Abstract
Background
Hyaluronidase has been used over many decades as an adjunct to local anaesthetic solution to improve the speed of onset of eye blocks and to provide better akinesia and analgesia. With the evolution of modern eye surgery techniques, fast onset and akinesia are not essential requirements anymore. The assumption that the addition of hyaluronidase to local anaesthetic injections confers better analgesia for the patient needs to be examined. There has been no recent systematic review to provide evidence that hyaluronidase actually improves analgesia.
Objectives
To ascertain if adding hyaluronidase to local anaesthetic solutions for use in ophthalmic anaesthesia in adults results in a reduction of perceived pain during the operation and to assess harms, participant and surgical satisfaction, and economic impact.
Search methods
We carried out systematic searches in Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and four other databases in June 2017. We searched the trial registers at www.ISRCTN.com, ClinicalTrials.gov and www.clinicaltrialsregister.eu for relevant trials. We imposed no language restrictions.
Selection criteria
We included randomized controlled trials (RCTs) that evaluated the effect of hyaluronidase on pain experienced by adults during intraocular surgery using a rating scale.
Data collection and analysis
Two review authors (HR and KA) independently extracted data and assessed methodological quality using standard procedures as expected by Cochrane.
Main results
We included seven trials involving 500 participants that studied the effect of hyaluronidase on intraoperative pain. Four of the seven trials with 289 participants reported the primary outcome in a dichotomous manner, and we proceeded to meta‐analyse the findings which showed a moderate heterogeneity that could not be explained (I2 = 41% ). The pooled risk ratio (RR) for these four trials was 0.83 with the 95% confidence interval ranging from 0.48 to 1.42. The reduction in intraoperative pain scores in the hyaluronidase group were not statistically significant. Among the three trials that reported the primary outcome in a continuous manner, the presence of missing data made it difficult to conduct a meta‐analysis. To further explore the data, we imputed standard deviations for the other studies from another included RCT (Sedghipour 2012). However, this resulted in substantial heterogeneity between study estimates (I² = 76% ). The lack of reported relevant data in two of the three remaining trials made it difficult to assess the direction of effect in a clinical setting.
Overall, there was no statistical difference regarding the intraoperative reduction of pain scores between the hyaluronidase and control group. All seven included trials had a low risk of bias.
According to GRADE, we found the quality of evidence was low and downgraded the trials for serious risk of inconsistency and imprecision. Therefore, the results should be analysed with caution.
Participant satisfaction scores were significantly higher in the hyaluronidase group in two high quality trials with 122 participants. Surgical satisfaction was also superior in two of three high quality trials involving 141 participants. According to GRADE, the quality of evidence was moderate for participant and surgical satisfaction as the trials were downgraded for imprecision due to the small sample sizes. The risk of bias in these trials was low.
There was no reported harm due to the addition of hyaluronidase in any of the studies. No study reported on the cost of hyaluronidase in the context of eye surgery.
Authors' conclusions
The effects of adding hyaluronidase to local anaesthetic fluid on pain outcomes in people undergoing eye surgery are uncertain due to the low quality of evidence available. A well designed RCT is required to address inconsistency and imprecision among the studies and to determine the benefit of hyaluronidase to improve analgesia during eye surgery. Participant and surgical satisfaction is higher with hyaluronidase compared to the control groups, as demonstrated in moderate quality studies. There was no harm attributed to the use of hyaluronidase in any of the studies. Considering that harm was only rarely defined as an outcome measure, and the overall small number of participants, conclusions cannot be drawn about the incidence of harmful effects of hyaluronidase. None of the studies undertook cost calculations with regards to use of hyaluronidase in local anaesthetic eye blocks.
Plain language summary
Addition of hyaluronidase to local anaesthetic eye blocks to reduce pain during eye surgery in adults.
Review question
We reviewed the evidence on the effectiveness of adding hyaluronidase to local anaesthetic eye block solutions (a numbing medicine injected into the eye to block nerves) to reduce pain and increase participant and surgical satisfaction during eye surgery in adults. We also looked for reports on side effects and cost.
Background
Hyaluronidase is an enzyme (a protein that regulates a chemical reaction in the body) that helps the spread of local anaesthetic through the tissues around the eye. It is widely used as an additive to local anaesthetic eye blocks to give more rapid onset of anaesthesia and reduce or block movement of the eye (called akinesia). With modern eye surgery techniques, fast onset and akinesia are no longer essential requirements, and often surgery can be undertaken pain‐free with topical (on the surface of the eye) anaesthesia alone. Hyaluronidase has been associated with infrequent side effects. Therefore, the use of hyaluronidase needs to be justified, which was the aim of this review.
Search date
The review is current to 30 June 2017.
Study characteristics
We included seven randomized controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) in our review. These involved 500 adults undergoing eye surgery under local anaesthesia. We looked at any additional effect of adding hyaluronidase to local anaesthetic on the pain experienced during eye surgery. We also looked at participant and surgical satisfaction scores and if any harms were reported after using hyaluronidase in the injection solution. None of the studies reported on costs.
Key results
Of the seven included trials, we pooled the results of four trials (289 participants) as the results were reported in a similar manner. They found that addition of hyaluronidase did not significantly reduce pain during surgery. Among the three remaining trials (211 participants) lack of data reporting in two trials made it difficult to pool the results. The overall result of looking at all these trials together suggests there was no significant reduction of pain with using hyaluronidase in eye nerve blocks.
We found moderate quality evidence from two trials (122 participants) to suggest that addition of hyaluronidase increased participant satisfaction scores. Three studies involving 141 participants looked at surgical satisfaction, which was reported as superior with hyaluronidase in the two larger studies and not significantly different in one small study (19 participants). None of the included studies reported any harmful effects of hyaluronidase.
Quality of evidence
The included trials that reported on pain during surgery were at low risk for bias. The overall quality of evidence was low because of variations in the effect on pain reduction. We contacted all trial authors to request more information on the trials, but the data were not available.
Moderate quality studies reported greater participant and surgical satisfaction with hyaluronidase.
Analgesia alone does not take into account the full spectrum of the beneficial effects of hyaluronidase. Patient comfort with the eye surgery is also likely to be improved by a speedy onset and reduced eye movements due to hyaluronidase.
Summary of findings
Summary of findings for the main comparison. Use of hyaluronidase as an adjunct to local anaesthetic eye blocks to reduce intraoperative pain in adults.
| Use of hyaluronidase as an adjunct to local anaesthetic eye blocks to reduce intraoperative pain in adults | ||||||
| Patients or population: adults (aged ≥ 18 years) undergoing ophthalmic surgery under local anaesthetic eye blocks. Setting: hospitals in the UK (4), Germany (1), Brazil (1) and Iran (1). Intervention: local anaesthetic eye blocks containing hyaluronidase. Comparison: local anaesthetic eye blocks containing no hyaluronidase. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no hyaluronidase | Risk with hyaluronidase | |||||
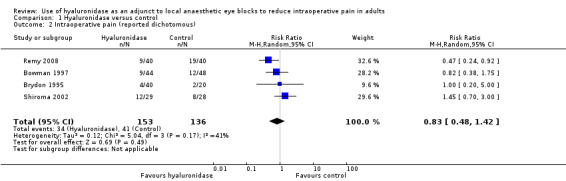
| Intraoperative pain (reported dichotomous) assessed with: analogue rating scales No follow‐up ‐ measured on day of surgery. | RR 0.83 (0.48 to 1.42) | 289 (4 RCTs) | ⊕⊕⊝⊝ Low1,2 | ‐ | ||
| 301 per 1000 | 250 per 1000 (145 to 428) | |||||
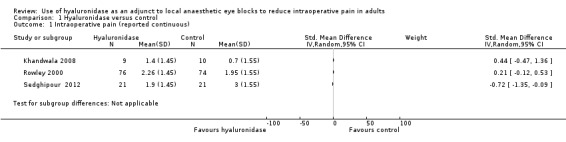
| Intraoperative pain (reported continuous) assessed with: analogue rating scales No follow‐up ‐ measured on day of surgery. | 3 trials looked at effect of hyaluronidase on reduction of intraoperative pain measured by rating scales. Results were reported as continuous data. 2 studies did not provide the SMD, which measures the effect in a clinical setting, the results could not be meta‐analysed and hence were reported narratively (Khandwala 2008; Rowley 2000). Among the 3 trials covering 211 participants (Khandwala 2008: Mean difference 0.70; Rowley 2000: Mean difference 0.31; Sedghipour 2012: Mean difference ‐1.10), only the Sedghipour study with 42 participants, which is a high quality study, showed a statistically significant (at the 5 % level) reduction in pain in the hyaluronidase group (P = 0.04). The remaining 2 studies with 169 participants showed no statistically significant (at the 5 % level) reduction of pain intraoperatively with hyaluronidase (Khandwala 2008: P = 0.5; Rowley 2000: n.s). These studies were also of high quality and low risk of bias. Khandwala and colleagues had an unclear attrition bias as 1/10 participants in the treatment group was dropped after randomization with no clear explanation. | ‐ | 211 (3 RCTs) | ⊕⊕⊝⊝ Low3 | ‐ | |
| Incidence of harm | None of the studies reported harms in relation to hyaluronidase. | ‐ | (0 studies) | ‐ | ‐ | |
| Participant satisfaction assessed with: scoring system No follow‐up ‐ measured on day of surgery. | Significantly better satisfaction in these well designed studies with low risk of bias (Remy 2008; Sedghipour 2012). The studies included 122 participants and showed higher satisfaction scores in the treatment group (P < 0.05). | ‐ | 122 (2 RCTs) | ⊕⊕⊕⊝ Moderate4 | ‐ | |
| Surgical satisfaction assessed with: scoring system No follow‐up ‐ measured on the day of surgery. | Surgical satisfaction was reportedly superior with hyaluronidase in the larger 2 studies (Remy 2008: P < 0.001; Sedghipour 2012: P = 0.02) and not significantly different in 1 small study (Khandwala 2008: P = 0.96). | ‐ | 141 (3 RCTs) | ⊕⊕⊕⊝ Moderate4 | ‐ | |
| Economic outcomes or cost calculations | None of the included studies reported economic outcomes or cost calculations | ‐ | (0 RCTs) | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; n.s: not statistically significant; RCT: randomized controlled trial; RR: risk ratio; SMD: standardized mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one level due to marked heterogeneity with a calculated I2 > 50%.
2Downgraded one level for imprecision due to wide 95% confidence intervals, reflecting uncertainty in the direction of effect estimate.
3Downgraded one level for imprecision and inconsistency in measurement, lack of data and small sample size.
4Downgraded one level because of imprecision secondary to small sample size.
Background
Description of the condition
Local anaesthesia for ophthalmic surgery can be provided by regional injection block or topical anaesthesia alone. The most frequently used anaesthetic injections are retrobulbar, peribulbar and sub‐Tenon's block.
During a local anaesthetic injection for ophthalmic surgery, the objective is to deliver local anaesthetic fluid to the sensory and motor nerve fibres in the orbit. There is a large variation of techniques, anaesthetic mixtures and instruments in use to achieve this. Some of these techniques have been compared in Cochrane Reviews; for example, peribulbar versus retrobulbar block (Alhassan 2015), and topical anaesthesia alone versus sub‐Tenon's block (Guay 2015). Schein 2000 undertook a comprehensive systematic review of anaesthetic interventions.
Unfortunately, there is always a proportion of blocks that fail to provide adequate analgesia or akinesia. To improve the quality of the anaesthetic block, various adjuncts to the local anaesthetic fluid have been introduced.
Description of the intervention
Atkinson 1949 first described the addition of hyaluronidase to local anaesthetic fluid with the intention of improving the speed of onset of analgesia and akinesia. Subsequently, hyaluronidase has commonly been added to local anaesthetic injection fluid for this purpose.
How the intervention might work
For any local anaesthetic block to work, the local anaesthetic fluid needs to spread through the orbital cavity to reach the relevant motor and sensor fibres. A complex system of connective tissue membranes divides the orbital space, thereby impeding the spread of local anaesthetic fluid (Koornneef 1988).
Buhren 2016 described the molecular mechanisms of how hyaluronidase spreads through this connective tissue barrier, in the most recent review on this topic. The main components of connective tissue are the fibrous proteins collagen and elastin as well as proteoglycans located in the extracellular matrix. Glycosamine‐glycans attach to proteoglycans in a characteristic manner giving the connective tissue its viscoelastic properties. The most common glycosamine‐glycan is hyaluronic acid, it is a linear glycosaminoglycan disaccharide composed of alternating units of N‐acetyl‐D‐glucosamine and D‐glucuronic acid via alternating ß(1‐4) and ß(1‐3) glycosidic bonds. Hyaluronidase (hyaluronoglucosaminidase) is an enzyme that facilitates the spread of local anaesthetic fluid through connective tissue by degrading hyaluronic acid into smaller fragments and hydrolyzing the disaccharides at hexosaminidic ß(1‐4) linkages.
Meyer 1934 first extracted hyaluronidase. Preparations contain purified ovine testicular hyaluronidase as a dehydrated sterilized solid for reconstitution before use. Brand names of animal‐derived hyaluronidase include Hydase, Vitrase, Amphadase, Wydase and Hyalase. Apart from a preparation of ovine testicular hyaluronidase, a recombinant human hyaluronidase is also available as Hylenex. It is produced by genetically engineered Chinese hamster ovary cells containing a DNA plasmid encoding for a soluble fragment of human hyaluronidase (Hylenex® Prescribing Information 2016). The exact chemical structure of this enzyme is unknown. The approximate molecular weight is 61,000 daltons (Borders 1968).
Hyaluronidase also alters the pH of a local anaesthetic due to the presence of phosphate buffers within the preparation. The pH of plain bupivacaine solution is changed from 5.3 to 6.3 following the addition of hyaluronidase, and it may maintain local anaesthetic solubility during the process of alkalinization (Roberts 1993). This alkalinization may also explain any improved anaesthesia and akinesia.
A reduction in time to onset of surgical anaesthesia is considered desirable to facilitate patient throughput. The action of hyaluronidase may promote rapid onset of anaesthesia and akinesia. The minimum and maximum effective doses of hyaluronidase are unknown. The doses used range from 0.75 IU/mL to 300 IU/mL (Dempsey 1997).
Why it is important to do this review
We conducted this systematic review to explore the uncertainty about the benefits of using hyaluronidase in local anaesthetic mixtures to provide analgesia during eye surgery. There is considerable variation of practice, and the studies in this area show conflicting results.
Furthermore, the use of hyaluronidase increases the cost of the anaesthetic and has been associated with adverse allergic reactions in a small number of cases. For example, Kempeneers 1992, described five people who developed an orbital pseudotumour as a complication of retrobulbar anaesthesia. Allergic reactions can range from local reactions to anaphylactic (systemic allergy) shock. When hyaluronidase is added to a local anaesthetic agent, the wider spread of the local anaesthetic solution also increases its absorption and removal in the bloodstream. This shortens the duration of action of the local anaesthetic and tends to increase the incidence of systemic reactions. Some hyaluronidase products contain bovine ingredients, and due to the theoretical concerns about transmissible spongiform encephalopathies, the World Health Organization (WHO) issued guidelines regarding the use of bovine materials in the manufacture of biological and pharmaceutical products (WHO 2010).
The absence of hyaluronidase in ophthalmic regional blockade has also been associated with adverse events. An interruption in hyaluronidase supply was associated with a cluster of postoperative diplopia (double vision) Brown 1999. It was postulated that the absence of hyaluronidase caused the local anaesthetic to loculate in close proximity to the extraocular muscles and cause clinically significant myotoxicity. When hyaluronidase was unavailable once again in 2000, Brown 2001 published a repeated cluster of diplopia cases. The omission of hyaluronidase in local anaesthesia fluid leads to clinically important rises in intraocular pressure. This is thought to be due to the decreased removal and dispersal of local anaesthetic fluid from the periocular compartment (Dempsey 1997).
Therefore, use of hyaluronidase must be justified, and data must be available for clinicians and patients to make an informed decision regarding the efficacy of hyaluronidase addition. The results of this review should allow justification (or not) for the use of adjuvant hyaluronidase to improve the quality of anaesthesia and analgesia.
Objectives
To ascertain if adding hyaluronidase to local anaesthetic solutions for use in ophthalmic anaesthesia in adults results in a reduction of perceived pain during the operation and to assess harms, participant and surgical satisfaction and economic impact.
Methods
Criteria for considering studies for this review
Types of studies
We included:
Randomized controlled trials (RCTs) and quasi‐randomized controlled clinical trials either published or unpublished;
Studies if they compared equal volumes and concentrations of local anaesthetic with and without adjuvant hyaluronidase administered with the injection;
Studies when other adjuvants such as adrenaline were used, only if the adjuvant was present in both the control and hyaluronidase intervention;
Types of participants
We included:
Adults (aged 18 years and older) presenting for ophthalmic surgery under ophthalmic anaesthetic block;
Participants receiving sub‐Tenon's, peribulbar, retrobulbar or other types of local anaesthetic;
Participants receiving sedation but documented this fact;
We excluded:
Participants receiving adnexal surgery and any other eye surgery that was not intraocular;
Participants who received general anaesthesia;
Types of interventions
Ophthalmic local anaesthetic blocks comparing adjuvant hyaluronidase to an otherwise equal anaesthetic and surgery without hyaluronidase.
We considered any dose of hyaluronidase in the intervention group and any dose or type of local anaesthetic agent.
We included studies that used any number of injections to anaesthetise the eye if the number of injections was equal in the treatment and control groups.
Types of outcome measures
Primary outcomes
Intraoperative pain, as measured by analogue rating scales. We excluded studies that reported pain, but did not measure pain formally using analogue rating scales, as they did not provide sufficiently useful information on the outcome. We excluded studies if they reported that 'supplementary injections' were primarily given to achieve akinesia, for example, if a certain immobility score was not reached.
Secondary outcomes
Incidence of harm (reported as a narrative).
Participant and surgical satisfaction, as documented by scoring systems.
Economic outcomes or cost calculations (reported as a narrative).
Search methods for identification of studies
Electronic searches
We carried out systematic searches in:
The Cochrane Central Register of Controlled Trials( CENTRAL, 2007 Issue 6; Appendix 1);
Ovid MEDLINE (1946 to 30 June 2017; Appendix 2);
Ovid Embase (1947 to 30 June 2017; Appendix 3);
Web of Science (1900 to 30 June 2017; Appendix 4);
Scopus (1823 to 30 June 2017; Appendix 5);
CINAHL Plus (EBSCOhost, 1937 to 30 June 2017; Appendix 6);
LILACS (1982 to 30 June 2017; Appendix 7);
We broke down our research question into four key searchable concepts: " Eye," "Surgery," " Local Anaesthesia" and "Hyaluronidase".This strategy ensured that we retrieved studies on eye surgery where local anaesthesia was applied along with hyaluronidase. We conducted searches for each concept using free text terms and MeSH terms wherever possible. When we carried out free text searches, we applied synonyms, derivative forms and singular/plural forms for each concept. Detailed search steps are documented in the 'Appendices'.
We applied no language restrictions.
Searching other resources
We searched the reference lists of all eligible trials and reviews and used any trials that fit the inclusion criteria.
We searched the registers at www.controlled‐trials.com, www.ISRCTN.com and www.clinicaltrialsregister.eu for relevant trials.
We contacted specialists in the field, authors of the included trials and pharmaceutical manufacturers for any unpublished data.
The search of these other resources was completed by 30 June 2017.
Data collection and analysis
Selection of studies
Two review authors (KA and HR) independently reviewed the trials identified from the search strategy, removed duplicates and documented the reason for each trial being excluded (see Characteristics of excluded studies table). We resolved any disagreements with the studies by input from a third review author CB). We presented information regarding methods, participants, setting, interventions and outcomes in the Characteristics of included studies table. Where studies had multiple publications, we planned to collate the reports of the same study so that each study, rather than each report, was the unit of interest for the review, and such studies had a single identifier with multiple references. (See Differences between protocol and review).
Data extraction and management
Two review authors (KA and HR) independently extracted and collected data on a paper form. After initial piloting this form was assessed and agreed for usability. A copy of this form is in Appendix 8. We (KA and HR) resolved any discrepancies in data extracted by discussion with a third review author (CB) as a final arbiter. In the case of additional information being required, HR or KA contacted the authors of the relevant trial.
Assessment of risk of bias in included studies
To assess the risk of bias, two review authors (KA and HR) independently assessed the studies included in the review according to the criteria described by Higgins 2011. We assessed the following aspects as being at either 'low risk', 'high risk' or 'unclear risk' of bias. We assessed the risk of bias for the following components of each trial.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Masking of participants and personnel (performance bias).
Masking of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other bias.
We included a 'Risk of bias' table as part of the Characteristics of included studies table based on Cochrane's tool for assessing the risk of bias, from the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 8:Higgins 2011). See Appendix 8 (data collection form) and Appendix 10 ('Risk of bias' table).
Measures of treatment effect
Intraoperative pain
We noted intraoperative pain measured using visual analogue scales (VAS) or verbal rating scales (VRS) when available and interpreted them as continuous data. We used the greatest intraoperative score reported. We planned to use the standardized mean difference (SMD) as an effect measure and 95% confidence intervals (CIs) to allow for the fact that different studies might have used different scales. However, because the only studies detected used the same scaling method, there was no need to use the SMD. If authors documented non‐normality of their data, we extracted medians and interquartile ranges and collated this information. (See Differences between protocol and review).
In the case of absolute numbers of participants experiencing pain where a rating scale was used but reported in a dichotomous manner (data as pain or no pain), we used risk ratios (RR) with 95% CI as a measure of effect. We collated this information and reported it. While there was evidence of moderate heterogeneity (I2 = 41%) we presented a meta‐analysis but urge caution interpreting this.
To avoid multiplicity, we restricted meta‐analyses to the primary outcomes but this would not be at the cost of presenting the totality of evidence should there be more RCTs providing information with regards to adverse effects.
Incidence of harm
We would have reported adverse events due to the use of hyaluronidase (e.g. allergic reactions) as a narrative, but as expected, there were no reports of such adverse events, probably because of their rarity and the relatively small number of participants in each trial.
Participant and surgical satisfaction
We reported participant and surgical satisfaction scores narratively. We would also have noted as narrative if other validated tools had been used.
Economic outcomes or cost calculation
We planned to report economic outcomes or cost calculations narratively.
Unit of analysis issues
We anticipated that most trials would involve one eye per participant, and even if both eyes were included, our outcomes were primarily measured at the participant rather than eye level. It was very unlikely that both eyes were operated on simultaneously with different anaesthetic procedures. (See Differences between protocol and review).
Dealing with missing data
We contacted authors and asked them to provide missing data. We imposed a time limit of two months and follow‐up on one occasion. Irrespective of the type of data, we reported dropout rates in the 'Risk of bias' tables within the Characteristics of included studies table and noted whether or not authors had compared characteristics of participants who had complete data sets against those that did not. We investigated studies that had missing data, whether or not imputing for missing cases impacted greatly on the interpretation of findings. We attempted to impute the data, but the values obtained would not be consistently reflected within the trials due to the variation in sample size.
Assessment of heterogeneity
We assessed all studies for clinical and methodological heterogeneity. We examined the I2 statistic and it's 95% CI to assess inconsistency between studies as recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used the thresholds advised by Higgins 2011, for the interpretation of the I2 statistic. We found substantial inconsistency in our primary outcome when assessed as a continuous score (I2 = 76%) but less when assessed as a dichotomous measure (I2 =41%). We attempted to investigate causes for this by exploring factors such as the type and duration of surgery, anaesthetic intervention (hyaluronidase dosage, type and volume of anaesthetic fluid) but the number of studies contributing to the meta‐analysis was small. Despite the heterogeneity, we presented a meta‐analysed outcome for the dichotomized outcome but urge caution in its interpretation.
Assessment of reporting biases
We assessed publication bias and small study effects in a qualitative manner using a funnel plot. We planned to test for funnel plot asymmetry if there had been a meta‐analysis with more than 10 studies included.
Data synthesis
We performed the analysis using Review Manager 5 (RevMan 2014).
While we found some evidence of heterogeneity (for the dichotomous outcome), we meta‐analysed results using a random‐effects model as per our original intentions. A random‐effects model was chosen because we believe that each trial estimates an intervention effect that follows a distribution across studies.
Subgroup analysis and investigation of heterogeneity
We planned no subgroup analyses.
Sensitivity analysis
We carried out sensitivity analyses to explore the robustness of the results to key methodological decisions that we made in our review. We examined whether or not excluding studies at risk of bias impacted on our findings, and since we had missing data, we attempted to impute and examine whether or not analysing intention‐to‐treat data differed considerably from the available case meta‐analysis.
GRADE assessment of quality of evidence
We adopted the GRADE system postprotocol (Rüschen 2013) to rate the quality of evidence for each outcome (Guyatt 2011). GRADE assessment classifies the quality of evidence into four categories; high, moderate, low and very low. The overall assessment considers the study design, risk of bias, imprecision, inconsistency, indirectness, publication bias, large effect size, dose‐response effect and presence of confounding factors to rate the evidence. We applied the principles of GRADE to assess the quality of evidence specific to each outcome in our review.
Intraoperative pain, as measured by analogue rating scales.
Incidence of harm.
Participant and surgical satisfaction, as documented by scoring systems.
Economic outcomes or cost calculations.
The GRADE software from GRADEpro GDT generated the 'Summary of findings' table. The GRADE approach ensures the confidence which one can have in the estimate of effect from the outcomes being assessed in the included studies.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
See Figure 1 for the study flow diagram.
1.
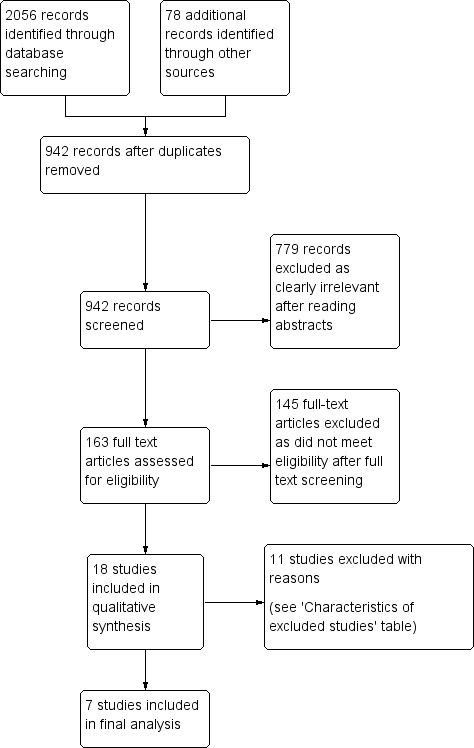
Study flow diagram.
We identified 942 references after removal of duplicates. Two review authors (HR and KA) independently read and analysed the abstracts of all references and if needed, full papers. We excluded 779 references as clearly irrelevant to the review. We obtained the full papers for the remaining 163 references and again analysed them independently. We identified 18 trials, and 11 trials were further excluded for reasons documented in the 'Characteristics of excluded studies' table. The review includes seven trials.
Included studies
Design
We included seven RCTs published from 1995 to 2012 with 500 participants (Bowman 1997; Brydon 1995; Khandwala 2008; Remy 2008; Rowley 2000; Sedghipour 2012; Shiroma 2002). One study was published in Portuguese and translated into English (Shiroma 2002).
Characteristics of study population
The review included 500 participants.The participants were adults (aged 18 years or older) presenting for ophthalmic surgery undergoing a retrobulbar, peribulbar or sub‐Tenon block. The mean age in the studies ranged from 66 to 77 years. Studies were balanced with regards to gender.
Setting
Four studies were conducted in the UK (Bowman 1997; Brydon 1995; Khandwala 2008; Rowley 2000). The remaining three studies were based in Germany (Remy 2008), Brazil (Shiroma 2002), and Iran (Sedghipour 2012).
Intervention
The seven trials studied the effect of adding hyaluronidase to a local anaesthetic mixture with the primary outcome measure of reduction of intraoperative pain. The participants were divided into a treatment group (hyaluronidase) and a control group (no hyaluronidase). The doses of hyaluronidase used ranged from 15 IU/mL to 150 IU/mL.
All seven trials assessed pain objectively using either the VAS or the VRS and compared a group with hyaluronidase to a group without hyaluronidase (Bowman 1997; Brydon 1995; Khandwala 2008; Remy 2008; Rowley 2000; Sedghipour 2012; Shiroma 2002).
One trial included three arms in their study looking at effects of no hyaluronidase and effects of hyaluronidase at 50 IU/mL and 150 IU/mL (Brydon 1995). We combined the results of the groups with different doses of hyaluronidase and compared them with the no hyaluronidase group.
Funding sources
Two trials reported no conflict of interest and received no funding support for the trials (Khandwala 2008; Remy 2008). There was no clear documentation of reported conflict of interest or funding support from the remaining five trials (Bowman 1997; Brydon 1995; Rowley 2000; Sedghipour 2012; Shiroma 2002).
We attempted to contact the authors of all the trials for additional data but received no clarification.
For more details about the included trials, see the Characteristics of included studies table.
Excluded studies
We excluded 11 studies after analysis (Berg 2001; Crawford 1994; Guise 1999; House 1991; Johansen 1993; Lange 1989; Moharib 2002; Morsman 1992; Ramanathan 1999; Sarvela 1992; Soares 2002).
Three studies reported that "supplementary injections" were primarily given to achieve akinesia, for example, if a certain immobility score was not reached. Pain during surgery was not assessed or reported specifically.(Crawford 1994; House 1991; Soares 2002).
One study did not mask the relevant part of the trial (Morsman 1992).
Four studies did not assess pain or discomfort using a rating scale, and the inclusion criteria were ultimately not met (Berg 2001; Guise 1999; Johansen 1993; Moharib 2002). We excluded these studies because they did not measure the outcome measure of "pain by rating scale". They reported an unstructured description of pain. This is unlikely to provide useful information about the levels of pain during the operation.
One study was published as a poster presentation (Ramanathan 1999). We contacted the author for more details about randomization, masking and results but none was made available.
We excluded one study because there was no randomization, this was not immediately obvious (Lange 1989).
We excluded one study because the relevant part of the study was not randomized and only compared different concentrations of hyaluronidase (Sarvela 1992).
See Characteristics of excluded studies table.
Awaiting classification
We found no studies awaiting classification.
Ongoing studies
We identified no ongoing studies.
Risk of bias in included studies
We judged the quality of studies according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Overall, the selected studies were of low risk; however, there was a lack of concise information in the methodology of randomization and withdrawal description. Please refer to Figure 2 and Figure 3 for a summary of risk of bias assessment for the selected studies and a 'Risk of bias' graph that represents the studies.
2.
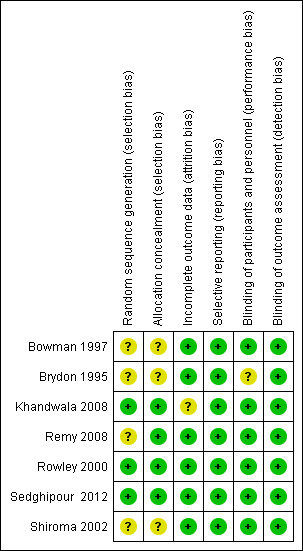
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
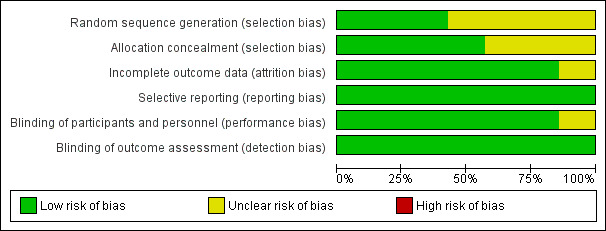
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
All seven included studies reported that allocation was randomized, but only three studies provided any detail about the method of random allocation (Khandwala 2008; Rowley 2000; Sedghipour 2012). These three studies were at low risk of allocation bias. The studies used random number tables, stratified lottery system or computer generated randomization. The remaining four studies were at unclear risk of bias.
With regard to allocation concealment, four of the seven studies used "coded syringes" (Khandwala 2008; Remy 2008; Rowley 2000; Sedghipour 2012). We classed these at low risk of bias, but an experienced operator might have recognized the formation of tiny bubbles in the mixture indicating hyaluronidase content.
Most studies implied that participants, personnel and assessors were unaware of the composition of the anaesthetic solution because the syringes were coded by an uninvolved third party, but exact details were rarely given.
In this context, only one study used a placebo control, with inactive Hyalase (Remy 2008).
Blinding
Five studies were classified as low risk for performance and detection bias (Bowman 1997; Khandwala 2008; Rowley 2000; Sedghipour 2012; Shiroma 2002). These studies had described double masking where neither the participant nor the caregiver was aware of the contents of the syringe. The outcome assessors were also masked, which further reduced the risk of bias by masking.
Incomplete outcome data
Withdrawal of participants after randomization was rarely reported in detail.
Six studies were at low risk as there was a description of no withdrawals; therefore, we had more confidence in the intention‐to‐treat analysis for these studies (Bowman 1997; Brydon 1995; Remy 2008; Rowley 2000; Sedghipour 2012; Shiroma 2002).
Of the included seven studies, one described the withdrawal of participants after randomization (Khandwala 2008). One participant was withdrawn from the treatment group due to incomplete data. No further details were given. Considering the low number of participants in this trial (10 in each of two arms) the exclusion may represent bias.
Selective reporting
We found all seven studies at low risk of reporting bias. Pain was stated as an outcome measure at the start of the trials. We did not attempt to obtain research protocols (Bowman 1997; Brydon 1995; Khandwala 2008; Remy 2008; Rowley 2000; Sedghipour 2012; Shiroma 2002).
Other potential sources of bias
We found no other potential sources of bias.
Effects of interventions
See: Table 1
Primary outcomes
1. Intraoperative pain, as measured by analogue rating scales
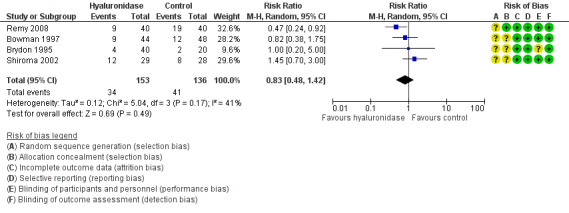
Seven trials with 500 participants looked at the effect of hyaluronidase on the reduction of intraoperative pain. Four of these trials with 289 participants reported intraoperative pain in a dichotomous manner (Bowman 1997; RR 0.82, 95% CI 0.38 to 1.75; Brydon 1995; RR 1.00, 95% CI 0.20 to 5.00; Remy 2008; RR 0.47, 95% CI 0.24 to 0.92; Shiroma 2002; RR 1.45, 95% CI 0.70 to 3.00). We calculated the (RR) as a measure of effect. The I2 statistic was 41%, which represents moderate heterogeneity. We proceeded to meta‐analyse the results and found that the pooled RR was 0.83 (95% CI 0.48 to 1.42). Therefore, there was no statistically significant reduction of pain scores in the hyaluronidase group.
Three studies involving 211 participants reported pain objectively using rating scales and presented continuous data (Khandwala 2008; Rowley 2000; Sedghipour 2012). Sedghipour 2012 described 42 participants in a high quality study and provided SDs for their data. They found a significant reduction of pain in the hyaluronidase group (P = 0.04). The other two trials did not report SDs (Khandwala 2008; Rowley 2000). We interchanged the SDs from Sedghipour 2012, but this resulted in significant heterogeneity among the studies (I²= 76%), so we did not perform a meta‐analysis. See Figure 4 and Figure 5.
4.
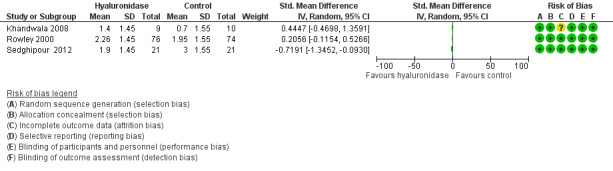
Forest plot of comparison: 1 Hyaluronidase versus control, outcome: 1.1 Intraoperative pain (measured by analogue rating scales; reported continuous).
5.
Forest plot of comparison: 1 Hyaluronidase versus control, outcome: 1.2 Intraoperative pain (measured by analogue rating scales; reported dichotomous).
Looking at the overall studies taking into account the results of the meta‐analysis and the individual studies that reported the continuous outcome, there was no statistically significant reduction in intraoperative pain with hyaluronidase in the local anaesthetic mixture. However, this has to be interpreted with caution.
We adopted the GRADEpro method of analysing the quality of evidence and produced Table 1. We found the quality of studies was low. We downgraded the quality of evidence due to concerns regarding inconsistency in the direction and magnitude of effect across the studies (I2 = 41% and 76%). We looked at the individual studies for factors that could have contributed to the heterogeneity and found that there was no wide variability between characteristics of participants, interventions and outcome measures. We tried to establish if the heterogeneity was due to the dose of hyaluronidase, the volume of injection or number of participants, but there was insufficient data provided to enable a valid analysis. The level of imprecision was another reason for downgrading the quality of evidence. Only Rowley 2000, provided a rationale for the selected sample size that would yield the specific effect measure.
Secondary outcomes
1. Incidence of harm
None of the included studies measured or reported the incidence of harm from hyaluronidase.
2. Participant and surgical satisfaction, as documented by scoring systems
Two studies analysing 122 participants looking at participant satisfaction reported that the investigator and participant assessment scores were significantly higher in the hyaluronidase group (P < 0.05)(Remy 2008; Sedghipour 2012). The studies assessed satisfaction in different ways so prohibiting meta‐analysis Remy 2008 used a five level VAS to assess participant efficacy and tolerability at the end of surgery and at the final visit, while Sedghipour 2012 captured data as a dichotomous 'satisfied' or 'unsatisfied'. Using the GRADE system to assess the quality of evidence, we found the studies were of moderate quality with low risk of bias.
We found three studies involving 141 participants that measured surgical satisfaction scores (Khandwala 2008; Remy 2008; Sedghipour 2012). Sedghipour 2012 reported that surgical satisfaction with intraoperative anaesthesia was 85.7% in the hyaluronidase group compared to 52.5% in the control group (P = 0.02). Remy 2008 reported a P value of less than 0.001 for surgical satisfaction in the hyaluronidase group. Khandwala 2008 found no difference in the quality of the surgical field between groups (P = 0.96). These studies assessed surgeon satisfaction as they had assessed participant satisfaction while Khandwala 2008 simply asked surgeons to rate surgical conditions on a VAS from 0 (worst) to 10 (best). Since each had assessed satisfaction using a different method, no meta‐analysis was conducted. There was low risk of bias with regards to the method of randomization with Sedghipour 2012, and there was incomplete outcome data reporting with Khandwala 2008. Overall, we found the quality of evidence with these two studies to be moderate. See Figure 2.
3. Economic outcomes or cost calculations
None of the included studies reported on the economic impact of using hyaluronidase.
Discussion
The use of hyaluronidase in ophthalmic surgery remains a topic of debate. The perceived advantages of adding hyaluronidase include shortened time to onset of the block and improved akinesia, and as investigated in this review: analgesia. The apparent disadvantages of hyaluronidase include the additional cost and possible adverse reactions.
Most modern surgical techniques are no longer essentially dependent on akinesia. Anaesthesia itself is readily provided by eye blocks without hyaluronidase. Even topical anaesthesia is often deemed sufficient during routine cataract surgery. We systematically reviewed the literature on the benefits of hyaluronidase for analgesia in ophthalmic surgery.
Summary of main results
We reviewed evidence from seven RCTs involving 500 participants regarding the reduction of pain during intraocular surgery by adding hyaluronidase to the local anaesthetic fluid. We found that the reduction of intraoperative pain by hyaluronidase was not statistically significant. The quality of evidence was low. We assessed the literature in this field as having a low risk of bias, but we had concerns regarding heterogeneity across the studies. With regards to the outcomes of participant and surgeon satisfaction, the moderate quality studies show an advantage of using hyaluronidase. (See Table 1).
Overall completeness and applicability of evidence
We are confident that our search strategy obtained all available studies. The results of this review are applicable to all adults undergoing intraocular surgery who would want to make an informed decision regarding the use of hyaluronidase as an adjunct in eye blocks to reduce intraoperative pain. We found that the use of hyaluronidase is beneficial in terms of participant and surgical satisfaction. Such benefit from using hyaluronidase was not statistically significant with regards to intraoperative reduction of pain.
We consider that most of the authors gave priority to akinesia as an outcome measure over analgesia, probably due to the perceived importance for the safe conduct of surgery. This priority has now receded as the majority of surgeons can carry out most operations without depending on fully established akinesia. Profound akinesia will still be necessary for more difficult operations and training situations. Hyaluronidase may be necessary to achieve akinesia in such situations.
Quality of the evidence
We found the overall quality of evidence to be low due imprecision and inconsistency of the results. There was moderate heterogeneity (I² = 41%). Therefore, we downgraded the quality of evidence by one level. We looked for possible causes for the variation such as sample size, dose of hyaluronidase or characteristics of participants but data were sparse.
We found the overall risk of bias in the studies to be low. However, there was an unclear risk with regards to methods of randomization and concealment in a few studies.
As for imprecision, failure to estimate the sample size needed to make an effect by six of the seven studies led to the quality of evidence to be downgraded by one level.
Potential biases in the review process
A potential bias arises from the narrow spectrum of the review question: "Does hyaluronidase improve pain control during eye surgery?" Hyaluronidase is used for a variety of indications. For example, if the speed of onset of anaesthesia is increased by hyaluronidase and the eye is much more akinetic at the beginning of the operation, participant and surgeon comfort will also likely be increased. Therefore, the narrow aspect of analgesia alone does not consider the full spectrum of beneficial effects from hyaluronidase.
This review found only a relatively small number of studies (seven) with a small number of participants (500). However, can systematic reviews with such sparse data be trusted (Afshari 2017)? During this Cochrane Review, we adhered to all essential requirements such as publishing a protocol, incorporating risk of bias assessment, searching for unpublished data and many other review tools as laid out in the Cochrane framework (Higgins 2011).
According to our protocol, we excluded studies that did not assess pain in a structured manner (using rating scales). Because of this, we excluded unstructured assessments that may have shown results. We consider that results from these trials would have a high risk of reporting bias and therefore, would not produce a reliable, useful effect.
Lack of reported data also led to certain included trials being excluded from the meta‐analysis. We were unable to obtain clarification about these issues from authors of the included studies.
Readers of this review may be interested in the incidence of adverse effects of hyaluronidase use. Adverse effects such as allergy to hyaluronidase are extremely rare. None of the trials we analysed reported any adverse events related to hyaluronidase, but we would like to highlight that our review would not have reliably captured the incidence of very rare adverse events due to an overall small number of participants.
We have acknowledged and taken into account the inherent methodological limitation of our systematic review and in our opinion addressed them adequately.
Agreements and disagreements with other studies or reviews
Our findings match that of the review of Schein 2000. Their review reported the same problems with the available literature that we found. Those are;
very few studies reported data on the effect of hyaluronidase on pain;
high levels of inconsistency among the included studies.
Schein's review was written in 2000, and despite many additional studies having been published since, there is still no certainty on the effect of hyaluronidase use for analgesia.
Authors' conclusions
Implications for practice.
The requirements for ophthalmic anaesthesia have changed considerably since the early 2000s. Nowadays, the majority of routine cataract surgery can be conducted pain free under topical anaesthesia alone. Injection blocks are used to provide more profound analgesia for some people and during some operations. The effects of adding hyaluronidase to local anaesthetic fluid on pain outcomes in people undergoing eye surgery are uncertain due to the low quality of the available evidence.
Implications for research.
To reach certainty on the question of hyaluronidase use for intraoperative analgesia, future studies should separate the various outcome parameters of speed of onset and akinesia from that of analgesia.
The importance of pain control is different for anterior and posterior segment eye surgery. This should be looked at in a well powered randomized controlled trial.
We found no studies that described the economical impact of using hyaluronidase. In the current climate of financial restrictions, this information would be very valuable, and any future study should incorporate this aspect.
What's new
| Date | Event | Description |
|---|---|---|
| 4 October 2018 | Amended | Acknowledgement section amended to include Co‐ordinating Editor |
Acknowledgements
We thank Jane Cracknell, Managing Editor of the Cochrane Anaesthesia, Critical and Emergency Care Group (ACE), and Karen Hovhannisyan (former ACE trials search co‐ordinator). We would like to thank Andrew Smith (Content Editor), Marialena Trivella (Statistical Editor), Vibeke E Horstmann (Statistical Editor), Ana Licina, Jacques Ripart, Mahmoud B Alhassan (Peer Reviewers), Janet Wale (Consumer Editor), and Andrew Smith (Co‐ordinating Editor) for their help and editorial advice during the preparation of the protocol (Rüschen 2013) and the systematic review.
Appendices
Appendix 1. The Cochrane Central Register of Controlled Trials (CENTRAL, 2017, Issue 6)
| Search lines | Search terms | Search results |
| 1 | eye* or Ophthalm* or ocular | 39,485 |
| cornea* or retin* or scler* or vitre* or iris or pupil or orbit* or chorod* | 33,449 | |
| 3 | intraocular or intra‐ocular or extraocular or extra‐ocular or monocular or oculo* or oculi or optic* | 16,808 |
| 4 | visual* or vision or sight or see* or view* or blind* | 380,089 |
| 5 | glaucoma or conjuncti* or uveitis or macula* or oedema or edema or strabismus or squint or astigmati* or myopi* or hypermetropia or trachoma | 33,656 |
| 6 | #1 or #2 or #3 or #4 or #5 | 413,088 |
| 7 | surg* or operat* | 203,926 |
| 8 | transplant* or graft* or extract* or cataract or refractive or oculoplast* or ophthalmosurg* | 92,037 |
| 9 | #7 or #8 | 258,017 |
| 10 | #6 and #9 | 112,631 |
| 11 | retrobulbar or peribulbar or sub‐tenon's block or sub‐tenon's block | 806 |
| 12 | "Nerve Block" or Lidocaine or Mepivacaine or Bupivacaine | 19,237 |
| 13 | (local anaesthe*) or (local anesthe*) | 15,573 |
| 14 | #11 or #12 or #13 | 25,341 |
| 15 | #10 and #14 | 11,101 |
| 16 | hyaluronidase or hyaluronoglucosaminidase or hyadase or vitrase or Amphadase or wydase or hyalase or hylenex | 533 |
| 17 | #10 and #14 and #16 | 230 |
| 18 | Limit to TRIALS database | 207 |
Appendix 2. MEDLINE (Ovid) from 1946 to 30 June 2017
| Search lines | Search terms | Search results |
| 1 | eye* or Ophthalm* or ocular | 578,410 |
| 2 | cornea* or retin* or scler* or vitre* or iris or pupil or orbit* or chorod* | 738,578 |
| 3 | intraocular or intra‐ocular or extraocular or extra‐ocular or monocular or oculo* or oculi or optic* | 478,576 |
| 4 | visual* or vision or sight or see* or view* or blind* | 2,691,438 |
| 5 | glaucoma or conjuncti* or uveitis or macula* or oedema or edema or strabismus or squint or astigmati* or myopi* or hypermetropia or trachoma | 436,501 |
| 6 | 1 or 2 or 3 or 4 or 5 | 3,928,131 |
| 7 | surg* or operat* | 2,516,135 |
| 8 | transplant* or graft* or extract* or cataract or refractive or oculoplast* or ophthalmosurg* | 1,578,347 |
| 9 | 7 or 8 | 3,810,842 |
| 10 | 6 and 9 | 724,695 |
| 11 | exp Ophthalmologic Surgical Procedures/ or exp Cataract Extraction/ | 99,847 |
| 12 | exp Refractive Surgical Procedures/ | 54,938 |
| 13 | exp Corneal Transplantation/ | 14,186 |
| 14 | exp Phacoemulsification/ or exp Lens Implantation, Intraocular/ | 13,574 |
| 15 | 11 or 12 or 13 or 14 | 99,847 |
| 16 | 10 or 15 | 753,408 |
| 17 | retrobulbar or peribulbar or sub‐tenon's block or subtenon's block | 4698 |
| 18 | local anaesthe* or local anesthe* | 38,258 |
| 19 | 17 or 18 | 42,504 |
| 20 | exp Anesthesia, Local/ | 16,213 |
| 21 | exp Anesthetics, Local/ | 99,177 |
| 22 | exp Lidocaine/ or exp Nerve Block/ | 30,602 |
| 23 | exp Mepivacaine/ or exp Bupivacaine/ | 12,786 |
| 24 | 20 or 21 or 22 or 23 | 120,407 |
| 25 | 19 or 24 | 140,352 |
| 26 | 16 and 25 | 13,782 |
| 27 | hyaluronidase or hyaluronoglucosaminidase or hyadase or vitrase or Amphadase or wydase or hyalase or hylenex | 10,943 |
| 28 | exp Hyaluronoglucosaminidase | 8216 |
| 29 | 27 or 28 | 10,943 |
| 30 | 16 and 25 and 29 | 319 |
| 31 | limit 30 to controlled clinical trial or randomised controlled trial | 139 |
| 32 | (((single adj (blind* or masked)) or double) adj (blind* or masked)).ab. or (((single adj (blind* or masked)) or double) adj (blind* or masked)).ti. | 142,612 |
| 33 | (randomized or randomly or placebo or trial or (controlled adj study)).ab. or (randomized or randomly or placebo or trial or (controlled adj study)).ti. | 1,054,662 |
| 34 | 32 or 33 | 1,066,944 |
| 35 | 30 and 34 | 135 |
| 36 | 31 or 35 | 155 |
Appendix 3. Embase (Ovid) 1947 to 30 June 2017
| Search lines | Search terms | Search results |
| 1 | eye* or Ophthalm* or ocular | 714,955 |
| 2 | cornea* or retin* or scler* or vitre* or iris or pupil or orbit* or chorod* | 1,053,140 |
| 3 | intraocular or intra‐ocular or extraocular or extra‐ocular or monocular or oculo* or oculi or optic* | 503,874 |
| 4 | visual* or vision or sight or see* or view* or blind* | 3,707,107 |
| 5 | glaucoma or conjuncti* or uveitis or macula* or oedema or edema or strabismus or squint or astigmati* or myopi* or hypermetropia or trachoma | 688,043 |
| 6 | 1 or 2 or 3 or 4 or 5 | 5,252,878 |
| 7 | surg* or operat* | 4,385,968 |
| 8 | transplant* or graft* or extract* or cataract or refractive or oculoplast* or ophthalmosurg* | 2,208,147 |
| 9 | 7 or 8 | 6,014,664 |
| 10 | 6 and 9 | 1,223,645 |
| 11 | exp eye surgery/ | 133,842 |
| 12 | exp cornea transplantation/ | 11,680 |
| 13 | exp cataract extraction/ or extracapsular cataract extraction/ | 43,300 |
| 14 | exp refractive surgery/ | 8947 |
| 15 | exp lens implant/ | 20,733 |
| 16 | exp eyelid reconstruction/ | 5242 |
| 17 | 11 or 12 or 13 or 14 or 15 or 16 | 138,278 |
| 18 | 10 or 17 | 1,241,841 |
| 19 | retrobulbar or peribulbar or sub‐tenon's block or subtenon's block | 7897 |
| 20 | local anaesthe* or local anesthe* | 80,577 |
| 21 | 19 or 20 | 87,361 |
| 22 | exp retrobulbar drug administration/ | 1081 |
| 23 | exp lidocaine/ or exp peribulbar anesthesia/ or exp local anesthetic agent/ or exp bupivacaine/ | 228,041 |
| 24 | exp local anesthesia/ or exp nerve block/ | 68,789 |
| 25 | exp levobupivacaine/ or exp procaine/ | 22,051 |
| 26 | ropivacaine/ or midazolam/ or propofol/ | 80,182 |
| 27 | 22 or 23 or 24 or 25 or 26 | 326,465 |
| 28 | 21 or 27 | 343,082 |
| 29 | 18 and 28 | 40,436 |
| 30 | hyaluronidase or hyaluronoglucosaminidase or hyadase or vitrase or Amphadase or wydase or hyalase or hylenex | 16,613 |
| 31 | exp recombinant hyaluronidase/ or exp hyaluronidase/ or exp hyaluronoglucosaminidase/ | 14,509 |
| 32 | 30 or 31 | 16,613 |
| 33 | 18 and 28 and 32 | 709 |
| 34 | limit 33 to (clinical trial or randomized controlled trial or controlled clinical trial or multicenter study or phase 1 clinical trial or phase 2 clinical trial or phase 3 clinical trial or phase 4 clinical trial) | 199 |
| 35 | (randomi?ed or randomly or placebo or trial or (controlled adj study)).ab. or (randomi?ed or randomly or placebo or trial or (controlled adj study)).ti. | 1,391,344 |
| 36 | (((single adj (blind* or masked)) or double) adj (blind* or masked)).ab. or (((single adj (blind* or masked)) or double) adj (blind* or masked)).ti. | 185,195 |
| 37 | 35 or 36 | 1,408,147 |
| 38 | 33 and 37 | 199 |
| 39 | 34 or 38 | 251 |
Appendix 4. Web of Science from 1900 to 30 June 2017
| Search lines | Search terms | Search results |
| 1 | eye* or Ophthalm* or ocular | 476,661 |
| 2 | cornea* or retin* or scler* or vitre* or iris or pupil or orbit* or chorod* | 1,045,927 |
| 3 | intraocular or intra‐ocular or extraocular or extra‐ocular or monocular or oculo* or oculi or optic* | 1,576,811 |
| 4 | visual* or vision or sight or see* or view* or blind* | 4,121,849 |
| 5 | glaucoma or conjuncti* or uveitis or macula* or oedema or edema or strabismus or squint or astigmati* or myopi* or hypermetropia or trachoma | 425,695 |
| 6 | #5 OR #4 OR #3 OR #2 OR #1 | 6,754,964 |
| 7 | surg* or operat* | 3,423,025 |
| 8 | transplant* or graft* or extract* or cataract or refractive or oculoplast* or ophthalmosurg* | 2,193,730 |
| 9 | #8 OR #7 | 5,349,001 |
| 10 | #9 AND #6 | 1,005,525 |
| 11 | (Lens Implantat*) OR (cornea transplantat*) OR (cataract extract*) OR (refractive surg*) OR (exp eyelid reconstruct*) | 26,003 |
| 12 | #11 OR #10 | 1,008,989 |
| 13 | (retrobulbar or peribulbar or sub‐tenon's block or subtenon's block) | 4057 |
| 14 | (retrobulbar or peribulbar or sub‐tenon's block or sub‐tenon's block) | 4056 |
| 15 | (local anaesthe*) OR (local anesthe*) | 41,088 |
| 16 | (lidocaine OR levobupivacaine OR procaine OR ropivacaine OR midazolam OR propofol) OR TOPIC: (nerve block) | 85,921 |
| 17 | #16 OR #15 OR #14 OR #13 | 117,842 |
| 18 | (hyaluronidase or hyaluronoglucosaminidase or hyadase or vitrase or Amphadase or wydase or hyalase or hylenex) OR TOPIC: (recombinant hyaluronidase) | 6294 |
| 19 | #18 AND #17 AND #12 | 214 |
Appendix 5. Scopus from 1823 to 30 June 2017
| Search lines | Search terms | Search results |
| 1 | eye* or Ophthalm* or ocular | 1,943,281 |
| 2 | cornea* or retin* or scler* or vitre* or iris or pupil or orbit* or chorod* | 3,136,708 |
| 3 | intraocular or intra‐ocular or extraocular or extra‐ocular or monocular or oculo* or oculi or optic | 872,852 |
| 4 | visual* or vision or sight or see* or view* or blind* | 13,637,048 |
| 5 | glaucoma or conjuncti* or uveitis or macula* or oedema or edema or strabismus or squint or astigmati* or myopi* or hypermetropia or trachoma | 1,292,318 |
| 6 | surg* or operat* | 11,977,249 |
| 7 | transplant* or graft* or extract* or cataract or refractive or oculoplast* or ophthalmosurg* | 6,168,543 |
| 8 | retrobulbar or peribulbar or (sub‐tenon's block) or (subtenon's block) | 15,268 |
| 9 | (local anaesthe*) or (local anesthe*) | 241,430 |
| 10 | Lidocaine or (Nerve Block) or Mepivacaine or Bupivacaine | 334,730 |
| 11 | hyaluronidase OR hyaluronoglucosaminidase OR hyadase OR vitrase OR amphadase OR wydase OR hyalase OR hylenex | 28,617 |
| 12 | #1 or #2 or #3 or #4 or #5 | 16,812,220 |
| 13 | #6 or #7 | 16,339,616 |
| 14 | #8 or #9 or #10 | 509,491 |
| 15 | #11 and #12 and #13 and #14 | 1,857 |
Appendix 6. CINAHL Plus (EBSCOhost) from 1937 to 30 June 2017
| Search lines | Search terms | Search results |
| 1 | eye* or Ophthalm* or ocular | 62,235 |
| 2 | cornea* or retin* or scler* or vitre* or iris or pupil or orbit* or chorod* | 68,825 |
| 3 | intraocular or intra‐ocular or extraocular or extra‐ocular or monocular or oculo* or oculi or optic* | 24,333 |
| 4 | visual* or vision or sight or see* or view* or blind* | 426,645 |
| 5 | glaucoma or conjuncti* or uveitis or macula* or oedema or edema or strabismus or squint or astigmati* or myopi* or hypermetropia or trachoma | 48,062 |
| 6 | S1 OR S2 OR S3 OR S4 OR S5 | 547,031 |
| 7 | surg* or operat* | 513,190 |
| 8 | transplant* or graft* or extract* or cataract or refractive or oculoplast* or ophthalmosurg* | 162,110 |
| 9 | S7 OR S8 | 625,438 |
| 10 | S6 AND S9 | 101,771 |
| 11 | (MH "Surgery, Eye+") OR (MM "Keratomileusis, Laser in Situ") OR (MM "Eye Enucleation") | 13,308 |
| 12 | (MM "Corneal Transplantation") | 1060 |
| 13 | (MH "Cataract Extraction+") | 5140 |
| 14 | (MM "Phacoemulsification") | 848 |
| 15 | S11 OR S12 OR S13 OR S14 | 13,308 |
| 16 | S10 OR S15 | 104,161 |
| 17 | retrobulbar or peribulbar or sub‐tenon's block or subtenon's block | 352 |
| 18 | local anaesthe* or local anesthe* | 10,097 |
| 19 | S17 OR S18 | 11,261 |
| 20 | (MM "Nerve Block") | 4680 |
| 21 | (MM "Anesthesia, Local") OR (MH "Anesthetics, Local+") | 14,442 |
| 22 | S20 OR S21 | 17,375 |
| 23 | S19 OR S22 | 20,462 |
| 24 | S16 AND S23 | 2377 |
| 25 | hyaluronidase or hyaluronoglucosaminidase or hyadase or vitrase or Amphadase or wydase or hyalase or hylenex | 371 |
| 26 | (MM "Hyaluronidase") | 129 |
| 27 | S25 OR S26 | 371 |
| 28 | S16 AND S23 AND S27 | 40 |
Appendix 7. LILACS from 1982 to 30 June 2017
| Search lines | Search terms | Search results |
| 1 | eye* or Ophthalm* or ocular or cornea* or retin* or scler* or vitre* or iris or pupil or orbit* or chorod* or intraocular or intra‐ocular or extraocular or extra‐ocular or monocular or oculo* or oculi or optic* or visual* or vision or sight or see* or view* or blind* or glaucoma or conjuncti* or uveitis or macula* or oedema or edema or strabismus or squint or astigmati* or myopi* or hypermetropia or trachoma | 5,238,714 |
| 2 | surg* or operat* or transplant* or graft* or extract* or cataract or refractive or oculoplast* or ophthalmosurg* | 4,831,015 |
| 3 | retrobulbar or peribulbar or sub‐tenon's block or sub‐tenon's block or (local anaesthe*) or (local anesthe*) or Lidocaine or (Nerve Block) or Mepivacaine or Bupivacaine | 320,196 |
| 4 | hyaluronidase OR hyaluronoglucosaminidase OR hyadase OR vitrase OR amphadase OR wydase OR hyalase OR hylenex | 8146 |
| 5 | 1 and 2 and 3 and 4 and 5 | 336 |
Appendix 8. Data collection form
Cochrane Anaesthesia Review Group
Study Selection, Quality Assessment & Data Extraction Form
| First author | Journal/Conference Proceedings etc | Year |
Study eligibility
| RCT/Quasi/CCT (delete as appropriate) | Relevant participants | Relevant interventions | Relevant outcomes |
| Yes / No / Unclear |
Yes / No / Unclear |
Yes / No / Unclear |
Yes / No* / Unclear |
* Issue relates to selective reporting when authors may have taken measurements for particular outcomes, but not reported these within the paper(s). Reviewers should contact trialists for information on possible non‐reported outcomes & reasons for exclusion from publication. Study should be listed in ‘Studies awaiting assessment’ until clarified. If no clarification is received after three attempts, study should then be excluded.
| Do not proceed if any of the above answers are ‘No’. If study to be included in ‘Excluded studies’ section of the review, record below the information to be inserted into ‘Table of excluded studies’. |
| Freehand space for comments on study design and treatment: |
References to trial
Check other references identified in searches. If there are further references to this trial link the papers now & list below. All references to a trial should be linked under one Study ID in RevMan.
| Code each paper | Author(s) | Journal/Conference Proceedings etc | Year |
| A | The paper listed above | ||
| B | Further papers | ||
| Participant characteristics | |
| Further details | |
| Age (mean, median, range, etc) | |
| Sex of participants (numbers / %, etc) | |
| Dose of hyaluronidase | |
Trial characteristics
Methodological quality
| Allocation of intervention | |
| State here method used to generate allocation and reasons for grading | Grade (circle) |
| |
Adequate (Random) |
| Inadequate (e.g. alternate) | |
| Unclear | |
|
Concealment of allocation Process used to prevent foreknowledge of group assignment in a RCT, which should be seen as distinct from masking | |
| State here method used to conceal allocation and reasons for grading | Grade (circle) |
| Adequate | |
| Inadequate | |
| Unclear | |
| Masking | |
| Person responsible for participants care | Yes / No |
| Participant | Yes / No |
| Outcome assessor | Yes / No |
| Other (please specify) | Yes / No |
|
Intention‐to‐treat An intention‐to‐treat analysis is one in which all the participants in a trial are analysed according to the intervention to which they were allocated, whether they received it or not. | |
| All participants entering trial | |
| 15% or fewer excluded | |
| More than 15% excluded | |
| Not analysed as ‘intention‐to‐treat’ | |
| Unclear | |
Were withdrawals described? Yes ? No ? not clear ?
Is attrition reported? Yes ? no ?
| Total number randomized | |
| Number in hyaluronidase group | |
| Number in control group |
Discuss if appropriate and note reasons for attrition
Is there a possibility of selective outcome reporting Yes? No?
Discuss if appropriate
Data extraction
| Outcomes relevant to your review | |
| Reported in paper (circle) | |
| Intraoperative pain | Yes / No |
| Adverse Events | Yes / No |
| Satisfaction scoring | Yes / No |
| For Continuous data | |||||||
| Code of paper |
Outcomes |
Unit of measurement |
Intervention group | Control group | Details if outcome only described in text | ||
| n | Mean (SD) | n | Mean (SD) | ||||
| Intraoperative VAS pain score (include range) |
|||||||
| For Dichotomous data | |||
| Code of paper | Outcomes | Intervention group (n) n = number of participants, not number of events |
Control group (n) n = number of participants, not number of events |
| Intraoperative Pain (where not VAS) | |||
|
Other information which you feel is relevant to the results Indicate if: any data were obtained from the primary author; if results were estimated from graphs etc; or calculated by you using a formula (this should be stated and the formula given). In general if results not reported in paper(s) are obtained this should be made clear here to be cited in review. |
|
Adverse events recorded here |
| Freehand space for writing actions such as contact with study authors and changes |
Surgical specialty: (CIRCLE)
1. Anterior segment.
2. Glaucoma.
3. Vitreoretinal surgery.
References to other trials
| Did this report include any references to published reports of potentially eligible trials not already identified for this review? | ||
| First author | Journal / Conference | Year of publication |
| Did this report include any references to unpublished data from potentially eligible trials not already identified for this review? If yes, give list contact name and details | ||
| | ||
| Trial characteristics Summary | |
| Further details | |
| Single centre / Multicentre | |
| Country / Countries | |
| How was participant eligibility defined? | |
| How many people were randomized? | |
| Number of participants in each intervention group | |
| Number of participants who received intended treatment | |
| Number of participants who were analysed | |
| Local anaesthetic Drug dose (s) used | |
| Dose of hyaluronidase | |
| Median (range) length of follow‐up reported in this paper (state weeks, months or years or if not stated) | |
| Time‐points when measurements were taken during the study | |
| Time‐points reported in the study | |
| Time‐points you are using in RevMan | |
| Trial design | |
| Other | |
Appendix 9. Characteristics of excluded studies
Reason for exclusion noted as narrative only:
Appendix 10. Risk of bias table
| Entry | Judgement Low risk/high risk/unclear risk |
Description |
| 1. Random sequence generation | ||
| 2. Allocation concealment | ||
| 3. Masking of participants and personnel | ||
| 4. Masking of outcome assessment | ||
| 5. Incomplete outcome data | ||
| 6. Selective reporting | ||
| 7. Other bias |
Data and analyses
Comparison 1. Hyaluronidase versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Intraoperative pain (reported continuous) | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Intraoperative pain (reported dichotomous) | 4 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.48, 1.42] |
1.1. Analysis.
Comparison 1 Hyaluronidase versus control, Outcome 1 Intraoperative pain (reported continuous).
1.2. Analysis.
Comparison 1 Hyaluronidase versus control, Outcome 2 Intraoperative pain (reported dichotomous).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bowman 1997.
| Methods | Parallel group, prospective, masked randomized controlled single centre study in the UK. Study dates not stated. |
|
| Participants | 92 adults (extracapsular cataract extraction phacoemulsification and trabeculectomy) received peribulbar block. Number of participants: hyaluronidase group; 44 control group ; 48. Mean age : hyaluronidase group: 72 years; control group : 75 years. |
|
| Interventions | Hyaluronidase group ; lignocaine 2% with adrenaline 1:200,000, + bupivacaine 0.5% + hyaluronidase 150 IU/mL. Control group: lignocaine 2% with adrenaline 1:200,000 + bupivacaine 0.5%. 10 mL peribulbar injection using a standardized technique. |
|
| Outcomes | Akinesia, objective analgesia assessed by surgeon, subjective analgesia assessed by participant after surgery. VAS 0 to 10 for subjective and objective pain scores were stratified into a dichotomous pain/no pain. |
|
| Notes | Conflict of interest and funding sources not documented. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of randomization described. |
| Allocation concealment (selection bias) | Unclear risk | Masked allocation but no details described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported. |
| Selective reporting (reporting bias) | Low risk | All groups were reported on. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Masking of participants described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double masking described. |
Brydon 1995.
| Methods | Parallel group, randomized double blind design in the UK. Study dates were not stated. |
|
| Participants | 60 consecutive adults for elective intra‐ocular surgery. Number of participants: 20 per group. Results for low dose and higher dose were combined for analysis. Mean age: hyaluronidase (low dose) group: 74 years; hyaluronidase (higher dose) group: 73 years; control group: 72 years. |
|
| Interventions | Hyaluronidase (low dose) group: peribulbar block with equal mixture of lignocaine 2% and bupivacaine 0.75% + hyaluronidase 50 IU/mL. Hyaluronidase (higher dose) group: peribulbar block with equal mixture of lignocaine 2% and bupivacaine 0.75% + hyaluronidase 150 IU/mL. Control group: peribulbar block with equal mixture of lignocaine 2% and bupivacaine 0.75%. No sedation and premedication given. |
|
| Outcomes | Speed of onset, akinesia, analgesia, top‐up frequency, incidence of harm. Analgesia measured by assessing participant's reaction to insertion of superior rectus suture and by direct questioning during procedure. 3 point scoring system used. Akinesia was the primary outcome measure. |
|
| Notes | Conflict of interest and funding sources not documented. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random assignment but no details described. |
| Allocation concealment (selection bias) | Unclear risk | Masked allocation but no details described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported on. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Composition of the local anaesthetic solution was not known to the anaesthetist", but no further details of masking described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor (surgeon) masked. |
Khandwala 2008.
| Methods | Parallel group, prospective randomized controlled double masked, single centre trial in Leeds, the UK. Study dates; not stated. |
|
| Participants | 20 adults undergoing routine cataract surgery. Data for 1 participant in hyaluronidase group were incomplete and excluded from analysis. Participants were ASA 1‐3. Number of participants in analysis: hyaluronidase group: 9; control group: 10. Mean age: hyaluronidase group: (73.8 years; control group: 74 years). Exclusion criteria; refusal, language problems, history of allergy to amide local anaesthetics or hyaluronidase or pre‐existing extra ocular muscle palsy. |
|
| Interventions | Hyaluronidase group: lignocaine 2% + hyaluronidase 15 IU/mL. Control group: lignocaine 2%. Sub‐Tenon's block. Total volume of local anaesthesia 5 mL with no premedication sedation. |
|
| Outcomes | Akinesia, depth of anaesthetic fluid spread on ultrasound, surgical conditions, pain during operation measured by visual analogue scale (VAS). SD pain scores unavailable. Attempts to contact authors for more clarification unsuccessful. |
|
| Notes | Authors declared no conflict of interest and received no funding from private or public bodies. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomization. |
| Allocation concealment (selection bias) | Low risk | Coded syringes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 participant in treatment group excluded after randomization, due to incomplete data. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel were masked. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor masked. |
Remy 2008.
| Methods | Parallel group, prospective randomized double masked placebo controlled trial with a multicentre design in Germany. Study dates: 29 July 2003 to 2 November 2004. |
|
| Participants | 80 adults undergoing elective cataract surgery with retrobulbar block. No participant dropped out. Number of participants: hyaluronidase group: 40; control group: 40. Mean (SD) age: hyaluronidase group: 76 ± 11 years; control group; 74±10 years. Inclusion criteria; adults aged >18 years, elective surgery, no active ocular disease and informed consent obtained in written form. Exclusion criteria; known intolerance to hyaluronidase, pregnancy, lack of co‐operation, history of alcohol or drug abuse, or local anaesthetic complications. |
|
| Interventions | Hyaluronidase group: 5 mL 1% mepivacaine + 75 IU/mL hyaluronidase. Control group: 5 mL 1% mepivacaine + placebo (special batch of Hyalase without active ingredient). |
|
| Outcomes | Primary end point; complete akinesia after 5 minutes. Secondary end points; akinesia at other times, top‐up injections, ptosis, time to anaesthesia, pain (VAS) immediately after surgery and 3 hours postsurgery and efficacy and tolerability for participant and surgeon. Adverse events recorded. First pain assessment point, planned before surgery then reported for immediately after surgery. |
|
| Notes | No conflict or financial interest reported by author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization not described in detail. |
| Allocation concealment (selection bias) | Low risk | Double masked as per German legal framework. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participant dropped out. |
| Selective reporting (reporting bias) | Low risk | All outcome measures were reported on. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo control, double masked. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Masked according to federal law. |
Rowley 2000.
| Methods | Parallel group, randomized double masked controlled trial in 1 centre in UK. Study dates: not stated. |
|
| Participants | 150 adults for elective cataract surgery with sub‐Tenon's block. Number of participants: hyaluronidase group: 76; control group: 74. Mean age; in hyaluronidase group; 77.14 years; control group; 76.51 years. Groups similar in terms of age, sex and proportion of blocks administered by each investigator. Exclusion criteria: people with learning difficulties, dementia, profound deafness and known adverse reaction to lignocaine or hyaluronidase. |
|
| Interventions | Hyaluronidase group: 3 mL 2% lignocaine/adrenaline + hyaluronidase 30 IU/mL. Control group: 3 mL 2% lignocaine/adrenaline. |
|
| Outcomes | Akinesia, post‐injection and immediate postoperative pain. Pain measured using VAS (0‐10 cm). SD pain scores not available with attempts to obtain more clarification from authors unsuccessful. |
|
| Notes | Declaration of funding sources and conflict of interest not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables. |
| Allocation concealment (selection bias) | Low risk | Masked syringes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported accordingly. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Masking of participants not described. Masking of personnel (operative surgeon, independent assistant and nursing staff) was described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double masked design. |
Sedghipour 2012.
| Methods | Parallel group, randomized double masked trial in 1 centre in Iran. Study dates: February 2011 to July 2011. |
|
| Participants | 44 adults initially recruited from a referral eye centre (Nikookari Eye Hospital) to undergo elective cataract surgery (phacoemulsification) under sub‐Tenon block. 2 participants did not meet the criteria and were excluded. Number of participants: hyaluronidase group: 21; control group: 21. Mean (SD) ages: hyaluronidase group: 65.62 ± 3.01 years; control group; 67 ± 4.4 years. Groups comparable for gender and age. Exclusion criteria: people with deafness and allergy to lidocaine or hyaluronidase. |
|
| Interventions | Hyaluronidase group: 2 mL 2% lidocaine + hyaluronidase 150 IU/mL Control group: 2 mL 2% lidocaine. Ampoules were identical in appearance with a printed code (A or B). Codes disclosed for statistical analysis only at end of study. |
|
| Outcomes | Akinesia, participant and surgical satisfaction with yes/no questions, postoperative pain via VAS scoring using a standard VAS chart. Participants were given appropriate explanation on usage of chart. | |
| Notes | No declaration of funding sources or conflict of interest reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Consecutive numbers assigned to participants on admission by a staff member not involved in study. |
| Allocation concealment (selection bias) | Low risk | Coded syringes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions after randomization reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Masking of participants, personnel by coded syringes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor masked by coded syringes. |
Shiroma 2002.
| Methods | Randomized double masked study conducted at State University of Campinas ‐ Unicamp, Brazil. Study dates: not stated. |
|
| Participants | 57 adults undergoing elective extracapsular cataract extraction on an outpatient basis. Participant's physical statuses described as ASA 1‐ 3. Number of participants: hyaluronidase group: 29; control group: 28. Sex: 31 men (54.4%); 26 women (45.6%). Age range (overall): 45‐ 89 years; mean (SD) overall: 67.73 (± 10.65). Groups homogeneous in relation to sex, age and physical condition. Peribulbar injection given as a block by double needle injection with 25x7 mm needle, administered 4 mL at lower temporal with super‐medial inclination of about 15°and 3 mL (nasal‐superior). Anaesthetic solution prepared without knowledge of ophthalmologist who performed the block. |
|
| Interventions | Hyaluronidase group: ropivacaine 1% + hyaluronidase 100 IU/mL. Control group: ropivacaine 1%. |
|
| Outcomes | Onset time to akinesia, need for supplementary injections and pain assessed by VAS (0‐10). | |
| Notes | No declaration of funding sources or conflict of interest reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not specified. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals documented, all participants included initially were assessed and reported. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Masking described in participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment masked. |
ASA: American Society of Anesthesiology Classification; SD: standard deviation; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Berg 2001 | Pain not assessed formally using a rating scale. Instead, augmentation eye drops given if participant complained of pain during procedure. Therefore, pain not measured but reported. |
| Crawford 1994 | 2 participants in the hyaluronidase group received local anaesthetic drops for pain intraoperatively. However, pain not formally measured, therefore, inclusion criteria not met. |
| Guise 1999 | Pain not formally measured using a rating scale. |
| House 1991 | Pain not measured as per rating scale and top‐ups primarily given to achieve akinesia initially. |
| Johansen 1993 | Surgeon assessed quality of analgesia and pain, not measured by asking participant. No formal method of assessing pain described. |
| Lange 1989 | On further inspection, not a randomized trial. |
| Moharib 2002 | Adequate pain relief defined as lack of complaint or response from participant. Did not constitute pain measurement and no formal assessment of pain described in the study. |
| Morsman 1992 | Presence or absence of hyaluronidase not masked. |
| Ramanathan 1999 | Poster presentation, Further details could not be obtained about randomization, masking and results. |
| Sarvela 1992 | Part one of third study was not randomized. Part two of this study compared two different concentrations of hyaluronidase only. |
| Soares 2002 | Intraoperative pain not measured (e.g. by asking participant). It was assumed that the participants would spontaneously voice their pain during operation. Therefore, study did not measure any of our stipulated outcomes. |
Differences between protocol and review
We made the following changes to the protocol (Rüschen 2013);
This review included only adults, we therefore stated "adults" in the title.
The phrase 'to reduce intraoperative pain' was added to the title to comply with PICO to reflect the review question.
Lee Adams was initially a registered author, but gave his agreement to be removed from the authors list at the protocol stage.
We added Kavitha Aravinth as second author.
We added Desta Bokre as fourth author.
We further stated in the primary objective that when considering studies for inclusion that we excluded studies that did not measure pain with a rating scale because spontaneous reporting of pain by the participant is not likely to produce reproducible results.
We stated the secondary objectives (incidence of harm, participant and surgical satisfaction and economic outcomes or cost calculations) in the Objectives section.
We stated in the protocol that intraoperative pain would be recorded using (VAS); however, we observed that included studies used either (VAS) or (VRS) to rate pain. As both are accepted and validated tools to measure pain, we analysed both these methods of measuring pain in the same manner.
We initially proposed to include only studies that described the first eye operation, but during the review process, we found no publication that described if the participants had a first or second eye operation.(See Types of participants and Unit of analysis issues).
We excluded adnexal surgery and any other eye surgery that was not intraocular. Anaesthetic techniques and surgical interventions for adnexal operations are inherently different from intraocular surgery and produce a very different pain profile.
We used GRADE‐pro to analyse the quality of evidence and to produce a 'Summary of Findings table'.
Collation of references: we added the following to the Selection of studies section. "Where studies had multiple publications, we planned to collate the reports of the same study so that each study, rather than each report, was the unit of interest for the review and such studies had a single identifier with multiple references.
Contributions of authors
Conceiving the review: HR.
Co‐ordinating the review: HR.
Undertaking manual searches: KA and HR.
Screening search results: KA and HR.
Organizing retrieval of papers: DB, KA and HR.
Screening retrieved papers against inclusion criteria: KA and HR.
Appraising quality of papers: KA and HR.
Abstracting data from papers: KA and HR.
Writing to authors of papers for additional information: KA and HR.
Providing additional data about papers: HR.
Obtaining and screening data on unpublished studies: HR.
Data management for the review: KA and HR.
Entering data into Review Manager (5): KA and HR.
Review Manager 5 statistical data: KA, HR and CB.
Other statistical analysis not using Review Manager 5: CB.
Double entry of data: KA and HR.
Interpretation of data: KA and HR.
Statistical inferences: CB.
Assessment of risk of bias: KA and HR.
Writing the review: KA and HR.
Securing funding for the review: HR and CB.
Guarantor for the review: HR.
Responsible for reading and checking review before submission: KA and HR.
Sources of support
Internal sources
-
Moorfields Eye Hospital, UK.
Salary and facilities
External sources
No sources of support supplied
Declarations of interest
HR: no known conflict of interest.
KA: no known conflict of interest.
CB : no known conflict of interest.
DB : no known conflict of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Bowman 1997 {published data only (unpublished sought but not used)}
- Bowman RJ, Newman DK, Richardson EC, Callear AB, Flanagan DW. Is hyaluronidase helpful for peribulbar anaesthesia?. Eye 1997;11(Pt 3):385‐8. [PUBMED: 9373482] [DOI] [PubMed] [Google Scholar]
Brydon 1995 {published data only (unpublished sought but not used)}
- Brydon CW, Basler M, Kerr WJ. An evaluation of two concentrations of hyaluronidase for supplementation of peribulbar anaesthesia. Anaesthesia 1995;50(11):998‐1000. [PUBMED: 8678264] [DOI] [PubMed] [Google Scholar]
Khandwala 2008 {published data only (unpublished sought but not used)}
- Khandwala M, Ahmed S, Goel S, Simmons IG, McLure HA. The effect of hyaluronidase on ultrasound‐measured dispersal of local anaesthetic following sub‐Tenon injection. Eye 2008;22(8):1065‐8. [PUBMED: 17525774] [DOI] [PubMed] [Google Scholar]
Remy 2008 {published data only (unpublished sought but not used)}
- Remy M, Pinter F, Nentwich MM, Kampik A, Schönfeld CL. Efficacy and safety of hyaluronidase 75 IU as an adjuvant to mepivacaine for retrobulbar anesthesia in cataract surgery. Journal of Cataract and Refractive Surgery 2008;34(11):1966‐9. [PUBMED: 19006746] [DOI] [PubMed] [Google Scholar]
Rowley 2000 {published data only (unpublished sought but not used)}
- Rowley SA, Hale JE, Finlay RD. Sub‐Tenon's local anaesthesia: the effect of hyaluronidase. British Journal of Ophthalmology 2000;84(4):435‐6. [PUBMED: 10729306] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sedghipour 2012 {published data only (unpublished sought but not used)}
- Sedghipour M, Mahdawifard A, Fouladi RF, Gharabaghi D, Rahbani M, Amiraslanzadeh G, et al. Hyaluronidase in subTenon's anaesthesia for phacoemulsification, a double blind randomized clinical trial. International Journal of Ophthalmology 2012;5(3):389‐92. [PUBMED: 22773994] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shiroma 2002 {published data only (unpublished sought but not used)}
- Shiroma HF, Ferreira EM, Isaac DLC, Ghanem VC, Arieta CEL. A comparison of 1% ropivacaine efficacy when associated or not with hyaluronidase in peribulbar anaesthesia in cataract surgery [Comparação da eficácia da ropivacaína 1% quando associada ou não à hialuronidase na anestesia peribulbar para cirurgia de catarata]. Arquivos Brasileiros de Oftalmologia 2002;65(5):525‐8. [0004‐2749; lil‐322156] [Google Scholar]
References to studies excluded from this review
Berg 2001 {published data only (unpublished sought but not used)}
- Berg AA, Montoya‐Pelaez LF. Comparison of lignocaine 2% with adrenaline, bupivacaine 0.5% with or without hyaluronidase and a mixture of bupivacaine, lignocaine and hyaluronidase for peribulbar block analgesia. Acta Anaesthesiologica Scandinavica 2001;45(8):961‐6. [PUBMED: 11576046] [DOI] [PubMed] [Google Scholar]
Crawford 1994 {published data only (unpublished sought but not used)}
- Crawford M, Kerr WJ. The effect of hyaluronidase on peribulbar block. Anaesthesia 1994;49(10):907‐8. [PUBMED: 7802194] [DOI] [PubMed] [Google Scholar]
Guise 1999 {published and unpublished data}
- Guise P, Laurent S. Sub‐Tenon's block: the effect of hyaluronidase on speed of onset and block quality. Anaesthesia and Intensive Care 1999;27(2):179‐81. [PUBMED: 10212716] [DOI] [PubMed] [Google Scholar]
House 1991 {published data only (unpublished sought but not used)}
- House PH, Hollands RH, Schulzer M. Choice of anesthetic agents for peribulbar anesthesia. Journal of Cataract and Refractive Surgery 1991;17(1):80‐3. [PUBMED: 2005563] [DOI] [PubMed] [Google Scholar]
Johansen 1993 {published data only (unpublished sought but not used)}
- Johansen J, Kjeldgård M, Corydon L. Retrobulbar anaesthesia: a clinical evaluation of four different anaesthetic mixtures. Acta Ophthalmologica 1993;71(6):787‐90. [PUBMED: 8154254] [DOI] [PubMed] [Google Scholar]
Lange 1989 {published data only (unpublished sought but not used)}
- Lange W, Denffer H, Honis M. Comparison of bupivacaine 0.75% with a mixture of bupivacaine 0.75% and mepivacaine 2% in retrobulbar anaesthesia: effects of hyaluronidase. Fortschritte der Ophthalmologie 1989;86(4):312‐5. [PUBMED: 2676792] [PubMed] [Google Scholar]
Moharib 2002 {published data only (unpublished sought but not used)}
- Moharib MM, Mitra S, Rizvi SG. Effect of alkalinization and/or hyaluronidase adjuvancy on a local anesthetic mixture for sub‐Tenon's ophthalmic block. Acta Anaesthesiologica Scandinavica 2002;46(5):599‐602. [PUBMED: 12027856] [DOI] [PubMed] [Google Scholar]
Morsman 1992 {published data only (unpublished sought but not used)}
- Morsman CD, Holden R. The effects of adrenaline, hyaluronidase and age on peribulbar anaesthesia. Eye 1992;6(3):290‐2. [PUBMED: 1446762] [DOI] [PubMed] [Google Scholar]
Ramanathan 1999 {published data only (unpublished sought but not used)}
- Ramanathan US, Sullivan CA, Thompson SM, McCauley D. A study of effect of hyaluronidase on subTenon anaesthesia. Cataract Surgery and Assessment 1999;40(4):S287. [Google Scholar]
Sarvela 1992 {published data only (unpublished sought but not used)}
- Sarvela J, Nikki P. Hyaluronidase improves regional ophthalmic anaesthesia with etidocaine. Canadian Journal of Anaesthesia 1992;39(9):920‐4. [PUBMED: 1451220] [DOI] [PubMed] [Google Scholar]
Soares 2002 {published data only (unpublished sought but not used)}
- Soares LF, Escovedo Helayel P, Conceição DB, Oliveira Filho GR. Peribulbar block with the association of 0.5% enantiomeric mixture of bupivacaine and 2% lignocaine: effects of hyaluronidase addition. Revista Brasileira de Anestesiologia 2002;52(4):420‐5. [PUBMED: 19479106] [DOI] [PubMed] [Google Scholar]
Additional references
Afshari 2017
- Afshari A, Wetterslev J, Smith AF. Can systematic reviews with sparse data be trusted?. Anaesthesia 2017;72(1):12‐6. [PUBMED: 27804113 ] [DOI] [PubMed] [Google Scholar]
Alhassan 2015
- Alhassan MB, Kyari F, Ejere HOD. Peribulbar versus retrobulbar anaesthesia for cataract surgery. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD004083.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkinson 1949
- Atkinson WS. Use of hyaluronidase with local anaesthesia in ophthalmology; preliminary report. Archives of Ophthalmology 1949;42(5):628‐33. [PUBMED: 15393388 ] [DOI] [PubMed] [Google Scholar]
Borders 1968
- Borders CL, Raferty MA. Purification and partial characterization of testicular hyaluronidase. Journal of Biological Chemistry 1968;243(13):3756‐62. [PUBMED: 5658550] [PubMed] [Google Scholar]
Brown 1999
- Brown S, Brooks S, Mazow M, Avilla C, Braverman D, Greenhaw S, et al. Cluster of diplopia cases after periocular anesthesia without hyaluronidase. Journal of Cataract and Refractive Surgery 1999;25(9):1245‐9. [PUBMED: 10476509 ] [DOI] [PubMed] [Google Scholar]
Brown 2001
- Brown S, Coats D, Collins M, Underdahl J. Second cluster of strabismus cases after periocular anesthesia without hyaluronidase. Journal of Cataract and Refractive Surgery 2001;27(11):1872‐5. [PUBMED: 11709263] [DOI] [PubMed] [Google Scholar]
Buhren 2016
- Buhren BA, Schrumpf H, Hoff NP, Bölke N, Hilton S, Gerber PA. Hyaluronidase: from clinical applications to molecular and cellular mechanisms. European Journal of Medical Research 2016;21(5). [PUBMED: 26873038] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dempsey 1997
- Dempsey GA, Barrett PJ, Kirby IJ. Hyaluronidase and peribulbar block. British Journal of Anaesthesia 1997;78(6):671‐4. [PUBMED: 9215017] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed August 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guay 2015
- Guay J, Sales K. Sub‐Tenon's anaesthesia versus topical anaesthesia for cataract surgery. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD006291.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583 ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hylenex® Prescribing Information 2016
- Halozyme Therapeutics, Inc. Hylenex® recombinant prescribing information, 2016. hylenex.com/downloads/approved‐uspi‐lbl301feb2016.pdf (accessed 27 January 2018).
Kempeneers 1992
- Kempeneers A, Dralands L, Ceuppens J. Hyaluronidase induced orbital pseudotumour as complication of retrobulbar anesthesia. Bulletin de la Societe Belge d'Ophtalmologie 1992;243:159‐66. [PUBMED: 1302146 ] [PubMed] [Google Scholar]
Koornneef 1988
- Koornneef L. Eyelid and orbital fascial attachments and their clinical significance. Eye 1988;2(2):130‐4. [PUBMED: 3197870] [DOI] [PubMed] [Google Scholar]
Meyer 1934
- Meyer K, Palmer FW. The polysaccharide of the vitreous humour. Journal of Biochemistry 1934;107:629‐34. [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 1993
- Roberts J, MacLeod B, Hollands RH. Improved peribulbar anaesthesia with alkalinization and hyaluronidase. Canadian Journal of Anaesthesia 1993;40(9):835‐8. [PUBMED: 8403178] [DOI] [PubMed] [Google Scholar]
Schein 2000
- Schein OD, Friedman DS, Fleisher LA, Lubomski LH, Magaziner J, Sprintz M, et al. Anaesthesia Management During Cataract Surgery. Evidence Report/Technology Assessment Number 16 AHRQ Publication No. 00‐E015 (Archived). Vol. 1, Rockville (MD): Agency for Healthcare Research and Quality, 2001. [Google Scholar]
WHO 2010
- World Health Organization. WHO guidelines on tissue infectivity distribution in transmissible spongiform encephalopathies, 2010. www.who.int/bloodproducts/tse/WHO%20TSE%20Guidelines%20FINAL‐22%20JuneupdatedNL.pdf (accessed 27 January 2018).
References to other published versions of this review
Rüschen 2013
- Rüschen H, Adams L, Bunce C. Use of hyaluronidase as an adjunct to local anaesthetic eye blocks. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD010368] [DOI] [PMC free article] [PubMed] [Google Scholar]


