Abstract
Background
Bronchiectasis is a chronic respiratory disease characterised by abnormal and irreversible dilatation and distortion of the smaller airways. Bacterial colonisation of the damaged airways leads to chronic cough and sputum production, often with breathlessness and further structural damage to the airways. Long‐term macrolide antibiotic therapy may suppress bacterial infection and reduce inflammation, leading to fewer exacerbations, fewer symptoms, improved lung function, and improved quality of life. Further evidence is required on the efficacy of macrolides in terms of specific bacterial eradication and the extent of antibiotic resistance.
Objectives
To determine the impact of macrolide antibiotics in the treatment of adults and children with bronchiectasis.
Search methods
We identified trials from the Cochrane Airways Trials Register, which contains studies identified through multiple electronic searches and handsearches of other sources. We also searched trial registries and reference lists of primary studies. We conducted all searches on 18 January 2018.
Selection criteria
We included randomised controlled trials (RCTs) of at least four weeks' duration that compared macrolide antibiotics with placebo or no intervention for the long‐term management of stable bronchiectasis in adults or children with a diagnosis of bronchiectasis by bronchography, plain film chest radiograph, or high‐resolution computed tomography. We excluded studies in which participants had received continuous or high‐dose antibiotics immediately before enrolment or before a diagnosis of cystic fibrosis, sarcoidosis, or allergic bronchopulmonary aspergillosis. Our primary outcomes were exacerbation, hospitalisation, and serious adverse events.
Data collection and analysis
Two review authors independently screened the titles and abstracts of 103 records. We independently screened the full text of 40 study reports and included 15 trials from 30 reports. Two review authors independently extracted outcome data and assessed risk of bias for each study. We analysed dichotomous data as odds ratios (ORs) and continuous data as mean differences (MDs) or standardised mean differences (SMDs). We used standard methodological procedures as expected by Cochrane.
Main results
We included 14 parallel‐group RCTs and one cross‐over RCT with interventions lasting from 8 weeks to 24 months. Of 11 adult studies with 690 participants, six used azithromycin, four roxithromycin, and one erythromycin. Four studies with 190 children used either azithromycin, clarithromycin, erythromycin, or roxithromycin.
We included nine adult studies in our comparison between macrolides and placebo and two in our comparison with no intervention. We included one study with children in our comparison between macrolides and placebo and one in our comparison with no intervention.
In adults, macrolides reduced exacerbation frequency to a greater extent than placebo (OR 0.34, 95% confidence interval (CI) 0.22 to 0.54; 341 participants; three studies; I2 = 65%; moderate‐quality evidence). This translates to a number needed to treat for an additional beneficial outcome of 4 (95% CI 3 to 8). Data show no differences in exacerbation frequency between use of macrolides (OR 0.31, 95% CI 0.08 to 1.15; 43 participants; one study; moderate‐quality evidence) and no intervention. Macrolides were also associated with a significantly better quality of life compared with placebo (MD ‐8.90, 95% CI ‐13.13 to ‐4.67; 68 participants; one study; moderate‐quality evidence). We found no evidence of a reduction in hospitalisations (OR 0.56, 95% CI 0.19 to 1.62; 151 participants; two studies; I2 = 0%; low‐quality evidence), in the number of participants with serious adverse events, including pneumonia, respiratory and non‐respiratory infections, haemoptysis, and gastroenteritis (OR 0.49, 95% CI 0.20 to 1.23; 326 participants; three studies; I2 = 0%; low‐quality evidence), or in the number experiencing adverse events (OR 0.83, 95% CI 0.51 to 1.35; 435 participants; five studies; I2 = 28%) in adults with macrolides compared with placebo.
In children, exacerbation frequency was reduced more with macrolides than with placebo (IRR 0.50, 95% CI 0.35 to 0.71; 89 children; one study; low‐quality evidence). However there was no significant difference in this age group with regard to: hospitalisations (OR 0.28, 95% CI 0.07 to 1.11; 89 children; one study; low‐quality evidence), serious adverse events, defined within the study as exacerbations of bronchiectasis or investigations related to bronchiectasis (OR 0.43, 95% CI 0.17 to 1.05; 89 children; one study; low‐quality evidence), or adverse events (OR 0.78, 95% CI 0.33 to 1.83; 89 children; one study), in those receiving macrolides compared to placebo. The same study reported an increase in macrolide‐resistant bacteria (OR 7.13, 95% CI 2.13 to 23.79; 89 children; one study), an increase in resistance to Streptococcus pneumoniae (OR 13.20, 95% CI 1.61 to 108.19; 89 children; one study), and an increase in resistance to Staphylococcus aureus (OR 4.16, 95% CI 1.06 to 16.32; 89 children; one study) with macrolides compared with placebo. Quality of life was not reported in the studies with children.
Authors' conclusions
Long‐term macrolide therapy may reduce the frequency of exacerbations and improve quality of life, although supporting evidence is derived mainly from studies of azithromycin, rather than other macrolides, and predominantly among adults rather than children. However, macrolides should be used with caution, as limited data indicate an associated increase in microbial resistance. Macrolides are associated with increased risk of cardiovascular death and other serious adverse events in other populations, and available data cannot exclude a similar risk among patients with bronchiectasis.
Plain language summary
Macrolide antibiotics for bronchiectasis
Background to the question
Bronchiectasis is a long‐term respiratory condition. The airways in the lungs are damaged, and people are prone to infection. Symptoms are chronic cough and the production of sputum (coughed‐up material (phlegm) from the lower airways). Moreover, bronchiectasis is associated with a mortality rate more than twice that of the general population.
Long‐term antibiotic therapy with macrolides (such as azithromycin, roxithromycin, erythromycin, and clarithromycin) may reduce the cycle of reinfection, reduce symptoms, and improve quality of life. We wanted to do this review to look at the evidence on use of macrolides in people with bronchiectasis. This review is intended to help people such as guideline producers, doctors, and patients make decisions about whether to use or recommend macrolides.
Study characteristics
We found 15 studies that compared macrolides with placebo (a substance or treatment with no benefit) or no intervention. Eleven studies involved 690 adults (aged 18 years and older) and four studies involved 190 children. Among adults, six used azithromycin, four roxithromycin, and one erythromycin. The four studies with children used azithromycin, clarithromycin, erythromycin, or roxithromycin. This review is current to January 2018.
Main results
The studies on azithromycin reported improved quality of life in adults. We do not have sufficient evidence from other macrolides to make a robust judgement on their use, and we similarly have insufficient evidence from children to draw clear conclusions.
Although we found only a few trials, they do show a possible increase in antibiotic resistance. Antibiotic resistance is seen when an antibiotic becomes less effective at killing the bacteria causing the chest infection.
We know that macrolides are associated with higher risk of cardiovascular death and other serious adverse events when they are used to treat other conditions. The data in our review suggest it is possible that people with bronchiectasis are at risk for these adverse effects when taking macrolides.
Quality of the evidence
Generally the limited number of studies evaluating macrolides and the variation among them indicate that we cannot be sure of the overall effect of their use in bronchiectasis. Further high‐quality studies are needed to examine the role of long‐term macrolide antibiotics in the treatment of adults and children with bronchiectasis.
Summary of findings
Background
Description of the condition
Bronchiectasis is a chronic respiratory disease characterised by abnormal and irreversible dilatation and distortion of the airways (Pasteur 2010). Bacterial colonisation of the damaged airways leads to chronic cough and sputum production, often with breathlessness and further structural damage to the airways. Diagnosis is made by computed tomography (CT) scanning of the chest when appropriate clinical symptoms are identified (Chang 2010), but asymptomatic radiological evidence of bronchiectasis may be noted (Kwak 2010).
Bronchiectasis has many causes, generally involving major or repeated insults to the lungs. Severe infections including pneumonia, tuberculosis, and pertussis may cause bronchiectasis, particularly if they occur during childhood while the lungs are still developing. Connective tissue disorders and defects in the immune system are other common causes of bronchiectasis, but many cases are idiopathic. Cystic fibrosis leads to severe, progressive bronchiectasis and usually is considered a separate entity from 'non‐cystic fibrosis' bronchiectasis. This review will exclude bronchiectasis secondary to cystic fibrosis.
Prevalence estimates are unclear owing to variable diagnostic strategies (Weycker 2005), a well as higher prevalence rates in developing countries (Habesoglu 2011), but the global disease burden is increasing, with mortality rising by 3% per year between 2001 and 2007, in England and in Wales (Roberts 2010), and hospitalisations by 3% per year in the United States (Seitz 2010). Prevalence is higher in women and those over 60 years of age (Roberts 2010; Seitz 2012). However, prevalence rates may be increasing more rapidly than was previously estimated, with 67 cases per 100,000 general population reported in Germany (Ringshausen 2015), and with UK prevalence rising from 350.5 to 566.1 in women and from 301.2 to 485.5 in men, affecting around 262,900 adults (Quint 2016). Similarly, UK incidence rates increased by 63% over nine years to 2013, rising from 21.2 to 35.2 in women and 8.2 to 26.9 in men, per 100,000 person‐years (Quint 2016). In paediatric populations, younger children and more frequent exacerbations are associated with worse quality of life (Kapur 2012). A higher prevalence of bronchiectasis has been reported among indigenous children in Australia (15:1000) and Alaska (16:1000) (Chang 2002). Incidence rates of 3.7 per 10,000 per year in children under 15 years of age have been reported in New Zealand (Twiss 2005). This equates to a prevalence of 1:3000 children overall and 1:625 children of Pacific Island descent (Twiss 2005). However, these increases may be due to improved diagnosis resulting from easier access to high‐quality CT scanners, rather than reflecting a true rise in prevalence (Goeminne 2016).
Estimated European mortality rates are 0.3 per 100,000 general population in EU countries (ranging from 0.01 in Germany to 1.18 in the UK) and 0.2 per 100,000 in non‐EU countries (ranging from 0.01 in Azerbaijan to 0.67 in Kyrgyzstan), based on data to 2009 (European Lung White Book 2013). Recent age‐adjusted mortality rates in the UK are estimated to be 2.26 times higher in women and 2.14 times higher in men compared with the wider population (Quint 2016). This information is based on estimated mortality rates for bronchiectasis of 1437.7 per 100,000 and for the general population of 635.9 per 100,000 (Quint 2016).
Description of the intervention
Chronic airway infection with pathogens such as Pseudomonas aeruginosa and Haemophilus influenzae and neutrophil‐mediated airway inflammation are key drivers of disease progression and poor outcomes in bronchiectasis (Chalmers 2012; Chalmers 2014; Finch 2015). Long‐term antibiotic therapy therefore is often prescribed with the intention of suppressing bacterial load and reducing airway inflammation (Chalmers 2012). This in turn aims to reduce exacerbations, improve symptoms, and improve quality of life (Haworth 2014). Prolonged antibiotic treatment can be administered in the form of oral or inhaled antibiotics. Inhaled antibiotics offer the advantage of delivering a higher dose of the drug directly to the site of bronchiectasis infection, with less potential for collateral damage and resistance; however, they are often time consuming to administer (Brodt 2014). Oral antibiotics by contrast are typically cheaper and easier to administer than inhaled antibiotics.
Oral antibiotics may be given at lower doses than those used to treat acute infection, with the aims of reducing adverse effects and promoting compliance (Haworth 2014). Macrolide antibiotics are antibacterial agents with anti‐inflammatory and immunomodulatory properties (Haworth 2014). Long‐acting macrolide antibiotics such as azithromycin can be given intermittently rather than requiring daily dosing. Penicillins, tetracyclines, and macrolides have all been tested as prolonged therapy in bronchiectasis (Pasteur 2010). National guidelines for bronchiectasis, such as those provided by the British Thoracic Society, suggest that use of long‐term antibiotic treatment should be considered for patients with three or more exacerbations per year (Pasteur 2010).
Long‐term use of macrolides in bronchiectasis is supported by their ease of administration, their effectiveness in cystic fibrosis and other neutrophilic lung diseases, and their reported anti‐inflammatory properties (Saiman 2003). Balanced against these traits is the potential for macrolides to induce antibiotic resistance and produce antibiotic‐related adverse effects, hearing impairment, and cardiotoxicity (Serisier 2013a).
How the intervention might work
Exacerbations, symptoms, and quality of life are directly linked to bacterial infection and airway inflammation in bronchiectasis (Chalmers 2012; Chalmers 2014). Macrolides are given as both antibacterial and anti‐inflammatory drugs, although it is unclear which of these properties is primarily responsible for the clinical effect observed in cystic fibrosis or bronchiectasis. Macrolides bind reversibly to the 50s ribosomal subunit, preventing bacterial protein synthesis (Haworth 2014). They therefore have broad activity against Gram‐positive organisms such as staphylococci and streptococci and exhibit a degree of activity against Gram‐negative organisms such as Haemophilus bacteria. It is interesting to note that macrolides show no bacteriocidal activity against P aeruginosa but may modify virulence by interfering with quorum sensing and virulence factors (Kohler 2010).
The anti‐inflammatory effects of macrolides have been known for decades and are classically demonstrated in their effectiveness against diffuse panbronchiolitis (Amsden 2005). Macrolides contain a macrocytic lactone ring that is thought to be responsible for most anti‐inflammatory effects (Haworth 2014). Macrolides are classified according to the number of lactone rings as 14‐, 15‐, and 16‐member ring macrolides. Macrolides confer potentially beneficial effects at every level of the 'vicious cycle' of bronchiectasis. They reduce the secretion of pro‐inflammatory cytokines from epithelial cells, inhibit leucocyte recruitment to the airway, inhibit neutrophil activation, and reduce oxidative stress (Zarogoulidis 2012).
Thus potential benefits of macrolides include suppression of bacterial infection, leading to reduced exacerbations, reduced cough and sputum production, and improved lung function and quality of life.
Why it is important to do this review
Bronchiectasis is associated with a mortality rate more than twice that of the general population ‐ 2.26 times higher in women and 2.14 times higher in men (Quint 2016). Data on the economic burden of bronchiectasis are few; however a 2001 US study reported 2.0 more days in hospital, 6.1 more outpatient appointments, and 27.2 more days of antibiotic treatment (Weycker 2005). It is estimated that additional annual costs associated with bronchiectasis ranged from USD 5681 to USD 7827 during the period between 2001 and 2009 (Joish 2013; Seitz 2010; Weycker 2005).
Frequent exacerbations lead to impaired quality of life and progressive lung damage with permanent loss of lung function (Martínez‐García 2007). Therefore, drug interventions that are effective in reducing the frequency of exacerbations should offer both short‐term and long‐term benefit for patients with bronchiectasis. A Cochrane Review of short‐term antibiotics provided little evidence on which to base a recommendation, with one small trial showing evidence of global improvement and pathogen eradication in sputum (Wurzel 2011). Another Cochrane Review of long‐term antibiotic therapy included 18 trials of moderate quality and provided evidence of reduced exacerbation frequency and hospitalisation but increased drug resistance (Hnin 2015). Neither of these Cochrane Reviews examined effects by class of antibiotics, and neither specifically created subgroups by macrolide therapy. A Cochrane Overview concluded that further evidence is required on the efficacy of antibiotics in terms of eradication of specific bacterial colonisation and the extent of antibiotic resistance (Welsh 2015). Recent recommendations from the European Task Force on Bronchiectasis further reinforced the importance of this question by identifying research on macrolide therapy as one of the key priorities in bronchiectasis (Aliberti 2016). Macrolides may potentially reduce exacerbations of bronchiectasis. Given their drawbacks, particularly cardiac toxicity as described by Ray 2012 and the potential for selecting antibiotic‐resistant organisms as discussed by Leclercq 2002, robust evidence on the effectiveness of macrolides is needed if they are to be used with confidence for this indication.
This review is being conducted alongside two other, closely related reviews: "Dual antibiotics for bronchiectasis" and"Head to head trials of antibiotics for bronchiectasis".
Objectives
To determine the impact of macrolide antibiotics in the treatment of adults and children with bronchiectasis.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) of at least four weeks' duration. We included cross‐over studies but used only data from the first pre‐cross‐over phase to eliminate potentially irreversible carry‐over effects (e.g. antibiotic resistance). We included studies reported as full text, those published as abstract only, and unpublished data.
Types of participants
We included adults and children with a diagnosis of bronchiectasis by bronchography, plain film chest radiography, or high‐resolution computed tomography who reported daily sputum expectoration for at least three months. We did not exclude participants whose condition was diagnosed by radiography alone. When a study included participants with different respiratory conditions, we included the study only if investigators performed a separate subgroup analysis for participants with bronchiectasis. We excluded studies if participants had been receiving continuous or high‐dose antibiotics immediately before enrolment, or if they had received a diagnosis of cystic fibrosis, sarcoidosis, or allergic bronchopulmonary aspergillosis. We defined children as individuals from six months to 18 years of age.
Types of interventions
We included studies comparing macrolide antibiotics with placebo, no intervention, or non‐macrolide antibiotics for long‐term management of stable bronchiectasis and reporting these different comparisons separately. We excluded studies looking at short‐term macrolides for management (as opposed to prevention) of exacerbations of bronchiectasis.
Types of outcome measures
We used exacerbation and hospitalisation rates as reported by study authors. We collected outcome data at a range of follow‐up points that best reflected available evidence from included studies (e.g. end of study, end of follow‐up, change from baseline).
Primary outcomes
Exacerbations (defined by study authors' criteria)
Hospitalisation (defined by study authors' criteria)
Serious adverse events defined by Hansen 2015, as follows: adverse events that result in death or life‐threatening events, requirement for hospitalisation or prolongation of existing hospitalisation, persistent or significant disability, or congenital anomalies; or events that are considered medically important
Secondary outcomes
Sputum volume and purulence
Measures of lung function (e.g. forced expiratory volume in one second (FEV1))
Systemic markers of infection (C‐reactive protein (CRP))
Adverse events (e.g. cardiac arrhythmias, gastrointestinal symptoms, hearing impairment)
Mortality (with this review indicating whether defined as all‐cause or bronchiectasis‐related in individual studies)
Emergence of resistance to antibiotics
Exercise capacity (e.g. the Six‐Minute Walk Distance test (6MWD))
Health‐related quality of life (e.g. St. George's Respiratory Questionnaire (SGRQ))
Reporting in the study one or more of the outcomes listed here was not an inclusion criterion for this review.
Search methods for identification of studies
Electronic searches
We identified studies from the Cochrane Airways Trials Register, which is maintained by the Information Specialist for the Group. The Cochrane Airways Trials Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies Online (crso.cochrane.org).
Weekly searches of MEDLINE Ovid SP 1946 to date.
Weekly searches of Embase Ovid SP 1974 to date.
Monthly searches of PsycINFO Ovid SP 1967 to date.
Monthly searches of Cumulative Index to Nursing and Allied Health Literature (CINAHL) EBSCO 1937 to date.
Monthly searches of Allied and Complementary Medicine (AMED) EBSCO.
Handsearches of the proceedings of major respiratory conferences.
Studies contained in the Cochrane Airways Trials Register are identified through search strategies based on the scope of Cochrane Airways. We have provided details of these strategies, as well as a list of handsearched conference proceedings, in Appendix 1. See Appendix 2 for search terms used to identify studies for this review.
We also conducted a search of the US National Institutes of Health Ongoing Trials Register, ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch).
We searched all databases from their inception to January 2018 and imposed no restrictions on language of publication.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references and searched relevant manufacturers' websites for study information. We searched PubMed (www.ncbi.nlm.nih.gov/pubmed) for errata or retractions from included studies published in full text and reported within the review the date this was done.
Data collection and analysis
Selection of studies
Two review authors (DE and LF) independently screened titles and abstracts for inclusion of all potential studies identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved full‐text study reports/publications, and two review authors (CK and LF) independently screened the full texts, identified studies for inclusion, and identified and recorded reasons for exclusion of ineligible studies. We encountered no disagreements, so the need to consult a third review author (SS or SJM) did not arise. We identified and excluded duplicates and collated multiple reports of the same trial, so that each trial rather than each report was the unit of interest in the review. We recorded the selection process in detail in the PRISMA flow diagram and the Characteristics of excluded studies table (Moher 2009).
Data extraction and management
We used a data collection form that was piloted on at least one study in the review to extract study characteristics and outcome data. One review author (LF) extracted the following characteristics from included studies.
Methods: study design, total duration, details of 'run‐in' period, number of centres and their locations, settings, withdrawals, and dates the study was carried out.
Participants: number, mean age and range, gender, bronchiectasis severity, diagnostic criteria, baseline lung function, smoking history, inclusion and exclusion criteria.
Interventions and comparisons: intervention, comparison, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes reported and follow‐up time points.
Notes: funding source and notable conflicts of interest of study authors.
Two review authors (LF and NR) independently extracted outcome data from the included studies. When investigators did not report outcome data in a usable way, we noted this in the Characteristics of included studies table. We resolved disagreements by reaching consensus or by involving a third review author (SS or SJM). One review author (LF) transferred data into the Review Manager 5 file (RevMan 2014), and a second review author (SS) verified and validated the information. One review author (CK) spot‐checked study characteristics for accuracy against the trial reports.
Assessment of risk of bias in included studies
Two review authors (NR and LF) independently assessed the risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), according to the domains below. We resolved disagreements by discussion or by consultation with another review author (SS or SJM).
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as bringing high, low, or unclear risk, provided a quote from the study report, and recorded our judgement in the 'Risk of bias' table. We summarised risk of bias judgements across different studies for each of the domains listed and reported these in a 'Risk of bias summary table' and a 'Risk of bias graph'. We considered blinding separately for different key outcomes when necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐reported pain scale). When information on risk of bias was related to unpublished data or correspondence with a study author, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the previously published protocol and have reported deviations from it in the Differences between protocol and review section.
Measures of treatment effect
We analysed dichotomous data as odds ratios (ORs) and continuous data as mean differences (MDs) or standardised mean differences (SMDs). We analysed hospitalisation and exacerbation rates as rate ratios when possible. We entered data as a scale with a consistent direction of effect. We undertook meta‐analyses only when these were meaningful (i.e. when treatments, participants, and the underlying clinical question were similar enough for pooling to make sense). We narratively described skewed data reported as medians and interquartile ranges, as well as data not suitable for meta‐analysis (e.g. data from mixed methods regression). Our review did not include trials with multiple intervention arms, but if future updates of the review should identify this type of trial, we will include only the intervention arms relevant to this review. When we combined two comparisons (e.g. drug A vs placebo and drug B vs placebo) in the same meta‐analysis, we halved the control group to avoid double‐counting.
Unit of analysis issues
The study participant was the unit of analysis in all included studies. For exacerbation and admission rates, we focused on the number of events experienced by the participant during the trial. For cross‐over trials, we used only data from the first pre‐cross‐over phase to minimise potential bias from carry‐over effects.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data when possible (e.g. only abstract available). When this was not possible and we thought that missing data might introduce serious bias, we explored the impact of including such studies in the overall assessment of results by performing a sensitivity analysis.
Assessment of heterogeneity
We used the I2 statistic to measure heterogeneity among the studies in each analysis. When we identified substantial heterogeneity (> 50%), we reported this in the text and explored possible causes by conducting prespecified subgroup analyses (e.g. adults vs children).
Assessment of reporting biases
We were not able to pool more than 10 studies for any comparison; therefore, we were unable to explore small‐study effects and publication biases by using a funnel plot.
Data synthesis
We included outcomes in meta‐analyses when we considered study designs, interventions, and outcomes as sufficiently similar. When we identified substantial heterogeneity (> 50%), we reported outcomes in the text, revealing the direction and size of the effect, along with the strength of the evidence (risk of bias). Antibiotic studies varied by population, design, and outcomes. However, we identified few studies for each comparison, and estimates from a random‐effects model therefore may have been unreliable, we used a fixed‐effect model, reported data with 95% confidence intervals (CIs), and evaluated the impact of model choice by performing a sensitivity analysis, when appropriate. We synthesised and reported dichotomous and continuous data separately for each outcome (e.g. exacerbation/no exacerbation or exacerbation duration), and when study authors reported both end‐of‐study point estimates and change from baseline scores, we analysed these separately.
'Summary of findings' tables
We created 'Summary of findings' tables by using the following primary and secondary outcomes: exacerbations, hospitalisations, serious adverse events, deaths, and quality of life. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of evidence from studies contributing data to meta‐analyses for these outcomes. We used methods and recommendations as described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and we used GRADEproGDT software (GRADEproGDT). We justified all decisions to downgrade or upgrade the quality of evidence provided by studies by using footnotes and adding comments to aid understanding when necessary.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses, although data were insufficient for comparisons of all subgroups. However, we chose to present the data for different macrolides as subgroups for all outcomes.
Macrolides versus other classes of long‐term antibiotics.
Types of macrolides.
Dose and frequency.
Duration.
We planned to use the following outcomes in conducting subgroup analyses.
Exacerbations.
Hospitalisations.
Serious adverse events.
We used the formal test for subgroup interactions provided in Review Manager 5 (RevMan 2014).
Sensitivity analysis
We evaluated effects of methodological study quality by removing studies at high or unclear risk of bias for the domains of random sequence generation and allocation concealment.
Results
Description of studies
Results of the search
A systematic search, conducted on 18 January 2018, identified 103 unique records of potentially relevant trials. Of these, we considered 63 records irrelevant following inspection of their titles and abstracts. We obtained and read full texts for the remaining 40 records and formally excluded eight records (documented in Excluded studies). We contacted the authors of one study (two records) awaiting classification (see Studies awaiting classification). A total of 15 trials, with 30 records, met our inclusion criteria for studies of macrolides for bronchiectasis. We have summarised the selection process in the study flow diagram (Figure 1).
1.
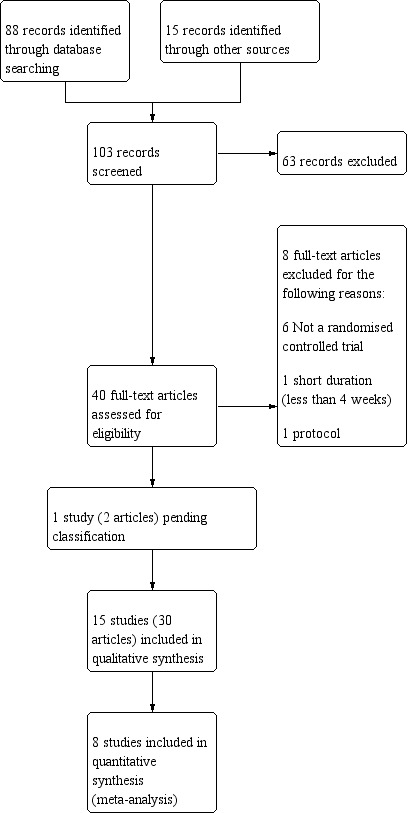
Study flow diagram.
Included studies
In 11 trials (Altenburg 2013; Asintam 2012; Cymbala 2005; Diego 2013; Juthong 2011; Liu 2012; Liu 2014; Lourdesamy 2014; Sadigov 2013; Serisier 2013; Wong 2012), study participants were adults, and in the remaining four trials (Koh 1997; Masekela 2013; Valery 2013; Yalcin 2006), participants were children. See Characteristics of included studies for further details. See Table 5 for an overview of study characteristics.
1. Study characteristics.
| Study |
Adults/ Children |
No. of participants | Type of macrolide | Macrolide dose | Frequency | Delivery mode | Combined weekly dose | Comparison | Duration (months unless stated) |
| Altenburg 2013 | Adults | 83 | Azithromycin | 250 mg | Once daily | Oral | 1750 mg | Placebo | 12 |
| Asintam 2012 | Adults | 30 | Roxithromycin | 300 mg | Once daily | Oral | 2100 mg | Placebo | 12 weeks |
| Cymbala 2005 | Adults | 12 | Azithromycin | 500 mg | 3 days per week | Oral | 1000 mg | No intervention | 6 |
| Diego 2013 | Adults | 36 | Azithromycin | 250 mg | 3 days per week | Oral | 750 mg | No intervention | 3 |
| Juthong 2011 | Adults | 26 | Roxithromycin | 300 mg | Once daily | Oral | 2100 mg | Placebo | 8 weeks |
| Koh 1997 | Children | 25 | Roxithromycin | 4 mg/kg | Twice daily | Oral | 56 mg/kg | Placebo | 12 weeks |
| Liu 2012 | Adults | 50 | Roxithromycin, ambroxol hydrochloride | 150 mg | Once daily | Oral | 1050 mg | Ambroxol hydrochloride (no intervention) | 6 |
| Liu 2014 | Adults | 52 | Roxithromycin | 150 mg | Once daily | Oral | 1050 mg | No intervention | 6 |
| Lourdesamy 2014 | Adults | 78 | Azithromycin | 1000 mg | Weekly | 1000 mg | Placebo | 3 | |
| Masekela 2013 | Children | 42 | Erythromycin | 125 mg for children weighing < 15 kg and 250 mg ≥ 15 kg | Daily | Oral | 875 mg for children weighing < 15 kg and 1750 mg ≥ 15 kg | Placebo | 12 |
| Sadigov 2013 | Adults | 65 | Azithromycin | 500 mg | 3 days per week | Oral | 1500 mg | Placebo | 6 |
| Serisier 2013 | Adults | 117 | Erythromycin | 250 mg | Twice daily | Oral | 3500 mg | Placebo | 11 |
| Valery 2013 | Children | 89 | Azithromycin | 30 mg/kg up to a maximum of 600 mg | Once a week | Oral | 30 mg/kg up to a maximum of 600 mg | Placebo | 24 |
| Wong 2012 | Adults | 141 | Azithromycin | 500 mg | 3 days per week | Oral | 1500 mg | Placebo | 6 |
| Yalcin 2006 | Children | 34 | Clarithromycin, supportive therapies | 15 mg/kg | Daily | Oral | 105 mg/kg | Supportive therapies (no intervention) | 3 |
Methods
Fourteen of the 15 included studies were parallel‐group RCTs, and the remaining study was an RCT with a cross‐over design (Cymbala 2005). Nine trials were double‐blind, five were open‐label, and one did not report information on study blinding. The intervention duration ranged from eight weeks in Juthong 2011 to 24 months in Valery 2013. The percentage of participants who withdrew after randomisation ranged from 0 in Juthong 2011and Yalcin 2006 to 27% in Masekela 2013, with an average withdrawal proportion of 8.7% across all included studies.
Seven trials were conducted in Asia (Asintam 2012; Juthong 2011; Koh 1997; Liu 2012; Liu 2014; Lourdesamy 2014; Sadigov 2013); three in Australia/New Zealand (Serisier 2013; Valery 2013; Wong 2012); three in Europe (Altenburg 2013; Diego 2013; Yalcin 2006); one in South Africa (Masekela 2013); and one in the USA (Cymbala 2005). Please see Figure 2 for the global distribution of trials. The oldest study concluded in 1996 (Koh 1997), and the most recent in 2013 (Lourdesamy 2014). Three studies recruited participants through multiple centres (Altenburg 2013; Valery 2013; Wong 2012); the remainder were conducted at single centres (Figure 2).
2.
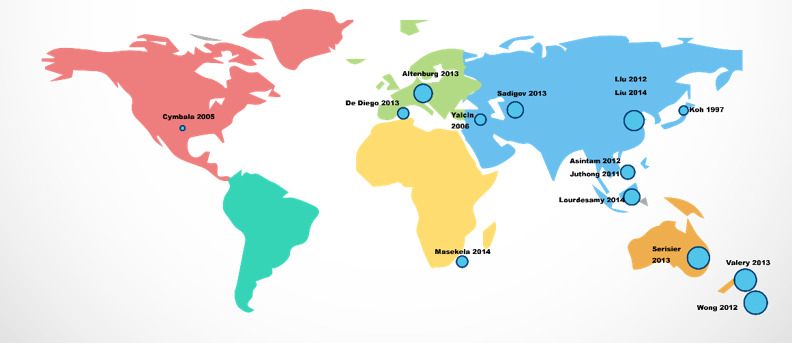
Global distribution of studies.
Six trials used intention‐to‐treat analyses (Altenburg 2013; Juthong 2011; Serisier 2013; Valery 2013; Wong 2012; Yalcin 2006), and seven trials included in analyses only participants who completed the study (Cymbala 2005; Diego 2013; Koh 1997; Liu 2012; Liu 2014; Lourdesamy 2014; Masekela 2013); the analyses performed in two studies were unclear (Asintam 2012; Sadigov 2013). Nine studies reported power calculations for sample size estimation (Altenburg 2013; Asintam 2012; Cymbala 2005; Diego 2013; Lourdesamy 2014; Masekela 2013; Serisier 2013; Valery 2013; Wong 2012), and all six remaining studies reported statistically significant results (Juthong 2011; Koh 1997; Liu 2012; Liu 2014; Sadigov 2013; Yalcin 2006).
Note:We could not include results fromCymbala 2005in the review, as data from the pre‐cross‐over phase alone were not available owing to ineffective randomisation procedures. SeeCharacteristics of included studiesand the associated risk of bias table for additional details.
Participants
We chose to present separately data from adults and children and data on different macrolides.
Adults
Eleven studies included a total of 690 adults aged 18 years and older, with a diagnosis of bronchiectasis confirmed by high‐resolution computed tomography (HRCT). Three studies specified the following numbers of exacerbations in the preceding year as screening criteria: at least three (Altenburg 2013); two (Serisier 2013); and one (Wong 2012). The number of randomised participants in each study ranged from 12 in Cymbala 2005 to 141 in Wong 2012, with a mean age range of 48 years in Liu 2012 to 70.8 years in Cymbala 2005, although one study did not report this information (Sadigov 2013). Data on gender were missing for 81 randomised participants: Three trials reported gender distribution only for those who completed the study (Cymbala 2005; Diego 2013; Liu 2014), and one did not report gender (Sadigov 2013). Of 601 participants for whom data were available, 373 were female and 236 were male, with the percentage of male participants ranging from 23% in Asintam 2012 to 54% in Juthong 2011, across individual studies.
Three studies reported baseline disease severity in terms of Bhalla score: 9.5 (Liu 2014), 12.5 (Asintam 2012), and 26.5 (Diego 2013). Seven studies reported baseline FEV1 % predicted ranging from moderate impairment at 57% of predicted (Diego 2013), to mild impairment at 80.7% of predicted (Altenburg 2013), and two further studies reported baseline FEV1 as 1.08 L in Lourdesamy 2014 and 1.42 L in Juthong 2011. The remaining two studies did not report baseline lung function (Liu 2012; Sadigov 2013). Seven studies reported smoking status, with the proportion of current smokers ranging from none in Asintam 2012 and Serisier 2013 to 28% in Lourdesamy 2014 one study reported 66% current or ex‐smokers (Cymbala 2005), and four studies did not report this information (Diego 2013; Liu 2012; Sadigov 2013; Wong 2012).
Children
Four studies included a total of 190 randomised children, consisting of 81 girls and 98 boys (gender of participants lost to follow‐up was not reported in Masekela 2013), younger than 18 years of age (Koh 1997; Masekela 2013; Valery 2013; Yalcin 2006). Four studies reported a diagnosis of bronchiectasis by HRCT, and Valery 2013 included children with chronic suppurative lung disease, thus meeting clinical criteria when HRCT was not available. Sample sizes ranged from 25 in Koh 1997 to 89 children in Valery 2013, with mean age ranging from four years in Valery 2013 to 13 years in Koh 1997. Participants in Valery 2013 were indigenous children from Australia and New Zealand. Children in Masekela 2013 had confirmed HIV infection, were receiving highly active antiretroviral therapy (HAART), and had undergone a sweat test to rule out cystic fibrosis. Children in Koh 1997 had increased airway responsiveness, confirmed by a metacholine challenge test. Valery 2013 specified at least one exacerbation in the preceding year as one of its inclusion criteria.
Three studies reported baseline FEV1 % predicted as follows: 54.8% (Masekela 2013), 76.5% (Yalcin 2006), and 83% (Koh 1997). Valery 2013 did not report lung function.
Interventions
Adults
The 11 adult studies evaluated three types of oral macrolides. Six studies used azithromycin with doses ranging from 750 to 1750 mg per week for a period of 12 to 52 weeks, four studies used roxithromycin with doses ranging from 1050 to 2100 mg per week for 8 to 24 weeks, and one study used erythromycin at a dose of 3500 mg per week for 48 weeks. Seven studies compared the intervention with placebo (Altenburg 2013; Asintam 2012; Juthong 2011; Lourdesamy 2014; Sadigov 2013; Serisier 2013; Wong 2012), three studies compared the intervention with no intervention (Cymbala 2005; Diego 2013; Liu 2014), and one study compared the intervention plus an antimucolytic with the antimucolytic alone (Liu 2012). We have summarised in Table 5 further details of interventions provided in individual studies.
Note: For all outcomes from Diego 2013, we have included only the mean change score, pending clarification by study authors of reported standard deviations. Therefore, we have included these data in the text narratively.
Outcomes
One study reported all of our prespecified outcomes (Altenburg 2013).
Seven adult studies reported the frequency of exacerbations (Altenburg 2013; Asintam 2012; Diego 2013; Liu 2014; Sadigov 2013; Serisier 2013; Wong 2012), and three reported the time to first exacerbation (Altenburg 2013; Sadigov 2013; Wong 2012). Two children's studies reported the frequency of exacerbations (Masekela 2013; Valery 2013), and one also reported the time to first exacerbation and the duration of the exacerbation (Valery 2013).
Two adult studies reported hospitalisations (Altenburg 2013; Lourdesamy 2014), as did one paediatric study (Valery 2013).
All three adult studies reported serious adverse events (Lourdesamy 2014; Serisier 2013; Wong 2012), as did one study in which the participants were children (Valery 2013).
Five of the adult studies reported sputum volume (Asintam 2012; Cymbala 2005; Diego 2013; Lourdesamy 2014; Serisier 2013), as did two paediatric studies (Koh 1997; Yalcin 2006). Data from Cymbala 2005 were not usable (see note above).
Nine adult studies reported lung function, measured as FEV1 or forced vital capacity (FVC), or both (Altenburg 2013; Asintam 2012; Cymbala 2005; Diego 2013; Juthong 2011; Lourdesamy 2014; Sadigov 2013; Serisier 2013; Wong 2012), as did all four paediatric studies (Koh 1997; Masekela 2013; Valery 2013; Yalcin 2006). Data from Cymbala 2005 were not usable (see note above).
Two adult studies reported FEV1 before and after bronchodilation (Diego 2013; Wong 2012), and one also reported FVC before and after bronchodilation (Wong 2012). The remaining studies did not specify whether lung function was measured before or after bronchodilation.
Three adult studies reported systemic markers such as C‐reactive protein (Altenburg 2013; Masekela 2013; Serisier 2013).
Five adult studies reported adverse events (Altenburg 2013; Juthong 2011; Lourdesamy 2014; Serisier 2013; Wong 2012), as did two studies with children (Liu 2014; Valery 2013).
All 15 studies directly reported or inferred all‐cause mortality due to completion of the study period by all participants (Altenburg 2013; Asintam 2012; Cymbala 2005; Diego 2013; Juthong 2011; Koh 1997; Liu 2012; Liu 2014; Lourdesamy 2014; Masekela 2013; Sadigov 2013; Serisier 2013; Valery 2013; Wong 2012; Yalcin 2006).
Four adult studies reported the emergence of resistance to antibiotics (Altenburg 2013; Juthong 2011; Serisier 2013; Wong 2012), as did one study that included children (Valery 2013).
Two adult studies reported exercise capacity as measured by the 6MWD test (Serisier 2013; Wong 2012).
Nine adult studies reported health‐related quality of life using SGRQ (Altenburg 2013; Asintam 2012; Diego 2013; Juthong 2011; Liu 2012; Liu 2014; Lourdesamy 2014; Serisier 2013; Wong 2012).
Note: Eight studies reported a formal sample size calculation (Altenburg 2013; Asintam 2012; Cymbala 2005; Diego 2013; Lourdesamy 2014; Serisier 2013; Valery 2013; Wong 2012), but two of these studies did not recruit the target number of participants (Asintam 2012; Cymbala 2005). Six studies provided details of online trial registration (Altenburg 2013; Diego 2013; Lourdesamy 2014; Serisier 2013; Valery 2013; Wong 2012). Eight studies included conflict of interest statements (Altenburg 2013; Cymbala 2005; Juthong 2011; Liu 2014; Lourdesamy 2014; Serisier 2013; Valery 2013; Wong 2012). Nine studies explicitly stated funding sources for the study (Altenburg 2013; Cymbala 2005; Diego 2013; Juthong 2011; Liu 2014; Lourdesamy 2014; Serisier 2013; Valery 2013; Wong 2012), but only six studies reported the role of funding sources in the trial (Altenburg 2013; Cymbala 2005; Lourdesamy 2014; Serisier 2013; Valery 2013; Wong 2012).
Subgroup analysis
One study with children conducted several post hoc subgroup analyses based on intervention compliance, intervention duration, bronchiectasis diagnosis, frequency of exacerbations at baseline, and positive bacterial infection at the beginning of the trial (Valery 2013).
Excluded studies
We excluded eight studies from this review. Six of these were not RCTs (Kudo 1988; Min 1988; Ming 2005; Rikitomi 1988; Saito 1988; Unoura 1986), one study was of less than four weeks' duration and therefore did not meet our inclusion criteria (Tagaya 2002), and one study served as the protocol for a trial (Chang 2013). Please see Characteristics of excluded studies for additional details.
Risk of bias in included studies
Full details of the risk of bias judgements can be found in the 'Risk of bias' section at the end of each Characteristics of included studies table. Figure 3 and Figure 4 also provide a summary of the risk of bias in all included studies. Two independent review authors (LF and NR) independently assessed the risk of bias for each of the included studies and reached agreement.
3.
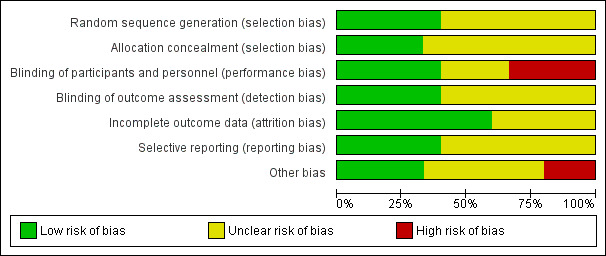
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.
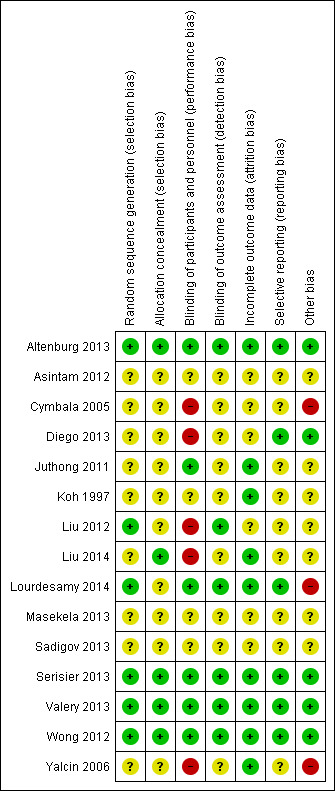
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Review authors considered the methods used to generate randomisation sequences as low risk in six studies (Altenburg 2013; Liu 2012; Lourdesamy 2014; Serisier 2013; Valery 2013; Wong 2012). Lourdesamy 2014 and Valery 2013 randomised participants using computer‐generated random numbers in a 1:1 ratio, and Valery 2013 also reported using a block design. Altenburg 2013 described an independently performed computer‐generated random allocation sequence that used a permuted block size of 10. Serisier 2013 also used a computer‐generated random allocation sequence but with block sizes of 2, 4, and 8, and stratified patients by baseline sputum Pseudomonas. Wong 2012 used a similar sequence generation with block size of 6 and stratified participants by centre. Liu 2012 randomised participants by using random number tables. The remaining nine studies provided unclear details regarding generation of the randomisation sequence (Asintam 2012; Cymbala 2005; Diego 2013; Juthong 2011; Koh 1997; Liu 2014; Masekela 2013; Sadigov 2013; Yalcin 2006).
We judged allocation concealment as having low risk of bias in four studies (Altenburg 2013; Serisier 2013; Valery 2013; Wong 2012), and we assigned unclear risk in 11 studies (Asintam 2012; Cymbala 2005; Diego 2013; Juthong 2011; Koh 1997; Liu 2012; Liu 2014; Lourdesamy 2014; Masekela 2013; Sadigov 2013; Yalcin 2006). Altenburg 2013 assigned identification codes with double‐blind allocation to treatment groups. Valery 2013 used sequentially numbered, double‐sealed, opaque envelopes to conceal group allocation. Serisier 2013 used an independent trial pharmacist to dispense blinded study drug according to the randomisation sequence. Wong 2012 randomly assigned participants to groups using a study‐independent statistician. Studies considered at unclear risk of allocation concealment bias did not provide adequate details of study methods to inform a clear judgement.
Blinding
We judged performance of the trial to be at low risk of bias in six studies (Altenburg 2013; Juthong 2011; Lourdesamy 2014; Serisier 2013; Valery 2013; Wong 2012). Juthong 2011 and Altenburg 2013 reported identical tablets in both groups. Juthong 2011Lourdesamy 2014, Serisier 2013Valery 2013, and Wong 2012 stated that study personnel (patients, supervisors, staff, researchers, investigators) were blinded to treatment allocation at all times. We judged three studies as having high risk of performance bias, as they were open‐label trials (Diego 2013; Liu 2012; Liu 2014). Investigators reported methods in the remaining studies in insufficient detail to permit a clear judgement of performance bias (Asintam 2012; Cymbala 2005; Koh 1997; Masekela 2013; Sadigov 2013; Yalcin 2006).
Six studies clearly stated blinding of outcome assessments (detection bias); we therefore judged these studies to be low risk of bias (Altenburg 2013; Liu 2012; Lourdesamy 2014; Serisier 2013; Valery 2013; Wong 2012). However, the remaining nine studies did not report methods in sufficient detail to inform a clear judgement of the risk of detection bias (Asintam 2012; Cymbala 2005; Diego 2013; Juthong 2011; Koh 1997; Liu 2014; Masekela 2013; Sadigov 2013; Yalcin 2006).
Incomplete outcome data
We judged incomplete outcome data (attrition bias) to introduce low risk of bias in nine studies (Altenburg 2013; Juthong 2011; Koh 1997; Liu 2014; Lourdesamy 2014; Serisier 2013; Valery 2013; Wong 2012; Yalcin 2006). Four studies reported no dropouts (Altenburg 2013; Juthong 2011; Serisier 2013; Yalcin 2006). Five studies clearly reported attrition rates and reasons for withdrawal (Koh 1997; Liu 2014; Lourdesamy 2014; Valery 2013; Wong 2012). We judged the remaining six studies to have unclear risk of attrition bias owing to insufficient reporting (Asintam 2012; Cymbala 2005; Diego 2013; Liu 2012; Masekela 2013; Sadigov 2013).
Selective reporting
We judged six of the included studies to have low risk of reporting bias (selective reporting) (Altenburg 2013; Diego 2013; Lourdesamy 2014; Serisier 2013; Valery 2013; Wong 2012), as the study protocols were available, and all outcomes of interest had been reported in the prespecified way. We judged the risk of reporting bias as unclear in nine studies (Asintam 2012; Cymbala 2005; Juthong 2011; Koh 1997; Liu 2012; Liu 2014; Masekela 2013; Sadigov 2013; Yalcin 2006), as a full trial protocol was not available.
Other potential sources of bias
We did not identify any other potential sources of bias in five studies (Altenburg 2013; Diego 2013; Serisier 2013; Valery 2013; Wong 2012), but we could not adequately assess this in seven other included studies (Asintam 2012; Juthong 2011; Koh 1997; Liu 2012; Liu 2014; Masekela 2013; Sadigov 2013). We judged three studies to have high risk of other potential sources of bias (Cymbala 2005Lourdesamy 2014; Yalcin 2006). Group allocation was ineffective in the pre‐cross‐over phase of Cymbala 2005, with eight of 11 participants receiving the intervention. In Lourdesamy 2014, baseline sputum volume (primary outcome) was significantly higher in the intervention arm compared with the placebo group. Similarly, in Yalcin 2006, baseline cytokine assay levels were again significantly higher in the intervention group compared with the placebo group.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Macrolides compared with placebo for adults with bronchiectasis.
| Macrolides compared with placebo for adults with bronchiectasis | ||||||
| Patient or population: adults with bronchiectasis Setting: outpatient clinics in Australia, Azerbaijan, Malaysia, Netherlands, New Zealand, and Thailand Intervention: macrolides Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with macrolides | |||||
| ≥ 1 exacerbation Follow‐up: range 24 weeks to 52 weeks | 714 per 1000 | 459 per 1000 (355 to 574) | OR 0.34 (0.22 to 0.54) | 341 (3 RCTs) | ⊕⊕⊕⊝ MODERATEa | 2 studies azithromycin (1750 mg/week for 52 weeks; 1500 mg/week for 6 months) 1 study erythromycin (3500 mg/week for 48 weeks) |
| Hospitalisation: all cause Follow‐up: range 12 weeks to 52 weeks | 133 per 1000 | 79 per 1000 (28 to 200) | OR 0.56 (0.19 to 1.62) | 151 (2 RCTs) | ⊕⊕⊝⊝ LOWb,c | 2 studies azithromycin (1000 mg/week for 12 weeks; 1750 mg/week for 52 weeks) |
| Serious adverse events Follow‐up: range 24 weeks to 48 weeks | 86 per 1000 | 44 per 1000 (18 to 104) | OR 0.49 (0.20 to 1.23) | 326 (3 RCTs) | ⊕⊕⊝⊝ LOWb,d | 2 studies azithromycin (1500 mg/week for 6 months; 1000 mg/week for 12 weeks) 1 study erythromycin (3500 mg/week for 48 weeks) |
| All‐cause mortality Follow‐up: range 8 weeks to 52 weeks | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | 540 (7 RCTs) | ⊕⊕⊝⊝ Lowe,f | 4 studies azithromycin (1000 to 1750 mg/week for 12 to 52 weeks) 2 studies roxithromycin (2100 mg/week for 8 to 12 weeks) 1 study erythromycin (3500 mg/week for 48 weeks) |
| Quality of life: endpoint assessed with SGRQ Scale from 0 to 100 Follow‐up: 12 weeks | Mean SGRQ score at endpoint in placebo groups was 39.1 points. | MD 8.90 lower (13.13 lower to 4.67 lower) | ‐ | 68 (1 RCTs) | ⊕⊕⊕⊝ Moderateb | 1 study azithromycin (1000 mg/week for 12 weeks) |
| Quality of life: change assessed with SGRQ Scale from 0 to 100 Follow‐up: range 8 weeks to 48 weeks | Mean change in SGRQ score ranged from ‐1.3 to ‐8.9 points. | MD 2.86 lower (5.67 lower to 0.04 lower) | ‐ | 305 (4 RCTs) | ⊕⊕⊝⊝ LOWg,h | 1 study azithromycin (1500 mg/week for 6 months) 1 study erythromycin (3500 mg/week for 48 weeks) 2 studies roxithromycin (2100 mg/week for 12 weeks; 2100 mg/week for 8 weeks) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio; SGRQ: St. George's Respiratory Questionnaire. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aEffect observed only with azithromycin (one point deducted in relation to design and implementation of available studies suggesting likelihood of bias).
bUnclear allocation concealment and baseline imbalances on Lourdesamy (one point deducted in relation to design and implementation of available studies suggesting likelihood of bias).
cTwo small studies and wide confidence interval (one point deducted for imprecision).
dWide confidence interval (one point deducted for imprecision).
eIn three of the seven studies, study methods were not clearly reported (one point deducted in relation to design and implementation of available studies suggesting likelihood of bias).
fA total of 28 participants across four studies were lost to follow‐up with no further details available and unclear details of withdrawals in one study (one point deducted in relation to design and implementation of available studies suggesting likelihood of bias).
gRandomisation, blinding, and other study methods unclear in two studies (Asintam; Juthong) (one point deducted in relation to design and implementation of available studies suggesting likelihood of bias).
hWide confidence interval and mean difference does not exceed the threshold for clinical significance (one point deducted for imprecision).
Summary of findings 2. Macrolides compared with no intervention for adults with bronchiectasis.
| Macrolides compared with no intervention for adults with bronchiectasis | ||||||
| Patient or population: adults with bronchiectasis Setting: outpatient clinics in China and Spain Intervention: macrolides Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no intervention | Risk with macrolides | |||||
| ≥ 1 exacerbation Follow‐up: 6 months | Study population | OR 0.31 (0.08 to 1.15) | 43 (1 RCT) | ⊕⊕⊕⊝ MODERATEa | Roxithromycin (1050 mg/week for 6 months) | |
| 762 per 1000 | 498 per 1000 (204 to 786) | |||||
| Hospitalisations ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Serious adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Mortality Follow‐up: range 3 months to 6 months | No deaths in two trials, although in 1 study (azithromycin), 6 participants were lost to follow‐up | not estimable | 88 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,c | 1 study azithromycin (750 mg/week for 3 months) 1 study roxithromycin (1050 mg/week for 6 months) | |
| QoL SGRQ: endpoint total score Scale from 0 to 100 Follow‐up: 6 months | Mean SGRQ: endpoint total score of 51.7 points | MD 8.81 lower (14.33 lower to 3.28 lower) | ‐ | 89 (2 RCT) | ⊕⊕⊕⊝ MODERATEa | 1 study roxithromycin (1050 mg/week for 6 months) 1 study roxithromycin (1050 mg/week for 6 months) |
| QoL SGRQ: change in total score Scale from 0 to 100 Follow‐up: 3 months | Mean SGRQ: change in total score of 4.1 | MD 12 lower (21.61 lower to 2.39 lower) | ‐ | 30 (1 RCT) | ⊕⊕⊕⊝ MODERATEa | Azithromycin (750 mg/week for 3 months) |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; OR: odds ratio; QoL: quality of life; SGRQ: St. George's Respiratory Questionnaire. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aOpen‐label study (one point deducted in relation to design and implementation of available studies suggesting likelihood of bias).
bUnclear randomisation and study methods (one point deducted in relation to design and implementation of available studies suggesting likelihood of bias).
c6 participants in one study lost to follow‐up and no further details reported (one point deducted in relation to design and implementation of available studies suggesting likelihood of bias).
Summary of findings 3. Macrolides compared with placebo for children with bronchiectasis.
| Macrolides compared with placebo for children with bronchiectasis | ||||||
| Patient or population: children with bronchiectasis Setting: outpatient clinics in Australia, New Zealand, and South Africa Intervention: macrolides Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with macrolides | |||||
| Exacerbation frequency | Number of exacerbations 195 (median: 4 range 0‐14) | Number of exacerbations 104 (median: 2 range 0‐9) | IRR 0.50 95% CI 0.35 to 0.71 | 89 (1 RCT) | ⊕⊕⊝⊝ LOWa,b | Azithromycin (30 mg/kg/week for up to 24 months) |
| Hospitalisation: all‐cause Follow‐up: 24 months | 205 per 1000 | 67 per 1000 (18 to 222) | OR 0.28 (0.07 to 1.11) | 89 (1 RCT) | ⊕⊕⊝⊝ LOWa,b | Azithromycin (30 mg/kg/week for 24 months) |
| Serious adverse events Follow‐up: 24 months | 432 per 1000 | 246 per 1000 (114 to 444) | OR 0.43 (0.17 to 1.05) | 89 (1 RCT) | ⊕⊕⊝⊝ LOWa,b | Azithromycin (30 mg/kg/week for 24 months) |
| Mortality | 1 child died but study group was not stated. | ‐ | 42 (1 RCT) | ⊕⊕⊝⊝ LOWc,d | Erythromycin (875 to 1750 mg/kg/week for 52 weeks) | |
| Quality of life not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; IRR: incidence rate ratio; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aWide confidence interval that includes 1 (no difference) (one point deducted for imprecision).
bLow event rates and low numbers (one point deducted in relation to design and implementation of available studies suggesting likelihood of bias).
cUnclear information on randomisation, blinding, and other study methods (one point deducted in relation to design and implementation of available studies suggesting likelihood of bias).
dNo information on participants lost to follow‐up (one point deducted in relation to design and implementation of available studies suggesting likelihood of bias).
Summary of findings 4. Macrolides compared with no intervention for children with bronchiectasis.
| Macrolides compared with no intervention for children with bronchiectasis | ||||||
| Patient or population: children with bronchiectasis Setting: outpatient clinic in Turkey Intervention: macrolides Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no intervention | Risk with macrolides | |||||
| Exacerbations ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Hospitalisation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Serious adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Mortality Follow‐up: 3 months | 0 per 1000 | 0 per 1000 (0 to 0) | not estimable | 34 (1 RCT) | ⊕⊕⊝⊝ LOWa,b | Clarithromycin (105 mg/kg/week for 3 months) |
| Quality of life ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aInsufficient information on study methods and procedures (one point deducted in relation to design and implementation of available studies suggesting likelihood of bias).
bNot blinded (one point deducted in relation to design and implementation of available studies suggesting likelihood of bias).
Macrolide versus placebo: adults
Primary outcomes
Exacerbations
Two adult studies of azithromycin ‐ Altenburg 2013 and Wong 2012 ‐ and one adult study of erythromycin ‐ Serisier 2013 ‐ with a total of 341 participants were included in a meta‐analysis. Results show that macrolides reduced the frequency of exacerbations to a greater extent than placebo (OR 0.34, 95% CI 0.22 to 0.54; I2 = 65%; Analysis 1.1; moderate‐quality evidence). This translates to 714 per 1000 in the placebo group experiencing one or more exacerbation compared with 459 per 1000 in the macrolide group (95% CI 355 to 574) or a number needed to treat for an additional beneficial outcome (NNTB) of 4 (95% CI 3 to 8) (Figure 5).
1.1. Analysis.
Comparison 1 Macrolide versus placebo: adults, Outcome 1 ≥ 1 exacerbation.
5.
Analysis 1.1. Cates plot showing the absolute reduction in numbers of participants experiencing one or more exacerbations in adults treated with macrolides compared with placebo (OR 0.34, 95% CI 0.22 to 0.54). 714 people per 1000 in the placebo group experienced one or more exacerbations compared with 459 (95% CI 355 to 574) per 1000 in the macrolide group.
As heterogeneity was substantial, we tested the impact of a random‐effects model on the pooled effect size, which remained unchanged (OR 0.34, 95% CI 0.15 to 0.75). However, we noted significant differences between azithromycin and erythromycin subgroups (test for subgroup differences: Chi2 = 5.63, df = 1 (P = 0.02), I2 = 82.2%) and beneficial effects related to the two azithromycin studies (OR 0.23, 95% CI 0.13 to 0.40; I2 = 0%) (Altenburg 2013; Wong 2012). Data show no differences between groups in the erythromycin study (OR 0.74, 95% CI 0.34 to 1.63). Two further studies did not report exacerbations in sufficient detail for inclusion in meta‐analyses. In one study of azithromycin (1500 mg/week for six months) involving 65 adults, trial authors reported that the intervention "significantly decreased the rate of event‐based exacerbations and significantly increased the time to the first event‐based exacerbation compared to placebo", but no further details were available (Sadigov 2013). In another study of roxithromycin involving 30 adults, two participants in the intervention group and one participant in the control group developed an exacerbation but researchers reported no further details (Asintam 2012).
Three adult studies reported significantly reduced incidence rate ratios in the intervention group as follows: 0.48 fewer exacerbations per year (95% CI 0.65 to 0.26) (Altenburg 2013); 0.57 fewer exacerbations per year (95% CI 0.77 to 0.42) (Serisier 2013); and 0.38 fewer exacerbations per year (95% CI 0.54 to 0.25) (Wong 2012).
One adult study reported time to first exacerbation following a post hoc analysis, with a hazard ratio of 0.29 (95% CI 0.16 to 0.51) favouring azithromycin (Altenburg 2013).
Hospitalisations
We included in a meta‐analysis two studies of azithromycin involving 151 adults (Altenburg 2013; Lourdesamy 2014); results show no evidence of a reduction in hospitalisations in the azithromycin group compared with the placebo group (OR 0.56, 95% CI 0.19 to 1.62; I2 = 0%; Analysis 1.2; low‐quality evidence), although these results should be interpreted with caution owing to the low event rate.
1.2. Analysis.
Comparison 1 Macrolide versus placebo: adults, Outcome 2 Hospitalisation: all‐cause.
Serious adverse events
Meta‐analysis included two studies of azithromycin involving 209 adults (Lourdesamy 2014; Wong 2012), along with one study of erythromycin with 117 adults (Serisier 2013). Serious adverse events included pneumonia, respiratory and non‐respiratory infections, haemoptysis, gastroenteritis, hernia, congestive heart failure, stroke, and skin carcinoma. Results show no difference in the numbers of participants with serious adverse events between study groups (OR 0.49, 95% CI 0.20 to 1.23; I2 = 0%; Analysis 1.3; low‐quality evidence) and no evidence of subgroup differences between azithromycin and erythromycin (test for subgroup differences: Chi2 = 0.48, df = 2 (P = 0.79), I2 = 0%), although results should be interpreted with caution owing to low event rates. Removing the study with unclear risk of bias for allocation concealment ‐ Lourdesamy 2014 ‐ from the meta‐analysis had little impact on the pooled treatment effect (OR 0.39, 95% CI 0.12 to 1.23; I2 = 0%).
1.3. Analysis.
Comparison 1 Macrolide versus placebo: adults, Outcome 3 Serious adverse events.
Secondary outcomes
Sputum volume and purulence
One study of azithromycin (1000 mg/week for 12 weeks) with 78 adults reported no difference in sputum volume between study groups (MD 3.70, 95% CI ‐5.78 to 13.18; Analysis 1.4) (Lourdesamy 2014). One study of erythromycin (3500 mg/week for 48 weeks) with 117 adults reported a significant reduction in the change from baseline in 24‐hour sputum weight, favouring the intervention (median change ‐4.4 grams, interquartile ratio (IQR) ‐7.8 to ‐1; P = 0.01) (Serisier 2013). One study of roxithromycin (2100 mg/week for 12 weeks) with 30 adults reported no improvement in sputum volume in either study group but provided no further details (Asintam 2012).
1.4. Analysis.
Comparison 1 Macrolide versus placebo: adults, Outcome 4 Sputum weight (g): endpoint.
Measures of lung function
Forced expiratory volume in one second (FEV1)
Seven adult studies reported FEV1 as litres, percent of predicted, or both (Altenburg 2013; Asintam 2012; Juthong 2011; Lourdesamy 2014; Sadigov 2013; Serisier 2013; Wong 2012). One trial of azithromycin (1000 mg/week for 12 weeks) with 78 participants showed no evidence of benefit in FEV1 % predicted from the intervention at the end of the study (MD 2.98, 95% CI ‐6.15 to 12.11; Analysis 1.5) (Lourdesamy 2014). One trial of azithromycin (1750 mg/week for 52 weeks) with 83 participants reported an increase of 1.03% in FEV1 % predicted in the intervention group every three months compared with a decrease of 0.10% in the placebo group (P = 0.047) (Altenburg 2013). One trial of erythromycin (3500 mg/week for 48 weeks) with 117 participants reported a significant difference in FEV1 %predicted change from baseline between groups, favouring macrolides (MD 2.40, 95% CI 0.34 to 4.46; Analysis 1.6) (Serisier 2013). One study of azithromycin (1500 mg/week for 6 months) with 65 participants reported significant improvements in prebronchodilator and postbronchodilator FEV1 but provided no further details (Sadigov 2013). One study of roxithromycin (2100 mg/week for 12 weeks) with 30 participants reported no improvement in either study group but provided no further details (Asintam 2012).
1.5. Analysis.
Comparison 1 Macrolide versus placebo: adults, Outcome 5 FEV1 (% predicted): endpoint.
1.6. Analysis.
Comparison 1 Macrolide versus placebo: adults, Outcome 6 FEV1 (% predicted): change (post bronchodilator).
A meta‐analysis of data from two studies showed no benefit from azithromycin or roxithromycin in FEV1 at the end of the study (MD 0.02 L, 95% CI ‐0.17 to 0.22; Analysis 1.7) (Juthong 2011; Lourdesamy 2014). Results show were no significant differences between the two macrolides (test for subgroup differences: Chi2 = 0.43, df = 1 (P = 0.51), I2 = 0%). Another study of azithromycin (1500 mg/week for 6 months) with 141 participants also showed no benefit from the intervention in change in FEV1 during the study (MD 0.04 L, 95% CI ‐0.03 to 0.11; Analysis 1.8) (Wong 2012).
1.7. Analysis.
Comparison 1 Macrolide versus placebo: adults, Outcome 7 FEV1 (L): endpoint.
1.8. Analysis.
Comparison 1 Macrolide versus placebo: adults, Outcome 8 FEV1 (L): change.
Forced vital capacity (FVC)
Four adult studies reported FVC as percent of predicted, in litres, or both ways (Altenburg 2013; Juthong 2011; Lourdesamy 2014; Wong 2012). One trial of azithromycin (1000 mg/week for 12 weeks) with 78 participants showed no benefit from the intervention at the end of the study in terms of FVC % predicted (MD 1.07, 95% CI ‐9.27 to 11.41; Analysis 1.9) (Lourdesamy 2014). Another study of azithromycin (1750 mg/week for 52 weeks) with 83 participants reported an increase in FVC of 1.33% predicted in the intervention group and a decrease of 0.30% predicted in the placebo group every three months (Altenburg 2013).
1.9. Analysis.
Comparison 1 Macrolide versus placebo: adults, Outcome 9 FVC (% predicted): endpoint.
A meta‐analysis of data from two studies with 94 participants showed no benefit at the end of the study from azithromycin or roxithromycin in terms of FVC (MD 0.08 L, 95% CI ‐0.19 to 0.36; Analysis 1.10) (Juthong 2011; Lourdesamy 2014). Results show no significant differences between the two macrolides (test for subgroup differences: Chi2 = 1.57, df = 1 (P = 0.21), I2 = 36.3%). One study of azithromycin (1500 mg/week for six months) with 141 participants showed no benefit from the intervention in changes in FVC (MD 0.08 L, 95% CI ‐0.53 to 0.69; Analysis 1.11) (Wong 2012).
1.10. Analysis.
Comparison 1 Macrolide versus placebo: adults, Outcome 10 FVC (L): endpoint.
1.11. Analysis.
Comparison 1 Macrolide versus placebo: adults, Outcome 11 FVC (L): change.
FEV1/FVC ratio
One study of azithromycin (1000 mg/week for 12 weeks) with 78 participants reported the FEV1/FVC ratio showing no evidence of benefit from the intervention at the end of the study (MD 3.57, 95% CI ‐3.89 to 11.03; Analysis 1.12) (Lourdesamy 2014).
1.12. Analysis.
Comparison 1 Macrolide versus placebo: adults, Outcome 12 FEV1/FVC: endpoint.
Systemic markers of infection
One trial of azithromycin (1750 mg/week for 52 weeks) with 83 participants reported no significant differences between median CRP values at the end of the study (azithromycin 2.6 mg/dL, IQR 1.5 ‐ 7; control 3.9 mg/dL, IQR 2 ‐ 6.15) and no changes in serum levels, although P values were not reported (Altenburg 2013). Similarly, one trial of erythromycin (3500 mg/week for 48 weeks) with 117 participants reported no differences between groups in CRP levels (median change difference ‐0.2 mg/L, IQR ‐1.5 to 1.2), although again significance values were not reported (Serisier 2013).
Adverse events
Five studies of three different macrolides (azithromycin, erythromycin, and roxithromycin) with 435 adult participants were included in a meta‐analysis (Altenburg 2013; Juthong 2011; Lourdesamy 2014; Serisier 2013; Wong 2012), showing no differences between study groups in the numbers of people experiencing adverse events (OR 0.83, 95% CI 0.51 to 1.35; I2 = 28%; Analysis 1.13). Trials provided no evidence of differences between the three different macrolides (test for subgroup differences: Chi2 = 2.07, df = 2 (P = 0.36), I2 = 3.3%). Removing two studies from the analysis with unclear risk of bias for sequence generation or allocation concealment had little impact on the pooled treatment effect (OR 0.83, 95% CI 0.50 to 1.39; I2 = 27%) (Juthong 2011; Lourdesamy 2014).
1.13. Analysis.
Comparison 1 Macrolide versus placebo: adults, Outcome 13 Adverse events.
All‐cause mortality
Data show no deaths during the intervention period in six of the adult studies (Altenburg 2013; Asintam 2012; Juthong 2011; Sadigov 2013; Serisier 2013; Wong 2012). One study of azithromycin (1000 mg/week for 12 weeks) with 78 participants reported no deaths in the placebo group and two deaths in the intervention group attributed to bronchopneumonia and not considered treatment‐related (Lourdesamy 2014). In performing our GRADE assessment, we judged this outcome to be of low quality (Table 1).
Emergence of resistance to antibiotics
One study of azithromycin with 83 adults reported no differences between groups in the emergence of resistance to antibiotics (OR 0.71, 95% CI 0.30 to 1.69; Analysis 1.14) (Altenburg 2013). In another study of roxithromycin (2100 mg/week for 8 weeks) with 26 adults, none of the participants experienced antibiotic resistance to any bacterial strain (Juthong 2011). However, a study of erythromycin (3500 mg/week for 48 weeks) with 117 participants reported a higher proportion of macrolide‐resistant oropharyngeal streptococci in the intervention group compared with the placebo group (median change difference 25.5%, IQR 15% to 33.7%; P = 0.001) (Serisier 2013).
1.14. Analysis.
Comparison 1 Macrolide versus placebo: adults, Outcome 14 Azithromycin‐resistant bacteria (any).
Exercise capacity
One study of erythromycin (3500 mg/week for 48 weeks) with 117 adults reported no differences in the change in 6MWD between study groups (MD ‐6.30, 95% CI ‐28.86 to 16.26; Analysis 1.15) (Serisier 2013). Similarly, a study of azithromycin (1500 mg/week for 6 months) with 141 adults reported no significance difference in change in 6MWD between study groups (mean change difference 6.48 m, 95% CI ‐11.28 to 24.22; P = 0.4) (Wong 2012).
1.15. Analysis.
Comparison 1 Macrolide versus placebo: adults, Outcome 15 6‐Minute walk test: change.
Health‐related quality of life
One study with 68 adults showed a significantly lower (better) SGRQ total score at the end of the study in the intervention group compared with the placebo group (MD ‐8.90, 95% CI ‐13.13 to ‐4.67; Analysis 1.16; moderate‐quality evidence) and an MD that exceeded the 4‐unit threshold of clinical significance (Lourdesamy 2014). We included in a meta‐analysis four adult studies with 305 participants showing greater improvements from baseline to study endpoint in quality of life with macrolides (MD ‐2.86, 95% CI ‐5.67 to ‐0.04; Analysis 1.17; low‐quality evidence) (Asintam 2012; Juthong 2011; Serisier 2013; Wong 2012). Although data show no significant differences between azithromycin and roxithromycin (test for subgroup differences: Chi2 = 0.01, df = 1 (P = 0.92), I2 = 0%), the beneficial effect was largely observed in the azithromycin group. Differences between groups in all four studies were below the threshold of clinical significance. Removing from the meta‐analysis the two studies with unclear risk of bias for sequence generation and allocation concealment had no impact on the pooled treatment effect (MD ‐2.97, 95% CI ‐5.94 to ‐0.00; I2 = 0%) (Asintam 2012; Juthong 2011).
1.16. Analysis.
Comparison 1 Macrolide versus placebo: adults, Outcome 16 Quality of life: endpoint.
1.17. Analysis.
Comparison 1 Macrolide versus placebo: adults, Outcome 17 Quality of life: change.
One study of azithromycin (1750 mg/week for 52 weeks) with 83 participants reported a significant improvement in quality of life (SGRQ total) with the intervention compared with placebo (intervention group mean change ‐6.09, control group mean change ‐2.06; P = 0.05) (Altenburg 2013).
Macrolide versus no intervention: adults
Primary outcomes
Exacerbations
One study of roxithromycin with 43 adults (1050 mg/week 6 months) did not find a clear difference in the frequency of exacerbations between groups (OR 0.31, 95% CI 0.08 to 1.15; Analysis 2.1; moderate‐quality evidence; Table 2) (Liu 2014).
2.1. Analysis.
Comparison 2 Macrolide versus no intervention: adults, Outcome 1 ≥ 1 exacerbation.
None of the included studies reported our other primary outcomes: hospitalisation and adverse or serious adverse events.
Secondary outcomes
Sputum volume and purulence
One study of azithromycin with 30 adults (750 mg/week 3 months) reported a decrease in sputum volume with macrolides (MD ‐11.00 mL/d; P < 0.05) (Diego 2013). Following the intervention, sputum volume decreased by 8.9 mL/d in the azithromycin group and increased by 2.1 mL/d in the control group by three months. Data show no differences in changes in sputum purulence scores between groups (mean change score: azithromycin 0.8, control 0.7) by three months.
Measures of lung function
One study of azithromycin with 30 adults (750 mg/week 3 months) found no differences between groups in FEV1 or FVC (Diego 2013). Relative to baseline, FEV1 increased by 0.06 L and 0.04 L in azithromycin and control groups, respectively, and FVC decreased by 0.07 L and 0.08 L in azithromycin and control groups, respectively, by three months.
Systemic markers of infection
One study of azithromycin with 30 adults (750 mg/week 3 months) found no differences between groups in CRP levels (Diego 2013). This study reported a reduction in CRP levels by three months compared with baseline in both groups: mean reduction: ‐17 in the azithromycin group, ‐11 in the control group.
Adverse events
One adult study reported adverse events as event rates but did not report the number of participants experiencing at least one adverse event (Liu 2014).
Mortality
Two adult studies reported no deaths during the study (Diego 2013; Liu 2014). Through GRADE assessment, we judged this outcome to be of very low quality (Table 2).
Quality of life
Two roxithromycin studies with 89 adults (1050 mg/week 6 months) reported significantly better quality of life with macrolides compared with no intervention at the end of six months (MD ‐8.81, 95% CI ‐14.33 to ‐3.28; Analysis 2.2; moderate‐quality evidence) (Liu 2012; Liu 2014). One study of azithromycin with 30 adults (750 mg/week for 3 months) reported a significant improvement in quality of life, measured by the SGRQ total score, after three months compared with no intervention (MD ‐12.00; P < 0.05; low‐quality evidence) (Diego 2013). The total score decreased by 7.9 units in the azithromycin group and increased by 4.1 units in the control group. It was not considered appropriate to combine these outcomes in a meta‐analysis owing to differences in macrolides, doses, and study duration.
2.2. Analysis.
Comparison 2 Macrolide versus no intervention: adults, Outcome 2 QoL SGRQ: endpoint total score.
The included studies did not report our other secondary outcomes ‐ exercise capacity and resistance to antibiotics.
Macrolide versus placebo: children
Primary outcomes
Exacerbations
Two studies reported exacerbation frequency in children. One study of azithromycin (30 kg/week for up to 24 months) reported that in children, exacerbation frequency was reduced more with macrolides than with placebo (IRR 0.50, 95% CI 0.35 to 0.71; 89 children; one study; low‐quality evidence). However, we feel this should be interpreted with a degree of caution as the same study reported no significant benefit from the intervention in the number of children with at least one exacerbation (OR 0.40, 95% CI 0.11 to 1.41; low‐quality evidence) nor in the time to first exacerbation (hazard ratio 0·63, 95% CI 0·40–1·00; log‐rank P = 0.12) (Valery 2013). Another study of erythromycin (875 < 15 kg > 1750 mg/week for 52 weeks) in 42 children reported that three children in the intervention group remained exacerbation‐free during the study, and all children in the placebo group had at least one exacerbation (Masekela 2013).
Hospitalisations
One study of azithromycin with 89 children showed no evidence of a reduction in numbers of children hospitalised for exacerbations between study groups (OR 0.28, 95% CI 0.07 to 1.11; Analysis 3.1; low‐quality evidence), although again these results should be interpreted with caution owing to the low event rate (Valery 2013).
3.1. Analysis.
Comparison 3 Macrolide versus placebo: children, Outcome 1 Hospitalisation: all‐cause.
Serious adverse events
One study of azithromycin with 89 children reported serious adverse events (Valery 2013), showing no differences between groups in the number of children experiencing at least one event (OR 0.43, 95% CI 0.17 to 1.05; Analysis 3.2; low‐quality evidence). The majority of the serious adverse events related either to an exacerbation or an investigation related to bronchiectasis (e.g., bronchoscopy).
3.2. Analysis.
Comparison 3 Macrolide versus placebo: children, Outcome 2 Serious adverse events.
Secondary outcomes
Sputum volume and purulence
One study of roxithromycin with 25 children reported a reduction in sputum purulence score with the intervention (MD ‐0.78, 95% CI ‐1.32 to ‐0.24; Analysis 3.3) (Koh 1997).
3.3. Analysis.
Comparison 3 Macrolide versus placebo: children, Outcome 3 Sputum purulence score: endpoint.
Measures of lung function
Forced expiratory volume in one second (FEV1)
A meta‐analysis of two studies with 65 participants showed no evidence of benefit from azithromycin or roxithromycin in FEV1 expressed as percent of predicted by the end of the study (MD 1.73, 95% CI ‐3.32 to 6.78; Analysis 3.4) (Koh 1997; Valery 2013). Removing the study, which had unclear risk of bias for sequence generation and allocation concealment from the meta‐analysis, had little impact on the treatment effect (MD 3.70, 95% CI ‐5.99 to 13.39) (Koh 1997). Another study of erythromycin (875 < 15 kg > 1750 mg/week for 52 weeks) with 42 children who had HIV and were all receiving highly active antiretroviral therapy (HAART) reported no difference in FEV1 % predicted between groups at the end of the study (MD 5.50, 95% CI ‐7.26 to 18.26) (Masekela 2013).
3.4. Analysis.
Comparison 3 Macrolide versus placebo: children, Outcome 4 FEV1 (% predicted): endpoint.
Forced vital capacity (FVC)
Masekela 2013 reported no significant difference in FVC % predicted between groups at the end of the study (MD 5.00, 95% CI ‐5.61 to 15.61).
Systemic markers of infection
One erythromycin study (875 mg < 15 kg > 1750 mg/week for 52 weeks) of 42 children with HIV who were receiving HAART reported no differences in CRP levels between groups (MD 1.60, 95% CI ‐38.38 to 41.58) (Masekela 2013).
Adverse events
One study of azithromycin (30 mg/kg/week for 24 months) in 89 participants reported no differences between study groups in the numbers of children experiencing adverse events (OR 0.78, 95% CI 0.33 to 1.83; Analysis 3.5) (Valery 2013).
3.5. Analysis.
Comparison 3 Macrolide versus placebo: children, Outcome 5 Adverse events.
All‐cause mortality
Masekela 2013 reported the death of one randomised participant but did not state the study group to which that participant had been assigned (low‐quality evidence).
Emergence of resistance to antibiotics
One study of azithromycin in 89 children reported an increase in macrolide‐resistant bacteria (OR 7.13, 95% CI 2.13 to 23.79; Analysis 3.6), an increase in resistance to Streptococcus pneumoniae (OR 13.20, 95% CI 1.61 to 108.19; Analysis 3.7), and an increase in resistance to Staphylococcus aureus (OR 4.16, 95% CI 1.06 to 16.32; Analysis 3.8) with macrolides compared with placebo (Valery 2013).
3.6. Analysis.
Comparison 3 Macrolide versus placebo: children, Outcome 6 Azithromycin‐resistant bacteria (any).
3.7. Analysis.
Comparison 3 Macrolide versus placebo: children, Outcome 7 Azithromycin‐resistant Streptococcus pneumoniae.
3.8. Analysis.
Comparison 3 Macrolide versus placebo: children, Outcome 8 Azithromycin‐resistant Staphylococcus aureus.
The included studies did not report our other secondary outcomes: exercise capacity and health‐related quality of life.
Macrolide versus no intervention: children
Primary outcomes
The included study did not report our primary outcomes: exacerbations, hospitalisations, and adverse and serious adverse events.
Secondary outcomes
Sputum volume and purulence
One study of clarithromycin with 34 children (105 mg/week 3 months) reported a significantly greater reduction in sputum volume with macrolides compared with placebo (P = 0.0001) but did not report exact values for each group (Yalcin 2006).
Measures of lung function
Yalcin 2006 also reported no differences in FEV1 between groups but did not report exact values and significance levels.
Mortality
Yalcin 2006 reported no deaths (low‐quality evidence).
The included study did not report our other secondary outcomes: systemic markers of infection, adverse events, resistance to antibiotics, exercise capacity, and quality of life.
Discussion
Summary of main results
Fifteen randomised trials met the inclusion criteria for this systematic review; 11 studies included adult participants only (Altenburg 2013; Asintam 2012; Cymbala 2005; Diego 2013; Juthong 2011; Liu 2012; Liu 2014; Lourdesamy 2014; Sadigov 2013; Serisier 2013; Wong 2012), and in four studies (Koh 1997; Masekela 2013; Valery 2013; Yalcin 2006), the participants were children. Considerable clinical heterogeneity was evident on a range of other factors, including four different types of macrolides, doses ranging from 750 mg/week to 3500 mg/week with regimens varying from twice daily to once a week, intervention duration ranging from eight weeks to 24 months, and background therapies administered to all groups in two studies. None of the included studies compared one type of macrolide versus another or versus a non‐macrolide antibiotic.
Evidence shows a reduction in exacerbations seen in our aggregation of data from four adult studies, including Altenburg 2013, Sadigov 2013Serisier 2013 and Wong 2012, and from one study of children ‐ Valery 2013 ‐ comparing macrolides with placebo, and we used GRADE criteria to assess the quality of this evidence as moderate. Most of these studies (with the exception of Serisier 2013) used azithromycin. Masekela 2013 reported no reduction in the number of exacerbations over 52 weeks with erythromycin compared with placebo. This study was carried out in South African children with HIV who were receiving antiretrovirals and showed varying degrees of HIV virological suppression. The specifics of this population make it difficult to generalise the findings of Masekela 2013 to individuals with bronchiectasis in less specialised circumstances. Studies comparing macrolides with no intervention were insufficient to establish clear effects. For hospitalisations, data show no evidence of benefit with azithromycin based on aggregation of data from two adult studies (Altenburg 2013; Lourdesamy 2014), along with one study of children (Valery 2013), and on evidence of low quality. We are unable to draw any conclusions on which macrolides may be most beneficial, as data were not available for all of our planned subgroups. Available low‐quality evidence from four adult studies, including Altenburg 2013Lourdesamy 2014Serisier 2013 and Wong 2012, and from one study of children ‐ Valery 2013 ‐ suggests that participants receiving macrolides experienced more adverse events. We are again unable to draw clear conclusions regarding the effectiveness of different macrolides, as four of the five studies used azithromycin. Studies comparing macrolides with no intervention did not report this outcome.
Overall our review provides promising, but inconclusive, results for our three predefined primary outcomes, but on the basis of currently available evidence, we are unable to present robust conclusions.
For our secondary outcomes, aggregated data from six studies comparing macrolides against placebo and yielding moderate‐ to low‐quality evidence (Altenburg 2013; Asintam 2012; Juthong 2011; Lourdesamy 2014; Serisier 2013; Wong 2012) indicate that macrolides have a positive impact on health‐related quality of life, as measured by St. George's Respiratory Questionnaire (SGRQ). Similarly, three studies comparing macrolides with no treatment showed improved quality of life with the intervention (Diego 2013; Liu 2012; Liu 2014). Data on sputum volume and purulence, measures of lung function, markers of infection, and demonstrated exercise capacity provided no indication of benefit from macrolides in adults. One of the largest adult studies of erythromycin provided limited evidence of increased resistance to macrolides (Serisier 2013).
None of the four children's studies measured quality of life. Macrolides were associated with improved sputum characteristics in two children's studies (Koh 1997; Yalcin 2006). Studies with children provided no evidence of benefit from macrolides in terms of measures of lung function, markers of infection, or demonstrated exercise capacity. One study of azithromycin with children provided limited evidence of increased resistance to macrolides (Valery 2013).
Evidence of moderate to very low quality from the 15 included studies provided no indication of a higher mortality rate with macrolides.
In relation to our predefined secondary outcomes, health‐related quality of life data further strengthen the impression noted in our primary outcomes that this intervention merits further exploration in high‐quality clinical trials.
Overall completeness and applicability of evidence
We have identified studies of macrolides in bronchiectasis reporting exacerbation and hospitalisation rates. Data for planned secondary outcomes, particularly adverse effects, are lacking. Our findings are based on studies totalling 690 adults and 190 children. Investigators used several different macrolide antibiotics (azithromycin, erythromycin, roxithromycin, and clarithromycin) in these populations in a variety of international settings. This breadth enhances the generalisability of findings but may conceal an advantage of, for example, one macrolide over another or use in adults over use in children, as none of the included studies reported direct comparisons between different macrolides or between adults and children. Small and short‐term studies mean that we may not detect small but clinically important increases in absolute risk for serious adverse events. Such adverse events, including mortality as reported in Ray 2012 and hearing loss as described in Albert 2011, have been reported in larger studies of macrolides for indications other than bronchiectasis. Although this review provides limited evidence of benefit associated with macrolide antibiotics, their relative benefit compared with benefit derived from other types of antibiotics remains unknown, as we did not identify any studies that included these comparisons.
Apart from Altenburg 2013, Juthong 2011, Serisier 2013, Wong 2012, and Valery 2013, we found a lack of information on microbial resistance associated with the macrolides used in the reports of included studies. No studies were designed to evaluate changes in resistance patterns in the wider community.
Quality of the evidence
The overall quality of studies included in this review ranges from very low to moderate for outcomes included in the GRADE assessment. From adult studies comparing macrolides versus placebo, the evidence for frequency of exacerbations and mortality from all causes is of moderate quality owing to imprecision of the effect (limited to azithromycin). Evidence for hospitalisations, serious adverse events, and quality of life was of low quality owing to study design limitations (unclear study methods, open‐label approach) and imprecision of the effect (few studies and wide confidence intervals), which resulted in downgrading of the quality of outcomes. Evidence for all‐cause mortality is of poor quality owing to unclear reporting and losses to follow‐up. From adult studies that compared macrolides versus no intervention, the evidence for frequency of exacerbations and quality of life as assessed by the SGRQ is of moderate quality owing to limitations in study design (open‐label study) and imprecision of the effect (few studies and no confidence intervals). The quality of evidence for mortality from all causes is very low owing to serious design limitations (open‐label study, unclear study methods) and inadequate reporting of participants lost to follow‐up.
Studies that compared macrolides versus placebo in children have provided low‐quality evidence on frequency of exacerbations, hospitalisations, serious adverse events, and mortality owing to design limitations (unclear methods), imprecision (wide confidence intervals and low event rates), and no information on participants lost to follow‐up. The single small study that compared macrolides versus no intervention in children provided low‐quality evidence on mortality from all causes owing to insufficient information on trial methods and imprecision.
We judged only four of our 15 included studies ‐ three with adults and one with children ‐ as having low risk of bias across all domains. Selection bias was unclear in nearly half of the included studies owing to lack of detailed reporting on random sequence generation and allocation concealment. Most studies blinded participants to group allocation, but several studies described investigator blinding in a way that was unclear, and most trials reported blinding of outcome assessment that was also unclear. None of the included studies had high risk of attrition or reporting bias, although some studies provided no information on participants who were lost to follow‐up. Furthermore, only six studies explicitly reported the role of funders in the trial; six studies registered their protocol on trial registries; and eight studies reported that investigators performed a formal sample size calculation before the start of the trial.
Potential biases in the review process
We used a comprehensive systematic search, conducted by a highly experienced information specialist, to identify potentially eligible studies. We searched multiple resources, including electronic databases, journals, conference proceedings, reference lists of included studies, citations of included studies, and trial registries. Nevertheless, we recognise the possibility of publication bias in this review that could either overestimate or underestimate effects of the intervention in terms of the different outcomes included in the review. Trials showing no, or negative, effects are less likely to be offered for publication, and if offered are less likely to be accepted, resulting in a biased set of data available for review. As we included only a few studies for each outcome, we were unable to assess the presence of publication bias through formal testing.
Furthermore, some papers may have been misclassified as not eligible for inclusion in this review. Two review authors independently assessed all studies, and a third review author verified the data; we are confident that we assessed studies excluded from analyses on the basis of consistent and appropriate criteria. For some full‐text reports, it is possible that we could have entered some data into analyses incorrectly, although we double‐checked all data to attempt to avoid extraction errors.
We contacted the investigators of three included studies based on conference proceedings that were available as an abstract, to obtain study characteristics and other numerical outcome data. Although we received responses from all of these investigators, we found that data from only two studies were provided. The same investigator was involved in both of these studies, which were conducted in similar settings and used similar interventions. Owing to the small number of included studies, we did not explore the impact of excluding studies with missing outcome data in the overall assessment of results by performing a sensitivity analysis. Finally, data were insufficient to permit all planned subgroup analyses, so we included only types of antibiotics, which we considered most clinically important.
Agreements and disagreements with other studies or reviews
Major findings of the present review are largely in agreement with the results of previously published meta‐analyses of the impact of macrolides on outcomes in bronchiectasis (Gao 2014), which have shown a reduced frequency of exacerbations, improved lung function, and improved quality of life with prolonged macrolide treatment. Differences in effect size between our present review and these previously published meta‐analyses reflect differences in the inclusion and exclusion criteria of studies, as well as the inclusion of some recent studies. In general, the strict methods applied in the present review have resulted in pooling of fewer studies and therefore more precise effect estimates.
The three largest macrolide trials have the longest duration of follow‐up and are concordant with overall results of the meta‐analysis, with each demonstrating benefit in terms of frequency of and/or time to first exacerbation (Altenburg 2013; Serisier 2013; Wong 2012). It is striking that all three studies are of high quality (Figure 4).
The benefit in terms of exacerbations was largely attributable to azithromycin. This should not be taken to indicate that azithromycin is superior to other macrolides because the characteristics of participants in the BLESS study of erythromycin were different from those of participants enrolled in azithromycin studies. Our analysis is not designed to determine whether the drug or the patient cohort is responsible for the apparent differential effect.
Authors' conclusions
Implications for practice.
This review includes 11 studies with 690 adults and four studies with 190 children. Long‐term macrolide therapy is an option for patients with bronchiectasis with the aim of reducing the rate of exacerbations and improving quality of life. Supporting evidence is derived mainly from studies of azithromycin, rather than other macrolides, and predominantly in adults rather than in children. However, macrolides should be used with caution, as limited data support an increase in microbial resistance with macrolides. Macrolides have been associated with excessive risk of cardiovascular death and other serious adverse events in populations other than individuals with bronchiectasis (Ray 2012), and available data cannot exclude a similar risk in patients with bronchiectasis. The presence of non‐tuberculous mycobacteria (NTM) should be identified in all patients before long‐term macrolide therapy is begun.
Implications for research.
The present review highlights several outstanding questions on long‐term macrolide treatment in clinical practice. Although macrolides significantly reduced exacerbations, studies used different macrolide drugs at different doses. As a result, we are unable to recommend the most appropriate agent, dose, or administration schedule (daily vs intermittent) for long‐term therapy. Doses ranging from 250 mg three times per week to 500 mg daily have been reported in clinical practice.
The European Bronchiectasis Network (EMBARC) recently published a series of research priorities, which included several related to macrolide treatment. Recognising the limitations of existing data, EMBARC recommended longer‐term studies to evaluate the development of antibiotic resistance as well as long‐term safety. Further studies conducted to determine whether macrolides should be administered continuously or in a cyclical pattern (as during the winter, when exacerbations occur more frequently) would help guide clinical practice. The optimal patient population to benefit from macrolides has not been identified, as each of the macrolide studies was too small to allow meaningful subgroup or 'responder' analyses. It is unclear whether macrolide therapy is suitable for all patients with bronchiectasis, and macrolides have important side effects, including the risk of inducing antibiotic resistance; thus, EMBARC has recommended further research to target these topics more effectively.
Existing studies and meta‐analyses have largely taken the view that macrolide efficacy in bronchiectasis has been proven, and that additional large studies are unnecessary. Our results suggest that substantial uncertainties about macrolide efficacy remain, particularly with regard to improvements in quality of life and lung function, as well as impact on antimicrobial resistance. In addition, the relative benefits of macrolides compared with those of other types of antibiotics are unknown, as we did not identify any studies that included these comparisons. Our analysis suggests that additional large, randomised, placebo controlled trials should be performed to confirm the efficacy and safety of macrolides.
Feedback
Reporting error ‐ exacerbations, 25 August 2018
Summary
We wish to point out a misreported result in the recently published Cochrane Review 'Macrolide antibiotics for bronchiectasis'. The first sentence of the fourth paragraph of the 'Main results' section of the abstract begins: "In children, there were no differences in exacerbation frequency (OR 0.40, 95% CI 0.11 to 1.41)". This was not a pre‐specified outcome in the protocol for this Review (Kelly 2016). The 'Types of outcome measures' subsection states "Where possible we will assess exacerbation and hospitalisation rates at 12 months. We will estimate annual rates in studies reporting shorter follow‐up times." The first primary outcome listed immediately below is "Exacerbations (defined using study authors' criteria)". So it clear that the primary pre‐specified outcome is the between‐group difference in exacerbation rates. The data that should have been extracted from Valery 2013 are reported in the first sentence of the first complete paragraph in the right hand column of page 614: "Compared with those receiving placebo, participants in the azithromycin group were significantly less likely to have pulmonary exacerbations (incidence rate ratio 0.50; 95% CI 0.35 to 0.71; p < 0.0001)." So there is clear evidence that, using the Cochrane Protocol's pre‐specified primary outcome, children who received azithromycin, as opposed to placebo, had significantly lower exacerbation rates.
The statistic reported in the abstract (OR 0.40, 95% CI 0.11 to 1.41) is extracted from the third row of Table 3 of Valery 2013. It is the odds of a child treated with azithromycin, as opposed to a child treated with placebo, being exacerbation‐free at 24 months. That is, it is the odds of azithromycin stopping any exacerbations. This outcome was not pre‐specified in the Cochrane Protocol as either a primary or secondary outcome. Furthermore it does not make sense clinically. While, in the population of children with either bronchiectasis or chronic suppurative lung disease, using long‐term macrolides could lead to a reduction of exacerbations (through either suppression of bacterial infections or inflammation modulation) no one would expect any intervention to completely eliminate exacerbations of their underlying chronic illness over a 24 month period. Thus, the results of Valery 2013 are incorrectly reported, and the review could more accurately say "In children, macrolides reduced exacerbation frequency to a greater extent than placebo (IRR 0.50, 95% CI 0.35 to 0.71)".
Prof. Robert Ware (Griffith University), Prof. Anne Chang (Children’s Health Queensland), and Prof. Keith Grimwood (Griffith University)
No conflicts of interest declared.
Cochrane Airways editorial team notes the feedback submitters were authors of the Valery 2013 study.
Reply
We thank Profs Ware, Chang and Grimwood for their feedback. Exacerbations were a primary outcome as specified in the protocol. We used the each study's definition of an exacerbation ("defined using the study authors' criteria") rather than trying to apply a standardised definition of exacerbation to each study retrospectively. The "study authors' criteria" does not refer to our choice of outcome measures (i.e. rates, etc of those exacerbations).
We did extract the incidence rate ratio from Valery 2013 and this is reported in our original review (second sentence following "Macrolides versus placebo: children … Exacerbations"). In response to this feedback we have revised the relevant sections of the abstract (main results), effects of interventions and summary of findings table 3 slightly to highlight this statistic more prominently. We believe our review is consistent with our initial protocol and that there aren't "reporting errors". The overall message of our review is unchanged.
Iain Crossingham, Sally Spencer, Steve Milan and James Chalmers.
Contributors
Feedback contributors: Prof. Robert Ware (Griffith University), Prof. Anne Chang (Children's Health Queensland), and Prof. Keith Grimwood (Griffith University)
Author contributors: Iain Crossingham, Sally Spencer, Steve Milan and James Chalmers.
What's new
| Date | Event | Description |
|---|---|---|
| 17 October 2018 | Feedback has been incorporated | Authors have responded to Feedback 1. They changed the emphasis slightly around reporting of exacerbations. They also reported the incidence rate ratio, rather than the odds of not experiencing an exacerbation over a year, in the abstract and summary of findings table. |
History
Protocol first published: Issue 10, 2016 Review first published: Issue 3, 2018
| Date | Event | Description |
|---|---|---|
| 8 October 2018 | Amended | Feedback added, but not incorporated. |
Acknowledgements
We would like to thank Edge Hill University for support provided for this review. We would also like to thank Cochrane Airways for its support. We would like to thank the following translators: Chunli Lu, Wangyu Cai, and Hiraku Tsujimoto, for their invaluable contributions to extraction of data from studies not published in English.
The Background and Methods sections of this protocol are based on a standard template used by Cochrane Airways.
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to Cochrane Airways. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group's Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (the Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| Embase (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Bronchiectasis search
1. exp Bronchiectasis/
2. bronchiect$.mp.
3. bronchoect$.mp.
4. kartagener$.mp.
5. (ciliary adj3 dyskinesia).mp.
6. (bronchial$ adj3 dilat$).mp.
7. or/1‐6
Filter to identify randomised controlled trials (RCTs)
1. exp "clinical trial [publication type]"/
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter (Lefebvre 2011) are adapted to identify trials in other electronic databases.
Appendix 2. Search strategy to identify relevant trials from the CAGR
#1 BRONCH:MISC1
#2 MeSH DESCRIPTOR Bronchiectasis Explode All
#3 bronchiect*
#4 #1 or #2 or #3
#5 MeSH DESCRIPTOR Macrolides Explode 1 2 3
#6 macrolide*
#7 azithromycin*
#8 clarithromycin*
#9 erythromycin*
#10 roxithromycin*
#11 spiramycin*
#12 telithromycin*
#13 troleandomycin*
#14 Josamycin*
#15 Midecamycin*
#16 Oleandomycin*
#17 Solithromycin*
#18 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17
#19 #4 AND #18
(Note: in search line #1, MISC1 denotes the field in the record where the reference has been coded for condition, in this case, bronchiectasis)
Data and analyses
Comparison 1. Macrolide versus placebo: adults.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ≥ 1 exacerbation | 3 | 341 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.22, 0.54] |
| 1.1 Azithromycin | 2 | 224 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.13, 0.40] |
| 1.2 Erythromycin | 1 | 117 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.34, 1.63] |
| 2 Hospitalisation: all‐cause | 2 | 151 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.19, 1.62] |
| 2.1 Azithromycin | 2 | 151 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.19, 1.62] |
| 3 Serious adverse events | 3 | 326 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.20, 1.23] |
| 3.1 Azithromycin | 2 | 209 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.20, 1.34] |
| 3.2 Erythromycin | 1 | 117 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 8.07] |
| 4 Sputum weight (g): endpoint | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Azithromycin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 FEV1 (% predicted): endpoint | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Azithromycin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 FEV1 (% predicted): change (post bronchodilator) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Erythromycin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 FEV1 (L): endpoint | 2 | 94 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.17, 0.22] |
| 7.1 Azithromycin | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.23, 0.21] |
| 7.2 Roxithromycin | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.27, 0.57] |
| 8 FEV1 (L): change | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Azithromycin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 FVC (% predicted): endpoint | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Azithromycin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 FVC (L): endpoint | 2 | 94 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.19, 0.36] |
| 10.1 Azithromycin | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.34, 0.30] |
| 10.2 Roxithromycin | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 0.38 [‐0.16, 0.92] |
| 11 FVC (L): change | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Azithromycin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 FEV1/FVC: endpoint | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 Azithromycin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Adverse events | 5 | 435 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.51, 1.35] |
| 13.1 Azithromycin | 3 | 292 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.41, 1.45] |
| 13.2 Erythromycin | 1 | 117 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.51, 2.62] |
| 13.3 Roxithromycin | 1 | 26 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.83] |
| 14 Azithromycin‐resistant bacteria (any) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14.1 Azithromycin | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 15 6‐Minute walk test: change | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15.1 Erythromycin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 Quality of life: endpoint | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐8.90 [‐13.13, ‐4.67] |
| 16.1 Azithromycin | 1 | 68 | Mean Difference (IV, Fixed, 95% CI) | ‐8.90 [‐13.13, ‐4.67] |
| 17 Quality of life: change | 4 | 305 | Mean Difference (IV, Fixed, 95% CI) | ‐2.86 [‐5.67, ‐0.04] |
| 17.1 Azithromycin | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | ‐3.25 [‐7.19, 0.69] |
| 17.2 Erythromycin | 1 | 117 | Mean Difference (IV, Fixed, 95% CI) | ‐2.60 [‐7.12, 1.92] |
| 17.3 Roxithromycin | 2 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐1.86 [‐10.63, 6.91] |
Comparison 2. Macrolide versus no intervention: adults.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ≥ 1 exacerbation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Roxithromycin | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 QoL SGRQ: endpoint total score | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐8.81 [‐14.33, ‐3.28] |
| 2.1 Roxithromycin | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐8.81 [‐14.33, ‐3.28] |
Comparison 3. Macrolide versus placebo: children.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hospitalisation: all‐cause | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Azithromycin | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Serious adverse events | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Azithromycin | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Sputum purulence score: endpoint | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Roxithromycin | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 FEV1 (% predicted): endpoint | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | 1.73 [‐3.32, 6.78] |
| 4.1 Azithromycin | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 3.70 [‐5.99, 13.39] |
| 4.2 Roxithromycin | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐4.91, 6.91] |
| 5 Adverse events | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Azithromycin | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Azithromycin‐resistant bacteria (any) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Azithromycin | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Azithromycin‐resistant Streptococcus pneumoniae | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Azithromycin | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Azithromycin‐resistant Staphylococcus aureus | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Azithromycin | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Altenburg 2013.
| Methods |
Aims: to investigate whether 1 year of long‐term low‐dose macrolide treatment added to standard therapy is effective in reducing exacerbation frequency in patients with non‐CF bronchiectasis Design: randomised, double‐blind, placebo‐controlled trial Total study duration: 30 months Number of study centres and locations: 14, Netherlands Study setting: outpatient clinics Methods of recruitment: outpatient clinics at each study centre by the pulmonary physician or the study investigator Withdrawals: 1 in each group owing to adverse events Study start/end dates: April 2008/September 2010 Analysis by intent‐to‐treat: yes |
|
| Participants | 83 adults randomised Inclusion criteria: individuals 18 years of age or older with non‐CF bronchiectasis diagnosed by plain bronchography or high‐resolution computed tomography, ≥ 3 lower respiratory tract infections (LRTIs) treated with oral or intravenous antibiotics in the preceding year, and ≥ 1 sputum culture yielding ≥ 1 bacterial respiratory pathogen in the preceding year Exclusion criteria: prolonged (> 4 weeks) macrolide therapy during the previous 3 months, oral or intravenous corticosteroids within 30 days of screening, or any antimicrobial treatment for an LRTI in the previous 2 weeks Mean age: intervention group: 59.9 years; control group: 64.6 years Gender: intervention group: 25 females, 18 males; control group: 28 females, 12 males Bronchiectasis diagnosis: plain bronchography or HRCT Severity of condition: not reported Baseline lung function: FEV1 (% predicted): intervention group: 77.7, control group: 82.7; FVC (% predicted): intervention group: 91.9, control group: 98.5 Smoking history: 2% current, 44% former, 54% never Baseline imbalances: no statistically significant differences between groups |
|
| Interventions |
Intervention group: azithromycin (n = 43) Dose: 250 mg; delivery mode: oral; frequency: 1/d; duration: 52 weeks Control group: placebo (n = 40) Placebo tablets indistinguishable from azithromycin were manufactured by a licensed trial pharmacy. Adherence: empty blister‐pack count: intervention group: 96.5%; control group: 98% Run‐in phase: following randomisation, participants observed for clinical stability for 2 weeks Run‐out phase: variable run‐out period of ≥ 90 days after 1 year of intervention |
|
| Outcomes |
Primary: number of infectious exacerbations, defined as an increase in respiratory symptoms requiring antibiotic treatment. Two types of exacerbations ‐ a protocol‐defined exacerbation (PDE) and a non‐PDE Secondary: lung function, CRP level, WBC count, microbiological evaluation, LRTI, HRQoL, and adverse events Post hoc analysis: time to a first exacerbation |
|
| Notes |
Power calculation: assuming that azithromycin would reduce the number of exacerbations by at least one‐third, a 1‐sided significance level of P = 0.05, with 80% power and estimated 20% dropout = total of 90 patients, for 36 per group Trial registration: clinicaltrials.gov Identifier: NCT00415350 Conflicts of interest: Dr. Boersma reported serving on an advisory board, and receiving payment from Pfizer, for an educational presentation. No other review authors reported COIs. Funders: Dr. Altenberg and Dr. Boersma were supported by a research grant from the Forest Medical School, an independent scientific institution based in the Alkmaar Medical Centre. The study was also supported by an unrestricted research grant from GlaxoSmithKline, and Teva Netherlands supplied the azithromycin tablets. Role of the sponsors: Funders had no role in the design and conduct of the study; collection, analysis, and interpretation of data; or preparation, review, or approval of the manuscript. Ethical approval: yes Conclusions: Macrolide maintenance therapy was effective in reducing exacerbations in patients with non‐CF bronchiectasis. In this trial, azithromycin treatment resulted in improved lung function and better quality of life but involved an increase in gastrointestinal adverse effects and high rates of macrolide resistance. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block randomisation was performed centrally with equally sized blocks of 10. |
| Allocation concealment (selection bias) | Low risk | Placebo and azithromycin tablets were provided in identical, individually numbered boxes, with each box containing a year's supply of study medication for 1 participant. Numbers on the boxes matched treatment allocation, in accordance with a computer‐generated allocation sequence that was kept in a safe place in the pharmacy providing the study medication. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants were seen by the investigator and were sequentially assigned a subject identification code through double‐blinded allocation to azithromycin or placebo treatment. Placebo tablets were indistinguishable from azithromycin tablets with respect to appearance, feel, and taste. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Attending physicians reporting study outcomes were blinded to group allocation. It is unlikely that blinding was ineffective. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Balanced between groups with similar reasons for withdrawal |
| Selective reporting (reporting bias) | Low risk | Study protocol prepublished and all prespecified outcomes reported |
| Other bias | Low risk | None identified |
Asintam 2012.
| Methods |
Aims: to determine whether roxithromycin would alter clinical outcomes Design: randomised, double‐blind, placebo‐controlled trial Total study duration: 6 months Number of study centres and locations: 1, Thailand Study setting: outpatient department, Songklanagarind Hospital Methods of recruitment: unclear Withdrawals: intervention group: 4, control group: 5 Study start/end dates: March 2011/September 2011 Analysis by intent‐to‐treat: unclear |
|
| Participants | 30 adults were randomised. Inclusion criteria: adults aged 15 to 75 years; symptomatic patients, with total symptoms score* ≥ 2 per day; stable clinical state; absence of deterioration in cough, dyspnoea, wheezing, fever, chest pain at least 2 weeks before randomisation Exclusion criteria: adverse drug reaction to macrolides; recent exacerbation within 2 weeks before randomisation; history of macrolide therapy within 2 weeks before randomisation; active malignancy and end‐stage disease, such as chronic heart failure, chronic renal failure, and cirrhosis; inability of patients to perform lung function tests due to haemoptysis, AFB positivity, aortic aneurysm, and unstable angina; women who were lactating Mean age: intervention group: 67 years; control group: 64 years Gender: intervention group: 9 women, 6 men; control group: 14 women, 1 man Bronchiectasis diagnosis: HRCT Severity of condition: intervention group: 13 (range 9‐19); control group: 12 (range 5‐19) (Bhalla) Baseline lung function: FEV1 (% predicted): intervention group: 53.5 ± 13.9; control group: 61.7 ± 19.2; FVC (% predicted): intervention group: 65.4 ± 20; control group: 66.9 ± 14.3 Smoking history: 20% former, 80% never; smoking history in pack‐years: intervention group: 6.7 years; control group: 0.7 years Baseline imbalances: no statistically significant differences between groups |
|
| Interventions |
Intervention group: roxithromycin (n = 15) Dose: 300 mg; delivery mode: oral; frequency: once daily; duration: 12 weeks Control group: placebo (n = 15) Co‐interventions: mucolytic drugs (93%), SABA (73%), theophylline (63%), and a combination of LABA/ICS (47%) Adherence: not reported Run‐in phase: not reported Run‐out phase: 12‐week wash |
|
| Outcomes |
Primary: quality of life (SGRQ) Secondary: exacerbations, sputum volume, pulmonary function tests Post hoc analysis: not reported |
|
| Notes |
Power calculation: estimated 61 patients needed to detect an increment in SGRQ scores of 12% with roxithromycin as compared with placebo with statistical power (1 minus the β value) of 80%, allowing for a type I (α) error of 0.05 Trial registration: not reported Conflicts of interest: not reported Funders: not reported Role of the sponsors: not reported Ethical approval: yes Conclusions: 12‐week roxithromycin 300 mg once daily in symptomatic stable bronchiectatic patients; did not show significant improvement in QoL by SGRQ scores, reduced sputum volume, or improved lung function |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Eligible subjects were randomized (1:1) into the treatment and control groups by block of four randomization method"; insufficient detail |
| Allocation concealment (selection bias) | Unclear risk | "30 patients were randomly allocated"; insufficient detail |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient detail |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient detail |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 9 patients withdrew (30%), but no further details were reported. |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not prespecified. |
| Other bias | Unclear risk | Details of funding sources were not reported. |
Cymbala 2005.
| Methods |
Aims: to determine whether long‐term, low‐dose azithromycin would improve pulmonary function and decrease incidences of infection and exacerbation Design: open‐label, cross‐over, randomised controlled (no intervention) trial Total study duration: 12 months Number of study centres and location: 1, USA Study setting: outpatient clinic Methods of recruitment: unclear Withdrawals: 1, who provided insufficient data for analysis Study start/end dates: January 2001/December 2001 Analysis by intent‐to‐treat: no |
|
| Participants | 12 adults were randomised. Inclusion criteria: patients aged > 18 years with a clinical diagnosis of bronchiectasis confirmed by HRCT, demonstrating airways larger than accompanying vessels Exclusion criteria: patients with a history of serious intolerance, allergy, or sensitivity to azithromycin or macrolides. In addition, if the investigator believed that the patient may not be able to follow instructions, the patient was excluded. Mean age: 70.8; SD 9.7 years Gender: 6 women, 5 men Bronchiectasis diagnosis: HRCT Severity of condition: not reported Baseline lung function (intervention group, control group): FEV1 (% predicted): 65.3, SD 15.1; FVC (% predicted): 48.5, SD 19.9 Smoking history: present or ex‐smoker: 8; never: 3 Baseline imbalances: not reported |
|
| Interventions |
Intervention group: azithromycin plus usual medications (n = 8) Dose: 500 mg; delivery mode: oral; frequency: 2/week (Monday and Thursday); duration: 6 months If participants complained of intolerable adverse events from the azithromycin regimen but wanted to continue in the study, their azithromycin regimen was reduced to 250 mg orally every Monday, Wednesday, and Friday. Control group: usual medications alone (n = 3) Adherence: 85% to 108% on azithromycin (1 person took an additional dose) Run‐in phase: 1‐month washout in participants who received azithromycin first Run‐out phase: unclear |
|
| Outcomes |
Primary: did not state which of the outcomes below was primary Secondary: pulmonary function tests (diary card), PF measurements, 24‐hour sputum volume Post‐hoc analysis: unclear |
|
| Notes |
Power calculation: By a paired t‐lest power calculation, the original proposed sample size of 30 participants would have provided 92.5% power at an alpha of 0.1 to identify a 50% change in 24‐hour sputum volume. However, only 11 of the 12 enrolled participants completed the study; therefore, the power to identify the same extent of change in 24‐hour sputum volume fell to 56%. Trial registration: not reported Conflicts of interest: no conflicts of interest for 6 study authors. One had received payments from several pharmaceutical companies including Pfizer, Bayer, Abbott, and Bristol Myers Squibb. Funders: The first year of the study was unfunded, although investigators received donations of study medication from local sales representatives. In the second year, a small unrestricted stipend was received from the manufacturer of azithromycin that covered participant incidentals (i.e. travel expenses, extra pulmonary function tests only). Role of the sponsors: unclear Ethical approval: yes Conclusions: The results of this pilot study support past data regarding probable disease‐modifying benefits of long‐term macrolide use in the treatment of individuals with chronic inflammatory pulmonary disorders. Long‐ term therapy with twice‐weekly azithromycin was well tolerated and may provide added benefit for patients with bronchiectasis without the adverse effect of immunosuppression, which is demonstrated with corticosteroids. Given that significant findings were identified in a study with such a limited sample size, additional large‐scale trials are warranted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Allocation concealment (selection bias) | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Selective reporting (reporting bias) | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Other bias | High risk | "Because of the randomization schedule and less than expected numbers at enrolment, the distribution of patients between the two study phases they received first was heavily biased, with 8 of II (73%) patients receiving the azithromycin phase first". The randomisation schedule was ineffective, with most receiving the active intervention in the first phase. |
Diego 2013.
| Methods |
Aims: to explore the effect of long‐term therapy with azithromycin on airway oxidative stress markers in exhaled breath condensate (EBC) Design: open‐label, randomised controlled (no intervention) trial Total study duration: 12 months Number of study centres and location: 1, Spain Study setting: outpatient clinic Methods of recruitment: invited patients with confirmed diagnosis of bronchiectasis attending clinic at University Hospital La Fe Withdrawals: 6. Numbers per study group not reported Study start/end date: 2005 Analysis by intent‐to‐treat: no |
|
| Participants | 36 adults were randomised. Inclusion criteria: stable, without change in medication or symptoms, emergency department visits or hospitalisations in the previous 4 weeks Exclusion criteria: positive sweat test for CF, bronchiectasis secondary to CF, pulmonary surgical processes, immunodeficiency secondary to HIV, malignancy, common variable immunodeficiency, emphysema, allergic bronchopulmonary aspergillosis or diffuse interstitial pulmonary disease, intolerance to macrolides, severe liver disease Mean age: 58 years; intervention group: 57 years; control group: 61 years Gender: intervention group: 9 women, 7 men; control group: 7 women, 7 men Bronchiectasis diagnosis: clinical data and HRCT Severity of condition: intervention group: 22; control group: 31 (Bhalla) Baseline lung function: FEV1 (% predicted): intervention group: 56, SD 6; control group: 58, SD 7 Smoking history: not reported Baseline imbalances: no statistically significant differences between groups |
|
| Interventions |
Intervention group: azithromycin plus usual care (n = 16) Dose: 250 mg; delivery mode: oral; frequency: 3/week; duration: 3 months Control group: usual care alone (n = 14) Participants in both groups continued taking their habitual treatment to the same doses, including inhaled steroids, bronchodilators, mucolytic therapy, and physiotherapy. In cases of severe exacerbations, steroids or antibiotics were recommended. Adherence: not reported Run‐in phase: unclear Run‐out phase: unclear |
|
| Outcomes |
Primary: changes in airway oxidative stress markers (FeNO, 8‐isoprostane, nitrites (NO2), and nitrates (NO3)) Secondary: changes in lung function (FVC, FEV1 (pre‐ and post‐BD), FEV1/FVC, total lung capacity, colour and volume of sputum, number of exacerbations, hospital admissions, functional capacity, health‐related quality of life Post hoc analysis: colonised vs not colonised with Pseudomonas aeruginosa |
|
| Notes |
Power calculation: based on expected 10% difference in FeNO between groups with 90% power and 5% statistical significance Trial registration: clinicaltrials.gov: NTC01463371 Conflicts of interest: not reported Funders: Fundacion Valenciana de Neumologia Role of the sponsors: not reported Ethical approval: yes Conclusions: 3‐month treatment with azithromycin; clinical benefit in patients with non‐CF bronchiectasis but no effect on airway oxidative stress markers |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Allocation concealment (selection bias) | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study investigators were blinded to group allocation, but this was an open‐label study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Six participants were lost to follow‐up. No reasons for missing data were provided. |
| Selective reporting (reporting bias) | Low risk | Study protocol was published and all prespecified (primary and secondary) outcomes were reported. |
| Other bias | Low risk | None identified |
Juthong 2011.
| Methods |
Aims: to investigate the efficacy of once‐daily roxithromycin for improving clinical outcomes Design: double‐blind, randomised, placebo‐controlled trial Total study duration: 6 months Number of study centres and location: 1, Thailand Study setting: outpatient department, Songklanagarind Hospital Methods of recruitment: not reported Withdrawals: none Study start/end dates: June 2010/November 2010 Analysis by intent‐to‐treat: yes |
|
| Participants | 26 adults were randomised. Inclusion criteria: aged 18 years and above, diagnosis of bronchiectasis, symptomatic bronchiectasis Exclusion criteria: macrolides in previous year, exacerbation of bronchiectasis in previous 3 months, allergy to macrolides, active malignancy, active or recent pulmonary infection within 3 months, pregnancy Mean age: intervention group: 55 years; control group: 60 years Gender: intervention group: 4 women, 8 men; control group: 8 women, 6 men Bronchiectasis diagnosis: chest radiograph and HRCT; diagnosis confirmed by pulmonologist Severity of condition: described as "severe" Baseline lung function: FEV1 (L): intervention group: 1.53 ± 0.62; control group: 1.31 ± 0.44; FVC (L): intervention group: 2.27 ± 0.79; control group: 1.98 ± 0.55 Smoking history: present 2 (8%),former: 6 (23%), never 18 (69%) Baseline imbalances: no statistically significant differences between groups |
|
| Interventions |
Intervention group: roxithromycin (n = 12) Dose: 300 mg; delivery mode: oral; frequency: once daily; duration: 8 weeks Control group: placebo (n = 14) Adherence: not reported Run‐in phase: not reported Run‐out phase: not reported |
|
| Outcomes |
Primary: symptoms scores, pulmonary function tests (FEV1 L, FVC L) Secondary: safety, tolerability, drug resistance Post hoc analysis: not reported |
|
| Notes |
Power calculation: not reported Trial registration: unclear Conflicts of interest: not stated Funders: Faculty and Hospital Fund for Research, Songklanagarind Hospital Role of the sponsors: not reported Ethical approval: not reported Conclusions: Once‐daily roxithromycin showed benefit for clinical outcomes as well as quality of life. Larger studies on the effects of macrolide in bronchiectasis treatment with longer follow‐up times should be done. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Allocation concealment (selection bias) | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind effectiveness was confirmed by contact with trial authors. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals were reported. |
| Selective reporting (reporting bias) | Unclear risk | Informaiton was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Other bias | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
Koh 1997.
| Methods |
Aims: to determine whether roxithromycin can reduce the degree of airway responsiveness in bronchiectasis Design: double‐blind, randomised, placebo‐controlled trial Total study duration: 12 weeks Number of study centres and location: 1, South Korea Study setting: outpatient clinic, Seoul National University Hospital Methods of recruitment: selected from the outpatient clinic list Withdrawals: 2 (1 in each group) removed by investigators owing to non‐compliance Study start/end dates: October 1995/February 1996 Analysis by intent‐to‐treat: no |
|
| Participants | 25 children were randomised. Inclusion criteria: increased airway responsiveness (defined as a provocative concentration of methacholine causing a 20% fall in FEV1 (PC20) < 25 mg/mL evaluated by the dosimeter method Exclusion criteria: not explicitly stated but patients with cystic fibrosis, humoral immune deficiency, bronchopulmonary aspergillosis, excluded; also, those who had taken antibiotics or corticosteroids or who had an upper respiratory tract infection in the past month Mean age: intervention group: 13.3 years; control group: 12.9 years Gender: intervention group: 6 girls, 7 boys; control group: 5 girls, 7 boys Bronchiectasis diagnosis: clinical features; confirmed by computed tomography, with bronchography when necessary Baseline lung function: FEV1 (% predicted): intervention group: 83 ± 6; control group: 84 ± 7 Smoking history: not applicable Severity of condition: not reported Baseline imbalances: 3 asthmatic patients in the intervention group and 4 in the control group. In the initial methacholine challenge test, 3 participants in the intervention group and 2 in the control group did not attain a maximal response plateau. No other significant differences were noted at baseline. |
|
| Interventions |
Intervention group: roxithromycin (n = 13) Dose: 4 mg/kg; delivery mode: oral; frequency: 2/d; duration: 12 weeks Control group: placebo (n = 12) Adherence: used packets or drug sachets monitored for compliance; 2 participants withdrew owing to non‐compliance Run‐in phase: not reported Run‐out phase: not reported |
|
| Outcomes |
Primary: unclear which of the outcomes below were primary Secondary: FEV1, sputum colour (sputum purulence score), sputum ‐ polymorphonuclear leucocyte (PMN) (sputum leucocyte score) Post hoc analysis: unclear |
|
| Notes |
Power calculation: not reported Trial registration: not reported Conflicts of interest: not reported Funders: Seoul National University Hospital Research Fund Role of the sponsors: not reported Ethical approval: yes Conclusions: Roxithromycin may decrease the degree of airway responsiveness in patients with bronchiectasis and increased airway responsiveness. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The study was conducted in a double‐blind, randomized, placebo‐controlled fashion after the preliminary methacholine challenge test". Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Allocation concealment (selection bias) | Unclear risk | "A doctor (not responsible for follow‐up or data analysis) was assigned the task of dividing the patients into two groups". Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The study was conducted in a double‐blind, randomized, placebo‐controlled fashion after the preliminary methacholine challenge test". Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants (1 in each group) were withdrawn from the study because of non‐compliance with medication or regular check‐up. Missing outcome data were balanced in numbers across intervention groups, and reasons for missing data were similar across groups. |
| Selective reporting (reporting bias) | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Other bias | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
Liu 2012.
| Methods |
Aims: to assess the effect of roxithromycin on inflammation media in induced sputum, dilated bronchial wall thickness, SGRQ scores, and exacerbation rates Design: open‐label, randomised controlled trial Total study duration: 6 months Number of study centres and location: 1, Qinzhou City, Guangxi Province, China Study setting: hospital Methods of recruitment: not reported Withdrawals: 4, number per group not reported; reasons for withdrawal not reported Study start/end dates: June 2007/June 2010 Analysis by intent‐to‐treat: no |
|
| Participants | 50 adults were randomised. Inclusion criteria: aged 18 to 65 years with bronchiectasis diagnosed by HRCT Exclusion criteria: allergy to macrolide, cirrhosis, liver dysfunction and exacerbation. Bronchiectasis exacerbation was defined as abnormalities in 4 of the following 9 symptoms, signs, or laboratory findings: change in sputum production (consistency, colour, volume, or hemoptysis); increased dyspnoea (chest congestion or shortness of breath); increased cough; fever (38° C); increased wheezing; decreased exercise tolerance, malaise, fatigue, or lethargy; FEV1 or FVC decreased 10% from a previously recorded value; radiographic changes indicative of a new pulmonary process; or changes in chest sounds. Concomitant medications unclear Mean age: intervention group: 47, SD 8; control group: 49, SD 9 (range 29‐67) Gender: intervention group: 12 male, 13 female; control group: 14 male, 11 female Bronchiectasis criteria: HRCT Severity of condition: not reported Baseline lung function (intervention group, control group): not reported Smoking history: intervention group, control group, pack‐years: not reported Baseline imbalances: not reported |
|
| Interventions |
Intervention group: roxithromycin + ambroxol hydrochloride (n = 25) Dose (Rox): 15 g (150 mg); delivery mode: oral; frequency: 1/d; duration: 6 months+ Dose (AH): 30 mg; delivery mode: oral; frequency: 3/d; duration: 6 months Control group: oral ambroxol hydrochloride (n = 25) Dose: 30 mg; delivery mode: oral; frequency: 3/d; duration: 6 months Adherence: not reported Run‐in phase: not reported Run‐out phase: not reported |
|
| Outcomes |
Primary: unclear Secondary: SGRQ and MRC Breathlessness Scale Time points: baseline, 6 months Post hoc analysis: unclear |
|
| Notes |
Power calculation: not reported Trial registration: not reported Conflicts of interest: unclear Funders: Chinese Medical Association Chronic Pulmonary Disease Fund (07010150023), Guangxi Province Department of Science Youth Fund (0991019), Guangxi Province Health Department Self‐funded Research Project (Z2007047) Role of the sponsors: unclear Ethical approval: unclear Conclusions: Scores for bronchial wall thickening of bronchiectasis were increased in participants with stable bronchiectasis. Low‐dose roxithromycin combined with ambroxol hydrochloride significantly improved degree of dyspnoea and reduced scores for extent of bronchiectasis, scores for bronchial wall thickening of bronchiectasis, and global CT scores as compared with treatment with ambroxol hydrochloride alone in participants with bronchiectasis who were in stable condition. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random number table" |
| Allocation concealment (selection bias) | Unclear risk | Information in study report was insufficient. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Reporting was unclear, but this was an open‐label study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 withdrawals were reported, but numbers for each group were not given. |
| Selective reporting (reporting bias) | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Other bias | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
Liu 2014.
| Methods |
Aims: to assess effects of roxithromycin on inflammation media in induced sputum, dilated bronchial wall thickness, SGRQ scores, and exacerbation of bronchiectasis in patients in stable condition Design: open‐label, randomised controlled trial Total study duration: 26 months Number of study centres and location: 1, China Study setting: Tenth Affiliated Hospital of Guangxi Medical University Methods of recruitment: unclear Withdrawals: intervention group: 4; control group: 5 Study start/end dates: May 2009/July 2011 Analysis by intent‐to‐treat: no |
|
| Participants | 52 adults randomised; 43 completed Inclusion criteria: between 18 and 65 years of age and hospitalised at the Tenth Affiliated Hospital of Guangxi Medical University directed by First Affiliated Hospital of Guangxi Medical University, Qinzhou, China, from May 2009 to July 2011 Exclusion criteria: protocol‐defined exacerbation (PDE) of bronchiectasis. PDE was prospectively defined as abnormalities in 4 of the following 9 symptoms, signs, or laboratory findings: change in sputum production (consistency, colour, volume, or haemoptysis); increased dyspnoea (chest congestion or shortness of breath); increased cough; fever (> 38° C); increased wheezing; decreased exercise tolerance, malaise, fatigue, or lethargy; FEV1 or FVC decreasing 10% from a previously recorded value; radiographic changes indicative of a new pulmonary process; or changes in chest sounds. Patients with CF who had documented clinical, radiological, and genotypic features and abnormal sweat test results (sweat sodium and chloride > 60 mmol/L) were excluded. Patients who were allergic to macrolides and patients with impaired hepatic disease or diabetes mellitus were also excluded. Mean age: intervention group: 47.1 years; control group: 49.2 years Gender: intervention group: 11 women, 11 men; control group: 9 women, 12 men Bronchiectasis diagnosis: standard chest radiograph compatible with bronchiectasis, for instance, fusiform infiltrates of mucoid impaction, "signet ring", or "tram tracks"; chest CT showing ectasia of peripheral bronchi, fluid‐filled airways, or thickening of the mucosa; daily chronic sputum production or haemoptysis ‐ all confirmed at baseline by HRCT Severity of condition: global CT score: intervention group: 9.47; control group: 9.54 Baseline lung function (intervention group, control group): FEV1 (L) 1.59, 1.63; FEV1 (% predicted): 66.8, 67.4; FVC (L) 2.27, 2.34; FVC (% predicted): not reported; FEV1/FVC: 70, 69.6 Smoking history: intervention group: 4.7 pack‐years; control group: 4.3 pack‐years Baseline imbalances: no significant differences between study groups at baseline |
|
| Interventions |
Intervention group: roxithromycin (n = 22) Dose: 150 mg; delivery mode: oral; frequency: 1/d; duration: 6 months Control group: no treatment (n = 21) Adherence: Treatment adherence was encouraged by telephone calls from the study co‐ordinator and by measurement of pill counts. Run‐in phase: 1‐month run‐in period free of exacerbation symptoms before baseline sampling Run‐out phase: not reported |
|
| Outcomes |
Primary: not specified Secondary: sputum production, lung function, inflammatory markers (including IL‐8, neutrophil elastase (NE), MMP‐9, tissue inhibitor of metalloproteinases‐1 (TIMP‐1), hyaluronidase (HA), and type IV collagen concentration in induced sputum), total and differential sputum cell counts, quality of life (SGRQ), dyspnoea, CT evaluation of the thorax Time points: baseline, 6 months Post hoc analysis: NA |
|
| Notes |
Power calculation: not reported Trial registration: not reported Conflicts of interest: none Funders: Trial authors acknowledge support from the Medical Experiment Center of Guangxi Medical University. The study was supported by grants from the Special Foundation for Chronic Respiratory Disease of Chinese Medical Association (no. 07010150023) and Youth Science Fund of Guangxi Zhuang Autonomous Region in China (no. 0991019). Role of the sponsors: not reported Ethical approval: yes Conclusions: Treatment with roxithromycin can decrease airway inflammation and reduce airway thickness of dilated bronchus, both of which are positively associated with chronic airway inflammation in steady‐state bronchiectasis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Eligible participants were randomly assigned to control and roxithromycin groups; information is insufficient to permit judgement of 'low risk' or 'high risk'. |
| Allocation concealment (selection bias) | Low risk | Study report information was insufficient. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Reporting was unclear but this was an open‐label study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal was balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Other bias | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
Lourdesamy 2014.
| Methods |
Aims: to demonstrate effects of azithromycin on sputum volume, quality of life, and independence, and to estimate duration of effects of azithromycin after cessation of therapy Design: double‐blind, randomised, placebo‐controlled trial Total study duration: 26 weeks Number of study centres and location: single, Malaysia Study setting: Respiratory Clinic, Hospital Taiping, Taiping; unclear whether in‐patient or out‐patient setting Methods of recruitment: not reported Withdrawals: 10 adults lost to follow‐up (intervention group: 6; control group: 4) Study start/end dates: November 2011/December 2013 Analysis by intent‐to‐treat: no |
|
| Participants | 78 adults were randomised. Inclusion criteria: over 18 years of age with diagnosis of bronchiectasis, reproducible spirometry and chronic sputum production documented in second week of the run‐in period; stable for 6 weeks before study entry Exclusion criteria: pregnant and lactating, active tuberculosis, malignancy Mean age: intervention group: 65.94 years; control group: 59.74 years Gender: intervention group: 24 women, 15 men; control group: 26 women, 13 men Bronchiectasis diagnosis: HRCT Severity of condition: not reported Baseline lung function (intervention group, control group): FEV1 (L): 1.09, 1.17; FVC (L): 1.56, 1.69; FEV1/FVC: 72.6, 70.90 Smoking history: intervention group: 11 current smokers, 28 non‐smokers; control group: 11 current smokers, 28 non‐smokers Baseline imbalances: no significant differences between treatment groups at baseline with respect to age, gender, weight, height, smoking status, serum albumin and creatinine levels, SGRQ scores, and lung function. Baseline sputum volume was significantly higher in the azithromycin group. |
|
| Interventions |
Intervention group: azithromycin (n = 39) Dose: 1000 mg; delivery mode: oral; frequency: weekly; duration: 12 weeks Control group: placebo (n = 39) Identical to Zithromax tablets Adherence: not reported Run‐in phase: 2 weeks Run‐out phase: 12 weeks; both groups received placebo |
|
| Outcomes |
Primary: 24‐hour sputum volume (percentage change from baseline) Secondary: SGRQ score, SGRQ (change in score from baseline) and spirometric assessment of FVC and FEV1, adverse events, serious adverse events Post hoc analysis: unclear |
|
| Notes |
Power calculation: "The study was powered to detect differences in sputum volume, quality of life and spirometry values with azithromycin treatment". Trial registration: clinicaltrials.gov: NCT02107274 Conflicts of interest: See role of sponsors below; conflicts of interest for individual trial authors not stated Funders: grant approved by the Ministry of Health of Malaysia. Study medications were manufactured and provided by Pfizer Inc. (Ann Arbor, Ml, USA). Role of the sponsors: Pfizer Ltd. (Sandwich, Kent, UK) was not involved in study design, data collection, or data interpretation. Ethical approval: yes (local institutional ethics committee) Conclusions: 12‐Week administration of 1000 mg azithromycin weekly in pulmonary bronchiectasis significantly reduced mean sputum volume, improved health status, and stabilised lung function. Azithromycin had a 'carryover effect' on sputum volume and health status for 12 weeks after cessation of therapy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence in a 1:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients were randomised to receive 12 weeks of placebo or azithromycin in a 1:1 ratio in a double‐blinded fashion. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was maintained from randomisation until database lock unless any patient emergencies arose. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Ten participants did not complete the study and were excluded from analyses. Four participants were lost to follow‐up for logistic reasons. Another 4 had gastrointestinal (GI) disturbances, which consisted predominantly of diarrhoea. Two deaths were recorded in the treatment arm. Both participants passed away owing to severe pneumonia. Missing outcome data were balanced in numbers across intervention groups, and reasons for missing data were similar across groups. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available, and all of the study's prespecified (primary and secondary) outcomes of interest in the review have been reported in the prespecified way. |
| Other bias | High risk | Groups were not balanced at baseline with regard to the primary outcome – sputum volume. |
Masekela 2013.
| Methods |
Aims: to evaluate the efficacy of erythromycin compared with placebo in reducing the number of pulmonary exacerbations among children with HIV‐related bronchiectasis over a period of 52 weeks Design: randomised, double‐blind, placebo‐controlled trial Total study duration: not reported Number of study centres and location: single, South Africa Study setting: Paediatric Chest Clinic, Steve Biko Academic Hospital, Pretoria Methods of recruitment: not reported Withdrawals: 1 child died after randomisation, but group allocation was not stated, and 10 were lost to follow‐up (intervention group: 6; control group: 4) Study start/end dates: January 2009/June 2012 Analysis by intent‐to‐treat: no |
|
| Participants | 42 children were randomised. Inclusion criteria: children aged 6 to 18 years with confirmed HIV infection. The presence of bronchiectasis was confirmed on HRCT scanning, with exclusion of other causes of bronchiectasis, including a sweat test to rule out CF. All children had to be able to perform reliable pulmonary function tests. Exclusion criteria: abnormal liver function tests (ALT/AST > 2.5 times normal); abnormal urea/creatinine; use of carbamazepine, warfarin, cyclosporine, or long‐term midazolam Mean age: intervention group: 8.4 years; control group: 9.1 years Gender: intervention group: 4 girls, 13 boys; control group: 9 girls, 5 boys Bronchiectasis diagnosis: HRCT scanning Severity of condition: Bhalla score: intervention group: 15; control group: 11.5 Baseline lung function (intervention group, control group): FEV1 (% predicted): 56, 53.6; FVC (% predicted): 49, 45 Baseline imbalances: Characteristics of the 2 study arms were generally balanced, with the exception of gender distribution, with more males (76%) in the erythromycin arm and more females in the placebo arm (64%). CD4 count (%) and CD4 (total × 106) were significantly lower and Bhalla score significantly higher in the intervention group than in the control group (worse). |
|
| Interventions | Intervention group: erythromycin (n = 17) Dose: 125 mg per oral suspension if < 15 kg body weight, or 250 mg per oral suspension if ≥ 15 kg body weight; delivery mode: oral; frequency: daily; duration: 52 weeks Control group: placebo (n = 14) Adherence: Compliance was assessed with use of a medication diary and verbal interviews. 90% of participants in each arm took their medication. Run‐in phase: unclear Run‐out phase: unclear |
|
| Outcomes |
Primary: exacerbations (protocol defined as the presence of ≥ 2 of the following: increased tachypnoea or dyspnoea, change in frequency of cough, increase in sputum productivity, fever, chest pain, new infiltrates on chest x‐ray) Secondary: pulmonary function parameters (FEV1, FVC, FEF), BMI z‐score, CD4 count (%), CD4 (total* 108), proinflammatory and anti‐inflammatory chemokines and cytokines, Bhalla score Post hoc analysis: unclear |
|
| Notes |
Power calculation: Sample size calculation was based on the number of pulmonary exacerbations requiring antibiotic therapy, estimated at 3 per year. A sample size of 20 participants per study arm was determined to have 90% power to detect a clinically relevant reduction in exacerbations of 30%, when a mean of 2 and a standard deviation of 1 exacerbation were assumed; and with a presumed dropout rate of 10% when testing was 1sided at the 0.05 level of significance. Trial registration: not reported Conflicts of interest: not declared Funders: unrestricted grant from the Research Development Program of the University of Pretoria. Adcock Ingram South Africa donated erythromycin. Role of the funders/sponsors: not reported Ethical approval: yes Conclusions: Administration of HAART and adjunctive care, which includes airway clearance and treatment of exacerbations, in children with HIV‐related bronchiectasis is associated with significant improvement in pulmonary function tests and IL‐8, with no additional benefit derived from the use of erythromycin. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned (1:1) to the erythromycin group (55%) or to the placebo group (45%). |
| Allocation concealment (selection bias) | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | All study personnel performing clinical evaluations were blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Two blinded radiologists carried out the CT scan. Additional details on outcome blinding were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10 participants were lost to follow‐up ‐ 4 in the placebo group and 6 in the intervention group; no reasons were provided. |
| Selective reporting (reporting bias) | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Other bias | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
Sadigov 2013.
| Methods |
Aims: to test the hypothesis that azithromycin would decrease the frequency of exacerbations, increase lung function, and decrease the severity of symptoms Design: randomised placebo‐controlled trial Total study duration: 12 months Number of study centres and location: single, Azerbaijan Study setting: hospital clinic Methods of recruitment: not reported Withdrawals: unclear Study start/end dates: February 2011/February 2012 Analysis by intent‐to‐treat: unclear |
|
| Participants | 65 adults were randomised. Inclusion criteria: not reported Exclusion criteria: not reported Mean age: not reported Gender: not reported Bronchiectasis diagnosis: not reported Severity of condition: not reported Baseline lung function: not reported Smoking history: not reported Baseline imbalances: not reported |
|
| Interventions |
Intervention group: azithromycin (n = 35) Dose: 500 mg; delivery mode: oral; frequency: 3 days per week; duration: 6 months Control group: placebo (n = 30) Adherence: unclear Run‐in phase: unclear Run‐out phase: unclear |
|
| Outcomes |
Primary: event‐based exacerbations, times of first exacerbation, adverse events, serious adverse events Secondary: sputum volume and purulence, FEV1, systemic and local markers of infection (leucocyte count, CRP, neutrophil count of induced sputum, interleukin‐6 (IL‐6) in induced sputum), adverse events (e.g. cardiac arrhythmias, gastrointestinal symptoms, hearing impairment) |
|
| Notes | Conference abstract only. Additional information provided by personal communication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Allocation concealment (selection bias) | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Selective reporting (reporting bias) | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Other bias | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk' as only data from the conference abstract were available. |
Serisier 2013.
| Methods |
Aims: to test the hypothesis that low‐dose erythromycin would reduce pulmonary exacerbations in patients with non‐CF bronchiectasis with a history of frequent exacerbations Design: randomised, double‐blind, placebo‐controlled study Total study duration: 26 months Number of study centres and location: single, Australia Study setting: regional adult CF centre, respiratory medicine department, Australian University Teaching Hospital; out‐patient setting Methods of recruitment: patients attending the centre, referral from respiratory physicians at other centres, and public radio advertisements Withdrawals: 10; intervention group: 5 (2 lost to follow‐up, 1 lost for possible QTc prolongation, 1 moved, 1 unable to continue); control group: 5 (2 lost to follow‐up, 1 with nausea, 1 withdrawn by physician, 1 unable to continue) Study start/end dates: October 2008/December 2011 Analysis by intent‐to‐treat: yes, using LOCF for missing data |
|
| Participants | 117 adults were randomised. Inclusion criteria: confirmed diagnosis of bronchiectasis and clinically stable for at least 4 weeks before enrolment (defined as no symptoms of exacerbation, no requirement for supplemental antibiotic therapy, and FEV1 within 10% of best recently recorded value when available) Exclusion criteria: CF, current mycobacterial disease or bronchopulmonary aspergillosis, any reversible cause for exacerbations, maintenance oral antibiotic prophylaxis, prior macrolide use except short‐term use, changes to medications in the preceding 4 weeks, cigarette smoking within 6 months, medications or comorbidities with the potential for important interactions with erythromycin Mean age: intervention group: 61.1 years; control group: 63.5 years Gender: intervention group: 38 women, 21 men; control group: 33 women, 25 men Bronchiectasis diagnosis: HRCT scan and clinical diagnosis (≥ 2 separate pulmonary exacerbations requiring supplemental systemic antibiotic therapy in the preceding 12 months, and daily sputum production) Severity of condition: 35% of adults had more than 5 exacerbations in the previous year. Bhalla score was not reported. Baseline lung function (intervention group, control group): FEV1 (postbronch % predicted): 70.2, 73.6 Smoking history: intervention group: ex‐smokers: 10, 2.3 pack‐years: non‐smokers: 49; control group: ex‐smokers: 15, 2.9 pack‐years: non‐smokers: 44 Baseline imbalances: no significant between‐group differences |
|
| Interventions |
Intervention group: erythromycin ethylsuccinate (n = 59) Dose: 400 mg (equivalent to 250 mg erythromycin base); delivery mode: oral; frequency: 2/d; duration: 48 weeks Control group: placebo (n = 58) spray‐dried lactose/magnesium stearate tablets Adherence: assessed at each visit by pill counts (intervention group: 95.6%; control group: 96.5%) Run‐in phase: unclear Run‐out phase: 4‐week washout period Erythromycin and placebo tablets were manufactured and supplied by Alpha Pharm and were identical in shape, appearance, and taste. |
|
| Outcomes |
Primary: mean rate of protocol‐defined pulmonary exacerbation (PDPE) per patient per year (required antibiotic administration for a sustained (> 24‐hour) increase in sputum volume or purulence accompanied by new deteriorations in ≥ 2 additional symptoms: sputum volume, sputum purulence, cough, dyspnoea, chest pain, or hemoptysis Secondary: rate of all pulmonary events (i.e. PDPEs plus non‐PDPEs) for which participants commenced antibiotics, total days of antibiotics, change in the proportion of commensal oropharyngeal streptococci resistant to macrolides, symptoms (LCQ), quality of life (SGRQ), 24‐hour sputum weight, FEV1 percent predicted, CRP level, exercise capacity (6MWT), sputum bacteriology, and sputum inflammatory cell counts. Safety endpoints included adverse events, liver function test results, and electrocardiogram findings. Post hoc analysis: unclear |
|
| Notes |
Power calculation: Assuming a baseline (SD) annual rate of exacerbations in the control group of 2.9 (1.2), 98 participants gave 90% power at the 5% significance level to show a 28% reduction in exacerbation rate with erythromycin ‐ a much more conservative estimate of efficacy than the 50% reduction seen in our uncontrolled pilot data. Assuming 20% attrition, the required sample size was increased to 118. Trial registration: anzctr.org.au Identifier: ACTRN12609000578202 Conflicts of interest: Dr. Serisier received honoraria, speakers' fees, and travel support from a range of pharmaceutical companies including GSK, Boehringer‐Ingelheim, AstraZeneca, Phebra, and Pharmaxis. Dr. Bowler received honoraria, speakers' fees, and travel support from a range of pharmaceutical companies including GSK, Boehringer‐Ingelheim, AstraZeneca, and Novartis. Other trial authors reported no conflicts of interest. Funders: Mater Adult Respiratory Research Trust Fund. No pharmaceutical company or other agency (including medical writers) had any role in this study. Role of the sponsors: The funding source had no role in design and conduct of the study; collection, analysis, and interpretation of data; or preparation, review, or approval of the manuscript. Ethical approval: yes Conclusions: Among patients with non‐CF bronchiectasis, 12‐ month use of erythromycin compared with placebo resulted in a modest decrease in the rate of pulmonary exacerbations and an increased rate of macrolide resistance. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequences, blocked in random groups of 2, 4, and 8 and stratified for the presence of sputum Pseudomonas aeruginosa at screening, were held by the Department of Pharmacy. |
| Allocation concealment (selection bias) | Low risk | The independent trial pharmacist dispensed blinded study drug according to the randomisation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial participants, trial supervisors, and all trial staff directly involved in participant care were unaware of treatment assignment at all times. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All participants and study personnel were masked to treatment assignment, including all investigators involved in sample processing and data entry. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of follow‐up was similar in both groups. |
| Selective reporting (reporting bias) | Low risk | All measurements stated in the methods were reported in the results section. Extended methods were available online (http://www.jama.com). |
| Other bias | Low risk | Trial authors used LOCF methods to impute missing data for ITT analyses, but robustness was confirmed via multiple imputation techniques to assess sensitivity. |
Valery 2013.
| Methods |
Aims: to establish whether long‐term (12 to 24 months) antibiotic treatment with azithromycin would reduce the rate of pulmonary exacerbations in indigenous children with non‐cystic fibrosis bronchiectasis; also to monitor for serious adverse events associated with azithromycin and examine its effect on nasopharyngeal carriage of bacterial pathogens. Design: double‐blind, randomised, placebo‐controlled trial Total study duration: 25 months Number of study centres and location: multi‐centre, Australia and New Zealand Study setting: community clinics in central and northern Australia and urban Maori and Pacific Island children from a tertiary paediatric hospital in Auckland Methods of recruitment: Children entered the study when they were clinically stable (≥ 8 weeks after their last exacerbation) as decided by clinic staff. Withdrawals: intervention group: 4 (1 was withdrawn by physician, 1 was withdrawn by parent, 1 refused meds, 1 fulfilled exit criteria); control group: 4 (2 withdrawn by physician, 1 moved out of study, 1 fulfilled exit criteria) Study start/end dates: November 2008/December 2010 Analysis by intent‐to‐treat: Analysis of the primary endpoint was by intention‐to‐treat. Analysis of secondary endpoints was by modified intention‐to‐treat, excluding participants with missing data, except for analysis of nasal swabs, which was done only for participants with swabs available from baseline and last clinic visits. |
|
| Participants | 89 children were randomised. Inclusion criteria: aged 1 to 8 years, living within the study area, had bronchiectasis confirmed radiographically by HRCT scans or chronic suppurative lung disease (bronchiectasis suspected clinically when HRCT scans were unavailable), and had ≥ 1 pulmonary exacerbation in the past 12 months Exclusion criteria: receiving chemotherapy, immunosuppressive treatment, or long‐term antibiotics; had an underlying cause for their bronchiectasis (e.g. cystic fibrosis, primary immunodeficiency), other chronic disorders (e.g. cardiac, neurological, renal, or hepatic abnormality), or macrolide hypersensitivity Mean age: intervention group: 3.99 years; control group: 4.22 years Gender: intervention group: 19 girls, 26 boys; control group: 23 girls, 21 boys Bronchiectasis diagnosis: HRCT scans or chronic suppurative lung disease (bronchiectasis suspected clinically when HRCT scans were unavailable) Severity of condition: Bhalla score not reported Baseline lung function: not reported Baseline imbalances: The most substantial difference was mechanical ventilation, with more children in the placebo group needing ventilation as neonates compared with those in the azithromycin group (22% vs 5%). However, participants in the azithromycin group were less likely to be premature (29% vs 39%), fewer had proven bronchiectasis (76% vs 89%), and their first admission to hospital for respiratory disease occurred later in life (mean of 6.5 vs 4.2 months). |
|
| Interventions |
Intervention group: azithromycin (n = 45) Dose: 30 mg/kg, maximum 600 mg; delivery mode: oral; frequency: once a week; duration: 24 months Study drug was administered under direct supervision at the community clinic (Australia) or at the child's home, preschool, or school (New Zealand). Control group: placebo (n = 44) Placebo medication was similar in appearance, taste, smell, and packaging to the active medication and had no active ingredients. Adherence: Research nurses contacted the community clinic and the child's caregiver, preschool, or school weekly to record drug adherence (children receiving medication and, if any, children who were absent from the community) and any issues with administration, such as the child spitting out the medication. These data were recorded in a participant medication logbook. Study personnel completed a medical review every 3 to 4 months. Intervention group: 88%; control group: 84% Run‐in phase: Children who were already receiving azithromycin (4 in each treatment group) had the antibiotic discontinued and underwent a 3‐month washout period before commencing the study. Run‐out phase: unclear Both azithromycin and placebo were provided in powder format and were reconstituted with 9 mL of sterile water to syrup for oral use (40 mg/mL). |
|
| Outcomes |
Primary: pulmonary exacerbation rate (treatment by clinic or hospital staff with antibiotics for any of the following (as recorded in the medical chart): increased cough, dyspnoea, increased sputum volume or colour intensity, new chest examination or radiographic findings, deterioration in predicted FEV1 percentage > 10%, or haemoptysis) Secondary: time to first pulmonary exacerbation, duration of exacerbation episode (discharge date minus admission date plus 1 day), severity (admission to hospital, oxygen supplementation), weight‐for‐age z‐scores (z‐score at last study clinic minus its value at baseline), respiratory signs and symptoms (assessed by study personnel on history and physical examination), sputum characteristics, school absenteeism, FEV1 % predicted in those aged 6 years and older, serious adverse events, and antibiotic resistance in bacterial pathogens cultured from deep nasal swabs Post hoc analysis: post hoc subgroup analyses for participants taking ≥ 70% of their expected doses, those who received the intervention for 23 to 24 months, children with HRCT‐proven bronchiectasis, children with ≥ 2 hospital‐managed pulmonary exacerbations before enrolment, children with ≥ 10 pulmonary exacerbations before enrolment, those without a history of mechanical ventilation, and those carrying any respiratory bacterial pathogens at baseline |
|
| Notes |
Power calculation: Sample size and power calculations were based on previous data; we anticipated that participants in the placebo group would have 4 episodes during the 24‐month trial period. Guided by results from an earlier randomised trial of azithromycin in patients with CF, we assumed pulmonary exacerbations would be reduced by 50% in the intervention group and by 15% in the placebo group. 51 participants per group would give 90% power to detect an average difference of 1.4 respiratory exacerbations per participant over a 2‐year period at the 5% level of significance. Trial registration: Australian New Zealand Clinical Trials Registry, number ACTRN12610000383066 Conflicts of interest: Trial authors declared they had no conflicts of interest. Funders: National Health and Medical Research Council (NHMRC) of Australia (project grant numbers 389837 (clinical component), 545223 (microbiology component), and CRE for lung health 1040830 (feedback)); Telstra Foundation (seeding grant ‐ Telstra Community Development Grant, 2004); Health Research Council of New Zealand (grant number 08/158); and Auckland Medical Research Foundation (grant number 81542) Role of the sponsors: Sponsors of the study had no role in study design, data collection, data analysis, or data interpretation, nor in writing of the report. Ethical approval: yes Conclusions: Once‐weekly azithromycin for up to 24 months decreased pulmonary exacerbations among indigenous children with non‐cystic fibrosis bronchiectasis or chronic suppurative lung disease. However, this strategy was accompanied by increased carriage of azithromycin‐resistant bacteria, the clinical consequences of which are uncertain, and will need careful monitoring and further study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent statistician used a computer‐generated permuted‐block design to provide the randomisation sequences. Children were allocated in a 1:1 ratio (stratified by study site and exacerbation frequency in the preceding 12 months (1‐2 vs > 3 episodes)) to azithromycin or placebo. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was achieved by use of sequentially numbered, double‐sealed, opaque envelopes. An independent person at the Queensland Institute of Medical Research (Brisbane, QLD, Australia) prepared the individual envelopes labelled with a randomisation number that contained the treatment code inside. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study drugs (powder for reconstitution to suspension) were provided in identical packaging, and the placebo (Institute of Drug Technology, Melbourne, VIC, Australia) was much the same in appearance, taste, and smell to azithromycin (Pfizer Australia, West Ryde, NSW, Australia). Participants, families, health professionals, and study personnel were unaware of treatment assignment until data analysis was completed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators collecting data were unaware of the treatment assigned to each child. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Eight children (4 per group) ceased the intervention early, mainly after they were withdrawn by their treating physician or because they experienced treatment failure (2 in the azithromycin group, 3 in the placebo group). |
| Selective reporting (reporting bias) | Low risk | The study protocol is available, and all of the study's prespecified (primary and secondary) outcomes of interest to the review were reported in the prespecified way. |
| Other bias | Low risk | Baseline imbalances were tested in post hoc subgroup analyses and showed increased efficacy for the intervention group, although as the trial authors note, analyses were not hypothesis driven and results should therefore be interpreted with caution. |
Wong 2012.
| Methods |
Aims: to test whether azithromycin decreases the frequency of exacerbations, increases lung function, and improves HRQoL in patients with non‐CF bronchiectasis Design: randomised, double‐blind, placebo‐controlled trial Total study duration: 18 months Number of study centres and location: 3, New Zealand Study setting: health centres Methods of recruitment: not reported Withdrawals: 4 withdrew from the azithromycin group (1 had adverse events, 2 were lost to follow‐up, 1 withdrew consent); 10 withdrew from the placebo group (2 had adverse events, 3 were lost to follow‐up, 4 withdrew consent, 1 had cultured Mycobacterium avium intracellulare in sputum). Study start/end dates: February 2008/October 2009 Analysis by intent‐to‐treat: yes |
|
| Participants | 141 adults were randomised. Inclusion criteria: ≥ 18 years of age, ≥ 1 pulmonary exacerbation requiring antibiotic treatment in the past year, and diagnosis of bronchiectasis defined by HRCT scan Exclusion criteria: history of CF; hypo‐gammaglobulinaemia; allergic bronchopulmonary aspergillosis; positive culture of non‐tuberculous mycobacteria in the past 2 years or at screening; macrolide treatment for more than 3 months in the past 6 months; or unstable arrhythmia Mean age: intervention group: 60.9 years; control group: 59 years Gender: intervention group: 48 women, 23 men; control group: 50 women, 20 men Bronchiectasis diagnosis: HRCT scan Severity of condition: Bhalla score not reported Baseline lung function (intervention group, control group): FEV1 (% predicted): 67.1, 67.3; FVC (% predicted): 77.7, 78.5; FEV1/FVC: 65.4%, 64.7% Smoking history: not reported Baseline imbalances: unclear |
|
| Interventions |
Intervention group: azithromycin (n = 71) Dose: 500 mg; delivery mode: oral; frequency: 3/week (Monday, Wednesday, and Friday); duration: 6 months Control group: placebo (n = 70) Adherence: intervention group: 97.9%; control group: 98.3%, assessed by pill counts Run‐in phase: not reported Run‐out phase: followed up for another 6 months without treatment |
|
| Outcomes |
Primary: rate of event‐based exacerbations in the first 6 months (increase in or new onset of ≥ 1 pulmonary symptom (sputum volume, sputum purulence, or dyspnoea) requiring treatment with antibiotics), FEV1 before bronchodilation, and SGRQ total score at the end of the treatment period Secondary: time to first exacerbation, rate of symptom‐based exacerbations (increase in or new onset of ≥ 1 pulmonary symptom reported on the daily diary card and mean of 3 symptom scores from the daily diary card on 2 consecutive days had to increase by ≥ 1 point (on a 5‐point scale) compared with the same calculation 1 week earlier), prebronchodilator and postbronchodilator FVC, postbronchodilator FEV1, exercise capacity (as measured by the 6MWT), SGRQ total score at 12 months, concentration of CRP (assessed only at 6 months), sputum cell counts and microbiology, and adverse events Post hoc analysis: unclear |
|
| Notes |
Power calculation: We estimated that about 134 patients would need to be enrolled for the study to have 80% power to detect a 33% difference between the 2 groups in the Poisson frequency of exacerbations during the 6‐month treatment period, assuming a 2‐sided level of 0 to 5 and a 10% dropout rate. With the assumption of normality, the study had power of 89% to detect a difference of 0 to 16 L in the prebronchodilator FEV1 and power of 87% to detect a difference of 8 units in SGRQ total score. Trial registration: Australian New Zealand Clinical Trials Registry, number ACTRN12607000641493 Conflicts of interest: Trial authors declared they had no conflicts of interest. Funders: Health Research Council of New Zealand and Auckland District Health Board Charitable Trust Role of the sponsors: The sponsor had no role in study design, data collection, data analysis, or data interpretation. The data monitoring committee of the sponsor provided feedback on the completed report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication. Ethical approval: yes Conclusions: Azithromycin, taken 3 times a week for 6 months, decreased the frequency of event‐based exacerbations and increased the time to first exacerbation in patients with non‐cystic fibrosis bronchiectasis. A treatment effect on exacerbations was evident 6 months after completion of treatment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐ generated random number list. Patients were randomly assigned in a 1:1 ratio with a permuted block size of 6 and sequential assignment, stratified by centre. |
| Allocation concealment (selection bias) | Low risk | Randomly assigned to receive azithromycin or placebo by a statistician independent of the reporting statistician |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, research assistants, and investigators were masked to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, research assistants, and investigators were masked to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 withdrew from the intervention group and 10 from the placebo group for similar reasons. |
| Selective reporting (reporting bias) | Low risk | All outcomes specified in the protocol were reported. |
| Other bias | Low risk | None identified |
Yalcin 2006.
| Methods |
Aims: to evaluate effects of macrolide antibiotics on the process of inflammation (by measuring IL‐8, TNF‐a, IL‐10 levels and cell profiles in BAL fluid), pulmonary function, and sputum production in children with steady‐state bronchiectasis, secondary to causes other than CF or primary immunodeficiencies Design: randomised controlled trial (open‐label, as no placebo) Total study duration: 12 months Number of study centres and location: single, Turkey Study setting: Department of Paediatric Chest Diseases at Hecettepe University Faculty of Medicine Methods of recruitment: unclear Withdrawals: none Study start/end dates: April 1999/March 2000 Analysis by intent‐to‐treat: yes |
|
| Participants | 34 children were randomised. Inclusion criteria: diagnosis of bronchiectasis not due to CF or primary immunodeficiencies, clinically stable with no evidence of acute pulmonary exacerbations; no history of upper or lower respiratory tract infection for at least 4 weeks before start of the study. No patients had received antibiotics within 4 months of study entry. None had taken oral or inhaled corticosteroids before or during the study. Exclusion criteria: not reported Mean age: intervention group: 13.1 years; control group: 11.9 years Gender: intervention group: 9 girls, 8 boys; control group: 6 girls, 11 boys Bronchiectasis diagnosis: clinical and high‐resolution computed tomography Severity of condition: not reported Baseline lung function (intervention group, control group): FEV1 (% predicted): 74, 79 Baseline imbalances: Data show no statistically significant differences between study and control groups in age, sex, FEV1, or oxygen saturation. But among inflammatory parameters, IL‐8 and TNF‐a levels in BAL fluid were significantly higher at the beginning of the study in the treatment group than in the control group (P = 0.02 and P = 0.02, respectively). |
|
| Interventions |
Intervention group: clarithromycin (CAM) + supportive therapies (n = 17) Dose: 15 mg/kg; delivery mode: oral; frequency: daily; duration: 3 months plus supportive therapies (mucolytic and expectorant medications and postural drainage) Control group: supportive therapies alone (mucolytic and expectorant medications and postural drainage) (NB: no placebo) (n = 17) Adherence: not reported Run‐in phase: unclear Run‐out phase: unclear |
|
| Outcomes |
Primary: unclear Secondary: unclear BAL cytokine levels (IL‐8, IL‐10, TNF‐alpha); BAL cell profiles (cell number, neutrophils, macrophages); culture test (aerobic and anaerobic bacteria, fungi, and mycobacteria); pulmonary function test (FEV1, FEF); oxygen saturation; sputum volume Post hoc analysis: unclear |
|
| Notes |
Power calculation: not reported Trial registration: not reported Conflicts of interest: not reported Funders: SANOVEL Pharmaceuticals Inc., supplied cytokine kits. Role of the sponsors: not reported Ethical approval: not reported Conclusions: Use of CAM in children with steady‐state bronchiectasis results in laboratory improvement by reducing inflammatory processes in the lungs. No corresponding clinical improvement could be shown, and although this is possible with long‐term use, trial validation is necessary. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information about the sequence generation process was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Allocation concealment (selection bias) | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded as trial was not placebo‐controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Treatment protocols for all participants were completed without interruption, as none experienced acute infection during follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Information was insufficient to permit judgement of 'low risk' or 'high risk'. |
| Other bias | High risk | Inflammatory markers were significantly higher in the intervention group at baseline; it is unclear whether this was controlled for in the change analysis. |
6MWT: six‐minute walking test; AFB: acid‐fast bacilli; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BAL: bronchoalveolar lavage; BMI: body mass index; CD4: cluster of differentiation 4; CF: cystic fibrosis; COI: conflict of interest; CRP: serum C‐reactive protein; CT: computed tomography; EBC: exhaled breath condensate; FEF: forced expiratory flow; FeNO: fractional exhaled nitric oxide; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; HAART: highly active antiretroviral therapy; HIV: human immunodeficiency virus; HRCT: high resolution computed tomography; HRQoL: health related quality of life; ICS: inhaled corticosteroids; IL‐6: interleukin‐6; IL‐8: Interleukin‐8; IL‐10: Interleukin‐10; ITT: intention to treat; LABA: long‐acting beta‐agonist; LCQ: Leicester Cough Questionnaire; LOCF: last observation carried forward; LRTI: lower respiratory tract Infection; MMP‐9: matrix metallopeptidase‐9; MRC: Medical Research Council; NE: neutrophil elastase; NO2: nitrite; NO3: nitrate; PC20: the Provocative Concentration of methacholine causing a 20% drop in FEV1; PDE: protocol‐defined exacerbation; PDPE: protocol‐defined pulmonary exacerbation; PF: pulmonary function; PMN: polymorphonuclear leucocyte; QoL: quality of life; QTc: the QT interval; SABA: short‐acting beta‐agonist; SD: standard deviation; SGRQ: St. George's Respiratory Questionnaire; TIMP‐1: tissue inhibitor of metalloproteinases‐1; TNF‐alpha: tumour necrosis factor‐alpha; WBC: white blood cell count.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chang 2013 | Protocol |
| Kudo 1988 | Not an RCT |
| Min 1988 | Not an RCT; not exclusively bronchiectasis; duration of treatment < 4 weeks |
| Ming 2005 | Not an RCT |
| Rikitomi 1988 | Not an RCT |
| Saito 1988 | Not an RCT |
| Tagaya 2002 | Macrolide used for treatment as opposed to prevention; duration of treatment < 4 weeks |
| Unoura 1986 | Not an RCT |
RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Tsang 1999.
| Methods |
Aims: to evaluate effects of low‐dose erythromycin on sputum volume and lung function indices in steady‐state bronchiectasis Design: double‐blind placebo‐controlled trial (trial authors contacted to determine whether randomised) Total study duration: 6 months Number of study centres and location: single, Hong Kong Study setting: outpatient clinics at the University of Hong Kong Methods of recruitment: not reported Withdrawals: intervention group: 3 withdrawals ‐ 2 were unreliable attenders, 1 developed a maculopapular rash 5 days after erythromycin therapy; control group: 0 withdrawals Study start/end dates: October 1996/April 1997 Analysis by intent‐to‐treat: no |
| Participants | 24 adults were randomised. Inclusion criteria: 24‐hour sputum volume > 10 mL; absence of unstable systemic disease; and "steady‐state" bronchiectasis (< 10% alteration of 24‐hour sputum volume, forced expiratory volume in 1 second (FEV1), and forced vital capacity (FVC); in the absence of deterioration in cough, dyspnoea, wheezing, fever, or chest pain at baseline visits) Exclusion criteria: unreliable clinic attendance, adverse reaction to macrolides, women who were lactating Mean age: intervention group: 50 years; control group: 59 years Gender: intervention group: 8 women, 3 men; control group; 8 women, 2 men Bronchiectasis diagnosis: high‐resolution computed tomography (HRCT) Severity of condition: not reported Baseline lung function: not reported Smoking history: intervention group: never: 10, ex‐smoker: 1; control group: never: 8, ex‐smoker: 2 Baseline imbalances: no significant differences between groups |
| Interventions |
Intervention group: erythromycin (n = 11) Dose: 500 mg; delivery mode: oral; frequency: 2/d; duration: 8 weeks Control group: placebo (n = 10) Adherence: not reported Run‐in phase: unclear Run‐out phase: unclear |
| Outcomes |
Primary: unclear which is the primary outcome Secondary: unclear 24‐Hour sputum volume; sputum leucocyte density (per mL); sputum pathogenic density (colony‐forming unit (cfu) ‐ mL˜); sputum (sol phase) IL‐la, TNF‐a, and LTB4; pulmonary function test (FEV1, FVC) Post hoc analysis: unclear |
| Notes |
Power calculation: Based on trial authors' experience, daily sputum volume might vary by as much as 10% between days in patients with stable bronchiectasis. With acceptance of a type I error of 0.05 and a type II error of 0.20 (power 0.80), study size for a randomised placebo‐controlled study of 20 participants (10 in each treatment group) would allow detection of 12% change in sputum volume. Trial registration: not reported Conflicts of interest: not declared Funders: CRCG grant from the University of Hong Kong Role of the sponsors: not reported Ethical approval: yes Conclusions: Results of this preliminary study, which is the first controlled study on the effects of erythromycin in chronic bronchial sepsis, show the efficacy of low‐dose and moderately long‐term erythromycin in steady‐state bronchiectasis. Low‐dose and long‐term erythromycin therapy might be a disease‐modifying treatment for idiopathic bronchiectasis. Additional studies should be performed to establish dose response, appropriate duration of therapy, and criteria for patient selection. |
cfu: colony‐forming unit; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; HRCT: high‐resolution computed tomography; IL: interleukin; LTB: leukotriene B; TNF: tumour necrosis factor.
Differences between protocol and review
We decided to present the results for adults and for children as separate comparisons. We also decided to present the results for different macrolides separately.
Regarding systemic markers of infection, during the course of the review, we decided to focus specifically on C‐reactive protein for the secondary outcome on systemic markers of infection, as it is the most widely used biomarker of systemic inflammation in clinical practice.
Contributions of authors
All review authors contributed to preparation of the Background section.
Lambert Felix, Nicola Relph, Stephen J Milan, and Sally Spencer contributed to the methods, results, discussion, and conclusions of the review.
David Evans, Carol Kelly, and Lambert Felix contributed to screening searches and identifying the included studies
James Chalmers, Iain Crossingham, and Carol Kelly contributed to the methods, discussion, and conclusions sections.
Sources of support
Internal sources
-
Edge Hill University, UK.
Funded Lambert Felix to provide support for a series of reviews on bronchiectasis. Carol Kelly and Sally Spencer were co‐applicants on the internal funding bid.
External sources
No sources of support supplied
Declarations of interest
Sally Spencer, Carol Kelly, and Nicola Relph were named co‐investigators on a study funded by Edge Hill University to develop a series of reviews on bronchiectasis. Lambert Felix was supported by that funding. No funding was received by any other review authors for participation in this systematic review.
David Evans provides freelance writing services to medical communication agencies. Steve Milan: none known.
Iain Crossingham received travel and training expenses from Hamilton Medical that are not connected to the topic of this review.
James D Chalmers declares grant support from Pfizer, AstraZeneca, and GlaxoSmithKline. In addition, he is part of an innovative medicines initiative consortium that includes Novartis and Basilea. He has participated in advisory boards for Bayer HealthCare, Chiesi, and Raptor Pharmaceuticals. He has received fees for speaking from Napp, AstraZeneca, BI, and Pfizer. None of these conflicts of interest are related to the work involved in this review, and these conflicts are unrelated to the topic of this review.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Altenburg 2013 {published data only}
- Altenburg J, Wilms E, Boersma W. The relationship between serum‐ and sputum levels of azithromycin and clinical endpoints in bronchiectasis patients in bronchiectasis patients using maintenance treatment. Pneumologie. 2016; Vol. 70:A3. [DOI: 10.1055/s-0036-1592227] [DOI]
- Altenburg J, Wolf R, Go S, Rijn P, Boersma W, Werf T. Changes of computed tomography features of bronchiectasis during one year of azithromycin treatment. European Respiratory Journal. 2016; Vol. 48:OA278. [DOI: 10.1183/13993003.congress-2016.OA278] [DOI]
- Altenburg J, Graaf C, Werf T, Boersma W. Long term azithromycin treatment: A randomised placebo‐controlled trial in non‐CF bronchiectasis; results from the BAT trial. European Respiratory Journal. 2011; Vol. 38:1924.
- Altenburg J, Graaf CS, Steinstra Y, Sloos JH, Haren EH, Koppers RJ, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non‐cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA 2013;309(12):1251‐9. [DOI] [PubMed] [Google Scholar]
- Boersma WG, Altenburg J, Werf TS. Evaluation of symptoms score and QoL in azithromycin maintenance treatment: results of a RCT trial in patients with bronchiectasis. American Journal of Respiratory and Critical Care Medicine. 2012; Vol. 185:A3658.
Asintam 2012 {published data only}
- Asintam P, Kiranantawat N, Juthong S. Can roxithromycin improve quality of life in bronchiectatic patients?. European Respiratory Journal. 2012; Vol. 40:P2168.
Cymbala 2005 {published data only}
- Cymbala AA, Edmonds LC, Bauer MA, Jederlinic PJ, May JJ, Victory JM, et al. The disease‐modifying effects of twice‐weekly oral azithromycin in patients with bronchiectasis. Treatments in Respiratory Medicine 2005;4(2):117‐22. [DOI] [PubMed] [Google Scholar]
Diego 2013 {published data only}
- Diego AD, Milara J, Martinez‐Moragón E, Palop M, León M, Cortijo J. Effects of long‐term azithromycin therapy on airway oxidative stress markers in non‐cystic fibrosis bronchiectasis. Respirology 2013;18(7):1056‐62. [DOI] [PubMed] [Google Scholar]
Juthong 2011 {published data only}
- Juthong S, Eiamsaard S. The effects of roxithromycin as anti‐inflammatory agent on clinical outcomes in patient with bronchiectasis: a double blinded randomized controlled study. European Respiratory Journal. 2011; Vol. 38(Suppl 55):455s.
Koh 1997 {published data only}
- Koh YY, Lee MH, Sun YH, Sung KW, Chae JH. Effect of roxithromycin on airway responsiveness in children with bronchiectasis: a double‐blind, placebo‐controlled study. European Respiratory Journal 1997;10(5):994‐9. [PUBMED: 9163637] [DOI] [PubMed] [Google Scholar]
Liu 2012 {published data only}
- Liu JF, Zhong XN, He ZY, Zhong DJ, Bai J, Zhang JQ, et al. [Impact of treatment with low dose roxithromycin on stable bronchiectasis]. Zhonghua Jie He He Hu Xi Za Zhi = Zhonghua jiehe he huxi zazhi = Chinese Journal of Tuberculosis and Respiratory Diseases 2012;35(11):824‐7. [PUBMED: 23290037] [PubMed] [Google Scholar]
Liu 2014 {published data only}
- Liu J, Zhong X, He Z, Wei L, Zheng X, Zhang J, et al. Effect of low‐dose, long‐term roxithromycin on airway inflammation and remodeling of stable noncystic fibrosis bronchiectasis. Mediators of Inflammation 2014;2014:708608. [PUBMED: 25580060] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lourdesamy 2014 {published data only}
- Lourdesamy Anthony AI, Muthukumaru U. Efficacy of azithromycin in the treatment of bronchiectasis. Respirology (Carlton, Vic.) 2014;19(8):1178‐82. [PUBMED: 25183304] [DOI] [PubMed] [Google Scholar]
Masekela 2013 {published data only}
- Masekela R, Anderson R, Gongxeka H, Steel HC, Green RJ. Lack of efficacy of erythromycin in children with human immunodeficiency virus‐related bronchiectasis – a randomised controlled trial. Paediatric Respiratory Reviews. 2013; Vol. 14, issue 2:S82/A009‐12. [DOI: ]
- Masekela R, Anderson R, Gongxeka H, Steel HC, et al. Lack of efficacy of an immunomodulatory macrolide in childhood HIV‐related bronchiectasis: a randomised, placebo‐controlled trial. Journal of Antivirals and Antiretrovirals 2013;5:44‐9. [Google Scholar]
Sadigov 2013 {published data only}
- Sadigov AS, Mammadov GT. Azythromycin for prevention of exacerbations in non‐cystic fibrosis bronchiectasis: how we can improve the clinical features of severe disease?. American Journal of Respiratory and Critical Care Medicine. 2013; Vol. 187:A3512.
Serisier 2013 {published data only}
- Burr L, Rogers G, Taylor S, McGuckin M, Serisier D. Sub inhibitory erythromycin reduces the expression of key P. Aeruginosa virulence determinants in non‐CF bronchiectasis subjects. Respirology. 2015:29.
- Burr LD, Rogers GB, Chen AC, Hamilton BR, Pool GF, Taylor SL, et al. Macrolide treatment inhibits Pseudomonas aeruginosa quorum sensing in non‐cystic fibrosis bronchiectasis. An analysis from the bronchiectasis and low‐dose erythromycin study trial. Annals of the American Thoracic Society 2016;13(10):1697‐703. [PUBMED: 27464029] [DOI] [PubMed] [Google Scholar]
- Chen AC, Martin MM, Burr L, Hasnain SZ, Lourie R, Bowler SD, et al. Clinical benefits of long‐term, low‐dose erythromycin in bronchiectasis are not due to anti‐inflammatory effects. American Journal of Respiratory and Critical Care Medicine. 2013; Vol. 187:A5970.
- Rogers GB, Bruce KD, Martin ML, Burr LD, Serisier DJ. Corrections. The effect of long‐term macrolide treatment on respiratory microbiota composition in non‐cystic fibrosis bronchiectasis: an analysis from the randomised, double‐blind, placebo‐controlled BLESS trial. Lancet Respiratory Medicine 2015; Vol. 3, issue 4:e15. [PUBMED: 25890660] [DOI] [PubMed]
- Rogers GB, Bruce KD, Martin ML, Burr LD, Serisier DJ. The effect of long‐term macrolide treatment on respiratory microbiota composition in non‐cystic fibrosis bronchiectasis: an analysis from the randomised, double‐blind, placebo‐controlled BLESS trial. Lancet Respiratory Medicine 2014;2(12):988‐96. [PUBMED: 25458200] [DOI] [PubMed] [Google Scholar]
- Serisier DJ, Bowler SD, McGuckin M, Chen A, Lourie A, Martin ML. Effect of long‐term, low‐dose erythromycin on pulmonary exacerbations among patients with non‐cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. American Journal of Respiratory and Critical Care Medicine. 2012; Vol. 185:A6862. [DOI] [PubMed]
- Serisier DJ, Martin ML, McGuckin MA, Lourie R, Chen AC, Brain B, et al. Effect of long‐term, low‐dose erythromycin on pulmonary exacerbations among patients with non‐cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA 2013;309(12):1260‐7. [PUBMED: 23532242] [DOI] [PubMed] [Google Scholar]
Valery 2013 {published data only}
- Hare KM, Grimwood K, Chang AB, Chatfield MD, Valery PC, Leach AJ, et al. Nasopharyngeal carriage and macrolide resistance in indigenous children with bronchiectasis randomized to long‐term azithromycin or placebo. European Journal of Clinical Microbiology and Infectious Diseases 2015;34(11):2275‐85. [PUBMED: 26363637] [DOI] [PubMed] [Google Scholar]
- Singleton R, Morris P, Leach A, Roseby R, White A, Valery P, et al. [Multicentre bronchiectasis study: an international observational and interventional study of bronchiectasis in indigenous children]. Respirology. 2008; Vol. 13(Suppl 2):A19.
- Singleton R, Morris P, Leach A, Roseby R, White A, Valery PC, et al. BIS ‐ Multi‐centre bronchiectasis study: a collaborative and international study of bronchiectasis in indigenous children. Respirology 2007; Vol. 12, issue 4:A‐192.
- Valery PC, Morris PS, Byrnes CA, Grimwood K, Torzillo PJ, Bauert PA, et al. Long‐term azithromycin for indigenous children with non‐cystic‐fibrosis bronchiectasis or chronic suppurative lung disease (Bronchiectasis Intervention Study): a multicentre, double‐blind, randomised controlled trial. Lancet Respiratory Medicine 2013;1(8):610‐20. [PUBMED: 24461664] [DOI] [PubMed] [Google Scholar]
Wong 2012 {published data only}
- Wong C, Jayaram L, Karalus N, Eaton T, Tong C, Hockey H, et al. Azithromycin for prevention of exacerbations in non‐cystic fibrosis bronchiectasis (EMBRACE): a randomised, double‐blind, placebo‐controlled trial. Lancet 2012;380(9842):660‐7. [PUBMED: 22901887] [DOI] [PubMed] [Google Scholar]
- Wong CA, Jayaram L, Karalus N, Eaton T, Tong C, Hockey H, et al. Azithromycin decreases exacerbations in non‐cystic fibrosis bronchiectasis. American Journal of Respiratory and Critical Care Medicine. 2012a; Vol. 185:A3657.
Yalcin 2006 {published data only}
- Yalcin E, Kiper N, Ozcelik U, Dogru D, Firat P, Sahin A, et al. Effects of clarithromycin on inflammatory parameters and clinical conditions in children with bronchiectasis. Journal of Clinical Pharmacy and Therapeutics 2006;31(1):49‐55. [PUBMED: 16476120] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chang 2013 {published data only}
- Chang AB, Grimwood K, Wilson AC, Asperen PP, Byrnes CA, O'Grady KA, et al. Bronchiectasis exacerbation study on azithromycin and amoxicillin‐clavulanate for respiratory exacerbations in children (BEST‐2): study protocol for a randomized controlled trial. Trials 2013;14(53):14‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kudo 1988 {published data only}
- Kudo K, Komase Y, Kowada A, Kabe J. Preclinical and clinical studies on TE‐031 (A‐56268) in treatment of bacterial respiratory tract infections. Chemotherapy 1988;36(Suppl 3):617‐22. [Google Scholar]
Min 1988 {published data only}
- Min KY, Kuriyama T, Fukuday Y. Clinical evaluation of TE‐031 in the chronic respiratory infections [Japanese]. Japanese Pharmacology and Therapeutics 1988;16(7):3027‐39. [Google Scholar]
Ming 2005 {published data only}
- Ming O, Yong L, Zhang W. Efficacy of macrolide and theophylline in the management of bronchiectasis. Respirology 2005;10:A168. [Google Scholar]
Rikitomi 1988 {published data only}
- Rikitomi N, Shishido H, Nagatake T, Mbaki U, Matsumoto K. Preclinical and clinical studies on TE‐031 (A‐56268) in treatment of bacterial respiratory tract infections. Chemotherapy 1988;36(Suppl 3):715‐28. [Google Scholar]
Saito 1988 {published data only}
- Saito A, Shimada J, Ohmori M, Shiba K, Yamaji K, Hojo T, et al. Clinical studies of TE‐031 (A‐56268). Chemotherapy 1988;38:576‐85. [Google Scholar]
Tagaya 2002 {published data only}
- Tagaya E, Tamaoki J, Kondo M, Nagai A. Effect of a short course of clarithromycin therapy on sputum production in patients with chronic airway hypersecretion. Chest 2002;122(1):213‐8. [PUBMED: 12114361] [DOI] [PubMed] [Google Scholar]
Unoura 1986 {published data only}
- Unoura T, Masuda M, Takeuchi K, Itoh T, Tamura M, Yuki T, et al. Clinical study on TE‐031(A‐56268) against respiratory infections. Physicians' Therapy Manual 1988;36:544‐8. [Google Scholar]
References to studies awaiting assessment
Tsang 1999 {published data only}
- Tsang KW, Ho PI, Chan KN, Ip MS, Lam WK, Ho CS, et al. A pilot study of low‐dose erythromycin in bronchiectasis. European Respiratory Journal 1999;13(2):361‐4. [PUBMED: 10065682] [DOI] [PubMed] [Google Scholar]
- Tsang KW, Ho PL, Chan KN, Ip M, Lam WK, Lam B, et al. Erythromycin (EM) reduces sputum volume and improves lung functions in bronchiectasis. American Thoracic Society International Conference; 1998 April 24‐29; Chicago. 1998; Vol. A58:A174.
Additional references
Albert 2011
- Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JAD, Criner GJ, et al. Azithromycin for prevention of exacerbations of COPD. New England Journal of Medicine 2011, 2011;365(8):689‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aliberti 2016
- Aliberti S, Masefield S, Polverino E, Soyza A, Loebinger MR, Menendez R, et al. Research priorities in bronchiectasis: a consensus statement from the EMBARC Clinical Research Collaboration. European Respiratory Journal 2016;48(3):632‐47. [DOI] [PubMed] [Google Scholar]
Amsden 2005
- Amsden GW. Anti‐inflammatory effects of macrolides ‐ an underappreciated benefit in the treatment of community‐acquired respiratory tract infections and chronic inflammatory pulmonary conditions?. Journal of Antimicrobial Chemotherapy 2005;55(1):10‐21. [DOI] [PubMed] [Google Scholar]
Brodt 2014
- Brodt AM, Stovold E, Zhang L. Inhaled antibiotics for stable non‐cystic fibrosis bronchiectasis: a systematic review. European Respiratory Journal 2014;44(2):382‐93. [DOI] [PubMed] [Google Scholar]
Chalmers 2012
- Chalmers JD, Smith MP, McHugh BJ, Doherty C, Govan JR, Hill AT. Short‐ and long‐term antibiotic treatment reduces airway and systemic inflammation in non–cystic fibrosis bronchiectasis. American Journal of Respiratory and Critical Care Medicine 2012;186(7):657‐65. [DOI] [PubMed] [Google Scholar]
Chalmers 2014
- Chalmers JD, Goeminne P, Aliberti S, Melissa J, McDonnell MJ, Lonni S, et al. The Bronchiectasis Severity Index: an international derivation and validation study. American Journal of Respiratory and Critical Care Medicine 2014;189(5):576‐85. [DOI: 10.1164/rccm.201309-1575OC] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2002
- Chang AB, Grimwood K, Mulholland EK, Torzillo PJ. Bronchiectasis in indigenous children in remote Australian communities. Medical Journal of Australia 2002;177(4):200‐4. [PUBMED: 12175325] [DOI] [PubMed] [Google Scholar]
Chang 2010
- Chang AB, Bell SC, Byrnes CA, Grimwood K, Holmes P, King PT, et al. Chronic suppurative lung disease and bronchiectasis in children and adults in Australia and New Zealand: a position statement from the Thoracic Society of Australia and New Zealand and the Australian Lung Foundation. Medical Journal of Australia 2010;193(6):356‐65. [DOI] [PubMed] [Google Scholar]
European Lung White Book 2013
Finch 2015
- Finch S, McDonnell MJ, Abo‐Leyah H, Aliberti S, Chalmers JD. A comprehensive analysis of the impact of Pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Annals of the American Thoracic Society 2015;12(11):1602‐11. [DOI] [PubMed] [Google Scholar]
Gao 2014
- Gao Y‐H, Guan W‐J, Xu G, Tang Y, Gao Y, Lin Z‐y, et al. Macrolide therapy in adults and children with non‐cystic fibrosis bronchiectasis: a systematic review and meta‐analysis. PLoS One 9;3:e90047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goeminne 2016
- Goeminne PC, Soyza A. Bronchiectasis: how to be an orphan with many parents?. European Respiratory Journal 2016;47(1):10‐3. [DOI] [PubMed] [Google Scholar]
GRADEproGDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEproGDT. Version accessed 14 July 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Habesoglu 2011
- Habesoglu MA, Ugurlu AO, Eyuboglu FO. Clinical, radiologic, and functional evaluation of 304 patients with bronchiectasis. Annals of Thoracic Medicine 2011;6(3):131‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansen 2015
- Hansen MP, Thorning S, Aronson JK, Beller EM, Glasziou PP, Hoffmann TC, et al. Adverse events in patients taking macrolide antibiotics versus placebo for any indication. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD011825] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haworth 2014
- Haworth CS, Bilton D, Elborn JS. Long term macrolide maintenance therapy in non‐CF bronchiectasis: evidence and questions. Respiratory Medicine 2014;108(1):1397‐408. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hnin 2015
- Hnin K, Nguyen C, Carson KV, Evans DJ, Greenstone M, Smith BJ. Prolonged antibiotics for non‐cystic fibrosis bronchiectasis in children and adults. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD001392.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Joish 2013
- Joish VN, Spilsbury‐Cantalupo M, Operschall E, Luong B, Boklage S. Economic burden of non‐cystic fibrosis bronchiectasis in the first year after diagnosis from a US health plan perspective. Applied Health Economics and Health Policy 2013;11(3):299‐304. [DOI] [PubMed] [Google Scholar]
Kapur 2012
- Kapur N, Masters IB, Newcombe P, Chang AB. The burden of disease in pediatric non‐cystic fibrosis bronchiectasis. Chest 2012;141(4):1018‐24. [PUBMED: 21885727] [DOI] [PubMed] [Google Scholar]
Kohler 2010
- Kohler T, Perron GG, Buckling A, Delden C. Quorum sensing inhibition selects for virulence and co‐operation in Pseudomonas aeruginosa. PLoS Pathogens 2010;6(5):e1000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kwak 2010
- Kwak HJ, Moon JY, Choi YW, Kim TH, Sohn JW, Yoon HJ, et al. High prevalence of bronchiectasis in adults: analysis of CT findings in a health screening program. Tohoku Journal of Experimental Medicine 2010;222(4):237‐42. [DOI] [PubMed] [Google Scholar]
Leclercq 2002
- Leclercq R. Mechanisms of resistance to macrolides and lincosamides: nature of the resistance elements and their clinical implications. Clinical Infectious Diseases 2002;34(4):482‐92. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Martínez‐García 2007
- Martínez‐García MA, Soler‐Cataluña JJ, Perpiñá‐Tordera M, Román‐Sánchez P, Soriano J. Factors associated with lung function decline in adult patients with stable non‐cystic fibrosis bronchiectasis. Chest 2007;132(5):1565‐72. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pasteur 2010
- Pasteur MC, Bilton D, Hill AT, British Thoracic Society Bronchiectasis (non‐CF) Guideline Group. British Thoracic Society Guidelines for Non‐CF Bronchiectasis. Thorax 2010;65(Suppl 1):i1‐58. [DOI] [PubMed] [Google Scholar]
Quint 2016
- Quint JK, Millett ERC, Joshi M, Navaratnam V, Thomas SL, Hurst JR, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population‐based cohort study. European Respiratory Journal 2016;47(1):186‐93. [DOI: 10.1183/13993003.01033-2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ray 2012
- Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. New England Journal of Medicine 2012;366:1881‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ringshausen 2015
- Ringshausen FC, Roux A, Diel R, Hohmann D, Welte T, Rademacher J. Bronchiectasis in Germany: a population‐based estimation of disease prevalence. European Respiratory Journal 2015;46(6):1805‐7. [PUBMED: 26293498] [DOI] [PubMed] [Google Scholar]
Roberts 2010
- Roberts HJ, Hubbard R. Trends in bronchiectasis mortality in England and Wales. Respiratory Medicine 2010;104:981‐5. [DOI] [PubMed] [Google Scholar]
Saiman 2003
- Saiman L, Marshall BC, Mayer‐Hamblett N, Burns JL, Quittner AL, Cibene DA, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 2003;290(13):1749‐56. [DOI] [PubMed] [Google Scholar]
Seitz 2010
- Seitz AE, Olivier KN, Steiner CA, Montes de Oca R, Holland SM, Prevots DR. Trends and burden of bronchiectasis‐associated hospitalizations in the United States. Chest 2010;138:944‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seitz 2012
- Seitz AE, Olivier KN, Adjemian J, Holland SM, Prevots DR. Trends in bronchiectasis among Medicare beneficiaries in the United States, 2000‐2007. Chest 2012;142(2):432‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Serisier 2013a
- Serisier DJ. Risk of population antimicrobial resistance associated with chronic macrolide use for inflammatory airway diseases. Lancet Respiratory Medicine 2013;1(3):262‐74. [DOI] [PubMed] [Google Scholar]
Twiss 2005
- Twiss J, Metcalfe R, Edwards E, Byrnes C. New Zealand national incidence of bronchiectasis "too high" for a developed country. Archives of Disease in Childhood 2005;90(7):737‐40. [PUBMED: 15871981] [DOI] [PMC free article] [PubMed] [Google Scholar]
Welsh 2015
- Welsh EJ, Evans DJ, Fowler SJ, Spencer S. Interventions for bronchiectasis: an overview of Cochrane systematic reviews. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD010337.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weycker 2005
- Weycker D, Edelsberg J, Oster G, Tino G. Prevalence and economic burden of bronchiectasis. Clinical Pulmonary Medicine 2005;12(4):205‐9. [Google Scholar]
Wurzel 2011
- Wurzel D, Marchant JM, Yerkovich ST, Upham JW, Masters IB, Chang AB. Short courses of antibiotics for children and adults with bronchiectasis. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD008695.pub2] [DOI] [PubMed] [Google Scholar]
Zarogoulidis 2012
- Zarogoulidis P, Papanas N, Kioumis I, Chatzaki E, Maltezos E, Zarogoulidis K. Macrolides from in vitro anti‐inflammatory and immunomodulatory properties to clinical practice in respiratory diseases. European Journal of Clinical Pharmacology 2012;68(5):479‐503. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kelly 2016
- Kelly C, Evans DJ, Chalmers JD, Crossingham I, Spencer S, Relph N, et al. Macrolide antibiotics for non‐cystic fibrosis bronchiectasis. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD012406] [DOI] [PMC free article] [PubMed] [Google Scholar]


