Abstract
This study sought to investigate the effects of placental laterality on the measurements of uterine artery (UtA) Doppler velocimetry and their application in predicting early-onset preeclampsia (PE).
We conducted a prospective cohort study on all women with singleton, uncomplicated pregnancies scheduled for first-trimester nuchal translucency at our institution. Pulsatility index (PI) for both UtAs was measured by Doppler velocimetry, and placental laterality was determined. Additionally, pregnancy outcome data were abstracted from the medical records. Receiver operating characteristic curves (ROCs) were plotted.
Of the 304 patients enrolled, 247 met the inclusion criteria. Among these patients, 240 had uncomplicated delivery, while 7 had early delivery at <34 weeks due to PE. For the uncomplicated pregnancies, PI measurements of the UtA ipsilateral to the placenta were similar (left versus right UtA: 1.06 ± 0.38 vs. 1.04 ± 0.40; P = .745). However, PI measurements of the UtA contralateral to the placenta differed significantly (left versus right UtA: 1.45 ± 0.51 vs. 1.3 ± 0.47; P = .027). In predicting early-onset PE, the ideal cut-off value for the placental side PI was 1.91, with sensitivity 100% and specificity 96.3%. For nonplacental side PI, the ideal cut-off value for PI was 1.975, with sensitivity 57.1% and specificity 79.2%. Using the mean of the left and right UtA PI, the ideal cut-off value was 1.63, with sensitivity 100% and specificity 74.2%.
ROC analysis confirmed that PI measurements of the UtA on the placental side were significantly lower than those on the contralateral side, PI measurements of the UtA ipsilateral to the placenta were similar.
Keywords: First trimester, placental laterality, preeclampsia, uterine artery Doppler
1. Introduction
Preeclampsia (PE) is a condition characterized by hypertension and proteinuria after gestational week 20; it reportedly affects 5% to 7.5% of all pregnancies and is a major cause of maternal, fetal, and neonatal morbidity and mortality worldwide.[1,2] The causes of PE remain obscure, although evidence increasingly appears to support the hypotheses of abnormal placentation.[3,4] The underlying pathological abnormality is probably present since the first trimester, but the symptoms of the disorder generally surface in the late second to third trimester.
Over the last few decades, extensive research has been conducted on the development of a reliable and safe screening test for PE. Efforts have been underway toward the identification of suitable biomarkers in the maternal serum and urine as well as the application of uterine artery (UtA) Doppler velocimetry. Alterations in the levels of certain biomarkers in the maternal blood and urine are reported to precede the onset of clinical PE by several weeks to months; these alterations correlate with disease severity and normalize after delivery.[5,6] However, assessment of the blood and urine levels of these biomarkers alone has low clinical utility in the prediction of PE disease.[7,8]
Another area of extensive research in this field is the application of ultrasound assessment of the UtA Doppler in predicting PE.[9–13] The underlying principle of UtA Doppler assessment in predicting PE is that impedance to flow in the UtAs normally decreases as the pregnancy progresses. Abnormal placentation leads to the defective invasion of spiral arteries and failure of their transformation into low-resistance vessels; this in turn increases the impedance in the UtA, which can be measured by ultrasound Doppler study.
Various UtA Doppler parameters have been evaluated for their effectiveness in predicting PE.[11–14] Although, traditionally, the pulsatility index (PI) is measured in both the UtAs and the mean of the 2 measurements is used to predict PE, the lowest UtA PI has been shown to be the best predictive value performance.[11] Nevertheless, studies on the value of UtA Doppler velocimetry in the prediction of PE have shown variable levels of sensitivity. This discrepancy may be attributed to the differences between the studies with respect to Doppler sampling techniques, definitions of abnormal flow velocity waveform, patient populations, and gestational age at examination.
The impedance to flow in the UtA on the side of implantation is lower than that on the contralateral side.[15] Despite the recognition of this difference, there have been no large prospective studies addressing the impact of placental laterality on UtA Doppler assessment in predicting PE.
This study aims to investigate the effects of placental laterality on the measurements of first-trimester UtA Doppler velocimetry and assess their relevance in the prediction of early-onset PE.
2. Materials and methods
A prospective cohort study was conducted at the Second Hospital of Jilin University, China. The study protocol was approved by the institutional review board of the hospital. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Written informed consent was obtained from all individual participants included in the study.
All women with singleton, uncomplicated pregnancies scheduled for first-trimester nuchal translucency assessment during the study period were invited to participate in the study. Written informed consent was obtained from all participants before the commencement of the study. The demographic characteristics (age; weight; height; and history of previous pregnancy, chronic hypertension, diabetes, and cigarette smoking during pregnancy) of all patients were recorded. Gestational age was calculated according to the first day of the last menstrual period and confirmed by measuring crown–rump length. All ultrasound examinations were performed on the same device, a Philips IU22 ultrasound system (Philips Healthcare Solutions, Bothell, WA) equipped with a 1 to 5 MHz curvilinear transabdominal transducer.
With the patient in the semirecumbent position, initial ultrasonographic measurements of crown–rump length and nuchal translucency thickness were obtained. UtA Doppler scan was performed according to the guidelines put forth by the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG).[16] Briefly, images of the uterus and cervical canal were obtained in the sagittal view, and color flow mapping was used to identify the UtA, which runs along the sides of the cervix and uterus. The pulsed-wave Doppler measurement was obtained from the ascending branch of the UtA, at the level of the internal os. With the gate set at 2 mm and the smallest angle of insonation set at <30°, 4 to 6 consecutive uniform waveforms were obtained (Table 1).
Table 1.
Demographic and clinical characteristics of the subject.

The PI was calculated by manually tracing the waveforms. The PI on each side as well as the average of the left and the right UtA measurements were recorded. The placental laterality was determined. A tendency for placental lateralization was defined by the presence of more than 50% of the placental mass beyond the long axis of the uterus (Fig. 1). All Doppler ultrasound scans of the UtA were performed by the same experienced examiner (WS).
Figure 1.
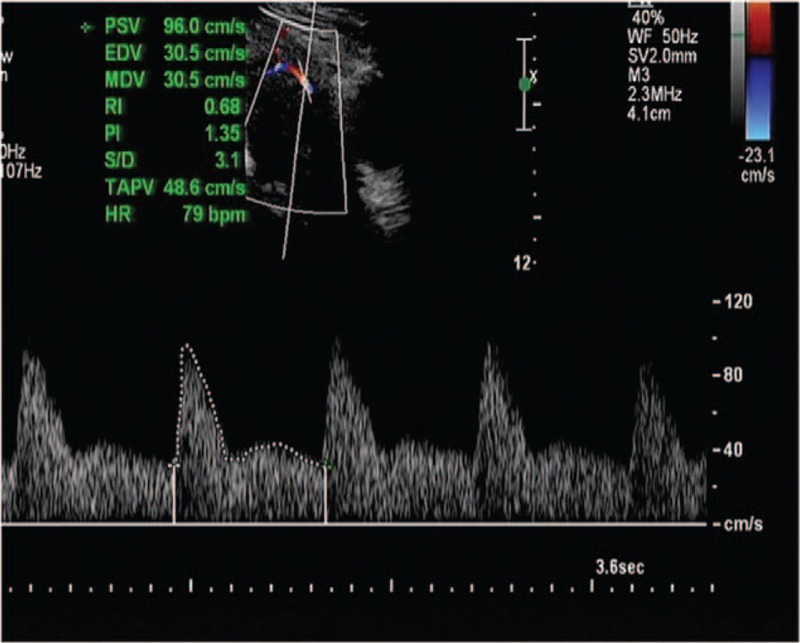
Uterine artery Doppler wave.
The pregnancy outcomes in all cases were obtained from the patients’ medical records. PE was defined by the new-onset of hypertension and proteinuria after gestational week 20, as defined by the International Society for the Study of Hypertension.[17]
All data were entered into an electronic database and assessed for normality of distribution. Descriptive statistics were presented as percentages for categorical data and as means for continuous data. Pearson χ2 tests were used for categorical comparisons and 2 sample t tests, for continuous comparisons.
All tests were 2-tailed, and a P value of less than .05 was considered significant. Receiver-operating characteristic (ROC) curves were generated for subjects developing PE at <34 weeks. The results are presented as the area under the curve (AUC) using the algorithms implemented in IBM SPSS Statistics version 23 software (SPSS Inc, Chicago, IL).
3. Results
A total of 304 patients were enrolled during the study period. Among them, 49 patients were excluded from the final analysis for any of the following reasons: loss of follow-up,[12] incomplete records,[11] fetal anomalies,[12] spontaneous miscarriage,[18] and spontaneous preterm birth.[5] We excluded 2 patients with gestational hypertension and 3 with late-onset PE who delivered at term and 3 cases of placental centralization since our aim was to evaluate the effect of the placental laterality in predicting early-onset of PE.
Thus, 247 patients were included in the final analysis. Among them, 240 patients had a normal pregnancy outcome, whereas 7 (2.83%) had delivery before 34 weeks of gestation due to early-onset PE. The maternal demographic characteristics and pregnancy outcomes of patients with normal pregnancy outcomes and those who developed PE were compared; no differences were noted between the 2 patient groups (Table 1). However, as compared to the cases with normal pregnancy outcomes, those with early-onset PE had significantly lower average gestational age at delivery and significantly lesser average neonatal weight.
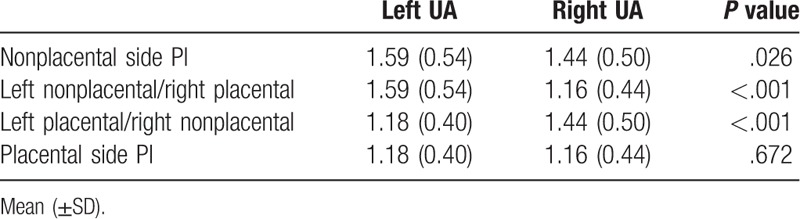
Interestingly, among patients with normal pregnancy outcomes, for the non-placenta-side, the left-side UtA PI was significantly higher than the right-side UtA PI (P = .026). Additionally, for the placental-side, PI measurements of the UtA ipsilateral to the placenta were similar without any significant effect of placental laterality (P = .672). Moreover, the PI was significantly lower for the placental-side UtA than for the non-placenta-side UtA (P < .001) (Table 2). The placental-side UtA PI was significantly lower than the mean UtA PI (P < .001).
Table 2.
Comparison of UtA (UA) pulsatility index (PI) on placental laterality.
ROC curve analysis showed that among the various PI values evaluated, the placental-side PI was the most useful in predicting PE, followed by the mean left- and right-side UtA PI and the non-placenta-side UtA PI (Fig. 2). Subsequently, an ideal cut-off value of 1.91 was identified for the placental-side PI on the basis of the maximal Youden's index, with an AUC of 0.987 (95% confidence interval [CI]: 0.972–1.00), sensitivity of 100%, and specificity of 96.3%. For the mean of the left and right UtA PI values, the optimal cut-off value was determined to be 1.63, with an AUC of 0.876 (95% CI: 0.796–0.956), sensitivity of 100%, and specificity of 74.2% (Fig. 2). Finally, the optimal cut-off value of PI for the non-placenta-side UtA was 1.975, with an AUC of 0.631 (95% CI: 0.387–0.875), sensitivity of 57.1%, and specificity of 79.2%. If the optimal cut-off value for predicting early onset PE was set at 1.91 on the basis of the vales obtained for the placental-side PI, then the mean of the left- and right-side PI had a sensitivity of 57.1% and specificity of 85.4%, whereas the non-placenta-side PI had a sensitivity of 57.1% and specificity of 76.3%.
Figure 2.
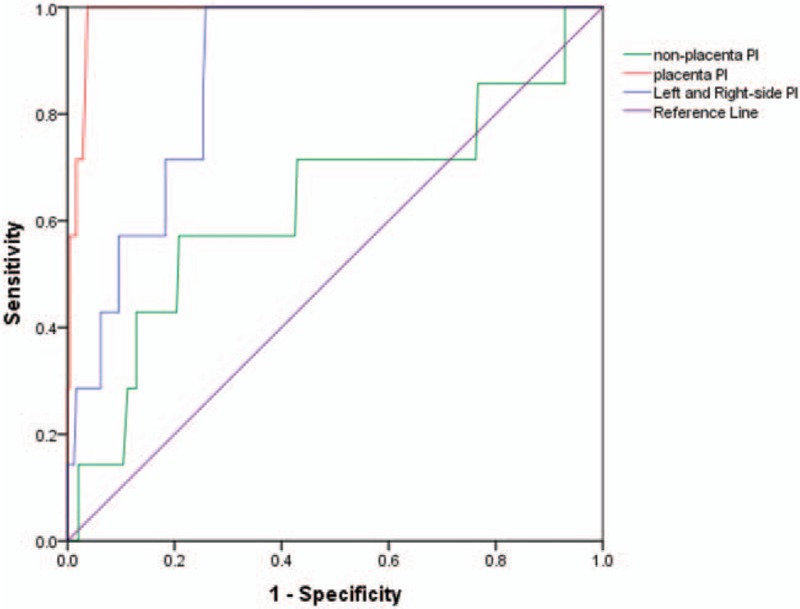
Receiver-operating characteristic curves demonstrating the accuracy of pulsatility index (PI) in predicating early-onset preeclampsia (PE). Red-line: placental PI; blue-line: mean PI; green-line: nonplacental side PI.
4. Discussion
Doppler ultrasound is a valuable modality in the evaluation of fetal and placental circulation as well as in the prediction of pregnancy outcomes. The current prospective study in the Chinese population showed that the first-trimester, placental-side UtA has a significantly lower PI than the corresponding value on the contralateral (non-placenta) side. Interestingly, our study also showed that the left-side UtA had a significantly higher PI than that of the right-side UtA (in the absence of placenta) and that the side on which the placenta was located did not influence the value of PI. Furthermore, we found that for the prediction of early-onset PE, the placental-side UtA PI was the most useful parameter, followed by the mean PI of the left and right-side UtAs and the PI value measured on the nonplacental side UtA, in that order.
Normal pregnancy outcomes are completely dependent on normal placental development, which in turn relies on adequate trophoblastic invasion and remodeling of the maternal spiral arteries during the early stages of gestation. In the normally developed placenta, the physiological changes in the UtA flow are reflected by a consistent decrease in the impedance between 6 and 24 weeks and the maintenance of a stable level thereafter.[19] The lower value of the placental-side UtA PI (as compared with the non-placental-side UtA PI observed in our study) was also noted by some previous studies.[19–23]
In our study, the placenta in the majority of the patients showed a tendency to be either on the left side or on the right side. In fact, in only 3 of our patients, we noted a tendency for placental centralization. This implies that in the first trimester, the placental distribution is not uniform, which simplifies the determination of the placental laterality.
Our study also showed that PI of the nonplacenta side the left-side UtA was higher than that of the right-sided UtA. The mechanism underlying this phenomenon remains unclear. The anatomic location of the arteries and normal uterine dextrorotation may be possible reasons for this difference. On the other hand, this phenomenon may also be related to the physiological differences between the blood pressure levels in the left side and right side. Considering these points, it would be interesting to determine whether there is any difference between the left and right UtA PI in nonpregnant women. This difference disappeared after placentation, since our data showed no difference between the PI of the left placental-side UtA and right placental-side UtA.
Traditionally, the PI is measured on the left- and right-side UtAs, and the mean of the 2 measurements is used in the prediction of PE.[14] A previous study has shown that the lowest UtA PI value was the most effective in predicting PE and that it has the best performance.[11] However, in that study,[11] the authors did not specify the placental laterality, assuming that the vessel with the lower PI is likely to be the placental one. Consistent with the findings of their study, our investigations clearly indicated that the placental-side PI was the most effective in predicating early-onset PE.
Some of the main mechanisms implicated in the development of early-onset PE are failure of trophoblastic invasion of the maternal spiral arteries and the lack of conversion from narrow muscular vessels into wide nonmuscular vessels.[19] In pregnancies that subsequently develop PE, the PI values of UtAs are increased (even during the first and second trimesters of pregnancy) as a result of the increase in the impedance to the blood flow in the UtA. Moreover, Doppler screening studies conducted in the first and second trimesters have reported variable detection rates, with the detection rate in the second trimester being higher, especially in high-risk populations.[12]
A recent meta-analysis that included 18 studies with 55,974 women concluded that first-trimester UtA Doppler assessment is useful in predicting early-onset pre-eclampsia as well as other adverse pregnancy outcomes.[13] In that study, the sensitivity and specificity for the prediction of early-onset PE using an abnormal UtA Doppler were reported as 47.8% (95% CI: 39.0–56.8) and 92.1% (95% CI: 88.6–94.6), respectively. However, another recent meta-analysis, which comprised 76 studies and included a sample of 298,329 prenatal Doppler screenings performed in nulliparous women during the first trimester and early period of the second trimester in singleton pregnancies, concluded that the first-trimester UtA Doppler study has limited value in predicting adverse perinatal outcomes.[14]
The sensitivity of UtA Doppler studies in the prediction of PE and fetal growth restriction increases after 16 weeks of gestation. Several explanations may be provided for the discrepancies between the results of the abovementioned meta-analyses as well as the low value of first-trimester UtA Doppler studies in predicting PE, including differences between the enrolled studies in terms of Doppler sampling techniques (especially earlier studies), definitions of abnormal flow velocity waveform, patient populations, and gestational age at examination. Further, a recent meta-analysis showed that the most effective predictive parameter for PE is the placental side UtA RI.[14] However, consistent with the findings of other studies,[20–22] our results indicated that placental-side PI can significantly improve the prediction of PE. Nevertheless, our study is the largest study conducted thus far in a homogenous Chinese population for the assessment of the value of placenta-side UtA PI in predicting early-onset PE. It may appear that the rate of early-onset pre-eclampsia is relatively higher in our study than in others. This discrepancy may be explained as follows. Firstly, our hospital (Second Hospital of Jilin University) is in northeast China where the prevalence of hypertensive disorders of pregnancy is about 6.09%—significantly higher than that in south China (2.59%).[24,25] Secondly, this study was conducted at a single regional center hospital because the Second Hospital of Jilin University is the main maternal care referral hospital in Jilin Province. Therefore, we possibly have higher percentages of patents with gestational hypertension than in other centers.
The main limitation of our study is the small sample size. Further studies of larger population groups are necessary to establish the actual potential of first trimester placental-side UtA PI in predicting PE, especially in combination with the measurement of biochemical markers. Another limitation is that there is no method to quantify the tendency for placental lateralization as well as NT measurement. Moreover, we currently cannot determine the widest nuchal translucency, and NT assessment is dependent on the examiner's subjective judgment. Similarly, lateralization of the placenta was subjectively assessed by the examiner. Further investigations are therefore necessary to evaluate the clinical applicability of this method.
The purpose of screening is to allow for timely preventive measures to avoid an adverse pregnancy outcome. Therefore, early prediction of late-pregnancy adverse outcomes (such as PE) has some clear advantages, such as the prompt initiation of appropriate management strategies that may prevent or mitigate these complications.
Several studies have shown that aspirin administered during pregnancy may prevent PE and adverse outcomes.[23,26] In addition, recent studies have shown that the combination of first-trimester UtA Doppler study and measurement of biochemical markers allows for a better prediction rate than the individual application of either investigation.[27–29] Therefore, using the effective UtA Doppler PI in conjunction with appropriate biochemical markers will further improve screening tests for PE.
We believe that with further improvement in screening methods and technology as well as earlier implementation of interventional strategies, the frequency of adverse pregnancy outcomes may decrease in the future.
Acknowledgments
The authors are grateful to Professor Saemundur Gudmundsson for proofreading this manuscript. The authors also thank Gui-Yun Wang, Ji-Ying Tian, and Yu-Hong Ma for their help in the recruitment of participants and data collection.
Author contributions
Conceptualization: Wen-Ling Song, Yan-Hui Zhao, Shu-Jing Shi, Xian-Ying Liu, Yang Jiao, Xiao-Jing Wang.
Data curation: Wen-Ling Song, Yan-Hui Zhao, Shu-Jing Shi, Xian-Ying Liu, Gui-Ying Zheng, Christopher Morosky, Yang Jiao, Xiao-Jing Wang.
Formal analysis: Wen-Ling Song, Yan-Hui Zhao, Shu-Jing Shi, Xian-Ying Liu, Gui-Ying Zheng, Christopher Morosky, Yang Jiao, Xiao-Jing Wang.
Funding acquisition: Wen-Ling Song, Yan-Hui Zhao, Shu-Jing Shi, Xian-Ying Liu, Yang Jiao, Xiao-Jing Wang.
Investigation: Wen-Ling Song, Yan-Hui Zhao, Shu-Jing Shi, Xian-Ying Liu, Gui-Ying Zheng, Christopher Morosky, Yang Jiao, Xiao-Jing Wang.
Methodology: Wen-Ling Song, Yan-Hui Zhao, Shu-Jing Shi, Xian-Ying Liu, Gui-Ying Zheng, Christopher Morosky, Yang Jiao, Xiao-Jing Wang.
Project administration: Wen-Ling Song, Yan-Hui Zhao, Shu-Jing Shi, Xian-Ying Liu, Gui-Ying Zheng, Yang Jiao, Xiao-Jing Wang.
Resources: Wen-Ling Song, Yan-Hui Zhao, Shu-Jing Shi, Xian-Ying Liu, Gui-Ying Zheng, Christopher Morosky, Yang Jiao, Xiao-Jing Wang.
Software: Wen-Ling Song, Yan-Hui Zhao, Shu-Jing Shi, Xian-Ying Liu, Gui-Ying Zheng, Christopher Morosky, Yang Jiao, Xiao-Jing Wang.
Supervision: Wen-Ling Song, Yan-Hui Zhao, Shu-Jing Shi, Xian-Ying Liu, Yang Jiao, Xiao-Jing Wang.
Validation: Wen-Ling Song, Yan-Hui Zhao, Shu-Jing Shi, Xian-Ying Liu, Gui-Ying Zheng, Christopher Morosky, Yang Jiao, Xiao-Jing Wang.
Visualization: Wen-Ling Song, Yan-Hui Zhao, Shu-Jing Shi, Xian-Ying Liu, Gui-Ying Zheng, Christopher Morosky, Yang Jiao, Xiao-Jing Wang.
Writing – original draft: Wen-Ling Song, Yan-Hui Zhao, Shu-Jing Shi, Xian-Ying Liu, Gui-Ying Zheng, Christopher Morosky, Yang Jiao, Xiao-Jing Wang.
Writing – review & editing: Wen-Ling Song, Yan-Hui Zhao, Shu-Jing Shi, Xian-Ying Liu, Gui-Ying Zheng, Christopher Morosky, Yang Jiao, Xiao-Jing Wang.
Footnotes
Abbreviations: PE = preeclampsia, PI = pulsatility index, ROCs = receiver operating characteristic curves, UtA = uterine artery.
This work was supported by Jilin Province Health Special Projects (JW2010-12-13), Jilin Provincial Development and Reform Commission (JF20122007-7).
The authors have no conflicts of interest to disclose.
References
- [1].Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ 2013;347:f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Abalos E, Cuesta C, Grosso AL, et al. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol 2013;170:1–7. [DOI] [PubMed] [Google Scholar]
- [3].Meekins JW, Pijnenborg R, Hanssens M, et al. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol 1994;101:669–74. [DOI] [PubMed] [Google Scholar]
- [4].Myatt L. Role of placenta in preeclampsia. Endocrine 2002;19:103–11. [DOI] [PubMed] [Google Scholar]
- [5].Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med 2006;355:992–1005. [DOI] [PubMed] [Google Scholar]
- [6].Levine RJ, Thadhani R, Qian C, et al. Urinary placental growth factor and risk of preeclampsia. JAMA 2005;293:77–85. [DOI] [PubMed] [Google Scholar]
- [7].Hui D, Okun N, Murphy K, et al. Combinations of maternal serum markers to predict preeclampsia, small for gestational age, and stillbirth: a systematic review. J Obstet Gynaecol Can 2012;34:142–53. [DOI] [PubMed] [Google Scholar]
- [8].Zeisler H, Llurba E, Chantraine F, et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med 2016;374:13–22. [DOI] [PubMed] [Google Scholar]
- [9].Papageorghiou AT, Yu CK, Cicero S, et al. Second-trimester uterine artery Doppler screening in unselected populations: a review. J Matern Fetal Neonatal Med 2002;12:78–88. [DOI] [PubMed] [Google Scholar]
- [10].Melchiorre K, Leslie K, Prefumo F, et al. First-trimester uterine artery Doppler indices in the prediction of small-for-gestational age pregnancy and intrauterine growth restriction. Ultrasound Obstet Gynecol 2009;33:524–9. [DOI] [PubMed] [Google Scholar]
- [11].Poon LC, Staboulidou I, Maiz N, et al. Hypertensive disorders in pregnancy: screening by uterine artery Doppler at 11-13 weeks. Ultrasound Obstet Gynecol 2009;34:142–8. [DOI] [PubMed] [Google Scholar]
- [12].Cnossen JS, Morris RK, ter Riet G, et al. Use of uterine artery Doppler ultrasonography to predict pre-eclampsia and intrauterine growth restriction: a systematic review and bivariable meta-analysis. CMAJ 2008;178:701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Velauthar L, Plana MN, Kalidindi M, et al. First-trimester uterine artery Doppler and adverse pregnancy outcome: a meta-analysis involving 55,974 women. Ultrasound Obstet Gynecol 2014;43:500–7. [DOI] [PubMed] [Google Scholar]
- [14].Matevosyan NR. Predictive accuracy of the first trimester Doppler scan: a meta-study. Wien Med Wochenschr 2015;165:199–209. [DOI] [PubMed] [Google Scholar]
- [15].Ito Y, Shouno H, Yamasaki M, et al. Relationship between the placental location and the flow velocity waveforms of bilateral uterine arteries. Asia Oceania J Obstet Gynaecol 1990;16:73–8. [DOI] [PubMed] [Google Scholar]
- [16].Bhide A, Acharya G, Bilardo CM, et al. ISUOG practice guidelines: use of Doppler ultrasonography in obstetrics. Ultrasound Obstet Gynecol 2013;41:233–9. [DOI] [PubMed] [Google Scholar]
- [17].Tranquilli AL. Introduction to ISSHP new classification of preeclampsia. Pregnancy Hypertens 2013;3:58–9. [DOI] [PubMed] [Google Scholar]
- [18].Kleinrouweler CE, Wiegerinck MM, Ris-Stalpers C, et al. Accuracy of circulating placental growth factor, vascular endothelial growth factor, soluble fms-like tyrosine kinase 1 and soluble endoglin in the prediction of pre-eclampsia: a systematic review and meta-analysis. BJOG 2012;119:778–87. [DOI] [PubMed] [Google Scholar]
- [19].Regnault TR, Galan HL, Parker TA, et al. Placental development in normal and compromised pregnancies: a review. Placenta 2002;23suppl A:S119–129. [DOI] [PubMed] [Google Scholar]
- [20].Bewley S, Campbell S, Cooper D. Uteroplacental Doppler flow velocity waveforms in the second trimester. A complex circulation. Br J Obstet Gynaecol 1989;96:1040–6. [DOI] [PubMed] [Google Scholar]
- [21].Liberati M, Rotmensch S, Zannolli P, et al. Uterine artery Doppler velocimetry in pregnant women with lateral placentas. J Perinat Med 1997;25:133–8. [DOI] [PubMed] [Google Scholar]
- [22].Tarzamni MK, Kefayati M, Maleki M, et al. Placental laterality and uterine blood flow at 20-40 weeks’ gestation in low-risk pregnancies. J Obstet Gynaecol 2016;36:24–30. [DOI] [PubMed] [Google Scholar]
- [23].Yousuf S, Ahmad A, Qadir S, et al. Utility of placental laterality and uterine artery Doppler abnormalities for prediction of preeclampsia. J Obstet Gynaecol India 2016;66:212–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Roberts CL, Ford JB, Algert CS, et al. Population-based trends in pregnancy hypertension and pre-eclampsia: an international comparative study. BMJ Open 2011;1:e000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ye C, Ruan Y, Zou L, et al. The 2011 survey on hypertensive disorders of pregnancy (HDP) in China: prevalence, risk factors, complications, pregnancy and perinatal outcomes. PLoS One 2014;9:e100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Meher S, Duley L, Hunter K, et al. Antiplatelet therapy before or after 16 weeks’ gestation for preventing preeclampsia: an individual participant data meta-analysis. Am J Obstet Gynecol 2017;216:121–8. [DOI] [PubMed] [Google Scholar]
- [27].Roberge S, Nicolaides K, Demers S, et al. The role of aspirin dose on the prevention of preeclampsia and fetal growth restriction: systematic review and meta-analysis. Am J Obstet Gynecol 2017;216:110–20. [DOI] [PubMed] [Google Scholar]
- [28].Kuc S, Wortelboer EJ, van Rijn BB, et al. Evaluation of 7 serum biomarkers and uterine artery Doppler ultrasound for first-trimester prediction of preeclampsia: a systematic review. Obstet Gynecol Surv 2011;66:225–39. [DOI] [PubMed] [Google Scholar]
- [29].Li L, Zheng Y, Zhu Y, et al. Serum biomarkers combined with uterine artery Doppler in prediction of preeclampsia. Exp Ther Med 2016;12:2515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]


