Abstract
PURPOSE
To evaluate comparative associations of breast magnetic resonance imaging (MRI) background parenchymal enhancement (BPE) and mammographic breast density with subsequent breast cancer risk.
PATIENTS AND METHODS
We examined women undergoing breast MRI in the Breast Cancer Surveillance Consortium from 2005 to 2015 (with one exam in 2000) using qualitative BPE assessments of minimal, mild, moderate, or marked. Breast density was assessed on mammography performed within 5 years of MRI. Among women diagnosed with breast cancer, the first BPE assessment was included if it was more than 3 months before their first diagnosis. Breast cancer risk associated with BPE was estimated using Cox proportional hazards regression.
RESULTS
Among 4,247 women, 176 developed breast cancer (invasive, n = 129; ductal carcinoma in situ,n = 47) over a median follow-up time of 2.8 years. More women with cancer had mild, moderate, or marked BPE than women without cancer (80% v 66%, respectively). Compared with minimal BPE, increasing BPE levels were associated with significantly increased cancer risk (mild: hazard ratio [HR], 1.80; 95% CI, 1.12 to 2.87; moderate: HR, 2.42; 95% CI, 1.51 to 3.86; and marked: HR, 3.41; 95% CI, 2.05 to 5.66). Compared with women with minimal BPE and almost entirely fatty or scattered fibroglandular breast density, women with mild, moderate, or marked BPE demonstrated elevated cancer risk if they had almost entirely fatty or scattered fibroglandular breast density (HR, 2.30; 95% CI, 1.19 to 4.46) or heterogeneous or extremely dense breasts (HR, 2.61; 95% CI, 1.44 to 4.72), with no significant interaction (P = .82). Combined mild, moderate, and marked BPE demonstrated significantly increased risk of invasive cancer (HR, 2.73; 95% CI, 1.66 to 4.49) but not ductal carcinoma in situ (HR, 1.48; 95% CI, 0.72 to 3.05).
CONCLUSION
BPE is associated with future invasive breast cancer risk independent of breast density. BPE should be considered for risk prediction models for women undergoing breast MRI.
INTRODUCTION
Mammographic breast density is now established as an imaging biomarker for breast cancer risk.1,2 Imaging biomarkers are representations of an in vivo biologic state and phenotype.3,4 The incorporation of breast density in breast cancer risk models,5,6 as well as state-mandated reporting7 of mammographic breast density to women, underscores the central role of imaging biomarkers in risk assessment. Recent studies have explored the predictive value of other breast imaging biomarkers, and accumulating evidence suggests elevated background parenchymal enhancement (BPE) assessed on breast magnetic resonance imaging (MRI) may predict primary breast cancer risk.8-10
BPE describes the phenomenon observed on breast MRI in which normal breast tissue demonstrates signal enhancement related to uptake of gadolinium-based intravenous contrast, which is used in routine MRI examinations.3,4,11 Biologically, BPE may represent increased tissue microvascularity and/or permeability5,6,12,13 regulated by endogenous hormones (primarily estrogen)7,11 and may represent tissue at risk for neoplasia.8-10,14 BPE is dynamic in appearance and distribution within a woman’s breast tissue and sensitive to the phase of menstrual cycle and lactation,1,2,15 as well as in response to antihormonal therapy,3,4,16,17 chemotherapy,5,6,10,18-20 and radiotherapy.7,21 Similar to mammographic breast density, BPE is qualitatively codified in the Breast Imaging Reporting and Data System (BI-RADS) Atlas8-10,22 as four ordinal levels of increasing enhancement—minimal, mild, moderate, and marked. In contrast to breast density, which is the relative quantity of fat and fibroglandular tissue assessed on mammograms, BPE indicates overall breast tissue contrast enhancement assessed on MRI.
BPE is used clinically to report the level of potential masking of suspicious lesions on MRI, which may impede diagnosis.3,4,11,23,24 In addition, recent single-center studies have demonstrated an association between high levels of BPE and increased breast cancer risk.5,6,8,9,12,13,25 In this study, we evaluate the association between BPE and future breast cancer risk among a population-wide cohort of women undergoing breast MRI from diverse practice settings in the United States. We compared BPE risk prediction relative to and in conjunction with mammographic breast density.
PATIENTS AND METHODS
Study Setting and Data Sources
We included breast MRIs conducted at 46 radiology facilities that participate in one of the following six regional Breast Cancer Surveillance Consortium (BCSC) registries: Carolina Mammography Registry, Kaiser Permanente Washington Registry, Metro Chicago Breast Cancer Registry, New Hampshire Mammography Network, San Francisco Mammography Registry, and Vermont Breast Cancer Surveillance System. BCSC registries link woman-level risk factors and clinical information to breast imaging examinations collected from community radiology facilities. Breast cancer diagnoses and tumor characteristics are obtained by linking with pathology databases, regional SEER programs, and state tumor registries. BCSC registries and the statistical coordinating center received institutional review board approval, and all procedures were Health Insurance Portability and Accountability Act compliant.
Participants and Examinations
We included all BPE measures from screening and diagnostic breast MRI examinations performed on women without a history of breast cancer from 2005 to 2015 (with one exam in 2000). MRI indication was defined as screening or diagnostic by the interpreting radiologist. MRIs were excluded if breast cancer was diagnosed within 3 months after the MRI examination.
Measures and Definitions
For five of the BCSC registries, BPE was assessed clinically as minimal, mild, moderate, or marked at the time of MRI interpretation (n = 116 radiologists). Although the concept of degrees of BPE was first published by Kuhl12 in 2007 and BPE was codified formally in the American College of Radiology BI-RADS in 2013,7,11,22 awareness and recording of the proposed BI-RADS BPE categories existed before official publication, with the first recorded assessments in 2000 in our database. Although most BPE assessments were prospectively assessed, a single BCSC registry did not consistently measure BPE clinically. Therefore, a radiologist (N.H.A.) blinded to cancer status retrospectively measured BPE in a subcohort of women with breast cancer and up to two matched controls (n = 271 MRIs total, of which 38 patients with 52 MRI examinations represented patients with cancer).
We dichotomized BPE into minimal versus mild, moderate, or marked BPE based on consensus among investigators and prior literature.8-10,14 This dichotomized definition was intended to decrease known inter-reader variability25-27 for BPE assessment. Breast density was dichotomized as low density for almost entirely fatty or scattered fibroglandular densities or as high density for heterogeneous or extremely dense tissue.
Breast density and risk factors were collected from the closest mammography examination within 5 years of the MRI examination and before any breast cancer diagnosis. Women completed a questionnaire at each mammography examination (which was usually performed within 6 months of an MRI) to collect information on race and ethnicity, history of first-degree relatives with breast cancer, menopausal status, and history of breast biopsy. Women were considered postmenopausal if they reported removal of both ovaries, periods that had stopped naturally or had not occurred for more than 365 days, use of current hormone therapy, or an age of 55 years or older.28 Women were considered pre- or perimenopausal if they reported currently having periods, using oral contraceptives, or not knowing if their periods had stopped.28 Women were considered to have surgical menopause, other amenorrhea, or unknown status if they were younger than age 55 years and reported hysterectomy without bilateral oophorectomy and no use of hormone therapy, if they reported their periods as stopped for other reasons, or if menopausal status could not be determined on the basis of available information. Prior diagnoses of benign breast disease were collected from pathology databases and grouped into the following four categories: nonproliferative, proliferative without atypia, proliferative with atypia, and lobular carcinoma in situ, as described previously.29-31 BCSC (version 2.0) 5-year risk score was based on age, race or ethnicity, BI-RADS breast density, first-degree family history, and history of breast biopsy and benign breast disease.5,6
Primary, Secondary, and Sensitivity Analyses
We described the participant population at baseline (ie, first BPE measure) by breast cancer status and BPE. Hazard ratios (HRs) and 95% CIs for breast cancer risk were estimated using Cox proportional hazards regression using both ordinal and dichotomized definitions of BPE. We modeled the data in the following two ways: restricting to each woman’s first BPE measure and including all eligible BPE measures for each woman. The second model was fit using a robust sandwich estimator for repeated measures survival data to account for multiple observations per woman.32 Women were observed from 3 months after date of BPE measure to breast cancer diagnosis or censoring as a result of death or end date of complete cancer capture. Models were adjusted for BCSC registry and MRI indication (screening v diagnostic) and for number of MRIs in models with multiple measures through stratification. All models were adjusted for age in years as a continuous variable.
BPE was further evaluated in secondary and sensitivity analyses. Associations of BPE with risk were evaluated separately for ductal carcinoma in situ (DCIS) and invasive cancer. Multiplicative interaction was tested by including product terms for BPE with breast density, first-degree family history, menopausal status, MRI indication, and BCSC risk score. Confounding was evaluated through adjustment using covariables from Table 1. For sensitivity analyses, we refit the model for dichotomous BPE with the following conditions: restricting to breast cancer diagnoses at least 1 year after BPE measurement and starting follow-up from this time; restricting to BPE measurements assessed in 2010 or later; restricting to nonsuspicious BI-RADS assessment categories 1, 2, and 3; and excluding the single registry that retrospectively evaluated BPE. Analyses were performed using SAS software, version 9.2 (SAS Institute, Cary, NC).
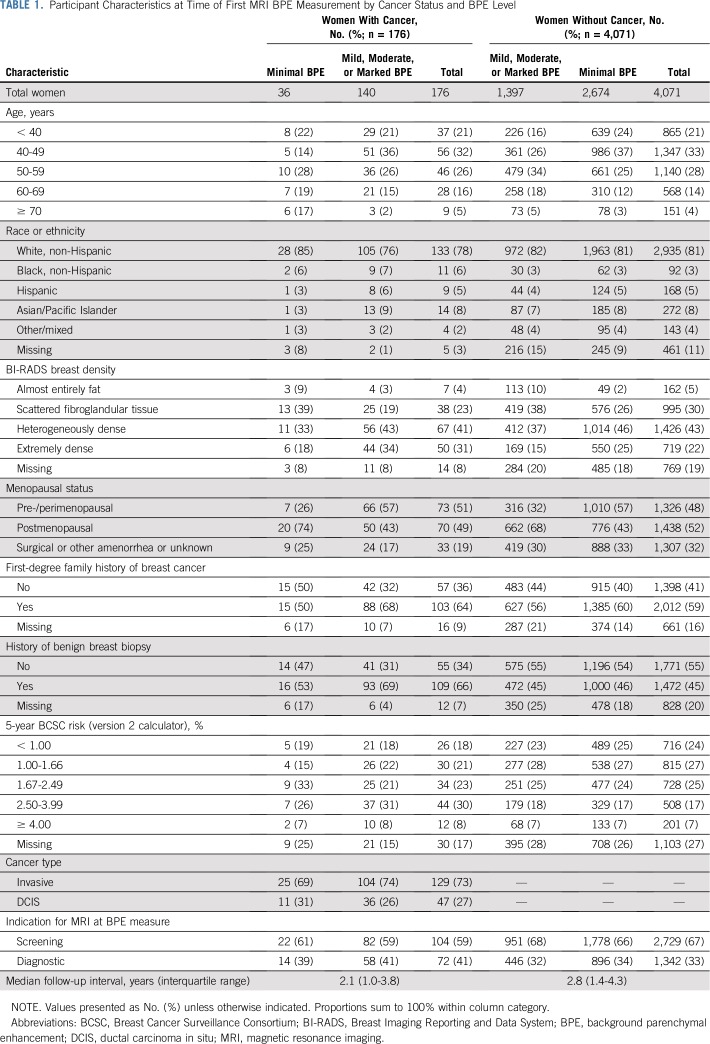
TABLE 1.
Participant Characteristics at Time of First MRI BPE Measurement by Cancer Status and BPE Level
RESULTS
Participant and MRI Examination Characteristics
Analysis included 6,640 eligible breast MRI examinations conducted in 4,247 women (Table 1). Breast MRI examinations were performed for a screening indication in 2,833 women (67%) and a diagnostic indication in 1,414 women (33%). A total of 176 women subsequently developed breast cancer, of whom 129 (73%) had invasive disease and 47 (27%) had DCIS. Median follow-up was 2.1 years for patients with cancer (interquartile range, 1.0 to 3.8 years) and 2.8 years for noncancer controls (interquartile range, 1.4 to 4.3 years).
Overall, 82% of women were younger than 60 years old, and 81% were of white or non-Hispanic race and ethnicity (Table 1). Women with breast cancer, compared with those without cancer, were slightly more likely to be premenopausal (51% v 48%, respectively), have a first-degree family history of breast cancer (64% v 59%, respectively), and have a 1.67% or greater 5-year breast cancer risk by the BCSC model (62% v 48%, respectively).
When comparing women without breast cancer by BPE group (Table 1), women with mild, moderate, or marked BPE, compared with women with minimal BPE, were more likely to be younger than age 60 years (85% v 76%, respectively), be premenopausal (57% v 32%, respectively), and have a first-degree family history of breast cancer (61% v 56%, respectively).
Association Between BPE and Cancer
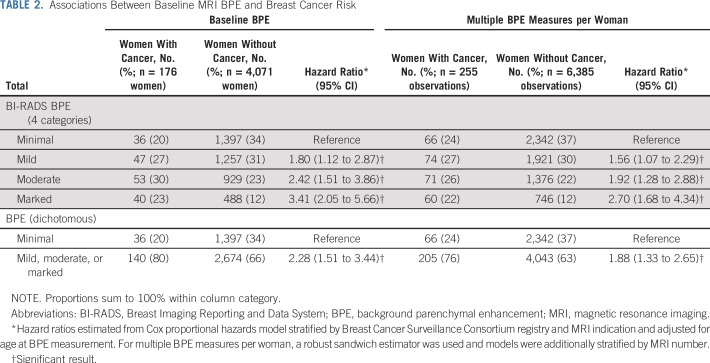
Women with cancer, compared with women without cancer, had a higher rate of mild, moderate, or marked BPE (80% v 66%, respectively; Table 2). In the primary analysis using baseline BPE measurement with minimal BPE as reference, increasing levels of BPE demonstrated significantly increased future breast cancer risk (mild: HR, 1.80; 95% CI, 1.12 to 2.87; moderate: HR, 2.42; 95% CI, 1.51 to 3.86; and marked: HR, 3.41; 95% CI, 2.05 to 5.66). These effects were attenuated but still statistically significant when including all BPE measures. Compared with minimal BPE, mild, moderate, or marked BPE was associated with significantly increased cancer risk using baseline BPE (HR, 2.28; 95% CI, 1.51 to 3.44) or repeated measures of BPE (HR, 1.88; 95% CI, 1.33 to 2.65).
TABLE 2.
Associations Between Baseline MRI BPE and Breast Cancer Risk
Comparative Association of BPE and Breast Density With Cancer
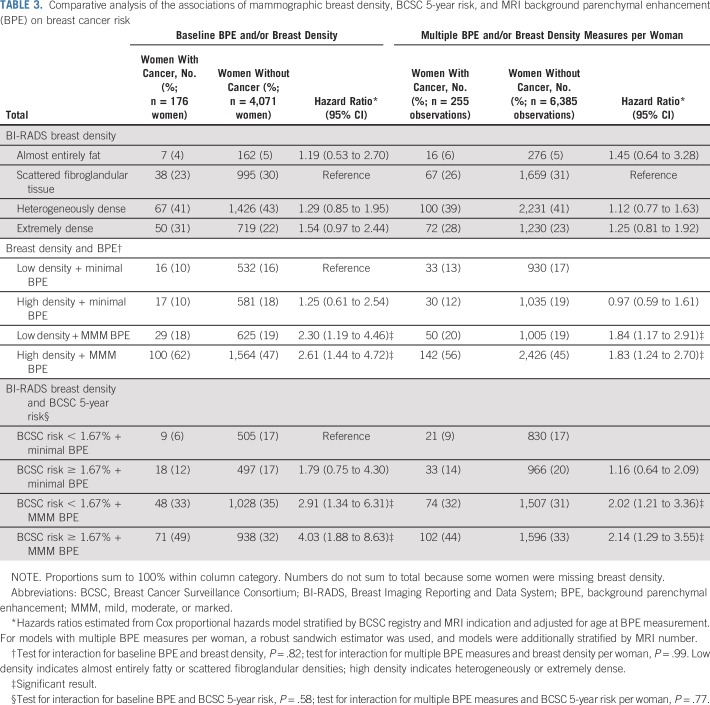
Women who developed breast cancer compared with women who remained cancer free had a higher proportion of dense breasts (72% v 65%, respectively; Table 3 and Fig 1).Compared with women with scattered fibroglandular tissue, women with extremely dense breasts demonstrated a nonsignificant increased risk of breast cancer (HR, 1.54; 95% CI, 0.97 to 2.44; Table 3), which was attenuated when using repeated measures of breast density.
TABLE 3.
Comparative analysis of the associations of mammographic breast density, BCSC 5-year risk, and MRI background parenchymal enhancement (BPE) on breast cancer risk
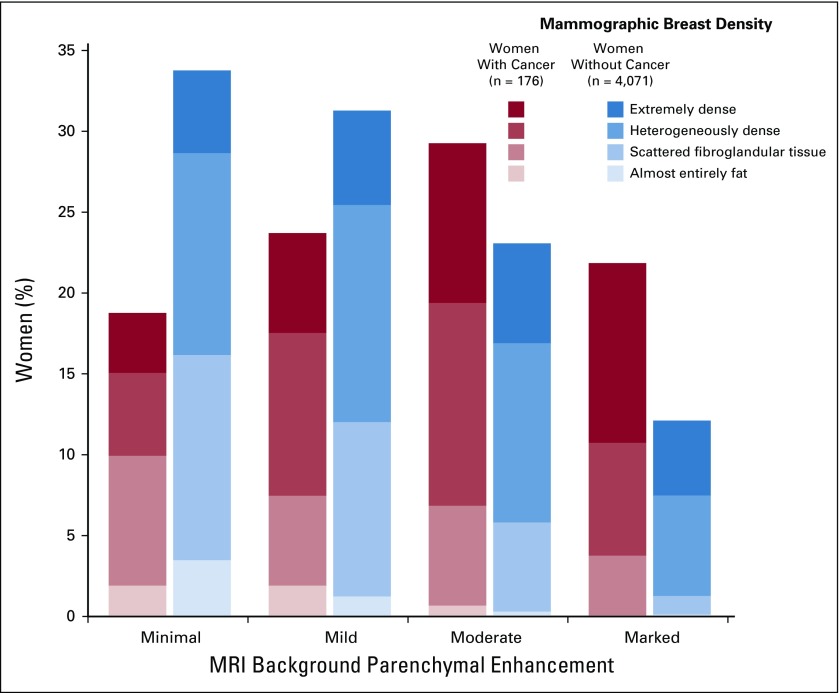
FIG 1.
Distribution of magnetic resonance imaging (MRI) background parenchymal enhancement (BPE) and mammographic breast density. Women with cancer, compared with women without cancer, had a higher proportion of mild, moderate, or marked BPE (80% v 66%, respectively) and of heterogeneously or extremely dense breasts (72% v 65%, respectively). When combining BPE and density, women with cancer had a higher proportion of both mild, moderate, or marked BPE and heterogeneously or extremely dense breasts compared with women without cancer (57% v 38%, respectively).
Women with breast cancer, compared with those without breast cancer, had a higher proportion of both mild, moderate, or marked BPE and high breast density (57% v 38%, respectively). Compared with women with low breast density and minimal BPE, women with high breast density and minimal BPE did not have a statistically significant increased risk of breast cancer (HR, 1.25; 95% CI, 0.61 to 2.54). In contrast, women with low breast density and mild, moderate, or marked BPE had a significantly increased risk of breast cancer (HR, 2.30; 95% CI, 1.19 to 4.46). Having both high breast density and high BPE significantly increased the risk of breast cancer compared with having either factor alone (HR, 2.61; 95% CI, 1.44 to 4.72). The test for interaction between dichotomized BPE and dichotomized breast density was nonsignificant (P = .82). Results for the repeated measures model demonstrated similar but attenuated effects.
Comparative Association of BPE and BCSC 5-Year Risk With Cancer
Women with a higher BPE and a high BCSC 5-year risk score of 1.67% or greater had a four-fold increased risk compared with women with minimal BPE and a low risk score (HR, 4.03; 95% CI, 1.88 to 8.63; Table 3). Risk was also elevated but to a lesser extent for higher BPE in the absence of a high risk score (HR, 2.91; 95% CI, 1.34 to 6.31) and marginally elevated for higher risk score in the absence of higher BPE (HR, 1.79; 95% CI, 0.75 to 4.30). There was no evidence of a multiplicative interaction between higher risk score and higher BPE on risk (P = .58). When including multiple examinations, similar statistically significant associations were noted, although with attenuated magnitudes of effect.
Secondary Analyses
Secondary and sensitivity analyses are fully described in Table 4. Mild, moderate, or marked BPE, compared with minimal BPE, was associated with a significantly increased risk of invasive cancer (HR, 2.73; 95% CI, 1.66 to 4.49) but not DCIS (HR, 1.48; 95% CI, 0.72 to 3.05). Higher BPE was associated with an increased risk of breast cancer among women with a first-degree family history (HR, 3.55; 95% CI, 1.93 to 6.53), but this was not statistically significant for women without a family history (HR, 1.29; 95% CI, 0.69 to 2.42; P for interaction = .02). When evaluating confounding through adjustment, BPE remained significantly associated with risk when adjusting for factors such as family history, benign breast biopsies, and postmenopausal status. Adjustment for breast density changed the magnitude of BPE effect by only 3% (HR, 2.22; 95% CI, 1.42 to 3.47).
TABLE 4.
Secondary and Sensitivity Analyses of Mild, Moderate, or Marked Versus Minimal MRI BPE on Breast Cancer Risk
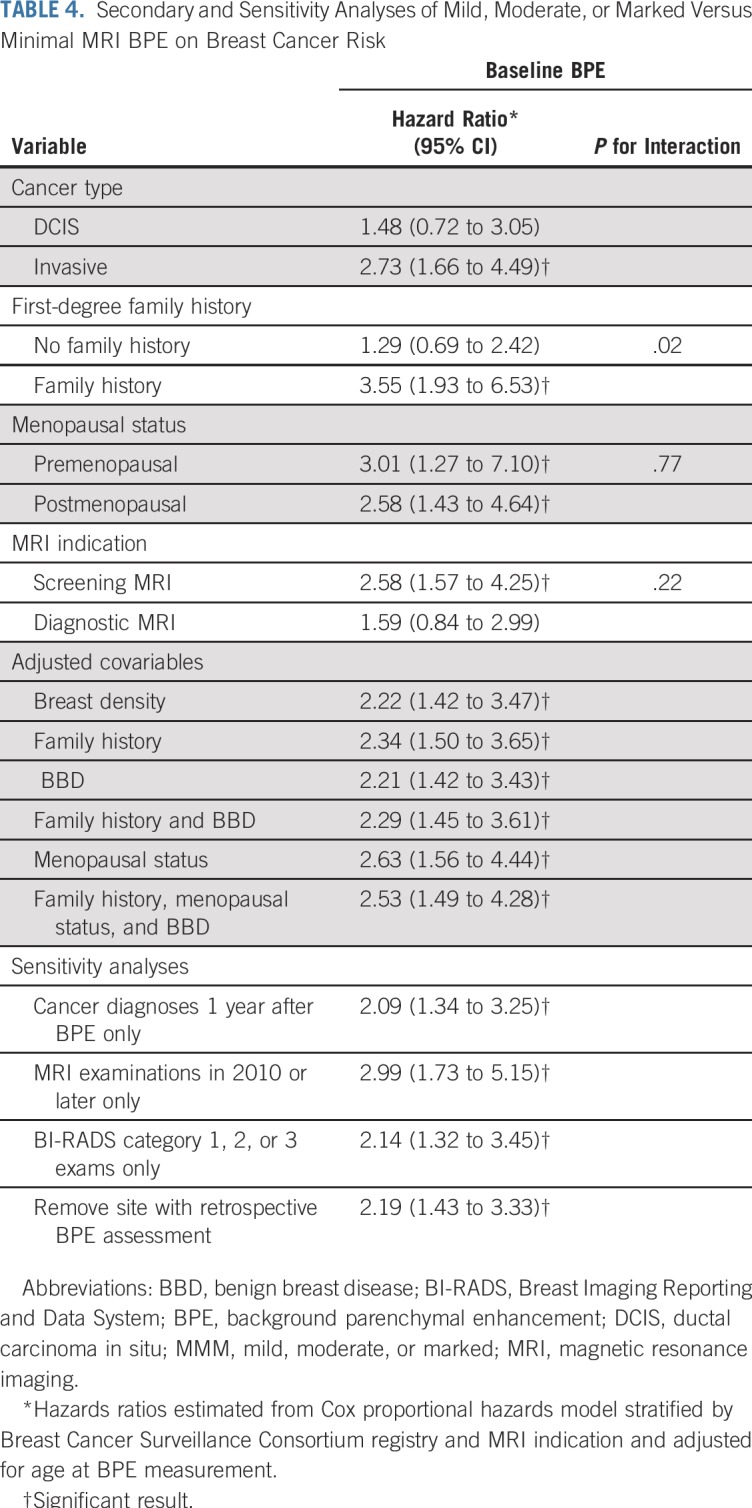
Sensitivity analyses demonstrated that dichotomous BPE remained significantly associated with cancer risk when restricting to cancer diagnoses made at least 1 year after BPE assessment (HR, 2.09; 95% CI, 1.34 to 3.25); restricting to BPE assessments made in or after 2010 (HR, 2.99; 95% CI, 1.73 to 5.15); limiting to negative MRI assessments of BI-RADS categories 1, 2, or 3 (HR, 2.14; 95% CI, 1.32 to 3.45); or removing the BCSC registry that retrospectively assessed BPE (HR, 2.19; 95% CI, 1.43 to 3.33).
DISCUSSION
In this population-based assessment of BPE, we demonstrate that among women undergoing screening or diagnostic breast MRI, elevated levels of BPE significantly increased risk of developing primary invasive breast cancer. BPE had a stronger association with breast cancer risk than breast density in this population. Moreover, BPE was independent of breast density in risk prediction, and the combination of BPE and breast density increased the overall risk for breast cancer more than either factor alone. Our results strengthen the findings of smaller, single-institution, retrospective studies8,9,25 and further validate the use of BPE as an imaging biomarker for primary breast cancer risk.
We also demonstrated BPE to be more strongly associated with invasive cancer than DCIS, suggesting it is a relevant biomarker for predicting clinically important breast cancer. Furthermore, BPE risk prediction remained significant when adjusting for other factors associated with increased breast cancer risk including increased age, family history, benign breast biopsies, and postmenopausal status. Our population represents a predominantly high-risk group, with 49% of women at intermediate to high 5-year risk (compared with 38% of women in a general screened population),33 which is the primary indication for screening breast MRI.34 However, in a subset of women with low or average risk (defined by a 5-year BCSC risk score of less than 1.67%), BPE continued to indicate a significantly increased breast cancer risk. Collectively, these findings suggest that BPE is a robust imaging biomarker for breast cancer risk that is independent of many established factors used in validated risk models.
Our study used the largest longitudinal, population-based cohort to date to confirm the association of BPE with primary breast cancer risk. The validity and robustness of this result are strengthened by our use of rigorously collected individual-level imaging and pathology data, the majority of which was prospectively obtained from diverse academic and community facilities across the United States. Our findings also validate prior single-institution studies. King et al8 initially found that moderate or marked BPE had a significantly increased odds ratio for cancer of 10.1 (95% CI, 2.9 to 35.3). However, this association may be biased because BPE was measured from MRIs that concurrently displayed enhancing cancer. Dontchos et al9 used BPE measurements that preceded cancer diagnosis and found that mild, moderate, or marked BPE was associated with an elevated cancer odds ratio of 9.0 (95% CI, 1.1 to 71.0). All studies, including ours, found that the significant associations between elevated BPE and breast cancer were greater than associations between breast density and cancer. However, only our study evaluated the interaction between breast density and BPE. Although breast density and BPE are not correlated among healthy women,35 we demonstrated that breast cancer risk was independently predicted by breast density and BPE.
Limitations of using BPE as an imaging biomarker for risk parallel the limitations of breast density. BPE is a qualitative assessment that is prone to interobserver and intraobserver differences that are comparable to or worse than assessment of breast density.26,27 BPE has physiologic variability, creating sources of measurement error and variation that tend to bias findings toward a null result, which may explain our attenuated results with a repeated measures model. Despite these limitations, BPE remained significantly predictive of cancer. Breast density did not significantly predict breast cancer risk despite being an established risk marker; however, this result may be a result of selection bias, because 68% of women in our study had dense breasts, compared with 52% in the general screening population.36 Women with dense breasts may be more likely to be referred for MRI because of increased individual risk or for supplementary screening related to dense tissue masking. Finally, BPE prediction remained robust through adjustment for confounders and sensitivity analyses to remove potential biases related to suggestive assessments on MRI, proximity in time of BPE assessment to cancer diagnosis, and evolving definitions of BPE.
The clinical applicability of BPE as a risk marker is limited to select populations who undergo MRI.34 Approximately 1% to 5% of all US women who have received breast imaging have undergone a breast MRI, although this modality may be inappropriately used for some and underused for others.37-39 The indications for and use of breast MRI may increase,40 particularly because it is a potential choice of supplemental screening for women with dense breasts41,42 and/or with recent developments in abbreviated MRI protocols.43,44 Information gained with BPE could be helpful for some women in future efforts to better define breast cancer risk and tailor supplemental screening strategies. For example, if an average-risk woman undergoes diagnostic MRI and demonstrates elevated BPE, her risk may be reassessed to determine if her absolute risk is sufficiently high to warrant screening MRI. Alternatively, high-risk women identified by standard risk prediction models who undergo screening MRI may demonstrate reduced risk if low BPE levels are considered in conjunction with standard risk models; these women may no longer require routine MRI screening.
In conclusion, we found BPE to be a strong predictor of future breast cancer risk, which was independent of breast density and other established risk factors. BPE should be considered for incorporation into risk prediction models for women undergoing MRI.
ACKNOWLEDGMENT
We thank the participating women, mammography facilities, and radiologists for the data they provided for this study.
Footnotes
Presented at the 8th International Workshop on Breast Densitometry and Cancer Risk Assessment, San Francisco, CA, June 7-9, 2017, and the 103rd Annual Meeting of the Radiological Society of North America, Chicago, IL, November 26-December 1, 2017.
Supported by the National Cancer Institute–funded Breast Cancer Surveillance Consortium program project (Grant No. P01CA154292), National Cancer Institute Grant No. U54CA163303, Patient-Centered Outcomes Research Institute Grant No. PCS-1504-30370, and Agency for Healthcare Research and Quality Grant No. R01 HS018366-01A1. The collection of cancer data used in this study was supported in part by several state public health departments and cancer registries throughout the United States. For a full description of these sources, please see http://www.bcsc-research.org/work/acknowledgement.html.
The statements in this publication are solely the responsibility of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute or its Board of Governors or Methodology Committee or the views of the National Cancer Institute or the National Institutes of Health.
See accompanying Editorial on page 943
AUTHOR CONTRIBUTIONS
Conception and design: Vignesh A. Arasu, Diana L. Miglioretti, Brian L. Sprague, Tracy Onega, Constance D. Lehman, Karla Kerlikowske
Financial support: Diana L. Miglioretti, Diana S.M. Buist, Louise M. Henderson, Karla Kerlikowske
Administrative support: Diana S.M. Buist, Karla Kerlikowske
Provision of study materials or patients: Diana S.M. Buist, Louise M. Henderson, Tracy Onega, Karla Kerlikowske
Collection and assembly of data: Diana L. Miglioretti, Brian L. Sprague, Diana S.M. Buist, Louise M. Henderson, Tracy Onega, Garth H. Rauscher, Karla Kerlikowske
Data analysis and interpretation: Vignesh A. Arasu, Diana L. Miglioretti, Brian L. Sprague, Nila H. Alsheik, Louise M. Henderson, Sally D. Herschorn, Janie M. Lee, Tracy Onega, Garth H. Rauscher, Karen J. Wernli, Constance D. Lehman, Karla Kerlikowske
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Population-Based Assessment of the Association Between Magnetic Resonance Imaging Background Parenchymal Enhancement and Future Primary Breast Cancer Risk
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Diana L. Miglioretti
Honoraria: Hologic
Nila H. Alsheik
Research Funding: Hologic
Diana S.M. Buist
Consulting or Advisory Role: Concure Oncology
Janie M. Lee
Employment: Day Zero Diagnostics (I)
Leadership: Day Zero Diagnostics (I)
Stock and Other Ownership Interests: Day Zero Diagnostics (I), Conformis (I)
Consulting or Advisory Role: GE Healthcare
Research Funding: GE Healthcare
Travel, Accommodations, Expenses: Day Zero Diagnostics (I)
Constance D. Lehman
Honoraria: GE Healthcare
Consulting or Advisory Role: GE Healthcare
Research Funding: GE Healthcare (Inst)
Travel, Accommodations, Expenses: GE Healthcare
No other potential conflicts of interest were reported.
REFERENCES
- 1.Vachon CM, Pankratz VS, Scott CG, et al. The contributions of breast density and common genetic variation to breast cancer risk. J Natl Cancer Inst. 2015;107:dju397. doi: 10.1093/jnci/dju397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan DC, Obuchowski NA, Kessler LG, et al. Metrology standards for quantitative imaging biomarkers. Radiology. 2015;277:813–825. doi: 10.1148/radiol.2015142202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hylton N. Dynamic contrast-enhanced magnetic resonance imaging as an imaging biomarker. J Clin Oncol. 2006;24:3293–3298. doi: 10.1200/JCO.2006.06.8080. [DOI] [PubMed] [Google Scholar]
- 5.Tice JA, Cummings SR, Smith-Bindman R, et al. Using clinical factors and mammographic breast density to estimate breast cancer risk: Development and validation of a new predictive model. Ann Intern Med. 2008;148:337–347. doi: 10.7326/0003-4819-148-5-200803040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tice JA, Miglioretti DL, Li C-S, et al. Breast density and benign breast disease: Risk assessment to identify women at high risk of breast cancer. J Clin Oncol. 2015;33:3137–3143. doi: 10.1200/JCO.2015.60.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dehkordy SF, Carlos RC. Dense breast legislation in the United States: State of the states. J Am Coll Radiol. 2016;13(suppl 11):R53–R57. doi: 10.1016/j.jacr.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 8.King V, Brooks JD, Bernstein JL, et al. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology. 2011;260:50–60. doi: 10.1148/radiol.11102156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dontchos BN, Rahbar H, Partridge SC, et al. Are qualitative assessments of background parenchymal enhancement, amount of fibroglandular tissue on MR images, and mammographic density associated with breast cancer risk? Radiology. 2015;276:371–380. doi: 10.1148/radiol.2015142304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Velden BHM, Dmitriev I, Loo CE, et al. Association between parenchymal enhancement of the contralateral breast in dynamic contrast-enhanced MR imaging and outcome of patients with unilateral invasive breast cancer. Radiology. 2015;276:675–685. doi: 10.1148/radiol.15142192. [DOI] [PubMed] [Google Scholar]
- 11.Giess CS, Yeh ED, Raza S, et al. Background parenchymal enhancement at breast MR imaging: Normal patterns, diagnostic challenges, and potential for false-positive and false-negative interpretation. Radiographics. 2014;34:234–247. doi: 10.1148/rg.341135034. [DOI] [PubMed] [Google Scholar]
- 12.Kuhl C. The current status of breast MR imaging: Part I. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology. 2007;244:356–378. doi: 10.1148/radiol.2442051620. [DOI] [PubMed] [Google Scholar]
- 13.Leung T-K, Huang P-J, Liang H-H, et al. Retrospective study of false-positive breast magnetic resonance images and pathological results in Taiwan. J Exp Clin Med. 2012;4:284–288. [Google Scholar]
- 14.Kumar AS, Chen DF, Au A, et al. Biologic significance of false-positive magnetic resonance imaging enhancement in the setting of ductal carcinoma in situ. Am J Surg. 2006;192:520–524. doi: 10.1016/j.amjsurg.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhl CK, Bieling HB, Gieseke J, et al. Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: Normal contrast medium enhancement and cyclical-phase dependency. Radiology. 1997;203:137–144. doi: 10.1148/radiology.203.1.9122382. [DOI] [PubMed] [Google Scholar]
- 16.King V, Goldfarb SB, Brooks JD, et al. Effect of aromatase inhibitors on background parenchymal enhancement and amount of fibroglandular tissue at breast MR imaging. Radiology. 2012;264:670–678. doi: 10.1148/radiol.12112669. [DOI] [PubMed] [Google Scholar]
- 17.Schrading S, Schild H, Kühr M, et al. Effects of tamoxifen and aromatase inhibitors on breast tissue enhancement in dynamic contrast-enhanced breast MR imaging: A longitudinal intraindividual cohort study. Radiology. 2014;271:45–55. doi: 10.1148/radiol.13131198. [DOI] [PubMed] [Google Scholar]
- 18.Hattangadi J, Park C, Rembert J, et al. Breast stromal enhancement on MRI is associated with response to neoadjuvant chemotherapy. AJR Am J Roentgenol. 2008;190:1630–1636. doi: 10.2214/AJR.07.2533. [DOI] [PubMed] [Google Scholar]
- 19.Jones EF, Sinha SP, Newitt DC, et al. MRI enhancement in stromal tissue surrounding breast tumors: Association with recurrence free survival following neoadjuvant chemotherapy. PLoS One. 2013;8:e61969. doi: 10.1371/journal.pone.0061969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Preibsch H, Wanner L, Bahrs SD, et al. Background parenchymal enhancement in breast MRI before and after neoadjuvant chemotherapy: Correlation with tumour response. Eur Radiol. 2016;26:1590–1596. doi: 10.1007/s00330-015-4011-x. [DOI] [PubMed] [Google Scholar]
- 21.Kim YJ, Kim SH, Choi BG, et al. Impact of radiotherapy on background parenchymal enhancement in breast magnetic resonance imaging. Asian Pac J Cancer Prev. 2014;15:2939–2943. doi: 10.7314/apjcp.2014.15.7.2939. [DOI] [PubMed] [Google Scholar]
- 22.D’Orsi CJ, Sickles EA, Mendelson EB, et al. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 23.DeMartini WB, Liu F, Peacock S, et al. Background parenchymal enhancement on breast MRI: Impact on diagnostic performance. AJR Am J Roentgenol. 2012;198:W373-W380. doi: 10.2214/AJR.10.6272. [DOI] [PubMed] [Google Scholar]
- 24.Ray KM, Kerlikowske K, Lobach IV, et al. Effect of background parenchymal enhancement on breast MR imaging interpretive performance in community-based practices. Radiology. 2018;286:822–829. doi: 10.1148/radiol.2017170811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grimm LJ, Saha A, Ghate SV, et al. Relationship between background parenchymal enhancement on high-risk screening MRI and future breast cancer risk. Acad Radiol. doi: 10.1016/j.acra.2018.03.013. epub ahead of print on March 27, 2018. [DOI] [PubMed] [Google Scholar]
- 26.Melsaether A, McDermott M, Gupta D, et al. Inter- and intrareader agreement for categorization of background parenchymal enhancement at baseline and after training. AJR Am J Roentgenol. 2014;203:209–215. doi: 10.2214/AJR.13.10952. [DOI] [PubMed] [Google Scholar]
- 27.Grimm LJ, Anderson AL, Baker JA, et al. Interobserver variability between breast imagers using the fifth edition of the BI-RADS MRI lexicon. AJR Am J Roentgenol. 2015;204:1120–1124. doi: 10.2214/AJR.14.13047. [DOI] [PubMed] [Google Scholar]
- 28.Phipps AI, Ichikawa L, Bowles EJA, et al. Defining menopausal status in epidemiologic studies: A comparison of multiple approaches and their effects on breast cancer rates. Maturitas. 2010;67:60–66. doi: 10.1016/j.maturitas.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312:146–151. doi: 10.1056/NEJM198501173120303. [DOI] [PubMed] [Google Scholar]
- 30.Page DL, Dupont WD, Rogers LW, et al. Atypical hyperplastic lesions of the female breast: A long-term follow-up study. Cancer. 1985;55:2698–2708. doi: 10.1002/1097-0142(19850601)55:11<2698::aid-cncr2820551127>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 31.Page DL, Schuyler PA, Dupont WD, et al. Atypical lobular hyperplasia as a unilateral predictor of breast cancer risk: A retrospective cohort study. Lancet. 2003;361:125–129. doi: 10.1016/S0140-6736(03)12230-1. [DOI] [PubMed] [Google Scholar]
- 32.Lee EW, Wei L. Cox-Type Regression Analysis for Large Numbers of Small Groups of Correlated Failure Time Observations. Norwell, MA: Kluwer Academic Publishers; 1992. [Google Scholar]
- 33.Kerlikowske K, Zhu W, Tosteson ANA, et al. Identifying women with dense breasts at high risk for interval cancer: A cohort study. Ann Intern Med. 2015;162:673–681. doi: 10.7326/M14-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 35.Hansen NL, Kuhl CK, Barabasch A, et al. Does MRI breast “density” (degree of background enhancement) correlate with mammographic breast density? J Magn Reson Imaging. 2014;40:483–489. doi: 10.1002/jmri.24495. [DOI] [PubMed] [Google Scholar]
- 36.Gierach GL, Ichikawa L, Kerlikowske K, et al. Relationship between mammographic density and breast cancer death in the Breast Cancer Surveillance Consortium. J Natl Cancer Inst. 2012;104:1218–1227. doi: 10.1093/jnci/djs327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wernli KJ, DeMartini WB, Ichikawa L, et al. Patterns of breast magnetic resonance imaging use in community practice. JAMA Intern Med. 2014;174:125–132. doi: 10.1001/jamainternmed.2013.11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller JW, Sabatino SA, Thompson TD, et al. Breast MRI use uncommon among U.S. women. Cancer Epidemiol Biomarkers Prev. 2013;22:159–166. doi: 10.1158/1055-9965.EPI-12-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill DA, Haas JS, Wellman R, et al. Utilization of breast cancer screening with magnetic resonance imaging in community practice. J Gen Intern Med. 2018;33:275–283. doi: 10.1007/s11606-017-4224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stout NK, Nekhlyudov L, Li L, et al. Rapid increase in breast magnetic resonance imaging use: Trends from 2000 to 2011. JAMA Intern Med. 2014;174:114–121. doi: 10.1001/jamainternmed.2013.11958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monticciolo DL, Newell MS, Moy L, et al. Breast cancer screening in women at higher-than-average risk: Recommendations from the ACR. J Am Coll Radiol. 2018;15:408–414. doi: 10.1016/j.jacr.2017.11.034. [DOI] [PubMed] [Google Scholar]
- 42.Horný M, Cohen AB, Duszak R, Jr, et al. Dense breast notification laws: Impact on downstream imaging after screening mammography. Med Care Res Rev. 2018;1:1077558717751941. doi: 10.1177/1077558717751941. [DOI] [PubMed] [Google Scholar]
- 43.Kuhl CK, Schrading S, Strobel K, et al. Abbreviated breast magnetic resonance imaging (MRI): First postcontrast subtracted images and maximum-intensity projection—A novel approach to breast cancer screening with MRI. J Clin Oncol. 2014;32:2304–2310. doi: 10.1200/JCO.2013.52.5386. [DOI] [PubMed] [Google Scholar]
- 44.Mango VL, Morris EA, Dershaw DD, et al. Abbreviated protocol for breast MRI: Are multiple sequences needed for cancer detection? Eur J Radiol. 2015;84:65–70. doi: 10.1016/j.ejrad.2014.10.004. [DOI] [PubMed] [Google Scholar]