Abstract
Background
Opioid therapy for chronic noncancer pain (CNCP) is controversial due to concerns regarding long‐term effectiveness and safety, particularly the risk of tolerance, dependence, or abuse.
Objectives
To assess safety, efficacy, and effectiveness of opioids taken long‐term for CNCP.
Search methods
We searched 10 bibliographic databases up to May 2009.
Selection criteria
We searched for studies that: collected efficacy data on participants after at least 6 months of treatment; were full‐text articles; did not include redundant data; were prospective; enrolled at least 10 participants; reported data of participants who had CNCP. Randomized controlled trials (RCTs) and pre‐post case‐series studies were included.
Data collection and analysis
Two review authors independently extracted safety and effectiveness data and settled discrepancies by consensus. We used random‐effects meta‐analysis' to summarize data where appropriate, used the I2 statistic to quantify heterogeneity, and, where appropriate, explored heterogeneity using meta‐regression. Several sensitivity analyses were performed to test the robustness of the results.
Main results
We reviewed 26 studies with 27 treatment groups that enrolled a total of 4893 participants. Twenty five of the studies were case series or uncontrolled long‐term trial continuations, the other was an RCT comparing two opioids. Opioids were administered orally (number of study treatments groups [abbreviated as "k"] = 12, n = 3040), transdermally (k = 5, n = 1628), or intrathecally (k = 10, n = 231). Many participants discontinued due to adverse effects (oral: 22.9% [95% confidence interval (CI): 15.3% to 32.8%]; transdermal: 12.1% [95% CI: 4.9% to 27.0%]; intrathecal: 8.9% [95% CI: 4.0% to 26.1%]); or insufficient pain relief (oral: 10.3% [95% CI: 7.6% to 13.9%]; intrathecal: 7.6% [95% CI: 3.7% to 14.8%]; transdermal: 5.8% [95% CI: 4.2% to 7.9%]). Signs of opioid addiction were reported in 0.27% of participants in the studies that reported that outcome. All three modes of administration were associated with clinically significant reductions in pain, but the amount of pain relief varied among studies. Findings regarding quality of life and functional status were inconclusive due to an insufficient quantity of evidence for oral administration studies and inconclusive statistical findings for transdermal and intrathecal administration studies.
Authors' conclusions
Many patients discontinue long‐term opioid therapy (especially oral opioids) due to adverse events or insufficient pain relief; however, weak evidence suggests that patients who are able to continue opioids long‐term experience clinically significant pain relief. Whether quality of life or functioning improves is inconclusive. Many minor adverse events (like nausea and headache) occurred, but serious adverse events, including iatrogenic opioid addiction, were rare.
Plain language summary
Opioids for long‐term treatment of noncancer pain
The findings of this systematic review suggest that proper management of a type of strong painkiller (opioids) in well‐selected patients with no history of substance addiction or abuse can lead to long‐term pain relief for some patients with a very small (though not zero) risk of developing addiction, abuse, or other serious side effects. However, the evidence supporting these conclusions is weak, and longer‐term studies are needed to identify the patients who are most likely to benefit from treatment.
Background
This systematic review differs in several ways from a previous systematic review on this topic that our group performed (Noble 2008). Because reviews in The Cochrane Library have fewer restrictions on the size of the review than a traditional peer‐reviewed article, we were able to include the outcomes health‐related quality of life and functional status in this review. The evidence base changed, with the inclusion of newly published studies (Collado 2008; Pascual 2007; Rauck 2008; Shaladi 2007; Thorne 2008) and non‐English‐language studies (Bettoni 2006; Klapetek 1971; Pimenta 1998), one study that we have reclassified as prospective (Kumar 2001), and one study that was not identified in our earlier searches (Thimineur 2004). In addition, two studies that were included in our previous review were excluded in this review, because we recently found that they were actually retrospective studies through personal communications with the study authors (Kanoff 1994; Tutak 1996). However, the differences in the studies that met general inclusion criteria did not impact the conclusions of the review in any important way. In addition, we updated our methodology to reflect more current methods by reducing the minimum number of studies needed to perform a meta‐regression from 10 to five, implementing an updated quality‐assessment approach using a revised instrument, not excluding studies with particularly low scores, and using each of the instrument items as a covariate to investigate heterogeneity where appropriate.
Chronic noncancer pain
The International Association for the Study of Pain (IASP) defines chronic pain as "pain which persists past the normal time of healing," which is considered to be pain lasting for three months or longer (IASP 1986). Chronic pain is a common problem worldwide. A World Health Organization survey of primary care patients seeking care at 15 centers in 14 countries across Asia, Africa, Europe, South America, and North America found that 22% of primary care patients reported pain lasting longer than 6 months (Gureje 1998). A systematic review of four international studies conducted in developed countries found prevalence rates of any type and severity level of chronic pain ranging from 10.5% to 55.2% of the population (Harstall 2003). The Pain in Europe survey of 46,000 individuals showed that one in five people suffer from chronic pain (Breivik 2006). In this survey, chronic pain sufferers reported 7 years of chronic pain on average, with some reporting pain lasting more than 20 years (Breivik 2006). An estimated 9% of Americans (Clark 2002) and 19% of Europeans (Breivik 2006) have moderate to severe chronic noncancer pain (CNCP). Women are more likely than men to experience chronic pain, and the overall prevalence of chronic pain increases with age (Ballantyne 2003; Blyth 2001; Breivik 2006; Elliott 1999). Although CNCP usually does not arise from an etiology that is inherently life‐threatening, suicide rates are elevated among individuals with chronic pain, especially when there is a sense of hopelessness about the pain (Fishbain 1991; Tang 2006). One study found that completed suicide was associated with higher pain severity (Kikuchi 2008). Suicide rates for people with chronic pain remain higher even when mental illness has been accounted for (Ratcliffe 2008).
Opioids for chronic noncancer pain
Opioids are a class of drugs that relieve pain by binding to and blocking certain receptors located in the brain and spinal cord. Although opioid use for acute pain, postsurgical pain, and palliative care is accepted in the United States. and many other countries, there is debate about whether opioids are appropriate for the treatment of CNCP. The efficacy of opioids for CNCP has been demonstrated in short‐term trials (Furlan 2006), including those for neuropathic pain (Eisenberg 2005), but little is known about whether these agents continue to be effective over the longer durations of treatment typical for CNCP. Concerns have also been raised about adverse effects that may arise with long‐term use, including the development of psychologic addiction or abuse, or both.
Exactly how many people take opioids long‐term for CNCP, and just what they are taking, has not been definitively defined. Chronic opioid use (for at least 6 months) has been estimated at 0.65% of a U.S.‐insured population (Cicero 2009), and regular or continuous use has been estimated at 0.27% of Dutch epidemiologic survey respondents (Eriksen 2006). An analysis of insurance database claims in the U.S. found that 10% of people who were prescribed opioids had at least a 3‐month supply (Williams 2008), and the Dutch epidemiologic survey found that 12% of people with pain due to causes other than cancer reported they take opioids "regularly or continuously." In the studies by Williams 2008 and Eriksen 2006, most people were prescribed weak opioids; in Cicero 2009 the opposite was true. In all three studies only a minority of patients were prescribed extended‐release opioids.
Objectives
The purpose of this systematic review is to summarize the evidence pertaining to the efficacy and safety of long‐term opioid therapy for CNCP. Aside from our previous work (Noble 2008) we are not aware of any published peer‐reviewed systematic review that provides this information and presents new syntheses. This review could be useful for identifying gaps in the literature and planning future research. Specifically, we seek to:
Determine the effectiveness of long‐term opioid therapy for CNCP;
Identify the adverse effects of long‐term opioid therapy for CNCP; and
Assess withdrawal rates from treatment by reasons for withdrawal based on patient statements.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) and nonrandomized controlled trials. However, we only found one controlled trial that evaluated the efficacy and safety of opioids for CNCP and reported long‐term outcomes. That study compared two opioids and was not controlled using placebo or a nonopioid treatment.
Pre‐post case‐series studies. Although the results from these studies may be less reliable than data from RCTs and controlled trials, they provide the best available evidence for this type of intervention at the present time. We defined long‐term open‐label continuations of short‐term RCTs as case‐series studies.
We searched for studies that were published in any language, and reported as full‐text articles (i.e., no meeting abstracts or poster presentations) that enrolled and administered opioids to at least 10 participants. We did not include redundant data on participants who were also reported on in other included studies, nor did we include studies with duplicate data. We wished to include prospective studies only to minimize the potential influence of selection bias. However, we included studies that could not definitively be determined to be prospective after unsuccessfully attempting to query their authors because we believe their exclusion could not be justified.
Types of participants
Participants of interest were adults aged at least 18 years with pain due to any cause other than cancer lasting for at least three months (that is, meeting the IASP definition for chronic pain) prior to trial enrolment, due to any noncancer cause. Previous nonopioid pharmacotherapy must have failed before beginning opioids.
Types of interventions
Any opioid taken by any route in any dose for at least six months.
Types of outcome measures
We assessed adverse events (side effects), discontinuation from study due to adverse events, discontinuation from study due to insufficient pain relief, average change in pain score, proportion of patients with at least 50% pain relief, health‐related quality of life, and function. Outcome measures must have been validated or used as a standard of care to be included in the analyses. In addition to these general inclusion criteria, we employed two criteria for pain outcomes:
pain and quality‐of‐life outcomes must have been patient‐reported;
outcome data must not have been collected retrospectively (for example, post‐treatment surveys/questionnaires), because reports based on memory of pain may differ from reports given at the time that pain is experienced (Eich 1985; Linton 1983).
For participants who discontinued participation before the end of the study, we intended to collect data on duration, dose, titration, and rotation of opioids from before they withdrew from the study. However, these data were generally not available in the publications we identified.
Search methods for identification of studies
Electronic searches
We searched the following databases:
The Cochrane Library, specifically: The Cochrane Central Register of Controlled Trials, The Cochrane Database of Systematic Reviews (Cochrane Reviews), Database of Abstracts of Reviews of Effects;
The ECRI Institute library, including: ECRI Institute Library Catalog, ECRI Institute Healthcare Standards, ECRI Institute International Health Technology Assessment from 1990;
MEDLINE from 1966;
EMBASE (Excerpta Medica) from 1980;
U.S. Food and Drug Administration (FDA) Web site from 1977;
U.S. National Guideline Clearinghouse (NGC) from 1998.
The date of last search update was May 19, 2009.
The search strategies employed combinations of freetext keywords as well as controlled vocabulary terms including (but not limited to) the concepts in Appendix 1. The strategy in Appendix 1 is presented in Ovid syntax; parallel strategies were created for searching the remaining databases.
Searching other resources
We also examined reference lists from identified studies and reviewed gray literature for additional studies not identified by electronic searches. Gray literature includes reports and studies produced by local government agencies, private organizations, educational facilities, and corporations that do not appear in the peer‐reviewed literature. Although we examined gray literature sources to identify relevant information, we only analyzed outcomes data from published, peer‐reviewed literature in this review.
Data collection and analysis
Selection of studies
Two review authors screened abstracts of all identified studies against the inclusion criteria (MN, PW). We retrieved all possibly relevant articles in full text for comprehensive assessment of internal validity (quality) and satisfaction of inclusion criteria.
Assessment of methodologic quality
Two review authors (MN, CA) independently assessed the internal validity of English‐language studies (non‐English language articles were assessed by volunteers affiliated with The Cochrane Collaboration). Discrepancies were resolved by consensus. We assessed the risk of bias in included studies using a 10‐question internal validity assessment instrument developed by methodologists at ECRI Institute for the assessment of case series using domains identified as important factors by experts in the field (Table 1; AHRQ 2002; Deeks 2003; Egger 2003). To evaluate long‐term, open‐label case series that were continuation studies of short‐term RCTs, we consulted the original publication of the RCT whenever necessary (see 'Characteristics of included studies' for original citations).
1. ECRI Institute pre‐post internal validity assessment scale (2008).
| Item Number | Item |
| 1 | For the outcome of interest, was the performance among patients at baseline similar among patients who entered the study as compared to patients who completed the study to the timepoint of interest? (For the same study, answer may vary by outcome. If attrition is less than 15% for this outcome, we answer 'yes' even if characteristics were not compared) |
| 2 | For all other important factors, were the characteristics of patients at baseline similar among patients who entered the study as compared to patients who competed the study to the timepoint of interest? (If attrition was less than 15% for the entire study, we answer 'yes' even if characteristics were not compared.) |
| 3 | Did the study enroll all, a consecutive series of, or a randomized sample of suitable patients within a time period? |
| 4 | Was the study prospectively planned? (Although redundant with inclusion criteria, this factor remains important for the assessment of bias and influences the internal validity category) |
| 5 | Did 5% or less of patients receive ancillary treatment(s) |
| 6 | Was compliance with treatment at least 85%? |
| 7 | Was the outcome measure of interest objective and was it objectively measured? (For the same study, answer may vary by outcome) |
| 8 | Was a standard instrument used to measure the outcome? (For the same study, answer may vary by outcome. Although redundant with inclusion criteria, this factor remains important for the assessment of bias and influences the internal validity category) |
| 9 | Did at least 85% of patients contribute data to this outcome? (For the same study, answer may vary by outcome and timepoint) |
| 10 | Was the funding for this study derived from a source that would not benefit financially from particular results? |
We assessed internal validity separately for each outcome using the instrument shown in Table 1, with the exception of adverse events, for which no internal validity assessment was conducted. Affirmative answers were scored +1, negative answers were scored ‐1, and answers "not reported" scored zero. Scores were normalized by adding the component question scores, adding 10, and dividing that sum by five. Scores of at least 7.5 were considered "moderate," and scores below 7.5 were considered "low." Given the inherent limitations of case series, we adjusted this scale so that no case series could be considered "high" in internal validity. We used the median internal validity score of the studies for each outcome to describe the overall evidence‐base quality. The overall quality of a body of evidence (for example, the median internal validity score of all studies addressing a particular outcome) influences the strength of qualitative conclusions and stability of quantitative conclusions drawn from it (Treadwell 2006).
We modified the scale to assess the internal validity of a study on the whole rather than by outcome and time point to complete the Risk of bias in included studies tables of this review. We assessed the comparability of participants at baseline and follow‐up for studies where continuous outcomes (e.g., quality‐of‐life scales) were reported only; for binary outcomes (e.g., proportion who discontinued) we always used the baseline number of participants as the denominator, factoring out attrition and making this item moot. We omitted the items regarding outcome objectivity (because the outcomes were universally subjective, so the answer is always "no") and standardization of instruments (because we required that the instrument be standard to meet inclusion criteria, so the answer is always "yes"). For studies that administered opioids using an automated implantable infusion pump, we always answered the item regarding compliance as yes.
Data extraction and management
Two review authors (MN, CA) independently extracted data from English‐language articles. Discrepancies were settled by consensus. Data from non‐English language articles were extracted by volunteers affiliated with The Cochrane Collaboration.
Assessment of heterogeneity
Whenever meta‐analysis was conducted, we used the I‐squared (I2) statistic to identify heterogeneity (Higgins 2003). Combined results with I2 > 50% were considered substantially heterogenous.
Measures of treatment effect and data synthesis (meta‐analysis)
We analyzed the available evidence using systematic a priori protocols, which are described as follows (and described in further detail in Treadwell 2006). We summarized data using meta‐analysis when at least two studies per mode of opioid administration addressed a particular outcome of interest. These protocols were used to attempt to formulate both qualitative (the direction of a treatment effect) and quantitative (the magnitude of a treatment effect) conclusions. We used random‐effects meta‐analyses to pool data (DerSimonian 1986).
For dichotomous data (for example, proportion of patients with at least a 50% reduction in pain), event rates (risk) were calculated. For continuous data (e.g., changes in visual analogue scale [VAS] pain scores from baseline), standardized mean differences (SMD) were calculated. A correlation coefficient of 0.50 between pre and post data was assumed for all continuous outcomes (this value was altered in sensitivity analyses). For the calculation of proportions of patients who had a certain outcome, when no events or no non‐events occurred, the conventional correction factor of 0.5 was added to the number of events or non‐events (numerator) and the total number of participants (denominator). Ninety‐five percent confidence intervals (95% CI) were calculated for all summary data.
Statistically significant changes may not always be large enough to be clinically meaningful. The minimum amount of change in pain score that we considered to be clinically meaningful was a 2‐point change on a scale of 0 to 10 (or, 20 percentage points), based on findings in trials studying general chronic pain (Farrar 2000), chronic musculoskeletal pain (Salaffi 2004), and chronic low‐back pain (Hagg 2003), which were commonly cited causes of pain among the participants enrolled in the studies included in the evidence base of this report. We also calculated the proportions of participants who attained at least 50% pain relief (that is, a 50% reduction from their baseline scores), which is generally accepted as being clinically significant.
The SMD enables pooling of data on the same outcome from different metrics (e.g., different pain scales or different quality‐of‐life measurement instruments) into a single statistic. However, interpreting the SMD can be difficult. When data were combined from studies that used different instruments and the summary effect size could not be interpreted in terms of the original metric, to facilitate interpretation we: (1) considered whether all studies comprising the evidence base showed statistically and/or clinically significant changes when considered individually; and (2) considered the general guidelines in Cohen 1988. Wherever possible we prefer to consider the outcome in terms of the original metric, but because different studies used different scales to measure the same outcome, this was not always possible in this review, and Cohen's guidelines enable some interpretation of the resulting summary effect size. Cohen's guidelines suggest that an SMD value of 0.2 is small, 0.5 is medium, and 0.8 is large; however, these guidelines are intended to represent between‐studies effect sizes, not pre‐post effect sizes. We stress that in the context of this report they are only intended to give a general sense of the size of change observed when considering the data in terms of the original metric is not possible and should not be used to draw firm conclusions regarding the overall effect size magnitude.
Dealing with missing data
If means and standard deviations were not available for continuous data, we attempted to determine an estimate of treatment effect from reported statistics (e.g., t‐values, F‐values, P‐values) (as described in Cooper 1994). However, where measures of variance were not reported, exact statistics were not reported either. In these instances, we excluded the study for the outcome(s) for which reporting was incomplete.
Investigation of heterogeneity
We divided the evidence base by mode of drug administration (i.e. oral, transdermal, intrathecal) to reduce clinical heterogeneity. We did not perform further subgroup analyses due to the paucity of data.
Statistical heterogeneity was explored using univariate meta‐regression (Harbord 2004). We investigated duration of treatment as a covariate whenever heterogeneity was detected, given our suspicion that length of treatment would be of particular importance (although we acknowledge that having only a small number of studies limits the power to detect an effect). Additional covariates were investigated when at least five studies comprised the evidence base and depended on data available for studies in the evidence bases of the different outcomes. They included publication date, sample size, predominant cause of pain, opioid dose, opioid administered, and quality assessment items. For oral opioids, we also investigated weak compared with strong opioids. (This was not necessary for intrathecal and transdermal opioids because they were all strong). P‐values of meta‐regressions were investigated using a permutation test, to avoid Type I errors (Higgins 2004). We did not explore possible sources of heterogeneity other than the duration of treatment when fewer than five studies per outcome were identified.
Sensitivity analyses
We performed sensitivity analyses on evidence bases comprised of at least three studies. Evidence bases consisting of only one or two studies were considered to inherently lack robustness. Our primary sensitivity analyses were cumulative meta‐analyses (Ioannidis 1999; Lau 1995), and influence (remove‐and‐replace‐one‐study) meta‐analyses (Olkin 1999). For cumulative meta‐analyses, we considered the evidence base for continuous outcomes robust if the point estimate did not change by more than one level of clinical significance (e.g., 20 percentage points for pain). When analyzing the proportion of participants with a particular outcome, we considered the evidence base to be robust if the point estimate did not change by more than 10 percentage points with the addition of the most recent two studies.
Because our data came from case series, we performed two additional sensitivity analyses for continuous outcomes for which conclusions were drawn. Assumptions about the assumed correlation coefficient (0.5) of pre and post data were tested by varying the correlation coefficient from ‐0.99 to 0.99, in the method of Thiessen Philbrook and colleagues (Thiessen Philbrook 2007).
If more than 15% attrition occurred at follow‐up for continuous outcomes (pain, quality of life, and function), we inflated the standard deviations of baseline scores by 100% and deflated them by 50% to test whether changing the baseline participant characteristics would overturn the conclusion, as the participants remaining long‐term may differ in important ways from the participants who dropped out. We used these methods to evaluate the evidence base for quantitative robustness (i.e., stability of the summary effect estimate), continuous/categorical outcomes, and qualitative robustness (i.e., consistency as to whether opioids show a benefit of any magnitude).
Assessment of reporting biases
For analyses without substantial heterogeneity (I² < 50%) and at least 10 studies, we planned to use the "trim and fill" method to test for funnel plot asymmetry, which suggests missing studies, possibly due to publication bias (Duval 2000a; Duval 2000b). However, none of the evidence bases for any of the outcomes met these criteria, precluding an investigation on publication bias.
Software used for assessment
Comprehensive meta‐analysis (Biostat, Englewood, NJ, USA) software was used for most statistical analyses. Meta‐regression with permutation methods (Harbord 2004) was performed using STATA (StataCorp LP, Bryan/College Station, TX, USA) software.
Results
Description of studies
Our searches identified 16,406 potentially relevant studies. However, the vast majority of these were not relevant (i.e., did not pertain to safety or effectiveness of opioids taken long‐term for noncancer pain), not clinical studies, or were duplicate identical citations (i.e., the same study identified more than once). Of the 130 potentially relevant studies identified by our searches of 11 databases (through to May 19, 2009), 54 appeared to be relevant and were retrieved and read in full, and 26 studies (n = 4893 enrolled), 1 with two treatment groups, met our inclusion criteria (the studies that did not meet inclusion criteria are listed with reasons for exclusion in the 'Characteristics of excluded studies' table). Consistent with our inclusion criteria, these studies represent the administration of any opioid in any dose by any mode of administration for at least six months in the context of any prospective trial of any design.
Participants in all treatment groups reported moderate or severe CNCP at baseline due to nociceptive or neuropathic pain, or both. The most prevalent painful conditions of participants enrolled in the studies were:
Back pain (number of studies [k] = 7) (Allan 2005; Anderson 1999; Anderson 2003; Angel 1998; Fredheim 2006; Mironer 2001; Rainov 2001).
Osteoarthritis (k = 6) (Caldwell 2002; McIlwain 2005; Portenoy 2007; Roth 2000; Rauck 2008; Thorne 2008, unspecified rheumatologic pain (k = 1) (Bettoni 2006), or arthrosis (k = 1) (Collado 2008).
Unspecified or a variety of types of CNCP (k = 5) (Kumar 2001; Milligan 2001; Mystakidou 2003; Thimineur 2004; Pascual 2007).
Neuropathic pain (k = 2) (Harati 2000; Hassenbusch 1995), neuropathic and/or back pain (k = 1) (Zenz 1992), or neuropathic or myofascial pain (k = 1) (Pimenta 1998).
Osteoporotic vertebral fracture (k =1) (Shaladi 2007).
Trigeminal neuralgia (k = 1) (Klapetek 1971).
Participants enrolled in these studies were generally middle‐aged, with mean ages ranging from 46 years (Hassenbusch 1995; Thimineur 2004) to 74 years (Shaladi 2007). The percentage of females enrolled was greater than 50% in most studies but ranged from 38% (Kumar 2001) to 86% (Bettoni 2006). The mean duration of pain prior to study enrollment was reported in only 10 studies, but where reported mean durations ranged from 19 months (Rainov 2001) to 10.3 years (Allan 2005). The mean duration of pain prior to enrollment in Rainov 2001 was much shorter than any of the other studies; the next shortest was a duration of pain of 5.3 years (Pimenta 1998), and most studies reported an average duration of pain of about 8 years (Anderson 1999; Klapetek 1971; Kumar 2001; Milligan 2001; Thorne 2008).
With a few exceptions, all of the included studies were conducted as open‐label case series. Five of the oral opioids studies began as short‐term RCTs, with continuations as long‐term open‐label case series (Caldwell 2002; Harati 2000; McIlwain 2005; Roth 2000; Thorne 2008). In these studies we only considered outcomes for the opioid treatment groups, because long‐term outcomes for the placebo groups were not available. Another study (Thimineur 2004) compared outcomes in chronic pain participants who underwent pump implantation for intrathecal delivery to outcomes of participants who were evaluated for pump implantation but did not meet all criteria to receive one. However, we did not consider this study to be adequately controlled due to these fundamental between‐group differences at baseline and therefore only considered the outcomes of the opioids treatment group, treating it as a case series. One RCT with long‐term outcomes (Allan 2005) included one group in which participants were treated with transdermal fentanyl and another in which participants were treated with oral extended‐release oxymorphone. No placebos were administered. To promote comparability with the other studies, we analyzed this study as two case series', one per mode of administration. Therefore, all studies were analyzed as case series to capture long‐term data, to promote comparability among studies, and enable meta‐analysis.
For analysis, this evidence base was stratified by route of opioid administration: oral (k = 12, n = 3040), transdermal (k = 5, n = 1628), or implantable infusion pump (k = 10, n = 225). Pain scores on a scale of 0‐10 on a VAS or numerical rating scale (NRS) can be generally categorized as mild (score of 1 to 4), moderate (5 to 6), or severe (7 to 10) pain (Farrar 2000). Among the studies that reported baseline pain scores, participants in the oral opioids studies had moderate to severe pain, and participants in the transdermal and intrathecal opioids studies had severe pain.
The scope of this review is to include any opioid administered long‐term. This includes both strong and weak opioids. We used this classification to investigate heterogeneity wherever possible. Opioids administered in the oral studies [and the duration they were administered for] included the following:
Strong opioids:
Extended‐release morphine (Allan 2005 [13 months]; Caldwell 2002 [6.9 months]).
Controlled‐release oxycodone (Portenoy 2007 [mean 24 months]; Roth 2000 [13 months]).
Extended‐release oxymorphone (Rauck 2008 [6 months]).
Extended‐release tramadol (Pascual 2007 [12 months]; Thorne 2008 [8 months]).
Methadone (Fredheim 2006 [9 months]).
Weak opioids:
Tramadol (Harati 2000 [7 months]).
Tilidine (valoron) (Klapetek 1971 [mean 7 months]).
Extended‐release oxymorphone (McIlwain 2005 [13 months]).
Dihydrocodeine (19%), buprenorphine (57%), or morphine (23%) (Zenz 1992 [mean 7 months]) (We categorized this study as "weak opioids," because the majority of patients took weak opioids).
In the transdermal studies, all participants received transdermal fentanyl (Allan 2005 [13 months]; Bettoni 2006 [mean 22.3 months]; Collado 2008 [6 months]; Milligan 2001 [12 months]; Mystakidou 2003 [48 months]).
Morphine was administered in all but one of the intrathecal studies.
Morphine alone was administered in some studies (Anderson 1999 [24 months]; Anderson 2003 [6 months]; Pimenta 1998 [mean 20 months]; Shaladi 2007 [12 months]).
Others offered adjuvants or alternatives, such as sufentanil citrate (Hassenbusch 1995 [mean 28.8 months]), clonidine (Kumar 2001 [mean 29 months]), bupivacaine, clonidine and/or midazolam (Rainov 2001 [mean 27 months]), hydromorphone, fentanyl, methadone, clonidine, baclofen, bupivacaine (Thorne 2008 [8 months]), dilaudid, fentanyl, clonidine, baclofen, bupivacaine, or methadone (Thimineur 2004 [36 months].
The exception was one study in which methadone was administered to participants for whom intrathecal morphine had failed (Mironer 2001 [6 months]).
Just as opioid and route of administration varied among studies, so too did dosage. Doses also varied considerably within studies due to individual differences in pain level, opioid tolerance, and titration. For information on dosing by study, please refer to the 'Characteristics of included studies' table.
Participants were treated on an outpatient basis, with the exception of screening and implantation phases of intrathecal studies.
Risk of bias in included studies
All of the evidence bases considered in this systematic review were of low internal validity and therefore potentially at high risk of bias. Reasons for low internal validity ratings varied by study, but included failure to describe participant recruitment methods; failure to compare the characteristics of participants who completed the study to participants who did not in studies with attrition; high prevalence of use of ancillary/adjuvant treatments; and use of funding from a source with a potential conflict of interest in the outcome. In oral and transdermal administration studies compliance was generally not reported. The risk of bias is probably highest for the continuous outcomes (e.g., pain relief, quality of life) and lowest for the outcomes on discontinuation from clinical study and adverse events. This is primarily because the high attrition rates affect both the risk of bias and the generalizability of the results from the continuous data outcomes. The reasoning for this is that only data from completer analyses were available at long‐term follow‐up, though this should not affect the dichotomous data because all enrolled participants are reflected in those data sets. Although participants had long‐term pain before enrolling in studies, regression to the mean remains possible. We attempted to minimize selection bias by requiring prospective studies, but this may still be a threat, particularly to open‐label continuations of RCTs.
Effects of interventions
The first outcome we present is adverse events, because many clinicians and patients are particularly concerned about the harms associated with opioids. We then present an assessment of discontinuation rates for the two most common reasons cited for leaving a study (adverse events and insufficient pain relief) before analyzing effectiveness outcomes. The rate of discontinuation from a clinical study represents the number of participants who discontinued participation in the study. The rate does not necessarily capture the rate of discontinuation of opioids. Participants may continue opioids under different protocols, change opioids, or change mode of administration. Likewise, participants who discontinued participating in trials that administered opioids using an implantable infusion pump did not necessarily have their pumps removed. Follow‐up data on participants who discontinued participating in the studies were not reported. In the one study that tracked satisfied departures, 87% of enrollees who discontinued participation left as satisfied departures, and at 48 months, 92% of enrollees were either still in the study or had a satisfied departure (Mystakidou 2003). The findings of this study suggest that high attrition is not necessarily synonymous with high rates of treatment failure.
For the four studies that were open‐label extensions of RCTs (Caldwell 2002; Harati 2000; McIlwain 2005; Roth 2000), we considered baseline data from the original RCTs. For discontinuation outcomes, we used the total number of participants who enrolled in the denominator, and the participants who discontinued from the drug groups (but not placebo during the RCT, although these participants could later discontinue opioids during the open‐label continuation) in the numerator. We also used the baseline pain scores from the beginning of the RCT rather than the beginning of the open‐label continuation to more accurately capture pre‐post data instead of maintenance data.
Adverse events (side effects)
The variability in thresholds for reporting adverse events, failure to report whether certain adverse events occurred in some studies, and inconsistent reporting or use of definitions of events/effects precluded our ability to pool data in meta‐analyses to determine rates. Absence of long‐term nonopioid control groups precluded determination of relative rates of adverse events.
The most commonly reported adverse events included gastrointestinal effects (i.e., constipation, nausea, dyspepsia), headache, fatigue/lethargy/somnolence, and urinary complications (i.e., retention, hesitancy, ''disturbance''). In at least one study, participants were proactively treated for common opioid side effects, such as nausea (Collado 2008). Studies for each of the modes of administration reported that adverse events/effects diminished over time (Bettoni 2006; Kumar 2001; Mystakidou 2003; Pascual 2007; Roth 2000; Zenz 1992). Most of the adverse events were minor. However, a few serious events were reported, including sedation (Fredheim 2006), hypoventilation/bradypnea (Milligan 2001), hallucinations (Kumar 2001), and abdominal pain (Rauck 2008).
There is much concern among patients and their families as well as clinicians that addiction may develop during long‐term opioid therapy. Of the 27 treatment groups, 18 specifically stated that participants were screened for a history of substance addiction or abuse before study enrollment (the remaining nine studies did not report whether participants with addiction/abuse histories were excluded). Six studies specifically stated that no cases of addiction were observed, and 18 studies did not report whether addiction was observed. Two studies did report possible cases of opioid addiction. In one prospective registry study in which participants were pre‐screened for addiction and abuse, six (3%) appeared to meet criteria for opioid abuse and/or addiction as defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV) (Portenoy 2007). In the other study, one (3%) case of drug‐seeking behavior was reported in a pre‐screened population (Anderson 1999). Among the studies where addiction or addiction and abuse rates are specifically reported, the total event rate is 0.27% (7/2613). If we assume that there were in fact no cases of addiction among the studies that did not report whether addiction occurred (as it seems likely that such an important adverse event would be reported if it were observed), then the rate is 0.14% (7/4884). Only one study reported on abuse separately from addiction (Milligan 2001), and reported signs of abuse in three participants. Most of the studies pre‐screened participants for abuse histories and all administered opioids under ongoing medical supervision, therefore, these rates should not be generalized to an unselected population or to individuals taking opioids without appropriate medical supervision.
Oral administration
Adverse event data were collected from 12 case series that examined the impact of oral opioids with a total of 2445 participants who began long‐term oral opioids. Two of the studies reported a total of 10 deaths. Eight participants in one study died within one year of beginning oral opioids for reasons that were not reported, suggesting that these deaths were not opoid‐related. The study authors reported that no serious opioid‐related adverse events occurred (Zenz 1992). In the other study, one death was attributed to an automobile accident and another to suicide (Pascual 2007).
Transdermal administration
Five studies that examined the impact of transdermal opioid administration and enrolled a total of 1626 participants provided adverse events data (Allan 2005; Bettoni 2006; Collado 2008; Milligan 2001; Mystakidou 2003). An adverse event that only occurred with transdermal administration was skin irritation at the application site. This was reported in two of the five transdermal studies at a rate of 9% in each of the studies (Allan 2005; Milligan 2001). Another study reported that two participants discontinued treatment due to dermatitis, but it is unclear whether the events were related to the site of application (Collado 2008). One study reported nine deaths, of which one, due to severe bronchopneumonia, was considered possibly attributable to transdermal opioids (Milligan 2001). The authors did not believe that the other eight deaths were opioid‐related.
Intrathecal administration
Adverse events were extracted from 10 studies on intrathecal opioid administration that enrolled a total of 228 participants. Adverse events unique to the intrathecal mode of administration included pump and catheter malfunctions and malpositioning, surgical complications, and postsurgical complications. The percentage of participants whose device complications required reoperation was high in some studies, such as 27% in Hassenbusch 1995, 25% in Kumar 2001, and 20% in Anderson 1999. The studies with the highest reoperation rates were older. It is unclear whether this might be due to use of older models of infusion pumps and accessories or less extensive surgeon experience with the pumps available at the time. Two studies reported a total of six deaths. In one study, the deaths were due to chronic obstructive pulmonary disease (n = 1), pericolonic abscess (n = 1), and myocardial infarction (n = 1) (Anderson 1999). In the other study, deaths were due to suicide (n = 1), myocardial infarction (n = 1), and an unknown cause (n = 1) (Thimineur 2004).
Discontinuation from clinical study due to adverse events
Oral administration
Eleven oral opioid studies that enrolled a total of 3026 participants, of whom 2473 were eligible for this analysis, with a variety of painful conditions and prescribed various different opioids reported the proportion of participants who discontinued participating in the clinical study due to adverse events (Allan 2005; Caldwell 2002; Harati 2000; Klapetek 1971; McIlwain 2005; Pascual 2007; Portenoy 2007; Rauck 2008; Roth 2000; Thorne 2008; Zenz 1992). Substantial heterogeneity was detected (I2 = 95.8%).
Duration of treatment, which ranged widely from about six to eight months (Caldwell 2002; Harati 2000; Rauck 2008; Thorne 2008; Zenz 1992) to about two years (Portenoy 2007), was not observed to be associated with the outcome. Year of study publication, drug administered, sample size, and cause of pain were not associated with the outcome either.
The two studies that did not appear to use funding from a source with a potential conflict of interest in the outcome had lower rates of discontinuation due to adverse events (P = 0.03). Testing the covariate using other meta‐regression models (i.e., fixed‐effect, method of moments, unrestricted maximum likelihood) led to detection of this effect as well. However, heterogeneity remained substantial when the source of funding that was known was controlled for (I2 = 96.4%), indicating unresolved differences among the studies. In addition, only two studies did not clearly have a funding source with a potential conflict of interest in the findings (e.g., drug company). Therefore, the robustness of this finding is unclear, and the finding may be spurious.
Whether strong or weak oral opioids were administered was also significantly associated with discontinuation from trial participation due to adverse events (P = 0.48), with weak opioids associated with lower discontinuation rates. When this factor is controlled for, heterogeneity remains significant (90.5%), so additional causes of heterogeneity remain important. We subdivided the evidence base and considered the rate for each opioid type. The rate for weak opioids was 11.4% (95% CI: 7.0% to 18.1%), and the rate for strong opioids was 34.1% (95% CI: 29.2% to 39.3%). Heterogeneity remained significant for both weak (I2 = 71.7%) and strong (I2 = 75.8) oral opioids.
The overall pooled rate was 22.9% (95% CI: 15.3% to 32.8%), but due to the remaining unexplained heterogeneity this estimate should be considered unstable. The subgroup rates for weak and strong opioids, while still substantially heterogenous, may be more useful. The all‐studies analysis is illustrated in Figure 1.
1.
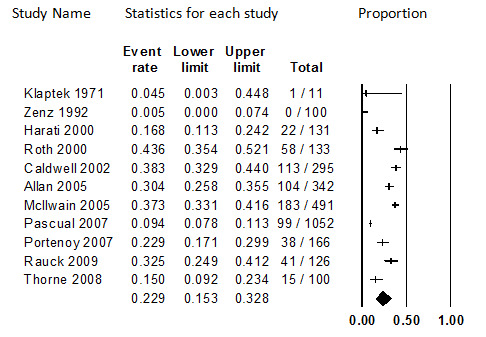
Discontinuation from Oral Opioids Studies due to Adverse Events, Follow‐up 6 months to 24 months (I2=95..8%)
Transdermal administration
Five transdermal fentanyl studies with 1628 participants with various painful conditions reported discontinuation rates due to adverse events (Allan 2005; Bettoni 2006; Collado 2008; Milligan 2001; Mystakidou 2003). When combined in meta‐analysis, the pooled proportion was 12.1% (95% CI: 4.9% to 27.0%). This estimate has substantial heterogeneity (I2 = 97.3%) that could not be explained by meta‐regression, and should therefore be considered unstable. Covariates investigated for association with the outcome were duration of the study (which ranged widely from six months [Collado 2008] to four years [Mystakidou 2003]), year of study publication, cause of pain, mean initial and follow‐up doses, sample size, and internal validity assessment items. This assessment is shown in Figure 2.
2.
Discontinuation from Transdermal Opioids Studies due to Adverse Events, Follow‐up 6 months to 48 months (I2=97.3%)
Intrathecal administration
Eighty‐six participants with predominantly failed back surgery syndrome or neuropathic pain from five studies contributed data on discontinuation from the trials due to adverse events (Anderson 1999; Angel 1998; Hassenbusch 1995; Kumar 2001; Pimenta 1998). The studies ranged in mean duration of treatment from 20 months (Pimenta 1998) to 29 months (Kumar 2001). The pooled proportion was 8.9% (95% CI: 4.0% to 18.6%), which was not substantially heterogenous (I2 < 0.001) and was robust to sensitivity analyses. The analysis is shown in Figure 3. Due to the low quality of the evidence base, the stability of this estimate should be considered low.
3.
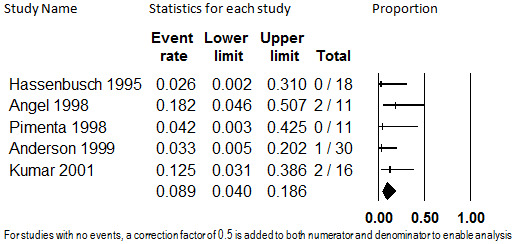
Discontinuation from Intrathecal Opioids Studies due to Adverse Events, Follow‐up 20 months (mean) to 29 months (mean) (I2<0.001)
Discontinuation from clinical study due to insufficient pain relief
Oral administration
The same 10 studies that comprised the evidence base for rate of discontinuation due to adverse events also reported the rate of discontinuation due to insufficient pain relief. The pooled rate of discontinuation due to insufficient pain relief was 10.3% (95% CI: 7.6% to 13.9%), as shown in Figure 4. Substantial heterogeneity was detected (I2 = 81.8%) and not explained by duration of treatment, drug administered, cause of pain, sample size, whether the opioid administered was weak or strong, or year of study publication. As with discontinuation from oral opioids due to adverse events, the studies with a source of funding with a potential financial interest in the outcome were associated with higher discontinuation rates (P = 0.03). However, this finding was not robust upon sensitivity analysis conducted by repeating the regression using different meta‐regression models, and may be spurious. Due to the unexplained heterogeneity, this estimate should be considered unstable.
4.
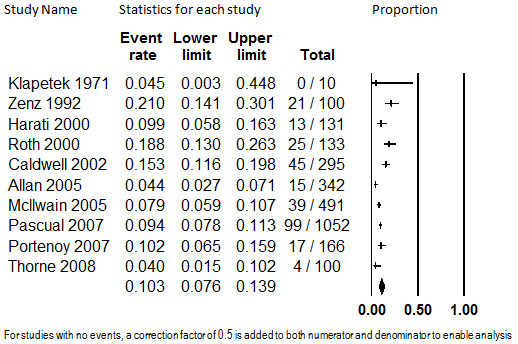
Discontinuation from Oral Opioids Studies due to Insufficient Pain Relief, Follow‐up 7 to 24 months (mean) (I2=81.8%)
Transdermal administration
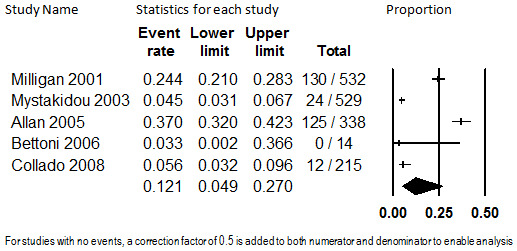
Three studies with 1391 participants with unspecified causes of chronic pain reported study discontinuation rates in participants prescribed transdermal fentanyl and collected data on discontinuation from the trial due to insufficient pain relief (Allan 2005; Milligan 2001; Mystakidou 2003). Duration of treatment ranged from 6 months to a mean of 29 months. Combined in meta‐analysis, the overall proportion of participants who discontinued study participation due to insufficient relief was 5.8% (95% CI: 4.2% to 7.9%). This is illustrated in Figure 5. Heterogeneity was substantial (I2 = 52.2%) and not explained by duration of treatment (which spanned from 12 months [Milligan 2001] to 48 months [Mystakidou 2003]), so the estimate should be considered unstable. We did not investigate other potential causes of heterogeneity because fewer than five studies comprised the evidence base.
5.
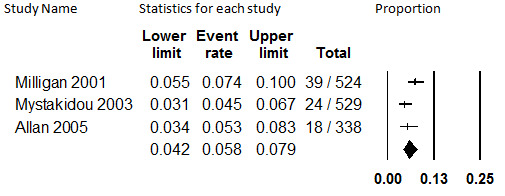
Discontinuation from Transdermal Opioids Studies due to Insufficient Pain Relief, Follow‐up 12 to 48 months (I2=52.2%)
Intrathecal administration
Six studies with 113 participants with mostly failed back surgery syndrome or neuropathic pain reported on the proportion who discontinued participating in the study due to insufficient pain relief (Anderson 1999; Anderson 2003; Angel 1998; Hassenbusch 1995; Kumar 2001; Pimenta 1998). The summary rate of discontinuation due to insufficient pain relief was 7.6% (95% CI: 3.7% to 14.8%). No substantial heterogeneity was detected (I2 < 0.001), and the estimate was robust to sensitivity analyses. This analysis is shown in Figure 6. This estimate should be regarded as low stability due to the low quality of the evidence base.
6.
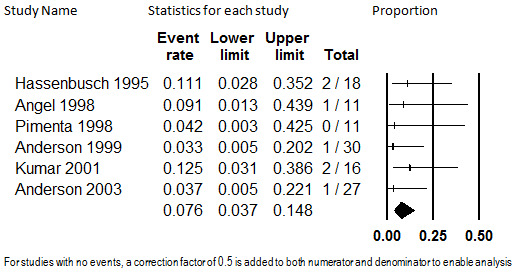
Discontinuation from Intrathecal Opioids Studies due to Insufficient Pain Relief, Follow‐up 6 to 29 months (mean) (I2<0.001)
Pain Relief
Oral administration
Continuous and categorical data
Three open‐label extension studies of RCTs (Harati 2000; McIlwain 2005; Roth 2000) and one nonrandomized open‐label case series (Rauck 2008) reported pretreatment pain scores and long‐term follow‐up pain scores. In the RCTs, a total of 755 participants were enrolled, and 376 (50%) continued treatment in the open‐label extension. In the nonrandomized study, 126 participants were enrolled in the titration phase, and 94 (75%) entered the maintenance phase after one week. The studies assessed the use of tramadol for neuropathic pain due to diabetes (Harati 2000), extended‐release oxymorphone for osteoarthritis pain (McIlwain 2005), extended‐release oxycodone for osteoarthritis pain (Roth 2000), and extended‐release oxymorphone for osteoarthritis or back pain (Rauck 2008). Each of the studies used a different scale to assess pain severity: 0 to 3 Likert scale (Roth 2000); a 0 to 4 Likert scale (Harati 2000); a 0 to 11 NRS (Rauck 2008), and a 0 to 100 VAS (McIlwain 2005). Use of the SMD allowed these studies to be combined in a meta‐analysis; however, the variation among instruments precludes translation of the pooled estimate into a single original metric.
We pooled data at 6 to 7.5 months to promote comparability of the data, because this was the only duration of follow‐up for which all three studies reported data. (Data at up to 12 months is reported in McIlwain 2005, and data at up to 18 months is reported in Roth 2000). At this time point, a total of only 273 participants, or 58% of those who began the long‐term extension or maintenance phase, remained. Because the attrition rate is so high, the participants are likely highly selected, and the data may be biased. It is possible that individuals with the poorest treatment response discontinued participating in the studies, in which case the pooled SMD would not represent the average treatment effect of all participants who began the study. Rather, it would represent the average treatment effect of those who were able to continue on oral opioids in the context of the studies for about six months. For those participants remaining in the studies at 6 months, the pooled SMD is 1.55 (95% CI: 0.85 to 2.25) (P < 0.001). Interpretation of the average amount of pain relief this represents in the original metric to attempt to determine whether the effect size is indeed large enough to be clinically significant is not possible, because three different pain scales were used. However, this SMD would be considered to represent a medium to large treatment effect between independent groups. More importantly, considered in isolation, each study suggests a clinically important reduction in pain from baseline.
Although all four studies reported significant reductions in pain among completers, they varied with respect to just how much pain reduction was achieved. The differences in these findings is measured as substantially heterogenous (I2 = 93.9%), and upon sensitivity analysis, this evidence base lacks quantitative robustness. Because only four studies comprise the evidence base, the source of this heterogeneity cannot be investigated, under our protocol. Because of the unexplained heterogeneity and lack of quantitative robustness, we believe the summary estimate may not accurately represent the average amount of pain relief among people able to continue opioids for about six months. Because of the high rate of attrition, it is not likely to capture the average amount of pain relief of all participants. However, because all four studies consistently show at least a moderate reduction in pain from baseline, and because this qualitative conclusion was robust to all sensitivity analyses, we conclude that pain relief that appears to be clinically important is achieved long‐term for patients able to remain on oral opioids for six months. The strength of the evidence supporting this conclusion is weak. This analysis is shown in Figure 7.
7.
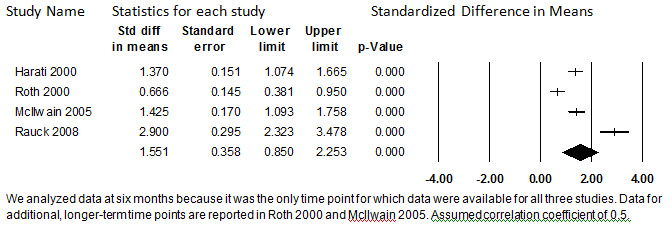
Change in Pain Score from Baseline, Oral Opioids, 6 to 7.5 months (I2=93.9%
Proportion of participants with at least 50% pain relief
Two studies with a total of 442 participants reported the proportion of participants who attained at least 50% pain relief on oral opioids. Allan 2005 studied extended‐release morphine in 342 participants with low‐back pain for 13 months, and Zenz 1992 studied extended‐release dihydrocodeine, buprenorphine, or extended‐release morphine in 100 participants with neuropathic or back pain for a mean of 7.5 months. The studies did not report data at more similar timepoints. One hundred thirty four (39%) participants in Allan 2005 and 51 participants (51%) in Zenz 1992 were reported to have at least 50% pain relief with long‐term opioid administration. Overall, 44.3% (95% CI: 33.3% to 55.9%) of participants had at least 50% pain relief, as shown in Figure 8. Because this number is substantially heterogenous (I2 = 77.3%), and because only two studies report this outcome, we do not believe that any firm conclusions regarding the precise proportion of participants who have at least 50% pain relief can be drawn at this time.
8.
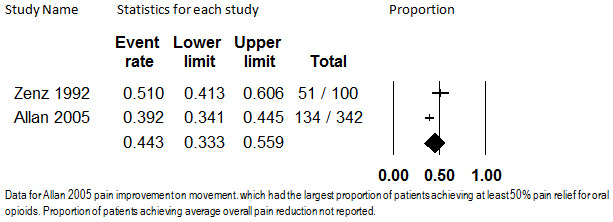
Proportion of Patients with at least 50% Pain Relief, Oral Opioids, Follow‐up 7.5 months (mean) to 13 months (I2=77.3%)
Transdermal administration
Continuous data
Two studies that administered transdermal fentanyl to participants with various chronic painful conditions reported long‐term pre‐post pain scores (Collado 2008; Mystakidou 2003). A total of 744 participants were enrolled in these studies. After six months of treatment, 668 participants, 90% of those who began treatment, remained. We analyzed outcomes at six months because that is the only long‐term duration of treatment reported by Collado 2008; however, follow‐up data at up to 48 months are reported in Mystakidou 2003. Both studies measured pain in terms of a VAS with a range of 0 to 10 (with higher values indicating greater pain).
Large reductions in pain were reported in both studies, with participants reporting severe pain at baseline (combined mean pain score 8.58, 95% CI: 6.07 to 11.09) and mild pain after six months of treatment (combined mean pain score 1.87, 95% CI: 1.53 to 2.21). In terms of the SMD calculated using a correlation coefficient of 0.5, the overall effect size was 7.56 (95% CI: 4.25 to 10.19), as shown in Figure 9. However, because there are only two studies currently available and because there is heterogeneity that cannot be explored due to the small size of the evidence base (I2 = 97.8%), the evidence base is currently too unstable to confidently draw conclusions regarding the average amount of pain relief patients who begin transdermal fentanyl might expect. However, these studies suggest that clinically significant pain relief is attained on average among patients who begin transdermal fentanyl for CNCP. This finding was robust to all sensitivity analyses but supported by weak evidence.
9.
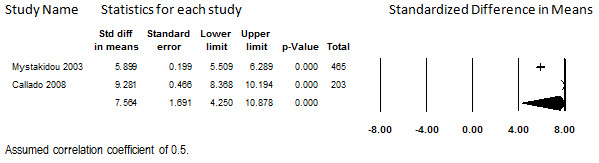
Change in Pain Score from Baseline, Transdermal Opioids, Follow‐up 6 months (I2=97.8%)
Proportion of patients with at least 50% pain relief
Only one study (Allan 2005) reported the proportion of participants who had at least a 50% reduction in pain from baseline. In this study, transdermal fentanyl was administered to 338 participants with chronic back pain for up to 13 months. One hundred and twenty nine (38.2% [95% CI: 33.1% to 43.5%]) participants had a 50% or greater reduction in pain during the course of the study. The finding from this study is shown in Figure 10. However, under our protocol, the results of a single study cannot be used to form evidence‐based conclusions as to how many individuals on transdermal fentanyl attain at least a 50% reduction in pain.
10.
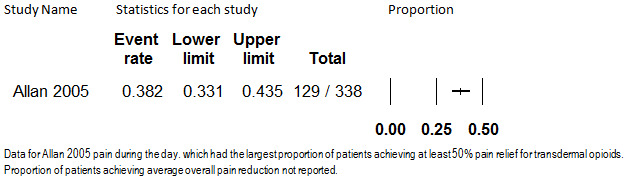
Proportion of Patients with at least 50% Pain Relief, Transdermal Opioids, Follow‐up 13 months
Intrathecal administration
Continuous/categorical
Nine studies that enrolled a total of 220 participants with CNCP due to a variety of causes, most frequently failed back surgery syndrome, reported pre‐ and post‐treatment pain scores (Anderson 1999; Anderson 2003; Angel 1998; Hassenbusch 1995; Kumar 2001; Mironer 2001; Rainov 2001; Shaladi 2007; Thimineur 2004). Most studies administered morphine alone or with an adjuvant, but three prescribed alternative opioids where deemed appropriate (Hassenbusch 1995; Mironer 2001; Thimineur 2004). Most of the studies reported outcome data on an intention‐to‐treat basis. Duration of treatment ranged widely, from 6 months to a mean of 29 months. Data for more compatible intermediate time points were not available.
We combined data at each studies' longest duration of follow‐up with the intent of using meta‐regression to determine whether duration of treatment is associated with reported pain scores. Only one study reported this outcome in terms of a NRS on a scale of 0 to 10 (Hassenbusch 1995), the rest reported the outcome in terms on VAS on a scale of 0 to 10. For both the NRS and the VAS, higher scores indicated greater pain. At longest follow‐up time, 201 (91%) participants remained to contribute data to this outcome. At baseline, the studies had a pooled score of 8.70 (95% CI: 8.37 to 9.04), indicating severe pain. At longest duration of treatment, the pooled score was 4.45 (95% CI: 3.44 to 5.47), indicating moderate pain. Calculated with a correlation coefficient of 0.5, the SMD was 2.01 (95% CI: 1.37 to 2.66). These data are shown in Figure 11. Importantly, when considered in isolation, each study shows clinically significant mean reductions in pain among the participants remaining in the study for long‐term follow‐up.
11.
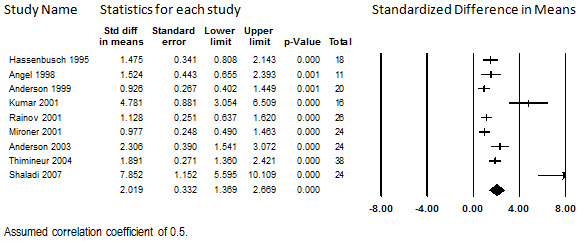
Change in Pain Score from Baseline, Intrathecal Opioids, Follow‐up 6 months to 29 months (mean) (I2=87.1%)
Although the studies consistently showed significant pain relief when considered individually, analysis revealed substantial heterogeneity in the amount of pain relief among the studies (I2 = 87.1%). We performed multiple univariate meta‐regressions to explore the heterogeneity. We investigated each of the items of the internal validity instrument, but none of these items were found to be significantly associated with the outcome. We also investigated year of publication, sample size, drug administered (morphine, morphine with adjuvant, or other), predominant cause of pain, and duration of treatment (follow‐up). Predominant cause of pain was the only factor significantly associated with the SMD (P = 0.01). As a robustness test, we repeated the regression using three other meta‐regression models (i.e., fixed‐effect, method of moments, unrestricted maximum likelihood), which consistently showed significant findings. Failed back surgery syndrome (k = 5) was associated with the least amount of pain relief, unspecified (k = 2) and neuropathic pain (k = 1) were associated with more pain relief, and osteoporotic vertebral fractures (k = 1) were associated with the most pain relief. However, the fact that few studies were available for three of the four causes of pain undermines the stability of this finding. In addition, previous work in this area that used different inclusion criteria did not detect a relationship (ECRI 2008). For these reasons the finding should be regarded with skepticism. When the model was replicated, heterogeneity remained substantial (I2 = 82.4%). Because a substantial portion of the heterogeneity remains unexplained, we have not drawn a quantitative conclusion regarding the amount of pain relief that patients beginning intrathecal opioids should expect. However, these data consistently show that participants experienced clinically significant pain relief with long‐term administration of opioids by an implantable infusion pump, and although supported by weak evidence, this finding is robust.
Proportion of patients with at least 50% pain relief
Seven studies that enrolled a total of 151 participants were included for this analysis (Anderson 1999; Anderson 2003; Angel 1998; Hassenbusch 1995; Kumar 2001; Mironer 2001; Shaladi 2007). Duration of follow‐up ranged from 6 months (Anderson 2003; Mironer 2001) to a mean of 29 months (Hassenbusch 1995; Kumar 2001). The summarized proportion of participants who had at least a 50% reduction in pain was 44.5% (95% CI: 27.2% to 63.2%), which is shown in Figure 12. This estimate was substantially heterogenous (I2 = 71.7%). We investigated the heterogeneity using study protocol factors and individual internal validity assessment responses. Size of enrollment sample, predominant cause of pain, drug(s) administered, and duration of treatment were not associated with the proportion of participants with at least 50% pain relief. However, year of study publication was, with more recently published studies showing larger proportions of participants with at least 50% pain relief (P = 0.02). This finding was consistent when other meta‐regression models were used (fixed‐effect, method of moments, and unrestricted maximum likelihood). Whether more recent therapy is truly related to improved outcomes is unclear; a relationship between year of study publication and average pain relief was not detected in the previous outcome. When the model was corrected for publication year, heterogeneity was reduced but remained substantial (I2 = 52.2%). One of the internal validity assessment items, whether the study was prospective or not, also had significant findings. Although prospective study design was an inclusion criterion for this assessment, for two of the studies it was unclear whether they were prospectively designed (Angel 1998; Shaladi 2007). These two studies had higher proportions of participants with at least 50% pain relief on average. Substantial heterogeneity remained despite correction of the model for this factor (I2 = 64.9%). When we dropped them and repeated the meta‐analysis, the point estimate was lower but the results were not significantly different (event rate 35.5%; 95% CI: 22.4% to 51.2%) and heterogeneity remained substantial (I2 = 58.8%). Due to the remaining unexplained heterogeneity, the exact proportion of participants who have at least 50% pain relief from intrathecal opioids cannot be accurately determined; however, it can be concluded that some patients do have this amount of pain relief.
12.
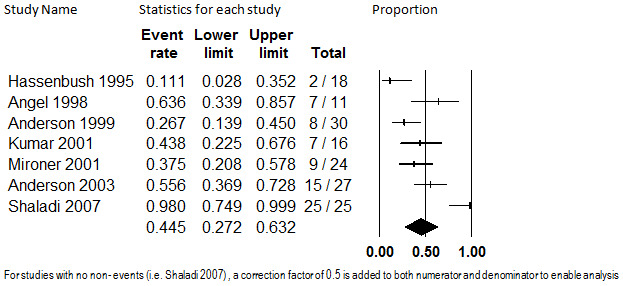
Proportion of Patients with at least 50% Pain Relief, Intrathecal Opioids, Follow‐up 6 months to 29 months (mean) (I2=71.7%)
Quality of life
Oral administration
One study that administered oral morphine to participants with low‐back pain reported a change in quality of life (Allan 2005). This study enrolled 335 participants and reported follow‐up data on the short form with 36 questions (SF‐36) at 13 months for 277 (83%) participants. Findings on both subscales are shown in Figure 13. A small improvement on the mental subscale and a larger improvement on the physical subscale were observed. However, a single study provides an insufficient quantity of data from which to draw conclusions.
13.
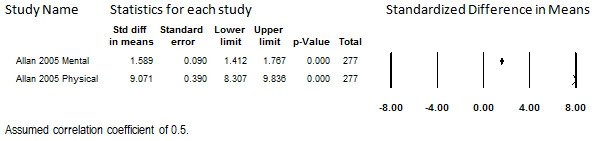
Change in Quality of Life from Baseline, Oral Opioids, Follow‐up 13 months
Transdermal administration
Three studies in which transdermal fentanyl was prescribed for participants with low‐back pain or various causes of pain reported quality‐of‐life outcomes in terms of the SF‐36 or the SF‐12 (Allan 2005; Milligan 2001; Mystakidou 2003). At 12 to 13 months, 753 (54%) of the 1394 total participants enrolled remained to report scores. (Longer‐term data are available for Mystakidou 2003, but we used their data at 12 months to promote comparability with the other two studies). Because a large portion of the participants dropped out, the findings from this assessment should be considered relevant only to participants who remain in treatment for a year, as participants who discontinued treatment before then may differ in important ways. One study showed minimal change on both the mental and physical subscales (Milligan 2001), one showed a large amount of improvement on both subscales (Mystakidou 2003), and one showed minimal improvement on the mental subscale but very large improvement on the physical subscale (Allan 2005).
When combined in meta‐analysis, heterogeneity was therefore substantial for both the mental subscale (I2 = 99.0%) and physical subscale (I2 = 99.1%), and could not be explored due to the small size of the evidence base. The pooled mental subscale SMD had inconclusive findings due to the lower limit of the 95% CI (SMD 0.78: 95% CI: ‐0.06 to 1.63), as shown in Figure 14. Because of this inconclusive statistical finding and the unexplained heterogeneity, no conclusions can be drawn regarding the association between long‐term administration of transdermal fentanyl and mental quality of life. The pooled physical subscale did show a large improvement from baseline, with a SMD of 4.46 (95% CI: 1.23 to 7.68); however, the effect size varied considerably between studies, as shown in Figure 15. Although this statistical finding precludes the accurate estimation of how much of an improvement in physical quality of life patients who remain on transdermal fentanyl may experience, these findings suggest that they do experience an improvement. This finding was weakly supported but robust to all sensitivity analyses
14.
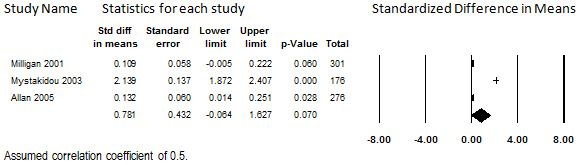
Change in Quality of Life from Baseline, Mental Subscale, Transdermal Opioids, Follow‐up 12 to 13 months (I2=99.0%)
15.
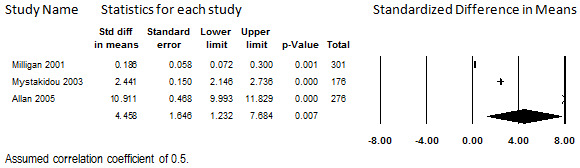
Change in Quality of Life from Baseline, Physical Subscale, Transdermal Opioids, Follow‐up 12 to 13 months (I2=99.1%)
Intrathecal administration
Three studies that reported quality of life in participants treated for CNCP due to various etiologies reported pre‐post quality‐of‐life data (Mironer 2001; Shaladi 2007; Thimineur 2004). A total of 92 participants were enrolled in these studies, of whom 86 (92%) remained at follow up to contribute data. Data from these studies were reported at different durations of treatment: six months (Mironer 2001); 12 months (Shaladi 2007); and 36 months (Thimineur 2004). Compatible intermediate time points were not reported. Instruments used were the Tollison Quality of Life Scale (Mironer 2001), the SF‐36 (Thimineur 2004), and the Questionnaire of the European Foundation for Osteoporosis (Shaladi 2007). One of the studies had inconclusive findings (Mironer 2001), one reported a small benefit (Thimineur 2004), and one reported a large benefit (Shaladi 2007).
We assessed their findings in terms of the SMD to enable side‐by‐side comparisons and calculation of an overall effect size, as shown in Figure 16. Heterogeneity among them was substantial (I2 = 93.4%). As only three studies comprised the evidence base, this heterogeneity could not be investigated. When the studies were combined in meta‐analysis, the overall finding was statistically inconclusive (SMD 1.02: 95% CI ‐0.04 to 2.09). Given these findings and the qualitatively different findings of the studies when considered individually, no conclusions can be drawn regarding whether intrathecal opioids are associated with an improvement in quality of life at this time.
16.
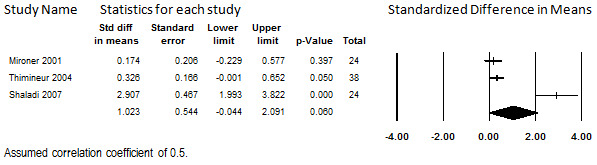
Change in Quality of Life from Baseline, Intrathecal Opioids, Follow‐up 6 to 36 months (I2=93.4%)
Function
Oral administration
No studies were identified for this outcome.
Transdermal administration
No studies were identified for this outcome.
Intrathecal administration
Three studies reported change in functional status after long‐term treatment with intrathecal opioids (Anderson 1999; Anderson 2003; Thimineur 2004). In these studies, a variety of opioids were infused for predominantly low‐back pain. These studies enrolled a total of 98 participants and reported follow‐up data on 82 (84%) of them. Each used a different instrument to assess function: the Oswestry Disability Index (Thimineur 2004), the short‐form Sickness Impact Profile (Anderson 2003), and the Chronic Illness Problem Inventory (Anderson 1999). The studies varied considerably in duration of follow‐up, reporting data at six months (Anderson 2003), 24 months (Anderson 1999), and 36 months (Thimineur 2004). Data at six months for the longer two studies were not reported.
The studies had inconsistent findings, showing inconclusive findings (Anderson 1999), a moderate effect size (Thimineur 2004), and a larger effect size (Anderson 2003). Analysis revealed substantial heterogeneity (I2 = 81.2%), for which the evidence base was too small to investigate. The estimate yielded by meta‐analysis was statistically inconclusive (SMD 0.56, 95% CI ‐0.02 to 1.13) (see Figure 17). Because of the inconsistencies among the studies, no conclusions can be drawn regarding the effect of intrathecal opioids on functional status at this time.
17.
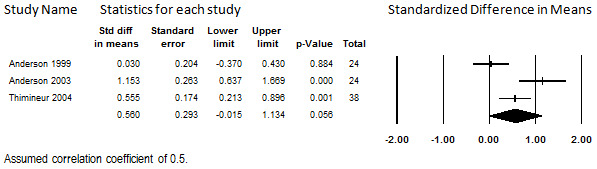
Change in Function Levels from Baseline, Intrathecal Opioids, Follow‐up 6 to 36 months (I2=81.2%)
Discussion
Many participants in the included studies, particularly those treated with orally‐administered opioids, were so dissatisfied with adverse events or insufficient pain relief that they discontinued participating in the studies. As no data are provided on these participants after they dropped out of the studies, it is impossible to say whether they continued opioid therapy under different protocols. For participants able to continue opioids in the studies, evidence (albeit weak) suggests that, for all analyzed modes of administration, their pain scores were lower on average than before therapy began, and that this relief could be maintained long‐term (> 6 months). However, data describing long‐term safety and efficacy of opioids for CNCP are limited in terms of quantity and quality. An evidence base consisting of low‐quality studies provides only weak evidence from which to draw qualitative conclusions and only low‐stability evidence from which to draw quantitative conclusions. Some of the quantitative estimates were not robust, meaning that an estimate of the treatment effect size cannot be accurately estimated with the currently available evidence, and the estimates may therefore be unstable and should be interpreted cautiously. The low internal validity ratings indicate that the evidence supporting our conclusions is highly subject to change, and that the likelihood is high that findings of future studies may overturn these conclusions. Furthermore, there may be a particular risk of publication bias in uncontrolled case‐series studies, as journals may be less likely to accept studies of this design, investigators may be less likely to submit them for publication (given the lesser financial investment in conducting them), and no clinical trial registry for uncontrolled studies is currently in widespread use. The small size of the evidence base for each mode of administration prevented us from using the trim‐and‐fill method of identifying potentially missing studies.
Among outcomes for which qualitative conclusions were possible, unexplained heterogeneity limited the number of quantitative conclusions we could make. We postulated that at least some of the heterogeneity might be explained by differences in duration of treatment; however, meta‐regression did not support this possibility for any outcome. For discontinuation from the study due to adverse events or insufficient pain relief, this may be because most people who discontinue participation in opioids studies do so within the first six months. For pain, this may be because optimum pain relief is achieved within six months and longer durations of treatments serve to maintain the effect, or it may be because participants with the most treatment‐resistant pain discontinue participating in the study early on. Alternatively, the evidence bases may simply have been underpowered to detect a relationship between duration of treatment and the outcomes. Investigation of heterogeneity for efficacy outcomes for oral and transdermal opioids was not possible due to the small size of the evidence bases. Investigation was possible for intrathecally‐administered opioids, and potential relationships between proportion of participants with clinically significant pain relief (> 50%) and year of publication and prospective study design, and between average pain relief and predominant cause of pain in the study, were identified. However, these relationships do not seem stable. The only convincing covariate we identified was a lower rate of discontinuation due to adverse events from clinical study in studies that administered oral weak opioids compared with studies that administered strong oral opioids.
The high attrition rates in some of the studies call into question whether the participants reporting continuous outcomes are representative of the patient population originally enrolled in those studies, and whether as a result, this attrition is a threat to internal or external validity. For oral opioids in particular, findings on amount of pain relief or change in quality of life strictly pertain to the participants remaining at longer durations of treatment, and do not necessarily indicate the average treatment effect for all patients who begin oral opioids. We found no studies that thoroughly attempted to identify prognostic factors for drop out. Measuring the proportion of participants who had clinically significant pain relief circumvented the problem of attrition, because individuals who dropped out of the study were assumed to be treatment failures.
Considering data from case series may be warranted in certain situations, including when a long duration of follow‐up is needed that an RCT cannot provide, when the technology appears promising and waiting for RCTs is unacceptable, and when a policy decision must be made in the absence of RCTs (Dalziel 2005). Case series are generally considered a lower level of evidence for measuring the impact of an intervention than controlled trials, because without a control group, there is no empirical estimate of what the patients’ outcomes might have been if they had not been treated. Control groups are essential when patients' future outcomes are highly uncertain. However, if the natural history of a disease is stable, substantive improvement would not be expected without the intervention in question. Also, we postulate that individuals who have failed multiple previous treatments may be less susceptible to placebo effects than the general population. As patients prescribed long‐term opioids have had pain long‐term, and have typically experienced failure of more conservative treatment options (e.g., simple analgesics, physical therapy), many have had a lack of success with surgery (e.g., patients with failed back surgery syndrome). Also, some research suggests that placebo effects in pain research are not large (Hrobjartsson 2004a; Hrobjartsson 2004b). We therefore believe it is justifiable to consider evidence from case series for this patient population. However, the fact that we considered data from uncontrolled case series should be kept in mind when considering the studies' results; influences from regression to the mean and selection bias cannot be ruled out. Furthermore, case series data cannot be extrapolated to draw conclusions regarding comparative effectiveness.
Authors' conclusions
Implications for practice.
Most of the participants in these studies had back pain, often following failed back surgery, osteoarthritis, or neuropathic pain. These findings should not be generalized to patients with chronic pain of other types, such as headache. Concern that an individual with CNCP may develop dependence on the drug during long‐term administration represents a potential barrier to treatment. However, the rate of observed signs of opioid addiction was extremely low in the body of evidence considered in this review (0.27%, conservatively). This rate would be 0.14% if no addictive behaviors occurred among the studies that did not mention addiction rates at all. Only three participants were reported as having potential abuse problems. These numbers do not support the contention that potential iatrogenic opioid addiction should limit therapy for well‐selected and well‐supervised patients. Because most studies screened out potential participants with histories of substance abuse or addiction, the rates of addiction reported in these studies are only generalizable to patients without a history of addictive/abusive behaviors. This finding is consistent with that of another review article on opioid abuse and addiction that calculated an abuse/addiction rate of 0.19% in studies that prescreened chronic pain patients for long‐term opioid use or addiction/abuse history, and 3.27% in all studies (Fishbain 2008). Given the complexity of definitively diagnosing opioid addiction (see Ballantyne 2006) and in the interest of capturing the overall effect of opioid therapy on quality of life, we sought to analyze health‐related quality‐of‐life outcomes in this review. However, findings on quality of life were inconclusive for all modes of administration.
Implications for research.
The Mayday Fund (www.maydayfund.org), a foundation sponsoring pain treatment research, asked ECRI Institute to review issues including the efficacy and safety of opioids for noncancer pain in 2004. The Milbank Memorial Fund (www.milbank.org) invited an international group of pain researchers to help formulate key questions. Representatives from these organizations and additional pain researchers critiqued the review and made recommendations for future research. Allowing rescue opioids (with the amount and frequency of dosage as an outcome) in placebo or nonopioid groups may circumvent ethical problems of withholding treatment from individuals with CNCP in long‐term RCTs. Protocols should specify uniform diagnostic and data collection criteria (e.g., pain etiology, drugs prescribed, dosing regimens) and mimic clinical practice (e.g., drug combinations could be used, drug changes could be made, drug dosage could be titrated slowly and adjusted, adverse effects could be aggressively managed). Reasons for discontinuation, including satisfied departures, must be documented. Long‐term poorly‐understood effects, including androgen deficiency and changes in immune function, must be documented. However, practical/pragmatic RCTs might be cost prohibitive (Tunis 2003). Adequately powered, well‐designed prospective cohort studies could provide useful information (Geborek 2002; MacDonald 2003; Mamdani 2002). Long‐term dose‐dependent efficacy and adverse effects could be researched in existing databases (e.g., European Medicines Evaluation Agency, FDA, Australia Therapeutic Goods Administration). Studies on U.S. Veterans Healthcare Administration data are examples of retrospective analyses (Hermos 2004; Mahowald 2005; Reid 2002; Ytterberg 1998).
Despite the identification of 26 treatment groups with 4768 participants, the evidence regarding the effectiveness of long‐term opioid therapy in CNCP is too sparse to draw firm conclusions, including quantity of mean of pain relief. Poor reporting reduced the amount of acceptable data. Studies should always report data needed for meta‐analysis (mean, standard deviation, number of participants, or data to calculate them), and authors of studies for which these data were not originally published should consider making them publicly available. Studies should also use validated scales, report intention‐to‐treat data in addition to completer analyses, and conduct post‐hoc analyses to identify prognostic factors for treatment success.
Feedback
Feedback submitted, 12 February 2018
Summary
Date of Submission: 12‐Feb‐2018
Name: Linda Burton
Email Address: lindaburton222@gmail.com
I had spinal surgery It left me with scar tissue I also suffer from various bulging discs that touch the nerves in the lumber thoracic and cervical regions This causes severe pain unbearable pain at times I was prescribed Mat 60 mg twice a day Rising to 120mg twice a day plus Oramorph I was then able to get out Do my own shopping Do the type of things people take for granted In trying to reduce the Mst I quickly found out that the pain this caused prevented me from leading any kind of normal life I have never experienced any kind of craving for any drug and if it didn't have pain I could happily give up any kind of Morphine or pain relief There is no high in taking Morphine for pain Your present policy is going to kill people like me We can't live with the level of pain so will commit suicide You will have left us with no choice and no life to live
Reply
Dear Linda Burton,
Thank you for submitting your comments and we are sorry to hear of your chronic pain condition. It is experiences such as yours that make us determined to help. You will have read that we state in the abstract: “weak evidence suggests that patients who are able to continue opioids long‐term experience clinically significant pain relief”. Cochrane reviews are not policy but seek to summarise the available evidence which we did at the time. “Weak evidence” just describes the quality of the evidence that we found at that time, and speaks to our confidence about the claims made about the value (efficacy and safety) of any medicine. We are aware that the review is now quite old and so are actively considering updating it or removing it from the library.
Best wishes,
Phil Wiffen
Contributors
Feedback Editor Hayley Barnes, Managing Editor Kerry Harding, and Co‐ordinating Editor Christopher Eccleston.
What's new
| Date | Event | Description |
|---|---|---|
| 7 March 2018 | Feedback has been incorporated | See Feedback |
History
Protocol first published: Issue 3, 2007 Review first published: Issue 1, 2010
| Date | Event | Description |
|---|---|---|
| 14 January 2016 | Amended | See Published notes. |
| 24 September 2010 | Amended | Contact details updated. |
| 8 April 2008 | Amended | Protocol converted to new review format. |
Notes
This review requires a new author team to complete the update. Please contact the PaPaS CRG for more information.
Acknowledgements
We thank Jeffrey Lerner, PhD, of ECRI Institute for overseeing the original project from inception to completion. We also thank Eileen Erinoff, MLIS, of ECRI Institute for designing and conducting the systematic literature searches, and Helen Dunn and Tracey Montasterno‐Stem for organizing retrieval of articles. For article translation and extraction of data from non‐English language articles, we thank Anette Blumle, PhD Simona Vecchi, and Maroussia Kostadinova Tzanova. We thank Charles M Turkelson, PhD, for his contributions to the original project.
We appreciate the members of the pain research, legal, social science, and regulatory community for their valuable insights during the earlier systematic review, especially Daniel Fox, PhD, formerly of the Milbank Memorial Fund, and Christina Spellman, PhD, of the Mayday Fund. We also thank James Campbell, MD, Dr Howard Fields, MD, PhD, and Rollin Gallagher, MD, MPH, for a number of helpful comments on the scientific and clinical context for the review. We also wish to thank the following people who reviewed and provided comments on the original draft report: Steven I Altchuler, PhD, MD; Daniel B Carr, MD; Roger Chou, MD, MPH; Mike Clarke, DPhil; Wilson M Compton, MD, MPE; Richard A Denisco, MD, MPH; Kathleen M Foley, MD; James Gigli; Geoff Gourlay, PhD; Marcia Meldrum, PhD; Robert Meyer, MD; Charles D Ponte, PharmD CDE, BCPS; Russell Portenoy, MD; Andrew SC Rice, MD; Linda Simoni‐Wastila, PhD; and James Zacny, PhD.
We also wish to thank the following people who participated in teleconferences on setting the research agenda: Mitchell Max, MD; Elon Eisenberg, MD; Ian Gilron, MD, MSc, FRCP (C); Geoff Gourlay, PhD; Arthur Lipman, PhD; Marcia Meldrum, PhD; Kathleen Foley, MD; Roger Goucke, FANZCA, FFPMANZCA, FAChPM; and Henry McQuay, MD.
Appendices
Appendix 1. Search strategy
| Ovid Syntax |
| 1.Exp pain/ 2.Pain$.ti,ab. 3.(Chronic or intractable or refractory or persistent).ti,ab. 4.Pain, intractable.de. 5.(#1 or #2) and #3 6.Soft tissue.ti,ab. 7.(Pancreatitis and chronic).ti,ab. 8.Arteriosclerosis obliterans.ti,ab. 9.Fibromyalgia.ti,ab. 10.Exp musculoskeletal disease/ 11.Exp musculoskeletal diseases/ 12.Fibrositis.ti,ab. 13.Arthrit$.ti,ab. 14.Back.ti,ab. 15.Neck.ti,ab. 16.Neck pain.de. 17.Tmj.ti,ab. 18.Exp joint diseases/ 19.Exp arthropathy/ 20.Exp back pain/ 21.Exp backache/ 22.Exp multiple sclerosis/ 23.MS.ti,ab. 24.phantom.ti,ab. 25.allodynia.ti,ab. 26.Sciatic$.ti,ab. 27.Neuralgia.ti,ab. 28.Neuropath$.ti,ab. 29.or/4‐28 30.exp analgesics, opioids/ 31.exp narcotics/ 32.exp opiates/ 33.exp narcotic analgesic agent/ 34.narcotic$.ti. 35.opiate$.ti. 36.opioid$.ti. 37.acemethadone.mp. 38.acetylmethadol.mp. 39.alfenta.mp. 40.alfentanil.mp. 41.amidone.mp. 42.anileridine.mp. 43.ardinex.mp. 44.benzomorphan$.mp. 45.buprenorphine.mp. 46.buprenex.mp. 47.butorphanol.mp. 48.carfentanil.mp. 49.codeine.mp. 50.codinovo.mp. 51.delsym.mp. 52.demerol.mp. 53.dextromoramide.mp. 54.dezocine.mp. 55.diacetyl morphine.mp. 56.diamorphine.mp. 57.dicodid.mp. 58.dihyrocodeinone.mp. 59.dihydroetorphine.mp. 60.dihydrohyroxycodeinone.mp. 61.dihyromorphine.mp. 62.dihydrone.mp. 63.dilaudid.mp. 64.dimepheptanol.mp. 65.dinarkon.mp. 66.dionine.mp. 67.diprenorphine.mp. 68.dolantin.mp. 69.dolargan.mp. 70.dolcontral.mp. 71.dolophine.mp. 72.dolosal.mp. 73.dolsin.mp. 74.duragesic.mp. 75.duramorph.mp. 76.dyhydromorphinone.mp. 77.dynorphin.mp. 78.endomorphin.mp. 79.eseroline.mp. 80.ethylketocyclazocine.de. 81.eucodal.mp. 82.fenoperidine.mp. 83.fentanyl.mp. 84.fioricet.mp. 85.fortral.mp. 86.hycodan.mp. 87.hycon.mp. 88.hydrocodon$.mp. 89.hydrocon.mp. 90.hydromorphon$.mp. 91.hydroxycodeinon.mp. 92.isocodeine.mp. 93.isonipecain.mp. 94.isopromedol.mp. 95.kaolin‐pectin.mp. 96.ketobemidone.mp. 97.laudacon.mp 98.lealgin.mp. 99.levallorphan.mp. 100.levamethadyl.mp. 101.levodroman.mp. 102.levomethadryl.mp. 103.levorphan$.mp. 104.lexir.mp. 105.lidol.mp. 106.lorfan.mp. 107.lofentain.mp. 108.lydol.mp. 109.meperidine.mp. 110.meptazinol.mp. 111.methadol.mp. 112.methadone.mp. 113.methadyl acetate.mp. 114.moradol.mp. 115.morphia.mp. 116.morphine.mp. 117.exp morphine derivatives/ 118.MS Contin.mp. 119.methynaloxone.mp. 120.nalbuphine.mp. 121.naloxiphan.mp. 122.nocistatin.mp. 123.nubain.mp. 124.numorphan.mp. 125.omnopon.mp. 126.operidine.mp. 127.opium.mp. 128.oramorph.mp. 129.oxycodein$.mp. 130.oxycodone.mp. 131.oxycone.mp. 132.oxyconum.mp. 133.oxycontin.mp. 134.oxymorph$.mp. 135.pancodiene.mp. 136.pantopon.mp. 137.papaveretum.mp. 138.paracymethadol.mp. 139.paramorfan.mp. 140.paramorphan.mp. 141.paregoric.mp. 142.pentazocine.mp. 143.percocet.mp. 144.pethidine.mp. 145.phenadone.mp. 146.phenazocine.mp. 147.phenbenzorphan.mp. 148.phenethylazocine.mp. 149.phenoperidine.mp. 150.physeptone.mp. 151.promedol.mp. 152.propoxyphene.mp. 153.protopine.mp. 154.pyrrolamidol.mp. 155.rapifen.mp. 156.remifentanil.mp. 157.revivon.mp. 158.robidone.mp. 159.stadol.mp. 160.sufentanil.mp. 161.sufentanyl.mp. 162.talwin.mp. 163.temgesic.mp. 164.thebaine.mp. 165.theocodin.mp. 166.tilidine.mp. 167.tramadol.mp. 168.trimeperidine.mp. 169.valoron.mp. 170.valerone.mp. 171.vicodin.mp. 172.or/30‐171 173.29 and 172 174.Editorial.de,pt. 175.News.de,pt. 176.Comment.de,pt. 177.Review.de,pt. 178.Note.de. 179.Conference paper.de. 180.or/174‐179 181.173 not180 182.Limit 181 human 183.Limit 182 humans 184.Remove duplicates from 183 185.Exp neoplasms/ or exp neoplasm/ 186.Cancer$.ti. 187.Carcinoma$.ti. 188.((Post adj 2 oper$) or postop$).ti. 189.or/185‐188 190.184 not 189 |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allan 2005.
| Methods | Randomized, controlled trial (RCT) comparing two strong opioids Duration: 13 months | |
| Participants | N = 680 enrolled
Primary condition: Low back pain Baseline pain score: Not reported Time since onset: 124.7 (SD 4.6) months Mean age: 54.0 years Female: 61% Previous opioid analgesia: No participants had previous strong opioid treatment during the four weeks before study commencement |
|
| Interventions | Oral sustained‐release morphine (n = 342) Initial dose: Mean 58 mg/day, Range 6 to130 mg Titrated to to 140 mg/day at endpoint Transdermal fentanyl (n = 338) Initial dose: Mean 25 ug/hour; Range 2.5 to 50 ug/hour Titrated to mean 57 ug/hour; Range 12.5 to 250 ug/hour at study end Supplemental analgesia: Permitted for breakthrough pain. Other non‐opioid medications maintained at pre‐study dose. |
|
| Outcomes | Discontinuation due to adverse events
Adverse events
Discontinuation due to insufficient pain relief
Pain relief (proportion with at least 50% relief) Quality of life |
|
| Notes | Categorical pain measures also reported but not included in analysis because number of people who contributed data at those time points not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Comparability of patients at baseline and follow‐up (continuous data) All outcomes | Unclear risk | Due to attrition |
| Selection method random or consecutive | Low risk | Consecutive series invited |
| Prospective | Low risk | Prospective |
| Free from confounding treatment(s) | High risk | Supplemental analgesia permitted. Other nonopioids medications permitted at constant doses. |
| Patient compliance monitored and reported at at least 85%? | Unclear risk | Not reported |
| Attrition and right censoring less than 15% (continuous data) All outcomes | High risk | Greater attrition |
| Funding from a source without financial conflict of interest? | High risk | Funding from Janssen Phamaceutica |
Anderson 1999.
| Methods | Case series Duration: 24 months | |
| Participants | N = 30 enrolled
Primary condition: Most had back and/or leg pain, In 47% associated with failed back surgery syndrome
Baseline pain score: Mean 78.5 (SD 15.9, Range 39 to 100)/100 VAS
Time since onset: 8 years (SD 9 years, Range 5 to 24 years)
Mean age: 58 years (SD 13 years)
Female: 53% Previous opioid analgesia: All patients had taken systemic narcotics before study enrolment, and 28/30 (93%) were taking them regularly at time of enrolment |
|
| Interventions | Intrathecal morphine Initial dose: Mean 1 mg/day Titration up to 25 mg/day at endpoint Supplemental analgesia: Allowed. At 24 months, 6/20 (30%) patients used supplemental oral narcotics |
|
| Outcomes | Discontinuation due to adverse events
Adverse events
Discontinuation due to insufficient pain relief
Pain (continuous) and pain relief (proportion with at least 50% relief) Function |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Comparability of patients at baseline and follow‐up (continuous data) All outcomes | High risk | Due to attrition |
| Selection method random or consecutive | Low risk | Consecutive |
| Prospective | Low risk | Prospective |
| Free from confounding treatment(s) | High risk | Supplemental analgesia allowed |
| Patient compliance monitored and reported at at least 85%? | Low risk | Pump administration |
| Attrition and right censoring less than 15% (continuous data) All outcomes | High risk | Higher attrition |
| Funding from a source without financial conflict of interest? | High risk | Funding from Medtronic |
Anderson 2003.
| Methods | Case series (Although the study was designed as a RCT, the comparison was between different pre‐implantation screening methods, not opioid treatment versus another treatment) Duration: 6 months | |
| Participants | N = 27 enrolled
Primary condition: Failed back surgery syndrome Baseline pain score: Mean 81.2 (SD 10.2) / 100 VAS Time since onset: Median of at least 5 years, minimum of 1 year Mean age: 55 years (SD 11 years, Range 32 to 80 years) Female: 46% Previous opioid analgesia: All patients took systemic opioids prior to enrolment; 86% (32/37) were taking systemic opioids at time of study enrolment |
|
| Interventions | Intrathecal morphine Initial dose: Mean 0.87 (SD 0.38) mg/day Titration to: Mean 4.1 (SD 2.7) mg/day Supplemental analgesia: Allowed. Systemic opioids were reduced overall. Non‐opioid systemic medication was constant through study |
|
| Outcomes | Adverse events Discontinuation due to insufficient pain relief Pain, continuous and proportion with at least 50% relief Function |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Comparability of patients at baseline and follow‐up (continuous data) All outcomes | Low risk | When completer‐only baseline data is used. |
| Selection method random or consecutive | Low risk | Consecutive patients screened |
| Prospective | Low risk | Prospective |
| Free from confounding treatment(s) | High risk | Supplemental medications and nonopioid analgesics allowed |
| Patient compliance monitored and reported at at least 85%? | Low risk | Pump administration |
| Attrition and right censoring less than 15% (continuous data) All outcomes | Low risk | Less attrition than 15% |
| Funding from a source without financial conflict of interest? | High risk | Funded by Medtronic |
Angel 1998.
| Methods | Case series Duration: Up to 36 months, median 28.5 months | |
| Participants | N=11 enrolled
Primary condition: Failed back syndrome Baseline pain score: Mean 9.5 (SD 0.4) / 10 VAS Time since onset: Not reported Mean age: Not reported; Range 29 to 81 Female: 55% |
|
| Interventions | Intrathecal morphine Initial dose 0.125 to 0.750 mg/day Titrated to 1.5 to 14.0 mg/day at endpoint Supplemental analgesia: Not reported |
|
| Outcomes | Discontinuation due to adverse events Adverse events Discontinuation due to insufficient pain relief Pain, continuous and proportion with at least 50% relief | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Comparability of patients at baseline and follow‐up (continuous data) All outcomes | Low risk | All patients followed up |
| Selection method random or consecutive | Low risk | Consecutive patients screened |
| Prospective | Unclear risk | Not reported |
| Free from confounding treatment(s) | Unclear risk | None reported |
| Patient compliance monitored and reported at at least 85%? | Low risk | Pump administration |
| Attrition and right censoring less than 15% (continuous data) All outcomes | Low risk | All patients followed up |
| Funding from a source without financial conflict of interest? | Unclear risk | Not reported |
Bettoni 2006.
| Methods | Case series Duration: Mean 22.3 months |
|
| Participants | N = 14 enrolled Primary condition: Rheumatological disease Baseline pain score: Mean 9.5 (Measure of variance not reported)/10 VAS Time since onset: Not reported Mean age: 71.9 years; Range 62 to 85 years Female: 85.7% Previous opioid analgesia: Not reported |
|
| Interventions | Transdermal fentanyl Initial dose: 25 micrograms/hour Titrated to 82.5 micrograms/hour (range 50 to 100) at endpoint Supplemental analgesia: Allowed |
|
| Outcomes | Discontinuation due to adverse events Adverse events |
|
| Notes | Pain and quality of life reported in study but not used in this analysis because measure of variance or data to estimate it not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Comparability of patients at baseline and follow‐up (continuous data) All outcomes | Low risk | (No continuous outcomes included in analysis) |
| Selection method random or consecutive | Unclear risk | Not reported |
| Prospective | Low risk | Prospective |
| Free from confounding treatment(s) | High risk | Supplemental analgesia allowed |
| Patient compliance monitored and reported at at least 85%? | Unclear risk | Not reported |
| Attrition and right censoring less than 15% (continuous data) All outcomes | Low risk | (No continuous outcomes included in analysis) |
| Funding from a source without financial conflict of interest? | Unclear risk | Not reported |
Caldwell 2002.
| Methods | RCT with case series open‐label continuation Duration: RCT 4 weeks, open‐label continuation another 26 weeks, total 6.9 months |
|
| Participants | N = 295 enrolled in RCT, 181 continued in case series Primary condition: Osteoarthritis of hip and/or knee Baseline pain score: 7.84 (SD 1.79) / 10 VAS Time since onset: NR Mean age: 62.4 years Female: 62% Previous opioid analgesia: Prior suboptimal response to NSAIDS and acetaminophen or previous intermittent opioid therapy required for inclusion |
|
| Interventions | Oral extended‐release morphine Initial Dose: 30 mg/day Titrated up to as high as 120 mg/day Supplemental analgesia: During RCT, only acetaminophen (up to 2 g/day) for non‐OA symptoms and aspirin for cardiovascular prophylaxis (up to 325 mg/day) allowed. During open‐label portion, acetaminophen (up to 4 g/day), NSAIDS, topical analgesics, and physical therapy allowed. |
|
| Outcomes | Discontinuation due to adverse events Adverse events Discontinuation due to insufficient pain relief |
|
| Notes | Pain and function also reported but not included in meta‐analysis because method of data reporting not statistically compatible with other studies. Both RCT and long‐term case series are reported in this citation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Comparability of patients at baseline and follow‐up (continuous data) All outcomes | Low risk | (No continuous outcomes included in analysis) |
| Selection method random or consecutive | Unclear risk | Not reported |
| Prospective | Low risk | Prospective |
| Free from confounding treatment(s) | High risk | Supplemental analgesia and pain treatments allowed |
| Patient compliance monitored and reported at at least 85%? | Unclear risk | Not reported |
| Attrition and right censoring less than 15% (continuous data) All outcomes | Low risk | (No continuous outcomes included in analysis) |
| Funding from a source without financial conflict of interest? | High risk | Appears to have been funded by the drug's manufacturer because trade name with trademark used in publication |
Collado 2008.
| Methods | Case series Duration: 6 months |
|
| Participants | N = 215 enrolled Primary condition: Various, including arthrosis (n = 51), ischemic vascular disease (n = 42), postlaminectomy syndrome (n = 34), vertebral compression fracture (n = 22), trigeminal neuralgia (n = 21), disk herniation (n = 18), rheumatoid arthritis (n = 9), diabetic polyneuropathy (n = 8), scoliosis (n = 4), central pain (n = 4) Baseline pain score: Mean 9.86 (SD 0.35) / 10 VAS Time since onset: Not reported, at least 6 months to meet inclusion criteria Mean age: 57.61 years; Range 32 to 75 Female: 54% Previous opioid analgesia: Not reported |
|
| Interventions | Transdermal fentanyl Initial dose:12 ug/hour Titrated to: Mean not reported. 50 ug/h in 48% of patients, 75 ug/hr in 18%, 100 ug/hr in 5% Supplemental analgesia:Oral transmucosal fentanyl citrate (Actiq) prescribed for breakthrough pain |
|
| Outcomes | Discontinuation due to adverse events Adverse events Pain, continuous |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Comparability of patients at baseline and follow‐up (continuous data) All outcomes | Low risk | All patients followed up |
| Selection method random or consecutive | Unclear risk | Not reported |
| Prospective | Low risk | Prospective |
| Free from confounding treatment(s) | High risk | Supplemental analgesics permitted |
| Patient compliance monitored and reported at at least 85%? | Unclear risk | Not reported |
| Attrition and right censoring less than 15% (continuous data) All outcomes | Low risk | All patients followed up |
| Funding from a source without financial conflict of interest? | Unclear risk | Not reported |
Fredheim 2006.
| Methods | Case series Duration: 9 months | |
| Participants | N = 12 enrolled
Primary Condition: Low back pain Baseline pain score: Mean 5.8 (Range 3 to 9; standard measure of variance not reported) / 10 NRS Time since onset: Not reported Mean age: 60.2 years Female: 42% Previous opioid analgesia: All patients had taken strong opioids without good pain control or with unacceptable side effects, including oral slow release morphine (n = 7), oral oxycodone (n = 2), and transdermal fentanyl (n = 3). All took morphine for 2 weeks prior to methadone to enable equianalgesic dosing of morphine to methadone. |
|
| Interventions | Oral methadone Initial dose: Based upon patients' pre‐study daily morphine dose Titrated up to a mean on 202 mg/day (range 50 to 800 mg.day) Supplemental analgesia: Not reported. Patients were instructed to contact the study physicians in case of breakthrough pain. |
|
| Outcomes | Adverse events | |
| Notes | Pain also reported but not included in analysis because outcome data were reported for fewer than 10 participants Data on discontinuation not included in analysis because patients discontinued study opioid but continued treatment on another opioid, therefore not discontinuing from the study or opioids altogether |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Comparability of patients at baseline and follow‐up (continuous data) All outcomes | Low risk | (No continuous outcomes included in analysis) |
| Selection method random or consecutive | Unclear risk | Not reported |
| Prospective | Low risk | Prospective |
| Free from confounding treatment(s) | Unclear risk | Not reported |
| Patient compliance monitored and reported at at least 85%? | High risk | Greater attrition |
| Attrition and right censoring less than 15% (continuous data) All outcomes | Low risk | (No continuous outcomes included in analysis) |
| Funding from a source without financial conflict of interest? | Unclear risk | Not reported, but since generic drugs were prescribed seems unlikely. |
Harati 2000.
| Methods | RCT with case series open‐label continuation Duration: 6‐week RCT plus 6 months of open‐label continuation; total 7.4 months |
|
| Participants | N = 131 enrolled in RCT, 117 enrolled in continuation Primary condition: Diabetic neuropathy Baseline pain score: Mean 2.5 (SD 0.61) / 4 on Likert scale (0 being no pain, 4 being extreme pain) Time since onset: NR; mean time since onset of diabetes, 13 years Mean age: 58 years (range 32 to 85 years) Female: 41.9% Previous opioid analgesics: Prospective patients on multiple daily doses of narcotics excluded |
|
| Interventions | Oral tramadol Initial dose: 50 mg/day Titrated to maximum of 400 mg/day Supplemental analgesia: In RCT portion, no other pain medications, including tricyclic antidepressants or anticonvulsants, were permitted. Not reported whether they were allowed in open‐label continuation. |
|
| Outcomes | Discontinuation due to adverse events Adverse events Discontinuation due to insufficient pain relief Pain, continuous |
|
| Notes | Continuation of: Harati Y, Gooch C, Swenson M, Edelman S, Greene D, Raskin P, Donofrio P, Cornblath D, Sachdeo R, Siu CO, Kamin M. Double‐blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology June 1998;50(6):1842‐6. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Comparability of patients at baseline and follow‐up (continuous data) All outcomes | Unclear risk | Due to attrition |
| Selection method random or consecutive | Unclear risk | Not reported |
| Prospective | Low risk | Prospective |
| Free from confounding treatment(s) | Low risk | Prohibited in RCT, unclear if this was enforced in continuation |
| Patient compliance monitored and reported at at least 85%? | Unclear risk | Not reported |
| Attrition and right censoring less than 15% (continuous data) All outcomes | High risk | Greater attrition |
| Funding from a source without financial conflict of interest? | High risk | Funded by Ortho‐McNeil Pharmaceuticals |
Hassenbusch 1995.
| Methods | Case series Duration: Up to 60 months, mean duration 28.8 months |
|
| Participants | N = 18 enrolled Primary condition: Neuropathic pain, including arachnoiditis (n = 9), chronic neuropathy (n = 3), traumatic intercostal neuralgia (n = 2), phantom pain (n = 1), lumbrosacral plexopathy (n = 1), spinal cord injury (n = 1), reflex sympathetic dystrophy (n = 1) Baseline pain score: Mean 8.5 (SD 0.92)/10 NRS Time since onset: Not reported Mean age: 46.6 years (range 40 to 77 years) Female: Percentage not reported Previous opioid analgesia: 15/18 took narcotics, including percocet (n = 6), Darvocet (n = 3), Dilaudid (n = 2), Vicodin (n = 2), morphine (n = 1), Tylox (n = 1) |
|
| Interventions | Intrathecal morphine Initial dose: Mean 0.49 (SD 0.24) mg/hour (n = 8) Titrated up mean 1.11 (SD 0.61) mg/hour (n = 7) Or, intrathecal sufentanil citrate Initial dose: Mean 0.67 (SD 0.22) ug/hour (n = 10) Titrated up to mean 2.39 (SD 0.95) ug/hour (n = 11) Supplemental analgesia: At last follow‐up 12/18 still used some supplemental opioid analgesia, and 11/18 used nonopioid medications |
|
| Outcomes | Discontinuation due to adverse events Adverse events Discontinuation due to insufficient pain relief Pain, continuous and proportion with at least 50% pain relief |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Comparability of patients at baseline and follow‐up (continuous data) All outcomes | Low risk | All patients followed up |
| Selection method random or consecutive | Low risk | Consecutive |
| Prospective | Low risk | Prospective |
| Free from confounding treatment(s) | High risk | Supplemental opioid and nonopioid analgesics allowed |
| Patient compliance monitored and reported at at least 85%? | Low risk | Pump administration |
| Attrition and right censoring less than 15% (continuous data) All outcomes | Low risk | All patients followed up |
| Funding from a source without financial conflict of interest? | Unclear risk | Not reported |
Klapetek 1971.
| Methods | Case series Duration: Mean 7.26 months; Range 1.5 to 17.7 months |
|
| Participants | N = 10 enrolled Primary condition: Trigeminal neuralgia Baseline pain score: Not reported, and not possible to calculate from qualitative scale used Time since onset: Mean 8 years; Range 1‐18 years Mean age: 48.9 years; Range 26 to 67 years Female: 70% Previous opioid analgesics: Not reported |
|
| Interventions | Oral valoron and nautrium‐diphenylhydantoin (n = 6), or valoron alone (n = 4) Initial dose: Not reported Titrated up to: Not reported. Total overall dose was 43.45 g; Range 6.53 to 125.73 grams. Mean daily dose was 196.1 g per patient. Supplemental analgesia: Six patients received additional natrium‐diphenylhydantoin |
|
| Outcomes | Discontinuation due to adverse events Adverse events Discontinuation due to insufficient pain relief |
|
| Notes | Pain outcomes not analyzed in this review because scale not standard | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Comparability of patients at baseline and follow‐up (continuous data) All outcomes | Low risk | (No continuous outcomes included in analysis) |
| Selection method random or consecutive | Unclear risk | Not reported |
| Prospective | Unclear risk | Not reported |
| Free from confounding treatment(s) | High risk | Additional treatment permitted |
| Patient compliance monitored and reported at at least 85%? | Unclear risk | Not reported |
| Attrition and right censoring less than 15% (continuous data) All outcomes | Low risk | (No continuous outcomes included in analysis) |
| Funding from a source without financial conflict of interest? | Unclear risk | Not reported |
Kumar 2001.
| Methods | Case series Duration: Mean 29.14 (SD 12.44) months; Range 13 to 49 months |
|
| Participants | N = 16 enrolled Primary condition: Various unspecified causes Baseline pain score: Mean 91.8 (SD 2.8) / 100 VAS Time since onset: 8 years (SD 4.2 years) Mean age: 42.1 (2.4) years, range 34 to 61 Female: 38% Previous opioid analgesics: All patients were on narcotic analgesics at enrolment |
|
| Interventions | Intrathecal morphine, with clonidine if needed (n=2) Initial dose: Mean 1.11 mg/day (SD 1.91 mg/day) Titrated to 7.42 mg/day (SD 4.2 mg/day) Supplemental analgesia: Six patients received no supplemental analgesics. The other 10 used antidepressants and/or analgesics. |
|
| Outcomes | Discontinuation from study due to adverse events Adverse events Discontinuation from study due to insufficient pain relief Pain |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Comparability of patients at baseline and follow‐up (continuous data) All outcomes | Low risk | All patients reported on |
| Selection method random or consecutive | Low risk | Appear to be consecutive referrals |
| Prospective | Low risk | Prospective |
| Free from confounding treatment(s) | High risk | Supplemental analgesia allowed |
| Patient compliance monitored and reported at at least 85%? | Low risk | Pump administration |
| Attrition and right censoring less than 15% (continuous data) All outcomes | Low risk | All patients followed up |
| Funding from a source without financial conflict of interest? | Unclear risk | Not reported |
McIlwain 2005.
| Methods | RCT with case series open‐label continuation Duration: 3‐week RCT plus 12 month open‐label continuation; total 12.7 months |
|
| Participants | N = 491 enrolled in RCT, N = 153 enrolled in open‐label continuation Primary condition: Osteoarthritis of hip or knee Baseline pain score: 52.1 (SD 23)/100 VAS Time since onset: Mean duration range among the randomized groups 9.1 to 10.3 years Mean age: 60 (SD 10) years Female: 62% Previous opioid analgesics: Not reported, but to meet inclusion criteria patients must have had previous unsuccessful treatment with an opioid, NSAID, or Cox‐2 inhibitor for at least 75 of 90 days prior to enrolment |
|
| Interventions | Oral extended‐release morphine Initial dose: 40 mg/day Titrated up to 80 mg/day. Doses divided. Supplemental analgesia: All analgesics were discontinued in washout phase prior to randomization for RCT. For open‐label continuation, NSAIDS (50.3% of patients), other analgesics and antipyretics (44.4%), antidepressants (34.0%), and antipsychotics (30.7%) were taken. |
|
| Outcomes | Discontinuation due to adverse events Adverse events Discontinuation due to insufficient pain relief |
|
| Notes | Continuation of: Matsumoto AK, Babul N, Ahdieh, H. Oxymorphone extended‐release tablets relieve moderate to severe pain and improve physical function in osteoarthritis: Results of a randomized, double‐blind, placebo‐and active‐controlled Phase III trial. Pain Medicine 2005;6(5):357‐66. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Comparability of patients at baseline and follow‐up (continuous data) All outcomes | High risk | Due to attrition |
| Selection method random or consecutive | Unclear risk | Not reported |
| Prospective | Low risk | Prospective |
| Free from confounding treatment(s) | High risk | Concominant drugs allowed for extension |
| Patient compliance monitored and reported at at least 85%? | Unclear risk | Not reported |
| Attrition and right censoring less than 15% (continuous data) All outcomes | High risk | Greater attrition |
| Funding from a source without financial conflict of interest? | High risk | Funded by Endo Pharmaceuticals and Penwest Pharmaceuticals |
Milligan 2001.
| Methods | Case series Duration: 12 months |
|
| Participants | N = 532 Primary condition: Unspecified. Pain affected nervous system (46.6%) or musculoskeletal/connective tissue (42.1%) and was most frequently in the lower back (44.5%) or lower limbs (21.6%) and due to degenerative/mechanical causes (40%) or trauma/operations/burns (25.4%) Baseline pain score: Not reported Time since onset: 8.8 years (SE 0.34 years) Mean age: 51.0 years (SE 0.56 years) Female: 51.7% Previous opioid analgesics: Patients must have had at least moderate pain relief with continuous treatment with a potent opioid |
|
| Interventions | Transdermal fentanyl Initial dose: Mean 48 (range 44 to 52) ug/hour Titrated to range 68 to 78 mg/hour Supplemental analgesics: Immediate‐release morphine 5mg every 4 hours as needed |
|
| Outcomes | Adverse events Discontinuation due to adverse events Quality of life |
|
| Notes | Pain also reported as an outcome but not included in this meta‐analysis because scale used not standard | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Comparability of patients at baseline and follow‐up (continuous data) All outcomes | Unclear risk | Due to attrition |
| Selection method random or consecutive | Unclear risk | Not reported |
| Prospective | Low risk | Prospective |
| Free from confounding treatment(s) | High risk | Rescue medication allowed |
| Patient compliance monitored and reported at at least 85%? | Unclear risk | Not reported |
| Attrition and right censoring less than 15% (continuous data) All outcomes | High risk | Greater attrition |
| Funding from a source without financial conflict of interest? | High risk | Funded by Janssen Research Foundation |
Mironer 2001.
| Methods | Case series Duration: 6 months | |
| Participants | N=24 enrolled
Primary condition: Failed back surgery syndrome Baseline pain score: Mean 8.5 (SD 1.1)/10 VAS Time since onset: NR Mean age: 51.4 years (Range 39 to 70 years) Female: 62.5% Previous opioid analgesics: All patients had previous intrathecal therapy with morphine, and also morphine with bupivacaine (n = 20), hydromorphone (n = 21), morphine plus clonidine (n = 4), meperidine (n = 4), SNX‐111 (n = 3), or other (n = 5) |
|
| Interventions | Intrathecal methadone Initial dose: Mean 9.2 mg/day (range 1.5 to 18 mg/day) Titrated to mean 16.8 mg/day (range 5 to 36 mg/day) at endpoint Supplemental analgesia: Not reported |
|
| Outcomes | Adverse events Pain, continuous and proportion with at least 50% relief Quality of Life | |
| Notes | Data on discontinuation not included in analysis because patients discontinued study opioid but continued treatment on another opioid, therefore not discontinuing from the study or opioids altogether | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Comparability of patients at baseline and follow‐up (continuous data) All outcomes | Low risk | All patients followed up |
| Selection method random or consecutive | Unclear risk | Not reported |
| Prospective | Low risk | Prospective |
| Free from confounding treatment(s) | Unclear risk | None reported |
| Patient compliance monitored and reported at at least 85%? | Low risk | Pump administration |
| Attrition and right censoring less than 15% (continuous data) All outcomes | Low risk | All patients followed up |
| Funding from a source without financial conflict of interest? | Unclear risk | Not reported, but since generic drugs were administered to patients who already had a pump it seems less likely |
Mystakidou 2003.
| Methods | Case series Duration: 48 months |
|
| Participants | N = 529 enrolled Primary condition: Neuropathic pain (41.2%), nociceptive (32.5%), or combined pain types (26.3). Most prevalent etiologies postherpetic neuralgia (17.2%) and low back pain (15.3%) Baseline pain score: Mean 7.3 (SD 1.04) / 10 VAS Time since onset: Not reported Mean age: 56.6 (SD 14.5) years, Range 21 to 88 years Female: 56.4% Previous opioid therapy: To meet inclusion criteria, patients must have had oral codeine with insufficient analgesia or oral morphine with insufficient analgesia or severe side effects |
|
| Interventions | Transdermal fentanyl Initial dose: Mean 25.8 (SD 0.58) ug/hour Titrated to 67.3 (SD 29.92) ug/hour Supplemental analgesics: Rescue morphine (5 mg) allowed, and NSAIDS prescribed as deemed necessary. Adjuvants included antidepressants, anticonvulsants, corticosteroids, and hydrocortisone. |
|
| Outcomes | Discontinuation due to adverse events Adverse events Discontinuation due to insufficient pain relief Pain, continuous Quality of life |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Comparability of patients at baseline and follow‐up (continuous data) All outcomes | Unclear risk | Due to attrition |
| Selection method random or consecutive | Unclear risk | Not reported |
| Prospective | Low risk | Prospective |
| Free from confounding treatment(s) | High risk | Adjuvants and rescue medication permitted |
| Patient compliance monitored and reported at at least 85%? | Unclear risk | Not reported |
| Attrition and right censoring less than 15% (continuous data) All outcomes | High risk | Greater attrition |
| Funding from a source without financial conflict of interest? | Unclear risk | Not reported, but no brand names mentioned |
Pascual 2007.
| Methods | Case series Duration: 12 months. Mean duration of treatment 5.4 months |
|
| Participants | N = 1067 enrolled and 1052 received at least one dose Primary condition: Not reported. Patient population included some patients with low back pain or osteoarthritis Baseline pain score: Mean 52.6 (SD 24.80)/100 VAS Time since onset: Not reported Mean age: 54.5 (SD 13.65) years Female: 62% Previous opioid analgesics: Patients taking other opioids were excluded. 7% of participants had previously been exposed to Tramadol ER. |
|
| Interventions | Oral Tramadol, extended‐release Initial dose: Day 1 100 mg once daily Titrated to: Day 4 200 mg once daily, week 2, 300 mg once daily. Maximum dose 300 mg/day for patients 75 and older and 400 mg/day for patients 75 and younger Supplemental analgesics: NSAIDS, Cox‐2 inhibitors, and acetaminophen were allowed. Patients taking MAOIs, tricyclic antidepressants (within 2 weeks of start date), other formulations of tramadol, neuroleptics, selective serotonin reuptake inhibitors, serotonin/norepinephrine reuptake inhibitors, carbamazepine, and quinidine were excluded. |
|
| Outcomes | Adverse events Discontinuation form study due to adverse events Discontinuation from study due to insufficient pain relief |
|
| Notes | Pain outcomes were also reported but not analyzed due to insufficient reporting (no measure of variance reported at follow‐up, insufficient data to enable imputation) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Comparability of patients at baseline and follow‐up (continuous data) All outcomes | Low risk | (No continuous outcomes included in analysis) |
| Selection method random or consecutive | Unclear risk | Not reported |
| Prospective | Low risk | Prospective |
| Free from confounding treatment(s) | High risk | Some additional analgesics allowed |
| Patient compliance monitored and reported at at least 85%? | Unclear risk | Not reported |
| Attrition and right censoring less than 15% (continuous data) All outcomes | Low risk | (No continuous outcomes included in analysis) |
| Funding from a source without financial conflict of interest? | High risk | Funding by Biovail Laboratories International |
Pimenta 1998.
| Methods | Case series Duration: Mean 19.6 months |
|
| Participants | N = 11 enrolled Primary condition: Neuropathic pain (n = 5), myofascial pain (n = 6) Baseline pain score: Mean 8.6 (Measure of variance no reported and not calculable from available data)/10 NRS Time since onset: Mean 5.3 years; Range 2 to 15 years Mean age: 50.8 years, range 32 to 72 years Female: Not reported Previous opioid analgesics: All patients were previously treated with opioids |
|
| Interventions | Intrathecal morphine clorhidrate (n = 10) or tramadol (n = 1) Initial dose: Morphine mean 2.00 mg, Range 1 to 4 mg per day Titrated to mean Morphine mean 1.55 mg, Range 0.5 to 2.0 mg Supplemental analgesics: Not reported |
|
| Outcomes | Discontinuation due to adverse events Adverse events Discontinuation due to insufficient pain relief |
|
| Notes | Pain outcomes also reported but not included in this analysis because measure of variance at baseline and follow‐up not reported and not calculable from available data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Comparability of patients at baseline and follow‐up (continuous data) All outcomes | Low risk | (Not included for any continuous outcomes) |
| Selection method random or consecutive | Low risk | Consecutive/all patients enrolled |
| Prospective | Low risk | Prospective |
| Free from confounding treatment(s) | Low risk | No confounding treatment permitted |
| Patient compliance monitored and reported at at least 85%? | Unclear risk | Not reported |
| Attrition and right censoring less than 15% (continuous data) All outcomes | Low risk | (Not included for any continuous outcomes) |
| Funding from a source without financial conflict of interest? | Unclear risk | Not reported |
Portenoy 2007.
| Methods | Open‐label prospective registry Duration: Up to 36 months. Mean duration of treatment 23.8 (SD 4.5) months (Some patients not able to participate for full three years due to right‐censoring caused by termination of study by sponsor) |
|
| Participants | N = 233 Primary conditions: Osteoarthritis (n = 87), neuropathic pain (n = 75), back pain (n = 71) Baseline pain score (of n = 219 considered intention‐to‐treat [ITT]): Mean 5.1 (SD 2.2) Time since onset: Not reported Mean age: 55.9 (13.2) years, ITT subpopulation Female: 57%, ITT subpopulation Previous opioid analgesics: 117 patients had previous controlled‐release oxycodone and required opioid analgesia and 60 received a short‐acting opioid |
|
| Interventions | Oral oxycodone tablets, controlled‐release Initial dose:Not reported Titrated to: Not reported for entire population. For ITT patients across all time points, mean 34.6 (SD 29.2) mg/day. Supplemental analgesia: Permitted at the discretion of the investigator. The most common concomitant opioids were hydrocodone/acetaminophen (10% of patients) and oxycodone/acetaminophen (6%). |
|
| Outcomes | Discontinuation due to adverse events Adverse events Discontinuation due to insufficient pain relief |
|
| Notes | For outcomes discontinuation due to adverse events and discontinuation due to insufficient pain relief, patients who were dismissed from the study when the sponsor terminated the study were not included in our analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Comparability of patients at baseline and follow‐up (continuous data) All outcomes | Low risk | (No continuous outcomes included in analysis) |
| Selection method random or consecutive | Unclear risk | Not reported |
| Prospective | Low risk | Prospective |
| Free from confounding treatment(s) | Unclear risk | None reported |
| Patient compliance monitored and reported at at least 85%? | Unclear risk | Not reported |
| Attrition and right censoring less than 15% (continuous data) All outcomes | Low risk | (No continuous outcomes included in analysis) |
| Funding from a source without financial conflict of interest? | High risk | Funding by Perdue Pharma, LP |
Rainov 2001.
| Methods | Case series Duration: Up to 38 months, Mean 42 months; Range 16 to 38 months | |
| Participants | N = 26 enrolled
Primary condition: Failed back surgery syndrome Baseline pain score: Mean 8.2 (SD 3.8)/10 VAS Time since onset: Mean 19 (SD 7) months Mean age: 54 years (Range 35 to 68 years) Female: 58% Previous Opioid Analgesics: All patients had opioids or antidepressants prior to study start |
|
| Interventions | Intrathecal morphine with bupivacaine (n = 20) and/or clonidine (n = 16) and/or midazolam (n = 10) Initial (test) dose: morphine 0.5 (SD 0.3) mg/day, midazolam 0.4 (SD 0.2) mg/day, clonidine 0.03 (SD 0.015) mg/day, bupivacaine 1.0 (0.4) mg/day Titrated to, morphine 5.2 (SD 0.3) mg/day, midazolam 0.08 (SD 0.4) mg/day, clonidine 0.06 (SD 0.03), bupivacaine 2.5 (1.5) mg/day at endpoint Supplemental Analgesics: Systemic opioids were weaned starting 4 days after pump implantation until discontinued on days 7 through 10. Andtidepressants were withdrawn 3 to 4 weeks after pump implantation. |
|
| Outcomes | Adverse events Pain, continuous | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Comparability of patients at baseline and follow‐up (continuous data) All outcomes | Low risk | All patients followed up |
| Selection method random or consecutive | Unclear risk | Not reported |
| Prospective | Low risk | Prospective |
| Free from confounding treatment(s) | Low risk | Supplemental analgesics, antidepressants, and neuroleptics withdrawn |
| Patient compliance monitored and reported at at least 85%? | Low risk | Pump administration |
| Attrition and right censoring less than 15% (continuous data) All outcomes | Low risk | All patients followed up |
| Funding from a source without financial conflict of interest? | High risk | Funded by Medtronic |
Rauck 2008.
| Methods | Open‐label case series Duration: 1 month titration phase plus 5 month maintenance phase; total 6 months |
|
| Participants | N=126 enrolled; 94 remained past first month titration phase for maintenance phase Primary condition: Osteoarthritis, with nearly as many people reporting back pain Baseline pain score: 6.2 (SD 1.3) / 0 to 10 NRS (10 being worst pain imaginable) Time since onset: Not reported, but all patients had pain for at least 3 months to meet inclusion criteria Mean age: 56.2 (SD 12.9) years Female: 56% Previous opioid analgesics: Could not have taken opioids during previous three months |
|
| Interventions | Oral controlled‐release oxymorphone Initial dose: 5 mg every 12 hours for 2 days, then titrated in 5 to 10‐mg increments every 3 to 7 days Titrated to 28 (SD 11; Range 5 to 100) mg/day Supplemental analgesics: During titration phase, no other opioids were allowed, and nonopioids could be decreased or discontinued but not increased. During maintenance phase, rescue medication was allowed. |
|
| Outcomes | Discontinuation due to adverse events Pain, continuous Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Comparability of patients at baseline and follow‐up (continuous data) All outcomes | Unclear risk | Due to attrition |
| Selection method random or consecutive | Unclear risk | Not reported |
| Prospective | Low risk | Prospective |
| Free from confounding treatment(s) | High risk | Rescue and supplemental analgesics allowed |
| Patient compliance monitored and reported at at least 85%? | Unclear risk | Data appears to have been collected but not reported |
| Attrition and right censoring less than 15% (continuous data) All outcomes | High risk | Greater attrition |
| Funding from a source without financial conflict of interest? | High risk | Funding by Endo Pharmacueticals |
Roth 2000.
| Methods | RCT followed by open‐label case series Duration: 14 day RCT plus 12 month case series; total 12.5 months | |
| Participants | N = 133 enrolled in RCT, 106 remained for long‐term follow up
Primary condition: Osteoarthritis Baseline pain score: Mean 2.5 (SD 1.2)/3 Likert scale (0 being no pain, 3 being severe pain) Time since onset: Mean 9 years Mean age: 62 years (Range 32 to 90) Female: 73.3% Previous opioid analgesics: Not reported |
|
| Interventions | Oral controlled‐release oxycodone Dose: 20 or 40 mg/day, divided Supplemental analgesics: NSAIDS were allowed to continue if dose was stable for 1 month prior to enrolment and not changed during the study. No other analgesics, including opioids or rescue analgesics, were allowed. |
|
| Outcomes | Discontinuation due to adverse events Discontinuation due to insufficient pain relief Pain, continuous/categorical | |
| Notes | Both RCT and open‐label continuation are reported on in this citation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Comparability of patients at baseline and follow‐up (continuous data) All outcomes | Unclear risk | Due to attrition |
| Selection method random or consecutive | Unclear risk | Not reported |
| Prospective | Low risk | Prospective |
| Free from confounding treatment(s) | High risk | Use of analgesics other than NSAIDS prohibited |
| Patient compliance monitored and reported at at least 85%? | Unclear risk | Not reported |
| Attrition and right censoring less than 15% (continuous data) All outcomes | High risk | Greater attrition |
| Funding from a source without financial conflict of interest? | High risk | Funding by Perdue Pharma LP |
Shaladi 2007.
| Methods | Case series Duration: 12 months |
|
| Participants | N = 24 enrolled Primary condition: Vertebral fractures due to osteoporosis Baseline pain score: 8.7 (SD 0.5)/10 VAS Time since onset: Not reported, but all patients failed conservative treatment for at least 3 months to meet inclusion criteria Mean age: 74.3 years (range 67 to 83 years) Female: 79% Previous opioid analgesics: All patients had taken systemic opioids and had severe side effects resistant to pharmacologic therapy |
|
| Interventions | Intrathecal morphine Initial dose: 1mg/day Titrated up to 25 mg/day, mean 16.32 mg/day Supplemental analgesics: No patients required oral or transdermal analgesics |
|
| Outcomes | Adverse Events Pain, proportion with at least 50% relief Pain, continuous Quality of life Function |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Comparability of patients at baseline and follow‐up (continuous data) All outcomes | Low risk | All patients followed up |
| Selection method random or consecutive | Unclear risk | Not reported |
| Prospective | Unclear risk | Not reported |
| Free from confounding treatment(s) | Low risk | No patients had additional analgesics |
| Patient compliance monitored and reported at at least 85%? | Low risk | Pump administration |
| Attrition and right censoring less than 15% (continuous data) All outcomes | Low risk | All patients followed up |
| Funding from a source without financial conflict of interest? | Unclear risk | Not reported |
Thimineur 2004.
| Methods | Case series Duration: 36 months |
|
| Participants | N = 38 enrolled Primary condition: Not reported. Likely back pain since Oswestry Disability Index (ODI) was administered. Baseline pain score: 8.4 (SD 1.4)/10 VAS Time since onset: 6.8 (SD 4) years Mean age: 46.1 (SD 12) years Female: Not reported Previous opioid analgesics: All patients had prior opioid exposure, but with dose‐limiting adverse events |
|
| Interventions | Initial doses not reported for any drug Intrathecal morphine (n = 9), Titrated to mean 10.8 mg/day Intrathecal Dilaudid (n = 21), Titrated to mean 13.5 mg/day Intrathecal fentanyl (n = 24), Titrated to mean 664 ug/day Intrathecal clonidine (n = 23), Titrated to mean 378 ug/day Intrathecal baclofen (n = 2), Titrated to mean 120 ug/day Intrathecal bupivacaine (n = 1), Titrated to 15.0 mg/day Intrathecal methadone (n = 1), Titrated to 10.0 mg/day Supplemental analgesics: All patients received other pain therapies, such as oral and transdermal medications, therapeutic injections, and physical therapy |
|
| Outcomes | Adverse Events Pain, continuous Quality of life Function |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Comparability of patients at baseline and follow‐up (continuous data) All outcomes | Low risk | Attrition rate relatively low |
| Selection method random or consecutive | Unclear risk | Unclear whether either all or consecutive patients were invited |
| Prospective | Low risk | Prospective |
| Free from confounding treatment(s) | High risk | All patients had additional pain therapies |
| Patient compliance monitored and reported at at least 85%? | Low risk | Pump administration |
| Attrition and right censoring less than 15% (continuous data) All outcomes | Low risk | Lower than 15% |
| Funding from a source without financial conflict of interest? | High risk | Funding by Medtronic |
Thorne 2008.
| Methods | RCT with open‐label case series continuation Duration: RCT 8 weeks, open‐label continuation 6 months; total 7.83 months |
|
| Participants | N = 100 enrolled in RCT, 53 continued on to open‐label extension Primary condition: Osteoarthritis of knee (82%), hip (9%) or both (9%) Baseline pain score: 50.9 (17.4)/100 VAS Time since onset: Mean 8.3 (SD 6.8) years Mean age: 61.0 (SD 10.3) years Female: 55% Previous Opioid Exposure: Patients had to be known to be opioid and tramadol‐tolerant prior to enrolment |
|
| Interventions | Oral tramadol Initial dose: Not reported Titrated to: 340.3 (SD 90.7) mg/day at end of 8‐week RCT, 313.2 (SD 100.1) mg/day for 29 patients who completed the study. Supplemental analgesics: Breakthrough pain was managed with acetaminophen. Patients taking MAOIs, carbamazepine, quinidine, selective serotonin reuptake inhibitors, tricyclic antidepressants, cyclobenzaprine, promethazine, neuroleptics, warfarin, or digoxin prior to the study were not enrolled. |
|
| Outcomes | Discontinuation due to adverse events Adverse events Discontinuation due to insufficient pain relief |
|
| Notes | Data for outcomes pain, quality of life, and function were collected and reported for the 8‐week RCT, but follow‐up data for the open‐label continuation were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Comparability of patients at baseline and follow‐up (continuous data) All outcomes | Low risk | (No continuous outcomes included) |
| Selection method random or consecutive | Unclear risk | Not reported |
| Prospective | Low risk | Prospective |
| Free from confounding treatment(s) | High risk | All analgesics besides acetaminophen prohibited |
| Patient compliance monitored and reported at at least 85%? | Unclear risk | Not reported |
| Attrition and right censoring less than 15% (continuous data) All outcomes | Low risk | (No continuous outcomes included) |
| Funding from a source without financial conflict of interest? | High risk | Funded by Perdue Pharma |
Zenz 1992.
| Methods | Time series Duration: Up to 48 months, mean 7.34 months | |
| Participants | N = 100 enrolled
Primary conditions: Neuropathic (n = 53) or low back pain (n = 24) Baseline Pain Score: 50.8 (SD 17.3)/100 VAS Time since onset: NR, but only 1 participant had pain for less than 1 year Mean age: 59.1 years (Range 29 to 81 years) Female: Not reported Previous opioid analgesics: Not reported |
|
| Interventions | Oral sustained‐release dihydrocodeine (n = 19), buprenorphine (n = 57), or sustained‐release morphine (n = 23). Mean dose buprenorphine 1.46 mg (range 0.4 to 3.2), morphine 255 mg/day (range 20 to 2,000), dihydrocodeine 170 (range 60 to 540) per day Supplemental analgesics: Not reported |
|
| Outcomes | Discontinuation due to adverse events Adverse events Discontinuation due to insufficient pain relief Pain, proportion with at least 50% relief |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Comparability of patients at baseline and follow‐up (continuous data) All outcomes | Unclear risk | (No continuous outcomes included in analysis) |
| Selection method random or consecutive | Unclear risk | Not reported |
| Prospective | Unclear risk | Not reported |
| Free from confounding treatment(s) | Unclear risk | None reported |
| Patient compliance monitored and reported at at least 85%? | Unclear risk | Not reported |
| Attrition and right censoring less than 15% (continuous data) All outcomes | Low risk | (No continuous outcomes included in analysis) |
| Funding from a source without financial conflict of interest? | Unclear risk | Not reported, but since all patients were prescribed generic drugs and drug changes were allowed, seems unlikely |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abs 2000 | Retrospective |
| Bouckoms 1992 | Retrospective |
| Chevlen 2000 | Substantial proportion of patients had cancer pain |
| Desai 1997 | Fewer than 10 patients remaining for long‐term evaluation |
| Dobscha 2009 | Not a study on opioids |
| Franco 2002 | Retrospective |
| Fredheim 2006b | No clinical outcomes reported |
| Gatti 2009 | Insufficient duration of treatment (< 6 months) |
| Griessinger 2005 | Substantial proportion of patients with cancer pain |
| Kalso 2007 | Secondary analysis only (no original data) |
| Kanoff 1994 | Retrospective |
| Krames 1993 | Retrospective |
| Likar 2001 | Retrospective |
| Likar 2006 | Substantial proportion of patients had cancer pain |
| Mariconti 2008 | Insufficient duration of treatment (< 6 months) |
| Milligan 1999 | Fewer than 10 participants enrolled |
| Mironer 1999 | Retrospective |
| Moulin 2005 | Retrospective |
| Pappagallo 1994 | Participants do not meet IASP definition of chronic pain (lasting at least 3 months) |
| Podichetty 2008 | Outcomes collected cross‐sectionally, pain reduction requires recall |
| Quang‐Cantagrel 2000 | Retrospective |
| Raphael 2004 | Retrospective |
| Rauck 2007 | Duration of treatment when outcomes were reported not long‐term (< 6 months) |
| Schulzek 1993 | Retrospective |
| Tutak 1996 | Retrospective |
| Valentino 1998 | Retrospective |
| Willis 1999 | Retrospective |
| Winkelmuller 1996 | Retrospective |
| Worz 1995 | Retrospective |
Differences between protocol and review
We have updated the methodology used in this review. First, we reduced the number of studies required to perform a meta‐regression from 10 to five. Second, we implemented a new approach to internal validity (quality) assessment by using a newer instrument updated to be more rigorous, and by not excluding studies if they met general inclusion criteria but scored poorly on the instrument. We used each of the responses as a potential covariate to perform meta‐regression in the presence of heterogeneity in sufficiently large evidence bases (those with at least five studies).
Contributions of authors
MN: conception and design, study selection, data extraction, quality assessment, statistical analysis and interpretation of data, drafting of manuscript, final approval, and responsibility for update. JRT: conception and design, critical revision of the manuscript for important intellectual content, statistical analyses and interpretation of data, and final approval. SJT: conception and design, critical revision of the manuscript for important intellectual content, statistical analyses and interpretation of data, and final approval. VHC: conception and design, securing funding for the review, final approval, and management. PJW: conception and design, and final approval. CA: study selection, data extraction, quality assessment, and final approval. KMS: conception and design, interpretation of data, critical revision of the manuscript for important intellectual content, supervision, and final approval.
Sources of support
Internal sources
ECRI Institute, USA.
External sources
The Mayday Fund, USA.
Declarations of interest
None known
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Allan 2005 {published data only}
- Allan L, Richarz U, Simpson K, Slappendel R. Transdermal fentanyl versus sustained release oral morphine in strong‐opioid naive patients with chronic low back pain. Spine 2005;30(22):2484‐90. [DOI] [PubMed] [Google Scholar]
Anderson 1999 {published data only}
- Anderson VC, Burchiel KJ. A prospective study of long‐term intrathecal morphine in the management of chronic nonmalignant pain. Neurosurgery 1999;44(2):289‐300. [DOI] [PubMed] [Google Scholar]
Anderson 2003 {published data only}
- Anderson VC, Burchiel KJ, Cooke B. A prospective, randomized trial of intrathecal injection vs. epidural infusion in the selection of patients for continuous intrathecal opioid therapy. Neuromodulation 2003;6(3):142‐52. [DOI] [PubMed] [Google Scholar]
Angel 1998 {published data only}
- Angel IF, Gould HJ Jr, Carey ME. Intrathecal morphine pump as a treatment option in chronic pain of nonmalignant origin. Surgical Neurology 1998;49(1):92‐8. [DOI] [PubMed] [Google Scholar]
Bettoni 2006 {published data only}
- Bettoni L. Transdermal fentanyl in rheumatology: two years' efficacy and safety in the treatment of chronic pain [Fentanyl transdermico in reumatologia: efficacia e sicurezza a due anni nel trattamento del dolore cronico]. Recenti Progressi in Medicina 2006;97(6):308‐10. [Google Scholar]
Caldwell 2002 {published data only}
- Caldwell JR, Rapoport RJ, Davis JC, Offenberg HL, Marker HW, Roth SH, et al. Efficacy and safety of a once‐daily morphine formulation in chronic, moderate‐to‐severe osteoarthritis pain: results from a randomized, placebo‐controlled, double‐blind trial and an open‐label extension trial. Journal of Pain and Symptom Management 2002;23(4):278‐91. [DOI] [PubMed] [Google Scholar]
Collado 2008 {published data only}
- Collado F, Torres LM. Association of transdermal fentanyl and oral transmucosal fentanyl citrate in the treatment of opioid naive patients with severe chronic noncancer pain. Journal of Opioid Management 2008;4(2):111‐5. [DOI] [PubMed] [Google Scholar]
Fredheim 2006 {published data only}
- Fredheim OM, Kaasa S, Dale O, Klepstad P, Landro NI, Borchgrevink PC. Opioid switching from oral slow release morphine to oral methadone may improve pain control in chronic non‐malignant pain: a nine‐month follow‐up study. Palliative Medicine 2006;20(1):35‐41. [DOI] [PubMed] [Google Scholar]
Harati 2000 {published data only}
- Harati Y, Gooch C, Swenson M, Edelman SV, Greene D, Raskin P, Donofrio P, Cornblath D, Olson WH, Kamin M. Maintenance of the long‐term effectiveness of tramadol in treatment of the pain of diabetic neuropathy. Journal of Diabetes Complications 2000;14(2):65‐70. [DOI] [PubMed] [Google Scholar]
Hassenbusch 1995 {published data only}
- Hassenbusch SJ, Stanton‐Hicks M, Covington EC, Walsh JG, Guthrey DS. Long‐term intraspinal infusions of opioids in the treatment of neuropathic pain. Journal of Pain and Symptom Management 1995;10(7):527‐43. [DOI] [PubMed] [Google Scholar]
Klapetek 1971 {published data only}
- Klapetek J. Long‐term treatment of trigeminal neuralgia using valoron [Langzeitanwendung von Valoron bei Trigenimusneuralgien]. Medizinische Welt 1971;39:1523‐6. [PubMed] [Google Scholar]
Kumar 2001 {published data only}
- Kumar K, Kelly M, Pirlot T. Continuous intrathecal morphine treatment for chronic pain of nonmalignant etiology: long‐term benefits and efficacy. Surgical Neurology 2001;55(2):86‐8. [DOI] [PubMed] [Google Scholar]
McIlwain 2005 {published data only}
- McIlwain H, Ahdieh H. Safety, tolerability, and effectiveness of oxymorphone extended release for moderate to severe osteoarthritis pain: a one‐year study. American Journal of Therapeutics 2005;12(2):106‐12. [DOI] [PubMed] [Google Scholar]
Milligan 2001 {published data only}
- Milligan K, Lanteri‐Minet M, Borchert K, Helmers H, Donald R, Kress HG, et al. Evaluation of long‐term efficacy and safety of transdermal fentanyl in the treatment of chronic noncancer pain. Journal of Pain 2001;2(4):197‐204. [DOI] [PubMed] [Google Scholar]
Mironer 2001 {published data only}
- Mironer YE, Tollison CD. Methadone in the intrathecal treatment of chronic nonmalignant pain resistant to other neuroaxial agents: the first experience. Neuromodulation 2001;4(1):25‐31. [DOI] [PubMed] [Google Scholar]
Mystakidou 2003 {published data only}
- Mystakidou K, Parpa E, Tsilika E, Mavromati A, Smyrniotis V, Georgaki S, Vlahos L. Long‐term management of noncancer pain with transdermal therapeutic system‐fentanyl. Journal of Pain 2003;4(6):298‐306. [DOI] [PubMed] [Google Scholar]
Pascual 2007 {published data only}
- Pascual MLG, Fleming RRB, Gana TJ, Vorsanger GJ. Open‐label study of the safety and effectiveness of long‐term therapy with extended‐release tramadol in the management of chronic nonmalignant pain. Current Medical Research and Opinion 2007;23(10):2531‐42. [DOI] [PubMed] [Google Scholar]
Pimenta 1998 {published data only}
- Pimenta CA, Teixeira MJ, Correa CF, Muller FD, Goes FC, Marcon RM. Intrathecal opioids in chronic non‐malignant pain: relief and life quality [Opiaceo intrathecal na dor cronica nao neoplastica]. Arquives de Neuro‐Psiquiatra 1998;56(3A):398‐405. [DOI] [PubMed] [Google Scholar]
Portenoy 2007 {published data only}
- Portenoy RK, Farrar JT, Backonja M, Cleeland CS, Yang K, Friedman M, Colucci SV, Richards P. Long‐term use of controlled‐release oxycodone for noncancer pain: results of a 3‐year registry study. Clinical Journal of Pain May 2007;23(4):287‐99. [DOI] [PubMed] [Google Scholar]
Rainov 2001 {published data only}
- Rainov NG, Heidecke V, Burkert W. Long‐term intrathecal infusion of drug combinations for chronic back and leg pain. Journal of Pain and Symptom Management 2001;22(4):862‐71. [DOI] [PubMed] [Google Scholar]
Rauck 2008 {published data only}
- Rauck R, Ma T, Kerwin R, Ahdieh H. Titration with oxymorphone extended release to achieve effective long term pain relief and improve tolerability in opioid‐naieve patients with moderate to severe pain. Pain Medicine 2008;9(7):777‐84. [DOI] [PubMed] [Google Scholar]
Roth 2000 {published data only}
- Roth SH, Fleischmann RM, Burch FX, Dietz F, Bockow B, Rapoport RJ, et al. Around‐the‐clock, controlled‐release oxycodone therapy for osteoarthritis‐related pain: placebo‐controlled trial and long‐term evaluation. Archives of Internal Medicine 2000;160(6):853‐60. [DOI] [PubMed] [Google Scholar]
Shaladi 2007 {published data only}
- Shaladi A, Saltari MR, Piva B, Crestani F, Tartari S, Pinato P, et al. Continuous intrathecal morphine infusion in patients with vertebral fractures due to osteoporosis. Clinical Journal of Pain 2007;23(6):511‐7. [DOI] [PubMed] [Google Scholar]
Thimineur 2004 {published data only}
- Thimineur MA, Kravitz E, Vodapally MS. Intrathecal opioid treatment for chronic non‐malignant pain: a 3‐year prospective study. Pain 2004;109(3):242‐9. [DOI] [PubMed] [Google Scholar]
Thorne 2008 {published data only}
- Thorne C, Beaulieu AD, Callaghan DJ, O'Mahony WF, Bartlett JM, Knight R, et al. A randomized, double‐blind, crossover comparison of the efficacy and safety of oral controlled‐release tramadol and placebo in patients with painful osteoarthritis. Pain Research & Management March/April 2008;13(2):93‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zenz 1992 {published data only}
- Zenz M, Strumpf M, Tryba M. Long‐term oral opioid therapy in patients with chronic nonmalignant pain. Journal of Pain and Symptom Management 1992;7(2):69‐77. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abs 2000 {published data only}
- Abs R, Verhelst J, Maeyaert J, Buyten JP, Opsomer F, Adriaensen H, et al. Endocrine consequences of long‐term intrathecal administration of opioids. Journal of Clinical Endocrinology & Metabolism 2000;85(6):2215‐22. [DOI] [PubMed] [Google Scholar]
Bouckoms 1992 {published data only}
- Bouckoms AJ, Masand P, Murray GB, Cassem EH, Stern TA, Tesar GE. Chronic nonmalignant pain treated with long‐term oral narcotic analgesics. Annals of Clinical Psychiatry 1992;4(3):185‐92. [Google Scholar]
Chevlen 2000 {published data only}
- Chevlen E. Morphine with dextromethorphan: conversion from other opioid analgesics. Journal of Pain and Symptom Management 2000;19(1‐Supplement):S42‐9. [DOI] [PubMed] [Google Scholar]
Desai 1997 {published data only}
- Desai PM. Pain relief in chronic pancreatitis with epidural buprenorphine injection. Indian Journal of Gastroenterology 1997;16(1):12‐3. [PubMed] [Google Scholar]
Dobscha 2009 {published data only}
- Dobscha SK, Corson K, Perrin NA, Hanson GC, Leibowitz RQ, Doak MN, Dickinson KC, Sullivan MD, Gerrity MS. Collaborative Care for Chronic Pain in Primary Care. JAMA March 25 2009;301(12):1242‐52. [DOI] [PubMed] [Google Scholar]
Franco 2002 {published data only}
- Franco Gay ML. Spinal morphine in nonmalignant chronic pain: a retrospective study in 39 patients. Neuromodulation 2002;5(3):150‐9. [DOI] [PubMed] [Google Scholar]
Fredheim 2006b {published data only}
- Fredheim OM, Borchgrevink PC, Hegrenaes L, Kaasa S, Dale O, Klepstad P. Opioid Switching from Morphine to Methadone Causes a Minor But Not Clinically Significant Increase in QTc Time: A Prospective 9‐Month Follow‐Up Study. Journal of Pain and Symptom Management 2006;32(2):180‐5. [DOI] [PubMed] [Google Scholar]
Gatti 2009 {published data only}
- Gatti A, Sabato AF, Occhioni R, Baldeschi GC, Reale C. Controlled‐release oxycodone and pregabalin in the treatment of neuropathic pain: Results of a Multicenter Italian Study. European Neurology 2009;61:129‐37. [DOI] [PubMed] [Google Scholar]
Griessinger 2005 {published data only}
- Griessinger N, Sittl R, Likar R. Transdermal buprenorphine in clinical practice‐‐a post‐marketing surveillance study in 13,179 patients. Current Medical Research & Opinion 2005;21(5):1147‐56. [DOI] [PubMed] [Google Scholar]
Kalso 2007 {published data only}
- Kalso E, Simpson KH, Slappendel R, Dejonckheere J, Richarz U. Predicting long‐term response to strong opioids in patients with low back pain: findings from a randomized, controlled trial of transdermal fentanyl and morphine. Biomed Central Medicine December 2007;5(39):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanoff 1994 {published data only}
- Kanoff RB. Intraspinal delivery of opiates by an implantable, programmable pump in patients with chronic, intractable pain of nonmalignant origin. Journal of the American Osteopathic Association 1994;94(6):487‐93. [PubMed] [Google Scholar]
Krames 1993 {published data only}
- Krames ES, Lanning RM. Intrathecal infusional analgesia for nonmalignant pain: analgesic efficacy of intrathecal opioid with or without bupivacaine. Journal of Pain and Symptom Management 1993;8(8):539‐48. [DOI] [PubMed] [Google Scholar]
Likar 2001 {published data only}
- Likar R, Koppert W, Blatnig H, Chiari F, Sittl R, Stein C, Schafer M. Efficacy of peripheral morphine analgesia in inflamed, non‐inflamed and perineural tissue of dental surgery patients. Journal of Pain and Symptom Management 2001;21(4):330‐7. [DOI] [PubMed] [Google Scholar]
Likar 2006 {published data only}
- Likar R, Kayser H, Sittl R. Long‐term management of chronic pain with transdermal buprenorphine: a multicenter, open‐label, follow‐up study in patients from three short‐term clinical trials. Clinical Therapeutics 2006;28(6):943‐52. [DOI] [PubMed] [Google Scholar]
Mariconti 2008 {published data only}
- Mariconti, M, Collini, R. Tramadol SR in arthrosic and neuropathic pain. Minerva Anesthesiologica 2008;74(3):63‐8. [PubMed] [Google Scholar]
Milligan 1999 {published data only}
- Milligan KA, Campbell C. Transdermal fentanyl in patients with chronic, nonmalignant pain: a case study series. Advances in Therapy 1999;16(2):73‐7. [PubMed] [Google Scholar]
Mironer 1999 {published data only}
- Mironer YE, Haasis JC III, Chapple IT, Somerville JJ, Barsanti JM. Successful use of methadone in neuropathic pain: a multicenter study by the National Forum of Independent Pain Clinicians. Pain Digest 1999;9(3):191‐3. [Google Scholar]
Moulin 2005 {published data only}
- Moulin DE, Palma D, Watling C, Schulz V. Methadone in the management of intractable neuropathic noncancer pain. Canadian Journal of Neurological Science 2005;32(3):340‐3. [DOI] [PubMed] [Google Scholar]
Pappagallo 1994 {published data only}
- Pappagallo M, Campbell JN. Chronic opioid therapy as alternative treatment for post‐herpetic neuralgia. Annals of Neurology 1994;35 Supplement:S54‐6. [DOI] [PubMed] [Google Scholar]
Podichetty 2008 {published data only}
- Podichetty VK, Varley ES, Secic M. Role of patient‐based health status outcome measurements in opioid management for low back pain. Journal of Opioid Management May/June 2008;4(3):153‐62. [DOI] [PubMed] [Google Scholar]
Quang‐Cantagrel 2000 {published data only}
- Quang‐Cantagrel ND, Wallace MS, Magnuson SK. Opioid substitution to improve the effectiveness of chronic noncancer pain control: a chart review. Anesthesia & Analgesia 2000;90(4):933‐7. [DOI] [PubMed] [Google Scholar]
Raphael 2004 {published data only}
- Raphael JH, Southall JL, Gnanadurai TV, Treharne GJ, Kitas GD. Multiple lead spinal cord stimulation for chronic mechanical low back pain: a comparative study with intrathecal opioid drug delivery. Neuromodulation 2004;7(4):260‐6. [DOI] [PubMed] [Google Scholar]
Rauck 2007 {published data only}
- Rauck, RL, Bookbinder, SA, Alftine, CD, Gershon, S, de Jong, E, Negro‐Vilar, A, Ghalie, R. A randomized, open‐label multicenter trial comparing once‐a‐day AVINZA (morphine sulfate extended‐release capsules) versus twice‐a‐day OxyContin (oxycodone hydrochloride controlled‐release tablets) for the treatment, of chronic, moderate to severe low back pain: Improved physical functioning in the ACTION trial. Journal of Opioid Management January/February 2007;3(1):35‐43. [DOI] [PubMed] [Google Scholar]
Schulzek 1993 {published data only}
- Schulzeck S, Gleim M, Maier C. Morphine tablets for chronic non‐tumor‐induced pain. Which factors modify the success or failure of a long‐term therapy?. Anaesthesist 1993;42(8):454‐6. [PubMed] [Google Scholar]
Tutak 1996 {published data only}
- Tutak U, Doleys DM. Intrathecal infusion systems for treatment of chronic low back and leg pain of noncancer origin. Southern Medical Journal 1996;89(3):295‐300. [DOI] [PubMed] [Google Scholar]
Valentino 1998 {published data only}
- Valentino L, Pillay KV, Walker J. Managing chronic nonmalignant pain with continuous intrathecal morphine. Journal of Neuroscience Nursing 1998;30(4):233‐9, 243‐4. [DOI] [PubMed] [Google Scholar]
Willis 1999 {published data only}
- Willis KD, Doleys DM. The effects of long‐term intraspinal infusion therapy with noncancer pain patients: evaluation of patient, significant‐other, and clinic staff appraisal. Neuromodulation 1999;2(4):241‐53. [DOI] [PubMed] [Google Scholar]
Winkelmuller 1996 {published data only}
- Winkelmuller M, Winkelmuller W. Long‐term effects of continuous intrathecal opioid treatment in chronic pain of nonmalignant etiology. Neurosurgery 1996;85(3):458‐67. [DOI] [PubMed] [Google Scholar]
Worz 1995 {published data only}
- Worz R, Worz E. Long‐term treatment with tilidine‐naloxon. An analysis of 50 patients with chronic pain conditions of non‐malignant origin [Langzeitbehandlung chronischer Schmerzen mit Tilidin‐Naloxon]. Fortschritte der Medizin 1995;113(27):388‐92. [PubMed] [Google Scholar]
Additional references
AHRQ 2002
- Systems to rate the strength of scientific evidence: summary. Agency for Healthcare Research and Quality (AHRQ) 2002; Vol. 47, accessed on 1st January 2007 from http://www.ahcpr.gov/clinic/tp/strengthtp.htm#Report.
Ballantyne 2003
Ballantyne 2006
- Ballantyne JC. Opioids for chronic pain: taking stock. Pain 2006;125(1‐2):3‐4. [DOI] [PubMed] [Google Scholar]
Blyth 2001
Breivik 2006
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. European Journal of Pain 2006;10(4):287‐333. [DOI] [PubMed] [Google Scholar]
Cicero 2009
- Cicero TJ, Wong G, Tian Y, Lynskey M, Todorov A, Isenberg K. Co‐morbidity and utilization of medical services by pain patients receiving opioid medications: data from an insurance claims database. Pain Jul 2009;144(1‐2):20‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clark 2002
Cohen 1988
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd Edition. Hillsdale (NJ): Lawrence Eribaum Associates, 1988. [Google Scholar]
Cooper 1994
- Cooper H, Hedges LV (eds). The handbook of research synthesis. New York: Russell Sage Foundation, 1994. [Google Scholar]
Dalziel 2005
- Dalziel K, Round A, Stein K, Garside R, Castelnuovo E, Payne L. Do the findings of case series studies vary significantly according to methodological characteristics?. Health Technology Assessment National Health Service Research and Development Programme 2005;9(2):1‐6. [DOI] [PubMed] [Google Scholar]
Deeks 2003
- Deeks JJ, Dinnes J, D'Amico R, Sowden AJ, Sakarovitch C, Song F, Petticrew M, Altman DG. Evaluating non‐randomised intervention studies. Health Technology Assessment 2003;7(27):1e186. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. English Title:. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Duval 2000a
- Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000;56(2):455‐63. [DOI] [PubMed] [Google Scholar]
Duval 2000b
- Duval SJ, Tweedie RL. A non‐parametric 'trim and fill' method of assessing publication bias in meta‐analysis. Journal of the American Statistical Association 2000;95(449):89‐98. [Google Scholar]
ECRI 2008
- ECRI Institute. Implanted infusion pumps for chronic noncancer pain. Prepared for the Washington State Health Care Authority (http://www.hta.hca.wa.gov) 2008 Jul 18.
Egger 2003
- Egger M, Juni P, Bartless C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technology Assessment 2003; Vol. 7, issue 1. [PubMed]
Eich 1985
- Eich E, Reeves JL, Jager B, Graff‐Radford SB. Memory for pain: relation between past and present pain intensity. Pain 1985;23(4):375‐80. [DOI] [PubMed] [Google Scholar]
Eisenberg 2005
Elliott 1999
Eriksen 2006
- Eriksen J, Sjogren P, Bruera E, Ekholm O, Rasmussen NK. Critical issues on opioids in chronic non‐cancer pain: an epidemiological study. Pain Nov 2006;125(1‐2):172‐9. [DOI] [PubMed] [Google Scholar]
Farrar 2000
- Farrar JT. What is clinically meaningful: outcome measures in pain clinical trials. Clinical Journal of Pain 2000;16(2 Supplement):S106‐12. [DOI] [PubMed] [Google Scholar]
Fishbain 1991
- Fishbain DA, Goldberg M, Rosomoff RS, Rosomoff H. Completed suicide in chronic pain. The Clinical Journal of Pain 1991;7:29‐36. [DOI] [PubMed] [Google Scholar]
Fishbain 2008
- Fishbain DA, Cole B, Lewis J, Rosomoff HL, Rosomoff RS. What percentage of chronic nonmalignant pain pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug‐related behaviours? A structured evidence‐based review. Pain Medicine 2008;9(4):444‐459. [DOI] [PubMed] [Google Scholar]
Furlan 2006
- Furlan AD, Sandoval JA, Mailis‐Gagnon A, Tunks E. Opioids for chronic noncancer pain: a meta‐analysis of effectiveness and side effects. Canadian Medical Association Journal 2006;174(11):1589‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Geborek 2002
- Geborek P, Crnkic M, Petersson IF, Saxne T. South Swedish Arthritis Treatment Group. Etanercept, infliximab, and leflunomide in established rheumatoid arthritis: clinical experience using a structured follow up programme in southern Sweden. Annuals of Rheumatic Disease 2002;61(9):793‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gureje 1998
- Gureje O, Korff M, Simon GE, Gater R. Persistent pain and well‐being: a World Heath Organization study in primary care. JAMA 1998;280(2):287‐51. [DOI] [PubMed] [Google Scholar]
Hagg 2003
- Hagg O, Fritzell P, Nordwall A. The clinical importance of changes in outcome scores after treatment for chronic low back pain. European Spine Journal 2003 Feb;12(1):12‐20. [DOI] [PubMed] [Google Scholar]
Harbord 2004
- Harbord R. METAREG: Stata module to perform meta‐analysis regression. EconPapers 2004 Sep 22.
Harstall 2003
- Harstall C, Ospina M. How prevalent is chronic pain?. Pain Clinical Update 2003; Vol. 11, issue 2:1‐4.
Hermos 2004
- Hermos JA, Young MM, Gagnon DR, Fiore LD. Characterizations of long‐term oxycodone/acetaminophen prescriptions in Veteran patients. Archives of Internal Medicine 2004;164(21):2361‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2004
- Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta‐regression. Statistics in Medicine 2004;23(11):1663‐82. [DOI] [PubMed] [Google Scholar]
Hrobjartsson 2004a
- Hrobjartsson A, Gotzsche PC. Placebo interventions for all clinical conditions. The Cochrane Database of Systematic Reviews [internet] 2004;2:808 pages. [DOI] [PubMed] [Google Scholar]
Hrobjartsson 2004b
- Hrobjartsson A, Gotzsche PC. Is the placebo powerless? Update of a systematic review with 52 new randomized trials comparing placebo with no treatment. Journal of Internal Medicine 2004;256(2):91‐100. [DOI] [PubMed] [Google Scholar]
IASP 1986
- International Association for the Study of Pain, Subcommittee on Taxonomy. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Pain 1986; Vol. Suppl 3:S1‐S225. [PubMed]
Ioannidis 1999
- Ioannidis JP, Contopoulos‐Ioannidis, Lau, J. Recursive cumulative meta‐analysis: a diagnostic for the evolution of total randomized evidence from group and individual patient data. Journal of Clinical Epidemiology 1999;52(4):281‐91. [DOI] [PubMed] [Google Scholar]
Kikuchi 2008
- Kikuchi N, Ohmori‐Matsuda K, Shimazu T, Sone T, Kakizaki M, Nakaya N, Kuriyama S, Tsuji I. Pain and risk of completed suicide in Japanese men: A population‐based cohort study in Japan (Ohsaki Cohort Study). Journal of Pain and Symptom Management 2008;37(3):316‐24. [DOI] [PubMed] [Google Scholar]
Lau 1995
- Lau J, Schmid CH, Chalmers TC. Cumulative meta‐analysis of clinical trials builds evidence for exemplary medical care. Journal of Clinical Epidemiology 1995;48(1):59‐60. [DOI] [PubMed] [Google Scholar]
Linton 1983
- Linton SJ, Gotestam KG. A clinical comparison of two pain scales: correlation, remembering chronic pain, and a measure of compliance. Pain 1983;17(1):57‐65. [DOI] [PubMed] [Google Scholar]
MacDonald 2003
- MacDonald TM, Morant SV, Goldstein JL, Burke TA, Pettitt D. Channelling bias and the incidence of gastrointestinal haemorrhage in users of meloxicam, coxibs, and older, non‐specific non‐steroidal anti‐inflammatory drugs. Gut 2003;52(9):1265‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahowald 2005
- Mahowald ML, Singh JA, Majeski P. Opioid use by patients in an orthopedics spine clinic. Arthritis & Rheumatism 2005;52(1):312‐21. [DOI] [PubMed] [Google Scholar]
Mamdani 2002
- Mamdani M, Rochon PA, Juurlink DN, Kopp A, Anderson GM, Naglie G, Austin PC, Laupacis A. Observational study of upper gastrointestinal haemorrhage in elderly patients given selective cyclo‐oxygenase‐2 inhibitors or conventional non‐steroidal anti‐inflammatory drugs. BMJ 2002;325(7365):624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olkin 1999
- Olkin, I. Diagnostic statistical procedures in medical meta‐analysis. Statistics in Medicine 1999;18(17‐18):2331‐41. [DOI] [PubMed] [Google Scholar]
Ratcliffe 2008
- Ratcliffe GE, Enns MW, Belik S, Sareen J. Chronic pain conditions and suicidal ideation and suicide attempts: An epidemiologic perspective. Clinical Journal of Pain 2008;24(3):204‐10. [DOI] [PubMed] [Google Scholar]
Reid 2002
- Reid MC, Engles‐Horton LL, Weber MB, Kerns RD, Rogers EL, O'Connor PG. Use of opioid medications for chronic noncancer pain syndromes in primary care. Journal of General Internal Medicine 2002;17(3):173‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salaffi 2004
- Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. European Journal of Pain 2004 Aug;8(4):283‐91. [DOI] [PubMed] [Google Scholar]
Tang 2006
- Tang NKY, Crane C. Suicidality in chronic pain: a review of the prevalence, risk factors, and psychological links. Psychological Medicine 2006;36:575‐86. [DOI] [PubMed] [Google Scholar]
Thiessen Philbrook 2007
- Thiessen Philbrook H, Barrowman N, Garg AX. Imputing variance estimates do not alter the conclusions of a meta‐analysis with continuous outcomes: a case study of changes in renal function after living kidney donation. Journal of Clinical Epidemiology 2007 Mar;60(3):228‐40. [DOI] [PubMed] [Google Scholar]
Treadwell 2006
- Treadwell, JT, Tregear SJ, Reston JT, Turkelson CM. A system for rating the stability and strength of medical evidence. BMC Medical Research Methodology 2006;6:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tunis 2003
- Tunis SR, Stryer DB, Clancy CMTunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA 2003;290(12):1624‐32. [DOI] [PubMed] [Google Scholar]
Williams 2008
- Williams RE, Sampson TJ, Kalilani L, Wurzelmann JI, Janning SW. Epidemiology of opioid pharmacy claims in the United States. Journal of Opioid Management May‐Jun 2008;4(3):145‐32. [DOI] [PubMed] [Google Scholar]
Ytterberg 1998
- Ytterberg SR, Mahowald ML, Woods SR. Codeine and oxycodone use in patients with chronic rheumatic disease pain. Arthritis & Rheumatism 1998;41(9):1603‐12. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
ECRI 2006
- Chapell R, Coates V, Noble M, Schoelles K, Treadwell J, Turkelson C. Evaluation of Efficacy/Effectiveness. Opioids for Chronic Noncancer Pain. Plymouth Meeting, PA: ECRI, 2006. [Google Scholar]
Noble 2008
- Noble M, Tregear SJ, Treadwell JR, Schoelles K. Long‐term opioid therapy for chronic noncancer pain: a systematic review and meta‐analysis of efficacy and safety. Journal of Pain and Symptom Management 2008 Feb;35(2):214‐28. [DOI] [PubMed] [Google Scholar]


