Abstract
Rationale:
Takotsubo syndrome (TTS) is a form of acute and usually reversible heart failure syndrome. Transient left ventricular dysfunction and electrocardiographic changes could mimic acute coronary syndrome but there are actually no obstructive coronary lesions.
Patient concerns:
A 76-year-old woman with chronic lung disease developed spontaneous tension pneumothorax with the presentation of severe dyspnea, respiratory failure, left ventricular dysfunction, and anterior wall ST-segment elevation on 12-lead electrocardiogram. Acute coronary syndrome was excluded by normal coronary angiograms.
Diagnosis:
The patient was diagnosed as tension pneumothorax complicated by TTS.
Interventions:
The woman underwent tubal thoracostomy for tension pneumothorax-induced obstructive shock. However, the patient further underwent ligation bullectomy for persistent air leakage 2 weeks later.
Outcomes:
The left ventricular dysfunction recovered 1 week after resolution of tension pneumothorax. Anterior wall ST-segment elevation resolved 25 days after admission.
Lessons:
Concurrent electrocardiograms and echocardiographic serial evaluations should be performed to provide more comprehensive information when dealing with tension pneumothorax patients.
Keywords: acute myocardial infarction, case report, stress cardiomyopathy, Takotsubo syndrome, tension pneumothorax
1. Introduction
Takotsubo syndrome (TTS) is a form of acute (but usually reversible) heart failure syndrome.[1] No obstructive coronary artery disease exists to explain the temporary left ventricular dysfunction and electrocardiographic ST-segment changes. Although the prognosis of TTS is comparable to that of age- and sex-matched patients with acute coronary syndrome (ACS), TTS related to physical rather than emotional stress has poorer outcomes as compared with ACS.[2] Consequently, the identification of patients with TTS is extremely important since the treatment strategies of TTS are totally different from those of ACS. We report herein a case of secondary TTS resulting from tension pneumothorax, indicating an interaction between reversible life-threatening thoracic and cardiac dysfunction. The patient has provided informed consent for publication of the case. The study was granted exemption from review by the Institutional Review Board of Chang Gung Foundation (application no: 201701100B0).
2. Case report
A 76-year-old woman with chronic obstructive pulmonary disease was referred to our hospital for persistent acute chest pain (duration approximately 16 hours) and associated dyspnea and diaphoresis. On arrival, her vital signs revealed a body temperature of 36.7°C, a heart rate of 117 beats/min, a respiration rate of 22 breaths/min and a blood pressure of 167/92 mm Hg. No cardiac murmur was detected and no other abnormalities were seen on physical examination. The hemogram was normal and serum biochemistry tests revealed a troponin I level of 0.258 ng/mL (reference, <0.3) and a brain natriuretic peptide level of 58.3 pg/mL (reference, <100). The 12-lead electrocardiogram showed sinus tachycardia and ST-segment elevation in the anterior precordial leads of V2-3 (Fig. 1A). Meanwhile, the chest X-ray showed hyperinflation bilaterally, consistent with chronic obstructive lung disease (Fig. 2A). The patient was intubated 80 minutes later due to acute hypoxemic respiratory failure (pulse oximeter oxygen saturation of 89%) and was put on intravenous dopamine for hypotension status post intubation (systolic blood pressure of 68 mm Hg). Acute coronary syndrome was impressed, and hence primary coronary angiography was arranged. The coronary arteriogram revealed 3 patent coronary arteries (Fig. 2B, C). Left ventriculography disclosed left ventricular systolic dysfunction with a left ventricular ejection fraction of 42% and hypokinetic middle to distal anteroseptal and inferoposterior walls and akinetic apex, which suggested TTS (Fig. 2D). The patient was admitted to the intensive care unit due to persistent hypotension with a systolic blood pressure of 49 mm Hg. Chest radiography and computed tomography (Fig. 2E) were arranged because of persistent hypoxemia and right tension pneumothorax was confirmed. The patient accordingly underwent tubal thoracostomy immediately (Fig. 2F) and resolution of hypoxemia and hypotension subsequently. A follow-up 12-lead electrocardiogram on day 2 showed sequential ST-T segment and T-wave changes, mimicking the changes of ACS (Fig. 1B). A diagnosis of tension pneumothorax complicated by possible TTS was made. Follow-up transthoracic echocardiography 7 days later showed a left ventricular ejection fraction of 77% without wall motion abnormalities. Extubation was performed successfully thereafter. Owing to persistent air leakage, the patient underwent ligation bullectomy 2 weeks later and was discharged uneventfully one month after admission. Follow-up 12-lead electrocardiograms showed poor R-wave progression in V1-3 without ST-segment changes on day 25 (Fig. 1C) and 5 months after discharge (Fig. 1D). The patient was still in a stable condition 2 years after discharge and showed no evidence of angina pectoris.
Figure 1.
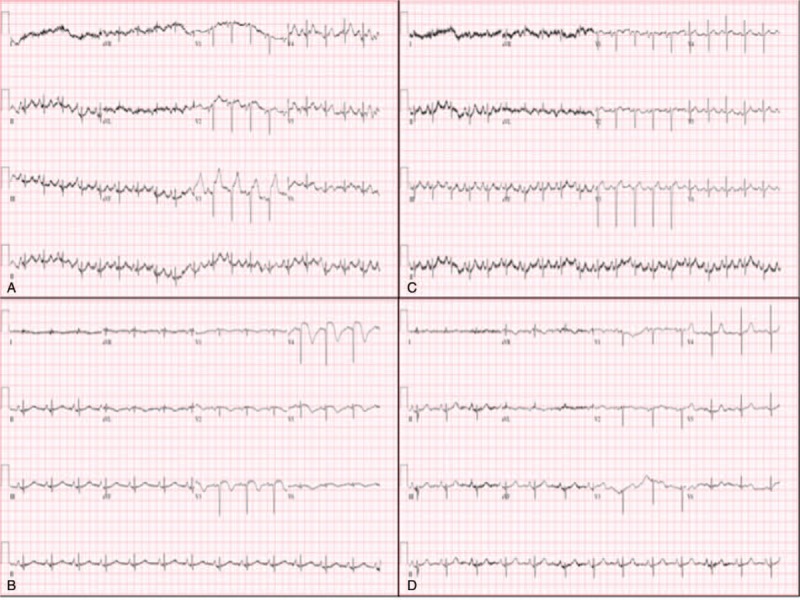
Serial electrocardiographic ST-segment and T-wave changes. Twelve-lead electrocardiogram at the emergency room showed ST-segment elevation in leads V2-3 and no reciprocal ST-segment depression (A). On the next day, persistent ST-segment elevation was observed in leads V3-4 with T-wave inversion in leads V1-6, I and aVL, resembling the sequential changes characteristic of acute coronary syndrome (B). On day 25, elevated ST-segment was apparent in lead V4 only and no more T-wave changes (C). No ST-segment elevation or T-wave changes were present 5 months after discharge (D).
Figure 2.
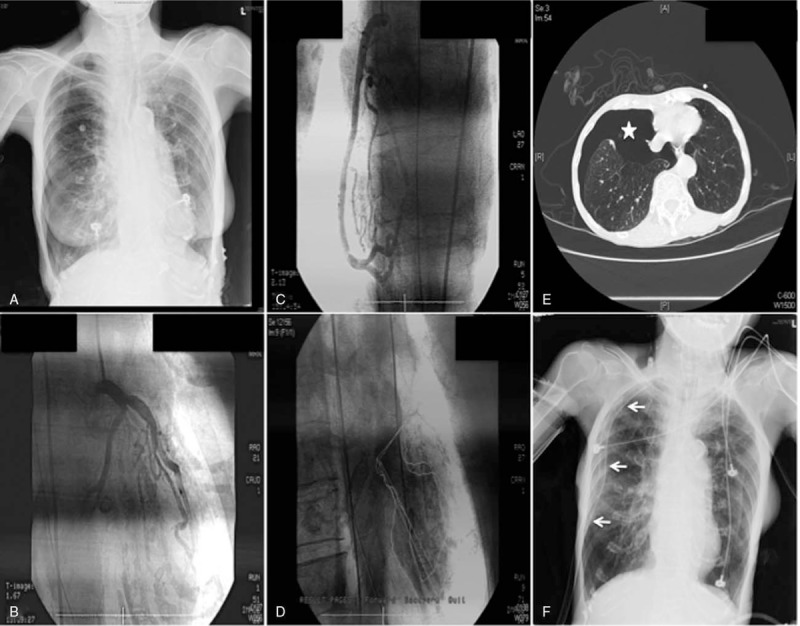
Chest radiograms, coronary angiograms, left ventriculogram, and chest computed tomogram. (A) First chest radiograph on arrival was ambiguous; (B) left coronary angiogram; (C) right coronary angiogram; (D) left ventriculogram; (E) right pneumothorax (asterisk) on chest computed tomogram; (F) chest radiograph after tubal thoracostomy (arrows).
3. Discussion
The term “takotsubo” as a sign of the disease was introduced in 1990, but was recognized as a disease entity long before 1990.[3–6] Sato et al[7] described TTS as stunned myocardium secondary to ischemia caused by multi-vessel coronary spasm, that is, post ischemic myocardial stunning. TTS, sometimes referred to as broken heart syndrome or stress cardiomyopathy, is increasingly recognized nowadays with greater access to urgent coronary angiography for patients with acute cardiac chest pain. It is characterized by a precipitating stressful event and is manifested as apical ballooning of the left ventricle with no obstructed coronary arteries. The left ventricular dysfunction and electrocardiographic abnormalities usually recover several weeks to months after the event onset. TTS occurs predominantly in postmenopausal women and 31% of patients with TTS have no identifiable trigger factor.[1,2] Based on current knowledge about TTS, the International Takotsubo Diagnostic Criteria (Inter TAK Diagnostic Criteria) was developed as: a transient left ventricular dysfunction beyond a single epicardial vascular distribution; an emotional, physical, or combined trigger may precede the TTS; neurologic disorder as well as pheochromocytoma may serve as triggers for TTS; new electrocardiographic abnormalities; elevation of cardiac troponin and brain natriuretic peptide; significant coronary artery disease is not a contradiction in TTS; absence of infectious myocarditis; and predominantly affect postmenopausal women.[8] Nevertheless, left ventriculography with coronary angiography is the gold standard diagnostic tool to confirm or exclude TTS.[8] A novel classification of TTS has been proposed based on the underlying trigger event to accurately risk stratify and predict outcomes for individual patients.[2] The case reported herein belongs to Class IIa, that is, TTS secondary to medical conditions (tension pneumothorax).
Several hypotheses have been proposed to explain the cardiac responses to severe emotional, physiological, or pathological stress.[1] These hypotheses can be broadly classified as vascular, myocardial, or coexistence of vascular and myocardial causes. However, there is no single proven pathophysiological mechanism to explain TTS. In fact, TTS can most likely be attributed to a synergistic combination of multiple factors since mechanistic studies have yielded conflicting results. Although obstructive shock is the most common pathological process of tension pneumothorax, there exist other undeterminant cardiac responses to tension pneumothorax in the present patient, including the relation between broken heart and broken lung.
The most common electrocardiographic finding of TTS is ST-segment elevation in the anterior leads. The associated symptoms include acute retrosternal chest pain, dyspnea and cold sweating. In the era of primary percutaneous coronary intervention, acute chest pain and ST-segment elevation would raise the highest concern of the critical care team members. In addition, electrocardiographic criteria have previously been used to differentiate ST-segment elevation myocardial infarction from TTS. In general, the absence of reciprocal ST depression in the inferior leads is a possible indication of TTS.[9] However, it is extremely challenging to verify true ST-segment elevation myocardial infarction and TTS in a patient's 1st medical contact. Consequently, the present patient was sent to the cardiac catheterization laboratory immediately, even though her electrocardiogram revealed anterior lead ST-segment elevation without ST depression in the opposite site. Intracoronary methylergonovine testing for excluding coronary vasospasm was not possible in the present case due to the unstable hemodynamic status of the patient. Nevertheless, this diagnostic strategy is the most important step of the TTS diagnosis algorithm since the formation of optimal treatment strategies depends on the outcomes of diagnostic coronary angiography and left ventriculography.[1]
The levels of cardiac troponin, B-type natriuretic peptide and N-terminal prohormone of brain natriuretic peptide could be elevated in TTS, suggesting myocardial necrosis and left ventricular dysfunction in TTS, respectively.[10,11] Some recent studies found that the circulating of certain microRNAs (miRNAs) is associated with TTS onset.[12,13] Jaguszewski and colleagues demonstrated that a signature of 4 circulating miRNAs comprising miR-1, miR-16, miR-26a, and miR-133a on admission as a robust biomarker to differentiating TTS from ST-segment elevation acute myocardial infarction.[13]
Echocardiography is the most important follow-up tool to confirm the diagnosis of TTS.[14] In addition to serial follow-up of left ventricular function and regional wall motion abnormalities, complication of TTS such as left ventricular outflow tract obstruction,[15] mitral regurgitation,[1] left ventricular free wall rupture,[16] and intracardiac thrombus[17] could be detected. Advanced echocardiographic modality such as myocardial deformation analysis could provide detailed information of myocardial contractility such as peak longitudinal strains in acute, subacute, and recovery stages.[18,19] In general, left ventricular contractility recovers completely within 4 to 8 weeks.[20] There are studies found that left ventricular contractility is not fully recovered after normalization of left ventricular ejection fraction.[21] Therefore, it is suggested that conventional echocardiography combined with myocardial deformation analysis should be used to assess cardiac function in TTS because the diagnosis needs serial evaluation.[22]
Some image studies could play important roles to exclude or confirm TTS. Cardiac computed tomography angiography could be a diagnostic alternative in patients who are in stable condition with low suspicion of ACS, suspected recurrent TTS, or associated with life-threatening comorbidity conditions, such as terminal cancer, advanced age with frailty, sepsis, intracranial disease, and bleeding tendency.[23] Though not routinely used in diagnosing TTS, cardiac magnetic resonance imaging criteria has been established that include typical regional wall motion abnormalities, edema, and the absence of late gadolinium enhancement (irreversible tissue injury).[24] The absence of late gadolinium enhancement help differentiate TTS from ACS and acute myocarditis. Therefore, cardiac magnetic resonance imaging provides more information in differential diagnosis and therapeutic plans in patients with suspected TTS.[23] Cardiac radionuclide imaging studies such as single photon emission computed tomography and position emission tomography have been used in TTS for evaluation of perfusion, metabolism, and innervation. These images have been used for research purpose to investigate the pathophysiology of TTS and their roles in clinical practice have not been established.[25]
In general, patients with TTS have long-term outcomes comparable to those of age- and sex-matched patients with ACS.[2] Although TTS is a reversible left ventricular dysfunction, hemodynamic and electrical instability make about one-fifth patients with TTS developing serious adverse in-hospital events.[10] Therefore, close monitoring and aggressive treatments are necessary in acute phase of TTS. Based on the largest TTS registry, death and major adverse cardiac and cerebrovascular event rates are estimated to be 5.6% and 9.9% per-patient year, respectively.[10] Furthermore, Sharkey et al found that 5% of the TTS survivors have the recurrent event, mostly occurring 3 weeks to 3.8 years after the 1st event.[26] Therefore, TTS is not a benign disease and patients with TTS needs long-term surveillance.
According to a literature review from PubMed, pneumothorax-complicated TTS has a favorable outcome after tubal thoracostomy and lung re-expansion.[27–31] The 1st chest radiograph in the present case did not disclose obvious pneumothorax, but deteriorated to shock status due to delayed diagnosis. Although the exact mechanisms in the patient remain elusive, it appears that TTS was induced by an increase in plasma catecholamines caused by pain and obstructive shock resulting from tension pneumothorax. Based on the limited experience provided by this case, it seems that pneumothorax-complicated TTS should be differentiated from ACS in the setting of postmenopausal females with a history of chronic lung disease, particularly when angiography does not reveal coronary obstructions.
4. Conclusion
Pneumothorax-complicated TTS may mimic ST-segment elevation ACS due to indistinguishable electrocardiographic findings and chief complaints. From the limited experience available in the present case, it is therefore suggested that concurrent electrocardiograms and echocardiographic serial evaluations should be performed to provide more information when dealing with tension pneumothorax patients.
Author contributions
Data curation: Wei-Siang Chen.
Investigation: Wei-Siang Chen, Ming-Jui Hung.
Methodology: Wei-Siang Chen, Ming-Jui Hung.
Project administration: Wei-Siang Chen.
Resources: Ming-Jui Hung.
Supervision: Ming-Jui Hung.
Writing – original draft: Wei-Siang Chen.
Writing – review & editing: Ming-Jui Hung.
Footnotes
Abbreviations: ACS = acute coronary syndrome, TTS = Takotsubo syndrome.
The authors have no conflicts of interest to disclose.
References
- [1].Lyon AR, Bossone E, Schneider B, et al. Current state of knowledge on Takotsubo syndrome: a position statement from the Taskforce on Takotsubo syndrome of the heart failure Association of the European Society of Cardiology. Eur J Heart Fail 2016;18:8–27. [DOI] [PubMed] [Google Scholar]
- [2].Ghadri JR, Kato K, Cammann VL, et al. Long-term prognosis of patients with Takotsubo syndrome. J Am Coll Cardiol 2018;72:874–82. [DOI] [PubMed] [Google Scholar]
- [3].Sato H, Tateishi H, Uchida T. Kodama K, Haze K, Hon M, et al. Takotsubo-type cardiomyopathy due to multivessel spasm. Clinical Aspect of Myocardial Injury: From Ischemia to Heart Failure. Tokyo: Kagakuhyouronsha; 1990. 56–64. [Google Scholar]
- [4].Parkes CM, Benjamin B, Fitzgerald RG. Broken heart: a statistical study of increased mortality among widowers. Br Med J 1969;1:740–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cebelin MS, Hirsch CS. Human stress cardiomyopathy. Myocardial lesions in victims of homicidal assaults without internal injuries. Hum Pathol 1980;11:123–32. [DOI] [PubMed] [Google Scholar]
- [6].Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 18-1986. A 44-year-old woman with substernal pain and pulmonary edema after severe emotional stress. N Engl J Med 1986;314:1240–7. [DOI] [PubMed] [Google Scholar]
- [7].Dote K, Sato H, Tateishi H, et al. Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases. J Cardiol 1991;21:203–14. [PubMed] [Google Scholar]
- [8].Ghadri JR, Wittstein IS, Prasad A, et al. International expert consensus document on Takotsubo syndrome (Part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J 2018;39:2032–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jim MH, Chan AO, Tsui PT, et al. A new ECG criterion to identify Takotsubo cardiomyopathy from anterior myocardial infarction: role of inferior leads. Heart Vessels 2009;24:124–30. [DOI] [PubMed] [Google Scholar]
- [10].Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med 2015;373:929–38. [DOI] [PubMed] [Google Scholar]
- [11].Nguyen TH, Neil CJ, Sverdlov AL, et al. N-terminal pro-brain natriuretic protein levels in Takotsubo cardiomyopathy. Am J Cardiol 2011;108:1316–21. [DOI] [PubMed] [Google Scholar]
- [12].Kuwabara Y, Ono K, Horie T, et al. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ Cardiovasc Genet 2011;4:446–54. [DOI] [PubMed] [Google Scholar]
- [13].Jaguszewski M, Osipova J, Ghadri JR, et al. A signature of circulating microRNAs differentiates Takotsubo cardiomyopathy from acute myocardial infarction. Eur Heart J 2014;35:999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Okura H. Echocardiographic assessment of Takotsubo cardiomyopathy: beyond apical ballooning. J Echocardiogr 2016;14:13–20. [DOI] [PubMed] [Google Scholar]
- [15].El Mahmoud R, Mansencal N, Pilliere R, et al. Prevalence and characteristics of left ventricular outflow tract obstruction in Tako-Tsubo syndrome. Am Heart J 2008;156:543–8. [DOI] [PubMed] [Google Scholar]
- [16].Ishida T, Yasu T, Arao K, et al. Images in cardiovascular medicine. Bedside diagnosis of cardiac rupture by contrast echocardiography. Circulation 2005;112:e354–5. [DOI] [PubMed] [Google Scholar]
- [17].Buchholz S, Ward MR, Bhindi R, et al. Cardiac thrombi in stress (Tako-Tsubo) cardiomyopathy: more than an apical issue? Mayo Clin Proc 2010;85:863–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kobayashi Y, Okura H, Kobayashi Y, et al. Left ventricular myocardial function assessed by three-dimensional speckle tracking echocardiography in Takotsubo cardiomyopathy. Echocardiography 2017;34:523–9. [DOI] [PubMed] [Google Scholar]
- [19].Dias A, Franco E, Rubio M, et al. Usefulness of left ventricular strain analysis in patients with Takotsubo syndrome during acute phase. Echocardiography 2018;35:179–83. [DOI] [PubMed] [Google Scholar]
- [20].Citro R, Rigo F, D’Andrea A, et al. Echocardiographic correlates of acute heart failure, cardiogenic shock, and in-hospital mortality in Tako-Tsubo cardiomyopathy. J Am Coll Cardiol Img 2014;7:119–29. [DOI] [PubMed] [Google Scholar]
- [21].Nowak R, Fijalkowska M, Gilis-Malinowska N, et al. Left ventricular function after Takotsubo is not fully recovered in long-term follow-up: a speckle tracking echocardiography study. Cardiol J 2017;24:57–64. [DOI] [PubMed] [Google Scholar]
- [22].Hung MJ. Role of echocardiography in Takotsubo cardiomyopathy. Int Cardiovasc Forum J 2016;5:70–1. [Google Scholar]
- [23].Ghadri JR, Wittstein IS, Prasad A, et al. International expert consensus document on Takotsubo syndrome (part II): diagnostic workup, outcome, and management. Eur Heart J 2018;39:2047–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (Takotsubo) cardiomyopathy. JAMA 2011;306:277–86. [DOI] [PubMed] [Google Scholar]
- [25].Manabe O, Naya M, Oyama-Manabe N, et al. The role of multimodality imaging in Takotsubo cardiomyopathy. J Nucl Cardiol 2018;doi: 10.1007/s12350-018-1312-x [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [26].Sharkey SW, Windenburg DC, Lesser JR, et al. Natural history and expansive clinical profile of stress (Tako-Tsubo) cardiomyopathy. J Am Coll Cardiol 2010;55:333–41. [DOI] [PubMed] [Google Scholar]
- [27].Akashi Y, Sakakibara M, Sasaki E, et al. “Takotsubo” cardiomyopathy with pneumothorax [in Japanese]. Nihon Naika Gakkai Zasshi 2001;90:2301–4. [DOI] [PubMed] [Google Scholar]
- [28].Akashi YJ, Sakakibara M, Miyake F. Reversible left ventricular dysfunction “Takotsubo” cardiomyopathy associated with pneumothorax. Heart 2002;87:E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kumar A, Padala S, Morales DC, et al. Broken lung and broken heart: a case of right pneumothorax resulting in Takotsubo cardiomyopathy. Conn Med 2013;77:99–102. [PubMed] [Google Scholar]
- [30].Gale M, Loarte P, Mirrer B, et al. Takotsubo cardiomyopathy in the setting of tension pneumothorax. Case Rep Crit Care 2015. 536931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ghanimeh MA, Bhardwaj B, Aly A, et al. Takotsubo cardiomyopathy secondary to spontaneous right-sided pneumothorax. BMJ Case Rep 2017;2017: [DOI] [PMC free article] [PubMed] [Google Scholar]


