Abstract
Background
Retinopathy of prematurity (ROP) is a vision‐threatening disease of preterm neonates. The use of beta‐adrenergic blocking agents (beta‐blockers), which modulate the vasoproliferative retinal process, may reduce the progression of ROP or even reverse established ROP.
Objectives
To determine the effect of beta‐blockers on short‐term structural outcomes, long‐term functional outcomes, and the need for additional treatment, when used either as prophylaxis in preterm infants without ROP, stage 1 ROP (zone I), or stage 2 ROP (zone II) without plus disease or as treatment in preterm infants with at least prethreshold ROP.
Search methods
We searched the Cochrane Neonatal Review Group Specialized Register; CENTRAL (in the Cochrane Library Issue 7, 2017); Embase (January 1974 to 7 August 2017); PubMed (January 1966 to 7 August 2017); and CINAHL (January 1982 to 7 August 2017). We checked references and cross‐references and handsearched abstracts from the proceedings of the Pediatric Academic Societies Meetings.
Selection criteria
We considered for inclusion randomised or quasi‐randomised clinical trials that used beta‐blockers for prevention or treatment of ROP in preterm neonates of less than 37 weeks' gestational age.
Data collection and analysis
We used the standard methods of Cochrane and the Cochrane Neonatal Review Group. We used the GRADE approach to assess the quality of evidence.
Main results
We included three randomised trials (N = 366) in this review. Two of these studies were at high risk of bias. All studies reported on prevention of ROP and compared oral propranolol with placebo or no treatment. We found no trials assessing beta‐blockers in infants with established stage 2 or higher ROP with plus disease.
In one trial, study medication was started after one week of life, i.e. prior to the first ROP screening. The other two trials included preterm infants if they had stage 2 or lower ROP without plus disease. Based on the GRADE assessment, we considered evidence to be of low quality for the following outcomes: rescue treatment with anti‐VEGF or laser therapy; and arterial hypotension or bradycardia requiring inotropic support. Evidence was of moderate quality for the following outcomes: progression to stage 2 with plus disease; progression to stage 3 ROP; and progression to stage 4 or 5 ROP.
Meta‐analysis of three trials (N = 366) suggested beneficial effects of oral beta‐blockers on the risk of requiring anti‐VEGF agents (typical risk ratio (RR) 0.32, 95% confidence interval (CI) 0.12 to 0.86; I² = 0%; typical risk difference (RD) −0.06, 95% CI −0.10 to −0.01; I² = 75%; number needed to treat for an additional beneficial outcome (NNTB) 18, 95% CI 14 to 84) and laser therapy (typical RR 0.54, 95% CI 0.32 to 0.89; typical RD −0.09, 95% CI −0.16 to −0.02; I² = 31%; NNTB 12, 95% CI 8 to 47). Meta‐analysis of two trials (N = 161) demonstrated a beneficial effect of oral beta‐blockers on progression to stage 3 ROP (typical RR 0.60, 95% CI 0.37 to 0.96; I² = 0%; typical RD −0.15, 95% CI −0.28 to −0.02; I² = 73%; NNTB 7, 95% CI 5 to 67). There was no significant effect of oral beta‐blockers on progression to stage 2 ROP with plus disease or to stage 4 or 5 ROP. Although meta‐analysis did not indicate a significant effect of beta‐blockers on arterial hypotension or bradycardia, propranolol dosage in one study was reduced by 50% in infants of less than 26 weeks' gestational age due to severe hypotension, bradycardia, and apnoea in several participants. Analyses did not indicate significant effects of beta‐blockers on complications of prematurity or mortality. None of the trials reported on long‐term visual impairment.
Authors' conclusions
Limited evidence of low‐to‐moderate quality suggests that prophylactic administration of oral beta‐blockers might reduce progression towards stage 3 ROP and decrease the need for anti‐VEGF agents or laser therapy. The clinical relevance of those findings is unclear as no data on long‐term visual impairment were reported. Adverse events attributed to oral propranolol at a dose of 2 mg/kg/d raise concerns regarding systemic administration of this drug for prevention of ROP at the given dose. There is insufficient evidence to determine the efficacy and safety of beta‐blockers for prevention of ROP due to high risk of bias in two included trials and the lack of long‐term functional outcomes. We would encourage researchers to conduct large, well‐designed trials to confirm or refute the role of beta‐blockers for prevention and treatment of ROP in preterm infants. Trials should report on long‐term visual impairment. Researchers should consider dose‐finding studies of systemic beta‐blockers and topical administration of beta‐blockers, in order to optimise drug delivery and minimise adverse events.
Plain language summary
Beta‐blockers for prevention and treatment of retinopathy of prematurity
Review question: We reviewed the evidence for the effect of beta‐blockers on retinopathy of prematurity (ROP) in preterm infants.
Background: Babies who are born early (preterm) are at risk of developing disordered growth of the blood vessels in the back of their eyes, a disease called retinopathy of prematurity. Severe stages of this condition may result in poor vision or even blindness. Early treatment of retinopathy may improve vision in the long term. Currently, laser therapy is the treatment of choice for severe stages of ROP. However, it is an invasive procedure requiring expert skills and anaesthesia, and is not available in all countries in the world. Safe and effective drugs to prevent the disease are thus desirable. Beta‐blockers are believed to be able to stop disordered growth of blood vessels in various parts of the body including the eye. Beta‐blockers are used in children to treat a variety of diseases and are generally well tolerated. Nevertheless, they also bear the risk of adverse effects like lowering heart rate and blood pressure. The main aim of this review was to find out whether beta‐blockers compared to placebo (an inactive drug) or no drug offer important advantages to preterm babies either by preventing severe stages of ROP or by treating the disease (when critical stages of ROP are already present).
Study characteristics: We examined the research published to 7 August 2017. We found three clinical trials recruiting 366 preterm babies. All three studies reported on preventing severe stages of retinopathy.
Key results: We found that orally administered beta‐blockers may offer short‐term benefits such as lower risk of progression to a more severe stage of retinopathy and less need for additional treatment. However, there were no data on long‐term vision; and studies did not show an effect of beta‐blockers on the most severe stages of retinopathy. On the other hand, serious adverse effects of beta‐blockers were reported in one of three studies.
Quality of evidence: The overall quality of evidence for outcomes in this review varied from low to moderate. Thus, our confidence in the results of this review is very limited. We cannot recommend routine use of beta‐blockers for prevention or treatment of ROP in preterm infants. Future high‐quality studies are necessary to determine whether benefits of beta‐blockers outweigh their risks in preventing or treating ROP in preterm infants.
Summary of findings
Summary of findings for the main comparison. Systemic administration of beta‐blockers compared to placebo or no treatment for prevention of retinopathy of prematurity in preterm infants.
| Systemic administration of beta‐blockers compared to placebo or no treatment for prevention of retinopathy of prematurity in preterm infants | ||||||
| Patient or population: Prevention of retinopathy of prematurity in preterm infants Setting: Neonatal clinical care units Intervention: Systemic beta‐blockers Comparison: Placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo or no treatment | Risk with Beta‐blockers | |||||
| Severe visual impairment or blindness at 6 to 12 months' corrected age | N/A | N/A | N/A | N/A | N/A | None of the studies reported on this outcome. |
| Rescue treatment with anti‐VEGF agents | 86 per 1000 | 27 per 1000 (10 to 74) | RR 0.32 (0.12 to 0.86) | 366 (3 RCTs) | ⊕⊕⊝⊝ LOW | Unblinded studies, incomplete outcome data, imprecision of point estimate, few events. |
| Treatment with laser photocoagulation or cryotherapy | 194 per 1000 | 105 per 1000 (62 to 173) | RR 0.54 (0.32 to 0.89) | 366 (3 RCTs) | ⊕⊕⊝⊝ LOW | Unblinded studies, incomplete outcome data, imprecision of point estimate. |
| Progression to stage 2 with plus disease | 38 per 1000 | 9 per 1000 (1 to 81) |
RR 0.25 (0.03 to 2.16) |
161 (2 RCTs) |
⊕⊕⊕⊝ MODERATE | Imprecision of point estimates, few events, 95% CI includes both 1) no effect and 2) appreciable benefit or appreciable harm. |
| Progression to stage 3 ROP | 375 per 1000 | 225 per 1000 (139 to 360) | RR 0.60 (0.37 to 0.96) | 161 (2 RCTs) | ⊕⊕⊕⊝ MODERATE | Imprecision of point estimate, few events. |
| Progression to stage 4 or 5 ROP | 50 per 1000 | 6 per 1000 (1 to 98) | RR 0.11 (0.01 to 1.96) | 161 (2 RCTs) | ⊕⊕⊕⊝ MODERATE | Few events, 95% CI includes both 1) no effect and 2) appreciable benefit or appreciable harm. |
| Arterial hypotension and/or bradycardia requiring treatment | 0 per 1000 | 0 per 1000 (0 to 0) | RR 24.35 (1.32 to 448.50) | 366 (3 RCTs) | ⊕⊕⊝⊝ LOW | Unblinded studies, few events. |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate — the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited — the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate — the true effect is likely to be substantially different from the estimate of effect | ||||||
Background
Description of the condition
Retinopathy of prematurity (ROP) is a vision‐threatening disease of preterm infants. Vascular endothelial growth factor (VEGF), a key factor in the pathogenesis of ROP, is essential for retinal blood vessel development and growth (Ferrara 1997). Inappropriately high retinal and vitreal VEGF levels seem to be important in the development of ROP. Retinopathy of prematurity proceeds in a biphasic fashion (Asthon 1954; Chen 2012; Mutlu 2013; Smith 2003). In the first phase, VEGF levels are reduced by a relatively hyperoxic environment, resulting in vessel obliteration. In the second proliferative phase, starting around 32 weeks' postmenstrual age (PMA), VEGF levels are elevated as a result of relatively hypoxic circumstances. This leads to neovascularisation in the retina with development of aberrant blood vessels from resident vasculature (Afzal 2007). These new aberrant vessels may grow into the vitreous body, causing retinal detachment and haemorrhage (Casini 2014; Hellström 2013). VEGF further interacts with other growth factors such as insulin‐like growth factor 1 (IGF‐1), which regulates expression and activation of VEGF in a positive feedback loop (Löfqvist 2006; Ristori 2011; Smith 1999). As VEGF plays an important role in neovascularisation, drugs that influence VEGF levels have been studied as treatment options for ROP. A protocol of a systematic review on anti‐VEGF drugs for treatment of ROP has been published (Sankar 2016).
A recently developed therapeutic approach for ROP focuses on use of beta‐adrenergic blocking agents (beta‐blockers), given that beta‐2 receptors are involved in the regulation of VEGF and IGF‐1 levels and play an important role in the pathogenesis of several neovascular retinal diseases (Casini 2014; Dal Monte 2013; Martini 2011).
Standard treatment for individuals at advanced stages of ROP includes laser photocoagulation and cryotherapy (ET‐ROP Group 2003). Despite the overall treatment success of such retinal ablation therapy, ROP has remained a major cause of potentially avoidable blindness and visual impairment among children worldwide, indicating the importance of new strategies for prevention and treatment of this disease (Blencowe 2013; Gilbert 2008).
Description of the intervention
Beta‐blockers, most commonly propranolol, have been suggested both for early prevention of ROP and for treatment of existing ROP in preterm neonates (summarised in Bührer 2015). Propranolol has been used for treatment of infantile haemangioma, for which it is thought to act by reducing VEGF levels (Léauté‐Labrèze 2008; Manunza 2010; Sans 2009). Similarities between regulation of growth of infantile haemangiomas and development of ROP have been postulated (Praveen 2009), and support the hypothesis that propranolol could be effective in the treatment of ROP due to beneficial effects on retinal neovascularisation.
Propranolol is used in children to treat a variety of diseases, including hypertension, cardiac arrhythmia, obstructive heart disease, thyrotoxicosis and migraine headache, and is generally well tolerated (Drolet 2013; Love 2004). Nevertheless, clinically relevant adverse events such as hypotension, bradycardia, heart block, hypoglycaemia and bronchospasm have been reported (Bonifazi 2010; de Graaf 2011; Fonseca 2010; Frishman 1988; Léauté‐Labrèze 2015). Published data on the use of propranolol in neonates are very limited. For a small number of cases of infantile haemangioma, systemic propranolol was given and was generally well tolerated, but harmful side effects such as hyperkalaemia and a single case of apnoea and bradycardia requiring resuscitation have been reported (Erbay 2010; Frost 2013; Pavlakovic 2010). Alternative routes of administration of propranolol have been considered to reduce the risk of adverse events. Recently, propranolol eye drops were applied in an animal model of ROP, resulting in high retinal and low plasma concentrations of propranolol (Padrini 2014).
Other beta‐blockers such as atenolol and timolol have been used for angioproliferative diseases in children, but valid data on their efficacy and safety profile in comparison with propranolol are not available (Abarzúa‐Araya 2014; Chakkittakandiyil 2012).
How the intervention might work
The current hypothesis suggests that beta‐blockers reduce the retinal expression of angioproliferative factors VEGF and IGF‐1 through blockade of beta‐adrenoreceptors (Ristori 2011). Animal studies on systemic propranolol application have yielded conflicting results, which have led to controversial discussions about the potential effects of propranolol in ROP (Chen 2012; Hård 2011; Ristori 2011). Ocular administration potentially reduces the risk of side effects as compared with systemic treatment (Dal Monte 2013; Padrini 2014).
Why it is important to do this review
Retinopathy of prematurity remains a significant complication of preterm birth, and non‐invasive treatment options are desirable. Beta‐blockers serve as a potential pharmacological intervention for preventing and treating preterm infants with ROP. Three narrative reviews on use of beta‐blockers for prevention and treatment of preterm infants with ROP have been published (Bührer 2015; Cavallaro 2014; Filippi 2014). No systematic reviews or meta‐analyses on this topic can be found in the literature. The use of beta‐blockers for prevention and treatment of ROP needs to be formally assessed in order to provide caregivers with clinically relevant data on their efficacy and safety.
The aim of this systematic review was to summarise current evidence on benefits and harms of beta‐blockers for prevention and treatment of ROP in preterm infants.
Objectives
For prevention studies, the primary objective was to determine whether early administration of beta‐blockers reduces the incidence and severity of ROP in preterm infants compared with placebo or no treatment. We classified studies as prevention trials if they enrolled preterm infants without ROP or with confirmed stage 1 ROP in zone I or stage 2 ROP without plus disease in zone II. We regarded trials with the aim of avoiding development of ROP as primary prophylaxis and trials with the aim of avoiding progression of ROP towards more severe stages as secondary prophylaxis.
For treatment studies, the primary objective of this review was to determine the effects of beta‐blockers compared with placebo or no treatment on severe visual impairment or blindness and on structural outcomes. We classified studies as treatment trials if they enrolled preterm infants with at least prethreshold ROP.
For both types of trials, the secondary objective was to determine the safety of beta‐blocker administration.
For both types of trials, we planned to perform subgroup analyses to consider whether gestational age (extremely preterm neonates < 28 weeks' gestational age, very preterm neonates 29 to 31 weeks' gestational age, preterm infants 32 to 36 weeks' gestational age) or specific beta‐blocking agents influence the outcomes of interventions.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐randomised clinical trials of beta‐blockers in preterm neonates were eligible for inclusion in the review. We planned to include parallel‐group but neither cross‐over nor cluster RCTs in this systematic review.
Types of participants
We considered preterm infants (< 37 weeks' gestation at birth) for inclusion in the review.
For prevention trials, infants without ROP or with confirmed stage 1 (zone I) or stage 2 ROP without plus disease (zone II) were eligible for inclusion in the review.
For treatment trials, we considered infants with at least prethreshold ROP for inclusion in the review.
For the purposes of this review, we planned to exclude studies that enrolled infants who had established retinal detachment before randomisation, or who had received anti‐VEGF treatment or retinal ablation therapy before randomisation.
Types of interventions
We considered the following interventions and control treatments for inclusion in this review.
Systemic administration of any beta‐blocker at any dose and duration versus placebo or no treatment.
Ocular administration of any beta‐blocker at any dose and duration versus placebo or no treatment.
Enrolled infants could receive intravitreal anti‐VEGF treatment, laser photocoagulation or cryotherapy, if threshold disease developed.
Types of outcome measures
Primary outcomes
Functional outcomes at six to 12 months' corrected age: severe visual impairment (acuity < 20/200) or blindness (acuity < 20/400) as defined by the World Health Organization (who.int/blindness/en/).
Need for additional treatment.
Anti‐VEGF agent.
Laser photocoagulation.
Cryotherapy.
Vitrectomy.
Structural outcomes (International Committee Retinopathy 2005).
-
For prevention trials.
Progression of ROP to stage 2 with plus disease.
Progression of ROP to stage 3 with or without plus disease.
Progression of ROP to stage 4 or 5 disease.
-
For treatment trials.
Progression of ROP to stage 4 or 5 disease.
-
Secondary outcomes
Functional outcomes at six to 12 months' corrected age include the following.
Amblyopia.
Nystagmus.
Refractive error in either eye.
Unfavourable structural outcomes, assessed at six to 12 months' corrected age, defined as follows (adapted from Andersen 2000).
Retinal fold involving the macula.
Retinal detachment involving zone I of the posterior pole.
Retrolental tissue or 'mass' obscuring the view of the posterior pole.
Early childhood unfavourable retinal structure, assessed at four to six years, defined as follows (adapted from Andersen 2000).
Retinal fold involving the macula.
Partial or complete cataract.
Partial retrolental membrane.
-
Obstructed view of macula from:
partial or complete cataract;
partial retrolental membrane;
partial or complete corneal opacity (due to ROP); or
enucleation from all causes.
-
Childhood mortality measured as:
death before discharge from primary hospital; or
death before one year of corrected age.
-
Adverse neurodevelopmental outcomes at 18 to 24 months' corrected age.
Cerebral palsy.
Moderate to severe developmental delay as assessed by validated neurodevelopmental tests such as Bayley Scales of Infant Development.
Complications of preterm birth, including:
intraventricular haemorrhage (IVH) grade 1 to 4 (Papile classification) (Papile 1978);
severe IVH grade 3 to 4 (Papile classification) (Papile 1978);
periventricular leukomalacia (PVL) (Mercier 2007; Valcamonico 2007);
mild, moderate or severe bronchopulmonary dysplasia (BPD) at 36 weeks' postmenstrual age (PMA) based on the classification suggested by Jobe and Bancalari (Jobe 2001) and calibrated by an oxygen reduction test, as described previously (Walsh 2003);
neurodevelopmental impairment (assessed at ≥ 12 months' corrected age by a validated scale, e.g. Griffiths or Bayley Scales of Infant Development) (Bayley 2006; Griffiths 1954; Mercier 2007); and
necrotising enterocolitis (NEC) ≥ stage 2 (modified Bell's criteria) (Bell 1978).
-
Potential adverse events, including:
hypotension requiring treatment with inotropic agents;
bradycardia (< 80 beats/min) requiring treatment with inotropic agents or endotracheal intubation, or both;
cardiac arrhythmia requiring treatment;
bronchospasm requiring treatment;
thrombocytopenia (platelet count < 100,000 × 10⁹/L);
hypoglycaemia (glucose level < 2.5 mmol/L); and
hyperkalaemia (potassium level > 6.2 mmol/L).
Search methods for identification of studies
We conducted systematic searches for randomised or quasi‐randomised controlled trials and considered only parallel‐group trials. We did not apply any language, publication year, or publication status restrictions.
Electronic searches
We searched the following sources: Cochrane Neonatal Review Group Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 7), PubMed (January 1966 to 7 August 2017), Embase (January 1974 to 7 August 2017), Cumulative Index to Nursing and Allied Health Literature (CINAHL) (January 1982 to 7 August 2017) and the Science Citation Index database (1984 to 7 August 2017).
Appendix 1 lists the search terms used.
Searching other resources
We checked references and cross‐references from identified studies and searched the Institute for Scientific Information (ISI) Web of Science using any known RCT as the starting point. We searched for unpublished studies from the proceedings of the Pediatric Academic Societies Meetings (from January 1990 to 7 August 2017) by hand‐ and online searching. We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; controlled‐trials.com and who.int/ictrp).
Data collection and analysis
We used the standard methods of Cochrane and the Cochrane Neonatal Review Group.
Selection of studies
Two review authors (SK and SMS) independently searched the literature and identified eligible trials as described above. We considered only RCTs and quasi‐RCTs that fulfilled the above criteria for inclusion in this review. We did not include studies published only in abstract form, unless final results of the trial had been reported and we could ascertain all necessary information from the abstract or the authors, or both. All review authors separately selected studies for inclusion. We resolved disagreements through discussion involving all review authors. We listed excluded studies in the 'Characteristics of excluded studies’ table, along with reasons for exclusion.
Data extraction and management
All review authors' independently extracted, assessed and coded data using standardised data extraction forms. One review author (SK) entered data into Review Manager 5 (Review Manager 2014). A second review author (SMS) checked data for accuracy. We reached consensus by discussion and contacted original study authors when questions arose or additional data were required.
Assessment of risk of bias in included studies
Two review authors (SK, SMS) independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane ‘Risk of bias’ tool (Higgins 2011) for the following domains.
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Any other bias.
Any disagreements were resolved by discussion or by a third assessor. See Appendix 2 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We performed statistical analyses using standard methods of the Cochrane Neonatal Review Group. We analysed the results of studies using Review Manager 5 software (Review Manager 2014); and presented results as risk ratios (RRs), risk differences (RDs), numbers needed to treat for an additional beneficial outcome (NNTBs) or numbers needed to treat for an additional harmful outcome (NNTHs) for categorical variables. We planned to use mean differences (MDs) for continuous variables. We replaced within‐group standard errors of the mean (SEMs) as reported in a trial by providing corresponding standard deviations (SDs) using the formula SD = SEM × √N. We reported 95% confidence intervals (CIs) for each statistic.
Unit of analysis issues
We included all RCTs and quasi‐RCTs in which the unit of randomisation and analysis was the individual infant. We did not include cluster RCTs.
Dealing with missing data
We addressed incomplete outcome data as mentioned above (Assessment of risk of bias in included studies). If outcomes specified in this review were measured but not reported, we contacted study authors to request such data.
Assessment of heterogeneity
We assessed the magnitude of heterogeneity of treatment effects using the I² statistic. I² (calculated as I² = 100% × (Q − df)/Q; where Q is the Cochrane heterogeneity statistic and df shows degrees of freedom) lies between 0% and 100%. I² cut‐offs and labels for heterogeneity were applied as follows.
I² less than 25% — no heterogeneity.
I² = 25% to 49% — low heterogeneity.
I² = 50% to 74% — moderate heterogeneity.
I² ≥ 75% — high heterogeneity.
In addition, we carefully inspected each forest plot for heterogeneity, as indicated by lack of overlapping CIs of individual trials.
Assessment of reporting biases
We intended to assess reporting and publication biases by examining the degree of asymmetry of a funnel plot in Review Manager 2014.
Data synthesis
We conducted statistical analyses according to recommendations of the Cochrane Neonatal Review Group (neonatal.cochrane.org/en/index.html) using Review Manager 5 software (Review Manager 2014). We used a fixed‐effect model to pool data for meta‐analyses and to analyse treatment effects in individual trials. We performed an analysis of all participants randomly assigned on an intention‐to‐treat basis. We used the Mantel‐Haenszel method for calculation of risk ratios (RRs) and risk differences (RDs). We planned to use the inverse variance method for measured quantities and standardised mean differences (SMDs) to combine trials that measure the same outcome using different methods with 95% CIs. For dichotomous outcomes, we estimated RRs with 95% CIs and NNTBs.
Quality of evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: severe visual impairment or blindness at six to 12 months' corrected age; the need for additional treatment (anti‐VEGF agents, laser photocoagulation, cryotherapy, vitrectomy); progression of ROP to stage 2 with plus disease or higher (prevention trials); and progression of ROP to stage 4 or 5 (treatment trials).
Two authors independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from randomised controlled trials as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate — the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited — the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate — the true effect is likely to be substantially different from the estimate of the effect.
Subgroup analysis and investigation of heterogeneity
We aimed to analyse potential sources of heterogeneity by performing prespecified subgroup analyses.
Potential subgroup analyses included the following.
Gestational age: extremely preterm neonates at less than 28 weeks' gestational age; very preterm neonates at 29 to 31 weeks' gestational age; preterm infants at 32 to 36 weeks' gestational age.
Specific beta‐blocking agents.
Sensitivity analysis
Differences in study design of included trials might affect the results of the systematic review. We aimed to explore the robustness of results for the primary outcome by performing sensitivity analysis based on methodological quality of included trials. For this, we aimed to exclude studies at high risk of bias. Additionally, we planned to perform a sensitivity analysis to compare the effects of beta‐blockers in truly randomised trials as opposed to quasi‐randomised trials.
Results
Description of studies
See: Characteristics of included studies
Results of the search
The search identified 27 reports (Figure 1). We detected six potentially eligible studies. Of these, three were excluded. Details of excluded studies along with the reasons for exclusion are listed in the 'Characteristics of excluded studies' table. Three studies were included in this review (Filippi 2013; Korkmaz 2017; Sanghvi 2017). Three ongoing trials were identified.
1.
Study flow diagram.
Included studies
Three studies incorporating a total of 366 preterm infants met inclusion criteria of this review (Filippi 2013; Korkmaz 2017; Sanghvi 2017). These trials were conducted as ROP prevention trials and included preterm infants diagnosed with stage 2 or lower ROP without plus disease (Filippi 2013; Korkmaz 2017) or preterm neonates in whom ROP was not assessed at enrolment but very unlikely to be present as they were less than 8 days old (Sanghvi 2017). A post hoc decision was made to accept eligibility of this study where the risk of ROP at entry was low but unknown. Details of included studies are given in the 'Characteristics of included studies' table. Of the three included trials, two trials were placebo‐controlled (Korkmaz 2017; Sanghvi 2017), while the third trial compared beta‐blocker administration to no treatment (Filippi 2013). All three included studies specified eligibility criteria for study participants.
Filippi 2013 was a two‐centre RCT conducted in Milan and Florence, Italy. Fifty‐two preterm infants with a gestational age of 23 to 31 weeks were enrolled immediately after detection of stage 2 ROP without plus disease. The mean postnatal age at study entry was 68 days. Infants were randomised to receive oral propranolol (1 mg/kg/d to 2 mg/kg/d) or no treatment. Propranolol was administered until complete retinal vascularisation and for a maximum of 90 days. Primary outcomes included progression to stage 2 ROP with plus disease and progression to stage 3 ROP. Secondary outcomes included progression to stage 4 or 5 ROP, incidence of laser therapy, rescue treatment with bevacizumab, need for vitrectomy, plasma VEGF, and sE‐selectin concentrations.
Korkmaz 2017 was a single‐centre study in preterm infants of less than 32 weeks' gestational age (mean gestational age 28 weeks) conducted in Kayseri, Turkey. This study enrolled 205 preterm infants diagnosed with stage 2 or lower ROP. They were randomly assigned to receive propranolol (2 mg/kg/d) or placebo (physiologic saline solution). Participants received study drug from 31 weeks' PMA at the earliest. Duration of treatment was not reported. Primary outcomes included the incidence of laser therapy and platelet mass index. Secondary outcomes were not defined.
Sanghvi 2017 was a two‐centre study in preterm neonates with a gestational age of 26 to 32 weeks, performed in Mumbai, India. This study enrolled 109 preterm neonates in the first week of life and randomly assigned them to oral propranolol (1 mg/kg/d) or placebo (calcium carbonate 1 mg/kg/d). Drug administration was started on day seven of life and continued until complete retinal vascularisation or 37 weeks' PMA (mean duration of drug administration, 32 days in the intervention group, 41 days in the control group). Primary outcome was all‐stage ROP. Further outcomes included the incidence of laser therapy, rescue treatment with anti‐VEGF agents, adverse events such as recurrent bradycardia, hypotension, hypoglycaemia, and visual outcome at 12 months' PMA.
Excluded studies
We excluded three identified studies from this review. Two trials were excluded because they were not randomised or quasi‐randomised trials (Bancalari 2016; Filippi 2016). The third trial was published as a research letter (Makhoul 2013). This trial was excluded because methodological details could not be fully clarified and 17/20 participants were lost to follow‐up. Details of excluded studies along with the reasons for exclusion are listed in the 'Characteristics of excluded studies' table.
Risk of bias in included studies
Overall, one trial was at low risk of bias (Sanghvi 2017); whereas the other two trials were at high risk of bias (Filippi 2013; Korkmaz 2017). Ratings of methodological quality are given in the 'Characteristics of included studies' table and are summarised in Figure 2.
2.
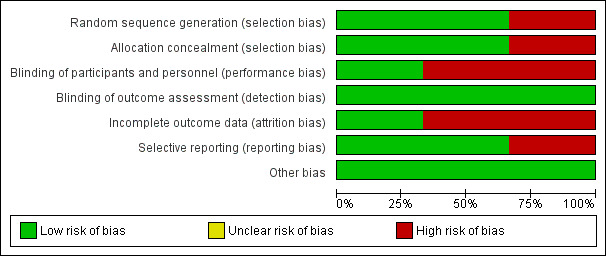
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation: Filippi 2013 and Sanghvi 2017 met this criterion. Randomisation procedure was not specified in Korkmaz 2017. Additional information obtained from the authors clarified that allocation method in Korkmaz 2017 was neither rigorous nor concealed.
Allocation concealment: this was deemed adequate in Filippi 2013. Additional information obtained from Sanghvi 2017 clarified that allocation method was concealed.
Blinding
Blinding of intervention: Filippi 2013 did not attempt to blind caregivers or parents towards the intervention. Additional information obtained from Korkmaz 2017 clarified that the intervention was unblinded. Blinding of caregivers and parents was deemed adequate in Sanghvi 2017.
Blinding of outcome assessments: all three trials masked assessors of primary outcomes (ophthalmologists), which was confirmed after obtaining additional information from two authors (Korkmaz 2017; Sanghvi 2017).
Incomplete outcome data
We assessed attrition bias as low risk in one trial (Sanghvi 2017); and as high risk in two trials (Filippi 2013; Korkmaz 2017). Reporting of outcomes was complete in Filippi 2013 as outcomes from 51/52 (98%) enrolled infants were reported. In 6/26 (23%) infants allocated to the intervention group, administration of beta‐blocker was discontinued early due to severe adverse effects. Three infants died prior to hospital discharge (5.7%); outcomes from one infant allocated to the intervention group, who died nine days after initiating treatment, were not reported. This was confirmed after obtaining additional information from the authors. The other two deceased infants had been allocated to the control group and had established stage 3 ROP with plus disease. Their data were included in the analysis. Korkmaz 2017 enrolled 205 preterm infants. A total of 34 infants (16.6%) were excluded after randomisation for various reasons. Outcome data were deemed incomplete due to unbalanced loss to follow‐up with the majority of exclusions occurring in the intervention group. In the intervention group, 27/110 (24.6%) participants were excluded; in the control group, 7/95 (7.4%) participants were excluded. Reasons for exclusion included irregularities in administration of study drug (n = 13, intervention group), parental request (n = 6, intervention group), potential adverse events such as bradycardia, apnoea, hypotension, hypoglycaemia, increasing respiratory support (n = 6, intervention group; n = 3, control group), and requirement of anti‐VEGF treatment (n = 2, intervention group; n = 4, control group). Additional information obtained from the authors clarified that outcome data from patients excluded after randomisation and study drug administration were not available. Sanghvi 2017, a trial of primary prophylaxis of ROP enrolling children less than 8 days of postnatal age, reported a mortality of 6.4% (7 of 109) prior to the first ROP screening. Primary outcome (all‐stage ROP) from these infants was thus not reportable. Complete outcome data were reported from the remaining 102 participants (94%).
Selective reporting
We assessed reporting bias as low risk in two out of three trials as they reported primary outcomes and main secondary outcomes (Filippi 2013; Sanghvi 2017), and as high risk in one trial (Korkmaz 2017). Filippi 2013 reported all outcomes specified in the report. Some of the secondary outcomes specified in the registered trial protocol (blindness and retinal detachment within six months from beginning of treatment were not reported). Sanghvi 2017 reported all outcomes specified in the report. Visual long‐term outcomes at 12 months' PMA were not prespecified in the trial protocol but reported. This trial was registered retrospectively . Korkmaz 2017 was not a registered trial and a trial protocol was not available.
Other potential sources of bias
None detected.
Effects of interventions
See: Table 1
See Table 1 for the comparison of beta‐blockers versus placebo or no treatment for prevention of ROP in preterm infants without ROP or prevention of ROP progression in infants with confirmed stage 1 or stage 2 ROP without plus disease. We found no eligible trials comparing beta‐blockers to placebo or no treatment in infants with established stage 2 or higher ROP with plus disease.
Primary outcomes
Functional outcomes at six to 12 months' corrected age
None of the included studies reported on severe visual impairment or blindness at six to 12 months' corrected age.
Need for additional treatment in prevention trials
Rescue treatment with anti‐VEGF agents (Outcome 1.1)
All three included studies reported on this outcome (Filippi 2013; Korkmaz 2017; Sanghvi 2017). Individual trials reported no difference in the rate of rescue treatment with anti‐VEGF agents between beta‐blocker and control groups. Meta‐analysis suggested a significant effect of beta‐blockers on rescue treatment with anti‐VEGF agents (typical RR 0.32, 95% CI 0.12 to 0.86; I² = 0%; typical RD −0.06, 95% CI −0.10 to −0.01; I² = 75%; N = 366 participants from three studies; NNTB 18, 95% CI 14 to 84) (Analysis 1.1). We graded the quality of evidence as low due to risk of bias and imprecision (Table 1).
1.1. Analysis.
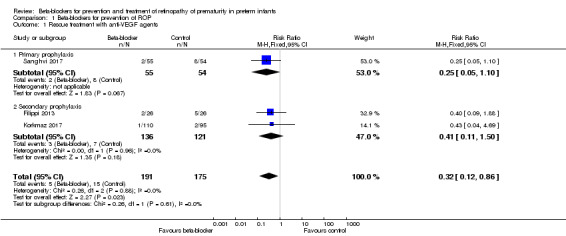
Comparison 1 Beta‐blockers for prevention of ROP, Outcome 1 Rescue treatment with anti‐VEGF agents.
Treatment with laser photocoagulation or cryotherapy (Outcome 1.2)
All three trials reported on the incidence of laser therapy (Filippi 2013; Korkmaz 2017; Sanghvi 2017). None of them reported a significant effect of beta‐blockers on this outcome. Meta‐analysis suggested a beneficial effect of beta‐blockers on this outcome (typical RR 0.54, 95% CI 0.32 to 0.89; I² = 0; typical RD −0.09, 95% CI −0.16 to −0.02; I² = 31%; N = 366 participants from three studies; NNTB 12, 95% CI 8 to 47) (Analysis 1.2). We graded the quality of evidence as low due to risk of bias and imprecision (Table 1)
1.2. Analysis.
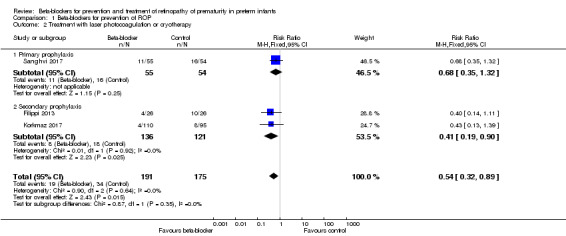
Comparison 1 Beta‐blockers for prevention of ROP, Outcome 2 Treatment with laser photocoagulation or cryotherapy.
Korkmaz 2017 provided additional data on the use of anti‐VEGF agents and laser photocoagulation allowing for intention‐to‐treat‐analysis of those outcomes.
Vitrectomy
In Filippi 2013 and Sanghvi 2017, no vitrectomy was performed. Korkmaz 2017 did not report on vitrectomy.
Structural outcomes
Progression to a more severe stage of ROP in prevention trials
Progression to stage 2 ROP with plus disease (Outcome 1.3)
Filippi 2013 and Sanghvi 2017 reported on progression to stage 2 ROP with plus disease. Neither found a significant effect of beta‐blockers on this outcome. Meta‐analysis of the results from these two trials did not indicate a significant effect of beta‐blockers (typical RR 0.25, 95% CI 0.03 to 2.16; I² = 0%; typical RD −0.04, 95% CI −0.09 to 0.02; I² = 0%; 161 participants from two studies) (Analysis 1.3). We graded the quality of evidence as moderate due to imprecision of results (Table 1).
1.3. Analysis.
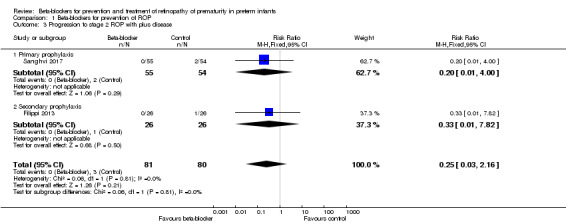
Comparison 1 Beta‐blockers for prevention of ROP, Outcome 3 Progression to stage 2 ROP with plus disease.
Progression to stage 3 ROP (Outcome 1.4)
Filippi 2013 and Sanghvi 2017 reported on this outcome. In Filippi 2013, the risk of progression to stage 3 ROP was significantly lower in the beta‐blocker versus control group, whereas in Sanghvi 2017, beta‐blockers had no significant effect on progression to stage 3 ROP. Meta‐analysis of data from these two trials indicated a beneficial effect of beta‐blockers on progression to stage 3 ROP compared to placebo or no treatment (typical RR 0.60, 95% CI 0.37 to 0.96; I² = 0%; typical RD −0.15, 95% CI −0.28 to −0.02; I² = 73%; N = 161 participants from two studies; NNTB 7, 95% CI 5 to 67) (Analysis 1.4). We graded the quality of evidence as moderate due to imprecision of results (Table 1).
1.4. Analysis.
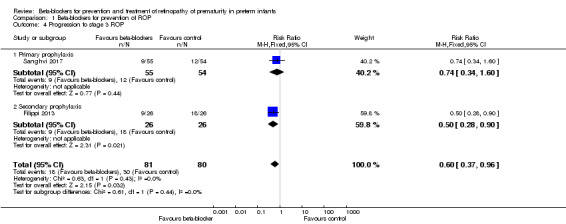
Comparison 1 Beta‐blockers for prevention of ROP, Outcome 4 Progression to stage 3 ROP.
Progression to stage 4 or 5 ROP (Outcome 1.5)
Filippi 2013 and Sanghvi 2017 reported on progression to stage 4 or 5 ROP. Filippi 2013 reported no difference in the risk of progression to stage 4 or 5 ROP in beta‐blocker versus control group. In Sanghvi 2017, no infant developed stage 4 or 5 ROP. Meta‐analysis did not indicate an effect of beta‐blockers on progression to stage 4 or 5 ROP (typical RR 0.11, 95% CI 0.01 to 1.96; I² = 0%; typical RD −0.05, 95% CI −0.11 to 0.01; I² = 90%; N = 161 participants from two studies) (Analysis 1.5). We graded the quality of evidence as moderate due to imprecision of results (Table 1).
1.5. Analysis.
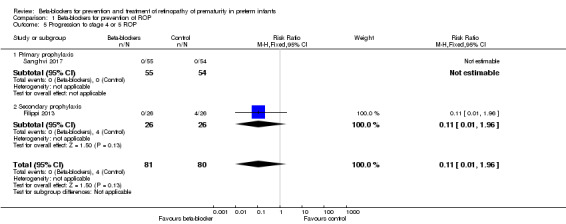
Comparison 1 Beta‐blockers for prevention of ROP, Outcome 5 Progression to stage 4 or 5 ROP.
Additional information obtained from Korkmaz 2017 clarified that no data were available on progression of ROP.
Secondary outcomes
Functional outcomes at six to 12 months' corrected age ‐ amblyopia, nystagmus and refractive error in either eye
Nystagmus at six to 12 months' corrected age (Outcome 1.6)
Sanghvi 2017 reported no difference in the incidence of nystagmus at 12 months' corrected age (RR 1.64, 95% CI 0.41 to 6.51; RD 0.04, 95% CI −0.06 to 0.13) (Analysis 1.6).
1.6. Analysis.
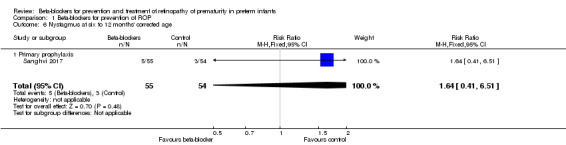
Comparison 1 Beta‐blockers for prevention of ROP, Outcome 6 Nystagmus at six to 12 months' corrected age.
Filippi 2013 mentioned preliminary results on nystagmus at 12 months' corrected age with a lower incidence in beta‐blocker versus control group (10% vs. 30%). However, no detailed values were published thus data could not be included in meta‐analysis. Korkmaz 2017 did not report on nystagmus at 12 months' corrected age.
Refractive error in either eye at six to 12 months' corrected age (Outcome 1.7)
Sanghvi 2017 reported no difference in the incidence of refractive error at 12 months' corrected age (RR 0.69, 95% CI 0.28 to 1.67; RD −0.06, 95% CI −0.19 to 0.08) (Analysis 1.7).
1.7. Analysis.
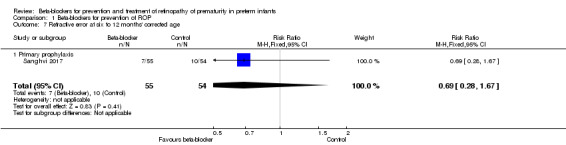
Comparison 1 Beta‐blockers for prevention of ROP, Outcome 7 Refractive error at six to 12 months' corrected age.
Filippi 2013 and Korkmaz 2017 did not report on refractive error.
Unfavourable structural outcomes at six to 12 months of age
None of the studies reported this outcome.
Early childhood unfavourable retinal structure at four to six years
None of the studies reported this outcome.
Mortality before discharge from the primary hospital (Outcome 1.8) or before one year of corrected age
Mortality before hospital discharge was not different between beta‐blocker and control groups in Filippi 2013 and Sanghvi 2017. Meta‐analysis of data from these trials did not indicate a significant effect of beta‐blockers versus placebo or no treatment on mortality (typical RR 0.99, 95% CI 0.30 to 3.29; I² = 0%; typical RD −0.00, 95% CI −0.08 to 0.07; I² = 0%; N = 161 participants from two studies) (Analysis 1.8). Korkmaz 2017 did not report on mortality. None of the studies reported on mortality after hospital discharge.
1.8. Analysis.
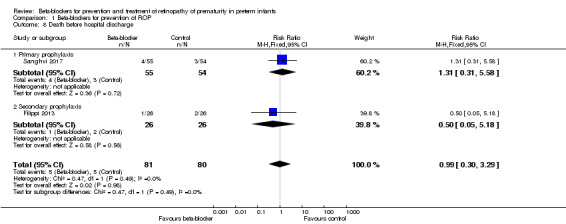
Comparison 1 Beta‐blockers for prevention of ROP, Outcome 8 Death before hospital discharge.
Complications of preterm birth
Bronchopulmonary dysplasia (Outcome 1.9)
Filippi 2013 and Korkmaz 2017 reported no difference in the risk of BPD between beta‐blocker and control groups. Similarly, meta‐analysis of data from these two trials did not show a significant effect of beta‐blockers on BPD (typical RR 1.14, 95% CI 0.75 to 1.73; I² = 0%; typical RD 0.03, 95% CI −0.07 to 0.13; I² = 10%; N = 257 participants from two studies) (Analysis 1.9).
1.9. Analysis.
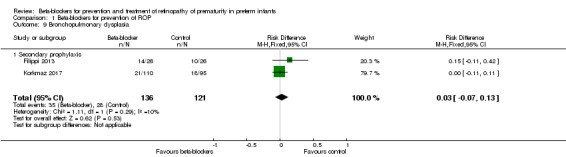
Comparison 1 Beta‐blockers for prevention of ROP, Outcome 9 Bronchopulmonary dysplasia.
Severe intraventricular haemorrhage (grade III to IV IVH) (Outcome 1.10)
All three included studies reported this outcome and none reported statistically significant differences in grade III to IV IVH in beta‐blocker versus control groups. Similarly, meta‐analysis of data from the three trials did not indicate a significant effect (typical RR 1.00, 95% CI 0.44 to 2.26; I² = 0%; typical RD 0.00, 95% CI −0.04 to 0.04; I² = 0%; N = 366 participants from three studies) (Analysis 1.10).
1.10. Analysis.
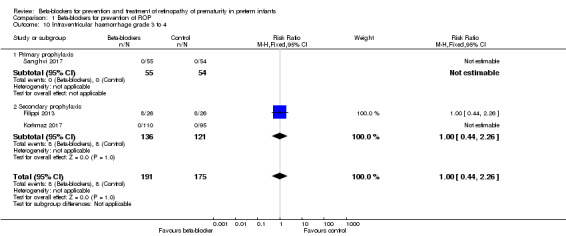
Comparison 1 Beta‐blockers for prevention of ROP, Outcome 10 Intraventricular haemorrhage grade 3 to 4.
Necrotising enterocolitis ≥ stage 2 (Outcome 1.11)
In Korkmaz 2017 and Sanghvi 2017, there were no significant differences in the incidence of stage 2 or greater NEC in beta‐blocker versus control groups. Meta‐analysis of data from these two studies did not show a significant effect of beta‐blockers on NEC (typical RR 2.45, 95% CI 0.50 to 12.11; I² = 0%; typical RD 0.02, 95% CI −0.02 to 0.05; I² = 77%; N = 314 participants from two studies) (Analysis 1.11).
1.11. Analysis.
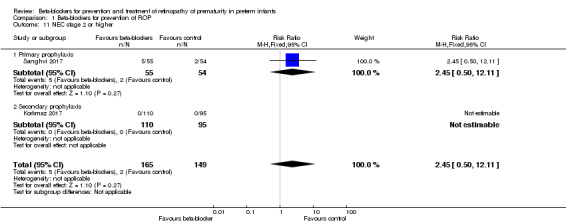
Comparison 1 Beta‐blockers for prevention of ROP, Outcome 11 NEC stage 2 or higher.
Additional data obtained from two authors enabled intention‐to‐treat‐analysis of outcomes 1.10 and 1.11 (Korkmaz 2017; Sanghvi 2017).
None of the studies reported on PVL or neurodevelopmental impairment.
Adverse events
Arterial hypotension requiring inotropic treatment (Outcome 1.12)
Filippi 2013 reported that three infants in the beta‐blocker group (gestational age 23 to 25 weeks) required inotropic agents due to hypotension, severe apnoea and bradycardia. Therefore, propranolol dose in this study was subsequently reduced by 50% for participants of less than 26 weeks' gestation. Overall, Filippi 2013 reported no statistically significant effect of beta‐blockers on the incidence of arterial hypotension. Korkmaz 2017 and Sanghvi 2017 reported no events. Meta‐analysis of data from three trials did not indicate a significant effect of beta‐blockers on arterial hypotension requiring inotropic agents (typical RR 7.00, 95% CI 0.38 to 129.11; I² = not applicable; typical RD 0.02, 95% CI −0.01 to 0.04; RD 0.04, 95% CI −0.01 to 0.09; I² = 82%; N = 366 participants from three studies) (Analysis 1.12). We graded the quality of evidence as low due to risk of bias and imprecision (Table 1).
1.12. Analysis.
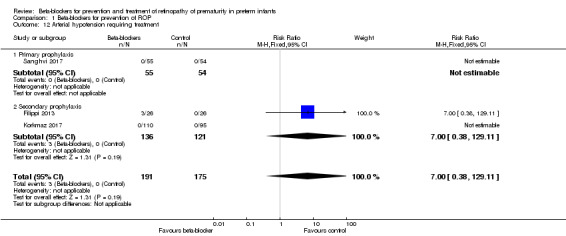
Comparison 1 Beta‐blockers for prevention of ROP, Outcome 12 Arterial hypotension requiring treatment.
Bradycardia requiring inotropic agents (Outcome 1.13)
Filippi 2013 reported no significant difference in the risk of bradycardia requiring inotropic agents between the beta‐blocker and control groups. In Korkmaz 2017 and Sanghvi 2017, no patient with bradycardia was reported. Meta‐analysis of data from three trials indicated no significant effect of beta‐blockers on bradycardia requiring inotropic agents (typical RR 11.00, 95% CI 0.64 to 189.31; I² = not applicable; typical RD 0.03, 95% CI −0.00 to 0.06; I² = 86%; N = 366 participants from three studies) (Analysis 1.13). The quality of evidence was graded low due to risk of bias and imprecision (Table 1).
1.13. Analysis.
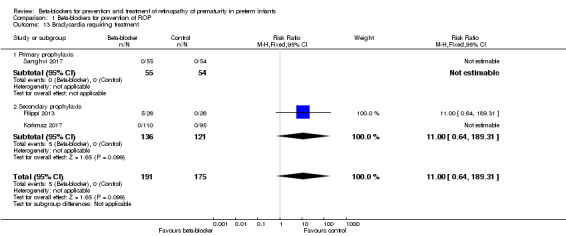
Comparison 1 Beta‐blockers for prevention of ROP, Outcome 13 Bradycardia requiring treatment.
Bronchospasm requiring treatment (Outcome 1.14)
Filippi 2013 reported no difference in risk of bronchospasm requiring treatment between beta‐blocker and control groups (RR 3.00, 95% CI 0.13 to 70.42; RD 0.04, 95% CI −0.06 to 0.14) (Analysis 1.14). Korkmaz 2017 and Sanghvi 2017 did not report on bronchospasm.
1.14. Analysis.
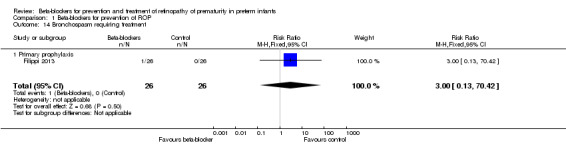
Comparison 1 Beta‐blockers for prevention of ROP, Outcome 14 Bronchospasm requiring treatment.
Hypoglycaemia (Outcome 1.15)
Korkmaz 2017 reported no significant difference in the risk of hypoglycaemia between the beta‐blocker and control groups. In Sanghvi 2017, no patient had hypoglycaemia. Meta‐analysis of two trials did not indicate a significant effect of beta‐blockers on hypoglycaemia (typical RR 6.05, 95% CI 0.32 to 115.73; I² = 0%; typical RD 0.02, 95% CI −0.01 to 0.04; I² = 20%; N = 314 participants from two studies) (Analysis 1.15).
1.15. Analysis.
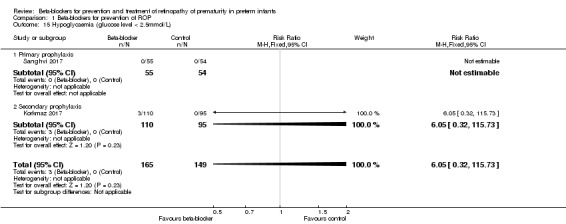
Comparison 1 Beta‐blockers for prevention of ROP, Outcome 15 Hypoglycaemia (glucose level < 2.5mmol/L).
Additional data obtained from one author allowed for intention‐to‐treat‐analysis of outcomes 1.12, 1.13, and 1.15 (Korkmaz 2017).
Filippi 2013 narratively reported that infants on oral propranolol had higher potassium levels compared to control infants but that values were within the normal range. No hyperkalaemia was reported. Korkmaz 2017 reported adverse events in nine patients (intervention group, n = 6; control group, n = 3) and excluded those patients from analysis after stopping study drug (see Incomplete outcome data (attrition bias)). Adverse events in Korkmaz 2017 included apnoea, arterial hypotension without treatment requirement and increase in respiratory support. Details of these events are unknown.
Data were not suitable for subgroup or sensitivity analyses.
Discussion
Summary of main results
Analysis of three RCTs suggests that prophylactic oral administration of beta‐blockers compared to placebo or no treatment may reduce the risk of progression to stage 3 ROP in preterm infants without ROP or confirmed stage 2 or lower ROP without plus disease, and may decrease the risk of requiring laser therapy or anti‐VEGF agents. Although meta‐analysis did not indicate a significant effect of oral beta‐blockers on the risk of hypotension or bradycardia requiring inotropic agents, propranolol dosage in Filippi 2013 was reduced by 50% in infants of less than 26 weeks' gestation due to severe hypotension, bradycardia, and apnoea in several extremely preterm infants. Meta‐analysis from two trials did not indicate an effect of prophylactic oral beta‐blockers on mortality or complications of preterm birth. We found no conclusive evidence to suggest or refute effects of prophylactic oral beta‐blockers on long‐term visual function, progression to stage 4 or 5 ROP, or long‐term neurodevelopment.
We identified no trials on the use of topical beta‐blockers for prevention of ROP and none that assessed the effect of beta‐blocker treatment in infants with established stage 2 or higher ROP with plus disease.
Overall completeness and applicability of evidence
The primary objective of this systematic review was to determine the effect of beta‐blockers on ROP progression, need for additional treatment, and functional long‐term outcomes in preterm infants without ROP or confirmed stage 2 or lower ROP without plus disease. Four RCTs on this topic were identified and three of them were included in this review. These three studies incorporated a total of 366 participants and all three were concerned with oral administration of propranolol compared to placebo or no treatment (Filippi 2013; Korkmaz 2017; Sanghvi 2017). Included trials provided incomplete evidence to address the objectives of this review. Beta‐blockers were found to reduce the risk of progression to stage 3 ROP but not to more severe stages of ROP (stage 4 or 5 ROP). Further, the use of beta‐blockers was associated with reduced rates of children undergoing laser therapy or anti‐VEGF treatment. The clinical importance of these results remains unknown as none of the trials reported on long‐term functional outcomes such as severe visual impairment or blindness. Given that adverse events including arterial hypotension and bradycardia were reported, safety concerns have to be addressed. Although meta‐analysis did not reveal a significantly increased risk of hypotension or bradycardia requiring inotropic agents in infants treated with beta‐blockers, those effects cannot be ruled out given that two out of three trials were at high risk of bias and there were few reported events. A fourth RCT, Makhoul 2013, was published as a research letter and we excluded it from this review as outcomes from 17/20 patients were not reported and methodological details could not be clarified. It is unlikely that inclusion of this trial would have led to relevant changes to the conclusions of this review.
Given the paucity of data available and the high risk of bias in Filippi 2013 and Korkmaz 2017, applicability and generalisability of the results of this review are very limited. We found no evidence to suggest or refute the use of beta‐blockers for treatment of at least prethreshold ROP as no trials were identified.
Quality of the evidence
The overall quality of evidence for outcomes in this review varied from low to moderate (Table 1). Thus, internal validity of the results of this review is very limited. The risk of selection and reporting bias was high in Korkmaz 2017. The risk of performance and attrition bias was high in two studies because caregivers and families were not blinded to the intervention and more than 20% of participants were withdrawn due to serious adverse events (Filippi 2013; Korkmaz 2017) (Figure 3). The risk of detection bias for the primary outcomes was low as outcome assessors (ophthalmologists) were masked in all included trials.
3.
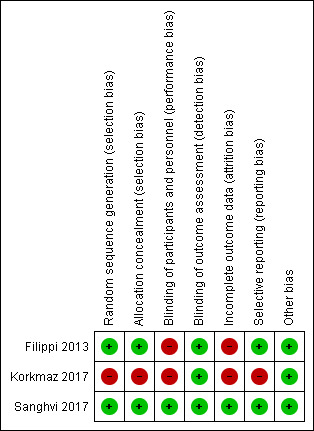
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Potential biases in the review process
We did not apply language restrictions to reduce publication bias and we contacted authors of potentially eligible studies to clarify study methodology. We further contacted authors of included studies to clarify methodological details of the trials and to obtain relevant outcome data.
Agreements and disagreements with other studies or reviews
We identified one systematic review on the use of propranolol for prophylaxis of ROP (Bührer 2015). This review included two RCTs incorporating a total of 72 preterm infants (Filippi 2013; Makhoul 2013). The authors of the review reported a non‐significant trend towards a reduced risk of laser treatment or bevacizumab injection in infants allocated to propranolol and found substantial differences in inclusion criteria and duration of propranolol administration in the two trials. They concluded that well‐designed trials were urgently needed to assess the clinical benefit‐to‐risk ratio of propranolol for prevention of vision‐threatening ROP. Makhoul 2013 was included in Bührer 2015 but excluded from the current review due to an 85% loss to follow‐up and unclear methodological details (study published as a research letter). The overall findings from the review of Bührer 2015 do not substantially differ from those of the current review.
Authors' conclusions
Implications for practice.
This review found limited evidence of low‐to‐moderate quality suggesting that oral beta‐blockers may reduce the risk of progression to stage 3 ROP (but not to stage 4 or 5 ROP) and reduce the risk of requiring laser treatment or anti‐VEGF agents. The clinical relevance of those findings is unclear as no data on long‐term vision impairment or blindness have been published. Serious adverse events attributed to propranolol at a dose of 2 mg/kg/d raise concerns regarding systemic administration of this drug for prevention of ROP at the given dose. There is insufficient evidence to determine the efficacy and safety of oral beta‐blockers for prevention of ROP due to high risk of bias in two out of three included trials and the lack of long‐term functional outcomes. We cannot recommend routine use of oral propranolol for prevention of ROP in preterm infants.
Implications for research.
We encourage researchers to undertake large, well‐designed RCTs to confirm or refute the role of beta‐blockers for prevention of ROP. In addition to outcomes such as progression of ROP and risk of requiring laser treatment or anti‐VEGF agents, these trials should report on adverse events and long‐term visual impairment. Due to observed adverse events in previous trials, dose‐finding studies of systemic use of beta‐blockers and ocular administration of beta‐blockers (given by standardised methods with particular attention to the nominal dose delivered to the eyes) should be considered in order to optimise drug delivery and minimise adverse events.
Acknowledgements
We thank Dr Baştuğ, Dr Filippi, Dr Makhoul and Dr Sanghvi for clarification of study methodology and provision of additional outcome data.
We thank the Cochrane Neonatal Review Group for providing valuable comments and advice at the protocol and review stages.
The methods section of this review is based on a standard template used by Cochrane Neonatal.
Appendices
Appendix 1. Search strategy
("retrolental fibroplasia" [MeSH] OR "retinopathy of prematurity" [MeSH]) AND ("Propranolol" [MeSH] OR "adrenergic beta‐antagonists" [MeSH] OR beta blocker) AND ("controlled clinical trial" [Publication Type] OR "randomised controlled trial" [Publication Type]).
Appendix 2. Risk of bias tool
1. Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated?
For each included study, we categorized the method used to generate the allocation sequence as:
low risk (any truly random process e.g. random number table; computer random number generator);
high risk (any non‐random process e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk.
2. Allocation concealment (checking for possible selection bias). Was allocation adequately concealed?
For each included study, we categorized the method used to conceal the allocation sequence as:
low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk
3. Blinding of participants and personnel (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study?
For each included study, we categorized the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or class of outcomes. We categorized the methods as:
low risk, high risk or unclear risk for participants; and
low risk, high risk or unclear risk for personnel.
4. Blinding of outcome assessment (checking for possible detection bias). Was knowledge of the allocated intervention adequately prevented at the time of outcome assessment?
For each included study, we categorized the methods used to blind outcome assessment. Blinding was assessed separately for different outcomes or class of outcomes. We categorized the methods as:
low risk for outcome assessors;
high risk for outcome assessors; or
unclear risk for outcome assessors.
5. Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorized the methods as:
low risk (< 20% missing data);
high risk (≥ 20% missing data); or
unclear risk.
6. Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. For studies in which study protocols were published in advance, we compared prespecified outcomes versus outcomes eventually reported in the published results. If the study protocol was not published in advance, we contacted study authors to gain access to the study protocol. We assessed the methods as:
low risk (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified outcomes of interest and are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk.
7. Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias?
For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk;
high risk;
unclear risk
If needed, we explored the impact of the level of bias through undertaking sensitivity analyses.
Data and analyses
Comparison 1. Beta‐blockers for prevention of ROP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rescue treatment with anti‐VEGF agents | 3 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.12, 0.86] |
| 1.1 Primary prophylaxis | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.05, 1.10] |
| 1.2 Secondary prophylaxis | 2 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.11, 1.50] |
| 2 Treatment with laser photocoagulation or cryotherapy | 3 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.32, 0.89] |
| 2.1 Primary prophylaxis | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.35, 1.32] |
| 2.2 Secondary prophylaxis | 2 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.19, 0.90] |
| 3 Progression to stage 2 ROP with plus disease | 2 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.16] |
| 3.1 Primary prophylaxis | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.00] |
| 3.2 Secondary prophylaxis | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.82] |
| 4 Progression to stage 3 ROP | 2 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.37, 0.96] |
| 4.1 Primary prophylaxis | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.34, 1.60] |
| 4.2 Secondary prophylaxis | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.28, 0.90] |
| 5 Progression to stage 4 or 5 ROP | 2 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.96] |
| 5.1 Primary prophylaxis | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Secondary prophylaxis | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.96] |
| 6 Nystagmus at six to 12 months' corrected age | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.41, 6.51] |
| 6.1 Primary prophylaxis | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.41, 6.51] |
| 7 Refractive error at six to 12 months' corrected age | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.28, 1.67] |
| 7.1 Primary prophylaxis | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.28, 1.67] |
| 8 Death before hospital discharge | 2 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.30, 3.29] |
| 8.1 Primary prophylaxis | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.31, 5.58] |
| 8.2 Secondary prophylaxis | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.18] |
| 9 Bronchopulmonary dysplasia | 2 | 257 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.07, 0.13] |
| 9.1 Secondary prophylaxis | 2 | 257 | Risk Difference (M‐H, Fixed, 95% CI) | 0.03 [‐0.07, 0.13] |
| 10 Intraventricular haemorrhage grade 3 to 4 | 3 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.44, 2.26] |
| 10.1 Primary prophylaxis | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 Secondary prophylaxis | 2 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.44, 2.26] |
| 11 NEC stage 2 or higher | 2 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.45 [0.50, 12.11] |
| 11.1 Primary prophylaxis | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.45 [0.50, 12.11] |
| 11.2 Secondary prophylaxis | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Arterial hypotension requiring treatment | 3 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.38, 129.11] |
| 12.1 Primary prophylaxis | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 Secondary prophylaxis | 2 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.38, 129.11] |
| 13 Bradycardia requiring treatment | 3 | 366 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.0 [0.64, 189.31] |
| 13.1 Primary prophylaxis | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Secondary prophylaxis | 2 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.0 [0.64, 189.31] |
| 14 Bronchospasm requiring treatment | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.42] |
| 14.1 Primary prophylaxis | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.42] |
| 15 Hypoglycaemia (glucose level < 2.5mmol/L) | 2 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.05 [0.32, 115.73] |
| 15.1 Primary prophylaxis | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Secondary prophylaxis | 1 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.05 [0.32, 115.73] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Filippi 2013.
| Methods | 2‐centre randomised controlled trial performed in Milano and Florence, Italy. Allocation concealment: yes. Randomisation: computer‐based random number generation (blocks of 8). Blinding of caregivers and personnel to intervention: no. Blinding of outcome ascertainment: yes (ophthalmologist). Complete follow‐up: yes. |
|
| Participants | Preterm neonates (n = 52 enrolled, n = 51 analysed). Inclusion criteria: gestational age < 32 weeks and stage 2 ROP without plus disease in zone II. Infants were stratified by centre and gestational age (23 to 25 weeks' gestational age and 26 to 31 weeks' gestational age). 1 neonate allocated to the intervention group was relocated to the control group on the first day of using study drug due to serious adverse effects from propranolol. Intervention group: mean (SD) postnatal age, 67 (±14) days; mean (SD) weight 1678 (± 393) grams. Control group: mean (SD) postnatal age, 68 (± 17) days; mean (SD) weight 1559 (± 431) grams. 67.3% of neonates were outborn and the majority of neonates were transferred to the 2 study centres for surgery. 15 (57.7%) participants in the intervention group and 13 (50%) participants in the control group had at least 1 major surgical intervention. Surgery was predominantly performed for closure of patent ductus arteriosus, hydrocephalus, and intestinal disease. Exclusion criteria: neonates with congenital or acquired cardiovascular anomalies, renal failure, cerebral haemorrhage; preterm infants with ROP in zone I or ≥ stage 2 ROP without plus disease in zone II. |
|
| Interventions | Intervention group (n = 25 analysed): oral propranolol (2 mg/ml syrup) at a dose of 0.5 mg/kg 6 hourly (n = 18); dosage was reduced to 0.25 mg/kg 6 hourly in 8 infants with a gestational age of 23 to 25 weeks. Duration of treatment: until complete retinal vascularisation, but no more than 90 days. Mean (range) treatment duration 66 (6 to 90) days. Control group (n = 26 analysed): no control treatment. Standard treatment for ROP was offered to all study participants if indicated (laser photocoagulation, rescue therapy with anti‐VEGF agents). |
|
| Outcomes | Primary outcome: Progression of ROP to stage 2 ROP with plus disease or to stage 3 ROP with or without plus disease. Secondary outcomes: Incidence of laser therapy, rescue treatment with bevacizumab, progression to stage 4 or 5 ROP, need for vitrectomy. Plasma VEGF and sE‐selectin concentrations. |
|
| Notes | Registered with clinicaltrials.gov NCT01079715. Dr Filippi (contact author) provided additional information on study methodology. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed by using sequentially numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of the intervention was not attempted. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors (ophthalmologist and data analyst) were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 52 patients were randomised. In the intervention group, 1/26 (4%) infants died after 9 days of treatment; in the control group, 2/26 (8%) infants died prior to complete retinal vascularisation. In 6/26 (23%) infants allocated to the intervention group, treatment was discontinued early due to severe adverse effects. Additionally, 1 patient from the intervention group was relocated and analysed in the control group due to severe adverse effects after 1 day of propranolol treatment. Reporting of outcomes from the single deceased infant in the intervention group was incomplete. |
| Selective reporting (reporting bias) | Low risk | Primary outcome reported as specified in the report. Some of the secondary outcomes specified in the registered trial protocol (blindness and retinal detachment within 6 months from beginning of treatment, see NCT01079715) were not reported. |
| Other bias | Low risk | None detected. |
Korkmaz 2017.
| Methods | Single‐centre randomised controlled trial performed in Kayseri, Turkey. Allocation concealment: no. Randomisation: nurse on clinical service flipped a coin. Depending on the result, she openly prepared propranolol or physiological saline solution. Blinding of caregivers and personnel to intervention: no. Blinding of outcome ascertainment: yes (ophthalmologist). Complete follow‐up: no. |
|
| Participants | Preterm neonates (n = 205 enrolled, n = 171 analysed). Inclusion criteria: gestational age < 32 weeks, birth weight < 1500 grams and ≤ stage 2 ROP. Participants were stratified into 3 groups: no ROP; stage 1 ROP; stage 2 ROP. Intervention group: mean (SD) gestational age 28.2 (± 2.04) weeks; mean (SD) birth weight 1069 (± 289) grams. Control group: mean (SD) gestational age 28.4 (± 1.91) weeks; mean (SD) birth weight 1068 (± 284) grams. Exclusion criteria: infants with cardiovascular anomalies, renal failure, IVH > stage 1, NEC ≥ stage 2. Withdrawal criteria: renal failure, apneas, hypoglycaemia, bradycardia, hypotension, insufficient weight gain, or on parental request. Participants in the intervention group were excluded post‐randomisation if administration of propranolol was interrupted for more than 24 hours and upon failure to thrive. |
|
| Interventions | Intervention group (n = 83 analysed): oral propranolol (1 mg/ml saline solution) at a dose of 0.5 mg/kg 6‐hourly. Control group (n = 88 analysed): placebo (physiological saline solution). Duration of treatment: initiation of treatment ≥ 31 weeks' PMA. Duration of treatment not standardised and not reported. |
|
| Outcomes | Primary outcome: Incidence of laser therapy; platelet mass index. Secondary outcomes: not defined. The following outcomes were reported: platelet count, BPD, IVH, PDA, NEC, sepsis. |
|
| Notes | Dr Baştuğ (contact author) provided additional information on study methodology and data analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation was accomplished by a clinical nurse who flipped a coin. Unbalanced numbers of participants in intervention (n = 110, 54%) and control groups (n = 95, 46%). |
| Allocation concealment (selection bias) | High risk | Study drug was prepared openly in the neonatal unit. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of the intervention was not attempted. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors (Ophthalmologists) were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A total of 34/205 (17%) infants were excluded post‐randomisation due to serious adverse events of propranolol (n = 9), withdrawal of parental consent (n = 6), poor adherence to administration of propranolol (n = 13), and use of anti‐VEGF (n = 6). The vast majority of exclusions occurred in the intervention group, resulting in unbalanced numbers of dropouts (study group, n = 27/110 (25%) excluded; control group, n = 7/95 (7%) excluded). |
| Selective reporting (reporting bias) | High risk | No study protocol available. Trial was not registered. |
| Other bias | Low risk | None detected. |
Sanghvi 2017.
| Methods | 2‐centre randomised, placebo‐controlled trial performed in Mumbai, India. Allocation concealment: yes. Randomisation: computer‐based random number generation (blocks of 2 or 4). Blinding of caregivers and personnel to intervention: yes. Blinding of outcome ascertainment: yes (ophthalmologists). Complete follow‐up: yes. |
|
| Participants | Preterm neonates (n = 109 enrolled, n = 102 analysed). Inclusion criteria: gestational age 26 to 32 weeks, postnatal age < 8 days. Participants were stratified into 2 groups according to gestational age (26 to 28 weeks' gestational age, 29 to 32 weeks' gestational age). Intervention group: mean (SD) gestational age 29.54 (± 1.69) weeks; mean (SD) postnatal age 5.78 (± 1.74) days; mean (SD) birth weight 1235 grams (± 280). Control group: mean (SD) gestational age 29.12 (± 1.74) weeks; mean (SD) postnatal age 5.96 (± 1.87) days; mean (SD) birth weight 1155 grams (± 284). Exclusion criteria: patients with recurrent bradycardia, second and third degree AV‐block, hypotension, refractory hypoglycaemia and major congenital malformations. |
|
| Interventions | Intervention group (n = 55 analysed): oral propranolol (1 mg/ml sterile water) at a dose of 0.5 mg/kg 12‐hourly. Control group (n = 54 analysed): placebo (calcium carbonate 1 mg/ml) at a dose of 0.5 mg/kg 12 hourly. Duration of treatment: from day 8 of life until 37 weeks' PMA or until complete retinal vascularisation; median (range) treatment duration 32 (7 to 72) days in the intervention group, 41 (1 to 107) days in the control group. |
|
| Outcomes | Primary outcome: All stages of ROP. Secondary outcomes: Adverse events such recurrent bradycardia, hypotension, hypoglycaemia. Incidence of laser therapy, rescue treatment with anti‐VEGF agents, visual outcome at 12 months' corrected age. |
|
| Notes | Registered retrospectively with ctri.nic.in CTRI/2013/11/004131. Dr Sanghvi (contact author) provided additional information on study methodology and data for subgroup analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Study coordinator nurse had access to the opaque binder containing the prespecified sequence of allocation, provided by a statistician. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Care givers (physicians and nurses) were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors (ophthalmologist and physicians) were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 109 patients were randomised. In the intervention group, 4/55 (7%) infants died and in the control group, 3/54 (6%) infants died prior to first ROP screening. Further 22 patients (20%) were lost to visual follow‐up at 12 months' corrected age (secondary outcome). |
| Selective reporting (reporting bias) | Low risk | Primary outcome reported as specified in the trial protocol. Visual long‐term outcomes at 12 months' PMA were not prespecified in the trial protocol but reported. Trial was registered retrospectively. |
| Other bias | Low risk | None detected. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bancalari 2016 | Data from VLBW infants (n = 47) with stage 2 or 3 ROP in zone II‐III were retrospectively analysed. Authors assessed the effect of propranolol on progression of ROP. Infants in the intervention group received propranolol. Outcomes of the intervention group were compared to a historical control group not receiving propranolol. Excluded because the study was not a randomised or quasi‐randomised trial. |
| Filippi 2016 | Preterm neonates (n = 23, gestational age < 32 weeks) with stage 2 ROP, zone II without plus disease received propranolol as ophthalmic solution until complete development of retinal vascularisation, but no more than 60 days. Trial was discontinued early. Excluded because the study was not a randomised or quasi‐randomised trial. |
| Makhoul 2013 | Preterm infants (n = 20, gestational age 23 to 28 weeks) with stage 1 (zone I) ROP, stage 2 or higher (zone I or II) ROP, and/or plus disease were randomised to receive propranolol or placebo. Outcomes included progression of ROP and need for invasive interventions. This study was published as a research letter. Excluded because methodological details could not be fully clarified and due to 85% loss to follow‐up (17/20 patients lost to follow‐up). |
Characteristics of ongoing studies [ordered by study ID]
NCT01660620 Ongoing.
| Trial name or title | Topical betaxolol for the prevention of retinopathy of prematurity. |
| Methods | Randomised, placebo‐controlled trial. |
| Participants | Preterm infants < 1251 grams birth weight. |
| Interventions | Topical betaxolol 0.25% or placebo twice daily between 32 and 35 weeks' PMA. |
| Outcomes | Primary outcome: development of apnoea and/or bradycardia; secondary outcome: ROP requiring treatment. |
| Starting date | 2012 |
| Contact information | Smith‐Kettlewell Eye Research Institute |
| Notes | Phase 1 interventional study/prevention study |
NCT03038295 Ongoing.
| Trial name or title | A prospective cohort study for propranolol treatment in retinopathy of prematurity. |
| Methods | Randomised, placebo‐controlled trial. |
| Participants | Preterm newborns (birth weight less than 1500 grams) with stage 1 or 2 ROP in zone II or III without plus. |
| Interventions | Propranolol ophthalmic solution (0.2%) 0.25 mg/kg/d. 3 microdrops propranolol solution or placebo in each eye 4 times daily until complete retinal vascularisation, but no more than 90 days. |
| Outcomes | Primary outcomes: ROP progression; rate of surgical treatment; adverse effects, steady state plasma concentrations of propranolol. |
| Starting date | 2017 |
| Contact information | chuannie@sina.com |
| Notes | Phase 2 interventional study |
NCT03083431 Ongoing.
| Trial name or title | Propranolol for prevention of threshold retinopathy of prematurity (ROPROP). |
| Methods | Randomised, placebo‐controlled trial. |
| Participants | Extremely preterm infants. |
| Interventions | Oral propranolol 1.6 mg/kg/d in 4 divided doses or placebo given for 4 to 10 weeks. |
| Outcomes | Primary outcome: survival until 48 weeks' PMA without ≥ stage 3 ROP. |
| Starting date | 2018 |
| Contact information | christoph.buehrer@charite.de, roman.weimann@charite.de |
| Notes | Phase 2 interventional study |
Differences between protocol and review
The differences between the protocol and review are listed below:
Data extraction and management
Deleted: "One review author (SK) entered data into Review Manager (RevMan 2014) using double data entry to reduce the risk of coding errors."
Added:"One review author (SK) entered data into Review Manager 5 (Review Manager 2014). A second review author (SMS) checked data for accuracy."
Contributions of authors
SK: designed and wrote the protocol and review. SMS: contributed to overall design, background, definitions of interventions and outcomes, and revision of protocol and review. RPN and KJ: contributed to background, definitions of interventions, outcomes, and revision of protocol and review.
Sources of support
Internal sources
-
University Children's Hospital Basel, Switzerland.
Protected research time
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
Declarations of interest
None.
New
References
References to studies included in this review
Filippi 2013 {published and unpublished data}
- Filippi L, Cavallaro G, Bagnoli P, Dal Monte M, Fiorini P, Donzelli G, et al. Oral propranolol for retinopathy of prematurity: risks, safety concerns, and perspectives. Journal of Pediatrics 2013;163(6):1570‐7. [DOI: 10.1016/j.jpeds.2013.07.049; PUBMED: 24054431] [DOI] [PubMed] [Google Scholar]
Korkmaz 2017 {published and unpublished data}
- Korkmaz L, Baştuğ O, Ozdemir A, Korkut S, Karaca C, Akin MA, et al. The efficacy of propranolol in retinopathy of prematurity and its correlation with the platelet mass index. Current Eye Research 2017;42(1):88‐97. [DOI: 10.3109/02713683.2016.1158272; PUBMED: 27260268] [DOI] [PubMed] [Google Scholar]
Sanghvi 2017 {published and unpublished data}
- Sanghvi KP, Kabra NS, Padhi P, Singh U, Dash SK, Avasthi BS. Prophylactic propranolol for prevention of ROP and visual outcome at 1 year (PreROP trial). Archives of Disease in Childhood. Fetal and Neonatal Edition 2017;102(5):F389‐94. [DOI: 10.1136/archdischild-2016-311548; CTRI/2013/11/004131; PUBMED: 28087723] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bancalari 2016 {published data only}
- Bancalari A, Schade R, Muñoz T, Lazcano C, Parada R, Peña R. Oral propranolol in early stages of retinopathy of prematurity. Journal of Perinatal Medicine 2016;44(5):499‐503. [DOI: 10.1515/jpm-2015-0357; PUBMED: 26845715] [DOI] [PubMed] [Google Scholar]
Filippi 2016 {published data only}
- Filippi L, Cavallaro G, Bagnoli P, Dal Monte M, Fiorini P, Berti E, et al. Propranolol 0.1% eye micro‐drops in newborns with retinopathy of prematurity: a pilot clinical trial. Pediatric Research 2017;81(2):307‐14. [DOI: 10.1038/pr.2016.230; PUBMED: 27814346] [DOI] [PubMed] [Google Scholar]
Makhoul 2013 {published and unpublished data}
- Makhoul IR, Peleg O, Miller B, Bar‐Oz B, Kochavi O, Mechoulam H, et al. Oral propranolol versus placebo for retinopathy of prematurity: a pilot, randomised, double‐blind prospective study. Archives of Disease in Childhood 2013;98(7):565‐7. [DOI: 10.1136/archdischild-2013-303951; PUBMED: 23632260] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01660620 Ongoing {published data only}
- NCT01660620. Topical betaxolol for the prevention of retinopathy of prematurity. clinicaltrials.gov/show/NCT01660620 (first received 08 August 2012).
NCT03038295 Ongoing {published data only}
- NCT03038295. A prospective cohort study for propranolol treatment in retinopathy of prematurity [Safety and efficacy of propranolol treatment in newborns with retinopathy of prematurity:a prospective cohort study]. clinicaltrials.gov/show/NCT03038295 (first received 19 January 2017).
NCT03083431 Ongoing {published data only}
- NCT03083431. Propranolol for prevention of threshold retinopathy of prematurity (ROPROP). clinicaltrials.gov/show/NCT03083431 (first received 20 March 2017).
Additional references
Abarzúa‐Araya 2014
- Ábarzúa‐Araya A, Navarrete‐Dechent CP, Heusser F, Retamal J, Zegpi‐Trueba MS. Atenolol versus propranolol for the treatment of infantile hemangiomas: a randomized controlled study. Journal of the American Academy of Dermatology 2014;70(6):1045‐9. [DOI: 10.1016/j.jaad.2014.01.905; PUBMED: 24656727] [DOI] [PubMed] [Google Scholar]
Afzal 2007
- Afzal A, Shaw LC, Ljubimov AV, Boulton ME, Segal MS, Grant MB. Retinal and choroidal microangiopathies: therapeutic opportunities. Microvascular Research 2007;74(2‐3):131‐44. [DOI: 10.1016/j.mvr.2007.04.011; PUBMED: 17585951] [DOI] [PubMed] [Google Scholar]
Andersen 2000
- Andersen CC, Phelps DL. Peripheral retinal ablation for threshold retinopathy of prematurity in preterm infants. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD001693] [DOI] [PMC free article] [PubMed] [Google Scholar]
Asthon 1954
- Asthon N, Ward B, Serpell G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. British Journal of Ophthalmology 1954;38(7):397‐432. [PUBMED: 13172417] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bayley 2006
- Bayley N. Bayley scales of infant and toddler development. 3rd Edition. San Antonio, TX: The Psychological Corporation, 2006. [Google Scholar]
Bell 1978
- Bell MJ, Ternberg JL, Feigin RD, Keating JP, Mashall R, Barton L, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Annals of Surgery 1978;187(1):1‐7. [PUBMED: 413500] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blencowe 2013
- Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C. Preterm‐associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatric Research 2013;74(Suppl 1):35‐49. [DOI: 10.1038/pr.2013.205; PUBMED: 24366462] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonifazi 2010
- Bonifazi E, Acquafredda A, Milano A, Montagna O, Laforgia N. Severe hypoglycemia during successful treatment of diffuse hemangiomatosis with propranolol. Pediatric Dermatology 2010;27(2):195‐6. [DOI: 10.1111/j.1525-1470.2009.01081.x; PUBMED: 20537072] [DOI] [PubMed] [Google Scholar]
Bührer 2015
- Bührer C, Bassler D. Oral propranolol: a new treatment for infants with retinopathy of prematurity?. Neonatology 2015;108(1):49‐52. [DOI: 10.1159/000381659; PUBMED: 25968340] [DOI] [PubMed] [Google Scholar]
Casini 2014
- Casini G, Dal Monte M, Fornaciari I, Filippi L, Bagnoli P. The β‐adrenergic system as a possible new target for pharmacologic treatment of neovascular retinal diseases. Progress in Retinal and Eye Research 2014;42:103‐29. [DOI: 10.1016/j.preteyeres.2014.06.001; PUBMED: 24933041] [DOI] [PubMed] [Google Scholar]
Cavallaro 2014
- Cavallaro G, Filippi L, Bagnoli P, Marca G, Cristofori G, Raffaeli G, et al. The pathophysiology of retinopathy of prematurity: an update of previous and recent knowledge. Acta Ophthalmologica 2014;92(1):2–20. [DOI: 10.1111/aos.12049; PUBMED: 23617889] [DOI] [PubMed] [Google Scholar]
Chakkittakandiyil 2012
- Chakkittakandiyil A, Phillips R, Frieden IJ, Siegfried E, Lara‐Corrales I, Lam J, et al. Timolol maleate 0.5% or 0.1% gel‐forming solution for infantile hemangiomas: a retrospective, multicenter, cohort study. Pediatric Dermatology 2012;29(1):28‐31. [DOI: 10.1111/j.1525-1470.2011.01664.x; PUBMED: 22150436 ] [DOI] [PubMed] [Google Scholar]
Chen 2012
- Chen J, Joyal JS, Hatton CJ, Juan AM, Pei DT, Hurst CG, et al. Propranolol inhibition of β‐adrenergic receptor does not suppress pathologic neovascularization in oxygen‐induced retinopathy. Investigative Ophthalmology & Visual Science 2012; Vol. 53, issue 6:2968‐77. [DOI: 10.1167/iovs.12-9691; PUBMED: 22491401] [DOI] [PMC free article] [PubMed]
Dal Monte 2013
- Dal Monte M, Casini G, Marca G, Isacchi B, Filippi L, Bagnoli P. Eye drop propranolol administration promotes the recovery of oxygen‐induced retinopathy in mice. Experimental Eye Research 2013;111:27‐35. [DOI: 10.1016/j.exer.2013.03.013; PUBMED: 23528535] [DOI] [PubMed] [Google Scholar]
de Graaf 2011
- Graaf M, Breur JM, Raphaël MF, Vos M, Breugem CC, Pasmans SG. Adverse effects of propranolol when used in the treatment of hemangiomas: a case series of 28 infants. Journal of the American Academy of Dermatology 2011;65(2):320‐7. [DOI: 10.1016/j.jaad.2010.06.048; PUBMED: 21601311] [DOI] [PubMed] [Google Scholar]
Drolet 2013
- Drolet BA, Frommelt PC, Chamlin SL, Haggstrom A, Bauman NM, Chiu YE, et al. Initiation and use of propranolol for infantile hemangioma: report of a consensus conference. Pediatrics 2013;131(1):128‐40. [DOI: 10.1542/peds.2012-1691; PUBMED: 23266923] [DOI] [PMC free article] [PubMed] [Google Scholar]
Erbay 2010
- Erbay A, Sarialioglu F, Malbora B, Yildirim SV, Varan B, Tarcan A, et al. Propranolol for infantile hemangiomas: a preliminary report on efficacy and safety in very low birth weight infants. Turkish Journal of Pediatrics 2010;52(5):450‐6. [PUBMED: 21434528] [PubMed] [Google Scholar]
ET‐ROP Group 2003
- Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Archives of Ophthalmology 2003;121(12):1684–94. [DOI: 10.1001/archopht.121.12.1684; PUBMED: 14662586] [DOI] [PubMed] [Google Scholar]
Ferrara 1997
Filippi 2014
- Filippi L, Dal Monte M, Casini G, Daniotti M, Sereni F, Bagnoli P. Infantile hemangiomas, retinopathy of prematurity and cancer: a common pathogenetic role of the β‐adrenergic system. Medicinal Research Reviews 2014;35(3):619‐52. [DOI: 10.1002/med.21336; PUBMED: 25523517] [DOI] [PubMed] [Google Scholar]
Fonseca 2010
- Fonseca VA. Effects of beta‐blockers on glucose and lipid metabolism. Current Medical Research and Opinion 2010;26(3):615‐29. [DOI: 10.1185/03007990903533681; PUBMED: 20067434] [DOI] [PubMed] [Google Scholar]
Frishman 1988
- Frishman WH. Beta‐adrenergic receptor blockers. Adverse effects and drug interactions. Hypertension 1988;11(3 Pt 2):II21‐9. [PUBMED: 2895072] [DOI] [PubMed] [Google Scholar]
Frost 2013
- Frost G, Relic J. Dangers of propranolol in preterm infants. Australasian Journal of Dermatology 2013;54(3):237‐8. [DOI: 10.1111/ajd.12079; PUBMED: 23905978] [DOI] [PubMed] [Google Scholar]
Gilbert 2008
- Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Human Development 2008;84(2):77‐82. [DOI: 10.1016/j.earlhumdev.2007.11.009; PUBMED: 18234457] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 15 November 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Griffiths 1954
- Griffiths R. The abilities of babies: a study in mental measurement. New York, US: McGraw‐Hill Book Company, Inc, 1954. [Google Scholar]
Hellström 2013
- Hellström A, Smith LE, Dammann O. Retinopathy of prematurity. Lancet 2013;382(9902):1445‐57. [DOI: 10.1016/S0140-6736(13)60178-6; PUBMED: 23782686] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hård 2011
- Hård AL, Hellström A. On the use of antiangiogenetic medications for retinopathy of prematurity. Acta Paediatrica 2011;100(8):1063‐5. [DOI: 10.1111/j.1651-2227.2011.02330.x; PUBMED: 21517962] [DOI] [PMC free article] [PubMed] [Google Scholar]
International Committee Retinopathy 2005
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Archives of Ophthalmology 2005;123(7):991–9. [DOI: 10.1001/archopht.123.7.991; PUBMED: 16009843] [DOI] [PubMed] [Google Scholar]
Jobe 2001
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine 2001;163(7):1723‐9. [DOI: 10.1164/ajrccm.163.7.2011060; PUBMED: 11401896] [DOI] [PubMed] [Google Scholar]
Love 2004
- Love JN, Sikka N. Are 1‐2 tablets dangerous? Beta‐blocker exposure in toddlers. Journal of Emergency Medicine 2004;26(3):309‐14. [DOI: 10.1016/j.jemermed.2003.11.015; PUBMED: 15028329] [DOI] [PubMed] [Google Scholar]
Léauté‐Labrèze 2008
- Léauté‐Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. New England Journal of Medicine 2008;358(24):2649‐51. [DOI: 10.1056/NEJMc0708819; PUBMED: 18550886] [DOI] [PubMed] [Google Scholar]
Léauté‐Labrèze 2015
- Léauté‐Labrèze C, Hoeger P, Mazereeuw‐Hautier J, Guibaud L, Baselga E, Posiunas G, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. New England Journal of Medicine 2015;372(8):735‐46. [DOI: 10.1056/NEJMoa1404710; NCT01056341; PUBMED: 25693013] [DOI] [PubMed] [Google Scholar]
Löfqvist 2006
- Löfqvist C, Andersson E, Sigurdsson J, Engström E, Hàrd AL, Niklasson A, et al. Longitudinal postnatal weight and insulin‐like growth factor I measurements in the prediction of retinopathy of prematurity. Archives of Ophthalomolgy 2006; Vol. 124, issue 12:1711‐8. [DOI: 10.1001/archopht.124.12.1711; PUBMED: 17159030] [DOI] [PubMed]
Manunza 2010
- Manunza F, Syed S, Laguda B, Linward J, Kennedy H, Gholam K, et al. Propranolol for complicated infantile haemangiomas: a case series of 30 infants. British Journal of Dermatology 2010;162(2):466‐8. [DOI: 10.1111/j.1365-2133.2009.09597.x; PUBMED: 20055816] [DOI] [PubMed] [Google Scholar]
Martini 2011
- Martini D, Monte MD, Ristori C, Cupisti E, Mei S, Fiorini P, et al. Antiangiogenic effects of β2‐adrenergic receptor blockade in a mouse model of oxygen‐induced retinopathy. Journal of Neurochemistry 2011;119(6):1317‐29. [DOI: 10.1111/j.1471-4159.2011.07530.x; PUBMED: 21988318] [DOI] [PubMed] [Google Scholar]
Mercier 2007
- Mercier CE, Dunn MS, Ferrelli KR, Howard DB, Soll RF, Vermont Oxford Network ELBW Infant Follow‐Up Study Group. Neurodevelopmental outcome of extremely low birth weight infants from the Vermont Oxford network: 1998‐2003. Neonatology 2010;97(4):329‐38 Erratum in: Neonatology 2010; 98(4):419. [DOI: 10.1159/000260136; PUBMED: 19940516] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mutlu 2013
- Mutlu FM, Sarici SU. Treatment of retinopathy of prematurity: a review of conventional and promising new therapeutic options. International Journal of Ophthalmology 2013;6(2):228‐36. [DOI: 10.3980/j.issn.2222-3959.2013.02.23; PUBMED: 23641347] [DOI] [PMC free article] [PubMed] [Google Scholar]
Padrini 2014
- Padrini L, Isacchi B, Bilia AR, Pini A, Lanzi C, Masini E, et al. Pharmacokinetics and local safety profile of propranolol eye drops in rabbits. Pediatric Research 2014;76(4):378‐85. [DOI: 10.1038/pr.2014.108; PUBMED: 25029260] [DOI] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529‐34. [PUBMED: 305471] [DOI] [PubMed] [Google Scholar]
Pavlakovic 2010
- Pavlakovic H, Kietz S, Lauerer P, Zutt M, Lakomek M. Hyperkalemia complicating propranolol treatment of an infantile hemangioma. Pediatrics 2010;126(6):1589‐93. [DOI: 10.1542/peds.2010-0077; PUBMED: 21115582] [DOI] [PubMed] [Google Scholar]
Praveen 2009
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ristori 2011
- Ristori C, Filippi L, Dal Monte M, Martini D, Cammalleri M, Fortunato P, et al. Role of the adrenergic system in a mouse model of oxygen‐induced retinopathy: antiangiogenic effects of beta‐adrenoreceptor blockade. Investigative Ophthalmology & Visual Science 2011;52(1):155‐70. [DOI: 10.1167/iovs.10-5536; PUBMED: 20739470] [DOI] [PubMed] [Google Scholar]
Sankar 2016
- Sankar MJ, Sankar J, Mehta M, Bhat V, Srinivasan R. Anti‐vascular endothelial growth factor (VEGF) drugs for treatment of retinopathy of prematurity. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD009734.pub2] [DOI] [PubMed] [Google Scholar]
Sans 2009
- Sans V, Roque ED, Berge J, Grenier N, Boralevi F, Mazereeuw‐Hautier J, et al. Propranolol for severe infantile hemangiomas: follow‐up report. Pediatrics 2009;124(3):e423‐31. [DOI: 10.1542/peds.2008-3458; PUBMED: 19706583] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Smith 1999
- Smith LE, Shen W, Perruzzi C, Soker S, Kinose F, Xu X, et al. Regulation of vascular endothelial growth factor‐dependent retinal neovascularization by insulin‐like growth factor‐1 receptor. Nature Medicine 1999;5(12):1390‐5. [DOI: 10.1038/70963; PUBMED: 10581081] [DOI] [PubMed] [Google Scholar]
Smith 2003
- Smith LE. Pathogenesis of retinopathy of prematurity. Seminars in Neonatology 2003;8(6):469‐73. [DOI: 10.1016/S1084-2756(03)00119-2; PUBMED: 15001119] [DOI] [PubMed] [Google Scholar]
Valcamonico 2007
- Valcamonico A, Accorsi P, Sanzeni C, Martelli P, Boria P, Cavazza A, et al. Mid‐ and long‐term outcome of extremely low birth weight (ELBW) infants: an analysis of prognostic factors. Journal of Maternal‐fetal & Neonatal Medicine 2007;20(6):465‐71. [DOI: 10.1080/14767050701398413; PUBMED: 17674256] [DOI] [PubMed] [Google Scholar]
Walsh 2003
- Walsh MC, Wilson‐Costello D, Zadell A, Newman N, Fanaroff A. Safety, reliability, and validity of a physiologic definition of bronchopulmonary dysplasia. Journal of Perinatology 2003;23(6):451‐6. [DOI: 10.1038/sj.jp.7210963; PUBMED: 13679930] [DOI] [PubMed] [Google Scholar]


