Abstract
Chronic pulmonary aspergillosis (CPA) is a slowly progressing pulmonary fungal infectious disease caused by Aspergillus. Aspergillus IgG, IgM are now considered to be valuable biomarkers in CPA diagnosing.
Our research attempts to evaluate the effectiveness of Aspergillus IgG, IgM in diagnosing CPA.
In our study, CPA patients were younger than the patients who suffered other pulmonary disease. The most common underlying disease in CPA patients was pulmonary tuberculosis. And the most common clinical symptom was hemoptysis. The comparison among the groups indicated statistical significance with regard to Aspergillus IgG and IgM between the CPA and other pulmonary disease groups (P < .01). The Aspergillus-specific IgG and IgM in infectious group exhibited higher levels than those in colonization group (P < .01). The area under the receiver operating characteristic curve of Aspergillus IgG was 0.762 (95% confidence interval: 0.664–0.860) (P < .01).
Aspergillus-specific IgG offers great diagnostic value with regard to CPA.
Keywords: Aspergillus IgG, Aspergillus IgM, chronic pulmonary aspergillosis
1. Introduction
Chronic pulmonary aspergillosis (CPA) is a slowly progressing pulmonary fungal infectious disease caused by Aspergillus. Approximately 3,000,000 people suffer from CPA worldwide, and the 5-year survival rate is estimated at 50%.[1] The onset of CPA is insidious, and the diagnosis is difficult. However, the early-stage diagnosis of CPA and the intervention therapy can significantly reduce the fatality of CPA. Therefore, a new effective serological marker is considered important for the confirmation of early-stage intervention. In 2016, the European Respiratory Society and the European Society of Clinical Microbiology and Infectious Diseases issued the first CPA guideline, which confirmed the value and significance of the use of Aspergillus-specific IgG for CPA diagnosis.[2]
Currently, the standardized assay for Aspergillus-specific IgG exhibits certain limitations. The majority of the clinical data is derived from European countries, and there have been no relevant data reported in China to date. There were few researches on Aspergillus-specific IgM and had not enough evidence to prove the diagnostic value. In the present study, the diagnostic value for CPA patients was confirmed via the measurement of the Aspergillus-specific IgG, IgM in CPA patients, which provided the basis for the development of CPA investigation and Aspergillus-specific IgG, IgM tests in China.
2. Materials and methods
2.1. Study objects
The patients selected were those who visited the outpatient department and/or were hospitalized in the Beijing Chao-yang Hospital, Capital Medical University, due to cough, hemoptysis. The patients were recruited from May 2016 to April 2018. The patient inclusion criteria were as follows: Patients with lesions on chest computed tomography, such as sarcoidosis, cavity, fibrosis, and aspergilloma; patients with Aspergillus detection in lower respiratory tract samples; patients with suspicion of CPA as demonstrated by clinical diagnosis. The exclusion criteria were the following: Patients with severe immune deficiency, namely agranulocytosis, receptor receiving allogeneic hematopoietic stem cell transplantation, long-term administration of large dose of steroid hormone, administration of T cells immunosuppressor in the past 90 days, severe hereditary immunodeficiency disease, and acquired immune deficiency syndrome. All the patients provided the complete clinical information required for the conduct of the study.
A total of 144 blood samples were collected for the detection of Aspergillus-specific IgG, IgM. In the cases selected into the study, there were 79 male and 65 female with an average age of (56.74 ± 15.67). In these cases, there were 22 cases with tuberculosis, 20 cases with bronchiectasis, 19 cases with chronic obstructive pulmonary disease (COPD), 7 cases with pulmonary sarcoidosis, 6 cases with pulmonary fibrosis, 4 cases with pneumoconiosis, 3 cases with lung tumor, 1 case with pulmonary vasculitis, and 1 case with nontuberculous mycobacterium pulmonary disease (6 cases coexist 2 kinds of diseases).
2.2. Methods
This was a prospective study and was approved by the Ethics Committee of Beijing Chao-yang Hospital, Capital Medical University. All the enrolled patients had signed the informed consent. The enrolled patients finished all the relevant tests according to the routine diagnosis of the disease, such as serum G test, serum and bronchoalveolar lavage fluid (BALF) GM test, serum specific IgE and Aspergillus fumigatus specific IgE, and pulmonary high-resolution computed tomography. The clinical information from the enrolled patients was collected, including laboratory examinations, imaging, microbiological examination, and therapeutic treatment. Aspergillus-specific IgG, IgM test were conducted following the collection of the blood samples. According to the diagnostic criteria, the cases were divided into 4 groups: Group 1a (proven CPA), Group 1b (possible CPA), Group 2 (Aspergillus colonization), Group 3 (other pulmonary disease).
2.2.1. Diagnostic criteria
Diagnosis of CPA[2]: proven CPA (meeting 1 of requirements as follows):
-
(1)
microscopic examination of sterile specimens: the specimens were collected by needle aspiration and/or biopsy. Histopathology, cytopathology, and/or direct microscopic examination indicated Aspergillus fungi infection, associated with corresponding tissue damage;
-
(2)
sterile specimen culture: Aspergillus was cultured from the samples derived from pulmonary lesions as demonstrated by clinical sampling and imaging using sterile operation (not including BALF). possible CPA (meeting (1) to (4) requirements at least):
-
(1)
clinical manifestation evidence: cough, expectoration, pyrexia, hemoptysis, chest pain, weight loss;
-
(2)
imaging evidence: CPA (including single or multiple pulmonary aspergillosis, new and/or continuously developing cavitary lesions with different cavity wall thickness, associated with pulmonary parenchyma injury around cavity and/or fibrosis, significant pleural thickening and A empyema;
-
(3)
the clinical manifestations of the patients and/or imaging evidence for at least 3 months;
-
(4)
the exclusion of other diseases namely, chronic cavitary pulmonary histoplasmosis, paracoccidioidomycosis, coccidioidomycosis, tuberculosis, nontuberculosis mycobacteria infection, necrotic lung cancer, pulmonary infarction, vasculitis, rheumatoid nodule, bacterial infection, including Streptococcus pneumonia, Haemophilus influenzae, Staphylococcus aureus, Pseudomonas aeruginosa, and anaerobic infections;
-
(5)
microbiological evidence: hypha was noted by direct microscopic examination; positive culture and/or serologic evidence in the phlegm, BALF, and bronchus brush.
-
(1)
Aspergillus colonization was established by positive Aspergillus detection from sputum culture for 2 or more times (≥2 times in the absence of compliance with the diagnostic criteria for CPA, allergic bronchopulmonary aspergillosis, invasive pulmonary aspergillosis and/or other diseases). And the pulmonary diseases absorbed without antifilamentous fungi agents.
2.2.2. Collection of Aspergillus-specific antibody samples
Following hospitalization, 5 mL of blood was obtained, and centrifuged to collect the supernatant for the Aspergillus-specific IgG, IgM measurement. This was carried out by enzyme-linked immunosorbent assay (ELISA). The experiment was conducted according to the instructions for A fumigate IgG, IgM antibody quantitative detection kit (Dynamiker, China, LOT No. 160801). The Aspergillus-specific IgG, IgM critical value that was lower than 50 AU/mL (<50 AU/mL) was considered negative, whereas a value of higher than 60 AU/mL (>60 AU/mL) was considered positive.
2.2.3. Statistical analysis
SPSS19.0 (SPSS Inc, Chicago, IL) was used to analyze the data. The measurement data were expressed as mean ± standard deviation or median (interquartile range), and analyzed by t test or Kruskal–Wallis test/1-way analysis of variance. The enumeration data were expressed as percentage, and analyzed by the chi-square test. P value of less than .05 (P < .05) was considered statistically significant.
3. Results
3.1. Characteristics of the study population
One hundred forty four cases were included. Seventy cases had CPA (16 cases had proven CPA), 28 cases found filamentous fungi and had no evidence to diagnosis mycotic infection. And there were 46 control cases (had other respiratory disease). The clinical characteristics of the different groups are shown in Table 1.
Table 1.
Clinical characteristics of different groups.
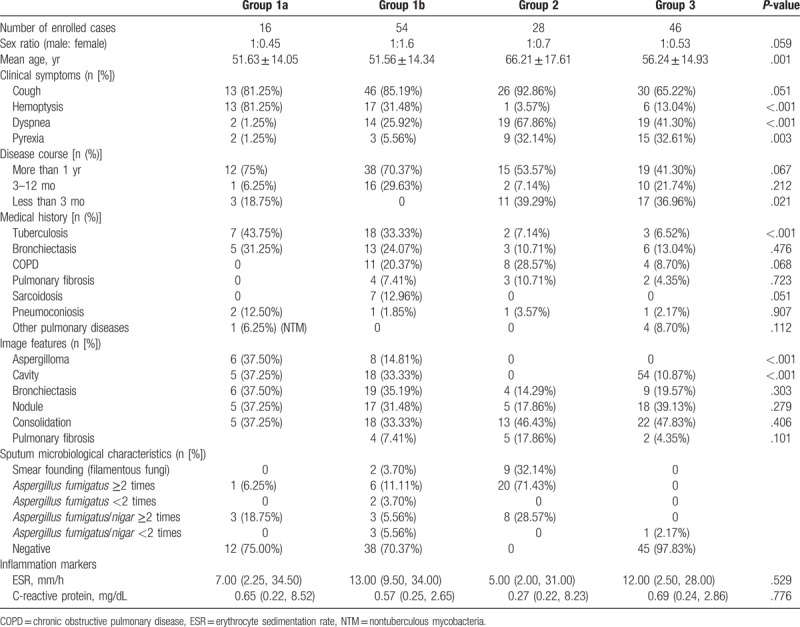
3.2. Diagnostic capability of Aspergillus-specific antibody testing based on pulmonary aspergillosis
The results of the serum G, GM, and BALF GM testing among the 3 groups exhibited no statistical significance. The differences in Aspergillus-specific IgG, IgM were significant (both P < .05) (Table 2). The comparison among the groups indicated statistical significance with regard to Aspergillus IgG, IgM between Group 1 and other groups (both P < .01).
Table 2.
The comparison of laboratory results of different groups.
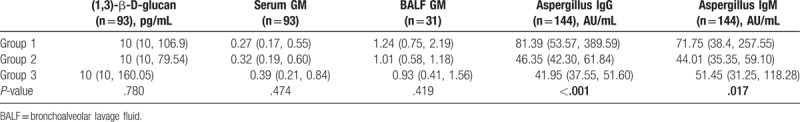
3.3. Identification capability of Aspergillus-specific antibody on Aspergillus infection and colonization
The comparison between Group 1 and Group 2 indicated that the variables Aspergillus-specific IgG, IgM in the infection group exhibited higher levels than those in the colonization group (P < .01). However, no statistical significance was noted in the serum G test, GM test, BALF GM detection (P > .05) (Table 2). There was no statistical significance between Group 2 and control group (Group 3).
3.4. Diagnostic capability evaluation of Aspergillus-specific antibody for the detection of disease
Receiver operating characteristic (ROC) curve was applied to evaluate the accuracy of Aspergillus IgG and IgM with regard to the diagnosis of CPA. The Y-axis represented the sensitivity, whereas the X-axis the 1-specificity. The ROC curve was plotted based on the X and Y axes and the area under the curve (AUC) of the ROC curve was estimated in order to evaluate the accuracy. The AUC of the ROC curve of the Aspergillus-specific IgG was 0.762 (95% confidence interval [CI] 0.664, 0.860). The difference was statistically significant (P < .01). The criteria for CPA with regard to Aspergillus-specific IgG were as follows: The sensitivity and specificity were 70.00% and 82.80%, respectively. The AUC of the ROC curve of the Aspergillus-specific IgM was 0.662 (95% CI 0.555, 0.769). The difference was statistically significant (P < .01). The sensitivity and specificity of Aspergillus-specific IgM were 58.82% and 68.00%, respectively. The ROC curve was shown in Figure 1.
Figure 1.
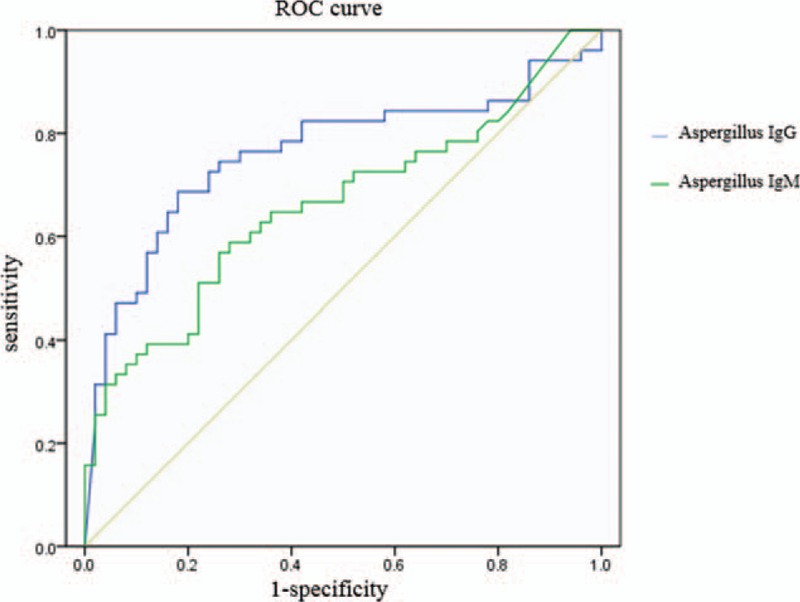
The ROC curve of aspergillus IgG and aspergillus IgM.
4. Discussion
The rate of Aspergillus infection in fungal infections has been shown to increase daily. Certain studies that were conducted in Japan indicated that the detection rate of deep fungi gradually increased, and that Aspergillus had been one of the main fungi responsible for respiratory tract infection, as demonstrated by specimen culture.[3,4] Currently, a paucity of investigations has been conducted with regard to CPA in China, compared with Japan.
Compared with the patients suffered from other pulmonary disease, the CPA patients had no differences in sex ratio. CPA patients were younger than the patients who suffered other pulmonary disease. The most common underlying disease in CPA patients was pulmonary tuberculosis. In Aspergillus colonization patients, the most common underlying disease was COPD, bronchiectasis and pulmonary fibrosis. These results were relevant to the high incidence of tuberculosis in China. In CPA patients, the most common clinical symptom was hemoptysis. Pyrexia and dyspnea were not familiar. The inflammation markers in CPA patients were similar to others. Lower respiratory tract specimens could seldom find Aspergillus in CPA patients. In our research, only 4 of 16 CPA patients had positive microbiological results. The most abnormal radiological changes in CPA patients were pulmonary aspergilloma and pulmonary cavitation.
The accurate diagnosis of CPA is challenging. Thus, the identification of a serologic marker of CPA is imperative for the timely treatment of Aspergillus infection. The serum GM test had low sensitivity to CPA diagnosis in the previous study.[5] In our study, the serum GM test is difficult to distinguish CPA from other pulmonary diseases, and there were no statistical significant differences between the groups. There were numerical differences in BALF GM between CPA group and other pulmonary disease group. The process of bronchoalveolar lavage is hard to standardize. BALF is very hard to obtain from critically ill patients, aged patients, or those with other bronchoscope contraindications. Thus, its application is limited. Only 31 BALF specimens were obtained in this study, so the diagnostic value of BALF GM still need to be verified with more larger sample-size studies.
In clinical practice, physicians frequently diagnose CPA based on clinical manifestations, although the diagnosis for CPA requires pathological specimen verification. Previous studies demonstrated that Aspergillus-specific IgG could be helpful to diagnose CPA. Dumollard et al tested 436 serum samples in a prospective multicentre study. A total of 3 different kits were used to test CPA that had high sensitivity and specificity. The AUC of the ROC curves were higher than 0.9.[6] Fujiuchi et al tested Aspergillus-specific IgG in 269 serum samples from the National Hospital Organization Asahikawa Medical Center. The analysis indicated that 96 samples were in compliance with the CPA diagnostic criteria, whereas the antibody levels were significantly higher than those in the control group. The AUC of the ROC curve was 0.94 (test kit was from Phadia, Uppsala, Sweden).[7]
In the present study, 16 CPA patients were confirmed cases, whereas the remaining 54 were clinically diagnosed cases. The Aspergillus-specific IgG levels were significantly higher than those determined in the other pulmonary disease groups. The AUC of the ROC curve of the Aspergillus-specific IgG was 0.762, whereas the sensitivity and specificity were 70.00% and 82.80%, respectively. Aspergillus-specific IgG provided optimal guidance for the clinical diagnosis of CPA. This study was the first that was conducted on Aspergillus-specific IgG testing of Chinese CPA patients. The data indicated the diagnostic value of Aspergillus-specific IgG on CPA among Chinese subjects, which further provided valuable insight for the diagnostic criteria of Aspergillus-specific IgG for the Chinese participants. In addition, the studies provided valuable information for the design of further CPA epidemiological studies in China.
The increase in the Aspergillus-specific IgG antibody is very common in CPA.[8,9] The reports that have examined the diagnostic value of specific IgM are limited. Certain studies indicated that the increase in the Aspergillus-specific IgM antibody was noted in over 50% of the CPA cases,[10] whereas specific CPA cases that did not reveal a significant increase in Aspergillus IgG, exhibited a specific IgM increase. The possible reason was attributed to the combination of IgM with different Aspergillus antigens as opposed to IgG.[11,12] Aspergillus-specific IgM exhibited supplementary function for the diagnosis of CPA. Until now, we do not clarify whether the duration and rise time is different between Aspergillus IgG and IgM. We want to determine that Aspergillus IgM has early diagnosis value which Aspergillus IgG did not have. Therefore, more researches with high quality, large samples, and adequate follow up are required for further verification. Although the Aspergillus-specific IgM exhibited statistical significance among the CPA, colonization and other pulmonary disease group, the sensitivity and specificity of this marker were limited. The positive likelihood ratio was relatively low. Furthermore, the combination of the Aspergillus IgG and IgM tests exhibited no advantages with regard to the diagnosis of CPA compared with the single use of the Aspergillus-specific IgG test. This might be related to the low sensitivity and specificity of IgM for the diagnosis of CPA. The diagnostic value of the Aspergillus-specific IgM for CPA was limited.
Several clinical challenges remain to be clarified with regard to the differentiation between the Aspergillus colonization and infection.[6] Aspergillus colonization has frequently been considered a precondition and/or primary stage of infection. In certain studies, these subjects were investigated in 1 group.[13] In the present study, the Aspergillus-specific IgG levels in CPA patients were significantly higher compared with those noted in the Aspergillus colonization patients. The latter patients exhibited similar Aspergillus-specific IgG levels with those corresponding to patients with other pulmonary diseases. Future studies should include a higher number of Aspergillus colonization patients.
The present study highlights the application of Aspergillus-specific IgG in the monitoring of CPA and/or the evaluation of the therapeutic response during follow-up. This is due to the collection of the serum samples for Aspergillus-specific antibody testing compared with BALF samples that are considerably difficult to obtain. In the present study, 2 cases were followed up and it was found that Aspergillus-specific IgG did not decrease within the 5-month of therapy, whereas it was significantly increased during the improvement of the clinical manifestations. Moreover, the levels of Aspergillus-specific IgG during therapy will be monitored during the follow up of all the enrolled patients for a long time in order to observe the changes during the disease progression. We hope to use the Aspergillus-specific IgG markers in the clinical therapy of CPA.
However, the majority of the kits are used to detect pulmonary aspergillosis that is caused by A fumigatus. With regard to the non-A fumigatus CPA patients, negative results of Aspergillus specific IgG testing may be observed. In the present study, 6 cases were diagnosed as CPA and exhibited Aspergillus flavus and/or Aspergillus niger that were cultured from lower respiratory tract samples. All 6 patients had low Aspergillus-specific IgG levels (<80 AU/m). A total of 4 out of 6 patients exhibited negative results of the Aspergillus IgG test (<50 AU/mL). Thus, the Aspergillus-specific IgG test exhibited certain limitations. Currently, no studies have been reported that have used the A flavus/Aspergillus nidulans specific IgG ELISA kit.[14]
The data of the present study indicated that Aspergillus-specific IgG, IgM could distinguish CPA from other pulmonary diseases effectively, and offers great diagnostic value with regard to CPA. Furthermore, the diagnostic value of Aspergillus-specific IgG, IgM on the identification of infection and colonization proved very invaluable. And the meaning of the long-term follow-up of CPA requires verification by large sample-size studies.
Author contributions
Investigation: Yiqun Guo, Yu Bai, Chunxia Yang.
Supervision: Li Gu.
Writing – original draft: Yiqun Guo.
Writing – review and editing: Yu Bai, Li Gu.
Footnotes
Abbreviations: AUC = area under the curve, BALF = bronchoalveolar lavage fluid, COPD = chronic obstructive pulmonary disease, CPA = chronic pulmonary aspergillosis, ELISA = enzyme-linked immunosorbent assay, NTM = nontuberculous mycobacteria, ROC = receiver operating characteristic.
The authors have no conflicts of interest to disclose.
References
- [1].Maddox AM, Keating MJ, Smith TL, et al. Prognostic factors for survival of 194 patients with low infiltrate leukemia. Leuk Res 1986;10:995–1006. [DOI] [PubMed] [Google Scholar]
- [2].Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J 2016;47:45–68. [DOI] [PubMed] [Google Scholar]
- [3].Kume H, Yamazaki T, Togano T, et al. Epidemiology of visceral mycoses in autopsy cases in Japan: comparison of the data from 1989, 1993, 1997, 2001, 2005 and 2007 in Annual of Pathological Autopsy Cases in Japan. Med Mycol J 2011;52:117–27. [DOI] [PubMed] [Google Scholar]
- [4].Tashiro T, Izumikawa K, Tashiro M, et al. Diagnostic significance of Aspergillus species isolated from respiratory samples in an adult pneumology ward. Med Mycol 2011;49:581–7. [DOI] [PubMed] [Google Scholar]
- [5].Kitasato Y, Tao Y, Hoshino T, et al. Comparison of Aspergillus galactomannan antigen testing with a new cut-off index and Aspergillus precipitating antibody testing for the diagnosis of chronic pulmonary aspergillosis. Respirology 2009;14:701–8. [DOI] [PubMed] [Google Scholar]
- [6].Dumollard C, Bailly S, Perriot S, et al. Prospective evaluation of a new Aspergillus IgG enzyme immunoassay kit for diagnosis of chronic and allergic pulmonary Aspergillosis. J Clin Microbiol 2016;54:1236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fujiuchi S, Fujita Y, Suzuki H, et al. Evaluation of a quantitative serological assay for diagnosing chronic pulmonary Aspergillosis. J Clin Microbiol 2016;54:1496–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Denning DW, Riniotis K, Dobrashian R, et al. Chronic cavitary and fibrosing pulmonary and pleural aspergillosis: case series, proposed nomenclature change, and review. Clin Infect Dis 2003;37Suppl 3:S265–80. [DOI] [PubMed] [Google Scholar]
- [9].Jhun BW, Jeon K, Eom JS, et al. Clinical characteristics and treatment outcomes of chronic pulmonary aspergillosis. Med Mycol 2013;51:811–7. [DOI] [PubMed] [Google Scholar]
- [10].Weig M, Frosch M, Tintelnot K, et al. Use of recombinant mitogillin for improved serodiagnosis of Aspergillus fumigatus-associated diseases. J Clin Microbiol 2001;39:1721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Centeno-Lima S, de Lacerda JM, Do CJ, et al. Follow-up of anti-Aspergillus IgG and IgA antibodies in bone marrow transplanted patients with invasive Aspergillosis. J Clin Lab Anal 2002;16:156–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kauffman HF, van der Heide S, Beaumont F, et al. Class-specific antibody determination against Aspergillus fumigatus by means of the enzyme-linked immunosorbent assay. III. Comparative study: IgG, IgA, IgM ELISA titers, precipitating antibodies and IgE binding after fractionation of the antigen. Int Arch Allergy Appl Immunol 1986;80:300–6. [DOI] [PubMed] [Google Scholar]
- [13].Oliva A, Flori P, Hennequin C, et al. Evaluation of the Aspergillus western blot IgG kit for diagnosis of chronic aspergillosis. J Clin Microbiol 2015;53:248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Page ID, Richardson M, Denning DW. Antibody testing in aspergillosis – quo vadis? Med Mycol 2015;53:417–39. [DOI] [PubMed] [Google Scholar]


