Abstract
PURPOSE
The aim of this open-label, first-in-setting, randomized phase III trial was to evaluate the efficacy of alisertib, an investigational Aurora A kinase inhibitor, in patients with relapsed/refractory peripheral T-cell lymphoma (PTCL).
PATIENTS AND METHODS
Adult patients with relapsed/refractory PTCL—one or more prior therapy—were randomly assigned 1:1 to receive oral alisertib 50 mg two times per day (days 1 to 7; 21-day cycle) or investigator-selected single-agent comparator, including intravenous pralatrexate 30 mg/m2 (once per week for 6 weeks; 7-week cycle), or intravenous gemcitabine 1,000 mg/m2 or intravenous romidepsin 14 mg/m2 (days 1, 8, and 15; 28-day cycle). Tumor tissue (disease subtype) and imaging were assessed by independent central review. Primary outcomes were overall response rate and progression-free survival (PFS). Two interim analyses and one final analysis were planned.
RESULTS
Between May 2012 and October 2014, 271 patients were randomly assigned (alisertib, n = 138; comparator, n = 133). Enrollment was stopped early on the recommendation of the independent data monitoring committee as a result of the low probability of alisertib achieving PFS superiority with full enrollment. Centrally assessed overall response rate was 33% for alisertib and 45% for the comparator arm (odds ratio, 0.60; 95% CI, 0.33 to 1.08). Median PFS was 115 days for alisertib and 104 days for the comparator arm (hazard ratio, 0.87; 95% CI, 0.637 to 1.178). The most common adverse events were anemia (53% of alisertib-treated patients v 34% of comparator-treated patients) and neutropenia (47% v 31%, respectively). A lower percentage of patients who received alisertib (9%) compared with the comparator (14%) experienced events that led to study drug discontinuation. Of 26 on-study deaths, five were considered treatment related (alisertib, n = 3 of 11; comparator, n = 2 of 15). Two-year overall survival was 35% for each arm.
CONCLUSION
In patients with relapsed/refractory PTCL, alisertib was not statistically significantly superior to the comparator arm.
INTRODUCTION
Peripheral T-cell lymphoma (PTCL) is a rare, heterogeneous group of non-Hodgkin lymphomas that comprises more than 29 distinct histologic subtypes.1 The most common subtype, PTCL not otherwise specified (PTCL-NOS), represents approximately 25% of cases (varying across ethnic groups).2,3 Although there is no standard of care, most patients receive frontline anthracycline-based chemotherapy, such as cyclophosphamide, doxorubicin, vincristine, prednisone; cyclophosphamide, doxorubicin, vincristine, prednisone plus etoposide; or infusional cyclophosphamide, doxorubicin, vincristine, prednisone plus etoposide. Patients who develop relapsed/refractory PTCL typically experience a dismal outcome, with median progression-free survival (PFS) and overall survival (OS) after first post–systemic therapy relapse or progression of 3.1 and 5.5 months, respectively.4
At the time of protocol finalization (2011), pralatrexate (antifolate) and romidepsin (histone deacetylase inhibitor) were approved in relapsed/refractory PTCL by the US Food and Drug Administration; however, there was no globally approved therapy in this setting.5,6 Brentuximab vedotin, a CD30-directed antibody-drug conjugate, had also been approved by the US Food and Drug Administration but only for systemic anaplastic large-cell lymphoma after failure of one or more prior multiagent chemotherapy regimen.7 On the basis of the literature showing single-agent activity with small numbers of patients, PTCL expert input, and drug use information outside the United States, gemcitabine (antimetabolite) was selected as a third option for the comparator arm of the trial in addition to pralatrexate and romidepsin.8,9 Previously reported overall response rates (ORRs) in relapsed/refractory PTCL were 29% with pralatrexate6 and 26% with romidepsin.10 Duration of response (DOR) was more than 1 year with each agent. Both exhibited relatively short PFS but produced durable remissions with acceptable safety profiles; however, given the low response rates, well-tolerated and active agents are still needed in this setting.
Aurora A kinase (AAK) is essential for mitosis,11 and studies have demonstrated overexpression and upregulation of aurora kinases in PTCL,12-14 which supports AAK inhibition as a novel therapeutic strategy.11,15 Alisertib (MLN8237) is an investigational, selective, small-molecule AAK inhibitor that demonstrated activity in human tumor cell lines,16-18 preclinical models of T-cell and B-cell lymphoma,19 and in vivo lymphoma models.20 Phase I studies established the recommended single-agent phase II dose as 50 mg two times per day for 7 days in 21-day cycles.21,22 Subsequent phase II studies reported efficacy and tolerability of alisertib across a range of malignancies,14,23-25 including relapsed/refractory B-cell and T-cell lymphoma, with ORRs of 27% (50% for a cohort of eight patients with noncutaneous T-cell lymphoma)23 and 30%,14 respectively.
Lumiere is the first randomized phase III trial in patients with relapsed/refractory PTCL. It aimed to differentiate alisertib from other approved or commonly used drugs and to hasten potential broader approval for patients with relapsed/refractory PTCL.
PATIENTS AND METHODS
Study Design and Patients
This randomized, two-arm, open-label, phase III trial enrolled patients at 105 centers in 27 countries (Data Supplement). The protocol was approved by institutional review boards and/or ethics committees at all sites and was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guideline for Good Clinical Practice, including written informed consent and data monitoring.
This study had two primary objectives: to determine whether alisertib improved ORR (complete response [CR] plus partial response) and/or PFS versus comparator on the basis of independent review committee (IRC) assessment using International Working Group 2007 criteria.26 OS was the key secondary end point. Other secondary objectives included safety and tolerability; CR rate; time-to progression (TTP); time-to partial response or better (TTR); DOR; and time to subsequent antineoplastic therapy.
Eligible patients were age 18 years or older; had PTCL, including PTCL-NOS, anaplastic large-cell lymphoma, angioimmunoblastic T-cell lymphoma, enteropathy associated T-cell lymphoma, hepatosplenic T-cell lymphoma, subcutaneous panniculitis-like T-cell lymphoma, transformed mycosis fungoides, or extranodal natural killer/T-cell lymphoma nasal type; and developed relapsed or refractory disease after one or more prior systemic therapy. Eligibility criteria are described in the Data Supplement.
Random Assignment and Treatment
Enrollment was stratified by nodal versus extranodal disease, International Prognostic Index score (low/intermediate [0 to 2] v intermediate/high [3 to 5]), and region (North America and the European Union v rest of the world). In arm A, patients received oral alisertib 50 mg two times per day on days 1 to 7 of 21-day cycles. Patients in arm B received investigator’s choice of single-agent comparator intravenously: gemcitabine 1000 mg/m2 over 30 minutes on days 1, 8, and 15 of 28-day cycles; pralatrexate 30 mg/m2 over 3 to 5 minutes one time per week for 6 weeks in 7-week cycles; or (United States only) romidepsin 14 mg/m2 over 4 hours on days 1, 8, and 15 of 28-day cycles. Pralatraxate-treated patients received intramuscular vitamin B12 1 mg every 8 to 10 weeks and oral folic acid 1.0 to 1.25 mg per day. Cycles were repeated if patients continued to benefit from/tolerate therapy. Alisertib dose reductions—by one dose level or more, with minimum 10-mg decrements per cycle and a maximum of two reductions—were permitted in cases of drug-related toxicities. Dose reductions were allowed for comparators according to prescribing information.
Assessments
Adequate tumor tissue was required from all patients for disease subtyping by central review (Cleveland Clinic). Extent of disease was evaluated by International Working Group 2007 criteria.26 Imaging scans were submitted for central independent review (IRC; BioClinica). Response was assessed every 8 weeks until 10 months (week 40), and every 12 weeks thereafter, using computed tomography and fluorodeoxyglucose-positron emission tomography until disease progression. Patients were observed for survival every 4 months. Adverse events (AEs) were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03).
Statistical Analysis
The intention-to-treat (ITT) population—all randomly assigned patients—was used for analysis of PFS, OS, TTR, and TTP. The per-protocol population, which consisted of ITT patients without major protocol violations, was used for sensitivity analyses. The safety population included all patients who received one or more doses of the study drug. The response-evaluable population—all patients with an eligible PTCL subtype (centrally confirmed), with measurable disease at baseline, who received one or more doses of the drug and who had one or more postbaseline response assessments by IRC—was used for analysis of ORR, CR rate, and DOR.
The study used an adaptive sample size re-estimation approach and exhaustive fallback procedure for type I error control. Study recruitment was capped at 354 patients to obtain a maximum of 261 PFS events, assuming 46 months of accrual, 8 months of additional follow-up, and an approximate 12% dropout rate, to detect a difference in median PFS of 6 months in the comparator arm and 9 months in the alisertib arm (85% power; α = .0125). This sample size also enabled testing of the assumption that ORR for the comparator arm was 30% and 55% for the alisertib arm (80% power; α = .0125), as well as to detect a 28.6% reduction in hazard ratio (HR) for OS (80% power; α = .025). Additional details of sample size calculation are provided in the Data Supplement. The study was not powered to identify statistically significant differences between alisertib and individual comparator treatments or between any two comparators.
An independent data monitoring committee reviewed unblinded safety and efficacy data at two planned interim analyses (IAs) and an additional ad hoc IA (Data Supplement). After the ad hoc IA, the independent data monitoring committee recommended halting enrolment as a result of a low probability of achieving superiority of alisertib over comparators.
RESULTS
Patients
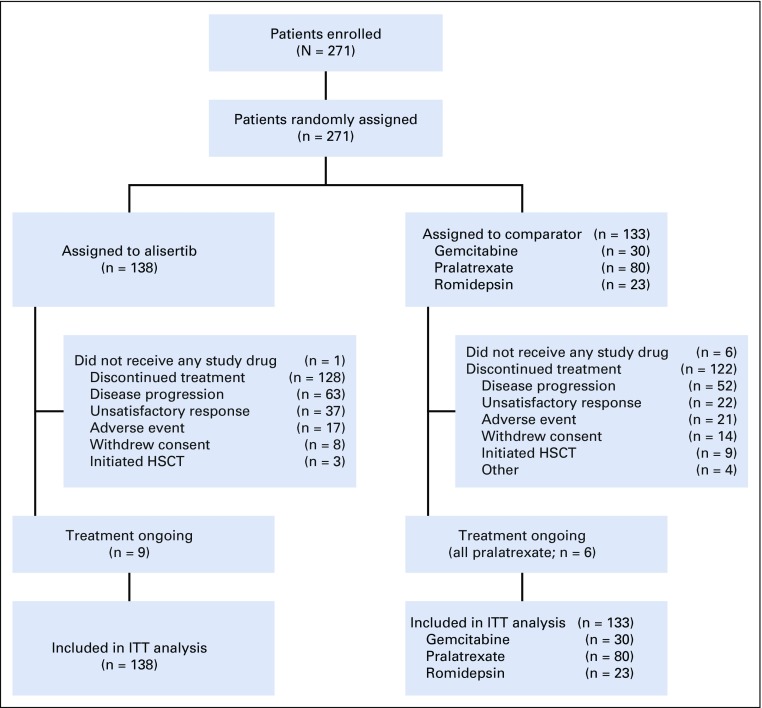
Between May 31, 2012, and October 20, 2014, 271 patients were enrolled and randomly assigned to receive alisertib (n = 138) or comparator (n = 133; gemcitabine, n = 30; pralatrexate, n = 80; romidepsin, n = 23). The safety population consisted of 264 patients (alisertib, n = 137; comparator n = 127). Seven patients had no eligible PTCL subtype and did not receive a study dose (alisertib, n = 1; gemcitabine, n = 1; pralatrexate, n = 4; romidepsin, n = 1; Fig 1).
FIG 1.
CONSORT diagram. HSCT, hematopoietic stem cell transplantation; ITT, intent to treat.
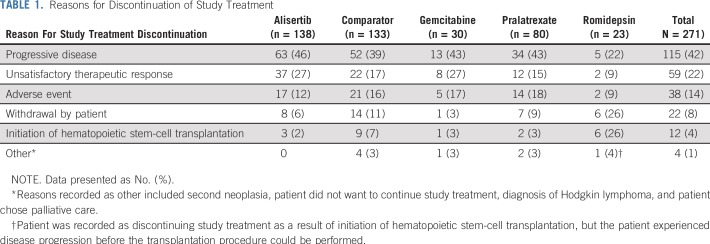
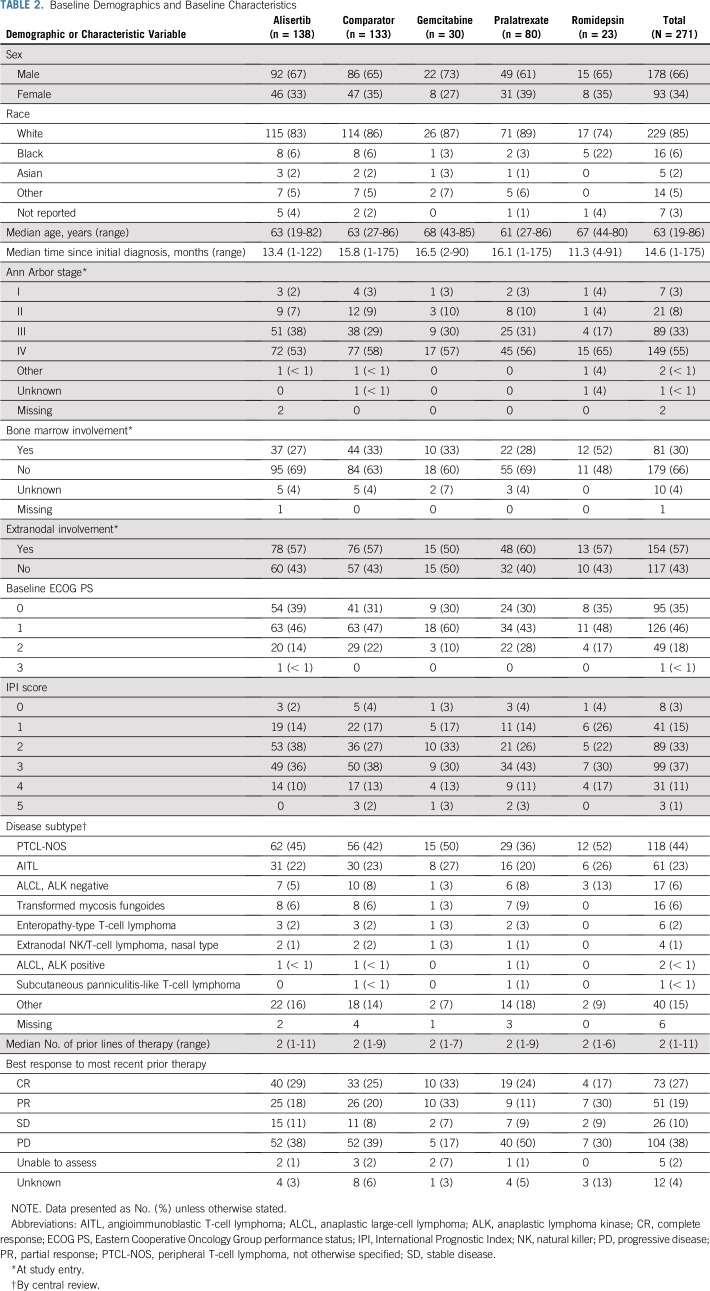
At final data cutoff (June 30, 2015), 15 patients were continuing treatment, (alisertib, n = 9; comparator, n = 6 [all pralatrexate]). Treatment discontinuation was mainly because of progressive disease (PD) reported for 63 (46%) alisertib-treated and 52 (39%) comparator-treated patients (gemcitabine, n = 13; pralatrexate, n = 34; romidepsin, n = 5; Table 1). Baseline demographics and disease characteristics were generally balanced across treatment arms (Table 2), including a median of two prior therapies.
TABLE 1.
Reasons for Discontinuation of Study Treatment
TABLE 2.
Baseline Demographics and Baseline Characteristics
Treatment Exposure
Alisertib-treated patients received a median of four (range, one to 50) 21-day treatment cycles, and 76 (55%) of 137 patients received four or more 4 cycles. Mean treatment duration was 20.8 weeks (range, 1 to 148 weeks). In the comparator arm, patients received a median of two (range, one to 17) cycles (28-day [gemcitabine and romidepsin] and 49-day [pralatrexate] cycles), and 38 (30%) of 127 patients received four or more cycles. Mean treatment duration was 16.6 weeks (range, 1 to 115 weeks). Treatment duration was 40 weeks or more for 24 patients (18%) on alisertib and 11 patients (9%) on comparators (all pralatrexate). Mean relative dose intensity was 92.9% for alisertib and 66.1% for comparators (gemcitabine, 73.5%; pralatrexate, 58.3%; romidepsin, 83.0%).
Efficacy
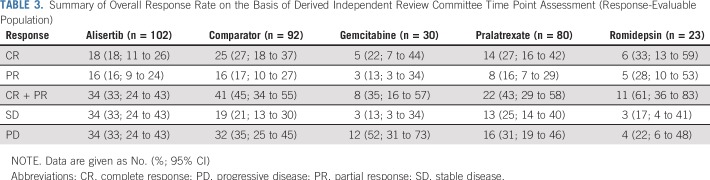
Central hematopathology confirmed only that 225 (83%) of 271 patients had an eligible PTCL subtype (discordance rate of 16% and 14% in alisertib and comparator arms, respectively). Forty-six patients who lacked PTCL or eligible PTCL subtype were excluded from the response-evaluable population. The Data Supplement provides additional details of diagnoses for these patients. An additional 31 patients were not response evaluable (did not receive one or more doses of the study drug and/or lacked postbaseline central response assessment). ORR was 33% (n = 34 of 102 patients) with alisertib versus 45% (n = 41 of 92 patients) with comparators (odds ratio, 0.60; 95% CI, 0.33 to 1.08; Table 3). CR rate with alisertib and comparators was 18% and 27%, respectively. ORR was 35% for gemcitabine (n = eight of 23 patients), 43% for pralatrexate (n = 22 of 51 patients), and 61% for romidepsin (n = 11 of 18 patients). ORR by PTCL subgroup with alisertib versus comparators was 37% versus 47% in PTCL-NOS, 28% versus 46% in angioimmunoblastic T-cell lymphoma, and 32% versus 38% in other eligible PTCL subtypes (patient numbers for the remaining individual subtypes were too low to assess separately for differences in ORR), respectively. The study was not powered to demonstrate significant response differences between comparator treatments; however, data are shown for ORR and PFS rates by the three major disease subtypes in the Data Supplement. Whereas the numbers of patients in the alisertib and comparator arms were fairly balanced across regions, there was a greater difference in ORR between treatment arms in North America (29% v 59% for alisertib v comparator) than in other regions (Western Europe: 33% v 36%; rest of world: 36% v 41%, respectively), possibly because romidepsin was available only in the United States (18 response-evaluable patients).
TABLE 3.
Summary of Overall Response Rate on the Basis of Derived Independent Review Committee Time Point Assessment (Response-Evaluable Population)
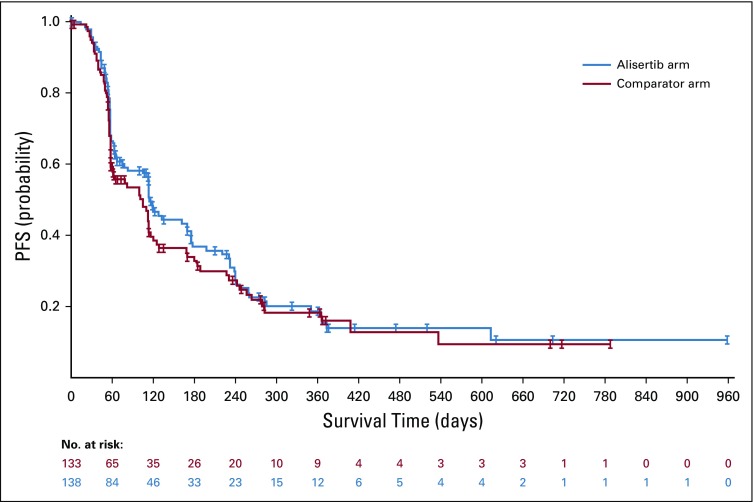
PD (57% v 47%) was a more common reason than death (11% v 18%) for a PFS event in both treatment arms. Median PFS was 115 versus 104 days for alisertib versus comparators, respectively (HR, 0.87; 95% CI, 0.644 to 1.162; Fig 2). Start of alternate treatment by the investigator before documented PD led to censoring of 21% and 17% of patients on alisertib and comparators, respectively. Median PFS was 57 days (gemcitabine), 101 days (pralatrexate), and 242 days (romidepsin). On the basis of sensitivity analyses (per protocol population), median PFS was 120 days versus 104 days for alisertib versus comparators, respectively (HR, 0.82; 95% CI, 0.593 to 1.136; Data Supplement), and 58 days, 99 days, and 242 days for gemcitabine, pralatrexate, and romidepsin, respectively.
FIG 2.
Progression-free survival (PFS) on the basis of derived independent review committee time point assessment (intent-to-treat population). Median PFS was 115 days for the alisertib arm versus 104 days for the comparator arm (hazard ratio, 0.87; 95% CI, 0.637 to 1.178).
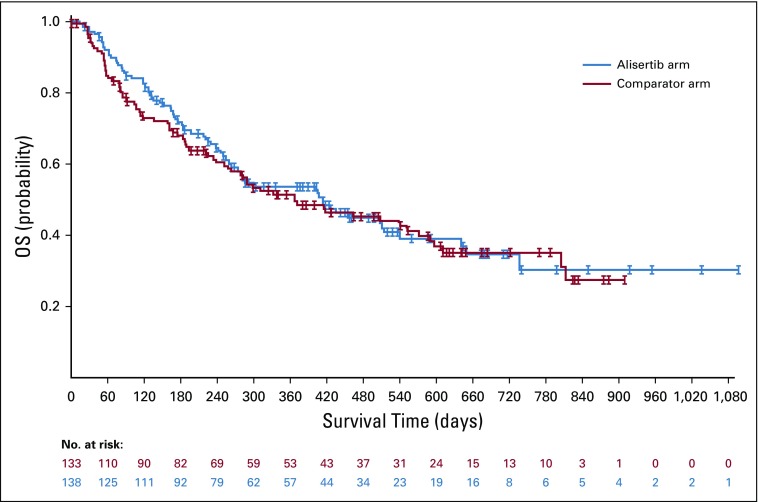
Median follow-up duration for OS was 519 days with alisertib and 586 days with comparators (75 deaths in each arm). Median OS was 415 days (13.7 months) with alisertib and 367 days (12.1 months) with comparators (HR, 0.98; 95% CI, 0.707 to 1.369; Fig 3). Twelve-month survival was 53.7% with alisertib and 51.5% with comparators, and 24-month survival was 35% in both arms. More mature survival data (62% deaths in each arm) were obtained in January 2016. Results were similar to the earlier data cutoff, with a median OS of 14.0 months versus 12.1 months for alisertib versus comparators (HR, 0.98; 95% CI, 0.698 to 1.373).
FIG 3.
Overall survival (OS; intent-to-treat population). Median OS was 415 days in the alisertib arm versus 367 days in the comparator arm (hazard ratio, 0.98; 95% CI, 0.707 to 1.369).
Median DOR was 225 days with alisertib versus 172 days with comparators (gemcitabine, 134 days; pralatrexate, 162 days; romidepsin, 473 days). Median TTR was 62 days for alisertib and 64 days for comparators (gemcitabine, 90 days; pralatrexate, 67 days; romidepsin, 61 days). Median TTP was 162 days with alisertib versus 116 days with comparators (HR, 0.95; 95% CI, 0.679 to 1.329). At 24 months, 12.8% of alisertib-treated patients and 14.3% on comparator drugs had not experienced disease progression. Median time to subsequent antineoplastic therapy was 336 days with alisertib and 233 days with comparators (gemcitabine, 144 days; pralatrexate, 233 days; romidepsin, not estimable; HR, 0.98; 95% CI, 0.692 to 1.385).
Safety
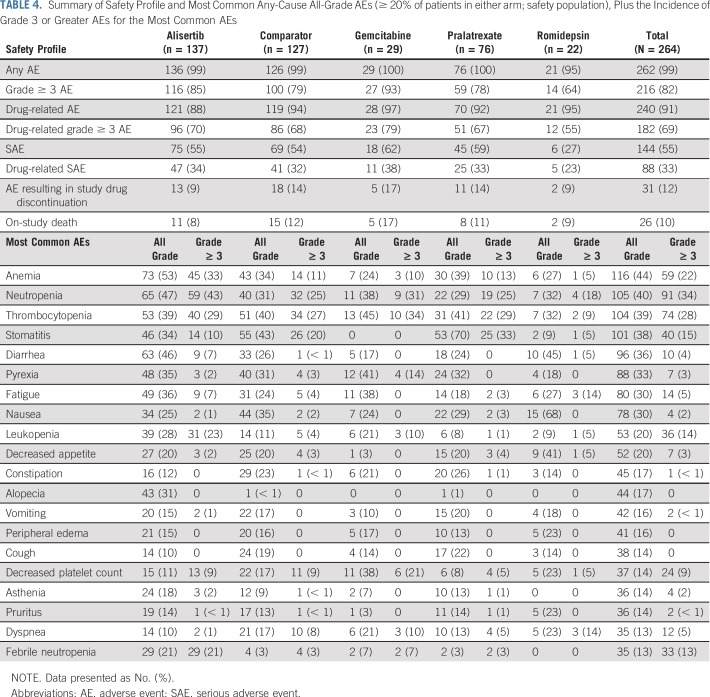
Almost all patients experienced one or more AE (alisertib, n = 136 [99%] of 137 patients; comparators, n = 126 [99%] of 127 patients); 88% and 94% of patients in the alisertib and comparator arms, respectively, experienced one or more treatment-related AE (Table 4). The most common all-cause grade 3 or greater AEs (alisertib v comparators) were neutropenia (43% v 25%, respectively), thrombocytopenia (29% v 27%, respectively,), and anemia (33% v 11%, respectively; Table 4), whereas the most common drug-related AEs included neutropenia (45% v 30%, respectively), stomatitis (31% v 42%, respectively), thrombocytopenia (34% v 38%, respectively), anemia (43% v 24%, respectively,), and diarrhea (32% v 19%, respectively; Data Supplement). In both arms, most patients experienced grade 3 or greater AEs (alisertib, n = 116 [85%] of 137 patients; comparators, n = 100 [79%] of 127 patients); 70% and 68% of patients, respectively, experienced drug-related grade 3 or greater AEs (Data Supplement), predominantly hematologic in nature—for example, anemia, leukopenia, neutropenia, and febrile neutropenia—with alisertib. Of note, only alisertib-treated patients reported drug-related alopecia (Data Supplement).
TABLE 4.
Summary of Safety Profile and Most Common Any-Cause All-Grade AEs (≥ 20% of patients in either arm; safety population), Plus the Incidence of Grade 3 or Greater AEs for the Most Common AEs
Thirteen alisertib-treated patients (9%) and 18 comparators-treated patients (14%) experienced AEs that resulted in study drug discontinuation. Drug-related AEs were acute generalized exanthematous pustulosis, asthenia, febrile neutropenia, plasma cell myeloma, and thrombocytopenia (all n = 1) with alisertib, and neutropenia, stomatitis, thrombocytopenia (all n = 2), adverse drug reaction, anaphylactoid reaction, granulocytopenia, leukopenia, mouth hemorrhage, and thrombosis (all n = 1) with comparators. Thirty-nine alisertib-treated patients (28%) and 42 comparator-treated patients (33%) experienced at least one AE-related dose reduction (gemcitabine, n = 16 [55%]; pralatrexate, n = 24 [32%]; romidepsin, n = 2 [9%]). Dose reduction was mostly required in cycle 1 for alisertib and cycle 1 or 2 for comparators. In addition, 13% of alisertib-treated patients and 57% of comparator-treated patients had their dose held as a result of an AE (gemcitabine, n = 14 [48%]; pralatrexate, n = 51 [67%]; romidepsin, n = 8 [36%]), and 36% and 19% of alisertib-treated and comparator-treated patients, respectively, had an AE-related dose delay.
Serious AEs occurred in 55% of patients on alisertib and 54% on comparators—febrile neutropenia (17% v 2%, respectively), pyrexia (9% v 10%, respectively), pneumonia (6% v 2%, respectively), stomatitis (5% v 9%, respectively), thrombocytopenia (5% v 4%, respectively), and anemia (5% v 2%, respectively) were the most common serious AEs with alisertib. Overall, 26 on-study deaths were recorded (alisertib, n = 11 [8%]; and comparator, n = 15 [12%; gemcitabine, n = 5; pralatrexate, n = 8; romidepsin, n = 2]; Table 4). Of these, three (septic shock, n = 2; pneumonia, n = 1) in the alisertib group, one (adverse drug reaction) in the gemcitabine group, one (multiorgan failure) in the pralatrexate group, and none in the romidepsin group were considered drug related.
DISCUSSION
This phase III, two-arm, open-label study was the first randomized trial in relapsed/refractory PTCL at the time it was conducted. Single-agent alisertib demonstrated activity in patients with relapsed/refractory PTCL but was not superior to comparators in terms of ORR or PFS. The study was terminated after the ad hoc IA as a result of a low probability of demonstrating superiority of alisertib over comparator. Alisertib was administered at 50 mg two times per day for 7 consecutive days in 21-day cycles (the maximum tolerated dose on this dosing schedule, providing pharmacologically active exposures evidenced by pharmacokinetic and tumor pharmacodynamic evaluations performed in early clinical development).27,28 As such, this phase III trial evaluated a dose and schedule of alisertib that is maximally tolerated and expected to achieve maximal tumor pharmacodynamic effects during multiple dose administrations to provide a robust test of the therapeutic hypothesis for AAK inhibition in PTCL.
ORR with alisertib (33%) is consistent with phase II studies in relapsed/refractory PTCL (ORR, 30%)14 and relapsed/refractory non-Hodgkin lymphomas (ORR, 27%).23 ORR with comparators (45%) was influenced by high ORR in patients on romidepsin (61%) and pralatrexate (43%) compared with gemcitabine (35%). Other studies in relapsed/refractory PTCL have demonstrated ORRs of 25% and 38% with romidepsin, and 31% with pralatrexate.10,29,30
Median PFS was numerically longer in alisertib-treated versus comparator-treated ITT patients (115 v 104 days, respectively). Although the ITT population included patients with ineligible PTCL disease subtypes, sensitivity analyses using the per-protocol population revealed similar results (median PFS, 120 days [alisertib] v 104 days [comparator]). The 0.87 HR that favored the alisertib arm, in which more patients had stable disease, indicated that alisertib actively controlled disease in patients with PTCL. A positive trend that favored alisertib was also observed for OS, DOR, and TTP, which further supported alisertib activity in this population. Median OS for alisertib (13.7 months) suggests that incorporating novel agents into treatment plans could improve outcomes in patients with PTCL, as their reported median OS was 5.5 months at first relapse before the introduction of novel agents.4
Nearly all patients on alisertib and comparators experienced one or more AE, and 88% and 94%, respectively, experienced drug-related AEs. Mean relative dose intensity for comparators was 66.1% (92.9% for alisertib), which means that more comparator-treated patients required AE-related dose modifications—33% of patients on comparators underwent one or more dose reductions (28% with alisertib). Taken together, these findings suggest that alisertib was better tolerated than comparators. Common alisertib-related toxicities were hematologic and GI, which is consistent with other single-agent alisertib studies,14,23-25 but manageable with dose reduction. One limitation of this study was that patients with a range of local diagnosis–based PTCL subtypes were enrolled, but 46 patients (17%) were subsequently identified by central expert review as lacking a protocol-eligible PTCL subtype—that is, no PTCL or inadequate tumor specimen. Therefore, the response-evaluable population was smaller than the safety population. Future studies should focus on selecting appropriate patient populations or confirming disease subtype before dosing while acknowledging that rapidly progressing diseases require prompt treatment initiation. Additional challenges in this trial included ensuring that investigators observed patients with required imaging to PD after the end of treatment and documented alternative therapy. This international phase III trial only enrolled patients in regions where the recommended dose of single-agent alisertib was 50 mg two times per day for 7 days in 21-day cycles.31 Previous studies in East Asian patients have identified a higher bioavailability of alisertib in these populations, translating to a lower alisertib dose of 30 mg two times per day.22,32 Thus, the design of future Asia-inclusive global clinical trials of alisertib will need to incorporate exposure-matched regional dosing with lower alisertib doses for East Asian patients.33
Although alisertib did not demonstrate superior efficacy over comparators, it showed activity and acceptable tolerability and safety in patients with relapsed/refractory PTCL, as well as positive trends in sensitivity analyses for PFS, OS, DOR, and TTP. The study was not powered for comparison with single-agent comparators; however, alisertib resulted in a similar ORR and numerically longer PFS versus those of gemcitabine while having the potential benefits of an oral administration route rather than an intravenous delivery. Additional studies are required to investigate whether alisertib provides greater benefit in a subgroup of patients with PTCL who responded poorly to comparator agents and the potential for treatment combinations of alisertib with novel agents.
ACKNOWLEDGMENT
The authors thank the patients who participated in this study and their families, as well as the Lumiere investigators and staff at all clinical sites. The authors also thank Prometrika (Cambridge, MA), who managed the independent data monitoring committee that reviewed safety and efficacy data at the interim analyses. In addition, the authors acknowledge medical writing support from Yosef Mansour and Dawn Lee (FireKite, an Ashfield company, part of UDG Healthcare), which was funded by Millennium Pharmaceuticals (Cambridge, MA), a wholly owned subsidiary of Takeda Pharmaceuticals, in compliance with Good Publication Practice 3 ethical guidelines (Battisti et al: Ann Intern Med 163:461-464, 2015).
Takeda Pharmaceuticals makes patient-level, de-identified data sets and associated documents available after applicable marketing approvals and commercial availability have been received, an opportunity for the primary publication of the research has been allowed, and other criteria have been met as set forth in Takeda’s Data Sharing Policy (see https://www.takedaclinicaltrials.com/ for details). To obtain access, researchers must submit a legitimate academic research proposal for adjudication by an independent review panel, who will review the scientific merit of the research and the requestor’s qualifications and conflict of interest that can result in potential bias. Once approved, qualified researchers who sign a data sharing agreement are provided access to these data in a secure research environment.
Footnotes
Presented at the 2012 American Society of Clinical Oncology Annual Meeting, Chicago, IL, USA, June 1-5, 2012; the Congresso Brasileiro de Hematologia, Hemoterapia e Terapia Celular, Rio de Janeiro, Brazil, November 8-11, 2012; the T-cell Lymphoma Forum, San Francisco, CA, January 24-26, 2013; the Pan Pacific Lymphoma Conference, Kohala Coast, HI, July 21-25, 2014; the 39th Congress of the European Society for Medical Oncology, Madrid, Spain, September 26-30, 2014; and the American Society of Hematology 57th Annual Meeting, Orlando, FL, December 5-8, 2015.
Funded by Millennium Pharmaceuticals, Cambridge, MA, a wholly owned subsidiary of Takeda Pharmaceutical Company Limited.
Clinical trial information: NCT01482962.
AUTHOR CONTRIBUTIONS
Conception design: Owen A. O’Connor, Eric D. Jacobsen, Francesco A. d’Amore, John P. Leonard, Hua Liu, Claudio Dansky Ullmann, Karthik Venkatakrishnan, E. Jane Leonard
Provision of study materials or patients: Owen A. O’Connor, Josep M. Roncero, Judith Trotman, Tamás Masszi, Juliana Pereira, Radhakrishnan Ramchandren, Dolores Caballero, Anne Lennard, Mehmet Turgut, Nelson Hamerschlak, Francesco A. d’Amore, Won-Seog Kim, Carlos S. Chiattone, Lorenz Trümper, Andrei R. Shustov
Collection and assembly of data: Owen A. O’Connor, Muhit Özcan, Josep M. Roncero, Judith Trotman, Judit Demeter, Tamás Masszi, Juliana Pereira, Radhakrishnan Ramchandren, Anne Beaven, Steven M. Horwitz, Anne Lennard, Nelson Hamerschlak, Francesco A. d’Amore, Francine Foss, Won-Seog Kim, Pier Luigi Zinzani, Carlos S. Chiattone, Eric D. His, Lorenz Trümper, Hua Liu, Claudio Dansky Ullmann, E. Jane Leonard, Andrei R. Shustov
Data analysis and interpretation: Owen A. O’Connor, Eric D. Jacobsen, Judit Demeter, Juliana Pereira, Radhakrishnan Ramchandren, Dolores Caballero, Steven M. Horwitz, Anne Lennard, Mehmet Turgut, Nelson Hamerschlak, Francine Foss, John P. Leonard, Eric D. Hsi, Lorenz Trümper, Hua Liu, Emily Sheldon-Waniga, Claudio Dansky Ullmann, Karthik Venkatakrishnan, Andrei R. Shustov
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Phase III Study of Alisertib or Investigator’s Choice (Selected Single Agent) in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Owen A. O’Connor
Honoraria: Mundipharma, Millennium Pharmaceuticals, Seattle Genetics
Consulting or Advisory Role: Mundipharma, Seattle Genetics, Millennium Pharmaceuticals, Spectrum Pharmaceuticals, Allos Therapeutics, TG Therapeutics
Research Funding: Millennium Pharmaceuticals, Seattle Genetics, Celgene, Spectrum Pharmaceuticals, TG Therapeutics
Travel, Accommodations, Expenses: Mundipharma, Seattle Genetics, Spectrum Pharmaceuticals, TG Therapeutics
Muhit Özcan
Honoraria: Takeda Pharmaceuticals, Amgen, Bristol-Myers Squibb
Research Funding: Janssen Pharmaceuticals, Celgene, Takeda Pharmaceuticals, Bayer, Merck, Archigen Biotech, Roche, Pharmacyclics
Travel, Accommodations, Expenses: Takeda Pharmaceuticals, Sanofi, Roche, Bristol-Myers Squibb, Abdi Ibrahim, Amgen
Eric D. Jacobsen
Consulting or Advisory Role: Merck, Seattle Genetics, Bayer
Research Funding: Merck, Celgene, Pharmacyclics, Janssen Pharmaceuticals
Travel, Accommodations, Expenses: Merck, Seattle Genetics, Bayer
Josep M. Roncero
Consulting or Advisory Role: Janssen-Cilag, Gilead Sciences
Speakers' Bureau: Janssen-Cilag, Gilead Sciences, AbbVie
Travel, Accommodations, Expenses: Roche, Janssen-Cilag
Judith Trotman
Research Funding: Beigene (Inst), Genentech (Inst), Pharmacyclics (Inst), Janssen-Cilag (Inst), Takeda Pharmaceuticals (Inst), Celgene (Inst)
Judit Demeter
Consulting or Advisory Role: Novartis, Amicus Pharma, Angelini Pharma, Pfizer, Roche, Takeda Pharmaceuticals, AbbVie
Travel, Accommodations, Expenses: Pfizer
Tamás Masszi
Consulting or Advisory Role: AbbVie, Bristol-Myers Squibb, Janssen-Cilag, Novartis, Pfizer, Takeda Pharmaceuticals
Juliana Pereira
Consulting or Advisory Role: AstraZeneca
Radhakrishnan Ramchandren
Consulting or Advisory Role: Seattle Genetics, Sandoz-Novartis, Pharmacyclics, Janssen Pharmaceuticals, Bristol-Myers Squibb
Research Funding: Merck (Inst), Seattle Genetics (Inst), Janssen Pharmaceuticals (Inst), Genentech (Inst)
Anne Beaven
Employment: GlaxoSmithKline (I)
Stock and Other Ownership Interests: GlaxoSmithKline (I)
Research Funding: Novartis (Inst), Celgene (Inst), CTI BioPharma Corp (Inst), Seattle Genetics (Inst), Teva Neuroscience (Inst)
Steven M. Horwitz
Consulting or Advisory Role: Celgene, Millennium Pharmaceuticals, Kyowa Hakko Kirin, Seattle Genetics, ADC Therapeutics, Corvus Pharmaceuticals, Innate Pharma, miRagen, Portola Pharmaceuticals, Verastem, Takeda Pharmaceuticals
Research Funding: Celgene, Seattle Genetics, Takeda Pharmaceuticals, Kyowa Hakko Kirin, Aileron Therapeutics, ADC Therapeutics, Verastem, Forty Seven
Anne Lennard
Employment: Specialist Medical Services
Leadership: Specialist Medical Services (I)
Consulting or Advisory Role: Roche, Janssen Pharmaceuticals
Travel, Accommodations, Expenses: Roche
Mehmet Turgut
Consulting or Advisory Role: Roche
Nelson Hamerschlak
Consulting or Advisory Role: Amgen, AbbVie, Sanofi
Speakers' Bureau: Celgene, Amgen, Jazz Pharmaceuticals
Francesco A. d’Amore
Consulting or Advisory Role: Servier (Inst)
Research Funding: Takeda Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Kyowa Hakko Kirin
Francine Foss
Consulting or Advisory Role: Celgene, Seattle Genetics, Spectrum Pharmaceuticals, Eisai, Millennium Pharmaceuticals, Verastem, miRagen
Speakers' Bureau: Seattle Genetics
Research Funding: Celgene
Won-Seog Kim
Consulting or Advisory Role: Celltrion
Research Funding: Takeda Pharmaceuticals, Kyowa Hakko Kirin, Donga, Johnson & Johnson, Celltrion, Novartis, Merck
John P. Leonard
Consulting or Advisory Role: Celgene, Juno Therapeutics, Genmab, NanoString Technologies, Regeneron, AbbVie, Sutter Medical Group, Biotest, Sunesis Pharmaceuticals, Bristol-Myers Squibb, Gilead Sciences, Epizyme, Pfizer, Bayer, Genentech, ADC Therapeutics, MEI Pharma, AstraZeneca, Novartis
Travel, Accommodations, Expenses: Juno Therapeutics
Pier Luigi Zinzani
Honoraria: Servier, Bristol-Myers Squibb, Gilead Sciences, Jansen Pharmaceuticals, MSD Oncology, Celltrion, Celgene, Roche
Speakers' Bureau: Verastem, Servier, Bristol-Myers Squibb, Gilead Sciences, Jansen Pharmaceuticals, MSD Oncology, Celltrion, Celgene, Roche
Carlos S. Chiattone
Consulting or Advisory Role: Roche, Takeda Pharmaceuticals, Janssen Pharmaceuticals
Eric D. Hsi
Honoraria: Seattle Genetics, Celgene, Jazz Pharmaceuticals
Research Funding: Wli Lilly (Inst), AbbVie (Inst), Cellerant Therapeutics (Inst)
Lorenz Trümper
Consulting or Advisory Role: Nordic Nanovector
Research Funding: Spectrum Pharmaceuticals
Hua Liu
Employment: Agios
Stock and Other Ownership Interests: Agios
Emily Sheldon-Waniga
Employment: Millennium Pharmaceuticals, Bluebird Bio
Stock and Other Ownership Interests: Bluebird Bio
Claudio Dansky Ullmann
Employment: MaxCyte
Karthik Venkatakrishnan
Employment: Takeda Pharmaceuticals
Stock and Other Ownership Interests: Takeda Pharmaceuticals
Patents, Royalties, Other Intellectual Property: Named inventor on published patent application US2015/0335668: “Method for Cancer Therapy”2; named inventor on published patent applications WO2013/142491 and US2013/0303519: “Methods of Treating Cancer Using Aurora Kinase Inhibitors”
E. Jane Leonard
Employment: Millennium Pharmaceuticals
Stock and Other Ownership Interests: Millennium Pharmaceuticals
Andrei R. Shustov
Honoraria: Kyowa Hakko Kirin
Consulting or Advisory Role: Kyowa Hakko Kirin
Research Funding: Spectrum Pharmaceuticals
Travel, Accommodations, Expenses: Spectrum Pharmaceuticals
No other potential conflicts of interest were reported.
REFERENCES
- 1.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams SV, Newcomb PA, Shustov AR. Racial patterns of peripheral T-cell lymphoma incidence and survival in the United States. J Clin Oncol. 2016;34:963–971. doi: 10.1200/JCO.2015.63.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vose J, Armitage J, Weisenburger D, et al. International peripheral T-cell and natural killer/T-cell lymphoma study: Pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 4.Mak V, Hamm J, Chhanabhai M, et al. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: Spectrum of disease and rare long-term survivors. J Clin Oncol. 2013;31:1970–1976. doi: 10.1200/JCO.2012.44.7524. [DOI] [PubMed] [Google Scholar]
- 5.Coiffier B, Pro B, Prince HM, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30:631–636. doi: 10.1200/JCO.2011.37.4223. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor OA, Pro B, Pinter-Brown L, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: Results from the pivotal PROPEL study. J Clin Oncol. 2011;29:1182–1189. doi: 10.1200/JCO.2010.29.9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: Results of a phase II study. J Clin Oncol. 2012;30:2190–2196. doi: 10.1200/JCO.2011.38.0402. [DOI] [PubMed] [Google Scholar]
- 8.Zinzani PL, Magagnoli M, Bendandi M, et al. Therapy with gemcitabine in pretreated peripheral T-cell lymphoma patients. Ann Oncol. 1998;9:1351–1353. doi: 10.1023/a:1008409601731. [DOI] [PubMed] [Google Scholar]
- 9.Zinzani PL, Venturini F, Stefoni V, et al. Gemcitabine as single agent in pretreated T-cell lymphoma patients: Evaluation of the long-term outcome. Ann Oncol. 2010;21:860–863. doi: 10.1093/annonc/mdp508. [DOI] [PubMed] [Google Scholar]
- 10.Coiffier B, Pro B, Prince HM, et al. Romidepsin for the treatment of relapsed/refractory peripheral T-cell lymphoma: Pivotal study update demonstrates durable responses. J Hematol Oncol. 2014;7:11. doi: 10.1186/1756-8722-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nikonova AS, Astsaturov I, Serebriiskii IG, et al. Aurora A kinase (AURKA) in normal and pathological cell division. Cell Mol Life Sci. 2013;70:661–687. doi: 10.1007/s00018-012-1073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahadevan D, Spier C, Della Croce K, et al. Transcript profiling in peripheral T-cell lymphoma, not otherwise specified, and diffuse large B-cell lymphoma identifies distinct tumor profile signatures. Mol Cancer Ther. 2005;4:1867–1879. doi: 10.1158/1535-7163.MCT-05-0146. [DOI] [PubMed] [Google Scholar]
- 13.Qi W, Spier C, Liu X, et al. Alisertib (MLN8237) an investigational agent suppresses aurora A and B activity, inhibits proliferation, promotes endo-reduplication and induces apoptosis in T-NHL cell lines supporting its importance in PTCL treatment. Leuk Res. 2013;37:434–439. doi: 10.1016/j.leukres.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barr PM, Li H, Spier C, et al. Phase II intergroup trial of alisertib in relapsed and refractory peripheral T-cell lymphoma and transformed mycosis fungoides: SWOG 1108. J Clin Oncol. 2015;33:2399–2404. doi: 10.1200/JCO.2014.60.6327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yakushijin Y, Hamada M, Yasukawa M. The expression of the aurora-A gene and its significance with tumorgenesis in non-Hodgkin’s lymphoma. Leuk Lymphoma. 2004;45:1741–1746. doi: 10.1080/10428190410001683615. [DOI] [PubMed] [Google Scholar]
- 16.Ding YH, Zhou ZW, Ha CF, et al. Alisertib, an aurora kinase A inhibitor, induces apoptosis and autophagy but inhibits epithelial to mesenchymal transition in human epithelial ovarian cancer cells. Drug Des Devel Ther. 2015;9:425–464. doi: 10.2147/DDDT.S74062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li JP, Yang YX, Liu QL, et al. The investigational aurora kinase A inhibitor alisertib (MLN8237) induces cell cycle G2/M arrest, apoptosis, and autophagy via p38 MAPK and Akt/mTOR signaling pathways in human breast cancer cells. Drug Des Devel Ther. 2015;9:1627–1652. doi: 10.2147/DDDT.S75378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Wang F, Li H, Yan XG, et al. Alisertib induces cell cycle arrest and autophagy and suppresses epithelial-to-mesenchymal transition involving PI3K/Akt/mTOR and sirtuin 1-mediated signaling pathways in human pancreatic cancer cells. Drug Des Devel Ther. 2015;9:575–601. doi: 10.2147/DDDT.S75221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zullo KM, Guo Y, Cooke L, et al. Aurora A kinase inhibition selectively synergizes with histone deacetylase inhibitor through cytokinesis failure in T-cell lymphoma. Clin Cancer Res. 2015;21:4097–4109. doi: 10.1158/1078-0432.CCR-15-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manfredi MG, Ecsedy JA, Chakravarty A, et al. Characterization of alisertib (MLN8237), an investigational small-molecule inhibitor of aurora A kinase using novel in vivo pharmacodynamic assays. Clin Cancer Res. 2011;17:7614–7624. doi: 10.1158/1078-0432.CCR-11-1536. [DOI] [PubMed] [Google Scholar]
- 21.Kelly KR, Shea TC, Goy A, et al. Phase I study of MLN8237—investigational aurora A kinase inhibitor—in relapsed/refractory multiple myeloma, non-Hodgkin lymphoma and chronic lymphocytic leukemia. Invest New Drugs. 2014;32:489–499. doi: 10.1007/s10637-013-0050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkatakrishnan K, Kim TM, Lin CC, et al. Phase 1 study of the investigational aurora A kinase inhibitor alisertib (MLN8237) in East Asian cancer patients: Pharmacokinetics and recommended phase 2 dose. Invest New Drugs. 2015;33:942–953. doi: 10.1007/s10637-015-0258-y. [DOI] [PubMed] [Google Scholar]
- 23.Friedberg JW, Mahadevan D, Cebula E, et al. Phase II study of alisertib, a selective aurora A kinase inhibitor, in relapsed and refractory aggressive B- and T-cell non-Hodgkin lymphomas. J Clin Oncol. 2014;32:44–50. doi: 10.1200/JCO.2012.46.8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matulonis UA, Sharma S, Ghamande S, et al. Phase II study of MLN8237 (alisertib), an investigational aurora A kinase inhibitor, in patients with platinum-resistant or -refractory epithelial ovarian, fallopian tube, or primary peritoneal carcinoma. Gynecol Oncol. 2012;127:63–69. doi: 10.1016/j.ygyno.2012.06.040. [DOI] [PubMed] [Google Scholar]
- 25.Melichar B, Adenis A, Lockhart AC, et al. Safety and activity of alisertib, an investigational aurora kinase A inhibitor, in patients with breast cancer, small-cell lung cancer, non-small-cell lung cancer, head and neck squamous-cell carcinoma, and gastro-oesophageal adenocarcinoma: A five-arm phase 2 study. Lancet Oncol. 2015;16:395–405. doi: 10.1016/S1470-2045(15)70051-3. [DOI] [PubMed] [Google Scholar]
- 26.Cheson BD. The International Harmonization Project for response criteria in lymphoma clinical trials. Hematol Oncol Clin North Am. 2007;21:841–854. doi: 10.1016/j.hoc.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Cervantes A, Elez E, Roda D, et al. Phase I pharmacokinetic/pharmacodynamic study of MLN8237, an investigational, oral, selective aurora a kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2012;18:4764–4774. doi: 10.1158/1078-0432.CCR-12-0571. [DOI] [PubMed] [Google Scholar]
- 28.Dees EC, Cohen RB, von Mehren M, et al. Phase I study of aurora A kinase inhibitor MLN8237 in advanced solid tumors: Safety, pharmacokinetics, pharmacodynamics, and bioavailability of two oral formulations. Clin Cancer Res. 2012;18:4775–4784. doi: 10.1158/1078-0432.CCR-12-0589. [DOI] [PubMed] [Google Scholar]
- 29.O’Connor OA, Horwitz S, Hamlin P, et al. Phase II-I-II study of two different doses and schedules of pralatrexate, a high-affinity substrate for the reduced folate carrier, in patients with relapsed or refractory lymphoma reveals marked activity in T-cell malignancies. J Clin Oncol. 2009;27:4357–4364. doi: 10.1200/JCO.2008.20.8470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piekarz RL, Frye R, Prince HM, et al. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood. 2011;117:5827–5834. doi: 10.1182/blood-2010-10-312603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venkatakrishnan K, Zhou X, Ecsedy J, et al. Dose selection for the investigational anticancer agent alisertib (MLN8237): Pharmacokinetics, pharmacodynamics, and exposure-safety relationships. J Clin Pharmacol. 2015;55:336–347. doi: 10.1002/jcph.410. [DOI] [PubMed] [Google Scholar]
- 32.Zhou X, Mould DR, Takubo T, et al. Global population pharmacokinetics of the investigational aurora A kinase inhibitor alisertib in cancer patients: Rationale for lower dosage in Asia. Br J Clin Pharmacol. 2018;84:35–51. doi: 10.1111/bcp.13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venkatakrishnan K, Burgess C, Gupta N, et al. Toward optimum benefit-risk and reduced access lag for cancer drugs in Asia: A global development framework guided by clinical pharmacology principles. Clin Transl Sci. 2016;9:9–22. doi: 10.1111/cts.12386. [DOI] [PMC free article] [PubMed] [Google Scholar]