Supplemental Digital Content is available in the text
Keywords: gestational diabetes mellitus, lipid profiles, triglycerides to high-density lipoprotein ratio
Abstract
To determine the longitude lipid profiles in women with and without gestational diabetes mellitus (GDM), and to investigate the relationship between lipid disturbances in the 1st trimester and GDM.
Blood samples were collected from 1283 normal pregnant women and 300 women with GDM. Serum lipids which include total cholesterol (TC), triglycerides (TGs), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were measured and the TG/HDL-C ratio was calculated in the 1st, 2nd, and 3rd trimesters of pregnancy and then we got the longitudinal lipid profiles. We compared the differences of lipid profiles between patients with GDM and normal pregnant women using 2-way repeated measures analysis of variance. Also additional propensity-based subgroup analyses were performed. The logistic regression analysis was used to determine the relationship between the lipid disturbances in the 1st trimester and GDM.
TG, TC, LDL-C concentrations, and TG/HDL-C ratio increased progressively throughout pregnancy; while HDL-C amounts increased from the 1st to the 2nd trimester with a slight decrease in the 3rd trimester. The GDM group showed higher TG concentrations, higher TG/HDL-C ratio, and lower HDL-C concentrations throughout pregnancy. There were no significant differences in TC and LDL-C concentrations in the 1st, 2nd, and 3rd trimesters (P > .05), between the GDM group and the control group. Logistic regression analysis showed that maternal age, prepregnancy body mass index (BMI), and TG/HDL ratio in the 1st trimester were associated with an increased risk of GDM.
The lipid profile alters significantly in patients with GDM, and maternal age, prepregnancy BMI, and TG/HDL ratio in the 1st trimester were associated with an increased risk of GDM.
1. Introduction
Gestational diabetes mellitus (GDM), defined as abnormal glucose tolerance firstly discovered during pregnancy, is the most common metabolic disease during pregnancy.[1] Its incidence is increasing worldwide and 15% to 22% of all pregnancies are affected. Due to the new diagnostic criteria, this prevalence may be higher.[2] GDM can cause many consequences, such as fetal macrosomia, preeclampsia and high cesarean section rate, and so on.[3,4] Also both women with GDM and their offspring experience a greater risk of obesity, type 2 diabetes, and cardiovascular disorders in the future.[5]
Maternal dyslipidemia which elevated over a physiologic range evidently is a common phenomenon during pregnancy.[6] Especially, hyperlipidemia is commonly discovered in the 2nd half of pregnancy, and it is regarded as a physiologically required mechanism to provide fuel and nutrients for the fetus.[7] The lipid level increases slightly in early pregnancy, but significantly in later pregnancy. However, to ascertain which level of lipid elevation is physiologic or pathologic is difficult nowadays.
The GDM is associated with increased lipid profiles. Although the literature describing lipid levels in pregnancy and GDM is extensive, results have been inconsistent.[8–11] Also there are few studies as to whether lipid patterns differ in women with GDM early in pregnancy and whether lipid abnormalities in the 1st trimester have potential clinical utility for identifying women at risk for subsequently developing GDM.[12] In this study, we sought to perform an observational study to compare the concentrations of total cholesterol (TC), triglycerides (TGs), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and TG/HDL-C ratio (TG to HDL-C ratio) in the 1st, 2nd, and 3rd trimesters of pregnancy in normal pregnant women and women with GDM. We aim to determine the longitudinal lipid levels by trimester and compare the differences between patients with GDM and normal pregnant women. Also we aim to determine whether lipid disturbances early in the 1st trimester of pregnancy are related to GDM.
2. Materials and methods
2.1. Setting and participants
This retrospective cohort study was conducted at Perking University International Hospital (PKUIH). This study was reviewed and approved by the ethics review board of PKUIH and the institutional review board approval number was 2018-020. A total of 1583 women who attended regular prenatal health care and delivered their babies in PKUIH from January to December 2017 were included in this study. Among them, 300 pregnancy women who were diagnosed as GDM in the 2nd trimester according to the American Diabetes Association (ADA) 2010 criteria[13] were classified as patients with GDM, while 1283 healthy pregnant women were included in the control group.
Inclusion criteria of pregnant women were: singleton pregnancy; had integrated medical records. Exclusion criteria of pregnant women were: multiple pregnancies; type 1 or type 2 diabetes mellitus before pregnancy, inherited metabolic diseases, cardiovascular disease, thyroid or liver dysfunction before pregnancy; use of drugs that could affect lipid levels, including corticosteroids, etc.
The information of maternal age, height, parity, prepregnancy body mass index (BMI), delivery mode, gestational age, birth weight, etc, were recorded. Prepregnancy BMI was derived as the weight (kg) divided by the square of the height (m), and the patients were classified as low weight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), or obese (≥30.0 kg/m2) on the basis of World Health Organization BMI classification.[14] Macrosomia is defined as birth weight ≥4000 g.
2.2. Biochemical analyses
Longitudinal lipid assessments were carried out during three periods: 6 to 8 gestational weeks (GWs) (the 1st trimester, T1), 24 to 28 GWs (the 2nd trimester, T2), and 32 to 34 GWs (the 3rd trimester, T3). TG/HDL-C ratio was calculated accordingly by trimester.
Blood samples were collected at the outpatient clinic by a trained nurse after a 10- to 12-hour fasting period. Serum TC, LDL-C, HDL-C, TG, and glucose concentrations were measured on an automatic biochemical analyzer (Beckman Coulter Co. Ltd., Tokyo, Japan) and monitored by a well-trained inspector. The inter- and intra-assay coefficients of variation were <1.6%, 0.6% (TC); <1.7%, 1.1% (TG); <1.1%, 0.6% (HDL-C); and <1.6%, 1.1% (LDL-C), respectively.
2.3. Statistical analysis
In our study, descriptive statistics included means and standard deviation (SD) for continuous variables, and numbers and percentages for categorical variables. First, we used 2-way repeated measures analysis of variance to estimate whether serum TC, TG, LDL-C, HDL-C concentrations, and TG/HDL-C ratio increased by trimesters (P for trend) and explore the differences in serum lipids concentrations in the 1st, 2nd, and 3rd trimesters between pregnant women with and without GDM (P for difference). Then, we used backward stepwise logistic regression analysis to test whether serum lipids measured in the 1st trimester could predict the risk of GDM in the 2nd trimester, and the candidate predictor variables included maternal age, prepregnancy BMI, and parity as confounding variables. Finally, to test robustness of our main results, we performed additional propensity-based subgroup analyses. Pregnant women with and without GDM were matched (1:1) on maternal age, prepregnancy BMI, and parity, using the Greedy matching macro.[15]
All the analyses were performed with SPSS version 20.0 for Windows (SPSS Inc, Chicago, IL). P values <.05 were defined as statistically significant.
3. Results
3.1. Characteristics of the study population
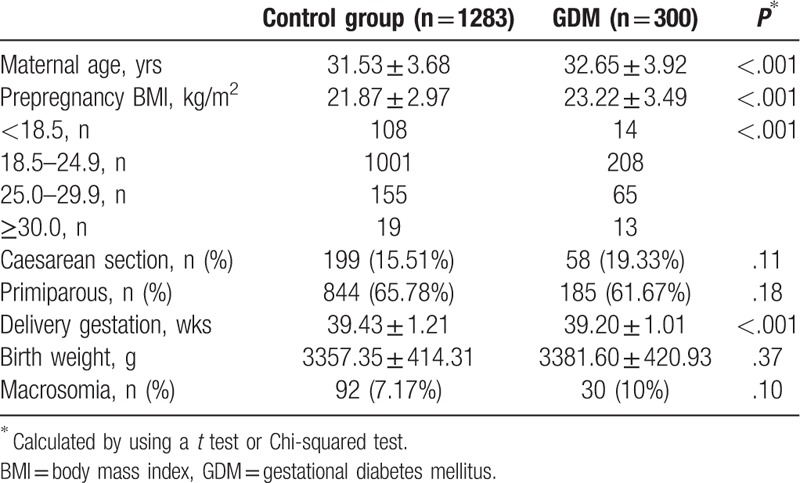
Table 1 presents maternal and neonatal characteristics of our study population. Among the 1583 mothers in the present study, the maternal age in the GDM group was 32.65 ± 3.92 years, while that of the control group was 31.53 ± 3.68 years, with significantly statistical difference between the 2 groups. The prepregnancy BMI in the GDM group was 23.22 ± 3.49 kg/m2, which was significantly higher than that of the control group (21.87 ± 2.97 kg/m2), P < .001. The mean age at delivery was 39.20 ± 1.01 weeks in the GDM group and 39.43 ± 1.21 weeks in the control group, with statistical difference between the 2 groups. There were no significantly statistical differences on the birth weight, the rate of primiparous, the rate of caesarean section, and the rate of macrosomia between the 2 groups.
Table 1.
Characteristics of study population.
3.2. Maternal lipid profiles by trimester
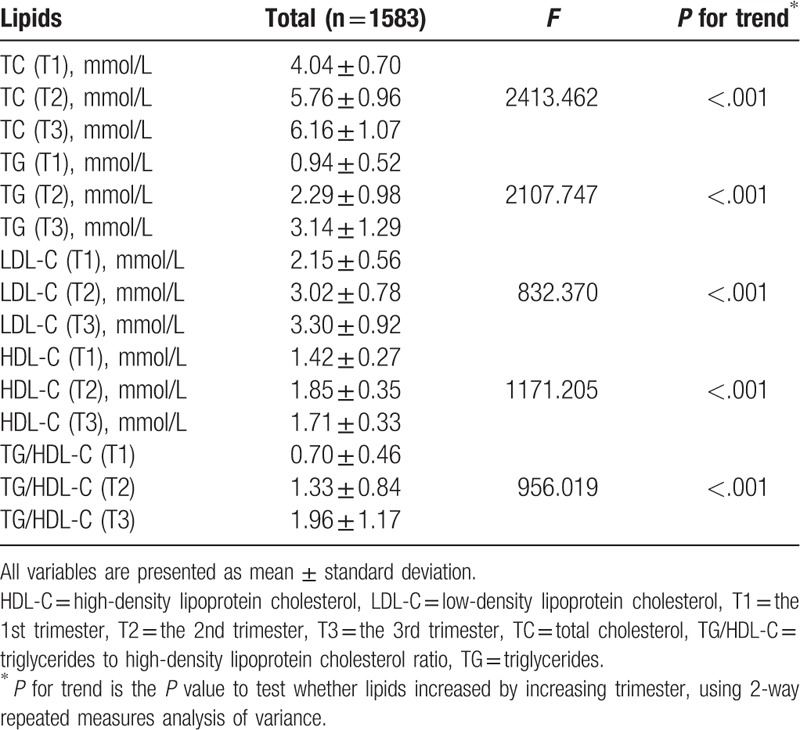
Table 2 shows maternal lipid profiles by trimester. Serum TG, TC, LDL-C concentrations, and TG/HDL-C ratio increased progressively throughout pregnancy (all P for trend <.001). However, HDL-C amounts increased from the 1st to the 2nd trimester with a slight decrease in the 3rd trimester. All the lipid parameters of the 3rd trimester were higher than those of the 1st and 2nd trimesters, except HDL-C. Similarly, the levels of lipids increased by increasing trimesters in both GDM and control groups (all P for trend <.001, Supplement Table 1).
Table 2.
Maternal lipid profiles by trimester.
3.3. Maternal lipid profiles between the 2 groups
Compared with the control group, the GDM group showed higher TG concentrations and higher TG/HDL-C ratio throughout pregnancy, while lower HDL-C concentrations throughout pregnancy (P < .05). However, there were no significant differences in TC and LDL-C concentrations in the 1st, 2nd, and 3rd trimesters between the GDM group and the control group (P > .05) (Table 3). Unadjusted values of lipids (TG, HDL-C, and TG/HDL-C ratio) in the 2 groups in different trimesters are depicted in Figure 1.
Table 3.
Maternal lipid profiles of the different trimesters in the 2 groups.
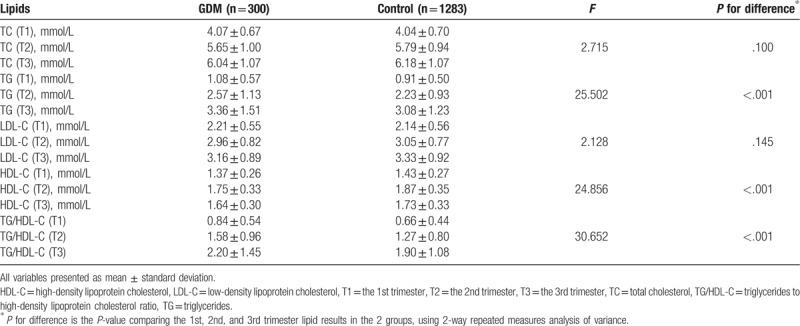
Figure 1.
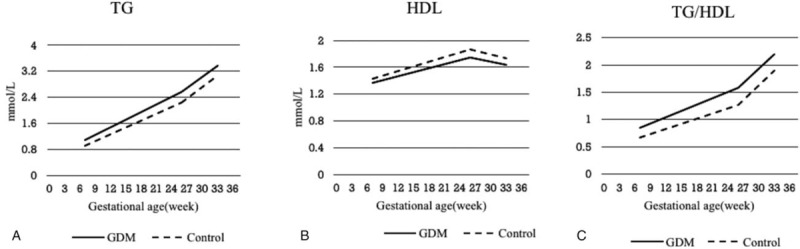
Unadjusted values of lipids (TG, HDL-C, and TG/HDL-C ratio) in the 2 groups in different trimesters. GDM = gestational diabetes mellitus, HDL-C = high-density lipoprotein cholesterol, TG = triglycerides, TG/HDL-C = triglycerides to high-density lipoprotein cholesterol ratio.
3.4. Longitudinal analysis
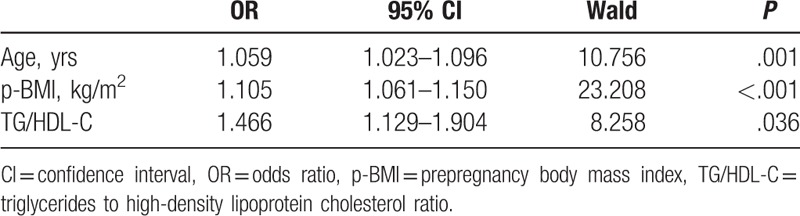
Logistic regression analysis showed that maternal age, prepregnancy BMI, and TG/HDL ratio measured in the 1st trimester were associated with an increased risk of GDM (Table 4).
Table 4.
Binary logistic regression analysis of the risk of GDM.
3.5. Propensity score analysis
The propensity-score matching identified 297 pregnant women with GDM and 297 pregnant without GDM. Maternal age, prepregnancy BMI, and parity were comparable between 2 groups (see Supplement Table 2). Similar to the main analysis, these analyses showed that TG, TC, LDL-C concentrations, and TG/HDL-C ratio increased progressively throughout pregnancy; while HDL-C amounts increased from the 1st to the 2nd trimester with a slight decrease in the 3rd trimester. Compared with the control group, the GDM group showed higher TG/HDL-C ratio and lower HDL-C concentrations throughout pregnancy, with no significant difference on TG concentrations. The difference of TG concentration was no longer significant probably because of the relatively small sample size (Supplement Tables 3–4).
4. Discussion
Lipid metabolism is essential for a healthy pregnancy development.[16] The plasma lipid profile including the levels of TC, HDL-C, LDL-C, and TG changes apparently during normal pregnancy.[17,18] Plasma lipid concentrations increase markedly during pregnancy due to estrogen stimulation and insulin resistance.[19] During the 1st two-thirds of gestation, there is an increase in maternal fat accumulation, associated with both hyperphagia and increased lipogenesis.[20–22] In the last 3rd trimester of gestation, however, as a result of increased lipolytic activity and declined lipoprotein lipase activity, maternal fat storage decreases or even ceases.[23,24] These changes are reflections of maternal physiologic adaptation to energy demand of the fetus, also they are necessary preparations for delivery and lactation.[16]
Circulating plasma lipid patterns during normal pregnancy have been widely studied, and most studies have found that serum TG, HDL-C, LDL-C, and TC levels are obviously elevated throughout the pregnancy.[25–27] Shen et al found that the levels of lipids, including TG, TC, and LDL-C, increased gradually during gestation and peaked before delivery; meanwhile, HDL-C amounts increased from the 1st to 2nd trimester with a slight decrease in the 3rd trimester.[8] Consistent with Shen's study, we also found the similar lipid profiles throughout the pregnancy. And this indicated that the elevation of lipid concentrations is a physiologic requirement for maintaining stable energy storage for the fetus. However, it is difficult to ascertain which level of lipid elevation is physiologic or pathologic and there are no worldwide standard criteria of lipid levels during pregnancy due to the heterogeneity of the population and territory.
Hypertriglyceridemia and low HDL-C levels are 2 important metabolic abnormalities associated with insulin resistance.[28,29] Moreover, the TG/HDL-C ratio was proved to be a good and sensitive indicator to identify insulin-resistant individuals of North American aboriginal, Chinese, and European.[29] The TG/HDL-C ratio was considered as an atherogenic index.[30] In our study, we found that the TG/HDL-C ratio increased significantly throughout the pregnancy, which indicated progressive insulin resistance during normal pregnancy.
The GDM is a complex condition that manifests as glucose intolerance and insulin resistance with onset or 1st recognition during pregnancy.[31] Women with GDM are at highly increased risk of developing metabolic diseases after pregnancy including hyperlipidemia, type 2 diabetes, and so on.[32,33]
There have been extensive studies about circulating lipid patterns in GDM vs normal pregnancy, with no consistent conclusions.[34] Except for a few studies in which no changes of serum TG levels were found in GDM group compared to nondiabetic group,[35–37] most studies suggested that women with GDM have increased levels of TG, LDL-C, and TC and lower levels of HDL-C. Savvidou et al found that women who developed GDM had higher TG, TC, LDL-C levels, and lower levels of HDL-C in univariate analysis.[38,39] Shen et al[8] found that high levels of TGs during pregnancy were associated with increased risk of GDM and gestational weight gain during gestation; TC and LDL-C levels were only higher in the 1st trimester for the GDM group, with no difference of HDL-C levels. A recent meta-analysis[9] showed that serum TG was significantly elevated among GDM women and the increase persisted across all the 3 trimesters of pregnancy. They also found that serum HDL-C levels were significantly lower in women with GDM in the 2nd and 3rd trimesters of pregnancy compared with women without insulin resistance. However, no elevated serum TC and LDL-C levels were found between women with GDM and women without insulin resistance in the study. Among the Chinese population, Jin et al[40] found that maternal high TG in late pregnancy was independently associated with increased risk of GDM; and relatively lower maternal HDL-C during pregnancy had a significant association with increased risk of GDM and macrosomia; indicating that high HDL-C was a protective factor for both of them. In a recent study, Khosrowbeygi et al found the atherogenic indexes including the LDL-C/HDL-C, TG/HDL-C, and TC/HDL-C ratios were higher in GDM compared with normal pregnancy and especially, the TG/HDL-C ratio showed a significantly positive correlation with insulin resistance, which might be used as a simple surrogate marker for assessing insulin resistance in pregnancy.[30] Similar to the results of the recent meta-analysis,[9] in our study, we found that compared with the control group, the GDM group showed higher TG concentrations, TG/HDL-C ratio, and lower HDL-C concentrations throughout pregnancy. In the matched-pairs analysis, after adjusting age and prepregnancy BMI, we found significant difference on HDL-C concentrations, but no significant difference on TG concentrations between the 2 groups. The phenomena may be probably because of the relatively small sample size, or because serum TG had a closer correlation with age and prepregnancy BMI, and serum HDL-C concentrations maybe more related to GDM than serum TG. But further studies with lager sample size in matched-pairs analysis will be needed.
This study indicated that lipid profiles were dramatically different between the GDM group and the control group and GDM women had more serious dyslipidemia and insulin resistance in pregnancy. Meanwhile, we found that there were no significant differences in TC and LDL-C concentrations in the 1st, 2nd, and 3rd trimesters between the 2 groups. However, the exact mechanism was unknown and further studies should be conducted to explore the role of dyslipidemia in the pathogenesis of GDM.
To examine whether lipid abnormalities in the 1st trimester have potential clinical utility for identifying women at risk for subsequently developing GDM, we analyzed the relationship between maternal lipid profile in the 1st trimester and GDM. Logistic regression analysis showed that maternal age, prepregnancy BMI, and TG/HDL ratio were associated with an increased risk of GDM. This indicated that TG/HDL-C ratio in the 1st trimester in combination with maternal age and prepregnancy BMI could be good markers to predict the risks of GDM. Similarly, Wang et al[41] found that TG/HDL-C ratio in combination with HbA1c and prepregnancy BMI were good markers to predict the risk of GDM and delivering large for gestational age infant. Therefore, the TG/HDL-C ratio might be useful for assessing insulin resistance in pregnancy, and its level in the early pregnancy may be a good predictor for occurrence of GDM. So, in clinical practice, elderly primipara should be reduced, and overweight or obese women are suggested to reduce weight to normal weight before pregnancy. Special attention should be paid to women with abnormal higher TG/HDL ratio in the 1st trimester, and obstetricians should inform them of necessary diet management, weight control, and proper exercise according to 2016 WHO recommendations on antenatal care.[42]
In the future, more research is needed to establish the lipid standard during pregnancy according to local maternal characteristics such as inheritance, ethnicity, region, etc. More studies will be needed to explore the correlation between dyslipidemia and the pathogenesis of GDM. Also for women with elderly maternal age, high prepregnancy BMI, elevated TG/HDL-C ratio in the 1st trimester, close surveillance and monitoring, necessary weight control, and diet management should be carried out to reduce the incidence of GDM as much as possible.
4.1. Main findings
This study yielded 3 main findings: maternal TG, TC, LDL-C concentrations, and TG/HDL-C ratio increased progressively throughout pregnancy; and HDL-C concentrations increased from the 1st to the 2nd trimester with a slight decrease in the 3rd trimester; Lipid profiles were dramatically different between the GDM group and the control group. Compared with the control group, the GDM group showed higher TG concentrations, TG/HDL-C ratio, and lower HDL-C concentrations throughout pregnancy. However, there were no significant differences in TC and LDL-C concentrations in the 1st, 2nd, and 3rd trimesters, between the GDM group and the control group; Maternal age, prepregnancy BMI, and TG/HDL ratio in the 1st trimester were associated with an increased risk of GDM and could predict the risk of GDM at an early stage.
4.2. Strengths and limitations
This was a longitudinal cohort study with large sample size. In this study, serum lipid levels were tested in 3 different trimesters. Although there were several studies about lipid profile during pregnancy, few studies have tested serum lipids longitudinally at multiple points during pregnancy and previous studies were usually small sample size. Moreover, this study performed additional propensity-based subgroup analyses to adjust prepregnancy age and BMI to avoid the influences on serum lipids concentrations in the 2 groups.
However, the present study still has some limitations. First of all, despite relatively large sample size, this study is a single-center study, and lacked population diversity. A multi-center study with different population and regions may be more comprehensive. Secondly, due to ethical reasons, all GDM women received dietary guidance and exercise management once the diagnosis was established, which may affect the lipid levels. Finally, the information of gestational weight gain was not collected, and we failed to get the relationship between gestational weight gain and the lipid changes during pregnancy.
5. Conclusion
Overall, in a large longitudinal cohort study, we found that TG, TC, LDL-C concentrations, and TG/HDL-C ratio increased progressively throughout pregnancy; meanwhile, HDL-C amounts increased from the 1st to the 2nd trimester with a slight decrease in the 3rd trimester. Lipid profiles were dramatically different between the GDM group and the control group, except of serum TC and LDL-C concentrations. Maternal age, prepregnancy BMI and TG/HDL ratio in the 1st trimester could predict the risk of GDM at an early stage.
Acknowledgments
The authors thank Liyuan Tao (Research Center of Clinical Epidemiology, Peking University Third Hospital) and Wuxiang Xie (Peking University Clinical Research Institute) for his their statistical assistance and advice.
Author contributions
Conceptualization: Li Lin.
Data curation: Jing Wang.
Methodology: Jing Wang, Zhi Li.
Writing – original draft: Jing Wang.
Writing – review & editing: Zhi Li.
jing WANG orcid: 0000-0002-1074-4279.
Supplementary Material
Footnotes
Abbreviations: ANOVA = analysis of variance, BMI = body mass index, GDM = gestational diabetes mellitus, GWs = gestational weeks, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, TC = total cholesterol, TGs = triglycerides, TG/HDL-C = triglycerides to high-density lipoprotein cholesterol ratio.
This study was funded by the National Natural Science Foundation of China (no: 81471476).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care 2007;30:S141–6. [DOI] [PubMed] [Google Scholar]
- [2].Cho NH. Gestational diabetes mellitus--challenges in research and management. Diabetes Res Clin Pract 2013;99:237–9. [DOI] [PubMed] [Google Scholar]
- [3].Catalano PM, McIntyre HD, Cruickshank JK, et al. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012;35:780–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Alberico S, Montico M, Barresi V, et al. The role of gestational diabetes, pre-pregnancy body mass index and gestational weight gain on the risk of newborn macrosomia: results from a prospective multicentre study. BMC Pregnancy Childbirth 2014;14:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hermes W, Franx A, van Pampus MG, et al. Cardiovascular risk factors in women who had hypertensive disorders late in pregnancy: a cohort study. Am J Obstet Gynecol 2013;208:474.e1–8. [DOI] [PubMed] [Google Scholar]
- [6].Leiva A, Guzmán-Gutiérrez E, Contreras-Duarte S, et al. Adenosine receptors: Modulators of lipid availability that are controlled by lipid levels. Mol Aspects Med 2017;55:26–44. [DOI] [PubMed] [Google Scholar]
- [7].Herrera E, Ortega-Senovilla H. Lipid metabolism during pregnancy and its implications for fetal growth. Curr Pharm Biotechnol 2014;15:24–31. [DOI] [PubMed] [Google Scholar]
- [8].Shen H, Liu X, Chen Y, et al. Associations of lipid levels during gestation with hypertensive disorders of pregnancy and gestational diabetes mellitus: a prospective longitudinal cohort study. BMJ Open 2016;6:e013509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ryckman KK, Spracklen CN, Smith CJ, et al. Maternal lipid levels during pregnancy and gestational diabetes: a systematic review and meta-analysis. BJOG 2015;122:643–51. [DOI] [PubMed] [Google Scholar]
- [10].Jia X, Wang S, Ma N, et al. Comparative analysis of vaspin in pregnant women with and without gestational diabetes mellitus and healthy non-pregnant women. Endocrine 2015;48:533–40. [DOI] [PubMed] [Google Scholar]
- [11].Li SM, Wang WF, Zhou LH, et al. Fibroblast growth factor 21 expressions in white blood cells and sera of patients with gestational diabetes mellitus during gestation and postpartum. Endocrine 2015;48:519–27. [DOI] [PubMed] [Google Scholar]
- [12].Wang C, Zhu W, Wei Y, et al. The predictive effects of early pregnancy lipid profiles and fasting glucose on the risk of gestational diabetes mellitus stratified by body mass index. J Diabetes Res 2016;2016:3013567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Metzger BE, Gabbe SG, et al. International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000;894:1–253. [PubMed] [Google Scholar]
- [15].Parsons LS. Performing a 1: N case-control match on propensity score. proceedings of the 29th Annual SAS users group international conference. 2004:165–29. [Google Scholar]
- [16].Di Cianni G, Miccoli R, Volpe L, et al. Maternal triglyceride levels and newborn weight in pregnant women with normal glucose tolerance. Diabet Med 2005;22:21–5. [DOI] [PubMed] [Google Scholar]
- [17].Chiang AN, Yang ML, Hung JH, et al. Alterations of serum lipid levels and their biological relevances during and after pregnancy. Life Sci 1995;56:2367–75. [DOI] [PubMed] [Google Scholar]
- [18].Toescu V, Nuttall SL, Martin U, et al. Changes in plasma lipids and markers of oxidative stress in normal pregnancy and pregnancies complicated by diabetes. Clin Sci 2004;106:93–8. [DOI] [PubMed] [Google Scholar]
- [19].Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr 2000;71:1256S–61S. [DOI] [PubMed] [Google Scholar]
- [20].Murphy SP, Abrams BF. Changes in energy intakes during pregnancy and lactation in a national sample of US women. Am J Public Health 1993;83:1161–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ghio A, Bertolotto A, Resi V, et al. Triglyceride metabolism in pregnancy. Adv Clin Chem 2011;55:133–53. [DOI] [PubMed] [Google Scholar]
- [22].Mankuta D, Elami-Suzin M, Elhayani A, et al. Lipid profile in consecutive pregnancies. Lipids Health Dis 2010;9:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Herrera E, Lasunción MA, Gomez-Coronado D, et al. Role of lipoprotein lipase activity on lipoprotein metabolism and the fate of circulating triglycerides in pregnancy. Am J Obstet Gynecol 1988;158(Pt 2):1575–83. [DOI] [PubMed] [Google Scholar]
- [24].Meyer BJ, Stewart FM, Brown EA, et al. Maternal obesity is associated with the formation of small dense LDL and hypoadiponectinemia in the third trimester. J Clin Endocrinol Metab 2013;98:643–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Farias DR, Franco-Sena AB, Vilela A, et al. Lipid changes throughout pregnancy according to pre-pregnancy BMI: results from a prospective cohort. BJOG 2016;123:570–8. [DOI] [PubMed] [Google Scholar]
- [26].Vahratian A, Misra VK, Trudeau S, et al. Prepregnancy body mass index and gestational age-dependent changes in lipid levels during pregnancy. Obstet Gynecol 2010;116:107–13. [DOI] [PubMed] [Google Scholar]
- [27].Emet T, Ustüner I, Güven SG, et al. Plasma lipids and lipoproteins during pregnancy and related pregnancy outcomes. Arch Gynecol Obstet 2013;288:49–55. [DOI] [PubMed] [Google Scholar]
- [28].Fan X, Liu EY, Hoffman VP, et al. Triglyceride/high-density lipoprotein cholesterol ratio: a surrogate to predict insulin resistance and low-density lipoprotein cholesterol particle size in nondiabetic patients with schizophrenia. J Clin Psychiatry 2011;72:806–12. [DOI] [PubMed] [Google Scholar]
- [29].Gasevic D, Frohlich J, Mancini GB, et al. The association between triglyceride to high-density-lipoprotein cholesterol ratio and insulin resistance in a multiethnic primary prevention cohort. Metabolism 2012;61:583–9. [DOI] [PubMed] [Google Scholar]
- [30].Khosrowbeygi A, Shiamizadeh N, Taghizadeh N. Maternal circulating levels of some metabolic syndrome biomarkers in gestational diabetes mellitus. Endocrine 2016;51:245–55. [DOI] [PubMed] [Google Scholar]
- [31].American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37:S81–90. [DOI] [PubMed] [Google Scholar]
- [32].Bo S, Monge L, Macchetta C, et al. Prior gestational hyperglycemia: a long-term predictor of the metabolic syndrome. J Endocrinol Invest 2004;27:629–35. [DOI] [PubMed] [Google Scholar]
- [33].Lauenborg J, Mathiesen E, Hansen T, et al. The prevalence of the metabolic syndrome in a Danish population of women with previous gestational diabetes mellitus is three-fold higher than in the general population. J Clin Endocrinol Metab 2005;90:4004–10. [DOI] [PubMed] [Google Scholar]
- [34].Herrera E, Ortega-Senovilla H. Disturbances in lipid metabolism in diabetic pregnancy - are these the cause of the problem? Best Pract Res Clin Endocrinol Metab 2010;24:515–25. [DOI] [PubMed] [Google Scholar]
- [35].Montelongo A, Lasunción MA, Pallardo LF, et al. Longitudinal study of plasma lipoproteins and hormones during pregnancy in normal and diabetic women. Diabetes 1992;41:1651–9. [DOI] [PubMed] [Google Scholar]
- [36].Marseille-Tremblay C, Ethier-Chiasson M, Forest JC, et al. Impact of maternal circulating cholesterol and gestational diabetes mellitus on lipid metabolism in human term placenta. Mol Reprod Dev 2008;75:1054–62. [DOI] [PubMed] [Google Scholar]
- [37].Rizzo M, Berneis K, Altinova AE, et al. Atherogenic lipoprotein phenotype and LDL size and subclasses in women with gestational diabetes. Diabet Med 2008;25:1406–11. [DOI] [PubMed] [Google Scholar]
- [38].Savvidou M, Nelson SM, Makgoba M, et al. First-trimester prediction of gestational diabetes mellitus: examining the potential of combining maternal characteristics and laboratory measures. Diabetes 2010;59:3017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Li G, Kong L, Zhang L, et al. Early pregnancy maternal lipid profiles and the risk of gestational diabetes mellitus stratified for body mass index. Reprod Sci 2015;22:712–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jin WY, Lin SL, Hou RL, et al. Associations between maternal lipid profile and pregnancy complications and perinatal outcomes: a population-based study from China. BMC Pregnancy Childbirth 2016;16:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wang D, Xu S, Chen H, et al. The associations between triglyceride to high-density lipoprotein cholesterol ratios and the risks of gestational diabetes mellitus and large-for-gestational-age infant. Clin Endocrinol 2015;83:490–7. [DOI] [PubMed] [Google Scholar]
- [42].World Health Organization. WHO recommendations on antenatal care for a positive pregnancy experience. Geneva, Switzerland: WHO; 2016. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


