Abstract
Intraventricular meningiomas are rather rare and only represent a small proportion of all intracranial meningiomas. Data are still limited toward this peculiar entity and surgical resection remains challenging for neurosurgeons. The purpose of present study is to demonstrate clinical features, surgical treatment, and potential risk factors determined long-term prognosis of intraventricular meningiomas.
A total of 89 surgically treated and histopathologically confirmed intraventricular meningiomas were identified in our institution from 2008 to 2018. Clinical features, neuroimaging findings, surgical records, and prognosis data were extracted and reviewed retrospectively. Group comparison and recurrence-free survival analysis were performed.
Female predominance was well established with a sex radio of 2.1:1. Raised intracranial pressure and decline of visual acuity were 2 chief symptoms that patients generally complained of. Preoperative magnetic resonance imaging (MRI) scans were performed in all patients and there was a trend toward lateral ventricular meningiomas were larger in size than others (P = .07). Superior parietal lobule and temporal approach were widely adopted and lateral/4th ventricular meningiomas were more easily to acquire total tumor resection as compared with 3rd ventricular meningiomas (P = .03). After an average follow-up of 57.3 months, 6 patients experienced recurrence of disease in our series. Individuals with subtotal resection (P < .001) and higher World Health Organization classification (P = .001) were more prone to relapse.
Intraventricular meningiomas presented with a wide variety of symptoms. Surgery remained 1st treatment of choice and optimal surgical approach should be planned individually based on preoperative MRI evaluation. Patients underwent subtotal tumor resection and with malignant tumor nature should be carefully monitored during follow-up.
Keywords: intraventricular, meningioma, prognosis, recurrence-free survival, surgical management
1. Introduction
Meningiomas are the 2nd most common primary tumors of central neural system in adults.[1] They originate from arachnoid cap cells and are reported to be found in any location of brain. However, meningiomas arising in the ventricular system are rather rare, representing a probability for only 0.5% to 3% of all intracranial meningiomas.[2] The available space of ventricle system is rather enormous; therefore, tumors can easily grow to a considerable size before becoming symptomatic. Besides, many complains are periodic and transient thus contributing to the delayed diagnosis in most patients. Surgical excision is considered the preferred treatment of choice; however, it remained a technically challenging problem in modern neurosurgery.
There are limited data concerning the clinical features, surgical treatment, and long-term prognosis regarding this rare and special entity. Herein, we retrospectively reviewed and analyzed the medical history, radiologic images, surgical records, and follow-up data of 89 intraventricular meningiomas which were operated in West China Hospital from 2008 to 2018 and specially focused on the factors influencing the long-term surviving.
2. Methods
2.1. Patient selection
We originally identified 97 patients who underwent evaluation and treatment for intraventricular meningioma between 2008 and 2018 in West China Hospital. After we thoroughly reviewed for the medical files and histopathologic reports, 1 patient was excluded for insufficient clinical records, 1 patient was excluded for reduplication, 6 patients were excluded for additional pathologic report confirmed nonmeningioma tumors, and a total of 89 intraventricular meningiomas remained in the present study. This retrospective research was approved by the ethics committee of West China Hospital.
2.2. Data collection
Clinical information such as age at diagnosis, gender, symptoms and signs, tumor location and size, World Health Organization (WHO) classification, extent of resection, relevant surgical complications, adjuvant therapy, etc, were extracted from medical archives, images files, and surgical records.
The tumor diameter was obtained by measuring the longest line of coronal, sagittal, and axial enhancement sections on magnetic resonance imaging (MRI). Tumor volume was verified by using the region of interest (ROI) function of image system and calculated as the sum of all tumor slabs area measured on each tumor slice multiplied by slice thickness.[3] The presence and extent of hydrocephalus was evaluated from preoperative MRI and tumor was divided into either homogeneous or heterogeneous in enhancement section. Moreover, the signal intensity of tumor on T1-weighted and T2-weighted MRI was categorized as hypointense, isointense, or hyperintense, and the shape of tumor was defined base on the solid component appearance.
In each case, extent of resection was analyzed and classified according to Simpson grading scale[4] by assessing surgeon's operation reports and postoperative radiologic images. Simpson grade I and grade II excisions were classified as gross total resection, then Simpson grade III to grade V excisions were labeled with subtotal resection. Complications occurring within 30 days after surgery were divided into 2 states according to Landriel Ibanez and his colleges’ classification[5] as follows: grade I and grade II complications were concluded as mild to moderate adverse postoperative events, while grade III and grade IV complications were regarded as severe postoperative life-threatening courses.
2.3. Surgical treatment
There are a variety of alternative approaches regarded the surgical treatment of ventricular meningiomas. Surgical approaches predominantly depended on tumor location, while patient's health condition and surgeon's experience were also taken into consideration when making the appropriate operational route. Superior parietal lobule approach and temporal approach were most commonly used for lateral ventricular meningiomas in our series (41 and 24 patients, respectively), while transcallosal approach and frontal approach were especially adopted for meningiomas located in the frontal horn or body of ventricle. Meningiomas in the 3rd ventricle required diverse surgical approaches depending on the size and location of the tumor, 4 patients were operated through transcallosal approach, 4 through infratentorial supracerebellar approach, 2 through superior parietal lobule approach, and 2 through frontal approach. All 7 patients with 4th ventricular meningiomas were resected via median suboccipital craniotomy (Table 1).
Table 1.
Summarized baseline characteristics of intraventricular meningioma.

2.4. Recurrence and progression
Recurrence was defined as documented significant growth of the tumor noted on routinely performed MRI during follow-up. Recurrence time was counted from the date after operation to the date of images found any changes of the tumor size in the enhancement array. Base on prior studies,[6–9] we brought extent of resection, histopathologic results, and tumor location into recurrence-free survival analysis. Patients with subtotal resection undergoing immediate postoperative adjuvant therapy with stereotactic radiosurgery for residual tumor were excluded from further analysis.
2.5. Statistical analysis
All data were cross-checked for accuracy before being subject to any statistical analysis. The Chi-squared test and Kruskal−Wallis test were used to compare categorical variables between subgroups. Whereas, the analysis of variance (ANOVA) and rank-sum (Mann–Whitney) test were used for group comparison for normally or non-normally distributed continued variables, respectively. Recurrence analysis was performed using the Kaplan–Meier method and survival curves were compared by nonparametric log-rank tests. Two-sided P values ≤.05 were considered to be statistically significant. All data were analyzed utilizing SPSS statistical program (Version 21.0; IBM Corp, Armonk, NY).
3. Results
3.1. Patient demographics
Between 2008 and 2018, a total of 89 patients who underwent 1st-time craniotomy for intraventricular meningiomas in West China Hospital were enrolled in present study cohort. Baseline characteristics are listed in Table 1. The mean age at diagnosis was 46.1 ± 14.5 years (range, 12–80 years). An apparently female predominance was discovered with a sex radio of 2.1:1 (60 women vs 29 men) in our research. Prior to hospitalization, patients experienced an average of 14.7 ± 30.5 months (range, 6 days to 16 years) of symptoms. Four people were lost in the follow-up and the rest 85 patients attributed to an average of 57.3 ± 32.8 months (range, 7–120 months) follow-up time. There was no significant difference between age, sex distribution, duration of main symptoms, or follow-up period between lateral, 3rd, and 4th ventricular meningiomas.
Six (7%) patients were accidentally found during routinely physical examination. Chief complains included raised intracranial pressure (e.g., headache, nausea, vomiting) in 63 patients (70%), decline of visual acuity (24 patients; 27%), dizzy (21 patients; 24%), motor deficits (16 patients; 18%), and sensory disturbances (11 patients; 12%). Cognitive and personality disturbances, hearing loss and tinnitus, gait ataxia were detected in 10 patients (11%) each. Seizure and dysphasia were found in 9 (10%) and 8 (9%) patients, respectively. One patient with 3rd ventricle meningioma developed polyuria (Table 2).
Table 2.
Symptoms and signs for ventricular meningiomas.

3.2. Image findings
Preoperative MRI scans with contrast enhancement were performed in all patients. Seventy (79%) intraventricular meningiomas occurred in lateral ventricle, 12 (13%) in the 3rd ventricle, and 7 (8%) in the 4th ventricle. Lateral ventricular meningiomas were more frequently occurred in left side than on the right (41 vs 29) and the majority of them were located in the trigone area. Tumors in frontal horn, temporal horn, or body of ventricle were seldom encountered (Table 3).
Table 3.
Location of ventricular meningiomas.

The mean maximal tumor diameter was 4.6 ± 1.5 cm (range, 1.7–8.3 cm) and the mean tumor volume calculated with an ROI algorithm was 41.6 ± 38.8 cm3 (range, 2.2–211.9 cm3). Though no statistical significance, there was a tendency toward lateral ventricular meningiomas were larger in size than others (P = .07) (Table 1). In terms of the shape of tumors, 54 were spherical (61%), 22 were irregular (25%), lobular and dumbbell shape could be found in 10 and 3 patients separately. Forty-eight patients (54%) were homogeneously enhanced on T1-weighted postcontrast images and 41 individuals were heterogeneously enhanced. A total of 73 patients developed different degrees of hydrocephalus (51 for local hydrocephalus, 17 for bilateral hydrocephalus, and 5 for whole ventricle obstructive hydrocephalus). Intraventricular meningiomas usually had a clear margin and were more likely to appear isointense or hypointense on T1-weighted images (61 and 27 patients, respectively) and isointense or hyperintense on T2-weighted images (45 and 34 patients, respectively) (Table 4).
Table 4.
Preoperative neuroimaging findings.

3.3. Surgical records and outcomes
All 89 intraventricular meningiomas underwent surgical resection through different approaches as described. Seventy-five patients gained total resection with no residual tumor detected on postoperative MRI evaluation, while 14 patients obtained subtotal resection and 8 of them were introduced to adjuvant gamma knife radiosurgery (GKS) to avoid recurrence. Intraventricular meningiomas were rich in blood supply and the mean blood loss during operation was 301.6 ± 258.9 mL (range, 40–1800 mL). After operation, intraventricular drainage tubes were inserted in 48 patients to prevent hematomas and release excessive cerebrospinal fluid (CSF). Drainage tube was removed 2 to 7 days after surgery depending on the patient's overall status and their computed tomography (CT) results. Four patients received ventriculoperitoneal shunt due to persistent postoperative hydrocephalus. Sixty patients experienced no perioperative complications and mild to moderate adverse events developed in 12 individuals. However, awareness should be raised that 17 patients presented with severe surgery-related complications which eventually led to 2 deaths. Histopathologic grading of intraventricular meningiomas was based on the current WHO classification: 72 patients were classified as grade I tumors, reflecting their benign nature, while 17 were defined as grade II tumors. We found no statistical significance between histologic results, blood loss volume, insertion of drainage tube, and perioperative surgical complications among intraventricular meningiomas with different tumor location. Lateral and 4th ventricular meningiomas were more easily to acquire total tumor resection as compared with 3rd ventricular meningiomas (P = .03) (Table 1).
3.4. Recurrence
During the long-time follow-up, 6 patients experienced recurrence of disease. Among them, 5 patients underwent subtotal resection and 4 patients were classified as WHO grade II meningioma. Four recurrence meningiomas were located in lateral ventricle and 2 were found in the 3rd ventricle. To study the possible factors that might influence meningioma recurrence, we took extent of resection, WHO classification and tumor locations into the recurrence-free survival analysis. Eight patients who received immediate postoperative adjuvant therapy with GKS for residual tumor were excluded from further analysis. Kaplan−Meier method revealed that there was a significant difference between patients with total or subtotal resection (P < .001) and patients with different histopathologically confirmed WHO grades (P = .001). No statistic significant difference (P = .09) was found in tumor location (lateral ventricle, 3rd ventricle or 4th ventricle) in Kaplan−Meier recurrence-free survival analysis (Fig. 1).
Figure 1.
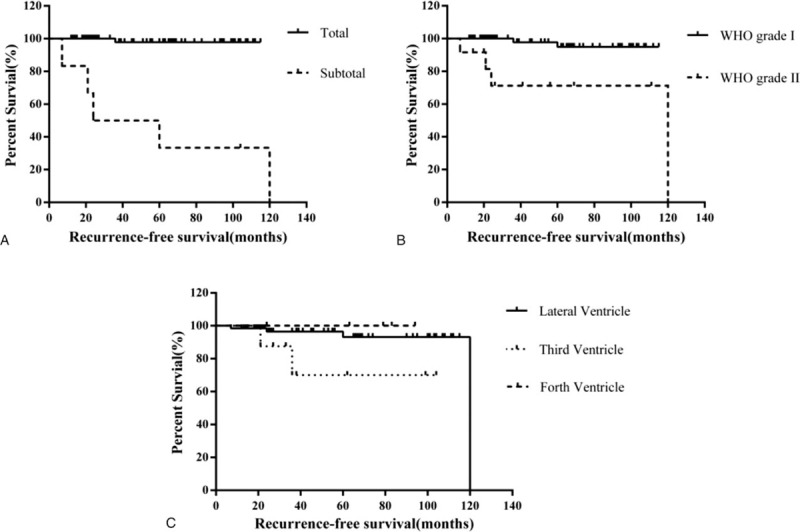
Kaplan–Meier recurrence-free survival analysis between subgroups, stratified by extent of resection, World Health Organization (WHO) classification, and tumor locations. (A) There was a significant difference of recurrence-free survival curves between total resection group and subtotal resection group (P < .001). (B) Recurrence-free survival curves were significantly different between WHO grade I group and WHO grade II group (P = .001). (C) Kaplan–Meier curves depicted no statistic significant difference between meningiomas in lateral ventricle, 3rd ventricle, and 4th ventricle (P = .09).
4. Discussion
Though meningiomas are the most common benign intracranial tumor, intraventricular meningiomas merely represent a rather small proportion of all presented cases. Since the 1st description of intraventricular meningioma by Shaw,[10] sporadic cases and case series had been discovered but with relatively few patients. Clinical features and surgical treatments had been discussed, and constitutionally benign nature contributed to the delightful long-term outcomes. However, neither recurrence-free survival data nor interrelated risk factors were well established in previous literature. We retrospectively reviewed medical histories of 89 surgically resected and histopathologically confirmed intraventricular meningiomas and brought long-time prognosis into analysis regarding this rare entity. To the best of our knowledge, this is the largest single institution cohort of entire ventricular system meningiomas.
Meningiomas can arise at any site of brain, but were seldom encountered in ventricular system. Three quarters of the intraventricular meningiomas arise from lateral ventricle[11] owing to more bulky choroid plexus and chorioid tela than other ventricles. In lateral ventricle, meningiomas appear in left side more frequently, and the majority of them originate from the trigone area. Female dominance was observed in present study which was similar to previous researches.[8,12,13] Most of intraventricular meningiomas were diagnosed after 3rd decade of life span and less common in pediatric patients which was contradictory to Germano and his colleagues’[14] opinions that the frequency was higher in child. Ventricular meningioma appeared large in size due to the extensively available space, which posed challenges to neurosurgeon regarding to gross total tumor resection. The tumors usually remained asymptomatic unless they perverted normal CSF circulation causing obstructive hydrocephalus, or gradually grew to a considerable size resulting in critical adjacent structure compression. Intraventricular meningiomas presented with a wide variety of symptoms, raised intracranial pressure and decline of visual acuity were 2 chief symptoms that patients generally complained of and the latter was caused either by oppressed optic radiation or papilledema. Focal neurologic deficits or disturbances could be detected and generally related to direct brain or cranial-nerve compression.
Accurate diagnosis was greatly facilitated by wider availability and utilization of preoperative neuroimaging examination like CT and, especially, MRI. On CT scans, intraventricular meningiomas usually contained calcification foci, and were slightly more hyperdense compared with normal brain tissue. MRI was the preferred choice, and intraventricular meningiomas were frequently isointense or slightly hypointense in T1-weighted images and isointense or hyperintense in T2-weighted images compared to cerebral gray matter. Contrast gadolinium enhancement sequences were especially useful in delineating the shape of tumor and half of tumors displayed heterogeneous appearance that probably due to intratumoral infarction. Dilatation of local ventricle and extent of obstructive hydrocephalus were sharply distinguished on MRI, which enabled us to assess the necessity of preoperative ventricular drainage.
Speaking to no regularly performed radiologic examinations, Ma et al used intraoperative ultrasound to identify trans-sulcus corridor, tumor feeding vasculature, and possible residual tumor.[15] Sun et al utilized functional neuronavigation to facilitate surgical planning by allowing a determination of optimal trajectory to the lesion.[16] Cerebral angiography is probably not necessary because intraventricular meningiomas were usually not amenable to preoperative embolization,[17] but it did provide us with information of predominant tumor blood supply and the position of prominent draining veins, which was useful to minimize intraoperative brain trauma and surgical-related complications.
Intraventricular meningiomas are substantially benign, and have a nonadherent margin much as favorable consistency, making complete microsurgical resection feasible. A great deal of approaches was described regarding to surgical resection of these tumors, but the optimal one was still controversial. We should take into consideration of various aspects when making the decision, such as distance of axis to lesion, early access to feeding artery, minimization of transcortical injury, adjacent critical anatomic structures, and patients’ preoperative symptoms as well as basic physical conditions.
Generous temporal-parietal-occipital bone flap could afford multiple transcortical approach options and define eloquent anatomic regions.[17] The superior parietal lobule approach was widely adopted in our series, especially for tumors in lateral trigone area. This approach give us direct exposure to tumor entity by opening superior parietal lobule or intraparietal sulcus, and it could be performed both in dominant or nondominant hemisphere. Although with late access to the vascular pedicle and long corridor to the lesion, it provided the best option for tumor evacuation without interrupting optic radiation, which maximally reduced the risk of contralateral visual deficits. It is advisable to perform intraoperative ultrasound as well as neuronavigation in locating small size tumor and ultrasonic knife aspirator is profitably useful in piecemeal decompression of tumor.
For tumors mainly located at temporal horn or inferior aspect of trigone, middle or inferior temporal gyrus approach provide the shortest trajectory to resection and earliest access to choroidal vessels, enabling an advanced control of tumor blood supply. Visual fibers run above and parallel to temporal horn which make optic radiation interruption inevitable though middle temporal pathway. However, a horizontal sulcus incision and gently cortical traction could minimize the possibility of postoperative visual deficits. Nevertheless, inferior temporal approach would be less likely to cause any visual disturbance for opening under the optic radiation, thus this route is preferred for dominant hemisphere in case of sensory aphasia.
Transcallosal approach could be adopted in a wide range of situations. Incision through the anterior corpus callosum could access tumor in frontal horn, body of lateral ventricle and the 3rd ventricle, while approaching through the posterior corpus callosum could resect tumor in trigone area. Interhemispheric fissure would be directly dissected and sagittal sinus injury or thrombosis is the potential surgical-related complications when using this approach. Moreover, disconnection syndrome could emerge due to opening callosum posteriorly.
Median suboccipital craniotomy serve as standard surgical approach for meningioma located in the 4th ventricle, and all 7 fourth ventricular meningiomas were surgically resected through this particular route. Cerebellomedullary fissure would be dissected to gain access to the ventricle and this fissure is generally developed by dissecting the lateral aspect of the tonsil and incising the tela choroidea extensively.[18] Most importantly, carefully preoperative planning of the optimal approach is highly advised, while rough maneuver, overvigorous retraction, bridging veins damage, and venous sinus injury could lead to catastrophic postoperative complications.
Though intraventricular meningiomas are generally benign, tumor recurrence could emerge in certain subsets of patients. Reported recurrence rate varied from 0% to 28%,[7–9,15,19,20] while none of them discussed the potential explanations and related risk factors. Six patients experienced tumor relapse during long period of follow-up that accounted for 9.3% recurrence rate when we excluded individuals receiving immediate adjuvant GKS therapy. Awareness should be reminded that the recurrence rate was inevitably overestimated, since all patients receiving additional GKS retained stable/shrunken tumor size and individuals who were lost in follow-up might have overall good surgical outcomes. MRI examination at 3 months after initial surgery was crucial to evaluate the necessity of adjuvant GKS therapy, and routine MRI reevaluation was recommended to perform annually or whenever necessary. Accordingly, patients undergoing total tumor resection or histopathologically confirmed WHO grade I tumor could benefit for a long period of time and acquire lower recurrence rate during follow-up. High-grade intraventricular meningiomas usually exhibit high proliferative activity and invasive growth pattern. Gross total resection is still the standard treatment for these tumors. However, subtotal tumor resection could happen owing to the unclear border with surrounding ependymal membrane and brain tissue. Adjuvant GKS therapy might have an overall good effect for tumor residues. The recurrence of WHO grade II tumors occurred within 2 years after surgery (Fig. 1B), MRI reevaluation was recommended to perform every 6 months for the first 2 years, and then yearly after. Reoperation is feasible for patients with recurrence. We demonstrated that more aggressively surgical attempts to accomplish total resection made intraventricular meningiomas a curable disease with a long time of recurrence-free survival. However, certain life-threatening circumstances should be taken into consideration and surgical decisions should be made precisely and individually toward total tumor resection. All together, we believed that the extent of resection and histopathology classifications possessed important utility in predicting the risk of recurrence in current neurosurgical practice. Patients who achieved subtotal tumor resection and with malignant tumor nature should be carefully monitored during follow-up.
Bias and loss of follow-up inevitably exist due to the retrospective nature of this study. Cognitive and neurologic function assessment was not performed during outcome evaluation. Still, clinical data in regard of tumor recurrence are far from enough, future multicenter cohorts with long time follow-up are warranted to verify current suggestions.
5. Conclusion
Meningiomas are rather rare in ventricular system and could present with a wide variety of symptoms. Surgery remained 1st treatment of choice for intraventricular meningiomas and preoperative MRI evaluation provided us with detailed information about tumors. Optimal surgical approach should be planned individually and surgery generally brought delightful long-term outcomes. Patients undergoing total tumor resection and with lower WHO grade classification were less likely to recur, while individuals who achieved subtotal tumor resection and with malignant tumor nature should be carefully monitored during follow-up.
Acknowledgment
The authors sincerely thank Prof Guanjian Liu (Department of Evidence-Based Center, West China School of Medicine, Sichuan University) for reviewing our statistical analyses.
Author contributions
Conceptualization: Cheng Chen, Liang Lv, Yu Hu, Senlin Yin, Peizhi Zhou.
Data curation: Cheng Chen, Liang Lv.
Formal analysis: Cheng Chen, Liang Lv.
Investigation: Cheng Chen, Liang Lv, Yu Hu, Senlin Yin.
Methodology: Cheng Chen, Liang Lv.
Project administration: Cheng Chen.
Resources: Cheng Chen, Senlin Yin.
Software: Cheng Chen, Yu Hu, Senlin Yin, Peizhi Zhou.
Supervision: Yu Hu, Senlin Yin, Peizhi Zhou, Shu Jiang.
Validation: Cheng Chen, Peizhi Zhou, Shu Jiang.
Visualization: Shu Jiang.
Writing – original draft: Cheng Chen, Shu Jiang.
Writing – review & editing: Cheng Chen, Peizhi Zhou, Shu Jiang.
Footnotes
Abbreviations: CSF = cerebrospinal fluid, CT = computed tomography, GKS = gamma knife radiosurgery, MRI = magnetic resonance imaging, ROI = region of interest, WHO = World Health Organization.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Kleihues P, Cavenee WK. International Agency for Research on Cancer. Pathology and Genetics of Tumours of the Nervous System. Lyon: IARC Press; 2000. [Google Scholar]
- [2].Nakamura M, Roser F, Bundschuh O, et al. Intraventricular meningiomas: a review of 16 cases with reference to the literature. Surg Neurol 2003;59:491–503. [DOI] [PubMed] [Google Scholar]
- [3].Hsu CY, Guo WY, Chien CP, et al. MIB-1 labeling index correlated with magnetic resonance imaging detected tumor volume doubling time in pituitary adenoma. Eur J Endocrinol 2010;162:1027–33. [DOI] [PubMed] [Google Scholar]
- [4].Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 1957;20:22–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Landriel Ibanez FA, Hem S, Ajler P, et al. A new classification of complications in neurosurgery. World Neurosurg 2011;75:709–15. [DOI] [PubMed] [Google Scholar]
- [6].Odegaard KM, Helseth E, Meling TR. Intraventricular meningiomas: a consecutive series of 22 patients and literature review. Neurosurg Rev 2013;36:57–64. [DOI] [PubMed] [Google Scholar]
- [7].Grujicic D, Cavallo LM, Somma T, et al. Intraventricular meningiomas: a series of 42 patients at a single institution and literature review. World Neurosurg 2017;97:178–88. [DOI] [PubMed] [Google Scholar]
- [8].Liu M, Wei Y, Liu Y, et al. Intraventricular meninigiomas: a report of 25 cases. Neurosurg Rev 2006;29:36–40. [DOI] [PubMed] [Google Scholar]
- [9].Nanda A, Bir SC, Maiti T, et al. Intraventricular meningioma: technical nuances in surgical management. World Neurosurg 2016;88:526–37. [DOI] [PubMed] [Google Scholar]
- [10].Shaw A. Fibrous tumour in the lateral ventricle of the brain, boney deposits in the arachnoid membrane of the right hemisphere. Trans Path Soc Lond 1854;5:18–21. [Google Scholar]
- [11].McDermott MW. Intraventricular meningiomas. Neurosurg Clinics N Am 2003;14:559–69. [DOI] [PubMed] [Google Scholar]
- [12].Nayar VV, DeMonte F, Yoshor D, et al. Surgical approaches to meningiomas of the lateral ventricles. Clin Neurol Neurosurg 2010;112:400–5. [DOI] [PubMed] [Google Scholar]
- [13].Wang Y, Lin Z, Li Z, et al. The incidence and risk factors of postoperative entrapped temporal horn in trigone meningiomas. World Neurosurg 2016;90:511–7. [DOI] [PubMed] [Google Scholar]
- [14].Germano IM, Edwards MS, Davis RL, et al. Intracranial meningiomas of the first two decades of life. J Neurosurg 1994;80:447–53. [DOI] [PubMed] [Google Scholar]
- [15].Ma J, Cheng L, Wang G, et al. Surgical management of meningioma of the trigone area of the lateral ventricle. World Neurosurg 2014;82:757–69. [DOI] [PubMed] [Google Scholar]
- [16].Sun GC, Chen XL, Yu XG, et al. Functional neuronavigation-guided transparieto-occipital cortical resection of meningiomas in trigone of lateral ventricle. World Neurosurg 2015;84:756–65. [DOI] [PubMed] [Google Scholar]
- [17].Fusco DJ, Spetzler RF. Surgical considerations for intraventricular meningiomas. World Neurosurg 2015;83:460–1. [DOI] [PubMed] [Google Scholar]
- [18].Matsushima T, Inoue T, Inamura T, et al. Transcerebellomedullary fissure approach with special reference to methods of dissecting the fissure. J Neurosurg 2001;94:257–64. [DOI] [PubMed] [Google Scholar]
- [19].Li Z, Li H, Jiao Y, et al. Clinical features and long-term outcomes of pediatric intraventricular meningiomas: data from a single neurosurgical center. Neurosurg Rev 2018;41:525–30. [DOI] [PubMed] [Google Scholar]
- [20].Menon G, Nair S, Sudhir J, et al. Meningiomas of the lateral ventricle - a report of 15 cases. Br J Neurosurg 2009;23:297–303. [DOI] [PubMed] [Google Scholar]


