Abstract
Background
Diabetic retinopathy (DR) is a chronic progressive disease of the retinal microvasculature associated with prolonged hyperglycaemia. Proliferative DR (PDR) is a sight‐threatening complication of DR and is characterised by the development of abnormal new vessels in the retina, optic nerve head or anterior segment of the eye. Argon laser photocoagulation has been the gold standard for the treatment of PDR for many years, using regimens evaluated by the Early Treatment of Diabetic Retinopathy Study (ETDRS). Over the years, there have been modifications of the technique and introduction of new laser technologies.
Objectives
To assess the effects of different types of laser, other than argon laser, and different laser protocols, other than those established by the ETDRS, for the treatment of PDR. We compared different wavelengths; power and pulse duration; pattern, number and location of burns versus standard argon laser undertaken as specified by the ETDRS.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (which contains the Cochrane Eyes and Vision Trials Register) (2017, Issue 5); Ovid MEDLINE; Ovid Embase; LILACS; the ISRCTN registry; ClinicalTrials.gov and the ICTRP. The date of the search was 8 June 2017.
Selection criteria
We included randomised controlled trials (RCTs) of pan‐retinal photocoagulation (PRP) using standard argon laser for treatment of PDR compared with any other laser modality. We excluded studies of lasers that are not in common use, such as the xenon arc, ruby or Krypton laser.
Data collection and analysis
We followed Cochrane guidelines and graded the certainty of evidence using the GRADE approach.
Main results
We identified 11 studies from Europe (6), the USA (2), the Middle East (1) and Asia (2). Five studies compared different types of laser to argon: Nd:YAG (2 studies) or diode (3 studies). Other studies compared modifications to the standard argon laser PRP technique. The studies were poorly reported and we judged all to be at high risk of bias in at least one domain. The sample size varied from 20 to 270 eyes but the majority included 50 participants or fewer.
Nd:YAG versus argon laser (2 studies): very low‐certainty evidence on vision loss, vision gain, progression and regression of PDR, pain during laser treatment and adverse effects.
Diode versus argon laser (3 studies): very‐low certainty evidence on vision loss, vision gain, progression and regression of PDR and adverse effects; moderate‐certainty evidence that diode laser was more painful (risk ratio (RR) troublesome pain during laser treatment (RR 3.12, 95% CI 2.16 to 4.51; eyes = 202; studies = 3; I2 = 0%).
0.5 second versus 0.1 second exposure (1 study): low‐certainty evidence of lower chance of vision loss with 0.5 second compared with 0.1 second exposure but estimates were imprecise and compatible with no difference or an increased chance of vision loss (RR 0.42, 95% CI 0.08 to 2.04, 44 eyes, 1 RCT); low‐certainty evidence that people treated with 0.5 second exposure were more likely to gain vision (RR 2.22, 95% CI 0.68 to 7.28, 44 eyes, 1 RCT) but again the estimates were imprecise . People given 0.5 second exposure were more likely to have regression of PDR compared with 0.1 second laser PRP again with imprecise estimate (RR 1.17, 95% CI 0.92 to 1.48, 32 eyes, 1 RCT). There was very low‐certainty evidence on progression of PDR and adverse effects.
'Light intensity' PRP versus classic PRP (1 study): vision loss or gain was not reported but the mean difference in logMAR acuity at 1 year was −0.09 logMAR (95% CI −0.22 to 0.04, 65 eyes, 1 RCT); and low‐certainty evidence that fewer patients had pain during light PRP compared with classic PRP with an imprecise estimate compatible with increased or decreased pain (RR 0.23, 95% CI 0.03 to 1.93, 65 eyes, 1 RCT).
'Mild scatter' (laser pattern limited to 400 to 600 laser burns in one sitting) PRP versus standard 'full' scatter PRP (1 study): very low‐certainty evidence on vision and visual field loss. No information on adverse effects.
'Central' (a more central PRP in addition to mid‐peripheral PRP) versus 'peripheral' standard PRP (1 study): low‐certainty evidence that people treated with central PRP were more likely to lose 15 or more letters of BCVA compared with peripheral laser PRP (RR 3.00, 95% CI 0.67 to 13.46, 50 eyes, 1 RCT); and less likely to gain 15 or more letters (RR 0.25, 95% CI 0.03 to 2.08) with imprecise estimates compatible with increased or decreased risk.
'Centre sparing' PRP (argon laser distribution limited to 3 disc diameters from the upper temporal and lower margin of the fovea) versus standard 'full scatter' PRP (1 study): low‐certainty evidence that people treated with 'centre sparing' PRP were less likely to lose 15 or more ETDRS letters of BCVA compared with 'full scatter' PRP (RR 0.67, 95% CI 0.30 to 1.50, 53 eyes). Low‐certainty evidence of similar risk of regression of PDR between groups (RR 0.96, 95% CI 0.73 to 1.27, 53 eyes). Adverse events were not reported.
'Extended targeted' PRP (to include the equator and any capillary non‐perfusion areas between the vascular arcades) versus standard PRP (1 study): low‐certainty evidence that people in the extended group had similar or slightly reduced chance of loss of 15 or more letters of BCVA compared with the standard PRP group (RR 0.94, 95% CI 0.70 to 1.28, 270 eyes). Low‐certainty evidence that people in the extended group had a similar or slightly increased chance of regression of PDR compared with the standard PRP group (RR 1.11, 95% CI 0.95 to 1.31, 270 eyes). Very low‐certainty information on adverse effects.
Authors' conclusions
Modern laser techniques and modalities have been developed to treat PDR. However there is limited evidence available with respect to the efficacy and safety of alternative laser systems or strategies compared with the standard argon laser as described in ETDRS
Plain language summary
Do newer laser treatments work better than standard laser treatments for proliferative diabetic retinopathy?
What was the aim of this review? The aim of this Cochrane Review was to find out if new ways of doing laser treatment for proliferative diabetic retinopathy (explained under 'What was studied in the review?' below) work better than standard treatment. Cochrane researchers collected and analysed all relevant studies to answer this question and found 11 studies.
Key messages There is limited evidence on the benefits and harms of different laser systems or strategies compared with the standard treatment.
What was studied in the review? People with diabetes can have problems in the back of their eyes that may affect their sight. One of these problems is the growth of harmful new blood vessels in the retina (the layer that covers the back of the eye that allows people to see); this is called proliferative diabetic retinopathy, referred to as ‘PDR’. Sight loss can occur as a result of PDR. Argon laser has been used to treat PDR for many years. New types of laser and new ways of doing laser treatment have been developed to treat PDR. The aim of this review was to assess the evidence for the benefits and harms of these new treatments.
What are the main results of the review? The Cochrane researchers found 11 relevant studies. Four studies were done in Italy, two studies were done in the US, one in South Korea, one in Iran, one in Slovenia, one in Greece and one in India. All the people included in these studies had PDR due to type 1 or type 2 diabetes. Most of these studies were small and provide limited evidence on which to base treatment decisions.
How up to date is this review? Cochrane researchers searched for studies that had been published up to 8 June 2017.
Summary of findings
Background
Description of the condition
Diabetic retinopathy (DR) is a chronic, progressive, potentially sight‐threatening disease of the retinal microvasculature associated with prolonged hyperglycaemia. As the leading cause of blindness among working‐aged adults around the world, DR is a major public health problem (Klein 2007). Its incidence is rising dramatically along with the incidence of type 2 diabetes, driven by greater longevity combined with sedentary lifestyles and increasing levels of obesity (Geiss 2011). Globally, there are approximately 93 million people with DR, including 17 million with proliferative DR, 21 million with diabetic macular oedema (DMO), and 28 million with vision‐threatening diabetic retinopathy (VTDR) (Yau 2012). A pooled analysis from diabetic population‐based studies around the world found overall prevalence rates of 34.6% for any DR, 6.96% for PDR, 6.81% for DMO and 10.2% for VTDR. All DR prevalence endpoints increased with diabetes duration, haemoglobin A(1c), and blood pressure levels and were higher in people with type 1 compared with type 2 diabetes (Yau 2012). These data highlight the substantial worldwide public health burden of DR and the importance of tackling modifiable risk factors to reduce its occurrence. The Diabetes Control and Complications Trial (DCCT) showed that intensive glycaemic control was effective in delaying the onset, as well as slowing the progression, of DR in patients with type 1 diabetes (DCCT Research Group 1993). The UK Prospective Diabetes Study (UKPDS) showed the risk of complications in type 2 diabetics was independently and additively correlated with hyperglycaemia and hypertension, with risk reductions of 21% per 1% decrease in HbA1c and 11% per 10 mmHg decrease in systolic blood pressure (Stratton 2006; UKPDS Group 1998). There are various classifications of DR, but all recognise the two basic mechanisms leading to loss of vision: retinopathy (risk of developing new vessels); and maculopathy (risk of damage to the central fovea). The differences between classifications relate mainly to levels of retinopathy and to terminology used. Severity is ranked into a stepwise scale from no retinopathy through various stages of non‐proliferative or pre‐proliferative disease to advanced proliferative disease (ETDRS Research Group 1991). This review is concerned with Vision Threatening Diabetic Retinopathy (VTDR) related to the development of PDR.
PDR is characterised by the development of new vessels and can be further defined by their location and severity. With regards to location, there may be: new vessels on the disc or within 1 disc diameter (DD) of the margin of the disc (NVD); elsewhere in the retina (NVE); on the iris (NVI); or anterior chamber angle (NVA). Classification of PDR severity includes: early PDR (NVD < 1/4 DD, NVE without haemorrhage); PDR with high risk characteristics such as NVD equal to or greater than 1/4 DD, any NVD‐ or NVE‐associated vitreous haemorrhage; florid (aggressive presentation) PDR; and gliotic (with the development of fibrotic tissue) PDR. 'Involutionary' PDR refers to new vessels that have regressed, usually in response to treatment but (rarely) spontaneously.
Description of the intervention
Laser photocoagulation reduces the oxygen demand of the outer layers of the retina and helps divert adequate oxygen and nutrients to the retina, favourably altering the haemodynamics (Stefánsson 2001). Laser photocoagulation appears also to act by reducing the expression of vasoactive factors such as vascular endothelial growth factor (VEGF) and protein kinase C (PKC) in the retina (Ghosh 2005). Indeed, different landmark studies have supported the efficacy of laser PRP in preventing vision loss. The Diabetic Retinopathy Study (DRS) demonstrated that laser photocoagulation of the retina reduced severe visual loss (defined as visual acuity of 5/200 or less on two consecutive visits at least four months apart) (DRS Research Group 1978); and the Early Treatment Diabetic Retinopathy Study (ETDRS) addressed the question of the appropriate time for performing laser photocoagulation, showing that PRP was beneficial only in cases where proliferative changes were present and specifically when high‐risk characteristics PDR were present (ETDRS Research Group 1985). It also showed that focal or grid photocoagulation was beneficial in reducing visual loss due to macular oedema (ETDRS Research Group 1985). As PRP may be associated with morbidity, the risk‒benefit ratio of PRP in people at higher risk would favour the performance of PRP. The visual loss due to PRP is much less debilitating at this stage compared with the high risk of severe vision loss in the near future if the retinopathy were to remain untreated (Feman 2004). The ETDRS also showed that focal or grid photocoagulation was beneficial in reducing the risk of visual loss due to DMO (ETDRS Research Group 1985).
How the intervention might work
It is believed that in the majority of cases, PDR represents an angiogenic response of the retina to extensive capillary closure. New vessels grow at the interface of perfused and non‐perfused retina. Peripheral retinal ischaemia, in the absence of surrogate markers or capillary drop‐out (blot haemorrhage, venous beading, intraretinal microvascular anomalies), may not always be readily discernible clinically, and hence fluorescein angiography — especially wide field fluorescein angiography — is especially useful in detecting ischaemic changes.
The aim of laser PRP treatment is to destroy the areas where there is capillary non‐perfusion and retinal ischaemia as it is in these ischaemic areas where VEGF, a permeability and angiogenic factor, is produced. Lasers act by inducing thermal damage after absorption of energy by tissue pigments. If there is an inadequate response after a standard PRP is undertaken and full regression of new vessels is not achieved, clinicians often supplement the treatment by undertaking further laser in untreated areas.
Following the guidelines published by the DRS and ETDRS, argon laser photocoagulation has been the gold standard for the treatment of PDR. Level 1 evidence from the DRS recommended multisession scatter PRP laser (800 to 1600 spots in one or two sittings, and follow‐up treatment applied as needed at 4‐month intervals) extending to or beyond the vortex vein ampullae (midperipheral retina) (DRS Research Group 1981). Practitioners still widely follow this guideline as a frame of reference. In general, ophthalmologists administer laser covering 360° of the midperipheral retina, with adequate spacing between laser burns (˜ 1 burn apart) to avoid compromising peripheral vision. In clinical practice, the power of the laser selected is set for each individual patient to achieve an adequate burn in the retina and is dependent on variables such as media clarity, fundus pigmentation, and method of delivery. Avoiding very intense white spots is advised to reduce possible complications such as haemorrhage and breaks through Bruch’s membrane which could lead to choroidal neovascularisation.
It has been suggested also that laser strategies other than single pulse argon laser peripheral PRP used by the ETDRS may help reduce ocular side effects, such as laser burn scarring and visual field loss (Muqit 2010). The newer 'yellow' wavelength lasers have the highest combined absorption in the melanin‐oxyhaemoglobin layers of the RPE/choriocapillaris complex and are thought to induce less scatter with increased efficiency compared to green laser photocoagulators (Castillejos‐Rios 1992). Diode laser may produce energy in the 532 nm (green) band, the 577 nm (yellow) band, or in the invisible infrared band (810 nm). These laser treatment strategies can target threshold level, or subthreshold level depending on the power used. MicroPulse mode is available for the 810 nm infrared band wavelength.
Laser PRP can be delivered as a single spot but now multispot laser delivery systems allow a reduced pulse duration compared with conventional argon laser (100 ms to 200 ms) with the aim of a quicker and less painful experience. Additionally, the procedure can be semiautomated by delivering multiple laser burns to the retina with a single depression of the foot pedal.
Why it is important to do this review
Current guidelines for the management of PDR recommend that an ophthalmologist promptly perform PRP until regression of neovascularisation is achieved (Ghanchi 2013). However, most of the evidence relies on the previously described landmark trials, which used older lasers from the 1980s. It does not provide enough evidence to recommend newer laser protocols which may be equally effective but safer and less uncomfortable for patients. Thus a high‐quality review, comparing the standard ETDRS laser treatment for PDR with alternative laser strategies and including modern lasers, was necessary. This systematic review was designed to examine efficacy and safety of alternative types of laser in people with PDR when compared with standard argon laser. It assessed the evidence base for alternative laser treatment strategies such as ischaemia‐targeted laser to the peripheral retina as seen on fluorescein angiography compared with standard argon laser. This review followed on from the preliminary work carried out by Evans 2014 in a recent Cochrane Review assessing the effects of laser photocoagulation for DR compared to no treatment or deferred treatment. PRP has been the mainstay of treatment of PDR for many years, but reviews on variations in the laser treatment protocol were recommended. A NIHR‐HTA project (12/71/01) addressed a similar question but in different populations, with earlier disease than in our review (Royle 2015).
Objectives
To assess the effects of different types of laser, other than argon laser, and different laser protocols, other than those established by the ETDRS, for the treatment of PDR. We compared different wavelengths; power and pulse duration; pattern, number and location of burns versus standard argon laser undertaken as specified by the ETDRS.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomised controlled trials (RCTs) in this review.
Types of participants
We included people with type 1 or 2 diabetes mellitus of all ages and both sexes with PDR as defined in the included studies. We included a subgroup of trials where participants have received previous pharmacological treatments for diabetic eye disease. We did not exclude studies that enrolled participants with other associated retinal diseases such as retinal vein occlusion as long as the diabetic subgroup with PDR is clearly identified and the reason for laser is PDR.
Types of interventions
We included RCTs that consider laser pan‐retinal photocoagulation (PRP) for PDR but only those with a comparator group of standard argon laser PRP.
Interventions
We compared variations of the following parameters to the standard argon laser single spot treatment (comparator). We excluded studies that considered lasers that are not in common use, such as the xenon arc photocoagulation, ruby or Krypton laser.
Wavelength
Any ophthalmic laser type (wavelength) including but not limited to:
810 nm
577 nm
532 nm
Laser burn application
Any laser burn application method including but not limited to:
variations in total number of burns required to induce regression of neovascularisation, including number of laser sessions required;
use of multispot pattern laser delivery;
use of conventional slit lamp or the fundus camera‐navigated laser system.
Location of laser burns
Any laser burn target location including but not limited to ischaemia‐targeted retinal location.
Laser combined with other treatments
We included studies in which participants may have also received non‐laser based therapies for other indications such as diabetic macular oedema (DMO), for example anti‐VEGF, intraocular steroid implants or traditional Chinese medicine; however, we considered these as a separate subset.
We excluded studies that compare laser versus laser plus another non‐laser intervention for PDR, as this is covered in another Cochrane Review (Martinez‐Zapata 2014)
Comparator
The comparator was standard argon laser single spot treatment according to ETDRS guidelines. Specifically, the recommendations in the ETDRS are an initial treatment of midperipheral scatter laser consisting of 1200 to 1600 burns of moderate intensity, 200 μm to 500 μm spot size, with one‐half spot to one‐spot diameter spacing. Argon pulse duration is 100 ms to 200 ms with power titrated to produce moderate‐intensity burns but with full treatment divided over at least two sessions according to different clinical scenarios (ETDRS Research Group 1987).
Types of outcome measures
Primary outcomes
The primary outcome was best‐corrected visual acuity (BCVA). Specifically we used the proportion of people who lost or gained at least 15 ETDRS letters (equivalent to 3 ETDRS lines) as measured on a LogMAR chart at the one‐ and five‐year time point.
Secondary outcomes
We considered the following secondary outcomes.
Change in mean BCVA (LogMAR) from baseline to 12 months and five years.
Change in mean best‐corrected near visual acuity (NVA) from baseline to 12 months and five years.
Progression of diabetic retinopathy and/or maculopathy from baseline to 12 months and five years as defined by trial investigators, including optical coherence tomography (OCT) mean central subfield thickness (CMT) where measured.
Visual field (VF) loss from baseline to 12 months and five years compared to baseline including mean deviation (MD).
Patient‐reported outcome measure (PROM) for pain associated with the treatment, vision‐related quality of life (QoL) measured using any validated questionnaire, or loss of driving licence at 12 months and five years.
Resource use and costs.
We recorded two additional outcomes (not planned at the protocol stage).
Regression of diabetic retinopathy.
Need for further laser treatment after 3 months.
The reason we recorded these additional two outcomes is because progression and regression of diabetic retinopathy, and also need for further laser PRP treatment, all represent the same domain, i.e. disease control. This is an important clinical outcome so we wanted to capture all possible data, and several trials do not report progression but report regression or need for further treatment.
Adverse events
Adverse events reported in the studies at any time including but not limited to: macular oedema, retinal detachment, vitreous haemorrhage, need for vitrectomy surgery, severe visual loss (BCVA < 6/60).
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist conducted systematic searches in the following databases for randomised controlled trials and controlled clinical trials. There were no language or publication year restrictions. The date of the search was 8 June 2017.
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 5) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 8 June 2017) (Appendix 1);
MEDLINE Ovid (1946 to 8 June 2017) (Appendix 2);
Embase Ovid (1980 to 8 June 2017) (Appendix 3);
ISRCTN registry (www.isrctn.com/editAdvancedSearch; searched 8 June 2017) (Appendix 4);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 8 June 2017) (Appendix 5);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 8 June 2017) (Appendix 6).
Searching other resources
We searched the reference lists of potentially includable studies to identify any additional trials. We did not handsearch conference proceedings for this review.
Data collection and analysis
Selection of studies
Two authors independently reviewed the titles and abstracts identified from the electronic and manual searches against the inclusion criteria using web‐based review management software (Covidence 2015). We obtained full‐text copies of all potentially or definitely relevant articles. We contacted trial investigators for further information if required. We resolved discrepancies between authors as to whether or not studies met inclusion criteria by discussion. We documented the excluded studies and the reasons for exclusion.
Data extraction and management
We extracted the following participant and trial characteristics and report them in a table format (Appendix 7).
Participant characteristics (age, sex, glycated haemoglobin (HbA1c), cholesterol, blood pressure, diagnostic criteria used for PDR, baseline visual acuity, OCT‐determined CMT, and areas of ischaemic retinal tissue according to fluorescein angiography).
Intervention (laser agent, laser settings, number of spots delivered, treatment interval and number, retinal target location).
Methodology (group size, randomisation, masking (blinding)).
Outcomes data as specified above.
We contacted trial investigators for key unpublished information that is missing from reports of included studies. Two review authors independently extracted the data, entering data into web‐based review management software (Covidence 2015), and using pre‐piloted data extraction templates. Covidence enabled us to compare discrepancies, which we resolved by discussion. We directly imported data from Covidence into Review Manager 5 (RevMan 5) (Review Manager 2014).
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of the included trials according to Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We considered the following main criteria.
Selection bias: random sequence generation, allocation concealment.
Performance bias: masking of participants, researchers and outcome assessors.
Attrition bias: loss to follow‐up, rates of adherence.
Reporting bias: selective outcome reporting. We reported each parameter as being at high, low, or unclear risk of bias, resolving any discrepancies between the authors by discussion. We contacted study authors to clarify study details relating to any unclear risk of bias. When there was no response from the authors, we classified the trial based on available information.
See Table 9 for additional information on assessment of risk of bias.
1. Risk of bias.
| Item | Low | Unclear | High |
| Sequence generation | Computer generated list, random table, other method of generating random list | Not reported how list was generated. Trial may be described as 'randomised' but with no further details | Alternate allocation, date of birth, records (review authors should exclude these RCTs) |
| Allocation concealment | Central centre (web/telephone access), sealed opaque envelopes | Not reported how allocation administered. Trial may be described as 'randomised' but with no further details | Investigator involved in treatment allocation or treatment allocation clearly not masked |
| Blinding (masking) of participants and personnel | Clearly stated that participants and personnel (apart from doctor) not aware of which lens received | Described as 'double‐masked' with no information on who was masked | No information on masking. As lenses different we will assume that in absence of reporting on this, participants and personnel were not masked |
| Blinding (masking) of outcome assessors | Clearly stated that outcome assessors were masked | Described as 'double‐masked' with no information on who was masked | No information on masking. As lenses different we will assume that in absence of reporting on this, outcome assessors were not masked |
| Incomplete outcome data* | Missing data less than 20% (i.e. more than 80% follow‐up) and equal follow‐up in both groups and no obvious reason why loss to follow‐up should be related to outcome | Follow‐up not reported or missing data > 20% (i.e. follow‐up < 80%) but follow‐up equal in both groups | Follow‐up different in each group and related to outcome |
| Selective outcome reporting | All outcomes in protocol, trials registry entry or both are reported | No access to protocol or trials registry entry | Outcomes in protocol or trials registry entry selectively reported |
We have specified a cut‐point of 20% loss to follow‐up to enable consistent assessment of studies. We considered loss to follow‐up of greater than this to represent a potential risk of attrition bias.
Measures of treatment effect
We measured treatment effect according to the data types described in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). These include the following.
Dichotomous data
Variables in this group included the primary outcome and the proportion of participants experiencing an adverse event during follow‐up. We reported dichotomous variables as risk ratios (RRs) with 95% confidence intervals (CIs).
Continuous data
We reported continuous variables including mean change in visual acuity as mean difference with 95% CIs (if normally distributed) or median and interquartile range (if not normally distributed).
Qualitative data
We reported the types of adverse event, resource use and quality of life data qualitatively as a narrative description of qualitative data.
Unit of analysis issues
Nine of the 10 studies included more than one eye per person. Han 1995 was the only study to include only one eye per person. None of the studies that included one or both eyes adjusted data analysis for within‐person correlation. We used the data as reported by the studies.
Dealing with missing data
We sought key unpublished information that was missing from reports of included studies by contacting study authors but this information was not usually available. We documented when loss to follow‐up was high (over 20%) or imbalanced between treatment groups as potential attrition bias.
Assessment of heterogeneity
We assessed heterogeneity by inspection of the forest plots and by calculating the I² value to assess the proportion of the variance that reflects variation in true effects (Higgins 2003). We considered I² values of greater than 50% to represent substantial inconsistency but also considered the Chi² P value. As this may have low power when there are few studies, we considered P values less than 0.1 to indicate statistical significance of the Chi² test.
Assessment of reporting biases
We were unable to look at reporting biases because there were only 10 studies found and not more than two studies available for each comparison. We considered selective outcome reporting bias as part of the assessment of risk of bias in the individual studies (see Assessment of risk of bias in included studies section).
Data synthesis
Where appropriate, we pooled data using a fixed‐effect model. None of the comparisons and outcomes had more than two trials contributing data.
Subgroup and sensitivity analyses
We were unable to perform planned subgroup and sensitivity analysis as there was not enough information available. See Differences between protocol and review section for details of planned analyses.
'Summary of findings' table
We reported absolute risks and measures of effect in a 'Summary of findings' (SOF) table providing key information concerning the certainty of the evidence, the magnitude of effect of the interventions examined, and the sum of available data on all specified review primary and secondary outcomes for a given comparison. Data was not available in suitable format for adverse events, so we provided a narrative summary within the SOF table.
The 'Summary of findings' table included the following key outcomes.
Proportion of people who lose 15 or more ETDRS letters (equivalent to 3 ETDRS lines) as measured on a LogMAR chart from baseline at one and five years.
Proportion of people who gain 15 or more ETDRS letters (equivalent to 3 ETDRS lines) as measured on a LogMAR chart from baseline at one and five years.
Progression of PDR from baseline at one and five years as defined by trial investigators, including OCT mean central subfield thickness (CMT) where measured.
Regression of PDR from baseline at one and five years as defined by trial investigators (new outcome).
Adverse events at any time such as: macular oedema, retinal detachment, vitreous haemorrhage, need for vitrectomy surgery, severe visual loss (BCVA < 6/60).
PROM: significant pain during the laser procedure.
Vision‐related quality of life (QoL) measure using any validated questionnaire at one and five years compared to baseline.
Two review authors independently used the GRADE approach to assess the certainty of the evidence in the included studies using GRADEpro GDT software (GRADEpro 2014). We resolved discrepancies by discussion.
We planned to calculate the assumed risk from the median risk in the comparator group of the included studies, but in the event there were not more than two or three studies per comparison so we used the pooled event risk in the comparator group.
Results
Description of studies
Results of the search
The electronic searches yielded a total of 4940 records (Figure 1). The Cochrane Information Specialist scanned the search results, removed 1472 duplicates and then removed 2977 references which were irrelevant to the scope of the review. We screened the remaining 491 reports and obtained 88 full‐text reports for further assessment. We included 13 reports of 11 studies (see Characteristics of included studies), and excluded 69 reports of 52 studies (see Characteristics of excluded studies). We did not identify any ongoing studies from our searches of the clinical trials registries. We have 6 studies awaiting classification for which we were unable to identify a full text report (Chaine 1986, Kianersi 2016; Wroblewski 1991) or were unable to obtain a translation (Uehara 1993, Yang 2010) or for which the full text did not provide enough information to judge inclusion (Salman 2011).
1.
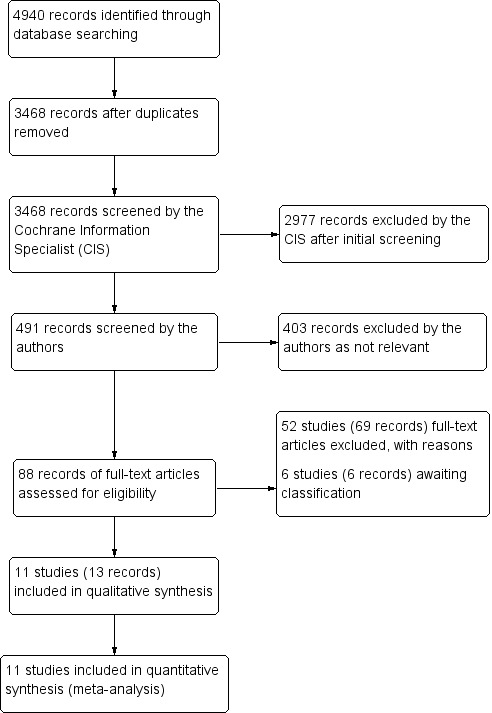
Study flow diagram.
Included studies
Types of study
We included a total of 11 studies in the review, all of which were randomised controlled trials. These studies were conducted in the US (2), Italy (4), South Korea (1), India (1), Iran (1), Slovenia (1), and Greece (1). There was generally poor recording of the sponsorship source, but two studies declared public funding (Blankenship 1988; Wade 1990.)
Participants
All studies in the review included both male and female adult participants with a clinical diagnosis of type 1 or type 2 diabetes mellitus, between the ages of 18 to 79 years, although age range of participants was not always reported. One or both eyes of each participant were required to have high risk proliferative diabetic retinopathy based on the ETDRS definition, Han 1995 being the only study to include only one eye per person. None of the studies that included one or both eyes adjusted the data analysis for within‐person correlation. There was one within‐person study (Tewari 2000), again with no appropriate, matched, analysis. Across all included studies the baseline mean age ranged from 40 to 58 years, and baseline mean visual acuity ranged from 0.12 to 0.89 LogMAR acuity. The size of studies varied from 20 to 270 eyes.
Interventions
All participants included in the review were treated with an alternative laser PRP strategy compared with standard argon laser PRP (defined as midperipheral scatter, panretinal photocoagulation with 0.1 second pulse duration of moderate laser intensity). We included a variety of alternative laser PRP interventions which included: double‐frequency Nd:YAG laser (532 nm) (Bandello 1996)(Brancato 1991); diode laser (810 nm) (Bandello 1993; Han 1995; Tewari 2000); longer exposure time of 0.5 second argon laser burn (Wade 1990); 'light intensity' lower energy treatment with standard argon laser pulse (Bandello 2001); 'mild scatter' argon laser pattern limited to only 400 to 600 laser burns in one sitting (Pahor 1998); 'central PRP' which compared a more central (mean number of 437 laser burns placed more posteriorly with sparing of a 2 DD area centred on the fovea and papillomacular bundle) versus a more standard 'peripheral' PRP (mean number of 441 laser burns placed more peripherally, anterior to the equator extending to the ora serrata when possible) treatment in addition to mid‐peripheral PRP (Blankenship 1988); 'central sparing' argon laser PRP distribution which stopped 3 DD from the upper temporal and lower margin of the fovea (Theodossiadis 1990); and an 'extended targeted' argon laser PRP to include the entire retina anterior to the equator and any capillary non‐perfusion areas between the vascular arcades and equator, including 1 DD beyond the ischaemic areas (Nikkhah 2017). See more details in the Characteristics of included studies table.
Outcomes
All studies except two studies (Bandello 1993; Tewari 2000) measured and reported our primary outcome of loss or gain of at least 15 ETDRS letters (equivalent to 3 ETDRS lines) as measured on a LogMAR chart. If the follow‐up was not recorded at the one‐ and five‐year time point we used the final time point provided.
Approximately half of the studies provided some measure of regression or progression of PDR. Visual field loss was only reported in one study and pain during laser treatment was reported in five studies.
No study recorded near visual acuity, or patient‐relevant outcomes such as loss of driving licence or vision‐related quality of life. No study discussed resource use and costs. Follow‐up time ranged from one month to two years.
Excluded studies
Fifty‐two studies were excluded after full text screening. Reasons for exclusion were as follows: intervention, i.e. evaluating a laser that is not currently available (n = 16); comparator, i.e. not compared with standard argon laser PRP (n = 15); study design, i.e. not randomised controlled trial (n = 12); outcome, i.e. study did not measure relevant outcomes (n =3); patient population, i.e. patients did not have PDR (n = 3), comparisons not pre‐specified by this review (n = 3). See Characteristics of excluded studies table for the list of exclusions with reasons.
Risk of bias in included studies
2.
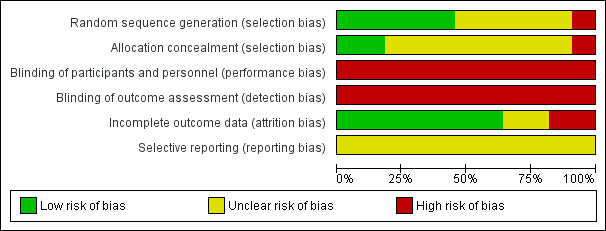
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
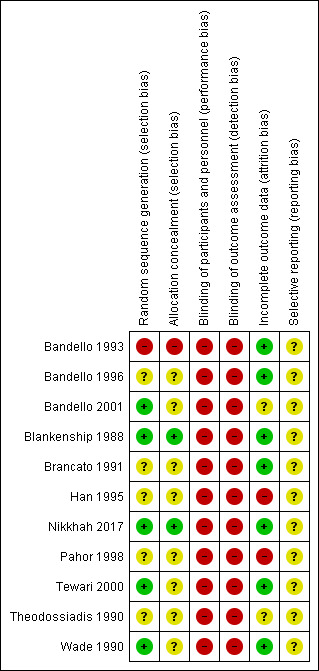
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Five studies reported an adequate method of random sequence generation: Bandello 2001 and Nikkhah 2017 used computer‐generated random numbers; Blankenship 1988, Tewari 2000 and Wade 1990 flipped a coin. In the remaining studies it was not possible to judge whether random sequence generation had been done properly. Bandello 1993 may have used alternate allocation.
Two studies reported allocation concealment. In Blankenship 1988 allocation was done after the participants were recruited. Nikkhah 2017 reported that the allocation sequence was kept concealed from the investigators.
Blinding
None of the studies masked participants, personnel or outcomes assessors so they were judged at high risk of bias for these domains.
Incomplete outcome data
Most studies (n = 7) had low risk of attrition bias.
In Han 1995 the number of participants randomised matched the number of participants analysed but the loss to follow‐up was not clearly reported. In Han’s exclusion criteria there was indication that people with adverse events were excluded after treatment but it was not reported how many people were excluded in this way.
In Pahor 1998 attrition was high (38%) after a follow‐up of one month after treatment, and it was not reported to which groups the loss to follow‐up occurred.
In a further two studies, not enough information was given to judge this (Bandello 2001; Theodossiadis 1990).
Selective reporting
We did not have access to trial protocols as the studies were conducted so long ago (Nikkhah 2017 was the only study on a clinical trial registry) so we were unable to judge whether or not selective reporting was likely to be a problem.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8
Summary of findings for the main comparison. Nd:YAG laser compared to argon‐green laser for proliferative diabetic retinopathy.
| Nd:YAG laser compared to argon‐green laser for proliferative diabetic retinopathy | ||||||
| Patient or population: people with proliferative diabetic retinopathy Setting: eye hospital Intervention: Nd:YAG laser Comparison: argon‐green laser | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with argon‐green laser | Risk with Nd:YAG laser | |||||
| BCVA: loss of 15 or more ETDRS letters follow‐up: 1 year | Study population | RR 0.80 (0.30 to 2.13) | 20 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | ||
| 500 per 1000 | 400 per 1000 (150 to 1000) | |||||
| BCVA: gain of 15 or more ETDRS letters | Study population | RR 0.33 (0.02 to 7.32) | 20 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | ||
| 100 per 1000 | 33 per 1000 (2 to 732) | |||||
| Progression of PDR follow‐up: 1 year | Study population | RR 1.00 (0.07 to 14.95) | 42 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 4 | ||
| 48 per 1000 | 48 per 1000 (3 to 712) | |||||
| Regression of PDR follow‐up: 1 year | Study population | RR 1.00 (0.87 to 1.14) | 42 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 5 | ||
| 952 per 1000 | 952 per 1000 (829 to 1000) | |||||
| Pain during laser treatment | Study population | RR 1.00 (0.36 to 2.76) |
62 (2 RCTs) |
⊕⊝⊝⊝ VERY LOW 1 6 | ||
| 190 per 1000 | 190 per 1000 (69 to 524) | |||||
| Vision‐related QoL ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse events | Vitreous haemorrhage, 13% of argon group, RR 1.22 (0.38 to 3.94); choroidal detachment, 19% of argon group, RR 0.23 (0.04 to 1.27); neurotrophic keratopathy, 10% of argon group, RR 1.29 (0.35 to 4.75). | 62 (2 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 7 | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High‐certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low‐certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded for risk of bias (−1): high risk of performance and detection bias.
2 Downgraded for imprecision (−1): small study, wide confidence intervals
3Downgraded for indirectness (−1): outcome was loss 2 or more lines of Snellen at 6 months
4 Downgraded for indirectness (−1): outcome was not clearly defined — "PDR worsened" — and was reported at 29 months
5 Downgraded for indirectness (−1): outcome was not clearly defined — "PDR improved" — and was reported at 29 months
6 Downgraded for imprecision (−2): small study, few events
7 Downgraded for inconsistency (−1): there was some inconsistency between studies
Summary of findings 2. Diode laser compared to argon laser for proliferative diabetic retinopathy.
| Diode laser compared to argon laser for proliferative diabetic retinopathy | ||||||
| Patient or population: people with proliferative diabetic retinopathy Setting: eye hospital Intervention: diode laser Comparison: argon laser | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with argon | Risk with Diode | |||||
| BCVA: loss of 15 or more ETDRS letters follow‐up: 1 year | Study population | RR 0.95 (0.66 to 1.36) |
134 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | ||
| 345 per 1000 | 379 per 1000 (231 to 628) | |||||
| BCVA: gain of 15 or more ETDRS letters follow‐up: 1 year | Study population | RR 0.60 (0.25 to 1.45) |
134 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 3 | ||
| 190 per 1000 | 119 per 1000 (47 to 302) | |||||
| Progression of PDR follow‐up: 1 year | Study population | RR 0.90 (0.41 to 2.00) |
66 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 4 | ||
| 286 per 1000 | 257 per 1000 (117 to 571) | |||||
| Regression of PDR follow‐up: 1 year | Study population | RR 0.75 (0.35 to 1.60) | 66 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 4 | ||
| 343 per 1000 | 257 per 1000 (120 to 549) | |||||
| Pain during laser treatment | 211 per 1000 | 688 per 1000 (428 to 1000) |
RR 3.07 (2.15 to 4.39) | 228 (4 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | |
| Vision‐related QoL ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse events | Vitreous haemorrhage, 15% of argon group. inconsistent results between two studies, RR 0.50 (0.05, 4.86) and RR 1.82 (0.76, 4.35); choroidal detachment, 8% of argon group RR 4.00 (0.51, 31.13; neurotrophic keratopathy, 0% of argon group, RR 3.00 (0.13, 67.51), maculopathy, 14% of argon group, RR 1.30 (0.54, 3.13), cataract, 16% of argon group, RR 0.52 (0.17, 1.57), pre‐retinal membrane, 5% of argon group, RR 1.16 (0.24, 5.49). | 134 (2 studies) | ⊕⊝⊝⊝ VERY LOW 1 5 | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High‐certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low‐certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded for risk of bias (−1): high risk of performance, detection and attrition bias
2 Downgraded for indirectness (−1): outcome was not clearly defined — "worsened" visual acuity and "improved" visual acuity
3 Downgraded for imprecision (−1): wide confidence interval
4 Downgraded for indirectness (−1): outcome was not clearly defined — "worsened" or "improved" neovascularisation
5 Downgraded for imprecision (−2): very wide confidence interval
Summary of findings 3. 0.5 compared to 0.1 second exposure for proliferative diabetic retinopathy.
| 0.5 compared to 0.1 second exposure for proliferative diabetic retinopathy | ||||||
| Patient or population: proliferative diabetic retinopathy Setting: eye hospital Intervention: 0.5 second exposure Comparison: 0.1 second exposure | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with 0.1 second exposure | Risk with 0.5 second exposure | |||||
| BCVA: loss of 15 or more EDTRS letters ‐ 1 year | Study population | RR 0.42 (0.08 to 2.04 |
44 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 200 per 1000 | 84 per 1000 (16 to 408) | |||||
| BCVA: gain of 15 or more EDTRS letters ‐ 1 year | Study population | RR 2.22 (0.68 to 7.28) |
44 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 150 per 1000 | 333 per 1000 (102 to 1000) | |||||
| Progression of PDR ‐ 1 year | Study population | RR 0.33 (0.02 to 7.14) |
16 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 | ||
| 125 per 1000 | 41 per 1000 (3 to 893) | |||||
| Regression of PDR ‐ 1 year | Study population | RR 1.17 (0.92 to 1.48) | 32 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 857 per 1000 | 1000 per 1000 (789 to 1000) | |||||
| Pain during laser treatment ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse events | Pre‐retinal or vitreous haemorrhage, 30% of 0.1 sec group, (RR 0.56 (0.18 to 1.70)); macular thickening, 2 cases in 0.1 sec group; combined rhegmatous and traction retinal detachment, 1 case in 0.5 sec group. | ‐ | 44 (1 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 3 | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High‐certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low‐certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded for risk of bias (−1): high risk of performance and detection bias
2 Downgraded for Imprecision (−1): wide confidence interval
3Downgraded for Imprecision (−2): small study, very wide confidence interval
Summary of findings 4. Light PRP compared to classic PRP for proliferative diabetic retinopathy.
| Light PRP compared to classic PRP for proliferative diabetic retinopathy | ||||||
| Patient or population: proliferative diabetic retinopathy Setting: eye hospital Intervention: light PRP Comparison: classic PRP | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with classic | Risk with Light | |||||
| BCVA: loss of 15 letters or more ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Mean difference in logMAR acuity at 1 year was −0.09, 95% CI −0.22 to 0.04; participants = 65; studies = 1. |
| BCVA: gain of 15 letters or more ‐ not reported | ||||||
| Progression of DR ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Regression of PDR ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Pain during laser treatment | Study population | RR 0.23 (0.03 to 1.93) | 65 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 129 per 1000 | 30 per 1000 (4 to 249) | |||||
| Adverse events | Vitreous haemorrhage, 19% in classic group, RR 0.07 (0.00 to 1.20); choroidal detachment 3 cases in classic group; neurotrophic keratopathy, 2 cases in classic group; clinically significant macular oedema, 23% of classic group, RR 0.13 (0.02 to 1.00). | ‐ | 65 (1 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 3 | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High‐certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low‐certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded for imprecision (−1): wide confidence interval
2 Downgraded for risk of bias (−1): high risk of performance and detection bias
3 Downgraded for imprecision (−2): few number of events
Summary of findings 5. Mild scatter PRP compared to full scatter PRP for proliferative diabetic retinopathy.
| Mild scatter PRP compared to full scatter PRP for proliferative diabetic retinopathy | ||||||
| Patient or population: proliferative diabetic retinopathy Setting: eye hospital Intervention: mild scatter PRP Comparison: full scatter PRP | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Full scatter PRP | Risk with Mild scatter PRP | |||||
| BCVA: loss of 15 or more ETDRS letters ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | Mean Snellen decimal acuity was similar in the two groups at 3 months. Mild scatter 0.93 (SD 0.11), full scatter 0.89 (SD 0.19). |
| BCVA: gain of 15 or more ETDRS letters ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Progression if PDR ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Regression of PDR ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Pain during laser treatment ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Vision‐related QoL ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High‐certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low‐certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
Summary of findings 6. Central PRP compared to peripheral PRP for proliferative diabetic retinopathy.
| Central compared to peripheral for proliferative diabetic retinopathy | ||||||
| Patient or population: proliferative diabetic retinopathy Setting: eye hospital Intervention: central Comparison: peripheral | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with peripheral | Risk with Central | |||||
| BCVA: loss of 15 or more ETDRS letters ‐ 1 year | Study population | RR 3.00 (0.67 to 13.46) | 50 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 80 per 1000 | 240 per 1000 (54 to 1000) | |||||
| BCVA: gain of 15 or more ETDRS letters ‐ 1 year | Study population | RR 0.25 (0.03 to 2.08) | 50 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 160 per 1000 | 40 per 1000 (5 to 333) | |||||
| Progression of DR ‐ not reported | ‐ | |||||
| Regression of PDR ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Pain during laser treatment ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Vision‐related QoL ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse events | Vitreous haemorrhage requiring additional PRP, 1 case in each group; macular traction detachment requiring pars plana vitrectomy, 3 cases in central group, 1 case in peripheral group; macular thickening associated with loss of 2 or more lines of visual acuity, 2 cases in each group. | ‐ | 50 (1 RCTs) |
⊕⊝⊝⊝ VERY LOW 1 3 | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High‐certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low‐certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Dowgraded for risk of bias (−1): high risk of performance and detection bias
2 Downgraded for imprecision (−1): wide confidence interval
3 Downgraded for imprecision (−2): few number of events
Summary of findings 7. Centre sparing PRP compared to full scatter PRP for proliferative diabetic retinopathy.
| Centre sparing PRP compared to full scatter PRP for proliferative diabetic retinopathy | ||||||
| Patient or population: proliferative diabetic retinopathy Setting: eye hospital Intervention: centre sparing PRP Comparison: full scatter PRP | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with full scatter PRP | Risk with Centre sparing | |||||
| BCVA: loss of 15 or more ETDRS letters follow‐up: 1 year | Study population | RR 0.67 (0.30 to 1.50) | 53 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 385 per 1000 | 258 per 1000 (115 to 577) | |||||
| BCVA: gain of 15 or more ETDRS letters follow‐up: 1 year ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Progression of DR ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Regression of PDR follow up: 1 year | Study population | RR 0.96 (0.73 to 1.27) | 53 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 808 per 1000 | 775 per 1000 (590 to 1000) | |||||
| Pain during laser treatment ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Vision‐related QoL ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High‐certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low‐certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded for risk of bias (−1): high risk of performance and detection bias
2 Downgraded for imprecision (−1): wide confidence intervals
Summary of findings 8. Extended targeted PRP compared to standard PRP.
| Extended targeted PRP compared with standard PRP for proliferative diabetic retinopathy | ||||||
|
Patient or population: people with diabetic retinopathy Settings: eye hospital Intervention: extended targeted PRP Comparison: standard PRP | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| standard PRP | extended targeted PRP | |||||
| BCVA: loss of 15 or more ETDRS letters: follow‐up 1 year | Study population | RR 0.94 (0.70 to 1.28) |
270 (1 RCT) | ⊕⊕⊝⊝ LOW1 2 | Mean difference in BCVA at 1 year was: 0.00 logMAR, (−0.05 to 0.05) | |
| 393 per 1000 | 369 per 1000 (275 to 503) | |||||
| BCVA: gain of 15 or more ETDRS letters: follow‐up 1 year, not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Progression of DR ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Regression of PDR follow‐up 1 year |
Study population | RR 1.11 (0.95 to 1.31) |
270 (1 RCT) |
⊕⊕⊝⊝ LOW1 2 | ||
| 644 per 1000 | 715 per 1000 (612 to 844) | |||||
| Pain during laser treatment ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Vision‐related QoL ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Adverse events | Quote: "None of the eyes developed tractional retinal detachment during the study. Additionally, no ocular or non‐ocular AEs related to the study intervention were detected by the investigators or reported by patients" | ⊕⊝⊝⊝ VERY LOW 3 | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; AE: adverse events | ||||||
| GRADE Working Group grades of evidence High‐certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate‐certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low‐certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low‐certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded for risk of bias (−1): high risk of performance and detection bias
2 Downgraded for imprecision (−1): wide confidence intervals
3 Downgraded for imprecision (−3): study was underpowered to detect rare adverse effects.
Nd:YAG (532 nm) laser PRP versus argon (514 nm) laser PRP
Two studies investigated this comparison (Bandello 1996; Brancato 1991). Bandello 1996 enrolled 42 eyes (33 participants) with PDR and followed up for 29 months. Brancato 1991 enrolled 20 eyes with PDR (16 people with NVD/NVE > 1/2 DA or associated with haemorrhage) and followed up for 6 months.
There was very low‐certainty evidence for all outcomes (Table 1).
People treated with Nd:YAG laser PRP were less likely to lose 15 or more letters of BCVA compared with argon laser PRP (risk ratio (RR) 0.80, 95% confidence interval (CI) 0.30 to 2.13; participants = 20; studies = 1) (Analysis 1.1).
People treated with Nd:YAG laser PRP were less likely to gain 15 or more letters BCVA compared with argon laser (RR 0.33, 95% CI 0.02 to 7.32; participants = 20; studies = 1; I² = 0%) (Analysis 1.2).
Both studies reported change in BCVA as decimal Snellen acuity which meant that it was not possible to provide a pooled analysis. There was little evidence of any important difference between the two groups (Analysis 1.3).
There was a similar risk of progression and regression of PDR in the two groups (RR progression 1.00, 95% CI 0.07 to 14.95 (Analysis 1.4); and RR regression 1.00, 95% CI 0.87 to 1.14 (Analysis 1.5) respectively).
Similar proportions of people reported pain during laser treatment (RR 1.00, 95% CI 0.36 to 2.76; participants = 62; studies = 2) (Analysis 1.6).
1.1. Analysis.
Comparison 1 Nd:YAG laser vs argon laser, Outcome 1 BCVA: loss of 15 or more ETDRS letters.
1.2. Analysis.
Comparison 1 Nd:YAG laser vs argon laser, Outcome 2 BCVA: gain of 15 or more ETDRS letters.
1.3. Analysis.
Comparison 1 Nd:YAG laser vs argon laser, Outcome 3 Change in BCVA.
| Change in BCVA | ||||
|---|---|---|---|---|
| Study | Nd: YAG: mean decimal Snellen acuity at follow‐up (SD) | N | Argon: mean decimal Snellen acuity at follow‐up (SD) | N |
| Bandello 1996 | 0.5 (0.25) | 21 | 0.45 (0.27) | 21 |
| Brancato 1991 | 0.5 (0.25) | 10 | 0.5 (0.25) | 10 |
1.4. Analysis.
Comparison 1 Nd:YAG laser vs argon laser, Outcome 4 Progression of PDR.
1.5. Analysis.
Comparison 1 Nd:YAG laser vs argon laser, Outcome 5 Regression of PDR.
1.6. Analysis.
Comparison 1 Nd:YAG laser vs argon laser, Outcome 6 Pain during laser treatment.
Other relevant outcomes such as NVA, VF loss, vision‐related QoL measure, details of any resource use and costs and need for further laser PRP treatment after three months were not reported.
Adverse events are set out in the following table. There were inconsistent results for vitreous haemorrhage and neurotrophic keratopathy. Choroidal detachment occurred less frequently in the YAG laser group but the estimates were imprecise and did not exclude no difference.
| Adverse event | Study | Nd:YAG n/N (%) |
Argon n/N (%) |
RR (95% CI) | Pooled RR (95% CI) |
| Vitreous haemorrhage | Bandello 1996 | 5/21 (24%) | 2/21 (10%) | 2.50 (0.54, 11.48) | 1.22 (0.38 to 3.94) I² = 57%) |
| Brancato 1991 | 0/10 (0%) | 2/10 (20%) | 0.20 (0.01, 3.70) | ||
| Choroidal detachment | Bandello 1996 | 1/21 (5%) | 5/21(24%) | 0.20 (0.03, 1.57) | 0.23 (0.04 to 1.27) I² = 0%) |
| Brancato 1991 | 0/10 (0%) | 1/10 (10%) | 0.33 (0.02, 7.32) | ||
| Neurotrophic keratopathy | Bandello 1996 | 3/21 (14%) | 3/21 (14%) | 1.00 (0.23, 4.40) | 1.29 (0.35 to 4.75) I² = 0%) |
| Brancato 1991 | 1/10 (10%) | 0/10 (0%) | 3.00 (0.14, 65.90) |
We graded the evidence for this comparison as very low‐certainty for all outcomes (Table 1). We downgraded 1 level for high risk of performance and detection bias as the studies were not masked; 1 level for imprecision as the studies were small and estimates of effect imprecise; and 1 level for indirectness as the outcomes were not reported at our pre‐specified time points and were not clearly defined.
Diode (810 nm) versus argon (514 nm) laser PRP
Three studies investigated this comparison (Bandello 1993; Han 1995; Tewari 2000). Han 1995 enrolled 108 eyes (108 people) with PDR and followed up for between 13 to 15 months. Bandello 1993 enrolled 34 people (44 eyes) with PDR and followed up for 2 years (on average). Tewari 2000 was a within‐person study of 22 people (44 eyes) with follow‐up of 6 months.
There was very low‐certainty evidence for the following outcomes (Table 2).
People treated with diode laser PRP had similar or slightly increased risk of "worsened" vision compared with argon green laser PRP (1.10, 95% CI 0.67 to 1.82; eyes = 108; studies = 1) (Analysis 2.1).
People treated with diode laser PRP were less likely to have "improved" vision compared with argon laser PRP (RR 0.63, 95% CI 0.25 to 1.59; eyes = 108; studies = 1) (Analysis 2.2).
Mean Snellen acuity was similar in both groups (Analysis 2.3).
People treated with diode laser PRP had a similar or slightly lower risk of progression of PDR compared with argon laser PRP (RR 0.90, 95% CI 0.41 to 2.00) (Analysis 2.4).
People treated with diode laser PRP were less likely to have regression of PDR compared with argon laser PRP (RR 0.75, 95% CI 0.35 to 1.60) (Analysis 2.5).
2.1. Analysis.
Comparison 2 Diode versus argon laser, Outcome 1 BCVA: loss of 15 or more ETDRS letters.
2.2. Analysis.
Comparison 2 Diode versus argon laser, Outcome 2 BCVA: gain of 15 or more ETDRS letters.
2.3. Analysis.
Comparison 2 Diode versus argon laser, Outcome 3 Change in BCVA.
| Change in BCVA | |||||
|---|---|---|---|---|---|
| Study | Follow‐up | Diode: mean Snellen decimal acuity at follow‐up (SD) | N | Argon: mean Snellen decimal acuity at follow‐up (SD) | N |
| Bandello 1993 | Average of approximately 2 years (24 months) | 0.4 (0.3) | 22 | 0.4 (0.3) | 22 |
| Tewari 2000 | 6 months | 3,62 (2.12) reciprocal of Snellen decimal acuity | 25 | 4.76 (2.83) reciprocal of Snellen decimal acuity | 25 |
2.4. Analysis.
Comparison 2 Diode versus argon laser, Outcome 4 Progression of PDR.
2.5. Analysis.
Comparison 2 Diode versus argon laser, Outcome 5 Regression of PDR.
There was moderate‐certainty evidence that diode laser was more painful (RR 3.12, 95% CI 2.16 to 4.51; participants = 202; studies = 3; I2 = 0%) Analysis 2.6.
2.6. Analysis.
Comparison 2 Diode versus argon laser, Outcome 6 Pain during laser treatment.
In the Han 1995 paper only the number of people (%) with "improved", "unchanged" or "worsened" visual acuity were provided; but there is no numerical definition for each of these and it was unclear which charts were used for visual acuity measurement.
Other relevant outcomes such as NVA, VF loss, pain during laser treatment, vision‐related QoL measure, details of any resource use and costs, and need for further laser PRP treatment after three months were not reported.
Adverse events are summarised in the following table and Analysis 2.7.
2.7. Analysis.
Comparison 2 Diode versus argon laser, Outcome 7 Adverse effects.
| Adverse event | Study | Diode n/N (%) |
Argon n/N (%) |
RR (95% CI) | Pooled RR (95% CI) |
| Vitreous haemorrhage | Bandello 1993 | 7/22 (32%) | 4/22 (18%) | 1.75 [0.60, 5.14] | 1.80 (0.91 to 3.53) |
| Han 1995 | 11/50 (22%) | 7/58 (12%) | 1.82 (0.76, 4.35) | ||
| Tewari 2000 | NR | NR | |||
| Choroidal detachment | Bandello 1993 | 4/22 (18%) | 1/22 (5%) | 4.00 [0.48, 33.00] | NA |
| Han 1995 | NR | NR | |||
| Tewari 2000 | NR | NR | |||
| Neurotrophic keratopathy | Bandello 1993 | 1/22 (5%) | 0/22 (0%) | 3.00 [0.13, 69.87] | NA |
| Han 1995 | NR | NR | |||
| Tewari 2000 | NR | NR | |||
| Maculopathy | Bandello 1993 | NR | NR | NA | |
| Brancato 1990 | NR | NR | |||
| Han 1995 | 9/50 (18%) | 8/58 (14%) | 1.30 (0.54, 3.13) | ||
| Tewari 2000 | NR | NR | |||
| Cataract | Bandello 1993 | NR | NR | NA | |
| Brancato 1990 | NR | NR | |||
| Han 1995 | 4/50 (8%) | 9/58 (16%) | 0.52 (0.17, 1.57) | ||
| Tewari 2000 | NR | NR | |||
| Pre‐retinal membrane | Bandello 1993 | NR | NR | NA | |
| Brancato 1990 | NR | NR | |||
| Han 1995 | 3/50 (6%) | 3/58 (5%) | 1.16 (0.24, 5.49) | ||
| Tewari 2000 | NR | NR |
NR = not reported; NA = not applicable.
We graded the evidence for this comparison as very low‐certainty for all outcomes (apart from pain) (Table 2). We downgraded all outcomes recorded in the studies by 1 level for high risk of performance and detection bias as it was unclear if the studies were masked and no details of randomisation were provided; and 1 level for imprecision due to wide confidence interval. The outcomes of BCVA and DR progression/regression were also downgraded by 1 level for indirectness as the outcomes were not clearly defined (both studies only stated "worsened" visual acuity, "worsened" or "improved" neovascularisation).
0.5‐second versus 0.1‐second duration of exposure of argon (514 nm) laser PRP
One study investigated this comparison (Wade 1990). Wade 1990 enrolled 50 eyes (41 participants) with high‐risk PDR (DRS Research Group 1978 criteria) and followed up for 6 months.
Low‐certainty and very low‐certainty evidence was available (Table 3).
Low‐certainty evidence that people treated with 0.5‐second laser PRP were less likely to have a loss of 15 or more letters of BVCA compared with 0.1‐second laser PRP. (RR 0.42, 95% CI 0.08 to 2.04) (Analysis 3.1).
Low‐certainty evidence that people treated with 0.5‐second laser PRP were more likely to have a gain of 15 or more letters of BVCA compared with 0.1‐second laser PRP (RR 2.22, 95% CI 0.68 to 7.28) (Analysis 3.2).
Very low‐certainty evidence of progression of PDR between the 0.5‐second group compared with standard 0.1‐second laser spot duration (RR 0.33, 95% CI 0.02 to 7.14) (Analysis 3.3).
Low‐certainty evidence that people treated with 0.5‐second laser PRP were less likely to have regression of PDR compared with 0.1‐second laser PRP (RR 1.17, 95% CI 0.92 to 1.48) (Analysis 3.4).
3.1. Analysis.
Comparison 3 0.5 versus 0.1 second exposure, Outcome 1 BCVA: loss of 15 or more EDTRS letters.
3.2. Analysis.
Comparison 3 0.5 versus 0.1 second exposure, Outcome 2 BCVA: gain of 15 or more EDTRS letters.
3.3. Analysis.
Comparison 3 0.5 versus 0.1 second exposure, Outcome 3 Progression of PDR.
3.4. Analysis.
Comparison 3 0.5 versus 0.1 second exposure, Outcome 4 Regression of PDR.
Other relevant outcomes such as NVA, VF loss, pain during laser treatment, vision‐related QoL measure, details of any resource use and costs were not reported.
Adverse events are summarised in the following table.
| Adverse event | 0.5 sec n/N (%) |
0.1 sec n/N (%) |
RR (95% CI) |
| Pre‐retinal or vitreous haemorrhage | 4/24 (17%) | 6/20 (30%) | 0.56 (0.18 to 1.70) |
| Macular thickening | 0/24 (0%) | 2/20 (10%) | 0.17 (0.01 to 3.31) |
| Combined rhegmatous and traction retinal detachment requiring pars plana vitrectomy | 1/24 (4%) | 0/20 (0%) | 2.52 (0.11 to 58.67) |
We graded the evidence for this comparison as low‐certainty for the outcomes of 'BCVA: loss of 15 or more ETDR letters' and 'regression of PDR' Table 3. We downgraded 1 level for high risk of imprecision due to wide confidence intervals, and downgraded 1 level for high risk of performance and detection bias. For the outcome of 'Progression of PDR' we downgraded an additional 1 level due to the very wide confidence interval.
'Light laser' intensity PRP versus 'classic' argon (514 nm) laser PRP
One study investigated this comparison (Bandello 2001). Bandello 2001 enrolled 65 eyes (50 people) with high‐risk PDR (DRS Research Group 1978 criteria) and followed up for an average of 22 months. Treatment included 'light intensity' lower energy argon laser PRP treatment to achieve a very light grey biomicroscopic effect on the retina versus 'classic' argon laser PRP to achieve an opaque, dusky, grey‐white, off‐white standard burn.
There was no difference in the change in BCVA between the light laser PRP and the classic laser PRP group (MD −0.09, 95% CI −0.22 to 0.04) (Analysis 4.1).
4.1. Analysis.
Comparison 4 Light versus classic, Outcome 1 Change in BCVA.
There was low‐certainty evidence that fewer people had pain during laser treatment in the light laser PRP compared with classic laser PRP group (RR 0.23, 95% CI 0.03 to 1.93) (Analysis 4.2) (Table 4).
4.2. Analysis.
Comparison 4 Light versus classic, Outcome 2 Pain during laser treatment.
Other relevant outcomes such as BCVA loss or gain of 15 or more letters, NVA, VF loss, vision‐related QoL measure, details of any resource use and costs and need for further laser PRP treatment after 3 months were not reported.
| Adverse event | Light n/N (%) |
Classic n/N (%) |
RR (95% CI) |
| Vitreous haemorrhage | 0/34 (0%) | 6/31 (19%) | 0.07 (0.00 to 1.20) |
| Choroidal detachment | 0/34 (0%) | 3/31 (10%) | 0.13, (0.01 to 2.43) |
| Neurotrophic keratopathy | 0/34 (0%) | 2/31 (6%) | 0.18 (0.01 to 3.67) |
| Clinically significant macular oedema | 1/34 (3%) | 7/31 ( 23%) | 0.13 (0.02 to 1.00) |
We graded the evidence for this comparison as low‐certainty for the outcomes of 'pain during laser treatment' (Table 4). We downgraded 1 level for high risk of imprecision due to wide confidence intervals, and downgraded 1 level for high risk of performance and detection bias.
'Mild scatter' versus 'full scatter' argon (514 nm) laser PRP
One study investigated this comparison (Pahor 1998). Pahor 1998 enrolled 40 eyes (32 people) with early PDR and followed up for one month. Treatment included 'mild scatter' argon laser PRP with pattern limited to 400 to 600 laser burns (500 μm, 0.1 sec) over one session versus 'full scatter' argon laser PRP with 1200 to 1600 laser burns (500 μm, 0.1 sec) over two sessions, two weeks apart.
Results are as follows.
There was no difference in the change in BCVA between the 'full scatter' PRP compared with the 'mild scatter' PRP group (MD 0.04, 95% CI −0.06 to 0.14) (Analysis 5.1).
Very low‐certainty evidence that people treated with 'full scatter' PRP were more likely to have visual field loss compared with the 'mild scatter' PRP group (MD −2.50, 95% CI −4.22 to −0.78) (Analysis 5.2) (Table 5).
5.1. Analysis.
Comparison 5 Mild scatter PRP vs full scatter PRP, Outcome 1 Change in BCVA.
| Change in BCVA | ||||
|---|---|---|---|---|
| Study | Mild scatter: mean Snellen decimal acuity (SD) | N | Full scatter: mean Snellen decimal acuity (SD) | N |
| Pahor 1998 | 0.93 (0.11) | 19 | 0.89 (0.19) | 21 |
5.2. Analysis.
Comparison 5 Mild scatter PRP vs full scatter PRP, Outcome 2 Visual field (mean deviation).
Other relevant outcomes such as BCVA loss or gain of 15 or more letters, NVA, progression or regression of DR, pain during laser treatment, vision‐related QoL measure, details of any resource use and costs and need for further laser PRP treatment after three months were not reported.
The authors made no comment regarding adverse events in this study.
We graded the evidence for this comparison as low‐certainty for the outcomes of 'Visual field loss at 1‐year follow‐up' Table 5. We downgraded 1 level for high risk of imprecision due to small study size and upper confidence interval close to 0 (null effect); and downgraded 1 level for high risk of performance, detection and attrition bias.
'Central' PRP versus 'peripheral' argon (514 nm) laser PRP
One study investigated this comparison (Blankenship 1988). Blankenship 1988 enrolled 50 eyes (40 participants) with high‐risk PDR (DRS Research Group 1978 criteria) and followed up for 6 months. This study compared more central PRP (mean number of 437 laser burns placed more posteriorly with sparing of a 2 DD area centred on the fovea and papillomacular bundle) versus a more standard 'peripheral' PRP (mean number of 441 laser burns placed more peripherally, anterior to the equator extending to the ora serrata when possible) treatment in addition to mid‐peripheral PRP.
The results were as follows.
Low‐certainty evidence that people treated with central PRP were more likely to lose 15 or more letters of BCVA compared with peripheral laser PRP (RR 3.00, 95% CI 0.67 to 13.46) (Analysis 6.1) (Table 6).
Low‐certainty evidence that people treated with central PRP were less likely to gain 15 or more letters of BCVA compared with peripheral laser PRP (RR 0.25, 95% CI 0.03 to 2.08) (Analysis 6.2).
Very low‐certainty evidence of a similar outcome between people treated with central PRP compared with peripheral laser PRP with regards needing further laser treatment after the initial treatment period (i.e. 3 months) (RR 1.00, 95% CI 0.07 to 15.12) (Analysis 6.3).
6.1. Analysis.
Comparison 6 Central versus peripheral, Outcome 1 BCVA: loss of 15 or more ETDRS letters.
6.2. Analysis.
Comparison 6 Central versus peripheral, Outcome 2 BCVA: gain of 15 or more ETDRS letters.
6.3. Analysis.
Comparison 6 Central versus peripheral, Outcome 3 Needing further laser treatment after initial treatment period i.e. after 3 months..
Other relevant outcomes such as NVA, progression or regression of DR, VF loss, pain during laser treatment, vision‐related QoL measure, details of any resource use and costs and need for further laser PRP treatment after three months were not reported.
| Adverse event | Central n/N (%) |
Peripheral n/N (%) |
RR (95% CI) |
| Vitreous haemorrhage (requiring additional PRP) | 1/25 (4%) | 1/25 (4%) | RR 1.00 (0.07 to 15.12) |
| Macular traction detachment (requiring pars plana vitrectomy) | 3/25 (12%) | 1/25 (4%) | RR 3.00 (0.33 to 26.92) |
| Macular thickening (associated with loss of 2 or more lines of visual acuity) | 2/25 (8%) | 2/25 (8%) | RR 1.00 (0.15 to 6.55) |
We graded the evidence for this comparison as low‐certainty for the outcomes of 'BCVA loss of 15 or more ETDRS letters at 1 year' (Table 6). We downgraded 1 level for high risk of imprecision due to wide confidence interval; and downgraded 1 level for high risk of performance and detection bias.
'Centre sparing' versus 'full scatter' argon (514 nm) laser PRP
One study investigated this comparison (Theodossiadis 1990). Theodossiadis 1990 enrolled 53 eyes (42 participants) with high‐risk PDR (DRS Research Group 1978 criteria) and followed up for 6 months. This study used argon laser PRP burns (1500 to 3000 burns of 200 μm to 500 μm diameter). In the centre sparing group, laser PRP covered the entire retinal periphery and midperiphery beginning 1 disc diameter from the pars plana, but the posterior pole was spared 2 disc diameter areas centred on the fovea and including the papillomacular bundle. In the full scatter group, laser PRP involved the periphery and midperiphery but stopped 1 disc diameter short of the nasal margins of the optic disc and 3 disc diameter away from the upper, lower and temporal margins of the fovea.
The results were as follows.
Low‐certainty evidence that people treated with centre sparing PRP were less likely to lose 15 or more ETDRS letters of BCVA compared with full scatter laser PRP (RR 0.67, 95% CI 0.30 to 1.50) (Analysis 7.1; Table 7).
Low‐certainty evidence that people treated with centre sparing PRP had similar regression of PDR compared with full scatter laser PRP (RR 0.96, 95% CI 0.73 to 1.27) (Analysis 7.2).
7.1. Analysis.
Comparison 7 Centre sparing versus full scatter PRP, Outcome 1 BCVA: loss of 15 or more ETDRS letters.
7.2. Analysis.
Comparison 7 Centre sparing versus full scatter PRP, Outcome 2 Regression of PDR.
This study concluded "no statistically significant difference regarding regression of neovascularisation and visual acuity" between the two groups. "There was a difference in retinal sensitivity in favour of group B at 15 and 30 degrees of visual field found" (Theodossiadis 1990).
Other relevant outcomes such as BCVA gain of 15 or more letters, NVA, regression of DR, vision‐related QoL measure, details of any resource use and costs and need for further laser PRP treatment after three months were not reported.
The authors made no comment regarding adverse events in this study.
We graded the evidence for this comparison as low‐certainty for the outcomes of 'BCVA loss of 15 or more ETDRS letters at 1 year' and 'Regression of PDR at 1‐year follow‐up' (Table 6). We downgraded 1 level for high risk of imprecision due to wide confidence interval, and downgraded 1 level for high risk of performance and detection bias.
'Extended targeted' PRP versus 'standard' argon (514 nm) laser PRP
One study investigated this comparison (Nikkhah 2017). Nikkhah 2017 enrolled 270 eyes (234 participants) with early or high‐risk PDR (DRS Research Group 1978 criteria) and followed up for three months. Treatment in both arms applied 1200 to 1600 argon laser burns with spot size of 200 μm, duration 200 ms and spacing of 0.5 burn width. In the extended targeted PRP (ETRP) group the laser was applied to the entire retina anterior to the equator as well as the capillary non‐perfusion areas between the vascular arcade and the equator. Conventional PRP (CPRP) laser was applied from the vascular arcade toward the midperiphery.
The results were as follows.
Low‐certainty evidence that people in the extended targeted PRP had similar or slightly reduced chance of loss of 15 or more letters of BCVA compared with the standard PRP group (RR 0.94, 95% CI 0.70 to 1.28) (Analysis 8.1) (Table 8).
Low‐certainty evidence that people in the extended targeted PRP had similar or slightly increased chance of regression of PDR compared with the standard PRP group (RR 1.11, 95% CI 0.95 to 1.31) (Analysis 8.3).
8.1. Analysis.
Comparison 8 Extended targeted PRP versus standard PRP, Outcome 1 BCVA: loss of 15 or more letters.
8.3. Analysis.
Comparison 8 Extended targeted PRP versus standard PRP, Outcome 3 Regression of PDR.
Other relevant outcomes such as BCVA gain of 15 or more letters, NVA, progression of DR, VF loss, pain during laser treatment, vision‐related QoL measure, details of any resource use and costs and need for further laser PRP treatment after three months were not reported.
No adverse events were observed. "None of the eyes developed tractional retinal detachment during the study. Additionally, no ocular or non‐ocular AEs related to the study intervention were detected by the investigators or reported by patients" (Nikkhah 2017).
We graded the evidence for this comparison as low‐certainty for the outcomes of 'BCVA loss of 15 or more ETDRS letters at 1 year' and 'Regression of PDR at 1‐year follow‐up' (Table 8). We downgraded 1 level for high risk of imprecision due to wide confidence interval; and downgraded 1 level for high risk of performance and detection bias.
Discussion
Summary of main results
There is recent interest in alternative laser photocoagulation strategies to the one used by the ETDRS in the treatment of PDR, using other lasers and different treatment approaches with, for example, less intense laser burns or more targeted laser strategies. We reviewed relevant studies with the aim to determine whether these new proposed treatment modalities are equally or more effective with potentially fewer side effects.
We identified 11 RCTs which compared alternative laser strategies for the treatment of PDR with standard argon laser PRP as performed in the ETDRS. We defined the comparator “standard argon laser PRP” as single spot treatment according to ETDRS guidelines. Specifically, the recommendations in the ETDRS were an initial treatment with peripheral scatter laser treatment consisting of 1200 to 1600 burns of moderate intensity, 200 μm to 500 μm spot size, with one‐half to one‐spot diameter spacing and duration of 100 ms to 200 ms, and power titrated to produce moderate‐intensity burns but with full treatment divided over at least two sessions according to different clinical scenarios (ETDRS Research Group 1987).
Five studies compared different lasers with standard argon laser PRP: two frequency double Nd:YAG (Bandello 1996; Brancato 1991); and three diode laser (Bandello 1993; Han 1995; Tewari 2000). One study evaluated a longer duration of laser pulse (0.5 second versus 0.1 second) (Wade 1990). One study compared a 'light intensity' lower energy argon laser PRP treatment to achieve a very light grey biomicroscopic effect on the retina versus 'classic' argon laser PRP to achieve an opaque, dusky, grey‐white, off‐white standard burn (Bandello 2001). One study compared 'mild scatter' argon laser PRP with pattern limited to 400 to 600 laser burns (500 μm, 0.1 sec) over one session versus 'full scatter' argon laser PRP with 1200 to 1600 laser burns (500 μm, 0.1 sec) over two sessions, two weeks apart (Pahor 1998).
Two studies compared different distribution of areas treated versus standard PRP. The first study compared 'central' versus 'peripheral' argon laser PRP treatment (Blankenship 1988). In this study argon laser PRP targeted the midperipheral fundus with the number of burns ranging from 410 to 500, but in addition the 'central' PRP group had burns (ranging in number from 400 to 470) more posteriorly, sparing a 2 DD area centred on the fovea, and papillomacular bundle. The 'peripheral' PRP (ranging from 400 to 500) placed burns more peripherally, anterior to the equator extending to the ora serrata. The second study compared 'centre sparing' versus 'full scatter' argon PRP (Theodossiadis 1990). This study used argon laser PRP burns (1500 to 3000 burns of 200 μm to 500 μm diameter) in two groups. In Group A 'centre sparing' argon laser PRP covered the entire retinal periphery and midperiphery beginning 1 DD from the pars plana, but the posterior pole was spared a 2 DD area centred on the fovea and including the papillomacular bundle. In Group B 'full scatter' argon laser PRP involved the periphery and midperiphery but stopped 1 DD short of the nasal margins of the optic disc and 3 DD away from the upper, lower and temporal margins of the fovea.
One study compared the 'extended targeted' argon laser PRP (ETRP) treatment of ischaemic areas of the retina versus 'standard' conventional PRP (CPRP) (Nikkhah 2017). Treatment in both arms applied 1200 to 1600 argon laser burns with spot size of 200 µm, duration 200 ms and spacing of 0.5 burn width. In the ETRP group the laser was applied to the entire retina anterior to the equator as well as the capillary non‐perfusion areas between the vascular arcade and the equator. CPRP laser was applied from the vascular arcade toward the midperiphery.
Overall completeness and applicability of evidence
There was no difference in the population included in these studies. All studies looked at both male and female adults with a clinical diagnosis of type 1 or type 2 diabetes mellitus, between the age of 18 to 79 years of age. One or both eyes of each participant were required to have high risk PDR.
Although all studies reported our primary outcome the research question was not fully answered as there were few RCTs for each of the comparisons (at most three studies), they were small in size (9 of the 11 studies included 50 participants or fewer) and with high risk of bias. None of the included RCTs reported the following outcomes of interest: near visual acuity; patient‐relevant outcomes such as loss of driving licence; vision‐related QoL measures. No details of any resource or cost implications were provided. Visual field loss was only reported in one study and pain during treatment was reported in three studies.
Recent developments in laser treatment of PDR include semi‐automated patterned scanning laser, with rapid application of multiple laser spots in an array with shorter pulse duration of 10 ms to 30 ms. We were unable to confirm if there are advantages of multispot laser over conventional argon laser PRP as no trial met our inclusion criteria of using standard argon laser PRP as a comparator. Of note: the two‐year results of the DRCR.net protocol reported outcomes of a subgroup of diabetic patients with PDR and treated with laser PRP receiving single spot or pattern laser treatments but as allocation to pattern or single‐spot laser was not randomised we could not include it in our review (Bressler 2017).
Navigated laser is another multispot laser modality with fundus imaging that utilises retinal navigation via computerized image capture and tracking assistance with high precision and reproducibility. No studies were identified that compared multispot laser with conventional argon laser.
Quality of the evidence
The evidence from the studies included in our review was mostly graded as low‐ or very low‐certainty.
The studies were poorly conducted and poorly reported and were judged to be at high risk of bias in at least one domain. All but one of the included studies included more than one eye per person but none of these studies adjusted for within‐person correlation.
The studies were small: the size varied from 20 to 270 eyes. The majority of studies included 50 or fewer particpants, with only two studies including more: 104 participants (Han 1995); and 234 participants (Nikkhah 2017). As a result the effect estimates were imprecise. We also downgraded for indirectness as some of the outcomes were not defined clearly and did not correspond directly to our review outcomes.
Potential biases in the review process
We followed standard methods expected by Cochrane. We have documented all departures from the protocol in Differences between protocol and review.
Agreements and disagreements with other studies or reviews
The ETDRS recommended multi‐session PRP laser extending into the midperipheral zones in high‐risk eyes (RCOphth Level 1) (DRS Research Group 1981). The current clinical Diabetic Retinopathy guidelines of the UK Royal College of Ophthalmologists state that PRP is recommended in high‐risk PDR (RCOphth Diabetic Retinopathy Guidelines 2012). It is recommended that “as retinopathy approaches the proliferative stage, laser scatter treatment (PRP) should be increasingly considered to prevent progression to high risk PDR”. However the preface to these College recommendations highlights that “technological advances in new laser technology using multispot and micropulse abilities have widened clinical knowledge and treatment options”. Our review was unable to find evidence to definitively support alternative modalities of treatment.
An NIHR health technology assessment evaluated the effectiveness and safety of laser PRP for people with pre‐PDR. This cohort was not included in our review but it is interesting to note that they found the current evidence is insufficient to recommend PRP for severe NPDR and that there was no robust evidence to determine whether new, more modern laser systems are more effective than the standard argon laser used in ETDRS although they appear to have fewer adverse effects (Royle 2015).
There has been interest in the use of anti‐VEGF for treatment of PDR. The Diabetic Retinopathy Clinical Research Network (DRCR.net protocol S 2015) was a non‐inferiority study to determine if intravitreal ranibizumab was non‐inferior to PRP for treatment of high‐risk PDR. The study authors concluded that treatment with intravitreal ranibizumab resulted in visual acuity that was non‐inferior to PRP at two years. CLARITY is a phase 2b, single‐masked, non‐inferiority multicentre trial of 232 participants with PDR and found that those treated with intravitreal Aflibercept had an improved BCVA outcome at one year compared with those treated with PRP standard care (CLARITY 2017). Another randomised clinical trial comparing ranibizumab and PRP reported two‐year outcomes in high‐risk PDR with and without macular oedema and showed ranibizumab monotherapy is non‐inferior to PRP, with less visual field loss and incident vitrectomy (Gross 2015).
However it is important to note that both the DRCR.net protocol S and CLARITY studies included diabetic participants predominantly without high‐risk characteristics (HRC) of PDR. This has implications in drawing clinically useful conclusions as short term there may be benefit in the use of anti‐VEGF therapy when only the side effects of the laser are seen. People without HRC may have remained stable with or without treatment. Only longer term outcomes of treatment targeting this group of people will quantify fully the efficacy, risk:benefit ratio and the long‐term compliance of patients.
Authors' conclusions
Implications for practice.
Modern laser techniques and modalities have been developed to treat PDR. However there is an evidence gap with respect to the efficacy and safety of alternative laser systems or strategies compared with the standard argon laser as described in ETDRS.
Implications for research.
Sight loss due to DR has already been identified as a public health priority by the Royal National Institute of Blind People (RNIB 2009) and as a research priority by the James Lind Alliance (“How can sight loss from diabetic retinal changes be prevented and reduced?”) (Rowe 2014).
Evaluating the most effective laser PRP treatment for PDR with least side effects is an important question. In particular larger high‐quality studies are needed to look at the benefits of newer lasers and techniques compared with conventional argon laser PRP for all outcomes, and in particular the long term outcomes most relevant to patients.
What's new
| Date | Event | Description |
|---|---|---|
| 26 March 2018 | Amended | Number in 'What was the aim of this review?' section of plain language summary corrected to 11. |
Acknowledgements
We thank Ms Anupa Shah and Prof Gianni Virgili at the Cochrane Eyes and Vision (CEV) UK editorial base for their assistance with the protocol; and Iris Gordon, Information Specialist at CEV UK. Thanks to the Diabetes UK Northern Ireland Research Partnership for review of the Plain Language Summary.
CEV created and executed the electronic search strategies.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Diabetic Retinopathy] explode all trees #2 diabet* near/3 retinopath* #3 proliferat* near/3 retinopath* #4 diabet* near/3 maculopath* #5 neovasculari?ation #6 #1 or #2 or #3 or #4 or #5 #7 MeSH descriptor: [Light Coagulation] explode all trees #8 MeSH descriptor: [Lasers, Gas] this term only #9 photocoagulat* #10 photo next coagulat* #11 (focal or grid or scatter) near/3 laser* #12 coagulat* or argon or krypton or YAG or diode or micropulse or Pascal or panretinal #13 #7 or #9 or #10 or #11 or #12 #14 #6 and #13
Appendix 2. MEDLINE Ovid search strategy
1. Randomised controlled trial.pt. 2. (Randomised or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp diabetic retinopathy/ 14. (diabet$ adj3 retinopath$).tw. 15. (proliferat$ adj3 retinopath$).tw. 16. (diabet$ adj3 maculopath$).tw. 17. neovasculari?ation.tw. 18. or/13‐17 19. exp light coagulation/ 20. lasers, gas/ 21. photocoagulat$.tw. 22. (photo adj1 coagulat$).tw. 23. ((focal or grid or scatter) adj3 laser$).tw. 24. (coagulat$ or argon or krypton or YAG or diode or micropulse or Pascal or panretinal).tw. 25. or/19‐24 26. 18 and 25 27. 12 and 26
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase Ovid search strategy
1. exp Randomised controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. exp diabetic retinopathy/ 34. (diabet$ adj3 retinopath$).tw. 35. (proliferat$ adj3 retinopath$).tw. 36. (diabet$ adj3 maculopath$).tw. 37. neovasculari?ation.tw. 38. or/33‐37 39. exp laser coagulation/ 40. argon laser/ 41. photocoagulat$.tw. 42. (photo adj1 coagulat$).tw. 43. ((focal or grid or scatter) adj3 laser$).tw. 44. (coagulat$ or argon or krypton or YAG or diode or micropulse or Pascal or panretinal).tw. 45. or/39‐44 46. 38 and 45 47. 32 and 46
Appendix 4. ISRCTN search strategy
diabetic retinopathy AND (laser OR photocoagulation OR coagulation OR argon OR krypton OR YAG OR diode OR micropulse OR Pascal OR panretinal)
Appendix 5. ClinicalTrials.gov search strategy
diabetic retinopathy AND (laser OR photocoagulation OR coagulation OR argon OR krypton OR YAG OR diode OR micropulse OR Pascal OR panretinal)
Appendix 6. WHO ICTRP search strategy
diabetic retinopathy = Condition AND laser OR photocoagulation OR coagulation OR argon OR krypton OR YAG OR diode OR micropulse OR Pascal OR panretinal = Intervention
Appendix 7. Data on study characteristics
| Mandatory items | Optional items | |
| Methods | ||
| Study design |
Parallel group RCT(i.e. people randomised to treatment) Within‐person RCT (i.e. eyes randomised to treatment) Cluster RCT (i.e. communities randomised to treatment) Cross‐over RCT Other, specify |
Exclusions after randomisation Losses to follow‐up Number randomised/analysed How were missing data handled? (e.g. available case analysis, imputation methods) Reported power calculation (Y/N), if yes, sample size and power Unusual study design/issues |
| Eyes or Unit of randomisation/unit of analysis |
One eye included in study, specify how eye selected Two eyes included in study, both eyes received same treatment, briefly specify how analysed (best/worst/average/both and adjusted for within person correlation/both and not adjusted for within person correlation) and specify if mixture one eye and two eye Two eyes included in study, eyes received different treatments,specify if correct pair‐matched analysis done |
|
| Participants | ||
| Country | Setting Ethnic group Equivalence of baseline characteristics (Y/N) |
|
| Total number of participants | This information should be collected for total study population recruited into the study. If these data are only reported for the people who were followed up only, please indicate. | |
| Number (%) of men and women | ||
| Average age and age range | ||
| Inclusion criteria | ||
| Exclusion criteria | ||
| Interventions | ||
| Intervention (n = ) Comparator (n = ) See MECIR 65 and 70 |
Number of people randomised to this group Drug (or intervention) name Dose Frequency Route of administration |
|
| Outcomes | ||
| Primary and secondary outcomes as defined in study reports See MECIR R70 |
List outcomes Adverse events reported (Y/N) Length of follow‐up and intervals at which outcomes assessed |
Planned/actual length of follow‐up |
| Notes | ||
| Date conducted | Specify dates of recruitment of participants mm/yr to mm/yr | Full study name: (if applicable) Reported subgroup analyses (Y/N) Were trial investigators contacted? |
| Sources of funding | ||
| Declaration of interest See MECIR 69 |
||
Data and analyses
Comparison 1. Nd:YAG laser vs argon laser.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 BCVA: loss of 15 or more ETDRS letters | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2 BCVA: gain of 15 or more ETDRS letters | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Change in BCVA | Other data | No numeric data | ||
| 4 Progression of PDR | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Regression of PDR | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Pain during laser treatment | 2 | 62 | Risk Ratio (IV, Fixed, 95% CI) | 1.0 [0.36, 2.76] |
| 7 Adverse effects | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Vitreous haemorrhage | 2 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.38, 3.94] |
| 7.2 Choroidal detachment | 2 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.04, 1.27] |
| 7.3 Troublesome pain | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.17, 5.77] |
| 7.4 Neurotrophic keratopathy | 2 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.35, 4.75] |
1.7. Analysis.
Comparison 1 Nd:YAG laser vs argon laser, Outcome 7 Adverse effects.
Comparison 2. Diode versus argon laser.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 BCVA: loss of 15 or more ETDRS letters | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2 BCVA: gain of 15 or more ETDRS letters | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Change in BCVA | Other data | No numeric data | ||
| 4 Progression of PDR | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Regression of PDR | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Pain during laser treatment | 3 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.12 [2.16, 4.51] |
| 7 Adverse effects | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Vitreous haemorrhage | 2 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.91, 3.53] |
| 7.2 Choroidal detachment | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.48, 33.00] |
| 7.3 Neurotrophic keratopathy | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.87] |
| 7.4 Maculopathy | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.54, 3.13] |
| 7.5 Cataract | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.17, 1.57] |
| 7.6 Pre‐retinal membrane | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.24, 5.49] |
Comparison 3. 0.5 versus 0.1 second exposure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 BCVA: loss of 15 or more EDTRS letters | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 BCVA: gain of 15 or more EDTRS letters | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Progression of PDR | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Regression of PDR | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Pre‐retinal or vitreous haemorrhage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Macular thickening | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Combined rhegmatous and traction retinal detachment requiring pars plana vitrectomy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.5. Analysis.
Comparison 3 0.5 versus 0.1 second exposure, Outcome 5 Adverse effects.
Comparison 4. Light versus classic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in BCVA | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Pain during laser treatment | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Vitreous haemorrhage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Choroidal detachment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Neurotrophic keratopathy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Clinically significant macular oedema | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
4.3. Analysis.
Comparison 4 Light versus classic, Outcome 3 Adverse effects.
Comparison 5. Mild scatter PRP vs full scatter PRP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in BCVA | Other data | No numeric data | ||
| 2 Visual field (mean deviation) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 6. Central versus peripheral.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 BCVA: loss of 15 or more ETDRS letters | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2 BCVA: gain of 15 or more ETDRS letters | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Needing further laser treatment after initial treatment period i.e. after 3 months. | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Vitreous haemorrhage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Macular traction detachment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Macular thickening | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
6.4. Analysis.
Comparison 6 Central versus peripheral, Outcome 4 Adverse effects.
Comparison 7. Centre sparing versus full scatter PRP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 BCVA: loss of 15 or more ETDRS letters | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Regression of PDR | 1 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected |
Comparison 8. Extended targeted PRP versus standard PRP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 BCVA: loss of 15 or more letters | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Change in BCVA | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Regression of PDR | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Change in central macular thickness | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
8.2. Analysis.
Comparison 8 Extended targeted PRP versus standard PRP, Outcome 2 Change in BCVA.
8.4. Analysis.
Comparison 8 Extended targeted PRP versus standard PRP, Outcome 4 Change in central macular thickness.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bandello 1993.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Diode laser
Argon‐green laser
Overall
Inclusion criteria: age at least 18 years; visual acuity (VA) of 0.3 or more; PDR with clinically obvious disc new vessels or neovascularisations elsewhere along the vascular arcades (equal to or more than one‐half the disc area or associated with hemorrhage). Exclusion criteria: vitreous hemorrhage obscuring more than 25 % of the fundus, maculopathy reducing the VA to below 0.3, tractional retinal detachment, Pretreatment: Possible differences in demographics, type of diabetes and duration of disease (Table 1). No statistical analysis on differences Eyes: 44 eyes of 34 people with 10 people having a within‐person design. No adjustment made in the analysis. |
|
| Interventions |
Intervention characteristics Diode laser
Argon‐green laser
|
|
| Outcomes | Visual acuity, PDR regression, vitreous haemorrhage, choroidal detachment, pain, complications Follow‐up: mean follow‐up 24 (SD 4) months in the diode laser group and 25 (SD 5) months in argon laser group |
|
| Notes |
Funding: NR Declaration: NR Country: Italy Setting: eye hospital Date of study: November 1989 to July 1990 Contacting of study investigator: not contacted Trial registration number: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote "Randomization assigned consecutively one eye to ALT and the next to DLT. Of the ten patients in which both eyes were included in the study, the right eye was assigned to DLT and the left to DLT" Judgement comment: Assignment appears to be by alternation. |
| Allocation concealment (selection bias) | High risk | Judgement comment: Allocation was not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no information on masking. We assume that in absence of reporting on this, participants and personnel were not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: no information on masking. We assume that in absence of reporting on this, outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: no apparent loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry. |
Bandello 1996.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Nd:YAG laser
Argon‐green laser
Overall
Inclusion criteria: age at least 18 years, visual acuity of 0.3 or more; PDR with clinically obvious disc new vessels, or neovascularisation elsewhere along the vascular arcades (equal to or more than 1/2 disc area or associated with haemorrhage). Exclusion criteria: lens opacities and/or vitreous haemorrhages obscuring the fundus; maculopathy reducing visual acuity below 0.3; tractional retinal detachment; previous laser treatment. Pretreatment: groups balanced with respect to age, type of diabetes and diabetes duration. More men in argon group but difficult to tell because of discrepancies in numbers and lack of information on denominators in terms of people rather than eyes. Eyes: a mixture of one and both eyes per person (42 eyes of 33 people). When both eyes were eligible (9 out of 33 participants) the laser selection was not random. |
|
| Interventions |
Intervention characteristics Nd:YAG laser
Argon‐green laser
|
|
| Outcomes | Best corrected visual acuity (Snellen decimal acuity); retinopathy by fundus photographs and panretinal fluorescein angiography. Follow‐up: 30 months |
|
| Notes |
Funding: NR Declaration: NR Country: Italy Setting: eye hospital Date of study: December 1990 to April 1992 Contacting of study investigator: not contacted Trial registration number: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation was administered. Trial was described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no information on masking. We assume that in absence of reporting on this, participants and personnel were not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: no information on masking. We assume that in absence of reporting on this, outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "In the ALT group one eye was excluded from the study after 28 months of follow‐up because of the development of a cataract, which made visualization of the fundus difficult and greatly reduced visual acuity. In the NdLT group one eye had to be excluded after 22 months of follow‐up because of central retinal artery occlusion" |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry. |
Bandello 2001.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Intervention
Comparator
Overall
Inclusion criteria: age at least 18 years; BCVA of 0.4 or more; PDR with 2 to 4 high‐risk characteristics (new vessels at disk greater than 1/4 to 1/3 disc area or vitreous or pre‐retinal haemorrhage associated with less extensive new vessels at disk, or with new vessels elsewhere 1/2 disc area or more in size). Exclusion criteria: vitreous haemorrhage obscuring more than 20% of the fundus; maculopathy reducing the BCVA to below 0.4; tractional retinal detachment; media clarity inadequate to permit completion of laser PRP; previous laser treatment. Pretreatment: classic PRP group a bit older (average age 57 versus 48); a bit more insulin dependent diabetes in light PRP group; more CSME in classic PRP group. Eyes: a mixture of one eye per person and within‐person study. 65 eyes of 50 people. Quote: "Of the 15 patients in which both eyes were included in the study, the right eye was randomly assigned to one of the two treatment techniques and the left eye to the other". |
|
| Interventions |
Intervention characteristics Intervention
Comparator
|
|
| Outcomes | Best corrected logMAR acuity, progression and regression, macular oedema, pain and complications, visual fields. Follow‐up: 22 months |
|
| Notes |
Funding: NR Declaration of interest: NR Country: Italy Setting: eye hospital Date study conducted: November 1995 to October 1996 Trial registration number: NR Contacting study investigators: not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: Quote: "Randomization assigned eyes either to light or to classic PRP on the basis of computer‐generated random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how allocation administered. Trial was described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no information on masking. We assume that in absence of reporting on this, particpants and personnel were not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: no information on masking. We assume that in absence of reporting on this, outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: follow‐up not reported. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry. |
Blankenship 1988.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline Characteristics Intervention
Comparator
Overall
Inclusion criteria: diabetes mellitus; available for follow‐up; 6/30 or better BCVA; 3 or 4 diabetic retinopathy risk factors; media clarity to permit argon laser PRP within a single session. Exclusion criteria: prior photocoagulation; substantial lens opacities or vitreous haemorrhages sufficient to prevent complete argon laser PRP. Pretreatment: groups were similar. Eyes: Both eyes were enrolled in 10 people (50 eyes of 40 people); both eyes reported but not adjusted for within‐person correlation. In comparator group 1 eye not randomised to make both arms equal. |
|
| Interventions |
Intervention characteristics Intervention
Comparator
|
|
| Outcomes | Best corrected visual acuity (Snellen lines) visual fields scores; macular thickening; neovascularisation of the disc and retina; intraocular pressure; vitreous haemorrhage. Follow‐up: 6 months |
|
| Notes |
Funding: "Supported in part by patients and contributors of the Bascom Palmer Eye Institute, Research to Prevent Blindness. Inc, New York, the Florida Lions Eye Bank, and the Brenn Green Diabetic Retinopathy Fund, Miami, Florida." Declaration of interest: NR Country: USA Setting: eye hospital Date study conducted: NR Trial registration number: not reported Contacting study investigators: not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After the patient had been informed and had consented to participate, a coin was flipped which determined whether the eye was to receive central or peripheral PRP argon laser treatment. The last two eyes were not Randomised but received central PRP treatment to make an equal number of 25 eyes in each group." |
| Allocation concealment (selection bias) | Low risk | Quote: "a coin was flipped which determined whether the eye was to receive central or peripheral PRP argon laser treatment. The last two eyes were not Randomised but received central PRP treatment to make an equal number of 25 eyes in each group." Judgement comment: allocation decided after informed and agreed to participate. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no information on masking. We assume that in the absence of reporting on this, participants and personnel were not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: no information on masking. We assume that in the absence of reporting on this, participants and personnel were not masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All of the patients returned for follow‐up evaluations 6 months after treatment." Judgement comment: 1‐ and 6‐month follow‐up available for all participants |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trial register entry. |
Brancato 1991.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline Characteristics Intervention
Comparator
Overall
Inclusion criteria: age at least 18 years; visual acuity ≥ 0.3; PDR with clinical obvious new vessels on disc or with new vessels elsewhere along vascular arcades, equal to and/or more than 1 disc area or associated with haemorrhage Exclusion criteria: Opacities of the lens and/or vitreous haemorrhage which would raise difficulties when performing PRP; tractional retinal detachment; previous laser treatment Pretreatment: NR: "After randomization, the two groups of patients were found to have comparable clinical characteristics such as age, sex, type and duration of diabetes mellitus and IVA". Eyes: 20 eyes of 16 people |
|
| Interventions |
Intervention characteristics Intervention
Comparator
|
|
| Outcomes | Visual acuity (Snellen); new vessel regression; side effects including pain; vitreous haemorrhage; and choroidal detachment. Follow‐up: 6 months |
|
| Notes |
Funding: NR Declaration: NR Country: Italy Setting: eye hospital Date study conducted: NR Trial registration number: NR Contacting study investigators: not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: no info on how the sequence generation list was done |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: no information provided on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no information on masking. We assume that in absence of reporting on this, participants and personnel were not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No information on masking. We assume that in absence of reporting on this, participants and personnel were not masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: it appears that they provide data for all participants in all outcomes (visual acuity and retinopathy) status — although they do not list the outcomes nor say which one is the primary outcome and which one secondary outcome. |
| Selective reporting (reporting bias) | Unclear risk | No access to trial protocol or registry entry |
Han 1995.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline Characteristics Intervention
Comparator
Overall
Inclusion criteria: Admitted into the Pusan University Hospital with a diagnosis of diabetic retinopathy. Exclusion criteria: more than ¼ of their eyes covered with blood due to vitreous haemorrhage after treatment; visual acuity below 0.3 due to diabetic maculopathy, as recorded before or after treatment; traction retinal detachment before or after treatment. Pretreatment: "Patients diagnosed with diabetic retinopathy (preproliferative or proliferative)". The mean ages and gender ratio of groups were comparable; however, no additional information about participants in the group such as stage of disease is provided. Eyes: unclear |
|
| Interventions |
Intervention Characteristics Intervention
Comparator
|
|
| Outcomes | Diabetic neovascular changes; visual acuity; complications including pain; vitreous haemorrhage; maculopathy; cataract; pre‐retinal membrane. Follow‐up: 13 to 15 months. |
|
| Notes |
Funding: NR Declaration of interest: NR Country: South Korea Setting: eye Hospital Date study conducted: May 1978 to August 1993 Trial registration number: NR Contacting study investigators: not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not reported how list was generated. Trial was described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: study was probably not masked. No information on masking. We assume that in absence of reporting on this, participants and personnel were not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: study was probably not masked. No information on masking. We assume that in absence of reporting on this, participants and personnel were not masked. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Judgement comment: the number of participants randomised matched the number of participants analysed. Loss to follow‐up not clearly reported. In exclusion criteria some indication that people with adverse events excluded after treatment but not reported how many this applied to: exclusion criteria "more than ¼ of their eyes covered with blood due to vitreous haemorrhage after treatment; visual acuity below 0.3 due to diabetic maculopathy, as recorded before or after treatment were excluded; traction retinal detachment before or after treatment". |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry. |
Nikkhah 2017.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Extended targeted retinal photocoagulation
Conventional PRP
Overall
Inclusion criteria: early or high‐risk PDR based on DRS definition Exclusion criteria: prior retinal laser treatment to the study eye; CMT of more than 300 μm as measured by OCT or the presence of sub‐ or intraretinal fluid at the centre of macula; prior vitreoretinal surgery; any other intraocular surgery within the last 6 months; ongoing neovascular glaucoma; recent anti‐VEGF treatment (in the last 6 months); severe cataract that could affect vision and precise laser treatment; vitreous haemorrhage severe enough to preclude peripheral retinal laser therapy; tractional retinal detachment; not enough dilatable pupil Pretreatment: there was more high‐risk PDR in the intervention group (109 eyes, 81%) than the comparator (94 eyes, 70%). Eyes: 285 eyes of 249 people |
|
| Interventions |
Intervention characteristics Extended targeted retinal photocoagulation
Conventional PRP
|
|
| Outcomes | Best corrected visual acuity (measured using Snellen chart and converted to logMAR for analysis); PDR regression; macular thickness; tractional retinal detachment; ocular or non‐ocular adverse events. Follow‐up: 3 months |
|
| Notes |
Funding: NR Declaration of interest: "The authors declare that they have no financial interest in the subject matter or materials discussed in this manuscript." Country: Iran Setting: eye Hospital Date study conducted: October 2011 to December 2014 Trial registration number: NCT01232179 Contacting study investigators: not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The permutated‐block randomization with varying length of 4, 6, 8 and 10 was selected as the method of randomization. Random allocation sequencing was performed by a biostatistician thorough a computer generated randomization list." |
| Allocation concealment (selection bias) | Low risk | Quote: "Details of the series were unknown to the investigators." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no information on masking. We assume that in absence of reporting on this, participants and personnel were not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "One senior faculty member vitreoretinal specialist other than the authors, judged PDR regression." Judgement comment: It was not clear if this person was masked or not. We assume that in absence of reporting on this, outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: follow‐up was high at approximately 95% and was equal follow‐up in both groups. There was no obvious reason why loss to follow‐up should be related to outcome. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: there were some changes from the trial registry entry and publication. Primary outcome on trial registry was "no leakage in widefield fluorescin angiography" at 3 months. Other outcomes were not specified. The primary outcome in the paper was as follows: "The primary outcome measure was early PDR regression, defined as reduction in neovascular process based on WFFA at three months after conclusion of laser therapy compared with baseline". |
Pahor 1998.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline Characteristics Mild scatter PRP
Full scatter PRP
Overall
Inclusion criteria: proliferative or pre‐proliferative DR Exclusion criteria: diseases known to affect visual field as aphakia, cataract, glaucoma, optic nerve and macular diseases; previous photocoagulation. Pretreatment: Similar visual acuity. Data on other characteristics not reported. "The mean age and diabetes duration were similar in the two groups." Eyes: 40 eyes of 32 participants. "The patient’s eyes were randomly assigned to... ". Eyes: 62 eyes of 47 people |
|
| Interventions |
Intervention characteristics Mild scatter PRP
Full scatter PRP
|
|
| Outcomes | Visual field, retinal sensitivity, Follow‐up: 1 month |
|
| Notes |
Funding: NR Declaration of interest: NR Country: Slovenia Setting: eye Clinic Date study conducted: NR Trial registration number: NR Contacting study investigators: not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patient’s eyes were randomly assigned to either full‐ or mild‐scatter panretinal laser coagulation." Judgement comment: method of generating the random allocation sequence not reported. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: nNot reported how allocation administered. Trial was described as “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no information on masking. We assume that in absence of reporting on this participants and personnel were not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: no information on masking. We assume that in absence of reporting on this outcome assessors were not masked |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "22 eyes of 15 patients were excluded from the study for following reasons: 7 patients (10 eyes) were unable to perform the automated perimetry reliability 1 month after treatment, 5 patients (9 eyes) came not to visual field examination after 1 month, in 3 patients (3 eyes) the macular region was treated." Judgement comment: total loss to follow‐up 38% and unclear to which group these people were allocated. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry. |
Tewari 2000.
| Methods |
Study design: randomised controlled trial Study grouping: within‐person study |
|
| Participants |
Baseline characteristics Diode laser
Argon‐green laser
Overall
Inclusion criteria: bilateral PDR Exclusion criteria: "We excluded patients who had previously received photocoagulation; who had hypertensive retinopathy, vascular block, or hazy media; and those in whom laser delivery was difficult" Pretreatment: Some difference in average visual acuity but difficult to assess how important that was. Eyes: within‐person study, eye to receive diode laser was randomly allocated, other eye received argon laser. Analysis was reported separately by eye i.e. was not matched appropriately. |
|
| Interventions |
Intervention characteristics Diode laser
Argon‐green laser
|
|
| Outcomes | Best‐corrected visual acuity, peripheral visual field, contrast sensitivity, pain. Follow‐up: 6 weeks, 6 months |
|
| Notes |
Funding: NR Declaration: NR Country: India Setting: eye hospital Date of study: NR Contacting of study investigator: not contacted Trial registration number: NR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "For each patient, the eye to receive diode laser was determined randomly with a coin toss," |
| Allocation concealment (selection bias) | Unclear risk | Quote "For each patient, the eye to receive diode laser was determined randomly with a coin toss, and the other eye received argon laser treatment" Judgement Comment. In theory this means that the allocation was unconcealed but as participants received both treatments it is unclear if this will have been an issue. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no information on masking. We assume that in absence of reporting on this participants and personnel were not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: no information on masking. We assume that in absence of reporting on this outcome assessors were not masked |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement Comment: all participants apparently followed up and follow‐up identical between groups because it is a within‐person study |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no access to protocol or trials registry entry. |
Theodossiadis 1990.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline characteristics Intervention
Comparator
Overall
Inclusion criteria: proliferative retinopathy with 3 or 4 risk factors; clear media; visual acuity of 0.5 and more; as little macular thickening as possible; had not been treated with focal laser treatment to the macula. Exclusion criteria: poor cooperation in visual field testing; development of vitreous haemorrhage; neovascular glaucoma; myocardiopathy. Pretreatment: baseline characteristics not reported. Eyes: 53 eyes of 42 people. |
|
| Interventions |
Intervention characteristics Intervention
Comparator
|
|
| Outcomes | Visual fields; retinal sensitivity; visual acuity; regression of neovascularisation. Follow‐up: 2 years |
|
| Notes |
Funding: NR Declaration: NR Country: Greece Setting: Eye Hospital Date study conducted: NR Trial registration number: NR Contacting study investigators: not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: randomly assigned but no detail given |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: NR not recorded so assume not done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: NR not recorded so assumed not masked |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgement comment: not recorded so assume not done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Judgement comment: unclear if excluded participants had been randomised or not before exclusion |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: no protocol available |
Wade 1990.
| Methods |
Study design: randomised controlled trial Study grouping: parallel group |
|
| Participants |
Baseline Characteristics Intervention (argon long exposure (0.5 s))
Comparator (argon standard exposure (0.1 s)
Overall
Included criteria: the participant had to have a diagnosis of diabetes mellitus, be able to return for post‐laser examinations at 1 week, 1 month, and 6 months, and be willing to participate in a random selection of either 0.l‐s or 0.5‐s burn exposure time. Ocular eligibility criteria of the involved eye required best corrected visual acuity of 6/30 or better, media clarity sufficient for PRP in a single session and three or four diabetic retinopathy risk factors. Excluded criteria: eyes with prior photocoagulation, or substantial lens or vitreous opacities sufficient to prevent PRP, were ineligible. Pretreatment: some imbalance in visual acuity but with small numbers probably by chance. Similar numbers of men and women and average age. Eyes: 50 eyes of 41 people |
|
| Interventions |
Intervention characteristics Intervention
Comparator
|
|
| Outcomes | Visual acuity (Snellen); changes in neovascularisation of the disc and retina; macular thickening; choroidal and retinal detachments; vitreous haemorrhages. Follow‐up: 6 months |
|
| Notes |
Funding: this project was supported in part by the Bascorn Palmer Eye Institute, Department of Ophthalmology, University of Miami, and the participants and contributors of the Department of Ophthalmology at Penn State College of Medicine; Research to Prevent Blindness. Inc., New York City; Florida Lions Eye Bank Laboratory and the Benn Green Diabetic Retinopathy Fund, Miami, Florida Declaration: NR Country: USA Setting: eye Hospital Date study conducted: NR Trial registration number: NR Contacting study investigators: not contacted |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Judgement comment: allocation was by coin toss |
| Allocation concealment (selection bias) | Unclear risk | Not reported how allocation administered. Trial was described “randomised” but with no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Judgement comment: no information on masking. We assume that in absence of reporting on this, participants and personnel were not masked. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Judgment comment: no information on masking. We assume that in absence of reporting on this, outcome assessors were not masked. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Judgement comment: loss to follow‐up was low (< 20%) and equal |
| Selective reporting (reporting bias) | Unclear risk | No access to trial protocol or registry entry |
SD: standard deviation NR: not reported PDR: proliferative diabetic retinopathy
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al Hussainy 2008 | No standard comparator group ‐ Nd:YAG laser only |
| Alvarez Verduzco 2010 | Not a randomised controlled trial |
| Beetham 1969 | Ruby laser no longer in common use |
| Belucio‐Neto 2015 | No standard (argon laser) comparator group |
| Blankenship 1986 | Krypton laser not in common use |
| Blankenship 1991 | No standard comparator group ‐ argon laser compared with xenon laser and untreated comparator group. |
| Canning 1991 | Relevant outcomes not reported and may not have been measured as study focuses on macular function. Study too old (<25 years) to obtain data from investigators. |
| Capoferri 1990 | Krypton laser not in common use |
| Chen 2004 | Krypton laser not in common use |
| Chen 2013 | No standard (argon laser) comparator group |
| Chew 1991 | Krypton laser no longer in common use |
| Chhablani 2014 | No standard (argon laser) comparator group |
| Crick 1978 | Xenon laser no longer in common use |
| Doft 1982 | Compared single versus multiple treatment sessions which was not one of our pre‐defined comparisons |
| Dong 2008 | Not a randomised controlled trial |
| Fankhauser 1972 | Not a randomised controlled trial |
| Francois 1971 | Xenon laser no longer in common use |
| Francois 1977 | Not a randomised controlled trial |
| Geltzer 1972 | Not a randomised controlled trial |
| Ghassemi 2013 | Measured nerve fibre layer thickness only |
| Guo 2013 | Compared one versus two sittings which was not one of our pre‐defined comparisons |
| Hamilton 1981 | Xenon laser no longer in common use |
| Inan 2016 | No standard comparator group |
| KARNS 1994 | Krypton laser no longer in common use |
| Khosla 1994 | Compared one versus two sittings which was not one of our pre‐defined comparisons |
| Kovacic 2012 | Not a randomised controlled trial |
| Li 1986 | Only measured electroretinographic changes |
| Liang 1983 | Xenon arc laser not in use any more |
| Lopez 2008 | Not a randomised controlled trial |
| Ludwig 1994 | Not a randomised controlled trial |
| MAPASS 2010 | No standard (argon) comparator group ‐ pascal laser only (532nm) |
| McLean 1976 | Xenon laser no longer in common use |
| Menchini 1990 | Krypton laser no longer in common use |
| Menchini 1995 | Krypton laser no longer in common use |
| Mirshahi 2013 | No standard (argon) comparator group ‐ Nd:YAG (532nm) laser only |
| Muraly 2011 | No standard (argon) comparator group ‐ Nd:YAG (532nm) laser only |
| Nagpal 2008 | No standard (argon) comparator group (conference abstract only). |
| Nagpal 2010 | No standard (argon) comparator group (conference abstract only). |
| Peng 2013 | No standard (argon) comparator group |
| PETER PAN 2011 | No standard (argon) comparator group ‐ PASCAL laser only |
| Plumb 1982 | Xenon arc laser no longer in common use. |
| Roohipoor 2016 | Intervention not in common use (red laser) |
| Sato 2012 | Participants had pre‐proliferative diabetic retinopathy |
| Schulenburg 1979 | Krypton laser not in common use |
| Seiberth 1987 | Participants in this study had pre‐proliferative diabetic retinopathy |
| Seiberth 1993 | Participants had pre‐proliferative diabetic retinopathy |
| Seymenoglu 2013 | Not a randomised controlled trial |
| Shiraya 2014 | Not a randomised controlled trial |
| Ulbig 1994 | Not a randomised controlled trial |
| Yassur 1980 | No standard (argon) comparator group ‐ comparator group was untreated. |
| Yilmaz 2016 | Not a randomised controlled trial |
| Zhang 2017 | No standard (argon) comparator group ‐ pattern scan (577nm) only |
Characteristics of studies awaiting assessment [ordered by study ID]
Chaine 1986.
| Methods | Prospective study |
| Participants | People with proliferative diabetic retinopathy |
| Interventions | Panretinal photocoagulation |
| Outcomes | Not known |
| Notes | We were unable to find either an abstract or a full text copy of this citation |
Kianersi 2016.
| Methods | Within‐person study |
| Participants | 146 eyes of 73 participants with proliferative diabetic retinopathy |
| Interventions |
Unclear what laser was used |
| Outcomes |
Follow‐up: 6 months Quote "Findings: There was no significant difference in the retinal neovascularization regression of disc and elsewhere in eyes treated with pattern scan (P = 0.26) or single spot laser (P = 0.31). While the areas of the retinal ischemia progression was significantly higher (9 cases) in group treated with pattern scan in comparison to other group (2 cases) (P = 0.02)." |
| Notes | We were unable to source a copy of this and did not receive a response from the author. |
Salman 2011.
| Methods | Parallel group study |
| Participants | 60 people with PDR |
| Interventions | Pascal versus conventional laser |
| Outcomes | Successful outcome |
| Notes | Investigator contacted but no reply. |
Uehara 1993.
| Methods | Within‐person study |
| Participants | People with bilateral early stage proliferative diabetic retinopathy (n=17) |
| Interventions | "Slight" photocoagulation (laser burns were 405 +/‐ 166) "Heavy" photocoagulation (laser burns were 1142 +/‐ 179) |
| Outcomes |
Follow‐up: more than 6 months after the last photocoagulation Quote "The results of judgement by fundus pictures and by vitreous fluorophotometric values were in perfect agreement. Eight cases (47%) in whom eyes received slight photocoagulation showed result better than the other eye. Two cases (12%) which received heavy photocoagulation were better than the other eye. Seven cases (41%) showed the same level of severity. No significant differences were found between slight and heavy photocoagulation." |
| Notes | Awaiting translation |
Wroblewski 1991.
| Methods | Prospective study, possibly randomised |
| Participants | Quote "30 eyes with PDR and presenting visual acuity of 20/100 or better. Eyes with vitreous hemorrhage or prior laser treatment were excluded." |
| Interventions | Central PRP (n=16 eyes) versus peripheral PRP (n=14 eyes) Quote "A Mainster panfunduscopic lens was used to deliver 1600 spots in 500 micron size in each group." |
| Outcomes | Macular oedema, visual acuity, retinal detachment, decrease in vision. Follow‐up: 6 weeks and 5 months |
| Notes | Quote "There was no significant difference in either group in. macular edema on IVFA. Three eyes (20%) have lost two lines of Snellen acuity at their six week follow up in the central group, but no significant difference in the final visual accuity was noted at the five month follow up. One eye in the central group and two in the peripheral group also developed fractional macular retinal detachment. Central PRP in PDR may carry a higher incidence of transient decrease in vision compared to peripheral PRP. An analysis of the risk factors in these 30 eyes is presented." Reported as abstract only. We contacted author for further clarification as to whether this was a randomised controlled trial but did not receive any reply. |
Yang 2010.
| Methods | Randomised controlled trial |
| Participants | People with diabetic retinopathy |
| Interventions |
|
| Outcomes |
Follow‐up: 1, 2, 4, 8 weeks and 1 year Quote "The progression of diabetic retinopathy was not different in both groups at the 1‐year follow‐up visit. The best‐corrected visual acuities at 1, 2, 4, and 8 weeks after PRP were decreased in both groups and, in the conventional PRP group, the decrements of visual acuity were greater than in the patterned PRP group. The increments of central macular thickness were also greater in the conventional PRP group than the patterned PRP group." |
| Notes | Awaiting translation |
Differences between protocol and review
We modified the inclusion criteria. We added krypton laser as an intervention to be excluded as it is not in common use.
We modified the outcomes as described Types of outcome measures.
We did not do the planned subgroup and sensitivity analyses because there were never more than 3 trials contributing to the analysis.
The planned analyses were as follows:
Subgroup analyses: Quote from protocol "We will consider clinical sources of heterogeneity including the type of diabetes, stability of glycaemic, lipid and blood pressure control, baseline visual acuity, baseline central macular thickness, and previous treatments for PDR. We will conduct subgroup analyses to investigate clinical heterogeneity. When parameters are available, we will stratify data according to baseline visual acuity worse than 6/24 Snellen equivalent (55 LogMAR letters), baseline CMT as measured by OCT greater than 400 μm, and type 1 or 2 diabetes. We will only perform these subgroup analyses for the primary outcome of this review."
Sensitivity analyses: Quote from protocol " We will conduct sensitivity analyses to determine the impact of exclusion of studies with lower methodological quality (defined as being at high risk of bias in one or more domains), unpublished data and industry‐funded studies. We will only perform the sensitivity analyses for the primary review outcomes."
We made the following amendments to the outcomes included in the summary of findings table.
We added in the outcome "gain of 15 or more EDTRS letters" on the advice of a peer reviewer because it is a primary outcome of our review and therefore was not appropriate to omit from the summary of findings table.
We added in the new outcome of regression of PDR (see Types of outcome measures)
We removed "visual field loss" because by adding these 2 new outcomes we had too many outcomes (8). We chose visual field loss because there was very limited data reported on this.
Contributions of authors
All the authors have made a substantial contribution to the review from conception and design of study, to drafting and commenting on the review including data analysis.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the NIHR to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- This review was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
Tanya Moutray has received educational travel grants from Novartis Pharmaceuticals and Bayer HealthCare Pharmaceuticals. Jennifer Evans has no conflicts of interest. David Armstrong has no conflicts of interest. Tunde Peto has no conflicts of interest. Noemi Lois has no conflicts of interest. Augusto Azuara‐Blanco has no conflicts of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Bandello 1993 {published data only}
- Bandello F, Brancato R, Trabucchi G, Lattanzio R, Malegori A. Diode versus argon‐green laser panretinal photocoagulation in proliferative diabetic retinopathy: a randomized study in 44 eyes with a long follow‐up time. Graefe's Archive for Clinical and Experimental Ophthalmology 1993;231(9):491‐4. [DOI] [PubMed] [Google Scholar]
- Brancato R, Bandello F, Trabucchi G, Leoni G, Lattanzio R. Argon and diode laser photocoagulation in proliferative diabetic retinopathy: A preliminary report. Lasers and Light in Ophthalmology 1990;3(3):233‐7. [Google Scholar]
Bandello 1996 {published data only}
- Bandello F, Brancato R, Lattanzio R, Trabucchi G, Azzolini C, Malegori A. Double‐frequency Nd:YAG laser vs. argon‐green laser in the treatment of proliferative diabetic retinopathy: randomized study with long‐term follow‐up. Lasers in Surgery and Medicine 1996;19(2):173‐6. [DOI] [PubMed] [Google Scholar]
Bandello 2001 {published data only}
- Bandello F, Brancato R, Menchini U, Virgili G, Lanzetta P, Ferrari E, et al. Light panretinal photocoagulation (LPRP) versus classic panretinal photocoagulation (CPRP) in proliferative diabetic retinopathy. Seminars in Ophthalmology 2001;16(1):12‐8. [DOI] [PubMed] [Google Scholar]
- Bandello FM, Brancato R, Menchini U, Lanzetta P. Light panretinal photocoagulation (LPRP) versus classic panretinal photocoagulation (CPRP) in proliferative diabetic retinopathy (PDR). American Academy of Ophthalmology. 1999:240. [DOI] [PubMed]
Blankenship 1988 {published data only}
- Blankenship GW. A clinical comparison of central and peripheral argon laser panretinal photocoagulation for proliferative diabetic retinopathy. Ophthalmology 1988;95(2):170‐7. [DOI] [PubMed] [Google Scholar]
Brancato 1991 {published data only}
- Brancato R, Bandello F, Trabucchi G, Lattanzio R. Frequency‐doubled Nd:YAG laser versus argon‐green laser photocoagulation in proliferative diabetic retinopathy: A preliminary report. Lasers and Light in Ophthalmology 1991;4(2):97‐102. [Google Scholar]
Han 1995 {published data only}
- Han HJ, Oum BS. Diode laser panretinal photocoagulation in diabetic retinopathy. Journal of the Korean Ophthalmological Society 1995;36(11):1972‐9. [Google Scholar]
Nikkhah 2017 {published data only}
- Nikkhah H, Ghazi H, Razzaghi M R, Karimi S, Ramezani A, Soheilian M. Extended targeted retinal photocoagulation versus conventional pan‐retinal photocoagulation for proliferative diabetic retinopathy in a randomized clinical trial. International Ophthalmology 2017 Feb 6 [Epub ahead of print]. [DOI: 10.1007/s10792-017-0469-7] [DOI] [PubMed]
Pahor 1998 {published data only}
- Pahor D. Visual field loss after argon laser panretinal photocoagulation in diabetic retinopathy: Full‐ versus mild‐scatter coagulation. International Ophthalmology 1998;22(5):313‐9. [DOI] [PubMed] [Google Scholar]
Tewari 2000 {published data only}
- Tewari HK, Ravindranath HM, Kumar A, Verma L. Diode laser scatter photocoagulation in diabetic retinopathy. Annals of Ophthalmology ‐ Glaucoma 2000;32(2):110‐2. [Google Scholar]
Theodossiadis 1990 {published data only}
- Theodossiadis GP, Boudouri A, Georgopoulos G, Koutsandrea C. Central visual field changes after panretinal photocoagulation in proliferative diabetic retinopathy. Ophthalmologica 1990;201(2):71‐8. [DOI] [PubMed] [Google Scholar]
Wade 1990 {published data only}
- Wade EC, Blankenship GW. The effect of short versus long exposure times of argon laser panretinal photocoagulation on proliferative diabetic retinopathy. Graefe's Archive for Clinical and Experimental Ophthalmology 1990;228(3):226‐31. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Al Hussainy 2008 {published data only}
- Al‐Husainy SS, Dodson PM, Gibson JM. Pain response and follow‐up of patients undergoing panretinal laser photocoagulation with reduced exposure times. Eye 2008;22(1):96‐9. [DOI] [PubMed] [Google Scholar]
- Al‐Husainy SS, Dodson PM, Gibson JM. Pain response in patients undergoing panretinal photocoagulation by continuous Wave Nd‐YAG laser. Investigative Ophthalmology and Visual Science 2002; Vol. 43:ARVO E‐abstract 3459.
Alvarez Verduzco 2010 {published data only}
- Alvarez‐Verduzco O, Garcia‐Aguirre G, Lopez‐Ramos Mde L, Vera‐Rodriguez S, Guerrero‐Naranjo JL, Morales‐Canton V. Reduction of fluence to decrease pain during panretinal photocoagulation in diabetic patients. Ophthalmic Surgery Lasers and Imaging 2010;41(4):432‐6. [DOI] [PubMed] [Google Scholar]
Beetham 1969 {published data only}
- Beetham WP, Aiello LM, Balodimos MC, Koncz L. Ruby‐laser photocoagulation of early diabetic neovascular retinopathy: preliminary report of a long‐term controlled study. Transactions of the American Ophthalmological Society 1969;67:39‐67. [PMC free article] [PubMed] [Google Scholar]
Belucio‐Neto 2015 {published data only}
- Belucio‐Neto J, Oliveira Xavier C, Oliveira Dias JR, Passos RM, Maia M, Maia A. Functional and anatomical outcomes in patients submitted to panretinal photocoagulation using 577nm multispot vs 532nm single‐spot laser: A clinical trial. Investigative Ophthalmology and Visual Science 2016;57:ARVO E‐abstract 5856. [Google Scholar]
- Belucio‐Neto J, Passos RM, Oliveira Xavier C, Oliveira Dias JR, Moraes N, Maia AM. Evaluation of 577nm multispot vs 532nm single‐spot panretinal photocoagulation for diabetic retinopathy: A clinical trial. Investigative Ophthalmology and Visual Science 2015; Vol. 56:ARVO E‐abstract 5686.
Blankenship 1986 {published data only}
- Blankenship GW. Red krypton and blue‐green argon panretinal laser photocoagulation for proliferative diabetic retinopathy: a laboratory and clinical comparison. Transactions of the American Ophthalmological Society 1986;84:967‐1003. [PMC free article] [PubMed] [Google Scholar]
- Blankenship GW, Gerke E, Batlle JF. Red krypton and blue‐green argon laser diabetic panretinal photocoagulation. Graefe's Archive for Clinical and Experimental Ophthalmology 1989;227(4):364‐8. [DOI] [PubMed] [Google Scholar]
Blankenship 1991 {published data only}
- Blankenship GW. Fifteen‐year argon laser and xenon photocoagulation results of Bascom Palmer Eye Institute's patients participating in the diabetic retinopathy study. Ophthalmology 1991;98(2):125‐8. [DOI] [PubMed] [Google Scholar]
Canning 1991 {published data only}
- Canning C, Polkinghorne P, Ariffin A, Gregor Z. Panretinal laser photocoagulation for proliferative diabetic retinopathy: the effect of laser wavelength on macular function. British Journal of Ophthalmology 1991;75(10):608‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Capoferri 1990 {published data only}
- Capoferri C, Bagini M, Chizzoli A, Pece A, Brancato R. Electroretinographic findings in panretinal photocoagulation for diabetic retinography. A randomized study with blue‐green argon and red krypton lasers. Graefe's Archive for Clinical and Experimental Ophthalmology 1990;228(3):232‐6. [DOI] [PubMed] [Google Scholar]
Chen 2004 {published data only}
- Chen Y, Dong Y, Guo X. Clinical research in the treatment of diabetic retinopathy using different wavelength Krypton lasers photocoagulation. Chinese Ophthalmic Research 2004;22(1):89‐91. [Google Scholar]
Chen 2013 {published data only}
- Chen Z, Song Y. Functional and structural changes after pattern scanning laser photocoagulation in diabetic retinopathy. Documenta Ophthalmologica 2013;127(1 Suppl. 1):37‐8. [Google Scholar]
Chew 1991 {published data only}
- Chew E, Ferris F, Singerman LJ, Brucker A, Murphy R, Mowery R. Clinical results of the Krypton‐Argon regression of Neovascularization Study (KARNS). The Macula Society 1991:40.
Chhablani 2014 {published data only}
- Chhablani J, Mathai A, Rani P, Gupta V, Arevalo JF, Kozak I. Comparison of conventional pattern and novel navigated panretinal photocoagulation in proliferative diabetic retinopathy. Investigative Ophthalmology and Visual Science 2014;55(6):3432‐8. [DOI] [PubMed] [Google Scholar]
- Chhablani J, Sambhana S, Mathai A, Gupta V, Arevalo JF, Kozak I. Clinical efficacy of navigated panretinal photocoagulation in proliferative diabetic retinopathy. American Journal of Ophthalmology 2015;159(5):884‐9. [DOI] [PubMed] [Google Scholar]
Crick 1978 {published data only}
- Crick MD, Chignell AH, Shilling JS. Argon laser v. xenon arc photocoagulation in proliferative diabetic retinopathy. Transactions of the Ophthalmological Societies of the United Kingdom 1978;98(1):170‐1. [PubMed] [Google Scholar]
Doft 1982 {published data only}
- Doft BH, Blankenship GW. Single versus multiple treatment sessions of argon laser panretinal photocoagulation for proliferative diabetic retinopathy. Ophthalmology 1982;89(7):772‐9. [DOI] [PubMed] [Google Scholar]
Dong 2008 {published data only}
- Dong L, Mu H, Feng ZL, Zhang XM, Wang BJ. Influence of different sequence of laser photocoagulation position on curative effect. International Journal of Ophthalmology 2008;8(4):760‐2. [Google Scholar]
Fankhauser 1972 {published data only}
- Fankhauser F, Gloor BP, Lotmar W, Roulier A. Results of the treatment of diabetic retinopathy by photocoagulation using the argon gas laser and the xenon high pressure lamp. Modern Problems in Ophthalmology 1972;10:558‐63. [PubMed] [Google Scholar]
- Fankhauser F, Lotmar W, Roulier A. Comparative management of diabetic retinopathy using photocoagulation with the Argon laser and Xenon high‐pressure lamp. I. Theoretical principles of phototherapy, side‐effects and apparatus design. Graefe's Archive for Clinical and Experimental Ophthalmology 1972;183(4):334‐7. [DOI] [PubMed] [Google Scholar]
Francois 1971 {published data only}
- Francois J, Cambie E. Retinal photocoagulation (xenon arc and lasers). Annals of Ophthalmology 1971;3(11):1201‐8. [PubMed] [Google Scholar]
Francois 1977 {published data only}
- Francois J, Cambie E. Argon laser photocoagulation in diabetic retinopathy. A comparative study of three different methods of treatment. Metabolic Ophthalmology 1977;1(2):125‐30. [Google Scholar]
Geltzer 1972 {published data only}
- Geltzer AI. Laser photocoagulation and diabetic retinopathy. Rhode Island Medical Journal 1972;55(9):275‐81. [PubMed] [Google Scholar]
Ghassemi 2013 {published data only}
- Ghassemi F, Ebrahimiadib N, Roohipoor R, Moghimi S, Alipour F. Nerve fiber layer thickness in eyes treated with red versus green laser in proliferative diabetic retinopathy: short‐term results. Ophthalmologica 2013;230(4):195‐200. [DOI] [PubMed] [Google Scholar]
Guo 2013 {published data only}
- Guo Y, Liu Y, Zhang SB. Proliferative diabetic retinopathy observed after laser photocoagulation. International Eye Science 2013;13(11):2333‐5. [Google Scholar]
Hamilton 1981 {published data only}
- Hamilton AM, Townsend C, Khoury D, Gould E, Blach RK. Xenon arc and argon laser photocoagulation in the treatment of diabetic disc neovascularization. Part 1. Effect on disc vessels, visual fields, and visual acuity. Transactions of the Ophthalmological Societies of the United Kingdom 1981;101(1):87‐92. [PubMed] [Google Scholar]
Inan 2016 {published data only}
- Inan UU, Polat O, Inan S, Yigit S, Baysal Z. Comparison of pain scores between patients undergoing panretinal photocoagulation using navigated or pattern scan laser systems. Arquivos Brasileiros de Oftalmologia 2016;79(1):15‐8. [DOI] [PubMed] [Google Scholar]
KARNS 1994 {published data only}
- Anonymous. Randomized comparison of krypton versus argon scatter photocoagulation for diabetic disc neovascularization. The Krypton Argon Regression Neovascularization Study report number 1. Ophthalmology 1993;100(11):1655‐64. [DOI] [PubMed] [Google Scholar]
- Anonymous. Randomized comparison of krypton versus argon scatter photocoagulation for diabetic disc neovascularizion. Laser and Light in Ophthalmology 1994; Vol. 6:204.
- Singerman LJ, Ferris FL, Chew EY, Murphy RP, Brucker AJ. Krypton Argon Regression of Neovascularization Study: results of a randomized controlled clinical trial. American Academy of Ophthalmology 1992:92.
- Singerman LJ, Ferris FL, Mowery RP, Brucker AJ, Murphy RP, Lerner BC, et al. Krypton Argon Regression of Neovascularization Study (KARNS). The Macula Society 1987:72. [DOI] [PubMed]
- Singerman LJ, Ferris FL, Mowery RP, Brucker AJ, Murphy RP, Lerner BC, et al. Krypton laser for proliferative diabetic retinopathy: the Krypton Argon Regression of Neovascularization Study. Journal of Diabetic Complications 1988;2(4):189‐96. [DOI] [PubMed] [Google Scholar]
Khosla 1994 {published data only}
- Khosla PK, Rao V, Tewari HK, Kumar A. Contrast sensitivity in diabetic retinopathy after panretinal photocoagulation. Ophthalmic Surgery 1994;25(8):516‐20. [PubMed] [Google Scholar]
Kovacic 2012 {published data only}
- Kovacic Z, Ivanisevic M, Bojic L, Hrgovic Z, Lesin M, Kurelovic D. Comparing two techniques of panretinal photocoagulation on visual acuity on patients with proliferative diabetic retinopathy. Medicinski Arhiv 2012;66(5):321‐3. [DOI] [PubMed] [Google Scholar]
Li 1986 {published data only}
- Li XX, Lapp ER, Bornfeld N, Gerke E, Foerster MH. Electroretinography findings in proliferative diabetic retinopathy following argon laser coagulation of the middle and exterior peripheral retina. Fortschritte der Ophthalmologie 1986; Vol. 83, issue 4:459‐61. [PubMed]
Liang 1983 {published data only}
- Liang JC, Fishman GA, Huamonte FU, Anderson RJ. Comparative electroretinograms in argon laser and xenon arc panretinal photocoagulation. British Journal of Ophthalmology 1983;67(8):520‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopez 2008 {published data only}
- Lopez MD, Velez‐Montoya R, Vera‐Rodriguez S, Martinez‐Castellanos M, Burgos‐Vejar O, Gonzalez‐Mijares CC, et al. Pattern‐scan laser system vs. conventional photocoagulation: changes in macular thickness after treatment. Investigative Ophthalmology and Visual Science 2008:ARVO E‐ abstract 2768.
- Perez Montesinos A, Velez‐Montoya R, Burgos O, Lopez‐Ramos L, Alvarez‐Verduzco O, Gonzalez‐Mijares C, et al. Pattern scan laser system vs. regular photocoagulation system: changes in contrast sensitivity post treatment in patients with diabetic retinopathy. Investigative Ophthalmology and Visual Science 2008:ARVO E‐ abstract 3507.
- Vera‐Rodriguez SE, Velez‐Montoya R, Lopez‐Ramos ML, Alvarez‐Verduzco O, Martinez‐Castellanos MA, Gonzalez‐Mijares CC, et al. Pattern scan laser system vs. conventional photocoagulation system: electroretinogram changes after treatment. Investigative Ophthalmology and Visual Science 2008:ARVO E‐ abstract 2766.
Ludwig 1994 {published data only}
- Ludwig K, Lasser T, Sakowski H, Abramowski H, Worz G. Continuous wave laser photocoagulation at different wavelengths: Equivalent power settings in edematous and non‐edematous retina. Lasers and Light in Ophthalmology 1994;6(3):159‐67. [PubMed] [Google Scholar]
MAPASS 2010 {published data only}
- Muqit MM, Gray JC, Marcellino GR, Henson DB, Fenerty CH, Stanga PE. Randomized clinical trial to evaluate the effects of Pascal panretinal photocoagulation on macular nerve fiber layer: Manchester Pascal Study report 3. Retina 2011;31(8):1699‐707. [DOI] [PubMed] [Google Scholar]
- Muqit MM, Gray JC, Marcellino GR, Henson DB, Young LB, Patton N, et al. In vivo laser‐tissue interactions and healing responses from 20‐ vs 100‐millisecond pulse Pascal photocoagulation burns. Archives of Ophthalmology 2010;128(4):448‐55. [DOI] [PubMed] [Google Scholar]
- Muqit MM, Marcellino GR, Gray JC, McLauchlan R, Henson DB, Young LB, et al. Pain responses of Pascal 20 ms multi‐spot and 100 ms single‐spot panretinal photocoagulation: Manchester Pascal Study, MAPASS report 2. British Journal of Ophthalmology 2010;94(11):1493‐8. [DOI] [PubMed] [Google Scholar]
- Muqit MM, Marcellino GR, Henson DB, Young LB, Patton N, Charles SJ, et al. Single‐session vs multiple‐session pattern scanning laser panretinal photocoagulation in proliferative diabetic retinopathy: The Manchester Pascal Study. Archives of Ophthalmology 2010;128(5):525‐33. [DOI] [PubMed] [Google Scholar]
- Muqit MM, Marcellino GR, Henson DB, Young LB, Turner GS, Stanga PE. Pascal panretinal laser ablation and regression analysis in proliferative diabetic retinopathy: Manchester Pascal Study Report 4. Eye 2011;25(11):1447‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
McLean 1976 {published data only}
- McLean EB. Argon and xenon photocoagulation in the treatment of diabetic retinopathy. Transactions of the Pacific Coast Oto‐Ophthalmological Society annual meeting 1976;57:183‐92. [PubMed] [Google Scholar]
Menchini 1990 {published data only}
- Menchini U, Scialdone A, Pietroni C, Carones F, Brancato R. Argon versus krypton panretinal photocoagulation side effects on the anterior segment. Ophthalmologica 1990;201(2):66‐70. [DOI] [PubMed] [Google Scholar]
Menchini 1995 {published data only}
- Menchini U, Lanzetta P, Soldano F, Ferrari E, Virgili G. Continuous wave Nd:YAG laser photocoagulation in proliferative diabetic retinopathy. British Journal of Ophthalmology 1995;79(7):642‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mirshahi 2013 {published data only}
- Mirshahi A, Lashay A, Roozbahani M, Fard MA, Molaie S, Mireshghi M, et al. Pain score of patients undergoing single spot, short pulse laser versus conventional laser for diabetic retinopathy. Graefe's Archive for Clinical and Experimental Ophthalmology 2013;251(4):1103‐7. [DOI] [PubMed] [Google Scholar]
Muraly 2011 {published data only}
- Muraly P, Limbad P, Srinivasan K, Ramasamy K. Single session of Pascal versus multiple sessions of conventional laser for panretinal photocoagulation in proliferative diabetic retinopathy: a comparitive study. Retina 2011;31(7):1359‐65. [DOI] [PubMed] [Google Scholar]
Nagpal 2008 {published data only}
- Nagpal M, Marlecha S, Nagpal, K. Comparative study of efficacy and of collateral damage of laser burns using single spot argon laser and pattern scan laser. American Academy of Ophthalmology 2008:263.
Nagpal 2010 {published data only}
- Nagpal M, Marlecha S, Nagpal, K. Comparison of laser photocoagulation for diabetic retinopathy using 532‐nm standard laser versus multispot pattern scan laser. Retina 2010;30(3):452‐8. [DOI] [PubMed] [Google Scholar]
Peng 2013 {published data only}
- Peng ZH, Cheng GM, Wu L. Observation of clinical efficacy of pattern scan laser photocoagulation on diabetic retinopathy. International Eye Science 2013;13(8):1639‐41. [Google Scholar]
PETER PAN 2011 {published data only}
- Muqit MM, Young LB, McKenzie R, John B, Marcellino GR, Henson DB, et al. Pilot randomised clinical trial of Pascal TargETEd Retinal versus variable fluence PANretinal 20 ms laser in diabetic retinopathy: PETER PAN study. British Journal of Ophthalmology 2013;97(2):220‐7. [DOI] [PubMed] [Google Scholar]
Plumb 1982 {published data only}
- Plumb AP, Swan AV, Chignell AH, Shilling JS. A comparative trial of xenon arc and argon laser photocoagulation in the treatment of proliferative diabetic retinopathy. British Journal of Ophthalmology 1982;66(4):213‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roohipoor 2016 {published data only}
- Roohipoor R, Dantism S, Ahmadraji A, Karkhaneh R, Zarei M, Ghasemi F. Subfoveal choroidal thickness after panretinal photocoagulation with red and green laser in bilateral proliferative diabetic retinopathy patients: short term results. Journal of Ophthalmology 2016;2016:9364861. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sato 2012 {published data only}
- Sato Y, Kojimahara N, Kitano S, Kato S, Ando N, Yamaguchi N, et al. Multicenter randomized clinical trial of retinal photocoagulation for preproliferative diabetic retinopathy. Japanese Journal of Ophthalmology 2012;56(1):52‐9. [DOI] [PubMed] [Google Scholar]
Schulenburg 1979 {published data only}
- Schulenburg WE, Hamilton AM, Blach RK. A comparative study of argon laser and krypton laser in the treatment of diabetic optic disc neovascularisation. British Journal of Ophthalmology 1979;63(6):412‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seiberth 1987 {published data only}
- Seiberth V, Alexandridis E. Function of the diabetic retina after panretinal argon laser photocoagulation. Influence of the intensity of the coagulation spots. Ophthalmologica 1991;202(1):10‐7. [DOI] [PubMed] [Google Scholar]
- Seiberth V, Alexandridis E. Feng W. Function of the diabetic retina after panretinal argon laser coagulation. Graefe's Archive for Clinical and Experimental Ophthalmology 1987;225(6):385‐90. [DOI] [PubMed] [Google Scholar]
Seiberth 1993 {published data only}
- Seiberth V, Schatanek S, Alexandridis E. Panretinal photocoagulation in diabetic retinopathy: argon versus dye laser coagulation. Graefe's Archive for Clinical and Experimental Ophthalmology 1993;231(6):318‐22. [DOI] [PubMed] [Google Scholar]
Seymenoglu 2013 {published data only}
- Seymenoglu G, Kayikcioglu O, Baser E, SamiIlker S. Comparison of pain response of patients undergoing panretinal photocoagulation for proliferative diabetic retinopathy: 532 nm standard laser vs. multispot pattern scan laser. Turk Oftalmoloiji Dergisi 2013;43(4):221‐4. [Google Scholar]
Shiraya 2014 {published data only}
- Shiraya T, Kato S, Shigeeda T, Yamaguchi T, Kaiya T. Comparison of burn size after retinal photocoagulation by conventional and high‐power short‐duration methods. Acta Ophthalmologica 2014;92(7):e585‐6. [DOI] [PubMed] [Google Scholar]
Ulbig 1994 {published data only}
- Ulbig MR, Arden GB, Hamilton AM. Color contrast sensitivity and pattern electroretinographic findings after diode and argon laser photocoagulation in diabetic retinopathy. American Journal of Ophthalmology 1994;117(5):583‐8. [DOI] [PubMed] [Google Scholar]
- Ulbig MW, Hamilton AM. Comparative use of diode and argon laser for panretinal photocoagulation in diabetic retinopathy. Der Ophthalmologe 1993;90(5):457‐62. [PubMed] [Google Scholar]
Yassur 1980 {published data only}
- Yassur Y, Pickle LW, Fine SL, Singerman L, Orth DH, Patz, A. Optic disc neovascularisation in diabetic retinopathy: II. Natural history and results of photocoagulation treatment. British Journal of Ophthalmology 1980;64(2):77‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yilmaz 2016 {published data only}
- Yilmaz I, Perente I, Saracoglu B, Yazici AT, Taskapili M. Changes in pupil size following panretinal retinal photocoagulation: conventional laser vs pattern scan laser (PASCAL). Eye 2016;30(10):1359‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2017 {published data only}
- Zhang S, Cao GF, Xu XZ, Wang CH. Pattern scan laser versus single spot laser in panretinal photocoagulation treatment for proliferative diabetic retinopathy. International Eye Science 2017; Vol. 17, issue 2:205‐8.
References to studies awaiting assessment
Chaine 1986 {published data only}
- Chaine G, Zerah I, Coscas G. Panretinal photocoagulation and proliferative diabetic retinopathy. Results of a long‐term prospective study [Photocoagulation pan‐retinienne et retinopathie diabetique proliferante. Resultats d'une etude prospective a long terme]. Bulletin des Sociétés d'Ophtalmologie de France 1986;86(11):1369‐72. [PubMed] [Google Scholar]
Kianersi 2016 {published data only}
- Kianersi F, Jamshidimadad A, Dehghani A, Golabchi K, Ghanbari H, Akhlaghi M. Comparison of the effects of the pattern scan and conventional single spot panretinal photocoagulation in patients with proliferative diabetic retinopathy; a randomized clinical trial. Journal of Isfahan Medical School 2016;33(365):2288‐95. [Google Scholar]
Salman 2011 {published data only}
- Salman AG. Pascal laser versus conventional laser for treatment of diabetic retinopathy. Saudi Journal of Ophthalmology 2011; Vol. 25, issue 2:175‐9. [DOI] [PMC free article] [PubMed]
Uehara 1993 {published data only}
- Uehara M, Tamura N, Kinjo M, Shinzato K, Fukuda M. A prospective study on necessary and sufficient retinal photocoagulation for diabetic retinopathy. Nippon Ganka Gakkai Zasshi 1993; Vol. 97, issue 1:83‐9. [PubMed]
Wroblewski 1991 {published data only}
- Wroblewski JJ, Anand R. Macular effects of central versus peripheral scatter laser treatment in proliferative‐diabetic retinopathy. Investigative Ophthalmology and Visual Science 1991; Vol. 32:ARVO abstract 1762.
Yang 2010 {published data only}
- Yang JW, Lee YC. Comparison of the effects of patterned and conventional panretinal photocoagulation on diabetic retinopathy. Journal of the Korean Ophthalmological Society 2010; Vol. 51, issue 12:1590‐7.
Additional references
Bressler 2017
- Bressler SB, Beaulieu WT, Glassman AR, Gross JG, Jampol LM, Melia M, et al. Factors associated with worsening proliferative diabetic retinopathy in eyes treated with panretinal photocoagulation or ranibizumab. Ophthalmology 2017;124(4):431‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castillejos‐Rios 1992
- Castillejos‐Rios D, Devenyl R, Moffat K, Yu E. Dye yellow vs argon green laser in panretinal photocoagulation for proliferative diabetic retinopathy: a comparison of minimum power requirements. Canadian Journal Ophthalmology 1992;27(5):243‐4. [PubMed] [Google Scholar]
CLARITY 2017
- Sivaprasad S, Prevost AT, Vasconcelos JC, Riddell A, Murphy C, Kelly J, et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single‐blinded, randomised, controlled, phase 2b, non‐inferiority trial. Lancet 2017;389(10085):2193‐2203. [DOI] [PubMed] [Google Scholar]
Covidence 2015 [Computer program]
- Veritas Health Innovation. Covidence. Version accessed after 3 October 2016. Melbourne, Australia: Veritas Health Innovation, 2015.
DCCT Research Group 1993
- DCCT Research Group. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. New England Journal of Medicine 1993;329(14):977‐86. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration 2011. Available from handbook.cochrane.org.
DRCR.net protocol S 2015
- Diabetic Retinopathy Clinical Research Network. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: A randomized trial. JAMA 2015;314(20):2137‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
DRS Research Group 1978
- Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy: the second report of diabetic retinopathy study findings. Ophthalmology 1978;85(1):82‐106. [DOI] [PubMed] [Google Scholar]
DRS Research Group 1981
- Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. The Diabetic Retinopathy Study Research Group. Ophthalmology 1981;88(7):583‐600. [PubMed] [Google Scholar]
ETDRS Research Group 1985
- ETDRS Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study Research Group. Archives of Ophthalmology 1985;103(12):1796‐1806. [PubMed] [Google Scholar]
ETDRS Research Group 1987
- ETDRS Research Group. Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early Treatment Diabetic Retinopathy Study Report Number 2. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1987;84(7):761‐74. [DOI] [PubMed] [Google Scholar]
ETDRS Research Group 1991
- ETDRS Research Group. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991;98(5 Suppl):766‐85. [PubMed] [Google Scholar]
Evans 2014
- Evans JR, Michelessi M, Virgili G. Laser photocoagulation for proliferative diabetic retinopathy. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD011234.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Feman 2004
- Feman SS. Diabetes and the eye. In: Tasman W, Jaeger EA editor(s). Duane's Clinical Ophthalmology. Vol. 5, Philadelphia, PA: Lippincott Williams & Wilkins, 2004. [Google Scholar]
Geiss 2011
- Geiss LS, Cowie C. Type 2 diabetes and persons at high risk of diabetes. In: Venkat Narayan KM, Williams D, Gregg EW, Cowie C editor(s). Diabetes Public Health: from Data to Policy. New York, NY: Oxford University Press, 2011:15–32. [Google Scholar]
Ghanchi 2013
- Ghanchi F, Diabetic Retinopathy Guidelines Working Group. The Royal College of Ophthalmologists’ clinical guidelines for diabetic retinopathy: a summary. Eye 2013;27(2):285–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghosh 2005
- Ghosh F, Gjorloff K. Protein kinase C expression in the rabbit retina after laser photocoagulation. Graefe's Archive for Clinical and Experimental Ophthalmology 2005;243(8):803–10. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 5 August 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2014.
Gross 2015
- Writing Committee for the Diabetic Retinopathy Clinical Research Network, Gross JG, Glassman AR, Jampol LM, Inusah S, Aiello LP, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA 2015;314(20):2137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Klein 2007
- Klein BE. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiology 2007;14(4):179–83. [DOI] [PubMed] [Google Scholar]
Martinez‐Zapata 2014
- Martinez‐Zapata MJ, Marti‐Carvajal AJ, Sola I, Pijoan JI, Buil‐Calvo JA, Cordero JA, et al. Anti‐vascular endothelial growth factor for proliferative diabetic retinopathy. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD008721.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muqit 2010
- Muqit MM, Marcellino GR, Henson DB, Young LB, Patton N, Charles SJ, et al. Single‐session vs multiple‐session pattern scanning laser panretinal photocoagulation in proliferative diabetic retinopathy. Archives of Ophthalmology 2010;128(5):525‐33. [DOI] [PubMed] [Google Scholar]
RCOphth Diabetic Retinopathy Guidelines 2012
- DR Guidelines Expert Working Party. Diabetic Retinopathy Guidelines ‐ Royal College of Ophthalmologists. www.rcophth.ac.uk/.../2013‐SCI‐301‐FINAL‐DR‐GUIDELINES‐DEC‐2012 (accessed 25 July 2017).
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
RNIB 2009
- RNIB. Future Sight Loss UK 1: The economic impact of partial sight and blindness in the UK adult population. www.rnib.org.uk/sites/default/files/FSUK_Report.pdf (accessed prior to 31 October 2017).
Rowe 2014
- Rowe F, Wormald R, Cable R, Acton M, Bonstein K, Bowen M, et al. The Sight Loss and Vision Priority Setting Partnership (SLV‐PSP): overview and results of the research prioritisation survey process. BMJ Open 2014;4(7):e004905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Royle 2015
- Royle P, Mistry H, Auguste P, Shyangdan D, Freeman K, Lois N, et al. Pan‐retinal photocoagulation and other forms of laser treatment and drug therapies for non‐proliferative diabetic retinopathy: systematic review and economic evaluation. Health Technology Assessment 2015; Vol. 19, issue 51:1‐247. [DOI] [PMC free article] [PubMed]
Stefánsson 2001
- Stefánsson E. The therapeutic effects of retinal laser treatment and vitrectomy: a theory based on oxygen and vascular physiology. Acta Ophthalmologica Scandinavica 2001;79(5):435‐40. [DOI] [PubMed] [Google Scholar]
Stratton 2006
- Stratton IM, Cull CA, Adler AI, Matthews DR, Neil HA, Holman RR. Additive effects of glycaemia and blood pressure exposure on risk of complications in type 2 diabetes: a prospective observational study (UKPDS 75). Diabetologia 2006;49(8):1761‐9. [DOI] [PubMed] [Google Scholar]
UKPDS Group 1998
- UK Prospective Diabetes Study Group. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352(9131):837‐53. [PubMed] [Google Scholar]
Yau 2012
- Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012;35(3):556‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Moutray 2016
- Moutray T, Evans JR, Armstrong DJ, Azuara‐Blanco A. Different lasers and techniques for proliferative diabetic retinopathy. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI: 10.1002/14651858.CD012314] [DOI] [PMC free article] [PubMed] [Google Scholar]


