Abstract
Background
Sickle cell disease (SCD) is a group of disorders that affects haemoglobin, which causes distorted sickle‐ or crescent‐shaped red blood cells. It is characterized by anaemia, increased susceptibility to infections and episodes of pain. The disease is acquired by inheriting abnormal genes from both parents, the combination giving rise to different forms of the disease. Due to increased erythropoiesis in people with SCD, it is hypothesized that they are at an increased risk for folate deficiency. For this reason, children and adults with SCD, particularly those with sickle cell anaemia, commonly take 1 mg of folic acid orally every day on the premise that this will replace depleted folate stores and reduce the symptoms of anaemia. It is thus important to evaluate the role of folate supplementation in treating SCD.
Objectives
To analyse the efficacy and possible adverse effects of folate supplementation (folate occurring naturally in foods, provided as fortified foods or additional supplements such as tablets) in people with SCD.
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group’s Haemoglobinopathies Trials Register comprising references identified from comprehensive electronic database searches and handsearches of relevant journals and abstract books of conference proceedings. We also conducted additional searches in both electronic databases and clinical trial registries.
Date of last search of the Cochrane Cystic Fibrosis and Genetic Disorders Group’s Haemoglobinopathies Trials Register: 17 November 2017.
Selection criteria
Randomised, placebo‐controlled trials of folate supplementation for SCD.
Data collection and analysis
Four review authors assessed We used the standard Cochrane‐defined methodological procedures.
Four review authors independently assessed the eligibility and risk of bias of the included trials and extracted and analysed the data included in the review. The quality of the evidence was assessed using GRADE.
Main results
One trial, undertaken in 1983, was eligible for inclusion in the review. This was a double‐blind placebo‐controlled quasi‐randomised triaI of supplementation of folic acid in people with SCD. A total of 117 children with homozygous sickle cell (SS) disease aged six months to four years of age participated over a one‐year period (analysis was restricted to 115 children).
Serum folate measures, obtained after trial entry at six and 12 months, were available in 80 of 115 (70%) participants. There were significant differences between the folic acid and placebo groups with regards to serum folate values above 18 µg/L and values below 5 µg/L (low‐quality evidence). In the folic acid group, values above 18 µg/L were observed in 33 of 41 (81%) compared to six of 39 (15%) participants in the placebo (calcium lactate) group. Additionally, there were no participants in the folic acid group with serum folate levels below 5 µg/L, whereas in the placebo group, 15 of 39 (39%) participants had levels below this threshold. Haematological indices were measured in 100 of 115 (87%) participants at baseline and at one year. After adjusting for sex and age group, the investigators reported no significant differences between the trial groups with regards to total haemoglobin concentrations, either at baseline or at one year (low‐quality evidence). It is important to note that none of the raw data for the outcomes listed above were available for analysis.
The proportions of participants who experienced certain clinical events were analysed in all 115 participants, for which raw data were available. There were no statistically significant differences noted; however, the trial was not powered to investigate differences between the folic acid and placebo groups with regards to: minor infections, risk ratio (RR) 0.99 (95% confidence interval (CI) 0.85 to 1.15) (low‐quality evidence); major infections, RR 0.89 (95% CI 0.47 to 1.66) (low‐quality evidence); dactylitis, RR 0.67 (95% CI 0.35 to 1.27) (low‐quality evidence); acute splenic sequestration, RR 1.07 (95% CI 0.44 to 2.57) (low‐quality evidence); or episodes of pain, RR 1.16 (95% CI 0.70 to 1.92) (low‐quality evidence). However, the investigators reported a higher proportion of repeat dactylitis episodes in the placebo group, with two or more attacks occurring in 10 of 56 participants compared to two of 59 in the folic acid group (P < 0.05).
Growth, determined by height‐for‐age and weight‐for‐age, as well as height and growth velocity, was measured in 103 of the 115 participants (90%), for which raw data were not available. The investigators reported no significant differences in growth between the two groups.
The trial had a high risk of bias with regards to random sequence generation and incomplete outcome data. There was an unclear risk of bias in relation to allocation concealment, outcome assessment, and selective reporting. Finally, There was a low risk of bias with regards to blinding of participants and personnel. Overall the quality of the evidence in the review was low.
There were no trials identified for other eligible comparisons, namely: folate supplementation (fortified foods and physical supplementation with tablets) versus placebo; folate supplementation (naturally occurring in diet) versus placebo; folate supplementation (fortified foods and physical supplementation with tablets) versus folate supplementation (naturally occurring in diet).
Authors' conclusions
One doubIe‐blind, placebo‐controlled triaI on folic acid supplementation in children with SCD was included in the review. Overall, the trial presented mixed evidence on the review's outcomes. No trials in adults were identified. With the limited evidence provided, we conclude that, while it is possible that folic acid supplementation may increase serum folate levels, the effect of supplementation on anaemia and any symptoms of anaemia remains unclear.
If further trials were conducted, these may add evidence regarding the efficacy of folate supplementation. Future trials should assess clinical outcomes such as folate concentration, haemoglobin concentration, adverse effects and benefits of the intervention, especially with regards to SCD‐related morbidity. Such trials should include people with SCD of all ages and both sexes, in any setting. To investigate the effects of folate supplementation, trials should recruit more participants and be of longer duration, with long‐term follow‐up, than the trial currently included in this review. However, we do not envisage further trials of this intervention will be conducted, and hence the review will no longer be regularly updated.
Keywords: Child, Preschool; Humans; Infant; Anemia, Sickle Cell; Anemia, Sickle Cell/blood; Anemia, Sickle Cell/drug therapy; Double-Blind Method; Erythrocyte Indices; Folic Acid; Folic Acid/administration & dosage; Folic Acid/blood; Growth; Hematinics; Hematinics/administration & dosage
Plain language summary
Folate supplementation in people with sickle cell disease
Review question
We wanted to assess how effective and safe folate supplementation (folate occurring naturally in foods, provided as fortified foods or additional supplements such as tablets) is in people with sickle cell disease (SCD).
Background
SCD is a group of disorders affecting haemoglobin (the molecule in red blood cells that delivers oxygen to cells throughout the body), leading to distorted sickle or crescent‐shaped red blood cells. It is characterized by anaemia (the blood cannot carry enough oxygen around the body), repeated infections and episodes of pain. While SCD was originally found in the tropics and subtropics, due to migration, it is now common worldwide. There are three widely‐used preventative measures for managing SCD, these include penicillin, immunisation against pneumococcal infection and folate supplementation. Folate is a water‐soluble B vitamin needed for erythropoiesis (the process which produces red blood cells). Given there is increased erythropoiesis in people with SCD, it is thought they may require increased folate intake, by supplements or through diet. However, a lack of evidence‐based research means it is still not clear whether the benefits of supplementation outweigh the risk of possible adverse effects.
Search date
The evidence is current to: 17 November 2017.
Study characteristics
We included one trial with 117 children with SCD aged between six months and four years. This was a one‐year doubIe‐blind (both participants and doctors did not know which treatment group the participants were allocated to) controlled triaI comparing children taking folic acid supplements to those taking a placebo (a 'dummy' treatment).
Key results
The trial investigators reported that folic acid supplementation led to higher levels of folic acid measured in the blood. However, there were no differences in haemoglobin concentrations at the end of one year.
The trial also reported on clinical factors linked to treatment, including growth, major and minor infections, acute splenic sequestration, episodes of bone or abdominal pains. The investigators reported no differences in these outcomes from baseline to the end of the trial; however, the trial was not large enough to detect any possible differences reported between the folic acid group and the placebo group.
Quality of the evidence
In the included trial it was not clear how participants were allocated to receive folic acid or placebo. The method of making sure that participants and trial staff did not know what treatment each person was receiving (called allocation concealment) was also not described. These two factors mean that the trial had a high risk of biased results.
The trial did not contain many participants. For many of its clinical endpoints, it was not designed to show differences between people taking folic acid and those taking a placebo. This means that the results from this trial are imprecise, and therefore hard to interpret.
Finally, our review was meant to investigate folate supplementation (folate occurring naturally in foods, provided as fortified foods or additional supplements such as tablets) in children and adults. Because we only identified one trial that investigated one form of supplementation in children, the results are not useful for other populations.
Therefore, we judged the evidence from the included trial to be of low quality. Based on just one low quality study with evidence only to show that folate supplementation raises the blood levels of folic acid, we cannot state whether this treatment is effective or not.
More trials with more people and longer treatment duration (and follow‐up) of folate supplementation in people with SCD are needed to strengthen this review; however, we do not envisage further trials of this intervention will be conducted, and hence the review will no longer be regularly updated.
Summary of findings
Summary of findings 1. Summary of findings.
| Folic acid compared with calcium lactate for sickle cell (SS) disease | ||||||
|
Patient or population: 117 children with homozygous sickle cell (SS) disease Settings: hospital Intervention: folic acid 5 mg Comparison: calcium lactate (placebo) | ||||||
| Outcomes1 | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| calcium lactate (placebo) | folic acid 5 mg | |||||
|
Folate concentration Serum folate levels, measured between 6 and 12 months after entry to the study |
1. values in excess of 18 pg/L occurring in 6/39 (15%) children in the placebo group. 2. levels below 5 pg/L occurring in 15/39 (39%) |
1. values in excess of 18 pg/L occurring in 33/41 (81 %) in
the folic acid group. 2. levels below 5 pg/L None in the folic acid group. |
NA | 80/115 (70%) (1 study) |
⊕⊕⊝⊝ low2 |
There were marked differences between trial groups in the distribution of serum folate levels. |
|
Haemoglobin concentration Haematological analyses were performed in the 100/115 (87%) children in whom baseline (within 2 months of entry to study) and 1 year (between 10 and 14 months after entry) |
See comment | See comment | NA | 100/115 (87%) (1 study) |
⊕⊕⊝⊝ low2 |
There were no significant differences in total haemoglobin (Hb) either at baseline or after 1 year. |
|
Adverse events ‐ acute splenic sequestration Clinical events experienced by children during the 1 year period commencing at entry to the trial |
8/56 Total episodes 15 |
9/59 Total episodes 12 |
RR 1.07 (95% CI 0.44 to 2.57) |
115 (1 study) |
⊕⊕⊝⊝ low2 |
There were no significant differences in these measures of growth between the folic acid and placebo groups. |
|
Adverse events ‐ painful episodes Clinical events experienced by children during the 1 year period commencing at entry to the trial |
18/56 Total episodes 27 |
22/59 Total episodes 39 |
RR 1.16 (95% CI 0.70 to 1.92) |
115 (1 study) |
⊕⊕⊝⊝ low2 |
There were no significant differences in painful episodes between the folic acid and placebo groups. |
|
Adverse events ‐ minor Infections Clinical events experienced by children during the 1 year period commencing at entry to the trial |
48 out of 56 children Total episodes/child: 2.3 |
50 out of 59 children Total episodes/child: 2.7 |
RR 0.99 (95% CI 0.85 to 1.15) |
115 (1 study) |
⊕⊕⊝⊝ low2 |
There were no differences in minor infections between the folic acid and placebo groups. |
|
Adverse events ‐ major infections Clinical events experienced by children during the 1 year period commencing at entry to the trial |
15 out of 56 children Total episodes/child: 19 |
14 out of 59 children Total episodes/child: 18 |
RR 0.89 (95% CI 0.47 to 1.66) |
115 (1 study) |
⊕⊕⊝⊝ low2 |
There were no differences in major infections between the folic acid and placebo group. |
|
Adverse events ‐ dactylitis Clinical events experienced by children during the 1 year period commencing at entry to the trial |
17 out of 56 children Total episodes: 32 |
12 out of 59 children Total episodes:15 |
RR 0.67 (95% CI 0.37 to 1.27) | 115 (1 study) |
⊕⊕⊝⊝ low2 |
There were no differences in dactylitis events between the folic acid and placebo group. |
| *The basis for the assumed risk is the risk in the control group. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1. Pre‐specified adverse events of 'Increased incidence of priapism' and 'The risk of masking cobalamin deficiency with consequent neuropsychiatric manifestations (nanogram per litre (ng/L))' were not assessed in the included trial (Rabb 1983). In the included trial, there was no difference between the folic acid and placebo groups for growth, determined by height‐for‐age and weight‐for‐age as well as height and growth velocity.
2. Reason for downgrading evidence to low ‐ very serious risk of bias (two domains of high risk of bias).
Background
Description of the condition
Sickle cell disease (SCD) is a group of disorders affecting haemoglobin leading to distorted sickle or crescent shaped red blood cells; it is characterized by anaemia, increased susceptibility to infections and episodes of pain. Pauling first showed the abnormal electrophoretic mobility of haemoglobin in an affected individual (Pauling 1949) and in 1957 Ingram discovered that the defect of the disease was a single amino acid substitution in the haemoglobin molecule of sickle cells (HbS) (Ingram 1957). The disease is acquired by inheriting abnormal genes from both parents, the combination giving rise to different forms of SCD. The SCD genotypes are as follows (Stuart 2004).
HbSS disease or sickle cell anaemia: homozygote for the βs globin with a severe or moderately severe phenotype.
HbS/β° thalassaemia: severe double heterozygote for HbS and βº thalassaemia, and almost indistinguishable from sickle cell anaemia phenotypically.
HbSC disease: double heterozygote for HbS and HbC with intermediate clinical severity.
HbS/β+ thalassaemia: double heterozygote for HbS and β+ thalassaemia with mild to moderate severity, but variable in different ethnic groups.
HbS/hereditary persistence of fetal Hb (S/HPHP): very mild phenotype or symptom‐free.
HbS/HbE syndrome: double heterozygote for HbS and HbE, very rare and generally very mild clinical course.
Rare combinations of HbS with HbD Los Angeles, HbO Arab, G‐Philadelphia, among others.
In most of sub‐Saharan Africa, SCD is very common, affecting up to 3% of births (Grosse 2011). While inherited haemoglobin disorders (SCD and thalassemias) were originally characteristic of the tropics and subtropics, due to migration, they are now common worldwide. The occurrence of the HbS gene is more common, spreading across parts of Sicily, Italy, Greece, Turkey, Africa, Saudi Arabia and India. In some regions of northern Greece, eastern Saudi Arabia and central India the prevalence varies from 20% to 30% (Serjeant 1997).
The cause of SCD is the substitution of valine for glutamic acid at the sixth position of beta‐globin.This leads to the production of an abnormal form of haemoglobin, haemoglobin S (HbS) (Aliyu 2006). The deleterious effects of SCD can affect nearly every organ system in the body. The two predominant pathologic features of SCD are haemolytic anaemia and vaso‐occlusion (Rees 2010). Important clinical manifestations of SCD are haemolysis, chronic anaemia, aplastic crises, vaso‐occlusion, acute chest syndrome, recurrent acute pain (e.g. dactylitis, musculoskeletal, or abdominal), functional asplenia, increased susceptibility to infections, jaundice, stroke, and pulmonary hypertension (Lane 1996).
Three measures have been widely used as prophylaxis for the management of SCD, these include penicillin prophylaxis, immunization against pneumococcal infection and folic acid supplementation (Aliyu 2006). Hydroxyurea is another highly efficacious prophylactic and therapeutic chemotherapeutic agent used in people with SCD, but it has limited international availability and accessibility (Wong 2014). Bone marrow transplantation (BMT) is the only cure for SCD; however, BMT requires a human lymphocyte antigen (HLA)‐identical donor. The difficulty in finding a suitable donor for BMT limits its widespread use. Therefore, the primary mode of disease management for SCD remains drug therapy aimed at decreasing the complications of this disease (Ndefo 2008).
In most people with homozygous SCD, the red blood cell (RBC) count is lower than normal because the average life span of sickled RBCs is about 17 days, in contrast to 120 days for normal RBCs (Schnog 2004). This high cell turnover may deplete the folate stores (Ndefo 2008). It is proposed that folate supplementation in the setting of anaemia raises haemoglobin levels and helps provide a healthy reticulocyte response. Hence, in the management of haemolytic anaemia in SCD, folic acid may replenish the depleted folate stores necessary for erythropoiesis (Stuart 2004).
Description of the intervention
Folic acid is available as multivitamin tablets in combination with other B‐complex vitamins (frequently at a dose of 400 micrograms (mcg)), or as a stand‐alone supplement. Paediatric doses commonly contain between 200 mcg and 400 mcg folic acid (Yeung 2011). Approximately 85% of supplemental folic acid, when taken with food, is bioavailable; however, when consumed without food, nearly 100% of supplemental folic acid is bioavailable (Carmel 2005; Standing Committee 1998). Folate is a water‐soluble B vitamin. The term folate includes both naturally occurring food folate and folic acid that is used in dietary supplements and fortified foods. Folic acid contains one molecule of glutamic acid, whereas the food folates are polyglutamates. The presence of polyglutamates in the food folates limits its bioavailability as compared to folic acid present in dietary supplements (Bailey 2004).
Folate is found naturally in a wide variety of foods, including vegetables (especially dark green leafy vegetables), fruits and fruit juices, nuts, beans, peas, dairy products, poultry and meat, eggs, seafood, and grains (Carmel 2005). Spinach, liver, yeast, asparagus, and Brussels sprouts are among the foods with the highest levels of folate.
The Food and Nutrition Board (FNB) developed dietary folate equivalents (DFE) to reflect the higher bioavailability of folic acid than that of food folate. At least 85% of folic acid is estimated to be bioavailable when taken with food, whereas only about 50% of folate naturally present in food is bioavailable (Carmel 2005; Standing Committee 1998).
Based on these values, the FNB defined DFE as follows:
1 mcg DFE = 1 mcg food folate;
1 mcg DFE = 0.6 mcg folic acid from fortified foods or dietary supplements consumed with foods;
1 mcg DFE = 0.5 mcg folic acid from dietary supplements taken on an empty stomach.
How the intervention might work
Folate acts as a co‐enzyme that carries and activates single carbons for the synthesis of purine nucleic acids (for DNA and RNA synthesis), thimidylate (for DNA synthesis) and for the conversion of homocysteine to methionine. Folate is required for DNA synthesis and hence proper cell division, the impairment of which can lead to megaloblastic anaemia (Bailey 2012; Carmel 2005; Standing Committee 1998). Due to increased erythropoiesis in people with SCD, it is hypothesized that they are at an increased risk for folate deficiency. For this reason, children and adults with SCD, particularly those with sickle cell anaemia, commonly take 1 mg of folic acid orally every day on the premise that this will replete depleted folate stores and reduce the symptoms of anaemia. In SCD, folate intake leads to a decrease in symptoms of anaemia (Falletta 1995). Other potential advantages of folate therapy in people with SCD include the prevention of hyperhomocysteinemia that may predispose to thrombotic events (Selhub 1995), which, in turn, may lead to painful episodes.
However, some studies note that in people with SCD, folate supplementation did not improve folate status or megaloblastic changes and folic acid supplementation did not elevate the serum and erythrocyte folate levels (Al‐Yassin 2012).
Possible side effects of folate supplementation include increased priapism and increased twin pregnancy rates in people with SCD (Al‐Yassin 2012). However, the relationship between twinning and folic acid supplementation is not supported (Wolff 2009). Early case reports suggested that a daily folic acid intake of 5000 μg or more could mask a vitamin B12 deficiency by preventing the development of anaemia. In turn, this could delay the diagnosis of an underlying vitamin B12 deficiency and thereby allow vitamin B12 deficiency‐associated neuropathies to progress (Dhar 2003). However, anaemia is no longer the recommended diagnostic indicator of vitamin B12 deficiency.
Potential side effects of folate supplementation have also been described in the general population. A randomised controlled trial (RCT) found adverse effects of routine supplementation of iron and folic acid in pre‐school children in a malaria‐prevalent area, with the supplemented group showing an increased risk of severe illness (malaria and other infections) and death (Sazawal 2006). Another side effect of supplementation may be an increased risk of some neoplasms depending on the dosage and timing of the exposure. For example, with regard to colorectal carcinoma, folate intervention after the microscopic neoplastic foci are established may promote colorectal carcinogenesis (Kim 2006). Another single study found a detrimental effect of high folate intake on cancer‐protective natural killer cells (Troen 2006). However, meta‐analyses of RCTs have not supported a role for folic acid supplementation in cancer risk (Vollset 2013).
There are also many potential benefits of folate supplementation within the general population. An RCT found folic acid helpful in preventing miscarriage and birth defects (spina bifida) in women who were pregnant or planning for pregnancy (Blencowe 2010). Folate supplementation combined with other B vitamins was shown to have some benefits in primary stroke prevention (Huo 2015; Lee 2010). A Cochrane review on the effectiveness of folate supplementation for depressive disorders concluded that folates may have a potential role when combined with other treatment (Taylor 2003). In a further RCT, it was observed that folic acid supplementation for three years significantly improved domains of cognitive function that tend to decline with age (Durga 2007).
An elevated homocysteine level has been associated with an increased risk of cardiovascular disease. Folate and other B vitamins are involved in homocysteine metabolism and researchers have hypothesized that they reduce cardiovascular disease risk by lowering homocysteine levels (Clarke 1998; Stott 2005). However, clinical trails indicate that folic acid supplementation does not reduce the risk of cardio‐vascular disease (CVD) (Clarke 2010).
Serum or erythrocyte folate concentration are used to diagnose folate deficiency. A serum folate value of more than 3 ng/mL and an erythrocyte folate concentration of more than 140 ng/mL indicates adequate folate status (Bailey 2012; Standing Committee 1998; Yetley 2011). Erythrocyte folate concentration provides a longer‐term measure of folate intake, unlike serum folate that varies with recent dietary intake (Standing Committee 1998; Yetley 2011). Another functional indicator of folate status is plasma homocysteine concentration. However, plasma homocysteine concentration is not specific for folate status, as it is influenced by other factors such as kidney dysfunction, vitamin B12 deficiency and levels of other micronutrients (Bailey 2012; Carmel 2005; Green 2011).
Why it is important to do this review
Folic acid supplementation in SCD is based on the premise that chronic haemolysis, inherent to the disease, leads to increased erythropoiesis which thereby depletes folate stores. It is therefore hypothesized that folate supplementation may replete these stores and thereby reduce the symptoms of anaemia. However, folic acid has been found to have possible deleterious effects in selected populations such as those with increased severity of malarial illness (Sazawal 2006). Furthermore, there is contrasting research‐based evidence regarding the efficacy of folate supplementation in SCD. It remains unclear whether the possible deleterious effects of high folic acid outweigh the potential benefits. For this reason we need a better understanding of the relevant biological and clinical efficacy of folate supplementation as compared to the potential adverse effects. Hence, the rationale for this review is to analyse the benefits and risks of folic acid supplementation (folic acid supplements or folate supplementation, or provision, through diet) in people with SCD.
Objectives
To analyse the efficacy and possible adverse effects of folate supplementation (folate occurring naturally in foods, provided as fortified foods or additional supplements such as tablets) in people with SCD.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) or quasi‐RCTs, including cluster‐randomised trials.
Types of participants
People with known SCD (SS, SC, Sβ+ and Sβº, proven by electrophoresis and sickle solubility test, with family studies or DNA tests as appropriate) of all ages and both sexes, in any setting.
Types of interventions
Folic acid supplements versus placebo
Folate supplementation, or provision, through diet versus placebo
Folic acid supplements versus folate supplementation through diet
Types of outcome measures
Primary outcomes
Folate concentration (using serum or plasma folate or erythrocyte folate)
Hemoglobin concentration (grams per decilitre (g/dL))
-
Adverse effects of the intervention, including but not limited to:
increased incidence of priapism;
the risk of masking cobalamin deficiency with consequent neuropsychiatric manifestations (nanogram per litre (ng/L));
any other side effects (e.g. twin pregnancy, etc.), including SCD‐related morbidities (e.g. pain, acute splenic sequestration, strokes, priapism, recurrent infections).
Secondary outcomes
Homocysteine levels (micromoles per litre (μmol/L))
Quality of life (as measured by validated scales, e.g. the Health‐related Quality of Life Assessment (HRQL) Scale)
Search methods for identification of studies
Electronic searches
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group's Haemoglobinopathies Trials Register using the terms: ((sickle cell OR (haemoglobinopathies AND general)) and (folate OR folic acid*).
The Haemoglobinopathies Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library) and weekly searches of MEDLINE. Unpublished work is identified by searching the abstract books of five major conferences: the European Haematology Association conference; the American Society of Hematology conference; the British Society for Haematology Annual Scientific Meeting; the Caribbean Public Health Agency Annual Scientific Meeting (formerly the Caribbean Health Research Council Meeting); and the National Sickle Cell Disease Program Annual Meeting. For full details of all searching activities for the register, please see the relevant section of the Cochrane Cystic Fibrosis and Genetic Disorders Group's website.
Date of the most recent search of the Cystic Fibrosis and Genetic Disorders Group's Haemoglobinopathies Trials Register: 17 November 2017.
Four review authors (R Dixit, S Nettem, HH Soe, L Vance) conducted additional searches to minimize publication and reporting biases. We searched the following electronic databases (with no language or date restrictions): Ovid Embase (http://ospguides.ovid.com/OSPguides/embase.htm) (02 August 2015) (Appendix 1); PubMed (http://www.ncbi.nlm.nih.gov/pubmed) (31 July 2015) (Appendix 2); CINAHL (https://www.ebscohost.com/nursing/cinahl-databases) (31 July 2015) (Appendix 3), (EBSCO Health) and CABI (http://www.cabi.org/publishing-products/resources-for-database-users/) (31 July 2015) (Web of Science) (Appendix 4). We also searched three trial registries: ISRCTN (http://www.isrctn.com/) (02 August 2015) (Appendix 5); ClinicalTrials.gov (http://clinicaltrials.gov/) (Appendix 6) (31 July 2015 and 02 February 2018) and; ICTRP (http://apps.who.int/trialsearch/) (03 Aug 2015 and 02 February 2018) (Appendix 7).
Searching other resources
We checked reference lists of articles and reviews for possible relevant trials. We also contacted other researchers or nutritional and SCD experts working in this field to identify additional trials (including unpublished and ongoing trials).
Data collection and analysis
Selection of studies
Four review authors (R Dixit, S Nettem, L Vance, P Stover) independently assessed the eligibility of the trials identified by the searches. When we did not find the relevant information in the abstract, we retrieved the relevant full text report(s) (if published) in order to complete this task. Two review authors (R Dixit, S Nettem) also assessed the eligibility criteria by completing the eligibility form that was designed in accordance with the inclusion criteria. We tabulated the excluded studies under ‘Characteristics of excluded studies’ and gave reasons for the exclusion.
The review authors were not blinded to the trial authors, institutions and trial results during their assessments.
We dealt with any issues or concerns by discussion or with help from the third review author whenever needed.
Data extraction and management
Two review authors (R Dixit, HH Soe) independently extracted data for primary and secondary outcomes in a customised data collection form developed by the Cochrane Cystic Fibrosis & Genetic Disorders Group. Following data extraction one author (R Dixit) entered the data into the Review Manager software (RevMan 2014) and a second author (SS Madan) cross checked for any errors or inconsistencies. We used a standard data extraction form which included at least the following items.
Method: year of the trial; trial duration; type of randomisation; allocation; concealment method; blinding; trial area; and sampling method.
Participants: number of participants in control and intervention groups; age; sex; similarity of group at baseline; and loss to follow‐up with reasons.
Interventions: interventions (dose, route and duration); comparison intervention (dose, route and duration); and co‐medication (dose, route and duration).
Outcomes: primary and secondary outcomes as mentioned above; any other outcomes assessed; times of assessment; and length of follow‐up.
Notes: published or unpublished data; title; authors; source; contact address; language of publication; year of publication; and funding sources, if any.
We resolved any issues and concerns in data extraction by discussion and consensus.
Assessment of risk of bias in included studies
Three review authors (R Dixit, S Nettem, L Vance) independently assessed the risk of bias of the included trials by using the criteria outlined in chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
We assessed the following five components for each trial:
random sequence generation (selection bias);
allocation concealment (selection bias);
masking (blinding) of participants and personnel (performance bias), and masking of outcome assessment;
incomplete outcome data (attrition bias) through withdrawals, dropouts and protocol deviations; and
selective reporting bias.
We also assessed for any other sources of bias as reported in the Cochrane Handbook for Systematic Reviews of Interventions (bias related to the specific trial design, early or abrupt end of trials, fraudulent trial).
For each of the mentioned components, we assigned judgements of either low, unclear or high risk of bias.
We recorded the results in the relevant Characteristics of included studies tables in Review Manager 5 (RevMan 2014), and summarised the findings in a ‘Risk of bias’ table or graph.
We resolved any concerns or issues by discussion or with opinion of a third review author (AL Abas).
Measures of treatment effect
For data analysis, we followed the guidelines set out in chapter 9 of the Cochrane Handbook for Systematic Review of Interventions (Deeks 2011).
For dichotomous data (cobalamin deficiency), we presented results as summary risk ratio (RR) with 95% confidence intervals (CI).
For event rates (recurrent infections and Increased incidence risk of priapism), we will present results as summary rate ratio.
For continuous data (folate concentration, haemoglobin concentration and homocysteine levels, quality of life), we will use the mean difference (MD) and corresponding 95% CIs. For future updates, if different scales have been used for assessing the quality of life outcome, we will present the results as a standardized mean difference (SMD). Also, if necessary, original outcome data will be transformed to substantially reduce skewness. If we find data that has not been transformed we will try to retrieve individual patient data (IPD) so that we can apply log transformation. Reports of trials may present results on a transformed scale (Higgins 2011b).
Given that meta‐analysis was not possible, we have presented a narrative summary along with tabulated data.
Unit of analysis issues
The unit of analysis was the participant with SCD.
Along with individually‐randomised trials, in future updates of this review, we plan to include (if found) cluster‐randomised trials. If we identify such trials for future updates, we will adjust the standard error (SE) of the effect estimate from cluster trials using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We will only make the necessary adjustments if that cluster trial has not been analysed correctly. We will undertake meta‐analyses using the generic inverse‐variance method available in Review Manager 5 (RevMan 2011). We will use an estimate of the intra‐cluster correlation co‐efficient (ICC) derived from the trial (if possible), or from another source. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC.
If, for future updates, we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the trial designs. We will also combine the results from both if the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a separate meta‐analysis if appropriate.
For cross‐over trials, we plan to always use a paired analysis from the two participants' periods in the meta‐analysis (Higgins 2011b).
Dealing with missing data
In order to allow an intention‐to‐treat analysis, we would have searched for full reports from the trial investigators if the included trial had only been published in abstract form, presented at meetings or reported to the co‐authors. In the future, if information is missing or unclear, we will contact the trial investigators for further details.
Although we recognise that intention‐to‐treat analysis in RCTs is the analysis methodology of choice, for future updates, in trials where data are missing due to participant dropout, we will conduct a primary analysis based on participants with complete data. It will be assumed that missing outcomes will not be a problem if loss to follow‐up is well documented and unrelated to outcomes in both trial arms, as per chapter 16 of the Cochrane Handbook for Systematic Review of Interventions (Higgins 2011b). In future updates with more trials, we will be able to perform a sensitivity analyses that will determine the effect of leaving out papers without complete data.
Assessment of heterogeneity
For future updates, if more trials are included we will use the Chi² test for assessing heterogeneity (significance level P < 0.1). We will quantify the degree of heterogeneity using the I² statistic (Deeks 2011).
The guidelines for interpretation of the I² values are as follows:
0% to 40% indicates unimportant levels of heterogeneity; 30% to 60% indicates moderate heterogeneity; 50% to 90% indicates substantial heterogeneity; 75% to 100% indicates considerable heterogeneity
We will also consider a visual inspection of the forest plot to see whether CIs overlap.
Assessment of reporting biases
Four review authors (R Dixit, S Nettem, HH Soe, L Vance) conducted extensive searches to minimize publication and reporting biases.
While searching trial registries we also looked for trial protocols; however, we did not find any for comparison with final trial reports. Within trials, we compared the 'Methods' section to the 'Results' section of the fully published paper to ensure that all of the outcomes that were mentioned in the objectives were reported in the results.
For future updates of the review, if 10 or more trials are included, we plan to use funnel plots to assess publication bias. In cases were asymmetry is detected, we will explore the causes(s).
Data synthesis
We carried out statistical analysis using Review Manager software (RevMan 2014). We meta‐analysed data where trials investigated similar comparisons and the same outcomes. We used a fixed‐effect meta‐analysis for combining data where it was reasonable to assume that trials were estimating the same underlying treatment effect, i.e. where trials are examining the same intervention, and the populations and methods are sufficiently similar.
For future updates, if we find significant heterogeneity, we plan to use a random‐effects model. No meta‐analysis was performed in this review, but meta‐analyses will be performed if appropriate studies are found in future.
Subgroup analysis and investigation of heterogeneity
We did not perform any subgroup analyses for this review.
Sensitivity analysis
If in the future, when we include more trials, we plan to carry out sensitivity analyses to investigate the robustness of the results regarding the various components of the risk of bias. We examined the effect on the primary outcome of excluding any trial judged to be at a high risk of bias by three of the domains: sequence generation; allocation concealment; and masking.
Summary of findings table
We have created a 'Summary of findings' table using the GRADE pro software (version 3.5) (Table 1). In the table we have assessed several parameters, such as limitations of design, inconsistency, indirectness, imprecision and publication bias. We included the primary outcomes of differences in red blood cell folate concentration level, haemoglobin concentration and adverse events including any reduction in recurrent infections.
Results
Description of studies
Please refer to the tables section of the review (Included studies; Excluded studies).
Results of the search
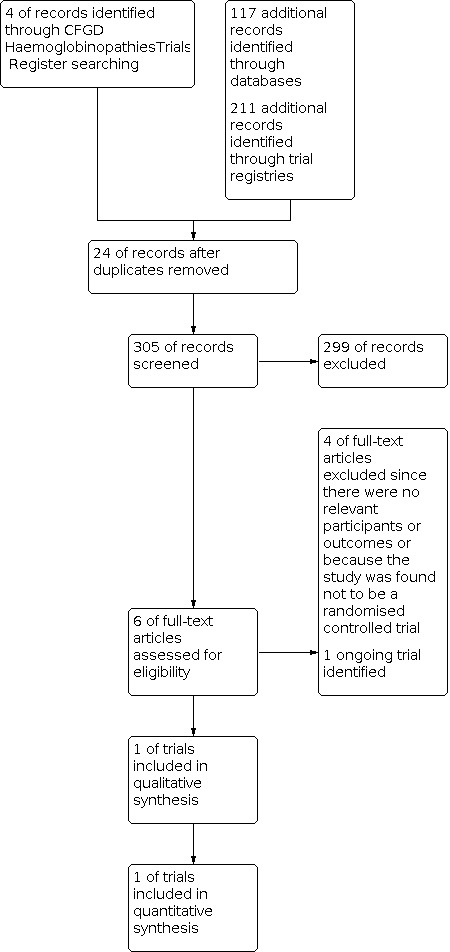
Of the four trials identified through the Cochrane Cystic Fibrosis and Genetic Disorders Group's Haemoglobinopathies Trials Register and the 328 trials and reports identified through other sources such as PubMed and ICTRP, we removed 24 duplicate records, leaving a total of 305 trials or reports. After initial screening of the 305 records, 300 were excluded. Then we proceeded to obtain the full text of all five trials out of which one trial including 117 participants met the inclusion criteria (Rabb 1983) and four studies were excluded (V‐FIT 2014; Hendrickse 1966; Liu 1975; Mojtahedzadeh 2006) (Figure 1).
1.
Study flow diagram
Included studies
We identified one trial that met our inclusion criteria (Rabb 1983). This trial reported on folic acid supplementation in children with sickle cell anaemia. No trials on folate supplementation or provision through diet versus placebo or folic acid supplements versus folate supplementation or provision through diet were found.
Folic acid supplements versus placebo
The included trial was in 117 children with sickle cell anaemia (Rabb 1983). This trial, conducted in Jamaica, was a quasi‐randomised double‐blind controlled triaI of supplementation with folic acid. Each participant was allocated to one of two treatment groups, one group receiving tablets designated 'tablet A' (containing calcium lactate), and the other receiving tablets designated 'tablet B' (containing folic acid 5 mg). Participants in both groups took one tablet daily for at least one year. The analyses were restricted to 115 of 117 participants who were followed for one or more years, 56 of 115 (49%) were allocated to take tablet A and 59 of 115 (51%) were allocated to take tablet B. A total of 64 of 115 (56%) of all participants were male, and 40 of 115 (35%) were 6 to 11 months, 23 of 115 (20%) 12 to 23 months, 24 of 115 (21%) 24 to 35 months, and 28 of 115 (24%) 36 to 47 months. Outcomes of this trial included serum folate, haemoglobin concentrations, growth, and clinical events including acute splenic sequestration, dactylitis or episodes of bone or abdominal pain.
Excluded studies
Four trials were excluded; for one trial, the participants and the outcomes were not relevant to our review (Mojtahedzadeh 2006) a further two trials did not have any control group without folate or even at a different dosage (V‐FIT 2014; Hendrickse 1966) and one was not a RCT (Liu 1975).
Ongoing trials
While we were unable to identify any ongoing RCTs involving folic acid supplementation or folate provision through diet, we did identify a Phase 2 clinical trial investigating the safety of beet juice (“Unbeetable”) in adults with sickle cell anemia (Kim‐Shapiro 2014). The trial is focused on the provision of nitrates through the beet juice, however, the beet juice also contains a substantial quantity of folate (23% to 46% of daily value, depending on amount consumed). As the trial may ultimately progress to a Phase 3 RCT, it is important to document in this review as potential future research. However, on 08 November 2017 this trial was suspended due to the fact that enrolment was very slow. The investigators hope to reopen this as a multi‐site study.
Risk of bias in included studies
Please refer to the figures for an overall assessment of the risk of bias (Figure 2; Figure 3)
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Generation of the randomisation sequence
The trial reported that randomisation ".... was achieved by allocating the children alternately to tablets A and B...." (Rabb 1983). Since alternative allocation is a form of quasi‐randomisation, introducing a source of selection bias, we have assessed this trial as having high risk of bias for this domain.
Allocation concealment
The method of allocation concealment was not clearly stated and we have therefore assessed this trial as having an unclear risk of bias for this domain (Rabb 1983).
Blinding
In the Rabb trial it was reported that "Tablets A and B were identical in appearance and neither the patients nor the paediatricians involved in the clinical management of the study were aware of the code" (Rabb 1983). We therefore classified this trial as low risk for performance bias. Since in the same trial it was not further mentioned about the blinding of outcome assessment, we have assessed this trial as having unclear risk for detection bias.
Incomplete outcome data
The included trial states that out of 117 participants recruited to the trial, only 115 were analysed further, with no reason cited for the missing numbers. In addition, there was variable availability of data regarding outcome measures for which the reasons were not cited. Folate measures were available in only 80 of 115 (70%) of the participants; hematological analyses for 100 of 115 (87%) of participants; and growth performance for 103 of 115 (90%) of participants. Additionally, the only outcome for which distribution of missing data was available was that of serum folate measures: approximately 70% of data were missing from each group. In contrast, information regarding clinical events was collected for all 115 participants (100%) included in the analyses. Due to the fact that there was a large proportion of missing data for one of our primary outcomes (folate measurements) and a substantial proportion of missing data for other primary and secondary outcomes (haemoglobin, growth), we have determined that there is a high risk of bias with regards to incomplete outcome data (Rabb 1983).
Selective reporting
All the parameters mentioned in the methodology section were included in results section. The trial included in the analysis had no protocol or like resource outlining previously defined outcomes, therefore, it was difficult to assess for reporting bias. For this reason, have determined the trial to have an unclear bias with regards to selective reporting (Rabb 1983).
Other potential sources of bias
The included trial did not report any other potential sources of bias. We also found no clear evidence of any other bias in the included trial, and we have therefore assessed this as having a low risk for other potential biases.
Effects of interventions
See: Table 1
We identified one trial that met our inclusion criteria (Rabb 1983). This trial reported on children with sickle cell anaemia with folic acid supplementation. No trials on folate supplementation through diet versus placebo or folic acid supplements versus folate supplementation through diet were found. Additionally, no trials undertaken on adults were found.
Folic acid supplements versus placebo
We identified one trial for inclusion (Rabb 1983). In this trial (n = 117), folic acid 5 mg per day was used as supplementation versus a placebo group receiving calcium lactate.
Primary outcomes
1. Folate concentration (using serum or plasma folate or erythrocyte folate)
This outcome was assessed in the included trial (Rabb 1983). The investigators state that serum folate levels, measured between six and 12 months after entry to the trial, were available in 80 of 115 (70%) participants. No data were available in the trial report that could be entered into the review, but it was reported in the paper that serum folate levels differed significantly between the two trial groups, with 33 of 41 (81%) of the folic acid supplementation group having values above 18 µg/L compared to 6 of 39 (15%) in the placebo group (low‐quality evidence). Additionally, while there were no participants in the folic acid group with serum folate levels below 5 µg/L, 15 of 39 (39%) participants in the placebo group had levels below this threshold (low‐quality evidence).
2. Hemoglobin concentration (grams per decilitre (g/dl))
This outcome was assessed in the included trial (Rabb 1983). Hematological indices were performed in 100 of 115 (87%) participants at baseline and at one year. No data were available in the trial report that could be entered in the review, but it was reported in the trial that there were no significant differences in total haemoglobin concentrations either at baseline or after one year baseline, after adjusting for age group and sex (low‐quality evidence).
3. Adverse effects of the intervention
a. Increased incidence of priapism
This was not assessed in the included trial (Rabb 1983).
b.The risk of masking cobalamin deficiency with consequent neuropsychiatric manifestations (nanogram per litre (ng/L))
This was not assessed in the included trial (Rabb 1983).
c. Any other side effects and SCD‐related morbidities
In the included trial, growth, determined by height‐for‐age and weight‐for‐age as well as height and growth velocity, was measured in 103 out of 115 (90%) participants. There were no significant differences in these measures of growth between the two groups. No data were available in the trial report that could be entered in the review, but the investigators reported that there were no significant differences in any growth parameters between the two trial groups (Rabb 1983).
Additionally, there was no difference between the folic acid and the placebo groups in the proportion of the participants experiencing acute splenic sequestration, RR 1.07 (95% CI 0.44 to 2.57) (low‐quality evidence) (Analysis 1.1). With regards to painful episodes (bone or abdominal pain), in the placebo group, 18 of 56 participants were affected and in the folic acid group, 22 of 59 were affected. However, no statistically significant differences noted in the trial since the trial was not powered to detect any potential differences between the two trial groups with regards to painful episodes, RR 1.16 (95% CI 0.70 to 1.92) (low‐quality evidence) (Analysis 1.2).
1.1. Analysis.

Comparison 1: Folic acid supplementation versus placebo, Outcome 1: Acute splenic sequestration
1.2. Analysis.

Comparison 1: Folic acid supplementation versus placebo, Outcome 2: Painful episodes
Also, no statistically significant differences noted in the trial (since it was not powered to detect any potential differences between the folic acid and placebo groups) with regards to the number of episodes of minor infections, RR 0.99 (95% CI 0.85 to 1.15) (low‐quality evidence) (Analysis 1.3); major infections, RR 0.89 (95% CI 0.47 to 1.66) (low‐quality evidence) (Analysis 1.4); or dactylitis episodes, RR 0.67 (95% CI 0.35 to 1.27) (low‐quality evidence) (Analysis 1.5). However, the investigators reported a higher proportion of repeat dactylitis episodes in the placebo group, with two or more attacks occurring in 10 of 56 participants compared to two of 59 in the folic acid group (P < 0.05) (Rabb 1983).
1.3. Analysis.

Comparison 1: Folic acid supplementation versus placebo, Outcome 3: Minor Infections
1.4. Analysis.

Comparison 1: Folic acid supplementation versus placebo, Outcome 4: Major Infection
1.5. Analysis.

Comparison 1: Folic acid supplementation versus placebo, Outcome 5: Dactylitis
Secondary outcomes
1. Homocysteine levels
This was not assessed in the included trial (Rabb 1983).
2. Quality of life
This outcome was not assessed in the included trial (Rabb 1983).
Discussion
Summary of main results
We identified one trial for inclusion in the review (Rabb 1983). At the end of this one‐year trial, the investigators reported a significant difference between the folic acid and placebo groups in relation to the serum folate level distribution, but no differences in haemoglobin concentrations. There were no summary data for these two parameters that could be included in the review. The trial also reported on various side effects, including growth performance (height for age, weight for age, height velocity, and weight velocity); however, the investigators reported that there were no differences in the above parameters at baseline or at the end of the trial. Again, there were no summary data for these two parameters that could be included in the review. Further side effects reported by this trial were on acute splenic sequestration and episodes of bone or abdominal pains; while summary data were available for these events, no statistically significant differences were noted in the trial since it was not powered to detect any potential differences between the folic acid and the placebo groups. This was also true for the number of episodes of minor as well as major infections between the folic acid and placebo groups.
Overall completeness and applicability of evidence
The single eligible trial presents mixed evidence on our pre‐defined outcomes with regards to folate supplementation in people with SCD. The trial was limited in its applicability because it was limited to one intervention (folic acid supplementation) and one population (children in Jamaica). The results of this review may not be applicable to other folate supplementation interventions or other populations.
While the recently published National Heart, Lung, and Blood Institute (NHLBI) guidelines only recommended folic acid supplementation in pregnant women with SCD, many providers in the USA and elsewhere still adhere to the practice of folate supplementation among all people with SCD. In Nigeria, the country with one of the highest burdens of SCD, a majority of SCD clinics provide folic acid supplementation while less than half provide penicillin, a well‐studied and life‐saving intervention (Galadanci 2014). It is important that the evidence behind folic acid supplementation is strong to improve the allocation of resources in areas with the highest burden of SCD. Since there is currently no supporting evidence for this common practice, we believe that it would not only be ethical, but also necessary, to conduct a randomized controlled trial to better answer the question of the need for folic acid supplementation in people with SCD.
Quality of the evidence
The included trial had a high risk of bias with regards to the randomisation of the treatment list (placebo or experimental), which is indicative of poor overall trial quality (Rabb 1983). The number of participants included in each of the analyses were not consistent with the total number randomised into the trial, with the reasons for those missing not being clearly reported, which further points to some potential for bias. However, the trial indicated in detail the blinding process and the included trial was a hospital‐based trial and hence ensured active case finding and compliance to the intervention. Furthermore, the trial report no sample size justification, and assuming the trial was powered to detect differences in folate levels, it was not powered to assess differences in morbidity.
For all of the above reasons, we have determined that the evidence from the included trial was of low quality (Rabb 1983) (Table 1).
Potential biases in the review process
We are confident that all relevant trials have been identified by the comprehensive search strategy and do not anticipate that the review methods could have introduced bias to the review.
Agreements and disagreements with other studies or reviews
We are unaware of similar reviews covering this topic.
We are aware of an article by Al Yassin; however, we could not retrieve the full text for the article (abstract only); we have contacted the author for further details, and await their reply (Al‐Yassin 2012). From the abstract available we could ascertain that it was a literature review which included only a small number of studies.
Authors' conclusions
Implications for practice.
There is some evidence that in sickle cell disease (SCD), folate supplementation may improve serum folate concentrations; however, it is unclear if folate supplementation has any affect on haemoglobin concentration, growth, minor infections, major infections, acute splenic sequestration, dactylitis or episodes of bone or abdominal pain.
At present, one low‐quality trial demonstrates that folate supplementation may lead to increased serum folate measurements, but further evidence is needed. The other effects (positive and negative) of folate supplementation remain unclear, and more trials with larger cohorts are needed to determine these.
Implications for research.
At present there is little evidence to support or refute the practice of folate supplementation in people with SCD. Further trials, involving more participants and longer treatment durations, are needed to enhance the validity of the review. if such trials were to be carried out, these should assess clinical outcomes such as folate concentration, haemoglobin concentration, adverse effects and benefits of the intervention, especially with regards to SCD‐related morbidity. Trials should include people with SCD of all ages and both sexes, in any setting. To investigate the effects of folate supplementation, these future trials should involve more participants and should have both a longer treatment duration and follow‐up than that reported in this review. However, we do not envisage further trials in this area will be conducted, and hence the review will no longer be regularly updated.
What's new
| Date | Event | Description |
|---|---|---|
| 8 April 2021 | Review declared as stable | A search was carried out on 17 Nov, 2017 and no potentially eligible trials were identified. No new studies are expected in this area. |
History
Protocol first published: Issue 5, 2014 Review first published: Issue 2, 2016
| Date | Event | Description |
|---|---|---|
| 8 March 2018 | New search has been performed | A search of the Cochrane Cystic Fibrosis and Geentic Disorders Group's Haemoglobinopathies Trials Register and two trial registries did not identify any potentially relevant trials for inclusion in the review. |
| 8 March 2018 | New citation required but conclusions have not changed | Minor changes have been made throughout this review. Given this is no longer an active area of research, this review will no longer be regularly updated. |
Acknowledgements
We would like to thank: Tracey Remmington, Managing Editor, Cochrane Cystic Fibrosis & Genetic Disorders Review Group for managing the editorial process for the protocol and reviews; Natalie Hall, Information Specialist of the Cochrane Cystic Fibrosis & Genetic Disorders Review Group, for comments on the search strategy; and the editors of the Cochrane Cystic Fibrosis & Genetic Disorders Review Group for their comments on the protocol and full reviews. We are very grateful to Professor Datuk Dr Abdul Razzak Chief Executive of Melaka‐Manipal Medical College, Malaysia and Professor Dr Jaspal Singh, Dean, Faculty of Medicine, Melaka‐Manipal Medical College, Malaysia for their support, constructive comments and encouragement in writing the protocol and review.
The review was partially developed during the World Health Organization (WHO)/Cochrane/ Cornell University Summer Institute for Systematic Reviews in Nutrition for Global Policy Making hosted by the Division of Nutritional Sciences, Cornell University, Ithaca, NY, USA, 27 July ‐ 7 August, 2015. WHO partially supported this programme through Cornell University Conferences Services.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
We would also like to thank the referees for their peer review comments on the protocol and review.
Appendices
Appendix 1. Embase search strategy
| Database | Date of Search | Search terms |
| Embase | 2 August 2015 | 1. exp sickle cell/ 2. sickle cell*:tw 3. haemoglobin S*:tw 4. HbS disease:tw 5. 1 OR 2 OR 3 OR 4 6. exp folic acid/ 7. exp folic acid deficiency/ 8. folic acid*:tw 9. folate:tw 10. vitamin B9:tw 11. B9 vitamin:tw 12. vitamin B‐9:tw 13. metafolin:tw 14. levomefolate:tw 15. folvite:tw 16. folacin:tw 17. 5‐methyltetrahydrofolate:tw 18. 5‐methylTHF:tw 19. 5‐MTHF:tw 20. L‐5‐MTHF:tw 21. 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 OR 19 OR 20 22. randomized controlled trial:tw 23. controlled clinical trial:tw 24. randomized:tw 25. placebo:tw 26. drug therapy/ 27. randomly:tw 28. trial:tw 29. groups [tiab] 30. 22 OR 23 OR 24 OR 25 OR 26 OR 27 OR 28 OR 29 31. 5 AND 21 AND 30 Results: 10 |
Appendix 2. PubMed search strategy
| Database | Date of Search | Search terms |
| PubMed | 31 July 2015 | #1 folic acid[MeSH] OR Folic Acid Deficiency [Mesh] OR folic acid[tw] OR folate[tw] OR vitamin B9[tw] OR B9 vitamin[tw] OR vitamin B‐9[tw] OR metafolin[tw] OR levomefolate[tw] OR folvite [tw] OR folacin [tw] OR 5‐methyltetrahydrofolate[tw] OR 5‐methylTHF[tw] OR 5‐MTHF[tw] OR L‐5‐MTHF[tw] #2 anaemia, sickle cell[MeSH] OR sickle cell[tw] OR haemoglobin S*[tw] OR HbS disease[tw] #3 (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) #1 AND #2 AND #3 Results: 73 |
Appendix 3. CINAHL search strategy
| Database | Date of Search | Search terms |
| CINAHL database | 31 July 2015 | #1 folic acid OR folate OR vitamin B9 OR B9 vitamin OR vitamin B‐9 OR metafolin OR levomefolate OR folvite OR folacin OR 5‐methyltetrahydrofolate OR 5‐methylTHF OR 5‐MTHF OR L‐5‐MTHF #2 sickle cell OR haemoglobin S dis* OR HbS dis* #3 randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR drug therapy OR randomly OR trial OR groups #1 AND #2 AND #3 Results: 7 |
Appendix 4. CABI, Web of Science search strategy
| Database | Date of Search | Search terms |
| CABI database | 31 July 2015 | TS = (("sickle cell" OR "haemoglobin S disease" OR "haemoglobin S disorder" OR "HbS disease") AND ("folic acid" OR folate OR "vitamin B9" OR "vitamin B‐9" OR metafolin OR levomefolate OR folvite OR folacin OR 5‐methyltetrahydrofolate OR 5‐methylTHF) AND (“randomized controlled trial” OR “controlled clinical trial” OR randomized OR placebo OR “drug therapy” OR randomly OR trial OR groups)) Results: 27 |
Appendix 5. ISRCTN search strategy
| Database | Date of Search | Search terms |
| ISRCTN | 2 August 2015 | #1 folic acid OR folate OR vitamin B9 OR B9 vitamin OR vitamin B‐9 OR metafolin OR levomefolate OR folvite OR folacin OR 5‐methyltetrahydrofolate OR 5‐methylTHF OR 5‐MTHF OR L‐5‐MTHF #2 sickle cell OR haemoglobin S OR HbS disease #1 AND #2 Results: 0 |
Appendix 6. ClinicalTrials.gov search strategy
| Database | Date of Search | Search terms |
| ClinicalTrials.gov | 31 July 2015 and 02 February 2018 | #1 "sickle cell" OR "haemoglobin S disease" OR "haemoglobin S disorder" OR "HbS disease" #2 "folic acid" OR folate OR "vitamin B9" OR "vitamin B‐9" OR metafolin OR levomefolate OR folvite OR folacin OR 5‐methyltetrahydrofolate OR 5‐methylTHF #1 AND #2 Results: 9 |
Appendix 7. ICTRP search strategy
| Database | Date of Search | Search terms |
| ICTRP Database | 3 Aug 2015 and 02 Fberuary 2018 | #1 folate #2 sickle cell #1 AND #2 Results: 202 |
Appendix 8. Glossary
| Term | Definition |
| Bioavailability | The extent to which a nutrient or medication can be used by the body. |
| Electrophoretic mobility | The migration of charged colloidal particles or molecules through a solution under the influence of an applied electric field usually provided by immersed electrodes. |
| Erythropoiesis | The formation or production of red blood cells. |
| Megaloblastic anaemia | A type of anaemia characterised by enlarged red cells and a relative reduction in leukocytes and platelets. |
| Neoplastic foci | Microscopic visualisation of the tumour cells. |
| Polyglutamates | A polymer of glutamic acid residues in the usual peptide linkage. |
| Mean cell volume | It is a measure of the average volume of a red blood corpuscle (or red blood cell). The measure is attained by multiplying a volume of blood by the proportion of blood that is cellular (the haematocrit (or hematocrit)), and dividing that product by the number of erythrocytes (red blood cells) in that volume. |
| Femtolitres (fL) | The femtolitre (US femtoliter) is the metric unit of volume equal to 10 −15 litres, or one thousand trillionth (European) or one quadrillionth (American) litre. It is abbreviated fL or fl. One femtolitre is the same as one cubic micrometre (μm). |
Data and analyses
Comparison 1. Folic acid supplementation versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Acute splenic sequestration | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.2 Painful episodes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3 Minor Infections | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4 Major Infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.5 Dactylitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Rabb 1983.
| Study characteristics | ||
| Methods | Double‐blind quasi‐RCT. | |
| Participants | 117 children admitted to the trial (115 analysed). Children with homozygous SCD aged 6 months to 4 years. | |
| Interventions | 5 mg folic acid (treatment B) versus a placebo of calcium lactate (treatment A) | |
| Outcomes | Folate concentration, haemoglobin concentration, recurrent infections, minor infections, major infections, acute splenic sequestration, dactylitis or episodes of bone or abdominal pain. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "A trial of pneumococcal vaccine and prophylactic penicillin, involving four treatment regimes. was already underway in this same group of children and it was important to ensure that the groups receiving tablets A and B were similarly distributed between these regimes. This was achieved by allocating the children alternately to tablets A and B within each of the four treatment regimes". |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned in the text. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Tablets A and B were identical in appearance and neither the participants nor the paediatricians involved in the clinical management of the study were aware of the code." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned in the text. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was a large proportion of missing data for one of our primary outcomes (folate measurements, only 70% data available) and a somewhat substantial proportion of missing data for other primary and secondary outcomes (haemoglobin, growth). Out of 117 children admitted to the trial, only 115 were analysed, and further no reason cited for missing numbers. |
| Selective reporting (reporting bias) | Unclear risk | There was no protocol or resource outlining previously defined outcomes. |
| Other bias | Low risk | No other biases were detected. |
RCT: randomized controlled trial SCD: sickle cell disease
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Hendrickse 1966 | RCT with 133 child participants with SCD (HbSS) in Nigeria. Participants were randomly allocated into 3 comparison groups, all of which took 1 to 2 tablets containing various therapeutic agents, either alone or in a specific formula preparation, including anti‐malarials, vitamins, and/or steroids. All 3 groups' treatment regimens included 5 mgm of folic acid. Outcomes included efficacy in malaria control, prevention of folic acid deficiency, infection control, growth indicators, and "overall course of the disease." The study did not contain a control group without folic acid supplementation, even at a different dosage, and was therefore not included in our analyses. |
| Liu 1975 | Non‐randomised, single‐arm trial with 61 children and adult participants with SCA and 2 socio‐economically matched control groups (61 with sickle cell trait, HbAS, and 69 race‐matched controls without sickle cell trait, HbAA). Participants did not take folic acid supplements had not experienced an infection or pain crisis within 2 weeks of enrolment. 24 participants with SCA who had low baseline serum or erythrocyte folate levels (or both) were supplemented with 1 mg folic acid per day for 3 to 12 months. Clinical and laboratory measures were taken for all participants with SCA twice prior to folate therapy and for 24 participants with SCA, twice after therapy. This study was not a RCT and was therefore not included in our review. |
| Mojtahedzadeh 2006 | Randomised placebo‐controlled trial with 51 adult participants (23 controls, 28 cases) in Iran with β‐thalassemia major on regular blood transfusions. Participants were stratified by baseline serum folate levels and then randomised to receive folic acid tablets (1 mg) or placebo, taken once daily for 4 weeks, at which point serum folate and other hematological indices were measured. Exclusion criteria included pregnant or lactating women, those with chronic liver disease, or those using folic acid supplements or medications interfering with folic acid metabolism. The study participants did not match our study eligibility criteria and was therefore not included in our analyses. |
| V‐FIT 2014 | RCT with 119 child participants with SCD (HbSS) in Tanzania. Children received in random order a daily RUSF providing 500 kcal, 1 RDA of vitamins and minerals & 1mg folate (Nutriset, France), plus weekly anti‐malarial prophylactic chloroquine syrup (150/225 mg base) (Wallace manufacturing chemicals, UK), or a vascular‐RUSF (RUSFv) fortified with arginine and citrulline (average 0.2 g/kg/d & 0.1 g/kg/d) plus daily chloroquine syrup (3 mg base/kg/d). Both the groups’ treatment regimens included 1 mg of folate. Outcomes included endothelium‐dependent and ‐independent vasodilatation, height, and weight and body composition. The study did not contain a control group without folic acid supplementation, even at a different dosage, and was therefore not included in our review. |
RCT: randomised controlled trial RDA: recommended daily allowance RUSF: ready‐to‐use supplementary food SCA: sickle cell anaemia SCD: sickle cell disease
Characteristics of ongoing studies [ordered by study ID]
Kim‐Shapiro 2014.
| Study name | Study of Beet Juice for Patients With Sickle Cell Anemia (NCT02162225). |
| Methods | Phase 2 clinical trial. Single group assignment. |
| Participants | Adults with sickle cell anaemia. |
| Interventions | Beet juice (dietary provision of folate). |
| Outcomes | Safety of intervention. |
| Starting date | June 2014. |
| Contact information | Natalia Dixon, MD ndixon@wakehealth.edu. |
| Notes | While we were unable to identify any ongoing randomised controlled trials involving folic acid supplementation or folate provision through diet, we did identify a Phase 2 clinical trial investigating the safety of beet juice (“Unbeetable”) in adults with sickle cell anemia (Kim‐Shapiro NCT02162225). The trial is focused on the provision of nitrates through the beet juice, however, the beet juice also contains a substantial quantity of folate (23% ‐ 46% of daily value, depending on amount consumed). As the trial may ultimately progress to a Phase 3 RCT, it is important to document in this review as potential future research. Note: on 08 November 2017 this trial was suspended due to the fact that enrolment was very slow. The investigators hope to reopen as multi‐site study. |
Contributions of authors
Conceiving the review: Cochrane Cystic Fibrosis & Genetic Disorders Review Group.
Literature search for background: RD, SN, SSM, PS, LV.
Writing the background, objectives and inclusion criteria sections: HH, RD, PS, LV.
Writing data collection and analysis sections: RD, AL A, SN, LV, PS.
Sources of support
Internal sources
No sources of support supplied
External sources
-
The National Institute for Health Research (NIHR), UK
United Kingdom Cochrane Centre
-
WHO/Cochrane/ Cornell University Summer Institute for Systematic Reviews in Nutrition for Global Policy Making, USA
The review was partially developed during the World Health Organization (WHO)/Cochrane/ Cornell University Summer Institute for Systematic Reviews in Nutrition for Global Policy Making hosted by the Division of Nutritional Sciences, Cornell University, Ithaca, NY, USA, 27 July to 7 August, 2015. WHO partially supported this programme through Cornell University Conferences Services.
Declarations of interest
Ruchita Dixit: none known. Sowmya Nettem: none known. Simerjit S Madan: none known. Htoo Htoo Kyaw Soe: none known Adinegara BL Abas: none known Leah Vance: has undertaken a research year that allowed for her participation in this review was supported by Grant 2014086 from the Doris Duke Charitable Foundation. She has no other conflicts to disclose. Patrick Stover: none known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Rabb 1983 {published data only}
- Rabb LM, Grandison Y, Mason K, Hayes RJ, Serjeant B, Serjeant GR. A trial of folate supplementation in children with homozygous sickle cell disease. British Journal of Haematology 1983;54(4):589-94. [CENTRAL: 31620] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Hendrickse 1966 {published data only}
- Hendrickse RG, Barnes PM. Sickle cell anaemia: report of a therapeutic trial. West African Medical Journal 1966;15(2):55-64. [CENTRAL: 477152] [PubMed] [Google Scholar]
Liu 1975 {published data only}
- Liu YK. Folic acid deficiency in sickle cell anemia. Scandanavian Journal of Haematology 1975;14(1):71-9. [DOI] [PubMed] [Google Scholar]
Mojtahedzadeh 2006 {published data only}
- Mojtahedzadeh F, Kosaryan M, Mahdavi MR, Akbari J. The effect of folic acid supplementation in beta-thalassemia major: a randomized placebo-controlled clinical trial. Archives of Iranian Medicine 2006;9(3):266-8. [CENTRAL: 566691] [EMBASE: 2006356607] [PMID: ] [PubMed] [Google Scholar]
V‐FIT 2014 {published data only}
- Cox SE, Makani J, Walter G, Mtunguja S, Kamala BA, Ellins E, et al. Ready-to-use supplementary food supplements improve endothelial function, hemoglobin and growth in Tanzanian children with sickle cell anaemia: the vascular function intervention study (V-FIT), a random order crossover trial. Proceedings of the 56th ASH Annual Meeting and Exposition; 2014 Dec 6-9; San Francisco, California 2014;89. [ABSTRACT NO: 4087] [CENTRAL: 1017322] [Google Scholar]
- Marealle A, Makani J, Kirkham F, Prentice A, Cox S. Amino acids in Tanzanian children with sickle cell disease: Baseline results of the vascular function intervention trial (VFIT). FASEB Journal 2015;29(1 Suppl Meeting Abstracts). [ABSTRACT NO: 729.14] [CENTRAL: 1080547] [EMBASE: 71863886] [Google Scholar]
References to ongoing studies
Kim‐Shapiro 2014 {published data only}
- Kim-Shapiro DB, Dixon N. Study of Beet Juice for Patients With Sickle Cell Anemia (NCT02162225). https://www.clinicaltrials.gov/ct2/show/NCT02162225 (accessed 03 September 2015).
Additional references
Aliyu 2006
- Aliyu ZY, Tumblin AR, Kato GJ. Current therapy of sickle cell disease. Haematologica 2006;91(1):7-10. [PMC free article] [PubMed] [Google Scholar]
Al‐Yassin 2012
- Al-Yassin A, Osei A, Rees D. Folic acid supplementation in children with sickle cell disease. Archives of Disease in Childhood 2012;97:A91-92. [Google Scholar]
Bailey 2004
- Bailey LB. Folate and vitamin B12 recommended intakes and status in the United States. Nutrition Reviews 2004;62(6):14-20. [DOI] [PubMed] [Google Scholar]
Bailey 2012
- Bailey LB, Caudill MA. Folate. In: Erdman Jr JW, MacDonald IA, Zeisel SH, editors(s). Present Knowledge in Nutrition. 10th edition. Washington, DC: Wiley-Blackwell, 2012:321-42. [Google Scholar]
Blencowe 2010
- Blencowe H, Cousens S, Modell B, Lawn J. Folic acid to reduce neonatal mortality from neural tube disorders. International Journal of Epidemiology 2010;39(1):110-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carmel 2005
- Carmel R. Folic Acid. In: Shils M, Shike M, Ross A, Caballero B, Cousins R, editors(s). Modern Nutrition in Health and Disease. 10th edition. Lippincott Williams & Wilkins, 2005:470-81. [Google Scholar]
Clarke 1998
- Homocysteine Lowering Trialists' Collaboration. Lowering blood homocysteine with folic acid based supplements: meta analysis of randomized trials. BMJ 1998;316(7135):894–8. [PMC free article] [PubMed] [Google Scholar]
Clarke 2010
- Clarke R, Halsey J, Lewington S, Lonn E, Armitage J, Manson JE, et al. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: Meta-analysis of 8 randomized trials involving 37 485 individuals. Archives of Internal Medicine 2010;170(18):1622-31. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks J, Higgins J, Altman D. Chapter 9: Analysing data and undertaking meta-analysis. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Dhar 2003
- Dhar M, Bellevue R, Carmel R. Pernicious anemia with neuropsychiatric dysfunction in a patient with sickle cell anemia treated with folate supplementation. New England Journal of Medicine 2003;348(22):2204–7. [DOI] [PubMed] [Google Scholar]
Durga 2007
- Durga J, Boxtel MP, Schouten EG, Kok FJ, Jolles J, Katan MB, et al. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet 2007;369(9557):208-16. [DOI] [PubMed] [Google Scholar]
Falletta 1995
- Falletta JM, Woods GM, Verter JI, Buchanan GR, Pegelow CH, Iyer RV, et al. Discontinuing penicillin prophylaxis in children with sickle cell anemia. Prophylactic Penicillin Study II. Journal of Pediatrics 1995;127(5):685-90. [DOI] [PubMed] [Google Scholar]
Galadanci 2014
- Galadanci N, Wudil BJ, Balogun TM, Ogunrinde GO, Akinsulie A, Hasan-Hanga F, Mohammed AS, Kehinde MO, Olaniyi JA, Diaku-Akinwumi IN, Brown BJ, Adeleke S, Nnodu OE, Emodi I, Ahmed S, Osegbue AO, Akinola N, Opara HI, Adegoke SA, Aneke J, Adekile AD. Current sickle cell disease management practices in Nigeria [Current sickle cell disease management practices in Nigeria]. International health 2014 Mar;6(1):23-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Green 2011
- Green R. Indicators for assessing folate and vitamin B-12 status and for monitoring the efficacy of intervention strategies. American Journal of Clinical Nutrition 2011 Aug;94(2):666S-72S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grosse 2011
- Grosse SD, Odame I, Atrash HK, Amendah DD, Piel FB, Williams TN. Sickle cell disease in Africa: a neglected cause of early childhood mortality. American Journal of Preventive Medicine 2011;41(6 Suppl 4):398-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March2011] The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Huo 2015
- Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman RF, et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA 2015;313(13):1325-35. [DOI] [PubMed] [Google Scholar]
Ingram 1957
- Ingram VM. Gene mutations in human haemoglobin: the chemical difference between normal and sickle haemoglobin. Nature 1957;180:326–8. [DOI] [PubMed] [Google Scholar]
Kim 2006
- Kim YI. Folate: a magic bullet or a double edged sword for colorectal cancer prevention? Gut 2006;55(10):1387–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lane 1996
- Peter A, Lane S. Sickle Cell Disease. Pediatric Hematology 1996;43(3):639-64. [DOI] [PubMed] [Google Scholar]
Lee 2010
- Lee M, Hong KS, Chang SC, Saver JL. Efficacy of homocysteine-lowering therapy with folic acid in stroke prevention: a meta-analysis. Stroke 2010;41(6):1205-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ndefo 2008
- Ndefo UA, Maxwell AE, Nguyen H, Chiobi TL. Pharmacological management of sickle cell disease. Pharmacy and Therapeutics 2008;33(4):238–43. [PMC free article] [PubMed] [Google Scholar]
Pauling 1949
- Pauling L, Itano HA, Singer SJ, Wells IC. Sickle cell anemia: a molecular disease. Science 1949;110:543–8. [DOI] [PubMed] [Google Scholar]
Rees 2010
- Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet 2010;376(9757):2018-31. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sazawal 2006
- Sazawal S, Black RE, Ramsan M, Chwaya HM, Stoltzfus RJ, Dutta A, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Present Knowledge in Nutrition 2006;367(9505):133-43. [DOI] [PubMed] [Google Scholar]
Schnog 2004
- Schnog JB, Duits AJ, Muskiet FAJ, ten Cate H, Rojer RA, Brandjes DPM. Sickle cell disease; a general overview. Netherlands Journal of Medicine 2004;62(10):364-71. [PubMed] [Google Scholar]
Selhub 1995
- Selhub J, Jacques PF, Bostom AG, D'Agostino RB, Wilson PW, Belanger AJ, et al. Association between plasma homocysteine concentrations and extracranial carotid-artery stenosis. New England Journal of Medicine 1995;332(5):286–91. [DOI] [PubMed] [Google Scholar]
Serjeant 1997
- Serjeant GR. Sickle-cell disease. Lancet 1997;350(9079):725–30. [DOI] [PubMed] [Google Scholar]
Standing Committee 1998
- Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. 10th edition. Washington, DC: National Academy Press, 1998. [PubMed] [Google Scholar]
Stott 2005
- Stott DJ, MacIntosh G, Lowe GD, Rumley A, McMahon AD, Langhorne P, et al. Randomized controlled trial of homocysteine-lowering vitamin treatment in elderly patients with vascular disease. American Journal of Clinical Nutrition 2005;82(6):1320-6. [DOI] [PubMed] [Google Scholar]
Stuart 2004
- Stuart MJ, Nagel RL. Sickle cell disease. Lancet 2004;364(9442):1343–60. [DOI] [PubMed] [Google Scholar]
Taylor 2003
- Taylor MJ, Carney SM, Geddes J, Goodwin G. Folate for depressive disorders. Cochrane Database of Systematic Reviews 2003, Issue 2. Art. No: CD003390. [DOI: 10.1002/14651858.CD003390] [DOI] [PMC free article] [PubMed] [Google Scholar]
Troen 2006
- Troen AM, Mitchell B, Sorensen B, Wener MH, Johnston A, Wood B, et al. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. Journal of Nutrition 2006;136(1):189-94. [DOI] [PubMed] [Google Scholar]
Vollset 2013
- Vollset SE, Clarke R, Lewington S, Ebbing M, Halsey J, Lonn E, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet 2013;381(9871):1029-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolff 2009
- Wolff T, Witkop CT, Miller T, Syed SB. Folic Acid Supplementation for the Prevention of Neural Tube Defects: An Update of the Evidence for the U.S. Preventive Services Task Force. http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/folic-acid-to-prevent-neural-tube-defects-preventive-medication (accessed 03 September 2015).
Wong 2014
- Wong TE, Brandow AM, Lim W, Lottenberg R. Update on the use of hydroxyurea therapy in sickle cell disease. Blood 2014;124(26):3850-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yetley 2011
- Yetley EA, Pfeiffer CM, Phinney KW, Fazili Z, Lacher DA, Bailey RL, et al. Biomarkers of folate status in NHANES: a roundtable summary. American Journal of Clinical Nutrition 2011;94(1):303-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yeung 2011
- Yeung LF, Cogswell ME, Carriquiry AL, Bailey LB, Pfeiffer CM, Berry RJ. Contributions of enriched cereal-grain products, ready-to-eat cereals, and supplements to folic acid and vitamin B-12 usual intake and folate and vitamin B-12 status in US children:National Health and Nutrition Examination Survey (NHANES), 2003-2006. American Journal of Clinical Nutrition 2011;93(1):172-85. [DOI] [PubMed] [Google Scholar]


