Abstract
Ubiquitin-conjugating enzyme E2C (UBE2C), a crucial part of the ubiquitin—conjugating enzyme complex, is reported to promote progression of various cancers. Leucine-rich repeated-containing G protein-coupled receptor (LGR5), a biomarker of cancer stem cells, is reported to be responsible for the initiation and progression of cancers. WW domain-containing oxidoreductase (WWOX), a suppressor of tumor, is reported to inhibit initiation and progression of cancers. Vasculogenic mimicry (VM), a new blood supply pattern, is associated with progression of cancers. However, the clinicopathological significance of UBE2C, LGR5, WWOX, and VM in invasive breast carcinoma (IBC) remains elusive. The aim of this study is to investigate the positive rate of UBE2C, LGR5, WWOX, and VM in IBC and their clinical significance.
Positive rates of UBE2C, LGR5, WWOX, and VM in 247 whole IBC samples were detected through immunohistochemistry. Patients data (including clinical, demography, follow-up) were collected.
Levels of UBE2C, LGR5, VM, and microvessel density (MVD) were significantly higher, and level of WWOX was significantly lower in IBC specimens when compared with normal mammary gland tissues. Levels of UBE2C, LGR5, VM, and MVD were all positively associated with tumor stages, lymph node metastasis (LNM) stages, tumor grades, and tumor-node-metastasis (TNM) stages, and unfavorably with patients’ overall survival (OS) and disease-free survival (DFS). Level of WWOX was negatively associated with tumor stages, LNM stages, grades, and TNM stages, and favorably with patients’ OS and DFS. Multivariate analysis indicated that levels of UBE2C, LGR5, VM, MVD, and WWOX, as well as TNM stages were independently prognostic factors for OS and DFS in patients with IBC.
UBE2C, LGR5, VM, MVD, and WWOX may be considered as promising indicator of IBC prognosis.
Keywords: invasive breast carcinoma, leucine-rich repeated-containing G protein-coupled receptor, microvessel density, ubiquitin-conjugating enzyme E2C, vasculogenic mimicry, WW domain-containing oxidoreductase
1. Introduction
Breast carcinoma was the most common diagnosed cancer among worldwide women and was estimated 520,000 deaths in the worldwide in 2012.[1] Breast cancer is a highly heterogeneous disease which makes it urgent to identify some biomarkers for early diagnosis, progression and prognosis judgement, and treatment. Indeed, estrogen receptor (ER), progesterone receptor (PR), and human epithelial growth factor receptor (HER2) amplification are related to distinct molecular subtypes and prognostic and therapeutic values.
Ubiquitin-conjugating enzyme E2C (UBE2C), also named as UbcH10, is a member of the E2 gene family and plays an important role in mitotic cyclin degradation and cell cycle progression.[2] UBE2C gene is located on chromosome 20q13 and encodes a 19.65 kDa protein. In normal tissues, UBE2C is almost undetectable,[3] whereas it overexpresses in many cancers.[4] The previous studies have demonstrated that UBE2C is involved in many biological behaviors, such as tumorigenesis, proliferation, cell cycle, and apoptosis.[5–8]
Tumor recurrence and metastasis should be related to a sub-population of cancer cells which names cancer stem cells (CSCs). Accumulating evidence has demonstrated that CSCs have the capacity to self-renew, multi-directional differentiate, and are responsible for the resistance of chemo- or radio-therapy. The leucine-rich repeat-containing G-protein-coupled receptors 5 (LGR5) is a biomarker of stem cells in many organs, such as intestine, hair follicle, stomach, mammary gland, and ovary.[9–13] LGR5, which was originally considered as a Wnt/Tcf4 target gene, is a member of glycoprotein hormone receptor family.[9] LGR5 is consisted of a large leucine-rich domain and N terminal of the peptide. Overexpression of LGR5 can promote cancer cells proliferation, progression, metastasis, and CSCs maintenance.[14,15]
WW domain-containing oxidoreducatase (WWOX) is considered as a suppressor and resides in one of the most active common fragile sites which named FRA16D.[16] WWOX, which encodes 2 N terminal WW domains and a central shot chain dehydrogenase/reductase domain, is located on human chromosome 16q23.3–24.1.[16] WWOX is widely expressed in normal tissues, whereas down- or loss-expression of WWOX is often found in cancers through heterozygosity and hypermethylation. Aberrant expression of WWOX is involved in the process of tumorigenesis, progression, and angiogenesis.[17–19] Moreover, overexpression of WWOX suppresses tumorigenesis and tumor metastasis.[19,20]
It is well known that angiogenesis should promote tumor growth, invasion, and metastasis. The most common indicator for evaluation angiogenesis is microvessel density (MVD). However, it is still unsatisfactory that the clinical benefits are from anti-angiogenic therapy of cancers.[21] Some researchers supposed that there was another mechanism of tumor blood supply. Vasculogenic mimicry (VM), which is considered a new pattern of tumor blood supply, is tube-like structure. VM is lining of tumor cells. VM can partly explain the poor effects of anti-angiogenic therapy. The typical VM is composed of 3 structures: highly aggressive cancer cells, plastic extracellular matrix, and tube-like structures which can connect the host microcirculation system.[22,23] Some highly aggressive cancer cells have cancer stem-like phenotype can mimic endothelial cells to form tube-like structure which can convey nutrient and oxygen.[22–24] VM also promote tumor cells growth, invasion, and metastasis.
Overall, studies have demonstrated that UBE2C, LGR5, WWOX, MVD, and VM are associated with tumor progression and prognosis. However, it is not widely reported for the associations among UBE2C, LGR5, WWOX, MVD, and VM. The purpose of this study is to examine the hypothesis that these biomarkers should be mutually associated and be associated with progression and prognosis in invasive breast carcinoma (IBC).
2. Methods
2.1. Patients and specimens
Two hundred forty-seven patients (median age: 54.7 years; range: 26–77 years) who were diagnosed IBC at the Department of Pathology of our hospital were collected, from January 2012 to December 2013, along with the corresponding adjacent normal mammary tissues (5 cm away from the tumor edges). Patients who had underwent any anti-cancer therapy were excluded. The clinicopathological characteristics of the 247 IBC tissue specimens were seen in Table 1. Patients’ follow-up data was also collected (at 3-month intervals through mobile phone and social applications). Overall survival (OS) time was calculated from surgery date to December 2017 or her death date (mean OS: 55.7 months; range: 10–83 months). Tumor stages and TNM stages both were evaluated in accordance with the 8th edition of the guidelines issued by American Joint Committee on Cancer (AJCC). Grades of differentiation were evaluated in accordance with the guidelines issued by World Health Organization (WHO).
Table 1.
Patients characteristics.

2.2. Reagent and immunohistochemistry
Mouse anti-human monoclonal antibody against UBE2C (1F5D3) and CD34 (ab54028), and rabbit anti-human polyclonal antibody against LGR5 (ab75732) and WWOX (ab74091) were purchased from the Abcam, Co., Ltd., Cambridge, Massachusetts, UK. Rabbit anti-human monoclonal antibody against human epidermal growth factor receptor 2 (HER2, EP3), estrogen receptor (ER, SP1), and progesterone receptor (PR, SP2) and other reagents were purchased from Fuzhou Maixin Biotechnology Development Co., Ltd., Fuzhou, Fujian, China. All specimens were fixed in 10% neutral formalin solution and embedded in paraffin. Continuous 4-μm-thick sections were cut. Immunohistochemistry was performed following the Elivision Plus method, and the procedure was performed following the kit instructions. All sections were deparaffinized and dehydrated using routine methods. Citrate buffer solution was used antigen repair, and endogenous peroxidase activity was quenched by methanol containing 3% H2O2 solution. Then were blocked with goat serum. UBE2C, LGR5, CD34, WWOX, HER2, ER, and PR primary antibodies were added, subsequently, all sections were incubated at 4 °C overnight. Then reagent A and reagent B were added. All sections were performed periodic acid-Schiff (PAS)-CD34 dual staining. All sections were developed in diaminobenzidine (DAB) substrate. Finally, sections were re-dyed with hematoxylin.
2.3. Assessment of immunohistochemistry
Ten randomly selected high-power-field (HPF) fields of every IBC section were to avoid potential intratumoral heterogeneity of any biomarker expression. The intensity of immunostaining was scored as follows: no staining was 0; weak staining was 1; moderate staining was 2; strong staining was 3. The percentage of positive cells was scored as follows: <11% was 1; 11% to 50% was 2; 51% to 75% was 3; >75% was 4. The final scores were yielded by multiplying the intensity score and the extent score (range 0–12). The eventual determination of the results was considered as positive (score >2). In accordance with 2013 ASCO/CAP guidelines, HER2 expression in 10% of cancer cells was considered as positive. ER and PR expression in no <1% of cancer cells were considered as positive.[25] If there was difference between assessment results from the 2 independent pathologists, the results were reassessed. MVD was determined by the mean number of small CD34-positive vessels counted. A modified Weidner method was used to assess the MVD of IBC by anti-CD34 immunostaining.[26] All tissues were performed by PAS-CD34 dual staining to show endothelial cells glycosylated basement membranes of vessels as well as vessel-like (VM) tubes.[27] The method was adopted from Yue and Chen[28] with some modifications.
2.4. Statistical analysis
Chi-square tests were used to assess the positive rates of UBE2C, LGR5, WWOX, MVD, and VM in IBC and the control tissues as well as the associations between these biomarkers expression and the clinicopathological characteristics of IBC. Correlation analysis was carried out by using Spearman correlation test. Univariate OS and DFS analyzes were performed using the Kaplan–Meier method with log-rank tests. Multivariate OS and DFS analyzes were performed using Cox regression model tests. P < .05 was defined statistically significance. All data of statistical analyzes were using SPSS 19.0 software (Chicago, IL).
3. Results
3.1. The positive rates of UBE2C, LGR5, and VM were significantly higher in IBC tissues than those in the control tissues, inversely to them, WWOX expression was significantly lower in IBC tissues
The positive expression of UBE2C was mainly confined nuclei and cytoplasm; the positive expression of LGR5 was mainly confined cytoplasm and membrane; the positive expression of WWOX was mainly confined cytoplasm. The positive rate of UBE2C expression in IBC (58.7%, 145/247) was significantly higher than that in the control group (0%, 0/247; P < .001; Fig. 1A and B). The positive rate of LGR5 expression in IBC (61.5%, 152/247) was significantly higher than that in the control group (8.9%, 22/247; P < .001; Fig. 1C and D). The positive rate of WWOX expression in IBC (47.0%, 116/247) was significantly lower than that in the control group (87.0%, 215/247; P < .001; Fig. 1E and F). The positive rate of VM in IBC (32%, 79/247) was significantly higher than that in the control group (0%, 0/247; P < .001; Fig. 1G and H).
Figure 1.

Immunostaining of UBE2C, or LGR5, or WWOX, or VM, or MVD in invasive breast carcinoma or the control tissue. A: Negative staining UBE2C in the control tissue (100 magnification, bar = 100 μm); B: Positive staining of UBE2C in the nuclei and cytoplasm of IBC cells (400 magnification, bar = 100 μm); C: Negative staining of LGR5 in the control tissues (100 magnification, bar = 100 μm); D: Positive staining of LGR5 in cytoplasm and membrane of the IBC cells (400 magnification, bar = 100 μm); E: Positive staining of WWOX in the cytoplasm of control tissues (100 magnification, bar = 100 μm); F: Negative staining of WWOX in the IBC cells (100 magnification, bar = 100 μm). G: Positive staining of VM in the IBC tissues (100 magnification; white arrow is VM structure, red arrow is microvessel, bar = 100 μm); H: Positive staining of MVD in the IBC cells (100 magnification, bar = 100 μm). IBC = invasive breast carcinoma, LGR5 = leucine-rich repeated-containing G protein-coupled receptor, MVD = microvessel density, UBE2C = ubiquitin-conjugating enzyme E2C, VM = vasculogenic mimicry.
3.2. The positive rates of UBE2C, LGR5, and VM were positively related to tumor size, histological grades, tumor stages, LNM stages as well as TNM stages, inversely to them, WWOX expression is negatively related to these clinicopathological characteristics
UBE2C expression in IBC was positively related to alcohol, tumor size, histological grades, tumor stages, LNM stages, and TNM stages. LGR5 expression in IBC was positively related to tumor size, histological grades, tumor stages, LNM stages as well as TNM stages. WWOX expression in IBC was negatively related to tumor size, histological grades, tumor stages, LNM stages, as well as TNM stages. The positive rate of VM in IBC was positively related to tumor size, histological grades, tumor stages, LNM stages, and TNM stages. The score of MVD in IBC was positively related to histological grades and TNM stages (Table 2).
Table 2.
The association between UBE2C, LGR5, WWOX, VM, MVD, and clinicopathological characteristics.

3.3. Spearman correlation test
Correlation analysis revealed that the positive rate of WWOX in IBC was negatively correlated with the positive rate of UBE2C (r = –0.512, P < .001), LGR5 (r = –0.473, P < .001), VM (r = –0.210, P = .001), MVD (r = –0.199, P = .002), and HER2 (r = –0.410, P < .001), and positively correlated with ER expression (r = 0.262, P < .001). The positive rate of UBE2C in IBC was positively associated with the positive rate of LGR5 (r = 0.436, P < .001), VM (r = 0.258, P < .001), MVD (r = 0.135, P = .034), HER2 (r = 0.268, P < .001) and negatively associated with ER expression (r = –0.193, P = .002). The positive rate of LGR5 in IBC was positively associated with VM (r = 0.167, P = .008), MVD (r = 0.145, P = .023), HER2 (r = 0.251, P < .001), and negatively associated with ER expression (r = –0.158, P = .013). The positive rate of VM in IBC was positively associated with MVD (r = 0.284, P < .001), HER2 (r = 0.227, P < .001), and negatively associated with PR expression (r = –0.192, P = .002). The score of MVD in IBC was positively associated with HER2 (r = 0.165, P = .010) (Table 3).
Table 3.
Associations among UBE2C, LGR5, WWOX, VM, and MVD in IBC.

3.4. Univariate and multivariate analyzes
As shown in Fig. 2A, univariate OS analysis suggested that OS time of UBE2C+ for patients with IBC were significantly lower than that of UBE2C– for patients (log-rank = 56.737, P < .001). As shown in Fig. 2B, the univariate OS time of LGR5+ patients were significantly lower than in LGR5– patients (log-rank = 60.951, P < .001). As shown in Fig. 2C, the univariate OS time of WWOX+ patients were significantly longer than in WWOX– patients (log-rank = 80.033, P < .001). As shown in Fig. 2D, the univariate OS time of VM+ patients were significantly shorter than in VM– patients (log-rank = 34.773, P < .001). As shown in Fig. 2E, the univariate OS time of MVDhigh score patients were significantly lower than in MVDlow score patients (log-rank = 22.534, P < .001). As shown in Fig. 2F and G, the univariate OS time of ER+ or PR+ patients were significantly longer than in ER– or PR– patients (log-rank = 18.999, P < .001; log-rank = 11.569, P = .001, respectively). As shown in Fig. 2H, the univariate OS time of HER2+ patients were significantly lower than in HER2– patients (log-rank = 37.689, P < .001) (Table 4). As shown in Fig. 3A, B, D, E, H, the univariate DFS time of UBE2C+, or LGR5+, or VM+, or MVDhigh score, or HER2+ patients were significantly lower than in UBE2C–, or LGR5–, or VM–, or MVDlow score, or HER2– patients (log-rank = 58.314, P < .0001; log-rank = 59.612, P < .001; log-rank = 36.745, P < .001; log-rank = 21.976, P < .001; log-rank = 41.686, P < .001, respectively). As shown in Fig. 3C, G, F, the univariate DFS time of WWOX+, or ER+, or PR+ patients were significantly higher than in WWOX–, or ER–, or PR– patients (log-rank = 82.818, P < .001; log-rank = 20.135, P < .001; log-rank = 13.735, P < .001, respectively) (Table 5).
Figure 2.
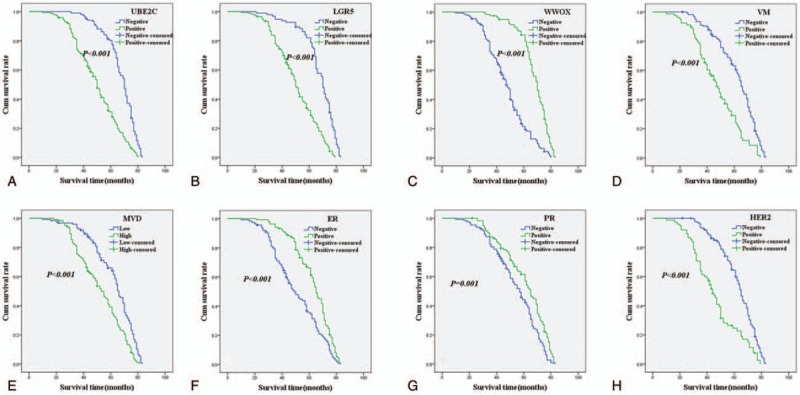
Kaplan–Meier analysis of overall survival time of patients with invasive breast carcinoma. A: Overall survival of all patients in relation to UBE2C expression (log-rank = 56.737, P < .001); B: OS of all patients in relation to LGR5 (log-rank = 60.951, P < .001); C: OS of all patients in relation to WWOX expression (log-rank = 80.033, P < .001); D: OS of all patients in relation to VM (log-rank = 34.773, P < .001). E: OS of all patients in relation to MVD (log-rank = 22.534, P < .001); F: OS of all patients in relation to ER (log-rank = 18.999, P < .001); G: OS of all patients in relation to PR (log-rank = 11.569, P = .001); H: OS of all patients in relation to HER2 (log-rank = 37.689, P < .001). In A, B, C, D, E, F, G, and H analyses, the green line represents positive staining of factors (MVD score ≥21 is positive) and the blue line represents negative staining factors (MVD score <21 is negative). LGR5 = leucine-rich repeated-containing G protein-coupled receptor, MVD = microvessel density, OS = overall survival, PR = progesterone receptor, UBE2C = ubiquitin-conjugating enzyme E2C, VM = vasculogenic mimicry.
Table 4.
Results of univariate analyses of overall survival (OS) time.

Figure 3.
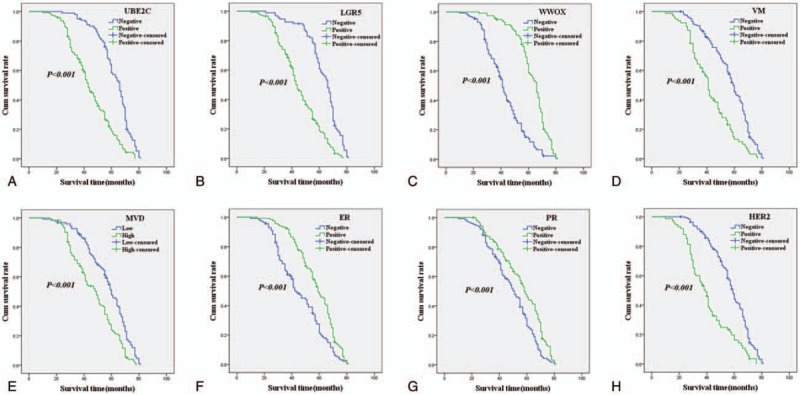
Kaplan–Meier analysis of disease-free survival time of patients with invasive breast carcinoma. A: Disease-free survival of all patients in relation to UBE2C expression (log-rank = 58.314, P < .0001); B: DFS of all patients in relation to LGR5 (log-rank = 59.612, P < .001); C: DFS of all patients in relation to WWOX expression (log-rank = 82.818, P < .001); D: DFS of all patients in relation to VM (log-rank = 36.745, P < .001). E: DFS of all patients in relation to MVD (log-rank = 21.976, P < .001); F: DFS of all patients in relation to ER (log-rank = 20.135, P < .001); G: DFS of all patients in relation to PR (log-rank = 13.735, P < .001); H: DFS of all patients in relation to HER2 (log-rank = 41.686, P < .001). In A, B, C, D, E, F, G, and H analyses, the green line represents positive staining of factors (MVD score ≥21 is positive) and the blue line represents negative staining factors (MVD score <21 is negative). DFS = disease-free survival, HER2 = human epithelial growth factor receptor 2, LGR5 = leucine-rich repeated-containing G protein-coupled receptor, MVD = microvessel density, PR = progesterone receptor, UBE2C = ubiquitin-conjugating enzyme E2C, VM = vasculogenic mimicry.
Table 5.
Results of univariate analyses of disease-free survival (DFS) time.

Multivariate analysis of OS suggested that positive expression of UBE2C, LGR5, WWOX, ER and VM, MVD as well as TNM stages were independent factors affecting patients’ OS (Table 6). Multivariate analysis of DFS suggested that positive expression of UBE2C, LGR5, WWOX, ER and VM, MVD, and TNM stages were independent factors affecting patients DFS (Table 7).
Table 6.
Results of multivariate analyses of overall survival (OS) time.

Table 7.
Results of multivariate analyses of disease-free survival (DFS) time.

4. Discussion
Breast cancer is one of the most common malignant tumors of women. Invasive breast carcinoma (IBC) is a highly heterogenous disease that leads to a serious threat to women's health and lives. It is urgent to investigate the pathogenesis of IBC and comprehensively evaluate biomarkers for IBC. UBE2C is considered to play an important role in the ubiquitin proteasome proteolytic pathway which is considered to be associated with occurrence and progression of tumors.[29] In this study, expression of UBE2C was significantly higher in IBC tissues than that in the normal mammary tissues, and its overexpression was positively associated with alcohol, tumor size, histological grades, tumor stages, LNM stages, and TNM stages. In addition, Kaplan–Meier survival analysis showed that IBC patients with UBE2C+ had significantly lower OS or DFS time than did UBE2C– patients. These results suggested that UBE2C overexpression promoted IBC progression and metastasis, as well as played a potential prognostic indicator for IBC.
LGR5, also named GPR49, is a common biomarker of CSCs. LGR5 knockdown CSCs showed lower capacity of proliferation and sphere formation.[30,31] In the present study, the data indicated that LGR5 expression was significantly related to tumor size, histological grades, tumor stages, LNM stages as well as TNM stages, similar to those reported previously studies.[32,33] These findings confirmed that LGR5 expression should play an important role in progression and metastasis of IBC. Moreover, the data showed that LGR5+ patients had lower OS or DFS time than LGR5– patients. LGR5 should be considered a useful biomarker for predicting prognosis of IBC.
WWOX is a suppressor gene and suppresses tumorigenesis through promoting apoptosis and maintaining genome integrity.[34,35] In our study, the data showed that WWOX expression was inversely related to tumor size, histological grades, tumor stages, LNM stages as well as TNM stages, parallel to those reported previously studies.[36,37] These results confirmed that loss of WWOX should promote invasion and metastasis of IBC. Furthermore, the data also confirmed that WWOX+ patients had longer OS or DFS time than WWOX– patients. Positive expression of WWOX should mean a favorable prognosis for IBC patients.
Several studies have demonstrated that angiogenesis promotes tumor cells proliferation, invasion, and metastasis in many human cancers. It is well known that anti-angiogenic therapy is a highlight method for anti-cancer therapy. However, it is still unsatisfactory for the benefit of anti-angiogenic therapy. VM is a channel which is lining cancer cells may partly explain this unsatisfactory benefit. In this study, the data showed that VM+ or MVDhigh score was significantly associated with histological grades and TNM stages, similar to those reported previously studies.[22,23] Kaplan–Meier analysis also indicated that VM+ or MVDhigh score patients had an unfavorable OS or DFS time than VM– or MVDlow score patients. VM and MVD are also considered a usefully potential indicator for prediction of IBC.
In this study, our data also demonstrated that expression of UBE2C, LGR5, and WWOX and VM, MVD, as well as TNM stages were independent prognostic factors of OS or DFS for patients with IBC. In addition, our data also indicated that WWOX expression was inversely associated with UBE2C, LGR5, VM, and MVD score; UBE2C, LGR5, VM, and MVD are positively associated with each other. The origin of breast cancer, in some studies, is considered to derive from putative CSCs.[9] It is believed that CSCs can promote the malignant transformation of cells in part by activation of Wnt/β-catenin signal pathway.[32] Overexpression of LGR5 is thought to promote breast cancer progression and CSCs maintenance.[14] UBE2C promotes degradation of mitotic cyclins and cell cycle progression and regulates anaphase-promoting complex.[37] Overexpression of UBE2C leads to chromosome missegregation and changes the cell cycle profile, therefore, promoting cell proliferation.[38] The microenvironment where CSCs reside are mainly composed of microvessel and microlymphatic vessels. It is reported that CSCs are able to differentiate tumor cells, endothelial cells, and other cells.[39,40] So, CSCs are able to mimic endothelial cells to form VM and differentiate endothelial cells to form vessel in order to meet tumor growth and invasiveness. In the meanwhile, loss of heterozygosity and hypermethylation of WWOX also promotes breast tumorigenesis and further facilitates cancer progression, and induces angiogenesis.[17–19] Overall, our findings confirmed that there is a complex relationship between the above biomarkers and IBC progression and prognosis. Combined investigation of these biomarkers, to a certain extent, the interaction of these biomarkers should be considered to reflect the progression and prognosis of IBC cells, so providing a potential choice of therapeutic target. The present study has already drawn out some conclusions, however, the size of samples in our study is relatively small and experimental method is relatively simply. The further studies with larger sized samples, such as in vitro, in vivo, and molecular experiment, are needed to support the present observations.
5. Conclusions
Our study demonstrated that UBE2C, LGR5, WWOX, VM, and MVD are associated with time of OS or DFS among patients with IBC. Therefore, UBE2C, LGR5, WWOX, VM, and MVD should be considered as useful and biomarkers in IBC, as well as potential targets for IBC.
Acknowledgments
The authors thank all staff members at the Department of Pathology of our hospital for assistance with the data collect and project management.
Author contributions
Miao Chen and Rong Shen carried out the design, analysis of pathology, and drafted the manuscript. Ting Wu and Pan Huang carried out sample collection and coordination. Qixiang Shao performed the immunohistochemical staining. All authors read and approved the manuscript.
Conceptualization: Miao Chen.
Data curation: Qixiang Shao.
Formal analysis: Qixiang Shao.
Methodology: Rong Shen, Ting Wu, Pan Huang.
Project administration: Rong Shen.
Resources: Ting Wu.
Software: Pan Huang.
Supervision: Miao Chen.
Validation: Ting Wu, Miao Chen.
Writing – review & editing: Rong Shen, Miao Chen.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, CSCs = cancer stem cells, DFS = disease-free survival, ER = estrogen receptor, HER2 = human epithelial growth factor receptor 2, HPF = high-power-field, IBC = invasive breast carcinoma, LGR5 = leucine-rich repeated-containing G protein-coupled receptor, LNM = lymph node metastasis, MVD = microvessel density, OS = overall survival, PR = progesterone receptor, TNM = tumor node metastasis, UBE2C = ubiquitin-conjugating enzyme E2C, VM = vasculogenic mimicry, WHO = World Health Organization, WWOX = WW domain-containing oxidoreductase.
This study was supported by the Science and Technology Support Program (Social Development) of Zhenjiang (No.SH2015055).
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable requests.
Consent for publication: Not applicable.
Ethics approval and consent to participate: Tissue samples for diagnostic and research aims were obtained with each patients’ consents and the research was approved by the ethical committee of Jiangsu University and performed in accordance with the guidelines of the Declaration of Helsinki.
The authors declare that they have no competing interests.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Shen Z, Jiang X, Zeng C, et al. High expression of ubiquitin-conjugating enzyme 2C (UBE2C) correlates with nasopharyngeal carcinoma progression. BMC Cancer 2013;13:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang R, Song Y, Liu X, et al. UBE2C induces EMT through Wnt/(-catenin and PI3K/Akt signaling pathways by regulating phosphorylation levels of Aurora-A. Int J Oncol 2017;50:1116–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Palumbo A, Jr, Da Costa NM, De Martino M, et al. UBE2C is overexpressed in ESCC tissues and its abrogation attenuates the malignant phenotype of ESCC cell lines. Oncotarget 2016;7:65876–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Li SZ, Song Y, Zhang HH, et al. UbcH10 overexpression increases carcinogenesis and blocks ALLN susceptibility in colorectal cancer. Sci Rep 2014;4:6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Endesfelder D, Burrell R, Kanu N, et al. Chromosomal instability selects gene copy-number variants encoding core regulators of proliferation in ER+ breast cancer. Cancer Res 2014;74:4853–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chang L, Zhang Z, Yang J, et al. Atomic structure of the APC/C and its mechanism of protein ubiquitination. Nature 2015;522:450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang Z, Liu P, Wang J, et al. Ubiquitin-conjugating enzyme E2C regulates apoptosis-dependent tumor progression of non-small cell lung cancer via ERK pathway. Med Oncol 2015;32:149. [DOI] [PubMed] [Google Scholar]
- [9].Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007;449:1003–7. [DOI] [PubMed] [Google Scholar]
- [10].Jaks V, Barker N, Kasper M, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet 2008;40:1291–9. [DOI] [PubMed] [Google Scholar]
- [11].Barker N, Huch M, Kujala P, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 2010;6:25–36. [DOI] [PubMed] [Google Scholar]
- [12].Plaks V, Brenot A, Lawson DA, et al. Lgr5-expressing cells are sufficient and necessary for postnatal mammary gland organogenesis. Cell Rep 2013;3:70–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Flesken-Nikitin A, Hwang CI, Cheng CY, et al. Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature 2013;495:241–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yang L, Tang H, Kong Y, et al. LGR5 promotes breast cancer progression and maintains stem-like cells through activation of Wnt/β-catenin signaling. Stem Cells 2015;33:2913–24. [DOI] [PubMed] [Google Scholar]
- [15].Wang X, Wang X, Liu Y, et al. LGR5 regulates gastric adenocarcinoma cell proliferation and invasion via activating Wnt signaling pathway. Oncogenesis 2018;7:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bednarek AK, Laflin KJ, Daniel RL, et al. WWOX, a novel WW domain-containing protein mapping to human chromosome 16q23.3-24. 1, a region frequently affected in breast cancer. Cancer Res 2000;60:2140–5. [PubMed] [Google Scholar]
- [17].Finnis M, Dayan S, Hobson L, et al. Common chromosomal fragile site FRA16D mutation in cancer cells. Hum Mol Genet 2005;14:1341–9. [DOI] [PubMed] [Google Scholar]
- [18].Yue X, Zhou L, Wong W, et al. ORAOV1 and WWOX are metastatic and prognostic biomarker for invasive breast cancer. Int J Clin Exp Med 2017;10:13607–15. [Google Scholar]
- [19].Wen J, Xu Z, Li J, et al. Decreased WWOX expression promotes angiogenesis in osteosarcoma. Oncotarget 2017;8:60917–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Abdeen SK, Ben-David U, Shweiki A, et al. Somatic loss of WWOX is associated with TP53 perturbation in basal-like breast cancer. Cell Death Dis 2018;9:832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Vredenburgh JJ, Desjardins A, Herndon JE, II, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 2007;13:1253–9. [DOI] [PubMed] [Google Scholar]
- [22].Wu S, Yu L, Wang D, et al. Aberrant expression of CD133 in non-small cell lung cancer and its relationship to vasculogenic mimicry. BMC Cancer 2012;12:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhu B, Zhou L, Yu L, et al. Evaluation of the correlation of vasculogenic mimicry, ALDH1, KAI1 and microvessel density in the prediction of metastasis and prognosis in colorectal carcinoma. BMC Surg 2017;17:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang SS, Gao XL, Liu X, et al. CD133+ cancer stem-like cells promote migration and invasion of salivary adenoid cystic carcinoma by inducing vasculogenic mimicry formation. Oncotarget 2016;7:29051–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Iwamoto T, Katagiri T, Niikura N, et al. Immunohistochemical Ki67 after short-term hormone therapy identifies low-risk breast cancers as reliably as genomic markers. Oncotarget 2017;8:26122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kumada T, Tsuneyama K, Hatta H, et al. Improved 1-h rapid immunostaining method using intermittent microwave irradiation: practicability based on 5 years application in Toyama Medical and Pharmaceutical University Hospital. Mod Pathol 2004;17:1141–9. [DOI] [PubMed] [Google Scholar]
- [27].Ahmadi SA, Moinfar M, Gohari Moghaddam K, et al. Practical application of angiogenesis and vasculogenic mimicry in prostatic adenocarcinoma. Arch Iran Med 2010;13:498–503. [PubMed] [Google Scholar]
- [28].Yue WY, Chen ZP. Does vasculogenic mimicry exist in astrocytoma? J Histochem Cytochem 2005;53:997–1002. [DOI] [PubMed] [Google Scholar]
- [29].Mo CH, Gao L, Zhu XF, et al. The clinicopathological significance of UBE2C in breast cancer: a study based on immunohistochemistry, microarray and RNA-sequencing data. Cancer Cell Int 2017;17:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chen X, Wei B, Han X, et al. LGR5 is required for the maintenance of spheroid-derived colon cancer stem cells. Int J Mol Med 2014;34:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tanaka K, Ikeda N, Miyashita K, et al. DEAD box protein DDX1 promotes colorectal tumorigenesis through transcriptional activation of the LGR5 gene. Cancer Sci 2018;109:2479–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hou MF, Chen PM, Chu PY. LGR5 overexpression confers poor relapse-free survival in breast cancer patients. BMC Cancer 2018;18:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chang NS, Doherty J, Ensign A, et al. WOX1 is essential for tumor necrosis factor-, UV light-, staurosporine-, and p53-mediated cell death, and its tyrosine 33-phosphorylated form binds and stabilizes serine 46-phosphorylated p53. J Biol Chem 2005;280:43100–8. [DOI] [PubMed] [Google Scholar]
- [34].Abu-Odeh M, Salah Z, Herbel C, et al. WWOX, the common fragile site FRA16D gene product, regulates ATM activation and the DNA damage response. Proc Natl Acad Sci U S A 2014;111:E4716–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chang R, Song L, Xu Y, et al. Loss of Wwox drives metastasis in triple-negative breast cancer by JAK2/STAT3 axis. Nat Commun 2018;9:3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Maroni P, Matteucci E, Bendinelli P, et al. Functions and epigenetic regulation of Wwox in bone metastasis from breast carcinoma: comparison with primary tumors. Int J Mol Sci 2017;18:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Summers MK, Pan B, Mukhyala K, et al. The unique N terminus of the UbcH10 E2 enzyme controls the threshold for APC activation and enhances checkpoint regulation of the APC. Mol Cell 2008;31:544–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fujita T, Ikeda H, Taira N, et al. Overexpression of UbcH10 alternates the cell cycle profile and accelerate the tumor proliferation in colon cancer. BMC Cancer 2009;9:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wang R, Chadalavad K, Wilshire J, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature 2010;468:829–33. [DOI] [PubMed] [Google Scholar]
- [40].Soda Y, Marumoto T, Friedmann-Morvinski D, et al. Transdifferentiation of glioblastoma cells into vascular endothelial cell. Proc Natl Acad Sci U S A 2011;108:4274–80. [DOI] [PMC free article] [PubMed] [Google Scholar]


