Abstract
Background
Asthma exacerbations in school‐aged children peak in autumn, shortly after children return to school following the summer holiday. This might reflect a combination of risk factors, including poor treatment adherence, increased allergen and viral exposure, and altered immune tolerance. Since this peak is predictable, interventions targeting modifiable risk factors might reduce exacerbation‐associated morbidity and strain upon health resources. The peak occurs in September in the Northern Hemisphere and in February in the Southern Hemisphere.
Objectives
To assess the effects of pharmacotherapy and behavioural interventions enacted in anticipation of school return during autumn that are designed to reduce asthma exacerbations in children during this period.
Search methods
We searched the Cochrane Airways Group Trials Register, ClinicalTrials.gov, the World Health Organization International Clinical Trials Registry Platform, reference lists of primary studies and existing reviews, and manufacturers’ trial registries (Merck, Novartis and Ono Parmaceuticals). We searched databases from their inception to 1 December 2017, and imposed no restriction on language of publication.
Selection criteria
We included all randomised controlled trials comparing interventions aimed specifically at reducing autumn exacerbations with usual care, (no systematic change in management in preparation for school return). We included studies providing data on children aged 18 years or younger.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Two review authors independently screened records identified by the search and then extracted data and assessed bias for trials meeting the inclusion criteria. A third review author checked for accuracy and mediated consensus on disagreements. The primary outcome was proportion of children experiencing one or more asthma exacerbations requiring hospitalisation or oral corticosteroids during the autumn period.
Main results
Our searches returned 546 trials, of which five met our inclusion criteria. These studies randomised 14,252 children to receive either an intervention or usual care. All studies were conducted in the Northern Hemisphere. Three interventions used a leukotriene receptor antagonist, one used omalizumab or a boost of inhaled corticosteroids, and the largest study, (12,179 children), used a medication reminder letter. Whilst the risk of bias within individual studies was generally low, we downgraded the evidence quality due to imprecision associated with low participant numbers, poor consistency between studies, and indirect outcome ascertainment.
A US study of 513 children with mild/severe asthma and allergic sensitisation was the only study to provide data for our primary outcome. In this study, the proportion of participants experiencing an exacerbation requiring oral corticosteroids or hospital admission in the 90 days after school return was significantly reduced to 11.3% in those receiving omalizumab compared to 21.0% in those receiving placebo (odds ratio 0.48, 95% confidence interval 0.25 to 0.92, moderate‐quality evidence). The remaining studies used alternative exacerbation definitions. When data from two leukotriene receptor antagonist studies with comparable outcomes were combined in a random‐effects model, there was no evidence of an effect upon exacerbations. There was no evidence that a seasonal medication reminder letter decreased unscheduled contacts for a respiratory diagnosis between September and December.
Four studies recorded adverse events. There was no evidence that the proportion of participants experiencing at least one adverse event differed between intervention and usual care groups. Lack of data prevented planned subgroup and sensitivity analyses.
Authors' conclusions
Seasonal omalizumab treatment from four to six weeks before school return might reduce autumn asthma exacerbations. We found no evidence that this strategy is associated with increased adverse effects other than injection site pain, but it is costly. There were no data upon which to judge the effect of this or other seasonal interventions on asthma control, quality of life, or asthma‐related death. In future studies definitions of exacerbations should be provided, and standardised where possible. To investigate possible differential effects according to subgroup, participants in future trials should be well characterised with respect to baseline asthma severity and exacerbation history in addition to age and gender.
Plain language summary
Interventions to prevent asthma attacks in children upon return to school in the autumn
Background
Asthma is a long‐term condition affecting the lungs. It is the most common long‐term condition affecting children. One in 11 children in the United Kingdom have asthma. People with asthma can experience asthma 'attacks' of coughing, wheezing, and difficulty breathing.
Each year there is a peak in asthma attacks after school restarts in autumn. The likely reason for this is that children are exposed to more viruses that can trigger asthma. Children may also have taken their regular medication less consistently with the break in routine over the summer.
As this increase in attacks at the start of the school year is predictable, and the reason for it is somewhat understood, it might be preventable. Approaches to reducing autumn asthma attacks include using extra medications when school restarts or medication reminders during the school holiday.
Main findings
Our searches found 546 trials, of which five were relevant. In total, 14,252 children were randomly assigned to receive either an intervention targeting autumn asthma attacks or usual care. Four small studies (approximately 200 to 1200 children in each) gave children extra asthma medication; these additional medications were omalizumab, leukotriene receptor antagonist tablets, or increased doses of inhaled steroids. One study sent a medication reminder letter over the summer holidays to parents of children with asthma.
One trial gave children either omalizumab or placebo. Omalizumab is an antibody designed to alter the immune response. It was given by injection regularly over four to six weeks before school return (i.e. over the bulk of the summer holidays). The children in this study had known allergic asthma. The study showed that omalizumab might reduce autumn attacks. Eleven per cent of those receiving omalizumab had an asthma attack during the first 90 days compared to 21% of those receiving placebo.
Three studies used leukotriene receptor antagonist tablets, either montelukast or pranlukast. Although the results of one study suggested that seasonal montelukast might reduce autumn attacks, there was no evidence of reduced attacks in the other two later trials, including a second larger trial of montelukast.
There was no evidence that sending a reminder letter reduces the number of children requiring an unplanned healthcare contact.
No study provided evidence that the total number of children experiencing adverse events was greater in the intervention than in the usual care group.
Limitations
Our findings were limited by the small numbers of studies identified and because these studies used different interventions and definitions of asthma exacerbations. Further research is needed to better understand how to prevent seasonal attacks, including interventions suitable for children with mild asthma, where expensive and painful treatments are not justified.
Summary of findings
Background
Description of the condition
Asthma is a chronic disease of the airways characterised by recurrent episodes of wheezing, breathlessness, and cough, together with variable expiratory airflow limitation. Symptoms are frequently associated with airway inflammation and bronchial hyper‐responsiveness (GINA 2017). Asthma can affect people of all ages, although childhood onset is common. Asthma is diagnosed clinically based upon evaluation of symptoms and response to pharmacotherapy. There is no specific diagnostic test, although spirometric measurement of reversible airflow limitation and indirect or direct tests of airway hyper‐responsiveness can be useful (GINA 2017).
The number of people with asthma globally is currently estimated to be approximately 300 million, and is expected to grow to closer to 400 million by 2025 (WHO 2007). Asthma is the most common chronic disease among children (Asher 2014). The International Study of Asthma and Allergies in Childhood (ISAAC), conducted between 2002 and 2003, found the highest prevalence of childhood wheeze in Latin and North America, and in English‐speaking countries in Australasia and Europe (Asher 2006). More than 1 million children (1 in 11) in the United Kingdom are currently believed to be living with asthma (Asthma UK 2016).
Symptom exacerbations can be triggered by a number of environmental challenges, including pollutants (Lierl 2003; Schildcrout 2006), physical activity (Randolph 2013), and respiratory infections or allergens (Brandt 2015; Ito 2015; Murray 2006; Olenec 2010). People whose airway inflammation is not adequately controlled are more vulnerable to exacerbations than those on adequate therapy with good treatment adherence. Poorly controlled day‐to‐day asthma symptoms can limit activities, including schooling, and impair sleep quality and overall quality of life (Kiotseridis 2013; Teyhan 2015; van Maanen 2013). However, it is asthma exacerbations or 'attacks' ‐ acute or subacute progressive worsening of symptoms ‐ which pose the greatest danger to people with asthma (NAEPP 2007). Asthma exacerbations are also associated with reduced school or work attendance and are the most important contributor to the economic and social costs of asthma (Bahadori 2009; Hoskins 2000; Ismaila 2013).
A seasonal peak in exacerbation rates has been consistently demonstrated in the autumn months (September to November) across multiple Northern Hemisphere countries (Fleming 2000; Gergen 2002). More specifically, exacerbation rates peak in September following the summer school holiday and in line with the start of the autumn term (Johnston 2006). Equivalent peaks during February have been reported in Southern Hemisphere countries (Lincoln 2006; Lister 2001). The autumn peak in asthma exacerbations is temporally linked to children returning to school and most pronounced in school‐aged children (Corne 2002). Hospitalisations and emergency department visits attributable to asthma demonstrate an initial peak in school‐aged children; however, this is followed within days by increased hospitalisations in preschool children and a more blunted peak in adults up to the age of 50 years (Sears 2008). There is evidence that viral infections, particularly rhinovirus, may contribute to this seasonality (Johnston 1996; Johnston 2005; Thumerelle 2003), but suboptimal asthma treatment and changes in tolerance may also be contributing factors (Johnston 2005; Tovey 2011). Not only do viral infections trigger asthma exacerbations, but there is also evidence that asthmatic individuals are more susceptible to rhinovirus infection than those without asthma (Baraldo 2012; Wark 2005). Individuals at particular risk of asthma exacerbation have been identified as those with more severe disease, greater degree of atopy, and recent exacerbations (Teach 2015b).
Description of the intervention
A number of interventions including asthma education programmes, action plans, self monitoring, and self initiation of oral corticosteroid (OCS) treatment have been shown to reduce both symptom exacerbations and need for unscheduled acute care in children with asthma (Bhogal 2006; Guevara 2003; Vuillermin 2011). Given that the seasonality of asthma exacerbations in school‐aged children is predictable and repeatable, it is reasonable to assume that management strategies that anticipate increased risk in the autumn might reduce exacerbation frequency during this period. Whilst the exact aetiology of the seasonal peak in asthma exacerbations is not fully understood, any change in management aimed at improving asthma control in anticipation of the autumn school return, if successful, could offer protection against the increased risk recognised to be associated with this event. Therapies that have been demonstrated to reduce the seasonal excess of exacerbations in the autumn, in addition to the annual number of exacerbations, include year‐round treatment with the anti‐immunoglobulin E (IgE) monoclonal antibody omalizumab (Busse 2011); or with high‐dose inhaled corticosteroids (ICS) (Szefler 2008). However, omalizumab is an expensive and sometimes painful treatment, whilst high‐dose ICS are associated with adverse effects upon growth and bone health (Pruteanu 2014; Wong 2000).
Given the pragmatic difficulties associated with minimising viral or allergen exposure, two main potential strategies remain that might reduce autumn asthma exacerbations whilst minimising treatment costs and adverse effects. The first strategy would be to add on, or increase, asthma pharmacotherapy before the autumn period; the second strategy would be to focus upon treatment adherence and achieving symptom control before and during the autumn. It is anticipated that school‐aged children would gain the greatest benefit from an intervention targeting seasonal exacerbations, since the autumn peak in exacerbations is most pronounced in this age group. Similarly, greater benefit might be demonstrable in those at increased risk of exacerbation due to poor treatment adherence, severe disease, allergic phenotype, or recent exacerbation.
Add‐on therapies include those aimed at reducing airway inflammation, such as corticosteroid preparations, macrolide antibiotics, or leukotriene receptor antagonists (LTRAs). Alternatively, agents such as biologics which more specifically target the interaction between the immune response, allergens, and viral infection might be selected (Beck 2004; Durrani 2012; Gill 2010). Important considerations with respect to choice of intervention include onset of action and ease of administration, in addition to cost and adverse effect profile.
Strategies to improve treatment adherence require adherence status to be assessed, and barriers leading to non‐adherence to be identified and addressed. The success of adherence interventions can be increased by a number of strategies, including the provision of biofeedback, Feldman 2012, and increasing motivation via motivational interviewing techniques (Borrelli 2007). Nevertheless, it is difficult to achieve sustained adherence (Jonasson 2000). Targeting adherence interventions to periods of increased exacerbation risk might increase their overall benefit.
How the intervention might work
Upon return to school in the autumn children are exposed to allergens and respiratory infections by close contact with their classmates (Cai 2011; Krop 2014). During the autumn months mould spores, which can act as a trigger for allergic asthma, are more abundant than at other times of the year (de Ana 2006). However, the sequential periods of peak risk demonstrated by school‐aged children, younger children, and adults suggest a transmissible agent is responsible. In support of this are findings from virological studies that demonstrate increased viral isolations during autumn, notably rhinovirus, from children with asthma compared to those without, with the highest rates of isolation measured in those admitted to hospital with an asthma exacerbation (Johnston 2005; Thumerelle 2003).
Changes in routine during the summer holidays and lower perceived risk of cold weather or respiratory infection might be associated with both intentional reduction in preventer medication and unintentional poor adherence (Johnston 2005; Sears 2008). A higher rate of exacerbation has been reported in people prescribed bronchodilator therapy alone than in those prescribed an inhaled steroid or other preventer medication (Johnston 2005; Murray 2006). Furthermore, within a trial of seasonal omalizumab treatment, school‐aged children with mild asthma but poor control, as evidenced by an exacerbation during the run‐in period of four to nine months, experienced a significant reduction in exacerbation frequency (Teach 2015a). Exacerbation frequency could not be significantly reduced in those with mild asthma but without a recent admission (Teach 2015a). Any intervention based upon reinforcing or increasing adherence to regular treatment, monitoring symptoms to assess control, or a seasonal enhancement of treatment might potentially reduce ongoing airway inflammation and the likelihood of viral infection triggering an exacerbation.
Why it is important to do this review
Although the asthma epidemic observed in the 1980s and 1990s appears to have stabilised, a study from the Northern Hemisphere demonstrates that emergency care contacts due to asthma remain significantly higher in September than in other months (Larsen 2016). Despite this, current national and international guidelines offer no guidance on strategies to reduce seasonal exacerbations after autumn school return. Following the recent successful trial of seasonal omalizumab, which demonstrated reduced exacerbations amongst children with severe or poorly controlled asthma (Teach 2015a), it is important to identify whether a similar effect can be achieved with less invasive and less expensive medications. This is particularly the case in countries such as the United Kingdom where omalizumab can only be prescribed to children meeting strict severity criteria. A quarter of annual hospitalisations for asthma are estimated to occur in September (Johnston 2001), and acute exacerbations are the principal driver of the economic and social costs of asthma (Bahadori 2009; Hoskins 2000; Ismaila 2013). Interventions based upon an anticipatory change in asthma management, if successful, could therefore substantially reduce both the overall exacerbation rate and the strain placed upon health services during autumn.
Objectives
To assess the effects of pharmacotherapy and behavioural interventions enacted in anticipation of school return during autumn that are designed to reduce asthma exacerbations in children during this period.
Methods
Criteria for considering studies for this review
Types of studies
We restricted inclusion to randomised controlled trials with a control arm of usual care since currently there is no recommended management strategy for autumn exacerbations. Studies reported as full text, those published as abstract only, and unpublished data were all eligible for inclusion.
Types of participants
We included studies presenting data relating to children with asthma. Studies needed to recruit children aged 18 years or younger, including preschool‐age as well as school‐aged children.
Types of interventions
We included studies comparing interventions aimed specifically at reducing autumn exacerbations with usual care where there is no systematic change in management in preparation for school return. Eligible interventions included pharmacotherapy trials and behavioural or educational‐based initiatives.
Types of outcome measures
Primary outcomes
The primary outcome was number of children (adjusted for the number of participants per group) experiencing one or more asthma exacerbations during the autumn period (the first three‐month period following the autumn school return) or during the intervention period if this included the autumn months. An exacerbation was defined as increased asthma symptoms requiring treatment with OCS or hospitalisation.
Secondary outcomes
Number of children experiencing exacerbations of asthma requiring hospitalisation.
Number of children experiencing exacerbations of asthma requiring paediatric intensive care unit admission.
Number of asthma‐related deaths.
Asthma control, measured by standardised tool (e.g. Childhood Asthma Control Test (cACT) or Asthma Control Test (ACT)).
Asthma‐related quality of life measured by standardised tool (e.g. Paediatric Asthma Quality of Life Questionnaire (PAQLQ) or Asthma Quality of Life Questionnaire (AQLQ)).
Days of schooling (or employment, for those beyond school age) missed.
Adverse events (including serious adverse events).
For each outcome data were collected throughout the autumn period or the intervention period (as for the primary outcome) in both the intervention group and the usual therapy group.
We did not require report of the primary outcome as an inclusion criterion for the review.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group's Trials Register, which is maintained by the Information Specialist for the Group. The Register contains trial reports identified through systematic searches of several sources:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies Online (crso.cochrane.org);
weekly searches of MEDLINE Ovid SP 1946 to date;
weekly searches of Embase Ovid SP 1974 to date;
monthly searches of PsycINFO Ovid SP 1967 to date;
monthly searches of CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature) 1937 to date;
monthly searches of AMED EBSCO (Allied and Complementary Medicine) all years to date;
handsearches of the proceedings of major respiratory conferences.
Studies contained in the Trials Register are identified through search strategies based on the scope of Cochrane Airways. Details of these strategies, as well as a list of handsearched conference proceedings, can be found in Appendix 1. See Appendix 2 for search terms used to identify studies for this review.
We also conducted a search of ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp/en/). We searched all databases from their inception to 1 December 2017, and imposed no restriction on language of publication.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We searched relevant manufacturers' websites for trial information (Merck, Novartis and Ono Pharmaceuticals).
On 1 December 2017 we searched for errata or retractions from included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed).
Data collection and analysis
Selection of studies
Two review authors (KCP, MA) independently screened for potential inclusion titles and abstracts of all the studies identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports/publications, and two review authors (KCP, MA) independently screened the full texts and identified studies for inclusion and recorded reasons for exclusion of the ineligible studies. Any disagreements were resolved through discussion or, if required, by consultation with a third review author (DK). We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process using EPPI‐Reviewer 4 and completed a PRISMA flow diagram and Characteristics of included studies table (EPPI‐Reviewer 4 2010; Moher 2009).
Data extraction and management
We used a data collection form for study characteristics and outcome data that was piloted on two studies in the review. Three review authors (KCP, KMH, MA) extracted study characteristics from included studies in triplicate. We extracted the following study characteristics.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and location, study setting, withdrawals, and date of study.
Participants: number, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, and exclusion criteria.
Interventions: intervention type, comparison, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
Two review authors (KCP, MA) independently extracted outcome data from the included studies. We noted in the Characteristics of included studies table if outcome data were not reported in a usable way. Any disagreements were resolved by consensus or by involving a third review author (KMH) when necessary. One review author (KCP) transferred data into the Review Manager 5 file (RevMan 2014). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the data in the study reports. A second review author (DK) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (KCP, MA) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreements were resolved by discussion or by involving another review author (KMH) when necessary. We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low, or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' tables included within the Characteristics of included studies table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for asthma‐related mortality may be very different than for a patient‐reported asthma control scale). Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the Characteristics of included studies table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol (Pike 2016) and reported deviations from it in the Differences between protocol and review section.
Measures of treatment effect
We analysed dichotomous data as odds ratios.
We undertook meta‐analyses only where this was meaningful, such as when the interventions, participants, outcomes, and underlying clinical question were similar enough for pooling to make sense.
Unit of analysis issues
We considered asthma exacerbation a dichotomous outcome using participants as the unit of analysis. The odds of exacerbation in the intervention group during the intervention were compared to the odds of exacerbation in individuals receiving usual therapy. Where multiple changes in management strategy were included in the original studies (e.g. seasonal omalizumab or a steroid boost in addition to usual therapy), the odds of exacerbation in each group that included a change in management were compared to the group receiving usual care only or usual care with a placebo. For large‐scale behavioural interventions (e.g. those involving contacting families in late summer to remind them of the need for treatment adherence), the unit of allocation may be at the level of primary care practice level rather than the individual. Where this was the case, we included results only if the original trial accounted for clustering or if it was possible to adjust for this by calculating a design effect.
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and to obtain missing numerical outcome data where possible (e.g. if an odds ratio was presented without a confidence interval).
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis. Where possible we intended to report the I² statistic, and if we identified substantial heterogeneity (I² > 50%) to explore possible causes by prespecified subgroup analyses.
Assessment of reporting biases
Had we been able to pool more than 10 trials, we intended to create and examine a funnel plot to explore possible small‐study and publication biases.
Data synthesis
We used an inverse variance model for outcomes where odds ratios from the original studies were adjusted for covariables. We used a Mantel‐Haenszel model for outcomes where confounding covariables were not identified and where absolute numbers of children experiencing the outcome were reported or could be calculated. We used Review Manager 5 software to calculate random‐effects models for all outcomes (RevMan 2014), as we expected variation in effects due to differences in study populations and methods (Mantel 1959). We performed a sensitivity analysis with a fixed‐effect model when we encountered significant heterogeneity.
'Summary of findings' tables
We created 'Summary of findings' tables for each intervention type using the following outcomes: exacerbation occurrence (requiring oral steroids or hospitalisation), exacerbation occurrence (defined according to alternative definition), and adverse events. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contributed data for the prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), employing GRADEpro GDT software. We justified all decisions to down‐ or upgrade the quality of studies using footnotes and made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We recognised that intervention type and/or disease severity might affect effect sizes, and therefore planned to carry out the following subgroup analyses for all outcomes.
An analysis separating studies based on pharmacological interventions from those based on non‐pharmacological interventions.
Analyses considering separately those with mild to moderate disease (intermittent bronchodilator only; or low/moderate ICS with or without a single add‐on therapy) and those with severe asthma (two or more add‐on therapies; or high‐dose ICS ‐ daily beclomethasone equivalents for children 5 to 12 years: ≥ 800 mcg; for children older than 12 years: ≥ 2000 mcg).
We planned to use identical primary and secondary outcomes in subgroup analyses as in the main analysis.
We planned to use the formal test for subgroup interactions in Review Manager 5 to determine statistical significance of subgroup analyses (RevMan 2014).
Sensitivity analysis
We planned to carry out the following sensitivity analyses.
An analysis including only studies without missing data.
An analysis excluding cluster‐randomised trials (in case any benefit in cluster‐randomised trials arises due to the 'herd' effect of an intervention).
We also planned to re‐run analyses and compare results after sequential exclusion of each study from any meta‐analysis.
Results
Description of studies
We included detailed descriptions of studies fulfilling the criteria specified in the protocol in the Characteristics of included studies section. Studies for which full texts were reviewed but were eventually excluded were collated along with reasons for exclusion in the Characteristics of excluded studies section.
Results of the search
Electronic searches run in December 2017 identified 546 records. We removed four duplicates and four abstracts where full texts describing the same study were also identified. After screening full texts and abstracts, we evaluated 31 full texts against the inclusion criteria. We assessed 22 as not meeting the inclusion criteria, leaving nine references to five studies for inclusion in the review (Figure 1).
1.

Study flow diagram.
Included studies
Five studies (nine citations) met the inclusion criteria. All five reported upon the effect of an intervention specifically designed to reduce asthma exacerbations in predominantly school‐aged children following return to school in the autumn. Two studies were funded by Merck (Johnston 2007; Weiss 2010), two by national funding bodies, (Julious 2016 (funded by the National Institute for Health Research) and Teach 2015a (funded by the National Institute of Allergy and Infectious Diseases, the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, Department of Health and Human Services)), and one did not list a funding source (Morita 2017). Between‐study variation in participant inclusion criteria, intervention type, and outcome definition limited the opportunity for meta‐analysis. For full details see the Characteristics of included studies tables.
Design and duration
The five included studies randomised 14,252 children to receive either an intervention or usual care. All studies were conducted in the Northern Hemisphere. The largest study randomised 12,179 children in a cluster‐randomised trial of a primary care‐based public health intervention (Julious 2016). Data were collected from August 2013 until the end of September 2014 from the Clinical Practice Research Datalink (CPRD), a computerised database of anonymised longitudinal medical records for primary care. The remaining studies were randomised controlled clinical trials employing a pharmacological intervention; three were blinded and one was an open study. Johnston 2007, Weiss 2010, and Morita 2017 studied interventions based upon LTRA administration from school return, whilst Teach 2015a compared administration of the anti‐IgE monoclonal omalizumab to a doubling of ICS dose or placebo initiated four to six weeks before school return. Three pharmacological studies recruited across multiple sites (Morita 2017; Teach 2015a; Weiss 2010), whilst in Johnston 2007 participation was at a single site. In Johnston 2007, 194 children were followed up for 45 days with no run‐in period. In Weiss 2010, 1162 children were followed up for 10 weeks after a 2‐ to 12‐week screening period. The 513 children in Teach 2015a received guideline‐based treatment to gain asthma control during a 4‐ to 9‐month run‐in period and were followed up until 90 days after school return. In Morita 2017, 204 children were enrolled and randomised two to six weeks before entering a 60‐day study period.
Inclusion and exclusion criteria
All studies referenced age and asthma diagnosis in their inclusion criteria. Julious 2016 included children aged 4 to 16 years with a coded diagnosis of asthma within their primary care record and a prescription for asthma medication within the last year. Four‐year‐old children were analysed separately since, whilst they are of school age in the United Kingdom, a diagnosis of asthma in this age group was judged to be more controversial than in older children. Children with neoplastic disease and those judged unsuitable for the intervention by their general practitioner were excluded. Johnston 2007 included children aged 2 to 14 years with doctor‐diagnosed asthma. Additional inclusion criteria were use of a reliever inhaler in the last year, school absence due to asthma in the last year or significant activity limitation, history of asthma exacerbations associated with respiratory viral infections, and ability to communicate in English. Children with significant cardiorespiratory comorbidity were excluded, as were those with an asthma exacerbation during the month before study inception and those using regular OCS or an LTRA. Weiss 2010 included children aged 6 to 14 years with a history of chronic asthma needing asthma medication in the six months preceding screening, at least one asthma exacerbation in the previous year in conjunction with a cold, and an alteration in environment differing from their typical school or education environment throughout August/September. Morita 2017 recruited 1‐ to 14‐year‐old children with physician‐diagnosed asthma, needing a rescue inhaler in the last year, and with a history of asthma exacerbations associated with apparent respiratory viral infections. Exclusion criteria were significant cardiorespiratory comorbidity, regular OCS use, or an asthma exacerbation in the month before the treatment period. Teach 2015a recruited children aged 6 to 17 years with an asthma diagnosis or symptoms for more than a year and at least one asthma exacerbation (requiring systemic corticosteroids or hospitalisation) within the prior 19 months. Additional inclusion criteria were positive perennial allergen skin test response, body weight and total serum IgE levels suitable for omalizumab, school attendance the following August or September, residence in a low‐income census tract, and insurance covering standard medications. There were no exclusions for this study beyond not meeting these inclusion criteria.
Baseline characteristics of participants
All five studies recruited more male than female participants: in each study 60% to 65% of participants were male. No study reported smoking status or exposure to environmental tobacco smoke. Only two studies reported baseline lung function: in Weiss 2010 mean forced expiratory volume in the first second of expiration (FEV1) was 89.8% predicted in the intervention group and 90.1% in the usual care group, and in Teach 2015a mean FEV1 across both groups at randomisation was 90.2% predicted. Only Teach 2015a systematically reported asthma severity: 195 randomised children were classified as step 5 according to a severity scale based on the National Heart, Lung and Blood Institute Expert Panel Report‐3 (severe persistent symptoms requiring high‐dose ICS and one adjunctive therapy), and 318 met the criteria for asthma severity steps 2 to 4 (mild‐moderate persistent symptoms requiring preventer medication but no more than medium‐dose ICS and one adjunctive therapy). Johnston 2007 reported that 90% of children were routinely receiving ICS, suggesting moderate severity, whilst only 30% of participants in Weiss 2010 and 50% in Morita 2017 routinely received ICS at randomisation. It is likely that the general practice‐based population in Julious 2016 included more people with mild asthma than the studies recruiting from secondary care.
Description of the intervention
The behavioural public health intervention was a letter sent to parents/carers of school‐aged children with asthma from the child's general practitioner reminding them to maintain their child’s medication and to collect a prescription if they were running low on medication. The letter was sent out during the week commencing 29 July and highlighted that school return is a time when asthma can worsen. The comparison group did not receive a letter (Julious 2016). In the pharmacological studies the interventions were added to usual care and compared with a placebo in addition to usual care. In Johnston 2007, an age‐specific dose of montelukast was given from 1 September to 15 October, whilst participants in Weiss 2010 received 5 mg montelukast from the night before the first day of school for eight weeks. Children in the intervention group in Morita 2017 received pranlukast 7 mg/kg twice daily between 15 September and 14 November. In Teach 2015a, children were randomised 3:3:1 to a standard dose of omalizumab based on serum IgE levels and weight, a doubling of their ICS dose, or placebo from 4 to 6 weeks before the start of the autumn term, continuing for 90 days after school return. Only children at steps 2 to 4 were entered into the ICS boost arm because of concerns that very high‐dose ICS provides limited additional efficacy and increases the risk of side effects.
Outcomes and analysis
Julious 2016 studied a number of outcomes, but the primary outcome was the proportion of children aged 5 to 16 years with unscheduled contacts during September 2013. Secondary outcomes measured in September included number of unscheduled contacts and proportion and total number of contacts (scheduled and unscheduled) and unscheduled contacts for a respiratory diagnosis. These outcomes were also measured throughout September to December 2013, September 2013 to August 2014, and in September 2014 in an 'echo study' to see if there was a maintained effect in the year following the main study and in which there was no study intervention. Between September 2013 and August 2014 time to first contact, first unscheduled contact, and first unscheduled contact for a respiratory diagnosis were also measured. The proportion of children with scheduled contacts was measured in August 2013, August 2014, and between August 2013 and July 2014. The number of participants collecting prescriptions was measured in August for both years. Quality‐adjusted life years (QALYs) gained and NHS health costs were measured between August 2013 and July 2014. Primary analyses were conducted on an intention‐to‐treat basis.
The primary outcome in Johnston 2007 was percentage of days during the intervention period with worsening asthma symptoms. Data were inputted daily by parents/carers into a prospectively completed sticker chart, and further data were collected by questionnaire two weeks after the end of the intervention period. The secondary outcome was number of unscheduled care visits. Analysis was intention‐to‐treat. In Weiss 2010, the primary outcome was percentage of days with worsening asthma symptoms, defined as one or more of increased beta‐agonist use, increased daytime symptoms score, night wakening, increased ICS use, OCS rescue or unanticipated visits to a doctor, emergency department, or hospital for asthma. Secondary outcomes were individual components of the composite primary endpoint and adverse events. Data were collected at 4, 8, and 10 weeks of the study and analysed in the full‐analysis population (all children who received at least one dose of study medication and had a valid measurement of the percentage of days with worsening asthma during the study period, derived from at least seven days of diary data). In Morita 2017, the primary outcome was total asthma score during the 60 study days, calculated based on asthma symptoms, need for medication, and need for an unscheduled physician visit or OCS. The secondary outcomes were days with worse asthma symptoms, number of colds, and days with fever. Data were analysed per protocol only from those compliant with treatment and returning adequate outcome data via a daily sticker chart. Teach 2015a conducted a modified intention‐to‐treat analysis, analysing data from children who were randomised, began study treatment, and had at least one study contact during the 90‐day outcome period. The primary outcome was asthma exacerbation, defined as worsening of asthma control requiring systemic corticosteroids or hospitalisation, during the 90‐day period from the first day of each child's school year. Secondary analyses considered exacerbations during the 90‐day intervention period according to subgroups based upon: exacerbation during run‐in, eosinophil count, total IgE, roach IgE, age, fractional exhaled nitric oxide (FeNO), FEV1, body mass index, ethnicity and gender. Interferon alpha responses to rhinovirus were measured in peripheral blood mononuclear cells from a subset of children.
Excluded studies
During screening of titles and abstracts, we excluded studies using a hierarchy of screening criteria. We asked first whether the study focused on asthma, followed by seasonal asthma exacerbations, and then considered whether the majority of the participants were school‐aged children, whether the paper focused on exacerbations at the beginning of the autumn school term, and compared an intervention to prevent these exacerbations to usual care. We excluded most studies due to no mention of seasonal asthma exacerbation or incorrect seasonal focus because the search terms picked up many studies of seasonal rhinitis in conjunction with asthma. Since a focus on asthma was the first stage in the screening hierarchy, this was also a common reason for exclusion. We excluded 22 records after viewing full texts, in most cases because the study did not focus on seasonal asthma exacerbations or did not present data from children. We excluded two studies because they did not employ an intervention specifically designed to reduce asthma exacerbations in children in autumn (Busse 2011; Gerald 2012), and two studies because they did not compare an intervention with usual care in which there is no systematic change in management in anticipation of children returning to school in the autumn (Prazma 2015; Yoshihara 2014); we prespecified both study designs as exclusionary in our protocol. We outlined details of reasons for exclusion of studies in the Characteristics of excluded studies section.
Risk of bias in included studies
Details of our 'Risk of bias' assessment for each included study and the reasoning behind our ratings can be found in the Characteristics of included studies section; a summary of 'Risk of bias' judgements by study and domain (selection bias, performance bias, detection bias, attrition bias, reporting bias, other bias) is presented in Figure 2. Most ratings in most domains for the included studies were low risk, with the exception of high risk of attrition bias in Morita 2017 as well as performance and detection bias due to lack of blinding. There was also unclear selection and performance bias in Julious 2016, unclear allocation bias in Weiss 2010 and Morita 2017, and unclear selective reporting bias in Johnston 2007.
2.
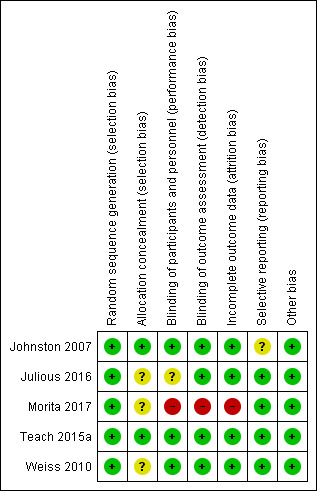
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All of the included studies were described as randomised. Each study described random sequence generation in sufficient detail in their report or in response to contact from the review authors to warrant a rating of low risk of bias. All included studies used computer‐generated randomisation. However, information on allocation concealment was incomplete for Weiss 2010 and Morita 2017, so this was rated unclear. Due to the nature of the intervention it was not possible in the primary care study to blind participating practices or children (Julious 2016), which might have led to some performance bias since practices were able to choose not to send the letter to individual patients or not to send any letters at all. There may have been systematic bias in the children or practices excluded in this manner, so we rated this study as at high risk of bias. Almost a quarter of the intervention group did not receive the intervention as intended. In contrast, since a letter reminding parents to pick up asthma medications for their child did not form part of usual care, all of the control group received the control intervention (no letter) according to the protocol.
Blinding
Morita 2017 was an open, unblinded study and was therefore at high risk of performance and detection bias. We found no evidence of risk of bias related to blinding of children or observers in the other pharmacological studies. These three studies were described as double‐blind, and study authors described measures such as matched placebos to hide group allocation from children and study personnel. In the primary care study (Julious 2016), the risk of detection bias was low since outcome data were collected via the Clinical Practice Research Datalink and designated as "scheduled", "unscheduled", or "irrelevant" by an independent adjudication panel comprised of experienced general practitioners who were blinded to the treatment group. However, there may have been some performance bias if coding of medical contacts was influenced by general practitioners knowing whether or not their practice was sending reminder letters. For this reason we rated performance bias for this study as unclear.
Incomplete outcome data
Risk of bias due to high or unbalanced dropout was low across all studies except Morita 2017. There was 14% attrition from the pranlukast group after commencing the study medication and only 3% attrition from the placebo arm. All children in Johnston 2007 completed the study, and rates of treatment adherence and diary card completion documenting outcome data were high. In Teach 2015a, the primary analysis was modified intention‐to‐treat, restricted to children who were randomised, began study treatment, and had one or more study contact during the outcome period. A number of sensitivity analyses were presented including best‐ and worst‐case analyses and an analysis using multiple imputation of missing data. There was good retention and similar dropout rates and reasons between groups. Weiss 2010 also conducted a modified intention‐to‐treat primary analysis, including all children who received at least one dose of study medication and had a valid measurement of the percentage of days with worsening asthma during the study period, derived from at least seven days of diary data. There was no imputation of missing data, but dropout rates and reasons were similar between groups. In Julious 2016, withdrawal rates were similar in the intervention and control arms. The trialists felt imputation was not required since outcome data were missing only where practices changed their computer system to one that did not support data collection. This was assumed to be unrelated to treatment allocation, however rates of withdrawal were at least 25% in both groups.
Selective reporting
All named outcomes were reported in the published reports of Weiss 2010, Teach 2015a, and Julious 2016; we rated these studies as at low risk of bias. For Johnston 2007, it was unclear if all a priori defined outcomes were reported. The protocol submitted at trial registration stated that OCS use would be an outcome considered separately from unscheduled medical contacts. Medical contacts were reported as an outcome, but OCS use was not. Although it was reported that all prescriptions of OCS occurred as a consequence of an unscheduled visit to a doctor, it was not clear whether all visits resulted in OCS prescription.
Other potential sources of bias
We identified no other sources of bias in any included study.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Omalizumab compared to usual care for autumn asthma exacerbations in children.
| Omalizumab compared to usual care for autumn asthma exacerbations in children | ||||||
|
Patient or population: autumn asthma exacerbations in children Setting: community Intervention: omalizumab Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with omalizumab | |||||
| Exacerbations assessed with: hospital admissions or oral steroid requirement in those with stage 2‐5 asthma follow‐up: 90 days | 210 per 1000 | 113 per 1000 (62 to 197) |
OR 0.48 (0.25 to 0.92) | 348 (1 RCT) |
⊕⊕⊕⊝ MODERATE 1 | Absolute effects calculated using control risk of 21.0% from Teach 2015a. |
| Exacerbations assessed with: hospital admissions or OCS requirement in those with stage 5 asthma follow‐up: 90 days | 326 per 1000 | 152 per 1000 (76 to 281) | OR 0.37 (0.17 to 0.81) | 184 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | Absolute effects calculated using control risk of 32.6% from Teach 2015a. |
| Exacerbations assessed with: hospital admissions or OCS requirement in those with stage 2‐4 asthma follow‐up: 90 days | 127 per 1000 | 83 per 1000 (31 to 207) | OR 0.63 (0.22 to 1.79) | 164 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | Absolute effects calculated using control risk of 12.7% from Teach 2015a. |
| Adverse events assessed with: number of children experiencing 1 or more adverse events asthma stage 2‐5 follow‐up: 17 to 19 weeks | 548 per 1000 | 546 per 1000 (425 to 657) | OR 0.99 (0.61 to 1.58) | 361 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OCS: oral corticosteroid; OR: odds ratio; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded once for imprecision because few children studied.
Summary of findings 2. A boost of inhaled corticosteroids compared to usual care for autumn asthma exacerbations in children.
| A boost of inhaled corticosteroids compared to usual care for autumn asthma exacerbations in children | ||||||
|
Patient or population: autumn asthma exacerbations in children Setting: community Intervention: a boost of inhaled corticosteroids Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with a boost of inhaled corticosteroids | |||||
| Exacerbations assessed with: hospital admission or oral corticosteroid requirement asthma stages 2‐4 follow‐up: 90 days | 127 per 1000 | 111 per 1000 (44 to 251) | OR 0.86 (0.32 to 2.30) | 173 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | Absolute effects calculated using control risk of 12.7% from Teach 2015a. |
| Adverse events assessed with: number of children experiencing 1 or more adverse events asthma stage 2‐4 follow‐up: 17 to 19 weeks | 533 per 1000 | 434 per 1000 (280 to 603) | OR 0.67 (0.34 to 1.33) | 176 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded once for imprecision because few children studied.
Summary of findings 3. Leukotriene receptor antagonist compared to usual care for autumn asthma exacerbations in children.
| Leukotriene receptor antagonist (LTRA) compared to usual care for autumn asthma exacerbations in children | ||||||
|
Patient or population: autumn asthma exacerbations in children Setting: community Intervention: LTRA Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with montelukast | |||||
| Exacerbations assessed with: oral corticosteroid or hospitalisation |
‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Exacerbations assessed with: unscheduled medical contacts follow‐up: range 45 days to 8 weeks | 146 per 1000 | 79 per 1000 (28 to 200) | OR 0.50 (0.17 to 1.46) | 1326 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Absolute effects calculated using control risk of 14.6% from Johnston 2007. |
| Adverse events assessed with: number of children experiencing 1 or more adverse events follow‐up: range 45 days to 10 weeks | 328 per 1000 | 307 per 1000 (235 to 392) | OR 0.91 (0.63 to 1.32) | 1326 (2 RCTs) | ⊕⊕⊕⊕ HIGH | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded once for inconsistency because asthma severity of children differed between included studies, and thresholds for medical contact or oral steroids appeared to differ between studies. 2Downgraded once for indirectness since studies contained no data on hospitalisation and need for oral steroids, so unscheduled medical contacts used as a proxy.
Summary of findings 4. Behavioural intervention compared to usual care for autumn asthma exacerbations in children.
| Behavioural intervention compared to usual care for autumn asthma exacerbations in children | ||||||
| Patient or population: autumn asthma exacerbations in children Setting: community Intervention: behavioural intervention Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with behavioural intervention | |||||
| Exacerbations assessed with: oral corticosteroid or hospitalisation | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Exacerbations assessed with: unscheduled contact for respiratory diagnosis follow‐up: 4 months | 167 per 1000 | 185 per 1000 (160 to 211) | OR 1.13 (0.95 to 1.33) | 10,481 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | Absolute effects calculated using control rate of 16.7% from Julious 2016. |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded once for indirectness because studies contained no data on hospitalisation and need for oral steroids, so unscheduled contacts for a respiratory diagnosis used as a proxy outcome.
Primary outcomes
Proportion of children experiencing one or more asthma exacerbations during the autumn period
Only Teach 2015a compared the number of children experiencing asthma exacerbations exactly as defined in the primary outcome of this review. Evidence relevant to this outcome is summarised in Table 1 and Table 2. During the 90‐day period from the first day of each child’s school year, the omalizumab intervention was associated with exacerbation (worsening of asthma control requiring systemic corticosteroids or hospitalisation) in 11.3% of children compared with 21.0% in the placebo arm, odds ratio (OR) 0.48, 95% confidence interval (CI) 0.25 to 0.92 (adjusted for study centre, dosing schedule, and asthma severity step) (Analysis 1.1, Figure 3). Considering those with stage 5 asthma, omalizumab was associated with a reduced odds of exacerbation (OR 0.37, 95% CI 0.17 to 0.81). In contrast, considering only steps 2 to 4 where children were allocated to omalizumab, placebo, or a third arm of a doubling of ICS, exacerbation rates were experienced by 8.4%, 12.7%, and 11.1% of children, respectively. The odds of exacerbation did not differ significantly between any pair of groups (omalizumab versus placebo OR 0.63, 95% CI 0.22 to 1.78; omalizumab versus inhaled steroid boost OR 0.73, 95% CI 0.33 to 1.64; inhaled steroid boost versus placebo OR 0.86, 95% CI 0.32 to 2.30). However, when those experiencing a recent exacerbation (during the four‐ to nine‐month run‐in ending four to six weeks before school return) were considered separately from those without a recent exacerbation, reduced odds of exacerbation were seen across all severity steps 2 to 5 in the omalizumab group compared to placebo (OR 0.12, 95% CI 0.02 to 0.64) and compared to ICS boost across steps 2 to 4 (OR 0.05, 95% CI 0.003 to 0.98). For those without an exacerbation during run‐in, the odds of exacerbation were OR 0.88, 95% CI 0.35 to 2.18 compared to placebo across steps 2 to 5 and OR 1.34, 95% CI 0.56 to 3.25 compared to ICS boost across steps 2 to 4.
1.1. Analysis.
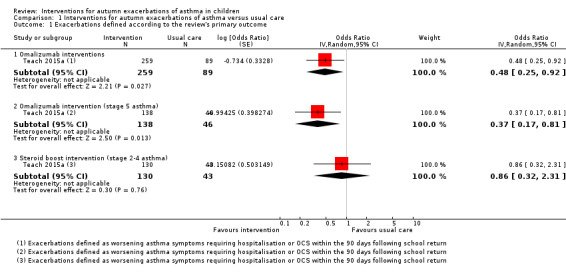
Comparison 1 Interventions for autumn exacerbations of asthma versus usual care, Outcome 1 Exacerbations defined according to the review's primary outcome.
3.
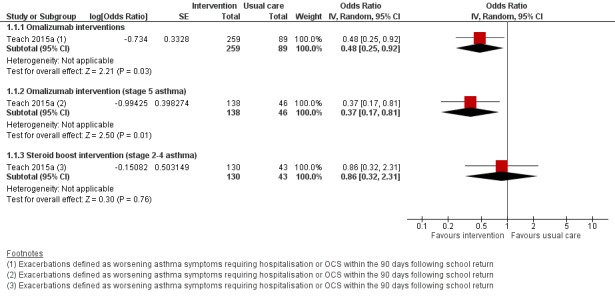
Forest plot of comparison: 1 Interventions for autumn exacerbations of asthma versus usual care, outcome: 1.1 Exacerbations defined according to the review's primary outcome.
Exacerbations reported according to alternative definitions
Johnston 2007 and Weiss 2010 considered the percentage of days with worsening asthma symptoms as the primary outcome, whilst Morita 2017 used a total asthma score based upon symptoms, medication need, and healthcare utilisation. These outcomes were not suitable for expression as an odds ratio. Johnston 2007 defined worsening asthma symptoms as symptoms that were worse than usual or needed extra asthma medication, or required an unscheduled visit to a doctor or treatment with oral corticosteroids; a 53% reduction in days with worsening asthma symptoms was reported compared with placebo during the 45‐day intervention (3.9% versus 8.3%, P = 0.02). Boys aged 2 to 5 years showed greater benefit from montelukast than did older boys, whereas among girls the treatment effect was most evident in 10‐ to 14‐year‐olds. The proportion of participants reporting one or more unscheduled visits to a doctor for asthma symptoms was markedly reduced in the montelukast group compared to the placebo group (4.1% versus 14.6%; OR 0.25, 95% CI 0.08 to 0.79), and it was reported that all prescriptions of OCS for asthma exacerbation occurred as a consequence of an unscheduled visit to a doctor. Weiss 2010 defined worsening asthma symptoms as one or more of the following actions: increased beta‐agonist use; increased daytime symptoms score; being awake 'all night' due to asthma; increased ICS use; OCS rescue; or unanticipated visits to a doctor, emergency department, or hospital for asthma. Analyses were adjusted for treatment, school start date, investigator site type, ICS use at entry, age, and sex. We found no significant difference in worsening symptoms between groups or for any component of this outcome, including OCS use (26.0% versus 30.3%; OR 0.79, 95% CI 0.59 to 1.06) and unanticipated medical contacts (11.8% versus 14.7%; OR 0.77, 95% CI 0.53 to 1.13). There was a consistent direction of effect favouring the intervention for five of the six outcomes, but none reached significance. Prespecified subgroup analyses found significantly fewer days of worsening symptoms in boys and in children aged 10 to 14 years, although interaction terms for age and gender were non‐significant. Morita 2017 based total asthma score on asthma symptoms, need for increased asthma medication, unscheduled physician visit or OCS; an adjustment was made in multivariable analysis for ICS use. There were no significant differences between pranlukast and control group in total asthma score (5.5 versus 7.8, P = 0.35) or days of worsening asthma symptoms (1.5 versus 1.8, P = 0.67). Significantly lower asthma scores and number of colds were seen for boys age one to five years. A higher number of colds and days of fever were seen in the control group compared to the pranlukast group, but only the latter reached significance (P = 0.06 and P = 0.04, respectively). Unscheduled visits to a doctor or OCS did not differ between groups (5.9% versus 8.6%; OR 0.67, 95% CI 0.16 to 2.80, Analysis 1.2, Figure 4). Evidence relevant to LTRA‐based interventions is summarised in Table 3.
1.2. Analysis.
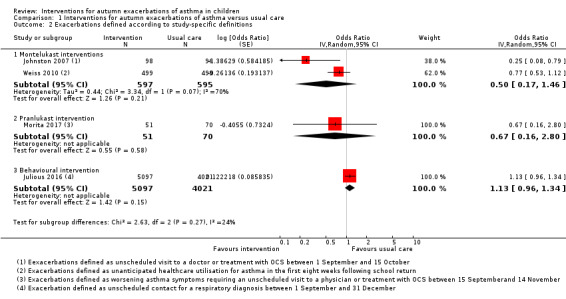
Comparison 1 Interventions for autumn exacerbations of asthma versus usual care, Outcome 2 Exacerbations defined according to study‐specific definitions.
4.
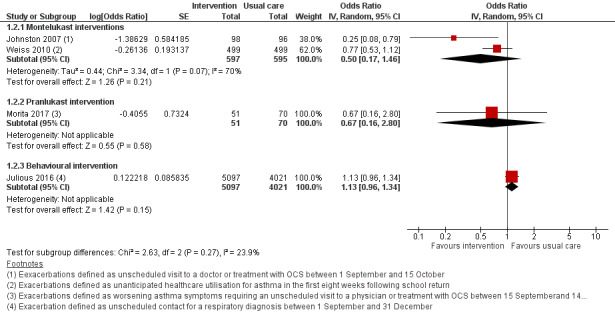
Forest plot of comparison: 1 Interventions for autumn exacerbations of asthma versus usual care, outcome: 1.2 Exacerbations defined according to study‐specific definitions.
In Julious 2016, exacerbations were not directly measured, but numbers of unscheduled contacts were reported as a proxy for this, and the study also reported unscheduled contacts coded with a respiratory diagnosis. Neither outcome significantly favoured the intervention. Data were reported between September and December rather than for the three months following school return; during September to December unscheduled contacts for a respiratory diagnosis were recorded for a greater proportion of children receiving the intervention letter than for those in the control group (18.4% versus 16.7%; OR 1.13, 95% CI 0.95 to 1.33), but this difference was not significant (Table 4). Unscheduled contacts for any diagnosis also did not differ significantly between the intervention and usual care groups (80.1% versus 79.1%; OR 1.10, 95% CI 0.96 to 1.26). The primary outcome period for this study was September; during this period no significant between‐group differences were reported for the proportion of children for whom any medical contact or any unscheduled contact was recorded. Unscheduled contacts for a respiratory diagnosis were recorded in significantly higher numbers in the intervention than in the usual care arm (5.3% versus 4.2%; OR 1.30, 95% CI 1.03 to 1.66). Analyses were modelled using age, sex, number of contacts the previous September and the trial arm as fixed‐effect, and the design/cluster effect of general practice as random‐effects. The study authors suggested that contacts following the intervention might have occurred as a result of appointments needed to assess children's need for preventer medication.
Asthma exacerbations and the period during which children were considered at risk of exacerbation after school return were defined differently in each trial. Moreover, the interventions trialled rarely used the same approach or medication. For these reasons, we limited meta‐analysis to studies with comparable interventions based upon seasonal administration of montelukast. Even amongst these three trials, participant populations and outcomes varied slightly: in Johnston 2007 the intervention period was fixed for 45 days from 1 September, whilst in Weiss 2010 the intervention period was for eight weeks from the night before each child's school return, and in Morita 2017 children were randomised two to six weeks before a fixed 60‐day study period starting from 15 September. The participant populations in these trials differed according to both age and asthma severity: participants were both younger and more likely to be receiving ICS in Johnston 2007 and Morita 2017 than in Weiss 2010. Despite the higher proportion of children receiving ICS at trial outset in Johnston 2007 and Morita 2017, higher rates of oral steroid prescription occurred in Weiss 2010. It was not possible to assess the review's primary outcome in these studies since, although each separately reported OCS prescription and unscheduled medical contact, the proportion of children with an exacerbation needing hospitalisation or OCS was not reported. Where evidence was based on single studies, the quality was moderate, downgraded due to small numbers of participants randomised or use of an indirect outcome (unscheduled respiratory contacts in Julious 2016 rather than hospitalisation or oral steroid requirement). When results from Johnston 2007 and Weiss 2010 were included in a random‐effects model, the odds ratio for unscheduled medical contacts was 0.50, 95% CI 0.17 to 1.46. We judged the evidence to be of low quality due to poor consistency between studies and concerns about the indirect outcome of unscheduled medical contact, the threshold for which appeared to differ between studies. The I² statistic was 70%, so we deemed a fixed‐effect model to be inappropriate.
Secondary outcomes
Hospital and paediatric intensive care unit admissions and asthma‐related deaths
Although Johnston 2007, Weiss 2010, Teach 2015a, and Morita 2017 reported medical contact data, including hospital admission, no study presented data on hospitalisation or paediatric intensive care unit admission separately from total unscheduled contacts or OCS use. No study reported any asthma‐related deaths.
Asthma control, quality of life, and impact on schooling
No study reported asthma control measured by a standardised tool (e.g. Childhood Asthma Control Test (cACT) or Asthma Control Test (ACT)). Weiss 2010 mentioned increased symptom score, but the scale on which this was measured was not defined. We found no significant between‐group differences for this outcome. No study measured asthma‐related quality of life or absence from school (or employment for those beyond school age).
Adverse events
In the pharmacological studies, there was no evidence that either adverse events or serious adverse event rate differed between the intervention and the usual care group. Adverse events were not formally reported in the primary care intervention study (Julious 2016). Morita 2017 reported that no children discontinued study medication due to an adverse event, and the authors of this study confirmed that no adverse events occurred in either group. In Johnston 2007, minor adverse events occurred in 20.4% of children in the montelukast group and in 28.1% of children in the placebo group (OR 0.66, 95% CI 0.34 to 1.27) (Table 3). Adverse events caused two children to discontinue the placebo: one child experienced behavioural change and the other tiredness and appetite changes. A significant behavioural disorder requiring emergency treatment was identified in a participant from the montelukast group at the follow‐up interview. No adverse events were described as serious. Teach 2015a reported adverse events during the period between randomisation and 30 days after the end of the intervention period. Of those children receiving at least one dose of the study drug, 54.5% in the omalizumab arm and 54.8% in the placebo arm experienced an adverse event (OR 0.99, 95% CI 0.61 to 1.58, Analysis 1.3, Figure 5) (Table 1). Considering only children eligible for the ICS boost (steps 2 to 4), one or more adverse events were reported by 43.5% of children in the ICS boost arm and 53.3% of children in the placebo arm (OR 0.67, 95% CI 0.34 to 1.33). Two serious adverse events occurred: a seventh nerve palsy in the placebo group and an episode of anaphylaxis in the ICS boost arm (Table 2). In Weiss 2010, 33.9% of children in the montelukast group and 33.6% of those in the placebo group reported at least one adverse event (OR 1.02, 95% CI 0.79 to 1.30) (Table 3); the most common adverse events were upper respiratory tract disorders and infections. Four serious adverse events occurred in the intervention group and one in the placebo group (0.7% versus 0.2%). Consequently, there was no evidence in any study that total adverse events occurred more frequently in the intervention than in the usual care arm. Moreover, we found no evidence of a significant difference between these groups when data from the montelukast studies were pooled in a random‐effects model (OR 0.91, 95% CI 0.63 to 1.32; I² = 32%). However, significantly more children experienced local administration site reactions in the intervention group in Teach 2015a compared to the usual care group (15.3% versus 6.5%, P = 0.03). We graded the quality of the evidence for this outcome as high for the pooled montelukast data and moderate for omalizumab or steroid boost intervention, downgrading the evidence due to the imprecision inherent to low participant numbers.
1.3. Analysis.
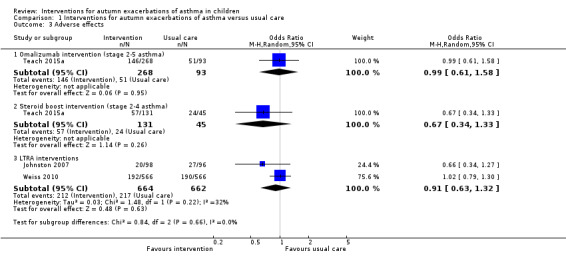
Comparison 1 Interventions for autumn exacerbations of asthma versus usual care, Outcome 3 Adverse effects.
5.
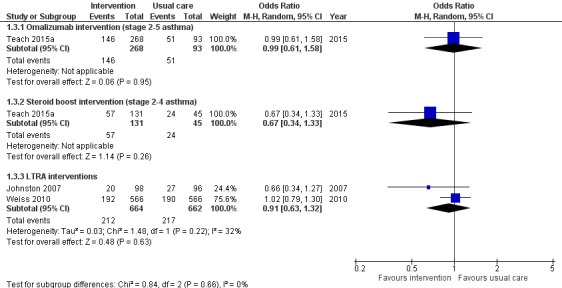
Forest plot of comparison: 1 Interventions for autumn exacerbations of asthma versus usual care, outcome: 1.3 Adverse effects.
Subgroup analyses
We had planned analyses separating studies of pharmacological interventions from studies of non‐pharmacological interventions, and to consider separately those with mild‐to‐moderate disease and those with severe asthma. Due to the low numbers of studies identified and the likely heterogeneity introduced by combining different pharmacological interventions, the planned subgroup analyses were not justified. We identified only one non‐pharmacological study, and baseline medication use and asthma severity were not always well described.
Sensitivity analyses
We had planned an analysis including only studies without missing data and an analysis excluding cluster‐randomised trials. Due to the low numbers of studies identified, these subgroup analyses were not possible.
Discussion
Summary of main results
Five randomised controlled trials met the inclusion criteria. Three were double‐blinded pharmacological studies; one was an open pharmacological study; and the remaining study was a cluster‐randomised trial of a public health intervention delivered in primary care. Three studies compared seasonal LTRA administration to a placebo, and one study compared seasonal omalizumab or an ICS boost to placebo. The primary care intervention was a letter sent to parents of children with asthma explaining the need to have adequate inhaled medication ready at the start of the autumn school term.
Two pharmacological studies reported a reduction in asthma exacerbations associated with the intervention. A 50% reduction (from 21% to 11.3%) in the proportion of children experiencing an exacerbation was found in allergen‐sensitised children with mild‐severe asthma and IgE > 30 IU/mL receiving omalizumab compared to placebo (Teach 2015a) (Table 1). In subgroup analyses within this study, a reduction in exacerbation risk was demonstrated in children receiving treatment for severe asthma where there is little scope for additional therapy other than OCS and in those with a recent exacerbation. A 70% reduction (from 14.6% to 4.1%) was found in children with moderate‐severe asthma receiving montelukast (Johnston 2007). However, neither a second larger trial of montelukast (Weiss 2010), nor pooled data from both studies found evidence for a significant between‐group difference in the proportion of children experiencing exacerbations (Table 3). Exacerbations requiring admission or a course of OCS were not reported in the primary care intervention study. However, there was no evidence that the proportion of participants who had at least one unscheduled medical contact between September and December differed between the intervention and the control group (Julious 2016) (Table 4). Of the planned secondary outcomes, we could only assess adverse events and serious adverse events; there was no evidence of a significant difference between intervention and usual care groups for either of these outcomes.
Overall completeness and applicability of evidence
Due to the small number of studies identified and variation in their inclusion criteria, interventions, and outcomes, it was not possible to perform subgroup analysis or sensitivity analyses. Insufficient data for subgroup analyses prevented us from reaching conclusions about the relative efficacy of pharmacological and non‐pharmacological interventions or about efficacy according to asthma severity or other characteristics such as age or gender. Whilst all included studies reported asthma exacerbations or worsening of symptoms, none considered the burden associated with worsening asthma symptoms in terms of absence from education or employment or used a validated measure of asthma control or quality of life. Consideration of these important clinical outcomes would have increased the applicability for a clinical audience. Outcomes such as paediatric intensive care unit admission and asthma‐related death are rare and were not reported in the included studies. All included studies were conducted in the Northern Hemisphere. Inclusion of studies from the Southern Hemisphere would increase the generalisability of the results. Similarly, it may not be possible to generalise the findings of Teach 2015a beyond the largely minority, low‐income population in which this study was conducted or to children with asthma who are not allergen‐sensitised. Lack of clarity regarding the efficacy of strategies aiming to prevent autumn exacerbations is reflected in current guidelines. Whilst the Global Initiative for Asthma guidelines recognise the autumn season as a risk period for exacerbation, and seasonality of symptoms is mentioned in the British Thoracic Society/Scottish Intercollegiate Guidelines Network guideline for the management of asthma, current guidelines do not offer management advice to tackle this problem (BTS 2016; GINA 2017).
Quality of the evidence
The five included studies randomised 14,252 children to receive either an intervention designed to reduce asthma exacerbations in children during autumn after school return or to usual care. The largest study randomised 12,179 children, and the smallest 194. Children were predominantly school‐age, although two studies enrolled a small number of preschool‐aged children (Johnston 2007; Morita 2017). Because the interventions investigated differed between studies, inconsistencies between the studies' results might reflect the relative efficacy of the interventions. For example, greater efficacy of pharmacological than non‐pharmacological interventions might explain why the intervention was found to be superior to placebo in Johnston 2007 and Teach 2015a and also approached significance for many outcomes in Weiss 2010 and Morita 2017, but no outcome favoured the intervention in Julious 2016. However, asthma severity and exacerbations also varied between and within studies. Differences in rates of asthma exacerbations did not always reflect difference in baseline severity. For example, higher rates of OCS use were reported in the population studied by Weiss 2010 than in those studied by Johnston 2007 and Morita 2017, despite lower baseline severity in the former study. Worsening asthma symptoms, inclusion criteria, intervention period, and outcomes were not uniformly defined across studies. Weiss 2010, Julious 2016, and Morita 2017 included children with relatively mild asthma, and this might have limited the potential for the interventions in these studies to reduce exacerbation rates below an already low baseline. Moreover, as a consequence of using routinely collected data, the study by Julious 2016 was also limited by considerable uncertainty around the adjudication of some of the contacts as scheduled, unscheduled, or irrelevant.
We assessed the quality of the evidence in this review using GRADEpro software and have presented this information in 'Summary of findings' tables. Overall, the evidence for exacerbation outcomes ranged from low to moderate according to the nature of the intervention, whilst the quality of the adverse event data was moderate or high. We downgraded evidence due to the small number of studies included and hence wide confidence intervals. Moreover, interventions differed qualitatively between studies, and in some cases surrogate outcomes were reported.
When pooling data from the montelukast studies, we used inverse variance random‐effects modelling for the exacerbation outcome due to constraints in the extracted data. While we would have preferred Mantel‐Haenszel modelling for both models, since it provides better estimates for infrequent events, this was not possible to implement with Review Manager 5 and the data available.
Potential biases in the review process
We used standard Cochrane methodology to conduct this review. We performed extensive literature searches and did not limit study selection by language of publication. Two review authors independently screened published data and conference abstracts. Discrepancies were resolved through discussion or, if necessary, by consultation with a third review author. Given our use of a thorough search strategy, it is unlikely that the study selection process missed any available published studies. We recognise that the clinical problem of asthma exacerbations associated with school return is complex and that consistent terminology does not exist to describe this problem or interventions designed to prevent it. To mitigate against this problem, the search terms used included 'February', 'autumn' or 'fall' and 'seasonal' in addition to 'September'. Two review authors independently extracted study characteristics and numerical data. Any discrepancies were resolved through discussion or, if necessary, by consultation with a third review author. Similarly, two review authors independently made decisions about risk of bias, resolving any discrepancies through discussion or, if necessary, by consultation with a third review author. We also attempted to contact all study authors to obtain additional information about outcomes and to clarify study methods to ensure accurate 'Risk of bias' decisions. We received three detailed replies and additional data from one study author, while one author was unable to provide the requested information relating to risk of bias. Review authors reported no conflicts of interest.
Agreements and disagreements with other studies or reviews
We identified no other systematic reviews relating to this issue.
Authors' conclusions
Implications for practice.
We found evidence from one relatively small study suggesting that add‐on seasonal omalizumab treatment commencing four to six weeks before school return might reduce asthma exacerbations in allergen‐sensitised children during the annual period of highest risk. Subgroups demonstrating benefit were children with severe asthma and those with frequent exacerbations. We found no evidence that this strategy is associated with significantly more adverse effects, other than administration site reactions, than placebo. Although results from one study suggest seasonal montelukast might reduce autumn exacerbations, there was no evidence for a reduction in exacerbations from either two subsequent trials based on leukotriene receptor antagonist therapy or pooled data from trials of montelukast. We found no data upon which to judge the effect of this or other interventions on asthma control, quality of life, or asthma‐related death.
Implications for research.
Further investigation of interventions to reduce the risk of asthma exacerbations in children after they return to school in the autumn is needed to reduce clinical impact and disease burden and also to better understand the mechanisms underlying asthma exacerbations. Analysis of interferon release from peripheral blood mononuclear cells of children receiving omalizumab within the Teach 2015a study suggest that omalizumab might improve the interferon response to rhinovirus, and in turn this might be one mechanism whereby exacerbations are reduced. Omalizumab appeared to be most effective in those with severe asthma, for whom treatment options are limited, and those at greatest risk of exacerbation. Whilst a seasonal approach would be cheaper than year‐round treatment, it remains expensive and can be painful to administer. Consequently, there is a need to identify relatively low‐expense interventions that could be useful to all those with asthma. To date, no studies have been conducted in the Southern Hemisphere, and only a limited number of pharmacological and non‐pharmacological strategies have been evaluated. In future studies, definitions of exacerbations should be provided, and where possible standardised. In order to support subgroup analysis according to asthma severity, children in future trials should be well characterised with respect to baseline asthma severity and previous exacerbation history, as well as age and gender.
Acknowledgements
Our thanks to the NIHR Collaborative Leadership in Applied Health Research and Care (CLAHRC) for their continued support.
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
Christian Osadnik was the Editor for this review and commented critically on the review.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Trials Register
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the Cochrane Airways Trials Register
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 2. Search strategy to identify relevant trials from the Cochrane Airways Trials Register
#1 AST:MISC1
#2 MeSH DESCRIPTOR Asthma Explode All
#3 asthma*:ti,ab
#4 #1 or #2 or #3
#5 MeSH DESCRIPTOR Seasons
#6 seasonal*:ti
#7 (season* NEAR (symptom* or vari* or exacerbat* or peak*)):ti,ab
#8 ((Autumn or Fall or summer or September or February) NEAR (symptom* or vari* or exacerbat* or peak*)):ti,ab
#9 MeSH DESCRIPTOR Schools Explode All
#10 (school* NEAR (holiday* or vacation* or return* or resume* or term* or year* or start*)):ti,ab
#11 #5 or #6 or #7 or #8 or #9 or #10
#12 #4 AND #11
[Note: in search line #1, MISC1 denotes the field in the record where the reference has been coded for condition, in this case, asthma]
Data and analyses
Comparison 1. Interventions for autumn exacerbations of asthma versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbations defined according to the review's primary outcome | 1 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Omalizumab interventions | 1 | 348 | Odds Ratio (Random, 95% CI) | 0.48 [0.25, 0.92] |
| 1.2 Omalizumab intervention (stage 5 asthma) | 1 | 184 | Odds Ratio (Random, 95% CI) | 0.37 [0.17, 0.81] |
| 1.3 Steroid boost intervention (stage 2‐4 asthma) | 1 | 173 | Odds Ratio (Random, 95% CI) | 0.86 [0.32, 2.31] |
| 2 Exacerbations defined according to study‐specific definitions | 4 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 Montelukast interventions | 2 | 1192 | Odds Ratio (Random, 95% CI) | 0.50 [0.17, 1.46] |
| 2.2 Pranlukast intervention | 1 | 121 | Odds Ratio (Random, 95% CI) | 0.67 [0.16, 2.80] |
| 2.3 Behavioural intervention | 1 | 9118 | Odds Ratio (Random, 95% CI) | 1.13 [0.96, 1.34] |
| 3 Adverse effects | 3 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Omalizumab intervention (stage 2‐5 asthma) | 1 | 361 | Odds Ratio (M‐H, Random, 95% CI) | 0.99 [0.61, 1.58] |
| 3.2 Steroid boost intervention (stage 2‐4 asthma) | 1 | 176 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.34, 1.33] |
| 3.3 LTRA interventions | 2 | 1326 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.63, 1.32] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Johnston 2007.
| Methods |
Study design: randomised, double‐blind, placebo‐controlled trial. Aim: to determine whether montelukast, added to usual asthma therapy, would reduce days with worse asthma symptoms and unscheduled physician visits of children during the September epidemic. Study centres and method of recruitment: recruited through advertising and through clinical practices in Hamilton and Brantford, Canada. Dates of study: 1 September 2005 to 15 October 2005. Run‐in period: no run‐in period. Duration of participation: 45 days. Consent: approved by the research ethics board at St. Joseph's Healthcare Hamilton. Informed consent from parents and assent from appropriately aged children. Power: a 40% reduction was expected in days with worse asthma symptoms in the montelukast group based upon results of a pilot study. Based upon 80% power and a 0.05 significance level, a sample‐size requirement of 88 per group was estimated. A 10% dropout rate was allowed for, so the final sample requirement was 97 per group. Imputation of missing data, i.e. assumptions made for ITT analysis: all randomised children completed the study and were included in analysis. |
|
| Participants |
Age (mean, range): not reported, 2 to 14 years. Gender: 65.0% male. Asthma severity: not explicitly mentioned, but 90% required inhaled corticosteroids (likely moderate to severe). Diagnostic criteria: physician‐diagnosed asthma. Number recruited: 196 Number randomised (intervention, control): 98, 96 Number completed (intervention, control): 98, 96 Number analysed (intervention, control): 98, 96 Withdrawals: 100% completed, no withdrawals. Inclusion criteria: 2 to 14 years old; physician‐diagnosed asthma needing a rescue inhaler in the last year; missing ≥ 1 day from school because of asthma in the last year or having significant limitation of normal activity; having a history of asthma exacerbations associated with apparent respiratory viral infections; ability to communicate in English. Exclusion criteria: significant cardiorespiratory comorbidity; using an LTRA; using regular OCS medication; asthma exacerbation in the month before study inception. |
|
| Interventions |
Intervention: montelukast age‐specific dose from 1 September to 15 October. Comparison: matched placebo. Concomitant medication: usual therapy. Excluded medication: already on montelukast. |
|
| Outcomes |
Primary outcome: percentage of days with worsening asthma symptoms during the intervention period (worsening symptoms defined as symptoms that were worse than usual or needed extra asthma medication, or requiring an unscheduled visit to a doctor or treatment with oral corticosteroids). Secondary outcome: number of unscheduled care visits. Time points measured: daily, then at the end of the study. Primary outcome result: the montelukast group experienced a 53% reduction in days with worse asthma symptoms compared with placebo (3.9% vs 8.3%, P = 0.02). Secondary outcome results: the montelukast group experienced a 78% reduction in unscheduled physician visits for asthma (4 for montelukast vs 18 for placebo, P = 0.011). Adverse events: minor adverse events occurred in 25 children in the montelukast group and in 35 children in the placebo group. 2 children discontinued study medication due to adverse events, 1 due to a personality change and 1 with change in appetite and increased tiredness; both children were taking placebo. The trial code was not broken, and symptom recording was continued. Another significant event was identified at the follow‐up interview after a child assigned to receive montelukast required emergency treatment for acute behaviour disorder. |
|
| Notes |
Funding: Merck Frosst Canada Ltd. Subgroups: subgroup analyses were exploratory risk of asthma worsening intervention vs control:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule. Randomly assigned in blocks of 4 according to gender and age. |
| Allocation concealment (selection bias) | Low risk | Randomisation schedule described as "concealed" and generated by an individual "not otherwise involved in the study". Mechanism of concealment described as based upon identical containers issued by third party (further information supplied by authors). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded. Intervention drug and placebo prepared by Merck Frosst, no reason to suspect parent or child could identify intervention drug from placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Given the use of a placebo, unlikely that the assessors would have knowledge of participant group. Subjective participant‐reported parent‐assessed symptoms and questionnaire used to assess other outcomes; these could have been affected if blinding inadequate, but no reason to suspect placebo led to incomplete blinding. Physician validated unscheduled care. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat primary analysis, 100% children completed the trial and returned 99.7% diary data. Adherence good in both groups (91.7% intervention vs 93.2% placebo). |
| Selective reporting (reporting bias) | Unclear risk | Unclear whether all prespecified outcomes included in the analysis |
| Other bias | Low risk | No baseline differences between groups, except more lifetime hospitalisations: 37.8% intervention vs 25.0% placebo |
Julious 2016.
| Methods |
Study design: cluster‐randomised controlled trial. Aim: to assess the impact of an NHS‐delivered public health intervention on unscheduled medical contacts in children with asthma during September and to perform a health economic analysis of the intervention. Study centres and method of recruitment: 142 UK general practices. Recruitment predominantly via the Clinical Practice Research Datalink (CPRD). A recruitment pack, including study information and an expression of interest form, was sent by post to the preferred contact at the practice to all 433 practices contributing to CPRD in England and Wales at the time of recruitment. Non‐responding practices were sent a reminder e‐mail, followed by a second reminder e‐mail and then final reminders by e‐mail and post. Some practices were also contacted by telephone, by CPRD or the study team at the Sheffield Clinical Trials Research Unit. Practices returned the completed expression of interest form, confirming or updating as necessary the information about the practice held by CPRD. Responses were tracked by CPRD to ensure practices that had replied were not contacted again. The expressions of interest were then forwarded to the study team to contact practices. Dates of study: 29 July 2013 to 30 September 2014. Run‐in period: none. Duration of participation: intervention commenced the week of 29 July 2013. Unscheduled care outcomes measured: September 2013, September to December 2013, September 2013 to August 2014, September 2014. Health economic outcomes measured: 1 August 2013 to 31 July 2014. Consent: ethics approval for the study was given by South Yorkshire Research Ethics Committee on 25 October 2012 (reference number: 12/YH/04). NHS permissions to conduct the study were obtained for all the primary care trusts in England and health boards in Wales. Power: the study was designed to detect a difference of 5% (30% vs 25%) with 90% power and a 2‐sided significance level of 5%, with an intraclass correlation of 0.03 to account for clustering. Based on this, 70 practices were estimated to be required per arm. It was expected that the sample size of 140 practices would equate to approximately 14,000 school‐aged children with asthma. Imputation of missing data, i.e. assumptions made for ITT analysis: analyses of effectiveness were performed as both ITT and PP, with the ITT being primary. If practices stopped submitting data to the CPRD before the end of a given follow‐up period, they were excluded from all analyses for that time period. The health economic analyses were based on the PP population. ITT analyses included all practices for which data were obtained by study period. The PP analyses were the subset of children in the ITT analyses to whom the intervention was delivered as intended by the protocol (i.e. individuals or practices not receiving a letter were excluded from PP analyses). |
|
| Participants |
Age (mean, range): 10.5 years, 5 to 16 years. 4‐year‐old children analysed separately. Gender: 60.0% male. Asthma severity: majority most likely mild (severity data not presented). Diagnostic criteria: coded diagnosis of asthma. Eligible participants identified in accordance with pre‐agreed diagnostic codes for asthma by the CPRD. Number recruited: 12,179 Number randomised (intervention, control): 5917, 6262 Number completed (intervention, control): 4411, 4438 Number analysed (intervention, control): 4411, 4438 (Note: figures above are for completing the entire trial until September 2014. ITT analyses of outcomes in September 2013, the primary outcome period, were based on 5305 intervention and 5586 control participants.) Withdrawals: from experimental group: discontinued intervention withdrawal before 30 September 2014: 13 practices, 506 children. From control group: discontinued intervention withdrawal before 30 September 2014: 18 practices, 1824 children. Inclusion criteria: aged between 4 and 16 years on 1 September 2013; coded diagnosis of asthma; prescribed asthma medication March 2012 to March 2013. Exclusion criteria: aged 4 years or under on 1 September 2013 or 16 years or over on 31 August 2013; not considered appropriate for this intervention by GP; not receiving asthma medication; coexisting neoplastic disease. |
|
| Interventions |
Intervention: NHS‐delivered public health intervention (a letter sent from the GP to parents/carers of school‐aged children with asthma reminding of the importance to take medications and the need to get sufficient medication sent out during the week commencing 29 July 2013). Comparison: no letter, control arm continue with standard care as usual, no other activity required. Concomitant medication: usual therapy. Excluded medication: none. |
|
| Outcomes |
Primary outcome: proportion of children with unscheduled contacts in September 2013. Secondary outcomes: number/proportion/time to first unscheduled contact; number/proportion/time to first unscheduled contacts for respiratory diagnosis; number/proportion/time to first all medical contacts; proportion scheduled contacts; number collecting prescriptions; QALYs gained; and NHS costs. Time points measured:
Primary outcome result: proportion of children with unscheduled contacts in September intervention vs control: 45.2 vs 43.7; OR 1.09, 95% CI 0.96 to 1.25. Secondary outcome results: intervention vs control multiple outcomes and subgroups assessed, most outcomes no significant difference between groups. Proportion prescriptions August 2013: OR 1.43, 95% CI 1.24 to 1.64; number of scheduled contacts per child August 2013: OR 95% CI 1.13, 0.84 to 1.52. No significant difference in unscheduled contacts September to December 2013, September 2013 to August 2014. Mean cost saving across the base case of GBP 36.07 per child and 96.3% probability that the intervention is cost‐saving. Intervention resulted in a QALY loss in 82.9% of samples and a mean loss of 0.00017 QALYs. Adverse events: not reported. |
|
| Notes |
Funding: National Institute for Health Research. Subgroups: the primary outcome was similar for 5‐ to 16‐year‐old children who had been prescribed preventative steroids compared to all 5‐ to 16‐year‐old children. Among children aged under 5 years, the differences were larger, and of borderline statistical significance, with the intervention being associated with more unscheduled visits for all subgroups. In all cases, the effect among the PP population was greater than that observed in the ITT population. Post hoc analyses demonstrated that for those who collected a prescription within the last 3 months, there was no difference in unscheduled contacts in September (55.2% vs 54.3% control), whilst for those whose last prescription was collected 3 to 6 months ago, there was an excess of unscheduled contacts in September (42.1% vs 39.7% control). (Data confirmed with study author since they differed between the summary and the main text of the report.) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by practice, stratified by size (confirmed by communication with author that the study statistician had no information about practices prior to randomisation other than list size). |
| Allocation concealment (selection bias) | Unclear risk | Sequence generated by 1 of 2 trial statisticians, then revealed to study manager and research assistant. Statisticians had no information about practice other than list size. However, characteristics of individual practices influenced whether the intervention was enacted or not. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study team and participants unblinded; this might have affected coding of contacts. Study team had no influence on data capture. Individual practices could choose not send the letter at all or not to send to selected patients. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Collected via CPRD. Contacts designated as "scheduled", "unscheduled", and "irrelevant" based on an independent adjudication panel comprised of experienced GPs who were blinded to the treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data due to change in computer system; presumed to be missing completely at random so no imputation. However, this was at least 25% in each group. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | No baseline difference in age, gender, and practice size |
Morita 2017.
| Methods |
Study design: randomised, open study. Aim: to investigate whether pranlukast added to usual asthma therapy in Japanese children during the autumn reduces asthma exacerbations. The effects of age and sex on the efficacy of pranlukast were also evaluated. Study centres and method of recruitment: multiple clinical sites in Chiba, Japan. Study participants were recruited between July 2007 and August 2007 through advertising and from the clinical practices in Chiba, Japan. Dates of study: 15 September 2007 to 14 November 2007. Run‐in period: from recruitment until 15 September 2007. Duration of participation: 60 days in addition to run‐in period. Consent: the investigation was approved by the Research Ethics Board of Chiba Universiy, Chiba (approval number: 631). Written informed consent was obtained from the parents of all participants and child assent when appropriate. Power: no a priori calculation. Imputation of missing data, i.e. assumptions made for ITT analysis: 13.6% of children excluded after randomisation in the pranlukast group (2.8% placebo), but no imputation made. |
|
| Participants |
Age (mean, range): 5.5 years (not reported but supplied by author), 1 to 14 years (divided into 2 age groups: 1 to 5 years and 6 to 14 years). Gender: 62.8% male. Asthma severity: 54.5% required inhaled corticosteroids. Diagnostic criteria: physician‐diagnosed asthma. Asthma was diagnosed by primary care doctors based on the Japanese paediatric guidelines for the treatment and management of bronchial asthma 2005. Number recruited: 204 Number randomised (intervention, control): 102, 102 Number completed (intervention, control): 59, 72 Number analysed (intervention, control): 51, 70 Withdrawals: 43 from intervention group and 30 from control group excluded before trial due to respiratory symptoms or insufficient diary recording by caregivers, or both, during the observation period. 8 from intervention group and 2 from control group excluded during the study period due to poor compliance or insufficient diary recording by caregivers, or both. Inclusion criteria: age 1 to 14 years old, physician‐diagnosed asthma needing a rescue inhaler in the last year, with a history of asthma exacerbations associated with apparent respiratory viral infections. Children who had been treated with LTRA were included after 14‐day washout period. Exclusion criteria: significant cardiorespiratory comorbidity; using regular oral corticosteroid; or had an asthma exacerbation in the month before treatment with pranlukast started. Children who had respiratory symptoms or problems with diary recording during observation, or both, were excluded from the study. |
|
| Interventions |
Intervention: regular pranlukast, an LTRA. 7 mg/kg, twice daily, in addition to their usual asthma therapy. Comparison: usual therapy. Concomitant medication: intervention taken in addition to usual asthma therapy. No restriction, but children who had been treated with LTRA were included after a 14‐day washout period. Excluded medication: no restriction, but 14‐day washout of LTRA. |
|
| Outcomes |
Primary outcome: total asthma score calculated during 8 weeks. Total asthma score was evaluated as follows: a blue sticker (score, 0) was applied on days when a child had no asthma symptoms; a green sticker (score, 1) indicated mild asthma symptoms; a yellow sticker (score, 2) indicated symptoms that were worse than usual or needed extra asthma medication, and an orange sticker (score, 3) was applied if a child's breathing symptoms required an unscheduled visit to a physician or treatment with oral corticosteroids. Secondary outcomes: days with worse asthma symptoms, number of colds, and days with fever. Days with worse asthma symptoms were defined as those with either an orange or a yellow sticker. A fever was defined as a temperature exceeding 38 °C. A “cold” was defined as the presence of more than 2 consecutive purple stickers indicating days with cold symptoms. At least 5 days with no cold symptoms were required before a subsequent new cold was identified. Time points measured: contemporaneous data collection at the end of 60 days. Primary outcome result: there were no significant differences between pranlukast and control group in total asthma score at 8 weeks (5.5 vs 7.8, P = 0.35), and in the days in which a child experienced a worsening of asthma symptoms (1.5 vs 1.8, P = 0.67) (data obtained through correspondence with the author). Secondary outcome results: higher number of colds in the control group compared to the pranlukast group (P = 0.06), and children taking pranlukast experienced fewer days with fever compared to the control group (P = 0.04). Adverse events: no children discontinued study medication due to adverse events. |
|
| Notes |
Funding: not stated. Subgroups: Boys vs girls. 1 to 5 years vs 6 to 14 years. Boys aged 1 to 5 years had the lower total asthma score at 8 weeks (P = 0.002), and experienced fewer cold episodes (P = 0.007). In boys, pranlukast significantly reduced total asthma score among 1‐ to 5‐year‐olds (P = 0.010), but did not reduce it among 6‐ to 14‐year‐olds. In girls, pranlukast did not affect total asthma score among 1‐ to 5‐year‐olds, but increased total asthma score among 6‐ to 14‐year‐olds (P = 0.027). 60 cold episodes were reported in the pranlukast group and 107 cases in the control group. A significant reduction in the number of cold episodes was observed in 1‐ to 5‐year‐old boys who were treated with pranlukast (P < 0.001). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment to either the pranlukast intervention group or the control group. Randomisation conducted according to sex and within the predefined age groups (1 to 5 years and 6 to 14 years). |
| Allocation concealment (selection bias) | Unclear risk | No description reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study was of open‐label design. The authors recognised this as a limitation of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Symptoms were reported subjectively by study participants. Participants and study observers were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rate of exclusions from pranlukast group after randomisation |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | Low risk | No baseline differences between groups. Comparisons of the baseline characteristics of the study groups were conducted using Chi² and Mann‐Whitney U‐tests. |
Teach 2015a.
| Methods |
Study design: 3‐arm, randomised, double‐blind, double placebo‐controlled, multicentre clinical trial. Aim: to compare (1) omalizumab with placebo and (2) omalizumab with an ICS boost with regard to autumn exacerbation rates when initiated 4 to 6 weeks before return to school. Study centres and method of recruitment: 8 US urban clinical research centres, no recruitment method information given. Dates of study: October 2011 to November 2013. Run‐in period: 2‐ to 12‐week screening. Duration of participation: from 4 to 6 weeks before school return until 90 days after school return. Consent: approved by all 8 institutional review boards. Consent from guardians and assent according to local guidelines. Power: enrolment of 453 participants (223 in the omalizumab arm, 155 in the inhaled corticosteroid boost arm, and 75 in the placebo arm (52 in steps 2 to 4 and 23 in step 5)) estimated to provide greater than 90% power to compare the omalizumab and placebo arms (11.8% vs 35.9% estimated effect) and 80% power to compare the omalizumab and ICS boost arms (12.9% vs 25.8% estimated effect). Imputation of missing data, i.e. assumptions made for ITT analysis: main analysis was based on modified ITT (children who were randomised, began study treatment, and had 1 or more study contact during the 90‐day outcome period were included in mITT). Supplemental volume included sensitivity analyses of mITT, PP, complete‐case, best‐case, worst‐case, and multiple imputation models. |
|
| Participants |
Age (mean, range): 10.2 years, 6 to 17 years. Gender: 63.4% male. Asthma severity: National Heart, Lung and Blood Institute Expert Panel Report‐3 based steps 2‐5 (mild‐severe). Diagnostic criteria: asthma diagnosis or symptoms for more than 1 year. Number recruited: 727 Number randomised steps 2‐4 (omalizumab, placebo, steroid boost): 133, 47, 138 Number randomised treatment step 5 (omalizumab, placebo): 145, 50 Number completed treatment: 439 total. Efficacy Number analysed steps 2‐4 (omalizumab, placebo, steroid boost): 121, 43, 130 Number analysed treatment step 5 (omalizumab, placebo): 138, 46 Safety Number analysed steps 2‐4 (steroid boost, placebo): 131, 45 Number analysed treatment steps 2‐5 (omalizumab, placebo): 268, 93 Withdrawals: 585 excluded pre‐enrolment, 214 excluded pre‐randomisation, 59 withdrew consent and were excluded pre‐enrolment, 35 withdrew consent and were excluded pre‐randomisation.
Inclusion criteria:
(Note: children requiring 500 μg of fluticasone or equivalent twice daily for control during the run‐in phase (step 5) were not entered into the ICS boost arm and instead were randomised at a ratio of 3:1 to omalizumab or injected placebo.) Exclusion criteria: not reported distinct from inclusion criteria. |
|
| Interventions |
Intervention: omalizumab standard dosing based on IgE and weight 4 to 6 weeks before, until 90 days after school start. Comparison: 1) placebo, or 2) ICS boost (doubled dose). Concomitant medication: ongoing guidelines‐based management EPR‐3. Excluded medication: none reported. |
|
| Outcomes |
Primary outcome: asthma exacerbation in the 90‐day period beginning on the first day of each child’s school year, defined as worsening of asthma control requiring systemic corticosteroids or hospitalisation. Secondary outcome: 11 prespecified, non‐mechanistic secondary outcomes (analysed exacerbation during 90‐day intervention according to subgroups based upon: exacerbation during run‐in, eosinophil count, total IgE, roach IgE, age, fraction FeNO, FEV1, BMI, ethnicity, and gender). IFNα responses to rhinovirus were measured in PBMCs from a subset of participants. Time points measured: 2 to 4 weekly during intervention. Primary outcome result: asthma exacerbation in the 90‐day period beginning on the first day of each child’s school year:
Secondary outcome results: exacerbation during 90‐day intervention according to subgroups. The following results differed significantly according to group: in those with an exacerbation during run‐in omalizumab vs placebo OR 0.12, 95% CI 0.02 to 0.64 (steps 2‐5), omalizumab vs ICS boost OR 0.05, 95% CI 0.002 to 0.98 (step 2‐4); in those with BMI centile ≥ 85 omalizumab vs ICS boost OR 0.13, 95% CI 0.03 to 0.61, (steps 2‐4); in those with BMI percentile < 85 ICS boost vs placebo OR 0.19, 95% CI 0.04 to 0.84 (steps 2‐4); in those with IgE < 255 kU/L omalizumab vs ICS boost OR 0.24, 95% CI 0.06 to 0.93 (steps 2‐4); in those with IgE 255 kU/L ICS boost vs placebo OR 0.24, 95% CI 0.06 to 0.87 (steps 2‐4); IFN‐α responses to rhinovirus were significantly increased in the omalizumab‐treated group (P = 0.03); among the omalizumab‐treated group, children with increases in ex vivo IFN‐α responses to rhinovirus to greater than the median value had a significantly lower rate of exacerbations during the outcome period OR 0.14, 95% CI 0.01 to 0.88. Adverse events: adverse events were reported by 54.5% of children in the omalizumab arm and 54.8% of children in the placebo arm (P > 0.99, steps 2–5) during the intervention phase. 1 or more adverse events were reported by 43.5% of children in the ICS boost arm and 53.3% of children in the placebo arm (P = 0.30, steps 2–4). 3 cases of grade 1 anaphylaxis occurred in the ICS boost, 2 in the placebo, and 3 in the omalizumab arm. Two serious AEs occurred during the intervention period, 1 each in the placebo (seventh nerve palsy) and ICS boost (anaphylaxis) arm. There were no deaths and no non–asthma‐related hospitalisations during the intervention phase. |
|
| Notes |
Funding: National institute for Allergy and Immune Diseases and an unrestricted grant from Novartis. Omalizumab and matching placebo were donated by Novartis. The ICS boost and matching placebo were donated by GlaxoSmithKline. Both companies had the opportunity to comment on the study design, but they had no role in the trial’s performance, data analysis, manuscript preparation, or decision to submit the manuscript for publication. Adrenaline auto injectors were provided by Mylan. Subgroups: 11 subgroups were based on: exacerbation during run‐in, eosinophil count, total IgE, roach IgE, age, FeNO, FEV1, BMI, ethnicity, and gender. A prespecified subgroup analysis was conducted considering children with an exacerbation during the run‐in phase. Omalizumab was more efficacious than both placebo (6.4% vs 36.3%; OR 0.12, 95% CI 0.02 to 0.64) and ICS boost (2.0% vs 27.8%; OR 0.05, 95% CI 0.002 to 0.98). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised, computer‐based random allocation scheme |
| Allocation concealment (selection bias) | Low risk | Described as centralised. No information on allocation concealment in report, but study authors confirmed that allocation was concealed using a third party and identical containers. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo, inhalers and injections. No evidence that adverse events differed between placebo and interventions, and no other reasons to suspect participants could identify to which group they had been assigned. Participants and other staff blinded. Unblinded nurses administered injections but not involved in outcome measurement. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Mix of objective and subjective outcomes, but assessors all blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary analysis was modified intention‐to‐treat restricted to children who were randomised, began study treatment, and had more than or equal to 1 study contact during the 90‐day outcome period. There was good retention (94%) and similar dropout rates and reasons between groups. |
| Selective reporting (reporting bias) | Low risk | Secondary outcomes predefined. All reported in online supplement. |
| Other bias | Low risk | Groups balanced according to baseline characteristics. |
Weiss 2010.
| Methods |
Study design: randomised, double‐blind, placebo‐controlled, multicentre study Aim: to determine the effectiveness of montelukast therapy in reducing asthma burden in children when initiated prophylactically on school return. Study centres and method of recruitment: 165 allergy and clinical paediatric practices in the United States and Canada. Hospital‐led recruitment. No recruitment information given. Dates of study: 28 June 2006 to 20 November 2006. Run‐in period: 2‐ to 12‐week screening. Duration of participation: 10 weeks. Consent: approved by local institutional review boards or ethical review committees with informed consent obtained from participants and parents or guardians. Power: assuming a treatment difference of 5% and a standard deviation of 24%, 495 evaluable participants in each treatment group was estimated to provide 90% power (2‐sided alpha 0.05) to demonstrate the superiority of montelukast. Imputation of missing data, i.e. assumptions made for ITT analysis: efficacy analysis was based on the analysis set population, which included all children who had received at least 1 dose of study medication and had a valid measurement of the percentage of days with worsening asthma during the study period (derived from at least 7 days of diary data). All randomised children who had received at least 1 dose of study drug were included in the safety analysis. |
|
| Participants |
Age (mean, range): 9.9 years, 6 to 14 years. Gender: 61.2% male montelukast group, 59.5% male placebo group. Asthma severity: 30% prescribed inhaled corticosteroids at randomisation (likely low/moderate). Diagnostic criteria: history of chronic asthma. Number recruited: 1162 Number randomised (intervention, control): 580, 582 Number completed (intervention, control): 536, 545 Number analysed (intervention, control): efficacy analysis 499, 499; safety analysis 566, 566. Withdrawals:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: montelukast 5 mg from the night before the first day of school for 8 weeks Comparison: matching placebo Concomitant medication: usual medications Excluded medication: none reported beyond exclusion criteria |
|
| Outcomes |
Primary outcome: percentage of days with worsening asthma symptoms, defined as 1 or more of: increased beta‐agonist use > 70% from baseline and a minimum increase of 2 puffs; increased daytime symptoms score > 50% from baseline; awake 'all night'; increased ICS use ≥ 100% from baseline or OCS rescue for worsening asthma; unanticipated visits to a doctor, emergency department, or hospital for asthma. Secondary outcomes:
Time points measured: 4, 8, and 10 weeks. Primary outcome result: percentage of days with worsening asthma symptoms: montelukast 24.3% vs placebo 27.2%; least squares means difference 3.0, 95% CI 6.21 to 0.29; P = 0.07 (OR for use of OCS obtained from authors and unpublished: OR 0.79, 95% CI 0.59 to 1.06). Secondary outcome results: no significant changes in components of primary outcome, safety outcomes, or interaction terms for subgroup analyses. Adverse events: 4 SAEs in the intervention group, 1 SAE in the placebo group. No SAE thought to be treatment related. The most common AEs were upper respiratory tract infections. |
|
| Notes |
Funding: Merck & Co. Subgroups: intervention better than control in boys and children 10 to 14 years, but interaction terms for age and gender non‐significant. No difference between groups according to inhaled corticosteroid use at entry, presence of cold symptoms, or according to individual components of the primary outcome.
Additional post hoc subgroup analyses suggested an increased percentage of days with asthma symptoms in the placebo compared to the intervention group at 3 to 4 weeks after school return and near‐significant superiority of intervention if school return is later than 15 August. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, randomisation schedule generated by study statistician. |
| Allocation concealment (selection bias) | Unclear risk | No description of schedule. Numbered containers, not specified whether identical. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical placebo used. Study double‐blinded including laboratory technicians, monitors, and study site personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded, outcome systematic but largely subjective participant‐reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary analysis based on a modified intention‐to‐treat design, including all children who had received at least 1 dose of study medication and had a valid measurement of the percentage of days with worsening asthma during the study period (derived from at least 7 days of diary data). There was no imputation of missing data, but similar dropout rates and reasons between groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | Generally balanced groups at baseline except inhaled corticosteroids last year intervention 54.1% vs placebo 48.7%. |
AE: adverse event BMI: body mass index CI: confidence interval CPRD: Clinical Practice Research Datalink EPR‐3: Expert Panel Report 3 GP: general practitioner ICS: inhaled corticosteroids IgE: immunoglobulin E IFNα: interferon alpha ITT: intention‐to‐treat FeNO: fractional exhaled nitric oxide FEV1: forced expiratory volume in the first second of expiration LABA: long‐acting beta‐agonist LTRA: leukotriene receptor antagonist mITT: modified intention‐to‐treat NHS: National Health Service OCS: oral corticosteroid OR: odds ratio PBMCs: peripheral blood mononuclear cells PP: per protocol SAE: serious adverse event QALY: quality‐adjusted life year
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anah 1980 | Not restricted to children (≤ 18 years). The average age of participants was 27.1 years. Also did not specifically address problems associated with school return. |
| Bruce 1977 | Not restricted to children (≤ 18 years). Sample group selected from adult volunteers. Also relates to the ragweed season rather than specifically addressing school return. |
| Bueving 2004 | Incorrect seasonal focus. Children participated during influenza season. Study lacks specific purpose of reducing school‐return exacerbations of asthma. |
| Busse 2011 | Incorrect methodology. Exacerbations after school return were reported as an outcome, but this was a post hoc analysis. The study was not a randomised controlled trial of an intervention specifically designed to reduce exacerbations after school return. |
| Coffman 1971 | Does not refer to asthma and incorrect seasonal focus. Study refers to hay fever grass pollen allergy during the summer months between May and July. |
| Corren 1992 | Study not restricted to children (≤ 18 years). Mean age for placebo group was 35.1 years. Mean age for nasal beclomethasone dipropionate group was 36.1 years. Also study was designed to reduce asthma and rhinitis symptoms during the autumn pollen season rather than addressing the problem of school return. |
| Crane 1998 | No mention of seasonal exacerbations of asthma |
| Engstrom 1970 | Incorrect seasonal focus. Main seasons of symptomatology extended from May to August. |
| Esquivel 2016 | No mention of seasonal exacerbations of asthma. This study examined data from the Preventative Omalizumab or Step‐up Therapy for Severe Fall Exacerbations (PROSE) study reported in Teach 2015a but considered 'colds' as the outcome. |
| Fang 2001 | Not limited to children (≤ 18 years). Mean age was 37 years. Also intervention not specifically designed to reduce exacerbations after school return. |
| Ford 1969a | Not restricted to children (≤ 18 years). All but one participant older than 30 years. Also intervention not specifically designed to reduce exacerbations after school return. |
| Ford 1969b | Incorrect seasonal focus, referred to pollinotic asthma in the height of spring |
| Gerald 2012 | Incorrect methodology. Purpose was not to compare intervention designed to reduce school‐return exacerbations of asthma with usual care. Randomised controlled cross‐over trial of year‐round hand sanitiser compared to normal hand hygiene |
| Grant 1995 | Not restricted to children (≤ 18 years). Aged 12 to 70 years. Also intervention not specifically designed to reduce exacerbations after school return but rather to prevent exacerbations associated with the pollen season. |
| Halterman 2002 | No mention of seasonal exacerbations of asthma |
| Halterman 2004 | No mention of seasonal exacerbations of asthma |
| Halterman 2005 | No mention of seasonal exacerbations of asthma |
| Joseph 2005 | No mention of seasonal exacerbations of asthma |
| Levy 2006 | No mention of seasonal exacerbations of asthma |
| Lewis 2012 | No mention of seasonal exacerbations of asthma |
| Prazma 2015 | Purpose was not to compare intervention designed to reduce school‐return exacerbations of asthma with usual care. Compared fluticasone propionate/salmeterol to fluticasone propionate rather than a usual care control. |
| Yoshihara 2014 | Purpose was not to compare intervention designed to reduce school‐return exacerbations of asthma with usual care. Compared suplatast tosilate to mequitazine rather than to a usual care control. |
Differences between protocol and review
Our original intention was to include randomised controlled trials, quasi‐randomised controlled trials, and observational studies. We believed observational trials presenting exacerbation data on a month‐by‐month basis might identify treatments or other potentially modifiable factors associated with a lessening of the autumn peak in asthma exacerbations. After conducting searches, we did not feel we could reliably identify all studies presenting these data since it was difficult to identify search terms to capture studies where seasonal differences were not the main focus. This review was therefore restricted to randomised controlled trials of interventions specifically designed to reduce asthma exacerbations in children after the return to school for the autumn term. The comparator was usual care since there are no established interventions for this problem. In a pragmatic change to our protocol due to the small number of studies returned, we decided not to restrict the review to school‐age children, since the autumn peak is less pronounced but still observed in preschool‐aged children, but does not occur appreciably in adults.
Unfortunately, due to the small number of studies identified and to differences in both interventions and outcomes, it was not possible to conduct subgroup or sensitivity analyses. We were also unable to assess any secondary outcomes except adverse events due to lack of data relating to these outcomes in the included trials. When pooling data from studies using a comparable intervention, we employed a Mantel‐Haenszel random‐effects model for the meta‐analysis of adverse effects, since these data were reported as absolute values in the included studies. We used an inverse variance model for the exacerbation outcome; however, as although odds ratios were reported or obtainable from study authors, the absolute number of children was not appropriate for use in Teach 2015a and Weiss 2010 studies, where the authors had adjusted for covariables in the odds ratio calculation.
Contributions of authors
KCP drafted the protocol.
KCP and MA identified studies for inclusion and extracted data from the included studies.
KCP performed the analyses and drafted the final review.
KMH extracted data from the included studies and resolved any disagreements between KCP and MA.
KCP, DK, and MA selected studies for inclusion in the review.
KCP, KMH, DK, and MA reviewed the protocol and the review for accuracy before submission.
Sources of support
Internal sources
-
The National Institute for Health Research (NIHR), through the Comprehensive Clinical Research Network and the NIHR Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London, UK.
Employment (Katharine Pike)
External sources
-
NIHR CLAHRC North Thames, UK.
Katherine Harris is in receipt of funding from the NIHR CLAHRC North Thames for her PhD. Katherine Harris was supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) North Thames at Bart’s Health NHS Trust. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
Declarations of interest
KCP: none known.
MA: none known.
DK: none known.
KMH: none known.
New
References
References to studies included in this review
Johnston 2007 {published data only}
- Johnston NW, Mandhane PJ, Dai J, Duncan JM, Greene JM, Lambert K, et al. Attenuation of the September epidemic of asthma exacerbations in children: a randomized, controlled trial of montelukast added to usual therapy. Pediatrics 2007;120(3):e702‐12. [DOI] [PubMed] [Google Scholar]
Julious 2016 {published data only}
- Julious SA, Horspool MJ, Davis S, Bradburn M, Norman P, Shephard N, et al. PLEASANT: Preventing and Lessening Exacerbations of Asthma in School‐age children Associated with a New Term ‐ a cluster randomised controlled trial and economic evaluation. Health Technology Assessment (Winchester, England) 2016;20(93):1‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morita 2017 {published data only}
- Morita Y, Campos AE, Suzuki S, Sato Y, Hoshioka A, Abe H, et al. Pranlukast reduces asthma exacerbations during autumn especially in 1‐ to 5‐year‐old boys. Asia Pacific Allergy 2017;7(1):10‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Teach 2015a {published data only}
- Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ Jr, Calatroni A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. Journal of Allergy and Clinical Immunology 2015;136(6):1476‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiss 2010 {published data only}
- Weiss KB, Gern JE, Johnston NW, Sears MR, Jones CA, Jia G, et al. The Back to School asthma study: the effect of montelukast on asthma burden when initiated prophylactically at the start of the school year. Annals of Allergy, Asthma & Immunology: official publication of the American College of Allergy, Asthma & Immunology 2010;105(2):174‐81. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anah 1980 {published data only}
- Anah CO, Jarike LN, Baig HA. High dose ascorbic acid in Nigerian asthmatics. Tropical and Geographical Medicine 1980;32(2):132‐7. [PubMed] [Google Scholar]
Bruce 1977 {published data only}
- Bruce CA, Norman PS, Rosenthal RR, Lichtenstein LM. The role of ragweed pollen in autumnal asthma. Journal of Allergy and Clinical Immunology 1977;59(6):449‐59. [DOI] [PubMed] [Google Scholar]
Bueving 2004 {published data only}
- Bueving HJ, Wouden JC, Raat H, Bernsen RMD, Jongste JC, Suijlekom‐Smit LWA, et al. Influenza vaccination in asthmatic children: effects on quality of life and symptoms. European Respiratory Journal 2004;24(6):925‐31. [DOI] [PubMed] [Google Scholar]
Busse 2011 {published data only}
- Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti‐IgE) for asthma in inner‐city children. New England Journal of Medicine 2011;364(11):1005‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coffman 1971 {published data only}
- Coffman DA. A controlled trial of disodium cromoglycate in seasonal allergic rhinitis. British Journal of Clinical Practice 1971;25(9):403‐6. [PubMed] [Google Scholar]
Corren 1992 {published data only}
- Corren J, Adinoff AD, Buchmeier AD, Irvin CG. Nasal beclomethasone prevents the seasonal increase in bronchial responsiveness in patients with allergic rhinitis and asthma. Journal of Allergy and Clinical Immunology 1992;90(2):250‐6. [DOI] [PubMed] [Google Scholar]
Crane 1998 {published data only}
- Crane J, Ellis I, Siebers R, Grimmet D, Lewis S, Fitzharris P. A pilot study of the effect of mechanical ventilation and heat exchange on house‐dust mites and Der p 1 in New Zealand homes. Allergy 1998;53(8):755‐62. [DOI] [PubMed] [Google Scholar]
Engstrom 1970 {published data only}
- Engstrom I, Vejmolova J. The effect of disodium cromoglycate on allergen challenge in children with bronchial asthma. Acta Allergologica 1970;25(5):382‐91. [DOI] [PubMed] [Google Scholar]
Esquivel 2016 {published and unpublished data}
- Esquivel AT, Busse WW, Calatroni A, Gergen PJ, Grindle K, Gruchalla RS, et al. Omalizumab decreases rates of cold symptoms in inner‐city children with allergic asthma. Annual Meeting of the American Academy of Allergy, Asthma and Immunology (AAAAI); 2016 Mar 4‐7; Los Angeles. ., 2016.
Fang 2001 {published data only}
- Fang Z, Cai Y, Wang L. The efficacy of controlling of house dusts in attacks of mite sensitive asthmatics. Zhonghua jie he he hu xi za zhi [Chinese Journal of Tuberculosis and Respiratory Diseases] 2001;24(11):685‐9. [PubMed] [Google Scholar]
Ford 1969a {published data only}
- Ford RM. Disodium cromoglycate in the treatment of seasonal and perennial asthma. Medical Journal of Australia 1969;2(11):537‐40. [DOI] [PubMed] [Google Scholar]
Ford 1969b {published data only}
- Ford RM. 'Intal' in the treatment of asthma. Medical Journal of Australia 1969;1(13):706. [DOI] [PubMed] [Google Scholar]
Gerald 2012 {published data only}
- Gerald LB, Gerald JK, Zhang B, McClure LA, Bailey WC, Harrington KF. Can a school‐based hand hygiene program reduce asthma exacerbations among elementary school children?. Journal of Allergy and Clinical Immunology 2012;130(6):1317‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grant 1995 {published data only}
- Grant JA, Nicodemus CF, Findlay SR, Glovsky MM, Grossman J, Kaiser H, et al. Cetirizine in patients with seasonal rhinitis and concomitant asthma: prospective, randomized, placebo‐controlled trial. Journal of Allergy and Clinical Immunology 1995;95(5 Pt 1):923‐32. [DOI] [PubMed] [Google Scholar]
Halterman 2002 {published data only}
- Halterman JS, McConnochie K, Yoos L, Conn KM, Kaczorowski J, Holzhauer R, et al. Year 1 results from a school‐based randomized trial for urban children with asthma. Pediatric Research 2002;51(4):1027. [Google Scholar]
Halterman 2004 {published data only}
- Halterman JS, Szilagyi PG, Yoos HL, Conn KM, Kaczorowski JM, Holzhauer RJ, et al. Benefits of a school‐based asthma treatment program in the absence of secondhand smoke exposure: results of a randomized clinical trial. Archives of Pediatrics and Adolescent Medicine 2004;158(5):460‐7. [DOI] [PubMed] [Google Scholar]
Halterman 2005 {published and unpublished data}
- Halterman JS, McConnochie KM, Conn KM, Yoos HL, Callahan PM, Neely TL, et al. A randomized trial of primary care provider prompting to enhance preventive asthma therapy. Archives of Pediatrics and Adolescent Medicine 2005;159(5):422‐7. [DOI] [PubMed] [Google Scholar]
Joseph 2005 {published data only}
- Joseph CLM, Havstad S, Anderson EW, Brown R, Johnson CC, Clark NM. Effect of asthma intervention on children with undiagnosed asthma. Journal of Pediatrics 2005;146(1):96‐104. [DOI] [PubMed] [Google Scholar]
Levy 2006 {published data only}
- Levy M, Heffner B, Stewart T, Beeman G. The efficacy of asthma case management in an urban school district in reducing school absences and hospitalizations for asthma. Journal of School Health 2006;76(6):320‐4. [DOI] [PubMed] [Google Scholar]
Lewis 2012 {published data only}
- Lewis E, Fernandez C, Nella A, Hopp R, Gallagher JC, Casale TB. Relationship of 25‐hydroxyvitamin D and asthma control in children. Annals of Allergy, Asthma & Immunology 2012;108(4):281‐2. [DOI] [PubMed] [Google Scholar]
Prazma 2015 {published data only}
- Prazma CM, Gern JE, Weinstein SF, Prillaman BA, Stempel DA. The association between seasonal asthma exacerbations and viral respiratory infections in a pediatric population receiving inhaled corticosteroid therapy with or without long‐acting beta‐adrenoceptor agonist: a randomized study. Respiratory Medicine 2015;109(10):1280‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoshihara 2014 {published data only}
- Yoshihara S, Yamada Y, Fukuda H, Tsuchiya T, Ono M, Fukuda N, et al. Prophylactic effectiveness of suplatast tosilate in children with asthma symptoms in the autumn: a pilot study. Allergology International 2014;63(2):199‐203. [DOI] [PubMed] [Google Scholar]
Additional references
Asher 2006
- Asher MI, Montefort S, Bjorksten B, Lai CKW, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross‐sectional surveys. Lancet 2006;368(9537):733‐43. [DOI] [PubMed] [Google Scholar]
Asher 2014
- Asher I, Pearce N. Global burden of asthma among children. International Journal of Tuberculosis and Lung Disease 2014;18(11):1269‐78. [DOI] [PubMed] [Google Scholar]
Asthma UK 2016
- Asthma UK. Asthma facts and statistics. www.asthma.org.uk/about/media/facts‐and‐statistics/ (accessed 1 September 2016).
Bahadori 2009
- Bahadori K, Doyle‐Waters MM, Marra C, Lynd L, Alasaly K, Swiston J, et al. Economic burden of asthma: a systematic review. BMC Pulmonary Medicine 2009;9:24. [PUBMED: 19454036] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baraldo 2012
- Baraldo S, Contoli M, Bazzan E, Turato G, Padovani A, Marku B, et al. Deficient antiviral immune responses in childhood: distinct roles of atopy and asthma. Journal of Allergy and Clinical Immunology 2012;130(6):1307‐14. [DOI] [PubMed] [Google Scholar]
Beck 2004
- Beck LA, Marcotte GV, MacGlashan D, Togias Al, Saini S. Omalizumab‐induced reductions in mast cell Fce psilon RI expression and function. Journal of Allergy and Clinical Immunology 2004;114(3):527‐30. [DOI] [PubMed] [Google Scholar]
Bhogal 2006
- Bhogal S, Zemek R, Ducharme FM. Written action plans for asthma in children. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD005306.pub2] [DOI] [PubMed] [Google Scholar]
Borrelli 2007
- Borrelli B, Riekert K, Weinstein A, Rathier L. Brief motivational interviewing as a clinical strategy to promote asthma medication adherence. Journal of Allergy and Clinical Immunology 2007;120(5):1023‐30. [DOI] [PubMed] [Google Scholar]
Brandt 2015
- Brandt EB, Biagini MJM, Acciani TH, Ryan PH, Sivaprasad U, Ruff B, et al. Exposure to allergen and diesel exhaust particles potentates secondary allergen‐specific memory responses, promoting asthma susceptibility. Journal of Allergy and Clinical Immunology 2015;136(2):295‐303.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
BTS 2016
- British Thoracic Society/Scottish Intercollegiate Guidelines Network. British Guideline on the Management of Asthma 2016. A national clinical guideline. brit‐thoracic.org.uk/document‐library/clinical‐information/asthma/btssign‐asthma‐guideline‐2016/ (accessed prior to 9 October 2017).
Cai 2011
- Cai G‐H, Hashim JH, Hashim Z, Ali F, Bloom E, Larsson L, et al. Fungal DNA, allergens, mycotoxins and associations with asthmatic symptoms among pupils in schools from Johor Bahru, Malaysia. Pediatric Allergy and Immunology 2011;22(3):290‐7. [DOI] [PubMed] [Google Scholar]
Corne 2002
- Corne JM, Marshall C, Smith S, Schreiber J, Sanderson G, Holgate ST, et al. Frequency, severity, and duration of rhinovirus infections in asthmatic and non‐asthmatic individuals: a longitudinal cohort study. Lancet 2002;359(9309):831‐4. [PUBMED: 11897281] [DOI] [PubMed] [Google Scholar]
de Ana 2006
- Ana SG, Torres‐Rodriguez JM, Ramirez EA, Garcia SM, Belmonte‐Soler J. Seasonal distribution of Alternaria, Aspergillus, Cladosporium and Penicillium species isolated in homes of fungal allergic patients. Journal of Investigational Allergology and Clinical Immunology 2006;16(6):357‐63. [PUBMED: 17153883] [PubMed] [Google Scholar]
Durrani 2012
- Durrani SR, Montville DJ, Pratt AS, Sahu S, DeVries MK, Rajamanickam V, et al. Innate immune responses to rhinovirus are reduced by the high‐affinity IgE receptor in allergic asthmatic children. Journal of Allergy and Clinical Immunology 2012;130(2):489‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
EPPI‐Reviewer 4 2010 [Computer program]
- Thomas J, Brunton J, Graziosi S. EPPI‐Reviewer 4: software for research synthesis. Version accessed prior to 9 October 2017. London, UK: Social Science Research Unit, UCL Institute of Education, 2010.
Feldman 2012
- Feldman J, Kutner H, Matte L, Lupkin M, Steinberg D, Sidora‐Arcoleo K, et al. Prediction of peak flow values followed by feedback improves perception of lung function and adherence to inhaled corticosteroids in children with asthma. Thorax 2012;67(12):1040‐5. [DOI] [PubMed] [Google Scholar]
Fleming 2000
- Fleming DM, Cross KW, Sunderland R, Ross AM. Comparison of the seasonal patterns of asthma identified in general practitioner episodes, hospital admissions, and deaths. Thorax 2000;55(8):662‐5. [PUBMED: 10899242] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gergen 2002
- Gergen PJ, Mitchell H, Lynn H. Understanding the seasonal pattern of childhood asthma: results from the National Cooperative Inner‐City Asthma Study (NCICAS). Journal of Pediatrics 2002;141(5):631‐6. [PUBMED: 12410190] [DOI] [PubMed] [Google Scholar]
Gill 2010
- Gill MA, Bajwa G, George TA, Dong CC, Dougherty II, Jiang N, et al. Counterregulation between the FcepsilonRI pathway and antiviral responses in human plasmacytoid dendritic cells. Journal of Immunology 2010;184(11):5999‐6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
GINA 2017
- Global Initiative for Asthma. 2017 GINA Report, Global Strategy for Asthma Management and Prevention. ginasthma.org/2017‐gina‐report‐global‐strategy‐for‐asthma‐management‐and‐prevention/ (accessed prior to 9 October 2017).
GRADEpro GDT [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro GDT. Version accessed prior to 9 October 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guevara 2003
- Guevara JP, Wolf FM, Grum CM, Clark NM. Effects of educational interventions for self management of asthma in children and adolescents: systematic review and meta‐analysis. BMJ (Clinical Research ed.) 2003;326(7402):1308‐9. [PUBMED: 12805167] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoskins 2000
- Hoskins G, McCowan C, Neville RG, Thomas GE, Smith B, Silverman S. Risk factors and costs associated with an asthma attack. Thorax 2000;55(1):19‐24. [PUBMED: 10607797] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ismaila 2013
- Ismaila AS, Sayani AP, Marin M, Su Z. Clinical, economic, and humanistic burden of asthma in Canada: a systematic review. BMC Pulmonary Medicine 2013;13:70. [PUBMED: 24304726] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ito 2015
- Ito K, Weinberger KR, Robinson GS, Sheffield PE, Lall R, Mathes R, et al. The associations between daily spring pollen counts, over‐the‐counter allergy medication sales, and asthma syndrome emergency department visits in New York City, 2002‐2012. Environmental Health: a Global Access Science Source 2015;14:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnston 1996
- Johnston SL, Pattemore PK, Sanderson G, Smith S, Campbell MJ, Josephs LK, et al. The relationship between upper respiratory infections and hospital admissions for asthma: a time‐trend analysis. American Journal of Respiratory and Critical Care Medicine 1996;154(3 Pt 1):654‐60. [PUBMED: 8810601] [DOI] [PubMed] [Google Scholar]
Johnston 2001
- Johnston NW, Sears MR. A national evaluation of geographic and temporal patterns of hospitalization of children for asthma in Canada. American Journal of Respiratory and Critical Care Medicine 2001;163:A359. [Google Scholar]
Johnston 2005
- Johnston NW, Johnston SL, Duncan JM, Greene JM, Kebadze T, Keith PK, et al. The September epidemic of asthma exacerbations in children: a search for etiology. Journal of Allergy and Clinical Immunology 2005;115(1):132‐8. [PUBMED: 15637559] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnston 2006
- Johnston NW, Sears MR. Asthma exacerbations. 1: epidemiology. Thorax 2006;61(8):722‐8. [PUBMED: 16877691] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jonasson 2000
- Jonasson G, Carlsen K‐H, Mowinckel P. Asthma drug adherence in a long term clinical trial. Archives of Disease in Childhood 2000;83(4):330‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kiotseridis 2013
- Kiotseridis H, Cilio CM, Bjermer L, Aurivillius M, Jacobsson H, Dahl A, et al. Quality of life in children and adolescents with respiratory allergy, assessed with a generic and disease‐specific instrument. Clinical Respiratory Journal 2013;7(2):168‐75. [DOI] [PubMed] [Google Scholar]
Krop 2014
- Krop EJM, Jacobs JH, Sander I, Raulf‐Heimsoth M, Heederik DJJ. Allergens and beta‐glucans in Dutch homes and schools: characterizing airborne levels. PLoS ONE 2014;9(2):e88871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Larsen 2016
- Larsen K, Zhu J, Feldman LY, Simatovic J, Dell S, Gershon AS, et al. The annual September peak in asthma exacerbation rates. Still a reality?. Annals of the American Thoracic Society 2016;13(2):231‐9. [PUBMED: 26636481] [DOI] [PubMed] [Google Scholar]
Lierl 2003
- Lierl MB, Hornung RW. Relationship of outdoor air quality to pediatric asthma exacerbations. Annals of Allergy, Asthma & Immunology 2003;90(1):28‐33. [DOI] [PubMed] [Google Scholar]
Lincoln 2006
- Lincoln D, Morgan G, Sheppeard V, Jalaludin B, Corbett S, Beard J. Childhood asthma and return to school in Sydney, Australia. Public Health 2006;120(9):854‐62. [PUBMED: 16904142] [DOI] [PubMed] [Google Scholar]
Lister 2001
- Lister S, Sheppeard V, Morgan G, Corbett S, Kaldor J, Henry R. February asthma outbreaks in NSW: a case control study. Australian and New Zealand Journal of Public Health 2001;25(6):514‐9. [PUBMED: 11824986] [DOI] [PubMed] [Google Scholar]
Mantel 1959
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22(4):719‐48. [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 2006
- Murray CS, Poletti G, Kebadze T, Morris J, Woodcock A, Johnston SL, et al. Study of modifiable risk factors for asthma exacerbations: virus infection and allergen exposure increase the risk of asthma hospital admissions in children. Thorax 2006;61(5):376‐82. [PUBMED: 16384881] [DOI] [PMC free article] [PubMed] [Google Scholar]
NAEPP 2007
- National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR‐3): Guidelines for the Diagnosis and Management of Asthma ‐ Summary Report 2007. Journal of Allergy and Clinical Immunology 2007;120(5 Suppl):S94‐138. [DOI] [PubMed] [Google Scholar]
Olenec 2010
- Olenec JP, Kim WK, Lee W‐M, Vang F, Pappas TE, Salazar LEP, et al. Weekly monitoring of children with asthma for infections and illness during common cold seasons. Journal of Allergy and Clinical Immunology 2010;125(5):1001‐6.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pruteanu 2014
- Pruteanu AI, Chauhan BF, Zhang L, Prietsch SOM, Ducharme FM. Inhaled corticosteroids in children with persistent asthma: dose‐response effects on growth. Evidence‐based Child Health: a Cochrane review journal 2014;9(4):931‐1046. [DOI] [PubMed] [Google Scholar]
Randolph 2013
- Randolph C. Pediatric exercise‐induced bronchoconstriction: contemporary developments in epidemiology, pathogenesis, presentation, diagnosis, and therapy. Current Allergy and Asthma Reports 2013;13(6):662‐71. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schildcrout 2006
- Schildcrout JS, Sheppard L, Lumley T, Slaughter JC, Koenig JQ, Shapiro GG. Ambient air pollution and asthma exacerbations in children: an eight‐city analysis. American Journal of Epidemiology 2006;164(6):505‐17. [DOI] [PubMed] [Google Scholar]
Sears 2008
- Sears MR. Epidemiology of asthma exacerbations. Journal of Allergy and Clinical Immunology 2008;122(4):662‐8; quiz 669‐70. [PUBMED: 19014756] [DOI] [PubMed] [Google Scholar]
Szefler 2008
- Szefler SJ, Mitchell H, Sorkness CA, Gergen PJ, O'Connor GT, Morgan WJ, et al. Management of asthma based on exhaled nitric oxide in addition to guideline‐based treatment for inner‐city adolescents and young adults: a randomised controlled trial. Lancet 2008;372(9643):1065‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Teach 2015b
- Teach SJ, Gergen PJ, Szefler SJ, Mitchell HE, Calatroni A, Wildfire J, et al. Seasonal risk factors for asthma exacerbations among inner‐city children. Journal of Allergy and Clinical Immunology 2015;135(6):1465‐73.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Teyhan 2015
- Teyhan A, Galobardes B, Henderson J. Child allergic symptoms and well‐being at school: findings from ALSPAC, a UK cohort study. PLoS ONE 2015;10(8):e0135271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thumerelle 2003
- Thumerelle C, Deschildre A, Bouquillon C, Santos C, Sardet A, Scalbert M, et al. Role of viruses and atypical bacteria in exacerbations of asthma in hospitalized children: a prospective study in the Nord‐Pas de Calais region (France). Pediatric Pulmonology 2003;35(2):75‐82. [PUBMED: 12526066] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tovey 2011
- Tovey ER, Rawlinson WD. A modern miasma hypothesis and back‐to‐school asthma exacerbations. Medical Hypotheses 2011;76(1):113‐6. [DOI] [PubMed] [Google Scholar]
van Maanen 2013
- Maanen A, Wijga AH, Gehring U, Postma DS, Smit HA, Oort FJ, et al. Sleep in children with asthma: results of the PIAMA study. European Respiratory Journal 2013;41(4):832‐7. [DOI] [PubMed] [Google Scholar]
Vuillermin 2011
- Vuillermin PJ, Robertson CF, South M. The role of parent‐initiated oral corticosteroids in preschool wheeze and school‐aged asthma. Current Opinion in Allergy and Clinical Immunology 2011;11(3):187‐91. [PUBMED: 21464710] [DOI] [PubMed] [Google Scholar]
Wark 2005
- Wark PAB, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza‐Stanca V, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. Journal of Experimental Medicine 2005;201(6):937‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2007
- Bousquet J, Khaltaev N (editors). World Health Organization: Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach. who.int/gard/publications/GARD%20Book%202007.pdf?ua=1 (accessed prior to 2 March 2018).
Wong 2000
- Wong CA, Walsh LJ, Smith CJ, Wisniewski AF, Lewis SA, Hubbard R, et al. Inhaled corticosteroid use and bone‐mineral density in patients with asthma. Lancet 2000;355(9213):1399‐403. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Pike 2016
- Pike KC, Harris K, Kneale D. Interventions for autumn exacerbations of asthma in children. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD012393] [DOI] [PMC free article] [PubMed] [Google Scholar]


