Cochrane Review) |
| Kindler 2011 | Biological agent (addressed in another Cochrane Review) |
| Kindler 2012 | Biological agent (addressed in another Cochrane Review) |
| Kindler 2015 | Biological agent ‐ addressed in another review |
| Klaassen 1985 | Participants with locally advanced pancreatic cancer (addressed in another Cochrane Review) |
| Ko 2012 | Biological agent (addressed in another Cochrane Review) |
| Ko 2016 | Biological agent ‐ addressed in another review |
| Lasalvia‐Prisco 2012 | Immunotherapy (addressed in another Cochrane Review) |
| Le (Ipilimumab) 2013 | Second‐line treatment (addressed in another Cochrane Review) |
| Le 2013 | Immunotherapy (addressed in another Cochrane Review) |
| Le 2015 | Immunotherapy agent ‐ addressed in another review |
| Li 2003 | Participants with locally advanced pancreatic cancer (addressed in another Cochrane Review) |
| Li 2016 | Locally advanced study (address in another Cochrane Review) |
| Linstadt 1988 | Participants with locally advanced pancreatic cancer (addressed in another Cochrane Review) |
| Loehrer 2011 | Participants with locally advanced pancreatic cancer (addressed in another Cochrane Review) |
| Lokich 1979 | Included participants with acinar pathology |
| Lygidakis 1995 | Not all participants had advanced pancreatic cancer |
| Mallinson 1980 | Not all participants had histologically confirmed pancreatic ductal adenocarcinoma (PDAC) |
| Meyer 2008 | Survival was not an endpoint |
| Middleton 2014 | Immunotherapy (addressed in another Cochrane Review) |
| Mitry 2006 | Non‐randomised study |
| Mizuno 2013 | May include adenosquamous participants. An attempt to contact authors was made |
| Modiano 2012 | Biological agent (addressed in another Cochrane Review) |
| Moertel 1981 | Participants with acinar and undifferentiated pathology were included |
| Moore 2003 | Biological agent (addressed in another Cochrane Review) |
| Moore 2007 | Biological agent (addressed in another Cochrane Review) |
| Mukherjee 2013 | Participants with locally advanced pancreatic cancer (addressed in another Cochrane Review) |
| Nakai 2012 | Not all participants had histologically confirmed PDAC |
| Nio 2010 | Retrospective study |
| O'Neil 2015 | Biological agent (addressed in another Cochrane Review) |
| O'Reilly 2013 | Biological agent ‐ addressed in another review (ongoing study) |
| O'Reilly 2015 | Second‐line study ‐ addressed in another review (ongoing study) |
| Oberic 2011 | Insufficient data published |
| Oster 1986 | Participants with acinar pathology were included |
| Palmer 1994 | Not all participants had a histologically confirmed PDAC |
| Pandya 2013 | Biological agent (addressed in another Cochrane Review) |
| Pelzer 2011 | Second‐line treatment (addressed in another Cochrane Review) |
| Philip 2010 | Biological agent (addressed in another Cochrane Review) |
| Philip 2014 | Biological agent (addressed in another Cochrane Review) |
| Propper 2014 | Second‐line treatment (addressed in another Cochrane Review) |
| Queisser 1979 | Insufficient information published |
| Ramanathan 2011 | Insufficient data published |
| Reni 2009 | Retrospective analysis |
| Reni 2013 | Second‐line treatment (addressed in another Cochrane Review) |
| Richards 2011 | Biological agent (addressed in another Cochrane Review) |
| Richly 2013 | Biological agent (addressed in another Cochrane Review) |
| Riess 2010 | Biological agent (addressed in another Cochrane Review) |
| Rougier 2013 | Biological agent (addressed in another Cochrane Review) |
| Ryan 2013 | Biological agent (addressed in another Cochrane Review) |
| Saif 2009 | Biological agent (addressed in another Cochrane Review) |
| Sakata 1992 | Insufficient information published ‐ pancreas cancer subgroup not reported separately |
| Schein 1978 | Participants with acinar and undifferentiated pathology were included |
| Schmitz‐Winnenthal 2013 | Overall survival not an endpoint |
| Senzer 2006 | Insufficient data published |
| Shapiro 2005 | Insufficient data published |
| Shinchi 2002 | Participants with locally advanced pancreatic cancer (addressed in another Cochrane Review) |
| Shinchi 2014 | Participants with locally advanced pancreatic cancer (addressed in another Cochrane Review) |
| Spano 2008 | Biological agent (addressed in another Cochrane Review) |
| Strumberg 2013 | Biological agent (addressed in another Cochrane Review) |
| Sudo 2014 | Included adenosquamous pathology |
| Sultana 2009 | Insufficient data published |
| Sun 2011 | Insufficient data published |
| Sunamura 2004 | Participants with locally advanced pancreatic cancer (addressed in another Cochrane Review) |
| Tagliaferri 2013 | Insufficient data published |
| Takada 1994 | Includes participants with biliary tract cancer. Subgroup analysis of pancreatic cancer participants not available |
| Topham 1993 | Preliminary results only |
| Trouilloud 2012 | Insufficient data published |
| Tuinmann 2008 | Interim analysis only. Full results not published |
| Ulrich‐Pur 2003 | Second‐line treatment (addressed in another Cochrane Review) |
| Van Cutsem 2004 | Biological agent (addressed in another Cochrane Review) |
| Van Cutsem 2009 | Biological agent (addressed in another Cochrane Review) |
| Van Cutsem 2013 | Insufficient data published |
| Van Cutsem 2014 | Biological agent (addressed in another Cochrane Review) |
| Van Cutsem 2015 | Biological agent (addressed in another Cochrane Review) |
| Von Hoff 1990 | Immunotherapy (addressed in another Cochrane Review) |
| Von Hoff 2014 | Second‐line treatment (addressed in another Cochrane Review) |
| Voorthuizen 2006 | Biological agent (addressed in another Cochrane Review) |
| Wagener 2002 | Immunotherapy (addressed in another Cochrane Review) |
| Wang 2000 | All participants had locally advanced PC (addressed in another Cochrane Review) |
| Wang 2004 | All participants had locally advanced PC (addressed in another Cochrane Review) |
| Wang 2015 | Biological agent (addressed in another Cochrane Review) |
| Wiedenmann 2008 | Biological agent (addressed in another Cochrane Review) |
| Wilkowski 2009 | Participants with locally advanced pancreatic cancer (addressed in another Cochrane Review) |
| Wolpin 2013 | Biological agent (addressed in another Cochrane Review) |
| Wright 2006 | Immunotherapy (addressed in another Cochrane Review) |
| Yamaue 2015 | Biological agent (addressed in another Cochrane Review) |
| Yongxiang 2001 | Non‐randomised study |
| Yoo 2009 | Second‐line treatment (addressed in another Cochrane Review) |
| Zemskov 2000 | Non‐randomised study |
| Zhang 2007 | All participants had locally advanced PC (addressed in another Cochrane Review) |
PC: pancreatic cancer; PDAC: pancreatic ductal adenocarcinoma.
Differences between protocol and review
Biological agents, second line therapies, locally advanced PC
The original protocol included studies addressing biological therapies, studies addressing second‐line treatment and people with locally advanced disease. We felt that due to the large number of comparisons, the review became unmanageable. We therefore decided to split the review and concentrate on chemotherapy and radiotherapy in the advanced setting. Separate reviews will report on biological and immunological agents, second‐line therapies and studies dealing exclusively with people with non‐metastatic, locally advanced disease.
Outcomes
The original protocol did not include adverse events, response rates and quality of life as secondary outcomes. Prior to data extraction, the review authors added those as secondary outcomes. We deleted disease‐specific survival as a secondary outcome.
Measures of treatment effect
The original protocol stated that fixed‐effect model meta‐analyses would be used to pool results for survival at 6 months and 12 months. It was never our intention to use 6‐ and 12‐month survival as endpoints in this review. We instead used HRs for overall and progression‐free survival. We employed random‐effect models for most analyses given the experimental arms were often very different within each comparison.
Dealing with multi‐armed studies
In such cases where studies reported the event rates for all arms, we divided the control arm accordingly and entered all arms of the studies into the analysis as appropriate. Where the event rates were not available, if the study had two arms that fell into a subgroup analysis, then we analysed only these two arms. We described any study that we could not analyse in the above two scenarios in table form only.
Number needed to treat (NNT) as a secondary endpoint
We replaced this outcome with GRADE 'Summary of findings' tables.
Contributions of authors
VC: protocol design, sourcing articles, reviewer one, data entry, analysis, results, discussion and conclusion, preparation of manuscript. AN: protocol design, reviewer two, analysis, statistics support, reviewing of manuscript. KS: protocol design, statistics support, reviewing of manuscript. CO: protocol design, radiotherapy trials/methodology support, reviewing of manuscript. LC: protocol design, reviewing of manuscript. AB: protocol design, reviewing of manuscript. RS: data analysis and statistical support, reviewing of the manuscript. DY: author of previous version of this review, protocol design, methodological support, reviewer three, reviewing of manuscript.
Sources of support
Internal sources
-
The Garvan Institute of Medical Research, Australia.
PhD stipend top up for Venessa Chin
External sources
-
The Royal Australasian College of Physicians, Australia.
PhD stipend for Venessa Chin
-
National Health and Medical Research Council, Australia.
PhD stipend for Venessa Chin
-
Pancare Australia, Australia.
PhD stipend for Venessa Chin
-
Sydney Catalyst, Australia.
PhD stipend for Venessa Chin
Declarations of interest
VC: Venessa Chin received scholarship funding from the Research and Education Foundation of the Royal Australasian College of Physicians, Pancare Australia, Sydney Catalyst, National Health and Medical Research Council, and the Garvan Institute of Medical Research for work related to this review.
AN: none known.
KS: Katrin Sjoquist received programme grant funding from the National Health and Medical Research Council for work related to this review. She has received consultancy fees, fees for expert testimony and travel support for work unrelated to this review.
CO: none known.
LC: Lorraine Chantrill is employed (part‐time) by NSW Health as a staff specialist in medical oncology and enrolled full‐time in PhD studies supported by the Australian Federal Government. She has been paid as an advisory board member in relation to chemotherapy for pancreas cancer and has been paid for formulating educational materials and presentations. She has received grants for the practice of clinical trials in pancreas and other cancers.
AB: Andew Biankin received grant funding from the Cancer Institute NSW for work related to this review. He also received consultancy fees from Celegene and Clovis Oncology for work unrelated to this review. His institution received consultancy fees from Roche for work unrelated to this review.
RS: Rob Scholten's institution has received grant funding from the Belgian Health Care Knowledge Centre for work related to this review. His institution has also received funding from the WHO and World Federation of Haemophilia for travel and consultancy unrelated to this review.
DY: Advisory Board Member, Specialised Therapeutics.
New
References
References to studies included in this review
Abou‐Alfa 2006 {published data only}
- Abou‐Alfa GKL, Harker R, Modiano G, Hurwitz M, Tchekmedyian H, et al. Randomized phase III study of exatecan and gemcitabine compared with gemcitabine alone in untreated advanced pancreatic cancer. Journal of Clinical Oncology 2006;24(27):4441‐7. [DOI] [PubMed] [Google Scholar]
Afchain 2009 {published data only}
- Afchain P, Chibaudel B, Lledo G, Selle F, Bengrine‐Lefevre L, Nguyen S, et al. First‐line simplified GEMOX (S‐GemOx) versus classical GEMOX in metastatic pancreatic cancer (MPA): results of a GERCOR randomized phase II study. Bulletin du Cancer 2009;96(5):E18‐22. [DOI] [PubMed] [Google Scholar]
Andren‐Sandberg 1983 {published data only}
- Andren‐Sandberg A, Holmberg J T, Ihse I. Treatment of unresectable pancreatic carcinoma with 5‐fluorouracil, vincristine, and CCNU. Scandinavian Journal of Gastroenterology 1983;18(5):609‐12. [DOI] [PubMed] [Google Scholar]
Berlin 2002 {published data only}
- Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson AB 3rd. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. Journal of Clinical Oncology 2002;20(15):3270‐5. [DOI] [PubMed] [Google Scholar]
Boeck 2008 {published data only}
- Boeck S, Hoehler T, Seipelt G, Mahlberg R, Wein A, Hochhaus A, et al. Capecitabine plus oxaliplatin (CapOx) versus capecitabine plus gemcitabine (CapGem) versus gemcitabine plus oxaliplatin (mGemOx): final results of a multicenter randomized phase II trial in advanced pancreatic cancer. Annals of Oncology 2008;19(2):340‐7. [DOI] [PubMed] [Google Scholar]
Bukowski 1983 {published data only}
- Bukowski RM, Balcerzak SP, O'Bryan RM, Bonnet JD, Chen TT. Randomized trial of 5‐fluorouracil and mitomycin C with or without streptozotocin for advanced pancreatic cancer. A Southwest Oncology Group study. Cancer 1983;52(9):1577‐82. [DOI] [PubMed] [Google Scholar]
Burris 1997 {published data only}
- Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first‐line therapy for patients with advanced pancreas cancer: a randomized trial. Journal of Clinical Oncology 1997;15(6):2403‐13. [DOI] [PubMed] [Google Scholar]
Cheverton 2004 {published data only}
- Cheverton P, Friess H, Andras C, Salek T, Geddes C, Bodoky G, et al. Phase III results of exatecan (DX‐8951f) versus gemcitabine (Gem) in chemotherapy‐naive patients with advanced pancreatic cancer (APC). Journal of Clinical Oncology 2004;22:4005. [Google Scholar]
Colucci 2002 {published data only}
- Colucci G, Giuliani F, Gebbia V, Biglietto M, Rabitti P, Uomo G, et al. Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: a prospective, randomized phase III study of the Gruppo Oncologia dell'Italia Meridionale. Cancer 2002;94(4):902‐10. [PubMed] [Google Scholar]
Colucci 2010 {published data only}
- Colucci G, Labianca R, Costanzo F, Gebbia V, Cartenì G, Massidda B, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with single‐agent gemcitabine as first‐line treatment of patients with advanced pancreatic cancer: the GIP‐1 study. Journal of Clinical Oncology 2010;28(10):1645‐51. [DOI] [PubMed] [Google Scholar]
Conroy 2011 {published data only}
- Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. New England Journal of Medicine 2011;364(19):1817‐25. [DOI] [PubMed] [Google Scholar]
Corrie 2017 {published data only}
- Corrie P, Qian W, Basu B, Valle JW, Falk S, Iwuji C, et al. A randomized phase II trial comparing different schedules of nab‐paclitaxel (nabP) combined with gemcitabine (GEM) as first line treatment for metastatic pancreatic adenocarcinoma (mPDAC). Journal of Clinical Oncology 2017;35(Suppl):4100. [Google Scholar]
Cullinan 1985 {published data only}
- Cullinan SA, Moertel CG, Fleming TR, Rubin JR, Krook JE, Everson LK, et al. A comparison of three chemotherapeutic regimens in the treatment of advanced pancreatic and gastric carcinoma. Fluorouracil vs fluorouracil and doxorubicin vs fluorouracil, doxorubicin, and mitomycin. JAMA 1985;253(14):2061‐7. [PubMed] [Google Scholar]
Cullinan 1990 {published data only}
- Cullinan S, Moertel CG, Wieand HS, Schutt AJ, Krook JE, Foley JF, et al. A phase III trial on the therapy of advanced pancreatic carcinoma. Evaluations of the Mallinson regimen and combined 5‐fluorouracil, doxorubicin, and cisplatin. Cancer 1990;65(10):2207‐12. [DOI] [PubMed] [Google Scholar]
Cunningham 2009 {published data only}
- Cunningham D, Chau I, Stocken DD. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. Journal of Clinical Oncology 2009;27(33):5513‐8. [DOI] [PubMed] [Google Scholar]
Di Costanzo 2005 {published data only}
- Costanzo F, Carlini P, Doni L, Massidda B, Mattioli R, Iop A, et al. Gemcitabine with or without continuous infusion 5‐FU in advanced pancreatic cancer: a randomised phase II trial of the Italian oncology group for clinical research (GOIRC). British Journal of Cancer 2005;93(2):185‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ducreux 2004 {published data only}
- Ducreux M, Mitry E, Ould‐Kaci M, Boige V, Seitz J F, Bugat R, et al. Randomized phase II study evaluating oxaliplatin alone, oxaliplatin combined with infusional 5‐FU, and infusional 5‐FU alone in advanced pancreatic carcinoma patients. Annals of Oncology 2004;15(3):467‐73. [DOI] [PubMed] [Google Scholar]
Frey 1981 {published data only}
- Frey C, Twomey P, Keehn R, Elliott D, Higgins G. Randomized study of 5‐FU and CCNU in pancreatic cancer: report of the Veterans Administration Surgical Adjuvant Cancer Chemotherapy Study Group. Cancer 1981;47(1):27‐31. [DOI] [PubMed] [Google Scholar]
Gansauge 2002 {published data only}
- Gansauge F, Ramadani M, Pressmar J, Gansauge S, Muehling B, Stecker K, et al. NSC‐631570 (Ukrain) in the palliative treatment of pancreatic cancer. Results of a phase II trial. Langenbeck's Archives of Surgery 2002;386(8):570‐4. [DOI] [PubMed] [Google Scholar]
Glimelius 1996 {published data only}
- Glimelius B, Hoffman K, Sjödén PO, Jacobsson G, Sellström H, Enander LK, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Annals of Oncology 1996;7(6):593‐600. [DOI] [PubMed] [Google Scholar]
Heinemann 2006 {published data only}
- Heinemann V, Quietzsch D, Gieseler F, Gonnermann M, Schönekäs H, Rost A, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. Journal of Clinical Oncology 2006;24(24):3946‐52. [DOI] [PubMed] [Google Scholar]
Herrmann 2007 {published data only}
- Bernhard J, Bietrich D, Scheithauer W, Gerber D, Bodoky G, Ruhstaller T, et al. Clinical benefit and quality of life in patients with advanced pancreatic cancer receiving gemcitabine plus capecitabine versus gemcitabine alone: a randomized multicenter phase III clinical trial—SAKK 44/00–CECOG/PAN.1.3.001. Journal of Clinical Oncology 2008;26(22):3695‐701. [DOI] [PubMed] [Google Scholar]
- Herrmann R, Bodoky G, Ruhstaller T, Glimelius B, Bajetta E, Schüller J, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. Journal of Clinical Oncology 2007;25(16):2212‐7. [DOI] [PubMed] [Google Scholar]
Hirao 2011 {published data only}
- Hirao K, Kawamoto H, Sakakihara I, Noma Y, Yamamoto N, Harada R, et al. A 4‐week versus a 3‐week schedule of gemcitabine monotherapy for advanced pancreatic cancer: a randomized phase II study to evaluate toxicity and dose intensity. International Journal of Clinical Oncology 2011;16(6):637‐45. [DOI] [PubMed] [Google Scholar]
Huguier 2001 {published data only}
- Huguier M, Barrier A, Valinas R, Flahault A, Adloff M, Pezet D, et al. Randomized trial of 5‐fluorouracil, leucovorin and cisplatin in advanced pancreatic cancer. Hepato‐gastroenterology 2001;48(39):875‐8. [PubMed] [Google Scholar]
Kelsen 1991 {published data only}
- Kelsen D, Hudis C, Niedzwiecki D, Dougherty J, Casper E, Botet J, et al. A phase III comparison trial of streptozotocin, mitomycin, and 5‐fluorouracil with cisplatin, cytosine arabinoside, and caffeine in patients with advanced pancreatic carcinoma. Cancer 1991;68(5):965‐9. [DOI] [PubMed] [Google Scholar]
Kovach 1974 {published data only}
- Kovach JS, Moertel CG, Schutt AJ, Hahn RG, Reitemeier RJ. Proceedings: a controlled study of combined 1,3‐bis‐(2‐chloroethyl)‐1‐nitrosourea and 5‐fluorouracil therapy for advanced gastric and pancreatic cancer. Cancer 1974;33(2):563‐7. [DOI] [PubMed] [Google Scholar]
Kulke 2009 {published data only}
- Kulke MH, Tempero MA, Niedzwiecki D, Hollis DR, Kindler HL, Cusnir M, et al. Randomized phase II study of gemcitabine administered at a fixed dose rate or in combination with cisplatin, docetaxel, or irinotecan in patients with metastatic pancreatic cancer: CALGB 89904. Journal of Clinical Oncology 2009;27(33):5506‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2017 {published data only}
- Lee HS, Chung MJ, Park JY, Bang S, Park SW, Kim HG, et al. A randomized, multicenter, phase III study of gemcitabine combined with capecitabine versus gemcitabine alone as first‐line chemotherapy for advanced pancreatic cancer in South Korea. Medicine 2017;96(1):5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levi 2004 {published data only}
- Levi FA, Tubiana‐Mathieu N, Focan C, Brezault‐Bonnet C, Coudert B, Carvalho C, et al. Chronomodulated (Chrono) vs constant (Cst) rate infusional 5‐fluorouracil (FU) with or without cisplatin (CDDP) in patients with advanced or metastatic pancreatic cancer. A multicenter randomized trial of the Chronotherapy Group of the European Organisation for Research and Treatment of Cancer (EORTC 05962). Journal of Clinical Oncology 2004;22:4117. [Google Scholar]
Li 2004 {published data only}
- Li CP, Chao Y. A prospective randomized trial of gemcitabine alone or gemcitabine + cisplatin in the treatment of metastatic pancreatic cancer. Journal of Clinical Oncology 2004;22:4144. [Google Scholar]
Lohr 2012 {published data only}
- Lohr JM, Haas SL, Bechstein WO, Bodoky G, Cwiertka K, Fischbach W, et al. Cationic liposomal paclitaxel plus gemcitabine or gemcitabine alone in patients with advanced pancreatic cancer: a randomized controlled phase II trial. Annals of Oncology 2011;23(5):1214‐22. [DOI] [PubMed] [Google Scholar]
Louvet 2005 {published data only}
- Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, André T, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. Journal of Clinical Oncology 2005;23(15):3509‐16. [DOI] [PubMed] [Google Scholar]
Lutz 2005 {published data only}
- Lutz MP, Cutsem E, Wagener T, Laethem JL, Vanhoefer U, Wils JA, et al. Docetaxel plus gemcitabine or docetaxel plus cisplatin in advanced pancreatic carcinoma: randomized phase II study 40984 of the European Organisation for Research and Treatment of Cancer Gastrointestinal Group. Journal of Clinical Oncology 2005;23(36):9250‐6. [DOI] [PubMed] [Google Scholar]
Maisey 2002 {published data only}
- Maisey N. There is an error in the published survival curves [personal communication]. Email to: V Chin 18 September 2014.
- Maisey N, Chau I, Cunningham D, Norman A, Seymour M, Hickish T, et al. Multicenter randomized phase III trial comparing protracted venous infusion (PVI) fluorouracil (5‐FU) with PVI 5‐FU plus mitomycin in inoperable pancreatic cancer. Journal of Clinical Oncology 2002;20(14):3130‐6. [DOI] [PubMed] [Google Scholar]
Meng 2012 {published data only}
- Meng Z, Garrett CR, Shen Y, Liu L, Yang P, Huo Y, et al. Prospective randomised evaluation of traditional Chinese medicine combined with chemotherapy: a randomised phase II study of wild toad extract plus gemcitabine in patients with advanced pancreatic adenocarcinomas. British Journal of Cancer 2012;107(3):411‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moertel 1977 {published data only}
- Moertel CG, Douglas HO Jr, Hanley J, Carbone P P. Treatment of advanced adenocarcinoma of the pancreas with combinations of streptozotocin plus 5‐fluorouracil and streptozotocin plus cyclophosphamide. Cancer 1977;40(2):605‐8. [DOI] [PubMed] [Google Scholar]
Moertel 1979 {published data only}
- Moertel CG, Engstrom P, Lavin PT, Gelber RD, Carbone PP. Chemotherapy of gastric and pancreatic carcinoma: a controlled evaluation of combinations of 5‐fluorouracil with nitrosoureas and "lactones". Surgery 1979;85(5):509‐13. [PubMed] [Google Scholar]
Oettle 2005 {published data only}
- Oettle H, Richards D, Ramanathan RK, Laethem JL, Peeters M, Fuchs M, et al. A phase III trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer. Annals of Oncology 2005;16(10):1639‐45. [DOI] [PubMed] [Google Scholar]
Ohkawa 2004 {published data only}
- Ohkawa S. Confirmed unpublished HR for OS (personal communication). Email to: V Chin 28 July 2014.
- Ohkawa S. Randomized controlled trial of gemcitabine in combination with UFT versus gemcitabine alone in patients with advanced pancreatic cancer. Journal of Clinical Oncology 2004;22(14 Suppl):4131. [Google Scholar]
Ozaka 2012 {published data only}
- Ozaka M, Matsumura Y, Ishii H, Omuro Y, Itoi T, Mouri H, et al. Randomized phase II study of gemcitabine and S‐1 combination versus gemcitabine alone in the treatment of unresectable advanced pancreatic cancer (Japan Clinical Cancer Research Organization PC‐01 study). Cancer Chemotherapy and Pharmacology 2012;69(5):1197‐204. [DOI] [PubMed] [Google Scholar]
Petrioli 2015 {published data only}
- Petrioli R, Roviello G, Fiaschi AI, Laera L, Marrelli D, Roviello F, et al. Gemcitabine, oxaliplatin, and capecitabine (GEMOXEL) compared with gemcitabine alone in metastatic pancreatic cancer: a randomized phase II study. Cancer Chemotherapy and Pharmacology 2015;75(4):683. [DOI] [PubMed] [Google Scholar]
Poplin 2009 {published data only}
- Poplin E, Feng Y, Berlin J, Rothenberg MLH, Mitchell H, et al. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed‐dose rate infusion) compared with gemcitabine (30‐minute infusion) in patients with pancreatic carcinoma E6201: a trial of the Eastern Cooperative Oncology Group. Journal of Clinical Oncology 2009;27(23):3778‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poplin 2013 {published data only}
- Poplin E, Wasan H, Rolfe L, Raponi M, Ikdahl T, Bondarenko I, et al. Randomized, multicenter, phase II study of CO‐101 versus gemcitabine in patients with metastatic pancreatic ductal adenocarcinoma: including a prospective evaluation of the role of hENT1 in gemcitabine or CO‐101 sensitivity. Journal of Clinical Oncology 2013;31(35):4453‐61. [DOI] [PubMed] [Google Scholar]
Reni 2005 {published data only}
- Reni M, Cordio S, Milandri C, Passoni P, Bonetto E, Oliani C, et al. Gemcitabine versus cisplatin, epirubicin, fluorouracil, and gemcitabine in advanced pancreatic cancer: a randomised controlled multicentre phase III trial. Lancet Oncology 2005;6(6):369‐76. [DOI] [PubMed] [Google Scholar]
Reni 2012 {published data only}
- Reni M, Cereda S, Rognone A, Belli C, Ghidini M, Longoni S, et al. A randomized phase II trial of two different 4‐drug combinations in advanced pancreatic adenocarcinoma: cisplatin, capecitabine, gemcitabine plus either epirubicin or docetaxel (PEXG or PDXG regimen). Cancer Chemotherapy and Pharmacology 2012;69(1):115‐23. [DOI] [PubMed] [Google Scholar]
Riess 2005 {published data only}
- Pelzer U. Unpublised OS and PFS data provided (personal communication). Email to: V Chin 30 July 2014.
- Riess H, Helm A, Niedergethmann M, Schmidt‐Wolf I, Moik M, Hammer K, et al. A randomised, prospective, multicenter, phase III trial of gemcitabine, 5‐fluorouracil (5‐FU), folinic acid vs. gemcitabine alone in patients with advanced pancreatic cancer. Journal of Clinical Oncology 2005;23(16 Suppl):LBA4009. [Google Scholar]
Rocha Lima 2004 {published data only}
- Rocha Lima CM, Green MR, Rotche R, Miller WH Jr, Jeffrey GM, Cisar LA. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. Journal of Clinical Oncology 2004;22(18):3776‐83. [DOI] [PubMed] [Google Scholar]
Scheithauer 2003 {published data only}
- Scheithauer W, Schull B, Ulrich‐Pur H, Schmid K, Raderer M, Haider, et al. Biweekly high‐dose gemcitabine alone or in combination with capecitabine in patients with metastatic pancreatic adenocarcinoma: a randomized phase II trial. Annals of Oncology 19/12/2002;14(1):97‐104. [DOI] [PubMed] [Google Scholar]
Singhal 2014 {published data only}
- Singhal MK, Kapoor A, Bagri PK, Narayan S, Singh D, Nirban RK, et al. A phase III trial comparing of FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. Annals of Oncology 2014;25:iv210. [Google Scholar]
Smith 2003 {published data only}
- Smith D, Gallagher N. A phase II/III study comparing intravenous ZD9331 with gemcitabine in patients with pancreatic cancer. European Journal of Cancer 2003;39(10):1377‐83. [DOI] [PubMed] [Google Scholar]
Stathopoulos 2006 {published data only}
- Stathopoulos GP, Syrigos K, Aravantinos G, Polyzos A, Papakotoulas P, Fountzilas G, et al. A multicenter phase III trial comparing irinotecan‐gemcitabine (IG) with gemcitabine (G) monotherapy as first‐line treatment in patients with locally advanced or metastatic pancreatic cancer. British Journal of Cancer 2006;95(5):587‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Takada 1998 {published data only}
- Takada T, Nimura Y, Katoh H, Nagakawa T, Nakayama T, Matsushiro T, et al. Prospective randomized trial of 5‐fluorouracil, doxorubicin, and mitomycin C for non‐resectable pancreatic and biliary carcinoma: multicenter randomized trial. Hepato‐gastroenterology 1998;45(24):2020‐6. [PubMed] [Google Scholar]
Tempero 2003 {published data only}
- Tempero M, Plunkett W, Ruiz Van Haperen V, Hainsworth J, Hochster H, Lenzi R, et al. Randomized phase II comparison of dose‐intense gemcitabine: thirty‐minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. Journal of Clinical Oncology 2003;21(18):3402‐8. [DOI] [PubMed] [Google Scholar]
Topham 1991 {published data only}
- Topham C, Glees J, Rawson NS, Woods EM, Coombes RC. Randomised trial of epirubicin alone versus 5‐fluorouracil, epirubicin and mitomycin C in locally advanced and metastatic carcinoma of the pancreas. British Journal of Cancer 1991;64(1):179‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ueno 2013 {published data only}
- Ueno H, Ioka T, Ikeda M, Ohkawa S, Yanagimoto H, Boku N, et al. Randomized phase III study of gemcitabine plus S‐1, S‐1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. Journal of Clinical Oncology 2013;31(13):1640‐8. [DOI] [PubMed] [Google Scholar]
Ueno 2013 – EPA study {published data only}
- Ueno M, Kobayashi S, Ohkawa S, Kameda R, Andou T, Sugimori K, et al. Randomized phase II study of gemcitabine monotherapy versus gemcitabine with an EPA‐enriched oral supplement in advanced pancreatic cancer. Journal of Clinical Oncology 2013;31:e15109. [Google Scholar]
Viret 2004 {published data only}
- Viret F, Ychou M, Lepille D, Mineur L, Navarro F, Topart D, et al. Gemcitabine in combination with cisplatin (GP) versus gemcitabine (G) alone in the treatment of locally advanced or metastatic pancreatic cancer: final results of a multicenter randomized phase II study. Journal of Clinical Oncology 2004;22(14 Suppl):4118. [Google Scholar]
Von Hoff 2013 {published data only}
- Hoff DD, Ervin TJ, Arena FP, Chiorean G, Infante JR, Moore MJ, et al. Randomized phase III study of weekly nab‐paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic adenocarcinoma of the pancreas (MPACT). Journal of Clinical Oncology 2013;31(4 Suppl):LBA148. [Google Scholar]
Wang 2002 {published data only}
- Wang X, Ni Q, Jin M, Li Z, Wu Y, Zhao Y, et al. Gemcitabine or gemcitabine plus cisplatin for in 42 patients with locally advanced or metastatic pancreatic cancer. Chinese Journal of Oncology 2002;24(4):404‐7. [PubMed] [Google Scholar]
Xinopoulos 2008 {published data only}
- Xinopoulos D, Dimitroulopoulos D, Karanikas I, Fotopoulou A, Oikonomou N, Korkolis D, et al. Gemcitabine as palliative treatment in patients with unresectable pancreatic cancer previously treated with placement of a covered metal stent. A randomized controlled trial. JBUON 2008;13(3):341‐7. [PubMed] [Google Scholar]
References to studies excluded from this review
Abdel Wahab 1999 {published data only}
- Abdel‐Wahab M, El‐Shennawy F, Agha S, Ragab E, Fathi O, Sultan A, et al. Evaluation of cell mediated immunity in advanced pancreatic carcinoma before and after treatment with interleukin‐2 (IL‐2). Hepato‐gastroenterology 1999; Vol. 46, issue Suppl 1:1293‐6. [PubMed]
Aigner 1998 {published data only}
- Aigner K R, Gailhofer S, Kopp S. Regional versus systemic chemotherapy for advanced pancreatic cancer: a randomized study. Hepato‐gastroenterology 1998;45(22):1125‐9. [PubMed] [Google Scholar]
Alberts 2005 {published data only}
- Alberts S R, Foster N R, Morton R F, Kugler J, Schaefer P, Wiesenfeld M, et al. PS‐341 and gemcitabine in patients with metastatic pancreatic adenocarcinoma: a North Central Cancer Treatment Group (NCCTG) randomized phase II study. Annals of Oncology 2005;16(10):1654‐61. [DOI] [PubMed] [Google Scholar]
Andersen 1981 {published data only}
- Andersen JR, Friis‐Moller A, Hancke S, Roder O, Steen J, Baden H. A controlled trial of combination chemotherapy with 5‐FU and BCNU in pancreatic cancer. Scandinavian Journal of Gastroenterology 1981;16(8):973‐5. [DOI] [PubMed] [Google Scholar]
Ardalan 1988 {published data only}
- Ardalan B, Singh G, Silberman H. A randomized phase I and II study of short‐term infusion of high‐dose fluorouracil with or without N‐(phosphonacetyl)‐L‐aspartic acid in patients with advanced pancreatic and colorectal cancers. Journal of Clinical Oncology 1988;6(6):1053‐8. [DOI] [PubMed] [Google Scholar]
Astsaturov 2011 {published data only}
- Astsaturov IA, Meropol NJ, Alpaugh RK, Burtness BA, Cheng JD, McLaughlin S, et al. Phase II and coagulation cascade biomarker study of bevacizumab with or without docetaxel in patients with previously treated metastatic pancreatic adenocarcinoma. American Journal of Clinical Oncology 2011;34(1):70‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 1976 {published data only}
- Baker LH, Vaitkevicius VK, Gehan E. Randomized prospective trial comparing 5‐fluorouracil (NSC‐19893) to 5‐fluorouracil and methyl‐CCNU (NSC‐95441) in advanced gastrointestinal cancer. Cancer Treatment Reports 1976;60(6):733‐7. [PubMed] [Google Scholar]
Benavides 2014 {published data only}
- Benavides M, Gallego Plazas J, Guillen C, Vera R, Iranzo V, Diaz I, et al. Gemcitabine (G)/erlotinib (E) versus gemcitabine/erlotinib/capecitabine (C) in the first‐line treatment of patients with metastatic pancreatic cancer (mPC): Efficacy and safety results of a phase IIb randomized study from the Spanish TTD Collaborative Group. Journal of Clinical Oncology 2014;32:5s. [DOI] [PubMed] [Google Scholar]
Benson 2014 {published data only}
- Benson A, Bendell J, Wainberg ZA, VyushkovD, Acs P, Kudrik F, et al. A phase 2 randomized, double‐blind, placebo controlled study of simtuzumab or placebo in combination with gemcitabine for the first line treatment of pancreatic adenocarcinoma. Annals of Oncology. 2014; Vol. 25:iv210. [DOI] [PMC free article] [PubMed]
Benson 2017 {published data only}
- Benson AB, Wainberg ZA, Hecht JR, Vyushkov D, Dong H, Bendell J, et al. A phase II randomized, double‐blind, placebo‐controlled study of simtuzumab or placebo in combination with gemcitabine for the first‐line treatment of pancreatic adenocarcinoma. Oncologist 2017;22(3):241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berglund 2010 {published data only}
- Berglund A, Bystrom P, Johansson B, Nygren P, Frodin JE, Pedersen D, et al. An explorative randomised phase II study of sequential chemotherapy in advanced upper gastrointestinal cancer. Medical Oncology 2010;27(1):65‐72. [DOI] [PubMed] [Google Scholar]
Bramhall 2001 {published data only}
- Bramhall SR, Rosemurgy A, Brown PD, Bowry C, Buckels JA. Marimastat as first‐line therapy for patients with unresectable pancreatic cancer: a randomized trial. Journal of Clinical Oncology 2001;19(15):3447‐55. [DOI] [PubMed] [Google Scholar]
Bramhall 2002 {published data only}
- Bramhall SR, Schulz J, Nemunaitis J, Brown PD, Baillet M, Buckels JA. A double‐blind placebo‐controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. British Journal of Cancer 2002;87(2):161‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buanes 2009 {published data only}
- Buanes T, Maurel J, Liauw W, Hebbar M, Nemunaitis J. A randomized phase III study of gemcitabine (G) versus GV1001 in sequential combination with G in patients with unresectable and metastatic pancreatic cancer (PC). Journal of Clinical Oncology 2009;27(15S):4601. [Google Scholar]
Bukowski 1993 {published data only}
- Bukowski RM, Fleming TR, Macdonald JS, Oishi N, Taylor SA, Baker LH. Evaluation of combination chemotherapy and phase II agents in pancreatic adenocarcinoma. A Southwest Oncology Group study. Cancer 1993;71(2):322‐5. [DOI] [PubMed] [Google Scholar]
Burtness 2016 {published data only}
- Burtness B, Powell M, Catalano P, Berlin J, Liles DK, Chapman AE, et al. Randomized phase II trial of irinotecan/docetaxel or irinotecan/docetaxel plus cetuximab for metastatic pancreatic cancer: an Eastern Cooperative Oncology Group Study. American Journal of Clinical Oncology 2016;39(4):340‐5. [10.1097/COC.0000000000000068] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cantore 2004 {published data only}
- Cantore M, Fiorentini G, Luppi G, Rosati G, Caudana R, Piazza E, et al. Gemcitabine versus FLEC regimen given intra‐arterially to patients with unresectable pancreatic cancer: a prospective, randomized phase III trial of the Italian Society for Integrated Locoregional Therapy in Oncology. Journal of Chemotherapy (Florence) 2004;16(6):589‐94. [DOI] [PubMed] [Google Scholar]
Cascinu 2008 {published data only}
- Cascinu S, Berardi R, Labianca R, Siena S, Falcone A, Aitini E, et al. Cetuximab plus gemcitabine and cisplatin compared with gemcitabine and cisplatin alone in patients with advanced pancreatic cancer: a randomised, multicentre, phase II trial. Lancet Oncology 2008;9(1):39‐44. [DOI] [PubMed] [Google Scholar]
Cascinu 2013 {published data only}
- Cascinu S, Berardi R, Sobrero A, Bidoli P, Labianca R, Siena S, et al. Sorafenib does not improve efficacy of chemotherapy in advanced pancreatic cancer: a GISCAD randomized phase II study. Digestive and Liver Disease 2013;46(2):182‐6. [DOI] [PubMed] [Google Scholar]
Catenacci 2013 {published data only}
- Catenacci DVT, Bahary N, Nattam SR, Marsh RW, Wallace JA, Rajdev L, et al. Final analysis of a phase IB/randomized phase II study of gemcitabine (G) plus placebo (P) or vismodegib (V), a hedgehog (Hh) pathway inhibitor, in patients (pts) with metastatic pancreatic cancer (PC): a University of Chicago phase II consortium study. Journal of Clinical Oncology 2013;31(15 Suppl):4012. [Google Scholar]
Chai 2013 {published data only}
- Chai K, Ai YQ, Jiang LW. Phase II study of dendritic cell vaccination combined with recombinant adenovirus‐p53 in treatment for patients with advanced pancreatic carcinoma. Journal of Clinical Oncology 2013;31:3049. [Google Scholar]
Chauffert 2008 {published data only}
- Chauffert B, Mornex F, Bonnetain F, Rougier P, Mariette C, Bouche O, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5‐FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000‐01 FFCD/SFRO study. Annals of Oncology 2008;19(9):1592‐9. [DOI] [PubMed] [Google Scholar]
Chen 2006 {published data only}
- Chen J, Röcken C, Nitsche B, Hosius C, Gschaidmeier H, Kahl S, et al. The tyrosine kinase inhibitor imatinib fails to inhibit pancreatic cancer progression. Cancer Letters 2006;233(2):328‐37. [DOI] [PubMed] [Google Scholar]
Chung 2004 {published data only}
- Chung HW, Bang SM, Park SW, Chung JB, Kang JK, Kim JW, et al. A prospective randomized study of gemcitabine with doxifluridine versus paclitaxel with doxifluridine in concurrent chemoradiotherapy for locally advanced pancreatic cancer. International Journal of Radiation Oncology, Biology, Physics 2004;60(5):1494‐501. [DOI] [PubMed] [Google Scholar]
Chung 2015 {published data only}
- Chung VM, McDonough SL, Philip PA, Cardin DB, Wang‐Gillam A, Hui L, et al. SWOG S1115: Randomized phase II trial of selumetinib (AZD6244; ARRY 142886) hydrogen sulfate (NSC‐748727) and MK‐2206 (NSC‐749607) vs. mFOLFOX in pretreated patients (Pts) with metastatic pancreatic cancer. Journal of Clinical Oncology. 2015; Vol. 33:4119.
Ciuleanu 2009 {published data only}
- Ciuleanu TE, Pavlovsky AV, Bodoky G, Garin AM, Langmuir VK, Kroll S, et al. A randomised Phase III trial of glufosfamide compared with best supportive care in metastatic pancreatic adenocarcinoma previously treated with gemcitabine. European Journal of Cancer 2009;45(9):1589‐96. [DOI] [PubMed] [Google Scholar]
Cohen 2005 {published data only}
- Cohen SJ, Dobelbower R Jr, Lipsitz S, Catalano PJ, Sischy B, Smith TJ, et al. A randomized phase III study of radiotherapy alone or with 5‐fluorouracil and mitomycin‐C in patients with locally advanced adenocarcinoma of the pancreas: Eastern Cooperative Oncology Group study E8282. International Journal of Radiation Oncology, Biology, Physics 2005;62(5):1345‐50. [DOI] [PubMed] [Google Scholar]
Cohen 2010 {published data only}
- Cohen SJ, Zalupski MM, Conkling P, Nugent FW, Ma W, Modiano M, et al. A phase II randomized double blind multicenter trial of gemcitabine (Gem) plus imexon (IMX) versus Gem plus placebo (P) in patients with chemotherapy‐naive pancreatic adenocarcinoma (PC). Journal of Clinical Oncology 2010;28:4076. [DOI] [PubMed] [Google Scholar]
Dahan 2010 {published data only}
- Dahan L, Bonnetain F, Ychou M, Mitry E, Gasmi M, Raoul JL, et al. Combination 5‐fluorouracil, folinic acid and cisplatin (LV5FU2‐CDDP) followed by gemcitabine or the reverse sequence in metastatic pancreatic cancer: final results of a randomised strategic phase III trial (FFCD 0301). Gut 2010;59(11):1527‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dalgleish 2015 {published data only}
- Dalgleish AG, IMAGE I Trial Investigators. A multicenter randomized, open‐label, proof‐of‐concept, phase II trial comparing gemcitabine with and without IMM‐101 in advanced pancreatic cancer. Journal of Clinical Oncology 2015;33:3051. [Google Scholar]
Deplanque 2015 {published data only}
- Deplanque G, Demarchi M, Hebbar M, Flynn P, Milchar B, Atkins J, et al. A randomized, placebo‐controlled phase III trial of masitinib plus gemcitabine in the treatment of advanced pancreatic cancer. Annals of Oncology 2015;26:1194‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ducreux 2002 {published data only}
- Ducreux M, Rougier P, Pignon JP, Douillard JY, Seitz JF, Bugat R, et al. A randomised trial comparing 5‐FU with 5‐FU plus cisplatin in advanced pancreatic carcinoma. Annals of Oncology 2002;13(8):1185‐91. [DOI] [PubMed] [Google Scholar]
Duffy 2015 {published data only}
- Duffy AG, Beg MS, Greten TF, Beatson MA, Mavroukakis S, Patel SP, et al. A multicenter randomized phase II study of NPC‐1C(N) in combination with gemcitabine (G) and nab‐paclitaxel (A) versus G and A alone in patients with metastatic or locally advanced pancreatic cancer (PC) previously treated with folfirinox (F). Journal of Clinical Oncology 2015;33(Suppl 3):TPS499. [Google Scholar]
El‐Khoueiry 2012 {published data only}
- El‐Khoueiry AB, Ramanathan RK, Yang DY, Zhang W, Shibata S, Wright JJ, et al. A randomized phase II of gemcitabine and sorafenib versus sorafenib alone in patients with metastatic pancreatic cancer. Investigational New Drugs 2012;30(3):1175‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 2014 {published data only}
- Evans J, Moore M, Cutsem E, Rock E, Strauss L, ODwyer P. Phase 2 Double‐blind, placebo‐controlled trial of dasatinib added to gemcitabine for subjects with locally‐advanced pancreatic cancer (LAPC). Annals of Oncology 2014;25:ii105. [DOI] [PubMed] [Google Scholar]
Friess 2006 {published data only}
- Friess H, Langrehr JM, Oettle H, Raedle J, Niedergethmann M, Dittrich C, et al. A randomized multi‐center phase II trial of the angiogenesis inhibitor Cilengitide (EMD 121974) and gemcitabine compared with gemcitabine alone in advanced unresectable pancreatic cancer. BMC Cancer 2006;6:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fuchs 2015 {published data only}
- Fuchs CS, Azevedo A, Okusaka T, Laethem JL, Lipton LR, Riess H, et al. A phase 3 randomized, double‐blind, placebo‐controlledtrial of ganitumab or placebo in combination withgemcitabine as first‐line therapy for metastaticadenocarcinoma of the pancreas: the GAMMA trial. Annals of Oncology 2015;26:921‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fukutomi 2015 {published data only}
- Fukutomi A, Mizusawa J, Katayama H, Nakamura S, Ito Y, Hiraoka N, et al. Randomized phase II study of S‐1 and concurrent radiotherapy with versus without induction chemotherapy of gemcitabine for locally advanced pancreatic cancer (JCOG 1106). Journal of Clinical Oncology 2015;33:4116. [Google Scholar]
Gill 2014 {published data only}
- Gill S, Ko YJ, Cripps C, Beaudoin A, Dhesy‐Thind SK, Zulfiqar M, et al. PANCREOX: A randomized phase 3 study of 5FU/LV with or without oxaliplatin for second‐line advanced pancreatic cancer (APC) in patients (pts) who have received gemcitabine (GEM)‐based chemotherapy (CT). Journal of Clinical Oncology 2014;32:5s. [DOI] [PubMed] [Google Scholar]
Gilliam 2012 {published data only}
- Gilliam AD, Broome P, Topuzov EG, Garin AM, Pulay I, Humphreys J, et al. An international multicenter randomized controlled trial of G17DT in patients with pancreatic cancer. Pancreas 2012;41(3):374‐9. [DOI] [PubMed] [Google Scholar]
GISTG 1985 (radiotherapy) {published data only}
- Gastrointestinal Tumour Study Group. Radiation therapy combined with Adriamycin or 5‐fluorouracil for the treatment of locally unresectable pancreatic carcinoma. Cancer 1985;56(11):2563‐8. [DOI] [PubMed] [Google Scholar]
GITSG 1979 {published data only}
- Gastrointestinal Tumor Study Group. A multi‐institutional comparative trial of radiation therapy alone and in combination with 5‐fluorouracil for locally unresectable pancreatic carcinoma. Annals of Surgery 1979;189(2):205‐8. [PMC free article] [PubMed] [Google Scholar]
GITSG 1985 {published data only}
- Gastrointestinal Trials Study Group. Phase II trials of maytansine, low‐dose chlorozotocin, and high‐dose chlorozotocin as single agents against advanced measurable adenocarcinoma of the pancreas. Gastrointestinal Tumor Study Group. Cancer Treatment Reports 1985;69(4):417‐20. [PubMed] [Google Scholar]
GITSG 1988 {published data only}
- Group Gastrointestinal Tumor Study. Treatment of locally unresectable carcinoma of the pancreas: comparison of combined‐modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. Journal of the National Cancer Institute 1988;80(10):751‐5. [PubMed] [Google Scholar]
Gonçalves 2012 {published data only}
- Gonçalves A, Gilabert M, François E, Dahan L, Perrier H, Lamy R, et al. BAYPAN study: a double‐blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancer. Annals of Oncology 2012;23(11):2799‐805. [DOI] [PubMed] [Google Scholar]
Gong 2007 {published data only}
- Gong JF, Zhang XD, Li J, Di LJ, Jin ML, Shen L. Efficacy of gemcitabine‐based chemotherapy on advanced pancreatic cancer. Ai Zheng [Chinese Journal of Cancer] 2007;26(8):890‐4. [PubMed] [Google Scholar]
Haas 2015 {published data only}
- Haas M, Boeck SH, Waldschmidt D, Reinacher‐Schick A, Freiberg‐Richter J, Seufferlein T, et al. ACCEPT: Afatinib as cancer therapy for exocrine pancreatic tumors‐An explorative randomized phase II trial. Journal of Clinical Oncology 2015;33:TPS4150. [Google Scholar]
Hammel 2013 {published data only}
- Hammel P, Huguet F, Laethem JL, Goldstein D, Glimelius B, Artru P, et al. Comparison of chemoradiotherapy (CRT) and chemotherapy (CT) in patients with a locally advanced pancreatic cancer (LAPC) controlled after 4 months of gemcitabine with or without erlotinib: final results of the international phase III LAP 07 study. Journal of Clinical Oncology 2013;31:LBA4003. [Google Scholar]
Han 2006 {published data only}
- Han GH, Yin ZX, Meng XJ, He CY, Zhang HB, Sun AH, et al. Prospective randomized clinical trial of two drug delivery pathway in the treatment of inoperable advanced pancreatic carcinoma. Chinese Journal of Digestive Diseases 2006;7(1):45‐8. [DOI] [PubMed] [Google Scholar]
Hazel 1981 {published data only}
- Hazel JJ, Thirlwell MP, Huggins M, Maksymiuk A, MacFarlane JK. Multi‐drug chemotherapy with and without radiation for carcinoma of the stomach and pancreas: a prospective randomized trial. Journal of the Canadian Association of Radiologists 1981;32(3):164‐5. [PubMed] [Google Scholar]
Heinemann 2013 {published data only}
- Heinemann V, Ebert MP, Laubender RP, Bevan P, Mala C, Boeck S. Phase II randomised proof‐of‐concept study of the urokinase inhibitor upamostat (WX‐671) in combination with gemcitabine compared with gemcitabine alone in patients with non‐resectable, locally advanced pancreatic cancer. British Journal of Cancer 2013;108(4):766‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heinemann 2013 (GUT) {published data only}
- Heinemann V, Vehling‐Kaiser U, Waldschmidt D, Kettner E, Marten A, Winkelmann C, et al. Gemcitabine plus erlotinib followed by capecitabine versus capecitabine plus erlotinib followed by gemcitabine in advanced pancreatic cancer: final results of a randomised phase 3 trial of the 'Arbeitsgemeinschaft Internistische Onkologie' (AIO‐PK0104). Gut 2013;62(5):751‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herman 2013 {published data only}
- Herman JM, Wild AT, Wang H, Tran PT, Chang KJ, Taylor GE, et al. Randomized phase III multi‐institutional study of TNFerade biologic with fluorouracil and radiotherapy for locally advanced pancreatic cancer: final results. Journal of Clinical Oncology 2013;31(7):886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hingorani 2015 {published data only}
- Hingorani SR, Proctor Harris W, Hendifar AE, Bullock AJ, Wu XW, Huang Y, et al. High response rate and PFS with PEGPH20 added to nab‐paclitaxel/gemcitabine in stage IV previously untreated pancreatic cancer patients with high‐HA tumors: Interim results of a randomized phase II study. Journal of Clinical Oncology 2015;33:4006. [Google Scholar]
Horton 1981 {published data only}
- Horton J, Gelber R D, Engstrom P, Falkson G, Moertel C, Brodovsky H, et al. Trials of single‐agent and combination chemotherapy for advanced cancer of the pancreas. Cancer Treatment Reports 1981;65(1‐2):65‐8. [PubMed] [Google Scholar]
Hurwitz 2015 {published data only}
- Hurwitz HI, Uppal N, Wagner SA, Bendell JC, Beck T, Wade SM 3rd, et al. Randomized, double‐blind, phase II study of ruxolitinib or placebo in combination with capecitabine in patients with metastatic pancreatic cancer for whom therapy with gemcitabine has failed. Journal of Clinical Oncology 2015;33(34):4039‐47. [DOI: 10.1200/JCO.2015.61.4578.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hurwitz 2015 (JANUS 1) {published data only}
- Hurwitz H, Garrett WM, Clark J, Brill KJ, Dawkins FW, Hidalgo M, et al. JANUS 1: A phase 3, placebo‐controlled study of ruxolitinib plus capecitabine in patients with advanced or metastatic pancreatic cancer (MPC) after failure or intolerance of first‐line chemotherapy. Journal of Clinical Oncology 2015;33:TPS4147. [Google Scholar]
Infante 2013 {published data only}
- Infante JR, Somer BG, Park JO, Li CP, Scheulen ME, Kasubhai SM, et al. A randomized, double‐blind, placebo‐controlled trial of trametinib, a MEK inhibitor, in combination with gemcitabine for patients with untreated metastatic adenocarcinoma of the pancreas. Journal of Clinical Oncology 2013;31(4 Suppl):291. [DOI] [PubMed] [Google Scholar]
Ioka 2009 {published data only}
- Ioka T, Takakura R, Nakaizumi A, Tanaka S, Iishi H, Nakamura S, et al. A multicenter randomized phase II study of full‐dose gemcitabine and concurrent radiotherapy comparing gemcitabine alone for the unresectable locally advanced pancreatic adenocarcinoma. Journal of Clinical Oncology 2009;27:e15512. [Google Scholar]
Ioka 2013 {published data only}
- Ioka T, Katayama K, Ishida N, Takada R, Yamai T, Fukutake N, et al. Randomized phase II study of best available fluoropyrimidine compared with continuation of gemcitabine (Gem) monotherapy in patients with Gem‐refractory pancreatic cancer. Journal of Clinical Oncology 2013;31:287. [Google Scholar]
Jacobs 2004 {published data only}
- A Jacobs. Full survival data not available. Email to V Chin 5 September 2014.
- Jacobs AD, Burris HA, Rivkin S, Ritch PS, Eisenberg PD, Mettinger KL. A randomized phase III study of rubitecan (ORA) vs. best choice (BC) in 409 patients with refractory pancreatic cancer report from a North‐American multi‐center study. Journal of Clinical Oncology 2004;22:4013. [Google Scholar]
Javle 2011 {published data only}
- Javle MM, Varadhachary GR, Fogelman DR, Shroff RT, Overman MJ, Ukegbu L, et al. Randomized phase II study of gemcitabine (G) plus anti‐IGF‐1R antibody MK‐0646, G plus erlotinib (E) plus MK‐0646 and G plus E for advanced pancreatic cancer. Journal of Clinical Oncology 2011;29:4026. [Google Scholar]
Johnson 2001 {published data only}
- Johnson CD, Puntis M, Davidson N, Todd S, Bryce R. Randomized, dose‐finding phase III study of lithium gamolenate in patients with advanced pancreatic adenocarcinoma. British Journal of Surgery 2001;88(5):662‐8. [DOI] [PubMed] [Google Scholar]
Kim 2011 {published data only}
- Kim GP, Foster NR, Salim M, Flynn PJ, Moore DF, Zon R, et al. Randomized phase II trial of panitumumab (P), erlotinib (E), and gemcitabine (G) versus erlotinib‐gemcitabine in patients with untreated, metastatic pancreatic adenocarcinoma. Journal of Clinical Oncology 2011;29:238. [Google Scholar]
Kindler 2008 {published data only}
- Kindler HL, Gangadhar T, Karrison T, Hochster HS, Moore MJ, Micetich K, et al. Final analysis of a randomized phase II study of bevacizumab (B) and gemcitabine (G) plus cetuximab (C) or erlotinib (E) in patients (pts) with advanced pancreatic cancer (PC). Journal of Clinical Oncology 2008;26:4502. [Google Scholar]
Kindler 2010 {published data only}
- KKindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). Journal of Clinical Oncology 2010;28(22):3617‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kindler 2011 {published data only}
- Kindler HL, Ioka T, Richel DJ, Bennouna J, Letourneau R, Okusaka T, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double‐blind randomised phase 3 study. Lancet Oncology 2011;12(3):256‐62. [DOI] [PubMed] [Google Scholar]
Kindler 2012 {published data only}
- Kindler HL, Richards DA, Garbo LE, Garon EB, Stephenson JJ Jr, Rocha‐Lima CM, et al. A randomized, placebo‐controlled phase 2 study of ganitumab (AMG 479) or conatumumab (AMG 655) in combination with gemcitabine in patients with metastatic pancreatic cancer. Annals of Oncology 2012;23(11):2384‐42. [DOI] [PubMed] [Google Scholar]
Kindler 2015 {published data only}
- Kindler HL, Locker GY, Mann H, Golan T. POLO: A randomized phase III trial of olaparib tablets in patients with metastatic pancreatic cancer (mPC) and a germline BRCA1/2 mutation (gBRCAm) who have not progressed following first‐line chemotherapy. Journal of Clinical Oncology. 2015; Vol. 33.
Klaassen 1985 {published data only}
- Klaassen DJ, MacIntyre JM, Catton GE, Engstrom PF, Moertel CG. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5‐fluorouracil alone with radiation plus concurrent and maintenance 5‐fluorouracil‐‐an Eastern Cooperative Oncology Group study. Journal of Clinical Oncology 1985;3(3):373‐8. [DOI] [PubMed] [Google Scholar]
Ko 2012 {published data only}
- Ko AH, Youssoufian H, Gurtler J, Dicke K, Kayaleh O, Lenz H J, et al. A phase II randomized study of cetuximab and bevacizumab alone or in combination with gemcitabine as first‐line therapy for metastatic pancreatic adenocarcinoma. Investigational New Drugs 2012;30(4):1597‐606. [DOI] [PubMed] [Google Scholar]
Ko 2016 {published data only}
- Ko AH, Murphy PB, Peyton JD, Shipley D, Al‐Hazzouri A, Rodrigeuz FA, et al. RAINIER: A randomized, double‐blinded, placebo‐controlled phase II trial of gemcitabine (gem) plus nab‐paclitaxel (nab‐P) combined with apatorsen (A) or placebo (Pl) in patients (pts) with metastatic pancreatic cancer (mPC). Journal of Clinical Oncology 2016;34(4 Suppl):419. [Google Scholar]
Lasalvia‐Prisco 2012 {published data only}
- Lasalvia‐Prisco E, Goldschmidt P, Galmarini F, Cucchi S, Vazquez J, Aghazarian M, et al. Addition of an induction regimen of antiangiogenesis and antitumor immunity to standard chemotherapy improves survival in advanced malignancies. Medical Oncology 2012;29(5):3626‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Le (Ipilimumab) 2013 {published data only}
- Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, et al. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM‐CSF gene in previously treated pancreatic cancer. Journal of Immunotherapy 2013;36(7):382‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Le 2013 {published data only}
- Le DT, Wang‐Gillam A, Picozzi VJ, Greten TF, Crocenzi TS, Springett GM, et al. Interim safety and efficacy analysis of a phase II, randomized study of GVAX pancreas and CRS‐207 immunotherapy in patients with metastatic pancreatic cancer. Journal of Clinical Oncology 2013;31:4040. [Google Scholar]
Le 2015 {published data only}
- Le DT, Crocenzi TS, Uram JN, Lutz ER, Laheru DA, Sugar EA, et al. Randomized phase II study of the safety, efficacy, and immune response of GVAX pancreas vaccine (with cyclophophamide) and CRS‐207 with or without nivolumab in patients with previously treated metastatic pancreatic adenocarcinomas (STELLAR). Journal for Immunotherapy of Cancer 2015;3(Suppl 2):155. [Google Scholar]
Li 2003 {published data only}
- Li CP, Chao Y, Chi KH, Chan WK, Teng HC, Lee RC, et al. Concurrent chemoradiotherapy treatment of locally advanced pancreatic cancer: gemcitabine versus 5‐fluorouracil, a randomized controlled study. International Journal of Radiation Oncology, Biology, Physics 2003;57(1):98‐104. [DOI] [PubMed] [Google Scholar]
Li 2016 {published data only}
- Li JQ, Yang JC, Liang JX, Wang SL. Pharmacokinetic study and clinical evaluation of a slow‐release 5‐fluorouracil implant in pancreatic cancer patients. Anticancer Drugs 2016;27(1):60‐5. [DOI] [PubMed] [Google Scholar]
Linstadt 1988 {published data only}
- Linstadt D, Quivey JM, Castro JR, Andejeski Y, Phillips TL, Hannigan J, et al. Comparison of helium‐ion radiation therapy and split‐course megavoltage irradiation for unresectable adenocarcinoma of the pancreas. Final report of a Northern California Oncology Group randomized prospective clinical trial. Radiology 1988;168(1):261‐4. [DOI] [PubMed] [Google Scholar]
Loehrer 2011 {published data only}
- Loehrer PJ Sr, Feng Y, Cardenes H, Wagner L, Brell JM, Cella D, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. Journal of Clinical Oncology 2011;29(31):4105‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lokich 1979 {published data only}
- Lokich J, Brooks J, Chaffey J. Comparative therapeutic trial of radiation with or without chemotherapy in pancreatic carcinoma. Gastrointestinal Tumor Study Group. International Journal of Radiation Oncology, Biology, Physics 1979;5(9):1643‐7. [DOI] [PubMed] [Google Scholar]
Lygidakis 1995 {published data only}
- Lygidakis NJ, Ziras FA, Kyparidou E, Parissis J, Papadopoulou P, Venetsanou B. Combined immunopharmaceutical therapy of patients with unresectable pancreatic carcinoma. Hepato‐gastroenterology 1995;42(6):1039‐52. [PubMed] [Google Scholar]
Mallinson 1980 {published data only}
- Mallinson CN, Rake MO, Cocking JB, Fox CA, Cwynarski MT, Diffey BL, et al. Chemotherapy in pancreatic cancer: results of a controlled, prospective, randomised, multicentre trial. BMJ 1980;281(6255):1589‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meyer 2008 {published data only}
- Meyer T, Caplin M, Palmer D, Valle JW, Larvin M, Waters J, et al. A phase IB/IIA, multicentre, randomised, double‐blind placebo controlled study to evaluate the safety and pharmacokinetics of Z‐360 in subjects with unresectable advanced pancreatic cancer in combination with gemcitabine. Journal of Clinical Oncology 2008;26:4636. [Google Scholar]
Middleton 2014 {published data only}
- Middleton G, Silcocks P, Cox T, Valle J, Wadsley J, Propper D, et al. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): an open‐label, randomised, phase 3 trial. Lancet Oncology 2014;15(8):829‐40. [DOI] [PubMed] [Google Scholar]
Mitry 2006 {published data only}
- Mitry E, Ducreux M, Ould‐Kaci M, Boige V, Seitz J F, Bugat R, et al. Oxaliplatin combined with 5‐FU in second line treatment of advanced pancreatic adenocarcinoma. Results of a phase II trial. Gastroenterologie Clinique et Biologique 2006;30(3):357‐63. [DOI] [PubMed] [Google Scholar]
Mizuno 2013 {published data only}
- Mizuno N, Yamao K, Komatsu Y, Munakata M, Ishiguro A, Yamaguchi, T, et al. Randomized phase II trial of S‐1 versus S‐1 plus irinotecan (IRIS) in patients with gemcitabine‐refractory pancreatic cancer. Journal of Clinical Oncology 2013;31:263. [Google Scholar]
Modiano 2012 {published data only}
- Modiano M, Keogh GP, Manges R, Stella PJ, Milne G, Looper E, et al. Apricot‐P: a randomized placebo‐controlled phase II study of COX‐2 inhibitor apricoxib or placebo in combination with gemcitabine and erlotinib in advanced or metastatic adenocarcinoma of the pancreas. Journal of Clinical Oncology 2012;30(4 Suppl):253. [Google Scholar]
Moertel 1981 {published data only}
- Moertel CG, Frytak S, Hahn RG, O'Connell MJ, Reitemeier RJ, Rubin J, et al. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5‐fluorouracil), and high dose radiation + 5‐fluorouracil: the Gastrointestinal Tumor Study Group. Cancer 1981;48(8):1705‐10. [DOI] [PubMed] [Google Scholar]
Moore 2003 {published data only}
- Moore MJ, Hamm J, Dancey J, Eisenberg PD, Dagenais M, Fields A, et al. Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12‐9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. Journal of Clinical Oncology 2003;21(17):3296‐302. [DOI] [PubMed] [Google Scholar]
Moore 2007 {published data only}
- Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. Journal of Clinical Oncology 2007;25(15):1960‐6. [DOI] [PubMed] [Google Scholar]
Mukherjee 2013 {published data only}
- Mukherjee S, Hurt CN, Bridgewater J, Falk S, Cummins S, Wasan H, et al. Gemcitabine‐based or capecitabine‐based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncology 2013;14(4):317‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakai 2012 {published data only}
- Nakai Y, Isayama H, Sasaki T, Sasahira N, Tsujino T, Toda N, et al. A multicentre randomised phase II trial of gemcitabine alone vs gemcitabine and S‐1 combination therapy in advanced pancreatic cancer: GEMSAP study. British Journal of Cancer 2012;106(12):1934‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nio 2010 {published data only}
- Nio K, Ueno H, Okusaka T, Morizane C, Hagihara A, Kondo S, et al. Chemoradiotherapy versus gemcitabine‐based chemotherapy in patients with unresectable, locally advanced pancreatic cancer. Journal of Clinical Oncology 2010;28:e14504. [Google Scholar]
O'Neil 2015 {published data only}
- O'Neil BH, Scott AJ, Ma WW, Cohen SJ, Aisner DL, Menter AR, et al. A phase II/III randomized study to compare the efficacy and safety of rigosertib plus gemcitabine versus gemcitabine alone in patients with previously untreated metastatic pancreatic cancer. Annals of Oncology 2015;26:1923‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Reilly 2013 {published data only}
- O'Reilly EM, Lowery MA, Yu KH, Capanu M, Stadler ZK, Epstein AS, et al. Randomized phase II study of gemcitabine (G), cisplatin (C) with or without veliparib (V) (arms A, B) and a phase II single‐arm study of single‐agent veliparib (arm C) in patients with BRCA or PALB2‐mutated pancreas adenocarcinoma (PC). Journal of Clinical Oncology 2013;31:TPS4144. [Google Scholar]
O'Reilly 2015 {published data only}
- O'Reilly EM, Walker C, Clark J, Brill KJ, Dawkins FW, Bendell JC, et al. JANUS 2: A phase III study of survival, tumor response, and symptom response with ruxolitinib plus capecitabine or placebo plus capecitabine in patients with advanced or metastatic pancreatic cancer (mPC) who failed or were intolerant to first‐line chemotherapy. Journal of Clinical Oncology 2015;33:TPS4146. [Google Scholar]
Oberic 2011 {published data only}
- Oberic L, Viret F, Baey C, Ychou M, Bennouna J, Adenis A, et al. Docetaxel‐ and 5‐FU‐concurrent radiotherapy in patients presenting unresectable locally advanced pancreatic cancer: a FNCLCC‐ACCORD/0201 randomized phase II trial's pre‐planned analysis and case report of a 5.5‐year disease‐free survival. Radiation Oncology (London) 2011;6:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oster 1986 {published data only}
- Oster MW, Gray R, Panasci L, Perry MC. Chemotherapy for advanced pancreatic cancer. A comparison of 5‐fluorouracil, adriamycin, and mitomycin (FAM) with 5‐fluorouracil, streptozotocin, and mitomycin (FSM). Cancer 1986;57(1):29‐33. [DOI] [PubMed] [Google Scholar]
Palmer 1994 {published data only}
- Palmer KR, Kerr M, Knowles G, Cull A, Carter DC, Leonard RC. Chemotherapy prolongs survival in inoperable pancreatic carcinoma. British Journal of Surgery 1994;81(6):882‐5. [DOI] [PubMed] [Google Scholar]
Pandya 2013 {published data only}
- Pandya SS, Wong L, Bullock AJ, Grabelsky SA, Shum MK, Shan J, et al. Randomized, open‐label, phase II trial of gemcitabine with or without bavituximab in patients with nonresectable stage IV pancreatic adenocarcinoma. Journal of Clinical Oncology 2013;31:4054. [Google Scholar]
Pelzer 2011 {published data only}
- Pelzer U, Schwaner I, Stieler J, Adler M, Seraphin J, Dorken B, et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5‐fluorouracil (OFF) plus BSC in patients for second‐line advanced pancreatic cancer: a phase III‐study from the German CONKO‐study group. European Journal of Cancer 2011;47(11):1676‐81. [DOI] [PubMed] [Google Scholar]
Philip 2010 {published data only}
- Philip PA, Benedetti J, Corless CL, Wong R, O'Reilly EM, Flynn PJ, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group‐directed intergroup trial S0205. Journal of Clinical Oncology 2010;28(22):3605‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Philip 2014 {published data only}
- Philip PA, Goldman B, Ramanathan RK, Lenz HJ, Lowy A M, Whitehead RP, et al. Dual blockade of epidermal growth factor receptor and insulin‐like growth factor receptor‐1 signaling in metastatic pancreatic cancer: Phase Ib and randomized phase II trial of gemcitabine, erlotinib, and cixutumumab versus gemcitabine plus erlotinib (SWOG S0727). Cancer 2014;120(19):2980‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Propper 2014 {published data only}
- Propper D, Davidenko I, Bridgewater J, Kupcinskas L, Flittipaldo A, Hillenbach C, et al. Phase II, randomized, biomarker identification trial (MARK) for erlotinib in patients with advanced pancreatic carcinoma. Annals of Oncology 2014;25(7):1384‐90. [DOI] [PubMed] [Google Scholar]
Queisser 1979 {published data only}
- Queisser W, Schaefer J, Arnold H. A prospective multi‐centre study of the response of metastatic gastrointestinal tumours. Deutsche Medizinische Wochenschrift 1979;104(35):1231‐6. [DOI] [PubMed] [Google Scholar]
Ramanathan 2011 {published data only}
- Ramanathan RK, Abbruzzese J, Dragovich T, Kirkpatrick L, Guillen JM, Baker AF, et al. A randomized phase II study of PX‐12, an inhibitor of thioredoxin in patients with advanced cancer of the pancreas following progression after a gemcitabine‐containing combination. Cancer Chemotherapy and Pharmacology 2011;67(3):503‐9. [DOI] [PubMed] [Google Scholar]
Reni 2009 {published data only}
- Reni M, Cereda S, Balzano G, Passoni P, Rognone A, Zerbi A, et al. Outcome of upfront combination chemotherapy followed by chemoradiation for locally advanced pancreatic adenocarcinoma. Cancer Chemotherapy and Pharmacology 2009;64(6):1253‐9. [DOI] [PubMed] [Google Scholar]
Reni 2013 {published data only}
- Reni M, Cereda S, Milella M, Novarino A, Passardi A, Mambrini A, et al. Maintenance sunitinib or observation in metastatic pancreatic adenocarcinoma: a phase II randomised trial. European Journal of Cancer 2013;49(17):3609‐15. [DOI] [PubMed] [Google Scholar]
Richards 2011 {published data only}
- Richards DA, Kuefler PR, Becerra C, Wilfong LS, Gersh RH, Boehm KA, et al. Gemcitabine plus enzastaurin or single‐agent gemcitabine in locally advanced or metastatic pancreatic cancer: results of a phase II, randomized, noncomparative study. Investigational New Drugs 29/08/2009;29(1):144‐53. [DOI] [PubMed] [Google Scholar]
Richly 2013 {published data only}
- Richly H, Maute L, Heil G, Russel J, Jager E, Koeberle D, et al. Prospective randomized phase II trial with gemcitabine versus gemcitabine plus sunitinib in advanced pancreatic cancer: a study of the CESAR Central European Society for Anticancer Drug Research‐EWIV. Journal of Clinical Oncology 2013;31:4035. [DOI] [PubMed] [Google Scholar]
Riess 2010 {published data only}
- Riess H, Pelzer U, Opitz B, Stauch M, Reitzig P, Hahnfeld S, et al. A prospective, randomized trial of simultaneous pancreatic cancer treatment with enoxaparin and chemotherapy: final results of the CONKO‐004 trial. Journal of Clinical Oncology 2010;28:4033. [Google Scholar]
Rougier 2013 {published data only}
- Rougier P, Riess H, Manges R, Karasek P, Humblet Y, Barone, et al. Randomised, placebo‐controlled, double‐blind, parallel‐group phase III study evaluating aflibercept in patients receiving first‐line treatment with gemcitabine for metastatic pancreatic cancer. European Journal of Cancer 2013;49(12):2633‐42. [DOI] [PubMed] [Google Scholar]
Ryan 2013 {published data only}
- Ryan DP, Reddy SG, Bahary N, Uronis HE, Sigal D, Cohn AL, et al. TH‐302 plus gemcitabine (G+T) versus gemcitabine (G) in patients with previously untreated advanced pancreatic cancer (PAC). Journal of Clinical Oncology 2013;31:325. [Google Scholar]
Saif 2009 {published data only}
- Saif MW, Oettle H, Vervenne WL, Thomas JP, Spitzer G, Visseren‐Grul C, et al. Randomized double‐blind phase II trial comparing gemcitabine plus LY293111 versus gemcitabine plus placebo in advanced adenocarcinoma of the pancreas. Cancer Journal 2009;15(4):339‐43. [DOI] [PubMed] [Google Scholar]
Sakata 1992 {published data only}
- Sakata Y, Chiba Y, Sato T, Kimura M, Fukushi G, Matsukawa M, et al. Comparative study of UFT plus mitomycin C and UFT plus doxorubicin in adenocarcinoma. Hirosaki Cooperative Group of Cancer Chemotherapy. Gan to Kagaku Ryoho. Cancer & Chemotherapy 1992;19(2):195‐201. [PubMed] [Google Scholar]
Schein 1978 {published data only}
- Schein PS, Lavin PT, Moertel CG, Frytak S, Hahn RG, O'Connell MJ, et al. Randomized phase II clinical trial of adriamycin, methotrexate, and actinomycin‐D in advanced measurable pancreatic carcinoma: a Gastrointestinal Tumor Study Group Report. Cancer 1978;42(1):19‐22. [DOI] [PubMed] [Google Scholar]
Schmitz‐Winnenthal 2013 {published data only}
- Schmitz‐Winnenthal FH, Grenacher L, Friedrich T, Lubenau H, Springer M, Breiner KM, et al. VXM01, an oral T‐cell vaccine targeting the tumor vasculature: results from a randomized, controlled, first‐in‐man study in pancreatic cancer patients. Journal of Clinical Oncology 2013;31:3090. [Google Scholar]
Senzer 2006 {published data only}
- Senzer N, Rosemurgy A, Javle M, Reid T, Posner MC, Chang KJ, et al. The PACT trial: Interim results of a randomized trial of TNFerade biologic plus chemoradiation (CRT) compared to CRT alone in locally advanced pancreatic cancer (LAPC). Journal of Clinical Oncology 2006;24:4102. [Google Scholar]
Shapiro 2005 {published data only}
- Shapiro J, Marshall J, Karasek P, Figer A, Oettle H, Couture F, et al. G17DT+gemcitabine [Gem] versus placebo+Gem in untreated subjects with locally advanced, recurrent, or metastatic adenocarcinoma of the pancreas: results of a randomized, double‐blind, multinational, multicenter study. Journal of Clinical Oncology 2005;23:LBA4012. [Google Scholar]
Shinchi 2002 {published data only}
- Shinchi H, Takao S, Noma H, Matsuo Y, Mataki Y, Mori S, et al. Length and quality of survival after external‐beam radiotherapy with concurrent continuous 5‐fluorouracil infusion for locally unresectable pancreatic cancer. International Journal of Radiation Oncology, Biology, Physics 2002;53(1):146‐50. [DOI] [PubMed] [Google Scholar]
Shinchi 2014 {published data only}
- Shinchi H, Takao S, Maemura K, Mataki Y, Kurahara H, Hiwatashi K, et al. Oral S‐1 with concurrent radiotherapy versus S‐1 alone in patients with locally unresectable pancreatic cancer. Pancreas. 45th Meeting of the American Pancreatic Association and Japan Pancreas Society 2014;43:1407. [Google Scholar]
Spano 2008 {published data only}
- Spano JP, Chodkiewicz C, Maurel J, Wong R, Wasan H, Barone C, et al. Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: an open‐label randomised phase II study. Lancet 2008;371(9630):2101‐8. [DOI] [PubMed] [Google Scholar]
Strumberg 2013 {published data only}
- Strumberg D, Schultheis B, Ebert MP, Kerkhoff A, Hofheinz RD, Behringer DM, et al. Phase II, randomized, double‐blind placebo‐controlled trial of nimotuzumab plus gemcitabine compared with gemcitabine alone in patients (pts) with advanced pancreatic cancer (PC). Journal of Clinical Oncology 2013;31:4009. [Google Scholar]
Sudo 2014 {published data only}
- Sudo K, Ishihara T, Hirata N, Ozawa F, Ohshima T, Azemoto R, et al. Randomized controlled study of gemcitabine plus S‐1 combination chemotherapy versus gemcitabine for unresectable pancreatic cancer. Cancer Chemotherapy and Pharmacology 2013;73(2):389. [DOI] [PubMed] [Google Scholar]
Sultana 2009 {published data only}
- Sultana A, Shore S, Raraty M G, Vinjamuri S, Evans J E, Smith C T, et al. Randomised Phase I/II trial assessing the safety and efficacy of radiolabelled anti‐carcinoembryonic antigen I(131) KAb201 antibodies given intra‐arterially or intravenously in patients with unresectable pancreatic adenocarcinoma. BMC Cancer 2009;9:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2011 {published data only}
- Sun Y, Li Y, Qin S, Ma D, Jiao SC, Yu SY, et al. A multicenter randomized phase II trial on Kanglaite Injection (KLT) plus gemcitabine hydrochloride (GEM) versus GEM in patients with local advanced and metastatic pancreatic cancer. Journal of Clinical Oncology 2011;29:e14510. [Google Scholar]
Sunamura 2004 {published data only}
- Sunamura M, Karasawa K, Okamoto A, Ogata Y, Nemoto K, Hosotani R, et al. Phase III trial of radiosensitizer PR‐350 combined with intraoperative radiotherapy for the treatment of locally advanced pancreatic cancer. Pancreas 2004;28(3):330‐4. [DOI] [PubMed] [Google Scholar]
Tagliaferri 2013 {published data only}
- Tagliaferri MA, Schwartzberg LS, Chen MM, Camacho LH, Kaplan EH, Arena FP, et al. A phase IIb trial of coix seed injection for advanced pancreatic cancer. Journal of Clinical Oncology 2013;31:e15023. [Google Scholar]
Takada 1994 {published data only}
- Takada T, Kato H, Matsushiro T, Nimura Y, Nagakawa T, Nakayama T. Comparison of 5‐fluorouracil, doxorubicin and mitomycin C with 5‐fluorouracil alone in the treatment of pancreatic‐biliary carcinomas. Oncology 1994;51(5):396‐400. [DOI] [PubMed] [Google Scholar]
Topham 1993 {published data only}
- Topham C, Glees J, Coombes R C. Comparison of single‐agent epirubicin and 5‐fluorouracil/epirubicin/mitomycin in patients with advanced adenocarcinoma of the pancreas. Oncology 1993;50(Suppl 1):78‐80. [DOI] [PubMed] [Google Scholar]
Trouilloud 2012 {published data only}
- Trouilloud I, Dupont‐Gossard AC, Artru P, Lecomte T, Gauthier M, Aparicio T, et al. FOLFIRI.3 (CPT‐11 plus folinic acid plus 5‐FU) alternating with gemcitabine or gemcitabine (G) alone in patients (pts) with previously untreated metastatic pancreatic adenocarcinoma (MPA): results of the randomized multicenter AGEO phase II trial FIRGEM. Journal of Clinical Oncology 2012;30:4018. [Google Scholar]
Tuinmann 2008 {published data only}
- Tuinmann G, Mueller L, Hossfeld D, Bokemeyer C. A randomised phase II study of gemcitabine versus mitomycin C versus gemcitabine/mitomycin C in patients with advanced pancreatic cancer. Journal of Clinical Oncology 2008;26:15658. [Google Scholar]
Ulrich‐Pur 2003 {published data only}
- Ulrich‐Pur H, Raderer M, Verena Kornek G, Schull B, Schmid K, Haider K, et al. Irinotecan plus raltitrexed vs raltitrexed alone in patients with gemcitabine‐pretreated advanced pancreatic adenocarcinoma. British Journal of Cancer 2003;88(8):1180‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Cutsem 2004 {published data only}
- Cutsem E, Velde H, Karasek P, Oettle H, Vervenne WL, Szawlowski A, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. Journal of Clinical Oncology 2004;22(8):1430‐8. [DOI] [PubMed] [Google Scholar]
Van Cutsem 2009 {published data only}
- Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Laethem JL, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. Journal of Clinical Oncology 2009;27(13):2231‐7. [DOI] [PubMed] [Google Scholar]
Van Cutsem 2013 {published data only}
- Cutsem E, Fram RJ, Schlichting M, Ryan DP. Efficacy and safety of gemcitabine in combination with TH‐302 compared with gemcitabine in combination with placebo in previously untreated patients with metastatic or locally advanced unresectable pancreatic adenocarcinoma: the MAESTRO trial. Journal of Clinical Oncology 2013;31:TPS4148. [Google Scholar]
Van Cutsem 2014 {published data only}
- Cutsem E, Li CP, Nowara E, Aprile G, Moore M, Federowicz I, et al. Dose escalation to rash for erlotinib plus gemcitabine for metastatic pancreatic cancer: the phase II RACHEL study. British Journal of Cancer 2014;111(11):2067‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Cutsem 2015 {published data only}
- Cutsem E, Hidalgo M, Bazin I, Canon JL, Pddubskaya E, Manojlovic N, et al. Phase II randomized trial of MEK inhibitor pimasertib or placebo combined with gemcitabine in the first‐line treatment of metastatic pancreatic cancer. Journal of Clinical Oncology 2015;33:344. [Google Scholar]
Von Hoff 1990 {published data only}
- Hoff DD, Fleming TR, Macdonald JS, Goodman PJ, Damme J, Brown TD, et al. Phase II evaluation of recombinant gamma‐interferon in patients with advanced pancreatic carcinoma: a Southwest Oncology Group study. Journal of Biological Response Modifiers 1990;9(6):584‐7. [PubMed] [Google Scholar]
Von Hoff 2014 {published data only}
- Hoff D, Li CP, Wang‐Gillam A, Bodoky G, Dean A, Jameson G, et al. Napoli‐1: Randomized phase 3 study of MM‐398 (Nal‐Iri), with or without 5‐fluorouracil and leucovorin, versus 5‐fluorouracil and leucovorin, in metastatic pancreatic cancer progressed on or following gemcitabine‐based therapy. Annals of Oncology 2014;25:2. [Google Scholar]
Voorthuizen 2006 {published data only}
- Voorthuizen TV, Vervenne WL, Daalen EH, Phoa SS, Gouma DJ, Bruno MJ, et al. A randomized phase II study comparing gemcitabine plus nadroparine versus gemcitabine in patients with locally advanced or metastatic pancreatic carcinoma: the GEMFRAX trial. Journal of Clinical Oncology 2006;24:4112. [Google Scholar]
Wagener 2002 {published data only}
- Wagener DJ, Wils JA, Kok TC, Planting A, Couvreur ML, Baron B. Results of a randomised phase II study of cisplatin plus 5‐fluorouracil versus cisplatin plus 5‐fluorouracil with alpha‐interferon in metastatic pancreatic cancer: an EORTC gastrointestinal tract cancer group trial. European Journal of Cancer 2002;38(5):648‐53. [DOI] [PubMed] [Google Scholar]
Wang 2000 {published data only}
- Wang B, Liu X, Wu Z. Effect of qi replenishing and blood circulation activating drugs in treatment of middle‐advanced pancreatic cancer with radio‐ and chemotherapy. Zhongguo Zhong Xi Yi Jie He za Zhi [Chinese Journal of Integrated Traditional and Western Medicine] 2000;20(10):736‐8. [PubMed] [Google Scholar]
Wang 2004 {published data only}
- Wang DM, Liu YH, Yu SP, Duan XN, Yang YM, Wan YL, et al. Intraoperative 125I brachytherapy combined with chemotherapy for pancreatic cancer. Zhonghua Zhong Liu za Zhi [Chinese Journal of Oncology] 2004;26(7):433‐6. [PubMed] [Google Scholar]
Wang 2015 {published data only}
- Wang JP, Wu CY, Yeh YC, Shyr YM, Wu YY, Kuo CY, et al. Erlotinib is effective in pancreatic cancer with epidermal growth factor receptor mutations: a randomized, open‐label, prospective trial. Oncotarget 2015;6(20):18162‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiedenmann 2008 {published data only}
- Wiedenmann B, Malfertheiner P, Friess H, Ritch P, Arseneau J, Mantovani G, et al. A multicenter, phase II study of infliximab plus gemcitabine in pancreatic cancer cachexia. Journal of Supportive Oncology 2008;6(1):18‐25. [PubMed] [Google Scholar]
Wilkowski 2009 {published data only}
- Wilkowski R, Boeck S, Ostermaier S, Sauer R, Herbst M, Fietkau R, et al. Chemoradiotherapy with concurrent gemcitabine and cisplatin with or without sequential chemotherapy with gemcitabine/cisplatin vs chemoradiotherapy with concurrent 5‐fluorouracil in patients with locally advanced pancreatic cancer‐‐a multi‐centre randomised phase II study. British Journal of Cancer 2009;101(11):1853‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolpin 2013 {published data only}
- Wolpin BM, O'Reilly EM, Ko YJ, Blaszkowsky LS, Rarick M, Rocha‐Lima CM, et al. Global, multicenter, randomized, phase II trial of gemcitabine and gemcitabine plus AGS‐1C4D4 in patients with previously untreated, metastatic pancreatic cancer. Annals of Oncology 2013;24(7):1792‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 2006 {published data only}
- Wright JA, Osterlee J, Fekete S, Lee Y, Young AH. A phase III trial of virulizin plus gemcitabine vs. gemcitabine alone in advanced pancreatic cancer: results of subgroup analysis. Journal of Clinical Oncology 2006;24:4116. [Google Scholar]
Yamaue 2015 {published data only}
- Yamaue H, Tsunoda T, Tani M, Miyazawa M, Yamao K, Mizuno N, et al. Randomized phase II/III clinical trial of elpamotide for patients with advanced pancreatic cancer: PEGASUS‐PC Study. Cancer Science 2015;106:883‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yongxiang 2001 {published data only}
- Yongxiang W, Tao W, Zongzheng J, Xi C, Liang G. Peripancreatic arterial ligation combined with arterial infusion regional chemotherapy for treating patients with advanced pancreatic carcinoma. Journal of Xi'an Medical University (English Edition) 2001;13(2):94‐7. [Google Scholar]
Yoo 2009 {published data only}
- Yoo C, Hwang JY, Kim JE, Kim TW, Lee JS, Park DH, et al. A randomised phase II study of modified FOLFIRI.3 vs modified FOLFOX as second‐line therapy in patients with gemcitabine‐refractory advanced pancreatic cancer. British Journal of Cancer 2009;101(10):1658‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zemskov 2000 {published data only}
- Zemskov VS, Procopchuk OL, Susak YM, Zemskov SV, Hodysh YY, Zemskova MV. Ukrain (NSC‐631570) in the treatment of pancreas cancer. Drugs under Experimental and Clinical Research 2000;26(5‐6):179‐90. [PubMed] [Google Scholar]
Zhang 2007 {published data only}
- Zhang Q, Wang XM, Chi HC. Effects of Guben Yiliu II combined with arterial perfusion with chemotherapeutic agent in treating advanced pancreatic cancer. Zhongguo Zhong Xi Yi Jie He za Zhi [Chinese Journal of Integrated Traditional and Western Medicine] 2007;27(5):400‐3. [PubMed] [Google Scholar]
Additional references
Bailey 2016
- Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016;531(7592):47‐52. [DOI] [PubMed] [Google Scholar]
Bernstein 2014
- Bernstien M, Kaubisch A, Rosenstein M, Aparo S, Garg MK, Kalnicki S, et al. Chemotherapy alone versus chemoradiation for unresectable pancreatic cancer: a meta‐analysis. International Journal of Radiation Oncology 2014;90(1 Suppl):S363‐4. [Google Scholar]
Callery 2009
- Callery MP, Chang KJ, Fishman EK. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Annals of Surgical Oncology 2009;16:1727‐33. [DOI] [PubMed] [Google Scholar]
Carpelan 2005
- Carpelan‐Holmstrom M, Nordling S, Pukkala E, Sankila R, Luttges J, Kloppel G, et al. Does anyone survive pancreatic ductal adenocarcinoma? A nationwide study re‐evaluating the data of the Finnish Cancer Registry. Gut 2005;54:385‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castellanos 2011
- Castellanos E, Berlin J, Cardin DB. Current treatment options for pancreatic carcinoma. Current Oncology Reports 2011;13(3):195‐205. [DOI] [PubMed] [Google Scholar]
Chan 2014
- Chan K, Shah K, Lien K, Coyle D, Lam H, Ko YJ. A Bayesian meta‐analysis of multiple treatment comparisons of systemic regimens for advanced pancreatic cancer. PLOS ONE 2014;9(10):e108749. [DOI: 10.1371/journal.pone.0108749] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2013
- Chen Y, Sun XJ, Jiang TH, Mao AW. Combined radiochemotherapy in patients with locally advanced pancreatic cancer: a meta‐analysis. World Journal of Gastroenterology 2013;19(42):7461‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Conlon 1996
- Conlon KC, Klimstra DS, Brennan MF. Long term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5‐year survivors. Annals of Surgery 1996;223:273‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ernst 2005
- Ernst E, Schmidt K. Ukrain ‐ a new cancer cure? A systematic review of randomised clinical trials. BMC Cancer 2005;5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gresham 2014
- Gresham GK, Wells GA, Gill S, Camerson C, Jonker DJ. Chemotherapy regimens for advanced pancreatic cancer: a systematic review and network meta‐analysis. BMC Cancer 2014;14:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Heinemann 2008
- Heinemann V, Boeck S, Hinke A, Labianca R, Louvet C. Meta‐analysis of randomized trials: evaluation of benefit from gemcitabine‐based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer 2008;8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Howard 1977
- Howard JM, Jordan GL. Cancer of the pancreas. Current Problems in Cancer 1977;2(3):5‐52. [DOI] [PubMed] [Google Scholar]
Kim 2007
- Kim R, Saif MW. Is there an optimal neoadjuvant therapy for locally advanced pancreatic cancer?. Journal of Oncology Practice 2007;8:279‐88. [PubMed] [Google Scholar]
Li 2014
- Li Q, Yan H, Liu W, Zhen H, Yang Y, Cao B. Efficacy and safety of gemcitabine‐fluorouracil combination therapy in the management of advanced pancreatic cancer: a meta‐analysis of randomized controlled trials. PLOS ONE 2014;9(8):e104346. [DOI: 10.1371/journal.pone.0104346] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nagrial 2015
- Nagrial AM, Chin VT, Sjoquist KM, Pajic M, Horvath LG, Biankin AV, et al. Second‐line treatment in inoperable pancreatic adenocarcinoma: a systematic review and synthesis of all clinical trials. Critical Reviews in Oncology/hematology 2015;96(3):483. [DOI] [PubMed] [Google Scholar]
Nishino 2010
- Nishino M, Jagannathan JP, Ramaiya NH, Abbeele AD. Revised RECIST guideline version 1.1: what oncologists want to know and what radiologists need to know. American Journal of Roentgenology 2010;195(2):281‐9. [DOI] [PubMed] [Google Scholar]
Petrelli 2014
- Petrelli F, Coinu A, Borgonovo K, Cabiddu M, Ghilardi M, Barni S. Polychemotherapy or gemcitabine in advanced pancreatic cancer: a meta‐analysis. Digestive and Liver Diseases 2014;46(5):452‐9. [DOI] [PubMed] [Google Scholar]
Philip 2011
- Philip PA. Locally advanced pancreatic cancer: where should we go from here?. Journal of Clinical Oncology 2011;29:4066‐8. [DOI] [PubMed] [Google Scholar]
Queensland Cancer Fund 2012
- Queensland Cancer Fund. Understanding Radiotherapy. A Guide for People with Cancer, their Families and Friends. Brisbane: Cancer Council Australia, 2012. [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html. The GRADE Working Group.
Shahrudin 1997
- Shahrudin MD. Carcinoma of the pancreas: resection outcome at the University Hospital Kuala Lumpur. International Surgery 1997;82:269‐74. [PubMed] [Google Scholar]
Siegel 2013
- Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2013. CA: A Cancer Journal for Clinicians 2013;63:11‐30. [DOI] [PubMed] [Google Scholar]
Sultana 2007
- Sultana A, Tudur Smith C, Cunningham D, Starling N, Neoptolemos JP, Ghaneh P. Meta‐analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. Journal of Clinical Oncology 2007;25(18):2607‐15. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8(June):16. [DOI: 10.1186/1745-6215-8-16] [DOI] [PMC free article] [PubMed] [Google Scholar]
Torre 2015
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global Cancer Statistics 2012. CA: A Cancer Journal for Clinicians 2015;65:87‐108. [DOI] [PubMed] [Google Scholar]
Tracey 2010
- Tracey E, Ling L, Baker D, Dobrovic A, Bishop J. Cancer in New South Wales: Incidence and Mortality 2007. Sydney: Cancer Institute NSW, 2010. [Google Scholar]
Von Hoff 2005
- Hoff DD, Evans DB, Hruban R. Pancreatic Cancer. Sudbury: Jones and Bartlett Publishers, 2005:158. [Google Scholar]
Warshaw 1992
- Warshaw AL, Fernandez‐del Castillo C. Pancreatic carcinoma. New England Journal of Medicine 1992;326:455‐65. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Yip 2009
- Yip D, Karapetis C, Strickland A, Steer CB, Goldstein D. Chemotherapy and radiotherapy for inoperable advanced pancreatic cancer. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD002093.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


