Abstract
Background
Combined oral contraceptives (COCs) have been associated with an increased risk of arterial thrombosis, i.e. myocardial infarction or ischemic stroke. However, as these diseases are rare in young women and as many types of combined oral contraception exist, the magnitude of the risk and the effect of different hormonal contents of COC preparations remain unclear.
Objectives
To estimate the risk of myocardial infarction or ischemic stroke in users compared with non‐users of different types, doses and generations of combined oral contraception.
Search methods
We searched electronic databases (MEDLINE (1966 to July 08, 2015), EMBASE (1980 to July 08, 2015), Popline (1970 to July 08, 2015) and LILACS (1985 to July 08, 2015) for eligible studies, without language restrictions.
Selection criteria
We included observational studies that recruited women in the reproductive age group (18 to 50 years) and compared the risk of myocardial infarction or ischemic stroke between users and non‐users of COCs.
Data collection and analysis
Two review authors independently selected relevant studies and extracted data. We pooled relative risks ()(combined odds ratios and one incidence rate ratio) and 95% confidence intervals (CIs) for myocardial infarction or ischemic stroke in users versus non‐users of COCs. We combined the outcomes of myocardial infarction and ischemic stroke and also analysed these outcomes separately. Analyses were stratified according to estrogen dose and progestagen type.
Main results
In total, we identified 1298 publications through the search strategy. We included 28 publications reporting on 24 studies. COC users were at increased risk of myocardial infarction or ischemic stroke compared with non‐users: relative risk (RR) 1.6 (95% CI 1.3‐1.9).These RRs were similar for myocardial infarction (1.6, 95% CI 1.2 to 2.1) and ischemic stroke (1.7, 95% CI 1.5 to 1.9). The risks did not vary clearly according to the generation of progestagen or according to progestagen type. When we stratified preparations according to estrogen dose, the risk of myocardial infarction or ischemic stroke seemed to increase with higher doses of estrogen.
Authors' conclusions
This meta‐analysis showed that the risk of myocardial infarction or ischemic stroke was 1.6‐fold increased in women using COCs . The risk was highest for pills with > 50 microgram estrogen. When combined with the results of studies on the risk of venous thrombosis in COC users, it seems that the COC pill containing levonorgestrel and 30 µg of estrogen is the safest oral form of combined oral hormonal contraception.
Plain language summary
The risk of heart attack and stroke in women using birth control pills
Background
Since their introduction, combined oral contraceptive pills have become one of the most popular birth control methods. These pills contain two types of female hormones, estrogen and progestagen. When used correctly, the failure rate (i.e. the occurrence of unwanted pregnancy) is less than one per 100 women per year. Despite their reliability, oral contraceptive pills have been found to increase the risk of a blood clot forming in an artery, i.e. arterial thrombosis (heart attack or stroke). As arterial thrombosis is rare in young women, and as many types of oral contraceptive pills exist, the size of the risk is unclear. Furthermore, the effect of different types of progestagens or different doses of estrogen on the risk of arterial thrombosis is unknown.
Review question
In this Cochrane Review we aimed to assess the risk of arterial thrombosis in different types of oral contraceptive pills. To do this, we searched the literature on July 8, 2015 for all studies that assessed the risk of arterial thrombosis associated with oral contraceptive pills in women under the age of 50 years.
Study characteristics
In total, 28 articles on 24 unique studies met the inclusion criteria.
Key results
Our results showed that the overall risk of arterial thrombosis was was 1.6‐fold increased in women using oral contraceptive pills compared with women who did not use oral contraceptive pills. The risk did not vary clearly according to progestagen type. However, we found that the risk of arterial thrombosis seemed to be twice as high in women taking pills with higher doses of estrogen. Also, the risk of other side effects of oral contraceptive pills (such as a blood clot in a vein‐venous thrombosis) should be considered before any type of oral contraceptive pill is prescribed. It is likely that the COC pill containing levonorgestrel and 30 µg of estrogen is the safest oral form of combined oral hormonal contraception.
Quality of the evidence
The overall quality of evidence in this review was moderate. Most studies (22 out of 28) correctly confirmed that patients had been diagnosed with arterial thrombosis. However, only four studies also checked that the type of pill a patient had been using was reported correctly. In addition, only half of the studies ensured that the correct comparisons were made between patients with and patients without arterial thrombosis. Also of importance is the fact that the analysis on progestagen type was based on few studies only.
Background
Description of the condition
Worldwide, myocardial infarction and ischemic stroke are two of the most important causes of morbidity and mortality (WHO 2013). It is estimated that over 17 million people die from cardiovascular diseases annually, with over 80% of deaths occurring in low‐ and middle‐income countries (Nabel 2012). Myocardial infarction most often occurs when an atherosclerotic plaque (a collection of fatty acids, fibrous tissue and leukocytes) ruptures in the wall of a coronary artery. The plaque contents obstruct the artery and deprive the downstream part of the heart muscle of oxygen (Nabel 2012). This can cause permanent cell damage or necrosis and leads to heart failure, stroke or death in up to 10% of cases (Brieger 2009; Roe 2010).
Ischemic stroke is characterized by brain ischemia due to the obstruction of a cerebral artery (Caplan 2009). As in myocardial infarction, a cerebral artery can be obstructed due to the rupture of an atherosclerotic plaque. Alternatively, a thrombus can embolize from elsewhere in the body and become lodged in the cerebral vasculature (Caplan 2009). The affected part of the brain rapidly loses function, leading to both transient and permanent disabilities such as loss of speech and movement (Di Carlo 2003).
The most important risk factors for myocardial infarction and ischemic stroke are hyper cholesterolemia, hypertension, diabetes and smoking (Wilson 1998). These risk factors generally accumulate over time, explaining why most patients with a cardiovascular event are aged 50 years or older (Berry 2012). Nevertheless, there are also risk factors that can contribute to myocardial infarction or ischemic stroke in younger (female) individuals. Studies in young women have shown an association between combined oral contraception use and an increased risk of myocardial infarction and ischemic stroke (Zakharova 2011). However, as these diseases are rare in young women and as many types of combined oral contraception exist, the magnitude of the risk and the effect of different hormonal contents of combined oral contraceptive (COC) preparations remain unclear.
Description of the intervention
COCs are the most commonly used reversible form of contraception in developed countries (United Nations 2011). They contain an estrogen and a progestagen that are usually taken together in one pill for the first 21 days of every menstrual cycle, followed by a pill‐free week (Speroff 2011). COCs prevent ovulation, mainly by suppressing the surge in luteinizing hormone (due to the progestagen content) (Speroff 2011). With full compliance, the failure rate (i.e. the occurrence of unwanted pregnancy) is less than one per 100 women per year (Trussell 2011).
How the intervention might work
COCs are associated with an increase in many coagulation factors (e.g. factor VII, VIII, X), increased activity of the fibrinolytic inhibitors Plasminogen Activator Inhibitor (PAI)‐1 and PAI‐2 and a reduced anticoagulant response (activated protein C resistance) (Tchaikovski 2010). Research has recently found hypercoagulability to be an important determinant of atherogenesis and atherosclerosis (Borissoff 2011), which in turn precedes myocardial infarction and ischemic stroke. In addition, COCs have been associated with increases in triglycerides, Low Density Lipoprotein cholesterol and insulin levels, and a reduced glucose tolerance (Godsland 1990), which are all well known risk factors for arterial cardiovascular disease (Wilson 1998).
Why it is important to do this review
In order to reduce the harmful thrombotic side‐effects, the dose of estrogen in COCs has been gradually reduced from 150 µg in the first preparations to ≤ 30 µg today (Speroff 2011). In addition to preparations with so‐called 'first generation' progestagens lynestrenol and norethisterone, preparations containing second (levonorgestrel and norgestrel) or third generation progestagens (desogestrel and gestodene) and preparations containing drospirenone were developed in an attempt to improve the cardio‐metabolic profile of COCs (Godsland 1990; Bringer 1992; Badimon 1999; Krattenmacher 2000). Still, results of studies on the risk of myocardial infarction and ischemic stroke associated with various types of COCs are conflicting. As over 100 million women use COCs worldwide (WHO 2004) and all combined preparations are equally effective for the prevention of unwanted pregnancies (Lawrie 2011), the issue of safety is paramount. Therefore, it is important to obtain a systematic overview of all available evidence on this topic in order to advise women as to the safest choice of combined oral contraception with regards to the risk of myocardial infarction and ischemic stroke.
Objectives
To estimate the risk of myocardial infarction and ischemic stroke in combined oral contraception users compared with non‐users.
To compare the risk of myocardial infarction and ischemic stroke associated with the three generations of COCs, as well as with preparations containing drospirenone or cyproterone acetate.
To compare the effect of varying doses of estrogen and types of progestagen in COCs on the risk of myocardial infarction and ischemic stroke.
Methods
Criteria for considering studies for this review
Types of studies
Myocardial infarction and ischemic stroke are potential side effects of COC use. Previous research has suggested that results of observational studies are credible when studying side effects of medication(Vandenbroucke 2004). This was supported by a meta‐analysis that showed no difference between the risk of side‐effects assessed in meta‐analyses of experimental data and the risk of side‐effects assessed in meta‐analyses of observational data(Golder 2011). Therefore in this Cochrane Review we included observational studies with a cohort, case‐control or nested case‐control design and, if available, data from randomised controlled trials (RCTs).
Types of participants
The participants were women in the reproductive age group (18 to 50 years) who either used or did not use COCs. We excluded studies on women using postmenopausal hormone therapy, non‐oral contraceptives or progestagen‐only contraceptives.
Types of interventions
We compared the risk of myocardial infarction and ischemic stroke between combined oral contraception users and non‐users. Both previous combined oral contraception users and never users were considered to be non‐users. The risk of myocardial infarction and ischemic stroke was assessed for different types of COC preparations. We categorized COCs according to their generation (progestagens). We classified preparations containing lynestrenol or norethindrone as first generation preparations, levonorgestrel and norethisterone acetate as second generation progestagens, and desogestrel and gestodene as third generation preparations. In addition, we categorized COCs separately according to the dose of estrogen and the type of progestagen used.
Types of outcome measures
The outcome measures of interest were objectively diagnosed fatal or non‐fatal first myocardial infarction or ischemic stroke. We classified a myocardial infarction as objectively confirmed if diagnosed based on a medical examination and pain assessment combined with an electrocardiogram (ECG), serum cardiac biomarkers or other specified strict diagnostic criteria of myocardial infarction, or by autopsy examination. Ischemic stroke was classified as objectively confirmed if a sudden onset focal neurological deficit was diagnosed on the basis of a medical history and neurological examination combined with brain imaging, or by autopsy examination.
We quantified the risk of developing either myocardial infarction or ischemic stroke in COC users compared with non‐users by obtaining crude numbers of users and non‐users of combined oral contraception, and crude numbers of women with and women without an arterial thrombotic event. We combined these numbers to compute an overall relative risk of myocardial infarction or ischemic stroke in COC users.
Primary outcomes
Fatal or non‐fatal arterial thrombosis (i.e. myocardial infarction or ischemic stroke).
Secondary outcomes
Fatal or non‐fatal myocardial infarction.
Fatal or non‐fatal ischemic stroke.
Search methods for identification of studies
We created the search in association with an expert librarian (C. Manion, Cochrane).
Electronic searches
We searched the following databases: MEDLINE (1966 to July 08, 2015), EMBASE (1980 to July 08, 2015), Popline (1970 to July 08, 2015) and LILACS (1985 to July 08, 2015). The study search was performed without language restrictions.
Searching other resources
The references of the selected studies and of reviews were additionally checked in case we did not capture any relevant studies through our search strategy.
Data collection and analysis
We used the study results to compare the relative risk of myocardial infarction and ischemic stroke between users and non‐users of COCs, and between women using different types and doses of COCs. Most studies included women without oral contraception or women who use preparations containing levonorgestrel with 30 µg of ethinylestradiol as a reference group. Standard meta‐analytic techniques were used, a random effect model being set as default.
Selection of studies
Two review authors (REJR, FMH) independently evaluated the title and abstract of all studies retrieved from the search strategy. This was done using standard piloted forms and specific inclusion and exclusion criteria. We resolved any disagreements by consensus and consulted a third review author (OMD) if necessary.
Data extraction and management
Two review authors (REJR and FMH) independently extracted data using standard, piloted data extraction forms and entered data into Review Manager (RevMan). We resolved all disagreements by consensus.
Assessment of risk of bias in included studies
Our 'Risk of bias' assessment was equipped for observational studies and adapted from the Newcaste Ottowa scale. We examined the risk of bias in the included observational studies based on four aspects that may affect the association between the exposure (COCs) and the outcome (myocardial infarction and ischemic stroke).
Firstly, exposure to combined oral contraception had to be confirmed through a prescription database in order for the risk of bias to be classified as 'low'. We classified other, less objective, methods such as interviews and questionnaires as a 'high' risk of bias, as research has shown that women have difficulty accurately recalling the type of preparations they used (Nischan 1993; Norell 1998).
Secondly, the diagnosis of a myocardial infarction or ischemic stroke had to be ascertained by objective measures. We classified studies in which myocardial infarction had been diagnosed on the basis of an ECG, serum cardiac biomarkers or other specified strict diagnostic criteria of myocardial infarction, or by autopsy examination as having a low risk of bias. For ischemic stroke these criteria were a neurological examination combined with brain imaging or other specified strict diagnostic criteria of ischemic stroke, or autopsy examination.
In cohort studies, loss to follow‐up can lead to biased risk estimates. We classified studies with < 10% loss to follow‐up as having a low risk of bias.
Finally, in case‐control selection, the selection of controls affects the validity of the results. We classified case‐control studies including controls from the source population of the cases (i.e. controls from the same neighbourhood as the cases who would most likely have been admitted to the same hospital as the cases if they had developed myocardial infarction or ischemic stroke) as low risk of bias (Grimes 2005).
As myocardial infarction and ischemic stroke are side effects of using COCs, and it is unethical to perform a RCT for side effects alone, we did not anticipate finding any RCTs on this topic. However, if such studies were found, we would have assessed the risk of bias according to recommended principles (Higgins 2011). We did not use an aggregate 'Risk of bias' score as this is generally discouraged (Jüni 1999).
Two review authors (REJR, FMH) independently assessed the risk of bias using a standard piloted form. Both review authors are trained in Clinical Epidemiology and Study Methodology. We resolved any persistent disagreement by consensus or discussion with a third review author (OMD). We did not use the 'Risk of bias' assessment to accept or reject studies.
Measures of treatment effect
From each matched case‐control study (matched on age and calendar time) included , we extracted the crude number of women who were exposed to combined oral contraception, the crude number of women not exposed to combined oral contraception, the crude number of women with myocardial infarction or ischemic stroke and the crude number of women without myocardial infarction or ischemic stroke event. In addition we extracted the crude numbers of women in separate subgroups (i.e. different doses of estrogen, different types of progestagen). We used these numbers to calculate odds ratios for COC users versus non‐users at the study level . For one cohort study (Lidegaard 2012a) this matching assumption was not met, and we extracted adjusted estimates (incidence rate ratio).
Unit of analysis issues
Current use of COCs, stratified according to the dose of ethinylestradiol and the type of progestagen, was analysed in women without a history of myocardial infarction or ischemic stroke. Also we studied the effect of previous combined oral contraception use on the risk of cardiovascular disease if data were available.
Dealing with missing data
We only included participants with complete data on exposure to COCs and the outcomes myocardial infarction or ischemic stroke, or both.
Assessment of heterogeneity
For results from standard meta‐analytic techniques, we presented statistical measures of heterogeneity (Chi² test and I² statistics).
Assessment of reporting biases
We made an overview of possible reporting biases for each included study. In addition, for the overall comparison of COC users with non‐users, we performed sensitivity analyses according to the presence or absence of various types of bias.
Data synthesis
We combined data from studies with similar designs, interventions and outcome measures. For the analysis on use versus non‐use and the analysis at the level of estrogen generations, we performed a random effects meta‐analysis based on data that were adjusted for age and calendar time by design or by analysis. For one cohort study (Lidegaard 2012a) we first performed a fixed effect meta‐analysis based on risk estimates for various contraceptives from that study. The pooled analysis was subsequently used in the random effects meta‐analysis. For analyses with 5 or less studies, both fixed and random effect analyses are presented as, in this case, the between study variability cannot be estimated reliably.
The matched case‐control studies provided odds ratios. As these studies were matched on calendar time the odds ratios are a valid estimate of the incidence rate ratio (Knol 2008, Vandenbroucke 2012). Moreover, as the outcome is very rare, relative risks, incidence rate ratios and odds ratios will be similar. We used the term relative risk to denote the pooled effect, even though formally speaking, this consisted of odds ratios and an incidence rate ratio.
We initially aimed to perform a formal network analysis. However, this requires the use of raw data in case of multiple arm studies. Raw data can only be used if unadjusted estimates provide meaningful effect estimates, which was not the case for the cohort study mentioned above Lidegaard 2012a. We therefore applied standard random effects meta‐analysis techniques throughout the review.
Sensitivity analysis
The outcomes myocardial infarction and ischemic stroke were pooled as well as analysed separately. To explore heterogeneity, we performed sensitivity analyses according to funding source and risk of bias. We defined funding as any financial support for the study from pharmaceutical companies.
Results
Description of studies
In total, we identified 1182 unique publications by searching electronic databases and checking the reference lists of the selected articles. Of these, we excluded 1047 publications based on the title or abstract, or both, and excluded a further 107 articles after detailed assessment of the full text (Figure 1). Twenty‐eight articles reporting on 24 separate studies met the inclusion criteria and were eligible for analysis. Heinemann 1998 and Lewis 1997 both presented results from the 'Transnational Study on Oral Contraceptives and the Health of Young Women' study, with Heinemann 1998 reporting on ischemic stroke and Lewis 1997 reporting on myocardial infarction. Similarly, van Kemmeren 2002 reported on ischemic stroke in the RATIO, whereas Tanis 2001 presented the data on myocardial infarction. Shapiro 1979 and Slone 1981 reported different analyses of the same study from the USA. The two articles by Mann 1975a and Mann 1975b reported on different data from a single British study. Finally, the article by Sidney 1998 on myocardial infarction described data from the Kaiser Permanente study and the University of Washington study, from which the risk of ischemic stroke is separately reported in Pettiti 1996 and Schwartz 1997. Thirteen studies were performed in Europe, eight in the USA and three studies in several countries around the world.
1.
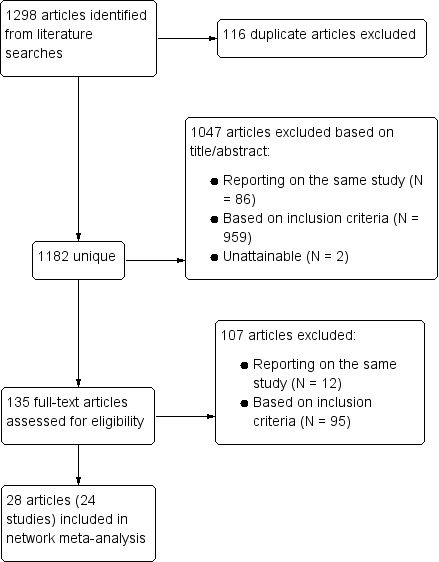
Study flow diagram.
The eligible publications included one cohort study (Lidegaard 2012a), 22 case‐control studies and one nested case‐control study. We did not find any RCTs on this topic. Sixteen of the 28 articles reported on the relationship between COCs and myocardial infarction. Eleven articles reported on ischemic stroke and one study assessed both the risk of myocardial infarction and the risk of ischemic stroke. All included articles were published between 1975 and 2010 and reported on data collected between 1968 and 2009. The pharmaceutical industry sponsored six of the 24 included studies.
Risk of bias in included studies
We have presented the risk of bias per publication in Figure 2. In total, only five studies confirmed the use of combined oral contraception through a prescription database and were classified as having a low risk of bias. All other papers assessed the exposure by questionnaire or interview only (high risk of bias).
2.
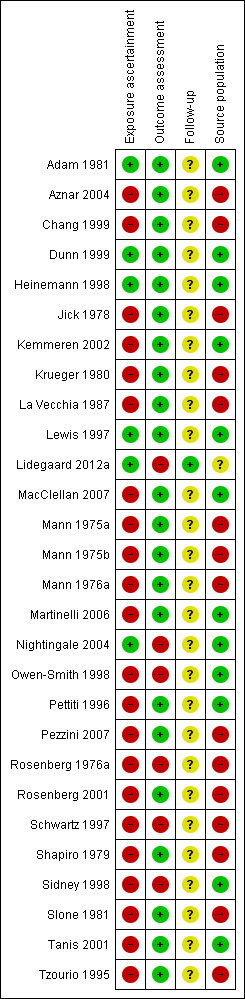
Risk of bias in the 28 included articles (reporting on 24 included studies). Green: low risk; red: high risk; yellow: unclear risk.
Regarding Lidegaard 2012a "Source population": not applicable as this was a population study.
The outcomes myocardial infarction and ischemic stroke were ascertained by objective measures in 22 studies. Six studies had a high risk of bias as the diagnoses were not objectively confirmed.
The only cohort study that was included in this Cochrane Review had almost complete follow‐up and so was classified as having a low risk of bias. Follow‐up was not applicable to the other included articles as they all reported on case‐control studies.
Thirteen studies had a low risk of bias as they included control subjects from the same source population as the cases. The 15 other studies included hospital controls as control subjects which is associated with a high risk of bias.
Effects of interventions
Current versus none use
All 24 included studies assessed the risk of myocardial infarction or ischemic stroke in current versus non‐users of combined oral contraception (Table 1). We performed a standard random‐effects meta‐analysis to assess the risk of myocardial infarction or ischemic stroke in these groups. (Figure 3) COC users were at increased risk of myocardial infarction or ischemic stroke compared with non‐users: relative risk 1.6 (95% CI 1.3‐1.9).These RRs were similar for myocardial infarction ( 1.6, 95% CI 1.2 to 2.1) and ischemic stroke (1.7, 95% CI 1.5 to 1.9).
1. Type of outcome in included studies.
| Study |
P ublication year |
Study design | Outcomea |
| Adam 1981. | 1981 | Case control | Myocardial infarction |
| Aznar 2004 | 2004 | Case control | Ischemic stroke |
| Chang 1999 | 1999 | Case control | Ischemic stroke |
| Dunn 1999 | 1999 | Case control | Myocardial infarction |
| Heinemann 1998/Lewis 1997. | 1998/1997 | Case control | Both |
| Jick 1978 | 1978 | Case control | Myocardial infarction |
| Kemmeren 2002/Tanis 2001 | 2002/2001 | Case control | Both |
| Krueger 1980 | 1981 | Case control | Myocardial infarction |
| La Vecchia 1987 | 1987 | Case control | Myocardial infarction |
| Lidegaard 2012a | 2012 | Cohort | Both |
| MacClellan 2007. | 2007 | Case control | Ischemic stroke |
| Mann 1975a/Mann 1975b | 1975/1976 | Case control | Myocardial infarction |
| Mann 1975b | 1975 | Case control | Myocardial infarction |
| Martinelli 2006 | 2006 | Case control | Ischemic stroke |
| Nightingale 2004 | 2004 | Nested case control | Ischemic stroke |
| Owen‐Smith 1998 | 1998 | Case control | Ischemic stroke |
| Pettiti 1996 | 1997 | Case control | Ischemic stroke |
| Pezzini 2007 | 2007 | Case control | Ischemic stroke |
| Rosenberg 1976a | 1976 | Case control | Myocardial infarction |
| Rosenberg 2001 | 2001 | Case control | Myocardial infarction |
| Schwartz 1997 | 1998 | Case control | Ischemic stroke |
| Sidney 1998 | 1998 | Case control | Myocardial infarction |
| Shapiro 1979/Slone 1981 | 1981/1981 | Case control | Myocardial infarction |
| Tzourio 1995 | 1995 | Case control | Ischemic stroke |
aDenotes both myocardial infarction and ischemic stroke.
3.
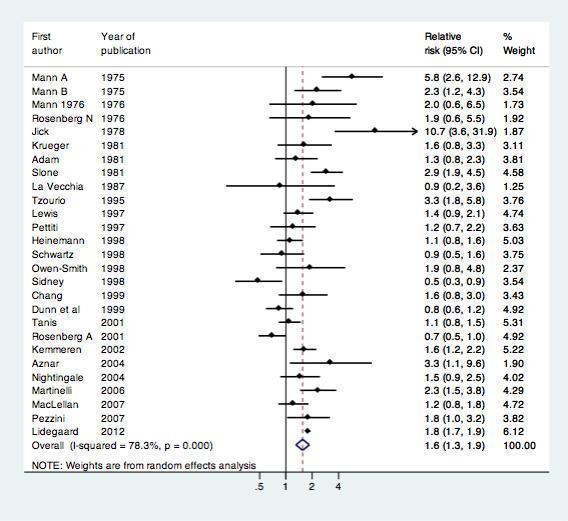
Effect of myocardial infarction and/or stroke in oral contraceptive users versus non‐users.
Progestagen generation
Overall, eight studes assessed the risk of myocardial infarction or ischemic stroke for different generations of COC preparations (Table 2, Figure 4). The results of the meta‐analysis showed that first generation preparations were not clearly associated with an increased risk of myocardial infarction or ischemic stroke: pooled RR 1.2 (95% CI 0.8 to 1.9) compared with non‐use. The pooled relative risks for second generation versus non‐use and third generation versus non‐use were 1.1 (95% CI 0.8‐1.7) and 1.1 (95% CI 0.7‐1.7) respectively.
2. Included studies with data on COC generation.
| Study | Design | Outcome |
Non‐use Event (n)/Total (n) |
1st generation Event (n)/Total (n) |
2nd generation Event (n)/Total (n) |
3rd generation Event (n)/Total (n) |
| Dunn 1999 | Case‐control | Myocardial infarction | 386/1853 | — | 20/139 | 20/81 |
| Kemmeren 2002 | Case‐control | Ischemic stroke | 101/669 | 7/38 | 52/225 | 32/142 |
| Lewis 1997 | Case‐control | Ischemic stroke | 125/397 | 14/28 | 27/62 | 8/41 |
| Lidegaard 2012a | Cohort | Both | * | * | * | * |
| Rosenberg 2001 | Case‐control | Myocardial infarction | 591/3301 | 11/79 | 4/46 | 2/17 |
| Schwartz 1998 | Case‐control | Ischemic stroke | 52/476 | 4/36 | 1/15 | — |
| Sidney 1998 | Case‐control | Myocardial infarction | 255/1159 | 8/60 | 3/27 | — |
| Tanis 2001 | Case‐control | Myocardial infarction | 146/714 | 11/42 | 59/232 | 20/130 |
Abbreviations: COC: combined oral contraceptives; n: number. 1st generation: preparations containing lynestrenol or norethisterone acetate. 2nd generation: preparations containing levonorgestrel. 3rd generation: preparations containing desogestrel or gestodene.
* Adjusted effect estimates extracted
4.
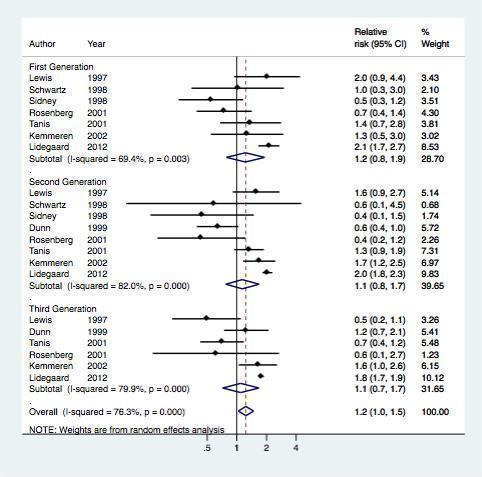
Effect of myocardial infarction and/or stroke in oral contraceptive users versus non‐users stratified per generation
Estrogen dose
Seven studies provided data on the risk of myocardial infarction or ischemic stroke according to the dose of estrogen (Table 3 , Figure 5). The risk of myocardial infarction or ischemic stroke increased with increasing doses of estrogen (RR 1.6, 95% CI 1.4 to 1.8) for preparations containing 20 µg of estrogen (RR 2.0, 95% CI 1.4 to 3.0) for 30 to 49 µg of estrogen and RR 2.4 (95% CI 1.8 to 3.3) for ≥ 50 µg of estrogen.
3. Included studies with data on oestrogen dose.
| Study | Design | Outcome |
Non‐use Event (n)/Total (n) |
20 µg E2 Event (n)/Total (n) |
30 to 49 µg E2 Event (n)/Total (n) |
≥ 50 µg E2 Event (n)/Total (n) |
| Chang 1999 | Case‐control | Ischemic stroke | 42/188 | — | — | 9/23 |
| Heinemann 1998 | Case‐control | Ischemic stroke | 96/353 | — | — | 15/38 |
| Lidegaard 2012a | Cohort | Both | * | * | * | * |
| Rosenberg 2001 | Case‐control | Myocardial infarction | 591/3301 | — | — | 4/7 |
| Shapiro 1979 | Case‐control | Myocardial infarction | 205/1812 | — | — | 18/107 |
| Sidney 1998 | Case‐control | Myocardial infarction | 255/1159 | — | — | 2/7 |
| Tzourio 1995 | Case‐control | Ischemic stroke | 25/135 | 2/7 | 30/76 | 8/15 |
Abbreviations: µg: micrograms; E2: ethinylestradiol; n: number.
* Adjusted effect estimates extracted
5.
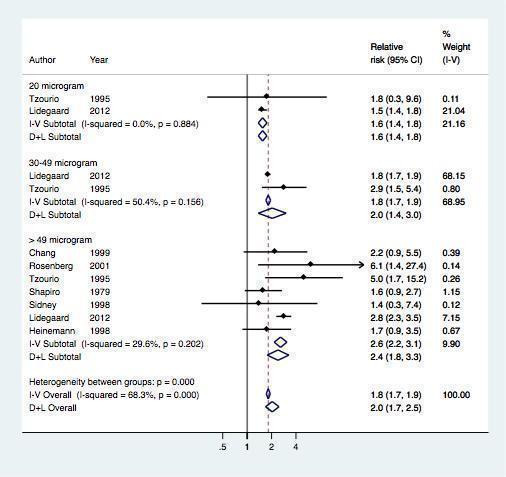
Effect of myocardial infarction and/or stroke in oral contraceptive users versus non‐users stratified by estrogen dose
Progestagen type
Finally, seven studies assessed the risk of myocardial infarction or ischemic stroke for various types of progestagen (Table 4, Figure 6). Of note, only few studies contributed data per progestagen type (maximum 5 studies, minimum 1 study).
4. Studies including data on progestagen type.
| Study | Design | Outcome | Event (n)/Total (n) | ||||||||
| Non‐use | Norethindron | Levonorgestrel | Norethisterone acetate | Desogestrel | Gestodene | Norgestimate | Drospirenone | Cyproterone acetate | |||
| Dunn 1999 | Case‐control | Myocardial infarction | 386/1853 | — | 18/123 | 2/16 | 9/46 | 11/35 | — | — | — |
| Kemmeren 2002 | Case‐control | Ischemic stroke | 101/669 | — | 52/225 | — | — | — | — | — | |
| Lewis 1997 | Case‐control | Myocardial infarction | 125/397 | — | 8/41 | — | — | — | — | — | — |
| Lidegaard 2012a | Cohort | Both | * | * | * | — | * | * | * | * | * |
| Rosenberg 2001 | Case‐control | Myocardial infarction | 591/ 3301 | 11/79 | 4/46 | — | — | — | — | — | — |
| Schwartz 1997 | Case‐control | Ischemic stroke | 156/ 921 | 10/64 | — | — | — | — | 4/24 | — | — |
| Sidney 1998 | Case‐control | Myocardial infarction | 255/ 1159 | 8/60 | — | — | — | — | 3/27 | — | — |
Abbreviations: n: number.
* Adjusted effect estimates extracted
6.
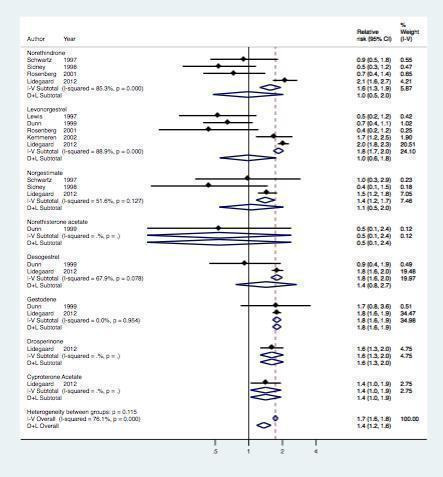
Effect of myocardial infarction and/or stroke in oral contraceptive users versus non‐users stratified per prosgestagen type
Check for consistency
Most analyses showed evidence of considerable heterogeneity. The overall analyses for use versus non‐use showed clear evidence of between study heterogeneity beyond chance (i2 78%).
Sensitivity analysis
We performed sensitivity analyses for the risk of myocardial infarction or ischemic stroke in users compared with non‐users of combined oral contraception according to funding source, and according to the risk of bias. Risk estimates were similar in studies sponsored by a pharmaceutical company: RR 1.6 (95% CI 0.9 to 2.4) versus non‐industry studies RR 1.5 (95% CI 1.2 to 1.9). Also no clear difference was found between studies with and without a objective diagnostic measures (p=0.3 from meta‐regression), or between studies with and without a biased selection of control subjects (p=0.2).
Discussion
Summary of main results
In this Cochrane Review, we performed a meta‐analysis of 28 publications to assess the risk of myocardial infarction or ischemic stroke in women using COCs. Overall, the risk of myocardial infarction or ischemic stroke was 1.6 fold increased in women that used COC compared with non‐users. This risk did also not vary according to the generation of progestagen or according to progestagen type. When we stratified preparations according to estrogen dose, the risk of myocardial infarction or ischemic stroke seemed to increase with higher doses of estrogen.
Overall completeness and applicability of the evidence
We have discussed this topic under the 'Potential biases in the review process' section.
Quality of the evidence
We have discussed this topic under the 'Potential biases in the review process' section and presented it in Figure 2.
Potential biases in the review process
We mostly included raw data for the analysis in our review. Therefore, we cannot rule out that our results may have been confounded by factors that influence both the type of COC preparation that is prescribed and the risk of myocardial infarction or ischemic stroke (e.g. age, body mass index, smoking and calendar time). However, all except one (Lidegaard 2012a) of the included studies were case‐control studies, in which adjustment for age and calendar time is usually dealt with by design (matching). In addition, body mass index and smoking are only weakly associated with COC use and so we do not expect that the lack of adjustment for these variables will have introduced important bias. Indeed, in the six studies that presented both crude and (extensively) adjusted risk estimates for myocardial infarction or ischemic stroke, adjustment only affected the results in two instances (Table 5). Furthermore, if certain types of COCs had been preferentially prescribed to 'less healthy' women (e.g. obese women or smokers) who have a higher risk of myocardial infarction of ischemic stroke, the number of arterial thrombotic events associated with these preparations would have been overestimated. As our results showed no increased risk of myocardial infarction or ischemic stroke in women using COC preparations, any further reduction in this risk estimate due to elimination of confounding would not change the conclusion of our meta‐analysis.
5. Adjusted risk of myocardial infarction or ischemic stroke in COC users versus non‐users per study.
| Study | Crude OR (95% CI) | Adjusted OR (95% CI) | Adjustment variables per study |
| Heinemann 1998 | 1.8 (1.3 to 2.5) | 2.8 (1.8 to 4.5) | Age, hypertension, body mass index, lipid levels, diabetes, smoking, alcohol, family history of stroke, duration of COC use, study centre |
| La Vecchia 1987 | 2.1 (0.7 to 7.1) | 1.8 (0.3 to 11.5) | Age, geographic area, marital status, education, social class, smoking, alcohol and coffee consumption, parity, age at menopause, diabetes, hypertension, obesity, hyperlipidemia, family history of ischemic heart disease |
| Martinelli 2006 | 2.3 (1.5 to 3.8) | 2.3 (1.4 to 3.8) | Age, educational level, hypertension, hypercholesterolemia, obesity, smoking |
| Nightingale 2004 | 1.6 (0.9 to 2.9) | 2.3 (1.2 to 4.6) | Heart disease, diabetes, hypertension, previous venous thrombosis, migraine, alcohol, smoking |
| Owen‐Smith 1998 | 2.9 (1.0 to 8.7) | 2.9 (0.9 to 9.6) | Social class, smoking status, history of hypertension |
| Pettiti 1996 | 1.0 (0.5 to 1.9) | 1.2 (0.5 to 2.6) | Hypertension, diabetes, smoking, race, body mass index |
Abbreviations: OR: odds ratio; COC: combined oral contraception. For all other studies, the crude OR or the adjusted OR, or both, were not presented in the manuscript.
A second point that warrants comment, is the lack of a generally accepted way of classifying oral contraceptives. For instance, in some studies, norgestimate is classified as a third generation preparation, whereas most studies only consider desogestrel and gestodene to be third generation preparations. In this meta‐analysis, we chose not to classify norgestimate as a third generation preparation, but only to include desogestrel and gestodene in this definition. However, as we did not find any large differences between the risk of myocardial infarction or ischemic stroke associated with desogestrel, gestodene and norgestimate in the analysis of each progestagen type separately , we do not expect that this choice has greatly influenced our results.
A third point is that some analyses (especially the analyses based on progestagen type) are based on very few studies only. It goes without saying that these results are therefore accompanied by much uncertainty.
Finally, in the analysis on estrogen dose, we did not take the type of progestagen into account. The reason for this was that most studies that provided data on the dose of estrogen did not specify the type of progestagen. However, we consider it unlikely that this classification greatly influenced our results, as preparations containing ≥ 50 µg of estrogen almost always contain levonorgestrel and we did not find this preparation to be associated with an increased risk of myocardial infarction or ischemic stroke in the analysis according to generation (second generation) or the analysis according to progestagen type. For the same reason, we could not take the estrogen dose into account in the analysis on progestagen type. However, as most preparations contained 30 to 49 µg of estrogen (Table 3) a large confounding effect of estrogen dose seems unlikely.
Agreements and disagreements with other studies or reviews
A previous systematic review on the relationship between COCs and myocardial infarction or ischemic stroke found the risk of myocardial infarction or ischemic stroke to be increased with COC use (Plu‐Bureau 2013). However, the review only included studies that were set up after 1990 and only assessed preparations containing low doses of estrogen, third generation progestagens (in which norgestimate was included) or progestin‐only contraceptives, making the two reviews incomparable. Four previous meta‐analyses, two on the risk of myocardial infarction or ischemic stroke (Baillargeon 2005; Peragallo‐Urrutia 2013), one on the risk of myocardial infarction alone (Khader 2003) and one on the risk of ischemic stroke alone (Gillum 2000), similarly found an increased risk in combined oral contraception users compared with non‐users. However, the inclusion criteria for these studies were less strict than for our current meta‐analysis, e.g. defining COC use within the past year as current use, including studies with non‐incident arterial events, analyzing studies with women up to 60 years of age, and including studies that did not present crude numbers of exposed or diseased cases and controls. To our knowledge, our Cochrane Review is the first meta‐analysis to assess the risk of incident myocardial infarction or ischemic stroke in women < 50 years of age who used COCs at or within one month before the date of inclusion.
Authors' conclusions
Implications for practice.
As all oral contraceptive preparations are equally effective for preventing unwanted pregnancies (Hardman 2009; van Hylckama Vlieg 2009), it is important that only the safest preparations are prescribed. The results of our meta‐analysis showed that the risk of myocardial infarction or ischemic stroke was 1.6‐fold increased in women using COCs. The risk was highest for pills containing ≥ 50 µg of estrogen.The risk of myocardial infarction or ischemic stroke did not differ clearly between progestogen generation in combination with estrogens However, COCs are especially known to increase the risk of venous thrombosis, and this risk should be kept in mind when counselling women about their choice of contraception (de Bastos 2014).When combined with the results of studies on the risk of venous thrombosis in COC users, it is likely that the COC pill containing levonorgestrel and 30 µg of estrogen is the safest oral form of combined oral hormonal contraception.
Implications for research.
The purpose of this meta‐analysis was to assess the risk of myocardial infarction or ischemic stroke in women using oral forms of hormonal contraception. However, there are also a number of (newer) non‐oral hormonal contraceptive preparations, such as the levonorgestrel‐releasing intrauterine device, transdermal contraceptive patches, subcutaneous hormonal implants and the vaginal ring. As yet, only a very small number of studies have assessed the risk of myocardial infarction or ischemic stroke in women using these preparations. In the coming years, information on (thrombotic) side‐effects of these preparations should be collected and analysed, so that the associated risk of myocardial infarction or ischemic stroke, if any, can be quantified.
For many women, such as the women included in the studies in our meta‐analysis, it is too late to prevent a first episode of myocardial infarction or ischemic stroke. However, advising women with a first myocardial infarction or ischemic stroke to discontinue the use of hormonal preparations seems important to prevent a recurrent event. To our knowledge, no studies have specifically compared the risk of recurrent myocardial infarction or ischemic stroke in women who continued or discontinued the use of oral contraceptive preparations after a first arterial event. The two studies to answer this question for venous thrombosis found that all oral hormonal preparations were associated with an increased risk of recurrent venous thrombosis compared with no hormone use (Christiansen 2005; Christiansen 2010). The only preparation that was not associated with an increased risk was the levonorgestrel‐releasing intrauterine device. It is possible that the levonorgestrel‐releasing intrauterine device is also a safe option for women after a first arterial thrombotic event, as, from the small amount of available research, this preparation does not seem to increase the risk of a first myocardial infarction or ischemic stroke (Lidegaard 2012b). However, large studies on women using a levonorgestrel‐releasing intrauterine device need to be performed before this preparation is recommended without reservation.
Feedback
Professor Øjvind Lidegaard's comment, 5 October 2015
Summary
The risk of arterial thrombosis with use of combined oral contraceptives is increased.
Roach RE, Helmerhorst FM, Lijfering WM, Stijnen T, Algra A, Dekkers OM. Combined oral contraceptives: the risk of myocardial infarction and ischemic stroke. Cochrane Database Syst Rev. 2015 Aug 27;8:CD011054.
Context
While the risk venous thrombosis with use of combined oral contraceptives (COC) is now convincingly quantified to be three to six fold increased depending mainly on the type of progestogen, studies on the risk of arterial end points are fewer and less consistent. Therefore, a meta‐analysis on available evidence might be relevant.
Methods
This Cochrane review includes data from 24 studies assessing the risk of thrombotic stroke and/or myocardial infarction in women of reproductive age using COC as compared with non‐users. Criteria for including and excluding studies were specified, as was information on categorising the studies’ risk of bias in ascertainment of exposure and assessment of end points.
Findings
The analysis included 23 case‐control studies with together 4,631 or 48% of included events and one cohort study with 5,036 (52%) events. The meta‐analysis concludes that low dose (<50 µg estrogen) COC do not confer an increased risk of neither thrombotic stroke nor myocardial infarction, that different progestogens don not confer a differential risk, but that high estrogen dose COC (50 µg estrogen) may double the risk of arterial thrombosis; RR = 2.0 (1.3‐2.9).
Commentary
In a field of 24 studies with a single study accounting for more than half of the included events, it is relevant to ask, what a meta‐analysis adds as compared to the one big study. First the one large cohort study assessed exposures daily through a 15‐year period (1), whereas 22 of the 23 others were case‐control studies assessing the exposure retrospectively (one was a nested case‐control study). Next, young women suspected for thrombotic stroke or myocardial infarction are generally extensively examined, with relatively clear criteria for judging whether the event is real. Therefore, the outcome diagnoses in this age group are generally fairly valid.
The results of the cohort study nevertheless differed from the conclusion of the meta‐analysis by concluding that COC conferred a significantly increased relative risk of ischemic stroke increasing from 1.6 (1.4‐1.9) for COC with 20 µg estrogen over 1.8 (1.6‐1.9) with 30‐40 µg estrogen to 2.0 (1.5‐2.7) with COC with 50 µg estrogen, the latter estimate in accordance with the meta‐analysis.
Therefore, a substantial part of the 23 case‐control studies must have found a protecting influence from COC on the risk of thrombotic stroke and myocardial infarction to achieve an overall relative risk of about unity. The problem is that all of the studies included in the meta‐analysis have found odds ratios of thrombotic stroke with COC substantially above one, typically between two and four. Therefore the overall estimate of the risk of thrombotic stroke with use of COC in the meta‐analysis is incompatible with the results of the included studies.
There are also some inconsistencies with the bias‐table. According to the method section, “Firstly, exposure to combined oral contraception had to be confirmed through a prescription database in order for the risk of bias to be classified as ’low’” (page 4, paragraph 4). Nevertheless, the Danish cohort study with such an ascertainment was classified as a study with “high risk” of bias. The same study was classified as having a high risk of bias in the outcome assessment, despite all women in Denmark suspected for thrombotic stroke go through CT and/or MR examinations.
Implications for practice
The influence from COC on the risk of thrombotic stroke is significant and in the order of 50‐100% increased for low‐dose COC. There are no consistent differences according to the progestogen type. Therefore the total thrombotic risk with use of COC is mainly a result of the substantially increased risk of venous thromboembolism. Therefore women generally are advised to use COC with 1st or 2nd generation progestogens, that is with norethisterone, levonorgestrel, or norgestimate, with the lowest possible dose of estrogen.
Reference
Lidegaard Ø, Løkkegaard E, Jensen A, Skovlund CW,Keiding N. Thrombotic stroke and myocardial infarction with hormonal contraception. New England Journal of Medicine 2012;366(24):2257–66.
Commentator details
Name: Øjvind Lidegaard, clinical Professor in Obstetrics & Gynaecology
Affiliation: Rigshospitalet, University of Copenhagen.
Mail correspondence: Oejvind.Lidegaard@regionh.dk.
Reply
Fra:F.M.Helmerhorst@lumc.nl [mailto:F.M.Helmerhorst@lumc.nl] Sendt: 5. Oktober 2015 19:39 Til: Øjvind Lidegaard Cc:R.E.J.Roach@lumc.nl; W.M.Lijfering@lumc.nl; A.Algra@umcutrecht.nl; O.M.Dekkers@lumc.nl Emne: BMJ
Dear Dr Lidegaard, dear Øjvind
Many thanks again for your renewed interest in our paper. We agree that your NEJM paper indeed is crucial in our review.
Our network meta‐analysis was based on raw data, and there were two reasons for this. The first reason was the theoretical consideration that confounding is generally not an issue when studying side‐effects (Golder Plos Med 2011). The second reason for including only raw data is technical, i.e. due to the nature of a network analysis.
For these two reasons, we extracted the unadjusted rates from your article and included them in our analyses. The main difference with other studies is that your paper did not adjust for age by design (matching). Interestingly, the unadjusted rate ratios in your paper are often below 1.0, in contrast to the adjusted ratios. Your results bear a considerable weight in the meta‐analyses which shifts the results towards the 1.0. So, the crucial issue is about confounding. It would be very helpful to this discussion if you could share data only adjusted for age. In our opinion, this is the only relevant confounding factor. If the age adjusted risk estimates are close to your overall adjusted estimates, we may have to reconsider some of the analyses presented.
On behalf of the team,
Frans M. Helmerhorst
Dept Clinical Epidemiology, Leiden University Medical Center
From: Øjvind Lidegaard [mailto:lidegaard@dadlnet.dk] Sent: 05 October 2015 23:25 To: Helmerhorst, F.M. (EPI) Cc: Roach, R.E.J. (COASS); Lijfering, W.M. (EPI); A.Algra@umcutrecht.nl; Dekkers, O.M. (EPI) Subject: SV: BMJ
Dear Frans, dear all.
Thanks for your rapid response.
Our analysis includes all Danish women in the relevant age group. Use of hormonal
contraception is high in young ages, whereas thrombotic events, especially arterial thrombosis increases exponentially with increasing age. An analysis not adjusting for age will ‐ therefore ‐ certainly bring heavily age‐confounded results, and therefore also ‐ in my opinion ‐ meaningless results. Of course an analysis not adjusted for age will bring about severely underestimated results due to the very screwed opposite development in use of contraception and incidence rate of thrombosis with increasing age.
So any analysis on this issue should always be adjusted for age, unless you match for age in the design, which we did not, and which many of the included case‐control studies did neither.
Best regards
Øjvind
Øjvind Lidegaard
Professor, DMSc
Head of Professors in Gyn‐Obs in East Denmark.
Department of Gynaecology 4232, Rigshospitalet,
Faculty of Health Science, University of Copenhagen
Fra:F.M.Helmerhorst@lumc.nl [mailto:F.M.Helmerhorst@lumc.nl] Sendt: 9. Oktober 2015 13:29 Til: Øjvind Lidegaard Cc:O.M.Dekkers@lumc.nl; R.E.J.Roach@lumc.nl; W.M.Lijfering@lumc.nl; A.Algra@umcutrecht.nl Emne: RE: BMJ
Dear Øjvind
After your critical comment for which we thank you very much indeed, we have to work.
Based on your comment we plan the following:
1. Redo the overall analysis ‘use vs non‐use’ based on adjusted effect estimates
2. Redo the analyses per generation based on standard meta‐analytic techniques using adjusted estimates
3. Rethink whether and how to adapt the network meta‐analysis on pill type. Here the network approach is preferred, and confounding might not be a huge issue when comparing pill types.
Plan 1 and 2 can be implemented soon, and will hopefully lead to a revised Cochrane manuscript in 2‐3 weeks.
From: Øjvind Lidegaard [mailto:lidegaard@dadlnet.dk] Sent: 13 October 2015 15:14 To: Helmerhorst, F.M. (EPI) Cc: Dekkers, O.M. (EPI); Roach, R.E.J. (COASS); Lijfering, W.M. (EPI); A.Algra@umcutrecht.nl Subject: SV: BMJ
Dear Frans (dear all).
I think 1. and 2. are straight forward.
About 3: As long as case‐control studies take cases and controls from about the same time period, and as long as they age match, it will probably be feasible to conduct a network analyses as you describe.
But if cases and controls are not matched on age, and if cases and controls are from different times, it will be crucial to adjust for both age and calendar year.
As you can find product specific adjusted estimates in the Danish NEJM publication, my suggestion is to apply these adjusted estimates also in the network model. This is because the different products were on the market at different times during a long study period, and because the age distribution of users of different product types are actually rather different. THerefore crude figures for also comparison between different product groups will be substantially confounded by age but also by year.
Best regards
Øjvind
Øjvind Lidegaard
Professor, DMSc
Head of Professors in Gyn‐Obs in East Denmark.
Department of Gynaecology 4232, Rigshospitalet,
Contributors
dr O. Lidegaard, dr A.Algra, dr O.M. Dekkers, dr F.M.Helmerhorst, dr W.M. Lijfering, dr R.E.J. Roach.
Feedback via Wiley, 31 January 2018
Summary
On January 31, 2018 the review group received comments from Wiley. The review group worked with authors to resolve the comment and update the review.
Original comment from Wiley:
The original comment from Wiley was: “I believe that the concluding phrase in your review of the risk of MI and Stroke in patients on COCs is open to misinterpretation. 'This meta‐analysis showed that the risk of myocardial infarction or ischemic stroke was 1.6‐fold increase in women using COCs. The risk was highest for pills with > 50 microgram estrogen. When combined with the results of studies on the risk of venous thrombosis in COC users, it seems that the COC pill containing levonorgestrel and 30 µg of estrogen is the safest oral form of hormonal contraception' The concluding phrase could be interpreted as meaning that the COC is safer than a POP.”
Reply
Review group worked with review authors to address the comment changing the sentence to: COC pill containing levonorgestrel and 30 µg of estrogen is the safest oral form of combined oral hormonal contraception?
Contributors
Jeanne‐Marie Guise, MD, MPH helped the review authors to address the comment
What's new
| Date | Event | Description |
|---|---|---|
| 28 February 2018 | Feedback has been incorporated | Feedback received from Wiley prompted the authors and review group to edit the following sentence throughout the review: COC pill containing levonorgestrel and 30 µg of estrogen is the safest oral form of combined oral hormonal contraception? |
History
Protocol first published: Issue 3, 2014 Review first published: Issue 8, 2015
| Date | Event | Description |
|---|---|---|
| 14 February 2016 | Amended | typo's changed, final check up. |
| 15 November 2015 | Feedback has been incorporated | See Feedback 1 1. Redo the overall analysis ‘use vs non‐use’ based on adjusted effect estimates. 2. Redo the analyses per generation based on standard meta‐analytic techniques using adjusted estimates. We abandoned therefore the network meta‐analysis as presented in the first version of this review. Conclusion adapted accordingly. |
Acknowledgements
We thank Carol Manion, Reference Librarian at FHI360, Durham, USA, and Jan W. Schoones, Walaeus Library, LUMC, Leiden, NL for developing the search strategies.
Appendices
Appendix 1. Search strategy
PubMed (http://www.ncbi.nlm.nih.gov/entrez/)
("Contraceptives, Oral"[mesh] OR "oral contraceptives" OR "oral contraceptive" OR "combined oral contraceptives" OR "combined oral contraceptive" OR (( norethisteron* OR norethindron* OR "ethynodiol diacetate" OR lynestrenol* OR norethynodrel* OR dienogest* OR levonorgestrel* OR norgestrel* OR dl‐norgestrel* OR desogestrel* OR norgestimat* OR gestoden* OR "medroxyprogesterone acetate" OR "chlormadinone acetate" OR nomegestrol* OR nestoron* OR "Cyproterone acetate" OR Drospirenon* OR oestrogen*[ti] OR estrogen[ti]) AND ("Ethinyl Estradiol" OR ethinylestradiol* OR Mestranol* OR "estradiol valerate" OR progestogen*[ti]))) AND (risk* OR risk factor*) AND ( "Intracranial Arterial Diseases/chemically induced"[Mesh] OR "Intracranial Arterial Diseases/epidemiology"[Mesh] OR “intracranial arterial disease”[tiab] OR "Cerebral Infarction/chemically induced"[Mesh] OR "Cerebral Infarction/epidemiology"[Mesh] OR “cerebrovascular accident”[tiab] OR “cerebral infarction”[tiab] OR "Brain Ischemia/chemically induced"[Mesh] OR "Brain Ischemia/epidemiology"[Mesh] OR “brain ischemia”[tiab] OR “brain ischaemia”[tiab] OR "Stroke/chemically induced"[Mesh] OR "Stroke/epidemiology"[Mesh] OR “acute ischemic stroke”[tiab] OR “acute ischaemic stroke”[tiab] OR “ischemic stroke”[tiab] OR “ischaemic stroke”[tiab] OR "Myocardial Infarction/chemically induced"[Mesh] OR "Myocardial Infarction/epidemiology"[Mesh] OR “myocardial infarction”[tiab])
Popline (http://www.popline.org)
(stroke OR "cerebrovascular effects" OR ischemia OR "myocardio infarction") AND ("oral contraceptive" OR "oral contraceptives") AND (clinical research OR random* OR trial*)
EMBASE (http://gateway.ovid.com/ovidweb.cgi?T=JS&MODE=ovid&NEWS=N&PAGE=main&D=emez)
("contraceptives, oral" OR "ontraceptives, oral" OR "oral contraceptives" OR "oral contraceptive" OR "combined oral contraceptives" OR "combined oral contraceptive" OR ((norethisterone OR norethisteron* OR norethindron* OR "ethynodiol diacetate" OR lynestrenol* OR norethynodrel* OR dienogest* OR levonorgestrel* OR norgestrel OR norgestrel* OR "dl‐norgestrel" OR desogestrel OR desogestrel* OR norgestimate OR norgestimat* OR gestodene OR gestoden* OR "medroxyprogesterone acetate" OR "chlormadinone acetate" OR nomegestrol OR nomegestrol* OR nestorone OR nestoron* OR "cyproterone acetate" OR Drospirenone OR Drospirenon* OR oestrogen* OR estrogen) AND ("Ethinyl Estradiol" OR "Ethinyl Estradiol" OR ethinylestradiol OR ethinylestradiol* OR Mestranol OR Mestranol* OR "estradiol valerate" OR "estradiol valerate" OR progestogen*))) AND (risk OR risks OR risk factor OR risk factors) AND (“intracranial arterial disease” OR "intracranial aneurysm" OR "cerebral infarction" OR “cerebrovascular accident” OR “cerebral infarction” OR “brain ischaemia” OR stroke OR “acute ischemic stroke” OR “acute ischaemic stroke” OR “ischemic stroke” OR “ischaemic stroke” OR “myocardial infarction”)
LILACs (https://mail.lumc.nl/owa/)
(contraceptives, oral OR anticonceptivos orales OR anticoncepcionais orais) AND (intracranial arterial diseases OR Enfermedades Arteriales Intracraneales OR Doenças Arteriais Intracranianas OR cerebral infarction OR infarto cerebral OR brain ischemia OR brain ischaemia OR isquemia encefalica OR stroke OR accidente cerebrovascular OR acidente vascular cerebral OR agudo isquemia accidente cerebrovascular OR agudo isquemia acidente vascular cerebral OR myocardial infarction OR infarto miocardio OR infarto do micardio)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adam 1981.
| Methods | Case‐control study | |
| Participants | 139 cases/276 controls Aged 15 to 44 years |
|
| Interventions | Combined oral contraception: current, past and none use | |
| Outcomes | Fatal myocardial infarction Events: current use 24/38, past use 35/70, none use 99/207 |
|
| Notes | UK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | Low risk | Myocardial infarction objectively confirmed. |
| Outcome assessment | Low risk | COC use objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | Low risk | Controls from the same source population as the cases. |
Aznar 2004.
| Methods | Case‐control study | |
| Participants | 29 cases/66 controls Aged 18 to 50 |
|
| Interventions | Combined oral contraception: current and none use | |
| Outcomes | Ischemic stroke Events: current use 9/8, none use 20/58 |
|
| Notes | Spain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | High risk | Ischemic stroke not objectively confirmed. |
| Outcome assessment | Low risk | COC use objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | High risk | Controls from a different source population to the cases. |
Chang 1999.
| Methods | Case‐control study | |
| Participants | 291 cases/736 controls Aged 20 to 44 years |
|
| Interventions | Combined oral contraception: current and none use, < 50 µg of oestrogen and ≥ 50 µg of oestrogen | |
| Outcomes | Ischemic stroke Events: current use 19/42, none use 41/146, <50 µg 10/28, ≥ 50 µg 9/14 |
|
| Notes | UK, Germany, Hungary, Serbia and Slovenia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | High risk | Ischemic stroke not objectively confirmed. |
| Outcome assessment | Low risk | COC use objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | High risk | Controls from a different source population to the cases. |
Dunn 1999.
| Methods | Case‐control study | |
| Participants | 448 cases/1728 controls Aged 16 to 44 |
|
| Interventions | Combined oral contraception: current and none use, 2nd generation (norethisterone acetate or levonorgestrel), norethisteron acetate, levonorgestrel, 3rd generation (desogestrel or gestodene), desogestrel, gestodene | |
| Outcomes | Myocardial infarction Events: current use 40/180, none use 386/1467, 2nd generation 20/119, levonorgestrel 18/105, norethisterone acetate 2/14, 3rd generation 20/61, desogestrel 9/37, gestodene 11/24 |
|
| Notes | UK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | Low risk | COC use objectively confirmed. |
| Outcome assessment | Low risk | COC use not objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | Low risk | Controls from the same source population as the cases. |
Heinemann 1998.
| Methods | Case‐control study | |
| Participants | 220 cases/775 controls Ages 16 to 44 |
|
| Interventions | Combined oral contraception: current and none use, 1st generation, 2nd generation, 3rd generation | |
| Outcomes | Ischemic stroke Events during: current use 124/182, none use 96/257, 1st generation 15/23, 2nd generation 58/97, 3rd generation 45/54 |
|
| Notes | UK, Germany, France, Switzerland and Austria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | Low risk | COC use objectively confirmed. |
| Outcome assessment | Low risk | Ischemic stroke objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | Low risk | Controls from the same source population as the cases. |
Jick 1978.
| Methods | Case‐control study | |
| Participants | 26 cases/59 controls Aged < 46 years |
|
| Interventions | Combined oral contraception: current, past and none use | |
| Outcomes | Myocardial infarction Events: current use 20/14, past use 4/16, none use 6/45 |
|
| Notes | USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | High risk | COC use not objectively confirmed. |
| Outcome assessment | Low risk | Myocardial infarction objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | High risk | Controls from a different source population to the cases. |
Kemmeren 2002.
| Methods | Case‐control study | |
| Participants | 203 cases/925 controls Aged 18 to 49 years |
|
| Interventions | Combined oral contraception: current use, none use, 1st generation, 2nd generation, 3rd generation | |
| Outcomes | Ischemic stroke Events: current use 102/348, none use 101/568, 1st generation 7/31, 2nd generation 52/173, 3rd generation 32/110 |
|
| Notes | The Netherlands | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | High risk | COC use not objectively confirmed |
| Outcome assessment | Low risk | Ischemic stroke objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | Low risk | Controls from the same source population as the cases. |
Krueger 1980.
| Methods | Case‐control study | |
| Participants | 75 cases/326 controls Aged 15 to 44 years |
|
| Interventions | Combined oral contraception: current and none use | |
| Outcomes | Fatal myocardial infarction Events: current use 12/34, none use 63/292 |
|
| Notes | USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | High risk | COC use not objectively confirmed |
| Outcome assessment | Low risk | Myocardial infarction objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | High risk | Controls from a different source population to the cases. |
La Vecchia 1987.
| Methods | Case‐control study | |
| Participants | 52 cases/91 controls Aged < 45 years |
|
| Interventions | Combined oral contraception: current, past and none use | |
| Outcomes | Myocardial infarction Events: current use 3/6, past use 15/12, none use 49/85 |
|
| Notes | Italy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | High risk | COC use not objectively confirmed |
| Outcome assessment | Low risk | Myocardial infarction objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | High risk | Controls from a different source population to the cases. |
Lewis 1997.
| Methods | case‐control study | |
| Participants | 75 cases/326 controls Aged 16 to 44 years |
|
| Interventions | Combined oral contraception: current use, none use, 1st generation, 2nd generation, 3rd generation, levonorgestrel | |
| Outcomes | Myocardial infarction Events: current use 57/89, none use 125/272, 1st generation 14/14, 2nd generation 27/35, 3rd generation 8/33, levonorgestrel 22/29 |
|
| Notes | UK, Germany, France, Switzerland, Austria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | Low risk | COC use objectively confirmed, |
| Outcome assessment | Low risk | Myocardial infarction objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | Low risk | Controls from the same source population as the cases. |
Lidegaard 2012a.
| Methods | Cohort study | |
| Participants | 5036 events/9,336,662 person years Aged 15 to 49 years |
|
| Interventions | Combined oral contraception: current use, none use, 1st generation, 2nd generation, 3rd generation, 20 µg oestrogen, 30 to 49 µg oestrogen, ≥ 50 µg oestrogen, levonorgestrel, norethindrone, norgestimate, desogestrel, gestodene, drospirenone, cyproterone acetate | |
| Outcomes | Myocardial infarction and ischemic stroke Events: current use 1548/4,528,151, none use 3488/9,336,662, 1st generation 62/170,218, 2nd generation 303/515,033, 3rd generation 920/3,345,929, levonorgestrel 303/515,033, norethindrone 62/170,218, norgestimate 106/453,536, desogestrel 287/1,009,163, gestodene 527/1,883,230, drospirenone 70/286,770, cyproterone acetate 41/187,145. |
|
| Notes | Denmark | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | Low risk | COC use objectively confirmed. |
| Outcome assessment | High risk | Information on myocardial infarction and ischemic stroke obtained from a diagnostic registry |
| Follow‐up | Low risk | No loss to follow up. |
| Source population | Unclear risk | Not applicable as this was a population study. |
MacClellan 2007.
| Methods | Case‐control study | |
| Participants | 386 cases/614 controls Aged 15 to 49 years |
|
| Interventions | Combined oral contraception: current use and none use | |
| Outcomes | Ischemic stroke Events: current use 48/64, none use 338/550 |
|
| Notes | USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | High risk | COC use not objectively confirmed. |
| Outcome assessment | Low risk | Ischemic stroke objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | Low risk | Controls from the same source population as the cases. |
Mann 1975a.
| Methods | Case‐control study | |
| Participants | 153 cases/196 controls Aged 15 to 49 years |
|
| Interventions | Combined oral contraception: current, past and none use | |
| Outcomes | Fatal myocardial infarction Events: current use 31/19, past use 10/20, none use 114/162 |
|
| Notes | UK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | High risk | COC use not objectively confirmed. |
| Outcome assessment | Low risk | Myocardial infarction objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | High risk | Controls from a different source population to the cases. |
Mann 1975b.
| Methods | Case‐control study | |
| Participants | 49 cases/166 controls Aged 15 to 49 years |
|
| Interventions | Combined oral contraception: current, past and none use | |
| Outcomes | Non‐fatal myocardial infarction Events: current use 20/16, past use 8/24, none use 52/174 |
|
| Notes | UK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | High risk | COC use not objectively confirmed. |
| Outcome assessment | Low risk | Myocardial infarction objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | High risk | Controls from a different source population to the cases. |
Mann 1976a.
| Methods | Case‐control study | |
| Participants | 54 cases/54 controls Aged 40 to 44 years |
|
| Interventions | Combined oral contraception: current, past and none use | |
| Outcomes | Fatal myocardial infarction Events: current use 10/5, past use 8/5, none use 44/45 |
|
| Notes | UK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | High risk | COC use not objectively confirmed. |
| Outcome assessment | Low risk | Myocardial infarction objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | High risk | Controls from a different source population to the cases. |
Martinelli 2006.
| Methods | Case‐control study | |
| Participants | 105 cases/293 controls Aged < 50 years |
|
| Interventions | Combined oral contraception: current and none use | |
| Outcomes | Ischemic stroke Events: current use 43/67, none use 62/226 |
|
| Notes | Italy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | High risk | COC use not objectively confirmed. |
| Outcome assessment | Low risk | Ischemic stroke objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | Low risk | Controls from the same source population as the cases. |
Nightingale 2004.
| Methods | Case‐control study | |
| Participants | 190 cases/1129 controls Aged < 50 years |
|
| Interventions | Combined oral contraception: current and none use | |
| Outcomes | Ischemic stroke Events: current use 19/80, none use 171/1049 |
|
| Notes | UK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | Low risk | COC use objectively confirmed. |
| Outcome assessment | High risk | Ischemic stroke not objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | Low risk | Controls from the same source population as the cases. |
Owen‐Smith 1998.
| Methods | Case‐control study | |
| Participants | 103 cases/309 controls Average age 29 years |
|
| Interventions | Combined oral contraception: current, past and none use | |
| Outcomes | Myocardial infarction Events: current use 8/13, past use 57/140, none use 95/296 |
|
| Notes | UK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | High risk | COC use not objectively confirmed. |
| Outcome assessment | High risk | Myocardial infarction not objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | Low risk | Controls from the same source population as the cases. |
Pettiti 1996.
| Methods | Case‐control study | |
| Participants | 142 cases/378 controls Aged 15 to 44 years |
|
| Interventions | Combined oral contraception: current, past and none use | |
| Outcomes | Ischemic stroke Events: current use 17/43, past use 82/271, none use 125/335 |
|
| Notes | USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | High risk | COC use not objectively confirmed. |
| Outcome assessment | Low risk | Ischemic stroke objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | Low risk | Controls from the same source population as the cases. |
Pezzini 2007.
| Methods | Case‐control study | |
| Participants | 108 cases/216 controls Aged < 45 years |
|
| Interventions | Combined oral contraception: current and none use | |
| Outcomes | Ischemic stroke Events: current use 43/31, none use 65/185 |
|
| Notes | Italy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | High risk | COC use not objectively confirmed. |
| Outcome assessment | Low risk | Ischemic stroke objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | High risk | Controls from a different source population to the cases. |
Rosenberg 1976a.
| Methods | Case‐control study | |
| Participants | 33 cases/1096 controls Aged 37 to 49 years |
|
| Interventions | Combined oral contraception: current and none use | |
| Outcomes | Myocardial infarction Events: current use 4/75, none use 29/1021 |
|
| Notes | USA, UK, New Zealand, Canada, Germany, Italy and Israel | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | High risk | COC use not objectively confirmed. |
| Outcome assessment | High risk | Myocardial infarction not objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | High risk | Controls from a different source population to the cases. |
Rosenberg 2001.
| Methods | Case‐control study | |
| Participants | 627 cases/2947 controls Aged < 45 years |
|
| Interventions | Combined oral contraception: current use, past use, none use, 30 to 49 µg oestrogen, ≥ 50 µg oestrogen, norethindrone, levonorgestrel, desogestrel/norgestimate | |
| Outcomes | Myocardial infarction Events: current use 36/237, past use 412/1926, none use 591/2710, 30 to 49 µg oestrogen 13/108, ≥ 50 µg oestrogen 4/20, norethindrone 11/68, levonorgestrel 4/42, desogestrel/norgestimate 2/15 |
|
| Notes | USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | High risk | COC use objectively confirmed. |
| Outcome assessment | Low risk | Myocardial infarction objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | High risk | Controls from a different source population to the cases. |
Schwartz 1997.
| Methods | Case‐control study | |
| Participants | 60 cases/485 controls Aged 18 to 44 years |
|
| Interventions | Combined oral contraception: current use, past use, none use, norethindrone, norgestrel | |
| Outcomes | Ischemic stroke Events: current use 6/46, past use 39/363, none use 52/424, norethindrone 4/32, norgestrel 1/14 |
|
| Notes | USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | High risk | COC use not objectively confirmed. |
| Outcome assessment | High risk | Ischemic stroke not objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | High risk | Controls from a different source population to the cases. |
Shapiro 1979.
| Methods | Case‐control study | |
| Participants | 234 cases/1742 controls Aged 25 to 49 years |
|
| Interventions | Combined oral contraception: < 50 µg oestrogen, 50 µg oestrogen, > 50 µg oestrogen | |
| Outcomes | Myocardial infarction Events: < 50 µg oestrogen 8/39, 50 µg oestrogen 16/78, > 50 µg oestrogen 2/11 |
|
| Notes | USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | High risk | COC use not objectively confirmed. |
| Outcome assessment | Low risk | Myocardial infarction objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | High risk | Controls from a different source population to the cases. |
Sidney 1998.
| Methods | Case‐control study | |
| Participants | 271 cases/993 controls Aged 18 to 44 years |
|
| Interventions | Combined oral contraception: current use, past use, none use, norethindrone, norgestrel, < 50 µg oestrogen, ≥ 50 µg oestrogen | |
| Outcomes | Myocardial infarction Events: current use 12/87, past use 214/769, none use 41/135, norethindrone 8/52, norgestrel 3/24, < 50 µg oestrogen 9/78, > 50 µg oestrogen 2/5 |
|
| Notes | USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | High risk | COC use not objectively confirmed. |
| Outcome assessment | High risk | Myocardial infarction not objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | Low risk | Controls from the same source population as the cases. |
Slone 1981.
| Methods | Case‐control study | |
| Participants | 234 cases/1742 controls Aged 25 to 49 years |
|
| Interventions | Combined oral contraception: current use, past use and none use | |
| Outcomes | Myocardial infarction Events: current use 41/51, past use 206/762, none use 556/2036 |
|
| Notes | USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | High risk | COC use not objectively confirmed |
| Outcome assessment | Low risk | Myocardial infarction objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | High risk | Controls from a different source population to the cases. |
Tanis 2001.
| Methods | Case‐control study | |
| Participants | 249 cases/925 controls Aged 18 to 49 years |
|
| Interventions | Combined oral contraception: current use, none use, 1st generation, 2nd generation, 3rd generation | |
| Outcomes | Myocardial infarction Events: current use 99/348, none use 146/568, 1st generation 11/31, 2nd generation 59/173, 3rd generation 20/110 |
|
| Notes | The Netherlands | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | High risk | COC use not objectively confirmed. |
| Outcome assessment | Low risk | Myocardial infarction objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | Low risk | Controls from the same source population as the cases. |
Tzourio 1995.
| Methods | Case‐control study | |
| Participants | 75 cases/173 controls Aged < 49 years |
|
| Interventions | Combined oral contraception: current use, past use, none use, 20 µg oestrogen, 30 to 49 µg oestrogen, 50 µg oestrogen | |
| Outcomes | Ischemic stroke Events: current use 47/63, past use 19/85, none use 25/110, 20 µg oestrogen 2/5, 30 to 49 µg oestrogen 30/46, 50 µg oestrogen 8/7 |
|
| Notes | France | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Exposure ascertainment | High risk | COC use not objectively confirmed. |
| Outcome assessment | Low risk | Ischemic stroke objectively confirmed. |
| Follow‐up | Unclear risk | Not applicable as this was a case‐control study. |
| Source population | High risk | Controls from a different source population to the cases. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adachi 2005 | No extractable number of exposed and non‐exposed cases/controls. |
| Ananijević‐Pandey 1989 | No extractable number of exposed and non‐exposed cases/controls. |
| Andersson 2012 | RATIO study, data included elswhere (Tanis 2001; Kemmeren 2002). |
| Arscott 2001 | Same data as Dunn 1999. |
| Azarpazhooh 2008 | No extractable number of exposed and non‐exposed cases/controls. |
| Barinagarrementeria 1998 | Arteritis included as ischemic stroke. |
| Beaumont 1986 | No control group. |
| Berheci 1975 | Review. |
| Bonnar 1986 | Review. |
| Borovská 1981 | Case series. |
| Burnhill 1999 | Included progestagen‐only contraceptives. |
| Chang 1986 | Not possible to distinguish between current and ever users. |
| Chasan‐Taber 2001 | Editorial. |
| Chen 2001 | Unattainable. |
| Colditz 1986 | Not possible to distinguish between current and ever users. |
| Collaborative Group for the Study of Stroke 1973 | Transient ischemic attack (TIA) included as ischemic stroke. |
| Corfman 1974 | Editorial. |
| Croft 1989 | Not possible to distinguish between current and ever users. |
| D'Avanzo 1994 | Same data as La Vecchia 1987. |
| Dalen 1981 | Review. |
| Elagib 2008 | No extractable number of exposed and non‐exposed cases/controls. |
| Farley 1998 | Simulated data. |
| Fortney 1986 | Review. |
| Gronich 2011 | Study on risk of venous thrombosis. |
| Haapaniemi 1997 | Women up to 60 years of age included. |
| Hannaford 1998 | Same data as Owen‐Smith 1998. |
| Heinemann 1997 | Current COC use defined as within 3 months before inclusion. |
| Heinemann 1999 | Same data as Heinemann 1998. |
| Heyman 1969 | No extractable number of exposed and non‐exposed cases/controls. |
| Heyman 1972 | No control subjects included. |
| Huang 2014 | No upper age limit: average age was 58 in both cases and controls. |
| Jain 1976 | No extractable number of exposed and non‐exposed cases/controls. |
| Jain 1977 | Meta‐analysis. |
| Jensen 1991 | No extractable number of exposed and non‐exposed cases/controls. |
| Jick 1978b | No control subjects included. |
| Jick 1996 | No extractable number of exposed and non‐exposed cases/controls. |
| Jick 2007 | Same data as Jick 1978. |
| Jugdutt 1983 | Case series. |
| Kisjanto 2005 | No extractable number of exposed and non‐exposed cases/controls. |
| Lewis 1996 | Study on postmenopausal hormone therapy. |
| Lewis 1997b | Same data as Lewis 1997. |
| Li 2002 | Intrauterine device as reference group. |
| Li 2006 | Risk of hemorrhagic stroke. |
| Li 2010 | Intrauterine device as reference group. |
| Lidegaard 1986 | TIA and cerebral apoplexy included as ischemic stroke. |
| Lidegaard 1998a | TIA and cerebral apoplexy included as ischemic stroke. |
| Lidegaard 1998b | TIA and cerebral apoplexy included as ischemic stroke. |
| Lidegaard 1999 | No extractable number of exposed and non‐exposed cases/controls. |
| Lidegaard 2001 | Review. |
| Lui 2003 | Data included elsewhere (Shapiro 1979). |
| Maguire 1979 | Thrombosis defined as arterial or venous thrombosis. |
| Mann 1975c | Same data as Mann 1975b. |
| Mann 1976b | Review. |
| Mant 1987 | Progestagen‐only preparations included as COC. |
| Mant 1998 | Current use defined as use within past 12 months. |
| Margolis 2007 | Current use defined as use within past 12 months. |
| Matias‐Guiu 1990 | Unclear stroke definition. |
| Matthews 1989 | Review. |
| Meinel 1988 | Thrombosis defined as arterial or venous thrombosis. |
| Parazzini 1991 | Women up to 55 years of age included. |
| Porter 1982 | Ischemic stroke and hemorrhagic stroke combined. |
| Porter 1985 | Ischemic stroke and hemorrhagic stroke combined. |
| Poulter 1996 | Methodological study. |
| Poulter 1999 | Current use defined as within previous 3 months. |
| Presl 1976 | Translated summary of Mann 1975a. |
| Pruissen 2008 | RATIO study, data included elsewhere (Tanis 2001; Kemmeren 2002). |
| Psaty 1994 | Study on postmenopausal hormone therapy. |
| Riedel 1993 | Commentary. |
| Rosenberg 1976b | Same data as Rosenberg 1976a. |
| Rosenberg 1980 | Women up to age 64 included. |
| Rosenberg 1990 | Same data as Rosenberg 1976a and Rosenberg 2001. |
| Royal College of General Practitioners 1983 | No extractable number of exposed and non‐exposed women. |
| Salobir 2003 | Follow‐up study of patients with arterial thrombosis. |
| Salobir 2004 | No risk of arterial thrombosis calculated for oral contraception users. |
| Salonen 1982 | No extractable number of exposed and non‐exposed cases. |
| Salvesen 2000 | Review. |
| Schoenberg 1970 | No extractable number of exposed and non‐exposed cases. |
| Schwartz 1998 | Same data as Schwartz 1997. |
| Sidney 1996 | Same data as Sidney 1998. |
| Siegerink 2010 | RATIO Study (Tanis 2001; Kemmeren 2002). |
| Siegerink 2011a | RATIO Study (Tanis 2001; Kemmeren 2002). |
| Siegerink 2011b | RATIO Study (Tanis 2001; Kemmeren 2002). |
| Siritho 2003 | Women up to age 55 included. |
| Slone 1978 | Same data as Slone 1981. |
| Slooter 2005 | RATIO Study (Tanis 2001; Kemmeren 2002). |
| Stiefelhagen 2003 | Medical quiz. |
| Stolley 1982 | Thrombosis defined as arterial or venous thrombosis. |
| Sun 2004 | Data on hemorrhagic stroke. |
| Sørensen 2002 | Review. |
| Tanis 2003a | Review. |
| Tanis 2003b | RATIO Study (Tanis 2001; Kemmeren 2002). |
| Tanis 2004 | RATIO Study (Tanis 2001; Kemmeren 2002). |
| Thompson 1989 | No extractable number of exposed and non‐exposed women. |
| Thorogood 1991 | Non‐incident myocardial infarction included. |
| Urbanus 2009 | RATIO Study (Tanis 2001; Kemmeren 2002). |
| Vessey 1969 | No extractable number of exposed and non‐exposed women. |
| Wang 2012 | Same data as Li 2006. |
| WHO 1995 | Description of background, pilot study, methods and the analyses carried out to validate the methods used in the study. |
| WHO 1996a | Current use defined as within previous 3 months. |
| WHO 1996b | Study on hemorrhagic stroke. |
| WHO 1997 | Current use defined as within previous 3 months. |
| WHO 1998 | Progestagen‐only preparations and combined injectable contraceptives included. |
| Yang 2009 | Current use defined as within previous 12 months. |
| Zamorski 1996 | Summary of Pettiti 1996. |
Differences between protocol and review
During data extraction we noticed that the age cut‐off for women included in the analyses differed per study. Some studies had an upper age limit of 45 years, whereas other studies had an upper limit of 60 or no upper age limit at all. In this review, we chose to include (mostly) pre‐menopausal women, and so chose an age cut‐off of 50 years. We did this by including studies that included women up to 50 years of age, and, where possible, also by extracting data on women < 50 years from studies that included older women.
In the protocol, Roach 2014, we stated that we would assess the risk of myocardial infarction or ischemic stroke for first, second and third generation contraceptive preparations in our analysis on COC generation, and also for preparations containing drospirenone. In the review we have now added cyproterone acetate to this sentence, as this is also a commonly used type of progestagen that cannot be assigned to a generation.
Information on how each generation of COC pill was classified was not present in the protocol 'Methods' section. This information is important as oral contraceptive generations can be classified in more than one way. We have added the definitions to the Types of interventions section.
We aimed to investigate the risk of reporting bias in a funnel plot. However, as our review included various different types of analysis (overall risk, and risk according to generation, estrogen dose and progestagen type), and four different types of bias were assessed along with confounding, making a funnel plot was not feasible. Instead, we presented the risk of bias in the review text, a 'Risk of bias' table and a 'Risk of bias' summary figure.
Contributions of authors
REJR, OMD and FMH developed the study design. TS provided statistical expertise. AA and WML provided clinical expertise. REJR drafted the protocol. OMD and FMH edited the protocol. REJR and FMH independently selected the publications and extracted the data. REJR interpreted the data and drafted the manuscript. FMH, OMD, TS, AA and WML critically reviewed drafts of the manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Netherlands Heart Foundation, Netherlands.
Dr Willem Lijfering is a postdoctoral researcher for the Netherlands Heart Foundation (2011T012). This organization did not play a role in the design and conduct of this study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Declarations of interest
REJR declares no conflict of interest.
FMH declares no conflict of interest.
WML declares no conflict of interest.
TS declares no conflict of interest.
AA declares no conflict of interest.
OMD declares no conflict of interest.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Adam 1981 {published data only}
- Adam SA, Thorogood M, Mann JI. Oral contraception and myocardial infarction revisited: the effects of new preparations and prescribing patterns. British Journal of Obstetrics and Gynaecology 1981;88(8):838‐45. [DOI] [PubMed] [Google Scholar]
Aznar 2004 {published data only}
- Aznar J, Mira Y, Vayá A, Corella D, Ferrando F, Villa P, et al. Factor V Leiden and prothrombin G20210A mutations in young adults with cryptogenic ischemic stroke. Thrombosis and Haemostasis 2004;91(5):1031‐4. [DOI] [PubMed] [Google Scholar]
Chang 1999 {published data only}
- Chang CL, Donaghy M, Poulter N. Migraine and stroke in young women: casecontrol study. The World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. BMJ 1999;318(7175):13‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunn 1999 {published data only}
- Dunn N, Thorogood M, Faragher B, Caestecker L, MacDonald TM, McCollum C, et al. Oral contraceptives and myocardial infarction:results of the MICA casecontrol study. BMJ 1999;318(7198):1579‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heinemann 1998 {published data only}
- Heinemann LA, Lewis MA, Spitzer WO, Thorogood M, Guggenmoos‐Holzmann I, Bruppacher R. Thromboembolic stroke in young women: a European case‐control study on oral contraceptives. Transnational Research Group on Oral Contraceptives and the Health of Young Women. Contraception 1998;57(1):29‐37. [DOI] [PubMed] [Google Scholar]
Jick 1978 {published data only}
- Jick H, Dinan B, Rothman KJ. Oral contraceptives and nonfatal myocardial infarction. JAMA 1978;239(14):1403‐6. [PubMed] [Google Scholar]
Kemmeren 2002 {published data only}
- Kemmeren JM, Tanis BC, Bosch MA, Bollen EL, Helmerhorst FM, Graaf Y, et al. Risk of arterial thrombosis in relation to oral contraceptives (RATIO) study: oral contraceptives and the risk of ischemic stroke. Stroke 2002;33(5):1202‐8. [DOI] [PubMed] [Google Scholar]
Krueger 1980 {published data only}
- Krueger DE, Ellenberg SS, Bloom S, Calkins BM. Maliza C, Nolan DC, et al. Fatal myocardial infarction and the role of oral contraceptives. Journal of Epidemiology 1980;111(6):655‐74. [DOI] [PubMed] [Google Scholar]
La Vecchia 1987 {published data only}
- Vecchia C, Franceschi S, Decarli A, Pampallona S, Tognoni G. Risk factors for myocardial infarction in young women. American Journal of Epidemiology 1987;125(5):832‐43. [DOI] [PubMed] [Google Scholar]
Lewis 1997 {published data only}
- Lewis MA, Heinemann LA, Spitzer WO, MacRae KD, Bruppacher R. The use of oral contraceptives and the occurrence of acute myocardial infarction in young women: results from the Transnational Study on Oral Contraceptives and the Health of Young Women. Contraception 1997;56(3):129‐40. [DOI] [PubMed] [Google Scholar]
Lidegaard 2012a {published data only}
- Lidegaard Ø, Løkkegaard E, Jensen A, Skovlund CW, Keiding N. Thrombotic stroke and myocardial infarction with hormonal contraception. New England Journal of Medicine 2012;366(24):2257‐66. [DOI] [PubMed] [Google Scholar]
MacClellan 2007 {published data only}
- MacClellan LR, Giles W, Cole J, Wozniak M, Stern B, Mitchell BD, et al. Probable migraine with visual aura and risk of ischemic stroke: the stroke prevention in young women study. Stroke 2007;38(9):2438‐45. [DOI] [PubMed] [Google Scholar]
Mann 1975a {published data only}
- Mann JI, Inman WH. Oral contraceptives and death from myocardial infarction. British Medical Journal 1975;2(5965):245‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mann 1975b {published data only}
- Mann JI, Thorogood M, Waters WE, Powell C. Oral contraceptives and myocardial infarction in young women: a further report. British Medical Journal 1975;3(5984):631‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mann 1976a {published data only}
- Mann JI, Inman WH, Thorogood M. Oral contraceptive use in older women and fatal myocardial infarction. BMJ 1976;2(6033):445‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinelli 2006 {published data only}
- Martinelli I, Battaglioli T, Burgo I, Domenico S, Mannucci PM. Oral contraceptive use, thrombophilia and their interaction in young women with ischemic stroke. Haematologica 2006;91(6):844‐7. [PubMed] [Google Scholar]
Nightingale 2004 {published data only}
- Nightingale AL, Farmer RD. Ischemic stroke in young women: a nested case‐control study using the UK General Practice Research Database. Stroke 2004;35(7):1574‐8. [DOI] [PubMed] [Google Scholar]
Owen‐Smith 1998 {published data only}
- Owen‐Smith V, Hannaford PC, Warskyj M, Ferry S, Kay CR. Effects of changes in smoking status on risk estimates for myocardial infarction among women recruited for the Royal College of General Practitioners' Oral Contraception Study in the UK. Journal of Epidemiology and Community Health 1998;52(7):420‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pettiti 1996 {published data only}
- Pettiti D, Sidney S, Bernstein A, Wolf S, Quesenberry C, Ziel HK. Stroke in users of low‐dose oral contraceptives. New England Journal of Medicine 1996;335(1):8‐15. [DOI] [PubMed] [Google Scholar]
Pezzini 2007 {published data only}
- Pezzini A, Grassi M, Iacoviello L, Zotto E, Archetti S, Giossi A, et al. Inherited thrombophilia and stratification of ischaemic stroke risk among users of oral contraceptives. Journal of Neurology, Neurosurgery, and Psychiatry 2007;78(3):271‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenberg 1976a {published data only}
- Rosenberg L, Armstrong B, Jick H. Myocardial infarction and estrogen therapy in premenopausal women. New England Journal of Medicine 1976;294(23):1290‐1. [DOI] [PubMed] [Google Scholar]
Rosenberg 2001 {published data only}
- Rosenberg L, Palmer JR, Rao RS, Shapiro S. Low‐dose oral contraceptive use and the risk of myocardial infarction. Archives of Internal Medicine 2001;161(8):1065‐70. [DOI] [PubMed] [Google Scholar]
Schwartz 1997 {published data only}
- Schwartz SM, Siscovick DS, Longstreth WT Jr, Psaty BM, Beverly RK, Raghunathan TE, et al. Use of low‐dose oral contraceptives and stroke in young women. Annals of Internal Medicine 1997;127(8 Pt 1):596‐603. [DOI] [PubMed] [Google Scholar]
Shapiro 1979 {published data only}
- Shapiro S, Slone D, Rosenberg L, Kaufman DW, Stolley PD, Miettinen OS. Oral‐contraceptive use in relation to myocardial infarction. Lancet 1979;1(8119):743‐7. [DOI] [PubMed] [Google Scholar]
Sidney 1998 {published data only}
- Sidney S, Siscovick DS, Petitti DB, Schwartz SM, Quesenberry CP, Psaty BM, et al. Myocardial infarction and use of low‐dose oral contraceptives: a pooled analysis of 2 US studies. Circulation 1998;98(11):1058‐63. [DOI] [PubMed] [Google Scholar]
Slone 1981 {published data only}
- Slone D, Shapiro S, Kaufman DW, Rosenberg L, Miettinen OS, Stolley PD. Risk of myocardial infarction in relation to current and discontinued use of oral contraceptives. NEJM 1981;305(8):420‐4. [DOI] [PubMed] [Google Scholar]
Tanis 2001 {published data only}
- Tanis BC, Bosch MA, Kemmeren JM, Cats VM, Helmerhorst FM, Algra A, et al. Oral contraceptives and the risk of myocardial infarction. New England Journal of Medicine 2001;345(25):1787‐93. [DOI] [PubMed] [Google Scholar]
Tzourio 1995 {published data only}
- Tzourio C, Tehindrazanarivelo A, Iglésias S, Alpérovitch A, Chedru F, d'Anglejan‐Chatillon J, Bousser MG. Case‐control study of migraine and risk of ischaemic stroke in young women. BMJ 1995;310(6983):830‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Adachi 2005 {published data only}
- Adachi T, Sakamoto S. Thromboembolism during hormone therapy in Japanese women. Seminars in Thrombosis and Haemostasis 2005;31(3):272‐80. [DOI] [PubMed] [Google Scholar]
Ananijević‐Pandey 1989 {published data only}
- Ananijević‐Pandey J, Vlajinac H. Myocardial Infarction in young women with reference to oral contraceptive use. International Journal of Epidemiology 1989;18(3):585‐8. [DOI] [PubMed] [Google Scholar]
Andersson 2012 {published data only}
- Andersson HM, Siegerink B, Luken BM, Crawley JT, Algra A, Lane DA, et al. High VWF, low ADAMTS13, and oral contraceptives increase the risk of ischemic stroke and myocardial infarction in young women. Blood 2012;119(6):1555‐60. [DOI] [PubMed] [Google Scholar]
Arscott 2001 {published data only}
- Arscott A, Nettelfield P. Myocardial infarction and oral contraceptives. Professional Nurse 2001;16(5):1117‐20. [PubMed] [Google Scholar]
Azarpazhooh 2008 {published data only}
- Azarpazhooh MR, Rafi S, Etemadi MM, Khadem N, Fazlinejad A. The relation between short‐term oral contraceptive consumption and cerebrovascular, cardiovascular disorders in Iranian women attending Hajj. Saudi Medical Journal 2008;29(7):1024‐7. [PubMed] [Google Scholar]
Barinagarrementeria 1998 {published data only}
- Barinagarrementeria F, Gonzàlez‐Duarte A, Miranda L, Cantú C. Cerebral infarction in young women: analysis of 130 cases. European Neurology 1998;40(4):228‐33. [DOI] [PubMed] [Google Scholar]
Beaumont 1986 {published data only}
- Beaumont V, Beaumont JL. Oral contraception and thrombosis: implication of anti sex‐steroid hormone antibodies. Myocardial Infarction in Women. 1st Edition. Vol. 1st, Springer Berlin Heidelberg, 1986:190‐9. [Google Scholar]
Berheci 1975 {published data only}
- Berheci AL. Oral contraceptives, a new risk factor in the production of cerbral arterial occlusive disease [Contraceptivele orale, un nou factor de risc in producerea bolii ocluzive cerebro‐arteriale la femei]. Neurologie, Psihiatrie, Neurochirurgie 1975;20(2):89‐92. [PubMed] [Google Scholar]
Bonnar 1986 {published data only}
- Bonnar J, Sabra AM. Oral contraceptives and blood coagulation. Journal of Reproductive Medicine 1986;31(6 Suppl):551‐6. [PubMed] [Google Scholar]
Borovská 1981 {published data only}
- Borovská D, Volejník V. Incidence of cerebrovascular lesions in users of oral contraceptives [Incidence cevnich lezi cerebralnich pri uzivani peroralnich antikonceptiv]. Ceskoslovenská Neurologie e Neurochirurgie 1981;44(2):116‐20. [PubMed] [Google Scholar]
Burnhill 1999 {published data only}
- Burnhill MS. The use of a large‐scale surveillance system in Planned Parenthood Federation of America clinics to monitor cardiovascular events in users of combination oral contraceptives. International Journal of Fertility and Women's Medicine 1999;44(1):19‐30. [PubMed] [Google Scholar]
Chang 1986 {published data only}
- Chang KK, Chow LP, Rider RV. Oral contraceptives and stroke: a preliminary report on an epidemiologic study in Taiwan, China. International Journal of Gynaecology and Obstetrics 1986;24(6):421‐30. [DOI] [PubMed] [Google Scholar]
Chasan‐Taber 2001 {published data only}
- Chasan‐Taber L, Stampfer M. Oral contraceptives and myocardial infarction ‐ the search for the smoking gun. New England Journal of Medicine 2001;345(25):1841‐2. [DOI] [PubMed] [Google Scholar]
Chen 2001 {published data only}
- Chen J, Li Y, Zhou J. A study of relationship between oral contraceptives and gene polymorphism and types of stroke [Chinese]. Chinese Journal of Epidemiology 2001;22(4):273‐6. [PubMed] [Google Scholar]
Colditz 1986 {published data only}
- Colditz GA, Stampfer MJ, Willett WC, Rosner B, Speizer FE, Hennekens CH. A prospective study of parental history of myocardial infarction and coronary heart disease in women. American Journal of Epidemiology 1986;123(1):48‐58. [DOI] [PubMed] [Google Scholar]
Collaborative Group for the Study of Stroke 1973 {published data only}
- Collaborative Group for the Study of Stroke in Young Women. Oral contraception and increased risk of cerebral ischaemia of thrombosis. New England Journal of Medicine 1973;288(17):871‐8. [DOI] [PubMed] [Google Scholar]
Corfman 1974 {published data only}
- Corfman PA. Coordinated studies of the effects of oral contraceptives. Contraception 1974;9(2):109‐22. [DOI] [PubMed] [Google Scholar]
Croft 1989 {published data only}
- Croft P, Hannaford P. Risk factors for acute myocardial infarction in women. BMJ 1989;298(6674):674. [DOI] [PMC free article] [PubMed] [Google Scholar]
D'Avanzo 1994 {published data only}
- D'Avanzo B, Vecchia C, Negri E, Parazzini F, Franceschi S. Oral contraceptive use and risk of myocardial infarction: an Italian case‐control study. Journal of Epidemiology and Community Health 1994;48(3):324‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dalen 1981 {published data only}
- Dalen JE, Hickler RB. Oral contraceptives and cardiovascular disease. American Heart Journal 1981;101(5):626‐39. [DOI] [PubMed] [Google Scholar]
Elagib 2008 {published data only}
- Elagib AH, Ahmed AE, Hussein A, Musa AM, Khalil EA, El‐Hassan AM. Possible predisposing factors for thrombotic cerebral accidents in Sudanese patients. Saudi Medical Journal 2008;29(2):304‐6. [PubMed] [Google Scholar]
Farley 1998 {published data only}
- Farley TM, Meirik O, Chang CL, Poulter NR. Combined oral contraceptives, smoking, and cardiovascular risk. Journal of Epidemiology and Community Health 1998;52(12):775‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fortney 1986 {published data only}
- Fortney JA, Harper JM, Potts M. Oral contraceptives and life expectancy. Studies in Family Planning 1986;17(3):117‐25. [PubMed] [Google Scholar]
Gronich 2011 {published data only}
- Gronich N, Lavi I, Rennert G. Higher risk of venous thrombosis associated with drospirenone‐containing oral contraceptives:a population‐based cohort study. Canadian Medical Association Journal 2011;183(18):E1319‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haapaniemi 1997 {published data only}
- Haapaniemi H, Hillbom M, Juvela S. Lifestyle‐associated risk factors for acute brain infarction among persons of working age. Stroke 1997;28(1):26‐30. [DOI] [PubMed] [Google Scholar]
Hannaford 1998 {published data only}
- Hannaford PC, Owen‐Smith V. Using epidemiological data to guide clinical practice: review of studies on cardiovascular disease and use of combined oral contraceptives. BMJ 1998;316(7136):984‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heinemann 1997 {published data only}
- Heinemann LA, Lewis MA, Thorogood M, Spitzer WO, Guggenmoos‐Holzmann I, Bruppacher R. Case‐control study of oral contraceptives and risk of thromboembolic stroke: results from International Study on Oral Contraceptives and Health of Young Women. BMJ 1997;315(7121):1502‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heinemann 1999 {published data only}
- Heinemann LA, Assmann A, DoMinh T, Garbe E, Transnational Research Group on Oral Contraceptives and the Health of Young Women. Oral progestogen‐only contraceptives and cardiovascular risk: results from the Transnational Study on Oral Contraceptives and the health of young women. European Journal of Contraception & Reproductive Health Care 1999;4(2):67‐73. [DOI] [PubMed] [Google Scholar]
Heyman 1969 {published data only}
- Heyman A, Arons M, Quinn M, Camplong L. The role of oral contraceptive agents in cerebral arterial occlusion. Neurology 1969;19(6):519‐24. [DOI] [PubMed] [Google Scholar]
Heyman 1972 {published data only}
- Heyman A, for the Collaborative Group for Study of Stroke in Young Women. Risk of stroke with use of oral contraceptives. Circulation. 1972; Vol. 155&156:185.
Huang 2014 {published data only}
- Huang X, Li Y, Huang Z, Wang C, Xu Z. Pai‐1 gene variants and COC use a reassociated with stroke risk: a case‐control study in the Han Chinese women. Journal of Molecular Neuroscience 2014;54(4):803‐10. [DOI] [PubMed] [Google Scholar]
Jain 1976 {published data only}
- Jain AK. Cigarette smoking, use of oral contraceptives, and myocardial infarction. American Journal of Obstetrics and Gynecology 1976;126(3):301‐7. [DOI] [PubMed] [Google Scholar]
Jain 1977 {published data only}
- Jain AK. Mortality risk associated with the use of oral contraceptives. Studies in Family Planning 1977;8(3):50‐4. [PubMed] [Google Scholar]
Jensen 1991 {published data only}
- Jensen G, Nyboe J, Appleyard M, Schnohr P. Risk factors for acute myocardial infarction in Copenhagen II: Smoking, alcohol intake, physical activity, obesity, oral contraception, diabetes, lipids, and blood pressure. European Heart Journal 1991;12(3):298‐308. [DOI] [PubMed] [Google Scholar]
Jick 1978b {published data only}
- Jick H, Dinan B, Herman R, Rothman KJ. Myocardial infarction and other vascular diseases in young women. Role of estrogens and other factors. JAMA 1978;240(23):2548‐52. [PubMed] [Google Scholar]
Jick 1996 {published data only}
- Jick H, Jick S, Myers MW, Vasilakis C. Risk of acute myocardial infarction and low‐dose combined oral contraceptives. Lancet 1996;347(9001):627‐8. [DOI] [PubMed] [Google Scholar]
Jick 2007 {published data only}
- Jick SS, Jick H. The contraceptive patch in relation to ischemic stroke and acute myocardial infarction. Pharmacotherapy 2007;27(2):218‐20. [DOI] [PubMed] [Google Scholar]
Jugdutt 1983 {published data only}
- Jugdutt BI, Stevens GF, Zacks DJ, Lee SJ, Taylor RF. Myocardial infarction, oral contraception, cigarette smoking, and coronary artery spasm in young women. American Heart Journal 1983;106(4 Pt 1):757‐61. [DOI] [PubMed] [Google Scholar]
Kisjanto 2005 {published data only}
- Kisjanto J, Bonneux L, Prihartono J, Ranakusuma TA, Grobbee DE. Risk factors for stroke among urbanised Indonesian women of reproductive age: a hospital‐based case‐control study. Cerebrovascular Diseases 2005;19(1):18‐22. [DOI] [PubMed] [Google Scholar]
Lewis 1996 {published data only}
- Lewis MA, Spitzer WO, Heinemann LA, MacRae KD, Bruppacher R, Thorogood M, Transnational Research Group on Oral Contraceptives and the Health of Young Women. Third generation oral contraceptives and risk of myocardial infarction: an international case‐control study. BMJ 1996;312(7023):88‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 1997b {published data only}
- Lewis MA, Spitzer WO, Heinemann LA, MacRae KD, Bruppacher R. Lowered risk of dying of heart attack with third generation pill may offset risk of dying of thromboembolism. BMJ 1997;315(7109):678‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2002 {published data only}
- Li Y, Gao E, Liu Y, Luo L, Wang X, Wang H, et al. Association between low‐dose oral contraceptive use and stroke in Chinese women. National Medical Journal of China 2002;82(15):1013‐7. [PubMed] [Google Scholar]
Li 2006 {published data only}
- Li Y, Zhou L, Coulter D, Gao E, Sun Z, Liu Y, et al. Prospective cohort study of the association between use of low‐dose oral contraceptives and stroke in Chinese women. Pharmacoepidemiology and Drug Safety 2006;15(10):726‐34. [DOI] [PubMed] [Google Scholar]
Li 2010 {published data only}
- Li Y, Chen F, Zhou L, Coulter D, Chen C, Sun Z, et al. COC use, ACE/AGT gene polymorphisms, and risk of stroke. Pharmacogenetics and Genomics 2010;20(5):298‐306. [DOI] [PubMed] [Google Scholar]
Lidegaard 1986 {published data only}
- Lidegaard O, Soe M, Andersen MV. Cerebral thromboembolism among young women and men in Denmark 1977‐1982. Stroke 1986;17(4):670‐5. [DOI] [PubMed] [Google Scholar]
Lidegaard 1998a {published data only}
- Lidegaard O. Thrombotic diseases in young women and the influence of oral contraceptives. American Journal of Obstetrics and Gynecology 1998;179(3 Pt 2):S62‐7. [DOI] [PubMed] [Google Scholar]
Lidegaard 1998b {published data only}
- Lidegaard O, Kreiner S. Cerebral thrombosis and oral contraceptives. A case control study. Contraception 1998;57(5):303‐14. [DOI] [PubMed] [Google Scholar]
Lidegaard 1999 {published data only}
- Lidegaard O. Smoking and use of oral contraceptives: impact on thrombotic diseases. American Journal of Obstetrics and Gynecology 1999;180(6 Pt 2):S357‐63. [DOI] [PubMed] [Google Scholar]
Lidegaard 2001 {published data only}
- Lidegaard O. Oral contraceptive pills and thrombosis [P‐piller og trombose]. Ugeskrift for Laeger 2001;163:4549‐53. [PubMed] [Google Scholar]
Lui 2003 {published data only}
- Lui KJ. Interval estimation of the attributable risk for multiple exposure levels in case‐control studies with confounders. Statistics in Medicine 2003;22(15):2443‐57. [DOI] [PubMed] [Google Scholar]
Maguire 1979 {published data only}
- Maguire MG, Tonascia J, Sartwell PE, Stolley PD, Tockman MS. Increased risk of thrombosis due to oral contraceptives: a further report. American Journal of Epidemiology 1979;110(2):188‐95. [DOI] [PubMed] [Google Scholar]
Mann 1975c {published data only}
- Mann JI, Vessey MP, Thorogood M, Doll SR. Myocardial infarction in young women with special reference to oral contraceptive practice. British Medical Journal 1975;2(5965):241‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mann 1976b {published data only}
- Mann JI, Doll R, Thorogood M, Vessey MP, Waters WE. Risk factors for myocardial infarction in young women. British Journal of Preventive & Social Medicine 1976;30(2):94‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mant 1987 {published data only}
- Mant D, Villard‐Mackintosh L, Vessey MP, Yeates D. Myocardial infarction and angina pectoris in young women. Journal of Epidemiology and Community Health 1987;41(3):215‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mant 1998 {published data only}
- Mant J, Painter R, Vessey M. Risk of myocardial infarction, angina and stroke in users of oral contraceptives: an updated analysis of a cohort study. British Journal of Obstetrics and Gynaecology 1998;105(8):890‐6. [DOI] [PubMed] [Google Scholar]
Margolis 2007 {published data only}
- Margolis KL, Adami HO, Luo J, Ye W, Weiderpass E. A prospective study of oral contraceptive use and risk of myocardial infarction among Swedish women. Fertility and Sterility 2007;88(2):310‐6. [DOI] [PubMed] [Google Scholar]
Matias‐Guiu 1990 {published data only}
- Matias‐Guiu J, Alvarez J, Insa R, Moltó JM, Martin R, Codina A, et al. Ischemic stroke in young adults II. Analysis of risk factors in the etiological subgroups. Acta Neurologica Scandinavica 1990;81(4):314‐7. [DOI] [PubMed] [Google Scholar]
Matthews 1989 {published data only}
- Matthews KA. Interactive effects of behavior and reproductive hormones on sex differences in risk for coronary heart disease. Health Psychology 1989;8(4):373‐87. [PubMed] [Google Scholar]
Meinel 1988 {published data only}
- Meinel VH, Goretzlehner G, Heinemann L. Hormonal contraception and cardiovascular risk. Results from a multicenter case‐control study [Hormonale Kontraceptiva und Kardiovaskulares Risiko. Ergebnisse einer multi zentrischen DDR‐Fall‐Kontrol‐Studie]. Zentralblatt fur Gynakologie 1988;110:1507‐14. [PubMed] [Google Scholar]
Parazzini 1991 {published data only}
- Parazzini F, Vecchia C, Negri E. Benefits and risks of the oral contraceptive pill ‐ a revision of the results from an Italian case‐control study [Benefici e rischi della pillola contraccettiva una revisione dei risultati da uno studio italiano]. Annali di Ostetricia, Ginecologia, Medicina Perinatale 1991;112(6):368‐75. [PubMed] [Google Scholar]
Porter 1982 {published data only}
- Porter JB, Hunter JR, Danielson DA, Jick H, Stergachis A. Oral contraceptives and nonfatal vascular disease ‐ recent experience. Obstetrics and Gynecology 1982;59(3):299‐302. [PubMed] [Google Scholar]
Porter 1985 {published data only}
- Porter JB, Hunter JR, Jick H, Stergachis A. Oral contraceptives and nonfatal vascular disease. Obstetrics and Gynecology 1985;66(1):1‐4. [PubMed] [Google Scholar]
Poulter 1996 {published data only}
- Poulter NR, Chang CL, Farley TM, Marmot MG. Reliability of data from proxy respondents in an international case‐control study of cardiovascular disease and oral contraceptives. World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Journal of Epidemiology and Community Health 1996;50(6):674‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poulter 1999 {published data only}
- Poulter NR, Chang CL, Farley TM, Marmot MG, Meirik O. Effect on stroke of different progestagens in low oestrogen dose oral contraceptives. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet 1999;354(9175):301‐2. [DOI] [PubMed] [Google Scholar]
Presl 1976 {published data only}
- Presl J. Myocardial infarction in young women and steroidal contraception [Infarkt myokardu u mladych zen a steroidni antikoncepce]. CeskaGynekologie 1976;41:156. [PubMed] [Google Scholar]
Pruissen 2008 {published data only}
- Pruissen DM, Slooter AJ, Rosendaal FR, Graaf Y, Algra A. Coagulation factor XIII gene variation, oral contraceptives, and risk of ischemic stroke. Blood 2008;111(3):1282‐6. [DOI] [PubMed] [Google Scholar]
Psaty 1994 {published data only}
- Psaty BM, Heckbert SR, Atkins D, Lemaitre R, Koepsell TD, Wahl PW, et al. The risk of myocardial infarction associated with the combined use of estrogens and progestins in postmenopausal women. Archives of Internal Medicine 1994;154(12):1333‐9. [PubMed] [Google Scholar]
Riedel 1993 {published data only}
- Riedel M, Lichtlen PR. Oral contraception and cerebral emboli [Orale kontrazeption und zerebrale embolien]. Deutsche Medizinische Wochenschrift 1993;118(41):1506‐7. [PubMed] [Google Scholar]
Rosenberg 1976b {published data only}
- Rosenberg L, Armstrong B, Jick H. Myocardial infarction and estrogen therapy in premenopausal women. New England Journal of Medicine 1976;294(23):1290‐1. [DOI] [PubMed] [Google Scholar]
Rosenberg 1980 {published data only}
- Rosenberg L, Hennekens CH, Rosner B, Belanger C, Rothman KJ, Speizer FE. Oral contraceptive use in relation to nonfatal myocardial infarction. American Journal of Epidemiology 1980;11(1):59‐66. [DOI] [PubMed] [Google Scholar]
Rosenberg 1990 {published data only}
- Rosenberg L, Palmer JR, Lesko SM, Shapiro S. Oral contraceptive use and the risk of myocardial infarction. American Journal of Epidemiology 1990;131(6):1009‐16. [DOI] [PubMed] [Google Scholar]
Royal College of General Practitioners 1983 {published data only}
- Royal College of General Practioners. Incidence of arterial disease among oral contraceptive users. Journal of the Royal College of General Practitioners 1983;33(247):75‐82. [PMC free article] [PubMed] [Google Scholar]
Salobir 2003 {published data only}
- Salobir B, Sabovic M, Peternel P, Stegnar M. Vascular bed specific alterations in coagulation and fibrinolytic parameters in young women following myocardial infarction, lacunar cerebral infarction and deep vein thrombosis. Pathophysiology of Haemostasis and Thrombosis 2003;33(2):96‐101. [DOI] [PubMed] [Google Scholar]
Salobir 2004 {published data only}
- Salobir B, Sabovic M. Possible vascular‐bed‐specific role of interleukin‐6 in young women with a history of myocardial infarction, lacunar cerebral infarction and deep vein thrombosis. Cytokine 2004;25(6):265‐72. [DOI] [PubMed] [Google Scholar]
Salonen 1982 {published data only}
- Salonen JT. Oral contraceptives, smoking and risk of myocardial infarction in young women. A longitudinul population study in eastern Finland. Acta Medica Scandinavica 1982;212(3):141‐4. [DOI] [PubMed] [Google Scholar]
Salvesen 2000 {published data only}
- Salvesen R. Migraine patients and birth control pills [Migrenepasienter og p‐piller]. Tidsskr Nor Lageforen 2000;120:1883‐4. [PubMed] [Google Scholar]
Schoenberg 1970 {published data only}
- Schoenberg BS, Whisnant JP, Taylor WF, Kempers RD. Strokes in women of childbearing age. A population study. Neurology 1970;20(2):181‐9. [DOI] [PubMed] [Google Scholar]
Schwartz 1998 {published data only}
- Schwartz SM, Petitti DB, Siscovick DS, Longstreth WT, Sidney S, Raghunathan TE, et al. Stroke and use of low‐dose oral contraceptives in young women: a pooled analysis of two US studies. Stroke 1998;29(11):2277‐84. [DOI] [PubMed] [Google Scholar]
Sidney 1996 {published data only}
- Sidney S, Petitti DB, Quesenberry CP Jr, Klatsky AL, Ziel HK, Wolf S. Myocardial infarction in users of low‐dose oral contraceptives. Obstetrics and Gynecology 1996;88(6):939‐44. [DOI] [PubMed] [Google Scholar]
Siegerink 2010 {published data only}
- Siegerink B, Govers‐Riemslag JW, Rosendaal FR, Cate H, Algra A. Intrinsic coagulation activation and the risk of arterial thrombosis in young women: results from the Risk of Arterial Thrombosis in Relation to Oral Contraceptives (RATIO) case‐control study. Circulation 2010;122(18):1854‐61. [DOI] [PubMed] [Google Scholar]
Siegerink 2011a {published data only}
- Siegerink B, Meltzer ME, Groot PG, Algra A, Lisman T, Rosendaal FR. Clot lysis time and the risk of myocardial infarction and ischaemic stroke in young women; results from the RATIO case–control study. British Journal of Haematology 2011;156(2):252‐8. [DOI] [PubMed] [Google Scholar]
Siegerink 2011b {published data only}
- Siegerink B, Andersson HM, Luken BM, Crawley JT, Algra A, Lane DA, et al. VWF and ADAMTS13 levels and the risk of myocardial infarction and ischaemic stroke in young women: results from the ratio case‐control study. Journal of Thrombosis and Haemstasis: Suppl 2. 2011; Vol. 9:209..
Siritho 2003 {published data only}
- Siritho S, Thrift AG, McNeil JJ, You RX, Davis SM, Donnan GA. Risk of ischemic stroke among users of the oral contraceptive pill: the Melbourne Risk Factor Study (MEFRS) Group. Stroke 2003;34(7):1575‐80. [DOI] [PubMed] [Google Scholar]
Slone 1978 {published data only}
- Slone D, Shapiro S, Rosenberg L, Kaufman DW, Hartz SC, Rossi AC, et al. Relation of cigarette smoking to myocardial infarction in young women. New England Journal of Medicine 1978;298(23):1273‐6. [DOI] [PubMed] [Google Scholar]
Slooter 2005 {published data only}
- Slooter AJ, Rosendaal FR, Tanis BC, Kemmeren JM, Graaf Y, Algra A. Prothrombotic conditions, oral contraceptives, and the risk of ischemic stroke. Journal of Thrombosis and Haemostasis 2005;3(6):1213‐7. [DOI] [PubMed] [Google Scholar]
Stiefelhagen 2003 {published data only}
- Stiefelhagen P. Prevention, diagnosis and therapy of stroke: time is brain!. MMW ‐ Fortschritte der Medizin 2003; Vol. 145. [PubMed]
Stolley 1982 {published data only}
- Stolley PD. The use of vital and morbidity statistics for the detection of adverse drug reactions and for monitoring of drug safety. J Clin Pharmacol 1982;22:499‐504. [DOI] [PubMed] [Google Scholar]
Sun 2004 {published data only}
- Sun Z, Li Y. Risk factors for haemorrhagic stroke and oral contraceptives among Chinese women: 1:1 Case‐control study. Chinese Journal of Family Planning 2004;12:606‐609. [Google Scholar]
Sørensen 2002 {published data only}
- Sørensen MB, Ottesen BS. Oral contraceptives and risk of myocardial infarction [P‐piller og risiko for myokardieinfarkt]. Ugeskrift for Laeger 2002;164(18):2415‐6. [PubMed] [Google Scholar]
Tanis 2003a {published data only}
- Tanis BC. Oral contraceptives and the risk of myocardial infarction. European Heart Journal 2003;24(5):377‐80. [DOI] [PubMed] [Google Scholar]
Tanis 2003b {published data only}
- Tanis BC, Bloemenkamp DG, Bosch MA, Kemmeren JM, Algra A, Graaf Y, et al. Prothrombotic coagulation defects and cardiovascular risk factors in young women with acute myocardial infarction. British Journal of Haematology 2003;122(3):471‐8. [DOI] [PubMed] [Google Scholar]
Tanis 2004 {published data only}
- Tanis BC, Blom HJ, Bloemenkamp DG, Bosch MA, Algra A, Graaf Y, et al. Folate, homocysteine levels, methylenetetrahydrofolate reductase (MTHFR) 677C ‐‐>T variant, and the risk of myocardial infarction in young women: effect of female hormones on homocysteine levels. Journal of Thrombosis and Haemostasis 2004;2(1):35‐41. [DOI] [PubMed] [Google Scholar]
Thompson 1989 {published data only}
- Thompson SG, Greenberg G, Meade TW. Risk factors for stroke and myocardial infarction in women in the United Kingdom as assessed in general practice: a case‐control study. British Heart Journal 1989;61(5):403‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorogood 1991 {published data only}
- Thorogood M, Mann J, Murphy M, Vessey M. Is oral contraceptive use still associated with an increased risk of fatal myocardial infarction? Report of a case‐control study. British Journal of Obstetrics and Gynaecology 1991;98(12):1245‐53. [DOI] [PubMed] [Google Scholar]
Urbanus 2009 {published data only}
- Urbanus RT, Siegerink B, Roest M, Rosendaal FR, Groot PG, Algra A. Antiphospholipid antibodies and risk of myocardialinfarction and ischaemic stroke in young women in the RATIO study: a case‐control study. Lancet Neurology 2009;8(11):998‐1005. [DOI] [PubMed] [Google Scholar]
Vessey 1969 {published data only}
- Vessey MP, Doll R. Investigation of relation between use of oral contraceptives and thromboembolic disease. A further report. British Medical Journal 1969;2(5658):651‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2012 {published data only}
- Wang C, Li Y, Li H, Sun T, Jin G, Sun Z, et al. Increased risk of stroke in oral contraceptive users carried replicated genetic variants: a population‐based case–control study in China. Human Genetics 2012;131(8):1337‐44. [DOI] [PubMed] [Google Scholar]
WHO 1995 {published data only}
- World Health Organization. A multinational case‐control study of cardiovascular disease and steroid hormone contraceptives. Description and validation of methods. World Health Organization Collaborative Study of Cardiovascular and Steroid Hormone Contraception. Journal of Clinical Epidemiology 1995;48(12):1513‐47. [DOI] [PubMed] [Google Scholar]
WHO 1996a {published data only}
- WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Ischaemic stroke and combined oral contraceptives: results of an international, multi centre, case‐control study. Lancet 1996;348(9026):498‐505. [PubMed] [Google Scholar]
WHO 1996b {published data only}
- WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Haemorrhagic stroke, overall stroke risk, and combined oral contraceptives: results of an international, multi centre, case‐control study. Lancet 1996;348(9026):505‐10. [PubMed] [Google Scholar]
WHO 1997 {published data only}
- WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Acute myocardial infarction and combined oral contraceptives: results of an international multi centre case‐control study. Lancet 1997;349(9060):1202‐9. [PubMed] [Google Scholar]
WHO 1998 {published data only}
- World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Cardiovascular disease and use of oral and injectable progestogen‐only contraceptives and combined injectable contraceptives: results of an international, multicenter, case‐control study. Contraception 1998;57(5):315‐24. [PubMed] [Google Scholar]
Yang 2009 {published data only}
- Yang L, Kuper H, Sandin S, Margolis KL, Chen Z, Adami HO, et al. Reproductive history, oral contraceptive use, and the risk of ischemic and hemorrhagic stroke in a cohort study of middle‐aged Swedish women. Stroke 2009;40(4):1050‐8. [DOI] [PubMed] [Google Scholar]
Zamorski 1996 {published data only}
- Zamorski M. Stroke in users of low‐dose oral contraceptives. Journal of Family Practice 1996;43(4):343‐4. [PubMed] [Google Scholar]
Additional references
Badimon 1999
- Badimon L, Bayés‐Genís A. Effects of progestogens on thrombosis and atherosclerosis. Human Reproduction Update 1999;5(3):191‐9. [DOI] [PubMed] [Google Scholar]
Baillargeon 2005
- Baillargeon JP, McClish DK, Essah PA, Nestler JE. Association between the current use of low‐dose oral contraceptives and cardiovascular arterial disease: a meta‐analysis. Journal of Clinical Endocrinology and Metabolism 2005;90(7):3863‐70. [DOI] [PubMed] [Google Scholar]
Berry 2012
- Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, et al. Lifetime risks of cardiovascular disease. New England Journal of Medicine 2012;366(4):321‐9. [PUBMED: 22276822] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borissoff 2011
- Borissoff JI, Spronk HM, Cate H. The haemostatic system as a modulator of atherosclerosis. New England Journal of Medicine 2011;364(18):1746‐60. [PUBMED: 21542745] [DOI] [PubMed] [Google Scholar]
Brieger 2009
- Brieger D, Fox KA, Fitzgerald G, Eagle KA, Budaj A, Avezum A, et al. Predicting freedom from clinical events in non‐ST‐elevation acute coronary syndromes: the Global Registry of Acute Coronary Events. Heart (British Cardiac Society) 2009;95(11):888‐94. [PUBMED: 19246481] [DOI] [PubMed] [Google Scholar]
Bringer 1992
- Bringer J. Norgestimate: a clinical overview of a new progestin. American Journal of Obstetrics and Gynecology 1992;166(6 Pt 2):1969‐77. [DOI] [PubMed] [Google Scholar]
Caplan 2009
- Caplan LR. Caplan's Stroke: A Clinical Approach. 4th Edition. Philadelphia: Saunders Elsevier, 2009. [Google Scholar]
Christiansen 2005
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA 2005;293(19):2352‐61. [DOI] [PubMed] [Google Scholar]
Christiansen 2010
- Christiansen SC, Lijfering WM, Helmerhorst FM, Rosendaal FR, Cannegieter SC. Sex difference in risk of recurrent venous thrombosis and the risk profile for a second event. Journal of Thrombosis and Haemostasis 2010;8(10):2159‐68. [DOI] [PubMed] [Google Scholar]
de Bastos 2014
- Bastos M, Stegeman BH, Rosendaal FR, Hylckama Vlieg A, Helmerhorst FM, Stijnen T, et al. Combined oral contraceptives: venous thrombosis. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD010813.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Di Carlo 2003
- Carlo A, Lamassa M, Baldereschi M, Pracucci G, Basile AM, Wolfe CD, et al. Sex differences in the clinical presentation, resource use, and 3‐month outcome of acute stroke in Europe: data from a multicenter multinational hospital‐based registry. Stroke 2003;34(5):1114‐9. [PUBMED: 12690218] [DOI] [PubMed] [Google Scholar]
Gillum 2000
- Gillum LA, Mamidipudi SK, Johnston SC. Ischemic stroke risk with oral contraceptives: a meta‐analysis. JAMA 2000;284(1):72‐8. [DOI] [PubMed] [Google Scholar]
Godsland 1990
- Godsland IF, Crook D, Simpson R, Proudler T, Felton C, Lees B, et al. The effects of different formulations of oral contraceptive agents on lipid and carbohydrate metabolism. New England Journal of Medicine 1990;323(20):1375‐81. [PUBMED: 2146499] [DOI] [PubMed] [Google Scholar]
Golder 2011
- Golder S, Loke YK, Bland M. Meta‐analyses of adverse effects data derived from randomised controlled trials as compared to observational studies: methodological overview. PLoS Medicine 2011;8(5):e1001026. [PUBMED: 21559325] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grimes 2005
- Grimes DA, Schulz KF. Compared to what? Finding controls for case‐control studies. Lancet 2005;365(9468):1429‐33. [PUBMED: 15836892] [DOI] [PubMed] [Google Scholar]
Hardman 2009
- Hardman SM, Gebbie AE. Hormonal contraceptive regimens in the perimenopause. Maturitas 2009;63(3):204‐12. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Jüni 1999
- Jüni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta‐analysis. JAMA 1999;282(11):1054‐60. [PUBMED: 10493204] [DOI] [PubMed] [Google Scholar]
Khader 2003
- Khader YS, Rice J, John L, Abueita O. Oral contraceptives use and the risk of myocardial infarction: a meta‐analysis. Contraception 2003;68(1):11‐7. [DOI] [PubMed] [Google Scholar]
Knol 2008
- Knol MJ, Vandenbroucke JP, Scott P, Egger M. What do case‐control studies estimate? Survey of methods and assumptions in published case‐control research. Am J Epidemiol 2008;168(9):1073‐1081. [DOI] [PubMed] [Google Scholar]
Krattenmacher 2000
- Krattenmacher R. Drospirenone: pharmacology and pharmacokinetics of a unique progestogen. Contraception 2000;62(1):29‐38. [DOI] [PubMed] [Google Scholar]
Lawrie 2011
- Lawrie TA, Helmerhorst FM, Maitra NK, Kulier R, Bloemenkamp K, Gülmezoglu AM. Types of progestogens in combined oral contraception: effectiveness and side‐effects. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD004861.pub2] [DOI] [PubMed] [Google Scholar]
Lidegaard 2012b
- Lidegaard O, Nielsen LH, Skovlund CW, Løkkegaard E. Venous thrombosis in users of non‐oral hormonal contraception: follow‐up study, Denmark 2001‐10. BMJ 2012;344:e2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nabel 2012
- Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. New England Journal of Medicine 2012;366(1):54‐63. [PUBMED: 22216842] [DOI] [PubMed] [Google Scholar]
Nischan 1993
- Nischan P, Ebeling K, Thomas DB, Hirsch U. Comparison of recalled and validated oral contraceptive histories. American Journal of Epidemiology 1993;138(9):697‐703. [PUBMED: 8237985] [DOI] [PubMed] [Google Scholar]
Norell 1998
- Norell SE, Boethius G, Persson I. Oral contraceptive use: interview data versus pharmacy records. International Journal of Epidemiology 1998;27(6):1033‐7. [PUBMED: 10024199] [DOI] [PubMed] [Google Scholar]
Peragallo‐Urrutia 2013
- Peragallo‐Urrutia R, Coeytaux RR, McBroom AJ, Gierisch JM, Havrilesky LJ, Moorman PG, et al. Risk of acute thromboembolic events with oral contraceptive use: a systematic review and meta‐analysis. Obstetrics and Gynecology 2013;122(2 Pt 1):380‐9. [DOI] [PubMed] [Google Scholar]
Plu‐Bureau 2013
- Plu‐Bureau G, Hugon‐Rodin J, Maitrot‐Mantelet L, Canonico M. Hormonal contraceptives and arterial disease: an epidemiological update. Best Practice & Research. Clinical & Endocrinology Metabolism 2013;27(1):35‐45. [DOI] [PubMed] [Google Scholar]
Review Manager (RevMan) [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roe 2010
- Roe MT, Messenger JC, Weintraub WS, Cannon CP, Fonarow GC, Dai D, et al. Treatments, trends, and outcomes of acute myocardial infarction and percutaneous coronary intervention. Journal of the American College of Cardiology 2010;56(4):254‐63. [PUBMED: 20633817] [DOI] [PubMed] [Google Scholar]
Speroff 2011
- Speroff L, Darney PH. A Clinical Guide for Contraception. 5th Edition. Wolters Kluwer, 2011. [Google Scholar]
Tchaikovski 2010
- Tchaikovski SN, Rosing J. Mechanisms of estrogen induced venous thromboembolism. Thrombosis Research 2010;126(1):5‐11. [PUBMED: 20163835] [DOI] [PubMed] [Google Scholar]
Trussell 2011
- Trussell J. Contraceptive failure in the United States. Contraception 2011;83(5):397‐404. [PUBMED: 21477680] [DOI] [PMC free article] [PubMed] [Google Scholar]
United Nations 2011
- United Nations. World Contraceptive Use 2011. http://www.un.org/esa/population/publications/contraceptive2011/contraceptive2011.htm (accessed 08 July 2015).
van Hylckama Vlieg 2009
- Hylckama Vlieg A, Helmerhorst FM, Vandenbroucke JP, Doggen CJ, Rosendaal FR. The venous thrombotic risk of oral contraceptives, effects of oestrogen dose and progestogen type: results of the MEGA case‐control study. BMJ 2009;339:b2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vandenbroucke 2004
- Vandenbroucke JP. When are observational studies as credible as randomised trials?. Lancet 2004;363(9422):1728‐31. [PUBMED: 15158638] [DOI] [PubMed] [Google Scholar]
Vandenbroucke 2012
- Vandenbroucke JP, Pearce N. Case‐control studies: basic concepts. Int J Epidemiol 2012;41(5):1480‐9. [DOI] [PubMed] [Google Scholar]
WHO 2004
- World Health Organization. Medical eligibility criteria for contraceptive use. http://apps.who.int/iris/bitstream/10665/42907/1/9241562668.pdf (accessed 08 July 2015). [PubMed]
WHO 2013
- World Health Organization. Cardiovascular diseases (CVDs). http://www.who.int/mediacentre/factsheets/fs317/en/ (accessed 08 July 2015).
Wilson 1998
- Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation 1998;97(18):1837‐47. [PUBMED: 9603539] [DOI] [PubMed] [Google Scholar]
Zakharova 2011
- Zakharova MY, Meyer RM, Brandy KR, Datta YH, Joseph MS, Schreiner PJ, et al. Risk factors for heart attack, stroke, and venous thrombosis associated with hormonal contraceptive use. Clinical and Applied Thrombosis/Hemostasis 2011;17(4):323‐31. [PUBMED: 20530058] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Roach 2014
- Roach REJ, Helmerhorst FM, Lijfering WM, Algra A, Dekkers OM. Combined oral contraceptives: the risk of myocardial infarction and ischemic stroke. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD011054] [DOI] [PMC free article] [PubMed] [Google Scholar]


