Abstract
This study evaluated the effectiveness of recombinant human interleukin-11 (rhIL-11) in the treatment of immune thrombocytopenia (ITP) and determined whether clinical and laboratory findings predicted the treatment response.
This retrospective, single-center, case–control study included 103 adult patients with ITP treated between July 2010 and April 2014 at Jiangxi Province People's Hospital. About 49 patients in the pred+IL group received prednisone (conventional dose) combined with an rhIL-11 regimen, and 54 patients in the pred alone group received prednisone (conventional dose) alone. Demographic data, initial and follow-up platelet counts, proportions of patients achieving platelet counts ≥30 × 109/L (response) and ≥100 × 109/L (complete response) at different time points, and adverse reactions were compared between groups.
Complete response rates were similar between groups overall but higher in the pred+IL group than in the pred alone group for newly diagnosed patients and those with severe ITP (P < .05). Proportions of patients achieving response or complete response at different time points were similar between groups overall but higher in the pred+IL group than in the pred alone group for newly diagnosed patients and those with severe ITP (P < .05). Posttreatment platelet count correlated negatively with platelet count at diagnosis and white blood cell (WBC) count at diagnosis in patients with newly diagnosed ITP (r = −0.337, P = .073 and r = −0.367, P = .050, respectively) or ITP with bleeding-related episodes (r = −0.357, P = .020 and r = −0.434, P = .004, respectively). No immediate or postinfusion severe adverse reactions were observed.
rhIL-11 increased CR and improved hemostasis in patients with newly diagnosed or severe ITP. Platelet and WBC counts at diagnosis can predict the response to rhIL-11.
Keywords: immune thrombocytopenia, platelet count, prednisone, recombinant human interleukin-11, white blood cell count
1. Introduction
Immune thrombocytopenia (ITP) is an autoimmune-mediated acquired disorder characterized by a transient or persistent reduction in platelet count due to decreased production and increased peripheral destruction of platelets secondary to antiplatelet antibodies, leading to an elevated risk of clinically significant bleeding.[1]
The goal of ITP therapy is to treat or prevent bleeding and maintain a platelet count above 30 × 109/L, a level that is considered safe and does not require further treatment.[1] Patients who are determined to be at risk of major bleeding or with active bleeding should be treated quickly to prevent or stop bleeding episodes. Conventional treatments for ITP include immunosuppressive therapies, such as corticosteroids, azathioprine, cyclosporine A, cyclophosphamide, mycophenolate mofetil, rituximab, vinca alkaloids, and intravenous (IV) immunoglobulin. Medical therapies primarily aim to reduce platelet destruction (e.g., Fc receptor blockade with IV immunoglobulin or IV anti-D prevents macrophage-mediated destruction of antibody-coated platelets), while surgical treatment (i.e., splenectomy) prevents platelet sequestration. However, patients with ITP do not always respond well to conventional “immunosuppressive” treatments. For those patients whose platelet counts do not respond to steroids, there is little consensus as to how to layer on additional therapies. Indeed, there are only a handful of options for which there is strong evidence regarding their utility.[2] Thrombopoietin (TPO) regulates the proliferation, differentiation, and maturation of hematopoietic progenitor cells into mature megakaryocytes through selective binding of the Mpl receptor and biologic effects.[3] TPO can stimulate the formation of platelets and increase the number of peripheral blood platelets in vivo.[4] Recombinant human TPO (rhTPO) can promote the production and release of platelets in patients with ITP and has been used to treat patients in China with corticosteroid-resistant and relapsed ITP.[5] Thrombopoietin-receptor agonists (TPO-RAs) are recommended for adult patients with ITP who relapse after splenectomy or who have contraindications for splenectomy. TPO-RAs, such as eltrombopag and romiplostim, can enhance platelet production and represent an effective 2nd-line medical option both in splenectomized and nonsplenectomized patients with ITP.[6,7] Besides stimulating platelet production from megakaryocytes, TPO-RAs have demonstrated additional immunomodulatory effects.[8–10]
Interleukin 11 (IL-11) directly stimulates the proliferation of hematopoietic stem cells and megakaryocytes to increase the platelet count.[11] IL-11 modulates autoimmunity by preventing Th1 polarization, which in turn inhibits IL-12 activation of Th1 cells to produce interferon (IFN), while enhancing Th2 cytokine production.[12,13] Recombinant human IL-11 (rhIL-11) has been shown to be effective at improving platelet counts following chemotherapy.[14–17] As an anti-inflammatory and immune-modulating agent, rhIL-11 has also been used successfully in the treatment of patients with psoriasis,[18] inflammatory bowel disease,[18] and ITP, and in the prevention of graft-vs-host disease in animal models.[19] Lin et al reported that rhIL-11 treatment resulted in a response rate (complete response [CR] rate and partial response rate) of 67.7% in patients with ITP, with significant decreases in Th1 and T-bet levels but increases in Th2 and GATA-3 levels, suggesting that rhIL-11 was effective in the treatment of ITP and had tolerable adverse effects.[20]
Although the safety and efficacy of rhIL-11 in the treatment of ITP have been extensively analyzed, it is not clear which patients are likely to respond well to this therapy. Patients with ITP at our hospital are routinely treated with prednisone, but some also receive rhIL-11 in combination with prednisone. Therefore, we retrospectively investigated the effectiveness of rhIL-11 in combination with prednisone for the treatment of ITP and evaluated whether clinical factors such as platelet and white blood cell (WBC) counts at diagnosis might predict the response to therapy.
2. Materials and methods
2.1. Patients
This retrospective, single-center, case–control study included consecutive adult patients with primary ITP who were treated with prednisone or prednisone combined with rhIL-11 at Jiangxi Province People's Hospital between July 2010 to and April 2014. The inclusion criteria were: a diagnosis of primary ITP; age >18 years; treated with prednisone or prednisone in combination with rhIL-11 between July 2010 and April 2014; and bone marrow examination had been carried out to exclude other differential diagnoses (such as infiltration or leukemia) and to confirm the presence of an adequate number of megakaryocytes. Patients were excluded from the analysis if any of the following criteria applied: history of allergy, infection during the past 4 weeks, or other autoimmune diseases; a diagnosis of refractory ITP; had received glucocorticosteroids, IV immunoglobulin, or other immunosuppressive treatments for at least 3 months prior to screening. Based on the inclusion and exclusion criteria, a total of 103 medical records were available that included demographic data, initial and follow-up platelet counts, platelet responses to treatment, and final outcomes from the time of initial diagnosis.
This study was approved by the ethics committee of the hospital, and all patients signed informed consent. For each patient, the observation period started on the day of the initial diagnosis. Patients were followed until the observation period ended on December 30, 2014 or until death (due to any cause) if it occurred before this date.
2.2. Patient grouping
The patients were divided into 2 groups according to their treatment regimen, which was chosen by each patient in consultation with the clinicians.
Patients in the pred+IL group (n = 49) received a conventional dose of prednisone (1 mg/kg/d PO with 10 mg/wk tapering and discontinuation after 6 weeks) combined with rhIL-11 (Qilu Pharmaceutical Co, Ltd, Jinan, China) at a dosage of 50 μg/kg body weight once daily for 7 to 14 consecutive days (this was based on the time required for the platelet count to recover to a normal level after treatment). Patients in the pred alone group (n = 54) received prednisone therapy alone.
2.3. Diagnosis and evaluations
The diagnosis of ITP was made according to the criteria in the Consensus of Chinese Experts on Diagnosis and Treatment of Adult Primary Immune Thrombocytopenia (version 2016)[21] and was based principally on the clinical history, physical examination, complete blood count, and examination of a peripheral blood smear in order to exclude other causes of thrombocytopenia. ITP was categorized as “newly diagnosed” if diagnosed within the previous 3 months, “persistent” if thrombocytopenia had lasted for 3 to 12 months, and “chronic” if it had lasted for longer than 12 months.[21] Moderate ITP was defined as an initial platelet count of between 30.0 × 109/L and 100.0 × 109/L without a further reduction to below 30.0 × 109/L during the first 3 months.[22] Severe thrombocytopenia was defined as a platelet count below 30.0 × 109/L at presentation or an initial platelet count between 30.0 × 109/L and 100.0 × 109/L with a reduction to below 30.0 × 109/L during the following 3 months.[22] Severe ITP was also defined as the presence of bleeding symptoms at presentation that required treatment, or the occurrence of new bleeding symptoms that required additional therapeutic interventions with a different platelet-enhancing agent or an increased dose.[21]
The peripheral blood smear and complete blood count were monitored daily during hospitalization and then weekly for 6 weeks. Response to treatment was classified as CR (platelet count >100 × 109/L), response (R, platelet count ≥30 × 109/L) and no response (NR, no rise in platelet count, a platelet count <30 × 109/L, or symptomatic bleeding).[21] A bleeding-related episode was defined as an actual bleeding event and/or use of rescue therapy (IV immunoglobulin administration, IV steroid administration, or platelet transfusion).[23]
Adverse events were noted because rhIL-11 is known to cause serious fluid retention that can result in peripheral edema, dyspnea on exertion, pulmonary edema, capillary leak syndrome, atrial arrhythmias, and exacerbation of preexisting pleural effusions.[24] Adverse responses investigated included: nausea, vomiting, abdominal distention, diarrhea, dizziness and lethargy; Cushing syndrome; abnormalities in blood pressure, blood potassium level, blood glucose level, liver function tests or kidney function tests.
2.4. Statistical analysis
SPSS version 19.0 (IBM Corp, Armonk, NY) was used for data analyses. Data are expressed as mean ± standard deviation. Statistical significance was determined by Student t test and the least significant difference (LSD) test unless the data were not normally distributed, in which case the Mann–Whitney U test and Kruskal–Wallis test were used. Pearson correlation was used for correlation analysis unless the data were not normally distributed, in which case Spearman correlation analysis was conducted. Pearson correlation analysis was used for posthoc analysis of the clinical factors at diagnosis and the response to treatment. P < .05 was considered to be statistically significant.
3. Results
3.1. Demographic features
A total of 103 patients were included in the analysis, with 49 patients in the pred+IL group and 54 patients in the pred alone group. Patient age was higher in the pred alone group than in the pred+IL group (P = .007), but the other demographic and clinical data did not differ significantly between groups (Table 1).
Table 1.
Patient characteristics.

3.2. Response to treatment with rhIL-11
There were no statistically significant differences between the 2 groups in CR rate or R rate at 28 days after treatment (Fig. 1A). However, in patients with severe ITP or newly diagnosed ITP (Fig. 1B and C), the CR rate was significantly higher in the pred+IL group than in the pred alone group (P < .05). CR rate was higher in patients with severe ITP than in patients with nonsevere ITP in the pred+IL group but not the pred alone group (Fig. 1D and E). These results suggest that rhIL-11 combined with prednisone may significantly increase the CR rate of patients with newly diagnosed ITP, especially severe ITP.
Figure 1.

Comparison of treatment response rates after 28 days of therapy between patients with immune thrombocytopenia (ITP) treated with prednisone alone and those treated with prednisone combined with recombinant human interleukin-11 (rhIL-11). (A) Treatment response rates in all patients. (B) Treatment response rates in patients with severe ITP. (C) Treatment response rates in patients with newly diagnosed ITP. (D) Treatment response rates in patients treated with prednisone alone, comparing severe ITP vs non-severe ITP. (E) Treatment response rates in patients treated with prednisone combined with rhIL-11, comparing severe ITP vs non-severe ITP. ∗P < .05. CR = complete response, NR = no response, R = response.
To determine whether rhIL-11 affected how quickly patients with ITP responded to prednisone, the response rates were compared between the 2 groups on days 3, 7, 14, and 28. Overall, the response rates showed no significant differences between groups at any time point (Fig. 2A and B). Interestingly, significant differences between groups were observed when subgroup analyses were performed based on patients with newly diagnosed ITP, severe ITP, and ITP with bleeding-related episodes (P < .05). In patients with newly diagnosed ITP, the proportion with platelet counts ≥30 × 109/L on day 7 after treatment was significantly higher in the pred+IL group (P < .05) than in the pred alone group (Fig. 2C), while the proportions with platelet counts ≥100 × 109/L on days 14 and 28 were significantly higher in the pred+IL group (P < .05) than in the pred alone group (Fig. 2D). In patients with ITP and bleeding-related episodes, there was a tendency (P = .056) for the proportion of patients with platelet counts ≥30 × 109/L to be higher in the pred+IL group than in the pred alone group on day 7 (Fig. 2E). In addition, the proportions of patients with platelet counts ≥100 × 109/L were significantly higher (P < .05) in the pred+IL group than in the pred alone group on days 7 and 14 (Fig. 2F). Based on the above data, we investigated the platelet responses in patients with severe ITP, as these patients require a faster and higher increase in platelet count. We found that the proportion of patients achieving a platelet count ≥30 × 109/L on day 7 was higher in the pred+IL group (P < .05) than in the pred alone group (Fig. 2G). Furthermore, the proportion of patients who achieved a platelet count ≥100 × 109/L was significantly higher (P < .05) in the pred+IL group than in the pred alone group on days 7, 14, and 28 (Fig. 2H).
Figure 2.
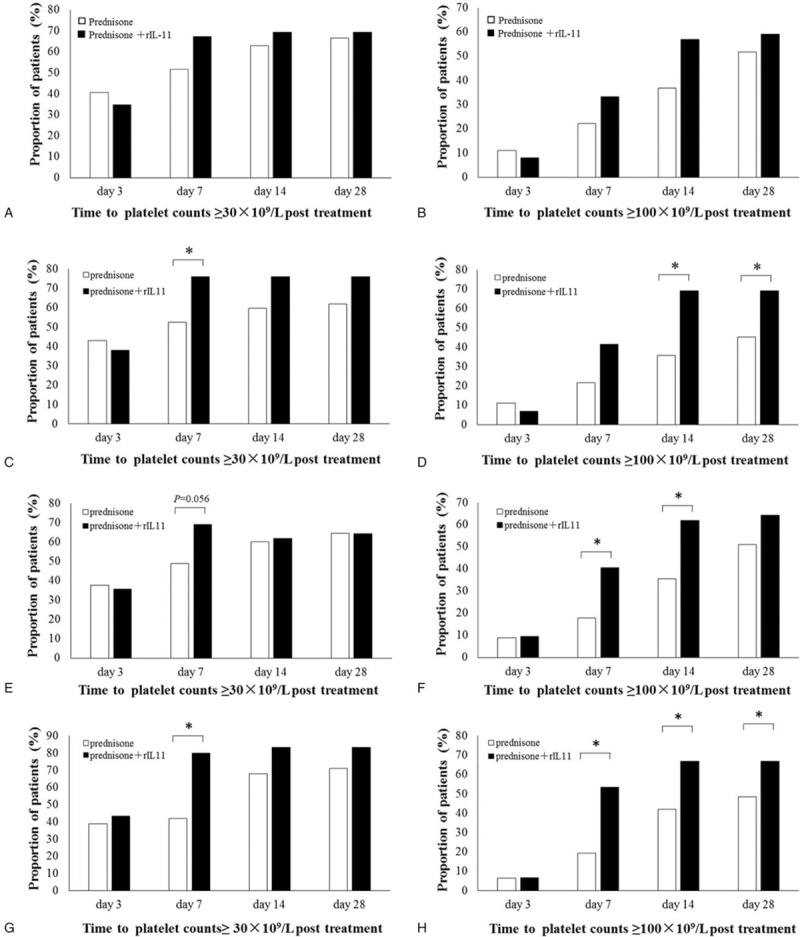
Comparison of treatment responses at 3, 7, 14, and 28 days posttreatment in patients with immune thrombocytopenia (ITP). (A, C, E, G) Proportion of patients in each group achieving a platelet count ≥30 × 109/L. (B, D, F, H) Proportion of patients in each group achieving a platelet count ≥100 × 109/L. (A, B) All patients with ITP. (C, D) Patients with newly diagnosed ITP. (E, F) Patients with ITP and bleeding-related episodes. (G, H) Patients with severe ITP. ∗P < .05.
3.3. Correlation between blood cell count and response to treatment with rhIL-11
We next investigated whether certain parameters measured at the time of diagnosis might predict the response to treatment with rhIL-11, including peripheral blood WBC count, peripheral blood platelet count, and peripheral blood hemoglobin level. As shown in Figure 3, the platelet count after treatment in the pred+IL group was negatively correlated with WBC count at diagnosis in patients with newly diagnosed ITP (r = −0.367, P = .050, Fig. 3B) or ITP with bleeding-related episodes (r = −0.434, P = .004, Fig. 3C), but no significant relationship was found in the pred alone group. We also found that the posttreatment platelet count in the pred+IL group was negatively correlated with platelet count at diagnosis in patients with bleeding-related episodes (r = −0.357, P = .020, Fig. 3I). Additionally, a trend toward a strong negative correlation was observed in patients with newly diagnosed ITP (r = −0.337, P = .073, Fig. 3H), while no correlations were observed in the pred alone group. No correlations between hemoglobin level at diagnosis and treatment response were observed in either group.
Figure 3.

Correlations between peripheral blood count before treatment and platelet count posttreatment in patients with immune thrombocytopenia (ITP). (A, D, G) Correlation between posttreatment platelet count and white blood cell count at diagnosis. (B, E, H) Correlation between posttreatment platelet count and hemoglobin level at diagnosis. (C, F, I) Correlation between posttreatment platelet count and platelet count at diagnosis in all patients with ITP. (A, E, G) Correlation between posttreatment platelet count and platelet count at diagnosis in patients with newly diagnosed ITP. (B, F, I) Correlation between posttreatment platelet count and platelet count at diagnosis in patients with ITP and bleeding-related episodes. r = correlation coefficient.
3.4. Safety
No immediate or postinfusion severe adverse reactions were observed. As shown in Table 2, the following adverse events occurred in the pred+IL group and were attributed to the rhIL-11 treatment: swelling of the hands and feet, red eyes, nausea and vomiting, allergy, and tachycardia. The incidences of swelling of the hands and feet (P = .048) and red eyes (P = .022) were significantly higher in the pred+IL group than in the pred alone group. No patient developed pulmonary edema, capillary leak syndrome, diarrhea with abdominal discomfort, or pleural effusion.
Table 2.
rhIL-11 therapy-related adverse events.

4. Discussion
The decision to treat ITP should be based on bleeding severity, bleeding risk, activity level, side effects, and patient preferences. The goal of all treatment strategies is to achieve a platelet count that is associated with adequate hemostasis rather than a normal platelet count.[21] IL-11 is a stromal-cell-derived cytokine with potent thrombopoietic and megakaryopoietic activity.[25–27] According to a clinical study, rhIL-11 demonstrated therapeutic effects in ameliorating chemotherapy-induced thrombocytopenia.[14–17] Some evidence on the use of rhIL-11 in adult ITP suggested an increased platelet count and reduced bleeding.[20,28,29]
Here, we performed a retrospective study on the efficacy and safety of combination therapy with prednisone and rhIL-11 in patients with ITP. This study demonstrated that prednisone/rhIL-11 combination treatment significantly increased CR and improved hemostasis in newly diagnosed patients with ITP and those with severe ITP. The platelet and WBC counts at the time of diagnosis also predicted the response to prednisone and rhIL-11 treatment in patients with ITP. Previous studies have reported differing clinical outcomes following treatment with rhIL-11. Lin et al[20] found rhIL-11 to be an effective treatment with tolerable adverse effects in patients with ITP, which is consistent with our study results. However, the study of Bussel et al[29] failed to replicate the therapeutic effect of rhIL-11 in patients with ITP and reported nearly intolerable side effects in all patients. This latter study[29] suggested that either rhIL-11 combined with prednisone (1 mg/kg/d PO with 10 mg/wk tapering and discontinuation after 6 weeks) or a shorter schedule of administration (rhIL-11 for only 7–14 days) might be more effective and less toxic. However, the study included only patients with refractory ITP, suggesting a need to explore the efficacy and safety of rhIL-11 in other types of ITP and further optimize the treatment strategy.
Primary ITP is caused by a complex mechanism involving auto-antibodies directed against platelets and T-cell-mediated platelet destruction.[28] Secondary ITP develops in a setting of autoimmune diseases, lymphoma, infection with Helicobacter pylori or infection with viruses such as HIV, hepatitis C, or cytomegalovirus.[30] Therefore, it is important to identify reliable predictors of ITP outcomes at the time of diagnosis as well as after initial therapy. For both the treating physicians and patients, there is a great need for reliable predictors of outcome in patients with ITP at the time of diagnosis. These predictors may in turn guide decision making regarding which therapeutic strategy to use to reduce or prevent further risk of bleeding. Several studies have tried to identify the right treatment strategy for individual patients with ITP after initial therapy of rhIL-11. Fontan et al[28] reported that rhIL-11 may be useful in patients with thrombocytopenia from chronic hepatitis C-induced cirrhosis when dosing was based on individual tolerance, with improvements in platelet counts observed in most patients. Lin et al[20] observed a significant therapeutic efficacy of rhIL-11 in the treatment of ITP, which was associated with restoration of Th1/Th2 and T-box/GATA-3 ratios, especially in patients who showed a good response. In this study, comparison of the results between patients treated with prednisone combined with rhIL-11 and those administered prednisone alone showed that rhIL-11 was likely to have a beneficial effect in patients with ITP, especially severe ITP.
In this study, the platelet and WBC counts at the time of diagnosis were significantly and negatively correlated with platelet recovery after combination treatment with rhIL-11 and prednisone in patients with ITP and bleeding-related episodes, suggesting that patients with a lower platelet count and bleeding tendency were more sensitive to treatment with rhIL-11. It is important to consider whether this was a true effect of rhIL-11 or whether it was due to confounding factors. This effect might be due to an action of rhIL-11 to improve platelet count and reduce or prevent further bleeding in patients. In regard to the mechanisms of rhIL-11, several points can be conjectured. IL-11 is a cytokine with thrombopoietic activity that increases plasma von Willebrand factor (vWF),[31] and vWF facilitates platelet adhesion to the subendothelium. Recently, it has been reported that cooperation and interactions with vWF enhance the adsorption probability in primary hemostasis (platelets adhere to the damaged endothelium to form a platelet plug).[32] In accordance with our results, patients with ITP and bleeding-related episodes, especially those with severe ITP, responded to combination therapy with rhIL-11 and prednisone with a significant increase in platelet count and vWF. IL-11 may directly stimulate the proliferation of hematopoietic stem cells and megakaryocytes to increase the platelet count.[11] IL-11's anti-inflammatory activity favors platelet survival in ITP. In our study, patients with ITP who responsd to rhIL-11 included those with newly diagnosed ITP and ITP with bleeding-related episodes, and the response was negatively correlated with WBC count at the time of diagnosis. But whether a low WBC count was associated with the development of infection is still unclear. However, a low WBC count might imply an immune-deficient status, which might in turn be a potential surrogate marker for the risk of infection. Indeed, viral infections such as rubella, varicella, mumps, cytomegalovirus, and Epstein–Barr virus are linked to the development of ITP and thrombocytopenia.[23] These infections can be relatively asymptomatic and trigger an autoimmune process. Whether or not a decreased WBC count is associated with infection in patients with ITP should be further studied.
With regards to the adverse effects of rhIL-11, most were grade I or II therapy-related side effects and resolved 1 to 2 weeks after the end of treatment. The extent of the toxicity appeared to be substantially less than that described by Bussel et al.[29] It is possible that short-term usage of rhIL-11 and prednisone reduces the reaction of the human body to rhIL-11. Taken together, the side effects were reversible within several days following discontinuation of rhIL-11. During rhIL-11 dosing, fluid balance should be monitored carefully, and appropriate medical management is advised.
The present study has some limitations. Firstly, the retrospective study design limits the strength of the evidence. Grouping of the patients was not randomized, and there were not clear guidelines for selection of the different treatment options. Therefore, there is likely to be some bias in the patient grouping. Secondly, the study involved a relatively small sample size. Thirdly, since each of the analyses contained a different number of patients, there exists a shift of biases between groups by virtue of incomplete inclusions. Fourthly, there were significant differences in age between the 2 treatment groups for all patients, although this was not the case for subgroup analyses of patients with newly diagnosed ITP, severe ITP or ITP with bleeding-related episodes. The lack of matching for age may have introduced some bias into the study. However, due to the small sample size, excluding some patients from both groups to match for age would have reduced the statistical power of the study to detect real differences in outcomes between groups. Importantly, there were no significant differences between groups in gender or other baseline clinical characteristics, indicating that the 2 groups were well-matched overall. To address these issues, we intend to carry out a randomized controlled trial in the near future to minimize these biases and obtain evidence of higher quality.
In conclusion, our results show that combination therapy with rhIL-11 and prednisone achieved CR and improved hemostasis in patients with ITP and may be especially useful in patients with severe ITP. The platelet and WBC counts at the time of diagnosis predicted the response to treatment with rhIL-11 and prednisone in ITP.
Author contributions
Conceptualization: Chenghao Jin.
Data curation: Yulu Wang, Tingting Liu, Anna Li, Liu Yang, Qingxiu Zhu, Minzhi Luo, Yujing Wei, Chunfang Kong.
Formal analysis: Yulu Wang, Hongbo Cheng, Haiyun Liu, Bo Ke, Weirong Ding.
Funding acquisition: Chenghao Jin.
Writing – original draft: Chenghao Jin, Yulu Wang.
Writing – review & editing: Chenghao Jin, Yulu Wang, Bo Ke.
Footnotes
Abbreviations: CR = complete response, ITP = immune thrombocytopenia, PLT = platelet, Pred = prednisone, rhIL-11 = recombinant human interleukin-11, TPO = thrombopoietin, WBC = white blood cell.
CJ and YW contributed equally to this work.
This work was supported by National Natural Science Foundation of China (81560026) as well as a Project on Social Development by Department of Science and Technology of Jiangxi Province.
The authors have no conflicts of interest to disclose.
References
- [1].Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood 2009;113:2386–93. [DOI] [PubMed] [Google Scholar]
- [2].Ghanima W, Godeau B, Cines DB, et al. How I treat immune thrombocytopenia: the choice between splenectomy or a medical therapy as a second-line treatment. Blood 2012;120:960–9. [DOI] [PubMed] [Google Scholar]
- [3].Tanimukai S, Kimura T, Sakabe H, et al. Recombinant human c-Mpl ligand (thrombopoietin) not only acts on megakaryocyte progenitors, but also on erythroid and multipotential progenitors in vitro. Exp Hematol 1997;25:1025–33. [PubMed] [Google Scholar]
- [4].Fibbe WE, Heemskerk DP, Laterveer L, et al. Accelerated reconstitution of platelets and erythrocytes after syngeneic transplantation of bone marrow cells derived from thrombopoietin pretreated donor mice. Blood 1995;86:3308–13. [PubMed] [Google Scholar]
- [5].Cui ZG, Liu XG, Qin P, et al. Recombinant human thrombopoietin in combination with cyclosporin A as a novel therapy in corticosteroid-resistant primary immune thrombocytopenia. Chin Med J (Engl) 2013;126:4145–8. [PubMed] [Google Scholar]
- [6].Kuter DJ, Bussel JB, Lyons RM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet 2008;371:395–403. [DOI] [PubMed] [Google Scholar]
- [7].Bussel JB, Provan D, Shamsi T, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:641–8. [DOI] [PubMed] [Google Scholar]
- [8].Bao W, Bussel JB, Heck S, et al. Improved regulatory T-cell activity in patients with chronic immune thrombocytopenia treated with thrombopoietic agents. Blood 2010;116:4639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li X, Zhong H, Bao W, et al. Defective regulatory B-cell compartment in patients with immune thrombocytopenia. Blood 2012;120:3318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Qu MM, Liu XN, Liu XG, et al. Cytokine changes in response to TPO receptor agonist treatment in primary immune thrombocytopenia. Cytokine 2017;92:110–7. [DOI] [PubMed] [Google Scholar]
- [11].Du X, Williams DA. Interleukin-11: review of molecular, cell biology, and clinical use. Blood 1997;89:3897–908. [PubMed] [Google Scholar]
- [12].Schwertschlag US, Trepicchio WL, Dykstra KH, et al. Hematopoietic, immunomodulatory and epithelial effects of interleukin-11. Leukemia 1999;13:1307–15. [DOI] [PubMed] [Google Scholar]
- [13].Curti A, Ratta M, Corinti S, et al. Interleukin-11 induces Th2 polarization of human CD4(+) T cells. Blood 2001;97:2758–63. [DOI] [PubMed] [Google Scholar]
- [14].Gordon MS, McCaskill-Stevens WJ, Battiato LA, et al. A phase I trial of recombinant human interleukin-11 (neumega rhIL-11 growth factor) in women with breast cancer receiving chemotherapy. Blood 1996;87:3615–24. [PubMed] [Google Scholar]
- [15].Tepler I, Elias L, Smith JW, et al. A randomized placebo-controlled trial of recombinant human interleukin-11 in cancer patients with severe thrombocytopenia due to chemotherapy. Blood 1996;87:3607–14. [PubMed] [Google Scholar]
- [16].Isaacs C, Robert NJ, Bailey FA, et al. Randomized placebo-controlled study of recombinant human interleukin-11 to prevent chemotherapy-induced thrombocytopenia in patients with breast cancer receiving dose-intensive cyclophosphamide and doxorubicin. J Clin Oncol 1997;15:3368–77. [DOI] [PubMed] [Google Scholar]
- [17].Zhang XL, Cheng XZ, Ye X, et al. Therapeutic effect of interleukin-11 on thrombocytopenia in patients with hematologic malignancies after chemotherapy. Zhonghua Zhong Liu Za Zhi 2010;32:713–5. [PubMed] [Google Scholar]
- [18].Trepicchio WL, Ozawa M, Walters IB, et al. Interleukin-11 therapy selectively downregulates type I cytokine proinflammatory pathways in psoriasis lesions. J Clin Invest 1999;104:1527–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hill GR, Cooke KR, Teshima T, et al. Interleukin-11 promotes T cell polarization and prevents acute graft-versus-host disease after allogeneic bone marrow transplantation. J Clin Invest 1998;102:115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lin Y, Zhou X, Guo W, et al. RhIL-11 treatment normalized Th1/Th2 and T-bet/GATA-3 imbalance in human immune thrombocytopenic purpura (ITP). Int Immunopharmacol 2016;38:40–4. [DOI] [PubMed] [Google Scholar]
- [21].Thrombosis and Hemostasis Group, Hematology Society, Chinese Medical Association. Consensus of Chinese experts on diagnosis and treatment of adult primary immune thrombocytopenia (version 2016) [in Chinese]. Zhonghua Xue Ye Xue Za Zhi 2016;37:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hu MH, Yu YB, Huang YC, et al. Absolute lymphocyte count and risk of short-term infection in patients with immune thrombocytopenia. Ann Hematol 2014;93:1023–9. [DOI] [PubMed] [Google Scholar]
- [23].Wright JF, Blanchette VS, Wang H, et al. Characterization of platelet-reactive antibodies in children with varicella-associated acute immune thrombocytopenic purpura (ITP). Br J Haematol 1996;95:145–52. [DOI] [PubMed] [Google Scholar]
- [24].Smith JW. Tolerability and side-effect profile of rhIL-11. Oncology (Williston Park) 2000;14:41–7. [PubMed] [Google Scholar]
- [25].Paul SR, Bennett F, Calvetti JA, et al. Molecular cloning of a cDNA encoding interleukin 11, a stromal cell-derived lymphopoietic and hematopoietic cytokine. Proc Natl Acad Sci U S A 1990;87:7512–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Turner KJ, Neben S, Weich N, et al. The role of recombinant interleukin 11 in megakaryocytopoiesis. Stem Cells 1996;14:53–61. [DOI] [PubMed] [Google Scholar]
- [27].Goldman SJ. Preclinical biology of interleukin 11: a multifunctional hematopoietic cytokine with potent thrombopoietic activity. Stem Cells 1995;13:462–71. [DOI] [PubMed] [Google Scholar]
- [28].Fontana V, Dudkiewicz P, Jy W, et al. Interleukin-11 for treatment of hepatitis C-associated ITP. Acta Haematol 2008;119:126–32. [DOI] [PubMed] [Google Scholar]
- [29].Bussel JB, Mukherjee R, Stone AJ. A pilot study of rhuIL-11 treatment of refractory ITP. Am J Hematol 2001;66:172–7. [DOI] [PubMed] [Google Scholar]
- [30].Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 2010;115:168–86. [DOI] [PubMed] [Google Scholar]
- [31].Gernsheimer T, James AH, Stasi R. How I treat thrombocytopenia in pregnancy. Blood 2013;121:38–47. [DOI] [PubMed] [Google Scholar]
- [32].Ragni MV, Jankowitz RC, Chapman HL, et al. A phase II prospective open-label escalating dose trial of recombinant interleukin-11 in mild von Willebrand disease. Haemophilia 2008;14:968–77. [DOI] [PMC free article] [PubMed] [Google Scholar]


