Abstract
Introduction:
Spinal gout is rarely encountered in clinical practice, is easily misdiagnosed, and often remains undiagnosed. This paper aims to provide some clues that are the salient diagnostic features of spinal gout, particularly axial pain, radiculopathy, and myelopathy, as determined on the basis of our experience with a few cases as well as a literature review.
Methods:
We retrospectively reviewed the clinical data of 5 patients that were treated for axial pain and neurological symptoms associated with spinal gout between 2014 and 2017 in our hospital. Herein, we systematically describe the clinical characteristics of 5 patients with spinal gout. The 5 patients included 4 men and 1 woman, aged between 24 and 75 years. The most common clinical presentation included spinal pain, radiculopathy, and myelopathy. Four of the 5 patients had a history of gout and elevated serum uric acid levels.
Results:
Four patients underwent surgery, while the remaining patient underwent conservative treatment and biopsy due to poor general condition. Pathological examination of the surgical samples in the 4 surgical cases and the biopsy sample in the remaining case confirmed the presence of spinal gout tophi. The neurological symptoms of all 5 patients were relieved after treatment.
Conclusion:
Due to its rarity and lack of typical defining criteria, the diagnosis of spinal gout is quite difficult. We recommend that patients presenting with axial pain; radicular pain or myelopathy; and especially high uric acid levels, with or without a history of gout, should be evaluated for spinal gout. Timely pathological examination of surgical or biopsy samples would help confirm the diagnosis and enable practitioners to provide the appropriate treatment to prevent disease progression.
Keywords: axial pain, myelopathy, radicular pain, spinal gout
1. Introduction
Gout is a systemic disease resulting from the deposition of monosodium urate crystals (MSU) in tissue. Gout affecting the spinal column is very rare. Hyperuricemia is one of the most important causative factors of gout; however, it can also occur in patients without the development of gout or formation of MSU crystals. Another important causative factor is a genetic predisposition for gout.[1,2] Gout affects 1% to 2% of adults in developed countries, where it is the most common type of inflammatory arthritis in men.[3] Several case reports on spinal gout have previously been published.[3–6] Since the publication of the first report on spinal gout in 1947, there has been considerable research on this topic.[6] However, few authors have summarized the clinical features of spinal gout attacks, including the clinical features, anatomical location, laboratory studies, and treatment choices in spinal gout. The primary aim of this study was to collect empirical evidence regarding the association between the symptoms of axial pain, radiculopathy, or myelopathy and spinal gout and evaluate the relevant clinical presentation and treatment options.
2. Methods
This study was approved by the ethics committee of China-Japan Union Hospital of Jilin University (the ethical approval number: 2018-NSFC-017), all cases enrolled in this study were agreed and signed written informed consent and its main including: cases were only used for medical communication and were not allowed for other purposes; the article includes pictures without the patient's name and other information, cannot reveal patient information. The patient agrees that the case is published. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from individual participants.
We screened the data of more than 5000 patients that were admitted to our hospital, for the treatment of spinal cord or nerve root compression, between 2014 and 2017. Only 5 of these patients presented with long-term axial pain, radiculopathy, or myelopathy, caused by spinal compression due to the presence of gout tophi. The positive value of diagnosis of spinal gout including symptoms and signs, laboratory tests, imaging studies, and pathological examination.[6,7]
We present case 2 to illustrate the clinical presentation of spinal gout involving the cervical and lumbar discs. The patient was a 45-year-old man who presented with low back pain radiating to the left lower extremity and limb weakness, which had persisted for over 2 years. For 3 months before presentation, the patient developed claudication, and for 1 month before presentation, the patient had been experiencing difficulty in walking and weakness of both lower limbs following exertion, as well as a worsening of the pain in the left lower limb. However, the patient did not have saddle paresthesia or bowel and/or bladder dysfunction. The patient reported that despite a 14-year history of hyperuricemia, he was not taking any medications for the condition. On physical examination, we identified several nodular, whitish deposits in several finger joints, the ears, and foot joints of the patient (Fig. 1) as well as a loss of cervical and lumbar lordosis and tenderness over the spinous processes and interspinous spaces, with limited spinal mobility. Evaluation of the patient's muscles according to the Medical Research Council Scale for muscle strength yielded the following results: left biceps, grade 2/5; right triceps, grade 2/5; and gastrocnemius muscles of both sides, grade 3/5. The knee jerk reflex was normal on both sides. The ankle jerk reflex was slow on both sides (+), and the left straight leg raising test was positive at 60°. No abnormal reflexes were detected.
Figure 1.
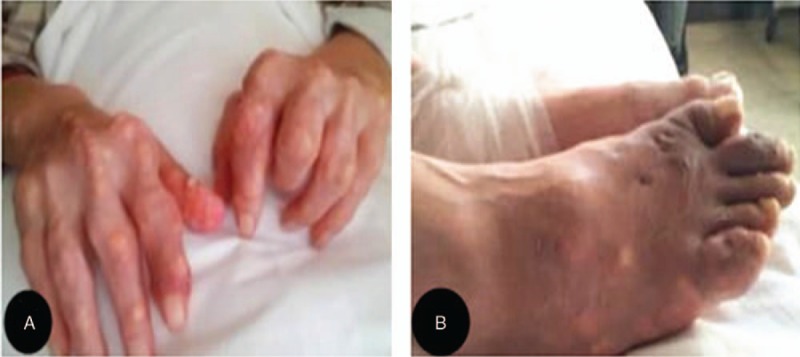
Images showing multiple tophi affecting various joints (A and B).
The results of the laboratory tests were as follows: white blood cell (WBC) count, 5.5 × 109/L (normal range: 4–10 × 109/L); erythrocyte sedimentation rate (ESR), 18 mm/h (normal range:<20 mm/h); serum creatinine level, 104.7 μmol/L (normal range 48–100 μmol/L); and serum uric acid level, 9.59 mg/dL (normal range: 2.60–7.20 mg/dL). The results of the imaging studies are shown in Figure 2.
Figure 2.
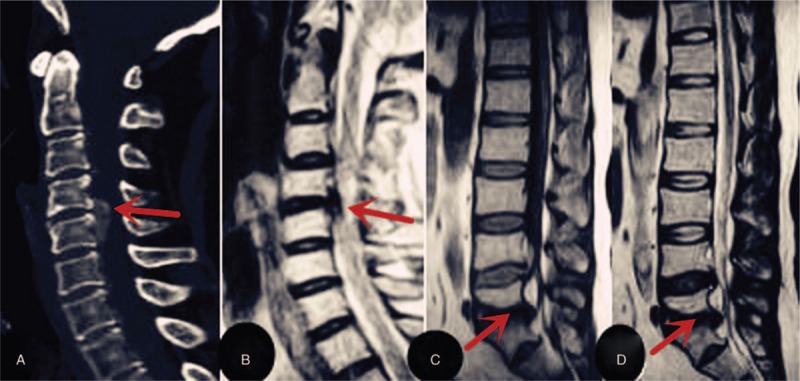
Non-contrast computed tomography (CT) and magnetic resonance imaging (MRI) scans obtained in case 2: Sagtital CT (2a) and T2-weighted images(2b) of C4/5 shows herniated lesion causing cord compression (indicated by arrows).A herniated lumbar disc at the L5-S1 level is seen on T1-weighted and T2-weighted sagittal MRI(2c and 2d indicated by arrowheads). CT = computed tomography, MRI = magnetic resonance imaging.
The patient underwent anterior cervical discectomy and fusion at the C4/5 level and minimally invasive transforaminal lumbar interbody fusion at the L5/S1 level. During the surgery, a nonadherent mass of chalky, white material was observed. This mass was easily separated from the dural sac of the cervical and lumbar intervertebral discs. Surgical excision of the mass compressing the spinal cord and the left S1 nerve root were performed for both diagnostic and treatment purposes. Pathological examination of the sample extracted from the intervertebral discs revealed the typical features of a gouty tophus, including the presence of amorphous material with a multinucleated giant-cell reaction (Fig. 3). Spinal gout with neurological symptoms generally require surgical decompression and medical treatment.[7] After surgery, the patient showed significant improvement in the weakness and radicular pain of limbs and was treated with colchicine (0.5 mg/dl, twice a day) after consultation with rheumatologist. The patient continued to remain symptom-free until the most recent follow-up, which was 3 years after the operation.
Figure 3.
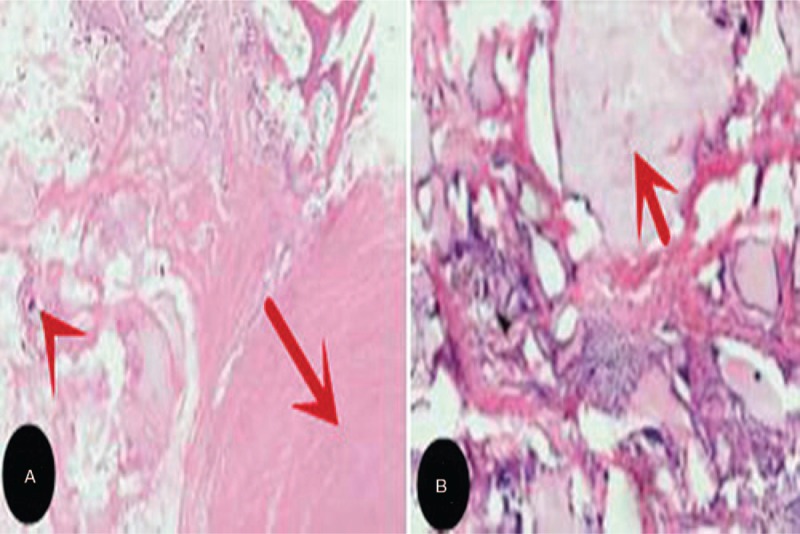
Photomicrographs of formalin-fixed surgically resected specimens obtained at the C4/5 (A) and L5/S1 (B) levels reveal chalk-colored tissue surrounded by multinucleated giant cells, lymphocytes, and fibroblasts (indicated by the red arrows). This appearance proves the deposition of tophaceous material (HE stain).
3. Results
The main clinical features observed in these patients were back/neck pain, weakness, numbness, loss of bladder or bowel control, and decreased sensation below the level of compression. After surgery and medication treatment, case 2 continued to remain symptom-free until the most recent follow-up. The patients were aged between 24 and 78 years (average age: 55.6 years) and included 4 men and 1 woman. Of the 5 patients that were reviewed, all but the youngest patient had a history of gout and elevated serum uric acid levels. Laboratory tests: the serum uric acid range from 7.69 to 12.86 mg/dL (average: 9.93 mg/dL, normal range: 2.60–7.20 mgL/dL); ESR range from 18 to 68 mm/h (normal range: <20 mm/h); WBC count range from 5.5 to 16.14 × 109/L (normal range: 4–10 × 109/L). In our study, only 2 patients had leukocytosis and elevated serum C-reaction protein (CRP) levels, whereas all of the patients had elevated serum uric acid levels and ESR. The data of all cases are depicted in Table 1. The lesions were detected in the ligamentum flavum, cervical/lumbar disc, facet joint, the posterior longitudinal ligament, and the lesions showed isointense or hypointense signals on T1-weighted (T1W) sections and were mostly hyperintense and isointense on T2-weighted (T2W) sections. The lesions were initially attributed to other conditions, such as calcification of the ligamentum flavum, degenerative disc disease, ossification of the posterior longitudinal ligament, and even tumors. Four patients underwent spinal decompression surgery, while 1 patient underwent biopsy for cervical lesions and received antigout medication because of severe acute gouty inflammation of the lower limb joints and fever. Pathological examination of the surgically extracted tissue and the biopsy sample showed the presence of chalky, crystalline material, thereby confirming that the lesions were spinal gout tophi.
Table 1.
Clinical information of 5 patients with spinal gout.
The follow-up time respectively at 3, 6, and 12 months treatment and once per year after the first year. Relevant treatment was then administered, which alleviated the patient's symptoms and was shown to continue to provide symptom relief to the patients until the most recent follow-up.
4. Discussion
Long-term exposure to elevated serum uric acid levels leads to the formation of MSU crystals, which accumulate in the synovium and articular cartilage of peripheral joint tissues, leading to the formation of nodules called tophi (Fig. 1).[5,7] Tophi are easily visible in the peripheral joints; however, lesions occurring in the spine are not easily apparent and are rare, whereby the current incidence of spinal gout is still not clear.
Between 2014 and 2017, we evaluated more than 5000 patients who were admitted to our department for axial pain, radicular pain, and myelopathy. However, these symptoms could be attributed to spinal gout in only 5 of these cases. Many studies have shown that the incidence of gout involving the axial bones may be greater than that recorded previously, as the actual diagnosis of gouty spine is mainly based on pathological examination.[8,9] The clinical manifestations of spinal gout are diverse and the symptoms are nonspecific. Patients with spinal gout may present with axial pain, fever, and a variety of neurological symptoms, such as radicular pain, myelopathy, cauda equina syndrome, and claudication.[4,5]
All of the patients that were included in this study had hyperbaric, and 4 patients had a history of gout, which is similar to the findings reported by Toprover et al. [10] The literature indicates that spinal gout in patients without a history of gout is very rare and that the condition is generally observed in young individuals.[11,12] Jegapragasan et al[13] recommend that spinal gout should be considered in patients with low back pain or nerve compression symptoms who have a history of gout manifesting with multiple nodules, hyperuricemia, and characteristic imaging features. The positive value of laboratory tests in the diagnosis of spinal gout is limited. WBC count, serum CRP level, and ESR are not specific in spinal gout, but elevated levels of serum uric acid are known to positively contribute to the development of spinal gout.[14,15] In our study, only 2 patients had leukocytosis and elevated serum CRP levels, whereas all of the patients had elevated serum uric acid levels and ESR. Therefore, the possibility of spinal gout should be considered when a patient presents with a history of gout or elevated uric acid level along with axial pain, radicular pain, or myelopathy.
Plain radiography has poor diagnostic value in spinal gout. Computed tomography (CT) and magnetic resonance imaging (MRI) are the most specific imaging methods in the differential diagnosis of gout, although CT is often more useful in peripheral gout. Common CT findings in spinal gout are as follows: bony erosion with sclerotic borders and normal bone density. The use of dual-energy CT has become increasingly popular in recent years.[16] However, Carr et al[16] recently concluded that the dual-energy CT findings in spinal gout were not disease specific and that MSU deposition in the axial skeleton may be a physiologic phenomenon in middle-aged men. MRI results also show no specific signs in cases of spinal gout. Typically, the mass lesion appears isointense on T1W images and hypointense on T2W images, while some patients show soft tissue of equal or slightly lower signal intensity on T1W imaging and low or high signal intensity on T2W imaging.[17] Intravenous administration of gadolinium results in heterogeneous peripheral enhancement of the mass lesion on T2W images, and 1 case report has indicated that gout tophi of the lumbar spine can mimic spinal meningioma. Consistent with these findings, in our study, the lesions showed isointense or hypointense signals on T1W sections and were mostly hyperintense and isointense on T2W sections.
Because the imaging findings in these cases are largely nonspecific, histologic examination is necessary to confirm a diagnosis of spinal gout. Histopathologic examination of the tophus is required for the definitive diagnosis of spinal gout.[7] The specimens are usually extracted during surgery, because most patients with spinal tophi causing spinal cord or nerve root compression require surgical treatment. In fact, we believe that if pathologic examination is not performed, the diagnosis may be incorrect or missed. In all of the 5 cases that are included in our study, the definitive diagnosis was established through histopathologic examination and the detection of MSU crystals in the tophus or surgically removed specimen.
Our research has some limitations. First, the duration of follow-up in our study was relatively short, and the longest 1 patient with follow-up time was just 3 years. Second, our study belongs to the retrospective study of cases, those cases were rare, and only were available in clinical. Therefore, its characteristics are difficult to analyze. Early diagnosis of spinal gout and prompt medical treatment may help preclude the need for spinal cord decompression and surgical treatment due to disease progression. However, patients with symptoms of spinal cord or nerve root compression require immediate surgical treatment. The symptoms of all 5 patients in our study were improved by surgical decompression and conservative treatment.
5. Conclusion
On the basis of our experiences in these cases and our review of the relevant literature, we recommend that clinicians should pay careful attention to patients that present with axial pain, especially those with a history of elevated serum uric acid levels and gout. However, an accurate diagnosis of gout can be established only through histopathological examination of the biopsy specimen. Timely diagnosis and prompt initiation of medical treatment can prevent the progression of the disease; meanwhile, patients with neurological symptoms may generally require surgical decompression as well as proper medical treatment.
Author contributions
Conceptualization: Shaolong Ma, Rui Gu.
Data curation: Quanming An.
Formal analysis: Quanming An.
Funding acquisition: Shaolong Ma, Rui Jiang.
Investigation: Jianhui Zhao, Quanming An.
Methodology: Jianhui Zhao, Quanming An.
Project administration: Shaolong Ma, Jianhui Zhao, Rui Jiang.
Resources: Shaolong Ma, Rui Jiang.
Software: Shaolong Ma, Rui Jiang.
Supervision: Rui Gu.
Validation: Rui Gu.
Visualization: Rui Gu.
Footnotes
Abbreviations: ACDF = anterior cervical discectomy and fusion, BBD = bowel/bladder dysfunction, CKD = chronic kidney diseases, CLF = cervical ligamentum flavum, CT = computed tomography, ESR = erythrocyte sedimentation rate, Hx = history, Hyper = hyperintense, Hypo = hypointense, Iso = isontense, Lam = laminectomy, LE = lower extremity, MEY = myelopathy, MIS-TLIF = minimally invasive transforaminal lumbar interbody fusion, MRI = magnetic resonance imaging, MSU = monosodium urate crystals, PLCL = poster longitudinal cervical ligament, RAD = radiculopathy, Sx = symptoms, T1W = T1-weighted, T2W = T2-weighted, UE = upper extremity, WBC = white blood cell.
This article is supported by the Department of Finance of Jilin province (project number: 3D516Q233430).
The authors have no conflicts of interest to disclose.
References
- [1].Dalbeth N, Merriman TR, Stamp LK. Gout. Lancet 2016;388:2039–52. [DOI] [PubMed] [Google Scholar]
- [2].Emmerson BT. The management of gout. N Engl J Med 1996;334:445–51. [DOI] [PubMed] [Google Scholar]
- [3].Woodward OM. ABCG2: the molecular mechanisms of urate secretion and gout. Am J Physiol Renal Physiol 2015;309:F485–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Beier CP, Hartmann A, Woertgen C, et al. A large, erosive intraspinal and paravertebral gout tophus. Case report. J Neurosurg Spine 2005;3:485–7. [DOI] [PubMed] [Google Scholar]
- [5].Draganescu M, Leventhal LJ. Spinal gout: case report and review of the literature. J Clin Rheumatol 2004;10:74–9. [DOI] [PubMed] [Google Scholar]
- [6].Kersley GD, Mandel L, Jeffrey MR. Gout; an unusual case with softening and subluxation of the first cervical vertebra and splenomegaly. Ann Rheum Dis 1950;9:282–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hasturk AE, Basmaci M, Canbay S, et al. Spinal gout tophus: a very rare cause of radiculopathy. Eur Spine J 2012;21Suppl 4:S400–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].de Mello FM, Helito PV, Bordalo-Rodrigues M, et al. Axial gout is frequently associated with the presence of current tophi, although not with spinal symptoms. Spine (Phila Pa 1976) 2014;39:E1531–6. [DOI] [PubMed] [Google Scholar]
- [9].Saketkoo LA, Robertson HJ, Dyer HR, et al. Axial gouty arthropathy. Am J Med Sci 2009;338:140–6. [DOI] [PubMed] [Google Scholar]
- [10].Toprover M, et al. Gout in the Spine: imaging, diagnosis, and outcomes. Curr Rheumatol Rep 2015;17:70. [DOI] [PubMed] [Google Scholar]
- [11].King JC, Nicholas C. Gouty arthropathy of the lumbar spine: a case report and review of the literature. Spine (Phila Pa 1976) 1997;22:2309–12. [DOI] [PubMed] [Google Scholar]
- [12].Varga J, Giampaolo C, Goldenberg DL. Tophaceous gout of the spine in a patient with no peripheral tophi: case report and review of the literature. Arthritis Rheum 1985;28:1312–5. [DOI] [PubMed] [Google Scholar]
- [13].Jegapragasan M, Calniquer A, Hwang WD, et al. A case of tophaceous gout in the lumbar spine: a review of the literature and treatment recommendations. Evid Based Spine Care J 2014;5:52–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Barrett K, Miller ML, Wilson JT. Tophaceous gout of the spine mimicking epidural infection: case report and review of the literature. Neurosurgery 2001;48:1170–2. discussion 1172-1173. [DOI] [PubMed] [Google Scholar]
- [15].Yamamoto M, Tabeya T, Masaki Y, et al. Tophaceous gout in the cervical spine. Intern Med 2012;51:325–8. [DOI] [PubMed] [Google Scholar]
- [16].Carr A, Doyle AJ, Dalbeth N, et al. Dual-energy CT of urate deposits in costal cartilage and intervertebral disks of patients with tophaceous gout and age-matched controls. AJR Am J Roentgenol 2016;206:1063–7. [DOI] [PubMed] [Google Scholar]
- [17].Yoon JW, Park KB, Park H, et al. Tophaceous gout of the spine causing neural compression. Korean J Spine 2013;10:185–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


