Abstract
Background:
Platelet-rich plasma (PRP) is used as an alternative therapy to reduce pain and improve functional restoration in patients with Achilles tendinopathy (AT). We evaluated the current evidence for the efficacy of PRP as a treatment for chronic AT.
Methods:
The PubMed, Embase, Web of Science, and The Cochrane Library databases were searched for articles on randomized controlled trials (RCTs) that compared the efficacy of PRP with that of with placebo injections plus eccentric training as treatment for AT. The articles were uploaded over the establishment of the databases to May 01, 2018. The Cochrane risk of bias (ROB) tool was used to assess methodological quality. Outcome measurements included the Victorian Institute of Sports Assessment-Achilles (VISA-A), visual analog scale (VAS) and Achilles tendon thickness. Statistical analysis was performed with RevMan 5.3.5 software.
Results:
Five RCTs (n = 189) were included in this meta-analysis. Significant differences in the VISA-A were not observed between the PRP and placebo groups after 12 weeks [standardized mean difference (SMD) = 0.2, 95% confidence interval (95% CI): 0.36 to 0.76, I2 = 71%], 24 weeks (SMD = 0.77, 95% CI: −0.10–1.65, I2 = 85%) and 1 year (SMD = 0.83, 95% CI: −0.76–2.42, I2 = 72%) of treatment. However, PRP exhibited better efficacy than the placebo treatment after 6 weeks (SMD = 0.46, 95% CI: 0.15–0.77, I2 = 34%). Two studies included VAS scores and tendon thickness. VAS scores after 6 weeks (SMD = 1.35, 95% CI: −0.1.04–3.74, I2 = 93%) and 24 weeks (SMD = 1.48, 95% CI: −0.1.59–4.55, I2 = 95%) were not significantly different. However, VAS scores at the 12th week (SMD = 1.10, 95% CI: 0.53–1.68, I2 = 83%) and tendon thickness (SMD = 1.51, 95% CI: 0.39–2.63, I2 = 53%) were significantly different.
Conclusion:
PRP injection around the Achilles tendon is an option for the treatment of chronic AT. Limited evidence supports the conclusion that PRP is not superior to placebo treatment. These results still require verification by a large number of well designed, heterogeneous RCT studies.
Keywords: Achilles tendinopathy, meta-analysis, platelet-rich plasma, randomized controlled trials
1. Introduction
Achilles tendinopathy (AT) is a disease that is commonly encountered in the outpatient department of orthopedics. This disease has serious complications and is mainly treated through conservative treatment in the clinic. However, this treatment approach has poor curative effect, and the disease easily relapses.[1] The root of the disease is long-term unreasonable or excessive exercise, which causes the Achilles tendon and its surrounding tissues to repeatedly rub or overstretch beyond the repair capability of the tendon itself and for inflammatory changes to occur in the tendon and periorbital tissue. Chronic inflammation leads to the degeneration of the hyaline and fatty tissues of the tendon. This effect weakens and even causes the spontaneous rupture of the Achilles tendon.[2]
AT is treated through nonsurgical approaches, such as steroid hormone-blocking therapy, oral nonsteroidal anti-inflammatory drugs, low-temperature external application, and low-frequency ultrasound stimulation. Steroids and lidocaine closure therapy, which exert strong anti-inflammatory and analgesic effects, are the most widely used treatment methods in clinical practice. However, repeated and multiple injections can lead to collagen necrosis and may degrade the mechanical properties of the Achilles tendon.[3–5] The long-term use of nonsteroidal anti-inflammatory drugs can easily lead to the occurrence of gastrointestinal ulcers. Thus, the clinical application of these drugs in the treatment of AT has been controversial.[6]
The tissue of the Achilles tendon is composed of tendon cells, fibrin collagen, and water. Given that the Achilles tendon lacks its own blood supply, its healing rate is significantly slower than that of other connective tissues when damaged.[7] As the related research continues to develop, scholars have found that growth factors play a crucial role in Achilles tendon repair and have considered using platelet-rich plasma (PRP) to treat chronic AT.[8,9] Animal experiments have demonstrated that PRP can promote the healing quality and process of Achilles tendons.[10,11]
PRP is blood rich in platelets and is derived from autologous whole blood through centrifugation. Highly concentrated platelets can release a large number of growth factors, such as transforming growth factor beta 1 (TGF-β1), insulin-like growth factor (IGF), epidermal growth factor (EGF), and platelet-derived growth factor (PDGF). PRP accelerates the repair of wound sites by stimulating vascular endothelial cell division, vascular proliferation, capillary growth, and collagen synthesis in the transplant area.[12,13] PRP has attracted considerable attention from researchers because of its obvious advantages, such as self-sufficiency, convenient extraction, and high safety. On the basis of the results of a large number of basic research and animal experiments, the local injection of autologous PRP has been used clinically to treat chronic AT. However, the conclusions drawn by numerous clinical studies on the efficacy and safety of PRP are inconsistent.
In this study, we comprehensively searched clinical literature related to the use of the local injection of PRP to treat AT. We combined relevant literature through systematic review and meta-analysis. We aimed to understand the efficacy of the local injection of PRP in AT treatment and compare the efficacy of PRP with that of conservative treatments.
2. Materials and methods
According to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) criteria, we created a prospective protocol, including objectives, literature-search strategies, inclusion and exclusion criteria, outcome measurements, and methods of statistical analysis before commencing the study. The study was approved by the ethics committee of The Third Affiliated Hospital of Hebei Medical University.
2.1. Literature search
PubMed (1950–May 2018), EMBASE (1974–May 2018), Web of Science (May 2018) and the Cochrane Library (May 2018) were systematically searched. The following MeSH or Emtree terms and their combinations were searched in the title and abstract: “plasma,” “platelet-rich,” “platelet rich plasma,” “PRP,” “plasma/platelet-rich,” “plasma/platelet-rich fibrin,” “tendon, Achilles,” “calcaneal tendon,” “calcaneal tendons,” “calcaneal tendons” and “tendo calcaneus.” The search deadline was May 1, 2018. We manually retrieved eligible references by reading the retrieved literature.
2.2. Literature inclusion criteria
Eligibility criteria were established on the basis of patient, intervention, comparison, outcome, and study design (PICOS) as follows: P: diagnosis of AT; I and C: injection of PRP around the tendon; O: the Victorian Institute of Sport Assessment-Achilles (VISA-A), visual analog scale (VAS) and Achilles tendon thickness measurement; S: randomized controlled clinical trial.
The details of the outcomes were as follows: The VISA-A score ranged from 0 to 100, where 0 denotes no activity and maximum pain, and 100 denotes maximum activity and no pain. The secondary outcome measures were pain during activity measured on the basis of a VAS score (where 0 equals no pain, and 100 is the worst pain imaginable; 0–100 mm). Tendon thickness was measured via ultrasonography.
2.3. Exclusion criteria
The exclusion criteria were as follows: PRP combined with surgery; lack of non-PRP controls; incomplete literature (such as only summary of meetings); duplication of literature (such as early and final papers of a clinical trial).
2.4. Literature quality evaluation
Two independent reviewers (CJL and JBB) evaluated the quality of the included studies by using the ROB tool provided by the Cochrane collaboration. Seven items, namely, random sequence generation, allocation concealment, participant and personnel blinding, outcome assessment blinding, incomplete outcome data, selective reporting and other bias, were assessed. Each item was judged as of “high risk,” “unclear risk,” and “low risk” on the basis of the data presented in the article. If the article provided sufficient and correct information, the judgement was “low risk.” If the article provided insufficient or unmentioned information, the judgement was “unclear risk.” If the article was reported incorrectly, the judgement was “high risk.” If disagreements occurred between the 2 researchers, a third researcher would join in the discussion until a consensus was reached.
2.5. Data extraction and analysis
The 2 researchers independently extracted the relevant data. If any dispute occurred, the third researcher would join the discussion, and they would decide together. The mean difference and 95% CI were calculated and analysed as the effect amounts in accordance with the ankle function scores of each study treatment group and control group. If multiple ankle joint function scores were used in the study, the priority sequence of VISA-A, VAS, and Achilles tendon thickness was calculated in case of complete data. Data extraction, transformation and analysis methods were performed in reference to the Cochrane system evaluation manual.
2.6. Statistical analysis
Meta-analysis was performed using the Review manager 5.3 software provided by Cochrane Collaboration and supplemented by Graphpad Prism 5.1 software for calculation and plotting. I2 was calculated to test heterogeneity amongst different studies. When I2 < 50%, the heterogeneity of the study was low, and the fixed-effect model (fixed effect) was used. I2 > 75% was suggestive of heterogeneity. Thus, heterogeneity was analysed, and the random-effect model (random effect) was used. Sensitivity analysis was performed by removing an article. P < .05 was considered statistically significant.
3. Results
3.1. Study selection
A total of 650 articles were obtained from the initial examination. After screening, 5 randomized controlled trials (RCTs) that included 189 patients with chronic AT were finally included for qualitative and quantitative analyses (Fig. 1). The general information included in the study is detailed in Table 1. One low bias, 2 medium biases and 2 high biases were determined in accordance with the Cochrane risk assessment criteria (Figs. 2 and 3).
Figure 1.
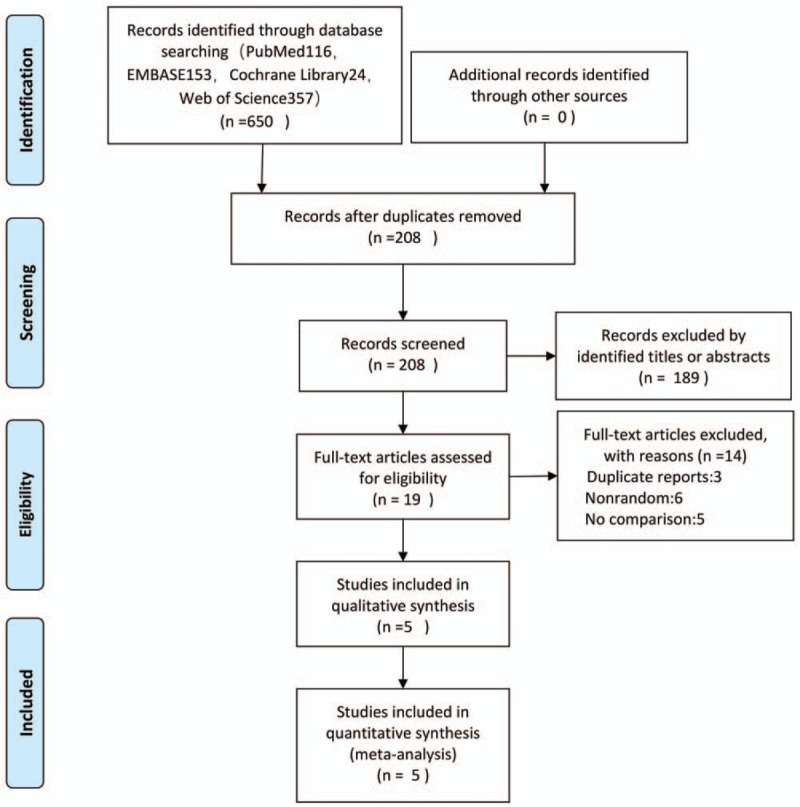
Flow diagram of studies identified, included, and excluded.
Table 1.
General characteristics of included studies.

Figure 2.
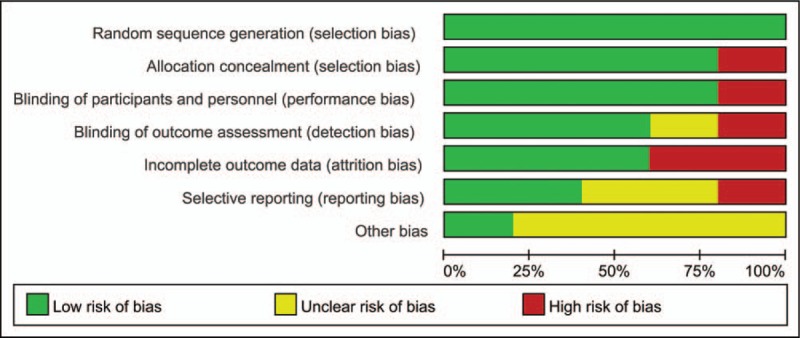
Risk of bias summary.
Figure 3.
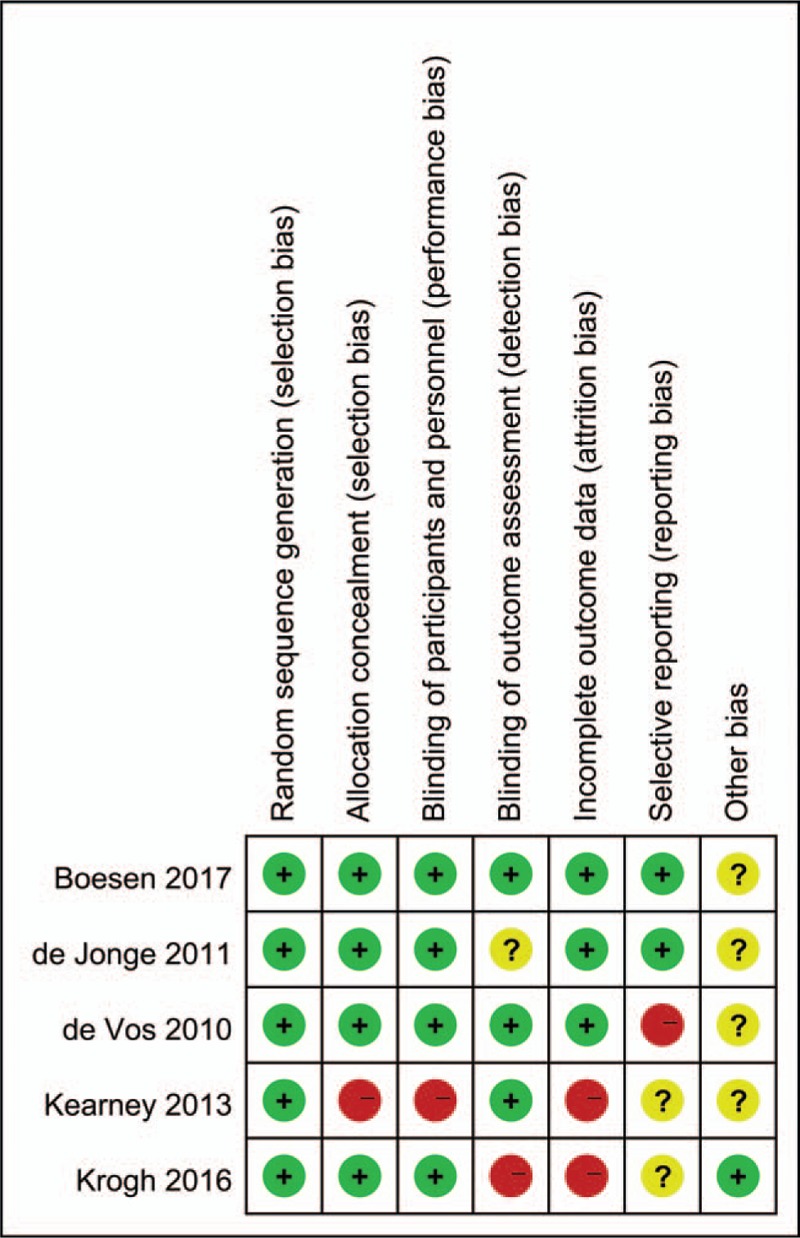
Risk of bias assessment.
3.2. Main outcome indicators, VISA-A score
Four, five and five articles included VISA-A scores at 6, 12 and 24 weeks after treatment. Except for the results at 6 weeks, those of other time points indicated high heterogeneity, and the random-effect model was used for combined analysis. The heterogeneous I2 < 40% was combined with the fixed-effect model. The results showed SMD = 0.46, 95% CI: 0.15, 0.77 and I2 = 34% at 6 weeks after treatment; SMD = 0.20, 95% CI: −0.36, 0.76, and I2 = 71% at 12 weeks after treatment; SMD = 0.77, 95% CI: −0.10, 1.65, and I2 = 85% at 24 weeks after treatment. No significant difference was found in improvement, but the VISA-A score of the PRP group was significantly higher than that of the control group 6 weeks after treatment. Patients were followed up 1 year after operation in only 2 studies (SMD = 0.83, 95% CI: −0.76, 2.42, and I2 = 72%). No significant difference existed between the experimental and control groups (Fig. 4).
Figure 4.
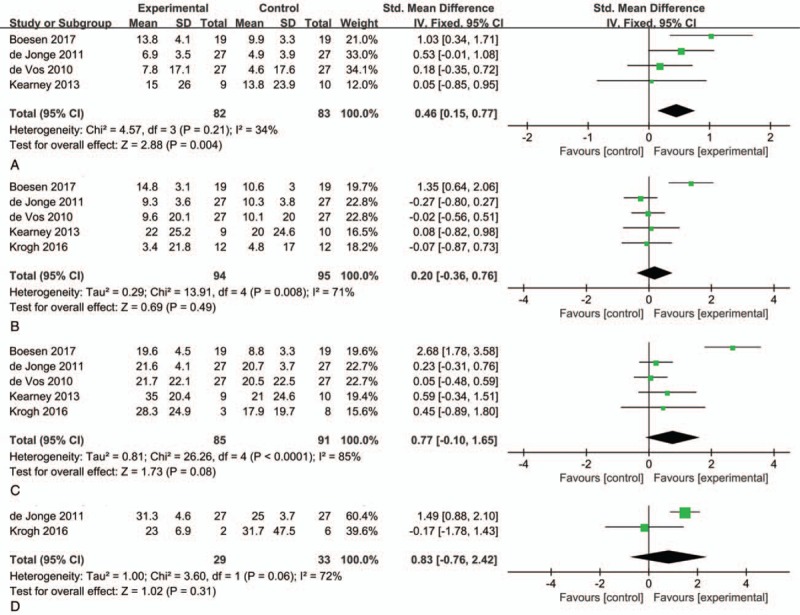
Forest plot for VISA-A score between PRP and placebo injections plus eccentric training. (A) 6 weeks after treatment; (B) 12 weeks after treatment; (C) 24 weeks after treatment; (D) 1 year after treatment. PRP = platelet-rich plasma, VISA-A = Victorian Institute of Sports Assessment-Achilles
3.3. VAS score
Two studies included VAS scores at 6, 12, and 24 weeks after treatment. The results were significantly heterogeneous, and a random-effect model was used for combined analysis. The results showed SMD = 1.35, 95% CI: −1.04, 3.74, and I2 = 93% at 6 weeks after treatment; SMD = 1.10, 95% CI: 0.53, 1.68, and I2 = 83% at 12 weeks after treatment; SMD = 1.48, 95% CI: −1.59, 4.55, and I2 = 95% at 24 weeks after treatment. The VAS scores of the PRP and control groups at 6 and 24 weeks after treatment were not significantly different. Improvement was not statistically significant, and the VAS scores of the PRP group were significantly higher than those of the control group at 12 weeks after treatment (Fig. 5).
Figure 5.
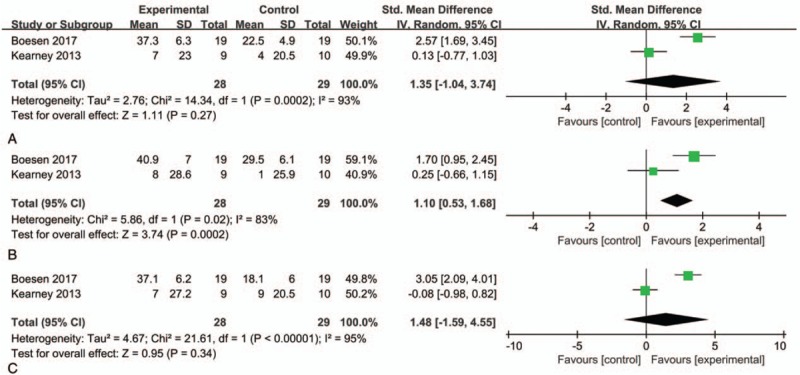
Forest plot for VAS score between PRP and placebo injections plus eccentric training. (A) 6 weeks after treatment; (B) 12 weeks after treatment; (C) 24 weeks after treatment. PRP = platelet-rich plasma, VAS = visual analog scale.
3.4. Measurement of Achilles tendon thickness
Two studies included the statistical analysis of the measurement of Achilles tendon thickness at 12 weeks after treatment. The results of random-effect model analysis showed that SMD = 1.51, 95% CI: 0.39, 2.63, and I2 = 53%, which suggested that the cerumen of the PRP group was significantly thinner than that of the control group (Fig. 6).
Figure 6.

Forest plot for the measurement of Achilles tendon thickness between PRP and placebo injections plus eccentric training.
3.5. Sensitivity analysis and publication bias
To identify the source of heterogeneity, the overall or subgroup with significant heterogeneity was subjected to sensitivity analysis (i.e., P < .10). The results showed that for the VISA-A score at 6 weeks after treatment, the I2 decreased from 34% to 0%, and the heterogeneity of P = 0.21 changed to P = 0.55 after Boesen's study was removed. In contrast to the VISA-A score of the control group, that of the PRP injection group was no longer statistically significant. For the VISA-A scores of all other time points, I2 decreased to 0%, and the study results did not significantly change. A single study was subjected to sensitivity analysis. The results showed that meta-analysis was unstable. The Cochrane Collaborative Systematic Review stated that when the number of research articles included in the meta-analysis is <10, the funnel plots used to evaluate publication bias are less effective and cannot be used to evaluate publication bias.
3.6. Quality of evidence
The GRADE system showed that the quality of evidence was moderate for VISA-A (12, 24 weeks) and was low for VAS and tendon thickness (Table 2).
Table 2.
GRADE assessment of outcomes.

4. Discussion
Although PRP has been clinically used for many years, its efficacy in AT treatment remains controversial. In this study, the quantitative analysis of the 5 items of grade-I clinical evidence showed that the efficacy of PRP does not significantly differ from that of the placebo.
Many factors contribute to the development of AT, and the exact mechanism that underlies this disease remains unclear. Most studies have suggested that AT is mainly caused by overwork, incorrect exercise training, stiff limbs and anatomic anomalies caused by weakness. Various factors first cause the local inflammation of the Achilles tendon. These effects then result in degenerative changes and finally in the partial or complete rupture of the Achilles tendon.[19]
The Achilles tendon itself lacks blood supply. Thus, its healing rate is significantly slower than that of other connective tissues. With the extension of relevant research, scholars have found that growth factors play a vital role in the repair of Achilles tendon and have considered the use of PRP to treat AT.
PRP is extracted from whole blood by a cell separation system. Platelets can secrete growth factors that are required for the repair of various tissues. These factors play an important role in tendon regeneration by increasing tendon cell proliferation, collagen synthesis and angiogenesis. A number of laboratory-based studies and limited clinical studies have confirmed that PRP exerts a good therapeutic effect on AT; therefore, PRP is widely used in clinical practice to treat AT.[20] Murawski[21] studies have shown that PRP provides good pain relief and satisfaction among patients with tendon diseases. However, such studies have limitations, i.e., they lack a control group or effective disease specificity, measurement and blinding methods. De Jonge compared the efficacy of PRP and placebo injection separately or in combination with centrifuge training for pain relief and functional improvement among patients with tendinitis. De Jonge found that treatment with PRP or the placebo did not significantly promote pain relief and function among patients with tendinitis. This finding has important clinical implications because PRP is increasingly used to treat chronic tendinopathy. Nevertheless, these conclusions are limited to laboratory and clinical studies. In vitro or in animal experiments have shown that PRP promotes tendon collagen synthesis and neovascularisation. However, these studies were performed using normal tendons or wounded tendons and not with an ideal tendinitis model. De Vos conducted a double-blind RCT in which 54 patients with chronic Achilles tendon inflammation received PRP treatment. Their results showed that PRP injection did not change the ultrasonic echo structure of the Achilles tendon lesion and the score of the neovascularisation. These studies do not support the clinical application of PRP.
Sensitivity analysis revealed that Boesen's article was highly heterogeneous from the other articles included in this meta-analysis. In contrast to previous RCTs, Boesen's study was the first RCT that showed that PRP has positive effects compared with the placebo. One explanation for the different results might be that Boesen provided 4 injections of PRP at 2-week intervals, whereas previous RCTs used only one injection of PRP. Abate et al[22] provided multiple injections of PRP to patients with chronic gingivitis, who showed positive clinical results in terms of function and pain. Although the potential mechanism of PRP in the treatment of tendonitis remains unclear, PRP has the potential to promote tendon healing. PRP contains various growth factors, including TGF-β1, IGF, EGF and PDGF, all of which are essential regulators of injured tissue repair. Repeated injection prolongs the time of exposure of the Achilles tendon to growth factors. This effect promotes the recovery of Achilles tendon tissue.[23]
Boesen's study employed a rehabilitation programme that was different from that employed by other RCTs. In the RCTs included in this study, the experimental and control groups received eccentric training. In Boesen's study, patients were allowed to recover gradually after 10 days. In contrast, in other studies, all patients avoided excessive exercise for 4 weeks to 6 weeks. Verrall et al[24] showed that during AT rehabilitation, movement did not provide inferior results compared with 6 weeks of stopping exercise. Insufficient evidence suggests that rest can improve prognosis. Van der Plas et al[25] conducted a 5-year follow-up study on eccentric training and found that 46 people (58 with AT) increased from baseline 49.2 to 83.6 in 5 years. Moreover, at follow-up, 39.7% reported complete pain relief, and the thickness of the sagittal Achilles tendon decreased from the baseline of 8.05 to 7.50 mm. Eccentric training, which can relieve pain and accelerate tendon remodelling and tissue repair, is effective in the treatment of chronic tendinitis.[26]
Our findings on chronic Achilles tendinopathy are in agreement with a recent meta-analysis by Chen et al,[27] which found that PRP treatment did not led to significantly improved VAS scores compared with alternative treatments in patients. However, our study also found that the thickness of the Achilles tendon was significantly thinner after PRP injection, which is an objective indicator of the relief of Achilles tendinitis symptoms.
PRP is derived from the patient and is administered after a short period of in vitro centrifugation. Thus, PRP itself does not cause immune rejection and lacks the risk of disease transmission. This finding is also confirmed in other diseases other than AT. The adverse reactions caused by PRP are nonspecific and heal within a short duration without serious consequences.[28] The literature included in this study did not report adverse reactions after PRP injection around the tendon.
5. Limitations
Although this study has established clear inclusion and exclusion criteria, only RCTs with the effectiveness of grade-I clinical evidence were included. Nevertheless, high heterogeneity remains amongst these RCT studies in quantitative analysis. Heterogeneity may originate from the extent of tendinitis, the method used to produce PRP, the cell component of PRP, the mode of activation, the dose and frequency of the injection and the control group. The scoring standards and methods used in the included studies also differed. Although subgroup analysis was performed at follow-up and the score of ankle joint function was unified and summed up, heterogeneity was ineffectively reduced, which is completely consistent with previous systematic reviews. The design of future RCTs should focus on reducing heterogeneity amongst existing research. In addition, this study failed to subdivide PRP into different types. Consequently, the efficacy and safety of a certain type of PRP treatment for tendinitis could not be emphasised. This limitation was related to the diversity of PRP production methods and the lack of a unified classification method. Furthermore, this study did not categorise the patient's age and disease severity given the limited patient information provided by the included RCTs. This inability precluded the identification of the patients who are most sensitive to the effects of PRP and the best indication for the treatment of AT with PRP.
6. Conclusion
In conclusion, this meta-analysis revealed that PRP injection around the Achilles tendon is a treatment option for chronic AT. Research on the mechanism of action of PRP has shown that PRP has unique potential for relieving pain and improving function among patients with AT. Thus, its application will be extensive. The present results still require verification with a large number of well-designed, homogeneous RCT studies.
Author contributions
DHT conceived and designed the experiments, KLY and CJL performed the experiments, JBB analyzed the data, GLL contributed reagents/materials/analysis tools, and CJL wrote the paper.
Conceptualization: Dehu Tian.
Data curation: Kun-lun Yu.
Formal analysis: Chun-jie Liu, Jiang-bo Bai.
Investigation: Guo-li Liu.
Methodology: Dehu Tian.
Resources: Jiang-bo Bai.
Software: Jiang-bo Bai.
Validation: Chun-jie Liu.
Visualization: Guo-li Liu.
Writing – original draft: Chun-jie Liu.
Writing – review & editing: Dehu Tian.
Dehu Tian orcid: 0000-0002-9409-9296.
Footnotes
Abbreviations: AT = Achilles tendinopathy, CI = confidence interval, EGF = epidermal growth factor, GRADE = the Grading of Recommendations Assessment,Developmen and Evaluation, IGF = insulin-like growth factor, PDGF = platelet-derived growth factor, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-analysis, PRP = platelet-rich plasma, RCTs = randomized controlled trials, ROB = Cochrane risk of bias, SMD = standardized mean difference, TGF-β1 = transforming growth factor beta-1, VAS = visual analog scale, VISA-A = Victorian Institute of Sports Assessment-Achilles.
The authors have no conflicts of interest to disclose.
References
- [1].McClinton S, Luedke L, Clewley D. Nonsurgical management of midsubstance Achilles tendinopathy. Clin Podiatr Med Surg 2017;34:137–60. [DOI] [PubMed] [Google Scholar]
- [2].Abbassian A, Khan R. Achilles tendinopathy: pathology and management strategies. Br J Hosp Med (Lond) 2009;70:519–23. [DOI] [PubMed] [Google Scholar]
- [3].Wetke E, Johannsen F, Langberg H. Achilles tendinopathy: a prospective study on the effect of active rehabilitation and steroid injections in a clinical setting. Scand J Med Sci Sports 2015;25:e392–9. [DOI] [PubMed] [Google Scholar]
- [4].Speed CA. Corticosteroid injections in tendon lesions. BMJ 2001;323:382–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Srivastava P, Aggarwal A. Ultrasound-guided retro-calcaneal bursa corticosteroid injection for refractory Achilles tendinitis in patients with seronegative spondyloarthropathy: efficacy and follow-up study. Rheumatol Int 2016;36:875–80. [DOI] [PubMed] [Google Scholar]
- [6].Caudell GM. Insertional Achilles tendinopathy. Clin Podiatr Med Surg 2017;34:195–205. [DOI] [PubMed] [Google Scholar]
- [7].Hess GW. Achilles tendon rupture: a review of etiology, population, anatomy, risk factors, and injury prevention. Foot Ankle Spec 2010;3:29–32. [DOI] [PubMed] [Google Scholar]
- [8].Najafbeygi A, Fatemi MJ, Lebaschi AH, et al. Effect of basic fibroblast growth factor on Achilles tendon healing in rabbit. World J Plast Surg 2017;6:26–32. [PMC free article] [PubMed] [Google Scholar]
- [9].Ai G, Shao X, Meng M, et al. Epidermal growth factor promotes proliferation and maintains multipotency of continuous cultured adipose stem cells via activating STAT signal pathway in vitro. Medicine (Baltimore) 2017;96:e7607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dietrich F, Hammerman M, Blomgran P. Effect of platelet-rich plasma on rat Achilles tendon healing is related to microbiota. Acta Orthop 2017;88:416–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang J, Yuan T, Zheng N, et al. The combined use of kartogenin and platelet-rich plasma promotes fibrocartilage formation in the wounded rat Achilles tendon entheses. Bone Joint Res 2017;6:231–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Alves R, Grimalt R. A review of platelet-rich plasma: history, biology, mechanism of action, and classification. Skin Appendage Disord 2018;4:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Noh KC, Liu XN, Zhuan Z, et al. Leukocyte-poor platelet-rich plasma-derived growth factors enhance human fibroblast proliferation in vitro. Clin Orthop Surg 2018;10:240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].De Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma Injection for chronic Achilles tendinopathy. A randomized controlled trial. JAMA 2010;303:144–9. [DOI] [PubMed] [Google Scholar]
- [15].De Jonge S, De Vos RJ, Weir A, et al. One-year follow-up of platelet-rich plasma treatment in chronic Achilles tendinopathy. Am J Sports Med 2011;39:1623–9. [DOI] [PubMed] [Google Scholar]
- [16].Kearney RS, Parsons N, Costa ML. Achilles tendinopathy management: A pilot randomised controlled trial comparing platelet-rich plasma injection with an eccentric loading programme. Bone Joint Res 2013;2:227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Krogh TP, Ellingsen T, Christensen R, et al. Ultrasound-guided injection therapy of Achilles tendinopathy with platelet-rich plasma or saline. Am J Sports Med 2016;44:1990–7. [DOI] [PubMed] [Google Scholar]
- [18].Boesen AP, Hansen R, Boesen MI, et al. Effect of high-volume injection, platelet-rich plasma, and sham treatment in chronic midportion Achilles tendinopathy. Am J Sports Med 2017;45:2034–43. [DOI] [PubMed] [Google Scholar]
- [19].Lu H, Yang H, Shen H, et al. The clinical effect of tendon repair for tendon spontaneous rupture after corticosteroid injection in hands. Medicine (Baltimore) 2016;95:e5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lin SL, Tsai CC, Wu SL, et al. Effect of arthrocentesis plus platelet-rich plasma and platelet-rich plasma alone in the treatment of temporomandibular joint osteoarthritis. Medicine (Baltimore) 2018;97:e0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Murawski CD, Smyth NA, Newman H, et al. A single platelet-rich plasma injection for chronic midsubst. Foot Ankle Spec 2014;7:372–6. [DOI] [PubMed] [Google Scholar]
- [22].Abate M, Di Carlo L, Verna S, et al. Synergistic activity of platelet-rich plasma and high volume image guided injection for patellar tendinopathy. Knee Surg Sports Traumatol Arthrosc 2018;26:3645–51. [DOI] [PubMed] [Google Scholar]
- [23].Charousset C, Zaoui A, Bellaiche L, et al. Are multiple platelet-rich plasma injections useful for treatment of chronic patellar tendinopathy in athletes?A prospective study. Am J Sports Med 2014;42:906–11. [DOI] [PubMed] [Google Scholar]
- [24].Verrall GM, Dolman BK, Best TM. Applying physical science principles to mid-substance Achilles tendinopathy and the relationship to eccentric lengthening exercises. Scand J Med Sci Sports 2018;28:1159–65. [DOI] [PubMed] [Google Scholar]
- [25].Van der Plas A, De Jonge S, De Vos RJ, et al. A 5-year follow-up study of Alfredson's heel-drop exercise programme in chronic midportion Achilles tendinopathy. Br J Sports Med 2012;46:214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].O’Neill S, Watson PJ, Barry S. Why are eccentric exercises effective for Achilles tendinopathy. Int J Sports Phys Ther 2015;10:552–62. [PMC free article] [PubMed] [Google Scholar]
- [27].Chen X, Jones IA, Park C, et al. The efficacy of platelet-rich plasma on tendon and ligament healing: a systematic review and meta-analysis with bias assessment. Am J Sports Med 2018;46:2020–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nikolopoulos KI, Pergialiotis V, Perrea D, et al. Restoration of the pubourethral ligament with platelet rich plasma for the treatment of stress urinary incontinence. Med Hypotheses 2016;90:29–31. [DOI] [PubMed] [Google Scholar]


