Abstract
Background:
Due to the resurgence of pertussis, many countries have revised the pertussis immunization schedules and recommended booster doses of pertussis component vaccine for adolescents and adults. Here we aim to investigate the effectiveness and safety of pertussis component vaccines in adolescents and adults.
Methods:
Based on a prospectively registered protocol, we reviewed the literature and selected trials in adolescents and adults using pertussis component vaccine. We followed Cochrane and GRADE (Grading of Recommendations, Assessment, Development and Evaluation) guidance to assess risk of bias, quality of evidence and to perform meta-analyses.
Results:
A total of 17 clinical trials were included. At post-vaccination with pertussis component vaccine, the vaccine protective rate of pertussis reached 88.89%, the vaccine response rate of pertussis antibodies in most trials were above 85%, and the antibody titers at post-vaccination were higher than at pre-vaccination. Reduced-antigen-content diphtheria-tetanus-acellular pertussis vaccine was associated with significantly higher incidences of nausea [RR = 1.26, 95%CI:1.01, 1.57] and vomiting [RR = 2.08, 95%CI:1.21, 3.58] in acellular pertussis vaccines combined with tetanus and diphtheria (Tdap) group than diphtheria tetanus-toxoid vaccines (Td) group. Higher dose of diphtheria toxoid and adjuvant in dTap might cause higher incidence of fever.
Conclusions:
Except for significant difference in gastrointestinal reaction (nausea, vomiting), acellular pertussis component vaccines are quite safe and has short-term effectiveness for the adolescents and adults. The adverse event of acellular pertussis component vaccine is similar to or safer than that of placebo or other vaccines without pertussis component.
Keywords: adolescents and adults, effectiveness, meta-analysis, pertussis component vaccines, safety
1. Introduction
Pertussis is a highly contagious upper respiratory infection caused by Bordetella pertussis and is a poorly controlled vaccine-preventable disease. Since 1980, the incidence of pertussis has been increasing periodically in the United States with a peak in every 3 or 4 years. There were 6568 cases reported in 1993 and 25,827 cases reported in 2004.[1,2] In Australia, 6000 pertussis cases were reported in 2000 and 9000 cases were reported in 2005.[3] Despite universal immunization of children with pertussis component vaccines, the incidence of pertussis has recently increased dramatically in many countries that previously achieved good control of pertussis.[1–7] The waning immunity of vaccinated individuals might contribute to the resurgence of pertussis.[4–7]
Besides high incidence of pertussis in infants, the burden of pertussis has recently increased considerably among adolescents and adults whose vaccine-induced immunity has waned.[8] This epidemiological feature is more obvious in the areas with higher coverage of pertussis immunization for infants. For example, pertussis cases in adolescents and adults in European and American countries accounted for more than 50% worldwide.[9–11] In Canada and Australia, adolescents and adults also became the most susceptible age groups.[12] Pediatric immunization has not decreased the incidence of pertussis in older individuals or the occurrence of outbreaks, nor has it eliminated the transmission of infections to non-immunized children. One study carried out in Canada, France, Germany and the USA showed that 76% to 83% infants with pertussis had been infected by their family members.[13] Adolescents and adults are hosts of Bordetella pertussis and can be sources of pertussis for young infants, who have the highest risk of pertussis-related complications, hospitalization, and death rate.[14–16]
The resurgence of pertussis has attracted attentions of many countries, and acellular pertussis vaccines combined with tetanus and diphtheria (Tdap) vaccine that can induce higher levels of immunogenicity in adults and adolescents were recommended to adults and adolescents by the American Committee on Immunization Practices (ACIP) in 2006.[17] Many developed counties such as USA, Australia, Canada, France, and Germany etc. have revised their pertussis immunization schedule on adolescents and adults in recent years and recommended boost immunization with at least 1 dose of pertussis component vaccine. For example, 2 additional boosts have been recommended for adolescents aging between 14 and 16 years of age and once again for adults in Canada.[3,18] In the United States, adolescents of 11 to 18 years of age are recommended 1 dose of Tdap, and another dose of Tdap for people of 19 to 64 years of age.[19] However, in China, there are only pertussis immunization schedules for infants, without immunization strategy for adolescents and adults.
Many countries have carried out clinical trials to evaluate the effectiveness and the safety after immunizing with pertussis containing vaccines in adolescents and adults,[20–24] but there is no systematic evaluation on adverse and protective effects. Recently, there are some assumptions showing that both the absolute and relative effectiveness of the pertussis containing vaccines might not be valid, and experts are even going to remove pertussis from its position as the leading vaccine-preventable disease in the United States.[25]
In this systematic review and meta-analysis, we assessed the effectiveness and safety of those pertussis-containing vaccines currently on the market for adolescents and adults, aiming to provide the optimal evidence-based immunization strategy for adolescents and adults. Our results will help the countries who are hesitating to recommend pertussis containing vaccine for adolescents and adults to make decisions and may help in selecting the optimal vaccination strategies.
2. Materials and methods
2.1. Search strategy
The study is a systematic review and so it doesn’t involve the ethical approval. The electronic databases including PubMed, Cochrane library, web of knowledge, MEDLINE, CNKI (Chinese National Knowledge Infrastructure databases), VIP Database for Chinese Technical Periodicals were searched for studies on pertussis vaccine from inception to November 2nd, 2018. The search strategy was as follows: whooping cough OR pertussis vaccine OR diphtheria-tetanus-pertussis vaccine OR diphtheria-tetanus-acellular pertussis vaccines OR diphtheria pertussis vaccines OR diphtheria–tetanus–pertussis whole cell. The search strategy varied according to the characteristics of different databases. Trials published in any language were included. In addition, the reference lists of relevant trials and reviews that we identified were screened to examine additional trials.
2.2. Inclusion criteria
All clinical trials that adopted acellular pertussis vaccine or reduced-antigen-content diphtheria-tetanus-acellular pertussis vaccine (intervention group) and non-pertussis component vaccine (control group) to vaccinate adolescents or adults were identified. Adolescents were defined as teenagers between 10 and 18 years old in this study.
2.3. Outcome measurement
The effectiveness related outcomes included vaccine protection, vaccine response, and geometric mean concentration calculation. Vaccine response for each pertussis antigen was defined as post-vaccination antibody concentration above the assay cut-off value in initially seronegative subjects, or a 2-fold or 4-fold increase in pre- to post-vaccination antibody concentrations in initially seropositive subject level. Adverse effects were also evaluated by comparing the incidences of local and general adverse events including swelling, chill or shiver, fever, vomiting, etc. after receiving the pertussis component vaccine.
2.4. Study selection
Two reviewers independently screened the citations and abstracts of all the identified trials and excluded trials that clearly did not meet the inclusion criteria. Full articles of potential eligible trials were retrieved for further assessment. Disagreements among 2 reviewers were resolved by discussion, according to Cochrane guidance.
2.5. Data extraction
The following data were extracted from each included trial using a pre-designed data extraction form:
-
1.
general information, including authors, publication year, and country;
-
2.
study design and methods;
-
3.
participant characteristics and sample size;
-
4.
intervention, including vaccination name, component, dose, manufacture, and follow-up time;
-
5.
outcome measures, including vaccine protective rate, vaccine response rate, antibody titers, and adverse effects.
Data extraction was done by 2 reviewers independently. Any disagreements were resolved through discussion or consulting a third independent reviewer.
2.6. Risk of bias assessment
Methodological quality of the included trials was assessed by 2 reviewers. In case of uncertainty during the assessment, a third reviewer was consulted. A total of 17 clinic trials were assessed according to Cochrane Collaboration checklist for assessing risk of bias.[26] The Cochrane risk of bias tool includes 6 items designed to assess sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other potential sources of bias. Each item was valued as “low risk”, “high risk” or “unclear risk”.
2.7. Statistical analysis
Because fewer studies in entered trials reported vaccine effectiveness and the antibody levels tested in these trials were expressed in different units, so we qualitatively described the effectiveness of variable pertussis component-containing vaccines by serological indicator. Vaccine response rate and antibody titer was used to qualitatively assess vaccine effectiveness. We also calculated rate ratio (RR) and 95% confidence intervals (CI) as well as vaccine protective rate to assess vaccine effectiveness. Meta analyses, performed by Review Manager 5.2.0 software, were used to assess the safety of pertussis component-containing vaccines. The outcomes of dichotomous data were assessed as mean difference (MD) and odds ratio (OR) respectively, with 95% CI. Assessment for heterogeneity was calculated using the Cochrane Q Chi-Squared and I[2] tests. When the test for heterogeneity showed P < .1 or I2 > 50%, the data was considered as high heterogeneity. A random effect model was used for high heterogeneity data, and a fixed effect model was used for homogeneous data. We conducted subgroup analysis according to different dose of the pertussis toxoid in the component of vaccine. All the outcome analyses were assessed for sensitivity using the leave-one-out sensitivity analysis by Stata 12.0 software. Because the number of included studies in each outcome was less than ten, publication bias was not assessed. For other analysis if not specifically mentioned, a P value of .05 was considered to be statistically significant.
3. Results
3.1. Search results
We identified 22,851potential articles from the 6 databases. After duplication check, the search yield was 19,347. Of which, 19,019 were excluded due to clearly irrelevant and 346 full-text articles were retrieved. A total of 17 clinic trials[9,20–24,27–32,16,33–36] met the inclusion criteria and were included in qualitative synthesis. Finally, 6 trials about vaccination safety comparing dTap with Td[20,22,23,27–29] and 4 trials about vaccination safety comparing acellular pertussis(ap) with placebo[9,24,30,32] were included in meta-analysis. Figure 1 shows the study selection process in a PRISMA flowchart.
Figure 1.
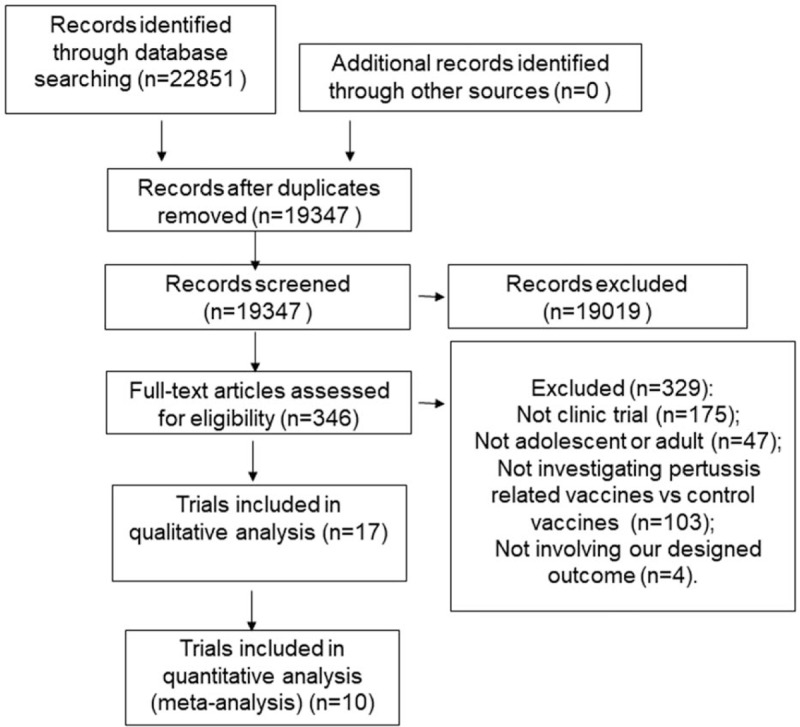
PRISMA flowchart of literature search.
3.2. Description of the included trials
The sample sizes of the included trials were ranged from 24 to 4241 participants, with a total of 14,878 participants in the 17 trials. Five[9,20,28,24,35] studies reported data on gender ratio between groups, while the others[21–23,27,29–32,16,34,35,37] studies did not mention the gender ratio. The participants were aged from 11 to 73 years old. All the trials were carried out in developed countries, of which 7 studies were conducted in USA, while the other 10 studies were in Belgium, Netherlands, Spain, Denmark, Canada, Japan, Sweden, Britain, German, France or Australia. A total of 16 clinical trials reported serological outcome data, but only one reported the vaccine effect. Seven trials compared dTap with Td,[20–23,27–29] 5 compared ap with placebo,[9,24,30–32] 3 compared ap with hepatitis A vaccine (HA),[16,34,35] 1 compared ap with meningococcal vaccine (MN),[36] and 1 compared dTpa-IPV with dT-IPV.[35] The longest observation time of adverse effects after vaccination was 6 months, and the follow-up time of effectiveness after vaccination was from 7 days to 18 months. The characteristics of the included studies are shown in Table 1. The risk of bias in included studies was assessed. Fifteen clinical trials mentioned that patients were randomly allocated to intervention group and control group, but only 9 trials described how the random sequences were generated. Eight trials mentioned the allocation concealment methods, 9 trials mentioned the blinding of participants while 8 trials mentioned the blinding of outcome assessment (Table 2).
Table 1.
Characteristics of the included studies.

Table 2.
Risk of bias assessment of the clinic trials.

3.3. Effectiveness (qualitative analysis)
3.3.1. Protective rate
Only 1 clinic trial with a sample size of 2781 healthy subjects aged from 15 to 65 years old[34] reported the outcome of vaccine. It defined the case with cough lasted for more than 5 days as the first case of pertussis. The intervention group received a dose of acellular pertussis vaccine and control group received a hepatitis A vaccine. All the subjects were followed up for 2.5 years. The results showed that the intervention group had lower incidence rate (72 cases of pertussis per 100,000 persons) than the control group (647 cases of pertussis per 100,000 persons) [RR = 0.11, 95% CI: 0.01–0.88, P = .04], and the vaccine efficacy (VE) of acellular pertussis vaccine reached 88.89%.
3.3.2. Vaccine response rate
Ten trials[9,20–22,24,28,29,33,35,36] compared dTap or ap group with control group that did not receive pertussis component vaccine. The seropositive rate of pertussis antibodies after interventions was analyzed. These trials reported that the seroprotection rate of anti-pertussis toxoid (anti-PT) was from 68% to 99.3%, and 9 trials reported the seroprotection rate of anti-filamentous haemagglutinin (anti-FHA) was from 76% to 100%. The seroprotection rate of anti-pertactin (anti-PRN) tested in 7 trials was from 94.5% to 100%. Only 3 trials mentioned the seroprotection rate of anti-fimbriae (anti-FIM), which was from 3% to 94.9% (Table 3). Although the 10 trials adopted different cut-off values in defining vaccine response, occurrence of seroconvertion can represent the seroprotection after immunization.
Table 3.
Vaccine response rate of pertussis antibodies after immunization of adolescents and adults.

3.3.3. Antibody titer
Six trials[22,24,29–31,33] reported the concentration of pertussis antibodies in serum at pre-vaccination and post-vaccination stages. The geometric mean titers in 4 trials were expressed by EU/ml, and expressed by μg/ml in the other 2 trials (Table 4). Because the antibody levels in these included trials were expressed by different units, the results were impossible to integrate. In trials expressed by EU/ml, the lowest mean concentrations of anti-PT, anti-FHA, anti-PRN, anti-FIM at post-vaccination with 1-month follow up were 38.38 EU/ml, 353.81EU/ml, 304.31EU/ml, and 5.06EU/ml, respectively, increasing 17.45, 27.90, 24.84, and 1.02 times compared with pre-vaccination, respectively. The highest mean concentrations of above antibodies at post-vaccination with 12-month follow up were 4.0, 9.9, 18.8, and 2.3 times higher than pre-vaccination, respectively. Only one trial extended the observation time for 18 months, and all of the antibody levels in this trial maintained at a stable level compared with the antibodies level at the 12th month. Additionally, all the antibodies titers gradually declined from 1 month to the 12th month in all trials, but the titers remained substantially higher than that in pre-vaccination.
Table 4.
Immunity to pertussis antibodies at pre-vaccination and post-vaccination.

3.4. Adverse effects (Meta analysis)
Nine trials reported the adverse effects, including 6 that comparing dTap with dT.[20,22,23,27–29]and 3 trials comparing ap with placebo among adolescents and adults.[24,30,31] In contrast, the trials comparing ap with HA and dTap-IPV with dT-IPV did not report any adverse effects.
3.4.1. dTap vs dT
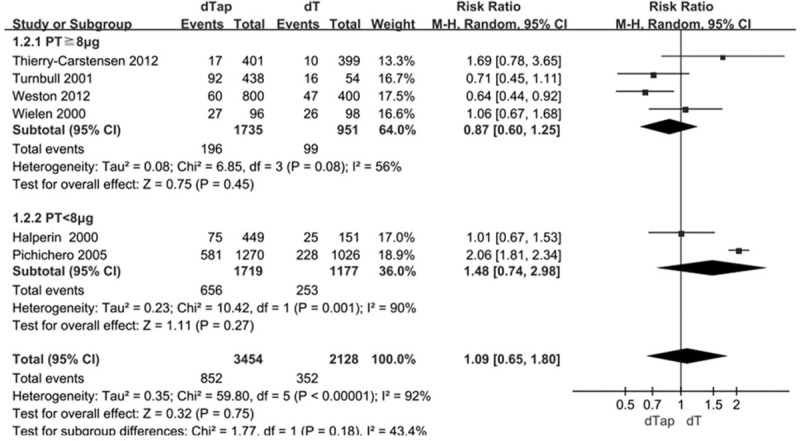
The results showed that the incidence of nausea[20,27,28] [RR = 1.26, 95%CI: 1.01, 1.57] and vomiting[20,23,27,29] [RR = 2.08, 95%CI: 1.21,3.58] in dTap group were significantly higher than those in dT group (Fig. 2E and F). The higher incidence of nausea and vomiting in dTap group was not changed in sensitivity analysis (Fig. 3A and B). The overall incidences of fever were not statistically significant different between dTap group and dT group [RR = 1.06, 95%CI: 0.58, 1.94] (Fig. 4A). In subgroup analysis according to different doses of pertussis toxoid, the incidence of fever in the dTap groups with PT < 8μg were significantly higher than those in dT group [RR = 1.61,95%CI:1.02,2.52], while the PT ≥ 8μg subgroups showed no statistically significant differences between dTap group and dT group (Fig. 4A). The incidence of fever was not statistically different between the 2 groups in sensitivity analysis (Fig. 3C). One clinical trial[20] reported the incidence of cough was 0.38% (11/2873) in dTap group and 1.93% (26/1348) in dT group [RR = 0.2, 95%CI: 0.10, 0.40]. There were no statistically significant differences between dTap group and dT group in the incidence of other adverse effects (Fig. 5, Fig. 2A–D and Fig. 4B and C). The pooled estimates of no significant differences were not changed in each individual sensitivity analysis by leaving one out approach (Fig. 3D–J).
Figure 2.

Forest plot of adverse effects in comparison of dTap with dT. (A) Injection site erythema or redness. (B) Headache. (C) Fatigue. (D) Myalgia. (E) Nausea. (F) Vomiting. dTap: reduced-antigen-content diphtheria-tetanus-acellular pertussis; Td: diphtheriatetanus-toxoid vaccines.
Figure 3.

Sensitivity analysis. (A) dTap vs dT in nausea. (B) dTap vs dT in vomiting. (C) dTap vs dT in fever. (D) dTap vs dT in injection site erythema or redness. (E) dTap vs dT in headache. (F) dTap vs dT in fatigue. (G) dTap vs dT in myalgia. (H) dTap vs dT in swollen joint. (I) dTap vs dT in chills. (J) dTap vs dT in injection site swelling. (K) ap vs placebo in headache. (L) ap vs placebo in injection site erythema or redness.
Figure 4.

Forest plot of adverse effects in comparison of dTap with dT. (A) Fever. (B) Swollen joint. (C) Chills: a injection site swelling. dTap: reduced-antigen-content diphtheria-tetanus-acellular pertussis; Td: diphtheriatetanus-toxoid vaccines.
Figure 5.
Forest plot of adverse effects in comparison of dTap with dT. Injection site swelling. dTap: reduced-antigen-content diphtheria-tetanus-acellular pertussis; Td: diphtheriatetanus-toxoid vaccines.
Only 1 trial[28] reported severe adverse events of dTap vaccination. The incidence of severe adverse events for dTap at 1-month and 6-month post-vaccination were 0.7% and 4.2%, respectively, however, the incidence of severe adverse events for dT was 0.9% [RR = 0.75, 95%CI:0.21,2.64] and 2.2% [RR = 1.85, 95%CI:0.93,3.68] at 1-month and 6-month post-vaccination, respectively. There was no statistically significant difference between dTap group and Td group in the incidence of severe adverse events.
3.4.2. ap vs placebo
There was no statistical significant difference between ap group and the placebo group in the incidence of headache [RR = 0.96, 95%CI: 0.59, 1.55, P = .87] (Fig. 6A) and injection site erythema or redness [RR = 0.62, 95%CI:0.27,1.42, P = .26] (Fig. 6B). The pooled estimates did not changed in sensitivity analysis in the incidence of headache and injection site erythema or redness (Fig. 3K and L).
Figure 6.

Forest plot of adverse effects in comparison of ap with placebo. (A) headache. (B) Injection site erythema or redness. ap: acellular pertussis.
4. Discussion
This study showed that acellular pertussis vaccine in adolescents and adults was effective. The vaccine efficacy reached 88.89%, which was higher than 84% yielded by 1 meta-analysis about ap vaccine effectiveness for children.[37] For the limited observation period of mean concentrations of pertussis antibodies in our entered trials, we only examined the short-term effectiveness of acellular pertussis containing vaccine for the adolescents and adults. Though the geometric mean titer of each pertussis antibody decreased over time, recent data indicate that even low levels of Ig antibody to PRN in children are highly protective and that antibodies to PT and FIM also contribute to protection.[33] Based on the assumption that similar levels of antibody in adolescents and adults can offer the same protection as do those observed in children, our study suggests that 1 recommended dosage of pertussis vaccine in adolescents and adults is sufficient.
In this meta-analysis, it appeared that acellular pertussis component containing vaccine was quite safe. Except for statistically higher incidence of nausea and vomiting in dTap group than dT group, there was no significantly higher incidence of local or systemic reactions in the pertussis component groups. The Centers for Disease Control and Prevent (CDC) in US has reported that the incidence of gastrointestinal reaction (nausea, vomiting, diarrhea, and stomach ache) after vaccinating dTap was up to 1 in 4 adolescents or 1 in 10 adults, whereas there were no gastrointestinal reactions after vaccinating dT.[38] In this meta-analysis, we found similar results, and the low incidence of nausea and vomiting suggests these gastrointestinal reactions are acceptable. The overall incidence of fever was not statistically significant different between dTap group and dT group, but subgroup analysis showed significant difference between dTap groups with PT ≤ 8 μg and dT groups [20,27]. We speculate that this may be caused by the different content of vaccine component from different manufacturers. The content of PT, FHA, PRN and FIM of dTap vaccine in SmithKline Beecham's trial was higher than Pasteur's, but the content of diphtheria toxoid and adjuvant in SmithKline Beecham's were lower than Pasteur's. These data demonstrate the potential routine use of pertussis component vaccine in adolescents and adults.
Heterogeneity was found in the pooled estimations of injection site swelling, fever, and chill. After we conducted subgroup analysis according to different dose of pertussis toxoid, the relative high heterogeneity in some pooled data were reduced significantly, suggesting that the dosage and composition in different vaccines might be the source of heterogeneity in the pooled estimations of adverse effects. It should be noted that all of the 17 trials were carried out in developed countries, and the pertussis component containing vaccines in trials were from the world-famous pharmaceutical enterprises such as GlaxoSmithKline, SmithKline Beecham, Pasteur, etc. Therefore, the results in this review might better guide the application in countries who adopt above vaccines or the countries who use the same component content vaccines as this study. Although we did not exclude the trials which were assessed as high risk of bias based on our inclusion criteria, we performed sensitivity analysis to check whether the trials with high risk of bias would affect the results of our meta-analysis. The pooled estimate with good stability was included. Our results by leave-one-out sensitivity analysis showed that the pooled estimates were not changed in each individual sensitivity analysis. Thus, the trials with high risk of bias were also included.
What we found can promote the trials and application of acellular pertussis among adolescents and adults in some countries, such as China. Moreover, current practice will stimulate more countries to carry out some trials based on our suggests including unified evaluation indexes (VE), unified measurement units (EU/ml) and more than 5 years of follow-up time.
4.1. Limitations
-
1.
The vaccine efficacy was the optimal index representing the effectiveness of pertussis containing vaccine, however, only 1 trial[37] reported the incidence of pertussis case at post-vaccine. In addition, because the control group was not immunized with pertussis content vaccines, we could not compare the serological outcome of pertussis between ap group and control group. Therefore, we are unable to analyze the effectiveness of pertussis vaccine by meta method in our research.
-
2.
Generally, the follow-up time of the vaccines in entered trials were too short, and the longest observation time in effectiveness was only 2.5 years. One trial[39] reported the average duration of protective immunity to pertussis after the fifth dose of children DTaP was 3 to 4 years, and thus the observation time of 2.5 years could not reflect the immune effect about pertussis vaccine enough.
-
3.
The cut-off values of antibody concentration were defined differently in entered trials and the antibody levels were expressed by different units, so we described the serological results in each study instead of comparing the results of all trials.
-
4.
We did not assess the publication bias because the number of included trials was less than 10 in every outcome[39].
-
5.
Given the limited number of studies included in the analysis, our results may have publication bias and the findings from our meta-analysis should be confirmed in future researches. These limitations above made it difficult to accurately estimate the long-term effects of the pertussis component vaccines.
4.2. Future direction
More studies based on developing countries with pertussis containing vaccines from different manufacturers are needed, in which VE should be reported as main outcome and a unified unit is needed to test antibody levels. And the follow-up time of immune effect should be lasted for more than 5 years.
5. Conclusions
In summary, our findings suggest that acellular pertussis component containing vaccines are quite safe and at least have short-term effect for adolescents and adults except for significant difference in gastrointestinal reaction (nausea, vomiting). The adverse event of acellular pertussis containing vaccines is similar to, even less than that of placebo or other vaccines. Acellular pertussis containing group had higher antibody titer at 18 months of post-vaccination and had higher vaccine efficacy of 88.89% in 2.5 years.
Author contributions
Conceptualization: Qing Wang.
Data curation: Jiawei Xu, Shudan Liu, Qing Wang.
Formal analysis: Jiawei Xu, Shudan Liu.
Funding acquisition: Qing Wang.
Investigation: Jiawei Xu, Qin Liu, Shanshan Kuang.
Methodology: Qin Liu, Rong Rong, Qing Wang, Shanshan Kuang.
Resources: Jiawei Xu, Shudan Liu, Rong Rong, Wenge Tang.
Software: Jiawei Xu, Shudan Liu, Qin Liu, Wenge Tang, Chunbei Zhou.
Validation: Rong Rong.
Writing – original draft: Jiawei Xu, Qing Wang.
Writing – review & editing: Qing Wang.
Footnotes
Abbreviations: ACIP = American Committee on Immunization Practices, anti-FHA = anti-filamentous haemagglutinin, anti-FIM = anti-fimbriae, anti-PT = anti-pertussis toxoid, Ap = acellular pertussis, CDC = The Centers for Disease Control and Prevent, CI = confidence intervals, CNKI = Chinese National Knowledge Infrastructure databases, dTap = reduced-antigen-content diphtheria-tetanus-acellular pertussis, dTap-IPV = combined reduced-antigen-content diphtheria-tetanus-acellular pertussis -polio vaccine, dT-IPV = combined reduced-antigen-content diphtheria-tetanus-polio vaccine, GRADE = Grading of Recommendations, Assessment, Development and Evaluation, HA = hepatitis A vaccine, MD = mean difference, MN = meningococcal vaccine, OR = odds ratio, RR = rate ratio, Td = diphtheria tetanus-toxoid vaccines, Tdap = tetanus and diphtheria, VE = vaccine efficacy.
The authors have no conflicts of interests to disclose.
References
- [1].Adams DA, Jajosky RA, Ajani U, et al. Summary of notifiable diseases-United States, 2012. MMWR Morb Mortal Wkly Rep 2014;61:1–21. [PubMed] [Google Scholar]
- [2].Broder KR, Cortese MM, Iskander JK, et al. Preventing tetanus, diphtheria and pertussis among adolescents:use of tetanusand diphtheria toxoids and acellular pertussis vaccines. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006;55:1–34. [PubMed] [Google Scholar]
- [3].NNDSS., Annual Report Writing Group. Australia's notifiable disease status, 2012: annual report of the National Notifiable Diseases Surveillance System. Commun Dis Intell Q Rep 2015;39:E46–136. [DOI] [PubMed] [Google Scholar]
- [4].Kathleen W, Jennifer Z, Kathleen H. Pertussis in California: a Tale of 2 epidemics. Pediatr Dis J 2018;37:324–8. [DOI] [PubMed] [Google Scholar]
- [5].Karagul A, Ogunc D, Midilli K, et al. Epidemiology of pertussis in adolescents and adult in Turkey. Epidemiol Infect 2015;143:2613–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lavine JS, Bjornstad ON, de Blasio BF, et al. Short-lived immunityagainst pertussis, age-specific routes of transmission, and the utility of a teenage booster vaccine. Vaccine 2012;30:544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Klein NP, Bartlett J, Rowhani-Rahbar A, et al. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med 2012;367:1012–9. [DOI] [PubMed] [Google Scholar]
- [8].Ntezayabo B, De Serres G, Duval B. Pertussis resurgence in Canada largely caused by a cohort effect. Pediatr Infect Dis J 2003;22:22–7. [DOI] [PubMed] [Google Scholar]
- [9].Cherry JD. Epidemic pertussis in 2012–the resurgence of a vaccine-preventable disease. N Engl J Med 2012;367:785–7. [DOI] [PubMed] [Google Scholar]
- [10].Centers for Disease Control, Prevention (CDC). Updated recommendations for use of dTaP vaccine from the Advisory Committee on immunization practices. MMWR Morb Mortal Wkly Rep 2011;60:13–5. [PubMed] [Google Scholar]
- [11].Cornia PB, Hersh AL, Lipsky BA, et al. Does this coughing adolescent or adult patient have pertussis? JAMA 2010;304:890–6. [DOI] [PubMed] [Google Scholar]
- [12].De Serres G, Shadmani R, Duval B, et al. Morbidity of pertussis in adolescents and adults. J Infect Dis 2000;182:174–9. [DOI] [PubMed] [Google Scholar]
- [13].Wendelboe AM, Hudgens MG, Poole C, et al. Estimating the role of casual contact from the community in transmission of Bordetella pertussis to young infants. Emerg Themes Epidemiol 2007;4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tanaka M, Vitek CR, Pascual FB, et al. Trends in pertussis among infants in the United States, 1980–1999. JAMA 2003;290:2968–75. [DOI] [PubMed] [Google Scholar]
- [15].Bisgard KM, Pascual FB, Ehresmann KR, et al. Infant pertussis:who was the source? Pediatric Infect Dis J 2004;23:985–9. [DOI] [PubMed] [Google Scholar]
- [16].Ward JI, Cherry JD, Chang SJ, et al. Efficacy of an acellular pertussis vaccine among adolescents and adults. New Engl J Med 2005;353:1555–63. [DOI] [PubMed] [Google Scholar]
- [17].Kretsinger K, Broder KR, Cortese MM, et al. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. Morb Mortal Wkly Rep 2006;55:1–37. [PubMed] [Google Scholar]
- [18].CDC. Tetanus and pertussis vaccination coverage among adults aged ≥18 years — United States, 1999 and 2008. MMWR 2010;59:1302–6. [PubMed] [Google Scholar]
- [19].WHO. Vaccine -prevention diseases: monitoring system, 2006 global summary. http: //www.who.int/vaccines-documents/Global Summary/Global Summery pdf Accessed February 4, 2019. [Google Scholar]
- [20].Pichichero ME, Rennels MB, Edwards KM, et al. Combined tetanus, diphtheria, and 5-component pertussis vaccine for use in adolescents and adults. JAMA 2005;293:3003–11. [DOI] [PubMed] [Google Scholar]
- [21].Theeten H, Rumke H, Hoppener FJ, et al. Primary vaccination of adults with reduced antigen-content diphtheria-tetanus-acellular pertussis or dTpa-inactivated poliovirus vaccines compared to diphtheria-tetanus-toxoid vaccines. Curr Med Res Opin 2007;23:2729–39. [DOI] [PubMed] [Google Scholar]
- [22].Turnbull FM, Heath TC, Jalaludin BB, et al. A randomized trial of two acellular pertussis vaccines (dTpa and pa) and a licensed diphtheria-tetanus vaccine (Td) in adults. Vaccine 2000;19:628–36. [DOI] [PubMed] [Google Scholar]
- [23].Weston WM, Friedland LR, Wu X, et al. Vaccination of adults 65 years of age and older with tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Boostrix?): results of two randomized trials. Vaccine 2012;30:1721–8. [DOI] [PubMed] [Google Scholar]
- [24].Rothstein EP, Anderson EL, Decker MD, et al. An acellular pertussis vaccine in healthy adults: safety and immunogenicity. Vaccine 1999;17:2999–3006. [DOI] [PubMed] [Google Scholar]
- [25].James D. Cherry, why do pertussis vaccines fail? Pediatrics 2012;129:968–70. [DOI] [PubMed] [Google Scholar]
- [26].The Cochrane Collaboration, Higgins JPT, Green S. Cochrane Handbook for Systematic Review of Interventions Version 5.1.0 [updated March 2011]. 2011. [Google Scholar]
- [27].Halperin SA, Smith B, Russell M, et al. An adult formulation of a five-component acellular pertussis vaccine combined with diphtheria and tetanus toxoids is safe and immunogenic in adolescents and adults. Pediatr Infect Dis J 2000;19:276–83. [DOI] [PubMed] [Google Scholar]
- [28].Thierry-Carstensen B, Jordan K, Uhlving HH, et al. A randomised, double-blind, non-inferiority clinical trial on the safety and immunogenicity of a tetanus, diphtheria and monocomponent acellular pertussis (TdaP) vaccine in comparison to a tetanus and diphtheria (Td) vaccine when given as booster vaccinations to healthy adults. Vaccine 2012;30:5464–71. [DOI] [PubMed] [Google Scholar]
- [29].Van der Wielen M, Van Damme P, Joossens E, et al. A randomised controlled trial with a diphtheria-tetanus-acellular pertussis (dTpa) vaccine in adults. Vaccine 2000;18:2075–82. [DOI] [PubMed] [Google Scholar]
- [30].Englund JA, Glezen WP, Barreto L. Controlled study of a new five-component acellular pertussis vaccine in adults and young children. J Infect Dis 1992;166:1436–41. [DOI] [PubMed] [Google Scholar]
- [31].Rutter DA, Ashworth LA, Day A, et al. Trial of a new acellular pertussis vaccine in healthy adult volunteers. Vaccine 1988;6:29–32. [DOI] [PubMed] [Google Scholar]
- [32].Thorstensson R, Trollfors B, Al-Tawil N, et al. Locht CA phase I clinical study of a live attenuated bordetella pertussis vaccine-BPZE1; a single centre, double-blind, placebo-controlled, dose-escalating study of BPZE1 given intranasally to healthy adult male volunteers. Plos One 2014;9:e83449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Le T, Cherry JD, Chang SJ, et al. Immune responses and antibody decay after immunization of adolescents and adults with an acellular pertussis vaccine: the APERT study. J Infect Dis 2004;190:535–44. [DOI] [PubMed] [Google Scholar]
- [34].Ward JI, Cherry JD, Chang SJ, et al. Bordetella pertussis infections in vaccinated and unvaccinated adolescents and adults, as assessed in a national prospective randomized acellular pertussis vaccine trial (APERT). Clin Infect Dis 2006;43:151–7. [DOI] [PubMed] [Google Scholar]
- [35].Grimprel E, von Sonnenburg F, Sanger R, et al. Combined reduced-antigen-content diphtheria–tetanus–acellular pertussis and polio vaccine (dTpa-IPV) for booster vaccination of adults. Vaccine 2005;23:3657–67. [DOI] [PubMed] [Google Scholar]
- [36].Christie CD, Garrison KM, Kiely L, et al. A trial of acellular pertussis vaccine in hospital workers during the cincinnati pertussis epidemic of 1993. Clin Infect Dis 2001;33:997–1003. [DOI] [PubMed] [Google Scholar]
- [37].Fulton TR, Phadke VK, Orenstein WA, et al. Protective effect of contemporary pertussis vaccines: a systematic review and meta-analysis. Clin Infect Dis 2016;62:1101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Centers of disease control and prevention. Possible side-effects from vaccine. http://www.cdc.gov/vaccines/vac-gen/side-effects.htm#tdap Accessed May 25, 2018. [Google Scholar]
- [39].Sutton AJ, Duval SJ, Tweedie RL, et al. Empirical assessment of effect of publication bias on meta-analyses. BMJ 2000;320:1574–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


