Abstract
Background
The first three years of a child's life are a key period of physical, physiological, cognitive and social development, and the caregiver‐infant relationship in early infancy plays an important role in influencing these aspects of development. Specifically, caregiver attunement facilitates the move from coregulation to self‐regulation; a parent's ability to understand their infant's behaviour as communication is a key part of this process. Early, brief interventions such as the Neonatal Behavioral Assessment Scale (NBAS) or Neonatal Behavioral Observation (NBO) system are potential methods of improving outcomes for both infant and caregiver.
Objectives
To assess the effects of the NBAS and NBO system for improving caregiver‐infant interaction and related outcomes in caregivers and newborn babies. Secondary objectives were to determine whether the NBAS and NBO are more effective for particular groups of infants or parents, and to identify the factors associated with increased effectiveness (e.g. timing, duration, etc.).
Search methods
In September 2017 we searched CENTRAL, MEDLINE, Embase, PsycINFO, 12 other databases and four trials registers. We also handsearched reference lists of included studies and relevant systematic reviews, and we contacted the Brazelton Institute and searched its websites to identify any ongoing and unpublished studies.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs that had used at least one standardised measure to assess the effects of the NBAS or NBO versus inactive control for improving outcomes for caregivers and their infants.
Data collection and analysis
Two reviewer authors independently assessed the records retrieved from the search. One reviewer extracted data, and a second checked them for accuracy. We presented the results for each outcome in each study as standardised mean differences (SMDs) or as risk ratios (RR) with 95% confidence intervals (CIs). When appropriate, we combined the results in a meta‐analysis using standard methodological procedures expected by Cochrane. We used the GRADE approach to assess the overall quality of the body of evidence for each outcome.
Main results
We identified and included 16 RCTs in this review: 13 assessing the NBAS and 3 the NBO for improving outcomes in 851 randomised participants, including parents and their premature or newborn (aged 4 to 12 weeks) infants. All studies took place in the USA, and we judged all of them to be at high risk of bias.
Seven studies involving 304 participants contributed data to one meta‐analysis of the impact of the NBAS or NBO for caregiver‐infant interaction, and the results suggest a significant, medium‐sized difference between intervention and control groups (SMD −0.53, 95% CI −0.90 to −0.17; very low‐quality evidence), with moderate heterogeneity (I2 = 51%). Subgroup analysis comparing the two types of programmes (i.e. NBAS and NBO) found a medium but non‐significant effect for the NBAS (−0.49, 95% CI −0.99 to 0.00, 5 studies), with high levels of heterogeneity (I2 = 61%), compared with a significant, large effect size for the NBO (−0.69, 95% CI −1.18 to −0.20, 2 studies), with no heterogeneity (I2 = 0.0%). A test for subgroup differences between the two models, however, was not significant. One study found a significant impact on the secondary outcome of caregiver knowledge (SMD −1.30, 95% CI −2.16 to −0.44; very low‐quality evidence). There was no evidence of an impact on maternal depression. We did not identify any adverse effects.
Authors' conclusions
There is currently only very low‐quality evidence for the effectiveness of the NBAS and NBO in terms of improving parent‐infant interaction for mostly low‐risk, first‐time caregivers and their infants. Further research is underway regarding the effectiveness of the NBO and is necessary to corroborate these results.
Plain language summary
The effectiveness of the Neonatal Behavioural Assessment Scale (NBAS) and Neonatal Behavioural Observation (NBO) system for parents and babies
Background
The first three years of a child's life comprise a key period of development, and the caregiver‐infant relationship in early infancy has been found to influence later outcomes. Caregiver sensitivity and ability to understand infant behaviours as communication is particularly important for infant development. Early, brief interventions such as the Neonatal Behavioural Assessment Scale (NBAS) or Neonatal Behavioural Observation (NBO) system can potentially improve outcomes for both infant and caregiver.
Review question
How effective is the NBAS or NBO for improving outcomes in parents and babies?
Included studies
We found 16 studies with a total of 851 participants. Of these, 13 studies evaluated the NBAS and 3 the NBO. All studies took place in the USA between 1981 and 2015.
Results
We were able to combine data from seven studies in a meta‐analysis (a statistical method of combining data from several studies to reach a single, more robust conclusion) measuring the impact of the NBAS on caregiver‐infant interaction. We found some evidence of effectiveness from very low‐quality studies. A comparison of the NBAS with the NBO for this outcome, however, suggested that while the NBO produced a larger effect size than the NBAS, this difference was not significant. One low‐quality study showed a positive impact on caregiver knowledge. We found no evidence of an impact on maternal depression. We did not identify any adverse effects as a result of the intervention.
Study quality
We considered the data for the main study outcomes to be of very low quality.
Authors' conclusion
There is currently very low‐quality evidence of the effectiveness that the NBAS and NBO improve parent‐infant interaction with low‐risk, first‐time parents and their infants. Ongoing studies with regard to the NBO will help to establish the accuracy of these results.
Summary of findings
Summary of findings for the main comparison. Summary of findings: NBAS or NBO versus control for caregiver‐infant interaction, caregiver mental health, and caregiver functioning.
| NBAS or NBO versus control for caregiver‐infant interaction, caregiver mental health, and caregiver functioning | |||||
|
Patient or population: caregiver‐infant dyads Settings: hospitals, clinics, home Intervention: NBAS or NBO Comparison: NBAS or NBO administered with no interaction | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control group | NBAS or NBO | ||||
|
Quality of caregiver‐infant interaction (parental sensitivity, attunement, etc.); continuous data Assessed at postintervention using validated assessment scales for caregiver‐infant interaction: higher scores indicate better outcome |
The mean score for quality of caregiver‐infant interaction ranged across control groups from 3.72 to 51.90 | The mean caregiver‐infant interaction score in the intervention groups was 0.53 lower (0.90 lower to 0.17 lower) | — | 304 (7 studies) | ⊕⊕⊝⊝ Very low1 |
|
Caregiver mental health (depression), dichotomous data Assessed at postintervention using the EPDS; lower scores indicate less depression |
Low‐risk population | RR 0.23 (0.05 to 1.04) | 106 (1 study) |
⊕⊕⊝⊝ Low2 | |
| 157 per 1000 |
36 per 1000 (8 to 163) |
||||
| Medium‐risk population | |||||
| NA | NA | ||||
| High‐risk population | |||||
| NA | NA | ||||
|
Infant social, emotional, cognitive and motor development Assessed when infant aged 4 months, using the BSID; higher scores indicate better development |
The mean score for infant mental development in the control group was 107.83 | The mean score for infant mental development in the intervention groups was0.13 lower (0.48 lower to 0.22 higher) | — | 125 (1 study) |
⊕⊕⊝⊝ Low2 |
|
Caregiver perception of infant (parents' perception of the degree of difficult temperament of the infant) Assessed at postintervention, 8 weeks after delivery; higher score indicates better outcome |
The mean score for caregiver perception of infant in the control group was 18.90 | The mean score for caregiver perception of the infant in the intervention group was 0.36 lower (0.95 lower to 0.24 higher) | — | 44 (1 study) |
⊕⊕⊝⊝ Low2 |
|
Caregiver stress (maternal perceptions of her adjustment to the parenting role) Assessed when infant aged 4 months, using the PSI |
The mean score for parent‐related caregiver stress in the control group was 2.19 | The mean score for parent‐related caregiver stress in the intervention groups was0.00 (0.35 lower to 0.35 higher) | — | 125 (1 study) |
⊕⊕⊝⊝ Low2 |
|
Caregiver knowledge (related to infants' physical capacities, including reflexes and senses) Assessed at postintervention, using multiple choice factual questions; higher scores indicate better outcome |
The mean score for caregiver knowledge in the control groups was8.30 | The mean score for caregiver knowledge in the intervention groups was 1.30 higher (0.44 to 2.16 higher) | — | 26 (1 study) |
⊕⊕⊝⊝ Very low1 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BSID: Bayley Scales of Infant Development; CI: confidence interval; EPDS: Edinburgh Postnatal Depression Scale; NA: not applicable; NBAS: Neonatal Behavioural Assessment Scale; NBO: Newborn Behavioural Observations system; PSI: Parenting Stress Index; RR: risk ratio; SMD: standardised mean difference. | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
1Downgraded due to risk of bias due to poor quality research (e.g. limitations in design, including inadequate randomisation or allocation procedures, together with attrition, which ranged from 4% to 20% in seven studies – none of which conducted an intention‐to‐treat analysis), inconsistency for the main outcome due to high levels of heterogeneity for the NBAS; and indirectness in terms of the low levels of generalisability to wider risk groups within the population.
2Downgraded due to risk of bias due to poor quality research (e.g. limitations in design, including inadequate randomisation or allocation procedures, together with attrition, which ranged from 4% to 20% in seven studies – none of which conducted an intention‐to‐treat analysis), and indirectness in terms of the low levels of generalisability to wider risk groups within the population.
Background
Description of the condition
The importance of attachment for children's development
The first three years of a child's life are a key period of physical, physiological, cognitive and social development (Richter 2004). The caregiver‐infant attachment relationship is important to a child's development (Bowlby 1969), and depending on its quality, the attachment relationship may confer a positive influence on a child or be a source of risk. For example, longitudinal research has identified some associations between children's social, emotional and cognitive outcomes and the caregiver‐infant attachment during the first year (Malekpour 2007; Sroufe 2005). Secure attachment in infancy has also been found to be associated with greater confidence and self‐regulation, more adaptive stress management, and more positive relationships in childhood and in later life (Sroufe 2005). Conversely, insecure and disorganised attachment in infancy is associated with unfavourable outcomes in later childhood, including problems with emotion regulation, social and behavioural issues, and lowered academic achievement (Berlin 2008; Granot 2001; Sroufe 2005), as well as an increased risk of a range of later forms of psychopathology, including externalising disorders (i.e. conduct and behaviour problems) (Fearon 2010); personality disorder (i.e. mental health problems characterised by enduring maladaptive patterns of emotional regulation, relating and behaviour) (Steele 2010); dissociation (Dutra 2005); post‐traumatic stress disorder (PTSD) (MacDonald 2008); and an increased likelihood of children experiencing symptoms that meet clinical criteria (Borelli 2010).
Attachment quality is not necessarily static (Belsky 1996; Vondra 2001), although it may remain stable between infancy and adulthood or change depending on a person's environment and experiences (Egeland 1984; Weinfield 2000). Therefore, the influence of attachment security on development may be best understood in terms of the risk or protection it delivers, rather than as a causal relationship, taking into account a given child's context and history (Belsky 2002). Although dynamic, and with the potential for discontinuity as well as continuity, attachment status in infancy is considered to be an important predictor of a child's long‐term development (Grossman 2002; Sroufe 2005; Waters 2000).
The neurobiology of attachment – regulation theory
Neurobiological research indicates that caregivers play a key role throughout the first year of life in the development of an infant's brain and physiology through regulation of the infant's developing stress response system (Gunnar 1996; Gunnar 2002; Schore 2002; Schore 2008). A caregiver who is able to interpret an infant's behavioural cues and respond appropriately to meet the child's emotional and physical needs, called an 'attuned' or 'responsive' caregiver, uses his or her vocalisations, facial expressions and physical handling to provide continuous psychobiological regulation of the infant's ever‐changing states of stress and arousal (Schore 2001). Over time, this dyadic regulation allows the infant to develop adaptive strategies for responding to and regulating stress independently, which then enables the child to be less vulnerable to future stress (Sroufe 2005). When a caregiver is severely and chronically misattuned, however, typical dyadic regulatory processes are disrupted, such that, in the face of chronically high levels of stress that are not successfully coregulated by the caregiver, the infant may develop only minimal or maladaptive strategies for self‐regulation (Gunnar 2007; Lyons‐Ruth 2005). Infant regulatory difficulties expressed as 'excessive crying' and problems with attachment, sleeping, and feeding are the primary reasons for referral to infant mental health services (Keren 2001). DeGangi 2000 found that all but five per cent of a group of infants who were experiencing moderate regulatory problems at seven months (i.e. problems with sleep and feeding; ability to self‐soothe and modulate affect states; ability to regulate mood, and emotional and behavioural control), were experiencing caregiver‐child relationship problems or developmental delays in the cognitive, motor, and language domains at age three. This finding highlights how important it is for children to have attuned caregivers to support them in developing adaptive stress response systems and regulatory capacity.
Understanding infant behaviour as communication
The successful shift from coregulation to self‐regulation happens within the context of a secure attachment relationship with a caregiver who can meet the infant's needs (Beebe 2010). In order to successfully identify and meet these needs, a caregiver must understand that infant behaviour is a communication of their needs, wants and preferences, and that these behavioural cues can be interpreted and merit a prompt appropriate response (Nugent 2007).
When caregivers understand that behaviour represents a communication of need, they can support their babies' growing ability to be sufficiently coregulated to enter into and remain in the quiet‐alert interactive state by ensuring all other physical needs are met (Hawthorne 2005; Nugent 2007). This facilitation may allow for longer or more frequent periods of contingent communication to take place. As early as five weeks of age, infants whose caregivers meet their needs and provide social interaction when the infants are available for it have been found to participate more readily during interactions by engaging in more "gazing, smiling and vocalizing" than infants of less responsive caregivers (Markova 2006). In turn, the more infants look, smile and vocalise at their mothers, the more affectionate the mothers' behaviours toward the infants become, reflecting the bi‐directional influence of both caregivers and infants to their interactive context (Clarke‐Stewart 1973).
Contingency in caregiver‐infant communication
A key factor influencing the quality of interaction patterns is the degree of contingency, or level of predictability and synchronicity of responses, between the parent‐infant dyad (Beebe 2012). Readiness of an infant to engage with the parents' emotional communications (i.e. by touch, sight or sound) are influenced by a variety of factors, including components of visceral motor regulation, which require subtle aspects of timing to achieve attunement (Porges 2011). A number of studies have now measured interpersonal contingent co‐ordination in mother‐infant dyads, some using the specialist technique microanalysing interaction (see Beebe 2010 for a summary). These show that interaction that is within what is defined as the midrange (i.e. infant and caregiver respond optimally to each others' cues without hypervigilance or withdrawal, and within repeated cycles characterised by synchrony, rupture and repair) is most optimal, in terms of parental sensitivity (Bornstein 2013); they also show an association with infant attachment security at 12 months (Beebe 2010; Beebe 2012). This contrasts with interaction that is either 'over‐contingent' (i.e. characterised by hypervigilance and intrusion) or 'under‐contingent' (i.e. characterised as inhibited and withdrawn), both of which are associated with insecure and disorganised attachment (Beebe 2010; Beebe 2012). This sensitivity is associated with the development of a secure attachment relationship (De Wolff 1997), which in turn is associated with positive developmental outcomes, including resilience and optimal social functioning (Lecce 2008).
Attempts to understand the correlates of infant attachment have more recently focused at the cognitive level on "reflective functioning" (Slade 2001), and "mind‐mindedness" (Meins 2012; Meins 2001), which refer to the caregiver's ability to accurately interpret the infant's thoughts and feelings. Different studies have associated reflective functioning with caregiver responsiveness (Greinenberger 2005; Slade 2001; Slade 2005), while low reflective functioning is associated with caregiver withdrawal, intrusiveness and hostility (Greinenberger 2005; Slade 2001; Slade 2005). Similarly, maternal mind‐mindedness has been found to be associated with infant attachment security at 12 months of age (Meins 2012; Meins 2001). Thus, when the caregiver views the infant as an individual with thoughts and feelings that can be imagined and interpreted through the infant's behaviour, the relationship is more likely to be characterised by responsive, contingent interactions to meet the infant's needs (Greinenberger 2005; Slade 2001; Slade 2005). Interventions that foster caregiver contingency or mind‐mindedness, therefore, have a role to play in fostering positive caregiver‐infant interaction and promoting optimal, long‐term, developmental outcomes in children.
Description of the intervention
Possibly one the earliest interventions that was developed to improve caregiver‐infant interaction at the behavioural level with a specific focus on caregiver responsiveness during the earliest days and months of the infant's life is the Brazelton Neonatal Behavioral Assessment Scale (NBAS) (Brazelton 1978), along with its shorter clinical variation, the Newborn Behavioral Observations (NBO) system. Paediatrician Dr T Berry Brazelton developed the NBAS in the 1970s as a way of assessing a newborn's neurological functioning and ability to participate actively in interaction (Brazelton 1995). In the 1980s, clinicians began using it as an intervention to support caregivers in understanding their infants' behaviour (Hawthorne 2004; Teti 1996). The NBAS and NBO direct the caregiver's attention toward the infant's behaviour; address the caregiver's awareness and understanding of newborn infants as unique individuals whose behaviours can be understood as meaningful communication of needs, wants, abilities, challenges, and preferences; and model contingent responses to these behaviours.
The NBAS can be used with healthy, full‐term infants from a few hours after birth to two months postpartum (Brazelton 1995), with stabilised preterm infants from 35 weeks' gestational age, and with otherwise developmentally delayed infants until much later (Hawthorne 2004). During an NBAS session, a qualified clinician (e.g. a paediatrician, midwife, psychologist, etc. trained to deliver the NBAS) administers as many as possible of the 28 behavioural and reflex items, given the time available and the infant's willingness and ability to engage, recording the infant's responses on a standardised scoring sheet. These items are designed to reveal the infant's unique social‐interactive and neurodevelopmental capabilities and difficulties, including orientation to human voices and other sounds, muscle tone, and self‐soothing (Brazelton 1995).
In 2007, the NBO – originally called the Clinical NBAS – was published, having been developed from the NBAS framework as a clinical tool for supporting caregivers in their relationships and interactions with their infants (Nugent 2007). The main difference during an NBO session is that the facilitator may select from 18 neurobehavioural items as opposed to 28, so an NBO session is typically much shorter than an NBAS session. The NBO can be used with healthy infants from a gestational age of 36 weeks to three months "post‐term", and with preterm or medically fragile infants who are stable enough to be close to hospital discharge or who are postdischarge at home (Nugent 2007).
Clinicians can administer both the NBAS and NBO at home, in hospital or in another clinical setting such as a children's centre. The instruments are designed to be flexibly tailored to each caregiver‐infant dyad. Both instruments have been used with caregivers who are considered low‐vulnerability (e.g. middle‐class, high educational attainment, married, etc.) and high‐vulnerability (e.g. low‐socioeconomic status, low educational attainment, young, depressed, drug‐using, etc.), with mothers and fathers, and with first‐time and experienced caregivers, with varying effectiveness on caregiver and infant outcomes (see Why it is important to do this review for further detail). The developers recommend that families participate in sessions anywhere from once to three or more times depending on resources and other factors such as infant vulnerability, positing that a greater number of sessions, spread over the first two to three months, provides a more detailed picture of the infant during a period of development.
Both the NBAS and NBO are used in research settings to explore associations between observed infant behaviours and antecedent variables or later outcomes and to evaluate their effectiveness as tools for assessing infant behaviour or enhancing given caregiver and infant outcomes. In clinical settings, they constitute intervention tools to support caregivers and their infants. Two key guidelines for primary care practice with caregivers and newborns in the United Kingdom, NHS England 2014 and WAVE Trust 2013, both recommend the NBAS and NBO.
How the intervention might work
A critical attachment‐related task for a caregiver in the newborn period is to understand the infant's behaviour (e.g. crying, body movement, sleeping, gaze toward or away from people or objects, etc.) as communication (Golas 1986). When a caregiver appropriately interprets and responds to this communication, the resulting prompt and contingent caregiving interactions are described as being responsive (e.g. Eshel 2006), attuned (e.g. Brazelton 1995), or sensitive (e.g. Ainsworth 1974). This type of caregiving, tailored to the needs and wants of the infant, has positive implications for the infant's developing stress response system and social abilities (Schore 2001; Sroufe 2005), enabling the caregiver to feel knowledgeable (Britt 1994a), confident (Hawthorne 2005; Ohgi 2004; Teti 1996), and satisfied in the caregiver role (Rauh 1988). This contributes to a caregiver‐infant relationship that is healthy and gratifying for both partners.
Alternatively, when a caregiver does not recognise the infant's behaviour as communication, they either may not respond to the behaviour, which in the extreme may constitute neglect, or may respond to these behaviours in a way that is non‐contingent (inappropriate to meet the infants' needs), abusive, frightening or unpredictable (Jacobvitz 1997; Lyons‐Ruth 2005). These interactive patterns, marked by a lack of responsiveness resulting from a mismatch of infant cues and caregiver interpretation and response, are associated with the development of insecure or disorganised attachment (Beebe 2010), with implications for a child's long‐term development (Berlin 2008; Granot 2001; Green 2002; Sroufe 2005).
Given that these patterns begin to become established by the time a child is three months old (Crockenberg 1982), the newborn period and early infancy is an opportune window for universal, early, preventive intervention with caregivers (Brazelton 1995). The NBAS or NBO may be universally and preventively used from the earliest moments in this window to support the emergence of positive caregiver‐infant interactions (Myers 1982b; Nugent 2007; Poley‐Strobel 1987), even in the face of caregiver challenges in adjusting and interacting with a child (Beeghly 1995b). Such intervention in the earliest days of the child's life have the potential to benefit the caregiver‐infant relationship, mediate potential risk factors for the child's development within this relationship (Myers 1982b; Poley‐Strobel 1987), and enhance the caregiver's satisfaction, confidence and experience of the relationship (Hawthorne 2005).
Why it is important to do this review
Proponents of the NBAS have suggested that its use may improve a caregiver's responsiveness (Das Eiden 1996), increase caregiver involvement (Worobey 1985), improve a caregiver's attitudes toward the infant and satisfaction in the caregiving role (Britt 1994b), and enhance caregiver confidence in their role and in understanding the infant (Hawthorne 2005). Although these suggested benefits are theoretically supported, in that caregiver responsiveness, involvement, satisfaction and confidence are related to caregiver‐infant interaction quality (see Eshel 2006; Gaertner 2007; Isabella 1994; Shea 1988, respectively), and the quality of the caregiver‐infant relationship is related to the developmental outcomes of the infant (Sroufe 2005), recent reviews of the effectiveness of the NBAS have been inconclusive (Britt 1994b; Das Eiden 1996; Worobey 1985). However, none of the three existing reviews used a systematic methodology, and therefore selection bias may have influenced their results.
These reviews also had further methodological and reporting problems, and all are now out‐dated, with new primary studies emerging since their publication. One meta‐analysis of 13 randomised controlled trials (RCTs) that found a small‐to‐moderate beneficial effect on "parenting quality" calculated an average global effect size for a single outcome across the 13 included studies (Das Eiden 1996). While seemingly providing conclusive evidence of effectiveness, the review presented the average effect size without explicitly noting that most of the included studies (k = 10) had effect sizes of less than 0.20, with just three studies increasing the average through small‐to‐medium effect sizes (0.48, 0.39 and 0.28). Britt 1994b also reviewed 13 studies, including unpublished reports, but only provided a narrative synthesis. Worobey 1985 only selected studies for inclusion that had demonstrated effectiveness in at least one of its outcomes.
Evidence about effectiveness and cost‐effectiveness are key to enabling decisions about how best to support caregivers of newborns. Despite the numerous evaluations of the effectiveness of the NBAS and NBO as an intervention, there is currently no reliable systematic review of this evidence to assist with decision‐making about its use. In the context of this lack of evidence, this review will enable us to assess the extent to which key recommendations for its use in primary care practice are valid (NHS England 2014; WAVE Trust 2013).
This review aims to improve upon previous reviews by using a systematic methodology to identify and appraise all rigorous studies published since the first NBAS manual in 1973 that have used the NBAS or NBO as an intervention, in order to provide an updated analysis of the effectiveness of the NBAS and NBO for enhancing a range of aspects of caregiving (see Bartram 2016 for study protocol).
Objectives
To assess the effects of the NBAS and NBO system for improving caregiver‐infant interaction and related outcomes in caregivers and newborn babies. Secondary objectives were to determine whether the NBAS and NBO are more effective for particular groups of infants or parents, and to identify the factors associated with increased effectiveness (e.g. timing, duration, etc.).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs (i.e. trials in which participants were allocated using a method that is not truly random, such as alternate allocation).
Types of participants
All caregiver and infant participants eligible to receive the Neonatal Behavioral Assessment Scale (NBAS) or Newborn Behavioral Observations (NBO) system.
Types of interventions
Experimental intervention
Studies using the NBAS or the NBO in full or as part of an intervention (as opposed to an assessment). We excluded studies in which the NBAS or NBO was provided as part of a suite of services, and in which independent effects could not be estimated.
We considered the NBAS to be used as an 'intervention' when investigators employed it with intent to influence an aspect of the infant's psychological or physical development, an aspect of caregiving (e.g. parental confidence, involvement, responsiveness, etc.) or a feature of the dyadic relationship (e.g. attunement, reciprocity, attachment quality, etc.).
Comparator intervention
Studies that utilised an inactive control group (e.g. wait‐list, placebo, care‐as‐usual, or no‐treatment) were eligible for inclusion, but not those using active control groups (e.g. receiving a variation of the NBAS or NBO, or an alternative intervention), in order to ensure that we could evaluate the primary rather than the relative effectiveness of the NBAS and NBO. We did not use other eligibility restrictions for the control group.
Types of outcome measures
All outcomes conceptually related to infant health and development, caregiver‐infant dyadic variables, and caregiver variables related to caregiving, including mental health, were of interest; we did not use outcomes as criteria for study inclusion. Trials could have measured outcomes at any time point in order to examine the short‐, medium‐, and long‐term effectiveness of the NBAS or NBO (Higgins 2016). For scales in which an increase (i.e. as opposed to a decrease) in score represents an improvement, we multiplied mean values by −1 to ensure that all scales pointed in the same direction. We provide examples of outcomes and measurements below.
Primary outcomes
Quality of caregiver‐infant interaction, including frequency and contingency (e.g. CARE index (Crittenden 2001), Nursing Child Assessment Teaching Scale (NCATS; Barnard 1978)), sensitivity and responsiveness (e.g. Nursing Child Assessment of Feeding Scale (NCAFS; Sumner 1994)), and responsiveness to infant distress (Britt 1994a).
Caregiver mental health (e.g. Edinburgh Postnatal Depression Scale (EPDS; Cox 1987)).
Adverse effects or unintended consequences (e.g. outcomes, such as caregiving quality, that worsen following the intervention).
Secondary outcomes
Infant social, emotional, cognitive, and motor development (e.g. Bayley Scales of Infant Development (BSID; Bayley 1969), Denver Developmental Screening Test (DDST; Frankenburg 1967), NBAS (motor and reflex items; Brazelton 1995), and McCarthy General Cognitive Index (GCI; McCarthy 1972)).
Infant physical health (e.g. anthropometric measures (see Cooper 2002), immunisation rates (see Koniak‐Griffin 2002), number of hospitalisations (see Koniak‐Griffin 2002), and health outcomes based on medical records (see Koniak‐Griffin 1999; Koniak‐Griffin 2000; Koniak‐Griffin 2003)).
Caregiver perception of infant (e.g. Broussard's Neonatal Perception Inventory (NPI; Broussard 1970)).
Caregiver stress (e.g. Parenting Stress Index (PSI; Abidin 1983)).
Caregiver reflective functioning (e.g. Parent Development Interview (PDI; Slade 2004)).
Caregiver satisfaction in the caregiving role (e.g. Myer's satisfaction estimation (Myers 1982b)).
Caregiver confidence, self‐efficacy, and self‐esteem in the caregiving role (e.g. Myer's confidence rating scale (Myers 1982b)).
Caregiver knowledge of infant behaviour (e.g. Maternal Developmental Expectations and Childrearing Attitudes Scale (MDECAS; Widmayer 1980).
Search methods for identification of studies
Electronic searches
Using the strategies in Appendix 1, we searched the electronic databases and trial registers listed below with no restrictions based on date of publication, language or publication status. We ran our initial searches in March 2015 and updated them in September 2016 and September 2017, except for the trials registers, which we searched on 13 October 2017.
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 8) in the Cochrane Library, and which includes the Cochrane Developmental, Psychosocial and Learning Problems Specialised Register (searched 14 September 2017).
MEDLINE Ovid (1946 to Week 37 2017).
Embase Ovid (1974 to Week 37 2017).
Cochrane Database of Systematic Reviews (CDSR; 2017, Issue 9) part of the Cochrane Library (searched 14 September 2017).
Database of Abstracts of Reviews of Effects (DARE; 2015, Issue 2) part of the Cochrane Library (searched 14 September 2017).
PsycINFO OVID (1806 to 14 September 2017).
CINAHL EBSCOhost (Cumulative Index to Nursing and Allied Health Literature; 1982 to 14 September 2017).
Science Citation Index Web of Science (SCI; 1970 to 14 September 2017).
Social Sciences Citation Index Web of Science (SSCI; 1970 to 14 September 2017).
Conference Proceedings Citation Index ‐ Science Web of Science (CPCI‐S; 1990 to 14 September 2017).
Conference Proceedings Citation Index ‐ Social Science & Humanities Web of Science (CPCI‐SS&H; 1990 to 14 September 2017).
BIOSIS (ISI) Web of Science (1969 to 12 September 2017; searched 14 September 2017).
ERIC EBSCOhost (Education Resources Information Center; 1966 to 14 September 2017).
SCOPUS Elsevier (1996 to 14 September 2017).
Sociological Abstracts ProQuest (1952 to 14 September 2017).
LILACS (Latin American and Caribbean Health Science Information database; lilacs.bvsalud.org/en; 1982 to 14 September 2017).
ClinicalTrials.gov (clinicaltrials.gov; searched 13 October 2017).
ISTRN (www.isrctn.com; searched 13 October 2017).
UK Clinical Research Network Study Portfolio (public.ukcrn.org.uk; searched 13 October 2017).
World Health Organisation International Clinical Trials Registry Platform (WHO ICTRP; who.int/ictrp/en; searched 13 October 2017).
Searching other resources
We also:
scanned reference lists of relevant reports identified through database searches to identify further relevant studies;
ran forward citation searches for relevant studies;
scanned bibliographies of systematic and non‐systematic reviews, plus the NBAS and NBO training manuals to identify relevant studies; and
contacted the Brazelton Institute and searched brazelton.co.uk and brazelton‐institute.com, to identify unpublished and ongoing studies.
Data collection and analysis
Selection of studies
Two review authors (JB and CBT) independently screened titles and abstracts of studies identified through electronic searches, to assess whether they met the inclusion criteria (see Criteria for considering studies for this review), and they excluded clearly irrelevant abstracts. Next, both review authors independently screened full‐text copies of reports that appeared to meet the inclusion criteria or for which more information was needed to decide on eligibility. We resolved any uncertainties by discussion with NH. CBT documented the search and selection processes, recording decisions in a PRISMA flow chart (Moher 2009).
Data extraction and management
NH extracted data from included studies using a piloted data extraction form and entered the data into Review Manager 5 (RevMan 5) (RevMan 2014). JB checked the data extraction, and the review authors resolved any uncertainties by discussion with a third review author (CBT). We contacted authors of studies that were not written in English to request a copy or the required information in English. We examined all relevant retraction statements and errata (Higgins 2016).
We extracted information on the following: study funding source, declarations of conflict of interest of the primary investigators, participant demographics, duration and content of each session, number of sessions, study design, group allocation, sample size, outcomes measured (and their validity, reliability, time points for assessment, and whether researchers were blinded), participant attrition, and results.
We assessed the extent to which studies had reported whether confounding factors may have influenced the results and to which these had been controlled for in the primary analysis: caregiver age, education, income or socioeconomic status, marital status, parity; and age and sex of infant (see also Dealing with missing data section).
Assessment of risk of bias in included studies
NH independently assessed the risk of bias of the included studies, using the Cochrane 'Risk of bias' assessment tool (Higgins 2017), across the following domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias. JB checked this process. We resolved differences in consultation with CBT.
For each included study, we described and assessed each domain as being at low, high or unclear risk of bias, with justification for these judgments (see also Dealing with missing data section). We list the 'Risk of bias' domains that we assessed in Appendix 2.
Measures of treatment effect
Dichotomous outcome data
Only one study reported dichotomous outcome data for one standardised measure (i.e. depression). We presented this outcome using the risk ratio (RR) and 95% confidence intervals (CI).
Continuous outcome data
All included studies measured at least one outcome using standardised tools that provided continuous data (i.e. means and standard deviations). We analysed the data using the standardised mean difference (SMD). We calculated the SMD by dividing the mean difference (MD) in postintervention scores between the intervention and control groups by the standard deviation of the outcome among participants. We presented the SMD with 95% CI for individual outcomes in individual studies. Where these data were not available, we presented significance levels as reported in the paper.
Unit of analysis issues
We did not encounter any unit‐of‐analysis issues. Please see Bartram 2016 and Table 2.
1. Unused methods table.
| Unit of analysis issues |
Cluster‐randomised trials The randomisation of clusters can result in an overestimate of the precision of the results (with a higher risk of a type I error) when their use has not been compensated for in the analysis. Had we included a cluster‐RCT, we planned to explore whether the authors had adequately controlled for the effects of clustering in the study. When they had, and when there was little difference between the study designs, and when there was unlikely to be an interaction between the effect of the intervention and the choice of randomisation method, we planned to combine the data from the cluster‐RCT with data from individual RCTs. When the effects of clustering had not been controlled for properly, we planned to derive an estimate of the intracluster correlation coefficient (ICC) from the study or that of a similar population, and to report whether an ICC had been used and conduct sensitivity analyses to determine the effect of using an ICC. We also planned to assess the impact of including data from a cluster‐RCT on the inclusion of the study in the meta‐analyses using a sensitivity analysis to explore the effects of the randomisation method. However, no cluster RCTs were identified or included. |
|
Trials with multiple treatments groups In the event that we had identified a multi‐arm study in which the NBAS and NBO had been compared with an alternative treatment and a control group, we planned to only extract data from two arms (e.g. NBAS and control group). In the event that we had identified a multi‐arm study in which the NBAS had been compared with the NBO and involved only one control group, we planned to combine the data from the NBAS and NBO arms for primary analyses and to conduct secondary, subgroup analyses and split the control group data. However, we identified no multiple treatment groups. | |
|
Cross‐over trials Cross‐over trials are not possible with this type of intervention, and none were identified. | |
| Assessment of reporting bias | We planned to draw funnel plots (estimated differences in treatment effects against their standard error) if there was a sufficient number of included studies (e.g. more than 10), to identify asymmetry due to publication bias and other small study effects. We also planned to assess whether there had been selective reporting of outcomes and to assess the impact of this using a sensitivity analysis. However, there were insufficient studies to undertake this analysis. |
| Subgroup analysis and investigation of heterogeneity | We planned to explore possible reasons for heterogeneity by undertaking the following, additional subgroup analyses, scrutinising studies to determine the extent of between‐trial differences.
However, it was only possible to undertake subgroup analysis for NBAS versus NBO, due to the small number of studies. |
| Sensitivity analysis | We planned to conduct sensitivity analyses on the basis of method of sequence generation only, to assess the robustness of the results, but this was not possible due to the small number of studies. |
NBAS: Neonatal Behavioral Assessment Scale; NBO: Newborn Behavioral Observations system; RCT: randomised controlled trial.
Dealing with missing data
When appropriate, we contacted study authors to request missing data. When we were unable to obtain them, we reported the available data only. We did not use imputation because the data were not presented in a suitable format (i.e. only the results of t‐tests of ANOVA's were provided).
We described the extent of the missing data and attrition for each included study and reported our findings in the 'Risk of bias' tables, beneath the Characteristics of included studies tables. We also reported the number of participants included in the final analysis as a proportion of all participants in each study. We provided a narrative summary of the reasons for missing data in the Results section, where we considered their possible impact on the results of the review.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by considering the extent of between‐trial differences, including methods, populations, interventions or outcomes. We assessed statistical heterogeneity using the I2 statistic. The importance of the observed value of I2 depends on the magnitude and direction of effects and strength of evidence for heterogeneity (e.g. P value from the Chi2 test, or a CI for I2) (Higgins 2002). An I2 with a significance level less than 0.10 is treated as evidence of heterogeneity. We also reported the estimate of between‐study variance for each meta‐analysis using Tau2 – an absolute measure of heterogeneity.
Assessment of reporting biases
We were unable to assess reporting biases. Please see Bartram 2016 and Table 2.
Data synthesis
We combined data for each outcome across studies using both the NBAS and the NBO, as these interventions were clinically homogenous enough in terms of their populations, methods of delivery and the outcomes assessed, to justify this procedure (Higgins 2016). We used the random‐effects model, weighted by the inverse of the variance for continuous data and the Mantel‐Haenszel method for dichotomous data, because we found high levels of variation in the results, possibly because the studies took place over a 30‐year period. When it was not possible to combine data, we presented results from single studies (i.e. individual effect sizes and 95% CI).
Subgroup analysis and investigation of heterogeneity
We explored possible reasons for heterogeneity by undertaking a subgroup analysis comparing one outcome (i.e. sensitivity) across the two different models of provision (i.e. NBAS versus NBO). No further subgroup analyses were possible. Please see Bartram 2016 and Table 2.
Sensitivity analysis
We were unable to conduct any of our preplanned sensitivity analyses. Please see Bartram 2016 and Table 2.
Summary of findings table
We applied the five GRADE considerations (limitations, effect consistency, imprecision, indirectness and publication bias) to the body of evidence for each outcome to assess its quality (Higgins 2016). When necessary, we downgraded the quality of the evidence by reducing the rating one or two levels for each factor, three levels being the maximum number of downgrades possible. The potential ratings for RCTs are listed below (Higgins 2016).
High quality: randomised trials.
Moderate quality: downgraded randomised trials.
Low quality: double‐downgraded randomised trials.
Very low quality: triple‐downgraded randomised trials.
JB and NH assessed the quality of the body of evidence for each of the following outcomes: quality of caregiver‐infant interaction; caregiver mental health; infant social, emotional, cognitive development; caregiver perception of infant; caregiver stress, and caregiver knowledge. We presented these outcomes and their ratings in Table 1, which we created using GRADEpro GDT (GRADEpro GDT 2015), for the following comparison: NBAS/NBO versus control for caregiver‐infant interaction, caregiver mental health, and caregiver functioning.
Results
Description of studies
Results of the search
In total, our searches yielded 9889 records, from which we identified and eliminated 2843 duplicates. Of the 7046 remaining records, we identified 40 as potentially relevant. We obtained the full texts of these records and reviewed them against our inclusion criteria (Criteria for considering studies for this review). We excluded 24 studies and included 16 (see Characteristics of included studies; Characteristics of excluded studies). We also identified three ongoing studies through contact with the Brazelton Centre (See 'Ongoing studies' below) (NCT03070652; Nicolson 2016; Nugent 2016).
See Figure 1.
1.
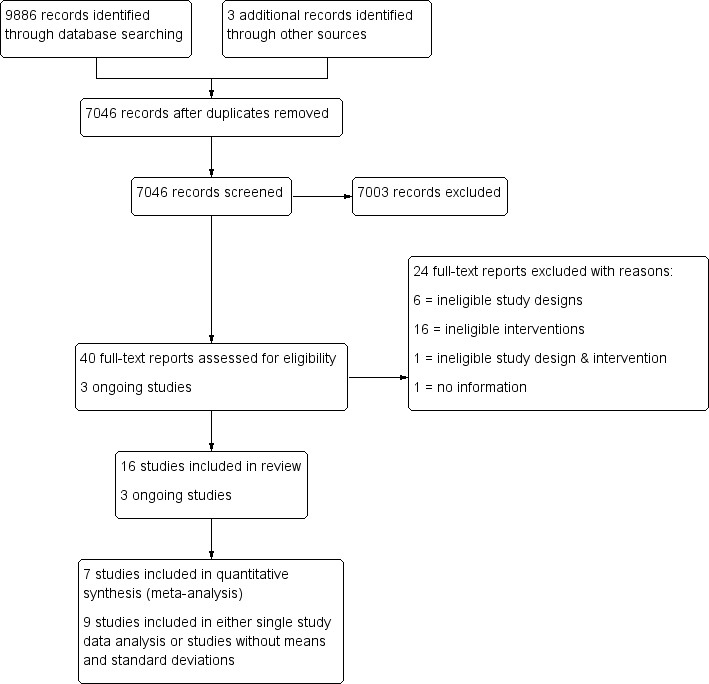
Study flow diagram.
Included studies
This review includes 16 studies (Anderson 1981; Beal 1989; Beeghly 1995a; Belsky 1985; Britt 1994; Furr 1982; Killough 2004; Liptak 1983; McManus 2012; Myers 1982a; Nugent 2014; Nugent 2015; Pannabecker 1982; Poley‐Strobel 1987; Szajnberg 1987; Worobey 1982). We provide further details about the included studies below and in the Characteristics of included studies tables.
Design
All 16 included studies were randomised controlled trials (RCTs). None of the included studies utilised a cluster design.
Ten studies were two‐arm comparisons of the NBAS or NBO against a single control group (Beal 1989; Beeghly 1995a; Belsky 1985; Killough 2004; McManus 2012; Myers 1982a; Nugent 2014; Nugent 2015; Poley‐Strobel 1987; Szajnberg 1987). The remaining six studies utilised more than one control/condition group (Anderson 1981; Britt 1994; Furr 1982; Liptak 1983; Pannabecker 1982; Worobey 1982).
Of the 10 studies that used only two conditions, control conditions in six studies consisted of treatment‐as‐usual (Killough 2004; McManus 2012; Nugent 2014; Nugent 2015; Poley‐Strobel 1987; Szajnberg 1987), in one the use of the NBAS in the physical absence of a caregiver (Beeghly 1995a), and in one the NBAS without involvement of the caregiver (Beal 1989); one study, Myers 1982a, had two intervention groups (mother treatment, father treatment) and one control group; and one study, Belsky 1985, had two intervention groups (mother treatment, mother and father treatment) and two control groups (mother control, mother and father control).
The six studies with more than one control group were as follows. Anderson 1981 had two control groups in addition to the experimental group. In one group, NBAS was performed on infants, but mothers did not receive immediate feedback and instead were offered a class on infant furnishings; for the second group, NBAS was performed on the infants and, following each assessment, the investigator met with the mother and explained the assessment. We included the first control condition in our analysis. Britt 1994 used three groups in addition to the NBAS group. In one group, the examiner talked with the mother about her baby; in the second group, mothers received a questionnaire assessment of the NBAS to complete; and the third group underwent an NBAS assessment of the baby as usual plus a questionnaire assessment. Our analysis included the control group where the experimenter talked with the mother about her baby. The remaining four studies did not provide means and standard deviations, so we presented data for all groups from analyses of variances (ANOVAs), which cannot be disaggregated for control group. In Furr 1982, the mothers were assigned to three other groups in addition to the intervention, which was maternal orientation to newborn behaviour using the Infant Behaviour Assessment Record (IBAR) derived from NBAS. In one group, feeding was observed on day two and IBAR derived from NBAS was then obtained; in the second group a feeding observation was carried out on the second day; the third group did not receive feeding observation or IBAR derived from NBAS. In Pannabecker 1982, the first control group was shown a videotape of a typical normal newborn, whereas the second control group received no educational information and no extra contact with their infants. Worobey 1982 had two control groups: for one group, immediately after the behavioural assessment of the newborn, the mother received a verbal summary of her infant's performance; for the second group, mothers observed a demonstration of NBAS on their newborns and were given the opportunity to talk about what they were seeing. In Liptak 1983, one control group of infants received the usual newborn care in the absence of parents, and the other control group infants received usual newborn care along with complete physical examination of baby in front of the mother.
Years of publication
Most studies were old in the sense that they took place between 1981 and 1995. Only four studies were more recent (Killough 2004; McManus 2012; Nugent 2014; Nugent 2015).
Setting
All included studies took place in a single centre in the USA.
Sample sizes
There was considerable variation in sample size between studies. Altogether the 16 included studies initially randomised 851 participants, with sample sizes ranging from 20 in Poley‐Strobel 1987 to 125 in Beeghly 1995a (mean N = 53.2 and median N = 43).
Recruitment
All participants were recruited from maternity wards in both rural and urban community settings. They included prenatal mothers (Belsky 1985), mothers postdelivery (Anderson 1981; Beeghly 1995a; Britt 1994; Furr 1982; Killough 2004; Liptak 1983; McManus 2012; Myers 1982a; Nugent 2014; Nugent 2015; Poley‐Strobel 1987; Szajnberg 1987; Worobey 1982), fathers who were present during labour and delivery (Beal 1989), and parents attending pre‐ and postnatal classes (Pannabecker 1982).
The intervention was initially delivered in a newborn nursery (Beeghly 1995a), mother's hospital room/anteroom (Anderson 1981; Beal 1989; Myers 1982a; Nugent 2014; Nugent 2015), a specially designated room (Belsky 1985), an unspecified place in the hospital (Britt 1994; Furr 1982; Killough 2004; Liptak 1983; Pannabecker 1982; Szajnberg 1987; Worobey 1982), or at home (McManus 2012; Poley‐Strobel 1987).
Participants
An inclusion criterion was that participants were all caregivers and infants eligible to receive the NBAS or NBO system. In 11 studies participants were mothers (Anderson 1981; Beeghly 1995a; Britt 1994; Furr 1982; Killough 2004; Liptak 1983; Nugent 2014; Nugent 2015; Poley‐Strobel 1987; Szajnberg 1987; Worobey 1982); 2 studies recruited fathers (Beal 1989; Pannabecker 1982); 2 studies, both parents as a couple (Belsky 1985; McManus 2012); and one study, mothers and fathers separately (Myers 1982a).
Seven studies included primiparous mothers (Anderson 1981; Furr 1982; Killough 2004; Liptak 1983; Nugent 2014; Nugent 2015; Poley‐Strobel 1987). Two studies included first‐time couples, although Myers 1982a included them separately, while Belsky 1985 included some couples and some mothers only. One study, McManus 2012, included both parents but did not describe their parity, and another, Britt 1994, included women who were abusing drugs irrespective of parity. Two studies included fathers: one specified including first‐time fathers (Beal 1989), and the second did not describe parity (Pannabecker 1982). Three studied did not describe mothers' parity (Beeghly 1995a; Szajnberg 1987; Worobey 1982).
The sex of participating infants was not specified in nine studies (Beal 1989; Belsky 1985; Britt 1994; Furr 1982; Liptak 1983; Nugent 2014; Nugent 2015; Pannabecker 1982; Szajnberg 1987). In six studies participating infants included both boys and girls (Beeghly 1995a; Killough 2004; McManus 2012; Myers 1982a; Poley‐Strobel 1987; Worobey 1982). One study included only female infants (Anderson 1981).
In 12 studies the age of participating infants was four days or less (Anderson 1981; Beal 1989; Beeghly 1995a; Belsky 1985; Furr 1982; Killough 2004; Myers 1982a; Nugent 2014; Nugent 2015; Pannabecker 1982; Poley‐Strobel 1987; Worobey 1982); age was less than 12 weeks in one study, McManus 2012, and less than 34 weeks gestational age in another (Szajnberg 1987). Two studies did not describe the age of the infant (Britt 1994; Liptak 1983).
The infants were full‐term and clinically normal in 10 studies (Anderson 1981; Furr 1982; Killough 2004; Liptak 1983; Myers 1982a; Nugent 2014; Nugent 2015; Pannabecker 1982; Poley‐Strobel 1987; Worobey 1982). In one study, Beeghly 1995a, half of the infants were small for gestational age (SGA) at the time of delivery and half were average for gestational age (AGA). In another study, Szajnberg 1987, infants of 28 to 32 weeks appropriate for gestational age were included when they reached 34 weeks. Infants were developmentally delayed in one study (McManus 2012), and in one study, Belsky 1985, perinatal assessment for neonatal functioning was insignificant. In two studies, details regarding the clinical presentation of the infant were not provided (Beal 1989; Britt 1994).
Interventions
We grouped the interventions into two categories: NBAS and NBO.
NBAS
Thirteen studies evaluated the effectiveness of NBAS (Anderson 1981; Beal 1989; Beeghly 1995a; Belsky 1985; Britt 1994; Furr 1982; Killough 2004; Liptak 1983; Myers 1982a; Pannabecker 1982; Poley‐Strobel 1987; Szajnberg 1987; Worobey 1982). Five studies involved demonstration only (Anderson 1981; Beal 1989; Liptak 1983; Poley‐Strobel 1987; Szajnberg 1987), and the remainder involved the parents as participants (Beeghly 1995a; Belsky 1985; Britt 1994; Furr 1982; Killough 2004; Myers 1982a; Pannabecker 1982; Worobey 1982).
NBO
Three studies evaluated the effectiveness of NBO, which was administered in the presence of the caregiver (McManus 2012; Nugent 2014; Nugent 2015).
Frequency and duration of the intervention
Nine studies administered the intervention only once (Anderson 1981; Beal 1989; Belsky 1985; Britt 1994; Furr 1982; Liptak 1983; Myers 1982a; Szajnberg 1987; Worobey 1982); five studies, twice (Killough 2004; Nugent 2014; Nugent 2015; Pannabecker 1982; Poley‐Strobel 1987); one study, three times (Beeghly 1995a); and one study, seven times (McManus 2012).
Seven studies did not describe the duration of intervention (Beal 1989; Belsky 1985; Britt 1994; Killough 2004; McManus 2012; Poley‐Strobel 1987; Szajnberg 1987). It was 30 minutes or less in five studies (i.e. 20 minutes in Liptak 1983; 12 to 25 minutes in Nugent 2014 and Nugent 2015; and 30 minutes in Furr 1982 and Pannabecker 1982), and about 45 to 60 minutes in four studies (i.e. about 45 minutes in Anderson 1981, Beeghly 1995a, and Worobey 1982; and 45 to 60 minutes in Myers 1982a).
Outcomes
Primary outcome measures
Quality of caregiver‐infant interaction
Thirteen studies assessed the impact of the NBAS or NBO on caregiver‐child interaction, using the scales listed below.
Anderson 1981 used the Price Adaptation Scale (PrAS; Price 1977).
Beeghly 1995a used the Maternal Sensitivity Scale (MSS; Barett 1988).
Belsky 1985 used the Reciprocal Interaction Measure (RIM: Belsky 1984).
Britt 1994 and Furr 1982 used the Nursing Child Assessment Feeding Scale (NCAFS; Barnard 1978; Sumner 1994).
Killough 2004 and Nugent 2015 used the CARE‐Index (Crittenden 2001).
Liptak 1983 used the Ainsworth Interaction Scale (AIS; Ainsworth 1971).
McManus 2012 used the Home Visiting Index (HVI; Nugent 2003).
Myers 1982a assessed the infant's state (asleep, quiet‐alert or fussing‐crying) and the presence or absence of caregiver behaviours (looks on face; talks to infant; smiles, touches or positions infant) every 10 seconds for 10 minutes.
Pannabecker 1982 used a list of behaviours (coding of behaviours using Klaus 1972 and Richard 1972 checklists).
Poley‐Strobel 1987 used the Assessment of Mother Infant Sensitivity (AMIS; Price 1983).
Worobey 1982 observed 16 behaviour categories using a pre‐coded checklist.
Caregiver mental health
Nugent 2014 assessed caregiver mental health using the Edinburgh Postnatal Depression Scale (EPDS; Cox 1987).
Secondary outcome measures
Infant social, emotional, cognitive and motor development
Beeghly 1995a and Szajnberg 1987 assessed infant development using the Bayley Scales of Infant Development (BSID; Bayley 1969).
Caregiver perception of infant
Four studies assessed the impact of the NBAS or NBO on caregiver perceptions of the infant.
Beal 1989 used the Infant Characteristic Questionnaire (ICQ; Bates 1979).
Myers 1982a and Liptak 1983 used the Neonatal Perception Inventory (NPI; Broussard 1970; Broussard 1971).
Szajnberg 1987 used the Carey Infant Temperament Questionnaire (ITQ; Carey 1978).
Caregiver stress
Two studies assessed the impact of the NBAS or NBO on caregiver stress.
Beeghly 1995a used the Parenting Stress Index (PSI; Abidin 1983).
Liptak 1983 used the Modified Degree of Bother Scale (DBS; Liptak 1977).
Caregiver reflective functioning
Killough 2004 assessed caregiver reflective functioning using the Maternal Representations Questionnaire (MRQ: Stern 1998).
Caregiver confidence, self‐efficacy and self‐esteem in the caregiving role
Poley‐Strobel 1987 assessed caregiver confidence using the Self‐Confidence Scales (SCS; Seashore 1973), which comprise paired‐comparison questionnaires in which the caregiver compares herself with five other possible caretakers.
Caregiver knowledge of infant behaviour
Two studies assessed the impact of the NBAS or NBO on caregiver knowledge of infant behaviour.
Myers 1982a used a set of 15 multiple‐choice, factual questions related to infants' physical capacities, including reflexes and senses.
Poley‐Strobel 1987 assessed maternal knowledge about infant behaviour using the Mother's Assessment of the Behaviour of her Infant scale (MABI; Field 1978).
No study assessed adverse effects, infant physical health or caregiver satisfaction in the caregiving role.
Excluded studies
We excluded 24 studies (in 24 reports) from this review, which are summarised in the Characteristics of excluded studies tables. The main reason for exclusion was that the NBAS/NBO was not used in isolation but as part of a wider intervention (10 studies: Cooper 2009; Golas 1986; Gomespedro 1995; Hart 1998; Nurcombe 1984; Ohgi 2004; Parker Loewen 1987; Teti 2009; Widmayer 1980; Widmayer 1981), or because they used variations of the NBAS but provided little further information (6 studies: Cardone 1990; Cooper 2015; Gomes 1987; Parker 1992; Sullivan 1980; Wendland Carro 1999). The remaining eight studies did not meet key inclusion criteria in that they were not RCTs, they included other intervention components, or both, or they did not provide sufficient information to make a judgment (Egeland 1984; Fowles 1999; Joshi 2013; Kiepura 2011; Kusaka 2007; Meisels 1993; Ogi 2001; Sanders 2006).
Ongoing studies
See Characteristics of ongoing studies.
We identified three ongoing studies (NCT03070652; Nicolson 2016; Nugent 2016). NCT03070652 is a cluster‐randomised trial assessing the effectiveness of the NBO (postbirth and over next three months) for improving interaction in with first‐time parents. Nicolson 2016 is an RCT of the effectiveness of the NBO (three sessions) for improving depression and mother‐infant interaction with first‐time parents. Nugent 2016 is an RCT examining the effectiveness of an NBO early intervention model of care for improving infant self‐regulation, parent‐infant interaction as well as maternal depression and socioemotional functioning in infants with developmental disabilities.
Risk of bias in included studies
We described and presented the risk of bias for each included study in the 'Risk of bias' tables, beneath the Characteristics of included studies. We also summarised our judgments about each risk of bias item for each included study below and graphically (see Figure 2 and Figure 3).
2.
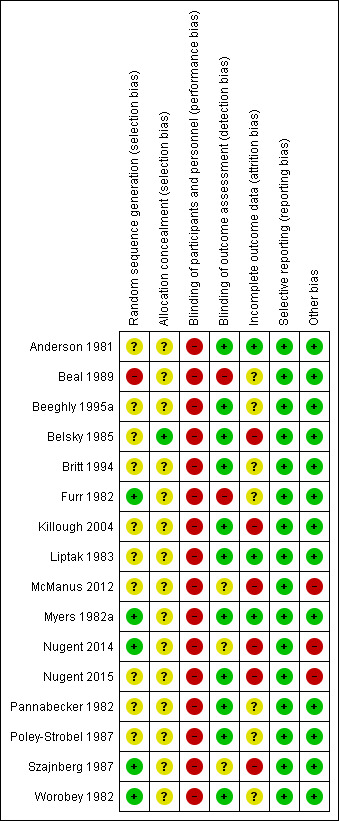
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
3.
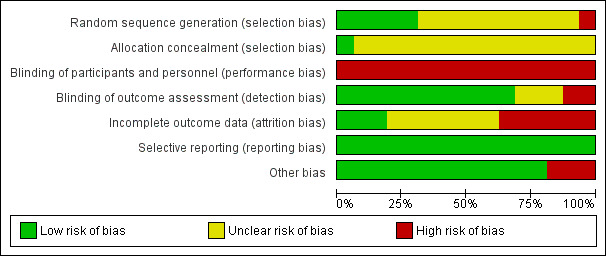
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation
In terms of random sequence generation, we rated only five studies at low risk of bias due to their use of adequate methods of sequence generation (e.g. toss of a coin and cyclic rotating table of numbers) (Furr 1982; Myers 1982a; Nugent 2014; Szajnberg 1987; Worobey 1982). We judged the 10 remaining studies to be at unclear or high risk of bias.
Allocation concealment
Only one study carried out allocation concealment where the individual scheduling a prenatal visit had no knowledge of the group to which each family was assigned or that assignment was taking place at that time (Belsky 1985). Blinding was achieved by providing the scheduler with a packet of manila folders in a specified order. Each time a prenatal visit was scheduled, the top folder in the stack was selected and the family's name and address was placed on the folder. The folders had been precoded for treatment condition. The only person who knew the group assignment was the author, who did not collect any of the data. We judged this study to be at low risk of bias. The remaining 15 studies did not mention allocation concealment, so we judged these to be at unclear risk of bias.
Blinding
Performance bias
In trials of newborn behaviour programmes it is not possible to blind either facilitators or parents to the type of treatment being implemented or received, although in three studies caregivers were unaware of the presence of other treatment conditions (Belsky 1985; Myers 1982a; Worobey 1982). Nevertheless, we rated all studies as being at high risk of performance bias.
Detection bias
We judged 11 studies to be at low risk of detection bias because observer‐rated outcomes were double‐blinded (Anderson 1981; Beeghly 1995a; Belsky 1985; Britt 1994; Killough 2004; Liptak 1983; Myers 1982a; Nugent 2015; Pannabecker 1982; Poley‐Strobel 1987; Worobey 1982).
The remaining five studies did not involve independent assessment of outcomes (Beal 1989; Furr 1982; McManus 2012; Nugent 2014; Szajnberg 1987). In Beal 1989, the principal investigator carried out the NBAS demonstration as well as postpartum home visits, so we rated this study as being at high risk of detection bias. In Furr 1982, the investigator was singularly involved in all phases of data collection: randomly assigning the sample of mothers, pre‐testing, providing the behavioural orientation and post‐testing. In addition, it was difficult to determine the effect of investigator bias in scoring the post‐test feeding episodes, so we judged this study to be at high risk of detection bias as well. In McManus 2012, the assessment was conducted as part of the last home visit when the infant was three months of age, but it was not clear whether this was administered by the early intervention service provider or by the researcher. Consequently, we judged it to be at unclear risk of detection bias. Two studies, Nugent 2014 and Szajnberg 1987, did not describe whether assessment was blinded and therefore we considered them to be at unclear risk of detection bias also.
Incomplete outcome data
No attrition occurred in one study, Anderson 1981, which we rated as being at low risk of bias.
Eight studies reported attrition (Belsky 1985; Killough 2004; Liptak 1983; McManus 2012; Myers 1982a; Nugent 2014; Nugent 2015; Szajnberg 1987), ranging from 4% in Liptak 1983 to 20% in Szajnberg 1987. None of these studies undertook intention‐to‐treat analyses, and we judged all studies with more than 5% attrition to be at high risk of attrition bias (Belsky 1985; Killough 2004; McManus 2012; Nugent 2014; Nugent 2015; Szajnberg 1987).
Seven studies did not mention attrition or the use of an intention‐to‐treat analysis, so we judged these to be at unclear risk of bias (Beal 1989; Beeghly 1995a; Britt 1994; Furr 1982; Pannabecker 1982; Poley‐Strobel 1987; Worobey 1982)
Selective reporting
We judged all 16 studies to be at low risk for reporting bias because they reported all pre‐specified outcomes.
Other potential sources of bias
All studies were RCTs, so confounding was not a source of bias; furthermore, no included studies used the NBAS or NBO as part of another intervention. Some studies imposed other restrictions as inclusion and exclusion criteria. Britt 1994 targeted drug‐abusing mothers; Poley‐Strobel 1987 included only mothers with low socioeconomic status and their newborns without any perinatal complications; McManus 2012 and Szajnberg 1987 targeted preterm deliveries; Beal 1989 involved first‐time fathers; Pannabecker 1982 involved first‐time fathers and their healthy newborns; Anderson 1981, Furr 1982, Killough 2004, Liptak 1983, Nugent 2014 and Nugent 2015 involved first‐time mothers with their healthy newborn babies; Belsky 1985 and Myers 1982a targeted first‐time parents and their healthy infants; Beeghly 1995a and Worobey 1982 targeted full‐term, normal healthy newborns.
We judged 13 studies to be at low risk for other potential sources of bias and rated three studies at high risk of bias due to the involvement of the authors with the NBAS Institute (McManus 2012; Nugent 2014; Nugent 2015).
Effects of interventions
See: Table 1
NBAS and NBO versus control
Primary outcomes
1. Quality of caregiver‐infant interaction
1.1 Meta‐analytic data
A meta‐analysis of data from seven studies revealed a statistically significant difference in caregiver‐interaction between intervention and control groups (SMD −0.53, 95% CI −0.90 to −0.17; 304 participants; Analysis 1.1; Anderson 1981; Beeghly 1995a; Britt 1994; Killough 2004; McManus 2012; Nugent 2015; Poley‐Strobel 1987). There was a moderate level of heterogeneity (Tau2 = 0.12; Chi2 = 12.23, df = 6, P = 0.06; I2 = 51%). We rated the quality of evidence as low because of methodological limitations in terms of blinding and allocation as well as follow‐up and dropout (see Table 1).
1.1. Analysis.
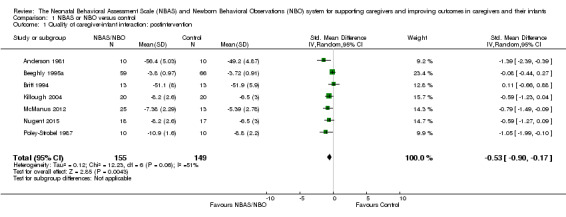
Comparison 1 NBAS or NBO versus control, Outcome 1 Quality of caregiver‐infant interaction: postintervention.
1.2 Studies without means or standard deviations
Six studies did not provide sufficient data to calculate an effect size for caregiver‐infant interaction; two of these reported significant results (Furr 1982; Worobey 1982), and four indicated no significant difference between intervention and control conditions (Belsky 1985; Liptak 1983; Myers 1982a; Pannabecker 1982).
2. Caregiver mental health: single study data
Data from a single study, Nugent 2014, indicated no statistically significant difference in caregiver depression between intervention and control groups when measuring depression either as a continuous (SMD −0.28, 95% CI −0.66 to 0.10; 106 participants; Analysis 1.2) or dichotomous outcome (RR 0.23, 95% CI 0.05 to 1.04; 106 participants; 1 study; Analysis 1.3). We rated the quality of evidence as low because of methodological limitations in terms of blinding and allocation as well as follow‐up and dropout. See Table 1.
1.2. Analysis.

Comparison 1 NBAS or NBO versus control, Outcome 2 Caregiver mental health (maternal depression): EPDS postintervention score.
1.3. Analysis.

Comparison 1 NBAS or NBO versus control, Outcome 3 Caregiver mental health (maternal depression): EPDS postintervention score (dichotomous data).
3. Adverse effects or unintended consequences
No studies provided data on this outcome.
Secondary outcomes
1. Infant social, emotional, cognitive and motor development
1.1 Single study data
One study involving 125 participants, Beeghly 1995a, found no statistically significant differences between intervention and control groups for mental development (SMD −0.13, 95% CI −0.48 to 0.22) or psychomotor development (SMD −0.11, 95% CI −0.47 to 0.24). See Analysis 1.4. We rated the quality of evidence as low because of methodological limitations in terms of blinding and allocation as well as follow‐up and dropout, inconsistency for the main outcome due to high levels of heterogeneity for the NBAS; and indirectness in terms of the low levels of generalisability to wider risk groups within the population. See Table 1.
1.4. Analysis.
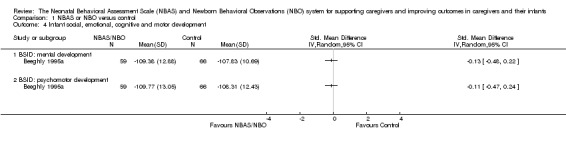
Comparison 1 NBAS or NBO versus control, Outcome 4 Infant social, emotional, cognitive and motor development.
1.2 Studies without means and standard deviations
One study reported no significant difference between intervention and control groups for infant development (Szajnberg 1987).
2. Infant physical health
No studies provided data on this outcome.
3. Caregiver perception of infant
3.1 Single study data
One study, Beal 1989, revealed no statistically significant difference in caregiver perceptions of the infant between intervention and control condition (SMD −0.36, 95% CI −0.95 to 0.24; 44 participants; Analysis 1.5). We rated the quality of evidence as low because of methodological limitations in terms of blinding and allocation as well as follow‐up and dropout, and indirectness in terms of the low levels of generalisability to wider risk groups within the population. See Table 1.
1.5. Analysis.

Comparison 1 NBAS or NBO versus control, Outcome 5 Caregiver perception of infant.
3.2 Studies without means and standard deviations
Three studies did not provide sufficient data to calculate an effect size (Liptak 1983; Myers 1982a; Szajnberg 1987), two of which reported no significant differences between intervention and control groups in caregiver perceptions of the infant (Myers 1982a; Szajnberg 1987), and one of which provided inadequate information (Liptak 1983).
4. Caregiver stress
4.1 Single study data
One study involving 125 participants, Beeghly 1995a, found no statistically significant differences between intervention and control conditions for parent‐related (SMD 0.00, 95% CI −0.35 to 0.35) or child‐related stress (SMD 0.06, 95% CI −0.29 to 0.41). See Analysis 1.7. We rated the quality of evidence as low because of methodological limitations in terms of blinding and allocation as well as follow‐up and dropout, and indirectness in terms of the low levels of generalisability to wider risk groups within the population. See Table 1.
1.7. Analysis.
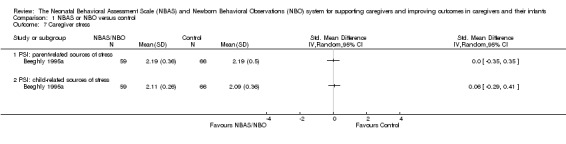
Comparison 1 NBAS or NBO versus control, Outcome 7 Caregiver stress.
4.2 Studies without means and standard deviations
One study reported no significant difference between intervention and control groups for caregiver stress (Liptak 1983).
5. Caregiver reflective functioning: studies without means and standard deviations
One study did not provide sufficient data to calculate an effect size for maternal reflective functioning and reported no statistically significant difference between the intervention and control groups (Killough 2004).
6. Caregiver satisfaction in the caregiving role
No studies provided data on this outcome.
7. Caregiver confidence, self‐efficacy and self‐esteem in the caregiving role: studies without means and standard deviations
One study reported no significant difference between intervention and control groups for caregiver confidence (Poley‐Strobel 1987).
8. Caregiver knowledge of infant behaviour
8.1 Single study data
One study, Myers 1982a, found a clear difference between intervention and control, favouring the NBAS/NBO group statistically for caregiver knowledge of infant behaviour (SMD −1.30, 95% CI −2.16 to −0.44; 26 participants; Analysis 1.6). We rated the quality of evidence as very low because of the use of a non‐standardised tool and methodological limitations in terms of blinding and allocation as well as follow‐up and dropout, and indirectness in terms of the low levels of generalisability to wider risk groups within the population. Table 1.
1.6. Analysis.

Comparison 1 NBAS or NBO versus control, Outcome 6 Caregiver knowledge of infant behaviour (multiple choice factual questions).
8.2 Studies without means and standard deviations
One study reported no significant difference between the intervention and control groups for caregiver knowledge of infant behaviour (Poley‐Strobel 1987).
Two studies reported no significant difference between intervention and control group for caregiver attitude (Beal 1989; Szajnberg 1987).
Subgroup analysis: quality of caregiver‐infant interaction
Subgroup analyses for the NBAS (five studies) and the NBO (two studies) indicated a medium but insignificant effect size for the former (SMD −0.49, 95% CI −0.99 to −0.00; 231 participants), with high levels of heterogeneity (Tau2 = 0.18; Chi2 = 10.31, df = 4, P = 0.04; I2 = 61%), while the NBO produced a large and significant effect size (−0.69, 95% CI −1.18 to −0.20; 73 participants) with no heterogeneity (Tau2 = 0.00; Chi2 = 0.16, df = 1, P = 0.69; I2 = 0%). A test for subgroup differences gave a Chi2 statistic of 0.31 with an associated P value of 0.58, suggesting that there is no evidence for different intervention effects between these two subgroups. We also conducted a meta‐regression to check this result. The meta‐regression for the intervention type (NBAS or NBO) is estimated as −0.22 (95% CI −1.34 to 0.90), which confirms the results from testing the subgroup difference by using the Chi2 test.
Discussion
Summary of main results
We included 16 studies (N = 851) in this review: 13 evaluated the effectiveness of the NBAS (Anderson 1981; Beal 1989; Beeghly 1995a; Belsky 1985; Britt 1994; Furr 1982; Killough 2004; Liptak 1983; Myers 1982a; Pannabecker 1982; Poley‐Strobel 1987; Szajnberg 1987; Worobey 1982), and 3 evaluated the NBO (McManus 2012; Nugent 2014; Nugent 2015). All studies provided postintervention data, although only 10 studies provided the necessary means and standard deviations to calculate an effect size. Furthermore, of these 10 studies, only one, Myers 1982a, also provided further assessment at a later time point, so we do not report follow‐up data as part of this review. Meta‐analysis was possible for only one primary outcome – quality of caregiver‐infant interaction. Single study data were available to assess one other primary outcome – caregiver mental health – and a number of secondary outcomes: infant social, emotional, cognitive and motor development; caregiver perception of infant; caregiver stress; and caregiver knowledge of infant behaviour.
The results of the meta‐analysis suggest there was a difference in the quality of caregiver‐infant interaction between intervention and control groups, with moderate heterogeneity (SMD −0.53, 95% CI −0.90 to −0.17; low‐quality evidence; Table 1). Although the effect size for the NBO was larger than that for the NBAS, results of a subgroup analysis found no statistically significant difference between the two models. This may reflect the small number of studies included in the meta‐analysis of the NBO, which was specifically developed as "a clinical relationship‐building tool, designed for pediatricians, nurses, infant mental health specialists, early intervention providers, home visitors and other allied health professionals, to help parents understand their baby's language" (www.brazeltontouchpoints.org/offerings/nbo‐and‐nbas), compared with the NBAS, which was developed as a neurobehavioural assessment tool. Nugent 2017 [pers comm] has described the NBO as: "an infant‐focused, family‐centered relationship‐based tool, designed to sensitize parents to their baby's competencies and individuality, in order to foster positive parent‐infant interactions and thus contribute to the development of the parent's confidence and to the quality of the parent‐infant relationship from the very beginning. It is conceived of as an interactive system in which parents play an active role in both the observations of their baby's behaviour and in the identification of appropriate caregiving strategies. Ideally, the NBO is used serially with the family across the first three months of life, in order to see how the baby is adapting to his or her new world and to enable the NBO practitioner to develop a relationship with the family". Although between‐group analysis showed no differences between the two models of intervention in terms of parent‐infant interaction, further studies of the NBO are needed to confirm this finding.
We conducted no meta‐analyses for any of the secondary outcomes due to an absence of suitable data for this purpose. Results from single studies did not show significant differences between intervention and control groups for the secondary outcomes of infant social, emotional, cognitive and motor development; caregiver perception of infant; or caregiver stress (low‐quality evidence). One study found a significant impact on the secondary outcome of caregiver knowledge (SMD −1.30, 95% CI −2.16 to −0.44; very low‐quality evidence).
Overall, this review did not find any evidence of adverse effects, in terms of deterioration in any of the outcomes measured.
Overall completeness and applicability of evidence
This review included data from 16 RCTs, many of which did not provide data appropriate for meta‐analysis, and our attempts to obtain the data from the authors were mostly unsuccessful, probably due to the fact that most studies were published in the 1980s and 1990s. Only three studies assessed the effectiveness of the NBO, and only two of these provided data amenable for pooling. Furthermore, most included studies were small, which may have had an impact on the power of the results and the confidence in the precision of the effect sizes. We also identified three ongoing RCTs of the effectiveness of the NBO on outcomes of relevance to the current review (NCT03070652; Nicolson 2016; Nugent 2016). This data will, in the long term, strengthen the overall analysis of the effectiveness of the NBO in particular.
Although the NBAS and NBO are highly standardised interventions in terms of their administration, the NBAS in particular was not designed as an intervention, and there is little guidance regarding wider aspects of their delivery with the result that the included studies varied in terms of the number of times that they were administered (i.e. ranging from one to four); the setting for administration (i.e. hospital or clinic; at home); whether the participant was the mother, father, or both; and the designation of the infant (e.g. preterm versus full‐term) and the parent (e.g. healthy and substance dependent). Generalisability is limited because all of the studies were conducted in the USA, and most of the participants were low‐risk dyads. As stated earlier, two‐thirds of the included studies were conducted in the 1980s and 1990s with only three studies being conducted since 2000.
Quality of the evidence
Using the GRADE approach, we rated the overall quality of the body evidence as low to very low (see Table 1). The three main reasons for downgrading the quality of the evidence were risk of bias due to poor quality research (e.g. limitations in design, including inadequate randomisation or allocation procedures, together with attrition, which ranged from 4% to 20% in seven studies – none of which conducted an intention‐to‐treat analysis), inconsistency for the main outcome due to high levels of heterogeneity for the NBAS; and indirectness in terms of the low levels of generalisability to wider risk groups within the population.
Potential biases in the review process
Our literature searches and screening process conformed strictly to Cochrane criteria, as defined by our Methods, with the most recent search being conducted in September 2017. We conducted systematic searches across a large number of highly relevant databases, including trials registers, to identify both completed and ongoing trials. Two review authors independently screened potentially eligible studies for inclusion and assessed risk of bias in included studies. None of the review authors had any significant conflicts of interest. The review itself, however, was limited in that it was aimed at assessing whether the NBAS and NBO were sufficiently powerful to be conceived of as a single treatment(s) rather than as brief interventions used alongside other methods, and, as such, excluded studies in which their independent impact could not be assessed.
Agreements and disagreements with other studies or reviews
This is the first review that has examined the effectiveness of the NBO in addition to the NBAS, so it is different from earlier reviews. The results of the current review are, to some extent, consistent with earlier reviews (Britt 1994b; Das Eiden 1996; Worobey 1985), all of which produced evidence suggesting that the NBAS was effective; however, the findings of the current review are much more limited in terms of the outcomes showing a beneficial effect. All of the earlier reviews had significant methodological limitations. The earliest review only included studies that demonstrated effectiveness in at least one of its outcomes (Worobey 1985). The second was a narrative review only (Britt 1994b). The third conducted a meta‐analysis of 13 RCTs but produced an average global effect size for 'parenting quality' (i.e. combining conceptually distinct aspects of parenting), which found a small‐to‐moderate beneficial effect (Das Eiden 1996); it also included studies that we excluded because they used modified versions of the NBAS. Furthermore, the most recent of these reviews is now over 20 years old (Das Eiden 1996), although only a small number of studies have been conducted since then.
Authors' conclusions
Implications for practice.
There is currently evidence from very low‐quality studies suggesting that the NBAS and NBO may be effective for improving caregiver‐infant interaction, albeit in mostly low‐risk caregiver‐infant dyads. The evidence regarding the NBAS showed a high level of heterogeneity, which may reflect the very different purposes for which the NBAS and the NBO were developed. This review has not examined the use of the NBAS or NBO as part of a broader package of interventions, so the recommendations made here relate to their standalone use only.
Implications for research.
The included studies assessed the impact of administering the NBAS or NBO only once (eight studies) or twice (four studies), with mostly low‐risk populations, where there may have been ceiling effects in terms of the possibility of showing change. It is currently not clear to what extent the NBAS or NBO are effective as a standalone intervention with higher risk populations. The current review has not examined the use of either the NBAS or the NBO as part of a broader package of interventions, so we make no recommendations about future research in terms of their use in that way.
Acknowledgements
We thank the editorial team of Cochrane Developmental, Psychosocial and Learning Problems (Margaret Anderson, Joanne Wilson, and Geraldine Macdonald) for their support and advice. We also thank Jo Abbott, who undertook the searches, and the editor, external referees and statistician, who reviewed and commented on the protocol and earlier drafts of the review.
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL), Cochrane Database of Systematic Reviews (CDSR), Database of Systematic Reviews (DARE); all in the Cochrane Library
#1 MeSH descriptor: [Parent‐Child Relations] explode all trees #2 MeSH descriptor: [Object Attachment] this term only #3 MeSH descriptor: [Early Intervention (Education)] this term only #4 ((parent* or mother* or father*) near/3 (demonstrat* or teach* or train*)):ti,ab,kw (Word variations have been searched) #5 Neonatal Behavio?r* Assessment Scale:ti,ab,kw (Word variations have been searched) #6 Newborn Behavio?r* Assessment Scale:ti,ab,kw (Word variations have been searched) #7 NBAS or BNAS or BNBAS:ti,ab,kw (Word variations have been searched) #8 (Brazelton* or Brazleton*):ti,ab,kw (Word variations have been searched) #9 Newborn Behavio?ral Observation* System:ti,ab,kw (Word variations have been searched) #10 NBO:ti,ab,kw (Word variations have been searched) #11 (newborn* or neonat* or baby or babies or infant*):ti,ab,kw (Word variations have been searched) #12 MeSH descriptor: [Infant] explode all trees #13 #11 or #12 #14 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 #15 #13 and #14
MEDLINE Ovid
1. Parent Child relations/ 2. Mother Child Relations/ 3. Father Child Relations/ 4. Object attachment/ 5. Early Intervention/ 6. ((parent$ or mother$ or father$) adj3 (demonstrat$ or teach$ or train*)).tw. 7. Neonatal Behavio?r$ Assessment Scale.mp. 8. Newborn Behavio?r$ Assessment Scale.mp. 9. NBAS.tw. 10. BNAS.tw. 11. BNBAS.tw. 12. (Brazelton$ or Brazleton$).mp. 13. Newborn Behavio?ral Observation$ System.mp. 14. NBO.tw. 15. (newborn$ or neonat$ or baby or babies or infant$).tw. 16. exp Infant/ 17. 15 or 16 18. or/1‐14 19. randomised controlled trial.pt. 20. controlled clinical trial.pt. 21. randomized.ab. 22. placebo.ab. 23. drug therapy.fs. 24. randomly.ab. 25. trial.ab. 26. groups.ab. 27. 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 28. exp animals/ not humans.sh. 29. 27 not 28 30. 17 and 18 and 29
Embase Ovid
1. Parent Child relations/ 2. Mother Child Relations/ 3. Father Child Relations/ 4. Object attachment/ 5. Early Intervention/ 6. ((parent$ or mother$ or father$) adj3 (demonstrat$ or teach$ or train*)).tw. 7. Neonatal Behavio?r$ Assessment Scale.mp. 8. Newborn Behavio?r$ Assessment Scale.mp. 9. NBAS.tw. 10. BNAS.tw. 11. BNBAS.tw. 12. (Brazelton$ or Brazleton$).mp. 13. Newborn Behavio?ral Observation$ System.mp. 14. NBO.tw. 15. (newborn$ or neonat$ or baby or babies or infant$).tw. 16. exp Infant/ 17. 15 or 16 18. or/1‐14 19. random$.tw. 20. factorial$.tw. 21. crossover$.tw. 22. cross over$.tw. 23. cross‐over$.tw. 24. placebo$.tw. 25. (doubl$ adj blind$).tw. 26. (singl$ adj blind$).tw. 27. assign$.tw. 28. allocat$.tw. 29. volunteer$.tw. 30. Crossover Procedure/ 31. double‐blind procedure.tw. 32. Randomized Controlled Trial/ 33. Single Blind Procedure/ 34. or/19‐33 35. (animal/ or nonhuman/) not human/ 36. 34 not 35 37. 17 and 18 and 36
PsycINFO Ovid
1. Parent Child relations/ 2. Mother Child Relations/ 3. Father Child Relations/ 4. Attachment Behavior/ 5. Early Intervention/ 6. Parent training/ 7. ((parent$ or mother$ or father$) adj3 (demonstrat$ or teach$ or train*)).tw. 8. Neonatal Behavio?r$ Assessment Scale.mp. 9. Newborn Behavio?r$ Assessment Scale.mp. 10. NBAS.tw. 11. BNAS.tw. 12. BNBAS.tw. 13. (Brazelton$ or Brazleton$).mp. 14. Newborn Behavio?ral Observation$ System.mp. 15. NBO.tw. 16. or/1‐15 17. (neonatal birth 1 mo or infancy 2 23 mo).ag. 18. (newborn$ or neonat$ or baby or babies or infant$).tw. 19. or/17‐18 20. clinical trials/ 21. random$.tw. 22. (allocat$ or assign$).tw. 23. ((clinic$ or control$) adj trial$).tw. 24. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 25. (crossover$ or "cross over$").tw. 26. random sampling/ 27. Experiment Controls/ 28. exp experimental methods/ 29. Placebo/ 30. placebo$.tw. 31. exp program evaluation/ 32. treatment effectiveness evaluation/ 33. ((effectiv$ or evaluat$) adj3 (intervention$ or stud$ or research$)).tw. 34. or/20‐33 35. 16 and 19 and 34
CINAHL EBSCOhost (Cumulative Index to Nursing and Allied Health Literature)
S26 S13 AND S16 AND S25 S25 S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 S24 (allocat* random*) S23 (MH "Quantitative Studies") S22 (MH "Placebos") S21 placebo* S20 (random* allocat*) S19 (MH "Random Assignment") S18 (Randomi?ed control* trial*) S17 (singl* blind* ) or (doubl* blind* ) or (tripl* blind* ) or (trebl* blind* ) or (trebl* mask* ) or (tripl* mask* ) or (doubl* mask* ) or (singl* mask* ) S16 S14 OR S15 S15 (MH "Infant+") S14 (newborn* or neonat* or baby or babies or infant*) S13 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 S12 NBO S11 Newborn Behavio?ral Observation* System S10 Newborn Behavio?ral Observation* System S9 (Brazelton* or Brazleton*) S8 NBAS or BNAS or BNBAS S7 Newborn Behavio?r* Assessment Scale S6 Newborn Behavio?r* Assessment Scale S5 Neonatal Behavio?r* Assessment Scale S4 ((parent* or mother* or father*) N3 (demonstrat* or teach* or train*)) S3 (MH "Early Intervention") S2 (MH "Attachment Behavior") S1 (MH "Parent‐Child Relations+")
Science Citation Index (SCI), Social Sciences Citation Index (SSCI), Conference Proceedings Citation Index ‐ Science (CPCI‐S); Conference Proceedings Citation Index ‐ Social Science & Humanities (CPCI‐SS&H), BIOSIS; all Web of Science
#1 TOPIC: ((((parent* or mother* or father*) near/3 (demonstrat* or teach* or train*)))) #2 TOPIC: ((Neonatal Behavio?r* Assessment Scale)) #3 TOPIC: ((Newborn Behavio?r* Assessment Scale)) #4 TOPIC: ((NBAS or BNAS or BNBAS)) #5 TOPIC: (((Brazelton* or Brazleton*))) #6 TOPIC: ((Newborn Behavio?ral Observation* System)) #7 TOPIC: ((NBO)) #8 TOPIC: (((newborn* or neonat* or baby or babies or infant*))) #9 #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 #10 #9 AND #8 #11 TS=Randomized clinical trial* OR TI=Randomized clinical #12 TI=randomi* OR TS=randomi* #13 TI=clin* OR TS=clin* #14 TS=trial* OR TI=trial* #15 #14 AND #13 #16 TS=(singl* OR Doubl* OR Tripl* OR Trebl*) OR TI=(singl* OR Doubl* OR Tripl* OR Trebl*) #17 TS=(mask* OR blind*) OR TI=(mask* OR blind*) #18 TS=crossover* OR TI=crossover* #19 TS=(allocate* OR assign*) OR TI=(allocate* OR assign*) #20 TS=random* OR TI=random* #21 #20 AND #19 #22 #21 OR #18 OR #17 OR #16 OR #15 OR #12 OR #11 #23 #22 AND #10
ERIC EBSCOhost (Education Resources Information Center)
S19 S17 AND S18 S18 TI ( trial* OR random* OR crossover OR blind*) ) OR AB ( trial* OR random* OR crossover OR blind*) ) S17 S13 AND S16 S16 S14 OR S15 S15 (newborn* or neonat* or baby or babies or infant*) S14 DE "Infants" OR DE "Neonates" OR DE "Premature Infants" S13 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 S12 DE "Early Intervention" S11 DE "Attachment Behavior" S10 DE "Parent Child Relationship" S9 NBO S8 Newborn Behavio?ral Observation* System S7 Newborn Behavio?ral Observation* System S6 (Brazelton* or Brazleton*) S5 NBAS or BNAS or BNBAS S4 Newborn Behavio?r* Assessment Scale S3 Newborn Behavio?r* Assessment Scale S2 Neonatal Behavio?r* Assessment Scale S1((parent* or mother* or father*) N3 (demonstrat* or teach* or train*))
SCOPUS Elsevier
TITLE‐ABS‐KEY ( ( nbas OR bnas OR bnbas OR brazelton* OR brazleton* OR nbo OR ( parent* train* ) OR ( parent* teach* ) OR ( parent* demonstrat* ) OR ( neonatal behavior* assessment scale ) OR ( neonatal behaviour* assessment scale ) ) OR ( newborn behavior* assessment scale ) OR ( newborn behaviour* assessment scale ) AND ( newborn* OR neonat* OR baby OR babies OR infant* ) AND ( trial* OR random* OR crossover OR blind* ) )
Sociological Abstracts ProQuest
(ab(trial* OR random* OR crossover OR blind*) OR ti(trial* OR random* OR crossover OR blind*)) AND ((SU.EXACT("Infants") OR ((newborn* OR neonate* OR baby OR babies OR infant*) OR SU.EXACT("Infants"))) AND (((parent* OR mother* OR father*) NEAR/3 (demonstrate* OR teach* OR train*)) OR (neonateal Behavio?r* Assessment Scale) OR (Newborn Behavio?r* Assessment Scale) OR (NBAS OR BNAS OR BNBAS) OR (bromelton* OR Brazleton*) OR (Newborn Behavio?ral Observation* System) OR NBO OR SU.EXACT("Parent Child Relations")))
LILACS (Lating American and Caribbean Health Science Information database)
lilacs.bvsalud.org/en
NBAS or BNAS or BNBAS or Brazelton$ or Brazleton$ or NBO or (parent$ train$) or (parent$ teach$) or (parent$ demonstrat$) or (Neonatal Behavior$ Assessment Scale) or (Neonatal Behaviour$ Assessment Scale) or (Newborn Behavior$ Assessment Scale) or (Newborn Behaviour$ Assessment Scale) [Words] and (newborn$ or neonat$ or baby or babies or infant$) [Words] and ((PT:"randomised controlled trial" OR PT:"controlled clinical trial" OR PT:"multicenter study" OR MH:"randomised controlled trials as topic" OR MH:"controlled clinical trials as topic" OR MH:"multicenter studies as topic" OR MH:"random allocation" OR MH:"double‐blind method" OR MH:"single‐blind method") OR ((ensaio$ OR ensayo$ OR trial$) AND (azar OR acaso OR placebo OR control$ OR aleat$ OR random$ OR enmascarado$ OR simpleciego OR ((simple$ OR single OR duplo$ OR doble$ OR double$) AND (cego OR ciego OR blind OR mask))) AND clinic$)) AND NOT (MH:animals OR MH:rabbits OR MH:rats OR MH:primates OR MH:dogs OR MH:cats OR MH:swine OR PT:"in vitro") [Words]
ClinicalTrials.gov
clinicaltrials.gov
Brazelton OR NBAS OR NBO
ISTRN
Brazelton OR NBAS OR NBO
UK Clinical Research Network Study Portfolio
public.ukcrn.org.uk
Brazelton OR NBAS OR NBO
World Health Organisation (WHO) International Clinical Trials Registry Platform (ICTRP)
who.int/ictrp/en
Brazelton OR NBAS OR NBO
Appendix 2. 'Risk of bias' domains and criteria for assigning ratings of low, high and unclear risk of bias
Sequence generation (selection bias)
Did the method used to generate the allocation sequence produce comparable groups?
Low risk of bias: used a truly random method of sequence generation such as a random number generator on a computer.
High risk of bias: used a quasi‐ or non‐random method of sequence generation such as assigning participants to groups based on case number or birth date.
Unclear risk of bias: the method of generation was not sufficiently described such as stating that 'participants were randomly assigned to treatment and control groups' without specifying the randomisation method.
Allocation concealment (selection bias)
Was the method used to conceal allocation sequence adequate, or could the allocation schedules have been foreseen in advance of, or during, recruitment? Could the schedules have been changed after assignment?
Low risk of bias: used adequate methods for allocation concealment such as central randomisation and sequentially numbered and sealed opaque envelopes.
High risk of bias: used methods that were inadequate for allocation concealment such as alternation, assignment by date of birth, and unsealed or non‐opaque envelopes.
Unclear risk of bias: the method of allocation concealment was not sufficiently described to allow assessment of whether it was adequate.
Blinding of participants and personnel (performance bias)
Were adequate steps taken to blind participants, intervention personnel, and investigators as to which treatment arm a given participant might have received?
Low risk of bias: used an adequate method of blinding or where lack of blinding was unlikely to affect results.
High risk of bias: used inadequate method of blinding or where no blinding method was used.
Unclear risk of bias: in cases where the method of blinding was not sufficiently described to allow assessment of whether it was adequate.
Blinding of outcome assessment (detection bias)
Were steps taken to blind outcome evaluators and data assessors as to which treatment a given participant might have received?
Low risk of bias: used adequate method of blinding or where lack of blinding was unlikely to affect results.
High risk of bias: used inadequate method of blinding or where no blinding method was used.
Unclear risk of bias: the method of blinding was not sufficiently described to allow assessment of whether it was adequate.
Incomplete outcome data (attrition bias)
Were incomplete data dealt with adequately by the study investigators? How were data on attrition and exclusions reported?
Low risk of bias: no outcome data were missing, or outcome data were missing for acceptable reasons such as attrition of healthy persons over a long‐term study, or missing outcome data were balanced across groups, or intention‐to‐treat analyses were used.
High risk of bias: a significant amount of outcome data were missing, or outcome data were missing for unacceptable reasons such as exclusion due to 'failure to improve', or missing outcome data were imbalanced across groups, or as‐treated analyses were used.
Unclear risk of bias: the reasons for missing outcome data were not sufficiently described to allow assessment of whether missing data were likely to affect the results.
Selective outcome reporting (reporting bias)
Was any attempt made to reduce the possibility of selective outcome reporting?
Low risk of bias: all of the expected outcomes were reported.
High risk of bias: not all the expected or pre‐specified outcomes were reported, or one or more reported primary outcomes were not pre‐specified, or outcomes were reported incompletely and could not be used.
Unclear risk of bias: it was unclear whether there was a risk of selective outcome reporting.
Other sources of bias
Was each study apparently free of other problems (e.g. baseline imbalance; contamination; fraudulence) that could put it at a high risk of bias? We examined, for example, baseline or pre‐treatment means, if available, to determine if any imbalance existed in those participant characteristics that were strongly related to outcome measures, as imbalance can cause bias in intervention effect estimates (Higgins 2017, section 8.14.1.2).
Low risk of bias: the study was apparently free of other sources of bias.
High risk of bias: the study was at risk of at least one other sources of bias.
Unclear risk of bias: there was insufficient information to assess if the study was at risk for other sources of bias.
Data and analyses
Comparison 1. NBAS or NBO versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of caregiver‐infant interaction: postintervention | 7 | 304 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.90, ‐0.17] |
| 2 Caregiver mental health (maternal depression): EPDS postintervention score | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Caregiver mental health (maternal depression): EPDS postintervention score (dichotomous data) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Infant social, emotional, cognitive and motor development | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 BSID: mental development | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 BSID: psychomotor development | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Caregiver perception of infant | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6 Caregiver knowledge of infant behaviour (multiple choice factual questions) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7 Caregiver stress | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 PSI: parent‐related sources of stress | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 PSI: child‐related sources of stress | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. NBAS or NBO versus control: subgroup analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of caregiver‐infant interaction | 7 | 304 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐0.90, ‐0.17] |
| 1.1 NBAS | 5 | 231 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.99, ‐0.00] |
| 1.2 NBO | 2 | 73 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐1.18, ‐0.20] |
2.1. Analysis.
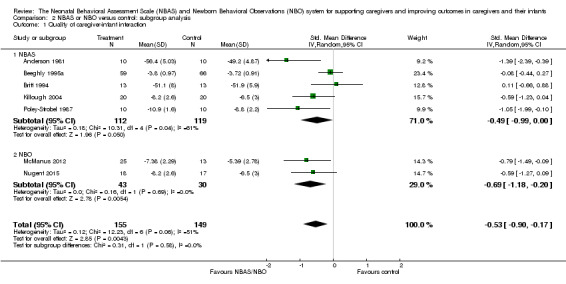
Comparison 2 NBAS or NBO versus control: subgroup analysis, Outcome 1 Quality of caregiver‐infant interaction.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anderson 1981.
| Methods |
Design: parallel‐group, randomised trial Unit of allocation: participant Study dates: not reported |
|
| Participants |
Total number randomised: 30 Mother‐infant pairs assigned to 1 of the 3 treatment groups: control (group I), explanation only (group II), demonstration only (group III) Number randomised to each group: 10 Participant mothers:
Participant infants:
|
|
| Interventions |
Control group: while the NBAS was performed on infants of mothers in this group, the mothers received no immediate feedback on their infants. Instead they received a class on infant furnishings. Explanation only: NBAS was performed on the infants, and following each assessment the investigator met with mother and explained the performance of the infant. Demonstration group: mothers observed the investigator performing NBAS on their infants and saw both stimuli and responses.The explanations were ongoing rather than at the end of session. |
|
| Outcomes |
Study outcomes: Price Adaptation Scale (PAS). A 21‐item instrument designed to measure the quality of reciprocity in mother‐infant interaction. Timing of outcome assessment: 10 days postpartum Outcome observer: a graduate student had been trained with the PAS rating system; this observer was unaware of treatment group to which the mothers were assigned. First assessment was 24‐48 h following delivery (pre‐test) and second observation was made at 10 days postpartum. Assessment was done between groups I vs III, groups I vs II, and group II vs III to find out whether instructions received about their neonate's capacities and behaviours influence the caregiver‐infant interaction at 10 to 12 days postpartum. |
|
| Notes |
Conflicts of interests: not mentioned Source of funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Eligible mother‐infant pairs were randomly assigned to 1 of 3 treatment groups; however, the method of sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information was provided about whether the mothers in the study knew about their own or other treatment conditions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The raters were unaware of the treatment group to which the mothers were assigned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | None |
Beal 1989.
| Methods |
Design: parallel‐group, randomised trial Unit of allocation: participant Study dates: not reported |
|
| Participants |
Total number randomised: 44 Fathers were assigned to 1 of the 2 groups: experimental, control Number randomised to each group: 22 Participant fathers:
Analysis of variance revealed no significant socioeconomic differences between the control and experimental groups. Participant infants: not specified |
|
| Interventions |
Experimental group: the researcher demonstrated the NBAS to father and mother together. Immediate and continuing feedback as well as a terminal summary statement was given to the parents during the examination. Control group: NBAS was performed on the infants by the researcher, and a summary statement was given to the parents. No actual demonstration was provided. |
|
| Outcomes |
Study instruments:
Timing of outcome assessment: 8 weeks postpartum (as a home visit). Outcome observer: first demonstration of NBAS was carried out 2 to 3 days following delivery (pre‐test), and second observation was made at 8 weeks postpartum. Assessment was undertaken to identify whether demonstrating NBAS would affect quality and frequency of father‐infant interaction, paternal attitude towards caretaking, paternal involvement in caretaking, perception of infant difficultness and quality of interaction. |
|
| Notes |
Conflicts of interests: not mentioned Source of funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Eligible fathers were randomly assigned to experimental or control group using alternating allocation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessors was not undertaken. NBAS demonstrations and postpartum home visits were both undertaken by the principal investigator. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported. |
| Other bias | Low risk | None |
Beeghly 1995a.
| Methods |
Design: parallel‐group, randomised trial Unit of allocation: participant Study dates: not reported |
|
| Participants |
Total number randomised: 125 Mothers were assigned to 1 of 2 groups: experimental (infant‐centred, NBAS‐based), control (mother‐centred; interview‐based) Number randomised to each group: 59 experimental, 66 control Participant mothers:
Participant infants:
|
|
| Interventions |
Experimental group: at day 3 in the hospital, and again at days 14 and 30 at home, the mother was invited to become a "participant observer" as the infant was examined with the NBAS by an experienced clinician with established reliability in using the NBAS as preventive intervention in the newborn period. After the administration of the NBAS, the examiner provided a narrative summary of the assessment, encouraging the parent to ask or discuss any questions, concerns or related issues. Control group: an experienced clinician interviewed the mother at days 3, 14 and 30, while a second, trained, reliable examiner administered and scored the NBAS with the infant in a separate room. After the interview, the mother was given a general summary of her infant's performance on the NBAS by the examiner, excluding specific guidelines for caregiving based on the observations of the infant during the NBAS. |
|
| Outcomes |
Study instruments:
Timing of outcome assessment: at 4 months age of the infant (at a laboratory). Outcome observer: Intervention protocols were administered on 3 successive days during the first month postpartum; at 3 days in the hospital and at 14 and 30 days at home. 3 touch points during the first month were implemented to allow 3 successive NBAS administration. Final outcome assessed at 4 months in the laboratory. |
|
| Notes |
Conflicts of interests: not mentioned Source of funding: grant R01 MH37234 from the National Institute of Mental Health, grant from the William Randolph Hearst Foundation and grant from the Mailman Family Foundation to TB Brazelton (PI) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Eligible mothers were randomly assigned to 1 of the 2 intervention groups; method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was undertaken; coders were blind to infant IUGR and maternal intervention status. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported. |
| Other bias | Low risk | None |
Belsky 1985.
| Methods |
Design: parallel‐group, randomised trial Unit of allocation: participant Study dates: not reported |
|
| Participants |
Total number randomised: 67 Participants assigned to 1 of 4 groups: mother and father (experimental group I); only mother (experimental group II), mother and father (control group I); only mother (control group II) Number randomised to each group: 15 in each experimental group; 15 in each control group (after attrition) Participant both parents or mothers only:
Participant infants: not described |
|
| Interventions | On 2nd, 3rd or 4th day of the infant's life and following the assessment of each newborn on the NBAS by a certified examiner, families participating in the project were allocated to 1 of 4 treatment groups. Experimental groups: actively elicited from their newborns a series of reflexes and behaviours culled from the NBAS under the guidance of the experimenter, now serving as facilitator – the same individual who had previously administered the NBAS to the infant. The facilitator neither touched nor held the infant during these sessions; rather she guided the parent through the procedure, describing what the parents were to do, alerting them as to what to look for, asking them what they observed, and discussing the significance of the behavioural phenomena under examination. Control group: received a detailed verbal report of the exam and their babies' performance from the examiner/facilitator. More specifically, the examiner described in step‐by‐step fashion exactly what she did during the exam, how the baby behaved, and the meaning of the infant's responses. |
|
| Outcomes |
Study instruments used
Timing of outcome assessment: during the prenatal phase of the project (preintervention) and again at 1, 3 and 9 months postpartum Outcome observer: Intervention protocols were administered on second, third or fourth day and outcomes observed at 1, 3 and 9 months postpartum to find out whether NBAS performed under the guidance of the experimenter yielded better results than control condition. |
|
| Notes |
Conflicts of interests: not mentioned Source of funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Eligible families were randomly assigned to 1 of 4 intervention groups; method of sequence generation was not described. |
| Allocation concealment (selection bias) | Low risk | The individual scheduling the prenatal visit was unaware of the group to which each family was assigned. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was achieved by providing the scheduler with a packet of manila folders in a specified order. Each time a prenatal visit was scheduled, the top folder in the stack was selected and the family's name and address were placed on the folder. The folders had been pre‐coded for treatment condition. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The only people with knowledge of group assignment were: the author, who did not collect any of the data in the investigation, and the prenatal visitor, who also administered the Brazelton exam and the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition: 7 out of 67 participants. Participant attrition was not selective, either as a function of group assignment or background factors. No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported. |
| Other bias | Low risk | None |
Britt 1994.
| Methods |
Design: parallel‐group, randomised trial Unit of allocation: participant Study dates: not reported |
|
| Participants |
Tota number randomised: 54 Participants assigned to 1 of 4 groups: experimental group I (used only NBAS), experimental group II (MABI), experimental group III (NBAS plus MABI), control group (NBAS not used on babies). Experimental groups II and III were not taken for calculation purposes. Number randomised to each group: 13 experimental, 13 control Participant mothers: all drug‐using mothers Characteristics of the participant both parents/mothers only:
Participant infants: no information |
|
| Interventions |
Experimental group: Brazelton only; mothers were taught how to elicit items from the Brazelton exam on their own infants. Control group: the experimenter talked with the mother about the conditions of the study and about her baby. |
|
| Outcomes |
Study instruments used:
Timing of outcome assessment: at 4 weeks (during a home visit) Outcome observer: a blind observer scored a feeding session and rated various aspects using the study instruments |
|
| Notes |
Conflicts of interests: not mentioned Source of funding: Virginia Commonwealth University (VCU) Faculty Grant‐in‐Aid awarded to the second author, and National Insitute on Drug Abuse (NIDA) Grant DA‐06094 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Eligible families were randomly assigned to 1 of the 4 intervention groups; no information provided about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A blinded observer scored feeding sessions and carried out the rating. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported. |
| Other bias | Low risk | None |
Furr 1982.
| Methods |
Design: parallel‐group, randomised trial Unit of allocation: participant Study dates: not reported |
|
| Participants |
Total number randomised: 40 Participants assigned to 1 of 4 groups: maternal orientation to newborn behaviour using Infant Behaviour Assessment Record (IBAR) adapted from NBAS (intervention‐ group III); feeding observation and maternal orientation to newborn behaviour using IBAR adapted from NBAS (group I); only feeding observation (group II); no action (group IV) Number randomised to each group: 10 in each group Participant mothers:
Participant infants:
|
|
| Interventions |
Experimental (group III): used IBAR adapted from NBAS, as a guideline for orienting the mothers to their newborn behaviour on third day. Group I: instructed to feed baby as if no one were present at day 2 and on day 3 maternal orientation as for group III. Group II: only feeding observation on day 2 Group IV: no action |
|
| Outcomes |
Study instruments used:
Timing of outcome assessment: at 2 weeks (during a home visit) Outcome observer: each mother was asked to breastfeed her baby. Scoring of NCAFS was completed following each observation |
|
| Notes |
Conflicts of interests: not mentioned Source of funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participant mothers were assigned a group number from a cyclic rotating table of numbers. |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Investigator singularly involved in all phases of data collection ‐ random allocation, pre‐testing, behavioural orientation and post‐testing |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported. |
| Other bias | Low risk | None |
Killough 2004.
| Methods |
Design: parallel‐group, randomised trial Unit of allocation: participant Study dates: not reported |
|
| Participants |
Total number randomised: 40 Participants were assigned to 1 of the 2 groups: experimental (used NBAS on babies), control (NBAS not used on babies) Number randomised to each group: 20 experimental, 20 control Participant mothers:
Participant infants:
|
|
| Interventions |
Experimental group: NBAS session was conducted on babies in the presence of the mother Control group: NBAS was not conducted |
|
| Outcomes |
Study instruments used:
Timing of outcome assessment: within 2 days of delivery, at 3 to 4 weeks after delivery and finally at 4 months after delivery Outcome observer: a researcher blind to the status of the family in the research assessed the final outcome at 4 months after delivery. |
|
| Notes |
Conflicts of interests: not mentioned Source of funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Eligible mother‐infant dyads were randomly assigned to 1 of the 4 intervention groups; sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A blinded researcher assessed the final session and carried out the rating. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 control dyads and 1 experimental dyad completed participation through time 2 but not time 3. Therefore, follow‐up sample size comprised of 17 participant dyads in the control group and 19 participant dyads in the experimental group. No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported. |
| Other bias | Low risk | None |
Liptak 1983.
| Methods |
Design: parallel‐group, randomised trial Unit of allocation: participant Study dates: not reported |
|
| Participants |
Total number randomised: 75 Mother‐infant pairs assigned to 1 of 3 treatment groups: experimental, control group I, control group II Number randomised to each group: 25 Participant mothers:
Participant infants:
|
|
| Interventions |
Experimental group: parents received NBAS; parts of this examination were demonstrated to the mothers on the morning of their discharge, approximately 30 minutes following a feeding. A semi‐structured discussion modified for the individuality of each child was held with mothers and included an assessment of the child's strengths, individual differences and the importance of mutual interaction. Control group I: infants received standard newborn care. Physical examinations were performed in the absence of parents. Mothers were visited by one of the authors every day during stay, and any concerns raised by the mothers were addressed. Control group II: infants received standard care + complete physical examinations in front of the mothers on the morning of discharge. During the examination the physician discussed the normal physical findings and emphasised the robustness of the infants. |
|
| Outcomes |
Study instruments used:
Timing of outcome assessment: at 1 and 3 months of age; for 35 minutes at 1 month and for 45 minutes at 3 months Outcome observer: an observer blind to the group status assessed the outcome. |
|
| Notes |
Conflicts of interests: not mentioned Source of funding: supported by a biomedical research support grant from the University of Rochester and by a grant from the Commonwealth Fund (No. 74‐26) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Eligible newborns were randomly assigned to 1 of the 3 groups; method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An observer blind to the group status assessed the outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Out of the 75 who agreed to participate, 3 families dropped out of the study (2 before the first home visit, and 1 after the first home visit). No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported. |
| Other bias | Low risk | None |
McManus 2012.
| Methods |
Design: parallel‐group, randomised trial Unit of allocation: participant Study dates: recruitment of infants January‐August 2004 |
|
| Participants |
Total number randomised: 41 Mother‐infant pairs assigned to 1 of 2 treatment groups: intervention, control Number randomised to each group: 25 intervention, 13 control (after attrition) Participant mothers: fluent in English Participant infants:
|
|
| Interventions |
Experimental group: received 7 home visits from an early‐intervention provider certified in the NBO system, who administered the NBO with the parents and discussed characteristics of the infant. The final visit took place no later than the end of 12th week of the infant's life. Control group: received traditional, early intervention, home‐based delivery; 7 home visits from an early‐intervention provider not certified in the NBO |
|
| Outcomes |
Study instruments used: Home Visiting Index (HVI). A 25‐item scale, which requests the parents to rate, on a 4‐point scale, their degree of agreement with statements about the quality of early intervention service delivery Timing of outcome assessment: at the end of the third month Outcome observer: the same early intervention service provider who gave the HVI to the parents at the end of the third month |
|
| Notes |
Conflicts of interests: both authors disclose their affiliation with the Brazelton Institute, Department of Newborn Medicine, Children's Hospital Boston. The second author is Director of the Brazelton Institute and assisted in the design of the neurobehavioural intervention under study. Source of funding: Robert Wood Johnson Health & Society Scholars Program at University of Wisconsin‐Madison and the Noonan Family Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to either NBO or usual care group using "simple randomisation procedures" – sequence generation was not provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of the 41 infants recruited, 3 were not followed due to inability to contact the family after initial referral. No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported. |
| Other bias | High risk | Both authors disclose their affiliation with the Brazelton Institute, Department of Newborn Medicine, Children's Hospital Boston. The second author is Director of the Brazelton Institute and assisted in the design of the neurobehavioural intervention under study. |
Myers 1982a.
| Methods |
Design: parallel‐group, randomised trial Unit of allocation: participant Study dates: not reported |
|
| Participants |
Total number randomised: 42 Mother‐infant pairs assigned to 1 of the 3 treatment groups: father treatment (group 1), mother treatment (group II), control (group III) Number randomised to each group: father treatment, 14; mother treatment, 14; control group, 12 (after attrition) Participant parents:
Participant infants:
|
|
| Interventions |
Experimental groups: a training session based on the NBAS with either the mother (mother treatment) or the father (father treatment) actively learning to administer the items and to observe her or his own infant's behaviour. Parents were told that these were some "games and exercises" to get to know their baby better. The treatment was given no sooner than the second day. The sessions were held in the mother's room for mothers and in a small room off the nursery for fathers. Control group: parents did not receive the training session or know that it existed. |
|
| Outcomes |
Study instruments used:
Timing of outcome assessment: in the hospital, at least 6 hours after the treatment, and at home at 4 weeks Outcome observer: the hospital measures were collected by 3 female assistants who were blind to group membership. The 4‐week measures consisted of questionnaires that were mailed to the participant's homes. Except for the assessment of father's caretaking, all measures were performed on both parents. |
|
| Notes |
Conflicts of interests: not mentioned Source of funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | All parents who met the criteria were randomly assigned to a treatment group by the throw of a die and did not differ in terms of parents' age and education or infant birth weight. |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Parents unaware of other treatment groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Measures were collected by assistants who were blind to group membership. 4‐week measure included a mailed questionnaire |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 members in the control group were lost to follow‐up at assessment at 4 weeks postpartum. No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported. |
| Other bias | Low risk | None |
Nugent 2014.
| Methods |
Design: parallel‐group, randomised trial Unit of allocation: participant Study dates: participants recruited between December and January 2001 |
|
| Participants |
Total number randomised: 112 Mother‐infant pairs assigned to 1 of the 2 groups: intervention, control Number randomised to each group: 57 (final stage 55) intervention, 55 (final stage 51) control Participant mothers: first‐time parent cohabiting with the father of the baby Participant infants:
|
|
| Interventions |
Intervention group: mothers received routine care plus NBO in the hospital at mothers' bedside and again at 1 month, postpartum home visit Control group: mothers received routine hospital care and a short‐attention, control home visit |
|
| Outcomes |
Study instruments used: Edinburgh Postnatal Depression Scale (EPDS) – a 10‐item, self‐report questionnaire to assess symptoms of postnatal depression during the previous week Timing of outcome assessment: at 1 month postpartum Outcome observer: EPDS was a self‐administered questionnaire at 1 month postpartum; not mentioned whether it was administered by an investigator blind to the group status or not |
|
| Notes |
No conflict of interest: author is director of the Brazelton Institute Source of funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A coin toss was used to randomly allocate eligible mothers to intervention and control groups. |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 112 mothers had enrolled for the study, but at 1 month postpartum, there were only 106 mothers. No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported. |
| Other bias | High risk | Author is director of the Brazelton Institute |
Nugent 2015.
| Methods |
Design: parallel‐group, randomised trial Unit of allocation: participant Study dates: not reported |
|
| Participants |
Total number randomised: 40 Mother‐infant pairs assigned to 1 of the 2 groups: intervention, control Number randomised to each group: 20 (final stage 18) intervention, 20 (final stage 17) control Participant mothers: first‐time parent living with the father of the baby Participant infants:
|
|
| Interventions |
Intervention group: mothers received routine hospital care plus NBO in the hospital and again at 1 month, postpartum home visit plus NBO (for second time). Home visit at 4 months postpartum. Control group: mothers received routine hospital care and home visit at 1 month and 4 months. |
|
| Outcomes |
Study instruments used: the CARE‐Index Timing of outcome assessment: at 4 months postpartum Outcome observer: CARE‐Index was administered in the home by an examiner who had been trained in its administration and coding and who was blind to the status of the dyad. |
|
| Notes |
Conclict of interest: author is director of the Brazelton Institute Source of funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mothers who consented to participate were randomly assigned either to intervention or control group. Method of sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Final outcome was assessed by an examiner who was blind to the status of the dyad |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 40 mothers were initially allocated to the study, but at 4 months postpartum only 35 mother‐infant dyads remained. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported. |
| Other bias | High risk | Author is director of the Brazelton Institute |
Pannabecker 1982.
| Methods |
Design: parallel‐group, randomised trial Unit of allocation: participant Study dates: not reported |
|
| Participants |
Total number randomised: 48 Father‐infant pairs assigned to 1 of the 3 groups: experimental (group I), hospital control (group II), office control (group III) Number randomised to each group: 16 Participant fathers: married, older, white and college educated; they were skilled or professionally employed. These fathers were well prepared for childbirth, since most couples had attended the Lamaze childbirth education classes, and all couples had attended some form of prenatal classes. Participant infants:
|
|
| Interventions |
Experimental group: 2 × 30‐minute sessions were held with each father during the postpartum hospitalisation. The first when the infant was 12‐36 hours old and the second approximately 24 hours after the first session. These sessions were expressly for fathers, and mothers were not included. At the first session, a number of physical characteristics of the infant were pointed out. Some exercises were demonstrated and the father was given time to hold and interact with his infant. At the second session, the father was given an opportunity to hold and interact with his infant after demonstration of a standard number of behavioural items from NBAS. Hospital control group: these fathers spent the same amount of time with the investigator at the same postnatal time points and discussed the same information, but with a major difference. All information was presented through a prepared videotape of a typical normal newborn. Office control group: these fathers had no prior contact with the experiment except for the recruiting visit in the hospital. No educational information and no extra contact with their infants. |
|
| Outcomes |
Study instruments used: direct observations of behaviours made during infant's physical examination and subsequent ratings of behaviours made from videotape segments – these were sensitive measures of father‐infant interaction. Timing of outcome assessment: at 4 weeks of age of the infant. Outcome observer: infant was examined by the paediatric nurse practitioner while the baby was with the father. Selected father and infant behaviours were observed during the physical examination by an observer blind to the group status. |
|
| Notes |
Conflicts of interests: not mentioned Source of funding: supported in part by a Nurse‐Scientist pre‐doctoral fellowship (US Public Health Service (USPHS), Division of Nursing, Grant NU‐5008‐05) and a grant from the Developmental Psychobiology Endowment Fund, Department of Psychiatry, University of Colorado Medical Center. Dr Emde is supported by Research Scientist Award. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | First 2 groups (I and II) were randomly assigned to an experimental group or a hospital control group; method of sequence generation was not described. |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessed by an observer unaware of the groups to which the participants belonged |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported. |
| Other bias | Low risk | None |
Poley‐Strobel 1987.
| Methods |
Design: parallel‐group, randomised trial Unit of allocation: participant Study dates: not reported |
|
| Participants |
Total number randomised: 20 Mother‐infant pairs assigned to 1 of 2 groups: experimental, control Number randomised to each group: 10 Participant mothers:
Participant infants:
|
|
| Interventions |
Experimental group: the intervention involved a teaching modelling session in which the investigator explained and demonstrated the infant's behavioural cues and responses to the mother using NBAS. The teaching plan was structured around 4 areas: interactive processes, motoric processes, organisation processes‐state control, and organisation processes‐response to stress. All of the interventions were scheduled around 1 of the infant's feeding times so that the infant would be awake and could be used in modelling the behaviours being discussed. Control group: NBAS was not used |
|
| Outcomes |
Study instruments used: assessment of Mother‐Infant Sensitivity. Mother‐infant interaction was measured using this instrument. Timing of outcome assessment: 10 to 14 days following delivery Outcome observer: study intervention was carried out 1 to 3 days after discharge from hospital in the first instance. An appointment was made with all mothers for a home visit 10‐14 days following delivery to observe another feeding session with the infant and obtain the post‐test data. The NBAS was also administered. |
|
| Notes |
Conflicts of interests: not mentioned Source of funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned to the control or the experimental group; no information provided about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | An observer unaware of the assignment of the mother to a group observed the feeding for the mother‐infant interaction. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | None |
Szajnberg 1987.
| Methods |
Design: parallel‐group, randomised trial Unit of allocation: participant Study dates: not reported |
|
| Participants |
Number randomised: 25 Mother‐infant pairs assigned to 1 of 2 groups: intervention, control Number randomised to each group: 12 intervention, 13 control Participant mothers: not described Participant infants:
|
|
| Interventions |
Intervention group: at 34 weeks of age, intervention group mothers were invited to observe NBAS performed on their infants by a trained examiner with a second observer/recorder. Mothers were invited to ask questions during and after the examination. Control group: mothers were invited to observe a standard complete physical performed by an attending neonatologist and a second observer/recorder. Mothers were invited to ask questions during and after the examination. |
|
| Outcomes |
Study instruments:
Timing of outcome assessment: at 6 months corrected age of the baby Outcome observer: at 6 months corrected age. Mothers were interviewed using a semi‐structured format. |
|
| Notes |
Conflicts of interests: not mentioned Source of funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Mother‐infant pairs were randomly assigned to control and intervention groups; no information provided about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | High risk | One control group participant refused follow‐up, and 4 were lost to follow‐up. They did not differ in income, demographic or medical characteristics. No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported. |
| Other bias | Low risk | None |
Worobey 1982.
| Methods |
Design: parallel‐group, randomised trial Unit of allocation: participant Study dates: not reported |
|
| Participants |
Total number randomised: 48 Mother‐infant pairs assigned to 1 of 3 groups: experimental treatment (group I), control group (group II), or contrast treatment (group III) Number randomised to each group: 16 Participant mothers: white, middle‐class Participant infants: healthy, full‐term newborns |
|
| Interventions | All infants were assessed using NBAS initially. Experiment treatment: active information through auditory, visual and tactile interaction. Mothers individually observed a 45‐minute demonstration of the NBAS conducted on their newborns, on second or third day of life after the examiner had assessed the newborn on NBAS before discharge from the hospital. The examiner, serving as facilitator, neither touched nor held the infant during these sessions; rather he talked the mother through the procedures and guided and encouraged her in eliciting infant responses. Control treatment: minimal information through auditory feedback. Immediately after the behavioural assessment of the newborn, the mother received a verbal summary of her infant's performance. The examiner described the baby's performance on the 4 categories of the profile, according to the priori cluster, and discussed the baby's performance with the new mother. This procedure allowed the investigator to alert the mother to characteristics of her newborn, based on its behavioural display, and provided the mother with the opportunity to talk about what she heard. Contrast treatment: passive observation with auditory and visual feedback. Mothers observed a 45‐minute demonstration of the NBAS conducted on their newborns. This procedure allowed the investigator to alert the mother to the characteristics of her newborn, based on its behavioural display, and also drew attention to specific infant behaviours and provided the mother with an opportunity to talk about what she was seeing. These mothers received a 1‐page summary of the demonstration 2 days later. |
|
| Outcomes |
Study instruments: pre‐coded checklist to assess mother‐infant interaction. Timing of outcome assessment: 4 and 6 weeks of age for the infant. Outcome observer: mother‐infant dyads were observed for 60 minutes on 1 occasion in the family's home, by 1 of the observers blind not only to experimental design but also to the purpose of the study. |
|
| Notes |
Conflicts of interests: not mentioned Source of funding: grant from the Penn State Division of Continuing Education |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned to 1 of the 3 treatment conditions; no information provided about sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The experimenter explicitly avoided telling mothers that the service provided would affect their parenting or their baby. However, one investigator administered all 3 treatments and thus was cognisant of group assignments. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Observers were blind not only to the experimental design but also to the purpose of the study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported. |
| Other bias | Low risk | None |
APGAR: appearance, pulse, grimace, activity and respiration;IUGR: intrauterine growth retardation;MABI: Mother's Assessment of the Behaviour of her Infant; NBAS: Neonatal Behavioural Assessment Scale; NBO: Newborn Behavioural Observations system; PI: principal investigator; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cardone 1990 | A derived "specialized application of NBAS" |
| Cooper 2009 | NBAS used as part of a wider intervention |
| Cooper 2015 | Used a variation of NBAS |
| Egeland 1984 | Not a randomised controlled trial |
| Fowles 1999 | Not a randomised controlled trial |
| Golas 1986 | NBAS used as part of intervention with the Amazing Newborn DVD |
| Gomes 1987 | Intervention 'based on' NBAS |
| Gomespedro 1995 | NBAS part of broader package of care |
| Hart 1998 | NBAS used in conjunction with MABI. |
| Joshi 2013 | Non‐randomised controlled trial; both control and intervention groups given NBAS |
| Kiepura 2011 | Non‐randomised controlled trial |
| Kusaka 2007 | Non‐randomised controlled trial |
| Meisels 1993 | Qualitative report of outcomes only (i.e. no date) |
| Nurcombe 1984 | NBAS used as part of intervention package – MITP. |
| Ogi 2001 | Intervention 'based on' NBAS; non‐randomised |
| Ohgi 2004 | NBAS used as part of wider package of care |
| Parker 1992 | Uses Newborn Individualized Developmental Care and Assessment Programme (NIDCAP) and Assessment of Preterm Infants' Behaviour (APIB) rather than NBAS |
| Parker Loewen 1987 | Does not appear to be the NBAS |
| Sanders 2006 | Not a randomised controlled trial |
| Sullivan 1980 | No information about how NBAS was used. |
| Teti 2009 | NBAS used as part of wider intervention |
| Wendland Carro 1999 | NBAS demonstrated on video |
| Widmayer 1980 | NBAS as part of package with the MABI. |
| Widmayer 1981 | NBAS as part of wider intervention package. |
MABI: Mother's Assessment of the Behaviour of her Infant; MITP: Mother Infant Transaction Program; NBAS: Neonatal Behavioural Assessment Scale.
Characteristics of ongoing studies [ordered by study ID]
NCT03070652.
| Trial name or title | What are the effects of supporting early parenting by increasing the understanding of the infant? A randomized community‐based trial |
| Methods | Design: cluster‐randomised trial |
| Participants |
Study population: new families.
Exclusion criteria: parents or infants affiliated to special treatment elsewhere |
| Interventions |
Intervention group: will receive NBO at 3 weeks after birth and in following visits up to 3 months Control group: will receive care as usual at 3 weeks after birth and in following visits up to 3 months |
| Outcomes |
|
| Starting date | 1 January 2017 |
| Contact information | Hanne Kronborg, Aarhus University, Denmark. E‐mail: HK@ph.au.dk |
| Notes |
Conflicts of interests: not mentioned Source of funding: not mentioned Trials register number:NCT03070652 |
Nicolson 2016.
| Trial name or title | Understanding your newborn and adapting to parenthood (UNA): a randomised clinical effectiveness trial of the Newborn Behavioural Observations (NBO) for new families with maternal antenatal risk factors for postnatal depression (PND) |
| Methods | Design: randomised controlled trial |
| Participants |
Study population: expectant first time single mothers and couples from the Royal Women’s Hospital and Bendigo Hospital in Australia. Target recuitment: this project aims to randomise n=150 expectant first time mothers screening positive for antenatal risk factors of PND. The study has so far screened n=218 first time mothers during pregnancy and of these women n=84 have screened positive for antenatal risk factors of PND and have been randomised into either the G1 comparison group or G2 intervention group. The project has also recruited n=80 first time fathers and of these participants, n=35 have been included in the randomised sample as above. |
| Interventions |
|
| Outcomes | G1 and G2 participants will complete the EPDS at 6 weeks and all three groups will complete an endpoint psychosocial questionnaire (including the EPDS and the PASS) at infant age 4 months. Additional endpoint data for G1 and G2 group participants will be collected on mother‐infant interaction, maternal depression diagnosis and infant development, using the Emotional Availability Scales, the SCID‐5 diagnostic interview depression module and the Bayley Scales of Infant Development respectively. |
| Starting date | 10 August 2017 |
| Contact information | Dr Susan Nicolson, Centre for Women’s Mental Health, Susan.Nicolson@thewomens.org.au |
| Notes |
Conflicts of interests: not mentioned Source of funding: not mentioned Trials register number: not registered |
Nugent 2016.
| Trial name or title | The effect of a Newborn Behavioral Observation System‐Early Intervention (NBO‐EI) model of care |
| Methods | Design: this is a pragmatic trial where infants from 5 early intervention programs in one state were randomized to receive an NBO‐EI model of care or usual care for the first 12 weeks corrected gestational age. Infants were followed up to 6 months corrected gestational age and a number of maternal and infant outcomes were measured. |
| Participants | Thirty‐two EI‐eligible infants participated. Of the total sample, 17 received the NBO‐EI model and 22 received usual EI care. |
| Interventions |
Intervention group: received an NBO‐EI model of care. The NBO is an 18‐item neurobehavioral relationship‐based intervention that involves a shared observation of the infant with the parent and includes elicited maneuvers with the purpose of 1) identifying infant neurobehaviors, 2) interpreting these neurobehaviors in the context of the parent‐infant interaction and infant self‐regulation and intentions, with the goal of enhancing the parent‐infant relationship, the cornerstone of optimal infant neurodevelopment and function. Specifically, the NBO‐EI group received weekly home visits from an EI provider certified in the Newborn Observational (NBO) System up to 12 weeks corrected gestational age. Content of NBO‐EI home visits consisted of intervention strategies guided by the NBO. Specifically, at each home visit the EI service provider administered the NBO with the parents and discussed 1) the infant’s attempts and successes at self‐regulation and requests for support, 2) how the infant’s neurobehaviors contribute to the parent‐infant social interactional encounter and caregiver bonding. The intervention group received the NBO‐EI during their weekly home visit for up to twelve successive weeks. The final intervention visit took place no later than the end of the twelfth week of the infant’s life, when the infant was three months of age, adjusted for prematurity. After the final study visit, in accordance with federal mandates governing EI service delivery, EI families chose to continue with weekly home visits or modified their home visit schedule. Usual care group: the usual care (UC) group received traditional EI home‐based service delivery. The UC group received weekly home visits from an EI provider not certified in the NBO. The content of UC group home visits consisted of therapeutic and developmental activities deemed appropriate for the infant’s adjusted age and typically included visual tracking, reaching and grasping toys of a variety of textures, and tolerance of developmental play. The final study visit took place no later than the end of the twelfth week of the infant’s life, when the infant was three months of age, adjusted for prematurity. After the final study visit, in accordance with federal mandates governing EI service delivery, EI families chose to continue with weekly home visits or modified their home visit schedule. |
| Outcomes |
Maternal depressive symptoms was collected using the Center for Epidemiologic Studies Depression Scale (CES‐D). The CES‐D asks mothers to report, on a 4‐point scale (0=rarely/none of the time to 3=all of the time), their frequency of symptoms for 20 scale items. Scores of 16 or greater indicate clinically significant depressive symptoms. The CES‐D was collected, via phone interview at the 3‐month and 6‐month follow‐up time, by an independent member of the study team who is blinded to the infant’s intervention group assignment. Parent‐infant interaction was collected using the Parent‐Child Early Relational Assessment (PCERA) . The PCERA measures parent’s and child’s affect and behavioral characteristics during a 5‐minute play observation using 29 parent items across 3 sub‐scales of parenting quality (positive affective involvement and verbalization; negative affect and behavior; and intrusiveness, insensitivity, and inconsistency) and 28 child items across 3 sub‐scales of infant emotional/behavioral regulation (positive affect, communicative, and social skills; quality of play, interest, and attentional skills; and dysregulation and irritability). For the this study, we utilized scores for the 3 parent and 3 child sub‐scales. Sub‐scale scores range from 1 (negative relational quality) to 5 (positive relational quality). PCERA scores were ascertained from a 5‐minute video clip taken by the EI home visitor (NBO‐EI and usual care groups) at 3 months and 6 months corrected gestational age. The 3‐month video will occur at the final study home visit. The 6‐month video coincided with the 6‐month eligibility / follow‐up evaluation that routinely occurs in MA EI programs. All videotapes will be reviewed and coded by an independent researcher trained in the PCERA. Parent’s perceptions of the quality of their interaction with their infant and the extent to which their home visitor facilitated optimal interaction was measured using the Home Visiting Index.[i] Home Visiting index (HVI) is a 25‐item scale that asks parents to rate, on a 4‐point scale (1=strongly agree and 4=strongly disagree) their degree of agreement with statements about the quality of EI service delivery. At the final study visit, parents were asked to complete the HVI and return it in a self‐addressed stamped envelope to the study team. Infant self‐regulation was collected using the Bayley Scales of Infants Development (BSID), Adaptive scale. The BSID, Adaptive Scale was collected, via phone interview at the 3‐month and 6‐month follow‐up time, by an independent member of the study team who is blinded to the infant’s intervention group assignment. Cognitive and personal‐social, function was collected using the Battelle Developmental Inventory (BDI‐2) cognitive and personal‐social sub‐scales. Batelle scores were collected via videotaped EI eligibility evaluation (at 6 months postterm) by an independent member of the study team who is blinded to the infant’s intervention group assignment. [i] NugentJK. Home visiting index. Brazelton Institute, Children’s Hospital, Boston; 2003 |
| Starting date | 1 November 2016 |
| Contact information | Beth M McManus, PT, MPH, ScD, Beth.mcmanus@ucdenver.edu, Mobile: 617‐529‐8138 |
| Notes |
Conflicts of interests: not mentioned Source of funding: not mentioned Trials register number: nor registered |
EPDS: Edinburgh Postnatal Depression Scale; NBO: Newborn Behavioral Observations system.
Differences between protocol and review
-
Review authors
Dieter Wolke left the review team, and Nadeeja Herath, who undertook a significant amount of the work on the review as part of her visiting fellowship to the UK, along with Cathy Bennett joined the review team.
-
Methods > Types of outcome measures
We refined our list of primary outcomes. We deleted 'infant attachment security' because the studies do not measure this outcome, and 'caregiver sensitivity and responsiveness', which duplicates 'caregiver interaction'. We moved 'caregiver reflective functioning' from our list of primary outcomes to our list of secondary outcomes because we felt it is not a primary outcome for change in studies of the NBAS/NBO.
We also refined our list of secondary outcomes by removing the outcome of 'caregiver involvement in caregiving activities and interaction' because it duplicated 'caregiver interaction'.
-
Methods > Searching other resources
We did not contact authors and experts in the field to identify unpublished studies because these studies are all registered with the National Brazelton Institute, who provided us with the necessary information.
-
Methods > Data extraction and management
Nadeeja Herath extracted the data, with Jane Barlow conducting a check of all extracted data due to time limitations.
-
Methods > Assessment of risk of bias in included studies
Nadeeja Herath assessed the risk of bias in included studies, with Jane Barlow conducting checks of all assessments due to time limitations.
-
It was not possible to populate Table 1 with data on 'infant attachment security' or 'caregiver reflective functioning' because these were not measured in the included studies. We did, however, provide information about 'quality of caregiver‐infant interaction', 'caregiver mental health', 'infant social, emotional, cognitive and motor development', 'caregiver perception of infant', and 'caregiver knowledge' because these were key outcomes of interest, all of which were measured in the included studies.
In addition, we were unable to use some of our methods, as specified in our protocol (Bartram 2016). These are reported in Table 2.
Contributions of authors
Contact author and guarantor of the review: JB. Drafting of protocol: JB and CBT. Study selection: JB, CBT and NH. Data extraction: JB, NH and CB. Assessment of study quality, including risk of bias: JB, NH and CBT. Data entry into RevMan 5: NH. Analyses: JB, NH and YW. Interpretation of analyses: JB and CBT. Drafting of final review: JB NH and CBT. Disagreement resolution: CBT. Updating of review: JB and CBT.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Jane Barlow is an Editor with Cochrane Developmental, Psychosocial and Learning Problems and was Co‐Chair of the Campbell Social Welfare Group until May 2015. Nadeeja INS Herath ‐ none known. Christine Bartram Torrance (CBT) is certified in the Neonatal Behavioral Assessment Scale (NBAS) and Newborn Behavioral Observations (NBO) system. CBT is facilitating the NBAS as part of her collaboration with an observational study in West Africa and will train colleagues in its delivery, for which she will receive payment. In this study, CBT is responsible for conducting NBAS sessions and ensuring handover of her role to local staff members, including ensuring they are trained and certified in the NBAS. Both this review and the observational study are being conducted as part of her PhD. CBT delivers NBAS occasionally on a voluntary basis. CBT is not currently being paid for conducting the NBAS or NBO in her study and has not been paid for conducting them previously in voluntary roles. CBT expects to continue offering these sessions in a voluntary capacity in the future. In terms of support in training others in the NBAS, CBT has not been paid previously, but as CBT is certified, it is possible that she could be paid in the future for training or support in training, or for delivering the sessions in research or support capacities. Cathy Bennett is the proprietor of Systematic Research Ltd. The company received a consultancy fee to enable CB to work as a co‐author of this review. CB has business relationships with other clients, who may provide consultancy fees for evidence‐based medicine reviews, projects and reports. Yinghui Wei ‐ none known.
New
References
References to studies included in this review
Anderson 1981 {published data only}
- Anderson CJ. Enhancing reciprocity between mother and neonate. Nursing Research 1981; Vol. 30, issue 2:89‐93. [PUBMED: 6907868] [PubMed]
Beal 1989 {published data only}
Beeghly 1995a {published data only}
- Beeghly M, Brazelton TB, Flannery KA, Nugent JK, Barrett DE, Tronick EZ. Specificity of preventative pediatric intervention effects in early infancy. Journal of Developmental and Behavioral Pediatrics 1995; Vol. 16, issue 3:158‐66. [PUBMED: 7560118] [PubMed]
Belsky 1985 {published data only}
- Belsky J. Experimenting with the family in the newborn period. Child Development 1985; Vol. 56:407‐14. [PUBMED: 3987415] [PubMed]
Britt 1994 {published data only}
- Britt GC, Myers BJ. Testing the effectiveness of BNAS intervention with a substance‐using population. Infant Mental Health Journal 1994;15(3):293‐304. [DOI: 10.1002/1097-0355(199423)15:3%3C293::AID-IMHJ2280150305%3E3.0.CO;2-E] [DOI] [Google Scholar]
Furr 1982 {published data only}
- Furr PA, Kirgis CA. A nurse‐midwifery approach to early mother‐infant acquaintance. Journal of Midwifery & Women's Health 1982;27(5):10‐4. [DOI: 10.1016/0091-2182(82)90004-0; PUBMED: 6922161] [DOI] [PubMed] [Google Scholar]
Killough 2004 {published data only}
- Killough RH. The Birth of Parenting: Maternal Representations, the Place of the Infant, and the Development of Sensitive Caring [PhD thesis]. Amherst: University of Massachusetts Amherst, 2004. [Google Scholar]
Liptak 1983 {published data only}
- Liptak GS, Keller BB, Feldman AW, Chamberlin RW. Enhancing infant development and parent‐practitioner interaction with the Brazelton Neonatal Assessment Scale. Pediatrics 1983; Vol. 72, issue 1:71‐8. [PUBMED: 6866594] [PubMed]
McManus 2012 {published data only}
- McManus BM, Nugent JK. A neurobehavioral intervention incorporated into a state early intervention program is associated with higher perceived quality of care among parents of high‐risk newborns. Journal of Behavioral Health Services and Research. 2012/04/25 2014; Vol. 41, issue 3:381‐9. [DOI: 10.1007/s11414-012-9283-1; PUBMED: 22529036] [DOI] [PubMed]
Myers 1982a {published data only}
Nugent 2014 {published data only}
- Nugent JK, Bartlett JD, Valim, C. Effects of an infant‐focused relationship‐based hospital and home visiting intervention on reducing symptoms of postpartum maternal depression: a pilot study. Infants and Young Children 2014; Vol. 27, issue 4:292‐304. [DOI: 10.1097/IYC.0000000000000017; 0896‐3746] [DOI]
Nugent 2015 {published data only}
- Nugent JK, Dym‐Bartlett J, Ende A, Killough J, Valim C. A randomized study of the effects of the Newborn Behavioural Observations (NBO) system on sensitivity in mother‐infant interaction. Society for Research in Child Development Biennial Meeting; 2015 Mar 19‐21; Philadelphia (PA). 2015.
Pannabecker 1982 {published data only}
Poley‐Strobel 1987 {published data only}
- Poley‐Strobel BA, Beckmann, CA. The effects of a teaching‐modeling intervention on early mother‐infant reciprocity. Infant Behavior and Development 1987; Vol. 10, issue 4:467‐76. [DOI: 10.1016/0163-6383(87)90043-9] [DOI]
Szajnberg 1987 {published data only}
Worobey 1982 {published data only}
- Worobey J, Belsky J. Employing the Brazelton scale to influence mothering: an experimental comparison of three strategies. Developmental Psychology 1982; Vol. 18, issue 5:736‐43. [DOI: 10.1037/0012-1649.18.5.736] [DOI]
References to studies excluded from this review
Cardone 1990 {published data only}
- Cardone IA, Gilkerson L. Family administered neonatal activities: an exploratory method for the integration of parental perceptions and newborn behaviour. Infant Mental Health Journal 1990;11(2):127‐41. [DOI: 10.1002/1097-0355(199022)11:2%3C127::AID-IMHJ2280110205%3E3.0.CO;2-A] [DOI] [Google Scholar]
Cooper 2009 {published data only}
- Cooper PJ, Tomlinson M, Swartz L, Landman M, Molteno C, Stein A, et al. Improving quality of mother‐infant relationship and infant attachment in socioeconomically deprived community in South Africa: randomised controlled trial. BMJ 2009;338:b1858. [DOI: 10.1136/bmj.b974] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2015 {published data only}
- Cooper PJ, Pascalis L, Woolgar M, Romaniuk H, Murray L. Attempting to prevent postnatal depression by targeting the mother‐infant relationship: a randomised controlled trial. Primary Health Care Research and Development 2015;16(4):383‐97. [DOI: 10.1017/S1463423614000401; PUBMED: 25381790] [DOI] [PubMed] [Google Scholar]
Egeland 1984 {published data only}
- Egeland B, Farber EA. Infant‐mother attachment: factors related to its development and changes over time. Child Development 1984;55(3):753‐71. [PUBMED: 6734316] [PubMed] [Google Scholar]
Fowles 1999 {published data only}
- Fowles ER. The Brazelton Neonatal Behavioural Assessment Scale and maternal identity. American Journal of Maternal Child Nursing 1999;24(6):287‐93. [PUBMED: 10565142] [DOI] [PubMed] [Google Scholar]
Golas 1986 {published data only}
- Golas GA, Parks P. Effect of early postpartum teaching on primiparas' knowledge of infant behavior and degree of confidence. Research in Nursing and Health 1986;9(3):209‐14. [DOI: 10.1002/nur.4770090305] [DOI] [PubMed] [Google Scholar]
Gomes 1987 {published data only}
- Gomes‐Pedro J. Early Intervention and Mother‐Infant Interaction During the First Three Months of Life. Washington DC: ERIC Clearinghouse, 1987. [Google Scholar]
Gomespedro 1995 {published data only}
- Gomes‐Pedro J, Patricio M, Carvalho A, Goldschmidt T, Torqal‐Garcia F, Monteiro MB. Early intervention with Portuguese mothers: a two year follow up. Journal of Developmental and Behavioral Pediatrics 1995;16(1):21‐8. [PUBMED: 7730453] [PubMed] [Google Scholar]
Hart 1998 {published data only}
- Hart S, Field T, Nearing G. Depressed mothers' neonates improve following the MABI and a Brazelton demonstration. Journal of Pediatric Psychology 1998;23(6):351‐6. [DOI: 10.1093/jpepsy/23.6.351] [DOI] [PubMed] [Google Scholar]
Joshi 2013 {published data only}
- Joshi K, Puntambekar A. Effects of early intervention on the behaviour of premature neonates ‐ a clinical trial. Journal of Physical Therapy 2013;8(1):12‐7. [Google Scholar]
Kiepura 2011 {published data only}
- Kiepura E, Kmita G, Cieślak‐Osik B, Urmańska W, Lewandowska D. Neonatal behavioural assessment of pre‐term and full‐term infants as experiences by parents [Ocena zachowania noworodka w doświadczeniu rodziców dzieci urodzonych przedwcześnie i o czasie]. Medycyna Wieku Rozwojowego 2011;15(3 Pt 2):414‐20. [PUBMED: 22253127] [PubMed] [Google Scholar]
Kusaka 2007 {published data only}
- Kusaka R, Ohgi S, Gima H, Fujimoto T. Short term effects of the neonatal behavioural assessment scale based intervention for infants with developmental disabilities. Journal of Physical Therapy Science 2007;19(1):1‐8. [DOI: 10.1589/jpts.19.1] [DOI] [Google Scholar]
Meisels 1993 {published data only}
- Meisels S, Dichtelmiller M, Liaw FR. A multidimensional analysis of early childhood intervention programme. In: Zeanah CH editor(s). Handbook of Infant Mental Health. 3rd Edition. Vol. 1, Guilford Press, 1993:361‐442. [Google Scholar]
Nurcombe 1984 {published data only}
- Nurcombe B, Howell DC, Rauh VA, Teti DM, Ruoff P, Brennan J. An intervention program for mothers of low‐birthweight infants: preliminary results. Journal of the American Academy of Child and Adolescent Psychiatry 1984;23(3):319‐25. [DOI: 10.1016/S0002-7138(09)60511-2] [DOI] [PubMed] [Google Scholar]
Ogi 2001 {published data only}
- Ogi S, Arisawa K, Takahashi T, Akiyama T, Goto Y, Fukuda M, et al. The developmental effects of an early intervention programme for very low birthweight infants. Brain and Development 2001;33(1):31‐6. [PUBMED: 11197893] [PubMed] [Google Scholar]
Ohgi 2004 {published data only}
- Ohgi S, Fukuda M, Akiyama T, Gima H. Effect of an early intervention programme on low birthweight infants with cerebral injuries. Journal of Paediatrics and Child Health 2004;40(12):689‐95. [DOI: 10.1111/j.1440-1754.2004.00512.x; PUBMED: 15569286 ] [DOI] [PubMed] [Google Scholar]
Parker 1992 {published data only}
- Parker S, Zahr LK, Cole JG, Brecht ML. Outcome after developmental intervention in the neonatal intensive care unit for mothers of preterm infants with low socioeconomic status. Journal of Paediatrics 1992;120(5):780‐5. [PUBMED: 1578316] [DOI] [PubMed] [Google Scholar]
Parker Loewen 1987 {published data only}
- Parker‐Loewen DL, Lytton H. Effects of short‐term interaction coaching with mothers of preterm infants. Infant Mental Health Journal 1987;8(3):277‐87. [DOI: 10.1002/1097-0355(198723)8:3%3C277::AID-IMHJ2280080310%3E3.0.CO;2-X] [DOI] [Google Scholar]
Sanders 2006 {published data only}
- Sanders LW, Buckner EB. The Newborn Behavioral Observations system as a nursing intervention to enhance engagement in first‐time mothers: feasibility and desirability. Pediatric Nursing 2006;32(5):455‐9. [PUBMED: 17100077] [PubMed] [Google Scholar]
Sullivan 1980 {published data only}
- Sullivan DL, Leake RD. Significant effect of the Brazelton Neonatal Behavioural Assessment Scale in maternal training. Pediatric Research 1980;14(4):439. [Google Scholar]
Teti 2009 {published data only}
- Teti DM, Black MM, Viscardi R, Glass P, O'Connell MA, Baker L, et al. Intervention with African American premature infants: four months results of an early intervention program. Journal of Early Intervention 2009;31(2):146‐66. [DOI: 10.1177/1053815109331864] [DOI] [Google Scholar]
Wendland Carro 1999 {published data only}
- Wendland‐Carro J, Piccinini CA, Millar WS. The role of an early intervention on enhancing the quality of mother‐infant interaction. Child Development 1999;70(3):713‐21. [PUBMED: 10368917] [DOI] [PubMed] [Google Scholar]
Widmayer 1980 {published data only}
- Widmayer SM, Field TM. Effects of Brazelton demonstrations on early interactions of preterm infants and their teenage mothers. Infant Behaviour and Development 1980;3(1):79‐89. [DOI: 10.1016/S0163-6383(80)80008-7] [DOI] [Google Scholar]
Widmayer 1981 {published data only}
- Widmayer SM, Field TM. Effects of Brazelton demonstrations for mother on the development of preterm infants. Pediatrics 1981;67(5):711‐4. [PUBMED: 7255001] [PubMed] [Google Scholar]
References to ongoing studies
NCT03070652 {unpublished data only}
- NCT03070652. What are the effects of supporting early parenting by increasing the understanding of the infant? [What are the effects of supporting early parenting by increasing the understanding of the infant? A randomized community based trial]. clinicaltrials.gov/ct2/show/NCT03070652 (first received 3 March 2017).
Nicolson 2016 {unpublished data only}
- Nicolson S. The UNA Study. Understanding your newborn and adapting to parenthood. Centre for Women's Mental Health. Unpublished manuscript 2016.
Nugent 2016 {unpublished data only}
- Nugent JK, McManus B. The effects of the NBO early intervention model of care on infants with developmental disabilities on infant self‐regulation, parent‐infant interaction and maternal depression and later social‐emotional functioning. Unpublished manuscript 2016.
Additional references
Abidin 1983
- Abidin RR. The Parenting Stress Index. Charlottesville (VA): Pediatric Psychology Press, 1983. [Google Scholar]
Ainsworth 1971
- Ainsworth MDS, Bell SM, Stayton DJ. Individual differences in strange situation behavior of one‐year‐olds. In: Schaffer HR editor(s). The Origins of Human Social Relations. New York (NY): Academic Press, 1971:17‐57. [Google Scholar]
Ainsworth 1974
- Ainsworth MDS, Bell SM, Stayton DJ. Infant‐mother attachment and social development: socialization as a product of reciprocal responsiveness to signals. In: Richards MPM editor(s). The Integration of a Child into a Social World. London: Cambridge University Press, 1974:97‐119. [Google Scholar]
Barett 1988
- Barett, D, Beeghly, M, Burrows, E, Brazelton TB. Effects of IUGR. Maternal Characteristics and Intervention on Maternal Behaviour with the 4‐month old infant. Infant Association for Infant Mental Health. Providence: RI, 1988. [Google Scholar]
Barnard 1978
- Barnard K. Nursing Child Assessment Teaching Scale. Seattle (WA): University of Washington, 1978. [Google Scholar]
Bates 1979
- Bates JE, Bennett Freeland CA, Lounsbury ML. Measurement of infant difficultness. Child Development 1979;50(3):794‐803. [DOI: 10.2307/1128946] [DOI] [PubMed] [Google Scholar]
Bayley 1969
- Bayley N. Bayley Scales of Infant Development: Birth to Two Years. New York: Psychological Corporation, 1969. [Google Scholar]
Beebe 2010
- Beebe B, Jaffe J, Markese S, Buck K, Chen H, Cohen P, et al. The origins of 12‐month attachment: a microanalysis of 4‐month mother‐infant interaction. Attachment and Human Development 2010;12(1‐2):3‐141. [DOI: 10.1080/14616730903338985; PUBMED: 20390524] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beebe 2012
- Beebe B, Lachmann F, Jaffe J, Markese S, Buck KA, Chen H, et al. Maternal postpartum depressive symptoms and 4‐month mother‐infant interaction. Psychoanalytic Psychology 2012;29(4):383‐407. [DOI: 10.1037/a0029387] [DOI] [Google Scholar]
Beeghly 1995b
- Beeghly M, Brazelton TB, Flannery KA, Nugent JK, Barrett DE, Tronick EZ. Specificity of preventative pediatric intervention effects in early infancy. Developmental and Behavioral Pediatrics 1995;16(3):158‐66. [PUBMED: 7560118] [PubMed] [Google Scholar]
Belsky 1984
- Belsky J, Rovine M, Taylor DG. The Pennsylvania Infant and Family Development Project, III: the origins of individual differences in infant‐mother attachment: maternal and infant contributions. Child Development 1984;55(3):718‐28. [DOI: 10.2307/1130124] [DOI] [PubMed] [Google Scholar]
Belsky 1996
- Belsky J, Campbell SB, Cohn JF, Moore G. Instability of infant‐parent attachment security. Developmental Psychology 1996;32(5):921‐4. [DOI: 10.1037//0012-1649.32.5.921] [DOI] [Google Scholar]
Belsky 2002
- Belsky J, Fearon RM. Infant‐mother attachment security, contextual risk, and early development: a moderational analysis. Development and Psychopathology 2002;14(2):293‐310. [PUBMED: 12030693] [DOI] [PubMed] [Google Scholar]
Berlin 2008
- Berlin LJ, Cassidy J, Appleyard KC, Shaver PR. The influence of early attachments on other relationships. Handbook of Attachment: Theory, Research, and Clinical Applications. 2nd Edition. New York (US): Guilford Press, 2008:333‐47. [Google Scholar]
Borelli 2010
- Borelli JL, David DH, Crowley MJ, Mayes LC. Links between disorganized attachment classification and clinical symptoms in school‐aged children. Journal of Child and Family Studies 2010;19(3):243‐56. [DOI: 10.1007/s10826-009-9292-8] [DOI] [Google Scholar]
Bornstein 2013
- Bornstein MH, Manian N. Maternal responsiveness and sensitivity re‐considered: some is more. Development and Psychopathology 2013;25(4 Pt 1):957‐71. [DOI: 10.1017/S0954579413000308; PMC3831361; PUBMED: 24229542] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowlby 1969
- Bowlby J. Attachment and Loss: Vol. 1. Attachment. New York: Basic Books, 1969. [Google Scholar]
Brazelton 1978
- Brazelton TB. The Brazelton Neonatal Behavior Assessment Scale: introduction. Monographs of the Society for Research in Child Development 1978;43(5‐6):1‐13. [PUBMED: 752799] [PubMed] [Google Scholar]
Brazelton 1995
- Brazelton TB. Neonatal Behavioral Assessment Scale. 3rd Edition. London: Mac Keith Press, 1995. [Google Scholar]
Britt 1994a
- Britt GC, Myers BJ. Testing the effectiveness of NBAS intervention with a substance‐using population. Infant Mental Health Journal 1994;15(3):293‐304. [DOI: 10.1002/1097-0355(199423)15:3%3C293::AID-IMHJ2280150305%3E3.0.CO;2-E] [DOI] [Google Scholar]
Britt 1994b
- Britt GC, Myers BJ. The effects of Brazelton intervention: a review. Infant Mental Health Journal 1994;15(3):278‐92. [DOI: 10.1002/1097-0355(199423)15:3%3C278::AID-IMHJ2280150304%3E3.0.CO;2-4] [DOI] [Google Scholar]
Broussard 1970
- Broussard E, Hartner MS. Maternal perception of the neonate as related to development. Child Psychiatry and Human Development 1970;1(1):16‐25. [DOI: 10.1007/BF01434585; PUBMED: 24178546] [DOI] [PubMed] [Google Scholar]
Broussard 1971
- Broussard E, Hartner M. Considerations regarding maternal perception of first born. In: Helmuth J editor(s). Exceptional Infants. Vol. 3, New York: Brunner/Mazel, 1971. [Google Scholar]
Carey 1978
- Carey WB, McDevitt SC. Revision of the Infant Temperament Questionnaire. Pediatrics 1978;61:735‐9. [PUBMED: 662513] [PubMed] [Google Scholar]
Clarke‐Stewart 1973
- Clarke‐Stewart KA. Interactions between mothers and their young children: characteristics and consequences. Monographs of the Society for Research in Child Development 1973;38(6‐7):1‐109. [DOI: 10.2307/1165928] [DOI] [PubMed] [Google Scholar]
Cooper 2002
- Cooper PJ, Landman M, Tomlinson M, Molteno C, Swartz L, Murray L. Impact of a mother‐infant intervention in an indigent peri‐urban South African context: pilot study. British Journal of Psychiatry 2002;180:76‐81. [PUBMED: 11772856] [DOI] [PubMed] [Google Scholar]
Cox 1987
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10‐item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry 1987;150(6):782‐6. [DOI: 10.1192/bjp.150.6.782] [DOI] [PubMed] [Google Scholar]
Crittenden 2001
- Crittenden PM. CARE‐Index: Coding Manual. Miami: Family Relations Institute, 2001. [Google Scholar]
Crockenberg 1982
- Crockenberg SB, Smith P. Antecedents of mother‐infant interaction and infant irritability in the first three months of life. Infant Behavior and Development 1982;5(2‐4):105‐19. [DOI: 10.1016/S0163-6383(82)80021-0] [DOI] [Google Scholar]
Das Eiden 1996
- Das Eiden R, Reifman A. Effects of Brazelton demonstrations on later parenting: a meta‐analysis. Journal of Pediatric Psychology 1996;21(6):857‐68. [PUBMED: 8990729] [DOI] [PubMed] [Google Scholar]
De Wolff 1997
- Wolff MS, Ijzendoorn MH. Sensitivity and attachment: a meta‐analysis on parental antecedents of infant attachment. Child Development 1997;68(4):571‐91. [PUBMED: 9306636] [PubMed] [Google Scholar]
DeGangi 2000
- DeGangi GA, Breinbauer C, Doussard Roosevelt JD, Porges S, Greenspan S. Prediction of childhood problems at three years in children experiencing disorders of regulation during infancy. Journal of Infant Mental Health 2000;21(3):156‐75. [DOI: 10.1002/1097-0355(200007)21:3%3C156::AID-IMHJ2%3E3.0.CO;2-D] [DOI] [Google Scholar]
Dutra 2005
- Dutra L, Lyons‐Ruth K. Maltreatment, maternal and child psychopathology, and quality of early care as predictors of adolescent dissociation. Society for Research in Child Development Biennial Meeting; 2005 Apr 7‐10; Atlanta (GA). 2005.
Eshel 2006
- Eshel N, Daelmans B, Mello MC, Martines J. Responsive parenting: interventions and outcomes. Bulletin of the World Health Organization 2006;84(12):991‐8. [PMC2627571; PUBMED: 17242836] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fearon 2010
- Fearon RP, Bakermans‐Kranenburg MJ, Ijzendoor MH, Lapsley AM, Roisman GI. The significance of insecure attachment and disorganization in the development of children's externalizing behavior: a meta‐analytic study. Child Development 2010;81(2):435‐56. [DOI: 10.1111/j.1467-8624.2009.01405.x; PUBMED: 20438450] [DOI] [PubMed] [Google Scholar]
Field 1978
- Field T, Rydberg Dempsey J, Hayward Hallock N, Shuman HH. The mother's assessment of the behavior of her infants. Infant Behaviour and Development 1978;1:156‐67. [DOI: 10.1016/S0163-6383(78)80026-5] [DOI] [Google Scholar]
Frankenburg 1967
- Frankenburg WK, Dodds JB. The Denver Developmental Screening Test. Journal of Pediatrics 1967;71(2):181‐91. [DOI: 10.1016/S0022-3476(67)80070-2] [DOI] [PubMed] [Google Scholar]
Gaertner 2007
- Gaertner BM, Spinrad TL, Eisenberg N, Greving KA. Parental childrearing attitudes as correlates of father involvement during infancy. Journal of Marriage and Family 2007;69(4):962‐76. [DOI: ; NIHMS32227; PMC2174267] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 10 August 2015. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Granot 2001
- Granot D, Mayseless O. Attachment security and adjustment to school in middle childhood. International Journal of Behavioral Development 2001;25(6):530‐41. [DOI: 10.1080/01650250042000366] [DOI] [Google Scholar]
Green 2002
- Green J, Goldwyn R. Annotation: attachment disorganisation and psychopathology: new findings in attachment research and their potential implications for developmental psychopathology in childhood. Journal of Child Psychology and Psychiatry 2002;43(7):835‐46. [PUBMED: 12405473] [DOI] [PubMed] [Google Scholar]
Greinenberger 2005
- Grienenberger JF, Kelly K, Slade A. Maternal reflective functioning, mother‐infant affective communication, and infant attachment: exploring the link between mental states and observed caregiving behavior in the intergenerational transmission of attachment. Attachment and Human Development 2005;7(3):299‐311. [DOI: 10.1080/14616730500245963; PUBMED: 16210241] [DOI] [PubMed] [Google Scholar]
Grossman 2002
- Grossmann K, Grossmann K, Winter M, Zimmermann P. Attachment relationships and appraisal of partnership: from early experience of sensitive support to later relationship representation. In: Pulkkinen L, Caspi A editor(s). Paths to Successful Development. Cambridge: Cambridge University Press, 2002:73‐105. [Google Scholar]
Gunnar 1996
- Gunnar M, Brodersen L, Nachmias M, Buss K, Rigatuso J. Stress reactivity and attachment security. Developmental Psychobiology 1996;29(3):191‐204. [DOI: 10.1002/(SICI)1098-2302(199604)29:3%26lt;191::AID-DEV1%26gt;3.0.CO;2-M; PUBMED: 8666128] [DOI] [PubMed] [Google Scholar]
Gunnar 2002
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology 2002;27(1‐2):199‐220. [PUBMED: 11750779] [DOI] [PubMed] [Google Scholar]
Gunnar 2007
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology 2007;58:145‐73. [DOI: 10.1146/annurev.psych.58.110405.085605; PUBMED: 16903808] [DOI] [PubMed] [Google Scholar]
Hawthorne 2004
- Hawthorne J. Training health professionals in the Neonatal Behavioral Assessment Scale (NBAS) and its use as an intervention. The Signal: Newsletter of the World Association for Infant Mental Health 2004; Vol. 12, issue 3 & 4:1‐36.
Hawthorne 2005
- Hawthorne J. Using the Neonatal Behavioral Assessment Scale to support parent‐infant relationships. Infant 2005;1(6):213‐8. [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI: 10.1002/sim.1186; PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2016
- Higgins JPT, Lasserson T, Chandler J, Tovey D, Churchill R. Methodological Expectations of Cochrane Intervention Reviews (MECIR) Version 1.05. Cochrane, 2016. Available from community.cochrane.org/mecir‐manual.
Higgins 2017
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Isabella 1994
- Isabella RA. Origins of maternal role satisfaction and its influences upon maternal interactive behavior and infant‐mother attachment. Infant Behavior and Development 1994;17(4):381‐7. [DOI: 10.1016/0163-6383(94)90030-2] [DOI] [Google Scholar]
Jacobvitz 1997
- Jacobvitz D, Hazen NL, Riggs S. Disorganized mental processes in mothers, frightened/frightening behavior in caregivers, and disoriented, disorganized behavior in infancy. Society for Research in Child Development Biennial Meeting; 1997; Washington (DC) 1997;17:329‐47. [Google Scholar]
Keren 2001
- Keren M, Feldman R, Tyano S. Diagnoses and interactive patterns of infants referred to a community‐based infant mental health clinic. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(1):27‐35. [DOI: 10.1097/00004583-200101000-00013; PUBMED: 11195558] [DOI] [PubMed] [Google Scholar]
Klaus 1972
- Klaus MH, Jerauld R, Kreger NC, McAlpine W, Steffa M, Kennell JH. Maternal attachment: importance of the first post partum days. New England Journal of Medicine 1972;286(9):460‐3. [DOI: 10.1056/NEJM197203022860904; PUBMED: 5009748] [DOI] [PubMed] [Google Scholar]
Koniak‐Griffin 1999
- Koniak‐Griffin D, Mathenge C, Anderson NL, Verzemnieks I. An early intervention program for adolescent mothers: a nursing demonstration project. Journal of Obstetric, Gynecologic and Neonatal Nursing 1999;28(1):51‐9. [PUBMED: 9924864] [DOI] [PubMed] [Google Scholar]
Koniak‐Griffin 2000
- Koniak‐Griffin D, Anderson NL, Verzemnieks I, Brecht ML. A public health nursing early intervention program for adolescent mothers: outcomes from pregnancy through 6 weeks postpartum. Nursing Research 2000;49(3):130‐8. [PUBMED: 10882317] [DOI] [PubMed] [Google Scholar]
Koniak‐Griffin 2002
- Koniak‐Griffin D, Anderson NL, Brecht ML, Verzemnieks I, Lesser J, Kim S. Public health nursing care for adolescent mothers: impact on infant health and selected maternal outcomes at 1 year postbirth. Journal of Adolescent Health 2002;30(1):44‐54. [PUBMED: 11755800] [DOI] [PubMed] [Google Scholar]
Koniak‐Griffin 2003
- Koniak‐Griffin D, Verzemnieks IL, Anderson NL, Brecht ML, Lesser J, Kim S, et al. Nurse visitation for adolescent mothers: two‐year infant health and maternal outcomes. Nursing Research 2003;52(2):127‐36. [PUBMED: 12657988] [DOI] [PubMed] [Google Scholar]
Lecce 2008
- Lecce S. Attachment and Subjective Well‐Being: the Mediating Role of Emotional Processing and Regulation [PhD thesis]. Toronto: University of Toronto, 2008. [Google Scholar]
Liptak 1977
- Liptak, GS, Hulka BS, Cassel JC. Effectiveness of physician‐mother interactions during infancy. Pediatrics 1977;60:186‐192. [PUBMED: 887332] [PubMed] [Google Scholar]
Lyons‐Ruth 2005
- Lyons‐Ruth K, Yellin C, Melnick S, Atwood G. Expanding the concept of unresolved mental states: hostile/helpless states of mind on the Adult Attachment Interview are associated with disrupted mother‐infant communication and infant disorganization. Development and Psychopathology 2005;17(1):1‐23. [PMC1857275; PUBMED: 15971757] [DOI] [PMC free article] [PubMed] [Google Scholar]
MacDonald 2008
- MacDonald HZ, Beeghly M, Grant‐Knight W, Augustyn M, Woods RW, Cabral H, et al. Longitudinal association between infant disorganized attachment and childhood posttraumatic stress symptoms. Development and Psychopathology 2008;20(2):493‐508. [DOI: 10.1017/S0954579408000242; PMC2430632; PUBMED: 18423091] [DOI] [PMC free article] [PubMed] [Google Scholar]
Malekpour 2007
- Malekpour M. Effects of attachment on early and later development. British Journal of Developmental Disabilities 2007;53 Part 2(105):81‐95. [DOI: 10.1179/096979507799103360] [DOI] [Google Scholar]
Markova 2006
- Markova G, Legerstee M. Contingency, imitation, and affect sharing: foundations of infants' social awareness. Developmental Psychology 2006;42(1):132‐41. [DOI: 10.1037/0012-1649.42.1.132; PUBMED: 16420123] [DOI] [PubMed] [Google Scholar]
McCarthy 1972
- McCarthy D. McCarthy Scales of Children's Abilities. New York: Psychological Corporation, 1972. [Google Scholar]
Meins 2001
- Meins E, Fernyhough C, Fradley E, Tuckey M. Rethinking maternal sensitivity: mothers' comments on infants' mental processes predict security of attachment at 12 months. Journal of Child Psychology and Psychiatry 2001;42(5):637‐48. [PUBMED: 11464968] [PubMed] [Google Scholar]
Meins 2012
- Meins E, Fernyhough C, Rosnay M, Arnott B, Leekam SR, Turner M. Mind‐mindedness as a multidimensional construct: appropriate and non‐attuned mind‐related comments independently predict infant‐mother attachment in a socially diverse sample. Infancy 2012;17(4):393‐415. [DOI: 10.1111/j.1532-7078.2011.00087.x] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLOS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097; PMC2707599 ; PUBMED: 19621072] [DOI] [PMC free article] [PubMed] [Google Scholar]
Myers 1982b
- Myers BJ. Early intervention using Brazelton training with middle‐class mothers and fathers of newborns. Child Development 1982;53(2):462‐71. [PUBMED: 7075329] [PubMed] [Google Scholar]
NHS England 2014
- NHS England. National Health Visiting Service Specification 2014/15. www.england.nhs.uk/wp‐content/uploads/2014/03/hv‐serv‐spec.pdf (accessed March 2014).
Nugent 2003
- Nugent, JK. Home Visiting Index. Boston: Brazelton Institute Children's Hospital, 2003. [Google Scholar]
Nugent 2007
- Nugent JK, Keefer CH, Minear S, Johnson LC, Blanchard Y. Understanding Newborn behaviour and Early Relationships: The Newborn Behavioral Observations (NBO) System Handbook. London: Paul H Brookes Publishing, 2007. [Google Scholar]
Nugent 2017 [pers comm]
- Nugent K. Request for information on potential unpublished studies [personal communication]. Email to: J Barlow 13 June 2017.
Porges 2011
- Porges SW. The Polyvagal Theory: Neurophysiological Foundations of Emotions, Attachment, Communication and Self‐regulation. New York (NY): WW Norton & Company, Inc, 2011. [Google Scholar]
Price 1977
- Price GM. The Price Adaptation Scale. Unpublished manuscript 1977.
Price 1983
- Price, GM. Sensitivity in mother‐infant interactions: the AMIS scale. Infant Behaviour and Development 1983;6(2‐3):353‐60. [DOI: 10.1016/S0163-6383(83)80043-5] [DOI] [Google Scholar]
Rauh 1988
- Rauh VA, Achenbach TM, Nurcombe B, Howell CT, Teti DM. Minimizing adverse effects of low birthweight: four‐year results of an early intervention. Child Development 1988;59(3):544‐53. [PUBMED: 2454783] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richard 1972
- Richard MPM, Bernal JF. An observational study of mother‐infant interaction. In: Blurton‐Jones N editor(s). Ethological studies of Child Behaviour. Cambridge: Cambridge University Press, 1972. [Google Scholar]
Richter 2004
- Richter L. The Importance of Caregiver‐Child Interactions for the Survival and Healthy Development of Young Children: A Review. Geneva: World Health Organization, 2004. [Google Scholar]
Schore 2001
- Schore AN. Effects of a secure attachment relationship on right brain development, affect regulation, and infant mental health. Infant Mental Health Journal 2001;22(1‐2):7‐66. [DOI: 10.1002/1097-0355(200101/04)22:1%3C7::AID-IMHJ2%3E3.0.CO;2-N] [DOI] [Google Scholar]
Schore 2002
- Schore AN. Advances in neuropsychoanalysis, attachment theory, and trauma research: implications for self psychology. Psychoanalytic Inquiry 2002;22(3):433‐84. [DOI: 10.1080/07351692209348996] [DOI] [Google Scholar]
Schore 2008
- Schore JR, Schore AN. Modern attachment theory: the central role of affect regulation in development and treatment. Clinical Social Work Journal 2008;36(1):9‐20. [DOI: 10.1007/s10615-007-0111-7] [DOI] [Google Scholar]
Seashore 1973
- Seashore MJ, Leifer AD, Barnett CR, Leiderman PH. The effects of denial of early mother‐infant interaction on maternal self‐confidence. Journal of Personality and Social Psychology 1973;26:369‐78. [DOI] [PubMed] [Google Scholar]
Shea 1988
- Shea E, Tronick Z. The maternal self‐report inventory: a research and clinical instrument for assessing maternal self‐esteem. In: Fitzgerald HE, Lester BE, Yogman MW editor(s). Theory and Research in Behavioral Pediatrics. New York: Plenum Press, 1988:101‐40. [Google Scholar]
Slade 2001
- Slade A, Grienenberger J, Bernbach E, Levy D, Locker A. Maternal reflective functioning and attachment: considering the transmission gap. Paper presented at the Biennial Meeting of the Society for Research on Child Development; 2001 April; Minneapolis (MN). 2001.
Slade 2004
- Slade AAJ, Berger B, Bresgi I, Kaplan M. The Parent Development Interview ‐ Revised. New York: The City University of New York, 2004. [Google Scholar]
Slade 2005
- Slade A, Grienenberger J, Bernbach E, Levy D, Locker A. Maternal reflective functioning, attachment and the transmission gap: a preliminary study. Attachment and Human Development 2005;7(3):283‐98. [DOI: 10.1080/14616730500245880; PUBMED: 16210240] [DOI] [PubMed] [Google Scholar]
Sroufe 2005
- Sroufe A, Egeland B, Carlson E, Collins W. Placing early attachment experiences in developmental context: the Minnesota Longitudinal Study. In: Grossman K, Grossmann K, Waters E editor(s). Attachment from Infancy to Adulthood. New York: The Guilford Press, 2005:48‐70. [Google Scholar]
Steele 2010
- Steele H, Siever L. An attachment perspective on borderline personality disorder: advances in gene‐environment considerations. Current Psychiatry Reports 2010;12(1):61‐7. [DOI: 10.1007/s11920-009-0091-0; PUBMED: 20425312] [DOI] [PubMed] [Google Scholar]
Stern 1998
- Stern DN, Bruschweiler‐Stern N, Freeland A. The Birth of a Mother: How the Motherhood Experience Changes You Forever. New York (NY): Basic Books, 1998. [Google Scholar]
Sumner 1994
- Sumner G, Spietz A. NCAST Caregiver/Parent‐Child Interaction Feeding Manual. Seattle: University of Washington, 1994. [Google Scholar]
Teti 1996
- Teti DM, O'Connell MA, Reiner CD. Parenting sensitivity, parental depression and child health: the mediational role of parental self‐efficacy. Infant and Child Development 1996;5(4):237‐50. [DOI: 10.1002/(SICI)1099-0917(199612)5:4%3C237::AID-EDP136%3E3.0.CO;2-5] [DOI] [Google Scholar]
Vondra 2001
- Vondra JI, Shaw DS, Swearingen L, Cohen M, Owens EB. Attachment stability and emotional and behavioral regulation from infancy to preschool age. Development and Psychopathology 2001;13(1):13‐33. [PUBMED: 11346048] [DOI] [PubMed] [Google Scholar]
Waters 2000
- Waters E, Merrick S, Treboux D, Crowell J, Albersheim L. Attachment security in infancy and early adulthood: a twenty‐year longitudinal study. Child Development 2000;71(3):684‐9. [PUBMED: 10953934] [DOI] [PubMed] [Google Scholar]
WAVE Trust 2013
- WAVE Trust, Department of Education. Conception to age 2 ‐ the age of opportunity: framework for local area service commissioners. www.wavetrust.org/sites/default/files/reports/conception‐to‐age‐2‐framework_0.pdf (accessed March 2013).
Weinfield 2000
- Weinfield NS, Sroufe LA, Egeland B. Attachment from infancy to early adulthood in a high‐risk sample: continuity, discontinuity, and their correlates. Child Development 2000;71(3):695‐702. [PUBMED: 10953936] [DOI] [PubMed] [Google Scholar]
Worobey 1985
- Worobey J. A review of Brazelton‐based interventions to enhance parent‐infant interaction. Journal of Reproductive and Infant Psychology 1985;3(2):64‐73. [DOI: 10.1080/02646838508403464] [DOI] [Google Scholar]
References to other published versions of this review
Bartram 2016
- Bartram SC, Barlow J, Wolke D. The Neonatal Behavioral Assessment Scale (NBAS) and Newborn Behavioral Observations system (NBO) for supporting caregivers and improving outcomes in caregivers and their infants. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD011754] [DOI] [PMC free article] [PubMed] [Google Scholar]


