Abstract
FOLFIRINOX is one of the most effective reference regimens in the 1st line treatment of locally advanced (LA) and metastatic pancreatic cancer (mPC), despite its high toxicity. We evaluated our real-life experience with “patient-tailored intent to treat FOLFIRINOX” in patients with LA or mPC compared to other reports along with the pivotal phase III trial.
We analyzed data from all consecutive patients with pancreatic ductal adenocarcinoma treated with dose-modified FOLFIRINOX in 2016 at Paul Brousse University Hospital. Irinotecan was administered whenever initial serum bilirubin was <1.5 × upper limit of normal. Oxaliplatin was stopped for severe sensory neuropathy. Initial dose reductions were made according to patient profile (eg, age, comorbidities) and later due to toxicity. The treatment was continued until surgery or disease progression. Endpoints were time to progression (TTP), overall survival (OS), objective response rate (ORR), and secondary complete resection (R0R1).
Thirty-seven patients with unresectable LA or mPC received patient-tailored FOLFIRINOX as 1st line chemotherapy. There were 22 male (59%) and 15 female patients (41%) aged 44 to 81 years with LA (18 patients, 49%) and mPC (19 patients, 51%). They had World Health Organization-performance status of 0 (59%) or 1 (41%). A total of 384 cycles were administered. Median dose intensities (mg/m2/w) were 28.9 for oxaliplatin, 56.8 for irinotecan, and 886.2 for 5-fluorouracil. Thirty-four patients were assessed for response; ORR and disease control rates were 47% and 85%, respectively. R0R1 rate was 30%. Median TTP and OS were 9.6 and 14.6 months. LA disease was associated with significantly longer TTP and OS (P < .001).
FOLFIRINOX with patient-tailored dose adaptations seems to offer better results in patients with advanced PC. This approach in the neoadjuvant setting results in a macroscopic R0R1 in 61% of patients with initially unresectable disease. It deserves prospective evaluation to further improve outcomes in the management of advanced PC.
Keywords: dose-modified FOLFIRINOX, pancreatic cancer, real-life practice
1. Introduction
Pancreatic adenocarcinoma is one of the most lethal neoplastic diseases with an incidence rate almost equivalent to the rate of mortality.[1] It is the eighth leading cause of cancer-related deaths worldwide[2] and the fourth leading cause of cancer-related deaths in developed countries.[3,4] The majority of patients present locally advanced or metastatic disease at initial diagnosis.[4] The median overall survival (OS) for locally advanced (LA) pancreatic cancer is approximately 12 months, while outcomes are worse for metastatic disease with median OS being estimated to be limited to 6 months.[5] In the last decade, the treatment of advanced pancreatic cancer has greatly evolved with positive results of numerous studies conducted in an effort to find more effective regimens.[6]
In 1990, after a randomized phase III trial that showed a modest but significant improvement in disease-related symptoms and median OS when compared to 5-fluorouracil (5FU) alone (5.6 vs 4.4 months, P = .002), gemcitabine remained as the standard chemotherapy regimen for advanced pancreatic cancer until 2011 when FOLFIRINOX was introduced as a new more effective first-line treatment.[7] FOLFIRINOX regimen improved median OS as compared to gemcitabine monotherapy (11.1 vs 6.8 months, hazard ratio [HR] for death 0.57; 95% confidence interval 0.45–0.73; P < .0001). However, it caused significant toxicities that limited its use to patients with a good performance status (PS) and frequently required hematopoietic growth factor support.[7] Recent studies proposed dose reduction of FOLFIRINOX in order to balance the benefit-risk ratio of this regimen.[8–11] Despite these modified FOLFIRINOX regimen reports, the optimal dosing for tolerance and response correlation remains unknown.
We report in this retrospective study 1-year experience with patient-tailored intent to treat FOLFIRINOX in patients with LA and metastatic pancreatic cancer (mPC) treated at Paul Brousse University Hospital, Villejuif, France, from January to December 2016.
2. Materials and methods
2.1. Patient selection
We analyzed data from all consecutive patients with pancreatic ductal adenocarcinoma treated with dose-modified FOLFIRINOX in 2016 at Paul Brousse University Hospital. There was no ethical concern since this was a retrospectively reviewed cohort of patients.
All consecutive patients with histologically- or cytologically-documented unresectable LA or mPC, who received as a first line therapy the FOLFIRINOX regimen combining irinotecan, oxaliplatin, 5FU, and leucovorin from January 1st to December 31st, 2016 were selected from hospital pharmacy log-out registries.
2.2. Study treatment
It was initially planned to deliver every 2 weeks, irinotecan, oxaliplatin, leucovorin, and 5FU as a conventional delivery scheme.[7] According to patient baseline assessment, chemotherapy was started with or without irinotecan and/or dose reductions. Thus, in case of hyperbilirubinemia, patients underwent for biliary stent placement and then started chemotherapy without irinotecan. Irinotecan was added when serum bilirubin decreased to ≤1.5 of upper limit of normal (Fig. 1). Oxaliplatin was stopped after severe oxaliplatin-related toxicities, especially grade 2 or more sensory neuropathy (Fig. 1). Chemotherapy was continued until surgery, progression, or occurrence of unacceptable toxicity. All patients received supportive care medications as prevention or treatment of chemo-induced toxicities.
Figure 1.
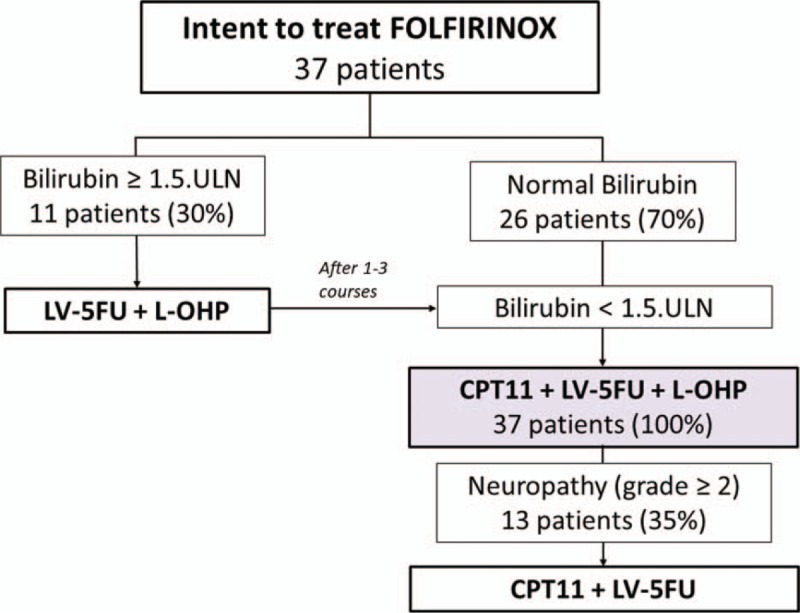
Intent to treat FOLFIRINOX in real life practice. Strategy flowchart: FOLFIRINOX combining 5FU, leucovorin, irinotecan CPT11, and oxaliplatin. All these drugs were administered when the bilirubin level was less than 1.5 ULN. If not, FOLFIRINOX was started without CPT11 until it became ≤1.5.ULN. L-OHP was stopped in case of grade 2 or more sensory neuropathy. 5FU = 5-fluorouracil, CPT11 = irinotecan, LV = leucovorin, L-OHP = oxaliplatin, ULN = upper limit of normal.
2.3. Assessments
All the data were collected from medical records by the investigator and then anonymously entered in a database. The data collected were: patient baseline characteristics, chemotherapy drugs administered and doses, number of cycles, antitumor response, time to progression (TTP), OS, and adverse events according to NCIC-CTCAE v3.0 toxicity.[12] A final independent review was made to ensure quality of data for consistency and plausibility. Errors were corrected in the database, whenever appropriate with the investigator.
2.4. Statistical consideration
Patient characteristics, toxicities, and objective response rates were compared using the Fisher exact test or Pearson Ki2-test. TTP and OS were computed using Kaplan–Meier method and compared according to clinical characteristics with Log-Rank test. TTP was defined as the time between the first day of the first chemotherapy course till progression or relapse, OS from the first day of the first course till last known to be alive or death. The cut-off date for follow-up was June 16th, 2018.
Predictive factors of TTP and OS were determined by computing HR calculated with Cox proportional HR model. All analyses were performed with intent-to-treat using SPSS v18.0 software (Chicago, IL). A P-value < .05 was considered statistically significant using bilateral tests.
3. Results
3.1. Patient characteristics
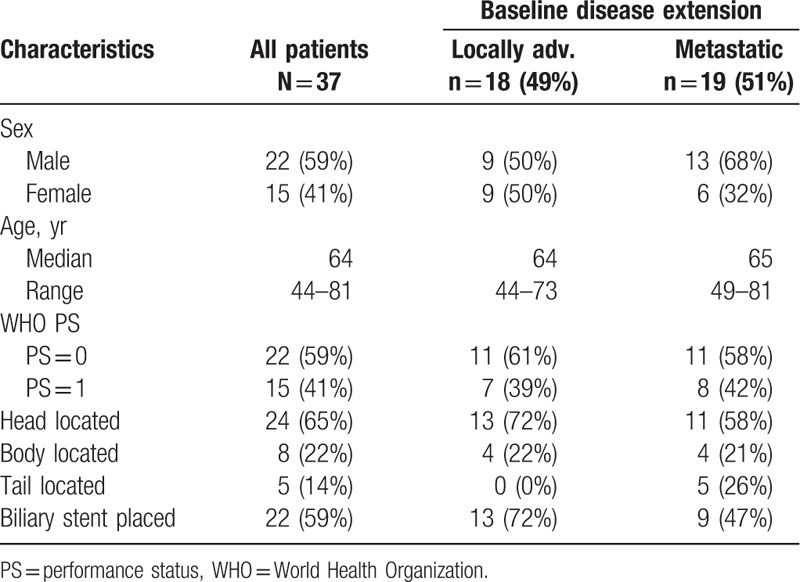
Thirty-seven patients treated with at least 1 cycle of FOLFIRINOX from January to December 2016 were retrieved in pharmacy registries. Patients’ main characteristics at baseline are reported in Table 1. Eight patients had previously known diabetes at the time of diagnosis (22%). Pancreatic tumor was LA in 18 patients (49%) and metastatic in 19 patients (51%). Metastases were liver only in 9 patients, peritoneum only in 1 patient, and multilocated in 9 patients. Eleven patients started intent to treat FOLFIRINOX without irinotecan. Thirteen patients stopped oxaliplatin for sensory neuropathy. A total of 384 treatment courses were given, resulting in a median number of 10 courses per patient (range: 2–38) for median treatment duration of 23 weeks (1st–3rd quartiles, 16–31). Thirty-four patients (92%) were evaluated for response (RECIST v1.1 criteria).[13] Three patients were lost to follow-up.
Table 1.
Main characteristics of all 37 patients with advanced pancreatic cancer (study population) and distinctly in locally advanced and metastatic populations.
3.2. Key safety data and dose intensities
Initially, 2 or 3 courses were administered without irinotecan in 30% of patients. Oxaliplatin was stopped in 35% of patients after a median of 9 courses (range: 2–16) because of sensory neuropathy. For patients who commenced their therapy with all protocol drugs, doses of each cytotoxic agent were reduced initially related to patient age and/or comorbidities and later according to tolerance, yet the median dose-intensities (mg/m2/wk) for all 384 courses received by the 37 included patients were 56.8 for irinotecan, 886.2 for 5FU and 28.9 for oxaliplatin. Thus, the median relative-dose-intensities were 63% for irinotecan, 63% for 5FU and 68% for oxaliplatin. There was no febrile neutropenia or toxic death. Nine patients (24%) experienced grade 3-4 toxicities. Grade 3-4 nonhematological toxicities were fatigue (14%), diarrhea (6%), nausea/vomiting (3%), and sensory neuropathy (3%).
3.3. Secondary tumor resection (R0/R1) and disease-free survival (DFS)
Secondary macroscopic tumor resection (R0 or R1) was achieved in 11/18 patients with LA disease (61% [95% confidence limits [CL], 38.6–83.6]), including 9 R0 and 2 R1, after a median of 9 treatment courses (range: 4–17). In this subgroup, median DFS was 12.0 months (9.2–14.7).
3.4. Antitumor response, TTP, and OS
Objective response rate (ORR) and disease control rate (DCR) were 43% (95% CL, 27–59) and 76% (62–90), respectively (Table 2). Antitumor responses were not significantly impacted by dose intensities or chemotherapy-related toxicities. After a median follow-up of 23 months (range, 18–29), median intent to treat TTP and OS were respectively 9.6 months (9.6–12.8) and 14.6 months (10.7–18.8). Kaplan–Meier analysis showed that baseline disease extension significantly impacted both TTP and OS (Fig. 2). Median TTP was 19.8 months (95% CL, 11.7–27.8) in the patients with LA disease, 6.7 months (3.6–10.1) in the patients with metastatic disease (P < .001) (Fig. 2A). Median OS of the 18 patients with LA disease was not reached, while it was 9.4 months (6.2–12.6) in the patients with metastatic disease (P < .001) (Fig. 2B).
Table 2.
Main efficacy parameters in all 37 intent to treat patients (study population) and separately in the subgroups defined by disease extension.
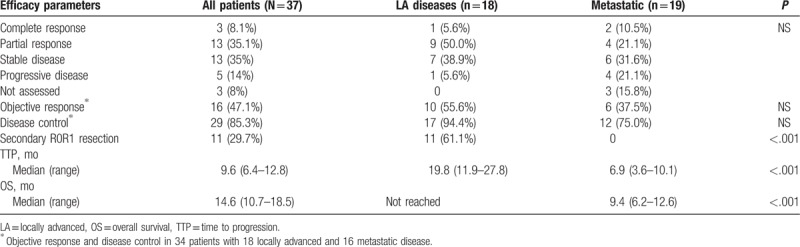
Figure 2.
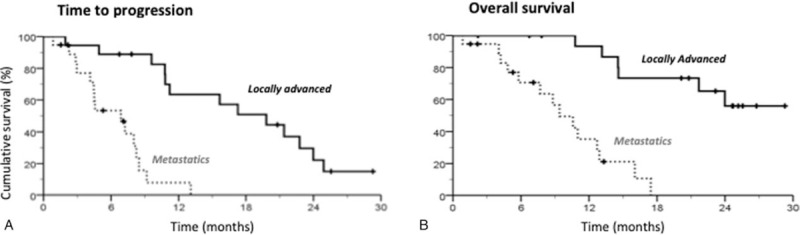
TTP and OS according to initial disease extension. (A) Kaplan–Meier curves of TTP according to initial disease extension. Median TTP was 19.8 mo (11.9–27.8) for locally advanced tumor and 6.7 mo (3.6–10.1) for metastatic disease; P (Log Rank) <.001. (B) Kaplan–Meier curves of OS according to initial disease extension. Median OS was not reached for locally advanced tumor. It was 9.4 mo (6.2–12.6) for metastatic disease; P (Log Rank) < .001. OS = overall survival, TTP = time to progression.
TTP and OS were statistically similar in patients with LA disease who underwent secondary surgery as compared to those who were not resected (Fig. 3). When we compared survival curves of metastatic patients to those of patients with LA but nonresected tumor, both TTP and OS were significantly better for patients with LA disease (Fig. 4). Multivariate Cox model analysis revealed that a male sex (P = .019), an age below the median of 64 years (P = .013), a World Health Organization (WHO) PS of 0 (P = .006), a LA disease (P < .001), and a tail located primary (P = .013) were the main joint predictive factors for achieving a longer TTP (Fig. 5A). A WHO PS = 0 (P = .005) and LA disease (P < .001) were identified as good prognostic factors for OS and pancreatic head primary (P = .028) was predicting worse OS (Fig. 5B).
Figure 3.
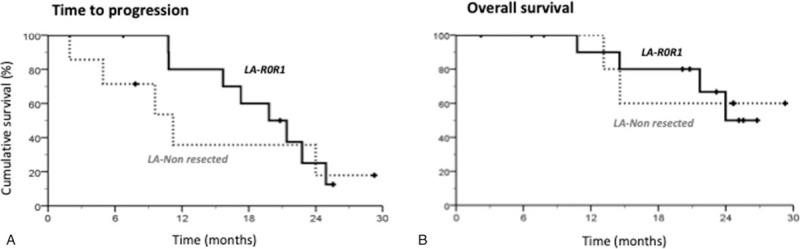
Intent to treat TTP and OS curves according to secondary tumor resection R0R1 in patients with LA disease. (A) Kaplan–Meier curves of TTP according to R0R1 resection, Median TTP was 19.8 mo (14.2–25.5) for resected patients and 11.2 mo (4.4–18.1) for nonresected patients, P (Log Rank) = .550. (B) Kaplan–Meier curves of OS according to R0R1 resection. Median OS was not reached for both resected and nonresected patients, P (Log Rank) = .936. LA = locally advanced, OS = overall survival, TTP = time to progression.
Figure 4.
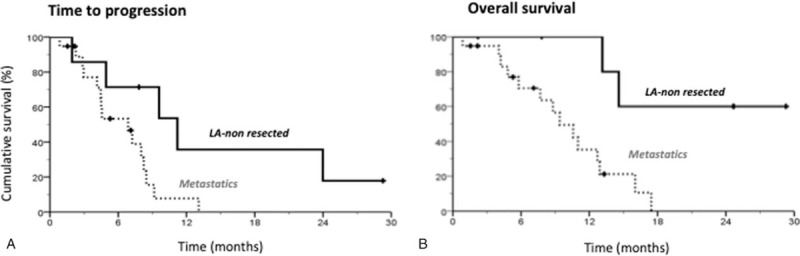
Comparison of TTP and OS curves of metastatic and LA nonresected patients. (A) Kaplan–Meier curves of TTP in metastatic and LA nonresected patients. Median TTP was 11.2 mo (4.4–18.0) for LA nonresected patients and 6.9 mo (3.6–10.1) for metastatic patients, P (Log Rank) = .030. (B) Kaplan–Meier curves of OS in metastatic and LA nonresected patients. Median OS was not reached for LA nonresected patients and 9.4 mo (6.2–12.6) for metastatic patients, P (Log Rank) = .007. LA = locally advanced, OS = overall survival, TTP = time to progression.
Figure 5.
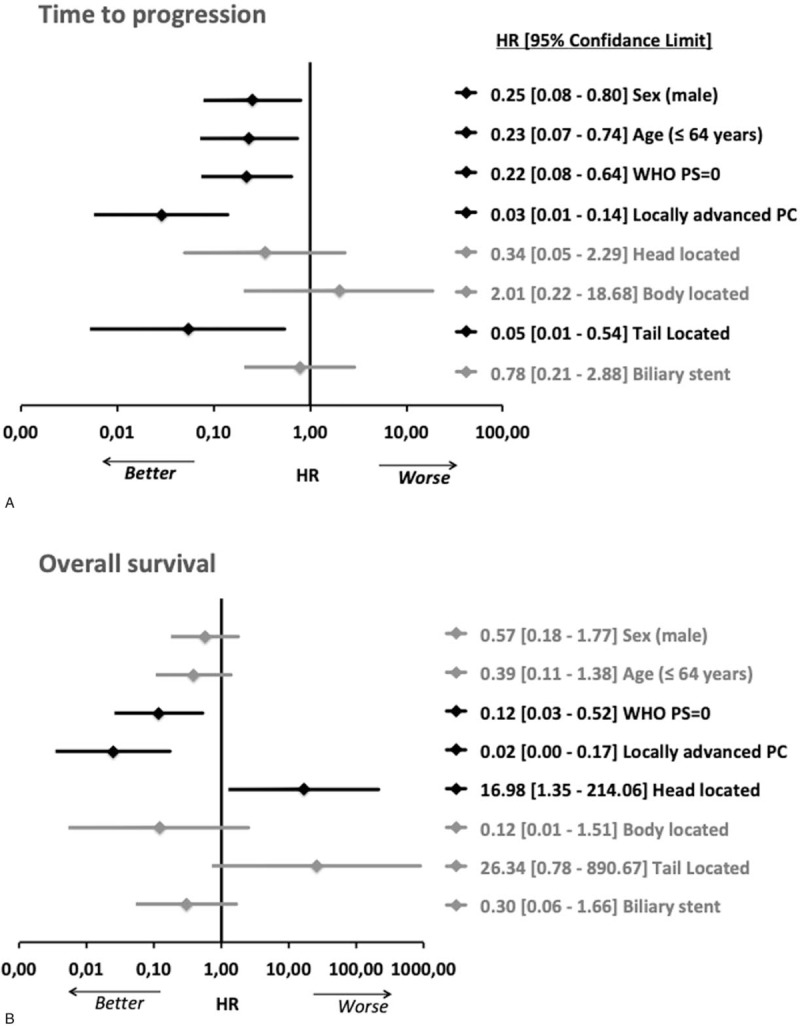
Forest-plot of prognostic factors for TTP and OS after multivariate Cox-Model hazard ratio computing. The upper plot displays the prognostic factors for intent to treat TTP (A); the plot below presents the prognostic factors of OS (B). Variables in bold are those that are significantly associated with TTP or OS. OS = overall survival, TTP = time to progression.
4. Discussion
Currently, FOLFIRINOX[14,15] and the combination of nab-paclitaxel and gemcitabine[16–19] are considered both as the standard regimens in the first line treatment of mPC. FOLFIRINOX has also showed its superiority compared to gemcitabine alone in the adjuvant treatment of pancreatic cancer.[20] It offers approximately an objective response rate of 32%, a median progression-free survival of 6 months and a median OS of 11 months in patients with mPC.[7,8,21–24] However, despite improved survival, this regimen has significant toxicities limiting its use.[7] Recent studies proposed modified-FOLFIRINOX versions with the goal to improve its tolerability by maintaining the efficacy.[8,11,23–25] However, the optimal dosing remains unknown.
Our approach in the present study was to evaluate our experience with FOLFIRINOX as routine intent-to-treat regimen with reduced-dose of each cytotoxic agent according to patient characteristics at the beginning and during therapy in order to decrease toxicity. Thus, in our series, 30% of patients started their treatment without irinotecan. Among the remaining patients (70%) who started chemotherapy with all individual drugs, 84% of them commenced FOLFIRINOX regimen with initial dose reduction of irinotecan. At the beginning of chemotherapy, most of the patients had dose reductions for both 5FU and oxaliplatin as compared to the pivotal phase III regimen.[7,26] Oxaliplatin was definitively stopped in 35% of patients because of sensory neuropathy, but the other drugs of the regimen were continued until disease progression.
In spite of decreased dosing, the outcomes were not compromised. After a median of 23 months follow-up, an ORR of 47%, a DCR of 85%, a median TTP of 9.6 months and OS of 14.6 months were achieved. These results are consistent with those reported by Chllamma et al[24] in a larger cohort involving 66 patients with metastatic and 36 with LA pancreatic cancer, in whom two-thirds of patients initiated their treatment with a dose reduction. Some studies have investigated the impact of dose modifications on response to FOLFIRINOX regimen[8,22] and did not find any correlation. In our cohort, there was no toxic death. Grade 3-4 toxicities were observed in 24% of patients. These results were comparable or even better in terms of efficacy and tolerance as compared to those reported with the original[7,22–24,27] or modified-FOLFIRINOX regimens.[8,9,25,28–30]
In our study, we looked for the influence of patient characteristics at initiation of FOLFIRINOX regimen on TTP and OS. We found that disease extension strongly impacted both TTP and OS. Head primary was predicting worse survival. Patients with LA disease had longer TTP and longer OS as compared to those with metastatic disease (median TTP 19.8 months vs 6.9 months, P < .001; median OS not reached vs 9.4 months, P < .001). LA disease was confirmed as independent prognostic factor in multivariate analysis (TTP: HR = 0.03, P < .001; OS: HR = 0.02, P < .001). Several studies reported similar results with longer TTP and longer OS in patients with LA disease than those with metastatic disease in larger cohorts.[8,24,27]
Hohla et al[31] suggested that female gender could positively predict response to FOLFIRINOX in patients with advanced pancreatic cancer. In our study, nonsignificant differences were observed between male and female patients in the univariate analysis regarding ORR, DCR, TTP, and OS. On the contrary, multivariate analysis has shown male sex as a good prognostic factor for TTP (HR = 0.25, P = .019). Given the fairly small size of both cohorts, the role of sex on outcomes remains controversial.
Because of high-level toxicity of FOLFIRINOX, the benefit-risk ratio of this regimen remains questionable in elderly patients. Our patients were aged 44 to 81 years with a median of 64 years. There were nonsignificant differences related to patient age in terms of grade 3 or 4 toxicities. Regarding efficacy parameters, Cox proportional HR model analysis identified an age below the median of 64 years as positive predictive factor for only TTP (HR = 0.23, P = .013). Similar results were observed by Berger et al[32] in a retrospective cohort assessing efficacy and toxicity of FOLFIRINOX regimen in an elderly population of 88 patients with pancreatic adenocarcinoma. In their study, older patients (≥65 years) compared to younger ones did not have significantly different toxicity of grade 3 or 4. The authors also found that older patients did not have worse PFS or OS. Elderly patients included in our cohort had longer survival than that reported by Berger et al. This difference could be explained by the fact that we considered the median age of our cohort (64 years) as the cut-off and the patients aged more than 76 years were excluded in Berger study, whereas we analyzed data from all consecutive patients for whom FOLFIRINOX was appropriate, regardless of age.
Previous studies had reported a high rate of head location of pancreatic adenocarcinoma.[8,11,27,33,34] Pancreatic head primaries are often diagnosed with jaundice and have increased risk of irinotecan-induced toxicity.[35] In our study, 65% of the patients had their tumors located in the pancreatic head, but this did not impact the safety because most of them underwent endoscopic or percutaneous biliary stent placement (83%). The smaller rate (38%) of head location reported by Conroy et al[7] may be due to the exclusion in their study of patients with initial high bilirubin levels resulting in a low proportion of enrolled patients with biliary stents (14.3%).
The main limitation of our study is the retrospective analysis of data in 37 patients; however, we included all consecutive patients to report real practice data.
In conclusion, FOLFIRINOX regimen delivered with initial dose-adaptations according to patients’ profiles improved safety without negative impact on response rate, TTP, and OS in LA or mPC. The efficacy of this modified version of FOLFIRINOX deserves to be assessed on a prospective cohort.
Acknowledgments
We sincerely acknowledge AK-SCIENCE, Vitry-Sur-Seine, France for its expert assistance in preparation of this manuscript and Dr Vincent Castagne for his precious help to obtain FOLFIRINOX-related data from hospital pharmacy registries.
Author contributions
Conceptualization: Ayhan Ulusakarya, Abdoulaye Karaboué, Mazen Haydar, Pamela Biondani, Yusuf Gumus, Jean-François Morère.
Data curation: Ayhan Ulusakarya, Nahla Teyar, Abdoulaye Karaboué, Sarra Krimi, Pamela Biondani, Yusuf Gumus, Amale Chebib, Wathek Almohamad, Jean-François Morère.
Formal analysis: Ayhan Ulusakarya, Abdoulaye Karaboué, Pamela Biondani.
Investigation: Ayhan Ulusakarya, Nahla Teyar, Abdoulaye Karaboué, Mazen Haydar, Sarra Krimi, Pamela Biondani, Yusuf Gumus, Amale Chebib, Wathek Almohamad.
Methodology: Ayhan Ulusakarya, Nahla Teyar, Abdoulaye Karaboué, Mazen Haydar, Sarra Krimi.
Project administration: Ayhan Ulusakarya.
Software: Abdoulaye Karaboué.
Supervision: Ayhan Ulusakarya.
Validation: Ayhan Ulusakarya, Nahla Teyar, Abdoulaye Karaboué, Mazen Haydar, Sarra Krimi, Pamela Biondani, Yusuf Gumus, Amale Chebib, Wathek Almohamad, Jean-François Morère.
Visualization: Ayhan Ulusakarya, Abdoulaye Karaboué.
Writing – original draft: Ayhan Ulusakarya, Abdoulaye Karaboué.
Writing – review and editing: Ayhan Ulusakarya, Nahla Teyar, Abdoulaye Karaboué, Mazen Haydar, Sarra Krimi, Pamela Biondani, Yusuf Gumus, Amale Chebib, Wathek Almohamad, Jean-François Morère.
Ayhan Ulusakarya orcid: 0000-0001-8683-6818.
Footnotes
Abbreviations: CI = confidence interval, CL = confidence limits, DCR = disease control rate, DFS = disease-free survival, HR = hazard ratio, LA = locally advanced, mPC = metastatic pancreatic cancer, ORR = objective response rate, OS = overall survival, PS = performance status, R0R1 = complete resection, TTP = time to progression.
This study was supported by the European-Lebanese association of oncologists.
This study was partially presented at ASCO-GI Meeting in January 2018 (San Francisco, USA).
All of the authors have no conflicts of interest or a source of funding relevant to this article.
References
- [1].Li BR, Hu LH, Li ZS. Chronic pancreatitis and pancreatic cancer. Gastroenterology 2014;147:541–2. [DOI] [PubMed] [Google Scholar]
- [2].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [3].Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- [4].Sant M, Allemani C, Santaquilani M, et al. EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999. Results and commentary. Eur J Cancer 2009;45:931–91. [DOI] [PubMed] [Google Scholar]
- [5].Peixoto RD, Speers C, McGahan CE, et al. Prognostic factors and sites of metastasis in unresectable locally advanced pancreatic cancer. Cancer Med 2015;4:1171–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Neuzillet C, Gaujoux S, Williet N, et al. Pancreatic cancer: French clinical practice guidelines for diagnosis, treatment and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, ACHBT, AFC). Dig Liver Dis 2018;50:1257–71. [DOI] [PubMed] [Google Scholar]
- [7].Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–25. [DOI] [PubMed] [Google Scholar]
- [8].Mahaseth H, Brutcher E, Kauh J, et al. Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas 2013;42:1311–5. [DOI] [PubMed] [Google Scholar]
- [9].Ghorani E, Wong HH, Hewitt C, et al. Safety and efficacy of modified FOLFIRINOX for advanced pancreatic adenocarcinoma: a UK single-centre experience. Oncology 2015;89:281–7. [DOI] [PubMed] [Google Scholar]
- [10].Blazer M, Wu C, Goldberg RM, et al. Neoadjuvant modified (m) FOLFIRINOX for locally advanced unresectable (LAPC) and borderline resectable (BRPC) adenocarcinoma of the pancreas. Ann Surg Oncol 2015;22:1153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lee JC, Kim JW, Ahn S, et al. Optimal dose reduction of FOLFIRINOX for preserving tumour response in advanced pancreatic cancer: using cumulative relative dose intensity. Eur J Cancer 2017;76:125–33. [DOI] [PubMed] [Google Scholar]
- [12].Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176–81. [DOI] [PubMed] [Google Scholar]
- [13].Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- [14].Liu GF, Li GJ, Zhao H. Efficacy and toxicity of different chemotherapy regimens in the treatment of advanced or metastatic pancreatic cancer: a network meta-analysis. J Cell Biochem 2018;119:511–23. [DOI] [PubMed] [Google Scholar]
- [15].Braiteh F, Patel MB, Parisi M, et al. Comparative effectiveness and resource utilization of nab-paclitaxel plus gemcitabine vs FOLFIRINOX or gemcitabine for the first-line treatment of metastatic pancreatic adenocarcinoma in a US community setting. Cancer Manag Res 2017;9:141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Borazanci E, Von Hoff DD. Nab-paclitaxel and gemcitabine for the treatment of patients with metastatic pancreatic cancer. Expert Rev Gastroenterol Hepatol 2014;8:739–47. [DOI] [PubMed] [Google Scholar]
- [18].Goldstein D, El-Maraghi RH, Hammel P, et al. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J Natl Cancer Inst 2015;107:dju413. [DOI] [PubMed] [Google Scholar]
- [19].Quinton AE, Gwynne SH, Yim KL. Nab-paclitaxel in combination with gemcitabine for the treatment of metastatic pancreas cancer: the South Wales experience. Med Oncol 2018;35:115. [DOI] [PubMed] [Google Scholar]
- [20].Conroy T, Hebbar M, Abdelghani MB, et al. Unicancer GI PRODIGE 24/CCTG PA.6 trial: a multicenter international randomized phase III trial of adjuvant mFOLFIRINOX versus gemcitabine (gem) in patients with resected pancreatic ductal adenocarcinomas. J Clin Oncol 2018;36:LBA4001. [Google Scholar]
- [21].Karim S, Zhang-Salomans J, Biagi JJ, et al. Uptake and effectiveness of FOLFIRINOX for advanced pancreatic cancer: a population-based study. Clin Oncol (R Coll Radiol) 2018;30:e16–21. [DOI] [PubMed] [Google Scholar]
- [22].Gunturu KS, Yao X, Cong X, et al. FOLFIRINOX for locally advanced and metastatic pancreatic cancer: single institution retrospective review of efficacy and toxicity. Med Oncol 2013;30:361. [DOI] [PubMed] [Google Scholar]
- [23].Moorcraft SY, Khan K, Peckitt C, et al. FOLFIRINOX for locally advanced or metastatic pancreatic ductal adenocarcinoma: the Royal Marsden experience. Clin Colorectal Cancer 2014;13:232–8. [DOI] [PubMed] [Google Scholar]
- [24].Chllamma MK, Cook N, Dhani NC, et al. FOLFIRINOX for advanced pancreatic cancer: the Princess Margaret Cancer Centre experience. Br J Cancer 2016;115:649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li X, Ma T, Zhang Q, et al. Modified-FOLFIRINOX in metastatic pancreatic cancer: a prospective study in Chinese population. Cancer Lett 2017;406:22–6. [DOI] [PubMed] [Google Scholar]
- [26].Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol 2013;31:23–9. [DOI] [PubMed] [Google Scholar]
- [27].Rombouts SJ, Mungroop TH, Heilmann MN, et al. FOLFIRINOX in locally advanced and metastatic pancreatic cancer: a single centre cohort study. J Cancer 2016;7:1861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Umemura A, Nitta H, Sasaki A, et al. Modified FOLFIRINOX for locally advanced and metastatic pancreatic cancer patients resistant to gemcitabine and S-1 in Japan: a single institutional experience. Hepatogastroenterology 2014;61:814–20. [PubMed] [Google Scholar]
- [29].Ozaka M, Ishii H, Sato T, et al. A phase II study of modified FOLFIRINOX for chemotherapy-naive patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol 2018;81:1017–23. [DOI] [PubMed] [Google Scholar]
- [30].Tong H, Fan Z, Liu B, et al. The benefits of modified FOLFIRINOX for advanced pancreatic cancer and its induced adverse events: a systematic review and meta-analysis. Sci Rep 2018;8:8666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hohla F, Hopfinger G, Romeder F, et al. Female gender may predict response to FOLFIRINOX in patients with unresectable pancreatic cancer: a single institution retrospective review. Int J Oncol 2014;44:319–26. [DOI] [PubMed] [Google Scholar]
- [32].Berger AK, Haag GM, Ehmann M, et al. Palliative chemotherapy for pancreatic adenocarcinoma: a retrospective cohort analysis of efficacy and toxicity of the FOLFIRINOX regimen focusing on the older patient. BMC Gastroenterol 2017;17:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bednar F, Zenati MS, Steve J, et al. Analysis of predictors of resection and survival in locally advanced stage III pancreatic cancer: does the nature of chemotherapy regimen influence outcomes? Ann Surg Oncol 2017;24:1406–13. [DOI] [PubMed] [Google Scholar]
- [34].Lakatos G, Petranyi A, Szucs A, et al. Efficacy and safety of FOLFIRINOX in locally advanced pancreatic cancer. a single center experience. Pathol Oncol Res 2017;23:753–9. [DOI] [PubMed] [Google Scholar]
- [35].Ueno H, Okusaka T, Funakoshi A, et al. A phase II study of weekly irinotecan as first-line therapy for patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol 2007;59:447–54. [DOI] [PubMed] [Google Scholar]


