Abstract
Background
This updated Cochrane Review of reminiscence therapy (RT) for dementia was first published in 1998, and last updated in 2005. RT involves the discussion of memories and past experiences with other people using tangible prompts such as photographs or music to evoke memories and stimulate conversation. RT is implemented widely in a range of settings using a variety of formats.
Objectives
To assess the effects of RT on people living with dementia and their carers, taking into account differences in its implementation, including setting (care home, community) and modality (group, individual).
Search methods
We searched ALOIS (the Cochrane Dementia and Cognitive Improvement Group's Specialized Register) on 6 April 2017 using the search term 'reminiscence.'
Selection criteria
We included all randomised controlled trials of RT for dementia in which the duration of the intervention was at least four weeks (or six sessions) and that had a 'no treatment' or passive control group. Outcomes of interest were quality of life (QoL), cognition, communication, behaviour, mood and carer outcomes.
Data collection and analysis
Two authors (LOP and EF) independently extracted data and assessed risk of bias. Where necessary, we contacted study authors for additional information. We pooled data from all sufficiently similar studies reporting on each outcome. We undertook subgroup analysis by setting (community versus care home) and by modality (individual versus group). We used GRADE methods to assess the overall quality of evidence for each outcome.
Main results
We included 22 studies involving 1972 people with dementia. Meta‐analyses included data from 16 studies (1749 participants). Apart from six studies with risk of selection bias, the overall risk of bias in the studies was low.
Overall, moderate quality evidence indicated RT did not have an important effect on QoL immediately after the intervention period compared with no treatment (standardised mean difference (SMD) 0.11, 95% confidence interval (CI) ‐0.12 to 0.33; I2 = 59%; 8 studies; 1060 participants). Inconsistency between studies mainly related to the study setting. There was probably a slight benefit in favour of RT in care homes post‐treatment (SMD 0.46, 95% CI 0.18 to 0.75; 3 studies; 193 participants), but little or no difference in QoL in community settings (867 participants from five studies).
For cognitive measures, there was high quality evidence for a very small benefit, of doubtful clinical importance, associated with reminiscence at the end of treatment (SMD 0.11, 95% CI 0.00 to 0.23; 14 studies; 1219 participants), but little or no difference at longer‐term follow‐up. There was a probable slight improvement for individual reminiscence and for care homes when analysed separately, but little or no difference for community settings or for group studies. Nine studies included the widely used Mini‐Mental State Examination (MMSE) as a cognitive measure, and, on this scale, there was high quality evidence for an improvement at the end of treatment (mean difference (MD) 1.87 points, 95% CI 0.54 to 3.20; 437 participants). There was a similar effect at longer‐term follow‐up, but the quality of evidence for this analysis was low (1.8 points, 95% CI ‐0.06 to 3.65).
For communication measures, there may have been a benefit of RT at the end of treatment (SMD ‐0.51 points, 95% CI ‐0.97 to ‐0.05; I2 = 62%; negative scores indicated improvement; 6 studies; 249 participants), but there was inconsistency between studies, related to the RT modality. At follow‐up, there was probably a slight benefit of RT (SMD ‐0.49 points, 95% CI ‐0.77 to ‐0.21; 4 studies; 204 participants). Effects were uncertain for individual RT, with very low quality evidence available. For reminiscence groups, evidence of moderate quality indicated a probable slight benefit immediately (SMD ‐0.39, 95% CI ‐0.71 to ‐0.06; 4 studies; 153 participants), and at later follow‐up. Community participants probably benefited at end of treatment and follow‐up. For care home participants, the results were inconsistent between studies and, while there may be an improvement at follow‐up, at the end of treatment the evidence quality was very low and effects were uncertain.
Other outcome domains examined for people with dementia included mood, functioning in daily activities, agitation/irritability and relationship quality. There were no clear effects in these domains. Individual reminiscence was probably associated with a slight benefit on depression scales, although its clinical importance was uncertain (SMD ‐0.41, 95% CI ‐0.76 to ‐0.06; 4 studies; 131 participants). We found no evidence of any harmful effects on people with dementia.
We also looked at outcomes for carers, including stress, mood and quality of relationship with the person with dementia (from the carer's perspective). We found no evidence of effects on carers other than a potential adverse outcome related to carer anxiety at longer‐term follow‐up, based on two studies that had involved the carer jointly in reminiscence groups with people with dementia. The control group carers were probably slightly less anxious (MD 0.56 points, 95% CI ‐0.17 to 1.30; 464 participants), but this result is of uncertain clinical importance, and is also consistent with little or no effect.
Authors' conclusions
The effects of reminiscence interventions are inconsistent, often small in size and can differ considerably across settings and modalities. RT has some positive effects on people with dementia in the domains of QoL, cognition, communication and mood. Care home studies show the widest range of benefits, including QoL, cognition and communication (at follow‐up). Individual RT is associated with probable benefits for cognition and mood. Group RT and a community setting are associated with probable improvements in communication. The wide range of RT interventions across studies makes comparisons and evaluation of relative benefits difficult. Treatment protocols are not described in sufficient detail in many publications. There have been welcome improvements in the quality of research on RT since the previous version of this review, although there still remains a need for more randomised controlled trials following clear, detailed treatment protocols, especially allowing the effects of simple and integrative RT to be compared.
Plain language summary
Reminiscence therapy for dementia
Review question
We wanted to find out what effect reminiscence therapy (RT) has on people with dementia. In particular, we were interested in effects on quality of life, communication, cognition (the general ability to think and remember), mood, daily activities and relationships. We were also interested in any effects on carers.
Background
RT involves discussing events and experiences from the past. It aims to evoke memories, stimulate mental activity and improve well‐being. Reminiscence is often assisted by props such as videos, pictures and objects. It can take place in a group or be done with a person on their own, when it often results in some form of life‐story book being created. RT helps older people with depression. It may be suitable for people with dementia both because depression is common in dementia and because people with dementia typically have a better memory for the distant past than for recent events.
Methods
We searched for randomised, controlled trials in which RT was compared with no treatment or with a non‐specific activity, such as time spent in general conversation. Our search covered all trials available up to April 2017.
Results
We found 22 trials with 1972 participants to include in the review. All the participants had dementia, mostly of mild or moderate severity. Some of the participants were living at home and some were in care homes. The length of the trials varied from four weeks to two years, and the overall amount of time spent on therapy varied from three to 39 hours. Overall, we thought most of the trials were well conducted.
Looking at all the trials together, there did not seem to be an effect of RT on the quality of life reported by the participants. However, there was probably a slight benefit of treatment in the trials done in care homes, which was not seen in the trials done in the community.
People having RT scored slightly better than the control group on tests of cognition immediately after the course of treatment, but not weeks to months later. It was not clear that the effect was large enough to be important. The effect was most evident in care home studies, which used individual RT, but not in community studies, which used group RT.
We found that group RT and RT in community settings may have a positive effect on the communication and interaction of the person with dementia immediately after the end of treatment, and probably also weeks to months later, although the effect was small.
Apart from a probable slight benefit of individual RT on scales measuring depressed mood, we found no evidence for effects of RT on other outcomes, such as agitation, ability to carry out daily activities or relationships with other people. We found no evidence of harmful effects of RT for the people with dementia themselves.
We found no effect of RT on family carers other than a suggestion that it made carers slightly more anxious in two large studies of joint reminiscence work. In this type of RT, the carers and the people with dementia were both directly involved in the reminiscence sessions.
Conclusions
We were encouraged to find that the amount and quality of research on RT for dementia has increased considerably since the last version of this review. We concluded that the effects of RT vary, depending on the way it is given and whether it takes place in care homes or the community. However, there is some evidence that RT can improve quality of life, cognition, communication and possibly mood in people with dementia in some circumstances, although all the benefits were small. More research is needed to understand these differences and to find out who is likely to benefit most from what type of RT.
Summary of findings
Background
Reminiscence therapy (RT) was introduced to dementia care in the late 1970s (Kiernat 1979; Norris 1986), and has taken a variety of forms. At its most basic, it involves the discussion of past activities, events and experiences, usually with the aid of tangible prompts (e.g. photographs, household and other familiar items from the past, music and archive sound recordings). More recently, digital storage and presentation of photographs, music and video clips have become widely used (Subramaniam 2010).
The development of reminiscence work is usually traced to Butler 1963's early work on "Life Review." Butler described Life Review as a naturally occurring process where the person looks back on his/her life and reflects on past experiences, including unresolved difficulties and conflicts. This concept was incorporated in psychotherapy for older people, which emphasises that life review can be helpful in promoting a sense of integrity and adjustment. Butler's seminal work contributed to the change in professional perspectives on reminiscence. Rather than being viewed as a problem, with the older person 'living in the past,' reminiscence was sees as a dynamic process of adjustment. This fitted well with Erikson 1950's late‐life stage of development, where the person is seen as reflecting on life, seeking to make sense and find meaning in a life lived.
Around the same time, increasing interest in oral history meant that the reminiscences of older people were valued more greatly. In the UK, the development of the 'Recall' tape‐slide package (Help the Aged 1981) meant that reminiscence triggers were widely available in day care centres, care homes and hospitals, leading many staff to establish some form of reminiscence work, of variable quality. There was also interest in using reminiscence to guide environmental design on the basis that, for example, a lounge of a care home which resembled a living room from earlier in the person's life would seem more familiar and might lead to better maintenance of independence.
It is evident that reminiscence work may take a number of different forms, from psychotherapy through to environmental redesign. There is an extensive literature on the various functions of reminiscence, with numerous classification systems proposed (e.g. Romaniuk 1981). Differences have emerged between reminiscence functions in their association with mental health, with seeking identity having a positive association and a focus on bitterness, boredom reduction and loss being associated with worse mental health (Ros 2016). In one general systematic review of reminiscence work, across a variety of populations, drawing from over 100 studies, Pinquart 2012 categorised the type of 'therapeutic work' undertaken into three broad categories: 'simple reminiscence,' involving the recall and sharing of selected personal and shared memories and stories; 'life review,' seen as a structured, evaluative process, usually conducted individually, covering the whole life story chronologically, seeking to integrate negative and positive memories; and 'life review therapy,' typically aimed at people with depression or other mental health difficulties where the aim is to re‐evaluate negative memories, promoting a more positive view of life. 'Life story work' is becoming increasingly used to describe aspects of reminiscence work, such as life review, where the emphasis is on developing a narrative biography, drawing together past, present and future. Life story books are common tangible outcomes from such work, but other media have also been used, such as a display box, portraying key elements of the person's life. Life story work has been employed with children and young people, people with learning disabilities and people with depression (Woods 2016). The type of reminiscence work undertaken has important implications for the training, supervision and support needed by those acting as facilitators or therapists.
Reminiscence work, including life review, has consistently been helpful for older people with depressed mood (Bohlmeijer 2003; Pinquart 2007). The effects are comparable to both medication and other psychosocial approaches. Life review may also be helpful in preventing depression in older adults (Pot 2010), and in improving life satisfaction and quality of life (QoL) in older adults in general (Bohlmeijer 2007). The effects are also seen in older people with depressed mood living in long‐term care environments (Zhang 2015). Given that depressed mood is more common in people with dementia, reminiscence work may be helpful in dementia in relation to improving mood.
In the context of dementia, reminiscence work can also be seen to have a cognitive rationale. People with dementia often appear able to recall events from their childhood, but not from more recent times, even earlier the same day. Drawing on the apparently preserved store of remote memories appears a sensible strategy, when dementia is typically accompanied by great difficulty in new learning. By linking with the person's cognitive strengths in this way, communication might be enhanced, allowing the person to talk confidently of their earlier life and experiences. In fact, studies of remote memory suggest that recall for specific events is not relatively preserved; performance across the lifespan is impaired but people with dementia, like all older people, have an 'autobiographical memory bump,' recalling more memories from youth and adolescence (Morris 1994). Some of the memories represent well‐rehearsed, much practised items or anecdotes. The almost complete absence of autobiographical memories from the person's middle years could lead to a disconnection of past and present, which could contribute to the person's difficulty in retaining a clear sense of personal identity. From a cognitive standpoint, autobiographical memory and level of communication appear key outcomes.
Since the first study on reminiscence work that was conducted with a group of older people with dementia was reported by Kiernat 1979, the approach has continued to be implemented widely, in a variety of forms. However, the research literature has developed more slowly. The 2005 version of this review included only four studies, and several of them were of low quality. In a more recent review, Cotelli 2012 also highlighted the absence of high quality studies. Subramaniam 2012 focused on individual reminiscence work in their systematic review, identifying five randomised controlled trials (RCT), mainly with small sample sizes. The distinction between 'simple' reminiscence and 'life review,' often leading to the production of a life story book, appeared salient in these reviews. Simple reminiscence may be on an individual or a group basis, whereas life review is typically conducted individually. The involvement of family carers in reminiscence groups jointly with people with dementia is a further development, using simple reminiscence but potentially having an effect on pre‐existing relationships (Bruce 1998; Thorgrimsen 2002).
The implications of this background for the current review are as follows.
The type of reminiscence work and its aims needs to be clearly defined. In considering reminiscence work with people with dementia, the key distinction is between 'simple' reminiscence work that has a focus on the individual making sense of their own life story, which is described as having an integrative function. This has implications for whether the work is carried out individually or in a group; life review/life story reminiscence is almost always individual, whereas simple reminiscence can be sustained in one‐to‐one settings or in a group. Life story work usually requires memory triggers specific to the person, whereas more general triggers may be sufficient to trigger a broad range of stories and memories, in simple reminiscence.
Different outcome measures may be appropriate according to the type of reminiscence work and its aims. The range of potential aims include: to enhance communication; to increase a sense of personal identity; to have an enjoyable activity in company with others; to improve mood and QoL; to stimulate memories; to increase the individualisation of care; or a combination of these. This list suggests that improvements in general cognition and behaviour might not be the most prominent of the changes expected, except as an indirect consequence of mood change perhaps.
The impact on others, in addition to the person with dementia, may also be important, particularly where family carers are involved in the reminiscence work. For example, Baines 1987 examined staff knowledge of those attending group sessions; this increased in reminiscence groups compared with no treatment. Knowledge regarding the person with dementia is of course a prerequisite for individualised care.
Memories from the person's earlier life will not all be sources of pleasure and happiness; indeed some may be distressing or traumatic. Evaluation of any negative impact of this approach is required to monitor whether the recall of such memories occurs, and, if it does, whether these can be managed safely within the particular therapeutic context.
Objectives
To assess the effects of RT on people living with dementia and their carers, taking into account differences in its implementation, including setting (care home, community) and modality (group, individual).
Methods
Criteria for considering studies for this review
Types of studies
Studies had to meet the following criteria.
RCTs including cluster randomised trials and cross‐over trials that used RT of any type as an intervention for people living with dementia.
Control activity was no treatment, treatment as usual or a passive treatment such as basic social contact.
Study was written in English and published in a peer‐reviewed journal.
Trials that did not publish (or later supply) adequate information about study design and results were included in the review but not in the meta‐analysis. Details are noted in Characteristics of included studies.
Types of participants
We included:
participants were people with a diagnosis of dementia, preferably a formal diagnosis according to the Diagnostic and Statistical Manual of Mental Disorders ‐ fourth edition (DSM‐IV), but other diagnostic criteria were considered and included if appropriate. There were no age limits. The main diagnostic categories were Alzheimer's disease (AD) and vascular dementia (VD). These were combined in the analysis;
all levels of severity. Severity of dementia was determined by group mean scores or score ranges on standardised scales such as the Clinical Dementia Rating (CDR) (Hughes 1982) or Mini‐Mental State Examination (MMSE) (Folstein 1975);
family or professional carers where studies recruited dyads (person with dementia and their carer together);
trials that investigated the effects of RT on different dementia diagnoses by allocating specific control groups for each diagnosis were analysed as separate studies.
We excluded:
participants with mild cognitive impairment (MCI) where the degree of cognitive impairment did not warrant a diagnosis of dementia.
Types of interventions
Studies were considered for this review if they described a reminiscence intervention (including life story work) targeting people living with dementia in any of the outcomes of interest. Outcomes of interest are described under Types of outcome measures.
Studies were included if the planned duration of the intervention was four weeks or longer or if at least six sessions were offered over a shorter time frame. There was no restriction on the maximum number of RT sessions.
Studies were included if a comparison was made to 'no treatment,' 'treatment as usual' or a basic passive control treatment. Passive treatments could consist of, for example, an equivalent number of sessions in which general conversation with participants took place. Comparisons with other activities or therapies such as music therapy were not considered in this review. 'Treatment as usual' was taken to mean standard health care, or activities in accordance with health or social care services' usual provision.
Types of outcome measures
Studies included assessments of any of the outcomes of interest, provided they used standardised measures, rating scales or questionnaires. Studies could have presented data on both outcomes for the person with dementia and carer outcomes.
Outcomes that measured post‐treatment (typically immediately after, or within one month after the intervention), and at follow‐up (typically one to six months' post‐intervention).
Maintaining the effects of the intervention over time was anticipated to be an issue for studies involving people with dementia, therefore, it was expected that post‐treatment data would be captured as close to the final session as possible, to identify immediate outcomes or changes that may have been lost to longer‐term follow‐up.
Attrition and the reasons for participants dropping out were noted.
Outcomes for the person with dementia
Primary outcome
Quality of life.
Secondary outcomes
Cognition.
Communication and interaction.
Quality of relationship with carer
Behaviour, including agitation and activities of daily living (ADL).
Mood‐related outcomes, including apathy, anxiety and depression.
Outcomes of interest for the person with dementia were measured using standardised instruments to determine if changes in these outcomes were observed following the intervention. This included self‐reported ratings, clinical ratings or carer ratings of the outcome.
Outcomes for the carer
'Carer' in these contexts refers to family carers and professional carers, although they were considered separately in the review.
Mood.
Stress/stain related to caring.
Quality of life.
Outcomes relating to the dyadic relationship.
Adverse outcomes
There is a potential risk that the process of recalling memories from the past may bring about difficult or emotional (or both) memories, which should be anticipated and managed sensitively by facilitators. The potential for adverse outcomes was monitored by observing negative responses on the outcome measures. Family carers or care staff hold their own perceptions of the intervention and its effect on the participant, as well as on themselves, which will be reflected in their carer‐rated outcome measures.
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois), the Cochrane Dementia and Cognitive Improvement Group's Specialized Register on 6 April 2017. The search term used was 'reminiscence.'
ALOIS was created in part thanks to a grant from the American Alzheimer's Association and is maintained by the Information Specialists of the Cochrane Dementia and Cognitive Improvement Group. It contains studies in the areas of dementia prevention, dementia treatment and cognitive enhancement in healthy older populations. The studies are identified from:
monthly searches of major healthcare databases: MEDLINE, Embase, CINAHL, PsycINFO and LILACS;
monthly searches of trial registers: ISRCTN; UMIN (Japan's Trial Register); the World Health Organization (WHO) portal (which covers ClinicalTrials.gov; ISRCTN; the Chinese Clinical Trials Register; the German Clinical Trials Register; the Iranian Registry of Clinical Trials and the Netherlands National Trials Register, plus others);
quarterly search of Central Register of Controlled Trials (CENTRAL);
six‐monthly searches grey literature sources: ISI Web of Knowledge Conference Proceedings; Index to Theses; Australasian Digital Theses.
To view a list of all sources searched for ALOIS see About ALOIS on the ALOIS website.
Details of the search strategies used for the retrieval of reports of trials from the healthcare databases, CENTRAL and conference proceedings can be viewed in the 'methods used in reviews' section within the editorial information about the Dementia and Cognitive Improvement Group.
Additional searches (6 April 2017) were performed in many of the sources listed above to cover the timeframe from the last searches performed for ALOIS to ensure that the search for the review was as up‐to‐date and comprehensive as possible. See Appendix 1 for search strategies.
Searching other resources
The Alzheimer's Society library.
Letters published in BPS Division of Clinical Psychology Faculty of Psychology of Older People and the BPS (British Psychological Society) magazines, requesting information on any controlled trials that may not have been easily discovered (e.g. unpublished papers).
Personal contact with specialists in the field.
Additionally, we searched the reference lists of all papers for further references, and review authors searched personal holdings of references to reports and trials. We sent letters/e‐mails to all authors of included RCTs asking for essential information, where this was not available in the publication (e.g. statistics or details of randomisation, or both).
Data collection and analysis
Selection of studies
Following deduplication, two review authors (LOP and EF) independently reviewed the abstracts and, if necessary, the manuscripts of potential studies identified by the search. These review authors were not involved in any of the studies produced by the searches. We excluded obviously irrelevant studies. We obtained the full text of remaining studies and excluded studies that did not meet the inclusion criteria with reasons outlined in the Characteristics of excluded studies table. If authors disagreed about the inclusion of a particular study, this was referred to another review author (BW or AS) for clarification. We collated multiple reports of the same study, so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1).
1.
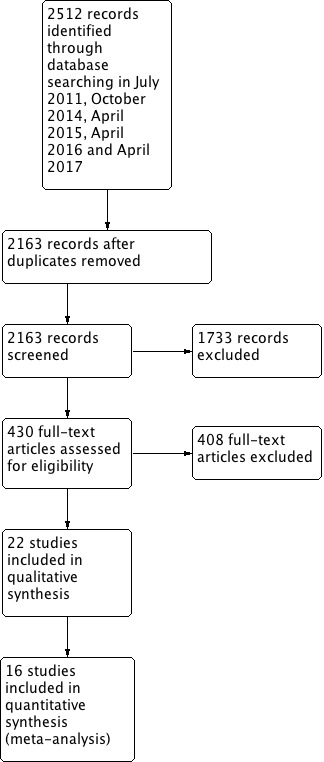
Study flow diagram.
Data extraction and management
Two review authors (EF and LOP) independently extracted descriptive characteristics, study methodology data and study results from the included studies, recorded them on a data collection form and entered them into Review Manager 5 (RevMan 2014). The form was piloted on ten studies. We compared the data to ensure accuracy. Where data did not match, one review author (LOP) checked the data of both authors and made changes if necessary with the agreement of another review author (EF).
For each outcome measure, the authors sought to obtain data on every participant randomised irrespective of whether the participant was excluded or dropped out of the intervention or research (i.e. data from an intention to treat (ITT) analysis). If these data were not available in the published studies, the review authors sought the data of those who completed the trials.
Where necessary, we sent emails to trial authors requesting additional information. If this was unsuccessful, we contacted authors through ResearchGate.
Assessment of risk of bias in included studies
Two review authors (LOP and EF) independently assessed the risk of bias of each trial using the Cochrane 'Risk of bias' tool (Higgins 2011). We attempted to obtain additional information from study authors when we required further information. Based on the methods detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we classified each category of bias as 'low risk of bias,' 'high risk of bias' or 'unclear risk of bias.' An outline of this can be seen in Table 1 below. The meta‐analysis included only trials with a low or unclear risk of bias, except in the case of random sequence generation where only trials with a low risk of bias were included. Any disagreements regarding risk of bias ratings were referred to an independent review author (AS) for clarification. Overall ratings were assigned with respect to each study's methodological quality and are described in the 'Risk of bias' table, Figure 2; and Figure 3.
2.
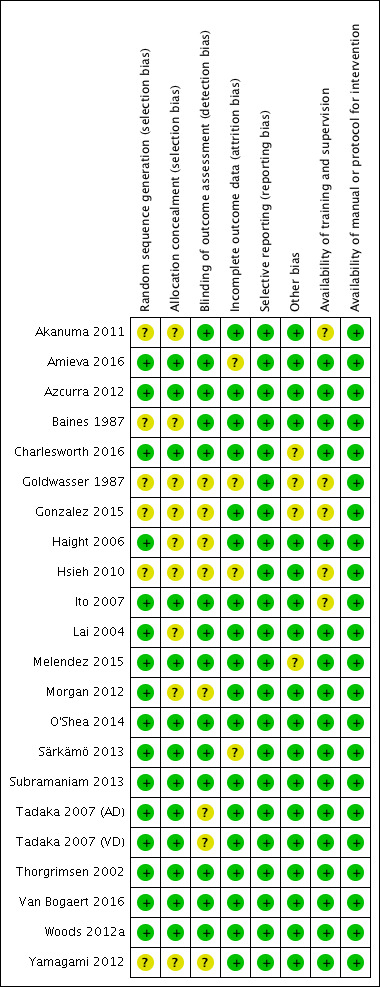
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
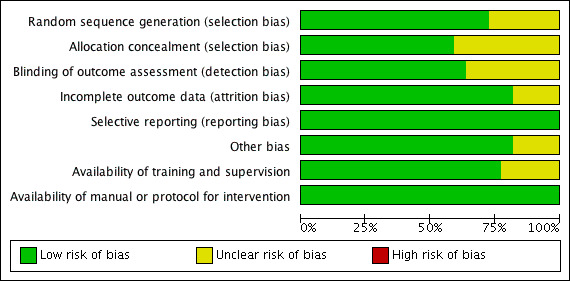
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
| Table 1. Risk of bias assessment table | |||
| Domain | Risk of bias judgement | ||
| Selection bias | Low | High | Unclear |
| Random sequence generation | Assigned if simple randomisation was used (e.g. computer‐generated random sequence, coin tossing). | Assigned if study reported an inadequate randomisation method (e.g. using date of birth or odd/even numbers). | Assigned if there was insufficient detail to judge the risk of bias as low or high. |
| Assigned if restricted randomisation was used (e.g. block randomisation, provided that within groups randomisation was not affected). | |||
| Allocation Concealment | Assigned if there was evidence of concealed allocation sequence in which allocations could not have been foreseen in advance of, or during, enrolment. | Assigned if those enrolling participants were aware of the group (or period in a cross‐over trial) to which the next enrolled participant would be allocated. | Assigned if there was insufficient detail to judge the risk of bias as low or high. |
| Detection bias | Low | High | Unclear |
| Blinding of outcome assessors (blinding of participants and facilitators is not possible in psychosocial interventions). | Assigned if outcome assessors were blind to treatment allocation. | Assigned if the outcome assessors were aware of treatment allocation (e.g. if the reminiscence facilitator was also an outcome assessor). | Assigned if there was insufficient detail to judge the risk of bias as low or high. |
| Attrition bias | Low | High | Unclear |
| Incomplete outcome data | Assigned if the study reported levels of attrition, reasons for attrition and how missing data were dealt with. Assigned if the impact of missing data was not believed to alter the conclusions and there were acceptable reasons for the missing data. | Assigned if there was inadequate information regarding the level of attrition in each group, reasons for attrition and if missing data were not handled correctly. | Assigned if there was insufficient detail to judge the risk of bias as low or high. |
| Reporting bias | Low | High | Unclear |
| Selective reporting | Assigned if study reported results of all outcome measures that were detailed in the methods section. If a study protocol was available, low risk of bias was assigned if the outcome assessments reported in the trial paper matched those detailed in the protocol. | Assigned if study did not report results of all outcome measures that were detailed in the methods section. Assigned if all outcome measures detailed in the protocol (if available) were not reported in the study. | Assigned if there was insufficient detail to judge the risk of bias as low or high. |
| Other bias | Low | High | Unclear |
| Availability of training and supervision | Assigned if RT sessions were facilitated by people who had received some form of training to ensure the necessary principles of RT were adhered to. The definition of training was inclusive and could range from a brief session to a longer, more intensive course. This also applied to interventions delivered by trained family carers. The opportunity for facilitators to access appropriate supervision was also desirable. | Assigned if there was no evidence of facilitator training or supervision. | Assigned if there was insufficient detail to judge the risk of bias as low or high. |
| Availability of manual, structure or protocol | Assigned if there was evidence of a documented intervention protocol, structure or manual outlining the content of each session to ensure the principles of RT were adhered to. | Assigned if there was no evidence of a treatment protocol, structure or manual for facilitators to follow. | Assigned if there was insufficient detail to judge the risk of bias as low or high. |
RT: reminiscence therapy.
Measures of treatment effect
Data from all included studies were continuous. This type of data required the mean change scores from baseline, the standard deviation of the mean change and the number of participants for each treatment group at each assessment. The majority of study authors did not report change scores from baseline. The baseline assessment was defined as the latest available assessment prior to randomisation, but no longer than two months prior. Where change scores were not reported, the review authors extracted the mean, standard deviation and number of participants for each treatment group at each time point and calculated the required summary statistics manually. In this case, a zero correlation between the measurements at baseline and assessment time was assumed. This method overestimates the standard deviation of the change from baseline, but this conservative approach is considered to be preferable in a meta‐analysis.
The meta‐analyses included the combination of data from trials that may not have used the same rating scale to measure a particular outcome. For example, cognition may have been measured by the MMSE in one study and the Autobiographical Memory Interview (AMI) in another. In this situation, the standardised mean difference (SMD; the absolute mean difference (MD) divided by the standard deviation) was used to measure the treatment difference. Where pooled trials used the same rating scale or test to measure an outcome, the MD was used.
To allow comparisons with other scales assessing similar outcomes, it was necessary to reverse the change scores on certain scales. For example, on measures of depression where a low score was indicative of a positive outcome on one scale and a high score was indicative of a positive outcome on another.
Unit of analysis issues
In studies using a cross‐over design, only data from the first treatment phase after randomisation were eligible for inclusion.
Where studies used cluster randomisation and were large, one review author (LOP) extracted the mean size of each cluster, the mean and standard deviation summary statistics, and the estimated intraclass correlation coefficient (ICC) in order to reduce the size of the trial to its effective sample size. This was carried out following Cochrane guidelines set out in the Cochrane Handbook for Systematic Reviews of Interventions. Where studies were not large enough, this could not be carried out.
Cluster trials were also assessed for additional biases associated with clustering, including recruitment bias; baseline imbalance; loss of clusters and comparability with individually randomised trials.
Dealing with missing data
Where possible, review authors extracted data on all participants randomised. Data from ITT analyses were preferred to per protocol or compliance analyses.
Assessment of heterogeneity
Assessments of heterogeneity were performed using both the Chi2 and I2 statistic. Review authors followed guidance in the Cochrane Handbook for Systematic Reviews of Interventions to interpret heterogeneity percentages (i.e. 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity; and 75% to 100% is considerable heterogeneity).
Assessment of reporting biases
If there were enough studies available, authors created a funnel plot to assess the risk of publication bias.
Data synthesis
The meta‐analyses presented overall estimates of the treatment difference using a fixed‐effect model. Where there was evidence of high heterogeneity of the treatment effect between trials, we used a random‐effects model (which results in broader CIs than a fixed‐effect model).
Subgroup analysis and investigation of heterogeneity
Subgroup analyses were performed where possible to assess for any important differences related to environmental context or the type/modality of the reminiscence intervention. Assessments of heterogeneity were performed using both the Chi2 test and I2 statistic. Where heterogeneity was high, we used a random‐effects model (rather than a fixed‐effect model).
Sensitivity analysis
Where necessary, sensitivity analyses were carried out. For example, when meta‐analysing carer scores on the Zarit Burden Interview (ZBI), a sensitivity analysis was carried out depending on the level of carer involvement in the intervention.
Presentation of results and 'Summary of findings' tables
We used GRADE methods to rate the quality of evidence (high, moderate, low or very low) behind each effect estimate in the review (Guyatt 2011). This rating referred to our level of confidence that the estimate reflected the true effect, taking account of the risk of bias in the included studies, inconsistency between studies, imprecision in the effect estimate, indirectness in addressing our review question and the risk of publication bias. We produced 'Summary of findings' tables for RT compared to no treatment to show the effect estimate and the quantity and quality of the supporting evidence for the following outcomes:
self‐reported QoL,
communication and interaction
cognition
functional behaviour
agitation
depressed mood
carer stress
We produced additional tables to summarise the effects on QoL, communication and interaction, and cognition for the two different settings for reminiscence work included in this review (community and care home settings) and for the two major modality types (individual reminiscence work and reminiscence groups). We prepared the 'Summary of findings' tables using the GRADEpro GDT 2015 (gradepro.org).
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies tables.
Results of the search
Systematic searches conducted since the previous review up to October 2014 identified 102 potentially eligible trials, of which 11 were included. A further search conducted in April 2015 identified 21 potential trials. One of these met the inclusion criteria, but was only available as a conference paper (Dwolatzky 2014). Later, in April 2016 another search returned 25 records, with three eligible for inclusion. A final search in April 2017 yielded 37 results, of which two were eligible for inclusion. This gave a total of 21 trials that met the inclusion criteria. Two trials by the same authors were identified (Tadaka 2004; Tadaka 2007), and further examination showed that the same data set and outcome measures were used in both papers. As the data from the earlier paper were not in usable form, the more recent paper, which presented the results as a comparison of AD and VD was included (Tadaka 2007), and the earlier paper (Tadaka 2004) was excluded. Because Tadaka 2007 analysed the two participant groups separately, with a different control group for each disease type, we entered this study into the meta‐analysis as two separate RCTs (Tadaka 2007 (AD); Tadaka 2007 (VD)), bringing the number of included studies to 22. More information can be found in the study flow diagram (Figure 1).
Included studies
The review included 22 studies, with 1972 participants: 1001 participants were randomised to treatment conditions and 971 participants to control conditions. In addition to the five studies in our previous 2005 review (Baines 1987; Goldwasser 1987; Lai 2004; Morgan 2012; Thorgrimsen 2002) (of which the Morgan 2012 study is now a published article rather than a doctoral thesis), 17 new studies met the inclusion criteria (Akanuma 2011; Amieva 2016; Azcurra 2012; Charlesworth 2016; Gonzalez 2015; Haight 2006; Hsieh 2010; Ito 2007; Melendez 2015; O'Shea 2014; Särkämö 2013; Subramaniam 2013; Tadaka 2007 (AD); Tadaka 2007 (VD); Woods 2012a; Yamagami 2012; Van Bogaert 2016).
We excluded six of the included studies from the meta‐analyses (Akanuma 2011; Baines 1987; Goldwasser 1987; Gonzalez 2015; Hsieh 2010; Yamagami 2012). All six studies were at unclear risk of selection bias due to inadequate information about random sequence generation and allocation concealment. This was the main reason they were excluded, although all six were also rated at unclear risk of bias in at least one other domain. The risk of bias details for each study are summarised in the Risk of bias in included studies tables while Figure 2 depicts the risk of bias summary.
Considering that the Baines 1987 and Goldwasser 1987 studies dated from the 1980s, we did not attempt to contact the study authors. Furthermore, in the previous versions of this review, we were unable to get in touch with the authors of the Goldwasser 1987 study. We attempted to contact the authors of the Akanuma 2011; Gonzalez 2015; Hsieh 2010; and Yamagami 2012 studies for more information, but there was no response.
Full details of included studies are presented in the Characteristics of included studies table and reasons for exclusion of studies in the Characteristics of excluded studies table.
Design
All studies were described by their authors as RCTs, although, as noted above, six studies did not provide enough information on the randomisation methods for us to be sure that the risk of selection bias was low. One study was a cross‐over trial (Baines 1987). There were three cluster randomised trials (Gonzalez 2015; Melendez 2015; O'Shea 2014).
Diagnosis
All studies recruited participants with a diagnosis of dementia. One study recruited both participants with MCI and dementia due to AD, but we extracted only data from participants with dementia (Melendez 2015). Four studies did not specify which diagnostic criteria were used (Baines 1987; Goldwasser 1987; Thorgrimsen 2002; Yamagami 2012). Twelve studies specified a diagnosis of dementia according the DSM‐IV (Azcurra 2012; Charlesworth 2016; Gonzalez 2015; Hsieh 2010; Lai 2004; Melendez 2015; O'Shea 2014; Subramaniam 2013; Tadaka 2007 (AD); Tadaka 2007 (VD); Van Bogaert 2016; Woods 2012a). Of these, four also used the CDR to support a diagnosis of dementia (Charlesworth 2016; Tadaka 2007 (AD); Tadaka 2007 (VD); Woods 2012a). One study enrolled participants with a diagnosis of AD based on the criteria of the National Institute of Neurological and Communicative Disorders and Stroke‐Alzheimer's Disease and Related Disorders Association (NINCDS‐ADRD) (Amieva 2016). One study reported that participants had undergone assessment and diagnosis at the neurology department of the General Hospital of Valencia and met the study inclusion criteria (Gonzalez 2015). Two studies recruited participants if they fulfilled the diagnostic criteria of ischaemic vascular disease with reference to computed tomography or magnetic resonance imaging (or both) findings, and if they scored between 10 and 24 on the MMSE (Akanuma 2011; Ito 2007). One study recruited care home staff volunteered residents who had a diagnosis of dementia (Haight 2006). In one study, staff members selected participants and then completed the Clifton Assessment Procedures for the Elderly (CAPE) scale (Baines 1987).
Dementia type
Six studies recruited participants with a specific type of dementia diagnosis. Four studies only recruited participants with a diagnosis of AD (Amieva 2016; Azcurra 2012; Gonzalez 2015; Melendez 2015), although Melendez 2015 also recruited a separate group of participants with amnesic MCI, and two studies sought only participants with a diagnosis of VD (Akanuma 2011; Ito 2007). Tadaka 2007 recruited both participants with AD and VD, but analysed the two groups separately, with a different control group for each disease type.
Dementia severity
The majority of included studies sought to recruit participants in the mild to moderate stages of dementia. However, Gonzalez 2015 and Melendez 2015 only included people with mild AD as measured by a score of 3 or 4 on the Geriatric Depression Scale (GDS; i.e. mild dementia was the maximum level). Five studies did not specify a particular level of severity in their inclusion/exclusion criteria (Goldwasser 1987; Haight 2006; Lai 2004; Thorgrimsen 2002; Yamagami 2012).
Six studies used the CDR as a screening measure to assess if participants met the inclusion criteria. Five studies required a score of between 1 and 2 (mild to moderate dementia) to participate (Morgan 2012; Subramaniam 2013; Tadaka 2007 (AD); Tadaka 2007 (VD); Woods 2012a), while potential participants in one study needed to score between 0.5 and 2 (questionable to moderate dementia) (Särkämö 2013). Nine studies reported baseline CDR scores. Azcurra 2012 reported a mean score of 1 and Särkämö 2013 reported a mean score of 1.35, indicating that participants had mild‐to‐moderate dementia. Seven studies reported (or sent the review authors) the number of participants who achieved each score. Across five studies, approximately 65% of participants obtained a score of 1 on the CDR indicating that they had mild dementia, while 35% scored 2 indicating moderate dementia (Hsieh 2010; Morgan 2012; Subramaniam 2013; Tadaka 2007 (AD); Tadaka 2007 (VD)). One study used the CDR sum of boxes as an outcome measure and baseline CDR scores indicated that nine participants had 'questionable dementia', 24 had mild dementia, 17 had moderate dementia and four had severe dementia (Yamagami 2012). Woods 2012a indicated that 6.2% of his participants scored 0.5, 67.4% scored 1 and 26.5% scored 2.
Sixteen studies reported MMSE scores at baseline. This included one study that used the Hasegawa Dementia Scale‐Revised (the authors reported this was similar to the MMSE) (Yamagami 2012). One study used the Cantonese version of the MMSE (Lai 2004), while two studies used the Spanish version (Gonzalez 2015; Melendez 2015). Although published cut‐off points on the MMSE should be interpreted cautiously, a widely cited study classified an MMSE score of less than 10 as severe impairment, 10 to 20 as moderate impairment and 20 to 25 as mild impairment (Folstein 1975). In 13 studies, the mean MMSE score fell within the moderate range (Azcurra 2012; Charlesworth 2016; Goldwasser 1987; Gonzalez 2015; Haight 2006; Ito 2007; Melendez 2015; O'Shea 2014; Särkämö 2013; Tadaka 2007 (AD); Tadaka 2007 (VD); Thorgrimsen 2002; Van Bogaert 2016; Yamagami 2012), and in one study the mean MMSE score fell within the severe range (Lai 2004).
Recruitment setting
Included studies recruited participants from a range of settings including residential care facilities, local hospitals, day hospital facilities and outpatient clinics. Fourteen studies recruited participants from residential/hospital care settings, while eight recruited community‐dwelling participants (Amieva 2016; Charlesworth 2016; Melendez 2015; Särkämö 2013; Tadaka 2007 (AD); Tadaka 2007 (VD); Thorgrimsen 2002; Woods 2012a). The interventions took place in the care homes where participants resided or community locations such as day centres.
Participant age
The mean age of participants was over 80 years, with the exception of participants in three studies where reported mean ages were 78 years (Akanuma 2011), 77 years (Särkämö 2013), and 78 years (Woods 2012a). One study reported age range of 60 to 99 years (Haight 2006), and one study reported the median participant age and interquartile range (IQR) as 84 years (78 to 90 years) (Van Bogaert 2016).
Length and duration of interventions
The length of reminiscence interventions ranged from four weeks (the minimum number for inclusion in the review) to 24 months. For studies that reported a range of time for each session (e.g. 60 to 90 minutes), we took the median time to calculate exposure time and session length.
The intervention delivered at the highest frequency each week was 30 minutes a day, five days a week, for four weeks (Baines 1987). Six other studies reported session frequencies of more than once a week (Azcurra 2012; Goldwasser 1987; Melendez 2015; O'Shea 2014; Van Bogaert 2016; Yamagami 2012). The greatest possible reminiscence exposure time was 39 hours (Amieva 2016). Participants received 90 minutes of reminiscence a week for 12 weeks, followed by six‐weekly maintenance sessions for the next 21 months. Two studies had a possible exposure time of 38 hours (Charlesworth 2016; Woods 2012a). In both studies, participants received weekly two‐hour reminiscence sessions for 12 weeks, followed by monthly reminiscence maintenance sessions for seven months, giving a total of 38 potential hours of RT. In the Charlesworth 2016 study, the family carers met separately from the main group for 45 minutes for four sessions, with the aim of developing listening and communication skills, and considering how the activities and strategies in the sessions could continue at home. The least intensive intervention was weekly 30‐minute sessions for six weeks, totalling three hours of possible exposure to reminiscence (Lai 2004). All other studies delivered the intervention once a week for varying lengths of time. For two studies, the length of reminiscence sessions, and, therefore, potential reminiscence exposure time was unclear (O'Shea 2014; Thorgrimsen 2002). Across the remaining included studies, the median intervention exposure time was 11.5 hours. The median individual session length was approximately 53 minutes with a range of 30 minutes to two hours per session.
Reminiscence therapy activities
Sixteen trials used simple reminiscence (Akanuma 2011; Amieva 2016; Baines 1987; Charlesworth 2016; Gonzalez 2015; Goldwasser 1987; Hsieh 2010; Ito 2007; Melendez 2015; O'Shea 2014; Särkämö 2013; Tadaka 2007 (AD); Tadaka 2007 (VD); Thorgrimsen 2002; Woods 2012a; Yamagami 2012), which is a form of unstructured autobiographical story telling (Gerben 2010). It involved discussions around specific themes of the past, such as school days, holidays, food and drink, and work, and was carried out in small groups. Five trials used the more structured approach of life review (Azcurra 2012; Haight 2006; Lai 2004; Morgan 2012; Subramaniam 2013), which aimed to reconstruct the participant's life in a sequential manner on a one‐to‐one basis (Haber 2006). One trial used a standardised reminiscence intervention based on the SolCos Model (Van Bogaert 2016).
One study trained staff across several care homes to deliver the interventions in small groups and gave data about their knowledge of the residents they cared for (O'Shea 2014). Three studies carried out reminiscence jointly with participants and their family carers living in the community (Charlesworth 2016; Thorgrimsen 2002; Woods 2012a). One study had a music listening group in which participants listened to songs from their past, and were encouraged to join in and share their memories of that period, such as "remembering the childhood through children's songs" (Särkämö 2013).
Control group activities
Participants in control conditions were either assigned to a 'treatment as usual' condition or a social contact group involving general unstructured conversion.
Some trials included additional conditions as well as a no‐treatment control condition. However, we used only the no‐treatment control in our analyses. For example, one study had an additional 'music singing group' (Särkämö 2013), while another study had included a counselling condition (Azcurra 2012). One study used a factorial design with four conditions, but we included only data from the RT only and treatment as usual groups (Charlesworth 2016). Similarly, another study had four conditions, but we extracted data only from the reminiscence and control conditions (Amieva 2016).
One study had a 'gift' condition whereby a family member of participants in the control group made a life story book for them without their knowledge. We included data from the first follow‐up time point (i.e. before the life story books were given to participants) in the review, as the 'gift' condition was effectively a no treatment control condition until the participants received their life story books (Subramaniam 2013).
Excluded studies
In preparing this up‐dated review, we excluded 63 studies that did not meet all necessary inclusion criteria (see Characteristics of excluded studies table).
Reasons for the exclusion of studies varied. The most common reasons were no or inadequate randomisation (meaning the study was not an RCT), intervention was not reminiscence or studies did not specifically recruit participants with a diagnosis of dementia.
Risk of bias in included studies
Specific details of the risk of bias for each study are outlined in the 'Risk of bias' table and are summarised in Figure 2 and Figure 3.
Allocation
13 studies were at low risk of selection bias, while nine were rated as unclear. Most studies reported randomisation methods although allocation concealment was rarely reported in detail. Where necessary, we contacted authors for clarification. Replies generally stated that adequate concealment of treatment allocation had been applied, without detailing the method. In these cases, good practice has been assumed, though it was regrettable that further details were not available. Three studies used an accredited trials unit to randomise and allocate participants to their respective conditions (Charlesworth 2016; Subramaniam 2013; Woods 2012a). Three studies used cluster randomisation. One large scale study used cluster randomisation stratified by public or private residential units (O'Shea 2014). Two studies recruited participants in two nursing homes and then randomly allocated the nursing homes to the treatment and control conditions (Gonzalez 2015; Melendez 2015).
Blinding
Performance bias
Participants cannot be blinded to the experience of taking part in an intervention and likewise, control participants will be aware that they have entered a research trial, but are not receiving any treatment. The person's expectations of potential benefits, or otherwise, may well influence outcome measures, which is difficult to control for.
Detection bias
Eight studies were at unclear risk of detection bias (Goldwasser 1987; Gonzalez 2015; Haight 2006; Hsieh 2010; Morgan 2012; Tadaka 2007 (AD); Tadaka 2007 (VD); Yamagami 2012). However, fourteen studies took adequate measures to blind outcome assessors and were at low risk of detection bias. Two studies asked assessors to record their prediction of which arm of the trial each participant belonged to, and their confidence in that prediction (Charlesworth 2016; Woods 2012a). In the Woods 2012a study, in 44% of cases, interviewers felt participants could equally have been assigned to control or treatment group, with 23% making a correct definite judgement. The proportion of correct definite judgements remained low at follow‐up, at about 25%, which reflected the considerable degree of uncertainty around treatment allocation. Charlesworth 2016 reported a similar prediction pattern. Measures of behaviour, functioning and carer‐rated outcomes of mood and QoL were typically completed by a person who knew the participant and could reliably comment.
Incomplete outcome data
Eighteen studies were at low risk of attrition bias, while four were at an unclear risk. Data extracted from several studies were from ITT analyses (Amieva 2016; Azcurra 2012; Charlesworth 2016; Lai 2004; Melendez 2015; O'Shea 2014; Woods 2012a). Eight studies used a per protocol analysis where the analysis was completed without data from participants who dropped out (Hsieh 2010; Ito 2007; Särkämö 2013; Subramaniam 2013; Tadaka 2007 (AD); Tadaka 2007 (VD); Van Bogaert 2016; Yamagami 2012). In the Ito 2007 study, both a per protocol and ITT analysis were completed, but we could only extract data from the per protocol analysis. Four studies reported zero withdrawals (Baines 1987; Haight 2006; Morgan 2012; Thorgrimsen 2002). In one study, one participant dropped out, so the authors randomly excluded one participant from each of the two other groups (Goldwasser 1987). All trials, apart from Gonzalez 2015, reported attrition rates.
The largest care home study, which was based in residential care homes across Ireland, reported 25/153 withdrawals (16%) in the intervention group and 27/151 (18%) in the control group, with withdrawals predominantly due to hospitalisation, transfer to a different residential home or the death of the participant (O'Shea 2014). The largest community‐based study reported a slightly higher attrition rate with 137 total withdrawals from the trial (23% from the treatment group and 34% from the control group) (Woods 2012a). Reasons cited were wide ranging and included death or illness of participant or carer, not enough time, or no explanation given. A total of 79/291 participants (27%) were lost over the duration of the Charlesworth 2016 study, for varying reasons including carer in poor health and loss to contact.
Selective reporting
There was no evidence of selective outcome reporting for any study. All studies reported the same outcome measures in the methods and results sections of papers. Four studies had a protocol and the outcome measures detailed in the protocol were reported in the completed papers (Charlesworth 2016; O'Shea 2014; Van Bogaert 2016; Woods 2012a).
Other potential sources of bias
Treatment protocol
The previous version of this review recommended that future trials should follow a clear treatment protocol, so that it is possible to define precisely the key elements of the different approaches to reminiscence work. The presence of a treatment protocol, or at least evidence of a session plan, is imperative to ensure that the intervention is delivered correctly, and to prevent intervention 'drift' (where the theme of the session may drift off‐topic), or introduce unintentional bias. Seventeen studies were at a low risk of bias relating to the presence of a treatment protocol, while five were at an unclear risk. Seven studies used a standardised reminiscence format. Three of these used the Haight 1992 Life Review Model and Life Review Experience Form, which provides a structured format for obtaining relevant information from participants (Haight 2006; Morgan 2012; Subramaniam 2013). The Woods 2012a, Charlesworth 2016 and Thorgrimsen 2002 studies followed 'Remembering Yesterday, Caring Today' (RYCT; Schweitzer 2008), which is a large group‐based approach, bringing people with dementia and family carers together with a focus on active reminiscence.The Van Bogaert 2016 study based their reminiscence intervention on the SolCos model (Soltys 1994).
Facilitator training and supervision
We considered the knowledge of staff delivering the interventions, total training hours and availability of supervision. All studies were at a low risk of bias in relation to facilitator training and supervision. Eleven studies did not specify training (Akanuma 2011; Goldwasser 1987; Gonzalez 2015; Hsieh 2010; Ito 2007; Melendez 2015; Morgan 2012; Särkämö 2013; Subramaniam 2013; Tadaka 2007 (AD); Tadaka 2007 (VD)), but all were reported to have been delivered by appropriate facilitators such as psychologists or gerontologists. Further details are available in the 'Risk of bias' table. The other studies provided four hours (Yamagami 2012), six hours (Baines 1987), 10 hours Haight 2006, 19.3 hours (Lai 2004), 22 hours (Thorgrimsen 2002), 30.4 hours (Azcurra 2012), one day (Charlesworth 2016), two and a half days (Woods 2012a), and three days (Amieva 2016; O'Shea 2014) of training. Facilitators in the Van Bogaert 2016 study received a training programme though the total number of hours was not specified.
Contamination
The main risk of contamination arose from trials located in care homes, in which control and intervention participants resided and socialised together. Two studies that included residential care participants seemed to use at least one member of staff or research team to carry out the intervention whilst also working in the home, potentially meaning that themes of reminiscence could be carried over into daily care and contaminate any control conditions (Goldwasser 1987; Haight 2006). However, correct adherence to the trial protocol would have minimised this risk.
Outcome measures
Where more than one measure of a single outcome domain was used in a study, data from the most common or the most extensive measure were included in the meta‐analysis. This was to avoid including data from the same participants more than once in each outcome analysis.
Most studies collected outcomes up to two weeks after the final session, but for some larger studies this may have been up to four weeks (Charlesworth 2016; Woods 2012a). For purposes of this review, the primary end points of the Amieva 2016; Charlesworth 2016; and Woods 2012a studies were after the 12 weeks of weekly reminiscence sessions (three months post‐baseline) while the later follow‐up time point was following completion of the monthly maintenance sessions.
Quality of life
Ten studies measured self‐reported QoL at the end of treatment time point, while six measured it at follow‐up. Two were excluded from the meta‐analysis for risk of selection bias (Baines 1987; Gonzalez 2015), while the Subramaniam 2013 follow‐up data were also not included because the control group condition had changed by then (participants had been given a life story book as a gift). In the meta‐analysis, all studies used the Quality of Life in Alzheimer's Disease (QoL‐AD), except for the Azcurra 2012 study, which used the Self‐Report Quality of Life (SR‐QoL) scale.
Seven studies measured proxy‐rated QoL. The Baines 1987 and Goldwasser 1987 studies were excluded from the meta‐analysis for risk of selection bias. All used the proxy scale on the QoL‐AD. Three studies went on to measure it at follow‐up.
Two studies measured observed QoL using the Well‐being/Ill‐being (WIB) scale at both end of treatment and follow‐up (Azcurra 2012; Lai 2004).
Cognition
Nineteen studies measured cognition at end of treatment. Five studies at unclear risk of selection bias (Akanuma 2011; Baines 1987; Goldwasser 1987; Gonzalez 2015; Yamagami 2012), and follow‐up data from Subramaniam 2013 were excluded. Eight studies were included in the meta‐analysis at follow‐up. The most commonly used measures in the meta‐analysis were the MMSE (nine studies) and the Autobiographical Memory Interview Extended Version (AMI‐E) (four studies).
Communication and Interaction
Eight studies measured communication and interaction at end of treatment with four assessing it at a later follow‐up time point. Two studies were excluded from the meta‐analysis for risk of selection bias (Baines 1987; Yamagami 2012). The meta‐analysis included data from four outcome measures; the Holden Communication Scale (Thorgrimsen 2002), Social Engagement Scale (SES) (Azcurra 2012; Lai 2004), Multidimensional Observation Scale for Elderly Subjects (MOSES) withdrawal subscale (Tadaka 2007 (AD); Tadaka 2007 (VD)), and the Communication Observation Scale for Cognitive Impaired (Haight 2006). The follow‐up meta‐analysis was comprised of data from the SES (Azcurra 2012; Lai 2004) and MOSES (Tadaka 2007 (AD); Tadaka 2007 (VD))
Quality of caring relationship
Three studies evaluated the quality of the relationship between the carer and the person with dementia (as rated by the person with dementia) at the end of treatment (Charlesworth 2016; Subramaniam 2013; Woods 2012a). All three used the Quality of Carer and Patient Relationship (QCPR), which has two subscales: warmth and absence of conflict. The Charlesworth 2016 and Woods 2012a studies measured this again at a follow‐up time point.
Behaviour
We divided measures of behaviour into measures of function (i.e. daily living skills) and measures of agitation/irritability. Four studies used scales which assess both of these domains (MBS, CAPE, Behavior Rating Scale for the Elderly (BRSE)) (Akanuma 2011; Baines 1987; Haight 2006; Thorgrimsen 2002). As the authors were unable to extract scores for each, data from these two outcome measures were not included in the meta‐analysis.
Behaviour: function
Seven studies measured functional behaviour at end of treatment and at follow‐up (Amieva 2016; Azcurra 2012; Charlesworth 2016; Goldwasser 1987; Haight 2006; Lai 2004; Woods 2012a) except for the O'Shea 2014 study. The Goldwasser 1987 study was excluded from the meta‐analysis for risk of selection bias. The most common outcome measure was the Activities of Daily Living Scale, though all studies used various ADL measures.
Behaviour: agitation/irritability
Four studies measured agitation/irritability (O'Shea 2014; Tadaka 2007 (AD); Tadaka 2007 (VD); Yamagami 2012), though one was excluded from the meta‐analysis as there was a high risk of selection bias (Yamagami 2012). The Tadaka 2007 (AD) and Tadaka 2007 (VD) studies used the irritability subscale of the MOSES at end of treatment and follow‐up, while the O'Shea 2014 study used the Cohen Mansfield Agitation Inventory (CMAI) at end of treatment only. The Ito 2007 study also measured agitation/irritability using the MOSES but did not report the scores obtained on each subscale. Therefore, the MOSES data from this study could not be included in this meta‐analysis.
Mood‐related outcomes (person with dementia)
Depression
Fifteen studies measured depression at end of treatment with ten contributing data to the meta‐analysis (Akanuma 2011; Amieva 2016; Charlesworth 2016; Goldwasser 1987; Gonzalez 2015; Haight 2006; Hsieh 2010; Morgan 2012; O'Shea 2014; Subramaniam 2013; Tadaka 2007 (AD); Tadaka 2007 (VD); Van Bogaert 2016; Woods 2012a; Yamagami 2012). Four used the Cornell Scale for Depression in Dementia (CSDD), which was the most common measure (Haight 2006; O'Shea 2014; Van Bogaert 2016; Woods 2012a). Other measures utilised were the Montgomery‐Åsberg Depression Rating Scale (MADRS), MOSES depression subscale, Geriatric Depression Scale ‐ Short Form (GDS‐SF), and 30‐question Geriatric Depression Scale (GDS‐30). Six of these studies also measured depression at follow‐up time points (Amieva 2016; Charlesworth 2016; Morgan 2012; Tadaka 2007 (AD); Tadaka 2007 (VD); Woods 2012a).
Anxiety
Two studies measured anxiety at end of treatment and follow‐up using the Hospital Anxiety and Depression Scale (HADS) ‐ Anxiety subscale (Charlesworth 2016) and Rating Anxiety In Dementia (RAID) scale (Woods 2012a).
Apathy
Two studies measured apathy at end of treatment; Amieva 2016 used a carer rated Apathy Index and Hsieh 2010 used the Apathy Evaluation Scale, but the latter study was excluded from the meta‐analysis for risk of selection bias.
Carer outcomes
Carer outcomes were divided into outcomes measuring stress related to caring, carer anxiety and depression, carer QoL, and the quality of the caring relationship.
Stress related to caring
Seven studies measured stress related to caring at end of treatment (Amieva 2016; Azcurra 2012; Charlesworth 2016; O'Shea 2014; Särkämö 2013; Thorgrimsen 2002; Woods 2012a) with five also measuring it at follow‐up (Amieva 2016; Azcurra 2012; Charlesworth 2016; Särkämö 2013; Woods 2012a). The most popular measures were the ZBI or Zarit Burden Interview ‐ Short Form (ZBI‐SF).
Carer depression and anxiety
Two studies measured carer depression and anxiety at end of treatment and follow‐up (Charlesworth 2016; Woods 2012a). Both studies used the HADS. These subscales were analysed separately.
Carer well‐being and quality of life
Four studies measured carer well‐being and QoL at end of treatment (Charlesworth 2016; Särkämö 2013; Thorgrimsen 2002; Woods 2012a). Only the Thorgrimsen 2002 study did not include a follow‐up measure. The meta‐analysis comprised of data from the 12‐item General Health Questionnaire (GHQ‐12), 28‐item General Health Questionnaire (GHQ‐28) and the 12‐item Short Form (SF‐12) Mental component.
Quality of caring relationship
Three studies evaluated the quality of the relationship between the carer and the person with dementia (as rated by the carer) at the end of treatment (Charlesworth 2016; Subramaniam 2013; Woods 2012a). All three used the Quality of Carer and Patient Relationship (QCPR), which has two subscales: warmth and absence of conflict. The Charlesworth 2016 and Woods 2012a studies measured this again at a follow‐up time point.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Reminiscence Therapy compared to no treatment for people living with dementia.
| Reminiscence Therapy compared to no treatment for people living with dementia | ||||||
| Patient or population: people living with dementia Setting: Care home and community settings Intervention: Reminiscence Therapy Comparison: no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no treatment | Risk with Reminiscence Therapy | |||||
| Quality of Life (self‐report) at end of treatment assessed with: QOL‐AD, SRQoL follow up: range 1 days to 6 weeks | SMD 0.11 higher (0.12 lower to 0.33 higher) | ‐ | 1060 (8 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | A higher score is indicative of improved quality of life. Subgroup analysis by setting likley explains the variation in effect size across the studies. | |
| Cognition at end of treatment assessed with: MMSE, AMI‐PSS, AMI(E)‐PSS, ADAS‐COG follow up: range 1 days to 6 weeks | SMD 0.11 higher (0 to 0.23 higher) | ‐ | 1219 (14 RCTs) | ⊕⊕⊕⊕ HIGH | A higher score is indicative of improved cognition | |
| Communication and Interaction at end of treatment assessed with: Social Engagement Scale, Communication Observation Scale, MOSES Withdrawal sub‐scale, Holden Communication Scale follow up: range 1 days to 2 weeks | SMD 0.51 lower (0.97 lower to 0.05 lower) | ‐ | 249 (6 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | A lower score is indicative of improved communication | |
| Functional behaviour at end of treatment assessed with: MDS‐ADL, FIM, ADL, BADLS, ADCS‐ADL, DAD follow up: range 1 days to 6 weeks | SMD 0.24 lower (0.69 higher to 0.21 higher) | ‐ | 1030 (6 RCTs) | ⊕⊝⊝⊝ VERY LOW 3 4 | A lower score is indicative of improved functional behaviour | |
| Agitation/irritability at end of treatment assessed with: CMAI, MOSES (irritability subscale) follow up: range 1 days to 6 weeks | SMD 0.03 higher (0.17 lower to 0.24 higher) | ‐ | 359 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | A lower score is indicative of improved agitation/irritability | |
| Depressed mood at end of treatment assessed with: CSDD, GDS, GDS‐SF,MOSES (depression subscale), HADS (depression subscale), MADRS follow up: range 1 days to 6 weeks | SMD 0.03 lower (0.15 lower to 0.1 higher) | ‐ | 973 (10 RCTs) | ⊕⊕⊕⊕ HIGH | A lower score is indicative of improved mood | |
| Stress related to caring (caregiver) assessed with: ZBI‐SF, RSS, NPI, Modified ZBI, ZBI follow up: range 1 days to 6 weeks | SMD 0.03 SD higher (0.21 lower to 0.14 higher) | ‐ | 1155 (7 RCTs) | ⊕⊕⊕⊝ MODERATE 5 | A lower score is indicative of less caregiver stress | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one point for inconsistency due to substantial heterogeneity
2 Downgraded one point for imprecision due to small sample size (<400 participants)
3 Downgraded one point for imprecision as the confidence interval contains null effect and the lower limit passes ‐0.5
4 Downgraded two points for inconsistency due to considerable heterogeneity
5 Downgraded one point for inconsistency due to moderate heterogeneity
Summary of findings 2. Reminiscence therapy compared to no treatment for people living with dementia (modality).
| Reminiscence therapy compared to no treatment for people living with dementia (modality) | ||||||
| Patient or population: people living with dementia (modality) Setting: care home and community settings Intervention: reminiscence therapy Comparison: no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no treatment | Risk with reminiscence therapy | |||||
| Individual: quality of life (self‐reported) at end of treatment assessed with: QoL‐AD Scale from: 13 to 52 follow‐up: range 1 to 7 days | ‐ | MD 7.00 points higher (0.14 lower to 14.14 higher) | ‐ | 23 (1 RCT) | ⊕⊕⊝⊝ Low1 | Higher score on quality of life measures indicated a more positive outcome. 3.0 points may be the minimum clinically important difference. |
| Individual: cognition at end of treatment assessed with: MMSE, AMI‐PSS follow‐up: range 1 day to 2 weeks | ‐ | SMD 0.32 higher (0.04 higher to 0.61 higher) | ‐ | 196 (5 RCTs) | ⊕⊕⊕⊝ Moderate2 | Higher score on cognitive measures indicated a more positive outcome. |
| Individual: communication at end of treatment assessed with: SES, COS follow‐up: range 1 day to 2 weeks | ‐ | SMD 0.74 lower (2.38 lower to 0.89 higher) | ‐ | 96 (2 RCTs) | ⊕⊝⊝⊝ Very low3,4 | Lower score on communication measures indicated a more positive outcome. |
| Group: quality of life (self‐reported) at end of treatment assessed with: QoL‐AD, SR‐QoL follow‐up: range 1 day to 6 weeks | ‐ | SMD 0.06 higher (0.15 lower to 0.28 higher) | ‐ | 1037 (7 RCTs) | ⊕⊕⊕⊕ High | Higher score on quality of life measures indicated a more positive outcome. |
| Group: cognition at end of treatment assessed with: MMSE, AMI‐PSS, ADAS‐Cog follow‐up: range 1 day to 6 weeks | ‐ | SMD 0.07 higher (0.05 lower to 0.20 higher) | ‐ | 1023 (9 RCTs) | ⊕⊕⊕⊕ High | Higher score on cognitive measures indicated a more positive outcome. |
| Group: communication at end of treatment assessed with: SES, COS, MOSES Withdrawal subscale follow‐up: range 1 day to 1 weeks | ‐ | SMD 0.39 lower (0.71 lower to 0.06 lower) | ‐ | 153 (4 RCTs) | ⊕⊕⊕⊝ Moderate2 | Lower score on communication measures indicated a more positive outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADAS‐Cog: Alzheimer's Disease Assessment Scale Cognitive subscale; AMI‐PSS: Autobiographical Memory Interview ‐ Perceived Stress Scale; CI: confidence interval; Communication Observation Scale; MD: mean difference; MMSE: Mini‐Mental State Examination; MOSES: Multidimensional Observation Scale for Elderly Subjects; QoL‐AD: Quality of Life in Alzheimer's Disease; RCT: randomised controlled trial; SES: Social Engagement Scale; SMD: standardised mean difference; SR‐QoL: Self‐Report Quality of Life. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded 2 levels for imprecision due to small sample size (< 400 participants) and including both null effect and an upper limit greater than 0.3.
2Downgraded 1 level for imprecision due to small sample size (< 400 participants).
3Downgraded 2 levels for inconsistency due to considerable unexplained heterogeneity.
4Downgraded 2 levels for imprecision due to small sample size (< 400 participants) and both confidence interval limits crossing 0.5.
Summary of findings 3. Reminiscence therapy compared to no treatment for people living with dementia (setting).
| Reminiscence therapy compared to no treatment for people living with dementia (setting) | ||||||
| Patient or population: people living with dementia (setting) Setting: community and care home settings Intervention: reminiscence therapy Comparison: no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with no treatment | Risk with reminiscence therapy | |||||
| Care home: quality of life (self‐reported) at end of treatment assessed with: QoL‐AD, SR‐QoL follow‐up: range 1 day to 6 weeks | ‐ | SMD 0.46 higher (0.18 higher to 0.75 higher) | ‐ | 193 (3 RCTs) | ⊕⊕⊕⊝ Moderate1 | Higher score on quality of life measures indicated a more positive outcome. |
| Care home: cognition at end of treatment assessed with: MMSE, AMI‐PSS follow‐up: range 1 day to 2 weeks | ‐ | SMD 0.29 higher (0.03 higher to 0.56 higher) | ‐ | 230 (6 RCTs) | ⊕⊕⊕⊝ Moderate1 | Higher score on cognitive measures indicated a more positive outcome. |
| Care home: communication at end of treatment assessed with: SES, Communication Scale for Cognitively Impaired follow‐up: range 1 day to 2 weeks | ‐ | SMD 0.52 lower (1.29 lower to 0.24 higher) | ‐ | 184 (3 RCTs) | ⊕⊝⊝⊝ Very low2,3 | Lower score on communication measures indicated a more positive outcome. |
| Community: quality of life (self‐reported) at end of treatment assessed with: QoL‐AD (self‐report) Scale from: 13 to 52 follow‐up: range 1 day to 6 weeks | ‐ | MD 0.57 points lower (1.37 lower to 0.22 higher) | ‐ | 867 (5 RCTs) | ⊕⊕⊕⊕ High | Higher score on quality of life measures indicated a more positive outcome. 3.0 points may be the minimum clinically important difference. |
| Community: cognition at end of treatment assessed with: MMSE, AMI‐PSS, AMI‐E‐PSS, ADAS‐Cog follow‐up: range 1 day to 6 weeks | ‐ | SMD 0.07 higher (0.05 lower to 0.20 higher) | ‐ | 989 (8 RCTs) | ⊕⊕⊕⊕ High | Higher score on cognitive measures indicated a more positive outcome. |
| Community: communication and interaction at end of treatment assessed with: Holden Communication Scale and MOSES (withdrawal subscale) follow‐up: range 1 day to 7 days | ‐ | SMD 0.57 lower (1.08 lower to 0.06 lower) | ‐ | 65 (3 RCTs) | ⊕⊕⊕⊝ Moderate1 | Lower score on communication measures indicated a more positive outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADAS‐Cog: Alzheimer's Disease Assessment Scale Cognitive subscale; AMI‐PSS: Autobiographical Memory Interview ‐ Perceived Stress Scale; AMI‐E‐PSS: Autobiographical Memory Interview ‐ Extended Version ‐ Perceived Stress Scale; MD: mean difference; MMSE: Mini‐Mental State Examination; MOSES: Multidimensional Observation Scale for Elderly Subjects; QoL‐AD: Quality of Life in Alzheimer's Disease; RCT: randomised controlled trial; SES: Social Engagement Scale; SMD: standardised mean difference; SR‐QoL: Self‐Report Quality of Life. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded 1 level for imprecision because of small sample size (< 400 participants).
2Downgraded 2 levels for imprecision because of small sample size (< 400) and the confidence interval including a null effect and a lower limit crossing ‐0.5.
3Downgraded 2 levels for inconsistency due to considerable unexplained heterogeneity.
Effect sizes
Evaluating the clinical meaningfulness of changes on the outcome measures used in studies of reminiscence interventions is challenging, as there are no internationally agreed standards to apply in this context. For SMDs, we have adopted the rule that an SMD of 0.5 or greater reflects an important difference, with SMDs less than 0.10 being negligible. For analyses using the MMSE, we judged a difference of 1.5 points or more as clinically important. The rate of decline on this measure has been estimated, in mild to moderate dementia, to be between 2 and 4 points per annum (Mohs 2000), and so 1.5 points is broadly equivalent to preventing six months of decline in cognition. For other measures, we did not have parallel criteria, so have applied the 0.5 of a standard deviation rule, taking the standard deviation from the baseline evaluations. Thus, for the QoL‐AD, we have taken a difference of 3 points or more to be clinically meaningful, reflecting approximately half the typical standard deviation in samples of people with mild to moderate dementia (e.g. Woods 2012a). For the SR‐QoL, this translates to 2.2 points or more (Azcurra 2012); for the WIB, 0.3 points or more (Azcurra 2012; Lai 2004); for the MOSES Withdrawal Scale, 3.1 points or more (Tadaka 2007); for the SES, 0.75 points or more (Azcurra 2012); for the QCPR warmth and absence of conflict scales, rated by the person with dementia, 1.8 points; for the MOSES Irritability Scale, 2.2 points or more (Tadaka 2007); for the ZBI 3 points or more (Azcurra 2012); for HADS ‐ Anxiety, 2.2 points or more; for HADS ‐ Depression, 1.8 points or more (Woods 2012a); for the QCPR rated by the carer: warmth 2.7 points or more, absence of conflict 2.2 points or more.
Outcomes for the person with dementia
Quality of life
(See Figure 4.)
4.
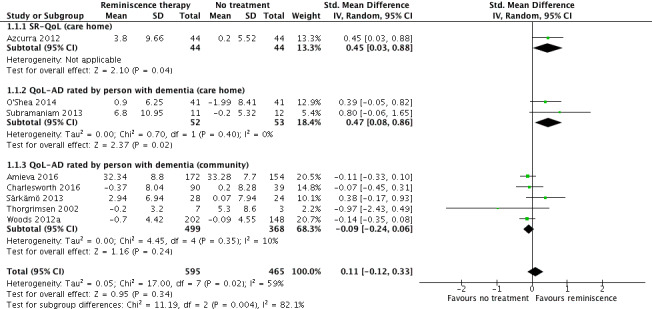
Forest plot of comparison: 1 Reminiscence therapy versus no treatment, outcome: 1.1 Self‐reported quality of life post‐treatment.
For the overall evaluation of the effects of reminiscence on QoL at the end of treatment, eight studies reporting a self‐report QoL measure were included in the meta‐analysis (Amieva 2016; Azcurra 2012; Charlesworth 2016; O'Shea 2014; Särkämö 2013; Subramaniam 2013; Thorgrimsen 2002; Woods 2012a). This included 1060 participants living with dementia; 595 received a reminiscence intervention and 465 received no treatment. Where studies used more than one measure of QoL, the analysis was conducted on the most common or extensive self‐report assessment; seven studies used the QoL‐AD (self‐report) and one study used the SR‐QoL. A random‐effects analysis resulted in a small overall effect size (SMD 0.11, 95% CI ‐0.12 to 0.33; I2 = 59%; moderate quality evidence; Analysis 1.1). This indicated that, across the eight included studies, reminiscence did not have an important effect on self‐reported QoL.
1.1. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 1 Self‐reported quality of life post‐treatment.
Five studies (all involving reminiscence groups) went on to measure the effects of reminiscence on QoL at later follow‐up of six to 21 months (Amieva 2016; Azcurra 2012; Charlesworth 2016; Särkämö 2013; Woods 2012a). This analysis involved 499 participants who received a reminiscence intervention and 375 who received a control intervention. We could not determine whether reminiscence was associated with any effect on self‐reported QoL at follow‐up. The results were inconsistent between studies and the result of the meta‐analysis was imprecise and compatible with either an improvement or a small detrimental effect (random‐effects analysis; SMD 0.35, 95% CI ‐0.11 to 0.80; I2 = 89%; moderate quality evidence; Analysis 1.17).
1.17. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 17 Self‐reported quality of life at follow‐up.
At both end of treatment and follow‐up there was substantial heterogeneity, which appeared to relate to different modalities and (particularly) contexts of reminiscence work being analysed together. Analyses were accordingly undertaken for different modalities and contexts separately.
Modality
One small study reported self‐report QoL outcomes for individual reminiscence interventions at end of treatment (Subramaniam 2013). This involved 23 participants, and indicated life story work may have improved self‐reported QoL‐AD (mean difference (MD) 7.00 points, 95% CI ‐0.14 to 14.14; low quality evidence).
Of the seven studies that implemented group reminiscence interventions, including 1037 participants, six used the self‐report QoL‐AD. There was little or no difference between the reminiscence and control groups (random‐effects analysis; SMD 0.06, 95% CI ‐0.15 to 0.28; I2 = 56%; high quality evidence). The different settings (community versus care home) appeared to be responsible for the substantial level of inconsistency identified in this analysis. The findings for reminiscence groups at longer‐term follow‐up were detailed in the previous section.
Setting
Three studies including 193 participants living in care home settings were included in the meta‐analysis of self‐report QoL indices (Azcurra 2012; O'Shea 2014; Subramaniam 2013). The analysis suggested that there was probably an improvement in self‐reported QoL following a reminiscence intervention in care homes, but we could not be sure that this was large enough to be clinically important (fixed‐effect analysis; SMD 0.46, 95% CI 0.18 to 0.75; I2 = 0%; moderate quality evidence). The single care‐home study that reported longer‐term (six months) follow‐up, with 88 participants, also showed a probable improvement on the SR‐QoL (9.8 points, 95% CI 7.05 to 12.55; moderate quality evidence) (Azcurra 2012).
Five studies with 867 participants included only community‐resident people with dementia (Amieva 2016; Charlesworth 2016; Särkämö 2013; Thorgrimsen 2002; Woods 2012a). All used the self‐report QoL‐AD. There was little or no difference between the reminiscence and control groups (fixed‐effect analysis; MD ‐0.57 points, 95% CI ‐1.37 to 0.22; I2 = 0%; high quality evidence). Four of these studies measured the effects of reminiscence on QoL of 786 participants living in the community at follow‐up time points of six to 21 months (Amieva 2016; Charlesworth 2016; Särkämö 2013; Woods 2012a). There was little or no difference between the reminiscence and control groups (QoL‐AD) (fixed‐effect analysis; MD 0.17 points, 95% CI ‐0.79 to 1.13; I2 = 0%; high quality evidence).
Proxy ratings
The above findings on QoL were based on self‐report measures. Five studies of reminiscence groups with 763 participants used proxy measures, where a family carer or member of care staff rated the person's QoL (Charlesworth 2016; O'Shea 2014; Särkämö 2013; Thorgrimsen 2002; Woods 2012a). All used the QoL‐AD proxy version. There was little or no difference in outcomes at the end of treatment (random‐effects analysis; MD 0.35 points, 95% CI ‐1.23 to 1.94; I2 = 45%; moderate quality evidence; Analysis 1.2). At longer‐term follow‐up of six to seven months postintervention, three studies with 505 participants, all community based and involving reminiscence groups, reported findings on the QoL‐AD proxy version (Charlesworth 2016; Särkämö 2013; Woods 2012a). There was little or no difference between the reminiscence and control groups (MD ‐0.15 points, 95% CI ‐1.14 to 0.83; I2 = 25%; high quality evidence; Analysis 1.18).
1.2. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 2 Proxy rated quality of life post‐treatment.
1.18. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 18 Proxy rated quality of life at follow‐up.
Observed quality of life
Two studies used the WIB, an observational measure of QoL, which was completed during a minimum of six hours' observation of the person undertaking their usual activities (Azcurra 2012; Lai 2004). The studies included 154 care home participants, and there was probably little or no difference on WIB scores at end of treatment (MD 0.00 points, 95% CI ‐0.17 to 0.18; I2 = 0%; moderate quality evidence). At longer‐term follow‐up of six to 24 weeks' postintervention, due to the imprecision of the results and the development of inconsistency between the studies, we were unable to determine whether there was any effect of reminiscence on observed QoL (random‐effects analysis; MD ‐0.40 points, 95% CI ‐1.34 to 0.54; I2 = 93%; very low quality evidence; Analysis 1.19).
1.19. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 19 Observed quality of life at follow‐up.
Cognition
(See Figure 5.)
5.
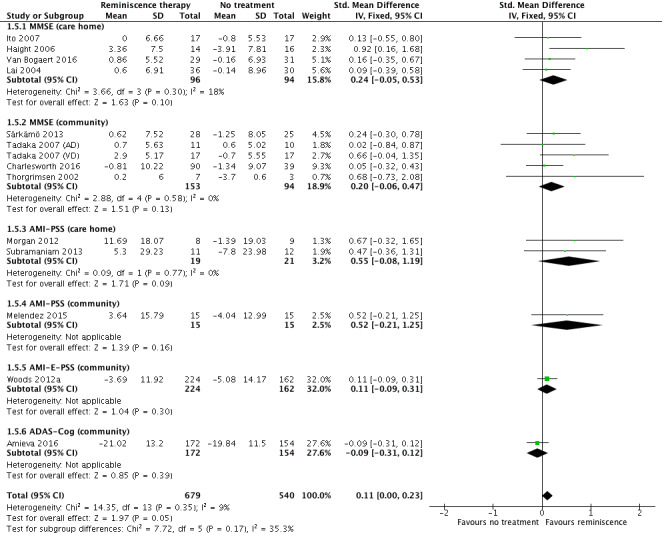
Forest plot of comparison: 1 Reminiscence therapy versus no treatment, outcome: 1.5 Cognition (overall) post‐treatment.
For cognition, we analysed data from 14 studies involving 1219 people living with dementia, in which 679 received some form of reminiscence and 540 were assigned to control groups. Where studies used more than one measure of cognition, we used the most common or extensive assessment (for the AMI and AMI‐E this was the Personal Semantic Memory Sub‐scale (PSS)). There was a slight improvement in cognition immediately following a reminiscence intervention, but the effect was small and of uncertain clinical importance (change scores between reminiscence and control conditions: SMD 0.11, 95% CI 0.00 to 0.23; I2 = 9%; high quality evidence; Analysis 1.5).
1.5. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 5 Cognition (overall) post‐treatment.
The MMSE was the most widely used cognitive measure, used in nine studies involving 437 participants. A fixed‐effect analysis found an improvement following reminiscence compared to the control group (MD 1.87 points, 95% CI 0.54 to 3.20; I2 = 0%; high quality evidence).
Nine studies measured the difference in cognition scores between reminiscence and control groups over a longer follow‐up period of six to 84 weeks postintervention. This involved 983 participants with 561 in the intervention groups and 422 in the control groups. There was little or no difference in outcome between groups (SMD 0.04, 95% CI ‐0.09 to 0.17; I2 = 3%; high quality evidence; Analysis 1.21). For the five studies reporting MMSE, there may have been an improvement at follow‐up of six to 36 weeks (MD 1.8 points, 95% CI ‐0.06 to 3.65; I2 = 0%; 282 participants; low quality evidence), with the quality rating reduced due to the relatively low sample size and imprecision.
1.21. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 21 Cognition (overall) at follow‐up.
Modality
There was a probable slight improvement with individual reminiscence compared with the control group in five studies with 196 participants, but we could not be sure that this was large enough to be clinically important (SMD 0.32, 95% CI 0.04 to 0.61; I2 = 6%; moderate quality evidence). For individual reminiscence, two studies (both in care homes) with 83 participants reported results at six weeks' follow‐up. There may have been some benefit, but the results were so imprecise that we could not be certain of this, or whether any effect was large enough to be clinically important (SMD 0.35, 95% CI ‐0.08 to 0.79; I2 = 0%; low quality evidence).
For the nine studies with 1023 participants of group reminiscence, there was little or no difference in cognition at the end of treatment between reminiscence and control groups (SMD 0.07, 95% CI ‐0.05 to 0.20; I2 = 0%; high quality evidence). For the six group reminiscence studies with 281 participants using the MMSE, there was a probable improvement in favour of reminiscence (MD 1.81 points, 95% CI 0.17 to 3.46; I2 = 0%; moderate quality evidence). For group reminiscence (all community‐based studies), there was little or no difference in cognition at longer‐term follow‐up of six to 84 weeks (SMD 0.01, 95% CI ‐0.12 to 0.14; I2 = 0%; 7 studies; 900 participants; high quality evidence).
Setting
Six studies in care homes, involving 230 participants, reported cognitive outcomes at end of treatment (Haight 2006; Ito 2007; Lai 2004; Morgan 2012; Subramaniam 2013; Van Bogaert 2016). There was a probable slight improvement in favour of the reminiscence intervention, but we could not be sure that this was large enough to be clinically important (SMD 0.29, 95% CI 0.03 to 0.56; I2 = 0%; moderate quality evidence).
Eight studies with 989 participants were carried out in community settings. There was little or no difference in cognition apparent at the end of treatment between reminiscence and control groups (SMD 0.07, 95% CI ‐0.05 to 0.20; I2 = 8%; high quality evidence).
All the community studies with long‐term follow‐up involved a group intervention and all those in care homes involved an individual intervention, and so these results have been detailed under 'Modality' above, with little or no difference for community/group studies, and uncertainty about possible benefit in care home/individual studies.
Communication and interaction
(See Figure 6.)
6.
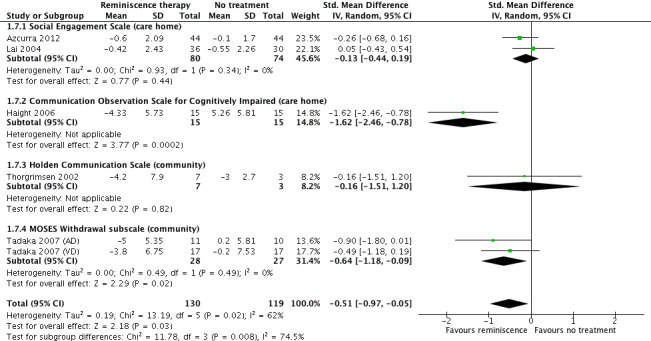
Forest plot of comparison: 1 Reminiscence therapy versus no treatment, outcome: 1.7 Communication and interaction post‐treatment.
Six studies with 249 participants, using a variety of different indicators of communication and interaction, were included in the end of treatment analysis (Azcurra 2012; Haight 2006; Lai 2004; Tadaka 2007 (AD); Tadaka 2007 (VD); Thorgrimsen 2002). (Note: in this analysis, negative scores indicated improved communication.) There may have been an improvement in communication and interaction following a reminiscence intervention, but, due to inconsistency between studies, we could not rule out a small or negligible effect (random‐effects analysis; SMD ‐0.51 points, 95% CI ‐0.97 to ‐0.05; I2 = 62%; low quality evidence; Analysis 1.7).
1.7. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 7 Communication and interaction post‐treatment.
Four of the six studies, with 204 participants, also reported data at six to 24 weeks' follow‐up (Azcurra 2012; Lai 2004; Tadaka 2007 (AD); Tadaka 2007 (VD)). There was probably an improvement in communication and interaction at longer‐term follow‐up after a reminiscence intervention, but we could not be sure that this was large enough to be clinically important (SMD ‐0.49 points, 95% CI ‐0.77 to ‐0.21; I2 = 0%; moderate quality evidence; Analysis 1.22).
1.22. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 22 Communication and interaction at follow‐up.
Modality
Two studies using individual reminiscence reported end of treatment measures of communication and interaction (Haight 2006; Lai 2004). We could not be certain whether there was an improvement as the quality of the evidence was very low, due to imprecision and serious inconsistency (random‐effects analysis; SMD ‐0.74, 95% CI ‐2.38 to 0.89; I2 = 91%; very low quality evidence). Longer‐term follow‐up data were only available in one study of individual reminiscence (Lai 2004), with effects uncertain due to imprecision.
Analysis of the four trials of group reminiscence, including 153 participants, indicated a probable slight improvement for participants receiving reminiscence compared with the control group at end of treatment, although we could not be certain of its clinical importance (SMD ‐0.39, 95% CI ‐0.71 to ‐0.06; I2 = 0%; moderate quality evidence) (Azcurra 2012; Tadaka 2007 (AD); Tadaka 2007 (VD); Thorgrimsen 2002). For group reminiscence, three studies with 138 participants reported data at six months' follow‐up (Azcurra 2012; Tadaka 2007 (AD); Tadaka 2007 (VD)). There was a probable improvement after this longer‐term follow‐up (SMD ‐0.63, 95% CI ‐0.97 to ‐0.29; I2 = 0%; moderate quality evidence).
Setting
Three studies, with 65 participants, were based in the community. There was a probable improvement on communication and interaction in favour of RT although we could not be certain it was clinically important (SMD ‐0.57, 95% CI ‐1.08 to ‐0.06; I2 = 0%; moderate quality evidence). At longer‐term follow‐up, only two studies, with 50 participants, took place in the community (Tadaka 2007 (AD); Tadaka 2007 (VD). There was a probable improvement in favour of RT, although we could not be certain it was clinically important (MOSES withdrawal subscale: MD ‐3.64 points, 95% CI ‐7.21 to ‐0.06; I2 = 0%; moderate quality evidence).
Three studies with 184 participants took place in care homes. Here, we could not ascertain from our results whether there was an important effect on communication and interaction due to imprecision and unexplained variation in results between studies (random‐effects analysis; SMD ‐0.52, 95% CI ‐1.29 to 0.24; I2 = 83%; very low quality evidence). The two care home studies, with 154 participants, with longer‐term follow‐up of six to 24 weeks used the SES (Azcurra 2012; Lai 2004). There may have been an improvement; however, imprecision and inconsistency between the studies means we could not be certain of a clinically important effect (random‐effects analysis; MD ‐0.93 points, 95% CI ‐1.77 to ‐0.09; I2 = 41%; low quality evidence).
Quality of relationship
Three studies, with 528 participants, included ratings by the person with dementia of his/her relationship with his/her family carer (Charlesworth 2016; Subramaniam 2013; Woods 2012a). All used the QCPR and reported results separately for its two subscales: warmth and absence of conflict. On both subscales, there was little or no difference between RT and control groups at the end of treatment (warmth: MD 0.16 points, 95% CI ‐0.53 to 0.84; I2 = 0%; high quality evidence; absence of conflict: MD ‐0.40 points, 95% CI ‐1.09 to 0.29; I2 = 15%; high quality evidence).
Two of the studies, involving 415 participants, both community‐based and using reminiscence groups, reported seven months' follow‐up post‐intervention. Due to the imprecision of the results and inconsistency between the two studies, we were unable to determine whether there was any effect of reminiscence on warmth at follow‐up (random‐effects analysis; MD ‐0.09 points, 95% CI ‐1.82 to 1.63; I2 = 62%; low quality evidence). There was little or no difference in absence of conflict (MD ‐0.38 points, 95% CI ‐1.28 to 0.51; I2 = 11%; high quality evidence).
Behaviour: function
Six studies, involving 1030 participants, assessed changes in the functional level of the person with dementia at the end of treatment (Amieva 2016; Azcurra 2012; Charlesworth 2016; Haight 2006; Lai 2004; Woods 2012a). In this analysis, a lower score indicated a more positive outcome. Due to imprecision and inconsistency between studies, we were uncertain whether RT improved function at the end of treatment (random‐effects analysis; SMD ‐0.24, 95% CI ‐0.69 to 0.21; I2 = 90%; very low quality evidence; Analysis 1.8). This uncertainty was present at longer‐term follow‐up (six to 84 weeks) in an analysis that involved five studies and 941 participants (random‐effects analysis; SMD ‐0.31, 95% CI ‐0.66 to 0.03; I2 = 83%; very low quality evidence; Analysis 1.24).
1.8. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 8 Behaviour (function) post‐treatment.
1.24. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 24 Behaviour (functional) at follow‐up.
Modality
Two studies, involving 96 participants, examined the effects of individual reminiscence on level of function (Haight 2006; Lai 2004). There was probably little or no difference in function between RT and control groups at the end of treatment (SMD ‐0.07, 95% CI ‐0.33 to 0.47; I2 = 0%; moderate quality evidence). Only the Lai 2004 study went on to assess participants at later follow‐up time points, with no effect evident at six weeks' post‐intervention.
Four studies implementing a group reminiscence intervention reproted a relevant outcome at end of treatment in 934 participants and at follow‐up in 875 participants (Amieva 2016; Azcurra 2012; Charlesworth 2016; Woods 2012a). From baseline to end of treatment, the difference in change scores between the RT and control groups showed a probable slight benefit of RT (random‐effects analysis; SMD ‐0.40, 95% CI ‐0.99 to 0.20; I2 = 94%; moderate quality evidence). There was a similar slight improvement at longer‐term follow‐up of six to 21 months (random‐effects analysis; SMD ‐0.38, 95% CI ‐0.78 to 0.03; I2 = 87%; moderate quality evidence). In both cases, we could not be sure that the improvement noted was large enough to be clinically important; the high inconsistency in each analysis was clearly attributable to the inclusion of the Azcurra 2012 study, the only one of the four to be located in a care home setting, and which reported more positive results in this domain than the community‐based group studies.
Setting
Three large group studies, including 846 participants, provided data on the effects of RT on functioning of community residents (Amieva 2016; Charlesworth 2016; Woods 2012a). There was little or no difference in function at the end of treatment between groups (SMD 0.05, 95% CI ‐0.09 to 0.18; I2 = 0%; high quality evidence), with a slight improvement in favour of RT, of uncertain clinical importance, at longer‐term follow‐up (SMD ‐0.12, 95% CI ‐0.27 to 0.02; I2 = 0%; high quality evidence).
Due to imprecision and inconsistency, we could not be certain of the effect of RT in care home settings (three studies with 184 participants; Azcurra 2012; Haight 2006; Lai 2004). This was true at the end of treatment (random‐effects analysis; SMD ‐0.53, 95% CI ‐1.87 to 0.80; I2 = 94%; very low quality evidence) and at longer‐term follow‐up (two studies with 154 participants; Azcurra 2012; Lai 2004) (random‐effects analysis; SMD ‐0.67, 95% CI ‐1.89 to 0.55; I2 = 92%; very low quality evidence). The inconsistency in results within these analyses was attributable to the more positive results reported by the one care home study using a group intervention (Azcurra 2012).
Behaviour: agitation/irritability
Three studies measured irritability and agitation using the MOSES irritability subscale (O'Shea 2014) and CMAI (Tadaka 2007 (AD); Tadaka 2007 (VD)). A lower score was indicative of improved agitation/irritability. The three studies included 359 participants, with 181 receiving an RT and 178 receiving control conditions, and all implemented a group reminiscence intervention. There was probably little or no difference in outcome between groups at the end of treatment (SMD 0.03, 95% CI ‐0.17 to 0.24; I2 = 0%; moderate quality evidence; Analysis 1.9). Two studies measured changes in behaviour scores again six months' post‐intervention (Tadaka 2007 (AD); Tadaka 2007 (VD)). There may have been a slight improvement, although we could not be certain of this, or of its clinical importance, due to imprecision (MD ‐1.52 points, 95% CI ‐4.07 to 1.03; I2 = 0%; low quality evidence; Analysis 1.25).
1.9. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 9 Behaviour (agitation/irritability) post‐treatment.
1.25. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 25 Behaviour (agitation/irritability) at follow‐up.
All the studies were group studies, and in the absence of heterogeneity between studies, analyses by setting were not undertaken.
Mood
(See Figure 7.)
7.
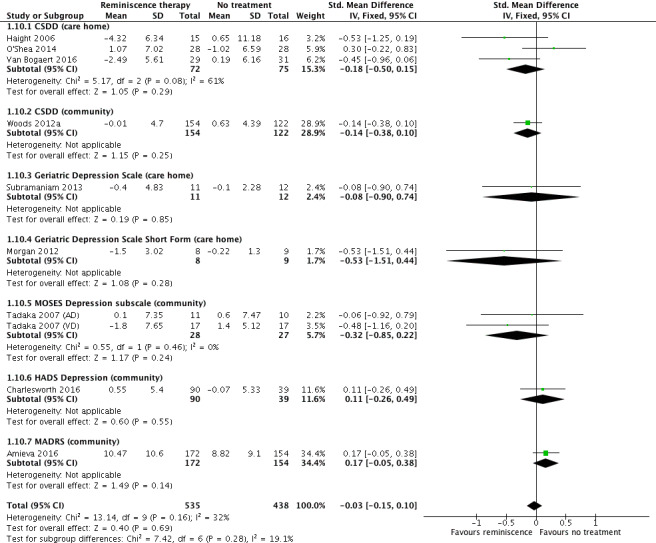
Forest plot of comparison: 1 Reminiscence therapy versus no treatment, outcome: 1.10 Mood‐related outcomes (depression) post‐treatment.
Ten studies included a mood scale administered at the end of treatment (Amieva 2016; Charlesworth 2016; Haight 2006; Morgan 2012; O'Shea 2014; Subramaniam 2013; Tadaka 2007 (AD); Tadaka 2007 (VD); Van Bogaert 2016; Woods 2012a). These included self‐report measures such as the HADS and the GDS, but four studies using the CSDD, where the researcher integrated reports from the carer and the person with dementia. In these analyses, negative scores indicated improved mood.
For depression, the 10 studies included 973 participants. There was little or no difference in depression between groups evident at the end of treatment (SMD ‐0.03, 95% CI ‐0.15 to 0.10; I2 = 32%; high quality evidence; Analysis 1.10). At longer‐term follow‐up (six to 84 weeks), six studies, including 747 participants, reported measures of depressed mood. We could not be certain of the effects of RT at follow‐up, as the results were consistent with improvement or with little or no effect, and there was inconsistency between studies attributable to the reminiscence modality (random‐effects analysis; SMD ‐0.16, 95% CI ‐0.43 to 0.11; I2 = 55%; moderate quality evidence; Analysis 1.26).
1.10. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 10 Mood‐related outcomes (depression) post‐treatment.
1.26. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 26 Mood‐related outcomes (depression) at follow‐up.
Two large community‐based studies of group reminiscence, including 436 participants, analysed anxiety (Charlesworth 2016; Woods 2012a). There was little or no difference in anxiety between groups at the end of treatment (SMD ‐0.03, 95% CI ‐0.22 to 0.16; I2 = 0%; high quality evidence; Analysis 1.11), and there was probably little or no difference in anxiety at seven months' follow‐up (SMD 0.01, 95% CI ‐0.20 to 0.21; I2 = 0%; moderate quality evidence; 391 participants; Analysis 1.27).
1.11. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 11 Mood‐related outcomes (anxiety) post‐treatment.
1.27. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 27 Mood‐related outcomes (anxiety) at follow‐up.
One study with 326 participants evaluated apathy, using a carer‐rated Apathy Index (Amieva 2016). There was probably little or no difference in apathy between groups at the end of treatment assessment or at 21 months' follow‐up.
Modality
The four studies using an individual reminiscence approach included 131 participants. There was probably a slight effect on depressed mood in favour of individual RT, although we could not be sure of its clinical importance (SMD ‐0.41, 95% CI ‐0.76 to ‐0.06; I2 = 0%; moderate quality evidence). The single study with an individual approach that included a longer‐term follow‐up (six weeks) showed a probable benefit, but the sample size (17 participants) was very small (Morgan 2012).
Six studies with 842 participants used a group approach. There was little or no difference between group RT and controls (SMD 0.03, 95% CI ‐0.10 to 0.17; I2 = 27%; high quality evidence). At longer‐term follow‐up of six to 21 months, five studies of group RT reported measures of depressed mood, but all were community based, so the results were confounded with the setting. There was little or no difference related to the RT (SMD ‐0.04, 95% CI ‐0.19 to 0.11; I2 = 0%; high quality evidence; 730 participants).
Setting
Five studies with 187 participants were based in care homes. There was probably a small benefit of RT, but we could not rule out little or no effect and could not be sure of the clinical importance of any effect (SMD ‐0.19, 95% CI ‐0.48 to 0.10; I2 = 30%; moderate quality evidence).
The five community‐based studies, all involving group interventions, included 786 participants and showed little or no effect of RT (SMD 0.01, 95% CI ‐0.13 to 0.16; I2 = 31%; high quality evidence).
The results for longer‐term follow‐up were discussed in the 'Modality' section above, with all the community studies being group studies, and the single care home study followed up involving individual reminiscence.
Outcomes for the carer
For all carer outcomes, lower scores indicated a more positive outcome.
Stress related to caring
Seven studies used measures such as the Relative Stress Scale (RSS) and ZBI that evaluated the carer's stress directly related to aspects of caring (Amieva 2016; Azcurra 2012; Charlesworth 2016; O'Shea 2014; Särkämö 2013; Thorgrimsen 2002; Woods 2012a). In six of the studies, the carer was a family member or friend, but in the O'Shea 2014 study, the carer was a healthcare assistant or nurse. At end of treatment, these studies involved 1155 participants. Overall, there was probably little or no difference in carer stress related to the reminiscence intervention (random‐effects analysis; SMD ‐0.03, 95% CI ‐0.21 to 0.14; I2 = 43%; moderate quality evidence; Analysis 1.12). There appeared to be some inconsistency between studies, related to the different settings. Excluding the O'Shea 2014 study, so that the 965 participants were all family or friend carers made little difference to the overall results (random‐effects analysis; SMD ‐0.08, 95% CI ‐0.32 to 0.16; I2 = 59%; high quality evidence). The inclusion of a single care home study, which reported much more positive findings, was responsible for the observed inconsistency (Azcurra 2012).
1.12. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 12 Carer outcomes (stress related to caring) post‐treatment.
Five studies, involving 895 participants, went on to measure carer stress at follow‐up of six to 21 months (Amieva 2016; Azcurra 2012; Charlesworth 2016; Särkämö 2013; Woods 2012a). The results were again inconsistent between studies (due to the inclusion of the single care home study) and the result of the meta‐analysis was imprecise being compatible with either improvement or a small or no effect (random‐effects analysis; SMD ‐0.19, 95% CI ‐0.54 to 0.16; I2 = 82%; moderate quality evidence; Analysis 1.28).
1.28. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 28 Carer outcomes (stress related to caring) at follow‐up.
Modality
All the seven studies that measured stress related to caring involved group reminiscence (Amieva 2016; Azcurra 2012; Charlesworth 2016; O'Shea 2014; Särkämö 2013; Thorgrimsen 2002; Woods 2012a). However, an additional aspect of modality that should be considered in relation to carer outcomes relates to whether the family carer was actively involved in the reminiscence group. This joint reminiscence was a key feature of the Charlesworth 2016; Särkämö 2013; Thorgrimsen 2002 and Woods 2012a studies, which included 551 participants. There was little or no difference in carer stress between the reminiscence intervention and control conditions at end of treatment in these studies (SMD 0.04, 95% CI ‐0.13 to 0.21; I2 = 16%; high quality evidence) or at longer‐term follow‐up of six to seven months (SMD ‐0.04, 95% CI ‐0.22 to 0.15; I2 = 0%; high quality evidence; 3 studies; 481 participants). In contrast, only one study including 88 participants evaluated family carer stress when family carers were not extensively involved in the RT (Azcurra 2012). The study used the ZBI and found that there was probably a benefit to carers at the end of treatment (MD ‐4.90 points, 95% CI ‐8.20 to ‐1.60; moderate quality evidence) and at six months' follow‐up (MD ‐7.90 points, 95% CI ‐10.97 to ‐4.83; moderate quality evidence).
Setting
Five studies, including 877 participants, were community‐based (Amieva 2016; Charlesworth 2016; Särkämö 2013; Thorgrimsen 2002; Woods 2012a). There was little or no difference in carer stress between groups at end of treatment (SMD 0.05, 95% CI ‐0.08 to 0.19; I2 = 0%; high quality evidence) or at longer‐term follow‐up of six to 21 months (SMD 0.02, 95% CI ‐0.12 to 0.16; I2 = 0%; high quality evidence; 4 studies; 807 participants).
There were two care home studies, both using the ZBI (Azcurra 2012; O'Shea 2014). The Azcurra 2012 study, which involved family carers, reported probable benefit at both end of treatment and longer‐term follow‐up, but, when combined with the O'Shea 2014 study, end of treatment data on staff carers, we could not be certain of any benefits due to inconsistency between the studies and imprecision (random‐effects analysis; MD ‐1.48 points, 95% CI ‐5.43 to 2.47; I2 = 70%; very low quality evidence; 278 participants).
Mood: depression and anxiety
Two large community‐based joint reminiscence group studies with 517 participants used the HADS to evaluate changes in anxiety and depressed mood following carers' participation in joint reminiscence groups with people with dementia (Charlesworth 2016; Woods 2012a). There was little or no difference between groups on the HADS Anxiety subscale at the end of treatment (MD 0.06 points, 95% CI ‐0.54 to 0.66; I2 = 0%; high quality evidence; Analysis 1.14). At seven months' follow‐up, there was probably a slight advantage for the control participants, but we could not be certain of the clinical implications of this result, which was also consistent with little or no difference (MD 0.56 points, 95% CI ‐0.17 to 1.30; I2 = 0%; moderate quality evidence; 464 participants; Analysis 1.30). There was little or no difference between reminiscence and control conditions for the HADS Depression subscale at the end of treatment (MD ‐0.08 points, 95% CI ‐0.59 to 0.44; I2 = 0%; high quality evidence; Analysis 1.13) and at seven months' follow‐up (MD ‐0.05 points, 95% CI ‐0.71 to 0.60; I2 = 0%; high quality evidence; Analysis 1.29).
1.14. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 14 Carer outcomes (anxiety) post‐treatment.
1.30. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 30 Carer outcomes (anxiety) at follow‐up.
1.13. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 13 Carer outcomes (depression) post‐treatment.
1.29. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 29 Carer outcomes (depression) at follow‐up.
Well‐being and quality of life
Four community‐based joint reminiscence group studies, including 530 participants, evaluated aspects of carer psychological well‐being (Charlesworth 2016; Särkämö 2013; Thorgrimsen 2002; Woods 2012a). Outcome scales included the GHQ‐12, GHQ‐28 and SF‐12 Mental component. There was little or no difference in carer well‐being between groups at end of treatment (SMD ‐0.04, 95% CI ‐0.22 to 0.13; I2 = 1%; high quality evidence; Analysis 1.15). Three studies provided longer‐term follow‐up data after six to seven months, and there was little or no difference in carer well‐being (SMD 0.01, 95% CI ‐0.18 to 0.19; I2 = 0%; high quality evidence; 467 participants; Analysis 1.31) (Charlesworth 2016; Särkämö 2013; Woods 2012a).
1.15. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 15 Carer outcomes (quality of life) post‐treatment.
1.31. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 31 Carer outcomes (quality of life) at follow‐up.
Quality of caring relationship
Three studies, including up to 528 participants, evaluated the quality of the relationship between the carer and the person with dementia (as rated by the carer), all using the QCPR with two subscales: warmth and absence of conflict (Charlesworth 2016; Subramaniam 2013; Woods 2012a). There was little or no difference related to RT on the warmth subscale at the end of treatment (MD ‐0.01 points, 95% CI ‐0.77 to 0.76; I2 = 0%; high quality evidence) or (for the Charlesworth 2016 and Woods 2012a studies) after seven months' follow‐up (MD ‐0.66 points, 95% CI ‐1.59 to 0.27; I2 = 0%; high quality evidence). Similarly, there was little or no difference on the absence of conflict subscale at end of treatment (MD ‐0.26 points, 95% CI ‐1.01 to 0.48; I2 = 0%; high quality evidence) or after longer‐term follow‐up (MD ‐0.37 points, 95% CI ‐1.23 to 0.50; I2 = 0%; high quality evidence).
Adverse outcomes
The only outcome identified that probably favoured control participants was carer anxiety at seven months' follow‐up, from an analysis involving two large community studies that involved family carers along with people with dementia in reminiscence groups. The estimated MD was small enough to be of uncertain clinical importance and the evidence was of moderate quality, downgraded due to imprecision. These two studies also reported a few incidences of specific adverse outcomes. The Charlesworth 2016 study recorded 159 'serious adverse events' during the trial, with three of these attributable to the RYCT intervention. Specific details were not given, though it reported that none of these three events led to withdrawal. The Woods 2012a study recorded one adverse event linked to participation in the trial. One participant became upset in one of the intervention sessions relating to marriage. There was a detailed protocol for dealing with distressing events that was implemented. While adverse events are regrettable, it is important to view them in context of the total number of participants and intervention sessions.
Discussion
Summary of main results
There has been a welcome increase in the volume of research on reminiscence in dementia care (and an improvement in its quality) since this review was last updated. It has now been possible to include large‐scale multicentre RCTs, using clearly defined interventions and protocols. It has also been possible to exclude studies where the risk of bias was rated as too high, without detracting greatly from the volume of research considered. For several outcomes, meta‐analyses included 800 or more participants. For the first time, it was possible to undertake analyses taking into account different modalities of reminiscence work and different contexts. Individual and group reminiscence work can now be considered separately for several outcomes, and community studies distinguished from those carried out in care homes.
The primary finding of the review was that reminiscence work was not associated consistently with improved well‐being and QoL for people with dementia assigned to receive it in research studies. Although its clinical importance was uncertain, in care homes, but not in community settings, there was a probable benefit on QoL measures immediately following the reminiscence intervention. This finding arose from a meta‐analysis of three studies, from different countries, involving 193 people with dementia. Notably, in four of the five community studies in this analysis (and none of the care home studies), the intervention involved joint group sessions, where people with dementia and their family carers participated together.
The extent to which reminiscence work would be predicted to improve cognitive functioning is debatable, but this review provides evidence (across 14 studies involving 1219 people with dementia) of a small benefit on cognitive tests evident immediately following the reminiscence work, but not sustained after a longer follow‐up period. The analyses separating individual and group reminiscence work indicated probable slight benefits in cognition related to individual work. There was a probable slight benefit to cognition in care homes but not the community. The overall effect size for cognition at the end of treatment (SMD) was 0.11 (95% CI 0.00 to 0.23); the comparable SMD from the Cochrane Review of cognitive stimulation (Woods 2012b) was 0.41 (95% CI 0.25 to 0.57). However, a direct comparison of the subset of studies using the MMSE in the two reviews indicated an MD of 1.74 points (95% CI 1.13 to 2.36), from 10 studies of cognitive stimulation involving 600 participants, compared with an MD of 1.87 points (95% CI 0.54 to 3.20) for nine studies of RT involving 437 participants. This suggests the effects may be comparable on the MMSE between the two types of interventions, but with a wider CI for the reminiscence result.
The quality of evidence relating to the communication and interaction outcome was lower, but there was probably a benefit of RT at longer‐term follow‐up, albeit of uncertain clinical importance. The number of studies including a relevant outcome measure was smaller (six at end of treatment, four at follow‐up, with 200 or more participants in each case), and the large studies of joint (with carer) reminiscence work were among those without a relevant outcome measure in this domain. Here, a probable effect was evident for community‐based studies at end of treatment and follow‐up, but was even less certain in the care home context. Group reminiscence was associated with a probable slight benefit in communication immediately and a benefit within the clinically important range at follow‐up, whereas (in smaller studies) there was considerable uncertainty in the results for individual reminiscence work.
Despite a body of evidence for the effects of reminiscence on depressed mood in older people without dementia (e.g. Bohlmeijer 2003; Pinquart 2007), only individual reminiscence work was associated with a probable improvement in mood for people with dementia in this review, and the size of the effect (SMD ‐0.41) was relatively small and of uncertain clinical importance. There were no indications of benefits associated with reminiscence work in relation to the other outcomes examined for the person with dementia. These included the person's level of function, extent of irritability and agitation, and their own rating of the quality of relationship with their family carer. Despite the inclusion of several large studies of joint reminiscence work, where family carers were fully involved in the reminiscence sessions, we identified no benefits for family carers in relation to reduced stress related to caring, well‐being and QoL, carer mood or the carer's rating of the quality of their relationship with the person with dementia. The only exception to this was a single care home study, with 88 participants, in which family carers probably experienced less stress after their relative had been involved in reminiscence groups, both immediately after treatment and at six months' follow‐up. Interestingly, this was the only study to examine family carer outcomes that did not have a focus on joint reminiscence work. There were some suggestions from the REMCARE (REMiniscence groups for people with dementia and their family CAREgivers) trial (Woods 2012a) of negative effects on carer anxiety, and this was evident to an extent in the analyses combining data from several studies of joint reminiscence work, where there was slightly higher carer anxiety (a difference of uncertain clinical importance) at the seven months' follow‐up assessment. One qualitative study explored potential factors in increased anxiety among carers taking part in joint reminiscence groups (Melunsky 2015). It identified issues such as the carer feeling disappointed when improvements in the group setting were not evident at home; the carer seeing people with more advanced dementia, resulting in increased fears for what the future might hold; and increased guilt from not being able to put into practice skills learned in the groups. These negative aspects were in the context of many positive experiences that carers reported from participation in the groups for themselves and the person with dementia.
Overall completeness and applicability of evidence
Although there is now a sufficient body of evidence to enable us to draw conclusions regarding reminiscence work in general, it remains difficult to consider fully different types of reminiscence work. For example, studies of individual reminiscence work have tended to be small‐scale and carried out in care homes, so we could not be certain of any difference in outcomes between individual and group approaches. Related to this, we were unable to draw a distinction in our analyses between simple and integrative reminiscence work. Although some studies have followed a very clear, published treatment protocol, reporting of details of interventions in other studies has been less complete, with even the distinction between individual and group reminiscence not always immediately apparent.
Little evidence has emerged regarding the characteristics of people with dementia that might be associated with better outcomes, with the exception of the suggestion that reminiscence has a stronger effect on QoL in a care home context, as opposed to community settings. There clearly are differences between studies in the extent (and direction of changes) demonstrated by the high levels of heterogeneity evident in several analyses. However, many of these differences are yet to be explained. Some studies included only people with AD; others only recruited people with VD; others included any form of dementia. No clear differences in outcomes related to dementia type emerged from the analyses undertaken, and similarly there were few indications of the effects of dementia severity.
It is unlikely that there is a simple 'dose' related effect, in that the studies offering the greatest exposure to reminiscence activities were among those with the least positive findings (Amieva 2016; Charlesworth 2016; Woods 2012a). However, within studies a 'dose' effect may have operated. It is clear that in community settings, a significant proportion of people randomised to receive a reminiscence intervention did not engage with the groups. For example, in Woods 2012a, 11% of participants did not attend a single reminiscence session, with over 25% attending three sessions or fewer. An even larger proportion of participants in the Charlesworth 2016 study (43%) did not attend any reminiscence sessions. In line with our protocol, we included the ITT results in our analyses in this review. While it is important to know that these groups may not, for a variety of reasons, be taken up by all people with dementia and their carers, their results must underestimate any actual direct effects of reminiscence. For example, in a compliance analysis, Woods 2012a showed an improvement in cognitive function (AMI‐E PSS) at the end of treatment and improved QoL (European Quality of Life 5 Dimensions; EQ‐5D) and quality of relationship (QCPR) at longer‐term follow‐up, but accompanied by an increase in carer stress (RSS). In contrast, Charlesworth 2016 reported no relationship between attendance and outcomes.
Quality of the evidence
There has been an overall significant improvement in the quality of included studies since the previous version of this review was undertaken. We are now able to include large‐scale studies, overseen by accredited clinical trials units, with quality assurance procedures and well‐developed remote randomisation procedures.
However, more generally, under‐reporting of details of trials meant that a number of authors had to be contacted for additional information regarding, for example, randomisation and allocation, and several studies were excluded because of their risk of bias.
Some risks of bias arise from the nature of psychosocial interventions such as RT. Participants and carers will be aware of the intervention being received, and, in general single‐blinding is the aim, with assessors being blind to treatment allocation. However, ratings completed by, for example, staff in care homes, may not be blinded, and the blinding of assessors may be compromised by participants and carers providing indications a treatment intervention has been received. Studies such as Woods 2012a asked assessors to indicate which group the participant was in, and their degree of certainty of their judgement so that the extent of bias could be estimated. Expectations of benefit from participation or resentment at not being allocated to the active treatment may occur, and may produce some additional bias. Treatment expectations may be seen in the context of a pragmatic trial as part of the overall 'treatment package,' of course.
Potential biases in the review process
Our search strategy was as comprehensive as possible, and we consulted with experts in the field to identify any further studies. Two review authors (LOP and EF) independently conducted selection of studies, data extraction and risk of bias assessments, and disagreements resolved by contacting authors and consultation with other members of the review author team. The present review included all outcomes detailed in the protocol, irrespective of whether or not the results identified improvements. It must be acknowledged that the included studies could represent a biased sample of the studies undertaken worldwide on RT. It may be the case that trials that are not 'successful' (i.e. do not produce the expected positive findings) are less likely to be published. This may be especially the case with smaller trials. The welcome trend to preregistration of trials, and the publication of trial protocols, makes this less likely to occur in the future in relation to larger, well‐funded trials. The meta‐analyses here have been influenced strongly by larger trials, several of which did not report any positive findings (e.g. Charlesworth 2016; Woods 2012a), but these were both of a group approach, in community settings. Our care home and individual reminiscence findings could perhaps have been more influenced by publication bias. A funnel plot of cognition at the end of treatment showed some asymmetry (Figure 8), but this was largely driven by smaller/lower quality community studies having positive findings, with care home studies showing a symmetrical pattern. For most of our outcomes, there are too few included studies for meaningful funnel plots to be plotted.
8.
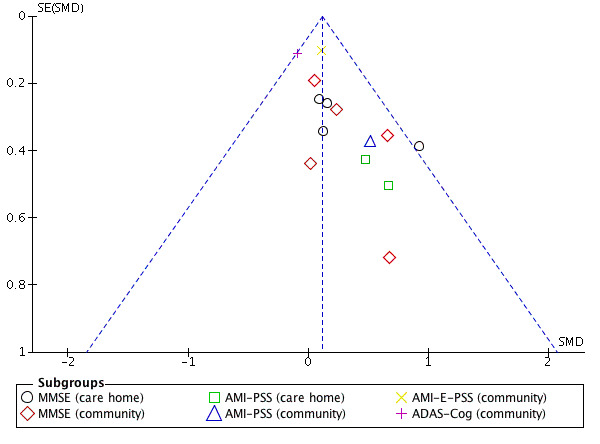
Funnel plot of comparison: 1 Reminiscence therapy versus no treatment, outcome: 1.5 Cognition (overall) post‐treatment.
ADAS‐Cog: Alzheimer’s Disease Assessment Scale for Cognition; AMI‐PSS: Autobiographical Memory Interview ‐ Perceived Stress Scale; AMI‐E‐PSS: Autobiographical Memory Interview Extended Version ‐ Perceived Stress Scale; MMSE: Mini‐Mental State Examination.
Agreements and disagreements with other studies or reviews
We identified five reviews that overlap with this one.
Cotelli 2012 included seven RCTs, with 218 participants, three of which were included in the current review. They identified some benefits for mood and cognitive function but pointed out that the number of trials 'remains very small and their quality is often poor' (two were excluded from this review on the basis of risk of bias). This review appeared to have predated the recent improvement in quality and size of trials.
Subramaniam 2012 focused on individual reminiscence work, identifying five RCTs, three of which were included in the current review. They concluded that there was a consistent pattern emerging, with those studies offering 'individual reminiscence work that includes a life review process, uses specific memory triggers and results in the production of a life story book' having positive psychosocial outcomes for people with dementia. In contrast, where reminiscence was more general, evidence for efficacy was not apparent. Unfortunately, there are still insufficient studies of integrative reminiscence work to confirm this early conclusion.
Kwon 2013 reported a meta‐analysis including 10 studies. The studies included are not referenced, so comparisons were difficult. They conclude that reminiscence had a positive effect on cognition, depression and QoL, all with large effect sizes, but not on problem behaviour.
Testad 2014 reported a broader review relating to people with dementia in care homes, with RT included as one of several psychosocial interventions. They included six studies involving RT, most of which did not meet the inclusion criteria for the current review (e.g. three were not RCTs). The authors concluded that reminiscence was associated with improved mood, but there was no consistent evidence regarding other outcomes.
Huang 2015 included 12 studies, with 1325 participants. Cognition and depressed mood were the main outcomes studied, with nine studies contributing to meta‐analyses in each case. As with the current review, they identified a small effect size for cognition (SMD 0.18, 95% CI 0.05 to 0.30) but unlike the current review, there was a moderate‐sized effect for depressed mood (SMD ‐0.48, 95% CI ‐0.70 to ‐0.28). There was evidence that the effect on mood was greater in care home settings than in the community, which is in accordance with our results. Notably the community studies included in the Huang 2015 review were all included in this review, but there were differences in the care home studies included, partly due to different exclusion criteria, but also because they were able to include studies published in Chinese. In general, the current review has adopted stricter quality standards than other reviews, and identified considerably more studies for inclusion, across different modalities and settings.
Authors' conclusions
Implications for practice.
Whilst this updated review has shown that reminiscence therapy (RT) can improve outcomes for people with dementia, its effects are inconsistent, often small in size and can differ considerably across settings and modalities. The outcomes for which some benefit has been identified are cognitive function, communication/interaction, quality of life (QoL) and mood. However, the effects are not consistent across different types of reminiscence work (group or individual, with or without family carer involvement), or across different contexts (care home or community), particularly where QoL is the outcome. The evidence relating to QoL is most promising in care homes; that relating to mood is most promising for individual RT.
RT can now be viewed alongside cognitive stimulation as an ecopsychosocial intervention with a credible evidence‐base. Individual and group approaches have some support, although the two large, well‐conducted UK studies of joint reminiscence group work involving family members alongside people with dementia have been the studies showing the smallest effects. Individual work has the potential benefit of resulting in some form of life story book, which provides a platform to enhance person‐centred care. However, to date it has not proven clearly superior to group work, which may have added value in terms of enhancing interaction and communication.
The lack of participation in the two UK studies of joint reminiscence work suggests that consideration should be given to offering it as one of a number of approaches, as participation does not appear to be valued by a significant number of people with dementia and carers. Where it is offered, benefits beyond the 'in the moment' enjoyment of a shared social group experience, should not be anticipated as general outcomes.
The diversity of approaches to reminiscence seen in the various studies suggests that there is a need for manuals and training to be developed so that approaches can be more readily shared, and common approaches developed, for both individual and group work. It is essential that the different functions of reminiscence and the different types of reminiscence work are recognised, to aid sharing of good practice and understanding of the training, support and resources needed for implementation.
Implications for research.
The research agenda in relation to reminiscence work now needs to address some of the discrepancies and uncertainties highlighted by this review, and more fully reflect and identify the differences in function and types of reminiscence work. A large scale randomised controlled trial (RCT) of individual, integrative reminiscence work, producing a conventional or digital life story book, would demonstrate whether the promising results from small studies could be replicated on a larger platform, with greater attention to the detail of randomisation and allocation concealment. Such a study should include enough people living with dementia in care home and community settings, and with a range of severities of impairment, so that more fine‐grained conclusions may be drawn. Research is also needed on the extent to which reminiscence work can drive person‐centred care, so that the person's biography becomes a rich resource for planning and action.
There has been increasing interest in digital reminiscence work (e.g. Subramaniam 2010; Subramaniam 2016), but to date there have been no studies meeting the criteria for inclusion in the current review. This is clearly an area where more research is justified, in developing the intervention and then delineating its effects.
The research to date has emphasised changes beyond the group, on measures carried out before and after a set number of reminiscence sessions. More emphasis on the experience of people with dementia (and carers) within the reminiscence session may be helpful. Is each session an enjoyable experience in itself, even if the lasting benefits are more elusive? Brooker 2000 used an observational method, Dementia Care Mapping, to demonstrate that people with dementia showed greater well‐being when participating in simple reminiscence groups than when undertaking other activities, and it would be helpful to take this 'in the moment' evaluation approach further.
Finally, in view of the significant number of people not taking up reminiscence interventions, research would be helpful delineating who does take it up and why, and what type of approach is beneficial for which people, so there can be better tailoring of interventions to individuals.
What's new
| Date | Event | Description |
|---|---|---|
| 6 April 2017 | New search has been performed | Top‐up searches were performed for this review in July 2011, October 2014, July 2015, April 2016 and April 2017. New studies were identified for inclusion within the review. |
| 6 April 2017 | New citation required and conclusions have changed | New studies added and content extensively revised. Conclusions changed. Additional authors brought in. |
History
Protocol first published: Issue 2, 1998 Review first published: Issue 3, 1998
| Date | Event | Description |
|---|---|---|
| 31 July 2011 | New search has been performed | An update search was performed for this review on 31 July 2011. New studies were identified for both inclusion and exclusion within the review. |
| 6 November 2008 | Amended | Converted to new review format. |
| 6 February 2005 | New citation required and conclusions have changed | The review has been substantially updated and rewritten following the publication of three new trials. |
Acknowledgements
The authors wish to thank Sue Marcus, Jenny McCleery and Anna Noel‐Storr of the Cochrane Dementia and Cognitive Improvement Group for their invaluable assistance. They are also grateful to the authors who provided additional data or clarification regarding their studies.
Appendices
Appendix 1. Update searches: July 2011, October 2014, April 2015, April 2016, April 2017
|
Source (searched 31 July 2011, 2 October 2014, and then 29 April 2015, 5 April 2016, 6 April 2017) |
Search strategy | Hits retrieved | |
| 1. ALOIS (www.medicine.ox.ac.uk/alois) [Date of most recent search: 6 April 2017] |
Keyword search: reminiscence OR RT |
July 2011: 40 Oct 2014: April 2015: 3 April 2016: 2 April 2017: 0 |
|
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1946‐present (OvidSP) [Date of most recent search: 6 April 2017] |
1. reminisc*.ti,ab. 2. RT.mp. and (dement* or alzheimer* or lewy or VCI).ti,ab. [mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier] 3. 1 or 2 4. randomized controlled trial.pt. 5. controlled clinical trial.pt. 6. randomized.ab. 7. placebo.ab. 8. randomly.ab. 9. trial.ab. 10. groups.ab. 11. or/4‐10 12. 3 and 11 13. (animals not (humans and animals)).sh. 14. 12 not 13 |
July 2011: 234 Oct 2014: 228 April 2015: 73 April 2016: 64 April 2017: 75 |
|
| 3. Embase 1980‐ 5 April 2017 (OvidSP) [Date of most recent search: 6 April 2017] |
1. reminisc*.ti,ab. 2. (RT and (dement* or alzheimer* or lewy or VCI or "cognit* impair*")).ti,ab. 3. or/1‐2 4. randomly.ab. 5. trial.ti,ab. 6. RCT.ti,ab. 7. ("single‐blind*" or "double‐blind*").ti,ab. 8. clinical trial/ 9. groups.ab. 10. or/4‐9 11. 3 and 10 12. limit 11 to human |
July 2011: 221 Oct 2014: 354 April 2015: 158 April 2016: 115 April 2017: 98 |
|
| 4. PsycINFO 1806‐April 2017 (OvidSP) [Date of most recent search: 6 April 2017] |
1. reminisc*.ti,ab. 2. (RT and (dement* or alzheimer* or lewy or VCI or "cognit* impair*")).ti,ab. 3. or/1‐2 4. randomly.ab. 5. groups.ab. 6. trial.ti,ab. 7. ("double‐blind*" or "single‐blind*").ti,ab. 8. Clinical Trials/ 9. RCT.ti,ab. 10. placebo.ab. 11. (randomised or randomized).ti,ab. 12. or/4‐11 13. 3 and 12 |
July 2011: 183 Oct 2014: 123 April 2015: 43 April 2016: 41 April 2017: 39 |
|
| 5. CINAHL (EBSCOhost) [Date of most recent search: 6 April 2017] |
S1 (MH "Reminiscence Therapy") S2 TX reminisc* S3 TX RT AND dement* S4 TX RT AND alzheimer* S5 TX RT AND lewy S6 TX RT AND "cognit* impair*" S7 S1 or S2 or S3 or S4 or S5 or S6 S8 TX random* S9 TX RCT OR CCT S10 (MH "Clinical Trials") S11 AB groups S12 TX "double‐blind*" OR "single‐blind*" S13 AB placebo* S14 S8 or S9 or S10 or S11 or S12 or S13 S15 S7 and S14 S16 EM 2004 S17 EM 2005 S18 EM 2006 S19 EM 2007 S20 EM 2008 S21 EM 2009 S22 EM 2010 S23 EM 2011 S24 S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 S25 S15 and S24 |
July 2011: 201 Oct 2014: 102 April 2015: 32 April 2016: 34 April 2017: 46 |
|
| 6. ISI Web of Science ‐ all databases [includes: Web of Science (1945‐present); BIOSIS Previews (1926‐present); MEDLINE (1950‐present); Journal Citation Reports] [Date of most recent search: 6 April 2017] |
Topic=(reminiscence therapy) AND Topic=(dementia* OR alzheimer*) AND Year Published=(2004‐2011) AND Topic=(random* OR trial OR RCT OR groups OR "double‐blind*" OR "single‐blind*") |
July 2011: 50 Oct 2014: 40 April 2015: 42 April 2016: 22 April 2017: 38 |
|
| 7. LILACS (BIREME) [Date of most recent search: 6 April 2017] |
Free form: reminiscence | July 2011: 7 Oct 2014: 12 April 2015: 0 April 2016: 0 April 2017: 13 |
|
| 8. CENTRAL (The Cochrane Library) (Issue 4 of 12, 2017) [Date of most recent search: 6 April 2017] |
#1 reminisc* #2 RT AND (dement* OR AD OR alzheimer* OR lewy OR "cognit* impair*") #3 (#1 OR #2), from 2004 to 2017 |
July 2011: 128 Oct 2014: 118 April 2015: 40 April 2016: 0 April 2017: 48 |
|
| 9. ClinicalTrials.gov (www.clinicaltrials.gov) [Date of most recent search: 6 April 2017] |
Interventional Studies | reminiscence | July 2011: 7 Oct 2014: 8 April 2015: 2 April 2016: April 2017: 6 |
|
| 10. ICTRP Search Portal (apps.who.int/trialsearch) [includes: Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] [Date of most recent search: 6 April 2017] |
Keyword search: reminiscence | July 2011: 19 Oct 2014: 20 April 2015: 3 April 2016: April 2017: 7 |
|
| PsycBITE [Date of most recent search: 6 April 2015] |
Keyword: Reminiscence AND Method: RCT | July 2011: 11 April 2015: 0 |
|
| TOTAL before deduplication | July 2011: 1101 Oct 2014: 1015 April 2015: 396 April 2016: April 2017: 370 |
||
| TOTAL after deduplication and first assessment by CDCIG information specialists | July 2011: Oct 2014: 102 April 2015: 21 April 2016: 279 April 2017: 37 |
||
Data and analyses
Comparison 1. Reminiscence therapy versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Self‐reported quality of life post‐treatment | 8 | 1060 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.12, 0.33] |
| 1.1 SR‐QoL (care home) | 1 | 88 | Std. Mean Difference (IV, Random, 95% CI) | 0.45 [0.03, 0.88] |
| 1.2 QoL‐AD rated by person with dementia (care home) | 2 | 105 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.08, 0.86] |
| 1.3 QoL‐AD rated by person with dementia (community) | 5 | 867 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.24, 0.06] |
| 2 Proxy rated quality of life post‐treatment | 5 | 763 | Mean Difference (IV, Random, 95% CI) | 0.35 [‐1.23, 1.94] |
| 2.1 QoL‐AD rated by carer (community) | 4 | 577 | Mean Difference (IV, Random, 95% CI) | 0.29 [‐1.75, 2.33] |
| 2.2 QoL‐AD rated by carer (care home) | 1 | 186 | Mean Difference (IV, Random, 95% CI) | 1.08 [‐1.33, 3.49] |
| 3 Observed quality of life (post‐treatment) | 2 | 154 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.17, 0.18] |
| 3.1 WIB (care home) | 2 | 154 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.17, 0.18] |
| 4 Cognition post‐treatment | 14 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 MMSE (care home) | 4 | 190 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.24 [‐0.05, 0.53] |
| 4.2 MMSE (community) | 5 | 247 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.06, 0.47] |
| 4.3 AMI‐PSS (care home) | 2 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.55 [‐0.08, 1.19] |
| 4.4 AMI‐PSS (community) | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.52 [‐0.21, 1.25] |
| 4.5 AMI‐E‐PSS (community) | 1 | 386 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.09, 0.31] |
| 4.6 AMI‐AIS (care home) | 2 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.52, 0.77] |
| 4.7 AMI‐AIS (community) | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.76 [0.02, 1.51] |
| 4.8 AMI‐E‐AIS (community) | 1 | 386 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.16, 0.25] |
| 4.9 ADAS‐Cog (community) | 1 | 326 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.31, 0.12] |
| 5 Cognition (overall) post‐treatment | 14 | 1219 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.00, 0.23] |
| 5.1 MMSE (care home) | 4 | 190 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.24 [‐0.05, 0.53] |
| 5.2 MMSE (community) | 5 | 247 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.06, 0.47] |
| 5.3 AMI‐PSS (care home) | 2 | 40 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.55 [‐0.08, 1.19] |
| 5.4 AMI‐PSS (community) | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.52 [‐0.21, 1.25] |
| 5.5 AMI‐E‐PSS (community) | 1 | 386 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.09, 0.31] |
| 5.6 ADAS‐Cog (community) | 1 | 326 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.31, 0.12] |
| 6 Quality of caring relationship post‐treatment | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 QCPR Warmth rated by person with dementia (care home) | 1 | 23 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.39 [‐0.44, 1.21] |
| 6.2 QCPR Warmth rated by person with dementia (community) | 2 | 505 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.17, 0.20] |
| 6.3 QCPR conflict rated by person with dementia (care home) | 1 | 23 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.67 [‐1.51, 0.18] |
| 6.4 QCPR conflict rated by person with dementia (community) | 2 | 500 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.22, 0.14] |
| 7 Communication and interaction post‐treatment | 6 | 249 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.97, ‐0.05] |
| 7.1 Social Engagement Scale (care home) | 2 | 154 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.44, 0.19] |
| 7.2 Communication Observation Scale for Cognitively Impaired (care home) | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.62 [‐2.46, ‐0.78] |
| 7.3 Holden Communication Scale (community) | 1 | 10 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐1.51, 1.20] |
| 7.4 MOSES Withdrawal subscale (community) | 2 | 55 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.18, ‐0.09] |
| 8 Behaviour (function) post‐treatment | 6 | 1030 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.69, 0.21] |
| 8.1 MDS‐ADL (care home) | 1 | 66 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.52, 0.45] |
| 8.2 Functional Independence Measure (care home) | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.41, 1.03] |
| 8.3 ADL (care home) | 1 | 88 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.83 [‐2.33, ‐1.33] |
| 8.4 Bristol Activities of Daily Living Scale (community) | 1 | 391 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.09, 0.31] |
| 8.5 ADCS‐ADL (community) | 1 | 129 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.41, 0.35] |
| 8.6 DAD (community) | 1 | 326 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.22, 0.21] |
| 9 Behaviour (agitation/irritability) post‐treatment | 3 | 359 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.17, 0.24] |
| 9.1 Cohen‐Mansfield Agitation Inventory (care home) | 1 | 304 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.14, 0.31] |
| 9.2 MOSES Irritability subscale (community) | 2 | 55 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.79, 0.27] |
| 10 Mood‐related outcomes (depression) post‐treatment | 10 | 973 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.15, 0.10] |
| 10.1 CSDD (care home) | 3 | 147 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.50, 0.15] |
| 10.2 CSDD (community) | 1 | 276 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.38, 0.10] |
| 10.3 Geriatric Depression Scale (care home) | 1 | 23 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.90, 0.74] |
| 10.4 Geriatric Depression Scale Short Form (care home) | 1 | 17 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.53 [‐1.51, 0.44] |
| 10.5 MOSES Depression subscale (community) | 2 | 55 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐0.85, 0.22] |
| 10.6 HADS Depression (community) | 1 | 129 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.26, 0.49] |
| 10.7 MADRS (community) | 1 | 326 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.05, 0.38] |
| 11 Mood‐related outcomes (anxiety) post‐treatment | 2 | 436 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.22, 0.16] |
| 11.1 HADS Anxiety (community) | 1 | 129 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.40, 0.35] |
| 11.2 RAID (community) | 1 | 307 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.25, 0.20] |
| 12 Carer outcomes (stress related to caring) post‐treatment | 7 | 1155 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.21, 0.14] |
| 12.1 Zarit Burden Interview Short Form (care home) | 1 | 88 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐1.04, ‐0.19] |
| 12.2 Zarit Burden Interview Short Form (community) | 1 | 37 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.73, 0.56] |
| 12.3 Relatives Stress Scale (community) | 2 | 385 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐1.82, 0.90] |
| 12.4 NPI (community) | 1 | 129 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.26, 0.49] |
| 12.5 Modified Zarit Burden Interview ‐ care assistant (care home) | 1 | 90 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.38, 0.44] |
| 12.6 Modified Zarit Burden Interview ‐ nurse report (care home) | 1 | 100 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.31, 0.47] |
| 12.7 Zarit Burden Interview | 1 | 326 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.14, 0.30] |
| 13 Carer outcomes (depression) post‐treatment | 2 | 517 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.59, 0.44] |
| 13.1 HADS ‐ Depression (community) | 2 | 517 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.59, 0.44] |
| 14 Carer outcomes (anxiety) post‐treatment | 2 | 517 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.54, 0.66] |
| 14.1 HADS ‐ Anxiety (community) | 2 | 517 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.54, 0.66] |
| 15 Carer outcomes (quality of life) post‐treatment | 4 | 530 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.22, 0.13] |
| 15.1 GHQ‐12 carer (community) | 2 | 47 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐0.82, 0.36] |
| 15.2 GHQ‐28 (community) | 1 | 354 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.18, 0.24] |
| 15.3 SF Mental (community) | 1 | 129 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.59, 0.16] |
| 16 Carer outcomes (quality of caring relationship) post‐treatment | 3 | 1051 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.67, 0.39] |
| 16.1 QCPR Absence of conflict carer (care home) | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐5.27, 4.87] |
| 16.2 QCPR Absence of conflict carer (community) | 2 | 500 | Mean Difference (IV, Fixed, 95% CI) | ‐0.27 [‐1.02, 0.48] |
| 16.3 QCPR Warmth carer (care home) | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐1.2 [‐6.07, 3.67] |
| 16.4 QCPR Warmth carer (community) | 2 | 505 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.75, 0.80] |
| 17 Self‐reported quality of life at follow‐up | 5 | 874 | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [‐0.11, 0.80] |
| 17.1 SR‐QoL (care home) | 1 | 88 | Std. Mean Difference (IV, Random, 95% CI) | 1.48 [1.00, 1.95] |
| 17.2 QoL‐AD rated by person with dementia (community) | 4 | 786 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.13, 0.15] |
| 18 Proxy rated quality of life at follow‐up | 3 | 505 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐1.14, 0.83] |
| 18.1 QoL‐AD rated by carer (community) | 3 | 505 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐1.14, 0.83] |
| 19 Observed quality of life at follow‐up | 2 | 154 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐1.34, 0.54] |
| 19.1 WIB (care home) | 2 | 154 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐1.34, 0.54] |
| 20 Cognition follow‐up | 9 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 20.1 MMSE (care home) | 1 | 66 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.31 [‐0.17, 0.80] |
| 20.2 MMSE (community) | 4 | 216 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.08, 0.48] |
| 20.3 AMI‐PSS (care home) | 1 | 17 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.51 [‐0.46, 1.48] |
| 20.4 AMI‐PSS (community) | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.55 [‐0.18, 1.28] |
| 20.5 AMI‐E‐PSS (community) | 1 | 328 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.26, 0.18] |
| 20.6 AMI‐AIS (care home) | 1 | 17 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐1.20, 0.71] |
| 20.7 AMI‐AIS (community) | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.63 [‐0.11, 1.36] |
| 20.8 AMI‐E‐AIS (community) | 1 | 325 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.21, 0.23] |
| 20.9 ADAS‐Cog (community) | 1 | 326 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.32, 0.11] |
| 21 Cognition (overall) at follow‐up | 9 | 983 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.09, 0.17] |
| 21.1 MMSE (care home) | 1 | 66 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.31 [‐0.17, 0.80] |
| 21.2 MMSE (community) | 4 | 216 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.08, 0.48] |
| 21.3 AMI‐PSS (care home) | 1 | 17 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.51 [‐0.46, 1.48] |
| 21.4 AMI‐PSS (community) | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.55 [‐0.18, 1.28] |
| 21.5 AMI‐E‐PSS (community) | 1 | 328 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.26, 0.18] |
| 21.6 ADAS‐Cog (community) | 1 | 326 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.32, 0.11] |
| 22 Communication and interaction at follow‐up | 4 | 204 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.49 [‐0.77, ‐0.21] |
| 22.1 Social Engagement Scale (care home) | 2 | 154 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.79, ‐0.14] |
| 22.2 MOSES withdrawal subscale (community) | 2 | 50 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.56 [‐1.12, 0.01] |
| 23 Quality of caring relationship at follow‐up | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 23.1 QCPR warmth rated by person with dementia (community) | 2 | 415 | Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.61, 1.11] |
| 23.2 QCPR conflict rated by person with dementia (community) | 2 | 409 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐1.28, 0.51] |
| 24 Behaviour (functional) at follow‐up | 5 | 941 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.66, 0.03] |
| 24.1 MDS‐ADL (care home) | 1 | 66 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.53, 0.44] |
| 24.2 ADL (care home) | 1 | 88 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.29 [‐1.75, ‐0.82] |
| 24.3 ADCS‐ADL (community) | 1 | 119 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.53, 0.25] |
| 24.4 Bristol Activities of Daily Living Scale (community) | 1 | 342 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.35, 0.08] |
| 24.5 DAD (community) | 1 | 326 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.32, 0.11] |
| 25 Behaviour (agitation/irritability) at follow‐up | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐1.52 [‐4.07, 1.03] |
| 25.1 MOSES Irritability subscale (community) | 2 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐1.52 [‐4.07, 1.03] |
| 26 Mood‐related outcomes (depression) at follow‐up | 6 | 747 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.43, 0.11] |
| 26.1 Geriatric Depression Scale Short Form (care home) | 1 | 17 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.63 [‐2.77, ‐0.49] |
| 26.2 CSDD (community) | 1 | 235 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.43, 0.09] |
| 26.3 MOSES Depression subscale (community) | 2 | 50 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.82, 0.30] |
| 26.4 HADS Depression (community) | 1 | 119 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.48, 0.30] |
| 26.5 MADRS (community) | 1 | 326 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.11, 0.32] |
| 27 Mood‐related outcomes (anxiety) at follow‐up | 2 | 391 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.20, 0.21] |
| 27.1 RAID (community) | 1 | 272 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.25, 0.23] |
| 27.2 HADS Anxiety (community) | 1 | 119 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.34, 0.44] |
| 28 Carer outcomes (stress related to caring) at follow‐up | 5 | 895 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.54, 0.16] |
| 28.1 Zarit Burden Interview Short Form (care home) | 1 | 88 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐1.51, ‐0.62] |
| 28.2 Zarit Burden Interview Short Form (community) | 1 | 35 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.74, 0.59] |
| 28.3 Relatives Stress Scale (community) | 1 | 327 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.28, 0.16] |
| 28.4 NPI (community) | 1 | 119 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.33, 0.45] |
| 28.5 Zarit Burden Interview (community) | 1 | 326 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.11, 0.32] |
| 29 Carer outcomes (depression) at follow‐up | 2 | 464 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.71, 0.60] |
| 29.1 HADS Depression (community) | 2 | 464 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.71, 0.60] |
| 30 Carer outcomes (anxiety) at follow‐up | 2 | 464 | Mean Difference (IV, Fixed, 95% CI) | 0.56 [‐0.17, 1.30] |
| 30.1 HADS Anxiety (community) | 2 | 464 | Mean Difference (IV, Fixed, 95% CI) | 0.56 [‐0.17, 1.30] |
| 31 Carer outcomes (quality of life) at follow‐up | 3 | 467 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.18, 0.19] |
| 31.1 GHQ‐12 (community) | 1 | 35 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.78, 0.55] |
| 31.2 GHQ‐28 (community) | 1 | 313 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.15, 0.29] |
| 31.3 SF Mental (community) | 1 | 119 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.54, 0.24] |
| 32 Carer outcomes (quality of caring relationship) at follow‐up | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 32.1 QCPR Conflict (community) | 2 | 495 | Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐1.23, 0.50] |
| 32.2 QCPR Warmth (community) | 2 | 456 | Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐1.59, 0.27] |
| 33 Mood‐related outcomes (apathy) post‐treatment | 1 | 326 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [‐1.30, 4.10] |
| 33.1 Apathy Index (carer rated) (community) | 1 | 326 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [‐1.30, 4.10] |
| 34 Mood‐related outcomes (apathy) at follow‐up (community) | 1 | 326 | Mean Difference (IV, Fixed, 95% CI) | 1.25 [‐1.89, 4.39] |
| 34.1 Apathy Index (carer rated) (community) | 1 | 326 | Mean Difference (IV, Fixed, 95% CI) | 1.25 [‐1.89, 4.39] |
1.3. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 3 Observed quality of life (post‐treatment).
1.4. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 4 Cognition post‐treatment.
1.6. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 6 Quality of caring relationship post‐treatment.
1.16. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 16 Carer outcomes (quality of caring relationship) post‐treatment.
1.20. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 20 Cognition follow‐up.
1.23. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 23 Quality of caring relationship at follow‐up.
1.32. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 32 Carer outcomes (quality of caring relationship) at follow‐up.
1.33. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 33 Mood‐related outcomes (apathy) post‐treatment.
1.34. Analysis.
Comparison 1 Reminiscence therapy versus no treatment, Outcome 34 Mood‐related outcomes (apathy) at follow‐up (community).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akanuma 2011.
| Methods | RCT. | |
| Participants | 24 care home residents with ischaemic VD according to ADDTC criteria with reference to CT and MRI, scoring 10‐24 on the MMSE. Care home situated in a rural area in northern Japan. Mean age: 78.25 years. |
|
| Interventions | Intervention: group RT. Control: treatment as usual. |
|
| Outcomes | Cognitive: MMSE. Behavioural: BRSE. Mood‐related outcomes: GDS. |
|
| Length and frequency of intervention | 1 hour per week for 3 months. | |
| Time points measured | Paper stated, "before and after the interventions." | |
| Number of participants who did not complete study | 0. | |
| Notes | Authors also measured PET and metabolic outcomes, voxel by voxel analysis, and ROI analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Paper stated participants randomly assigned to 2 arms but did not specify how. |
| Allocation concealment (selection bias) | Unclear risk | Not specified, though paper reported the allocator was blind when performing allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "the assessment of cognitive function and behavioral activities was conducted by well‐trained neuropsychologists who were blinded to the assignment. Nursing staff engaged in daily care, and who were blinded to the study protocol, assessed the patients' behavioral activities." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in methods section were reported and there was no evidence of selective outcome reporting. |
| Other bias | Low risk | |
| Availability of training and supervision Objective outcome measures | Unclear risk | 3 specialists were part of each group (a psychologist, speech therapist, and occupational therapist). |
| Availability of manual or protocol for intervention All outcomes | Low risk | The facilitators followed a clear protocol detailed in Akanuma 2006. |
Amieva 2016.
| Methods | Multicentre RCT. | |
| Participants | 653 community‐dwelling people who attended day centres or memory clinics in France, diagnosed with AD, 16‐26 on MMSE, 2‐5 on GDS, and with an identified family carer. 326 participants were in groups relevant to this review. Mean age: 78.75 years |
|
| Interventions | Intervention 1: group RT. Control: treatment as usual. Intervention 2: cognitive training (not included in the current review). Intervention 3: individual cognitive rehabilitation (not included in the current review). |
|
| Outcomes | Quality of life: QoL‐AD. Cognitive: MMSE, ADAS‐Cog. Behavioural: DAD, AGGIR. Mood‐related outcomes: Apathy Inventory, MADRS. Carer outcomes: ZBI. |
|
| Length and frequency of intervention | 1 × 90‐minute session per week for the first 3 months, and once every 6 weeks for next 21 months. | |
| Time points measured | Baseline, 3 months' postbaseline (i.e. after the weekly sessions) and 24 months' postbaseline (i.e. after 6 weekly sessions). (MMSE and GDS measures only taken/reported at 24 months). |
|
| Number of participants who did not complete study | 56/326 (17.18%). | |
| Notes | Primary outcome was rate of participants alive and without moderately severe to severe dementia at 2 years. The NPI was also used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The list of randomization was prepared by a statistician using permuted blocks, stratified by site." |
| Allocation concealment (selection bias) | Low risk | Participants randomised through an independent and remote telephone randomisation service provided by the clinical trial unit. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All assessment interviews done by physicians and psychologists blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 33 dropouts from RT and 23 from control group. Paper distinguished between participants who died and participants who withdrew, though specific reasons for these withdrawals were not reported. Results from a 'Missing Equals Failure' analysis was carried out. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported and there was no evidence of selective outcome reporting. |
| Other bias | Low risk | n/a |
| Availability of training and supervision Objective outcome measures | Low risk | All therapists received a 3‐day training session where the therapy programmes were thoroughly presented. Therapists were given contact details for the researchers who designed the programmes so they could contact them if necessary. |
| Availability of manual or protocol for intervention All outcomes | Low risk | "Each therapy program was developed according to current scientific data, standardized by a leader known to have scientific and clinical expertise in the field. To guarantee homogeneity in the way interventions were applied, a standardized procedure was followed." Therapists were given a manual detailing the intervention. |
Azcurra 2012.
| Methods | RCT. | |
| Participants | 135 participants from privately funded nursing homes in Argentina, with a diagnosis of AD according to DSM‐IV. 90 participants were in groups relevant to the current review. Mean age: 85 years. |
|
| Interventions | Intervention 1: group RT. Control: unstructured social contact. Intervention 2: counselling (not included in the current review). |
|
| Outcomes | Quality of life: SR‐QoL, WIB. Communication: SES. Behavioural: ADL scale. Carer: ZBI. |
|
| Length and frequency of intervention | 1 hour twice per week for 12 weeks. | |
| Time points measured | Paper stated, "The data were collected at baseline (T0), twelve weeks (T1), and six months post‐intervention (T2)." | |
| Number of participants who did not complete study | 5/90 (5.56%). | |
| Notes | Some participants were on psychotropic medication and physically restrained. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised block design. Participants meeting enrolment criteria were randomly allocated to 1 of 3 groups by the assignment of a unique kit number using a permuted block design at each investigational site (block size of 6) (stated by e‐mail). |
| Allocation concealment (selection bias) | Low risk | Paper reported, "we used an appropriate method of randomisation with adequate concealment of the participant allocation to treatment groups." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independent raters (social workers) completed outcome measures blinded to group allocation. The facilitators carrying out the intervention blinded to the outcome measures. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis. Missing data replaced with the mean value of the outcome variables for each group. Withdrawals due to death, moving and believing their allocated condition (control) was "useless." |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported and there was no evidence of selective outcome reporting. |
| Other bias | Low risk | n/a. |
| Availability of training and supervision Objective outcome measures | Low risk | Team trained by principal investigator to deliver the corresponding sessions in a structured manner. Facilitators had 15 training sessions totalling 30.4 hours. |
| Availability of manual or protocol for intervention All outcomes | Low risk | Clear protocols developed in the training sessions. |
Baines 1987.
| Methods | RCT. Cross‐over design. |
|
| Participants | 15 people living in a care home with moderate to severe impairment of cognitive functioning, as measured using the CAPE. 10 of these participants were in groups that were included in the current review. Mean age: 81.5 years. |
|
| Interventions | Intervention 1: group RT. Control: no treatment. Intervention 2: reality orientation(not included in the current review). |
|
| Outcomes | Quality of life: Life Satisfaction Index. Cognitive: CAPE (information/orientation subscale). Communication: Holden Communication Scale. Behavioural: CAPE (behaviour subscale). |
|
| Length and frequency of intervention | 30 minutes per day, 5 days per week, for 4 weeks. | |
| Time points measured | Before and immediately after the 4‐week intervention. | |
| Number of participants who did not complete study | 0/10 (from groups relevant to the current review). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Paper stated, "participants were randomly assigned to one of three groups," but did not report method used. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessments made by an independent psychologist, and staff who knew the residents well, but were not involved with the therapy groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | n/a. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported and there was no evidence of selective outcome reporting. |
| Other bias | Low risk | n/a. |
| Availability of training and supervision Objective outcome measures | Low risk | Paper reported, "preliminary training for staff included six hours of introductory talks, videos, discussions and hand outs." Staff training was carried out by a clinical psychologist. |
| Availability of manual or protocol for intervention All outcomes | Low risk | Paper reported, "the reminiscence therapy sessions were based on the format suggested by Andrew Norris (Norris 1986)." |
Charlesworth 2016.
| Methods | Factorial pragmatic RCT. | |
| Participants | 291 community‐dwelling people in the UK, with a diagnosis of dementia according to DSM‐IV and their family carers. 144 participants were in groups relevant to the current review. Mean age: 74.21 years. |
|
| Interventions | Intervention 1: group reminiscence (RYCT) for people with dementia and their carers (RYCT program) Control: treatment as usual Intervention 2: carer support programme. Intervention 3: both intervention 1 and intervention 2 The current review used data from participants in the RYCT (only) and treatment as usual (only) groups. |
|
| Outcomes | Quality of life: QoL‐AD, EQ‐5D, DEMQOL. Behavioural: ADCS‐ADL. Mood‐related outcomes: HADS, NPI, QCPR. Carer: mental component score of the UK Short Form‐12 Health Survey (UK SF‐12), EQ‐5D, HADS, Emotional Loneliness Scale, NPI‐D, PANAS, COPE‐PAC, PGI, QCPR. |
|
| Length and frequency of intervention | 1 session, 2 hours per week, for 12 weeks. After the weekly sessions, monthly sessions continued for 7 months, giving a possible 19 sessions over 10 months. | |
| Time points measured | Baseline, 5 months' postrandomisation and 12 months postrandomisation. | |
| Number of participants who did not complete study | 50/291 (17%); across all groups, including those not relevant to the current review. Specific attrition for each group not reported. | |
| Notes | In group reminiscence sessions, family carers met separately from the main group for 45 minutes during 4 sessions, with the aim of developing listening and communication skills, and considering how the activities and strategies in the sessions could continue at home. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A 2‐stage sequential dynamic algorithm was used to randomise participants. Randomisation was web based and was developed in collaboration with an accredited trials unit. |
| Allocation concealment (selection bias) | Low risk | The combination of the 2 randomisation stages resulted in participant allocation. An (unblinded) administrator then informed carers of their allocation by letter. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All research interviewers who assessed outcomes were blinded. After interview, researchers recorded their perceptions of participants' allocation. This showed no evidence of bias due to non‐blinded researchers. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The authors reported "missing scores were imputed, with multiple imputations calculated using a linear regression model, taking into account demographic variables, treatment group and other scores provided at a given time point." Withdrawals due to carer time constraints, poor health of carer or relative with dementia. Specific dropouts from each group not reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section and study protocol were reported. There was no evidence of selective outcome reporting. |
| Other bias | Unclear risk | n/a. |
| Availability of training and supervision Objective outcome measures | Low risk | Trial protocol reported "each group session is led by two experienced facilitators, supported by a team, including volunteers, health and social care staff and trainees to facilitate small group discussion and activities and engage the people with dementia. All members of the RYCT team attended a training day led by one of the original RYCT programme authors." |
| Availability of manual or protocol for intervention All outcomes | Low risk | The group reminiscence intervention followed the RYCT programme for people with dementia and their family carers. |
Goldwasser 1987.
| Methods | RCT. | |
| Participants | 30 participants with clinical diagnosis of dementia based on subjective criteria and MMSE scores. Recruited from a single nursing home in Virginia, USA. 20 participants were in groups relevant to this review. Mean age: 82.3 years. |
|
| Interventions | Intervention: group RT. Control: no treatment. Supported group therapy (not included in this review). |
|
| Outcomes | Cognitive: MMSE. Behavioural: Katz Index Activities of Daily Living. Mood‐related outcomes: Beck Depression Inventory. |
|
| Length and frequency of intervention | 30 minutes, twice per week, for 5 weeks. | |
| Time points measured | Preintervention, 1 week' postintervention and 5 weeks' postintervention. | |
| Number of participants who did not complete study | 2/20 (10%). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method unspecified, though paper stated, "thirty participants were initially selected...and randomly assigned to three groups of ten people." |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessments carried out by a psychology graduate, a registered nurse and a 'practical nurse,' none of whom were aware of the groups to which participants were assigned. As staff were involved in carrying out the intervention, there may have been a risk of contamination. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 person in the intervention group died so the authors randomly excluded 1 person from each of the other 2 groups. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported and there was no evidence of selective outcome reporting. |
| Other bias | Unclear risk | n/a. |
| Availability of training and supervision Objective outcome measures | Unclear risk | Not formally specified in paper but facilitators seemed to have been coached on some factors, such as rapport, "non‐verbal expression" and ways to "help participants generate internal cues." |
| Availability of manual or protocol for intervention All outcomes | Low risk | Paper reported that a structured reminiscence protocol was developed with user involvement. |
Gonzalez 2015.
| Methods | Cluster RCT. | |
| Participants | 42 participants with a diagnosis of AD living in 2 nursing homes in Valencia, Spain. Diagnosis determined by DSM‐IV‐TR, MMSE < 23 and impairment on a neuropsychological examination. Mean age: 80.24 years. |
|
| Interventions | Intervention: group integrative reminiscence programme. Control: treatment as usual. |
|
| Outcomes | Quality of life: Ryff Psychological Well‐Being scales. Cognitive: MMSE (Spanish Version). Mood‐related outcomes: CES‐D. |
|
| Length and frequency of intervention | 10 weekly sessions, each lasting 60 minutes. | |
| Time points measured | Pretest (2 weeks before the intervention) and post‐test (immediately after intervention). | |
| Number of participants who did not complete study | 0/42. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Cluster randomisation. "Nursing homes were randomised to determine where the intervention program would be administered." No explanation was given of the randomisation procedure. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who administered assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | n/a. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported and there was no evidence of selective outcome reporting. |
| Other bias | Unclear risk | Cluster RCT. The study had 2 clusters but was not large enough to apply the methods detailed in the Cochrane Handbook for Systematic Reviews of Interventions to reduce it to its effective sample size. No evidence of baseline imbalanced, missing clusters from the analysis or recruitment bias. |
| Availability of training and supervision Objective outcome measures | Unclear risk | Programme led by a psychologist but no information regarding current/previous training/supervision. |
| Availability of manual or protocol for intervention All outcomes | Low risk | Paper stated that the authors "implemented a programme based on earlier research." No manual but paper described detailed aims and activities for each individual session. |
Haight 2006.
| Methods | RCT. | |
| Participants | 30 participants with dementia diagnosis (no diagnosis specification) recruited from 6 care homes in Northern Ireland. Age range: 60‐99 years. |
|
| Interventions | Intervention: individual life review with the production of a Life Story Book. Control: treatment as usual. |
|
| Outcomes | Cognitive: MMSE. Communication: COS. Behavioural: MBS, FIM. Mood‐related outcomes: CSDD, AMS. |
|
| Length and frequency of intervention | 1 hour per week for 6 weeks. | |
| Time points measured | Baseline and 8 weeks' postbaseline. | |
| Number of participants who did not complete study | 0. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors stated by e‐mail that residents were randomised to each condition by blinded researchers. |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | n/a. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported and there was no evidence of selective outcome reporting. |
| Other bias | Low risk | n/a. |
| Availability of training and supervision Objective outcome measures | Low risk | Existing care home staff delivered the intervention. They engaged in 2 hours of preliminary training + weekly supervision with the researchers accounting for 10 hours of ongoing training. |
| Availability of manual or protocol for intervention All outcomes | Low risk | Used The Life Review Experiences Form (Haight 1992). |
Hsieh 2010.
| Methods | RCT. | |
| Participants | 61 residents from 2 nursing homes in Northern Taiwan diagnosed with dementia using the DSM‐IV. Mean age: 77 years. |
|
| Interventions | Intervention: group RT. Control: no treatment. |
|
| Outcomes | Mood‐related outcomes: GDS, AES‐C, NPI (Apathy subscale and Depression subscale). | |
| Length and frequency of intervention | 1 × 40‐ to 50‐minute session per week for 12 weeks. | |
| Time points measured | Baseline and postintervention (12 weeks' postbaseline). | |
| Number of participants who did not complete study | 5/61 (8.2%). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated that participants were randomised to either control or treatment condition. Method not specified. Attempted to contact author but no response received. |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Nursing home staff completed the NPI. Single investigator administered the other scales but no details regarding blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 withdrawals from intervention group and 1 from control group. 1 participant died but no reasons given for the withdrawal of the other 4 participants. Authors carried out analysis with remaining 56 participants. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported and there was no evidence of selective outcome reporting. |
| Other bias | Low risk | n/a. |
| Availability of training and supervision Objective outcome measures | Unclear risk | Not specified, though research teams who specialised in geriatric psychiatric nursing served as leaders and coleaders in the intervention group. |
| Availability of manual or protocol for intervention All outcomes | Low risk | Paper stated, "the components of all the sessions had clear structures and guidelines for the leaders and co‐leaders to facilitate the group interventions' and a 'research protocol was designed to include 18 activities suitable for all elderly patients residing in long‐term care." |
Ito 2007.
| Methods | RCT. | |
| Participants | 60 participants with clinical diagnosis of VD recruited from 2 nursing homes and 1 hospital in Japan. 40 were in groups that were included in the current review. Mean age: 82 years. |
|
| Interventions | Intervention 1: group RT. Control: supportive care. Intervention 2: social contact (not included in this review). |
|
| Outcomes | Cognitive: MMSE (Japanese Version), CASI. Mood‐related outcomes: MOSES.* |
|
| Length and frequency of intervention | 1 hour per week for 12 weeks. | |
| Time points measured | Paper stated, "before and after the interventions." | |
| Number of participants who did not complete study | 6/40 (15%) (from groups relevant to the current review). | |
| Notes | *MOSES data not included as no subscale data available, only the overall score. Contacted author by email requesting further information but we have not received a response. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 2‐step randomised allocation of participants stratified by age and education conducted by blinded researchers. Groups of 12 participants randomly divided into 3 subgroups by a computer, based on education and age. Subgroups were then randomly allocated to 3 arms by blinded researchers. |
| Allocation concealment (selection bias) | Low risk | Group allocation. Paper reported allocators were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessments were carried out by neuropsychologists blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants dropped out of both the control and reminiscence groups. Attrition due to ill health or transfer out of the care home. 1 participant withdrew consent. Data from the ITT analysis was not extractable. Instead, authors extracted data from the per protocol analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported and there was no evidence of selective outcome reporting. |
| Other bias | Low risk | n/a. |
| Availability of training and supervision Objective outcome measures | Unclear risk | Not specified though paper reports each group included a care provider and 3 specialists, who were chosen among a psychologist, 2 speech therapists, 3 occupational therapists, 3 medical social workers and a nurse. |
| Availability of manual or protocol for intervention All outcomes | Low risk | Paper provided detailed schedule for each session and was based on that proposed by Akanuma and colleagues (Akanuma 2006). |
Lai 2004.
| Methods | RCT. | |
| Participants | 101 participants with a diagnosis of dementia according to the DSM‐IV recruited from 2 nursing homes in Hong Kong. 66 of these were in groups that were included in the present review. The remainder were in a comparison group. Mean age: 85.7 years. |
|
| Interventions | Intervention 1: individual RT (specific reminiscence and life story). Control: no treatment. Intervention 2: social support (not included in this review). |
|
| Outcomes | Quality of life: WIB. Cognitive: MMSE (Cantonese Version). Communication: SES. Behavioural: Minimum Data Set ‐ Activities of Daily Living. |
|
| Length and frequency of intervention | 1 × 30‐minute session per week for 6 weeks. | |
| Time points measured | Assessments carried out immediately before and after the 6‐week treatment period, and at 6 weeks' follow‐up. | |
| Number of participants who did not complete study | 10/66 (15.15%) (from groups relevant to the current review). | |
| Notes | Most participants were restrained either intermittently or continuously. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Paper stated that participants were randomly assigned to groups using fixed random allocation methods. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters and assessors blinded to participant allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT and per protocol analysis reported in study paper. Authors extracted data from the ITT analysis. Reasons for withdrawal included participants being wrongly included, ill health, death or participant feeling depressed during the sessions. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported and there was no evidence of selective outcome reporting. |
| Other bias | Low risk | n/a. |
| Availability of training and supervision Objective outcome measures | Low risk | Interventions delivered by professional staff with additional training. Mean number of hours of training provided to assessors was 25 (SD 3.6). |
| Availability of manual or protocol for intervention All outcomes | Low risk | The development, testing and refining of the intervention programme took place in 5 cycles. The contents of an LSB as proposed by Hellen 1998 were adopted. |
Melendez 2015.
| Methods | Cluster RCT. | |
| Participants | 30 participants with AD according to DSM‐IV with MMSE scores < 19 who were attending day centres in Valencia, Spain. Mean age: 84.2 years. |
|
| Interventions | Intervention: group RT. Control: treatment as usual. |
|
| Outcomes | Cognitive: AMI. | |
| Length and frequency of intervention | 2 × 30‐minute sessions per week for 10 weeks. | |
| Time points measured | Paper stated, "pre‐test, post‐test and 2‐month follow‐up tests were performed." | |
| Number of participants who did not complete study | 2/30 (6.66%). | |
| Notes | Study also recruited participants with amnestic MCI but data from these participants were excluded from the present review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cluster randomisation. Paper reported, "The day centres were randomised to determine where the intervention programme would be administered. To randomise the groups, the names of centres were introduced into a spreadsheet and programme output file presented name of centre to receive treatment." |
| Allocation concealment (selection bias) | Low risk | Randomised on level of day centre. Allocation decided using spreadsheet and corresponding programme output file. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear who interviewed participants, but they were recorded and 2 psychologists then independently analysed the scores. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant from each group dropped out. The participant from the reminiscence intervention left the day centre to be "institutionalised." Reason for control drop out unspecified. ITT analysis completed. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported and there was no evidence of selective outcome reporting. |
| Other bias | Unclear risk | Cluster RCT. The study had 2 clusters but was not large enough to apply the methods detailed in the Cochrane Handbook for Systematic Reviews of Interventions to reduce it to its effective sample size. No evidence of baseline imbalanced, missing clusters from the analysis or recruitment bias. |
| Availability of training and supervision Objective outcome measures | Low risk | Led by a psychologist. Author stated by email that "an official master gerontologist" oversaw the teaching and training of the reminiscence processes. |
| Availability of manual or protocol for intervention All outcomes | Low risk | Clear protocol outlined in the paper. |
Morgan 2012.
| Methods | RCT. | |
| Participants | 17 participants living in care homes in North Wales with mild to moderate dementia (CDR used to determine severity). Mean age: 80 years. |
|
| Interventions | Intervention: structured individual life review. Control: treatment as usual. |
|
| Outcomes | Cognitive: AMI. Mood‐related outcomes: GDS‐SF. |
|
| Length and frequency of intervention | 30‐ to 60‐minute session once per week for 12 weeks (or more, depending on progress through the Life Review Experiencing Form, Haight 1992). | |
| Time points measured | Baseline, postintervention and 6 weeks' postintervention. | |
| Number of participants who did not complete study | 0/17. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Paper reported, "initial participants were randomly assigned alternately to the groups. Subsequent participants were allocated using the randomisation by minimization method (Altman 2005), which allocates the next participant in the trial according to the characteristics of those already participating, so that each allocation reduces any imbalance in the stratifying variables...even when the sample size is small." |
| Allocation concealment (selection bias) | Unclear risk | Not specified. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Half of the measures taken by primary researcher, unblinded to allocation, the other half taken by blinded assistant psychologist. There were no significant differences in scores of those assessed by the researcher and the blind assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | n/a. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported and there was no evidence of selective outcome reporting. |
| Other bias | Low risk | n/a. |
| Availability of training and supervision Objective outcome measures | Low risk | Therapist was a clinical psychologist in her final year of doctoral training under supervision of an experienced clinical psychologist. Total training time not specified. |
| Availability of manual or protocol for intervention All outcomes | Low risk | Structured life review based on Haight's Life Review Experience form (Haight 1992). |
O'Shea 2014.
| Methods | Cluster RCT. | |
| Participants | 304 long‐stay care home residents in Ireland living with dementia according to DSM‐IV or any other diagnosis by a clinician, nurses judgement, nurses records (or a combination) or prescribed any medication for AD. Mean age: 85.4 years. |
|
| Interventions | Intervention: group RT. Control: treatment as usual. |
|
| Outcomes | Quality of life: QoL‐AD. Behavioural: Cohen Mansfield Agitation Inventory. Mood‐related outcomes: CSDD. Carer: Modified ZBI. |
|
| Length and frequency of intervention | 3‐4 sessions per week for a mean of 14 weeks (range 12‐17 weeks). Session duration unspecified. | |
| Time points measured | Baseline and 18‐22 weeks' postrandomisation. | |
| Number of participants who did not complete study | 76/304 (25%). | |
| Notes | Approximately 75% of care homes recorded close to the mean target of 3 or 4 sessions per week. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation at the level of the long‐stay residential unit. Randomisation was on a ratio of 1:1 and was stratified by public and private residential units (one‐third public to two‐thirds private, reflecting the overall distribution of beds in the region). |
| Allocation concealment (selection bias) | Low risk | Concealment of group allocation achieved by giving the responsibility for sequence generation and group allocation to a researcher who was independent of the study and its investigators. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research nurses involved in data generation and collection were blinded to group allocation of participating units. Data analysis undertaken by researchers and statisticians blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 25 residents lost to follow‐up in intervention group (18 died, 1 was transferred, 2 in hospital, 1 withdrew and 3 too ill to participate) and 27 in control group (18 died, 3 too ill, 2 in hospital and 1 was transferred). Paper reported that all results were insensitive to the inclusion of missing data using multivariate imputation by chained equations. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section and study protocol were reported and there was no evidence of selective outcome reporting. |
| Other bias | Low risk | Cluster RCT. 18 clusters (9 intervention and 9 control). Although study authors analysed data appropriately, review authors needed to extract data (from a table) that did not account for clustering. The study authors reported the ICC for each measure, which the review authors extracted and used to calculate the effective sample size to enter into Review Manager 5 (RevMan 2014). No evidence of recruitment bias or missing clusters after randomisation. Cluster‐specific baseline adjustment was implemented to prevent baseline imbalance creating bias. |
| Availability of training and supervision Objective outcome measures | Low risk | Staff training involved a structured education programme, facilitated by experienced nurse educators, delivered over 3 days and augmented by telephone support and onsite visits. |
| Availability of manual or protocol for intervention All outcomes | Low risk | A structured education programme for staff was delivered and staff were trained in intervention design. Staff target was 1 planned formal session and 3 spontaneous sessions per week. |
Subramaniam 2013.
| Methods | RCT. | |
| Participants | 24 participants with dementia as assessed by DSM‐IV recruited from care homes in North Wales, UK. Mean age: 86 years. |
|
| Interventions | Intervention: individual life review/life story book (participants were involved in the creation). Control: life story book given to participants as a gift 12 weeks into the intervention (just after post‐treatment assessment). |
|
| Outcomes | Quality of life: QoL‐AD. Cognitive: AMI‐E. Mood‐related outcomes: GDS. Carer: QCPR. |
|
| Length and frequency of intervention | 1 hour per week for 12 weeks. Mean of 12 sessions (range 11‐16). | |
| Time points measured | Baseline (T0), 12 weeks' postbaseline (T2) and 6 weeks later (T3). (T3 data not included in this review*). |
|
| Number of participants who did not complete study | 1/24 (4.17%). | |
| Notes | *Data from T3 not included in the current review as participants in the control condition were given life story books and used them between T2 and T3. Once they received their books, the control condition was no longer treatment as usual or passive. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated using a sequential individual‐based randomisation, which randomised participants into parallel groups using a dynamic stratification algorithm. The randomisation process was carried out by an accredited clinical trials unit. |
| Allocation concealment (selection bias) | Low risk | Randomisation process was undertaken by an accredited trials unit. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome measures carried out by 2 assessors blinded to treatment allocation with no other involvement in the process of the research. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant died part way through the trial, their data were excluded. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported and there was no evidence of selective outcome reporting. |
| Other bias | Low risk | n/a. |
| Availability of training and supervision Objective outcome measures | Low risk | Therapist was a clinical psychologist trained in reminiscence work. Weekly supervision was provided with consultant clinical psychologist. |
| Availability of manual or protocol for intervention All outcomes | Low risk | Life review intervention was based on Haight's Life Review model and Life Review Experiencing Form (Haight 1992). |
Särkämö 2013.
| Methods | RCT. | |
| Participants | 89 participants with mild‐to‐moderate dementia (assessed using CDR) recruited as person with dementia ‐ carer dyads from day units and inpatient facilities in Finland. Of these, 59 dyads belonged to groups relevant to the current study. Mean age: 78.91 years. |
|
| Interventions | Intervention: music listening and reminiscing in a group setting. Control: care as usual. |
|
| Outcomes | Quality of life: QoL‐AD and Cornell‐Brown Scale for Quality of Life in Dementia. Cognitive: MMSE, Frontal Assessment Battery and Modified Version of the Autobiographical Fluency Task. Carer: ZBI, GHQ‐12. |
|
| Length and frequency of intervention | 1.5 hours per week for 10 weeks. | |
| Time points measured | Baseline, postintervention (3 months from baseline) and 9 months postbaseline. | |
| Number of participants who did not complete study | 9/57 (13.56%). | |
| Notes | Study also contained a singing coaching group (27 dyads). Data from these participants were not included in the current review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation carried out by a blinded staff member using a random number generator. |
| Allocation concealment (selection bias) | Low risk | An independent researcher was responsible for sequence generation and group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All assessments were carried out blinded to the group allocation of the participants. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 participants dropped out from the intervention group and 7 from the control group. Reasons for withdrawals were not given. Authors analysed the data with the remaining participants. There were no statistically significant differences between participants who completed the study and who dropped out. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported and there was no evidence of selective outcome reporting. |
| Other bias | Low risk | n/a. |
| Availability of training and supervision Objective outcome measures | Low risk | Paper reported that sessions were led by a trained music therapist. |
| Availability of manual or protocol for intervention All outcomes | Low risk | The paper detailed each session clearly. |
Tadaka 2007 (AD).
| Methods | RCT. | |
| Participants | 24 participants from a geriatric health services facility in Tokyo, Japan with a diagnosis of AD according to the DSM‐IV. Mean age: 81.85 years. |
|
| Interventions | Intervention: group RT. Control: treatment as usual. |
|
| Outcomes | Cognitive: MMSE. Communication: MOSES (Withdrawal subscale). Behavioural: MOSES (Irritability subscale). Mood‐related outcomes: MOSES (Depression subscale). |
|
| Length and frequency of intervention | 1 × 60‐ to 90‐minute session per week for 8 weeks. | |
| Time points measured | Baseline, immediately postintervention and 6 months postintervention. | |
| Number of participants who did not complete study | 4/24 (16.67%). | |
| Notes | Study also investigated effects of RT on 36 people with VD (Tadaka 2007 (VD)). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list within each subset of dementia type (AD or VD). |
| Allocation concealment (selection bias) | Low risk | 2 social workers from the facility with no connection to the study allocated participants based on the computer‐generated list. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | MMSE administered by a psychiatrist blinded to the allocation of participants at all 3 time points. MOSES was completed by family members who were not blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Per protocol analysis. 1 dropout from reminiscence group and 3 from control group. All 4 dropped out because they were admitted to hospital. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported and there was no evidence of selective outcome reporting. |
| Other bias | Low risk | n/a. |
| Availability of training and supervision Objective outcome measures | Low risk | 'Specialists were trained public health nurses or clinical psychologists who had MA or PhD degrees and several years’ experience in the care of elderly people with dementia and trained in the reminiscence group program techniques. Specialists performed roles of group leader or co‐leader to facilitate the reminscence group program'. |
| Availability of manual or protocol for intervention All outcomes | Low risk | No evidence of a written protocol or manual although a clear structure was described in the paper. |
Tadaka 2007 (VD).
| Methods | RCT. | |
| Participants | 36 participants from a geriatric health services facility. Diagnosis of VD according to the DSM‐IV. Mean age: 84.25 years. |
|
| Interventions | Intervention: structured group RT. Control: treatment as usual. |
|
| Outcomes | Cognitive: MMSE. Communication: MOSES (Withdrawal subscale). Behavioural: MOSES (Irritability subscale). Mood‐related outcomes: MOSES (Depression subscale). |
|
| Length and frequency of intervention | 1 × 60‐ to 90‐minute session per week for 8 weeks. | |
| Time points measured | Prior to intervention, immediately postintervention and 6 months postintervention. | |
| Number of participants who did not complete study | 6/36 (16.67%). | |
| Notes | Study also investigated effects of RT on 24 people with AD (Tadaka 2007 (AD)). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list within each subset of dementia type (AD or VD). |
| Allocation concealment (selection bias) | Low risk | 2 social workers from the facility with no connection to the study allocated participants based on the computer‐generated list. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | MMSE administered by a psychiatrist blinded to the allocation of participants at all 3 time points. MOSES completed by family members who were not blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Per protocol analysis. 3 dropouts from intervention group and 3 from control group. 1 participant from each group died while the other 4 were admitted to hospital. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported and there was no evidence of selective outcome reporting. |
| Other bias | Low risk | n/a. |
| Availability of training and supervision Objective outcome measures | Low risk | 'Specialists were trained public health nurses or clinical psychologists who had MA or PhD degrees and several years’ experience in the care of elderly people with dementia and trained in the RT group program techniques. Specialists performed roles of group leader or co‐leader to facilitate the reminsicence group program'. |
| Availability of manual or protocol for intervention All outcomes | Low risk | No evidence of a written protocol or manual, although a clear structure was described in the paper. |
Thorgrimsen 2002.
| Methods | RCT. | |
| Participants | 11 community‐dwelling dyads (11 people with dementia and their carers) who had been referred to a RYCT reminiscence group by community psychiatric nurses and occupational therapists. No other diagnostic information specified. Mean age of person with dementia: 76.3 years. | |
| Interventions | Intervention: joint reminiscence groups for the person with dementia and their carers. Control: no treatment. |
|
| Outcomes | Quality of life: QoL‐AD. Cognitive: MMSE. Communication: Holden Communication Scale. Behavioural: CAPE‐BRS. Carer: GHQ‐12, Relatives Stress Scale. |
|
| Length and frequency of intervention | Programme comprised 18 weekly reminiscence sessions, 11 of which were attended only by the informal carers and the volunteers involved in the project. | |
| Time points measured | Baseline and 1 follow‐up 18 weeks postbaseline assessment. | |
| Number of participants who did not complete study | 2/22 (9.09%), i.e. 1 dyad. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised to the intervention and control groups using sealed envelopes. |
| Allocation concealment (selection bias) | Low risk | Used sealed envelopes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blind to group allocation. Participants were informed about the importance of the assessor being blind with respect to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data excluded for missing dyad. Dropout due to ill health of person with dementia. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported and there was no evidence of selective outcome reporting. |
| Other bias | Low risk | n/a. |
| Availability of training and supervision Objective outcome measures | Low risk | Age Exchange (internationally known for its work in all areas of reminiscence) conducted the intervention. Carers were taught the applications of reminiscence by Age Exchange in 11 weekly sessions. |
| Availability of manual or protocol for intervention All outcomes | Low risk | Intervention was the RYCT programme, based on the standardised manual Reminiscing with People with Dementia ‐ a Handbook for Carers (Bruce 1999). |
Van Bogaert 2016.
| Methods | RCT. | |
| Participants | 72 care home residents with a diagnosis of dementia according to the DSM‐V criteria and MMSE of 10‐24. Mean age: 83.75 years. |
|
| Interventions | Intervention: individual reminiscence based on the SolCos Model. Control: treatment as usual. |
|
| Outcomes | Cognitive: MMSE, Frontal Assessment Battery. Mood‐related outcomes: CSDD. Other: NPI. |
|
| Length and frequency of intervention | 2 × 45‐minute sessions per week for 8 weeks. | |
| Time points measured | Preintervention (week 0) and postintervention (week 9). | |
| Number of participants who did not complete study | 12/72 (16.66%). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly selected into the intervention group or control group using sequentially numbered, opaque sealed envelope for each resident. |
| Allocation concealment (selection bias) | Low risk | A person not involved with the study divided the envelopes into 2 blinded boxes manually and randomly. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A researcher who was not involved with any aspect of the intervention programme, collected the study participants' assessment scales and other data before and after the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12 withdrawals (7 from intervention group and 5 from control group) because of sudden illness leading to admission to hospital (1) or palliative care (1) and death (6), disruptive or aggressive behaviour during the sessions (2) and withdrawal of consent after baseline (2). |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section and study protocol were reported. No evidence of selective outcome reporting. |
| Other bias | Low risk | n/a. |
| Availability of training and supervision Objective outcome measures | Low risk | 1 researcher ran a training programme with 18 nursing home volunteers as facilitators. This researcher also provided support and advice to the facilitators throughout the intervention. |
| Availability of manual or protocol for intervention All outcomes | Low risk | The standardised individual reminiscence intervention was based on the SolCos model (Soltys 1994). A clear and standardised structure was reported in the paper. |
Woods 2012a.
| Methods | Multicentre RCT. | |
| Participants | 488 participants living in the community meeting DSM‐IV criteria for dementia. Participants participated in dyads with informal carers, most of whom were spouses. Mean age: 77.5 years. |
|
| Interventions | Intervention: joint reminiscence groups for the person with dementia and their carer. Control: treatment as usual. |
|
| Outcomes | Quality of life: QoL‐AD, QCPR, EQ‐5D. Cognitive: AMI‐E. Behavioural: BADLS. Mood‐related outcomes: CSDD, RAID. Carer: GHQ‐28, HADS, RSS, QCPR, EQ‐5D. |
|
| Length and frequency of intervention | 1 × 2‐hour session per week for 12 weeks followed by 1 maintenance session per month for 7 months. | |
| Time points measured | Baseline before randomisation, 3 months postbaseline (following completion of the weekly reminiscence sessions) and 10 months postbaseline (following completion of the monthly maintenance reminiscence sessions). | |
| Number of participants who did not complete study | 138/488 dyads (28.28%). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was completed using a dynamic allocation method stratifying for spousal or non‐spousal relationship of the dyad. Complete list randomisation for each wave of recruitment within each centre was completed by an accredited trials unit. |
| Allocation concealment (selection bias) | Low risk | By undertaking a complete list randomisation for each wave at each centre, allocation knowledge of the next assignment would be irrelevant as all participants for a centre would be randomised together. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unblinded researchers (responsible for allocation and running sessions) were the only staff informed at each of the centres of the participant's allocation. Researchers blinded to group allocation carried out all follow‐up assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 137 total withdrawals from trial including 29 deaths. Reasons included death, ill health, no wish to continue, family circumstances, no time and no reason given. 1 dyad was excluded due to re‐recruitment. A linear regression model was applied to take missing data into account. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section and study protocol were reported. There was no evidence of selective outcome reporting. |
| Other bias | Low risk | n/a. |
| Availability of training and supervision Objective outcome measures | Low risk | 2 × half day training sessions for volunteers and facilitators took place before each group commenced. |
| Availability of manual or protocol for intervention All outcomes | Low risk | Intervention followed the RYCT manual. |
Yamagami 2012.
| Methods | RCT. | |
| Participants | 54 participants from 4 residential care homes in Japan with a diagnosis of dementia. Mean age: 85.2 years. |
|
| Interventions | Intervention: group RT. Group: no treatment. |
|
| Outcomes | Cognitive: CDR‐SB, Hasegawa Dementia Scale Revised. Communication: MOSES (Withdrawal subscale). Behavioural: MOSES (Irritability subscale). Mood‐related outcomes: MOSES (Depression subscale). |
|
| Length and frequency of intervention | 1 hour, twice per week, for 12 weeks. | |
| Time points measured | Paper described, "before test and after test." | |
| Number of participants who did not complete study | 1/54 (1.85%). | |
| Notes | Paper reported intervention "Brain Activating Rehabilitation" but inspection of paper indicates that this was mainly reminiscence activities). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated to conditions, stratifying for severity of dementia. No further details. |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Paper reported, "care staff who did not participate in the intervention primarily evaluated participants." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant dropped out of the control group due to sickness. Analysis carried out with 53 remaining participants. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the methods section were reported and there was no evidence of selective outcome reporting. |
| Other bias | Low risk | n/a. |
| Availability of training and supervision Objective outcome measures | Low risk | Paper reported that staff of each residential care home had studied the principles of the intervention and received 4 hours training. After each session of the intervention, an evaluation meeting was held to improve the skills of the staff. |
| Availability of manual or protocol for intervention All outcomes | Low risk | No evidence of a manual although a clear structure was described in paper. |
AD: Alzheimer's disease; ADAS‐Cog: Alzheimer’s Disease Assessment Scale for Cognition; ADCS‐ADL: Alzheimer’s Disease Cooperative Study ‐ Activities of Daily Living; ADDTC: Alzheimer's Disease Diagnostic and Treatment Centers; ADL: activities of daily living; AES‐C: Apathy Evaluation Scale ‐ Clinician; AGGIR: Grille d'Autonomie Gérontologique Groups Iso‐Ressources; AMI: Autobiographical Memory Interview; AMI‐E: Autobiographical Memory Interview Extended Version; AMS: Alzheimer's Mood Scale; BADLS: Bristol Activities of Daily Living Scale; BRSE: Brief Retirement Self‐Efficacy; CAPE: Clifton Assessment Procedures for the Elderly; CASI: Cognitive Abilities Screening Instrument; CDR: Clinical Dementia Rating; CDR‐SB: Clinical Dementia Rating ‐ Sum of Boxes; CES‐D: Center for Epidemiological Studies ‐ Depression; COPE‐PAC: a multidimensional coping inventory; COS: Communication Observation Scale; CSDD: Cornell Scale for Depression in Dementia; CT: computer tomography; DAD: Disablement Assessment for Dementia; DEMQOL: a self‐reported outcome measure designed to enable the assessment health‐related quality of life of people with dementia; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders ‐ fourth edition; DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders ‐ fourth edition, Text Revision; EQ‐5D: European Quality of Life 5 Dimensions; FIM: Functional Independence Measure; GDS: Geriatric Depression Scale; GDS‐SF: Geriatric Depression Scale ‐ Short Form; GHQ‐12: 12‐item General Health Questionnaire; HADS: Hospital Anxiety and Depression Scale; ICC: intraclass correlation coefficient; ITT: intention to treat; LSB: life story book; MADRS: Montgomery‐Åsberg Depression Rating Scale; MBS: Memory and Behaviour Problems; MCI: mild cognitive impairment; MMSE: Mini‐Mental State Examination; MOSES: Multidimensional Observation Scale for Elderly Subjects; MRI: magnetic resonance imaging; n/a: not available; NPI: Neuropsychiatric Inventory; NPI‐D: Neuropsychiatric Inventory ‐ Caregiver Distress; PANAS: Positive and Negative Affect Schedule; PET: positron emission tomography; PGI: Patient Global Impression; QCPR: Quality of Carer and Patient Relationship; QoL‐AD: Quality of Life in Alzheimer's Disease; RAID: Rating Anxiety In Dementia; RCT: randomised controlled trial; ROI: return on investment; RSS: Relative Stress Scale; RT: reminiscence therapy; RYCT: Remembering Yesterday, Caring Today; SD: standard deviation; SES: Social Engagement Scale; SolCos: a transformational reminiscence model on depressive symptoms; SR‐QoL: Self‐Report Quality of Life; VD: vascular dementia; WIB: Well‐being/Ill‐being Scale; ZBI: Zarit Burden Interview.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Afonso 2009 | Older population with no diagnosis of dementia. |
| Akhoondzadeh 2014 | Healthy older adults and no control group. |
| Allen 2014 | Not specific to dementia. |
| Asiret 2016 | Not an RCT. Authors stated "Patients were listed through their mini mental test scores, and randomized as odd numbers to control group and even numbers to intervention group." This is allocation rather than randomisation. |
| Baillon 2004 | Only 3 sessions of reminiscence therapy. No control group. |
| Baillon 2005 | Only 3 sessions of reminiscence therapy. No control group. |
| Barban 2016 | Reminiscence therapy combined with process‐based cognitive training. |
| Bogaert 2013 | Inadequate generation of a randomised sequence meaning study was not an RCT. |
| Bohlmeijer 2008 | Not specific to dementia. |
| Brooker 2000 | Not an RCT. |
| Burckhardt 1987 | Not an intervention study and not specific to reminiscence and dementia. |
| Chao 2006 | An MMSE score < 24 was an exclusion criteria, i.e. population did not have a clear diagnosis of dementia. |
| Chenoweth 2009 | Not reminiscence therapy. |
| Chiang 2010 | MMSE score > 20 was necessary to be included in the study, i.e. population did not have a clear diagnosis of dementia. |
| Choy 2016 | Participants did not have dementia. |
| Chueh 2014 | No mention of diagnosis of dementia. |
| Chung 2009 | Single group experimental design, i.e. not an RCT. |
| Crook 2016 | Not an RCT. |
| Curto Prieto 2015 | No passive control group. |
| Eritz 2016 | Study used a life history intervention which was not carried out directly with the person with dementia |
| Gudex 2010 | Residential home population that specifically excluded residents with severe dementia. No diagnostic information provided; no data given for people with dementia. |
| Haight 2003 | Randomisation not mentioned |
| Haslam 2010 | Comparison was against an activity (playing skittles) rather than no treatment/social contact. |
| Haslam 2014 | No passive control condition. |
| Head 1990 | No randomisation. |
| Hilgeman 2014 | Mixed intervention, mostly focusing on advance care planning. |
| Hsu 2009 | Population did not have a clear diagnosis of dementia. |
| Hutson 2014 | Not sufficient reminiscence therapy content. |
| Jo 2015 | Not an RCT. |
| Lalanne 2015 | Control condition was a cognitive training programme. |
| Lancioni 2014 | Feasibility study, not an RCT. |
| Lin 2011 | Not an RCT. |
| Liu 2007 | Excluded people with a diagnosis of dementia. |
| Lopes 2016 | Inclusion criteria appeared to include people with MCI, according to cut‐off scores on Montreal Cognitive Assessment which is used to determine eligibility for the study. Data not available separately for participants meeting criteria for mild dementia. Less than 10% of participants had formal diagnosis of dementia. |
| MacKinlay 2009 | Qualitative study. |
| Mackinlay 2010 | Intervention was not reminiscence therapy. |
| McKee 2003 | Reminiscence used with a general care home population. Data for people with dementia not presented separately. |
| McMurdo 2000 | Residential home population with MMSE score >12; no diagnostic information provided; no data given for people with dementia. |
| Melendez‐Moral 2013 | Not specific to dementia. |
| Morris 2015 | No mention of dementia. |
| Nakamae 2014 | Not sufficient reminiscence therapy content, correspondence with authors stated that participants engaged in 5 minutes of reminiscence per session. |
| Nakatsuka 2015 | Only included participants with MCI. |
| Orten 1989 | Population without clear diagnosis of dementia. |
| Politis 2004 | Not enough reminiscence therapy content |
| Rattenbury 1989 | Cognitive impairment was an exclusion factor for this study, i.e. population did not have a clear diagnosis of dementia. |
| Rawtaer 2015 | Inclusion criteria include MMSE of ≥ 24, i.e. not a population with dementia. |
| Sabir 2016 | People with dementia excluded. |
| Serrano 2004 | Diagnosis of dementia was an exclusion factor. |
| Stinson 2006 | Significant cognitive impairment was an exclusion factor for this study, i.e. population did not have a clear diagnosis of dementia. |
| Tadaka 2004 | Study used the same participants and measures as the Tadaka 2007 (AD); Tadaka 2007 (VD) studies which were included in this review. The 2004 version was excluded to avoid double counting the same data in the analysis. |
| Tanaka 2017 | Intervention includes reality orientation and physical exercise as well as reminiscence. Described as cognitive rehabilitation. |
| Thornton 1987 | Not an intervention study. |
| Tolson 2012 | Intervention was not reminiscence therapy. |
| Van Dijk 2012 | Not enough reminiscence therapy (1 session only). |
| Wang 2004 | Cognitive impairment was an exclusion factor for this study, i.e. population did not have a clear diagnosis of dementia. |
| Wang 2007 | Inadequate generation of a randomised sequence meaning not an RCT. |
| Wang 2009 | Inadequate generation of a randomised sequence meaning not an RCT. |
| Wingbermuehle 2014 | Not an intervention study. |
| Wu 2016 | Intervention described as spiritual reminiscence but the content does not reflect reminiscence work. |
| Yamagami 2007 | Not an RCT. |
| Yasuda 2009 | 1 group, not an RCT. |
| Yousefi 2015 | No mention of dementia in paper. |
| Zauszniewski 2004 | A diagnosis of dementia was an exclusion factor for this study. |
MCI: mild cognitive impairment; MMSE: Mini‐Mental State Examination; RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
Dwolatzky 2014.
| Trial name or title | Computer‐Supported Personal Interventions for Older People with Cognitive Impairment and Dementia. |
| Methods | RCT. |
| Participants | Participants in adult daycare centres aged ≥ 65 years with cognitive impairment or dementia (only data from participants with dementia will be extracted). |
| Interventions | Intervention: computerised personal reminiscence. Control: treatment as usual. Intervention 2: computerised cognitive training*. |
| Outcomes | Quality of life: QoL‐AD, Will To Live and NPI. Cognitive: Mindstreams computerised cognitive assessment battery. Carer outcomes: short version of Zarit Caregiver Burden Interview. |
| Starting date | Unknown. |
| Contact information | Name: Tzvi Dwolatzky. Contact e‐mail: Tzvidov@bgu.ac.il. |
| Notes | Data from computerised cognitive arm will not be included in this review. |
NPI: Neuropsychiatric Inventory; QoL‐AD: Quality of Life in Alzheimer's Disease; RCT: randomised controlled trial.
Differences between protocol and review
Current review includes updated use of 'Risk of bias' tool, use of GRADE approach, inclusion of 'Summary of findings' tables, methods to deal with data from cluster randomised controlled trials and subgroup analyses. In this review, unlike the previous versions, we were able to exclude studies from meta‐analyses on the grounds of quality.
Contributions of authors
Original version and first update:
AS: all correspondence; drafting of review versions; updating of review; selection of trials; extraction of data; interpretation of data analyses.
BW: drafting of review versions.
Update 2005:
BW: all correspondence; drafting of review versions; updating of review; selection of trials; extraction of data; interpretation of data analyses.
Update 2017:
LOP and EF: all correspondence; drafting of review sections; selection of trials; extraction of data; preparation of tables and figures.
BW: data analyses and interpretation; drafting of review background, results and discussion.
AS: comments on draft review.
Sources of support
Internal sources
Bangor University, Wales, UK.
External sources
Centre for Ageing & Dementia Research, Health & Care Research Wales, UK.
KESS 2 (European Social Fund), UK.
-
NIHR, UK.
This update was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health
Declarations of interest
BW: None known
LOP: None known
EMF: None known
AES: None known
MO: None known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Akanuma 2011 {published data only}
- Akanuma K, Meguro K, Meguro M, Sasaki E, Chiba K, Ishii H, et al. Improved social interaction and increased anterior cingulate metabolism after group reminiscence with reality orientation approach for vascular dementia. Psychiatry Research 2011;192(3):183‐7. [DOI] [PubMed] [Google Scholar]
Amieva 2016 {published data only}
- Amieva H, Robert PH, Grandoulier AS, Meillon C, Rotrou J, Andrieu S, et al. Group and individual cognitive therapies in Alzheimer’s disease: the ETNA3 randomized trial. International Psychogeriatrics / IPA 2016;28(5):707‐17. [DOI] [PubMed] [Google Scholar]
Azcurra 2012 {published and unpublished data}
- Azcurra DJLS. A reminiscence program intervention to improve the quality of life of long‐term care residents with Alzheimer's disease. A randomized controlled trial. Revista Brasileira de Psiquiatria (Sao Paulo, Brazil : 1999) 2012;34:422‐33. [DOI: 10.1016/j.rbp.2012.05.008] [DOI] [PubMed] [Google Scholar]
Baines 1987 {published data only}
- Baines S, Saxby P, Ehlert K. Reality orientation and reminiscence therapy: a controlled cross‐over study of elderly confused people. British Journal of Psychiatry 1987;151:222‐31. [DOI] [PubMed] [Google Scholar]
Charlesworth 2016 {published and unpublished data}
- Charlesworth G, Burnell K, Crellin N, Hoare Z, Hoe J, Knapp M, et al. Peer support and reminiscence therapy for people with dementia and their family carers: a factorial pragmatic randomised trial. Journal of Neurology, Neurosurgery, and Psychiatry 2016;87(11):1218‐28. [DOI: 10.1136/jnnp-2016-313736] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrell M, Hoe J, Charlesworth G, Russell I, Challis D, Moniz‐Cook E, et al. Support at Home: Interventions to Enhance Life in Dementia (SHIELD) ‐ evidence, development and evaluation of complex interventions. Programme Grants for Applied Research 2017;5:5. [DOI: 10.3310/pgfar05050] [DOI] [PubMed] [Google Scholar]
Goldwasser 1987 {published data only}
- Goldwasser AN, Auerbach SM, Harkins SW. Cognitive, affective and behavioural effects of reminiscence group therapy on demented elderly. International Journal of Aging & Human Development 1987;25(3):209‐22. [DOI] [PubMed] [Google Scholar]
Gonzalez 2015 {published data only}
Haight 2006 {published and unpublished data}
- Haight B, Gibson F, Michel Y. The Northern Ireland life review / life storybook project for people with dementia. Alzheimer's & Dementia 2006;2(1):56‐8. [DOI: 10.1016/j.jalz.2005.12.003] [DOI] [PubMed] [Google Scholar]
Hsieh 2010 {published data only (unpublished sought but not used)}
- Hsieh CJ, Chang C, Su SF, Hsiao YL, Shih YW, Han WH, et al. Reminiscence group therapy on depression and apathy in nursing home residents with mild to moderate dementia. Journal of Experimental Clinical Medicine 2010;2(2):72‐8. [Google Scholar]
Ito 2007 {published data only}
Lai 2004 {published and unpublished data}
- Lai CKY, Chi I, Kayser‐Jones J. A randomised controlled trial of a specific reminiscence approach to promote the well‐being of nursing home residents with dementia. International Psychogeriatrics / IPA 2004;16(1):33‐49. [DOI] [PubMed] [Google Scholar]
Melendez 2015 {published and unpublished data}
Morgan 2012 {published data only}
- Morgan S, Woods RT. Life review with people with dementia in care homes: a preliminary randomised controlled trial. Dementia: Non‐Pharmacological Therapies 2012;1(1):43‐59. [Google Scholar]
O'Shea 2014 {published and unpublished data}
- O'Shea E, Devane D, Cooney A, Casey D, Jordan F, Hunter A, et al. The impact of reminiscence on the quality of life of residents with dementia in long‐stay care. International Journal of Geriatric Psychiatry 2014;29(10):1062‐70. [DOI: 10.1002/gps.4099] [DOI] [PubMed] [Google Scholar]
Särkämö 2013 {published and unpublished data}
- Särkämö T, Tervaniemi M, Laitinen S, Numminen A, Kurki M, Johnson JK, et al. Cognitive, emotional and social benefits of regular musical activities in early dementia: randomised controlled study. Gerontologist 2013;54(4):634‐50. [DOI] [PubMed] [Google Scholar]
Subramaniam 2013 {published data only}
- Subramaniam P, Woods B, Whitaker C. Life review and life story books for people with mild to moderate dementia: a randomised controlled trial. Aging & Mental Health 2013;18(3):363‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tadaka 2007 (AD) {published data only}
- Tadaka E, Kanagawa K. Effects of reminiscence group in elderly people with Alzheimer disease and vascular dementia in a community setting. Geriatrics & Gerontology International 2007; Vol. 7, issue 2:167‐73.
Tadaka 2007 (VD) {published data only}
- Tadaka E, Kanagawa K. Effects of reminiscence group in elderly people with Alzheimer disease and vascular dementia in a community setting. Geriatrics & Gerontology International 2007; Vol. 7, issue 2:167‐73.
Thorgrimsen 2002 {published data only}
- Thorgrimsen L, Schweitzer P, Orrell M. Evaluating reminiscence for people with dementia: a pilot study. The Arts in Psychotherapy 2002;29(1):93‐7. [Google Scholar]
Van Bogaert 2016 {published data only}
- Bogaert P, Tolson D, Eerlingen R, Carvers D, Wouters K, Paque K, et al. SolCos model‐based individual reminiscence for older adults with mild to moderate dementia in nursing homes: a randomized controlled intervention study. Journal of Psychiatric and Mental Health Nursing 2016;23(9‐10):568‐75. [DOI] [PubMed] [Google Scholar]
Woods 2012a {published and unpublished data}
- Woods RT, Bruce E, Edwards RT, Elvish R, Hoare Z, Hounsome B, et al. REMCARE: reminiscence groups for people with dementia and their family caregivers ‐ effectiveness and cost‐effectiveness pragmatic multicentre randomised trial. Health Technology Assessment 2012;16(48):v‐xv, 1‐116. [DOI: 10.3310/hta16480] [DOI] [PubMed] [Google Scholar]
- Woods RT, Orrell M, Bruce E, Edwards RT, Hoare Z, Hounsome B, et al. REMCARE: pragmatic multi‐centre randomised trial of reminiscence groups for people with dementia and their family carers: effectiveness and economic analysis. PloS One 2016;11(4):e0152843. [DOI: 10.1371/journal.pone.0152843] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamagami 2012 {published data only (unpublished sought but not used)}
- Yamagami T, Takayama Y, Maki Y, Yamaguchi H. A randomized controlled trial of brain‐activating rehabilitation for elderly participants with dementia in residential care homes. Dementia and Geriatric Cognitive Disorders 2012;2:372‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Afonso 2009 {published data only}
Akhoondzadeh 2014 {published data only}
- Akhoondzadeh G, Jalalmanesh S, Hojjati H. Effect of reminiscence on cognitive status and memory of the elderly people. Iranian Journal of Psychiatry and Behavioral Sciences 2014; Vol. 8, issue 3:75‐80. [PMC free article] [PubMed]
Allen 2014 {published data only}
- Allen RS, Harris GM, Burgio LD, Azuero CB, Miller LA, Shin HJ, et al. Can senior volunteers deliver reminiscence and creative activity interventions? results of the legacy intervention family enactment randomized controlled trial. Journal of Pain and Symptom Management 2014;48(4):590‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Asiret 2016 {published data only}
- Asiret GD, Kapucu S. The effect of reminiscence therapy on cognition, depression, and activities of daily living for patients with Alzheimer disease. Journal of Geriatric Psychiatry and Neurology 2016 ;29(1):31‐7. [DOI] [PubMed] [Google Scholar]
Baillon 2004 {published data only}
- Baillon S, Diepen E, Prettyman R, Redman J, Rooke N, Campbell R. A comparison of the effects of Snoezelen and reminiscence therapy on the agitated behaviour of patients with dementia. International Journal of Geriatric Psychiatry 2004;19:1047‐52. [DOI] [PubMed] [Google Scholar]
Baillon 2005 {published data only}
- Baillon S, Diepen E, Prettyman R, Redman J, Rooke N, Campbell R. Variability in response of older people with dementia to both snoezelen and reminiscence. British Journal of Occupational Therapy 2005;68:367‐74. [Google Scholar]
Barban 2016 {published data only}
- Barban F, Annicchiarico R, Pantelopoulos S, Federici A, Perri R, Fadda L, et al. Protecting cognition from aging and Alzheimer's disease: a computerized cognitive training combined with reminiscence therapy. International Journal of Geriatric Psychology 2016;31(4):340‐8. [DOI] [PubMed] [Google Scholar]
Bogaert 2013 {published data only}
- Bogaert P, Grinsven R, Tolson D, Wouters K, Engelborghs S, Mussele S. Effects of SolCos model‐based individual reminiscence on older adults with mild to moderate dementia due to Alzheimer disease: a pilot study. Journal of the American Medical Directors Association 2013;14(7):528.e9‐528.e13. [DOI] [PubMed] [Google Scholar]
Bohlmeijer 2008 {published data only}
- Bohlmeijer ET, Westerhof GJ, Jong ME. The effects of integrative reminiscence on meaning in life: results of a quasi‐experimental study. Aging & Mental Health 2008;12(5):639‐46. [DOI] [PubMed] [Google Scholar]
Brooker 2000 {published data only}
- Brooker D, Duce L. Wellbeing and activity in dementia: a comparison of group reminiscence therapy, structured goal‐directed group activity and unstructured time. Aging & Mental Health 2000;4(4):354‐8. [Google Scholar]
Burckhardt 1987 {published data only}
- Burckhardt CS. The effect of therapy on the mental health of the elderly. Research in Nursing & Health 1987;10(4):277‐85. [DOI] [PubMed] [Google Scholar]
Chao 2006 {published data only}
- Chao SY, Liu HY, Wu CY, Jin SF, Chu TL, Huang TS, et al. The effects of group reminiscence therapy on depression, self esteem, and life satisfaction of elderly nursing home residents. Journal of Nursing Research 2006;14(1):36‐45. [DOI] [PubMed] [Google Scholar]
Chenoweth 2009 {published data only}
- Chenoweth L, King MT, Jeon YH, Brodaty H, Stein‐Parbury J, Norman R, et al. Caring for Aged Dementia Care Resident Study (CADRES) of person‐centred care, dementia care mapping, and usual care in dementia: a cluster‐randomised trial. Lancet Neurology 2009;8(4):317‐25. [DOI] [PubMed] [Google Scholar]
Chiang 2010 {published data only}
- Chiang KJ, Chu H, Chang HJ, Chung MH, Chen CH, Chiou HY, et al. The effects of reminiscence therapy on psychological well‐being, depression, and loneliness among the institutionalized aged. International Journal of Geriatric Psychiatry 2010;25(4):380‐8. [DOI] [PubMed] [Google Scholar]
Choy 2016 {published data only}
- Choy JCP, Lou VWQ. Effectiveness of the modified instrumental reminiscence intervention on psychological well‐being among community‐dwelling Chinese older adults: a randomized controlled trial. American Journal of Geriatric Psychiatry 2016;24(1):60‐9. [DOI] [PubMed] [Google Scholar]
Chueh 2014 {published data only}
- Chueh KH, Chang TY. Effectiveness of group reminiscence therapy for depressive symptoms in male veterans: 6‐month follow‐up. International Journal of Geriatric Psychiatry 2014;29(4):377‐83. [DOI] [PubMed] [Google Scholar]
Chung 2009 {published data only}
- Chung JCC. An intergenerational reminiscence programme for older adults with early dementia and youth volunteers: values and challenges. Scandinavian Journal of Caring Sciences 2009;23(2):259‐64. [DOI] [PubMed] [Google Scholar]
Crook 2016 {published data only}
- Crook N, Adams M, Shorten N, Langdon PE. Does the well‐being of individuals with Down syndrome and dementia improve when using life story books and rummage boxes? A randomized single case series experiment. Journal of Applied Research in Intellectual Disabilities 2016;29(1):1‐10. [DOI] [PubMed] [Google Scholar]
Curto Prieto 2015 {published data only}
- Curto Prieto D, Sole Serrano C, Castro M, Mercadal Brotons M, Asensio FM. Effects of music therapy in institutionalized elderly with dementia. European Geriatric Medicine 2015;6:S48. [Google Scholar]
Eritz 2016 {published data only}
- Eritz H, Hadjistavropoulos T, Williams J, Kroeker K, Martin RR, Lix LM, et al. A life history intervention for individuals with dementia: a randomised controlled trial examining nursing staff empathy, perceived patient personhood and aggressive behaviours. Ageing and Society 2016;36(10):2061‐89. [Google Scholar]
Gudex 2010 {published data only}
- Gudex C, Horsted C, Jensen AM, Kjer M, Surensen J. Consequences from use of reminiscence ‐ a randomised intervention study in ten Danish nursing homes. BMC Geriatrics 2010;10:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haight 2003 {published data only}
- Haight BK, Bachman DL, Hendrix S, Wagner MT, Meeks A, Johnson J. Life review: treating the dyadic family unit with dementia. Clinical Psychology & Psychotherapy 2003;10:165‐74. [Google Scholar]
Haslam 2010 {published and unpublished data}
- Haslam C, Haslam SA, Jetten J, Bevins A, Ravenscroft S, Tonks J. The social treatment: the benefits of group interventions in residential care settings. Psychology and Aging 2010;25(1):157‐67. [DOI] [PubMed] [Google Scholar]
Haslam 2014 {published data only}
- Haslam C, Haslam SA, Ysseldyk R, McCloskey L, Pfisterer K, Brown SG. Social identification moderates cognitive health and well‐being following story‐ and song‐based reminiscence. Aging & Mental Health 2014;18(4):425‐34. [DOI] [PubMed] [Google Scholar]
Head 1990 {published data only}
- Head DM, Portnoy S, Woods RT. The impact of reminiscence groups in two different settings. International Journal of Geriatric Psychiatry 1990;5(1):295‐302. [Google Scholar]
Hilgeman 2014 {published data only}
- Hilgeman MM, Allen RS, Snow A, Durkin DW, DeCoster J, Burgio LD. Preserving Identity and Planning for Advance Care (PIPAC): preliminary outcomes from a patient‐centered intervention for individuals with mild dementia. Aging & Mental Health 2014;18(4):411‐24. [DOI] [PubMed] [Google Scholar]
Hsu 2009 {published data only}
- Hsu YC, Wang JJ. Physical, affective, and behavioral effects of group reminiscence on depressed institutionalized elders in Taiwan. Nursing Research 2009;58(4):294‐9. [DOI] [PubMed] [Google Scholar]
Hutson 2014 {published data only}
- Hutson C, Orrell M, Dugmore O, Spector A. Sonas: a pilot study investigating the effectiveness of an intervention for people with moderate to severe dementia. American Journal of Alzheimer's Disease and Other Dementias 2014;29(8):696‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jo 2015 {published data only}
- Jo H, Song E. The effect of reminiscence therapy on depression, quality of life, ego‐Integrity, social behavior function, and activities of daily living in elderly patients with mild dementia. Educational Gerontology 2015;41:1‐13. [Google Scholar]
Lalanne 2015 {published data only}
- Lalanne J, Gallarda T, Piolino P. The Castle of Remembrance: new insights from a cognitive training programme for autobiographical memory in Alzheimer's disease. Neuropsychological Rehabilitation 2015;25(2):254‐82. [DOI] [PubMed] [Google Scholar]
Lancioni 2014 {published data only}
- Lancioni GE, Singh NN, O'Reilly MF, Sigafoos J, Ferlisi G, Zullo V, et al. A computer‐aided program for helping patients with moderate Alzheimer's disease engage in verbal reminiscence. Research in Developmental Disabilities 2014;35(11):3026‐33. [DOI] [PubMed] [Google Scholar]
Lin 2011 {published data only}
- Lin LJ, Li KY, Tabourne CE. Impact of the life review program on elders with dementia: a preliminary study at a day care center in southern Taiwan. Journal of Nursing Research 2011;19(3):199‐209. [DOI] [PubMed] [Google Scholar]
Liu 2007 {published data only}
- Liu S‐J, Lin C‐J, Chen Y‐M, Huang X‐Y. The effects of reminiscence group therapy on self‐esteem, depression, loneliness and life satisfaction of elderly people living alone. Mid‐Taiwan Journal of Medicine 2007;12(3):133‐42. [Google Scholar]
Lopes 2016 {published data only}
- Lopes TS, Afonso R, Ribeiro ÓM. A quasi‐experimental study of a reminiscence program focused on autobiographical memory in institutionalized older adults with cognitive impairment. Archives of Gerontology and Geriatrics 2016;66:183‐92. [DOI] [PubMed] [Google Scholar]
MacKinlay 2009 {published data only}
- MacKinlay E. Using spiritual reminiscence with a small group of Latvian residents with dementia in a nursing home: a multifaith and multicultural perspective. Journal of Religion, Spirituality & Aging 2009;21(4):318‐29. [Google Scholar]
Mackinlay 2010 {published data only}
- Mackinlay E, Trevitt C. Living in aged care: using spiritual reminiscence to enhance meaning in life for those with dementia. International Journal of Mental Health Nursing 2010;19(6):394‐401. [DOI] [PubMed] [Google Scholar]
McKee 2003 {published data only}
- McKee K, Wilson F, Elford H, Hinchliff S, Bolton G, Cheung Chung M, et al. Reminiscence: is living in the past good for wellbeing?. Nursing & Residential Care 2003;5(10):489‐91. [Google Scholar]
McMurdo 2000 {published data only}
- McMurdo ME, Millar AM, Daly F. A randomized controlled trial of fall prevention strategies in old peoples' homes. Gerontology 2000;46(2):83‐7. [DOI] [PubMed] [Google Scholar]
Melendez‐Moral 2013 {published data only}
- Melendez‐Moral JC, Charco‐Ruiz L, Mayordomo‐Rodriguez T, Sales‐Galan A. Effects of a reminiscence program among institutionalized elderly adults. Psicothema 2013;25(3):319‐23. [DOI] [PubMed] [Google Scholar]
Morris 2015 {published data only}
- Morris BH. Savor the memory: a reminiscence exercise to increase positive emotions and reduce depression risk in anxious individuals. Graduate Theses and Dissertations 2015:5278.
Nakamae 2014 {published and unpublished data}
- Nakamae T, Yotsumoto K, Tatsumi E, Hashimoto T. Effects of productive activities with reminiscence in occupational therapy for people with dementia: a pilot randomised controlled study. Hong Kong Journal of Occupational Therapy 2014;24(1):13‐9. [Google Scholar]
Nakatsuka 2015 {published data only}
- Nakatsuka M, Nakamura K, Hamanosono R, Takahashi Y, Kasai M, Sato Y, et al. A cluster randomized controlled trial of nonpharmacological interventions for old‐old subjects with a Clinical Dementia Rating of 0.5: the Kurihara project. Dementia and Geriatric Cognitive Disorders 2015;5(2):221‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Orten 1989 {published data only}
- Orten JD, Allen M, Cook J. Reminiscence groups with confused nursing centre residents: an experimental study. Social Work in Health Care 1989;14(1):73‐86. [DOI] [PubMed] [Google Scholar]
Politis 2004 {published data only}
- Politis AM, Vozzella S, Mayer LS, Onyike CU, Baker AS, Lyketsos CG. A randomized, controlled, clinical trial of activity therapy for apathy in patients with dementia residing in long‐term care. International Journal of Geriatric Psychiatry 2004;19(11):1087‐94. [DOI] [PubMed] [Google Scholar]
Rattenbury 1989 {published data only}
- Rattenbury C, Stones MJ. A controlled evaluation of reminiscence and current topics discussion groups in a nursing home context. Gerontologist 1989;29(6):768‐71. [DOI] [PubMed] [Google Scholar]
Rawtaer 2015 {published data only}
- Rawtaer I, Mahendran R, Yu J, Fam J, Feng L, Kua EH. Psychosocial interventions with art, music, Tai Chi and mindfulness for subsyndromal depression and anxiety in older adults: a naturalistic study in Singapore. Asia‐Pacific Psychiatry 2015;7(3):240‐50. [DOI] [PubMed] [Google Scholar]
Sabir 2016 {published data only}
- Sabir M, Henderson CR, Kang SY, Pillemer K. Attachment‐focused integrative reminiscence with older African Americans: a randomized controlled intervention study. Aging & Mental Health 2016;20(5):517‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Serrano 2004 {published data only}
Stinson 2006 {published data only}
- Stinson CK, Kirk E. Structured reminiscence: an intervention to decrease depression and increase self‐transcendence in older women. Journal of Clinical Nursing 2006;15(2):208‐18. [DOI] [PubMed] [Google Scholar]
Tadaka 2004 {published data only}
- Tadaka E, Kanagawa K. Randomized controlled trial of a group care program for community‐dwelling elderly people with dementia. Japan Journal of Nursing Science 2004;1(1):19‐25. [Google Scholar]
Tanaka 2017 {published data only}
- Tanaka S, Honda S, Nakano H, Sato Y, Araya K, Yamaguchi H. Comparison between group and personal rehabilitation for dementia in a geriatric health service facility: single‐blinded randomized controlled study. Psychogeriatrics 2017;17(3):177‐85. [DOI] [PubMed] [Google Scholar]
Thornton 1987 {published data only}
- Thornton S, Brotchie J. Reminiscence: a critical review of the empirical literature. British Journal of Clinical Psychology 1987;26(2):93‐111. [DOI] [PubMed] [Google Scholar]
Tolson 2012 {published data only}
- Tolson D, Schofield I. Football reminiscence for men with dementia: lessons from a realistic evaluation. Nursing Inquiry 2012;19(1):63‐70. [DOI] [PubMed] [Google Scholar]
Van Dijk 2012 {published data only}
- Dijk AM, Weert JCM, Droes RM. Does theatre improve the quality of life of people with dementia?. International Psychogeriatrics / IPA 2012;24(3):367‐81. [DOI] [PubMed] [Google Scholar]
Wang 2004 {published data only}
- Wang JJ. The comparative effectiveness among institutionalized and non‐institutionalized elderly people in Taiwan of reminiscence therapy as a psychological measure. Journal of Nursing Research 2004;12(3):237‐45. [DOI] [PubMed] [Google Scholar]
Wang 2007 {published data only}
- Wang JJ. Group reminiscence therapy for cognitive and affective function of demented elderly in Taiwan. International Journal of Geriatric Psychiatry 2007;22(12):1235‐40. [DOI] [PubMed] [Google Scholar]
Wang 2009 {published data only}
- Wang JJ, Yen M, OuYang WC. Group reminiscence intervention in Taiwanese elders with dementia. Archives of Gerontology and Geriatrics 2009;49(2):227‐32. [DOI] [PubMed] [Google Scholar]
Wingbermuehle 2014 {published data only}
- Wingbermuehle C, Bryer D, Berg‐Weger M, Tumosa N, McGillick J, Rodriguez C, et al. Baseball reminiscence league: a model for supporting persons with dementia. Journal of the American Medical Directors Association 2014;15(2):85‐9. [DOI] [PubMed] [Google Scholar]
Wu 2016 {published data only}
- Wu LF, Koo M. Randomized controlled trial of a six‐week spiritual reminiscence intervention on hope, life satisfaction, and spiritual well‐being in elderly with mild and moderate dementia. International Journal of Geriatric Society 2016;31(2):120‐7. [DOI] [PubMed] [Google Scholar]
Yamagami 2007 {published data only}
- Yamagami T, Oosawa M, Ito S, Yamaguchi H. Effect of activity reminiscence therapy as brain‐activating rehabilitation for elderly people with and without dementia. Psychogeriatrics 2007;7(2):69‐75. [Google Scholar]
Yasuda 2009 {published data only}
- Yasuda K, Kuwabara K, Kuwahara N, Abe S, Tetsutani N. Effectiveness of personalised reminiscence photo videos for individuals with dementia. Neuropsychological Rehabilitation 2009;19(4):603‐19. [DOI] [PubMed] [Google Scholar]
Yousefi 2015 {published data only}
- Yousefi Z, Sharifi K, Tagharrobi Z, Akbari H. The effect of narrative reminiscence on happiness of elderly women. Iranian Red Crescent Medical Journal 2015;17(11):e19612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zauszniewski 2004 {published data only}
- Zauszniewski JA, Eggenschwiler K, Preechawong S, Chung CW, Airey TF, Wilke Patricia A, et al. Focused reflection reminiscence group for elders: implementation and evaluation. Journal of Applied Gerontology 2004;23(4):429‐42. [Google Scholar]
References to ongoing studies
Dwolatzky 2014 {published data only}
- Dwolatzky T, Tractinsky N, Sarne‐Fleischmann V. Computer‐supported personal interventions for older people with cognitive impairment and dementia. Alzheimer's Association International Conference 2014; 2014 Jul 12‐17; Copenhagen, Denmark 2014:P158.
Additional references
Akanuma 2006
- Akanuma K, Kasai M, Chiba K, Nakashio D, Sasaki R, Meguro K. The effects of group work including reality orientation and reminiscence for institutionalized patients with vascular dementia. Japanese Journal of Geriatric Psychiatry 2006;17:317‐25. [Google Scholar]
Altman 2005
- Altman DG, Bland JM. Treatment allocation by minimisation.. British Medical Journal 2005;330:843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bohlmeijer 2003
- Bohlmeijer E, Smit F, Cuijpers P. Effects of reminiscence and life review on late‐life depression: a meta‐analysis. International Journal of Geriatric Psychiatry 2003;18:1088‐94. [DOI] [PubMed] [Google Scholar]
Bohlmeijer 2007
- Bohlmeijer ET, Roemer M, Cuijpers P, Smit F. The effects of reminiscence on psychological well‐being in older adults: a meta‐analysis. Aging & Mental Health 2007;11:291‐300. [DOI] [PubMed] [Google Scholar]
Bruce 1998
- Bruce E, Gibson F. Remembering Yesterday, Caring Today: Evaluators' Report. London (UK): Age Exchange, 1998. [Google Scholar]
Bruce 1999
- Bruce E, Hodgson S, Schweitzer P. Reminiscing with People with Dementia: a Handbook for Carers. London (UK): Age Exchange, 1999. [Google Scholar]
Butler 1963
- Butler RN. The life review: an interpretation of reminiscence in the aged. Psychiatry 1963;26:65‐76. [DOI] [PubMed] [Google Scholar]
Cotelli 2012
- Cotelli M, Manenti R, Zanetti O. Reminiscence therapy in dementia: a review [Review]. Maturitas 2012;72(3):203‐5. [DOI] [PubMed] [Google Scholar]
DSM‐IV
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington (DC): APA, 1994. [Google Scholar]
Erikson 1950
- Erikson, E. H. Childhood and Society. New York: Norton, 1950. [Google Scholar]
Folstein 1975
- Folstein MF, Folstein SE, McHugh PR. 'Mini Mental State': a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12:189‐98. [DOI] [PubMed] [Google Scholar]
Gerben 2010
- Gerben JW, Bohlmeijer E, Webster JD. Reminiscence and mental health: a review of recent progress in theory, research and interventions. Ageing and Society 2010;30:697‐721. [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐394. [DOI] [PubMed] [Google Scholar]
Haber 2006
- Haber D. Life review: implementation, theory, research, and therapy. International Journal of Aging and Human Development 2006;63(2):153‐71. [DOI] [PubMed] [Google Scholar]
Haight 1992
- Haight B. The structured life‐review process: a community approach to the ageing client. In: GMM Jones, BML Miesen editor(s). Caregiving in Dementia. London (UK): Routledge, 1992:277‐92. [Google Scholar]
Hellen 1998
- Hellen CR. Alzheimer's Disease: Activity‐Focused Care. 2nd Edition. Boston (MA): Butterworth‐Heinemann, 1998. [Google Scholar]
Help the Aged 1981
- Help the Aged. Recall. London (UK): Help the Aged, 1981. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Huang 2015
- Huang HC, Chen YT, Chen PY, Hu SHL, Liu F, Kuo YL, et al. Reminiscence therapy improves cognitive functions and reduces depressive symptoms in elderly people with dementia: a meta‐analysis of randomized controlled trials. Journal of the American Medical Directors Association 2015;16(12):1087‐94. [DOI] [PubMed] [Google Scholar]
Hughes 1982
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. British Journal of Psychiatry 1982;140:566‐72. [DOI] [PubMed] [Google Scholar]
Kiernat 1979
- Kiernat JM. The use of life review activity with confused nursing home residents. American Journal of Occupational Therapy 1979;33:306‐10. [PubMed] [Google Scholar]
Kwon 2013
- Kwon M‐H, Cho B‐H, Lee J‐S. Reminiscence therapy for dementia ‐ meta analysis. Advanced Science and Technology Letters 2013;40:10‐5. [DOI: 10.14257/astl.2013.40.03] [DOI] [Google Scholar]
Melunsky 2015
- Melunsky N, Crellin N, Dudzinski E, Orrell M, Wenborn J, Poland F, et al. The experience of family carers attending a joint reminiscence group with people with dementia: a thematic analysis. Dementia 2015;14(6):842‐59. [DOI: 10.1177/1471301213516332] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohs 2000
- Mohs R. Neuropsychological assessment of patients with Alzheimer’s disease. In: Bloom FE, Kupfer DJ editor(s). Psychopharmacology: 4th Generation of Progress. Brentwood (TN): American College of Neuropsychopharmacology, 2000. [www.acnp.org/publications/psycho4generation.aspx] [Google Scholar]
Morris 1994
- Morris RG. Recent developments in the neuropsychology of dementia. International Review of Psychiatry 1994;6:85‐107. [Google Scholar]
Norris 1986
- Norris AD. Reminiscence with Elderly People. London (UK): Winslow, 1986. [Google Scholar]
Pinquart 2007
- Pinquart M, Duberstein PR, Lyness JM. Effects of psychotherapy and other behavioral interventions on clinically depressed older adults: a meta‐analysis. Aging & Mental Health 2007;11(6):645‐57. [DOI] [PubMed] [Google Scholar]
Pinquart 2012
- Pinquart M, Forstmeier S. Effects of reminiscence interventions on psychosocial outcomes: a meta‐analysis. Aging & Mental Health 2012;16(5):541‐58. [DOI] [PubMed] [Google Scholar]
Pot 2010
- Pot AM, Bohlmeijer ET, Onrust S, Melenhorst AS, Veerbeek M, Vries W. The impact of life review on depression in older adults: a randomized controlled trial. International Psychogeriatrics / IPA 2010;22(4):572‐81. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Romaniuk 1981
- Romaniuk M, Romaniuk J. Looking back: an analysis of reminiscence functions and triggers. Experimental Aging Research 1981;7:477‐89. [DOI] [PubMed] [Google Scholar]
Ros 2016
- Ros L, Melendez JC, Webster JD, Butler T, Sales A, Latorre JM, et al. Reminiscence functions scale: factorial structure and its relation with mental health in a sample of Spanish older adults. International Psychogeriatrics / IPA 2016;28(9):1521‐32. [DOI] [PubMed] [Google Scholar]
Schweitzer 2008
- Schweitzer P, Bruce E. Remembering Yesterday, Caring Today. Reminiscence in Dementia Care: a Guide to Good Practice. London (UK): Jessica Kingsley Publishers, 2008. [Google Scholar]
Soltys 1994
- Soltys F, Coats L. The SolCos model: facilitating reminiscence therapy. Journal of Gerontological Nursing 1994;20:11‐6. [DOI] [PubMed] [Google Scholar]
Subramaniam 2010
- Subramaniam P, Woods B. Towards the therapeutic use of information and communication technology in reminiscence work for people with dementia: a systematic review. International Journal of Computers in Healthcare 2010;1(2):106‐25. [Google Scholar]
Subramaniam 2012
- Subramaniam P, Woods B. The impact of individual reminiscence therapy for people with dementia: systematic review [Review]. Expert Review of Neurotherapeutics 2012;12(5):545‐55. [DOI] [PubMed] [Google Scholar]
Subramaniam 2016
- Subramaniam P, Woods B. Digital life storybooks for people with dementia living in care homes: an evaluation. Clinical Interventions in Aging 2016;11:1263‐76. [DOI: 10.2147/CIA.S111097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tadaka 2007
- Tadaka E, Kanagawa K. Effects of reminiscence group in elderly people with Alzheimer disease and vascular dementia in a community setting. Geriatrics & Gerontology International 2007;7(2):167‐73. [Google Scholar]
Testad 2014
- Testad I, Corbett A, Aarsland D, Lexow KO, Fossey J, Woods B, et al. The value of personalized psychosocial interventions to address behavioral and psychological symptoms in people with dementia living in care home settings: a systematic review. International Psychogeriatrics / IPA 2014;26(7):1083‐98. [DOI] [PubMed] [Google Scholar]
Woods 2012b
- Woods B, Aguirre E, Spector AE, Orrell M. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD005562.pub2] [DOI] [PubMed] [Google Scholar]
Woods 2016
- Woods B, Subramaniam P. The evidence base for lifestory work so far. In: Kaiser P, Eley R editor(s). Life Story Work with People with Dementia; Ordinary Lives, Extraordinary People. London (UK): Jessica Kingsley, 2016:83‐94. [Google Scholar]
Zhang 2015
- Zhang SJ, Hwu YJ, Wu PI, Chang CW. The effects of reminiscence therapy on depression, self‐esteem and life satisfaction on institutionalized older adults: a meta‐analysis. Journal of Nursing & Healthcare Research 2015;11(1):33‐42. [Google Scholar]
References to other published versions of this review
Woods 2005
- Woods B, Spector A, Jones C, Orrell M, Davies S. Reminiscence therapy for dementia. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001120.pub2] [DOI] [PubMed] [Google Scholar]


