Abstract
Background:
Renal replacement therapy (RRT) is the main treatment for patients with sepsis-induced acute kidney injury (SAKI). However, the choice of RRT strategy remains controversial.
Objective:
This study assessed the effectiveness of RRT variants in SAKI patients by a network meta-analysis.
Methods:
This study searched the literature in the PubMed, EmBase, and Cochrane Library databases up to August 18, 2018. The outcomes of the analysis were the survival rate, renal recovery rate, intensive care unit (ICU) duration, and hospital duration.
Results:
Twenty-two articles were included in the analysis. The results showed that only the negative control was inferior to the regimens of RRT with polymyxin B-immobilized fiber (PMXF), PMXF alone, continuous venovenous hemofiltration (CVVH), CVVH plus alkaline phosphatase (AP), continuous venovenous hemodialysis (CVVHD), high-volume CVVH, and extra high-volume CVVH in terms of the survival rate. According to the surface under the cumulative ranking , RRT with PMXF (84.4%) and PMXF (84.3%) were the treatments most likely to improve the survival rate among SAKI patients, followed by CVVH plus AP (69%). Continuous venovenous hemodiafiltration (CVVHDF), extra high-volume CVVHDF, intermittent venovenous hemodiafiltration (IVVHDF), and low-volume CVVHDF resulted in very similar survival rates. CVVH plus AP conferred relative advantages in the renal recovery rate and ICU duration.
Conclusion:
CVVH, CVVHD, and their derived RRT strategies can improve survival rates in SAKI patients, but there is no significant difference among the RRT strategies. There was also no significant difference in the survival rate among CVVHDF, IVVHDF, and their derived strategies. More high-quality randomized controlled trials with large sample sizes are needed for further research.
Keywords: acute kidney injury, acute renal injury, meta-analysis, renal replacement therapy, sepsis
1. Introduction
Acute kidney injury (AKI) is a very common, life-threatening disease occurring in critically ill patients with high mortality, and up to 57% of intensive care unit (ICU) patients have AKI.[1] The main cause of AKI is sepsis, and approximately 50% of patients with septic shock experience AKI. Although the pathophysiological mechanism of sepsis-induced AKI (SAKI) remains unclear, kidney hypoperfusion is generally considered the main reason. Inflammatory factors, oxidative stress, and coagulation cascade activation are also involved in the development of SAKI. SAKI differs from ischemic AKI and is regarded as an independent disease involving inflammatory mediators, and cell infiltration leads to a decrease in the nonrenal blood supply associated with microcirculatory disturbances, rendering treatment more complex.[2]
Renal replacement therapy (RRT) is the main treatment for SAKI patients. RRT treatment can maintain the stability of hemodynamics, including the mean arterial pressure, cerebral perfusion pressure, and renal perfusion pressure. In addition, RRT can correct internal environmental disorders and maintain and support multiple organ functions to improve mortality and prevent complications. Despite recent improvements in RRT technology, the mortality of AKI patients remains high.
Previous meta-analyses have focused on whether SAKI patients needed early RRT intervention and whether high-volume hemofiltration was more beneficial than standard volume hemofiltration. Early RRT intervention had no significant benefit among AKI patients,[3,4] and high-volume hemofiltration did not exhibit a significant advantage over standard volume hemofiltration.[5] A meta-analysis comparing intensive dose and low intensive dose CRRT also showed that a high dose of CRRT could not reduce the mortality of AKI patients but increased complications.[6] The anticoagulation of the CRRT circuit is also an important technical aspect in critically ill patients. Analyses comparing the regional citrate and heparin anticoagulation in RRT strategy have shown that citrate and heparin have a similar anticoagulation effect and maintain circuit patency, but regional citrate anticoagulation (RCA) could significant reduce the risk of bleeding. Therefore, RCA is recommended in high bleeding risk CRRT patients. However, the safety of RCA in patients with liver failure remains unclear. Therefore, RCA application is recommended in the case of perfect metabolic monitoring.[7,8]
Variant RRT strategies are available, and the various RRT strategies have different abilities to remove solutes in SAKI patients. However, the optimal RRT strategy that has the best therapeutic effect on SAKI patients is unclear, and the choice of RRT strategy remains controversial in the clinic. The disadvantage of a traditional meta-analysis is that this analysis cannot indirectly or comprehensively compare the therapeutic effects of RRT strategies with different solute clearance abilities in SAKI patients. In this study, all potential RRT strategies are included according to existing RCT studies, and the RRT strategies are distinguished based on dosing and modality. A network meta-analysis is used to analyze the differing effects of the different types of CRRT in patients with SAKI.
2. Methods
This meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews guidelines (Supporting Information). Ethical approval was not necessary because this study was a meta-analysis. Therefore, our data were based on published studies only.
2.1. Data search strategy
We systematically searched the PubMed, EmBase, and Cochrane Library databases for articles published by August 18, 2018 without any language restrictions using keywords, including “acute renal failure,” “acute kidney injury,” “acute kidney failure,” “sepsis,” “septic,” “septicemia,” “blood poisoning,” “systemic inflammatory response syndrome,” and “random∗.” The bibliographies of the obtained publications and the references of relevant reviews were also checked to ensure that no relevant studies were inadvertently omitted.
2.2. Data selection and extraction
The literature search and selection were independently performed by 2 authors, and all disagreements were resolved by discussion. The inclusion criteria were as follows:
-
(1)
studies involving adult SAKI patients or mostly (≥80%) adult SAKI patients;
-
(2)
studies with a randomized controlled trial (RCT) design; and
-
(3)
studies describing the outcomes of the survival rate, renal recovery rate, ICU duration, and hospital duration.
The exclusion criteria were as follows:
-
(1)
studies involving only sepsis or AKI patients, studies with less than 80% of enrolled patients having SAKI, and no separate outcomes reported for SAKI patients;
-
(2)
studies without an RCT design; and
-
(3)
studies that did not report the above outcomes.
Additionally, reviews, conference presentations, basic research articles, and editorials were excluded. Studies that failed to present original data were also eliminated.
We extracted information regarding the author, publication year, sample size, age, intervention type, control treatment, dialysate flow rate or ultrafiltration rate, and follow-up period. To facilitate comparisons among the studies, we predefined a dialysate flow rate or an ultrafiltration rate <30 mL/kg/h as low-volume RRT, a rate ≥30 mL/kg/h, and <50 mL/kg/h as normal volume RRT, a rate ≥50 mL/kg/h and ≤70 mL/kg/h as high-volume RRT, and a rate >70 mL/kg/h as extra high-volume RRT. If the unit was not in mL/kg/h, the value was calculated according to the average 60 kg weight of the patients. We assessed the methodological quality of the included trials using a risk of bias approach according to the methods described by the Cochrane Collaboration.
In our analysis, the major outcome was the survival rate of septic AKI patients, which was defined as the 28-day survival rate. The survival rate was obtained from a Kaplan–Meier curve. If the 28-day survival rate was not reported, the ICU survival rate, hospital survival rate, or longest follow-up survival rate were used. The secondary outcomes were the renal recovery rate or RRT-free rate, ICU duration, and hospital duration.
2.3. Statistical analysis
We performed a random-effects network meta-analysis using the frequentist framework for mixed multiple treatment comparisons.[9] Network plots were produced for each outcome in which the nodes are weighted according to the number of studies evaluating each treatment, and the edges are determined according to the precision of the direct estimate for each pairwise comparison. The consistency within every closed triangle or quadratic loop was investigated using a loop-specific approach to evaluate the coherency of the direct and indirect comparisons.[6] To address global inconsistency from all possible sources, we used a design-by-treatment interaction model when adjusting the results for the entire publication bias.[9] To rank the treatments per each outcome, we used surface under the cumulative ranking (SUCRA) probabilities.[9] Comparison-adjusted funnel plots were used to determine whether small-study effects were present in our analysis.[9] We also performed a pairwise meta-analysis using a random-effects model as needed. For the dichotomous outcomes, the odds ratios (ORs) with 95% confidence intervals (CIs) were calculated to determine the sizes of the effects. For the continuous data, the standard mean differences (SMDs) with 95% CIs were calculated. For median (interquartile) results, the values of the mean and standard deviation were estimated for the analysis. All tests were 2-tailed, and a P-value less than.05 was considered statistically significant. The data analyses were performed using STATA software (version 14.0; STATA Corporation, College Station, TX).
3. Results
3.1. Literature search
In our search, 1563 articles were identified after removing duplications. In total, 1450 articles were excluded after screening the titles and abstracts. The full text of the remaining 113 articles was assessed, and the following types of studies were excluded: studies with no SAKI patients or studies in which the SAKI patients accounted for less than 80% of all patients (48); reviews (12); crossover studies involving a similar RRT strategy but only changed the order of the sequence (9); studies with undesired results (8); studies without an RCT design (3); protocols (3); conference abstracts (3); secondary research studies (2); studies with pediatric SAKI patients (1); duplicate publications (1); and basic research studies (1). Finally, 22 articles involving 3360 patients were included in our meta-analysis [10–30] (Table 1).
Table 1.
Characters of included studies.
3.2. Study characteristics
The included studies were published between 2000 and 2017. Most studies included less than 100 SAKI patients, and the average/median age of the patients was mostly between 50 and 70 years. One study did not report the patients’ age.[17] The main RRT treatment strategies included intermittent hemodialysis (IHD), continuous venovenous hemofiltration (CVVH), continuous venovenous hemodiafiltration (CVVHDF), continuous venovenous hemodialysis (CVVHD), and their related derivative strategies (Table 1). When the RRT strategy was not specified or a physician-decision RRT was used, the RRT group was included in the analysis. Because of the particularity of RRT treatment, it is difficult to implement blinding methods in research. The blinding method was only used in studies investigating adjuvant drugs, such as alkaline phosphatase (AP). All included studies had an RCT design; thus, the quality of the research is ideal (Fig. 1).
Figure 1.

Risk-of-bias graph of each included study. The assessments of each risk-of-bias item are presented for all included studies.
3.3. Results of the network and traditional meta-analyses
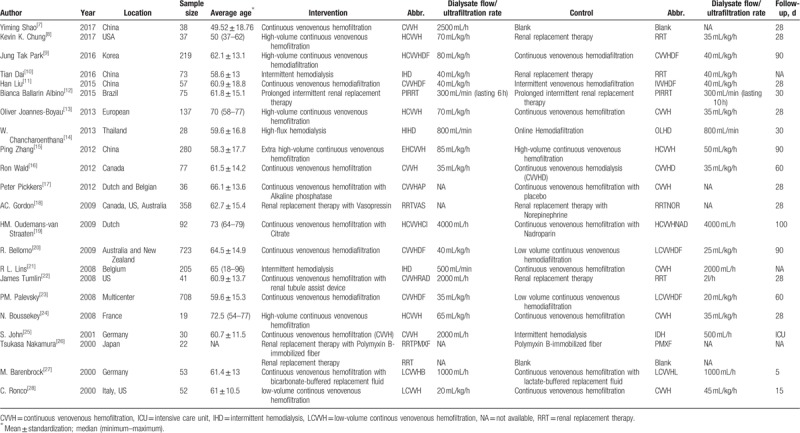
In the network meta-analysis of the survival rate, because the treatment strategies cannot form a mutual relationship, they were divided into 2 parts. The strategies in the first part of the analysis included CVVH, CVVH plus AP, CVVHD, CVVH with a renal tubule assist device (RAD), extra high-volume CVVH (EHCVVH), high-volume CVVH (HCVVH), IHD, low-volume CVVH (LCVVH), polymyxin B-immobilized fiber (PMXF), RRT, and RRT plus PMXF. CVVH was the most frequently utilized therapy and had the highest precision compared to that of EHCVVH (Fig. 2A). The global inconsistency analysis showed that there was significant inconsistency among the studies (P = .5663). The inconsistency plot including 1 quadratic loop shows an exp(IF) larger than 0 (Fig. 3). According to the network pairwise comparison results, only the negative control was inferior to RRT with PMXF, PMXF, CVVH, CVVH plus AP, CVVHD, HCVVH, and EHCVVH in terms of the survival rate (Table 2). There were no significant differences in the other comparisons. Regarding the SUCRA rank, the RRT with PMXF (84.4%) and PMXF (84.3%) treatments were the most likely to improve the patients’ survival rate, but the results are excessively dependent on a single study with a very small sample size; thus, this result is unreliable. Then, the next best treatments in terms of survival rate were CVVH plus AP (69%), CVVHD (63.9%), and CVVH plus RAD (61.8%). The comparison-adjusted funnel plot used to assess publication bias and determine the presence of small-study effects did not suggest that there was any publication bias (Fig. 4A).
Figure 2.
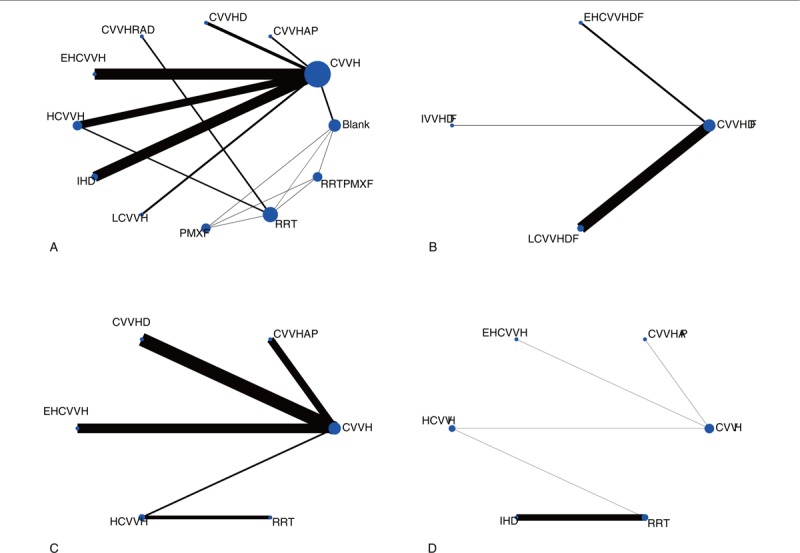
Network of the comparisons of the RRT strategies included in the analyses. (A) First part of the survival rate; (B) Second part of the survival rate; (C) Renal recovery rate; (D) ICU duration. In the network plot, the line between 2 interventions indicates a direct comparison, and the thickness indicates the precision of the direct estimate in each pairwise comparison. The nodes are weighted according to the number of studies. The abbreviation definitions are listed in Table 1. ICU = intensive care unit, RRT = renal replacement therapy.
Figure 3.
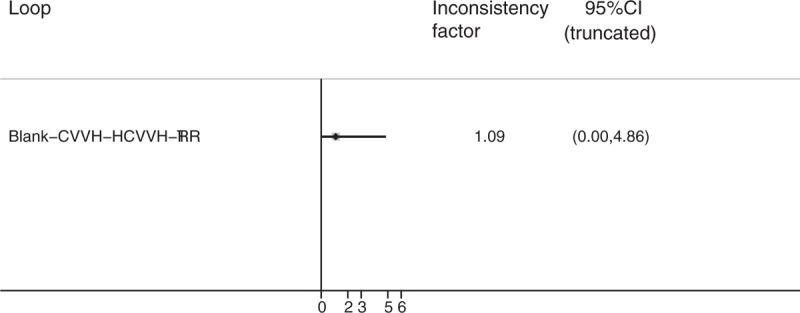
Inconsistency plot of loop-specific heterogeneity for the survival rate result.
Table 2.
The league table for first part of survival rate result estimates RRT strategies according to their relative effects.
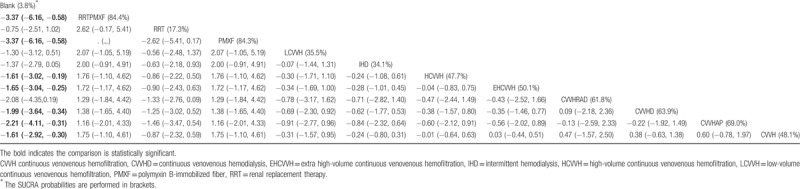
Figure 4.
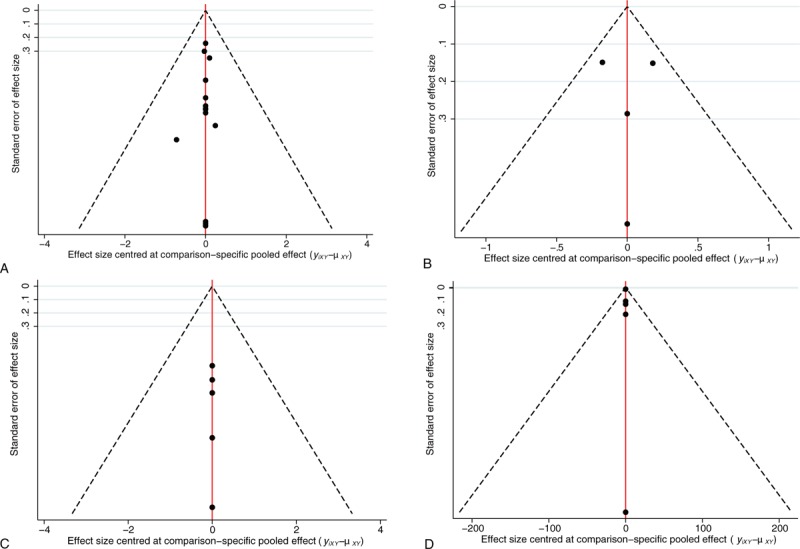
Comparison-adjusted funnel plot used to assess the first (A) and second parts of the survival rate results (B), renal recovery rate (C) and ICU duration results (D). ICU = intensive care unit.
In the second part of the survival rate analysis, the strategies included CVVHDF, extra high-volume CVVHDF (EHCVVHDF), intermittent venovenous hemodiafiltration (IVVHDF), and low-volume CVVHDF (LCVVHDF). Among these treatments, CVVHDF is the most frequently studied and shows the highest precision with LCVVHDF (Fig. 2B). There is no circular comparison; thus, a consistency model was used for the analysis. There were no significant differences in the network comparisons (Table 3). According to the SUCRA ranking, the results of the 4 interventions were very close, and only CVVHDF (58.4%) showed a comparative advantage. There was no publication bias in the comparison-adjusted funnel plot (Fig. 4B). The RRT strategies that were not involved in the network analysis were assessed by a traditional analysis. Only CVVH with citrate was significantly superior to CVVH with nadroparin in terms of the survival rate (OR: 0.33; 95% CI: 0.14–0.79; P = .013). The other treatment comparisons showed no significant differences (Fig. 5).
Table 3.
The league table for second part of survival rate result estimates RRT strategies according to their relative effects.

Figure 5.
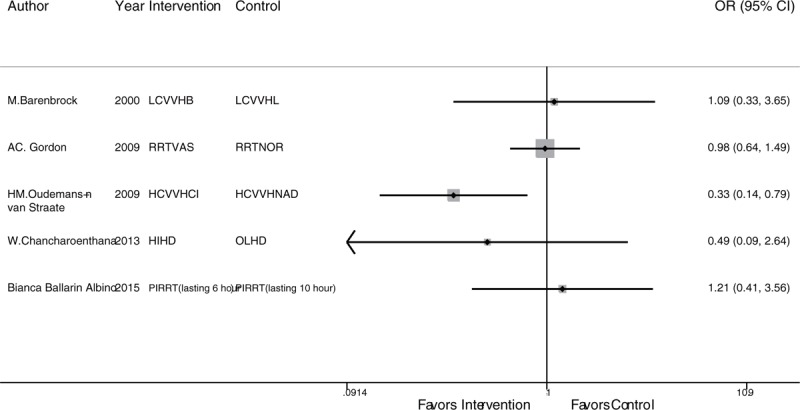
Traditional analysis of the survival rate of the RRT strategies not included in the network analysis. RRT = renal replacement therapy.
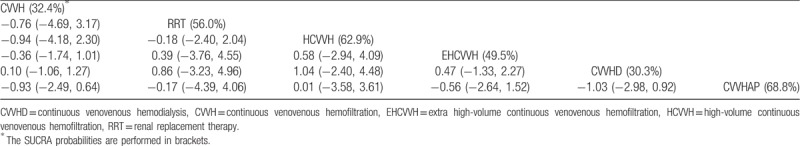
In the network analysis of the renal recovery rate, the RRT strategies included CVVH, CVVH plus AP, CVVHD, EHCVVH, HCVVH, and RRT. CVVH is the most frequently studied and shows the highest precision with CVVHD (Fig. 2C). There were no significant differences in the network comparisons (Table 4). According to the SUCRA results, CVVH plus AP (68.8%), HCVVH (62.9%), and RRT (56.0%) were comparatively advantageous. There was no publication bias in the comparison-adjusted funnel plot (Fig. 4C). There are also no other significant differences in the results of the traditional meta-analysis.
Table 4.
The league table for renal recovery rate result estimates RRT strategies according to their relative effects.
In the ICU duration analysis, the treatment strategies included CVVH, CVVH plus AP, EHCVVH, HCVVH, IHD, and RRT. CVVH was the most frequently studied; however, IHD and RRT had the highest precision (Fig. 2D). According to the network comparison results, only RRT was superior to IHD (Table 5). According to the SUCRA ranking, CVVH plus AP (74.3%), RRT (59.2%), and EHCVVH (51.8%) showed relative advantages. There was no publication bias in the comparison-adjusted funnel plot (Fig. 4D). According to the traditional meta-analysis, RRT with Vas resulted in a significantly shorter ICU time among end-stage SAKI patients (SMD: 1.22; 95% CI: 0.61–1.83; P < .001) (Fig. 6). In addition, a network meta-analysis could not be performed to analyze the hospital duration, and the results of the traditional meta-analysis showed no significant comparative differences.
Table 5.
The league table for ICU duration result estimates RRT strategies according to their relative effects.

Figure 6.
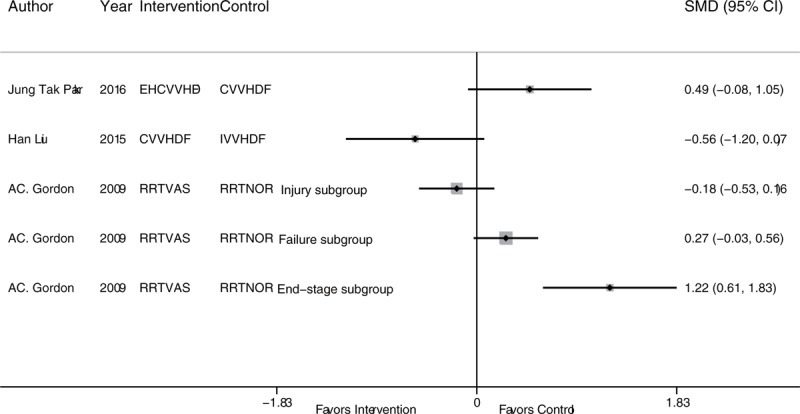
Traditional analysis of the ICU duration of the RRT strategies not included in the network analysis. ICU = intensive care unit, RRT = renal replacement therapy.
4. Discussion
Among SAKI patients, the application of RRT strategies is still controversial. This study analyzed RRT strategies for SAKI treatment by a network meta-analysis. The survival rate results showed that RRT with PMXF, PMXF, CVVH, CVVH plus AP, CVVHD, HCVVH, and EHCVVH were superior to the negative control, but there was no significant difference among the RRT strategies. CVVH with citrate was significantly superior to CVVH with nadroparin in the traditional meta-analysis of adjuvant drug use. Regarding the secondary outcomes, there was no significant difference in the renal recovery rate among the RRT strategies. According to the ICU duration results, the network comparison showed that only physician-decision RRT was superior to IHD, and the traditional comparison showed that RRT with Vas resulted in a significantly shortened ICU time among end-stage SAKI patients based on a single study. The above results show that there is still no absolutely advantageous RRT strategy for SAKI patients. CVVH plus AP, CVVHD, and CVVH plus RAD showed nonstatistical advantages, and more studies are needed to confirm the efficacy of PMXF in SAKI treatment. The effects of the CVVHDF, EHCVVHDF, IVVHDF, and LCVVHDF interventions all exhibited very close improvements in the patient survival rate. In addition, RCT studies comparing CVVH and CVVHDF remain lacking.
RRT treatment can simulate renal function, eliminate toxic substances and metabolites, correct excessive capacity load and acid–base balance, and maintain internal environment stability. In addition, RRT can remove inflammatory mediators of large molecules in the body and prevent damage to the kidneys. Although there was no significant difference among the RRT methods in our study, the survival rate of the patients receiving RRT treatment was significantly improved compared to that of the patients without RRT treatment.
The main RRT strategies included in this study were CVVH, CVVHD, CVVHDF, and their derivative strategies. Hemofiltration removes solutes mainly by convection, while hemodialysis removes solutes mainly by diffusion. CVVH is the standard RRT treatment in the ICU. Venipuncture avoids complications associated with arterial puncture, but the clearance rate of small molecules by CVVH is slow. CVVHDF can increase the diffusion effect of CVVH, thus improving the scavenging efficiency of small molecules. CVVHD scavenges solutes mainly by diffusion and has an advantage over CVVH in scavenging small molecules. Compared with intermittent treatment, continuous treatment has a less disrupted effect on hemodynamics, thereby reducing the cardiac load and complications. In addition, adjusting the speed of the dialysate and filtrate also changes the efficiency of RRT, such as HCVVH and EHCVVH. However, there was still no significant difference in the patient survival rate among these strategies. The following reasons may explain the negative results:
-
(1)
the sample size is still insufficient, and a larger sample size is needed to confirm the differences among the strategies, and
-
(2)
the difference among RRT strategies is not the main factor influencing death among SAKI patients but rather the AKI stage or infection degree could be the main factor.[1]
The purpose of RRT treatment is to replace renal function to remove solutes, such as immunoregulation, metabolic, endocrine functions, and so on, that are lacking. RAD is composed of a conventional hemofilter lined by monolayers of renal cells. RRT with RAD treatment is closer to the natural function of the kidney. In an included RCT, RRT with RAD significantly improved the AKI patients’ 28-day survival rate, but there was no significant difference in the results in the SAKI subgroup population.[25] AP is an endogenous enzyme that can remove endotoxins and proinflammatory substances by dephosphorylation. The local AP concentrations affect the kidney's resistance to endotoxins and are associated with the progression of AKI. In SAKI patients, AP can reduce the innate immune response. However, clinical results showed significant improvement only in endogenous creatinine clearance, but no significant improvement in survival or RRT-dependence was observed.[31] PMXF is also used to remove endotoxins from the body to improve the survival of SAKI patients. The results confirmed that PMXF can improve the survival rate, and the treatment effect does not depend on RRT application.[17] A systematic review of PMXF treatment for sepsis showed that PMXF had beneficial effects on the mean arterial pressure, dopamine use, PaO2/FiO2 ratio, and mortality rates.[32] Although these 2 drugs have therapeutic effects in SAKI patients, the sample size of related research is still far from sufficient, and more studies are needed to confirm the efficiency.
In conclusion, CVVH, CVVHD, and their derived RRT strategies can improve the survival rates of SAKI patients, but there was no significant difference among the RRT strategies. There was also no significant difference in the survival rate among CVVHDF, IVVHDF, and their derived strategies. More high-quality RCTs with larger sample sizes are still needed in further research.
4.1. Limitations
There were several limitations in this analysis. First, this meta-analysis is based on study-level data and not individual-level data. Second, regarding the primary outcome, the results include the 28-day survival rate and ICU/hospital survival rate, which contributes to the study heterogeneity. Third, SAKI has a high risk of death, and the differences in disease stage and severity in patients might cause instability in the results. Therefore, it is important to explicitly analyze patients’ risk, injury, failure, loss, and end-stage renal failure and/or the Kidney Disease Improving Global Outcomes scores and analyze them within subgroups in further research. Fourth, this study did not analyze the results of the clearance rate of inflammatory factors. Fifth, this study did not analyze the effect of the different order of RRT strategy sequences or intervention time points on the treatment outcome. Sixth, a small sample size effect may have affected the results as related meta-analyses of critically ill patients involving small sample size studies tend to have low methodological quality and overestimate the effect size compared with larger sample trials.[33] In this study, many small sample studies were included, and the quality of the research was limited by the design defects of the blind method. Although the application of the comparison-adjusted funnel plot did not find an effect of the small sample on the results, more high-quality RCTs with large sample sizes are needed to further confirm the results of this study.
Author contributions
Conceptualization: Junjing Zha, Changtai Fang.
Data curation: Junjing Zha, Chuan Li.
Investigation: Chuan Li.
Methodology: Chuan Li.
Resources: Gaoxiang Cheng, Lijuan Huang.
Software: Gaoxiang Cheng, Lijuan Huang, Zhaoqing Bai.
Visualization: Zhaoqing Bai.
Writing – original draft: Junjing Zha, Changtai Fang.
Writing – review and editing: Changtai Fang.
Footnotes
Abbreviations: AKI = acute kidney injury, AP = alkaline phosphatase, CIs = confidence intervals, CRRT = continuous renal replacement therapy, CVVH = continuous venovenous hemofiltration, CVVHD = continuous venovenous hemodialysis, CVVHDF = continuous venovenous hemodiafiltration, EHCVVH = extra high-volume continuous venovenous hemofiltration, EHCVVHDF = extra high-volume continuous venovenous hemodiafiltration, HCVVH = high-volume continuous venovenous hemofiltration, ICU = intensive care unit, IHD = intermittent hemodialysis, IVVHDF = intermittent venovenous hemodiafiltration, LCVVH = low-volume continuous venovenous hemofiltration, LCVVHDF = low-volume continuous venovenous hemodiafiltration, ORs = odds ratios, PMXF = polymyxin B-immobilized fiber, RAD = renal tubule assist device, RCA = regional citrate anticoagulation, RCT = randomized controlled trial, RRT = renal replacement therapy, SAKI = sepsis-induced acute kidney injury, SMDs = standard mean differences, SUCRA = surface under the cumulative ranking.
JZ and CL contributed equally to this work.
The Preferred Reporting Items for Systematic Reviews Network Meta-Analysis (PRISMA-NMA) Checklist.
The authors have no conflicts of interest to disclose.
References
- [1].Zhang A, Cai Y, Wang PF, et al. Diagnosis and prognosis of neutrophil gelatinase-associated lipocalin for acute kidney injury with sepsis: a systematic review and meta-analysis. Crit Care 2016;20:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Morrell ED, Kellum JA, Pastor-Soler NM, et al. Septic acute kidney injury: molecular mechanisms and the importance of stratification and targeting therapy. Crit Care 2014;18:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang H, Li L, Chu Q, et al. Early initiation of renal replacement treatment in patients with acute kidney injury: a systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bhatt GC, Das RR. Early versus late initiation of renal replacement therapy in patients with acute kidney injury-a systematic review & meta-analysis of randomized controlled trials. BMC Nephrol 2017;18:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Clark E, Molnar AO, Joannes-Boyau O, et al. High-volume hemofiltration for septic acute kidney injury: a systematic review and meta-analysis. Crit Care 2014;18:R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhang Z, Xu X, Zhu H. Intensive- vs less-intensive-dose continuous renal replacement therapy for the intensive care unit-related acute kidney injury: a meta-analysis and systematic review. J Crit Care 2010;25:595–600. [DOI] [PubMed] [Google Scholar]
- [7].Zhang Z, Hongying N. Efficacy and safety of regional citrate anticoagulation in critically ill patients undergoing continuous renal replacement therapy. Intensive Care Med 2012;38:20–8. [DOI] [PubMed] [Google Scholar]
- [8].Wu MY, Hsu YH, Bai CH, et al. Regional citrate versus heparin anticoagulation for continuous renal replacement therapy: a meta-analysis of randomized controlled trials. Am J Kidney Dis 2012;59:810–8. [DOI] [PubMed] [Google Scholar]
- [9].Park JT, Lee H, Kee YK, et al. High-dose versus conventional-dose continuous venovenous hemodiafiltration and patient and kidney survival and cytokine removal in sepsis-associated acute kidney injury: a randomized controlled trial. Am J Kidney Dis 2016;68:599–608. [DOI] [PubMed] [Google Scholar]
- [10].Dai T, Cao S, Yang X. Comparison of clinical efficacy between continuous renal replacement therapy and intermittent haemodialysis for the treatment of sepsis-induced acute kidney injury. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2016;28:277–80. [PubMed] [Google Scholar]
- [11].Liu H, Liu Y, Sun JK, et al. Extravascular lung water monitoring of renal replacement therapy in lung water scavenging for septic acute kidney injury. Int J Clin Exp Med 2015;8:18907–16. [PMC free article] [PubMed] [Google Scholar]
- [12].Albino BB, Balbi AL, Abrao JM, et al. Dialysis complications in acute kidney injury patients treated with prolonged intermittent renal replacement therapy sessions lasting 10 versus 6 hours: results of a randomized clinical trial. Artif Organs 2015;39:423–31. [DOI] [PubMed] [Google Scholar]
- [13].Joannes-Boyau O, Honore PM, Perez P, et al. High-volume versus standard-volume haemofiltration for septic shock patients with acute kidney injury (IVOIRE study): a multicentre randomized controlled trial. Intensive Care Med 2013;39:1535–46. [DOI] [PubMed] [Google Scholar]
- [14].Chancharoenthana W, Tiranathanagul K, Srisawat N, et al. Enhanced vascular endothelial growth factor and inflammatory cytokine removal with online hemodiafiltration over high-flux hemodialysis in sepsis-related acute kidney injury patients. Ther Apher Dial 2013;17:557–63. [DOI] [PubMed] [Google Scholar]
- [15].Zhang P, Yang Y, Lv R, et al. Effect of the intensity of continuous renal replacement therapy in patients with sepsis and acute kidney injury: a single-center randomized clinical trial. Nephrol Dial Transplant 2012;27:967–73. [DOI] [PubMed] [Google Scholar]
- [16].Wald R, Friedrich JO, Bagshaw SM, et al. Optimal Mode of clearance in critically ill patients with acute kidney injury (OMAKI) – a pilot randomized controlled trial of hemofiltration versus hemodialysis: a Canadian Critical Care Trials Group project. Crit Care 2012;16:R205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pickkers P, Heemskerk S, Schouten J, et al. Alkaline phosphatase for treatment of sepsis-induced acute kidney injury: a prospective randomized double-blind placebo-controlled trial. Crit Care 2012;16:R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gordon AC, Russell JA, Walley KR, et al. The effects of vasopressin on acute kidney injury in septic shock. Intensive Care Med 2010;36:83–91. [DOI] [PubMed] [Google Scholar]
- [19].Oudemans-van Straaten HM, Bosman RJ, Koopmans M, et al. Citrate anticoagulation for continuous venovenous hemofiltration. Crit Care Med 2009;37:545–52. [DOI] [PubMed] [Google Scholar]
- [20].Bellomo R, Cass A, Cole L, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 2009;361:1627–38. [DOI] [PubMed] [Google Scholar]
- [21].Lins RL, Elseviers MM, Van der Niepen P, et al. Intermittent versus continuous renal replacement therapy for acute kidney injury patients admitted to the intensive care unit: results of a randomized clinical trial. Nephrol Dial Transplant 2009;24:512–8. [DOI] [PubMed] [Google Scholar]
- [22].Tumlin J, Wali R, Williams W, et al. Efficacy and safety of renal tubule cell therapy for acute renal failure. J Am Soc Nephrol 2008;19:1034–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Palevsky PM, Zhang JH, O’Connor TZ, et al. Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 2008;359:7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Boussekey N, Chiche A, Faure K, et al. A pilot randomized study comparing high and low volume hemofiltration on vasopressor use in septic shock. Intensive Care Med 2008;34:1646–53. [DOI] [PubMed] [Google Scholar]
- [25].John S, Griesbach D, Baumgartel M, et al. Effects of continuous haemofiltration vs intermittent haemodialysis on systemic haemodynamics and splanchnic regional perfusion in septic shock patients: a prospective, randomized clinical trial. Nephrol Dial Transplant 2001;16:320–7. [DOI] [PubMed] [Google Scholar]
- [26].Nakamura T, Ushiyama C, Suzuki S, et al. Effect of polymyxin B-immobilized fiber hemoperfusion on sepsis-induced rhabdomyolysis with acute renal failure. Nephron 2000;86:210. [DOI] [PubMed] [Google Scholar]
- [27].Barenbrock M, Hausberg M, Matzkies F, et al. Effects of bicarbonate- and lactate-buffered replacement fluids on cardiovascular outcome in CVVH patients. Kidney Int 2000;58:1751–7. [DOI] [PubMed] [Google Scholar]
- [28].Ronco C, Bellomo R, Homel P, et al. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet 2000;356:26–30. [DOI] [PubMed] [Google Scholar]
- [29].Cruz DN, Perazella MA, Bellomo R, et al. Effectiveness of polymyxin B-immobilized fiber column in sepsis: a systematic review. Crit Care 2007;11:R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang Z, Xu X, Ni H. Small studies may overestimate the effect sizes in critical care meta-analyses: a meta-epidemiological study. Crit Care 2013;17:R2. [DOI] [PMC free article] [PubMed] [Google Scholar]


