Abstract
Background
Fluvastatin is thought to be the least potent statin on the market, however, the dose‐related magnitude of effect of fluvastatin on blood lipids is not known.
Objectives
Primary objective To quantify the effects of various doses of fluvastatin on blood total cholesterol, low‐density lipoprotein (LDL cholesterol), high‐density lipoprotein (HDL cholesterol), and triglycerides in participants with and without evidence of cardiovascular disease.
Secondary objectives To quantify the variability of the effect of various doses of fluvastatin. To quantify withdrawals due to adverse effects (WDAEs) in randomised placebo‐controlled trials.
Search methods
The Cochrane Hypertension Information Specialist searched the following databases for randomised controlled trials up to February 2017: the Cochrane Central Register of Controlled Trials (CENTRAL) (2017, Issue 1), MEDLINE (1946 to February Week 2 2017), MEDLINE In‐Process, MEDLINE Epub Ahead of Print, Embase (1974 to February Week 2 2017), the World Health Organization International Clinical Trials Registry Platform, CDSR, DARE, Epistemonikos and ClinicalTrials.gov. We also contacted authors of relevant papers regarding further published and unpublished work. No language restrictions were applied.
Selection criteria
Randomised placebo‐controlled and uncontrolled before and after trials evaluating the dose response of different fixed doses of fluvastatin on blood lipids over a duration of three to 12 weeks in participants of any age with and without evidence of cardiovascular disease.
Data collection and analysis
Two review authors independently assessed eligibility criteria for studies to be included, and extracted data. We entered data from placebo‐controlled and uncontrolled before and after trials into Review Manager 5 as continuous and generic inverse variance data, respectively. WDAEs information was collected from the placebo‐controlled trials. We assessed all trials using the 'Risk of bias' tool under the categories of sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other potential biases.
Main results
One‐hundred and forty‐five trials (36 placebo controlled and 109 before and after) evaluated the dose‐related efficacy of fluvastatin in 18,846 participants. The participants were of any age with and without evidence of cardiovascular disease, and fluvastatin effects were studied within a treatment period of three to 12 weeks. Log dose‐response data over doses of 2.5 mg to 80 mg revealed strong linear dose‐related effects on blood total cholesterol and LDL cholesterol and a weak linear dose‐related effect on blood triglycerides. There was no dose‐related effect of fluvastatin on blood HDL cholesterol. Fluvastatin 10 mg/day to 80 mg/day reduced LDL cholesterol by 15% to 33%, total cholesterol by 11% to 25% and triglycerides by 3% to 17.5%. For every two‐fold dose increase there was a 6.0% (95% CI 5.4 to 6.6) decrease in blood LDL cholesterol, a 4.2% (95% CI 3.7 to 4.8) decrease in blood total cholesterol and a 4.2% (95% CI 2.0 to 6.3) decrease in blood triglycerides. The quality of evidence for these effects was judged to be high. When compared to atorvastatin and rosuvastatin, fluvastatin was about 12‐fold less potent than atorvastatin and 46‐fold less potent than rosuvastatin at reducing LDL cholesterol. Very low quality of evidence showed no difference in WDAEs between fluvastatin and placebo in 16 of 36 of these short‐term trials (risk ratio 1.52 (95% CI 0.94 to 2.45).
Authors' conclusions
Fluvastatin lowers blood total cholesterol, LDL cholesterol and triglyceride in a dose‐dependent linear fashion. Based on the effect on LDL cholesterol, fluvastatin is 12‐fold less potent than atorvastatin and 46‐fold less potent than rosuvastatin. This review did not provide a good estimate of the incidence of harms associated with fluvastatin because of the short duration of the trials and the lack of reporting of adverse effects in 56% of the placebo‐controlled trials.
Plain language summary
Fluvastatin for lowering lipids
Review question
What is the effect of various doses of fluvastatin on blood lipids?
The effects of various doses of fluvastatin on blood lipids were quantified in 145 studies.
Background
Fluvastatin is thought to be the least potent statin but the precise dose‐related effect of fluvastatin on lipids is unknown. It would be interesting to know how much fluvastatin lowers blood lipids in the 145 studies retrieved.
Search date
The evidence is current to February 2017.
Study characteristics
Randomised placebo‐controlled and uncontrolled before and after trials of different fixed doses of fluvastatin. The studies were of three to 12 weeks duration.
Participants could be of any age and gender with or without evidence of cardiovascular disease.
One‐hundred and forty‐five included trials involved 18,846 participants.
Key results
Fluvastatin 10 mg/day to 80 mg/day reduced LDL cholesterol by 15% to 33%. There were strong linear dose‐related effects on blood total cholesterol and LDL cholesterol and a weak linear dose‐related effect on blood triglycerides. There was no dose‐related effect of fluvastatin on blood HDL cholesterol.
Based on the effect on LDL cholesterol, fluvastatin is 12‐fold less potent than atorvastatin and 46‐fold less potent than rosuvastatin.
Of the 36 placebo‐controlled trials only 16 reported withdrawals due to adverse effects (WDAEs). WDAEs were higher, risk ratio 1.52 (95% confidence interval (CI) 0.94 to 2.45), demonstrating uncertainty, but the possibility of an increase in adverse effects.
Quality of the evidence
The quality of evidence was high for the lipid levels. For WDAEs the quality of evidence was very low because 20 (55.6%) out of 36 placebo‐controlled trials did not report WDAEs.
Summary of findings
Background
Description of the condition
Cardiovascular disease is the major cause of death and disability in the developed world (Eisenberg 1998). Existing evidence shows a weak association in young adults between adverse cardiovascular events and concentration of total cholesterol or low‐density lipoprotein (LDL) cholesterol in the serum (NCEP 1993).
The current recommended treatment for secondary prevention of adverse cardiovascular events after diet and lifestyle changes is drug therapy with the drug class widely known as "statins".
Description of the intervention
Fluvastatin is the least potent widely prescribed statin in the world. Fluvastatin and the seven other statins are prescribed to prevent adverse cardiovascular events and to lower total cholesterol and LDL cholesterol. Importantly, statins have been shown in individual randomised controlled trials (RCTs), and in a systematic review and meta‐analysis of RCTs to reduce mortality and major vascular events in people with occlusive vascular disease (CTT 2005).
How the intervention might work
Statins act on the liver by inhibiting the rate‐limiting enzyme for cholesterol synthesis, 3‐hydroxy‐3‐methyl‐glutaryl‐CoA (HMG Co‐A) reductase. This enzyme is the first step in a sequence of reactions resulting in the production of cholesterol and its derivatives, LDL cholesterol and very low‐density lipoprotein (VLDL cholesterol) particles. The prevailing hypothesis is that statins reduce mortality and morbidity in people with occlusive vascular disease by reducing the production of cholesterol. However, the HMG Co‐A reductase enzyme is also responsible for the production of coenzyme Q₁₀, vitamin D, steroid hormones, and many other compounds. It therefore remains possible that the beneficial effects of statins are due to an action other than the reduction of cholesterol, often referred to as the pleiotropic effects of statins (Liao 2005).
Most important for this review is the fact that a fasting blood lipid profile consisting of total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides is used clinically to monitor the effect of a prescribed statin. The outcome therefore in this review, presented as the percentage reduction in the five serum lipids, represents the best available pharmacological marker of the magnitude of the statin effect.
Why it is important to do this review
Statins are the most widely prescribed class of drugs in the world. Statin prescribing and the average prescribed doses are increasing. Clinicians currently have an approximate sense of the different potency of the different statins, but a systematic assessment of the potency, the slope of the dose‐response relationship, and the variability of the effect has not been completed for any of the statins. It is possible that in addition to differences in potency, the slope of the dose‐response relationship or the variability of response differs between different statins. A small number of previous systematic reviews have assessed the effect of statins on serum lipids (Bandolier 2004; Edwards 2003; Law 2003; Ward 2007). They have demonstrated that different statins have different potencies in terms of lipid lowering, and that higher doses of statins cause greater lowering of serum lipids than lower doses (Kellick 1997; Schaefer 2004; Schectman 1996). However, none of these systematic reviews has calculated the slope of the dose response or the variability of effect, and none of them is up‐to‐date. The most comprehensive systematic review to date (Law 2003) has the limitation that it presents the data based on the average reduction in LDL cholesterol concentration rather than on the percentage reduction from baseline. The purpose of our systematic review is to build on Law's work. Since fluvastatin is the least potent statin, we have chosen this as the third drug to study in this class, to complement the reviews we published on the lipid‐lowering efficacy of atorvastatin (Adams 2014) and rosuvastatin (Adams 2015). We used the surrogate marker to measure the pharmacological effect of statins, the percentage reduction from baseline, to describe the dose‐response relationship of the effect of fluvastatin on total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides. We plan to use the methodology established for atorvastatin (Adams 2014) and rosuvastatin (Adams 2015) to study the other drugs in the class (cerivastatin, lovastatin, pravastatin, simvastatin, and pitavastatin) in subsequent reviews, and to compare the results with fluvastatin, rosuvastatin and atorvastatin.
Objectives
Primary objective
To quantify the effects of various doses of fluvastatin on the surrogate markers: blood total cholesterol, LDL cholesterol, triglycerides and HDL cholesterol in people with and without evidence of cardiovascular disease.
We recognise that the outcomes important to patients are mortality and cardiovascular morbidity, however, that is not the objective of this systematic review. We want to learn more about the pharmacology of fluvastatin by characterising the dose‐related effect and variability of the effect of fluvastatin on the surrogate markers.
Secondary objective
To quantify the variability of the effect of various doses of fluvastatin on withdrawals due to adverse effects (WDAEs).
Methods
Criteria for considering studies for this review
Types of studies
Randomised placebo‐controlled trials. We have also included uncontrolled before and after trials because it has been shown that there is no placebo effect of statins on lipid parameters. Therefore in this case a placebo control is not essential (Tsang 2002). We did not include cross‐over trials, but if the outcomes were reported for the parallel arms prior to the cross‐over we did include that data.
Types of participants
Participants could be of any age, with and without evidence of cardiovascular disease. They could have normal lipid parameters or any type of hyperlipidaemia or dyslipidaemia. We accepted participants with various comorbid conditions, including type 2 diabetes mellitus, hypertension, metabolic syndrome, chronic renal failure or cardiovascular disease.
Types of interventions
Fluvastatin must have been administered at a constant daily dose compared to placebo or alone for a period of three to 12 weeks. We have chosen this administration time window to allow at least three weeks for a steady‐state effect of fluvastatin to occur and to keep it short enough to minimise participants dropping out. We included studies where fluvastatin was administered once daily in the morning or evening, twice daily or where it was not specified. Trials required a washout baseline dietary stabilisation period of at least three weeks, where all previous lipid‐altering medication was withdrawn. This baseline phase ensured participants follow a standard lipid‐regulating diet and helped to stabilise baseline lipid values prior to treatment. In trials where participants were not receiving lipid‐altering medications or dietary supplements before receiving the test drug, we did not require washout baseline dietary stabilisation periods.
Types of outcome measures
Fluvastatin 10 mg/day, 20 mg/day, 40 mg/day and 80 mg/day are the doses predominantly prescribed. Because of this and because most of the trials studied these doses we have presented these doses in the 'Summary of findings' tables.
Lipid parameters: For the placebo‐controlled trials we present the mean percentage change from baseline for different doses of fluvastatin minus the mean percentage change from baseline with placebo for each of the lipid parameters below. For the before and after trials we present the mean percentage change from baseline of different doses of fluvastatin. RCT data and before and after data were combined because it was shown for most data that there was a lack of difference in the mean differences between the two types of studies.
Primary outcomes
LDL cholesterol.
Secondary outcomes
Total cholesterol.
HDL cholesterol.
Triglycerides.
End of treatment variability (standard deviation (SD)) and coefficient of variation of LDL cholesterol measurements for each dose of fluvastatin. It is important to know whether fluvastatin has an effect on the variability of lipid measures and ultimately to compare this with the effect of other statins.
Withdrawals due to adverse effects (WDAEs) limited to the placebo‐controlled trials.
Search methods for identification of studies
Electronic searches
The Cochrane Hypertension Information Specialist conducted systematic searches in the following databases for randomised controlled trials (RCTs) without language, publication year or publication status restrictions:
the Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies (CRS‐Web) (searched 10 February 2017);
MEDLINE Ovid (1946 to February Week 2 2017), MEDLINE Ovid Epub Ahead of Print, and MEDLINE Ovid In‐Process & Other Non‐Indexed Citations (searched 10 February 2017);
Embase Ovid (searched 10 February 2017);
ClinicalTrials.gov (www.clinicaltrials.gov) searched 10 February 2017);
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch) searched 10 February 2017).
The Information Specialist modelled subject strategies for databases on the search strategy designed for MEDLINE. We present search strategies for major databases in Appendix 1.
Searching other resources
The Cochrane Hypertension Information Specialist searched the Cochrane Database of Systematic Reviews (CDSR) via Wiley, the Database of Abstracts of Reviews of Effects (DARE) via Wiley, and Epistemonikos to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials.
We checked the bibliographies of included studies and any relevant systematic reviews identified for further references to relevant trials.
We contacted experts/organisations in the field to obtain additional information on relevant trials.
We contacted original authors for clarification and further data if trial reports were unclear.
We performed an initial search of Web of Science on 4 April 2016 and omitted this database from subsequent searches, as it did not yield any unique included studies.
We included grey literature by searching other resources:
ProQuest Dissertations and Theses (search.proquest.com/pqdtft/);
Novartis (www.novartis.ca/products/en/pharmaceuticals‐az.shtml);
US Food and Drug Administration (www.fda.gov/);
European Patent Office (worldwide.espacenet.com.
Data collection and analysis
Selection of studies
Initial selection of trials involved retrieving and reading the titles and abstracts of each paper found from the electronic search databases or bibliographic citations. We have provided a PRISMA flow diagram (Figure 1). Two review authors (SA and SS) analysed the full‐text papers independently, to decide on the trials to be included. We resolved disagreements by recourse to a third review author (JMW). Two review authors (SA and SS) independently extracted the appropriate data from each of the included trials. If there was disagreement over a value, we reached consensus by data recalculation to determine the correct value.
1.
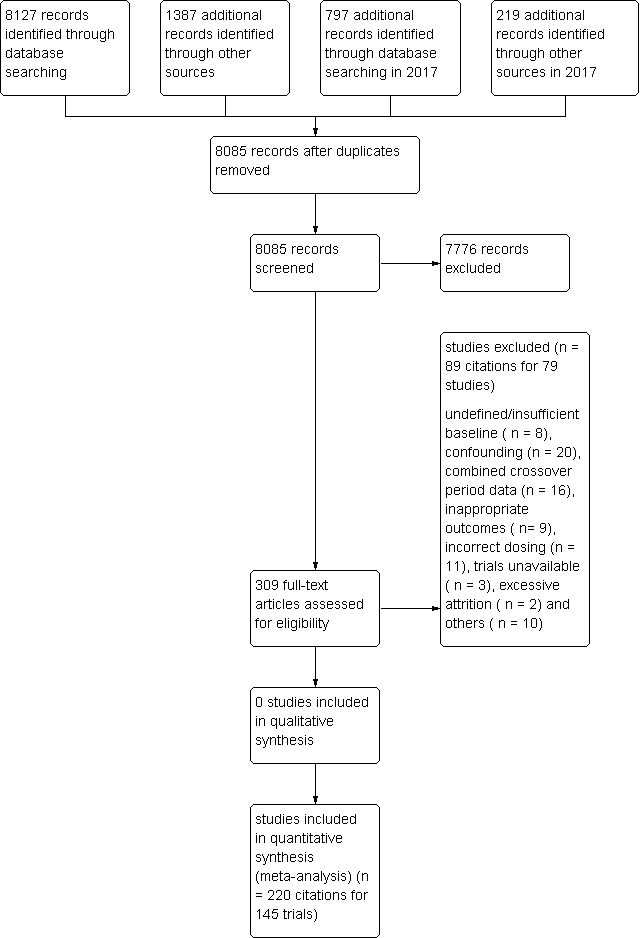
Fluvastatin flow diagram
Data extraction and management
We directly extracted the mean percentage change from the data or calculated it from the baseline and endpoint values. We added the calculated data to the Data and analyses section of the review. When the calculated data differed from the given data by more than 10%, we judged the data set as not being reliable and these data were not included in the review. We extracted standard deviations (SDs) and standard errors (SEs) from the report or calculated them when possible. We entered data from placebo‐controlled and uncontrolled before and after trials into Review Manager 5 (RevMan 2014) as continuous and generic inverse variance data, respectively.
Assessment of risk of bias in included studies
We assessed all trials using the 'Risk of bias' tool under the categories of adequate sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other potential biases. We appreciate that the first three items are inappropriate for before and after trials and that this is a limitation. However, because the lipid parameters were measured in a remote laboratory they were considered unlikely to be affected by the trial design. We produced 'Risk of bias' tables' as outlined in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 8 (Higgins 2011).
Measures of treatment effect
We analysed the treatment effects as mean difference for each dose in the placebo‐controlled RCTs and generic inverse variance for each dose in the before and after uncontrolled trials separately. In the event that the mean effects from the two trial designs were not different, we re‐analysed all efficacy study data using the generic inverse variance to determine the overall weighted treatment effects and their 95% confidence intervals (CIs) for serum total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides.
Unit of analysis issues
The unit of analysis is the mean values for the people completing the trial for each trial. We expected follow‐up to be reasonably high for these short‐term trials. The data however represent treatment efficacy and not real‐world effectiveness of fluvastatin on these lipid parameters.
Dealing with missing data
When data were missing, we requested them from the authors. The most common type of value that was not reported was the SD of the change.
In the case of a missing SD for the change in lipid parameters, we imputed the SD using the following hierarchy (listed from high to low preference).
SD calculated either from the t statistics corresponding to the exact P value reported or from the 95% CI of the mean difference between treatment groups.
Average weighted SD of the change from other trials in the review (Furukawa 2006).
Because it is common for the SD to be miscalculated, and in order not to overweight trials where it is inaccurately calculated and lower than expected, when SD values were less than 40% of the average weighted SDs, we used the imputed value by the method of Furukawa (Furukawa 2006).
Assessment of heterogeneity
The Chi2 test to identify heterogeneity is not appropriate because it has low power when there are few studies, but has excessive power to detect clinically unimportant heterogeneity when there are many studies. The I² is a better statistic. The I² calculates between‐study variance/(between‐study variance + within‐study variance). This measures the proportion of total variation in the estimate of the treatment effect that is due to heterogeneity between studies. This statistic is also independent of the number of studies in the analysis (Higgins 2002).
Assessment of reporting biases
We assessed publication bias using funnel plots, as outlined in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 10 (Sterne 2011).
Data synthesis
We entered all placebo‐controlled studies into Review Manager 5 (RevMan 2014) as mean difference fixed‐effect model data to determine the weighted treatment effect and 95% CIs for serum total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides. We entered all uncontrolled before and after studies as generic inverse variance fixed‐effect model data to determine the weighted treatment effect. If the effect in the placebo‐controlled trials was not statistically significantly different from the before and after trials, we entered all trials for each dose as generic inverse variance to determine the best overall weighted treatment effect for each dose.
If an I² was ≥50%, we used the random‐effects model to assess whether the pooled effect was statistically significant.
We recorded trial data of each study and dose in GraphPad Prism 4, to yield a weighted least squares analysis based on the inverse of the square of the standard error (SE) for each lipid parameter, to generate weighted log dose response curves. We entered the number of participants in placebo‐controlled trials who prematurely withdrew due to at least one adverse effect in Review Manager 5 (RevMan 2014) as dichotomous data for each dose and all combined doses of fluvastatin.
The relative potency of fluvastatin with respect to atorvastatin and rosuvastatin, was determined as the ratio of the milligram (mg) amount of fluvastatin to the mg amount of atorvastatin or rosuvastatin needed to produce the same specified effect. These values were calculated from the log dose response curves of fluvastatin, atorvastatin and rosuvastatin for total cholesterol, LDL cholesterol and triglycerides. The relative potencies were estimated from these dose ratios.
Data presentation ‐ ’Summary of findings’ tables
We used the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach to assess the quality of the supporting evidence behind each estimate of treatment effect (Schünemann 2011a; Schünemann 2011b). We presented key findings of the review, including a summary of the amount of data, the magnitude of the effect size and the overall quality of the evidence, in Table 1. We preselected the following outcomes: LDL cholesterol lowering efficacy of fluvastatin (by dose), and WDAEs.
for the main comparison.
| LDL cholesterol lowering efficacy of fluvastatin | ||||||
|
Patient or population: participants with normal or abnormal lipid profiles Settings: ambulatory care Intervention: fluvastatin Comparison: LDL cholesterol percentage change from baseline for all trials | ||||||
| Outcomes |
Anticipated absolute effects mmol/L (95%CI) |
Percent reduction
(95% CI) % |
No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Before exposure to fluvastatin1 | After exposure to fluvastatin | |||||
|
LDL‐cholesterol fluvastatin 10 mg/day |
4.81 (4.44 to 5.17) |
4.08 (3.98 to 4.16) |
15.2 (17.1 to 13.4) |
595 (6) | ⊕⊕⊕⊕ high | Effect predicted from log dose‐response equation is 14.8%. Randomised and before and after design not different P = 0.94. |
|
LDL‐cholesterol fluvastatin 20 mg/day |
4.87 (4.54 to 5.21) |
3.90 (3.88 to 3.91) |
20.0 (19.7 to 20.3) |
9010 (55) | ⊕⊕⊕⊕ high | Effect predicted from log dose‐response equation is 20.8%. Randomised and before and after design not different P = 0.16. |
|
LDL‐cholesterol fluvastatin 40 mg/day |
4.74 (4.41 to 5.06) |
3.51 (3.48 to 3.54) |
25.9 (25.3 to 26.5) |
3658 (57) | ⊕⊕⊕⊕ high | Effect predicted from log dose‐response equation is 26.8%. Randomised and before and after design not different P = 0.58. |
|
LDL‐cholesterol fluvastatin 80 mg/day |
4.80 (4.47 to 5.13) |
3.13 (3.10 to 3.15) |
34.9 (35.5 to 34.3) |
4928 (32) | ⊕⊕⊕⊕ high | Effect predicted from log dose‐response equation is 32.8%. Randomised and before and after design not different P = 0.07. |
| CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. Mean baseline values.
Subgroup analysis and investigation of heterogeneity
The main subgroup analyses are the different doses of fluvastatin. We assessed heterogeneity using I² (Higgins 2002). If the I² was ≥ 50%, we attempted to identify possible causes for this by carrying out a number of planned subgroup analyses, provided there were sufficient numbers of trials (see below).
We analysed subgroups based on the following factors.
Placebo‐controlled trials versus before and after trials (described above).
Men versus women.
Morning administration time versus evening administration time.
Novartis funded versus non‐Novartis funded trials.
Sensitivity analysis
We conducted sensitivity analyses to assess the effect of different co morbidities, such as familial hyperlipidaemia, on the treatment effect. We compared the treatment effects as generic inverse variance between trials whose participants were reported to have type IIa or familial hypercholesterolaemia versus trials whose participants were not reported to have genetic hypercholesterolaemia. Trials were not included in the comparison if the participants had both familial and non‐familial hypercholesterolaemia. We conducted sensitivity analyses to assess the effect of different methods of dosing, such as twice daily versus single dose, on the treatment effect.
Results
Description of studies
This review included 145 trials involving 18,846 people. There were 109 before and after trials, 35 randomised double‐blind placebo‐controlled trials, one randomised single‐blind placebo‐controlled trial. The number of placebo and fluvastatin participants were 2925 and 15,921, respectively. The number of male and female participants reported in 125 of the 145 trials were 9836 and 8845, respectively. Participants could be of any age. There were 13 familial hypercholesterolaemia trials and 99 non‐familial hypercholesterolaemia trials.
Results of the search
Database searching identified a total of 10,530 records. After the duplicates were removed, 8085 records remained. The number of irrelevant records was 7776. From these remaining records, 309 were obtained as full‐text articles and assessed for eligibility. The number of excluded records with reasons was 79 trials. The final number of included studies was 145. (Figure 1).
Included studies
Two hundred and twenty citations to 145 trials met the inclusion criteria and had extractable data to evaluate the dose‐related blood lipid‐lowering effect of fluvastatin. Each included study is summarised in the Characteristics of included studies table. The publication languages of the 145 included studies were 119 (82.1%) English, seven (4.8%) Japanese, six (4.1%) Russian, three (2.1%) Chinese, three (2.1%) German, three (2.1%) Polish, one (0.7%) Czech, French, Hungarian and Spanish, respectively. Of the 36 placebo‐controlled trials, 33 (91.7%) were double‐blind, one (2.8%) was single‐blind, and two (5.6%) were open‐label trials. Trials evaluating the lipid‐altering efficacy of fluvastatin were first published in 1994. Between 1994 and 2014, the number of available studies increased and then decreased. The year with the most available studies was 1995 (Figure 2).
2.
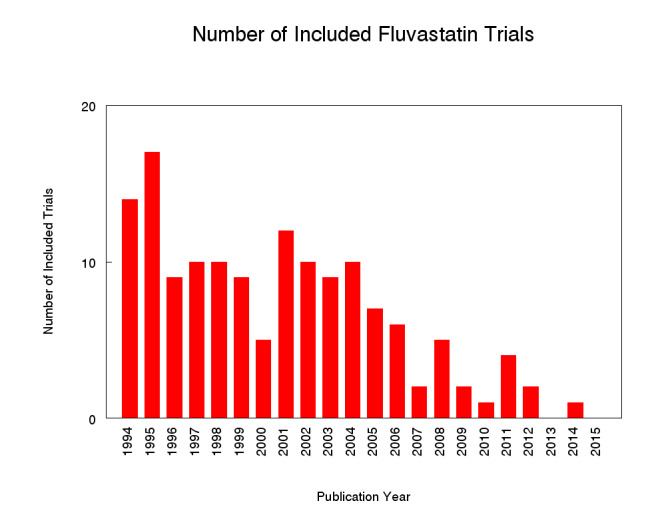
Number of included studies according to publication year
The baseline mean (range) lipid parameters were as follows: total cholesterol, 7.01 mmol/L (3.88 mmol/L to 10.52 mmol/L), 271 mg/dL (150 mg/dL to 407 mg/dL); LDL‐cholesterol, 4.93 mmol/L (2.07 mmol/L to 8.00 mmol/L), 191 mg/dL (80 mg/dL to 309 mg/dL); HDL‐cholesterol 1.24 mmol/L (0.87 mmol/L to 1.77 mmol/L), 47.9 mg/dL (33.6 mg/dL to 68.4 mg/dL) and triglycerides, 2.04 mmol/L (0.8 to mmol/L 5.9 mmol/L), 181 mg/dL (71 mg/dL to 523 mg/dL). Trials were available for the dose range of 2.5 mg to 80 mg fluvastatin daily and were sufficient to generate dose‐response regression lines for total cholesterol, LDL cholesterol and triglycerides (Figure 3; Figure 4; Figure 5).
3.
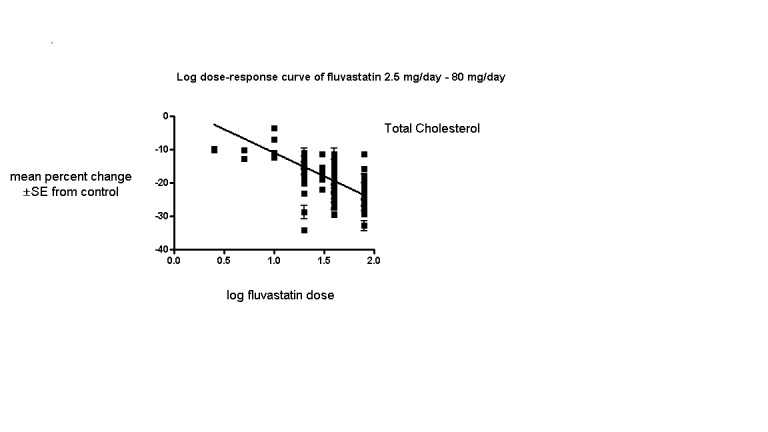
Log dose fluvastatin response curve for total cholesterol
Values represent the results of each trial for each dose comparison. The standard error bars cannot be seen because they all lie within the points
4.
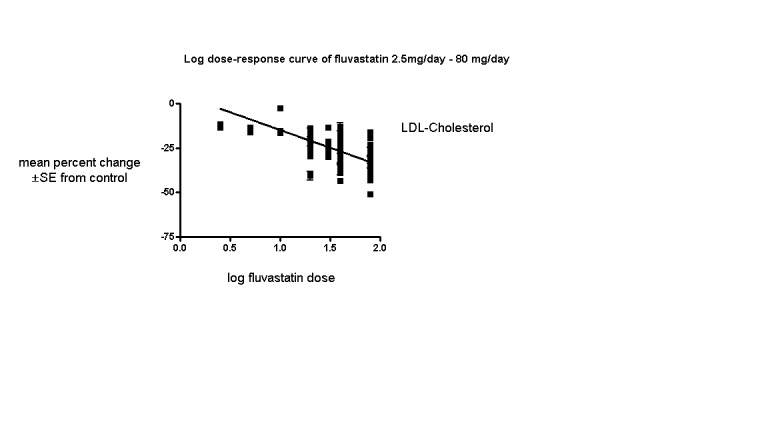
Log dose fluvastatin response curve for LDL cholesterol
Values represent the results of each trial for each dose comparison. The standard error bars cannot be seen because they all lie within the points
5.
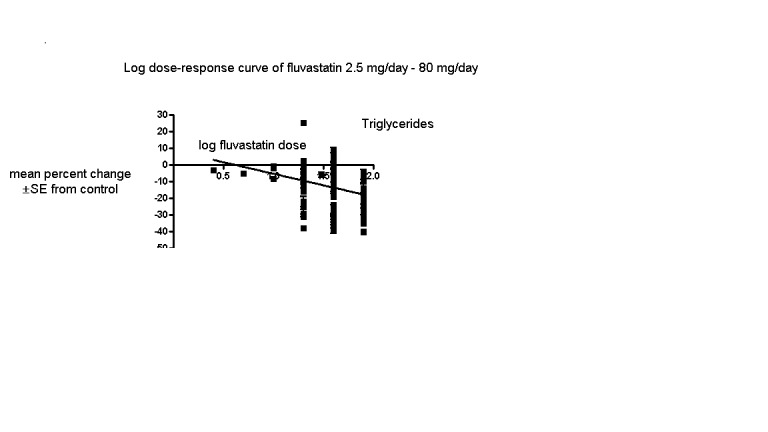
Log dose fluvastatin response curve for triglycerides
Values represent the results of each trial for each dose comparison. The standard error bars cannot be seen because they all lie within the points
Excluded studies
Seventy‐nine studies were excluded. Reasons for exclusion included confounding, inappropriate dosing, pooled data, attrition bias if more than 25% participants were not included in the efficacy analysis, inappropriate outcomes such as median percentage change from baseline or absolute change from baseline that could not be converted to percentage change from baseline, inadequate dietary baseline stabilisation period and combined data for all cross‐over periods. Trials in which participants were receiving drugs that affect blood lipid level concentrations, for example immunosuppressants such as cyclosporine and protease inhibitors such as ritonavir and indinavir were classified as excluded trials. The reasons for excluding each trial are listed in the Characteristics of excluded studies table.
Risk of bias in included studies
Sequence generation was not applicable to the 109 before‐and‐after trials. Of the 36 randomised placebo‐controlled trials, four (11.1%) were judged to have low risk of bias for sequence generation. The others were judged unclear.
Allocation
Allocation concealment was not applicable to the 109 before‐and‐after trials. The single‐blinded trial was judged a high risk of bias for this category. Of the 35 double‐blind randomised placebo‐controlled trials, three (8.6%) were judged a low risk of bias for allocation concealment.
Blinding
We judged the risk of performance and detection bias for lipid parameters to be low for all the trials as they were done in remote laboratories and unlikely to influenced by the investigators.
There was a high risk of detection bias of withdrawals due to adverse effects (WDAEs) assessment in the two open‐label randomised placebo‐controlled trials and in the single‐blind randomised placebo‐controlled trial. Of the 33 double‐blind randomised placebo‐controlled trials, six (18.2%) were judged a low risk of detection bias for WDAEs.
Incomplete outcome data
Incomplete outcome reporting leading to attrition bias was not a problem in this review as few participants were lost to follow‐up and were balanced across the groups in the placebo‐controlled trials. Overall, 91.9% of the participants completed the treatment.
Selective reporting
Out of 145 trials, 143 (98.6%) reported the primary lipid outcome LDL‐C, thus selection bias was not a potential source of bias for this outcome.
Out of 36 placebo‐controlled trials, only 16 (44.4%) reported WDAEs. The trials that did not report could have deliberately not done so because WDAEs were increased. Therefore, selective reporting bias was judged an important source of bias for this outcome. See 'Risk of bias' tables in Characteristics of included studies, and for the overall risk of bias, see (Figure 6).
6.

'Risk of bias' graph: Summary of overall risk of bias for the lipid parameters according to each item.
Other potential sources of bias
The main other potential source of bias was industry funding. Out of the 145 trials, 48 (33.1%) reported funding by industry, 14 (9.7%) reported no industry funding and in 83 (57.2%) trials, the source of funding was not reported. Out of 48 industry funded trials, 35 (72.9%) were funded by Novartis, marketers of fluvastatin and 13 (27.1%) were funded by other pharmaceutical companies. The Novartis funded trials might be biased in favour of fluvastatin and would be expected to overestimate the treatment effect while trials funded by rival pharmaceutical companies might be biased against fluvastatin and be expected to underestimate the treatment effect. In trials where the source of funding was not reported, bias could be for or against fluvastatin. Novartis funded versus non‐Novartis funded LDL cholesterol efficacy data were available for the doses of 10 mg/day, 20 mg/day, 40 mg/day and 80 mg/day. These data were analysed separately using the generic inverse variance fixed‐effect model in RevMan 5. The sensitivity analysis revealed that the lipid‐lowering efficacy of fluvastatin in Novartis‐funded versus non‐Novartis funded trials were not different for most doses analysed; 10 mg/day (‐16.6% versus ‐16.2%; P = 0.94), 20 mg/day (‐19.77% versus ‐18.94%; P = 0.05), 40 mg/day (‐23.25% versus ‐25.65%; P = 0.007), and 80 mg/day (‐34.80% versus ‐33.88%; P = 0.28). Assessment for publication bias was done by reviewing the funnel plots for all lipid outcomes with 10 or more trials. None of these funnel plots suggested publication bias.
The determination of lipids in the blood samples were done by laboratories not connected to the trial personnel or participants, therefore we judged the overall risk of bias to be low for both the placebo‐controlled RCTs and for the before and after design trials (see Figure 6).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
2.
| Total cholesterol lowering efficacy of fluvastatin | ||||||
|
Patient or population: participants with normal or abnormal lipid profiles Settings: ambulatory care Intervention: fluvastatin Comparison: Total cholesterol percentage change from baseline for all trials | ||||||
| Outcomes |
Anticipated absolute effects mmol/L (95%CI) |
Percent reduction
(95% CI) % |
No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Before exposure to fluvastatin1 | After exposure to fluvastatin | |||||
|
Total cholesterol fluvastatin 10 mg/day |
6.90 (6.47 to 7.33) |
6.16 (6.02 to 6.30) |
10.7 (12.7 to 8.6) |
287 (4) | ⊕⊕⊕⊕ high | Effect predicted from log dose‐response equation is 10.9%. Randomised and before and after design not different P = 0.86. |
|
Total cholesterol fluvastatin 20 mg/day |
6.99 (6.61 to 7.37) |
5.96 (5.94 to 5.98) |
14.8 (15.1 to 14.5) |
6309 (50) | ⊕⊕⊕⊕ high | Effect predicted from log dose‐response equation is 15.2%. Randomised versus before and after design borderline different P = 0.044. |
|
Total cholesterol fluvastatin 40 mg/day |
6.91 (6.54 to 7.27) |
5.60 (5.57 to 5.64) |
18.9 (19.3 to 18.4) |
2966 (55) | ⊕⊕⊕⊕ high | Effect predicted from log dose‐response equation is 19.4%. Randomised and before and after design not different P = 0.106. |
|
Total cholesterol fluvastatin 80 mg/day |
6.97 (6.62, 7.32) |
5.24 (5.12 to 5.27) |
24.9 (25.5 to 24.4) |
3943 (27) | ⊕⊕⊕⊕ high | Effect predicted from log dose‐response equation is 23.6%. Randomised and before and after design not different P = 0.595. |
| CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. Mean baseline values.
3.
| Triglyceride lowering efficacy of fluvastatin | ||||||
|
Patient or population: participants with normal or abnormal lipid profiles Settings: ambulatory care Intervention: fluvastatin Comparison: Triglyceride percentage change from baseline for all trials | ||||||
| Outcomes |
Anticipated absolute effects mmol/L (95%CI) |
Percent Reduction
(95% CI) % |
No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Before exposure to fluvastatin1 | After exposure to fluvastatin | |||||
|
Triglycerides fluvastatin 10 mg/day |
1.93 (1.63 to 2.22) |
1.87 (1.73 to 2.01) |
3.0 (10.1 to ‐4.2) |
259 (3) | ⊕⊕⊕⊕ high | Effect predicted from log dose‐response equation is 5.2%. Only RCT data. |
|
Triglycerides fluvastatin 20 mg/day |
1.98 (1.68 to 2.28) |
1.76 (1.74 to 1.77) |
11.1 (11.8 to 10.3) |
7510 (39) | ⊕⊕⊕⊕ high | Effect predicted from log dose‐response equation is 9.4%. Randomised and before and after design not different P = 0.277. |
|
Triglycerides fluvastatin 40 mg/day |
1.94 (1.70 to 2.17) |
1.72 (1.69 to 1.75) |
11.1 (12.6 to 9.6) |
2646 (48) | ⊕⊕⊕⊕ high | Effect predicted from log dose‐response equation is 13.6% Randomised and before and after design not different P = 0.186. |
|
Triglycerides fluvastatin 80 mg/day |
1.92 (1.67 to 2.17) |
1.59 (1.56 to 1.62) |
17.5 (19.1 to 15.9) |
3623 (23) | ⊕⊕⊕⊕ high | Effect predicted from log dose‐response equation is 17.7% Randomised and before and after design not different P = 0.496. |
| CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. Mean baseline values.
4.
| Withdrawal due to adverse events due to fluvastatin | ||||||
|
Patient or population: participants with normal or abnormal lipid profiles Settings: ambulatory care Intervention: fluvastatin Comparison: WDAEs fluvastatin versus placebo | ||||||
| Outcomes | Illustrative Comparative Risks* (95%CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo | fluvastatin | |||||
|
WDAEs within 3‐12 weeks |
RR 1.52 (0.94 to 2.45) | 3023 (16) | ⊕⊝⊝⊝ very low1,2 | only 16 out of 36 placebo controlled trials reported withdrawals due to adverse effects. | ||
| 18 per 1000 |
27 per 1000 (17 to 44) |
|||||
|
*The basis for the assumed risk is the measure of absolute effect with the placebo group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. Downgraded 2 levels due to high risk of selective reporting and other biases.
2. Downgraded 1 level due to wide confidence intervals.
See: Table 1 for the main comparison LDL‐cholesterol lowering efficacy of fluvastatin for all trials.
Overall efficacy of fluvastatin
Values from all data describing the efficacy of fluvastatin to lower the lipid parameters from placebo and before and after trials from the Data and analyses section were entered as generic inverse variance data separately into GraphPad Prism 4 to yield log dose‐response curves for placebo and before and after trials. To compare slope results of placebo‐controlled versus before and after trials, t‐tests from the formula t = (Placebo Slope‐Before and After Slope)/SQRT(SE2placebo slope+SE2before and after slope) were performed from the slopes and standard errors of the curves for total cholesterol, LDL cholesterol, HDL cholesterol and triglycerides. The results showed that for most lipid parameters there were no differences between placebo‐controlled trials and before and after trials for total cholesterol P = 0.118, LDL cholesterol P = 0.0077, HDL cholesterol P = 0.115 and triglycerides P = 0.624. This demonstrates that the two trial designs provide similar estimates of the lipid‐lowering efficacy of fluvastatin except for LDL cholesterol.
In addition, two‐tailed one sample t‐tests were performed from the placebo‐controlled trials to test for the difference between placebo mean effects and zero.The results of these tests demonstrated the placebo means were not different from zero except for the triglycerides: total cholesterol: 0.61 (95% CI ‐0.54 to 1.76) P = 0.3057, LDL cholesterol: 0.59 (95% CI ‐0.97 to 2.15) P = 0.4627, HDL cholesterol 0.68 (95%CI ‐1.116 to 2.47) P = 0.5028 and triglycerides: 5.59 (95%CI 2.51 to 8.68) P = 0.001. The triglyceride placebo mean appears to be different because blood triglyceride measurements are extremely variable and are not as reliable because there is a broad biological variability both within and among individuals. The evidence of lack of a placebo effect provided further justification for combining all the trials to determine the overall efficacy.
Validation for combining the results from the two trial designs was previously shown in the atorvastatin and rosuvastatin reviews (Adams 2014; Adams 2015).
Combining the results from the two trial designs was done by entering all data into the RevMan 5 using the generic inverse variance model outside of this review (data and analysis are not shown). The mean parameters from this analysis are summarised in Table 5.
1. Fluvastatin Overall Efficacy.
| Fluvastatin dose (mg/day) | 2.5 | 5 | 10 | 20 | 30 | 40 | 80 |
| Total Cholesterol (mean percentage change from control) |
‐9.8 | ‐11.7 | ‐10.7 | ‐14.8 | ‐18.0 | ‐18.85 | ‐24.9 |
| 95% confidence interval |
(‐12.0 to ‐7.7) | (‐14.2 to ‐9.2) | (‐12.7 to ‐8.6) | (‐15.1 to ‐14.5) | (‐19.2 to ‐16.7) | (‐19.3 to ‐18.4) | (‐25.4 to ‐24.4) |
| LDL‐Ca (mean percentage change from control) |
‐12.1 | ‐14.5 | ‐15.2 | ‐20.0 | ‐25.3 | ‐25.9 | ‐34.9 |
| 95% confidence interval |
(‐14.2 to ‐10.1) | (‐16.3 to ‐12.7) | (‐17.1 to ‐13.3) | (‐20.3 to ‐19.7) | (‐26.9 to ‐23.7) | (‐26.5 to ‐25.3) | (‐35.5 to ‐34.3) |
| Triglycerides (mean percentage change from control) |
‐3.3 | ‐5.3 | ‐3.0 | ‐11.1 | ‐5.9 | ‐11.1 | ‐17.5 |
| 95% confidence interval |
(‐14.6 to 8.0) | (‐13.1 to 2.5) | (‐10.1 to 4.2) | (‐11.8 to ‐10.3) | (‐20.1 to 8.3) | (‐12.6 to ‐9.6) | (‐19.1 to ‐15.9) |
aLDL‐C: low‐density lipoprotein cholesterol
Primary Outcome: LDL cholesterol
In total 143/145 (98.6%) trials and 18,606/18,846 (97%) participants contributed to the LDL cholesterol data analysis. The effect of different doses of fluvastatin on LDL cholesterol are shown in the Data and analyses section (Analysis 1.1; Analysis 2.1; Analysis 2.2; Analysis 3.1; Analysis 4.1; Analysis 4.5; Analysis 5.1; Analysis 6.1; Analysis 6.5; Analysis 7.1; Analysis 7.5). The analysis for LDL cholesterol yielded the log dose‐response straight‐line equation, y = ‐19.98 log(x) + 5.181. This equation provides the best estimate of the mean reductions in blood LDL‐cholesterol from baseline for fluvastatin doses ranging from 2.5 mg/day to 80 mg/day as it uses all the available data. Using this formula, the calculated reductions in total blood LDL‐cholesterol for doses of 2.5 mg per day to 80 mg per day ranged from 2.8% to 32.8%. For every two‐fold dose increase there was a 6.01% (95% CI 5.43 to 6.60) percentage decrease in blood LDL cholesterol (Figure 4).
1.1. Analysis.
Comparison 1 2.5 mg vs control, Outcome 1 LDL‐cholesterol.
2.1. Analysis.
Comparison 2 5 mg vs control, Outcome 1 LDL‐cholesterol.
2.2. Analysis.
Comparison 2 5 mg vs control, Outcome 2 LDL‐cholesterol.
3.1. Analysis.
Comparison 3 10 mg vs control, Outcome 1 LDL‐cholesterol.
4.1. Analysis.
Comparison 4 20 mg vs control, Outcome 1 LDL‐cholesterol.
4.5. Analysis.
Comparison 4 20 mg vs control, Outcome 5 LDL‐cholesterol.
5.1. Analysis.
Comparison 5 30 mg vs control, Outcome 1 LDL‐cholesterol.
6.1. Analysis.
Comparison 6 40 mg vs control, Outcome 1 LDL‐cholesterol.
6.5. Analysis.
Comparison 6 40 mg vs control, Outcome 5 LDL‐cholesterol.
7.1. Analysis.
Comparison 7 80 mg vs control, Outcome 1 LDL‐cholesterol.
7.5. Analysis.
Comparison 7 80 mg vs control, Outcome 5 LDL‐cholesterol.
Secondary Outcome: Total cholesterol
In total 131/145 (90.3%) trials and 13,797/18,846 (73.2%) participants contributed to the total cholesterol data analysis. The effect of different doses of fluvastatin on total cholesterol are shown in the Data and analyses section (Analysis 3.2; Analysis 4.2; Analysis 4.6; Analysis 5.2; Analysis 6.2; Analysis 6.6; Analysis 7.2; Analysis 7.6). The analysis for total cholesterol yielded the log dose‐response straight‐line equation, y = ‐14.08 log(x) + 3.155. This equation provides the best estimate of the mean reductions in blood total cholesterol from baseline for fluvastatin doses ranging from 2.5 mg/day to 80 mg/day as it uses all the available data. Using this formula, the calculated reductions in total blood cholesterol for doses of 2.5 mg per day to 80 mg per day ranged from 2.45% to 23.6%. For every two‐fold dose increase there was a 4.24% (95% CI 3.68 to 4.8) percentage decrease in blood total cholesterol (Figure 3).
3.2. Analysis.
Comparison 3 10 mg vs control, Outcome 2 Total cholesterol.
4.2. Analysis.
Comparison 4 20 mg vs control, Outcome 2 Total cholesterol.
4.6. Analysis.
Comparison 4 20 mg vs control, Outcome 6 Total cholesterol.
5.2. Analysis.
Comparison 5 30 mg vs control, Outcome 2 Total cholesterol.
6.2. Analysis.
Comparison 6 40 mg vs control, Outcome 2 Total cholesterol.
6.6. Analysis.
Comparison 6 40 mg vs control, Outcome 6 Total cholesterol.
7.2. Analysis.
Comparison 7 80 mg vs control, Outcome 2 Total cholesterol.
7.6. Analysis.
Comparison 7 80 mg vs control, Outcome 6 Total cholesterol.
Secondary Outcome: HDL cholesterol
The GraphPad Prism 4 analysis showed that fluvastatin doses ranging from 2.5 mg/day to 80 mg/day had no dose‐related effect on blood HDL cholesterol. All doses of fluvastatin caused a small increase in HDL cholesterol. When all trials and doses were pooled using generic inverse variance the magnitude of the increase was 3.7% (95% CI 3.4 to 4.0).
Secondary Outcome: Triglycerides
In total 112/145 (77.2%) trials and 14,324/18,846 (76%) participants contributed to the triglyceride data analysis. The effect of different doses of fluvastatin on triglycerides are shown in the Data and analyses section (Analysis 3.4; Analysis 4.4; Analysis 4.8; Analysis 6.4; Analysis 6.8; Analysis 7.4; Analysis 7.8). The analysis for triglycerides yielded the log dose‐response straight‐line equation, y = ‐13.83 log(x) + 8.602. This equation provides the best estimate of the mean reductions in blood triglycerides from baseline for fluvastatin doses ranging from 2.5 mg/day to 80 mg/day as it uses all the available data. Using this formula, the calculated reductions in total blood triglycerides for doses of 5 mg per day to 80 mg per day ranged from 1.1% to 17.7%. For every two‐fold dose increase there was a 4.16% (95% CI 1.98 to 6.34) percentage decrease in blood triglycerides (Figure 5).
3.4. Analysis.
Comparison 3 10 mg vs control, Outcome 4 Triglycerides.
4.4. Analysis.
Comparison 4 20 mg vs control, Outcome 4 Triglycerides.
4.8. Analysis.
Comparison 4 20 mg vs control, Outcome 8 Triglycerides.
6.4. Analysis.
Comparison 6 40 mg vs control, Outcome 4 Triglycerides.
6.8. Analysis.
Comparison 6 40 mg vs control, Outcome 8 Triglycerides.
7.4. Analysis.
Comparison 7 80 mg vs control, Outcome 4 Triglycerides.
7.8. Analysis.
Comparison 7 80 mg vs control, Outcome 8 Triglycerides.
Secondary Outcome: End of treatment variability
End‐of‐treatment variabilities of fluvastatin and placebo were compared to determine the effect of fluvastatin on variability of blood lipids when expressed as a co‐efficient of variation. Compared with placebo, fluvastatin (all doses) increased the co‐efficient of variation of blood LDL cholesterol (24.75 versus 30.1; P = 0.03 N = 55). Fluvastatin did not significantly affect the end‐of‐treatment variabilities of total cholesterol, HDL‐cholesterol and triglycerides.
Secondary Outcome: Withdrawal data
Sixteen (44.4%) of the 36 placebo‐controlled trials reported WDAEs during the three to 12 week treatment period. In seven trials, no participant discontinued treatment due to adverse effects or died during the study, therefore a risk ratio was not estimable. There was no fluvastatin dose‐response relationship for WDAEs. The effect of different doses of fluvastatin on withdrawal due to adverse effects (WDAEs) are shown in the Data and analyses section (Analysis 1.2; Analysis 2.3; Analysis 3.5; Analysis 4.9; Analysis 6.9; Analysis 7.9). WDAEs were not different between fluvastatin and placebo for any of the fluvastatin doses. The pooled estimate for all doses compared to placebo showed a risk ratio (RR) of 1.52 (95% CI 0.94 to 2.45) for WDAEs in these short‐term trials (Analysis 8.1).
1.2. Analysis.
Comparison 1 2.5 mg vs control, Outcome 2 WDAEs.
2.3. Analysis.
Comparison 2 5 mg vs control, Outcome 3 WDAEs.
3.5. Analysis.
Comparison 3 10 mg vs control, Outcome 5 WDAEs.
4.9. Analysis.
Comparison 4 20 mg vs control, Outcome 9 WDAE.
6.9. Analysis.
Comparison 6 40 mg vs control, Outcome 9 WDAE.
7.9. Analysis.
Comparison 7 80 mg vs control, Outcome 9 WDAEs.
8.1. Analysis.
Comparison 8 all doses vs control, Outcome 1 WDAEs.
Subgroup Analyses
Male versus female participant data were available for the 5 mg/day, 20 mg/day and 40 mg/day doses. These data were analysed separately for LDL‐cholesterol lowering efficacy using the generic inverse variance fixed‐effect model in RevMan 5 outside of this review. The subgroup analysis revealed that the efficacy of fluvastatin in male participants and female participants were not different. The efficacy for the 5 mg/day dose (male versus female participant) was: (‐13.9 versus ‐13.2; P = 0.79); for the 20 mg/day dose (male versus female participant) was: (‐21.83 versus ‐18.15; P = 0.21); and for the 40 mg/day dose (male versus female participant) was: (‐25.61 versus ‐27.82; P = 0.43).
A comparison of morning administration time versus evening administration time was not possible because only one trial provided appropriate data. Twice‐daily administration versus single‐dose administration were available for doses of 20 mg/day, 40 mg/day and 80 mg/day. These data were compared for LDL cholesterol lowering efficacy. The percentage reductions in twice‐daily versus single‐dose regimens showed no difference: 20 mg/day ‐20.01 (95 % CI ‐20.33 to ‐19.69) versus ‐19.99 (95 % CI ‐20.31 to ‐19.68) P = 0.965; 40 mg/day ‐25.90 (95 % CI ‐26.45 to ‐25.35) versus ‐26.07 (95 % CI ‐26.62 to ‐25.51) P = 0.670; and 80 mg/day ‐34.89 (95 % CI ‐35.45 to ‐34.33) versus ‐34.33 (95 % CI ‐34.93 to ‐33.73) P = 0.224.
Sensitivity Analyses
Familial versus non‐familial hypercholesterolaemia participant data were available for the doses 5 mg/day, 20 mg/day, 30 mg/day and 40 mg/day. These data were analysed separately for LDL cholesterol lowering efficacy using the generic inverse variance fixed‐effect model in RevMan 5. The efficacy of fluvastatin in familial patients tended to be less than in non‐familial patients: 5 mg/day ‐13.6 (95% CI ‐16.0 to ‐11.2) versus ‐15.9 (95% CI ‐20.2 to ‐11.6) P = 0.36; 20 mg/day ‐18.8 (95% CI ‐22.8 to ‐14.8) versus ‐19.8 (95% CI ‐20.2 to ‐19.4) P = 0.37; 30 mg/day ‐13.4 (95% CI ‐19.0 to ‐7.8) versus ‐26.9 (95% CI ‐30.4 to ‐23.5) P = 0.003; and 40 mg/day ‐26.2 (95% CI ‐28.1 to ‐24.4) versus ‐24.3 (95% CI ‐24.8 to ‐23.9) P = 1.00.
Discussion
Summary of main results
Long‐term, daily fluvastatin intake is effective at lowering blood LDL cholesterol concentrations and does so in a predictable dose‐related manner. The 'Summary of findings' table documents that fluvastatin lowers LDL cholesterol by 15% at 10 mg/day and by 33% at 80 mg/day (Table 1). These moderate reductions reflect a reduction in synthesis of cholesterol by the liver and indicate that liver HMG CoA reductase is being inhibited by up to one third over this dose range. This has significant implications beyond circulating LDL cholesterol, as LDL cholesterol is only one of many important biochemical products that are produced by the HMG CoA reductase pathway. Those other products, including co‐enzyme Q10, heme A, vitamin D, steroid hormones and many other compounds, are also likely to be reduced by about one third with the 80 mg dose of fluvastatin. It is important to recognise that the long‐term consequences of reduction of these products is presently unknown.
In the data and analysis section it can be seen that there are more trials and data with the before and after design than from placebo‐controlled trials. For the doses where there is a large number of trials and participants, it can be seen that estimates of the effect of fluvastatin on the lipid parameters are similar with the two different trial designs. This, plus the demonstration that the placebo effect was not different from zero, justified using generic inverse variance to pool and display the combined estimates in Table 5. In addition, all trial data were entered into GraphPad Prism 4 to calculate the regression lines shown in Figure 4; Figure 3 and Figure 5. The overall efficacy results from GraphPad Prism 4 provide the best estimate of the treatment effect, because it is based on a regression line calculated from all the data for all the doses. The estimates of the average treatment effect from the regression lines are similar to the mean value for all the data for each dose (see Table 1).
In this review, it was established using regression analysis that there was a correlation between the baseline value and fluvastatin effect on LDL cholesterol when the effect was expressed as absolute change from baseline (P < 0.0001). There was no correlation between the baseline value and the fluvastatin effect when the effect was expressed as per cent reduction from baseline (P = 0.21). This finding provides strong support for the fact that systematic reviews reporting the effect of statins on absolute changes in lipid parameters are problematic and potentially misleading.
What is the effect of fluvastatin on the end of treatment variability?
End‐of‐treatment variabilities of fluvastatin and placebo were compared to determine the effect of fluvastatin on variability of blood lipids when expressed as a co‐efficient of variation. Compared with placebo, fluvastatin at all doses increased the co‐efficient of variation of blood LDL cholesterol. Fluvastatin did not statistically significantly affect the variability of total cholesterol, HDL‐cholesterol and triglyceride measurements. In order to increase the power to answer this question we identified 66 placebo‐controlled trials from the atorvastatin (Adams 2015), rosuvastatin (Adams 2014) and fluvastatin reviews. In this comparison, the end‐of‐treatment variability expressed as the coefficient of variation for the statin was significantly increased as compared to placebo: total cholesterol (19.5 versus 15.9; P = 0.0005 N = 150) and LDL cholesterol (29.0 versus 23.3; P = 0.0004 N = 171). There was no increase in the end‐of‐treatment variability for the statin compared with placebo for HDL cholesterol (25.28 versus 25.32; P = 0.977 N = 142) and triglycerides (52.8 versus 51.1; P = 0.776 N = 123). The most plausible explanation for the increase in end of treatment variability for total cholesterol and LDL cholesterol with statins is that it reflects some individual variability in response to the statin that would not be present in the people receiving placebo.
Does fluvastatin increase withdrawals due to adverse effects?
Of 36 placebo‐controlled trials, 16 (44%) reported withdrawals due to adverse effects (WDAEs). This analysis represented only 3023 participants, 1759 of whom received fluvastatin and 1264 of whom received placebo. The pooled estimate for all doses provided a risk ratio (RR) of 1.52 (95% CI 0.94 to 2.45), demonstrating uncertainty, but the possibility of an increase in adverse effects even in these short‐term trials. As 20 (56%) of 36 placebo‐controlled trials did not report WDAEs, risk of selective reporting bias for this outcome is high, and the null effect may be a result of that bias. Furthermore, this analysis was limited to trials of three to 12 weeks’ duration and thus does not reflect adverse effects of fluvastatin that occur after intake of longer duration. Risk of participant selection bias is also high in these trials, as many of the participants studied could have been selected because they were known to tolerate statins at baseline.
Overall completeness and applicability of evidence
This review included 145 trials with 18,846 participants. As such it provided us with robust evidence of the dose‐related lipid‐lowering effects of fluvastatin. It was unknown when we did the review whether the time of fluvastatin administration is important with respect to lipid lowering. Only one trial (Scharnagl 2006) compared morning and evening administration and did not show a difference. A sensitivity analysis comparing twice‐daily versus single‐dose regimen data were available for the doses 20 mg/day, 40 mg/day and 80 mg/day. The percentage reductions in twice‐daily versus single‐dose regimens showed no difference. We therefore felt justified in combining data from both dosing regimens. Recently a Cochrane review has attempted to answer this question and concluded that statin lipid‐lowering effect is the same for morning and evening administration (Izquiero‐Palomares 2016).
Practitioners can use this evidence to calculate the expected effect of doses of fluvastatin commonly utilised in society. It is unlikely that further research will change these estimates appreciably. However, there was a fair amount of heterogeneity in many of the estimates and it is possible that this was due to differences in the populations being studied (e.g. gender or genetic differences) (Thompson 2005). To explore this, where it was possible, we compared the effect of fluvastatin in males and females plus in patients with familial and non‐familial hypercholesterolaemia. A subgroup analysis comparing male versus female participant data was available for the doses 5 mg/day, 20 mg/day and 40 mg/day and no difference was proven. However, we judged the amount of data available were insufficient to answer whether the lipid‐lowering effect of fluvastatin differed in males and females. If anything, it would be anticipated that the effect would be greater in females because on average they weigh less than males. It is important for authors to report data separately by sex and if this had been done in all these trials, we likely would have been able to answer this important question. The results of this subgroup analysis for both atorvastatin and rosuvastatin suggested a larger effect in females than males: atorvastatin 10 mg/day (Adams 2015) male versus female ‐39.2 (95% CI ‐41.6 to ‐36.9) versus ‐41.8 (95% CI ‐43.4 to ‐40.2) P = 0.08 and rosuvastatin 10 mg/day (Adams 2014) male versus female ‐45.1 (95% CI ‐47.9 to ‐42.2) versus ‐49.4 (95% CI ‐51.7 to ‐47.2) P = 0.02.
Familial versus non‐familial hypercholesterolaemia participant data were available for the fluvastatin doses 5 mg/day, 20 mg/day, 30 mg/day and 40 mg/day. These data were analysed separately for LDL cholesterol‐lowering efficacy using the generic inverse variance fixed‐effect model in RevMan 5. The percentage reduction in familial patients was less than non‐familial for all doses except 40 mg/day (see results). These findings of a lesser affect in familial hypercholesterinaemic participants is consistent with what was found for atorvastatin (Adams 2015): atorvastatin 10 mg/day ‐34.7 (95% CI ‐36.6 to ‐32.8) versus ‐36.3 (95% CI ‐36.7 to ‐35.8) P = 0.12 and 20 mg/day ‐38.0 (95% CI ‐39.8 to ‐36.2) versus ‐43.6 (95% CI ‐44.4 to ‐42.8) P < 0.00001.
The profound and relatively consistent effect of fluvastatin on lipid parameters shown in this review is probably appreciated by clinicians who treat patients with these drugs. The ability to know whether a patient is taking a statin or not is also most likely evident to investigators involved in statin placebo‐controlled randomised controlled trials (RCTs). Knowledge of the lipid parameters almost certainly leads to loss of blinding in statin RCTs. The present review calls attention to that problem and efforts to prevent this loss of blinding are needed in future statin RCTs (Higgins 2011).
We have used data from the Cholesterol Treatment Trialists’ (CTT) publications to determine the effects of fluvastatin, atorvastatin and rosuvastatin on LDL cholesterol lowering and reduction of myocardial infarction. In two RCTs a mean fluvastatin dose of 72 mg/day reduced LDL cholesterol by 31.9%, and reduced myocardial infarction, relative risk, 0.68 (95% CI 0.55 to 0.85) as compared to placebo. In five RCTs a mean atorvastatin dose of 26 mg/day reduced LDL cholesterol by 44.0% and reduced myocardial infarction, relative risk, 0.67 (95% CI 0.58 to 0.77) as compared to placebo. In four RCTs a mean rosuvastatin dose of 16 mg/day reduced LDL cholesterol by 48.8% and reduced myocardial infarction, relative risk, 0.82 (95% CI 0.73 to 0.93) as compared to placebo. Thus despite reducing LDL cholesterol by a much lesser amount with fluvastatin than atorvastatin and rosuvastatin, fluvastatin reduced myocardial infarction similarly to atorvastatin and to a greater degree than rosuvastatin. Fluvastatin 72 mg is equivalent to about 6 mg of atorvastatin and about 1.6 mg of rosuvastatin in LDL cholesterol lowering. These findings call into question the commonly held belief that the effect of statins to reduce myocardial infarction is solely due to lipid lowering. It certainly suggests that statins could be acting by some other mechanism to reduce myocardial infarction and calls for more head‐to‐head RCTs comparing different statins.
Quality of the evidence
The summary of all ’Risk of bias’ parameters for the lipid effects suggests a high risk of bias (Figure 6). However, the lipid parameter outcomes are probably relatively resistant to bias. If anything, a high risk of bias would lead to an overestimate of the lipid‐lowering effects rather than an underestimate. However, because of the objectivity of the lipid measurements we think that the estimates of effects are reasonably accurate. This view is strengthened by the fact that the two different trial designs, placebo‐controlled RCTs and before and after design produced similar results. Furthermore, we could not show evidence of funding bias. Comparing Novartis‐funded trials where an overestimate of the effect might be expected and non‐Novartis‐funded trials where a bias towards underestimating the effect of fluvastatin may be expected did not show any difference in the effect of fluvastatin on lipid parameters. Furthermore, review of funnel plots did not suggest evidence of publication bias.
Low risk of bias is not true for the harm outcome, withdrawals due to adverse effects (WDAE). This was reported in 16 (44.4%) of the 36 placebo‐controlled trials. There is therefore a high risk of selective reporting bias for this outcome and this combined with the high risk of other biases means that we cannot be confident that not finding a significant increase in WDAEs is correct (Table 4).
Potential biases in the review process
Combining the placebo‐controlled trials with the before and after trials is a limitation of the review. We have explained why the increased risk of bias associated with the before and after design is less in this instance because the lipid parameters were measured in a remote laboratory. Another limitation of this review is that many trials did not report standard deviations for the lipid‐lowering effects. Where possible these values were determined by the method of (Furukawa 2006), from t‐statistics corresponding to the exact P values reported or from the 95% CI of the mean difference between treatment groups. In trials where the standard deviation was not reported and could not be calculated, the standard deviations were imputed as the average of this parameter from trials that reported it. Such imputation might weight some studies more or less; however, this has been shown in other reviews not to have much effect on the estimate of the effect size (Heran 2008; Musini 2014). Another limitation is that few studies were available to demonstrate the lipid‐lowering effect of fluvastatin at very low and very high doses. We did not downgrade the quality of evidence due to heterogeneity of LDL cholesterol because the confidence intervals for the pooled result estimates were narrow.
Agreements and disagreements with other studies or reviews
The best estimate of the mean per cent reduction in blood LDL‐cholesterol for any dose of fluvastatin can be calculated from the log dose‐response equation. Using this equation y = ‐19.73 log(x) + 4.869, a fluvastatin dose of 40 mg/day reduces LDL cholesterol by an average of 26.7%. This is close to the range of 22.0% to 26.0% reduction in LDL cholesterol from the six comparative trials from the Drug Effectiveness Review Project (DERP) (Smith 2009) and a range of 24.8% to 29.4% reduction in LDL cholesterol in 23 placebo‐controlled trials from (Law 2003).
Comparison of the effect with other statins
The greatest value in doing this type of review is the ability to compare fluvastatin to other statins. At present we can compare fluvastatin to atorvastatin and rosuvastatin, which have been reviewed using the same protocol. The most important finding in this review is that the slope of the dose response effect for fluvastatin on LDL, total cholesterol and triglycerides is not different from the slopes of the dose response curve for atorvastatin (Adams 2015) and rosuvastatin (Adams 2014). This provides some confirmation that the three statins are all causing lipid lowering by a similar mechanism. However, it also demonstrates that fluvastatin is much less potent than the other two drugs: fluvastatin is 12‐fold less potent than atorvastatin in lowering LDL cholesterol and 46‐fold less potent than rosuvastatin. This means that fluvastatin 80 mg/day reduces LDL cholesterol on average by 32.7 %; the dose of atorvastatin and rosuvastatin to achieve the same reduction in LDL cholesterol is 7 mg/day and 2 mg/day, respectively.
Authors' conclusions
Implications for practice.
Specific findings of the review
Fluvastatin 2.5 mg/day to 80 mg/day causes a linear dose‐response reduction in the per cent change from control of blood total cholesterol, LDL cholesterol, and triglycerides, but not for HDL cholesterol. Manufacturer‐recommended fluvastatin doses of 10 mg/day to 80 mg/day resulted in a range of 14.9% to 32.7% decrease of LDL cholesterol. From the slope of the lines for every two‐fold dose increase, there was a 4.2%, 6.0%, and 4.2% decrease in blood total cholesterol, LDL cholesterol, and triglycerides, respectively.
To determine the relative potency of fluvastatin with respect to atorvastatin and rosuvastatin, the ratio of the mg amount of fluvastatin to the mg amount of atorvastatin or rosuvastatin needed to produce the same effect was determined. These values were calculated from the log dose response curves of fluvastatin, atorvastatin and rosuvastatin for total cholesterol and LDL cholesterol. Fluvastatin was determined to be about 12‐fold less potent than atorvastatin and 46‐fold less potent than rosuvastatin in reducing LDL cholesterol.
Fluvastatin was shown to increase the variability of LDL cholesterol measurements which confirms what has been shown for atorvastatin and rosuvastatin.
We are uncertain about the risk of withdrawal due to adverse events from all doses of fluvastatin as compared to placebo (RR 1.52; 95% CI 0.94 to 2.45). The evidence for this outcome is very low quality and thus it cannot be considered reliable.
Implication of these findings Fluvastatin lowers lipid parameters in a dose‐related fashion that is similar to but much less potent than atorvastatin and rosuvastatin; 80 mg fluvastatin lowers LDL cholesterol about as much as 2 mg of rosuvastatin and 7 mg of atorvastatin.
Implications for research.
More randomised controlled trials (RCTs) for fluvastatin at doses of 2.5 and 80 mg/day are needed as well as for higher and lower doses to improve the estimate of the dose‐response efficacy of fluvastatin.
All placebo‐controlled RCTs must accurately report withdrawals due to adverse effects (WDAEs).
All trials should report the effects separately in men and women so it is possible to determine if there are any clinically significant dose‐related sex differences.
Acknowledgements
The review authors would like to acknowledge assistance provided by Gavin Wong, Dr Benji Heran, and Dr David Godin, who assisted with validation of the data provided by included studies.
Appendices
Appendix 1. Search strategies
Database: Ovid MEDLINE(R) 1946 to Present with Daily Update Search Date: 10 February 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 fluvastatin.mp. 2 fluindostatin.mp. 3 canef.mp. 4 cranoc.mp. 5 lescol.mp. 6 lochol.mp. 7 or/1‐6 8 animals/ not (humans/ and animals/) 9 7 not 8 10 remove duplicates from 9 ***************************
Database: Cochrane Central Register of Controlled Trials <2017, Issue 2> via Cochrane Register of Studies (CRS‐Web) Search Date: 10 February 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1fluvastatin #2fluindostatin #3canef #4cranoc #5lescol #6lochol #7#1 OR #2 OR #3 OR #4 OR #5 OR #6 ***************************
Database: Cochrane Database of Systematic Reviews (CDSR) and Database of Abstracts of Reviews of Effects (DARE) via Wiley Search Date: 10 February 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ #1All Text fluindostatin OR fluvastatin *************************** Database: Embase <1974 to 2017 February 09> Search Date: 10 February 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 fluvastatin.mp. 2 fluindostatin.mp. 3 canef.mp. 4 cranoc.mp. 5 lescol.mp. 6 lochol.mp. 7 or/1‐6 8 cholesterol$.mp. 9 (HDL or LDL).mp. 10 lipoprotein?.mp. 11 lipid$.mp. 12 triglyceride$.mp. 13 triacylglycerol.mp. 14 or/8‐13 15 7 and 14 16 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 17 15 not 16 18 remove duplicates from 17 *************************** Database: ClinicalTrials.gov Search Date: 10 February 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Interventions: fluindostatin OR fluvastatin Study type: Interventional Studies *************************** Database: WHO International Clinical Trials Registry Platform (ICTRP) Search Date: 10 February 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ fluindostatin OR fluvastatin *************************** Database: Epistemonikos Search Date: 10 February 2017 ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Search terms: fluindostatin OR fluvastatin Publication type: Systematic review
Data and analyses
Comparison 1. 2.5 mg vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 LDL‐cholesterol | 2 | 338 | Mean Difference (IV, Fixed, 95% CI) | ‐11.91 [‐14.14, ‐9.69] |
| 2 WDAEs | 1 | 173 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.98] |
Comparison 2. 5 mg vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 LDL‐cholesterol | 2 | 332 | Mean Difference (IV, Fixed, 95% CI) | ‐15.76 [‐18.91, ‐12.60] |
| 2 LDL‐cholesterol | 2 | 91 | Mean Difference (Fixed, 95% CI) | ‐13.85 [‐16.02, ‐11.69] |
| 3 WDAEs | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.16] |
Comparison 3. 10 mg vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 LDL‐cholesterol | 5 | 570 | Mean Difference (IV, Random, 95% CI) | ‐14.49 [‐17.95, ‐11.02] |
| 2 Total cholesterol | 3 | 259 | Mean Difference (IV, Random, 95% CI) | ‐8.44 [‐13.95, ‐2.93] |
| 3 HDL‐cholesterol | 3 | 259 | Mean Difference (IV, Fixed, 95% CI) | 1.86 [‐1.28, 5.00] |
| 4 Triglycerides | 3 | 259 | Mean Difference (IV, Fixed, 95% CI) | ‐2.96 [‐10.19, 4.28] |
| 5 WDAEs | 2 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.16] |
3.3. Analysis.
Comparison 3 10 mg vs control, Outcome 3 HDL‐cholesterol.
Comparison 4. 20 mg vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 LDL‐cholesterol | 14 | 2329 | Mean Difference (IV, Fixed, 95% CI) | ‐20.82 [‐21.88, ‐19.77] |
| 2 Total cholesterol | 12 | 2023 | Mean Difference (IV, Fixed, 95% CI) | ‐15.81 [‐16.75, ‐14.88] |
| 3 HDL‐cholesterol | 10 | 1727 | Mean Difference (IV, Fixed, 95% CI) | 2.33 [0.90, 3.77] |
| 4 Triglycerides | 10 | 1712 | Mean Difference (IV, Fixed, 95% CI) | ‐9.67 [‐12.61, ‐6.73] |
| 5 LDL‐cholesterol | 41 | 6681 | Mean Difference (Random, 95% CI) | ‐20.92 [‐21.83, ‐20.02] |
| 6 Total cholesterol | 38 | 4286 | Mean Difference (Random, 95% CI) | ‐15.68 [‐16.67, ‐14.68] |
| 7 HDL‐cholesterol | 32 | 6239 | Mean Difference (Random, 95% CI) | 5.34 [4.51, 6.17] |
| 8 Triglycerides | 29 | 5798 | Mean Difference (Random, 95% CI) | ‐9.15 [‐11.36, ‐6.94] |
| 9 WDAE | 7 | 1060 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.14, 5.46] |
4.3. Analysis.
Comparison 4 20 mg vs control, Outcome 3 HDL‐cholesterol.
4.7. Analysis.
Comparison 4 20 mg vs control, Outcome 7 HDL‐cholesterol.
Comparison 5. 30 mg vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 LDL‐cholesterol | 7 | 336 | Mean Difference (Random, 95% CI) | ‐24.03 [‐27.72, ‐20.34] |
| 2 Total cholesterol | 6 | 285 | Mean Difference (Random, 95% CI) | ‐17.23 [‐19.68, ‐14.78] |
| 3 HDL‐cholesterol | 2 | 47 | Mean Difference (Random, 95% CI) | 7.86 [‐0.36, 16.07] |
5.3. Analysis.
Comparison 5 30 mg vs control, Outcome 3 HDL‐cholesterol.
Comparison 6. 40 mg vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 LDL‐cholesterol | 11 | 1275 | Mean Difference (IV, Random, 95% CI) | ‐27.04 [‐30.69, ‐23.40] |
| 2 Total cholesterol | 11 | 1276 | Mean Difference (IV, Random, 95% CI) | ‐18.21 [‐21.17, ‐15.26] |
| 3 HDL‐cholesterol | 6 | 716 | Mean Difference (IV, Fixed, 95% CI) | 5.14 [2.86, 7.41] |
| 4 Triglycerides | 10 | 1198 | Mean Difference (IV, Fixed, 95% CI) | ‐13.53 [‐17.27, ‐9.78] |
| 5 LDL‐cholesterol | 46 | 2383 | Mean Difference (Random, 95% CI) | ‐26.41 [‐27.67, ‐25.14] |
| 6 Total cholesterol | 44 | 1690 | Mean Difference (Random, 95% CI) | ‐19.52 [‐20.60, ‐18.45] |
| 7 HDL‐cholesterol | 35 | 1354 | Mean Difference (Random, 95% CI) | 3.87 [2.06, 5.68] |
| 8 Triglycerides | 38 | 1448 | Mean Difference (Random, 95% CI) | ‐11.23 [‐14.07, ‐8.40] |
| 9 WDAE | 4 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.47 [0.75, 16.11] |
6.3. Analysis.
Comparison 6 40 mg vs control, Outcome 3 HDL‐cholesterol.
6.7. Analysis.
Comparison 6 40 mg vs control, Outcome 7 HDL‐cholesterol.
Comparison 7. 80 mg vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 LDL‐cholesterol | 10 | 2727 | Mean Difference (IV, Random, 95% CI) | ‐34.62 [‐38.60, ‐30.64] |
| 2 Total cholesterol | 10 | 2757 | Mean Difference (IV, Random, 95% CI) | ‐25.76 [‐28.10, ‐23.41] |
| 3 HDL‐cholesterol | 9 | 2644 | Mean Difference (IV, Random, 95% CI) | 1.06 [‐2.26, 4.38] |
| 4 Triglycerides | 10 | 2756 | Mean Difference (IV, Fixed, 95% CI) | ‐17.28 [‐19.63, ‐14.92] |
| 5 LDL‐cholesterol | 22 | 2201 | Mean Difference (Random, 95% CI) | ‐33.04 [‐35.17, ‐30.90] |
| 6 Total cholesterol | 17 | 1186 | Mean Difference (Random, 95% CI) | ‐23.27 [‐24.99, ‐21.55] |
| 7 HDL‐cholesterol | 13 | 828 | Mean Difference (Random, 95% CI) | 3.36 [‐0.50, 7.22] |
| 8 Triglycerides | 13 | 867 | Mean Difference (Random, 95% CI) | ‐20.04 [‐26.35, ‐13.73] |
| 9 WDAEs | 4 | 1430 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.71, 2.51] |
7.3. Analysis.
Comparison 7 80 mg vs control, Outcome 3 HDL‐cholesterol.
7.7. Analysis.
Comparison 7 80 mg vs control, Outcome 7 HDL‐cholesterol.
Comparison 8. all doses vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 WDAEs | 16 | 3023 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.94, 2.45] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abetel 1998.
| Methods | 4‐week washout period 12‐week before and after trial |
|
| Participants | 23 hypertensive patients with hypercholesterolaemia age 20‐65 years old TC/HDL‐C > 4.5 TC > 5.2 mmol/L (201 mg/dL) with CHD TC > 6.5 mmol/L (251 mg/dL) without CHD BP is 140‐160/90‐110 no exclusion criteria Fluvastatin 40 mg/day baseline TC : 7.98 mmol/L (309 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 5.63 mmol/L (218 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.15 mmol/L (44 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 2.66 mmol/L (236 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day | |
| Outcomes | per cent change from baseline at 12 weeks of blood TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | all lipid parameters were included in the efficacy analysis SDs were determined from P values |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4.2% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
ACCESS 2001.
| Methods | 5‐8 week washout period 54‐week before and after trial |
|
| Participants | 3887 men and women with hypercholesterolaemia LDL‐C 190‐350 mg/dL (4.91‐9.05 mmol/L) in patients with no CHD or peripheral vascular disease and 1 or no risk factors 160‐300 mg/dL (4.14‐7.76 mmol/L) in patients with no CHD or peripheral vascular disease and > 1 risk factor 130‐250 mg/dL (3.36‐6.47 mmol/L) in patients with clinically evident CHD or peripheral vascular disease Triglycerides < 400 mg/dL (4.52 mmol/L) exclusion criteria: statin hypersensitivity, use of prohibited medications, acute liver disease, uncontrolled diabetes mellitus age < 18 and > 80 years Fluvastatin 20 mg/day baseline TC : 6.83 mmol/L (264 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 4.63 mmol/L (179 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.21 mmol/L (47 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 2.14 mmol/L (190 mg/dL) |
|
| Interventions | 493 patients received Fluvastatin 20 mg/day for 0‐6 weeks 493 patients received Fluvastatin 20‐40 mg/day for 6‐12 weeks 493 patients received Fluvastatin 20‐80 mg/day for 12‐18 weeks 493 patients received Fluvastatin 20‐80 mg/day for 18‐54 weeks 494 patients received Lovastatin 20 mg/day for 0‐6 weeks 494 patients received Lovastatin 20‐40 mg/day for 6‐12 weeks 494 patients received Lovastatin 20‐80 mg/day for 12‐18 weeks 494 patients received Lovastatin 20‐80 mg/day for 18‐54 weeks 478 patients received Pravastatin 10 mg/day for 0‐6 weeks 478 patients received Pravastatin 10‐20 mg/day for 6‐12 weeks 478 patients received Pravastatin 10‐40 mg/day for 12‐18 weeks 478 patients received Pravastatin 10‐40 mg/day for 18‐54 weeks 478 patients received Simvastatin 10 mg/day for 0‐6 weeks 478 patients received Simvastatin 10‐20 mg/day for 6‐12 weeks 478 patients received Simvastatin 10‐40 mg/day for 12‐18 weeks 478 patients received Simvastatin 10‐40 mg/day for 18‐54 weeks 1944 patients received Atorvastatin 10 mg/day for 0‐6 weeks 1944 patients received Atorvastatin 10‐20 mg/day for 6‐12 weeks 1944 patients received Atorvastatin 10‐40 mg/day for 12‐18 weeks 1944 patients received Atorvastatin 10‐80 mg/day for 18‐54 weeks |
|
| Outcomes | per cent change from baseline at 6 weeks of blood TC, LDL‐C and HDL‐C | |
| Source of Funding | Pfizer | |
| Notes | Lovastatin, pravastatin, simvastatin, atorvastatin groups were not included in the efficacy analysis Fluvastatin time periods of 6‐12, 12‐18 and 18‐54 weeks were also not included in the efficacy analysis because some participants had a doubling of dose at weeks 6, 12 and 18. blood triglycerides were not included in the efficacy analysis because the calculated value and the given values differed by 29.8% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19/493 (3.9%) participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Pfizer‐funded the trial |
AlvarezSala 2008.
| Methods | 10‐week dietary washout period 12‐week before and after trial |
|
| Participants | 82 men and women 18‐75 years old with primary hypercholesterolaemia LDL‐C ≥130 mg/dL (≥ 3.4 mmol/L) triglycerides ≤ 400 mg/dL (≤ 4.5 mmol/L) exclusion criteria: congestive heart failure III‐IV; uncontrolled arrhythmia; MI; unstable angina or severe or unstable peripheral artery disease in the preceding 3 months; uncontrolled diabetes; uncontrolled endocrine or metabolic diseases, renal or hepatic dysfunction; myopathic disorders, coagulation disorders; and /or any condition that would make protocol compliance unlikely pregnancy or lactation and confounding drugs 44 participants received fluvastatin 80 mg/day 38 participants received fluvastatin 80 mg/day + ezetimibe 10 mg/day Fluvastatin 80 mg/day baseline TC : 7.7 mmol/L (298 mg/dL) Fluvastatin 80 mg/day baseline LDL‐C : 5.6 mmol/L (217 mg/dL) Fluvastatin 80 mg/day baseline HDL‐C : 1.5 mmol/L (58 mg/dL) Fluvastatin 80 mg/day baseline triglycerides: 1.6 mmol/L (142 mg/dL) |
|
| Interventions | Fluvastatin XL 80 mg/day Fluvastatin XL 80 mg/day + ezetimibe 10 mg/day |
|
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | Novartis | |
| Notes | Fluvastatin XL 80 mg/day + ezetimibe 10 mg/day group was not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11.4% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Novartis funded the study |
Baggio 1994a.
| Methods | 4‐week single‐blind placebo washout period 6‐week before and after trial |
|
| Participants | 22 elderly women with primary phenotype IIa hypercholesterolaemia LDL‐cholesterol ≥ 160 mg/dL ( ≥ 4.14 mmol/L) triglycerides ≤ 250 mg/dL (≤ 2.82 mmol/L) exclusion criteria: secondary forms of dyslipidaemia, actively treated diabetes mellitus, obesity, liver and renal dysfunction, acute MI, previous coronary bypass surgery or malignancy Fluvastatin 40 mg/day baseline TC : 8.4 mmol/L (325 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 6.1 mmol/L (236 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.6 mmol/L (62 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 1.4 mmol/L (124 mg/dL) |
|
| Interventions | fluvastatin 40 mg/day | |
| Outcomes | per cent change from baseline at 3‐6 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | SDs were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Baggio 1994b.
| Methods | 4‐week single‐blind placebo washout period 6‐week before and after trial |
|
| Participants | 39 men and women with type IIA primary hypercholesterolaemia mean age 67 years LDL‐cholesterol ≥ 160 mg/dL ( ≥ 4.14 mmol/L) triglycerides ≤ 250 mg/dL (≤ 2.82 mmol/L) exclusion criteria: secondary dyslipidaemia, diabetes mellitus controlled with drugs, obesity BMI ≥29 abnormal liver and renal function, cancer, MI and coronary bypass surgery Fluvastatin 40 mg/day baseline TC : 8.17 mmol/L (316 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 5.92 mmol/L (229 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.5 mmol/L (58 mg/dL Fluvastatin 40 mg/day baseline triglycerides: 1.64 mmol/L (145 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day | |
| Outcomes | per cent change from baseline at 3‐6 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | SDs were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 15.4% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding not reported |
Bard 1995.
| Methods | 8‐week cholesterol‐lowering diet 6‐week placebo washout period 6‐week before and after trial |
|
| Participants | 101 men and women aged 18‐75 with primary hypercholesterolaemia received fluvastatin 20 mg/day for 6 weeks then 40 mg/day from 6‐12 weeks 50 men and women aged 18‐75 with primary hypercholesterolaemia received cholestyramine for 12 weeks exclusion criteria:MI in the 6 months preceding the study, unstable anginal pectoris, diabetes, impaired renal and liver function, familial hypercholesterolaemia, type I, III, IV or V hyperlipoproteinaemia excessive alcohol consumption and ingestion of probucol within 1 year of study Fluvastatin 20 mg/day baseline TC : 8.4 mmol/L (325 mg/dL Fluvastatin 20 mg/day baseline LDL‐C : 6.5 mmol/L (251 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.3 mmol/L (50 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 1.5 mmol/L (133 mg/dL) |
|
| Interventions | fluvastatin 20 mg/day for 6 weeks fluvastatin 40 mg/day for 6‐12 weeks cholestyramine 16 g/day for 6 weeks cholestyramine 16 g/day for 6‐12 weeks |
|
| Outcomes | per cent change from baseline at 6 weeks of plasma TC and LDL‐C | |
| Source of Funding | unknown | |
| Notes | fluvastatin 40 mg/day for 6‐12 weeks cholestyramine 16 g/day for 6 weeks cholestyramine 16 g/day for 6‐12 weeks groups were not analysed HDL‐C and triglycerides were not included in the efficacy analysis because the calculated values were different by more than 10% from the given data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not provided |
Berger 1996.
| Methods | at least a 4‐week washout period 6‐week before and after trial |
|
| Participants | 270 men and women mean age 56 years with primary hypercholesterolaemia 136 participants received fluvastatin serum TG < 400 mg/dL (4.52 mmol/L) LDL‐C ≥ 190 mg/dL (4.91 mmol/L) and less than 2 CHD risk factors LDL‐C ≥ 160 mg/dL (4.14 mmol/L) and two or more CHD risk factors LDL‐C ≥ 130 mg/dL (3.36 mmol/L) and definite CHD or other atherosclerotic disease exclusion criteria: none reported Fluvastatin 20 mg/day baseline TC : 7.11 mmol/L (275 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 4.83 mmol/L (187 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.21 mmol/L (47 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 2.34 mmol/L (207 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day Lovastatin 20 mg/day |
|
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | Lovastatin 20 mg/day group was not included in the efficacy analysis SDs were imputed by the method of Furukawa 2006 for serum HDL‐C and serum triglycerides |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | First author works for Merck and Co. |
Betteridge 1994.
| Methods | 8‐week cholesterol lowering diet 4‐week single‐blind placebo washout period 8‐week before and after trial |
|
| Participants | 82 male and female patients aged 18‐75 years with primary hypercholesterolaemia received fluvastatin LDL‐cholesterol ≥ 160 mg/dL ( ≥ 4.1 mmol/L) in association with premature CAD or ≥ 2 defined risk factors for CAD or LDL‐cholesterol ≥ 190 mg/dL ( ≥ 4.9 mmol/L) with no CAD and < 2 risk factors and plasma TG levels ≤ 350 mg/dL (4.0 mmol/L) exclusion criteria: familial hypercholesterolaemia, type I, III, IV or V hyperlipoproteinaemia, pregnant or lactating women, child bearing potential secondary dyslipidaemia, GI impairment, MI, angioplasty, major surgery within 6 months of trial, congestive heart failure, severe or unstable angina pectoris untreated hypertension, obesity, medication use that might interfere with study results ingestion of probucol within 1 year of study Fluvastatin 20 mg/day baseline TC : 7.86 mmol/L (304 mg/dL Fluvastatin 20 mg/day baseline LDL‐C : 5.83 mmol/L (225 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.25 mmol/L (48 mg/dL Fluvastatin 20 mg/day baseline triglycerides: 1.71 mmol/L (151 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day for 8 weeks Gemfibrozil 600 mg twice daily for 8 weeks |
|
| Outcomes | per cent change from baseline at 8 weeks of plasma TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | Gemfibrozil 600 mg twice daily for 8 weeks group was not analysed sed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not provided |
Bevilacqua 1997.
| Methods | 4‐week placebo run‐in dietary washout period 20‐week double‐blind randomised placebo‐controlled trial |
|
| Participants | 48 men and women mean age 59 years with a history of angina pectoris, previous MI or coronary bypass surgery total cholesterol of 200‐300 mg/dL (5.17‐7.46 mmol/L) and concomitant high lipoprotein(a) > 30 mg/dL all women were postmenopausal exclusion criteria: secondary hypercholesterolaemia, serum triglycerides > 300 mg/dL (3.39mmol/L) liver or renal dysfunction, obesity, smoking Placebo baseline TC : 6.34 mmol/L (325 mg/dL) Placebo baseline LDL‐C : 4.27 mmol/L (236 mg/dL) Placebo baseline HDL‐C : 1.16 mmol/L (62 mg/dL) Placebo baseline triglycerides: 1.49 mmol/L (124 mg/dL) Fluvastatin 40 mg/day baseline TC : 6.36 mmol/L (325 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 4.47 mmol/L (236 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.23 mmol/L (62 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 1.32 mmol/L (124 mg/dL) |
|
| Interventions | placebo 8‐12 weeks placebo 12‐20 weeks fluvastatin 40 mg/day 8‐12 weeks fluvastatin 40 mg/day 12‐20 weeks |
|
| Outcomes | per cent change from baseline at 8‐12 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and WDAEs | |
| Source of Funding | Sandoz pharmaceuticals | |
| Notes | placebo 12‐20 weeks fluvastatin 40 mg/day 12‐20 weeks time period was not analysed SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind treatment Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | Unclear risk | Blinding method was not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4.2% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Sandoz pharmaceuticals funded the trial |
Bevilacqua 2004.
| Methods | 4‐week dietary run‐in period 12‐week before and after trial |
|
| Participants | 100 men and postmenopausal women age 45‐71 years old with type 2 diabetes mellitus, mixed dyslipidaemia LDL‐C, 150‐300 mg/dL (3.88‐7.76 mmol/L) 50 received fluvastatin triglycerides > 200 mg/dL ( 2.26 mmol/L) and HDL‐C < 50 mg/dL (1.29 mmol/L) exclusion criteria: surgery, MI, or angioplasty during the 6 months before randomisation, uncontrolled hypertension, liver disease, renal dysfunction myopathy, alcohol/drug abuse, statin hypersensitivity, pregnancy or lactation, use of oral contraceptives at the start of the study Fluvastatin 80 mg/day baseline LDL‐C : 3.85 mmol/L (149 mg/dL) Fluvastatin 80 mg/day baseline HDL‐C : 1.06 mmol/L (41 mg/dL) Fluvastatin 80 mg/day baseline triglycerides: 4.93 mmol/L (437 mg/dL) |
|
| Interventions | Fluvastatin XL 80 mg/day for 12 weeks Atorvastatin 20 mg/day for 12 weeks |
|
| Outcomes | per cent change from baseline at 12 weeks of serum LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | Atorvastatin 20 mg/day group was not analysed SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Bevilacqua 2005.
| Methods | 8‐week dietary run‐in period 8‐week before and after trial |
|
| Participants | 94 men and women aged 48‐79 years with type 2 diabetes mellitus and the lipid triad triglycerides > 2.3 mmol/L (204 mg/dL) HDL‐C < 1.3 mmol/L (50 mg/dL), and elevated levels of sdLDL exclusion criteria: surgery, MI, or angioplasty during the 6 months before randomisation, uncontrolled hypertension, liver disease, renal dysfunction myopathy, alcohol/drug abuse, statin hypersensitivity, pregnancy or lactation, use of oral contraceptives at the start of the study Fluvastatin 80 mg/day baseline LDL‐C : 4.7 mmol/L (182 mg/dL) Fluvastatin 80 mg/day baseline HDL‐C : 1.05 mmol/L (41 mg/dL) Fluvastatin 80 mg/day baseline triglycerides: 5.0 mmol/L (443 mg/dL) |
|
| Interventions | Fluvastatin XL 80 mg/day for 8 weeks ( 48 participants) Simvastatin 20 mg/day for 8 weeks (46 participants) |
|
| Outcomes | per cent change from baseline at 8 weeks of serum LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | Simvastatin 20 mg/day for 8 weeks group was not analysed SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on all participants were reported |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Bjarnason 2001.
| Methods | no participant was receiving lipid medications known to interfere with the trial washout not required 12‐week before and after trial |
|
| Participants | 23 women aged 65 years received vitamin C 500 mg/day and 45 women received fluvastatin 40 mg/day and vitamin C 500 mg/day TC > 5.2 mmol/L (201 mg/dL) TC = 7.34 mmol/L (284 mg/dL) LDL‐C = 4.86 mmol/L (188 mg/dL) HDL‐C = 1.89 mmol/L (73 mg/dL) exclusion criteria:BMI > 40, severe or chronic diseases, conditions that may interfere with the trial lack of consent, allergy to statins, participation in another trial within 3 months of the trial |
|
| Interventions | Vitamin C 500 mg/day Fluvastatin 40 mg/day + Vitamin C 500 mg/day |
|
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C and HDL‐C | |
| Source of Funding | Novo Nordisk A/S | |
| Notes | Vitamin C 500 mg/day group was not included in the efficacy analysis SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 36.8% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Novo Nordisk A/S funded the trial |
Branchi 1999.
| Methods | 3‐month dietary period none receiving drugs known to affect lipid metabolism 2‐month run‐in period 2‐month before and after trial |
|
| Participants | 200 hypercholesterolaemic men and women with LDL‐cholesterol levels of 160 mg/dL (4.14 mmol/L) (range 160‐426 mg/dL) (range 4.14‐11.0 mmol/L) or greater age ranged from 24‐75 years mean age 58 years serum triglyceride levels of less than 400 mg/dL (4.52 mmol/L) (range 52‐398 mg/dL) (range 0.59‐4.49 mmol/L) exclusion criteria: diabetes, hypothyroidism,renal and liver dysfunction Fluvastatin 40 mg/day baseline TC : 8.0 mmol/L (309 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 5.8 mmol/L (224 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.3 mmol/L (50 mg/dL |
|
| Interventions | 50 participants received 10 mg/day atorvastatin 50 participants received 40 mg/day fluvastatin 50 participants received 20 mg/day pravastatin 50 participants received 10 mg/day simvastatin |
|
| Outcomes | per cent change from baseline at 2 months of serum TC, LDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | baseline serum triglycerides was reported as a median 10 mg/day atorvastatin 20 mg/day pravastatin 10 mg/day simvastatin groups were not analysed HDL‐C was not included in the efficacy analysis because the calculated value was different by more than 10% from the given value |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not provided |
Broncel 2007.
| Methods | 8‐week dietary washout period 4‐week before and after trial |
|
| Participants | 22 male and female patients with hyperlipidaemia TC > 200 mg/dL (5.17 mmol/L) LDL‐C > 130 mg/dL (3.36 mmol/L) TG < 400 mg/dL (4.52 mmol/L) exclusion criteria: homozygous familial hypercholesterolaemia, hyperlipidaemia type I, III, IV, V, secondary hyperlipidaemia, diabetes, arterial hypertension, obesity BMI 30, renal and hepatic dysfunction, heart failure, systemic diseases, alcohol abuse, acute and chronic inflammation Fluvastatin 80 mg/day baseline TC : 7.0 mmol/L (271 mg/dL Fluvastatin 80 mg/day baseline LDL‐C : 4.67 mmol/L (181 mg/dL) Fluvastatin 80 mg/day baseline HDL‐C : 1.48 mmol/L (57 mg/dL Fluvastatin 80 mg/day baseline triglycerides: 1.94 mmol/L (172 mg/dL) |
|
| Interventions | Fuvastatin XL 80 mg/day | |
| Outcomes | per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | SDs were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Brown 1998.
| Methods | 4‐week run‐in period 54‐week before and after trial |
|
| Participants | 318 men and women with documented atherosclerosis age 18‐80 years old BMI not greater than 32 80 participants received fluvastatin LDL‐C ≥ 130 mg/dL (3.36 mmol/L) and ≤ 250 mg/dL (6.5 mmol/L) exclusion criteria: statin or resin hypersensitivities, taking prohibited medications, pregnant or lactation secondary hyperlipoproteinaemia such as uncontrolled hypothyroidism, nephrotic syndrome, severe renal dysfunction or uncontrolled diabetes mellitus; active liver disease or hepatic dysfunction; had a MI, coronary angioplasty, coronary artery bypass graft surgery and/or severe or unstable angina pectoris within 1 month of screening; had participated in another clinical trial within 30 days of screening for this study significant abnormalities that might compromise this study Fluvastatin 20 mg/day baseline TC : 6.465 mmol/L (250 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 4.4 mmol/L (170 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.06 mmol/L (41 mg/dL Fluvastatin 20 mg/day baseline triglycerides: 2.15 mmol/L (190 mg/dL) |
|
| Interventions | 10 mg/day atorvastatin for 0‐12 weeks 20 mg/day atorvastatin for 12‐24 weeks 40 mg/day atorvastatin for 24‐36 weeks 80 mg/day atorvastatin for 36‐48 weeks 80 mg/day atorvastatin + 5 g colestipol twice daily for 48‐54 weeks 10 mg/day simvastatin for 0‐12 weeks 20 mg/day simvastatin for 12‐24 weeks 40 mg/day simvastatin for 24‐36 weeks 40 mg/day simvastatin + 5 g colestipol twice daily for 36‐48 weeks 40 mg/day simvastatin + 10 g colestipol twice daily for 48‐54 weeks 20 mg/day lovastatin for 0‐12 weeks 40 mg/day lovastatin for 12‐24 weeks 40 mg lovastatin twice daily for 24‐36 weeks 40 mg lovastatin twice daily + 5 g colestipol twice daily for 36‐48 weeks 40 mg lovastatin twice daily + 10 g colestipol twice daily for 48‐54 weeks 20 mg/day fluvastatin for 0‐12 weeks 40 mg/day fluvastatin for 12‐24 weeks 40 mg/day fluvastatin + 5 g colestipol twice daily for 24‐36 weeks 40 mg/day fluvastatin + 10 g colestipol twice daily for 36‐54 weeks |
|
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | 10 mg/day atorvastatin for 0‐12 weeks 20 mg/day atorvastatin for 12‐24 weeks 40 mg/day atorvastatin for 24‐36 weeks 80 mg/day atorvastatin for 36‐48 weeks 80 mg/day atorvastatin + 5 g colestipol twice daily for 48‐54 weeks 10 mg/day simvastatin for 0‐12 weeks 20 mg/day simvastatin for 12‐24 weeks 40 mg/day simvastatin for 24‐36 weeks 40 mg/day simvastatin + 5 g colestipol twice daily for 36‐48 weeks 40 mg/day simvastatin + 10 g colestipol twice daily for 48‐54 weeks 20 mg/day lovastatin for 0‐12 weeks 40 mg/day lovastatin for 12‐24 weeks 40 mg lovastatin twice daily for 24‐36 weeks 40 mg lovastatin twice daily + 5 g colestipol twice daily for 36‐48 weeks 40 mg lovastatin twice daily + 10 g colestipol twice daily for 48‐54 weeks 40 mg/day fluvastatin for 12‐24 weeks 40 mg/day fluvastatin + 5 g colestipol twice daily for 24‐36 weeks 40 mg/day fluvastatin + 10 g colestipol twice daily for 36‐54 weeks groups were not analysed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Bruckert 2003.
| Methods | 4‐week washout period for those receiving lipid‐lowering agents 2‐month randomised double‐blind placebo‐controlled trial |
|
| Participants | 1229 men and women aged 70‐85 years with primary hypercholesterolaemia Total cholesterol ≥ 251 mg/dL ( ≥ 6.49 mmol/L) LDL‐cholesterol ≥ 159 mg/dL ( ≥ 4.11 mmol/L) triglycerides ≤ 407 mg/dL (≤ 4.595 mmol/L) exclusion criteria: type I or type V hyperlipoproteinaemia, secondary hyperlipidaemia, renal dysfunction symptomatic heart failure; history of MI, angina pectoris, stroke, severe peripheral artery disease and muscle disease Placebo baseline TC : 7.27 mmol/L (281 mg/dL) Placebo baseline LDL‐C : 5.17 mmol/L (200 mg/dL) Placebo baseline HDL‐C : 1.36 mmol/L (53 mg/dL) Placebo baseline triglycerides: 1.43 mmol/L (127 mg/dL Fluvastatin 80 mg/day baseline TC : 7.27 mmol/L (281 mg/dL) Fluvastatin 80 mg/day baseline LDL‐C : 5.17 mmol/L (200 mg/dL) Fluvastatin 80 mg/day baseline HDL‐C : 1.37 mmol/L (53 mg/dL) Fluvastatin 80 mg/day baseline triglycerides: 1.63 mmol/L (144 mg/dL) |
|
| Interventions | placebo Fluvastatin 80 mg/day |
|
| Outcomes | per cent change from baseline at 2 months of serum TC, LDL‐C, HDL‐C, triglycerides and WDAEs | |
| Source of Funding | Novartis Pharma AG | |
| Notes | SDs were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | all lipids were measured at a central laboratory (Pasteur Institute, Lille, France) |
| Blinding of outcome assessment (detection bias) WDAEs | Unclear risk | Blinding method not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 18.7% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Novartis Pharma AG funded the trial with a grant |
Bruni 2003.
| Methods | 6‐week dietary run‐in period 6‐week before and after trial |
|
| Participants | 64 men and women with hypercholesterolaemia age 36‐63 years old 16 participants received fluvastatin mean values were as followed: TC = 6.86 mmol/L (265 mg/dL) HDL‐C = 1.24 mmol/L (48 mg/dL) TG = 1.13 mmol/L (100 mg/dL) BMI = 24.7 no participant was taking hypolipidaemic, antiplatelet, anticoagulant or pro fibrinolytic drugs all females were not receiving hormone therapy exclusion criteria: cardiovascular events in the clinical history and hypertension, diabetes,liver renal thyroid, infectious, immunological or malignant disease 16 participants received each of the drugs Fluvastatin 40 mg/day baseline TC : 6.86 mmol/L (265 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 5.13 mmol/L (198 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.26 mmol/L (49 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 1.14 mmol/L (101 mg/dL) |
|
| Interventions | atorvastatin 10 mg/day simvastatin 20 mg/day fluvastatin 40 mg/day pravastatin 40 mg/day |
|
| Outcomes | per cent change from baseline at 3‐6 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | simvastatin 20 mg/day fluvastatin 40 mg/day pravastatin 40 mg/day groups were not included in the efficacy analysis SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Buzkova 2012.
| Methods | no washout required because no participants was receiving lipid‐altering substances within 8 weeks of the study 12‐week before and after trial |
|
| Participants | 48 men and women of Czech nationality with hypercholesterolaemia exclusion criteria:diabetes mellitus, liver disease, metabolic disease, previous treatment with fluvastatin, concomitant therapy with strong CYP2C9 inducers or inhibitors, history of stomach or gut surgery, cancer,immunosuppressant therapy, pregnancy , lactation, alcoholism Fluvastatin 80 mg/day baseline TC : 6.56 mmol/L (254 mg/dL) Fluvastatin 80 mg/day baseline LDL‐C : 3.86 mmol/L (149 mg/dL) Fluvastatin 80 mg/day baseline triglycerides: 2.34 mmol/L (207 mg/dL) |
|
| Interventions | Fluvastatin 80 mg/day | |
| Outcomes | per cent change from baseline at 12 weeks of plasma TC, LDL‐C, and triglycerides | |
| Source of Funding | Charles University (PRVOUK) | |
| Notes | SDs were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Low risk | Research program of the Charles University (PRVOUK) |
Buzzi 1997.
| Methods | 4‐week low‐fat dietary washout period 42‐84 days before and after trial |
|
| Participants | men and women 18 years or older with confirmed primary hypercholesterolaemia and TG levels ≤ 400 mg/dL (4.52 mmol/L) TC levels ≥ 300 mg/dL (7.76 mmol/L) LDL‐cholesterol level ≥ 130 mg/dL (3.36 mmol/L) exclusion criteria:pregnant women, child bearing potential,active liver disease, severe renal insufficiency |
|
| Interventions | 42‐day fluvastatin 20 mg/day 42‐84 day fluvastatin 20‐40 mg/day |
|
| Outcomes | per cent change from baseline at 48 days of serum LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | 42‐84 day time period was not analysed because some patients remained on 20 mg/day while others had their dose raised to 40 mg/day Total cholesterol data were not included in the efficacy analysis because the calculated value was different by more than 10% from the given value SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16.3% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not provided |
Ceska 1996.
| Methods | at least a 4‐week dietary washout period 12‐week before and after trial |
|
| Participants | 18 men and women are broken into 2 groups: 8 participants have heterozygous familial hypercholesterolaemia and 10 participants have familial combined hyperlipidaemia age is 34‐55 years exclusion criteria: none reported Fluvastatin 20 mg/day baseline TC : 7.95 mmol/L (307 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 5.5 mmol/L (213 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.25 mmol/L (48 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 2.4 mmol/L (213 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day for 0‐6 weeks Fluvastatin 40 mg/day for 6‐12 weeks |
|
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | government grant IGA MZ CR 2351 | |
| Notes | Fluvastatin 40 mg/day for 6‐12 weeks group was not included in the efficacy analysis SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Low risk | Work was supported by grant IGA MZ CR 2351 |
Cingozbay 2002.
| Methods | no washout required because no participant was receiving any medication or dietary restriction 3‐month before and after trial |
|
| Participants | 20 men and women with hyperlipidaemia age 31‐53 years BMI 25.9 patients with other causes of peripheral insulin resistance were excluded Fluvastatin 40 mg/day baseline TC : 7.5 mmol/L (290 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 5.9 mmol/L (523 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC and triglycerides | |
| Source of Funding | unknown | |
| Notes | LDL‐C and HDL‐C lipid data were not included in the efficacy analysis SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No data for LDL‐C |
| Selective reporting (reporting bias) | High risk | LDL‐C outcome was not reported |
| Other bias | Unclear risk | Source of funding was not reported |
CURVES 1998.
| Methods | 6‐week dietary run‐in period 8‐week before and after trial |
|
| Participants | Men and women 18‐80 years old with hypercholesterolaemia 25 participants received fluvastatin plasma LDL cholesterol ≥ 160 mg/dL ( ≥ 4.14 mmol/L) plasma triglycerides ≤ 400 mg/dL (4.5 mmol/L) exclusion criteria:primary hypothyroidism, nephrotic syndrome, type 1 or uncontrolled type 2 diabetes, hepatic dysfunction, BMI > 32 uncontrolled hypertension; MI, coronary angioplasty, coronary bypass graft, severe or unstable angina pectoris within 3 months, statin hypersensitivities significant abnormalities that could affect the study Fluvastatin 20 mg/day baseline TC : 8.3 mmol/L (321 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 6.1 mmol/L (236 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.3 mmol/L (50 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 2.1 mmol/L (186 mg/dL) Fluvastatin 40 mg/day baseline TC : 7.1 mmol/L (275 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 5.0 mmol/L (193 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.3 mmol/L (50 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 2.0 mmol/L (177 mg/dL) |
|
| Interventions | atorvastatin 10, 20, 40 and 80 mg/day simvastatin 10, 20, and 40 mg/day pravastatin 10, 20, and 40 mg/day lovastatin 20, 40 mg/day and 40 mg twice daily fluvastatin 20 mg/day fluvastatin 40 mg/day |
|
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | atorvastatin 10, 20, 40 and 80 mg/day simvastatin 10, 20, and 40 mg/day pravastatin 10, 20, and 40 mg/day lovastatin 20, 40 mg/day and 40 mg twice daily groups were not analysed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | source of funding was not provided |
Dallongeville 1994a.
| Methods | lipid‐lowering treatment was discontinued for 10 weeks (1 year for probucol) prior to the study start 6‐week dietary placebo run‐in period 6‐week randomised placebo‐controlled double‐blind trial |
|
| Participants | 429 men and women LDL‐C > 160 mg/dL (4.14 mmol/L) and premature CHD and/or two associated risk factors LDL‐C > 190 mg/dL (4.91 mmol/L) and no CHD, plus triglycerides < 300 mg/dL (3.39 mmol/L) Placebo baseline LDL‐C : 6.53 mmol/dL (253 mg/dL) Fluvastatin 2.5 mg/day baseline LDL‐C : 6.74 mmol/L (261 mg/dL) Fluvastatin 5 mg/day baseline LDL‐C : 6.76 mmol/L (261 mg/dL) Fluvastatin 10 mg/day baseline LDL‐C : 6.24 mmol/L (241 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 6.24 mmol/L (241 mg/dL) |
|
| Interventions | Placebo for 6 weeks Fluvastatin 2.5 mg/day for 6 weeks Fluvastatin 5 mg/day for 6 weeks Fluvastatin 10 mg/day for 6 weeks Fluvastatin 20 mg/day for 6 weeks |
|
| Outcomes | per cent change from baseline at 6 weeks of LDL‐C | |
| Source of Funding | unknown | |
| Notes | TC, HDL‐C, triglycerides and WDAEs were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | LDL‐C was determined by the Pasteur Institute Central Laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | WDAEs were not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 0.2% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Dallongeville 1994b.
| Methods | 6‐week diet plus placebo run‐in period 6‐week double‐blind placebo‐controlled trial |
|
| Participants | 423 men and women with hypercholesterolaemia LDL‐Cholesterol > 160 mg/dL (4.14 mmol/L) and premature CAD and/or two associated risk factors; LDL‐Cholesterol > 190 mg/dL (4.91 mmol/L) and no CAD triglycerides < 300 mg/dL (3.39 mmol/L) Placebo baseline TC : 8.4 mmol/L (325 mg/dL) Placebo baseline LDL‐C : 6.3 mmol/L (244 mg/dL) Placebo baseline HDL‐C : 1.3 mmol/L (50 mg/dL) Placebo baseline triglycerides: 1.6 mmol/L (142 mg/dL) Fluvastatin 20 mg/day baseline TC : 8.3 mmol/L (321 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 6.2 mmol/L (240 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.4 mmol/L (54 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 1.6 mmol/L (142 mg/dL) Fluvastatin 40 mg/day baseline TC : 8.0 mmol/L (309 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 6.0 mmol/L (232 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.3 mmol/L (50 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 1.6 mmol/L (142 mg/dL) |
|
| Interventions | Placebo for 6 weeks Fluvastatin 20 mg/day for 6 weeks Fluvastatin 40 mg/day for 6 weeks |
|
| Outcomes | per cent change from baseline at 6 weeks of serum TC and LDL‐C for the 20 mg/day data set per cent change from baseline at 6 weeks of serum TC, LDL‐C and triglycerides for the 40 mg/day data set |
|
| Source of Funding | unknown | |
| Notes | HDL‐C and triglycerides were not included in the efficacy analysis of the 20 mg/day data set because the calculated data were different by more than 10% from the given data HDL‐C was not included in the efficacy analysis of the 40 mg/day data set because the calculated value was different by more than 10% from the given value WDAEs were not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo and fluvastatin capsule appearances were not reported as appearing identical Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | LDL‐C was determined by the Pasteur Institute Central Laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No WDAES were reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2.6% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Davidson 2003.
| Methods | 6‐week dietary run‐in washout period 6‐week before and after trial |
|
| Participants | 838 men and women aged > 20 years with primary hypercholesterolaemia 337 received fluvastatin triglycerides ≤ 4.5 mmol/L (399 mg/dL) LDL‐C ≥ 3.4 mmol/L (131mg/dL) with evidence of CHD or other atherosclerotic disease LDL‐C ≥ 4.1 mmol/L (159mg/dL) with ≥2 other CHD risk factors but no CHD or other atherosclerotic disease LDL‐C ≥ 4.9 mmol/L (189mg/dL) without CHD or other atherosclerotic disease and < 2 other CHD risk factors exclusion criteria: MI, coronary bypass surgery or angioplasty in the prior 3 months current coronary insufficiency, clinically significant ventricular arrhythmias, potential childbearing, pregnancy Fluvastatin 20 mg/day baseline TC : 7.1 mmol/L (275 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 4.9 mmol/L (189 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.2 mmol/L (46 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 2.1 mmol/L (186 mg/dL) Fluvastatin 40 mg/day baseline TC : 7.0 mmol/L (271 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 4.8 mmol/L (186 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.2 mmol/L (46 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 2.2 mmol/L (195 mg/dL) |
|
| Interventions | Lovastatin 10 mg/day for 6 weeks Lovastatin 20 mg/day for 6 weeks Lovastatin 40 mg/day for 6 weeks Fluvastatin 20 mg/day for 6 weeks Fluvastatin 40 mg/day for 6 weeks |
|
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | Lovastatin 10 mg/day for 6 weeks Lovastatin 20 mg/day for 6 weeks Lovastatin 40 mg/day for 6 weeks groups were not analysed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Dergunov 2003.
| Methods | 8‐week dietary run‐in period 16‐week before and after trial |
|
| Participants | 67 men with controlled hypertension LDL‐C > 4.1 mmol/L (159 mg/dL) TG 0.49‐3.26 mmol/L (43‐289 mg/dL) exclusion criteria: none reported Fluvastatin 20 mg/day baseline TC : 6.93 mmol/L (268 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 5.07 mmol/L (196 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.06 mmol/L (41 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 1.765 mmol/L (156 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day for 0‐4 weeks Fluvastatin 20‐40 mg/day for 4‐8 and 8‐12 weeks Off fluvastatin for 12‐16 weeks |
|
| Outcomes | per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | partially by Russian Foundation for Basic Research grant 01‐04‐48140 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Partially funded by Russian Foundation for Basic Research grant 01‐04‐48140 |
Di Lullo 2005.
| Methods | 2‐month washout period 6‐month before and after trial |
|
| Participants | 130 men and women between 18‐80 years old, 80 participants received fluvastatin mild to moderate chronic renal failure creatinine clearance 45‐55 mL/min CRP between 3 mg/dL and 14 mg/dL total cholesterol 250‐350 mg/dL (6.465‐9.05 mmol/L) HDL‐C 50‐70 mg/dL (1.29‐1.81 mmol/L) LDL‐C 100‐190 mg/dL (2.59‐4.91 mmol/L) triglycerides 160‐450 mg/dL (1.81‐5.08 mmol/L) exclusion criteria: severe heart failure, familial hypercholesterolaemia, hypertriglyceridaemia creatinine clearance < 15 mL/hr on dialysis severe hepatic, hematologic, respiratory, cardiac and psychiatric illnesses childbearing potential, pregnancy |
|
| Interventions | Fluvastatin 80 mg XL /day for 6 months | |
| Outcomes | per cent change from baseline at 3 months of serum TC, LDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | HDL‐C data were not included in the efficacy analysis because the calculated value was different by more than 10% from the given value SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Ding 1997.
| Methods | participants could not be taking any lipid‐lowering drugs and had to adhere to a low‐cholesterol diet for at least 6 weeks 12‐week randomised double‐blind placebo‐controlled trial |
|
| Participants | 46 type 2 diabetic patients stable diabetes control Placebo baseline TC : 6.2 mmol/L (240 mg/dL) Placebo baseline LDL‐C : 4.3 mmol/L (166 mg/dL) Placebo baseline HDL‐C : 1.2 mmol/L (46 mg/dL) Placebo baseline triglycerides: 1.6 mmol/L (142 mg/dL) Fluvastatin 20 mg/day baseline TC : 6.3 mmol/L (244 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 4.5 mmol/L (174 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.1 mmol/L (42.5 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 1.5 mmol/L (132 mg/dL) |
|
| Interventions | Placebo 0‐6 weeks Placebo 6‐12 weeks Fluvastatin 20 mg/day 0‐6 weeks Fluvastatin 40 mg/day 6‐12 weeks |
|
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and WDAEs | |
| Source of Funding | unknown | |
| Notes | Placebo 6‐12 weeks Fluvastatin 40 mg/day 6‐12 weeks groups were not analysed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | Low risk | no patient discontinued medication because of adverse effects |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 13% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Dujovne 1994.
| Methods | 8‐week dietary washout period 18 week randomised double‐blind placebo‐controlled cross‐over trial |
|
| Participants | 44 men and women with primary hypercholesterolaemia LDL‐C ≥4.14 mmol/L (160 mg/dL) exclusion criteria: homozygous familial hypercholesterolaemia, secondary hypercholesterolaemia cardiovascular disease, statin hypersensitivity, concomitant medication that could influence the analysis of safety or efficacy no baseline data |
|
| Interventions | Placebo Fluvastatin 20 mg/day |
|
| Outcomes | per cent change from baseline at 3‐6 weeks of serum TC, LDL‐C and triglycerides | |
| Source of Funding | unknown | |
| Notes | cross‐over phase 2 weeks 7‐12 and phase 3 weeks 13‐18 were not included in the efficacy analysis WDAEs were not reported in the first phase reported in phase 2 week 15 of the trial |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo and treatment capsules were identical in appearance |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | LDL‐C was determined by a central laboratory (Medical Research Laboratories, Cincinnati, Ohio) |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | WDAEs were not reported within the 12‐week treatment period |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2.2% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Ertugrul 2011.
| Methods | no washout required because no participant was receiving any lipid medication 8‐week before and after trial |
|
| Participants | 134 men and women with hyperlipidaemia LDL‐C > 100 mg/dL (2.59 mmol/L) 69 patients received rosuvastatin and 65 patients received fluvastatin exclusion criteria: alcoholism, malignancy, hyper and hypocalcaemia and hyperparathyroidism participants receiving phosphorus‐calcium modifying drugs, statins or fibrates Fluvastatin 80 mg/day baseline LDL‐C : 4.4 mmol/L (170 mg/dL Fluvastatin 80 mg/day baseline HDL‐C: 1.2 mmol/L (46 mg/dL) |
|
| Interventions | Rosuvastatin 10 mg/day Fluvastatin XL 80 mg/day |
|
| Outcomes | per cent change from baseline at 8 weeks of serum LDL‐C and HDL‐C | |
| Source of Funding | unknown | |
| Notes | Rosuvastatin 10 mg/day group was not included in the efficacy analysis SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Fanghanel 1995.
| Methods | 8‐week dietary stabilisation run‐in period 12‐week before and after trial |
|
| Participants | 40 men and women with type IIa and IIb primary hypercholesterolaemia mean age 46 years range 25‐79 years 20 received fluvastatin and 20 received bezafibrate Total cholesterol > 6.2 mmol/L (240 mg/dL) exclusion criteria:MI or coronary angioplasty within 3 months of trial severe cardiac insufficiency, severe angina pectoris, uncontrolled arterial hypertension possibility of pregnancy, pregnant use of investigational drugs within 6 months, drug abuse excessive alcohol consumption Fluvastatin 40 mg/day baseline TC : 7.0 mmol/L (309 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 5.12 mmol/L (232 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.48 mmol/L (50 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 1.69 mmol/L (142 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day for 0‐12 weeks Bezafibrate 400 mg/day for 0‐12 weeks |
|
| Outcomes | per cent change from baseline at 6‐12 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | Bezafibrate 400 mg/day for 0‐12 weeks was not analysed SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | The source of funding was not reported |
Fanghanel Salmon 1996.
| Methods | 8‐week dietary run‐in period 12‐week before and after trial |
|
| Participants | 40 men and women with type IIa hypercholesterolaemia LDL‐C > 190 mg/dL(4.91 mmol/L) or LDL‐C > 160 mg/dL (4.14 mmol/L) with one or more risk factors TG < 250 mg/dL (2.82mmol/L) exclusion criteria: secondary lipidaemia, cardiac abnormalities hepatic or renal dysfunction, use of birth control pills and statin hypersensitivity Fluvastatin 40 mg/day baseline TC : 7.04 mmol/L (272 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 4.74 mmol/L (183 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.3 mmol/L (50 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 2.13 mmol/L (189 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day | |
| Outcomes | per cent change from baseline at 6‐12 weeks of serum TC, LDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | HDL‐C data were not included in the efficacy analysis because the calculated value was different by more than 10% from the given value SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Fernandez 2001.
| Methods | 4‐week run‐in washout period 8‐week before and after trial |
|
| Participants | 70 women age 60‐80 years old with primary hypercholesterolaemia LDL‐C ≥3.4 mmol/L (131 mg/dL) TC ≥5.2 mmol/L (201 mg/dL) and TG <4.52 mmol/L(400 mg/dL) exclusion criteria:active renal of hepatic disease, cancer, severe hypertension and uncontrolled diabetes mellitus unstable angina, MI, stroke, TIAs, coronary surgery within 3 months of trial Fluvastatin 20 mg/day baseline TC : 6.67 mmol/L (258 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 4.93 mmol/L (191 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.05 mmol/L (41 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 1.86 mmol/L (165 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day Policosanol 10 mg/day |
|
| Outcomes | per cent change from baseline at 4‐8 weeks of serum TC, LDL‐C and HDL‐C | |
| Source of Funding | unknown | |
| Notes | Policosanol 10 mg/day group was not analysed Triglyceride data were not included in the efficacy analysis because the calculated value was different by more than 10% from the given value SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Filippova 1997.
| Methods | 8‐week lipid lowering diet washout period 12‐week before and after trial |
|
| Participants | 20 patients with CAD Total cholesterol ≥ 5.2 mmol/L (201 mg/dL) one patient died 6 weeks before the start of the fluvastatin dosing no exclusion criteria reported 19 patients were included in the efficacy analysis Fluvastatin 20 mg/day baseline TC : 7.62 mmol/L (295 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 5.17 mmol/L (200 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.07 mmol/L (41 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 2.84 mmol/L (252 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day for 6 weeks Fluvastatin 40 mg/day for 6‐12 weeks |
|
| Outcomes | per cent change from baseline at 6 weeks of blood LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | Fluvastatin 40 mg/day for 6‐12 weeks group was not included in the efficacy analysis Total cholesterol data were not included in the efficacy analysis because the calculated value was different by more than 10% from the given value SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
FSGJ 1995.
| Methods | 4‐week dietary washout period 12‐week before and after trial |
|
| Participants | 381 men and women with type IIa and IIb hypercholesterolaemia age 20‐70 years old Total cholesterol 190‐504 mg/dL (4.91‐13.0 mmol/L) LDL‐C 75.6‐436.2 mg/dL (1.96‐11.3 mmol/L) HDL‐C 25‐115 mg/dL (0.65‐2.97 mmol/L) Triglycerides 38‐618 mg/dL (0.42‐6.98 mmol/L) 192 participants received fluvastatin 189 participants received pravastatin exclusion criteria: hypothyroidism, Cushings disease, gallbladder disease, pancreatitis, cancer, unstable diabetes, severe hypertension, alcohol abuse, obese people on diet, renal, liver dysfunction, brain disease, heart disease statin hypersensitivity, MI within 6 months of trial and childbearing potential Fluvastatin 30 mg/day baseline TC : 7.15 mmol/L (276 mg/dL) Fluvastatin 30 mg/day baseline LDL‐C : 4.93 mmol/L (191 mg/dL) Fluvastatin 30 mg/day baseline HDL‐C : 1.44 mmol/L (56 mg/dL) Fluvastatin 30 mg/day baseline triglycerides: 1.74 mmol/L (154 mg/dL) |
|
| Interventions | Fluvastatin 30 mg/day Pravastatin 10 mg/day |
|
| Outcomes | per cent change from baseline at 4‐12 weeks of serum TC and LDL‐C | |
| Source of Funding | unknown | |
| Notes | Pravastatin 10 mg/day group was not included in the efficacy analysis HDL‐C and triglyceride data were not included in the efficacy analysis because the calculated values were different by more than 10% from the given values for all the doses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 24% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Fujimoto 2004.
| Methods | no washout required because they were not on any hypolipidaemic treatments 3 month before and after trial |
|
| Participants | 16 men and women with hypercholesterolaemia mean age 56 years old no chronic or metabolic disease, no acute coronary events total cholesterol level > 220 or > 180 mg/dL ( > 5.69 or > 4.65mmol/L) if angiography documented coronary artery disease exclusion criteria: MI within 6 months of trial, wall motion abnormality in the area of the left anterior descending coronary artery severe valvular disease, history of coronary bypass surgery, a left ventricular ejection fraction <40%, left ventricular hypertrophy, atrial fibrillation, BP > 160/90 taking antioxidants, premenopausal and severe concomitant illness Doppler recordings for CFR measurement were inadequate CFR was < 2.0 because of suspected significant left anterior descending coronary artery stenosis Fluvastatin 20 mg/day baseline TC : 6.21 mmol/L (240 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 4.14 mmol/L (160 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.4 mmol/L (54 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 1.43 mmol/L (127 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day | |
| Outcomes | per cent change from baseline at 3 months of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | SDs were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Galal 1997.
| Methods | 8‐week dietary run‐in period 12‐week before and after trial |
|
| Participants | 467 men and women 18 years or older confirmed primary hypercholesterolaemia with TG < 4.5 mmol/L (400 mg/dL) TC of 6.5‐7.8 mmol/L (250‐300 mg/dL) with at least 2 non‐lipid risk factors such as hypertension, smoking, diabetes mellitus, obesity and family history of coronary heart disease patients with CHD or peripheral artery disease or TC > 7.8 mmol/L (300 mg/dL) LDL‐C > 3.4 mmol/L (130 mg/dL) exclusion criteria: pregnancy or lactation, child bearing potential, active liver disease, renal dysfunction, fluvastatin hypersensitivity Fluvastatin 20 mg/day baseline TC : 7.86 mmol/L (304 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 5.334 mmol/L (206 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 0.897 mmol/L (35 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 2.82 mmol/L (250 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day for 0‐6 weeks Fluvastatin 40 mg/day for 7‐12 weeks |
|
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Source of Funding | unknown | |
| Notes | SDs were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 32.5% participants were not included in the efficacy analysis` |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | One of the authors is the Product Manager for Sandoz |
Gao 2003.
| Methods | 4‐week washout period 4‐week before and after |
|
| Participants | 60 men and women with CAD and hyperlipidaemia age 53‐85 years TC ≥ 5.2 mmol/L (201 mg/dL) LDL‐C ≥ 3.12 mmol/L (121 mg/dL) TG ≥ 1.70 mmol/L (151 mg/dL) exclusion criteria: kidney and endocrine diseases secondary hyperlipidaemia Fluvastatin 20 mg/day baseline TC : 5.5 mmol/L (213 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 3.5 mmol/L (135 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.2 mmol/L (46 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 2.06 mmol/L (182 mg/dL) |
|
| Interventions | 30 patients received fluvastatin 20 mg/day 30 patients received xuehikang pill 2 times per day |
|
| Outcomes | per cent change from baseline at 4 weeks of blood TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | xuehikang group was not included in the efficacy analysis SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding not reported |
Ghods 1995.
| Methods | 4‐week dietary washout period 12‐week before and after trial |
|
| Participants | 10 men and women with nephrotic syndrome and hypercholesterolaemia TC > 240 mg/dL (6.21 mmol/L) LDL‐C > 160 mg/dL (4.14 mmol/L) exclusion criteria: liver disease, participants, 18 years, pregnancy potential Fluvastatin 20 twice daily baseline TC : 9.982 mmol/L (386 mg/dL) Fluvastatin 20 twice daily baseline LDL‐C : 6.025 mmol/L (233 mg/dL) Fluvastatin 20 twice daily baseline HDL‐C : 1.32 mmol/L (51 mg/dL) Fluvastatin 20 twice daily baseline triglycerides: 5.837 mmol/L (517 mg/dL) |
|
| Interventions | Fluvastatin 20 twice daily | |
| Outcomes | per cent change from baseline at 4‐12 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | SDs were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding not reported |
Goedecke 2002.
| Methods | no washout required because they were not on any hypolipidaemic treatments within 3 months of the trial 4‐week dietary run‐in period 12‐week randomised double‐blind placebo‐controlled trial |
|
| Participants | 48 men and women age 18‐75 years old with hypercholesterolaemia 160 mg/dL ≤ LDL‐C ≤ 300 mg/dL (4.14 mmol/L ≤ LDL‐C ≤ 7.76 mmol/L) triglycerides ≤ 350 mg/dL (3.95 mmol/L) exclusion criteria: pregnancy or lactation, childbearing potential without safe contraceptive protection therapy with lipid‐lowering agents within the last 3 months prior to study entry alcohol abuse, autoimmune diseases, nephrotic syndrome, obstructive liver disease, multiple myeloma hypothyroidism, chronic pancreatitis, porphyria or myopathy type 1 or uncontrolled type 2 diabetes mellitus, patients with atrial fibrillation and AV Block (grade II or higher) statin hypersensitivity, participation in another drug study within 3 months of this trial Diseases and conditions that may affect the pharmacokinetics or pharmacodynamics of the test substances, e.g. gastrointestinal diseases liver disease, kidney disease MI within 3 months of trial, disallowed medications, drug abuse, non compliant patients Placebo baseline TC : 7.805 mmol/L (302 mg/dL) Placebo baseline LDL‐C : 5.535 mmol/L (214 mg/dL) Placebo baseline HDL‐C : 1.44 mmol/L (56 mg/dL) Placebo baseline triglycerides: 2.44 mmol/L (216 mg/dL) Fluvastatin 40 mg/day baseline TC : 7.615 mmol/L (294 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 5.43 mmol/L (210 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.41 mmol/L (55 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 1.67 mmol/L (148 mg/dL) |
|
| Interventions | Placebo Fluvastatin 40 mg/day |
|
| Outcomes | per cent change from baseline at 6‐12 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | WDAEs were not reported SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind treatment placebo and fluvastatin capsule appearances were not reported as appearing identical Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | WDAEs were not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Gotoh 2011.
| Methods | No washout required because no participant received lipid‐lowering agents 3‐month before and after trial |
|
| Participants | 28 non‐diabetic normotensive postmenopausal type IIA hypercholesterolaemic women exclusion criteria: drugs known to interfere with bone metabolism, amenorrhoea for less than 12 months, secondary hypercholesterolaemia hypertension, diabetes mellitus Fluvastatin 30 mg/day baseline TC : 6.51 mmol/L (251 mg/dL) Fluvastatin 30 mg/day baseline LDL‐C : 4.22 mmol/L (163 mg/dL) Fluvastatin 30 mg/day baseline HDL‐C : 1.31 mmol/L (51 mg/dL) |
|
| Interventions | Fluvastatin 30 mg/day | |
| Outcomes | per cent change from baseline at 8‐12 weeks of serum TC, LDL‐C and HDL‐C | |
| Source of Funding | unknown | |
| Notes | Triglycerides were not included in the efficacy analysis SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Greten 1994.
| Methods | 8‐week dietary stabilisation period with the last four weeks of washout of previous lipid‐lowering therapy 12‐week before and after trial |
|
| Participants | 64 male and female patients with primary hypercholesterolaemia (familial heterozygous hypercholesterolaemia, familial combined hyperlipidaemia or polygenic type IIa hypercholesterolaemia) age 18‐75 years received fluvastatin and 67 bezafibrate. LDL‐C ≥160 mg/dL (4.1 mmol/L) and TG ≤ 300 mg/dL (3.4 mmol/L) body weight within 40% ideal normal liver and renal function exclusion criteria: other dyslipidaemic phenotypes, secondary hypercholesterolaemia, condition that might affect drug handling, safety or evaluation of results MI, angioplasty within the last 3 months, congestive heart failure, severe angina pectoris,untreated hypertension, use of either medications known to interact with the study drugs, use of probucol within 6 months of study, pregnancy change of pregnancy, drug and alcohol abuse Fluvastatin 40 mg/day baseline TC : 9.12 mmol/L (353 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 6.95 mmol/L (269 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.43 mmol/L (55 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 1.62 mmol/L (143 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day for 3‐12 weeks Bezafibrate 400 mg/day for 3‐12 weeks |
|
| Outcomes | per cent change from baseline at 3‐12 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | Sandoz AG Nürnberg | |
| Notes | Bezafibrate 400 mg/day for 3‐12 weeks group was not analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | The study was supported by Sandoz AG Nürnberg |
Guan 2004.
| Methods | no washout required because no participant was receiving any lipid medication 12‐week before and after trial |
|
| Participants | 6 men and women with type 2 diabetes mellitus and hyperlipidaemia mean age 56.2 years BMI 23.0 TC 208‐316 mg/dL (5.38‐8.17 mmol/L) LDL‐C 125‐225 mg/dL (3.23‐5.82 mmol/L) HDL‐C 30.1‐76.5 mg/dL (0.78‐1.98 mmol/L) TG 105‐249 mg/dL ( 1.19‐2.81 mmol/L) exclusion criteria:uncontrolled hypertension, liver disease, renal dysfunction, lipid‐lowering therapy before study, insulin use at start of study Fluvastatin 20 mg/day baseline TC : 6.18 mmol/L (239 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 4.01 mmol/L (155 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.31 mmol/L (51 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 1.83 mmol/L (162 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day | |
| Outcomes | per cent change from baseline at 4‐12 weeks of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Source of Funding | unknown | |
| Notes | SDs were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Haak 2001.
| Methods | 4‐week dietary run‐in period 12‐week randomised, double‐blind, placebo‐controlled trial |
|
| Participants | 64 men and women with hyperlipidaemia with LDL‐C > 160 mg/dL (4.14 mmol/L) TG < 350 mg/dL (3.95 mmol/L) who were inadequately controlled by diet Placebo baseline TC : 7.78 mmol/L (301 mg/dL) Placebo baseline LDL‐C : 5.30 mmol/L (205 mg/dL) Placebo baseline HDL‐C : 1.45 mmol/L (56 mg/dL) Placebo baseline triglycerides: 2.35 mmol/L (208 mg/dL) Fluvastatin 80 mg/day baseline TC : 7.58 mmol/L (293 mg/dL) Fluvastatin 80 mg/day baseline LDL‐C : 5.46 mmol/L (211 mg/dL) Fluvastatin 80 mg/day baseline HDL‐C : 1.42 mmol/L (55 mg/dL) Fluvastatin 80 mg/day baseline triglycerides: 1.67 mmol/L (148 mg/dL) |
|
| Interventions | Placebo for 12 weeks Fluvastatin 40 mg twice daily for 12 weeks |
|
| Outcomes | per cent change from baseline at 8‐12 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and WDAEs | |
| Source of Funding | Novartis | |
| Notes | SDs were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind treatment placebo and fluvastatin capsule appearances were not reported as appearing identical Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | Unclear risk | Blinding method not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 25% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Novartis funded the trial |
Hailer 1996.
| Methods | 8‐week washout period 6‐week placebo run‐in period 12‐week before and after trial |
|
| Participants | 8 heterozygous patients with familial LDL‐receptor defective hypercholesterolaemia phenotypic IIa or IIb hyperlipoproteinaemia Fluvastatin 40 mg/day baseline TC : 9.7 mmol/L (375 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 7.9 mmol/L (305 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.2 mmol/L (46 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 1.8 mmol/L (159 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day for 12 weeks Bezafibrate 400 mg/day for 12 weeks |
|
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | SDs were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Homma 2003.
| Methods | 8‐week washout period 24‐week before and after trial |
|
| Participants | 30 men and women with non familial type 2 hyperlipoproteinaemia exclusion criteria: familial hypercholesterolaemia and familial combined hyperlipoproteinaemia TG > 350 mg/dL (3.95 mmol/L) and those treated with probucol, diabetes mellitus, CHD, or cerebrovascular disease Fluvastatin 20 mg/day baseline TC : 7.76 mmol/L (300 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 5.25 mmol/L (203 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.66 mmol/L (64 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 1.9 mmol/L (168 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day for 12 weeks Fluvastatin 40 mg/day 12‐24 weeks |
|
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | Fluvastatin 40 mg/day 12‐24 weeks was not analysed SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Huhle 1999.
| Methods | 4‐week dietary placebo run‐in period 8‐week randomised, double‐blind, placebo‐controlled trial |
|
| Participants | 22 men and women age 30‐70 years serum LDL‐C > 160 mg/dL (4.14 mmol/L) serum TG < 300 mg/dL (3.39 mmol/L) exclusion criteria: type 1 diabetes mellitus, pregnancy severe liver and/or pancreatic disease renal failure, MI within 2 months of trial, heart failure, uncontrolled hypertension and medications that affect lipids within 3 weeks of trial |
|
| Interventions | Placebo 0‐8 weeks Fluvastatin 40 mg twice daily for 0‐8 weeks |
|
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | WDAEs were not reported SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Aallocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind treatment placebo and fluvastatin capsule appearances were not reported as appearing identical Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | WDAEs were not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Hunninghake 1998.
| Methods | 4‐week run‐in period 54‐week before and after trial |
|
| Participants | 344 men and women at risk for CHD aged 18‐80 years old BMI ≤ 32 85 participants received fluvastatin triglycerides ≤ 400 mg/dL (4.52 mmol/L) total cholesterol ≥190 mg/dL (4.91 mmol/L) and less than 2 risk factors for CHD LDL‐C ≥160 mg/dL (4.14 mmol/L) and 2 or more CHD risk factors exclusion criteria: statin or resin hypersensitivities, taking prohibited medications, pregnant or lactation secondary hyperlipoproteinaemia such as uncontrolled hypothyroidism, nephrotic syndrome, severe renal dysfunction or uncontrolled diabetes mellitus; active liver disease or hepatic dysfunction; had a MI, coronary angioplasty, coronary artery bypass graft surgery and/or severe or unstable angina pectoris within 1 month of screening; had participated in another clinical trial within 30 days of screening for this study significant abnormalities that might compromise this study Fluvastatin 20 mg/day baseline TC : 7.40 mmol/L (286 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 5.2 mmol/L (201 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.11 mmol/L (43 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 2.36 mmol/L (209 mg/dL) |
|
| Interventions | 10 mg/day atorvastatin for 0‐12 weeks 20 mg/day atorvastatin for 12‐24 weeks 40 mg/day atorvastatin for 24‐36 weeks 80 mg/day atorvastatin for 36‐48 weeks 80 mg/day atorvastatin + 5 g colestipol twice daily for 48‐54 weeks 10 mg/day simvastatin for 0‐12 weeks 20 mg/day simvastatin for 12‐24 weeks 40 mg/day simvastatin for 24‐36 weeks 40 mg/day simvastatin + 5 g colestipol twice daily for 36‐48 weeks 40 mg/day simvastatin + 10 g colestipol twice daily for 48‐54 weeks 20 mg/day lovastatin for 0‐12 weeks 40 mg/day lovastatin for 12‐24 weeks 40 mg lovastatin twice daily for 24‐36 weeks 40 mg lovastatin twice daily + 5 g colestipol twice daily for 36‐48 weeks 40 mg lovastatin twice daily + 10 g colestipol twice daily for 48‐54 weeks 20 mg/day fluvastatin for 0‐12 weeks 40 mg/day fluvastatin for 12‐24 weeks 40 mg/day fluvastatin + 5 g colestipol twice daily for 24‐36 weeks 40 mg/day fluvastatin + 10 g colestipol twice daily for 36‐54 weeks |
|
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | Parke‐Davis Pharmaceutical Research | |
| Notes | 10 mg/day atorvastatin for 0‐12 weeks 20 mg/day atorvastatin for 12‐24 weeks 40 mg/day atorvastatin for 24‐36 weeks 80 mg/day atorvastatin for 36‐48 weeks 80 mg/day atorvastatin + 5 g colestipol twice daily for 48‐54 weeks 10 mg/day simvastatin for 0‐12 weeks 20 mg/day simvastatin for 12‐24 weeks 40 mg/day simvastatin for 24‐36 weeks 40 mg/day simvastatin + 5 g colestipol twice daily for 36‐48 weeks 40 mg/day simvastatin + 10 g colestipol twice daily for 48‐54 weeks 20 mg/day lovastatin for 0‐12 weeks 40 mg/day lovastatin for 12‐24 weeks 40 mg lovastatin twice daily for 24‐36 weeks 40 mg lovastatin twice daily + 5 g colestipol twice daily for 36‐48 weeks 40 mg lovastatin twice daily + 10 g colestipol twice daily for 48‐54 weeks 40 mg/day fluvastatin for 12‐24 weeks 40 mg/day fluvastatin + 5 g colestipol twice daily for 24‐36 weeks 40 mg/day fluvastatin + 10 g colestipol twice daily for 36‐54 weeks groups were not analysed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3.5% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Parke‐Davis Pharmaceutical Research funded the trial |
Hunninghake 2002.
| Methods | 4‐week placebo run‐in period 24‐week before and after trial |
|
| Participants | 555 men and women with primary hypercholesterolaemia (IIa or IIb) LDL‐C ≥ 4.1 mmol/L (159 mg/dL) triglycerides ≤ 4.5 mmol/L (399 mg/dL) exclusion criteria: homozygous familial hypercholesterolaemia, Type I, III, IV, V hyperlipoproteinaemia, secondary hyperlipidaemia pregnancy, childbearing potential, any current condition that might affect drug pharmacokinetics, acute illness or trauma during the previous 3 months, uncontrolled hyperthyroidism MI, major cardiac surgery or angioplasty during the prior 6 months severe or unstable angina pectoris, uncontrolled congestive heart failure or hypertension, musculoskeletal disease history of drug abuse, probucol use within 1 year of trial and statin hypersensitivity |
|
| Interventions | Fluvastatin IR 40 mg/day for 24 weeks Fluvastatin XL 80 mg/day for 24 weeks |
|
| Outcomes | per cent change from baseline at 4‐12 weeks of blood LDL‐C | |
| Source of Funding | Novartis | |
| Notes | 12‐24 week data were not included in the efficacy analysis SD was imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Novartis funded the trial |
Hussein 2002.
| Methods | no patient received lipid medications prior to entrance into the study 6‐week low lipid diet period 4‐month before and after trial |
|
| Participants | 21 patients with hypertension and hyperlipidaemia exclusion criteria:chronic renal disease, hepatic disease, unstable angina, congestive heart failure cancer or hematologic disease, alcohol or drug abuse,psychiatric disease pregnancy patients currently under treatment with statins, angiotensin II antagonists or ACE inhibitors |
|
| Interventions | 7 patients received fluvastatin 40 mg/day for 2 months 7 patients received fluvastatin 40 mg/day + valsartan 80 mg/day for 2‐4 months 8 patients received valsartan 80 mg/day for 2 months 8 patients received valsartan 80 mg/day + fluvastatin 40 mg/day for 2‐4 months 6 patients received fluvastatin 40 mg/day for 4 months |
|
| Outcomes | per cent change from baseline at 2 months of plasma TC and LDL‐C | |
| Source of Funding | unknown | |
| Notes | 7 patients received fluvastatin 40 mg/day + valsartan 80 mg/day for 2‐4 months 8 patients received valsartan 80 mg/day for 2 months 8 patients received valsartan 80 mg/day + fluvastatin 40 mg/day for 2‐4 months 6 patients received fluvastatin 40 mg/day for 4 months groups were not included in the efficacy analysis SDs were imputed by the method of Furikawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Ichihara 2002.
| Methods | no washout required because no participants were receiving any lipid medication 6‐month randomised double‐blind placebo‐controlled trial |
|
| Participants | 22 haemodialysis patients with type 2 diabetes mellitus on haemodialysis for 6‐60 months no clinical cardiovascular disease, no secondary hyperparathyroidism or adynamic bone disease exclusion criteria: pre‐menopausal women, HRT,dietary supplements, endocrine‐metabolic disorders other than diabetes or drugs that may effect lipid metabolism smokers, ethanol consumption > 40 g for men > 20 g for women Placebo baseline TC : 3.88 mmol/L (150 mg/dL) Placebo baseline LDL‐C : 2.07 mmol/L (80 mg/dL) Placebo baseline HDL‐C : 1.16 mmol/L (45 mg/dL) Placebo baseline triglycerides: 1.05 mmol/L (93 mg/dL) Fluvastatin 20 mg/day baseline TC : 4.34 mmol/L (168 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 2.38 mmol/L (92 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.29 mmol/L (50 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 1.06 mmol/L (94 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day | |
| Outcomes | per cent change from baseline at 3 months of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Source of Funding | unknown | |
| Notes | WDAEs were not reported SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo and fluvastatin capsule appearances were not reported as appearing identical Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | WDAEs were not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Inoue 2011.
| Methods | no washout period required because no patient was receiving hypolipidaemic treatment 3‐month before and after trial |
|
| Participants | 10 men and women with hypertension and hypercholesterolaemia TC ≥ 220 mg/dL (5.69 mmol/L) LDL‐C ≥ 120 mg/dL (3.10 mmol/L) exclusion criteria: none reported Fluvastatin 20 mg/day baseline TC : 5.61 mmol/L (217 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 3.83 mmol/L (148 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.42 mmol/L (55 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 1.59 mmol/L (141 mg/dL) |
|
| Interventions | fluvastatin 20 mg/day | |
| Outcomes | per cent change from baseline at 3‐6 weeks of serum TC, LDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | HDL‐C data were not included in the efficacy analysis because the calculated value was different by more than 10% from the given value SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | all participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Insull 1994.
| Methods | 6‐week placebo washout period 6‐week randomised double‐blind placebo‐controlled trial |
|
| Participants | 207 men and women with primary hypercholesterolaemia (type IIa of IIb) LDL‐C ≥4.15 mmol/L (160 mg/dL) triglycerides levels of ≤ 3.38 mmol/L (299 mg/dL) exclusion criteria: unstable or severe angina pectoris, MI,coronary angioplasty or coronary artery surgery within 6 months of trial, congestive heart failure, secondary hypercholesterolaemia, uncontrolled hypertension,liver dysfunction, steroid treatment, use of anticoagulant drugs other than aspirin or dipyridamole in stable doses, women of childbearing potential and HRT Placebo baseline TC : 7.5 mmol/L (290 mg/dL) Placebo baseline LDL‐C : 5.5 mmol/L (213 mg/dL) Placebo baseline HDL‐C : 1.3 mmol/L (50 mg/dL) Placebo baseline triglycerides: 1.5 mmol/L (133 mg/dL) Fluvastatin 20 mg/day baseline TC : 7.6 mmol/L (294 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 5.6 mmol/L (217 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.3 mmol/L (50 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 1.55 mmol/L (137 mg/dL) |
|
| Interventions | placebo for 6 weeks Fluvastatin 10 mg twice daily for 6 weeks Fluvastatin 20 mg/day for 6 weeks |
|
| Outcomes | per cent change from baseline at 3‐6 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides and WDAEs | |
| Source of Funding | Sandoz | |
| Notes | Fluvastatin 10 mg twice daily for 6 weeks Fluvastatin 20 mg/day for 6 weeks groups were combined |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo and fluvastatin were formulated in identical‐appearing capsules |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | LDL‐C was determined at a central laboratory (Medical Research laboratories [MRL], Cincinnati,Ohio) |
| Blinding of outcome assessment (detection bias) WDAEs | Unclear risk | Blinding method not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Sandoz funded the trial |
Isaacsohn 1999.
| Methods | 10‐week washout period 12‐week before and after trial |
|
| Participants | 197 men and women aged 18 to 75 years with documented primary hypercholesterolaemia LDL‐C ≥ 157.5 mg/dL (4.07 mmol/L) or ≥ 130 mg/dL (3.36 mmol/L) with documented coronary artery disease of two or more cardiovascular risk factors plasma triglycerides ≤ 400 mg/dL (4.52 mmol/L) have a food rating score ≤ 15 exclusion criteria: clinically active cardiovascular disease, hypertension with alterations in diuretic or beta blocker therapy within two months of entry uncontrolled diabetes mellitus or other endocrine abnormalities and uncontrolled hypothyroidism ophthalmic abnormalities, cancer other than basil cell or squamous cell carcinoma, psychosis hepatic dysfunction, weight . 140% ideal body weight, statin hypersensitivity, significant GI tract disorders, child‐bearing potential homozygous familial hypercholesterolaemia, renal dysfunction, current use of other medications that would interfere with the trial treatment with other hypolipidaemic drugs within 10 weeks of entry, drug or alcohol abuse, night shift workers therapy with another investigational product within 30 days, other medical conditions which might interfere with the trial Fluvastatin 20 mg/day baseline TC : 6.89 mmol/L (268 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 4.76 mmol/L (184 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.25 mmol/L (48 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 1.94 mmol/L (172 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day for 0‐6 weeks Fluvastatin 40 mg/day for 6‐12 weeks Cerivastatin 0.2 mg/day for 0‐6 weeks Cerivastatin 0.3 mg/day for 6‐12 weeks |
|
| Outcomes | per cent change from baseline at 6 weeks of plasma TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | Novartis | |
| Notes | Fluvastatin 40 mg/day for 6‐12 weeks Cerivastatin 0.2 mg/day for 0‐6 weeks Cerivastatin 0.3 mg/day for 6‐12 weeks groups were not included in the efficacy analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13.7% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Novartis funded the trial |
Isaacsohn 2003.
| Methods | 4‐week run‐in period 12‐week before and after trial |
|
| Participants | 173 men and women at least 18 years of age primary hypercholesterolaemia TG ≤ 400 mg/dL (4.52 mmol/L) and LDL‐C levels ≥ pre established levels that were based on the presence or absence of atherosclerotic disease and other risk factors for CHD exclusion criteria: active liver disease or hepatic dysfunction, impaired renal function Fluvastatin 40 mg/day baseline TC : 7.01 mmol/L (271 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 4.78 mmol/L (185 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.27 mmol/L (49 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 2.15 mmol/L (190 mg/dL) Fluvastatin 80 mg/day baseline TC : 6.83 mmol/L (264 mg/dL) Fluvastatin 80 mg/day baseline LDL‐C : 4.68 mmol/L (181 mg/dL) Fluvastatin 80 mg/day baseline HDL‐C : 1.24 mmol/L (48 mg/dL) Fluvastatin 80 mg/day baseline triglycerides: 2.01 mmol/L (178 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day Fluvastatin 80 mg/day |
|
| Outcomes | per cent change from baseline at 4‐12 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | SDs were imputed by the method of Furikawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1.2% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Itakura 1995.
| Methods | 6‐week dietary period with last 4 weeks placebo run‐in period 8‐week before and after trial |
|
| Participants | 114 men and women age 39‐60 years old with type IIa or IIb hypercholesterolaemia Total cholesterol 220‐430 mg/dL (5.69‐11.12 mmol/L) LDL‐C 133.4‐355.6 mg/dL (3.45‐9.20 mmol/L) HDL‐C 26‐94 mg/dL (0.67‐2.43 mmol/L) Triglycerides 35‐1239 mg/dL (0.40‐14.0 mmol/L) exclusion criteria: hypothyroidism, Cushings disease, gallbladder disease, pancreatitis, cancer, unstable diabetes, severe hypertension, alcohol abuse, obese people on diet, renal, liver dysfunction, brain disease, heart disease statin hypersensitivity and lupus Fluvastatin 2.5 mg/day baseline TC : 7.42 mmol/L (287 mg/dL) Fluvastatin 2.5 mg/day baseline LDL‐C : 5.15 mmol/L (199 mg/dL) Fluvastatin 2.5 mg/day baseline HDL‐C : 1.33 mmol/L (51 mg/dL) Fluvastatin 2.5 mg/day baseline triglycerides: 2.50 mmol/L (221 mg/dL) Fluvastatin 5 mg/day baseline TC : 7.52 mmol/L (291 mg/dL) Fluvastatin 5 mg/day baseline LDL‐C : 5.56 mmol/L (215 mg/dL) Fluvastatin 5 mg/day baseline HDL‐C : 1.27 mmol/L (49 mg/dL) Fluvastatin 5 mg/day baseline triglycerides: 1.54 mmol/L (136 mg/dL) Fluvastatin 10 mg/day baseline TC : 7.34 mmol/L (284 mg/dL) Fluvastatin 10 mg/day baseline LDL‐C : 5.22 mmol/L (202 mg/dL) Fluvastatin 10 mg/day baseline HDL‐C : 1.32 mmol/L (51 mg/dL) Fluvastatin 10 mg/day baseline triglycerides: 1.92 mmol/L (170 mg/dL) Fluvastatin 20 mg/day baseline TC : 7.53 mmol/L (291 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 5.40 mmol/L (209 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.33 mmol/L (51 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 2.04 mmol/L (181 mg/dL) |
|
| Interventions | Fluvastatin 2.5 mg/day for 4‐8 weeks Fluvastatin 5 mg/day for 4‐8 weeks Fluvastatin 10 mg/day for 4‐8 weeks Fluvastatin 20 mg/day for 4‐8 weeks |
|
| Outcomes | per cent change from baseline at 4‐8 weeks of serum TC and LDL‐C | |
| Source of Funding | unknown | |
| Notes | HDL‐C and triglyceride data were not included in the efficacy analysis because the calculated values were different by more than 10% from the given values for all the doses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11.4% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Ito 1995.
| Methods | 4‐week washout period 52‐week before and after trial |
|
| Participants | 45 men and women aged 20‐70 years of age with type IIa and IIb hypercholesterolaemia with BMI = 24.1 23 participants received fluvastatin Total cholesterol 224.0‐376.0 mg/dL (5.79‐9.72 mmol/L) LDL‐C 117.6‐255.6 mg/dL (3.04‐6.61 mmol/L) HDL‐C 32.5‐77.0 mg/dL (0.84‐1.99 mmol/L) Triglycerides 78.5‐451.5 mg/dL (0.89‐5.10 mmol/L) 23 participants were randomised to fluvastatin and 22 participants were randomised to probucol exclusion criteria: hypothyroidism, Cushings disease, gallbladder disease, pancreatitis, cancer, unstable diabetes, severe hypertension, alcohol abuse, obese people on diet, renal, liver dysfunction, brain disease, heart disease statin hypersensitivity and lupus Fluvastatin 20 mg/day baseline TC : 7.15 mmol/L (276 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 4.79 mmol/L (185 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.43 mmol/L (55 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 1.89 mmol/L (167 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day for 0‐12 weeks Fluvastatin 30 mg/day for 12‐24 weeks Fluvastatin 20‐40 mg/day for 24‐52 weeks Probucol 500 mg/day for 52 weeks |
|
| Outcomes | per cent change from baseline at 4‐12 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | Fluvastatin 30 mg/day for 12‐24 weeks Fluvastatin 20‐40 mg/day for 24‐52 weeks Probucol 500 mg/day for 52 weeks groups were not included in the efficacy analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4.3% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Jacobson 1994.
| Methods | 8‐week drug washout/dietary initiation period 6‐week dietary/placebo washout period 6‐week randomised double‐blind placebo‐controlled trial |
|
| Participants | 74 men and women aged 21‐70 years LDL cholesterol levels ≥160 mg/dL (4.14 mmol/L) triglycerides ≤350 mg/dL (3.95 mmol/L) exclusion criteria:homozygous familial hypercholesterolaemia, active peptic ulcer or gout, recent MI, congestive heart failure, severe or unstable angina pectoris, uncontrolled hypertension and secondary hyperlipidaemia Placebo baseline TC : 7.5 mmol/L (290 mg/dL) Placebo baseline LDL‐C : 5.3 mmol/L (205 mg/dL) Placebo baseline HDL‐C : 1.3 mmol/L (50 mg/dL) Placebo baseline triglycerides: 0.8 mmol/L (71 mg/dL) Fluvastatin 20 mg/day baseline TC : 7.6 mmol/L (294 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 5.5 mmol/L (213 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.4 mmol/L (54 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 0.8 mmol/L (71 mg/dL) |
|
| Interventions | Placebo for 6 weeks Placebo and 3 gram niacin/day for 6‐15 weeks Fluvastatin 20 mg/day for 6 weeks Fluvastatin 20 mg/day and 3 gram niacin/day for 6‐15 weeks |
|
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides and WDAEs | |
| Source of Funding | unknown | |
| Notes | Placebo and 3 g niacin/day for 6‐15 weeks Fluvastatin 20 mg/day and 3 g niacin/day for 6‐15 weeks groups were not analysed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind treatment placebo and fluvastatin capsule appearances were not reported as appearing identical Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | Low risk | There were no withdrawals for subjects receiving fluvastatin monotherapy |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding not reported |
Jacotot 1994.
| Methods | 8‐week dietary stabilisation period 6‐week placebo phase 6‐week randomised, double‐blind placebo‐controlled trial |
|
| Participants | 431 randomised men and women age 18‐70 years LDL‐C ≥4.1 mmol/L (159 mg/dL) TG ≤ 3.4 mmol/L (301 mg/dL) exclusion criteria: childbearing potential, homozygous familial hypercholesterolaemia, type I, III, IV and V hyperlipoproteinaemia serious surgical of medical conditions (cardiovascular, GI, ophthalmic, hepatic , renal dysfunction) Placebo baseline TC : 8.8 mmol/L (340 mg/dL) Placebo baseline LDL‐C : 6.5 mmol/L (251 mg/dL) Placebo baseline HDL‐C : 1.4 mmol/L (54 mg/dL) Placebo baseline triglycerides: 1.6 mmol/L (142 mg/dL) Fluvastatin 2.5 mg/day baseline TC : 9.0 mmol/L (348 mg/dL) Fluvastatin 2.5 mg/day baseline LDL‐C : 6.7 mmol/L (259 mg/dL) Fluvastatin 2.5 mg/day baseline HDL‐C : 1.4 mmol/L (54 mg/dL) Fluvastatin 2.5 mg/day baseline triglycerides: 1.6 mmol/L (142 mg/dL) Fluvastatin 5 mg/day baseline TC : 8.9 mmol/L (344 mg/dL) Fluvastatin 5 mg/day baseline LDL‐C : 6.8 mmol/L (263 mg/dL) Fluvastatin 5 mg/day baseline HDL‐C : 1.4 mmol/L (54 mg/dL) Fluvastatin 5 mg/day baseline triglycerides: 1.4 mmol/L (124 mg/dL) Fluvastatin 10 mg/day baseline TC : 8.5 mmol/L (329 mg/dL) Fluvastatin 10 mg/day baseline LDL‐C : 6.2 mmol/L (240 mg/dL) Fluvastatin 10 mg/day baseline HDL‐C : 1.4 mmol/L (54 mg/dL) Fluvastatin 10 mg/day baseline triglycerides: 1.6 mmol/L (142 mg/dL) Fluvastatin 20 mg/day baseline TC : 8.6 mmol/L (333 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 6.3 mmol/L (244 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.4 mmol/L (54 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 1.6 mmol/L (142 mg/dL) |
|
| Interventions | Placebo for 6 weeks Fluvastatin 2.5 mg/day for 6 weeks Fluvastatin 5 mg/day for 6 weeks Fluvastatin 10 mg/day for 6 weeks Fluvastatin 20 mg/day for 6 weeks |
|
| Outcomes | per cent change from baseline at 4‐6 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides and WDAEs | |
| Source of Funding | unknown | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind treatment placebo and fluvastatin capsule appearances were not reported as appearing identical Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | LDL‐C was determined at a central laboratory (SERLIA, Institut‐Pasteur, Lille, France) |
| Blinding of outcome assessment (detection bias) WDAEs | Unclear risk | Blinding method not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1.4 % participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding not reported |
Jacotot 1995.
| Methods | 6‐week placebo run‐in period 16‐week before and after trial |
|
| Participants | 68 male and female participants aged 18‐75 years with LDL‐C ≥ 160 mg/dL (4.14 mmol/L) received fluvastatin and 66 received pravastatin triglycerides ≤ 400 mg/dL (4.52 mmol/L) exclusion criteria: homozygous familial hypercholesterolaemia, hyperlipidaemia type I, III, IV or V impaired renal or liver function Fluvastatin 40 mg/day baseline TC : 7.7 mmol/L (298 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 5.6 mmol/L (217 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.3 mmol/L (50 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 1.7 mmol/L (151 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day for 4 weeks Fluvastatin 40 mg twice daily for 4‐16 weeks Pravastatin 20 mg/day for 4 weeks Pravastatin 40 mg/day for 4‐16 weeks |
|
| Outcomes | per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | Fluvastatin 40 mg twice daily for 4‐16 weeks Pravastatin 20 mg/day for 4 weeks Pravastatin 40 mg/day for 4‐16 weeks groups were not analysed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4.4% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Jarai 1996.
| Methods | 8‐week dietary washout period 12‐week before and after trial |
|
| Participants | 43 patients with hypercholesterolaemia and essential hypertension BMI = 24.7 TC ≥ 6.5 mmol/L (251 mg/dL) TG < 4.6 mmol/L (407 mg/dL) exclusion criteria: secondary hypertension, familial hypercholesterolaemia, type I, III, IV, V hyperlipidaemia hyperlipoproteinaemia with TG > 4.6 mmol/L (407 mg/dL) Obstructive liver or biliary tract disease, gallbladder disease pancreatitis, autoimmune disease, alcoholism, macroglobulinaemia chronic porphyria, musculoskeletal disorders, renal dysfunction MI or angioplasty within 6 months of study, congestive heart failure II‐IV, unstable angina pectoris uncontrolled hypertension, diabetes mellitus , extreme obesity, statin hypersensitivity Fluvastatin 20 mg/day baseline TC : 7.22 mmol/L (279 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 5.13 mmol/L (198 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 2.02 mmol/L (179 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day | |
| Outcomes | per cent change from baseline at 3‐6 weeks of serum TC, LDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | SDs were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16.3% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Jokubaitis 1994.
| Methods | 4‐week washout and an 8 week dietary stabilisation phase 12‐week randomised double‐blind placebo‐controlled trial |
|
| Participants | 66 men and women with hyperlipidaemia age 40‐70 years with NIDDM TC > 200 mg/dL (5.17 mmol/L) 130 mg/dL < LDL‐C ≤ 300 mg/dL (3.36mmol/L < LDL‐C ≤ 7.76 mmol/L) 200 mg/dL < TG ≤ 1000 mg/dL ( 2.26 mmol/L < TG ≤ 11.3 mmol/L) exclusion criteria: secondary or hereditary lipid disease, cardiovascular disease, prohibited medication use, organ dysfunction, childbearing potential Placebo baseline TC : 7.3 mmol/L (282 mg/dL) Placebo baseline LDL‐C : 4.4 mmol/L (170 mg/dL) Placebo baseline HDL‐C : 1.0 mmol/L (39 mg/dL) Placebo baseline triglycerides: 3.9 mmol/L (345 mg/dL) Fluvastatin 20 mg/day baseline TC : 7.4 mmol/L (286 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 4.4 mmol/L (170 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.0 mmol/L (39 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 3.9 mmol/L (345 mg/dL) |
|
| Interventions | Placebo for 6 weeks Placebo for 6‐12 weeks Fluvastatin 20 mg/day for 6 weeks Fluvastatin 20 mg twice daily for 6‐12 weeks |
|
| Outcomes | per cent change from baseline at 0‐6 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and WDAEs | |
| Source of Funding | Sandoz | |
| Notes | Placebo for 6‐12 weeks Fluvastatin 20 mg twice daily for 6‐12 weeks groups were not analysed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo and fluvastatin capsule appearances were not reported as appearing identical Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | Low risk | No discontinuations were as a result of adverse events |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4.5% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Sandoz funded the study |
Khan 1999.
| Methods | no patient was receiving lipid‐lowering agents therefore washout not required 24‐week before and after trial |
|
| Participants | 27 patients with hypercholesterolaemia and lower limb PAOD of these 17 participants received fluvastatin LDL cholesterol > 4.1 mmol/L (159 mg/dL) exclusion criteria:diabetes mellitus, hypertension Fluvastatin 40 mg/day baseline TC : 7.3 mmol/L (292 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 5.4 mmol/L (208 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.2 mmol/L (46 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 1.5 mmol/L (133 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day | |
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | Sir Jules Thorn Charitable Trust | |
| Notes | SDs were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Low risk | Funded by the Sir Jules Thorn Charitable Trust |
Klosiewicz‐Latoszek 2003.
| Methods | no washout period required because no patient was receiving hypolipidaemic treatment 12‐week before and after trial |
|
| Participants | 180 men and women with mixed hyperlipidaemia and high risk for coronary heart disease age 35‐70 years old 20 participants received fluvastatin TC 5.2‐10.0 mmol/L (201‐387 mg/dL) TG 2.3‐10.0 mmol/L (204‐886 mg/dL) exclusion criteria: participants receiving drugs that may affect the lipid profile such as diuretics beta blockers use of glucocorticoids and if BMI changed by 2 during the trial Fluvastatin 40 mg/day baseline TC : 7.9 mmol/L (305 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 5.7 mmol/L (220 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.1 mmol/L (43 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 4.3 mmol/L (381 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day Simvastatin 20 mg/day Lovastatin 20 mg/day Atorvastatin 10 mg/day Fluvastatin 40 mg/day + fibrate Simvastatin 20 mg/day + fibrate Lovastatin 20 mg/day + fibrate Atorvastatin 10 mg/day + fibrate |
|
| Outcomes | per cent change from baseline at 8‐12 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | Simvastatin 20 mg/day Lovastatin 20 mg/day Atorvastatin 10 mg/day Fluvastatin 40 mg/day + fibrate Simvastatin 20 mg/day + fibrate Lovastatin 20 mg/day + fibrate Atorvastatin 10 mg/day + fibrate groups were not included in the efficacy analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Koren 1999.
| Methods | 4‐week washout period 54‐week before and after trial |
|
| Participants | 308 men and women age 18‐80 years BMI ≤32, documented atherosclerosis and LDL‐C 130‐250 mg/dL (3.36‐6.465 mmol/L) exclusion criteria: none stated no lipid baseline values reported |
|
| Interventions | Fluvastatin 20 mg/day for 0‐12 weeks Fluvastatin 20‐40 mg/day for 12‐24 or 54 weeks Atorvastatin 10 mg/day Atorvastatin 20 mg/day Atorvastatin 40 mg/day Atorvastatin 80 mg/day Lovastatin 20 mg/day Lovastatin 40 mg/day Lovastatin 80 mg/day Simvastatin 10 mg/day Simvastatin 20 mg/day Simvastatin 40 mg/day |
|
| Outcomes | per cent change from baseline at 12 weeks of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Source of Funding | unknown | |
| Notes | Fluvastatin 20‐40 mg/day for 12‐24 or 54 weeks Atorvastatin 10 mg/day Atorvastatin 20 mg/day Atorvastatin 40 mg/day Atorvastatin 80 mg/day Lovastatin 20 mg/day Lovastatin 40 mg/day Lovastatin 80 mg/day Simvastatin 10 mg/day Simvastatin 20 mg/day Simvastatin 40 mg/day groups were not included in the efficacy analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Kowalski 2006.
| Methods | 4‐week dietary run‐in period 6‐week before and after trial |
|
| Participants | 35 men and women with mixed hyperlipidaemia age 35‐47 and BMI >25, low physical activity and family history of CHD 18 participants received fluvastatin TC > 300 mg/dL (7.76 mmol/L) LDL‐C 170 mg/dL (4.4 mmol/L) TG > 200 mg/dL (2.26 mmol/L) exclusion criteria: childbearing potential no baseline values |
|
| Interventions | Fluvastatin 40 mg/day Atorvastatin 10 mg/day |
|
| Outcomes | per cent change from baseline at 8‐12 weeks of serum TC, LDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | Atorvastatin group was not included in the efficacy analysis SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Kozlov 2000.
| Methods | 8‐week dietary run‐in period 12‐week before and after trial |
|
| Participants | 40 men and women with type 2 diabetes mellitus with hypercholesterolaemia and combined hyperlipidaemia age 40‐60 years old 21 patients had hypercholesterolaemia and 19 had combined hyperlipidaemia 40 patients received fluvastatin Hypercholesterolemia defined as LDL cholesterol more than 2.6 mmol /L (100 mg/L) with normal triglyceride levels less than 2.3 mmol / L (204 mg/dL), combined hyperlipidaemia with LDL cholesterol more than 2.6 mmol/L (100 mg/dL) and triglycerides more than 2.3 mmol /L(204 mg/dL). exclusion criteria: patients younger than 35 or older than 70 years, unstable angina, MI, balloon dilatation or coronary artery bypass surgery within 6 months from the start of the study AST/ALT levels ≥ 20% ULN, TG > 4.5 mmol/L (400 mg/dL), elevated creatinine, congestive heart failure, type 1 diabetes mellitus, homozygous familial hypercholesterolaemia women that may become pregnant, ventricular arrhythmias, drugs that might affect lipid metabolism and disposition Fluvastatin 40 mg/day baseline TC : 7.54 mmol/L (292 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 5.47 mmol/L (212 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.02 mmol/L (39 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 2.3 mmol/L (204 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day Fenofibrate 200 mg/day |
|
| Outcomes | per cent change from baseline at 4‐12 weeks of blood TC, LDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | Fenofibrate 200 mg/day group was not included in the efficacy analysis HDL‐C data were not included in the efficacy analysis because the calculated value was different by more than 10% from the given value SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Lan 2001.
| Methods | 4‐6 week washout period before screening 4‐week placebo run‐in period 24‐week before and after trial |
|
| Participants | 72 men and women with familial hypercholesterolaemia age 20‐75 years old LDL‐C ≥3.5 mmol/L (135 mg/dL) with an additional cardiovascular risk factor LDL‐C ≥3.0 mmol/L (116 mg/dL) with known CHD or other atherosclerotic disease TG level ≥2.3 mmol/L (204 mg/dL) exclusion criteria: pregnancy or lactation or childbearing potential alcohol consumption greater than 10 drinks per week confounding medications acute or chronic liver disease MI, severe or unstable angina pectoris, PTCA, CABG, stroke, carotid endarterectomy, or other major vascular surgery within the previous 3 months type 1 or uncontrolled type 2 diabetes mellitus uncontrolled hypertension, secondary hypercholesterolaemia, BMI ≥30 partial ileal bypass and statin hypersensitivity or any other condition or therapy that might compromise patient safety or successful study participation Fluvastatin 20 mg/day baseline TC : 8.0 mmol/L (309 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 6.4 mmol/L (247 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.165 mmol/L (45 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day from week 4‐8 Fluvastatin 40 mg/day from week 8‐16 Fluvastatin 40 mg/day + 300 mg twice daily gemfibrozil from week 16‐20 Fluvastatin 40 mg/day + 600 mg twice daily gemfibrozil from week 20‐24 |
|
| Outcomes | per cent change from baseline at 4‐8 weeks of serum TC, LDL‐C and HDL‐C | |
| Source of Funding | unknown | |
| Notes | Fluvastatin 40 mg/day from week 8‐16 Fluvastatin 40 mg/day + 300 mg twice daily gemfibrozil from week 16‐20 Fluvastatin 40 mg/day + 600 mg twice daily gemfibrozil from week 20‐24 groups were not included in the efficacy analysis triglycerides were not included in the efficacy analysis because the values were expressed as a geometric mean SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 12.5% of participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
LCAS 1997.
| Methods | 6‐week diet stabilisation and placebo washout period 130‐week randomised double‐blind placebo‐controlled trial |
|
| Participants | 429 men and women age 35‐75 years LDL‐C 115‐190 mg/dL (2.97‐4.91 mmol/L) TG ≤300 mg/dL (3.39 mmol/L) in all patients and ≤ 250 mg/dL (2.82 mmol/L) inpatients who would be assigned cholestyramine angiographic evidence of ≥1 coronary lesion causing 30% to 75% diameter stenosis by calliper measurement in a coronary artery untreated by angioplasty and not 100% occluded ≥2 of the 3 major coronary arteries be evaluable by angiography, untreated by angioplasty and <100% occluded exclusion criteria:>50% stenosis in he left main coronary artery, prior CABG, uncontrolled hypertension, type 1 diabetes or treated type 2 diabetes mellitus probucol could not have been taken within 1 year of randomisation Placebo baseline TC : 5.45 mmol/L (211 mg/dL) Placebo baseline LDL‐C : 3.52 mmol/L (136 mg/dL) Placebo baseline HDL‐C : 1.14 mmol/L (44 mg/dL) Placebo baseline triglycerides: 1.76 mmol/L (156 mg/dL) Fluvastatin 40 mg/day baseline TC : 5.51 mmol/L (213 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 3.54 mmol/L (137 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.11 mmol/L (43 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 1.86 mmol/L (165 mg/dL) |
|
| Interventions | Placebo twice daily for 0‐12 weeks Fluvastatin 20 mg twice daily for 0‐12 weeks Placebo twice daily + CME 4 g/day for 12‐18 weeks Fluvastatin 20 mg twice daily + CME 4 g/day for 12‐18 weeks Placebo twice daily + CME 8 g/day for 18‐24 weeks Fluvastatin 20 mg twice daily + CME 8 g/day for 18‐24 weeks Placebo twice daily + CME 12 g/day for 24‐130 weeks Fluvastatin 20 mg twice daily + CME 12 g/day for 24‐130 weeks |
|
| Outcomes | per cent change from baseline at 3‐6 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | Sandoz | |
| Notes | Placebo twice daily + CME 4 g/day for 12‐18 weeks Fluvastatin 20 mg twice daily + CME 4 g/day for 12‐18 weeks Placebo twice daily + CME 8 g/day for 18‐24 weeks Fluvastatin 20 mg twice daily + CME 8 g/day for 18‐24 weeks Placebo twice daily + CME 12 g/day for 24‐130 weeks Fluvastatin 20 mg twice daily + CME 12 g/day for 24‐130 weeks groups were not included in the efficacy analysis WDAEs were not reported for the 0‐12 week time period |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | WDAEs were not reported for the 0‐12 week time period |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Sandoz funded the study |
Leitersdorf 1994.
| Methods | 4‐week placebo run‐in period 16‐week before and after trial |
|
| Participants | 63 men and women > 18 years old dominant inherited hypercholesterolaemia (familial) LDL‐C > 4.9 mmol/L (189 mg/dL) triglycerides levels < 3.4 mmol/L (301 mg/dL) participants had to have tendon xanthomas or ischaemic heart disease LDL receptor gene mutation or a co segregating LDL receptor haplotype and hypercholesterolaemia in the patient's families Fluvastatin 5 mg/day baseline LDL‐C : 7.3 mmol/L (282 mg/dL) Fluvastatin 5 mg/day baseline HDL‐C : 0.89 mmol/L (34 mg/dL) |
|
| Interventions | Fluvastatin 5 mg/day for 4 weeks Fluvastatin 10 mg/day for 4‐8 weeks Fluvastatin 20 mg/day for 8‐12 weeks Fluvastatin 40 mg/day for 12‐16 weeks |
|
| Outcomes | per cent change from baseline at 4 weeks of serum LDL‐C and HDL‐C | |
| Source of Funding | unknown | |
| Notes | Fluvastatin 10 mg/day for 4‐8 weeks Fluvastatin 20 mg/day for 8‐12 weeks Fluvastatin 40 mg/day for 12‐16 weeks groups were not analysed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1.6% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | The source of funding was not reported |
Leitersdorf 1995.
| Methods | 8‐week washout dietary stabilisation period 60‐week before and after trial |
|
| Participants | 22 men and women with heterozygous familial hypercholesterolaemia who completed 3 previous studies and whose plasma LDL‐C levels did not, at any time, reach the target of 155 mg/dL (4.0 mmol/L) exclusion criteria: serious drug‐related adverse event or deterioration of liver or kidney function during the previous studies Fluvastatin 40 mg/day baseline TC : 9.36 mmol/L (362 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 7.66 mmol/L (296 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 0.94 mmol/L (36 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 1.66 mmol/L (147 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day for 6 weeks Fluvastatin 40 mg/day + 400 mg/day bezafibrate for 6‐12 weeks Fluvastatin 40 mg/day + 400 mg/day bezafibrate + 8 g/day cholestyramine for 12‐60 weeks |
|
| Outcomes | per cent change from baseline at 6 weeks of plasma TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | Fluvastatin 40 mg/day + 400 mg/day bezafibrate for 6‐12 weeks Fluvastatin 40 mg/day + 400 mg/day bezafibrate + 8 g/day cholestyramine for 12‐60 weeks were not included in the efficacy analysis SDs were imputed by the method of Furukawa 2006 for LDL‐C and HDL‐C because the given SDs were < 9 for LDL‐C and < 9.6 for HDL‐C |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Leonhardt 1997.
| Methods | 4‐week dietary washout period 8‐week randomised, double‐blind placebo‐controlled trial |
|
| Participants | 20 men and women age 50‐60 years with hypercholesterolaemia TC > 5.2 mmol/L (201 mg/dL) LDL‐C > 4.1 mmol/L ( 159 mg/dL) TG < 3.5 mmol/L (310 mg/dL) exclusion criteria:therapy with lipid‐lowering supplements, steroid hormones except oral contraceptives, immunosuppressants, aluminium antacids erythromycin, ketoconazole or analogs, p‐aminoacetic acid Placebo baseline TC : 8.89 mmol/L (343 mg/dL) Placebo baseline LDL‐C : 6.91 mmol/L (267 mg/dL) Placebo baseline HDL‐C : 1.15 mmol/L (44 mg/dL) Placebo baseline triglycerides: 1.82 mmol/L (161 mg/dL) Fluvastatin 40 mg twice daily baseline TC : 8.13 mmol/L (314 mg/dL) Fluvastatin 40 mg twice daily baseline LDL‐C : 5.94 mmol/L (230 mg/dL) Fluvastatin 40 mg twice daily baseline HDL‐C : 1.26 mmol/L (49 mg/dL) Fluvastatin 40 mg twice daily baseline triglycerides: 1.91 mmol/L (169 mg/dL) |
|
| Interventions | Placebo for 8 weeks Fluvastatin 40 mg twice daily for 8 weeks |
|
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | no WDAEs were reported SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Method of random sequence generation was not reported |
| Allocation concealment (selection bias) | High risk | No allocation concealment was reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo and fluvastatin capsule appearances were not reported as appearing identical Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No WDAEs were reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Leu 2004.
| Methods | no washout period required because no patient was receiving hypolipidaemic treatment 12‐week randomised, double‐blind, placebo‐controlled trial |
|
| Participants | 43 patients with hypercholesterolaemia and LDL‐C > 160 mg/dL (4.14 mmol/L) triglycerides < 400 mg/dL (4.52 mmol/L) exclusion criteria: uncontrolled hypertension, diabetes mellitus chronic liver disease, renal dysfunction, current tobacco smokers and a history of other cardiovascular disease significant coronary artery disease Placebo baseline TC : 6.742 mmol/L (261 mg/dL) Placebo baseline LDL‐C : 4.841 mmol/L (187 mg/dL) Placebo baseline HDL‐C : 1.239 mmol/L (48 mg/dL) Placebo baseline triglycerides: 1.839 mmol/L (163 mg/dL) Fluvastatin 80 mg/day baseline TC : 7.024 mmol/L (272 mg/dL) Fluvastatin 80 mg/day baseline LDL‐C : 4.919 mmol/L (190 mg/dL) Fluvastatin 80 mg/day baseline HDL‐C : 1.283 mmol/L (50 mg/dL) Fluvastatin 80 mg/day baseline triglycerides: 1.887 mmol/L (167 mg/dL) |
|
| Interventions | Placebo Fluvastatin 80 mg/day |
|
| Outcomes | per cent change from baseline at 12 weeks of plasma TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | WDAEs were not reported SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random sequence generation method not reported |
| Allocation concealment (selection bias) | High risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind treatment placebo and fluvastatin capsule appearances were not reported as appearing identical Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | All lab samples were analysed in duplicate by an individual blinded to treatment protocol |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | WDAEs were not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Leu 2005.
| Methods | no washout period required because no patient was receiving hypolipidaemic treatment or supplements 12‐week randomised, double‐blind, placebo‐controlled trial |
|
| Participants | 51 men and women with hypercholesterolaemia and LDL‐C > 160 mg/dL (4.14 mmol/L) triglycerides < 400 mg/dL (4.52 mmol/L) exclusion criteria: uncontrolled hypertension and diabetes mellitus chronic liver disease, acute infectious/inflammatory status, renal dysfunction current tobacco smokers and had acute coronary syndrome within 1 month Placebo baseline TC : 6.812 mmol/L (263 mg/dL) Placebo baseline LDL‐C : 4.833 mmol/L (187 mg/dL) Placebo baseline HDL‐C : 1.272 mmol/L (49 mg/dL) Placebo baseline triglycerides: 1.692 mmol/L (150 mg/dL) Fluvastatin 80 mg/day baseline TC : 7.264 mmol/L (281 mg/dL) Fluvastatin 80 mg/day baseline LDL‐C : 4.983 mmol/L (193 mg/dL) Fluvastatin 80 mg/day baseline HDL‐C : 1.358 mmol/L (53 mg/dL) Fluvastatin 80 mg/day baseline triglycerides: 1.629 mmol/L (144 mg/dL) |
|
| Interventions | Placebo Fluvastatin 80 mg/day |
|
| Outcomes | per cent change from baseline at 3‐6 weeks of plasma TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | WDAEs were not reported SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind treatment placebo and fluvastatin capsule appearances were not reported as appearing identical Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | All lab samples were analysed in duplicate by an individual blinded to treatment protocol |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | WDAEs were not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Lin 2000.
| Methods | 6‐week dietary washout period 8‐week before and after trial |
|
| Participants | 29 men and women with hypercholesterolaemia age 20‐70 years LDL‐C ≥ 160 mg/dL ( ≥ 4.14 mmol/L) or ≥ 130 mg/dL ( ≥ 3.36 mmol/L) with at least two atherosclerosis risk factors exclusion criteria: familial hypercholesterolaemia, type I, III or V hyperlipidaemia childbearing potential, congestive heart failure III and IV, statin hypersensitivity under therapy with non registered drugs or participating in another trial confounding disease and conditions, liver and kidney disease, receiving immunosuppressants, steroids except contraceptives, aluminium antacids erythromycin,some antifungals, and para‐aminosalicylic acid Fluvastatin 40 mg/day baseline TC : 6.773 mmol/L (262 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 4.965 mmol/L (192 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.27 mmol/L (49 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 1.91 mmol/L (169 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day | |
| Outcomes | per cent change from baseline at 4‐8 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | SDs were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20.7% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Lintott 1995.
| Methods | 4‐week washout period and a 6 week placebo run‐in period 12‐week randomised double‐blind placebo‐controlled trial |
|
| Participants | 42 hyperlipidaemic men and women TC ≥ 6.2 mmol/L (240 mg/dL) HDL‐C ≤ 0.90 mmol/L (35 mg/dL) exclusion criteria: active cardiac, GI, hepatic, or renal disease hypothyroidism unless treated or controlled, secondary hyperlipidaemia, MI or coronary bypass surgery within 3 months of trial or unstable angina confounding drugs childbearing potential no baseline values |
|
| Interventions | Placebo for 12 weeks Fluvastatin 40 mg/day for 12 weeks |
|
| Outcomes | per cent change from baseline at 12 weeks of plasma TC, LDL‐C, triglycerides and WDAEs | |
| Source of Funding | unknown | |
| Notes | no HDL‐C data reported SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind fashion placebo and fluvastatin capsule appearances were not reported as appearing identical Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | Low risk | No patient had to be withdrawn from the study due to adverse events |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | High risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
LIPS 2003.
| Methods | no patient received lipid‐lowering medications for at least 6 weeks 3‐4 year randomised double‐blind placebo‐controlled trial |
|
| Participants | 1677 men and women age 18‐80 years with unstable angina, stable angina, silent ischaemia who had undergone successful first PCI procedure of 1 or more lesions in the native coronary arteries during the same hospitalisation patients having a re stenosed target lesion within 6 months of first angioplasty were to be included 844 were randomised to fluvastatin and 833 to placebo TC ≥ 3.5 mmol/L (135 mg/dL) and < 7.0mmol/L (270 mg/dL) and a fasting TG < 4.5 mmol/L (400 mg/dL) after at least 6 week without lipid‐lowering therapy For patients status post MI within 24 hours to 4 weeks, TC > 3.5 to , 5.5 mmol/L, or for those patients with type 1 or type 2 diabetes mellitus, TC must have been ≥3.5 to ≤6.0 mmol/L exclusion criteria:BP > 180/100 despite medical therapy, undiagnosed hypertension, left ventricular ejection fraction < 30%, medical history of PCI or CABG procedure more than 6 months previous, or with severe non‐CHD such as valvular disease, idiopathic cardiomyopathy or congenital heart disease severe renal dysfunction, obesity BMI > 35, cancer or other disease with life expectancy of less than 4 years, with death, MI, or CABG between TCT procedure and hospital discharge, GI or liver impairment or major surgery within 3 months of randomisation treatment with probucol within 12 months prior to randomisation or with lipid‐lowering agents other than study medication, erythromycin, ketoconazole or anticonvulsant therapies currently participating in a study of any device or drug requiring clinical or angiographic follow‐up except in stent or a diagnostic registry with no angiographic follow‐up, or who had previously participated in this study Placebo baseline TC : 5.2 mmol/L (201 mg/dL) Placebo baseline LDL‐C : 3.4 mmol/L (131 mg/dL) Placebo baseline HDL‐C : 1.0 mmol/L (39 mg/dL) Placebo baseline triglycerides: 1.7 mmol/L (151 mg/dL) Fluvastatin 40 mg/day baseline TC : 5.2 mmol/L (201 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 3.4 mmol/L (131 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.0 mmol/L (39 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 1.8 mmol/L (159 mg/dL) |
|
| Interventions | Placebo Fluvastatin 40 mg twice daily |
|
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Source of Funding | Novartis | |
| Notes | SDs were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was done by central allocation |
| Allocation concealment (selection bias) | Low risk | Dispensing of sequentially numbered sets of study medication distributed to each site, and eligible patients received the next sequential medication pack at that site randomisation may have been done by central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Investigators were blinded to the lipid results from week 0 through the duration of the study LDL‐C was determined at a central laboratory (Analytico Medinet, Breda, the Netherlands) |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | WDAEs reported were for the 3.9 year time period not the 6 week time period |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 17.2`% of participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Novartis funded the trial |
Lorena 1997.
| Methods | 1‐month dietary run‐in period 2‐month before and after trial |
|
| Participants | 20 men and women with type IIa and IIb hypercholesterolaemia age 40‐50 years exclusion criteria:diabetes mellitus, impaired hepatic and renal function, secondary hypercholesterolaemia, drug or alcohol abuse concomitant treatment with anticoagulants and antiplatelet drugs macrovascular complications history Fluvastatin 40 mg/day baseline TC : 7.2 mmol/L (278 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 5.1 mmol/L (197 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.3 mmol/L (50 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 2.3 mmol/L (204 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day for 8 weeks | |
| Outcomes | per cent change from baseline at 8 weeks of plasma TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | SDs were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Lunder 2011.
| Methods | washout not required because participants were not receiving any lipid‐lowering medication 30‐day randomised double‐blind placebo‐controlled trial |
|
| Participants | 50 men age 30‐50 years non‐smokers, normotensive, non‐obese no clinical cardiovascular disease, no other chronic disease and without any regular medication therapy exclusion criteria: none Placebo baseline TC : 6.1 mmol/L (236 mg/dL) Placebo baseline LDL‐C : 4.1 mmol/L (159 mg/dL) Placebo baseline HDL‐C : 1.3 mmol/L (50 mg/dL) Placebo baseline triglycerides: 1.2 mmol/L (106 mg/dL) Fluvastatin 10 mg/day baseline TC : 5.7 mmol/L (220 mg/dL) Fluvastatin 10 mg/day baseline LDL‐C : 3.7 mmol/L (143 mg/dL) Fluvastatin 10 mg/day baseline HDL‐C : 1.2 mmol/L (46 mg/dL) Fluvastatin 10 mg/day baseline triglycerides: 1.6 mmol/L (142 mg/dL) |
|
| Interventions | Placebo Fluvastatin 10 mg/day |
|
| Outcomes | per cent change from baseline at 1 month of blood TC, LDL‐C, HDL‐C and triglycerides | |
| Source of Funding | unknown | |
| Notes | WDAEs were not reported SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | WDAEs were not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Lunder 2012.
| Methods | washout not required because participants were not on any regular medication 1‐month randomised double‐blind placebo‐controlled trial |
|
| Participants | 40 apparently healthy men age 30‐50 years old exclusion criteria: smoking, hypertension, hypercholesterolaemia, diabetes mellitus,, other cardiovascular diseases, chronic medical conditions and regular medication therapy women Placebo baseline TC : 5.7 mmol/L (220 mg/dL) Placebo baseline LDL‐C : 3.6 mmol/L (139 mg/dL) Placebo baseline HDL‐C : 1.4 mmol/L (54 mg/dL) Placebo baseline triglycerides: 1.3 mmol/L (115 mg/dL) Fluvastatin 10 mg/day baseline TC : 5.6 mmol/L (217 mg/dL) Fluvastatin 10 mg/day baseline LDL‐C : 3.7 mmol/L (143 mg/dL) Fluvastatin 10 mg/day baseline HDL‐C : 1.4 mmol/L (54 mg/dL) Fluvastatin 10 mg/day baseline triglycerides: 1.4 mmol/L (124 mg/dL) |
|
| Interventions | Placebo Fluvastatin 10 mg/day‐Valsartan 20 mg/day |
|
| Outcomes | per cent change from baseline at 1 month of blood TC, LDL‐C, HDL‐C, triglycerides and WDAEs | |
| Source of Funding | Slovenian research agency | |
| Notes | SDs were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number generator |
| Allocation concealment (selection bias) | Low risk | Envelopes were kept in possession of an independent medical student and packed in opaque containers |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo or active ingredients were identical in appearance |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | Low risk | No adverse events reported by participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Low risk | Slovenian research agency |
Lye 1998.
| Methods | 8‐week dietary run‐in period 12‐week randomised double‐blind placebo‐controlled |
|
| Participants | 69 men and women older that 60 years with type IIa, IIb and IV hypercholesterolaemia LDL‐C > 4.1 mmol/L (159 mg/dL) BMI < 55 exclusion criteria:type I, III or V dyslipidaemia, GI, renal impairment, MI within 3 months of trial obstructive hepatic or biliary disease, pancreatitis, gall bladder disease, abnormal liver enzymes, congestive heart failure grades III or IV severe or unstable angina pectoris, hypertension, severe retinopathy cataracts and other confounding factors Placebo baseline TC : 7.5 mmol/L (290 mg/dL) Placebo baseline LDL‐C : 5.3 mmol/L (205 mg/dL) Placebo baseline HDL‐C : 1.3 mmol/L (50 mg/dL) Placebo baseline triglycerides: 2.0 mmol/L (177 mg/dL) Fluvastatin 40 mg/day baseline TC : 7.4 mmol/L (286 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 5.2 mmol/L (201 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.4 mmol/L (54 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 1.6 mmol/L (142 mg/dL) |
|
| Interventions | Placebo for 12 weeks Fluvastatin 40 mg/day for 12 weeks |
|
| Outcomes | per cent change from baseline at 8 weeks of plasma TC, LDL‐C, HDL‐C, triglycerides and WDAEs | |
| Source of Funding | Sandoz | |
| Notes | SDs were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind treatment placebo and fluvastatin capsule appearances were not reported as appearing identical Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | Unclear risk | Blinding method not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4.3% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Sandoz funded the trial |
Mark 2001.
| Methods | 2‐month dietary washout period 12‐month before and after trial |
|
| Participants | 23 men and women with hypercholesterolaemia mean age 59 years exclusion criteria: MI history, mitral valve prolapse, arrhythmias of branch blocks, taking psychotropic drugs or antiarrhythmic drugs except beta blockers Fluvastatin 40 mg/day baseline TC : 6.59 mmol/L (255 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 4.33 mmol/L (167 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.35 mmol/L (52 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 2.00 mmol/L (177 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day | |
| Outcomes | per cent change from baseline at 3 months of blood TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | SDs were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Martin 2002.
| Methods | no washout period required because no patient was receiving hypolipidaemic treatment within 3 months of the trial 12‐week randomised double‐blind placebo‐controlled trial |
|
| Participants | 48 men and women with hypercholesterolaemia 160 mg/dL < LDL‐C < 300 mg/dL (4.14 mmol/L < LDL‐C < 7.76 mmol/L) triglycerides < 350 mg/dL (3.95 mmol/L) exclusion criteria: childbearing potential history of drug or alcohol abuse nephrotic syndrome, autoimmune diseases, obstructive liver disease, multiple myeloma glycogen storage disease, hypothyroidism, chronic pancreatitis, porphyria, myopathy, MI within 3 months of the trial type 1 or uncontrolled type 2 diabetes mellitus, atrial fibrillation or AV‐block grade 2 or higher statin hypersensitivity or receiving drugs that might affect pharmacodynamics or pharmacokinetics of statins hepatic or renal dysfunction, participation in another human trial within 3 months of this trial patients receiving steroid hormones, immunosuppressants, ketoconazole, erythromycin, vitamin E, or probucol Placebo baseline TC : 7.67 mmol/L (297 mg/dL) Placebo baseline LDL‐C : 5.28 mmol/L (204 mg/dL) Placebo baseline HDL‐C : 1.45 mmol/L (56 mg/dL) Placebo baseline triglycerides: 2.35 mmol/L (208 mg/dL) Fluvastatin 80 mg/day baseline TC : 7.69 mmol/L (297 mg/dL) Fluvastatin 80 mg/day baseline LDL‐C : 5.44 mmol/L (210 mg/dL) Fluvastatin 80 mg/day baseline HDL‐C : 1.41 mmol/L (55 mg/dL) Fluvastatin 80 mg/day baseline triglycerides: 1.67 mmol/L (148 mg/dL) |
|
| Interventions | Placebo Fluvastatin 80 mg/day |
|
| Outcomes | per cent change from baseline at 6‐12 weeks of serum TC, LDL‐C, HDL‐C, triglycerides and WDAEs | |
| Source of Funding | unknown | |
| Notes | SDs were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind treatment placebo and fluvastatin capsule appearances were not reported as appearing identical Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | Unclear risk | Blinding method not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Marz 2001.
| Methods | 8‐week run‐in washout period 12‐week double‐blind randomised placebo‐controlled trial |
|
| Participants | 52 postmenopausal women mean age 44‐75 years old LDL‐C >150 mg/dL (3.88 mmol/L) triglycerides > 120 mg/dL 1.35 mmol/L) exclusion criteria: LDL‐C ≥ 300 mg/dL (7.76 mmol/L), triglycerides ≥ 500 mg/dL (5.65 mmol/L) acute MI within 3 months of trial, type 1 diabetes, uncontrolled type 2 diabetes, severe obesity, overt liver disease, chronic renal failure, myopathy alcohol or drug abuse, several other significant disease, HRT, immunosuppressants, erythromycin and/or neomycin, ketoconazole Placebo baseline TC : 8.20 mmol/L (317 mg/dL) Placebo baseline LDL‐C : 4.03 mmol/L (156 mg/dL) Placebo baseline HDL‐C : 1.16 mmol/L (45 mg/dL) Placebo baseline triglycerides: 3.09 mmol/L (274 mg/dL) Fluvastatin 40 mg/day baseline TC : 8.56 mmol/L (331 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 4.50 mmol/L (174 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.22 mmol/L (47 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 2.56 mmol/L (227 mg/dL) |
|
| Interventions | Placebo for 12 weeks Fluvastatin for 12 weeks |
|
| Outcomes | per cent change from baseline at 8‐12 weeks of serum TC, LDL‐C and triglycerides | |
| Source of Funding | Novartis | |
| Notes | WDAEs were not reported HDL‐C data were not included in the efficacy analysis because the calculated value was different by more than 10% from the given value SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo and fluvastatin capsule appearances were not reported as appearing identical Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | All laboratory assessments were performed centrally at the Department of Medicine, University of Freiburg, Germany |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | WDAEs were not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Novartis funded the study |
Milani 1995.
| Methods | 4‐week single‐blind placebo run‐in period 4‐week before and after trial |
|
| Participants | 20 men and women with type IIa primary hypercholesterolaemia age 53 years LDL‐C ≥ 160 mg/dL (4.14 mmol/L) Triglycerides ≤ 250 mg/dL (2.82 mmol/L) exclusion criteria: secondary forms of dyslipidaemia, obesity, abnormal liver or renal function, patients with neoplasms, acute MI, coronary bypass surgery Fluvastatin 40 mg/day baseline TC : 7.6 mmol/L (294 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 5.5 mmol/L (213 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.1 mmol/L (42.5 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 2.1 mmol/L (186 mg/dL) |
|
| Interventions | 40 mg/day fluvastatin for 4 weeks 40 mg/day pravastatin for 4 weeks |
|
| Outcomes | per cent change from baseline at 4 weeks of plasma TC, LDL‐C, HDL‐C and triglycerides | |
| Source of Funding | unknown | |
| Notes | 40 mg/day pravastatin for 4 weeks group was not analysed SDs were imputed by the method of Furikawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | Not a blinded trial WDAEs were not reported compared to placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Mirdamadi 2008.
| Methods | no washout required no participant received lipid‐lowering therapy 6‐week dietary run‐in period 3‐month before and after trial |
|
| Participants | 164 men and women with type IIb hyperlipidaemia non‐smokers between 21‐70 years old 57 participants received fluvastatin exclusion criteria: hepatic, endocrine renal disorders, diabetes mellitus, glucose intolerance, alcoholism,drug abuse, gallstones, cancer pregnancy or lactation, receiving anticoagulants Fluvastatin 80 mg/day baseline TC : 7.61 mmol/L (294 mg/dL) Fluvastatin 80 mg/day baseline LDL‐C : 5.17 mmol/L (200 mg/dL) Fluvastatin 80 mg/day baseline HDL‐C : 1.24 mmol/L (48 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/day Simvastatin 10/20 mg/day Fluvastatin XR 80 mg/day |
|
| Outcomes | per cent change from baseline at 6‐12 weeks of serum TC and LDL‐C | |
| Source of Funding | grants from OTKA (K63025), OMFB‐1613 and ETT 243/2006 | |
| Notes | Atorvastatin 10 mg/day Simvastatin 10/20 mg/day groups were not included in the efficacy analysis HDL‐C data were not included in the efficacy analysis because the calculated value was different by more than 10% from the given value triglycerides of the fluvastatin group were not included in the efficacy analysis because they are expressed as medians SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Low risk | Grants from OTKA (K63025), OMFB‐1613 and ETT 243/2006 |
Moradmand 1998.
| Methods | 6‐month dietary washout run‐in period 12‐week randomised single‐blind placebo‐controlled trial |
|
| Participants | 120 men and women with hypercholesterolaemia TC ≥ 220 mg/dL ( 5.69 mmol/L) LDL‐cholesterol ≥ 160 mg/dL ( ≥ 4.14 mmol/L) TG < 350 mg/dL (3.95 mmol/L) exclusion criteria: none reported Placebo baseline TC : 6.91 mmol/L (267 mg/dL) Placebo baseline LDL‐C : 4.75 mmol/L (184 mg/dL) Placebo baseline HDL‐C : 1.28 mmol/L (49 mg/dL) Placebo baseline triglycerides: 2.295 mmol/L (203 mg/dL) Fluvastatin 40 mg/day baseline TC : 7.12 mmol/L (275 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 5.12 mmol/L (198 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.13 mmol/L (44 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 2.52 mmol/L (223 mg/dL) |
|
| Interventions | Placebo Fluvastatin 40 mg/day Lovastatin 20 mg/day |
|
| Outcomes | per cent change from baseline at 6‐12 weeks of plasma TC, LDL‐C, HDL‐C, and triglycerides and WDAEs | |
| Source of Funding | unknown | |
| Notes | Lovastatin 20 mg/day group was not included in the efficacy analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | Single‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
MUST 2001.
| Methods | 6‐week washout period 18‐week before and after trial |
|
| Participants | 478 men and women between ages of 20 and 70 years with type IIa or IIb primary hypercholesterolaemia 237 received simvastatin and 241 received fluvastatin LDL‐C ≤6.0 mmol/L (232 mg/dL) (CHD group, 3.5‐6.0 mmol/L) (135.3‐232.0 mg/dL) MRF group, 4.0‐6.0 mmol/L (154.7‐232.0 mg/dL), triglyceride levels <4.5 mmol/L (< 398.6 mg/dL) exclusion criteria: statin hypersensitivity, pregnancy or lactation, inadequate contraception, active liver disease, hepatic dysfunction homozygous familial hypercholesterolaemia, uncontrolled diabetes mellitus, alcohol or drug abuse MI, coronary bypass surgery or angioplasty within the past 3 months unstable angina, ventricular arrhythmia, confounding drugs or medical conditions Fluvastatin 20 mg/day baseline LDL‐C : 4.70 mmol/L (182 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.18 mmol/L (46 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 1.98 mmol/L (175 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day for 6 weeks Simvastatin 10 mg/day for 6 weeks Fluvastatin 20‐40 mg/day for 6‐12 weeks Simvastatin 10‐20 mg/day for 6‐12 weeks Fluvastatin 20‐80 mg/day for 12‐18 weeks Simvastatin 10‐40 mg/day for 12‐18 weeks |
|
| Outcomes | per cent change from baseline at 6 weeks of blood LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | Merck | |
| Notes | Simvastatin 10 mg/day for 6 weeks Fluvastatin 20‐40 mg/day for 6‐12 weeks Simvastatin 10‐20 mg/day for 6‐12 weeks Fluvastatin 20‐80 mg/day for 12‐18 weeks Simvastatin 10‐40 mg/day for 12‐18 weeks groups were not analysed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Merck funded the trial |
Nakaya 1995.
| Methods | 4‐week dietary washout period 8‐week randomised double‐blind placebo‐controlled trial |
|
| Participants | 40 men and 1 women with type IIa or IIb hypercholesterolaemia age 25‐64 years Total cholesterol 221.0‐423.0 mg/dL (5.72‐10.94 mmol/L) LDL‐C 123.4‐334.6 mg/dL (3.19‐8.65 mmol/L) HDL‐C 31‐87 mg/dL (0.80‐2.25 mmol/L) Triglycerides 47‐1005 mg/dL (0.53‐11.35 mmol/L) 20 participants received placebo 20 participants received fluvastatin exclusion criteria: hypothyroidism, Cushings disease, gallbladder disease, pancreatitis, cancer, unstable diabetes, severe hypertension, alcohol abuse, obese people on diet, renal, liver dysfunction, brain disease, heart disease statin hypersensitivity and lupus Placebo baseline TC : 6.71 mmol/L (259 mg/dL) Placebo baseline LDL‐C : 4.36 mmol/L (169 mg/dL) Placebo baseline HDL‐C : 1.39 mmol/L (54 mg/dL) Placebo baseline triglycerides: 2.36 mmol/L (209 mg/dL) Fluvastatin 20 mg/day baseline TC : 6.76 mmol/L (261 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 4.64 mmol/L (179 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.39 mmol/L (54 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 2.09 mmol/L (185 mg/dL) |
|
| Interventions | placebo fluvastatin 20 mg/day |
|
| Outcomes | per cent change from baseline at 4‐8 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Rrandomised block design randomised according to a series set |
| Allocation concealment (selection bias) | Low risk | Centrally allocated via telephone web‐based pharmacy‐controlled randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind and placebo tablets looked identical to the treatment tablets |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid analysis was done at a central laboratory (Medical Research laboratories [MRL], Cincinnati,Ohio) |
| Blinding of outcome assessment (detection bias) WDAEs | Low risk | No participant withdrew from the study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 17.5% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Nash 1996.
| Methods | 6‐week dietary stabilisation/placebo washout period 8‐week before and after trial |
|
| Participants | 137 men and women with hypercholesterolaemia controlled with lovastatin therapy at washout period LDL cholesterol levels must be > 160 mg/dL (4.14 mmol/L) but ≤ 200 mg/dL (5.17 mmol/L) triglycerides levels ≤ 350 mg/dL ( 3.95 mmol/L) exclusion criteria: homozygous familial hypercholesterolaemia, MI, severe or unstable angina, major surgery or angioplasty within 6 months of study uncontrolled hypertension, secondary hyperlipidaemia, childbearing potential, pregnant, other lipid‐altering agents no baseline data reported |
|
| Interventions | Fluvastatin 20 mg/day for 4 weeks Fluvastatin 40 mg/day for 4‐8 weeks Lovastatin 20 mg/day for 8 weeks |
|
| Outcomes | per cent change from baseline at 8 weeks of plasma TC, LDL‐C, HDL‐C and triglycerides | |
| Source of Funding | Sandoz | |
| Notes | Fluvastatin 40 mg/day for 4‐8 weeks Lovastatin 20 mg/day for 8 weeks groups were not analysed SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2.9% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Sandoz funded the trial |
NOVARTIS 2005b.
| Methods | 4‐week dietary washout period and 2‐week run‐in period 16‐week before and after trial |
|
| Participants | 98 men and women aged 18‐65 years with mild to moderate hypertension 48 participants received fluvastatin dyslipidaemia LDL‐C up to 160 mg/dL (4.13 mmol/L) exclusion criteria: stroke or MI within 3 months, angina, 3rd or 4th degree encephalopathy, congestive heart failure, diabetes mellitus requiring treatment hepatic and renal dysfunction, gastric or duodenal ulcer exacerbation during prior 12 months, receiving regular antihypertensive or lipid‐lowering treatment or other excluded medication Fluvastatin 80 mg/day baseline TC : 5.75 mmol/L (222 mg/dL) Fluvastatin 80 mg/day baseline LDL‐C : 3.36 mmol/L (130 mg/dL) Fluvastatin 80 mg/day baseline triglycerides: 1.77 mmol/L (157 mg/dL) |
|
| Interventions | fluvastatin 80 mg/day for 0‐8 weeks valsartan 80 mg/day for 0‐8 weeks fluvastatin 80 mg/day + valsartan 160 mg/day for 8‐16 weeks |
|
| Outcomes | per cent change from baseline at 8 weeks of serum TC and LDL‐C | |
| Source of Funding | Novartis | |
| Notes | valsartan 80 mg/day for 0‐8 weeks fluvastatin 80 mg/day + valsartan 160 mg/day for 8‐16 weeks groups were not included in the efficacy analysis Triglyceride data were not included in the efficacy analysis because the calculated value differed by 30% from the given value SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Novartis funded the trial |
NOVARTIS 2006b.
| Methods | 4‐week placebo dietary run‐in period 12‐week before and after trial |
|
| Participants | 319 men and women with mixed dyslipidaemia and primary hypercholesterolaemia ≥18 years old TC ≥220 mg/dL (5.72 mmol/L) mixed dyslipidaemia: LDL‐C ≥140 mg/dL (3.64 mmol/L) and serum TG ≥170 mg/dL (1.9 mmol/L) and ≤ 400 mg/dL (4.52 mmol/L) primary hypercholesterolaemia: LDL‐C ≥140 mg/dL (3.64 mmol/L) and serum TG < 150 mg/dL (1.7 mmol/L) exclusion criteria: pregnancy or pregnancy potential, secondary dyslipidaemia GI tract surgery, bowel conditions, upper GI tract disease, pancreas disease, hepatic dysfunction, renal dysfunction urinary tract problems, plasma CPK > 1.5 X ULN, TSH levels outside normal range, acute illness or trauma within 3 months of trial entry unstable congestive heart failure , severe or unstable angina pectoris MI, major surgery or angioplasty during the 6 months prior to trial entry severe or uncontrolled hypertension, muscle disease, drug or alcohol abuse investigational drug exposure and ingestion of any lipid altering agents within 4 weeks of study entry immunosuppressants or continuous systemic erythromycin statin intolerance or hypersensitivity excessive obesity and mental dysfunction or language problems Fluvastatin 40 mg/day baseline TC : 6.69 mmol/L (259 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 4.42 mmol/L (171 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.4 mmol/L (54 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 1.92 mmol/L (170 mg/dL) Fluvastatin 80 mg/day baseline TC : 6.69 mmol/L (259 mg/dL) Fluvastatin 80 mg/day baseline LDL‐C : 4.42 mmol/L (171 mg/dL) Fluvastatin 80 mg/day baseline HDL‐C : 1.38 mmol/L (53 mg/dL) Fluvastatin 80 mg/day baseline triglycerides: 1.94 mmol/L (172 mg/dL) |
|
| Interventions | fluvastatin IR 40 mg/day fluvastatin SR 80 mg/day |
|
| Outcomes | per cent change from baseline at 4‐12 weeks of plasma TC, LDL‐C, HDL‐C and triglycerides | |
| Source of Funding | Novartis | |
| Notes | SDs were imputed by the method of Furukawa 2006 except for LDL‐C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1.6% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Novartis funded the trial |
Okopien 2005.
| Methods | no washout period required because no patient was receiving hypolipidaemic treatment within 3 months of the trial 3‐month before and after trial |
|
| Participants | 131 men and women with type IIa and IIb dyslipidaemia 33 type IIa participants received fluvastatin type IIa plasma TC > 200 mg/dL ( 5.17 mmol/L), LDL‐C >135 mg/dL (3.49 mmol/L) TG < 200 mg/dL (2.26 mmol/L) type IIb plasma TC > 200 mg/dL ( 5.17 mmol/L), LDL‐C >135 mg/dL (3.49 mmol/L) TG > 200 mg/dL (2.26 mmol/L) ineffective dietary treatment for at least 3 months common carotid intima‐media thickness ≥0.7 mm exclusion criteria: age > 65 years or < 35 years, other types of primary dyslipidaemias, secondary dyslipidaemia, acute or chronic inflammation, symptomatic congestive heart failure, unstable coronary artery disease, MI or stroke within 6 month of trial, moderate or severe arterial hypertension, hepatic or renal dysfunction, malabsorption syndromes, received other drugs that may affect trial, HRT or oral contraception and poor patient compliance Fluvastatin 40 mg/day baseline TC : 7.15 mmol/L (276 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 4.71 mmol/L (182 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.27 mmol/L (49 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 1.614 mmol/L (143 mg/dL) |
|
| Interventions | Type IIa Fluvastatin 40 mg/day Type IIa Simvastatin 20 mg/day Type IIb Ciprofibrate 100 mg/day Type IIb Fenofibrate 200 mg/day |
|
| Outcomes | per cent change from baseline at 1‐3 months of plasma TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | statuary grant NN‐4‐061/98 of the Medical University of Silesia | |
| Notes | Type IIa Simvastatin 20 mg/day Type IIb Ciprofibrate 100 mg/day Type IIb Fenofibrate 200 mg/day groups were not included in the efficacy analysis SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Low risk | statuary grant NN‐4‐061/98 of the Medical University of Silesia |
Olsson 2001.
| Methods | 4‐week placebo dietary run‐in washout period 12‐week before and after trial |
|
| Participants | 695 men and women with type IIa or IIb hypercholesterolaemia aged ≥18 years LDL‐C ≥ 160 mg/dL ( ≥ 4.14 mmol/L) triglycerides ≤ 400 mg/dL (≤ 4.52 mmol/L) exclusion criteria: homozygous familial hypercholesterolaemia type I, III, IV, V or secondary hyperlipoproteinaemia, diabetes, renal or hepatic impairment MI or undergone major surgery or angioplasty in the previous 6 months, unstable angina pectoris unstable congestive heart failure, poorly or uncontrolled hypertension and muscle disease Fluvastatin 40 mg/day baseline LDL‐C : 5.24 mmol/L (203 mg/dL) Fluvastatin 40 mg twice daily and 80 mg/day baseline LDL‐C : 5.15 mmol/L (199 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day Fluvastatin 40 mg before or with breakfast and at bedtime Fluvastatin 80 mg at bedtime |
|
| Outcomes | per cent change from baseline at 4‐12 weeks of blood LDL‐C | |
| Source of Funding | Novartis Pharma AG | |
| Notes | data were combined from fluvastatin 40 mg twice daily and fluvastatin 80 mg 'every afternoon' groups SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Novartis Pharma AG funded the trial |
Osamah 1997.
| Methods | no washout period required because no patient was receiving hypolipidaemic treatment 24‐week before and after trial |
|
| Participants | 30 men 40‐70 years old 6 mmol/L < plasma TC < 8 mmol/L (232 mg/dL < plasmaTC < 309 mg/dL) plasma TG < 3 mmol/L (266 mg/dL) with no chronic or metabolic diseases, no acute coronary event Fluvastatin 40 mg/day baseline TC : 7.675 mmol/L (297 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 5.295 mmol/L (205 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 2.76 mmol/L (244 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day | |
| Outcomes | per cent change from baseline at 4‐12 weeks of serum TC, LDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | 12‐24 week time period was not included in the efficacy analysis SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16.7% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Ose 1995.
| Methods | 6‐week placebo washout period 6‐week before and after trial |
|
| Participants | 432 adults men and women patients total cholesterol ≥6.5 mmol/L (≥250 mg/dL) 213 received fluvastatin and 219 received simvastatin LDL cholesterol ≥ 4.9 mmol/L (≥190 mg/dL) for those without CHD and < 2 CHD risk factors; ≥ 4.1 mmol/L (≥160 mg/dL) for those without CHD but with ≥ 2 CHD risk factors; ≥3.4 mmol/L (≥ 130 mg/dL) for those with CHD exclusion criteria: patients > 70 years of age, secondary hypercholesterolaemia, unstable or Prinzmetal angina, MI or CABG within previous 2 months plasma triglyceride ≥4.0 mmol/L (≥ 350 mg/dL), childbearing potential, history of substance abuse, patients with poor mental function recent history of hepatitis, impaired hepatic function, uncontrolled diabetes mellitus, concurrent use of immunosuppressants or of investigational drug therapy prohibited within 30 days of study entry Fluvastatin 20 mg/day baseline TC : 8.3 mmol/L (321 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 6.2 mmol/L (240 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.3 mmol/L (50 mg/dL) Fluvastatin 40 mg/day baseline TC : 8.2 mmol/L (317 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 8.0 mmol/L (309 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.3 mmol/L (50 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day for 6 weeks Fluvastatin 40 mg/day for 6 weeks Simvastatin 5 mg/day for 6 weeks Simvastatin 10 mg/day for 6 weeks |
|
| Outcomes | per cent change from baseline at 6 weeks of plasma TC and LDL‐C | |
| Source of Funding | Merck & Co. Inc | |
| Notes | Simvastatin 5 mg/day for 6 weeks Simvastatin 10 mg/day for 6 weeks groups were not analysed HDL‐C data were not included in the efficacy analysis because the calculated value was different by more than 10% from the given value Triglyceride data were not included in the efficacy analysis because it was expressed as a median percent change from baseline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4.2% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Merck & Co. Inc. funded the trial |
Parks 2006.
| Methods | 6‐week placebo run‐in period 24‐week before and after trial |
|
| Participants | 29 boys 9‐12 years old with heterozygous familial hypercholesterolaemia LDL‐C > 90th percentile for age a parent with primary hypercholesterolaemia and either a family history of premature ischaemic heart disease of tendon xanthoma exclusion criteria: homozygous familial hypercholesterolaemia, obesity BMI > 30, significant liver, kidney or muscle disease Fluvastatin 20 mg/day baseline LDL‐C : 5.85 mmol/L (226 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day for 0‐6 weeks Fluvastatin 20 mg twice daily for 6‐12 weeks Fluvastatin 40 mg twice daily for 12‐24 weeks |
|
| Outcomes | per cent change from baseline at 6 weeks of blood LDL‐C | |
| Source of Funding | Novartis | |
| Notes | Fluvastatin 20 mg twice daily for 6‐12 weeks Fluvastatin 40 mg twice daily for 12‐24 weeks groups were not included in the efficacy analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Novartis funded the study |
Perova 1996.
| Methods | 8‐week dietary run‐in period 12‐week before and after study |
|
| Participants | 70 hypertensive patients with LDL‐C ≥ 4.1 mmol/L ( 158.5 mg/dL) 51 patients had type IIa and 19 patients had type IIb hypercholesterolaemia no exclusion criteria Fluvastatin 20 mg/day baseline TC : 6.98 mmol/L (270 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 5.12 mmol/L (198 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.06 mmol/L (41 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 1.79 mmol/L (159 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day for 0‐4 weeks Fluvastatin 20‐40 mg/day for 4‐8 weeks Fluvastatin 20‐40 mg/day for 8‐12 weeks |
|
| Outcomes | per cent change from baseline at 4 weeks of plasma TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | Fluvastatin 20‐40 mg/day for 4‐8 weeks Fluvastatin 20‐40 mg/day for 8‐12 weeks time periods were not included in the efficacy analysis SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | a All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | source of funding was not reported |
Pinon 2002.
| Methods | 4‐6 week washout period 6‐month before and after trial |
|
| Participants | 27 men and women with polygenic hypercholesterolaemia 20‐65 years old serum cholesterol >240 mg/dL; LDL‐C > 160 mg/dL; triglycerides < 200 mg/dL (serum cholesterol > 6.21mmol/L; LDL‐C > 4.14 mmol/L; triglycerides < 2.26 mmol/L) exclusion criteria: renal and hepatic dysfunction, cancer, inflammatory or infectious diseases,, previous ischaemic event, thyroid hormone alterations obesity, chronic alcoholism, diabetes mellitus, hypertension or pregnancy and surgery within 3 months of study |
|
| Interventions | Fluvastatin 40 mg/day | |
| Outcomes | per cent change from baseline at 3 months of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | SDs were imputed by the method of Furukawa 2006 except for triglycerides which was determined from the P value | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Porsch‐Ozcurumez 2001.
| Methods | 6‐week dietary run‐in period 12‐week randomised double‐blind placebo‐controlled trial |
|
| Participants | 21 men and women age 18‐70 years with LDL‐C > 120 mg/dL (3.10 mmol/L) triglycerides ≤ 350 mg/dL (≤ 3.95 mmol/L) BMI 27.5‐29.3 history of current radiolucent gallstones or cholecystectomy due to gallstone disease exclusion criteria: cancer, renal, hepatic, thyroid diseases, diabetes mellitus, drug or alcohol abuse treatment with lipid‐lowering drugs or substances that might influence biliary lipid composition Placebo baseline TC : 5.82 mmol/L (225 mg/dL) Placebo baseline LDL‐C : 4.01 mmol/L (155mg/dL) Placebo baseline HDL‐C : 1.32 mmol/L (51 mg/dL) Placebo baseline triglycerides: 1.5 mmol/L (133 mg/dL) Fluvastatin 80 mg/day baseline TC : 6.13 mmol/L (237 mg/dL) Fluvastatin 80 mg/day baseline LDL‐C : 4.29 mmol/L (166 mg/dL) Fluvastatin 80 mg/day baseline HDL‐C : 1.345 mmol/L (52 mg/dL) Fluvastatin 80 mg/day baseline triglycerides: 1.31 mmol/L (116 mg/dL) |
|
| Interventions | Placebo Fluvastatin 40 mg twice daily |
|
| Outcomes | per cent change from baseline at 3‐6 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | partially by Novartis Pharma GmbH | |
| Notes | WDAEs were not reported SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind treatment placebo and fluvastatin capsule appearances were not reported as appearing identical Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | WDAEs were not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Novartis Pharma GmbH partially funded the trial |
Puccetti 2001.
| Methods | no washout period required because no patient was receiving hypolipidaemic treatment 12‐week before and after trial |
|
| Participants | 144 men and women with type IIa hypercholesterolaemia age 33‐64 years 25 participants received fluvastatin TC = 6.93 mmol/L (268 mg/dL) HDL‐C = 1.25 mmol/L (48 mg/dL) TG = 1.15 mmol/L (102 mg/dL) exclusion criteria: history of cardiovascular events, current hypertension,diabetes,liver, renal, thyroid, infectious, immunological or malignant diseases Fluvastatin 20 mg/day baseline TC : 6.55 mmol/L (253 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 4.86 mmol/L (188 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.21 mmol/L (47 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 1.05 mmol/L (93 mg/dL) |
|
| Interventions | Simvastatin 20 mg/day Cerivastatin 0.2 mg/day Atorvastatin 10 mg/day Pravastatin 20 mg/day for 0‐6 weeks Pravastatin 20‐40 mg/day for 6‐12 weeks Fluvastatin 20 mg/day for 0‐6 weeks Fluvastatn 20‐40 mg/day for 6‐12 weeks |
|
| Outcomes | per cent change from baseline at 6 weeks of plasma TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | University of Siena | |
| Notes | Simvastatin 20 mg/day Cerivastatin 0.2 mg/day Atorvastatin 10 mg/day Pravastatin 20 mg/day for 0‐6 weeks Pravastatin 20‐40 mg/day for 6‐12 weeks Fluvastatin 20‐40 mg/day for 6‐12 weeks groups were not included in the efficacy analysis SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Low risk | Grant from the University of Siena |
Puccetti 2002.
| Methods | no washout period required because no patient was receiving hypolipidaemic treatment 4‐week before and after trial |
|
| Participants | 64 men and women with hypercholesterolaemia age 36‐64 years 16 participants received fluvastatin TC = 6.86 mmol/L (265 mg/dL) HDL‐C = 1.24 mmol/L (48 mg/dL) TG = 1.13 mmol/L (100 mg/dL) BMI = 24.7 exclusion criteria: history of cardiovascular events, current hypertension,diabetes,liver, renal, thyroid, infectious, immunological or malignant diseases Fluvastatin 40 mg/day baseline TC : 6.54 mmol/L (253 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 4.8 mmol/L (186 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.24 mmol/L (48 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 1.1 mmol/L (97 mg/dL) |
|
| Interventions | Atorvastatin 10 mg/day Simvastatin 20 mg/day Pravastatin 40 mg/day Fluvastatin 40 mg/day |
|
| Outcomes | per cent change from baseline at 4 weeks of plasma TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | Atorvastatin 10 mg/day Simvastatin 20 mg/day Pravastatin 40 mg/day groups were not included in the efficacy analysis SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Riegger 1999.
| Methods | 4‐week dietary run‐in period 6‐week before and after trial |
|
| Participants | 365 men and women with hyperlipidaemia aged 40‐70 years total cholesterol ≥ 250 mg/dL (6.465 mmol/L) LDL‐C > 160 mg/dL (4.14 mmol/L) and triglycerides ≤ 300 mg/dL(3.39 mmol/L) after run‐in period proven coronary stenosis of > 70% exclusion criteria: PTCA within the last 6 months, planned PTCA or CABG, congestive heart failure type III or IV hypersensitivity or intolerance to HMG‐CoA reductase inhibitors, therapy with non registered drugs or other experimental studies within 3 months diseases or condition that could influence the pharmacokinetics or pharmacodynamics of the trial medication, GI, liver or renal diseases, childbearing potential, pregnancy drug or alcohol abuse, non compliance and no written consent |
|
| Interventions | Fluvastatin 40 mg/day | |
| Outcomes | per cent change from baseline at 6 weeks of serum LDL‐C | |
| Source of Funding | unknown | |
| Notes | SD were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Rywik 1997.
| Methods | 8‐week dietary run‐in period 12‐week before and after trial |
|
| Participants | 62 men and women with type II hyperlipidaemia age 18‐70 years old women were post menopause or had a hysterectomy hypertension controlled with diuretics, beta adrenergic agents, ACE inhibitors or Calcium channel blockers TC ≥ 6.5 mmol/L (251 mg/dL) LDL‐C ≥ 4.1 mmol/L (159 mg/dL) exclusion criteria: homozygous hypercholesterolaemia, heterozygous familial hyperlipidaemia, hyperlipidaemia type I, III, IV or V, secondary lipidaemia TG > 6.0 mmol/L (531 mg/dL) chronic disease or surgery that may affect the assessment of the trial MI, angioplasty or coronary bypass within 6 months of trial congestive heart failure (II‐IV) or unstable angina uncontrolled hypertension diabetes mellitus or extreme obesity (BMI ≥ 35) Fluvastatin 20 mg/day baseline TC : 7.8 mmol/L (302 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 5.5 mmol/L (213 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.3 mmol/L (50 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 2.1 mmol/L (186 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day for 0‐4 weeks Fluvastatin 20‐40 mg/day for 4‐12 weeks |
|
| Outcomes | per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | Fluvastatin 20‐40 mg/day for 4‐12 weeks period was not included in the efficacy analysis SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Saito 1995.
| Methods | 4‐week dietary placebo washout period 8‐week before and after trial |
|
| Participants | 170 men and women with type IIa, IIb and III hypercholesterolaemia age 24‐79 years old Total cholesterol 221‐435 mg/dL (5.72‐11.2 mmol/L) LDL‐C 112.6‐338.4 mg/dL (2.9‐8.75 mmol/L) HDL‐C 28‐123 mg/dL (0.72‐3.18 mmol/L) Triglycerides 44‐795 mg/dL (0.5‐9.0 mmol/L) 50 participants received 20 mg/day 47 participants received 30 mg/day 53 participants received 40 mg/day exclusion criteria: hypothyroidism, Cushings disease, gallbladder disease, pancreatitis, cancer, unstable diabetes, severe hypertension, alcohol abuse, obese people on diet, renal, liver dysfunction, brain disease, heart disease statin hypersensitivity and lupus Fluvastatin 20 mg/day baseline TC : 7.30 mmol/L (282 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 4.99 mmol/L (193 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.56 mmol/L (60 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 1.81 mmol/L (160 mg/dL) Fluvastatin 30 mg/day baseline TC : 7.35 mmol/L (284 mg/dL) Fluvastatin 30 mg/day baseline LDL‐C : 5.13 mmol/L (198 mg/dL) Fluvastatin 30 mg/day baseline HDL‐C : 1.34 mmol/L (52 mg/dL) Fluvastatin 30 mg/day baseline triglycerides: 1.93 mmol/L (171 mg/dL) Fluvastatin 40 mg/day baseline TC : 7.42 mmol/L (287 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 5.22 mmol/L (202 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.39 mmol/L (54 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 1.97 mmol/L (174 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day Fluvastatin 30 mg/day Fluvastatin 40 mg/day |
|
| Outcomes | per cent change from baseline at 4‐8 weeks of serum TC, LDL‐C and HDL‐C | |
| Source of Funding | unknown | |
| Notes | HDL‐C data were not included in the efficacy analysis because the calculated value was different by more than 10% from the given value for doses 20 mg/day and 30 mg/day Triglyceride data were not included in the efficacy analysis because the calculated value was different by more than 10% from the given value for all doses |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7.3% OF participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | source of funding was not reported |
Saitta 2000.
| Methods | 4‐6 week run‐in period and a 4 week placebo period 12‐week randomised placebo‐controlled trial |
|
| Participants | 40 men and women with familial hypercholesterolaemia with BMI < 27 TC > 280 mg/dL (7.24 mmol/L) LDL‐C > 190 mg/dL (4.91 mmol/L) TG < 180 mg/dL (2.03 mmol/L) exclusion criteria: arterial hypertension, cardiovascular, thyroid and/or kidney disease and diabetes mellitus Placebo baseline TC : 7.69 mmol/L (297 mg/dL) Placebo baseline LDL‐C : 5.42 mmol/L (210 mg/dL) Placebo baseline HDL‐C : 1.48 mmol/L (57 mg/dL) Placebo baseline triglycerides: 1.32 mmol/L (117 mg/dL) Fluvastatin 40 mg/day baseline TC : 7.63 mmol/L (295 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 5.4 mmol/L (209 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.53 mmol/L (59 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 1.296 mmol/L (115 mg/dL) |
|
| Interventions | Placebo Fluvastatin 40 mg/day |
|
| Outcomes | per cent change from baseline at 4‐12 weeks of blood TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | no WDAEs reported SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding is not mentioned Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No WDAEs reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Sarano 2003.
| Methods | 8‐week dietary washout period 16‐week before and after trial |
|
| Participants | 56 men and women with coronary artery disease with type 2 diabetes mellitus controlled with oral medication LDL‐C = 4.1 mmol/L (159 mg/dL) Triglycerides > 2.3 mmol/L (204 mg/dL) 30 men and women with coronary artery disease and mixed hyperlipidaemia without diabetes mellitus all participants ranged in age from 40‐70 years 40 participants received fluvastatin Included patients were on a standard lipid‐lowering diet and those with type 2 diabetes a diet with reduced carbohydrate content Inclusion criteria: unstable angina, MI, coronary bypass surgery, balloon angioplasty within 6 months of study, type 1 diabetes mellitus, uncontrolled diabetes, renal dysfunction, hepatic dysfunction no exclusion criteria cholelithiasis and triglycerides > 4.5 mmol/L (399 mg/dL) Fluvastatin 40 mg/day baseline TC : 7.88 mmol/L (305 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 5.45 mmol/L (211 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.01 mmol/L (39 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 3.14 mmol/L (278 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day Fenofibrate 200 mg/day |
|
| Outcomes | per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | Fenofibrate 200 mg/day group was not included in the efficacy analysis SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Sasaki 1995a.
| Methods | 4‐week run‐in period 20‐week before and after trial |
|
| Participants | 42 men and women with type IIa and IIb hypercholesterolaemia age 34‐69 years old 22 participants received fluvastatin Total cholesterol 232‐361 mg/dL (6.0‐9.3 mmol/L) LDL‐C 128.6‐279.6 mg/dL (3.3‐7.2 mmol/L) HDL‐C 37‐93 mg/dL (0.96‐2.4 mmol/L) Triglycerides 46‐505 mg/dL (0.5‐5.7 mmol/L) 18 patients received fluvastatin 18 patients received niceritrol exclusion criteria: hypothyroidism, Cushings disease, gallbladder disease, pancreatitis, cancer, unstable diabetes, severe hypertension, alcohol abuse, obese people on diet, renal, liver dysfunction, brain disease, heart disease statin hypersensitivity and lupus Fluvastatin 30 mg/day baseline TC : 7.48 mmol/L (289 mg/dL) Fluvastatin 30 mg/day baseline LDL‐C : 5.22 mmol/L (202 mg/dL) Fluvastatin 30 mg/day baseline HDL‐C : 1.48 mmol/L (57 mg/dL) |
|
| Interventions | Fluvastatin 30 mg/day for 0‐8 weeks Fluvastatin 30 mg/day + Niceritrol 750 mg/day for 8‐16 weeks Fluvastatin 30 mg/day for 16‐20 weeks Niceritrol 750 mg/day for 0‐8 weeks Niceritrol 750 mg/day + Fluvastatin 30 mg/day for 8‐16 weeks Niceritrol 750 mg/day for 16‐20 weeks |
|
| Outcomes | per cent change from baseline at 8 weeks of blood TC and LDL‐C | |
| Source of Funding | unknown | |
| Notes | Fluvastatin 30 mg/day + Niceritrol 750 mg/day for 8‐16 weeks Fluvastatin 30 mg/day for 16‐20 weeks Niceritrol 750 mg/day for 0‐8 weeks Niceritrol 750 mg/day + Fluvastatin 30 mg/day for 8‐16 weeks Niceritrol 750 mg/day for 16‐20 weeks groups were not included in the efficacy analysis HDL‐C data were not included in the efficacy analysis because the calculated value was different by more than 10% from the given value |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5.6% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | source of funding was not reported |
Sasaki 1995b.
| Methods | at least a 4‐week washout period 16‐week before and after trial |
|
| Participants | 42 men and women with primary hypercholesterolaemia with total cholesterol ≥220 mg/dL (5.69 mmol/L) and Lp(a) ≥15 mg/dL exclusion criteria: poorly controlled diabetes mellitus or severe hypertension, alcohol abuse, obese participants on weight reduction programs any clinically critical conditions 22 patients in the fluvastatin preceding group 20 patients in the niceritrol preceding group Fluvastatin 30 mg/day baseline TC : 7.27 mmol/L (281 mg/dL) Fluvastatin 30 mg/day baseline LDL‐C : 5.01 mmol/L (194 mg/dL) Fluvastatin 30 mg/day baseline HDL‐C : 1.51 mmol/L (58 mg/dL) Fluvastatin 30 mg/day baseline triglycerides: 1.76 mmol/L (156 mg/dL) |
|
| Interventions | Fluvastatin 30 mg/day for 0‐8 weeks Fluvastatin 30 mg/day + Niceritrol 750 mg/day for 8‐16 weeks Niceritrol 750 mg/day for 0‐8 weeks Niceritrol 750 mg/day + Fluvastatin 30 mg/day for 8‐16 weeks |
|
| Outcomes | per cent change from baseline at 8 weeks of serum TC and LDL‐C | |
| Source of Funding | Sandoz | |
| Notes | Fluvastatin 30 mg/day + Niceritrol 750 mg/day for 8‐16 weeks Niceritrol 750 mg/day for 0‐8 weeks Niceritrol 750 mg/day + Fluvastatin 30 mg/day for 8‐16 weeks groups were not included in the efficacy analysis HDL‐C and triglyceride data were not included in the efficacy analysis because the calculated values were different by more than 10% from the given values |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4.8% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Sandoz pharmaceuticals funded the study |
Scharnagl 2006.
| Methods | no washout period required because no patient was receiving hypolipidaemic treatment 4‐week dietary placebo run‐in period 8‐week before and after trial |
|
| Participants | 236 men and women age 35‐80 years old type IIa/IIb hypercholesterolaemia LDL‐C ≥ 160 mg/dL ( ≥ 4.14 mmol/L) and triglycerides < 400 mg/dL (4.52 mmol/L) exclusion criteria: secondary dyslipidaemia, active liver disease, myopathy, thyroid stimulating hormone ≥ 2X ULN significant cardiovascular disease 6 months prior to the study, uncontrolled type 2 diabetes within 3 months of study entry statin hypersensitivity, prohibited concomitant therapy or receiving supplements known to alter lipid metabolism Fluvastatin 80 mg/day baseline TC : 7.3 mmol/L (282 mg/dL) Fluvastatin 80 mg/day baseline LDL‐C : 4.89 mmol/L (189 mg/dL) Fluvastatin 80 mg/day baseline HDL‐C : 1.52 mmol/L (59 mg/dL) Fluvastatin 80 mg/day baseline triglycerides: 1.99 mmol/L (176 mg/dL) |
|
| Interventions | Fluvastatin 80 mg/day | |
| Outcomes | per cent change from baseline at 4‐8 weeks of serum TC and LDL‐C | |
| Source of Funding | Astellas Pharma | |
| Notes | HDL‐C and triglyceride data were not included in the efficacy analysis because the calculated values were different by more than 10% from the given values SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | Not a blinded trial WDAEs were not reported compared to placebo |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16.5% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Astellas Pharma funded the trial |
Schulte 1996.
| Methods | 4‐week (3 months in the case of statin pretreatment) run‐in period 4‐week before and after trial |
|
| Participants | 120 men and women between 26‐74 years of age with hypercholesterolaemia 60 received fluvastatin and 60 received simvastatin LDL‐C > 185 mg/dL (4.78 mmol/L), serum triglycerides < 300 mg/dL (3.39 mmol/L) exclusion criteria: active liver or gall bladder disease, elevated aminotransferases or other severe/disabling diseases, childbearing potential, drug or alcohol abuse, musculoskeletal diseases treatment with rifampicin, cyclosporin and erythromycin Fluvastatin 40 mg/day baseline TC : 7.8 mmol/L (302 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 5.7 mmol/L (220 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.2 mmol/L (46 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 1.7 mmol/L (151 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day for 4 weeks Simvastatin 20 mg/day for 4 weeks |
|
| Outcomes | per cent change from baseline at 4 weeks of plasma TC, LDL‐C, HDL‐C and triglycerides | |
| Source of Funding | Astra GmbH | |
| Notes | Simvastatin 20 mg/day for 4‐week group was not analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Astra GmbH supported this study with a grant |
Sejda 2006.
| Methods | no washout period required because no patient was receiving hypolipidaemic treatment 3‐month before and after trial |
|
| Participants | 14 men and women with dyslipidaemia age 60 years BP of 135/81 fasting plasma glycaemia 5.56 mmol/L TC 5.4‐7.9 mmol/L (209‐305 mg/dL), triglycerides < 3 mmol/L (266 mg/dL) exclusion criteria:thyroid disease, pregnancy or lactation,cancer, serious hepatic or renal function, consumption of >40 g/day of alcohol and/or the intake of lipid‐lowering drugs Fluvastatin 80 mg/day baseline TC : 6.21 mmol/L (240 mg/dL) Fluvastatin 80 mg/day baseline LDL‐C : 4.02 mmol/L (155 mg/dL) Fluvastatin 80 mg/day baseline HDL‐C : 1.13 mmol/L (44 mg/dL) Fluvastatin 80 mg/day baseline triglycerides: 2.56 mmol/L (227 mg/dL) |
|
| Interventions | Fluvastatin 80 mg/day | |
| Outcomes | per cent change from baseline at 3 months of plasma TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | government grant | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Low risk | Supported by grant No LN00A069 from the Ministry of Education, Youth and Sports Czech Republic |
Seres 2005.
| Methods | no washout period required because no patient was receiving hypolipidaemic treatment 6‐week before and after trial |
|
| Participants | 21 men with hypercholesterolaemia exclusion criteria:liver , thyroid and kidney diseases diabetes mellitus, infective disorders, fever, and lipid‐lowering medication use Fluvastatin 40 mg/day baseline TC : 5.9 mmol/L (228 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 4.11 mmol/L (159 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.4 mmol/L (54 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 1.7 mmol/L (132 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day | |
| Outcomes | per cent change from baseline at 6 weeks of plasma TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | Medical Research Council, Budapest, Hungary, ETT(11AO24/0003) | |
| Notes | SDs were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Low risk | Study funded by the Medical Research Council, Budapest, Hungary, ETT(11AO24/0003) |
Sigurdsson 1998.
| Methods | 8‐week dietary washout period 16‐week before and after trial |
|
| Participants | 113 men and women with moderate hypercholesterolaemia age 59.8 years history of typical angina pectoris lasting at least 3 months or a MI at least 6 months before entry 57 participants received fluvastatin and 56 received simvastatin serum cholesterol between 5.5 and 8.0 mmol/L (213 and 309 mg/dL) inclusive and serum triglyceride value of ≤2.5 mmol/L (221mg/dL) exclusion criteria:patients with concomitant conditions such as a MI or a CVA within the past 6 months, planned angioplasty, coronary bypass surgery during the previous 6 months, unstable angina, cardiac or renal failure, hepatic disease, uncontrolled hypertension, partial ileal bypass, secondary hypercholesterolaemia, HMG‐CoA reductase inhibitor hypersensitivity, childbearing potential, alcohol and drug abuse and concomitant use of lipid ‐lowering agents within 6 weeks Fluvastatin 20 mg/day baseline TC : 6.73 mmol/L (260 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 4.96 mmol/L (192 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.12 mmol/L (43 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day for 10 weeks Fluvastatin 20‐40 mg/day from weeks 10‐16 Simvastatin 20 mg/day for 10 weeks Simvastatin 20‐40 mg/day from weeks 10‐16 |
|
| Outcomes | per cent change from baseline at 6‐10 weeks of serum TC, LDL‐C and HDL‐C | |
| Source of Funding | Merck & Co Inc | |
| Notes | Fluvastatin 20‐40 mg/day from weeks 10‐16 Simvastatin 20 mg/day for 10 weeks Simvastatin 20‐40 mg/day from weeks 10‐16 groups were not analysed Triglyceride data were not reported because they were median percent change from baseline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1.8% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Merck & Co Inc funded the study |
Singer 2002.
| Methods | no washout required because no participants was receiving any lipid‐lowering medication 6‐month before and after trial |
|
| Participants | 55 men and women with combined hyperlipidaemia (type IIb) age 56‐58 years old exclusion criteria: none reported Fluvastatin 40 mg/day baseline TC : 7.51 mmol/L (290 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 4.64 mmol/L (179 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.23 mmol/L (48 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 2.935 mmol/L (260 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day for 0‐2 months Fluvastatin 40 mg/day + fish oil or olive oil for 2‐4 months Fluvastatin 40 mg/day for 4‐6 months |
|
| Outcomes | per cent change from baseline at 2 months of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Source of Funding | unknown | |
| Notes | Fluvastatin 40 mg/day + fish oil or olive oil for 2‐4 months Fluvastatin 40 mg/day for 4‐6 months groups were not included in the efficacy analysis SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Smit 1999.
| Methods | 8‐week dietary run‐in period 6‐week before and after trial |
|
| Participants | 21 men and women with combined hyperlipidaemia age 54 years BMI = 26.6 LDL‐C ≥4.14 mmol/L (160 mg/dL) and triglycerides ≥ 2.3 mmol/L (178 mg/dL) exclusion criteria: diabetes mellitus, renal hepatic,muscle or cardiac disease participants receiving drugs that accompany myopathy or elevated muscle proteins were also excluded Fluvastatin 40 mg/day baseline TC : 8.4 mmol/L (325 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 5.4 mmol/L (209 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.1 mmol/L (43 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 4.3 mmol/L (381 mg/dL) |
|
| Interventions | 7 participants received fluvastatin 40 mg/day 7 participants received gemfibrozil 600 mg twice daily 7 participants received fluvastatin 40 mg/day + gemfibrozil 600 mg twice daily |
|
| Outcomes | per cent change from baseline at 6 weeks of plasma TC, LDL‐C and HDL‐C | |
| Source of Funding | in part by a Pioneer Grant from the Dutch Foundation for Scientific Research | |
| Notes | 7 participants received gemfibrozil 600 mg twice daily 7 participants received fluvastatin 40 mg/day + gemfibrozil 600 mg twice daily groups were not included in the efficacy analysis Triglyceride data were not included in the efficacy analysis because the calculated value was different by more than 10% from the given value SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Low risk | Funded in part by a Pioneer Grant from the Dutch Foundation for Scientific Research |
Sonmez 2003.
| Methods | no washout period required because no patient was receiving hypolipidaemic treatment 8‐week before and after trial |
|
| Participants | 35 men and women age , 60 years, BMI < 29, fasting glucose < 107 mg/dL, plasma triglyceride 150‐350 mg/dL (1.69‐3.95 mmol/L) LDL‐C > 160 mg/dL (4.14 mmol/L) exclusion criteria: none reported Fluvastatin 40 mg/day baseline TC : 7.176 mmol/L (277 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 5.264 mmol/L (204 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.186 mmol/L (46 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 1.829 mmol/L (162 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day | |
| Outcomes | per cent change from baseline at 8 weeks of plasma TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | SDs were imputed by the method of Furukawa 2006 for LDL‐C , HDL‐C and triglycerides | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Sonmez 2006.
| Methods | no washout period required because no patient was receiving hypolipidaemic treatment within 3 months of the study 12‐week before and after trial |
|
| Participants | 43 men and women with dyslipidaemia age < 60 years old BMI < 29 fasting blood glucose, 107 mg/dL 24 participants received fluvastatin plasma triglycerides 150‐350 mg/dL (1.69‐3.95 mmol/L) plasma LDL‐C > 160 mg/dL (4.14 mmol/L) creatinine < 1.2 mg/dL no evidence of hypertension or other metabolic diseases BP < 140/90 exclusion criteria: none reported Fluvastatin 80 mg/day baseline TC : 6.37 mmol/L (246 mg/dL) Fluvastatin 80 mg/day baseline LDL‐C : 4.0 mmol/L (155 mg/dL) Fluvastatin 80 mg/day baseline HDL‐C : 1.39 mmol/L (54 mg/dL) Fluvastatin 80 mg/day baseline triglycerides: 2.17 mmol/L (192 mg/dL) |
|
| Interventions | Fluvastatin 80 mg/day plus TLC TLC |
|
| Outcomes | per cent change from baseline at 12 weeks of plasma TC, LDL‐C and HDL‐C | |
| Source of Funding | unknown | |
| Notes | TLC group is not a placebo therefore this group was not included in the efficacy analysis Triglyceride data were not included in the efficacy analysis because the calculated value was different by more than 10% from the given value |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Spieker 2000.
| Methods | 4‐week run‐in period 16‐week randomised double‐blind placebo‐controlled trial cross‐over |
|
| Participants | 454 men and women aged 20‐70 years plasma total cholesterol > 6.5 mmol/L (251 mg/dL) TC/HDL‐C ratio > 5 exclusion criteria:pregnancy, lactation, renal and hepatic disease, secondary hypercholesterolaemia, alcohol and drug abuse and current use of lipid‐lowering agents Placebo baseline TC : 8.73 mmol/L (338 mg/dL) Placebo baseline LDL‐C : 5.65 mmol/L (218 mg/dL) Placebo baseline HDL‐C : 1.1 mmol/L (43 mg/dL) Placebo baseline triglycerides: 4.59 mmol/L (407 mg/dL) Fluvastatin 20 mg/day baseline TC : 8.55 mmol/L (331 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 5.8 mmol/L (224 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.15 mmol/L (44 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 3.93 mmol/L (348 mg/dL) |
|
| Interventions | Group A: Fluvastatin 20 mg/day for 0‐4 weeks Group A: Placebo for 4‐12 weeks Group A: Fluvastatin 20 mg/day + Bezafibrate 400 mg/day for 12‐16 weeks Group B: Placebo for 0‐4 weeks Group B: Placebo for 4‐8 weeks Group B: Fluvastatin 20 mg/day for 8‐12 weeks Group B: Fluvastatin 20 mg/day + Bezafibrate 400 mg/day for 12‐16 weeks Group C: Fluvastatin 20 mg/day for 0‐4 weeks Group C: Fluvastatin 20 mg/day for 4‐8 weeks Group C: Fluvastatin 20 mg/day for 8‐12 weeks Group C: Fluvastatin 20 mg/day + Bezafibrate 400 mg/day for 12‐16 weeks |
|
| Outcomes | per cent change from baseline at 0‐4 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | Sandoz | |
| Notes | Group A: Fluvastatin 20 mg/day for 0‐4 weeks Group B: Placebo for 0‐4 weeks Group C: Fluvastatin 20 mg/day for 0‐4 weeks groups or time periods were analysed WDAEs were not reported for the 0‐4 and 4‐8 week time periods only SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo and fluvastatin capsule appearances were not reported as appearing identical Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | WDAEs were not reported for the appropriate time periods |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Sandoz funded the trial |
Sprecher 1994.
| Methods | 6‐week single‐blind placebo washout period 24‐week randomised double‐blind placebo‐controlled trial |
|
| Participants | 224 randomised patients with hypercholesterolaemia 150 participants received fluvastatin and 74 received placebo LDL‐C ≥4.14 mmol/L (160 mg/dL) plasma triglycerides ≤ 3.39 mmol/L (300 mg/dL) exclusion criteria: homozygous familial hypercholesterolaemia, secondary hyperlipidaemia, liver and renal disease,diabetes, MI or angioplasty within 6 months of study, uncontrolled hypertension Placebo baseline LDL‐C : 5.4 mmol/L (209 mg/dL) Fluvastatin 10 mg/day baseline LDL‐C : 5.4 mmol/L (209 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 5.4 mmol/L (209 mg/dL) |
|
| Interventions | 1 Placebo from 0‐8 weeks, 8‐16 weeks and 16‐24 weeks 2 Placebo from 0‐8 weeks 2 Placebo + cholestyramine 8 grams/day from 8‐16 weeks 2 Placebo + cholestyramine 16 grams/day from 16‐24 weeks 3 Fluvastatin 10 mg/day for 0‐8 weeks, 8‐16 weeks and 16‐24 weeks 4 Fluvastatin 10 mg/day for 0‐8 weeks 4 Fluvastatin 10 mg/day + cholestyramine 8 g/day from 8‐16 weeks 4 Fluvastatin 10 mg/day + cholestyramine 16 g/day from 16‐24 weeks 5 Fluvastatin 20 mg/day for 0‐8 weeks, 8‐16 weeks and 16‐24 weeks 6 Fluvastatin 20 mg/day for 0‐8 weeks 6 Fluvastatin 20 mg/day + cholestyramine 8 g/day from 8‐16 weeks 6 Fluvastatin 20 mg/day + cholestyramine 16 g/day from 16‐24 weeks |
|
| Outcomes | LDL‐Cholesterol data were reported | |
| Source of Funding | Sandoz | |
| Notes | all 6 groups were included in the efficacy analysis from 0‐8 weeks WDAEs were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo and fluvastatin capsule appearances were not reported as appearing identical Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid analysis was done at a central laboratory (Medical Research laboratories [MRL], Cincinnati,Ohio) |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | WDAEs were not analysed because participants may have been withdrawn during the phase 2 (8‐16 weeks) or phase 3 (16‐24 weeks) periods |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2.8% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Sandoz funded the study |
Stein 2008.
| Methods | 5‐week lead‐in drug washout dietary stabilization period 12‐week before and after trial |
|
| Participants | 199 men and women age ≥18 years with dyslipidaemia who had previously documented muscle‐related side effects exclusion criteria: homozygous familial hypercholesterolaemia, type I, IV, and V dyslipoproteinemias myopathy, unexplained serum creatine kinase levels > 3 X ULN history of rhabdomyolysis or any congenital muscular disease, fluvastatin and ezetimibe hypersensitivity, hepatic dysfunction, renal dysfunction acute coronary syndrome, arterial revascularization, CABG surgery and stroke within 6 months of study patients receiving drugs metabolized by cytochrome P450 2C9 69 participants received fluvastatin Fluvastatin 80 mg/day baseline TC : 6.8 mmol/L (263 mg/dL) Fluvastatin 80 mg/day baseline LDL‐C : 4.505 mmol/L (174 mg/dL) Fluvastatin 80 mg/day baseline HDL‐C : 1.386 mmol/L (54 mg/dL) Fluvastatin 80 mg/day baseline triglycerides: 1.98 mmol/L (175 mg/dL) |
|
| Interventions | Fluvastatin 80 mg/day Ezetimibe 10 mg/day Fluvastatin 80 mg/day + ezetimibe 10 mg/day |
|
| Outcomes | per cent change from baseline at 4‐12 weeks of blood TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | Novartis Pharma AG | |
| Notes | Ezetimibe 10 mg/day Fluvastatin 80 mg/day + ezetimibe 10 mg/day groups were not included in the efficacy analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Novartis Pharma AG funded the study |
Stojakovic 2010.
| Methods | no washout period required because no patient was receiving hypolipidaemic treatment within 3 months of trial entry 4‐week run‐in phase 12‐week before and after trial |
|
| Participants | 84 men and women with CHD or CHD risk equivalent with LDL‐C between 100‐160 mg/dL (2.59‐4.14 mmol/L) 28 participants received fluvastatin exclusion criteria: heart failure stage III‐IV, age older than 80 years, previous acute coronary syndrome or CABG within the last 8 weeks of study |
|
| Interventions | Fluvastatin 80 mg/day Fluvastatin 80 mg/day + ezetimibe 10 mg/day |
|
| Outcomes | per cent change from baseline at 12 weeks of serum TC and LDL‐C | |
| Source of Funding | Astellas/Novartis and MSD | |
| Notes | HDL‐C and triglyceride data were not included in the efficacy analysis because the calculated values were different by more than 10% from the given values | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Astellas/Novartis and MSD funded the study |
Susekov 1998.
| Methods | 8‐week dietary run‐in period 12‐week before and after trial |
|
| Participants | 61 men and women with type 2 diabetes mellitus have TC > 6.5 mmol/L (251 mg/dL) LDL‐C > 3.5 mmol/L (135 mg/dL) and triglycerides < 4.5 mmol/L (399 mg/dL) were included in the screening phase and 24 were complied with the inclusion/exclusion criteria The active phase of the study included 24 patients aged 57.7 years with type 2 diabetes and primary hyperlipidaemia type 11b and 23 patients completed the study and were included in the efficacy analysis 24 patients received fluvastatin 20 mg/day for 6 weeks in patients where LDL‐C remained above 2.6 mmol/L (101 mg/dL) the dose was doubled to 40 mg/day for the next 6 weeks exclusion criteria: none reported Fluvastatin 20 mg/day baseline TC : 7.0 mmol/L (271 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 4.75 mmol/L (184 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.04 mmol/L (40 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 2.66 mmol/L (236 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day for 0‐6 weeks Fluvastatin 20‐40 mg/day for 6‐12 weeks |
|
| Outcomes | per cent change from baseline at 6 weeks of serum TC, LDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | Fluvastatin 20‐40 mg/day for 6‐12 weeks time period was not included in the efficacy analysis HDL‐C data were not included in the efficacy analysis because the calculated value was different by more than 10% from the given value SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Tambaki 2004.
| Methods | 3‐month dietary washout period 3‐month before and after trial |
|
| Participants | 90 dyslipidaemic patients were divided into those with type IIa dyslipidaemia LDL‐C > 160 mg/dL (4.14 mmol/L) and type IIb dyslipidaemia LDL‐C > 160 mg/dL (4.14 mmol/L) and triglycerides > 200 mg/dL (2.26 mmol/L) type IIa received fluvastatin and type IIb received ciprofibrate 50 participants received fluvastatin exclusion criteria: liver disease, renal failure, diabetes mellitus, thyroid disease, cardiovascular disease or smoking history, birth control pills and HRT Fluvastatin 40 mg/day baseline TC : 7.76 mmol/L (300 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 5.43 mmol/L (210 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.37 mmol/L (53 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 1.95 mmol/L (173 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day Ciprofibrate 100 mg/day |
|
| Outcomes | per cent change from baseline at 3 months of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | Ciprofibrate 100 mg/day group was not included in the efficacy analysis SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Tan 1999.
| Methods | 9‐week single‐blind placebo run‐in phase with diet stabilisation 12‐week randomised, double‐blind, placebo‐controlled trial |
|
| Participants | Men and women age 35‐70 years LDL‐C > 4.1 mmol/L (159 mg/dL) TG < 4 mmol/L (354 mg/dL) exclusion criteria:significant renal or hepatic impairment, uncontrolled hypertension congestive heart failure patients taking lipid‐lowering agents within 3 months of trial Placebo baseline TC : 6.61 mmol/L (256 mg/dL) Placebo baseline LDL‐C : 4.83 mmol/L (187 mg/dL) Placebo baseline HDL‐C : 1.1 mmol/L (43 mg/dL) Fluvastatin 20 mg/day baseline TC : 6.74 mmol/L (261 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 4.84 mmol/L (187 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.13 mmol/L (44 mg/dL) |
|
| Interventions | Placebo for 0‐6 weeks Placebo for 6‐12 weeks Fluvastatin 20 mg/day for 0‐6 weeks Fluvastatin 40 mg/day for 6‐12 weeks |
|
| Outcomes | per cent change from baseline at 8‐12 weeks of serum TC, LDL‐C and HDL‐C | |
| Source of Funding | Novartis | |
| Notes | Placebo for 6‐12 weeks Fluvastatin 40 mg/day for 6‐12 weeks groups were not analysed Triglycerides were not measured because they were expressed as geometric mean percent change WDAEs were not reported SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind treatment placebo and fluvastatin capsule appearances were not reported as appearing identical Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | WDAEs were not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Novartis funded the trial |
Tazuma 1995.
| Methods | 4‐6 week placebo washout period 12‐week before and after trial |
|
| Participants | 19 men and women with type IIa and IIb hypercholesterolaemia serum total cholesterol ≥ 220 mg/dL (5.69 mmol/L) aged 40‐75 years exclusion criteria: none reported Fluvastatin 30 mg/day baseline TC : 7.22 mmol/L (279 mg/dL) Fluvastatin 30 mg/day baseline LDL‐C : 5.25 mmol/L (203 mg/dL) Fluvastatin 30 mg/day baseline HDL‐C : 1.22 mmol/L (47 mg/dL) Fluvastatin 30 mg/day baseline triglycerides: 1.64 mmol/L (145 mg/dL) |
|
| Interventions | Fluvastatin 30 mg/day | |
| Outcomes | per cent change from baseline at 4‐12 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | SDs were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Tekin 2008.
| Methods | no washout required because no participant was on any lipid medication within 1 month of trial 12‐week before and after trial |
|
| Participants | 29 men and women from Turkey with type II and III chronic heart failure participants received heart failure medications for at least 3 months before trial entrance LDL‐C > 100 mg/dL (2.59 mmol/L) exclusion criteria: receiving statins within 3 months of study and uncontrolled diabetes mellitus no baseline data reported |
|
| Interventions | Fluvastatin 80 mg/day | |
| Outcomes | per cent change from baseline at 12 weeks of plasma TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | SDs were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Tomlinson 1995.
| Methods | 4‐week placebo run‐in washout period 8‐week before and after trial |
|
| Participants | 31 Chinese patients with hypercholesterolaemia received fluvastatin plasma total cholesterol > 7.5 mmol/L (290 mg/dL) plasma TG ≤ 3.5 mmol/L (310 mg/dL) exclusion criteria: plasma TG > 3.5 mmol/L (310 mg/dL) uncontrolled diabetes Fluvastatin 20 mg/day baseline TC : 8.4 mmol/L (325 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 6.1 mmol/L (236 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.4 mmol/L (54 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 2.2 mmol/L (195 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day for 0‐4 weeks Fluvastatin 40 mg/day for 4‐8 weeks |
|
| Outcomes | per cent change from baseline at 4 weeks of plasma TC and LDL‐C | |
| Source of Funding | Sandoz | |
| Notes | Fluvastatin 40 mg/day for 4‐8 weeks group was not analysed HDL‐C and triglyceride data were not included in the efficacy analysis because the calculated values were different by more than 10% from the given values |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Sandoz provided financial support |
Tsirpanlis 2004.
| Methods | no washout period required because no patient was receiving hypolipidaemic treatment 4‐week before and after trial |
|
| Participants | 69 men and women hyperlipidaemic or normolipidaemic haemodialysis patients exclusion criteria: inflammatory events due to infection trauma, surgery, MI, active collagen disease, neoplasia, hepatic dysfunction Fluvastatin 40 mg/day baseline TC : 5.6 mmol/L (217 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 3.35 mmol/L (130 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.09 mmol/L (42 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 2.1 mmol/L (186 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day | |
| Outcomes | per cent change from baseline at 4 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | SDs were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 26.1% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
TULIPS 2007.
| Methods | no washout period required because no patient was receiving hypolipidaemic treatment within 3 months of the trial entry 12‐week before and after trial |
|
| Participants | 224 patients aged 21‐75 years with primary hypercholesterolaemia LDL‐C levels >3.37 to < 5.70 mmol/L (> 143 to < 220 mg/dL) and triglyceride levels < 4.52 mmol/L (< 400 mg/dL) exclusion criteria: homozygous familial hypercholesterolaemia, type II, III, IV, V hyperlipidaemia secondary hyperlipidaemia, type 1 diabetes mellitus, serious renal failure, hepatic disease, pregnancy or lactation, MI, unstable angina pectoris, serious arrhythmias, syncope, heart failure III and IV cardiac surgery within last 3 months, prior or current myalgia and cancer Fluvastatin 80 mg/day baseline TC : 6.5 mmol/L (251 mg/dL) Fluvastatin 80 mg/day baseline LDL‐C : 4.3 mmol/L (166 mg/dL) Fluvastatin 80 mg/day baseline HDL‐C : 1.3 mmol/L (50 mg/dL) Fluvastatin 80 mg/day baseline triglycerides: 1.8 mmol/L (159 mg/dL) |
|
| Interventions | Fluvastatin XL 80 mg/day | |
| Outcomes | per cent change from baseline at 3‐6 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | Novartis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2.2% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Novartis funded the trial |
Tvorogova 1998.
| Methods | no washout period required because no patient was receiving hypolipidaemic treatment within 3 months of trial entry 1‐month dietary stabilisation period 3‐month before and after trial |
|
| Participants | 61 patients with primary hyperlipoproteinaemia, CAD with stable angina class II and III Total cholesterol > 6.5 mmol/L (250 mg/dL) LDL‐C > 4.3 mmol/L (165 mg/dL) 36 patients mean age of 45.9 years received simvastatin 25 patients mean age of 47.2 years received fluvastatin exclusion criteria: diabetes, nephrotic syndrome, chronic renal failure, liver disease, hypothyroidism, congestive heart failure, obesity grade II and III, worsening of diseases of the gastrointestinal tract Fluvastatin 20 mg/day baseline TC : 10.52 mmol/L (407 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 7.92 mmol/L (306 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.27 mmol/L (49 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 2.5 mmol/L (221 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day Simvastatin 10 mg /day |
|
| Outcomes | per cent change from baseline at 3 months of blood TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | Simvastatin 10 mg /day group was not included in the efficacy analysis SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Valdivielso 2009.
| Methods | 6‐week dietary washout period 8‐week before and after trial |
|
| Participants | 8 men and women with type 2 diabetes mellitus and mixed hyperlipidaemia age 57 years old exclusion criteria: known vascular disease Fluvastatin 80 mg/day baseline TC : 6.7 mmol/L (259 mg/dL) Fluvastatin 80 mg/day baseline LDL‐C : 4.06 mmol/L (157 mg/dL) Fluvastatin 80 mg/day baseline HDL‐C : 0.88 mmol/L (34 mg/dL) Fluvastatin 80 mg/day baseline triglycerides: 3.8 mmol/L (337 mg/dL) |
|
| Interventions | Fluvastatin 80 mg/day for 0‐8 weeks Fluvastatin 80 mg/day + Omacor 4 g/day for 8‐16 weeks |
|
| Outcomes | per cent change from baseline at 8 weeks of plasma TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | Ferrer‐Novag company | |
| Notes | Fluvastatin 80 mg/day + Omacor 4 g/day for 8‐16 weeks group was not included in the efficacy analysis SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11.1% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Ferrer‐Novag company funded the study |
Visseren 2001.
| Methods | 8‐week dietary run‐in period 12‐week randomised double‐blind, placebo‐controlled trial |
|
| Participants | 87 men and women aged 40‐75 years with type 2 diabetes mellitus for at least 6 months and receiving stable insulin therapy plasma LDL‐C > 4.1 mmol/L (159 mg/dL) glycated haemoglobin < 8% exclusion criteria:ketoacidosis, MI or coronary angioplasty within 6 months prior to the study severe congestive heart failure, unstable angina pectoris, uncontrolled severe hypertension, retinopathy, alcoholism, hypothyroidism, BMI>35, receiving glucose‐lowering drugs, beta‐blockers, diuretics LDL‐C > 7.0 mmol/L (271 mg/dL) TG > 8.0 mmol/L (709 mg/dL), raises transaminase levels or proteinuria, pregnancy Placebo baseline TC : 6.4 mmol/L (247 mg/dL) Placebo baseline LDL‐C : 4.8 mmol/L (186 mg/dL) Placebo baseline HDL‐C : 1.2 mmol/L (46 mg/dL) Placebo baseline triglycerides: 1.7 mmol/L (151 mg/dL) Fluvastatin 40 mg/day baseline TC : 6.7 mmol/L (259 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 5.1 mmol/L (197 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.2 mmol/L (46 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 1.9 mmol/L (168 mg/dL) |
|
| Interventions | Placebo of 12 weeks Fluvastatin 40 mg/day for 12 weeks |
|
| Outcomes | per cent change from baseline at 8‐12 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | Novartis | |
| Notes | WDAEs were not reported in the 0‐12 week time period of interest SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was based upon a computer‐generated random number programme without stratification |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind all medications were given as identical capsules |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | All laboratory investigations were carried out by a central laboratory, neither the investigators nor the patients were informed about serum cholesterol or other lipid levels throughout the study |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | WDAEs were not reported in the 0‐12 week time period of interest |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Novartis funded the trial |
Wang 2004.
| Methods | no washout required because no participant received lipid‐lowering agents within 3 months of trial 8‐week before and after trial |
|
| Participants | 35 men and women with hypercholesterolaemia age 18‐75 years TC ≥ 6.5 mmol/L (251 mg/dL) LDL‐C ≥ 3.4 mmol/L (131 mg/dL) TG > 2.3 mmol/L ( 204 mg/dL) exclusion criteria: coronary heart disease, congestive heart failure, cardiac arrhythmia, diabetes, alcohol abuse, pregnancy, oestrogen use Fluvastatin 40 mg/day baseline TC : 7.04 mmol/L (272 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 4.12 mmol/L (159 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.25 mmol/L (48 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 2.11 mmol/L (187 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day | |
| Outcomes | per cent change from baseline at 8 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | SDs were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Wang 2008.
| Methods | no washout required because no participant received lipid‐lowering agents 2‐month randomised placebo‐controlled trial |
|
| Participants | 120 men and women with acute cerebral infarction and hyperlipidaemia TC > 5.72 mmol/L (221 mg/dL) LDL‐C > 3.64 mmol/L (141 mg/dL) HDL‐C < 1.0 mmol/L (39 mg/dL) TG > 1.7 mmol/L ( 151 mg/dL) exclusion criteria: severe liver disease, renal disease, statin hypersensitivity and lack of compliance Placebo baseline TC : 5.47 mmol/L (212 mg/dL) Placebo baseline LDL‐C : 2.89 mmol/L (112 mg/dL) Placebo baseline triglycerides: 2.27 mmol/L (201 mg/dL) Fluvastatin 40 mg/day baseline TC : 5.48 mmol/L (212 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 2.91 mmol/L (113 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 2.29 mmol/L (203 mg/dL) |
|
| Interventions | Placebo Fluvastatin 40 mg every night Xuezhikang 0.6 mg twice daily |
|
| Outcomes | per cent change from baseline at 2 months of blood TC, LDL‐C and triglycerides | |
| Source of Funding | unknown | |
| Notes | Xuezhikang 0.6 mg twice daily group was not included in the efficacy analysis WDAEs were not reported SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | WDAEs were not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Watanabe 2001.
| Methods | no washout required because no participant received lipid‐lowering agents 12‐month before and after trial |
|
| Participants | 31 women with hyperlipidaemia serum total cholesterol > 220 mg/dL (5.69 mmol/L) exclusion criteria: none Fluvastatin 20 mg/day baseline TC : 6.23 mmol/L (241 mg/dL) |
|
| Interventions | 15 participants received fluvastatin 20 mg/day 16 participants received pravastatin 10 mg/day |
|
| Outcomes | per cent change from baseline at 1 month of blood total cholesterol | |
| Source of Funding | unknown | |
| Notes | pravastatin 10 mg/day group was not included in the efficacy analysis SD was imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No participants had there LDL‐C measured |
| Selective reporting (reporting bias) | High risk | LDL‐C outcome was not reported |
| Other bias | Unclear risk | The source of funding was not reported |
Weiss 1998.
| Methods | 8‐week dietary‐stabilisation drug washout period 12‐week before and after trial |
|
| Participants | 1776 men and women 18‐75 years old with moderate hypercholesterolaemia LDL‐C ≥150 mg/dL (3.88 mmol/L) exclusion criteria: triglycerides ≥ 350 mg/dL (3.95 mmol/L) SGOT > 1.2 X ULN type 1 diabetes mellitus, participants were 40% above ideal weight Fluvastatin 20 mg/day baseline TC : 6.81 mmol/L (263 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 4.59 mmol/L (177 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.29 mmol/L (50 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 2.06 mmol/L (182 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day for 0‐6 weeks Fluvastatin could be titrated to 40 mg/day for 6‐12 weeks |
|
| Outcomes | per cent change from baseline at 6 weeks of plasma TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | the titrated time period of 6‐12 weeks was not included in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8.3% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Winkler 2002.
| Methods | 4‐week dietary run‐in period 8‐week randomised, double‐blind placebo‐controlled |
|
| Participants | 89 men and women with type 2 diabetes and hyperlipidaemia LDL‐C 3.37‐5.96 mmol/L (130‐230 mg/dL) TG 1.37‐6.84 mmol/L (121‐606 mg/dL) exclusion criteria:surgery MI or angioplasty during the 6 months before randomisation, uncontrolled hypertension, liver disease, chronic renal failure myopathy, alcohol/drug abuse, statin hypersensitivity, pregnancy, insulin or oral contraceptives Placebo baseline TC : 6.17 mmol/L (239 mg/dL) Placebo baseline LDL‐C : 3.29 mmol/L (127 mg/dL) Placebo baseline HDL‐C : 1.09 mmol/L (42 mg/dL) Placebo baseline triglycerides: 2.43 mmol/L (215 mg/dL) Fluvastatin 80 mg/day baseline TC : 6.32 mmol/L (244 mg/dL) Fluvastatin 80 mg/day baseline LDL‐C : 3.37 mmol/L (130 mg/dL) Fluvastatin 80 mg/day baseline HDL‐C : 1.17 mmol/L (45 mg/dL) Fluvastatin 80 mg/day baseline triglycerides: 2.41 mmol/L (213 mg/dL) |
|
| Interventions | Placebo for 8 weeks Fluvastatin 80 mg/day for 8 weeks |
|
| Outcomes | per cent change from baseline at 8 weeks of plasma TC, LDL‐C, and triglycerides | |
| Source of Funding | Novartis | |
| Notes | no WDAEs reported HDL‐C data were not included in the efficacy analysis because the calculated value was different by more than 10% from the given value SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo and fluvastatin capsule appearances were not reported as appearing identical Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | WDAEs were not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Novartis funded the trial |
Wittke 1999.
| Methods | 4‐week run‐in period 3‐month before and after trial |
|
| Participants | 18 men with a lipid disorder age 38‐65 years BMI 24.2‐33.5 HDL‐C 40 mg/dL (1.03 mmol/L) exclusion criteria: none reported Fluvastatin 20 mg/day baseline TC : 8.3 mmol/L (321 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 6.4 mmol/L (247 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.15 mmol/L (44 mg/dL) |
|
| Interventions | 6 men did not receive any treatment before the exercise period (control group) 6 men received fluvastatin 20 mg/day 3 months before the exercise period (pretreatment group) 6 men received fluvastatin 20 mg/day after the 4 week run‐in period from the start of the exercise period (treatment group) |
|
| Outcomes | per cent change from baseline at 3 months of serum total cholesterol, LDL‐C and HDL‐C | |
| Source of Funding | unknown | |
| Notes | the control and pretreatment groups were not included in the efficacy analysis for triglycerides the calculated value was different from the given data by more than 10% SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Wu 2005.
| Methods | 4‐week placebo dietary run‐in period 12‐week before and after trial |
|
| Participants | 61 men and women ≥18 years with primary hypercholesterolaemia LDL‐C ≥ 160 mg/dL (4.14 mmol/L) triglycerides ≤ 400 mg/dL (4.52 mmol/L) exclusion criteria: pregnant or lactating, uncontrolled hypertension, congestive heart failure severe or unstable angina pectoris, diabetes mellitus, uncontrolled hypothyroidism, renal impairment, chronic liver disease acute illness or severe trauma within 3 months of study MI, major surgery, coronary angioplasty within 6 months before study Fluvastatin 40 mg/day baseline TC : 6.96 mmol/L (269 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 4.94 mmol/L (191 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.24 mmol/L (48 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 1.7 mmol/L (151 mg/dL) Fluvastatin 80 mg/day baseline TC : 6.88 mmol/L (266 mg/dL) Fluvastatin 80 mg/day baseline LDL‐C : 4.81 mmol/L (186 mg/dL) Fluvastatin 80 mg/day baseline HDL‐C : 1.22 mmol/L (47 mg/dL) Fluvastatin 80 mg/day baseline triglycerides: 1.89 mmol/L (167 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day IR for 12 weeks Fluvastatin 80 mg/day XR for 12 weeks |
|
| Outcomes | per cent change from baseline at 12 weeks of serum TC and LDL‐C | |
| Source of Funding | Novartis | |
| Notes | HDL‐C and triglyceride data were not included in the efficacy analysis because the calculated values were different by more than 10% from the given values for the 40 mg/day and 80 mg/day doses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | High risk | Novartis funded the trial |
Yamagishi 2009.
| Methods | no washout period required because no patient was receiving hypolipidaemic treatment 3‐month before and after trial |
|
| Participants | 72 participants with hypercholesterolaemia TC ≥ 220 mg/dL ( 5.69 mmol/L) LDL‐C ≥ 140 mg/dL ( 3.62 mmol/L) triglycerides ≥ 150 mg/dL ( 1.69 mmol/L) no participant had hypertension, diabetes, recent cardiovascular events, ischaemic heart disease, atrial fibrillation, arteriosclerosis obliterans renal of hepatic dysfunction 62 participants received fluvastatin 10 participants received no statin treatment (control group) exclusion criteria: congestive heart failure Fluvastatin 30 mg/day baseline LDL‐C : 4.02 mmol/L (155 mg/dL) |
|
| Interventions | Fluvastatin 30 mg/day no statin treatment |
|
| Outcomes | per cent change from baseline at 1‐3 months of plasma LDL‐C | |
| Source of Funding | unknown | |
| Notes | control group was not included in the efficacy analysis SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13.9% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Yamamoto 1995.
| Methods | 4‐week dietary run‐in period 52‐week before and after trial |
|
| Participants | 49 men and women with type IIa and IIb hypercholesterolaemia total cholesterol > 220 mg/dL (5.69 mmol/L) exclusion criteria: hypothyroidism, Cushings disease, gallbladder disease, pancreatitis, cancer, unstable diabetes, severe hypertension, alcohol abuse, obese people on diet, renal, liver dysfunction, brain disease, heart disease statin hypersensitivity and lupus 25 participants received fluvastatin Fluvastatin 20 mg/day baseline TC : 7.11 mmol/L (275 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 4.74 mmol/L (183 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.61 mmol/L (62 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 1.988 mmol/L (176 mg/dL) |
|
| Interventions | Fluvastatin 20 mg/day from 0‐12 weeks Fluvastatin 20‐30 mg/day from 12‐24 weeks Fluvastatin 20‐40 mg/day from 24‐52 weeks Pravastatin 10 mg/day from 0‐12 weeks Pravastatin 10‐20 mg/day from 12‐24 weeks Pravastatin 10‐20 mg/day from 24‐52 weeks |
|
| Outcomes | per cent change from baseline at 12 weeks of blood TC, LDL‐C and HDL‐C | |
| Source of Funding | unknown | |
| Notes | Fluvastatin 20‐30 mg/day from 12‐24 weeks Fluvastatin 20‐40 mg/day from 24‐52 weeks Pravastatin 10 mg/day from 0‐12 weeks Pravastatin 10‐20 mg/day from 12‐24 weeks Pravastatin 10‐20 mg/day from 24‐52 weeks groups were not included in the efficacy analysis Triglyceride data were not included in the efficacy analysis because the calculated value was different by more than 10% from the given value |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Yasuda 2004.
| Methods | 4‐week dietary run‐in period 48‐week before and after trial |
|
| Participants | 80 Japanese men and women with type 2 diabetes mellitus and hyperlipidaemia age 38‐75 years with advanced nephropathy Total cholesterol > 6.2 mmol/L (240 mg/dL) triglycerides < 4.52 mmol/L (400 mg/dL) urinary protein excretion 0.5‐3.0 g/day serum creatinine concentration < 440 μmol/L creatinine clearance 20‐70 mL/min/1.73m2 exclusion criteria: endocrinological, haematological or hepatic disease; cerebral infarction or haemorrhage; homozygous familial hypercholesterolaemia; MI occurring within the previous 6 months; unstable angina, nephrotic syndrome; or other major diseases Fluvastatin 20 mg/day baseline TC : 6.8 mmol/L (263 mg/dL) Fluvastatin 20 mg/day baseline LDL‐C : 4.4 mmol/L (170 mg/dL) Fluvastatin 20 mg/day baseline HDL‐C : 1.3 mmol/L (50 mg/dL) Fluvastatin 20 mg/day baseline triglycerides: 2.46 mmol/L (218 mg/dL) |
|
| Interventions | 39 participants received fluvastatin 20 mg/day for 48 weeks 41 participants received diet only for 48 weeks |
|
| Outcomes | per cent change from baseline at 4‐12 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | SDs were imputed by the method of Furukawa 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Zavoral 1996.
| Methods | 6‐week placebo washout period 9‐week randomised double‐blind placebo‐controlled trial |
|
| Participants | 602 patients with primary hypercholesterolaemia with LDL‐C 160‐400 mg/dL (4.14‐10.3 mmol/L) and TG ≤ 350 mg/dL (3.95 mmol/L) no exclusion criteria no baseline values reported |
|
| Interventions | Placebo Fluvastatin 20 mg/day |
|
| Outcomes | per cent change from baseline at 6‐9 weeks of serum TC, LDL‐C, HDL‐C, and triglycerides | |
| Source of Funding | unknown | |
| Notes | SDs were imputed by the method of Furukawa 2006 WDAEs were not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random sequence generation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind treatment placebo and fluvastatin capsule appearances were not reported as appearing identical Lipid parameter measurements unlikely influenced by lack of proper blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | WDAEs were not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Unclear risk | Source of funding was not reported |
Zhang 2014.
| Methods | no washout period required because no patient was receiving hypolipidaemic treatment 3‐month before and after trial |
|
| Participants | 68 men and women with cardiac syndrome X exclusion criteria: MI, valvular heart disease, left ventricular hypertrophy, hypertension, congestive heart failure, oestrogen replacement therapy and participants receiving lipid‐lowering agents 23 participants received fluvastatin Fluvastatin 40 mg/day baseline TC : 5.65 mmol/L (218 mg/dL) Fluvastatin 40 mg/day baseline LDL‐C : 4.18 mmol/L (162 mg/dL) Fluvastatin 40 mg/day baseline HDL‐C : 1.25 mmol/L (48 mg/dL) Fluvastatin 40 mg/day baseline triglycerides: 2.02 mmol/L (179 mg/dL) |
|
| Interventions | Fluvastatin 40 mg/day Diltiazem 90 mg/day Fluvastatin 40 mg/day + Diltiazem 90 mg/day |
|
| Outcomes | per cent change from baseline at 3 months of serum TC, LDL‐C, HDL‐C and triglycerides | |
| Source of Funding | National Natural Science Foundation of China (No. 81100207) | |
| Notes | Diltiazem 90 mg/day Fluvastatin 40 mg/day + Diltiazem 90 mg/day groups were not included in the efficacy analysis SDs were imputed by the method of Furukawa 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before and after design |
| Allocation concealment (selection bias) | High risk | Controlled before and after design |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Lipid parameter measurements unlikely influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) LDL‐cholesterol | Low risk | Lipid parameters were measured in a remote laboratory |
| Blinding of outcome assessment (detection bias) WDAEs | High risk | No comparison possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4.3% participants were not included in the efficacy analysis |
| Selective reporting (reporting bias) | Low risk | LDL‐C outcome was reported |
| Other bias | Low risk | National Natural Science Foundation of China (No. 81100207) |
ACE: angiotensin‐converting‐enzyme ,ALT: alanine aminotransferase, AST: aspartate aminotransferase, BMI: basal metabolic index, BP: blood pressure, CABG: coronary artery bypass grafting; CAD: coronary artery disease, CFR: coronary flow reserve, CHD: coronary heart disease,CPK: creatine phosphokinase, CRP: C‐reactive protein, CYP: cytochrome P‐450, , g: gram, GI: gastrointestinal, HDL‐C: high‐density lipoprotein cholesterol, HRT: hormone replacement therapy, LDL‐C: low‐density lipoprotein cholesterol, mg/d; milligram per day, mmol/L: millimoles per litre, MI: myocardial infarction, NIDDM: non–insulin‐dependent diabetes mellitus, p: probability, PAOD: peripheral arterial obstructive disease, PCI: percutaneous coronary intervention, PTCA: percutaneous transluminal coronary angioplasty, SD: standard deviation, sdLDL: small dense low‐density lipoprotein, SGOT: serum glutamic oxaloacetic transaminase, TC: total cholesterol, TG: triglycerides, TIA: transient ischaemic attack, TSH: thyroid stimulating hormone, WDAEs: withdrawal due to adverse events, ULN: upper limit of normal, XL: extended release
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Afzal 1999 | given LDL‐C values were significantly different from Friedewald calculated values |
| Akiyama 2001 | confounding factors immunosuppressants |
| Alaupovic 2006 | combined data for all cross‐over periods |
| Ambrosi 2000 | confounding factor immunosuppressants |
| Anderssen 2005 | placebo data were subtracted from the treatment data |
| Asztalos 2002 | combined data for all cross‐over periods |
| Austen 1996 | confounding factor cyclosporine |
| Ballantyne 2000 | endpoint after week 2 is variable undefined endpoint as to time period |
| Benesic 2004 | confounding factor indinavir an antiretroviral agent |
| Blann 2001 | fluvastatin dosing not specific 20 mg/day or 40 mg/day |
| Brorholt‐Petersen 2001 | data were combined for all cross‐over periods |
| Broyles 1995 | all lipids were reported as median per cent change from baseline |
| Chen 2001 | no library has this journal for 1997 |
| Eagles 1996 | lipid data were combined for all cross‐over periods |
| Eichstadt 1995 | lipid data were for titrated doses of 40 mg/day to 80 mg/day |
| Ersoy 2014 | confounding factor immunosuppressants |
| EudraCt 2006 | trial results are not available EMA does not hold the CSR, study sponsor Abteilung Klinsche Chemie, UKL Freiburg and BfArM (National Competent Authority) did not respond to our request for trial results |
| Ghods 1995a | confounding factor immunosuppressants |
| Goldberg 1996 | confounding factor is cyclosporine |
| Gomez 1999 | confounding factors immunosuppressants |
| Gottsater 1999 | median per cent change from baseline |
| Guethlin 1999 | some participants received a fluvastatin dose increase at one month, 2‐month data dosing is 40 mg/day to 80 mg/day |
| Gurgun 2008 | run‐in period too short, 2 weeks |
| Haasis 1996 | median per cent reduction in LDL‐C |
| Hagen 1994 | no 3‐12 week lipid data for the 20 mg/day dose and no washout between the 20 mg/day and 40 mg/day dose |
| Haramaki 2007 | data from cross‐over periods were combined |
| He 2001 | not available from any library |
| He 2007 | Insufficient baseline 2‐week dietary washout period |
| Hilleman 2000 | data were combined for both cross‐over periods |
| Holdaas 1995 | confounding factor cyclosporine |
| Hongo 2008 | dose is 20 mg/day to 40 mg/day, dose is not specific |
| Illingworth 1996 | data were combined for all cross‐over periods |
| Inoue 2011a | dosing is 10 mg/day to 30 mg/day, not a specific dose |
| Koizumi 1995 | participants increased dosage from 20 mg/day to 30 mg/day at week 8, only week 12‐24 week data were reported |
| Kuril'skaia 1997 | 18 participants received 20 mg/day and 12 participants received 40 mg/day fluvastatin; data for both groups were combined |
| Lal 1997 | confounding factor immunosuppressants |
| Li 1995 | confounding factors immunosuppressants |
| Locsey 1997 | confounding factors immunosuppressants |
| Marcus 1994 | 31% participants were not included in the efficacy analysis |
| Mattu 2000 | no library has this volume and issue not available |
| Matzkies 1999 | some participants were on the immunosuppressant cyclosporine |
| Merck Sharp & Dohme 2015 | this is a general statin study not a fluvastatin study |
| Miwa 2005 | combined data for all cross‐over periods |
| Murdock 1999 | data from non‐specific HMG CoA Reductase Inhibitors |
| NOVARTIS 2003 | could not calculate the per cent change from baseline, absolute change was reported |
| NOVARTIS 2004 | could not calculate the per cent change from baseline, absolute change was reported; all cross‐over period data were combined |
| NOVARTIS 2006a | absolute change was reported no baseline values were given therefore the per cent change from baseline could not be calculated |
| NOVARTIS 2012 | some participants were receiving lipid‐lowering monotherapy improperly prior to visit 1 and some participants received fluvastatin immediate release capsule 40 mg once daily during the 6‐week open‐label study phase |
| O'Rourke 2004 | confounding factor participants received immunosuppressants such a cyclosporine |
| Ostadal 2010 | data is expressed as fluvastatin minus placebo |
| Paragh 1999 | 28% participants were not included in the efficacy analysis |
| Peters 1994 | Evident Bias Introduced Drug Company Data |
| Podder 1997 | confounding factor participants received immunosuppressants such a cyclosporine |
| Rindone 1998 | all lipid data were combined for all cross‐over points |
| Robertsen 2014 | confounding factor immunosuppressant everolimus in renal transplant patients |
| Romano 2000 | TC ≤ 23.7 ± 7% and LDL‐C ≤ 32 ± 12%, lipid values are not specific |
| Samuelsson 2002 | combined data for both cross‐over periods |
| Sasaki 1997 | confounding factor is probucol |
| Schaefer 2004 | lipid data combined, periods 1 and 2 may be a cross‐over trial |
| Schobel 1998 | dose is 40 mg/day to 80 mg/day, dosing was not specific |
| Schrama 1998 | confounding factor immunosuppressant cyclosporine |
| Setiawati 2008 | no proper washout period for those patients who received previous medications for dyslipidaemia and change in total cholesterol and LDL cholesterol went down by about another 11.3% from week 4 to week 8 |
| Sheridan 2014 | fluvastatin 40 mg/day for 0‐4 weeks, 80 mg/day for 5‐12 weeks lipid data at 12 weeks only reported titrated dose trial |
| Smit 1995 | data were combined for both cross‐over periods |
| Teramoto 1995 | variable dosing |
| Turk 2001 | confounding factor immunosuppressants |
| van der Graaf 2006 | median per cent change |
| van der Linde 2006 | all data combined from both cross‐over periods |
| van Haelst 2001 | 1 patient was receiving a fibrate drug at baseline |
| Westphal 2008 | data were combined for all cross‐over periods |
| Westphal 2009 | data were combined for all cross‐over periods |
| Widimsky 1997 | length of period where all lipid‐lowering agents were withdrawn before the trial was not reported |
| Widimsky 1999 | patients received 20 mg per day fluvastatin during the 6‐week run‐in period |
| Wu 2014 | lipid labelling is incorrect |
| Yamawaki 2007 | data from all statins were combined |
| Yang 2000 | lipid washout of lipid altering agents of 5 half‐lives not 3‐week washout period |
| Yuan 1991 | lipid data were from all fluvastatin doses combined |
| Zhang 2005 | lipid data were combined for all cross‐over periods |
| Zhao 2014 | 6 week run‐in with fluvastatin 40 mg/day |
EMA: European Medicine Agency, CSR: clinical study report, HMG‐CoA: 3‐hydroxy‐3‐methyl‐glutaryl‐coenzyme A, LDL‐C: low‐density lipoprotein cholesterol, TC: total cholesterol,
Differences between protocol and review
Trials in which participants were receiving drugs that affect blood lipid level concentrations such as immunosuppressants such as cyclosporine and protease inhibitors such as ritonavir and indinavir were classified as excluded trials. Trials where more than 25% of the participants were not included in the efficacy analysis were classified as excluded trials. These were not mentioned in the protocol. We conducted sensitivity analyses to assess the effect of different methods of dosing, such as twice daily versus single dose, on the treatment effect. This sensitivity analysis was not mentioned in the protocol.
Contributions of authors
JMW, MT and SPA contributed to the design of the protocol.
MT, SPA and SSS extracted the data
SPA analysed the data and made contributions to the discussion
JMW interpreted the data, made contributions to the discussion and conclusions
Sources of support
Internal sources
-
Department of Anesthesiology, Pharmacology & Therapeutics, University of BC, Canada.
Office space
External sources
-
BC Ministry of Health grant to the Therapeutics Initiative, Canada.
Salary support
Declarations of interest
None known.
New
References
References to studies included in this review
Abetel 1998 {published data only}
- Abetel G, Poget PN, Bonnabry JP. Antihypertensive effect of a cholesterol‐lowering agent (fluvastatin). Pilot study. Schweizerische Medizinische Wochenschrift 1998;128(7):272‐7. [MEDLINE: ] [PubMed] [Google Scholar]
ACCESS 2001 {published data only}
- Andrews TC, Ballantyne CM, Hsia JA, Kramer JH. Achieving and maintaining National Cholesterol Education Program low‐density lipoprotein cholesterol goals with five statins. American Journal of Medicine 2001;111(3):185‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ballantyne CM, Andrews TC, Hsia JA, Kramer JH, Shear C, the ACCESS Study Group. Correlation of non‐high‐density lipoprotein cholesterol with apolipoprotein B: effect of 5 hydroxymethylglutaryl coenzyme A reductase inhibitors on non‐high‐density lipoprotein cholesterol levels. American Journal of Cardiology 2001;88(3):265‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Smith DG, McBurney CR. An Economic Analysis of the Atorvastatin Comparative Cholesterol Efficacy and Safety Study (ACCESS). Pharmacoeconomics 2003;21(Supplement 1):13‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
AlvarezSala 2008 {published data only}
- AlvarezSala LA, Cachofeiro V, Masana L, Suarez C, Pinilla B, Plana N, et al. Effects of fluvastatin extended‐release (80 mg) alone and in combination with ezetimibe (10 mg) on low‐density lipoprotein cholesterol and inflammatory parameters in patients with primary hypercholesterolemia: a 12‐week, multicenter, randomized, open‐label, parallel‐group study. Clinical Therapeutics 2008;30(1):84‐97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Novartis. A multicenter, randomized, open‐label, parallel‐group study to evaluate the efficacy and safety of the combination of extended‐release fluvastatin with ezetimibe versus extended‐release fluvastatin alone on low density lipoprotein ( LDL) cholesterol levels. Study Number CXUO320BES03 2005.
- Novartis. Efficacy and safety study of fluvastatin and ezetimibe combined versus fluvastatin alone. ClinicalTrials.gov 2011. [NCT00171288]
Baggio 1994a {published data only}
- Baggio G, Candia O, Forte PL, Mello F, Crepaldi G. Efficacy and safety of fluvastatin, a new HMG CoA reductase inhibitor, in elderly hypercholesterolemic women. Drugs 1994;47(Suppl 2):59‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Baggio 1994b {published data only}
- Baggio G, Candia O, Forte P L, Mello F, Andriolli A, Donazzan S, et al. Efficacy and safety of fluvastatin in elderly hypercholesterolemic patients: A pilot study. Current Therapeutic Research ‐ Clinical and Experimental 1994;55(4):401‐7. [EMBASE: 24176118] [Google Scholar]
Bard 1995 {published data only}
- Bard JM, Dallongeville J, Hagen E, Pfister P, Ose L, Fruchart JC, et al. Comparison of the effect of fluvastatin, an hydroxymethyl glutaryl coenzyme A reductase inhibitor, and cholestyramine, a bile acid sequestrant, on lipoprotein particles defined by apolipoprotein composition. Metabolism: Clinical and Experimental 1995;44(11):1447‐54. [CENTRAL: CN‐00120297 UPDATE] [DOI] [PubMed] [Google Scholar]
Berger 1996 {published data only}
- Berger ML, Wilson HM, Liss CL. A comparison of the tolerability and efficacy of lovastatin 20 mg and fluvastatin 20 mg in the treatment of primary hypercholesterolemia. Journal of Cardiovascular Pharmacology and Therapeutics 1996;1(2):101‐6. [1996:597464 CAN125:316905] [DOI] [PubMed] [Google Scholar]
Betteridge 1994 {published data only}
- Betteridge DJ, Durrington PN, Fairhurst GJ, Jackson G, McEwan MSR, McInnes GT, et al. Comparison of lipid‐lowering effects of low‐dose fluvastatin and conventional‐dose gemfibrozil in patients with primary hypercholesterolemia. American Journal of Medicine 1994;96(6 PART A):45S‐54S. [BIOSIS :PREV199497408612] [DOI] [PubMed] [Google Scholar]
Bevilacqua 1997 {published data only}
- Bevilacqua M, Bettica P, Milani M, Vago T, Rogolino A, Righini V, et al. Effect of fluvastatin on lipids and fibrinolysis in coronary artery disease. American Journal of Cardiology 1997;79(1):84‐7. [CENTRAL: CN‐00136455 UPDATE] [DOI] [PubMed] [Google Scholar]
Bevilacqua 2004 {published data only}
- Bevilacqua M, Guazzini B, Righini V, Barrella M, Chebat E. Metabolic effects of fluvastatin extended release 80 mg and atorvastatin 20 mg in patients with type 2 diabetes mellitus and low serum high‐density lipoprotein cholesterol levels: A 4‐month, prospective, open‐label, randomized, blinded‐end point (Probe) trial. Current Therapeutic Research ‐ Clinical & Experimental 2004;65(4):330‐44. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bevilacqua 2005 {published data only}
- Bevilacqua M, Righini V, Barrella M, Vago T, Chebat E, Dominguez LJ. Effects of fluvastatin slow‐release (XL 80 mg) versus simvastatin (20 mg) on the lipid triad in patients with type 2 diabetes. Advances in Therapy 2005;22(6):527‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bjarnason 2001 {published data only}
- Bjarnason NH, Riis BJ, Christiansen C. The effect of fluvastatin on parameters of bone remodeling. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA 2001;12(5):380‐4. [DOI] [PubMed] [Google Scholar]
Branchi 1999 {published data only}
- Branchi A, Fiorenza AM, Rovellini A, Torri A, Muzio F, Macor S, et al. Lowering effects of four different statins on serum triglyceride level. European Journal of Clinical Pharmacology 1999;55(7):499‐502. [CENTRAL: CN‐00167879 UPDATE] [DOI] [PubMed] [Google Scholar]
Broncel 2007 {published data only}
- Broncel M, Balcerak M, Cieslak D, Duchnowicz P, Koter‐Michalak M, Sikora J, et al. [Effect of fluvastatin extended release on the protein‐lipid structure of erythrocyte membrane and C‐reactive protein in patients with hyperlipidemia]. Polski Merkuriusz Lekarski 2007;22(128):107‐11. [MEDLINE: ] [PubMed] [Google Scholar]
Brown 1998 {published data only}
- Brown AS, Bakker‐Arkema RG, Yellen L, Henley RW Jr, Guthrie R, Campbell CF, et al. Treating patients with documented atherosclerosis to National Cholesterol Education Program‐recommended low‐density‐lipoprotein cholesterol goals with atorvastatin, fluvastatin, lovastatin and simvastatin. Journal of the American College of Cardiology 1998;32(3):665‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bruckert 2003 {published data only}
- Bruckert E, Lievre M, Giral P, Crepaldi G, Masana L, Vrolix M, et al. Short‐term efficacy and safety of extended‐release fluvastatin in a large cohort of elderly patients. American Journal of Geriatric Cardiology 2003;12(4):225‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Couvert P, Giral P, Dejager S, Gu J, Huby T, Chapman MJ, et al. Association between a frequent allele of the gene encoding OATP1B1 and enhanced LDL‐lowering response to fluvastatin therapy. Pharmacogenomics 2008;9(9):1217‐27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bruni 2003 {published data only}
- Bruni F, Puccetti L, Pasqui AL, Pastorelli M, Bova G, Cercignani M, et al. Different effect induced by treatment with several statins on monocyte tissue factor expression in hypercholesterolemic subjects. Clinical and Experimental Medicine 2003;3(1):45‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Buzkova 2012 {published data only}
- Buzkova H, Pechandova K, Danzig V, Vareka T, Perlik F, Zak A, et al. Lipid‐lowering effect of fluvastatin in relation to cytochrome P450 2C9 variant alleles frequently distributed in the Czech population. Medical Science Monitor : International Medical Journal of Experimental and Clinical Research 2012;18(8):CR512‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buzzi 1997 {published data only}
- Buzzi AP, Pastore MA, Argentine and Multicenter Evaluation Investigators. Argentine multicenter evaluation of fluvastatin in the treatment of patients with hypercholesterolemia. Current Therapeutic Research ‐ Clinical and Experimental 1997;58(12):1013‐28. [DOI: 10.1016/S0011-393X(97)80068-9] [DOI] [Google Scholar]
Ceska 1996 {published data only}
- Ceska R. Fluvastatin in the treatment of hyperlipoproteinemia, preliminary results. Vnitrni Lekarstvi 1996;42(8):533‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Cingozbay 2002 {published data only}
- Cingozbay BY, Top C, Terekeci H, Keskin O, Onde ME. Effects of fluvastatin treatment on insulin sensitivity in patients with hyperlipidaemia. Journal of International Medical Research 2002;30(1):21‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
CURVES 1998 {published data only}
- Hilleman DE, Heineman SM, Foral PA. Pharmacoeconomic assessment of HMG‐CoA reductase inhibitor therapy: analysis based on the CURVES study. Pharmacotherapy:The Journal of Human Pharmacology & Drug Therapy 2000;20(7):819‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hilleman DE, Mohiuddin SM, Wurdeman R L, Holmberg MJ, Woodruff MP. The curves trial: A pharmacoeconomic evaluation. American Journal of Managed Care 1997;3(10):1573. [WOS:A1997YJ15800026] [Google Scholar]
- Hilleman DE, Mohiuddin Syed M, Woodruff MP, Holmberg MJ, Pedersen CA, Wurdeman RL. The CURVES trial: A pharmacoeconomic evaluation. Pharmacotherapy 1997;17(5):1107‐8. [Google Scholar]
- Jones P, Kafonek S, Laurora I, Hunninghake D. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (The CURVES study) (vol 81, pg 582, 1998). American Journal of Cardiology 1998;82(1):128. [WOS:000074705000028] [DOI] [PubMed] [Google Scholar]
- Jones P, Kafonek S, Laurora I, Hunninghake D, CURVES Investigators. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (The CURVES study). American Journal of Cardiology 1998;81(5):582‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jones P, Kafonek S, Laurora I, Hunninghake D, CURVES investigators. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin and fluvastatin in patients with hypercholesterolemia (the CURVES study). Perfusion 1998;11(4):202‐8. [WOS:000073474000005] [DOI] [PubMed] [Google Scholar]
- Jones PH. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (The CURVES study) ‐ Reply. American Journal of Cardiology 1998;82(3):407. [WOS:000075193900035] [DOI] [PubMed] [Google Scholar]
- Jones PH. Comparison of the dose efficacy of atorvastatin with pravastatin, simvastatin, fluvastatin and lovastatin: The CURVES study. European Heart Journal 1997;18(ABSTR. SUPPL.):371. [Google Scholar]
- Jones PH, Blumenthal RS. Comparative dose efficacy of statins. Cardiology Review. United States: MRA Publications Inc., 1998; Vol. 15, issue 12:25‐9. [EMBASE: 29155698]
- Kafonek S, CURVES Investigators. The CURVES study ‐ A comparison of the dose efficacy of atorvastatin with pravastatin, simvastatin, fluvastatin and lovastatin. Journal of Hypertension 1998;16:S232. [CENTRAL: CN‐00866607] [Google Scholar]
- Pincus J. Comparative dose efficacy study of atorvastatin versus simvastatin, pravastatin, lovastatin, and fluvastatin in patients with hypercholesterolemia (the CURVES study). American Journal of Cardiology 1998;82(3):406‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Russell MW, Huse DM, Miller J D, Kraemer DF, Hartz SC. Cost effectiveness of HMG‐CoA reductase inhibition in Canada. Canadian Journal of Clinical Pharmacology = Journal Canadien de Pharmacologie Clinique 2001;8(1):9‐16. [MEDLINE: ] [PubMed] [Google Scholar]
Dallongeville 1994a {published data only}
- Dallongeville J, Fruchart J ‐C, Pfister P, Bard J ‐M. Fluvastatin reduces levels of plasma Apo B‐containing particles and increases those of LpA‐I. American Journal of Medicine 1994;96(6 PART A):32S‐6S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dallongeville 1994b {published data only}
- Dallongeville J, Fruchart J ‐C, Pfister P, Bard J ‐M. Fluvastatin reduces levels of plasma Apo B‐containing particles and increases those of LpA‐I. American Journal of Medicine 1994;96(6 PART A):32S‐6S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Dallongeville J, Fruchart JC, Pfister P, Bard JM. Effect of fluvastatin on plasma apolipoprotein‐B‐containing particles, including lipoprotein(a). European Fluvastatin Study Group. Journal of Internal Medicine. Supplement 1994;736:95‐101. [MEDLINE: ] [PubMed] [Google Scholar]
Davidson 2003 {published data only}
- Davidson MH, Palmisano J, Wilson H, Liss C, Dicklin MR. A multicenter, randomized, double‐blind clinical trial comparing the low‐density lipoprotein cholesterol‐lowering ability of lovastatin 10, 20, and 40 mg/d with fluvastatin 20 and 40 mg/d. Clinical Therapeutics 2003;25(11):2738‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dergunov 2003 {published data only}
- Dergunov AD, Perova NV, Visvikis S, Siest G. Time‐dependent lipid response on fluvastatin therapy of patients with hypercholesterolemia sensitive to apoE phenotype. Vascular Pharmacology 2003;40(5):237‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Di Lullo 2005 {published data only}
- Lullo L, Addesse R, Comegna C, Firmi G, Polito P. Effects of fluvastatin treatment on lipid profile, C‐reactive protein trend, and renal function in dyslipidemic patients with chronic renal failure. Advances in Therapy 2005;22(6):601‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ding 1997 {published data only}
- Ding PYA, Sheu WHH, Hu CA, Pei D. Efficacy and safety of fluvastatin in patients with non‐insulin‐ dependent diabetes mellitus and hypercholesterolemia. Acta Cardiologica Sinica 1997;13(3):138‐44. [EMBASE: 28068982] [Google Scholar]
Dujovne 1994 {published data only}
- Dujovne CA, Davidson MH. Fluvastatin administration at bedtime versus with the evening meal: a multicenter comparison of bioavailability, safety, and efficacy. American Journal of Medicine 1994;96(6A):37S‐40S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ertugrul 2011 {published data only}
- Ertugrul DT, Yavuz B, Cil H, Ata N, Tutal E. STATIN‐D Study: Comparison of the influences of rosuvastatin and fluvastatin treatment on the levels of 25 hydroxyvitamin D. Cardiovascular Therapeutics 2011;29(2):146‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fanghanel 1995 {published data only}
- Fanghanel G, Espinosa J, Olivares D, Sanchez L, Morales M, Martinez L, et al. Open‐label study to assess the efficacy, safety, and tolerability of fluvastatin versus bezafibratefor hypercholesterolemia. American Journal of Cardiology 1995;76(2):57A‐61A. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fanghanel Salmon 1996 {published data only}
- Fanghanel Salmon G, Salgado Loza JL, Sanchez Reyes L, Padilla Retana JA, Espinosa Campos J. The efficacy, safety and tolerance of fluvastatin sodium 40 mg in patients with hyperlipidemia type IIA. Archivos del Instituto de Cardiologia de Mexico 1996;66(2):151‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Fernandez 2001 {published data only}
- Fernandez JC, Mas R, Castan˜o G, Menendez R, Amor AM, Gonzalez RM, et al. Comparison of the efficacy, safety and tolerability of policosanol versus fluvastatin in elderly hypercholesterolaemic women. Clinical Drug Investigation 2001;21(2):103‐13. [CENTRAL: CN‐00393466] [Google Scholar]
Filippova 1997 {published data only}
- Filippova VG, Mantsurova AV, Zadionchenko VS, Zaporozhets TP. [The effect of a new hypolipemic preparation fluvastatin (Lescol) on rheological indices and hemostatic parameters]. Terapevticheskii Arkhiv 1997;69(4):43‐5. [MEDLINE: ] [PubMed] [Google Scholar]
FSGJ 1995 {published data only}
- Fluvastatin Study Group in Japan. [Clincal efficacy of fluvastatin (XU62‐320) in hyperlipidemia: A comparative study with pravastatin in a double‐blind Comparative method]. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1995;11(8):1679‐726. [CENTRAL: CN‐00582328] [Google Scholar]
Fujimoto 2004 {published data only}
- Fujimoto K, Hozumi T, Watanabe H, Shimada K, Takeuchi M, Sakanoue Y, et al. Effect of fluvastatin therapy on coronary flow reserve in patients with hypercholesterolemia. American Journal of Cardiology 2004;93(11):1419‐21, A10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Galal 1997 {published data only}
- Galal MS, Abdel Wahed M, Salem KA. Saudi Arabia experience trial of fluvastatin (Lescol) in the treatment of hyperlipidemia. Journal of the Egyptian Public Health Association 1997;72(3‐4):285‐302. [MEDLINE: ] [PubMed] [Google Scholar]
Gao 2003 {published data only}
- Gao LS, Liao Y. [Effective observation of Xue Zhi Kang and Fluvastatin for adjusting blood fat of coronary patient]. Modern Journal of Integrated Traditional Chinese and Western Medicine [Xian Dai Zhong Xi Yi Jie He za Zhi] 2003;12(23):2528. [CENTRAL: CN‐00975248] [Google Scholar]
Ghods 1995 {published data only}
- Ghods AJ, Milanian I, Ghadiri G. The effect of fluvastatin on hypercholesterolemia in patients with nephrotic syndrome. Iranian Journal of Medical Sciences 1995;20(3‐4):110‐2. [EMBASE: 26191201] [Google Scholar]
Goedecke 2002 {published data only}
- Goedecke C. Microscopic examination of the capillary microcirculation under the influence of the HMG‐CoA reductase inhibitor fluvastatin in patients with hypercholesterolemia [Kapillarmikroskopische Untersuchung der mikrozirkulation unter dem Einfluss des HMG‐CoA‐Reduktasehemmers Fluvastatin bei Patienten mit Hypercholesterinamie]. ProQuest Dissertations and Theses. Germany: Johann Wolfgang Goethe‐Universitaet Frankfurt am Main (Germany), 2002. [ProQuest document ID 305435666]
Gotoh 2011 {published data only}
- Gotoh M, Mizuno K, Ono Y, Takahashi M. Fluvastatin increases bone mineral density in postmenopausal women. Fukushima Journal of Medical Science 2011;57(1):19‐27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Greten 1994 {published data only}
- Greten H, Beil FU, Schneider J, Weisweiler P, Armstrong VW, Keller C, et al. Treatment of primary hypercholesterolemia: fluvastatin versus bezafibrate. American Journal of Medicine 1994;96(6A):55S‐63S. [PUBMED: 8017468] [DOI] [PubMed] [Google Scholar]
Guan 2004 {published data only}
- Guan JZ, Murakami H, Yamato K, Tanabe J, Matsui J, Tamasawa N, et al. Effects of fluvastatin in type 2 diabetic patients with hyperlipidemia: reduction in cholesterol oxidation products and VCAM‐1. Journal of Atherosclerosis and Thrombosis 2004;11(2):56‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Haak 2001 {published data only}
- Haak E, Abletshauser C, Weber S, Goedicke C, Martin N, Hermanns N, et al. Fluvastatin therapy improves microcirculation in patients with hyperlipidaemia. Atherosclerosis 2001;155(2):395‐401. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hailer 1996 {published data only}
- Hailer S, Pogarell O, Keller C, Wolfram G. Effect of fluvastatin or bezafibrate on the distribution of high density lipoprotein subpopulations in patients with familial hypercholesterolemia. Arzneimittel‐Forschung 1996;46(9):879‐83. [MEDLINE: ] [PubMed] [Google Scholar]
Homma 2003 {published data only}
- Homma Y, Homma K, Iizuka S, Iigaya K. Effects of fluvastatin on plasma levels of low‐density lipoprotein subfractions, oxidized low‐density lipoprotein, and soluble adhesion molecules: A twenty‐four‐week, open‐label, dose‐increasing study. Current Therapeutic Research ‐ Clinical & Experimental 2003;64(4):236‐47. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huhle 1999 {published data only}
- Huhle G, Abletshauser C, Mayer N, Weidinger G, Harenberg J, Heene D L. Reduction of platelet activity markers in type II hypercholesterolemic patients by a HMG‐CoA‐reductase inhibitor. Thrombosis Research 1999;95(5):229‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hunninghake 1998 {published data only}
- Hunninghake D, Bakker‐Arkema RG, Wigand JP, Drehobl M, Schrott H, Early JL, et al. Treating to meet NCEP‐recommended LDL cholesterol concentrations with atorvastatin, fluvastatin, lovastatin, or simvastatin in patients with risk factors for coronary heart disease. Journal of Family Practice 1998;47(5):349‐56. [MEDLINE: ] [PubMed] [Google Scholar]
Hunninghake 2002 {published data only}
- Hunninghake DB, Davidson M, Knapp HR, Schrott HG, Manfreda S, Angelo J, et al. Extended‐release fluvastatin 80 mg shows greater efficacy, with comparable tolerability, versus immediate‐release fluvastatin 40 mg for once daily treatment of primary hypercholesterolaemia. British Journal of Cardiology 2002;9(8):469‐75. [CENTRAL: CN‐00642353] [Google Scholar]
Hussein 2002 {published data only}
- Hussein O, Shneider J, Rosenblat M, Aviram M. Valsartan therapy has additive anti‐oxidative effect to that of fluvastatin therapy against low‐density lipoprotein oxidation: studies in hypercholesterolemic and hypertensive patients. Journal of Cardiovascular Pharmacology 2002;40(1):28‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ichihara 2002 {published data only}
- Ichihara A, Hayashi M, Ryuzaki M, Handa M, Furukawa T, Saruta T. Fluvastatin prevents development of arterial stiffness in haemodialysis patients with type 2 diabetes mellitus. Nephrology, Dialysis, Transplantation : official publication of the European Dialysis and Transplant Association ‐ European Renal Association 2002;17(8):1513‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Inoue 2011 {published data only}
- Inoue T, Taguchi I, Abe S, Toyoda S, Node K. Inhibition of intestinal cholesterol absorption might explain cholesterol‐lowering effect of telmisartan. Journal of Clinical Pharmacy & Therapeutics 2011;36(1):103‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Insull 1994 {published data only}
- Insull W Jr, Black D, Dujovne C, Hosking JD, Hunninghake D, Keilson L, et al. Efficacy and safety of once‐daily vs twice‐daily dosing with fluvastatin, a synthetic reductase inhibitor, in primary hypercholesterolemia. Archives of Internal Medicine 1994;154(21):2449‐55. [MEDLINE: ] [PubMed] [Google Scholar]
Isaacsohn 1999 {published data only}
- Isaacsohn J, Stein E, Orchard T, Weinstein R, Huh C, Whalen E, et al. Comparative efficacy, safety , and tolerability of cerivastatin A new HMG‐CoA reductase inhibitor, and fluvastatin in subjects with primary hypercholesterolemia. American Journal of Health‐System Pharmacy 1999;56(Supplement 1):50. [Google Scholar]
Isaacsohn 2003 {published data only}
- Isaacsohn JL, LaSalle J, Chao G, Gonasun L. Comparison of treatment with fluvastatin extended‐release 80‐mg tablets and immediate‐release 40‐mg capsules in patients with primary hypercholesterolemia. Clinical Therapeutics 2003;25(3):904‐18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Itakura 1995 {published data only}
- Itakura H, Goto Y, Nakamura H, Yoshida S, Saito Y, Yasugi T, et al. [Clinical effect of fluvastatin (XU62‐320) on hyperlipidemia: double‐blind comparative preliminary dose‐finding study in four parallel groups]. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1995;11:103‐29. [CENTRAL: CN‐00545865] [Google Scholar]
Ito 1995 {published data only}
- Ito H, Nakano T, Yoshida S, Saito Y, Murano S, Orimo H, et al. [The effects of the long‐term treatment of fluvastatin, a newly developed HMG‐CoA reductase inhibitor, on serum lipid and endocrine function in hyperlipidemic subjects]. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1995;11:45‐78. [CENTRAL: CN‐00546389] [Google Scholar]
Jacobson 1994 {published data only}
- Jacobson TA, Amorosa LF. Combination therapy with fluvastatin and niacin in hypercholesterolemia: a preliminary report on safety. American Journal of Cardiology 1994;73(14):25D‐9D. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jacobson TA, Chin MM, Fromell GJ, Jokubaitis LA, Amorosa LF. Fluvastatin with and without niacin for hypercholesterolemia. American Journal of Cardiology 1994;74(2):149‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Jacobson TA, Jokubaitis LA, Amorosa LF. Fluvastatin and niacin in hypercholesterolemia: a preliminary report on gender differences in efficacy. American Journal of Medicine 1994;96(6A):64S‐8S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jacotot 1994 {published data only}
- Jacotot B, Banga JD, Pfister P, Mehra M. Efficacy of a low dose‐range of fluvastatin (Xu‐62‐320) in the treatment of primary hypercholesterolemia ‐ a dose‐response study in 431 patients. British Journal of Clinical Pharmacology 1994;38(3):257‐63. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacotot 1995 {published data only}
- Jacotot B, Benghozi R, Pfister P, Holmes D. Comparison of fluvastatin versus pravastatin treatment of primary hypercholesterolemia. American Journal of Cardiology 1995;76(2):54A‐6A. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jarai 1996 {published data only}
- Jarai Z, Kapocsi J, Farsang C, Detki K, Pados G, Sebestyen Z, et al. [Effect of fluvastatin on serum lipid levels in essential hypertension]. Orvosi Hetilap 1996;137(34):1857‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Jokubaitis 1994 {published data only}
- Jokubaitis LA, Knopp RH, Frohlich J. Efficacy and safety of fluvastatin in hyperlipidaemic patients with non‐insulin‐dependent diabetes mellitus. Journal of Internal Medicine. Supplement 1994;736:103‐7. [MEDLINE: ] [PubMed] [Google Scholar]
- Knopp RH, Frohlich J, Jokubaitis LA, Dawson K, Broyles FE, Gomez‐Coronado D. Efficacy and safety of fluvastatin in patients with non‐insulin‐dependent diabetes mellitus and hyperlipidemia. American Journal of Medicine 1994;96(6A):69S‐78S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Knopp RH, Frolich JJ. Efficacy and safety of fluvastatin in patients with non‐insulin‐dependent diabetes mellitus and hyperlipidemia: preliminary report. American Journal of Cardiology 1994;73(14):39D‐41D. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Khan 1999 {published data only}
- Khan F, Litchfield SJ, Stonebridge PA, Belch JJ. Lipid‐lowering and skin vascular responses in patients with hypercholesterolaemia and peripheral arterial obstructive disease. Vascular Medicine (London, England) 1999;4(4):233‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Klosiewicz‐Latoszek 2003 {published data only}
- Klosiewicz‐Latoszek L, Respondek W, Bialobrzeska‐Paluszkiewicz J, Grzybowska B, Jakobisiak‐Ostasz B, Stolarska I. Efficacy and safety of combined statin‐fenofibrates therapy compared with monotherapy in patients with mixed hyperlipidemia and high risk of coronary heart disease. Polski Merkuriusz Lekarski : Organ Polskiego Towarzystwa Lekarskiego 2003;15(85):42‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Koren 1999 {published data only}
- Koren M J, BakkerArkema RG. Achieving LDL goals: A comparison of four statins. Cardiology Review 1999;16(9):34‐7. [CENTRAL: CN‐00414495] [Google Scholar]
Kowalski 2006 {published data only}
- Kowalski J, Barylski M, Banach M, Grycewicz J, Irzmanski R, Pawlicki L. Neutrophil superoxide anion generation during atorvastatin and fluvastatin therapy used in coronary heart disease primary prevention. Journal of Cardiovascular Pharmacology 2006;48(4):143‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kozlov 2000 {published data only}
- Kozlov SG, Lyakishev AA, Tvorogova MG. Assessment of efficacy of fluvastatin and fenofibrate in patients with noninsulin‐dependent diabetes with hypercholesterolemia and combined hyperlipidemia. Kardiologiya 2000;40(5):4‐9. [WOS:000088205600001] [Google Scholar]
Lan 2001 {published data only}
- Lan W. Clinical features and risk of coronary heart disease in familial hypercholesterolaemia and studies on hypolipidaemic drug treatment in Hong Kong Chinese. ProQuest Dissertations and Theses Global Ph.D.. Hong Kong: The Chinese University of Hong Kong (Hong Kong), 2001. [ProQuest document ID 231403100]
LCAS 1997 {published data only}
- Aldrete VJ. Effects of fluvastatin on atherosclerosis [A propósito del estudio LCAS, efectos de la fluvastatina sobre la aterosclerosis]. Medicina Interna de Mexico 1998;14(3):I‐IV. [Google Scholar]
- Ballantyne CM, Herd JA, Ferlic LL, Dunn JK, Farmer JA, Jones PH, et al. Influence of low HDL on progression of coronary artery disease and response to fluvastatin therapy. Circulation 1999;99(6):736‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ballantyne CM, Schein JR, Jones P H, Gotto AM Jr. Treatment of patients with mild to moderate hypercholesterolemia: Lipoprotein and Coronary Atherosclerosis Study (LCAS). Cardiovascular Reviews and Reports 1998;19(1):12‐24. [EMBASE: 1998028193] [Google Scholar]
- Chen S, Tsybouleva N, Ballantyne CM, Gotto AM Jr, Marian AJ. Effects of PPARalpha, gamma and delta haplotypes on plasma levels of lipids, severity and progression of coronary atherosclerosis and response to statin therapy in the lipoprotein coronary atherosclerosis study. Pharmacogenetics 2004;14(1):61‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Herd JA. Lessons from clinical trials: LCAS and other studies. Medical Science Symposia Series; Multiple Risk Factors in Cardiovascular Disease: Strategies of Prevention of Coronary Heart Disease, Cardiac Failure, and Stroke. Vol. 12, Dordrecht, Netherlands: Springer, 1998:267‐74. [WOS:000073874700031] [Google Scholar]
- Herd JA. The effect of fluvastatin on coronary atherosclerosis: The Lipoprotein and Coronary Atherosclerosis Study (LCAS). Herz Kreislauf 1997;29(2):36. [WOS:A1997WN29300001] [Google Scholar]
- Herd JA. The lipoprotein and coronary atherosclerosis study (LCAS): lipid and metabolic factors related to atheroma and clinical events. American Journal of Medicine 1998;104(6A):42S‐9S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Herd JA, Ballantyne CM, Farmer JA, Ferguson JJ 3rd, Jones PH, West MS, et al. Effects of fluvastatin on coronary atherosclerosis in patients with mild to moderate cholesterol elevations (Lipoprotein and Coronary Atherosclerosis Study [LCAS]). American Journal of Cardiology 1997;80(3):278‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Herd JA, Ballantyne CM, Farmer JA, Ferguson JJ, Gould KL, Jones PH, et al. The effect of fluvastatin on coronary atherosclerosis: The lipoprotein and coronary atherosclerosis study (LCAS). Circulation 1996;94(8):3496. [WOS:A1996VN11903486] [Google Scholar]
- Herd JA, West MS, Ballantyne C, Farmer J, Gotto AM. Base‐line characteristics of subjects in the Lipoprotein and Coronary Atherosclerosis Study (Lcas) with fluvastatin. American Journal of Cardiology 1994;73(14):D42‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Herd JA, West MS, Ballantyne C, Farmer J, Gotto AM Jr. Baseline characteristics of subjects in the Lipoprotein and Coronary Atherosclerosis Study (LCAS) with fluvastatin. American Journal of Cardiology 1994;73(14):42D‐9D. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Herd JA, West MS, Ballantyne CM, Farmer JA, Ferlic LL, Jones PH, et al. Predictors of clinical cardiac and all fatal events in the Lipoprotein and Coronary Atherosclerosis Study (LCAS). Circulation 1997;96(8 SUPPL.):I27‐8. [MEDLINE: ] [Google Scholar]
- Herd JA, West MS, Ferlic L, Ballantyne CM, Gotto AM Jr. Influence of age and sex on angiographic change in the lipoprotein and coronary atherosclerosis study (LCAS). Journal of the American College of Cardiology 1997;29(2 SUPPL. A):139A. [MEDLINE: ] [Google Scholar]
- Lutucuta S, Ballantyne CM, Elghannam H, Gotto AM Jr, Marian AJ. Novel polymorphisms in promoter region of atp binding cassette transporter gene and plasma lipids, severity, progression, and regression of coronary atherosclerosis and response to therapy. Circulation Research 2001;88(9):969‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Marian AJ, Safavi F, Ferlic L, Dunn JK, Gotto AM, Ballantyne CM. Interactions between angiotensin‐I converting enzyme insertion/deletion polymorphism and response of plasma lipids and coronary atherosclerosis to treatment with fluvastatin: the lipoprotein and coronary atherosclerosis study. Journal of the American College of Cardiology 2000;35(1):89‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Salek L, Lutucuta S, Ballantyne CM, Gotto AM Jr, Marian AJ. Effects of SREBF‐1a and SCAP polymorphisms on plasma levels of lipids, severity, progression and regression of coronary atherosclerosis and response to therapy with fluvastatin. Journal of Molecular Medicine (Berlin, Germany) 2002;80(11):737‐44. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sing K, Ballantyne CM, Ferlic L, Brugada R, Cushman I, Dunn JK, et al. Lipoprotein lipase gene mutations, plasma lipid levels, progression/regression of coronary atherosclerosis, response to therapy, and future clinical events: Lipoproteins and Coronary Atherosclerosis Study. Atherosclerosis 1999;144(2):435‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Turban S, Fuentes F, Ferlic L, Brugada R, Gotto AM, Ballantyne CM, et al. A prospective study of paraoxonase gene Q/R192 polymorphism and severity, progression and regression of coronary atherosclerosis, plasma lipid levels, clinical events and response to fluvastatin. Atherosclerosis 2001;154(3):633‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vollmer H. The Lipoprotein and Coronary Atherosclerosis Study (LCAS) confirms the benefit of lipid lowering for example with fluvastatin [FLUVASTATIN. LCAS‐STUDIE BESTATIGT NUTZEN DER LIPIDSENKUNG]. Therapie und Erfolg 1998;2(1):76‐7. [EMBASE: 1998066877] [Google Scholar]
- West MS, Herd J A, Ballantyne CM, Pownall HJ, Simpson S, Gould L, et al. The Lipoprotein and Coronary Atherosclerosis Study (LCAS): design, methods, and baseline data of a trial of fluvastatin in patients without severe hypercholesterolemia. Controlled ClinicalTtrials 1996;17(6):550‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- West MS, Herd JA, Ballantyne CM, Pownall HJ, Simpson S, Gould L, et al. Erratum: The lipoprotein and coronary atherosclerosis study (LCAS): Design, methods and baseline data of a trial of fluvastatin in patients without severe hypercholesterolemia. Controlled Clinical Trials 1997;18(1):90. [EMBASE: 1997103405] [DOI] [PubMed] [Google Scholar]
Leitersdorf 1994 {published data only}
- Leitersdorf E. Gender‐related response to fluvastatin in patients with heterozygous familial hypercholesterolaemia. Drugs 1994;47(Supplement 2):54‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Leitersdorf 1995 {published data only}
- Leitersdorf E, Eisenberg S, Eliav O, Berkman N, Dann EJ, Landsberger D, et al. Efficacy and safety of high dose fluvastatin in patients with familial hypercholesterolaemia. European Journal of Clinical Pharmacology 1993;45(6):513‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Leitersdorf E, Eisenberg S, Eliav O, Friedlander Y, Berkman N, Dann EJ, et al. Genetic determinants of responsiveness to the HMG‐CoA reductase inhibitor fluvastatin in patients with molecularly defined heterozygous familial hypercholesterolemia. Circulation 1993;87(4 Suppl):III35‐44. [MEDLINE: ] [PubMed] [Google Scholar]
- Leitersdorf E, Muratti EN, Eliav O, Meiner V, Eisenberg S, Dann EJ, et al. Efficacy and safety of a combination fluvastatin‐bezafibrate treatment for familial hypercholesterolemia: comparative analysis with a fluvastatin‐cholestyramine combination. American Journal of Medicine 1994;96(5):401‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Leitersdorf E, Muratti EN, Eliva O, Peters TK. Efficacy and safety of triple therapy (fluvastatin‐bezafibrate‐cholestyramine) for severe familial hypercholesterolemia. American Journal of Cardiology 1995;76(2):A84‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Leonhardt 1997 {published data only}
- Leonhardt W, Kurktschiev T, Meissner D, Lattke P, Abletshauser C, Weidinger G, et al. Effects of fluvastatin therapy on lipids, antioxidants, oxidation of low density lipoproteins and trace metals. European Journal of Clinical Pharmacology 1997;53(1):65‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Leu 2004 {published data only}
- Leu HB, Wu CC, Wu TC, Lin SJ, Chen JW. Fluvastatin reduces oxidative stress, decreases serum monocyte chemotactic protein‐1 level and improves endothelial function in patients with hypercholesterolemia. Journal of the Formosan Medical Association = Taiwan yi zhi 2004;103(12):914‐20. [MEDLINE: ] [PubMed] [Google Scholar]
Leu 2005 {published data only}
- Leu H B, Chen J W, Wu T C, Ding Y A, Lin S J, Charng M J. Effects of fluvastatin, an HMG‐CoA reductase inhibitor, on serum levels of interleukin‐18 and matrix metalloproteinase‐9 in patients with hypercholesterolemia. Clinical Cardiology 2005;28(9):423‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2000 {published data only}
- Lin TH, Huang CH, Voon WC, Yen HW, Lai HM, Liang HY, et al. The effect of fluvastatin on fibrinolytic factors in patients with hypercholesterolemia. Kaohsiung Journal of Medical Sciences 2000;16(12):600‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Lintott 1995 {published data only}
- Lintott CJ, Scott RS, Bremer JM, Shand BI. Fluvastatin for dyslipoproteinemia, with or without concomitant chronic renal insufficiency. American Journal of Cardiology 1995;76(2):97A‐101A. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
LIPS 2003 {published data only}
- Arampatzis CA, Goedhart D, Serruys PW, Saia F, Lemos PA, Feyter P, LIPS Investigators. Fluvastatin reduces the impact of diabetes on long‐term outcome after coronary intervention‐‐a Lescol Intervention Prevention Study (LIPS) substudy. American Heart Journal 2005;149(2):329‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chaplin S, Scuffham PA, Alon M, Boom G. Secondary prevention after PCI: the cost‐effectiveness of statin therapy in the Netherlands associated included citation for the LIPS trial. Netherlands Heart Journal 2004;12:331‐6. [PMC free article] [PubMed] [Google Scholar]
- Delea TE, Jacobson TA, Serruys PW, Edelsberg JS, Oster G. Cost‐effectiveness of fluvastatin following successful first percutaneous coronary intervention. Annals of Pharmacotherapy 2005;39(4):610‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lee CH, Feyter P, Serruys PW, Saia F, Lemos PA, GoedHart D, et al. Beneficial effects of fluvastatin following percutaneous coronary intervention in patients with unstable and stable angina: results from the Lescol intervention prevention study (LIPS). Heart (London) 2004;90(10):1156‐61. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos PA, Serruys PW. Fluvastatin treatment after first PCI . Cardiology Review 2003;20(9):12‐5. [CENTRAL: CN‐00474363] [Google Scholar]
- Lemos PA, Serruys PW, Feyter P, Mercado NF, Goedhart D, Saia F, et al. Long‐term fluvastatin reduces the hazardous effect of renal impairment on four‐year atherosclerotic outcomes (a LIPS substudy). American Journal of Cardiology 2005;95(4):445‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lemos PA, Feyter PJ, Serruys PW, Saia F, Arampatzis CA, Disco C, et al. Fluvastatin reduces the 4‐year cardiac risk in patients with multivessel disease. International Journal of Cardiology 2005;98(3):479‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lesaffre E, Kocmanova D, Lemos PA, Disco C M, Serruys PW. A retrospective analysis of the effect of noncompliance on time to first major adverse cardiac event in LIPS. Clinical Therapeutics 2003;25(9):2431‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Messerli AW, Aronow HD, Sprecher DL. The Lescol Intervention Prevention Study (LIPS): start all patients on statins early after PCI. Cleveland Clinic Journal of Medicine 2003;70(6):561‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Novartis. Clinical Study Report SNDA #20‐261 SE1 033 C and #21‐192 SE1 005 C (LIPS). assessed: https://fda.opentrials.net/search URL: file:///C:/Users/Stephen%20Adams/Documents/LIPS%20Clinical%20Study%20Report%20from%20the%20FDA%20opentrials.pdf 2003.
- Saia F, Feyter P, Serruys PW, Lemos PA, Arampatzis CA, Hendrickx GR, et al. Lescol Intervention Prevention Study (LIPS) Investigators. Effect of fluvastatin on long‐term outcome after coronary revascularization with stent implantation. American Journal of Cardiology 2004;93(1):92‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Scuffham P. Use of fluvastatin following percutaneous coronary intervention. Expert Review of Pharmacoeconomics & Outcomes Research 2005;5(2):113‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Scuffham PA, Chaplin S. A cost‐effectiveness analysis of fluvastatin in patients with diabetes after successful percutaneous coronary intervention. Clinical Therapeutics 2005;27(9):1467‐77. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Scuffham PA, Chaplin S. An economic evaluation of fluvastatin used for the prevention of cardiac events following successful first percutaneous coronary intervention in the UK. Pharmacoeconomics 2004;22(8):525‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Scuffham PA, Kosa J. The cost‐effectiveness of fluvastatin in Hungary following successful percutaneous coronary intervention. Cardiovascular Drugs & Therapy 2006;20(4):309‐17. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Serruys PWJC, Feyter PJ, Benghozi R, Hugenholtz PG, Lesaffre E. The Lescol Intervention Prevention Study (LIPS): A double‐blind, placebo‐controlled, randomized trial of the long‐term effects of fluvastatin after successful transcatheter therapy in patients with coronary heart disease. International Journal of Cardiovascular Interventions. United Kingdom: Martin Dunitz Ltd, 2001; Vol. 4, issue 4:165‐72. [CENTRAL: CN‐00425771] [DOI] [PubMed]
- Serruys PWJC, Feyter P, Macaya C, Kokott N, Puel J, Vrolix M, et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA 2002;287(24):3215‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lorena 1997 {published data only}
- Lorena M, Perolini S, Casazza F, Milani M, Cimminiello C. Fluvastatin and tissue factor pathway inhibitor in type IIA and IIB hyperlipidemia and in acute myocardial infarction. Thrombosis Research 1997;87(4):397‐403. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lunder 2011 {published data only}
- Lunder M, Janic M, Habjan S, Sabovic M. Subtherapeutic, low‐dose fluvastatin improves functional and morphological arterial wall properties in apparently healthy, middle‐aged males‐‐a pilot study. Atherosclerosis 2011;215(2):446‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lunder 2012 {published data only}
- Lunder M, Janic M, Jug B, Sabovic M. The effects of low‐dose fluvastatin and valsartan combination on arterial function: a randomized clinical trial. European Journal of Internal Medicine 2012;23(3):261‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lye 1998 {published data only}
- Lye M, Valacio R, Reckless JP, Ghosh AK, Findlay IN, Ghosh MK, et al. Elderly patients with hypercholesterolaemia: a double‐blind study of the efficacy, safety and tolerability of fluvastatin. Coronary Artery Disease 1998;9(9):583‐90. [MEDLINE: ] [PubMed] [Google Scholar]
Mark 2001 {published data only}
- Mark L, Simondan G, Nagy E, Katona A. The effect of fluvastatin on QT dispersion and lipid levels. Kardiologia Polska 2001;55(11):386‐8. [EMBASE: 2001415646] [Google Scholar]
Martin 2002 {published data only}
- Martin NI. [Untersuchungen zum Einfluss von Fluvastatin auf die Mikrozirkulation bei Patienten mit Hypercholesterinamie durchgefuhrt mittels Laser Doppler Technik]. ProQuest Dissertations and Theses Dr.. Germany: Johann Wolfgang Goethe‐Universitaet Frankfurt am Main (Germany), 2002. [ProQuest document ID 305472562]
Marz 2001 {published data only}
- Marz W, Scharnagl H, Abletshauser C, Hoffmann MM, Berg A, Keul J, et al. Fluvastatin lowers atherogenic dense low‐density lipoproteins in postmenopausal women with the atherogenic lipoprotein phenotype. Circulation 2001;103(15):1942‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Milani 1995 {published data only}
- Milani M, Cimminiello C, Lorena M, Arpaia G, Soncini M, Bonfardeci G. Effects of two different HMG‐CoA reductase inhibitors on thromboxane production in type IIA hypercholesterolemia. Biomedicine & Pharmacotherapy 1996;50(6‐7):269‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Milani M, Cimminiello C, Merlo B, Lorena M, Arpaia G, Bonfardeci G. Effects of fluvastatin and pravastatin on lipid profiles and thromboxane production in type IIa hypercholesterolemia. American Journal of Cardiology 1995;76(2):51A‐53A. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mirdamadi 2008 {published data only}
- Mirdamadi HZ, Sztanek F, Derdak Z, Seres I, Harangi M, Paragh G. The human paraoxonase‐1 phenotype modifies the effect of statins on paraoxonase activity and lipid parameters. British Journal of Clinical Pharmacology 2008;66(3):366‐74. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moradmand 1998 {published data only}
- Moradmand S, Shafiee A, Niakan MR, Fouladkou F. A comparison between fluvastatin and lovastatin effects in Iranian patients with hypercholesterolemia. Acta Medica Iranica 1998;36(2):97‐101. [CENTRAL: CN‐00664188] [Google Scholar]
MUST 2001 {published data only}
- Dam MJ, Penn HJ, Hartog FR, Kragten HA, Trip Buirma RJ, Kastelein JJ, et al. A comparison of the efficacy and tolerability of titrate‐to‐goal regimens of simvastatin and fluvastatin: a randomized, double‐blind study in adult patients at moderate to high risk for cardiovascular disease. Clinical Therapeutics 2001;23(3):467‐78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nakaya 1995 {published data only}
- Nakaya N, Goto Y, Inoue T. [Clinical Effect of Fluvastatin (XU62‐320) on Hyperlipidemia: Comparative Study with Placebo in a Double‐Blind Method]. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1995;11:213‐34. [CENTRAL: CN‐00545174] [Google Scholar]
Nash 1996 {published data only}
- Nash DT. Meeting national cholesterol education goals in clinical practice‐‐a comparison of lovastatin and fluvastatin in primary prevention. American Journal of Cardiology 1996;78(6A):26‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NOVARTIS 2005b {unpublished data only}
- NOVARTIS. A 16 week open label randomised prospective study on vasoprotective efficacy and safety if monotherapy fluvastatin extended release (XL) 80 mg or valsartan 160 mg and their combination in dyslipidemic patients with arterial hypertension and endothelial dysfunction. Study Number CXUO320BRU01 2005.
NOVARTIS 2006b {published data only}
- NOVARTIS. A sixteen‐week, double‐blind, double‐dummy, randomized, parallel‐group, multicenter, active controlled study to assess the efficacy and safety of fluvastatin 80 mg slow release (SR) tablet compared to fluvastatin 40 mg immediate release capsule both once daily at bedtime in patients with mixed dyslipidemia or primary hypercholesterolemia. Study Number CXUO320B2302 2006.
Okopien 2005 {published data only}
- Okopien B, Krysiak R, Kowalski J, Madej A, Belowski D, Zielinski M, et al. Monocyte release of tumor necrosis factor‐alpha and interleukin‐1beta in primary type IIa and IIb dyslipidemic patients treated with statins or fibrates. Journal of Cardiovascular Pharmacology 2005;46(3):377‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Okopien B, Krysiak R, Kowalski J, Madej A, Belowski D, Zielinski M, et al. The effect of statins and fibrates on interferon‐gamma and interleukin‐2 release in patients with primary type II dyslipidemia. Atherosclerosis 2004;176(2):327‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Okopien B, Krysiak R, Madej A, Belowski D, Zielinski M, Kowalski J, et al. Effect of simvastatin and fluvastatin on plasma fibrinogen levels in patients with primary hypercholesterolemia. Polish Journal of Pharmacology 2004;56(6):781‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Olsson 2001 {published data only}
- Olsson AG, Pauciullo P, Soska V, Luley C, Pieters RE, Broda G, et al. Comparison of the efficacy and tolerability of fluvastatin extended‐release and immediate‐release formulations in the treatment of primary hypercholesterolemia: a randomized trial. Clinical Therapeutics 2001;23(1):45‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Osamah 1997 {published data only}
- Hussein O, Schlezinger S, Rosenblat M, Keidar S, Aviram M. Reduced susceptibility of low density lipoprotein (LDL) to lipid peroxidation after fluvastatin therapy is associated with the hypocholesterolemic effect of the drug and its binding to the LDL. Atherosclerosis 1997;128(1):11‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Osamah H, Mira R, Sorina S, Shlomo K, Michael A. Reduced platelet agregation after fluvastatin therapy is associated with altered platelelt lipid composition and drug binding to the platelets. British Journal of Clinical Pharmacology 1997;44(1):77‐83. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ose 1995 {published data only}
- Ose L, Scott R, Brusco O, Ugarte AC, Descamps O, Heller F, et al. Double‐blind comparison of the efficacy and tolerability of simvastatin and fluvastatin in patients with primary hypercholesterolaemia. Clinical Drug Investigation 1995;10(3):127‐38. [EMBASE: 1995283964] [DOI] [PubMed] [Google Scholar]
Parks 2006 {unpublished data only}
- Parks MH. Study CXUO320BZA01. NDA 20‐261 and 21‐192 2006.
Perova 1996 {published data only}
- Aronov DM, Akhmedzhanov NM, Vartanova OA, Volchkova TM, Gratsianskii NA, Ivleva A Ia, et al. [The hypolipidemic effect of and tolerance for Lescol in treating hypercholesterolemia in hypertension patients (an analysis of the data from a multicenter study)]. Terapevticheskii Arkhiv 1995;67(1):45‐8. [MEDLINE: ] [PubMed] [Google Scholar]
- Perova NV, Olfer'ev AM, Ozerova IN, Paramonova IV, Akhmedzhanov NM, Gratsianskii NA, et al. The association between the ability of fluvastatin (lescol) to correct atherogenic dyslipoproteinemias and human plasma lipoprotein spectra and drug dosage [Svyaz' mezhdu sposobnost'yu fluvastatina ( Lescol ) dlya korrektsii aterogennoy dyslipoproteinemias i spektry plazmy lipoproteinov cheloveka i dozy narkotikov]. Clinical Pharmacology and Therapeutics (Russia) 1996;5(3):23‐8. [Google Scholar]
Pinon 2002 {published data only}
- Pinon F, Merino JF, Ferrer JC, Martinez M, Vaya A, Aznar J. Plasma lipids and blood fluidity in patients with polygenic hypercholesterolaemia treated with fluvastatin. Clinical Hemorheology and Microcirculation 2002;27(3‐4):193‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Porsch‐Ozcurumez 2001 {published data only}
- Porsch‐Ozcurumez M, Hardt PD, Schnell‐Kretschmer H, Bergmann K, Darui C, Nonhoff J, et al. Effects of fluvastatin on biliary lipids in subjects with an elevated cholesterol saturation index. European Journal of Clinical Pharmacology 2001;56(12):873‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Puccetti 2001 {published data only}
- Puccetti L, Bruni F, Bova G, Cercignani M, Palazzuoli A, Console E, et al. Effect of diet and treatment with statins on platelet‐dependent thrombin generation in hypercholesterolemic subjects. Nutrition Metabolism & Cardiovascular Diseases 2001;11(6):378‐87. [MEDLINE: ] [PubMed] [Google Scholar]
Puccetti 2002 {published data only}
- Puccetti L, Pasqui A L, Pastorelli M, Bova G, Cercignani M, Palazzuoli A, et al. Time‐dependent effect of statins on platelet function in hypercholesterolaemia. European Journal of Clinical Investigation 2002;32(12):901‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Riegger 1999 {published data only}
- Riegger G, Abletshauser C, Ludwig M, Schwandt P, Widimsky J, Weidinger G, et al. The effect of fluvastatin on cardiac events in patients with symptomatic coronary artery disease during one year of treatment. Atherosclerosis 1999;144(1):263‐70. [DOI] [PubMed] [Google Scholar]
Rywik 1997 {published data only}
- Rywik S, Klosiewicz‐Latoszek L, Broda G, Szostak WB. [Efficacy and safety of treating hyperlipidemia type II with fluvastatin in patients with arterial hypertension]. Polski Merkuriusz Lekarski 1997;3(13):13‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Saito 1995 {published data only}
- Saito Y, Goto Y, Yasugi T, Hata Y, Nakaya N, Nakashima M. [Clinical effect of fluvastatin (XU62‐320) on hyperlipidemia: double‐blind comparative dose‐finding study in three parallel groups]. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1995;11:153‐80. [CENTRAL: CN‐00545455] [Google Scholar]
Saitta 2000 {published data only}
- Saitta A, Castaldo M, Sardo A, Saitta MN, Cinquegrani M, Bonaiuto M, et al. Effects of fluvastatin treatment on red blood cell Na+ transport systems in hypercholesterolemic subjects. Journal of Cardiovascular Pharmacology 2000;35(3):376‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sarano 2003 {published data only}
- Sarano NE, Kozlov SG, Tvorogova MG, Lyakishev AA, Naumov VG. Combination therapy with fluvastatin and fenofibrate in ischemic heart disease patients with combined hyperlipidemia and type 2 diabetes. Kardiologiia 2003;4(1):30‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Sasaki 1995a {published data only}
- Sasaki J, Yamamoto K, Kobori Shozo, Setoguchi Y, Sakakida N, Matsunaga A, et al. [Effects of fluvastatin (XU62‐320), a new inhibitor of HMG‐CoA reductase, combined with niceritrol in primary hypercholesterolemic patients]. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1995;11:279‐306. [CENTRAL: CN‐00542668] [Google Scholar]
Sasaki 1995b {published data only}
- Sasaki J, Yamamoto K, Kobori S, Setoguchi Y, Arakawa K. Effects of fluvastatin, a new inhibitor of HMG‐CoA reductase, and niceritrol on serum lipids, lipoproteins and cholesterol ester transfer activity in primary hypercholesterolemic patients. International Journal of Clinical Pharmacology & Therapeutics 1995;33(7):420‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Scharnagl 2006 {published data only}
- Scharnagl H, Vogel M, Abletshauser C, Freisinger F, Stojakovic T, Marz W. Efficacy and safety of fluvastatin‐extended release in hypercholesterolemic patients: morning administration is equivalent to evening administration. Cardiology 2006;106(4):241‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schulte 1996 {published data only}
- Levin LA, Schmidt A, Schulte K‐L, Beil S, Fager G. A comparison of clinical and pharmacoeconomic properties of fluvastatin and simvastatin in the management of primary hypercholesterolaemia. British Journal of Medical Economics. United Kingdom, 1997; Vol. 11, issue 1‐2:23‐35. [CENTRAL: CN‐00198776]
- Schulte K.‐L, Beil S. Efficacy and tolerability of fluvastatin and simvastatin in hypercholesterolaemic patients: A double‐blind, randomised, parallel‐ group comparison. Clinical Drug Investigation 1996;12(3):119‐26. [EMBASE: 1996304147] [Google Scholar]
Sejda 2006 {published data only}
- Sejda T, Jedliekova V, Svandova E, Poledne R. The effect of fluvastatin on cICAM‐1 as a biomarker of endothelial dysfunction in patients with dyslipidemia. International Angiology 2006;25(4):414‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Seres 2005 {published data only}
- Seres I, Foris G, Pall D, Kosztaczky B, Paragh G Jr, Varga Z, et al. Angiotensin II‐induced oxidative burst is fluvastatin sensitive in neutrophils of patients with hypercholesterolemia. Metabolism: Clinical and Experimental 2005;54(9):1147‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sigurdsson 1998 {published data only}
- Sigurdsson G, Haraldsdottir SO, Melberg T H, Tikkanen MJ, Miettinen TE, Kristianson KJ. Simvastatin compared to fluvastatin in the reduction of serum lipids and apolipoproteins in patients with ischaemic heart disease and moderate hypercholesterolaemia. Acta Cardiologica 1998;53(1):7‐14. [MEDLINE: ] [PubMed] [Google Scholar]
Singer 2002 {published data only}
- Singer P. Fluvastatin and fish oil are more effective on cardiovascular risk factors than fluvastatin alone. Medizinische Welt 2002;53(9):298‐302. [CENTRAL: CN‐00443733] [DOI] [PubMed] [Google Scholar]
Smit 1999 {published data only}
- Smit JW, Bruin TW, Eekhoff EM, Glatz J, Erkelens DW. Combined hyperlipidemia is associated with increased exercise‐induced muscle protein release which is improved by triglyceride‐lowering intervention. Metabolism: Clinical and Experimental 1999;48(12):1518‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Smit JW, Jansen GH, Bruin TW, Erkelens DW. Treatment of combined hyperlipidemia with fluvastatin and gemfibrozil, alone or in combination, does not induce muscle damage. American Journal of Cardiology 1995;76(2):126A‐8A. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sonmez 2003 {published data only}
- Sonmez A, Baykal Y, Kilic M, Yilmaz MI, Saglam K, Bulucu F, et al. Fluvastatin improves insulin resistance in nondiabetic dyslipidemic patients. Endocrine 2003;22(2):151‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sonmez 2006 {published data only}
- Sonmez A, Dogru T, Tasci I, Yilmaz M I, Pinar M, Naharci I, et al. The effect of fluvastatin on plasma adiponectin levels in dyslipidaemia. Clinical Endocrinology 2006;64(5):567‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Spieker 2000 {published data only}
- Spieker LE, Noll G, Hannak M, Luscher TF. Efficacy and tolerability of fluvastatin and bezafibrate in patients with hyperlipidemia and persistently high triglyceride levels. Journal of Cardiovascular Pharmacology 2000;35(3):361‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sprecher 1994 {published data only}
- Jacobson TA. Combination drug therapy for hyperlipidemia associated included citation for the Sprecher 1994 trial. Clinical Cardiology 1994;17:IV28‐34. [Google Scholar]
- Sprecher DL, Abrams J, Allen JW, Keane WF, Jokubaitis L. Low‐dose combined therapy with fluvastatin and cholestyramine in hyperlipidemic patients. Annals of Internal Medicine 1994;120(7):537‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stein 2008 {published data only}
- Novartis Pharma Services AG. A 12‐week multicentre, double blind, double dummy, randomized, parallel group, active controlled study to evaluate the efficacy and tolerability of fluvastatin extended release (Lescol XL® 80 mg) alone or in combination with ezetimibe10 mg as compared to ezetimibe monotherapy, in dyslipidemic patients with previous history of muscular complaints with other statins. EU Clinical Trials Register 2005. [EUCTR2004‐004208‐19‐NO]
- Stein EA, Ballantyne CM, Windler E, Sirnes PA, Sussekov A, Yigit Z, et al. Efficacy and tolerability of fluvastatin XL 80 mg alone, ezetimibe alone, and the combination of fluvastatin XL 80 mg with ezetimibe in patients with a history of muscle‐related side effects with other statins. American Journal of Cardiology 2008;101(4):490‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Stein EA, Ruben C, Gimpelewicz R. Fluvastatin for the treatment of patients with a history of muscle related side effects with other statins. Patent US 2009/0275551 A1 Nov 5, 2009.
Stojakovic 2010 {published data only}
- EudraCt. Fluvastatin 80 mg ret. vs combination with ezetimib 10 mg. EU Clinical Trials Register Number 2004‐002535‐12 2004.
- Stojakovic T, Campo A, Scharnagl H, Sourij H, Schmolzer I, Wascher TC, et al. Differential effects of fluvastatin alone or in combination with ezetimibe on lipoprotein subfractions in patients at high risk of coronary events. European Journal of Clinical Investigation 2010;40(3):187‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Susekov 1998 {published data only}
- Susekov AV, Tvorogova MG, Surkova EV, Antsiferov MB, Kozlov SG, Titov VN, et al. Fluvastatin treatment of hyperlipoproteinemia in patients with non‐insulin‐dependent diabetes mellitus. Kardiologiya 1998;38(3):33‐6. [WOS:000073083200007] [Google Scholar]
Tambaki 2004 {published data only}
- Tambaki AP, Rizos E, Tsimihodimos V, Tselepis AD, Elisaf M. Effects of antihypertensive and hypolipidemic drugs on plasma and high‐density lipoprotein‐associated platelet activating factor‐acetylhydrolase activity. Journal of Cardiovascular Pharmacology and Therapeutics 2004;9(2):91‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tan 1999 {published data only}
- Tan KC, Janus ED, Lam KS. Effects of fluvastatin on prothrombotic and fibrinolytic factors in type 2 diabetes mellitus. American Journal of Cardiology 1999;84(8):934‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tazuma 1995 {published data only}
- Tazuma S, Ohya T, Mizuno T, Takizawa I, Kunita T, Takata K, et al. Effects of fluvastatin on human biliary lipids. American Journal of Cardiology 1995;76(2):110A‐3A. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tekin 2008 {published data only}
- Tekin A, Sezgin N, Katircibasi MT, Tekin G, Colkesen Y, Sezgin AT, et al. Short‐term effects of fluvastatin therapy on plasma interleukin‐10 levels in patients with chronic heart failure. Coronary Artery Disease 2008;19(7):513‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tomlinson 1995 {published data only}
- Tomlinson B, Mak T, Tsui JY, Woo J, Shek CC, Critchley JA, et al. Effects of fluvastatin on lipid profile and apolipoproteins in Chinese patients with hypercholesterolemia. American Journal of Cardiology 1995;76(2):136A‐9A. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tsirpanlis 2004 {published data only}
- Tsirpanlis G, Boufidou F, Manganas S, Chantzis K, Bleta A, Stamatelou K, et al. Treatment with fluvastatin rapidly modulates, via different pathways, and in dependence on the baseline level, inflammation in hemodialysis patients. Blood Purification 2004;22(6):518‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
TULIPS 2007 {published data only}
- Ilerigelen B, Uresin Y, San M, Kultursay H, Guneri S, Serdar OA, et al. Efficacy and safety of extended‐release fluvastatin in Turkish patients with hypercholesterolaemia: TULIPS (Turkish Lipid Study). Current Medical Research and Opinion 2007;23(5):1093‐102. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tvorogova 1998 {published data only}
- Tvorogova MG, Susekov AV, Semenova OA, Kukharchuk VV, Titov VN. [Variability of the hypolipidemic action of simvastatin and fluvastatin in patients with primary hyperlipoproteinemia]. Terapevticheskii Arkhiv 1998;70(12):8‐13. [MEDLINE: ] [PubMed] [Google Scholar]
Valdivielso 2009 {published data only}
- Valdivielso P, Rioja J, Garcia‐Arias C, Sanchez‐Chaparro MA, Gonzalez‐Santos P. Omega 3 fatty acids induce a marked reduction of apolipoprotein B48 when added to fluvastatin in patients with type 2 diabetes and mixed hyperlipidemia: a preliminary report. Cardiovascular Diabetology 2009;8:1. [DOI: 10.1186/1475-2840-8-1; MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Visseren 2001 {published data only}
- Visseren FL, Bouter PK, Potter Van Loon BJ, Erkelens WD. Treatment of dyslipidemia with fluvastatin in patients with type 2 diabetes mellitus: effects on lipids, mental state and fibrinolysis. Clinical Drug Investigation 2001;21(10):671‐8. [CENTRAL: CN‐00394834] [Google Scholar]
Wang 2004 {published data only}
- Wang JQ, Gao LH, Liu GN, Qi GX, Zhou Y. Observation on effects of short term cholesterol‐lowering therapy with low‐dosage Fluvastatin on NO and endothelial function in patients with hypercholesterolemia. [Chinese]. Chinese Pharmacological Bulletin 2004;20(11):1284‐6. [EMBASE: 2005085572] [Google Scholar]
Wang 2008 {published data only}
- Wang X Y, Teng Y L, Xin H X. [The Effect of Fluvastatin and Xuezh ik ang on Serum Lipid in Patients with Cerebral Infarction and Hyper Lipidemia]. China Foreign Medical Treatment [Zhong Wai Yi Liao] 2008;27(25):11‐2. [CENTRAL: CN‐00976678] [Google Scholar]
Watanabe 2001 {published data only}
- Watanabe S, Fukumoto S, Takeuchi Y, Fujita H, Nakano T, Fujita T. Effects of 1‐year treatment with fluvastatin or pravastatin on bone. American Journal of Medicine 2001;110(7):584‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Weiss 1998 {published data only}
- Weiss RJ. Fluvastatin titrate‐to‐goal clinical practice study. American Journal of Therapeutics 1998;5(5):281‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Winkler 2002 {published data only}
- Winkler K, Abletshauser C, Friedrich I, Hoffmann MM, Wieland H, Marz W. Fluvastatin slow‐release lowers platelet‐activating factor acetyl hydrolase activity: A placebo‐controlled trial in patients with type 2 diabetes. Journal of Clinical Endocrinology & Metabolism 2004;89(3):1153‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Winkler K, Abletshauser C, Hoffmann MM, Friedrich I, Baumstark MW, Wieland H, et al. Effect of fluvastatin slow‐release on low density lipoprotein (LDL) subfractions in patients with type 2 diabetes mellitus: baseline LDL profile determines specific mode of action. Journal of Clinical Endocrinology and Metabolism 2002;87(12):5485‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wittke 1999 {published data only}
- Wittke R. Effect of fluvastatin in combination with moderate endurance training on parameters of lipid metabolism. Sports Medicine 1999;27(5):329‐35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wu 2005 {published data only}
- Wu Chih‐Cheng, Hsu Tsui‐Lieh, Chiang Hung‐Ting, Ding Philip Yu‐An. Efficacy and safety of slow‐release fluvastatin 80 mg daily in Chinese patients with hypercholesterolemia. Journal of the Chinese Medical Association : JCMA 2005;68(8):353‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yamagishi 2009 {published data only}
- Yamagishi T, Kato M, Koiwa Y, Omata K, Hasegawa H, Kanai H. Evaluation of plaque stabilization by fluvastatin with carotid intima‐ medial elasticity measured by a transcutaneous ultrasonic‐based tissue characterization system. Journal of Atherosclerosis and Thrombosis 2009;16(5):662‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yamamoto 1995 {published data only}
- Yamamoto T, Abe E, Kawahara J, Hamano K, Kagawa E, Kotake E. [Clinical safety and tolerability of long‐term treatment with fluvastatin (XU62‐320) in hypercholesterolemia: ophthalmological effect]. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1995;11(3):651‐71. [CENTRAL: CN‐00599138] [Google Scholar]
Yasuda 2004 {published data only}
- Yasuda G, Kuji T, Hasegawa K, Ogawa N, Shimura G, Ando D, et al. Safety and efficacy of fluvastatin in hyperlipidemic patients with chronic renal disease. Renal Failure 2004;26(4):411‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zavoral 1996 {published data only}
- Deslypere JP. Clinical implications of the biopharmaceutical properties of fluvastatin. American Journal of Cardiology 1994;73(14):12D‐17D. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Troendle AJ. Clinical reviews of fluvastatin: Short‐term and long‐term data. Clinical Cardiology 1994;17(12 SUPPL.):IV11‐5. [EMBASE: 1994370091] [Google Scholar]
- Zavoral JH, Winick AG, Bergmann SD, Toth J, Haggerty BJ. Clinical experience with fluvastatin‐‐‐‐the first synthetic HMG‐CoA reductase inhibitor. P & T Journal 1996;21(2):63‐78. [EMBASE: 1996069085] [Google Scholar]
Zhang 2014 {published data only}
- Zhang X, Li Q, Zhao J, Li X, Sun X, Yang H, et al. Effects of combination of statin and calcium channel blocker in patients with cardiac syndrome X. Coronary Artery Disease 2014;25(1):40‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Afzal 1999 {published data only}
- Afzal N, Raza SN, Nadeem MA, Khan JA, Israr M, Malik MA. Effect of fluvastatin on dyslipidaemia associated with type 2 diabetes mellitus. Specialist 1999;15(3):241‐8. [EMBASE: 29334056] [Google Scholar]
Akiyama 2001 {published data only}
- Akiyama T, Ishii T, Imanishi M, Nishioka T, Matsuura T, Kurita T. Efficacy and safety of treatment with low‐dose fluvastatin in hypercholesterolemic renal transplant recipients. Transplantation Proceedings 2001;33(3):2115‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Alaupovic 2006 {published data only}
- Alaupovic P, Attman P ‐O, Knight‐Gibson C, Mulec H, Weiss L, Samuelsson O. Effect of fluvastatin on apolipoprotein‐defined lipoprotein subclasses in patients with chronic renal insufficiency. Kidney International 2006;69(10):1865‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ambrosi 2000 {published data only}
- Ambrosi P, Aillaud MF, Habib G, Kreitmann B, Metras D, Luccioni R, et al. Fluvastatin decreases soluble thrombomodulin in cardiac transplant recipients. Thrombosis & Haemostasis 2000;83(1):46‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Anderssen 2005 {published data only}
- Anderssen SA, Hjelstuen AK, Hjermann I, Bjerkan K, Holme I. Fluvastatin and lifestyle modification for reduction of carotid intima‐media thickness and left ventricular mass progression in drug‐treated hypertensives. Atherosclerosis 2005;178(2):387‐97. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Asztalos 2002 {published data only}
- Asztalos BF, Horvath KV, McNamara JR, Roheim PS, Rubinstein JJ, Schaefer EJ. Comparing the effects of five different statins on the HDL subpopulation profiles of coronary heart disease patients. Atherosclerosis 2002;164(2):361‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Austen 1996 {published data only}
- Austen JL, Shifrin FA, Bartucci MR, Knauss TC, Schulak JA, Hricik DE. Effects of fluvastatin on hyperlipidemia after renal transplantation: influence of steroid therapy. Annals of Pharmacotherapy 1996;30(12):1386‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ballantyne 2000 {published data only}
- Ballantyne CM, McKenney J, Trippe BS. Efficacy and safety of an extended‐release formulation of fluvastatin for once‐daily treatment of primary hypercholesterolemia. American Journal of Cardiology 2000;86(7):759‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Benesic 2004 {published data only}
- Benesic A, Zilly M, Kluge F, Weissbrich B, Winzer R, Klinker H, et al. Lipid lowering therapy with fluvastatin and pravastatin in patients with HIV infection and antiretroviral therapy: comparison of efficacy and interaction with indinavir. Infection 2004;32(4):229‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Blann 2001 {published data only}
- Blann AD, Belgore F M, Constans J, Conri C, Lip GY. Plasma vascular endothelial growth factor and its receptor Flt‐1 in patients with hyperlipidemia and atherosclerosis and the effects of fluvastatin or fenofibrate. American Journal of Cardiology 2001;87(10):1160‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brorholt‐Petersen 2001 {published data only}
- Brorholt‐Petersen JU, Jensen HK, Raungaard B, Gregersen N, Faergeman O. LDL‐receptor gene mutations and the hypocholesterolemic response to statin therapy. Clinical Genetics 2001;59(6):397‐405. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Broyles 1995 {published data only}
- Broyles FE, Walden CE, Hunninghake DB, Hill‐Williams D, Knopp RH. Effect of fluvastatin on intermediate density lipoprotein (remnants) and other lipoprotein levels in hypercholesterolemia. American Journal of Cardiology 1995;76(2):129A‐35A. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chen 2001 {published data only}
- Chen L Q, Sun X F, Fang MJ. [Effect of fluvastatin on plasma endothelin and platelet aggregation in elderly hypertensive patients with hypercholeoterolemia]. Geriatrics and Health Care 2001;3(1):5‐6. [CENTRAL: CN‐00429264] [Google Scholar]
Eagles 1996 {published data only}
- Eagles CJ, Kendall MJ, Maxwell S. A comparison of the effects of fluvastatin and bezafibrate on exercise metabolism: a placebo‐controlled study in healthy normolipidaemic subjects. British Journal of Clinical Pharmacology 1996;41(5):381‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eichstadt 1995 {published data only}
- Eichstadt HW, Abletshauser CB, Stork T, Weidinger G. Beneficial effects of fluvastatin on myocardial blood flow at two time‐points in hypercholesterolemic patients with coronary artery disease. Journal of Cardiovascular Pharmacology 2000;35(5):735‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Eichstadt HW, Eskotter H, Hoffman I, Amthauer HW, Weidinger G. Improvement of myocardial perfusion by short‐term fluvastatin therapy in coronary artery disease. American Journal of Cardiology 1995;76(2):122A‐5A. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ersoy 2014 {published data only}
- Ersoy A, Eryilmaz S, Yildiz A, Ersoy C, Cuma Gul B, Baran I. Does fluvastatin improve arterial functions in dyslipidemic renal transplant recipients?. Biomedical Research (India) 2014;25(2):157‐60. [EMBASE: 2014179775] [Google Scholar]
EudraCt 2006 {published data only}
- EudraCt. Niaspan in combination with fluvastatin compared to fluvastatin‐monotherapy fpr patients with metabolic syndrome ‐ niaspan in combination with fluvasttin compared to fluvastatin monotherapy for metabolic syndrome. EudraCT Number 2005‐003812‐31 2006.
Ghods 1995a {published data only}
- Ghods A J, Milanian I, Arghani H, Ghadiri G. The efficacy and safety of fluvastatin in hypercholesterolemia in renal transplant recipients. Transplantation Proceedings 1995;27(5):2579‐80. [MEDLINE: ] [PubMed] [Google Scholar]
Goldberg 1996 {published data only}
- Goldberg R, Roth D. Evaluation of fluvastatin in the treatment of hypercholesterolemia in renal transplant recipients taking cyclosporine. Transplantation 1996;62(11):1559‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Goldberg RB, Roth D. A preliminary report of the safety and efficacy of fluvastatin for hypercholesterolemia in renal transplant patients receiving cyclosporine. American Journal of Cardiology 1995;76(2):107A‐9A. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gomez 1999 {published data only}
- Gomez G, Alvarez ML, Errasti P, Lavilla FJ, Garcia N, Ballester B, et al. Fluvastatin in the treatment of hypercholesterolemia in renal transplantation. Transplantation Proceedings 1999;31(6):2326‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gottsater 1999 {published data only}
- Gottsater A, Anwaar I, Lind P, Mattiasson I, Lindgarde F. Increasing plasma fibrinogen, but unchanged levels of intraplatelet cyclic nucleotides, plasma endothelin‐1, factor VII, and neopterin during cholesterol lowering with fluvastatin. Blood Coagulation & Fibrinolysis 1999;10(3):133‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guethlin 1999 {published data only}
- Guethlin M, Kasel AM, Coppenrath K, Ziegler S, Delius W, Schwaiger M. Delayed response of myocardial flow reserve to lipid‐lowering therapy with fluvastatin. Circulation 1999;99(4):475‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gurgun 2008 {published data only}
- Gurgun C, Ildizli M, Yavuzgil O, Sin A, Apaydin A, Cinar C, et al. The effects of short term statin treatment on left ventricular function and inflammatory markers in patients with chronic heart failure. International Journal of Cardiology 2008;123(2):102‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Haasis 1996 {published data only}
- Haasis R, Berger J. Fluvastatin vs. lovastatin in primary hypercholesterolemia associated excluded citation for the Haasis 1996 trial. Herz Kreislauf 1995;27(11):375‐80. [CENTRAL: CN‐00170685] [Google Scholar]
- Haasis R, Berger J, Andersson F, Kartman B, Juan J. A pharmacoeconomic evaluation of fluvastatin and lovastatin in primary hypercholesterolaemia. British Journal of Medical Economics 1996;10(2):145‐57. [CENTRAL: CN‐00181303] [Google Scholar]
Hagen 1994 {published data only}
- Hagen E, Istad H, Ose L, Bodd E, Eriksen HM, Selvig V, et al. Fluvastatin efficacy and tolerability in comparison and in combination with cholestyramine. European Journal of Clinical Pharmacology 1994;46(5):445‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Haramaki 2007 {published data only}
- Haramaki N, Ikeda H, Takenaka K, Katoh A, Sugano R, Yamagishi S, et al. Fluvastatin alters platelet aggregability in patients with hypercholesterolemia: possible improvement of intraplatelet redox imbalance via HMG‐CoA reductase. Arteriosclerosis, Thrombosis, and Vascular Biology 2007;27(6):1471‐7. [DOI] [PubMed] [Google Scholar]
He 2001 {published data only}
- He X L. Fluvastatin versus pravastatin in treating hyperlipidemia. China Pharmacist 2001;20(3):165‐7. [Google Scholar]
He 2007 {published data only}
- He BR, Jiang LY, Xiong JF, Li D M. [Effect of Xue Zhi Kang on blood fat and C‐reactive protein of aged people]. Zhejiang Journal of Integrated Traditional Chinese and Western Medicine [zhe Jiang Zhong Xi Yi Jie He za Zhi] 2007;17(10):608. [CENTRAL: CN‐00976312 NEW] [Google Scholar]
Hilleman 2000 {published data only}
- Hilleman DE, Woodruff MP, Holmberg MJ, Wurdeman RL, Seyedroudbari A. Comparative cost effectiveness of fluvastatin and lovastatin in patients with hypercholesterolemia. Journal of Managed Care Pharmacy 2000;6(May‐Jun):241‐6. [Google Scholar]
Holdaas 1995 {published data only}
- Fellstrom B, Holdaas H, Jardine AG, Holme I, Nyberg G, Fauchald P, et al. Effect of fluvastatin on renal end points in the Assessment of Lescol in Renal Transplant (ALERT) trial. Kidney international 2004;66(4):1549‐55. [DOI] [PubMed] [Google Scholar]
- Holdaas H, Fellstrom B, Jardine AG, Holme I, Nyberg G, Fauchald P, et al. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo‐controlled trial. Lancet (London, England) 2003;361(9374):2024‐31. [DOI] [PubMed] [Google Scholar]
- Holdaas H, Hartmann A, Stenstrom J, Dahl K J, Borge M, Pfister P. Effect of fluvastatin for safely lowering atherogenic lipids in renal transplant patients receiving cyclosporine. American Journal of Cardiology 1995;76(2):102A‐6A. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hongo 2008 {published data only}
- Hongo M, Tsutsui H, Mawatari E, Hidaka H, Kumazaki S, Yazaki Y, et al. Fluvastatin improves arterial stiffness in patients with coronary artery disease and hyperlipidemia: a 5‐year follow‐up study. Circulation Journal : official journal of the Japanese Circulation Society 2008;72(5):722‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Illingworth 1996 {published data only}
- Illingworth DR, Stein EA, Knopp RH, Hunninghake DB, Davidson MH. A randomized multicenter trial comparing the efficacy of simvastatin and fluvastatin. Journal of Cardiovascular Pharmacology & Therapeutics 1996;1(1):23‐30. [DOI] [PubMed] [Google Scholar]
Inoue 2011a {published data only}
- Inoue T, Ikeda H, Nakamura T, Abe S, Taguchi I, Kikuchi M, et al. Potential benefit of statin therapy for dyslipidemia with chronic kidney disease: Fluvastatin Renal Evaluation Trial (FRET). Internal Medicine 2011;50(12):1273‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Koizumi 1995 {published data only}
- Koizumi J, Haraki T, Yagi K, Inazu A, Kajinami K, Miyamoto S, et al. Clinical efficacy of fluvastatin in the long‐term treatment of familial hypercholesterolemia. American Journal of Cardiology 1995;76(2):47A‐50A. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kuril'skaia 1997 {published data only}
- Kuril'skaia TE, Tarabrin AL, Kuznetsova EE, Leont'eva VG, Chizhova EA, Misharina NP, et al. [The effect of fluvastatin (Lescol) treatment on the clinical status and function of the liver in patients with ischemic heart disease]. Terapevticheskii Arkhiv 1997;69(2):55‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Lal 1997 {published data only}
- Lal SM, Gupta N, Georgiev O, Ross G Jr. Lipid‐lowering effects of fluvastatin in renal transplant patients. A clinical observation. International Journal of Artificial Organs 1997;20(1):18‐21. [MEDLINE: ] [PubMed] [Google Scholar]
Li 1995 {published data only}
- Li PK, Mak TW, Chan TH, Wang A, Lam CW, Lai KN. Effect of fluvastatin on lipoprotein profiles in treating renal transplant recipients with dyslipoproteinemia. Transplantation 1995;60(7):652‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Locsey 1997 {published data only}
- Locsey L, Asztalos L, Kincses Z, Balazs G. Fluvastatin (Lescol) treatment of hyperlipidaemia in patients with renal transplants. International Urology & Nephrology 1997;29(1):95‐106. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marcus 1994 {published data only}
- Marcus A. Fluvastatin titrate‐to‐goal clinical practice study: Interim results. Clinical Cardiology 1994;17(12 Suppl):IV16‐IV20. [EMBASE: 1994370092] [Google Scholar]
Mattu 2000 {published data only}
- Mattu Siddiqui AM, Khalil A, Durrani N, Ahmad H, M A Mattu A M Siddiqui A Khalil NDurrani. A clinical trial of fluvastatin in hypertensive patients. Pakistan Heart Journal 2000;33(3‐4):11‐15. [CENTRAL: CN‐00746496] [Google Scholar]
Matzkies 1999 {published data only}
- Matzkies FK, Bahner U, Teschner M, Hohage H, Heidland A, Schaefer RM. Efficiency of 1‐year treatment with fluvastatin in hyperlipidemic patients with nephrotic syndrome. American Journal of Nephrology 1999;19(4):492‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Merck Sharp & Dohme 2015 {published data only}
- Merck Sharp, Dohme Corp. Observational study of approaches to lipid‐lowering therapy in Russian patients with coronary heart disease <<Treat to Goal>> (Study P05464). ClinicalTrials.gov Identifier: NCT00730132 2015.
Miwa 2005 {published data only}
- Miwa S, Watada H, Omura C, Takayanagi N, Nishiyama K, Tanaka Y, et al. Anti‐oxidative effect of fluvastatin in hyperlipidemic type 2 diabetic patients. Endocrine Journal 2005;52(2):259‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Murdock 1999 {published data only}
- Murdock DK, Murdock AK, Murdock RW, Olson KJ, Frane AM, Kersten ME, et al. Long‐term safety and efficacy of combination gemfibrozil and HMG‐CoA reductase inhibitors for the treatment of mixed lipid disorders. American Heart Journal 1999;138(1 Pt 1):151‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NOVARTIS 2003 {unpublished data only}
- NOVARTIS. An open‐label prospective multicentre study to determine the efficacy, tolerability and effect on patientcompliance of fluvastatin 80 mg treatment in patients with primary hypercholesterolemia. Study Number CXUO320BTR02 2003.
NOVARTIS 2004 {published data only}
- NOVARTIS. A double‐blind, randomised, placebo ‐controlled, 4‐armed, 3‐period cross‐over study to investigatethe effect on endothelial function of the combination therapy fluvastatin 80 mg SR and valsartan 160 mg,monotherapy fluvastatin 80 mg SR, and monotherapy valsartan 160 mg in comparison to placebo inpatients with type 2 diabetes mellitus. Study Number CXUO320DE21 2004.
NOVARTIS 2006a {published data only}
- NOVARTIS. A multicenter, randomized, double‐blind, 5‐week cross‐over study to investigate pleiotropic effectsof fluvastatin 80 mg ER and fluvastatin 40 mg bid IR in comparison to placebo in patientswith Metabolic Syndrome. Study Number CXUO230BDE30 2006.
NOVARTIS 2012 {published data only}
- NOVARTIS. Efficacy and safety of fluvastatin sodium extended release tablets 80 mg once daily in Chinese patients with primary hypercholesterolemia or mixed dyslipidemia. ClinicalTrials.gov Identifier: NCT01551173 2012.
O'Rourke 2004 {published data only}
- O'Rourke B, Barbir M, Mitchell AG, Yacoub MH, Banner NR. Efficacy and safety of fluvastatin therapy for hypercholesterolemia after heart transplantation: results of a randomised double blind placebo controlled study. International Journal of Cardiology 2004;94(2‐3):235‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ostadal 2010 {published data only}
- Novartis. Fluvastatin in patients with Acute Coronary Syndrome (FACS study). Study Number: CXUO320BCZ01 2006.
- Novartis. Fluvastatin in the therapy of Acute Coronary Syndrome. ClinicalTrials.gov 2010. [NCT00171275]
- Ostadal P, Alan D, Vejvoda J, Kukacka J, Macek M, Hajek P, et al. Fluvastatin in the First‐line therapy of Acute Coronary Syndrome: results of the multicenter, randomized, double‐blind, placebo‐controlled trial (the FACS‐trial). Trials [Electronic Resource] 2010;11:61. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paragh 1999 {published data only}
- Paragh G, Balogh Z, Kakuk G, Kovacs P. Comparison of the lipid‐lowering effects of fluvastatin, lovastatin and simvastatin in patients with hyperlipoproteinaemia. An internally controlled clinical study. Clinical Drug Investigation 1999;18(3):209‐15. [EMBASE: 1999338979] [Google Scholar]
Peters 1994 {published data only}
- Peters TK, Muratti EN, Mehra M. Efficacy and safety of fluvastatin in women with primary hypercholesterolemia. Drugs 1994;47(Suppl 2):64‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Podder 1997 {published data only}
- Podder H, Gero L, Foldes K, Szabo J, Lazar N, Jaray J. Treatment of metabolic disorders with fluvastatin after renal transplantation. Transplantation Proceedings 1997;29(1‐2):216‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rindone 1998 {published data only}
- Rindone JP, Hiller D, Arriola G. A comparison of fluvastatin 40 mg every other day versus 20 mg every day in patients with hypercholesterolemia. Pharmacotherapy 1998;18(4):836‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Robertsen 2014 {published data only}
- Robertsen I, Asberg A, Granseth T, Vethe NT, Akhlaghi F, Ghareeb M, et al. More potent lipid‐lowering effect by rosuvastatin compared with fluvastatin in everolimus‐treated renal transplant recipients. Transplantation 2014;97(12):1266‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Romano 2000 {published data only}
- Romano M, Mezzetti A, Marulli C, Ciabattoni G, Febo F, Ienno S, et al. Fluvastatin reduces soluble P‐selectin and ICAM‐1 levels in hypercholesterolemic patients: role of nitric oxide. Journal of Investigative Medicine : the official publication of the American Federation for Clinical Research 2000;48(3):183‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Samuelsson 2002 {published data only}
- Samuelsson O, Attman PO, Knight‐Gibson C, Mulec H, Weiss L, Alaupovic P. Fluvastatin improves lipid abnormalities in patients with moderate to advanced chronic renal insufficiency. American Journal of Kidney Diseases 2002;39(1):67‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sasaki 1997 {published data only}
- Sasaki S, Nakagawa M, Nakata T, Endo N, Miyao K, Kitamura K, et al. Efficacy and safety of the 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase inhibitor fluvastatin in hyperlipidemic patients treated with probucol. Cardiology 1997;88(2):160‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schaefer 2004 {published data only}
- Schaefer EJ, McNamara JR, Tayler T, Daly JA, Gleason JL, Seman LJ, et al. Comparisons of effects of statins (atorvastatin, fluvastatin, lovastatin, pravastatin, and simvastatin) on fasting and postprandial lipoproteins in patients with coronary heart disease versus control subjects. American Journal of Cardiology 2004;93(1):31‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schobel 1998 {published data only}
- Schobel HP, Schmieder RE. Vasodilatory capacity of forearm resistance vessels is augmented in hypercholesterolemic patients after treatment with fluvastatin. Angiology 1998;49(9):743‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schrama 1998 {published data only}
- Schrama YC, Hene RJ, Jonge N, Joles JA, Rijn HJ, Bar DR, et al. Efficacy and muscle safety of fluvastatin in cyclosporine‐treated cardiac and renal transplant recipients: an exercise provocation test. Transplantation 1998;66(9):1175‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Setiawati 2008 {published data only}
- Setiawati A, Darmansjah I. Safety and tolerability of fluvastatin XL in the treatment of hyper‐cholesterolemia: A postmarketing surveillance conducted in Indonesia. Medical Journal of Indonesia 2008;17:88‐95. [Google Scholar]
Sheridan 2014 {published data only}
- Sheridan DA, Bridge SH, Crossey MME, Felmlee DJ, Fenwick FI, Thomas HC, et al. Omega‐3 fatty acids and/or fluvastatin in hepatitis C prior non‐responders to combination antiviral therapy ‐ a pilot randomised clinical trial. Liver International 2014;34(5):737‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Smit 1995 {published data only}
- Smit JW, Wijnne HJ, Schobben F, Sitsen A, Erkelens D W. Effects of alcohol and fluvastatin on lipid metabolism and hepatic function. Annals of Internal Medicine 1995;122(9):678‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Teramoto 1995 {published data only}
- Teramoto T, Goto Y, Kurokawa K, Nakamura H, Yoshida S, Saito Y, et al. Clinical efficacy of fluvastatin for hyperlipidemia in Japanese patients. American Journal of Cardiology 1995;76(2):33A‐6A. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Turk 2001 {published data only}
- Turk S, Yildiz A, Tukek T, Akkaya V, Aras U, Turkmen A, et al. The effect of fluvastatin of hyperlipidemia in renal transplant recipients: a prospective, placebo‐controlled study. International Urology & Nephrology 2001;32(4):713‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van der Graaf 2006 {published data only}
- Novartis. EXECUTIVE SUMMARY FOR PEDIATRIC SUPPLEMENT. FDA 2006.
- Novartis. Efficacy and safety of fluvastatin in children with heterozygous familial hypercholesterolemia. ClinicalTrials.gov 2005. [NCT00171236]
- Novartis. Open‐label, phase III, dose‐titration, multicenter study to assess the efficacy and safety offluvastatin sodium capsules and fluvastatin sodium extended ‐release (XL) tablets (20, 40 and 80 mg)given orally at bedtime for 114 weeks in pediatric patients with heterozygous familial hypercholesterolemia. Study Number: CXUO320B2301 2006.
- Graaf A, Nierman MC, Firth JC, Wolmarans KH, Marais AD, Groot E. Efficacy and safety of fluvastatin in children and adolescents with heterozygous familial hypercholesterolaemia. Acta Paediatrica 2006;95(11):1461‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van der Linde 2006 {published data only}
- Linde NA, Sijbrands EJ, Boomsma F, Meiracker AH. Effect of low‐density lipoprotein cholesterol on angiotensin II sensitivity: a randomized trial with fluvastatin . Hypertension 2006;47(6):1125‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
van Haelst 2001 {published data only}
- Haelst PL, Doormaal JJ, May JF, Gans RO, Crijns HJ, Tervaert JW. Secondary prevention with fluvastatin decreases levels of adhesion molecules, neopterin and C‐reactive protein. European Journal of Internal Medicine 2001;12(6):503‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Westphal 2008 {published data only}
- Westphal S, Abletshauser C, Luley C. Fluvastatin treatment and withdrawal: effects on endothelial function. Angiology 2008;59(5):613‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Westphal 2009 {published data only}
- Westphal S, Abletshauser C, Luley C. Different galenic formulations of fluvastatin have equal lipid‐lowering potential but differ in reducing lipemia‐induced endothelial dysfunction. Coronary Artery Disease 2009;20(1):81‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Widimsky 1997 {published data only}
- Widimsky J, Hulinsky V, Lanska V, Balazovjech I. Czech and Slovak fluvastatin study in patients with severe hyperlipidemia. [Czech]. Vnitrni Lekarstvi 1997;43(7):419‐24. [EMBASE: 1997267327] [Google Scholar]
Widimsky 1999 {published data only}
- Widimsky J, Hulinsky V, Balazovjech I, Lanska V. [Long‐term treatment of combined hyperlipidemia with a combination of fluvastatin and fenofibrate]. Vnitrni Lekarstvi 1999;45(4):210‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Wu 2014 {published data only}
- Wu J, Ma Z Ding X Zhao X, Du X. Clinical efficacy of different doses of fluvastatin on patients with coronary heart disease combined with hyperlipidemia. Progress in Modern Biomedicine 2014;14:2936‐8. [Google Scholar]
Yamawaki 2007 {published data only}
- Yamawaki T, Yamada A, Fukumoto Y, Kishi T, Sobashima A, Kuwata K, et al. Statin therapy may prevent Restenosis after successful coronary intervention, independent of lipid‐lowering effect and CRP level. Fukuoka Acta Medica 2007;98(6):260‐9. [PubMed] [Google Scholar]
Yang 2000 {published data only}
- Yang Z, Bo X, Zhu J. Effects of fluvastatin on platelet activation and insulin resistance in patients with primary hypercholesterolemia. Zhonghua Xinxueguanbing Zazhi 2000;28(4):264‐6. [Google Scholar]
Yuan 1991 {published data only}
- Yuan J, Tsai MY, Hegland J, Hunninghake DB. Effects of fluvastatin XU 62‐320 an HMG‐Coenzyme a reductase inhibitor on the distribution and composition of low density lipoprotein subspecies in humans. Atherosclerosis 1991;87(2‐3):147‐58. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhang 2005 {published data only}
- Zhang B, Noda K, Matsunaga A, Kumagai K, Saku K. A comparative crossover study of the effects of fluvastatin and pravastatin (FP‐COS) on circulating autoantibodies to oxidized LDL in patients with hypercholesterolemia. Journal of Atherosclerosis and Thrombosis 2005;12(1):41‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhao 2014 {published data only}
- Zhao S, Wang F, Yang K, Hao Y, Li G, Yang M, et al. [Efficacy and safety of fluvastatin extended‐release tablets in Chinese patients with hyperlipidemia: a multi‐center, randomized, double‐blind, double dummy, active‐controlled, parallel‐group study]. Chung‐Hua Nei Ko Tsa Chih Chinese Journal of Internal Medicine 2014;53(6):455‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Additional references
Adams 2014
- Adams SP, Sekhon SS, Wright JM. Rosuvastatin for lowering lipids. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD010254.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adams 2015
- Adams SP, Tsang M, Wright JM. Atorvastatin for lowering lipids. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD008226.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bandolier 2004
- Bandolier. Cholesterol lowering with statins. Bandolier 2004:121‐2.
CTT 2005
- Cholesterol Treatment Trialists' (CTT). Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366(9493):1267‐78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Edwards 2003
- Edwards JE, Moore RA. Statins in hypercholesterolaemia: a dose‐specific meta‐analysis of lipid changes in randomised, double blind trials. BMC Family Practice 2003;4:18. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eisenberg 1998
- Eisenberg DA. Cholesterol lowering in the management of coronary artery disease: the implications of recent trials. American Journal of Medicine 1998;104(2A):2S‐5S. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Heran 2008
- Heran BS, Wong MMY, Heran IK, Wright JM. Blood pressure lowering efficacy of angiotensin converting enzyme (ACE) inhibitors for primary hypertension. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003823.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐1558. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, (editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Izquiero‐Palomares 2016
- Izquierdo‐Palomares JM, Fernandez‐Tabera JM, Plana MN, Añino Alba A, Gómez Álvarez P, Fernandez‐Esteban I, et al. Chronotherapy versus conventional statins therapy for the treatment of hyperlipidaemia. Cochrane Database of Systematic Reviews 2016, Issue 11. [DOI: 10.1002/14651858.CD009462.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kellick 1997
- Kellick KA, Burns K, McAndrew E, Haberl E, Hook N, Ellis AK. Focus on atorvastatin: An HMG‐CoA reductase inhibitor for lowering both elevated LDL cholesterol and triglycerides in hypercholesterolemic patients. Formulary 1997;32(4):352‐363. [EMBASE: 1997129035] [Google Scholar]
Law 2003
- Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta‐analysis. BMJ 2003;326(7404):1423. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liao 2005
- Liao JK, Laufs U. Pleiotropic effects of statins. Annual Review of Pharmacology and Toxicology 2005;45:89‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Musini 2014
- Musini VM, Nazer M, Bassett K, Wright JM. Blood pressure‐lowering efficacy of monotherapy with thiazide diuretics for primary hypertension. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD003824.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCEP 1993
- NCEP Expert Panel. Summary of the second report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel II). JAMA 1993;269(23):3015‐23. [MEDLINE: ] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordice Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordice Cochrane Centre, The Cochrane Collaboration, 2014.
Schectman 1996
- Schectman G, Hiatt J. Dose‐response characteristics of cholesterol‐lowering drug therapies: Implications for treatment. Annals of Internal Medicine 1996;125(12):990‐1000. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH (editors). Chapter 11: Presenting results and ’Summary of findings’ tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Smith 2009
- Smith MEB, Lee NJ, Haney E, Carson S. Drug Class Review HMG‐CoA Reductase Inhibitors (Statins) and Fixed‐dose Combination Products Containing a Statin. Final Report Update 5, 2009. www.ncbi.nlm.nih.gov/books/NBK47273/pdf/TOC.pdf (last accessed 8 November 2012). [PubMed]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Thompson 2005
- Thompson JF, Man M, Johnson KJ, Wood LS, Lira ME, Lloyd DB, et al. An association study of 43 SNPs in 16 candidate genes with atorvastatin response. Pharmacogenomics Journal 2005;5(6):352‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tsang 2002
- Tsang MB, Adams SP, Jauca C, Wright JM. In some systematic reviews placebos may not be necessary: an example from a statin dose‐response study. 10th Cochrane Colloquium. Stavanger, 31 July ‐ 3 August 2002; Vol. Abstracts:Poster 29.
Ward 2007
- Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technology Assessment (Winchester, England) 2007;11(14):1‐160, iii‐iv. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Adams 2016
- Adams SP, Sekhon SS, Wright JM, Tsang M. Fluvastatin for lowering lipids. Cochrane Database of Systematic Reviews 2016, Issue 7. [DOI: 10.1002/14651858.CD012282] [DOI] [PMC free article] [PubMed] [Google Scholar]


