Abstract
Background
Factor Xa inhibitors and vitamin K antagonists (VKAs) are now recommended in treatment guidelines for preventing stroke and systemic embolic events in people with atrial fibrillation (AF). This is an update of a Cochrane review previously published in 2013.
Objectives
To assess the effectiveness and safety of treatment with factor Xa inhibitors versus VKAs for preventing cerebral or systemic embolic events in people with AF.
Search methods
We searched the trials registers of the Cochrane Stroke Group and the Cochrane Heart Group (September 2016), the Cochrane Central Register of Controlled Trials (CENTRAL) (August 2017), MEDLINE (1950 to April 2017), and Embase (1980 to April 2017). We also contacted pharmaceutical companies, authors and sponsors of relevant published trials. We used outcome data from marketing authorisation applications of apixaban, edoxaban and rivaroxaban that were submitted to regulatory authorities in Europe and the USA.
Selection criteria
We included randomised controlled trials (RCTs) that directly compared the effects of long‐term treatment (lasting more than four weeks) with factor Xa inhibitors versus VKAs for preventing cerebral and systemic embolism in people with AF.
Data collection and analysis
The primary efficacy outcome was the composite endpoint of all strokes and systemic embolic events. Two review authors independently extracted data, and assessed the quality of the trials and the risk of bias. We calculated a weighted estimate of the typical treatment effect across trials using the odds ratio (OR) with 95% confidence interval (CI) by means of a fixed‐effect model. In case of moderate or high heterogeneity of treatment effects, we used a random‐effects model to compare the overall treatment effects. We also performed a pre‐specified sensitivity analysis excluding any open‐label studies.
Main results
We included data from 67,688 participants randomised into 13 RCTs. The included trials directly compared dose‐adjusted warfarin with either apixaban, betrixaban, darexaban, edoxaban, idraparinux, idrabiotaparinux, or rivaroxaban. The majority of the included data (approximately 90%) was from apixaban, edoxaban, and rivaroxaban.
The composite primary efficacy endpoint of all strokes (both ischaemic and haemorrhagic) and non‐central nervous systemic embolic events was reported in all of the included studies. Treatment with a factor Xa inhibitor significantly decreased the number of strokes and systemic embolic events compared with dose‐adjusted warfarin in participants with AF (OR 0.89, 95% CI 0.82 to 0.97; 13 studies; 67,477 participants; high‐quality evidence).
Treatment with a factor Xa inhibitor significantly reduced the number of major bleedings compared with warfarin (OR 0.78, 95% CI 0.73 to 0.84; 13 studies; 67,396 participants; moderate‐quality evidence). There was, however, statistically significant and high heterogeneity (I2 = 83%). When we repeated this analysis using a random‐effects model, it did not show a statistically significant decrease in the number of major bleedings (OR 0.88, 95% CI 0.66 to 1.17). A pre‐specified sensitivity analysis excluding all open‐label studies showed that treatment with a factor Xa inhibitor significantly reduced the number of major bleedings compared with warfarin (OR 0.75, 95% CI 0.69 to 0.81), but high heterogeneity was also observed in this analysis (I2 = 72%). The same sensitivity analysis using a random‐effects model also showed a statistically significant decrease in the number of major bleedings in participants treated with factor Xa inhibitors (OR 0.76, 95% CI 0.60 to 0.96).
Treatment with a factor Xa inhibitor significantly reduced the risk of intracranial haemorrhages (ICHs) compared with warfarin (OR 0.50, 95% CI 0.42 to 0.59; 12 studies; 66,259 participants; high‐quality evidence). We observed moderate, but statistically significant heterogeneity (I2 = 55%). The pre‐specified sensitivity analysis excluding open‐label studies showed that treatment with a factor Xa inhibitor significantly reduced the number of ICHs compared with warfarin (OR 0.47, 95% CI 0.40 to 0.56), with low, non‐statistically significant heterogeneity (I2 = 27%).
Treatment with a factor Xa inhibitor also significantly reduced the number of all‐cause deaths compared with warfarin (OR 0.89, 95% 0.83 to 0.95; 10 studies; 65,624 participants; moderate‐quality evidence).
Authors' conclusions
Treatment with factor Xa inhibitors significantly reduced the number of strokes and systemic embolic events compared with warfarin in people with AF. The absolute effect of factor Xa inhibitors compared with warfarin treatment was, however, rather small. Factor Xa inhibitors also reduced the number of ICHs, all‐cause deaths and major bleedings compared with warfarin, although the evidence for a reduction in the latter is less robust.
Plain language summary
Comparing two types of blood‐thinning drugs, factor Xa inhibitors and vitamin K antagonists, to prevent blood clots in people with atrial fibrillation
Review question
We compared the benefits and harms of two types of so‐called "blood‐thinning" drugs (factor Xa inhibitors and vitamin K antagonists) in people with atrial fibrillation.
Background
People with atrial fibrillation, a condition that causes the heart to beat irregularly, are at an increased risk of getting blood clots. Such clots can block blood vessels and cause severe organ damage in the brain (stroke) or other organs. Various guidelines recommend that people with atrial fibrillation should be treated with "blood‐thinning" drugs such as factor Xa inhibitors or vitamin K antagonists (e.g. warfarin) because these drugs can prevent the formation of blood clots. Serious side effects of these drugs are bleedings (e.g. into the brain) that can cause serious disability or even death.
Study characteristics
We searched various sources up to 29 August 2017 and included 13 studies that involved 67,688 people with atrial fibrillation who received either a factor Xa inhibitor or a vitamin K antagonist. All included people were adults and on average aged between 65 and 74 years. Approximately one‐third were women.
Key results
We found that factor Xa inhibitors when compared with warfarin, which was used as comparator in all trials, reduced the number of strokes in people with atrial fibrillation. This reduction was, however, rather small. Factor Xa inhibitors also appeared to reduce the number of serious bleedings (including those into the brain) and the number of people dying from any cause compared with warfarin.
Quality of evidence
We considered the quality of evidence in our review as moderate to high. The studies that we included were generally large to very large. We found that the results from the larger studies were generally similar and this strengthened our findings. Finally, we are confident that we included all relevant studies in our review and did not miss any important studies.
Summary of findings
for the main comparison.
| Factor Xa inhibitors compared with vitamin K antagonists for preventing stroke and other systemic embolic events in patient with atrial fibrillation | ||||||
|
Patient or population: people with atrial fibrillation deemed eligible for long‐term anticoagulant treatment Settings: hospital‐based setting Intervention: factor Xa inhibitor1 Comparison: dose‐adjusted vitamin K antagonist2 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Warfarin | Factor Xa inhibitors | |||||
|
Stroke and other systemic embolic events Follow‐up: 12 weeks to 2.8 years |
34 per 1000 |
32 per 1000 (33 to 28) |
OR 0.89 (0.82 to 0.97) |
67477 (13) | ⊕⊕⊕⊕ high | Most data (90%) from studies of apixaban, edoxaban and rivaroxaban |
|
All strokes Follow‐up: 12 weeks to 2.8 years |
30 per 1000 |
28 per 1000 (29 to 24) |
OR 0.89 (0.81 to 0.97) |
67449 (13) | ⊕⊕⊕⊕ high | Most data (90%) from studies of apixaban, edoxaban rivaroxaban |
|
Major bleedings Follow‐up: 12 weeks to 2.8 years |
51 per 1000 |
41 per 1000 (43 to 38) |
OR 0.78 (0.73 to 0.84) |
67396 (13) | ⊕⊕⊕⊝ moderate3 | Most data (90%) from studies of apixaban, edoxaban and rivaroxaban |
|
Intracranial haemorrhages Follow‐up: 12 weeks to 2.8 years |
13 per 1000 |
7 per 1000 (8 to 6) |
OR 0.50 (0.42 to 0.59) |
66259 (12) | ⊕⊕⊕⊕ high4 | Most data (90%) from studies of apixaban, edoxaban and rivaroxaban |
|
All‐cause deaths Follow‐up: 12 weeks to 2.8 years |
67 per 1000 |
66 per 1000 (67 to 57) |
OR 0.89 (0.83 to 0.95) |
65624 (10) | ⊕⊕⊕⊝ moderate5 | Most data (90%) from studies of apixaban, edoxaban and rivaroxaban |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The 13 studies included in this review studied the following types of oral and parenteral factor Xa inhibitors: edoxaban, rivaroxaban, apixaban, edoxaban, betrixaban, darexaban, idraparinux and idrabiotaparinux. Assumed risk calculated from pooled data from warfarin treatment arms (mean) of included studies.
2 All included studies used warfarin with a target INR 2.0‐3.0 as active comparator. Three studies performed in Japan had a target INR of 1.6‐2.6 and 2.0‐2.6 in participants aged > 70 years.
3 High, statistically significant heterogeneity was observed in the initial analysis and in pre‐specified sensitivity analysis excluding all open‐label studies (i.e. the prematurely halted AMADEUS trial). Some other heterogeneity might be explained by baseline differences in the included populations in the three largest trials (ROCKET AF, ENGAGE TIMI‐AF and ARISTOTLE). See section Effects of Interventions, Major bleedings for further discussion.
4 High, statistically significant heterogeneity was observed in the initial analysis. No statistically significant heterogeneity was observed in a pre‐specified sensitivity analysis in which data from all open‐label studies were excluded (i.e. prematurely halted AMADEUS trial).
5 Outcome not reported in three trials and was missing/unknown for some randomised participants (up to 2.1% ) in some of the other included trials.
Background
Description of the condition
Atrial fibrillation (AF) is the most common type of arrhythmia in adults and becomes more common with increased age (Go 2001). The prevalence of AF is estimated at around 2% to 3% of the adult population (Kirchhof 2007; Haim 2015). The lifetime risk for developing AF is approximately one in four for people aged 40 years and older (Lloyd‐Jones 2004; Heeringa 2006). Furthermore, with an increasing elderly population in most countries, the incidence of AF is set to rise substantially during the coming decades (Wattigney 2003; Miyasaki 2006; Krijthe 2013).
Individuals with AF have an increased risk of thromboembolic events (e.g. ischaemic stroke, deep venous thrombosis, pulmonary embolism). The mechanisms behind this increased risk are complex and seem to be related to abnormal changes in blood flow, vessel walls and blood constituents that lead to a hypercoagulable or prothrombotic state (Watson 2009). The risk of stroke is about four to five times greater than for people of the same age who are in sinus rhythm, and it is estimated that about 15% to 30% of all strokes are caused by AF (Wolf 1991; Henriksson 2012). Ischaemic strokes in people with AF are more often disabling and fatal, and generally occur at a higher age compared with strokes in people with a normal sinus rhythm (Marini 2005).
Description of the intervention
In people with AF, prevention of thromboembolic events relies mainly on adequate antithrombotic therapy with a vitamin K antagonist (VKA), direct thrombin inhibitor or factor Xa inhibitor (ACC/AHA/HRS 2014; ESC 2016).
VKAs, such as warfarin, are a class of anticoagulants that reduce blood clotting by inhibiting the action of vitamin K. Treatment with warfarin, with an International Normalised Ratio (INR) target range of 2.0 to 3.0, has been shown to reduce the risk of stroke by about two‐thirds in people with AF and is more effective than antiplatelet agents (Hart 2007). Antithrombotic therapy with a VKA is still recommended as a treatment option in both European and American clinical guidelines for people with AF, who have an increased risk of thromboembolic complications (ACC/AHA/HRS 2014; ESC 2016). However, it is estimated that only about 50% to 60% of eligible people with AF actually receive treatment with a VKA, and of those who receive treatment many are treated suboptimally (Boulanger 2006; Connolly 2007). One important reason for this is that patients or their physicians fear bleeding complications, especially among the elderly (Sudlow 1997; Hylek 2007). Other reasons are that VKAs exhibit considerable variability in dose response among patients, are subject to multiple food and drug interactions, and have a narrow therapeutic window. Treatment with VKAs thus necessitates frequent laboratory monitoring and dose adjustments, which can be burdensome and difficult in clinical practice.
The under‐use of VKAs for stroke prevention in people with AF has prompted the development of other types of anticoagulant drugs. In 2012 a new class of anticoagulants, the factor Xa inhibitors, became available on the market. These factor Xa inhibitors have similar mechanisms of action: binding reversibly to the active site of factor Xa thereby inhibiting the formation of thrombin and fibrin. At least for the orally administered agents, the pharmacokinetic profile appears to be more or less comparable, with a relatively short half‐life leading to once or twice daily dosing of the oral agents (Mousa 2010). Factor Xa inhibitors appear to offer practical advantages over VKAs, with fewer food and drug interactions, a fixed daily or even weekly dose, and no need for monitoring of the anticoagulant effect (Mousa 2010). There are currently no approved antidotes to counteract the anticoagulation effect of factor Xa inhibitors.
Several factor Xa inhibitors (apixaban, rivaroxaban and edoxaban) and one oral direct thrombin inhibitor (dabigatran) have already been compared with VKAs in large, phase III randomised controlled trials (RCTs). Based on the results from these large studies, recently updated guidelines now also recommend such agents as treatment options for preventing stroke and other thromboembolic events in people with AF (EHRA 2013; ACC/AHA/HRS 2014; ESC 2016).
Why it is important to do this review
The prevalence and incidence of AF will most likely continue to increase and will cause more strokes during the coming decades (Wattigney 2003; Miyasaki 2006; Krijthe 2013). Factor Xa inhibitors appear to have several pharmacological and practical advantages over VKAs (Eikelboom 2010; Mousa 2010). They also have the potential to increase the proportion of people with AF who receive effective anticoagulant therapy. Despite the fact that recently updated European and American guidelines now also recommend apixaban, dabigatran, edoxaban and rivaroxaban, many people will continue to be treated with the relatively cheap and effective VKAs in the coming years, although this may vary considerably between countries and regions (EHRA 2013; ACC/AHA/HRS 2014; ESC 2016). A comparison of the efficacy and safety of the factor Xa inhibitors versus VKAs is therefore considered highly relevant.
Objectives
To assess the effectiveness and safety of treatment with factor Xa inhibitors versus vitamin K antagonists (VKAs) for preventing cerebral or systemic embolic events in people with AF.
Methods
Criteria for considering studies for this review
Types of studies
We sought to identify all unconfounded randomised controlled trials (RCTs) that directly compared the effects of long‐term treatment (more than four weeks) with factor Xa inhibitors with that of VKAs for preventing cerebral and systemic embolism in people with AF.
Types of participants
People with AF who were eligible for treatment with anticoagulants in order to reduce the risk of cerebral and systemic embolism. We included people with and without a previous stroke or transient ischaemic attack (TIA).
Types of interventions
Treatment with an oral or parenteral factor Xa inhibitor (e.g. antistasin, apixaban, betrixaban, darexaban, DU176b, edoxaban, eribaxaban, fondaparinux, idraparinux, idrabiotaparinux, otamixaban, razaxaban, rivaroxaban, yagin, YM150, LY517717, SSR126517E) versus oral vitamin K antagonists (warfarin and congeners) with the intensity monitored using the International Normalised Ratio (INR).
Types of outcome measures
Primary outcomes
The composite endpoint of all strokes (both ischaemic and haemorrhagic) and other systemic embolic events.
Secondary outcomes
All strokes (both ischaemic and haemorrhagic)
Ischaemic strokes
All disabling or fatal strokes (both ischaemic and haemorrhagic). The definition of a disabling stroke depends on the varying criteria in the included studies. Strokes are deemed fatal when death ensues within 30 days of the onset of stroke
Systemic embolic events (excluding embolic events in the central nervous system)
Major bleedings (defined by the International Society on Thrombosis and Haemostasis (ISTH) criteria or modified ISTH criteria)
Intracranial haemorrhages. This includes all intraparenchymal, subdural and epidural haematomas, and subarachnoid haemorrhages confirmed by neuroimaging or post‐mortem examination
Non‐major clinically relevant bleedings (defined by ISTH‐criteria or modified ISTH‐criteria)
Myocardial infarction. The diagnosis of myocardial infarction was based upon electrocardiographic changes, elevation of enzymes or confirmation during post‐mortem examination
Vascular deaths (deaths due to stroke, heart disease, haemorrhage and sudden deaths of unknown cause)
All‐cause deaths
Other adverse events (i.e. non‐bleeding adverse events)
Search methods for identification of studies
See the 'Specialised register' section in the Cochrane Stroke Group module. We searched for trials in all languages and arranged translation of relevant papers where required.
Electronic searches
We searched the trials registers of the Cochrane Stroke Group and the Cochrane Heart Group (September 2016). In addition, we searched the following electronic databases and trials registers:
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 8) in the Cochrane Library (searched August 2017) (Appendix 2);
MEDLINE Ovid (from 1950 to April 2017) (Appendix 2);
Embase Ovid (from 1980 to April 2017) (Appendix 3);
Stroke Trials Directory (www.strokecenter.org/trials) (June 2012 and September 2016);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched September 2016);
ISRCTN Registry Current Controlled Trials (www.isrctn.com) (September 2016);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch) (August 2017).
We developed the initial MEDLINE and Embase search strategies with the help of the Cochrane Stroke Group Information Specialist (2013) and updated search strategies with help from a librarian at the Norwegian Institute of Public Health (2016). We adapted the MEDLINE strategy for the searches of the other databases listed above.
Searching other resources
In an effort to identify further published, unpublished, ongoing and planned trials we:
screened reference lists of relevant trials;
-
contacted the following relevant pharmaceutical companies:
Sanofi (July 2012), responded and additional data were received for AMADEUS 2008;
Bristol Myers Squibb (July 2012), no response;
Daiichi Sankyo (July 2012), no response;
Portola Pharmaceuticals (July 2012), no response;
Bayer (July 2012), no response;
Astellas Europe (July 2012), no response;
-
contacted the following authors, colleagues and researchers active in the field:
HR Büller (June and July 2012), responded, additional data subsequently provided by sponsor of AMADEUS 2008 (Sanofi Aventis);
CB Granger (June and July 2012), responded, but no additional data provided for ARISTOTLE 2011;
S Ogawa (June and July 2012), no response and no additional data provided for ARISTOTLE‐J 2011;
N Chung (June and July 2012), no response and no additional data provided for Edoxaban Asia 2011;
JI Weitz (June and July 2012), responded, but no additional data provided for Edoxaban US/Europe 2010;
MD Ezekowitz (June and July 2012), responded, but no additional data provided for EXPLORE‐Xa 2013;
M Hori (June and July 2012), responded, additional data were provided for J‐ROCKET AF 2012;
M Patel (June and July 2012), no response and no additional data provided for ROCKET AF 2011;
AGG Turpie (June and July 2012), no response and no additional data provided for OPAL‐1 2010;
GYH Lip (June and July 2012), no response and no additional data provided for OPAL‐2 2011;
searched Google Scholar (http://scholar.google.co.uk/) (latest search September 2016);
used Science Citation Index Cited Reference search for forward tracking of relevant references;
used clinical outcome data from apixaban, rivaroxaban and edoxaban published in publicly available reports published on the website of the Food and Drug Administration (FDA);
contacted the European Medicines Agency (EMA) to gain access to the clinical trials study reports that were submitted by pharmaceutical companies for the marketing authorisation approval procedures of apixaban, rivaroxaban and edoxaban. Access to clinical study data of these compounds was granted by the EMA in 2015 and relevant outcome data have been included in this update.
Data collection and analysis
Selection of studies
One of the review authors (KBS) independently screened titles and abstracts of references identified by the searches and excluded obviously irrelevant citations. We obtained the full paper copies of the remaining articles, and both authors assessed these for inclusion. We resolved any uncertainties or disagreements on whether papers were eligible for inclusion by discussion with external experts. If we excluded a trial we kept a record of both the report and the reason for exclusion.
We did not use a scoring system to assess the quality of each trial, but for each included trial we collected information about:
the method of randomisation (including concealment of allocation);
blinding (care provider, patient, outcome assessment);
the number of participants lost to follow‐up;
whether or not the trial data were analysed according to the 'intention‐to‐treat' principle.
Data extraction and management
Both review authors independently extracted data from the report of each eligible trial and recorded the information on a specially designed data extraction form. We were not blinded to journal or institution and extracted the following data from each report:
inclusion and exclusion criteria;
method of randomisation;
masked versus open‐label intervention;
diagnostic criteria used for the assessment of major vascular events, stroke (both ischaemic and haemorrhagic), vascular death (including fatal haemorrhages), myocardial infarction or systemic embolism;
number of participants in each treatment group with outcome events;
generic name and dose(s) of factor Xa inhibitor used;
duration of anticoagulant therapy in the trial, the intensity of anticoagulation dose‐adjusted using INR, and adherence to anticoagulant treatment;
concomitant treatment with other anticoagulants, antiplatelets, or both, or any non‐steroidal anti‐inflammatory drugs;
outcomes (as listed above).
One review author (KBS) entered the data into the Cochrane Review Manager software, Review Manager 5 (RevMan 2014). The other review author (EB) checked these data against the hard‐copy data extraction forms to correct any clerical data entry errors. If any relevant data were missing from the available publications, we directly contacted the principal investigators or sponsor concerned, or used data from clinical study reports submitted to the EMA or FDA, or both.
Assessment of risk of bias in included studies
We used Cochrane's recommended tool for assessing the risk of bias in included studies (Cochrane Handbook 2011). Both review authors scored the potential for bias of specific features of each study as 'low', 'unclear' or 'high' risk. We resolved any disagreements by discussion with external experts.
Measures of treatment effect
For dichotomous outcomes, we calculated a weighted estimate of the treatment effects across trials (odds ratio, OR).
Dealing with missing data
In cases where the published information did not allow for an intention‐to‐treat analysis, we contacted the authors to get as complete follow‐up data as possible on all randomised participants for the originally proposed period of follow‐up, or used data from clinical study reports submitted to the European Medicines Agency (EMA) and Food and Drug Administration (FDA).
Assessment of heterogeneity
We tested for heterogeneity between trial results with the Cochrane Q statistic and I2 statistic (percentage of total variation across studies due to heterogeneity). We interpreted the amount of heterogeneity as 'low', 'moderate' and 'high' for I2 cut‐off values of 25%, 50% and 75%, respectively. We also assessed heterogeneity qualitatively.
Assessment of reporting biases
We used funnel plots to assess reporting bias. We also assessed these plots qualitatively.
Data synthesis
We calculated a weighted estimate of the typical treatment effect across trials using OR, by means of a fixed‐effect model. However, in case of moderate to high heterogeneity of treatment effects, we used a random‐effects model to enable further comparison of the overall treatment effects.
Subgroup analysis and investigation of heterogeneity
We performed pre‐planned subgroup analyses for:
type of factor Xa inhibitor;
dose of factor Xa inhibitor;
route of administration of factor Xa inhibitor;
previous stroke versus no previous stroke;
participants who received VKA treatment with time‐in‐therapeutic range (TTR) equal to or greater than 60% ("high" quality) versus less than 60% ("low" quality) (Connolly 2008; ESC 2016);
VKA treatment‐experienced participants versus treatment‐naive participants;
participants who received concomitant antiplatelet therapy (aspirin) versus those who did not;
age less than 75 years versus age 75 years or over;
race;
sex;
baseline stroke risk factors (assessed by the CHADS2 score).
We used the method described by Deeks for performing subgroup analyses (Deeks 2001).
Sensitivity analysis
In the case of any evidence of heterogeneity that could not be explained by study quality, we conducted a sensitivity analysis excluding any open‐label trials.
GRADE and 'Summary of findings'
We assessed the quality of evidence by using the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias). Overall quality of the evidence was assessed as either high, moderate, low or very low (Higgins 2011).
We also included a Table 1, comparing factor Xa inhibitors with vitamin K antagonists for the important outcomes of:
stroke and other systemic embolic events;
all strokes;
major bleedings;
intracranial haemorrhages;
all‐cause death.
Results
Description of studies
For detailed descriptions see the Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
The literature search identified a total of 1900 reports (updated search April 2017; see Figure 1 for further details). After removing duplicates and screening titles and abstracts, we identified 21 reports that we retrieved in full text and evaluated for eligibility.
1.
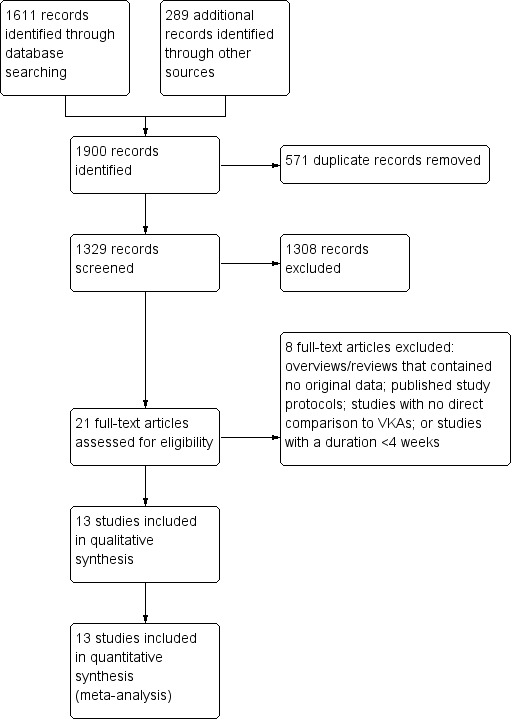
Study flow diagram.
Eight of the 21 reports were either: overviews or expert reviews that contained no new outcome data; publications of study protocols; studies with no direct comparison of the study drug to vitamin K antagonists (VKAs); or studies with a duration shorter than four weeks (see Characteristics of excluded studies for further details).
We included the remaining 13 reports, which were original publications of randomised controlled trials (RCTs) enrolling a total of 67,688 participants with atrial fibrillation (AF) who were considered eligible for long‐term (more than four weeks) anticoagulation treatment with a VKA (AMADEUS 2008; Edoxaban US/Europe 2010; OPAL‐1 2010; ARISTOTLE 2011; ARISTOTLE‐J 2011; Edoxaban Asia 2011; OPAL‐2 2011; ROCKET AF 2011; Edoxaban Japan 2012; J‐ROCKET AF 2012; ENGAGE AF‐TIMI 48 2013; EXPLORE‐Xa 2013; BOREALIS AF STUDY 2014).
In AMADEUS 2008 97 participants recruited by a single centre were excluded from the intention‐to‐treat analyses; the reason for this was not clearly stated in the publication. In ROCKET AF 2011 93 participants, all recruited by one centre, were excluded from the intention‐to‐treat analyses because of good clinical practice (GCP) violations that rendered the data unreliable. After excluding these 190 participants, we had data from 67,498 participants for further analysis.
The included studies directly compared various types of factor Xa inhibitors with VKAs. One trial studied the compound idraparinux, which was administered subcutaneously once weekly (AMADEUS 2008). One trial studied idrabiotaparinux—a biotin moiety to idraparinux—that was also administered subcutaneously once weekly (BOREALIS AF STUDY 2014) . The remaining trials all studied oral factor Xa inhibitors (i.e. apixaban, betrixaban, darexaban, edoxaban and rivaroxaban), that were administered once or twice daily.
All studies randomised participants to more than one dose of the studied factor Xa inhibitor. Studies of the compounds apixaban (ARISTOTLE 2011; ARISTOTLE‐J 2011), rivaroxaban (ROCKET AF 2011; J‐ROCKET AF 2012) and edoxaban (Edoxaban US/Europe 2010; Edoxaban Asia 2011; Edoxaban Japan 2012; ENGAGE AF‐TIMI 48 2013) contributed to approximately 90% of all data included in this review.
Dose‐adjusted warfarin was the active comparator in all included trials. In most trials the target INR for warfarin treatment was between 2.0 and 3.0. In ARISTOTLE‐J 2011, participants aged 70 years and over had a target INR of 2.0 to 2.6, whereas in OPAL‐1 2010, Edoxaban Japan 2012 and J‐ROCKET AF 2012, participants in this age category had a target INR of 1.6 to 2.6. The quality of the anticoagulation with warfarin (time in therapeutic range (TTR) calculated using the Rosendaal method) was reported in 11 trials (AMADEUS 2008; Edoxaban US/Europe 2010; ARISTOTLE 2011; ARISTOTLE‐J 2011; Edoxaban Asia 2011; ROCKET AF 2011; Edoxaban Japan 2012; J‐ROCKET AF 2012; ENGAGE AF‐TIMI 48 2013; EXPLORE‐Xa 2013; BOREALIS AF STUDY 2014). Reported (median) TTR values ranged from 45% to 83% in these studies. TTR values were not reported in two studies (OPAL‐1 2010; OPAL‐2 2011).
The mean baseline CHADS2 score in the included studies ranged from 1.9 to 3.5. Mean baseline CHADS2 scores were not reported in four studies (AMADEUS 2008; Edoxaban US/Europe 2010; OPAL‐1 2010; BOREALIS AF STUDY 2014).
All participants were aged 18 years or older. Mean and median ages of randomised participants ranged between 65 and 74 years, and approximately one‐third of all randomised participants were women. Mean ages and gender were not stated in one study (OPAL‐1 2010).
The median duration of follow‐up ranged from 12 weeks to 2.8 years. Six trials (AMADEUS 2008; ARISTOTLE 2011; ROCKET AF 2011; J‐ROCKET AF 2012; ENGAGE AF‐TIMI 48 2013; BOREALIS AF STUDY 2014) were all designed as event‐driven studies, whereas the remaining smaller studies all had predefined durations of follow‐up.
The included studies used different definitions of 'disabling stroke'. One study used the modified Rankin scale to score stroke outcome; scores from 0 to 2 were defined as 'non‐disabling', and scores from 3 to 5 as 'disabling' (ROCKET AF 2011). The outcome of stroke was only assessed by the investigator in this study. Data on disabling strokes (defined as 'strokes with serious residual disability') were also reported in J‐ROCKET AF 2012, though it was not stated which functional outcome scale and which cut‐off value, if any, were used to define 'serious residual disability'. In one study it was unclear which scale was used for assessing functional outcome in one participant who suffered an ischaemic stroke during the study period (OPAL‐1 2010). It was, however, stated that this stroke was 'resolved' and we have therefore chosen not to count this as a disabling stroke.
Risk of bias in included studies
For detailed information see: Characteristics of included studies, Figure 2 ('Risk of bias' graph) and Figure 3 ('Risk of bias' summary).
2.
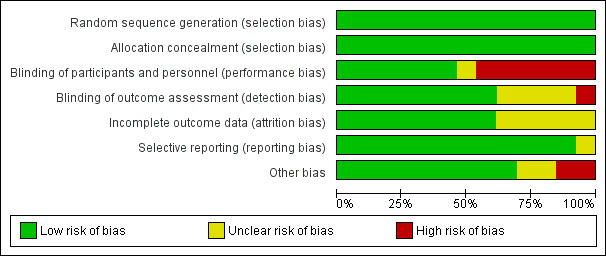
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
3.
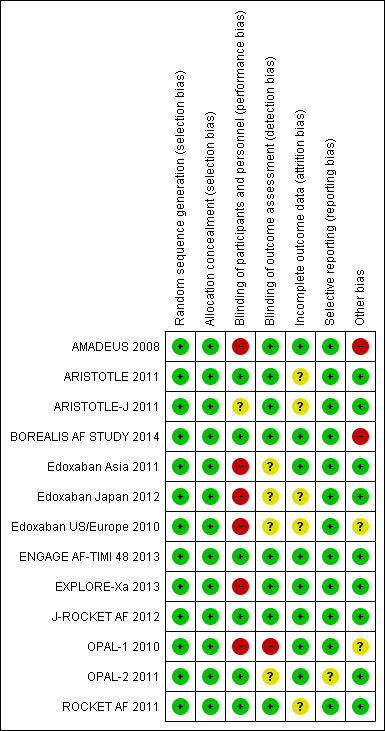
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
All 13 included trials randomly assigned participants to treatment groups, using either a computerised interactive voice response system (AMADEUS 2008; Edoxaban US/Europe 2010; ROCKET AF 2011; ENGAGE AF‐TIMI 48 2013; BOREALIS AF STUDY 2014), block randomisation schedule (Edoxaban Asia 2011), or a non‐specified randomisation method (OPAL‐1 2010; ARISTOTLE 2011; ARISTOTLE‐J 2011; OPAL‐2 2011; Edoxaban Japan 2012; J‐ROCKET AF 2012; EXPLORE‐Xa 2013).
In six studies, randomisation was stratified for previous warfarin use (warfarin experienced versus warfarin naive) or clinical site, or both, (AMADEUS 2008; ARISTOTLE 2011; ARISTOTLE‐J 2011; Edoxaban Japan 2012; EXPLORE‐Xa 2013; BOREALIS AF STUDY 2014). One study stratified participants according to their baseline CHADS2 score (scores 2 to 3 versus 4 to 6) and need for a reduction in the edoxaban dose (30 mg versus 60 mg) (ENGAGE AF‐TIMI 48 2013). The remaining trials did not report stratification for any baseline variables.
Blinding
Six studies were fully double‐masked (blinded) trials (ARISTOTLE 2011; OPAL‐2 2011; ROCKET AF 2011; J‐ROCKET AF 2012; ENGAGE AF‐TIMI 48 2013; BOREALIS AF STUDY 2014): we judged these studies as having low risk of performance bias. Another six studies were partially‐masked trials, i.e. the different doses of factor Xa inhibitors were administered in a double‐masked fashion, but warfarin was administered open‐label (Edoxaban US/Europe 2010; OPAL‐1 2010; ARISTOTLE‐J 2011; Edoxaban Asia 2011; Edoxaban Japan 2012; EXPLORE‐Xa 2013): these studies were judged as having unclear or high risk of performance bias. We included only one fully open‐label study with a high risk of performance bias (AMADEUS 2008).
Adjudication of outcome events was performed by blinded, centralised committees in 11 studies (AMADEUS 2008; Edoxaban US/Europe 2010; ARISTOTLE 2011; ARISTOTLE‐J 2011; OPAL‐2 2011; ROCKET AF 2011; Edoxaban Japan 2012; J‐ROCKET AF 2012; ENGAGE AF‐TIMI 48 2013; EXPLORE‐Xa 2013; BOREALIS AF STUDY 2014). A centralised adjudication committee was also used in Edoxaban Asia 2011, but it was unclear whether this committee was blinded or not, since this was not specified in the publication. No details on the adjudication of outcome events were provided for OPAL‐1 2010.
Incomplete outcome data
The reported analysis for efficacy outcomes was a (modified) intention‐to‐treat analysis in 10 studies (AMADEUS 2008; OPAL‐1 2010; ARISTOTLE 2011; ARISTOTLE‐J 2011; OPAL‐2 2011; ROCKET AF 2011; Edoxaban Japan 2012; ENGAGE AF‐TIMI 48 2013; EXPLORE‐Xa 2013; BOREALIS AF STUDY 2014). In one study the primary efficacy outcome (composite of stroke and systemic embolic events) was reported for the intention‐to‐treat population; other efficacy outcomes were analysed in the per protocol population, defined as participants without any major study protocol violations (J‐ROCKET AF 2012). This definition led to the exclusion of 6/1280 randomised participants (0.5%) from all secondary efficacy analyses in J‐ROCKET AF 2012. Two studies only analysed efficacy outcomes in the 'safety population', defined as participants who received at least one dose of the study drug and had at least one post‐dose assessment (Edoxaban US/Europe 2010; Edoxaban Asia 2011). This led to the exclusion of 1/235 participants (0.4%) in Edoxaban Asia 2011, and 3/1146 (0.3%) participants in Edoxaban US/Europe 2010.
Four studies analysed safety outcomes in the intention‐to‐treat population (AMADEUS 2008; OPAL‐1 2010; OPAL‐2 2011; EXPLORE‐Xa 2013). Nine studies only analysed safety outcomes in the 'safety population', defined as the participants who received at least one dose of the study drug (Edoxaban US/Europe 2010; ARISTOTLE 2011; ARISTOTLE‐J 2011; Edoxaban Asia 2011; ROCKET AF 2011; Edoxaban Japan 2012; J‐ROCKET AF 2012; ENGAGE AF‐TIMI 48 2013; BOREALIS AF STUDY 2014). This led to the exclusion of one (0.4%); three (0.3%); 61 (0.3%); five (2.3%); 28 (0.2%); two (0.2%); 11 (2.1%) and 79 (0.4%) participants in Edoxaban Asia 2011, Edoxaban US/Europe 2010, ARISTOTLE 2011, ARISTOTLE‐J 2011, ROCKET AF 2011, J‐ROCKET AF 2012, Edoxaban Japan 2012 and ENGAGE AF‐TIMI 48 2013, respectively.
We used data from a subgroup analysis in people with previous stroke and TIA who were randomised into the ROCKET AF trial (Hankey 2012).
Loss to follow‐up in the included studies was generally low, ranging from 0% (ARISTOTLE‐J 2011; J‐ROCKET AF 2012) to 2.7% (AMADEUS 2008) of all randomised participants. The number of participants lost to follow‐up was not reported in OPAL‐1 2010.
Selective reporting
There was no indication of selective reporting in any of the included studies. All predefined efficacy and safety outcomes stated in the study protocols were reported in the publications, abstracts or clinical study reports submitted to regulatory authorities.
Other potential sources of bias
One study was terminated prematurely after a recommendation from the trial's Data and Safety Monitoring Board because of excess bleeding complications in the idraparinux group (AMADEUS 2008). Another study was also halted prematurely by the sponsor (Sanofi Aventis), but for commercial or strategic reasons (BOREALIS AF STUDY 2014). None of the other included trials were stopped prematurely.
Enrolment into the treatment arm receiving darexaban 240 mg once daily (OPAL‐1 2010) and the arm receiving edoxaban 60 mg twice daily (Edoxaban US/Europe 2010) was halted after recommendations by the trials' respective Data and Safety Monitoring Boards due to an excess of bleeding complications.
Effects of interventions
See: Table 1
Although we included 13 studies in this review, most of the following analyses include data from less than 13 studies.
Primary outcome
Composite endpoint of all strokes (both ischaemic and haemorrhagic) and other systemic embolic events
This outcome was reported in all 13 included studies (number of participants (n) = 67,477). Most data (approximately 90%) came from studies that used the agents apixaban (ARISTOTLE 2011; ARISTOTLE‐J 2011), rivaroxaban (ROCKET AF 2011; J‐ROCKET AF 2012) and edoxaban (Edoxaban US/Europe 2010; Edoxaban Asia 2011; Edoxaban Japan 2012; ENGAGE AF‐TIMI 48 2013). Treatment with a factor Xa inhibitor significantly decreased the number of strokes and other systemic embolic events compared with dose‐adjusted warfarin in participants with atrial fibrillation (AF) (odds ratio (OR) 0.89, 95% confidence interval (CI) 0.82 to 0.97; 13 studies; 67,477 participants; high‐quality evidence: Analysis 1.1). We observed low, non‐statistically significant heterogeneity (I2 = 13%). The total number of non‐central nervous system systemic embolic events was very low, contributing to approximately 5% of the total outcomes of the composite endpoint, meaning that the primary outcome was largely driven by the stroke component (ischaemic and haemorrhagic strokes).
1.1. Analysis.
Comparison 1 Factor Xa inhibitors versus VKA, Outcome 1 Stroke and other systemic embolic events.
We calculated the number needed to treat (NNT), or number need to treat for an additional harmful outcome (NNTH), for studies with median follow‐up periods of one year or more (ARISTOTLE 2011; ROCKET AF 2011; ENGAGE AF‐TIMI 48 2013). We used a web‐based NNT calculator to calculate the NNTs/NNTHs with 95% CIs (graphpad.com/quickcalcs/NNT2) and entered the outcome data used in Analysis 1.1. The NNT for apixaban was 169 (95% CI 94.5 to 772.7) for a median treatment/follow‐up period of 1.8 years (ARISTOTLE 2011). Assuming that the risk of these outcome events was constant during treatment, this translates into a NNT of 304 for one year of treatment. This NNT indicates that 304 people need to be treated with apixaban for one year in order to prevent one stroke or systemic embolic event compared with warfarin. For rivaroxaban the NNT was 194 for a median treatment/follow‐up period of 1.9 years, which translates into a NNT of 376 per year. Because the 95% CI for the absolute risk reduction (0.52%) extends from a negative number (‐0.13%) to a positive number (1.17%), it is difficult to compute a 95% CI for the NNT in this case.
We could only calculate a NNTH for edoxaban, since the OR was greater than 1 in ENGAGE AF‐TIMI 48 2013 (OR 1.01, 95% CI 0.88 to 1.15). The NNTH was 2736 for a median treatment/follow‐up period of 2.8 years, which is equivalent to a NNTH of 7661 per year. This NNTH indicates that 7661 people need to be treated for one year with edoxaban to cause one more stroke or systemic embolic embolism compared with warfarin.
Secondary outcomes
All strokes (ischaemic and haemorrhagic)
The composite endpoint of 'all strokes' was reported in all 13 included studies (n = 67,449). Treatment with a factor Xa inhibitor significantly decreased the number of strokes compared with warfarin (OR 0.89, 95% CI 0.81 to 0.97; 13 studies; 67,449 participants; high‐quality evidence: Analysis 1.2). There was low, non‐statistically significant heterogeneity (I2 = 18%).
1.2. Analysis.
Comparison 1 Factor Xa inhibitors versus VKA, Outcome 2 All strokes.
Ischaemic stroke
We were able to calculate the effect of study versus control treatment on the number of ischaemic strokes for 12 studies (n = 66,306). No data were available for Edoxaban US/Europe 2010. The analysis showed no statistically significant decrease or increase in the number of ischaemic strokes in participants treated with a factor Xa inhibitor compared with warfarin (OR 1.03, 95% CI 0.92 to 1.14: Analysis 1.3). There was moderate, non‐statistically significant heterogeneity between the analysed studies (I2 = 37%).
1.3. Analysis.
Comparison 1 Factor Xa inhibitors versus VKA, Outcome 3 Ischaemic stroke.
Disabling or fatal strokes
Seven studies that included 39,026 participants reported data on disabling or fatal strokes. Treatment with a factor Xa inhibitor was not associated with a statistically significant reduction in the number of disabling or fatal strokes compared with warfarin (OR 0.91, 95% CI 0.77 to 1.06: Analysis 1.4). We observed low, non‐statistically significant heterogeneity (I2 = 16%).
1.4. Analysis.
Comparison 1 Factor Xa inhibitors versus VKA, Outcome 4 Disabling or fatal stroke.
Non‐central nervous system (CNS) systemic embolic events
The occurrence of non‐CNS systemic embolic events was reported in all 13 included studies (n = 67,449). Treatment with a factor Xa inhibitor significantly reduced the number of non‐CNS systemic embolic events compared with warfarin (OR 0.68, 95% CI 0.48 to 0.96: Analysis 1.5). There was low, non‐statistically significant heterogeneity (I2 = 15%).
1.5. Analysis.
Comparison 1 Factor Xa inhibitors versus VKA, Outcome 5 Systemic embolic events (non‐CNS).
Major bleedings
All 13 included studies (n = 67,396) reported the number of major bleedings defined either by the ISTH‐criteria, or a slight modification of these criteria. Treatment with a factor Xa inhibitor significantly reduced the number of major bleedings compared with warfarin (OR 0.78, 95% CI 0.73 to 0.84; moderate‐quality evidence: Analysis 1.6). There was, however, high and statistically significant heterogeneity (I2 = 83%). Because of this, we performed an analysis using a random‐effects model. Contrary to the results from the fixed‐effect model, this analysis did not show a statistically significant decrease in the number of major bleedings in people treated with factor Xa inhibitors compared with warfarin (OR 0.88, 95% CI 0.66 to 1.17).
1.6. Analysis.
Comparison 1 Factor Xa inhibitors versus VKA, Outcome 6 Major bleedings.
To further explore the observed statistical heterogeneity we also performed a pre‐specified sensitivity analysis in which we excluded open‐label studies (sensitivity analyses not shown in forest plots). The only included open‐label trial was AMADEUS 2008, which was stopped prematurely due to an excess of major bleeding in the idraparinux arm (OR 2.62, 95% CI 1.70 to 4.03). The sensitivity analysis excluding AMADEUS 2008, using a fixed‐effect model, showed that treatment with a factor Xa inhibitor significantly reduced the number of major bleedings compared with warfarin (OR 0.75, 95% CI 0.69 to 0.81). However, we still observed moderate and statistically significant heterogeneity (I2 = 72%). A sensitivity analysis excluding AMADEUS 2008, but using a random‐effects model, also showed a statistically significant decrease in the number of major bleedings in people treated with factor Xa inhibitors (OR 0.76, 95% CI 0.60 to 0.96).
Intracranial haemorrhages (ICHs)
Data on ICHs were reported in 12 studies that randomised 66,259 participants. No data were reported for Edoxaban US/Europe 2010. Treatment with a factor Xa inhibitor significantly reduced the risk of ICH compared with warfarin (OR 0.50, 95% CI 0.42 to 0.59; 12 studies; 66,259 participants; high‐quality evidence: Analysis 1.7). We observed statistically significant, moderate heterogeneity (I2 = 55%). An analysis using a random‐effects model also indicated a statistically significant reduction of ICHs in participants treated with a factor Xa inhibitor compared with warfarin (OR 0.57, 95% CI 0.40 to 0.82).
1.7. Analysis.
Comparison 1 Factor Xa inhibitors versus VKA, Outcome 7 Intracranial haemorrhages.
We performed a pre‐specified sensitivity analysis in which we excluded open‐label studies to further explore the observed moderate heterogeneity (sensitivity analyses not shown in forest plots). As mentioned previously, the only open‐label study was the prematurely halted AMADEUS 2008, in which a statistically significant increase in the risk of ICHs was observed (OR 11.10, 95% CI 1.43 to 86.02). The sensitivity analysis with a fixed‐effect model showed that treatment with a factor Xa inhibitor significantly reduced the number of ICHs compared with warfarin (OR 0.47, 95% CI 0.40 to 0.56). We observed low, non‐statistically significant heterogeneity in this analysis (I2 = 27%).
Non‐major clinically relevant bleedings
All 13 studies reported the number of non‐major clinically relevant bleeding defined by either ISTH criteria or a modification of these criteria. Data on 67,396 randomised participants were available for analysis. We found a statistically significant difference in the number of non‐major clinically relevant bleedings, which favoured factor Xa inhibitors over warfarin (OR 0.86, 95% CI 0.82 to 0.91: Analysis 1.8). We observed statistically significant and high heterogeneity (I2 = 88%). An analysis with a random‐effects model showed no statistically significant difference in the number of non‐major clinically relevant bleedings that were observed in the two treatment groups (OR 0.87, 95% CI 0.70 to 1.07).
1.8. Analysis.
Comparison 1 Factor Xa inhibitors versus VKA, Outcome 8 Non‐major clinically relevant bleeds.
We performed a pre‐specified sensitivity analysis in which we excluded open‐label studies (sensitivity analysis not shown in forest plots). This sensitivity analysis again excluded the prematurely halted AMADEUS 2008 study, in which a statistically significant increase in the risk of non‐major clinically relevant bleedings was reported (OR 1.48, 95% CI 1.23 to 1.79). This analysis, using a fixed‐effect model, showed that treatment with factor Xa inhibitors did significantly reduce the number of non‐major clinically relevant bleedings compared with warfarin (OR 0.83, 95% CI 0.79 to 0.87). However, we observed statistically significant, high heterogeneity (I2 = 84%). We repeated the same sensitivity analysis using a random‐effects model, and found similar results (OR 0.80, 95% CI 0.65 to 0.98).
Myocardial infarction
The number of myocardial infarctions that occurred during the study period was reported in 10 studies that randomised 62,703 participants. No data were available for OPAL‐2 2011, Edoxaban Japan 2012 and BOREALIS AF STUDY 2014. There was no statistically significant difference between the number of myocardial infarctions in participants treated with factor Xa inhibitors compared with warfarin (OR 0.96, 95% CI 0.84 to 1.10: Analysis 1.9). We observed no heterogeneity in the analysis (I2 = 0%).
1.9. Analysis.
Comparison 1 Factor Xa inhibitors versus VKA, Outcome 9 Myocardial infarction.
Vascular deaths
Vascular deaths were reported in 10 studies (n = 45,027). No data were available for OPAL‐1 2010, ARISTOTLE 2011 and BOREALIS AF STUDY 2014. The analysis showed a statistically significant difference between the number of vascular deaths, which favoured factor Xa inhibitors over warfarin (OR 0.86, 95% CI 0.79 to 0.94: Analysis 1.10). There was no sign of any heterogeneity between the included studies (I2 = 0%).
1.10. Analysis.
Comparison 1 Factor Xa inhibitors versus VKA, Outcome 10 Vascular deaths.
All‐cause deaths
The number of participants who died from any cause was reported in 10 studies (n = 65,624). No data were available for Edoxaban US/Europe 2010, OPAL‐1 2010 and Edoxaban Asia 2011. Treatment with a factor Xa inhibitor significantly reduced the number of deaths compared with warfarin (OR 0.89, 95% 0.83 to 0.95; 10 studies; 65,624 participants; moderate‐quality evidence: Analysis 1.11). We observed no heterogeneity (I2 = 0%).
1.11. Analysis.
Comparison 1 Factor Xa inhibitors versus VKA, Outcome 11 All‐cause deaths.
Other adverse events
We did not analyse this outcome because of a paucity of data on adverse events other than the ones reported above. Sufficient data on other adverse events were only systematically presented for the large phase III studies of apixaban, rivaroxaban and edoxaban (ARISTOTLE 2011; ROCKET AF 2011, ENGAGE AF‐TIMI 48 2013) and are listed in the appendices of the original publications, in the clinical study reports of these three compounds that were submitted to regulatory authorities, and in the approved product labels. Of note, there was no evidence for an increased risk of hepatotoxicity associated with apixaban, rivaroxaban or edoxaban compared with warfarin based on these data.
Subgroup analyses
We performed 22 pre‐specified subgroup analyses for the primary efficacy outcome (composite of stroke and systemic embolic events) and safety outcome (major bleedings). Results of these subgroup analyses are presented below.
Interpretation of subgroup data requires caution because the trials were not powered to detect differences in these subgroups. Furthermore, randomisation into the included studies was in most cases not stratified by the factors that were analysed in these subgroup analyses; it is therefore not possible to ensure that potential confounders were balanced across treatment arms.
Different factor Xa inhibitors
Subgroup analysis 1: stroke and systemic embolic events
An analysis of the different factor Xa inhibitors showed that only the agents apixaban (OR 0.78, 95% CI 0.65 to 0.93) and rivaroxaban (OR 0.85, 95% CI 0.72 to 1.00) significantly decreased the number of strokes and systemic embolic events compared with warfarin (Analysis 1.1). The agents idraparinux, idrabiotaparinux, edoxaban, darexaban and betrixaban did not show a statistically significant difference, but there was no evidence of any statistically significant differences between the risk estimates of these agents and those of apixaban or rivaroxaban (test for subgroup differences: P = 0.36; I2 = 10%).
Subgroup analysis 2: major bleedings
. Major bleedings occurred significantly less often in participants that were treated with apixaban (OR 0.69, 95% CI 0.60 to 0.80), betrixaban (OR 0.19, 95% CI 0.05 to 0.82) and edoxaban (OR 0.63, 95% CI 0.56, 0.71) compared with warfarin (Analysis 1.6). More major bleedings were observed in participants treated with idraparinux compared with warfarin (OR 2.62, 95% CI 1.70 to 4.03). We observed no statistically significant differences for idrabiotaparinux, rivaroxaban and darexaban compared with warfarin. There was evidence of high heterogeneity and statistically significant differences between the risk estimates of the various agents (test for subgroup differences: P < 0.00001; I2 = 95%).
Different doses of factor Xa inhibitors
Subgroup analysis 3: stroke and systemic embolic events
A total of 22 different doses of the included factor Xa inhibitors were assessed in the studies (Analysis 3.1). Unfortunately, we could not use data from ROCKET AF 2011, since the number of events were not reported for the different dose levels used in this study. Overall, we observed no statistically significant differences in the number of stroke or systemic embolic events between the various tested doses of the factor Xa inhibitors compared with warfarin (test for subgroup differences: P = 0.22; I2 = 19.1%). Both apixaban doses (2.5 mg and 5 mg twice daily) and the rivaroxaban dose (15 mg once‐daily) showed a statistically significant decreased number of strokes and other systemic embolic events compared with warfarin. Differences in thromboembolic events between the other doses and types of factor Xa inhibitors compared with warfarin were not statistically significant.
3.1. Analysis.
Comparison 3 Factor Xa inhibitors versus VKA: dose of Factor Xa inhibitor, Outcome 1 Stroke and other systemic embolic events.
Subgroup analysis 4: major bleedings
We could use data about 21 different doses of factor Xa inhibitors for this subgroup analysis (Analysis 3.2). Outcome data for the two different idraparinux doses were pooled because we could not obtain data for two separate doses that were used in AMADEUS 2008. Overall, we observed statistically significant differences in the number of major bleedings between the doses of factor Xa inhibitors compared with warfarin (test for subgroup differences: P < 0.00001; I2 = 78%). For both idraparinux doses and the edoxaban dose of 60 mg twice daily, there was a statistically significant increase in the number of major bleedings compared with warfarin. We observed a statistically significant decrease in the number of major bleedings compared with warfarin for two doses of edoxaban (30 mg and 60 mg once daily) and for apixaban (5 mg twice‐daily dose).
3.2. Analysis.
Comparison 3 Factor Xa inhibitors versus VKA: dose of Factor Xa inhibitor, Outcome 2 Major bleedings.
Route of administration
Subgroup analysis 5: stroke and systemic embolic events
We compared the number of stroke and systemic embolic events in people treated with oral versus parenteral factor Xa inhibitors (Analysis 2.1). Of note, only the agents idraparinux and idrabiotaparinux were administered parenterally. We only observed a statistically significant decrease for this outcome in people treated with oral factor Xa inhibitors compared with warfarin (OR 0.87, 95% CI 0.80 to 0.96). Overall, we observed no statistically significant differences in the number of strokes and systemic embolic events between the oral and parenteral factor Xa inhibitors (test for subgroup differences: P = 0.59; I2 = 0%).
2.1. Analysis.
Comparison 2 Factor Xa inhibitors versus VKA: route of administration, Outcome 1 Stroke and other systemic embolic events.
Subgroup analysis 6: major bleedings
The number of major bleedings was lower in people receiving orally administered factor Xa inhibitors compared with warfarin (OR 0.74, 95% CI 0.69 to 0.80: Analysis 2.2). For the parenterally administered factor Xa inhibitors, we observed a statistically significant increase in major bleedings when compared with warfarin (OR 1.82, 95% CI 1.30 to 2.55). We observed a statistically significant difference in the number of major bleedings between the oral and parenteral factor Xa inhibitors (test for subgroup differences: P < 0.00001; I2 = 96%).
2.2. Analysis.
Comparison 2 Factor Xa inhibitors versus VKA: route of administration, Outcome 2 Major bleedings.
Quality of anticoagulation with warfarin
Subgroup analysis 7: stroke and systemic embolic events
We performed a subgroup analysis in people who received warfarin treatment with median time‐in‐therapeutic range (TTR) ≤ 60% versus ≥ 60%, i.e. 'low/bad' versus 'high/good' quality treatment (Analysis 5.1). To perform this analysis for the primary efficacy endpoint, we had sufficient data from the phase III trials of apixaban, rivaroxaban and edoxaban (ARISTOTLE 2011; ROCKET AF 2011; ENGAGE AF‐TIMI 48 2013), which enrolled 52,695 participants in total. The number of strokes and systemic embolic events was lower among people treated with factor Xa inhibitors compared with warfarin in centres with TTRs ≥ 60%, although this difference did not reach statistical significance (OR 0.89, 95% CI 0.77 to 1.04). In centres with TTRs ≤ 60% the number of strokes and systemic embolic events was again lower in people treated with factor Xa inhibitors and this difference was statistically significant (OR 0.81, 95% CI 0.70 to 0.93). Overall, we did not observe any statistically significant differences between the three compounds in this subgroup analysis (test for subgroup differences: P = 0.34; I2 = 0%).
5.1. Analysis.
Comparison 5 Factor Xa inhibitors versus VKA: quality of anticoagulation with VKA (TTR), Outcome 1 Stroke and other systemic embolic events.
Subgroup analysis 8: major bleedings
We performed a similar subgroup analysis based on the quality of warfarin treatment for major bleedings (Analysis 5.2). Again, we could only include data for apixaban, edoxaban and rivaroxaban from their respective phase III studies (n = 52,730). We observed a lower number of major bleedings with factor Xa inhibitors compared with warfarin (OR 0.80, 95% CI 0.72 to 0.90) in centres with TTRs ≥ 60%. There was, however, high and statistically significant heterogeneity in this analysis (I2 = 90%; P < 0.0001). A sensitivity analysis using a random‐effects model did not show a statistically significant decrease (OR 0.87, 95% CI 0.60 to 1.27). In centres with TTRs ≤ 60% the risk of major bleedings was lower for the factor Xa inhibitors compared with warfarin (OR 0.69, 95% CI 0.62 to 0.77), but we observed high heterogeneity (I2 = 88%; P = 0.0002). A sensitivity analysis using a random‐effects model also showed a statistically significant lower risk for the factor Xa inhibitors compared with 'low' quality warfarin treatment (OR 0.67, 95% CI 0.49 to 0.93). Overall, there were no significant differences between the three compounds in this subgroup analysis (test for subgroup differences: P = 0.05; I2 = 73%).
5.2. Analysis.
Comparison 5 Factor Xa inhibitors versus VKA: quality of anticoagulation with VKA (TTR), Outcome 2 Major bleedings.
Previous stroke and transient ischaemic attack (TIA)
Subgroup analysis 9: stroke and systemic embolic events
We compared the efficacy of factor Xa inhibitors in participants with or without a previous stroke or TIA (Analysis 4.1). We had data from four compounds (n = 59,247) for this for this subgroup analysis: idraparinux, apixaban, edoxaban and rivaroxaban. The risk of stroke and systemic embolic events was significantly lower in people treated with factor Xa inhibitors who had previously suffered a stroke or TIA (OR 0.87, 95% CI 0.76 to 1.00), and in people who did not have a previous stroke or TIA (OR 0.85, 95% CI 0.74 to 0.97), compared to warfarin. We observed no statistically significant differences in these analyses (test for subgroup differences: P = 0.78; I2 = 0%).
4.1. Analysis.
Comparison 4 Factor Xa inhibitors versus VKA: previous stroke or TIA, Outcome 1 Stroke and other systemic embolic events.
Subgroup analysis 10: major bleedings
We performed a similar subgroup analysis for the major bleedings outcome (Analysis 4.2). We were able to include data on three factor Xa inhibitors: apixaban, edoxaban and rivaroxaban (n = 54,615). The number of major bleedings was significantly lower in participants with or without a previous stroke or TIA who received treatment with factor Xa inhibitors compared with warfarin: OR 0.79 (95% CI 0.69 to 0.90) and OR 0.73 (95% CI 0.67 to 0.80), respectively. Overall, we observed no statistically significant differences in these analyses (test for subgroup differences: P = 0.35; I2 = 0%).
4.2. Analysis.
Comparison 4 Factor Xa inhibitors versus VKA: previous stroke or TIA, Outcome 2 Major bleedings.
Prior VKA treatment
Subgroup analysis 11: stroke and systemic embolic events
We analysed the efficacy of factor Xa inhibitors in people who had received prior treatment with a VKA versus those who had not (total n = 59,247). The number of strokes and systemic embolic events was significantly lower with factor Xa inhibitors in people who had not received prior VKA treatment (OR 0.80, 95% CI 0.69 to 0.93), but not in people who had received treatment with a VKA prior to randomisation (OR 0.90, 95% CI 0.79 to 1.02) (Analysis 6.1). We found low, non‐statistically significant heterogeneity in these analyses (test for subgroup differences: P = 0.24; I2 = 26%).
6.1. Analysis.
Comparison 6 Factor Xa inhibitors versus VKA: previous VKA use, Outcome 1 Stroke and other systemic embolic events.
Subgroup analysis 12: major bleedings
A similar analysis for the primary safety endpoint showed a statistically significant lower risk of major bleedings with factor Xa inhibitors in people who had not received prior VKA treatment (OR 0.74, 95% CI 0.66 to 0.84) and in those who had prior VKA treatment (OR 0.75, 95% CI 0.68 to 0.83) (Analysis 6.2). High and statistically significant heterogeneity was observed in the latter analysis (I2 = 89%; P < 0.00001). Overall however, we observed no statistically significant differences in the analyses (test for subgroup differences: P = 0.85; I2 = 0%).
6.2. Analysis.
Comparison 6 Factor Xa inhibitors versus VKA: previous VKA use, Outcome 2 Major bleedings.
Concomitant antiplatelet therapy
Subgroup analysis 13: stroke and systemic embolic events
We had data to explore the efficacy of the factor Xa inhibitors apixaban and rivaroxaban in people receiving concomitant antiplatelet therapy (Analysis 7.1). The number of strokes and systemic embolic events in people treated with the factor Xa inhibitors was significantly lower regardless of concomitant antiplatelet use at baseline or not: OR 0.81 (95% CI 0.66 to 0.98) and OR 0.69 (95% CI 0.61 to 0.78), respectively. We did, however, observe high and statistically significant heterogeneity in the analysis of the latter subgroup (I2 = 84%; P = 0.002). A sensitivity analysis with a random‐effects model also showed a statistically significant lower risk of strokes and systemic embolic events in this group (OR 0.58, 95% CI 0.36 to 0.93). Overall, we observed statistically significant differences in the number of stroke and systemic embolic events in this subgroup analysis (P = 0.05; I2 = 74%).
7.1. Analysis.
Comparison 7 Factor Xa inhibitors versus VKA: concomitant antiplatelet use, Outcome 1 Stroke and other systemic embolic events.
Subgroup analysis 14: major bleedings
The number of major bleedings was significantly lower in people treated with a factor Xa inhibitor who did not receive concomitant antiplatelet therapy at baseline (OR 0.80, 95% CI 0.70 to 0.91) (Analysis 7.2), however, we observed high, statistically significant heterogeneity (I2 = 75%; P = 0.0008). There was no statistically significant difference in the risk of major bleeding events in people receiving concomitant antiplatelet therapy (OR 0.93, 95% CI 0.80 to 1.09). Again, we observed high heterogeneity (I2 = 61%; P = 0.08). Sensitivity analyses with a random‐effects model did not show statistically significant differences in these two subgroups: OR 0.78 (95% CI 0.57 to 1.08) versus OR 0.94 (95% CI 0.70 to 1.26), respectively.
7.2. Analysis.
Comparison 7 Factor Xa inhibitors versus VKA: concomitant antiplatelet use, Outcome 2 Major bleedings.
Age
Subgroup analysis 15: stroke and systemic embolic events
We analysed the efficacy of treatment with some factor Xa inhibitors in two age categories: age less than 75 years versus 75 years or more (Analysis 8.1). The number of strokes and systemic embolic events was significantly lower in people aged 75 years or more with factor Xa inhibitors (OR 0.76, 95% CI 0.66 to 0.88). We found no statistically significant difference in participants aged less than 75 years (OR 0.96, 95% CI 0.84 to 1.09). Overall, there was a statistically significant difference in the number of strokes and systemic embolic events in this subgroup analysis (P = 0.02; I2 = 80%).
8.1. Analysis.
Comparison 8 Factor Xa inhibitors versus VKA: age, Outcome 1 Stroke and other systemic embolic events.
Subgroup analysis 16: major bleedings
The number of major bleedings was significantly lower with factor Xa inhibitors in both age categories (Analysis 8.2). Still, high and statistically heterogeneity was observed in both analyses (I2 = 81% and 89%; P < 0.0001, respectively). Sensitivity analyses with a random‐effects model showed a statistically significant difference in the number of major bleedings in people aged less than 75 years (OR 0.73, 95% CI 0.57 to 0.94), but not in people aged 75 years or more (OR 0.80, 95% CI 0.57 to 1.11).
8.2. Analysis.
Comparison 8 Factor Xa inhibitors versus VKA: age, Outcome 2 Major bleedings.
Sex
Subgroup analysis 17: stroke and systemic embolic events
We had data from four compounds to assess the efficacy of factor Xa inhibitors compared with warfarin in women and men. There was a similar and statistically significant reduction of the risk of strokes and systemic embolic events for both women (OR 0.84, 95% CI 0.73 to 0.98) and men (OR 0.87, 95% CI 0.76 to 0.98) treated with factor Xa inhibitors, and no statistically significant differences (Analysis 10.1). Overall, there was no statistically significant difference in the number of strokes and systemic embolic events in this subgroup analysis (P = 0.80; I2 = 0%).
10.1. Analysis.
Comparison 10 Factor Xa inhibitors versus VKA: sex, Outcome 1 Stroke and other systemic embolic events.
Subgroup analysis 18: major bleedings
An analysis of major bleeding events showed a statistically significantly lower risk in both women and men in favour of factor Xa inhibitors compared with warfarin (Analysis 10.2). There was, however, high and statistically significant heterogeneity in both analyses (I2 = 80% and 78%; P < 0.001, respectively). Pre‐specified sensitivity analyses with a random‐effects model showed no statistically significant differences in the number of bleedings in both sexes (men: OR 0.79, 95% CI 0.63 to 1.01; women: OR 0.72, 95% CI 0.52 to 1.00).
10.2. Analysis.
Comparison 10 Factor Xa inhibitors versus VKA: sex, Outcome 2 Major bleeding.
Baseline stroke risk (CHADS2 score)
Subgroup analysis 19: stroke and systemic embolic events
We had data from four agents (apixaban, edoxaban, idraparinux and rivaroxaban) to assess the efficacy in people with different baseline stroke risks based on their CHADS2 scores (Analysis 11.1). The risk of stroke and systemic embolic events was significantly lower in people with baseline CHADS2 scores of 3 or more (i.e. high risk of stroke) who were treated with factor Xa inhibitors compared with warfarin (OR 0.81, 95% CI 0.72 to 0.92). No statistically significant differences were observed in people with baseline CHADS2 scores of 0 to 1 (OR 0.90, 95% CI 0.61 to 1.32) and 2 (OR 0.92, 95% CI 0.78 to 1.08). Overall, there was no statistically significant difference in the number of strokes and systemic embolic events in this subgroup analysis (P = 0.49; I2 = 0%).
11.1. Analysis.
Comparison 11 Factor Xa inhibitors versus VKA: baseline CHADS2 score, Outcome 1 Stroke and other systemic embolic events.
Subgroup analysis 20: major bleedings
We also analysed the risk of major bleedings by baseline CHADS2 score (Analysis 11.2). In all three subcategories there were significantly lower numbers of major bleedings in participants treated with factor Xa inhibitors compared with warfarin (CHADS2 score 0 to 1: OR 0.69 (95% CI 0.53 to 0.90); CHADS2 score 2: OR 0.71 (95% CI 0.63 to 0.80); and CHADS2 score 3 or more: OR 0.88 (95% CI 0.79 to 0.98)). There was, however, high and statistically significant heterogeneity in these analyses (I2 ranging from 77% to 95%; P values < 0.001). Sensitivity analyses with a random‐effects model did not show any statistically significant differences in the number of bleedings between factor Xa inhibitors and warfarin in any of the subgroups: CHADS2 score 0 to 1: OR 1.34 (95% CI 0.20 to 9.09); CHADS2 score 2: OR 0.92 (95% CI 0.63 to 1.35); and CHADS2 score 3 or more: OR 0.91 (95% CI 0.70 to 1.19).
11.2. Analysis.
Comparison 11 Factor Xa inhibitors versus VKA: baseline CHADS2 score, Outcome 2 Major bleedings.
Race
Subgroup analysis 21: stroke and systemic embolic events
Most people included in the studies were either Asian (22%) or White (74%). We only observed a statistically significant lower number of stroke and systemic embolic events in Asian people treated with factor Xa inhibitors compared with warfarin (OR 0.73, 95% CI 0.56 to 0.94: Analysis 9.1). Overall, there was no statistically significant difference in the number of strokes and systemic embolic events in this subgroup analysis (P = 0.36; I2 = 62%).
9.1. Analysis.
Comparison 9 Factor Xa inhibitors versus VKA: race, Outcome 1 Stroke and other systemic embolic events.
Subgroup analysis 22: major bleedings
We observed statistically significant lower numbers of major bleedings in all races included in the studies treated with factor Xa inhibitors, but not in Black people (OR 0.91, 95% CI 0.45 to 1.87: Analysis 9.2).
9.2. Analysis.
Comparison 9 Factor Xa inhibitors versus VKA: race, Outcome 2 Major bleedings.
Discussion
Summary of main results
Primary and secondary analyses
We analysed data from 67,688 people with a confirmed diagnosis of atrial fibrillation (AF), who were included in 13 trials that examined the efficacy and safety of long‐term anticoagulation with a factor Xa inhibitor compared with vitamin K antagonists (VKAs).
Treatment with factor Xa inhibitors significantly reduced the number of strokes and systemic embolic events compared with dose‐adjusted warfarin. Still, the absolute overall effect in the reduction of stroke and systemic embolic events with factor Xa inhibitors was rather small, as shown by the high NNTs and NNTHs observed in the phase III studies of apixaban, rivaroxaban and edoxaban. The number of ischaemic strokes only was not significantly different when comparing the various factor Xa inhibitors with warfarin, although studies were not adequately powered for this subgroup analysis.
An analysis of all factor Xa inhibitors showed a statistically significant reduced risk of major bleedings compared with warfarin. However, there was a moderate to high degree of heterogeneity between the included studies. A pre‐specified sensitivity analysis excluding all open‐label studies showed that part of the observed heterogeneity was caused by the increased risk of major bleedings in one open‐label study of subcutaneously administered idraparinux (AMADEUS 2008). This study was stopped prematurely because of an increased risk of bleeding in the idraparinux treatment arm. Due to the premature termination of this study it is difficult to assess whether this was a false positive finding, or whether there is indeed an increased risk of bleeding associated with idraparinux. Other heterogeneity might be explained by baseline differences in the risk of bleeding between the study populations enrolled into the three largest trials that accounted for approximately 90% of the data included in this review (ARISTOTLE 2011; ROCKET AF 2011; ENGAGE AF‐TIMI 48 2013). When compared with people enrolled into ARISTOTLE 2011 and ENGAGE AF‐TIMI 48 2013, people enrolled into ROCKET AF 2011 were generally older (median age 70 and 72 years, versus 73 years), had higher baseline CHADS2 scores (mean 2.1 and 2.8, versus 3.8), had more often suffered a previous stroke or transient ischaemic attack (TIA) (19% and 28%, versus 55%), and more often used aspirin at baseline (31% and 29%, versus 38%). These are all known risk factors for (major) bleedings during anticoagulant treatment (Pisters 2010). In addition, the observed heterogeneity might also be partly explained by differences in the characteristics and bleeding profiles of apixaban, edoxaban and rivaroxaban.
Importantly, the number of intracranial haemorrhages (ICHs) was significantly lower in people treated with factor Xa inhibitors compared with warfarin. We observed high and statistically significant heterogeneity in this analysis, which appeared to be mainly caused by the increased number of ICHs in the prematurely halted open‐label study of idraparinux (AMADEUS 2008). A pre‐specified sensitivity analysis, in which we excluded data from this open‐label study, showed a clinically relevant and statistically significant reduction of the number of ICHs in people treated with a factor Xa inhibitor compared with warfarin without any signs of heterogeneity.
Treatment with factor Xa inhibitors also significantly reduced the number of deaths compared with warfarin, though this outcome was not reported in three smaller trials and was missing or unknown for a small number of participants (up to 2.1% ) in some of the other trials. Treatment with factor Xa inhibitors also did not appear to be associated with an increased risk of acute myocardial infarctions or vascular deaths compared with warfarin.
Subgroup analyses
Generally, the subgroup analyses for stroke and systemic embolic events supported the results from the main analysis, though no statistically significant reduction was seen in some important subgroups (e.g. people who had previously received VKA treatment, people aged under 75 years, and people with baseline CHADS2 scores of less than 3). The difference in major bleedings in most subgroups was also in line with findings from the main analysis that showed a statistically significant reduction with factor Xa inhibitors. High heterogeneity was, however, observed in many of the subgroup analyses for this outcome. Furthermore, many of the included subgroups (i.e. different dose levels of factor Xa inhibitors) contained no, or only very few, events and should therefore be interpreted cautiously.
We also performed a subgroup analysis comparing outcomes in people treated with apixaban, edoxaban and rivaroxaban versus 'high' and 'low' quality warfarin treatment. This analysis showed that a statistically significant reduction of thromboembolic events in people receiving these factor Xa inhibitors was only observed in comparison with 'low' quality warfarin treatment, although overall there was no statistically significant difference between these two subgroups. The number of major bleedings was significantly lower with these three factor Xa inhibitors regardless of the quality of the warfarin treatment. It should, however, be noted that the evidence for a reduction in bleedings compared with 'high' quality warfarin treatment is less robust due to high and statistically significant heterogeneity between the three factor Xa inhibitors included in this subgroup analysis. Interpretation of these analyses requires further caution since none of the included studies were adequately powered to detect differences between these subgroups.
Summary
In summary, factor Xa inhibitors appear to be an effective treatment for the prevention of stroke or systemic embolic events in people with AF who are eligible for long‐term anticoagulation. The absolute effect of factor Xa inhibitors compared with warfarin treatment was, however, rather small. Treatment with factor Xa inhibitors was associated with a reduction of deaths, ICHs and major bleedings compared with warfarin, although the evidence for a statistically significant reduction in major bleedings is less robust due to high heterogeneity between the included studies. Finally, even though effect estimates varied for the different factor Xa inhibitors, it is currently not possible to accurately determine which factor Xa inhibitor is the most effective and safe as head‐to‐head studies have not been performed.
Overall completeness and applicability of evidence
All data that were used in this review are derived from studies that randomised people with a confirmed diagnosis of AF, who were all deemed eligible for long‐term anticoagulation with warfarin by the randomising physician. The mean CHADS2 score of the randomised participants was around 2.7 (range 1.9 to 3.5), suggesting that few, if any, people with AF who did not need anticoagulation for prevention of thromboembolic events (a so‐called 'truly low risk population') were included in the studies. Reported median time in therapeutic range (TTR) values ranged from 45% to 89% in the included studies, but varied between regions and study centres. In general, the observed TTRs are comparable with those seen in older studies that used dose‐adjusted warfarin for preventing stroke and systemic embolic events in people with AF, and with data from clinical practice. The majority of included studies did not state an upper age limit as a contraindication and the mean and median ages of randomised participants ranged between 65 and 74 years. Based on these observations, we can be reasonably confident that this review covers a clinically relevant population of people with AF eligible for long‐term anticoagulation that also appears generalisable to a 'real world' setting.
The CHA2DS2‐VASc score was not used in any of the included studies to assess the risk of stroke. Recent guidelines prefer the use of this score over the simpler CHADS2 score and recommend that anticoagulation with either an oral anticoagulant (i.e. apixaban, dabigatran, edoxaban or rivaroxaban) or a VKA should be considered in people with a CHA2DS2‐VASc score of 1 or more in men and 2 or more in women (ACC/AHA/HRS 2014; ESC 2016). There is some evidence that the CHA2DS2‐VASc scale is better at identifying people with a 'very low' risk of stroke than the older CHADS2 score (ESC 2016). Consequently, data from people who are at a 'very low' risk of stroke are most probably not included in this review. Caution is thus needed when drawing any conclusions on the effectiveness and safety of factor Xa inhibitors in people that fall into this 'very low risk' category.
Data on participants with severe renal failure (i.e. creatinine clearance < 30 ml/minute) and on haemodialysis, who are known to have a higher risk of both thromboembolic events and bleedings, are also scarce in this review because these people were excluded from participation in most of the included trials.
Finally, we have included data from seven different factor Xa inhibitors in this review. Still, the majority of the data (approximately 90%) are from only three factor Xa inhibitors: apixaban, edoxaban and rivaroxaban. Results from the presented analyses of the other factor Xa inhibitors are based on smaller data sets and therefore considered less robust.
Quality of the evidence
The studies included in this review were generally large to very large; the smallest study enrolled 222 participants. Only one of the 13 included studies was conducted in an open‐label fashion. The remaining studies were either double‐masked or partially‐masked. Most studies used centralised, blinded adjudication committees for the assessments of the primary safety and efficacy outcomes. Furthermore, outcome data from the (larger) studies appear generally consistent. Based on these considerations, the overall quality of the body of evidence assessed in this review is considered high.
Potential biases in the review process
We contacted lead authors and sponsors in order to gather any non‐reported data from relevant studies. Disappointingly, such additional data were only provided for two studies (AMADEUS 2008 and J‐ROCKET AF 2012).
We also used data from licensing applications that were submitted to the European Medicines Agency (EMA) and the Food and Drug Administration (FDA) for the compounds apixaban, edoxaban and rivaroxaban in our analyses. Data from these three compounds comprise approximately 90% of all data that were analysed in our review. The use of these unpublished data has significantly decreased the potential for publication biases in our review.
We also carried out thorough searches of several different databases to further avoid selection bias. There is, however, a small possibility that we still might have missed some (smaller) studies.
Agreements and disagreements with other studies or reviews
We compared our findings with the results from several other recently published meta‐analyses and systematic reviews that examined the efficacy and safety of factor Xa inhibitors compared with warfarin in people with AF (Biondi‐Zoccai 2013; Capodanno 2013; Dogliotti 2013; Kwong 2013; Lega 2013; Mitchell 2013; Tahir 2013; Cameron 2014; Fu 2014; Providencia 2014; Ruff 2014; Caldeira 2015; Gomez‐Outes 2015; Lin 2015; Morimoto 2015; Almutairi 2016; Garg 2016; Lip 2016; Liu 2016; Tawfik 2016; Xiang 2016). Findings from these studies are generally in agreement with the findings and conclusions from our review, notably for the primary efficacy and safety outcomes. These meta‐analyses and reviews were, however, less comprehensive than our review because they all included data from fewer studies and fewer factor Xa inhibitors, performed fewer (subgroup) analyses and also did not appear to have used unpublished data that were submitted to regulatory bodies (i.e. EMA and FDA).
Authors' conclusions
Implications for practice.
Overall, there is a net clinical benefit of treatment with factor Xa inhibitors in people with atrial fibrillation (AF), as treatment seems to be associated with a relatively small reduction in the number of strokes and systemic embolic events and a more pronounced reduction in the number of intracranial haemorrhages, other major bleedings and deaths compared with dose‐adjusted warfarin.
Despite the apparent overall net clinical benefit, the currently available efficacy and safety data do not provide sufficient evidence to determine the most optimal factor Xa inhibitor, since head‐to‐head studies have not yet been performed.
Caution is needed when drawing any firm conclusions concerning the net clinical benefit for people with a 'very low risk' for thromboembolic events (i.e. low CHA2DS2‐VASc scores), people with severe renal impairment, or people on haemodialysis, as these populations were generally not included in the studies analysed in our review.
Implications for research.
Future studies could aim to:
further determine the effectiveness and safety of long‐term anticoagulation treatment with a factor Xa inhibitor (i.e. beyond two years) in a 'real world' population of people with AF;
directly compare the efficacy and safety of two (or more) factor Xa inhibitors;
identify means of further minimising the risk of major and non‐major bleedings without reducing the benefit of factor Xa inhibitors;
further assess the efficacy and safety of factor Xa inhibitors in people with a 'very low risk' for thromboembolic events (i.e. low CHA2DS2‐VASc scores);
provide more data on the effectiveness, health‐related quality of life and cost‐effectiveness of treatment with factor Xa inhibitors compared with VKAs for prevention of thromboembolic events in people with AF, also in various settings (e.g. comparing centres with 'high' versus 'low' quality warfarin administration);
further develop and validate reliable assays to monitor the effect of factor Xa inhibitors (e.g. prior to acute surgery or to assess adherence to therapy).
Feedback
New Feedback, 20 June 2014
Summary
In reviewing "Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation (Review)"1 it seems to us that the choice of outcomes may have been driven in part by how they were defined within the clinical trials, rather than via a hierarchy of outcomes pre‐specified by the reviewers. For example, the "diagnosis of myocardial infarction was based upon electrocardiographic changes, elevation of enzymes or confirmation during post‐mortem examination." Simply choosing the outcome definition as reported in clinical trials may compel readers to draw conclusions on outcomes even if they are not defined in a clinically meaningful way. In this particular case one could argue that biomarker or ECG defined myocardial infarctions (MIs) are less important to patients than symptomatic MIs resulting in hospitalization and disability. In this review the conclusion is that MI risk is not increased with this class of drugs. However, it remains entirely possible that symptomatic MI risk is increased while asymptomatic MI is decreased, leading to a conclusion of "no difference". It might be preferable to collect information on all MIs and discuss and analyze the implications for differences in the way clinical trials actually report and define MI.
While the review1 concludes that "[t]here was no indication of selective reporting in any of the included studies", we suggest that there is indeed selective outcome reporting in the identified trials and that this has serious implications for drug‐ therapy decision making. First, it is reported that disabling or fatal strokes were only reported in four of 10 trials, i.e., for 16,099 participants. It would seem highly unlikely that a clinical trial would not categorize strokes in this way and that it is possible that this information might be available in sources other than the published clinical trial report. Did the review authors attempt to contact the trial authors? Have the relevant FDA drug approval packages been searched for this data?2 Given the ARISTOTLE trial3 enrolled over 18,000 participants, but is not counted in this outcome analysis, a more comprehensive search for this data seems warranted. Last, the addition of nine more disabling or fatal strokes to the Factor Xa inhibitor side of the analysis renders the finding non‐statistically significant and highlights that the magnitude of the reported effect is small relative to the size of the missing data. Until this data is acquired we feel it is premature to draw any conclusions on these types of strokes.
We have equal concern with respect to the all‐cause death analysis. Only six of 10 trials reported on this outcome, yet the review authors fail to recommend caution in the interpretation of their finding that "Factor Xa inhibitors significantly reduced the number of all‐cause deaths compared with warfarin"1. In addition, we note in the risk of bias assessment for the ARISTOTLE trial3 that a judgment of low risk is assigned for incomplete outcome data (attrition bias). The support for judgment reads "[e]fficacy and safety outcomes analysed in ITT population. Number of participants with missing data on vital status and reasons reported. Number of participants that discontinued during study and reasons are reported". It is true that in the main publication of the ARISTOTLE trial3 that the number of participants with missing data on vital status is reported: 380 patients or 2.1% of the originally randomized population. It is unclear to us though how this might equate a low risk of bias given that the proportion of participants with missing vital status (2.1%) is five times greater than the difference in mortality reported between the two groups in this trial (absolute difference 0.4%). This missingness of course was of great importance in the US FDA review of apixaban4. One reviewer concluded that "[t]he alleged death benefit of apixaban compared to warfarin is fragile as reported by the sponsor, i.e., p = 0.046, a change in only one death rendering the difference significantly insignificant. Furthermore, the validity of this fragile benefit depends upon having 100% valid data. The substantial missing vital status follow‐up, the problems with data recordings, and the lack of a significant death benefit for warfarin destroy confidence that apixaban reduces all‐cause mortality" 4. Given that the ARISTOTLE trial contributes substantially to the all‐cause death analysis we feel the findings from the US FDA review should serve to temper confidence in this suggested benefit of Factor Xa inhibitors as compared to warfarin.In the main publications of the ARISTOTLE and ROCKET‐ AF trials3,5 it is indicated that major bleeds were only counted if they occurred within 48 hours of the last dose of assigned study drug. This truncated duration of follow up raises some concerns. First, this is not congruent with the principle of intention‐to‐treat analysis. A bleed occurring 60 hours after the last dose of study drug, for example, would not be counted as a consequence of the intervention even though it could indeed be a result of treatment with the study drug. Thus all major bleeds that happened to patients enrolled in these two large RCTs may not have been captured. This should be addressed in the risk of bias assessment, discussion, and conclusion sections of the review.
Further, the review reports that intracranial hemorrhages "includes all intraparenchymal, subdural and epidural hematomas, subarachnoid haemorrhages confirmed by neuroimaging or post‐mortem examination". Were the reviewers able to find this detailed level of diagnostic criteria in each of the studies included in this outcome analysis? We could not.
Last, while the FDA reviewers performed a sensitivity analysis to hypothetically estimate the impact of dispensing errors that occurred in the ARISTOTLE trial4 on some outcomes, this was not performed for all outcomes (i.e., it was not performed for intracranial hemorrhages). This should be discussed given the importance of this outcome in drug‐therapy decision making and it surely warrants consideration in the risk of bias assessment of this large trial. In fact, we find no mention of the dispensing errors that occurred in the ARISTOTLE trial in this review and we suggest referral to the "Submission Quality and Integrity" section of FDA drug approval packages be considered an essential starting point in the systematic review of this data6.
We were extremely pleased to see attention drawn to the inability to extrapolate the results of these trials to very low risk people given the lowering threshold for treatment occurring in current clinical practice guidelines.
Thank you for the opportunity to share our concerns.
Cait O'Sullivan (PharmD, BScPh, BA) Clinical Pharmacist Island Health Clinical Pharmacy Programs Aaron M Tejani (BSc(Pharm), PharmD) Researcher Therapeutics Initiative, University of British Columbia
We certify that we have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of this feedback.
References
Bruins Slot KMH, Berge E. Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation (Review). Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No.:CD008980.
Turner EH. How to access and process FDA drug approval packages for use in research. BMJ 2013;347:f5992.
Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. New England Journal of Medicine 2011;365:981‐92.
U.S. Food and Drug Administration. Apixaban Medical Review. NDA 202155 [Internet]. 2012 [cited 2014 May 11]. Available from http://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202155Orig1s000MedR.pdf.
Patel MR, Mahaffey KW, Garg Y, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. New England Journal of Medicine 2011;365;883‐91.
U.S. Food and Drug Administration. Drugs@FDA. FDA Approved Drug Products. [Internet]. Available from http://www.accessdata.fda.gov/Scripts/cder/drugsatfda/index.cfm.
Reply
We thank the authors for their comprehensive and constructive feedback on our review. We have addressed their comments below.
[1] In reviewing "Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation (Review)" it seems to us that the choice of outcomes may have been driven in part by how they were defined within the clinical trials, rather than via a hierarchy of outcomes pre‐specified by the reviewers. For example, the "diagnosis of myocardial infarction was based upon electrocardiographic changes, elevation of enzymes or confirmation during post‐mortem examination". Simply choosing the outcome definition as reported in clinical trials may compel readers to draw conclusions on outcomes even if they are not defined in a clinically meaningful way. In this particular case one could argue that biomarker or ECG defined myocardial infarctions (MIs) are less important to patients than symptomatic MIs resulting in hospitalization and disability. In this review the conclusion is that MI risk is not increased with this class of drugs. However, it remains entirely possible that symptomatic MI risk is increased while asymptomatic MI is decreased, leading to a conclusion of "no difference". It might be preferable to collect information on all MIs and discuss and analyze the implications for differences in the way clinical trials actually report and define MI.
Reply: The outcomes that were used in our review were all pre‐specified in the protocol that was published in 20111, and the definitions were not driven by the definitions used in the trials. Outcome definitions were based on those that were used in Cochrane reviews in the same therapeutic area2,3. Use of similar outcome definitions might enable and facilitate a cross‐review comparison of the results. Furthermore, we used outcome definitions that are widely used in clinical practice and in other clinical trials.
The definition of myocardial infarctions (MIs) that was used in our review is similar to the one used by the World Health Organisation (WHO) to define MIs, i.e. a patient is diagnosed with MI if two (probable) or three (definite) of the following criteria are satisfied:
Clinical history of ischaemic type chest pain lasting for more than 20 minutes
Changes in serial ECG tracings
Rise and fall of serum cardiac biomarkers
This definition captures the symptomatic MIs (as pointed out by the reviewers), but also underlines that the diagnosis should not be based on symptoms only, as these might vary considerably between patients and might even be absent in others (i.e., "silent" MI). This definition may not capture the small proportion of patients with truly "silent" MIs, but we agree with the comment that the symptomatic MIs are what are most relevant for patients, and are the ones that should be assessed in the review.
[2] While the review concludes that "[t]here was no indication of selective reporting in any of the included studies", we suggest that there is indeed selective outcome reporting in the identified trials and that this has serious implications for drug‐therapy decision making. First, it is reported that disabling or fatal strokes were only reported in four of 10 trials, i.e., for 16,099 participants. It would seem highly unlikely that a clinical trial would not categorize strokes in this way and that it is possible that this information might be available in sources other than the published clinical trial report. Did the review authors attempt to contact the trial authors? Have the relevant FDA drug approval packages been searched for this data? Given the ARISTOTLE trial enrolled over 18,000 participants, but is not counted in this outcome analysis, a more comprehensive search for this data seems warranted. Last, the addition of nine more disabling or fatal strokes to the Factor Xa inhibitor side of the analysis renders the finding non‐statistically significant and highlights that the magnitude of the reported effect is small relative to the size of the missing data. Until this data is acquired we feel it is premature to draw any conclusions on these types of strokes.
Reply: We agree that it is important to identify the disabling and fatal strokes. We did contact the sponsors of the clinical trials and the principle investigators to provide more data on disabling or fatal strokes, but in nearly all cases this was not provided. According to published protocols this outcome was not systematically collected in some studies (e.g. the large ARISTOTLE trial), and this is not unusual for large clinical cardiovascular prevention trials (mixed primary and secondary prevention).
We did not search the drug approval packages that have been submitted to regulatory authorities (US FDA and the European Medicines Agency), but plan to do this when we update the review later this year. We also plan to re‐contact the sponsors/principle investigators of all studies to collect these data. Hopefully, we will then be able to draw better conclusions on this clinically relevant outcome.
[3] We have equal concern with respect to the all‐cause death analysis. Only six of 10 trials reported on this outcome, yet the review authors fail to recommend caution in the interpretation of their finding that “Factor Xa inhibitors significantly reduced the number of all‐cause deaths compared with warfarin".
Reply: Again, we agree that this is a very important effect variable and we regret that it was not possible to get data from four of the included studies that enrolled 3160 patients in total. As with the disabling stroke‐outcome we plan to collect more data for the planned update of the review later this year. Still, considering that we had data on 92.5% of all included patients (n = 38,924) for this outcome, we consider that our interpretation is valid and supported by relatively robust data.
[4] In addition, we note in the risk of bias assessment for the ARISTOTLE trial that a judgment of low risk is assigned for incomplete outcome data (attrition bias). The support for judgment reads "[e]fficacy and safety outcomes analysed in ITT population. Number of participants with missing data on vital status and reasons reported. Number of participants that discontinued during study and reasons are reported". It is true that in the main publication of the ARISTOTLE trial that the number of participants with missing data on vital status is reported: 380 patients or 2.1% of the originally randomized population. It is unclear to us though how this might equate a low risk of bias given that the proportion of participants with missing vital status (2.1%) is five times greater than the difference in mortality reported between the two groups in this trial (absolute difference 0.4%). This missingness of course was of great importance in the US FDA review of apixaban. One reviewer concluded that “[t]he alleged death benefit of apixaban compared to warfarin is fragile as reported by the sponsor, i.e., P = 0.046, a change in only one death rendering the difference significantly insignificant. Furthermore, the validity of this fragile benefit depends upon having 100% valid data. The substantial missing vital status follow‐up, the problems with data recordings, and the lack of a significant death benefit for warfarin destroy confidence that apixaban reduces all‐cause mortality", Given that the ARISTOTLE trial contributes substantially to the all‐cause death analysis we feel the findings from the US FDA review should serve to temper confidence in this suggested benefit of Factor Xa inhibitors as compared to warfarin.
Reply: We thank you for pointing this out and agree that this hampers the interpretation of mortality data from the ARISTOTLE trial. We will address this issue in the update of the review.
[5] In the main publications of the ARISTOTLE and ROCKET‐AF trials it is indicated that major bleeds were only counted if they occurred within 48 hours of the last dose of assigned study drug. This truncated duration of follow up raises some concerns. First, this is not congruent with the principle of intention‐to‐treat analysis. A bleed occurring 60 hours after the last dose of study drug, for example, would not be counted as a consequence of the intervention even though it could indeed be a result of treatment with the study drug. Thus all major bleeds that happened to patients enrolled in these two large RCTs may not have been captured. This should be addressed in the risk of bias assessment, discussion, and conclusion sections of the review.
Reply: We thank the reviewers for this comment. We will look more critically into the various definitions of (major) bleedings that were used in the included studies and discuss this in more detail in the update of the review.
[6] Further, the review reports that intracranial hemorrhages "includes all intraparenchymal, subdural and epidural hematomas, subarachnoid haemorrhages confirmed by neuroimaging or post‐mortem examination". Were the reviewers able to find this detailed level of diagnostic criteria in each of the studies included in this outcome analysis? We could not.
Reply: Neuroimaging and/or autopsy was performed in all of the included studies to diagnose intracranial haemorrhages. According to the study protocols and/or publications of the results that we reviewed intracranial haemorrhages were diagnosed based on the identification of a haemorrhage in the parenchymal, subdural, epidural and subarachnoid regions. In the larger studies, which contributed the majority of intracranial haemorrhages, blinded neuroimaging committees also adjudicated this outcome. We have no reason to doubt that the haemorrhages were classified in accordance with the protocols, and therefore consider the available data on intracranial haemorrhages to be robust.
[7] Last, while the FDA reviewers performed a sensitivity analysis to hypothetically estimate the impact of dispensing errors that occurred in the ARISTOTLE trial on some outcomes, this was not performed for all outcomes (i.e., it was not performed for intracranial hemorrhages). This should be discussed given the importance of this outcome in drug‐therapy decision making and it surely warrants consideration in the risk of bias assessment of this large trial. In fact, we find no mention of the dispensing errors that occurred in the ARISTOTLE trial in this review and we suggest referral to the "Submission Quality and Integrity" section of FDA drug approval packages be considered an essential starting point in the systematic review of this data.
Reply: As mentioned previously, we only used published data in our review and did not have access to drug approval packages that have been submitted to regulatory authorities or assessment reports of these data by regulatory authorities. At the time of our analysis we were thus not aware of this issue (dispensing errors in the ARISTOTLE trial) and were not able to assess and further discuss this. We will take this issue into account in the update of our review.
[8] We were extremely pleased to see attention drawn to the inability to extrapolate the results of these trials to very low risk people given the lowering threshold for treatment occurring in current clinical practice guidelines.
Reply: Thanks for your feedback.
References:
Bruins Slot KMH, Berge E. Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation (Protocol). Cochrane Database of Systematic Reviews 2011, Issue 2. Art. No.: CD008980. DOI: 10.1002/14651858.CD008980.
Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non‐valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database of Systematic Reviews 2007, Issue 3. Art. No.: CD006186. DOI: 10.1002/14651858.CD006186.pub2.
Saxena R, Koudstaal PJ. Anticoagulants versus antiplatelet therapy for preventing stroke in patients with nonrheumatic atrial fibrillation and a history of stroke or transient ischemic attack. Cochrane Database of Systematic Reviews 2004, Issue 4. Art. No.: CD000187. DOI: 10.1002/14651858.CD000187.pub2.
Contributors
Feedback: Cait O'Sullivan, Aaron M Tejani Responses: Karsten MH Bruins Slot, Eivind Berge
New feedback added, 16 February 2015
Summary
We read with interest the review on factor Xa Inhibitors versus vitamin K antagonists (VKA) for preventing cerebral or systemic embolism in patients with atrial fibrillation (1) and there are a few points we wish to address.
[1] The majority of the outcome data for major bleeding included in the review comes from the results of single, randomized controlled trials of apixaban (2: ARISTOTLE) and rivaroxaban (3: ROCKET‐AF). For the primary outcome of stroke and systemic embolism, the review calculates a number‐needed‐to‐treat (NNT) for rivaroxaban of 369 per year, indicating that 369 people need to be treated with rivaroxaban instead of dose‐adjusted warfarin for one year to prevent one stroke or systemic embolism. We find the inclusion of an NNT in this case inappropriate. In the ROCKET‐AF trial, the rates of the primary efficacy outcome between rivaroxaban and warfarin were found to be not statistically significant and met the criteria for non‐inferiority, not superiority. Presenting a NNT infers that rivaroxaban is superior to warfarin and that an estimated number of patients can be treated with rivaroxaban instead of warfarin to prevent the primary outcome, which is misleading. If an NNT is calculated on non‐statistically significant results, one of the confidence intervals will indicate benefit and the other harm (Cochrane Handbook). We would recommend removing the NNT data presented altogether to avoid misinterpretation. In the case you disagree, confidence intervals should be added for qualification. In addition, a description of how the NNT was calculated would be helpful as we could not replicate the findings given the available trial data.
[2] In the conclusion of the review, it states that “factor Xa inhibitors lower the risk of major bleedings”. We disagree with this statement. In the included ROCKET‐AF trial (3), rivaroxaban was found to have similar rates of all‐cause mortality and major and clinically relevant non‐major bleeding. However, the main limitation of the ROCKET‐AF trial was the poor INR control in the warfarin groups, with the mean time in therapeutic range (TTR) reported as 55%. The poor INR control in the warfarin arm biases the results in favor of rivaroxaban and questions whether or not rivaroxaban was adequately compared to the standard of care. Efficacy of warfarin in preventing thrombotic events and safety in terms of bleeding risk is dependent on the quality of INR control. The review planned a subgroup analysis in patients that received VKA treatment and had data regarding TTR greater than or less than 60%, however, only data from the ROCKET‐AF trial was available and presented. An analysis of center‐TTR quartile in the ROCKET‐AF trial found in the supplementary appendix, the authors state that the effect of rivaroxaban did not differ across quartiles and that at the highest quartile with the best INR control, the hazard ratio of 0.74 (0.49‐1.12) still favored rivaroxaban compared to warfarin (Table 5 in the Supplementary Appendix). In contrast, the analysis in the FDA medical review (4) demonstrates that when warfarin administration was associated with TTR greater than 68%, there was actually a relative increase in primary outcome events in the rivaroxaban group with point estimates of the hazard ratio greater than 1 and wide confidence intervals. Similarly, the FDA’s United States subgroup analysis (mean TTR 63%) showed a statistically significant increase in the number of major bleeding events in the rivaroxaban arm (4). In addition, major bleeding events were recorded in the ROCKET‐AF up until two days after the last dose which we feel would lead to missed bleeding events. The FDA medical review also includes major bleeding events up to thirty days after the last dose which is more likely to capture events and we are curious as to what data the authors included in their analysis. Overall, we are not convinced that rivaroxaban decreases major bleeding risk. Given that the relatively poor INR control in the warfarin arm is a major limitation of the ROCKET‐AF trial, we recommend that it be included in the discussion when interpreting the results. The following statement, “the benefits of rivaroxaban in preventing stroke and systemic embolic events compared to warfarin are more or less consistent regardless of warfarin administration” should also be revised. Another consideration is that the finding of a significant benefit in major bleeding risk with the factor Xa inhibitors in the review depended on what type of analysis was conducted; fixed‐effect or random‐effect. Even with a sensitivity analysis that removed the open‐label trial, there was still considerable heterogeneity. We appreciate the reviewers for exploring the possible causes of the heterogeneity (e.g. bleeding risk was higher in ROCKET‐AF given patient population), however, given that the results were not robust or consistent with various analyses, we would avoid a blanket statement of reduced major bleeding risk with factor Xa inhibitors in all patients as it is misleading.
[3] Even if the analyses were straightforward, the fact that both the apixaban and rivaroxaban RCTs have yet to be replicated should caution any firm conclusions on the safety and efficacy of these agents. The inclusion of serious adverse events (SAEs) would have been helpful to represent the overall net impact of factor Xa Inhibitors, including rivaroxaban. Unfortunately, the ROCKET‐AF trial inappropriately excluded the clinical efficacy endpoints of ischemic stroke, systemic embolism or myocardial infarction as SAEs, making an accurate interpretation of the net benefit or harm difficult.
[4] Finally, since the data from the ROCKET‐AF trial does not adequately support that rivaroxaban is as effective as warfarin used skilfully (4), it may be more appropriate to conclude that rivaroxaban may be an alternative to those who refuse warfarin therapy or cannot comply with warfarin monitoring.
Thank you for your attention to our concerns.
References:
Bruins Slot KMH, Berge E. Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation (Review). Cochrane Database of Systematic Reviews 2013, Issue 8. Art. No.:CD008980.
Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. New England Journal of Medicine 2011; 365:981‐92.
Patel MR, Mahaffey KW, Garg Y, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in non‐valvular atrial fibrillation. New England Journal of Medicine 2011;365:883‐91.
U.S Food and Drug Administration. Drugs@FDA. FDA Approved Drug Products. [Internet]. Available from http://www.accessdata.fda.gov/scripts/cder/drugsatfda/
Patel MR, Mahaffey KW, Garg Y, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in non‐valvular atrial fibrillation. Supplementary appendix. New England Journal of Medicine 2011;365:883‐91. Available at http://www.nejm.org/doi/full/10.1056/NEJMoa1009638.
Therapeutics Initiative. Serious Adverse Event Analysis: Lipid‐Lowering Therapy Revisited. Therapeutics Letter Issue 42, Aug‐Oct 2001.
Reply
We thank the authors for the critical review of our meta‐analysis. We have addressed their comments and feedback below.
[1] The NNT for rivaroxaban was calculated with an online NNT‐calculator (http://www.calctool.org/CALC/prof/medical/NNT). We entered the following variables to calculate the NNT for the entire trial period of 1.9 years: 269 events for 7081 patients treated with rivaroxaban, and 306 events for 7090 patients treated with warfarin. This gave a NNT for the 1.9 year period of 193.409, which then was rounded up to 194 as is customary and recommended in the literature (e.g. Sackett et al 1996). Since the NNT of 194 is for a 1.9 year treatment period, the NNT per year is calculated by multiplying 194 with 1.9. By doing this we assumed that the event rate is constant over time.
We agree with the comment that this NNT should be interpreted with caution, since superiority of rivaroxaban over warfarin for the primary efficacy outcome was not shown in the ROCKET‐AF trial and will add confidence intervals and a note of caution (i.e. non‐significance of the results) to the next version of the review. We feel that mentioning the NNT is of interest for the reader, as it indicates that any differences in the number of stroke and SEEs of factor Xa inhibitors (including rivaroxaban) compared with warfarin are rather marginal, since (well‐regulated) warfarin appears to be a highly effective drug.
[2] In our review we have not concluded that "factor Xa inhibitors lower the risk of major bleedings". Instead, we concluded in the Summary of main results that "factor Xa inhibitors appear to reduce the number of major bleedings and intracranial haemorrhages compared with warfarin, though the evidence for a statistically significant reduction in major bleedings is less robust." We have also stated in the Authors’ conclusions section that "… overall, there is a small net clinical benefit of treatment with factor Xa inhibitors in people with AF as it leads to a reduction of strokes and systemic embolic events and also seems to lower the risk of major bleedings (including intracranial haemorrhages) compared with dose‐adjusted warfarin." We have thus not categorically stated that factor Xa inhibitors lower the risk of major bleedings, as is suggested in the comment, but have used more careful wordings on this important issue in our review.
Unfortunately, we only had data from the ROCKET‐AF study for the pre‐specified subgroup analysis in patients who received VKA treatment with time‐in‐therapeutic range (TTR) equal to or greater than 60% versus less than 60%, and have not used data from the FDA medical review. We agree with the comments concerning the limitations of this subgroup analysis and plan to include a revised analysis on the quality of anticoagulation with warfarin in the next version of the review. This new analysis will hopefully include data of more factor Xa inhibitors (i.e. apixaban and edoxaban) and also non‐published data that was submitted to the European Medicines Agency (EMA). If poor INR control continues to be a limitation we will include this in the discussion when interpreting the results. We will also carefully reconsider the reasons for heterogeneity and our statement that "the benefits of rivaroxaban in preventing stroke and systemic embolic events compared to warfarin are more or less consistent regardless of warfarin administration".
[3] We planned to perform an analysis of 'Other adverse events' in our review, as stated in the review’s protocol and Methods section. Unfortunately, there were very few studies that presented these data systematically. We therefore have chosen to focus on bleeding and major cardiovascular adverse events and deaths in our review. Depending on the availability of more specific data on 'other' adverse events (e.g. hepatotoxicity) in non‐published data submitted to the EMA for the compounds rivaroxaban, edoxaban and apixaban we plan to update this analysis.
[4] In our conclusions of the review, we have given the results for all factor Xa inhibitors combined, and not for the individual compounds, such as rivaroxaban. We conclude that factor Xa inhibitors appear to be an effective treatment for the prevention of stroke or other systemic embolic events in people with AF who are eligible for long‐term anticoagulation. This conclusion is based on the available data for all six included factor Xa inhibitors that showed a statistically significant reduction of the number of strokes and SEEs compared with warfarin (OR 0.81, 95% CI 0.72 to 0.91). We have not specifically mentioned that rivaroxaban is as effective as warfarin. We did, however, state that based on the assessed data we are currently not able to determine which of the available factor Xa inhibitors is the most effective and safe.
Contributors
Feedback: Sarah Burgess and Aaron M Tejani Responses: Karsten MH Bruins Slot, Eivind Berge
What's new
| Date | Event | Description |
|---|---|---|
| 29 August 2017 | New search has been performed | We performed updated literature searches of the Cochrane Stroke Group and the Cochrane Heart Group Trials Registers (September 2016), the Cochrane Central Register of Controlled Trials (CENTRAL) (August 2017), MEDLINE (1950 to April 2017) and Embase (1980 to April 2017). We have added three new trials that included 25,064 people. We have now included data from 67,688 people randomised into 13 trials. |
| 24 April 2017 | New citation required but conclusions have not changed | No changes in main conclusions of this updated review compared with the previous version (2013). |
History
Protocol first published: Issue 2, 2011 Review first published: Issue 8, 2013
| Date | Event | Description |
|---|---|---|
| 1 October 2014 | Amended | Correction to name in Feedback section |
| 20 June 2014 | Feedback has been incorporated | See the Feedback section |
Acknowledgements
We thank Brenda Thomas (Cochrane Stroke Group) for her help in developing the original search strategies, and the peer reviewers of the original manuscript and update for their constructive feedback.
We also thanks Marita Heintz (Norwegian Institute of Public Health) for updating the search strategies and performing new literature searches in 2016/2017.
Appendices
Appendix 1. CENTRAL (The Cochrane Library)
#1 MeSH descriptor: [Coumarins] explode all trees
#2 4‐Hydroxycoumarin* or warfarin* or acenocoumarol or nicoumalone or sinthrome or Sintrom or phenindione or dicoumarol or coumadin or phenprocoumon or phepromaron or ethyl‐biscoumacetate or phenindione or Diphenadione or Tioclomarol or Racumi or Marcoumar or Marcumar or Falithrom or Jantoven or vitamin K antagonist* or VKA or fluindione or difenacoum or coumatetralyl)
#3 factor Xa inhibitor*
#4 antistastin or rivaroxaban or Xarelto or apixaban or Eliquis or Edoxaban or Savaysa or Betrixaban or fondaparinux or idraparinux or lixiana or exanta or Darexaban or Otamixaban* or Razaxaban or YM 150 or DU‐176b or LY517717 or SSR126517E
#4 #1 or #2 or #3
#5 MeSH descriptor: [atrial fibrillation or atrial flutter] explode all trees
#6 #5 or #6
#7 #6 and #4
Appendix 2. MEDLINE (Ovid) search strategy
1. atrial fibrillation/ or atrial flutter/ 2. ((atrial or auricular) adj5 (fibrillation$ or flutter$)).tw. 3. AF.tw. 4. 1 or 2 or 3 5. factor Xa/ai 6. ((factor Xa or factor 10a or fXa or autoprothrombin c or thrombokinase) adj5 inhib$).tw. 7. (activated adj5 (factor X or factor 10) adj5 inhib$).tw. 8. xabans.tw. 9. (antistasin or apixaban or betrixaban or du 176b or eribaxaban or fondaparinux or idraparinux or otamixaban or razaxaban or rivaroxaban or yagin or ym 150 or ym150 or LY517717 or darexaban or edoxaban or SSR126517E).tw. 10. (antistasin or apixaban or betrixaban or du 176b or eribaxaban or fondaparinux or idraparinux or otamixaban or razaxaban or rivaroxaban or yagin or ym 150 or ym150 or LY517717 or darexaban or edoxaban or SSR126517E).nm. 11. 5 or 6 or 7 or 8 or 9 or 10 12. Warfarin/ 13. (warfarin$ or adoisine or aldocumar or athrombin$ k or carfin or coumadin$ or coumafene or coumaphene or jantoven or kumatox or lawarin or marevan or panwarfarin or panwarfin or prothromadin or sofarin or tedicumar or tintorane or waran or warfant or warfilone or warnerin).tw. 14. (warfarin$ or adoisine or aldocumar or athrombin$ k or carfin or coumadin$ or coumafene or coumaphene or jantoven or kumatox or lawarin or marevan or panwarfarin or panwarfin or prothromadin or sofarin or tedicumar or tintorane or waran or warfant or warfilone or warnerin).nm. 15. exp Vitamin K/ai [Antagonists & Inhibitors] 16. (vitamin K antagonist$ or VKA or VKAs).tw. 17. 4‐hydroxycoumarins/ or acenocoumarol/ or coumarins/ or dicumarol/ or ethyl biscoumacetate/ or phenindione/ or phenprocoumon/ 18. (coumarin$ or cumarin$ or phenprocoum$ or phenprocum$ or dicoumar$ or dicumar$ or acenocoumar$ or acenocumar$ or fluindione or phenindione or clorindione or diphenadione).tw. 19. (coumarin$ or cumarin$ or phenprocoum$ or phenprocum$ or dicoumar$ or dicumar$ or acenocoumar$ or acenocumar$ or fluindione or phenindione or clorindione or diphenadione).nm. 20. 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 21. 4 and 11 and 20
Appendix 3. Embase (Ovid) search strategy
1. exp heart atrium fibrillation/ or heart atrium flutter/ 2. ((atrial or auricular) adj5 (fibrillation$ or flutter$)).tw. 3. AF.tw. 4. 1 or 2 or 3 5. exp blood clotting factor 10a inhibitor/ 6. ((factor Xa or factor 10a or fXa or autoprothrombin c or thrombokinase) adj5 inhib$).tw. 7. (activated adj5 (factor X or factor 10) adj5 inhib$).tw. 8. xabans.tw. 9. (antistasin or apixaban or betrixaban or du 176b or eribaxaban or fondaparinux or idraparinux or otamixaban or razaxaban or rivaroxaban or yagin or ym 150 or ym150 or LY517717 or darexaban or edoxaban or SSR126517E).tw. 10. 5 or 6 or 7 or 8 or 9 11. Warfarin/ 12. (warfarin$ or adoisine or aldocumar or athrombin$ k or carfin or coumadin$ or coumafene or coumaphene or jantoven or kumatox or lawarin or marevan or panwarfarin or panwarfin or prothromadin or sofarin or tedicumar or tintorane or waran or warfant or warfilone or warnerin).tw. 13. antivitamin K/ 14. (vitamin K antagonist$ or VKA or VKAs).tw. 15. exp coumarin anticoagulant/ 16. (coumarin$ or cumarin$ or phenprocoum$ or phenprocum$ or dicoumar$ or dicumar$ or acenocoumar$ or acenocumar$ or fluindione or phenindione or clorindione or diphenadione).tw. 17. 11 or 12 or 13 or 14 or 15 or 16 18. 4 and 10 and 17
Data and analyses
Comparison 1. Factor Xa inhibitors versus VKA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stroke and other systemic embolic events | 13 | 67477 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.82, 0.97] |
| 1.1 Apixaban versus VKA | 2 | 18423 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.65, 0.93] |
| 1.2 Darexaban versus VKA | 2 | 1745 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.29, 4.80] |
| 1.3 Edoxaban versus VKA | 4 | 23007 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.88, 1.14] |
| 1.4 Idraparinux versus VKA | 1 | 4576 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.37, 1.21] |
| 1.5 Rivaroxaban versus VKA | 2 | 15445 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.72, 1.00] |
| 1.6 Betrixaban versus VKA | 1 | 508 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.08, 35.22] |
| 1.7 Idrabiotaparinux versus VKA | 1 | 3773 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.49, 1.67] |
| 2 All strokes | 13 | 67449 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.81, 0.97] |
| 2.1 Apixaban versus VKA | 2 | 18423 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.64, 0.93] |
| 2.2 Betrixaban versus VKA | 1 | 508 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.08, 35.22] |
| 2.3 Darexaban versus VKA | 2 | 1745 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.31, 10.10] |
| 2.4 Edoxaban versus VKA | 4 | 23007 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.88, 1.15] |
| 2.5 Idraparinux versus VKA | 1 | 4576 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.38, 1.27] |
| 2.6 Rivaroxaban versus VKA | 2 | 15417 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.66, 0.97] |
| 2.7 Idrabiotaparinux versus VKA | 1 | 3773 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.51, 1.79] |
| 3 Ischaemic stroke | 12 | 66306 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.92, 1.14] |
| 3.1 Apixaban versus VKA | 2 | 18423 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.73, 1.12] |
| 3.2 Darexaban versus VKA | 2 | 1745 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.18, 13.22] |
| 3.3 Edoxaban versus VKA | 3 | 21864 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.04, 1.42] |
| 3.4 Idraparinux versus VKA | 1 | 4576 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.32, 1.31] |
| 3.5 Rivaroxaban versus VKA | 2 | 15417 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.71, 1.09] |
| 3.6 Betrixaban versus VKA | 1 | 508 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.08, 35.22] |
| 3.7 Idrabiotaparinux versus VKA | 1 | 3773 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.29, 1.62] |
| 4 Disabling or fatal stroke | 7 | 39026 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.77, 1.06] |
| 4.1 Darexaban versus VKA | 2 | 1745 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.14, 10.94] |
| 4.2 Edoxaban versus VKA | 3 | 21864 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.86, 1.29] |
| 4.3 Rivaroxaban versus VKA | 2 | 15417 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.54, 0.92] |
| 5 Systemic embolic events (non‐CNS) | 13 | 67449 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.48, 0.96] |
| 5.1 Apixaban versus VKA | 2 | 18423 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.44, 1.76] |
| 5.2 Darexaban versus VKA | 2 | 1745 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.04, 2.13] |
| 5.3 Edoxaban versus VKA | 4 | 23007 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.59, 1.59] |
| 5.4 Idraparinux versus VKA | 1 | 4576 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.18] |
| 5.5 Rivaroxaban versus VKA | 2 | 15417 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.11, 0.64] |
| 5.6 Betrixaban versus VKA | 1 | 508 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.7 Idrabiotaparinux versus VKA | 1 | 3773 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.52] |
| 6 Major bleedings | 13 | 67396 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.73, 0.84] |
| 6.1 Apixaban versus VKA | 2 | 18358 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.60, 0.80] |
| 6.2 Darexaban versus VKA | 2 | 1745 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.27, 1.45] |
| 6.3 Edoxaban versus VKA | 4 | 22922 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.56, 0.71] |
| 6.4 Idraparinux versus VKA | 1 | 4576 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.62 [1.70, 4.03] |
| 6.5 Rivaroxaban versus VKA | 2 | 15514 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.88, 1.17] |
| 6.6 Betrixaban versus VKA | 1 | 508 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.05, 0.82] |
| 6.7 Idrabiotaparinux versus VKA | 1 | 3773 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.52, 1.63] |
| 7 Intracranial haemorrhages | 12 | 66259 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.42, 0.59] |
| 7.1 Apixaban versus VKA | 2 | 18358 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.30, 0.58] |
| 7.2 Darexaban versus VKA | 2 | 1745 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.15, 5.93] |
| 7.3 Edoxaban versus VKA | 3 | 21785 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.30, 0.50] |
| 7.4 Idraparinux versus VKA | 1 | 4576 | Odds Ratio (M‐H, Fixed, 95% CI) | 11.10 [1.43, 86.02] |
| 7.5 Rivaroxaban versus VKA | 2 | 15514 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.46, 0.88] |
| 7.6 Betrixaban versus VKA | 1 | 508 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.7 Idrabiotaparinux versus VKA | 1 | 3773 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.45, 2.23] |
| 8 Non‐major clinically relevant bleeds | 13 | 67396 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.70, 1.07] |
| 8.1 Apixaban versus VKA | 2 | 18358 | Odds Ratio (M‐H, Random, 95% CI) | 0.67 [0.58, 0.78] |
| 8.2 Darexaban versus VKA | 2 | 1745 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.14, 5.70] |
| 8.3 Edoxaban versus VKA | 4 | 22922 | Odds Ratio (M‐H, Random, 95% CI) | 0.88 [0.60, 1.30] |
| 8.4 Idraparinux versus VKA | 1 | 4576 | Odds Ratio (M‐H, Random, 95% CI) | 1.48 [1.23, 1.79] |
| 8.5 Rivaroxaban versus VKA | 2 | 15514 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.95, 1.18] |
| 8.6 Betrixaban versus VKA | 1 | 508 | Odds Ratio (M‐H, Random, 95% CI) | 0.66 [0.20, 2.23] |
| 8.7 Idrabiotaparinux versus VKA | 1 | 3773 | Odds Ratio (M‐H, Random, 95% CI) | 0.54 [0.39, 0.75] |
| 9 Myocardial infarction | 10 | 62703 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.84, 1.10] |
| 9.1 Apixaban versus VKA | 2 | 18423 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.66, 1.17] |
| 9.2 Edoxaban versus VKA | 3 | 22482 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.88, 1.32] |
| 9.3 Idraparinux versus VKA | 1 | 4576 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.59, 2.58] |
| 9.4 Rivaroxaban versus VKA | 2 | 15417 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.63, 1.06] |
| 9.5 Betrixaban versus VKA | 1 | 508 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.6 Darexaban vs VKA | 1 | 1297 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.28, 6.32] |
| 10 Vascular deaths | 10 | 45027 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.79, 0.94] |
| 10.1 Apixaban versus VKA | 1 | 222 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Edoxaban versus VKA | 4 | 23007 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.95] |
| 10.3 Idraparinux versus VKA | 1 | 4576 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.43, 1.20] |
| 10.4 Rivaroxaban versus VKA | 2 | 15417 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.73, 1.11] |
| 10.5 Betrixaban versus VKA | 1 | 508 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 5.34] |
| 10.6 Darexaban vs VKA | 1 | 1297 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.01 [0.16, 56.10] |
| 11 All‐cause deaths | 10 | 65624 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.83, 0.95] |
| 11.1 Apixaban versus VKA | 2 | 18423 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.79, 1.00] |
| 11.2 Betrixaban versus VKA | 1 | 508 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 5.34] |
| 11.3 Idraparinux versus VKA | 1 | 4576 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.71, 1.46] |
| 11.4 Rivaroxaban versus VKA | 2 | 15417 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.70, 1.01] |
| 11.5 Edoxaban versus VKA | 2 | 21630 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.81, 0.97] |
| 11.6 Darexaban vs VKA | 1 | 1297 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.36 [0.24, 77.61] |
| 11.7 Idrabiotaparinux versus VKA | 1 | 3773 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.66, 1.22] |
Comparison 2. Factor Xa inhibitors versus VKA: route of administration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stroke and other systemic embolic events | 13 | 67477 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.80, 0.95] |
| 1.1 Oral administration | 11 | 59128 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.80, 0.96] |
| 1.2 Parenteral administration | 2 | 8349 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.51, 1.19] |
| 2 Major bleedings | 13 | 67396 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.73, 0.84] |
| 2.1 Oral administration | 11 | 59047 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.69, 0.80] |
| 2.2 Parenteral administration | 2 | 8349 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.30, 2.55] |
Comparison 3. Factor Xa inhibitors versus VKA: dose of Factor Xa inhibitor.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stroke and other systemic embolic events | 12 | 64283 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.85, 1.01] |
| 1.1 Apixaban 2.5mg twice daily | 2 | 979 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.23, 0.90] |
| 1.2 Apixaban 5 mg twice daily | 2 | 17518 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.67, 0.98] |
| 1.3 Edoxaban 30mg once daily | 4 | 14964 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.98, 1.33] |
| 1.4 Edoxaban 45mg once daily | 1 | 259 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.82 [0.11, 69.87] |
| 1.5 Edoxaban 60mg once daily | 4 | 14965 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.74, 1.02] |
| 1.6 Edoxaban 30mg twice daily | 1 | 494 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.20, 5.13] |
| 1.7 Edoxaban 60mg twice daily | 1 | 430 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.05, 4.46] |
| 1.8 Rivaroxaban 10mg once daily | 1 | 781 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.38, 2.78] |
| 1.9 Rivaroxaban 15mg once daily | 1 | 1136 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.14, 0.85] |
| 1.10 Darexaban 15mg twice daily | 1 | 486 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.09, 11.11] |
| 1.11 Darexaban 30mg once daily | 2 | 669 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.09, 11.18] |
| 1.12 Darexaban 30mg twice daily | 1 | 486 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.09, 11.11] |
| 1.13 Darexaban 60mg once daily | 2 | 674 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.25, 9.59] |
| 1.14 Darexaban 60mg twice daily | 1 | 486 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.28, 14.42] |
| 1.15 Darexaban 120mg once daily | 2 | 673 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.15, 6.62] |
| 1.16 Darexaban 240mg | 1 | 172 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.66 [0.15, 91.07] |
| 1.17 Idraparinux 1,5mg once weekly | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.18 Idraparinux 2,5mg once weekly | 1 | 4508 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.37, 1.21] |
| 1.19 Betrixaban 40 mg | 1 | 254 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.20 Betrixaban 60 mg | 1 | 254 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.02 [0.12, 74.93] |
| 1.21 Betrixaban 80 mg | 1 | 254 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.02 [0.12, 74.93] |
| 1.22 Idrabiotaparinux 3mg once weekly | 1 | 3773 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.49, 1.67] |
| 2 Major bleedings | 11 | 63950 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.65, 0.75] |
| 2.1 Apixaban 2.5mg twice daily | 2 | 973 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.38, 1.16] |
| 2.2 Apixaban 5mg twice daily | 2 | 17460 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.61, 0.82] |
| 2.3 Edoxaban 30mg once daily | 4 | 14741 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.40, 0.54] |
| 2.4 Edoxaban 45mg once daily | 1 | 259 | Odds Ratio (M‐H, Fixed, 95% CI) | 6.68 [0.34, 130.64] |
| 2.5 Edoxaban 60mg once daily | 4 | 14918 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.69, 0.90] |
| 2.6 Edoxaban 30mg twice daily | 1 | 494 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.21 [0.60, 44.92] |
| 2.7 Edoxaban 60mg twice daily | 1 | 430 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.59 [1.02, 71.95] |
| 2.8 Darexaban 15mg twice daily | 1 | 486 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 1.98] |
| 2.9 Darexaban 30mg once daily | 2 | 669 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.01, 2.01] |
| 2.10 Darexaban 30mg twice daily | 1 | 486 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.20, 2.85] |
| 2.11 Darexaban 60mg once daily | 2 | 674 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.10, 2.34] |
| 2.12 Darexaban 60mg twice daily | 1 | 486 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.40, 3.91] |
| 2.13 Darexaban 120mg once daily | 2 | 674 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.29, 3.35] |
| 2.14 Darexaban 240mg once daily | 1 | 172 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.66 [0.15, 91.07] |
| 2.15 Rivaroxaban 10mg once daily | 1 | 780 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [0.71, 3.36] |
| 2.16 Rivaroxaban 15mg once daily | 1 | 1137 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.43, 1.49] |
| 2.17 Betrixaban 40 mg | 1 | 254 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.60] |
| 2.18 Betrixaban 60 mg | 1 | 254 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.60] |
| 2.19 Betrixaban 80 mg | 1 | 254 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.14, 2.52] |
| 2.20 Idrabiotaparinux 3mg once weekly | 1 | 3773 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.52, 1.63] |
| 2.21 Idraparinux 1.5/2.5mg once weekly | 1 | 4576 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.62 [1.70, 4.03] |
Comparison 4. Factor Xa inhibitors versus VKA: previous stroke or TIA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stroke and other systemic embolic events | 5 | 59247 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.78, 0.94] |
| 1.1 Previous stroke or TIA | 5 | 18721 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.76, 1.00] |
| 1.2 No previous stroke or TIA | 5 | 40526 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.74, 0.97] |
| 2 Major bleedings | 4 | 54615 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.70, 0.81] |
| 2.1 Previous stroke or TIA | 4 | 17616 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.69, 0.90] |
| 2.2 No previous stroke or TIA | 4 | 36999 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.67, 0.80] |
Comparison 5. Factor Xa inhibitors versus VKA: quality of anticoagulation with VKA (TTR).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stroke and other systemic embolic events | 3 | 52695 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.94] |
| 1.1 Good quality | 3 | 27714 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.04] |
| 1.2 Bad quality | 3 | 24981 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.70, 0.93] |
| 2 Major bleedings | 3 | 52730 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.68, 0.80] |
| 2.1 Good quality | 3 | 22288 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.72, 0.90] |
| 2.2 Bad quality | 3 | 30442 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.62, 0.77] |
Comparison 6. Factor Xa inhibitors versus VKA: previous VKA use.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stroke and other systemic embolic events | 5 | 59247 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.78, 0.94] |
| 1.1 VKA naive | 5 | 22969 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.69, 0.93] |
| 1.2 VKA experienced | 5 | 36278 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.79, 1.02] |
| 2 Major bleedings | 4 | 54679 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.69, 0.81] |
| 2.1 VKA naive | 4 | 21863 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.66, 0.84] |
| 2.2 VKA experienced | 4 | 32816 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.68, 0.83] |
Comparison 7. Factor Xa inhibitors versus VKA: concomitant antiplatelet use.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stroke and other systemic embolic events | 3 | 31774 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.61, 0.78] |
| 1.1 Concomitant antiplatelet use | 3 | 11279 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.66, 0.98] |
| 1.2 No concomitant antiplatelet use | 3 | 20495 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.54, 0.73] |
| 2 Major bleedings | 3 | 33654 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.77, 0.94] |
| 2.1 Concomitant antiplatelet use | 3 | 11267 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.80, 1.09] |
| 2.2 No concomitant antiplatelet use | 3 | 22387 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.70, 0.91] |
Comparison 8. Factor Xa inhibitors versus VKA: age.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stroke and other systemic embolic events | 4 | 57974 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.79, 0.95] |
| 1.1 Age < 75 years | 4 | 36089 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.84, 1.09] |
| 1.2 Age ≥ 75 years | 4 | 21885 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.66, 0.88] |
| 2 Major bleedings | 3 | 53402 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.69, 0.81] |
| 2.1 Age < 75 years | 3 | 33100 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.64, 0.80] |
| 2.2 Age ≥ 75 years | 3 | 20302 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.70, 0.87] |
Comparison 9. Factor Xa inhibitors versus VKA: race.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stroke and other systemic embolic events | 6 | 41449 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.79, 0.99] |
| 1.1 Asian participants | 6 | 6432 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.56, 0.94] |
| 1.2 White participants | 3 | 33279 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.82, 1.06] |
| 1.3 Black participants | 3 | 486 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.36, 2.50] |
| 1.4 Other races | 3 | 1252 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.41, 1.58] |
| 2 Major bleedings | 6 | 55056 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.69, 0.80] |
| 2.1 Asian participants | 6 | 8931 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.49, 0.71] |
| 2.2 White participants | 3 | 44010 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.73, 0.86] |
| 2.3 Black participants | 3 | 665 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.45, 1.87] |
| 2.4 Other races | 3 | 1450 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.34, 0.81] |
Comparison 10. Factor Xa inhibitors versus VKA: sex.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stroke and other systemic embolic events | 5 | 59247 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.78, 0.94] |
| 1.1 Women | 5 | 21808 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.73, 0.98] |
| 1.2 Men | 5 | 37439 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.76, 0.98] |
| 2 Major bleeding | 4 | 54680 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.69, 0.81] |
| 2.1 Men | 4 | 34388 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.71, 0.85] |
| 2.2 Women | 4 | 20292 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.61, 0.79] |
Comparison 11. Factor Xa inhibitors versus VKA: baseline CHADS2 score.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stroke and other systemic embolic events | 5 | 59244 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.78, 0.94] |
| 1.1 CHADS2‐score 0‐1 | 2 | 8061 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.61, 1.32] |
| 1.2 CHADS2‐score 2 | 5 | 26309 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.78, 1.08] |
| 1.3 CHADS2‐score ≥ 3 | 5 | 24874 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.72, 0.92] |
| 2 Major bleedings | 5 | 59253 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.73, 0.85] |
| 2.1 CHADS2‐score 0‐1 | 2 | 8047 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.53, 0.90] |
| 2.2 CHADS2‐score 2 | 5 | 26284 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.63, 0.80] |
| 2.3 CHADS2‐score ≥ 3 | 5 | 24922 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.79, 0.98] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
AMADEUS 2008.
| Methods | Randomised, open‐label, active‐controlled trial | |
| Participants | 4673 people with documented AF and an indication for long‐term anticoagulation based on the presence of at least 1 of the following risk factors: previous ischaemic stroke, TIA or systemic embolism; hypertension requiring drug treatment; left ventricular dysfunction; age over 75 years; age 65 to 75 years with either diabetes mellitus or symptomatic coronary artery disease | |
| Interventions | Idraparinux (2.5 mg weekly or 1.5 mg weekly subcutaneously in participants with a calculated creatinine clearance at baseline of 10 to 30 ml/minute; n = 2283), or dose‐adjusted warfarin (target INR 2.0 to 3.0; n = 2293) | |
| Outcomes | Primary efficacy outcome: composite of stroke or systemic embolic event Secondary efficacy outcomes: ischaemic stroke; non‐ischaemic stroke; haemorrhagic stroke; undefined stroke; non‐CNS systemic embolism; venous thromboembolic events; myocardial infarction Primary safety outcome: major bleeding (defined by ISTH criteria) Secondary safety outcomes: any clinically relevant bleeding; fatal bleeding; non‐fatal bleeding; non‐fatal intracranial haemorrhage; bleeding into critical organ; bleeding associated with fall in haemoglobin of more than 20 g/L or leading to transfusion of more than 2 units of blood; intracranial haemorrhage; intracranial events (ischaemic or haemorrhagic stroke or other intracranial haemorrhage); non‐major clinically relevant bleeding; mortality |
|
| Notes | Study sponsored by Sanofi | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment groups |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned to treatment groups with a computerised interactive voice‐response system. Stratification by study centre and prior use of VKA |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study (both participants and study personnel were aware of the assigned treatment) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All suspected outcome events were adjudicated by a central adjudication committee unaware of the treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Efficacy and safety outcomes analysed in ITT population. Number of participants that discontinued were reported. Reasons for discontinuation were listed |
| Selective reporting (reporting bias) | Low risk | All predefined efficacy and safety outcomes were reported for the ITT population |
| Other bias | High risk | Study terminated prior to finalisation after recommendation by the DSMB due to excess bleeding complications in the idraparinux group |
ARISTOTLE 2011.
| Methods | Randomised, double‐blind, active controlled trial | |
| Participants | 18,201 people with documented AF or atrial flutter and at least 1 additional risk factor for stroke: at least 75 years old; previous stroke, TIA or systemic embolic event; symptomatic heart failure within previous 3 months or left ventricular ejection fraction of no more than 40%; diabetes mellitus; hypertension requiring pharmacologic treatment | |
| Interventions | Apixaban (5 mg twice daily, or 2.5 mg twice daily in participants with at least 2 or more of the following criteria: age at least 80 years, body weight of no more than 60 kg, or serum creatinine level of 1.5 mg/dl or more; n = 9120) versus dose‐adjusted warfarin (target INR 2.0 to 3.0; n = 9081) | |
| Outcomes | Primary efficacy outcome: composite of stroke or systemic embolic events Secondary efficacy outcomes: death from any cause, myocardial infarction Primary safety outcome: major bleeding (ISTH criteria) Secondary safety outcomes: composite of major bleeding and clinically relevant non‐major bleeding; any bleeding; other adverse events; liver function abnormalities |
|
| Notes | Study co‐sponsored by Bristol‐Myers Squibb and Pfizer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment groups |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned to treatment groups. Stratification by clinical site and prior VKA use |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Efficacy and safety outcomes were adjudicated by a clinical events committee whose members were not aware of study group assignments |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Efficacy and safety outcomes analysed in ITT population. Number of participants that discontinued during study and reasons are reported. Number of participants with missing data on vital status rather high (n = 380; 2.1%) and could have had an impact on the robustness of the mortality data from this study |
| Selective reporting (reporting bias) | Low risk | All predefined efficacy and safety outcomes reported for ITT population |
| Other bias | Low risk | _ |
ARISTOTLE‐J 2011.
| Methods | Randomised, partially‐blinded, active controlled trial | |
| Participants | 222 Japanese people with a history of documented non‐valvular AF and 1 or more additional risk factors for stroke: age at least 75 years; congestive heart failure with left ventricular ejection fraction of no more than 40%; hypertension requiring medication; diabetes mellitus deemed to require medication on physicians' discretion; history of cerebral infarction or TIA | |
| Interventions | Apixaban (5 mg twice daily or 2.5 mg twice daily; n = 148) versus dose‐adjusted warfarin (target INR 2.0 to 3.0 or 2.0 to 2.6 if age 70 or more years; n = 74) during a predefined 12‐week treatment period | |
| Outcomes | Primary safety outcome: composite of major bleeding and clinically relevant non‐major bleeding (defined by ISTH criteria) Secondary safety and efficacy outcomes: major bleeding; clinically relevant non‐major bleeding; composite of total bleeding events (including minor bleedings); composite of stroke or systemic embolism; composite of stroke, systemic embolism and myocardial infarction or all‐cause death |
|
| Notes | Study co‐sponsored by Pfizer and Bristol‐Myers Squibb | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment groups |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned to treatment groups. Stratification by trial site and prior use of VKA |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Partially blinded design: open‐label warfarin and double‐blind apixaban administration |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Reported efficacy and safety outcomes were adjudicated by an independent committee whose members were not aware of study group assignments |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Primary efficacy outcome analysed in ITT population. All other efficacy and safety outcomes analysed in 'safety population'. Number of participants that discontinued during study and reasons not stated |
| Selective reporting (reporting bias) | Low risk | All predefined efficacy and safety outcomes reported for safety population |
| Other bias | Low risk | _ |
BOREALIS AF STUDY 2014.
| Methods | Randomised, double‐blind, double‐dummy non‐inferiority clinical trial | |
| Participants | 3773 participants with AF documented by ECG and a history of previous stroke/TIA or non‐CNS systemic embolic events | |
| Interventions | Idrabiotaparinux subcutaneous injection of 3 mg once a week for the first 7 weeks, thereafter followed by 2 mg once a week, except in participants with a creatinine clearance of 30 to 50 mL min (‐1) or aged ≥ 75 years who received 1.5 mg once a week after the first 7 weeks (n = 1886), or warfarin (dose‐adjusted to INR 2 to 3; n = 1887) | |
| Outcomes | Primary efficacy outcome: composite of all fatal or non‐fatal strokes and systemic embolism Primary safety outcome: clinically relevant bleeding (major and clinically relevant non‐major bleeding) defined by ISTH‐criteria |
|
| Notes | Study sponsored by Sanofi‐Aventis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment groups |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned to treatment groups |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded central adjudication of all primary safety and efficacy outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary safety and efficacy outcome analysed in ITT population. Number of participants that discontinued during study and reasons reported |
| Selective reporting (reporting bias) | Low risk | All predefined efficacy and safety outcomes reported for ITT population |
| Other bias | High risk | Study was prematurely halted (3773 participants randomised of planned 9600 participants) by the sponsor for commercial/strategic reasons |
Edoxaban Asia 2011.
| Methods | Randomised, partially‐blinded, active controlled trial | |
| Participants | 235 Asian people with documented non‐valvular AF | |
| Interventions | Edoxaban (30 mg daily or 60 mg daily; n = 159) versus dose‐adjusted warfarin (target INR 2.0 to 3.0) during a predefined 3‐month period (n = 75) | |
| Outcomes | Primary safety outcome: composite of major, clinically relevant non‐major, and minor bleeding (by ISTH definitions) Secondary safety outcome: all adverse events, laboratory variables Secondary efficacy outcome: composite of stroke, systemic embolic events, myocardial infarction, cardiovascular death and hospitalisation for any other cardiac condition |
|
| Notes | Study sponsored by Daiichi Sankyo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment groups |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned to treatment groups |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label administration of both edoxaban and warfarin. Different edoxaban doses administered in double‐blind fashion |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Adjudication of outcomes by investigator and Clinical Events Committee. Unclear whether Clinical Events Committee was blinded to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Safety outcomes analysed in safety population (participants who received at least 1 dose of study drug and had at least 1 post‐dose safety assessment) Efficacy outcomes analysed in full analysis set (all participants who received at least 1 dose of study drug and had at least 1 post‐dose efficacy assessment) Number of participants that discontinued during study and reasons for discontinuation stated |
| Selective reporting (reporting bias) | Low risk | All predefined safety and efficacy outcomes reported for safety population and full analysis set, respectively |
| Other bias | Low risk | _ |
Edoxaban Japan 2012.
| Methods | Randomised, partially‐blinded, active controlled trial | |
| Participants | 536 Japanese people with documented atrial fibrillation by ECG at least twice within 12 months and a CHADS2‐score ≥ 1 | |
| Interventions | Edoxaban (30 mg, 45 mg or 60 mg once‐daily; n = 396) versus dose‐adjusted warfarin (INR 2 to 3 for participants aged < 70 years and 1.6 to 2.6 for participants aged ≥70 years; n = 129) | |
| Outcomes | Primary safety outcome: all bleeding events (major, clinically relevant non‐major and minor bleedings defined by slightly modified ISTH‐criteria) Secondary safety outcomes: asymptomatic intracranial haemorrhages, other adverse events and adverse drug reactions Primary efficacy outcome: thromboembolic events |
|
| Notes | Study sponsored by Daiichi Sankyo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment groups |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned to treatment groups. Stratification by warfarin use at time of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label administration of both edoxaban and warfarin. Different edoxaban doses administered in double‐blind fashion |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Adjudication of outcome events by investigator, an independent "event assessment committee" (all bleeding and thromboembolic events) and an independent, blinded "asymptomatic intracranial haemorrhages committee". Unclear whether the "event assessment committee" also was blinded to treatment assignments |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Bleeding outcomes reported in a "modified" full analysis set, defined as all participants who proceeded to the treatment period after the run‐in period and received at least ≥ 1 dose of study treatment Efficacy outcomes reported in full analysis set, defined as all participants who received at least ≥ 1 dose of study treatment Number of participants that discontinued during run‐in and study period stated, but reasons for discontinuation not stated |
| Selective reporting (reporting bias) | Low risk | All predefined safety and efficacy outcomes reported for "modified" full analysis set or full analysis set |
| Other bias | Low risk | _ |
Edoxaban US/Europe 2010.
| Methods | Randomised, partially‐blinded, active controlled trial | |
| Participants | 1146 people aged between 18 and 65 years with documented non‐valvular AF and a CHADS2 score of at least 2 | |
| Interventions | Edoxaban (30 mg once daily, 30 mg twice daily, 60 mg once daily, or 60 mg twice daily; n = 893) versus dose‐adjusted warfarin (target INR 2.0 to 3.0) during a predefined 12‐week period (n = 250) | |
| Outcomes | Primary safety outcome: major bleeding (defined by modified ISTH criteria) Secondary safety outcomes: clinically relevant non‐major bleeding; minor bleeding; liver function tests Secondary efficacy outcomes: composite of stroke (ischaemic or haemorrhagic), systemic embolic event, myocardial infarction, cardiovascular death and hospitalisation for any cardiac conditions |
|
| Notes | Study sponsored by Daiichi Sankyo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment groups |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned to treatment groups |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label administration of both edoxaban and warfarin. Different doses of edoxaban administered in double‐blind fashion |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Adjudication of bleeding events by independent central adjudication committee that was blinded to treatment assignment. Unclear whether efficacy outcomes were centrally adjudicated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Safety and efficacy outcomes analysed in 'safety population' (participants who received at least 1 dose of study drug and had at least 1 post‐dose safety assessment). Number of participants that discontinued during study and reasons for discontinuation not stated |
| Selective reporting (reporting bias) | Low risk | All predefined safety and efficacy outcomes reported for safety population |
| Other bias | Unclear risk | Randomisation into edoxaban 60 mg twice‐daily treatment arm prematurely terminated after enrolment of 180 participants based on recommendation of independent DSMB due to an excess of bleedings |
ENGAGE AF‐TIMI 48 2013.
| Methods | Randomised, double‐blind, double‐dummy, active controlled trial | |
| Participants | 21,105 people aged 21 years or older with AF documented by electrical tracing within the 12 months preceding randomisation, a CHADS2‐score of 2 or higher and anticoagulation therapy planned for the duration of the trial | |
| Interventions | Edoxaban (30 mg or 60 mg once daily; n = 14,069) versus dose‐adjusted warfarin (target INR 2.0 to 3.0; n = 7036). Edoxaban doses were halved in either group if people had an estimated creatinine clearance 30‐50 ml/min, body weight < 60 kg or concomitant use of verapamil, quinidine or dronedarone at time of randomisation or during the study period |
|
| Outcomes | Primary efficacy outcome: composite of stroke (ischaemic or haemorrhagic) or systemic embolic event Secondary efficacy outcomes: stroke, systemic embolic event, myocardial infarction Primary safety outcome: major bleeding (defined by ISTH‐criteria) Secondary safety outcomes: death from cardiovascular causes (including bleedings), death from any cause, minor bleeding, hepatic events |
|
| Notes | Study sponsored by Daiichi Sankyo | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment groups |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned to treatment groups. Stratification by CHADS2‐score 2‐3 versus 4‐6, and status with respect to need for reduction of edoxaban dose. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Key efficacy and safety outcomes were adjudicated by an independent clinical end‐point committee whose members were not aware of study assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Efficacy and safety outcomes analysed in modified ITT population (all randomised participants who received at least one dose of study drug during treatment period). Number of participants with missing data on vital status and reasons reported. Number of participants that discontinued during study and reasons were reported |
| Selective reporting (reporting bias) | Low risk | All predefined efficacy and safety outcomes reported for modified ITT population |
| Other bias | Low risk | N/A |
EXPLORE‐Xa 2013.
| Methods | Randomised, partially‐blinded, active controlled trial | |
| Participants | 508 people with documented non‐valvular AF and an indication for anticoagulation with VKA | |
| Interventions | Betrixaban (40 mg, 60 mg or 80 mg daily; n = 381) or dose‐adjusted warfarin (target INR 2.0 to 3.0) for at least 3 months (n = 127) | |
| Outcomes | Primary safety outcome: composite of major or clinically relevant non‐major bleeding (ISTH criteria) Secondary safety and efficacy outcomes: stroke (fatal and non‐fatal); myocardial infarction; systemic embolic events; pulmonary embolism; all‐cause death; other adverse events |
|
| Notes | Study sponsored by Portola Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment groups |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned to treatment groups with a computerised interactive voice response system |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label administration of both betrixaban and warfarin. Separate dosages of betrixaban administered in double‐blind fashion |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adjudication of safety and efficacy outcomes by an independent clinical endpoint committee that was blinded to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported in ITT population. Number of participants that discontinued during study stated with reason |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes reported for ITT population |
| Other bias | Low risk | _ |
J‐ROCKET AF 2012.
| Methods | Randomised, double‐blind, active controlled trial | |
| Participants | 1280 Japanese people aged ≥ 20 years with documented AF and either a risk of stroke, TIA or systemic embolic embolism or ≥ 2 of the following risk factors for thromboembolism: congestive heart failure and/or left ventricular ejection fraction < 35%, hypertension, age ≥ 75 years or diabetes mellitus | |
| Interventions | Rivaroxaban (15 mg once daily, or 10 mg once daily in participants with a creatinine clearance of 30 to 49 ml/minute; n = 637), or dose‐adjusted warfarin (target INR 1.6 to 2.6 for participants ≥ 70 years and 2.0 to 3.0 for participants < 70 years; n = 637) | |
| Outcomes | Primary safety outcome: composite of major bleeding (defined by ISTH definition) or non‐major clinically relevant bleeding Primary efficacy outcome: composite of stroke or non‐CNS systemic embolic events Secondary safety and efficacy outcomes: composite of stroke, non‐CNS systemic embolic events or vascular death; composite of stroke, non‐CNS systemic embolic events, vascular death or myocardial infarction; stroke; myocardial infarction; vascular death; non‐CNS systemic embolic events; disabling stroke; all‐cause death; major bleeding; non‐major clinically relevant bleeding; other adverse events; liver function tests |
|
| Notes | Study sponsored by Johnson & Johnson Pharmaceutical Research and Development, and Bayer HealthCare | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment groups |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned to treatment groups |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Adjudication of safety and efficacy outcomes by an independent clinical endpoint committee that was blinded to treatment assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported in safety analysis population, ITT population or per protocol analysis population. Number of participants that discontinued due to adverse events stated |
| Selective reporting (reporting bias) | Low risk | All predefined efficacy and safety outcomes reported |
| Other bias | Low risk | _ |
OPAL‐1 2010.
| Methods | Randomised, partially blind, active controlled trial | |
| Participants | 448 people recruited in the Asian‐Pacific region with documented non‐valvular AF | |
| Interventions | Darexaban (30 mg, 60 mg, 120 mg or 240 mg once daily; n = 354) versus dose‐adjusted warfarin (target INR 2.0 to 3.0 in participants less than 70 years and 1.6 to 2.6 in participants 70 years or older) during a predefined period of 12 weeks (n = 94) | |
| Outcomes | Primary safety outcome: composite of major or clinically relevant non‐major bleeding Secondary safety outcome: adverse events, liver function tests (ALT and AST) and renal function (serum creatinine) Primary efficacy outcome: composite of stroke, TIA, systemic embolic events and all‐cause death |
|
| Notes | Study sponsored by Astellas | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment groups |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned to treatment groups |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label administration of both darexaban and warfarin. Different doses of darexaban administered in double‐blind fashion |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Adjudication of outcomes not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported in ITT population. Number of participants that discontinued during study and reasons for discontinuation stated |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes reported for ITT population |
| Other bias | Unclear risk | Randomisation into 240 mg darexaban arm terminated early after recommendation from DSMB due to increased bleeding |
OPAL‐2 2011.
| Methods | Randomised, double‐blind, active controlled trial | |
| Participants | 1297 people with documented AF and a CHADS2 score of 1 to 6 | |
| Interventions | Darexaban (15 mg twice daily, 30 mg once daily, 30 mg twice daily, 60 mg once daily, 60 mg twice daily or 120 mg once daily; n = 973) versus dose‐adjusted warfarin (target INR 2.0 to 3.0) during a period of 24 to 52 weeks (n = 324) | |
| Outcomes | Primary safety endpoint: composite of major or clinically relevant non‐major bleeding (ISTH definitions) Secondary safety endpoint: major bleeding (ISTH definition) Primary efficacy endpoint: composite of ischaemic stroke, TIA, systemic embolic embolism, vascular death |
|
| Notes | Study sponsored by Astellas | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment groups |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned to treatment groups |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Adjudication of outcomes not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported in ITT population. Number of participants that discontinued during study stated |
| Selective reporting (reporting bias) | Unclear risk | All predefined outcomes reported for ITT population |
| Other bias | Low risk | _ |
ROCKET AF 2011.
| Methods | Randomised, double‐blind, active controlled trial | |
| Participants | 14,264 people with documented AF and a CHADS2 score ≥ 2 | |
| Interventions | Rivoraxaban (20 mg daily, or 15 mg daily in participants with a creatinine clearance of 30 to 49 ml/minute; n = 7081) versus dose‐adjusted warfarin (target INR 2.0 to 3.0; n = 7090) | |
| Outcomes | Primary efficacy outcome: composite of stroke or systemic embolic events Secondary efficacy outcomes: composite of stroke, systemic embolism or death from cardiovascular cause; composite of stroke, systemic embolism, death from cardiovascular cause or myocardial infarction; individual components of composite efficacy endpoints Primary safety outcome: composite of major bleedings (defined by ISTH criteria) and non‐major clinically relevant bleedings Secondary safety outcomes: major bleedings, intracranial haemorrhages, minor bleedings |
|
| Notes | Study sponsored by Johnson & Johnson Pharmaceutical Research and Development, and Bayer HealthCare | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment groups |
| Allocation concealment (selection bias) | Low risk | Participants were randomly assigned to treatment groups with the use of a central, computerised, automated voice‐response system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, double‐dummy design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Efficacy and safety outcomes were adjudicated by a blinded and independent clinical endpoint committee |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Primary efficacy outcome analysed in ITT population. Primary and secondary efficacy also analysed in the as treated, per protocol population during treatment. Safety outcomes only analysed in safety, on‐treatment population. Number of participants that discontinued during study and reasons are reported |
| Selective reporting (reporting bias) | Low risk | All predefined efficacy and safety outcomes reported, but not all for ITT population |
| Other bias | Low risk | _ |
AF: atrial fibrillation; ALT: alanine transaminase; AST: aspartate aminotransferase; CNS: central nervous system; DSMB: Data Safety and Monitoring Board; ECG: electrocardiogram; INR: International Normalised Ratio; ISTH: International Society on Thrombosis and Haemostasis; ITT: intention‐to‐treat; TIA: transient ischaemic attack; VKA: vitamin K antagonist
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| BAY59‐7939 Japan 2006 | Duration too short for inclusion (< 28 days) |
| Camm 2011 | Expert review of edoxaban; no outcome data |
| Connolly 2011 | Primary results from the AVERROES study, in which apixaban was not directly compared with a VKA |
| Harenberg 2010 | Expert review of idraparinux and idrabiotaparinux; no additional outcome data |
| Lopes 2010 | Study protocol and rationale for ARISTOTLE 2011; no outcome data |
| Partida 2011 | Expert review of edoxaban; no outcome data |
| ROCKET investigators 2010 | Study protocol and rationale for ROCKET AF 2011; no outcome data |
| Study‐11866 2007 | Study duration too short for inclusion (28 days) |
VKA: vitamin K antagonist
Differences between protocol and review
In light of the available data we have chosen to modify two secondary outcomes that were defined in the protocol; we changed:
"Major extracranial haemorrhages, defined as severe enough to lead to hospitalisation, blood transfusion or surgery" into "Major bleedings (defined by ISTH criteria or modified ISTH criteria)"; and
"Minor bleeding and bruising" into "Non‐major clinically relevant bleeding (defined by ISTH criteria or modified ISTH criteria)".
A handsearch of conference proceedings has not been performed systematically. We believe it is unlikely that any important trial in this field would not be reported in full.
Contributions of authors
KBS: conception and design of the review; writing of the protocol, first version of the review and the current update. EB: commenting on the protocol and reviewing draft versions of the first and updated review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
South‐Eastern Norway Regional Health Authority, Norway.
Educational grant
Declarations of interest
KBS is currently employed by F. Hoffmann‐La Roche (Roche Norge AS). The data included in this review are based on research which has had no influence or involvement by F. Hoffmann‐La Roche by any means. The views expressed in this review are the personal views of KBS and should not be understood or quoted as being made on behalf of or reflecting the position of F. Hoffmann‐La Roche.
EB chaired a symposium organised by Bayer (entitled “Real World Data”) during the Nordic Stroke Conference in August 2017, after the review had been submitted. He received EUR 900 in compensation for this work. This was in breach of Cochrane's Conflicts of Interest policy, but this has been discussed with the Funding Arbiters, who agreed to allow publication of this updated review.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
AMADEUS 2008 {published and unpublished data}
- The Amadeus Investigators. Comparison of idraparinux with vitamin K antagonists for prevention of thromboembolism in patients with atrial fibrillation: a randomised, open‐label, non‐inferiority trial. Lancet 2008;371:315‐21. [DOI] [PubMed] [Google Scholar]
ARISTOTLE 2011 {published and unpublished data}
- Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. New England Journal of Medicine 2011;365:981‐92. [DOI] [PubMed] [Google Scholar]
ARISTOTLE‐J 2011 {published data only (unpublished sought but not used)}
- Ogawa S, Shinohara Y, Kanmuri K. Safety and efficacy of the oral direct factor Xa inhibitor apixaban in Japanese patients with non‐valvular atrial fibrillation. Circulation Journal 2011;75:1852‐9. [DOI] [PubMed] [Google Scholar]
BOREALIS AF STUDY 2014 {published data only}
- Buller HR, Halperin J, Hankey GJ, Pillion G, Prins MH, Raskob GE. Comparison of idrabiotaparinux with vitamin K antagonists for prevention of thromboembolism in patients with atrial fibrillation: the Borealis‐Atrial Fibrillation Study. Journal of Thrombosis and Haemostasis 2014;12(6):824‐30. [DOI] [PubMed] [Google Scholar]
Edoxaban Asia 2011 {published data only (unpublished sought but not used)}
- Chung N, Jeon HK, Lien LM, Lai WT, Tse HF, Chung WS, et al. Safety of edoxaban, an oral factor Xa inhibitor, in Asian patients with non‐valvular atrial fibrillation. Thrombosis and Haemostasis 2011;105:535‐45. [DOI] [PubMed] [Google Scholar]
Edoxaban Japan 2012 {published data only}
- Yamashita T, Koretsune Y, Yasaka M, Inoue H, Kawai Y, Yamaguchi T, et al. Randomized, multicenter, warfarin‐controlled phase II study of edoxaban in Japanese patients with non‐valvular atrial fibrillation. Circulation Journal 2012;76:1840‐7. [DOI] [PubMed] [Google Scholar]
Edoxaban US/Europe 2010 {published data only (unpublished sought but not used)}
- Weitz JI, Connolly SJ, Patel I, Salazar D, Rohatagi S, Mendell J, et al. Randomised, parallel‐group, multicentre, multinational phase 2 study comparing edoxaban, an oral factor Xa inhibitor, with warfarin for stroke prevention in patients with atrial fibrillation. Thrombosis and Haemostasis 2010;104:633‐41. [DOI] [PubMed] [Google Scholar]
ENGAGE AF‐TIMI 48 2013 {published and unpublished data}
- Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. New England Journal of Medicine 2013;369:2093‐104. [DOI] [PubMed] [Google Scholar]
EXPLORE‐Xa 2013 {published data only (unpublished sought but not used)}
- Connoly SJ, Eikelboom J, Dorian P, Hohnloser SH, Gretler DD, Sinha U, et al. Betrixaban compared with warfarin in patients with atrial fibrillation: results of a phase 2, randomized, dose‐ranging study (Explore‐Xa). European Heart Journal 2013;34(20):1498‐505. [DOI: 10.1093/eurheartj/eht/039] [DOI] [PMC free article] [PubMed] [Google Scholar]
J‐ROCKET AF 2012 {published and unpublished data}
- Hori M, Matsumoto M, Tanahashi N, Momomura SI, Uchiyama S, Goto S, J‐ROCKET AF study investigators. Rivaroxaban vs warfarin in Japanese patients with atrial fibrillation. Circulation Journal 2012;76(9):2104‐11. [DOI: 10.1253/circj.CJ-12-0454] [DOI] [PubMed] [Google Scholar]
OPAL‐1 2010 {published and unpublished data}
- Turpie AGGF, Lip GYH, Minematsu K, Goto S, Renfurm RW, Wong KSL. Safety and tolerability of YM150 in subjects with non‐valvular atrial fibrillation: a phase II study. European Heart Journal 2010;31 Suppl 1:173. [Google Scholar]
OPAL‐2 2011 {published data only}
- Lip GYH, Halperin JL, Petersen P, Rodger GM, Renfurm RW. Safety and tolerability of the oral factor Xa inhibitor YM150 vs warfarin in 1297 patients with non‐valvular atrial fibrillation: a dose confirmation study (OPAL‐2). Journal of Thrombosis and Haemostatis 2011;9 Suppl 2:748. [DOI] [PubMed] [Google Scholar]
ROCKET AF 2011 {published data only (unpublished sought but not used)}
- Patel MR, Mahaffey KW, Garg Y, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. New England Journal of Medicine 2011;365:883‐91. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
BAY59‐7939 Japan 2006 {unpublished data only}
- BAY59‐7936. ClinicalTrials.gov.
Camm 2011 {published data only}
- Camm AJ, Bounameaux H. Edoxaban: a new oral direct factor Xa inhibitor. Drugs 2011;71(12):1503‐26. [DOI] [PubMed] [Google Scholar]
Connolly 2011 {published data only}
- Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. New England Journal of Medicine 2011;364(9):806‐17. [DOI] [PubMed] [Google Scholar]
Harenberg 2010 {published data only}
- Harenberg J. Idraparinux and idrabioparinux. Expert Review of Clinical Pharmacology 2010;3(1):9‐16. [DOI] [PubMed] [Google Scholar]
Lopes 2010 {published data only}
- Lopes RD, Alexander JH, Al‐Khatib SM, the ARISTOTLE investigators. Apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial: design and rationale. American Heart Journal 2010;159(3):331‐9. [DOI] [PubMed] [Google Scholar]
Partida 2011 {published data only}
- Partida RA, Guigliano RP. Edoxaban: pharmacological principles, preclinical and early‐phase clinical testing. Future Cardiology 2011;7(4):459‐70. [DOI] [PubMed] [Google Scholar]
ROCKET investigators 2010 {published data only}
- ROCKET AF Study Investigators. Rivaroxaban once‐daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation: rationale and design of the ROCKET AF study. American Heart Journal 2010;159(3):340‐7. [DOI] [PubMed] [Google Scholar]
Study‐11866 2007 {unpublished data only}
- Study synopsis.
Additional references
ACC/AHA/HRS 2014
- January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Cigarroa JE, et al. Guidelines for the management of patients with atrial fibrillation. Circulation 2014; Vol. 129. [DOI: 10.1161/CIR.000000000000000041] [DOI]
Almutairi 2016
- Almutairi A, Zhou L, Lee JK, Marion S, Martin JR, Lo‐Ciganic W. Comparative effectiveness and safety of novel oral anticoagulants (NOACS) versus vitamin K antagonists (VKA) in atrial fibrillation: a meta‐analysis of observational studies. Value Health 2016;3(19):A41. [Google Scholar]
Biondi‐Zoccai 2013
- Biondi‐Zoccai G, Malavasi V, D’Ascenzo F, Abbate A, Agostoni P, Lotrionte M, et al. Comparative effectiveness of novel oral anticoagulants for atrial fibrillation: evidence from pair‐wise and warfarin‐controlled network meta‐analyses. HSR Proceedings in Intensive Care and Cardiovascular Anesthesia 2013;5(1):40‐54. [PMC free article] [PubMed] [Google Scholar]
Boulanger 2006
- Boulanger L, Kim J, Friedman M, Hauch O, Foster T, Menzin J. Patterns of use of antithrombotic therapy and quality of anticoagulation monitoring among patients with non‐valvular atrial fibrillation in clinical practice. International Journal of Clinical Practice 2006;60:258‐64. [DOI] [PubMed] [Google Scholar]
Caldeira 2015
- Caldeira D, Rodrigues FB, Barra M, Santos AT, Abreu D, Goncalves N. Non‐vitamin K antagonist oral anticoagulants and major bleeding‐related fatality in patients with atrial fibrillation and venous thromboembolism: a systematic review and meta‐analysis. Heart 2015;15(101):1204‐11. [DOI] [PubMed] [Google Scholar]
Cameron 2014
- Cameron C, Coyle D, Richter T, Kelly S, Gauthier K, Steiner S, et al. Systematic review and network meta‐analysis comparing antithrombotic agents for the prevention of stroke and major bleeding in patients with atrial fibrillation. BMJ Open 2014;4(6):e004301. [DOI: 10.1136/bmjopen-2013-004301] [DOI] [PMC free article] [PubMed] [Google Scholar]
Capodanno 2013
- Capodanno D, Capranzano P, Giacchi G, Calvi V, Tamburino C. Novel oral anticoagulants versus warfarin in non‐valvular atrial fibrillation: a meta‐analysis of 50,578 patients. International Journal of Cardiology 2013;167(4):1237‐41. [DOI: 10.1016/j.ijcard.2012.03.148] [DOI] [PubMed] [Google Scholar]
CHADS2
- Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 2001;285(22):2864‐70. [DOI] [PubMed] [Google Scholar]
Cochrane Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Connolly 2007
- Connolly SJ, Eikelboom J, O'Donnell M, Pogue J, Yusuf S. Challenges of establishing new antithrombotic therapies in atrial fibrillation. Circulation 2007;116:449‐55. [DOI] [PubMed] [Google Scholar]
Connolly 2008
- Connolly SJ, Pogue J, Eikelboom J, Flaker G, Commerford P, Franzosi G, the ACTIVE W Investigators. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of International Normalized Ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation 2008;118:2029‐37. [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care. Meta‐analysis in Context. London: BMJ Books, 2001:285‐312. [Google Scholar]
Dogliotti 2013
- Dogliotti A1, Paolasso E, Giugliano RP. Novel oral anticoagulants in atrial fibrillation: a meta‐analysis of large, randomized, controlled trials vs warfarin. Clinical Cardiology 2013;36(2):61‐7. [DOI: 10.1002/clc.22081] [DOI] [PMC free article] [PubMed] [Google Scholar]
EHRA 2013
- Heidbuchel H, Verhamme P, Alings M, Antz M, Hacke W, Oldgren J, et al. European Heart Rhythm Association practical guide on the use of new oral anticoagulants in patients with non‐valvular atrial fibrillation. Europace 2013;15:625‐51. [DOI] [PubMed] [Google Scholar]
Eikelboom 2010
- Eikelboom JW, Weitz JI. New anticoagulants. Circulation 2010;121:1523‐32. [DOI] [PubMed] [Google Scholar]
ESC 2016
- Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. European Heart Journal 2016;37:2893‐962. [DOI] [PubMed] [Google Scholar]
Fu 2014
- Fu W, Guo H, Guo J, Lin K, Wang H, Zhang Y, et al. Relative efficacy and safety of direct oral anticoagulants in patients with atrial fibrillation by network meta‐analysis. Journal of Cardiovascular Medicine 2014;12(15):873‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garg 2016
- Garg J, Chaudhary R, Krishnamoorthy P, Palaniswamy C, Shah N, Bozorgnia B, et al. Safety and efficacy of oral factor‐Xa inhibitors versus vitamin K antagonist in patients with non‐valvular atrial fibrillation. International Journal of Cardiology 2016;218:235‐9. [DOI] [PubMed] [Google Scholar]
Go 2001
- Go AS, Hylek EM, Philips KA, Henault, LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults. National implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370‐5. [DOI] [PubMed] [Google Scholar]
Gomez‐Outes 2015
- Gomez‐Outes A, Suarez‐Gea ML, Terleira‐Fernandez AI, Vargas‐Castrillon E, Calvo‐Rojas G. Direct oral anticoagulants versus warfarin for stroke prevention in atrial fibrillation: a subgroup meta‐analysis in European patients. Clinical Therapeutics 2015;37(8 Suppl):e10. [Google Scholar]
Haim 2015
- Haim M, Hoshen M, Reges O, Rabi Y, Balicer R, Leibowitz M. Prospective national study of the prevalence, incidence, management and outcome of a large contemporary cohort of patients with incident non‐valvular atrial fibrillation. Journal of the American Heart Association 2015;4:e001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hankey 2012
- Hankey GJ, Patel MR, Stevens SR, Becker RC, Breithardt G, Carolei A. Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of ROCKET AF. Lancet Neurology 2012;11:315‐22. [DOI] [PubMed] [Google Scholar]
Hart 2007
- Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have non‐valvular atrial fibrillation. Annals of Internal Medicine 2007;146:857‐67. [DOI] [PubMed] [Google Scholar]
Heeringa 2006
- Heeringa J, Kuip DA, Hofman A, Kors JA, Herpen G, Stricker BH, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. European Heart Journal 2006;27:949‐53. [DOI] [PubMed] [Google Scholar]
Henriksson 2012
- Henriksson KM, Farahmand B, Asberg S, Edvardsson N, Terent A. Comparison of cardiovascular risk factors and survival in patients with ischemic or hemorrhagic stroke. International Journal of Stroke 2012;7:276‐81. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hylek 2007
- Hylek EM, Evans‐Molina C, Shea C, Henault LE, Regan S. Major haemorrhage and tolerability of warfarin in the first year of therapy among elderly patients with atrial fibrillation. Circulation 2007;115:2689‐96. [DOI] [PubMed] [Google Scholar]
Kirchhof 2007
- Kirchhof P, Auricchio A, Bax J, Crijns H, Camm J, Diener H‐C, et al. Outcome parameters for trials in atrial fibrillation: executive summary. Recommendations from a consensus conference organized by the German Atrial Fibrillation Competence NETwork (AFNET) and the European Heart Rhythm Association (EHRA). European Heart Journal 2007;28:2803‐17. [DOI] [PubMed] [Google Scholar]
Krijthe 2013
- Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. European Heart Journal 2013;34:2746‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kwong 2013
- Kwong JS, Lam YY, Yan BP, Yu CM. Bleeding of new oral anticoagulants for stroke prevention in atrial fibrillation: a meta‐analysis of randomized controlled trials. Cardiovascular Drugs and Therapy 2013;27(1):23‐35. [DOI: 10.1007/s10557-012-6426-9] [DOI] [PubMed] [Google Scholar]
Lega 2013
- Lega JC, Mismetti P, Cucherat M, et al. Impact of double‐blind vs. open study design on the observed treatment effects of new oral anticoagulants in atrial fibrillation: a meta‐analysis. Journal of Thrombosis and Haemostasis 2013;11(7):1240‐50. [DOI: 10.1111/jth.12294] [DOI] [PubMed] [Google Scholar]
Lin 2015
- Lin L, Lim WS, Zhou HJ, Khoo AL, Tan KT, Chew AP, et al. Clinical and safety outcomes of oral antithrombotics for stroke prevention in atrial fibrillation: a systematic review and network meta‐analysis. Journal of the American Medical Directors Association 2015;16(12):1103.e1‐19. [DOI] [PubMed] [Google Scholar]
Lip 2016
- Lip GYH, Mitchell SA, Liu X, Liu LZ, Phatak H, Kachroo S, et al. Relative efficacy and safety of non‐Vitamin K oral anticoagulants for non‐valvular atrial fibrillation: network meta‐analysis comparing apixaban, dabigatran, rivaroxaban and edoxaban in three patient subgroups. Internaitonal Journal of Cardiology 2016;204:88‐94. [DOI] [PubMed] [Google Scholar]
Liu 2016
- Liu L, Weber B, Oesterle A, Nayak HM, Upadhyay GA. A meta‐analysis of the efficacy, effectiveness, and safety of novel oral anticoagulants compared with vitamin K antagonists for stroke prevention in atrial fibrillation. Heart Rhythm 2016;1:S599. [Google Scholar]
Lloyd‐Jones 2004
- Lloyd‐Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, et al. Lifetime risk for development of atrial fibrillation: the Framingham heart study. Circulation 2004;110:1042‐6. [DOI] [PubMed] [Google Scholar]
Marini 2005
- Marini C, Santis F, Sacco S, Russo T, Olivieri L, Totaro R, et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke. Results from a population study. Stroke 2005;36:1115‐9. [DOI] [PubMed] [Google Scholar]
Mitchell 2013
- Mitchell SA, Simon TA, Raza S, Jakouloff D, Orme ME, Lockhart I, et al. The efficacy and safety of oral anticoagulants in warfarin‐suitable patients with nonvalvular atrial fibrillation: systematic review and meta‐analysis. Clinical and Applied Thrombosis/Hemostasis 2013;19(6):619‐31. [DOI: 10.1177/1076029613486539] [DOI] [PubMed] [Google Scholar]
Miyasaki 2006
- Miyasaki Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projection for future prevalence. Circulation 2006;114:119‐25. [DOI] [PubMed] [Google Scholar]
Morimoto 2015
- Morimoto T, Crawford B, Wada K, Ueda S. Comparative efficacy and safety of novel oral anticoagulants in patients with atrial fibrillation: a network meta‐analysis with the adjustment for the possible bias from open label studies. Journal of Cardiology 2015;66(6):466‐74. [DOI] [PubMed] [Google Scholar]
Mousa 2010
- Mousa SA. Oral direct factor Xa inhibitors, with special emphasis on rivaroxaban. Anticoagulants, Antiplatelets, and Thrombolytics. Springer Science+Business Media, 2010:181‐201. [DOI] [PubMed] [Google Scholar]
Pisters 2010
- Pisters R, Lane DA, Nieuwlaat R, Vos CB, Crijns HJ, Lip GY. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest 2010;138(5):1093‐100. [DOI] [PubMed] [Google Scholar]
Providencia 2014
- Providência R, Grove EL, Husted S, Barra S, Boveda S, Morais J. A meta‐analysis of phase III randomized controlled trials with novel oral anticoagulants in atrial fibrillation: comparisons between direct thrombin inhibitors vs. factor Xa inhibitors and different dosing regimens. Thrombosis Research 2014;134(6):1253‐64. [DOI: 10.1016/j.thromres.2014.10.002] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ruff 2014
- Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta‐analysis of randomised trials. Lancet 2014;15(383):955‐62. [DOI: 10.1016/S0140-6736(13)62343-0] [DOI] [PubMed] [Google Scholar]
Sudlow 1997
- Sudlow C, Rodgers H, Kenny RA, Thomson R. Population‐based study of the use of anticoagulants among patients with atrial fibrillation in the community. BMJ 1997;314:1529‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tahir 2013
- Tahir F, Riaz H, Riaz T, Badshah MB, Riaz IB, Hamza A, et al. The new oral anti‐coagulants and the phase 3 clinical trials ‐ a systematic review of the literature. Thrombosis Journal 2013;11(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tawfik 2016
- Tawfik A, Bielecki JM, Krahn M, Dorian P, Hoch JS, Boon H, et al. Systematic review and network meta‐analysis of stroke prevention treatments in patients with atrial fibrillation. Clinical Pharmacology 2016;8:93‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watson 2009
- Watson T, Shantsila E, Lip GYH. Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet 2009;373:155‐66. [DOI] [PubMed] [Google Scholar]
Wattigney 2003
- Wattigney WA, Mensah GA, Croft JB. Increasing trends in hospitalisation for atrial fibrillation in the United States, 1985 through 1999: implications for primary prevention. Circulation 2003;108:711‐6. [DOI] [PubMed] [Google Scholar]
Wolf 1991
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983‐8. [DOI] [PubMed] [Google Scholar]
Xiang 2016
- Xiang CL, Gong YZ, Zeng LJ, Wang R, Kea S, Chaudhary N, et al. Efficacy and safety or oral direct factor Xa inhibitors versus warfarin in patients with atrial fibrillation: a meta‐analysis of randomized controlled trials. Acta Cardiologica 2016;71(3):349‐57. [DOI] [PubMed] [Google Scholar]


