Abstract
Background
Posterior vaginal wall prolapse (also known as 'posterior compartment prolapse') can cause a sensation of bulge in the vagina along with symptoms of obstructed defecation and sexual dysfunction. Interventions for prevention and conservative management include lifestyle measures, pelvic floor muscle training, and pessary use. We conducted this review to assess the surgical management of posterior vaginal wall prolapse.
Objectives
To evaluate the safety and effectiveness of any surgical intervention compared with another surgical intervention for management of posterior vaginal wall prolapse.
Search methods
We searched the Cochrane Incontinence Group Specialised Register of controlled trials, which contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (searched April 2017). We also searched the reference lists of relevant articles, and we contacted researchers in the field.
Selection criteria
We included randomised controlled trials (RCTs) comparing different types of surgery for posterior vaginal wall prolapse.
Data collection and analysis
We used Cochrane methods. Our primary outcomes were subjective awareness of prolapse, repeat surgery for any prolapse, and objectively determined recurrent posterior wall prolapse.
Main results
We identified 10 RCTs evaluating 1099 women. Evidence quality ranged from very low to moderate. The main limitations of evidence quality were risk of bias (associated mainly with performance, detection, and attrition biases) and imprecision (associated with small overall sample sizes and low event rates).
Transanal repair versus transvaginal repair (four RCTs; n = 191; six months' to four years' follow‐up)
Awareness of prolapse is probably more common after the transanal approach (risk ratio (RR) 2.78, 95% confidence interval (CI) 1.00 to 7.70; 2 RCTs; n = 87; I2 = 0%; low‐quality evidence). If 10% of women are aware of prolapse after transvaginal repair, between 10% and 79% are likely to be aware after transanal repair.
Repeat surgery for any prolapse: Evidence is insufficient to show whether there were any differences between groups (RR 2.42, 95% CI 0.75 to 7.88; 1 RCT; n = 57; low‐quality evidence).
Recurrent posterior vaginal wall prolapse is probably more likely after transanal repair (RR 4.12, 95% CI 1.56 to 10.88; 2 RCTs; n = 87; I2 = 35%; moderate‐quality evidence). If 10% of women have recurrent prolapse on examination after transvaginal repair, between 16% and 100% are likely to have recurrent prolapse after transanal repair.
Postoperative obstructed defecation is probably more likely with transanal repair (RR 1.67, 95% CI 1.00 to 2.79; 3 RCTs; n = 113; I2 = 10%; low‐quality evidence).
Postoperative dyspareunia: Evidence is insufficient to show whether there were any differences between groups (RR 0.32, 95% CI 0.09 to 1.15; 2 RCTs; n = 80; I2 = 5%; moderate‐quality evidence).
Postoperative complications: Trials have provided no conclusive evidence of any differences between groups (RR 3.57, 95% CI 0.94 to 13.54; 3 RCTs; n = 135; I2 = 37%; low‐quality evidence). If 2% of women have complications after transvaginal repair, then between 2% and 21% are likely to have complications after transanal repair.
Evidence shows no clear differences between groups in operating time (in minutes) (mean difference (MD) 1.49, 95% CI ‐11.83 to 8.84; 3 RCTs; n = 137; I2 = 90%; very low‐quality evidence).
Biological graft versus native tissue repair
Evidence is insufficient to show whether there were any differences between groups in rates of awareness of prolapse (RR 1.09, 95% CI 0.45 to 2.62; 2 RCTs; n = 181; I2 = 13%; moderate‐quality evidence) or repeat surgery for any prolapse (RR 0.60, 95% CI 0.18 to 1.97; 2 RCTs; n = 271; I2 = 0%; moderate‐quality evidence). Trials have provided no conclusive evidence of a difference in rates of recurrent posterior vaginal wall prolapse (RR 0.55, 95% CI 0.30 to 1.01; 3 RCTs; n = 377; I2 = 6%; moderate‐quality evidence); if 13% of women have recurrent prolapse on examination after native tissue repair, between 4% and 13% are likely to have recurrent prolapse after biological graft. Evidence is insufficient to show whether there were any differences between groups in rates of postoperative obstructed defecation (RR 0.96, 95% CI 0.50 to 1.86; 2 RCTs; n = 172; I2 = 42%; moderate‐quality evidence) or postoperative dyspareunia (RR 1.27, 95% CI 0.26 to 6.25; 2 RCTs; n = 152; I2 = 74%; low‐quality evidence). Postoperative complications were more common with biological repair (RR 1.82, 95% CI 1.22 to 2.72; 3 RCTs; n = 448; I2 = 0%; low‐quality evidence).
Other comparisons
Single RCTs compared site‐specific vaginal repair versus midline fascial plication (n = 74), absorbable graft versus native tissue repair (n = 132), synthetic graft versus native tissue repair (n = 191), and levator ani plication versus midline fascial plication (n = 52). Data were scanty, and evidence was insufficient to show any conclusions about the relative effectiveness or safety of any of these interventions. The mesh exposure rate in the synthetic group compared with the native tissue group was 7%.
Authors' conclusions
Transvaginal repair may be more effective than transanal repair for posterior wall prolapse in preventing recurrence of prolapse, in the light of both objective and subjective measures. However, data on adverse effects were scanty. Evidence was insufficient to permit any conclusions about the relative effectiveness or safety of other types of surgery. Evidence does not support the utilisation of any mesh or graft materials at the time of posterior vaginal repair. Withdrawal of some commercial transvaginal mesh kits from the market may limit the generalisability of our findings.
Plain language summary
Surgical management of pelvic organ prolapse in women
Review question
Which surgical interventions for posterior vaginal wall prolapse have the best outcomes, and what are the complications of each intervention?
Background
Posterior vaginal wall prolapse is descent of the rectum or small bowel, causing the back wall of the vagina to bulge into the vagina. This condition can be treated conservatively with pelvic floor muscle training or vaginal pessaries, or it can be managed surgically. Several different operations are currently performed to manage prolapse of the posterior vaginal wall. This review aims to compare these different operations in terms of their effectiveness and safety. Surgery for prolapse of the posterior vaginal wall can be done through the back passage or through the vagina. Different vaginal techniques aim to restore the strong fascial layer at the midline along the whole length of the posterior vaginal wall (midline fascial plication), or to identify and repair specific defects in this strong fascial layer (site‐specific repair). Those who perform repairs can use a woman’s own native tissue alone or can add a graft. The graft can be absorbable, biological, or synthetic.
Study characteristics
This review identified 10 randomised controlled trials including 1099 women with posterior vaginal wall prolapse. Four trials compared transanal repairs with transvaginal repairs. One study compared site‐specific repair with midline fascial plication ‐ two different techniques for transvaginal native tissue repair. One trial compared absorbable graft and native tissue vaginal repair. Four trials compared biological graft with native tissue, and one trial compared synthetic graft with native tissue. The evidence is current to April 2017.
Key results
Repair through the vagina may be more effective than repair through the back passage for posterior vaginal wall prolapse. However, data on adverse effects are scanty. Evidence was insufficient to permit conclusions about the relative effectiveness or safety of other types of surgery. Evidence does not support using mesh or biological grafts at the time of posterior vaginal repair. Withdrawal of some commercial transvaginal mesh kits from the market may limit the generalisability of our findings.
Quality of the evidence
Evidence quality ranged from very low to moderate. The main limitations in evidence quality were risk of bias (associated mainly with performance, detection, and attrition biases) and imprecision (associated with small overall sample sizes and low event rates).
Summary of findings
Background
Pelvic organ prolapse is common and is seen on examination in 40% to 60% of parous women (Handa 2004; Hendrix 2002). The annual aggregated rate of associated surgery in the United States is in the range of 10 to 30 per 10,000 women (Brubaker 2002). Pelvic organ prolapse is the descent of one or more of the pelvic organs (uterus, vagina, bladder, or bowel). Types of prolapse include:
upper vaginal prolapse (i.e. uterus, vaginal vault (after hysterectomy when the top of the vagina drops down));
anterior vaginal wall prolapse (i.e. cystocele (bladder descends), urethrocele (urethra descends), paravaginal defect (pelvic fascia defect)); and
posterior vaginal wall prolapse (i.e. enterocele (small bowel descends), rectocele (rectum descends), perineal deficiency).
A woman can present with prolapse at one or more of these sites. Posterior vaginal wall prolapse can cause the sensation of bulge in the vagina and can also cause symptoms of obstructed defecation, sometimes requiring splinting or digitation to facilitate bowel emptying. As with prolapse in other compartments of the vagina, posterior wall prolapse can cause sexual dysfunction. Prevention and conservative management of posterior wall prolapse is consistent with all types of vaginal prolapse and involves lifestyle measures, pelvic floor muscle training, and pessary use. The topic of this systematic review and meta‐analysis is the surgical management of posterior vaginal wall prolapse.
Description of the condition
Posterior wall prolapse is usually caused by prolapse of the rectum into the vagina (rectocele), but it can also be caused by prolapse of the small bowel into the vagina (enterocele).
The aetiology of pelvic organ prolapse (POP) is complex and multi‐factorial. Known risk factors include pregnancy, childbirth, congenital or acquired connective tissue abnormalities, denervation or weakness of the pelvic floor, ageing, hysterectomy, menopause, and factors associated with chronically raised intra‐abdominal pressure (Bump 1998; Gill 1998; MacLennan 2000).
Women with prolapse commonly have a variety of pelvic floor symptoms, only some of which are directly related to the prolapse. Generalised symptoms of prolapse include pelvic heaviness; bulge, lump, or protrusion coming down from the vagina; a dragging sensation in the vagina; and backache. Symptoms of bladder, bowel, or sexual dysfunction are frequently present. For example, women may need to use their fingers to reduce the prolapse to aid defecation. These symptoms may be directly related to the prolapsed organ, for example, obstructed defecation when a rectocele is present. They may also be independent of the prolapse, for example, faecal urgency when a rectocele is present.
Description of the intervention
Treatment of women with prolapse depends on the severity of the prolapse, its symptoms, the woman's general health, and surgeon preference and capabilities. Options available for treatment include conservative, mechanical, and surgical interventions.
Generally, conservative or mechanical treatments are considered for women with a mild degree of prolapse, those who wish to have more children, frail women, and women unwilling to undergo surgery. Conservative and mechanical interventions have been considered in separate Cochrane reviews (Adams 2004; Hagen 2011). These reviews provided no good evidence to guide management. The current review considers all surgical procedures for women with posterior vaginal wall prolapse.
Surgical management of posterior wall prolapse can be transvaginal or transanal. Different techniques can be used transvaginally, and repairs can utilise native tissue and biological or synthetic graft materials. Appendix 1 describes the various surgical techniques that are available.
Over the past five years and following significant litigation regarding outcomes of prolapse surgery after use of transvaginal polypropylene mesh, many of the products evaluated in this review have been voluntarily removed from the market (Prolift ‐ Gynecare/Ethicon, Somerville, NJ, USA; Perigee ‐ American Medical Systems, Minnetonka, MN, USA; Avaulta ‐ Bard, Covington, LA, USA), or companies have excluded transvaginal utilisation of the mesh product (Gynemesh PS ‐ Gynecare/Ethicon). When reading this review, one must be mindful that the data presented include some products that are no longer available for use.
To aid assessment of surgery, clinicians should record clear preoperative and postoperative site‐specific vaginal grading, details of the operative intervention, and impact of the surgery on functional aspects of bladder, bowel, and sexual function.
How the intervention might work
Aims of surgery include:
restoration of normal vaginal anatomy;
restoration or maintenance of normal bowel function; and
restoration or maintenance of normal sexual function.
Why it is important to do this review
Surgical management of posterior vaginal wall prolapse remains non‐standardised. The wide variety of surgical treatments available for prolapse indicates lack of consensus as to optimal treatment. Provided that sufficient numbers of trials of adequate quality have been conducted, the most reliable evidence is likely to come from randomised controlled trials, which serve as the basis for this review. The aim of this review is to identify optimal practice while highlighting topics requiring further research.
Objectives
To evaluate the safety and effectiveness of any surgical intervention compared with another intervention for management of posterior vaginal wall prolapse.
Methods
Criteria for considering studies for this review
Types of studies
We included published and unpublished randomised controlled trials (RCTs) in which investigators compared any surgery for posterior vaginal wall prolapse against any other surgery for posterior vaginal wall prolapse. We excluded quasi‐randomised studies (e.g. studies with evidence of inadequate sequence generation such as alternate days, patient numbers) as they are associated with high risk of bias. As this is a systematic review of surgical interventions, we excluded cross‐over studies, as the design is not valid in this context. Review inclusion criteria require that trials provide follow‐up for at least six months.
Types of participants
Eligible studies included adult women seeking treatment for symptomatic posterior vaginal wall prolapse ‐ primary or recurrent.
Types of interventions
Eligible studies compared different types of surgery for posterior vaginal wall prolapse by looking at the following.
-
Differences in route.
Transanal.
Transvaginal.
-
Differences in type of repair.
Any surgical technique to repair posterior vaginal wall prolapse compared with any other surgical technique to repair posterior vaginal wall prolapse.
Types of outcome measures
Primary outcomes
1. Awareness of prolapse: any affirmative response to questions related to awareness of prolapse or vaginal bulge (subjective failure)
2. Repeat surgery for any prolapse
3. Recurrent posterior vaginal wall prolapse, defined as any stage 2 or greater prolapse (Pelvic Organ Prolapse Quantification (POP‐Q): Ap or Bp assessed to be prolapsed to 1cm above the hymen or lower (more distal)(objective failure)
Ap is a point on the posterior vaginal wall 3 cm from the vaginal entrance, range ‐3 to +3 cm
Bp is approximately at the midpoint of the posterior vaginal wall, range ‐3 to +10 cm
C describes the vaginal apex, ranging from ‐10 to non‐determined limit
Ba is approximately at the midpoint of the anterior vaginal wall, range ‐3 to +10 cm
Secondary outcomes
4. Bowel function
4.1 Postoperative obstructed defecation
4.2 Postoperative anal incontinence
4.3 Postoperative constipation
5. Sexual function
5.1 De novo dyspareunia
5.2 Postoperative dyspareunia
5.3 No improvement in dyspareunia
6. Prolapse outcomes (POP‐Q scores present nine measurements of the vagina to quantify and describe vaginal prolapse). For simplicity, we have reported four of these basic measurements
6.1 Mean postoperative change in Ap
6.2 Mean postoperative change in Bp
6.3 Mean postoperative change C
6.4 Mean postoperative change in Ba
7. Quality of life (QOL) and satisfaction
7.1 Postoperative Pelvic Floor Impact Questionnaire (PFIQ)‐7
7.2 Postoperative Pelvic Floor Distress Inventory (PFDI)‐20
7.3 Postoperative pelvic Organ Prolapse Symptom Score (POP‐SS)
7.4 Postoperative Pelvic organ prolapse/urinary Incontinence Sexual Questionnaire (PISQ)‐12
8. Adverse events
8.1 Mesh exposure
8.2 Reoperation for mesh exposure
8.3 Intraoperative complications including bowel injury and haemorrhage
8.4 Postoperative complications including wound infection
9. Perioperative outcomes ‐ continuous
9.1 Estimated blood loss (EBL; mL)
9.2 Operation time (minutes)
9.3 Length of stay (days)
9.4 Postoperative narcotic use (mg equivalent of morphine)
10. Perioperative outcomes ‐ dichotomous
10.1 Persistent postoperative pain
10.2 Discharge from hospital within 48 hours
10.3 Blood transfusion
11. Investigations
11.1 Defecogram: mean postoperative rectocele size (cm)
11.2 Anal manometry: postoperative mean maximum anal resting pressure (MARP) (mmHg)
Search methods for identification of studies
We did not impose any language or other limits on any of the searches detailed below.
Electronic searches
We searched the Cochrane Incontinence Group Specialised Register of controlled trials, which contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (searched April 2017) (Appendix 1). We handsearched conference proceedings for the International Urogynecology Society (IUGA) and the International Continence Society (ICS) for podium presentations up until June 2016. We searched the reference lists of relevant articles and contacted researchers in the field.
Searching other resources
We handsearched conference proceedings for the International Urogynecology Society (IUGA) and the International Continence Society (ICS) for podium presentations from 2012 to June 2016. We searched the reference lists of relevant articles and contacted researchers in the field.
Data collection and analysis
Selection of studies
Two review authors assessed the titles and, if available, abstracts of all possibly eligible studies for compliance with the inclusion criteria for this review. At least two review authors then independently assessed full‐text reports for each study likely to be eligible. We have listed excluded studies along with reasons for their exclusion in the Characteristics of excluded studies table. We have presented the selection process in a PRISMA flow chart (Figure 1).
1.
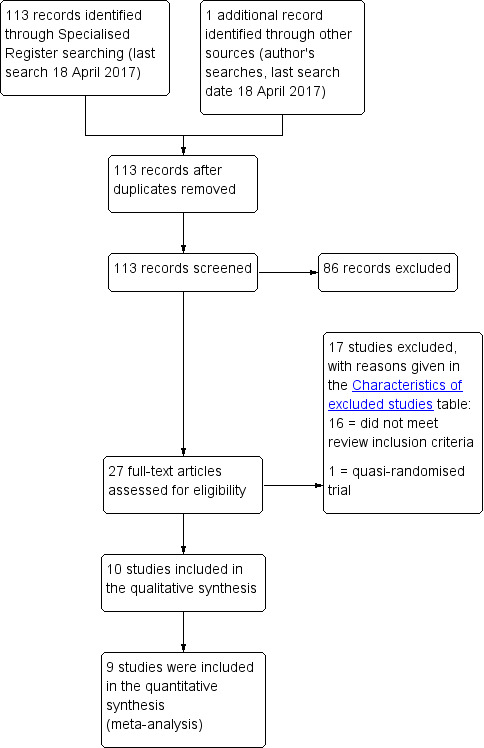
PRISMA study flow diagram.
Data extraction and management
At least two review authors extracted data and performed comparisons to ensure accuracy. We resolved discrepancies by discussion or by consultation with a third party. When trial data were not reported adequately, we attempted to acquire the necessary information from the trialist.
Assessment of risk of bias in included studies
Two review authors independently assessed included studies for risk of bias using the Cochrane risk of bias assessment tool (Higgins 2011b) to evaluate selection (random sequence generation and allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome assessors); attrition (incomplete outcome data); reporting (selective reporting); and other biases. We resolved disagreements by discussion or by consultation with a third review author. We will describe all judgements fully and will present our conclusions in the risk of bias table that we will incorporate into our interpretation of review findings by performing sensitivity analyses (see below).
We considered that robust methods of sequence generation and allocation concealment would prevent bias related to differing surgical skills, even when studies were not stratified by surgeon.
We considered that all our primary outcomes were at risk of detection and/or performance bias unless both personnel and outcome assessors were clearly blinded, as even repeat surgery may be influenced by knowledge of which type of surgery was conducted initially.
We rated studies with over 15% loss to follow‐up as having high risk of attrition bias.
We rated studies that reported outcomes according to a published protocol as having low risk of selection bias. Among trials for which a published protocol was not available, we rated those that reported at least one of our primary outcomes as having unclear risk of bias and those that did not report any of our primary outcomes as having high risk.
Measures of treatment effect
For dichotomous data, we used numbers of events in control and intervention groups of each study to calculate Mantel‐Haenszel risk ratios (RRs). For continuous data, if all studies reported exactly the same outcomes, we calculated mean differences (MDs) between treatment groups. If investigators reported similar outcomes on different scales, we calculated standardised mean differences (SMDs). We presented 95% confidence intervals (CIs) for all outcomes. We compared the magnitude and direction of effect reported by studies with how they are presented in the review, while accounting for legitimate differences.
Unit of analysis issues
All analyses were per woman randomised.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis as far as possible and attempted to obtain missing data from the original trialists. When these were unobtainable, we analysed only available data.
Assessment of heterogeneity
We considered whether clinical and methodological characteristics of included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by using the I2 measure. We considered I2 greater than 50% to indicate substantial heterogeneity (Higgins 2011; Higgins 2003).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, review authors aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by staying alert for duplication of data. If we included more than 10 studies in a single analysis, we planned to construct a funnel plot to assess reporting bias.
Data synthesis
If studies were sufficiently similar, we combined data using a fixed‐effect model in Review Manager software (Revman 2014) for the following comparisons.
Transanal versus vaginal.
Site‐specific versus midline fascial plication.
Absorbable graft versus native tissue.
Biological graft versus native tissue.
Synthetic graft versus native tissue.
Levator ani plication versus native tissue repair.
Subgroup analysis and investigation of heterogeneity
If we detected substantial heterogeneity, we explored possible explanations by conducting sensitivity analyses. We took any statistical heterogeneity into account when interpreting results, especially if we noted any variation in the direction of effect.
We combined trials only if interventions were similar enough in terms of clinical criteria. When we suspected important heterogeneity through visual inspection of results, we used the Chi2 test for heterogeneity (at 10%) or the I2 statistic to look for further differences between trials (Higgins 2003). When concern about heterogeneity persisted, we used a random‐effects model.
We identified trials separately and combined if they addressed other secondary objectives of the review related to prevention or treatment of complications or evaluation of urinary, bowel, or sexual function.
Sensitivity analysis
We conducted sensitivity analyses for the primary outcomes to determine whether our conclusions were robust to arbitrary decisions made regarding eligibility and analysis. These analyses included consideration of whether review conclusions would have differed if:
we had restricted eligibility to studies at low risk of bias (defined as low risk of selection bias and not as high risk of bias in any domain);
we had adopted a random‐effects model; or
the summary effect measure used had been odds ratio rather than risk ratio.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared a 'Summary of findings' table using GRADEproGDT 2015 and Cochrane methods. This table evaluates the overall quality of the body of evidence for the main review outcomes (awareness of prolapse, repeat surgery for prolapse, recurrent posterior vaginal wall prolapse) and for additional clinically relevant outcomes (postoperative obstructed defecation, postoperative dyspareunia, postoperative complications, estimated blood loss, and operating time) for the main review comparison (transanal repair vs transvaginal repair).
We prepared an additional 'Summary of findings' table for another important comparison (biological graft vs native tissue), which evaluates the main review outcomes (awareness of prolapse, repeat surgery for prolapse, recurrent posterior vaginal wall prolapse) and additional clinically relevant outcomes (postoperative obstructed defecation, postoperative dyspareunia, postoperative complications, and estimated blood loss).
We considered other comparisons clinically less important, and although we assessed the quality of evidence by using GRADE methods, we did not construct 'Summary of findings' tables for these comparisons. In particular, all synthetic meshes used in the included studies have been withdrawn from the commercial market, decreasing the clinical significance of these outcomes.
We assessed the quality of evidence using GRADE criteria: risk of bias, consistency of effect, imprecision, indirectness, and publication bias. Two review authors worked independently to provide judgements about evidence quality (high, moderate, low, or very low) and resolved disagreements by discussion. We justified, documented, and incorporated judgements into reporting of results for each outcome.
Results
Description of studies
Results of the search
We screened 113 abstracts and excluded 86 of them. We screened 27 full texts and included 10 studies (Farid 2010; Glazener 2017; Kahn 1999; Nieminen 2004; Paraiso 2006; Park 2014 Abstract; Sand 2001; Sung 2012; Vijaya 2011 Abstract; Wei 2015). We excluded 17 studies and found no studies that are ongoing or are awaiting classification.
We provided full details of the included trials in the Characteristics of included studies table.
We presented the flow of literature through the assessment process in a PRISMA flow chart (Figure 1).
Included studies
Study design and setting
We included 10 RCTs from five countries (Egypt, Finland, UK, USA, and China). All studies used a parallel design.
Participants
The 10 trials randomised a total of 1099 women, all of whom received a surgical intervention. Studies reported mean participant age of between 54 and 65 (Farid 2010; Glazener 2017; Kahn 1999; Nieminen 2004; Paraiso 2006; Sand 2001; Sung 2012; Vijaya 2011 Abstract), except Farid 2010, which reported mean age of 48 years. Wei 2015 reported age ranges of 26 to 71 years for the transvaginal group and 30 to 69 years for the transanal group. Six trials reported mean parity of 2 to 3 (Farid 2010; Glazener 2017; Kahn 1999; Nieminen 2004; Paraiso 2006; Sand 2001), and Farid 2010 reported mean parity of 4.4.
Interventions
Included trials compared the following interventions.
Transanal versus transvaginal repair. Four trials made this comparison and randomised 191 women (Farid 2010; Kahn 1999; Nieminen 2004; Wei 2015). All four trials included women with posterior vaginal wall prolapse who had symptoms of prolapse or obstructed defecation, or both. We have provided the description of techniques used in theses studies in Appendix 2.
Site‐specific repair versus midline fascial plication. One trial made this comparison and randomised 74 women with stage 2 or greater posterior vaginal wall prolapse (Paraiso 2006). We have described these two techniques in Appendix 2.
Absorbable graft versus native tissue. One trial made this comparison in 132 women with rectocele (Sand 2001). Investigators used polyglactin 910 knitted mesh (Ethicon, Somerville, New Jersey, and Cincinnati, Ohio, USA). For women randomly assigned to the absorbable mesh group, researchers placed mesh just cephalad to the deep transverse perineal muscles during posterior vaginal wall repair.
-
Biological graft versus native tissue. Four studies made this comparison in 420 women (Glazener 2017; Paraiso 2006; Park 2014 Abstract; Sung 2012).
Glazener 2017 was a large trial that randomised 735 women to fascial or graft anterior, posterior, or both repairs. A total of 191 randomised women underwent a posterior repair only and are included in this review. Inclusion criteria required that women must be booked for anterior, posterior, or both repairs. Biological graft materials were porcine acellular collagen matrix, porcine small intestine submucosa, or bovine dermal grafts. Study personnel inserted the graft below the fascial layer if possible and secured it with peripheral sutures.
Paraiso 2006 included women with stage 2 or greater posterior vaginal wall prolapse and randomised them to receive native tissue plus augmentation with porcine subintestinal submucosal graft or native tissue alone. Investigators secured the graft superiorly to the posterior vaginal fibromuscularis and epithelium with 2.0 delayed absorbable polydioxanone sutures. Laterally, they attached the mesh to the levator ani fascia with interrupted 2.0 braided polyester sutures. In cases for which concomitant uterosacral vaginal vault suspension or iliococcygeus fascial suspension was performed, they secured the graft to the perineal body by using 2.0 polyglycolic acid suture.
Park 2014 Abstract randomised 109 women with symptomatic grade 2 or greater prolapse undergoing laparoscopic sacrocolpopexy to native tissue repair augmented with porcine biograft or native tissue repair alone.
Sung 2012 included women with grade 2 or greater posterior wall prolapse with defecatory or prolapse symptoms and randomised participants to native tissue repair plus augmentation with porcine subintestinal submucosal graft or native tissue repair alone. Investigators trimmed the graft to appropriate size and secured it over the native tissue repair, suturing it laterally to the levator ani fascia using interrupted 2.0 polyglycolic acid sutures bilaterally. They secured the graft superiorly to the rectovaginal connective tissue and inferiorly to the perineal body using 2.0 polyglycolic acid sutures.
Synthetic graft versus native tissue. One trial made this comparison in 191 women with rectocele (Glazener 2017). In this trial, investigators used non‐absorbable type 1 monofilament macroporous polypropylene mesh. Weight of the mesh ranged from 19 g/m2 to 44 g/m2 and hybrid (coated mesh) was allowed. Researchers inserted the mesh below the fascial layer if possible and secured it with peripheral sutures.
Levator ani plication versus midline fascial plication. One trial made this comparison in 52 women but did not report on any of our primary or secondary outcomes (Vijaya 2011 Abstract); thus we were unable to include trial data in our meta‐analysis.
Follow‐up
Two trials reported median follow‐up of less than one year (Farid 2010; Vijaya 2011 Abstract); five reported median follow‐up of 12 months (Nieminen 2004; Paraiso 2006; Sand 2001; Sung 2012; Wei 2015); three reported median follow‐up of 24 months (Glazener 2017; Kahn 1999; Park 2014 Abstract); and no trials reported outcomes at greater than five years.
Outcomes
Eight studies reported data in a form suitable for analysis for at least one of the primary outcomes.
Four reported awareness of prolapse (Kahn 1999; Nieminen 2004; Paraiso 2006; Sung 2012).
Three reported reoperation for any prolapse (Glazener 2017; Kahn 1999; Paraiso 2006).
Six reported recurrent posterior wall prolapse (Glazener 2017; Kahn 1999; Nieminen 2004; Paraiso 2006; Sand 2001; Sung 2012).
Seven reported adverse events as an outcome (Farid 2010; Glazener 2017; Kahn 1999; Nieminen 2004; Paraiso 2006; Park 2014 Abstract; Sung 2012).
The primary outcome in Glazener 2017 ‐ the largest included trial ‐ was patient‐reported prolapse symptoms based on POP‐SS.
Excluded studies
Overall we excluded 17 studies from this review. We have provided full details in the Characteristics of excluded studies table.
Risk of bias in included studies
We have summarised review authors' assessments of risk of bias across included studies in Figure 2 and Figure 3.
2.
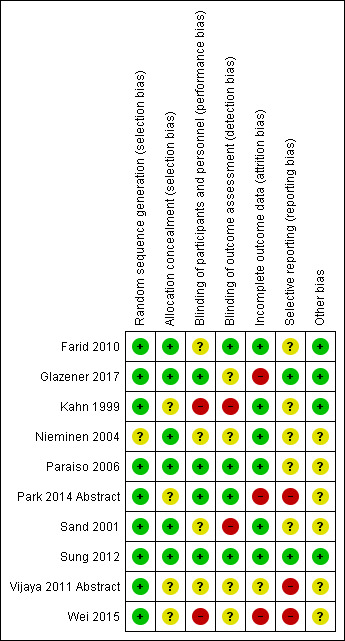
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
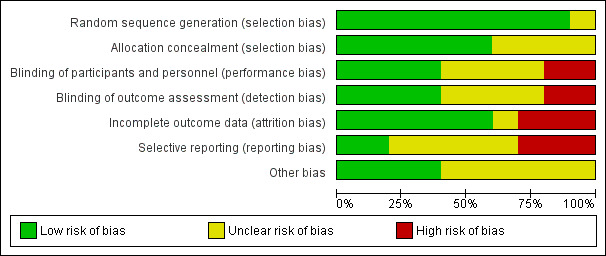
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation was adequate in all trials, so risk of selection bias was low in all 10 trials. Sufficient detail was provided in five of 10 RCTs, which adequately described the randomisation process and confirmed that the randomisation process was securely concealed, for example, allocation by a remote person or sealed envelopes (Farid 2010; Glazener 2017; Nieminen 2004; Paraiso 2006; Sand 2001; Sung 2012).
In the other four trials, it is unclear whether allocation was concealed before assignment (Kahn 1999; Nieminen 2004; Vijaya 2011 Abstract; Wei 2015).
Blinding
Four trials blinded patients (Glazener 2017; Paraiso 2006; Park 2014 Abstract; Sung 2012), meaning that they had low performance bias. Two trials had high risk of performance bias (Kahn 1999; Wei 2015), and reporting was unclear in the remaining four studies. Reviewers remained blinded in four trials (Farid 2010; Paraiso 2006; Park 2014 Abstract; Sung 2012), meaning that risk of detection bias was low. Two trials had high risk of detection bias (Kahn 1999; Sand 2001), and reporting was unclear in the remaining four trials.
Incomplete outcome data
Loss to follow‐up was a variable problem, ranging from zero in Kahn 1999, Nieminen 2004, and Paraiso 2006, to 28% in Park 2014 Abstract at 24 months. Farid 2010 had a 2% attrition rate at six months, Sand 2001 and Sung 2012 had a 12% attrition rate at 12 months, Wei 2015 had a 16% attrition rate at 50 months, and Glazener 2017 had a 20% attrition rate at 24 months. Vijaya 2011 Abstract did not state an attrition rate. Therefore, we assessed risk of attrition as low in nine trials and as unclear in Vijaya 2011 Abstract.
Selective reporting
Seven of the 10 trials reported on at least one primary outcome. We identified trial protocols for two trials (Glazener 2017; Sung 2012), which we rated as having low risk of reporting bias because they reported on all intended primary outcomes and did not switch outcomes. Three studies did not report any of the primary outcomes, and we rated them as having high risk of selection bias, as we could not find the trial protocols (Park 2014 Abstract; Vijaya 2011 Abstract; Wei 2015). We rated the five trials that reported on primary outcomes but did not have accessible protocols as having unclear risk of reporting bias (Farid 2010; Kahn 1999; Nieminen 2004; Paraiso 2006; Sand 2001).
Other potential sources of bias
We found no other potential sources of bias.
Effects of interventions
Summary of findings for the main comparison. Transanal repair versus transvaginal repair.
| Transanal repair versus transvaginal repair for women with posterior vaginal wall prolapse | |||||
|
Patient or population: women with posterior vaginal wall prolapse Setting: hospital operating theatre Intervention: transanal repair Control: transvaginal repair | |||||
|
Outcomes (follow‐up time) |
Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with transvaginal repair | Risk with transanal repair | ||||
| Awareness of prolapse (subjective failure) (12‐25 months) |
103 per 1000 | 285 per 1000 (103 to 790) | RR 2.78 (1.00 to 7.70) | 87 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b |
| Repeat surgery for any prolapse (25 months) |
125 per 1000 | 303 per 1000 (94 to 985) | RR 2.42 (0.75 to 7.88) | 57 (1 RCT) | ⊕⊕⊝⊝ LOWa,c |
| Recurrent posterior vaginal wall prolapse (objective failure) (12‐25 months) |
103 per 1000 | 423 per 1000 (160 to 1000) | RR 4.12 (1.56 to 10.88) | 87 (2 RCTs) | ⊕⊕⊝⊝ MODERATEa |
| Postoperative obstructed defecation (6‐25 months) |
254 per 1000 | 424 per 1000 (254 to 709) | RR 1.67 (1.00 to 2.79) | 113 (3 RCTs) | ⊕⊕⊝⊝ LOWa,b |
| Postoperative dyspareunia (12‐25 months) |
194 per 1000 | 62 per 1000 (17 to 224) | RR 0.32 (0.09 to 1.15) | 80 (2 RCTs) | ⊕⊕⊝⊝ LOWa,c |
| Postoperative complications (6‐25 months) |
16 per 1000 | 56 per 1000 (15 to 212) |
RR 3.57 (0.94 to 13.54) |
135 (3 RCTs) | ⊕⊕⊕⊝ LOWa,b |
| Operating time (12‐50 months) |
Mean operating time in control groups ranged from 32 to 74 minutes. | MD 1.49 minutes lower in the transanal group (11.83 lower to 8.84 higher) | ‐ | 137 (3 RCTs) | ⊕⊕⊝⊝ VERY LOWd,e,f |
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level owing to serious risk of bias; one study at high risk of performance and detection bias, second study at unclear risk of bias in several domains.
bDowngraded one level owing to serious imprecision; findings compatible with benefit in transvaginal group or with no difference between groups.
cDowngraded one level owing to serious imprecision; single trial and/or very few events.
dDowngraded one level owing to serious risk of bias, two of three studies at high risk of performance and detection bias.
eDowngraded one level owing to inconsistency as I2 = 90%.
fDowngraded one level owing to serious imprecision; findings compatible with benefit in either group or with no difference between groups.
Summary of findings 2. Biological graft versus native tissue repair for posterior vaginal wall prolapse.
| Biological graft versus native tissue repair for posterior vaginal wall prolapse | |||||
|
Patient or population: women with posterior vaginal wall prolapse Setting: hospital operating theatre Control: native tissue fascial Comparison: biological graft | |||||
|
Outcomes (follow‐up time) |
Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with native tissue | Risk with biological graft | ||||
| Awareness of prolapse (subjective failure) (16‐24 months) |
87 per 1000 | 95 per 1000 (39 to 222) | RR 1.09 (0.45 to 2.62) | 181 (2 RCTs) | ⊕⊕⊕⊝ MODERATEa |
| Repeat surgery for any prolapse (24 months) |
50 per 1000 | 30 per 1000 (9 to 98) | RR 0.60 (0.18 to 1.97) | 271 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b |
| Recurrent posterior vaginal wall prolapse (objective failure) (16‐24 months) |
130 per 1000 | 72 per 1000 (39 to 132) | RR 0.55 (0.30 to 1.01) | 377 (3 RCTs) | ⊕⊕⊝⊝ LOWb,c |
| Postoperative obstructed defecation (16‐24 months) |
171 per 1000 | 164 per 1000 (85 to 318) | RR 0.96 (0.50 to 1.86) | 172 (2 RCTs) | ⊕⊕⊕⊝ MODERATEa |
| Postoperative dyspareunia (16‐24 months) |
133 per 1000 | 169 per 1000 (35 to 833) | RR 1.26 (0.59 to 2.68) | 152 (2 RCTs) | ⊕⊕⊕⊝ LOWa,d |
| Postoperative complications (including wound infection) (16‐24 months) |
118 per 1000 | 215 per 1000 (144 to 321) | RR 1.82 (1.22 to 2.72) | 448 (3 RCTs) | ⊕⊕⊕⊝ MODERATEb |
| Operating time (24 months) |
Mean operating time in the control group was 169 minutes. | MD 19 minutes lower in the biological graft group (range 49.93 minutes lower to 11.93 minutes higher) | ‐ | 68 (1 RCTs) | ⊕⊕⊝⊝ LOWe |
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||||
aDowngraded one level owing to serious imprecision; findings compatible with benefit in either group or with no difference between groups.
bDowngraded one level owing to serious risk of bias due to high attrition rates in one study.
cDowngraded one level owing to serious imprecision; findings compatible with benefit in biological graft group or with no difference between groups.
dDowngraded one level owing to serious inconsistency: I2 = 74%.
eDowngraded one level owing to serious imprecision, with wide confidence intervals. Findings compatible with benefit in either group or with no effect.
1. Transanal versus transvaginal
Four trials reported on this comparison (Farid 2010; Kahn 1999; Nieminen 2004; Wei 2015).
Primary outcomes
1.1 Awareness of prolapse
Awareness of prolapse may be more common after transanal repair (risk ratio (RR) 2.78, 95% confidence interval (CI) 1.00 to 7.70; 2 RCTs; n = 87; I2 = 0%; low‐quality evidence; Analysis 1.1; Figure 4). This suggests that if 10% of women are aware of prolapse after transvaginal repair, between 10% and 79% are likely to be aware after transanal repair.
1.1. Analysis.
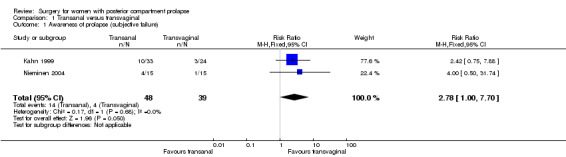
Comparison 1 Transanal versus transvaginal, Outcome 1 Awareness of prolapse (subjective failure).
4.
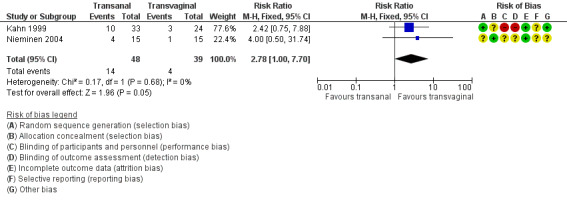
Forest plot of comparison: 1 Transanal versus transvaginal, outcome: 1.1 Awareness of prolapse (subjective failure).
A sensitivity analysis using odds ratios instead of risk ratios showed benefit for the transvaginal group with higher rates of prolapse awareness in the transanal group (Peto OR 3.05, 95% CI 1.08 to 8.60; I2 = 0%; Mantel Haenszel OR 3.52, 95% CI 1.05 to 11.78).
1.2 Repeat surgery for prolapse
Evidence was insufficient to show whether there was any difference between transanal and transvaginal groups (RR 2.42, 95% CI 0.75 to 7.88; 1 RCT; n = 57; low‐quality evidence; Analysis 1.2; Figure 5).
1.2. Analysis.
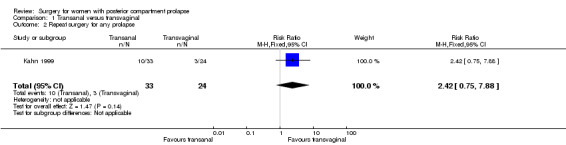
Comparison 1 Transanal versus transvaginal, Outcome 2 Repeat surgery for any prolapse.
5.
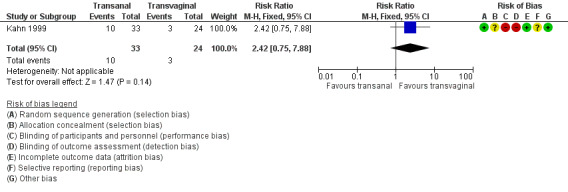
Forest plot of comparison: 1 Transanal versus transvaginal, outcome: 1.2 Repeat surgery for any prolapse.
1.3 Recurrent posterior vaginal wall prolapse
After one to two years' follow‐up, recurrent posterior wall prolapse was more likely after transanal repair (RR 4.12, 95% CI 1.56 to 10.88; 2 RCTs; n = 87; I2 = 35%; moderate‐quality evidence; Analysis 1.3; Figure 6). This suggests that if 10% of women have recurrent prolapse on examination after transvaginal repair, between 16% and 100% are likely to have recurrent prolapse after transanal repair.
1.3. Analysis.

Comparison 1 Transanal versus transvaginal, Outcome 3 Recurrent posterior vaginal wall prolapse (objective failure).
6.
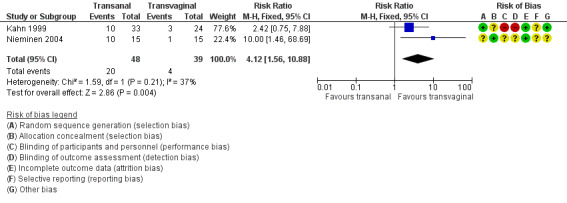
Forest plot of comparison: 1 Transanal versus transvaginal, outcome: 1.3 Recurrent posterior vaginal wall prolapse (objective failure).
Secondary outcomes
1.4 Bowel function
1.4.1 Postoperative obstructive defecation
Data show possibly more women with postoperative obstructed defecation in the transanal group (RR 1.66, 95% CI 1.00 to 2.79; 3 RCTs; n = 113; I2 = 10%; low‐quality evidence; Analysis 1.4). Our findings suggest that if 25% of women undergoing transvaginal repair have postoperative obstructed defecation, between 25% and 71% undergoing transanal repair will have postoperative obstructed defecation.
1.4. Analysis.
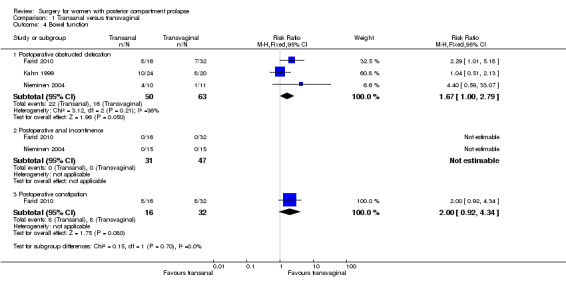
Comparison 1 Transanal versus transvaginal, Outcome 4 Bowel function.
1.4.2 Postoperative anal incontinence
Two studies reported no cases of de novo postoperative anal incontinence (Farid 2010;Nieminen 2004).
1.4.3 Postoperative constipation
Evidence was insufficient to show whether there was any difference in rates of postoperative constipation between transanal and transvaginal groups (RR 2.00, 95% CI 0.92 to 4.34; 1 RCT; n = 48; Analysis 1.4).
1.5 Sexual function
1.5.1 De novo dyspareunia
Evidence was insufficient to show whether there was any difference between groups, because two studies reporting this outcome described only a single occurrence of de novo dyspareunia, which occurred in the transanal group (RR 3.00, 95% CI 0.13 to 68.26; 2 RCTs; n = 78; Analysis 1.5) (Farid 2010;Nieminen 2004).
1.5. Analysis.
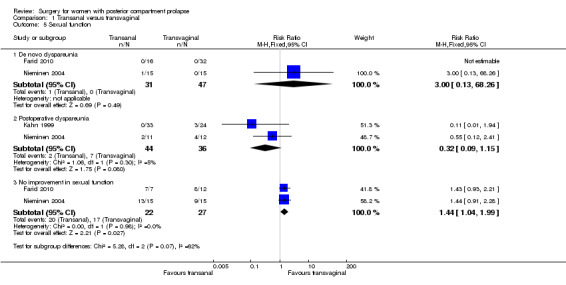
Comparison 1 Transanal versus transvaginal, Outcome 5 Sexual function.
1.5.2 Postoperative dyspareunia
Trials provided no evidence of a significant difference between the two groups in rates of postoperative dyspareunia (RR 0.32, 95% CI 0.09 to 1.15; 2 RCTs; n = 80; I2 = 5%; moderate‐quality evidence). If 19% of women have postoperative dyspareunia after a transvaginal repair, between 2% and 22% are likely to do so after transanal repair (Analysis 1.5).
1.5.3 No improvement in sexual function
Women were more likely to have no improvement in sexual function after a transanal repair than after a transvaginal repair (RR 1.44, 95% CI 1.04 to 1.99; 2 RCTs; n = 49; I2 = 0%; low‐quality evidence; Analysis 1.5).
1.6 Prolapse outcomes
1.6.1 Mean postoperative Ap
The postoperativeAp value was better in the transvaginal group (mean difference (MD) 1.44 cm, 95% CI 0.81 to 2.07; 1 RCT; n = 30; Analysis 1.6).
1.6. Analysis.
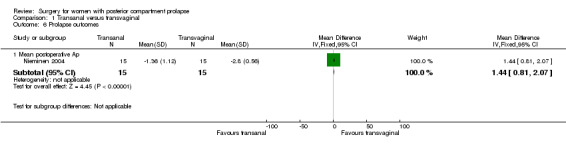
Comparison 1 Transanal versus transvaginal, Outcome 6 Prolapse outcomes.
1.6.2 Mean postoperative Bp
Trials provided no data for this outcome.
1.6.3 Mean postoperative C
Trials provided no data for this outcome.
1.6.4 Mean postoperative Ba
Trials provided no data for this outcome.
1.7 Quality of life and satisfaction measures
Trials provided no data for these outcomes.
1.8 Adverse events
1.8.1 Mesh exposure
Trials provided no data for this outcome.
1.8.2 Repeat surgery for mesh exposure
Trials reported no data for this outcome.
1.8.3 Intraoperative complications including bowel injury and haemorrhage
Two trials reported no intraoperative complications (Farid 2010;Nieminen 2004; n = 80).
1.8.4 Postoperative complications
Trials provided no conclusive evidence of a difference between transanal and transvaginal groups (RR 3.57, 95% CI 0.94 to 13.54; 3 RCTs; n = 135; I2 = 37%; low‐quality evidence; Analysis 1.7).
1.7. Analysis.
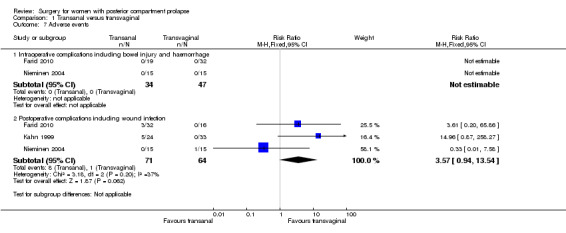
Comparison 1 Transanal versus transvaginal, Outcome 7 Adverse events.
1.9 Perioperative outcomes ‐ dichotomous
1.9.1 Persistent postoperative pain
Evidence was insufficient to show whether there was a difference in persistent postoperative pain between the two groups (RR 0.12, 95% CI 0.02 to 0.94; 1 RCT; n = 57; Analysis 1.8).
1.8. Analysis.
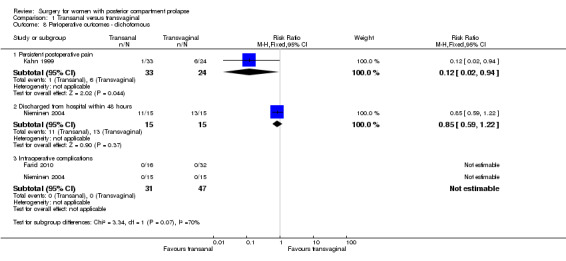
Comparison 1 Transanal versus transvaginal, Outcome 8 Perioperative outcomes ‐ dichotomous.
1.9.2 Discharge from hospital within 48 hours
Evidence was insufficient to show whether there was a difference in the number of women discharged from hospital within 24 hours (RR 0.85, 95% CI 0.59 to 1.22; 1 RCT; n = 30).
1.9.3 Blood transfusion
Trials provided no data for this outcome.
1.10 Perioperative outcomes ‐ continuous
1.10.1 Estimated blood loss
Mean estimated blood loss was less in the transanal group than in the transvaginal group (MD ‐79.38 mL, 95% CI ‐119.08 to ‐39.69; 2 RCTs; n = 87; moderate‐quality evidence; Analysis 1.9).
1.9. Analysis.
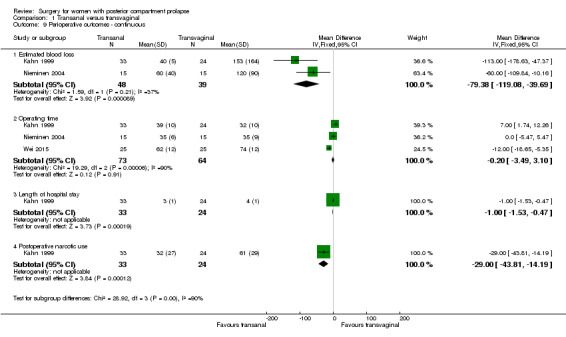
Comparison 1 Transanal versus transvaginal, Outcome 9 Perioperative outcomes ‐ continuous.
1.10.2 Operating time
Evidence was insufficient to show whether there was a difference between groups in operating time (MD ‐0.20 minutes, 95% CI ‐3.49 to 3.10; 3 RCTs; n = 137; moderate‐quality evidence; Analysis 1.10).
1.10. Analysis.
Comparison 1 Transanal versus transvaginal, Outcome 10 Investigations.
1.10.3 Postoperative narcotic use
Evidence was insufficient to show whether there was a difference in narcotic use between the two groups (MD ‐29.00, 95% CI ‐5.12 to 10.98; 1 RCT; n = 57; mg equivalent of morphine).
1.10.4 Length of stay in hospital
Length of stay was shorter in the transanal group in the only study that reported this outcome (MD 1 day, 95% CI 0.47 to 1.53; 1 RCT; n = 57).
1.11 Investigations
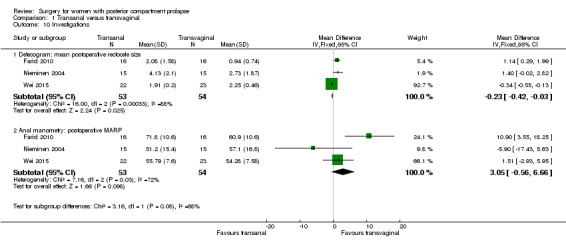
1.11.1 Defecogram: mean postoperative rectocele size
Evidence was insufficient to show whether there was a difference between groups in mean postoperative rectocele size (MD 0.62 cm, 95% CI ‐0.64 to 1.89; 3 RCTs; n = 107).
1.11.2 Anal manometry: postoperative MARP
Evidence was insufficient to show whether there was any difference in MARP between transanal and transvaginal groups (MD 2.93 mmHg, 95% CI ‐5.12 to 10.98; 3 RCTs; n = 107).
2. Site‐specific repair versus midline fascial plication
A single trial reported outcomes for this comparison (Paraiso 2006).
Primary outcomes
2.1 Awareness of prolapse
Evidence was insufficient to show whether there was any difference between groups in rates of awareness of prolapse (RR 0.86, 95% CI 0.25 to 2.88; 1 RCT; n = 60; low‐quality evidence; Analysis 2.1).
2.1. Analysis.
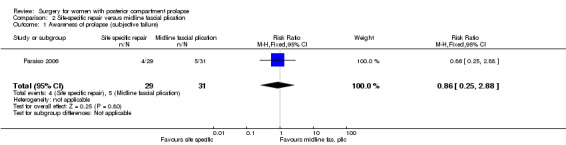
Comparison 2 Site‐specific repair versus midline fascial plication, Outcome 1 Awareness of prolapse (subjective failure).
2.2 Repeat surgery for prolapse
Evidence was insufficient to show whether there was any difference between groups (RR 1.78, 95% CI 0.17 to 18.78; 1 RCT; n = 70; low‐quality evidence; Analysis 2.2).
2.2. Analysis.
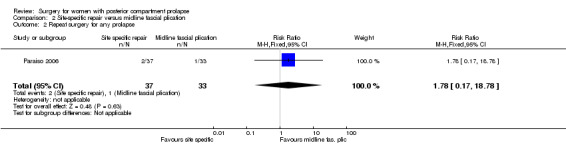
Comparison 2 Site‐specific repair versus midline fascial plication, Outcome 2 Repeat surgery for any prolapse.
2.3 Recurrent posterior vaginal wall prolapse
Evidence was insufficient to show whether there was any difference between groups (RR 1.56, 95% CI 0.49 to 4.91; 1 RCT; n = 55; low‐quality evidence; Analysis 2.3).
2.3. Analysis.
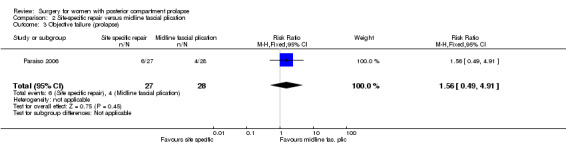
Comparison 2 Site‐specific repair versus midline fascial plication, Outcome 3 Objective failure (prolapse).
Secondary outcomes
2.4 Bowel function
2.4.1 Postoperative obstructive defecation
Evidence was insufficient to show whether there was any difference between groups (RR 1.11, 95% CI 0.53 to 2.31; 1 RCT; n = 56; low‐quality evidence; Analysis 2.4).
2.4. Analysis.
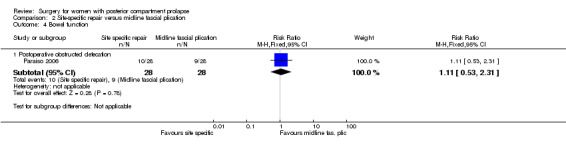
Comparison 2 Site‐specific repair versus midline fascial plication, Outcome 4 Bowel function.
2.4.2 Postoperative anal incontinence
Trials provided no data for this outcome.
2.4.3 Postoperative constipation
Trials provided no data for this outcome.
2.5 Sexual function
2.5.1 De novo dyspareunia
Trials provided no data for this outcome.
2.5.2 Postoperative dyspareunia
Evidence was insufficient to show whether there was any difference between groups (RR 1.07, 95% CI 0.28 to 4.06; 1 RCT; n = 34; low‐quality evidence; Analysis 2.5).
2.5. Analysis.

Comparison 2 Site‐specific repair versus midline fascial plication, Outcome 5 Sexual function.
2.5.3 No improvement in sexual function
Trials provided no data for this outcome.
2.6 Prolapse outcomes
Trials provided no data for these outcomes.
2.7 Quality of life and satisfaction measures
2.7.1 PFIQ‐7 scores
Evidence was insufficient to show whether there was a difference between groups in PFIQ‐7 scores (MD 0 points, 95% CI ‐21.9 to 21.9; 1 RCT; n = 32; Analysis 2.6).
2.6. Analysis.
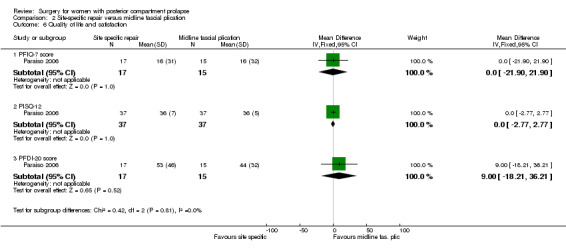
Comparison 2 Site‐specific repair versus midline fascial plication, Outcome 6 Quality of life and satisfaction.
2.7.2 PFDI‐20 scores
Evidence was insufficient to show whether there was a difference between groups in PFDI‐20 scores (MD 9 points, 95% CI ‐18.21 to 36.21; 1 RCT; n = 32; Analysis 2.6).
2.7.3 PISQ‐12
Evidence was insufficient to show whether there was a difference between groups in PISQ‐12 scores (MD 0 points, 95% CI ‐2.77 to 2.77; 1 RCT; n = 32; Analysis 2.6).
2.7.4 POP‐SS
Trials provided no data for this outcome.
2.8 Adverse events
2.8.1 Mesh exposure
Trials provided no data for this outcome.
2.8.2 Repeat surgery for mesh exposure
Trials provided no data for this outcome.
2.8.3 Intraoperative complications including bowel injury and haemorrhage
Evidence was insufficient to show whether there was any difference between groups (RR 5.0, 95% CI 0.25 to 100.7; 1 RCT; n = 74; Analysis 2.7).
2.7. Analysis.
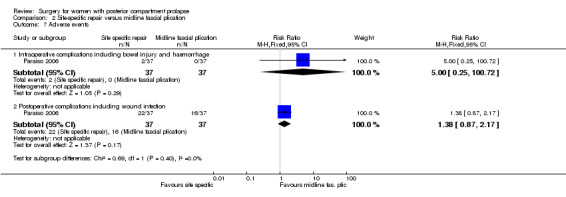
Comparison 2 Site‐specific repair versus midline fascial plication, Outcome 7 Adverse events.
2.8.4 Postoperative complications
Evidence was insufficient to show whether there was a difference between groups in rates of postoperative complications (RR 1.38, 95% CI 0.87 to 2.17; 1 RCT; n = 74; Analysis 2.7).
2.9 Perioperative outcomes ‐ dichotomous
2.9.1 Persistent postoperative pain
Trials provided no data for this outcome.
2.9.2 Discharge from hospital within 48 hours
Trials provided no data for this outcome.
2.9.3 Blood transfusion
Evidence was insufficient to show whether there was a difference between groups in blood transfusion rates (RR 0.14, 95% CI 0.01 to 2.67; 1 RCT; n = 74; Analysis 2.8).
2.8. Analysis.
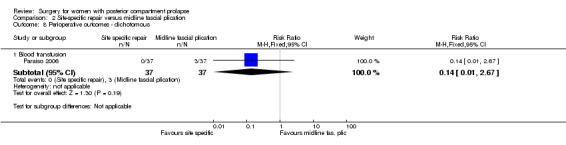
Comparison 2 Site‐specific repair versus midline fascial plication, Outcome 8 Perioperative outcomes ‐ dichotomous.
2.10 Perioperative outcomes ‐ continuous
2.10.1 Estimated blood loss
Trials provided no data for this outcome.
2.10.2 Operating time
Evidence was insufficient to show whether there was a difference in operating time between groups (MD 1 minute, 95% CI ‐30.22 to 32.22; 1 RCT; n = 74; Analysis 2.9).
2.9. Analysis.
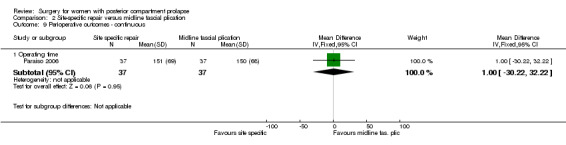
Comparison 2 Site‐specific repair versus midline fascial plication, Outcome 9 Perioperative outcomes ‐ continuous.
2.10.3 Postoperative narcotic use
Trials provided no data for this outcome.
2.10.4 Length of stay in hospital
Trials provided no data for this outcome.
2.11 Investigations
2.11.1 Defecogram: mean postoperative rectocele size
Trials provided no data for this outcome.
2.11.2 Anal manometry: postoperative MARP
Trials provided no data for this outcome.
3. Absorbable graft versus native tissue
A single study reported outcomes for this comparison (Sand 2001).
Primary outcomes
3.1 Awareness of prolapse
Trials provided no data for this outcome.
3.2 Repeat surgery for prolapse
Trials provided no data for this outcome.
3.3 Recurrent posterior vaginal wall prolapse (objective failure)
Evidence was insufficient to show whether there was any difference between groups in rates of objective failure (RR 0.88, 95% CI 0.31 to 2.49; 1 RCT; n = 104; low‐quality evidence; Analysis 3.1).
3.1. Analysis.
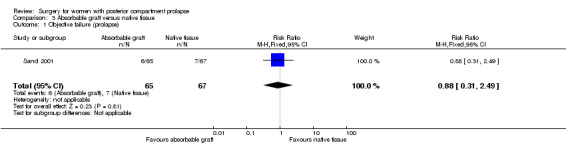
Comparison 3 Absorbable graft versus native tissue, Outcome 1 Objective failure (prolapse).
Secondary outcomes
3.4 Bowel function
Trials provided no data for these outcomes.
3.5 Sexual function
Trials provided no data for these outcomes.
3.6 Prolapse outcomes
Trials provided no data for these outcomes.
3.7 Quality of life and satisfaction measures
Trials provided no data for these outcomes.
3.8 Adverse events
Trials provided no data for these outcomes.
3.9 Perioperative outcomes ‐ dichotomous
Trials provided no data for these outcomes.
3.10 Perioperative outcomes ‐ continuous
Trials provided no data for these outcomes.
3.11 Investigations
Trials provided no data for these outcomes.
4. Biological graft versus native tissue
Four trials reported outcomes for this comparison (Glazener 2017; Paraiso 2006; Park 2014 Abstract; Sung 2012).
Primary outcomes
4.1 Awareness of prolapse
Evidence was insufficient to show whether there was any difference between groups in rates of awareness of prolapse (RR 1.09, 95% CI 0.45 to 2.62; 2 RCTs; n = 181; I2 = 13%; moderate‐quality evidence; Analysis 4.1;Figure 7).
4.1. Analysis.

Comparison 4 Biological graft versus native tissue, Outcome 1 Awareness of prolapse (subjective failure).
7.

Forest plot of comparison: 4 Biological graft versus native tissue, outcome: 4.1 Awareness of prolapse (subjective failure).
4.2 Repeat surgery for prolapse
Evidence was insufficient to show whether there was any difference between groups in rates of repeat surgery for prolapse (RR 0.60, 95% CI 0.18 to 1.97; 2 RCTs; n = 271; I2 = 0%; low‐quality evidence; Analysis 4.2;Figure 8).
4.2. Analysis.
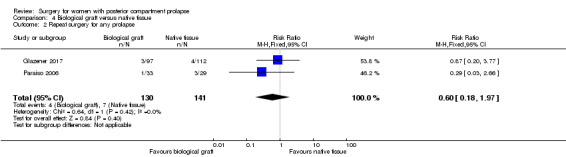
Comparison 4 Biological graft versus native tissue, Outcome 2 Repeat surgery for any prolapse.
8.

Forest plot of comparison: 4 Biological graft versus native tissue, outcome: 4.2 Repeat surgery for any prolapse.
4.3 Recurrent posterior vaginal wall prolapse (objective failure)
Trials provided no conclusive evidence of a difference between groups in rates of objective failure (RR 0.55, 95% CI 0.30 to 1.01; 3 RCTs; n = 377; I2 = 6%; low‐quality evidence; Analysis 4.3;Figure 9). If 13% of women have recurrent prolapse on examination after native tissue repair, between 4% and 13% are likely to have recurrent prolapse after biological graft. Limiting the analysis to studies at low risk of bias suggested benefit for the biological graft group (RR 0.47, 95% CI 0.24 to 0.94; 2 RCTs; n = 191; I2 = 26%).
4.3. Analysis.
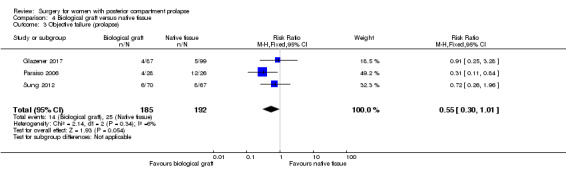
Comparison 4 Biological graft versus native tissue, Outcome 3 Objective failure (prolapse).
9.

Forest plot of comparison: 4 Biological graft versus native tissue, outcome: 4.3 Objective failure (prolapse).
Secondary outcomes
4.4 Bowel function
4.4.1 Postoperative obstructed defecation
Evidence was insufficient to show whether there was any difference between groups in rates of postoperative obstructive defecation (RR 0.96, 95% CI 0.50 to 1.86; 2 RCTs; n = 172; I2 = 42%; moderate‐quality evidence; Analysis 4.4).
4.4. Analysis.
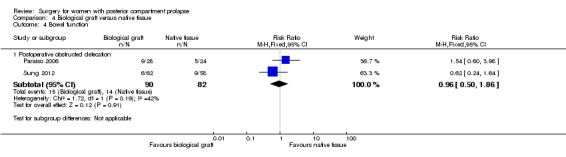
Comparison 4 Biological graft versus native tissue, Outcome 4 Bowel function.
4.4.2 Postoperative anal incontinence
Trials provided no data for this outcome.
4.4.3 Postoperative constipation
Trials provided no data for this outcome.
4.5 Sexual function
4.5.1 De novo dyspareunia
Trials provided no data for this outcome.
4.5.2 Postoperative dyspareunia
Evidence was insufficient to show whether there was any difference between groups (RR 1.27, 95% CI 0.26 to 6.25; 2 RCTs; n = 152; I2 = 74%; low‐quality evidence; Analysis 4.5).
4.5. Analysis.
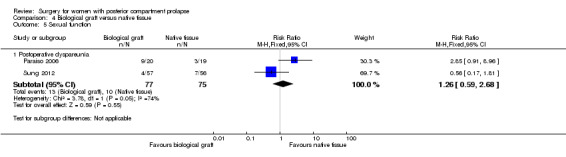
Comparison 4 Biological graft versus native tissue, Outcome 5 Sexual function.
4.5.3 No improvement in sexual function
Trials provided no data for this outcome.
4.6 Prolapse outcomes
4.6.1 Mean postoperative Ap
Trials provided no data for this outcome.
4.6.2 Mean postoperative Bp
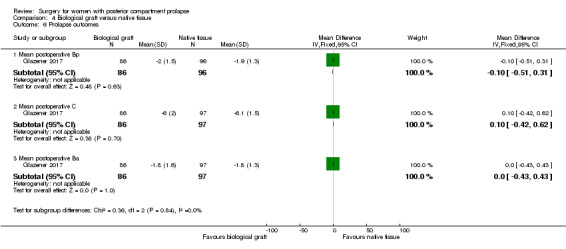
Evidence was insufficient to show whether there was a difference between groups in postoperative Bp values (MD 0.1 cm, 95% CI ‐0.31 to 0.51; 1 RCT; n = 182; Analysis 4.6).
4.6. Analysis.
Comparison 4 Biological graft versus native tissue, Outcome 6 Prolapse outcomes.
4.6.3 Mean postoperative C
Evidence was insufficient to show whether there was a difference between groups in postoperative C values (MD ‐0.1 cm, 95% CI ‐0.62 to 0.42; 1 RCT; n = 183; Analysis 4.6).
4.6.4 Mean postoperative Ba
Evidence was insufficient to show whether there was a difference between groups in postoperative Ba values (MD 0 cm, 95% CI ‐0.43 to 0.43; 1 RCT; n = 183; Analysis 4.6).
4.7 Quality of life and satisfaction measures
4.7.1 PFIQ‐7
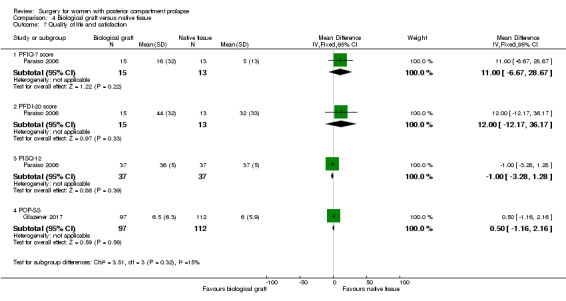
Evidence was insufficient to show whether there was a difference between groups in postoperative PFIQ‐7 scores (MD ‐11 points, 95% CI ‐28.67 to 6.67; 1 RCT; n = 28; Analysis 4.7).
4.7. Analysis.
Comparison 4 Biological graft versus native tissue, Outcome 7 Quality of life and satisfaction.
4.7.2 PFDI‐20
Evidence was insufficient to show whether there was a difference between groups in PFDI‐20 values (MD ‐12 points, 95% CI ‐35.26 to 11.26; 1 RCT; n = 30; Analysis 4.7).
4.7.3 PISQ
Evidence was insufficient to show whether there was a difference between groups in PISQ scores (MD 1 point, 95% CI ‐1.28 to 3.28; 1 RCT; n = 74; Analysis 4.7).
4.7.4 POP‐SS
Evidence was insufficient to show whether there was a difference between groups in POP‐SS scores (MD‐0.5 points, 95% CI ‐2.16 to 1.16; 1 RCT; n = 209; Analysis 4.7).
4.8 Adverse events
4.8.1 Mesh exposure
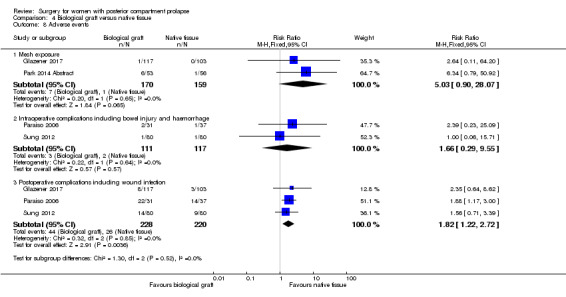
Evidence was insufficient to show whether there was any difference between groups in mesh exposure rates (RR 5.0, 95% CI 0.9 to 28.07; 2 RCTs; n = 329; Analysis 4.8).
4.8. Analysis.
Comparison 4 Biological graft versus native tissue, Outcome 8 Adverse events.
4.8.2 Repeat surgery for mesh exposure
Trials provided no data for this outcome.
4.8.3 Intraoperative complications including bowel injury and haemorrhage
Evidence was insufficient to show whether there was any difference between biological graft and native tissue groups (RR 1.66, 95% CI 0.29 to 9.55; 2 RCTs; n = 228; Analysis 4.8).
4.8.4 Postoperative complications
Trials reported more postoperative complications in the biological graft group than in the native tissue group (RR 1.82, 95% CI 1.22 to 2.72; 3 RCTs; n = 448; high‐quality evidence).
4.9 Perioperative outcomes ‐ dichotomous
4.9.1 Persistent postoperative pain
Trials provided no data for this outcome.
4.9.2 Discharge from hospital within 48 hours
Trials provided no data for this outcome.
4.9.3 Blood transfusion
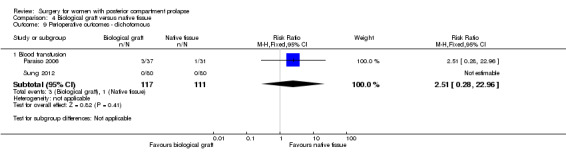
Evidence was insufficient to show whether there was any difference between biological graft and native tissue groups (RR 2.5, 95% CI 0.28 to 22.96; 2 RCTs; n = 228; Analysis 4.9).
4.9. Analysis.
Comparison 4 Biological graft versus native tissue, Outcome 9 Perioperative outcomes ‐ dichotomous.
4.10 Perioperative outcomes ‐ continuous
4.10.1 Estimated blood loss
Trials provided no data for this outcome.
4.10.2 Operating time
Evidence was insufficient to show whether there was a difference in operating times (MD 19 minutes lower in the biological graft group, 95% CI ‐49.93 to 11.93; 1 RCT; n = 68; Analysis 4.10).
4.10. Analysis.

Comparison 4 Biological graft versus native tissue, Outcome 10 Perioperative outcomes ‐ continuous.
4.10.3 Postoperative narcotic use
Trials provided no data for this outcome.
4.10.4 Length of stay in hospital
Trials provided no data for this outcome.
4.11 Investigations
Trials provided no data for these outcomes.
5. Synthetic graft versus native tissue
A single study reported on outcomes for this comparison (Glazener 2017).
Primary outcomes
5.1 Awareness of prolapse
Trials provided no data for this outcome.
5.2 Repeat surgery for prolapse
Evidence was insufficient to show whether there was any difference between groups (RR 1.0, 95% CI 0.14 to 6.98; 1 RCT; n = 232; moderate‐quality evidence; Analysis 5.1;Figure 10).
5.1. Analysis.
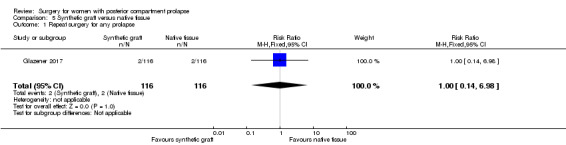
Comparison 5 Synthetic graft versus native tissue, Outcome 1 Repeat surgery for any prolapse.
10.
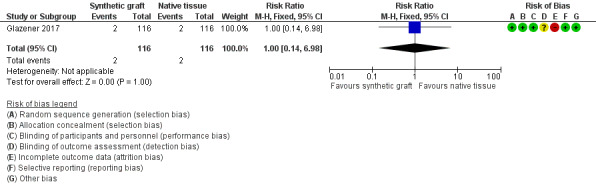
Forest plot of comparison: 5 Synthetic graft versus native tissue, outcome: 5.1 Repeat surgery for any prolapse.
5.3 Recurrent posterior vaginal wall prolapse
Evidence was insufficient to show whether there was any difference between groups in rates of recurrent posterior wall prolapse (RR 0.68, 95% CI 0.2 to 2.34; 1 RCT; n = 200; moderate‐quality evidence; Analysis 5.2;Figure 11).
5.2. Analysis.
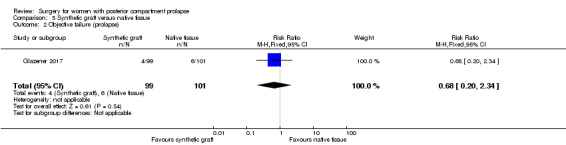
Comparison 5 Synthetic graft versus native tissue, Outcome 2 Objective failure (prolapse).
11.
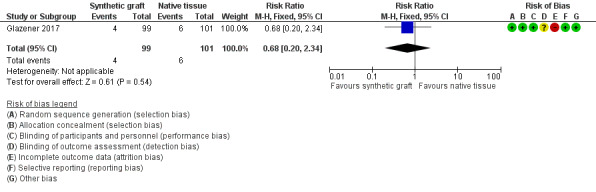
Forest plot of comparison: 5 Synthetic graft versus native tissue, outcome: 5.2 Objective failure (prolapse).
Secondary outcomes
5.4 Bowel function
Trials provided no data for these outcomes.
5.5 Sexual function
Trials provided no data for these outcomes.
5.6 Prolapse outcomes
5.6.1 Mean postoperative Ap
Trials provided no data for this outcome.
5.6.2 Mean postoperative Bp
Evidence was insufficient to show whether there was a difference between groups in Bp values (MD 0.2 cm, 95% CI ‐0.18 to 0.58; 1 RCT; n = 191; Analysis 5.3).
5.3. Analysis.
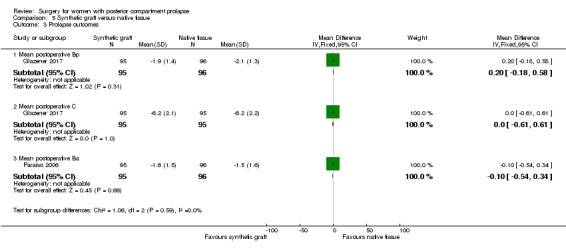
Comparison 5 Synthetic graft versus native tissue, Outcome 3 Prolapse outcomes.
5.6.3 Mean postoperative C
Evidence was insufficient to show whether there was a difference between groups in C values (MD 0 cm,, 95% CI ‐0.61 to 0.61; 1 RCT; n = 190; Analysis 5.3).
5.6.4 Mean postoperative Ba
Evidence was insufficient to show whether there was a difference between groups in Ba values (MD ‐0.1 cm, 95% CI ‐0.54 to 0.34; 1 RCT; n = 191; Analysis 5.3).
5.7 Quality of life and satisfaction measures
5.7.1 POP‐SS
Evidence was insufficient to show whether there was a difference between groups in POPP‐SS scores (MD 0.7 points, 95% CI 0.75 to 2.15; 1 RCT; n = 232; Analysis 5.4).
5.4. Analysis.
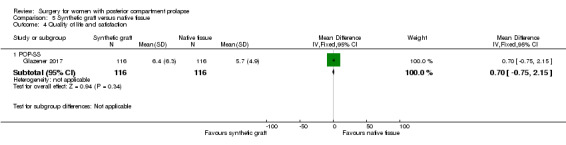
Comparison 5 Synthetic graft versus native tissue, Outcome 4 Quality of life and satisfaction.
5.8 Adverse events
5.8.1 Mesh exposure
Data show more mesh exposures in the synthetic graft group, with a rate of 7% compared with 0% in the native tissue group (RR 18.7, 95% CI 1.10 to 317.94; 1 RCT; n = 252; Analysis 5.5).
5.5. Analysis.
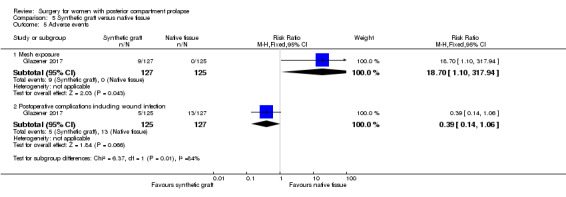
Comparison 5 Synthetic graft versus native tissue, Outcome 5 Adverse events.
5.8.2 Repeat surgery for mesh exposure
Trials provided no data for this outcome.
5.8.3 Intraoperative complications including bowel injury and haemorrhage
Trials provided no data for this outcome.
5.8.4 Postoperative complications
Evidence was insufficient to show whether there was a difference between groups in rates of postoperative complications (RR 0.39, 95% CI 0.14 to 1.06; 1 RCT; n = 252; Analysis 5.5).
5.9 Perioperative outcomes ‐ dichotomous
5.9.1 Persistent postoperative pain
Trials provided no data for this outcome.
5.9.2 Discharge from hospital within 48 hours
Trials provided no data for this outcome.
5.9.3 Blood transfusion
Evidence was insufficient to show whether there was any difference between synthetic graft and native tissue groups in blood transfusion rates (RR 2.51, 95% 0.28 to 22.96; 1 RCT; n = 228; Analysis 5.6).
5.6. Analysis.
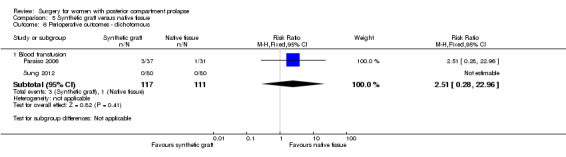
Comparison 5 Synthetic graft versus native tissue, Outcome 6 Perioperative outcomes ‐ dichotomous.
5.10 Perioperative outcomes ‐ continuous
Trials provided no data for these outcomes.
5.11 Investigations
Trials provided no data for these outcomes.
6. Levator ani plication versus midline fascial plication
One trial reported outcomes for this comparison but provided no data suitable for analysis (Vijaya 2011 Abstract).
This small study of 52 women reported superior objective outcomes with fascial plication alone as compared with levator ani plication with midline fascial plication at six months, with mean difference in the preoperative and postoperative Ap scores greater in the fascial plication group. The abstract states that quality of life assessment based on a Prolapse Quality of Life (P‐QOL) questionnaire was significantly improved in both groups, with no differences between groups. Trial authors also reported no differences between groups in sexual function before or after the intervention, and data show that bowel function was improved by the intervention in the fascial repair group but not in the levator plication group, as assessed by the Birmingham Bowel and Urinary Symptoms Questionnaire.
Other analyses
Sensitivity analysis by risk of bias did not substantially change review findings, except for one analysis (Analysis 4.3). As noted above, analysis restricted to studies at lower risk of bias suggested that biological graft may be associated with lower rates of objective failure (recurrent vaginal wall prolapse) than native tissue repair.
Sensitivity analysis using Mantel‐Haenszel or Peto odds ratios as the effect estimate did not substantially change review findings, except for one analysis (Analysis 1.1). As noted above, use of odds ratios revealed that transanal repair was associated with higher rates of subjective prolapse than transvaginal repair. This finding did not reach statistical significance when risk ratios were used.
Sensitivity analysis based on a random‐effects rather than a fixed‐effect model did not substantially change any review findings.
We were unable to conduct our planned assessment of reporting bias, as insufficient studies in any one comparison precluded construction of a funnel plot.
Discussion
Summary of main results
Four trials compared transanal and transvaginal approaches for management of posterior vaginal wall prolapse. Both subjective and objective success appeared to be greater in the transvaginal group. The transvaginal group was probably less likely to have obstructed defecation and was more likely to have improvement in sexual function. Postoperiative complications may be less likely after transvaginal surgery, although findings for this outcome were inconclusive. However, intraoperative blood loss was greater in the transvaginal group.
Four trials compared biological graft and native tissue repair and found no clear evidence of a difference between groups for measures of effectiveness. However, postoperative complications were more common with biological repair.
In comparisons of site‐specific vaginal repair versus midline fascial plication, absorbable graft versus native tissue repair, synthetic graft versus native tissue repair, and levator ani plication versus midline fascial plication, evidence was insufficient to permit any conclusions about their relative effectiveness or safety.
Overall completeness and applicability of evidence
Generally well‐designed randomised controlled trials comparing surgical interventions for posterior vaginal wall prolapse are scarce. Of the four trials comparing transanal repair versus transvaginal repair, investigators in either one or two trials reported each of the primary outcomes. One randomised controlled trial provided data for our primary outcomes for site‐specific repair versus midline fascial plication. In the other comparison versus midline fascial plication, one randomised controlled trial provided data for one of our primary outcomes. In the absorbable graft versus native tissue comparison, one randomised controlled trial presented data for one of our primary outcomes. Of the four trials that address biological graft versus native tissue, two to three trials reported each of the primary outcomes. A single trial provided data for two of our primary outcomes for the synthetic graft versus native tissue comparison.
Well‐designed randomised controlled trials are needed to examine all of our comparisons. None of the included trials performed cost analysis.
Quality of the evidence
Using GRADEpro software, we assessed risk of bias, imprecision, inconsistency, and indirectness for each of the review comparisons, and we used these assessments to grade the quality of evidence assigned to each outcome, ranging from moderate to very low (see Effects of interventions section). We were unable to assess risk of publication bias owing to lack of data.
The main limitations in evidence quality were serious risk of bias (associated mainly with performance, detection, and attrition biases) and serious imprecision (associated with small overall sample sizes and low event rates).
The quality of evidence related to comparisons of transvaginal versus transanal approach ranged from very low to moderate. The quality of evidence related to comparisons of biological graft versus native tissue ranged from low to moderate.
Potential biases in the review process
Systematic searches of the literature for published and unpublished trials were rigorous, and we do not believe that any publications have been omitted. The large number of secondary outcomes reported in this review increases the potential for spurious positive findings (type 1 error). Therefore in drawing our conclusions, we limited our focus to primary outcomes (awareness of prolapse, repeat surgery for prolapse, recurrent posterior vaginal wall prolapse) and the four most clinically important secondary outcomes (postoperative obstructed defecation, postoperative dyspareunia, postoperative complications, and operating time).
A persistent limitation of meta‐analysis of studies of pelvic floor disorders is that many different validated questionnaires are utilised, which makes collation of data challenging.
Agreements and disagreements with other studies or reviews
Another comprehensive meta‐analysis of level one evidence for surgical management of posterior vaginal wall prolapse can be found in the 2017 International Consultation on Incontinence (ICI 2017) proceedings. The ICI document concludes that transvaginal repair of posterior wall defects is more successful when midline fascial plication is used with or without levatorplasty than when a site‐specific repair technique is used in terms of objective success; however we did not find a significant difference between these two types of repair. The ICI reported a finding of higher rates of dyspareunia with levatorplasty than with midline fascial plication alone.
Our findings are consistent with those provided by the ICI for transanal versus transvaginal repair, and our evidence suggests that the transvaginal approach may be superior to the transanal approach.
Our findings are consistent with the ICI finding that no conclusive evidence shows that biological or synthetic mesh repair is more effective than native tissue repair in the posterior vaginal wall.
Authors' conclusions
Implications for practice.
Transvaginal repair may be more effective than transanal repair for posterior wall prolapse for preventing recurrence of prolapse when both objective and subjective measures are considered. However, data on adverse effects are scanty. Evidence was insufficient to permit any conclusions about the relative effectiveness or safety of other types of surgery. Evidence does not support utilisation of any mesh or graft materials at the time of posterior vaginal repair. Withdrawal of some commercial transvaginal mesh kits from the market may limit the generalisability of review findings.
Implications for research.
Long‐term follow‐up in current trials will establish whether long‐term benefits are derived from transvaginal graft or mesh, provided that adequate follow‐up rates can be achieved. Research on graft or mesh products that may be effective, without the complications associated with current meshes, is of paramount importance.
What's new
| Date | Event | Description |
|---|---|---|
| 23 February 2018 | New citation required but conclusions have not changed | The addition of 3 new studies has not led to a change in the conclusions of this review. |
| 23 February 2018 | New search has been performed | A comparison of surgical interventions for management of posterior vaginal wall prolapse was formerly part of the 2013 Cochrane review "Surgical management of pelvic organ prolapse in women". We now present this as a separate review. Three new trials are included that were not in the previous review: Glazener 2017; Park 2014 Abstract; Wei 2015. |
History
Review first published: Issue 3, 2018
| Date | Event | Description |
|---|---|---|
| 12 June 2014 | New citation required but conclusions have not changed | Review updated with 1 new trial incorporated |
| 14 April 2010 | Amended | Citation changed, conflicts added |
| 17 November 2009 | New citation required but conclusions have not changed | Full reports of 59 potentially eligible studies assessed; for this update, 23 new eligible studies assessed (Al‐Nazer 2007a; Ali 2006a; Allahdin 2008; Barber 2006; Biller 2008; Borstad 2008; Braun 2007a; Carramao 2008a; Constantini 2008; de Tayrac 2008; Dietz 2008a; Glavind 2007; Guerette 2006a; Lim 2007a; Meschia 2007a; Natale 2007; Natale 2009; Nguyen 2008; Nieminen 2008; Pantazis 2008a; Schierlitz 2007a; Segal 2007; Sivaslioglu 2008). Overall, 17 studies excluded from the review ‐ 6 during this update (Barber 2006; Biller 2008; Carramao 2008a; Glavind 2007; Meschia 2007a; Segal 2007). Full details given in Characteristics of excluded studies tables In this, the second update, 18 new trials added (Al‐Nazer 2007; Ali 2006; Allahdin 2008; Borstad 2008; Braun 2007a; Constantini 2007; Constantini 2008; de Tayrac 2008; Dietz 2008a; Guerette 2006; Lim 2007; Natale 2007; Natale 2009; Nguyen 2008; Nieminen 2008; Pantazis 2008; Schierlitz 2007; Sivaslioglu 2008) and 3 previously included studies updated (Brubaker 2008; Meschia 2007; Roovers 2004) |
| 9 February 2009 | New search has been performed | New search conducted February 2009 |
| 10 October 2008 | Amended | Converted to new review format |
| 17 April 2007 | New citation required and conclusions have changed | Substantive update (Issue 3, 2007). 22 RCTs (8 new included trials). Findings still insufficient to provide robust evidence to support current and new practice (such as whether to perform a concurrent continence operation, or whether to use mesh or grafts) |
Acknowledgements
We acknowledge the work of Elisabeth J. Adams and Suzanne Hagen as co‐authors on the original review, and Charis Glazener as co‐author on the original review and update.
We also acknowledge Minglan Li for help in translating one of the included studies from Chinese into English (Wei 2015).
The authors of the 2017 update would like to thank Sheila Wallace, Information Specialist of the Cochrane Incontinence Review Group, for designing the search strategy and running the searches for this review.
Appendices
Appendix 1. Searches
Search strategy: The Incontinence Group Specialised Register was searched using the Group's own keyword system (all searches were of the keyword field of Reference Manager 2012). The search terms used were: ({design.cct*} OR {design.rct*}) AND ({topic.posterior vaginal prolapse*}) OR ({topic.rectocele*} AND ({intvent.surg*}) Date of the most recent search of the register for this review: April 2017.
Appendix 2. Types of operations
Transvaginal repair of posterior vaginal wall prolapse ‐ midline fascial plication
Indications
Treatment of rectocele (rectum bulges or herniates forward into the vagina) and defects of the perineum (area separating entrance of the vagina and anus).
Aim
Correct defects in the rectovaginal fascia separating rectum and vagina while allowing bowel function to be maintained or corrected without interfering with sexual function.
Surgical technique
An incision is made on the posterior wall of the vagina starting at the entrance and finishing at the top of the vagina.
Vagina and rectovaginal fascia are dissected from the vagina until the pelvic floor muscles (puborectalis) are located.
Defects in the fascia are corrected by central plication of the fascia with delayed absorption sutures.
Perineal defects are repaired by placing deep sutures into the perineal muscles to build up the perineal body.
Overlying vaginal and vulval skin is then closed.
A pack is usually placed into the vagina and a catheter into the bladder at the end of surgery.
Transanal repair of posterior vaginal wall prolapse
Indications
Treatment of rectocele (rectum bulges or herniates forward into the vagina).
Aim
Correct defects in the rectovaginal fascia separating rectum and vagina while allowing bowel function to be maintained or corrected without interfering with sexual function.
Surgical technique
Transverse incision is made at the dentate line, followed by two vertical incisions at either end of the transverse incision and extended about 7 cm proximally.
A mucomuscular flap with a broader base was created and haemostasis was obtained by electrocoagulation.
Around four vertical sutures with delayed absorbable sutures are placed in the rectovaginal fascia.
Around two horizontal sutures were then placed in the rectovaginal fascia.
Any excess of the mucomuscular flap was excised and closed with a running suture.
A haemostatic sponge was left in the anal canal and was removed on the first postoperative day.
Site‐specific versus midline fascial plication
These two techniques differ at step 3 above. Midline fascial plication is a global repair of the fascia from proximal to distal. The fascia on right and left of the rectum is brought together at the midline, providing global support to the rectum.
The site‐specific technique involved identifying specific defect in the rectovaginal fascia by inspection and repairing each defect individually. It can involve repairing fascia to fascia or fascia to arcus tendineus fascia pelvis (the lateral pelvic side wall support structure for the vagina) depending on where the defects are.
Levator ani plication with midline fascial plication.
Vijaya 2011 Abstract compared levator ani plication with midline fascial plication with midline fascial plication alone. This means that the bilateral levator ani muscles were brought together at the midline with the fascial repair.
Graft repair
If a graft (absorbable, biological, synthetic mesh) is used, then graft is placed overlying the fascial repair (step 3 of transvaginal repair above) before the vaginal mucosa is closed (step 5 above). Some synthetic meshes are anchored to the sacrospinous ligaments and some are not anchored.
Data and analyses
Comparison 1. Transanal versus transvaginal.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Awareness of prolapse (subjective failure) | 2 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.78 [1.00, 7.70] |
| 2 Repeat surgery for any prolapse | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.42 [0.75, 7.88] |
| 3 Recurrent posterior vaginal wall prolapse (objective failure) | 2 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.12 [1.56, 10.88] |
| 4 Bowel function | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Postoperative obstructed defecation | 3 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.00, 2.79] |
| 4.2 Postoperative anal incontinence | 2 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Postoperative constipation | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.92, 4.34] |
| 5 Sexual function | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 De novo dyspareunia | 2 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.26] |
| 5.2 Postoperative dyspareunia | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.09, 1.15] |
| 5.3 No improvement in sexual function | 2 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [1.04, 1.99] |
| 6 Prolapse outcomes | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Mean postoperative Ap | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 1.44 [0.81, 2.07] |
| 7 Adverse events | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Intraoperative complications including bowel injury and haemorrhage | 2 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 Postoperative complications including wound infection | 3 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.57 [0.94, 13.54] |
| 8 Perioperative outcomes ‐ dichotomous | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Persistent postoperative pain | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.02, 0.94] |
| 8.2 Discharged from hospital within 48 hours | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.59, 1.22] |
| 8.3 Intraoperative complications | 2 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Perioperative outcomes ‐ continuous | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Estimated blood loss | 2 | 87 | Mean Difference (IV, Fixed, 95% CI) | ‐79.38 [‐119.08, ‐39.69] |
| 9.2 Operating time | 3 | 137 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐3.49, 3.10] |
| 9.3 Length of hospital stay | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐1.53, ‐0.47] |
| 9.4 Postoperative narcotic use | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐29.00 [‐43.81, ‐14.19] |
| 10 Investigations | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Defecogram: mean postoperative rectocele size | 3 | 107 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.42, ‐0.03] |
| 10.2 Anal manometry: postoperative MARP | 3 | 107 | Mean Difference (IV, Fixed, 95% CI) | 3.05 [‐0.56, 6.66] |
Comparison 2. Site‐specific repair versus midline fascial plication.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Awareness of prolapse (subjective failure) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.25, 2.88] |
| 2 Repeat surgery for any prolapse | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.17, 18.78] |
| 3 Objective failure (prolapse) | 1 | 55 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.49, 4.91] |
| 4 Bowel function | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Postoperative obstructed defecation | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.53, 2.31] |
| 5 Sexual function | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Postoperative dyspareunia | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.28, 4.06] |
| 6 Quality of life and satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 PFIQ‐7 score | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐21.90, 21.90] |
| 6.2 PISQ‐12 | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.77, 2.77] |
| 6.3 PFDI‐20 score | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 9.0 [‐18.21, 36.21] |
| 7 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Intraoperative complications including bowel injury and haemorrhage | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 100.72] |
| 7.2 Postoperative complications including wound infection | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.87, 2.17] |
| 8 Perioperative outcomes ‐ dichotomous | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Blood transfusion | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.67] |
| 9 Perioperative outcomes ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Operating time | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐30.22, 32.22] |
Comparison 3. Absorbable graft versus native tissue.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Objective failure (prolapse) | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.31, 2.49] |
Comparison 4. Biological graft versus native tissue.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Awareness of prolapse (subjective failure) | 2 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.45, 2.62] |
| 2 Repeat surgery for any prolapse | 2 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.18, 1.97] |
| 3 Objective failure (prolapse) | 3 | 377 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.30, 1.01] |
| 4 Bowel function | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Postoperative obstructed defecation | 2 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.50, 1.86] |
| 5 Sexual function | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Postoperative dyspareunia | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.59, 2.68] |
| 6 Prolapse outcomes | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Mean postoperative Bp | 1 | 182 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.51, 0.31] |
| 6.2 Mean postoperative C | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.42, 0.62] |
| 6.3 Mean postoperative Ba | 1 | 183 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.43, 0.43] |
| 7 Quality of life and satisfaction | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 PFIQ‐7 score | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 11.0 [‐6.67, 28.67] |
| 7.2 PFDI‐20 score | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 12.0 [‐12.17, 36.17] |
| 7.3 PISQ‐12 | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐3.28, 1.28] |
| 7.4 POP‐SS | 1 | 209 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐1.16, 2.16] |
| 8 Adverse events | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Mesh exposure | 2 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.03 [0.90, 28.07] |
| 8.2 Intraoperative complications including bowel injury and haemorrhage | 2 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.29, 9.55] |
| 8.3 Postoperative complications including wound infection | 3 | 448 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.22, 2.72] |
| 9 Perioperative outcomes ‐ dichotomous | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Blood transfusion | 2 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.51 [0.28, 22.96] |
| 10 Perioperative outcomes ‐ continuous | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Operating time | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐19.0 [‐49.93, 11.93] |
Comparison 5. Synthetic graft versus native tissue.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Repeat surgery for any prolapse | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.98] |
| 2 Objective failure (prolapse) | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.20, 2.34] |
| 3 Prolapse outcomes | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Mean postoperative Bp | 1 | 191 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.18, 0.58] |
| 3.2 Mean postoperative C | 1 | 190 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.61, 0.61] |
| 3.3 Mean postoperative Ba | 1 | 191 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.54, 0.34] |
| 4 Quality of life and satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 POP‐SS | 1 | 232 | Mean Difference (IV, Fixed, 95% CI) | 0.70 [‐0.75, 2.15] |
| 5 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Mesh exposure | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 18.70 [1.10, 317.94] |
| 5.2 Postoperative complications including wound infection | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.14, 1.06] |
| 6 Perioperative outcomes ‐ dichotomous | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Blood transfusion | 2 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.51 [0.28, 22.96] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Farid 2010.
| Methods | Multi‐surgeon dual‐centre RCT Randomisation: nurse taking card from envelope ‐ blinded Reviewers blinded No significant differences between groups at the beginning 6‐Month follow‐up |
|
| Participants | 3 groups of 16 participants December 2002‐2005 62 multi‐parous with symptomatic rectocele (obstructed defecation) Not described re blinding of participants 48 after exclusions Inclusion criteria: rectocele larger than 2 cm symptomatic for obstructed defecation (1 or more of the following symptoms: need for digital manipulation during defecation, sense of incomplete evacuation, excessive straining, or dyspareunia) Exclusion criteria: recurrent rectocele, rectal intussusception, anismus, diabetes, previous anal surgery, systemic steroid treatment, connective tissue disease, slow‐transit constipation, compromised anal sphincter function, abnormal thyroid function |
|
| Interventions | A (n = 16): transperineal repair (3.0 Vicryl) with levatorplasty (0.0 Vicryl) B (n = 16): transperineal repair alone C (n = 16): transanal approach (2.0 Vicryl) (Delorme procedure) |
|
| Outcomes |
|
|
| Notes | Trial authors conclude that transperineal repair is superior to transanal repair in structural and functional outcomes; Egypt Ethics granted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Examiners blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for, 1 lost to follow‐up (left the country) ‐ not stated in paper the group to which this participant was assigned |
| Selective reporting (reporting bias) | Unclear risk | Reported on 2 of this review's primary outcomes |
| Other bias | Low risk | NO COI reported Groups similar at the start No concomitant procedures |
Glazener 2017.
| Methods | RCT: 2 parallel comparing A:B (mesh trial) A:C (graft trial) 35 centres in UK 65 surgeons Remote Web‐based randomisation 2‐Year follow‐up Modified ITT analysis |
|
| Participants | 1352 randomised, 320 randomised within posterior repair subgroup n = 1348 Mesh trial (overall not just posterior) A: 111 (430) B: 111 (435) Graft trial A: 93 (367) C: 98 (368) 35 centres Primary anterior or posterior repairs |
|
| Interventions | A: native tissue B: synthetic mesh C: biological graft |
|
| Outcomes |
|
|
| Notes | HTA‐funded study in UK | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated online programme |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients were blinded until 12 months unless asked |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Reviewers attempted to remain blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 20% at 2 years. Specific attrition rates for posterior repair groups not given |
| Selective reporting (reporting bias) | Low risk | Reported on all primary outcomes of this review, protocol available |
| Other bias | Low risk | Funding declared and no COI Concomitant procedures included anterior and apical repair procedures ‐ same numbers in comparison groups had concomitant procedures |
Kahn 1999.
| Methods | Single‐centre RCT (number table randomisation, concealment unclear)
Follow‐up: 25 months (8‐37) A+B 89% followed up |
|
| Participants | 63 randomised, 57 underwent surgery (2 from each group decided on conservative management, 1 had no prolapse at time of surgery, and 1 delayed surgery past duration of study owing to personal circumstances) Withdrawal: 4 (A 2, B 2) Excluded: 2 (1 no rectocele surgery because posterior vaginal wall cyst, 1 did not have surgery performed) Inclusion: symptomatic rectocele (bulge or impaired defecation with > 15% trapping on isotope defecography), failing conservative treatment | |
| Interventions | A (24): posterior colporrhaphy with levator plication, enterocele repair, hysterectomy, anterior repair as required B (33): transanal repair by single colorectal surgeon, circular muscle plicated longitudinally, permanent suture | |
| Outcomes |
|
|
| Notes | Abstract No blinding No stratification No CONSORT Individual who reviewed outcomes unclear No validated symptom or QOL questionnaires USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Number table randomisation |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients who underwent surgery lost to follow‐up ‐ see above |
| Selective reporting (reporting bias) | Unclear risk | Reported on all primary outcomes of this review, protocol not available |
| Other bias | Low risk | COI not reported No concomitant procedures |
Nieminen 2004.
| Methods | Single‐centre RCT (nurse took card from envelope with 15 vaginal and 15 transanal cards) Blinding ‐ unclear Follow‐up: A 12 months, B 12 months Groups similar at the start, so no selection bias No loss to follow‐up |
|
| Participants | 30 women Inclusion: symptomatic rectocele not responding to conservative treatment Exclusion: any other prolapse or compromised anal sphincter function 42 eligible women participated 12 excluded owing to compromised anal sphincter function 30 analysed | |
| Interventions | A (15): midline rectovaginal fascia plication Vicryl repair, excess mucosa trimmed, no levatorplasty, enterocele repaired, perineorrhaphy B (15): transanal repair performed by 2 colorectal surgeons Vertical and horizontal Vicryl sutures, enterocele repaired, anal mucosa trimmed | |
| Outcomes |
|
|
| Notes | Full text Ethics granted January 1998‐March 2001 Outcomes not clearly defined or laid out Validated questionnaires not used No intention to treat No CONSORT Finland |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specifically stated |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Reported on 2 of review's primary outcomes, protocol not found |
| Other bias | Unclear risk | Groups similar at the start No concomitant procedures |
Paraiso 2006.
| Methods | Single‐centre RCT (computer‐generated randomisation by sealed envelopes with blinded research nurse)
106 randomised to: Posterior colporrhaphy (37) Site‐specific repair (37) Site‐specific repair augmented with porcine small intestine submucosa (32: Fortagen, Organogenesis) June 2002‐December 2004 ITT analysis 93% follow‐up with mean 17.5 months Assessed at 6 weeks, 6 months, 1 and 2 years |
|
| Participants | 106 women Inclusion: 21 years and over, stage 2 or greater posterior vaginal wall prolapse with or without other prolapse or incontinence or gynaecological procedures Exclusion: concomitant colorectal procedures, allergy to pork | |
| Interventions | A (37): posterior colporrhaphy as per Maher 2‐0 Ethibond B (37): site‐specific repair Cundiff 2‐0 Ethibond C (32): as in B with 4x8 cm porcine small intestine submucosa graft inlay (Fortagen) | |
| Outcomes |
|
|
| Notes | Ongoing study: initial full‐text review after 1 year
ITT basis
CONSORT statement
Independent nurse review
Limited sample size USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded up to 6 weeks |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded non‐surgeon reviewer |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 17.5 months, 33/37 (11% attrition) native tissue, 37/37 (0% attrition) site‐specific, 29/32 biological graft (10% attrition) |
| Selective reporting (reporting bias) | Unclear risk | Reported on all of review's primary outcomes, protocol not found |
| Other bias | Unclear risk | Declared unrestricted research grant from Organogenesis, whose product was being evaluated. Company had no involvement in designing or running trial. No concomitant procedures |
Park 2014 Abstract.
| Methods | Single‐centre RCT Computer‐generated randomisation schedule Patients were blinded to examiner (nurse). Two years' follow‐up Sample size of 50 per group provides 80% power to detect a 20% difference between groups. ITT analysis Groups same at the start Follow‐up 6 weeks, 6 months, 12 months, 24 months Completed 2 years' follow‐up: Gp A 43/53 (77%); Gp B 38/53 (72%) |
|
| Participants | 172 eligible 109 randomised (63 excluded) Inclusion: women aged 31 to 77 years with symptomatic prolapse (≥ stage 2) undergoing laparoscopic sacral colpopexy Exclusion: none A: 56 B: 53 Completed analysis in 2 years: Gp A 43 (77%); Gp B 38 (72%) |
|
| Interventions | A: Native tissue repair 56 2. Biological graft 53 |
|
| Outcomes |
|
|
| Notes | t‐tests and regression analyses used to determine statistical significance USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block, similar groups |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants blinded for 2 years |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded examiner |
| Incomplete outcome data (attrition bias) All outcomes | High risk | At 2 years, native tissue 43/53 (77%) (23% attrition) Biological graft 38/53 (72%) (28% attrition) |
| Selective reporting (reporting bias) | High risk | No primary outcomes of this review reported, protocol not found |
| Other bias | Unclear risk | Not stated Trial done in women undergoing a different operation ‐ laparoscopic sacral colpopexy |
Sand 2001.
| Methods | Prospective, randomised, controlled trial Participant selection by computer‐generated random number tables 12‐Month follow‐up |
|
| Participants | 161 women enrolled in trial Anterior/posterior colporrhaphy with polyglactin 910 mesh (80) Anterior/posterior colporrhaphy without polyglactin 910 mesh (80) (1 woman excluded) 17 lost to follow‐up at 52 months Inclusion: cystocele protruding to or beyond the hymenal ring in the standing position while coughing or straining, regardless of other concurrent prolapse. Participants had to be > 18 years old, ambulatory, and willing to comply with return visits. Exclusion: pregnant or contemplating pregnancy in the next 12 months. Had only an anterior enterocele or only a paravaginal defect with no need for central cystocele repair at the time of reconstructive surgery Patients seen at 2, 6, 12, and 52 weeks after surgery |
|
| Interventions | Anterior/posterior colporrhaphy with polyglactin 910 mesh (80) Anterior/posterior colporrhaphy without polyglactin 910 mesh (80) (1 woman excluded) |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number tables |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Examiner not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 17 lost to follow‐up at 52 months (11%). Not stated in study to which groups these were assigned, but it is stated that loss to follow‐up was not significantly different between the 2 groups |
| Selective reporting (reporting bias) | Unclear risk | Reported on 1 of this review's primary outcomes, protocol not found |
| Other bias | Unclear risk | Not stated Concomitant procedure was anterior vaginal repair; same numbers in both comparison groups had concomitant procedures. |
Sung 2012.
| Methods | Two‐site double‐blinded randomised controlled trial: Allocation concealment sealed envelopes Randomisation block and stratified site Patients and assessors blinded (patients unblinded 12 months) ITT analysis (1 did not receive graft) 16‐Month follow‐up |
|
| Participants | 160 women randomised 137 12‐month follow‐up anatomical, 133 subjective data January 2004, 5 years Inclusion criteria: women with stage 2 or greater symptomatic rectocele (defined as vaginal bulge, defecatory symptoms, or both) Exclusion criteria: < 18 years, women undergoing concomitant sacrocolpopexy or colorectal procedures, history of porcine allergy, connective tissue disease, pelvic malignancy, pelvic radiation, inability to understand English, or inability or unwillingness to consent or comply with follow‐up. All other vaginal prolapse repairs and anti‐incontinence procedures included |
|
| Interventions | Biological graft vs native tissue repair | |
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence, groups similar at start |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded reviewers |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 12 months, 70/80 (13%) in native tissue and 68/80 in graft group (15%) |
| Selective reporting (reporting bias) | Low risk | Reported on 2 of this review's primary outcomes, protocol found |
| Other bias | Low risk | No financial conflict of interest; grant funding National Institute of Child and Human Health No concomitant procedures |
Vijaya 2011 Abstract.
| Methods | RCT with block randomisation Allocation concealment, power and consort not stated Before and 6 months postoperatively, anatomical outcome assessed by POP‐Q, subjective outcomes by P‐QOL, sexual dysfunction by FSFI, and bowel‐associated symptoms by BBUSQ‐22 16‐Month follow‐up |
|
| Participants | Inclusion: symptomatic posterior wall prolapse Exclusion: concomitant surgery NS |
|
| Interventions | A (26): standard posterior colporrhaphy (with plication of the levator ani muscle) B (26): fascial and vaginal plication repair |
|
| Outcomes |
|
|
| Notes | Very scant raw data United Kingdom |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation, groups similar at the start |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | High risk | Primary outcomes of this review not reported, no protocol found |
| Other bias | Unclear risk | Not stated No concomitant procedures |
Wei 2015.
| Methods | RCT Random number table No blinding No ITT analysis Unclear how many women were screened to get the 50 included Follow‐up 50 months 5 lost to follow‐up at 12 months, 8 at 50 months |
|
| Participants | 50 women Inclusion criteria: women with rectocele with defecatory difficulty, imaging showing rectocele at least 4 cm in depth with at least 6 months conservative treatment first Exlusion criteria: intussusception, puborectalis syndrome, any other obstructive constipation, gastrointestinal motor dysfunction disease, colorectal cancer, constipation caused by psychological or endocrine disorder |
|
| Interventions | Transvaginal 25 f/u 12 months: 23; 50 months: 22 Transanal 25 f/u 12 months: 22; 50 months: 20 |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | At 50 months, 20/25 (80%) in transanal group, 22/25 (78%) in transvaginal group |
| Selective reporting (reporting bias) | High risk | Primary outcomes of this review not reported, protocol not found |
| Other bias | Unclear risk | Not stated No concomitant procedures |
BBUSQ: Birmingham Bowel and Urinary Symptoms Questionnaire. COI: conflict of interest. EBL: estimated blood loss. FSFI: Female Sexual Function Index. HTA: Health Technology Assessment. ITT: intention‐to‐treat. MARP: mean anal resting pressure. PFDI: Pelvic Floor Distress Inventory. PFIQ: Pelvic Floor Impact Questionnaire. PISQ: Pelvic organ prolapse/urinary Incontinence Sexual Questionnaire. POP‐Q: Pelvic Organ Prolapse Quantification (according to ICS). POP‐SS: Pelvic Organ Prolapse Symptom Score. P‐QOL: Prolapse Quality of Life Questionnaire. QOL: quality of life. RCT: randomised controlled trial.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allahdin 2008 | RCT evaluating the 3 surgical techniques for pelvic organ prolapse surgery. Pelvic organ prolapse data were grouped, and data specific to posterior prolapse were not available. |
| Boccasanta 2004 | RCT on 2 transanal stapled techniques for outlet obstruction. Outlet obstruction caused not only by rectoceles but also by descending perineum and intussusception. Prolapse data not explicitly presented |
| Boccasanta 2011 | RCT investigating clinical and functional outcomes of the stapled transanal rectal resection in patients with obstructed defecation caused by rectal intussusception and rectocele. Participants with rectal intussusception and rectocele were grouped together, and rectocele data were not explicitly presented. |
| Dahlgren 2011 | RCT comparing use of a porcine skin graft (Pelvicol) vs conventional colporrhaphy in recurrent pelvic organ prolapse surgery. Data regarding participants with cystocele and rectocele were presented together, and information specific to rectocele was not explicitly presented. |
| Derpapas 2013 | Study comparing posterior colpoperineorrhaphy or fascial and vaginal epithelial plication (FEP) of the posterior vaginal wall. Participants were already included in the Vijaya study. |
| Detollenaere 2013 | Study comparing sacrospinous hysteropexy (SH) against vaginal hysterectomy in patients with uterine descent POP‐Q stage > 2. Study was not included because patients with anterior and posterior vaginal wall prolapse were grouped together. Posterior prolapse data were not explicitly presented. |
| Gentile 2014 | Study comparing effectiveness and safety of endorectal proctopexy against the STARR procedure in patients with mucosal prolapse or anorectal intussusception (types of rectal prolapse) rather than vaginal prolapse |
| Glazener 2016 | Study looking at clinical effectiveness and cost‐effectiveness when comparing surgical methods of vaginal wall prolapse. No data on pelvic organ prolapse were given. |
| Leanza 2013 | Quasi‐randomised controlled trial |
| Lehur 2008 | RCT not comparing 2 surgical techniques. Study compared conservative management vs surgical management of rectal mucosal prolapse, which is not the topic of our meta‐analysis. |
| Liu 2016 | RCT focusing on treatment of obstructed bowel syndrome associated with rectocele and internal rectal intussusception. The 2 pathologies were not differentiated. Trial concerned 2 different transanal approaches to deal primarily with rectal mucosal prolapse. |
| Mahmoud 2012 | RCT evaluating transanal repair with and without use of a stapler for the procedure. This comparison is not relevant to our meta‐analysis. |
| Noe 2014 | RCT comparing pectopexy with sacral colpopexy for correction of vaginal prolapse POP‐Q stage 2 or greater. Data for posterior vaginal prolapse were not available as this was grouped broadly with anterior and apical prolapse. |
| Nygaard 2013 | RCT investigating anatomic and symptomatic outcomes of abdominal sacrocolpopexy. Study was not included because patients with anterior and posterior vaginal wall prolapse were grouped together. Data for posterior wall prolapse could not be separated from rest of trial data. |
| Svabik 2016 | RCT investigating 2 surgical procedures for post‐hysterectomy vaginal vault prolapse (anterior and posterior Prolift mesh vs sacrospinous colpopexy with anterior and/or posterior native tissue vaginal repair). Apical prolapse covered in separate Cochrane review |
| Tang 2006 | Excluded on the basis of comparing 2 different incision techniques for the posterior vaginal wall rather than 2 different repair techniques |
| Wang 2010 | Study investigating the value of co‐treatment with rectal wall repair and procedure for prolapse and haemorrhoids (PPH) for outlet obstruction constipation (OOC) induced by rectocele in woman. This comparison was not relevant to our meta‐analysis. |
FEP: fascial epithelial plication. OOC: outlet obstruction constipation. POP‐Q: Pelvic Organ Prolapse Quantification (according to ICS). PPH: procedure for prolapse and haemorrhoids. RCT: randomised controlled trial. STARR: stapled transanal rectal resection.
Differences between protocol and review
None.
Contributions of authors
All review authors contributed to writing the protocol. Four review authors (A. Mowat, D. Maher, C Christmann‐Schmid, K. Baessler) assessed the relevance and eligibility of studies for inclusion in this review. They then assessed the quality of included studies; three review authors (A. Mowat, C. Maher, N. Haya) independently extracted data from trial reports, interpreted the results, and contributed to writing of the draft version of this review.
Sources of support
Internal sources
None, Other.
External sources
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to the Cochrane Incontinence Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS, or the Department of Health.
Declarations of interest
AM, DM, KB, CC, NH, and CM have no interests to declare.
New
References
References to studies included in this review
Farid 2010 {published data only}
- Farid M, Madbouly KM, Hussein A, Mahdy T, Moneim HA, Omar W. Randomized controlled trial between perineal and anal repairs of rectocele in obstructed defecation. World Journal of Surgery 2010;34:822‐9. [DOI] [PubMed] [Google Scholar]
Glazener 2017 {published data only}
- Glazener C, Breeman S, Elders A, Hemming C, Cooper KG, Freeman RM, et al. Mesh, graft, or standard repair for women having primary transvaginal anterior or posterior compartment prolapse surgery: two parallel‐group, multicentre, randomised, controlled trials (PROSPECT). Lancet 2017;389:381‐92. [DOI] [PubMed] [Google Scholar]
Kahn 1999 {published and unpublished data}
- Kahn MA, Kumar D, Stanton SL. Posterior colporrhaphy vs transanal repair of the rectocele: an initial follow up of a prospective randomized controlled trial. British Journal of Obstetrics and Gynaecology 1998;105 Suppl 17:57. [6675] [Google Scholar]
- Kahn MA, Stanton SL, Kumar D, Fox SD. Posterior colporrhaphy is superior to the transanal repair for treatment of posterior vaginal wall prolapse. Neurourology and Urodynamics 1999;18(4):329‐30. [Google Scholar]
- Kahn MA, Stanton SL, Kumar DA. Anorectal physiological effects of rectocele correction by posterior colporrhaphy or the transanal approach. Proceedings of the International Continence Society (ICS), 27th Annual Meeting; 1997 Sept 23‐26; Yokohama, Japan. 1997:285‐6. [5853]
- Kahn MA, Stanton SL, Kumar DA. Randomised prospective trial of posterior colporrhaphy vs transanal repair of rectocele: preliminary findings. Proceedings of the International Continence Society (ICS), 27th Annual Meeting, 1997 Sept 23‐26; Yokohama, Japan. 1997:82‐3. [5863]
Nieminen 2004 {published and unpublished data}
- Nieminen K, Hiltunen K, Laitinen J, Oksala J, Heinonen P. Transanal or vaginal approach to rectocele repair: a prospective, randomized pilot study. Diseases of the Colon and Rectum 2004;47(10):1636‐42. [DOI] [PubMed] [Google Scholar]
Paraiso 2006 {published data only}
- Paraiso M, Barber M, Muir T, Walters M. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. American Journal of Obstetrics and Gynecology 2006;195:1762‐71. [DOI] [PubMed] [Google Scholar]
Park 2014 Abstract {published data only}
- Park J, Kassis NC, Steele GK, Woodman PJ, Hale DS. Biograft addition to posterior synthetic mesh during laparoscopic sacral colpoperineopexy: a randomized controlled clinical trial. International Urogynecology Journal 2014;25:S24‐5. [Google Scholar]
Sand 2001 {published and unpublished data}
- Sand PK, Koduri S, Lobel RW, Winkler HA, Tomezsko J, Culligan PJ. Prospective randomized trial of polyglactin910 mesh to prevent recurrence of cystoceles and rectoceles. American Journal of Obstetrics and Gynecology 2001;184(7):1357‐64. [DOI] [PubMed] [Google Scholar]
Sung 2012 {published data only}
- Sung VW, Rardin CR, Raker CA, Lasala CA, Myers DL. Porcine subintestinal submucosal graft augmentation for rectocele repair: a randomized controlled trial. Obstetrics and Gynecology 2012;119(1):125‐33. [42876] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vijaya 2011 Abstract {published data only}
- Vijaya G, Dell'Utri C, Derpapas A, Digesu A, Gallo P, Hendricken C, et al. A prospective randomised trial comparing two surgical techniques for posterior vaginal wall prolapse using subjective and objective measures (Abstract number 52). Neurourology and Urodynamics 2011;30(6):872‐3. [42172] [Google Scholar]
Wei 2015 {published data only}
- Wei W, Rong‐Xian L, Xin P, Jiu‐Di LV. Transanal vs transvaginal repair of symptomatic rectocele: analysis of 50 cases. World Chinese Journal of Digestology 2015;23(9):1521. [Google Scholar]
References to studies excluded from this review
Allahdin 2008 {published data only}
- Allahdin S, Glazener C, Bain C. A randomised controlled trial evaluating the use of polyglactin mesh, polydioxanone and polyglactin sutures for pelvic organ prolapse surgery. Journal of Obstetrics and Gynaecology 2008;28(4):427‐31. [DOI] [PubMed] [Google Scholar]
- Madhuvrata P, Glazener C, Boachie C, Allahdin S, Bain C. A randomised controlled trial evaluating the use of polyglactin (Vicryl) mesh, polydioxanone (PDS) or polyglactin (Vicryl) sutures for pelvic organ prolapse surgery: outcomes at 2 years. Journal of Obstetrics and Gynaecology 2011;31(5):429‐35. [DOI] [PubMed] [Google Scholar]
Boccasanta 2004 {published data only}
- Boccasanta P, Venturi M, Salamina G, Cesana BM, Bernasconi F, Roviaro G. New trends in the surgical treatment of outlet obstruction: clinical and functional results of two novel transanal stapled techniques from a randomised controlled trial. International Journal of Colorectal Disease 2004;19:359‐69. [DOI] [PubMed] [Google Scholar]
Boccasanta 2011 {published data only}
- Boccasanta P, Venturi M, Roviaro G. What is the benefit of a new stapler device in the surgical treatment of obstructed defecation?. Diseases of the Colon and Rectum 2011;54(1):77‐84. [DOI] [PubMed] [Google Scholar]
Dahlgren 2011 {published data only}
- Dahlgren E, Kjølhede P, RPOP‐PELVICOL Study Group. Long‐term outcome of porcine skin graft in surgical treatment of recurrent pelvic organ prolapse. Acta Obstetricia et Gynecologica Scandinavica 2011;90(12):1393‐401. [DOI] [PubMed] [Google Scholar]
Derpapas 2013 {published data only}
- Derpapas A, Vijaya G, Digesu AG, Fernando R, Khullar V. Clinical and ultrasonographic assessment of two different surgical techniques for posterior vaginal wall repair. International Urogynecology Journal 2013;24:127. [Google Scholar]
Detollenaere 2013 {published data only}
- Detollenaere RJ, Boon J, Stekelenburg J, Kluivers KB, Vierhout ME, Vaneijndhoven HW. Short term anatomical results of a randomized controlled non inferiority trial comparing sacrospinous hysteropexy and vaginal hysterectomy in treatment of uterine prolapse stage 2 or higher. International Urogynecology Journal 2013;24:S1‐S2. [Google Scholar]
Gentile 2014 {published data only}
- Gentile M, Rosa M, Cestaro G, Vitiello C, Sivero L. Internal Delorme vs. STARR procedure for correction of obstructed defecation from rectocele and rectal intussusception. Annali Italiani di Chirurgia 2014;85(2):177‐83. [PubMed] [Google Scholar]
Glazener 2016 {published data only}
- Glazener C, Breeman S, Elders A, Hemming C, Cooper K, Freeman R, et al. Clinical effectiveness and cost‐effectiveness of surgical options for the management of anterior and/or posterior vaginal wall prolapse. Health Technology Assessment 2016;20(95):452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leanza 2013 {published and unpublished data}
- Leanza V, Intagliata E, Leanza G, Vecchio R. Pelvic posterior compartment defects: comparative study of two vaginal surgical procedures. Urogynaecologia 2013;27(1):11‐3. [Google Scholar]
Lehur 2008 {published data only}
- Lehur PA, Stuto A, Fantoli M, Villani RD, Queralto M, Lazorthes F, et al. Outcomes of stapled transanal rectal resection vs. biofeedback for the treatment of outlet obstruction associated with rectal intussusception and rectocele. Diseases of the Colon and Rectum 2008;51(11):1611‐8. [DOI] [PubMed] [Google Scholar]
Liu 2016 {published data only}
- Liu Z, Yang G, Deng Q, Yang Q. Efficacy observation of partial stapled transanal rectal resection combined with Bresler procedure in the treatment of rectocele and internal rectal intussusception. Chinese Journal of Gastrointestinal Surgery 2016;19(5):566‐70. [PubMed] [Google Scholar]
Mahmoud 2012 {published data only}
- Mahmoud SA, Omar W, Farid M. Transanal repair for treatment of rectocele in obstructed defaecation: manual or stapled. Association of Coloproctology of Great Britain and Ireland 2012;14(1):104‐10. [DOI] [PubMed] [Google Scholar]
Noe 2014 {published data only}
- Noe GK, Anapolski M. A randomized trial of laparoscopic sacral colpopexy versus laparoscopic pectopexy for vaginal and uterine prolapse. Journal of Minimally Invasive Gynecology 2014;21(6):S56. [Google Scholar]
Nygaard 2013 {published data only}
- Nygaard I, Brubaker L, Zyczynski HM, Cundiff G, Richter H, Gantz M, et al. Long‐term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse. JAMA 2013;309(19):2016‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Svabik 2016 {published data only}
- Svabik K, Masata J, Hubka P, Martan A. Randomized trial comparing vaginal mesh repair (Prolift total) versus sacrospinous vaginal colpopexy (SSF) in the management of vaginal vault prolapse after hysterectomy for patients with levator ani avulsion injury ‐ 6 years follow‐up. International Urogynecology Journal 2016;27:S59‐60. [Google Scholar]
Tang 2006 {published data only}
- Tang XG, Wu ZJ, Du LJ. Clinical trial of rectocele repair with longitudinal incision and transverse suture on the vaginal posterior wall. Chinese Journal of Gastrointestinal Surgery 2006;9(4):311‐3. [PubMed] [Google Scholar]
Wang 2010 {published data only}
- Wang J, Zheng J‐Z, Wang T‐F, Zhang K. Clinical evaluation of co‐treatment with rectal wall repair and procedure for prolapse and haemorrhoids on outlet obstruction constipation induced by rectocele in woman. Journal of Jilin University Medicine Edition 2010;36(4):763‐6. [Google Scholar]
Additional references
Adams 2004
- Adams E, Thomson A, Maher C, Hagen S. Mechanical devices for pelvic organ prolapse in women. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD004010.pub2] [DOI] [PubMed] [Google Scholar]
Brubaker 2002
- Brubaker L, Bump R, Jacquetin B, Schuessler B, Weidner A, Zimmern P, et al. [Pelvic organ prolapse.]. Incontinence: 2nd International Consultation on Incontinence. 2nd Edition. Plymouth: Health Publication Ltd, 2002:243‐65. [Google Scholar]
Bump 1998
- Bump R, Norton P. Epidemiology and natural history of pelvic floor dysfunction. Obstetrics and Gynecology Clinics of North America 1998;25(4):723‐46. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gill 1998
- Gill EJ, Hurt WG. Pathophysiology of pelvic organ prolapse. Obstetrics and Gynecology Clinics of North America 1998;25(4):759‐69. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADEproGDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEproGDT. Version accessed 10 May 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available from gradepro.org.
Hagen 2011
- Hagen S, Stark D. Conservative prevention and management of pelvic organ prolapse in women. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD003882.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Handa 2004
- Handa VL, Garrett E, Hendrix S, Gold E, Robbins J. Progression and remission of pelvic organ prolapse: a longitudinal study of menopausal women. [Progression and remission of pelvic organ prolapse: a longitudinal study of menopausal women]. American Journal of Obstetrics and Gynecology 2004;190(1):27‐32. [DOI] [PubMed] [Google Scholar]
Hendrix 2002
- Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women's Health Initiative: gravity and gravidity [Pelvic organ prolapse in the Women's Health Initiative: gravity and gravidity]. American Journal of Obstetrics and Gynecology 2002;186(6):1160‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011. Available from http://handbook.cochrane.org..
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
ICI 2017
- Maher C, Baessler K, Barber M, Cheon C, Consten E, Cooper K, et al. Pelvic organ prolapse surgery. In: Adams P, Cardoza L, Khoury S, Wein A editor(s). Incontinence: 5th International Consultation on Incontinence. Paris: ICUD‐EAU, 2017. [Google Scholar]
MacLennan 2000
- MacLennan AH, Taylor AW, Wilson DH, Wilson D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. British Journal of Obstetrics and Gynaecology 2000;107(12):1460‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Revman 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Review Manager (RevMan) [Computer program]. Version . .. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
References to other published versions of this review
Maher 2003
- Maher C, Carey CM, Adams EJ, Hagen S. Surgical management of pelvic organ prolapse in women. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD004014] [DOI] [Google Scholar]
Maher 2004
- Maher C, Baessler K, Glazener CMA, Adams EJ, Hagen S. Surgical management of pelvic organ prolapse in women. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004014.pub2] [DOI] [PubMed] [Google Scholar]


