Abstract
PURPOSE
Pegylated recombinant human hyaluronidase (PEGPH20) degrades hyaluronan (HA) and, in combination with chemotherapy, prolongs survival in preclinical models. The activity of PEGPH20 with modified fluorouracil, leucovorin, irinotecan, and oxaliplatin (mFOLFIRINOX) was evaluated in patients with metastatic pancreatic cancer (mPC).
MATERIALS AND METHODS
Patients had untreated mPC, a performance status of 0 to 1, and adequate organ function. Tumor HA status was not required for eligibility. After a phase Ib dose-finding study of mFOLFIRINOX plus PEGPH20, the phase II open-label study randomly assigned patients (1:1) to the combination arm or to mFOLFIRINOX alone (n = 138). The primary end point was overall survival (OS).
RESULTS
PEGPH20 dosages of 3 µg/kg every 2 weeks were more tolerable than twice-weekly dosages used in the phase I study, so 3 µg/kg every 2 weeks was the phase II dosage. An amendment instituted enoxaparin prophylaxis in the PEGPH20 combination arm as a result of increased thromboembolic (TE) events. The planned interim futility analysis when 35 deaths (of 103 analyzable patients) occurred resulted in an OS hazard ratio (HR) of 2.07 that favored the control arm, and the study was closed to accrual. The treatment-related grade 3 to 4 toxicity was significantly increased in the PEGPH20 combination arm relative to control (odds ratio, 2.7; 95% CI, 1.1 to 7.1). The median OS in the mFOLFIRINOX arm was 14.4 months (95% CI, 10.1 to 15.7 months) versus 7.7 months (95% CI, 4.6 to 9.3 months) in the PEGPH20 combination arm.
CONCLUSION
Addition of PEGPH20 to mFOLFIRINOX seems to be detrimental in patients unselected for tumor HA status. This combination caused increased toxicity (mostly GI and TE events) and resulted in decreased treatment duration compared with mFOLFIRINOX alone. The median OS in the mFOLFIRINOX control arm (14.4 months) is, to our knowledge, the longest yet reported and can be considered for patients with good PS.
INTRODUCTION
The regimens of fluorouracil (FU), leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) and of gemcitabine plus nab-paclitaxel (GA) are the optimal choices for patients with metastatic pancreatic cancer (mPC) who have a good performance status (PS). FOLFIRINOX and GA have a median overall survival (OS) of 11.4 and 8.5 months, respectively, compared with 5.9 to 6.4 months with gemcitabine alone.1-3 Despite these recent advances, the outcome remains poor, and fewer than 10% of patients survive 2 years.2,3 A number of new agents are in active investigation, and the tumor stroma has emerged as a key focus of research in pancreatic cancer.4-7 Primary and metastatic lesions are characterized by a dense desmoplastic stroma in which cancer cells are sparsely scattered.8 Hyaluronic acid (hyaluronan, or HA) is a major component of this extracellular matrix. Preclinical studies have shown that high levels of HA in the extracellular matrix promote tumorigenesis.7-9 Unmodified hyaluronidase has been used clinically, but a short half-life limits cancer applications. The pegylated form of human recombinant hyaluronidase (PEGPH20) has superior pharmacologic characteristics and, in preclinical models, depletes HA in the cancer extracellula matrix.9,10 A phase I study of PEGPH20 in solid tumors established the tolerable dosage as 3 µg/kg given intravenously (IV) twice per week. In patients with pre- and post-dose tumor biopsies (n = 6), a decrease in tumor HA levels was observed, and Dynamic Contrast Enhanced magnetic resonance imaging (n = 11) showed changes in tumor perfusion consistent with the postulated mechanism of action.11 Studies in genetically engineered mouse models of pancreatic cancer sparked interest in evaluation of PEGPH20 in clinical trials for mPC.12,13 Rapid depletion of tumor HA after PEGPH20 administration was seen and resulted in significantly improved survival for the combination of gemcitabine and PEGPH20 compared with gemcitabine alone.12,13 Subsequently, a phase Ib study evaluated gemcitabine and PEGPH20 in patients with mPC (n = 28). The phase II dosage was gemcitabine 1,000 mg/m2 per week with the PEGPH20 dosage of 3.0 µg/kg twice per week for 4 weeks then once per week for 3 weeks. The median progression-free survival (PFS) was 5.0 months, and the median OS was 6.6 months. High HA levels were seen in 35% of 17 tumor samples analyzed using a validated immunohistochemical assay. The response rate (RR) of 67%, the PFS (7.2 months), and the OS (13.0 months) favored the subset of patients with high HA levels (n = 6).14 Subsequently, a randomized phase II study of combined PEGPH20 and GA was initiated (ClinicalTrials.gov identifier: NCT01839487 [Halo-109-202]). In parallel, the Southwest Oncology Group initiated a randomized, phase Ib/II study of PEGPH20 and FOLFIRINOX (ClinicalTrials.gov identifier: NCT01959139 [S1313]). Neither study selected patients according to HA tumor status.
MATERIALS AND METHODS
Eligibility Criteria
Pertinent eligibility criteria are as follows: age 18 to 75 years, Zubrod PS of 0 to 1, and measurable mPC. Adequate organ function was required (normal serum creatinine and bilirubin level). A pretreatment tumor sample was required for registration. Exclusion criteria included the following: prior therapy for mPC, exposure to oxaliplatin or irinotecan at least 3 years before registration, prior abdominal radiation, receipt of warfarin or megestrol acetate, and history of pre-existing carotid artery disease or arterial thromboembolic (TE) events that required intervention or treatment.
Treatments
Each 2-week cycle of mFOLFIRINOX consisted of oxaliplatin 85 mg/m2, leucovorin 400 mg/m2, irinotecan 180 mg/m2, and FU 2,400 mg/m2 (by 46-h IV infusion) given according to published guidelines.3 The FU bolus was omitted (the FOLFIRINOX modification). All patients received pegfilgrastim or filgrastim after disconnection of the FU ambulatory pump. PEGPH20 was provided by Halozyme Pharmaceuticals (San Diego, CA). PEGPH20 was administered IV over 10 minutes, 24 hours before each dose of mFOLFIRINOX. The administration of PEGPH20 required corticosteroid premedication before and after dosing (ie, dexamethasone 8 mg twice per day by mouth or IV on day 1 of dosing, then 4 mg twice per day for the 3 subsequent days) to reduce the incidence of myalgias and arthralgias. The protocol was subsequently amended to require the use of prophylactic enoxaparin 1 mg/kg per day beginning 1 day before treatment with PEGPH20 because of an imbalance in TE events in this and the parallel Halo-109-202 study.
Phase Ib Study Design
The phase I part was a standard 3 plus 3, limited dose de-escalation study to evaluate safety and to determine a recommended phase II dose of PEGPH20. Potential PEGPH20 dose levels in a 2-week cycle included 3 µg/kg on day 1 and day 3 or 4; 3 µg/kg on day 1 only; and 1.6 µg/kg on day 1 only. The study schema and dose-limiting toxicity (DLT) definitions are listed in Appendix Table A1 (online only).
Phase II Study Design
The primary end point of the phase II study was OS in patients treated with PEGPH20 plus mFOLFIRINOX compared with those treated with mFOLFIRINOX alone. We hypothesized a hazard ratio (HR) of 0.67 in a comparison of the two arms, with the assumption that the investigational arm would improve median survival from 10 months with mFOLFIRINOX alone to 15 months in combination. With a one-sided type I error of 10% and 80% power, this design required 138 eligible patients. Secondary objectives were to assess the PFS, the RR, and the toxicity profile. Translational objectives were to correlate cancer antigen 19-9 (CA 19-9) levels, plasma HA levels, and tumor HA levels with clinical outcomes of treated patients. The study did not require stratification or selection on the basis of tumor HA status.
Patients were seen every 2 weeks for a history and physical examination with routine blood tests. The CA19-9 level was measured at baseline and every 4 weeks. Dose reductions for mFOLFIRINOX followed published guidelines.3 The PEGPH20 dose could be reduced to 1.6 µg/kg on day 1 if specific drug-related grade 3 or 4 toxicity occurred (eg, musculoskeletal toxicity). Patients were removed from the study if an arterial TE event or a grade 4 venous TE event occurred during the study or if a patient was receiving enoxaparin at the start of study. Adverse events (AEs) were assessed for severity using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.15 Patients were evaluated for tumor response every 8 weeks by RECIST, version 1.1, criteria.16
Tumoral and Serum HA Studies
A total of 10 mL of whole blood was collected at baseline, 48 hours after the first PEGPH20 dose, before cycle 2, and before cycle 3. Serum HA analysis was performed by Micro Constants (San Diego, CA). Paraffin-embedded tissue or five unstained slides from diagnosis of metastatic tumor were required for tumor HA assay. A Clinical Laboratory Improvement Amendments certified laboratory performed the assays (Ventana Medical Systems, Tucson, AZ), and high HA was defined as extracellular matrix HA tumor staining ≥ 50%).
Random Assignment
In the open-label, phase II study, patients were randomly assigned in a 1:1 manner into the two treatment groups. Assignment used a dynamic balancing algorithm17 with stratification by Zubrod PS (0 v 1).
Statistical Analysis
The primary analysis of OS was conducted in all eligible patients according to a modified intent-to-treat principle. Probabilities of OS and PFS were estimated using the Kaplan-Meier method. Statistical differences in event rates between treatment arms were assessed via stratified Cox regression model. An interim futility analysis of OS was planned when one third of the expected events (approximately 37 deaths) was observed. Evidence to suggest early closure would consist of rejection of the alternative hypothesis (HR of 0.67 for OS) at a one-sided alpha level of .07. RR was compared by treatment arm via χ2 test in patients with measurable disease. The mean number of treatment cycles per patient was compared across treatment arms using a Wilcoxon rank-sum test. Proportions of patients who experienced grades 3 to 5 toxicities were compared by treatment arm using an odds ratio estimated by simple logistic regression. Statistical tests were reported as two sided.
RESULTS
This was a study conducted by the Southwest Oncology Group (SWOG) and National Clinical Trials Network. Participating site institutional review board approval and patient written informed consent were obtained before trial enrollment.
Phase I
The study opened to accrual in January 2014 at limited SWOG institutions. At dose-level 1 (PEGPH20 3 μg/kg on day 1 and day 3 or 4 of each cycle), two DLTs (myalgia and fatigue/mucositis) were observed in the first five patients enrolled. This cohort was closed because of intolerability and dose-level 2 (PEGPH20 at 3 μg/kg on day 1 only, every 2 weeks) accrued six patients. One patient experienced DLTs (AST/ALT elevation, fatigue, dehydration, sepsis), and the phase II dosage was established as PEGPH20 at 3 μg/kg on day 1 with mFOLFIRINOX on days 2 to 4 every 2 weeks.
Study Amendments
The study temporarily closed to accrual in April 2014 after two patients were enrolled in the phase I part, when the parallel Halo-109-202 study was suspended because of the higher-than-expected TE rate in the PEGPH20-and-GA arm. To reduce the risk of TE events, a study amendment (October 2014) required patients to receive aspirin 81 mg/d, and those with a history of TE events or who received warfarin were excluded. After the phase II study was activated in May 2015, a preplanned interim safety analysis after 40 patients were randomly assigned and completed two cycles was performed in March 2016. Imbalance in all-grade TE events was noted in the PEGPH20-plus-mFOLFIRINOX arm compared with the control arm (38% v 11%). There were no arterial TE events. Aspirin was replaced by enoxaparin prophylaxis (1mg/kg subcutaneously per day or equivalent) for the PEGPH20-plus-mFOLFIRINOX arm only. Ongoing toxicity analysis revealed higher-than-expected levels of nausea, vomiting, and diarrhea in the PEGPH20-plus-mFOLFIRINOX arm. An amendment (October 2016) recommended that patients on the mFOLFIRINOX-plus-PEGPH20 arm receive antiemetics suitable for a highly emetogenic regimen, prophylactic use of loperamide per guidelines for the first sign of diarrhea, and a lower threshold grade 2 or greater diarrhea) for dose reduction and delay of chemotherapy. On March 14, 2017, accrual was suspended on the basis of the results of a planned interim futility analysis; the study was permanently closed in July 2017.
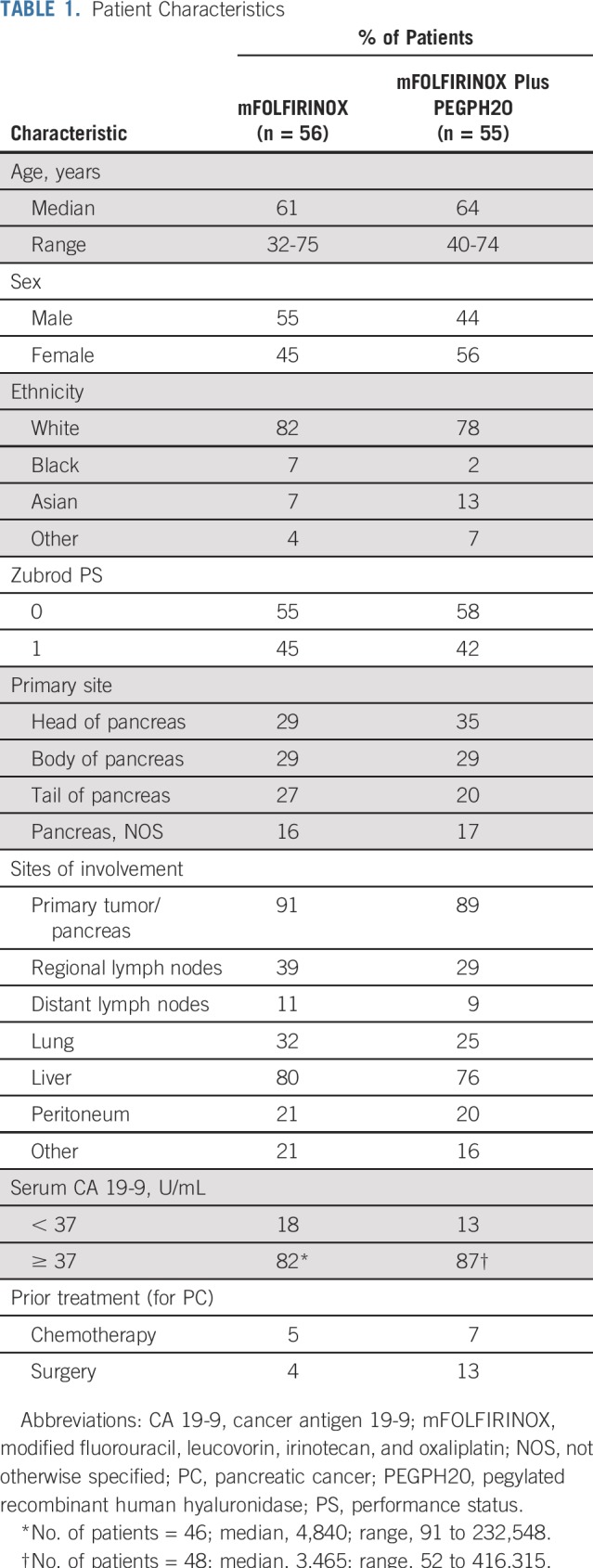
Patient Characteristics
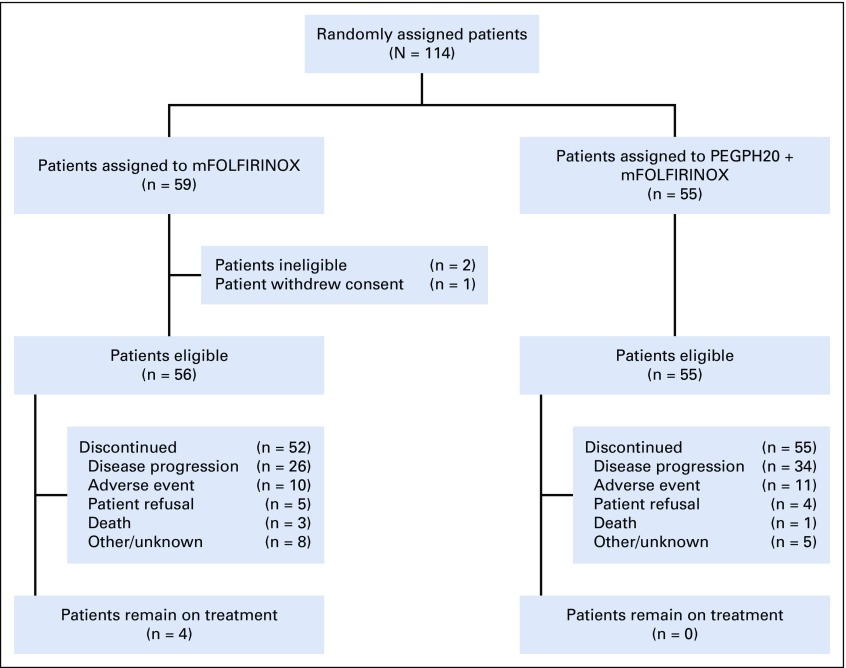
The randomized, phase II study accrued 114 patients from May 2015 to March 2017 at 30 SWOG member institutions. Three patients were excluded from the efficacy analysis: two patients were ineligible, and one withdrew consent (Fig 1). Patient characteristics (n = 111) are listed in Table 1.
FIG 1.
CONSORT flow diagram. mFOLFIRINOX, modified fluorouracil, leucovorin, irinotecan, and oxaliplatin; PEGPH20, pegylated recombinant human hyaluronidase.
TABLE 1.
Patient Characteristics
Safety and Toxicity
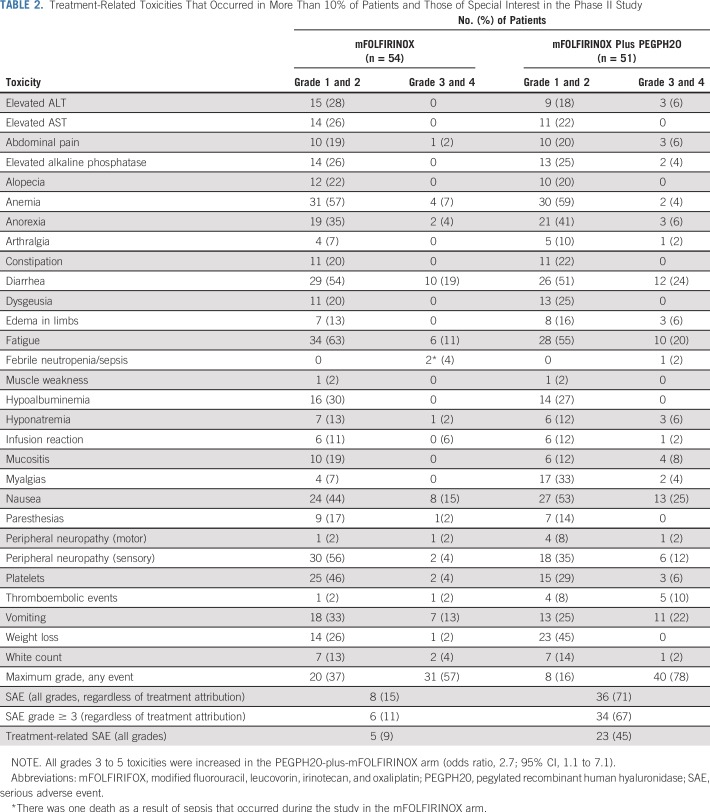
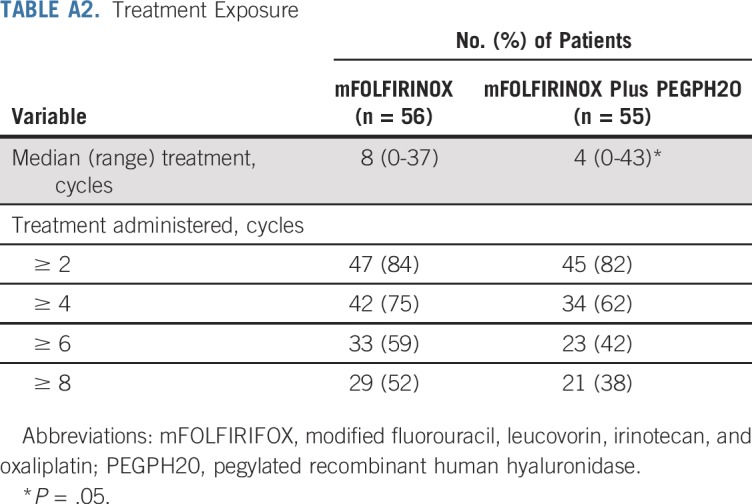
The safety analysis included 105 patients, because six did not receive protocol therapy. In the mFOLFIRINOX arm (n = 54), there was one death as a result of sepsis. Four patients had grade 4 treatment-related AEs: neutropenia, hypokalemia, decreased platelets, and sepsis. An additional 26 patients had grade 3 toxicities (Table 2). Of the 51 patients assessed for AEs on the PEGPH20-plus-mFOLFIRINOX arm, seven patients had grade 4 treatment-related AEs, including hypokalemia, hyperglycemia, infusion-related reaction, decreased lymphocytes, sepsis, TE, and vomiting. Thirty-three additional patients experienced grade 3 AEs, including four TE events and one GI bleed (Table 2). The incidence of TE events in the PEGPH20 arm decreased from 33% to 9% after enoxaparin prophylaxis. The treatment exposure was higher in the mFOLFIRINOX arm compared with the combined arm (Appendix Table A2, online only). Dose delays and severe AEs were all increased in the PEGPH20-plus-mFOLFIRINOX arm compared with the mFOLFIRINOX arm (Table 2).
TABLE 2.
Treatment-Related Toxicities That Occurred in More Than 10% of Patients and Those of Special Interest in the Phase II Study
Efficacy
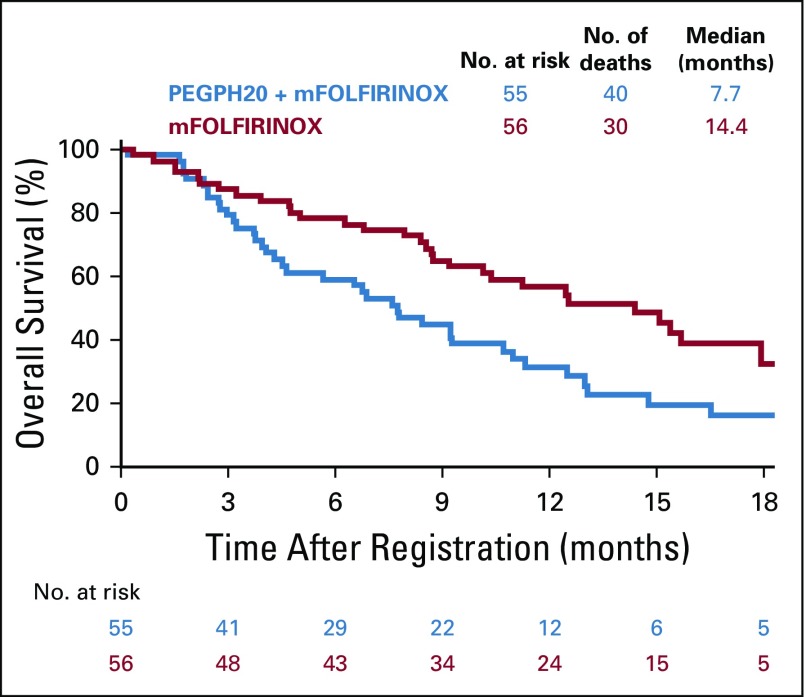
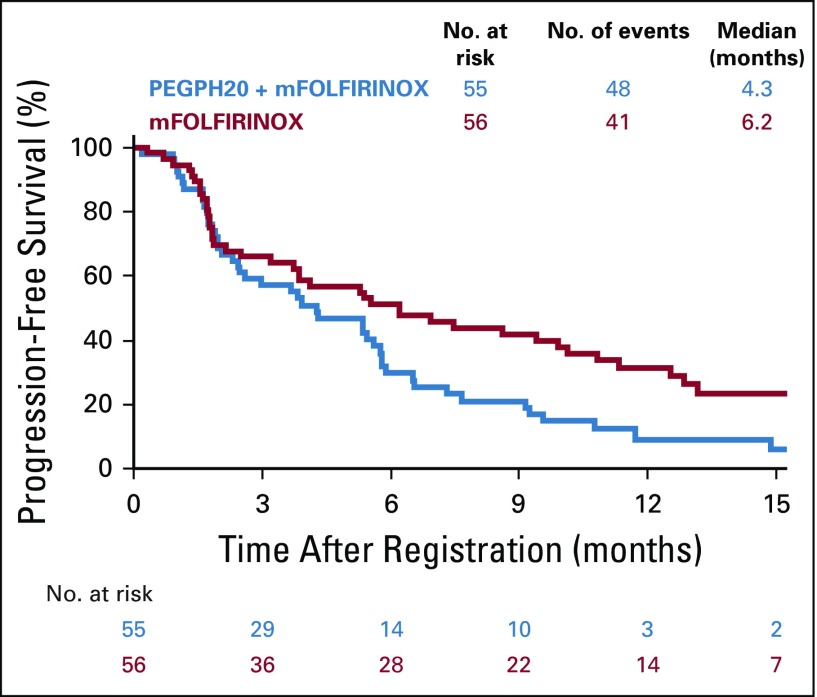
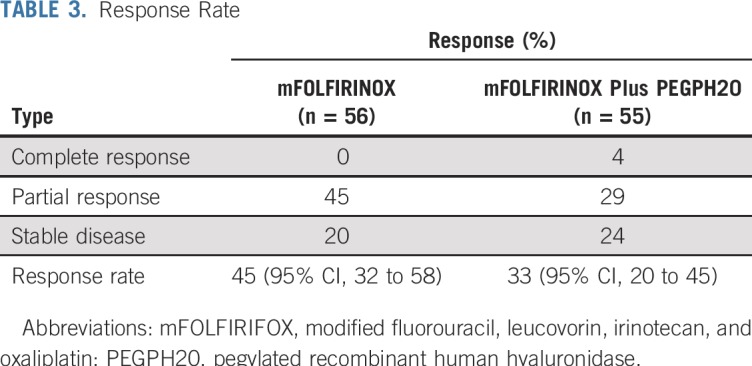
OS was statistically significantly longer in the mFOLFIRINOX arm (n = 56, and 30 deaths) compared with the PEGPH20-plus-mFOLFIRINOX arm (n = 55, and 40 deaths). The median OS durations were 14.4 versus 7.7 months, respectively (HR, 2.07; 95% CI, 1.28 to 3.34; P < .01; Fig 2). Similarly, the median PFS was longer in the control (n = 41 events) versus experimental arm (n = 48 events): 6.2 versus 4.3 months, respectively (HR, 1.74; 95% CI, 1.14 to 2.66; P = .01; Fig 3). The control arm of mFOLFIRINOX had a higher RR of 45% (95% CI, 32% to 58%) than the PEGPH20-plus-mFOLFIRINOX arm RR of 33% (which included two complete responses; 95% CI, 20% to 45%), although this difference did not reach statistical significance (P = .20; Table 3). In the mFOLFIRINOX arm, 73% received second-line therapy: 62% received GA, and a smaller number (11%) continued with a modification of FOLFIRINOX. In the PEGPH20-plus-mFOLFIRINOX arm, 58% received second-line therapy: 47% received GA, and 11% continued with a modification of FOLFIRINOX.
FIG 2.
Overall survival. mFOLFIRINOX, modified fluorouracil, leucovorin, irinotecan, and oxaliplatin; PEGPH20, pegylated recombinant human hyaluronidase.
FIG 3.
Progression-free survival. mFOLFIRINOX, modified fluorouracil, leucovorin, irinotecan, and oxaliplatin; PEGPH20, pegylated recombinant human hyaluronidase.
TABLE 3.
Response Rate
CA 19-9 levels.
The CA 19-9 levels were checked at baseline and every 4 weeks. The maximum percentage decrease in CA 19-9 from baseline was defined as high (≥ 50% decrease) versus low (< 50% decrease). Of the 20 patients on the PEGPH20-plus-mFOLFIRINOX arm with follow-up data, nine (45%) had a 50% or greater decrease in CA 19-9 that was associated with improved RR (P = .04) and PFS (P = .04) but not OS (P = .27). Of the 32 patients on the mFOLFIRINOX arm with follow-up data, 17 (53%) had a 50% or greater decrease in CA 19-9 that was associated with improved RR (P = .04), PFS (P = .01), and OS (P = .07).
Tumoral and Plasma HA
Plasma HA.
Samples were assayed for total HA concentration using a validated method that measures pharmacodynamic response to PEGPH20 (MicroConstants, San Diego, CA) in the PEGPH20 + mFFOX arm only. Baseline circulating HA levels ranged from 29-838 ng/mL (n = 49, median 99). Consistent with previously published results (11), HA levels increased by a median of 33,708 ng/mL (n = 42, range 49-290,896 ng/mL), or over 300-fold at the 48-hour blood draw, demonstrating PEGPH20 activity.
Tumor HA.
Tumor HA analysis and scoring was done with a validated immunohistochemistry test (Ventana Medical Systems, Tucson, AZ), as used in the Halo-109-202 study. This test was Clinical Laboratory Improvement Amendments certified for slides fewer than 90 days old; only 11 specimens in the SWOG biospecimen bank met these requirements, and eight were evaluable (results not shown).
DISCUSSION
The inferior outcomes in all efficacy measures (OS, PFS, and RR) with the addition of PEGPH20 to mFOLFIRINOX was unexpected, and the negative effect on survival (median OS of 7.7 months for the combination arm compared with 14.4 months for the mFOLFIRINOX-alone arm) is striking. The observed rate of expected AEs for the control arm of mFOLFIRINOX, such as nausea, diarrhea, neuropathy, fatigue, and neutropenic fever, was remarkably low compared with published data and was partly explained by the mFOLFIRINOX regimen and growth factor support.3 In contrast, the addition of PEGPH20 to mFOLFIRINOX significantly increased GI toxicity and TE events. Enoxaparin prophylaxis was required to reduce TE events comparable to control arm occurrences. Because of toxicity, patients in the combination arm had increased dose delays, dose reductions, and reduced drug exposure of FOLFIRINOX. It is likely that lower overall drug exposure in patients who received the combination (median, four cycles v eight for mFOLFIRINOX alone) contributed to inferior survival. Another hypothesis is that PEGPH20 combined with the mFOLFIRINOX backbone had an unexpected negative effect on the tumor microenvironment or an adverse drug interaction with one or more of the FOLFIRINOX components, such as granulocyte colony-stimulating growth factor. Despite the incomplete tumor HA analysis because a majority of slides were more than 90 days old, it is unlikely, but not definitive that the median OS of patients with high-HA tumors (percentage not determined) treated in the combination arm would equal or exceed the 14.4 months seen in the mFOLFIRINOX-alone arm.
In contrast to this study (ie, S1313), the recently published, randomized Halo-109-202 study shows promise with the addition of PEGPH20 to GA.18 Similar to the S1313 study, TE events increased with the addition of PEGPH20, and patients required enoxaparin. In our study, we also determined that enoxaparin was necessary, because low-dose aspirin (in the first 40 randomly assigned patients) did not completely eliminate TEs. The AE profile of the PEGPH20-plus-GA arm was acceptable. The tumor high-HA rate was 34%. In this subset of high-HA tumors, efficacy parameters favored the addition of PEGPH20; the RR was 45% versus 31%, the median PFS was 9.2 versus. 5.2 months (HR, 0.51; 95% CI, 0.26 to 1.00; P = .048), and the median OS was 11.5 versus 8.5 months (HR, 0.96; 95% CI, 0.57 to 1.61). There are several important differences between S1313 and Halo-109-202. PEGPH20 (3 ug/kg) could be administered on a twice-a-week schedule with weekly GA (days 1, 8, and 15); thus, patients received six doses in the first 4 weeks compared with only three doses of mFOLFIRINOX (days 1, 14 and 28). In the phase IB part of S1313, twice-a-week administration of PEGPH20 (six doses in the first 4 weeks) with mFOLFIRINOX was toxic and caused enhanced fatigue and general intolerance, so it required reduction in the frequency of dosing. Other differences between Halo-109-202 and S1313 are accrual (279 v 114), end points (PFS v OS), HA tumor analysis (246 v 11), and interim analysis (no v yes). Halo-109-301 is a multinational, double-blind, phase III, randomized study to evaluate PEGPH20 with GA in high-HA tumors only (ClinicalTrials.gov identifier: NCT02715804). The study is ongoing at this time and is without any safety issues reported by the sponsor.
The control arm of mFOLFIRINOX was remarkably well tolerated and resulted in a median OS of 14.4 months, which is—to our knowledge—the highest reported for this regimen. Because of concerns of toxicity, many oncologists reduce the dose of one or more of the components (FU, oxaliplatin, or irinotecan) by 15% to 25%.19-22 We chose to modify the regimen by omitting bolus FU (which is a common modification of the FOLFOX regimen) without any other modifications, and we opted to give prophylactic growth factor support. On the basis of the results of S1313, mFOLFIRINOX can be considered for patients with good PS. The use of prophylactic granulocyte growth factor support with cycle 1 may not be needed in all cases and can be given per institutional guidelines.23 TE events should be identified early and appropriately treated. There was one death as a result of sepsis in the mFOLFIRINOX arm, so oncologists are cautioned about the need for careful selection and monitoring of patients with mPC during therapy.
Stromal targeting agents should be evaluated cautiously given the negative results of Hedgehog inhibitors (vismodegib and saredigib) when combined with gemcitabine in phase II, randomized studies.24,25 In summary, the results of S1313 highlight the importance of preclinical research when multiple agents are evaluated as well as the importance of robust clinical trial design and conduct with appropriate safety reviews. With close monitoring of toxicities as they emerged and appropriate study interim analyses, we stopped the study early for inferior survival in the investigational arm. We expect that other ongoing clinical and preclinical studies will give additional insights.
ACKNOWLEDGMENT
We thank all patients and families for participating in this study, and we thank the staff at Southwest Oncology Group and investigators/staff at member institutions for their assistance.
Appendix
TABLE A1.
Dose-Limiting Toxicity and Study Schema
TABLE A2.
Treatment Exposure
Footnotes
Presented in part at the Gastrointestinal Cancers Symposium, San Francisco, CA, January 18-20, 2018.
Supported by National Institutes of Health/National Cancer Institute Grants No. CA180888, CA180819, CA180826, CA180828, CA180835, CA189830, CA189853, CA189953, CA180830, CA189848, CA189858, CA180846, CA13612, and CA46368; and in part by Halozyme (drug supply only).
Clinical trial information: NCT01959139.
See accompanying Editorial on page 1041
AUTHOR CONTRIBUTIONS
Conception and design: Ramesh K. Ramanathan, Shannon L. McDonough, Philip A. Philip, Sunil R. Hingorani, Katherine A. Guthrie, Howard S. Hochster
Collection and assembly of data: Ramesh K. Ramanathan, Shannon L. McDonough, Philip A. Philip, Jill Lacy, E. Gabriela Chiorean, Deepti Behl, Rakesh Gaur, Katherine A. Guthrie
Data analysis and interpretation: Ramesh K. Ramanathan, Shannon L. McDonough, Philip A. Philip, Jill Lacy, Jeremy S. Kortmansky, Jaykumar Thumar, E. Gabriela Chiorean, Anthony F. Shields, Paul T. Mehan, Rakesh Gaur, Tara Seery, Katherine A. Guthrie
Provision of study materials or patients: Ramesh K. Ramanathan, Philip A. Philip, Jill Lacy, Jeremy S. Kortmansky, E. Gabriela Chiorean, Anthony F. Shields, Howard S. Hochster
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST AND DATA AVAILABILITY STATEMENT
Phase IB/II Randomized Study of FOLFIRINOX Plus Pegylated Recombinant Human Hyaluronidase Versus FOLFIRINOX Alone in Patients With Metastatic Pancreatic Adenocarcinoma: SWOG S1313
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Ramesh K. Ramanathan
Honoraria: Pharmacyclics, Insys Therapeutics, Novocure
Employment: Merck
Consulting or Advisory Role: Cerulean Pharma, Novocure, Insys Therapeutics, Pharmacyclics
Research Funding: AbbVie (Inst), Celgene (Inst), Merck (Inst), Schering-Plough (Inst), Merrimack (Inst), Boston Biomedical (Inst), Berg Pharma (Inst), Superlab Far East (Inst), Ipsen (Inst)
Philip A. Philip
Honoraria: Celgene, Amgen, Sanofi, Bayer, Bristol-Myers Squibb, Novartis, Ipsen, Halozyme, Rafael Pharmaceuticals, Merck, Aslan Pharmaceuticals, Lexi, Biologics
Consulting or Advisory Role: Celgene, Novartis, Halozyme, Merrimack, Ipsen, Merck, Rafael Pharmaceuticals
Speakers' Bureau: Celgene, Bayer, Ipsen, Merck, Bristol-Myers Squibb
Research Funding: Bayer (Inst), Incyte (Inst), Karyopharm Therapeutics (Inst), Merck (Inst), Taiho Pharmaceutical (Inst), Momenta Pharmaceuticals (Inst), Novartis (Inst), Plexxikon (Inst), Immunomedics (Inst), Regeneron (Inst), Genentech (Inst), Tyme (Inst), Caris Life Sciences (Inst), Aslan Pharmaceuticals (Inst), QED (Inst), Halozyme (Inst), Boston Biomedical (Inst), Advanced Accelerator Applications (Inst), Lilly (Inst)
Other Relationship: Halozyme
Sunil R. Hingorani
Consulting or Advisory Role: Halozyme, Aduro Biotech
Research Funding: Halozyme (Inst)
Jill Lacy
Consulting or Advisory Role: Sirtex Medical, Celgene, KeyQuest Health, Navigant Consulting, AstraZeneca
Travel, Accommodations, Expenses: Lilly
E. Gabriela Chiorean
Consulting or Advisory Role: Celgene, Five Prime Therapeutics, Halozyme, Vicus Therapeutics, Genentech, Roche, Ipsen, AstraZeneca, Eisai, Seattle Genetics, Array Biopharma.
Research Funding: Celgene (Inst), Incyte (Inst), Stemline Therapeutics (Inst), Ignyta (Inst), Boehringer Ingelheim (Inst), Lilly (Inst), Merck (Inst)
Travel, Accommodations, Expenses: Genentech, Roche, Celgene, AstraZeneca
Anthony F. Shields
Consulting or Advisory Role: GE Healthcare, Endocyte, Bayer, Bristol-Myers Squibb, Siemens Healthcare Diagnostics, Merck, Merrimack, Taiho Pharmaceutical, Personal Genome Diagnostics
Speakers' Bureau: GE Healthcare, Karyopharm Therapeutics, Merrimack, Taiho Pharmaceutical, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, OncoMed, Plexxikon, Novartis, Eisai, Inovio Pharmaceuticals
Research Funding: H3 Biomedicine, Caris Life Sciences
Travel, Accommodations, Expenses: GE Healthcare, Merrimack, Endocyte, Caris Life Sciences
Deepti Behl
Consulting or Advisory Role: Novartis, Boehringer Ingelheim, Genentech
Speakers' Bureau: Bristol-Myers Squibb, Merck, Novartis
Rakesh Gaur
Research Funding: Amgen (Inst), Celgene (Inst), Bristol-Myers Squibb (Inst), Cyclacel (Inst)
Tara Seery
Employment: Chan Soon Shiong Institute for Medicine
Consulting or Advisory Role: Bayer
Speakers' Bureau: Bayer, Ipsen
Howard S. Hochster
Consulting or Advisory Role: Bayer, Genentech, Amgen, Exelixis
No other potential conflicts of interest were reported.
REFERENCES
- 1.Sohal DP, Mangu PB, Khorana AA, et al. Metastatic pancreatic cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34:2784–2796. doi: 10.1200/JCO.2016.67.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 4.Krantz BA, O’Reilly EM. Biomarker-based therapy in pancreatic ductal adenocarcinoma: An emerging reality? Clin Cancer Res. 2018;24:2241–2250. doi: 10.1158/1078-0432.CCR-16-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tesfaye AA, Kamgar M, Azmi A, et al. The evolution into personalized therapies in pancreatic ductal adenocarcinoma: Challenges and opportunities. Expert Rev Anticancer Ther. 2018;18:131–148. doi: 10.1080/14737140.2018.1417844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahn DH, Ramanathan RK. Targeting the stroma in pancreatic cancer. Linchuang Zhongliuxue Zazhi. 2017;6:65. doi: 10.21037/cco.2017.11.02. [DOI] [PubMed] [Google Scholar]
- 7.Vennin C, Murphy KJ, Morton JP, et al. Reshaping the tumor stroma for treatment of pancreatic cancer. Gastroenterology. 2018;154:820–838. doi: 10.1053/j.gastro.2017.11.280. [DOI] [PubMed] [Google Scholar]
- 8.Whatcott CJ, Diep CH, Jiang P, et al. Desmoplasia in Primary tumors and metastatic lesions of pancreatic cancer. Clin Cancer Res. 2015;21:3561–3568. doi: 10.1158/1078-0432.CCR-14-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong KM, Horton KJ, Coveler AL, et al. Targeting the tumor stroma: The biology and clinical development of pegylated recombinant human hyaluronidase (PEGPH20) Curr Oncol Rep. 2017;19:47. doi: 10.1007/s11912-017-0608-3. [DOI] [PubMed] [Google Scholar]
- 10.Thompson CB, Shepard HM, O’Connor PM, et al. Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol Cancer Ther. 2010;9:3052–3064. doi: 10.1158/1535-7163.MCT-10-0470. [DOI] [PubMed] [Google Scholar]
- 11.Infante JR, Korn RL, Rosen LS, et al. Phase 1 trials of PEGylated recombinant human hyaluronidase PH20 in patients with advanced solid tumours. Br J Cancer. 2018;118:153–161. doi: 10.1038/bjc.2017.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobetz MA, Chan DS, Neesse A, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–120. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hingorani SR, Harris WP, Beck JT, et al. Phase Ib study of PEGylated recombinant human hyaluronidase and gemcitabine in patients with advanced pancreatic cancer. Clin Cancer Res. 2016;22:2848–2854. doi: 10.1158/1078-0432.CCR-15-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Cancer Institute https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm Cancer Therapy Evaluation Program: Common Terminology Criteria for Adverse Events.
- 16.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
- 18.Hingorani SR, Zheng L, Bullock AJ, et al. HALO 202: Randomized phase II study of PEGPH20 plus nab-paclitaxel/gemcitabine versus nab-paclitaxel/gemcitabine in patients with untreated, metastatic pancreatic ductal adenocarcinoma. J Clin Oncol. 2018;36:359–366. doi: 10.1200/JCO.2017.74.9564. [DOI] [PubMed] [Google Scholar]
- 19.Usón PLS, Jr, Rother ET, Maluf FC, et al. Meta-analysis of modified FOLFIRINOX regimens for patients with metastatic pancreatic cancer. Clin Colorectal Cancer. 2018;17:187–197. doi: 10.1016/j.clcc.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Ozaka M, Ishii H, Sato T, et al. A phase II study of modified FOLFIRINOX for chemotherapy-naïve patients with metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2018;81:1017–1023. doi: 10.1007/s00280-018-3577-9. [DOI] [PubMed] [Google Scholar]
- 21.Chllamma MK, Cook N, Dhani NC, et al. FOLFIRINOX for advanced pancreatic cancer: The Princess Margaret Cancer Centre experience. Br J Cancer. 2016;115:649–654. doi: 10.1038/bjc.2016.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahaseth H, Brutcher E, Kauh J, et al. Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas. 2013;42:1311–1315. doi: 10.1097/MPA.0b013e31829e2006. [DOI] [PubMed] [Google Scholar]
- 23.Smith TJ, Bohlke K, Lyman GH, et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2015;33:3199–3212. doi: 10.1200/JCO.2015.62.3488. [DOI] [PubMed] [Google Scholar]
- 24.Catenacci DV, Junttila MR, Karrison T, et al. Randomized phase Ib/II study of gemcitabine plus placebo or vismodegib, a Hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J Clin Oncol. 2015;33:4284–4292. doi: 10.1200/JCO.2015.62.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The Olive Laboratory https://www.olivelab.org/ipi-926-03.html Clinical trial: IPI-926-03.