Abstract
PURPOSE
The BRIGHT study (ClinicalTrials.gov identifier: NCT00877006) was initiated to compare the efficacy and safety of bendamustine plus rituximab (BR) with either rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or rituximab plus cyclophosphamide, vincristine, and prednisone (R-CVP) for treatment-naive patients with indolent non-Hodgkin lymphoma or mantle-cell lymphoma. This publication provides long-term follow-up data.
PATIENTS AND METHODS
Patients were monitored for a minimum of 5 years after completion of study treatment for the time-to-event end points of progression-free survival (PFS), event-free survival, duration of response, and overall survival per investigator assessment. Data on the number of patients who received second-line anticancer treatment and the occurrence of other malignancies were also collected.
RESULTS
The medians were not reached for any of the time-to event end points for either the BR or R-CHOP/R-CVP study treatment groups by study completion. PFS rates at 5 years were 65.5% in the BR treatment group and 55.8% in the R-CHOP/R-CVP group. The difference in PFS was considered significant with a hazard ratio of 0.61 (95% CI, 0.45 to 0.85; P = .0025). The hazard ratio for event-free survival and duration of response (P = .0020 and .0134, respectively) also favored the BR regimen over R-CHOP/R-CVP. However, no significant difference in overall survival was observed. The overall safety profiles of BR, R-CHOP, and R-CVP were as expected; no new safety data were collected during long-term follow-up. A higher number of secondary malignancies was noted in the BR treatment group.
CONCLUSION
Overall, BR demonstrated better long-term disease control than R-CHOP/R-CVP and should be considered as a first-line treatment option for patients with indolent and mantle-cell lymphoma.
INTRODUCTION
The BRIGHT study (ClinicalTrials.gov identifier: NCT00877006) was initiated to compare the efficacy and safety of bendamustine plus rituximab (BR) with either rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or rituximab plus cyclophosphamide, vincristine, and prednisone (R-CVP) for treatment-naive patients with indolent non-Hodgkin lymphoma (iNHL) or mantle-cell lymphoma (MCL). The primary objective of the study was met because BR was shown to be noninferior to R-CHOP/R-CVP in complete response (CR) rate (P = .0225 for noninferiority).1
BR remains a preferred regimen for the initial treatment of patients with indolent lymphoma.2 This use is based on the initial results of the BRIGHT study and the Study Group of Indolent Lymphomas (StiL) Non-Hodgkin Lymphoma (NHL) 1 study.3 Understandably, there has been interest in the long-term outcomes for patients who received BR rather than the commonly used regimens of R-CHOP and R-CVP. Long-term follow-up data for the BRIGHT and StiL NHL1 studies and an analysis of maintenance rituximab in the BRIGHT study have been presented at international meetings.4-6 This publication provides these long-term follow-up data for the BRIGHT study. The overall response rate data remained unchanged from the initial report, and no new response data are presented.
PATIENTS AND METHODS
The patients and methods for this study have been described previously.1 In the study, treatment-naive patients with iNHL or MCL were preassigned to receive standard treatment (R-CHOP or R-CVP) on the basis of their performance status, comorbidities, and general health and were then randomly assigned to receive standard or experimental treatment (BR). In total, 447 patients were enrolled, with 224 patients randomly assigned to receive BR and 223 patients randomly assigned to receive R-CHOP (n = 104) or R-CVP (n = 119). There were 371 patients with iNHL, 74 patients with MCL, and two patients whose disease was unassigned. Patients were scheduled to receive six cycles of treatment, and up to two additional cycles could be given at the investigator’s discretion. After completion of treatment, patients were observed for a minimum of 5 years. Follow-up data were initially collected every 12 months; after a protocol amendment in December 2012, data were collected every 6 months. Maintenance rituximab was permitted during the follow-up period.
At each follow-up assessment, the date of relapse or disease progression was recorded together with the means by which this was determined. Any further anticancer treatment was recorded, including maintenance rituximab. The occurrence and date of any new cancer diagnosis were recorded, as was any transformation of disease. Histopathologic verification was not required as source data. The patient’s survival status was recorded, as were the date and cause of death.
Results for the secondary efficacy end points of progression-free survival (PFS), event-free survival (EFS), duration of response (DOR), and overall survival (OS) were based on investigator assessments. Investigators were instructed to follow standard institutional practice for iNHL follow-up, and no interval for imaging was prescribed. PFS was defined as the time from random assignment to disease progression, relapse, or death from any cause, whichever occurred first. EFS was defined as the time from random assignment to disease progression, death, or discontinuation of treatment for any reason, whichever occurred first. DOR was defined as the time from first response (CR or partial response) to disease progression, relapse, or death as a result of any cause. OS was defined as the time from random assignment to death from any cause. Adverse events (AEs) were not collected for the long-term follow-up period. A single spontaneously reported event of hepatitis B reactivation in a patient in the BR treatment group was recorded.
Statistical Methods
Comparisons between treatment groups of time-to-event end points were prospectively defined as secondary objectives and were analyzed in the intent-to-treat (ITT) population. Time-to-event variables were compared between the treatment groups using stratified log-rank test by prespecified standard therapy and lymphoma type for overall treatment effect. Hazard ratios (HRs) and their 95% CIs were estimated using a proportional hazards model adjusting for prespecified standard therapy and lymphoma type. Medians and their 95% CIs were estimated using the Kaplan-Meier method. Patients without an event in these analyses were censored at the last known valid tumor assessment date. In the analysis of OS, patients who were still alive at the time of last follow-up were censored at the date last known to be alive. An ad hoc Cox proportional hazards regression was performed for PFS and OS in the ITT population. A comparison of baseline parameters and clinical outcomes in patients with and without maintenance rituximab was conducted in the ITT population. Analyses of new cancer diagnoses and time to new treatment (TTNT) were performed in the treated population. TTNT was analyzed as described for the other time-to-event variables. All data were processed and summarized using SAS version 9.1.3 (SAS Institute, Cary, NC) or later versions.
RESULTS
The last patient follow-up occurred on December 15, 2016. The median follow-up time for the entire study was 65.0 months (range, 1.9 to 70.9 months) for patients in the BR group and 64.1 months (range, 0.8 to 68.2 months) for patients in the R-CHOP/R-CVP group. Of the total number of follow-up intervals, 59% and 56% approximated to 6 months (30 weeks or less) in the BR and R-CHOP/R-CVP treatment groups, respectively.
Study Patients
The ITT population consisted of 224 BR patients and 223 R-CHOP/R-CVP patients. A total of 221 BR patients and 215 R-CHOP/R-CVP patients received at least one dose of treatment and formed the treated population. Thirty-seven patients in each group had a diagnosis of MCL. Patient characteristics for the iNHL and MCL subgroups are shown in the Data Supplement. Forty-three percent of BR patients and 45% of R-CHOP/R-CVP patients received rituximab maintenance therapy.
PFS
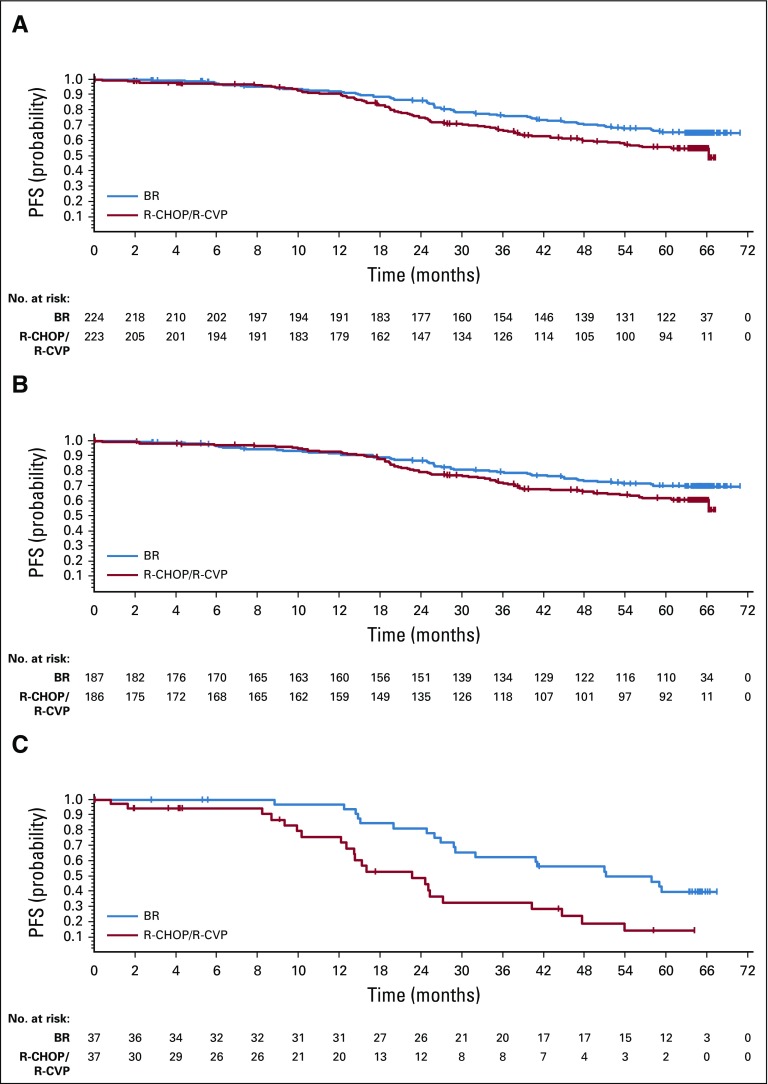
The median time to progression was not reached for either the BR or R-CHOP/R-CVP treatment groups. PFS rates at 5 years after random assignment were 65.5% in the BR treatment group and 55.8% in the R-CHOP/R-CVP group. The difference in PFS was considered significant with an HR of 0.61 (95% CI, 0.45 to 0.85; P = .0025; Fig 1A, Data Supplement). An ad hoc analysis was conducted in subsets of patients who did and did not receive maintenance rituximab. Maintenance rituximab was infrequent in patients with MCL and was less common in patients with an International Prognostic Index status of high-intermediate or high risk (Data Supplement). The treatment effect of BR was comparable between patients who received maintenance rituximab (HR, 0.60; 95% CI, 0.34 to 1.03) and patients who did not (HR, 0.60; 95% CI, 0.41 to 0.89; Data Supplement). The HR for PFS was 0.70 (95% CI, 0.49 to 1.01) in favor of BR in patients with iNHL (P = .0582; Fig 1B, Data Supplement) and 0.40 (95% CI, 0.21 to 0.75) in favor of BR in patients with MCL (P = .0035; Fig 1C, Data Supplement).
FIG 1.
Progression-free survival (PFS) by treatment group for (A) all patients, (B) patients with indolent non-Hodgkin lymphoma, and (C) patients with mantle-cell lymphoma. Note: Censored values are represented by a vertical bar. BR, bendamustine plus rituximab; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CVP, rituximab plus cyclophosphamide, vincristine, and prednisone.
PFS analyses were also performed by preassigned treatment group (Data Supplement). The HR for PFS was 0.65 (95% CI, 0.4 to 1.06) in favor of BR in patients preassigned to R-CHOP treatment (P = .0800) and 0.59 (95% CI, 0.38 to 0.90) in favor of BR in patients preassigned to R-CVP (P = .0128).
OS
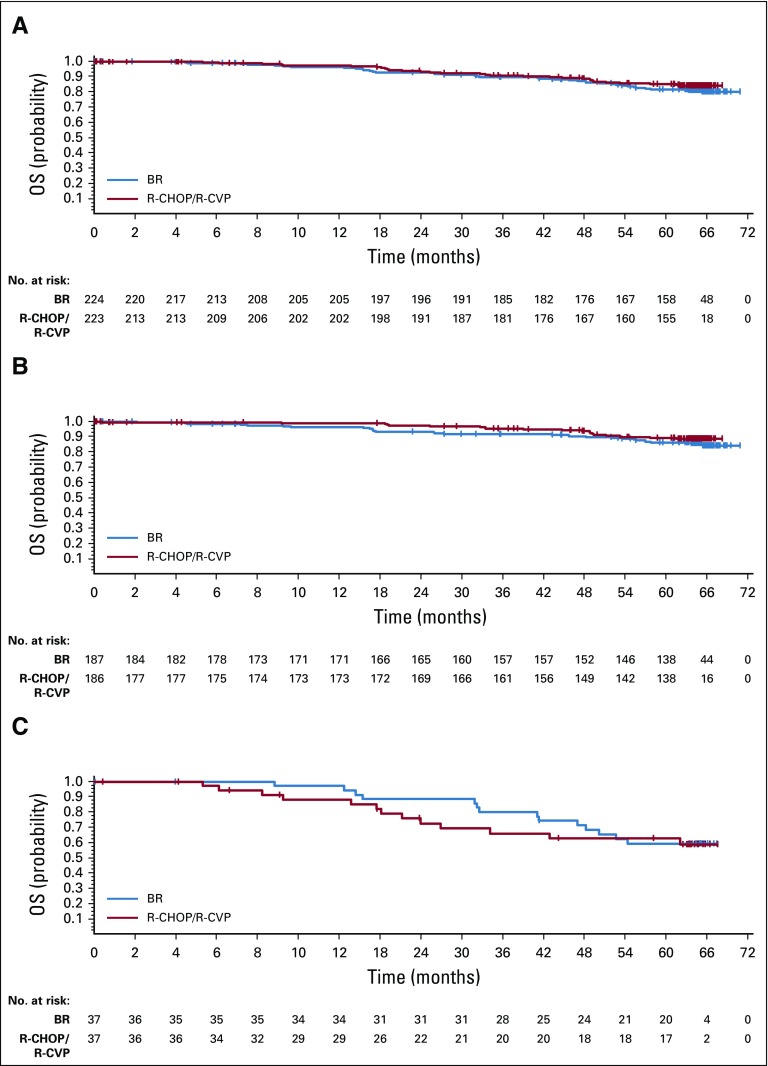
The OS of patients treated with BR was similar to that of patients treated with R-CHOP/R-CVP (HR, 1.15; 95% CI, 0.72 to 1.84; P = .5461; Fig 2A, Data Supplement). There were 40 deaths (18%) in the BR group and 32 deaths (14%) in the R-CHOP/R-CVP group. The percentages of patients alive at 5 years were 81.7% and 85.0% in the BR and R-CHOP/R-CVP treatment groups, respectively. There was no significant difference in OS between the BR and R-CHOP/R-CVP groups in patients who received (HR, 1.71; 95% CI, 0.62 to 4.72; P = .2973) and did not receive (HR, 0.80; 95% CI, 0.44 to 1.45; P = .4550) maintenance rituximab (Data Supplement).
FIG 2.
Overall survival (OS) by treatment group for (A) all patients, (B) patients with indolent non-Hodgkin lymphoma, and (C) patients with mantle-cell lymphoma. Note: Censored values are represented by a vertical bar. BR, bendamustine plus rituximab; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CVP, rituximab plus cyclophosphamide, vincristine, and prednisone.
Evaluations of OS by lymphoma type demonstrated similar results, with no significant difference in OS between treatment groups. The HR for OS was 1.34 (95% CI, 0.74 to 2.42) for patients with iNHL (P = .3316; Fig 2B, Data Supplement) and 0.86 (95% CI, 0.40 to 1.83) for patients with MCL (P = .6894; Fig 2C, Data Supplement). No differences in OS between treatment groups were observed in the R-CHOP and R-CVP preassigned subgroups (Data Supplement).
EFS and DOR
Results for EFS followed the same trend as those for PFS and were in favor of BR treatment when compared with R-CHOP/R-CVP (HR, 0.63; 95% CI, 0.46 to 0.84; P = .0020; Data Supplement). As for PFS, the difference in EFS in favor of BR was greater for patients with MCL (HR, 0.35; 95% CI, 0.16 to 0.60; P < .001) than for patients with iNHL (HR, 0.75; 95% CI, 0.53 to 1.05; P = .0944; Data Supplement).
Evaluations of DOR were also in favor of BR treatment, with an HR of 0.66 (95% CI, 0.47 to 0.92) in favor of BR (P = .0134; Data Supplement). Greater benefit was observed among patients with MCL (HR, 0.47; 95% CI, 0.24 to 0.91; P = .0231) and in patients preassigned to R-CVP treatment (HR, 0.63; 95% CI, 0.40 to 0.99; P = .0457; Data Supplement).
Ad Hoc Analysis of Second-Line Therapy
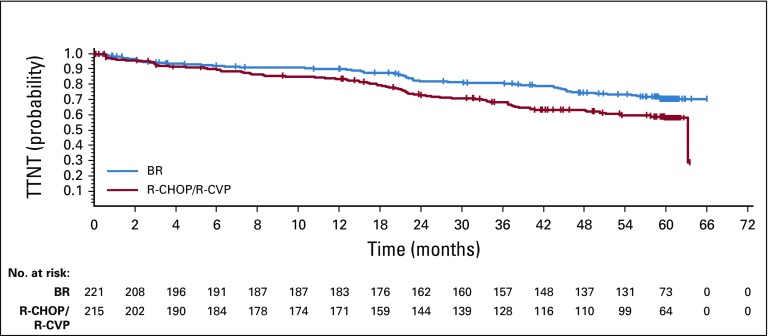
An ad hoc analysis for TTNT, defined as the time from the last dose of study drug to the first new lymphoma treatment, was performed in the treated population. A total of 58 patients (26%) in the BR group and 83 patients (39%) in the R-CHOP/R-CVP group received new lymphoma treatment. The HR for TTNT was 0.57 (95% CI, 0.41 to 0.81) in favor of BR (P = .0012; Fig 3). Of the 88 patients in the R-CHOP/R-CVP treatment group with disease relapse or progression, 32 (36%) received bendamustine in a second- or third-line regimen, most commonly in the form of BR.
FIG 3.
Time to first new lymphoma treatment (TTNT) by treatment group. Note: Censored values are represented by a vertical bar. BR, bendamustine plus rituximab; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CVP, rituximab plus cyclophosphamide, vincristine, and prednisone.
Multivariable Analysis
Ad hoc Cox proportional hazards regression analyses were performed to evaluate the potential impact of prognostic factors on PFS and OS (Data Supplement). The variables examined were study treatment, age, lactate dehydrogenase level, International Prognostic Index score, presence or absence of “B” symptoms, bone marrow involvement, and level of β2-microglobulin. The factors of age, lactate dehydrogenase and β2-microglobulin levels, and B symptoms were significant in the analysis for PFS and OS, whereas study treatment was significant in the analysis for PFS.
Safety
AEs were not planned to be monitored or collected during the follow-up period, and the safety assessments of the BR and R-CHOP/R-CVP regimens have not changed from those first published.1 One new serious AE of hepatitis B was spontaneously reported in the BR group. This patient developed hepatitis B reactivation 3 years after the last dose of the study drug and subsequently died of acute hepatic failure. This patient had received maintenance rituximab for approximately 5 months after the end of study treatment. The patient was not receiving prophylactic hepatitis treatment.
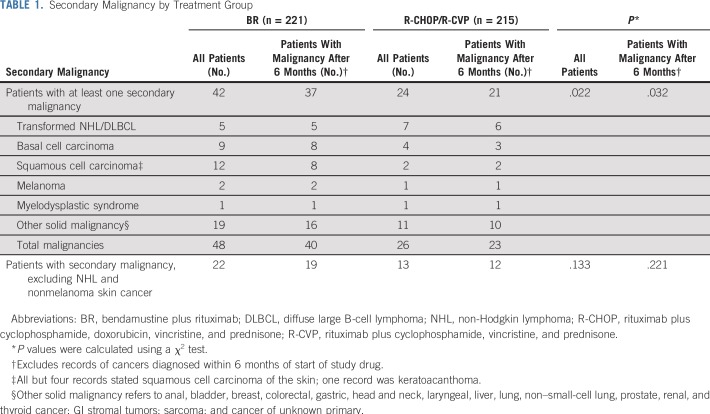
A significantly higher incidence of secondary malignancy was observed in the BR treatment group relative to the R-CHOP/R-CVP treatment group, most notably in the incidence of squamous and basal cell carcinomas of the skin (Table 1). Five patients (2.2%) in the BR group and seven patients (3.2%) in the R-CHOP/R-CVP group had transformed disease or diffuse large B-cell lymphoma during follow-up. For four patients in the BR treatment group and nine patients in the R-CHOP/R-CVP treatment group, the new cancer diagnosis occurred after the start of second-line therapy. The occurrence of new cancer diagnosis did not seem to be related to the use of maintenance rituximab. The incidence of new cancer diagnosis in patients with and without maintenance rituximab was 16.5% and 20.5%, respectively, for the BR treatment group and 10.0% and 11.4%, respectively, for the R-CHOP/R-CVP treatment group. The difference in the incidence in new cancer diagnoses was smallest for patients treated in the United States (11.5% for BR v 9.3% for R-CHOP/R-CVP), and the incidence was lower in Hispanic or Latino patients (3.6%) than in non-Hispanic and non-Latino patients (16.9%). The median time to secondary malignancies was 29.2 months in the BR group (n = 42) and 30.2 months in the R-CHOP/R-CVP group (n = 24; HR, 0.83; 95% CI, 0.49 to 1.39; P = .4757).
TABLE 1.
Secondary Malignancy by Treatment Group
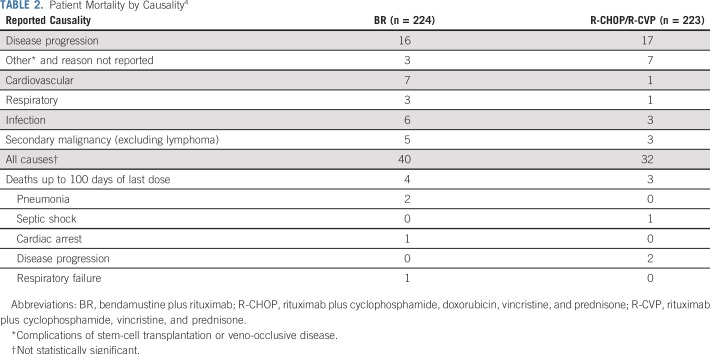
Differences in the number and causes of death were noted between the treatment groups (Table 2). In the BR group, nine patients died of infections, sepsis, or respiratory failure compared with four patients in the R-CHOP/R-CVP treatment group. One of these patients had received maintenance rituximab, and four of the BR treatment group patients had received second-line regimens, as had two of the R-CHOP/R-CVP treatment group patients. There were seven cardiovascular-related deaths (cardiac arrest, cardiopulmonary arrest, infarct, and arrhythmia) in the BR group compared with one (infarct) in the R-CHOP/R-CVP group. None of these patients had received maintenance rituximab, but five of the BR treatment group patients had received at least second-line regimens, as had the R-CHOP/R-CVP treatment group patient.
TABLE 2.
Patient Mortality by Causality4
Five patients in the BR group died of malignancies other than lymphoma, compared with three patients in the R-CHOP/R-CVP group. Only one of these patients had received second-line treatment of lymphoma (BR in a patient in the R-CHOP/R-CVP treatment group). The time relative to the last dose of study drug is shown in the Data Supplement for deaths other than those attributed to disease progression.4
DISCUSSION
The BRIGHT study was powered to test for noninferiority between the treatment regimens in the primary end point of CR rate in the efficacy-evaluable population. Comparisons between the treatment groups in the time-to-event end points of DOR, EFS, PFS, and OS were prospectively defined as secondary objectives of the study, but the study was not powered for these end points. Analyses of PFS, EFS, and DOR were uniformly in favor of the BR regimen compared with R-CHOP/R-CVP, with the strongest trends observed in the subgroup of patients with MCL and those preassigned to R-CVP. Benefit from BR treatment did not translate to prolonged OS, possibly because of the subsequent lines of therapy, including the use of BR in patients in the R-CHOP/R-CVP group. The use of maintenance rituximab was similar in the two treatment groups and was unlikely to contribute to the small differences observed in the time-to-event outcomes. These findings are consistent with those in the StiL NHL1 study, which reported a significant prolongation of TTNT by BR treatment but no significant difference in OS.5
In both treatment groups, patients who received maintenance rituximab had longer PFS and OS than those who did not. However, the contribution of maintenance rituximab to this improved outcome is difficult to assess because the maintenance treatment was not assigned on a randomized basis but was rather at the discretion of the investigator.6
The greater sensitivity of patients with MCL to bendamustine observed in this study is consistent with previous experience as reported by Czuczman et al.7 The high response observed in this population with relapsed or refractory MCL was durable, with a median DOR of 18.9 months.7 The mechanism for bendamustine’s cytotoxic effects on MCL cells is unclear but may be attributed to activation of mitochondrial apoptotic pathways.8 The magnitude of the treatment difference was diminished in patients with iNHL; however, there was still a strong trend in favor of BR in PFS (HR, 0.70; P = .0582).
The overall safety profiles of BR, R-CHOP, and R-CVP were as expected. There was no indication of a significant difference in early non–disease-related mortality between treatment groups. Nine of 16 deaths as a result of infection, respiratory failure, or cardiovascular events occurred more than 1,000 days after the last dose of study treatment in the BR treatment group, as did one of the five such events in the R-CHOP/R-CVP treatment group. The late nature of these deaths and the intervening lines of therapy in approximately half of these patients complicate any analysis of causality.
More new cancer diagnoses were observed with BR than with R-CHOP/R-CVP. This finding is at odds with the lack of a difference reported for the STiL NHL1 study.3,5 The reason for the higher rates of secondary malignancy in the BR group in the BRIGHT study is unclear. Both treatment groups were well balanced for age. The number of patients with a prior malignancy other than the study disease who subsequently developed a secondary malignancy was small and was similar between groups. The time to a secondary malignancy was also similar. The percentage of patients who completed study treatment and the duration of follow-up were similar between groups. The greatest difference in incidence between the treatment groups was in squamous and basal cell carcinomas of the skin. Further review of this potential risk is warranted because periodic dermatologic examination would be an effective way to manage it.
The incidence of transformed disease in the BRIGHT study (2.7%) was lower than the estimates of 2% to 3% per year that have been obtained from a range of retrospective and prospective studies.9,10 This lower than expected rate may be a result of a lack of a prescribed method for observing patients for transformation. The incidence of transformed disease was higher in the subset of BRIGHT patients with follicular disease (3.7%).
Major limitations to this study that may have impacted the PFS comparison include the open-label design, the lack of prespecified imaging follow-up at prescribed intervals, and the lack of Independent Review Committee review. However, the investigators were instructed to adhere to their institutional practice in observing patients with iNHL, and the differences observed between the treatment groups in DOR, EFS, and PFS were substantial. Other factors that limited data interpretation include the change in follow-up schedule from annual to every 6 months, subsequent lines of therapy, the small number of patients with MCL, and lack of a requirement for histologic confirmation for secondary malignancies.
Overall, the data suggest that BR provides greater disease control than R-CHOP/R-CVP with fewer patients requiring second-line treatment during the follow-up period. The absence of a significant improvement in OS in both the BRIGHT and StiL NHL1 studies suggests that the sequence of BR and R-CHOP or R-CVP may not be critical. Rather, both regimens offer patients with iNHL effective treatment and, together with the use of newer agents and regimens, mean that patients have a much wider range of treatment options than was the case 10 years ago. Therefore, the choice of regimen for the initial treatment of iNHL may be driven more by patient preferences regarding the differences in toxicity profile. In conclusion, the cumulative, long-term evidence supports BR as a first-line treatment option for patients with iNHL and MCL.
Footnotes
Clinical trial information: NCT00877006.
Presented in part at the 53rd Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 2-6, 2017.
Supported by Teva Pharmaceuticals, Malvern, PA.
AUTHOR CONTRIBUTIONS
Conception and design: Ian W. Flinn, Richard van der Jagt, Kathryn Kolibaba
Provision of study materials or patients: Richard van der Jagt, Brad Kahl, David MacDonald, Samar Issa, Julie Chang, John M. Burke
Collection and assembly of data: Ian W. Flinn, Richard van der Jagt, Brad Kahl, David MacDonald, Kathryn Kolibaba, Samar Issa, Julie Chang, Judith Trotman, Doreen Hallman, John M. Burke
Data analysis and interpretation: Ian W. Flinn, Richard van der Jagt, Brad Kahl, Peter Wood, Tim Hawkins, David MacDonald, David Simpson, Kathryn Kolibaba, Ling Chen, John M. Burke
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
First-Line Treatment of Patients With Indolent Non-Hodgkin Lymphoma or Mantle-Cell Lymphoma With Bendamustine Plus Rituximab Versus R-CHOP or R-CVP: Results of the BRIGHT 5-Year Follow-Up Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Ian W. Flinn
Consulting or Advisory Role: AbbVie (Inst), Seattle Genetics (Inst), TG Therapeutics (Inst), Verastem (Inst)
Research Funding: Acerta Pharma (Inst), Agios (Inst), Calithera Biosciences (Inst), Celgene (Inst), Constellation Pharmaceuticals (Inst), Genentech (Inst), Gilead Sciences (Inst), Incyte (Inst), Infinity Pharmaceuticals (Inst), Janssen (Inst), Karyopharm Therapeutics (Inst), Kite Pharma (Inst), Novartis (Inst), Pharmacyclics (Inst), Portola Pharmaceuticals (Inst), Roche (Inst), TG Therapeutics (Inst), Trillium Therapeutics (Inst), AbbVie (Inst), ArQule (Inst), BeiGene (Inst), Curis (Inst), FORMA Therapeutics (Inst), Forty Seven (Inst), Merck (Inst), Pfizer (Inst), Takeda (Inst), Teva (Inst), Verastem (Inst), Gilead Sciences (Inst), AstraZeneca (Inst), Juno Therapeutics (Inst), Unum Therapeutics (Inst), MorphoSys (Inst), AbbVie (Inst)
Richard van der Jagt
Honoraria: Roche, Lundbeck, Gilead
Consulting or Advisory Role: Lundbeck, Roche
Speakers' Bureau: Teva, Lundbeck, Gilead, Roche
Research Funding: Teva (Inst), Gilead (Inst), Roche (Inst)
Brad Kahl
Consulting or Advisory Role: Celgene, Juno Therapeutics, AbbVie, Pharmacyclics, Acerta Pharma, ADC Therapeutics, Pharmacyclics, Genentech, Roche
Research Funding: Genentech (Inst), Acerta Pharma (Inst), ADC Therapeutics (Inst)
Travel, Accommodations, Expenses: Celgene, Juno Therapeutics, Genentech, AbbVie, Millennium, Seattle Genetics
Peter Wood
Honoraria: Bayer, Bristol-Myers Squibb
David MacDonald
Honoraria: Roche Canada, Teva
Consulting or Advisory Role: AstraZeneca, Merck, Sandoz, AbbVie
Expert Testimony: Janssen
Travel, Accommodations, Expenses: Roche
David Simpson
Honoraria: Celgene, AbbVie, Janssen-Cilag, Roche, Merck Sharp & Dohme
Consulting or Advisory Role: Celgene, Merck, AbbVie, Janssen-Cilag
Research Funding: Amgen
Travel, Accommodations, Expenses: Celgene, Gilead Sciences
Kathryn Kolibaba
Employment: Compass Oncology
Honoraria: TG Therapeutics
Consulting or Advisory Role: Gilead Sciences
Research Funding: Acerta Pharma (Inst), Celgene (Inst), Cell Therapeutics (Inst), Genentech (Inst), Gilead Sciences (Inst), Janssen (Inst), Pharmacyclics (Inst), Novartis (Inst), Seattle Genetics (Inst), TG Therapeutics (Inst)
Travel, Accommodations, Expenses: TG Therapeutics
Samar Issa
Travel, Accommodations, Expenses: Hoffmann-La Roche
Julie Chang
Research Funding: Celgene, Genentech, Adaptive Biotechnologies
Judith Trotman
Research Funding: BeiGene (Inst), Genentech (Inst), Pharmacyclics (Inst), Janssen-Cilag (Inst), Takeda (Inst), Celgene (Inst)
Doreen Hallman
Employment: Teva
Stock and Other Ownership Interests: Teva
Ling Chen
Employment: Teva, CSL Behring
Stock and Other Ownership Interests: Teva
John M. Burke
Consulting or Advisory Role: Celgene, Genentech, AbbVie, Seattle Genetics, Tempus Labs, Kite/Gilead, Juno Therapeutics, Bayer, AstraZeneca
Speakers' Bureau: Seattle Genetics
Research Funding: Janssen
No other potential conflicts of interest were reported.
REFERENCES
- 1.Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: The BRIGHT study. Blood. 2014;123:2944–2952. doi: 10.1182/blood-2013-11-531327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): B-Cell Lymphoma, Version 2.2018. https://www.nccn.org/professionals/physician_gls/default.aspx. [Google Scholar]
- 3.Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: An open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–1210. doi: 10.1016/S0140-6736(12)61763-2. [DOI] [PubMed] [Google Scholar]
- 4.Flinn I. First-line treatment of iNHL or MCL patients with BR or R-CHOP/R-CVP: Results of the BRIGHT 5-year follow-up study. J Clin Oncol. 2017;35(suppl 15; abstr 7500) doi: 10.1200/JCO.18.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rummel M, Maschmeyer G, Ganser A, et al. Bendamustine plus rituximab (B-R) versus CHOP plus rituximab (CHOP-R) as first-line treatment in patients with indolent lymphomas: Nine-year updated results from the StiL NHL1 study. J Clin Oncol. 2017;35(suppl 15; abstr 7501) [Google Scholar]
- 6.Kahl B, Burke JM, van der Jagt R, et al. Assessment of maintenance rituximab after first-line bendamustine-rituximab in patients with follicular lymphoma: An analysis from the BRIGHT trial. Blood. 2017;130:484. [Google Scholar]
- 7.Czuczman MS, Goy A, Lamonica D, et al. Phase II study of bendamustine combined with rituximab in relapsed/refractory mantle cell lymphoma: Efficacy, tolerability, and safety findings. Ann Hematol. 2015;94:2025–2032. doi: 10.1007/s00277-015-2478-9. [DOI] [PubMed] [Google Scholar]
- 8.Roué G, López-Guerra M, Milpied P, et al. Bendamustine is effective in p53-deficient B-cell neoplasms and requires oxidative stress and caspase-independent signaling. Clin Cancer Res. 2008;14:6907–6915. doi: 10.1158/1078-0432.CCR-08-0388. [DOI] [PubMed] [Google Scholar]
- 9.Casulo C, Burack WR, Friedberg JW. Transformed follicular non-Hodgkin lymphoma. Blood. 2015;125:40–47. doi: 10.1182/blood-2014-04-516815. [DOI] [PubMed] [Google Scholar]
- 10.Casulo C, Friedberg J. Transformation of marginal zone lymphoma (and association with other lymphomas) Best Pract Res Clin Haematol. 2017;30:131–138. doi: 10.1016/j.beha.2016.08.029. [DOI] [PubMed] [Google Scholar]