Abstract
Background
Current guidelines recommend that patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) should be treated with systemic corticosteroid for seven to 14 days. Intermittent systemic corticosteroid use is cumulatively associated with adverse effects such as osteoporosis, hyperglycaemia and muscle weakness. Shorter treatment could reduce adverse effects.
Objectives
To compare the efficacy of short‐duration (seven or fewer days) and conventional longer‐duration (longer than seven days) systemic corticosteroid treatment of adults with acute exacerbations of COPD.
Search methods
Searches were carried out using the Cochrane Airways Group Specialised Register of Trials, MEDLINE and CENTRAL (Cochrane Central Register of Controlled Trials) and ongoing trials registers up to March 2017.
Selection criteria
Randomised controlled trials comparing different durations of systemic corticosteroid defined as short (i.e. seven or fewer days) or longer (i.e. longer than seven days). Other interventions—bronchodilators and antibiotics—were standardised. Studies with participants requiring assisted ventilation were excluded.
Data collection and analysis
We used standard methodological procedures as expected by The Cochrane Collaboration.
Main results
Eight studies with 582 participants met the inclusion criteria, of which five studies conducted in hospitals with 519 participants (range 28 to 296) contributed to the meta‐analysis. Mean ages of study participants were 65 to 73 years, the proportion of male participants varied (58% to 84%) and COPD was classified as severe or very severe. Corticosteroid treatment was given at equivalent daily doses for three to seven days for short‐duration treatment and for 10 to 15 days for longer‐duration treatment. Five studies administered oral prednisolone (30 mg in four, tapered in one), and two studies provided intravenous corticosteroid treatment. Studies contributing to the meta‐analysis were at low risk of selection, performance, detection and attrition bias. In four studies we did not find a difference in risk of treatment failure between short‐duration and longer‐duration systemic corticosteroid treatment (n = 457; odds ratio (OR) 0.72, 95% confidence interval (CI) 0.36 to 1.46)), which was equivalent to 22 fewer per 1000 for short‐duration treatment (95% CI 51 fewer to 34 more). No difference in risk of relapse (a new event) was observed between short‐duration and longer‐duration systemic corticosteroid treatment (n = 457; OR 1.04, 95% CI 0.70 to 1.56), which was equivalent to nine fewer per 1000 for short‐duration treatment (95% CI 68 fewer to 100 more). Time to the next COPD exacerbation did not differ in one large study that was powered to detect non‐inferiority and compared five days versus 14 days of systemic corticosteroid treatment (n = 311; hazard ratio 0.95, 95% CI 0.66 to 1.37). In five studies no difference in the likelihood of an adverse event was found between short‐duration and longer‐duration systemic corticosteroid treatment (n = 503; OR 0.89, 95% CI 0.46 to 1.69, or nine fewer per 1000 (95% CI 44 fewer to 51 more)). Length of hospital stay (n = 421; mean difference (MD) ‐0.61 days, 95% CI ‐1.51 to 0.28) and lung function at the end of treatment (n = 185; MD FEV1 ‐0.04 L; 95% CI ‐0.19 to 0.10) did not differ between short‐duration and longer‐duration treatment.
Authors' conclusions
Information from a new large study has increased our confidence that five days of oral corticosteroids is likely to be sufficient for treatment of adults with acute exacerbations of COPD, and this review suggests that the likelihood is low that shorter courses of systemic corticosteroids (of around five days) lead to worse outcomes than are seen with longer (10 to 14 days) courses. We graded most available evidence as moderate in quality because of imprecision; further research may have an important impact on our confidence in the estimates of effect or may change the estimates. The studies in this review did not include people with mild or moderate COPD; further studies comparing short‐duration systemic corticosteroid versus conventional longer‐duration systemic corticosteroid for treatment of adults with acute exacerbations of COPD are required.
Plain language summary
Are shorter courses of systemic steroids as effective as conventional longer courses in the treatment of patients with flare‐ups of COPD?
Why is this question important?
Chronic obstructive pulmonary disease (COPD), which includes emphysema and chronic bronchitis, is a long‐term lung condition that is commonly associated with smoking. Patients with COPD may experience flare‐ups (exacerbations), often precipitated by infection, in which symptoms such as breathlessness, cough and phlegm become markedly worse, and extra treatment or admission to hospital is required.
Systemic (i.e. not inhaled) corticosteroids, such as prednisolone, prednisone and cortisone, are commonly used in the treatment of patients with these flare‐ups (exacerbations). We wanted to assess whether a shorter course (seven or fewer days) of this treatment was as good as a course of usual length (longer than seven days) and caused fewer side effects.
How did we answer the question?
We looked for all studies that compared oral or injected corticosteroid treatment given for seven or fewer days versus treatment given for longer than seven days in people with acute exacerbations of COPD.
What did we find?
We found eight studies that included 582 people with COPD who experienced a flare‐up that required extra treatment in hospital. These studies compared oral or injected corticosteroid treatment given for seven or fewer days versus treatment for longer than seven days. Most of the people in these studies were in their late sixties and had severe or very severe symptoms of COPD; more men than women took part. The last search for studies to be included in the review was conducted in March 2017.
No differences were observed between shorter and longer courses of treatment. People treated for seven or fewer days did not have a higher rate of treatment failure or longer time to their next exacerbation; the number of people who avoided treatment failure ranged from 51 fewer to 34 more per 1000 treated (average 22 fewer people per 1000). Time in hospital and lung function (blowing tests) at the end of treatment were not different. No differences in side effects or death were noted between treatments. Information on quality of life, which is an important outcome for people with COPD, is limited, as only one study measured it.
The eight studies included in this review were generally well designed, and the quality of the evidence was rated as moderate because of imprecision in results; more research, especially involving people with less severe COPD, is needed.
Summary of findings
Summary of findings for the main comparison. SCS treatment for 7 or fewer days compared with SCS treatment for longer than 7 days for acute exacerbations of COPD.
| SCS treatment for 7 or fewer days compared with SCS treatment for longer than 7 days for acute exacerbations of COPD | ||||||
| Patient or population: patients with acute exacerbations of COPD Settings: hospital‐initiated treatment Intervention: SCS treatment for 7 or fewer days Comparison: SCS treatment for longer than 7 days | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| SCS treatment for longer than 7 days | SCS treatment for 7 or fewer days | |||||
| Treatment failure Need for additional treatment Follow‐up: 10 to 14 days | 83 per 1000 | 61 per 1000 (32 to 117) | OR 0.72 (0.36 to 1.46) | 457 (4 studies) | ⊕⊕⊕⊝ Moderatea | Equivalent to 22 fewer (95% CI 51 fewer to 34 more) |
| Relapse New acute exacerbation or COPD‐related admission Follow‐up: 14 to 180 days | 295 per 1000 | 304 per 1000 (227 to 395) | OR 1.04 (0.7 to 1.56) | 478 (4 studies) | ⊕⊕⊕⊝ Moderatea | In one study (Leuppi 2013), hazard ratio for time to re‐exacerbation was 0.95 (95% CI 0.66 to 1.37) |
| Adverse drug effect—hyperglycaemia Follow‐up: 3 to 14 days | 442 per 1000 | 439 per 1000 (336 to 548) | OR 0.99 (0.64 to 1.53) | 345 (2 studies) | ⊕⊕⊕⊝ Moderatea | |
| Adverse drug effects Gastrointestinal tract bleeding, symptomatic gastrointestinal reflux, symptoms of congestive heart failure or ischaemic heart disease, sleep disturbance, fractures, depression Follow‐up: 10 to 180 days | 84 per 1000 | 75 per 1000 (40 to 135) | OR 0.88 (0.46 to 1.7) | 503 (5 studies) | ⊕⊕⊝⊝ Lowa,b | |
| Mortality Follow‐up: 14 to 180 days | 77 per 1000 | 71 per 1000 (32 to 147) | OR 0.91 (0.4 to 2.06) | 336 (2 studies) | ⊕⊕⊕⊝ Moderatea | |
| Length of hospitalisation (days) Follow‐up: 3 to 14 days | Mean length of hospitalisation in control groups was 10 days | Mean length of hospitalisation in intervention groups was 0.61 lower (1.51 lower to 0.28 higher) | 421 (3 studies) | ⊕⊕⊕⊝ Moderatea | ||
| Lung function (end of treatment) FEV1 (L) Follow‐up: 10 to 14 days | Mean FEV1 in control groups ranged from 0.84 to 1.14 L | Mean lung function (end of treatment) in intervention groups was 0.04 lower (0.19 lower to 0.10 higher) | 187 (4 studies) | ⊕⊝⊝⊝ Very lowa,c,d,e | ||
|
Health‐related quality of life (QOL) Overall score (includes activity limitations, symptoms, fatigue, emotional functioning); scale 0 best to 6 worst; minimum important difference 0.5 Follow‐up: 30 days |
Mean QOL score in control groups was 1.24 | Mean QOL score in intervention groups was 0.06 higher (‐0.16 lower to 0.28 higher) | 271 (1 study) | ⊕⊕⊕⊝ Moderatea | ||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aWide confidence intervals include significant benefit or harm (‐1 for imprecision). bParticipants and physicians not blinded to treatment in one study (‐1 for risk of bias). cParticipants and physicians not blinded to treatment in one study; however risk of bias for the outcome measurement is considered low. dHigh risk of attrition bias in one study (‐1 for risk of bias). eSignificant unexplained heterogeneity (‐1 for inconsistency).
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD) is characterised by persistent airflow limitation that is usually progressive and is associated with an enhanced chronic inflammatory response to noxious particles or gases in the airways and the lung (GOLD 2014). A diagnosis of COPD is considered on a clinical basis in the presence of symptoms such as dyspnoea, chronic cough or sputum production and exposure to known risk factors. Confirmation of COPD diagnosis is based on demonstration of persistent airflow limitation with spirometry, according to the criterion of postbronchodilator forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratio less than 0.7, as specified in guidelines included in GOLD (Global Strategy for Diagnosis, Management, and Prevention of COPD) (GOLD 2014). However, heterogeneity (Agusti 2010) and phenotypical variation in COPD (Weatherall 2009) are increasingly recognised. COPD and asthma overlap syndrome are known to have a higher prevalence in older individuals and are associated with more frequent exacerbations (Hardin 2014).
COPD is an important and increasing cause of mortality; it was estimated to be the fifth leading cause of death in the year 2000 and was responsible for 2.75 million deaths (Lopez 2006). Worldwide mortality due to COPD is projected to rise to 4.5 million deaths in 2020, becoming the third leading cause (Murray 1997). Worldwide COPD resulted in 16.5 million years of life lost in 2000, almost 10 million years lived with disability and 26.5 million disability‐adjusted life‐years (Lopez 2006).
The Burden of Lung Disease (BOLD) study (Buist 2007) reported a worldwide prevalence of stage II or greater severity (FEV1 < 80% predicted) of 10.1% (standard error (SE) 4.8) overall, 11.8% (SE 7.9) for men and 8.5% (SE 5.8) for women. International variation in the prevalence and severity of COPD is partially explained by variation in smoking prevalence and other risk factors (Buist 2007).
Exacerbations and co‐morbidities contribute to the varying natural history of COPD in individual patients (GOLD 2014). Exacerbations contribute to long‐term decline in lung function (Donaldson 2002) and reduced physical activity (Donaldson 2005) and are associated with increased risk of death (Soler‐Cataluna 2005). They also have a profound and long‐lasting effect on quality of life (Groenewegen 2001; Wilkinson 2004); in 10% of exacerbations, pre‐exacerbation quality of life was not recovered after three months (Seemungal 2000).
Exacerbations of COPD are a frequent cause of hospitalisation (Donaldson 2006), although exacerbations with less severe symptoms and signs are often managed on an outpatient basis (NICE 2010). Hospital‐at‐home or early discharge services, if available, may be used as an alternative way of caring for patients with exacerbations of COPD who otherwise would need to be admitted or to remain in hospital (NICE 2010). Treatment of patients with exacerbations is a large contributor to the economic burden of COPD (Schermer 2002; Sullivan 2000), and a high proportion of costs is due to hospitalisation (Oostenbrink 2004).
Studies on the frequency of exacerbations usually use an “event‐based” definition based on healthcare utilization (Effing 2009), and different events may be a proxy for severity, with unscheduled clinic or emergency room visits rated “moderate,” and those requiring hospitalisation labelled “severe” (Rodriguez‐Roisin 2000). However, the clinical onset of an acute exacerbation is defined according to symptoms, although no universally agreed upon definition is known (Rodriguez‐Roisin 2000). Type 1 exacerbations were originally defined by Anthonisen on the basis of three major symptoms: increased dyspnoea, sputum volume and sputum purulence; type 2 exacerbations were associated with only two of the major symptoms, and type 3 exacerbations involved one major symptom plus cough, wheeze or symptoms of an upper respiratory tract infection (Anthonisen 1987). A later definition required an increase in two of the "major symptoms" of dyspnoea—sputum volume or sputum purulence—or in one major symptom and one "minor symptom" (wheeze, sore throat, cough or common cold symptoms) for two days (Seemungal 2000). More recently, a standardised measure for assessing frequency, severity and duration of exacerbations of COPD using patient‐reported outcomes has been developed for use in clinical studies: the EXACT (exacerbations of chronic pulmonary disease tool) diary, which consists of 14 items (Leidy 2010; Leidy 2011).
The inflammatory mechanisms underlying the onset and development of COPD exacerbations are complex (Bathoorn 2008), and exacerbations can be precipitated by several factors, and the most common causes are infective (Papi 2006); bacterial pathogens are identified in just over 50% of cases, and viral causes in around 25% (Sethi 2002; Sherk 2000). Non‐infective causes such as air pollution and other environmental conditions that increase airway inflammation may account for 15% to 20% of exacerbations (Sethi 2008). Exacerbations become more frequent and more severe as the severity of COPD increases (Suissa 2012), although the rate at which they occur may reflect an independent susceptibility phenotype—the “frequent exacerbator” (Hurst 2010).
Description of the intervention
The acute inflammatory response to airway infection is influenced by both pathogenic and host factors, resulting in increased airway and systemic inflammation (Sethi 2008). Airway inflammation is significantly increased during exacerbations of COPD, and evidence of increased numbers of neutrophils, lymphocytes and eosinophils is seen in airways and in sputum (Falk 2008; Papi 2006). Systemic inflammation is also present in COPD; many circulating inflammatory mediators are elevated, both in stable COPD and during exacerbations. C‐reactive protein (CRP) is a known marker of systemic inflammation whose levels are elevated during exacerbations; it is a likely participant in the inflammatory cascade (Falk 2008). Theoretical mechanisms for clinical improvement in lung function among patients treated with corticosteroids during exacerbations may include reduction in airway inflammation or a decrease in airway oedema (Wedzicha 2000).
Why it is important to do this review
Systemic corticosteroid use in COPD is associated with potential adverse drug effects, including fluid retention, hypertension, diabetes mellitus, adrenal suppression and osteoporosis (McEvoy 1996). Fracture risk is increased (De Vries 2007; Vestergaard 2007); this is potentially important, as the rate of existing vertebral compression fractures in the COPD population experiencing acute exacerbations, especially among older people with longer duration of disease, is high (Majumdar 2010). Short‐term use of systemic corticosteroids in the treatment of patients with exacerbations of COPD also has adverse effects on respiratory and peripheral muscle strength (Decramer 1994). The risk of adverse drug effects of systemic corticosteroids in COPD rises with increasing frequency of high‐dose systemic corticosteroid use and with increasing cumulative exposure (more than 1 g) (De Vries 2007).
Systemic corticosteroid treatment of patients with acute exacerbations of COPD decreases treatment failure and is associated with early improvement in lung function, breathlessness and arterial hypoxaemia and reduced length of hospital stay (Walters 2014), although in this Cochrane review, the duration of systemic corticosteroid treatment varied, usually described as between three days and 15 days. Guidelines for the management of COPD specify a duration of treatment ranging from seven days to 10 days (GOLD 2014), from seven days to 14 days (NICE 2010) and from 10 days to 14 days (McKenzie 2003). Thus, it is important to define the optimum duration of corticosteroid treatment and to take into account limiting potential adverse effects.
Objectives
To compare the efficacy of short‐duration (seven or fewer days) and conventional longer‐duration (longer than seven days) systemic corticosteroid treatment of adults with acute exacerbations of COPD.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing short‐duration systemic corticosteroid treatment (seven or fewer days) versus conventional, longer‐duration treatment (longer than seven days).
Types of participants
Adults with an acute exacerbation of COPD. The definition of an acute exacerbation could include any combination of an increase in breathlessness, sputum volume, sputum purulence, cough or wheeze. We excluded studies that included patients with asthma and other lung diseases (e.g. interstitial lung disease, bronchiectasis), unless separate data on participants with COPD alone were available.
Types of interventions
Systemic corticosteroid (SCS) given for a period of seven or fewer days (SCS ≤ 7) versus systemic corticosteroid given for longer than seven days (SCS > 7). Co‐interventions were required to be standardised across groups. We excluded studies in which participants received assisted ventilation (invasive or non‐invasive).
Types of outcome measures
Primary outcomes
Treatment failure (e.g. the need for additional treatment, hospital admission/re‐admission for index episode, return to emergency department, unscheduled physician visit for the index episode).
Relapse after treatment (e.g. treatment for new acute exacerbation, re‐admission or hospitalisation for COPD).
Adverse drug effects.
Secondary outcomes
Mortality.
Lung function.
Length of hospital stay.
Arterial blood gases.
Symptom scores.
Quality of life.
Time period for assessment of continuous outcomes: Early response was measured on or before day seven of treatment, and end of treatment response measurements were made at the time point equivalent to the end of the longer treatment period.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Trials Register, which is maintained by the Information Specialist for the Group. The Cochrane Airways Trials Register contains studies identified from several sources:
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies Online (crso.cochrane.org);
Weekly searches of MEDLINE Ovid SP 1946 to date;
Weekly searches of Embase Ovid SP 1974 to date;
Monthly searches of PsycINFO Ovid SP 1967 to date;
Monthly searches of CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature) 1937 to date;
Monthly searches of AMED EBSCO (Allied and Complementary Medicine);
Handsearches of the proceedings of major respiratory conferences.
Studies contained in the Trials Register are identified through search strategies based on the scope of Cochrane Airways. Details of these strategies, as well as a list of handsearched conference proceedings are in Appendix 1.
We conducted additional supplementary searches of MEDLINE (PubMed platform, dates covered 1950 to March 2017) and CENTRAL (2017, Issue 3). EMBASE (Embase.com platform, dates covered 1947 to July 2014) searches were conducted in previous versions of this review.. Search strategies used in the Cochrane Airways Register of Trials and other databases are shown in Appendix 2.
For this review update, searches were conducted in the Cochrane Airways Group Register of Trials, and in the other databases in March 2017.
Searching other resources
Bibliographies of RCTs and review articles identified for inclusion were searched for additional papers. Registers of ongoing trials at Clinicaltrials.gov and the WHO trial portal were searched in March 2017.
Data collection and analysis
Selection of studies
All potentially relevant trials were assessed for relevance by at least two review authors (DT, CW), who screened the full text to independently select trials for inclusion and to identify and record reasons for exclusion of ineligible studies. We resolved disagreements through discussion or, if required, we consulted a third person (JW). We identified and excluded duplicates and collated multiple reports of the same study, so that each study (rather than each report) was the unit of interest in the review. We recorded the selection process as a PRISMA flow diagram and in the Characteristics of excluded studies table.
We initially screened all studies using the following criteria.
Acute exacerbation of COPD?
Systemic corticosteroids as intervention?
Comparison of short‐duration (seven or fewer days) versus longer‐duration (longer than seven days) corticosteroid therapy?
Randomised controlled trial?
We noted other arms and reasons for study exclusion when appropriate. We determined exclusion using the criteria listed above and categorised the following as applicable.
Not different durations of steroids.
Not exacerbations of COPD.
Not treatment with systemic corticosteroids.
Review or other type of article.
We also assessed each study for accuracy of the diagnosis of COPD using the following criteria (GOLD 2014).
Did the patient have dyspnoea, chronic cough or sputum production?
Was there a history of exposure to risk factors including tobacco smoke; occupational dusts and chemicals; and smoke from home cooking and heating fuel?
Was a spirometry measurement of the FEV1/FVC ratio less than 0.7 post bronchodilator?
Data extraction and management
We used a data collection form to document study characteristics and outcome data. Two review authors (DT, CW) extracted the following study characteristics from included studies.
Methods: study design, total duration of study, number of study centres and locations, study setting, withdrawals and date of study.
Participants: N, mean age, age range, gender, COPD diagnostic criteria, diagnostic criteria for exacerbation, baseline lung function, smoking history, inclusion criteria and exclusion criteria.
Interventions: systemic corticosteroid treatment dose/schedule, concomitant medications and excluded medications.
Outcomes: primary and secondary outcomes specified and collected and time points reported.
Notes: funding for trial and notable conflicts of interest of trial authors.
Two review authors (two of JW, DT, CW) independently extracted outcome data from included studies. Results presented in graphical form were extracted using the software Plot Digitizer. When results were available as means with interquartile ranges, standard deviation was based on the width of the interquartile range—approximately 1.35. standard deviations.
Data were entered into Review Manager (by JW, DT, CW) and were double‐checked by a second review author. We checked that data were entered correctly by comparing data presented in the systematic review against data provided in the study reports.
Assessment of risk of bias in included studies
Two review authors (of JW, CW, DT) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion or by involving another review author. We assessed risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Presence of other bias(es).
We graded each potential source of bias as high, low or unclear, and provided a quote from the study report, together with a justification for our judgement, in the 'Risk of bias' table. We summarised risk of bias judgements across different studies for each of the domains listed. When information on risk of bias was related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for studies that contributed to this outcome.
Measures of treatment effect
For continuous variables, data were analysed as mean differences (MDs) with 95% confidence intervals (CIs). Standardised mean differences (SMDs) with 95% CIs were used when different scales of measurement had been used for an outcome. The SMD is a statistic that expresses the difference in means between treatment groups in units of the pooled standard deviation. Dichotomous outcomes were analysed using Mantel‐Haenszel odds ratios with 95% CIs. When events were rare, we employed the Peto odds ratio. Scale data were entered with a consistent direction of effect.
We undertook meta‐analyses only when these were meaningful—when treatments, participants and the underlying clinical question were similar. When data were skewed, we described them narratively.
For 'time‐to‐event' outcomes such as log hazard ratios, the fixed‐effect generic inverse variance outcome was used to combine results. This method provides a weighted average of the effect estimates of separate studies (Higgins 2011). Number needed to treat for an additional beneficial outcome was calculated from the pooled odds ratio and its confidence interval, using baseline risk in the control group.
Unit of analysis issues
We analysed dichotomous data using participants as the unit of analysis. For continuous data, the MD based on change from baseline was preferred over the MD based on absolute values when both were available.
Dealing with missing data
We contacted investigators to obtain missing numerical outcome data when possible (e.g. when a study was reported as an abstract only). When this was not possible, and when missing data were thought to introduce serious bias, we performed a sensitivity analysis to explore the impact of including such studies in the overall assessment of results.
Assessment of heterogeneity
An assessment of possible heterogeneity, when the null hypothesis is that all studies are evaluating the same effect, was carried out for pooled effects using a Breslow‐Day test of heterogeneity; the Chi2 test measures the deviation of observed effect sizes from an underlying overall effect. This test has low power for detecting true heterogeneity when studies have small sample size or are few in number, hence we used a P value of 0.10 (Deeks 2008). In addition, the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than to chance (Higgins 2003), was used. Statistical heterogeneity was interpreted as follows: 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity and 50% to 90% may represent substantial heterogeneity (Higgins 2011).
We assessed clinical and methodological heterogeneity by recording differences in study design and participant characteristics between individual studies. When we found substantial heterogeneity, we reported this and explored possible causes by performing prespecified subgroup analysis.
Assessment of reporting biases
We tried to minimise reporting bias from non‐publication of studies or selective outcome reporting by using a broad search strategy. We checked references of included studies and relevant systematic reviews, and we contacted study authors to request additional outcome data. To assess the presence of publication bias, we visually inspected funnel plots when 10 or more studies contributed to the analysis for a specified outcome.
Data synthesis
Data were pooled using Review Manager 5.3 (RevMan 2014). We used a fixed‐effect model, and when heterogeneity could not be explained, we performed a sensitivity analysis using a random‐effects model. We presented the findings of our primary outcomes in a 'Summary of findings' table according to recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) (generated with the use of GradePro software).
Subgroup analysis and investigation of heterogeneity
When we detected statistically significant heterogeneity (P value < 0.10), several options were available. We explored the clinical diversity of studies through prespecified subgroup analyses by comparing the following factors.
Definition of COPD.
Aspects of study quality (e.g. concealment of allocation).
Characteristics of participants.
We considered random‐effects analysis methods (DerSimonian 1986) when heterogeneity was unexplained and study sizes were large, or when study findings were not pooled.
We performed subgroup analyses, stratified by the following variables, when possible.
Inpatient versus outpatient.
Studies that included participants previously treated with corticosteroids (inhaled and systemic).
Sensitivity analysis
In assessment of heterogeneity, possible causes arising from details of study design were considered. Sensitivity analyses were performed using a random‐effects model versus a fixed‐effect model for risk of bias and other potential confounders.
Results
Description of studies
Results of the search
Of 644 unique records obtained in the 2011 searches (Figure 1), seven studies met the inclusion criteria, of which four reported data and contributed to the original meta‐analysis (Walters 2011). One ongoing study (Reid 2010) suspended recruitment in 2010, but no data are available. Searches from 2011 to 2014 yielded 284 results in the Cochrane Airways Group Register of Trials, 39 results in CINAHL, 168 in EMBASE and 102 in CENTRAL. When the inclusion criteria were applied, one new study was identified for inclusion (Leuppi 2013), which was listed as an ongoing trial in 2011.
1.
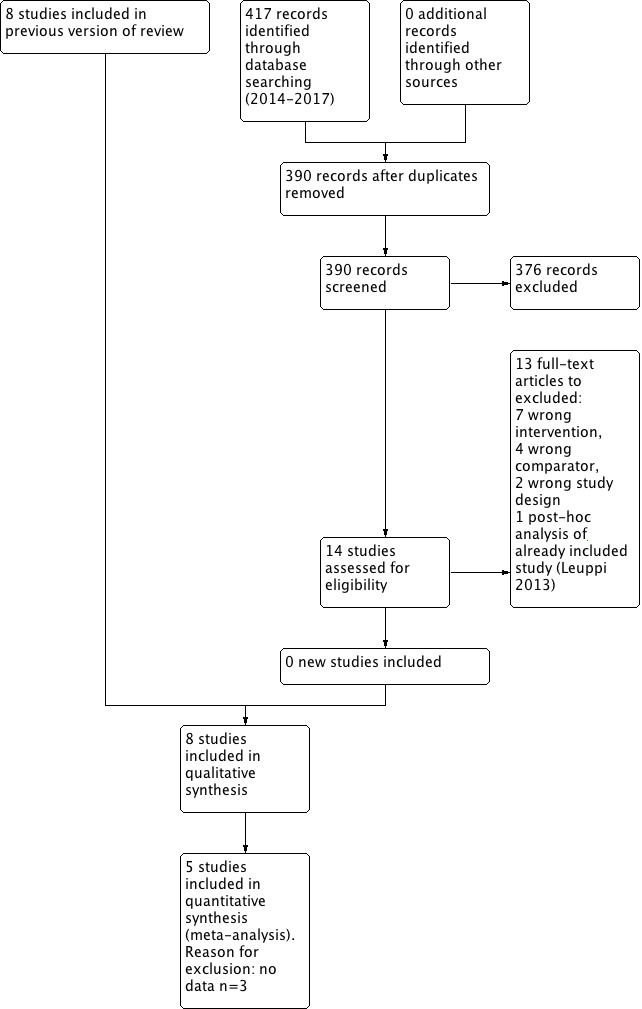
1 Study flow diagram.
In March 2017, we ran an update search. This returned 417 references of which 13 were duplicate, 390 were judged as irrelevant based on title and abstract alone. 14 full text articles were assessed, 13 excluded (7 Wrong intervention, 4 Wrong comparator, 2 Wrong study design), 1 citation belonged to an already included study (Leuppi 2013).
One on‐going study was identified as of March 2017 (Leuppi 2017).
Included studies
We have provided details of the eight included studies in the Characteristics of included studies table and a comparison of their main inclusion criteria and characteristics in Table 2.
1. Study characteristics.
| Study ID/Setting | Inclusion criteria | AE definition | N participants included/Completing | Mean age, years | % males/ % smokers | Prestudy SCS use, % | SCS ≤ 7 days | SCS > 7 days | Definition of treatment failure |
| Chen 2005 China/ inpatients | 2 years of continuous productive cough, FEV1/FVC post BD < 0.7, FEV1 < 80% predicted. No respiratory failure, diabetes or bronchial asthma | At least 2 of 3 symptoms: increased sputum or dyspnoea or purulent sputum | 86/81 | 71 | 75/44 | Not stated | Prednisolone 30 mg 7 days | Prednisolone 30 mg/d 10 days + 15 mg/d 5 days | Not known |
| Gomaa 2008 (abstract only) | FEV1 < 50% predicted, no respiratory acidosis | Not stated | 42/Not known | Not stated | Not stated | Not stated | Prednisolone 30 mg 7 days | Prednisolone 30 mg 15 days | Outcome not reported |
| Leuppi 2013 Switzerland/5 sites | Age > 40 years, smoking history ≥ 20 pack‐years | At least 2 of the following: change in baseline dyspnoea, cough or sputum quantity/purulence | 314/296 | 69 | 60/45 | 20 | 5 days: days 1 to 4 methylprednisolone 40 mg, days 2 to 5 oral prednisolone 40 mg | 14 days: days 1 to 4 methylprednisolone 40 mg, days 2 to 14 oral 40 mg prednisolone | Received open‐label glucocorticoids during index exacerbation |
| Rahman 2004 (abstract only) Bangladesh/1 site | Not stated | Not stated | Not stated | Not stated | Not stated | Not stated | Prednisolone 30 mg 7 days | Prednisolone 30 mg 14 days | Outcome not reported |
| Salam 1998 (abstract only) USA/Unknown sites | Not stated | Not stated | 21/Not known | Not stated | Not stated | None in previous month | IV CS 3 days | IV CS 3 days + 7 days OCS | Outcome not reported |
| Sayiner 2001 Turkey/1 site | FEV1 < 35% predicted, PYH > 20, no respiratory failure requiring ventilation | Not stated, requiring admission | 36/34 | 65 | 94/Not stated | None in previous month | IV methylprednisone 0.5 mg/kg qds 3 days | IV methylprednisone 0.5 mg/kg qds 3 days, bd 3 days, od 4 days | Required open‐label steroid treatment |
| Sirichana 2008 (abstract and author data) Thailand/1 site | Age > 40 years, symptoms > 24 hours | Increase in at least 2 of 3 symptoms—dyspnoea, sputum volume, sputum purulence; requiring admission | 48/42 | 73 | 88/Not stated | None in previous month | Prednisolone 30 mg 5 days | Prednisolone 30 mg 10 days | Outcome not reported |
| Wood‐Baker 1997 (abstract and author data) Australia & New Zealand/2 sites | Age > 40 years; > 10 pack‐year smoking history; FEV1 < 50% predicted | Not stated, requiring admission | 38/28 | 71 | 63/Not stated | None for current AEs, no long‐term OS > 5 mg/d | Prednisolone 2.5 mg/kg orally daily for 3 days | Prednisolone 0.6 mg/kg orally daily for 7 days followed by prednisolone 0.3 mg/kg orally daily for 7 days | Lack of progress according to attending physician during treatment |
All eight studies with a total of 582 participants were parallel‐group RCTs comparing systemic corticosteroid treatment for seven or fewer days versus treatment for longer than seven days in people with an acute exacerbation of COPD; all were conducted in hospital settings. These studies were published between 1997 and 2013. Two studies reported the definition for COPD diagnosis (Chen 2005; Leuppi 2013), and two studies specified the inclusion of participants with an FEV1 below 50% predicted (Gomaa 2008; Wood‐Baker 1997) or below 35% predicted (Sayiner 2001). Two studies (Leuppi 2013; Sirichana 2008) provided a specific symptom‐based definition of an acute exacerbation.
Studies varied in size from 21 to 314 participants; the mean age of participants was between 65 and 73 years, and the proportion of female participants ranged from 6% to 42% (in four studies that reported these data). Participants had severe to very severe COPD; in two studies baseline mean FEV1 ranged from 0.5 to 0.8 litres, and in two other studies baseline mean FEV1 ranged from 25% to 33% predicted.
Five studies used only oral prednisolone (Chen 2005; Gomaa 2008; Rahman 2004; Sirichana 2008; Wood‐Baker 1997) at a dose of 30 mg daily, except Wood‐Baker 1997, which provided oral prednisolone 2.5 mg/kg in the short‐duration arm and a tapered dose (0.6 mg/kg/d for seven days followed by 0.3 mg/kg/d for seven days) in the longer‐duration arm. Three studies used intravenous corticosteroid initially in both arms for 24 or 72 hours: methylprednisolone in Leuppi 2013 and Sayiner 2001, and an unspecified intravenous corticosteroid in Salam 1998. Participants then received systemic corticosteroids intravenously for seven days in Sayiner 2001 and orally for seven days in Salam 1998 in the longer‐duration arm. All participants in Leuppi 2013 received oral prednisone from day 2 onwards for the appropriate treatment duration.
Duration of treatment in the short‐course arm was three days in Salam 1998, Sayiner 2001 and Wood‐Baker 1997; five days in Leuppi 2013 and Sirichana 2008; and seven days in Chen 2005, Gomaa 2008 and Rahman 2004. The duration of longer‐course treatment was 10 days in Salam 1998, Sayiner 2001 and Sirichana 2008; 14 days in Gomaa 2008, Leuppi 2013, Rahman 2004 and Wood‐Baker 1997; and 15 days in Chen 2005.
Three studies were published as full papers (Chen 2005; Leuppi 2013; Sayiner 2001), and additional data were supplied by the study author for Chen 2005. Of five studies published only as abstracts in conference proceedings, data were supplied by authors for Sirichana 2008 and Wood‐Baker 1997. Despite at least two requests, no data were made available for Gomaa 2008, Rahman 2004 and Salam 1998.
Excluded studies
A total of 23 studies with reasons for exclusion are listed in Characteristics of excluded studies.
Risk of bias in included studies
The methodological quality of the published included trials was good; they were determined to have low risk of selection, performance, detection and attrition bias. Risk of bias was unclear in studies available only as abstracts (Figure 2 and Figure 3).
2.
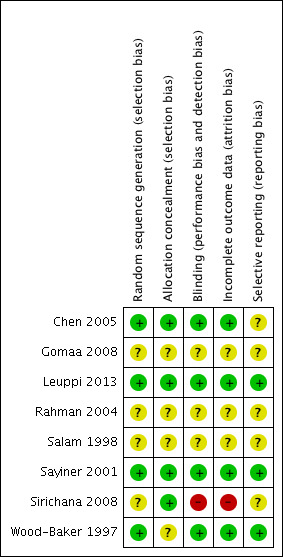
Risk of bias summary: review authors' judgements about each item for each included study.
3.
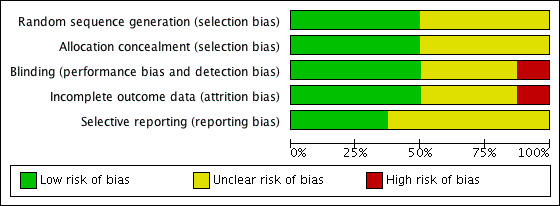
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
In four studies (Chen 2005; Leuppi 2013; Sayiner 2001; Wood‐Baker 1997) the randomisation procedure was adequate, but sequence generation was unclear. Allocation concealment was adequate in four studies (Chen 2005; Leuppi 2013; Sayiner 2001; Sirichana 2008) but was not described in four studies.
Blinding
Four studies were double blind (Chen 2005; Leuppi 2013; Sayiner 2001; Wood‐Baker 1997). One study (Sirichana 2008) was not blinded to participants or investigators. Blinding was unclear in the three other studies, although one study (Gomaa 2008) stated that the study was single blinded but provided no further details.
Incomplete outcome data
Incomplete data were adequately addressed in four studies (Chen 2005; Leuppi 2013; Sayiner 2001; Wood‐Baker 1997), but risk of bias was judged high in Sirichana 2008 and was unclear in three studies.
Selective reporting
Three studies were assessed as free of selective reporting (Leuppi 2013; Sayiner 2001; Wood‐Baker 1997), but risk of bias was unclear in five studies.
Effects of interventions
See: Table 1
Primary outcomes
1.1 Treatment failure
Four studies reported treatment failure that occurred during the index exacerbation, and the definition was provided in three studies (Table 2). No significant difference was noted in the likelihood of treatment failure between systemic corticosteroid for seven or fewer days and longer than seven days (n = 457; odds ratio (OR) 0.72, 95% confidence interval (CI) 0.36 to 1.46; Analysis 1.1; Figure 4), and no statistical heterogeneity was observed. This result is equivalent to 22 fewer treatment failures per 1000 (95% CI 51 fewer to 34 more). The outcome was downgraded for imprecision and was rated as having moderate quality because the confidence intervals for the pooled effect included important benefit and potential harm.
1.1. Analysis.
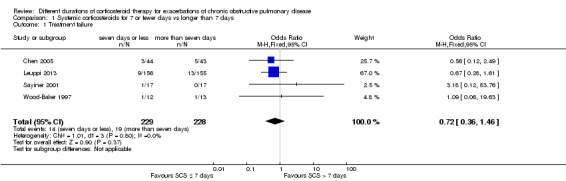
Comparison 1 Systemic corticosteroids for 7 or fewer days vs longer than 7 days, Outcome 1 Treatment failure.
4.
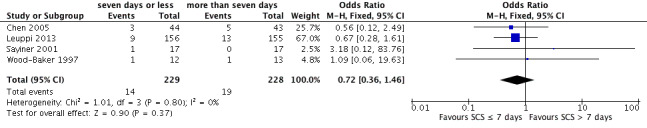
Forest plot outcome: treatment failure comparing systemic corticosteroids for ≤ 7 days vs > 7 days.
1.2 Relapse
Relapse was measured as a new acute exacerbation or hospital admission for COPD in four studies: Chen 2005 (follow‐up period not stated), Sayiner 2001 and Leuppi 2013 (six‐month follow‐up) and Sirichana 2008 (30‐day follow‐up). No significant difference was reported between systemic corticosteroid use for seven or fewer days and for longer than seven days (n = 478; OR 1.04, 95% CI 0.7 to 1.56; Analysis 1.2; Figure 5), and no statistical heterogeneity was observed. The outcome was downgraded for imprecision and was rated as having moderate quality because the confidence intervals for the pooled effect included important benefit and potential harm.
1.2. Analysis.
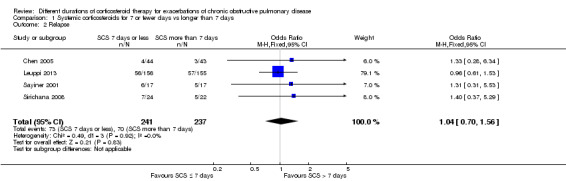
Comparison 1 Systemic corticosteroids for 7 or fewer days vs longer than 7 days, Outcome 2 Relapse.
5.
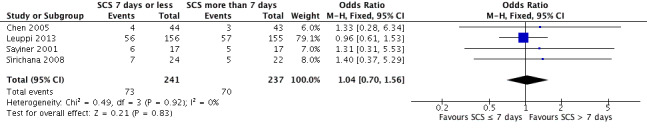
Forest plot of comparison: 1 Systemic corticosteroids for 7 or fewer days vs longer than seven days, outcome: 1.2 Relapse.
1.3 Time to re‐exacerbation
Time to next COPD exacerbation, defined as "acute‐onset worsening of the patient’s condition beyond day‐to‐day variations requiring interaction with a healthcare provider which could occur during the index exacerbation or during follow‐up," over six months of follow‐up was reported in one study (Leuppi 2013). This study adopted an a priori non‐inferiority definition, by which the proportion of participants with re‐exacerbation during follow‐up should be no more than 65% compared with 50% for standard treatment for an exacerbation, yielding a critical hazard ratio of 1.515. Time to the next exacerbation did not differ in this one large study, which was powered to detect non‐inferiority and compared five days versus 14 days of systemic corticosteroid treatment (n = 311; hazard ratio (HR) 0.95, 95% CI 0.66 to 1.37; Analysis 1.3).
1.3. Analysis.

Comparison 1 Systemic corticosteroids for 7 or fewer days vs longer than 7 days, Outcome 3 Time to re‐exacerbation.
1.4 to 1.6 Adverse effects
1.4 Hyperglycaemia: In two studies (Leuppi 2013; Sayiner 2001) no difference was noted in the likelihood of hyperglycaemia between systemic corticosteroid for seven or fewer days and longer than seven days (n = 345; OR 0.99, 95% CI 0.64 to 1.53; Analysis 1.4), and no heterogeneity was observed. The outcome was downgraded for imprecision and was rated as having moderate quality because the confidence intervals for the pooled effect included important benefit and potential harm.
1.4. Analysis.
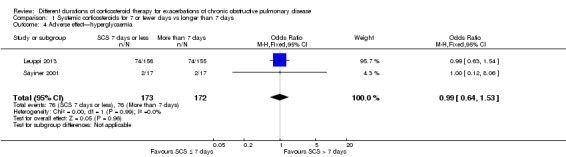
Comparison 1 Systemic corticosteroids for 7 or fewer days vs longer than 7 days, Outcome 4 Adverse effect—hyperglycaemia.
1.5 Hypertension: In one trial (Leuppi 2013)no difference was noted in the likelihood of hypertension between systemic corticosteroid for seven or fewer days and longer than seven days (n = 311; OR 0.61, 95% CI 0.31 to 1.22; Analysis 1.5).
1.5. Analysis.
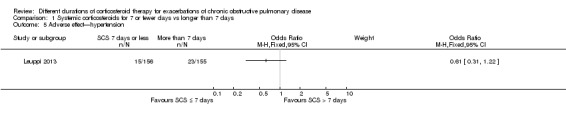
Comparison 1 Systemic corticosteroids for 7 or fewer days vs longer than 7 days, Outcome 5 Adverse effect—hypertension.
1.6 Other adverse effects were reported in five studies and included gastrointestinal tract bleeding, symptomatic gastrointestinal reflux, symptoms of congestive heart failure or ischaemic heart disease, sleep disturbance, fractures and depression. No difference was noted in the likelihood of another adverse event between systemic corticosteroids for seven or fewer days and longer than seven days (n = 503; OR 0.89, 95% CI 0.46 to 1.69; Analysis 1.6). The outcome was downgraded for imprecision and was rated as having moderate quality because the confidence intervals for the pooled effect included important benefit and potential harm.
1.6. Analysis.
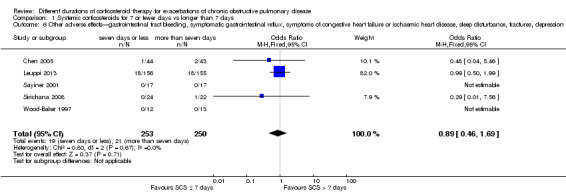
Comparison 1 Systemic corticosteroids for 7 or fewer days vs longer than 7 days, Outcome 6 Other adverse effects—gastrointestinal tract bleeding, symptomatic gastrointestinal reflux, symptoms of congestive heart failure or ischaemic heart disease, sleep disturbance, fractures, depression.
Secondary outcomes
1.7 Mortality
In two studies the likelihood of mortality did not differ between systemic corticosteroid for seven or fewer days and longer than seven days (n = 336; OR 0.91, 95% CI 0.40 to 2.06; Analysis 1.7). The outcome was downgraded for imprecision and was rated as having moderate quality because the confidence intervals for the pooled effect included important benefit and potential harm.
1.7. Analysis.
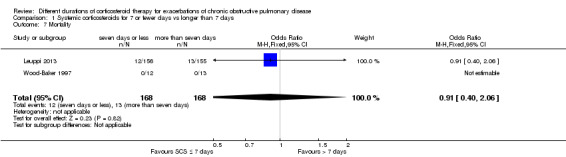
Comparison 1 Systemic corticosteroids for 7 or fewer days vs longer than 7 days, Outcome 7 Mortality.
1.8 Length of hospitalisation
In three studies no difference was noted in length of hospital stay between systemic corticosteroid for seven or fewer days and longer than seven days (n = 421; mean difference (MD) ‐0.61 days, 95% CI ‐1.51 to 0.28; Analysis 1.8). The outcome was downgraded for imprecision and was rated as having moderate quality because the confidence intervals for the pooled effect included important benefit and potential harm.
1.8. Analysis.
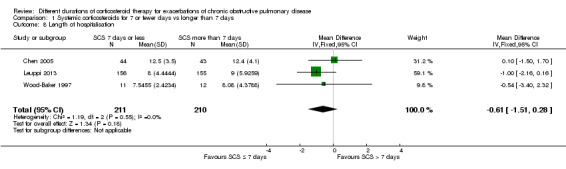
Comparison 1 Systemic corticosteroids for 7 or fewer days vs longer than 7 days, Outcome 8 Length of hospitalisation.
1.9 to 1.14 Lung function
In three studies no difference was noted in absolute FEV1 measured within seven days between systemic corticosteroid for seven or fewer days and longer than seven days (n = 96; MD ‐0.07 litres, 95% CI ‐0.19 to 0.05; Analysis 1.9), and no heterogeneity was observed. Leuppi 2013 reported lung function as FEV1 percentage predicted and noted no difference between systemic corticosteroid for seven or fewer days and longer than seven days (n = 289; MD 0.76%, 95% CI ‐3.15 to 4.66; Analysis 1.10). In three studies no difference was noted in early response of FVC between systemic corticosteroid for seven or fewer days and longer than seven days (n = 97; MD 0.01 L, 95% CI ‐0.20 to 0.22; Analysis 1.11), and no statistical heterogeneity was observed.
1.9. Analysis.
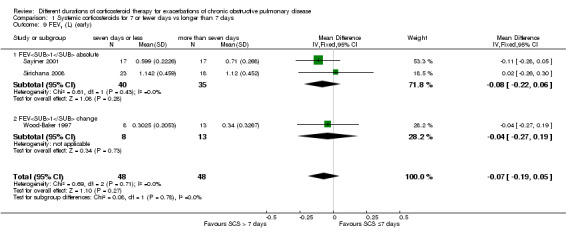
Comparison 1 Systemic corticosteroids for 7 or fewer days vs longer than 7 days, Outcome 9 FEV1 (L) (early).
1.10. Analysis.

Comparison 1 Systemic corticosteroids for 7 or fewer days vs longer than 7 days, Outcome 10 FEV1 % predicted (6 days).
1.11. Analysis.
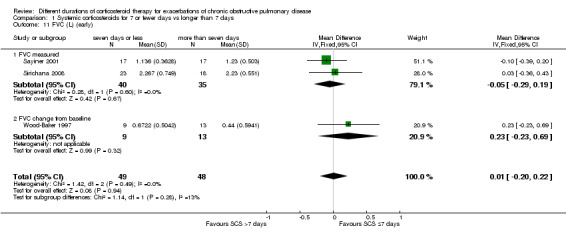
Comparison 1 Systemic corticosteroids for 7 or fewer days vs longer than 7 days, Outcome 11 FVC (L) (early).
For lung function measured at the end of treatment in four studies, no difference was noted in FEV1 between systemic corticosteroid for seven or fewer days and longer than seven days (n = 187; MD ‐0.04 litres, 95% CI ‐0.19 to 0.10; Analysis 1.12); important heterogeneity was observed (Chi² = 7.17, df = 3 (P value 0.07), I² = 58%). The outcome was rated as having very low quality and was downgraded for imprecision because the confidence intervals for the pooled effect included important benefit and potential harm, and risk of attrition bias and inconsistency were due to unexplained heterogeneity. In Leuppi 2013, no difference was noted in FEV1 percentage predicted at 30 days between systemic corticosteroid for seven or fewer days and more than seven days (n = 289; MD 1.29%, 95% CI ‐3.35 to 5.93; Analysis 1.13). In three studies no difference was noted in FVC at the end of treatment between systemic corticosteroid for seven or fewer days and longer than seven days (n = 99; MD ‐0.12, 95% CI ‐0.33 to 0.09; Analysis 1.14), and no heterogeneity was observed.
1.12. Analysis.
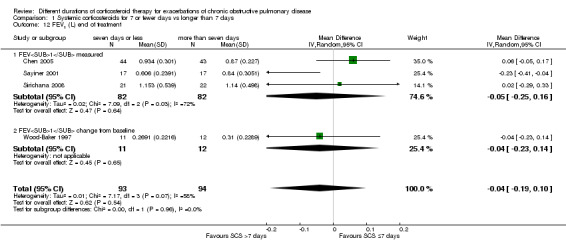
Comparison 1 Systemic corticosteroids for 7 or fewer days vs longer than 7 days, Outcome 12 FEV1 (L) end of treatment.
1.13. Analysis.

Comparison 1 Systemic corticosteroids for 7 or fewer days vs longer than 7 days, Outcome 13 FEV1 % predicted 30 days.
1.14. Analysis.
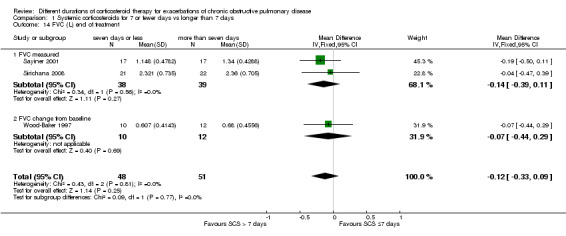
Comparison 1 Systemic corticosteroids for 7 or fewer days vs longer than 7 days, Outcome 14 FVC (L) end of treatment.
1.15 to 1.17 Arterial blood gases
Sayiner 2001 reported partial pressure of oxygen in arterial blood (PaO2) before seven days (n = 34). No difference was noted between treatment with systemic corticosteroid for seven or fewer days and longer than seven days (MD ‐0.20 mmHg; 95% CI ‐9.21 to 8.81; Analysis 1.15).
1.15. Analysis.

Comparison 1 Systemic corticosteroids for 7 or fewer days vs longer than 7 days, Outcome 15 PaO2 (mmHg) (early).
In two studies (n = 121) at completion of treatment, no difference was noted in PaO2 after 10 to 14 days of follow‐up between systemic corticosteroid for seven or fewer days and longer than seven days (MD ‐1.38 mmHg; 95% CI ‐4.96 to 2.21; Analysis 1.16).
1.16. Analysis.
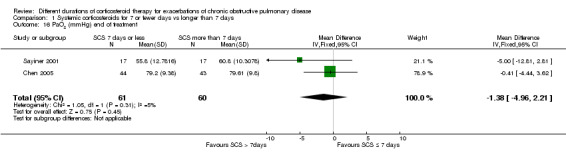
Comparison 1 Systemic corticosteroids for 7 or fewer days vs longer than 7 days, Outcome 16 PaO2 (mmHg) end of treatment.
In Sayiner 2001 (n = 34) no difference was noted in partial pressure of carbon dioxide in arterial blood (PaCO2) after three days (MD ‐1.80 mmHg, 95% CI ‐11.14 to 7.54) or after 10 days (MD 1.50 mmHg, 95% CI ‐5.20 to 8.20) between treatment with systemic corticosteroid for seven or fewer days and longer than seven days (Analysis 1.17).
1.17. Analysis.
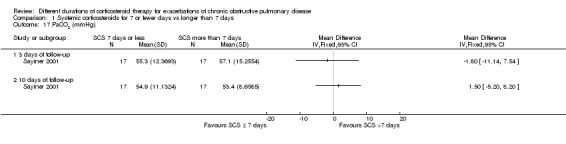
Comparison 1 Systemic corticosteroids for 7 or fewer days vs longer than 7 days, Outcome 17 PaCO2 (mmHg).
1.18 to 1.19 Symptom scores
In two studies no differences were noted in dyspnoea scores when measured before seven days between systemic corticosteroid for seven or fewer days and longer than seven days (n = 320; standardised mean difference (SMD) ‐0.08, 95% CI ‐0.29 to 0.14; Analysis 1.18), and no statistical heterogeneity was observed. When expressed on the Medical Research Council (MRC) scale (1 to 5), this would equate to 0.1 units better (95% CI 0.4 better to 0.2 worse).
1.18. Analysis.
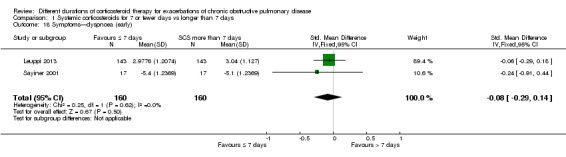
Comparison 1 Systemic corticosteroids for 7 or fewer days vs longer than 7 days, Outcome 18 Symptoms—dyspnoea (early).
In four studies at the end of treatment, no difference was noted in dyspnoea scores between systemic corticosteroid for seven or fewer days and longer than seven days (n = 404; SMD 0.16, 95% CI ‐0.03 to 0.36; Analysis 1.19),and moderate heterogeneity was observed (Chi² = 5.89, df = 3 (P value 0.12), I² = 49%). When expressed on the MRC scale (1 to 5), this would equate to 0.2 units worse (95% CI 0.04 better to 0.45 worse).
1.19. Analysis.
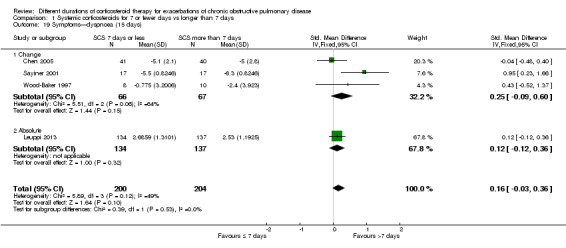
Comparison 1 Systemic corticosteroids for 7 or fewer days vs longer than 7 days, Outcome 19 Symptoms—dyspnoea (15 days).
1.20 to 1.21 Quality of life
Health‐related quality of life was assessed in Leuppi 2013 by using the acute bronchitis health‐related quality‐of‐life interview, which measures changes in health‐related quality of life among patients treated for chronic lung disease; it consists of 22 items in four domains: activity‐related limitations (six items); effects of symptoms (eight items); fatigue (four items); and emotional functioning (four items). Each item is rated on a 7‐point scale, and a difference of 0·5 points is taken to be the smallest important difference (Evans 2002). No difference was reported in overall score between systemic corticosteroid for seven or fewer days and longer than seven days when measured before seven days (n = 290; MD 0.03, 95% CI ‐0.15 to 0.21; Analysis 1.20) or after 30 days (n = 263; MD 0.07, 95% CI ‐0.11 to 0.25; Analysis 1.21).
1.20. Analysis.
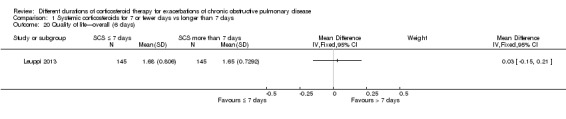
Comparison 1 Systemic corticosteroids for 7 or fewer days vs longer than 7 days, Outcome 20 Quality of life—overall (6 days).
1.21. Analysis.

Comparison 1 Systemic corticosteroids for 7 or fewer days vs longer than 7 days, Outcome 21 Quality of life—overall (30 days).
Discussion
Summary of main results
SCS treatment for seven or fewer days compared with SCS treatment for longer than seven days for acute exacerbations of COPD
Please see Table 1.
The likelihood of treatment failure did not differ between treatment with systemic corticosteroids for seven or fewer days and longer than seven days over 10 to 14 days of follow‐up in four studies, although wide confidence intervals around the estimate were reported (457 participants; odds ratio (OR) 0.72, 95% confidence interval (CI) 0.36 to 1.46). This difference equates to 22 fewer people experiencing treatment failure per 1000 (95% CI 51 fewer to 34 more) for short‐duration corticosteroid treatment. Similarly, no difference was noted for re‐exacerbation over 14 to 180 days of follow‐up in four studies (478 participants; OR 1.04, 95% CI 0.7 to 1.56); this equates to nine fewer people with re‐exacerbation per 1000 (95% CI 68 fewer to 100 more) for longer‐duration corticosteroid treatment. Time to the next COPD exacerbation did not differ in one large study, which was powered to detect non‐inferiority and compared five days versus 14 days of systemic corticosteroid treatment (n = 311; hazard ratio 0.95, 95% CI 0.66 to 1.37).
The likelihood of adverse drug effects when systemic corticosteroids given for seven or fewer days were compared with systemic corticosteroids given for longer than seven days did not differ for hyperglycaemia in two studies with data of moderate quality (n = 345; OR 0.99, 95% CI 0.64 to 1.53), nor for a range of other drug effects in five studies with data of low quality (n = 503; OR 0.88, 95% CI 0.46 to 1.69). Mortality during follow‐up of between 14 days and 180 days did not differ in two studies (n = 336; OR 0.91, 95% CI 0.40 to 2.06) with data of moderate quality.
No differences in length of hospitalisation were noted in three studies (n = 421; MD ‐0.61 days, 95% CI ‐1.51 to 0.28) with data of moderate quality, and no differences in lung function at the end of treatment were reported in four studies (n = 187; mean difference (MD) in forced expiratory volume in one second (FEV1) ‐0.04 L, 95% CI ‐0.19 to 0.10) with data of very low quality. No difference in health‐related quality of life was observed in one study with data of moderate quality.
Overall completeness and applicability of evidence
Five of the eight included studies were not published as full articles. Meta‐analyses were limited by the need to omit three studies for which no data could be extracted from abstracts, or for which no data were supplied by study authors (Gomaa 2008; Rahman 2004; Salam 1998). The small number of trials meant that no subgroup analyses were performed.
One study (Wood‐Baker 1997) closed prematurely because of the difficulty of recruiting participants who met the inclusion criterion stating that participants should not have commenced oral steroid treatment before recruitment. This stipulation also caused low recruitment for the ongoing study (Reid 2010). In many countries this is likely to be a barrier to conducting inpatient studies comparing corticosteroid treatment of shorter duration versus conventional duration of up to 14 days, as guidelines and action plans recommend early initiation of therapy.
As this review excluded studies in which participants received assisted ventilation (invasive or non‐invasive), the findings are not generalisable to people on assisted ventilation. Doses of systemic corticosteroid given to patients admitted to an intensive care unit (ICU) who may require assisted ventilation usually are much higher than those given in the studies included in this review (Kiser 2014). An observational study evaluating corticosteroid use in patients admitted to the ICU with an exacerbation of chronic obstructive pulmonary disease (COPD) concluded that fewer deaths and shorter stays in hospital occurred among people treated with systemic corticosteroid at doses equivalent to less than 240 mg per day of methylprednisolone. An observational study involving patients admitted to a non‐intensive care setting with an exacerbation of COPD who received systemic corticosteroids did not assess duration of treatment; this study found that low‐dose corticosteroids (20 to 80 mg/d prednisone equivalent) administered orally were not associated with worse outcomes than were seen with high‐dose intravenous therapy (120 to 800 mg/d prednisone equivalent) during the first two hospital days (Lindenauer 2010).
Quality of the evidence
Studies that contributed to the meta‐analysis were at low risk of selection bias, although one study was at high risk of performance and detection bias from lack of blinding. Some heterogeneity between studies was observed with regard to the inclusion criteria. Many studies did not provide details of their inclusion criteria, including their definition of an acute exacerbation of COPD. Only three studies specified the severity of COPD in participants based on their lung function reading (FEV1) upon admission. Thresholds to define severity included FEV1 less than 50% predicted in Gomaa 2008 and Wood‐Baker 1997, and less than 35% predicted in Sayiner 2001. Variation in the systemic corticosteroid used in the included studies was reported: Five used oral prednisolone, one at high then tapering dose (Wood‐Baker 1997), and two used intravenous corticosteroid. However, oral and intravenous corticosteroids have been shown to have equal efficacy and should not, therefore, contribute to any heterogeneity (Therapeutic Guidelines 2009). Sensitivity analyses according to risks of bias due to lack of blinding or route of corticosteroid administration had no significant effects on pooled results (Table 3). Duration of follow‐up varied between studies and was not stated in four studies. Follow‐up periods may have been too short to allow detection of potential differences in relapse, mortality and readmission. Lack of data for pooling limited the analysis of some outcomes that are important to patients, such as quality of life.
2. Sensitivity analyses.
| Excluding Sirichana (not blinded) | All studies | |
| Relapse | 1.01 (0.66 to 1.55) | 1.04 (0.70 to 1.56) |
| FEV1 early | ‐0.09 (‐0.22 to 0.05) | ‐0.07 (‐0.19 to 0.05) |
| FEV1 end | ‐0.06 (‐0.23 to 0.11) | ‐0.04 (‐0.19 to 0.10) |
| FVC early | ‐0.00 (‐0.25 to 0.25) | 0.01 (‐0.20 to 0.22) |
| FVC end | ‐0.14 (‐0.38 to 0.09) | ‐0.12 (‐0.33 to 0.09) |
| Adverse event—other | 0.94 (0.48 to 1.82) | 0.89 (0.46 to 1.69) |
| Excluding Sayiner (IV SCS) | All studies | |
| Treatment failure | 0.66 (0.32 to 1.37) | 0.72 (0.36 to 1.46) |
| Relapse | 1.02 (0.67 to 1.56) | 1.04 (0.70 to 1.56) |
| Adverse event—hyperglycaemia | 0.99 (0.63 to 1.54) | 0.99 (0.64 to 1.53) |
| Adverse event—other | 0.89 (0.46 to 1.69) | 0.89 (0.46 to 1.69) |
| FEV1 early | ‐0.02 (‐0.19 to 0.16) | ‐0.07 (‐0.19 to 0.05) |
| FEV1 end | 0.03 (‐0.06 to 0.12) | ‐0.04 (‐0.19 to 0.10) |
| FVC early | 0.12 (‐0.18 to 0.42) | 0.01 (‐0.20 to 0.22) |
| FVC end | ‐0.06 (‐0.34 to 0.22) | ‐0.12 (‐0.33 to 0.09) |
Potential biases in the review process
The review authors made every effort to identify all relevant published and unpublished studies by using additional methods to identify studies that might not have been found during the main electronic search (e.g. searching drug company databases and clinical trial registration sites, checking reference lists). We did not routinely contact individual trial authors for additional data unless outcomes were clearly selectively reported. All review authors adhered to the most recent best practice guidelines in terms of study selection, resolution of disagreements, data extraction and analysis to reduce bias and error. We included all data obtained from the included studies, even those from studies at higher risk of bias with regard to participant withdrawals, such as Sirichana 2008, but we performed sensitivity analysis to check whether omitting the study changed the results. We obtained data for one study (Wood‐Baker 1997) from an author from this review team; however, data extraction and analysis were performed in the same way as for the other studies.
Authors' conclusions
Implications for practice.
We have obtained information from one new large study that has increased our confidence that five days of oral corticosteroids is likely to be sufficient for treatment of patients with exacerbations of COPD, and this review suggests that the likelihood that shorter courses of systemic corticosteroids (of around five days) lead to worse outcomes compared with longer (10 to 14 days) courses is low. Most evidence was graded as having moderate quality as the result of imprecision; further research may have an important impact on our confidence in the estimates of effect or may change these estimates.
Implications for research.
The studies in this review did not include people with mild or moderate COPD, and further studies are required to compare short‐duration systemic corticosteroid versus conventional, longer‐duration treatment for people with acute exacerbations in this group. Studies should use validated patient‐reported exacerbation outcomes (Jones 2014; Leidy 2014), and this review reinforces the need for researchers to publish results of all completed clinical trials. However, difficulties are associated with recruiting participants to studies with this clinical question in many countries in which early treatment in the community is promoted.
What's new
| Date | Event | Description |
|---|---|---|
| 11 March 2017 | New citation required but conclusions have not changed | No new studies identified. The only change was to add the search results. |
| 11 March 2017 | New search has been performed | 417 references, 13 duplicates. 390 irrelevant on title/abstract; 13 excluded. 1 citation for an already included study (Leuppi 2013). 1 ongoing study identified (Leuppi 2017). No new studies included, no change to conclusions. |
History
Protocol first published: Issue 1, 2008 Review first published: Issue 10, 2011
| Date | Event | Description |
|---|---|---|
| 11 March 2017 | New search has been performed | New literature search run. |
| 5 June 2014 | New search has been performed | New literature search run |
| 5 June 2014 | New citation required and conclusions have changed | One new study (Leuppi 2013) with 314 participants added, conclusions redrafted. Background and PLS redrafted and 'Summary of findings' table added. Methods amended to bring them up‐to‐date, but no substantial alterations in how we conducted the review. See 'Differences between protocol and review' for a description |
| 14 February 2012 | Amended | Review republished to rectify copyediting error. Corrections made to signs of treatment effects and confidence intervals in the abstract and in the main text |
Acknowledgements
We acknowledge the work of Wendy Wang, Carla Morley and Amir Soltani, as authors of the previous version of this review, published in 2011.
We acknowledge the assistance of the following individuals.
Dr Worawan Sirichana, Department of Medicine, Chulalongkorn University, Bangkok, Thailand, who kindly supplied study information and data.
Dr C Xie and Dr G Chen, Department of Respiratory Medicine, First Affiliated Hospital of Zhongshan University, Guangzhou, China, who kindly supplied study information and data.
Dr Abdullah Sayiner of Ege University, Ismir, Turkey, who kindly supplied study information.
Dr W Chou and Dr M Sung, who wrote the first draft of the protocol, and Yi Foong, who provided translation from Mandarin.
Chris Cates was the Editor for this review and commented critically on the review.
TheBackground and Methods sections of this protocol/review are based on a standard template used by the Cochrane Airways Group.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL | Monthly |
| MEDLINE (Ovid) | Weekly |
| Embase (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
COPD search
1. Lung Diseases, Obstructive/
2. exp Pulmonary Disease, Chronic Obstructive/
3. emphysema$.mp.
4. (chronic$ adj3 bronchiti$).mp.
5. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
6. COPD.mp.
7. COAD.mp.
8. COBD.mp.
9. AECB.mp.
10. or/1‐9
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases
Appendix 2. Database search strategies
| Database | Search |
| EMBASE.com (with RCT and humans as limits) |
*steroid* or *corticoid* or "adrenal cortex hormone*" or beclomethasone or betamethasone or cortisone or deflazacort or dexamethasone or fluticasone or hydrocortisone or methylprednisolone or methylprednisolone or prednis* or pregnenediones or sterapred or triamcinolone AND exacerb* or acute* or status* or sever* or wors* or emergenc* or attack* or crisis |
| Medline via PubMed | #1 COPD[MeSH Terms] #2 "adrenal cortex hormone*" #3 steroid #4 steroids #5 glucocorticoid* #6 corticoid* #7 corticosteroid* #8 beclomethasone #9 betamethasone #10 fluticasone #11 cortisone #12 dexamethasone #13 hydrocortisone #14 prednisolone #15 prednisone #16 methylprednisolone #17 methylprednisone #18 triamcinolone #19 (#2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18) #20 randomised controlled trial [pt] #21 controlled clinical trial [pt] #22 randomised [tiab] #23 placebo [tiab] #24 clinical trials as topic [mesh: noexp] #25 randomly [tiab] #26 trial [ti] #27 (#20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26) #28 (animals [mh] NOT humans [mh]) #29 (#27 NOT #28) #30 ("2012/01/01"[Date ‐ Publication] : "3000"[Date ‐ Publication]) #31 (#1 AND #19 AND #29 AND #30) |
| ClinicalTrials.gov | steroid AND exacerbation AND COPD |
| Current controlled trials | steroid* AND exacerb* AND COPD |
| CINAHL (with RCTs and humans as limits) | COPD or COAD or emphysema* or chronic bronchitis* AND *steroid* or corticosteroid* or glucocorticoid* or corticoid* or "adrenal cortex hormone*" or beclomethasone or betamethasone or cortisone or deflazacort or dexamethasone or fluticasone or hydrocortisone or methylprednisolone or methylprednisolone or prednis* or pregnenediones or sterapred or triamcinolone AND (exacerb* or acute* or status* or sever* or wors* or emergenc* or attack* or crisis) AND date restriction 2009‐2010 |
| Australia New Zealand Clinical Trials Registry | COPD AND exacerb* |
| CENTRAL (the Cochrane Library) | *steroid* or corticosteroid* or glucocorticoid* or corticoid* or "adrenal cortex hormone*" or beclomethasone or betamethasone or cortisone or deflazacort or dexamethasone or fluticasone or hydrocortisone or methylprednisolone or methylprednisolone or prednis* or pregnenediones or sterapred or triamcinolone AND (exacerb* or acute* or status* or sever* or wors* or emergenc* or attack* or crisis) |
| Cochrane Airways Group Register of Trials (via CRS Web) | #1 MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All #2 MeSH DESCRIPTOR Bronchitis, Chronic #3 (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*) #4 COPD:MISC1 #5 (COPD OR COAD OR COBD):TI,AB,KW #6 #1 OR #2 OR #3 OR #4 OR #5 #7 MeSH DESCRIPTOR Adrenal Cortex Hormones Explode All #8 MeSH DESCRIPTOR Adrenal Cortex Hormones Explode All #9 steroid* or corticosteroid* or corticoid* #10 beclomethasone #11 betamethasone #12 cortisone #13 deflazacort #14 dexamethasone #15 fluticasone #16 hydrocortisone #17 methylprednisolone #18 methylprednisone #19 prednis* #20 pregnenediones #21 sterapred #22 triamcinolone #23 MeSH DESCRIPTOR Acute Disease #24 exacerb* or acute* or sever* or wors* or emergenc* or attack* or crisis #25 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 #26 #23 or #24 #27 #6 and #25 and #26 |
Data and analyses
Comparison 1. Systemic corticosteroids for 7 or fewer days vs longer than 7 days.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure | 4 | 457 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.36, 1.46] |
| 2 Relapse | 4 | 478 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.70, 1.56] |
| 3 Time to re‐exacerbation | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 4 Adverse effect—hyperglycaemia | 2 | 345 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.64, 1.53] |
| 5 Adverse effect—hypertension | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6 Other adverse effects—gastrointestinal tract bleeding, symptomatic gastrointestinal reflux, symptoms of congestive heart failure or ischaemic heart disease, sleep disturbance, fractures, depression | 5 | 503 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.46, 1.69] |
| 7 Mortality | 2 | 336 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.40, 2.06] |
| 8 Length of hospitalisation | 3 | 421 | Mean Difference (IV, Fixed, 95% CI) | ‐0.61 [‐1.51, 0.28] |
| 9 FEV1 (L) (early) | 3 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.19, 0.05] |
| 9.1 FEV1 absolute | 2 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.22, 0.06] |
| 9.2 FEV1 change | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.27, 0.19] |
| 10 FEV1 % predicted (6 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11 FVC (L) (early) | 3 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.20, 0.22] |
| 11.1 FVC measured | 2 | 75 | Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.29, 0.19] |
| 11.2 FVC change from baseline | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.23, 0.69] |
| 12 FEV1 (L) end of treatment | 4 | 187 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.19, 0.10] |
| 12.1 FEV1 measured | 3 | 164 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.25, 0.16] |
| 12.2 FEV1 change from baseline | 1 | 23 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.23, 0.14] |
| 13 FEV1 % predicted 30 days | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14 FVC (L) end of treatment | 3 | 99 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.33, 0.09] |
| 14.1 FVC measured | 2 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.39, 0.11] |
| 14.2 FVC change from baseline | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.44, 0.29] |
| 15 PaO2 (mmHg) (early) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 16 PaO2 (mmHg) end of treatment | 2 | 121 | Mean Difference (IV, Fixed, 95% CI) | ‐1.38 [‐4.96, 2.21] |
| 17 PaCO2 (mmHg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 17.1 3 days of follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 17.2 10 days of follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 18 Symptoms—dyspnoea (early) | 2 | 320 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.29, 0.14] |
| 19 Symptoms—dyspnoea (15 days) | 4 | 404 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.03, 0.36] |
| 19.1 Change | 3 | 133 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.09, 0.60] |
| 19.2 Absolute | 1 | 271 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.12, 0.36] |
| 20 Quality of life—overall (6 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 21 Quality of life—overall (30 days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chen 2005.
| Methods | STUDY DESIGN: parallel group LOCATION/NUMBER OF CENTRES: inpatients in China, unknown number of centres DURATION OF STUDY: 14 days |
|
| Participants | N SCREENED = not stated N RANDOMISED = SCS ≤ 7 days n = 44, SCS > 7 days n = 43 N COMPLETED = SCS ≤ 7 days n = 41, SCS > 7 days n = 40 M = 98 F = 32 AGE INT = 70.3, CONTROL = 71.7 (NS diff) SMOKERS: INT = 18/44 (41%), CONTROL = 20/43 (47%) (NS diff) BASELINE DETAILS: Baseline comparison of groups showed no significant differences in age, gender, course of disease, proportion of current smokers or the prestudy course of exacerbation INCLUSION CRITERIA: exacerbation of COPD: type 2 AEs (i.e. at least 2/3 increased dyspnoea, increased sputum, purulent sputum); use of diagnostic criteria for COPD: 2 years of continuous productive cough, FEV1/FVC post bronchodilator < 0.7, FEV1 < 80% predicted EXCLUSION CRITERIA: respiratory failure, diabetes, bronchial asthma |
|
| Interventions | SCS ≤ 7 days: prednisolone 30 mg/d 7 days + placebo 7 days SCS > 7 days: prednisolone 30 mg/d 10 days + 15 mg/d 5 days Delivery: oral Co‐interventions permitted: not stated Co‐interventions not permitted: not stated Follow‐up period: not stated |
|
| Outcomes | Outcomes measured: lung function, arterial blood gas measurement, days of hospitalisation Composite symptom score: highest 18 (worse) to lowest 0 (no symptoms) for breathlessness, sputum volume, cough, amount of sleep, exercise capacity, wheezing Treatment failure: no definition stated Rate of relapse: no definition stated Side effects: corticosteroids |
|
| Notes | INVESTIGATOR‐SUPPLIED DATA: outcomes reported: absolute values and change in FEV1, FEV1/FVC ratio, PEF, PaO2 # relapse, # failed treatment, # side effect | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stochastic function used to create 150 numbers 0 to 1 Randomisation code prepared by an assistant not involved in other parts of the study Sealed envelopes prepared before study initiation by assistant |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes used and allocated by third party |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants: use of identical boxes and medications Researchers: blinded to medications |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Numbers of failed treatment given, numbers with side effects given and information included in publication Publication details, reasons for withdrawals as worsening disease, poor compliance, treatment failure |
| Selective reporting (reporting bias) | Unclear risk | Results given by study author for FEV1, PaO2, symptom scores, rate of relapse, number of side effects, number with failed treatment No information on hospital duration Unclear what symptom score scale was used |
Gomaa 2008.
| Methods | STUDY DESIGN: parallel LOCATION, NUMBER OF CENTRES: Cairo, Egypt DURATION OF STUDY: 14 days |
|
| Participants | N SCREENED: not stated N RANDOMISED: 42 N COMPLETED: not stated M not stated/F not stated AGE: not stated BASELINE DETAILS: not stated INCLUSION CRITERIA: COPD exacerbation with postbronchodilator FEV1 less than 50% of predicted and without respiratory acidosis EXCLUSION CRITERIA: not stated |
|
| Interventions | SCS ≤ 7 days: prednisolone 30 mg 7 days SCS > 7 days: prednisolone 30 mg 14 days Co‐interventions: not stated FOLLOW‐UP PERIOD: 30 days |
|
| Outcomes | FEV1 day 1, day 7, day 14 and day 30 Symptom score evaluation (breathlessness, cough and sputum) |
|
| Notes | Information from abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | States single blinded but no details on blinding provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Specific data not provided |
| Selective reporting (reporting bias) | Unclear risk | Inadequate information to address |
Leuppi 2013.
| Methods | STUDY DESIGN: parallel LOCATION, NUMBER OF CENTRES: Switzerland, recruited in 5 teaching hospitals DURATION OF STUDY: 14 days, follow‐up 180 days |
|
| Participants | N SCREENED: 717 N RANDOMISED: 314 N COMPLETED: 296 M = 188, F = 123 BASELINE DETAILS: Gender F: SCS ≤ 7 days 33%/SCS > 7 days 47% (P value 0.02) CURRENT SMOKERS: SCS ≤ 7 days 49%/SCS > 7 days 40% LTOT: SCS ≤ 7 days 16%/SCS > 7 days 11% AGE: SCS ≤ 7 days: 69.8 (11.3)/SCS > 7 days 69.8 (10.6) FEV1 % predicted: SCS ≤ 7 days 32 (SD 15)/SCS > 7 days 31 (SD 13) PYH SMOKING median years: SCS ≤ 7 days 50/SCS > 7 days 45 INCLUSION CRITERIA: age > 40 years, smoking history ≥ 20 pack‐years EXCLUSION CRITERIA: history of asthma, FEV1/FVC ratio > 0.7 (post bronchodilator), radiological diagnosis of pneumonia, estimated survival < 6 months (due to severe co‐morbidity), pregnancy or lactation, inability to give written consent |
|
| Interventions | SCS ≤ 7 days: 5 days: day 1: IV methylprednisolone 40 mg, days 2 to 5: oral prednisolone 40 mg, days 6to 14: placebo SCS > 7 days: 14 days: day 1: IV methylprednisolone 40 mg, days 2 to 14: oral 40 mg prednisolone Co‐interventions:
Physiotherapy, supplemental oxygen and ventilator support (administered according to international guidelines) Additional glucocorticoids administered at the discretion of treating physicians TREATMENT PERIOD: 5 days vs 14 days TREATMENT SETTING: inpatient after presentation to ED. Treated as outpatients after discharge direct from ED; INT: 12 participants (7.7%); CONTROL: 13 participants (8.4%) FOLLOW‐UP PERIOD: 180 days |
|
| Outcomes | FEV1 % predicted Adverse effects: (infection, hyperglycaemia, hypertension, other) Relapse Duration of hospital stay Mortality Quality of life (bronchitis‐associated quality‐of‐life score, 0 to 6 = worst) Patient‐reported overall performance (visual analogue scale) Dyspnoea (Medical Research Council Dyspnoea score 1 to 5 = worst) |
|
| Notes | More women in the short‐term arm (46.5% vs 32.7%; P value 0.02). ISRCTN19646069. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned to one of two treatment regimens using a centralised, computerbased randomisation procedure with fixed blocks of 6. Study subjects randomised after stratification. |
| Allocation concealment (selection bias) | Low risk | Each study subject randomly assigned to receive a prepared set of study medication, consisting of two drug vials. The drugs prepared prior to the initiation of the study and packed by the Pharmacology Department, University Hospital, Basel, according to a randomisation list. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients, caregivers, outcome assessors, data collectors, the biostatistician, and all other investigators remained blinded to group allocation until the primary analysis was completed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three patients excluded after randomisation in a blinded fashion because of erroneous initial COPD diagnoses. Data from remaining 311 patients were used for intention‐to‐treat analyses. 296 patients completed 14‐day treatment period according to study protocol and were included in the perprotocol analysis. |
| Selective reporting (reporting bias) | Low risk | Prior to its initiation, the study was registered (ISRCTN19646069). The protocol summary published on the LANCET website (http://www.thelancet.com/protocol‐reviews/05PRT‐17), planned outcomes reported. |
Rahman 2004.
| Methods | STUDY DESIGN: parallel LOCATION, NUMBER OF CENTRES: Bangladesh, single tertiary‐care centre DURATION OF STUDY: 14 days |
|
| Participants | N SCREENED: not stated N RANDOMISED: not stated N COMPLETED: not stated M = not stated F = not stated AGE: claimed no significant difference between groups BASELINE DETAILS: claimed no significant difference between groups INCLUSION CRITERIA: not stated EXCLUSION CRITERIA: not stated |
|
| Interventions | SCS ≤ 7 days: 7‐day group received oral prednisolone 30 mg/d for 7 days SCS > 7 days: 14‐day group administered 30 mg/d oral prednisolone for 14 days Co‐interventions: not stated TREATMENT PERIOD: 7 days vs 14 days FOLLOW‐UP PERIOD: not stated |
|
| Outcomes | FEV1, FVC, symptom score (specific symptoms not stated) | |
| Notes | No reply from study author to request for more information about study and results | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Claimed to be single blind; however did not state whether 7‐day group received placebo for the extra 7 days |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
Salam 1998.
| Methods | STUDY DESIGN: parallel LOCATION, NUMBER OF CENTRES: unclear DURATION OF STUDY: 10 days |
|
| Participants | N SCREENED: not stated N RANDOMISED: 21 N COMPLETED: not clear M = no details available F = no details available AGE: no details available BASELINE DETAILS: study authors stated no significant differences between groups at baseline INCLUSION CRITERIA: patients admitted with an exacerbation of COPD who had not received steroids within the preceding month EXCLUSION CRITERIA: not stated |
|
| Interventions | SCS ≤ 7 days: 3 days of IV CS dose not stated and 7 days of placebo SCS > 7 days: 3 days of intravenous (IV) CS and 7 days oral CS dose not stated Co‐interventions: not stated TREATMENT PERIOD: 10 days vs 3 days FOLLOW‐UP PERIOD: not stated |
|
| Outcomes | FEV1, FVC, dyspnoea, cough | |
| Notes | Request to study author for more information about the study and results—no data supplied | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Yes, but more information required to decide if adequate |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Not stated |
Sayiner 2001.
| Methods | STUDY DESIGN: parallel LOCATION, NUMBER OF CENTRES: Izmir, Turkey. 1 tertiary centre DURATION OF STUDY: 6 months |
|
| Participants | N SCREENED: 198 N RANDOMISED: 36 N COMPLETED: 34 (SCS ≤ 7 n = 17; SCS > 7 n = 17) M = SCS ≤ 7: 16, SCS > 7: 16 F = SCS ≤ 7: 1, SCS > 7: 1 AGE: SCS ≤ 7: 67.4 SCS > 7: 64.1 BASELINE DETAILS: no significant differences between the 2 groups for age, duration of COPD, smoking history, blood eosinophilia, baseline levels of FEV1 Number of exacerbations within 12 months (31 participants): no differences between groups LTOT SCS ≤ 7: 5 (29%); SCS > 7: 2 (12%) Prior medication: ipratropium bromide, long‐acting beta2‐agonists and theophylline INCLUSION CRITERIA: current or ex‐smokers with a smoking history > 20 pack‐years and severe airway obstruction (FEV1 < 35% predicted), who presented with an exacerbation necessitating hospitalisation EXCLUSION CRITERIA: personal or family history of asthma, atopy, allergic disease, presence of eosinophilia, use of systemic steroids within the preceding month, presence of severe hypertension, uncompensated congestive heart failure or uncontrolled (or difficult to control) diabetes mellitus and respiratory failure necessitating mechanical ventilation therapy |
|
| Interventions | SCS ≤ 7: methylprednisolone, 0.5 mg/kg IV 6‐hourly for 3 days, followed by normal saline solution as placebo treatment IV twice daily for the following 3 days and once daily for the final 4 days SCS > 7: methylprednisolone, 0.5 mg/kg IV 6‐hourly for the first 3 days, followed by 0.5 mg/kg 12‐hourly for 3 days and 0.5 mg/kg once daily for 4 more days (total 10 days) Co‐interventions: all given high doses of inhaled beta2‐agonists, ipratropium bromide, theophylline (dose determined according to serum level measurements), antibiotics when indicated (presence of increased dyspnoea, sputum volume and sputum purulence) for 10 days, and H2‐receptor antagonists for 10 days, during which they received the study medication TREATMENT PERIOD: 10 days. All participants remained hospitalised for at least 10 days FOLLOW‐UP PERIOD: following 6 months |
|
| Outcomes | FEV1and PaO2 levels on day 3 and day 10, absolute group means Arterial blood gas levels day 1, day 3, day 5, day 7 and day 10 (arterial blood gases taken on room air after cessation of supplemental oxygen 30 minutes before, if the participant was on oxygen) Symptom scores (dyspnoea, cough) Recurrence of exacerbation in the following 6 months (collected by review of participants' records) Adverse events. Symptom scores (using 7‐point scale on which higher scores represented better function) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence was generated before recruitment of participants. This was done by allocating study participants to odd numbers and the control group to even numbers Randomisation code was broken only after all data were analysed and statistical analysis was performed |
| Allocation concealment (selection bias) | Low risk | Randomisation code was set up and sealed opaque envelopes were prepared before study initiation by one of the study authors (AS), who also prepared the study medications along with the head nurse |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind study: participants and data collectors blinded Only the principal investigator (AS) and the head nurse were informed about the randomisation list Participants were blinded to the nature of the IV medications. On inclusion of a participant in the study, the related envelope was opened and the drug package was given to the nurse in charge of treatment Physician/investigator who followed the participant, recorded arterial blood gas results, performed bedside spirometry and assessed symptom scores remained blinded to the study medication |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 withdrawals. 1 from each group, reason given |
| Selective reporting (reporting bias) | Low risk | Days 5 and 7 spirometry and ABG results not included. Reason:A comparison of 3 days vs 10 days of treatment was chosen for the 2 groups because these times were roughly the treatment duration in previous studies showing that steroids were effective. Both regimens were of relatively short duration, and the potential for adverse events was taken into account |
Sirichana 2008.
| Methods | STUDY DESIGN: parallel LOCATION, NUMBER OF CENTRES: Thailand, single tertiary‐care centre (King Chulalongkorn Memorial Hospital) DURATION OF STUDY: 30 days |
|
| Participants | N SCREENED: 107 N RANDOMISED: 48 N COMPLETED: 42 M = SCS ≤ 7: 22; SCS > 7: 20 F = SCS ≤ 7: 3; SCS > 7: 3 AGE: SCS ≤ 7: 72.45 ± 9.62 years; SCS > 7: 73.39 ± 9.16 years BASELINE DETAILS: not stated INCLUSION CRITERIA: participant with COPD who presented with acute exacerbation and age > 40 years Definition of acute exacerbation of COPD: at least 2 of 3 of these symptoms: increased dyspnoea, increased sputum volume, increased purulent sputum Persistence of symptoms at least 24 hours EXCLUSION CRITERIA: patient with history of asthma, atopic, allergy, bronchiectasis, lung cancer, pneumonia Respiratory failure Previously used systemic corticosteroids within preceding 30 days Poorly controlled DM, severe infection |
|
| Interventions | SCS ≤ 7: 5‐day group received prednisolone 30 mg/d for 5 days SCS > 7: 10‐day group was administered 30 mg/d prednisolone for 10 days Co‐interventions: not stated TREATMENT PERIOD: 5 days vs 10 days FOLLOW‐UP PERIOD: 30 days |
|
| Outcomes | FEV1, FVC, IC, clinical symptoms, fasting plasma glucose and CRP Recurrent exacerbation within 30 days | |
| Notes | Investigators supplied group mean data for FEV1 (L), FVC (L), IC (L), fasting plasma glucose(mg/dL), CRP (mg/L) and recurrent exacerbation within 30 days | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes used |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants, investigators, outcome assessors not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of participants in group 1 was 25, and the number completing the study was 19 1 participant was reported to withdraw Unknown why the other 5 did not complete the study |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
Wood‐Baker 1997.
| Methods | STUDY DESIGN: parallel 3‐group design
LOCATION, NUMBER OF CENTRES: hospitalised patients, 2 centres in Australia/New Zealand DURATION OF STUDY: 2 weeks |
|
| Participants | N SCREENED: 47 N RANDOMISED: 38 N COMPLETED: 28 (20 withdrawals, 1 death) M = 24 F = 14 AGE: SCS ≤ 7: 69.3, SD 5.5: SCS > 7: 71.1, SD 9.7 BASELINE DETAILS: not stated INCLUSION CRITERIA: > 40 years of age; > 10 pack‐year smoking history; FEV1 < 50% of predicted value EXCLUSION CRITERIA: long‐term corticosteroid therapy > 5 mg prednisolone per day or taking oral prednisolone for the current exacerbation; other co‐existent lung disease, including pneumonia; previous adverse reaction to corticosteroids; inability to cooperate with investigators; endoscopically or radiographically proven peptic ulcer disease within the past 2 years; history of cardiac failure, current hepatic or renal failure; inadequately treated hypertension |
|
| Interventions | SCS ≤ 7: prednisolone 2.5 mg/kg orally daily for 3 days followed by 11 days placebo SCS > 7: prednisolone 0.6 mg/kg orally daily for 7 days followed by prednisolone 0.3 mg/kg orally daily for 7 days Co‐interventions: inhaled bronchodilators, oral antibiotics, oral/intravenous xanthines and oxygen | |
| Outcomes | Measured: spirometry, 6‐minute walking distance, duration of hospitalisation, PaO2, PaCO2, visual analogue scale of breathlessness (in mm on a 100‐mm scale, more breathless represented by a higher number), treatment failure (no definition), mortality, adverse events Reported or data available: spirometry, 6‐minute walking distance, duration of hospitalisation, visual analogue scale of breathlessness; mortality: 1 death in the placebo arm Morbidity: no adverse biochemical effects | |
| Notes | Study stopped early after 36 months because of poor enrolment. Results published as abstract, and study data available to review authors. Data analysed by review authors in 3 groups. Data from 3‐day and 14‐day treatment groups used in the review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers table |
| Allocation concealment (selection bias) | Unclear risk | information not available |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants blinded to allocation until completion |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Full data on outcomes listed in protocol are available |
| Selective reporting (reporting bias) | Low risk | None identified |
ABG: arterial blood gas; COPD: chronic obstructive pulmonary disease; CRP: C‐reactive protein; CS: corticosteroid; ED: emergency department; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; GOLD: Global Strategy for Diagnosis, Management, and Prevention of COPD; IC: inspiratory capacity; INT: intervention; LTOT: long‐term oxygen therapy; PaCO2: partial pressure of carbon dioxide in arterial blood; PaO2: partial pressure of oxygen in arterial blood; PEF: peak expiratory flow; SCS: systemic corticosteroid; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aaron 2003 | Did not assess different durations of corticosteroid treatment |
| Abroug 2014 | Compared systemic corticosteroids vs placebo |
| Albert 1980 | Compared intravenous methylprednisolone vs placebo for 3 days in exacerbations of COPD |
| Albert 2008 | Article was a comment on RCT of carbocysteine |
| Ardestani 2017 | Compared two courses of IV steroids over the same duration |
| Brusse‐Keizer 2014 | Compared amoxicillin/clavulanic acid vs placebo |
| Ceviker 2014 | Compared oral vs parenteral steroids |
| Cordero 1996 | Compared systemic corticosteroids vs placebo |
| Courtney 2003 | Compared oral steroid vs placebo in stable COPD |
| Davies 1999 | Compared oral steroid vs placebo in exacerbations of COPD |
| De Jong 2007 | Compared oral vs IV corticosteroid given for 5 days |
| Ding 2016 | Compared neublised vs parenteral steroids |
| GlaxoSmithKline 2005 | Study of stable COPD not exacerbation |
| Kalkoti 2014 | Compared Deriphyllin + standard treatment vs standard treatment. |
| Li 2003 | Comparison of methylprednisolone vs dexamethasone in the treatment of patients with acute exacerbations of COPD |
| Makarova 2016 | Compared neublised vs IV steroids |
| McCrory 2000 | Article was a letter on a study by Bullard 1996 |
| Odonchimeg 2015 | Compared MDI budesonide vs nebulised budesonide vs IV prednisolone |
| Sharma 2014 | Commentary on a study by Abroug 2014 |
| Sun 2015 | Compared IV hydrocortisone vs placebo |
| Sun 2015a | Compared inhaled budesonide vs IV methylprednisolone |
| Woods 2014 | Systematic review of systemic corticosteroids in AECOPD |
| Yilmazel Ucar 2014 | Compared IV methylprednisolone with two strengths of neublised budesonide |
Characteristics of ongoing studies [ordered by study ID]
Leuppi 2017.
| Trial name or title | Reduction of Corticosteroid Use in Outpatient Treatment of Exacerbated COPD (RECUT) |
| Methods | Prospective, randomized, double‐blind, placebo‐controlled, non‐inferiority trial in an out‐patient setting |
| Participants | Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | Intervention group 1: Three days prednisolone 40mg, two days placebo Intervention group 2: Five days prednisolone 40mg |
| Outcomes | Primary:
Secondary:
|
| Starting date | March 2015 (Recruiting) |
| Contact information | Prof. Joerg Leuppi. +41 61 925 21 80 joerg.leuppi@ksbl.ch Kantonsspital Baselland, Liestal, BL, Switzerland, 4410 |
| Notes |
Reid 2010.
| Trial name or title | A randomised double blind study of short course systemic steroids versus conventional 14 days course in acute exacerbations of COPD |
| Methods | Randomised controlled trial, blinded, parallel |
| Participants | Inclusion criteria: All patients admitted to the Royal Hobart Hospital with the primary diagnosis of acute exacerbation of COPD No age limit, males and females Exclusion criteria:
|
| Interventions | Intervention group 1: prednisolone 0.5 mg/kg for 14 days Intervention group 2: prednisolone of 1 mg/kg for 3 days (max 50 mg) + 11 days placebo Control group: placebo |
| Outcomes | Length of stay in hospital, rate of treatment failure, symptom score, lung function tests, sputum cellularity |
| Starting date | 07/07/2007. Recruiting not completed |
| Contact information | Dr David Reid, Prof EH Walters. Menzies Research Institute, Tasmania, Hobart TAS 7001, Australia. +61 3 62267043; D.E.C.Reid@utas.edu.au |
| Notes |
COPD: chronic obstructive pulmonary disease; CS: corticosteroid; GP: general practitioner; ICS: inhaled corticosteroid.
Differences between protocol and review
Adverse events were included as a primary outcome, and the order of the secondary outcomes was changed. Methods were updated to apply the latest intervention methods, as required for a Cochrane review, and a 'Summary of findings' table was included.
Contributions of authors
JAE Walters contributed to searches and study selection, data extraction, data entry and analysis and interpretation of results. D Tan and C White contributed to searches and study selection, data extraction, data entry and analysis and interpretation of results. This review was drafted and revised by JAE Walters, and D Tan, C White and R Wood‐Baker contributed to revisions.
Sources of support
Internal sources
-
Breathe Well Centre of Research Excellence, University of Tasmania, Australia.
Logistical support
External sources
-
NHMRC, Australia.
Funding support for the Australian Satellite of the Cochrane Airways Group
Declarations of interest
R Wood‐Baker was chief investigator in a study contributing data to this review. Data from this study were verified and analysed by JAE Walters.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Chen 2005 {published and unpublished data}
- Chen G, Xie C. The efficacy and treatment length of oral corticosteroids in patients with acute exacerbations of chronic obstructive pulmonary disease [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 1966. [Google Scholar]
- Chen G, Xie CM, Luo YF. [The effects and therapeutic duration of oral corticosteroids in patients with acute exacerbation of chronic obstructive pulmonary diseases]. [Chinese]. Chinese Journal of Tuberculosis and Respiratory Diseases [Chung‐Hua Chieh Ho Ho Hu Hsi Tsa Chih] 2008;31(8):577‐80. [PubMed] [Google Scholar]
Gomaa 2008 {published data only (unpublished sought but not used)}
- Gomaa M, Faramawy M, Ibrahim H. Duration of systemic corticosteroids treatment in COPD exacerbations [Abstract]. European Respiratory Society 18th Annual Congress; 2008 Oct 3‐7; Berlin. 2008:[P3601].
Leuppi 2013 {published data only}
- Leuppi JD, Schuetz P, Bingisser R, Bodmer M, Briel M, Drescher, et al. Short‐term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA 2013;309(21):2223‐31. [DOI] [PubMed] [Google Scholar]
- Schuetz P, Leuppi JD, Bingisser R, Bodmer M, Briel M, Drescher T, Duerring U, Henzen C, Leibbrandt Y, Maier S, Miedinger D, Mueller B, Scherr A, Schindler C, Stoeckli R, Viatte S, Garnier C, Tamm M, Rutishauser J. Prospective analysis of adrenal function in patients with acute exacerbations of COPD: the Reduction in the Use of Corticosteroids in Exacerbated COPD (REDUCE) trial. Eur J Endocrinol 2015;173(1):19‐27. [DOI] [PubMed] [Google Scholar]
- Schuetz P, Leuppi JD, Tamm M, Briel M, Bingisser R, Dürring U, et al. Short versus conventional term glucocorticoid therapy in acute exacerbation of chronic obstructive pulmonary disease—the "REDUCE" trial. Swiss Medical Weekly 2011;140(18 October 2010):w13109. [DOI] [PubMed] [Google Scholar]
Rahman 2004 {published data only (unpublished sought but not used)}
- Abdullah Al Mamun SM, Rahman S. Role of 7‐day and 14‐day courses of oral prednisolone treatment in acute exacerbation of COPD [Abstract]. Thorax 2011;66(Suppl 4):A172. [Google Scholar]
- Rahman M, Abdullah A, Mamun SM, Haque MD. Role of 7‐day and 14‐day courses of oral prednisolone treatment in acute exacerbation of COPD [Abstract]. Chest 2004;126(4 Suppl):839S‐a. [Google Scholar]
Salam 1998 {published data only (unpublished sought but not used)}
- Salam T, Akers SM, Lotano R, Arnold GK, Bartter T, Pratter MR, et al. Optimal duration of corticosteroid therapy in the treatment of exacerbations of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Suppl):A801. [Google Scholar]
Sayiner 2001 {published data only}
- Sayiner A, Aytemur ZA, Cirit M, Unsal I. Systemic glucocorticoids in severe exacerbations of COPD. Chest 2001;119(3):726‐30. [DOI] [PubMed] [Google Scholar]
Sirichana 2008 {published and unpublished data}
- Sirichana W, Sittipunt C, Kawkitinarong K, Wongtim S. Comparison between 5 days and 10 days of prednisolone in treatment of acute exacerbation of chronic obstructive pulmonary disease [Abstract]. Respirology 2008;13(Suppl 5):A120 [012‐01]. [Google Scholar]
Wood‐Baker 1997 {published and unpublished data}
- Wood‐Baker R, Wilkinson J, Pearce M, Ryan G. A double‐blind, randomised, placebo‐controlled trial of corticosteroids for acute exacerbations of chronic obstructive pulmonary disease [Abstract]. Australian & New Zealand Journal of Medicine 1998;28:262. [Google Scholar]
References to studies excluded from this review
Aaron 2003 {published data only (unpublished sought but not used)}
- Aaron SD, Vandemheen KL, Hebert P, Dales R, Stiell IG, Ahuja J, et al. Outpatient oral prednisone after emergency treatment of chronic obstructive pulmonary disease [comment]. New England Journal of Medicine 2003;348(26):2618‐25. [DOI] [PubMed] [Google Scholar]
Abroug 2014 {published data only}
- Abroug F, Ouanes‐Besbes L, Fkih‐Hassen M, Ouanes I, Ayed S, Dachraoui F, Brochard L, Elatrous S. Prednisone in COPD exacerbation requiring ventilatory support: an open‐label randomised evaluation. Eur Respir J 2014;43(3):717‐24. [DOI] [PubMed] [Google Scholar]
Albert 1980 {published data only}
- Albert RK, Martin TR, Lewis SW. Controlled clinical trial of methylprednisolone in patients with chronic bronchitis and acute respiratory insufficiency. Annals of Internal Medicine 1980;92(6):753‐8. [DOI] [PubMed] [Google Scholar]
Albert 2008 {published data only}
- Albert P, Calverley P. A PEACE‐ful solution to COPD exacerbations? [comment]. Lancet 2008;371(9629):1975‐6. [DOI] [PubMed] [Google Scholar]
Ardestani 2017 {published data only}
- Ardestani E, Kalantary E, Samaiy, Taherian K. Methyl prednisolone vs dexamethasone in management of COPD exacerbation; a randomized clinical trial. Emergency 2017;5(1):195‐200. [PMC free article] [PubMed] [Google Scholar]
Brusse‐Keizer 2014 {published data only}
- Brusse‐Keizer M, VanderValk P, Hendrix R, Kerstjens H, Palen J. Necessity of amoxicillin clavulanic acid in addition to prednisolone in mild‐to‐moderate COPD exacerbations. BMJ open respiratory research 2014;1(1):e000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ceviker 2014 {published data only}
- Ceviker Y, Sayiner A. Comparison of two systemic steroid regimens for the treatment of COPD exacerbations. Pulm Pharmacol Ther Apr 2014;27(2):179‐83. [DOI] [PubMed] [Google Scholar]
Cordero 1996 {published data only}
- Cordero PJ, Benlloch E, Solè A, Morales P, Menèndez R, Cebri·NJ. Corticosteroid therapy for COPD exacerbations at inpatient settings: a controlled‐randomized study. Archivos de Bronconeumologia 1996;32(Suppl 2):17. [Google Scholar]
- Cordero PJ, Solè A, Benlloch E, Morales P, Vallterra J, Menèndez R, et al. A randomized controlled study of prednisone in outpatients with acute exacerbation of COPD. European Respiratory Journal Supplement 1996;9 Suppl 23:110s‐1s. [Google Scholar]
Courtney 2003 {published data only}
- Courtney AU. Oral prednisone prevents relapse in COPD exacerbations. Journal of Family Practice 2003;52(10):762‐4. [PubMed] [Google Scholar]
Davies 1999 {published data only}
- Davies L, Angus R, Mand Calverley PMA. A prospective, randomised, double‐blind, placebo controlled study of oral corticosteroids in patients admitted with normocapnic acute exacerbations of chronic obstructive pulmonary disease (COPD). Thorax 1997;52(Suppl 6):A5 S18. [Google Scholar]
- Davies L, Angus RM, Calverley PM. Oral corticosteroids in patients admitted to hospital with exacerbations of chronic obstructive pulmonary disease: a prospective randomised controlled trial. Lancet 1999;354(9177):456‐60. [DOI] [PubMed] [Google Scholar]
De Jong 2007 {published data only}
- Jong YP, Uil M, Grotjohan P, Postma S, Kerstjens M, Berg JWK. A comparison of intravenous versus oral administration of corticosteroids in the treatment of patients admitted to the hospital with an exacerbation of COPD [Abstract]. European Respiratory Journal 2004;24(Suppl 48):64s. [Google Scholar]
- Jong YP, Uil SM, Grotjohan HP, Postma DS, Kerstjens HA, Berg JW. Oral or IV prednisolone in the treatment of COPD exacerbations: a randomized, controlled, double‐blind study.[see comment]. Comment in: Chest. 2007 Dec;132(6):1728‐9; PMID: 18079214. Chest 2007;132(6):1741‐7. [DOI] [PubMed] [Google Scholar]
Ding 2016 {published data only}
- Ding Z, Li X, Lu Y, Rong G, Yang R, Zhang R, Wang G, Wei X, Ye Y, Qian Z, Liu H, Zhu D, Zhou R, Zhu K, Ni R, Xia K, Luo N, Pei C. A randomized, controlled multicentric study of inhaled budesonide and intravenous methylprednisolone in the treatment on acute exacerbation of chronic obstructive pulmonary disease. Respiratory medicine 2016;121:39‐47. [DOI] [PubMed] [Google Scholar]
GlaxoSmithKline 2005 {published data only}
- GlaxoSmithKline. A single centre, randomised, double‐blind, parallel group study to compare the efficacy of nebulised fluticasone propionate 2 mg twice daily (bd) with placebo in patients with moderate to severe chronic obstructive pulmonary disease. GlaxoSmithKline Clinical Trial Register 2005.
Kalkoti 2014 {published data only (unpublished sought but not used)}
- Kalkoti V, Kanani NJ. A prospective study for evaluating the clinical efficacy of methylxanthines in patients of acute exacerbation of chronic obstructive pulmonary disease (AECOPD) admitted in a tertiary care hospital. Indian journal of pharmacology 2014;46(7 SUPPL. 1):S25. [Google Scholar]
Li 2003 {published data only}
- Li H, He G, Chu H, Zhao L, Yu H. A step‐wise application of methylprednisolone versus dexamethasone in the treatment of acute exacerbations of COPD. Respirology 2003;8(2):199‐204. [DOI] [PubMed] [Google Scholar]
- Li HP, He GJ, Chu HQ. The application of methylprednisolone in acute exacerbations of COPD. European Respiratory Journal 2000;16(Suppl 31):50s. [Google Scholar]
Makarova 2016 {published data only (unpublished sought but not used)}
- Makarova EV, Varvarina GN, Menkov NV, Tsapaeva MY, Lazareva ES, Kazatskaya ZhA, Novikov VV, Karaulov AV. Nebulized budesonide in the treatment of exacerbations of chronic obstructive pulmonary disease: Efficacy, safety, and effects on the serum levels of soluble differentiation molecules. Terapevticheskii arkhi 2016;88(3):24‐31. [DOI] [PubMed] [Google Scholar]
McCrory 2000 {published data only}
- McCrory DC, Hasselblad V. The results of a randomized controlled trial of hydrocortisone in acute exacerbation of COPD. American Journal of Emergency Medicine 2000;18(1):122. [DOI] [PubMed] [Google Scholar]
Odonchimeg 2015 {published data only}
- Odonchimeg P, Ichinnorov D, Sarantuyaa Zh, Tumur‐Ochir Ts, Choyzhamts G. Efficacy of different regimens of steroid therapy in patients with exacerbation of chronic obstructive pulmonary disease. Pulmonologiya 2015;25(1):58‐63. [Google Scholar]
Sharma 2014 {published data only}
- Sharma N, Venado A, Morrison J. Protocol‐based treatment of septic shock, fibrinolysis for submassive pulmonary embolism, and use of corticosteroids in acute exacerbations of chronic obstructive pulmonary disease requiring mechanical ventilation. Am J Respir Crit Care Med 2014;190(7):827‐8. [DOI] [PubMed] [Google Scholar]
Sun 2015 {published data only}
- Sun WP, Yuan GX, Hu YJ, Liao LZ, Fu L. Effect of low‐dose glucocorticoid on corticosteroid insufficient patients with acute exacerbation of chronic obstructive pulmonary disease. World Journal of Emergency Medicine 2015;6(1):34‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2015a {published data only}
- Sun X, He Z, Zhang J, Deng J, Bai J, Li M, Zhong X. Compare the efficacy of inhaled budesonide and systemic methylprednisolone on systemic inflammation of AECOPD. Pulmonary pharmacology & therapeutics 2015;31:111‐116. [DOI] [PubMed] [Google Scholar]
Woods 2014 {published data only}
- Woods JA, Wheeler JS, Finch CK, Pinner NA. Corticosteroids in the treatment of acute exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2014;9:421‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yilmazel Ucar 2014 {published data only}
- Yilmazel Ucar E, Araz O, Meral M, Sonkaya E, Saglam L, Kaynar H, Gorguner AM, Akgun M. Two different dosages of nebulized steroid versus parenteral steroid in the management of COPD exacerbations: a randomized control trial. Med Sci Monit 2014;20:513‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Leuppi 2017 {unpublished data only}
- Leuppi J. Reduction of Corticosteroid Use in Outpatient Treatment of Exacerbated COPD (RECUT). Clinicaltrials.gov 2015: NCT02386735.
Reid 2010 {unpublished data only}
- Reid D, Soltani A, Wood‐Baker R, Walters EH. Personal correspondence 2010.
Additional references
Agusti 2010
- Agusti A, Calverley PM, Celli B, Coxson HO, Edwards LD, Lomas DA, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respiratory Research 2010;11:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anthonisen 1987
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Annals of Internal Medicine 1987;106(2):196‐204. [DOI] [PubMed] [Google Scholar]
Bathoorn 2008
- Bathoorn E, Kerstjens H, Postma D, Timens W, MacNee W. Airways inflammation and treatment during acute exacerbations of COPD. International Journal of Chronic Obstructive Pulmonary Disease 2008;3(2):217‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buist 2007
- Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD study): a population‐based prevalence study. Lancet 2007;370(9589):741‐50. [DOI] [PubMed] [Google Scholar]
De Vries 2007
- Vries F, Bracke M, Leufkens HGM, Lammers JWJ, Cooper C, Staa TP. Fracture risk with intermittent high‐dose oral glucocorticoid therapy. Arthritis and Rheumatism 2007;56(1):208‐14. [DOI] [PubMed] [Google Scholar]
Decramer 1994
- Decramer M, Lacquet LM, Fagard R, Rogiers P. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. American Journal of Respiratory and Critical Care Medicine 1994;150(1):11‐6. [DOI] [PubMed] [Google Scholar]
Deeks 2008
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. www.cochrane‐handbook.org. The Cochrane Collaboration, 2008. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Donaldson 2002
- Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57(10):847‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Donaldson 2005
- Donaldson GC, Wilkinson TMA, Hurst JR, Perera WR, Wedzicha JA. Exacerbations and time spent outdoors in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2005;171:446‐52. [DOI] [PubMed] [Google Scholar]
Donaldson 2006
- Donaldson GC, Wedzicha JA. COPD exacerbations 1: epidemiology. Thorax 2006;61:164‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Effing 2009
- Effing TW, Kerstjens HAM, Monninkhof EM, Valk PDLPM, Wouters EFM, Postma DS, et al. Definitions of exacerbations: does it really matter in clinical trials on COPD?. Chest 2009;136(3):918‐23. [DOI] [PubMed] [Google Scholar]
Evans 2002
- Evans AT, Husain S, Durairaj L, Sadowski LS, Charles‐Damte M, Wang Y. Azithromycin for acute bronchitis: a randomised, double‐blind, controlled trial. Lancet 2002;359(9318):1648‐54. [DOI] [PubMed] [Google Scholar]
Falk 2008
- Falk JA, Minai OA, Mosenifar Z. Inhaled and systemic corticosteroids in chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society 2008;5(4):506‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
GOLD 2014
- GOLD. Global Strategy for the Diagnosis, Management and Prevention of COPD, 2014. http://www.goldcopd.org/.
Groenewegen 2001
- Groenewegen K, Schols A, Wouters E. Prognosis after hospitalization for acute exacerbations of COPD. European Respiratory Journal 2001;8(Suppl 33):209s. [Google Scholar]
Hardin 2014
- Hardin M, Cho M, McDonald ML, Beaty T, Ramsdell J, Bhatt S, et al. The clinical and genetic features of COPD‐asthma overlap syndrome. European Respiratory Journal 2014;44(2):341‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JD, Altman G. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins J, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hurst 2010
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal‐Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. New England Journal of Medicine 2010;363(12):1128‐38. [DOI] [PubMed] [Google Scholar]
Jones 2014
- Jones PW, Lamarca R, Chuecos F, Singh D, Agusti A, Bateman ED, et al. Characterisation and impact of reported and unreported exacerbations: results from ATTAIN. European Respiratory Journal 2014;epub ahead of print:doi:10.1183/09031936.00038814. [DOI] [PubMed] [Google Scholar]
Kiser 2014
- Kiser TH, Allen RR, Valuck RJ, Moss M, Vandivier RW. Outcomes associated with corticosteroid dosage in critically ill patients with acute exacerbations of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2014;189(9):1052‐64. [DOI] [PubMed] [Google Scholar]
Leidy 2010
- Leidy NK, Wilcox TK, Jones PW, Murray L, Winnette R, Howard K, et al. Development of the EXAcerbations of chronic obstructive pulmonary disease Tool (EXACT): a patient‐reported outcome (PRO) measure. Value in Health 2010;13(8):965‐75. [DOI] [PubMed] [Google Scholar]
Leidy 2011
- Leidy NK, Wilcox TK, Jones PW, Roberts L, Powers JH, Sethi S. Standardizing measurement of chronic obstructive pulmonary disease exacerbations. American Journal of Respiratory and Critical Care Medicine 2011;183(3):323‐9. [DOI] [PubMed] [Google Scholar]
Leidy 2014
- Leidy NK, Murray LT, Jones P, Sethi S. Performance of the EXAcerbations of Chronic Pulmonary Disease Tool Patient‐reported Outcome Measure in three clinical trials of chronic obstructive pulmonary disease. Annals of the American Thoracic Society 2014;11(3):316‐25. [DOI] [PubMed] [Google Scholar]
Lindenauer 2010
- Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA 2010;303(23):2359‐67. [DOI] [PubMed] [Google Scholar]
Lopez 2006
- Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, et al. Chronic obstructive pulmonary disease: current burden and future projections. European Respiratory Journal 2006;27(2):397‐412. [DOI] [PubMed] [Google Scholar]
Majumdar 2010
- Majumdar SR, Villa‐Roel C, Lyons KJ, Rowe BH. Prevalence and predictors of vertebral fracture in patients with chronic obstructive pulmonary disease. Respiratory Medicine 2010;104(2):260‐6. [DOI] [PubMed] [Google Scholar]
McEvoy 1996
- McEvoy CE, Niewoehner DE. Adverse effects of corticosteroid therapy for COPD. Chest 1997;111:732‐43. [DOI] [PubMed] [Google Scholar]
McKenzie 2003
- McKenzie DK, Frith PA, Burdon JG, Town GI. The COPDX Plan: Australian and New Zealand guidelines for the management of chronic obstructive pulmonary disease 2003. Medical Journal of Australia 2003;178(Suppl):S7‐S39. [DOI] [PubMed] [Google Scholar]
Murray 1997
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990‐2020: global burden of disease study. Lancet 1997;349(9064):1498‐504. [DOI] [PubMed] [Google Scholar]
NICE 2010
- National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease: national clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care [update 2010]. http://guidance.nice.org.uk/CG101 (accessed 18 July 2011).
Oostenbrink 2004
- Oostenbrink JB, Rutten‐van Molken MP. Resource use and risk factors in high‐cost exacerbations of COPD. Respiratory Medicine 2004;98:883‐91. [DOI] [PubMed] [Google Scholar]
Papi 2006
- Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. American Journal of Respiratory and Critical Care Medicine 2006;173(10):1114‐21. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rodriguez‐Roisin 2000
- Rodriguez‐Roisin R. Toward a consensus definition for COPD exacerbations. Chest 2000;117(5 Suppl 2):398S‐401S. [DOI] [PubMed] [Google Scholar]
Schermer 2002
- Schermer T, Chavannes N, Saris C, Akkermans R, Schayck C, Weel C. Exacerbations and associated health care cost in patients with chronic obstructive pulmonary disease in general practice. Results from the COOPT trial. European Respiratory Journal 2002;20(Suppl 38):398s. [Google Scholar]
Seemungal 2000
- Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2000;161(5):1608‐13. [DOI] [PubMed] [Google Scholar]
Sethi 2002
- Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. New England Journal of Medicine 2002;347(7):465‐71. [DOI] [PubMed] [Google Scholar]
Sethi 2008
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. New England Journal of Medicine 2008;359(22):2355‐65. [DOI] [PubMed] [Google Scholar]
Sherk 2000
- Sherk PA, Grossman RF. The chronic obstructive pulmonary disease exacerbation. Clinics in Chest Medicine 2000;21(4):705‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Soler‐Cataluna 2005
- Soler‐Cataluna JJ, Martinnez‐Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005;60(11):925‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Suissa 2012
- Suissa S, Dell'Aniello S, Ernst P. Long‐term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax 2012;67(11):957‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sullivan 2000
- Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest 2000;117:5s‐9s. [DOI] [PubMed] [Google Scholar]
Therapeutic Guidelines 2009
- Therapeutic Guidelines Ltd. Therapeutic Guidelines: Respiratory. Version 4. Melbourne: Therapeutic Guidelines Ltd, 2009. [Google Scholar]
Vestergaard 2007
- Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk in patients with chronic lung diseases treated with bronchodilator drugs and inhaled and oral corticosteroids. Chest 2007;132(5):1599‐607. [PUBMED: 17890464 ] [DOI] [PubMed] [Google Scholar]
Walters 2011
- Walters JA, Wang W, Morley C, Soltani A, Wood‐Baker R. Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD006897.pub2] [DOI] [PubMed] [Google Scholar]
Walters 2014
- Walters JAE, Tan DJ, White CJ, Gibson PG, Wood‐Baker R, Walters EH. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD001288.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weatherall 2009
- Weatherall M, Travers J, Shirtcliffe PM, Marsh SE, Williams MV, Nowitz MR, et al. Distinct clinical phenotypes of airways disease defined by cluster analysis. European Respiratory Journal 2009;34(4):812‐8. [DOI] [PubMed] [Google Scholar]
Wedzicha 2000
- Wedzicha JA. Oral corticosteroids for exacerbations of chronic obstructive pulmonary disease. Thorax 2000;55(Suppl 1):S23‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilkinson 2004
- Wilkinson TM, Donaldson GC, Hurst JR, Seemungal TA, Wedzicha JA. Early therapy improves outcomes of exacerbations of chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2004;169(12):1298‐303. [DOI] [PubMed] [Google Scholar]
Wouters 2003
- Wouters EFM. Economic analysis of the confronting COPD survey: an overview of results. Respiratory Medicine 2003;97(Suppl 3):S3‐S14. [DOI] [PubMed] [Google Scholar]


