Abstract
Background
Post‐extraction bleeding (PEB) is a recognised, frequently encountered complication in dental practice, which is defined as bleeding that continues beyond 8 to 12 hours after dental extraction. The incidence of post‐extraction bleeding varies from 0% to 26%. If post‐extraction bleeding is not managed, complications can range from soft tissue haematomas to severe blood loss. Local causes of bleeding include soft tissue and bone bleeding. Systemic causes include platelet problems, coagulation disorders or excessive fibrinolysis, and inherited or acquired problems (medication induced). There is a wide array of techniques suggested for the treatment of post‐extraction bleeding, which include interventions aimed at both local and systemic causes. This is an update of a review published in June 2016.
Objectives
To assess the effects of interventions for treating different types of post‐extraction bleeding.
Search methods
Cochrane Oral Health’s Information Specialist searched the following databases: Cochrane Oral Health’s Trials Register (to 24 January 2018), the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, 2017, Issue 12), MEDLINE Ovid (1946 to 24 January 2018), Embase Ovid (1 May 2015 to 24 January 2018) and CINAHL EBSCO (1937 to 24 January 2018). The US National Institutes of Health Trials Registry (ClinicalTrials.gov) and the World Health Organization International Clinical Trials Registry Platform were searched for ongoing trials. We searched the reference lists of relevant systematic reviews.
Selection criteria
We considered randomised controlled trials (RCTs) that evaluated any intervention for treating PEB, with male or female participants of any age, regardless of type of teeth (anterior or posterior, mandibular or maxillary). Trials could compare one type of intervention with another, with placebo, or with no treatment.
Data collection and analysis
Three pairs of review authors independently screened search records. We obtained full papers for potentially relevant trials. If data had been extracted, we would have followed the methods described in the Cochrane Handbook for Systematic Reviews of Interventions for the statistical analysis.
Main results
We did not find any randomised controlled trial suitable for inclusion in this review.
Authors' conclusions
We were unable to identify any reports of randomised controlled trials that evaluated the effects of different interventions for the treatment of post‐extraction bleeding. In view of the lack of reliable evidence on this topic, clinicians must use their clinical experience to determine the most appropriate means of treating this condition, depending on patient‐related factors. There is a need for well designed and appropriately conducted clinical trials on this topic, which conform to the CONSORT statement (www.consort‐statement.org/).
Keywords: Female, Humans, Male, Oral Hemorrhage, Oral Hemorrhage/etiology, Oral Hemorrhage/therapy, Postoperative Hemorrhage, Postoperative Hemorrhage/etiology, Postoperative Hemorrhage/therapy, Tooth Extraction, Tooth Extraction/adverse effects
Plain language summary
Interventions for managing bleeding after tooth removal
Review question
We conducted this review to assess different interventions for treating bleeding after tooth removal.
Background
After tooth extraction, it is normal for the area to bleed and then clot, generally within a few minutes. It is abnormal if bleeding continues without clot formation, or lasts beyond 8 to 12 hours; this is known as post‐extraction bleeding (PEB). Such bleeding incidents can cause distress for patients, who might need emergency dental consultations and interventions. The causes of PEB can be local, a systemic disease, or a medication. To control this bleeding, many local and systemic methods have been practised, based on the clinician's expertise. To inform clinicians about the best treatment, evidence is needed from studies where people have been randomly allocated to one of at least two different groups, which receive different treatments, or no treatment (i.e. 'randomised controlled trials' or RCTs).
Study characteristics
Authors working with Cochrane Oral Health updated this review of RCTs that assess interventions to treat bleeding after tooth removal. The original review was published in June 2016. For this version, we searched the medical and dental literature to 24 January 2018. We found no RCTs that met the inclusion criteria for our review.
Key results and quality of the evidence
This review revealed that there is no RCT evidence for the effectiveness of any available intervention for treating post‐extraction bleeding. High quality RCTs are needed to help clinicians and patients make informed choices about treatment options.
Summary of findings
Summary of findings for the main comparison. Summary of findings for interventions to treat post‐extraction bleeding.
| Interventions for treating post‐extraction bleeding | ||||||
|
Patient or population: people with post‐extraction bleeding Settings: hospital or dental practice | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
Bleeding, as measured by:
|
No data are available as no RCTs have been conducted on interventions to treat post‐extraction bleeding |
|||||
| Patient‐reported outcomes related to pain or discomfort during the procedure | ||||||
| Treatment‐associated average cost | ||||||
| Adverse events | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Tooth removal, or extraction, is one of the most common invasive oral surgical procedures carried out in routine dental practice (Van Galen 2014), and post‐extraction bleeding is a recognised, frequently encountered complication (McCormick 2014a). Immediately following the removal of a tooth, bleeding or oozing commonly occurs. This bleeding can be easily controlled in most cases (Amer 2014), and almost completely stops within eight hours of extraction. However, sometimes it may continue, resulting in a life‐threatening situation (Funayama 1994). It is important to distinguish between active bleeding from the surgical site and oozing. The active bleeding complication is commonly termed 'post‐extraction bleeding' (PEB) or 'post‐operative bleeding after extraction'. Amer 2014 has described PEB as "evidence of bleeding beyond the pressure pack". Lockhart 2003 has provided four criteria to define PEB, namely that it:
continues beyond 12 hours;
causes the patient to call or return to the dental practitioner, or go to the accident and emergency department;
results in the development of a large haematoma or ecchymosis within the oral soft tissues; or
requires a blood transfusion, hospitalisation, or both.
The rate of postoperative bleeding after extraction of mandibular third molars is 0.6% and after extraction of maxillary third molars is 0.4% (Chiapasco 1993). Jensen 1974 reviewed 103 cases of postoperative prolonged bleeding after oral surgery and reported that 75% of PEB occurred within eight hours of the surgery, and only four patients had coagulation deficiencies. He also reported that postoperative prolonged bleeding from the mandibular molars is more common (80%) than bleeding from the maxillary molars (20%) because of the highly vascular floor of the mouth. Wells 2000 reported that the risk of prolonged bleeding was 0.2% to 1.4% in cases of third molar removal surgery. Iwabuchi 2014 reported 2.77% clinically‐significant PEB in patients receiving warfarin therapy, and 0.39% in non‐warfarin groups, regardless of the type of teeth (95% CI 0.65% to 4.10%). Kataoka 2016 reported that the incidence of PEB ranged from 0 to 26% in their cohort study. Yagyuu 2017 reported that the risk of post‐extraction bleeding was similar for patients on direct oral anticoagulants and Vitamin K antagonist extractions.
Post‐extraction bleeding has been attributed to various factors that can be broadly classified as local and systemic (McDonnell 2013; Van Galen 2014). Post‐extraction bleeding can be caused locally, from soft tissue or bone bleeding. Soft tissue bleeding can be due to traumatic extraction, leading to laceration of blood vessels (arterial, venous or capillary). Bone or osseous bleeding can be from either the nutrient canals or from the central vessels. Inflammation at the site of extraction, the presence of infection, traumatic extraction, and failure of the patient to follow post‐extraction instructions have also been associated with PEB. Systemic factors include platelet problems, coagulation disorders or excessive fibrinolysis, and inherited or acquired problems (medication induced).
Post‐extraction bleeding can be categorised as primary prolonged bleeding, intermediate or reactionary prolonged bleeding, and secondary prolonged bleeding. Primary prolonged bleeding occurs during the extraction procedure, and may be due to traumatic extraction leading to laceration of blood vessels, infections, such as periapical granuloma, or injury to the bone. Patients with primary prolonged bleeding present with their mouth actively filling with blood immediately after removing the haemostatic dressing. Reactionary bleeding occurs a few hours post‐extraction and is more common in patients with systemic disorders or patients on anticoagulant therapy. Secondary bleeding (liver clots) usually occurs 7 to 10 days after extraction, and is a complication rarely encountered in dental practice (Malik 2008; Table 2). Abdullah 2014 has classified PEB as mild (oozing), moderate (bleeding persisting on the second day of extraction), and severe (any bleeding that needed hospitalisation).
1. Types of bleeding after dental extractions.
| Normal bleeding | Post‐extraction bleeding | ||
| Primary | Reactionary | Secondary | |
|
|
|
|
In this review, we considered all the definitions and classifications described above as PEB.
Description of the intervention
The treatment of bleeding complications following a dental extraction is an essential skill for the dental practitioner (McCormick 2014b). Clinical decision making on how to control PEB depends on multiple factors, including the surgical location and site of bleeding, wound size, extent of bleeding, accessibility of the bleeding site, and timing of bleeding (Howe 2013). Furthermore, the selection of an intervention strategy to achieve haemostasis (blood clot formation at the site of vessel injury (Traver 2006)) also depends upon whether the patient is taking any medication or is suffering from any systemic disease, such as cirrhosis, that can affect bleeding and coagulation (McCormick 2014b).
Interventions for treating PEB can be broadly categorised into local and systemic interventions. Local interventions can be further subdivided into surgical interventions, non‐surgical interventions and a combination of both.
Local interventions
Surgical intervention mainly involves suturing the extraction or bleeding site (Bajkin 2014; Van Galen 2014).
Non‐surgical haemostatic measures, or styptics, encompass an array of pharmacotherapies, sealants, adhesives, absorbable agents, biologics, and combination products (Howe 2013). Common haemostatic agents used in oral surgery in extraction sites include the following (Al‐Belasy 2003; Mingarro‐de‐León A 2014): local pressure application with gauze, oxidised cellulose (Abdullah 2014), gel foam, thrombin, collagen fleeces (Baumann 2009), cyanoacrylate glue, acrylic or surgical splints (Anderson 2013), local antifibrinolytic solutions, such as tranexamic acid mouthwash (Carter 2003), fibrin glue or adhesive (Cocero 2015), resorbable gelatin sponge, collagen sponge, gauze soaked with tranexamic acid (Perdigão 2012), chlorhexidine bio‐adhesive gel, calcium alginate (Scarano 2014), Haemocoagulase (Joshi 2014), Ankaferd Blood Stopper (Amer 2014), green tea extract (Soltani 2014), Chitosan‐based dressings (Pippi 2015; Sharma 2017), and bone wax.
Various combinations of surgical and non‐surgical interventions have also been used, such as tranexamic acid mouthwash along with gelatin sponge and sutures, and fibrin glue with collagen fleece and sutures (Al‐Belasy 2003).
Systemic interventions
Systemic interventions are especially important in patients who have an associated systemic cause for bleeding. The role of local haemostatics is limited in these cases, because their use results in only temporary cessation of bleeding (Auluck 2004). Systemic interventions include administration of fresh frozen plasma (FFP), platelets, or both (Cocero 2015), factor replacement therapy, using recombinant or plasma‐derived anti‐haemophilic factor A (FVIII) or anti‐haemophilic factor B or Christmas factor (FIX) in the case of haemophilia, and plasma‐derived Von Willebrand factor (VWF)/FVIII concentrates in the case of Von Willebrand disease (Anderson 2013), intranasal desmopressin (Stanca 2010), intravenous synthetic vasopressin (Minkin 2015), oral or intravenous tranexamic acid (Morimoto 2004), oral or intravenous epsilon amino‐d‐caproic acid (Van Galen 2014). There are contradictory opinions on discontinuation of antithrombotic medications; for example, Aframian 2007 suggests the discontinuation of these medications, whereas Wahl 2016 suggests these medications for dental procedures should not be interrupted, as the prognosis of potential post‐extraction bleeding that could result from antithrombotic continuation is better than the prognosis of a potential stroke or heart attack that could follow antithrombotic interruption.
How the intervention might work
Haemostasis, or control of bleeding, in the oral cavity is dependent on the dynamic balance between fibrin formation and resolution and is influenced by the external environment, which contains both plasminogen and plasminogen activators (Carter 2003). It is a complex interaction between platelets, plasma proteins, and coagulation and fibrinolytic pathways. The clotting cascade involves the sequential activation of proenzymes in a stepwise response, which ultimately provides local generation of fibrin lattices that reinforce the platelet plug (Traver 2006). The coagulation process consists of three main phases: initiation, amplification, and propagation (Figure 1; Glick 2013). The initiation phase begins with injury to the endothelium and tissue factor release, ultimately leading to thrombin formation. Platelet aggregation and activation occur during the amplification phase (Glick 2013), and provide the initial haemostatic response (Traver 2006). Finally, fibrin formation and stabilisation of the platelet clot occur during the propagation phase (Glick 2013).
1.
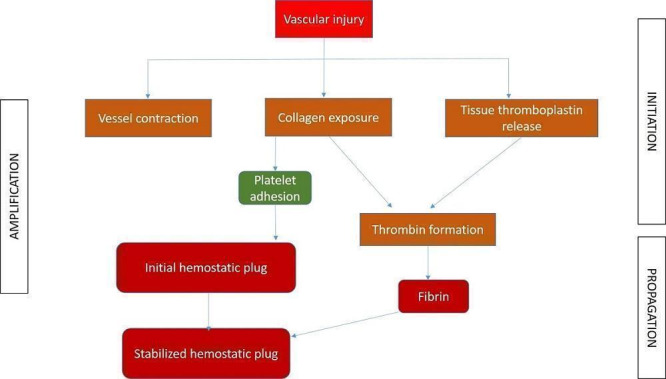
Different phases of coagulation
Different interventions to control PEB basically interfere with the clotting cascade at different levels, resulting in cessation of bleeding. The mechanism of action of various interventions can be broadly summarised, based on the different phases.
Initiation phase: pressure packs, suture, bone wax, cellulose, styptics, gel foam, tranexamic acid, aminocaproic acid, cryoprecipitate, desmopressin (DDAVP), factor VIII concentrate and prothrombin complex concentrate (PCC) act at different stages of this phase.
Amplification phase: ethamsylate, haemostatic collagen and Actcell® act during this phase.
Propagation phase: cryoprecipitate and recombinant factor (VIIa) act during this phase.
Local interventions work either mechanically or by augmenting the coagulation cascade. Haemostatic agents act to stop bleeding by causing vasoconstriction or promoting platelet aggregation, whereas tissue adhesives or sealants bind to and close defects in tissue (Traver 2006). Systemic interventions work by inhibiting fibrinolysis or promoting coagulation (Van Galen 2014).
Why it is important to do this review
Cochrane Oral Health undertook an extensive prioritisation exercise in 2014 to identify a core portfolio of titles that were the most clinically important ones to maintain in the Cochrane Library (Worthington 2015). This review was identified as a priority title by the oral and maxillofacial surgery expert panel (Cochrane OHG priority review portfolio).
A wide array of techniques are suggested for the treatment of PEB and different guidelines have been published (Higginson 2007; University of Cambridge). Until our first version of this review in June 2016, there had been no Cochrane review to summarise the effects of the various interventions available to treat PEB and provide evidence to guide clinical dental practice. Considering the different complex interventions addressing various outcome measures, it seems important to attempt to describe the components of interventions and to identify effective intervention strategies. A systematic review can inform the implementation of different approaches and trigger the development of new interventions on the basis of current best evidence. A systematic review on this topic is also needed since interventions of questionable effectiveness and unclear consequences might be in use.
Objectives
To assess the effects of interventions for treating different types of post‐extraction bleeding.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials (RCTs) evaluating any intervention for treating post‐extraction bleeding (PEB). We excluded quasi‐RCTs, cross‐over trials and preventive trials. We had planned to include split‐mouth studies, provided there was no possibility of contamination. Split‐mouth design is one of the self‐controlled study designs that is unique to dentistry. The design is characterised by subdividing the mouth of the subject into homogeneous within‐patient experimental units such as quadrants, sextants, contralateral or ipsilateral quadrants or sextants, or a symmetrical combination of these.
Types of participants
We considered trials with participants of any age and either gender, who reported PEB, regardless of the type of teeth (anterior or posterior, mandibular or maxillary).
Types of interventions
We considered any surgical or non‐surgical technique or material used for the treatment of PEB. We had planned to make the following comparisons.
Direct comparisons of different interventions
Intervention versus placebo or no treatment
Types of outcome measures
Primary outcomes
Bleeding ‐ measured by:
amount of blood loss;
complete cessation of bleeding, as assessed clinically by the investigator;
time required for the control of bleeding.
Secondary outcomes
Patient‐reported outcomes related to pain or discomfort during the procedure;
Treatment‐associated average cost;
Adverse effects.
Search methods for identification of studies
Electronic searches
Cochrane Oral Health’s Information Specialist conducted systematic searches in the following databases for randomised controlled trials and controlled clinical trials without language or publication status restrictions:
Cochrane Oral Health’s Trials Register (searched 24 January 2018) (Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 12) in the Cochrane Library (searched 24 January 2018) (Appendix 2);
MEDLINE Ovid (1946 to 24 January 2018) (Appendix 3);
Embase Ovid (1 May 2015 to 24 January 2018) (Appendix 4);
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 24 January 2018) (Appendix 5).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6 (Lefebvre 2011). Due to the Cochrane Centralised Search Project to identify all clinical trials in the database and add them to CENTRAL, only most recent months of the Embase database were searched at the review's inception, and this search was updated for this version of the review. See the searching page on the Cochrane Oral Health website for more information. No other restrictions were placed on the date of publication when searching the electronic databases.
Searching other resources
The following trial registries were searched for ongoing studies:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 24 January 2018) (Appendix 6);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 24 January 2018) (Appendix 7).
We searched the reference lists of relevant systematic reviews for further studies.
We did not perform a separate search for adverse effects of interventions.
Data collection and analysis
Selection of studies
Three pairs of review authors (Ashok L (AL) and Prashanti Eachempati (PE), Himanshi Aggarwal (HA) and Kiran Kumar (KK), and Muthu MS (MMS) and Haszelini Hassan (HH)), independently and in duplicate, screened the titles and abstracts from the electronic searches to identify potentially eligible studies. The search was designed to be sensitive and include controlled clinical trials; these were filtered out early in the selection process if they were not randomised. We obtained full‐text copies of all eligible and potentially eligible studies, which were independently evaluated by two authors (Sumanth Kumbargere Nagraj (SKN) and PE) . We resolved disagreements by discussion. When resolution was not possible, we consulted an arbiter (Adinegara Lutfi Abas). We assessed articles in languages other than English after the abstracts had been translated (Table 2). We did not find any trials that fulfilled the inclusion criteria.
Data extraction and management
We had planned that two review authors (SKN, PE) would independently extract the data; and would not have been blinded to the authors. We would have resolved any disagreements by discussion between the two review authors, if necessary, consulting a third review author (PE) in order to reach consensus. We had planned to extract data using a customised data extraction form. All the items in the data extraction form were designed following guidance from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We would have entered study details into the 'Characteristics of included studies' table in Review Manager (RevMan) software (RevMan 2014), recording the following details for each included trial:
publication details such as year of publication, language;
demographic details of the report;
inclusion and exclusion criteria;
type of trial, sample size, method of randomisation, allocation concealment, blinding, method of assessing the outcomes and drop‐outs, if any;
type of intervention;
details of the outcomes reported;
duration of follow‐up;
results of the intervention;
funding details.
We had planned to write, email or telephone the contact author of included studies when clarification of details or additional data were required.
Assessment of risk of bias in included studies
Two review authors (SKN and PE) had planned to independently assess the risk of bias in the included trials in seven domains:
random sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias);
selective outcome reporting (reporting bias);
other biases.
For each of these components, we had planned to assign a judgment regarding the risk of bias as either high, low, or unclear based on guidance in Higgins 2011. We had planned to contact the trial authors if details were missing or unclear, and resolve disagreements through consensus. We had planned to record our judgements and justifications in 'Risk of bias' tables for each included study and generate a 'Risk of bias' summary graph and figure. We would have used these judgements to grade the overall quality of evidence for each comparison and outcome in the 'Summary of findings' tables.
We had planned to summarise the risk of bias according to Higgins 2011; see Table 3.
2. Summarising the risk of bias for a body of evidence.
| Risk of bias | Interpretation | In outcome | In included studies |
| Low risk of bias | Plausible bias unlikely to seriously alter the results | Low risk of bias for all key domains | Most information is from studies at low risk of bias |
| Unclear risk of bias | Plausible bias that raises some doubt about the results | Unclear risk of bias for one or more key domains | Most information is from studies at low or unclear risk of bias |
| High risk of bias | Plausible bias that seriously weakens confidence in the results | High risk of bias for one or more key domains | The proportion of information from studies at high risk of bias is sufficient to affect the interpretation of results |
Measures of treatment effect
We had expected our primary outcome to be expressed in dichotomous data. We would have expressed the effect estimate as a risk ratio (RR) with 95% confidence intervals (CI). We had expected our secondary outcomes to be presented as dichotomous data, continuous data, or ordinal scales. We had planned to use means and standard deviations to calculate mean differences and 95% CI for continuous data measured with the same scales and standardised mean differences if studies used different scales to measure the same outcome.
If data were expressed in ordinal scales, we had planned to explore the possibility of converting them to dichotomous outcomes. If outcomes were reported at baseline, trial endpoints, or follow‐up, we had planned to extract the mean change and standard deviation from baseline for each treatment group. We had intended to pool either end scores or change scores, but preferred end scores when available; we would have combined end and change scores where necessary.
We had planned to analyse data expressed as counts (number of bleeding incidents) in the same way as continuous data.
Unit of analysis issues
We expected two types of non‐standard study designs in this review:
multiple treatment groups;
split‐mouth design
We were expecting data related to repeated observation on participants for our secondary outcome of time required for the control of bleeding. In this case, we had planned to follow the method described in section 9.3.4 of the Cochrane Handbook (Higgins 2011).
In trials where adverse effects were described as counts, we wanted to follow the method described in section 9.2.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In the case of dropouts, we had planned to use what the paper reports and deal with it in the 'Risk of bias' assessment. We were not expecting to find any cluster‐randomised trials for this condition.
Dealing with missing data
We had planned to contact study authors to obtain missing data. We would have used the methods outlined in section 16.1.2 of the Cochrane Handbook for Systematic Reviews of Interventions to calculate missing standard deviations. If it had not been possible to calculate the SDs, we would have described the outcomes qualitatively (Higgins 2011).
Assessment of heterogeneity
If meta‐analyses were performed, we would have assessed heterogeneity using a Chi² test, where a P value < 0.1 indicated statistically significant heterogeneity. We would have quantified heterogeneity using the I² statistic as follows:
0% to 40% implies slight heterogeneity;
30% to 60% moderate heterogeneity;
50% to 90% substantial heterogeneity;
75% to 100% considerable heterogeneity.
If there was considerable heterogeneity (I² > 75%), which could not be explained by the subgroup analyses, we planned not to conduct meta‐analysis. We had planned to interpret I² values between 0% to 40% as possibly insignificant, 30% to 60% as possibly significant, 50% to 90% as possibly substantial, and 75% to 100% as possibly very substantial ('considerable'); depending on whether the inconsistency in results was due to differences in the direction of effect estimates between trials rather than due to differences in the magnitude of effect estimates favouring an intervention (Deeks 2011).
Assessment of reporting biases
If there were more than 10 studies included in a meta‐analysis, we had planned to assess the possible presence of reporting bias by testing for asymmetry in a funnel plot. If present, we would have carried out statistical analyses using the methods described by Egger 1997.
Data synthesis
We had planned to analyse the data using RevMan software (RevMan 2014). If the data available from the studies had similar comparisons and outcomes, we would have undertaken a meta‐analysis. Our general approach would have been to use a random‐effects model. With this approach, the CIs for the average intervention effect are wider than those obtained using a fixed‐effect approach, leading to a more conservative interpretation. We wanted to use all end scores or all change scores when available, but would have combined end and change scores where necessary, using the criteria in section 9.4.5.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We had planned to report the results from studies not included in a meta‐analysis in additional tables.
Subgroup analysis and investigation of heterogeneity
If there was significant heterogeneity, we had planned to explore the reasons by performing the following subgroup analyses based on different groups of patients. The subgroups were to be divided based on:
type of PEB (primary, reactionary, or secondary);
type of extraction (surgical or forceps);
type of underlying bleeding or clotting disorder (deficiency of factors, qualitative disorders, vessel disorders);
drug history (anticoagulant, antiplatelet, or combination);
type of teeth (deciduous or permanent, mobile or firm, maxillary or mandibular, anterior or posterior).
Sensitivity analysis
If there were sufficient included studies, we would have undertaken sensitivity analysis based on risk of bias, including only studies at low risk of bias.
Summarising findings and assessing the quality of the evidence
We had planned to use the GRADE approach to interpret findings (Schunemann 2008). We had planned to use GRADE Profiler software (GRADEpro GDT 2014), and import data from RevMan 2014 to create 'Summary of findings' tables for each comparison included in the review. These tables were to provide information concerning the overall quality of the evidence from the trials, the magnitude of effect of the interventions examined, and the sum of available data on the primary and secondary outcomes. The GRADE approach considers ‘quality’ to be a judgment of the extent to which we can be confident that the estimates of effect are correct (Schunemann 2008). A body of evidence from randomised controlled studies is initially graded as high and downgraded by one or two levels on each of five domains, after full consideration of: any limitations in the design of the studies, the directness (or applicability) of the evidence, the consistency of results, precision of the results, and the possibility of publication bias. A quality level of 'high' reflects confidence that the true effect lies close to that of the estimate of the effect for an outcome. A judgement of 'moderate' quality indicates that the true effect is likely to be close to the estimate of the effect, but acknowledges the possibility that it could be substantially different. 'Low' and 'very low' quality evidence limit our confidence in the effect estimate (Balshem 2011).
Results
Description of studies
We found no published or ongoing randomised controlled trials that evaluated interventions for treating post‐extraction bleeding.
Results of the search
Our search strategy identified 1916 titles and abstracts of studies up to 24 January 2018. These were independently assessed for relevance by three pairs of review authors (AL and PE; HA and KK; MMS and HH). We checked the reference lists of the excluded studies and added another 24 references. After removal of duplicates, we had a total of 1187 records. We rejected 1147 based on the abstracts. We obtained full texts for other 40 trials. Twenty‐eight of these were not RCTs (studies not in English that we translated are listed in Appendix 8). We excluded the other 12 trials for reasons described below. None of the trials met the inclusion criteria for our review (Figure 2).
2.
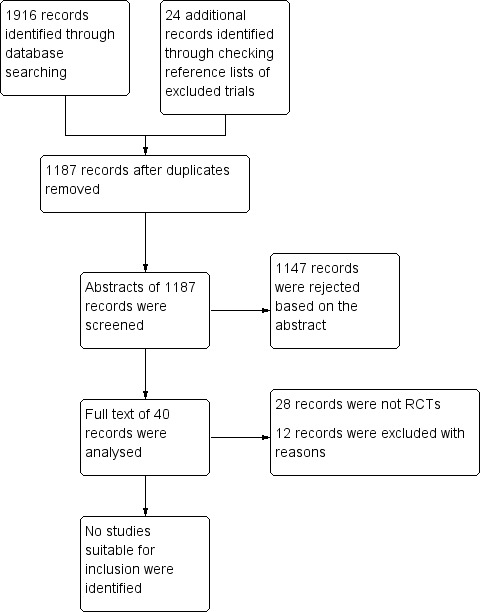
Study flow diagram
Included studies
We did not find any studies suitable for inclusion.
Excluded studies
We excluded 12 trials for the following reasons. Al‐Mubarak 2006 evaluated the safety of dental extractions in patients taking oral anticoagulants. The study evaluated the efficacy of suturing and the effect of warfarin on prevention of post‐extraction bleeding. Ak 2012; Carter 2003; Howard 1973; Henderson 1998; Pinsent 1986; CTRI/2017/09/009784; NCT02918045; NCT03108365 and Zhou 1985 were preventive trials. Kjellman 1973 assessed pain management after removal of impacted third molars. Medeiros 2011 assessed the amount of intraoperative and postoperative bleeding with acetylsalicylic acid versus acetylsalicylic acid therapy that was suspended seven days preoperatively.
Risk of bias in included studies
No trials were included.
Effects of interventions
See: Table 1
No studies fulfilled our inclusion criteria.
Discussion
Post‐extraction bleeding (PEB) is one of the treatment complications of dental extraction that might make a patient panic and seek immediate dental consultation. With the increasing number of patients on anticoagulant therapy with aspirin, warfarin, and clopidogrel, the chance of encountering PEB is increasing. Usually, these people are aware of their medical condition and report their medical history. It is normal to use precautionary measures to prevent PEB in such patients. However, this may not be the situation in low‐ and middle‐income countries, where the majority of patients may not give a proper medical and drug history, and medical records may not be accessible. Hence, it is important to know how to control PEB in cases where no preventive measures were used.
Post‐extraction bleeding can also occur due to local or systemic problems that are not expected in routine dental extractions. We do not have any evidence‐based guidelines to manage such cases. The present review aimed to assess the effects of various interventions for the treatment of different types of PEB.
We did not find any suitable trials to include in our review. This is because most of the trials advocated preventive measures prior to and immediately after extraction, and reported either the incidence of PEB or tested preventive measures. Most of these trials reported the management of PEB based on clinician experience. Several trials tested whether anticoagulants, antiplatelets, or antifibrinolytics should be stopped before dental extraction, and reported varying incidence rates of PEB in control and intervention groups.
The majority of the preventive trials randomised extraction cases into intervention groups. An ideal trial for this review would randomise PEB (primary or reactionary or secondary) cases instead.
This topic seems to be an unexplored area of primary research. We found one observational trial in a German clinical trial register in which postoperative bleeding incidents after dental treatment in patients with and without anticoagulant therapy were studied (DRKS00009286). We found no non‐Cochrane systematic reviews on this topic. We identified two narrative reviews based on the authors' opinions (Leonard 1995; McCormick 2014a). We found two websites that have published guidelines to manage PEB (Emed handbook by Higginson 2007; University of Cambridge).
We observed the term 'post‐extraction bleeding' being used in different ways. Joshi 2014 used PEB terminology to describe the normal bleeding that happens after dental extraction in their study. Al‐Bahlani 2001 describes any type of bleeding after dental extraction as postoperative bleeding. This can create confusion and there is a need to standardise PEB terminology and its definition.
Authors' conclusions
Implications for practice.
We identified no published or ongoing randomised controlled clinical trials on interventions to treat post‐extraction bleeding, so it is not possible to present evidence to clinicians or patients. In the absence of any evidence from randomised controlled trials, clinicians should base their decisions on clinical experience, in conjunction with evidence from preventive trials.
Implications for research.
There is a need for robust clinical trials to evaluate the effects of interventions for the treatment of PEB. Future randomised controlled trials should focus on interventions to control bleeding in patients after the extraction, and in whom no preventive interventions have been advocated. This will help us understand the best intervention strategy to be used if an emergency situation arises post‐extraction, to control primary, secondary or reactionary haemorrhage. Considering the varying incidence of PEB (0% to 26%), multicentric trials to achieve appropriate sample size (power of the study) should be conducted. Any future trials should be well designed and reported according to the CONSORT statement (http://www.consort‐statement.org/).
What's new
| Date | Event | Description |
|---|---|---|
| 14 May 2018 | Review declared as stable | There are no completed or ongoing randomised controlled trials on this topic. |
History
Protocol first published: Issue 10, 2015 Review first published: Issue 6, 2016
| Date | Event | Description |
|---|---|---|
| 21 February 2018 | New citation required but conclusions have not changed | We excluded an additional three trials. |
| 24 January 2018 | New search has been performed | We updated our search to 20 February 2018. We made minor changes to update the Background. |
Notes
This review will no longer be updated as there are no completed or ongoing randomised controlled trials evaluating treatments for post‐extraction bleeding.
Acknowledgements
The authors would like to thank Pradeep Kumar for screening the titles and abstracts for the previous version of this review.
The authors thank Ms. Anne Littlewood, Information Specialist, Ms. Janet Lear, Administrator, Ms. Laura CI MacDonald, Managing Editor, and Ms. Helen Wakeford, Deputy Managing Editor, all of Cochrane Oral Health. We thank Philip Riley and Ruth Floate for their comments. We also thank Ms. Shazana Binti Mohd Selva, Chief Librarian, Dr. Venkatachalapathy Suram, Professor, Professor Dr.Adinegara Lutfi Abas, Dean, Faculty of Medicine, Professor Dr. Abdul Rashid Haji Ismail, Dean, Faculty of Dentistry, Melaka Manipal Medical College, Manipal Academy of Higher Education, Melaka, Malaysia, and Professor Dr. Ravi Kant, Vice Chancellor, King George's Medical University, Lucknow, India for all the suggestions and help during the review preparation. We also thank Dr. Naresh Yedthare Shetty, Senior Lecturer, International Medical University, Kuala Lumpur for his valuable suggestions.
We acknowledge the help of foreign language translators Joanna Zajac, Malgorzata Bala, Paul Tramini, Lilia Ziganshina, Karin Rau, Anette Blümle, Anna Misyail Abdul Rashid, Loh Zheng Tao, Giovanni Lodi, Andrea Pokorna, Maddalena Manfredi, Ubai Alsharif, Dr Liyuan Ma, Professor Chengge Hua, and Professor Zongdao Shi.
Appendices
Appendix 1. Cochrane Oral Health's Trials Register search strategy
((tooth or teeth or molar* or bicuspid* or cuspid* or incisor*):ti,ab) AND (INREGISTER)
((extract* or remov* or surgery):ti,ab) AND (INREGISTER)
(#1 and #2) AND (INREGISTER)
((bleed* or "blood loss" or hemorrhag* or haemorrhag* or hemorrag* or haemorrag*):ti,ab) AND (INREGISTER)
(#3 and #4) AND (INREGISTER)
Appendix 2. The Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 [mh ^"Tooth extraction"] #2 [mh Tooth] #3 (tooth or teeth or molar* or bicuspid* or cuspid* or incisor*) #4 [mh ^"Tooth, impacted"] #5 {or #2‐#4} #6 (extract* or remov* or surgery) #7 #5 and #6 #8 #1 or #7 #9 [mh ^"Blood loss, surgical"] #10 [mh ^"Postoperative hemorrhage"] #11 [mh ^"Hemorrhagic disorders"] #12 [mh ^"Oral hemorrhage"] #13 (bleed* or "blood loss" or hemorrhag* or haemorrhag* or hemorrag* haemorrag*) #14 {or #9‐#13} #15 #8 and #14
Appendix 3. MEDLINE Ovid search strategy
1. Tooth extraction/ 2. exp Tooth/ 3. (tooth or teeth or molar$ or bicuspid$ or cuspid$ or incisor$).ti,ab. 4. Tooth, impacted/ 5. or/2‐4 6. (extract$ or remov$ or surgery).ti,ab. 7. 5 and 6 8. 1 or 7 9. Blood loss, surgical/ 10. Postoperative hemorrhage/ 11. Hemorrhagic disorders/ 12. Oral hemorrhage/ 13. (bleed$ or "blood loss" or hemorrhag$ or haemorrhag$ or hemorrag$ or haemorrag$).ti,ab. 14. or/9‐13 15. 8 and 14
This subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity‐maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of The Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011] (Lefebvre 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 4. Embase Ovid search strategy
1. Tooth extraction/ 2. exp Tooth/ 3. (tooth or teeth or molar$ or bicuspid$ or cuspid$ or incisor$).ti,ab. 4. 2 or 3 5. (extract$ or remov$ or surgery).ti,ab. 6. 4 and 5 7. 1 or 6 8. Bleeding/ 9. Bleeding disorder/ 10. Oral bleeding/ 11. (bleed$ or "blood loss" or hemorrhag$ or haemorrhag$ or hemorrag$ or haemorrag$).ti,ab. 12. or/8‐11 13. 7 and 12 This subject search was linked to an adapted version of the Cochrane Centralised Search Project filter for identifying RCTs in Embase Ovid (see http://www.cochranelibrary.com/help/central‐creation‐details.html for information):
1. Randomized controlled trial/ 2. Controlled clinical study/ 3. Random$.ti,ab. 4. randomization/ 5. intermethod comparison/ 6. placebo.ti,ab. 7. (compare or compared or comparison).ti. 8. ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 9. (open adj label).ti,ab. 10. ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 11. double blind procedure/ 12. parallel group$1.ti,ab. 13. (crossover or cross over).ti,ab. 14. ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 15. (assigned or allocated).ti,ab. 16. (controlled adj7 (study or design or trial)).ti,ab. 17. (volunteer or volunteers).ti,ab. 18. trial.ti. 19. or/1‐18 20. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 21. 19 not 20
Appendix 5. CINAHL EBSCO search strategy
S13 S8 and S12 S12 S9 or S10 or S11 S11 (bleed* or "blood loss" or hemorrhag* or haemorrhag* or hemorrag* haemorrag*) S10 (MH "Postoperative Hemorrhage") S9 (MH "Blood Loss, Surgical") S8 S1 or S7 S7 S5 and S6 S6 (extract* or remov* or surgery) S5 S2 or S3 or S4 S4 (MH "Tooth, Impacted") S3 (tooth or teeth or molar* or bicuspid* or cuspid* or incisor*) S2 (MH "Tooth+") S1 (MH "Tooth Extraction")
This subject search was linked to Cochrane Oral Health’s filter for CINAHL EBSCO:
S1 MH Random Assignment or MH Single‐blind Studies or MH Double‐blind Studies or MH Triple‐blind Studies or MH Crossover design or MH Factorial Design S2 TI ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or AB ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or SU ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") S3 TI random* or AB random* S4 AB "latin square" or TI "latin square" S5 TI (crossover or cross‐over) or AB (crossover or cross‐over) or SU (crossover or cross‐over) S6 MH Placebos S7 AB (singl* or doubl* or trebl* or tripl*) or TI (singl* or doubl* or trebl* or tripl*) S8 TI blind* or AB mask* or AB blind* or TI mask* S9 S7 and S8 S10 TI Placebo* or AB Placebo* or SU Placebo* S11 MH Clinical Trials S12 TI (Clinical AND Trial) or AB (Clinical AND Trial) or SU (Clinical AND Trial) S13 S1 or S2 or S3 or S4 or S5 or S6 or S9 or S10 or S11 or S12
Appendix 6. US National Institutes of Health Trials Registry (ClinicalTrials.gov) search strategy
“oral surgery” and bleed
“oral surgery” and hemorrhage
tooth and extraction and bleed
tooth and extraction and hemorrhage
Appendix 7. The WHO International Clinical Trials Registry Platform search strategy
Oral surgery and bleed
Oral surgery and hemorrhage
Oral surgery and haemorrhage
Tooth extraction and bleed
Tooth extraction and hemorrhage
Tooth extraction and haemorrhage
Appendix 8. Studies we translated and rejected as ineligible for inclusion
| Trial identification | Title | Language | Translator/s | Reasons for rejection |
| Trentalancia 1967 | The use of 5‐oxytryptamine in post‐extraction haemorrhages | Italian | Giovanni Lodi | 1. The study is not randomised. Randomisation is never mentioned. The author stated that "the patients have been divided as follows" (page 1386). 2. Patients were at risk of bleeding, and not with post‐extraction bleeding. |
| Pavek 1976 | Evaluation of the haemostatic effect of Dicynon in dentoalveolar surgery | Czech | Andrea Pokorna | The study does not fulfil criteria as it describes application of Dicynone in four groups of patients – no randomisation, no detailed description of the patient groups. |
| Szpirglas 1979 | Stomatological haemorrhages; haemostasis with GRF (gelatin‐resorcin‐formol) | Italian | Maddalena Manfredi | This study is a description of a topical haemostasis technique without any report about patients. |
| Marini 1966 | Therapy of post‐extraction haemorrhages in haemophiliac patients with epsilon‐aminocaproic acid (EACA). | Italian | Maddalena Manfredi | This is a case series. |
| Torteli 1965 | Use of "reptilase" in postoperative haemorrhage of the dental apparatus | German | Ubai Alsharif | This is a case series of 14 patients who were treated with Raptilase, and does not fulfil the inclusion criteria. |
| Zhou 1985 | Prevention and treatment of haemorrhage after extraction of teeth by using the pulvis of cibotium barometz‐alum burn | Chinese | Dr Liyuan Ma, Professors Chengge Hua, Zongdao Shi and Mr.Loh Zheng Tao | The trial is a RCT with two arms; however, the randomisation method is not reported. This is a preventive study for reducing post‐extraction complications including PEB, instead of managing post‐extraction bleeding. |
| Antoszewski 1972 | Cepevit‐K preparation in controlling haemorrhages and bleeding following tooth extraction | Polish | Joanna Zajac and Malgorzata Bala | Not a randomised controlled trial. They used Cepevit‐K: ‐ after extraction for 22 patients (for 17, bleeding was stopped within eight minutes; chirurgical treatment was provided for the others). ‐ two days before extraction in 18 patients with low coagulation time (for four patients in this group, additional chirurgical treatment was needed). The only comparison is 20 other people with bleeding after tooth extraction, where the author used other techniques (other than chirurgical treatment); it only worked for eight patients, so the other 12 were given Cepevit‐K. |
| Fetkowska‐Mielnik 1969 | Clinical evaluation of the results of treatment of post‐extraction bleeding with new drugs E.A.C.A., styptanon | Polish | Joanna Zajac and Malgorzata Bala | Not a randomised controlled trial. Study was based on observation of 69 patients, all with blood coagulation problems: some after extraction with bleeding, some before extraction. For all blood analyses were done and according to results: ‐ for some: epsilon aminocaproic acid (EACA) was used ‐ for others: Styptanon was used ‐ for others: EACA and Styptanon together |
| Khomiachenko 1978 | Use of aminocaproic acid for stopping the haemorrhage after tooth extraction | Russian | Lilia Ziganshina and Anna Misyail Abdul Rashid | This is an abstract describing 100% success of 5% aminocapronic acid in 135 patients. This was not a trial; there was no comparison. |
| Neuner 1968 | Therapy of haemorrhage following extractions | German | Karin Rau and Anette Bluemle | This is not a randomised control trial. In this publication, the interventions for post‐extraction bleeding are explained in detail, but not within the scope of a clinical study. |
| Saltykova 1974 | The use of new haemostatic drug in dental practice | Russian | Lilia Ziganshina and Anna Misyail Abdul Rashid | This is a single case report. |
| Martineau 1989 | Hemorrhage in the dental office. Study of local haemostatic treatment | French | Paul Tramini | This paper is just a recommendation for practitioners and students in case of PEB problems. No data are available. |
| Rokicka‐Milewska 1966 | Application of epsilon‐aminocaproic acid for oral mucosal bleeding in haemophiliacs | Polish | Joanna Zajac and Malgorzata Bala | This article is not a RCT. All participants (13 children with haemophilia: nine type A and four type B) received epsilon‐aminocaproic (20% solution) acid 24 hours before tooth extraction. Intolerance developed in some children, so the dose was changed (from 0.1 g/kg of body weight to 0.05g/kg), or the drug was administered intravenously. |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ak 2012 | Preventive trial |
| Al‐Mubarak 2006 | Preventive trial |
| Carter 2003 | Preventive trial |
| CTRI/2017/09/009784 | Trial for management of bleeding immediately after deciduous teeth extraction |
| Henderson 1998 | Preventive trial |
| Howard 1973 | Preventive trial |
| Kjellman 1973 | Trial for pain management after removal of impacted third molar |
| Medeiros 2011 | No treatment. It measured the amount of intraoperative and postoperative bleeding during and after the suspension of aspirin |
| NCT02918045 | Preventive trial |
| NCT03108365 | Preventive trial |
| Pinsent 1986 | Preventive trial |
| Zhou 1985 | Preventive trial |
Differences between protocol and review
We changed the title from 'managing' to 'treating'. We made this change throughout as 'managing' could be interpreted as including prevention, which was not our intention.
Figure 1 in the protocol has been modified to Table 1 in the review ('Types of bleeding after dental extractions').
Primary and secondary outcomes are re‐worded without changing the meaning.
We had mentioned under selection of studies that two pairs of review authors would independently screen the titles and abstracts to identify potentially eligible studies. However, three pairs of review authors screened the titles and abstracts.
As we did not have any included trials, the contribution of authors differed from what we had planned.
Contributions of authors
Sumanth Kumbargere Nagraj: drafting the protocol, evaluation of full text articles for inclusion or exclusion, drafting the final review, and updating the review.
Eachempati Prashanti: drafting the protocol, screening titles and abstracts, evaluation of full text articles for inclusion or exclusion and updating the review.
Himanshi Aggarwal: drafting the protocol, screening titles and abstracts, and drafting the final review.
Ashok Lingappa: content expert, screening titles and abstracts.
Murugan S Muthu: content expert, screening titles and abstracts, and drafting the final review.
Salian Kiran Kumar Krishanappa: undertaking searches, screening titles and abstracts.
Haszelini Hassan: content expert, screening titles and abstracts, and drafting the final review.
Sources of support
Internal sources
-
Faculty of Dentistry, Melaka Manipal Medical College, Manipal University, Melaka Campus, Malaysia.
Library support and providing training in Cochrane Systematic Reviews
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
-
Cochrane Oral Health Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships‐alliances). Contributors over the past year have been the American Association of Public Health Dentistry, USA; AS‐Akademie, Germany; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; and NHS Education for Scotland, UK; Swiss Society of Endodontology, Switzerland.
Declarations of interest
Sumanth Kumbargere Nagraj: no interests to declare Eachempati Prashanti: no interests to declare Himanshi Aggarwal: no interests to declare Ashok Lingappa: no interests to declare Murugan S Muthu: no interests to declare Salian Kiran Kumar Krishanappa: no interests to declare Haszelini Hassan: no interests to declare
Stable (no update expected for reasons given in 'What's new')
References
References to studies excluded from this review
Ak 2012 {published data only}
- Ak G, Alpkılıç Başkırt E, Kürklü E, Koray M, Tanyeri H, Zülfikar B. The evaluation of fibrin sealants and tissue adhesives in oral surgery among patients with bleeding disorders. Turkish Journal of Hematology 2012;29(1):40‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Al‐Mubarak 2006 {published data only}
- Al‐Mubarak S, Rass MA, Alsuwyed A, Alabdulaaly A, Ciancio S. Thromboembolic risk and bleeding in patients maintaining or stopping oral anticoagulant therapy during dental extraction. Journal of Thrombosis and Haemostasis 2006;4(3):689‐91. [DOI] [PubMed] [Google Scholar]
Carter 2003 {published data only}
- Carter G, Goss A, Lloyd J, Tocchetti R. Tranexamic acid mouthwash versus autologous fibrin glue in patients taking warfarin undergoing dental extractions: a randomized prospective clinical study. Journal of Oral and Maxillofacial Surgery 2003;61(12):1432‐5. [DOI] [PubMed] [Google Scholar]
CTRI/2017/09/009784 {unpublished data only}
- CTRI/2017/09/009784. Effectiveness of topical haemocoagulase as a haemostatic agent in children undergoing extraction of deciduous teeth – a split‐mouth, randomized, double‐blind, clinical trial. www.ctri.nic.in (first received 15 September 2017).
Henderson 1998 {published data only}
- Henderson NJ, Crawford PJ, Reeves BC. A randomised trial of calcium alginate swabs to control blood loss in 3‐5‐year‐old children. British Dental Journal 1998;184(4):187‐90. [DOI] [PubMed] [Google Scholar]
Howard 1973 {published data only}
- Howard D, Whitehurst VE, Bingham R, Stanback J. The use of bucrylate to achieve hemostasis in tooth extraction sites. Oral Surgery 1973;35(6):762‐5. [DOI] [PubMed] [Google Scholar]
Kjellman 1973 {published data only}
- Kjellman O. Apernyl as alveolar inlay in connection with the removal of impacted third molars of the lower jaw. A clinical double blind investigation of 100 patients. Swedish Dental Journal 1973;66(2):197‐200. [PubMed] [Google Scholar]
Medeiros 2011 {published data only}
- Medeiros FB, Andrade AC, Angelis GA, Conrado VC, Timerman L, Farsky P, et al. Bleeding evaluation during single tooth extraction in patients with coronary artery disease and acetylsalicylic acid therapy suspension: a prospective, double‐blinded, and randomized study. Journal of Oral and Maxillofacial Surgery 2011;69(12):2949‐55. [DOI] [PubMed] [Google Scholar]
NCT02918045 {published data only}
- NCT02918045. Dental extractions in patients under dual antiplatelet therapy (DUALex). clinicaltrials.gov/ct2/show/NCT02918045 (first received 28 September 2016).
NCT03108365 {published data only}
- NCT03108365. Evaluating the effectiveness of axiostat hemostatic dressing material versus conventional method of hemostasis and healing of extraction wounds in patients on oral anti‐platelet drugs ‐ a comparative study. clinicaltrials.gov/show/NCT03108365 (first received 11 April 2017).
Pinsent 1986 {published data only}
- Pinsent RJ, Baker GP, Ives G, Davey RW, Jonas S. Does arnica reduce pain and bleeding after dental extraction?. British Homeopathic Research Group Communications 1986;15:3‐11. [Google Scholar]
Zhou 1985 {published data only}
- Zhou RJ. 1985 Aug 5 ( 8) 483‐484. Prevention and treatment of hemorrhage after extraction of teeth by using the pulvis of cibotium barometz‐alum burn. Zhong xi yi jie he za zhi [Chinese Journal of Modern Developments in Traditional Medicine] 1985;5(8):483‐4. [PubMed] [Google Scholar]
Additional references
Abdullah 2014
- Abdullah WA, Khalil H. Dental extraction in patients on warfarin treatment. Journal of Clinical, Cosmetic and Investigational Dentistry 2014;6:65‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aframian 2007
- Aframian DJ, Lalla RV, Peterson DE. Management of dental patients taking common hemostasis‐altering medications. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 2007;103 Suppl:S45 e1‐11. [DOI] [PubMed] [Google Scholar]
Al‐Bahlani 2001
- Al‐Bahlani S, Sherriff A, Crawford PJ. Tooth extraction, bleeding and pain control. Journal of the Royal College of Surgeons of Edinburgh 2001;46(5):261‐4. [PubMed] [Google Scholar]
Al‐Belasy 2003
- Al‐Belasy FA, Amer MZ. Hemostatic effect of n‐Butyl‐2‐Cyanoacrylate (Histoacryl) glue in warfarin‐treated patients undergoing oral surgery. Journal of Oral and Maxillofacial Surgery 2003;61:1405‐9. [DOI] [PubMed] [Google Scholar]
Amer 2014
- Amer MZ, Mourad SI, Salem AS, Abdelfadil E. Correlation between International Normalized Ratio values and sufficiency of two different local hemostatic measures in anticoagulated patients. European Journal of Dentistry 2014;8:475‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2013
- Anderson JAM, Brewer A, Creagh D, Hook S, Mainwaring J, McKernan A, et al. Guidance on the dental management of patients with haemophilia and congenital bleeding disorders. British Dental Journal 2013;215:497‐504. [DOI] [PubMed] [Google Scholar]
Antoszewski 1972
- Antoszewski Z. Cepevit‐K preparation in controlling hemorrhages and bleeding following tooth extraction. Polski tygodnik lekarski 1972;27(47):1861‐62. [PubMed] [Google Scholar]
Auluck 2004
- Auluck A, Pai KM, Bhat KS, Thoppil PS. Unusual post‐extraction hemorrhage in a cardiac patient: a case report. Journal of the Canadian Dental Association 2004;70(11):769‐73. [PubMed] [Google Scholar]
Bajkin 2014
- Bajkin BV, Selaković SD, Mirković SM, Šarčev IN, Tadič AJ, Milekič BR. Comparison of efficacy of local hemostatic modalities in anticoagulated patients undergoing tooth extractions. Vojnosanitetski pregled 2014;71(12):1097‐101. [DOI] [PubMed] [Google Scholar]
Baumann 2009
- Baumann P, Schumacher H, Hüsing J, Luntz S, Knaebel HP. A randomized, controlled, prospective trial to evaluate the haemostatic effect of Lyostypt versus Surgicel in arterial bypass anastomosis: "COBBANA" trial. Trials 2009;10:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiapasco 1993
- Chiapasco M, Cicco L, Marrone G. Side effects and complications associated with third molar surgery. Oral Surgery, Oral Medicine, and Oral Pathology 1993;76(4):412‐20. [DOI] [PubMed] [Google Scholar]
Cocero 2015
- Cocero N, Pucci F, Messina M, Pollio B, Mozzati M, Bergamasco L. Autologous plasma rich in growth factors in the prevention of severe bleeding after teeth extractions in patients with bleeding disorders: a controlled comparison with fibrin glue. Blood Transfusion 2015;13(2):287‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
DRKS00009286
- Ermer M. Post‐operative bleeding in dento‐alveolar surgery. Available from http://www.drks.de/DRKS00009286 (accessed 31 March 2016).
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fetkowska‐Mielnik 1969
- Fetkowska‐Mielnik K, Komorowska A. Clinical evaluation of the results of treatment of postextraction bleeding with new drugs E.A.C.A., styptanon. Czasopismo stomatologiczne 1969;22(2):179‐83. [PubMed] [Google Scholar]
Funayama 1994
- Funayama M, Kumagai T, Saito K, Watanabe T. Asphyxial death caused by postextraction hematoma. American Journal of Forensic Medicine and Pathology 1994;15:87–90. [DOI] [PubMed] [Google Scholar]
Glick 2013
- Glick JB, Kaur RR, Siegel D. Achieving hemostasis in dermatology‐Part II: Topical hemostatic agents. Indian Dermatology Online Journal 2013;4(3):172‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 22 March 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [March 2011], The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higginson 2007
- Higginson I. Post Extraction Dental Haemorrhage. EMed Handbook ‐ Cork Emergency Medicine, Available from http://www.emed.ie/HE‐ENT/Dental/Dental_Haemorrhage_Post_Extraction.php#typ (accessed 16 March 2016).
Howe 2013
- Howe N, Cherpelis B. Obtaining rapid and effective hemostasis: Part I. Update and review of topical hemostatic agents. Journal of the American Academy of Dermatology 2013;69(5):659.e1‐17. [DOI] [PubMed] [Google Scholar]
Iwabuchi 2014
- Iwabuchi H, Imai Y, Asanami S, Shirakawa M, Yamane GY, Ogiuchi H, et al. Evaluation of postextraction bleeding incidence to compare patients receiving and not receiving warfarin therapy: a cross‐sectional, multicentre, observational study. BMJ Open 2014;4(12):e005777. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 1974
- Jensen S. Hemorrhage after oral surgery. An analysis of 103 cases. Oral Surgery, Oral Medicine, and Oral Pathology 1974;37(1):2‐16. [DOI] [PubMed] [Google Scholar]
Joshi 2014
- Joshi SA, Gadre KS, Halli R, Shandilya R. Topical use of Hemocoagulase (Reptilase): a simple and effective way of managing post‐extraction bleeding. Annals of Maxillofacial Surgery 2014;4(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kataoka 2016
- Kataoka T, Hoshi K, Ando T. Is the HAS‐BLED score useful in predicting post‐extraction bleeding in patients taking warfarin? A retrospective cohort study. BMJ Open 2016;6(3):e010471. [DOI: 10.1136/bmjopen-2015-010471] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khomiachenko 1978
- Khomiachenko VP. Use of aminocaproic acid for stopping the hemorrhage after tooth extraction. Stomatologiia (Mosk) 1978;57(1):91. [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Leonard 1995
- Leonard MS. An approach to some dilemmas and complications of office oral surgery. Australian Dental Journal 1993;40(3):159‐63. [DOI] [PubMed] [Google Scholar]
Lockhart 2003
- Lockhart PB, Gibson J, Pond SH, Leitch J. Dental management considerations for the patient with an acquired coagulopathy. Part 1: Coagulopathies from systemic disease. British Dental Journal 2003;195(8):439‐45. [DOI] [PubMed] [Google Scholar]
Malik 2008
- Malik NA. Textbook of Oral and Maxillofacial Surgery. Second Edition. New Delhi: Jaypee Brothers Medical Publishers, 2008. [Google Scholar]
Marini 1966
- Marini MP, Arturi F, Crolle G. Therapy of post‐extraction hemorrhages in hemophiliac patients with epsilon‐aminocaproic acid (EACA). Haematologica 1966;51(7):553‐68. [PubMed] [Google Scholar]
Martineau 1989
- Martineau C. Hemorrhage in the dental office. Study of local hemostatic treatment. Information dentaire 1989;71(40):3861‐69. [PubMed] [Google Scholar]
McCormick 2014a
- McCormick NJ, Moore UJ, Meechan JG, Norouzi M. Haemostasis. Part 2: Medications that affect haemostasis. Dental Update 2014;41(5):395‐405. [DOI] [PubMed] [Google Scholar]
McCormick 2014b
- McCormick NJ, Moore UJ, Meechan JG. Haemostasis. Part 1: The management of post‐extraction haemorrhage. Dental Update 2014;41(4):290‐2, 294‐6. [DOI] [PubMed] [Google Scholar]
McDonnell 2013
- McDonnell D. Bleeding sockets. British Dental Journal 2013;215:104. [DOI] [PubMed] [Google Scholar]
Mingarro‐de‐León A 2014
- Mingarro‐de‐León A, Chaveli‐López B, Gavaldá‐Esteve C. Dental management of patients receiving anticoagulant and/or antiplatelet treatment. Journal of Clinical and Experimental Dentistry 2014;6(2):e155‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Minkin 2015
- Minkin P, Bertetti R, Lindsey S, Bovino B. Management of tooth extraction in a patient with a rare bleeding disorder associated with Hermansky‐Pudlak syndrome: a case report. Journal of Oral and Maxillofacial Surgery 2015;73(2):219‐23. [DOI] [PubMed] [Google Scholar]
Morimoto 2004
- Morimoto Y, Yoshioka A, Imai Y, Takahashi Y, Minowa H, Kirita T. Haemostatic management of intraoral bleeding in patients with congenital deficiency of alpha2‐plasmin inhibitor or plasminogen activator inhibitor‐1. Haemophilia 2004;10(5):669‐74. [DOI] [PubMed] [Google Scholar]
Neuner 1968
- Neuner O, Schegg HK. Therapy of hemorrhage following extractions. Schweizerische Monatsschrift fur Zahnheilkunde 1968;78(10):974‐82. [PubMed] [Google Scholar]
Pavek 1976
- Pavek V. Evaluation of the hemostatic effect of Dicynon in dentoalveolar surgery. Ceskoslovenska stomatologie 1976;76(1):56‐64. [PubMed] [Google Scholar]
Perdigão 2012
- Perdigão JP, Almeida PC, Rocha TD, Mota MR, Soares EC, Alves AP, et al. Postoperative bleeding after dental extraction in liver pretransplant patients. Journal of Oral and Maxillofacial Surgery 2012;70:e177‐84. [DOI] [PubMed] [Google Scholar]
Pippi 2015
- Pippi R, Santoro M, Cafolla A. The effectiveness of a new method using an extra‐alveolar hemostatic agent after dental extractions in older patients on oral anticoagulation treatment: an intrapatient study. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology 2015 [Epub ahead of print];120(1):15‐21. [DOI: 10.1016/j.oooo.2015.02.482] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rokicka‐Milewska 1966
- Rokicka‐Milewska R, Krajewska‐Martel A, Dyszy‐Laube B. Application of epsilon‐aminocaproic acid for oral mucosal bleeding in hemophiliacs. Polskie Archiwum Medycyny Wewnetrznej 1966;37(4):445‐50. [PubMed] [Google Scholar]
Saltykova 1974
- Saltykova ZA, Tarasova LN. The use of new hemostatic drug in dental practice. Stomatologiia 1974;53(1):85‐6. [PubMed] [Google Scholar]
Scarano 2014
- Scarano A, Sinjari B, Murmura G, Mijiritsky E, Iaculli F, Mortellaro C, et al. Hemostasis control in dental extractions in patients receiving oral anticoagulant therapy: an approach with calcium sulfate. Journal of Craniofacial Surgery 2014;25(3):843‐6. [DOI] [PubMed] [Google Scholar]
Sharma 2017
- Sharma S, Kale TP, Balihallimath LJ, Motimath A. Evaluating effectiveness of axiostat hemostatic material in achieving hemostasis and healing of extraction wounds in patients on oral antiplatelet drugs. Journal of Contemporary Dental Practice 2017;18(9):802‐6. [PUBMED: 28874645] [DOI] [PubMed] [Google Scholar]
Soltani 2014
- Soltani R, Haghighat A, Fanaei M, Asghari G. Evaluation of the effect of green tea extract on the prevention of gingival bleeding after posterior mandibular teeth extraction: a randomized controlled trial. Evidence‐Based Complementary and Alternative Medicine 2014; Vol. 2014, issue article 857651. [DOI: 10.1155/2014/857651] [DOI] [PMC free article] [PubMed]
Stanca 2010
- Stanca CM, Montazem AH, Lawal A, Zhang JX, Schiano TD. Intranasal eesmopressin versus blood transfusion in cirrhotic patients with coagulopathy undergoing dental extraction: a randomized controlled trial. Journal of Oral and Maxillofacial Surgery 2010;68:138‐43. [DOI] [PubMed] [Google Scholar]
Szpirglas 1979
- Szpirglas H, Gotte P, Tsamis J, Fraccari F. Stomatological hemorrhages; hemostasis with GRF (gelatin‐resorcin‐formol). Minerva stomatologica 1979;28(4):285‐7. [PubMed] [Google Scholar]
Torteli 1965
- Torteli A, Hattyasy D. Use of "reptilase" in postoperative hemorrhages of the dental apparatus. Schweizerische Monatsschrift fur Zahnheilkunde 1965;75(11):1214‐21. [PubMed] [Google Scholar]
Traver 2006
- Traver MA, Assimos DG. New generation tissue sealants and hemostatic agents: innovative urologic applications. Reviews in Urology 2006;8(3):104‐11. [PMC free article] [PubMed] [Google Scholar]
Trentalancia 1967
- Trentalancia M. The use of 5‐oxytryptamine in post‐extraction hemorrhages. Dental Cadmos 1967;35(10):1377‐90. [PubMed] [Google Scholar]
University of Cambridge
- University of Cambridge University Dental Practice. Persistent bleeding following dental extraction. Available from http://www.dental.cam.ac.uk/info‐nhs/dis‐post‐extraction‐bleeding. (accessed on 16 March 2016).
Van Galen 2014
- Galen KPM, Engelen ET, Mauser‐Bunschoten EP, Es RJJ, Schutgens REG. Antifibrinolytic therapy for preventing oral bleeding in patients with a hemophilia or Von Willebrand disease undergoing oral or dental procedures. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD011385] [DOI] [Google Scholar]
Wahl 2016
- Wahl MJ, Schmitt MM. Postextraction bleeding in a patient taking antithrombotics: report of a case. General Dentistry 2016;64(3):60‐3. [PUBMED: 27148659] [PubMed] [Google Scholar]
Wells 2000
- Wells D, Capes J, Powers M. Complications of dentoalveolar surgery. In: Fonseca R editor(s). Oral and Maxillofacial Surgery. Vol. 1, Philadelphia: WB Saunders, 2000:421‐38. [Google Scholar]
Worthington 2015
- Worthington H, Clarkson J, Weldon J. Priority oral health research identification for clinical decision‐making. Evidence‐based Dentistry 2015;16(3):69‐71. [DOI] [PubMed] [Google Scholar]
Yagyuu 2017
- Yagyuu T, Kawakami M, Ueyama Y, Imada M, Kurihara M, Matsusue Y, et al. Risks of postextraction bleeding after receiving direct oral anticoagulants or warfarin: a retrospective cohort study. BMJ Open 2017;7:e015952. [DOI: 10.1136/bmjopen-2017-015952] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Sumanth 2015
- Sumanth KN, Prashanti E, Aggarwal H, Kumar P, Kiran Kumar Krishanappa S. Interventions for managing post‐extraction bleeding. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD011930] [DOI] [PubMed] [Google Scholar]
Sumanth 2016
- Kumbargere Nagraj S, Prashanti E, Aggarwal H, Lingappa A, Muthu MS, Kiran Kumar Krishanappa S, et al. Interventions for treating post‐extraction bleeding. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD011930.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


