Abstract
Background
The surgical stress response plays an important role on the pathogenesis of perioperative cardiac complications. Alpha‐2 adrenergic agonists attenuate this response and may help prevent postoperative cardiac complications.
Objectives
To determine the efficacy and safety of α‐2 adrenergic agonists for reducing mortality and cardiac complications in adults undergoing cardiac surgery and non‐cardiac surgery.
Search methods
We searched CENTRAL (2017, Issue 4), MEDLINE (1950 to April Week 4, 2017), Embase (1980 to May 2017), the Science Citation Index, clinical trial registries, and reference lists of included articles.
Selection criteria
We included randomized controlled trials that compared α‐2 adrenergic agonists (i.e. clonidine, dexmedetomidine or mivazerol) against placebo or non‐α‐2 adrenergic agonists. Included trials had to evaluate the efficacy and safety of α‐2 adrenergic agonists for preventing perioperative mortality or cardiac complications (or both), or measure one or more relevant outcomes (i.e. death, myocardial infarction, heart failure, acute stroke, supraventricular tachyarrhythmia and myocardial ischaemia).
Data collection and analysis
Two authors independently assessed trial quality, extracted data and independently performed computer entry of abstracted data. We contacted study authors for additional information. Adverse event data were gathered from the trials. We evaluated included studies using the Cochrane 'Risk of bias' tool, and the quality of the evidence underlying pooled treatment effects using GRADE methodology. Given the clinical heterogeneity between cardiac and non‐cardiac surgery, we analysed these subgroups separately. We expressed treatment effects as pooled risk ratios (RR) with 95% confidence intervals (CI).
Main results
We included 47 trials with 17,039 participants. Of these studies, 24 trials only included participants undergoing cardiac surgery, 23 only included participants undergoing non‐cardiac surgery and eight only included participants undergoing vascular surgery. The α‐2 adrenergic agonist studied was clonidine in 21 trials, dexmedetomidine in 24 trials and mivazerol in two trials.
In non‐cardiac surgery, there was high quality evidence that α‐2 adrenergic agonists led to a similar risk of all‐cause mortality compared with control groups (1.3% with α‐2 adrenergic agonists versus 1.7% with control; RR 0.80, 95% CI 0.61 to 1.04; participants = 14,081; studies = 16). Additionally, the risk of cardiac mortality was similar between treatment groups (0.8% with α‐2 adrenergic agonists versus 1.0% with control; RR 0.86, 95% CI 0.60 to 1.23; participants = 12,525; studies = 5, high quality evidence). The risk of myocardial infarction was probably similar between treatment groups (RR 0.94, 95% CI 0.69 to 1.27; participants = 13,907; studies = 12, moderate quality evidence). There was no associated effect on the risk of stroke (RR 0.93, 95% CI 0.55 to 1.56; participants = 11,542; studies = 7; high quality evidence). Conversely, α‐2 adrenergic agonists probably increase the risks of clinically significant bradycardia (RR 1.59, 95% CI 1.18 to 2.13; participants = 14,035; studies = 16) and hypotension (RR 1.24, 95% CI 1.03 to 1.48; participants = 13,738; studies = 15), based on moderate quality evidence.
There was insufficient evidence to determine the effect of α‐2 adrenergic agonists on all‐cause mortality in cardiac surgery (RR 0.52, 95% CI 0.26 to 1.04; participants = 1947; studies = 16) and myocardial infarction (RR 1.01, 95% CI 0.43 to 2.40; participants = 782; studies = 8), based on moderate quality evidence. There was one cardiac death in the clonidine arm of a study of 22 participants. Based on very limited data, α‐2 adrenergic agonists may have reduced the risk of stroke (RR 0.37, 95% CI 0.15 to 0.93; participants = 1175; studies = 7; outcome events = 18; low quality evidence). Conversely, α‐2 adrenergic agonists increased the risk of bradycardia from 6.4% to 12.0% (RR 1.88, 95% CI 1.35 to 2.62; participants = 1477; studies = 10; moderate quality evidence), but their effect on hypotension was uncertain (RR 1.19, 95% CI 0.87 to 1.64; participants = 1413; studies = 9; low quality evidence).
These results were qualitatively unchanged in subgroup analyses and sensitivity analyses.
Authors' conclusions
Our review concludes that prophylactic α‐2 adrenergic agonists generally do not prevent perioperative death or major cardiac complications. For non‐cardiac surgery, there is moderate‐to‐high quality evidence that these agents do not prevent death, myocardial infarction or stroke. Conversely, there is moderate quality evidence that these agents have important adverse effects, namely increased risks of hypotension and bradycardia. For cardiac surgery, there is moderate quality evidence that α‐2 adrenergic agonists have no effect on the risk of mortality or myocardial infarction, and that they increase the risk of bradycardia. The quality of evidence was inadequate to draw conclusions regarding the effects of alpha‐2 agonists on stroke or hypotension during cardiac surgery.
Plain language summary
Using alpha‐2 adrenergic agonists to prevent heart complications after major surgery
Review question
Do alpha‐2 adrenergic agonists (clonidine, dexmedetomidine and mivazerol) reduce the number of deaths and heart complications when given around the time of surgery?
Background
Heart‐related complications can lead to death and long hospital stays after surgery. Each year, about 300 million people undergo major surgery, of whom nine million experience serious heart complications. These complications may occur, in part, because surgery places a large stress on the heart. This stress can lead to high blood pressure and high heart rates during surgery, neither of which are good for the heart. Alpha‐2 adrenergic agonists are a group of medicines that can prevent the blood pressure and heart rate from increasing during surgery. Thus, these medicines may also protect the heart from the stress of surgery. We wanted to find out if giving these medicines around the time of surgery could protect the heart from the stress of surgery and thus prevent major heart complications.
Study characteristics
We found 47 studies that were published up to May 2017. These studies involved 17,039 adults who had major surgery. Twenty‐four studies involved 2672 adults having heart surgery. Twenty‐three studies involved 14,367 adults undergoing major operations other than heart surgery. Forty studies compared alpha‐2 adrenergic agonists to dummy treatment (placebo). The other seven studies compared them to other medicines. Twenty‐one studies tested an alpha‐2 adrenergic agonist medicine called clonidine, 24 studied another medicine called dexmedetomidine and two studied another medicine called mivazerol. The duration of alpha‐2 adrenergic agonist medicine studied varied from one dose before surgery to three days of treatment. Most people who took part in these studies were men, and their average age was 60 to 70 years old. Fourteen studies reported receiving money from the company that manufactured the medicine being tested in the same study. Another 15 studies did not report where they received the money needed to fund the study. The number of people who took part in each study varied between 20 participants to as many as 10,000 participants. Nineteen studies included more than 100 participants.
Key results
We found that alpha‐2 adrenergic agonists generally had no clear benefits for preventing death or major complications after surgery. For people having major operations other than heart surgery, alpha‐2 adrenergic agonists did not lower their chances of dying, having a heart attack or having a stroke after surgery. We did not find sufficient evidence that, in people having heart surgery, alpha‐2 adrenergic lowered the risk of dying or having a heart attack after surgery. There was some very limited evidence that these medicines might prevent strokes after heart surgery. Nonetheless, more research is needed before we can be certain that alpha‐2 adrenergic agonists truly have this benefit. These medicines also had some important side effects. People who received alpha‐2 adrenergic agonists were much more likely to have low blood pressures or low heart rates during or after surgery.
Quality of evidence
We assessed the quality of all studies we identified using a specialized tool called the GRADE criteria. In general, we found that most of the evidence in these studies was moderate or high quality. Thus, based on our results, we can be reasonably certain that alpha‐2 adrenergic agonists are not helpful for reducing the numbers of deaths or major heart complications that happen after surgery.
Summary of findings
Background
Description of the condition
Perioperative cardiac complications are a major health concern for the 312 million people who annually undergo major surgery worldwide (Meara 2015). For example, about 3% of people who undergo major non‐cardiac surgery experience perioperative myocardial infarction (MI) (VISION 2014). Major cardiac complications, such as MI, lead to increased mortality, hospital stay and costs (Fleischmann 2003; Force 1990; VISION 2014). The surgical stress response may play an important role in the pathogenesis of these complications. Specifically, surgical stress stimulates the sympathetic nervous system, which in turns leads to increased plasma levels of norepinephrine and epinephrine (Halter 1997). These effects increase blood pressure and heart rate, which can predispose the myocardium to ischaemia, especially in people with decreased coronary blood flow reserve.
Description of the intervention
Alpha‐2 (α‐2) adrenergic agonists selectively bind to presynaptic α‐2 adrenergic receptors to activate a negative feedback mechanism that inhibits central sympathetic outflow (Muzi 1992). These receptors are mainly located in the central nervous system, specifically in the brain stem and locus coeruleus. Activation of these receptors lead to hypotension, bradycardia, central sedation, anxiolysis and analgesia. Three specific α‐2 adrenergic agonists that have been evaluated in people undergoing surgery, namely clonidine, dexmedetomidine and mivazerol. Clonidine and dexmedetomidine are available for clinical use, while the use of mivazerol has been restricted to clinical trials. Clonidine has a half‐life of 12 to 18 hours with excellent bioavailability, lending to its suitability for once daily administration in oral tablet or transdermal patch forms. An intravenous (IV) formulation of clonidine is also available. Dexmedetomidine has a shorter half‐life of only two hours and variable bioavailability, consequently making it more suited for administration as a continuous IV infusion (Flood 2015). Similarly, mivazerol is also administered as a continuous IV infusion (Oliver 1999).
How the intervention might work
As indicated above, α‐2 adrenergic agonists inhibit central sympathetic outflow. Hence, they can attenuate perioperative haemodynamic abnormalities (Ellis 1994; McSPI‐Europe 1997; Talke 1995), and perhaps also prevent cardiac complications. Furthermore, clonidine has the unique ability to reduce sympathetic activity without blunting the baroreflex, which is critical for responding to the fluctuations in circulating blood volume often encountered during surgery (Muzi 1992). Nonetheless, α‐2 adrenergic agonists have important adverse effects, including hypotension and bradycardia (Biccard 2008). These haemodynamic effects may have clinically important consequences for people undergoing surgery. For example, in the Perioperative Ischemic Evaluation ‐ 1 (POISE‐1) randomized controlled trial (RCT), acute perioperative β‐blockade increased risks of bradycardia, hypotension, acute stroke, and death (POISE 2008). Given that α‐2 adrenergic agonists have both potential benefits and adverse effects, a quantitative systematic review may help determine their overall efficacy and safety.
Why it is important to do this review
Previous systematic reviews of perioperative α‐2 adrenergic agonists have been published (Biccard 2008; Nishina 2002; Stevens 2003). However, two of them were restricted to individual α‐2 adrenergic agonists, namely clonidine (Nishina 2002), and dexmedetomidine (Biccard 2008). The other review was restricted to studies published before 2002 (Stevens 2003). A systematic review according to the Cochrane methodology is therefore justified. The current review is an update to a previous Cochrane Review that included studies published before August 2008 (Wijeysundera 2009). This update was deemed necessary, in part, given the publication of the largest RCT to‐date of perioperative α‐2 adrenergic agonists, the Perioperative Ischemic Evaluation ‐ 2 (POISE‐2) trial (Devereaux 2014a).
Objectives
To determine the efficacy and safety of α‐2 adrenergic agonists for reducing mortality and cardiac complications in adults undergoing cardiac surgery and non‐cardiac surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included published RCTs.
Types of participants
We included adults (aged 18 years or older) undergoing surgery under general anaesthesia, neuraxial anaesthesia, or both. We excluded surgery performed under local anaesthesia or peripheral nerve blockade alone because such procedures are generally associated with a very low risk of mortality and morbidity. We also excluded surgery performed on pregnant women, organ transplant recipients, or people with substance withdrawal. Organ transplantation procedures may be associated with a high risk of mortality unrelated to cardiovascular causes, thereby masking any potential benefit from α‐2 adrenergic agonists.
Types of interventions
The experimental intervention must have included clonidine, mivazerol or dexmedetomidine administration before surgery (within 24 hours), during surgery, or after surgery (within 48 hours). The medications must have been administered via IV, intramuscular, oral or transdermal routes. There were no restrictions on the dose, duration or frequency of the intervention.
We permitted active interventions in the comparator group only if the comparator was judged to have minimal to no effect on the primary or secondary outcomes. For example, in a trial where dexmedetomidine was being primarily evaluated for the role of providing postoperative sedation after major surgery, comparison to propofol was judged to be reasonable.
Types of outcome measures
Included trials had to evaluate the efficacy or safety of α‐2 adrenergic agonists in reducing perioperative mortality or cardiac complications, or both. Studies were included if they measured one or more relevant outcomes, which included death, MI, heart failure (HF), acute stroke, supraventricular tachyarrhythmia (SVT) or myocardial ischaemia. In addition, studies with similar objectives to our review were included, even if these same studies did not report any relevant outcome events (i.e. death, MI, HF, acute stroke, SVT, myocardial ischaemia).
Primary outcomes
All‐cause mortality within 30 days after surgery: any reported death. The time period for outcome ascertainment in each trial was also documented.
Secondary outcomes
Cardiac mortality within 30 days after surgery: sudden death or death resulting from a primarily identifiable cardiac cause. The time period for outcome ascertainment in each trial was also documented.
MI within 30 days after surgery: definition as per individual study (specific criteria employed were documented). The time period for outcome ascertainment in each trial was also documented.
Myocardial ischaemia within 30 days after surgery: as detected on an electrocardiogram (ECG) or trans‐oesophageal echocardiogram (specific criteria employed were documented). The time period for outcome ascertainment in each trial was also documented.
SVT within 30 days after surgery: SVT, atrial fibrillation or atrial flutter. The time period for outcome ascertainment in each trial was also documented.
HF within 30 days after surgery: clinical diagnosis of HF or need for postoperative intra‐aortic balloon pump support (applicable only for cardiac surgery). The time period for outcome ascertainment in each trial was also documented.
Adverse effects from treatment
Acute stroke within 30 days after surgery: new focal neurological deficit with signs and symptoms lasting longer than 24 hours. The time period for outcome ascertainment in each trial was also documented.
Physiological effects of treatment
Bradycardia requiring pharmacological or pacemaker treatment during the period of study drug administration.
Hypotension requiring treatment with inotropes or vasopressors during the period of study drug administration.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL, 2017, Issue 4), MEDLINE (1950 to April week 4 2017), Embase (1980 to May 2017), the Science Citation Index and reference lists of articles. The Ovid platform was used for searching the electronic databases.
We searched MEDLINE using the search terms presented in Appendix 1. We then limited the studies to those identified simultaneously by a highly sensitive search strategy for identifying RCTs in MEDLINE (Dickersin 1994). Our search strategies for CENTRAL and Embase are presented in Appendix 1.
Searching other resources
We entered all trials selected for inclusion into the Science Citation Index to identify any additional relevant articles. The bibliographies of all included articles and published reviews were searched to identify any other potentially relevant studies for inclusion. Additionally, we searched clinical trial registries, namely ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), for published studies meeting our inclusion criteria. These additional searches were completed in May 2017.
Data collection and analysis
Selection of studies
Two authors (DD, AS) independently performed literature searches for potentially relevant RCTs. All identified published full papers and abstracts were assessed independently for inclusion by the same two authors. We applied no language restrictions. We documented the reasons for exclusion for all excluded studies. We resolved all disagreements by consensus or involvement of a third author (DNW).
Data extraction and management
Two authors (DD, AS) independently extracted data from the included studies on a predesigned data abstraction form (Appendix 2). These same two authors independently entered all data into Review Manager 5 (RevMan 2014). We were not blinded to study authors, institution or journal when performing data abstraction. Where necessary, we contacted authors of published trials to provide any additional information required for the analyses (see Methods of the review).
Assessment of risk of bias in included studies
Two authors (DD, AS) independently evaluated the quality of all included trials using the criteria recommended by the Cochrane Anaesthesia, Critical and Emergency Care (ACE) Group. These criteria emphasize the adequacy of allocation concealment, randomization, blinding and intention‐to‐treat (ITT) analysis. Each included study was evaluated using the Cochrane 'Risk of bias' tool (Higgins 2011a). We were not blinded to study authors, institution or journal when performing quality assessment.
Measures of treatment effect
We performed all statistical analyses using Review Manager 5 (RevMan 2014). Given that all outcomes and adverse effects were dichotomous, all treatment effects were expressed as pooled risk ratios (RR) with 95% confidence intervals (CI).
Unit of analysis issues
We excluded cross‐over trials and cluster randomized trials in this review. If a study had multiple treatment arms, comparisons were made between α‐2 adrenergic agonist and placebo, or between α‐2 adrenergic agonist and inactive control.
Dealing with missing data
If a study had missing relevant data in the published report, we attempted to contact the study authors up to three times to obtain these data. If data were missing due to participant attrition, and imputation methods were not used in the published report, we employed complete case analysis when importing the data.
Assessment of heterogeneity
We measured heterogeneity using the I2 statistic: the proportion of total variation explained by between‐study variation as opposed to chance (Higgins 2002; Higgins 2003). Higher I2 statistics imply more heterogeneity between studies than would be expected by chance alone.
Assessment of reporting biases
We carried out funnel plot analyses to assess for publication bias (Egger 1997), with formal tests for asymmetry being performed only if meta‐analyses pooled data from 10 or more studies (Higgins 2011b).
Data synthesis
Given the clinical heterogeneity between cardiac and non‐cardiac surgery, we conducted analyses for these two subgroups separately. If an individual study included both cardiac and non‐cardiac surgery procedures, we attempted to obtain subgroup‐specific results from the authors. If such data were not available, and greater than 75% of participants underwent cardiac surgical procedures, the specific study was allocated to the cardiac surgery subgroup. Conversely, the study was allocated to the non‐cardiac surgery subgroup if greater than 75% of participants underwent non‐cardiac surgical procedures. In all other cases, the specific study was excluded from the review. In the presence of low heterogeneity (I2 statistic 25% or less) (Higgins 2003), pooled RRs were calculated using the fixed‐effect model. In the presence of moderate‐to‐significant heterogeneity (I2 statistic greater than 25%) (Higgins 2003), we used the random‐effects model and carried out post‐hoc analyses to attempt to explain the heterogeneity.
Subgroup analysis and investigation of heterogeneity
A priori, we planned several subgroup analyses to determine the potential influence of the surgical procedure, the specific α‐2 adrenergic agonist employed and coexistent therapies on the overall results. Subgroup‐specific results were only calculated if there were two or more studies within the subgroup. These subgroup analyses were as follows.
Treatment effects of α‐2 adrenergic agonists on mortality (all‐cause and cardiac‐cause), MI and ischaemia based on the type of non‐cardiac surgical procedure, namely vascular versus non‐vascular non‐cardiac surgery. If a variety of surgical procedures were included in a study, we attempted to obtain subgroup‐specific results from the authors. If such data were not available, and greater than 75% of participants underwent the same class of surgery, the specific study was allocated to that specific subgroup. Failing that, the specific study was excluded from the subgroup analysis based on procedure type. We used statistical tests of interaction to assess for the presence of any subgroup effects.
We calculated treatment effects for each of clonidine, mivazerol and dexmedetomidine on mortality (all‐cause and cardiac‐cause), and MI in non‐cardiac surgery. Statistical tests of interaction were used to assess for the presence of any subgroup effects.
Sensitivity analysis
We planned several sensitivity analyses a priori to characterize the influence of study quality and outcome definitions on the overall results.
We restricted the meta‐analyses to the subset of studies that clearly reported methods for blinding and allocation concealment.
We determined the effect of α‐2 adrenergic agonists on MI in the subset of RCTs that strictly defined MI a priori as either significant new Q waves on an ECG or significant elevations in enzymatic markers of cardiac injury (MB isoenzyme of creatinine kinase, troponin‐I, troponin‐T).
We determined the effect of α‐2 adrenergic agonists on myocardial ischaemia in the subset of RCTs that strictly defined ischaemia a priori as ST segment depression or elevation of 0.1 mV or greater for one minute or longer.
In addition, we performed four additional post‐hoc analyses.
Significant statistical heterogeneity was identified when calculating the pooled effect of α‐2 adrenergic agonists on hypotension during non‐cardiac surgery. To explore potential explanations for this heterogeneity, we conducted subgroup analyses based on the specific agent (i.e. clonidine, mivazerol or dexmedetomidine) in the included trials. A statistical test of interaction was used to assess for the presence of a subgroup effect.
During the course of the review, we identified several very large included RCTs that might have highly influenced the overall pooled estimates. Therefore, we conducted a sensitivity analysis that excluded these very large RCTs.
Mivazerol is an experimental α‐2 adrenergic agonist that was studied in several relatively large trials, but never proceeded through the approval process for clinical use. At the request of external peer reviewers of this review, we conducted a sensitivity analysis that excluded trials that evaluated mivazerol.
Several relevant studies were conducted prior to 1997, during a period when perioperative practice might not necessarily be generalizable to contemporary practice. At the request of external peer reviewers of this review, we conducted a sensitivity analysis that excluded trials where data were collected prior to 1997.
'Summary of findings' tables and GRADE
To characterize the confidence in the pooled estimated treatment effects better, we used GRADE methodology to assess the quality of evidence (Guyatt 2008). We generated 'Summary of findings' tables that separately presented pooled treatment effect estimates for the subgroups of participants who underwent non‐cardiac surgery and cardiac surgery. To facilitate this process, data from the meta‐analyses in Review Manager 5 (RevMan 2014), were initially exported into GRADEpro. The GRADE approach rates quality of evidence as high, moderate, low or very low (GRADE Handbook 2013). Since all data included in this review were from RCTs, the quality of evidence for each outcome of interest was initially rated as high level, and then potentially downgraded up to three levels based on any deficiencies in the quality of the underlying evidence. The quality of evidence underlying each pooled treatment effect estimate was assessed with respect to the risk of bias, inconsistency, indirectness, imprecision and publication bias (Balshem 2011). The anticipated risk for comparison of each outcome was determined based on the event rate in the control group. Only outcomes judged as critically important, based on their impact on patient health or clinical decision‐making, were chosen for presentation in the 'Summary of findings' tables. In this present review, we included the following outcomes in the 'Summary of findings' tables, provided that relevant estimated pooled treatment effects were present: all‐cause mortality, cardiac mortality, MI, acute stroke, bradycardia and hypotension.
Results
Description of studies
Results of the search
Our search results are presented in Figure 1. The authors identified 3099 separate papers in the literature search and three additional papers from other sources and in total read 389 papers in full.
1.
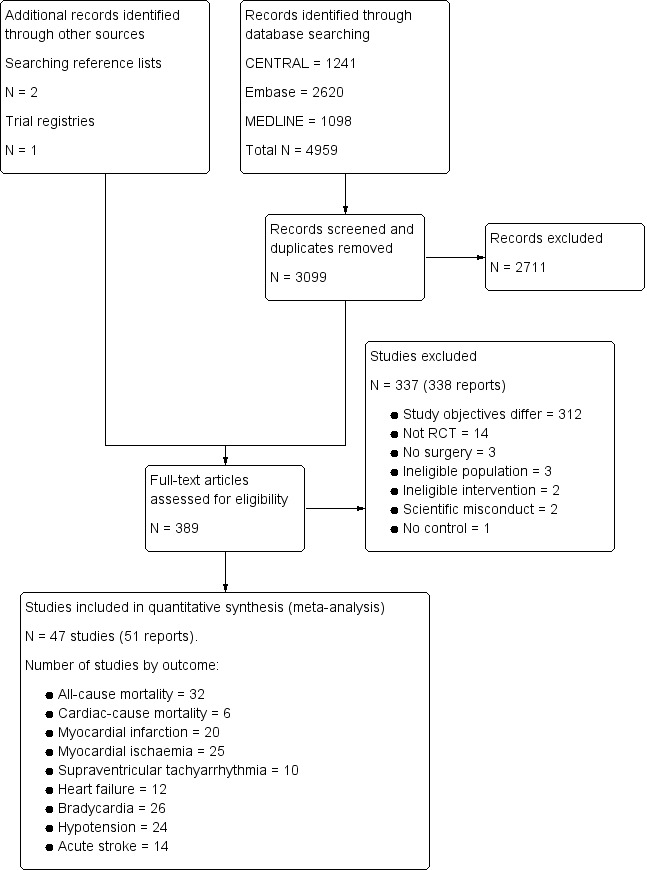
Study flow diagram.
Included studies
We included 47 trials, which encompassed 17,039 participants (Abi‐Jaoude 1993; Ammar 2016; Bergese 2010; Chi 2016; Cho 2016; Corbett 2005; Devereaux 2014a; Djaiani 2016; Dorman 1993; El‐Kerdawy 2004; Ellis 1994; Ghignone 1986; Ghignone 1987; Helbo‐Hansen 1986; Herr 2003; Jalonen 1997; Khalil 2013; Kim 2014a; Lee 2013a; Li 2017; Lipszyc 1991; Liu 2016; Loick 1999; Matot 2000; McSPI‐Europe 1997; Myles 1999; Oliver 1999; Park 2014; Patel 2016; Pawlik 2005; Pluskwa 1991; Quintin 1993; Quintin 1996; Ren 2013; Shehabi 2009; Soliman 2016; Stuhmeier 1996; Su 2016; Talke 1995; Talke 2000; Venn 1999; Venn 2001; Viviano 2012; Wallace 2004; Wijeysundera 2014a; Xu 2014; Yin 2002). These studies are described in detail in the Characteristics of included studies tables.
Twenty four studies with 2672 participants involved cardiac surgery alone (Abi‐Jaoude 1993; Ammar 2016; Chi 2016; Cho 2016; Corbett 2005; Djaiani 2016; Dorman 1993; El‐Kerdawy 2004; Ghignone 1986; Helbo‐Hansen 1986; Herr 2003; Jalonen 1997; Khalil 2013; Kim 2014a; Li 2017; Liu 2016; Loick 1999; Myles 1999; Park 2014; Patel 2016; Quintin 1993; Ren 2013; Shehabi 2009; Venn 1999). In all cases, the procedure involved was coronary artery bypass graft surgery or valve replacement surgery.
Of the 23 studies with 14,367 participants that involved non‐cardiac surgery (Bergese 2010; Devereaux 2014a; Ellis 1994; Ghignone 1987; Lee 2013a; Lipszyc 1991; Matot 2000; McSPI‐Europe 1997; Oliver 1999; Pawlik 2005; Pluskwa 1991; Quintin 1996; Soliman 2016; Stuhmeier 1996; Su 2016; Talke 1995; Talke 2000; Venn 2001; Viviano 2012; Wallace 2004; Wijeysundera 2014a; Xu 2014; Yin 2002), eight involved vascular procedures exclusively (Lipszyc 1991; McSPI‐Europe 1997; Pluskwa 1991; Quintin 1996; Soliman 2016; Stuhmeier 1996; Talke 1995; Talke 2000), and seven involved non‐vascular procedures exclusively (Ghignone 1987; Lee 2013a; Matot 2000; Pawlik 2005; Venn 2001; Viviano 2012; Xu 2014). One non‐cardiac surgery study presented subgroup‐specific results for both vascular and non‐vascular procedures (Oliver 1999).
Sample size
The sample sizes of the included trials ranged from 20 participants to 10,010 participants. Fourteen studies had fewer than 50 participants (Abi‐Jaoude 1993; Dorman 1993; Ghignone 1986; Ghignone 1987; Helbo‐Hansen 1986; Lipszyc 1991; Matot 2000; Pawlik 2005; Pluskwa 1991; Quintin 1993; Quintin 1996; Talke 1995; Talke 2000; Venn 2001), 14 studies had 50 to 100 participants (Ammar 2016; Chi 2016; Corbett 2005; El‐Kerdawy 2004; Ellis 1994; Jalonen 1997; Khalil 2013; Lee 2013a; Liu 2016; Loick 1999; Patel 2016; Viviano 2012; Xu 2014; Yin 2002), and 19 studies had greater than 100 participants (Bergese 2010; Cho 2016; Devereaux 2014a; Djaiani 2016; Herr 2003; Kim 2014a; Li 2017; McSPI‐Europe 1997; Myles 1999; Oliver 1999; Park 2014; Ren 2013; Shehabi 2009; Soliman 2016; Stuhmeier 1996; Su 2016; Venn 1999; Wallace 2004; Wijeysundera 2014a).
Demographics of sample
The mean age of participants in most studies was 60 to 70 years. In addition, the ratio of men to women in the included studies was skewed, with trials generally recruiting disproportionally more men (Characteristics of included studies table).
Intervention and comparators
The number of studies that assessed dexmedetomidine was 24, clonidine was 21 and mivazerol was two. Treatment duration ranged from a single preoperative dose to a 72‐hour course of treatment. With the exception of seven studies (Corbett 2005; Djaiani 2016; Herr 2003; Liu 2016; Park 2014; Shehabi 2009; Venn 2001), all trials compared α‐2 adrenergic agonists against inactive control. Of the four studies with active controls, one compared dexmedetomidine to morphine (Shehabi 2009), whereas the remainder were comparisons of dexmedetomidine versus propofol.
All studies that evaluated dexmedetomidine employed the IV route of administration. Dexmedetomidine was administered intraoperatively in 15 studies, with administration being continued postoperatively in nine of them. The duration of postoperative administration varied across these nine studies, ranging from continuation until arrival to the critical care unit, to continuation for 48 hours. Nine additional studies investigated dexmedetomidine that was administered entirely after surgery in the critical care unit. Both studies of mivazerol administered the drug IV starting from the intraoperative period, with continuation until 72 hours after surgery.
There was considerable variation in the administration regimens used in trials that assessed clonidine. It was administered intraoperatively by the IV route in three studies, with one of these studies also administering an oral loading dose before surgery. A single study used IV clonidine that was administered only preoperatively (i.e. 30 minutes prior to surgery). Four studies employed clonidine administered using the combination of an oral preoperative loading dose, and subsequent maintenance via the transdermal route for 72 hours. Finally, 12 studies administered clonidine orally before surgery, with three of them administering an additional intraoperative dose via the nasogastric route.
Funding
Thirty‐two studies reported their funding sources, whereas 15 did not (Abi‐Jaoude 1993; Chi 2016; Cho 2016; El‐Kerdawy 2004; Ghignone 1986; Ghignone 1987; Lipszyc 1991; Loick 1999; Myles 1999; Park 2014; Pluskwa 1991; Quintin 1993; Ren 2013; Stuhmeier 1996; Viviano 2012). Fourteen studies reported operational funding from pharmaceutical companies (Bergese 2010; Djaiani 2016; Helbo‐Hansen 1986; Herr 2003; Jalonen 1997; Li 2017; McSPI‐Europe 1997; Oliver 1999; Quintin 1996; Su 2016; Talke 1995; Talke 2000; Venn 1999; Venn 2001), and the remaining 18 studies reported that no pharmaceutical funds were used to complete the research (Ammar 2016; Corbett 2005; Devereaux 2014a; Dorman 1993; Ellis 1994; Khalil 2013; Kim 2014a; Lee 2013a; Liu 2016; Matot 2000; Patel 2016; Pawlik 2005; Shehabi 2009; Soliman 2016; Wallace 2004; Wijeysundera 2014a; Xu 2014; Yin 2002). Several studies in the latter group reported that a pharmaceutical company supplied the study drug as in‐kind support, and explicitly stated no further funds were received from the company.
Excluded studies
After the full‐text articles were reviewed, we excluded 337 studies. The reasons for these exclusions are presented in the Characteristics of excluded studies table, as well as the study flow diagram (Figure 1). The most common reason for exclusion was study objectives that differed from this present review (312 excluded studies). In these cases, the focus of these studies was to answer a question unrelated to the efficacy or safety of α‐2 adrenergic agonists for reducing mortality or cardiac complications (e.g. assessing the efficacy of these drugs for providing analgesia). Of the remaining articles, 14 were excluded because the experimental design was not an RCT, three were excluded since participants did not undergoing surgery and three were excluded due to an ineligible population. Two studies could not be classified into either the cardiac or non‐cardiac surgery subgroups, and were therefore excluded (Martin 2003; Triltsch 2002). A further two studies were excluded because the intervention was administered via an ineligible route (Nader 2009; Tzortzopoulou 2009), while one study was excluded due to lack of a control arm (Moghadam 2012). Three reports of two individual studies were excluded due to concerns about scientific misconduct (Boldt 1996; Wahlander 2005). In each of these cases, a lead author was found to have conducted scientific misconduct (Anon 2013; Rasmussen 2011; Wise 2013). Notably, both studies had been included in the previous 2009 version of this review (Wijeysundera 2009), at which point these issues with scientific misconduct had not yet been identified (Boldt 1996; Wahlander 2005).
Studies awaiting classification
No studies are currently awaiting classification.
Ongoing studies
We found no ongoing studies.
Risk of bias in included studies
The methodological quality of included studies is shown in the 'Risk of bias' figures (Figure 2; Figure 3). A visual summary of judgements about the quality and risk of bias for each trial is presented in Figure 3. Details explaining the judgements for each domain are presented in the 'Risk of bias' tables (Characteristics of included studies).
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Of the 47 included trials, only 28 were judged to have adequate methods of generating allocation sequences. Of the remaining 19 studies, two trials used methods likely to produces bias (Helbo‐Hansen 1986; Lipszyc 1991), while the remaining trials were classified as having unclear risk of bias because the methods were not described in adequate detail. Concealment of allocation sequence was generally poor with only 18 studies reporting methods associated with low risk of bias, while six studies described methods associated with a high risk of bias (Bergese 2010; Corbett 2005; Khalil 2013; Lee 2013a; Lipszyc 1991; Liu 2016). Only 16 studies reported adequate allocation sequence generation and allocation concealment.
Blinding
Although 31 studies described themselves as double‐blind, only 21 clearly reported adequate methods for how blinding was achieved. Of the remaining 26 studies, 14 were open‐label and therefore assessed to be high risk of bias, while the others were judged to have an unclear risk of bias. Outcome assessment was blinded in 21 trials, and therefore judged to be at low risk of bias. Only 16 trials demonstrated blinding of participants, personnel and outcome assessors.
Incomplete outcome data
Thirty trials reported no exclusions, exclusions deemed to be appropriate and ITT analysis. For four trials, exclusions (Lee 2013a; Oliver 1999; Quintin 1996; Stuhmeier 1996), were either not reported or judged as being excessive enough to likely cause bias. The remainder either failed to use ITT analysis or adequately account for exclusions. Only 11 studies reported a flow diagram of participants in the trial (Bergese 2010; Chi 2016; Devereaux 2014a; Kim 2014a; Lee 2013a; Li 2017; Liu 2016; Shehabi 2009; Su 2016; Viviano 2012; Wijeysundera 2014a), as is recommended in the CONSORT statement (Schulz 2010).
Selective reporting
Of the 47 trials, 37 demonstrated concordance between outcomes discussed in the methods or protocol and the outcomes reported. Four studies were judged to be of unclear risk of bias because they reported adverse events without discussing any surveillance methods (Liu 2016; Park 2014; Soliman 2016; Viviano 2012). The remaining six studies either failed to report major outcomes, or reported major outcomes not discussed in the relevant methods sections (Corbett 2005; Dorman 1993; Helbo‐Hansen 1986; Khalil 2013; Oliver 1999; Stuhmeier 1996).
Other potential sources of bias
Five trials had other sources of bias classified as unclear risk or high risk. Two of the trials had high risk of bias due to significant changes in their methods during the trial recruitment phase. One trial terminated early (Ellis 1994), while the other changed its selection criteria (Oliver 1999). Three trials were classified as having unclear risk of bias, because two trials (Lipszyc 1991; Quintin 1993), were being published only in abstract form (therefore lacking complete peer‐review), and another lacked reproducible selection criteria (Lee 2013a).
Effects of interventions
Summary of findings for the main comparison. Alpha‐2 adrenergic agonists compared to control in non‐cardiac surgery.
| Alpha‐2 adrenergic agonists compared to control in non‐cardiac surgery | ||||||
| Patient or population: adults undergoing non‐cardiac surgery Setting: hospital inpatient care Intervention: α‐2 adrenergic agonist Comparison: placebo or inactive control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Risk ratio (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control | Risk with α‐2 adrenergic agonists | |||||
|
All‐cause mortality (within 30‐days after surgery: any reported death) |
Study population | RR 0.80 (0.61 to 1.04) | 14,081 (16 RCTs) | ⊕⊕⊕⊕ High1,2 | ‐ | |
| 17 per 1000 | 13 per 1000 (10 to 17) | |||||
|
Cardiac mortality (within 30‐days after surgery: sudden death or death resulting from a primarily identifiable cardiac cause.) |
Study population | RR 0.86 (0.60 to 1.23) | 12,525 (5 RCTs) | ⊕⊕⊕⊕ High1,2 | ‐ | |
| 10 per 1000 | 8 per 1000 (6 to 12) | |||||
|
Myocardial infarction (within 30‐days after surgery: as detected on an electrocardiogram or trans‐oesophageal echocardiogram) |
Study population | RR 0.94 (0.69 to 1.27) | 13,907 (12 RCTs) | ⊕⊕⊕⊝ Moderate1,2,3 | ‐ | |
| 59 per 1000 | 55 per 1000 (41 to 75) | |||||
|
Acute stroke (within 30‐days after surgery: new focal neurologic deficit with signs and symptoms lasting longer than 24 hours) |
Study population | RR 0.93 (0.55 to 1.56) | 11,542 (7 RCTs) | ⊕⊕⊕⊕ High1 | ‐ | |
| 5 per 1000 | 4 per 1000 (3 to 8) | |||||
|
Bradycardia (requiring pharmacological or pacemaker treatment during the period of study drug administration) |
Study population | RR 1.59 (1.18 to 2.13) | 14,035 (16 RCTs) | ⊕⊕⊕⊝ Moderate1,2,4 | ‐ | |
| 75 per 1000 | 119 per 1000 (89 to 160) | |||||
|
Hypotension (requiring treatment with inotropes or vasopressors during the period of study drug administration) |
Study population | RR 1.24 (1.03 to 1.48) | 13,738 (15 RCTs) | ⊕⊕⊕⊝ Moderate1,2,4 | ‐ | |
| 304 per 1000 | 377 per 1000 (313 to 450) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
1Risk of bias was not serious. Although multiple studies lacked proper allocation concealment and blinding, outcome unlikely to be influenced. Not downgraded.
2Indirectness not serious. Intervention (mivazerol) used in one large study not available for clinical use. Not downgraded.
3Evidence of publication bias in funnel plot of analysis. Downgraded by one level.
4Serious inconsistency between studies indicated by substantial heterogeneity. Downgraded by one level.
Summary of findings 2. Alpha‐2 adrenergic agonists compared to control in cardiac surgery.
| Alpha‐2 adrenergic agonists compared to control in cardiac surgery | ||||||
| Patient or population: adults undergoing cardiac surgery Setting: hospital inpatient care Intervention: α‐2 adrenergic agonist Comparison: placebo or inactive control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control | Risk with α‐2 adrenergic agonists | |||||
|
All‐cause mortality (within 30‐days after surgery: any reported death) |
Study population | RR 0.52 (0.26 to 1.04) | 1947 (16 RCTs) | ⊕⊕⊕⊝ Moderate1,2 | ‐ | |
| 21 per 1000 | 11 per 1000 (5 to 21) | |||||
|
Cardiac mortality (within 30‐days after surgery: sudden death or death resulting from a primarily identifiable cardiac cause) |
1 death from 12 participants in clonidine arm, and no deaths in 10 participants in control arm. | Not estimable | 22 (1 RCT) |
Not estimable | We did not GRADE evidence for this outcome as accurate estimation of RRs is not possible for such low event rates. | |
|
Myocardial infarction (within 30‐days after surgery: sudden death or death resulting from a primarily identifiable cardiac cause) |
Study population | RR 1.01 (0.43 to 2.40) | 782 (8 RCTs) | ⊕⊕⊕⊝ Moderate1,2 | ‐ | |
| 20 per 1000 | 21 per 1000 (9 to 49) | |||||
|
Acute stroke (within 30‐days after surgery: new focal neurologic deficit with signs and symptoms lasting longer than 24 hours) |
Study population | RR 0.37 (0.15 to 0.93) | 1175 (7 RCTs) | ⊕⊕⊝⊝ Low1,3 | Total of 18 acute stokes reported, with 14 in control group and 4 in treatment group. | |
| 24 per 1000 | 9 per 1000 (4 to 22) | |||||
|
Bradycardia (requiring pharmacological or pacemaker treatment during the period of study drug administration) |
Study population | RR 1.88 (1.35 to 2.62) | 1477 (10 RCTs) | ⊕⊕⊕⊝ Moderate1,4 | ‐ | |
| 64 per 1000 | 120 per 1000 (86 to 167) | |||||
|
Hypotension (requiring treatment with inotropes or vasopressors during the period of study drug administration) |
Study population | RR 1.19 (0.87 to 1.64) | 1413 (9 RCTs) | ⊕⊕⊝⊝ Low1,2,5 | ‐ | |
| 332 per 1000 | 395 per 1000 (289 to 544) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
1Risk of bias was not serious. Although multiple studies lack proper allocation concealment and blinding, outcome unlikely to be influenced. Not downgraded.
2Serious imprecision, because analysis was below optimal information size and confidence interval includes significant benefit and harm. Downgraded by one level.
3Very serious imprecision, because analysis is below optimal information size and number of events was very small. Downgraded by two levels.
4Serious imprecision, because analysis was below optimal information size. Downgraded by one level
5Serious inconsistency between studies indicated by substantial heterogeneity. Downgraded one level.
Non‐cardiac surgery
Primary outcome
1. All‐cause mortality within 30 days after surgery
Sixteen studies reported all‐cause mortality, with 210 events (1.5%) among 14,081 participants. Alpha‐2 adrenergic agonists had no statistically significant reduction in all‐cause mortality (RR 0.80, 95% CI 0.61 to 1.04, P = 0.10), without any measurable heterogeneity (I2 = 0%) (Analysis 1.1). The quality of this evidence was high (Table 1).
1.1. Analysis.
Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 1 All‐cause mortality.
Secondary outcomes
1. Cardiac mortality within 30 days after surgery: sudden death or death resulting from a primarily identifiable cardiac cause
Five studies reported cardiac‐related deaths, with 114 events (0.9%) among 12,525 participants. Alpha‐2 adrenergic agonists did not cause a statistically significant reduction in cardiac‐related mortality (RR 0.86, 95% CI 0.60 to 1.23, P = 0.41) with low measurable heterogeneity (I2 = 16%) (Analysis 1.2). The quality of evidence was high (Table 1).
1.2. Analysis.
Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 2 Cardiac mortality.
2. Myocardial infarction within 30 days after surgery (definition as per individual study)
Twelve studies reported MIs, with 835 events (6.0%) among 13,907 participants. Alpha‐2 adrenergic agonists were not associated with any statistically significant difference in the risk of MI (RR 0.94, 95% CI 0.69 to 1.27, P = 0.67) with moderate heterogeneity (I2 = 37%) (Analysis 1.3). The quality of evidence was moderate (Table 1).
1.3. Analysis.
Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 3 Myocardial infarction.
3. Myocardial ischaemia within 30 days after surgery: as detected on an electrocardiogram or transoesophageal echocardiogram (definition as per individual study)
Twelve studies reported myocardial ischaemia, with 291 events (21.1%) among 1379 participants. Alpha‐2 adrenergic agonists did not significantly reduced the risk of ischaemia (RR 0.73, 95% CI 0.53 to 1.02, P = 0.06; I2 = 45%) (Analysis 1.4).
1.4. Analysis.
Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 4 Myocardial ischaemia.
4. Supraventricular tachycardia within 30 days after surgery: supraventricular tachycardia, atrial fibrillation or atrial flutter
Two studies reported SVTs, with one event (2.3%) among 44 participants. Both studies evaluated dexmedetomidine. Since there was no events reported in one of the studies (Venn 2001), pooled estimates were not calculated. The remaining trial showed no effect of α‐2 adrenergic agonists on SVT (RR 1.11, 95% CI 0.05 to 24.07) (Analysis 1.5) (Talke 1995).
1.5. Analysis.
Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 5 Supraventricular tachyarrhythmia.
5. Heart failure within 30 days after surgery: clinical diagnosis of heart failure
Eight studies reported episodes of HF, with 107 events (1.0%) among 10,802 participants. There was no significant reduction in congestive heart failure (CHF) with perioperative α‐2 adrenergic agonist use (RR 1.21, 95% CI 0.83 to 1.75, P = 0.32), with negligible heterogeneity (I2 = 3%) (Analysis 1.6).
1.6. Analysis.
Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 6 Heart failure.
Adverse effects from treatment
1. Acute stroke within 30 days after surgery: new focal neurological deficit with signs and symptoms lasting longer than 24 hours
Seven studies reported acute strokes, with 56 strokes (0.5%) among 11,542 participants. Alpha‐2 adrenergic agonists had no significant effect on acute stroke (RR 0.93, 95% CI 0.55 to 1.56 P = 0.79) with no measurable heterogeneity (I2 = 0%) (Analysis 1.7). The quality of evidence for effects on acute stroke was high (Table 1).
1.7. Analysis.
Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 7 Acute stroke.
Physiological effects of treatment
1. Bradycardia requiring pharmacological or pacemaker treatment
Sixteen studies reported bradycardia, with 1349 events (9.6%) in 14,035 participants. Within these 16 studies, α‐2 adrenergic agonists significantly increased the risk of bradycardia (RR 1.59, 95% CI 1.18 to 2.13, P = 0.002), albeit with substantial heterogeneity (I2 = 53%) (Analysis 1.8). The quality of evidence for treatment effects on bradycardia was moderate (Table 1).
1.8. Analysis.
Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 8 Bradycardia.
2. Hypotension requiring treatment with inotropes or vasopressors
Fifteen studies reported hypotension, with 4766 events (34.7%) in 13,738 participants. Alpha‐2 adrenergic agonists caused a significant increase in the risk of perioperative hypotension (RR 1.24, 95% CI 1.03 to 1.48, P = 0.02), albeit with substantial heterogeneity (I2 = 54%) (Analysis 1.9). Based on a post‐hoc subgroup analysis, the choice of drug may explain this heterogeneity (Analysis 4.4). Specifically, there was statistically significant evidence of subgroup effects based on whether the studies evaluated clonidine, dexmedetomidine or mivazerol (test of interaction P < 0.001). Clonidine significantly increased the risk of hypotension (RR 1.29, 95% CI 1.23 to 1.35, P < 0.001). Dexmedetomidine was also associated with an increased risk (RR 1.81, 95% CI 1.07 to 3.06, P = 0.03). Conversely, mivazerol did not increase the risk of hypotension (RR 0.95, 95% CI 0.82 to 1.10, P = 0.48). Clonidine and mivazerol subgroup analyses had no measurable heterogeneity (I2 = 0%), whereas the dexmedetomidine subgroup analysis demonstrated significant heterogeneity (I2 = 50%). The quality of evidence for treatment effects on hypotension was moderate (Table 1).
1.9. Analysis.
Comparison 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, Outcome 9 Hypotension.
4.4. Analysis.
Comparison 4 Alpha‐2 adrenergic agonists (stratified by drug) versus control in non‐cardiac surgery, Outcome 4 Hypotension.
Cardiac surgery
Primary outcome
1. All‐cause mortality within 30 days after surgery
Sixteen studies reported all‐cause mortality, with 29 events (1.5%) among 1949 participants. Alpha‐2 adrenergic agonists did not result in a statistically significant reduction in all‐cause mortality (RR 0.52, 95% CI 0.26 to 1.04, P = 0.06), without any measurable heterogeneity (I2 = 0%) (Analysis 2.1). The quality of this evidence was moderate (Table 2).
2.1. Analysis.
Comparison 2 Alpha‐2 adrenergic agonists versus control in cardiac surgery, Outcome 1 All‐cause mortality.
Secondary outcomes
1. Cardiac mortality within 30 days after surgery: sudden death or death resulting from a primarily identifiable cardiac cause
Only one study reported cardiac mortality, with 1 event among the 12 participants in the clonidine arm and no events among the 10 participants in the control arm (Loick 1999). Thus, no pooled analysis was performed.
2. Myocardial infarction within 30 days after surgery: definition as per individual study
Eight studies reported MIs, with 16 events (2.0%) among 782 participants. Alpha‐2 adrenergic agonists were not associated with reduced risk of MI (RR 1.01, 95% CI 0.43 to 2.40, P = 0.98) in an analysis with no heterogeneity (I2 = 0%) (Analysis 2.2). The quality of evidence was moderate (Table 2).
2.2. Analysis.
Comparison 2 Alpha‐2 adrenergic agonists versus control in cardiac surgery, Outcome 2 Myocardial infarction.
3. Myocardial ischaemia within 30 days after surgery: as detected on an electrocardiogram or transoesophageal echocardiogram (definition as per individual study)
Thirteen studies reported myocardial ischaemia, with 243 events (21.4%) among 1134 participants. Alpha‐2 adrenergic agonists significantly reduced the risk of ischaemia (RR 0.69, 95% CI 0.56 to 0.86, P < 0.001) with no heterogeneity (I2 = 0%) (Analysis 2.3).
2.3. Analysis.
Comparison 2 Alpha‐2 adrenergic agonists versus control in cardiac surgery, Outcome 3 Myocardial ischaemia.
4. Supraventricular tachycardia within 30 days after surgery: supraventricular tachycardia, atrial fibrillation or atrial flutter
Six studies reported SVTs, with 79 events (7.7%) among 1044 participants. Alpha‐2 adrenergic agonists had no significant effect on the risk of SVT (RR 0.77, 95% CI 0.50 to 1.16, P = 0.21) with low measurable heterogeneity (I2 = 24%) (Analysis 2.4).
2.4. Analysis.
Comparison 2 Alpha‐2 adrenergic agonists versus control in cardiac surgery, Outcome 4 Supraventricular tachyarrhythmia.
5. Heart failure within 30 days after surgery: clinical diagnosis of heart failure or need for postoperative intra‐aortic balloon pump support
Four studies reported 38 HF events (6.9%) among 549 participants. Alpha‐2 adrenergic agonists had no statistically significant effect on the risk of HF (RR 0.90, 95% CI 0.49 to 1.63, P = 0.72) with no measurable heterogeneity (I2 = 0%) (Analysis 2.5).
2.5. Analysis.
Comparison 2 Alpha‐2 adrenergic agonists versus control in cardiac surgery, Outcome 5 Heart failure.
Adverse effects from treatment
1. Acute stroke within 30 days after surgery: new focal neurological deficit with signs and symptoms lasting longer than 24 hours
Seven studies reported acute stroke, with 18 events (1.5%) among 1175 participants. Alpha‐2 adrenergic agonists significantly reduced the risk of acute stroke (RR 0.37, 95% CI 0.15 to 0.93, P = 0.03; I2 = 0%) (Analysis 2.6). The quality of evidence was low (Table 2).
2.6. Analysis.
Comparison 2 Alpha‐2 adrenergic agonists versus control in cardiac surgery, Outcome 6 Acute stroke.
Physiological effects of treatment
1. Bradycardia requiring pharmacological or pacemaker treatment
Ten studies reported episodes of bradycardia, with 136 events (9.2%) among 1477 participants. Pooled analysis demonstrated that α‐2 adrenergic agonists significantly increased the risk of bradycardia (RR 1.88, 95% CI 1.35 to 2.62, P = 0.0002) with no heterogeneity (I2 = 0%) (Analysis 2.7). The quality of evidence was moderate (Table 2).
2.7. Analysis.
Comparison 2 Alpha‐2 adrenergic agonists versus control in cardiac surgery, Outcome 7 Bradycardia.
2. Hypotension requiring treatment with inotropes or vasopressors
Nine studies reported 494 episodes of hypotension (35%) among 1413 participants. Alpha‐2 adrenergic agonists did not significantly increase the risk of hypotension (RR 1.19, 95% CI 0.87 to 1.64, P = 0.28) in an analysis with substantial heterogeneity (I2 = 72%) (Analysis 2.8). The quality of evidence was low (Table 2).
2.8. Analysis.
Comparison 2 Alpha‐2 adrenergic agonists versus control in cardiac surgery, Outcome 8 Hypotension.
Subgroup analyses
Vascular versus non‐vascular non‐cardiac surgery
There was no statistically significant evidence of subgroup effects based on procedure type (i.e. vascular versus non‐vascular procedures) with respect to the outcomes of all‐cause mortality (test of interaction P = 0.17; Analysis 3.1), cardiac mortality (test of interaction P = 0.13; Analysis 3.2), MI (test of interaction P = 0.13; Analysis 3.3), and myocardial ischaemia (test of interaction P = 0.17; Analysis 3.4).
3.1. Analysis.
Comparison 3 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery ‐ stratified by vascular versus non‐vascular surgery, Outcome 1 All‐cause mortality.
3.2. Analysis.
Comparison 3 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery ‐ stratified by vascular versus non‐vascular surgery, Outcome 2 Cardiac mortality.
3.3. Analysis.
Comparison 3 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery ‐ stratified by vascular versus non‐vascular surgery, Outcome 3 Myocardial infarction.
3.4. Analysis.
Comparison 3 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery ‐ stratified by vascular versus non‐vascular surgery, Outcome 4 Myocardial ischaemia.
Drug (i.e. clonidine, mivazerol or dexmedetomidine) evaluated in non‐cardiac surgery
There was no statistically significant evidence of subgroup effects based on the specific α‐2 adrenergic agonist evaluated with respect to the outcomes of all‐cause mortality (test of interaction P = 0.50) (Analysis 4.1), and MI (test of interaction P = 0.48) (Analysis 4.3). Conversely, there was a statistically significant subgroup effect with respect to cardiac mortality (test of interaction P = 0.05) (Analysis 4.2). In these subgroup analyses, mivazerol significantly reduced cardiac mortality (RR 0.51, 95% CI 0.27 to 0.98, P = 0.04), whereas clonidine did not (RR 1.12, 95% CI 0.71 to 1.75, P = 0.63). There were insufficient studies that reported myocardial ischaemia as an outcome for dexmedetomidine or mivazerol to facilitate drug‐specific subgroup analysis for the outcome.
4.1. Analysis.
Comparison 4 Alpha‐2 adrenergic agonists (stratified by drug) versus control in non‐cardiac surgery, Outcome 1 All‐cause mortality.
4.3. Analysis.
Comparison 4 Alpha‐2 adrenergic agonists (stratified by drug) versus control in non‐cardiac surgery, Outcome 3 Myocardial infarction.
4.2. Analysis.
Comparison 4 Alpha‐2 adrenergic agonists (stratified by drug) versus control in non‐cardiac surgery, Outcome 2 Cardiac mortality.
Sensitivity analyses
Studies that clearly reported blinding and concealed allocation
The pooled effects of α‐2 adrenergic agonists on all‐cause mortality (RR 0.68, 95% CI 0.41 to 1.11, P = 0.12; participants = 13,066; studies = 7; Analysis 5.1), MI (RR 1.08, 95% CI 0.95 to 1.23, P = 0.26; participants = 13,026; studies = 6; Analysis 5.2), and myocardial ischaemia (RR 0.77, 95% CI 0.40 to 1.48, P = 0.43; participants = 412; studies = 3; Analysis 5.3) were qualitatively similar when analyses were restricted to trials that clearly reported methods for blinding and allocation concealment.
5.1. Analysis.
Comparison 5 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery studies with blinding and concealed allocation, Outcome 1 All‐cause mortality.
5.2. Analysis.
Comparison 5 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery studies with blinding and concealed allocation, Outcome 2 Myocardial infarction.
5.3. Analysis.
Comparison 5 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery studies with blinding and concealed allocation, Outcome 3 Myocardial ischaemia.
Strict definitions of myocardial infarction and ischaemia
When analyses were restricted to trials that strictly defined MI on ECG or enzymatic criteria, pooled treatment effects in non‐cardiac surgery (RR 0.98, 95% CI 0.70 to 1.36, P = 0.90; participants = 13,003; studies = 8) and cardiac surgery (RR 0.76, 95% CI 0.19 to 2.98, P = 0.69; participants = 275; studies = 3) were qualitatively unchanged (Analysis 6.1). When analyses were restricted to studies that strictly defined events of myocardial ischaemia, the effects in non‐cardiac surgery remained non‐significant (RR 0.76, 95% CI 0.54 to 1.07, P = 0.12; participants = 1175; studies = 9). In cardiac surgery, the sensitivity analysis continued to demonstrate a reduction in the risk of ischaemia (RR 0.71, 95% CI 0.55 to 0.91, P = 0.007; participants = 820; studies = 8) (Analysis 6.2).
6.1. Analysis.
Comparison 6 Alpha‐2 adrenergic agonists versus control in studies that used strict definitions of myocardial infarction or ischaemia, Outcome 1 Myocardial infarction.
6.2. Analysis.
Comparison 6 Alpha‐2 adrenergic agonists versus control in studies that used strict definitions of myocardial infarction or ischaemia, Outcome 2 Myocardial ischaemia.
Influence of two large trials
The overall results of this review are likely highly influenced by two large RCTs in non‐cardiac surgery, one of which assessed mivazerol (Oliver 1999), while the other assessed clonidine (Devereaux 2014a). Therefore, we performed a post‐hoc sensitivity analysis that excluded these studies. After excluding these two trials, treatment effect on all‐cause mortality became statistically significant (RR 0.45, 95% CI 0.22 to 0.93, P = 0.03; participants = 2174; studies = 14; Analysis 7.1). Conversely, the effect on cardiac mortality (RR 0.47, 95% CI 0.10 to 2.25, P = 0.35; participants = 618; studies = 3; Analysis 7.2), and MI (RR 0.56, 95% CI 0.25 to 1.25, P = 0.16; participants = 2000; studies = 10; Analysis 7.3) were statistically non‐significant, albeit with more optimistic individual point estimates (i.e. pooled treatment effects shifted towards larger risk reductions).
7.1. Analysis.
Comparison 7 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery (excluding Oliver 1999 and Devereaux 2014), Outcome 1 All‐cause mortality.
7.2. Analysis.
Comparison 7 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery (excluding Oliver 1999 and Devereaux 2014), Outcome 2 Cardiac mortality.
7.3. Analysis.
Comparison 7 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery (excluding Oliver 1999 and Devereaux 2014), Outcome 3 Myocardial infarction.
Excluding drugs not introduced into clinical practice (i.e. mivazerol)
In post‐hoc sensitivity analyses excluding the two trials that evaluated mivazerol (McSPI‐Europe 1997; Oliver 1999), there was no change in pooled treatment effects pertaining to all‐cause mortality, cardiac mortality, MI, SVT, HF, stroke, bradycardia, or hypotension (Analysis 8.1; Analysis 8.2; Analysis 8.3; Analysis 8.5; Analysis 8.6; Analysis 8.7; Analysis 8.8; Analysis 8.9). Conversely, the pooled treatment effect on ischaemia became statistically significant (RR 0.68, 95% CI 0.48 to 0.97, P = 0.03; participants = 1079; studies = 11; I2 = 40%) (Analysis 8.4), albeit in an analysis with moderate heterogeneity and relatively few participants.
8.1. Analysis.
Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 1 All‐cause mortality.
8.2. Analysis.
Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 2 Cardiac mortality.
8.3. Analysis.
Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 3 Myocardial infarction.
8.5. Analysis.
Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 5 Supraventricular tachyarrhythmia.
8.6. Analysis.
Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 6 Heart failure.
8.7. Analysis.
Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 7 Acute stroke.
8.8. Analysis.
Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 8 Bradycardia.
8.9. Analysis.
Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 9 Hypotension.
8.4. Analysis.
Comparison 8 Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery, Outcome 4 Myocardial ischaemia.
Restricting studies more representative of contemporary perioperative practice
When analyses pertaining to non‐cardiac surgery were restricted to studies that collected data within the previous 20 years, there was no change in the pooled treatment effects pertaining to all‐cause mortality, cardiac mortality, MI, HF or stroke (Analysis 9.1; Analysis 9.2; Analysis 9.3; Analysis 9.5; Analysis 9.6). Nonetheless, exclusion of older studies resulted in a significant reduction in the risk of myocardial ischaemia (RR 0.51, 95% CI 0.28 to 0.93, P = 0.03; participants = 634; studies = 6; I2 = 48%) in an analysis with moderate heterogeneity (Analysis 9.4). In cardiac surgery, exclusion of older studies resulted in no substantive effect on the pooled treatment effects for MI, myocardial ischaemia, SVT, HF or stroke (Analysis 10.2; Analysis 10.3; Analysis 10.4; Analysis 10.5; Analysis 10.6). Conversely, the pooled treatment effect on all‐cause mortality became statistically significant (RR 0.47, 95% CI 0.23 to 0.97; participants = 1782; studies = 13; I2 = 0%) (Analysis 10.1).
9.1. Analysis.
Comparison 9 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery within past 20 years, Outcome 1 All‐cause mortality.
9.2. Analysis.
Comparison 9 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery within past 20 years, Outcome 2 Cardiac mortality.
9.3. Analysis.
Comparison 9 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery within past 20 years, Outcome 3 Myocardial infarction.
9.5. Analysis.
Comparison 9 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery within past 20 years, Outcome 5 Heart failure.
9.6. Analysis.
Comparison 9 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery within past 20 years, Outcome 6 Acute stroke.
9.4. Analysis.
Comparison 9 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery within past 20 years, Outcome 4 Myocardial ischaemia.
10.2. Analysis.
Comparison 10 Alpha‐2 adrenergic agonists versus control in cardiac surgery within past 20 years, Outcome 2 Myocardial infarction.
10.3. Analysis.
Comparison 10 Alpha‐2 adrenergic agonists versus control in cardiac surgery within past 20 years, Outcome 3 Myocardial ischaemia.
10.4. Analysis.
Comparison 10 Alpha‐2 adrenergic agonists versus control in cardiac surgery within past 20 years, Outcome 4 Supraventricular tachyarrhythmia.
10.5. Analysis.
Comparison 10 Alpha‐2 adrenergic agonists versus control in cardiac surgery within past 20 years, Outcome 5 Heart failure.
10.6. Analysis.
Comparison 10 Alpha‐2 adrenergic agonists versus control in cardiac surgery within past 20 years, Outcome 6 Acute stroke.
10.1. Analysis.
Comparison 10 Alpha‐2 adrenergic agonists versus control in cardiac surgery within past 20 years, Outcome 1 All‐cause mortality.
Funnel plots
Funnel plots of included studies revealed no obvious publication bias with regard to the outcome of mortality (Figure 4), but some possible bias with regard to MI (Figure 5). Since this analysis pooled results from only nine studies, formal statistical testing for asymmetry was not conducted.
4.

Funnel plot of comparison: 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, outcome: 1.1 All‐cause mortality.
5.

Funnel plot of comparison: 1 Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery, outcome: 1.3 Myocardial infarction.
Discussion
Summary of main results
Our present review found high‐quality evidence that perioperative α‐2 adrenergic agonists did not reduce the risk of all‐cause mortality, cardiac mortality or MI in people undergoing non‐cardiac or cardiac surgery (Table 1; Table 2). These findings remained stable in sensitivity analyses restricted to studies that either demonstrated low risks of bias or employed strict definitions of MI. Aside from lacking any beneficial effect on these clinical outcomes, α‐2 adrenergic agonists also conferred important risks, specifically increased rates of hypotension and bradycardia. While these haemodynamic effects were not associated with an increased risk of acute stroke, the 95% CIs for this pooled effect were wide (RR 0.93, 95% CI 0.55 to 1.56), thereby not excluding the possibility of a moderate increase in stroke risk with perioperative α‐2 adrenergic agonists.
Overall completeness and applicability of evidence
The 47 RCTs included in this systematic review encompassed 17,039 participants, a wide range of relevant surgical procedures performed in several different countries internationally and clinically relevant dosing regimens of currently available α‐2 adrenergic agonists (i.e. clonidine, dexmedetomidine). Furthermore, a significant number of participants from the included studies underwent surgery within the past decade. Thus, we are confident that the overall findings of our systematic review, namely that α‐2 adrenergic agonists do not significantly reduce risks of cardiovascular complications or mortality when given prophylactically before major non‐cardiac or cardiac surgery, can be reasonably extrapolated to contemporary perioperative practice.
Nonetheless, there were insufficient participants within specific subgroups to conclusively evaluate several potential benefits of α‐2 adrenergic agonists, namely prevention of stroke, myocardial ischaemia and all‐cause mortality after cardiac surgery. The subgroup analysis evaluating effects on stroke during cardiac surgery was small, with only seven included studies that encompassed 1175 participants (Analysis 2.6). The pooled estimate was based on low‐quality data, calculated using very few outcome events (i.e. 18 strokes). Previous research has found that treatment effects are generally overestimated in meta‐analyses that include relatively few outcome events (Thorlund 2011). Consistent with this possibility, the magnitude of the pooled estimate was somewhat implausible (RR 0.37, 95% CI 0.15 to 0.93), in that it suggested a 63% relative reduction in the risk of stroke from a single perioperative intervention. Therefore, further research is needed to determine whether α‐2 adrenergic agonists can truly reduce the risk of acute stroke after cardiac surgery.
Similarly, the statistically significant pooled treatment effect on all‐cause mortality was observed only in a post‐hoc subset analysis restricted to cardiac surgery trials conducted after 1997 (Analysis 10.1). This subset was relatively small (13 studies encompassing 1782 participants), the pooled estimated was calculated using very few outcome events (i.e. 28 deaths) and the magnitude of the pooled estimate was somewhat implausible (RR 0.47, 95% CI 0.23 to 0.97) for a single intervention. More studies are needed to assess the effect of α‐2 adrenergic agonists on all‐cause mortality after cardiac surgery.
In a separate subgroup analysis in cardiac surgery, α‐2 adrenergic agonists also caused a significant reduction in perioperative myocardial ischaemia (Analysis 2.3). Nonetheless, myocardial ischaemia is a surrogate outcome with important associated limitations (Svensson 2013). Especially in the absence of associated reductions in clinical important and patient‐relevant outcomes such as mortality or MI, isolated reductions in perioperative myocardial ischaemia are not sufficient justifications for employing α‐2 adrenergic agonists in clinical practice.
Quality of the evidence
This systematic review was supported by 47 RCTs that recruited 17,039 participants. The sample size of the included RCTs varied greatly, ranging from 20 to over 10,000 participants. Nineteen studies had over 100 participants, with only two studies involving over 1000 participants (Devereaux 2014a; Oliver 1999). The vast majority of these participants (14,367) were recruited into the 23 included trials in non‐cardiac surgery. By comparison, the remaining 24 RCTs in cardiac surgery involved 2672 participants.
Only 16 studies reported adequate methods for random sequence generation and allocation concealment. Furthermore, although 31 studies described themselves as 'double‐blinded,' only 21 studies reported appropriate methods to achieve blinding. Nonetheless, the majority of participants were from well‐designed studies with adequate methods for allocation and blinding, thereby rendering them low risk to be influenced by selection bias, performance bias and detection bias.
The analyses pertaining to the primary and secondary outcomes in people undergoing non‐cardiac surgery were generally robust. These findings were judged as moderate to high quality by GRADE methodology (Table 1). Although many studies failed to report adequate methods to avoid risk of bias, the specific outcomes were unlikely to be influenced and thus no downgrading of quality was necessary. In addition, there was a potential threat of indirectness since the second largest RCT evaluated mivazerol (Oliver 1999), which is not available for clinical use. However, given the similarity of mivazerol to dexmedetomidine (which is available for clinical use), we reasoned that this risk was likely not serious. Conversely, funnel plots suggested that the pooled treatment effects on MI was affected by publication bias (Figure 5). The asymmetry in the funnel plots was produced by two small studies with seemingly unrealistic effect sizes (Ellis 1994; Stuhmeier 1996). While the combined weight of these studies was less than 3% of the pooled analysis, we downgraded the evidence by one level because of suspicion of publication bias for an outcome known to be influenced by performance bias (Analysis 1.3). Finally, the quality of evidence for the physiological effects of bradycardia and hypotension were both downgraded because of substantial heterogeneity (I2 greater than 50%) in the analyses (Analysis 1.8; Analysis 1.9).
The quality of evidence for the effects of α‐2 adrenergic agonists in cardiac surgery was generally lower, largely due to imprecision resulting from significantly fewer participants in the pooled analyses (Table 2). The quality of evidence for all outcomes was downgraded because the optimal information size of 2000 participants was not achieved, and the 95% CIs of the pooled estimates did not rule out clinically significant effects (GRADE Handbook 2013). Thus, the quality of evidence for the analyses pertaining to all‐cause mortality and MI was moderate. As there were very few outcomes events in the analysis of acute stroke (i.e. 18 strokes), it was downgraded another level and judged as low quality. Finally, the presence of substantial imprecision in pooled analysis pertaining to hypotension led to the quality of this evidence being downgraded to low (I2 = 72%; Analysis 2.8). Nonetheless, the magnitude of the association between α‐2 adrenergic agonists and hypotension in cardiac surgery (RR 1.19, 95% CI 0.87 to 1.64) was qualitatively very similar to that observed in non‐cardiac surgery (RR 1.24, 95% CI 1.03 to 1.48), where the quality of evidence was moderate.
Potential biases in the review process
There were several limitations to our review process. First, while our search was exhaustive in that it covered all major medical indexes and clinical trial registries, we might have missed some published trials only listed in other less commonly used indices. Nonetheless, we believe it unlikely that our search strategy missed any relevant studies of at least moderate size and quality. Second, we only included studies reporting subgroup‐specific outcome data based on surgical procedure type (i.e. cardiac surgery versus non‐cardiac surgery). Consequently, we excluded any study that did not predominantly include procedures (greater than 75%) from either of these surgical procedure subgroup, unless subgroup‐specific outcome data could be obtained from the authors. Consequently, two otherwise eligible studies could not be included in this systematic review (Martin 2003; Triltsch 2002). In excluding these studies, we balanced the risk of biasing the analyses with the loss of additional data, and chose the latter to maintain the integrity of our analyses.
Agreements and disagreements with other studies or reviews
There are several potential reasons why the theoretically beneficial physiological effects of α‐2 adrenergic agonists did not translate into reduced rates of major postoperative cardiac complications. First, α‐2 adrenergic agonists might not have sufficiently reduced heart rates to mitigate the risks of perioperative MI, as has been previously proposed (Devereaux 2014a). This hypothesis is supported by the observation that, while perioperative β‐blockers caused more significant bradycardia than α‐2 adrenergic agonists, rates of perioperative MI were reduced with β‐blockers but not α‐2 adrenergic agonists (Wijeysundera 2014b). Second, the predominant mechanism underlying perioperative MI in many affected people might not be increases in blood pressure and heart rate induced by the surgical stress response. For example, almost 30% of people with postoperative MI do not have significant obstructive coronary artery disease (Sheth 2015). It is unlikely that limiting perioperative increases in heart rate would help prevent MI in such people. Third, at a population‐level, the beneficial effects of heart rate reduction in some people undergoing surgery might have been offset by equal numbers of people who experienced deleterious effects from significant perioperative hypotension.
Notably, our overall findings with respect to the absence of major reductions in perioperative cardiovascular risk from α‐2 adrenergic agonists was somewhat consistent with results seen with prophylactic therapy with other sympatholytic agents. Specifically, β‐adrenergic blockers have been shown to cause a net harmful effect in non‐cardiac surgery (Wijeysundera 2014b). It remains to be seen whether any strategy of prophylactically attenuating haemodynamic abnormalities with sympatholytic agents can safely reduce perioperative cardiac risk. Indeed, if future RCTs with either alternative regimens of previously evaluated sympatholytic agents (i.e. α‐2 adrenergic agonists, β‐adrenergic blockers) or alternative negative chronotropic agents (e.g. ivabradine) fail to show net overall benefit, the general strategy for perioperative cardiac risk reduction may have to shift from prophylactic therapy to early treatment. Specifically, as opposed to administering these medications to a broad group of people before surgery, clinicians might instead consider targeting treatment in high‐risk people identified on the basis of ischaemic ECG changes or elevated cardiac troponin concentrations early after surgery.
Comparison of our present systematic review on perioperative α‐2 adrenergic agonists with a prior systematic review of perioperative β‐adrenergic blockers in non‐cardiac surgery also provides some potential insights into the mechanisms underlying perioperative stroke (Wijeysundera 2014b). Specifically, α‐2 adrenergic agonists (RR 1.24, 95% CI 1.03 to 1.48) and β‐adrenergic blockers (RR 1.47, 95% CI 1.34 to 1.60) conferred approximately similar risks of perioperative hypotension. Despite this similarity in haemodynamic effects, β‐blockers (RR 1.86, 95% CI 1.09 to 3.16) conferred significantly increased risks of perioperative acute stroke while α‐2 adrenergic agonists did not (RR 0.93, 95% CI 0.55 to 1.56). These contrasting effects are also evident when comparing two large individual perioperative RCTs of these two different drug classes, namely the POISE‐1 trial of metoprolol (POISE 2008) and the POISE‐2 trial of clonidine (Devereaux 2014a). Specifically, while metoprolol (hazard ratio (HR) 1.55, 95% CI 1.38 to 1.74) and clonidine (HR 1.32, 95% CI 1.24 to 1.40) caused qualitatively similar increases in rates of hypotension, metoprolol significantly increased risks of stroke (HR 2.17, 95% CI 1.26 to 3.74) while clonidine did not (HR 1.06, 95% CI 0.54 to 2.05). These findings suggest that, despite the previously observed association between perioperative hypotension and stroke (POISE 2008), the mechanisms underlying perioperative acute stroke are likely more complex than simply a decrease in perfusion pressure. Further research is needed to better delineate these mechanisms, and thereby inform the development of strategies to help prevent this often devastating perioperative complication (POISE 2008).
Importantly, perioperative α‐2 adrenergic agonists do have other potential benefits that may justify their selective use in some people undergoing surgery. Specifically, in a previous systematic review of 30 small RCTs encompassing 1792 participants, these agents decreased both postoperative pain intensity and morphine consumption (Blaudszun 2012). Our present study provided additional data supporting the safety of using α‐2 adrenergic agonists as an adjunct therapy for managing postoperative acute pain, provided that there is adequate attention to the associated risks of hypotension and bradycardia.
Strengths
Our present review had several strengths. The literature search was extensive and encompassed all languages. In addition, our study utilized a substantially larger data set than previous reviews (Biccard 2008; Nishina 2002; Stevens 2003; Wijeysundera 2003; Wijeysundera 2009). Finally, we performed multiple sensitivity analyses to assess the potential influence of study quality, outcome definition and publication bias on our overall conclusions.
Limitations
Our review had several limitations that should be considered. First, the results were heavily influenced by two large trials of mivazerol (Oliver 1999) and clonidine (Devereaux 2014a) in non‐cardiac surgery. While excluding these large trials from the meta‐analysis resulted in somewhat more optimistic point estimates for individual pooled treatment effects, these pooled estimates remained statistically non‐significant. Second, as with any systematic review, our results may have been affected by publication bias. Though funnel plots indicated no obvious bias in the reporting of death (all‐cause and cardiac‐cause), they did suggest reporting bias may be present for MI. Third, our present analysis pooled trials of α‐2 adrenergic agonists with differing selectivity for their target receptors, namely α‐2 adrenoceptors and non‐adrenergic imidazoline receptors (Khan 1999). Specifically, both mivazerol and dexmedetomidine have considerably greater selectivity for these target receptors than clonidine. Furthermore, when comparing the two large individual trials that dominated this meta‐analysis (Devereaux 2014a; Oliver 1999), clonidine had no effect on mortality and increased rates of significant perioperative hypotension, while mivazerol, a more selective α‐2 adrenergic agonist that is not currently available for clinical use, was associated with trends towards reduced mortality and had no effect on rates of hypotension. Importantly, these contrasting findings might have also been due to the different time periods when the studies were conducted, differences in study design, differences in participant characteristics or chance. Nonetheless, the contrasting findings still suggest that any future RCT of α‐2 adrenergic agonists for cardiac risk reduction in people undergoing surgery should focus on agents with higher selectivity for α‐2 adrenoceptors, such as dexmedetomidine or mivazerol.
Authors' conclusions
Implications for practice.
Our study found high‐quality data to firmly conclude that there are no compelling reasons for employing perioperative α‐2 adrenergic agonists to reduce the risks of perioperative death or major cardiac complications in people undergoing surgery. These agents do not reduce the risks of death, myocardial infarction (MI) or acute stroke after surgery. Furthermore, they are associated with important adverse effects, namely increased risks of hypotension and bradycardia.
Implications for research.
First, randomized controlled trials (RCTs) of clonidine for reducing perioperative cardiovascular risk should not be performed in the future because the Perioperative Ischemic Evaluation ‐ 2 (POISE‐2) trial provides compelling evidence that this specific agent lacks benefit. Second, while it is presently unclear whether more selective α‐2 adrenergic agonists, such as dexmedetomidine or mivazerol, have differing effects on clinical outcomes in comparison to clonidine, there are at least theoretical reasons to pursue this hypothesis in future trials. Thus, future RCTs of perioperative α‐2 adrenergic agonists should focus on these more selective agents. Any such future trial should also adhere to quality standards for RCTs including blinding (participants, caregivers, outcome adjudicators), allocation concealment and intention‐to‐treat analysis. Third, appropriately designed future RCTs are needed to determine whether more selective α‐2 adrenergic agonists can help prevent perioperative stroke or all‐cause death after cardiac surgery.
Feedback
Why pool results, 1 April 2018
Summary
I am troubled by the summary statements, because analysing the three drugs together is problematic.
Why mix dexmedetomidine studies with clonidine studies and present their pooled results? They have vastly different specificities for the alpha‐2 receptor.
Why not include three subgroups, analysing studies of each of the drugs individually?
Meta‐analysis of these drugs when used only in the postoperative setting would have been especially useful to Intensivists, especially dexmedetomidine alone.
In this study, page 17, especially reading from “The influence of two large studies” on, seems to show BENEFITS of alpha ‐2 blockers when two large studies (which did NOT include dexmedetomidine) were removed. Additional “subgroup” analyses after this section make for interesting reading which is not really reflected in the summary statements.
The summary of papers does not include results ‐ so a super‐quick meta‐analysis of the dexmedetomidine ‐ only studies is made more difficult.
In summary: this paper, Prima facie, debunks the use of these drugs perioperatively, but the details show that dexmedetomidine may well be very useful.
Reply
The author has raised several important issues, especially methodological concerns pertaining to subgroup analyses.
The effects of clonidine, dexmedetomidine, and mivazerol may indeed plausibly differ based on their different selectivity for alpha‐2 adrenoceptors and non‐adrenergic imidazoline receptors. Consequently, we specifically conducted formal statistical testing for such subgroup differences. This testing revealed no significant between‐drug differences with respect to effects on death (P = 0.50) and myocardial infarction (P = 0.48), and borderline differences with respect to effects on cardiac death (P = 0.05). Consequently, our primary approach to present pooled treatment effects for all three drugs is consistent with existing published study data. Similarly, we advocate against over interpreting the sensitivity analyses that excluded that two large studies, especially since there was only one resulting statistically significant pooled treatment effect, which was itself based on 27 outcome events.
Conversely, we do agree that dexmedetomidine holds some promise as a beneficial perioperative intervention, especially when reviewing more recent studies and studies restricted to cardiac surgery. Nonetheless, the findings of these subset analyses in our review may not be robust, especially due to the risk of inflated Type 1 error (i.e., false positive findings) from repeated statistical testing. Thus, we chose against including these potential benefits (e.g., significant reduction in mortality in contemporary cardiac surgery trials) in the summary statement that represents the strength of current evidence. Instead, these potential benefits warrant rigorous assessment in future research involving larger trials of specific drugs (i.e., dexmedetomidine) in targeted subgroups (i.e., cardiac surgery).
Contributors
Summary
David Collins. Intensivist / Anaesthetist. I do not have any affiliation with or involvement in any organisation with a financial interest in the subject matter of my comment.
Reply
Duminda N. Wijeysundera, MD PhD FRCPC
Li Ka Shing Knowledge Institute, St. Michael’s Hospital
30 Bond Street, Toronto, Ontario
Canada
What's new
| Date | Event | Description |
|---|---|---|
| 12 September 2018 | Feedback has been incorporated | Reply to feedback summary incorporated (Feedback 1) |
History
Protocol first published: Issue 2, 2003 Review first published: Issue 4, 2009
| Date | Event | Description |
|---|---|---|
| 13 August 2018 | Feedback has been incorporated | Feedback summary incorporated (Feedback 1). |
| 13 March 2018 | Amended | Typo corrected in Acknowledgements |
| 4 May 2017 | New citation required and conclusions have changed | No benefit of α‐2 adrenergic agonists was identified with respect to the prevention of cardiac complications or death after surgery. The study methods were updated to include summary of findings tables, using GRADE methodology. The main analyses were subdivided into cardiac and non‐cardiac studies. The inclusion criteria were broadened to include studies that only reported on acute stroke and heart failure outcomes. All studies excluded in the prior review were re‐evaluated using the new criteria. The former author JS Bender has left the team and two new authors have joined, namely D Duncan and A Sankar. |
| 4 May 2017 | New search has been performed | 1721 abstracts were screened, 199 full‐texts were assessed for eligibility and 19 additional studies were included. A search of clinical trial registries identified one additional published study that was included. Three previously included reports of two studies (Boldt 1996; Wahlander 2005) are now excluded because of reported scientific misconduct by lead authors. Two other previously included studies (Martin 2003; Triltsch 2002) are now excluded because the 2017 review conducted separate analyses for cardiac and non‐cardiac surgical procedures, and these two studies could not be classified into either subgroup. Sensitivity analyses were added to assess for the influence of (i) studies that evaluated mivazerol and (ii) studies with data collection occurring more than 20 years previously. |
| 1 August 2016 | Amended | This review has two included studies that have been retracted (Boldt 1996; Wahlander 2005). The Cochrane authors are in the process of updating this review. |
| 22 May 2008 | Amended | Converted to new review format. |
Notes
August 2016
Two studies (Boldt 1996;Wahlander 2005), which were included in the previous 2009 version of this review (Wijeysundera 2009), were removed from the 2016 version of the review due to concerns about scientific conduct.
Acknowledgements
We would like to thank Rodrigo Cavallazz (content editor), Cathal D Walsh (statistical editor), Ben Gibbison, Pierre Foex, Ernst‐Peter Horn (peer reviewers), Anne Lyddiatt (consumer referee) for their help and editorial advice during the preparation of this updated systematic review.
Dr Wijeysundera is supported in part by a New Investigator Award from the Canadian Institutes of Health Research (Ottawa, Ontario, Canada). Dr Beattie is the Fraser Elliot Chair of Cardiac Anesthesia at the University Health Network (Toronto, Ontario, Canada). Both Dr Wijeysundera and Dr Beattie are supported in part by Merit Awards from the Department of Anesthesia at the University of Toronto (Toronto, Ontario, Canada). We are indebted to the following authors who responded to our questions regarding their publications: Drs M Fischler, RM Grounds, HM Loick, I Matot, P Myles, M Oliver, L Quintin, C Spies, P Talke and A Wallace.
Appendices
Appendix 1. Search terms for electronic databases
| MEDLINE (OvidSP) search terms:
1. postoperative complications/ or perioperative care/ or intraoperative complications/ or (intraoperative or perioperative or postoperative).mp. 2. exp clonidine/ or exp dexmedetomidine/ or mivazerol.mp. 3. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 4. 1 and 2 and 3 Embase (OvidSP) search terms 1. postoperative complication/ or postoperative period/ or perioperative period/ or intraoperative period/ or peroperative care/ or peroperative complication/ or (perioperative or intraoperative or postoperative).ti,ab. 2. clonidine/ or dexmedetomidine/ or mivazerol.ti,ab. 3. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. 4. 1 and 2 and 3 CENTRAL search terms #1 clonidine OR dexmedetomidine OR mivazerol #2 perioperative OR preoperative OR postoperative #3 #1 AND #2 |
Appendix 2. Data abstraction form
Study ID:
Reviewer:
Title:
Authors:
Journal:
Year: Volume:
Issue: Pages
Study quality
Randomized?
Allocation concealed?
How?
Blinded?
Intention‐to‐treat?
Drop‐outs accounted?
Include?
Overall features
Surgical population (inclusion/exclusion):
Anesthesia type:
Alpha‐2 agonist regimen(s) (dose, frequency)
Control arm regimens
Follow‐up duration
Main outcome data
Dose & regimen:
Patients (n):
All‐cause mortality:
Cardiac death:
Myocardial infarction:
Myocardial ischaemia:
Heart failure:
Supraventricular tachyarrhythmia:
Definition of myocardial infarction:
Definition of myocardial ischaemia:
Side effects data
Hypotension:
Bradycardia:
Acute stroke:
Other (specify):
Comments:
Subgroup results
Subgroup type:
Patients (n):
All‐cause mortality:
Cardiac death:
Myocardial infarction:
Myocardial ischaemia:
Heart failure:
Supraventricular tachyarrhythmia:
Hypotension:
Bradycardia:
Acute stroke:
Other (specify):
Add further pages for any other relevant subgroups.
Data and analyses
Comparison 1. Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 16 | 14081 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.61, 1.04] |
| 2 Cardiac mortality | 5 | 12525 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.60, 1.23] |
| 3 Myocardial infarction | 12 | 13907 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.69, 1.27] |
| 4 Myocardial ischaemia | 12 | 1379 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.53, 1.02] |
| 5 Supraventricular tachyarrhythmia | 2 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.05, 24.07] |
| 6 Heart failure | 8 | 10802 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.83, 1.75] |
| 7 Acute stroke | 7 | 11542 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.55, 1.56] |
| 8 Bradycardia | 16 | 14035 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [1.18, 2.13] |
| 9 Hypotension | 15 | 13738 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.03, 1.48] |
Comparison 2. Alpha‐2 adrenergic agonists versus control in cardiac surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 16 | 1947 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.26, 1.04] |
| 2 Myocardial infarction | 8 | 782 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.43, 2.40] |
| 3 Myocardial ischaemia | 13 | 1134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.56, 0.86] |
| 4 Supraventricular tachyarrhythmia | 6 | 1044 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.50, 1.16] |
| 5 Heart failure | 4 | 549 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.49, 1.63] |
| 6 Acute stroke | 7 | 1175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.15, 0.93] |
| 7 Bradycardia | 10 | 1477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.35, 2.62] |
| 8 Hypotension | 9 | 1413 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.87, 1.64] |
Comparison 3. Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery ‐ stratified by vascular versus non‐vascular surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 13 | 3713 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.40, 0.98] |
| 1.1 Vascular surgery | 8 | 1798 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.25, 0.88] |
| 1.2 Non‐vascular surgery | 6 | 1915 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.46, 1.67] |
| 2 Cardiac mortality | 4 | 2515 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.27, 0.93] |
| 2.1 Vascular surgery | 4 | 1522 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.16, 0.79] |
| 2.2 Non‐vascular surgery | 1 | 993 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.35, 2.77] |
| 3 Myocardial infarction | 9 | 3539 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.44, 1.17] |
| 3.1 Vascular surgery | 7 | 1766 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.27, 1.00] |
| 3.2 Non‐vascular surgery | 3 | 1773 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.55, 2.15] |
| 4 Myocardial ischaemia | 9 | 961 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.52, 1.17] |
| 4.1 Vascular surgery | 6 | 865 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.54, 1.29] |
| 4.2 Non‐vascular surgery | 3 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.04, 1.34] |
Comparison 4. Alpha‐2 adrenergic agonists (stratified by drug) versus control in non‐cardiac surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 16 | 14081 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.61, 1.04] |
| 1.1 Clonidine | 7 | 10787 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.64, 1.23] |
| 1.2 Dexmedetomidine | 7 | 1097 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.15, 1.58] |
| 1.3 Mivazerol | 2 | 2197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.42, 1.15] |
| 2 Cardiac mortality | 5 | 12525 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.60, 1.23] |
| 2.1 Clonidine | 3 | 10328 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.71, 1.75] |
| 2.2 Mivazerol | 2 | 2197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.27, 0.98] |
| 3 Myocardial infarction | 12 | 13907 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.69, 1.27] |
| 3.1 Clonidine | 6 | 10756 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.57, 1.92] |
| 3.2 Dexmedetomidine | 4 | 954 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.20, 1.49] |
| 3.3 Mivazerol | 2 | 2197 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.17, 2.08] |
| 4 Hypotension | 15 | 13738 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.03, 1.48] |
| 4.1 Clonidine | 7 | 10485 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [1.23, 1.35] |
| 4.2 Dexmedetomidine | 6 | 1056 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [1.07, 3.06] |
| 4.3 Mivazerol | 2 | 2197 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.82, 1.10] |
Comparison 5. Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery studies with blinding and concealed allocation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 7 | 13066 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.41, 1.11] |
| 2 Myocardial infarction | 6 | 13026 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.95, 1.23] |
| 3 Myocardial ischaemia | 3 | 412 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.40, 1.48] |
Comparison 6. Alpha‐2 adrenergic agonists versus control in studies that used strict definitions of myocardial infarction or ischaemia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Myocardial infarction | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Non‐cardiac surgery | 8 | 13003 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.70, 1.36] |
| 1.2 Cardiac surgery | 3 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.19, 2.98] |
| 2 Myocardial ischaemia | 17 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Non‐cardiac surgery | 9 | 1175 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.54, 1.07] |
| 2.2 Cardiac surgery | 8 | 820 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.55, 0.91] |
Comparison 7. Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery (excluding Oliver 1999 and Devereaux 2014).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 14 | 2174 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.22, 0.93] |
| 2 Cardiac mortality | 3 | 618 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.10, 2.25] |
| 3 Myocardial infarction | 10 | 2000 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.25, 1.25] |
Comparison 8. Alpha‐2 adrenergic agonists (excluding mivazerol) versus control in non‐cardiac surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 14 | 11884 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.62, 1.16] |
| 2 Cardiac mortality | 3 | 10328 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.71, 1.75] |
| 3 Myocardial infarction | 10 | 11710 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.59, 1.53] |
| 4 Myocardial ischaemia | 11 | 1079 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.48, 0.97] |
| 5 Supraventricular tachyarrhythmia | 2 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.05, 24.07] |
| 6 Heart failure | 7 | 10502 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.85, 1.84] |
| 7 Acute stroke | 6 | 11242 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.49, 1.63] |
| 8 Bradycardia | 14 | 11838 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.17, 2.36] |
| 9 Hypotension | 13 | 11541 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.15, 1.55] |
Comparison 9. Alpha‐2 adrenergic agonists versus control in non‐cardiac surgery within past 20 years.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 11 | 13378 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.61, 1.06] |
| 2 Cardiac mortality | 2 | 11907 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.61, 1.29] |
| 3 Myocardial infarction | 7 | 13195 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.93, 1.24] |
| 4 Myocardial ischaemia | 6 | 634 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.28, 0.93] |
| 5 Heart failure | 5 | 10424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.89, 2.03] |
| 6 Acute stroke | 5 | 11218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.50, 1.70] |
Comparison 10. Alpha‐2 adrenergic agonists versus control in cardiac surgery within past 20 years.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 13 | 1782 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.23, 0.97] |
| 2 Myocardial infarction | 4 | 593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.22, 3.03] |
| 3 Myocardial ischaemia | 7 | 908 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.54, 0.96] |
| 4 Supraventricular tachyarrhythmia | 5 | 964 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.44, 1.19] |
| 5 Heart failure | 2 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.48, 1.77] |
| 6 Acute stroke | 6 | 1095 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.14, 0.98] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abi‐Jaoude 1993.
| Methods | Randomized controlled trial comparing clonidine versus placebo. | |
| Participants | 24 participants undergoing CABG surgery. Age (yr): mean (SD): clonidine group: 59 (7.5); placebo group: 56 (12). Sex: 17 men, 7 women. Exclusion criteria: emergency surgical procedures, LVEF < 0.5, and chronic clonidine treatment. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: source not disclosed. Declarations of interest: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Authors described study as 'double‐blind.' Reported use of a placebo in control arm, and described that "management was double blind throughout the study period." No other details reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Ammar 2016.
| Methods | Randomized controlled trial comparing dexmedetomidine versus placebo. | |
| Participants | 50 ASA class II or III people scheduled for cardiac surgery using CPB. Age (yr): mean (SD): dexmedetomidine group: 55 (7); placebo group: 59 (6). Sex: 38 men, 12 women. Exclusion criteria: aged > 75 yr, LVEF < 55%, pre‐existing severe LV hypertrophy, cardiomyopathies, Grade II (pseudonormal filling) and Grade III (restrictive filling) diastolic dysfunction, preoperative AF, pericardial disease, drug dependence, cerebrovascular diseases, use of α‐2 agonists, type I diabetes mellitus, renal disease, significant pulmonary disease and hepatic insufficiency. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: Minoufiya University. Declaration of interest: no conflict of interest. Recruitment dates: June 2012 to February 2014. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization using random number table. |
| Allocation concealment (selection bias) | Low risk | Independent statistician assigned to perform central randomization to ensure proper concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and clinicians blinded to assignment throughout study period. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Bergese 2010.
| Methods | Randomized controlled trial comparing dexmedetomidine versus placebo. | |
| Participants | 124 people undergoing elective awake fibreoptic intubation for anticipated difficult airway. Age (yr): range 19‐78. Sex: 69 men, 36 women. Exclusion criteria: pregnant or lactating women, use of α‐2‐adrenergic agonist or antagonist within 14 days, use of opioid administered orally or IV within 1 hr or intramuscularly within 4 hr, presence of increased intracranial pressure or cerebrospinal fluid leak, acute alcoholic intoxication, uncontrolled seizure disorder, history of acute unstable angina, laboratory‐confirmed acute MI within past 6 weeks, HR < 50 bpm, SBP < 90 mmHg, complete heart block unless person had a pacemaker, or liver transaminase enzymes > 2 times upper normal limit. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: Hospira, Inc. Declarations of interest: authors received personal fees from pharmaceutical company. Recruitment dates: 7 August 2006 to 26 January 2007 at 17 medical centres in US. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 1:1 randomization, stratified by Mallampati and ASA classification. No details on methods provided. |
| Allocation concealment (selection bias) | High risk | Authors stated randomization schedule used. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Authors described study as 'double‐blind.' Reported use of placebo in control arm. No other details reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 9 participants in dexmedetomidine and 11 participants in placebo group did not receive study drug because surgery was cancelled. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Chi 2016.
| Methods | Randomized controlled trial comparing dexmedetomidine (high dose and low dose) versus placebo. | |
| Participants | 100 people undergoing OPCAB for 3‐vessel disease. With 34 participants in high‐dose, 33 in low‐dose and 33 in placebo groups. Age (yr): mean (SD): high‐dose: 56 (7); low‐dose: 54 (7); placebo: 56 (8). Sex: 60 men, 40 women. Exclusion criteria: LVEF < 40%, LV aneurysm, acute MI in 2 weeks before OPCAB surgery, AF, need for cardiac valve replacement, associated vascular diseases, severe systemic diseases involving renal and hepatic systems, respiratory disease (forced vital capacity < 50% of predicted values) and preoperative left bundle‐branch block. |
|
| Interventions |
|
|
| Outcomes | No outcomes reported (study not included in analyses). | |
| Notes | Funding: not stated. Declarations of interest: not stated. Recruitment dates: June 2012 to December 2013. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 105 participants enrolled and randomized; however, 5 excluded from analysis because of conversion to CPB (n = 3), reoperation for major bleeding within 4 hr (n = 1) and incomplete data acquisition (n = 1). While these were a relatively small number of participants, there was no indication that post‐hoc exclusion was planned. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Cho 2016.
| Methods | Randomized controlled trial comparing dexmedetomidine versus placebo. | |
| Participants | 200 people scheduled for cardiac surgery with CPB. Age (yr): mean (SD): dexmedetomidine group: 64 (12); saline group: 62 (13). Sex: 96 men, 104 women. Exclusion criteria: left main coronary artery occlusion > 50%, haemodynamically significant arrhythmia, LVEF < 30%, intra‐aortic balloon pump or ventricular assist device, estimated GFR < 15 mL/min/1.73 m2, use of α‐2 adrenergic agonist to treat hypertension, untreated hypertension, previous exposure to dexmedetomidine or history of severe allergy to drugs. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: not directly stated; however, they stated no commercial associations. Declarations of interest: no conflict of interest. Recruitment dates: June 2013 to January 2015. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Matched placebo and intervention, prepared by independent member of staff. All staff involved in direct care were blinded to allocation arm. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Corbett 2005.
| Methods | Randomized controlled trial comparing dexmedetomidine versus propofol. | |
| Participants | 89 people undergoing non‐emergent CABG surgery with an expected length of intubation < 24 hr. Age (yr): mean (SD): 63 (10.4). Sex: 73 men, 16 women Exclusion criteria: hypersensitivity to either drug or any component of drugs; severe hypotension immediately before initiation of study drug; HR 40 bpm immediately before initiation of study drug; renal insufficiency; hepatic dysfunction; requirement for continued neuromuscular blocking agents postoperatively; requirement for epidural or spinal anaesthesia; gross obesity; history of alcohol or drug abuse. Participants withdrawn from study if length of intubation exceeded 48 hr. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: Society of Critical Care Medicine, Clinical Pharmacy and Pharmacology Section, Ortho‐Biotech Fellowship Grant, and departmental funds. Declaration of interest: not stated. Recruitment dates: October 2002 to April 2004. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors described use of random‐number table. |
| Allocation concealment (selection bias) | High risk | Methods of concealment not discussed. Allocation took place in operating room at end of operation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. |
| Selective reporting (reporting bias) | High risk | Do not discuss length of intubation or ICU stay as outcomes in methods; however, they were reported. |
| Other bias | Low risk | None. |
Devereaux 2014a.
| Methods | Randomized controlled trial comparing clonidine versus placebo. | |
| Participants | 10,010 people undergoing elective non‐cardiac surgery (38% orthopaedic, 27% general, 6% vascular). Age (yr): mean (SD): clonidine group: 68.5 (10.4); placebo group: 68.6 (10.3). Sex: 5283 men, 4727 women. Exclusion criteria: see extensive list in published protocol (Devereaux 2014b). |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: grants from Canadian Institutes of Health Research, Commonwealth Government of Australia's National Health and Medical Research Council, Spanish Ministry of Health and Social Policy, and Boehringer Ingelheim. In this 2‐by‐2 factorial design trial, Bayer Pharma provided the aspirin study drug while Boehringer Ingelheim provided the clonidine study drug. Declarations of interest: several authors received personal fees from pharmaceutical companies but declared that these fees had no relation or influence on the study. Recruitment dates: July 2010 to December 2013. Although some participants underwent surgery with only a nerve block, this accounted for < 1% of total sample. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomization stratified by ASA stratum and centre. |
| Allocation concealment (selection bias) | Low risk | Central 24‐hr randomization centre. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors reported, "Patients, health care providers, data collectors, and outcome adjudicators are blinded to treatment allocation." Matched placebo used in control arm. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome adjudicators blinded. Detailed definitions of outcomes provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis used and all missing data and participants accounted for. |
| Selective reporting (reporting bias) | Low risk | Outcomes determined a priori and reported in a separate publication. |
| Other bias | Low risk | None. |
Djaiani 2016.
| Methods | Randomized controlled trial comparing dexmedetomidine versus propofol. | |
| Participants | 185 participants aged > 60 yr undergoing elective complex cardiac surgery and aged > 70 yr undergoing either isolated coronary revascularization or single‐valve surgery (repair or replacement) with use of CPB. Age (yr): mean (SD): dexmedetomidine group: 73 (6.4); propofol group: 72 (6.2). Sex: 138 men, 45 women. Exclusion criteria: serious mental illness, delirium, severe dementia or emergency procedures. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: funded, in part, by Hospira Inc, and Department of Anesthesia and Pain Management, Toronto General Hospital, Toronto, Ontario, Canada. Declaration of interest: authors declared no potential conflicts. Recruitment dates: July 2011 to July 2014. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization in blocks of 4. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes generated according to randomization schedule and opened by a study co‐ordinator. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Only participants blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unblinded outcome assessment. Unclear if it would affect outcome of death. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 185 participants randomized and 183 analysed. 1 participant died in operating room, and 1 participant underwent off‐pump coronary revascularization surgery based on intraoperative decision and was excluded from analysis. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Dorman 1993.
| Methods | Randomized controlled trial comparing clonidine versus placebo. | |
| Participants | 43 people undergoing CABG surgery. Age (yr): mean (SD): clonidine group: 65 (2); placebo group: 61 (2). Sex: 37 men, 6 women. Exclusion criteria: LVEF < 45%, LV end‐diastolic pressure > 18 mmHg, or chronic clonidine exposure. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: grant from Society of Cardiovascular Anesthesiologists. Declaration of interest: not discussed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors described prospective randomization but did not describe methods. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Reported use of placebo in control arm but did not describe details regarding blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded investigator used to independently assess ST changes. Blinding for other outcomes not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. |
| Selective reporting (reporting bias) | High risk | No prespecified outcomes discussed in methods. |
| Other bias | Low risk | None. |
El‐Kerdawy 2004.
| Methods | Randomized controlled trial comparing dexmedetomidine versus control. | |
| Participants | 50 people undergoing OPCAB graft surgery. Age (yr): mean (SD): dexmedetomidine group: 61 (8); control group: 62 (4). Sex: 30 men, 22 women (error in manuscript, 2 additional participants) Exclusion criteria: LVEF < 45%, LV end‐diastolic pressure > 18 mmHg, cardiac valvular abnormality, atrioventricular block, chronic clonidine or methyldopa treatment, or SBP < 90 mmHg. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: not discussed. Declaration of interest: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors stated participants randomly allocated but methods not described. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Ellis 1994.
| Methods | Randomized controlled trial comparing clonidine versus placebo. | |
| Participants | 61 people, with a diagnosis or risk factors of coronary artery disease, who were undergoing major non‐cardiac surgery (82% vascular) Age (yr): median (IQR): clonidine group: 69 (61‐74); placebo group: 68 (63‐75). Sex: 29 men, 32 women. Exclusion criteria: chronic methyldopa or clonidine therapy, serum creatinine > 30 mg/dL, planned carotid endarterectomy surgery, planned thoracic aortic aneurysm surgery, pulse < 50 bpm, or PR interval > 0.24 sec. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Study was terminated early at 61 participants due to low incidence of ischaemia. It was originally designed to recruit 160 participants in 2 arms. Funding: Anesthesiology Young Investigator Award from the Foundation for Anesthesia Education and Research. Declaration of interest: not stated. Recruitment dates: November 1990 to May 1992. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors reported use of computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors reported that all participants and clinicians were blinded to treatment assignment throughout study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment by an investigator not involved in care of participant, blinded to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | High risk | Study terminated early because of a lower than expected rate of myocardial ischaemia; unclear whether this unblinded interim analysis was prespecified. |
Ghignone 1986.
| Methods | Randomized controlled trial comparing clonidine versus placebo. | |
| Participants | 24 people with hypertension who were NYHA class 3‐4 with LVEF > 0.5 and undergoing CABG surgery. Age (yr): mean (SD): clonidine group: 60 (9); control group: 58 (5). Sex: 13 men, 11 women. Exclusion criteria: none. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: not discussed. Declaration of interest: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors stated participants were randomly assigned but did not describe methods. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Criteria for outcome assessment not prespecified. Open‐label. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Inconsistency in reporting number of participants in each arm (i.e. Figure 2). |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Ghignone 1987.
| Methods | Randomized controlled trial comparing clonidine versus placebo | |
| Participants | 30 people ASA II‐III with hypertension undergoing non‐cardiac surgery (abdominal, head and neck, orthopaedic). Age (yr): mean (SD): clonidine group: 49 (15); control group: 48 (13). Sex: 14 men, 16 women. Exclusion criteria: severe hypertension (DBP > 110 mmHg), heart failure, chronic airway obstruction, MI with 2 yr, active angina pectoris. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: not discussed. Declaration of interest: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described use of random‐number table. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Criteria for outcome assessment not prespecified. Open‐label. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Helbo‐Hansen 1986.
| Methods | Randomized controlled trial comparing clonidine versus control. | |
| Participants | 40 people undergoing CABG surgery. Age (yr): mean (SD): clonidine group: 52 (10); control group: 55 (7). Sex: 36 men, 4 women Exclusion criteria: concurrent valve replacement or aneurysmectomy planned, disseminated disease other than essential arterial hypertension or diabetes, preoperative medication included α‐adrenergic receptor blockers, ganglion blockers, loop‐diuretics, clonidine or methyldopa, LVEF < 40%, AF, atrioventricular block, SBP < 80 mmHg at time of study drug administration. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: Boehringer Ingelheim International. Declaration of interest: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Authors described use of 'stratified randomisation.' Stratification abandoned for last few participants to even out groups. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Placebo used in control arm; however, details of blinding not discussed. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not discussed, |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported, |
| Selective reporting (reporting bias) | High risk | MI and mortality not prespecified as outcomes in methods, |
| Other bias | Low risk | None. |
Herr 2003.
| Methods | Randomized controlled trial comparing dexmedetomidine versus propofol. Analyses performed on intention‐to‐treat basis. | |
| Participants | 295 people undergoing CABG surgery. Age (yr): mean (SD): dexmedetomidine group: 62 (10); propofol group: 62 (9). Sex: 265 men, 30 women. Exclusion criteria: pregnant women, neurological condition preventing evaluation, unstable or uncontrolled diabetes, grossly obese, ejection fraction < 30%, hospitalized for drug overdose. People who received neuromuscular block, epidural or spinal anaesthesia in postoperative period or any other factor that investigator determined would affect study data (i.e. haemodynamic instability) were discontinued from study. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: supported by Abbot Laboratories. Declaration of interest: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors described use of blocked randomization. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Jalonen 1997.
| Methods | Randomized controlled trial comparing dexmedetomidine versus placebo. | |
| Participants | 80 people undergoing CABG surgery. Age (yr): mean (SD): dexmedetomidine group: 55 (9), placebo group: 56 (8). Sex: 67 men, 13 women. Exclusion criteria: left main coronary artery stenosis > 50%, significant valvular dysfunction, severe concurrent systemic disorders, preoperative medication with clonidine or α‐methyldopa, strong susceptibility to allergic reactions, and uninterpretable results of ECG (e.g. left bundle branch block). |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: supported by Orion Corporation. Declaration of interest: not stated. Recruitment dates: June 1992 to March 1993. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors described use of permuted block randomization. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Authors described study as 'double‐blind.' Reported use of a placebo in control arm. No other details reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded ECG interpretation but no other outcomes discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Khalil 2013.
| Methods | Randomized controlled trial comparing dexmedetomidine versus placebo. | |
| Participants | 50 people undergoing OPCAB graft surgery. Age (yr): mean (SD): dexmedetomidine group: 58.1 (10): placebo group: 58.9 (10). Sex: 31 men, 19 women. Exclusion criteria: body mass index > 35 kg/m2; left main coronary artery disease; left bundle branch block; severe combined renal, hepatic or respiratory disorders; or any contraindication to use of dexmedetomidine. Post‐hoc exclusion of people who developed severe haemodynamic alterations or arrhythmias requiring CPB. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: reported no financial support received for research. Declaration of interest: stated no conflict of interest. Recruitment dates: January to September 2008. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors reported use of a 'random number table.' |
| Allocation concealment (selection bias) | High risk | Concealment not discussed. Use of random number table high risk. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors described study as blinded. Intensivist and anaesthesiologist caring for participants were blinded. Described use of matched placebo in control arm. Separate members of staff responsible for managing the infusion, who were not involved in care of participant. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment not discussed. Outcome of all‐cause mortality low risk to be influenced. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. |
| Selective reporting (reporting bias) | High risk | Adverse event data not reported (stated as not significant). |
| Other bias | Low risk | None. |
Kim 2014a.
| Methods | Randomized controlled trial comparing dexmedetomidine versus lidocaine versus combination versus control. | |
| Participants | 153 people (40 dexmedetomidine, 36 lidocaine, 39 combined, and 38 control) under‐going OPCAB. Only participants in dexmedetomidine and control group included in meta‐analyses. Age (yr): median (IQR): dexmedetomidine group: 63 (56‐68); control group: 65 (57‐72). Sex: 60 men, 18 women. Exclusion criteria: planned CPB and people diagnosed with arrhythmia with medication or pacemaker. Post‐hoc exclusion of cases with unexpected conversion to CPB during surgery. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: no external funding. Declaration of interest: not stated. Recruitment dates: September 2012 to August 2013. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Internet‐based computer‐generated randomization sequence. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Anaesthesiologist not blinded to study drug. Participants and surgeon kept blinded; however, no placebo used in control group. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Data analyst blinded. No discussion of blinding of outcome assessment. Outcomes definition for myocardial ischaemia not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 160 participants initially randomized, 4 participants excluded due to unexpected conversion to surgery with CPB. 3 participants further excluded from analysis because there was a missing laboratory value; however, groups they were allocated to not disclosed. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Lee 2013a.
| Methods | Randomized controlled trial comparing dexmedetomidine versus remifentanil versus placebo. | |
| Participants | 85 participants (28 dexmedetomidine, 28 low‐dose remifentanil, 29 placebo) ASA physical status I‐II and aged 20‐65 yr, undergoing laparoscopic‐assisted vaginal hysterectomy. Only dexmedetomidine and placebo group were included in meta‐analyses. Age (yr): mean (SD): dexmedetomidine group: 49 (6); placebo group: 48 (5). Sex: women. Exclusion criteria: allergy to dexmedetomidine, clinically significant medical or psychiatric conditions, pregnancy, history of alcohol or drug abuse, or opioid‐containing pain or sedative medications. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: study supported by Wonkwang University, Iksan, South Korea. Declaration of interest: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors reported use of a 'random number table.' |
| Allocation concealment (selection bias) | High risk | Concealment not discussed. Use of a random number table was high risk. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Reported use of placebo in control group. Blinding not discussed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed and outcomes not defined. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 participants lost to follow‐up for conversion to open surgery or re‐exploration for postoperative bleeding. Not predefined in exclusion criteria or methods. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Unclear risk | Selection bias: exclusion criteria vague (i.e. "clinically significant medical or psychiatric condition"). |
Li 2017.
| Methods | Randomized controlled trial comparing dexmedetomidine versus placebo. | |
| Participants | 285 elderly people (age ≥ 60 yr) undergoing elective CABG or valve replacement surgery, or both. Age (yr): mean (SD): dexmedetomidine group: 66.4 (5.4); placebo group: 67.5 (5.3). Sex: 197 men, 88 women. Exclusion criteria: history of schizophrenia, epilepsy, Parkinson's disease or severe dementia; inability to communicate because of severe visual/auditory dysfunction or language barrier; history of functional neurosurgery or brain injury; preoperative sick sinus syndrome, severe bradycardia (HR < 50 bpm), second‐degree or above atrioventricular block without pacemaker; severe hepatic insufficiency (Child‐Pugh grades C); severe renal insufficiency (requirement of renal replacement therapy); person refused to participate in study. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: Scientific Research Fund (2015) from Peking University First Hospital. Study drugs manufactured and supplied by Jiangsu Hengrui Medicine Co, Ltd, Jiangsu, China. Declaration of interest: Dr DX Wang received lecture fees or travel expenses (or both) for lectures given at domestic academic meetings from Jiangsu Hengrui Medicine Co, Ltd, China. Prof D Ma was supported by BOC Chair grant, Royal College of Anaesthetists, and BJA Fellowship grant, London, UK. Other authors reported no conflict of interests. Recruitment: 1 December 2014 to 19 July 2015. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centre‐stratified randomization with a block size of 4 using SAS statistical package by an independent biostatistician. |
| Allocation concealment (selection bias) | Low risk | Randomization results sealed in sequentially numbered letters and stored at site of investigation until end of study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All investigators, healthcare team members and participants blinded to treatment group assignment throughout study period. Study drugs prepared and coded by independent pharmacist. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not formally discussed but outcomes unlikely to be influenced. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | More participants in dexmedetomidine group (8) were lost to follow‐up than in control group (3), which may influence results given low frequency of outcomes. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Lipszyc 1991.
| Methods | Randomized controlled trial comparing clonidine versus placebo. | |
| Participants | 40 people undergoing vascular (carotid artery) surgery. Age: not reported. Sex: not reported. Exclusion criteria: not reported. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Published as an abstract. Funding: not discussed. Declaration of interest: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not described as randomized trial. |
| Allocation concealment (selection bias) | High risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Authors described study as 'double‐blind.' Reported use of placebo in control arm. No other details reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Unclear risk | Study published as abstract and not formally peer reviewed. |
Liu 2016.
| Methods | Randomized controlled trial comparing dexmedetomidine versus propofol. | |
| Participants | 88 participants, aged ≥18 yr, undergoing elective cardiac surgery with CPB, admitted to ICU while intubated and ventilated, and lack of prior AF or flutter before receiving sedation in ICU. Age (yr): mean (IQR): dexmedetomidine group: 53 (46.0‐63.0); propofol group: 57 (49.3‐62.0). Sex: 35 men, 53 women. Exclusion criteria: HR < 50 bpm, atrioventricular conduction block grade II or III (unless a pacemaker had been installed), MAP < 55 mmHg (despite appropriate IV volume replacement and vasopressor treatment), acute severe neurological disorder, propofol or dexmedetomidine allergy or other contraindications. In addition, people who had received ≥ 2 sedatives within 24 hr postoperatively excluded. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: authors stated, "not applicable." Declaration of interest: declared no conflict of interest. Recruitment dates: January 2015 to December 2015. First Affiliated Hospital of Zhejiang University. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table used. |
| Allocation concealment (selection bias) | High risk | Used random number table but no discussion of concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 participants in each group excluded because they received sedatives. |
| Selective reporting (reporting bias) | Unclear risk | Mortality reported but not listed as outcome in methods. |
| Other bias | Low risk | None. |
Loick 1999.
| Methods | Randomized controlled trial comparing clonidine versus thoracic epidural anaesthesia versus control. | |
| Participants | 70 people (24 clonidine, 25 epidural, 21 control) undergoing CABG surgery. Age (yr): mean (SD): clonidine group: 62 (11); placebo group: 63 (7). Sex: 36 men, 9 women. Exclusion criteria: disorders of intestine and liver, gastritis, duodenal ulcer, autonomic neuropathy, diabetes mellitus. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Only participants in clonidine and control arms included in analyses. Data on ischaemia available for only 29 participants (clonidine group: 14; control group: 15). Funding: not discussed. Declaration of interest: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors stated participants randomly allocated but methods not described. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 participants in control arm excluded because of repeat thoracotomy for bleeding. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Matot 2000.
| Methods | Randomized controlled trial comparing clonidine versus placebo. | |
| Participants | 36 people ASA I‐III, aged ≥ 50 yr undergoing microlaryngoscopy and rigid bronchoscopy under general anaesthesia. Age (yr): mean (SD): clonidine group: 63 (9); placebo group: 61 (10). Sex: 26 men, 10 women. Exclusion criteria: preoperative use of clonidine, HR < 50 bpm, atrioventricular block, left bundle‐branch block or gastrointestinal disturbance that would hinder absorption medication. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: institutional funding. Declaration of interest: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors stated participants randomly allocated but methods not described. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors described use of coded oral preparations with placebo in control arm. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinician who analysed ECGs was blinded to treatment assignment. Criteria were prespecified to define ischaemia, hypotension and bradycardia. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
McSPI‐Europe 1997.
| Methods | Randomized controlled trial comparing mivazerol (high and low dose) versus placebo. | |
| Participants | 317 people (17 excluded) with coronary artery disease undergoing vascular surgery under general anaesthesia for > 1 hr. (high‐dose group: 98; low‐dose group: 99; placebo: 103). Age (yr): mean (SD): high‐dose group: 67 (10); low‐dose group: 65 (10); placebo group: 66 (8). Sex: 233 men, 67 women. Exclusion criteria: taking methyldopa, α‐2 adrenergic agonists or tricyclic antidepressants; in cardiogenic shock and had clinical signs of heart failure or required chronic inotropic support for ventricular dysfunction; unstable angina or uncontrolled hypertension; conduction defects that precluded electrocardiographic analysis of ST segments; pregnant or ASA physical status V. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Mivazerol arms were combined in analyses. Funding: grants from UCB Pharma and the Ischemia Research and Education Foundation. Declaration of interest: stated no conflict of interest. Recruitment dates: March to December 1993. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors described use of blocked stratified randomization. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors reported use of placebo in control arm. Reported that staff who undertook clinical care were blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Authors reported that staff who interpreted ECG recordings, diagnosed MI by ECG criteria and performed statistical analyses were blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Excluded 7 participants for 'technical reasons,' which is ambiguous. Table 2 only reported results for 98/103 participants in placebo group. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Myles 1999.
| Methods | Randomized controlled trial comparing clonidine versus placebo | |
| Participants | 156 people (6 excluded) undergoing CABG surgery. Age (yr): mean (SD): clonidine group: 65 (11); placebo group: 65 (9). Sex: 128 men, 28 women. Exclusion criteria: receiving clonidine or alpha‐methyldopa, allergic to clonidine, considered very high risk (clinical severity score > 9), hypotensive (SBP < 120 mmHg), heart failure, ejection fraction < 25%, AF or atrioventricular block, left bundle branch block or had a pacemaker. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: not discussed. Declaration of interest: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors described use of stratified randomization. |
| Allocation concealment (selection bias) | Low risk | Independent randomization by research pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors reported use of a placebo in control arm. In addition, they described that "investigators and patients" were blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Diagnosis of MI by ECG and biochemical criteria was made by a blinded clinician. Statistical analyses performed by staff blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions reported with valid explanations provided. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Oliver 1999.
| Methods | Randomized controlled trial comparing mivazerol versus placebo. Analyses performed on intention‐to‐treat basis. | |
| Participants | 2854 people with a diagnosis or risk factors for coronary artery disease who were undergoing vascular, abdominal, thoracic or orthopaedic surgery. Results only presented for 1897 with coronary artery disease. Age (yr): 48% aged 65‐75. Sex: 1403 men, 494 women. Exclusion criteria: unstable angina, MI in past 14 days, uninterpretable ECG Q‐waves, cardiogenic shock, prescribed alpha‐methyldopa or clonidine, severe hepatic disorders, renal insufficiency, emergency surgery, pregnant or nursing women. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | 2857 participants were recruited in total, but only results for 1897 participants with coronary artery disease were reported. Funding: UCB SA Pharma Sector. Declaration of interest: State steering committee not sponsored by UCB Pharma but further potential conflicts not stated. Recruitment dates: June 1994 to February 1997. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors described use of a computer‐generated randomization schedule with stratification by institution. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors described study as 'double‐blind.' Reported use of a placebo in control arm. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Authors reported that all outcomes were adjudicated by staff who were blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only presented data for 1897/2854 recruited participants. |
| Selective reporting (reporting bias) | High risk | Reported primary outcome on participants with known coronary heart disease and excluded 957/2854 participants who were at risk. |
| Other bias | High risk | Adaptive study design. Study inclusion criteria were modified after monitoring of blinded data (1304 participants recruited). Since event rates in participants with risk factors for coronary artery disease were lower than expected, trial protocol was amended to focus only on participants with pre‐existing coronary artery disease. |
Park 2014.
| Methods | Randomized controlled trial comparing dexmedetomidine versus remifentanil. | |
| Participants | 142 people undergoing open heart surgery with CPB. Age (yr): mean (SD): dexmedetomidine group: 51 (16); remifentanil group: 54 (13). Sex: 79 men, 63 women. Exclusion criteria: re‐do and emergency surgery; severe pulmonary or systemic disease; LVEF < 40%; pre‐existing renal dysfunction (serum creatinine level > 2.0 mg/dL); documented preoperative dementia, Parkinson's disease or recent stroke; and aged > 90 yr or < 17 yr. In addition, people who had psychotropic medications, evidence of progressed heart block and surgery requiring deep hypothermic circulatory arrest involving thoracic aorta were excluded. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: not discussed. Declaration of interest: declared no conflict of interest. Recruitment dates: April 2012 to March 2013. Konkuk University Medical Center. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated participants randomly assigned, but no further description provided. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not discussed. Presumed to be open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not discussed. Presumed to be open‐label. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. |
| Selective reporting (reporting bias) | Unclear risk | Reported adverse events not discussed in methods. |
| Other bias | Low risk | None. |
Patel 2016.
| Methods | Randomized controlled study comparing clonidine versus clonidine + ketamine versus placebo. | |
| Participants | 50 people undergoing OPCAB with stable angina and preserved myocardial function Age (yr): mean (SD): clonidine group: 58 (8); placebo group: 62 (6). Sex: 46 men, 4 women. Exclusion criteria: diabetes mellitus, renal or liver disease, rhythm disorders, concomitant heart valve surgery, ejection fraction < 40%, and emergency surgery. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: stated no financial support for research. Declaration of interest: declared no conflict of interest. Recruitment dates: January 2015 to September 2015. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized randomization table. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants, data collector and data processor kept blinded. Clinicians blinding not discussed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, data collector and data processor were kept blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Pawlik 2005.
| Methods | Randomized controlled trial comparing clonidine versus placebo. | |
| Participants | 30 people with a diagnosis of obstructive sleep apnoea who were undergoing head‐and‐neck surgery. Age (yr): mean (SD): clonidine group: 49 (5); placebo group: 54 (8). Sex: 28 men, 2 women. Exclusion criteria: history of MI within 6 months, resting room air saturation < 90%, taking clonidine to treat hypertension preoperatively. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: support from institutional departments. Declaration of interest: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors described use of a computer program to perform randomization. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Authors described study as 'double‐blind.' Reported use of a placebo in control arm. No other details reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 participant excluded from haemodynamic analysis because of an angiotensin‐converting enzyme inhibitor overdose on postoperative ward. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Pluskwa 1991.
| Methods | Randomized controlled trial comparing clonidine versus placebo. | |
| Participants | 30 people (1 excluded) undergoing vascular (carotid artery) surgery. Age (yr): mean (SD): clonidine group: 67 (8); placebo group: 67 (10). Sex: 21 men, 8 women. Exclusion criteria: unstable angina pectoris or on long‐term clonidine therapy. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: not discussed. Declaration of interest: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors described use of a random number table. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors reported study was double‐blind and that matched placebo was used in control arm. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 participant excluded after randomization when a thrombosis in carotid artery was discovered intraoperatively. 1 participant had data partially excluded because they required reoperation. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Quintin 1993.
| Methods | Randomized controlled trial comparing clonidine versus placebo. | |
| Participants | 26 people undergoing CABG surgery. Age: not reported. Sex: not reported. Exclusion criteria: not reported. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Results of a pilot study published as a letter to editor. Funding: not discussed. Declaration of interest: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors reported allocating participants in a randomized manner but provided no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Authors described study as 'double‐blind.' Reported use of a placebo in control arm. No other details reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | ECG changes assessed by 2 independent observers blinded to treatment arm. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Unclear risk | Not formally peer reviewed. |
Quintin 1996.
| Methods | Randomized controlled trial comparing clonidine versus placebo. | |
| Participants | 24 people (3 excluded) with hypertension who were undergoing vascular (aortic) surgery. Age (yr): mean (SD): clonidine group: 64 (8); placebo group: 69 (5). Sex: 17 men, 4 women. Exclusion criteria: treatment with clonidine, gaunabenz, rilmelidine, methyldopa or reserpine. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: national research foundations and Boehringer‐Ingelheim. Declaration of interest: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors reported prospective randomization but provided no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Authors described study as 'double‐blind.' Reported use of placebo in control arm. No other details reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 participants excluded for surgical reasons (2) or inadequate data collection (1). |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Ren 2013.
| Methods | Randomized controlled trial comparing dexmedetomidine versus placebo. | |
| Participants | 162 people undergoing off‐pump CABG surgery. Age: mean (SD): dexmedetomidine group: 60 (4); placebo group: 58 (6). Sex: 53 men, 109 women. Exclusion criteria: aged > 75 yr, ejection fraction < 40% or bradycardia based on preoperative diagnosis (HR < 50 bpm), preoperative history of arrhythmia, preoperative SBP < 90 mmHg, obesity, type I or type II diabetes mellitus, drug dependence, and history of psychiatry and cerebrovascular diseases. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: not discussed. Declaration of interest: not stated. Recruitment dates: January 2010 to January 2011. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors reported allocating participants in a randomized manner but provide no further details. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not discussed. Reported use of matched placebo in control arm. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not done; however, outcomes unlikely to be affected given predefined criteria. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Shehabi 2009.
| Methods | Randomized controlled trial comparing dexmedetomidine versus morphine. | |
| Participants | 306 people aged ≥ 60 yr undergoing on‐pump cardiac surgery (including CABG, valve replacement surgery, or both). Age (yr): median (IQR): dexmedetomidine group: 72 (66‐76); morphine group: 71 (65‐75). Sex: 225 men, 81 women. Exclusion criteria: allergic to any of study medications, receiving other α‐2 adrenergic agonists such as clonidine or psychoactive agents other than night‐time hypnotics, preoperative HR < 55 bpm or SBP < 90 mmHg (or both), bodyweight > 150 kg or a preoperative creatinine > 140 µmol/L (1.6 mg/dL) or creatinine clearance < 50 mL/min (calculated by Cockcroft Gault formula). In addition, people with documented preoperative dementia, Parkinson's disease, recent seizures and unable to understand English and thus unable to participate in delirium assessment were excluded. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: support solely from institutional or departmental (or both) sources, though study drug provided by Hospira. Declaration of interest: authors declared no conflict of interest. Recruitment dates: August 2004 to December 2007. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors described use of computer‐generated randomization with blocks of 10. |
| Allocation concealment (selection bias) | Low risk | Centralized randomization with a research pharmacist who prepared study drugs. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors reported study was double blinded and stated "surgeons, anaesthetists, and intensive care medical and nursing staff were blinded." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not discussed but outcomes clearly defined and unlikely to be influenced. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Soliman 2016.
| Methods | Randomized controlled trial comparing dexmedetomidine versus placebo. | |
| Participants | 150 people undergoing elective aortic vascular surgery (aortic aneurysm or aortobifemoral anastomosis). Age (yr): mean (SD): dexmedetomidine group: 58 (7); placebo group: 58 (8). Sex: 75 men, 75 women. Exclusion criteria: acute MI, congestive heart failure, heart block, obese people or emergency. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: authors stated no support. Declaration of interest: declare no conflict of interest. Recruitment dates: 2013‐2015. Kasr El‐Aini Hospital, Cairo University, Egypt. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients classified randomly (by simple randomization)." Details not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The medication was prepared by the nursing staff and given to anaesthetist blindly." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All date reported. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Stuhmeier 1996.
| Methods | Randomized controlled trial comparing clonidine versus placebo. Analyses were performed on intention‐to‐treat basis. | |
| Participants | 297 people undergoing vascular surgery. Age (yr): mean (range): overall: 64 (28‐82). Sex: 206 men, 91 women. Exclusion criteria: chronic myocardial ischaemia, preoperative digitalis or chronic clonidine medication, AF, bundle branch block, second degree or greater atrioventricular‐nodal block on preoperative ECG. Criteria for post hoc exclusion were transfer to another hospital within 4 days, redo surgery, another surgery within 1 week, missing data. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: not discussed. Declaration of interest: not stated. Recruitment dates: June 1993 to December 1994. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors described use of a random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors described study as 'double‐blind.' Reported use of a matched placebo in control arm. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinicians who evaluated all ECG recordings were blinded to treatment assignment. No other details on blinding provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 47 participants excluded from outcome analysis (from 297 total) because of transfers to other hospitals (14) or departments (10) within 4 days after surgery, redo surgery required within 1 week (8), other surgery within 1 week (8) and missing outcome data (7). |
| Selective reporting (reporting bias) | High risk | Primary and secondary outcomes of interest were not prespecified in methods. |
| Other bias | Low risk | None. |
Su 2016.
| Methods | Randomized controlled trial comparing dexmedetomidine versus placebo. | |
| Participants | 700 people aged ≥ 65 yr undergoing elective non‐cardiac surgery under general anaesthesia and were admitted to ICU after surgery. Age (yr): mean (SD): dexmedetomidine group: 74 (7); placebo: 74 (7). Sex: 432 men, 268 women. Exclusion criteria: preoperative history of schizophrenia, epilepsy, Parkinsonism or myasthenia gravis; inability to communicate in preoperative period (coma, profound dementia or language barrier); brain injury or neurosurgery; known preoperative LVEF < 30%, sick sinus syndrome, severe sinus bradycardia (< 50 bpm), or second‐degree or greater atrioventricular block without pacemaker; serious hepatic dysfunction (Child‐Pugh class C); serious renal dysfunction (undergoing dialysis before surgery) or low likelihood of survival for > 24 hr. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: Braun Anaesthesia Scientific Research Fund and Wu Jieping Medical Foundation. A Chinese pharmaceutical company provided drugs used in study. Declaration of interest: several authors, including first author, received funding from pharmaceutic companies. Recruitment dates: 17 August 2011 to 20 November 2013. Peking University First Hospital and Peking University Third Hospital in Beijing. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Biostatistician, who was independent of data management and statistical analyses, generated random numbers (in a 1:1 ratio)." |
| Allocation concealment (selection bias) | Low risk | "The results of randomisation were sealed in sequentially numbered envelopes and stored at the site of investigation until the end of the study." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Clinicians, participants and study members blinded to treatment group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Talke 1995.
| Methods | Randomized controlled trial comparing 3 dexmedetomidine arms (low, medium and high doses), and 1 placebo arm. | |
| Participants | 25 participants, with a diagnosis or risk factors of coronary artery disease, undergoing vascular surgery. Age (yr): mean (SD): dexmedetomidine group (combined): 65 (9); placebo group: 66 (6). Sex: not reported. Exclusion criteria: unstable angina, uninterpretable ECG, taking clonidine or tricyclic antidepressant preoperatively or did not received study drug for first 24 hr postoperatively. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Dexmedetomidine arms were combined for purpose of analyses. Funding: support grant from Orion Corporation, a pharmaceutical company. Declaration of interest: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but details not provided. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Authors described study as 'double‐blind.' Reported use of a placebo in control arm. No other details reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All staff who analysed ECG recordings were blinded to treatment assignment. No other details of blinding reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 participant in dexmedetomidine arm excluded because of emergent reoperation. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Talke 2000.
| Methods | Randomized controlled trial comparing dexmedetomidine versus placebo. Analyses performed on intention‐to‐treat basis. | |
| Participants | 41 people undergoing vascular surgery. Age (yr): mean (SD): dexmedetomidine group: 66 (9); placebo group: 65 (9). Sex: 37 men, 4 women. Exclusion criteria: pregnant, taking clonidine or tricyclic antidepressants, or had second or third‐degree heart block. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: grant from Orion Corporation, a pharmaceutical company. Declaration of interest: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors described use of random permuted blocks with stratification by centre. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Authors described study as 'double‐blind.' Reported use of placebo in control arm. No other details reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Venn 1999.
| Methods | Randomized controlled trial comparing dexmedetomidine versus placebo. | |
| Participants | 105 people (7 excluded) who had undergone cardiac (83%) and non‐cardiac (17%) surgery, and needed > 6 hr of mechanical ventilation and sedation after surgery. Age (yr): mean (SD): dexmedetomidine group: 63 (14); placebo group: 64 (12). Sex: 73 men, 25 women. Exclusion criteria: serious central nervous system trauma or undergoing neurosurgery; requirement for neuromuscular blocking agents, epidural or spinal anaesthesia; any contraindications or allergy to any of trial drugs; gross obesity (> 50% above ideal bodyweight); admission for a drug overdose, prior enrolment in a trial with any experimental drug in last 30 days; uncontrolled diabetes and excessive bleeding that would be likely to require reoperation. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Excluded participants receiving epidural or spinal anaesthesia. Funding: Abbott Laboratories. Declaration of interest: 1 author performs consultancy work for Abbott Laboratories. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors described study as randomized but no other details reported. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors described study as 'double‐blind.' Reported use of a matched placebo in control arm. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7 participants withdrawn from study because of reoperation for bleeding (3), bradycardia and hypotension (2), residual neuromuscular blockade (1) and surgeon's request (1). These participants were included in safety data. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Venn 2001.
| Methods | Randomized controlled trial comparing dexmedetomidine versus propofol. | |
| Participants | 20 people undergoing non‐vascular non‐cardiac surgery and required > 8 hr of mechanical ventilation after surgery. Age (yr): mean (range): dexmedetomidine group: 65 (60‐77), propofol group: 67 (64‐74). Sex: not reported. Exclusion criteria: none reported. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: supported in part by Abbott Laboratories, a pharmaceutical company. Declaration of interest: 1 author performed consultancy work for Abbott Laboratories. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors described study as randomized but no other details reported. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Viviano 2012.
| Methods | Randomized controlled trial comparing clonidine versus ropivacaine epidural versus placebo. | |
| Participants | 60 people undergoing elective lung resection. Age (yr): median (IQR): clonidine group: 67 (61‐73); saline group: 67 (50‐71). Sex: 24 men, 16 women. Exclusion criteria: aged < 18 yr; guardianship/conservatorship (people who were in a coma, had advanced Alzheimer's disease or had other serious illnesses or injuries); refusal to participate; pre‐existing alterations of immune system or undergoing treatments or having disorders with direct influence on immune system; pregnancy; contraindications for epidural catheter insertion; contraindications for clonidine, ropivacaine or remifentanil treatment; previous treatment with trial drugs or drugs belonging to same pharmacological group; NYHA Functional Classification ≥ class III heart failure; and MI in 8 weeks before surgery. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: not reported. Declaration of interest: declare no conflict of interest. Recruitment dates: January 2006 to May 2007. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization with block size of 6. |
| Allocation concealment (selection bias) | Low risk | Central randomization with an independent research pharmacist preparing all study drugs. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors reported "all personnel and participants were blinded to treatment assignment for the duration of the study." A research pharmacist supplied study drugs in coded syringes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not discussed; however, outcome unlikely to be influenced. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Authors stated that all randomized participants completed trial. 10/70 participants included were later excluded. Participants withdrawn during operation, and not included in randomization which was technically not possible. 1 participant removed as they experienced a cardiac arrest resulting in unbinding and excluded from final analysis. |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not discussed in methods but reported. |
| Other bias | Low risk | None. |
Wallace 2004.
| Methods | Randomized controlled trial comparing clonidine versus placebo. Analyses performed on intention‐to‐treat basis. | |
| Participants | 190 people with a diagnosis or risk factors of coronary artery disease, undergoing elective non‐cardiac surgery (26% vascular, 18% abdominal, 5% thoracic). Age (yr): mean (SD): clonidine group: 68 (8); placebo group: 69 (9). Sex: not reported. Exclusion criteria: unstable angina in month prior to surgery; uninterpretable Holter ECG secondary to left bundle‐branch block, cardiac pacemaker dependency or marked resting ST‐segment and T‐wave abnormalities that precluded ECG ST‐segment interpretation; preoperative use of clonidine, methyldopa or tricyclic antidepressants; symptomatic aortic stenosis; SBP < 100 mmHg and refusal or inability to give informed consent. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | 10.5% prevalence of epidural use. Funding: supported by a grant‐in‐aid from American Heart Association, Veterans Administration Merit Review Funding, Ischemia Research and Education Foundation, Northern California Institute for Research and Education. Declaration of interest: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors described use of a computer‐generated randomization schedule. |
| Allocation concealment (selection bias) | Low risk | Allocation by an independent pharmacist. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors described study as 'double‐blind.' Reported use of a matched placebo in control arm. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | ECG and safety data analysed by independent clinicians blinded to treatment arm. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 12 participants withdrawn from study for cancellation of procedure (10), hypotension (1) and chest pain (1). |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Wijeysundera 2014a.
| Methods | Randomized controlled trial comparing clonidine versus placebo. Analyses performed on intention‐to‐treat basis. | |
| Participants | 168 people considered to be an intermediated to high risk of perioperative cardiac complications undergoing elective non‐cardiac surgery (28% vascular) with an expected hospital length of stay ≥ 48 hr and receiving oral β‐blocker therapy for ≥ 30 days prior to surgery. Age (yr): mean (SD): clonidine group: 70 (8); placebo group: 72 (8). Sex: 118 men, 50 women. Exclusion criteria: pre‐existing use of α‐2 adrenergic agonists, prior adverse reaction to α‐2 adrenergic agonists, decompensated heart failure, LVEF < 40%, SBP < 90 mmHg, known clinically significant aortic stenosis and concomitant life‐threatening disease likely to limit life expectancy to < 30 days. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: Heart and Stroke Foundation of Ontario and Canadian Anesthesia Research Foundation. Declaration of interest: declared no conflicts of interest. The lead (DNW) and senior (WSB) authors of this study were authors on this present systematic review; however, neither author was involved in primary data abstraction or quality assessment process in this review. Recruitment dates: June 2006 to November 2007 (Toronto General Hospital), January 2008 to August 2009 (Vancouver General Hospital), September 2007 to November 2008 (Victoria Hospital). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors reported use of computer‐generated randomization schedule. |
| Allocation concealment (selection bias) | Low risk | Permuted blocks with varying size used and randomization lists only available to research pharmacists. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors stated participants, clinicians and data collectors blinded to treatment assignment. Reported use of a matched placebo in control arm. Drugs were prepared by research pharmacists. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Authors stated data collectors and outcome adjudicators blinded to treatment assignment. Outcomes defined with explicit criteria. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes reported. Intention‐to‐treat analysis used with 7% dropout (6 clonidine and 4 placebo). |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Xu 2014.
| Methods | Randomized controlled trial comparing dexmedetomidine versus placebo. | |
| Participants | 80 participants aged 41‐75 yr, ASA II‐III and diagnosis of coronary heart disease undergoing elective hip surgery with an expected duration > 2 hr. Age (yr): mean (SD): dexmedetomidine group: 60 (5); placebo: 59 (6). Sex: 37 men, 43 women. Exclusion criteria: history of hypertension, hypotension, diabetes mellitus, arrhythmia, cerebrovascular disease, severe arrhythmia, heart failure or a combination; taking non‐steroidal anti‐inflammatory drugs and hormonal medications for underlying diseases; any known sensitivity to study medications or previous anaesthetic exposure within 1 year; abnormal preoperative liver and kidney function; abnormal levels of cTnI, GP‐BB and myocardial enzymes; and LVEF < 40%. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: grants from National Natural Science Foundation of China, Science and Technology Agency, Bureau of Chinese Medicine, Project of Medical Technology, Clinical Scientific Research of Medical Association and clinical scientific research fund of Chinese Medical Association. Declaration of interest: declared no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "This study was a prospective randomized double‐blind trial." Random sequence generation not discussed. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes used. Opened by anaesthesiologist not involved in care of participant. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Drug prepared by separate anaesthesiologist uninvolved in participant care or study. Equal volume of normal saline used in control, and likely it was matched. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded outcome adjudicator. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
Yin 2002.
| Methods | Randomized controlled trial comparing clonidine versus placebo. | |
| Participants | 60 people with coronary artery disease undergoing non‐cardiac surgery (10% vascular, 50% intraperitoneal, 27% orthopaedic). Age (yr): mean (SD): clonidine group: 63 (5); placebo group: 60 (6). Sex: 49 men, 11 women. Exclusion criteria: physical status other than ASA III, systemic hypotension (SBP < 90 mmHg), severe atrioventricular conduction block including second‐degree Mobitz type II and third‐degree AV block, left bundle branch block, implantation of cardiac pacemaker or chronic clonidine exposure. |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Funding: grant of National Science Council, Taiwan. Declaration of interest: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors described participants being prospectively randomized but provided no other details. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Authors described study as 'double‐blind.' Reported use of a placebo in control arm. Authors described that "anaesthesia providers in the study were blind to all research information." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Interpretation of all ECGs was performed by staff blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. |
| Selective reporting (reporting bias) | Low risk | Outcomes reported were concordant with methods. |
| Other bias | Low risk | None. |
AF: atrial fibrillation; ASA: American Society of Anesthesiologists; bpm: beats per minute; CABG: coronary artery bypass graft; CK‐MB: creatinine kinase ‐ MB; CPB: cardiopulmonary bypass; cTnI: cardiac troponin I; DBP: diastolic blood pressure; ECG: electrocardiogram; ECHO: echocardiogram; GP‐BB: glycogen phosphorylase BB; GFR: glomerular filtration rate; HR: heart rate; hr: hours; ICU: intensive care unit; IQR: interquartile range; IU: international units; IV: intravenous; LV: left ventricular; LVEF: left ventricular ejection fraction; MAP: mean arterial pressure; MI: myocardial infarction; min: minute; n: number; NYHA: New York Heart Association; OPCAB: off‐pump coronary artery bypass; PCI: percutaneous coronary intervention; RASS: Richmond Agitation‐Sedation Scale; SD: standard deviation; SBP: systolic blood pressure; sec: second; yr: year.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abd Aziz 2011 | Study objectives differed. |
| Abdalla 2003 | Study objectives differed. |
| Abdel‐Meguid 2013 | Study objectives differed. |
| Abdelmageed 2011 | Study objectives differed. |
| Aho 1991a | Study objectives differed. |
| Aho 1991b | Study objectives differed. |
| Aho 1992 | Study objectives differed. |
| Akin 2008 | Quasi‐randomized trial design (participants divided into 2 groups based on order in which they were admitted to the intensive care unit). |
| Akkaya 2014 | Study objectives differed. |
| Aldehayat 2011 | Study objectives differed. |
| Aliyeva 2009 | Study objectives differed. |
| Altan 2005 | Study objectives differed. |
| Altindis 2008 | Study objectives differed. |
| Amminikutty 2015 | Study objectives differed. |
| Anvaroglu 2008 | Study objectives differed. |
| Apitzsch 2000 | Study objectives differed. |
| Arain 2002 | Study objectives differed. |
| Arain 2004 | Study objectives differed. |
| Arora 2015 | Not randomized design. |
| Ayoglu 2007 | Study objectives differed. |
| Ayoglu 2008 | Study objectives differed. |
| Babu 2013 | Study objectives differed. |
| Bajwa 2011 | Study objectives differed. |
| Bajwa 2012 | Study objectives differed. |
| Bakan 2015 | Study objectives differed. |
| Bakhamees 2007 | Study objectives differed. |
| Bakri 2015 | Study objectives differed. |
| Balaraju 2013 | Study objectives differed. |
| Basar 2008 | Study objectives differed. |
| Batista 2015 | Study objectives differed. |
| Bayram 2011 | Study objectives differed. |
| Bayram 2012 | Study objectives differed. |
| Beg 2001 | Study objectives differed. |
| Beigh 2003 | Study objectives differed. |
| Bekker 2008 | Study objectives differed. |
| Bekker 2013 | Study objectives differed. |
| Benhamou 1994 | Study objectives differed. |
| Bernard 1991a | Study objectives differed. |
| Bernard 1991b | Study objectives differed. |
| Bernard 1993 | Study objectives differed. |
| Bernard 1994 | Study objectives differed. |
| Bhanderi 2014 | Study objectives differed. |
| Bharti 2010 | Study objectives differed. |
| Bharti 2013 | Study objectives differed. |
| Bhattacharjee 2010 | Study objectives differed. |
| Bicer 2006 | Study objectives differed. |
| Bindu 2013 | Study objectives differed. |
| Boldt 1996 | Scientific misconduct (Rasmussen 2011; Wise 2013). |
| Bouslama 2013 | Study objectives differed. |
| Bozgeyik 2014 | Study objectives differed. |
| Buggy 1997 | Study objectives differed. |
| Bulow 2007 | Study objectives differed. |
| Bulow 2016 | Study objectives differed. |
| But 2006 | Study objectives differed. |
| Campagni 1999 | Study objectives differed. |
| Carabine 1991a | Study objectives differed. |
| Carabine 1991b | Study objectives differed. |
| Carabine 1992 | Study objectives differed. |
| Caumo 2009 | Study objectives differed. |
| Ceballos 2011 | Study objectives differed. |
| Celebi 2013 | Study objectives differed. |
| Chadha 1992 | Study objectives differed. |
| Chaoba 2011 | Study objectives differed. |
| Chaturvedi 2014 | Not randomized design. |
| Chen 2013 | Study objectives differed. |
| Chen 2014 | Study objectives differed. |
| Chen 2014a | Study objectives differed. |
| Cheung 2011 | Study objectives differed. |
| Cheung 2014 | Study objectives differed. |
| Cho 2015 | Study objectives differed. |
| Chua 2010 | Study objectives differed. |
| Cindea 2012 | Study objectives differed. |
| Curtis 2002 | Study objectives differed. |
| De Deyne 2000 | Study objectives differed. |
| De Kock 1992 | Study objectives differed. |
| De Kock 1994 | Study objectives differed. |
| De Kock 1995 | Study objectives differed. |
| De la Mora‐Gonzalez 2012 | Not randomized design. |
| Delaunay 1991 | Study objectives differed. |
| Demirhan 2011 | Study objectives differed. |
| Dhorigol 2010 | Study objectives differed. |
| Dimou 2003 | Study objectives differed. |
| Doak 1993 | Study objectives differed. |
| Dobrydniov 1999 | Study objectives differed. |
| Dobrydnjov 2002 | Study objectives differed. |
| Dogan 2008 | Study objectives differed. |
| Dorman 1997 | Study objectives differed. |
| Durmus 2007 | Study objectives differed. |
| Eberhart 2000 | Study objectives differed. |
| El 2012 | Not randomized design. |
| Elkassem 2008 | Study objectives differed. |
| Elliott 1997 | Study objectives differed. |
| Ellis 1998 | Study objectives differed. |
| ElSheikh 2010 | Study objectives differed. |
| Elvan 2008 | Study objectives differed. |
| Engelman 1989 | Study objectives differed. |
| Eremenko 2014a | Study objectives differed. |
| Eremenko 2014b | Not randomized design. |
| Erkola 1994 | Study objectives differed. |
| Ezri 1998 | Study objectives differed. |
| Favre 1995 | Study objectives differed. |
| Fehr 2001 | Study objectives differed. |
| Feld 2003 | Study objectives differed. |
| Feld 2006 | Study objectives differed. |
| Feld 2007 | Study objectives differed. |
| Flacke 1987 | Study objectives differed. |
| Frank 1999 | Study objectives differed. |
| Frank 2000a | Study objectives differed. |
| Frank 2000b | Study objectives differed. |
| Frank 2002 | Study objectives differed. |
| Galindo 2008 | Study objectives differed. |
| Gandhi 2017 | Study objectives differed. |
| Ganter 2005 | Study objectives differed. |
| Gao 2012 | Study objectives differed. |
| Garcia‐Guiral 1994 | Study objectives differed. |
| Ghatak 2010 | Study objectives differed. |
| Ghosh 2008 | Study objectives differed. |
| Gomez‐Vazquez 2007 | Study objectives differed. |
| Goyagi 1996 | Study objectives differed. |
| Grottke 2003 | Study objectives differed. |
| Grundmann 1997 | Study objectives differed. |
| Guglielminotti 1998 | Study objectives differed. |
| Gupta 2011a | Study objectives differed. |
| Gupta 2011b | Study objectives differed. |
| Gupta 2011c | Study objectives differed. |
| Gupta 2012 | Study objectives differed. |
| Guven 2011 | Study objectives differed. |
| Hahm 2002 | Study objectives differed. |
| Hall 2006 | Participants did not undergo surgery. |
| Handa 2000 | Study objectives differed. |
| Harsoor 2013 | Study objectives differed. |
| Harsoor 2014 | Study objectives differed. |
| Hashemian 2017 | Study objectives differed. |
| Hazra 2014 | Study objectives differed. |
| Hidalgo 2005 | Study objectives differed. |
| Higuchi 2002 | Study objectives differed. |
| Honarmand 2007 | Study objectives differed. |
| Horn 1997 | Study objectives differed. |
| Horng 2007 | Study objectives differed. |
| Hwang 2015 | Study objectives differed. |
| Ishiyama 2006 | Study objectives differed. |
| Jaakola 1994 | Study objectives differed. |
| Jabalameli 2005 | Study objectives differed. |
| Javaherfroosh 2009 | Study objectives differed. |
| Jeffs 2002 | Study objectives differed. |
| Jellish 2001 | Study objectives differed. |
| Ji 2013 | Not randomized design. |
| Joao 2014 | Study objectives differed. |
| Joris 1993 | Study objectives differed. |
| Joris 1998 | Study objectives differed. |
| Joshi 2012 | Study objectives differed. |
| Juarez‐Pichardo 2009 | Study objectives differed. |
| Kajiyama 2009 | Not randomized design. |
| Kalajdzija 2011 | Study objectives differed. |
| Kang 2012 | Study objectives differed. |
| Kang 2013 | Study objectives differed. |
| Kang 2015 | Study objectives differed. |
| Karaman 2013 | Study objectives differed. |
| Karaman 2015 | Study objectives differed. |
| Kawasaki 2014 | Not randomized design. |
| Kaya 2010 | Study objectives differed. |
| Kaymak 2008 | Study objectives differed. |
| Ke 2013 | Study objectives differed. |
| Keniya 2011 | Study objectives differed. |
| Khafagy 2012 | Study objectives differed. |
| Kim 2012 | Study objectives differed. |
| Kim 2013a | Study objectives differed. |
| Kim 2013b | Study objectives differed. |
| Kim 2013c | Study objectives differed. |
| Kim 2014b | Not randomized design. |
| Korkmaz 2013 | Study objectives differed. |
| Koyuncu 2009 | Study objectives differed. |
| Kulka 1996 | Study objectives differed. |
| Kumari 2012 | Study objectives differed. |
| Lang 2011 | Study objectives differed. |
| Lattermann 2001 | Study objectives differed. |
| Launo 1991 | Study objectives differed. |
| Laurito 1991 | Study objectives differed. |
| Laurito 1993 | Study objectives differed. |
| Lawrence 1997 | Study objectives differed. |
| Le Guen 2014 | Study objectives differed. |
| Lee 2012 | Study objectives differed. |
| Lee 2013b | Study objectives differed. |
| Leino 2011 | Study objectives differed. |
| Levanen 1995 | Study objectives differed. |
| Li 2010 | Study objectives differed. |
| Li 2013 | Study objectives differed. |
| Liu 2013 | Study objectives differed. |
| Lu 2013 | Study objectives differed. |
| Lyons 1997 | Study objectives differed. |
| Ma 2013 | Study objectives differed. |
| Mahendru 2013 | Study objectives differed. |
| Maldonado 2009 | Study objectives differed. |
| Malek 2009 | Study objectives differed. |
| Malek 2010a | Not randomized design. |
| Malek 2010b | Study objectives differed. |
| Manne 2014 | Study objectives differed. |
| Mannion 2005 | Study objectives differed. |
| Marangoni 2005 | Study objectives differed. |
| Marchal 2001 | Study objectives differed. |
| Mariappan 2014 | Study objectives differed. |
| Marinangeli 2002 | Study objectives differed. |
| Martin 2003 | Half the participants underwent cardiac surgery and half underwent non‐cardiac surgery; therefore, it did not meet inclusion criteria for either subgroup (cardiac surgery versus non‐cardiac surgery subgroups). |
| Massad 2009 | Study objectives differed. |
| Mishra 2012 | Study objectives differed. |
| Mizrak 2010 | Study objectives differed. |
| Mizrak 2012 | Study objectives differed. |
| Mizrak 2013 | Study objectives differed. |
| Moghadam 2012 | Participants were known substance abusers. |
| Mohamed 2012 | Study objectives differed. |
| Mohamed 2013 | Control group did not meet inclusion criteria. |
| Mohammadi 2007 | Study objectives differed. |
| Mohammadi 2008 | Study objectives differed. |
| Mousa 2013 | Study objectives differed. |
| Muhammad 2012 | Study objectives differed. |
| Murari Sudre 2004 | Study objectives differed. |
| Myatra 2010 | Participants did not undergo surgery. |
| Nader 2001 | Study objectives differed. |
| Nader 2009 | Treatment given by alternative route. |
| Nakagawa 2001 | Study objectives differed. |
| Nitta 2013 | Study objectives differed. |
| Nour El‐Din 2004 | Study objectives differed. |
| Nunez‐Bacarreza 2006 | Study objectives differed. |
| Oddby‐Muhrbeck 2002 | Study objectives differed. |
| Ohata 1999 | Study objectives differed. |
| Ohtani 2008 | Study objectives differed. |
| Ohtani 2011 | Study objectives differed. |
| Okuyama 2005 | Study objectives differed. |
| Omote 1995 | Study objectives differed. |
| Onodera 2011 | Study objectives differed. |
| Owen 1997 | Study objectives differed. |
| Ozbakis 2008 | Study objectives differed. |
| Ozkose 2006 | Study objectives differed. |
| Panda 2012a | Study objectives differed. |
| Panda 2012b | Study objectives differed. |
| Pandazi 2011 | Study objectives differed. |
| Pant 2012 | Study objectives differed. |
| Parameswara 2012 | Study objectives differed. |
| Paris 2009 | Study objectives differed. |
| Park 1996 | Study objectives differed. |
| Park 2012 | Study objectives differed. |
| Parlow 1999 | Study objectives differed. |
| Patel 2012 | Study objectives differed. |
| Patil 2012 | Participants did not undergo surgery. |
| Pestilci 2015 | Study objectives differed. |
| Piper 1999 | Study objectives differed. |
| Piper 2004 | Study objectives differed. |
| Porkkala 1998 | Study objectives differed. |
| Pouttu 1987 | Study objectives differed. |
| Procaccini 1993 | Study objectives differed. |
| Quintin 1990 | Study objectives differed. |
| Quintin 1991a | Study objectives differed. |
| Quintin 1991b | Study objectives differed. |
| Raouf 2004 | Study objectives differed. |
| Ray 2010 | Study objectives differed. |
| Reddy 2013 | Study objectives differed. |
| Richa 2008 | Study objectives differed. |
| Rohrbach 1999 | Study objectives differed. |
| Rosenfeld 1993 | Study objectives differed. |
| Ruan 2011 | Study objectives differed. |
| Rubino 2010 | Study objectives differed. |
| Salgado 2008 | Study objectives differed. |
| Salgado Filho 2013 | Study objectives differed. |
| Samantaray 2012 | Study objectives differed. |
| Sassi 2013 | Study objectives differed. |
| Scheinin 1992 | Study objectives differed. |
| Schlimp 2011 | Study objectives differed. |
| Schreiberova 2008 | Study objectives differed. |
| Segal 1991 | Study objectives differed. |
| Selina 2011 | Not randomized design. |
| Senses 2013 | Study objectives differed. |
| Shams 2013 | Study objectives differed. |
| Shin 2012 | Study objectives differed. |
| Shin 2013 | Study objectives differed. |
| Shrestha 2012 | Study objectives differed. |
| Shukla 2011 | Study objectives differed. |
| Si 2011 | Study objectives differed. |
| Simoni 2009 | Study objectives differed. |
| Singh 2011 | Study objectives differed. |
| Singh 2013 | Study objectives differed. |
| Singh Bajwa 2012 | Study objectives differed. |
| Sitilci 2010 | Study objectives differed. |
| Solanki 2013 | Study objectives differed. |
| Soliman 2011 | Study objectives differed. |
| Stapelfeldt 2005 | Study objectives differed. |
| Stocche 2004 | Study objectives differed. |
| Striebel 1993 | Study objectives differed. |
| Sudar 2013 | Study objectives differed. |
| Sulemanji 2007 | Study objectives differed. |
| Sun 2013 | Study objectives differed. |
| Sung 2000 | Study objectives differed. |
| Taheri 2010 | Study objectives differed. |
| Taittonen 1997a | Study objectives differed. |
| Taittonen 1997b | Study objectives differed. |
| Taittonen 1998 | Study objectives differed. |
| Talke 1997 | Study objectives differed. |
| Tanskanen 2006 | Study objectives differed. |
| Techanivate 2012 | Study objectives differed. |
| Tekin 2007 | Study objectives differed. |
| Thomson 1998 | Study objectives differed. |
| Toz 2010 | Study objectives differed. |
| Traill 1993 | Study objectives differed. |
| Triltsch 2002 | Half the participants underwent cardiac surgery and half underwent non‐cardiac surgery; therefore, it did not meet inclusion criteria for either subgroup (cardiac surgery versus non‐cardiac surgery subgroups). |
| Tufanogullari 2008 | Study objectives differed. |
| Turgut 2009 | Study objectives differed. |
| Tzortzopoulou 2009 | Treatment given by alternative route. |
| Unlugenc 2005 | Study objectives differed. |
| Usta 2011 | Study objectives differed. |
| Uyar 2008 | Study objectives differed. |
| Vanderstappen 1996 | Study objectives differed. |
| von Dossow 2006 | Study objectives differed. |
| Vukovic 2012 | Study objectives differed. |
| Wahlander 2005 | Scientific misconduct (Anon 2013). |
| Wallenborn 2008 | Not randomized design. |
| Wan 2011 | Study objectives differed. |
| Wang 2012 | Study objectives differed. |
| Wawrzyniak 2013 | Study objectives differed. |
| Weilbach 2009 | Study objectives differed. |
| Wright 1990 | Study objectives differed. |
| Xu 2010 | Study objectives differed. |
| Xue 2014 | Not randomized design. |
| Yacout 2012 | Study objectives differed. |
| Yadav 2013 | Study objectives differed. |
| Yang 2013 | Study objectives differed. |
| Yektaz 2011a | Study objectives differed. |
| Yektaz 2011b | Study objectives differed. |
| Yldrm 2012 | Study objectives differed. |
| Yoganarasimha 2012 | Study objectives differed. |
| Yotsui 2001 | Study objectives differed. |
| Yu 2003 | Study objectives differed. |
| Zalunardo 2000 | Study objectives differed. |
| Zalunardo 2002 | Study objectives differed. |
| Zalunardo 2010 | Study objectives differed. |
| Zhang 2013a | Study objectives differed. |
| Zhang 2013b | Study objectives differed. |
| Zhou 2011 | Study objectives differed. |
Differences between protocol and review
Several changes have been made since the publication of the original protocol (Wijeysundera 2003).
Changes for the 2018 review.
The target population of 'all major surgery' was divided into the subgroups of cardiac and non‐cardiac surgical procedures for all analyses. This alteration was in response to comments from an editorial board member, who raised concerns about the significant clinical heterogeneity between cardiac versus non‐cardiac surgical procedures.
We did not include a planned subgroup analysis comparing α‐2 adrenergic agonists to control in people receiving epidural or spinal anaesthesia as there was only one trial (Oliver 1999).
The inclusion criteria were broadened to include studies that only reported the outcomes of acute stroke and HF, based on comments from an editorial board member.
Given the large influence of two large RCTs (Devereaux 2014a; Oliver 1999), we performed a post‐hoc sensitivity analysis that excluded these specific studies.
Based on based on comments received during the peer‐review process, we performed post‐hoc sensitivity analyses that excluded the two RCTs of mivazerol (McSPI‐Europe 1997; Oliver 1999) since it is not available for clinical use.
Based on comments received during the peer‐review process, we performed post‐hoc sensitivity analyses that excluded studies where data collection or enrolment occurred more than 20 years ago, specifically to assess for the potential influence of temporal advances in perioperative on pooled treatment effects.
The quality of evidence underlying the main estimated pooled treatment effects was assessed based on the GRADE methodology and presented in 'Summary of findings' tables.
Changes for 2009 review (Wijeysundera 2009):
Based on comments from a peer reviewer (Peter Alston), the title was changed from 'Alpha‐2 adrenergic agonists for the prevention of cardiovascular complications among patients undergoing cardiac or non‐cardiac surgery' to 'Alpha‐2 adrenergic agonists for the prevention of cardiac complications among patients undergoing surgery.'
-
We performed several post‐hoc analyses that were not specified in the original protocol.
A subgroup analysis was performed based on drug type to explain the moderate heterogeneity for the pooled effect of α‐2 adrenergic agonists on perioperative hypotension.
We performed a post‐hoc subgroup analysis based on surgical procedure to explain the significant heterogeneity for the pooled effect of α‐2 adrenergic agonists on perioperative bradycardia.
We have added acute stroke as a secondary outcome (side‐effect from treatment) based on comments from a peer‐reviewer (Helen Higham), and the results of the POISE‐1 trial (POISE 2008). Specifically, the POISE‐1 trial found that perioperative beta‐blockers caused a significantly increased risk of perioperative acute stroke.
Contributions of authors
Conceiving the review: DNW, WSB.
Coordinating the review: DNW.
Undertaking manual searches: DD, AS.
Screening search results: DD, AS.
Organizing retrieval of papers: DD, AS.
Screening retrieved papers against inclusion criteria: DD, AS.
Appraising quality of papers: DD, AS.
Abstracting data from papers: DD, AS.
Writing to authors of papers for additional information: DD, AS.
Data management for the review: DD.
Entering data into Review Manager 5 (RevMan 2014): DD, AS.
RevMan statistical data: DD.
Interpretation of data: DD, AS, WSB, DNW.
Statistical inferences: DD, AS, WSB, DNW.
Writing the review: DD, AS, WSB, DNW.
Performing previous work that was the foundation of the present study: DNW, WSB.
Guarantor for the review (one author): DNW.
Person responsible for reading and checking review before submission: DNW.
Sources of support
Internal sources
Department of Anesthesia, University of Toronto, Canada.
External sources
Canadian Institutes of Health Research, Canada.
Declarations of interest
DD: no conflict of interest.
AS: no conflicts of interest.
WSB: the senior author of one included study (Wijeysundera 2014a); however, he had no involvement in either the data abstraction or quality assessment process. This author had no other relevant conflicts of interest.
DNW: the lead author of one included study (Wijeysundera 2014a); however, he had no involvement in either the primary data abstraction or quality assessment process. This author had no other relevant conflicts of interest.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Abi‐Jaoude 1993 {published data only}
- Abi‐Jaoude F, Brusset A, Ceddaha A, Schlumberger S, Raffin L, Dubois C, et al. Clonidine premedication for coronary artery bypass grafting under high‐dose alfentanil anesthesia: intraoperative and postoperative hemodynamic study. Journal of Cardiothoracic and Vascular Anesthesia 1993;7(1):35‐40. [PUBMED: 8431573] [DOI] [PubMed] [Google Scholar]
- Brusset A, Abi‐Jaoude F, Ceddaha A, Schlumberger S, Raffin L, Fischler M. Clonidine aggravates the postoperative hemodynamic profile after coronary bypass: randomized, double‐blind study. Annales Françaises d'Anesthèsie et de Rèanimation 1989;8 Suppl:R215. [PUBMED: 2690688] [PubMed] [Google Scholar]
- Brusset A, Abi‐Jaoude F, Raffin L, Ceddaha A, Schlumberger S, Fischler M. Clonidine modifies the hemodynamic profile in coronary bypass: double‐blind, randomized study. Annales Françaises d'Anesthèsie et de Rèanimation 1989;8 Suppl:R214. [PUBMED: 2690687] [PubMed] [Google Scholar]
Ammar 2016 {published data only}
- Ammar A, Mahmoud K, Kasemy Z, Helwa M. Cardiac and renal protective effects of dexmedetomidine in cardiac surgeries: a randomized controlled trial. Saudi Journal of Anaesthesia 2016;10(4):395‐401. [EMBASE: 612358144] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bergese 2010 {published data only}
- Bergese S, Candiotti K, Bokesch P, Zura A, Wisemandle W, Bekker A. A phase IIIb, randomized, double‐blind, placebo‐controlled, multicenter study evaluating the safety and efficacy of dexmedetomidine for sedation during awake fiberoptic intubation. American Journal of Therapeutics 2010;17(6):586‐95. [PUBMED: 20535016] [DOI] [PubMed] [Google Scholar]
Chi 2016 {published data only}
- Chi X, Liao M, Chen X, Zhao Y, Yang L, Luo A, et al. Dexmedetomidine attenuates myocardial injury in off‐pump coronary artery bypass graft surgery. Journal of Cardiothoracic and Vascular Anesthesia 2016;30(1):44‐50. [PUBMED: 26429360] [DOI] [PubMed] [Google Scholar]
Cho 2016 {published data only}
- Cho JS, Shim JK, Soh S, Kim MK, Kwak YL. Perioperative dexmedetomidine reduces the incidence and severity of acute kidney injury following valvular heart surgery. Kidney International 2016;89(3):693‐700. [PUBMED: 26444030] [DOI] [PubMed] [Google Scholar]
Corbett 2005 {published data only}
- Corbett SM, Rebuck JA, Greene CM, Callas PW, Neale BW, Healey MA, et al. Dexmedetomidine does not improve patient satisfaction when compared with propofol during mechanical ventilation. Critical Care Medicine 2005;33(5):940‐5. [PUBMED: 15891317] [DOI] [PubMed] [Google Scholar]
Devereaux 2014a {published data only (unpublished sought but not used)}
- Devereaux PJ, Sessler DI, Leslie K, Kurz A, Mrkobrada M, Alonso‐Coello P, et al. Clonidine in patients undergoing noncardiac surgery. New England Journal of Medicine 2014;370(16):1504‐13. [PUBMED: 24679061] [DOI] [PubMed] [Google Scholar]
Djaiani 2016 {published data only}
- Djaiani G, Silverton N, Fedorko L, Carroll J, Styra R, Rao V, et al. Dexmedetomidine versus propofol sedation reduces delirium after cardiac surgery: a randomized controlled trial. Anesthesiology 2016;124(2):362‐8. [PUBMED: 26575144] [DOI] [PubMed] [Google Scholar]
Dorman 1993 {published data only}
- Dorman BH, Zucker JR, Verrier ED, Gartman DM, Slachman FN. Clonidine improves perioperative myocardial ischemia, reduces anesthetic requirement, and alters hemodynamic parameters in patients undergoing coronary artery bypass surgery. Journal of Cardiothoracic and Vascular Anesthesia 1993;7(4):386‐95. [PUBMED: 8400091] [DOI] [PubMed] [Google Scholar]
El‐Kerdawy 2004 {published data only}
- El‐Kardawy H, Gouda N, Kamal H, Doss L. Dexmedetomidine as anaesthetic adjunct for patients undergoing off‐pump coronary artery bypass grafting. Egyptian Journal of Anaesthesia 2004;20(1):29‐34. [EMBASE: 2004089519] [Google Scholar]
Ellis 1994 {published data only}
- Ellis JE, Drijvers G, Pedlow S, Laff SP, Sorrentino MJ, Foss JF, et al. Premedication with oral and transdermal clonidine provides safe and efficacious postoperative sympatholysis. Anesthesia and Analgesia 1994;79(6):1133‐40. [PUBMED: 7978438] [DOI] [PubMed] [Google Scholar]
Ghignone 1986 {published data only}
- Ghignone M, Quintin L, Duke PC, Kehler CH, Calvillo O. Effects of clonidine on narcotic requirements and hemodynamic response during induction of fentanyl anesthesia and endotracheal intubation. Anesthesiology 1986;64(1):36‐42. [PUBMED: 3942335] [DOI] [PubMed] [Google Scholar]
Ghignone 1987 {published data only}
- Ghignone M, Calvillo O, Quintin L. Anesthesia and hypertension: the effect of clonidine on perioperative hemodynamics and isoflurane requirements. Anesthesiology 1987;67(1):3‐10. [PUBMED: 3605732] [PubMed] [Google Scholar]
Helbo‐Hansen 1986 {published data only}
- Helbo‐Hansen S, Fletcher R, Lundberg D, Nordstrom L, Werner O, Stahl E, et al. Clonidine and the sympatico‐adrenal response to coronary artery by‐pass surgery. Acta Anaesthesiologica Scandinavica 1986;30(3):235‐42. [PUBMED: 3017039] [DOI] [PubMed] [Google Scholar]
Herr 2003 {published data only}
- Herr DL, Sum‐Ping ST, England M. ICU sedation after coronary artery bypass graft surgery: dexmedetomidine‐based versus propofol‐based sedation regimens. Journal of Cardiothoracic and Vascular Anesthesia 2003;17(5):576‐84. [PUBMED: 14579210] [DOI] [PubMed] [Google Scholar]
Jalonen 1997 {published data only}
- Jalonen J, Hynynen M, Kuitunen A, Heikkila H, Perttila J, Salmenpera M, et al. Dexmedetomidine as an anesthetic adjunct in coronary artery bypass grafting. Anesthesiology 1997;86(2):331‐45. [PUBMED: 9054252] [DOI] [PubMed] [Google Scholar]
Khalil 2013 {published data only}
- Khalil M, Abdel Azeem M. The impact of dexmedetomidine infusion in sparing morphine consumption in off‐pump coronary artery bypass grafting. Seminars in Cardiothoracic and Vascular Anesthesia 2013;17(1):66‐71. [PUBMED: 23108415] [DOI] [PubMed] [Google Scholar]
Kim 2014a {published data only}
- Kim HJ, Kim WH, Kim G, Kim E, Park MH, Shin BS, et al. A comparison among infusion of lidocaine and dexmedetomidine alone and in combination in subjects undergoing coronary artery bypass graft: a randomized trial. Contemporary Clinical Trials 2014;39(2):303‐9. [PUBMED: 25447444] [DOI] [PubMed] [Google Scholar]
Lee 2013a {published data only}
- Lee C, Kim YD, Kim JN. Antihyperalgesic effects of dexmedetomidine on high‐dose remifentanil‐induced hyperalgesia. Korean Journal of Anesthesiology 2013;64(4):301‐7. [PUBMED: 23646238] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2017 {published data only}
- Li X, Yang J, Nie XL, Zhang Y, Li XY, Li LH, et al. Impact of dexmedetomidine on the incidence of delirium in elderly patients after cardiac surgery: a randomized controlled trial. PloS One 2017;12(2):e0170757. [NCT02267538; PUBMED: 28182690] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lipszyc 1991 {published data only}
- Lipszyc M, Engelman E. Clonidine does not prevent myocardial ischemia during noncardiac surgery. Anesthesiology 1991;75:A93. [Google Scholar]
Liu 2016 {published data only}
- Liu X, Zhang K, Wang W, Xie G, Fang X. Dexmedetomidine sedation reduces atrial fibrillation after cardiac surgery compared to propofol: a randomized controlled trial. Critical Care 2016;20(1):298. [EMBASE: 612244138] [DOI] [PMC free article] [PubMed] [Google Scholar]
Loick 1999 {published data only}
- Loick HM, Schmidt C, Aken H, Junker R, Erren M, Berendes E, et al. High thoracic epidural anesthesia, but not clonidine, attenuates the perioperative stress response via sympatholysis and reduces the release of troponin T in patients undergoing coronary artery bypass grafting. Anesthesia and Analgesia 1999;88(4):701‐9. [PUBMED: 10195508] [DOI] [PubMed] [Google Scholar]
Matot 2000 {published data only}
- Matot I, Sichel JY, Yofe V, Gozal Y. The effect of clonidine premedication on hemodynamic responses to microlaryngoscopy and rigid bronchoscopy. Anesthesia and Analgesia 2000;91(4):828‐33. [PUBMED: 11004033] [DOI] [PubMed] [Google Scholar]
McSPI‐Europe 1997 {published data only}
- McSPI‐Europe Research Group. Perioperative sympatholysis: beneficial effects of the alpha 2‐adrenoceptor agonist mivazerol on hemodynamic stability and myocardial ischemia. Anesthesiology 1997;86(2):346‐63. [PUBMED: 9054253] [PubMed] [Google Scholar]
Myles 1999 {published data only}
- Myles PS, Hunt JO, Holdgaard HO, McRae R, Buckland MR, Moloney J, et al. Clonidine and cardiac surgery: haemodynamic and metabolic effects, myocardial ischaemia and recovery. Anaesthesia and Intensive Care 1999;27(2):137‐47. [PUBMED: 10212709] [DOI] [PubMed] [Google Scholar]
Oliver 1999 {published data only}
- Oliver MF, Goldman L, Julian DG, Holme I. Effect of mivazerol on perioperative cardiac complications during non‐cardiac surgery in patients with coronary heart disease: the European Mivazerol Trial (EMIT). Anesthesiology 1999;91(4):951‐61. [PUBMED: 10519497] [DOI] [PubMed] [Google Scholar]
Park 2014 {published data only}
- Park J, Bang S, Chee H, Kim J, Lee S, Shin J. Efficacy and safety of dexmedetomidine for postoperative delirium in adult cardiac surgery on cardiopulmonary bypass. Korean Journal of Thoracic and Cardiovascular Surgery 2014;47(3):249‐54. [CENTRAL: 01305779] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2016 {published data only}
- Patel J, Thosani R, Kothari J, Garg P, Pandya H. Clonidine and ketamine for stable hemodynamics in off‐pump coronary artery bypass. Asian Cardiovascular and Thoracic Annals 2016;24(7):638‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pawlik 2005 {published data only}
- Pawlik MT, Hansen E, Waldhauser D, Selig C, Kuehnel TS. Clonidine premedication in patients with sleep apnea syndrome: a randomized, double‐blind, placebo‐controlled study. Anesthesia and Analgesia 2005;101(5):1374‐80. [PUBMED: 16243997] [DOI] [PubMed] [Google Scholar]
Pluskwa 1991 {published data only}
- Pluskwa F, Bonnet F, Saada M, Macquin‐Mavier I, Becquemin JP, Catoire P. Effects of clonidine on variation of arterial blood pressure and heart rate during carotid artery surgery. Journal of Cardiothoracic and Vascular Anesthesia 1991;5(5):431‐36. [PUBMED: 1932647] [DOI] [PubMed] [Google Scholar]
Quintin 1993 {published data only}
- Quintin L, Cicala R, Kent M, Thomsen B. Effect of clonidine on myocardial ischaemia: a double‐blind pilot trial. Canadian Journal of Anaesthesia 1993;40(1):85‐6. [PUBMED: 7980723] [DOI] [PubMed] [Google Scholar]
Quintin 1996 {published data only}
- Quintin L, Bouilloc X, Butin E, Bayon MC, Brudon JR, Levron JC, et al. Clonidine for major vascular surgery in hypertensive patients: a double‐blind, controlled, randomized study. Anesthesia and Analgesia 1996;83(4):687‐95. [PUBMED: 8831304] [DOI] [PubMed] [Google Scholar]
Ren 2013 {published data only}
- Ren J, Zhang H, Huang L, Liu Y, Liu F, Dong Z. Protective effect of dexmedetomidine in coronary artery bypass grafting surgery. Experimental and Therapeutic Medicine 2013;6(2):497‐502. [PUBMED: 24137215] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shehabi 2009 {published data only}
- Shehabi Y, Grant P, Wolfenden H, Hammond N, Bass F, Campbell M, et al. Prevalence of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: a randomized controlled trial (DEXmedetomidine compared to morphine‐DEXCOM study). Anesthesiology 2009;111(5):1075‐84. [PUBMED: 19786862] [DOI] [PubMed] [Google Scholar]
Soliman 2016 {published data only}
- Soliman R, Zohry G. The myocardial protective effect of dexmedetomidine in high‐risk patients undergoing aortic vascular surgery. Annals of Cardiac Anaesthesia 2016;19(4):606‐13. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stuhmeier 1996 {published data only}
- Stuhmeier KD, Mainzer B, Cierpka J, Sandmann W, Tarnow J. Small, oral dose of clonidine reduces the incidence of intraoperative myocardial ischemia in patients having vascular surgery. Anesthesiology 1996;85(4):706‐12. [PUBMED: 8873539] [DOI] [PubMed] [Google Scholar]
Su 2016 {published data only}
- Su X, Meng ZT, Wu XH, Cui F, Li HL, Wang DX, et al. Dexmedetomidine for prevention of delirium in elderly patients after non‐cardiac surgery: a randomised, double‐blind, placebo‐controlled trial. Lancet 2016;388(10054):1893‐902. [EMBASE: 613271791] [DOI] [PubMed] [Google Scholar]
Talke 1995 {published data only}
- Talke P, Li J, Jain U, Leung J, Drasner K, Hollenberg M, et al. Effects of perioperative dexmedetomidine infusion in patients undergoing vascular surgery. Anesthesiology 1995;82(3):620‐33. [PUBMED: 7879930] [DOI] [PubMed] [Google Scholar]
Talke 2000 {published data only}
- Talke P, Chen R, Thomas B, Aggarwall A, Gottlieb A, Thorborg P, et al. The hemodynamic and adrenergic effects of perioperative dexmedetomidine infusion after vascular surgery. Anesthesia and Analgesia 2000;90(4):834‐9. [PUBMED: 10735784] [DOI] [PubMed] [Google Scholar]
Venn 1999 {published data only}
- Venn RM, Bradshaw CJ, Spencer R, Brealey D, Caudwell E, Naughton C, et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia 1999;54(12):1136‐42. [PUBMED: 10594409] [DOI] [PubMed] [Google Scholar]
- Venn RM, Hell J, Grounds RM. Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Critical Care 2000;4(5):302‐8. [PUBMED: 11056756] [DOI] [PMC free article] [PubMed] [Google Scholar]
Venn 2001 {published data only}
- Venn RM, Bryant A, Hall GM, Grounds RM. Effects of dexmedetomidine on adrenocortical function, and the cardiovascular, endocrine and inflammatory responses in post‐operative patients needing sedation in the intensive care unit. British Journal of Anaesthesia 2001;86(5):650‐6. [PUBMED: 11575340] [DOI] [PubMed] [Google Scholar]
- Venn RM, Grounds RM. Comparison between dexmedetomidine and propofol for sedation in the intensive care unit: patient and clinician perceptions. British Journal of Anaesthesia 2001;87(5):684‐90. [PUBMED: 11878517] [DOI] [PubMed] [Google Scholar]
Viviano 2012 {published data only}
- Viviano E, Renius M, Ruckert J, C, Bloch A, Meisel C, Harbeck‐seu A, et al. Selective neurogenic blockade and perioperative immune reactivity in patients undergoing lung resection. Journal of International Medical Research 2012;40(1):141‐56. [EMBASE: 2012137611; PUBMED: 22429354] [DOI] [PubMed] [Google Scholar]
Wallace 2004 {published data only}
- Wallace AW, Galindez D, Salahieh A, Layug EL, Lazo EA, Haratonik KA, et al. Effects of clonidine on cardiovascular morbidity and mortality after noncardiac surgery. Anesthesiology 2004;101(2):284‐93. [PUBMED: 15277909] [DOI] [PubMed] [Google Scholar]
Wijeysundera 2014a {published data only}
- Wijeysundera DN, Choi PT, Badner NH, Brasher PM, Dresser GK, Delgado DH, et al. A randomized feasibility trial of clonidine to reduce perioperative cardiac risk in patients on chronic beta‐blockade: the EPIC study. Canadian Journal of Anaesthesia 2014;61(11):995‐1003. [PUBMED: 25189430] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 2014 {published data only}
- Xu L, Hu Z, Shen J, McQuillan PM. Does dexmedetomidine have a cardiac protective effect during non‐cardiac surgery? A randomised controlled trial. Clinical and Experimental Pharmacology and Physiology 2014;41(11):879‐83. [EMBASE: 2014925423; PUBMED: 25132247] [DOI] [PubMed] [Google Scholar]
Yin 2002 {published data only}
- Yin YC, Chow LH, Tsao CM, Chu CC, Tsou MY, Chan KH, et al. Oral clonidine reduces myocardial ischemia in patients with coronary artery disease undergoing noncardiac surgery. Acta Anaesthesiologica Sinica 2002;40(4):197‐203. [PUBMED: 12596619] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdalla 2003 {published data only}
- Abdalla N, Soliman AH. The effects of dexmedetomidine premedication on cortisol and interleukin‐6 in patients undergoing major abdominal surgery. Egyptian Journal of Anaesthesia 2003;19(3):283‐90. [EMBASE: 2003448440] [Google Scholar]
Abd Aziz 2011 {published data only}
- Abd Aziz N, Chue MC, Yong CY, Hassan Y, Awaisu A, Hassan J, et al. Efficacy and safety of dexmedetomidine versus morphine in post‐operative cardiac surgery patients. International Journal of Clinical Pharmacy 2011;33(2):150‐4. [PUBMED: 21744187] [DOI] [PubMed] [Google Scholar]
Abdelmageed 2011 {published data only}
- Abdelmageed WM, Elquesny KM, Shabana RI, Abushama HM, Nassar AM. Analgesic properties of a dexmedetomidine infusion after uvulopalatopharyngoplasty in patients with obstructive sleep apnea. Saudi Journal of Anaesthesia 2011;5(2):150‐6. [EMBASE: 2011405475; PUBMED: 21804794] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Abdel‐Meguid 2013 {published data only}
- Abdel‐Meguid ME. Dexmedetomidine as anesthetic adjunct for fast tracking and pain control in off‐pump coronary artery bypass. Saudi Journal of Anaesthesia 2013;7(1):6‐8. [EMBASE: 2013272421; PUBMED: 23717223] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Aho 1991a {published data only}
- Aho M, Lehtinen A‐M, Erkola O, Kallio A, Korttila K. The effect of intravenously administered dexmedetomidine on perioperative hemodynamics and isoflurane requirements in patients undergoing abdominal hysterectomy. Anesthesiology 1991;74(6):997‐1002. [PUBMED: 1675042] [DOI] [PubMed] [Google Scholar]
Aho 1991b {published data only}
- Aho MS, Erkola OA, Scheinin H, Lehtinen AM, Korttila KT. Effect of intravenously administered dexmedetomidine on pain after laparoscopic tubal ligation. Anesthesia and Analgesia 1991;73:112‐8. [PUBMED: 1854025] [DOI] [PubMed] [Google Scholar]
Aho 1992 {published data only}
- Aho M, Scheinin M, Lehtinen AM, Erkola O, Vuorinen J, Korttila K. Intramuscularly administered dexmedetomidine attenuates hemodynamic and stress hormone responses to gynecologic laparoscopy. Anesthesia and Analgesia 1992;75(6):932‐9. [PUBMED: 1359808] [PubMed] [Google Scholar]
Akin 2008 {published data only}
- Akin S, Aribogan A, Arslan G. Dexmedetomidine as an adjunct to epidural analgesia after abdominal surgery in elderly intensive care patients: A prospective, double‐blind, clinical trial. Current Therapeutic Research ‐ Clinical and Experimental 2008;69(1):16‐28. [EMBASE: 2008152548; PUBMED: 24692779] [DOI] [PMC free article] [PubMed] [Google Scholar]
Akkaya 2014 {published data only}
- Akkaya A, Tekelioglu UY, Demirhan A, Bilgi M, Yildiz I, Apuhan T, et al. Comparison of the effects of magnesium sulphate and dexmedetomidine on surgical vision quality in endoscopic sinus surgery: Randomized clinical study. [Portuguese]. Revista Brasileira de Anestesiologia 2014;64(6):406‐12. [EMBASE: 2015921311; PUBMED: 25437697] [DOI] [PubMed] [Google Scholar]
Aldehayat 2011 {published data only}
- Aldehayat G. Intraoperative dexmedetomidine administration at the end of surgery prevents post anesthetic shivering. Rawal Medical Journal 2011;36(4):274‐6. [EMBASE: 2011658011] [Google Scholar]
Aliyeva 2009 {published data only}
- Aliyeva A, Gunusen I, Karaman S, Firat V. Effects of two different doses of dexmedetomidine on intraoperative desflurane consumption, hemodynamic parameters and neuromuscular blockade. [Turkish]. Erciyes Tip Dergisi 2009;31(2):110‐8. [EMBASE: 2010018778] [Google Scholar]
Altan 2005 {published data only}
- Altan A, Turgut N, Yildiz F, Turkmen A, Ustun H. Effects of magnesium sulphate and clonidine on propofol consumption, haemodynamics and postoperative recovery. British Journal of Anaesthesia 2005;94(4):438‐41. [PUBMED: 15653705] [DOI] [PubMed] [Google Scholar]
Altindis 2008 {published data only}
- Altindis NT, Karaaslan D, Peker TT, Ozmen S, Bulbul M. Comparison of meperidine alone with meperidine plus dexmedetomidine for postoperative patient‐controlled analgesia. Neurosciences 2008;13(2):117‐21. [EMBASE: 2008302337; PUBMED: 21063303] [PubMed] [Google Scholar]
Amminikutty 2015 {published data only}
- Amminikutty CM, Biji KP. Study comparing preemptive analgesic effects of oral Gabapentin and Clonidine against placebo in total abdominal hysterectomy under combined spinal epidural anaesthesia. International Journal of Pharma and Bio Sciences 2015;6(3):P290‐5. [EMBASE: 2015220621] [Google Scholar]
Anvaroglu 2008 {published data only}
- Anvaroglu R, Kelsaka E, Sarihasan B, Demirkaya M, Ustun E, Ulger F. The effect of intravenous dexmedetomidine premedication on hemodynamic response during endotracheal intubation and extubation and postoperative analgesic consumption. [Turkish]. Anestezi Dergisi 2008;16(4):201‐5. [EMBASE: 2009075187] [Google Scholar]
Apitzsch 2000 {published data only}
- Apitzsch H, Olthoff D, Thieme V, Vetter V, Wiegel M. The effects of perioperative continuous administration of mivazerol on early postoperative haemodynamics and plasma catecholamines after major surgery. Anasthesiologie, Intensivmedizin, Notfallmedizin, Schmerztherapie 2000;35(8):515‐22. [PUBMED: 10992963] [DOI] [PubMed] [Google Scholar]
Arain 2002 {published data only}
- Arain SR, Ebert TJ. The efficacy, side effects, and recovery characteristics of dexmedetomidine versus propofol when used for intraoperative sedation. Anesthesia and Analgesia 2002;95(2):461‐6. [PUBMED: 12145072] [DOI] [PubMed] [Google Scholar]
Arain 2004 {published data only}
- Arain SR, Ruehlow RM, Uhrich TD, Ebert TJ. The efficacy of dexmedetomidine versus morphine for postoperative analgesia after major inpatient surgery. Anesthesia and Analgesia 2004;98(1):153‐8. [PUBMED: 14693611] [DOI] [PubMed] [Google Scholar]
Arora 2015 {published data only}
- Arora S, Kulkarni A, Bhargava AK. Attenuation of hemodynamic response to laryngoscopy and orotracheal intubation using intravenous clonidine. Journal of Anaesthesiology Clinical Pharmacology 2015;31(1):110‐4. [EMBASE: 2015736900; PUBMED: 25788783] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ayoglu 2007 {published data only}
- Ayoglu H, Altunkaya H, Ozer Y, Yapakci O, Cukdar G, Ozkocak I. Does dexmedetomidine reduce the injection pain due to propofol and rocuronium?. European Journal of Anaesthesiology 2007;24(6):541‐5. [PUBMED: 17241503] [DOI] [PubMed] [Google Scholar]
Ayoglu 2008 {published data only}
- Ayoglu H, Yapakci O, Ugur MB, Uzun L, Altunkaya H, Ozer Y, et al. Effectiveness of dexmedetomidine in reducing bleeding during septoplasty and tympanoplasty operations. Journal of Clinical Anesthesia 2008;20(6):437‐41. [EMBASE: 2008461522; PUBMED: 18929284] [DOI] [PubMed] [Google Scholar]
Babu 2013 {published data only}
- Babu M, Verma A, Agarwal A, Tyagi C, Upadhyay M, Tripathi S. A comparative study in the post‐operative spine surgeries: Epidural ropivacaine with dexmedetomidine and ropivacaine with clonidine for post‐operative analgesia. Indian Journal of Anaesthesia 2013;57(4):371‐6. [EMBASE: 2013673831; PUBMED: 24163451] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bajwa 2011 {published data only}
- Bajwa SJS, Arora V, Kaur J, Singh A, Parmar SS. Comparative evaluation of dexmedetomidine and fentanyl for epidural analgesia in lower limb orthopedic surgeries. Saudi Journal of Anaesthesia 2011;5(4):365‐70. [EMBASE: 2011640719; PUBMED: 22144922] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bajwa 2012 {published data only}
- Bajwa S, Kaur J, Singh A, Parmar S, Singh G, Kulshrestha A, et al. Attenuation of pressor response and dose sparing of opioids and anaesthetics with pre‐operative dexmedetomidine. Indian Journal of Anaesthesia 2012;56(2):123‐8. [EMBASE: 2012406418; PUBMED: 22701201] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bakan 2015 {published data only}
- Bakan M, Umutoglu T, Topuz U, Uysal H, Bayram M, Kadioglu H, et al. Opioid‐free total intravenous anesthesia with propofol, dexmedetomidine and lidocaine infusions for laparoscopic cholecystectomy: a prospective, randomized, double‐blinded study. [Portuguese]. Revista Brasileira de Anestesiologia 2015;65(3):191‐9. [EMBASE: 2015796275; PUBMED: 25990496] [DOI] [PubMed] [Google Scholar]
Bakhamees 2007 {published data only}
- Bakhamees HS, El‐Halafawy YM, El‐Kerdawy HM, Gouda NM, Altemyatt S. Effects of dexmedetomidine in morbidly obese patients undergoing laparoscopic gastric bypass. Middle East Journal of Anesthesiology 2007;19:537‐51. [PUBMED: 18044282] [PubMed] [Google Scholar]
Bakri 2015 {published data only}
- Bakri MH, Ismail EA, Ibrahim A. Comparison of dexmedetomidine and dexamethasone for prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy. Korean Journal of Anesthesiology 2015;68(3):254‐60. [EMBASE: 2015102998; PUBMED: 26045928] [DOI] [PMC free article] [PubMed] [Google Scholar]
Balaraju 2013 {published data only}
- Balaraju TC, Ramdas B, Thomas R, Garg A, Sreekantha, Yogish B, et al. Comparative evaluation of oral clonidine and intravenous clonidine premedication in functional endoscopic sinus surgery. International Journal of Pharma and Bio Sciences 2013;4(1):B587‐91. [EMBASE: 2013473514] [Google Scholar]
Basar 2008 {published data only}
- Basar H, Akpinar S, Doganci N, Buyukkocak U, Kaymak C, Sert O, et al. The effects of preanesthetic, single‐dose dexmedetomidine on induction, hemodynamic, and cardiovascular parameters. Journal of Clinical Anesthesia 2008;20(6):431‐6. [EMBASE: 2008461521; PUBMED: 18929283] [DOI] [PubMed] [Google Scholar]
Batista 2015 {published data only}
- Batista HMT, Bezerra IMP, Abreu LC. Effect of clonidine on the target dose of propofol: Bispectral index evaluation. International Archives of Medicine 2015;8(1):1‐9. [CENTRAL: CN‐01074279; EMBASE: 2015058251] [Google Scholar]
Bayram 2011 {published data only}
- Bayram A, Esmaoglu A, Akin A, Baskol G, Aksu R, Bicer C, et al. The effects of intraoperative infusion of dexmedetomidine on early renal function after percutaneous nephrolithotomy. Acta Anaesthesiologica Scandinavica 2011;55(5):539‐44. [EMBASE: 2011204716; PUBMED: 21827441] [DOI] [PubMed] [Google Scholar]
Bayram 2012 {published data only}
- Bayram A, Altuntas R, Ulgey A, Gunes I, Akin A, Esmaoglu A, et al. Comparison between dexmedetomidine and remifentanil for controlled hypotension in patients scheduled for tympanoplasty. Erciyes Tip Dergisi 2012;34(2):65‐8. [EMBASE: 2012410017] [Google Scholar]
Beg 2001 {published data only}
- Beg AA, Saleena K, Naqeeb AJ, Dar BA, Sofi FA. Effect of oral clonidine premedication on anxiety and sedation in patients undergoing TURP under spinal anaesthesia. JK Practitioner : a Journal of Current Clinical Medicine & Surgery 2001;8:15‐7. [EMBASE: 2001101034] [Google Scholar]
Beigh 2003 {published data only}
- Beigh A, Naqeeb A, Khan FA. Haemodynamic and analgesic effect of oral clonidine on subarachanoid block with lidocaine. Journal of Anaesthesiology Clinical Pharmacology 2003;19:389‐93. [EMBASE: 2005003169] [Google Scholar]
Bekker 2008 {published data only}
- Bekker A, Sturaitis M, Bloom M, Moric M, Golfinos J, Parker E, et al. The effect of dexmedetomidine on perioperative hemodynamics in patients undergoing craniotomy. Anesthesia and Analgesia 2008;107(4):1340‐7. [EMBASE: 2009306675; PUBMED: 18806050] [DOI] [PubMed] [Google Scholar]
Bekker 2013 {published data only}
- Bekker A, Haile M, Kline R, Didehvar S, Babu R, Martiniuk F, et al. The effect of intraoperative infusion of dexmedetomidine on the quality of recovery after major spinal surgery. Journal of Neurosurgical Anesthesiology 2013;25(1):16‐24. [EMBASE: 2013041174; PUBMED: 22824921] [DOI] [PMC free article] [PubMed] [Google Scholar]
Benhamou 1994 {published data only}
- Benhamou D, Narchi P, Hamza J, Marx M, Peyrol MT, Sembeil F. Addition of oral clonidine to postoperative patient‐controlled analgesia with i.v. morphine. British Journal of Anaesthesia 1994;72(5):537‐40. [PUBMED: 8198904] [DOI] [PubMed] [Google Scholar]
Bernard 1991a {published data only}
- Bernard JM, Hommeril J‐L, Passuti N, Pinaud M. Postoperative analgesia by intravenous clonidine. Anesthesiology 1991;75(4):577‐82. [PUBMED: 1928767] [DOI] [PubMed] [Google Scholar]
Bernard 1991b {published data only}
- Bernard JM, Bourreli B, Hommeril JL, Pinaud M. Effects of oral clonidine premedication and postoperative i.v. infusion on haemodynamic and adrenergic responses during recovery from anaesthesia. Acta Anaesthesiologica Scandinavica 1991;35(1):54‐9. [PUBMED: 2006600] [DOI] [PubMed] [Google Scholar]
Bernard 1993 {published data only}
- Bernard JM, Hommeril JL, Legendre MP, Passuti N, Pinaud M. Spinal or systemic analgesia after extensive spinal surgery: comparison between intrathecal morphine and intravenous fentanyl plus clonidine. Journal of Clinical Anesthesia 1993;5(3):231‐6. [PUBMED: 8318243] [DOI] [PubMed] [Google Scholar]
Bernard 1994 {published data only}
- Bernard JM, Lagarde D, Souron R. Balanced postoperative analgesia: effect of intravenous clonidine on blood gases and pharmacokinetics of intravenous fentanyl. Anesthesia and Analgesia 1994;79(6):1126‐32. [PUBMED: 7978437] [DOI] [PubMed] [Google Scholar]
Bhanderi 2014 {published data only}
- Bhanderi D, Shah C, Shah B, Mandowara N. Comparison of iv dexmedetomidine V/S iv clonidine In hemodynamic stability in laparoscopic surgery. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2014;5(4):910‐7. [EMBASE: 2014495017] [Google Scholar]
Bharti 2010 {published data only}
- Bharti N, Bala SDI, Singh G. Effect of clonidine on postoperative pain: comparison of intravenous administration versus wound infiltration. Anaesthesia and Intensive Care 2010;38(6):1110‐1. [EMBASE: 71053337] [Google Scholar]
Bharti 2013 {published data only}
- Bharti N, Dontukurthy S, Bala I, Singh G. Postoperative analgesic effect of intravenous (i.v.) clonidine compared with clonidine administration in wound infiltration for open cholecystectomy. British Journal of Anaesthesia 2013;111(4):656‐61. [PUBMED: 23704191] [DOI] [PubMed] [Google Scholar]
Bhattacharjee 2010 {published data only}
- Bhattacharjee DP, Nayek SK, Dawn S, Bandopadhyay G, Gupta K. Effects of dexmedetomidine on haemodynamics in patients undergoing laparoscopic cholecystectomy‐ A comparative study. Journal of Anaesthesiology Clinical Pharmacology 2010;26(1):45‐8. [EMBASE: 2010114400] [Google Scholar]
Bicer 2006 {published data only}
- Bicer C, Esmaoglu A, Akin A, Boyaci A. Dexmedetomidine and meperidine prevent postanaesthetic shivering. European Journal of Anaesthesiology 2006;23:149‐53. [PUBMED: 16426470] [DOI] [PubMed] [Google Scholar]
Bindu 2013 {published data only}
- Bindu B, Pasupuleti S, Gowd UP, Gorre V, Murthy RR, Laxmi MB. A double blind, randomized, controlled trial to study the effect of dexmedetomidine on hemodynamic and recovery responses during tracheal extubation. Journal of Anaesthesiology Clinical Pharmacology 2013;29(2):162‐7. [EMBASE: 2013349348; PUBMED: 23878434] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boldt 1996 {published data only}
- Boldt J, Rothe G, Schindler E, Doll C, Gorlach G, Hempelmann G. Can clonidine, enoximone, and enalaprilat help to protect the myocardium against ischaemia in cardiac surgery?. Heart 1996;76(3):207‐13. [PUBMED: 8868976] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bouslama 2013 {published data only}
- Bouslama A, Echehoumi H, Smairi S, Ben Jazia K. A single intravenous dose of clonidine (4 mug/kg) given before induction reduces postoperative nausea and vomiting in patients after myomectomy under general anesthesia. European Journal of Anaesthesiology 2013;30:148. [EMBASE: 71315902] [Google Scholar]
Bozgeyik 2014 {published data only}
- Bozgeyik S, Mizrak A, Kilic E, Yendi F, Ugur B. The Effects of Preemptive Tramadol and Dexmedetomidine on Shivering during Arthroscopy. Saudi Journal of Anaesthesia 2014;8(2):238‐43. [EMBASE: 2014306876; PUBMED: 24843340] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buggy 1997 {published data only}
- Buggy D, Higgins P, Moran C, O'Donovan F, McCarroll M. Clonidine at induction reduces shivering after general anaesthesia. Canadian Journal of Anaesthesia 1997;44(3):263‐7. [PUBMED: 9067044] [DOI] [PubMed] [Google Scholar]
Bulow 2007 {published data only}
- Bulow NM, Barbosa NV, Rocha JB. Opioid consumption in total intravenous anesthesia is reduced with dexmedetomidine: a comparative study with remifentanil in gynecologic videolaparoscopic surgery. Journal of Clinical Anesthesia 2007;19(4):280‐5. [PUBMED: 17572323] [DOI] [PubMed] [Google Scholar]
Bulow 2016 {published data only}
- Bulow NMH, Colpo E, Pereira RP, Correa EFM, Waczuk EP, Duarte MF, et al. Dexmedetomidine decreases the inflammatory response to myocardial surgery under mini‐cardiopulmonary bypass. Brazilian Journal of Medical and Biological Research 2016;49(4):e4646. [EMBASE: 20160167871; PUBMED: 26909786] [DOI] [PMC free article] [PubMed] [Google Scholar]
But 2006 {published data only}
- But AK, Ozgul U, Erdil F, Gulhas N, Toprak HI, Durmus M, et al. The effects of pre‐operative dexmedetomidine infusion on hemodynamics in patients with pulmonary hypertension undergoing mitral valve replacement surgery. Acta Anaesthesiologica Scandinavica 2006;50(10):1207‐12. [PUBMED: 16978159] [DOI] [PubMed] [Google Scholar]
Campagni 1999 {published data only}
- Campagni MA, Howie MB, White PF, McSweeney TD. Comparative effects of oral clonidine and intravenous esmolol in attenuating the hemodynamic response to epinephrine injection. Journal of Clinical Anesthesia 1999;11(3):208‐15. [PUBMED: 10434216] [DOI] [PubMed] [Google Scholar]
Carabine 1991a {published data only}
- Carabine UA, Milligan KR, Moore JA. Adrenergic modulation of preoperative anxiety: a comparison of temazepam, clonidine, and timolol. Anesthesia and Analgesia 1991;73(5):633‐7. [PUBMED: 1683183] [DOI] [PubMed] [Google Scholar]
Carabine 1991b {published data only}
- Carabine UA, Wright PMC, Moore J. Preanaesthetic medication with clonidine: a dose‐response study. British Journal of Anaesthesia 1991;67(1):79‐83. [PUBMED: 1859765] [DOI] [PubMed] [Google Scholar]
Carabine 1992 {published data only}
- Carabine UA, Allen RW, Moore J. Partial attenuation of the pressor response to endotracheal intubation. A comparison of the effects of intravenous clonidine and fentanyl. European Journal of Anaesthesiology 1992;9(4):325‐9. [PUBMED: 1628636] [PubMed] [Google Scholar]
Caumo 2009 {published data only}
- Caumo W, Levandovski R, Hidalgo MPL. Preoperative anxiolytic effect of melatonin and clonidine on postoperative pain and morphine consumption in patients undergoing abdominal hysterectomy: a double‐blind, randomized, placebo‐controlled study. Journal of Pain 2009;10(1):100‐8. [EMBASE: 2009000997; PUBMED: 19010741] [DOI] [PubMed] [Google Scholar]
Ceballos 2011 {published data only}
- Ceballos Caballero M, Osorio Rodriguez G. Anesthesia for bariatric surgery: fentanyl versus fentanyl and dexmedetomidina. [Spanish]. Neurologia, Neurocirugia y Psiquiatria 2011;44(4):114‐21. [EMBASE: 2012108220] [Google Scholar]
Celebi 2013 {published data only}
- Celebi N, Canbay O, Cil H, Ayaz A. Effects of dexmedetomidine on succinylcholine‐induced myalgia in the early postoperative period. Saudi Medical Journal 2013;34(4):369‐73. [EMBASE: 2013229731; PUBMED: 23552589] [PubMed] [Google Scholar]
Chadha 1992 {published data only}
- Chadha R, Padmanabhan V, Joseph A, Mohandas K. Oral clonidine pretreatment for haemodynamic stability during craniotomy. Anaesthesia and Intensive Care 1992;20(3):341‐4. [PUBMED: 1524175] [DOI] [PubMed] [Google Scholar]
Chaoba 2011 {published data only}
- Singh CL, Devi AN, Singh RN, Laithangbam P, Singh MK. The effect of intrathecal clonidine on hyperbaric bupivacaine for postoperative analgesia. JMS ‐ Journal of Medical Society 2011;25(3):29‐33. [EMBASE: 2013558752] [Google Scholar]
Chaturvedi 2014 {published data only}
- Chaturvedi A, Jain V, Pandaya MP. Effect of dexmedetomidine on postoperative pain and recovery in patients undergoing cervical spine surgery. Pain Practice 2014;14:88. [EMBASE: 71512674] [Google Scholar]
Chen 2013 {published data only}
- Chen J, Yan J, Han X. Dexmedetomidine may benefit cognitive function after laparoscopic cholecystectomy in elderly patients. Experimental and Therapeutic Medicine 2013;5(2):489‐94. [EMBASE: 2012742995; PUBMED: 23403854] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2014 {published data only}
- Chen J, Zhou JQ, Chen ZF, Huang Y, Jiang H. Efficacy and safety of dexmedetomidine versus propofol for the sedation of tube‐retention after oral maxillofacial surgery. Journal of Oral and Maxillofacial Surgery 2014;72(2):285.e1‐7. [EMBASE: 2014044109; PUBMED: 24438599] [DOI] [PubMed] [Google Scholar]
Chen 2014a {published data only}
- Chen J, Tong XG. Application of dexmedetomidine with total intravenous anesthesia on perioperative period of carotid endarterectomy. [Chinese]. Chinese Journal of Contemporary Neurology and Neurosurgery 2014;14(2):87‐92. [EMBASE: 2014252119] [Google Scholar]
Cheung 2011 {published data only}
- Cheung CW, Ng KF, Choi WS, Chiu WK, Aaron Ying CL, et al. Evaluation of the analgesic efficacy of local dexmedetomidine application. Clinical Journal of Pain 2011;27(5):377‐82. [EMBASE: 2011258679; PUBMED: 21317777] [DOI] [PubMed] [Google Scholar]
Cheung 2014 {published data only}
- Cheung CW, Qiu Q, Ying AC, Choi SW, Law WL, Irwin MG. The effects of intra‐operative dexmedetomidine on postoperative pain, side‐effects and recovery in colorectal surgery. Anaesthesia 2014;69(11):1214‐21. [PUBMED: 24915800] [DOI] [PubMed] [Google Scholar]
Cho 2015 {published data only}
- Cho JS, Kim HI, Lee KY, An JY, Bai SJ, Cho JY, et al. Effect of intraoperative dexmedetomidine infusion on postoperative bowel movements in patients undergoing laparoscopic gastrectomy: a prospective, randomized, placebo‐controlled study. Medicine 2015;94(24):e959. [PUBMED: 26091461] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chua 2010 {published data only}
- Chua KS, Wang FY, Hsu HT, Lua IC, Wang HM, Tsai CJ. The effectiveness of dexmedetomidine infusion for sedating oral cancer patients undergoing awake fibreoptic nasal intubation. European Journal of Anaesthesiology 2010;27(1):36‐40. [EMBASE: 2010345545; PUBMED: 19550337] [DOI] [PubMed] [Google Scholar]
Cindea 2012 {published data only}
- Cindea I, Balcan A, Samoila B, Gherghina V, Popescu R, Iordache I. The impact of intravenous clonidine on management of acute postoperative pain following colorectal surgery. European Journal of Anaesthesiology 2012;29:204. [EMBASE: 71084666] [Google Scholar]
Curtis 2002 {published data only}
- Curtis FG, Castiglia YMM, Stolf AA, Ronzella E, Vanni SMD, Do Nascimento P Jr. Dexmedetomidine and sufentanil as intraoperative analgesics. Comparative study. Revista Brasileira de Anestesiologia 2002;52(5):525‐34. [EMBASE: 2002342279; PUBMED: 19475222] [PubMed] [Google Scholar]
De Deyne 2000 {published data only}
- Deyne C, Struys M, Heylen R, Jongh R, Laenen M, Buyse L, et al. Influence of intravenous clonidine pretreatment on anesthetic requirements during bispectral EEG‐guided sevoflurane anesthesia. Journal of Clinical Anesthesia 2000;12(1):52‐7. [PUBMED: 10773509] [DOI] [PubMed] [Google Scholar]
De Kock 1992 {published data only}
- Kock MF, Pichon G, Scholtes JL. Intraoperative clonidine enhances postoperative morphine patient‐controlled analgesia. Canadian Journal of Anaesthesia 1992;39(6):537‐44. [PUBMED: 1643675] [DOI] [PubMed] [Google Scholar]
De Kock 1994 {published data only}
- Kock M, Lavandhomme P, Scholtes JL. Intraoperative and postoperative analgesia using intravenous opioid, clonidine and lignocaine. Anaesthesia and Intensive Care 1994;22(1):15‐21. [PUBMED: 8160943] [DOI] [PubMed] [Google Scholar]
De Kock 1995 {published data only}
- Kock M, Merello L, Pendeville P, Maiter D, Scholtes JL. Effects of intravenous clonidine on the secretion of growth hormone in the perioperative period. Acta Anaesthesiologica Belgica 1995;45(4):167‐74. [PUBMED: 7887119] [PubMed] [Google Scholar]
De la Mora‐Gonzalez 2012 {published data only}
- Mora‐Gonzalez JF, Robles‐Cervantes JA, Mora‐Martinez JM, Barba‐Alvarez F, Cruz Llontop‐Pisfil E, Gonzalez‐Ortiz M, et al. Hemodynamic effects of dexmedetomidine‐fentanyl vs. nalbuphine‐propofol in plastic surgery. Middle East Journal of Anesthesiology 2012;21(4):553‐7. [PUBMED: 23327028] [PubMed] [Google Scholar]
Delaunay 1991 {published data only}
- Delaunay L, Bonnet F, Duvaldestin P. Clonidine decreases postoperative oxygen consumption in patients recovering from general anaesthesia. British Journal of Anaesthesia 1991;67(4):397‐401. [PUBMED: 1931396] [DOI] [PubMed] [Google Scholar]
Demirhan 2011 {published data only}
- Demirhan A, Gul R, Ganidagli S, Koruk S, Mizrak A, Sanli M, et al. Combination of dexmedetomidine and tramadol in the treatment of pain after thoracotomy. [Turkish]. Gogus‐Kalp‐Damar Anestezi ve Yogun Bakim Dernegi Dergisi 2011;17(2):34‐41. [EMBASE: 2012130652] [Google Scholar]
Dhorigol 2010 {published data only}
- Dhorigol MG, Dhulkhed VK, Biyani A, Desai N. Randomized controlled, double‐blind study to evaluate oral clonidine to prevent post‐subarachnoid block shivering in patients undergoing elective urological surgery. Journal of Anaesthesiology Clinical Pharmacology 2010;26(1):15‐8. [EMBASE: 2010114394] [Google Scholar]
Dimou 2003 {published data only}
- Dimou P, Paraskeva A, Papilas K, Fassooulaki A. Transdermal clonidine: does it affect pain after abdominal hysterectomy?. Acta Anaesthesiologica Belgica 2003;54:227‐32. [PUBMED: 14598620] [PubMed] [Google Scholar]
Doak 1993 {published data only}
- Doak GJ, Duke PC. Oral clonidine premedication attenuates the haemodynamic effects associated with ketamine anaesthetic induction in humans. Canadian Journal of Anaesthesia 1993;40(7):612‐8. [PUBMED: 8403135] [DOI] [PubMed] [Google Scholar]
Dobrydniov 1999 {published data only}
- Dobrydniov I, Samarutel J. Enhancement of intrathecal lidocaine by addition of local and systemic clonidine. Acta Anaesthesiologica Scandinavica 1999;43(5):556‐62. [PUBMED: 10342005] [DOI] [PubMed] [Google Scholar]
Dobrydnjov 2002 {published data only}
- Dobrydnjov I, Axelsson K, Samarutel J, Holmstrom B. Postoperative pain relief following intrathecal bupivacaine combined with intrathecal or oral clonidine. Acta Anaesthesiologica Scandinavica 2002;46(7):806‐14. [PUBMED: 12139535] [DOI] [PubMed] [Google Scholar]
Dogan 2008 {published data only}
- Dogan Y, Alptekin A, Ozkan D, Arik E, Gumus H. Comparison of the effect of dexmedetomidine and remifentanyl on the hemodynamic response to intubation. Anestezi Dergisi 2008;16(3):136‐41. [EMBASE: 2008557577] [Google Scholar]
Dorman 1997 {published data only}
- Dorman T, Clarkson K, Rosenfeld BA, Shanholtz C, Lipsett PA, Breslow MJ. Effects of clonidine on prolonged postoperative sympathetic response. Critical Care Medicine 1997;25(7):1147‐52. [PUBMED: 9233740] [DOI] [PubMed] [Google Scholar]
Durmus 2007 {published data only}
- Durmus M, But AK, Dogan Z, Yucel A, Miman MC, Ersoy MO. Effect of dexmedetomidine on bleeding during tympanoplasty or septorhinoplasty. European Journal of Anaesthesiology 2007;24(5):447‐53. [PUBMED: 17241505] [DOI] [PubMed] [Google Scholar]
Eberhart 2000 {published data only}
- Dorman T, Clarkson K, Rosenfeld BA, Shanholtz C, Lipsett PA, Breslow MJ. Effects of clonidine on prolonged postoperative sympathetic response. Critical Care Medicine 1997;25(7):1147‐52. [PUBMED: 9233740] [DOI] [PubMed] [Google Scholar]
- Eberhart LH, Novatchkov N, Schricker T, Georgieff M, Baur CP. Intravenous premedication with clonidine and midazolam before ambulatory surgery. A controlled double‐blind study in ASA 1 patients. Anasthesiologie, Intensivmedizin, Notfallmedizin, Schmerztherapie 2000;35(6):388‐93. [PUBMED: 10900497] [DOI] [PubMed] [Google Scholar]
El 2012 {published data only}
- Ayad AE, Masry A. Epidural steroid and clonidine for chronic intractable post‐thoracotomy pain: a pilot study. Pain Practice 2012;12(1):7‐13. [PUBMED: 21615856] [DOI] [PubMed] [Google Scholar]
Elkassem 2008 {published data only}
- Elkassem SA. Dexmedetomidine vs remifentanil in abdominoplasty surgery (BIS guided): a comparative study. Egyptian Journal of Anaesthesia 2008;24(3):261‐9. [EMBASE: 2010651443] [Google Scholar]
Elliott 1997 {published data only}
- Elliott S, Eckersall S, Fligelstone L, Jothilingam S. Does the addition of clonidine affect duration of analgesia of bupivacaine wound infiltration in inguinal hernia surgery?. British Journal of Anaesthesia 1997;79(4):446‐9. [PUBMED: 9389260] [DOI] [PubMed] [Google Scholar]
Ellis 1998 {published data only}
- Ellis JE, Pedlow S, Bains J. Premedication with clonidine does not attenuate suppression of certain lymphocyte subsets after surgery. Anesthesia and Analgesia 1998;87(6):1426‐30. [PUBMED: 9842842] [DOI] [PubMed] [Google Scholar]
ElSheikh 2010 {published data only}
- ElSheikh S, Gamal G. The effect of thoracic epidural clonidine in the management of patients with intra‐abdominal hypertension. Egyptian Journal of Anaesthesia 2010;26(2):89‐96. [EMBASE: 2010648951] [Google Scholar]
Elvan 2008 {published data only}
- Elvan EG, Oc B, Uzun S, Karabulut E, Coskun F, Aypar U. Dexmedetomidine and postoperative shivering in patients undergoing elective abdominal hysterectomy. European Journal of Anaesthesiology 2008;25(5):357‐64. [PUBMED: 18205960] [DOI] [PubMed] [Google Scholar]
Engelman 1989 {published data only}
- Engelman E, Lipszyc M, Gilbart E, Linden P, Bellens B, Romphey A, et al. Effects of clonidine on anesthetic drug requirements and hemodynamic response during aortic surgery. Anesthesiology 1989;71(2):178‐87. [PUBMED: 2502935] [DOI] [PubMed] [Google Scholar]
Eremenko 2014a {published data only}
- Eremenko AA, Chemova EV. Comparison of dexmedetomidine and propofol for short‐term sedation in early postoperative period after cardiac surgery. Anesteziologiia i Reanimatologiia 2014;Mar‐Apr(2):37‐41. [PUBMED: 25055491] [PubMed] [Google Scholar]
Eremenko 2014b {published data only}
- Eremenko AA, Chernova EV. Treatment of delirium in the early postoperative period after cardiac surgery. Anesteziologiia i Reanimatologiia 2014;May‐Jun(3):30‐4. [PUBMED: 25306681] [PubMed] [Google Scholar]
Erkola 1994 {published data only}
- Erkola O, Korttila K, Aho M, Haasio J, Aantaa R, Kallio A. Comparison of intramuscular dexmedetomidine and midazolam premedication for elective abdominal hysterectomy. Anesthesia and Analgesia 1994;79(4):646‐53. [PUBMED: 7943770] [DOI] [PubMed] [Google Scholar]
Ezri 1998 {published data only}
- Ezri T, Szmuk P, Shklar B, Katz J, Geva D. Oral clonidine premedication does not prolong analgesia after herniorrhaphy under subarachnoid anesthesia. Journal of Clinical Anesthesia 1998;10(6):474‐81. [PUBMED: 9793811] [DOI] [PubMed] [Google Scholar]
Favre 1995 {published data only}
- Favre JB, Gardaz JP, Ravussin P. Effect of clonidine on ICP and on the hemodynamic responses to nociceptive stimuli in patients with brain tumors. Journal of Neurosurgical Anesthesiology 1995;7(3):159‐67. [PUBMED: 7549366] [DOI] [PubMed] [Google Scholar]
Fehr 2001 {published data only}
- Fehr SB, Zalunardo MP, Seifert B, Rentsch KM, Rohling RG, Pasch T, et al. Clonidine decreases propofol requirements during anaesthesia: effect on bispectral index. British Journal of Anaesthesia 2001;86(5):627‐32. [PUBMED: 11575336] [DOI] [PubMed] [Google Scholar]
Feld 2003 {published data only}
- Feld JM, Laurito CE, Beckerman M, Vincent J, Hoffman WE. Non‐opioid analgesia improves pain relief and decreases sedation after gastric bypass surgery. Canadian Journal of Anaesthesia 2003;50(4):336‐41. [PUBMED: 12670809] [DOI] [PubMed] [Google Scholar]
Feld 2006 {published data only}
- Feld JM, Hoffman WE, Stechert MM, Hoffman IW, Ananda RC. Fentanyl or dexmedetomidine combined with desflurane for bariatric surgery. Journal of Clinical Anesthesia 2006;18(1):24‐8. [PUBMED: 16517328] [DOI] [PubMed] [Google Scholar]
Feld 2007 {published data only}
- Feld J, Hoffman WE, Paisansathan C, Park H, Ananda RC. Autonomic activity during dexmedetomidine or fentanyl infusion with desflurane anesthesia. Journal of Clinical Anesthesia 2007;19(1):30‐6. [PUBMED: 17321924] [DOI] [PubMed] [Google Scholar]
Flacke 1987 {published data only}
- Flacke JW, Bloor BC, Flacke WE, Wong D, Dazza S Stead SW, et al. Reduced narcotic requirement by clonidine with improved hemodynamic and adrenergic stability in patients undergoing coronary bypass surgery. Anesthesiology 1987;67(1):11‐9. [PUBMED: 3496811] [DOI] [PubMed] [Google Scholar]
Frank 1999 {published data only}
- Frank T, Thieme V, Olthoff D. Preoperative clonidine comedication within the scope of balanced inhalation anesthesia with sevoflurane in oral surgery procedures. Anaesthesiologie und Reanimation 1999;24(3):65‐70. [PUBMED: 10472699] [PubMed] [Google Scholar]
Frank 2000a {published data only}
- Frank T, Thieme V, Olthoff D. Clonidine within the scope of balanced inhalation anesthesia with sevoflurane ‐ effects on pEEG parameters. Anaesthesiologie und Reanimation 2000;25(2):32‐6. [PUBMED: 10816895] [PubMed] [Google Scholar]
Frank 2000b {published data only}
- Frank T, Thieme V, Radow L. Premedication in maxillofacial surgery under total intravenous anesthesia. Effects of clonidine compared to midazolam on the perioperative course. Anasthesiologie, Intensivmedizin, Notfallmedizin, Schmerztherapie 2000;35(7):428‐34. [PUBMED: 10949680] [DOI] [PubMed] [Google Scholar]
Frank 2002 {published data only}
- Frank T, Wehner M, Heinke W, Schmadicke L. Clonidine vs. midazolam for premedication ‐ comparison of the anxiolytic effect by using the STAI‐test. Anasthesiologie, Intensivmedizin, Notfallmedizin, Schmerztherapie 2002;37(2):89‐93. [PUBMED: 11865386] [DOI] [PubMed] [Google Scholar]
Galindo 2008 {published data only}
- Galindo Palazuelos M, Diaz Setien NA, Rodriguez Cundin P, Manso Marin FJ, Castro Ugalde A. Premedication with intraoperative clonidine and low‐dose ketamine in outpatient laparoscopic cholecystectomy. [Spanish]. Revista Espanola de Anestesiologia y Reanimacion 2008;55(7):414‐7. [PUBMED: 18853679] [DOI] [PubMed] [Google Scholar]
Gandhi 2017 {published data only}
- Gandhi KA, Panda NB, Vellaichamy A, Mathew PJ, Sahni N, Batra YK. Intraoperative and postoperative administration of dexmedetomidine reduces anesthetic and postoperative analgesic requirements in patients undergoing cervical spine surgeries. Journal of Neurosurgical Anesthesiology 2017;29(3):258‐63. [EMBASE: 609460791] [DOI] [PubMed] [Google Scholar]
Ganter 2005 {published data only}
- Ganter MT, Hofer CK, Spahn DR, Bruggisser M, Bombeli T, Seifert B, et al. The effect of clonidine on perioperative blood coagulation. Journal of Clinical Anesthesia 2005;17(6):456‐62. [PUBMED: 16171667] [DOI] [PubMed] [Google Scholar]
Gao 2012 {published data only}
- Gao GJ, Xu YY, Wang B, Lv HM, Yang WY, Shang Y. Feasibility of dexmedetomidine assisting sevoflurane for controlled hypotension in endoscopic sinus surgery. [Chinese]. Medical Journal of Chinese People's Liberation Army 2012;37(1):45‐8. [EMBASE: 2014601501] [Google Scholar]
Garcia‐Guiral 1994 {published data only}
- Garcia‐Guiral M, Carrera A, Lora‐Tamayo JI, Luengo C, Pascual E, Quintana B, et al. Premedication with clonidine in the neurosurgical patient: sedation, anesthetic requirements and hemodynamic perfusion. Revista Española de Anestesiología y Reanimación 1994;41(2):77‐81. [PUBMED: 8041979] [PubMed] [Google Scholar]
Ghatak 2010 {published data only}
- Ghatak T, Chandra G, Malik A, Singh D, Bhatia VK. Evaluation of the effect of magnesium sulphate vs. clonidine as adjunct to epidural bupivacaine. Indian Journal of Anaesthesia 2010;54(4):308‐13. [EMBASE: 2011056851; PUBMED: 20882172] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghosh 2008 {published data only}
- Ghosh I, Bithal PK, Dash HH, Chaturvedi A, Prabhakar H. Both clonidine and metoprolol modify anesthetic depth indicators and reduce intraoperative propofol requirement. Journal of Anesthesia 2008;22(2):131‐4. [EMBASE: 2008262464; PUBMED: 18500609] [DOI] [PubMed] [Google Scholar]
Gomez‐Vazquez 2007 {published data only}
- Gomez‐Vazquez ME, Hernandez‐Salazar E, Hernandez‐Jimenez A, Perez‐Sanchez A, Zepeda‐Lopez VA, Salazar‐Paramo M. Clinical analgesic efficacy and side effects of dexmedetomidine in the early postoperative period after arthroscopic knee surgery. Journal of Clinical Anesthesia 2007;19(8):576‐82. [PUBMED: 18083469] [DOI] [PubMed] [Google Scholar]
Goyagi 1996 {published data only}
- Goyagi T, Nishikawa T. Oral clonidine premedication enhances the quality of postoperative analgesia by intrathecal morphine. Anesthesia and Analgesia 1996;82(6):1192‐6. [PUBMED: 8638790] [DOI] [PubMed] [Google Scholar]
Grottke 2003 {published data only}
- Grottke O, Muller J, Dietrich PJ, Krause TH, Wappler F. Comparison of premedication with clonidine and midazolam combined with TCI for orthopaedic shoulder surgery. Anasthesiologie, Intensivmedizin, Notfallmedizin, Schmerztherapie 2003;38(12):772‐80. [PUBMED: 14666440] [DOI] [PubMed] [Google Scholar]
Grundmann 1997 {published data only}
- Grundmann U, Berg K, Stamminger U, Juckenhofel S, Wilkem W. Comparison of pethidine and clonidine in the prevention of postoperative shivering. A prospective, randomized, placebo‐controlled, double‐blind study. Anasthesiologie, Intensivmedizin, Notfallmedizin, Schmerztherapie 1997;32(1):36‐42. [PUBMED: 9138543] [DOI] [PubMed] [Google Scholar]
Guglielminotti 1998 {published data only}
- Guglielminotti J, Descraques C, Petitmaire S, Almenza L, Grenapin O, Mantz J. Effects of premedication on dose requirements for propofol: comparison of clonidine and hydroxyzine. British Journal of Anaesthesia 1998;80(6):733‐6. [PUBMED: 9771298] [DOI] [PubMed] [Google Scholar]
Gupta 2011a {published data only}
- Gupta D, Srivastava S, Dubey R, Prakash P, Singh P, Singh U. Comparative evaluation of atenolol and clonidine premedication on cardiovascular response to nasal speculum insertion during trans‐sphenoid surgery for resection of pituitary adenoma: A prospective, randomised, double‐blind, controlled study. Indian Journal of Anaesthesia 2011;55(2):135‐40. [EMBASE: 2012059701; PUBMED: 21712869] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2011b {published data only}
- Gupta K, Sharma D, Gupta PK. Oral premedication with pregabalin or clonidine for hemodynamic stability during laryngoscopy and laparoscopic cholecystectomy: A comparative evaluation. Saudi Journal of Anaesthesia 2011;5(2):179‐84. [EMBASE: 2011405481; PUBMED: 21804800] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2011c {published data only}
- Gupta R, Verma R, Bogra J, Kohli M, Raman R, Kushwaha JK. A comparative study of intrathecal dexmedetomidine and fentanyl as adjuvants to bupivacaine. Journal of Anaesthesiology Clinical Pharmacology 2011;27(3):339‐43. [EMBASE: 2011448409; PUBMED: 21897504] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2012 {published data only}
- Gupta K, Jain M, Gupta PK, Rastogi B, Saxena SK, Manngo A. Dexmedetomidine premedication for fiberoptic intubation in patients of temporomandibular joint ankylosis: A randomized clinical trial. Saudi Journal of Anaesthesia 2012;6(3):219‐23. [EMBASE: 2012596318; PUBMED: 23162393] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guven 2011 {published data only}
- Guven DG, Demiraran Y, Sezen G, Kepek O, Iskender A. Evaluation of outcomes in patients given dexmedetomidine in functional endoscopic sinus surgery. Annals of Otology, Rhinology and Laryngology 2011;120(9):586‐92. [EMBASE: 2011502324; PUBMED: 22032072] [DOI] [PubMed] [Google Scholar]
Hahm 2002 {published data only}
- Hahm TS, Cho HS, Lee KH, Chung IS, Kim JA, Kim MH. Clonidine premedication prevents preoperative hypokalemia. Journal of Clinical Anesthesia 2002;14(1):6‐9. [PUBMED: 11880014] [DOI] [PubMed] [Google Scholar]
Hall 2006 {published data only}
- Hall DL, Rezvan E, Tatakis DN, Walters JD. Oral clonidine pretreatment prior to venous cannulation. Anesthesia Progress 2006;53(2):34‐42. [PUBMED: 16863391] [DOI] [PMC free article] [PubMed] [Google Scholar]
Handa 2000 {published data only}
- Handa F, Tanaka M, Nishikawa T, Toyooka H. Effects of oral clonidine premedication on side effects of intravenous ketamine anesthesia: a randomized, double‐blind, placebo‐controlled study. Journal of Clinical Anesthesia 2000;12(1):19‐24. [PUBMED: 10773503] [DOI] [PubMed] [Google Scholar]
Harsoor 2013 {published data only}
- Harsoor SS, Rani DD, Yalamuru B, Sudheesh K, Nethra SS. Effect of supplementation of low dose intravenous dexmedetomidine on characteristics of spinal anaesthesia with hyperbaric bupivacaine. Indian Journal of Anaesthesia 2013;57(3):265‐9. [EMBASE: 2013477136; PUBMED: 23983285] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harsoor 2014 {published data only}
- Harsoor S, Rani D, Lathashree S, Nethra S, Sudheesh K. Effect of intraoperative dexmedetomidine infusion on sevoflurane requirement and blood glucose levels during entropy‐guided general anesthesia. Journal of Anaesthesiology Clinical Pharmacology 2014;30(1):25‐30. [EMBASE: 2014095808; PUBMED: 24574589] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hashemian 2017 {published data only}
- Hashemian M, Ahmadinejad M, Mohajerani SA, Mirkheshti A. Impact of dexmedetomidine on hemodynamic changes during and after coronary artery bypass grafting. Annals of Cardiac Anaesthesia 2017;20(2):152‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hazra 2014 {published data only}
- Hazra R, Manjunatha SM, Babrak Manuar MD, Basu R, Chakraborty S. Comparison of the effects of intravenously administered dexmedetomidine with clonidine on hemodynamic responses during laparoscopic cholecystectomy. Anaesthesia, Pain and Intensive Care 2014;18(1):25‐30. [EMBASE: 2015025031] [Google Scholar]
Hidalgo 2005 {published data only}
- Hidalgo MP, Auzani JA, Rumpel LC, Moreira NL Jr, Cursino AW, Caumo W. The clinical effect of small oral clonidine doses on perioperative outcomes in patients undergoing abdominal hysterectomy. Anesthesia and Analgesia 2005;100(3):795‐802. [PUBMED: 15728070] [DOI] [PubMed] [Google Scholar]
Higuchi 2002 {published data only}
- Higuchi H, Adachi Y, Dahan A, Olofsen E, Arimura S, Mori T, et al. The interaction between propofol and clonidine for loss of consciousness. Anesthesia and Analgesia 2002;94(4):886‐91. [PUBMED: 11916791] [DOI] [PubMed] [Google Scholar]
Honarmand 2007 {published data only}
- Honarmand A, Safavi MR. Preoperative oral dextromethorphan vs. clonidine to prevent tourniquet‐induced cardiovascular responses in orthopaedic patients under general anaesthesia. European Journal of Anaesthesiology 2007;24(6):511‐5. [PUBMED: 17202010] [DOI] [PubMed] [Google Scholar]
Horn 1997 {published data only}
- Horn EP, Werner C, Sessler DI, Steinfath M, Schulte am Esch J. Late intraoperative clonidine administration prevents postanesthetic shivering after total intravenous or volatile anesthesia. Anesthesia and Analgesia 1997;84(3):613‐7. [PUBMED: 9052312] [DOI] [PubMed] [Google Scholar]
Horng 2007 {published data only}
- Horng HC, Wong CS, Hsiao KN, Huh BK, Kuo CP, Cherng CH, et al. Pre‐medication with intravenous clonidine suppresses fentanyl‐induced cough. Acta Anaesthesiologica Scandinavica 2007;51(7):862‐5. [PUBMED: 17578464] [DOI] [PubMed] [Google Scholar]
Hwang 2015 {published data only}
- Hwang W, Lee J, Park J, Joo J. Dexmedetomidine versus remifentanil in postoperative pain control after spinal surgery: a randomized controlled study. BMC Anesthesiology 2015;15:21. [EMBASE: 2015845589; PUBMED: 25750586] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ishiyama 2006 {published data only}
- Ishiyama T, Kashimoto S, Oguchi T, Furuya A, Fukushima H, Kumazawa T. Clonidine‐ephedrine combination reduces pain on injection of propofol and blunts hemodynamic stress responses during the induction sequence. Journal of Clinical Anesthesia 2006;18(3):211‐5. [PUBMED: 16731324] [DOI] [PubMed] [Google Scholar]
Jaakola 1994 {published data only}
- Jaakola ML, Kanto J, Scheinin H, Kallio A. Intramuscular dexmedetomidine premedication ‐ an alternative to midazolam‐fentanyl‐combination in elective hysterectomy?. Acta Anaesthesiologica Scandinavica 1994;38(3):238‐43. [PUBMED: 7912877] [DOI] [PubMed] [Google Scholar]
Jabalameli 2005 {published data only}
- Jabalameli M, Hashemi M, Soltani H, Hashemi J. Oral clonidine premedication decreases intraoperative bleeding in patients undergoing endoscopic sinus surgery. Journal of Research in Medical Sciences 2005;10(1):25‐30. [EMBASE: 2005178855] [Google Scholar]
Javaherfroosh 2009 {published data only}
- Javaherfroosh F, Pipelzadeh MR, Namazi M. Clonidine reduces post operative nausea and vomiting in laparoscopic gynecological surgery. Pakistan Journal of Medical Sciences 2009;25(5):782‐5. [EMBASE: 2009571437] [Google Scholar]
Jeffs 2002 {published data only}
- Jeffs SA, Hall JE, Morris S. Comparison of morphine alone with morphine plus clonidine for postoperative patient‐controlled analgesia. British Journal of Anaesthesia 2002;89(3):424‐7. [PUBMED: 12402720] [PubMed] [Google Scholar]
Jellish 2001 {published data only}
- Jellish WS, Theard MA, Cheng MA, Leonetti JP, Crowder CM, Tempelhoff R. The effects of clonidine premedication and scalp infiltration of lidocaine on hemodynamic responses to laryngoscopy and skull pin head‐holder insertion during skull base procedures. Skull Base 2001;11(3):169‐76. [PUBMED: 17167618] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ji 2013 {published data only}
- Ji F, Li Z, Nguyen H, Young N, Shi P, Fleming N, et al. Perioperative dexmedetomidine improves outcomes of cardiac surgery. Circulation 2013; Vol. 127, issue 15:1576‐84. [EMBASE: 2013243940; PUBMED: 23513068] [DOI] [PMC free article] [PubMed]
Joao 2014 {published data only}
- Joao BB, do Amaral JL, Bueno RM, Ferez D, Falcao LF, Perez MV, et al. Intravenous clonidine administration and its ability to reduce pulmonary arterial pressure in patients undergoing heart surgery. Brazilian Journal of Anesthesiology 2014;64(1):40‐8. [PUBMED: 24565387] [DOI] [PubMed] [Google Scholar]
Joris 1993 {published data only}
- Joris J, Banache M, Bonnet F, Sessler DI, Lamy M. Clonidine and ketanserin both are effective treatment for postanesthetic shivering. Anesthesiology 1993;79(3):532‐9. [PUBMED: 8363079] [DOI] [PubMed] [Google Scholar]
Joris 1998 {published data only}
- Joris JL, Chiche JD, Canivet JL, Jacquet NJ, Legros JJ, Lamy LL. Hemodynamic changes induced by laparoscopy and their endocrine correlates: effects of clonidine. Journal of the American College of Cardiology 1998;32(5):1389‐96. [PUBMED: 9809953] [DOI] [PubMed] [Google Scholar]
Joshi 2012 {published data only}
- Joshi SA, Khadke VV, Subhedar RD, Patil AW, Motghare VM. Comparative evaluation of intrathecal midazolam and low dose clonidine: efficacy, safety and duration of analgesia. A randomized, double blind, prospective clinical trial. Indian Journal of Pharmacology 2012; Vol. 44, issue 3:357‐61. [EMBASE: 2012322476; PUBMED: 22701246] [DOI] [PMC free article] [PubMed]
Juarez‐Pichardo 2009 {published data only}
- Juarez‐Pichardo JS, Avila‐Lopez A, Serrano‐Herrera MA. Preventive postoperative analgesia with dexmetomidine iv compared to lidocaine iv in cholecystectomy. [Spanish]. Revista Mexicana de Anestesiologia 2009;32(2):81‐8. [EMBASE: 2009468805] [Google Scholar]
Kajiyama 2009 {published data only}
- Kajiyama S, Nakagawa I, Hidaka S, Okada H, Kubo T, Nao Y. Effect of dexmedetomidine on intraoperative somatosensory evoked potential monitoring. [Japanese]. Japanese Journal of Anesthesiology 2009;58(8):966‐70. [EMBASE: 2009464313] [PubMed] [Google Scholar]
Kalajdzija 2011 {published data only}
- Kalajdzija M, Cero I, Prnjavorac B, Ljuca S. Influence of clonidine on the chemodynamic stability and stress response in the course of surgery on general anesthesia. Medicinski arhiv 2011;65(4):210‐2. [PUBMED: 21950225] [DOI] [PubMed] [Google Scholar]
Kang 2012 {published data only}
- Kang WS, Kim SY, Son JC, Kim JD, Muhammad HB, Kim SH, et al. The effect of dexmedetomidine on the adjuvant propofol requirement and intraoperative hemodynamics during remifentanil‐based anesthesia. Korean Journal of Anesthesiology 2012;62(2):113‐8. [EMBASE: 2012125528; PUBMED: 22379564] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 2013 {published data only}
- Kang SH, Kim YS, Hong TH, Chae MS, Cho ML, Her YM, et al. Effects of dexmedetomidine on inflammatory responses in patients undergoing laparoscopic cholecystectomy. Acta Anaesthesiologica Scandinavica 2013;57(4):480‐7. [EMBASE: 2013243000; PUBMED: 23240685] [DOI] [PubMed] [Google Scholar]
Kang 2015 {published data only}
- Kang MH, Lee HJ, Lim YJ, Jeon YT, Hwang JW, Park HP. Preoperative dexmedetomidine attenuates hemodynamic responses to hydrodissection in patients undergoing robotic thyroidectomy. Journal of Anesthesia 2015;29(2):191‐7. [PUBMED: 25262475] [DOI] [PubMed] [Google Scholar]
Karaman 2013 {published data only}
- Karaman S, Gunusen I, Ceylan MA, Karaman Y, Cetin EN, Derbent A, et al. Dexmedetomidine infusion prevents postoperative shivering in patients undergoing gynecologic laparoscopic surgery. Turkish Journal of Medical Sciences 2013;43(2):232‐7. [EMBASE: 2013171247] [Google Scholar]
Karaman 2015 {published data only}
- Karaman Y, Abud B, Tekgul ZT, Cakmak M, Yildiz M, Gonullu M. Effects of dexmedetomidine and propofol on sedation in patients after coronary artery bypass graft surgery in a fast‐track recovery room setting. Journal of Anesthesia 2015;29(4):522‐8. [PUBMED: 25617159] [DOI] [PubMed] [Google Scholar]
Kawasaki 2014 {published data only}
- Kawasaki T, Kawasaki C, Sata T. Dexmedetomidine suppresses inflammatory mediators level in patients of cardiac surgery using cardiopulmonary bypass. Shock 2014;41:59. [EMBASE: 71497732] [Google Scholar]
Kaya 2010 {published data only}
- Kaya FN, Yavascaoglu B, Turker G, Yildirim A, Gurbet A, Mogol EB, et al. Intravenous dexmedetomidine, but not midazolam, prolongs bupivacaine spinal anesthesia. Canadian Journal of Anesthesia 2010;57(1):39‐45. [EMBASE: 2010046678; PUBMED: 20039211] [DOI] [PubMed] [Google Scholar]
Kaymak 2008 {published data only}
- Kaymak C, Basar H, Doganci N, Sert O, Apan A. The effects of perioperative low ‐ moderate doses of dexmedetomidine infusion on hemodynamic and neuroendocrine parameters. Turkish Journal of Medical Sciences 2008;38(1):65‐71. [EMBASE: 2008051508] [Google Scholar]
Ke 2013 {published data only}
- Ke J, Pen X. The effect of dexmedetomidine on post‐operative blood pressure after controlled hypotension in endoscopic sinus surgery. [Chinese]. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke za Zhi [Journal of Clinical Otorhinolaryngology, Head, and Neck Surgery] 2013;27(10):478‐80. [PUBMED: 23937012] [PubMed] [Google Scholar]
Keniya 2011 {published data only}
- Keniya VM, Ladi S, Naphade R. Dexmedetomidine attenuates sympathoadrenal response to tracheal intubation and reduces perioperative anaesthetic requirement. Indian Journal of Anaesthesia 2011;55(4):352‐7. [EMBASE: 2011568239; PUBMED: 22013250] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khafagy 2012 {published data only}
- Khafagy HF, Ebied RS, Osman ES, Ali MZ, Samhan YM. Perioperative effects of various anesthetic adjuvants with TIVA guided by bispectral index. Korean Journal of Anesthesiology 2012;63(2):113‐9. [EMBASE: 2012524970; PUBMED: 22949977] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2012 {published data only}
- Kim WO, Yoon Y, Choi Y, Kim SH, Kil HK. Effects of intravenous dexmedetomidine on low‐dose bupivacaine spinal anaesthesia in elderly patients. Acta Anaesthesiologica Scandinavica 2012;56(3):382‐7. [PUBMED: 22220945] [DOI] [PubMed] [Google Scholar]
Kim 2013a {published data only}
- Kim SH, Oh YJ, Park BW, Sim J, Choi YS. Effects of single‐dose dexmedetomidine on the quality of recovery after modified radical mastectomy: a randomised controlled trial. Minerva Anestesiologica 2013;79(11):1248‐58. [EMBASE: 2014029902; PUBMED: 23698545] [PubMed] [Google Scholar]
Kim 2013b {published data only}
- Kim SY, Kim JM, Lee JH, Song BM, Koo BN. Efficacy of intraoperative dexmedetomidine infusion on emergence agitation and quality of recovery after nasal surgery. British Journal of Anaesthesia 2013;111(2):222‐8. [EMBASE: 2013463896; PUBMED: 23524149] [DOI] [PubMed] [Google Scholar]
Kim 2013c {published data only}
- Kim Y‐I, Seo K‐H, Kang H‐R. Optimal dose of prophylactic dexmedetomidine for preventing postoperative shivering. International Journal of Medical Sciences 2013;10(10):1327‐32. [PUBMED: 23983593] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2014b {published data only}
- Kim WH, Shim HS, Kim G, Lee JE, Lee YT, Cho HS. A comparison of the infusion of dexmedetomidine versus remifentanil in patients undergoing off‐pump coronary artery bypass graft: a randomized trial. Experimental and Clinical Cardiology 2014;20(6):4234‐51. [EMBASE: 2014448289] [Google Scholar]
Korkmaz 2013 {published data only}
- Korkmaz Disli Z, Celebi N, Canbay O, Celebioglu B. Comparison of morphine and dexmedetomidine delivered by patient‐controlled analgesia device during the postoperative period of coronary artery bypass surgery. [Turkish]. Anestezi Dergisi 2013;21(1):29‐36. [EMBASE: 2013167966] [Google Scholar]
Koyuncu 2009 {published data only}
- Koyuncu O, Alagol A, Turan A. Comparison of dexmedetomidine and ketamine infusions in patients undergoing lower extremity surgery with regional anesthesia. European Journal of Anaesthesiology 2009;26:122. [EMBASE: 70162271] [Google Scholar]
Kulka 1996 {published data only}
- Kulka PJ, Tryba M, Zenz M. Preoperative alpha2‐adrenergic receptor agonists prevent the deterioration of renal function after cardiac surgery: results of a randomized, controlled trial. Critical Care Medicine 1996;24(6):947‐52. [PUBMED: 8681596] [DOI] [PubMed] [Google Scholar]
Kumari 2012 {published data only}
- Kumari I, Naithni U, Bedi V, Gupta S, Gupta R, Bhuie. Comparison of clonidine versus midazolam in monitored anesthesia care during ENT surgery ‐ a prospective, double blind, randomized clinical study. Anaesthesia, Pain and Intensive Care 2012;16(2):157‐64. [EMBASE: 2012643410] [Google Scholar]
Lang 2011 {published data only}
- Lang Y, Wang TL, Wu XM, Ding LG. Application of sedation with a low dose of dexmedetomidine during intrathecal anesthesia in elderly patients. [Chinese]. Zhonghua yi xue hui 2011; Vol. 91, issue 28:1953‐6. [EMBASE: 2012710260; PUBMED: 22093888] [PubMed]
Lattermann 2001 {published data only}
- Lattermann R, Schricker T, Georgieff M, Schreiber M. Low dose clonidine premedication accentuates the hyperglycemic response to surgery. Canadian Journal of Anaesthesia 2001;48(8):755‐9. [PUBMED: 11546715] [DOI] [PubMed] [Google Scholar]
Launo 1991 {published data only}
- Launo C, Palermo S, Germi MR, Lanfrit C, Frasca A, Simonetti F. Clonidine and postoperative shivering. Minerva Anestesiologica 1991;57(7‐8):427‐31. [PUBMED: 1944967] [PubMed] [Google Scholar]
Laurito 1991 {published data only}
- Laurito CE, Baughman VL, Becker GL, DeSilva TW, Carranza CJ. The effectiveness of oral clonidine as a sedative/anxiolytic and as a drug to blunt the hemodynamic responses to laryngoscopy. Journal of Clinical Anesthesia 1991;3(3):186‐93. [PUBMED: 1878231] [DOI] [PubMed] [Google Scholar]
Laurito 1993 {published data only}
- Laurito CE, Baughman VL, Becker GL, Cunnigham F, Pygon BH, Citron GM. Oral clonidine blunts the hemodynamic responses to brief but not prolonged laryngoscopy. Journal of Clinical Anesthesia 1993;5(1):54‐7. [PUBMED: 8442970] [DOI] [PubMed] [Google Scholar]
Lawrence 1997 {published data only}
- Lawrence CJ, Lange S. Effects of a single pre‐operative dexmedetomidine dose on isoflurane requirements and peri‐operative haemodynamic stability. Anaesthesia 1997;52(8):736‐44. [PUBMED: 9291757] [DOI] [PubMed] [Google Scholar]
Lee 2012 {published data only}
- Lee HK, Kim YC, Yim JY. Assessment of the effect of dexmedetomidine in the management of postoperative pain when combined with fentanyl in the patient‐controlled analgesia. European Journal of Anaesthesiology 2012;29:196‐7. [EMBASE: 71084641] [Google Scholar]
Lee 2013b {published data only}
- Lee J, Kim Y, Park C, Jeon Y, Kim D, Joo J, et al. Comparison between dexmedetomidine and remifentanil for controlled hypotension and recovery in endoscopic sinus surgery. Annals of Otology, Rhinology and Laryngology 2013;122(7):421‐6. [EMBASE: 2013495596; PUBMED: 23951692] [DOI] [PubMed] [Google Scholar]
Le Guen 2014 {published data only}
- Guen M, Liu N, Tounou F, Auge M, Tuil O, Chazot T, et al. Dexmedetomidine reduces propofol and remifentanil requirements during bispectral index‐guided closed‐loop anesthesia: a double‐blind, placebo‐controlled trial. Anesthesia and Analgesia 2014;118(5):946‐55. [PUBMED: 24722260] [DOI] [PubMed] [Google Scholar]
Leino 2011 {published data only}
- Leino K, Hynynen M, Jalonen J, Salmenpera M, Scheinin H, Aantaa R. Renal effects of dexmedetomidine during coronary artery bypass surgery: a randomized placebo‐controlled study. BMC Anesthesiology 2011; Vol. 11:9. [EMBASE: 2011348574; PUBMED: 21605394] [DOI] [PMC free article] [PubMed]
Levanen 1995 {published data only}
- Levanen J, Makela M‐L, Scheinin H. Dexmedetomidine premedication attenuates ketamine‐induced cardiostimulatory effects and postanesthetic delirium. Anesthesiology 1995;82(5):1117‐25. [PUBMED: 7741286] [DOI] [PubMed] [Google Scholar]
Li 2010 {published data only}
- Li H, She S, Yan Y, Zhu S. [Effect of dexmedetomidine on bispectral index and auditory evoked potential index during anesthesia with target controlled infusion of propofol and remifentanyl]. Zhejiang da Xue Xue Bao. Yi Xue Ban = Journal of Zhejiang University. Medical Sciences 2010;39(1):84‐8. [PUBMED: 20175241] [DOI] [PubMed] [Google Scholar]
Li 2013 {published data only}
- Li H, Chen YH, Gong HJ. Effects of dexmedetomidine on inflammatory cytokines and renal function in patients underwent radical nephrectomy during perioperative period. [Chinese]. Journal of Jilin University Medicine Edition 2013;39(3):588‐91. [EMBASE: 2013391674] [Google Scholar]
Liu 2013 {published data only}
- Liu HL, Qian YN. [Effects of dexmedetomidine on perioperative inflammatory response in patients undergoing valve replacement]. [Chinese]. Zhongguo Ying Yong Sheng li Xue Za Zhi = Zhongguo Yingyong Shenglixue Zazhi = Chinese Journal of Applied Physiology 2013; Vol. 29, issue 4:316‐7. [PUBMED: 24175551] [PubMed]
Lu 2013 {published data only}
- Lu Y, Zhang Y, Dong CS, Yu JM, Wong GT. Preoperative dexmedetomidine prevents tourniquet‐induced hypertension in orthopedic operation during general anesthesia. Kaohsiung Journal of Medical Sciences 2013;29(5):271‐4. [EMBASE: 2013279941; PUBMED: 23639514] [DOI] [PubMed] [Google Scholar]
Lyons 1997 {published data only}
- Lyons FM, Bew S, Sheeran P, Hall GM. Effects of clonidine on the pituitary hormonal response to pelvic surgery. British Journal of Anaesthesia 1997;78(2):134‐7. [PUBMED: 9068327] [DOI] [PubMed] [Google Scholar]
Ma 2013 {published data only}
- Ma PP, Piao MH, Wang YS, Ma HC, Feng CS. Influence of dexmedetomidine and sub‐anesthetic dose of ketamine on postoperative delirium in elderly orthopedic patients under total intravenous anesthesia. [Chinese]. Journal of Jilin University Medicine Edition 2013;39(1):128‐32. [EMBASE: 2013147857] [Google Scholar]
Mahendru 2013 {published data only}
- Mahendru V, Tewari A, Katyal S, Grewal A, Singh MR, Katyal R. A comparison of intrathecal dexmedetomidine, clonidine, and fentanyl as adjuvants to hyperbaric bupivacaine for lower limb surgery: a double blind controlled study. Journal of Anaesthesiology Clinical Pharmacology 2013;29(4):496‐502. [EMBASE: 2013651749] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maldonado 2009 {published data only}
Malek 2009 {published data only}
- Malek J, Hess L, Votava M, Marecek F, Kurzova A. The combination of dexmedetomidine, fentanyl, midazolam and atropine may result in dangerous respiratory depression and hypoxemia. European Journal of Anaesthesiology 2009;26:136. [EMBASE: 70162317] [Google Scholar]
Malek 2010a {published data only}
- Malek J, Marecek F, Hess L, Votava M, Kurzova A. Dexmedetomidine in combination with fentanyl and midazolam can induce serious respiratory depression. [Czech]. Bolest 2010;13(2):80‐7. [EMBASE: 2010397845] [Google Scholar]
Malek 2010b {published data only}
- Malek J, Marecek F, Hess L, Kurzova A, Ocadlik M, Votava M. A combination of dexmedetomidine with ketamine and opioids results in significant inhibition of hemodynamic changes associated with laparoscopic cholecystectomy and in prolongation of postoperative analgesia. [Czech]. Rozhledy v Chirurgii : Mesicnik Ceskoslovenske Chirurgicke Spolecnosti 2010;89(5):275‐81. [EMBASE: 20666328] [PubMed] [Google Scholar]
Manne 2014 {published data only}
- Manne GR, Upadhyay MR, Swadi VN. Effects of low dose dexmedetomidine infusion on haemodynamic stress response, Sedation and post‐operative analgesia requirement in patients undergoing laparoscopic cholecystectomy. Indian Journal of Anaesthesia 2014;58(6):726‐31. [PUBMED: 25624537] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mannion 2005 {published data only}
- Mannion S, Hayes I, Loughnane F, Murphy DB, Shorten GD. Intravenous but not perineural clonidine prolongs postoperative analgesia after psoas compartment block with 0.5% levobupivacaine for hip fracture surgery. Anesthesia and Analgesia 2005;100(3):873‐8. [PUBMED: 15728081] [DOI] [PubMed] [Google Scholar]
Marangoni 2005 {published data only}
- Marangoni MA, Castiglia YM, Pechutti TP. Analgesic efficacy of dexmedetomidine as compared to sufentanil in intraperitoneal surgeries. Comparative study. Revista Brasileira de Anestesiologia 2005;55(1):19‐27. [EMBASE: 2005078359; PUBMED: 19471805] [DOI] [PubMed] [Google Scholar]
Marchal 2001 {published data only}
- Marchal JM, Gomez‐Luque A, Martos‐Crespo F, Sanchez De La Cuesta F, Martinez‐Lopez MC, Delgado‐Martinez AD. Clonidine decreases intraoperative bleeding in middle ear microsurgery. Acta Anaesthesiologica Scandinavica 2001;45(5):627‐33. [PUBMED: 11309017] [DOI] [PubMed] [Google Scholar]
Mariappan 2014 {published data only}
- Mariappan R, Ashokkumar H, Kuppuswamy B. Comparing the effects of oral clonidine premedication with intraoperative dexmedetomidine infusion on anesthetic requirement and recovery from anesthesia in patients undergoing major spine surgery. Journal of Neurosurgical Anesthesiology 2014;26(3):192‐7. [EMBASE: 2014397409; PUBMED: 23887684] [DOI] [PubMed] [Google Scholar]
Marinangeli 2002 {published data only}
- Marinangeli F, Ciccozzi A, Donatelli F, Pietro A, Iovinelli G, Rawal G, et al. Clonidine for treatment of postoperative pain: a dose‐finding study. European Journal of Pain 2002;6(1):35‐42. [PUBMED: 11888226] [DOI] [PubMed] [Google Scholar]
Martin 2003 {published data only}
- Mantz J, French Dexmedetomidine Phase III Group. Phase III study on dexmedetomidine used for postoperative sedation of patients requiring mechanical ventilation for less than 24 hours: the French experience. Middle East Journal of Anesthesiology 2002;16(6):597‐606. [PUBMED: 12503263] [PubMed] [Google Scholar]
- Martin E, Ramsay G, Mantz J, Sum‐Ping ST. The role of the alpha2‐adrenoceptor agonist dexmedetomidine in postsurgical sedation in the intensive care unit. Journal of Intensive Care Medicine 2003;18(1):29‐41. [PUBMED: 15189665] [DOI] [PubMed] [Google Scholar]
Massad 2009 {published data only}
- Massad IM, Mohsen WA, Basha AS, Al‐Zaben KR, Al‐Mustafa MM, Alghanem SM. A balanced anesthesia with dexmedetomidine decreases postoperative nausea and vomiting after laparoscopic surgery. Saudi Medical Journal 2009;30(12):1537‐41. [EMBASE: 2010055533; PUBMED: 19936416] [PubMed] [Google Scholar]
Mishra 2012 {published data only}
- Mishra S. Prospective, randomized study to assess the role of dexmedetomidine in patients with posterior fossa tumors undergoing craniotomy and requiring post operative ventilation in ICU. Intensive Care Medicine 2012;38:S36‐7. [EMBASE: 71013335] [Google Scholar]
Mizrak 2010 {published data only}
- Mizrak A, Koruk S, Bilgi M, Kocamer B, Erkutlu I, Ganidagli S, et al. Pretreatment with dexmedetomidine or thiopental decreases myoclonus after etomidate: a randomized, double‐blind controlled trial. Journal of Surgical Research 2010;159(1):e11‐6. [EMBASE: 2010115952; PUBMED: 20018300] [DOI] [PubMed] [Google Scholar]
Mizrak 2012 {published data only}
- Mizrak A, Sanli M, Bozgeyik S, Gul R, Ganidagli S, Baysal E, et al. Dexmedetomidine use in direct laryngoscopic biopsy under TIVA. Middle East Journal of Anesthesiology 2012;21(4):605‐12. [PUBMED: 23327034] [PubMed] [Google Scholar]
Mizrak 2013 {published data only}
- Mizrak A, Ganidagli S, Cengiz MT, Oner U, Saricicek V. The effects of DEX premedication on volatile induction of mask anesthesia (VIMA) and sevoflurane requirements. Journal of Clinical Monitoring and Computing 2013;27(3):329‐34. [EMBASE: 2013337331; PUBMED: 23400425] [DOI] [PubMed] [Google Scholar]
Moghadam 2012 {published data only}
- Moghadam MJ, Ommi D, Mirkheshti A, Shadnoush M, Dabbagh A. The effect of pretreatment with clonidine on propofol consumption in opium abuser and non‐abuser patients undergoing elective leg surgery. Journal of Research in Medical Sciences 2012;17(8):728‐31. [EMBASE: 2012537334; PUBMED: 23798938] [PMC free article] [PubMed] [Google Scholar]
Mohamed 2012 {published data only}
- Mohamed AA, Fares KM, Mohamed SA. Efficacy of intrathecally administered dexmedetomidine versus dexmedetomidine with fentanyl in patients undergoing major abdominal cancer surgery. Pain Physician 2012;15(4):339‐48. [EMBASE: 2012453151; PUBMED: 22828688] [PubMed] [Google Scholar]
Mohamed 2013 {published data only}
- Mohamed HS, Asida SM, Salman OH. Dexmedetomidine versus nimodipine for controlled hypotension during spine surgery. Egyptian Journal of Anaesthesia 2013;29(4):325‐31. [EMBASE: 2013684468] [Google Scholar]
Mohammadi 2007 {published data only}
- Mohammadi SS, Seyedi M. Effects of oral clonidine in preventing postoperative shivering after general anesthesia. International Journal of Pharmacology 2007;3(5):441‐3. [EMBASE: 2008112839] [Google Scholar]
Mohammadi 2008 {published data only}
- Mohammadi SS, Seyedi M. Comparing oral gabapentin versus clonidine as premedication on early postoperative pain, nausea and vomiting after general anesthesia. International Journal of Pharmacology 2008;4(2):153‐6. [EMBASE: 2009077239] [Google Scholar]
Mousa 2013 {published data only}
- Mousa SA, Abd Elfatah Alsobky H. Efficacy and effect of TIVA with propofol or dexmedetomidine versus sevoflurane without muscle relaxant during repair of the brachial plexus. Egyptian Journal of Anaesthesia 2013;29(1):31‐40. [EMBASE: 2013099363] [Google Scholar]
Muhammad 2012 {published data only}
- Muhammad HB, Esa N, Idris NH, Sidik H. Is dexmedetomidine with patient controlled analgesia morphine better than continuous morphine infusion as a post operative analgesia in off pump coronary artery bypass grafting surgery? A preliminary study. British Journal of Anaesthesia 2012;108:ii398‐9. [EMBASE: 70719618] [Google Scholar]
Murari Sudre 2004 {published data only}
- Murari Sudre EC, MDo Salvador C, Elena Bruno G, Valentim Vassallo D, Rocha Lauretti G, Sudre Filho GN. Remifentanil versus dexmedetomidine as coadjutants of standardized anesthetic technique in morbidly obese patients. Revista Brasileira de Anestesiologia 2004;54(2):178‐89. [EMBASE: 2004138134; PUBMED: 19471725] [DOI] [PubMed] [Google Scholar]
Myatra 2010 {published data only}
- Myatra SN, Dalvi N, Divatia JV, Mohite S, Pethkar T, Patil V, et al. A observer blind, randomized, parallel group, comparative, study to evaluate safety and efficacy of dexmedetomidine hcl versus propofol for postoperative sedation in the intensive care unit. Intensive Care Medicine 2010;36:S327. [EMBASE: 70291183] [Google Scholar]
Nader 2001 {published data only}
- Nader ND, Ignatowski TA, Kurek CJ, Knight PR, Spengler RN. Clonidine suppresses plasma and cerebrospinal fluid concentrations of TNF‐alpha during the perioperative period. Anesthesia and Analgesia 2001;93(2):363‐9. [PUBMED: 11473862] [DOI] [PubMed] [Google Scholar]
Nader 2009 {published data only}
- Nader ND, Li CM, Dosluoglu HH, Ignatowski TA, Spengler RN. Adjuvant therapy with intrathecal clonidine improves postoperative pain in patients undergoing coronary artery bypass graft. Clinical Journal of Pain 2009;25(2):101‐6. [EMBASE: 2009320482; PUBMED: 19333153] [DOI] [PubMed] [Google Scholar]
Nakagawa 2001 {published data only}
- Nakagawa M, Mammoto T, Sakai T, Kishi Y, Mashimoto T. Premedication modifies the quality of sedation with propofol during regional anesthesia. Canadian Journal of Anaesthesia 2001;48(3):284‐7. [PUBMED: 11305831] [DOI] [PubMed] [Google Scholar]
Nitta 2013 {published data only}
- Nitta R, Goyagi T, Nishikawa T. Combination of oral clonidine and intravenous low‐dose ketamine reduces the consumption of postoperative patient‐controlled analgesia morphine after spine surgery. Acta Anaesthesiologica Taiwanica 2013;51(1):14‐7. [EMBASE: 2013334847; PUBMED: 23711600] [DOI] [PubMed] [Google Scholar]
Nour El‐Din 2004 {published data only}
- Nour El‐Din BM. Clinical evaluation of dexmedetomidine following ultra‐fast track off‐pump coronary artery bypass grafting. Egyptian Journal of Anaesthesia 2004;20(3):253‐9. [EMBASE: 2004379459] [Google Scholar]
Nunez‐Bacarreza 2006 {published data only}
- Nunez‐Bacarreza JJ, Portela‐Ortiz JM, Magro‐Ibanez E, Garcia‐Hernandez L, Cabrera‐Jardines R, Alarcon‐Rodriguez J. Hypertension induced by pneumoperitoneum and its treatment with dexmedetomidine. Revista Mexicana de Anestesiologia 2006;29(2):70‐3. [EMBASE: 2006386472] [Google Scholar]
Oddby‐Muhrbeck 2002 {published data only}
- Oddby‐Muhrbeck E, Eksborg S, Bergendahl HTG, Muhrbeck O, Lonnqvist PA. Effects of clonidine on postoperative nausea and vomiting in breast cancer surgery. Anesthesiology 2002;96(5):1109‐14. [PUBMED: 11981150] [DOI] [PubMed] [Google Scholar]
Ohata 1999 {published data only}
- Ohata H, Iida H, Watanabe Y, Dohi S. Hemodynamic responses induced by dopamine and dobutamine in anesthetized patients premedicated with clonidine. Anesthesia and Analgesia 1999;89(4):843‐8. [PUBMED: 10512253] [DOI] [PubMed] [Google Scholar]
Ohtani 2008 {published data only}
- Ohtani N, Kida K, Shoji K, Yasui Y, Masaki E. Recovery profiles from dexmedetomidine as a general anesthetic adjuvant in patients undergoing lower abdominal surgery. Anesthesia and Analgesia 2008;107(6):1871‐4. [EMBASE: 2009307257; PUBMED: 19020132] [DOI] [PubMed] [Google Scholar]
Ohtani 2011 {published data only}
- Ohtani N, Yasui Y, Watanabe D, Kitamura M, Shoji K, Masaki E. Perioperative infusion of dexmedetomidine at a high dose reduces postoperative analgesic requirements: a randomized control trial. Journal of Anesthesia 2011;25(6):872‐8. [EMBASE: 2011705273; PUBMED: 21953329] [DOI] [PubMed] [Google Scholar]
Okuyama 2005 {published data only}
- Okuyama K, Inomata S, Toyooka H. The effects of prostaglandin E1 or oral clonidine premedication on blood loss during paranasal sinus surgery. Canadian Journal of Anaesthesia 2005;52(5):546‐7. [PUBMED: 15872138] [DOI] [PubMed] [Google Scholar]
Omote 1995 {published data only}
- Omote K, Satoh O, Sonoda H, Kumeta Y, Yamaya K, Namiki A. Effects of oral alpha 2 adrenergic agonists, clonidine and tizanidine, on tetracaine spinal anesthesia. Masui 1995;44(6):816‐23. [PUBMED: 7637157] [PubMed] [Google Scholar]
Onodera 2011 {published data only}
- Onodera Y, Yamagishi A, Kunisawa T, Kurosawa A, Takahata O, Iwasaki H. Postoperative analgesia by continuous intravenous fentanyl or dexmedetomidine in patients receiving anticoagulant therapy. [Japanese]. Japanese Journal of Anesthesiology 2011;60(8):936‐40. [EMBASE: 2011473798; PUBMED: 21861419] [PubMed] [Google Scholar]
Owen 1997 {published data only}
- Owen MD, Fibuch EE, McQuillan R, Millington WR. Postoperative analgesia using a low‐dose, oral‐transdermal clonidine combination: lack of clinical efficacy. Journal of Clinical Anesthesia 1997;9(1):8‐14. [PUBMED: 9051539] [DOI] [PubMed] [Google Scholar]
Ozbakis 2008 {published data only}
- Ozbakis Akkurt BC, Inanoglu K, Turhanoglu S, Asfuroglu Z. Effects of dexmedetomidine and tramadol administered before induction of anesthesia on postoperative pain. [Turkish]. Anestezi Dergisi 2008;16(4):183‐7. [EMBASE: 2009075183] [Google Scholar]
Ozkose 2006 {published data only}
- Ozkose Z, Demir FS, Pampal K, Yardim S. Hemodynamic and anesthetic advantages of dexmedetomidine, an a2‐agonist, for surgery in prone position. Tohoku Journal of Experimental Medicine 2006;210(2):153‐60. [PUBMED: 17023769] [DOI] [PubMed] [Google Scholar]
Panda 2012a {published data only}
- Panda BK, Singh P, Marne S, Pawar A, Keniya V, Ladi S, et al. A comparison study of dexmedetomidine vs clonidine for sympathoadrenal response, perioperative drug requirements and cost analysis. Asian Pacific Journal of Tropical Disease 2012;2(SUPPL2):S815‐21. [EMBASE: 2013011143] [Google Scholar]
Panda 2012b {published data only}
- Panda NB, Amutha V. Dexmedetomidine‐an ideal anesthetic adjuvant in cervical spine surgery. European Journal of Anaesthesiology 2012;29:S18‐9. [EMBASE: 71077477] [Google Scholar]
Pandazi 2011 {published data only}
- Pandazi A, Karamanis P, Sidiropoulou T, Matsota P, Papasideris C, Niokou D, et al. Low‐dose (1 mug/kg) clonidine premedication and hypotension after carotid artery surgery. Vascular and Endovascular Surgery 2011;45(7):614‐8. [EMBASE: 2011577079; PUBMED: 21984028] [DOI] [PubMed] [Google Scholar]
Pant 2012 {published data only}
- Pant KC, Gandhi S. Intravenous clonidine improves glycemic control in type‐2 diabetic patients undergoing laparoscopic cholecystectomy. British Journal of Anaesthesia 2012;108:ii350‐1. [EMBASE: 70719541] [Google Scholar]
Parameswara 2012 {published data only}
- Parameswara G, Badgandi A, Murthy HK. Clonidine vs dexmeditomidine in intracranial tumor surgeries: comparison of haemodynamic stability, brain relaxation and emergence. British Journal of Anaesthesia 2012;108:ii74. [EMBASE: 70719073] [Google Scholar]
Paris 2009 {published data only}
- Paris A, Kaufmann M, Tonner PH, Renz P, Lemke T, Ledowski T, et al. Effects of clonidine and midazolam premedication on bispectral index and recovery after elective surgery. European Journal of Anaesthesiology 2009;26(7):603‐10. [EMBASE: 2010242180; PUBMED: 19367170] [DOI] [PubMed] [Google Scholar]
Park 1996 {published data only}
- Park J, Forrest J, Kolesar R, Bhola D, Beattie S, Chu C. Oral clonidine reduces postoperative PCA morphine requirements. Canadian Journal of Anaesthesia 1996;43(9):900‐6. [PUBMED: 8874906] [DOI] [PubMed] [Google Scholar]
Park 2012 {published data only}
- Park JK, Cheong SH, Lee KM, Lim SH, Lee JH, Cho K, et al. Does dexmedetomidine reduce postoperative pain after laparoscopic cholecystectomy with multimodal analgesia?. Korean Journal of Anesthesiology 2012;63(5):436‐40. [EMBASE: 2012714558] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parlow 1999 {published data only}
- Parlow JL, Begou G, Sagnard P, Cottet‐Emard JM, Levron JC, Annat G, et al. Cardiac baroreflex during the postoperative period in patients with hypertension: effect of clonidine. Anesthesiology 1999;90(3):681‐92. [PUBMED: 10078667] [DOI] [PubMed] [Google Scholar]
Patel 2012 {published data only}
- Patel CR, Engineer SR, Shah BJ, Madhu S. Effect of intravenous infusion of dexmedetomidine on perioperative haemodynamic changes and postoperative recovery: a study with entropy analysis. Indian Journal of Anaesthesia 2012;56(6):542‐6. [EMBASE: 2012747718; PUBMED: 23325938] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patil 2012 {published data only}
- Patil PM, Patil SP. Is clonidine an adequate alternative to epinephrine as a vasoconstrictor in patients with hypertension?. Journal of Oral and Maxillofacial Surgery 2012;70(2):257‐62. [EMBASE: 2012038438; PUBMED: 21940091] [DOI] [PubMed] [Google Scholar]
Pestilci 2015 {published data only}
- Pestilci Z, Sergin D, Alper I, Kocabas S, Ulukaya S, Askar FZ. Propofol versus dexmedetomidine for postoperative sedation in fast‐track cardiac anesthesia. [Turkish]. Gogus‐Kalp‐Damar Anestezi ve Yogun Bakim Dernegi Dergisi 2015;21(1):8‐15. [EMBASE: 2015077461] [Google Scholar]
Piper 1999 {published data only}
- Piper SN, Suttner SW, Schmidt CC, Maleck WH, Kumle B, Boldt J. Nefopam and clonidine in the prevention of postanaesthetic shivering. Anaesthesia 1999;54:695‐9. [PUBMED: 10417466] [DOI] [PubMed] [Google Scholar]
Piper 2004 {published data only}
- Piper SN, Rohm KD, Suttner SW, Maleck WH, Kranke P, Boldt J. A comparison of nefopam and clonidine for the prevention of postanaesthetic shivering: a comparative, double‐blind and placebo‐controlled dose‐ranging study. Anaesthesia 2004;59:559‐64. [PUBMED: 15144295] [DOI] [PubMed] [Google Scholar]
Porkkala 1998 {published data only}
- Porkkala T, Jantti V, Hakkinen V, Kaukinen S. Colonidine does not attenuate median nerve somatosensory evoked potentials during isoflurane anesthesia. Journal of Clinical Monitoring and Computing 1998;14:165‐70. [PUBMED: 9676863] [DOI] [PubMed] [Google Scholar]
Pouttu 1987 {published data only}
- Pouttu J, Scheinin B, Rosenberg PH, Viinamaki O, Scheinin M. Oral premedication with clonidine: effects on stress responses during general anaesthesia. Acta Anaesthesiologica Scandinavica 1987;31(8):730‐4. [PUBMED: 3434164] [DOI] [PubMed] [Google Scholar]
Procaccini 1993 {published data only}
- Procaccini B, Clementi G, Varrassi G. Effects of clonidine vs trinitroglycerin on myocardial oxygen balance and on pulmonary gas exchange after myocardial revascularization. Minerva Anestesiologica 1993;59(5):235‐45. [PUBMED: 8355864] [PubMed] [Google Scholar]
Quintin 1990 {published data only}
- Quintin L, Bonnet F, Macquin I, Szekely B, Becquemin JP, Ghignone M. Aortic surgery: effect of clonidine on intraoperative catecholaminergic and circulatory stability. Acta Anaesthesiologica Scandinavica 1990;34(2):132‐7. [PUBMED: 2407044] [DOI] [PubMed] [Google Scholar]
Quintin 1991a {published data only}
- Quintin L, Roudot F, Roux C, Macquin I, Basmaciogullari A, Guyene T, et al. Effect of clonidine on the circulation and vasoactive hormones after aortic surgery. British Journal of Anaesthesia 1991;66(1):108‐15. [PUBMED: 1997045] [DOI] [PubMed] [Google Scholar]
Quintin 1991b {published data only}
- Quintin L, Viale JP, Annat G, Hoen JP, Butin E, Cottet‐Emard JM, et al. Oxygen uptake after major abdominal surgery: effect of clonidine. Anesthesiology 1991;74(2):236‐41. [PUBMED: 1990899] [DOI] [PubMed] [Google Scholar]
Raouf 2004 {published data only}
- Raouf A, Aziz MA. Use of dexmedetomidine as an adjuvant during sodium nitroprusside‐induced hypotension in middle ear surgery. Egyptian Journal of Anaesthesia 2004;20(2):127‐34. [EMBASE: 2004207703] [Google Scholar]
Ray 2010 {published data only}
- Ray M, Bhattacharjee DP, Hajra B, Pal R, Chatterjee N. Effect of clonidine and magnesium sulphate on anaesthetic consumption, haemodynamics and postoperative recovery: a comparative study. Indian Journal of Anaesthesia 2010;54(2):137‐41. [EMBASE: 2011051651; PUBMED: 20661352] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reddy 2013 {published data only}
- Reddy VS, Shaik NA, Donthu B, Sannala VKR, Jangam V. Intravenous dexmedetomidine versus clonidine for prolongation of bupivacaine spinal anesthesia and analgesia: a randomized double‐blind study. Journal of Anaesthesiology Clinical Pharmacology 2013;29(3):342‐7. [EMBASE: 2013584803; PUBMED: 24106359] [DOI] [PMC free article] [PubMed] [Google Scholar]
Richa 2008 {published data only}
- Richa F, Yazigi A, Sleilaty G, Yazbeck P. Comparison between dexmedetomidine and remifentanil for controlled hypotension during tympanoplasty. European Journal of Anaesthesiology 2008;25(5):369‐74. [PUBMED: 18294411] [DOI] [PubMed] [Google Scholar]
Rohrbach 1999 {published data only}
- Rohrbach A, Jage J, Olthoff D. Intraoperative clonidine modulates sympathetic tone in the early postoperative period after remifentanil. A double blind, placebo‐controlled study. Acute Pain 1999;2(3):129‐38. [EMBASE: 1999373401] [Google Scholar]
Rosenfeld 1993 {published data only}
- Rosenfeld BA, Faraday N, Campbell D, Dorman T, Clarkson K, Siedler A, et al. Perioperative platelet reactivity and the effects of clonidine. Anesthesiology 1993;79(2):255‐61. [PUBMED: 8342838] [DOI] [PubMed] [Google Scholar]
Ruan 2011 {published data only}
- Ruan XC, Ruan X, He L, She S. Dexthomedine does not increase risk of trigemino‐Cardiac reflex during microvascular trigeminal decompression in cases of trigeminal neuralgia. Journal of Neurosurgical Anesthesiology 2011;23(4):423. [EMBASE: 70538809] [PubMed] [Google Scholar]
Rubino 2010 {published data only}
- Rubino AS, Onorati F, Caroleo S, Galato E, Nucera S, Amantea B, et al. Impact of clonidine administration on delirium and related respiratory weaning after surgical correction of acute type‐A aortic dissection: results of a pilot study. Interactive Cardiovascular and Thoracic Surgery 2010;10(1):58‐62. [EMBASE: 2010057909; PUBMED: 19854793] [DOI] [PubMed] [Google Scholar]
Salgado 2008 {published data only}
- Salgado PFS, Sabbag AT, Silva PC, Brienze SLA, Dalto HP, Modolo NSP, et al. Synergistic effect between dexmedetomidine and 0.75% ropivacaine in epidural anesthesia. [Portuguese]. Revista da Associacao Medica Brasileira 2008;54(2):110‐5. [PUBMED: 18506317] [DOI] [PubMed] [Google Scholar]
Salgado Filho 2013 {published data only}
- Salgado Filho IPM. The influence of pre‐anesthetic medication with clonidine on glycemic response in coronary artery bypass graft surgery with cardiopulmonary by‐pass. Cardiology (Switzerland) 2013;126:306. [EMBASE: 71231861] [Google Scholar]
Samantaray 2012 {published data only}
- Samantaray A, Rao M, Chandra A. The effect on post‐operative pain of intravenous clonidine given before induction of anaesthesia. Indian Journal of Anaesthesia 2012;56(4):359‐64. [EMBASE: 2012539842; PUBMED: 23087458] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sassi 2013 {published data only}
- Sassi M, Zekaj E, Grotta A, Pollini A, Pellanda A, Borroni M, et al. Safety in the use of dexmedetomidine (Precedex) for deep brain stimulation surgery: our experience in 23 randomized patients. Neuromodulation 2013;16(5):401‐6. [EMBASE: 2013695343; PUBMED: 22780449] [DOI] [PubMed] [Google Scholar]
Scheinin 1992 {published data only}
- Scheinin B, Lindgren L, Randell T, Scheinin H, Scheinin M. Dexmedetomidine attenuates sympathoadrenal responses to tracheal intubation and reduces the need for thiopentone and peroperative fentanyl. British Journal of Anaesthesia 1992;68(2):126‐31. [PUBMED: 1347229] [DOI] [PubMed] [Google Scholar]
Schlimp 2011 {published data only}
- Schlimp CJ, Pipam W, Wolrab C, Ohner C, Kager HI, Likar R. Clonidine for remifentanil‐induced hyperalgesia: a double‐blind randomized, placebo‐controlled study of clonidine under intra‐operative use of remifentanil in elective surgery of the shoulder. [German]. Schmerz 2011;25(3):290‐5. [EMBASE: 2011388625; PUBMED: 21594659] [DOI] [PubMed] [Google Scholar]
Schreiberova 2008 {published data only}
- Schreiberova J, Hess L, Krajina A, Lojik M. Dexmedetomidine‐ketamine‐midazolam combination for sedation in endovascular treatment of cerebral arterio‐venousmalformations and carotid artery stenosis. [Czech]. Ceska a Slovenska Neurologie a Neurochirurgie 2008;71(4):446‐52. [EMBASE: 2008446158] [Google Scholar]
Segal 1991 {published data only}
- Segal IS, Jarvis DJ, Duncan SR, White PF, Maze M. Clinical efficacy of oral‐transdermal clonidine combinations during the perioperative period. Anesthesiology 1991;74(2):220‐5. [PUBMED: 1990896] [DOI] [PubMed] [Google Scholar]
Selina 2011 {published data only}
- Selina F, Akhtaruzzaman KM, Talha KA, Hossain MM, Iqbal KM. Role of oral clonidine on intraoperative haemodynamic stability for craniotomies of intracranial space occupying lesion. Mymensingh medical journal : MMJ 2011;20(2):257‐63. [PUBMED: 21522097] [PubMed] [Google Scholar]
Senses 2013 {published data only}
- Senses E, Apan A, Arzu Kouml, se E, Oz G, Rezak H. The effects of midazolam and dexmedetomidine infusion on peri‐operative anxiety in regional anesthesia. Middle East Journal of Anesthesiology 2013;22(1):35‐40. [EMBASE: 23833848] [PubMed] [Google Scholar]
Shams 2013 {published data only}
- Shams T, Bahnasawe NS, Abu‐Samra M, El‐Masry R. Induced hypotension for functional endoscopic sinus surgery: a comparative study of dexmedetomidine versus esmolol. Saudi Journal of Anaesthesia 2013;7(2):175‐80. [EMBASE: 2013447907; PUBMED: 23956719] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shin 2012 {published data only}
- Shin YS, Ko YK. Preemptive analgesia by intravenous morphine combined with fentanyl and clonidine in spinal surgery. Pain Practice 2012;12:140. [EMBASE: 70654969] [Google Scholar]
Shin 2013 {published data only}
- Shin HW, Yoo HN, Kim DH, Lee H, Shin HJ, Lee HW. Preanesthetic dexmedetomidine 1 mug/kg single infusion is a simple, easy, and economic adjuvant for general anesthesia. Korean Journal of Anesthesiology 2013;65(2):114‐20. [EMBASE: 2013571775] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shrestha 2012 {published data only}
- Shrestha BR, Gautam B, Shrestha S, Maharjan SK. Study of haemodynamic and endocrine stress responses following carbon dioxide pneumoperitonium. Journal of Nepal Health Research Council 2012;10(1):41‐6. [PUBMED: 22929636] [PubMed] [Google Scholar]
Shukla 2011 {published data only}
- Shukla D, Verma A, Agarwal A, Pandey HD, Tyagi C. Comparative study of intrathecal dexmedetomidine with intrathecal magnesium sulfate used as adjuvants to bupivacaine. Journal of Anaesthesiology Clinical Pharmacology 2011;27(4):495‐9. [EMBASE: 2011599873; PUBMED: 22096283] [DOI] [PMC free article] [PubMed] [Google Scholar]
Si 2011 {published data only}
- Si YN, Zhang Y, Lu YL, Chen H, Bao HG. Influences of dexmedetomidine in doses of propofol and fentanyl and recovery from anesthesia. [Chinese]. Journal of Jilin University Medicine Edition 2011;37(1):135‐8. [EMBASE: 2013739730] [Google Scholar]
Simoni 2009 {published data only}
- Simoni RF, Cangiani LM, Pereira AMSA, Abreu MP, Cangiani LH, Zemi G. Efficacy of intraoperative methadone and clonidine in pain control in the immediate postoperative period after the use of remifentanil. [Portuguese, English]. Revista Brasileira de Anestesiologia 2009;59(4):421‐30. [EMBASE: 2009503681; PUBMED: 19669016] [DOI] [PubMed] [Google Scholar]
Singh 2011 {published data only}
- Singh S, Arora K. Effect of oral clonidine premedication on perioperative haemodynamic response and postoperative analgesic requirement for patients undergoing laparoscopic cholecystectomy. Indian Journal of Anaesthesia 2011;55(1):26‐30. [EMBASE: 2011246356; PUBMED: 21431049] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2013 {published data only}
- Singh M, Choudhury A, Kaur M, Liddle D, Verghese M, Balakrishnan I. The comparative evaluation of intravenous with intramuscular clonidine for suppression of hemodynamic changes in laparoscopic cholecystectomy. Saudi Journal of Anaesthesia 2013;7(2):181‐6. [EMBASE: 2013447905; PUBMED: 23956720] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh Bajwa 2012 {published data only}
- Singh Bajwa SJ, Gupta S, Kaur J, Singh A, Parmar SS. Reduction in the incidence of shivering with perioperative dexmedetomidine: a randomized prospective study. Journal of Anaesthesiology Clinical Pharmacology 2012;28(1):86‐91. [EMBASE: 2012120704; PUBMED: 22345953] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sitilci 2010 {published data only}
- Sitilci AT, Ozyuvaci E, Alkan Z, Demirgan S, Yigit O. The effect of perioperative infused dexmedetomidine on postoperative analgesic consumption in mastoidectomy operations. [Turkish]. Agri 2010;22(3):109‐16. [PUBMED: 20865582] [PubMed] [Google Scholar]
Solanki 2013 {published data only}
- Solanki SI, Bharti NA, Batra YK, Jain A, Kumar P, Nikhar S. The analgesic effect of intrathecal dexmedetomidine or clonidine, with bupivacaine, in trauma patients undergoing lower limb surgery: a randomised, double‐blind study. Anaesthesia and Intensive Care 2013;41(1):51‐6. [PUBMED: 23362890] [DOI] [PubMed] [Google Scholar]
Soliman 2011 {published data only}
- Soliman RN, Hassan AR, Rashwan AM, Omar AM. Prospective, randomized controlled study to assess the role of dexmedetomidine in patients with supratentorial tumors undergoing craniotomy under general anesthesia. Middle East Journal of Anesthesiology 2011;21(1):23‐33. [PUBMED: 21991729] [PubMed] [Google Scholar]
Stapelfeldt 2005 {published data only}
- Stapelfeldt C, Lobo EP, Brown R, Talke PO. Intraoperative clonidine administration to neurosurgical patients. Anesthesia and Analgesia 2005;100(1):226‐32. [PUBMED: 15616082] [DOI] [PubMed] [Google Scholar]
Stocche 2004 {published data only}
- Stocche RM, Garcia LV, Klamt JG, Dos Reis MP, Gil DR, Mesquita KL. Influence of Iintravenous clonidine in the cost of sevoflurane anesthesia for outpatient middle ear procedures. Revista Brasileira de Anestesiologia 2004;54:91‐8. [EMBASE: 2004033413; PUBMED: 19471716] [DOI] [PubMed] [Google Scholar]
Striebel 1993 {published data only}
- Striebel WH, Koenigs DI, Kramer JA. Intravenous clonidine fails to reduce postoperative meperidine requirements. Journal of Clinical Anesthesia 1993;5(3):221‐5. [PUBMED: 8318241] [DOI] [PubMed] [Google Scholar]
Sudar 2013 {published data only}
- Sudar Codi R, Selvarajan N, Manimekalai K, Salwe KJ. Effect of oral clonidine premedication on the duration of analgesia produced by spinal bupivacaine. International Journal of Pharma and Bio Sciences 2013;4(3):P1017‐24. [EMBASE: 2013500054] [Google Scholar]
Sulemanji 2007 {published data only}
- Sulemanji DS, Donmez A, Aldemir D, Sezgin A, Turkoglu S. Dexmedetomidine during coronary artery bypass grafting surgery: is it neuroprotective? A preliminary study. Acta Anaesthesiologica Scandinavica 2007;51(8):1093‐8. [PUBMED: 17697305] [DOI] [PubMed] [Google Scholar]
Sun 2013 {published data only}
- Sun S, Huang SQ. Effects of pretreatment with a small dose of dexmedetomidine on sufentanil‐induced cough during anesthetic induction. Journal of Anesthesia 2013;27(1):25‐8. [PUBMED: 22923258] [DOI] [PubMed] [Google Scholar]
Sung 2000 {published data only}
- Sung CS, Lin SH, Chan KH, Chang WK, Chow LH, Lee TY. Effect of oral clonidine premedication on perioperative hemodynamic response and postoperative analgesic requirement for patients undergoing laparoscopic cholecystectomy. Acta Anaesthesiologica Sinica 2000;38(1):23‐9. [EMBASE: 2000193331; PUBMED: 11000660] [PubMed] [Google Scholar]
Taheri 2010 {published data only}
- Taheri A, Javadimanesh MA, Ashraf H. The effect of oral clonidine premedication on nausea and vomiting after ear surgery. Middle East Journal of Anesthesiology 2010;20(5):691‐4. [PUBMED: 20803858] [PubMed] [Google Scholar]
Taittonen 1997a {published data only}
- Taittonen M, Kirvela O, Aantaa R, Kanto J. Cardiovascular and metabolic responses to clonidine and midazolam premedication. European Journal of Anaesthesiology 1997;14(2):190‐6. [PUBMED: 9088819] [DOI] [PubMed] [Google Scholar]
Taittonen 1997b {published data only}
- Taittonen MT, Kirvela OA, Aantaa R, Kanto JH. Effect of clonidine and dexmedetomidine premedication on perioperative oxygen consumption and haemodynamic state. British Journal of Anaesthesia 1997;78(4):400‐6. [PUBMED: 9135361] [DOI] [PubMed] [Google Scholar]
Taittonen 1998 {published data only}
- Taittonen MT, Kirvela OA, Aantaa R, Kanto JH. The effect of clonidine or midazolam premedication on perioperative responses during ketamine anesthesia. Anesthesia and Analgesia 1998;87(1):161‐7. [PUBMED: 9661567] [DOI] [PubMed] [Google Scholar]
Talke 1997 {published data only}
- Talke P, Tong C, Lee HW, Caldwell J, Eisenach JC, Richardson CA. Effect of dexmedetomidine on lumbar cerebrospinal fluid pressure in humans. Anesthesia and Analgesia 1997;85(2):358‐64. [PUBMED: 9249114] [DOI] [PubMed] [Google Scholar]
Tanskanen 2006 {published data only}
- Tanskanen PE, Kytta JV, Randell TT, Aantaa RE. Dexmedetomidine as an anaesthetic adjuvant in patients undergoing intracranial tumour surgery: a double‐blind, randomized and placebo‐controlled study. British Journal of Anaesthesia 2006;97(5):658‐65. [PUBMED: 16914460] [DOI] [PubMed] [Google Scholar]
Techanivate 2012 {published data only}
- Techanivate A, Dusitkasem S, Anuwattanavit C. Dexmedetomidine compare with fentanyl for postoperative analgesia in outpatient gynecologic laparoscopy: a randomized controlled trial. Journal of the Medical Association of Thailand 2012;95(3):383‐90. [EMBASE: 2012162406; PUBMED: 22550837] [PubMed] [Google Scholar]
Tekin 2007 {published data only}
- Tekin M, Kati I, Tomak Y, Kisli E. Effect of dexmedetomidine IV on the duration of spinal anesthesia with prilocaine: a double‐blind, prospective study in adult surgical patients. Current Therapeutic Research ‐ Clinical and Experimental 2007;68(5):313‐24. [EMBASE: 2007611059; PUBMED: 24692763] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomson 1998 {published data only}
- Thomson IR, Peterson MD, Hudson RJ. A comparison of clonidine with conventional preanesthetic medication in patients undergoing coronary artery bypass grafting. Anesthesia and Analgesia 1998;87(2):292‐9. [PUBMED: 9706918] [DOI] [PubMed] [Google Scholar]
Toz 2010 {published data only}
- Toz ZP, Sergin D, Alper I, Kocabas S, Ulukaya S, Askar FZ. The comparison of dexmedetomidine and propofol for postoperative sedation in fast‐track cardiac anaesthesia. Journal of Cardiothoracic and Vascular Anesthesia 2010;24(3 suppl 1):23. [EMBASE: 70157149] [Google Scholar]
Traill 1993 {published data only}
- Traill R, Gillies R. Clonidine premedication for craniotomy: effects on blood pressure and thiopentone dosage. Journal of Neurosurgical Anesthesiology 1993;5(3):171‐7. [PUBMED: 8400756] [DOI] [PubMed] [Google Scholar]
Triltsch 2002 {published data only}
- Triltsch AE, Welte M, Homeyer P, Grobe J, Genahr A, Moshirzadeh M, et al. Bispectral index‐guided sedation with dexmedetomidine in intensive care: a prospective, randomized, double blind, placebo‐controlled phase II study. Critical Care Medicine 2002;30(5):1007‐14. [PUBMED: 12006795] [DOI] [PubMed] [Google Scholar]
Tufanogullari 2008 {published data only}
- Tufanogullari B, White PF, Peixoto MP, Kianpour D, Lacour T, Griffin J, et al. Dexmedetomidine infusion during laparoscopic bariatric surgery: the effect on recovery outcome variables. Anesthesia and Analgesia 2008;106(6):1741‐8. [PUBMED: 18499604] [DOI] [PubMed] [Google Scholar]
Turgut 2009 {published data only}
- Turgut N, Turkmen A, Ali A, Altan A. Remifentanil‐propofol vs dexmedetomidine‐propofol‐anesthesia for supratentorial craniotomy. Middle East Journal of Anesthesiology 2009;20(1):63‐70. [PUBMED: 19266828] [PubMed] [Google Scholar]
Tzortzopoulou 2009 {published data only}
- Tzortzopoulou A, Kolotoura A, Koudouna E, Kyralidou A, Adreotti B, Tagara M, et al. Continuous surgical wound infusion with either ropivacaine or combination of ropivacaine and clonidine via elastomeric pump. A randomized double‐blind study. Pain Practice 2009;9:153‐4. [EMBASE: 70207423] [Google Scholar]
Unlugenc 2005 {published data only}
- Unlugenc H, Gunduz M, Guler T, Yagmur O, Isik G. The effect of pre‐anaesthetic administration of intravenous dexmedetomidine on postoperative pain in patients receiving patient‐controlled morphine. European Journal of Anaesthesiology 2005;22(5):386‐91. [PUBMED: 15918389] [DOI] [PubMed] [Google Scholar]
Usta 2011 {published data only}
- Usta B, Gozdemir M, Demircioglu RI, Muslu B, Sert H, Yaldiz A. Dexmedetomidine for the prevention of shivering during spinal anesthesia. Clinics 2011;66(7):1187‐91. [PUBMED: 21876972] [DOI] [PMC free article] [PubMed] [Google Scholar]
Uyar 2008 {published data only}
- Uyar AS, Yagmurdur H, Fidan Y, Topkaya C, Basar H. Dexmedetomidine attenuates the hemodynamic and neuroendocrinal responses to skull‐pin head‐holder application during craniotomy. Journal of Neurosurgical Anesthesiology 2008;20(3):174‐9. [PUBMED: 18580347] [DOI] [PubMed] [Google Scholar]
Vanderstappen 1996 {published data only}
- Vanderstappen I, Vandermeersch E, Vanacker B, Mattheusen M, Herijgers P, Aken H. The effect of prophylactic clonidine on postoperative shivering. A large prospective double‐blind study. Anaesthesia 1996;51(4):351‐5. [PUBMED: 8686824] [DOI] [PubMed] [Google Scholar]
von Dossow 2006 {published data only}
- Dossow V, Baehr N, Moshirzadeh M, Heymann C, Braun JP, Hein OV, et al. Clonidine attenuated early proinflammatory response in T‐cell subsets after cardiac surgery. Anesthesia and Analgesia 2006;103(4):809‐14. [PUBMED: 17000786] [DOI] [PubMed] [Google Scholar]
Vukovic 2012 {published data only}
- Vukovic J, Ramakrishnan P, Milan Z. Does epidural clonidine improve postoperative analgesia in major vascular surgery?. Medicinski Glasnik 2012;9(1):49‐55. [EMBASE: 2012129422; PUBMED: 22631908] [PubMed] [Google Scholar]
Wahlander 2005 {published data only}
- Frumento RJ, Logginidou HG, Wahlander S, Wagener G, Playford HR, Sladen RN. Dexmedetomidine infusion is associated with enhanced renal function after thoracic surgery. Journal of Clinical Anesthesia 2006;18(6):422‐6. [PUBMED: 16980158] [DOI] [PubMed] [Google Scholar]
- Wahlander S, Frumento RJ, Wagener G, Saldana‐Ferretti B, Joshi RR, Playford HR, et al. A prospective, double‐blind, randomized, placebo‐controlled study of dexmedetomidine as an adjunct to epidural analgesia after thoracic surgery. Journal of Cardiothoracic and Vascular Anesthesia 2005;19:630‐5. [PUBMED: 16202898] [DOI] [PubMed] [Google Scholar]
Wallenborn 2008 {published data only}
- Wallenborn J, Thieme V, Hertel‐Gilch G, Grafe K, Richter O, Schaffranietz L. Effects of clonidine and superficial cervical plexus block on hemodynamic stability after carotid endarterectomy. Journal of Cardiothoracic and Vascular Anesthesia 2008;22(1):84‐9. [EMBASE: 2008056133; PUBMED: 18249336] [DOI] [PubMed] [Google Scholar]
Wan 2011 {published data only}
- Wan LJ, Huang QQ, Yue JX, Lin L, Li SH. Comparison of sedative effect of dexmedetomidine and midazolam for post‐operative patients undergoing mechanical ventilation in surgical intensive care unit. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue/Chinese Critical Care Medicine/Zhongguo Weizhongbing Jijiuyixue 2011;23(9):543‐6. [PUBMED: 21944176] [PubMed] [Google Scholar]
Wang 2012 {published data only}
- Wang BS, Yu JB, Wang F, Zhang L, Zhang Y, Li SQ. Effect of dexmedetomidine on stress responses during extubation in patients undergoing uvulopalatopharyngoplasty. [Chinese]. Zhonghua er bi yan hou tou jing wai ke za zhi = Chinese journal of otorhinolaryngology head and neck surgery 2012;47(6):498‐501. [PUBMED: 22932146] [PubMed] [Google Scholar]
Wawrzyniak 2013 {published data only}
- Wawrzyniak K, Kusza K, Cywinski JB, Burduk PK, Kazmierczak W. Premedication with clonidine before TIVA optimizes surgical field visualization and shortens duration of endoscopic sinus surgery ‐ Results of a clinical trial. Rhinology 2013;51(3):259‐64. [PUBMED: 23943734] [DOI] [PubMed] [Google Scholar]
Weilbach 2009 {published data only}
- Weilbach C, Vangerow H, Karst M, Bernateck M, Piepenbrock S, Braun J, et al. Respiratory depression following general anaesthesia involving clonidine combined with fentanyl ‐ a prospective randomized trial. [German]. Anasthesiologie und Intensivmedizin 2009;50(5):269‐75. [EMBASE: 2009288404] [Google Scholar]
Wright 1990 {published data only}
- Wright PM, Carabine UA, McClune S, Orr DA, Moore J. Preanaesthetic medication with clonidine. British Journal of Anaesthesia 1990;65(5):628‐32. [PUBMED: 2248839] [DOI] [PubMed] [Google Scholar]
Xu 2010 {published data only}
- Xu ZL, Xu XG, Cui SQ. Effects of dexmedetomidine on blood glucose, beta‐endorphin, tumor necrosis factor‐alpha and interleukin‐6 in patients undergoing radical esophagectomy. [Chinese]. Academic Journal of Second Military Medical University 2010;31(12):1330‐2. [EMBASE: 2011027447] [Google Scholar]
Xue 2014 {published data only}
- Xue FS, Wang SY, Cui XL, Li RP. Does perioperative dexmedetomidine improve mortality after coronary artery bypass surgery?. Journal of Cardiothoracic and Vascular Anesthesia 2014;28(5):e46‐7. [EMBASE: 2014708231; PUBMED: 25130426] [DOI] [PubMed] [Google Scholar]
Yacout 2012 {published data only}
- Yacout AG, Osman HA, Abdel‐Daem MH, Hammouda SA, Elsawy MM. Effect of intravenous dexmedetomidine infusion on some proinflammatory cytokines, stress hormones and recovery profile in major abdominal surgery. Alexandria Journal of Medicine 2012;48(1):3‐8. [EMBASE: 2012077551] [Google Scholar]
Yadav 2013 {published data only}
- Yadav G, Pratihary BN, Jain G, Paswan AK, Mishra LD. A prospective, randomized, double blind and placebo‐control study comparing the additive effect of oral midazolam and clonidine for postoperative nausea and vomiting prophylaxis in granisetron premedicated patients undergoing laparoscopic cholecystecomy. Journal of Anaesthesiology Clinical Pharmacology 2013;29(1):61‐5. [EMBASE: 2013091118; PUBMED: 23493482] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2013 {published data only}
- Yang ZJ, Zhang XA, Hu B, Shao WD, Xu B, Tu WF. Different doses of dexmedetomidine used for sedation in lower limb operation under combined spinal‐epidural anesthesia. [Chinese]. Chinese Journal of New Drugs 2013;22(3):326‐30. [EMBASE: 2013499185] [Google Scholar]
Yektaz 2011a {published data only}
- Yektaz A. The postoperative analgesic properties of intrathecal 2 Kg and 4 Kg dexmedetomidine combined with hyperbaric bupivacaine and its effects on the spinal anaesthesia. Regional Anesthesia and Pain Medicine 2011;36(5 suppl.2):E226. [EMBASE: 70735895] [Google Scholar]
Yektaz 2011b {published data only}
- Yektaz A. Postoperative analgesic characteristics of intrathecal adjuvant agents including ketamine, fentanyl, sufentanyl, neostigmine, dexmedetomidine, midazolame and droperidole and their effects on spinal anesthesia. Regional Anesthesia and Pain Medicine 2011;36(5 suppl.2):E226. [EMBASE: 70735893] [Google Scholar]
Yldrm 2012 {published data only}
- Yldrm A, Kaya FN, Yavaccaolu B, Baaan‐Mool E. The effects of preanesthetic, different two single‐doses dexmedetomidine on the onset time of rocuronium. British Journal of Anaesthesia 2012;108:ii361‐2. [EMBASE: 70719559] [Google Scholar]
Yoganarasimha 2012 {published data only}
- Yoganarasimha N, Raghavendra TR, Sridhar K, Amitha S, Radha MK. Intrathecal clonidine as an adjuvant for postoperative analgesia. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2012; Vol. 3, issue 4:589‐96. [EMBASE: 2013138569]
Yotsui 2001 {published data only}
- Yotsui T. Clonidine premedication prevents sympathetic hyperactivity but does not prevent hypothalamo‐pituitary‐adrenocortical responses in patients undergoing laparoscopic cholecystectomy. Journal of Anesthesia 2001;15(2):78‐82. [PUBMED: 14566527] [DOI] [PubMed] [Google Scholar]
Yu 2003 {published data only}
- Yu HP, Hseu SS, Yien HW, Teng YH, Chan KH. Oral clonidine premedication preserves heart rate variability for patients undergoing larparoscopic cholecystectomy. Acta Anaesthesiologica Scandinavica 2003;47(2):185‐90. [PUBMED: 12631048] [DOI] [PubMed] [Google Scholar]
Zalunardo 2000 {published data only}
- Zalunardo MP, Zollinger A, Spahn DR, Seifert B, Pasch T. Preoperative clonidine attenuates stress response during emergence from anesthesia. Journal of Clinical Anesthesia 2000;12(5):343‐9. [PUBMED: 11025232] [DOI] [PubMed] [Google Scholar]
Zalunardo 2002 {published data only}
- Zalunardo MP, Serafino D, Szelloe P, Weisser F, Zollinger A, Seifert B, et al. Preoperative clonidine blunts hyperadrenergic and hyperdynamic responses to prolonged tourniquet pressure during general anesthesia. Anesthesia and Analgesia 2002;94(3):615‐8. [PUBMED: 11867385] [DOI] [PubMed] [Google Scholar]
Zalunardo 2010 {published data only}
- Zalunardo MP, Ivleva‐Sauerborn A, Seifert B, Spahn DR. Quality of premedication and patient satisfaction after premedication with midazolam, clonidine or placebo: randomized double‐blind study with age‐adjusted dosage. [German]. Anaesthesist 2010;59(5):410‐8. [EMBASE: 2010381440; PUBMED: 20224951] [DOI] [PubMed] [Google Scholar]
Zhang 2013a {published data only}
- Zhang XD, Piao MH, Wang YS, Feng CS. Influence of sub‐anesthetic dose of ketamine and dexmedetomidine on early postoperative cognitive function in elderly orthopedic patients under total intravenous anesthesia. [Chinese]. Journal of Jilin University Medicine Edition 2013;39(1):133‐7. [EMBASE: 2013147858] [Google Scholar]
Zhang 2013b {published data only}
- Liu C, Zhang Y, She S, Xu L, Ruan X. A randomised controlled trial of dexmedetomidine for suspension laryngoscopy. Anaesthesia 2013;68(1):60‐6. [PUBMED: 23106186] [DOI] [PubMed] [Google Scholar]
Zhou 2011 {published data only}
- Wu ZL, Zhou ZF, Xu LX, She SZ. [Effect of dexmedetomidine on patient‐controlled intravenous analgesia with fentanyl in elderly patients after total hip replacement]. Nan Fang Yi Ke Da Xue Xue Bao = Journal of Southern Medical University 2011;31(4):701‐4. [PUBMED: 21515474] [PubMed] [Google Scholar]
Additional references
Anon 2013
- Anon. Retraction notice to "Dexmedetomidine infusion is associated with enhanced renal function after thoracic surgery" (J Clin Anesth 2006;18:422‐6). Journal of Clinical Anesthesia 2013;25(5):432. [PUBMED: 24228273] 24228273 [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [PUBMED: 21208779] [DOI] [PubMed] [Google Scholar]
Biccard 2008
- Biccard BM, Goga S, Beurs J. Dexmedetomidine and cardiac protection for non‐cardiac surgery: a meta‐analysis of randomised controlled trials. Anaesthesia 2008;63(1):4‐14. [PUBMED: 18086064] [DOI] [PubMed] [Google Scholar]
Blaudszun 2012
- Blaudszun G, Lysakowski C, Elia N, Tramèr MR. Effect of perioperative systemic α2 agonists on postoperative morphine consumption and pain intensity: systematic review and meta‐analysis of randomized controlled trials. Anesthesiology 2012;116(6):1312‐22. [PUBMED: 22546966] [DOI] [PubMed] [Google Scholar]
Devereaux 2014b
- Devereaux PJ. Rationale and design of the PeriOperative ISchemic Evaluation‐2 (POISE‐2) trial: an international 2 x 2 factorial randomized controlled trial of acetyl‐salicylic acid vs. placebo and clonidine vs. placebo in patients undergoing noncardiac surgery. American Heart Journal 2014;167(6):804‐9.e4. [PUBMED: 24890528] [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309:1286‐91. [PUBMED: 7718048] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleischmann 2003
- Fleischmann KE, Goldman L, Young B, Lee TH. Association between cardiac and noncardiac complications in patients undergoing noncardiac surgery: outcomes and effects on length of stay. American Journal of Medicine 2003;115(7):515‐20. [PUBMED: 14599629] [DOI] [PubMed] [Google Scholar]
Flood 2015
- Flood P, Rathmell JP, Shafer S. Stoelting’s Pharmacology and Physiology in Anesthetic Practice. 5th Edition. Philadelphia (PA): Wolters Kluwer Health, 2015. [Google Scholar]
Force 1990
- Force T, Hibberd P, Weeks G, Kemper AJ, Bloomfield P, Tow D, et al. Perioperative myocardial infarction after coronary artery bypass surgery. Clinical significance and approach to risk stratification. Circulation 1990;82(3):903‐12. [PUBMED: 2394010] [DOI] [PubMed] [Google Scholar]
GRADE Handbook 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). GRADE handbook for grading quality of evidence and strength of recommendations. Available from guidelinedevelopment.org/handbook: The GRADE Working Group, October 2013. [Google Scholar]
GRADEpro [Computer program]
- GRADE Working Group. GRADEpro Guideline Development Tool. McMaster University (developed by Evidence Prime Inc.), 2015.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ (Clinical Research Ed.) 2008;336(7651):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Halter 1997
- Halter JB, Pflug AE, Porte DJ. Mechanism of plasma catecholamine increases during surgical stress in man. Journal of Clinical Endocrinology and Metabolism 1977;45(5):936‐44. [PUBMED: 925142] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks J, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [PUBMED: 22008217] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011b
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Khan 1999
- Khan ZP, Ferguson CN, Jones RM. Alpha‐2 and imidazoline receptor agonists. Anaesthesia 1999;54(2):146‐65. [PUBMED: 10215710] [DOI] [PubMed] [Google Scholar]
Meara 2015
- Meara JG, Leather AJM, Hagander L, Alkire BC, Alonso N, Ameh EA, et al. Global Surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet 21 April 2015;386(99993):569‐624. [PUBMED: 25924834] [DOI] [PubMed] [Google Scholar]
Muzi 1992
- Muzi M, Goff DR, Kampine JP, Roerig DL, Ebert TJ. Clonidine reduces sympathetic activity but maintains baroreflex responses in normotensive humans. Anesthesiology 1992;77(5):864‐71. [PUBMED: 1443738] [DOI] [PubMed] [Google Scholar]
Nishina 2002
- Nishina K, Mikawa K, Uesugi T, Obara H, Maekawa M, Kamae I, et al. Efficacy of clonidine for prevention of perioperative myocardial ischemia. A critical appraisal and meta‐analysis of the literature. Anesthesiology 2002;96(2):323‐9. [PUBMED: 11818763] [DOI] [PubMed] [Google Scholar]
POISE 2008
- POISE Study Group. Effects of extended‐release metoprolol succinate in patients undergoing non‐cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008;371(9627):1839‐47. [PUBMED: 18479744] [DOI] [PubMed] [Google Scholar]
Rasmussen 2011
- Rasmussen LS, Yentis SM, Aken H, Shafer SL, Eisenach JC, Edmunds LH, et al. Editors‐in‐Chief statement regarding published clinical trials conducted without IRB approval by Joachim Boldt. Minerva Anestesiologica 2011;77(5):562‐3. [PUBMED: 21540815] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schulz 2010
- Schulz KF, Altman DG, Moher D, the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [PUBMED: 20332509 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheth 2015
- Sheth T, Chan M, Butler C, Chow B, Tandon V, Nagele P, et al. Prognostic capabilities of coronary computed tomographic angiography before non‐cardiac surgery: prospective cohort study. BMJ 2015;350:h1907. [PUBMED: 25902738] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stevens 2003
- Stevens RD, Burri H, Tramer MR. Pharmacologic myocardial protection in patients undergoing noncardiac surgery: a quantitative systematic review. Anesthesia and Analgesia 2003;97(3):623‐33. [PUBMED: 12933373] [DOI] [PubMed] [Google Scholar]
Svensson 2013
- Svensson S, Menkes DB, Lexchin J. Surrogate outcomes in clinical trials: a cautionary tale. JAMA Internal Medicine 2013;173(8):611‐12. [PUBMED: 23529157] [DOI] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Imberger G, Walsh M, Chu R, Gluud C, Wetterslev J, et al. The number of patients and events required to limit the risk of overestimation of intervention effects in meta‐analysis ‐ a simulation study. PloS One 2011;6(10):e25491. [PUBMED: 22028777] [DOI] [PMC free article] [PubMed] [Google Scholar]
VISION 2014
- Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Writing Group. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30‐day outcomes. Anesthesiology 2014;120(3):564‐78. [PUBMED: 24534856] [DOI] [PubMed] [Google Scholar]
Wijeysundera 2014b
- Wijeysundera D, Duncan D, Nkonde‐Price C, Virani S, Washam J, Fleischmann K, et al. Perioperative beta blockade in noncardiac surgery: a systematic review for the 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation 2014;130(24):2246‐64. [PUBMED: 25085964] [DOI] [PubMed] [Google Scholar]
Wise 2013
- Wise J. Boldt: the great pretender. BMJ (Clinical Research Ed.) 2013;346:f1738. [PUBMED: 23512099] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Wijeysundera 2003
- Wijeysundera DN, Naik JS, Beattie WS. Alpha‐2 adrenergic agonists prevent perioperative cardiovascular complications: a meta‐analysis. American Journal of Medicine 2003;114:742‐52. [DOI] [PubMed] [Google Scholar]
Wijeysundera 2009
- Wijeysundera DN, Bender JS, Beattie WS. Alpha‐2 adrenergic agonists for the prevention of cardiac complications among patients undergoing surgery. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD004126.pub2] [DOI] [PubMed] [Google Scholar]


