Abstract
Background
Survivors of critical illness often experience a multitude of problems that begin in the intensive care unit (ICU) or present and continue after discharge. These can include muscle weakness, cognitive impairments, psychological difficulties, reduced physical function such as in activities of daily living (ADLs), and decreased quality of life. Early interventions such as mobilizations or active exercise, or both, may diminish the impact of the sequelae of critical illness.
Objectives
To assess the effects of early intervention (mobilization or active exercise), commenced in the ICU, provided to critically ill adults either during or after the mechanical ventilation period, compared with delayed exercise or usual care, on improving physical function or performance, muscle strength and health‐related quality of life.
Search methods
We searched CENTRAL, MEDLINE, Embase and CINAHL. We searched conference proceedings, reference lists of retrieved articles, databases of trial registries and contacted experts in the field on 31 August 2017. We did not impose restrictions on language or location of publications.
Selection criteria
We included all randomized controlled trials (RCTs) or quasi‐RCTs that compared early intervention (mobilization or active exercise, or both), delivered in the ICU, with delayed exercise or usual care delivered to critically ill adults either during or after the mechanical ventilation period in the ICU.
Data collection and analysis
Two researchers independently screened titles and abstracts and assessed full‐text articles against the inclusion criteria of this review. We resolved any disagreement through discussion with a third review author as required. We presented data descriptively using mean differences or medians, risk ratios and 95% confidence intervals. A meta‐analysis was not possible due to the heterogeneity of the included studies. We assessed the quality of evidence with GRADE.
Main results
We included four RCTs (a total of 690 participants), in this review. Participants were adults who were mechanically ventilated in a general, medical or surgical ICU, with mean or median age in the studies ranging from 56 to 62 years. Admitting diagnoses in three of the four studies were indicative of critical illness, while participants in the fourth study had undergone cardiac surgery. Three studies included range‐of‐motion exercises, bed mobility activities, transfers and ambulation. The fourth study involved only upper limb exercises. Included studies were at high risk of performance bias, as they were not blinded to participants and personnel, and two of four did not blind outcome assessors. Three of four studies reported only on those participants who completed the study, with high rates of dropout. The description of intervention type, dose, intensity and frequency in the standard care control group was poor in two of four studies.
Three studies (a total of 454 participants) reported at least one measure of physical function. One study (104 participants) reported low‐quality evidence of beneficial effects in the intervention group on return to independent functional status at hospital discharge (59% versus 35%, risk ratio (RR) 1.71, 95% confidence interval (CI) 1.11 to 2.64); the absolute effect is that 246 more people (95% CI 38 to 567) per 1000 would attain independent functional status when provided with early mobilization. The effects on physical functioning are uncertain for a range measures: Barthel Index scores (early mobilization: median 75 control: versus 55, low quality evidence), number of ADLs achieved at ICU (median of 3 versus 0, low quality evidence) or at hospital discharge (median of 6 versus 4, low quality evidence). The effects of early mobilization on physical function measured at ICU discharge are uncertain, as measured by the Acute Care Index of Function (ACIF) (early mobilization mean: 61.1 versus control: 55, mean difference (MD) 6.10, 95% CI ‐11.85 to 24.05, low quality evidence) and the Physical Function ICU Test (PFIT) score (5.6 versus 5.4, MD 0.20, 95% CI ‐0.98 to 1.38, low quality evidence). There is low quality evidence that early mobilization may have little or no effect on physical function measured by the Short Physical Performance Battery score at ICU discharge from one study of 184 participants (mean 1.6 in the intervention group versus 1.9 in usual care, MD ‐0.30, 95% CI ‐1.10 to 0.50), or at hospital discharge (MD 0, 95% CI ‐1.00 to 0.90). The fourth study, which examined postoperative cardiac surgery patients did not measure physical function as an outcome.
Adverse effects were reported across the four studies but we could not combine the data. Our certainty in the risk of adverse events with either mobilization strategy is low due to the low rate of events. One study reported that in the intervention group one out of 49 participants (2%) experienced oxygen desaturation less than 80% and one of 49 (2%) had accidental dislodgement of the radial catheter. This study also found cessation of therapy due to participant instability occurred in 19 of 498 (4%) of the intervention sessions. In another study five of 101 (5%) participants in the intervention group and five of 109 (4.6%) participants in the control group had postoperative pulmonary complications deemed to be unrelated to intervention. A third study found one of 150 participants in the intervention group had an episode of asymptomatic bradycardia, but completed the exercise session. The fourth study reported no adverse events.
Authors' conclusions
There is insufficient evidence on the effect of early mobilization of critically ill people in the ICU on physical function or performance, adverse events, muscle strength and health‐related quality of life at this time. The four studies awaiting classification, and the three ongoing studies may alter the conclusions of the review once these results are available. We assessed that there is currently low‐quality evidence for the effect of early mobilization of critically ill adults in the ICU due to small sample sizes, lack of blinding of participants and personnel, variation in the interventions and outcomes used to measure their effect and inadequate descriptions of the interventions delivered as usual care in the studies included in this Cochrane Review.
Plain language summary
Early intervention (movement or active exercise) for critically ill adults in the intensive care unit
Review question
Does helping critically ill adults to move or exercise early in their stay in the intensive care unit (ICU) improve their ability to perform everyday activities such as walking, and the ability to perform daily self care on discharge from hospital? We reviewed the evidence for this question, to see if there are benefits to early exercise, including the amount of time spent in the ICU or hospital, muscle strength, feelings of well‐being, and also to see if there are harms, such as the occurrence of falls. The movement or exercise could include things such as moving in, or sitting out of bed, practicing standing up, walking, arm exercises, and self‐care activities such as eating or brushing hair.
Background
Adults who are critically ill, and spend time in an ICU, can develop muscle weakness and other problems. This can occur because of the illness that led to their admission to the ICU, treatments associated with this illness, the impact of ongoing health conditions, and their lack of movement while in the ICU. They may also have ongoing problems when they leave ICU (or hospital) such as having trouble doing daily activities (for example dressing, bathing and mobility); feeling depressed or anxious and having difficulty returning to work.
We wanted to evaluate if assisting these people to move early in their ICU stay would allow them to be better able to look after themselves, be stronger and feel better about life.
Study characteristics
We found four studies that included a total of 690 adults who had been in the ICU. Patients were randomized to receive exercises and assistance to move early in their stay in the ICU or to usual care. All participants had been on a breathing machine at some point during their time in the ICU. Three studies included adults with critical illness involving severe disease of the lungs or severe body response to infection and one study involved adults who had undergone cardiac surgery.
Study funding sources
One study was funded by the Intensive Care Foundation, Royal Brisbane and Women's Hospital, Australia and the investigator was supported by a Postgraduate Award from Singapore.
Key results
We were unable to determine whether early movement or exercise of critically ill people in the ICU improves their ability to do daily activities, muscle strength, or quality of life. There were mixed results on the effect of early movement or exercise on physical function. One study found that on some measures of physical function, participants who received the intervention could get out of bed earlier and walk greater distances. However, the same study found no differences in the number of daily activities they could do when leaving ICU. Early movement or exercise appears safe as the number of adverse events was very low. There was no difference between groups in time spent in hospital, muscle strength or death rates.
Quality of the evidence
Overall there was low‐quality evidence from these studies. The main reasons were that only a small number of studies have examined this intervention. Most studies included only a small number of participants, and participants and study staff were aware of group assignment. In addition, in two studies, staff assessing outcomes were aware of group assignment. There were also differences in participant diagnoses, interventions and the way that outcomes were measured. The four studies awaiting classification, and the three ongoing studies may alter the conclusions of the review once these results are available.
Currency of the evidence
Evidence in this review is current to August 2017.
Summary of findings
Summary of findings for the main comparison. Early intervention (mobilization or active exercise) versus usual care for critically ill adults.
| Early intervention (mobilization or active exercise) versus usual care for critically ill adults | ||||||
|
Patient or population: critically ill, mechanically ventilated adults
Settings: general, medical or surgical ICU in Australia/China/USA
Intervention: early intervention (mobilization or active exercise) Control: usual care (defined as no mobilization/active exercise while in ICU, or mobilization/active exercise given later than the intervention group) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Early intervention (mobilization or active exercise) | |||||
| Physical function ‐ return to independent functional status at hospital discharge Defined as ability to perform 6 ADLs (bathing, dressing, eating, grooming, transferring from bed to chair, using the toilet) and walk independently, measured with Functional Independence Measure | Study population | RR 1.71 (1.11 to 2.64) | 104 (1 study) | ⊕⊕⊝⊝ low1,2 | ||
| 345 per 1000 | 591 per 1000 (383 to 912) | |||||
| Physical function ‐ independent ADLs total at ICU discharge Functional Independence Measure (0 ‐ 6) | Study population | 104 (1 study) | ⊕⊕⊝⊝ low1,2 | |||
| Median 0 (IQR 0 to 5) | Median 3 (IQR 0 to 5) | |||||
| Physical function ‐ Independent ADL total at hospital discharge Functional Independence Measure (0 ‐ 6) | Study population | 104 (1 study) | ⊕⊕⊝⊝ low1,2 | |||
| Median 4 (IQR 0 to 6) | Median 6 (IQR 0 to 6) | |||||
|
Physical function Barthel score (0 ‐ 100) |
Study population | 104 (1 study) |
⊕⊕⊝⊝ low1,2 | |||
| Median 55 (IQR 0 to 85) | Median 75 (IQR 7.5 to 95) | |||||
| Physical performance Acute Care Index of Function score at ICU discharge (0 ‐ 100) | Study population | 42 (1 study) | ⊕⊕⊝⊝ low2,3 | |||
| Mean score 55.0 (45.0 to 65.0) | Mean score 61.1 (46.2 to 76.0) MD 6.10 (‐11.85 to 24.05) |
|||||
|
Physical performance Physical Function ICU Test score at ICU discharge |
Study population | 42 (1 study) |
⊕⊕⊝⊝ low2,3 | |||
| Mean score 5.4 (4.7 to 6.1) | Mean score 5.6 (4.7 to 6.5) MD 0.20 (‐0.98 to 1.38) |
|||||
|
Physical performance Short Physical Performance Battery score at ICU discharge |
Study population | 184 (1 study) |
⊕⊕⊝⊝ low2,3 | |||
| Mean score 1.9 (1.3 to 2.4) | Mean score 1.6 (1.0 to 2.2) MD ‐0.30 (‐1.10 to 0.50) |
|||||
|
Physical performance Short Physical Performance Battery score at hospital discharge |
Study population | 204 (1 study) |
⊕⊕⊝⊝ low2,3 | |||
| Mean score 4.7 (4.0 to 5.4) | Mean score 4.7 (4.0 to 5.4) MD 0 (‐1.00 to 0.90) |
|||||
|
Adverse events Proportion of participants with one or more events, or proportion of intervention sessions where an event occurred (falls, accidental dislodgement of attachments, haemodynamic instability, oxygen desaturation or any other adverse events defined by study authors) |
One study reported that in the intervention group 1/49 (2%) experienced oxygen desaturation < 80% and 1/49 (2%) had accidental dislodgement of the radial catheter. This study also found cessation of therapy due to patient instability occurred in 19/498 (4%) of the intervention sessions. In another study 5/101 (5%) of the intervention group and 5/109 (4.6%) of the control group had postoperative pulmonary complications. These were deemed to be unrelated to intervention. A third study found 1/150 in the intervention group had an episode of asymptomatic bradycardia, but completed the exercise session. The fourth study reported no adverse events. | 690 (4 studies) | ⊕⊕⊝⊝ low2,4 | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADLs: activities of daily living; CI: confidence interval; ICU: intensive care unit; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded one point for high risk of bias. Risk of bias was high for blinding of participants and personnel (performance bias). 2Downgraded for imprecision (only one small study). 3Downgraded one point for high risk of bias. Risk of bias was high for blinding of participants and personnel (performance bias) and incomplete outcome data (attrition bias; for all outcomes except mortality). 4Downgraded for imprecision, as there were very few adverse events of each type.
Background
Description of the condition
Critically ill patients are admitted to the intensive care unit (ICU) so that physiological responses to illness and injury can be monitored and stabilised in a sophisticated manner, and respiration can be assisted with mechanical ventilation if needed. Multiple factors, including haemodynamic instability, altered sleep patterns, the presence of vascular attachments and sedation to improve patient comfort during mechanical ventilation, can limit mobilization of these patients (Adler 2012).
Intensive care unit‐acquired weakness (ICUAW) may be described as clinically identified weakness that develops during an ICU admission with no other known cause except the acute illness or its treatment (Hermans 2015). ICUAW is a common complication for critically ill patients and is associated with extended duration of mechanical ventilation (DeJonghe 2002), sepsis, systemic inflammatory response syndrome, multi‐organ failure and hyperglycaemia (Desai 2011). Incidence of ICUAW in this patient population has been found to be as high as 46% (95% CI 43% to 49%) (Stevens 2007). Critically ill patients can sustain loss of muscle mass within the first week of admission to the ICU (Parry 2015a; Puthucheary 2013). ICUAW has also been associated with worse acute outcomes, higher healthcare‐related costs, and the persistence of weakness is associated with a higher mortality one year after ICU admission (Hermans 2014a). The long‐term weakness appears to result from heterogeneous muscle pathophysiology, with both muscle atrophy and decreased contractile capacity involved (Dos Santos 2016).
Among critically ill patients in ICU, some may have or develop acute lung injury or acute respiratory distress syndrome (Herridge 2005). Patients with acute lung injury demonstrate rapid onset of infiltrates in bilateral lungs and mild to moderate hypoxaemia of noncardiac origin (Herridge 2005). In a two‐year follow‐up on people with this condition, the presence of ICUAW was associated with impairments in physical function; six‐minute walk distance (Crapo 2002), and the physical function subscale scores of the Short F‐36 survey (Ware 1992), were significantly lower (52% to 69% of predicted value) at six, 12 and 24 months' follow‐up (Fan 2014). ICUAW has also been related to a higher incidence of hospital mortality (Ali 2008), and the persistence of weakness is associated with a higher mortality one year after ICU admission (Hermans 2014a).
The term 'post‐intensive care syndrome' was developed to describe new or residual problems that are often experienced by survivors of critical illness. These include cognitive impairments (such as altered memory, attention and executive functioning); psychological difficulties (such as depression, anxiety and post‐traumatic stress disorder) and physical impairments in pulmonary, neuromuscular and physical function (Needham 2012). These problems can affect the performance of activities of daily living (ADLs) and lead to decreased quality of life for these people. In addition, similar psychological difficulties may occur in families of people with critical illness (Needham 2012).
In an attempt to improve outcomes for the survivors of critical illness, there have been efforts to interrupt sedation (Kress 2000), to allow patients to choose their own level of sedation (Chlan 2010), and to cease sedation (Strøm 2011), for patients who are mechanically ventilated. As patients become increasingly responsive, they are better able to participate in active exercise and to mobilize outside of bed, even when mechanically ventilated. Bailey 2007, demonstrated infrequent adverse events in participants who mobilized while mechanically ventilated and concluded that early mobility of patients in the ICU is feasible and safe. To assist in the assessment of patient readiness and appropriateness to commence early mobility in the ICU, a panel of multidisciplinary experts reached consensus on safety recommendations concerning respiratory, cardiovascular, neurological, medical or surgical and other factors (Hodgson 2014).
Description of the intervention
We considered interventions that commenced earlier than the intervention received by the control group while the patient was in the ICU and may have included any of the following activities.
Cycle ergometer: (a stationary cycle where work intensity can be adjusted by varying pedal resistance and cycling rate)
Active‐assisted exercises (exercises performed by the participant with manual assistance of another person)
Active range‐of‐motion exercises (exercises moving a joint(s) through its range of motion, that are performed independently by the participant)
Bed mobility activities (activities including rolling, bridging and transfer to upright sitting)
ADLs (self‐care tasks such as eating, bathing, dressing and toileting)
Transfer training (repetition of transfers such as sitting to standing and bed to chair or commode)
Pre‐gait exercises (improving postural stability, static and dynamic balance and marching on the spot)
Ambulation (gait training and walking with or without mobility aids).
(See Types of interventions for additional details.)
Characteristics of the intervention such as type, provider skills and training, timing of delivery, dose/duration, tailoring and progression of intervention, and resources used in the delivery can greatly influence an intervention's efficacy as well as the heterogeneity of the population receiving the intervention. Evaluation of the impact of the intervention across studies is dependent on adequate reporting in the included studies so that variations in its delivery may be identified and analysed. To facilitate understanding of the components of the interventions across studies, we used the Template for Intervention Description and Replication (TIDieR) checklist to report intervention details (see Table 2).
1. Characteristics of each intervention ‐ summarized using TIDieR criteria*.
| Item | Morris 2016 | Kayambu 2015 | Patman 2001 | Schweickert 2009 |
| Brief name | Standardized rehabilitation therapy | Early physical rehabilitation in ICU | Physiotherapy | Early physical and occupational therapy in mechanically ventilated, critically ill patients |
|
What
(Materials and Procedures) |
3 exercise types:
Both the physiotherapy and resistance training targeted lower extremity functional tasks and ADLs. See trial protocol (supplement to article) for more details. |
Specific equipment not reported. Procedures mentioned include: arm or leg ergometry; passive, active and resisted ROM exercises; bed mobility activities; sitting and standing balance exercises; transfer training; pre‐gait exercises; ambulation with assistance; electrical muscle stimulation; and Tilt table |
Specific equipment was not reported. Procedures mentioned include: positioning; manual hyperinflation; endotracheal suctioning; thoracic expansion exercises; and upper limb exercises |
Specific equipment was not reported. Procedures mentioned included: passive, active‐assisted and active ROM exercises; bed mobility activities; sitting balance exercises; ADL exercises to promote increased independence with functional tasks; transfer training; pre‐gait exercises; and ambulation |
| Who provided | Physiotherapist, ICU nurse, and nursing assistant | ICU research physiotherapist | Team of physiotherapists under the guidance of the principal investigator | An occupational therapist and a physical therapist |
| Where | One medical ICU, Medical Centre, North Carolina, USA | Quaternary‐level general ICU, Australia | Surgical ICU, Perth, Australia |
Two medical ICUs: Chicago, USA and Iowa City, USA |
| When and how much | 3 separate sessions every day of hospitalization for 7 days per week, from enrolment through to hospital discharge | 30 min 1‐2 times/day within 48 h of diagnosis of sepsis until discharge from the ICU |
1‐2 interventions during the first 24 h of mechanical ventilation |
Interventions were synchronized with daily interruption of sedation. Each morning within 48‐72 h of intubation until return to previous level of function or discharge from the ICU |
| Tailoring and progression | The participant's level of consciousness determined whether they were considered suitable to receive the physiotherapy or progressive resistance exercise, as did their ability to complete the exercises. When participants were unconscious, the 3 sessions consisted of passive ROM. As consciousness was gained, physiotherapy and progressive resistance exercise was commenced. Participants did not need to be free of mechanical ventilation to begin any of the exercise sessions | Interventions were tailored, planned, administered and progressed at the discretion of the physiotherapist, participant acuity of illness and level of co‐operation |
Not reported | Progression of interventions depended on participant tolerance and stability. |
|
Modification of intervention throughout trial |
Not reported | Not reported | Not reported | Not reported |
|
Fidelity (strategies to improve) |
Not reported | Not reported | Not reported | Not reported |
|
Fidelity (extent) |
The mean percentage of study days that participants received therapy was 87.1% (SD 18.4%) for passive ROM; 54.6% (SD 27.2%) for physiotherapy; and 35.7% (SD 23%) for progressive resistive exercise. The median days of delivery of therapy per participant was 8.0 (IQR, 5.0‐14.0) for passive ROM, 5.0 (IQR, 3.0‐8.0) for physiotherapy, and 3.0 (IQR, 1.0‐5.0) for progressive resistance exercise | All participants adhered and remained enrolled for an average of 11.4 days. No further details. |
Not reported | Therapy occurred on 87% of the days on the study for all participants in the intervention group and on 95% of the days on the study for 22/55 (40%) of participants in the control group |
*See Hoffmann 2014 for TIDieR criteria
ADLs: activities of daily living; ICU: intensive care unit; ROM: range of motion
How the intervention might work
The consequences of bed rest are well documented and include adverse effects on the cardiovascular system (through decreased functional capacity), the respiratory system (through difficulty weaning from mechanical ventilation) and the neuromuscular system (through ICUAW) (Koo 2011). Beneficial effects of exercise training are widespread and can include improvements in skeletal muscle function, respiration (increased tidal volume and oxygen transport capacity) and cardiovascular function (including prevention of age‐related diastolic dysfunction and decreased oxidative stress) (Gielen 2010). Prolonged immobilization is one of the risk factors for ICUAW (Hermans 2015), and hence reducing the duration of immobilization has been suggested as one of the actions that can be taken to prevent it (Hermans 2015). It has been suggested that early mobilization might reduce muscle injury through its effect on muscle unloading (Hermans 2015), but the pathophysiological mechanisms through which this intervention might work are complex and not clearly understood. As the recovery from ICUAW can take weeks or months, its impact on function and quality of life can last for years. In a five‐year follow‐up study of survivors of acute respiratory distress syndrome, generalized weakness and fatigue were chief complaints and still present in many survivors at this time (Herridge 2011). Hence preventing or lessening the impact of ICUAW may have consequent effects on patients’ function and quality of life in the weeks, months, and years following an ICU admission.
Why it is important to do this review
A Cochrane protocol for a systematic review (Greve 2012), and one Cochrane systematic review (Connolly 2015), relevant to the impact of mobilization of critically ill patients have been published. Greve 2012, has a focus on preventing ICU delirium and will assess the impact of any multicomponent (behavioural, cognitive, psychological, physical training) or pharmacological interventions, or both. Connolly 2015, evaluated the efficacy of exercise rehabilitation or training for functional exercise capacity and health‐related quality of life in adult ICU survivors who had been mechanically ventilated longer than 24 hours. However, neither of these reviews plans to examine or has investigated mobilization delivered early in the participants' admission to ICU. The protocol (Greve 2012), has not specified the timing of the intervention while the systematic review (Connolly 2015), investigated the impact of exercise rehabilitation once participants had been discharged from the ICU.
Early mobilization and active exercise of critically ill patients are increasingly being provided in some ICUs. However, the effectiveness of early interventions that are being used is not clear. This review aims to guide clinicians and intensive care unit policy makers regarding the timing of mobilization and active exercise for critically ill patients.
Objectives
To assess the effects of early intervention (mobilization or active exercise), commenced in the ICU, provided to critically ill adults either during or after the mechanical ventilation period, compared with delayed exercise or usual care, on improving physical function and performance, muscle strength and health‐related quality of life.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCTs) or quasi‐RCTs that compared early intervention (mobilization or active exercise) of critically ill participants either during or after the mechanical ventilation period in the ICU with delayed exercise or usual care (see Types of interventions).
Types of participants
We included adults who had been admitted to an ICU and were mechanically ventilated. We excluded studies with participants who had pre‐existing or rapidly developing neuromuscular disease, spinal cord injury, cardiopulmonary arrest, raised intracranial pressure, advanced dementia or irreversible disorders with expected six‐month mortality.
Types of interventions
Interventions
The intervention must have been conducted within the ICU and must have consisted of mobilization or active exercise, or both, that was designed to commence earlier than the care received by the control group.
We considered any combination of one or more of the following types of exercise modalities.
Cycle ergometer
Active‐assisted exercises
Active range‐of‐motion (ROM) exercises
Bed mobility activities (e.g. bridging, rolling, lying to sitting)
ADLs or exercises related to increasing independence with functional tasks
Transfer training
Pre‐gait exercises (including marching on the spot)
Ambulation
Any other type of active exercise modality that commenced while the participant was in the ICU
Comparators
The comparator may have consisted of:
delayed intervention (mobilization/active exercise the same as the intervention group, but given later, either in the ICU, or after the participant left the ICU);
usual care (no mobilization/active exercise while in ICU);
inspiratory/respiratory muscle training only.
Types of outcome measures
Primary outcomes
Physical function (the ability to perform everyday activities such as basic ADLs) as measured by a validated scale (e.g. Barthel Index, Functional Independence Measure (FIM)) or physical performance tasks (as measured by a scale such as the Physical Function ICU Test (PFIT), Acute Care Index of Functional Status (ACIF), Short Physical Performance Battery, walking tests)
Adverse events (falls, accidental dislodgement of attachments, haemodynamic instability, oxygen desaturation or any other adverse events defined by study authors)
Secondary outcomes
Length of stay (ICU and hospital)
Muscle strength (e.g. Medical Research Council (MRC) score (Medical Research Council 1942), cross‐sectional diameter, handgrip strength)
Health‐related quality of life or well‐being (e.g. The Medical Outcome Study (MOS) 36‐item Short‐Form Health Survey (SF‐36) questionnaire (Ware 1992)
Delirium
Death from any cause at any measured time point
Hospital costs
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 8) via the Cochrane Library, MEDLINE (Ovid SP) (1946 to August week 4, 2017), Embase (Elsevier) (2010 to August 2017) and CINAHL (1981 to August 2017).
We used the search strategy described in Appendix 1 to search CENTRAL and Appendix 2 to search MEDLINE. We combined the MEDLINE search terms with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐and precision‐maximizing version (2008 revision); Ovid format (Lefebvre 2011). We adapted the search strategy to search Embase (see Appendix 3), and CINAHL (see Appendix 4).
We did not impose a language restriction.
Searching other resources
We searched the Controlled Trials registry www.controlled‐trials.com/ (August 2017) (see Appendix 5 for detailed search strategy), ClinicalTrials.gov registry clinicaltrials.gov/ (August 2017) (see Appendix 6), and the World Health Organization International Clinical Trials Registry Platform www.who.int/ictrp/en/ (August 2017) (see Appendix 7), for studies that may have been missed or unpublished and reviewed relevant conference proceedings and abstract presentations of important symposia.
We corresponded with authors of studies that had been completed but not published and with content experts to identify unpublished research and trials still under way.
We did not impose a language restriction.
Data collection and analysis
Selection of studies
Two researchers (review author, KAD and a research assistant) independently screened titles and abstracts and assessed full‐text articles that were identified from the search. We resolved any disagreement through discussion or consultation with a third review author (TCH or EMB, or both) as required.
Data extraction and management
Two researchers (KAD, and a research assistant) independently used the data collection form shown in our protocol (Doiron 2013), to extract data from all included studies. We resolved any disagreement through discussion or consultation with a third review author (EMB or TCH, or both) as required.
We examined trials that met the inclusion criteria and recorded the following information.
Methods: a description of study design, randomization and treatment setting
Participants: number of participants, age, gender, race/ethnicity, body mass index, inclusion and exclusion criteria, ICU days before inclusion, primary presenting diagnosis, biochemical data, health and well‐being status scores and functional scale scores
Interventions: description of experimental and comparator interventions and relevant co‐interventions (e.g. medications)
Outcomes: baseline and end of study measurement of functional status (e.g. functional independence measure (FIM) (Keith 1987), Barthel Index (Mahoney 1965)), health‐related quality of life or well‐being (the MOS 36‐item Short‐Form Health Survey (SF‐36) questionnaire (Ware 1992)), and muscle strength, as well as adverse events, length of stay (ICU and hospital), delirium, death from any cause and hospital costs
Notes: language of the study and any other information relevant to this review
We commented briefly about the reasons for exclusion of studies identified in the search strategy but not subsequently included.
Assessment of risk of bias in included studies
Two review authors (EMB and KAD) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving a third review author (TCH). We assessed the risk of bias according to the following domains.
Allocation sequence generation
Concealment of allocation
Blinding of study participants and personnel
Incomplete outcomes data
Selective outcomes reporting
Other biases
We graded each potential source of bias as yes, no or unclear according to whether the potential for bias was high, low or unknown.
We considered a trial as having a high risk of bias if we assessed either of the domains of concealment of allocation or blinding of study participants and personnel as inadequate or unclear.
We included a 'Risk of bias' summary (see Figure 1), and a 'Risk of bias' graph (see Figure 2), as part of the Characteristics of included studies table, which detailed all of the judgements made for all included studies in the review.
1.
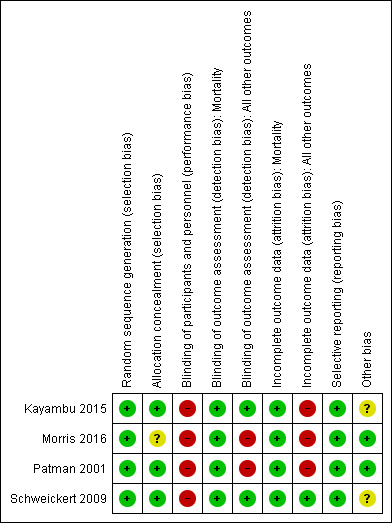
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
2.
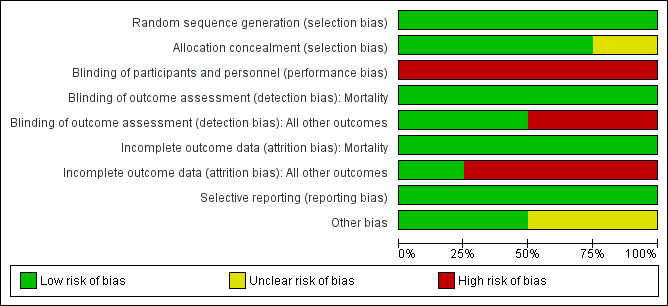
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Measures of treatment effect
Primary outcomes
Functional status
Where studies reported this as a dichotomous outcome (e.g. return to independent functional status at hospital discharge), we used a risk ratio to compare the intervention group with the control group. Where studies reported ADL composite measures and functional component measures using continuous scales (e.g. Barthel Index (Mahoney 1965)), we reported results using means (standard deviations (SDs)) or medians (interquartile range).
Adverse events
We reported the proportion of participants who experienced any adverse event that was reported by the study authors. We also descriptively reported the numbers of particular types of adverse events.
Secondary outcomes
Length of stay
This was reported in days, and we therefore reported the mean difference (MD) where possible, or the median (interquartile range) in each group.
Muscle strength, health‐related quality of life
These outcomes were measured using continuous scales and we reported the MD where possible, or the median (interquartile range) in each group.
Delirium
This was reported as days with delirium in the ICU and in hospital. We reported the median (interquartile range) scores in each group.
Death from any cause
This was reported as the percentage of participants in each group who died and reported as risk ratio to compare groups.
Hospital costs
None of the included studies reported this outcome. If they had done so, we planned to report the MD in costs between intervention and control groups.
Unit of analysis issues
Individual participants were the unit of analysis.
Dealing with missing data
We wrote to investigators to verify key study characteristics and details of the outcomes data as needed. We contacted a study author in Patman 2001, to identify group allocation for participants who died. We subsequently reported this information in Effects of interventions (death from any cause) and Table 3. We corresponded with authors in one study to identify the timing of the interventions received by the intervention and control groups (Kayambu 2015). We reported this information in Included studies (comparators). We also requested clarification on the methods used to calculate results from this study. We intended to conduct intention‐to‐treat (ITT) analysis and to impute missing standard deviations but this was not required.
2. Death/Survival.
| n (%) in intervention group | n (%) in control group | Risk ratio (95% CI) | P value | Reference of studies | |
| ICU mortality | 3/26 (12%) | 1/24 (4%) | RR 2.77 (0.31 to 24.85) |
0.36 | Kayambu 2015 |
| ICU mortality | 0/101 (0%) | 3/109 (2.8%) | RR 0.16 (0.008 to 3.03) |
0.22 | Patman 2001 |
| Hospital mortality | 9/49 (18%) | 14/55 (25%) | RR 0.72 (0.34 to 1.52) |
0.53 | Schweickert 2009 |
| 90‐day mortality | 8/26 (31%) | 2/24 (8%) | RR 3.69 (0.87 to 15.69) |
0.08 | Kayambu 2015 |
|
6‐month hospital‐free survival |
73/150 (48.7%) | 67/150 (44.7%) | RR 1.09 (0.86 to 1.39) |
0.69 | Morris 2016 |
CI: confidence interval; ICU: intensive care unit; n: number; RR: risk ratio
Assessment of heterogeneity
We noted clinical heterogeneity in studies relating to the participants, interventions, and outcome measures. We did not measure statistical heterogeneity as we did not perform a meta‐analysis.
Assessment of reporting biases
We did not create a funnel plot to investigate potential publication bias as only four studies were included in this review.
Data synthesis
If sufficient studies for meta‐analysis had been found, we planned to use a random‐effects model because of the varying nature of potential interventions in this review. However, as there were insufficient studies to perform a meta‐analysis, we descriptively reported the results of included studies.
Subgroup analysis and investigation of heterogeneity
If heterogeneity of studies had been observed, we planned to investigate possible sources of heterogeneity such as age group, cause of ICU stay, length of mechanical ventilation, comorbidities such as diabetes and use of corticosteroids using subgroup analyses. However, as there were insufficient studies identified, we reported these factors descriptively.
Sensitivity analysis
We planned to perform a sensitivity analysis by omitting the studies judged at high risk of bias, defined as lack of concealment of allocation and blinding of study participants and personnel. However we were unable to do this as no meta‐analysis was performed.
'Summary of findings' table and GRADE
We used the principles of the GRADE system (Guyatt 2008), to assess the quality of the body of evidence associated with specific outcomes (functional status and adverse events) in our review and constructed Table 1 using the GRADEpro software (GRADEpro GDT 2015). The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers within‐study risk of bias (methodological quality), the directness of the evidence, the heterogeneity of the data, the precision of effect estimates and the risk of publication bias.
Two review authors (KAD and EMB) independently performed the GRADE assessment of quality of the evidence. We resolved disagreements by consensus. We planned to consult the third review author if we had been unable to resolve disagreements. We rated the quality of the evidence for each outcome as high, moderate, low or very low.
Results
Description of studies
We included RCTs that compared early intervention (mobilization or active exercise) commenced in the ICU (either during or after the mechanical ventilation period) with delayed exercise or usual care for critically ill adults.
Results of the search
We identified a total of 7185 references from our searches of CENTRAL, MEDLINE (Ovid SP), Embase (Ovid SP) and CINAHL, and one reference after searching trials registries (to August 2017). We identified 2303 duplicates and excluded 4858 further references as they were not eligible for this review. We examined 25 full‐text articles, and identified four studies that fulfilled our inclusion criteria (Kayambu 2015; Morris 2016; Patman 2001; Schweickert 2009). See Figure 3 for further information.
3.
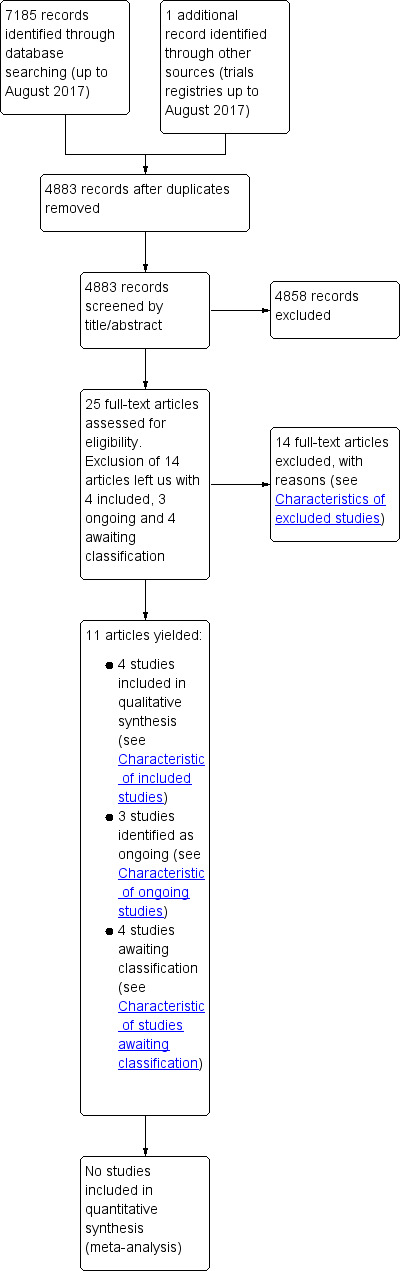
Study flow diagram
Included studies
We included four RCTs in this review (Kayambu 2015; Morris 2016; Patman 2001; Schweickert 2009).
Participants
We report participant details in the Characteristics of included studies section. The total number of participants enrolled in all four trials was 690. All were aged over 18 years; the mean or median age of participants ranged from 56 to 62 years. Sample size varied across studies; Kayambu 2015 (50 participants), Morris 2016 (300 participants), Patman 2001 (236 participants), and Schweickert 2009 (104 participants).
One study reported that all participants in the intervention group were mechanically ventilated for the duration of the intervention (Patman 2001), while the remaining three studies did not report the percentages of those who were intubated during the intervention period (Kayambu 2015; Morris 2016; Schweickert 2009).
The most common reason for ICU admission varied across the studies. In Kayambu 2015, 19 of 26 (73%) participants in the intervention group and 17 of 24 (71%) in the control group were admitted with septic shock; in Morris 2016 68% had acute respiratory failure without chronic lung disease, 31% had acute respiratory failure with chronic lung disease and 2% had an ICU diagnosis of coma; in Patman 2001, 71 of 108 (66%) participants in the intervention group and 68 of 109 (62%) of those in the control group had undergone coronary artery surgery; and in Schweickert 2009 27 of 49 (55%) participants in the intervention group and 31 of 55 (56%) in the control group were admitted with acute lung injury.
Two studies were conducted in a single ICU (Kayambu 2015; Morris 2016), one study in a surgical ICU (Patman 2001), and one study in medical ICUs at two sites (Schweickert 2009).
Please refer to the Characteristics of included studies for more detail.
Interventions
There was variation in most aspects of the interventions between the four studies: electrical muscle stimulation (EMS), tilt table therapy, arm or leg ergometry and activities ranging from passive to active to resisted range‐of‐motion exercises, transfers, balance training (sitting and standing) through to ambulation with assistance were part of the intervention in Kayambu 2015; passive range‐of‐motion, physical therapy including bed mobility, transfer training and balance training, and progressive resistance exercise using elastic resistance bands were used in Morris 2016; upper limb exercises were performed with the intervention group in the trial by Patman 2001; and activities ranging from passive to active‐assisted exercises through to transfer training, ADL tasks and ambulation were implemented in Schweickert 2009.
The time to commencement of the intervention was variable across studies. in Kayambu 2015 the intervention group commenced therapy within 48 hours of admission to ICU and in Morris 2016 a median of 1 day after admission to ICU. In Patman 2001 the intervention group commenced therapy during the first 24 hours of intubation and in Schweickert 2009 at a median of 1.5 days, interquartile range (IQR) (1.0 to 2.1) after intubation had commenced.
Frequency and duration of the delivery of the intervention also varied across studies. Kayambu 2015 reported that the intervention was delivered for 30 minutes, once or twice per day until the participant was discharged from the ICU and that participants remained in the study for a mean of 11.4 days. In Morris 2016, the intervention sessions were given three times per day, with a goal of achievement of repetitions, rather than a specified time for each session. The intervention was continued until discharge from hospital. In the study by Patman 2001, the intervention was delivered as required during the intubated phase, which lasted 24 hours (participants were withdrawn from the study if mechanical ventilation was required for more than 24 hours). No further details regarding the frequency and duration of the intervention were provided. Schweickert 2009 reported that the intervention was delivered every morning until participants returned to their previous level of function or were discharged. Information on the discharge location (ICU or hospital) was not stated. Study authors reported that the median duration of therapy for the intervention group during mechanical ventilation was 0.32 hours per day, IQR (0.17 to 0.48) and a median of 0.21 hours per day IQR (0.08 to 0.33) while not being ventilated.
The intervention was provided by physiotherapists in Kayambu 2015, Morris 2016 and Patman 2001; and by a physiotherapist and an occupational therapist in Schweickert 2009. (Refer to the Characteristics of included studies for more detail.) Key characteristics of the interventions in each trial are listed in Table 2, according to the TIDieR components (Hoffmann 2014).
Comparators
Information about the timing of treatment in the control group was reported in three studies (Morris 2016, Patman 2001; Schweickert 2009). In Morris 2016, the usual care group participants could receive weekday physical therapy if it was ordered by the clinical team. This started a median of seven days after admission, compared with one day in the intervention group. The control group received physical therapy on a mean 11.7% of study days, compared with 87.1% in the intervention group. In Patman 2001, participants received the same intervention as those in the intervention group but 24 hours later (after they were extubated from mechanical ventilation). In Schweickert 2009, participants in the control group received physical and occupational therapy as ordered by the primary care team and active physiotherapy treatment occurred only after they had been mechanically ventilated for two weeks. Study authors reported that the control group received an intervention a median of 7.4 days after intubation. After correspondence with study authors, Kayambu 2015 reported that 10 of 24 (42%) participants in the control group received the same intervention as those in the intervention group (with the exception of EMS, tilt table therapy and arm or leg ergometry) at the same time as those in the intervention group (within 48 hours of admission) while 14 out of 24 (58%) of the participants in this group received it later (after 48 hours of admission).
Refer to the Characteristics of included studies for more detail.
Primary outcomes
Physical function and performance
Three studies measured physical function or performance, and each used different measures (Kayambu 2015; Morris 2016; Schweickert 2009). Kayambu 2015, used the acute care index of function (ACIF) (Van Dillen 1988), and the physical function ICU test (PFIT) (Skinner 2009), and Morris 2016 used the Short Physical Performance Battery score (SPPB). Schweickert 2009 reported the percentage of participants returning to independent functional status at discharge, the number of independent ADLs achieved at ICU and hospital discharge, the time from intubation to out of bed, standing, marching in place, transferring to a chair, and walking, and the Barthel Index. These study authors used the functional independence measure (FIM) (Keith 1987), to measure return to independent functional status and ADLs. Schweickert 2009 also measured time to achieve milestones (e.g. time from intubation to marching in place) and walking distance achieved at hospital discharge.
Adverse events
All studies reported adverse events but only two studies defined this outcome (Patman 2001; Schweickert 2009). Patman 2001 used the presence of four or more of the following criteria to identify the incidence of postoperative pulmonary complications: oral temperature greater than 38˚C, hypoxia (oxygen saturation < 92% on room air), abnormal findings on chest X‐ray reported by blinded experienced senior radiologists, abnormal white cell count (< 2 or > 10 x 109 cells per litre) and positive sputum culture on microscopy. Schweickert 2009 described adverse events as a fall to knees, endotracheal tube removal, systolic blood pressure more than 200 mmHg, diastolic blood pressure less than 90 mmHg, and desaturation to less than 80% . In the protocol for their study, Kayambu 2015 reported that an adverse event checklist would be used to assist in clinical decisions regarding cessation or modification of the intervention but did not provide further details. Morris 2016 collected adverse events of any kind, and classified them by severity and likelihood of being related to the intervention sessions.
Secondary outcomes
Length of stay (LOS) ICU or hospital
This outcome was reported by all four included studies.
Muscle strength
Three studies reported muscle strength (Kayambu 2015; Morris 2016; Schweickert 2009). Kayambu 2015 at ICU discharge, Morris 2016 at ICU discharge, hospital discharge and at follow‐up visits, and Schweickert 2009 at hospital discharge. Kayambu 2015 and Schweickert 2009 used the Medical Research Council score (Medical Research Council 1942), to measure this outcome. Morris 2016 used dynamometer and hand grip strength; Schweickert 2009 measured hand‐grip strength and reported the incidence of ICUAW at hospital discharge.
Health‐related quality of life
THis outcome was reported by two studies. Kayambu 2015 used the 36‐item Short Form Health Survey (SF‐36) questionnaire (Ware 1992), for 11 of 26 (42%) of the participants in the intervention group and 19 of 24 (79%) of the participants in the control group to measure physical function, role physical, bodily pain, general health, vitality, social functioning, role emotional and mental health at six months post‐hospital discharge. Morris 2016 used the SF‐36 physical health summary score and mental health summary scores at hospital discharge and follow‐up visits.
Delirium
Two studies reported the number of ICU days and the number of hospital days with delirium (Morris 2016; Schweickert 2009).
Death from any cause
All four included studies reported death from any cause using the percentage in the intervention group compared with the percentage in the control group. Kayambu 2015 and Patman 2001 reported ICU mortality, and Schweickert 2009 reported hospital mortality. Morris 2016 reported six‐month mortality and Kayambu 2015 reported 90‐day mortality.
Hospital costs
None of the included studies reported costs.
Funding
One study was funded by the Intensive Care Foundation and the principal investigator was supported by a postgraduate award from Singapore (Kayambu 2015); two studies did not report funding (Morris 2016; Patman 2001) and one study author declared that no funding was received (Schweickert 2009).
For further descriptive information, see Characteristics of included studies.
Excluded studies
We excluded 14 studies for the reasons identified in the Characteristics of excluded studies table. These included study design, comparators and timing of the intervention between groups. One study was not a RCT (Morris 2008), one study was conducted in a respiratory care centre (not the ICU) (Chen 2012); four studies used comparators that did not match those in this review; active or passive ROM, or both (Burtin 2009); passive chair transfer (Collings 2015); active and passive mobilization (Médrinal 2013), and active intervention once versus twice per day (Yosef‐Brauner 2015). Seven studies did not compare early versus later interventions (Brummel 2014; Chiang 2006; Denehy 2013; ISRCTN20436833; Moss 2016; Nava 1998; NCT01058421; Porta 2005).
Awaiting classification
We identified four studies that are awaiting classification (Dong 2014; Files 2013; Malicdem 2010; Susa 2004). The reasons we placed these studies in this category varied. The contact author in one study declined to clarify methods and eligibility criteria as they expected to publish their results (Files 2013), and we have not received a response to our correspondence regarding eligibility criteria or timing of intervention from authors in the remaining three studies (Dong 2014; Malicdem 2010; Susa 2004). See Characteristics of studies awaiting classification for further information.
Ongoing studies
We identified three ongoing studies in trial registries (NCT01927510; NCT01960868; RBR‐6sz5dj). One study has been completed but not yet published (NCT01927510), and we were unable to identify publications for the remaining two studies (NCT01960868; RBR‐6sz5dj). In addition, study authors did not respond to our correspondence regarding the status of their trials. See Characteristics of ongoing studies for further information.
Risk of bias in included studies
See Figure 1 for 'Risk of bias summary' and Figure 2 for 'Risk of bias' graph for the studies included in this review.
Allocation
All four studies demonstrated adequate random sequence generation and allocation concealment except Morris 2016, which was unclear for allocation concealment, and therefore we considered them at low risk of selection bias.
Blinding
Blinding of participants and personnel
Although (Kayambu 2015), stated that they blinded participants, we considered all studies to be at high risk of performance bias as these interventions could not have been blinded for either participants or trial personnel in the ICU.
Blinding of outcome assessment
Two studies (Morris 2016; Patman 2001), demonstrated a high risk of detection bias for all outcomes except mortality; both reported that the outcome assessor was aware of group allocation. Kayambu 2015 and Schweickert 2009 blinded outcome assessors; therefore we considered these studies to be at low risk of detection bias. As the event of mortality would have been evaluated by personnel outside all of the studies, we considered them all to be at low risk of detection bias for this outcome.
Incomplete outcome data
Three studies demonstrated a high risk of attrition bias for all outcomes except mortality (Kayambu 2015; Morris 2016; Patman 2001). These studies reported results only for participants who completed the study, rather than for all randomized participants. Morris 2016 achieved outcome measurement in approximately 67% of those randomized. In addition, we noted a high dropout rate for the intervention group in the study conducted by Kayambu 2015. Only one study demonstrated a low risk of attrition bias (Schweickert 2009), as study authors presented outcome data for all outcomes including mortality for all enrolled participants.
Selective reporting
We considered all included studies to have a low risk of bias for selective reporting.
Kayambu 2015 and Morris 2016 reported all outcomes specified in the protocols for their study, and the remaining two studies reported all outcomes specified in the methods section of the text (Patman 2001; Schweickert 2009).
Other potential sources of bias
Two studies demonstrated an unclear risk of bias associated with the reporting of standard care as the control condition was not well described (Kayambu 2015; Schweickert 2009). These studies compared the intervention with standard care and the components of care delivered to the control group was not discussed in Schweickert 2009. Therefore we feel that elements of the intervention may have been delivered to the control groups. Although Kayambu 2015 described the components of the intervention delivered to standard care, the frequency and duration of the exercise strategy were not well explained and the dosage and intensity were not reported.
Effects of interventions
See: Table 1
Primary outcomes
1. Physical function and performance (the ability to perform everyday activities such as basic ADLs, and physical performance tasks)
Three studies, involving a total of 454 participants, reported aspects of physical functional status (Kayambu 2015; Morris 2016; Schweickert 2009).
Kayambu 2015, reported results for 42 of 50 (84%) of the participants for the acute care index of function (ACIF) and the physical function ICU test (PFIT) at discharge from ICU, and there was no clear difference between groups. However the evidence is of low quality, due to high risk of performance and attrition biases and imprecision (one small study); ACIF: (61.1 versus 55, MD 6.10, 95% CI ‐11.85 to 24.05; P = 0.45), and PFIT (5.6 versus 5.4, MD 0.20, 95% CI ‐0.98 to 1.38; P = 0.61).
Morris 2016 reported SPPB score as a measure of physical performance, with a mean score of 1.6 in the intervention group and 1.9 in the control group (MD ‐0.3, 95% CI ‐1.1 to 0.5; P = 0.46) at ICU discharge, and a MD of 0 (95% CI ‐1.0 to 0.9) at hospital discharge.
The study by Schweickert 2009, reported a number of outcomes associated with functional status for all of the 104 participants in this study. More of those in the intervention group returned to independent functional status at hospital discharge (59% versus 35%, RR 1.71, 95% CI 1.11 to 2.64; P = 0.01). Participants in the intervention group achieved a greater number of independently performed ADL on discharge from the ICU (median of 3 versus 0; P = 0.15) and hospital (median of 6 versus 4; P = 0.06), but these results were not statistically significantly different. There was no clear difference between the intervention and control groups for the Barthel Index score at hospital discharge (median score of 75 versus 55; P = 0.05). Schweickert 2009, reported other outcomes related to physical performance, with results favouring the intervention group for time from intubation to out of bed (median of 1.7 versus 6.6 days), standing (median of 3.2 versus 6 days), marching in place (median of 3.3 versus 6.2 days), transferring to a chair (median of 3.1 versus 6.2 days) and walking (median of 3.8 versus 7.3 days). Results for each of these outcomes were clinical important in size. Study authors also reported a difference favouring the intervention group for the greatest walking distance at hospital discharge (median of 33.4 versus 0 metres; P = 0.004).
Study samples were generally small, there was no blinding of participants or personnel, there was heterogeneity in the interventions and the outcomes used to measure their effect and inadequate descriptions of the interventions delivered as standard care for this outcome. Therefore we downgraded evidence to low. See Table 4 for further information about physical function and performance outcomes.
3. Functional status measures.
| Outcome measure | Intervention group | Control group | Effect size (95% CI) where possible | P value | Reference of studies |
| Return to independent functional status at hospital discharge ‐ n (%) in each group | 29/49 (59%) | 19/55 (35%) | RR 1.71 (1.11 to 2.64) | 0.01 | Schweickert 2009 |
| Independent ADL total at ICU discharge ‐ median (IQR) | 3 (0‐5) | 0 (0‐5) | 0.15 | Schweickert 2009 | |
| Independent ADL total at hospital discharge ‐ median (IQR) | 6 (0‐6) | 4 (0‐6) | 0.06 | Schweickert 2009 | |
| Acute Care Index of Function (ACIF) at ICU discharge ‐ mean (SD) | 61.1 (33.1) | 55 (24.4) | MD 6.10 (‐11.85 to 24.05) | 0.45 | Kayambu 2015 |
| Physical Function ICU Test (PFIT) at ICU discharge ‐ mean (SD) | 5.6 (2.1) | 5.4 (1.7) | MD 0.20 (‐0.98 to 1.38) | 0.61 | Kayambu 2015 |
| Short Physical Performance Battery at ICU discharge ‐ mean (SD) | 1.6 (3.1) | 1.9 (2.8) | MD ‐0.3 (‐1.1 to 0.5) | 0.46 | Morris 2016 |
| Time from intubation to out of bed (days) ‐ median (IQR) | 1.7 (1.1 to 3.0) |
6.6 (4.2‐8.3) |
< 0.0001 | Schweickert 2009 | |
| Time from intubation to standing (days) ‐ median (IQR) | 3.2 (1.5 to 5.6) |
6.0 (4.5‐8.9) |
< 0.0001 | Schweickert 2009 | |
| Time from intubation to marching in place (days) ‐ median (IQR) | 3.3 (1.6 to 5.8) |
6.2 (4.6‐9.6) |
< 0.0001 | Schweickert 2009 | |
| Time from intubation to transferring to a chair (days) ‐ median (IQR) | 3.1 1.8 to 4.5) |
6.2 (4.5‐8.4) |
< 0.0001 | Schweickert 2009 | |
| Time from intubation to walking (days) ‐ median (IQR) | 3.8 (1.9 to 5.8) |
7.3 (4.9‐9.6) |
< 0.0001 | Schweickert 2009 | |
| Barthel Index score at hospital discharge (score 0‐100) ‐ median (IQR) | 75 (7.5 to 95) | 55 (0‐85) | 0.05 | Schweickert 2009 | |
| Greatest walking distance (metres) at hospital discharge ‐ median (IQR) (metres) | 33.4 (0 to 91.4) | 0 (0‐30.4) | 0.004 | Schweickert 2009 |
ADL: activities of daily living; CI: confidence interval; ICU: intensive care unit; IQR: interquartile range; MD: mean difference; n: number; RR: risk ratio; SD: standard deviation
2. Adverse events (falls, accidental dislodgement of attachments, haemodynamic instability, oxygen desaturation or any other adverse events stated by trial authors)
All studies reported adverse events for a total of 690 participants and the incidence was low.
Kayambu 2015, reported that no adverse events occurred during exercise sessions.
Morris 2016, reported no adverse events specific to physical therapy (e.g. endotracheal removal, vascular access device removal, fall, cardiac arrest). There were four events in the intervention group and three in the control group considered severe, and one life‐threatening event in the intervention group. All were deemed unrelated to physical therapy. There was also an episode of asymptomatic bradycardia lasting less than one minute, with the participant completing the exercise session afterwards.
Schweickert 2009, reported the following serious adverse events for the intervention group: accidental dislodgement of the radial arterial catheter in one of 49 (2%) participants, and one of 49 (2%) participants experienced oxygen desaturation less than 80%. This study also reported cessation of therapy due to patient instability in 19 of 498 (4%) of the intervention sessions.
Patman 2001, reported 10 serious adverse events in that five of 101 (5%) participants in the intervention group compared to five of 109 (4.6%) participants in the control group met the criteria for postoperative pulmonary complications, however these were deemed unrelated to intervention.
As there was no blinding of participants and personnel, and heterogeneity in the interventions, small numbers of participants and inadequate descriptions of the interventions delivered as standard care, there was low‐quality evidence for this outcome.
Secondary outcomes
1a Length of stay (ICU)
All studies involving a total of 690 participants reported length of stay in the ICU.
Schweickert 2009 (104 participants), reported that those in the intervention group stayed a shorter time in the ICU (median of 5.9 versus 7.9 days; P = 0.08). Morris 2016, reported similar times in ICU for the two groups (7.5 versus 8.0, median difference 0, 95% CI ‐2.5 to 2.0; P = 0.68). In contrast, two studies involving a total of 260 participants reported that those in the intervention group stayed longer in the ICU: Patman 2001; (42.7 versus 36.7 days, MD 6, 95% CI ‐3.58 to 15.58; P = 0.56), and Kayambu 2015; (median of 12 versus 8.5 days; P = 0.43).
As there was no blinding of participants and personnel, heterogeneity in the interventions, small numbers of participants and inadequate descriptions of the interventions delivered as standard care, there was low‐quality evidence for this outcome.
1b Length of stay (hospital)
All four included studies reported length of stay in the hospital. Participants in the intervention group spent less time in hospital in two studies involving a total of 260 participants but the evidence was of low quality so we cannot be sure of this result: Patman 2001 (9.2 versus 9.6 days, MD ‐0.40, 95% CI ‐1.97 to 1.17; P = 0.25) and Kayambu 2015 (median of 41 versus 45 days; P = 0.80). Morris 2016 found that both groups spent similar time in hospital (10.0 days, median difference 0, 95% CI ‐1.5 to 3.0; P = 0.41. Schweickert 2009 reported on 104 participants and found no clear difference between groups (median of 13.5 versus 12.9 days; P = 0.93).
As there was no blinding of participants and personnel, heterogeneity in the interventions and the outcomes used to measure their effect, small numbers of participants and inadequate descriptions of the interventions delivered as standard care, there was low‐quality evidence for this outcome.
See Table 5 for further information for length of stay in the ICU and hospital.
4. Length of stay (ICU and hospital).
| Outcome measure | Intervention group | Control group | Mean difference (95% CI) where possible | P value | Reference of studies |
| Mean (SD) LOS (h) in ICU ‐ mean (SD) | 42.7 (42.4) | 36.7 (26.8) | 6.00 (‐3.58 to 15.58) | 0.56 | Patman 2001 |
| Median (IQR) LOS (days) in ICU ‐ median (IQR) | 12.0 (4‐45) | 8.5 (3‐36) | 0.43 | Kayambu 2015 | |
| 7.5 (4‐14) | 8.0 (4‐13) | 0.68 | Morris 2016 | ||
| 5.9 (4.5‐13.2) | 7.9 (6.1‐12.9) | 0.08 | Schweickert 2009 | ||
| Mean (SD) LOS (days) in hospital ‐ mean (SD) | 9.2 (4.5) | 9.6 (6.7) | ‐0.40 (‐1.97 to 1.17) | 0.25 | Patman 2001 |
| Median (IQR) LOS (days)in hospital ‐ median (IQR) | 41 (9‐158) | 45 (14‐308) | 0.80 | Kayambu 2015 | |
| 10.0 (6‐17) | 10.0 (7‐16) | 0.41 | Morris 2016 | ||
| 13.5 (8.0‐23.1) | 12.9 (8.9‐19.8) | 0.93 | Schweickert 2009 |
CI: confidence interval; ICU: intensive care unit; IQR: interquartile range; LOS: length of stay; SD: standard deviation
2. Muscle strength (Medical Research Council (MRC) score, cross‐sectional diameter)
Two studies involving a total of 146 participants used the MRC sum score to measure muscle strength (Kayambu 2015; Schweickert 2009). Kayambu 2015 reported results for 42 of 50 (84%) participants and found that those in the intervention group attained slightly higher MRC scores at ICU discharge (51.9 versus 47.3, MD 4.60, 95% CI ‐3.11 to 12.31; P = 0.24) but with a confidence interval that suggests the effect could favour either intervention or control. The study by Schweickert 2009 involved 104 participants and the intervention group scored higher in this outcome (median of 52 versus 48; P = 0.38) but not statistically significantly so. Two studies involving 404 participants measured hand grip strength using dynamometry (Morris 2016; Schweickert 2009). There were no clear differences between groups for this outcome. Schweickert 2009 reported that the percentage of participants who had ICU‐acquired paresis at hospital discharge was lower in the intervention group but we cannot be sure of this effect, due to small numbers of participants; (15/49 (31%) versus 27/55 (49%), RR 0.62, 95% CI 0.38 to 1.03; P = 0.09).
As there was no blinding of participants and personnel, heterogeneity in the interventions and the outcomes used to measure their effect, small numbers of participants and inadequate descriptions of the interventions delivered as standard care, there was low‐quality evidence for this outcome.
See Table 6 for further information on muscle strength.
5. Muscle strength.
| Intervention group | Control group | Effect size (95% CI) where possible | P value | Reference of studies | |
| Muscle strength (MRC score, 0‐60) at ICU discharge ‐ mean (SD) | 51.9 (10.5) | 47.3 (13.6) | MD 4.60 (‐3.11 to 12.31) | 0.24 | Kayambu 2015 |
| Hand‐grip strength (kg) at ICU discharge ‐ mean (SD) | 20.0 | 20.9 | MD ‐0.8 (‐4.0 to 2.3) | 0.60 | Morris 2016 |
| Muscle strength (MRC score, 0‐60) at hospital discharge ‐ median (IQR) | 52 (25‐58) | 48 (0‐58) | 0.38 | Schweickert 2009 | |
| Hand‐grip strength (kg) at hospital discharge ‐ median (IQR) | 39 (10‐58) | 35 (0‐57) | 0.67 | Schweickert 2009 | |
| Hand‐grip strength (kg) at hospital discharge ‐ mean (SD) | 22.6 (10.4) | 24.3 (16.3) | MD ‐1.7 (‐4.6 to 1.2) | 0.25 | Morris 2016 |
| ICU‐acquired paresis at hospital discharge ‐ n (%) | 15/49 (31%) | 27/55 (49%) | RR 0.62 (0.38 to 1.03) | 0.09 | Schweickert 2009 |
CI: confidence interval; ICU: intensive care unit; IQR: interquartile range; MD: mean difference; MRC: medical research council; SD: standard deviation
3. Health‐related quality of life or well‐being (e.g. MOS 36‐item Short‐Form Health Survey (SF‐36) questionnaire)
Two studies involving 350 participants reported this outcome. Kayambu 2015 reported results for eight subsets of the SF‐36 questionnaire at six months post‐hospital discharge for 30 of 50 (60%) participants. Participants in the intervention group achieved clinically meaningfully higher scores in physical function; (81.8 versus 60, MD 21.8, 95% CI 0.81 to 42.79; P = 0.04); and role physical; (61.4 versus 17.1, MD 44.3, 95% CI 14.79 to 73.81; P = 0.005). There were no important between‐group differences for the remaining six domains of the SF‐36 in this study. Morris 2016 reported results for the physical function summary score and the mental health summary score of the SF‐36 at hospital discharge and at follow‐up visits. There were no clinically meaningful differences between groups at any time point except at six months, where the intervention group had significantly higher scores. However, no mention was made of adjusting for repeated testing on this measure. The MD in physical function score at six months was 12.2 units, a clinically important difference.
As there was no blinding of participants and personnel, heterogeneity in the interventions small numbers of participants and inadequate descriptions of the interventions delivered as standard care, there was low‐quality evidence for this outcome.
See Table 7 for further information for health‐related quality of life outcomes at 6 months.
6. Health‐related quality of life.
| Outcome measure at 6 months' follow‐up | Mean (SD) of intervention group | Mean (SD) of control group | Mean difference (95% CI) | P value | Reference of studies |
| SF‐36 physical function | 81.8 (22.2) | 60.0 (29.4) | 21.8 (0.81 to 42.79) | 0.04 | Kayambu 2015 |
| 55.9 (27.3) | 43.6 (27.7) | 12.2 (3.9 to 20.7) | 0.001 | Morris 2016 | |
| SF‐36 role physical | 61.4 (43.8) | 17.1 (34.4) | 44.3 (14.79 to 73.81) | 0.005 | Kayambu 2015 |
| SF‐36 bodily pain | 70.9 (20.7) | 64.7 (22.5) | 6.20 (‐10.78 to 23.18) | 0.46 | Kayambu 2015 |
| SF‐36 general health | 50.5 (11.9) | 41.8 (11.3) | 8.70 (‐0.24 to 17.64) | 0.06 | Kayambu 2015 |
| SF‐36 vitality | 45.9 (12.0) | 39.2 (7.7) | 6.70 (‐0.22 to 13.62) | 0.07 | Kayambu 2015 |
| SF‐36 social functioning | 71.6 (37.1) | 73.7 (37.2) | ‐2.10 (‐30.94 to 26.74) | 0.88 | Kayambu 2015 |
| SF‐36 role emotional | 63.6 (40.7) | 33.3 (45.8) | 30.30 (‐3.88 to 64.48) | 0.08 | Kayambu 2015 |
| SF‐36 mental health | 38.6 (11.5) | 37.3 (7.4) | 1.30 (‐5.75 to 8.35) | 0.71 | Kayambu 2015 |
| 48.8 | 46.4 | 2.4 (‐1.2 to 6.0) | 0.19 | Morris 2016 |
CI: confidence interval; SD: standard deviation; SF‐36: Short Form 36
4. Delirium
Two studies involving 404 participants (Morris 2016; Schweickert 2009), reported this outcome in the ICU, and one study reported this outcome for the entire hospital stay (Schweickert 2009). Schweickert 2009 found that those in the intervention group spent a lower number of days with delirium while in ICU; (median of 2 versus 4 days; P = 0.03) and in hospital; (median of 2 versus 4 days; P = 0.02). However, Morris 2016 found no difference between groups (median of 0 versus 0 days; P = 0.71).
As there was no blinding of participants and personnel, heterogeneity in the interventions, small numbers of participants and inadequate descriptions of the interventions delivered as standard care, there was low‐quality evidence for this outcome.
See Table 8 for further information about delirium.
7. Delirium.
| Intervention group | Control group | P value | Reference of studies | |
| ICU (days) with delirium ‐ median (IQR) | 2.0 (0.0‐6.0) | 4.0 (2.0‐7.0) | 0.03 | Schweickert 2009 |
| 0 (0 ‐12.5) | 0 (0‐9.1) | 0.71 | Morris 2016 | |
| Hospital (days) with delirium ‐ median (IQR) | 2.0 (0.0‐6.0) | 4.0 (2.0‐8.0) | 0.02 | Schweickert 2009 |
ICU: intensive care unit; IQR: interquartile range
5. Death from any cause
Two studies involving a total of 260 participants measured the percentage of participants who died in the ICU, but the numbers were too small to be confident in this result (Kayambu 2015 3/26 (12%) versus 1/24 (4%), RR 2.77, 95% CI 0.31 to 24.85; P = 0.36, and Patman 2001 (0/101 (0%) versus 3/109 (2.8%), RR 0.16, 95% CI 0.008 to 3.03; P = 0.22). One study involving a total of 104 participants measured mortality while participants were in the hospital (Schweickert 2009). There was no clear difference between groups, but again the numbers are too small to be confident of this result (9/49 (18%) versus 14/55 (25%), RR 0.72, 95% CI 0.34 to 1.52; P = 0.53). One study involving 50 participants measured 90‐day mortality (Kayambu 2015), and reported that the percentage of those in the intervention group who died within 90 days of admission to this study was 8/26 (31%) versus 2/24 (8%) in the control group (RR 3.69, 95% CI 0.87 to 15.69; P = 0.08). Morris 2016 reported only on the proportion alive and hospital‐readmission‐free at six months (48.7% versus 44.7%, RR 1.09, 95% CI 0.86 to 1.39; P = 0.69). As there was heterogeneity in the interventions, small numbers of participants and inadequate descriptions of the interventions delivered as standard care, there was low‐quality evidence for this outcome.
See Table 3 for further information about this outcome.
6. Hospital costs
No studies measured this outcome.
7. Other outcomes not pre‐specified in this review ‐ Duration of intubation/mechanical ventilation
Three studies, including a total of 390 participants, reported duration of mechanical ventilation. Schweickert 2009 found that those in the intervention group spent less time on mechanical ventilation and this difference was of clinically important size (median of 3.4 versus 6.1 days; P = 0.02). Two studies reported that those in the intervention group spent a longer time intubated but there were no clear differences between groups (Kayambu 2015; Patman 2001). Patman 2001 reported results for 210 of 236 (89%) participants; (13 versus 12.7 hours, MD 0.20, 95% CI ‐1.1 to 1.65; P = 0.85) and Kayambu 2015 on 50 participants; (median of 8 versus 7 days; P = 0.22).
As there was no blinding of participants and personnel, heterogeneity in the interventions, small numbers of participants and inadequate descriptions of the interventions delivered as standard care, there was low‐quality evidence for this outcome.
See Table 9 for further information about this outcome.
8. Other outcomes not specified in this review.
| Intervention group | Control group | Mean difference (95% CI) where possible | P value | Reference of studies | |
| Duration (h) of mechanical ventilation ‐ mean (SD) | 13 (4.8) | 12.7 (4.7) | 0.20 (‐1.1 to 1.65) | 0.85 | Patman 2001 |
| Duration (days) of mechanical ventilation ‐ median (IQR) | 8.0 (4‐64) | 7.0 (2‐30) | 0.22 | Kayambu 2015 | |
| 3.4 (2.3‐7.3) | 6.1 (4.0‐9.6) | 0.02 | Schweickert 2009 |
Abbreviations: CI: confidence interval. IQR: interquartile range. SD: standard deviation.
As this outcome was not listed in the protocol for this review (Doiron 2013), we have indicated this change in the section Differences between protocol and review.
Discussion
Summary of main results
There were mixed results for the effect of early mobilization or active exercise on the primary outcome of physical function or performance. Benefits from the intervention were found for return to independent functional status at hospital discharge in one study, and for greatest walking distance at hospital discharge and time from intubation to functional mobility in the same study (Schweickert 2009). However, no significant effect was found for other measures of this outcome in this study, including the number of independent ADLs achieved at ICU or hospital discharge and the Barthel Index Score at hospital discharge. The other two studies that measured physical performance status did not find any clinically important differences between groups, although confidence intervals were wide, and quality of evidence was low.
All four studies measured adverse events. Three studies reported a low incidence of adverse events in the intervention groups (Morris 2016; Patman 2001; Schweickert 2009), and one study (Kayambu 2015), reported no adverse events. This finding appears to support the safety and feasibility of early mobilization for mechanically ventilated, critically ill patients in the ICU, however the quality of the evidence was low due to small numbers of participants and events, and this result requires confirmation in other studies.
Length of stay in the ICU and in hospital was measured in all studies but no differences between groups were observed. In the three studies that measured muscle strength (Kayambu 2015; Morris 2016; Schweickert 2009), no significant differences were reported except at six months, favouring the intervention group, in one study (Schweickert 2009). Two studies (Kayambu 2015; Morris 2016), measured health‐related quality of life and found significant differences favouring the intervention group in two of the 36‐item Short Form Health Survey subscales in one study (Kayambu 2015) but not the other. Schweickert 2009 was the only study that measured delirium and reported a significant difference with the intervention group having less time with delirium while in the ICU and hospital. No differences in mortality were found by any of the studies.
Overall completeness and applicability of evidence
There are limitations in the applicability of the existing evidence and its completeness. Admission diagnoses in three of the studies signified critical illness and the majority of the participants were intubated for longer than three days (Kayambu 2015; Morris 2016; Schweickert 2009). While participants in the study by Patman 2001 were considered routine ICU patients after cardiac surgery, they were withdrawn from the study if mechanical ventilation was required for more than 24 hours. This is the only included study in which participants were withdrawn from the study on the basis of a predefined length of mechanical ventilation. This study also used only a small range of interventions and did not measure any functional outcomes (Patman 2001). Hence, the results from this study and its contribution to the body of evidence should be interpreted with these differences in mind (Patman 2001).
The sample size was small in all studies and less than the calculated minimum needed in Kayambu 2015. There were differences in the content of the interventions, the providers, the timing, dosage, tailoring, and exercise progression across all studies. No two studies tested the same intervention. Additional evidence from multicentre RCTs is needed to inform clinical decision‐making about the effectiveness of early mobilization and active exercise in the critically ill population. The limited range of and variation in the interventions trialled does not allow us to draw conclusions about the essential components of interventions. There was no agreement between the studies on what is 'early' intervention, and 'late', however the studies all began exercise in the intervention group at a median of one day after admission to ICU. The comparator of 'late' ranged from a median of two days to seven days.
There was also heterogeneity in the types of outcome measures used to evaluate the impact of early mobilization and active exercise across the studies. Three studies measured functional status, however they used different measurement tools and methods (Kayambu 2015; Morris 2016; Schweickert 2009). Schweickert 2009 reported on a number of outcomes of interest to clinicians, but used the functional independence measure (FIM) (Keith 1987), and the Barthel Index (Mahoney 1965), to measure functional status. As no studies have investigated the reliability and validity of the FIM and the Barthel Index in critically ill patients (Adler 2012; Tipping 2012), caution is needed in the interpretation of these results. A scoping review looking at outcome measures in critical illness found eight measures of physical activity limitation used in 25 studies (Turnbull 2016). There is a project registered with the COMET initiative (www.comet‐initiative.org), aimed at attaining consensus on a core outcome set for trials of physical rehabilitation after critical illness. As muscle weakness contributes to impaired physical function and the ability to perform ADLs, interventions to achieve significant gains in muscle strength are potentially important (Mehrholz 2015). The review by Turnbull (Turnbull 2016), found that 43% of studies investigating ICU survivorship used the MRC scale to measure muscle strength.
Thus further investigation is required to examine the type, frequency, intensity and dosage of early mobilization required in this population. No studies reported on costs or cost‐savings of providing the intervention.
Quality of the evidence
Meta‐analyses of data from the four studies in this review were not possible, as different measures of the primary outcome were used in each study. Risk of bias was low for methods used to randomize and allocate participants in three studies and unclear in the fourth. All studies were at high risk of performance bias due to the lack of blinding of participants and personnel. This finding was a contributing factor to the downgrading of the quality of evidence to low for all functional status outcomes in Table 1, as they are subjectively measured. While it is understandable that blinding of participants and personnel is challenging in the ICU environment, this introduces the potential for altered participant response to the interventions and adjustment of the intervention by study personnel (Schultz 2002). Risk of detection and attrition bias was low in all studies for mortality but high in two for the subjectively rated outcomes due to lack of blinding of outcome assessors. This introduces the potential for amplification of estimates of treatment effect, and therefore results from studies with detection bias need to be interpreted with caution. Risk of reporting bias was low across all studies. We downgraded the quality of evidence for all outcomes for imprecision, as the results came from only one small study for most outcomes, and there was a very small number of events for the outcome of adverse events. There were too few studies to assess inconsistency of results or publication bias. The reported studies used direct patient‐related outcomes of physical function and performance.
Potential biases in the review process
We completed a comprehensive search in multiple stages, two review authors independently screened references and all review authors screened full‐text articles before they were chosen for inclusion in the review. Data entry and calculations were checked by two review authors. However, we did not search conference proceedings unless citations were found in the search. We added the outcome of duration of mechanical ventilation to the review as we thought it would be of interest to clinicians working with critically ill adults in the ICU. We are unsure of what level of physiotherapy or exercise intervention the control group received in one study (Patman 2001). We were unable to correspond with the authors of some of the studies in Characteristics of studies awaiting classification (Malicdem 2010; Susa 2004) and Characteristics of ongoing studies (NCT01960868; RBR‐6sz5dj).
Agreements and disagreements with other studies or reviews
We found nine systematic reviews examining mobilization of patients in the ICU (Adler 2012; Castro‐Avila 2015; Choi 2008; Hermans 2014b; Kayambu 2013; Li 2013; Pinheiro 2012; Stiller 2013; Thomas 2011). Five of these reviews included observational study designs as well as RCTs, and four included other settings, such as high‐dependency units, respiratory intensive care units and respiratory care centres.
All reviews included studies that used active exercise. However, in contrast to this Cochrane Review, additional interventions were included; inspiratory muscle training (Adler 2012; Choi 2008; Thomas 2011), breathing exercises (Choi 2008; Li 2013), chest physiotherapy (Li 2013; Stiller 2013), and electrical muscle stimulation (Choi 2008; Pinheiro 2012; Thomas 2011). We examined passive interventions only if delivered in combination with active mobilization or active exercise. Interventions were delivered at a variety of stages (early and late) during participant admission in most of the reviews and one examined participants who received interventions only after being intubated for prolonged periods (Choi 2008). In contrast, we included only studies delivering interventions earlier than those received in standard care. All of the reviews included the primary outcomes specified in this current review (functional status and adverse events).
All reviews supported the use of early mobility for increasing walking distance, and two reviews found improved return to independent functional status (Li 2013; Thomas 2011). Similarly, we found improvements in these outcomes in the only study that measured them (Schweickert 2009). We found no difference between the groups in other measures of functional status, as used by Kayambu 2015 and Morris 2016. All reviews reported a low incidence of adverse events as did this Cochrane Review. In the six reviews that reported length of stay, only one (Choi 2008), found no difference between groups for length of stay. Five reviews reported increased muscle strength (Adler 2012; Choi 2008; Kayambu 2013; Pinheiro 2012; Thomas 2011). These results were found in RCTs in which the interventions were cycle ergometers and electrical muscle stimulation, retrospective studies and a quasi‐RCT. In contrast, none of the RCTs that measured muscle strength in this Cochrane Review found a significant difference between groups (Kayambu 2015; Morris 2016; Schweickert 2009). Six reviews reported health‐related quality of life and all found improvements in the physical function item of the SF‐36 (Adler 2012; Castro‐Avila 2015; Kayambu 2013; Li 2013; Pinheiro 2012; Thomas 2011). We also found improvements in the physical function and role physical components of this outcome in two RCTs that measured it (Kayambu 2015; Morris 2016). Two of the reviews reported decreased delirium in the ICU and hospital, which is similar to the result reported in this review (Adler 2012; Thomas 2011). Four reviews, reported no significant differences between groups for mortality at hospital discharge (Hermans 2014b; Kayambu 2013; Li 2013; Pinheiro 2012). We also found no significant differences between groups for ICU, hospital or 90‐day mortality.
Our review concentrated on the timing of exercise intervention. Some of the other reviews included studies that evaluated different intensities of exercise interventions, or a combination of timing and intensity.
Authors' conclusions
Implications for practice.
The evidence for the effectiveness of early mobilization of mechanically ventilated, critically ill patients in the intensive care unit (ICU) on measures of physical function and performance is inconsistent and uncertain due to its low quality. The evidence on adverse events is also of low quality. There is wide variation in the type, timing, intensity and progression of the interventions delivered to this population (Jolley 2014), and there is insufficient, high‐quality evidence to disentangle these factors currently. We assessed that there is currently low‐quality evidence for the effect of early mobilization of critically ill adults in the ICU due to small sample sizes, lack of blinding of participants and personnel, variation in the interventions and outcomes used to measure their effect and inadequate descriptions of the interventions delivered as usual care in the studies included in this Cochrane Review. The four studies awaiting classification, and the three ongoing studies may alter the conclusions of the review once these results are available.
Implications for research.
Results from ongoing studies across multiple sites will provide some evidence regarding the impact of this intervention in critically ill patients in the ICU (NCT01927510; NCT01960868; RBR‐6sz5dj). However, these three studies are small. We have also identified four studies awaiting classification which may also contribute to the volume of evidence in this area (Dong 2014; Files 2013; Malicdem 2010; Susa 2004). Although the interventions in these studies are not well described at this stage, all contain some of the outcomes specified in this review, and will potentially contribute data from a further 500 participants. We hope to be able to assess and include these studies in an update of this review. In order to be confident of the safety of early intervention, more randomized controlled trials with larger sample sizes, clearly reported interventions and control conditions, and blinded outcome assessment are needed. Standardization of outcome measures between studies would permit meta‐analysis of outcomes. It is also important to disentangle early intervention from intensity of intervention in the design of new studies, in order to be able to confidently recommend either early intervention, or more intensive intervention, irrespective of timing.
What's new
| Date | Event | Description |
|---|---|---|
| 20 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
Acknowledgements
We would like to thank Bronagh Blackwood (content editor), Vibeke E Horstmann (statistical editor), Linda Denehy, Sue Berney, Tom J Overend, Andrea Lemos (peer reviewers) and Odie Geiger (consumer referee) for their help and editorial advice during the preparation of this systematic review.
We would like to thank Matthew Zacharias (content editor), Cathal Walsh (statistical editor), Andrea Lemos, Andrezza L Bezzera, Linda Denehy and Sue Berney (peer reviewers) for their help and editorial advice during the preparation of the Cochrane protocol (Doiron 2013).
We would like to thank Karen Hovhannisyan, former Trials Search Co‐ordinator, Cochrane Anaesthesia, Critical and Emergency Care (ACE), and Sarah Thorning, previous Trials Search Co‐ordinator, Cochrane Acute Respiratory Infections, for their assistance in identifying search terms for this review, and Justin Clark, Information Specialist at the Centre for Research in Evidence‐Based Practice for running search updates. We also thank Leanne McGregor and Rebecca Sims (research assistants at Centre for Research in Evidence‐Based Practice, Bond University) for their assistance with screening search results and initial data extraction.
Appendices
Appendix 1. CENTRAL, Cochrane Library search strategy
([mh "Intensive Care Units"] OR [mh ^"Critical Illness"] OR [mh "Critical Care"] OR (critical* NEAR3 (ill* OR care*)):ti,ab OR "intensive care":ti,ab OR (icu OR icuaw):ti,ab)
AND
([mh "Exercise Therapy"] OR [mh "Physical Therapy Modalities"] OR [mh "Occupational Therapy"] OR (mobilizat* OR mobilisat* OR mobility):ti,ab OR exercis*:ti,ab OR (therap* NEAR3 (physical OR exercise OR occupation*)):ti,ab OR ((bed OR "daily living") NEAR3 activit*):ti,ab OR (training OR pregait OR pre‐gait OR walk* OR adl OR physiotherap* OR ambulation):ti,ab OR ((cycle OR bicycle) NEAR1 ergomet*):ti,ab)
Appendix 2. MEDLINE (Ovid SP) search strategy
(exp Intensive Care Units/ OR Critical Illness/ OR exp Critical Care/ OR (critical* adj3 (ill* or care*)).tw. OR intensive care.tw. OR (icu or icuaw).tw.)
AND
(exp Exercise Therapy/ OR exp Physical Therapy Modalities/ OR Occupational Therapy/ OR (mobilizat* or mobilisat* or mobility).tw. OR exercis*.tw. OR (therap* adj3 (physical or exercise or occupation*)).tw. OR ((bed or daily living) adj3 activit*).tw. OR (training or pregait or pre‐gait or walk* or adl or physiotherap* or ambulation).tw. OR ((cycle or bicycle) adj1 ergomet*).tw.)
AND
((randomized controlled trial OR controlled clinical trial).pt. OR randomized.ab. OR randomised.ab. OR placebo.ab. OR drug therapy.fs. OR randomly.ab. OR trial.ab. OR groups.ab.) not (exp animals/ not humans.sh.)
Appendix 3. Embase (Ovid SP) search strategy
(icu:ab,ti OR icuaw:ab,ti OR 'intensive care':ab,ti OR ((critical* NEAR/3 (ill* OR care)):ab,ti) OR 'intensive care'/exp OR 'critical illness'/de OR 'intensive care unit'/de)
AND
(training:ab,ti OR pregait:ab,ti OR 'pre‐gait':ab,ti OR walk*:ab,ti OR adl:ab,ti OR physiotherapy*:ab,ti OR (((cycle OR bicycle) NEAR/1 ergomet*):ab,ti) OR ambulation:ab,ti OR (((bed OR 'daily living') NEAR/3 activity):ab,ti) OR ((therap* NEAR/3 (physical* OR exercise OR occupation*)):ab,ti) OR exercis*:ab,ti OR mobiliz*:ab,ti OR mobilis*:ab,ti OR mobility:ab,ti OR 'occupational therapy'/de OR 'physiotherapy'/exp OR 'kinesiotherapy'/exp)
AND
((random*:ab,ti OR placebo*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR allocat*:ab,ti OR trial:ti OR ((doubl* NEXT/1 blind*):ab,ti) OR 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp) NOT (('animal'/exp OR 'animal'/de OR 'nonhuman'/exp OR 'nonhuman'/de OR 'animal experiment'/exp OR 'animal experiment'/de) NOT (('animal'/exp OR 'animal'/de OR 'nonhuman'/exp OR 'nonhuman'/de OR 'animal experiment'/exp OR 'animal experiment'/de) AND 'human'/de)))
Appendix 4. CINAHL (EBSCOhost) search strategy
TI ((cycle or bicycle) N1 ergomet*) OR AB ((cycle or bicycle) N1 ergomet*) OR TI (training or pregait or pre‐gait or walk* or adl or physiotherap* or ambulation) OR AB (training or pregait or pre‐gait or walk* or adl or physiotherap* or ambulation) TI ((bed or daily living) N3 activit*) OR AB ((bed or daily living) N3 activit*) TI (therap* N3 (physical or exercise or occupation*)) OR AB (therap* N3 (physical or exercise or occupation*)) TI exercis* OR AB exercis* TI (mobilizat* or mobilisat* or mobility) OR AB (mobilizat* or mobilisat* or mobility) (MH "Occupational Therapy+") (MH "Physical Therapy+") (MH "Therapeutic Exercise+")
AND
TI (icu or icuaw) OR AB (icu or icuaw) OR TI intensive care OR AB intensive care OR TI (critical* N3 (ill* or care*)) OR AB (critical* N3 (ill* or care*)) OR (MH "Critical Care") OR (MH "Critical Illness") OR (MH "Intensive Care Units+")
AND
(MH "Quantitative Studies") OR TI placebo* OR AB placebo* OR (MH "Placebos") OR (MH "Random Assignment") OR TI random* OR AB random* OR TI ((singl* or doubl* or tripl* or trebl*) W1 (blind* or mask*)) OR AB ((singl* or doubl* or tripl* or trebl*) W1 (blind* or mask*)) OR TI clinic* trial* OR AB clinic* trial* OR PT clinical trial OR (MH "Clinical Trials+")
Appendix 5. Controlled Trials Registry search strategy
intensive care unit AND critically ill AND mobilisation
intensive care unit AND critically ill AND mobilization
intensive care unit AND critically ill AND exercise
intensive care unit AND critically ill AND physiotherapy
intensive care unit AND critically ill AND physical therapy
intensive care unit AND mechanical ventilation AND mobilisation
intensive care unit AND mechanical ventilation AND mobilization
intensive care unit AND mechanical ventilation AND exercise
intensive care unit AND mechanical ventilation AND physiotherapy
intensive care unit AND mechanical ventilation AND physical therapy
ICU AND critically ill AND mobilisation
ICU AND critically ill AND mobilization
ICU AND critically ill AND exercise
ICU AND critically ill AND physiotherapy
ICU AND critically ill AND physical therapy
ICU AND mechanical ventilation AND mobilisationICU AND mechanical ventilation AND mobilization
ICU AND mechanical ventilation AND exercise
ICU AND mechanical ventilation AND physiotherapy
ICU AND mechanical ventilation AND physical therapy
Appendix 6. ClinicalTrials.gov Registry search strategy
critically ill AND mobilisation
critically ill AND mobilization
critically ill AND exercise
critically ill AND physiotherapy
critically ill AND physical therapy
mechanical ventilation AND mobilisation
mechanical ventilation AND mobilization
mechanical ventilation AND exercise
mechanical ventilation AND physiotherapy
mechanical ventilation AND physical therapy
intensive care unit AND mobilisation
intensive care unit AND mobilization
intensive care unit AND exercise
intensive care unit AND physiotherapy
intensive care unit AND physical therapy
ICU AND mobilisation
ICU AND mobilization
ICU AND exercise
ICU AND physiotherapy
ICU AND physical therapy
Appendix 7. WHO International Clinical Trials Registry Platform search strategy
critically ill AND mobilisation
critically ill AND mobilization
critically ill AND exercise
critically ill AND physiotherapy
critically ill AND physical therapy
mechanical ventilation AND mobilisation
mechanical ventilation AND mobilization
mechanical ventilation AND exercise
mechanical ventilation AND physiotherapy
mechanical ventilation AND physical therapy
intensive care unit AND mobilisation
intensive care unit AND mobilization
intensive care unit AND exercise
intensive care unit AND physiotherapy
intensive care unit AND physical therapy
ICU AND mobilisation
ICU AND mobilization
ICU AND exercise
ICU AND physiotherapy
ICU AND physical therapy
Appendix 8. Original search strategy from protocol MEDLINE (Ovid SP)
1. exp Respiration, Artificial/ or (mechanical* adj3 ventila*).af. 2. exp Exercise Therapy/ or exp Physical Therapy Modalities/ or exp Occupational Therapy/ or mobilizat*.mp. or mobilisat*.mp. or mobility.mp or exercis*.mp. or (therap* adj3 (physical or exercise or occupational)).mp. or ((bed or daily living) adj3 activit*).mp. or training.mp. or pre?gait.mp. or walk*.mp. or ADL*.ti,ab. or physiotherap*.mp. 3. exp Intensive Care Units/ or ICU.mp. or exp Critical Illness/ or exp Critical Care/ or (critical* adj3 (ill* or care)).mp. or intensive care.mp. or ICUAW.mp. 4. 1 and 2 and 3
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kayambu 2015.
| Methods | Single‐centre, parallel‐group RCT Trial dates: December 2010‐August 2012 Objective: to assess the impact of early physical rehabilitation therapy on physical function and self‐reported quality of life in patients admitted to the ICU with sepsis syndromes compared to standard care Randomization: participants were randomly assigned to either the intervention or control groups using computer‐generated randomization. Consent: study authors obtained consent from each participant's next of kin or substitute decision maker. |
|
| Participants |
Inclusion criteria: ≥ 18 years, mechanically ventilated ≥ 48 h, diagnosed with sepsis (≥ 2 criteria of a systemic inflammatory response plus confirmed or strongly suspected infection), severe sepsis (sepsis with organ failure), or septic shock (severe sepsis with hypotension not responding to the provision of fluid) Exclusion criteria: head injuries, burns, spinal injuries, multiple fractured lower limbs and patients diagnosed with septic shock who were unresponsive to maximal treatment, moribund or had an expected mortality within 48 h Participants: 50 participants were randomized; 26 (M:F 18:8, median age 62.5 years) to the intervention group and 24 (M:F 14:10, median age 65.5 years) to the control group. Baseline characteristics were similar across groups with the exception of DNR status; 9/26 (35%) of those in the intervention group compared to 4/24 (17%) in the control group were given a DNR order. The primary diagnosis on admission to the ICU for both the intervention and standard care groups was septic shock. |
|
| Interventions |
Intervention group What (materials and procedures): specific equipment used during the interventions was not reported. Interventions included arm or leg ergometry; passive, active and active resisted ROM exercises; sitting up in bed; sitting out of bed; sitting and standing balance exercises; sit to stand, marching on the spot, ambulation with assistance, tilt table therapy and electrical muscle stimulation (vastus medialis, vastus lateralis, tibialis anterior, brachioradialis) Who provided: the ICU research physiotherapist; study authors did not report reimbursement of trial personnel, their expertise/usual role, assessment of competence and components of training (if needed) to perform the interventions Where: a single, quaternary‐level, university‐affiliated, general ICU in a hospital in Brisbane, Australia When and how much: 30 min 1‐2 times daily within 48 h of being diagnosed with sepsis until discharge from the ICU Tailoring: study authors stated that interventions were individualised but no further information was reported. Interventions were planned, administered and progressed at the discretion of the physiotherapist and the participant's acuity of illness and level of co‐operation based on the Ramsay sedation score. To ensure that interventions were delivered safely, data from participant Intellivue bedside monitors MO70 (Phillips) was collected every 10 s and printed out for ten min prior, during and postinterventions; the intra‐arterial line was put to zero 10 min prior to the intervention and withdrawal criteria were developed to assist decision making regarding cessation or modification of the intervention. Modifications: not reported Fidelity (strategies to improve): not reported Fidelity (extent): there no withdrawals during the conduct of the trial and all participants adhered to the intervention for an average of 11.4 days. Study authors reported the frequency and duration of interventions received by the intervention and the control group but this is difficult to interpret. Control group What (materials and procedures): specific equipment used during the interventions was not reported. Interventions included passive, active and active resisted ROM exercises; sitting up in bed; sitting out of bed; sitting and standing balance exercises; sit to stand; marching on the spot and ambulation with assistance. Who provided: ICU therapists who were not involved in the research team; study authors did not report reimbursement of trial personnel, their expertise/usual role, assessment of competence and components of training (if needed) to perform the interventions Where: same location as the intervention group When and how much: study authors confirmed via email that participants in the control group received the intervention less regularly and later than those in the intervention group; 10/24 (42%) participants received usual care within 48 h of diagnosis of sepsis after ICU admission while 14/24 (58%) subjects received it 48 h after sepsis was diagnosed in ICU. Tailoring: not reported Modification: not reported Fidelity (strategies to improve): not reported Fidelity (extent): same as the intervention group |
|
| Outcomes |
|
|
| Potential conflicts of interest | This study was funded by the Intensive Care Foundation and the principal investigator was supported by a postgraduate award from Singapore (no further details were reported). Study authors declared that there were no conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to either the intervention or control groups using computer‐generated randomization. |
| Allocation concealment (selection bias) | Low risk | Sequencing of randomization was generated and serial numbers were assigned by research staff not involved in the study. Re‐identifiable serial numbers were concealed from research staff for group allocation and protected by an electronic password. |
| Blinding of participants and personnel (performance bias) All other outcomes | High risk | Study authors stated that participants but not treating therapists were blinded. However, we considered this study to be at high risk of bias as we feel that the interventions could not have been blinded for either participants or personnel. |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | The incidence of mortality would have been evaluated by personnel outside this study. |
| Blinding of outcome assessment (detection bias) All other outcomes | Low risk | Investigators blinded outcome assessors, substitute decision makers and health care staff. |
| Incomplete outcome data (attrition bias) Mortality | Low risk | Mortality is an objective outcome and appeared to be measured on all participants in this study. |
| Incomplete outcome data (attrition bias) All other outcomes | High risk | All other outcomes were reported only for the remaining participants in the intervention and control groups. More participants withdrew from the intervention group than the control group both during admission and after hospital discharge. For example, there was a loss of 15/26 (58%) participants from the intervention group compared to 5/24 (21%) in the control group at 6 months post‐hospital discharge. |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the protocol for this study were reported. |
| Other bias | Unclear risk | This study described the components, frequency and duration of the exercise strategy delivered as standard care but the dosage and intensity was unknown. Therefore we considered this study to have an unclear bias associated with the reporting of standard care. |
Morris 2016.
| Methods | A single‐centre parallel‐group RCT conducted in an ICU in Winston‐Salem, North Carolina, USA to investigate the impact on hospital length of stay of standardized rehabilitation therapy initiated in the ICU versus usual care. | |
| Participants | 300 participants. Mean (SD) age: 56 (15) years. Intervention group: 55 (17) years; usual care: 58 (14) years Inclusion criteria: age ≥ 18 years, mechanically ventilated via an endotracheal tube or Bipap, PaO2/FIO ratio < 300 Exclusion criteria: inability to walk without assistance prior to acute ICU illness (use of a cane or walker not exclusion), cognitive impairment prior to acute ICU illness (non‐verbal), acute stroke, BMI > 50, neuromuscular disease that could impair weaning, hip fracture, unstable cervical spine or pathological fracture, mechanically ventilated > 80 h, current hospitalization or transferring hospital stay > 7 days, moribund, DNR/DNI on admission, involvement in other research study |
|
| Interventions |
Intervention group What (materials and procedures): passive ROM included 5 repetitions for each upper and lower extremity joint. Physiotherapy included bed mobility, transfer training, and balance training. The exercises included transfer to the edge of the bed; safe transfers to and from bed, chair, or commode; seated balance activities; pre‐gait standing activities (forward and lateral weight shifting, marching in place); and ambulation. Progressive resistance exercise included dorsiflexion, knee flexion and extension, hip flexion, elbow flexion and extension, and shoulder flexion. Resistance was added through the use of elastic resistance bands (TheraBand, Hygienic Corporation). Both the physiotherapy and resistance training targeted lower extremity functional tasks and ADL (further details are available in the supplement of the article). Who provided: the rehabilitation team consisted of a physiotherapist, an ICU nurse, and a nursing assistant; study authors did not report their expertise/usual role, assessment of competence and components of training (if needed) to perform the interventions Where: a single medical centre in North Carolina, USA When and how much: 3 separate sessions every day of hospitalization for 7 days per week, from enrolment through to hospital discharge. Tailoring: the participant's level of consciousness determined whether they were considered suitable to receive the physiotherapy or progressive resistance exercise, as did their ability to complete the exercises. When participants were unconscious, the 3 sessions consisted of passive ROM. As consciousness was gained, physiotherapy and progressive resistance exercise was commenced. Participants did not need to be free of mechanical ventilation to begin any of the exercise sessions. Modifications: not reported Fidelity (strategies to improve): not reported Fidelity (extent): the mean percentage of study days that participants received therapy was 87.1% (SD 18.4%) for passive ROM; 54.6% (SD 27.2%) for physiotherapy; and 35.7% (SD 23%) for progressive resistive exercise. The median days of delivery of therapy per participant was 8.0 (IQR, 5.0‐14.0) for passive ROM, 5.0 (IQR, 3.0‐8.0) for physiotherapy, and 3.0 (IQR, 1.0‐5.0) for progressive resistance exercise Control group: usual care, which could include physical therapy if ordered, between Monday and Friday. The mean percentage of study days that participants received physiotherapy was 11.7% (SD, 14.5%). The median days of delivery of physiotherapy for the usual care group was 1.0 (IQR, 0.0‐8.0). |
|
| Outcomes |
|
|
| Potential conflicts of interest | The study was supported by the National Institutes of Health, National Institute of Nursing Research, and the National Heart, Lung & Blood Institute. The study authors reported that the sponsors did not participate in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned, using a computer‐generated variably sized approach (in block sizes of 2, 4, 6, or 8), to standardized rehabilitation therapy or usual care |
| Allocation concealment (selection bias) | Unclear risk | No description of any allocation concealment |
| Blinding of participants and personnel (performance bias) All other outcomes | High risk | Physiotherapists delivering the intervention and participants receiving the intervention were not blinded. |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | The incidence of mortality would have been evaluated by personnel outside this study. |
| Blinding of outcome assessment (detection bias) All other outcomes | High risk | The research team were not involved in the decision for hospital discharge, however it appears that study personnel measured other outcomes. |
| Incomplete outcome data (attrition bias) Mortality | Low risk | Mortality is an objective outcome and appeared to be measured on all participants in this study. |
| Incomplete outcome data (attrition bias) All other outcomes | High risk | All data available for primary outcome (LOS), however only 55% of patients completed 6‐month follow‐up |
| Selective reporting (reporting bias) | Low risk | Study authors reported all outcomes specified in the methods section of the text and in the protocol. |
| Other bias | Low risk | |
Patman 2001.
| Methods | Single‐centre, parallel‐group RCT Trial dates: May 1998‐May 1999 Objective: to investigate the impact of an active physiotherapy intervention to participants in the intubation period after cardiac surgery compared to usual care Randomization: by an independent person who utilized a randomized numbers table (Portney 1993) Consent: the requirement for participant consent was waived |
|
| Participants |
Inclusion criteria: all participants admitted consecutively to the ICU following elective or semi‐urgent cardiac surgery. No further details were provided. Exclusion criteria: participants with a history of a condition that could affect their participation in the intervention (e.g. severe asthma, chronic airflow limitation, bronchiectasis or ankylosing spondylitis), and the following findings occurring in the postoperative phase: unstable cardiovascular status (systolic blood pressure < 100 and > 180 mmHg or mean arterial pressure < 60 or > 110 mmHg), arrhythmias compromising cardiovascular function, excessive blood loss (> 100 mL/h) or neurological complications Participants: 236 participants were randomized; 108 to the intervention group and 128 to the control group. 7 participants in the intervention group and 19 in the control group were withdrawn as they required mechanical ventilation > 24 h. Outcomes were reported for 101 (M:F 81:20, mean age 62.8 years) in the intervention group and 109 (M:F 77:32, mean age 63.9 years) in the control group. Baseline characteristics across groups were similar with the exception of the percentage of current smokers; 46/101 (46%) of those in the intervention group versus 14/109 (13%) of the control group continued to smoke pre‐admission. The most common diagnosis on ICU admission was coronary artery surgery. |
|
| Interventions |
Intervention group What (materials and procedures): specific equipment used during the interventions was not reported. Physiotherapy interventions included positioning, manual hyperinflation, endotracheal suctioning, thoracic expansion exercises and upper limb exercises. Who provided: a team of physiotherapists under the direct guidance of the principal investigator; study authors did not report reimbursement of trial personnel, their expertise/usual role, assessment of competence and components of training (if needed) to perform the interventions Where: surgical intensive care unit (ICU) in a major tertiary hospital in Perth, Australia When and how much: interventions were delivered during the intubated phase of the postoperative period; participants received a mean of 1.84 interventions Tailoring: interventions were not specifically standardised or controlled. Modifications: not reported Fidelity (strategies to improve): not reported Fidelity (extent): not reported Control group What (materials and procedures): nursed in the supine and then semi‐erect position; endotracheal suctioning as required Who provided: nursing staff Where: same as intervention group When and how much: not reported Tailoring: not reported Modifications: not reported Fidelity (strategies to improve): not reported Fidelity (extent): not reported |
|
| Outcomes |
*no information on the timing of this measurement was reported |
|
| Potential conflicts of interest | Study authors did not report if funding had been obtained during this study or if there were any conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to either the intervention or control group using a random number table in this study. |
| Allocation concealment (selection bias) | Low risk | Participants were randomized by an independent person and nursing and medical staff were blind to group allocation. |
| Blinding of participants and personnel (performance bias) All other outcomes | High risk | Physiotherapists delivering the intervention and participants receiving the intervention were not blinded. |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | The incidence of mortality would have been evaluated by personnel outside this study. |
| Blinding of outcome assessment (detection bias) All other outcomes | High risk | Measurement of all other outcomes was performed on a daily basis by the principal investigator who was not blinded to group allocation. |
| Incomplete outcome data (attrition bias) Mortality | Low risk | Mortality is an objective outcome and appeared to be measured on all participants in this study. |
| Incomplete outcome data (attrition bias) All other outcomes | High risk | Although study authors reported that participants withdrawn when mechanical ventilation exceeded 24 h would be included in the ITT analysis, outcomes were reported only for the remaining participants in the intervention and control groups. |
| Selective reporting (reporting bias) | Low risk | Study authors reported all outcomes specified in the methods section of the text. |
| Other bias | Low risk | |
Schweickert 2009.
| Methods | Multi‐centre, parallel‐group, RCT Trial dates: June 2005‐October 2007 Objective: to investigate the efficacy of combining daily interruption of sedation with physical and occupational therapy on functional and neuropsychiatric outcomes in patients receiving mechanical ventilation in intensive care compared to usual care. Randomization: in a 1:1 ratio using a computer‐generated, permuted block randomization scheme to either exercise and mobilization ‐ physical and occupational therapy (intervention group) or to standard care as ordered by the primary care team (the control group) Consent: study authors obtained written consent from participants or authorised representatives. |
|
| Participants |
Inclusion criteria: adults (≥ 18 years of age), mechanically ventilated for < 72 h and expected to continue for at least 24 h who met criteria for baseline functional independence (defined a priori as a Barthel Index score ≥ 70 obtained from a proxy describing patient function 2 weeks before admission) Exclusion criteria: rapidly developing neuromuscular disease, cardiopulmonary arrest, irreversible disorders with 6‐month mortality estimated at > 50%, raised intracranial pressure, absent limbs, or enrolment in another trial Participants: 104 participants were randomized; 49 (M:F 20:29, aged a median of 57.7 years) to the intervention group and 55 (M:F 32:23, aged a median of 54.4 years) to the control group. Baseline characteristics across groups were similar and the primary diagnosis on admission to the ICU was acute lung injury. Many participants developed sepsis ‐ 42/49 (86%) in the intervention group and 45/55 ((82%) in the control group. |
|
| Interventions |
Intervention group What (materials and procedures): specific equipment was not reported. Interventions included passive, active‐assisted and active ROM exercises, bed mobility activities, sitting balance exercises, ADL, exercises to promote increased independence with functional tasks, transfer training, pre‐gait exercises, and ambulation. Who provided: an occupational and a physical therapist; study authors did not report reimbursement of trial personnel, their expertise/usual role and assessment of competence/components of training (if needed) to perform the interventions Where: 2 medical ICUs at 2 medical centres in Chicago, IL and Iowa City, IA, USA When and how much: each morning within 48‐72 h of intubation (a median of 1.5 days after intubation) until return to previous level of function or discharge from the ICU. The median duration of the intervention during mechanical ventilation was 0.32 h per day and after extubation was a median of 0.21 h per day. Tailoring: interventions were synchronized with daily interruption of sedation or narcotics and progression of interventions depended on patient tolerance and stability. Interventions were discontinued or not initiated in the presence of signs of clinical instability. Participants received daily independent neurological assessments through the use of the Richmond agitation‐sedation scale (RASS) for level of arousal and the confusion assessment method (CAM) for the ICU for delirium and coma. Modifications: not reported Fidelity (strategies to improve): not reported Fidelity (extent): therapy occurred on 87% of the days on the study for all participants in the intervention group. Reasons for therapy interruption with participants included instability, inability to attend therapy and a change in the care goals to comfort measures only. Control group What: standard care as ordered by the primary care team (study authors confirmed via email that the interventions were based on the findings of a physical and occupational therapy assessment and on each participant's deficits) Who provided: physical and occupational therapists When and how much: participants usually received the intervention a median of 7.4 days after intubation (study authors confirmed via email that this was typically after extubation or tracheostomy). The median duration of the intervention during mechanical ventilation was 0 h per day and after extubation was a median of 0.19 h per day. Tailoring: interventions were synchronized with daily interruption of sedation or narcotics and progression of interventions depended on participant tolerance and stability. Modifications: not reported Fidelity (strategies to improve): not reported Fidelity (extent): therapy occurred on 95% of the days on the study for 22/55 (40%) of participants in the control group. Reasons for interruption of therapy were the same as those for the intervention group. |
|
| Outcomes |
|
|
| Potential conflicts of interest | Study authors reported that no funding was received for this study, and declared that there were no conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | At both sites, a computer‐generated, permuted block randomization scheme was used to allocate participants to each study group. |
| Allocation concealment (selection bias) | Low risk | Each assignment was designated in a consecutively numbered, sealed, opaque envelope by an investigator with no further involvement in the trial. |
| Blinding of participants and personnel (performance bias) All other outcomes | High risk | It was not possible to blind the physical and occupational therapists providing the interventions or the participants in this study. |
| Blinding of outcome assessment (detection bias) Mortality | Low risk | The incidence of mortality would have been evaluated by personnel outside this study. |
| Blinding of outcome assessment (detection bias) All other outcomes | Low risk | All other outcomes were assessed by physical and occupational therapists who were unaware of randomization assignment. Before each assessment, the participants and any visitors were instructed (via a structured introductory statement) not to discuss previous interventions. Furthermore, assessments occurred in the afternoon at a time distant from the morning therapy intervention. |
| Incomplete outcome data (attrition bias) Mortality | Low risk | Mortality is an objective outcome and appeared to be measured on all participants in this study. |
| Incomplete outcome data (attrition bias) All other outcomes | Low risk | Data were analysed by an ITT approach for 104 participants (total participants randomized to the intervention and control groups). Participants who died during the study were assigned scores of 0 for ventilator‐free days, strength testing (MRC examination and hand grip), ADL total, walk distance, and Barthel Index score. |
| Selective reporting (reporting bias) | Low risk | Study authors reported all outcomes specified in the methods section of the text. |
| Other bias | Unclear risk | Study authors defined the control condition as 'standard care with physical and occupational therapy delivered as ordered by the primary care team and explained that neither site designated a physical therapist to patients mechanically ventilated for < 2 weeks'. As the timing and components of care were not discussed and as elements of the intervention may have been delivered to the control group in this study, we feel there is an unclear risk of bias associated with this description of standard care. |
ADL: activities of daily living; APACHE: Acute Physiology and Chronic Health Evaluation; BMI: body mass index; CAM: confusion assessment method; DNI: do not intubate; DNR: do not resuscitate; ECG: electrocardiogram F: female; FiO2: fraction of inspired oxygen; FPI: Functional Performance Inventory; ICU: intensive care unit; IQR: interquartile range; LOS: length of stay; M: male; MMSE: Mini‐Mental State Examiniation; MO70: model 70; MRC: Medical Research Council; PHS: physical health summary; PaO2: partial pressure of oxygen; QoL: quality of life; RASS: Richmond Agitation‐Sedation Scale; RCT: randomized controlled trial; ROM: range of motion; SD: standard deviation; SF‐36: Short Form 36; SPPB: Short Physical Performance Battery
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brummel 2014 | While the control group may have received the intervention later than the intervention group, this was not incorporated into the study design. |
| Burtin 2009 | Comparators did not match those in this review |
| Chen 2012 | Usual care consisted of encouragement to exercise, which participants may or may not have done. |
| Chiang 2006 | There was no difference in the timing of the intervention between groups. |
| Collings 2015 | Comparators did not match those in this review |
| Denehy 2013 | There was no difference in the timing of mobilization between groups. |
| ISRCTN20436833 | There was no difference in the timing of the intervention between groups. |
| Morris 2008 | Not a RCT |
| Moss 2016 | Trial of intensity, not timing |
| Médrinal 2013 | Comparators did not match those in this review |
| Nava 1998 | There was no difference in the timing of the intervention between groups. |
| NCT01058421 | There was no difference in the timing of the intervention between groups. |
| Porta 2005 | Comparators did not match those in this review plus there was no difference in the timing of the intervention between groups. |
| Yosef‐Brauner 2015 | Comparators did not match those in this review |
RCT: randomized controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Dong 2014.
| Methods | Single‐centre, parallel‐group RCT Trial dates: May 2010‐May 2012 Objective: to assess the feasibility and effects of early rehabilitation therapy in mechanically ventilated patients in the ICU compared to routine care Randomization: Participants were randomly assigned in a 1:1 ratio to either the rehabilitation or control group ‐ further details were not reported. Consent: obtained from participants or their authorized representatives |
| Participants |
Inclusion criteria: age > 18 years, mechanically ventilated > 48 h but < 72 h with duration of expected mechanical ventilation ≥ 1 week, clear consciousness, cardiovascular and respiratory stability and absence of an unstable fracture Exclusion criteria: inability to independently perform functional activities, requiring long‐term mechanical ventilation, rapid development of neuromuscular disease, irreversible disorders with an estimated 6‐month mortality of > 50%, increased intracranial pressure, absent limbs, preadmission glucocorticoids applied for at least 20 days, ICU admission after cardiopulmonary resuscitation, tumour radiotherapy and chemotherapy within 6 months of admission and acute myocardial infarction or unstable ischaemia within 3 weeks of admission Participants: 60 participants were randomized; 30 (M:F 21:9, mean age of 55.3 years) to the intervention group and 30 (M:F 20:10, mean age 55.5 years) to the control group. Baseline characteristics across groups was similar and the primary diagnosis for both groups on admission to the ICU was acute respiratory distress syndrome. |
| Interventions |
Intervention group What (materials and procedures): specific equipment used during the interventions was not reported. Interventions included transfer from supine to edge of bed, sitting to standing, bed to chair and walking at the bedside. Who provided: 1 physician and 1 nurse; study authors did not report reimbursement of trial personnel, their expertise/usual role, assessment of competence and components of training (if needed) to perform the interventions. Where: ICU in a hospital in Qingdao, China When and how much: twice daily within 48‐72 h of tracheal intubation or tracheostomy until return to previous level of function or discharge from the ICU (discharge destination was confirmed via email communication with study authors) Tailoring: intervention training time and intensity was adjusted in response to participant status and progression of activities depended on participant tolerance and stability. While the interventions were occurring, each participant's position was changed every 2 h passively or actively. Where possible, sedation was given only at night but if needed during the day was ceased 1‐2 h before the intervention was delivered; the intervention was delivered once the participants could follow instructions. Enteral nutrition was stopped during the intervention and participants' oxygen saturation, ECG and blood pressure were monitored if necessary. Interventions were discontinued or not initiated in the presence of signs of clinical instability. Modifications: not reported Fidelity (strategies to improve): not reported Fidelity (extent): not reported Control group Study authors reported that participants in the control group received routine care and gave no further details. |
| Outcomes |
* no information on the timing of these measurements was reported |
| Notes |
Files 2013.
| Methods | Single‐centre RCT in the USA investigating the impact of rehabilitation on patients with acute respiratory failure in the ICU |
| Participants | 100 participants with acute respiratory failure were randomized to receive early rehabilitation or usual care. Participants were divided into two cohorts of 50 but study authors did not describe the allocation of participants within each group. |
| Interventions | Participants in cohort 1 received early rehabilitation once per day versus usual care and cohort 2 received early rehabilitation twice per day (with the second session including resistance training) versus usual care. Components of the intervention were not reported and usual care did not include early rehabilitation. Details regarding the number of participants receiving the intervention versus usual care in each cohort were not reported. |
| Outcomes |
|
| Notes | Elizabeth Chmelo (one of the authors of this study) was contacted by email to clarify its method and eligibility. However, she declined to provide more information as she hoped the study would be submitted for publication soon. |
Malicdem 2010.
| Methods | Single‐centre RCT conducted in an ICU in The Philippine Heart Centre, Quezon City, Philippines investigating the outcome of pulmonary rehabilitation on difficult‐to‐wean patients |
| Participants | 24 participants were randomized; 12 to the intervention group and 12 to the control group. |
| Interventions |
Intervention group: breathing exercises, cycle ergometry and upper body exercises Control group: usual care (further details are not known) |
| Outcomes |
|
| Notes | Searches failed to identify publications associated with this study and therefore we contacted study authors regarding publication of this study. We have not received a reply. |
Susa 2004.
| Methods | Single‐centre RCT conducted in a hospital in Trecenta, Italy to evaluate the feasibility and effectiveness of a multi‐faceted intensive rehabilitation program on participants post‐major colorectal surgery. This trial started in December 2000 and finished in December 2002. |
| Participants | 40 participants were randomized; 20 (M:F 14/6, mean age 66 years) to the intervention group and 20 (M:F 13:7, mean age 69 years) to the control group. |
| Interventions |
Intervention group: assistance with mobilization 24 to 48 h after surgery Control group: mobilized to a chair after the first 48 h |
| Outcomes |
The unit of measure is not known for these outcomes. |
| Notes | We contacted study authors regarding details about the participants and the intervention and we did not receive a response |
ADLs: activities of daily living; APACHE: Acute Physiology and Chronic Health Evaluation; F: female; FiO2: fraction of inspired oxygen; ICU: intensive care unit; M: male; PaO2: partial pressure of oxygen; SPPB: Short Physical Performance Battery
Characteristics of ongoing studies [ordered by study ID]
NCT01927510.
| Trial name or title | Pilot randomized controlled trial of early mobilisation in critically ill patients to improve functional recovery and quality of life |
| Methods | Multi‐centre, parallel‐group, pilot RCT conducted across five facilities in Australia and New Zealand to investigate the impact of early mobilization of critically ill patients in the ICU on functional recovery. |
| Participants | Study investigators plan to recruit 50 participants. Inclusion criteria: > 18 years old, admitted to the ICU, mechanically ventilated 48 h, written informed consent from next of kin or as per each individual ethics committee if delayed or telephone consent is unacceptable. Exclusion criteria: instability (cardiovascular or respiratory), acute brain injury, acute spinal cord injury, Guillain‐Barré Syndrome, second ICU admission during a single hospital admission, unable to follow simple verbal commands in English, death inevitable and imminent, inability to walk without assistance prior to onset of acute illness necessitating ICU admission, cognitive impairment prior to current acute illness, agitation which precludes safe implementation of intervention, written rest‐in‐bed orders due to documented injury or process that precludes mobilization, deemed unsafe to commence the intervention by treating clinician, has met all the inclusion criteria with no concomitant exclusion criteria for a period of more than 48 h |
| Interventions |
Intervention group: early mobilization Control group: standard care |
| Outcomes |
|
| Starting date | August 2013 |
| Contact information | Carol Hodgson, PhD Monash University |
| Notes | From ClinicalTrials.gov |
NCT01960868.
| Trial name or title | Early rehabilitation is feasible and safe in ICU in liver transplanted patients |
| Methods | Single‐centre, parallel‐group RCT being conducted in an ICU in Marseille, France to investigate the impact of early mobilization on length of stay and to validate the feasibility of this intervention with patients with liver transplantation |
| Participants | Study investigators plan to recruit 40 participants Inclusion criteria: age > 18 years, needing a liver transplant, informed consent provided Exclusion criteria: declining to consent, major contraindications to the intervention (paralysis neuromyopathy majeure), haemodynamic instability or severe infection, pregnancy, nursing mothers, persons deprived of their liberty by a judicial or administrative decision or under legal protection, urgent need for liver transplant |
| Interventions |
Intervention group: experimental physical therapy 5 days per week from 1 to several times per day Control group: standard physical therapy |
| Outcomes |
|
| Starting date | October 2013 |
| Contact information | Loic Mondoloni (loic.mondoloni@ap‐hm.fr) Assistance Publique Hopitaux De Maseille |
| Notes | We contacted the study author but did not receive a response. From ClinicalTrials.gov |
RBR‐6sz5dj.
| Trial name or title | Use of game therapy to assess functionality and upper limb muscle strength in critical patients |
| Methods | Single‐centre, parallel‐group RCT being conducted in an ICU in Curitiba, Brazil to investigate the impact of game therapy on function and upper limb muscle strength in critically ill patients |
| Participants | Study investigators planned to recruit 15 participants admitted to the ICU with loss of muscle strength in the upper limbs. Inclusion criteria: admitted to ICU, minimum age ≥ 16 years, Glasgow Coma Scale 15, handgrip dynamometry < normal for their age and sex, able to actively carry out the proposed activity, absence of thrombosis in the upper limbs, fractures, dislocations, muscle strains or ligament requiring the use of immobilizers in upper limbs, unable to mobilize the upper limbs, recent thoracotomy (< 40 days), absence of visual impairment (blindness); not pregnant, absence of upper‐limb amputation, absence of diagnosis of neuromuscular disease, trauma, spinal cord tumours or abscesses, hemiplegia/paresis, encephalopathy plexus injury or brain injury. Exclusion criteria: haemodynamically unstable (MAP < 60 mmHg or > 130 mmHg), refusal to do 1 or 2 daily sessions of the proposed activity, decreased level of consciousness, 30% worsening of dynamometry measure (when measured every 7 days) |
| Interventions |
Intervention group: 2 sets of 15 repetitions of active ROM exercises to the upper limbs using Gameterapia Nintendo® Wii game with a Samsung 26‐inch (66cm) screen and the game Wii Sports Tennis Control group: not reported |
| Outcomes | Hand grip strength (dynamometer), range of motion (goniometer), motivation (satisfaction survey) |
| Starting date | May 2011 |
| Contact information | Maira Maturana (mairamaturana@yahoo.com.br) Curitiba, Brazil |
| Notes | We contacted the study author and did not receive a response. From Registro Brasileiro de Ensaios Clinicos via the International Clinical Trials Registry Platform World Health Organization |
DNI: do not intubate; DNR: do not resuscitate; EQ5D: EuroQol 5 domains; ICU: intensive care unit; MAP: mean arterial pressure; MRC: Medical Research Council; QoL: quality of life: RCT: randomized controlled trial; ROM: range of motion
Differences between protocol and review
We made the following changes to the published protocol (Doiron 2013).
We changed the title from 'Early intervention (mobilization or active exercise) for critically ill patients in the intensive care unit' to 'Early intervention (mobilization or active exercise) for critically ill adults in the intensive care unit' because we only assessed studies investigating the adult population in this clinical setting.
We changed 'participants' to 'adults' in the objective section in the Abstract and the Objectives section in the review.
We expanded the initial search strategy because the first search (Appendix 8), did not return an expected study.
We removed the Acute Physiology and Chronic Health Evaluation (APACHE) score and SOFA score from the examples listed for the outcome Health‐related quality of life or well‐being (see Types of outcome measures and Data extraction and management), because they do not measure quality of life.
We clarified the definition of our primary outcome.
We removed length of stay in the ICU and hospital from the 'Summary of findings' table as they are not primary outcomes in this review (see Table 1).
We added the outcome 'duration of mechanical ventilation' to Table 9 (Other outcomes not specified in this review) because most included studies reported this outcome and we felt this to be of interest to clinicians.
We intended to conduct intention‐to‐treat (ITT) analysis and impute missing standard deviations but this was not required.
If we had done a meta‐analysis, we planned to use the I2 statistic (Higgins 2003) to measure heterogeneity in the participants, interventions and outcomes. As there were insufficient studies to do a meta‐analysis, we reported possible sources of heterogeneity descriptively.
We planned to investigate possible sources of heterogeneity such as age group, cause of ICU stay, length of mechanical ventilation, comorbidities such as diabetes and use of corticosteroids using subgroup analyses. However, as there were insufficient studies identified, we reported these factors descriptively.
Contributions of authors
Conceiving the review: Katherine A Doiron (KAD), Tammy C Hoffmann (TCH) Co‐ordinating the review: KAD Undertaking manual searches: KAD Screening search results: KAD, research assistant Organizing retrieval of papers: KAD Screening retrieved papers against inclusion criteria: KAD, research assistant Appraising quality of papers: KAD, Elaine M Beller (EMB), TCH Abstracting data from papers: KAD, EMB, research assistant Writing to authors of papers for additional information: KAD Providing additional data about papers: KAD Obtaining and screening data on unpublished studies: KAD Providing data management for the review: KAD, EMB Entering data into Review Manager 5 (RevMan 5) (RevMan 2014): KAD Entering RevMan 5 statistical data: EMB, KAD Performing other statistical analysis not using RevMan 5: not applicable Interpreting data: KAD, EMB Making statistical inferences: KAD, EMB Writing the review: KAD, TCH, EMB Securing funding for the review: no funding received Performing previous work that was the foundation of the present study: none Serving as guarantor for the review (one review author): KAD Taking responsibility for reading and checking the review before submission: EMB
Sources of support
Internal sources
No sources of support supplied, Other.
External sources
-
National Health and Medical Research Council (NHMRC), Australia.
Elaine M Beller's work on this review was supported by an Australia Fellowship Grant from the NHMRC, Australia, to the Centre for Evidence‐Based Practice, Bond University.
Declarations of interest
Katherine A Doiron: none known Tammy C Hoffmann: none known Elaine M Beller: work on this review was supported by an Australia Fellowship Grant from the National Health and Medical Research Council (NHMRC), Australia, to the Centre for Evidence‐Based Practice, Bond University.
Edited (no change to conclusions)
References
References to studies included in this review
Kayambu 2015 {published data only}
- Kayambu G, Boots R, Paratz J. Early physical rehabilitation in intensive care patients with sepsis syndromes: a pilot randomised controlled trial. Intensive Care Medicine 2015;41:865‐74. [PUBMED: 25851383] [DOI] [PubMed] [Google Scholar]
Morris 2016 {published data only}
- Morris PE, Berry MJ, Files DC, Thompson JC, Hauser J, Flores L, et al. Standardized rehabilitation and hospital length of stay among patients with acute respiratory failure: a randomized clinical trial. JAMA 2016;315(4):2694‐702. [PUBMED: 27367766] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patman 2001 {published data only}
- Patman S, Sanderson D, Blackmore M. Physiotherapy following cardiac surgery: is it necessary during the intubation period?. Australian Journal of Physiotherapy 2001;47:7‐16. [PUBMED: 11552858] [DOI] [PubMed] [Google Scholar]
Schweickert 2009 {published data only}
- Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CE, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009;373:1874‐82. [PUBMED: 19446324 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Brummel 2014 {published data only}
- Brummel NE, Girard TD, Ely EW, Pandharipande PP, Morandi A, Hughes CG, et al. Feasibility and safety of early combined cognitive and physical therapy for critically ill medical and surgical patients: the activity and cognitive therapy in ICU (ACT‐ICU) trial. Intensive Care Medicine 2014;40(3):370‐9. [PUBMED: 24257969] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burtin 2009 {published data only}
- Burtin C, Clerckx B, Robbeets C, Ferdinande P, Langer D, Troosters T, et al. Early exercise in critically ill patients enhances short‐term functional recovery. Critical Care Medicine 2009;37(9):2499‐505. [PUBMED: 19623052] [DOI] [PubMed] [Google Scholar]
Chen 2012 {published data only}
- Chen YH, Lin HL, Hsiao HF, Chou LT, Kao KC, Huang CC, et al. Effects of exercise training on pulmonary mechanics and functional status in patients with prolonged mechanical ventilation. Respiratory Care 2012;57(5):727‐34. [PUBMED: 22152978] [DOI] [PubMed] [Google Scholar]
Chiang 2006 {published data only}
- Chiang LL, Wang LY, Wu CP, Wu HD, Wu YT. Effects of physical training on functional status in patients with prolonged mechanical ventilation. Physical Therapy 2006;86(9):1271‐81. [PUBMED: 16959675] [DOI] [PubMed] [Google Scholar]
Collings 2015 {published data only}
- Collings N, Cusack R. A repeated measures, randomised cross‐over trial, comparing the acute exercise response between passive and active sitting in critically ill patients. BMC Anesthesiology 2015;15(1):1‐8. [PUBMED: 25670916] [DOI] [PMC free article] [PubMed] [Google Scholar]
Denehy 2013 {published data only}
- Denehy L, Skinner EH, Edbrooke L, Haines K, Warrillow S, Hawthorne G, et al. Exercise rehabilitation for patients with critical illness: a randomised controlled trial with 12 months of follow‐up. Critical Care 2013;17(4):1‐12. [PUBMED: 23883525] [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN20436833 {unpublished data only}
- ISRCTN20436833. Extra physiotherapy in critical care: intensive versus standard physical rehabilitation therapy in the critically ill. apps.who.int/trialsearch/default.aspx/ISRCTN20436833 (accessed 5 April 2015).
Médrinal 2013 {published data only}
- Médrinal C, Lebret M, Bousta M, Nassaj A, Colas G. Effects of sitting at the bedside of the patient with mechanical ventilation [Efffets de la station assise au bord du lit du patient intubé et ventilé]. Kinésithérapie, la Revue 2013;13(138):43‐9. [DOI: 10.1016/j.kine.2012.12.073] [DOI] [Google Scholar]
Morris 2008 {published data only}
- Morris PE, Goad A, Thompson C, Taylor K, Harry B, Passmore L, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Critical Care Medicine 2008;36(8):2238‐43. [PUBMED: 18596631] [DOI] [PubMed] [Google Scholar]
Moss 2016 {published data only}
- Moss M, Nordon‐Craft A, Malone D, Pelt D, Frankel SK, Warner ML, et al. A randomized trial of an intensive physical therapy program for patients with acute respiratory failure. American Journal of Respiratory and Critical Care Medicine 2016;193(10):1101‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nava 1998 {published data only}
- Nava S. Rehabilitation of patients admitted to a respiratory intensive care unit. Archives of Physical Medicine and Rehabilitation 1998;79(7):849‐54. [PUBMED: 9685104] [DOI] [PubMed] [Google Scholar]
NCT01058421 {unpublished data only}
- NCT01058421. Treatment of critical illness polyneuromyopathy. apps.who.int/trialsearch/default.aspx/NCT01058421 (accessed 5 April 2015).
Porta 2005 {published data only}
- Porta R, Vitacca M, Gilè LS, Clini E, Bianchi L, Zanotti E, et al. Supporting arm training in patients recently weaned from mechanical ventilation. Chest 2005;128(4):2511‐20. [PUBMED: 16236917] [DOI] [PubMed] [Google Scholar]
Yosef‐Brauner 2015 {published data only}
- Yosef‐Brauner O, Adi N, Ben Shahar T, Yehezkel E, Carmeli E. Effect of physical therapy on muscle strength, respiratory muscles and functional parameters in patients with intensive care unit‐acquired weakness. The Clinical Respiratory Journal 2015;9(1):1‐6. [PUBMED: 24345055] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Dong 2014 {published data only (unpublished sought but not used)}
- Dong Z, Yu B, Sun Y, Fang W, Li L. Effects of early rehabilitation therapy on patients with mechanical ventilation. World Journal of Emergency Medicine 2014;5(1):48‐52. [PUBMED: 25215147] [DOI] [PMC free article] [PubMed] [Google Scholar]
Files 2013 {published data only (unpublished sought but not used)}
- Files D, Morris P, Shrestha S, Dhar S, Young M, Hauser J, et al. Randomized, controlled pilot study of early rehabilitation strategies in acute respiratory failure. Critical Care 2013;17 (Suppl 2):540. [DOI: 10.1186/cc12478] [DOI] [Google Scholar]
Malicdem 2010 {published data only (unpublished sought but not used)}
- Malicdem MG, Cruz BO‐D, Punzal P, Guia T. Outcome of pulmonary rehabilitation among difficult to wean patients admitted at the Philippine Heart Center ‐ a randomized controlled study. Respirology 2010;15:99. [Google Scholar]
Susa 2004 {published data only (unpublished sought but not used)}
- Susa A, Roveran A, Bocchi A, Carrer S, Tartari S. FastTrack approach to major colorectal surgery [Approccio FastTrack alla chirurgia colorettale maggiore]. Chirurgia Italiana 2004;56(6):817‐24. [PubMed] [Google Scholar]
References to ongoing studies
NCT01927510 {published data only}
- NCT01927510. TEAM: a trial of early activity and mobility in ICU (TEAM‐RCT). clinicaltrials.gov/ct2/show/NCT01927510 first received 22 August 2013.
NCT01960868 {published data only}
- NCT01960868. Early rehabilitation program is feasible and safe in ICU in liver transplanted patients. clinicaltrials.gov/ct2/show/NCT01960868 first received 11 October 2013.
RBR‐6sz5dj {published data only}
- RBR‐6sz5dj. Use of de game therapy to assess functionality and upper limb muscle strength in critical patients. www.ensaiosclinicos.gov.br/rg/RBR‐6sz5dj/ first received 29 May 2012.
Additional references
Adler 2012
- Adler J, Malone D. Early mobilization in the intensive care unit: a systematic review. Cardiopulmonary Physical Therapy Journal 2012;23(1):5‐13. [PUBMED: 22807649] [PMC free article] [PubMed] [Google Scholar]
Ali 2008
- Ali NA, O'Brien JM, Hoffman SP, Phillips G, Garland A, Finley JC, et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. American Journal of Respiratory and Critical Care Medicine 2008;178(3):261‐8. [PUBMED: 18511703] [DOI] [PubMed] [Google Scholar]
Bailey 2007
- Bailey P, Thomsen GE, Spuhler VJ, Blair R, Jewkes J, Bezdjian L, et al. Early activity is feasible and safe in respiratory failure patients. Critical Care Medicine 2007;35(1):139‐45. [PUBMED: 17133183] [DOI] [PubMed] [Google Scholar]
Castro‐Avila 2015
- Castro‐Avila AC, Seron P, Fan E, Gaete M, Mickan S. Effect of early rehabilitation during intensive care unit stay on functional status: systematic review and meta‐analysis. PLoS One 2015;10(7):e0130722. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chlan 2010
- Chlan LL, Weinert CR, Skaar DJ, Tracy MF. Patient‐controlled sedation: a novel approach to sedation management for mechanically ventilated patients. Chest 2010;138(5):1045‐53. [PUBMED: 20299632] [DOI] [PubMed] [Google Scholar]
Choi 2008
- Choi J, Tasota FJ, Hoffman LA. Mobility interventions to improve outcomes in patients undergoing prolonged mechanical ventilation: a review of the literature. Biological Research for Nursing 2008;10(1):21‐33. [PUBMED: 18647758] [DOI] [PMC free article] [PubMed] [Google Scholar]
Connolly 2015
- Connolly B, Salisbury L, O'Neill B, Geneen L, Douiri A, Grocott MP, et al. Exercise rehabilitation following intensive care unit discharge for recovery from critical illness. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD008632.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Crapo 2002
- Crapo RO, Casaburi R, Coates AL, Enright PL, MacIntyre NR, McKay RT, et al. ATS statement: guidelines for the six‐minute walk test. American Journal of Respiratory and Critical Care Medicine 2002;166(1):111‐7. [PUBMED: 12091180] [DOI] [PubMed] [Google Scholar]
DeJonghe 2002
- DeJonghe B, Sharshar T, Lefaucheur JP, Authier FJ, Durand‐Zaleski I, Boussarsar M, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA 2002;288(22):2859‐67. [PUBMED: 12472328] [DOI] [PubMed] [Google Scholar]
Desai 2011
- Desai SV, Law TJ, Needham DM. Long‐term complications of critical care. Critical Care Medicine 2011;39(2):371‐9. [PUBMED: 20959786] [DOI] [PubMed] [Google Scholar]
Dos Santos 2016
- Dos Santos C, Hussain SN, Mathur S, Picard M, Herridge M, Correa J, et al MEND ICU Group, RECOVER Program Investigators, Canadian Critical CareTranslational Biology Group. Mechanisms of chronic muscle wasting and dysfunction after an Intensive Care Unit stay: a pilot study. American Journal of Respiratory and Critical Care Medicine 2016;194:821‐30. [DOI] [PubMed] [Google Scholar]
Fan 2014
- Fan E, Dowdy DW, Colantuoni E, Mendez‐Tellez PA, Sevransky JE, Shanholtz C, et al. Physical complications in acute lung injury survivors: a two‐year longitudinal prospective study. Critical Care Medicine 2014;42(4):849‐59. [PUBMED: 24247473] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gielen 2010
- Gielen S, Schuler G, Adams V. Cardiovascular effects of exercise training: molecular mechanisms. Circulation 2010;122:1221‐38. [PUBMED: 20855669] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version Accessed August 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Greve 2012
- Greve I, Vasilevskis EE, Egerod I, Bekker Mortensen C, Møller AM, Svenningsen H, et al. Interventions for preventing intensive care unit delirium. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD009783] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians. BMJ 2008;336:995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hermans 2014a
- Hermans G, Mechelen H, Clerckx B, Vanhullebusch T, Mesotten D, Wilmer A, et al. Acute outcomes and 1‐year mortality of intensive care unit–acquired weakness: a cohort study and propensity‐matched analysis. American Journal of Respiratory and Critical Care Medicine 2014;190(4):410‐20. [PUBMED: 24825371] [DOI] [PubMed] [Google Scholar]
Hermans 2014b
- Hermans G, Jonghe B, Bruyninckx F, Berghe G. Interventions for preventing critical illness polyneuropathy and critical illness myopathy. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD006832.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hermans 2015
- Hermans G, Berghe, G. Clinical review: intensive care unit acquired weakness. Critical Care 2015;19:274. [PUBMED: 26242743 ; PUBMED: 10.1186/s13054‐015‐0993‐7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Herridge 2005
- Herridge MS, Angus DC. Acute lung injury ‐ affecting many lives. New England Journal of Medicine 2005;353(16):1736‐8. [PUBMED: 16236746] [DOI] [PubMed] [Google Scholar]
Herridge 2011
- Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz‐Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. New England Journal of Medicine 2011;364(14):1293‐304. [PUBMED: 21470008] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003; Vol. 327:557‐60. [DOI] [PMC free article] [PubMed]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hodgson 2014
- Hodgson CL, Stiller K, Needham DM, Tipping CJ, Harrold M, Baldwin CE, et al. Expert consensus and recommendations on safety criteria for active mobilization of mechanically ventilated critically ill adults. Critical Care 2014;18(6):658. [PUBMED: 25475522] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348(g1687):1‐12. [DOI: 10.1136/bmj.g1687] [DOI] [PubMed] [Google Scholar]
Jolley 2014
- Jolley SE, Regan‐Baggs J, Dickson RP, Hough CL. Medical intensive care unit clinician attitudes and perceived barriers towards early mobilization of critically ill patients: a cross‐sectional survey study. BMC Anesthesiology 2014;14(84):1‐9. [PUBMED: 25309124 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kayambu 2013
- Kayambu G, Boots R, Paratz J. Physical therapy for the critically ill in the ICU: a systematic review and meta‐analysis. Critical Care Medicine 2013;41:1543‐54. [DOI] [PubMed] [Google Scholar]
Keith 1987
- Keith RA, Granger CV, Hamilton BB, Sherwin FS. The functional independence measure: a new tool for rehabilitation. Advances in Clinical Rehabilitation 1987;1:6‐18. [PUBMED: 3503663] [PubMed] [Google Scholar]
Koo 2011
- Koo KK, Choong K, Fan E. Prioritizing rehabilitation strategies in the care of the critically ill. Critical Care Rounds 2011;8(4):1‐7. [Google Scholar]
Kress 2000
- Kress JP, Pohlman AS, O'Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. New England Journal of Medicine 2000;342(20):1471‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Li 2013
- Li Z, Peng X, Zhu B, Zhang Y, Xi X. Active mobilization for mechanically ventilated patients: a systematic review. Archives of Physical Medicine and Rehabilitation 2013;94(3):551‐61. [PUBMED: 23127305] [DOI] [PubMed] [Google Scholar]
Mahoney 1965
- Mahoney Fl, Barthel DW. Functional evaluation: the Barthel Index. Maryland State Medical Journal 1965;14(2):61‐5. [PUBMED: 14258950] [PubMed] [Google Scholar]
Medical Research Council 1942
- Medical Research Council. Nerve Injuries Research Committee. Aids to examination of the peripheral nervous system. His Majesty's Stationery Office 1942:48 (iii).
Mehrholz 2015
- Mehrholz J, Pohl M, Kugler J, Burridge J, Mückel S, Elsner B. Physical rehabilitation for critical illness myopathy and neuropathy. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD010942.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Needham 2012
- Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, et al. Improving long‐term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Critical Care Medicine 2012;40(2):502‐9. [PUBMED: 21946660] [DOI] [PubMed] [Google Scholar]
Parry 2015a
- Parry SM, El‐Ansary D, Cartwright MS, Sarwal A, Berney S, Koopman R, et al. Ultrasonography in the intensive care setting can be used to detect changes in the quality and quantity of muscle and is related to muscle strength and function. Journal of Critical Care 2015;30(5):1151.e9‐14. [PUBMED: 26211979] [DOI] [PubMed] [Google Scholar]
Pinheiro 2012
- Pinheiro AR, Christofoletti G. Motor physical therapy in hospitalized patients in an intensive care unit: a systematic review [Fisioterupia motora em pacientes internados na unidade de terapia intensiva: uma revisão sistemática]. Revista Brasileira de Terapia Intensiva 2012;24(2):188‐96. [PUBMED: 23917769] [PubMed] [Google Scholar]
Portney 1993
- Portney L, Watkins M. Foundations of Clinical Research: Applications to Practice. Norwalk, Connecticut: Appleton and Lange, 1993. [Google Scholar]
Puthucheary 2013
- Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, et al. Acute skeletal muscle wasting in critical illness. JAMA 2013;310(15):1591‐600. [PUBMED: 24108501] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schultz 2002
- Schulz KF, Grimes DA. Blinding in randomised trials: hiding who got what. Lancet 2002;359(9307):696‐700. [PUBMED: 11879884] [DOI] [PubMed] [Google Scholar]
Skinner 2009
- Skinner EH, Berney S, Warrillow S, Denehy L. Development of a physical function outcome measure (PFIT) and a pilot exercise training protocol for use in intensive care. Critical Care and Resuscitation: Journal of the Australasian Academy of Critical Care Medicine 2009;11(2):110‐5. [PUBMED: 19485874] [PubMed] [Google Scholar]
Stevens 2007
- Stevens RD, Dowdy DW, Michaels RK, Mendez‐Tellez PA, Pronovost PJ, Needham DM. Neuromuscular dysfunction acquired in critical illness: a systematic review. Intensive Care Medicine 2007;33:1876‐91. [PUBMED: 17639340] [DOI] [PubMed] [Google Scholar]
Stiller 2013
- Stiller K. Physiotherapy in intensive care: an updated systematic review. Chest 2013;144(3):825‐47. [DOI: 10.1378/chest.12-2930; PUBMED: 23722822] [DOI] [PubMed] [Google Scholar]
Strøm 2011
- Strøm T, Stylsvig M, Toft P. Long‐term psychological effects of a no‐sedation protocol in critically ill patients. Critical Care 2011 [Epub ahead of print];15(6):R293. [PUBMED: 22166673] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2011
- Thomas A. Physiotherapy led early rehabilitation of the patient with critical illness. Physical Therapy Reviews 2011;16(1):46‐57. [DOI: 10.1179/1743288X10Y.0000000022] [DOI] [Google Scholar]
Tipping 2012
- Tipping CJ, Young PJ, Romero L, Saxena MK, Dulhunty J, Hodgson CL. A systematic review of measurements of physical function in critically ill adults. Critical Care and Resuscitation 2012;14(4):302‐11. [PUBMED: 23230880] [PubMed] [Google Scholar]
Turnbull 2016
- Turnbull AE, Rabiee A, Davis WE, Nasser MF, Venner VR, Lolitha R, et al. Outcome measurement in ICU survivorship research from 1970‐2013: a scoping review of 425 publications. Critical Care Medicine July 2016;44(7):1267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Dillen 1988
- Dillen LR, Roach KE. Reliability and validity of the Acute Care Index of Function for patients with neurologic impairment. Physical Therapy 1988;68(7):1098‐101. [PUBMED: 3387465] [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Concept framework and item selection. Medical Care 1992;30(6):473‐83. [PUBMED: 1593914] [PubMed] [Google Scholar]
References to other published versions of this review
Doiron 2013
- Doiron KA, Hoffmann T, Beller EM. Early intervention (mobilization or active exercise) for critically ill patients in the intensive care unit. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD010754] [DOI] [PMC free article] [PubMed] [Google Scholar]


