Abstract
Background
Bronchiectasis is a chronic inflammatory disease characterised by a recurrent cycle of respiratory bacterial infections associated with cough, sputum production and impaired quality of life. Antibiotics are the main therapeutic option for managing bronchiectasis exacerbations. Evidence suggests that inhaled antibiotics may be associated with more effective eradication of infective organisms and a lower risk of developing antibiotic resistance when compared with orally administered antibiotics. However, it is currently unclear whether antibiotics are more effective when administered orally or by inhalation.
Objectives
To determine the comparative efficacy and safety of oral versus inhaled antibiotics in the treatment of adults and children with bronchiectasis.
Search methods
We identified studies through searches of the Cochrane Airways Group's Specialised Register (CAGR), which is maintained by the Information Specialist for the group. The Register contains trial reports identified through systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, CINAHL, AMED, and PsycINFO, and handsearching of respiratory journals and meeting abstracts. We also searched ClinicalTrials.gov and the WHO trials portal. We searched all databases in March 2018 and imposed no restrictions on language of publication.
Selection criteria
We planned to include studies which compared oral antibiotics with inhaled antibiotics. We would have considered short‐term use (less than four weeks) for treating acute exacerbations separately from longer‐term use as a prophylactic (4 weeks or more). We would have considered both intraclass and interclass comparisons. We planned to exclude studies if the participants received continuous or high‐dose antibiotics immediately before the start of the trial, or if they have received a diagnosis of cystic fibrosis (CF), sarcoidosis, active allergic bronchopulmonary aspergillosis or active non‐tuberculous Mycobacterial infection.
Data collection and analysis
Two review authors independently applied study inclusion criteria to the searches and we planned for two authors to independently extract data, assess risk of bias and assess overall quality of the evidence using GRADE criteria. We also planned to obtain missing data from the authors where possible and to report results with 95% confidence intervals (CIs).
Main results
We identified 313 unique records through database searches and a further 21 records from trial registers. We excluded 307 on the basis of title and abstract alone and a further 27 after examining full‐text reports. No studies were identified for inclusion in the review.
Authors' conclusions
There is currently no evidence indicating whether orally administered antibiotics are more beneficial compared to inhaled antibiotics. The recent ERS bronchiectasis guidelines provide a practical approach to the use of long‐term antibiotics. New research is needed comparing inhaled versus oral antibiotic therapies for bronchiectasis patients with a history of frequent exacerbations, to establish which approach is the most effective in terms of exacerbation prevention, quality of life, treatment burden, and antibiotic resistance.
Plain language summary
Oral versus inhaled antibiotics for people with bronchiectasis
Review question
We wished to know whether oral antibiotics (taken by mouth) or inhaled antibiotics are more effective for reducing the duration and frequency of infective episodes of bronchiectasis, admissions to hospital and side effects, as well as reducing the risk of chest infections not responding to treatment with antibiotics.
Background
Bronchiectasis is a long‐term incurable condition where people get repeated bacterial chest infections that lead to frequent cough, breathlessness and mucus production. These often occur three or more times a year and require treatment with antibiotics, either short‐term for the presenting chest infection, or long‐term to prevent chest infections recurring. It was once thought to be an uncommon disease but recent figures show that up to 5 people in every 1000 may have bronchiectasis and the death rate for people with the condition may be more than twice that of the general population.
Antibiotics are commonly used to treat chest infections in people with bronchiectasis, to eliminate the specific types of bacteria that cause the infection. Some antibiotics are more effective against particular types of bacteria compared to others, and these different types of bacteria can develop resistance to treatment with antibiotics, making them less effective and reducing the subsequent choice of antibiotic. Antibiotics can also be given to people in different ways, such as by mouth in pill form or breathed in as an inhalation.
We do not currently know which method of administering antibiotics, orally or by inhalation, is the most effective for treating recurrent chest infections in terms of eliminating the bacteria, reducing the chances of people developing resistance to antibiotics and reducing the symptoms of bronchiectasis.
We searched for all the published and unpublished available evidence, up until March 2018, which compared orally administered antibiotics versus inhaled antibiotics.
Study characteristics
While there have been a few studies investigating the benefits of antibiotics for people with bronchiectasis, none have compared orally administered antibiotics with inhaled antibiotics.
Quality of the evidence
There is no high‐quality evidence available to determine whether oral or inhaled antibiotics are more helpful for people with bronchiectasis. More research studies are needed to evaluate the effectiveness of oral antibiotics compared to inhaled antibiotics for reducing the rate of chest infections and the chances of developing resistance to antibiotic therapy.
Background
Description of the condition
Bronchiectasis is a chronic inflammatory lung disease that presents with cough, sputum production and recurrent respiratory tract infections (Pasteur 2010). It is defined radiologically by the presence of permanently dilated airways usually visualised on computed tomography (CT). Bronchiectasis represents a final common pathway of multiple disorders with the most common associations being with severe infections (pneumonia, childhood infection and Mycobacterial infection), allergic bronchopulmonary aspergillosis, rheumatological diseases, inflammatory bowel disease and disorders of mucociliary clearance such as primary ciliary dyskinesia (Lonni 2015). Treatments for bronchiectasis have historically been extrapolated from cystic fibrosis with a focus on antibiotic treatments and physiotherapy (Chalmers 2016).
Although previously considered a relatively rare disease (Kolbe 1996), bronchiectasis appears to be increasing, with higher rates in developing countries, women and those aged over 60 years (Chang 2003; Weycker 2005; Habesoglu 2011; Seitz 2012). Prevalence rates may also be higher in children from ethnic populations, for example indigenous Australians (up to 14 per 1000) and Native Alaskan children (up to 20.5 per 1000) (Singleton 2000; Chang 2002). International prevalence rates in the general population are variable, ranging from 0.5 per 100,000 in Finland to 3.7 per 100,000 in New Zealand (European Lung White Book 2013). More recent data from the UK reported an increase of over 60% in prevalence over a nine‐year period, with 263,000 adults living with bronchiectasis in 2013 (Quint 2016). Incidence rates increased by 63% to 35 per 100,000 in women and 27 per 100,000 in men, with over 15,000 new cases in 2013. However, increased prevalence may be partly attributable to increased awareness of bronchiectasis and more efficient detection through CT scanning (Goeminne 2016).
Mortality rates increased by 3% per year during a six‐year period to 2007 in England and Wales (Roberts 2010). In the USA, hospitalisations also rose annually by 3% over a nine‐year period (Seitz 2010). Average European mortality rates from 2005 to 2009 are estimated at 0.3 per 100,000 general population in EU countries (from 0.01 in Germany to 1.18 in the UK) and 0.2 per 100,000 in nine non‐EU countries (from 0.01 in Azerbaijan to 0.67 in Kyrgyzstan) (European Lung White Book 2013). Recent age‐adjusted mortality rates for the UK were more than twice that of the general population (2.26 times higher in women; 2.14 times higher in men) (Quint 2016).
Description of the intervention
Bronchiectasis is characterised by a common pathophysiological pathway that consists of a vicious cycle. Three elements play a pivotal role in this cycle: inflammation, infection and airway damage by enzymatic components. In this cycle, infection or colonisation by various micro‐organisms cause an inflammatory response. When this inflammation is not able to clear the micro‐organism, the inflammation can become chronic and even excessive compared to the bacterial burden. This can then finally result in airway damage and remodelling (Goeminne 2010).
Interventions aiming to reduce or break this vicious cycle often focus on the treatment of the chronic bacterial infection. Data show that these chronic infections are most often caused by Gram‐negatives, with a special focus on Pseudomonas aeruginosa as this has been linked with more severe disease and increased morbidity and mortality (Wilson 2016). To treat or eradicate these chronic infections, long courses and high dosage of systemic antibiotic treatment are often required. This is frequently accompanied by side effects and can also result in resistance. Therefore, inhaled antibiotics are increasingly being considered, as they can deliver high concentrations of the antibiotic at the site of infection with less systemic absorption and toxicity, but can result in increased airway irritation or bronchospasm (Geller 2009).
How the intervention might work
A recent Cochrane review of 18 trials in patients with bronchiectasis receiving prolonged antibiotics, showed that there was a significant reduction of exacerbation risk (Hnin 2015). Furthermore, recent data clearly suggest an important relationship between inflammation and bacterial load/presence in bronchiectasis. Chronic Pseudomonas aeruginosa infection was associated with increased matrix metalloprotease activity and a higher bacterial load was associated with an increase in hospitalisations, exacerbations and symptom severity (Chalmers JD 2012; Goeminne 2014). Chalmers et al also showed that both short‐ and long‐term antibiotic treatment significantly reduced airway and systemic inflammation. This is in line with a series of long‐term systemic antibiotic therapy trials with macrolides, proving that long‐term oral macrolides are useful for patients with bronchiectasis in reducing exacerbations and improving clinical symptoms (Wong 2012; Altenburg 2013; Serisier 2013). It is speculated that macrolides not only act through their antibacterial activity but also have anti‐inflammatory and immunomodulatory effect (Altenburg 2011a). These long‐term oral macrolide treatments, however, raise some concerns as to safety and bacterial resistance (Altenburg 2011b).
Inhaled antibiotics may provide an effective suppressive antibiotic therapy with an acceptable safety profile in adult patients with stable bronchiectasis and chronic bronchial infection. Their use has been widespread in CF since the early 1990s, as inhaled antibiotics improve lung function and reduce exacerbation rates (Ryan 2011). For inhaled antibiotics, different antibiotic regimens have been investigated in non‐CF bronchiectasis, including inhaled amikacin, aztreonam, ciprofloxacin, gentamicin, colistin and tobramycin. The antibiotics chosen often have a concentration‐dependent effect, where a greater area under the curve/minimum inhibitory concentration ratio improves bacterial killing (Restrepo 2015). As resistance is one of the concerns in chronic antibiotic treatment, these inhaled antibiotics may achieve very high concentrations of the drug in the airways, overcoming bacterial resistance (Dudley 2008; Rubin 2008; Quon 2014). On the other hand, inhalation antibiotic treatment is hampered by a delivery that is not uniform, creating a concentration gradient with lower concentrations in deeper parts of the lung (Rubin 2008). In non‐CF bronchiectasis, a recent review found that long‐term inhaled antibiotics can effectively reduce the sputum bacterial density, increase Pseudomonas aeruginosa eradication and attenuate the risk of exacerbation, but with higher risk of wheeze and bronchospasm (Yang 2016).
Why it is important to do this review
In meta‐analyses of trials involving participants with non‐CF bronchiectasis, authors have concluded that inhaled antibiotics reduced sputum bacterial load and the risk of acute exacerbation, with an acceptable safety profile, when compared to symptomatic treatment or placebo (Brodt 2014; Yang 2016). However, in reality, clinicians will often be faced with the choice between various routes of delivering antibiotics, not only the choice of whether or not to give them. A comparison between the oral and inhaled route was highlighted as a priority in a recently published overview of interventions for bronchiectasis (Welsh 2015).The potential benefits of improved bacterial killing and reduced risk of bacterial resistance described above need to be weighed against the cost of drug delivery via inhalation and specific side effects associated with this route, such as bronchospasm and wheeze (BNF (online); Brodt 2014; Yang 2016).
Therefore in this review we will include studies that directly compare the effectiveness and safety of delivering antibiotics by inhalation or orally, both in an acute setting and for longer‐term prophylaxis in people with bronchiectasis. We intend to summarise the evidence to provide the most up‐to‐date information for guideline developers, clinicians and patients, and highlight future research needs. This review is being conducted alongside four other closely related Cochrane reviews on macrolide antibiotics for bronchiectasis (Kelly 2016); dual antibiotics for bronchiectasis (Felix 2017); head‐to‐head trials of antibiotics for bronchiectasis (Kaehne 2017); and continuous versus intermittent antibiotics for bronchiectasis (Donovan 2017).
Objectives
To determine the comparative efficacy and safety of oral versus inhaled antibiotics in the treatment of adults and children with bronchiectasis.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised controlled trials (RCTs), reported as full‐text or published as abstract only or unpublished data.
Types of participants
We planned to include adults and children diagnosed with bronchiectasis by bronchography, plain film chest radiograph, or high‐resolution computed tomography. We planned to exclude studies if the participants received continuous or high‐dose antibiotics immediately before the start of the trial, or if they had received a diagnosis of cystic fibrosis (CF), sarcoidosis, active allergic bronchopulmonary aspergillosis or active non‐tuberculous Mycobacterial infection.
Types of interventions
We planned to include studies comparing oral antibiotics with inhaled antibiotics. We would have considered short‐term use (less than 4 weeks) for treating acute exacerbations separately from longer‐term use as a prophylactic (4 weeks or more). We would have considered both intraclass and interclass comparisons. We planned to include the following comparison groups.
Inhaled aminoglycosides versus oral antibiotics
Inhaled polymyxin versus oral antibiotics
Inhaled beta‐lactam versus oral antibiotics
Types of outcome measures
Primary outcomes
We planned to include the following primary outcomes for short‐term therapy, longer‐term therapy or both, as indicated.
Duration of exacerbation (short‐term)
Exacerbation (both), e.g. frequency during follow‐up or time to first exacerbation
Hospitalisations due to exacerbations (both)
Serious adverse events (both)
Secondary outcomes
Response rates as defined by study authors, e.g. diary cards of physician global assessment
Sputum volume and purulence
Measures of lung function, e.g. forced expiratory volume in one second (FEV1)
Adverse events, e.g. cardiac arrhythmias, gastrointestinal symptoms, hearing impairment, bronchospasm
Mortality
Emergence of resistance to antibiotics or treatment emergent pathogens
Exercise capacity, e.g. Six‐Minute Walk Test (6MWT)
Quality of life (QOL), e.g. St George Respiratory Questionnaire (SGRQ) or alternative QOL tools
Eradication of pathogens
Reporting one or more of the outcomes listed here in the study was not used as an inclusion criterion for the review.
We planned to include the above secondary outcomes for both short‐term and long‐term therapy.
Search methods for identification of studies
Electronic searches
We identified studies from the Cochrane Airways Specialised Register, which is maintained by the Information Specialist for the group. The Cochrane Airways Specialised Register contains studies identified from several sources:
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies Online (crso.cochrane.org);
Weekly searches of MEDLINE Ovid SP, 1946 to date;
Weekly searches of Embase Ovid SP, 1974 to date;
Monthly searches of PsycINFO Ovid SP, 1967 to date;
Monthly searches of CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature), 1937 to date;
Monthly searches of AMED EBSCO (Allied and Complementary Medicine);
Handsearches of the proceedings of major respiratory conferences.
Studies contained in the Specialised Register are identified through search strategies based on the scope of Cochrane Airways. Details of these strategies, as well as a list of handsearched conference proceedings are in Appendix 1. See Appendix 2 for search terms used to identify studies for this review.
We also conducted a search of US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/). We searched all databases from their inception to 5 March 2018, and we imposed no restriction on language of publication.
Searching other resources
We planned to check the reference lists of all primary studies and review articles for additional references. As there are multiple manufacturers of both oral and inhaled antibiotics and many are off‐patent, we did not conduct a search of manufacturers' websites for study information.
We planned to search for errata or retractions from included studies published in full text on PubMed and report the date of this search within the review.
Data collection and analysis
Selection of studies
Two review authors (TD and RN) screened the titles and abstracts of the search results independently and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We then retrieved the full‐text study reports of all potentially eligible studies and two review authors (TD and RN) independently screened them for inclusion, recording the reasons for exclusion of ineligible studies. We did not have any disagreements, but if we had then we planned to resolve this through discussion or, if required, by consulting a third person/review author (SS/SJM). We planned to identify and exclude duplicates and collate multiple reports of the same study so that each study, rather than each report, would be the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and 'Characteristics of excluded studies' table (Moher 2009).
Data extraction and management
We planned to use a data collection form for study characteristics and outcome data, which was used for a similar review on a closely related topic. For future updates, we will extract the following study characteristics from included studies.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and location, study setting, withdrawals and date of study.
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria and exclusion criteria.
Interventions: intervention, comparison, concomitant medications and excluded medications.
Outcomes: baseline exacerbation data (e.g. frequency, duration), primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for studies and notable conflicts of interest of trial authors.
If we identify eligible trials in future updates of this review, two review authors (RN and TD) will independently extract outcome data from included studies. We will note in the 'Characteristics of included studies' table if outcome data were not reported in a usable way. We will resolve disagreements by consensus or by involving a third person/review author (SS/SJM). One review author will transfer data into the Review Manager file (RevMan 2014). This entered data will be double‐checked for accuracy by another review author by comparing the data presented in the systematic review with the study reports.
Assessment of risk of bias in included studies
In future updates, if any studies meet the inclusion criteria, then two review authors will assess risk of bias independently for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We will resolve any disagreements by discussion or by involving another author (SS/SJM). We will assess the risk of bias according to the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting; and
other bias.
If we identify eligible trials in future updates of this review, we will judge each potential source of bias as high, low or unclear risk, and provide a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We will summarise the 'Risk of bias' judgements across different studies for each of the domains listed. We plan to consider blinding separately for different key outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐reported quality of life scale). Where information on risk of bias relates to unpublished data or correspondence with a trialist, we will note this in the 'Risk of bias' table.
When considering treatment effects, we will take into account the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to this published protocol and planned to justify any deviations from it in the 'Differences between protocol and review' section of the systematic review.
Measures of treatment effect
If we identify eligible trials in future updates of this review, we will adhere to the following data analysis plan.
We will analyse dichotomous data as odds ratios (ORs) and continuous data as mean differences (MDs) or standardised mean differences (SMDs). We will enter data presented as a scale (e.g. quality of life measures) with a consistent direction of effect. We will describe skewed data narratively (for example, as medians and interquartile ranges for each group).
We will undertake meta‐analyses only where this is meaningful; that is, if the treatments, participants and the underlying clinical question are similar enough for pooling to make sense.
Where multiple trial arms are reported in a single study, we will include only the relevant arms. If two comparisons (e.g. drug A versus placebo and drug B versus placebo) are combined in the same meta‐analysis, we will either combine the active arms or halve the control group to avoid double‐counting.
If adjusted analyses are available (ANOVA or ANCOVA) we will use these as a preference in our meta‐analyses. If both change from baseline and endpoint scores are available for continuous data, we will use change from baseline scores unless there is low correlation between measurements in individuals. If a study reports outcomes at multiple time points (repeated observations), we will perform separate analyse for different periods of follow‐up.
We will use intention‐to‐treat (ITT) analyses where they are reported (i.e. all those who were randomised are analysed) instead of completer or per protocol analyses.
Unit of analysis issues
If we identify eligible trials in future updates of this review, we will adhere to the following plan. For dichotomous outcomes, we will use participants, rather than events, as the unit of analysis (i.e. number of children admitted to hospital, rather than number of admissions per child). However, if rate ratios are reported in a study, we will analyse them on this basis. We will only meta‐analyse data from cluster‐RCTs if the available data have been adjusted (or can be adjusted), to account for the clustering.
Dealing with missing data
If we identify eligible trials in future updates of this review, we will contact investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study is identified as an abstract only). Where this is not possible, and the missing data are thought to introduce serious bias, we will take this into consideration in the GRADE rating for affected outcomes.
Assessment of heterogeneity
If we identify eligible trials in future updates of this review, we will use the I² statistic to measure heterogeneity among the studies in each analysis. If we identify substantial heterogeneity we will report it and explore the possible causes by prespecified subgroup analysis.
Assessment of reporting biases
If we identify eligible trials in future updates of this review, and are able to pool more than 10 studies, we will create and examine a funnel plot to explore possible small study and publication biases.
Data synthesis
If we identify eligible trials in future updates of this review, we will use a random‐effects model and perform a sensitivity analysis with a fixed‐effect model.
'Summary of findings' table
If we identify eligible trials in future updates of this review, we will create a 'Summary of findings' table using the following outcomes: duration of exacerbations, exacerbations (frequency and time to first exacerbation), frequency of hospitalisations due to exacerbations, serious adverse events, response rates, mortality and quality of life. We will use the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to the studies that contribute data for the prespecified outcomes. We will use the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using GRADEpro software (GRADEpro GDT). We will justify all decisions to downgrade the quality of studies using footnotes and we will make comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
If we identify eligible trials in future updates of this review, we plan to carry out the following subgroup analyses.
Adults versus children (18 years or younger)
Patients chronically infected with Pseudomonas aeruginosa versus those not infected with Pseudomonas aeruginosa
Macrolide versus non‐macrolide oral antibiotic
We will use the following outcomes in subgroup analyses.
Exacerbation duration (short‐term therapy)
Exacerbation, e.g. frequency during follow‐up or time to first exacerbation
Hospitalisation due to exacerbations
Adverse events
We will use the formal test for subgroup interactions in Review Manager (RevMan 2014).
Sensitivity analysis
If we identify eligible trials in future updates of this review, we plan to carry out the following sensitivity analyses, removing the studies judged as high risk of bias from the primary outcome analyses.
Exacerbation duration (short‐term therapy)
Exacerbation, e.g. frequency during follow‐up or time to first exacerbation (both)
Hospitalisation due to exacerbations
Adverse events
We will compare the results from a fixed‐effect model with the random‐effects model.
Results
Description of studies
Results of the search
We identified 313 unique records through database searches and a further 21 records from trial registries (www.ClinicalTrials.gov and www.who.int/ictrp/en/). We excluded 307 records on the basis of title and abstract and a further 27 after examining the full text. Details of the search are shown in the flow diagram Figure 1. The searches were conducted in March 2018.
1.
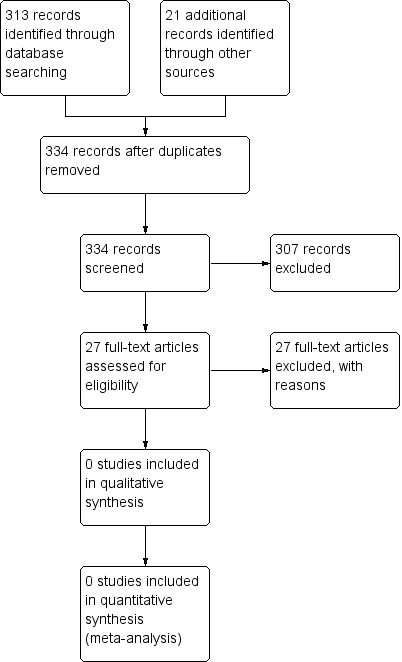
Study flow diagram
Included studies
We did not identify any randomised controlled trials (RCTs) meeting our prespecified inclusion criteria.
Excluded studies
We excluded 27 studies after reviewing the full‐text publication. Reasons for exclusion were: trial compared an inhaled or nebulised antibiotic to placebo or usual care (n = 20); the record was a letter to editor (n = 2); trial compared addition of inhaled antibiotics to oral antibiotics (n = 2); trial compared intravenous followed by oral antibiotics for an acute exacerbation of bronchiectasis to a full intravenous course of antibiotics (n = 1); trial was not randomised and investigated impact of long‐ and short‐term antibiotic treatment on inflammation (n = 1); and trial compared addition of inhaled antibiotics to intravenous antibiotics versus intravenous antibiotics alone (n = 1). See Characteristics of excluded studies.
Risk of bias in included studies
We did not identify any RCTs meeting our prespecified inclusion criteria.
Effects of interventions
We did not identify any RCTs meeting our prespecified inclusion criteria.
Discussion
Summary of main results
It was not possible to achieve our aim to provide an overview of the effectiveness of oral versus inhaled antibiotics for bronchiectasis with respect to our predefined outcomes; our comprehensive search found no randomised controlled trials meeting our predefined inclusion criteria (see: Criteria for considering studies for this review). The absence of evidence addressing this question is a cause for considerable concern as uncertainties remain regarding the most effective route of administration for reducing exacerbations and minimising the development of antibiotic resistance .
Overall completeness and applicability of evidence
Unfortunately we were unable to assess the completeness and applicability of evidence on oral versus inhaled antibiotics for bronchiectasis as no studies met our inclusion criteria.
Quality of the evidence
We were unable to consider the quality of evidence comparing oral versus inhaled antibiotics for bronchiectasis as no relevant clinical trials were available.
Potential biases in the review process
Our searches for relevant clinical trials were extensive and comprehensive, with expert support from the Cochrane Airways Group.
Agreements and disagreements with other studies or reviews
We did not identify any systematic reviews or clinical trials relevant to the comparison between oral versus inhaled antibiotics for bronchiectasis.
European Respiratory Society guidelines for the management of bronchiectasis suggest use of an inhaled antibiotic over oral antibiotics for adults with bronchiectasis and chronic P. aeruginosa infection, unless inhaled antibiotics are contraindicated, not tolerated or ineffective (Polverino 2017). Macrolides (azithromycin, erythromycin) are recommended in preference to inhaled antibiotics for adults with bronchiectasis and no infection with P. aeruginosa. When macrolides are contraindicated, not tolerated or ineffective, other oral antibiotics are recommended (Polverino 2017).
A Cochrane review on cystic fibrosis reported a reduction in pulmonary exacerbations and a small improvement in lung function over six months with macrolide antibiotics (Southern 2012). However, there are no randomised controlled trials of oral versus inhaled antibiotic treatments to prevent exacerbations in cystic fibrosis, and clinical guideline recommendations are inconsistent. The USA Cystic Fibrosis Foundation guidelines recommend both oral azithromycin and inhaled tobramycin in patients with cystic fibrosis bronchiectasis chronically infected with P. aeruginosa (Flume 2007). In contrast, UK guidelines on cystic fibrosis recommend nebulised antipseudomonal antibiotics for patients with chronic P. aeruginosa infection, and azithromycin as an additional therapy for deteriorating patients (Cystic Fibrosis Trust 2009). In both cystic fibrosis and bronchiectasis guidelines, most of the evidence for treating patients with P. aeruginosa infection is from studies of inhaled antimicrobials and so these are often used first.
Authors' conclusions
Implications for practice.
There is currently no evidence from randomised controlled trials to indicate whether orally administered antibiotics are more or less beneficial than inhaled antibiotics. Until such evidence is available, practitioners may consider consulting local, national and international guidelines, such as the European Respiratory Society guidelines for the management of bronchiectasis (Polverino 2017).
Implications for research.
The above recommendations from the European Respiratory Society guidelines are not based on direct evidence as there are no studies that compare inhaled versus oral antibiotics, either in patients with P. aeruginosa infection or in other populations of patients (Polverino 2017). The recommendations are based largely on the experience of inhaled antibiotics in cystic fibrosis, and are influenced by the fact that most trials to date have used inhaled antibiotics for patients with P. aeruginosa infection and oral antibiotics in populations without P. aeruginosa (Chalmers 2015). The primary objective of both oral and inhaled antibiotic therapy in bronchiectasis is the prevention of exacerbations (Hill 2017).
It would therefore be desirable to see randomised controlled trials comparing the administration of inhaled versus oral antibiotic therapies for bronchiectasis patients with a history of frequent exacerbations, with the aim of establishing which approach is most effective in terms of exacerbation prevention, quality of life, treatment burden, and antibiotic resistance (Aliberti 2016b). Since bronchiectasis is a clinically heterogeneous disease with four potential disease clusters (pseudomonas infection, other chronic infection, daily sputum, dry bronchiectasis), it is likely that different patient populations will response differently to inhaled or oral antibiotic treatments, and so it is a research priority to identify clinical phenotypes of subgroups likely to respond to each therapy (Aliberti 2016a).
Acknowledgements
We thank Edge Hill University and Lancaster University for their support in the development of this review. Dr Chalmers and Dr Goeminne acknowledge support from the European Bronchiectasis Network (EMBARC), which is funded by the European Respiratory Society.
We would also like to thank the Cochrane Airways Group for their support.
The Background and Methods sections of this protocol are based on a standard template used by Cochrane Airways.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service, or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Trials Register
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (The Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| Embase (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the Trials Register
Bronchiectasis search
1. exp Bronchiectasis/
2. bronchiect$.mp.
3. bronchoect$.mp.
4. kartagener$.mp.
5. (ciliary adj3 dyskinesia).mp.
6. (bronchial$ adj3 dilat$).mp.
7. or/1‐6
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 2. Search strategy to identify relevant studies from the Cochrane Airways Trials Register
#1 BRONCH:MISC1
#2 MeSH DESCRIPTOR Bronchiectasis Explode All
#3 bronchiect*
#4 #1 or #2 or #3
#5 MeSH DESCRIPTOR Anti‐Bacterial Agents Explode 1
#6 antibiotic* or anti‐biotic*
#7 anti‐bacteri* or antibacteri*
#8 *cillin
#9 *mycin OR *micin
#10 *oxacin
#11 *tetracycline
#12 macrolide*
#13 quinolone*
#14 trimethoprim
#15 ceph*
#16 sulpha*
#17 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16
#18 #4 and #17
Note: in search line #1, MISC1 denotes the field in the record where the reference has been coded for condition, in this case, bronchiectasis.
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aksamit 2016 | Wrong comparator. The trial compares inhaled ciprofloxacin to placebo. |
| Alder 2011 | Wrong comparator. The trial compares inhaled ciprofloxacin to placebo. |
| Antoniu 2011 | Wrong comparator. The trial compares inhaled gentamicin to placebo. |
| Bilton 2006 | Wrong intervention. The trial compares the addition of inhaled tobramycin to ciprofloxacin versus ciprofloxacin alone. |
| Chalmers 2012 | Not a randomised controlled trial. The study sought to investigate the impact of long‐ and short‐term antibiotic treatment on inflammation. |
| Fiel 2000 | Wrong comparator. The trial compares inhaled tobramycin to placebo. |
| Flume 2013 | Wrong comparator. The trial compares inhaled aztreonam to placebo. |
| Hampel 2011 | Wrong comparator. The trial compares inhaled ciprofloxacin to placebo. |
| Labiris 1999 | Wrong comparator. The trial compared inhaled and nebulised gentamicin to intravenous gentamicin in a triple cross‐over design. |
| Ledson 2000 | Letter to editor regarding "n of 1" trial of inhaled taurolidine versus placebo |
| Murray 2009 | Wrong comparator. The trial compares inhaled gentamicin to placebo. |
| NCT00749866 2008 | Wrong comparator. The trial compares inhaled gentamicin to placebo. |
| NCT01313624 2011 | Wrong comparator. The trial compares inhaled aztreonam to placebo. |
| NCT01677403 2012 | Wrong comparator. The trial compares inhaled tobramycin to placebo. |
| O'Donnell 1999 | Wrong comparator. The trial compares inhaled tobramycin to placebo. |
| O'Donnell 2016 | Wrong comparator. The trial compares inhaled ciprofloxacin to placebo. |
| Orriols 1999 | Wrong comparator. The trial compares inhaled ceftazidime and tobramycin to usual care. |
| Santiveri 1995 | Wrong comparator. The trial compares addition of inhaled antibiotics to intravenous antibiotics versus intravenous antibiotics alone. |
| Serisier 2012 | Letter to editor regarding trial of inhaled gentamicin compares to placebo |
| Shrewsbury 2004 | Wrong intervention. The trial compares the addition of inhaled tobramycin to ciprofloxacin to ciprofloxacin alone. |
| Soyza 2015 | Wrong comparator. The trial compares inhaled ciprofloxacin to placebo. |
| Tabernero 2012 | Wrong comparator. The trial compares inhaled colistin to usual care. |
| Terpstra 2016 | Wrong comparator. The trial compares two different doses of inhaled tobramycin to placebo. |
| Twiss 2008 | Wrong comparator. The trial compares inhaled gentamicin to placebo. |
| Twiss 2009 | Wrong comparator. The trial compares inhaled gentamicin to placebo. |
| Wilson 2011 | Wrong comparator. The trial compares inhaled ciprofloxacin to placebo. |
| Wong 2004 | Wrong intervention and wrong comparator. Trial compares intravenous followed by oral antibiotics for an acute exacerbation of bronchiectasis, versus a full intravenous course of antibiotics. |
Differences between protocol and review
As there are multiple manufacturers of both oral and inhaled antibiotics and many are off‐patent, we did not conduct a search of manufacturers websites.
Contributions of authors
PG and LF independently screened the search in consultation with SS, SJM and JDC. LF, SS and SJM completed the analyses and Results section. All review authors contributed to the Discussion, Conclusions and remaining sections of the review.
Sources of support
Internal sources
-
Edge Hill University, UK.
Provided funding for a part‐time review author (LF) to support a series of Cochrane Reviews on bronchiectasis.
External sources
-
All authors, UK.
This review was completed, in part, through a grant of £5,000 from the Cochrane Review Support Programme.
Declarations of interest
SS: is the lead applicant on a grant from Edge Hill University that provides support staff for a number of bronchiectasis reviews. She is also an editor with the Cochrane Airways Group.
LF: none known
SM: none known
RN: is Joint Co‐ordinating Editor with the Cochrane Airways Group.
PCG: has received lecture fees from Novartis, Chiesi, Eurogenerics, Astra Zeneca and Boehringer and received travel accommodation from Chiesi and Novartis.
JDC: has received research funding from Astrazeneca and Pfizer, and has received lecture fees or served on advisory boards for Bayer, Griffols, Astrazeneca, Pfizer, Napp and Chiesi.
New
References
References to studies excluded from this review
Aksamit 2016 {published data only}
- Aksamit TR, Soyza A, Operschall E, Bandel T‐J, Krahn U, Montegriffo E, et al. Baseline demographic profile of subjects of the phase 3 RESPIRE 2 trial of ciprofloxacin dry powder for inhalation (DPI) in non‐cystic fibrosis bronchiectasis (NCFB). American Thoracic Society 2016 International Conference 2016;193:A1768. [https://www.atsjournals.org/doi/abs/10.1164/ajrccm‐conference.2016.193.1_MeetingAbstracts.A1768] [Google Scholar]
Alder 2011 {published data only}
- Alder J, Wilson R, Welte T, Polverino E, Soyza A, Greville H. Antimicrobial efficacy of ciprofloxacin dry powder for inhalation in patients with non‐cystic fibrosis bronchiectasis [Abstract]. European Respiratory Journal 2011;38(Suppl 55):1930. [Google Scholar]
Antoniu 2011 {published data only}
- Antoniu SA, Trofor AC. Inhaled gentamicin in non‐cystic fibrosis bronchiectasis: Effects of long‐term therapy. Expert Opininion in Pharmacotherapy 2011;12(7):1191‐4. [DOI] [PubMed] [Google Scholar]
Bilton 2006 {published data only}
- Bilton D, Henig N, Morrissey B, Gotfried M. Addition of inhaled tobramycin to ciprofloxacin for acute exacerbations of Pseudomonas aeruginosa infection in adult bronchiectasis. Chest 2006;130(5):1503‐10. [DOI] [PubMed] [Google Scholar]
Chalmers 2012 {published data only}
- Chalmers JD, Smith MP, McHugh BJ, Doherty C, Govan JR, Hill AT. Short‐ and long‐term antibiotic treatment reduces airway and systemic inflammation in non‐cystic fibrosis bronchiectasis. American Journal of Respiratory and Critical Care Medicine 2012;186(7):657‐65. [DOI] [PubMed] [Google Scholar]
Fiel 2000 {published data only}
- Fiel SB. The relationship between antimicrobial efficacy and improved medical condition in tobramycin solution for inhalation therapy in bronchiectasis. European Respiratory Journal 2000;16(Suppl 31):S494. [Google Scholar]
Flume 2013 {published data only}
- Flume P, Barker AF, Ruzi J, Thompson P, Lewis S, Shao L, et al. Baseline demographic profile of subjects from a study of inhaled aztreonam lysine for chronic gram negative infection with non‐cystic fibrosis bronchiectasis. American Journal of Respiratory and Critical Care Medicine 2013;187:A4536. [Google Scholar]
Hampel 2011 {published data only}
- Hampel B, Schoeman O, Reimnitz P, Jones P, Wilson R. Health status impact of ciprofloxacin dry powder for inhalation in patients with non‐cystic fibrosis bronchiectasis. European Respiratory Journal 2011;38(Suppl 55):2534. [Google Scholar]
Labiris 1999 {published data only}
- Crowther Labiris NR, Holbrook AM, Chrystyn H, Macleod SM, Newhouse MT. Dry powder versus intravenous and nebulized gentamicin in cystic fibrosis and bronchiectasis. A pilot study. American Journal of Respiratory and Critical Care Medicine 1999;160(5):1711‐6. [DOI] [PubMed] [Google Scholar]
Ledson 2000 {published data only}
- Ledson MJ, Cowperthwaite C, Walshaw MJ, Gallagher MJ, Williets T, Hart CA. Nebulised taurolidine and B cepacia bronchiectasis. Thorax 2000;55(1):91‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 2009 {published data only}
- Murray MP, Govan RW, Doherty CJ, Greening AP, Gray RD, Simpson AJ, et al. Long‐term nebulised gentamicin in non‐cystic fibrosis bronchiectasis improves microbial load, exercise tolerance, exacerbation frequency and health‐related quality of life. Thorax 2009;64(Suppl 4):S125. [Google Scholar]
NCT00749866 2008 {published data only}
- NCT00749866. Long term nebulised gentamicin in patients with bronchiectasis [Can long term nebulised gentamicin reduce the bacterial burden, break the vicious cycle of inflammation and improve quality of life in patients with bronchiectasis]. clinicaltrials.gov/ct2/show/NCT00749866 (first received 9 September 2008).
NCT01313624 2011 {published data only}
- NCT01313624. A study to see if AZLI (an inhaled antibiotic) is effective in treating adults with non‐cf bronchiectasis ‐ AIR‐BX1 [A phase 3, double‐blind, multicenter, randomized, placebo‐controlled trial evaluating repeated courses of aztreonam for inhalation solution in subjects with non‐cf bronchiectasis and gram‐negative endobronchial infection]. clinicaltrials.gov/ct2/show/NCT01313624 (first received 14 March 2011).
NCT01677403 2012 {published data only}
- NCT01677403. A study to access safety and efficacy of nebulized tobramycin in patients with bronchiectasis [A randomized,controlled study to evaluate the efficacy,indications,adverse reactions and resistance of combined administration of nebulized tobramycin compared with systemic administration alone in patients with bronchiectasis]. clinicaltrials.gov/ct2/show/NCT01677403 (first received 3 September 2012).
O'Donnell 1999 {published data only}
- O'Donnell A, Tully H, Kylstra JW, Wells C, Schaeffler B, Barker AF. Tobramycin solution for inhalation (TOBI) as maintenance treatment for bronchiectasis patients with pseudomonas aeruginosa. Thorax 1999;54(Suppl 3):A68. [Google Scholar]
O'Donnell 2016 {published data only}
- O'Donnell AE, Bilton D, Serisier D, Wanner A, Froehlich J, Bruinenberg P, et al. ORBIT‐3 and ORBIT‐4: design of a phase 3 program to investigate safety and efficacy of pulmaquin in non‐cystic fibrosis bronchiectasis (NCFBE) patients chronically colonized with pseudomonas aeruginosa (PA). American Journal of Respiratory and Critical Care Medicine 2016;193:A1775. [Google Scholar]
Orriols 1999 {published data only}
- Orriols R, Roig J, Ferrer J, Sampol G, Rosell A, Ferrer A, et al. Inhaled antibiotic therapy in non‐cystic fibrosis patients with bronchiectasis and chronic bronchial infection by pseudomonas aeruginosa. Respiratory Medicine 1999;93(7):476‐80. [DOI] [PubMed] [Google Scholar]
Santiveri 1995 {published data only}
- Santiveri C, Orriols R, Roig J, Balcells E, Bellver P, Ferrer A, et al. Effectiveness of inhaled antibiotic treatment for pseudomonas aeruginosa in outpatients with colonized bronchiectasis without mucoviscidosis. Archivos de Bronconeumologia 1995;31(Suppl):42. [Google Scholar]
Serisier 2012 {published data only}
- Serisier DJ, Bowler SD. Randomized controlled trial of nebulized gentamicin in non‐cystic fibrosis bronchiectasis...without patient blinding. American Journal of Respiratory and Critical Care Medicine 2012;186(5):461; author reply 461‐2. [DOI] [PubMed] [Google Scholar]
Shrewsbury 2004 {published data only}
- Shrewsbury SB, Bilton D, Gotfried M, Jones SA. The TABLE study (TOBI in acute bronchiectasis: additional treatment for exacerbations): rationale and methodology [Abstract]. American Thoracic Society 100th International conference; 2004 May 21‐26; Orlando. 2004:A42 Poster A53.
Soyza 2015 {published data only}
- Soyza A De, T Aksamit, E Operschall, T J Bandel, U Krahn, M Criollo, et al. Baseline demographic profile of subjects of the phase 3 RESPIRE 1 trial of ciprofloxacin dry powder for inhalation (DPI) in non‐cystic fibrosis bronchiectasis (NCFB). European Respiratory Journal 2015;46(Suppl 59):PA2617. [Google Scholar]
Tabernero 2012 {published data only}
- Tabernero E, Alkiza R, Gil P, Garros J, Cantero D, Artola JL, et al. Inhaled colistin in elderly patients with bronchiectasis and chronic bronchial infection with pseudomonas. European Respiratory Journal 2012;40(Suppl 56):S215. [Google Scholar]
Terpstra 2016 {published data only}
- Terpstra L, Altenburg J, Duijkers R, Boersma WG. Study design: effects of long term tobramycin inhalation solution (TIS) once daily on exacerbation rate in patients with non‐cystic fibrosis bronchiectasis a double blind, randomized, placebo and TIS twice daily (open label) controlled trial. The BATTLE study. American Journal of Respiratory and Critical Care Medicine 2016;193:A1788. [Google Scholar]
Twiss 2008 {published data only}
- Twiss J, Byrnes CA. Nebulized antibiotics reduce symptoms, bacterial density and oral antibiotic usage in children with non cystic fibrosis bronchiectasis. American Thoracic Society International Conference; 2008 May 16‐21; Toronto. 2008:A681.
Twiss 2009 {published data only}
- J Twiss, C Byrnes. Nebulised antibiotics reduce symptoms, bacterial density and oral antibiotic usage in children with non cystic fibrosis bronchiectasis. Respirology 2009;14(Suppl 1):A76. [Google Scholar]
Wilson 2011 {published data only}
- Wilson R, Welte T, Polverino E, Soyza A, Greville HW, O’Donnell AE, et al. Efficacy and safety of ciprofloxacin dry powder for inhalation in patients with non‐cystic fibrosis bronchiectasis. European Respiratory Journal 2011;38(Suppl 55):S334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2004 {published data only}
- Wong M, Lam J, Ip S, Lam CL, Ip M, Lam WK, et al. Quality of life effect of levofloxacin compared to ceftazidime treatment in infective exacerbation of bronchiectasis. Respirology 2004;9(Suppl 3):A135. [Google Scholar]
Additional references
Aliberti 2016a
- Aliberti S, Lonni S, Dore S, McDonnell MJ, Goeminne PC, Dimakou K, et al. Clinical phenotypes in adult patients with bronchiectasis. European Respiratory Journal 2016;47(4):1113‐22. [DOI: 10.1183/13993003.01899-2015] [DOI] [PubMed] [Google Scholar]
Aliberti 2016b
- Aliberti S, Masefield S, Polverino E, Soyza A, Loebinger MR, Menendez R, et al. Research priorities in bronchiectasis: a consensus statement from the EMBARC Clinical Research Collaboration. European Respiratory Journal 2016;48(3):632‐47. [DOI: 10.1183/13993003.01888-2015] [DOI] [PubMed] [Google Scholar]
Altenburg 2011a
- Altenburg J, Graaff CS, Werf TS, Boersma WG. Immunomodulatory effects of macrolide antibiotics ‐ part 1: biological mechanisms. Respiration 2011;81(1):67‐74. [DOI] [PubMed] [Google Scholar]
Altenburg 2011b
- Altenburg J, Graaff CS, Werf TS, Boersma WG. Immunomodulatory effects of macrolide antibiotics ‐ part 2: advantages and disadvantages of long‐term, low‐dose macrolide therapy. Respiration 2011b;81(1):75‐87. [DOI] [PubMed] [Google Scholar]
Altenburg 2013
- Altenburg J, Graaff CS, Stienstra Y, Sloos JH, Haren EH, Koppers RJ, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non‐cystic fibrosis bronchiectasis: the BAT randomized controlled trial. Journal of the American Medical Association 2013;309(12):1251‐9. [DOI] [PubMed] [Google Scholar]
BNF (online)
- Joint Formulary Committee. British National Formulary. www.medicinescomplete.com/mc/?utm_source=bnforg&utm_medium=homepage&utm_campaign=medicinescomplete (accessed 13 October 2016).
Brodt 2014
- Brodt AM, Stovold E, Zhang L. Inhaled antibiotics for stable non‐cystic fibrosis bronchiectasis: a systematic review. European Respiratory Journal 2014;44(2):382‐93. [DOI: 10.1183/09031936.00018414] [DOI] [PubMed] [Google Scholar]
Chalmers 2015
- Chalmers JD, Aliberti S, Blasi F. Management of bronchiectasis in adults. European Respiratory Journal 2015;45(5):1446‐62. [DOI: 10.1183/09031936.00119114] [DOI] [PubMed] [Google Scholar]
Chalmers 2016
- Chalmers JD, Aliberti S, Polverino E, Vendrell M, Crichton M, Loebinger M, et al. The EMBARC European Bronchiectasis Registry: protocol for an international observational study. European Respiratory Journal 2016;2(1):00081‐2015. [DOI: 10.1183/23120541.00081-2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chalmers JD 2012
- Chalmers JD, Smith MP, McHugh BJ, Doherty C, Govan JR, Hill AT. Short‐ and long‐term antibiotic treatment reduces airway and systemic inflammation in non‐cystic fibrosis bronchiectasis. American Journal of Respiratory and Critical Care Medicine 2012;186(7):657‐65. [DOI] [PubMed] [Google Scholar]
Chang 2002
- Chang AB, Grimwood K, Mulholland K, Torzillo P. Bronchiectasis in Indigenous children in remote Australian communities. Medical Journal of Australia 2002;177:200‐4. [PUBMED: 12175325] [DOI] [PubMed] [Google Scholar]
Chang 2003
- Chang AB, Bell SC, Byrnes CA, Grimwood K, HolmesP, King PT, et al. Chronic suppurative lung disease and bronchiectasis in children and adults in Australia and New Zealand: a position statement from the Thoracic Society of Australia and New Zealand and the Australian Lung Foundation. Medical Journal of Australia 2010;193(6):356‐65. [DOI] [PubMed] [Google Scholar]
Cystic Fibrosis Trust 2009
- Cystic Fibrosis Trust. Antibiotic treatment for cystic fibrosis. Third Edition. London: Cystic Fibrosis Trust, 2009. [Google Scholar]
Donovan 2017
- Donovan T, Felix LM, Chalmers JD, Milan SJ, Mathioudakis AG, Spencer S. Continuous versus intermittent antibiotics for non‐cystic fibrosis bronchiectasis. Cochrane Database of Systematic Reviews 2017, Issue 7. [DOI: 10.1002/14651858.CD012733] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dudley 2008
- Dudley MN, Loutit J, Griffith DC. Aerosol antibiotics: considerations in pharmacological and clinical evaluation. Current Opinion in Biotechnology 2008;19(6):637‐43. [DOI] [PubMed] [Google Scholar]
European Lung White Book 2013
- Gibson GJ, Loddenkemper R, Lundback Bo, Sibille Y (eds). Bronchiectasis. European Lung White Book: Respiratory Health and Disease in Europe. European Respiratory Society, 2013. [http://www.erswhitebook.org/] [DOI] [PubMed] [Google Scholar]
Felix 2017
- Felix Lambert M, Grundy S, Milan Stephen J, Armstrong R, Harrison H, Lynes D, et al. Dual antibiotics for non‐cystic fibrosis bronchiectasis. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD012514] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flume 2007
- Flume PA, O’Sullivan BP, Robinson KA, Goss CH, Mogayzel PJ Jr, Willey‐Courand DB, et al. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. American Journal of Respiratory and Critical Care Medicine 2007;176(10):957–69. [DOI: 10.1164/rccm.200705-664OC] [DOI] [PubMed] [Google Scholar]
Geller 2009
- Geller DE. Aerosol antibiotics in cystic fibrosis. Respiratory Care 2009;54(5):658‐70. [DOI] [PubMed] [Google Scholar]
Goeminne 2010
- Goeminne P, Dupont L. Non‐cystic fibrosis bronchiectasis: diagnosis and management in 21st century. Postgraduate Medical Journal 2010;86(1018):493‐501. [DOI] [PubMed] [Google Scholar]
Goeminne 2014
- Goeminne PC, Vandooren J, Moelants EA, Decraene A, Rabaey E, Pauwels A, et al. The sputum colour chart as a predictor of lung inflammation, proteolysis and damage in non‐cystic fibrosis bronchiectasis: a case‐control analysis. Respirology 2014;19(2):203‐10. [DOI] [PubMed] [Google Scholar]
Goeminne 2016
- Pieter G, Soyza A. Bronchiectasis: how to be an orphan with many parents?. European Respiratory Journal 2016;47(1):10‐3. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 1 March 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Habesoglu 2011
- Habesoglu MA, Ugurlu AO, Eyuboglu FO. Clinical, radiologic, and functional evaluation of 304 patients with bronchiectasis. Annals of Thoracic Medicine 2011;6(3):131‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hill 2017
- Hill AT, Haworth CS, Aliberti S, Barker A, Blasi F, Boersma W, et al. Pulmonary exacerbation in adults with bronchiectasis: a consensus definition for clinical research. European Respiratory Journal 2017;49(6):pii: 1700051. [DOI: 10.1183/13993003.00051-2017] [DOI] [PubMed] [Google Scholar]
Hnin 2015
- Hnin K, Nguyen C, Carson KV, Evans DJ, Greenstone M, Smith BJ. Prolonged antibiotics for non‐cystic fibrosis bronchiectasis in children and adults. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD001392.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaehne 2017
- Kaehne A, Milan SJ, Felix LM, Spencer S, Sheridan E, Marsden PA. Head‐to‐head trials of antibiotics for non‐cystic fibrosis bronchiectasis. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD012590] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2016
- Kelly C, Evans David J, Chalmers James D, Crossingham I, Spencer S, Relph N, et al. Macrolide antibiotics for non‐cystic fibrosis bronchiectasis. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD012406] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kolbe 1996
- Kolbe J, Wells AU. Bronchiectasis: a neglected cause of respiratory morbidity and mortality. Respirology 1996;1(4):221‐5. [DOI] [PubMed] [Google Scholar]
Lonni 2015
- Lonni S, Chalmers JD, Goeminne PC, McDonnell MJ, Dimakou K, Soyza A, et al. Etiology of non‐cystic fibrosis bronchiectasis in adults and its correlation to disease severity. Annals of the American Thoracic Society 2015;12(12):1764‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pasteur 2010
- Pasteur MC, Bilton D, Hill AT, British Thoracic Society Bronchiectasis non‐CF Guideline Group. British Thoracic Society guideline for non‐CF bronchiectasis. Thorax 2010;65 Suppl 1:i1‐58. [DOI] [PubMed] [Google Scholar]
Polverino 2017
- Polverino E, Goeminne PC, McDonnell MJ, Aliberti S, Marshall SE, Loebinger MR, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. European Respiratory Journal 2017;50(3):pii: 1700629. [10.1183/13993003.00629‐2017] [DOI] [PubMed] [Google Scholar]
Quint 2016
- Quint JK, Millett ER, Joshi M, Navaratnam V, Thomas SL, Hurst JR, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population‐based cohort study. European Respiratory Journal 2016;47(1):186‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Quon 2014
- Quon BS, Goss CH, Ramsey BW. Inhaled antibiotics for lower airway infections. Annals of the American Thoracic Society 2014;11(3):425‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Restrepo 2015
- Restrepo MI, Keyt H, Reyes LF. Aerolized antibiotics. Respiratory Care 2015;60(6):762‐1; discussion 771‐3. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roberts 2010
- Roberts HJ, Hubbard R. Trends in bronchiectasis mortality in England and Wales. Respiratory Medicine 2010;104:981‐5. [DOI] [PubMed] [Google Scholar]
Rubin 2008
- Rubin BK. Aerosolized antibiotics for noncystic fibrosis bronchiectasis. Journal of Aerosol Medicine and Pulmonary Drug Delivery 2008;21(1):71‐6. [DOI] [PubMed] [Google Scholar]
Ryan 2011
- Ryan G, Singh M, Dwan K. Inhaled antibiotics for long‐term therapy in cystic fibrosis. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD001021.pub2] [DOI] [PubMed] [Google Scholar]
Seitz 2010
- Seitz AE, Olivier KN, Steiner CA, Montes de Oca R, Holland SM, Prevots DR. Trends and burden of bronchiectasis‐associated hospitalizations in the United States, 1993‐2006. Chest 2010;138:944‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seitz 2012
- Seitz AE, Olivier KN, Adjemian J, Holland SM, Prevots DR. Trends in bronchiectasis among Medicare beneficiaries in the United States, 2000‐2007. Chest 2012;142(2):432‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Serisier 2013
- Serisier DJ, Martin ML, McGuckin MA, Lourie R, Chen AC, Brain B, et al. Effect of long‐term, low‐dose erythromycin on pulmonary exacerbations among patients with non‐cystic fibrosis bronchiectasis: the BLESS randomised controlled trial. JAMA 2013;309(12):1260‐7. [DOI] [PubMed] [Google Scholar]
Singleton 2000
- Singleton R, Morris A, Redding G, Poll J, Holck P, Martinez P, et al. Bronchiectasis in Alaska Native children: causes and clinical courses. Pediatric Pulmonology 2000;29(3):182‐7. [DOI: 10.1002/(SICI)1099-0496(200003)29:3%3C182::AID-PPUL5%3E3.0.CO;2-T] [DOI] [PubMed] [Google Scholar]
Southern 2012
- Southern KW, Barker PM, Solis‐Moya A, Patel L. Macrolide antibiotics for cystic fibrosis. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD002203.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Welsh 2015
- Welsh EJ, Evans DJ, Fowler SJ, Spencer S. Interventions for bronchiectasis: an overview of Cochrane systematic reviews. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD010337.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weycker 2005
- Weycker D, Edelsberg J, Oster G, Tino G. Prevalence and economic burden of bronchiectasis. Clinical Pulmonary Medicine 2005;12(4):205‐9. [Google Scholar]
Wilson 2016
- Wilson R, Aksamit T, Aliberti S, Soyza A, Elborn JS, Goeminne P, et al. Challenges in managing pseudomonas aeruginosa in non‐cystic fibrosis bronchiectasis. Respiratory Medicine 20165;117:179‐89. [DOI] [PubMed] [Google Scholar]
Wong 2012
- Wong C, Jayaram L, Karalus N, Eaton T, Tong C, Hockey H, et al. Azithromycin for prevention of exacerbations in non‐cystic fibrosis bronchiectasis (EMBRACE): a randomised, double‐blind, placebo‐controlled trial. Lancet 2012;380(9842):660‐7. [DOI] [PubMed] [Google Scholar]


