Abstract
Background
Follicular aspiration under transvaginal ultrasound guidance is routinely performed as part of assisted reproductive technology (ART) to retrieve oocytes for in vitro fertilisation (IVF). However, controversy as to whether follicular flushing following aspiration yields a larger number of oocytes and hence is associated with greater potential for pregnancy than aspiration only is ongoing.
Objectives
To assess the safety and efficacy of follicular flushing as compared with aspiration only performed in women undergoing ART.
Search methods
We searched the following electronic databases up to 18 July 2017: Cochrane Gynaecology and Fertility Group (CGF) Specialised Register of Controlled Trials, the CENTRAL Register of Studies Online (CRSO), MEDLINE, Embase, PsycINFO, and the Cumulative Index to Nursing and Allied Health Literature (CINAHL). We also searched the trial registries ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform to identify ongoing and registered trials up to 4 July 2017. We reviewed the reference lists of reviews and retrieved studies to identify further potentially relevant studies.
Selection criteria
We included randomised controlled trials (RCTs) that compared follicular aspiration and flushing with aspiration alone in women undergoing ART using their own gametes. Primary outcomes were live birth rate and miscarriage rate per woman randomised.
Data collection and analysis
Two independent review authors assessed studies against the inclusion criteria, extracted data, and assessed risk of bias. A third review author was consulted if required. We contacted study authors as required. We analysed dichotomous outcomes using Mantel‐Haenszel odds ratios (ORs), 95% confidence intervals (CIs), and a fixed‐effect model, and we analysed continuous outcomes using mean differences (MDs) between groups presented with 95% CIs. We examined the heterogeneity of studies via the I2 statistic. We assessed the quality of evidence by using GRADE (Grades of Recommendation, Assessment, Development and Evaluation) criteria.
Main results
We included ten studies, with a total of 928 women. All included studies reported outcomes per woman randomised. We assessed no studies as being at low risk of bias across all domains and found that the main limitation was lack of blinding. Using the GRADE method, we determined that the quality of the evidence ranged from moderate to very low, and we identified issues arising from risk of bias, imprecision, and inconsistency.
Comparing follicular flushing to aspiration alone revealed probably little or no difference in the live birth rate (OR 0.95, 95% CI 0.58 to 1.56; three RCTs; n = 303; I2 = 30%; moderate‐quality evidence). This suggests that with a live birth rate of approximately 41% with aspiration alone, the equivalent live birth rate with follicular flushing is likely to lie between 29% and 52%. None of the included studies reported on the primary outcome of miscarriage rate.
Data show probably little or no difference in oocyte yield (MD ‐0.28 oocytes, 95% CI ‐0.64 to 0.09; six RCTs; n = 708; I2 = 0%; moderate‐quality evidence). Very low‐quality evidence suggests that the duration of oocyte retrieval was longer in the follicular flushing group than in the aspiration only group (MD 166.01 seconds, 95% CI 141.96 to 190.06; six RCTs; n = 714; I2 = 88%). We found no evidence of a difference in the total number of embryos per woman randomised (MD ‐0.10 embryos, 95% CI ‐0.34 to 0.15; two RCTs; n = 160; I2 = 58%; low‐quality evidence) and no evidence of a difference in the number of embryos cryopreserved (meta‐analysis not possible). Data show probably little or no difference in the clinical pregnancy rate (OR 1.07, 95% CI 0.78 to 1.46; five RCTs; n = 704; I2 = 49%; moderate‐quality evidence). Only two studies reported on adverse outcomes: One reported no differences in patient‐reported adverse outcomes (depression, anxiety, and stress), and the other reported no differences in needle blockage, vomiting, and hypotension. No studies reported on safety.
Authors' conclusions
This review suggests that follicular flushing probably has little or no effect on live birth rates compared with aspiration alone. None of the included trials reported on effects of follicular aspiration and flushing on the miscarriage rate. Data suggest little or no difference between follicular flushing and aspiration alone with respect to oocyte yield, total embryo number, or number of cryopreserved embryos. In addition, follicular flushing probably makes little or no difference in the clinical pregnancy rate. Evidence was insufficient to allow any firm conclusions with respect to adverse events or safety.
Plain language summary
Follicular flushing during oocyte retrieval in assisted reproductive technology
Review question
Cochrane authors sought to assess the safety and efficacy of flushing follicles as part of egg collection in women undergoing interventions to help them get pregnant, termed assisted reproductive technology (ART).
Background
Couples who have difficulty becoming pregnant naturally may choose to have interventions to help them get pregnant. These interventions are known as assisted reproductive technology (ART). One of these interventions is in vitro fertilisation (IVF), or a variant of IVF, called intracytoplasmic sperm injection (ICSI). During IVF, controlled ovarian stimulation uses hormones to stimulate multiple eggs to develop in the ovaries. After ovarian stimulation, a needle guided by ultrasound is used to collect these eggs that are inside follicles. Instead of using only suction to obtain the contents of follicles (aspiration), it has been proposed that flushing the follicles after aspiration may lead to collection of more eggs and higher chances of becoming pregnant and having a baby. This technique is called follicular flushing.
Study characteristics
This review included ten research studies that randomly assigned a total of 928 women to follicular aspiration alone or follicular flushing after aspiration. To see if there was a difference between the two techniques, we wanted to look at the main results of live birth rate (number of babies born per 1000 women) and miscarriage rate (number of miscarriages per 1000 women). We carried out a comprehensive search to identify all relevant research in this field available in July 2017.
Key results
Three studies reported on the main result of live birth rate and noted that follicular flushing probably has little or no effect on live birth rate compared with aspiration alone (moderate‐quality evidence). This suggests that if a live birth rate of approximately 41% is seen with aspiration alone, the equivalent live birth rate with follicular flushing is likely to lie between 29% and 52%. None of the included studies reported on the miscarriage rate.
Studies also found that follicular flushing probably makes little or no difference in the number of eggs retrieved, the number of embryos, or the clinical pregnancy rate compared with aspiration alone. Although the quality of evidence was very low, it appears that follicular flushing takes much longer to perform than aspiration alone. Evidence was insufficient to permit any firm conclusions with respect to adverse events or safety.
More research is needed to find out whether any specific patient groups would benefit from follicular flushing.
Quality of the evidence
The quality of evidence for the main outcome of live birth rate was moderate. The quality of evidence for the other outcomes ranged from very low to moderate. The main limitations of included studies were lack of blinding (the process whereby women participating in the trial as well research staff are not aware of the intervention used), inconsistency (differences between different studies), and imprecision (insufficient data).
Summary of findings
Summary of findings for the main comparison. Follicular flushing compared with placebo in assisted reproductive techniques.
| Follicular flushing compared with placebo in assisted reproductive techniques | ||||||
| Patient or population: assisted reproductive techniques Setting: ART clinic Intervention: follicular flushing Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with aspiration only | Risk with follicular flushing | |||||
| Live birth rate | 414 per 1000 | 401 per 1000 (290 to 524) | OR 0.95 (0.58 to 1.56) | 303 (3 RCTs) | ⊕⊕⊕⊝ MODERATEa | |
| Miscarriage rate | not estimable | (0 studies) | ‐ | No data for this outcome were reported in any of the included studies. | ||
| Oocyte yield | Mean oocyte yield was 7.13. | MD 0.02 lower (0.1 lower to 0.07 higher) | ‐ | 708 (6 RCTs) | ⊕⊕⊕⊝ MODERATEa | |
| Duration of oocyte retrieval | Mean duration of oocyte retrieval was 285.33 seconds. | MD 70.29 higher (62.15 higher to 78.44 higher) | ‐ | 714 (6 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,c | |
| Total number of embryos | Mean total number of embryos was 1.50. | MD 0 (0.05 lower to 0.04 higher) | ‐ | 160 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b | |
| Clinical pregnancy rate | 362 per 1000 | 378 per 1000 (307 to 453) | OR 1.07 (0.78 to 1.46) | 704 (5 RCTs) | ⊕⊕⊕⊝ MODERATEa | |
| Adverse events | ‐ | (2 RCTs) | ⊕⊕⊕⊝ MODERATEa | One study reported no differences in patient‐reported adverse outcomes (depression, anxiety, and stress). Another study reported higher doses of analgesia required in the follicular flushing group compared with the aspiration alone group (median 100 mg (range 50 to 100 mg) vs 50 mg (range 50 to 100 mg)). |
||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; OR: odds ratio; RCT, randomised controlled trial. | ||||||
| GRADE Working Group grades of evidence. High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aRisk of bias ‐ incorporates at least one open‐label study. Downgraded one level
bInconsistency ‐ high degree of heterogeneity. Downgraded one level
cImprecision ‐ wide confidence intervals. Downgraded one level
Background
Description of the condition
Assisted reproductive technology (ART) requires handling oocytes and embryos outside the woman's body. The technique involves ovarian stimulation, monitoring of follicular growth, oocyte recovery, sperm preparation and insemination, embryo culture, embryo transfer, and luteal support. Other variables, particularly female age, can significantly affect the number of oocytes retrieved and the success rate of ART.
Description of the intervention
Once maturity of the follicles is achieved, human chorionic gonadotropin (hCG) or recombinant luteinising hormone (rLH) is used to trigger oocyte maturation. Oocyte pickup is performed approximately 36 hours later. Technical details of oocyte recovery vary between fertility centres, especially with regard to type of anaesthesia (local, sedation, or general), type of aspiration needle (wide or narrow bore, single or double channel), route of retrieval (transvaginal or abdominal), aspiration alone or aspiration with follicular flushing, type of flushing medium, and the collecting system.
The number of embryos obtained is dependent on the number of oocytes retrieved (Wood 2000). To maximise the number of oocytes recovered, investigators have suggested follicular aspiration followed by a single 2‐mL flush (el Hussein 1992). Waterstone and Parson reported that use of double‐lumen needles and flushing yielded 20% more oocytes (Waterstone 1992). On the contrary, other studies found no difference in number of oocytes collected, fertilisation rate, embryo quality, or pregnancy rate (Haydardedeoglu 2011; Haydardedeoglu 2017; Kara 2012; Kingsland 1991; Knight 2001; Levens 2009; Mok‐Lin 2013; Tan 1992; von Horn 2017). Tan 1992 suggested that aspiration without flushing reduced operative time and decreased the amount of anaesthetic required.
How the intervention might work
The place of follicular flushing during oocyte recovery in ART remains uncertain. Benefits of flushing include the possibility of obtaining more oocytes and subsequently more embryos. Whether this translates into higher pregnancy and live birth rates remains controversial. However, flushing may be associated with longer operative time and larger amounts of required anaesthetics and analgesics. From the patient's perspective, it could also mean higher costs. Moreover, anaesthetics such as propofol could have detrimental effects on embryos, at least in the mouse model (Janssenwillen 1997; Tatone 1998). Flushing could remove some of the follicular cells that might have an important endocrine luteal support function.
Why it is important to do this review
The prevalence of subfertility and the significant costs of assisted conception make assessment of ART techniques imperative to establish which are more effective in terms of attaining a live birth, and which are cost‐beneficial, with a view toward improving treatment outcomes. This review provides information for women and clinicians and identifies aspects that require future study.
Objectives
To assess the safety and efficacy of follicular flushing as compared with aspiration only performed in women undergoing ARTs.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) were eligible for inclusion. We included cross‐over trials only when pre‐cross‐over data were extractable for analysis. We included conference abstracts and handled these in the same way as full publications.
Types of participants
Participants were women who underwent assisted conception treatment by in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI) using their own gametes.
Types of interventions
We included trials if they investigated any form of follicular aspiration and flushing during oocyte retrieval and compared effects of follicular flushing versus a control procedure by which only aspiration was performed.
We included trials in which investigators replaced embryos resulting from oocytes derived from mixed groups of flushed and unflushed follicles in the same woman. We performed sensitivity analysis for inclusion or exclusion of these trials, when appropriate.
For inclusion in the review, trials had to report that all recruited women had undergone only one cycle of treatment within the context of the trial and had had embryos replaced in the uterine cavity in fresh or frozen‐thawed cycles. We did not exclude trials where embryo replacement did not take place because of failure of fertilisation or failure of the embryo to divide further (cleavage arrest).
We excluded trials that directly compared different methods of follicular flushing (without an aspiration only control group).
Types of outcome measures
Primary outcomes
Live birth rate per woman randomised, with live birth defined as per the International Committee for Monitoring Assisted Reproductive Technology (ICMART) as "the complete expulsion or extraction from a woman of a product of fertilization, after 22 completed weeks of gestational age; which, after such separation, breathes or shows any other evidence of life, such as heart beat, umbilical cord pulsation or definite movement of voluntary muscles, irrespective of whether the umbilical cord has been cut or the placenta is attached. A birth weight of 500 grams or more can be used if gestational age is unknown" (Zegers‐Hochschild 2017)
Miscarriage rate per woman randomised
Secondary outcomes
Oocyte yield, defined as number of oocytes retrieved per woman randomised
Duration of oocyte retrieval
Total number of embryos per woman randomised
Number of cryopreserved embryos per woman randomised
Clinical pregnancy rate per woman randomised, defined per ICMART as the presence of one or more gestational sacs on ultrasound or definitive clinical signs of pregnancy (Zegers‐Hochschild 2017)
Ongoing pregnancy rate per woman randomised, defined as a pregnancy of 12 or more weeks' gestation
Adverse events as defined by trialists (patient‐reported outcomes and surgical complications including needle blockage, vomiting, and hypotension)
Search methods for identification of studies
Electronic searches
We used the following search strategy to obtain all reports that described (or might have described) RCTs of follicular flushing.
Cochrane Gynaecology and Fertility Group (CGFG) Specialised Register of Controlled Trials, PROCITE platform (searched 18 July 2017) (Appendix 1).
Cochrane Central Register of Controlled Trials; via the Cochrane Register of Studies Online, CRSO Web platform (searched 18 July 2017) (Appendix 2).
MEDLINE Ovid (searched from 1946 to 18 July 2017) (Appendix 3).
Embase Ovid (searched from 1980 to 18 July 2017) (Appendix 4).
PsycINFO Ovid (searched from 1806 to 18 July 2017) (Appendix 5).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) Ebsco (from 1961 to 18 July 2017) (Appendix 6).
Searching other resources
We handsearched reference lists of relevant trials and systematic reviews retrieved by the search and contacted experts in the field to obtain additional data
We handsearched relevant journals and conference abstracts that were not covered in the CGFG register, in liaison with the Information Specialist
We searched for ongoing and registered trials on clinical trials.gov (http://www.clinicaltrials.gov) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) search portal (http://www.who.int/trialsearch/Default.aspx) (all "other resources" searched 4 July 2017)
Data collection and analysis
Selection of studies
After an initial screen of titles and abstracts retrieved by the search (EG and PM), review authors retrieved the full text of all potentially eligible studies. Two review authors (EG and PM) independently examined these full texts for compliance with the inclusion criteria (Appendix 7) and selected eligible studies. We corresponded with study investigators as required to clarify study eligibility. We resolved disagreements by discussion or through arbitration by a third review author (IG). We documented the selection process using a PRISMA flow chart (Figure 1).
1.

Study flow diagram.
Data extraction and management
Two review authors (EG and PM) independently extracted data from eligible studies using the data extraction proforma that had been designed and pilot‐tested by the review authors (Appendix 8) and resolved disagreements by discussion or through arbitration by a third review author (IG). Data extracted included study characteristics and outcome data. When studies had multiple publications, we collated multiple reports of the same trial under a single study ID with multiple references. We corresponded with study investigators to ask for further data or methods and/or results, as required.
Assessment of risk of bias in included studies
Two review authors (EG and PM) independently assessed the included studies for risk of bias using the Cochrane risk of bias assessment tool to assess the following: selection (random sequence generation and allocation concealment); performance (blinding of participants and personnel); detection (blinding of outcome assessors); attrition (incomplete outcome data); reporting (selective reporting); and other bias (Higgins 2011). We assigned judgement as recommended in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 8.5 (Higgins 2011b) and resolved disagreements by discussion or through arbitration by a third review author (IG). We described all judgements fully, presented conclusions in the risk of bias tables, and incorporated this information into our interpretation of review findings by performing sensitivity analyses.
Measures of treatment effect
We performed statistical analysis in accordance with Cochrane guidelines. For dichotomous data (e.g. live births), we used the number of events in each group to calculate Mantel‐Haenszel odds ratios (ORs) with 95% confidence intervals (CIs), then combined these for meta‐analysis using RevMan 5.3 software and a fixed‐effect model. For continuous data, we calculated mean differences (MDs) between treatment groups and presented these along with 95% CIs.
Unit of analysis issues
The primary analysis was per woman randomised. We summarised in an additional table data that did not allow valid analysis (e.g. 'per cycle' data, per pregnancy data) but did not include these data in a meta‐analysis. We counted a multiple birth as a single live birth event. If we identified any cross‐over trials, we planned to use only first‐phase data.
Dealing with missing data
We analysed data on an intention‐to‐treat basis as far as possible and attempted to obtain missing data from the original trialists. When these were unobtainable, we undertook imputation of individual values for live birth only: We assumed that live birth did not occur in participants without reported outcomes. We analysed other outcomes using only available data. Any imputation undertaken was subjected to sensitivity analysis.
When studies reported sufficient detail to allow calculation of mean differences but provided no information on associated standard deviation (SD), we assumed that the outcome had a standard deviation equal to the highest standard deviation provided by other studies included within the same analysis.
Assessment of heterogeneity
We used statistical heterogeneity, ascertained by measure of the I2, to determine whether the clinical and methodological characteristics of included studies were sufficiently similar for meta‐analysis. We regarded an I2 measurement greater than 50% as indicating substantial heterogeneity (Higgins 2011). We explored substantial heterogeneity by conducting planned subgroup analyses as detailed below.
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, review authors aimed to minimise the potential for bias by searching multiple databases and grey literature. We planned that if at least 10 studies were included in the same analysis, we would produce a funnel plot to assess publication bias.
Data synthesis
We combined data from the primary studies using a fixed‐effect model for the comparison of aspiration/flush versus aspiration only.
We did not stratify data. In meta‐analyses, we graphically displayed an increase in the risk of a particular outcome that may be beneficial (e.g. live birth) or detrimental (e.g. miscarriage) to the right of the centre line and a decrease in the risk of an outcome to the left of the centre line.
Subgroup analysis and investigation of heterogeneity
When data were available, we conducted subgroup analyses to obtain separate evidence for primary outcomes within the following subgroups.
Age: women younger or older than 40 years.
Poor ovarian reserve: as determined by follicle‐stimulating hormone (FSH) levels, anti‐Müllerian hormone (AMH) levels, and/or antral follicle count (AFC). We used cutoff values for subgrouping as defined by trialists, or, in cases for which individual data were reported, we used the following cutoffs: FSH 10 IU/L, AMH 0.8 ng/mL, and AFC 6 follicles.
Poor response to ovarian stimulation: development of fewer than five mature follicles following controlled ovarian stimulation for IVF or ICSI versus normal response; alternatively, poor response as defined by trialists.
When possible, we extracted data on these subgroups directly from the included trials. When not reported, we used mean trial data (e.g. mean trial FSH level) to place the whole trial into one of these subgroups.
We performed a post hoc subgroup analysis for the outcomes of live birth rate and miscarriage rate, while taking statistical heterogeneity into account. When we detected substantial heterogeneity (I2 > 50%), we sought clinical and methodological differences between studies that may have accounted for this.
Sensitivity analysis
We conducted sensitivity analyses for primary outcomes to determine whether conclusions were robust to arbitrary decisions made regarding eligibility and analysis. These analyses included consideration of whether review conclusions would have differed if:
eligibility had been restricted to studies without high risk of bias (defined as studies at low risk of selection bias and with no domains at high risk of bias);
a random effects model had been adopted;
alternative imputation strategies had been implemented; or
we had included only fully published trials.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared a 'Summary of findings' table using GRADEpro and Cochrane methods to evaluate the overall quality of the body of evidence found for main review outcomes for the main review comparison of follicular aspiration + flushing versus aspiration only (GRADEpro GDT 2015). We assessed the quality of evidence by using GRADE criteria: risk of bias, consistency of effect, imprecision, indirectness, and publication bias. Two review authors (EG and PM) who worked independently made judgements about evidence quality (high, moderate, low, or very low) and resolved disagreements by discussion or through arbitration by a third review author (IG). We justified, documented, and incorporated judgements into reporting of results for each outcome.
We used the following outcomes for GRADE assessment.
Live birth rate.
Miscarriage rate.
Oocyte yield.
Duration of oocyte retrieval.
Total number of embryos.
Clinical pregnancy rate.
Adverse events.
Results
Description of studies
Results of the search
For the 2018 update, our electronic search on 18 July 2017 yielded 330 articles. We identified 11 additional articles via other sources: 10 by searching trial registries and 1 by reviewing the reference list of another article. After removal of duplicates, we kept 233 articles for screening. Of these, we excluded 202 records as they were clearly not relevant (Characteristics of excluded studies). Three studies were ongoing trials that had not yet reported their results (Characteristics of ongoing studies). We obtained and reviewed the full text for the remaining 28 articles.
We identified 28 trials that provided data on follicular flushing during oocyte retrieval in assisted reproductive cycles (Avila 2013; Aydin 2017; Bagtharia 2005; Biljan 1997; Dean 1997; el Hussein 1992; Faller 2010; Ghosh 2002; Gordon 2002; Haines 1989; Haydardedeoglu 2011; Haydardedeoglu 2017; Kara 2012; Khalifa 1999; Kingsland 1991; Knight 2001; Lenz 1987; Levens 2009; Mehri 2014; Mendez Lozano 2008; Mok‐Lin 2013; Neyens 2016; Pirrello 2011; Scott 1989; Tan 1992; von Horn 2017; Waterstone 1992; Ziebe 2000), along with three ongoing trials (NCT01329302; NCT02277210; NCT02641808). See Characteristics of ongoing studies.
Ten studies met the inclusion criteria for this review (Haines 1989; Haydardedeoglu 2011; Haydardedeoglu 2017; Kara 2012; Kingsland 1991; Levens 2009; Mok‐Lin 2013; Scott 1989; Tan 1992; von Horn 2017). Of these, we included four in our qualitative synthesis (Haines 1989; Kingsland 1991; Mok‐Lin 2013; Tan 1992), and we included nine in our quantitative analysis (meta‐analysis) (Haydardedeoglu 2011; Haydardedeoglu 2017; Kara 2012; Kingsland 1991; Levens 2009; Mok‐Lin 2013; Scott 1989; Tan 1992; von Horn 2017). We have presented the trial flow diagram in Figure 1.
Included studies
Study design and setting
We included ten parallel‐design randomised controlled trials, all of which have been published as full articles. All were single‐centre studies and three were carried out in the USA (Levens 2009; Mok‐Lin 2013; Scott 1989); three in Turkey (Haydardedeoglu 2011; Haydardedeoglu 2017; Kara 2012); two in the UK (Kingsland 1991; Tan 1992); one in Australia (Haines 1989); and one in Germany (von Horn 2017).
Participants
The ten included studies included a total of 928 participants: 477 women in the intervention group and 451 in the control group.
Four studies recruited women with poor ovarian response (Haydardedeoglu 2017; Levens 2009; Mok‐Lin 2013; von Horn 2017), and each study defined poor ovarian response differently. Levens 2009 defined it as a cumulative follicle count of 4‐8 follicles greater than or equal to 12 mm with at least 2 follicles greater than 16 mm; Mok‐Lin 2013, it was defined as 4 or fewer follicles greater than or equal to 12 mm; in Haydardedeoglu 2017, it was defined as 5 or fewer follicles greater than or equal to 13mm in size and serum progesterone less than 1.5 ng/ml; and von Horn 2017 as five or fewer follicles greater than 10 mm. Apart from poor ovarian response, all patients included in Haydardedeoglu 2017 also had poor ovarian reserve, as defined by an antral follicle count (AFC) less than 6 and an anti‐Müllerian hormone (AMH) level less than 0.8 ng/mL. One study recruited patients with tubal damage (Kingsland 1991). Five studies did not specify any inclusion criteria (Haines 1989; Haydardedeoglu 2011; Kara 2012; Scott 1989; Tan 1992).
Two studies excluded patients with poor ovarian response or high ovarian response (Haydardedeoglu 2011; Tan 1992). Other exclusion factors were natural IVF ‐ as reported in Mok‐Lin 2013 and Haydardedeoglu 2017 ‐ and absent ovary or ovary(/ies) predicted to be difficult to access ‐ as reported in von Horn 2017. Four studies did not specify any exclusion criteria (Haines 1989; Kara 2012; Kingsland 1991; Scott 1989).
Interventions
One study used clomiphene citrate to achieve ovarian hyperstimulation (Haines 1989). All other studies employed gonadotropin‐releasing hormone agonist in a long‐luteal protocol (Kingsland 1991), a low‐dose luteal agonist protocol (Haydardedeoglu 2017; Kara 2012), an antagonist protocol (Haydardedeoglu 2011; Haydardedeoglu 2017; Mok‐Lin 2013), a long‐follicular protocol (Tan 1992), an FSH antagonist protocol (Haydardedeoglu 2017), a long‐luteal or microdose follicular flare protocol (Levens 2009), or a long unspecified protocol (Scott 1989).
Some studies induced final oocyte maturation with 5000 IU hCG (Haines 1989; Kingsland 1991; von Horn 2017), and others with 10,000 IU hCG (Haydardedeoglu 2017; Kara 2012; Levens 2009; Mok‐Lin 2013; Tan 1992). One study did not specify the dose of hCG used (Haydardedeoglu 2011), and another did not clarify whether hCG was used (Scott 1989).
Two studies used the same type of double‐lumen needle: Kingsland 1991 without and Tan 1992 with removal of the inner channel to convert it to a single‐channel needle. The other studies used single‐ or double‐lumen needles that were ‐ in Levens 2009, Haydardedeoglu 2011, Mok‐Lin 2013, and Haydardedeoglu 2017 ‐ or were not ‐ in Haines 1989, Scott 1989, and Kara 2012 ‐ standardised for length plus/minus diameter, to control for flow dynamics within the needle. One study used a 17G Steiner‐Tan Needle, which is described as a single‐lumen needle surrounded by a plastic tube that allows passage of flushing medium for follicular flushing, and a 17G Gynetics single‐lumen needle in the control arm (von Horn 2017).
Three studies used IVF (Kingsland 1991; Scott 1989; Tan 1992), and three studies used ICSI (Haydardedeoglu 2011; Haydardedeoglu 2017; Kara 2012) for fertilisation. Three studies used both IVF and ICSI (Levens 2009; Mok‐Lin 2013; von Horn 2017). One study did not specify how fertilisation occurred (Haines 1989).
Two studies transferred up to three embryos (Kingsland 1991; Tan 1992), and two studies up to four embryos (Haydardedeoglu 2011; Kara 2012). Six studies did not specifically comment on the number of embryos transferred (Haines 1989; Haydardedeoglu 2017; Levens 2009; Mok‐Lin 2013; Scott 1989; von Horn 2017), although the mean number in Haydardedeoglu 2017 was less than two, and in Levens 2009 and Mok‐Lin 2013 was less than three.
Outcomes
Primary outcomes
Three studies reported on the primary outcome of live birth rate per woman randomised (Haydardedeoglu 2011; Mok‐Lin 2013; Haydardedeoglu 2017). None of the included studies reported on the primary outcome of miscarriage per woman randomised.
Secondary outcomes
Six studies reported on oocyte yield (Haydardedeoglu 2011; Haydardedeoglu 2017; Kara 2012; Levens 2009; Scott 1989; von Horn 2017), and eight studies on duration of oocyte retrieval (Haydardedeoglu 2011; Haydardedeoglu 2017; Kara 2012; Kingsland 1991; Levens 2009; Mok‐Lin 2013; Tan 1992; von Horn 2017).
Two studies reported on the total number of embryos per woman randomised (Haydardedeoglu 2017; von Horn 2017); two on the number of cryopreserved embryos per woman randomised (Haydardedeoglu 2011; Mok‐Lin 2013); five on the clinical pregnancy rate (Haydardedeoglu 2011; Haydardedeoglu 2017; Kara 2012; Mok‐Lin 2013; Tan 1992); four on the ongoing pregnancy rate (Kara 2012; Kingsland 1991; Levens 2009; von Horn 2017); one on adverse events including blockage of the needle, vomiting, and hypotension (Tan 1992); and one on adverse events including patient depression, anxiety, and stress (von Horn 2017).
Author correspondence
We contacted Haydardedeoglu 2017 and von Horn 2017 to further clarify some of the data included in the original papers. To date, we have received a response only from von Horn 2017.
Excluded studies
The previously published version of this systematic review excluded 11 studies (Bagtharia 2005; Biljan 1997; Dean 1997; el Hussein 1992; Gordon 2002; Khalifa 1999; Knight 2001; Lenz 1987; Mendez Lozano 2008; Waterstone 1992; Ziebe 2000). This update excluded seven additional studies (Avila 2013; Aydin 2017; Faller 2010; Ghosh 2002; Mehri 2014; Neyens 2016; Pirrello 2011).
Of the studies excluded from this update, five were not RCTs (Avila 2013; Aydin 2017; Ghosh 2002; Mehri 2014; Neyens 2016). Two studies incorporated the same population of patients, and we excluded both owing to trial author‐reported issues with study ethics and inclusion criteria (Faller 2010; Pirrello 2011).
Risk of bias in included studies
We assessed risk of bias in all included studies as demonstrated in Figure 2 and Figure 3. Detailed information can be found in Characteristics of included studies.
2.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Random sequence generation
Six studies used adequate methods for random sequence generation, such as random numbers tables or computer‐generated randomisation sequences, and hence we deemed these studies to be at low risk of bias (Haydardedeoglu 2011; Haydardedeoglu 2017; Kara 2012; Levens 2009; Mok‐Lin 2013; von Horn 2017). The remaining four studies did not provide details on whether or how they carried out randomisation, and hence we judged them to be at unclear risk of bias (Haines 1989; Kingsland 1991; Scott 1989; Tan 1992).
Allocation concealment
Six studies reported adequate methods used for allocation concealment, such as numbered sealed, opaque envelopes, and hence we deemed these trials to be at low risk of bias (Haydardedeoglu 2011; Haydardedeoglu 2017; Levens 2009; Mok‐Lin 2013; Tan 1992; von Horn 2017). The remaining four studies provided no relevant details, and hence were judged them to be at unclear risk of bias (Haines 1989; Kara 2012; Kingsland 1991; Scott 1989).
Blinding
Blinding of participants and personnel (performance bias)
Two studies reported blinding of participants and personnel (Levens 2009; Mok‐Lin 2013). Two studies were open‐label, and hence we judged these studies to be high risk of bias (Haydardedeoglu 2011; von Horn 2017). The remaining studies did not report on blinding, and hence we judged them to be at unclear risk of bias (Haines 1989; Haydardedeoglu 2017; Kara 2012; Kingsland 1991; Scott 1989; Tan 1992).
Blinding of outcome assessment (detection bias)
With the exception of Haydardedeoglu 2011 and von Horn 2017, both of which were open‐label and hence at high risk of bias, none of the other studies reported on blinding of outcome assessors, and hence we judged these studies to be at uncertain risk of bias.
Incomplete outcome data
All trials analysed all randomised women.
Selective reporting
Seven studies reported on a priori outcomes, and we judged these studies to be at low risk of bias (Haydardedeoglu 2011; Haydardedeoglu 2017; Kingsland 1991; Levens 2009; Mok‐Lin 2013; Tan 1992; von Horn 2017). The remaining three studies did not include an a priori statement of outcomes to be studied, and hence we deemed them to be at unclear risk of bias (Haines 1989; Kara 2012; Scott 1989).
Other potential sources of bias
We deemed six studies to be at low risk of other bias (Haydardedeoglu 2011; Haydardedeoglu 2017; Kara 2012; Levens 2009; Mok‐Lin 2013; von Horn 2017), whereas we deemed the remaining studies to be at unclear risk of bias owing to lack of information (Haines 1989; Kingsland 1991; Scott 1989; Tan 1992).
Effects of interventions
See: Table 1
1. Follicular flushing versus aspiration alone
See Table 1.
Primary outcomes
1.1 Live birth rate
We found no evidence of a difference in live birth rate (OR 0.95, 95% CI 0.58 to 1.56; three RCTs; n = 303; I2 = 30%; moderate‐quality evidence). This suggests that with a live birth rate of approximately 41% (414 per 1000) with aspiration alone, the equivalent live birth rate with follicular flushing lies between 29% and 52% (290 to 524 per 1000).
Sensitivity analysis that excluded studies at high risk of bias ‐ Haydardedeoglu 2011 ‐ similarly showed no evidence of a difference in live birth rate (OR 0.60, 95% CI 0.25 to 1.47; two RCTs; n = 130; I2 = 44%; high‐quality evidence). Sensitivity analysis based on a random‐effects model showed estimates similar to those obtained with the fixed‐effect model (OR 0.89, 95% CI 0.44 to 1.81; three RCTs; I2 = 30%; moderate‐quality evidence). We carried out no sensitivity analyses on alternative imputation strategies nor only on fully published trials, as these were not applicable. See Analysis 1.1 and Figure 4.
1.1. Analysis.

Comparison 1 Follicular flushing, Outcome 1 Live birth rate.
4.

Forest plot of comparison: 1 Follicular flushing, outcome: 1.1 Live birth rate.
1.1.1. Subgroup analysis: age
No studies reported on this outcome.
1.1.2. Subgroup analysis: poor ovarian reserve
No studies specifically reported on this comparison. The women included in Haydardedeoglu 2017 had both poor ovarian reserve and poor response to ovarian stimulation. The review authors agreed to include them under 'poor response to ovarian stimulation' for the purposes of subgroup analysis.
1.1.3. Subgroup analysis: poor response to ovarian stimulation
We found no evidence of a difference in live birth rate among participants with poor ovarian response (OR 0.60, 95% CI 0.25 to 1.47; two RCTs; n = 130; I2 = 44%; high‐quality evidence).
1.2. Miscarriage rate
No studies reported on this outcome.
Secondary outcomes
1.3. Oocyte yield
We found no evidence of a difference in oocyte yield per woman randomised (mean difference (MD) ‐0.28 oocytes, 95% CI ‐0.64 to 0.09; six RCTs; n = 708; I2= 0%; moderate‐quality evidence). See Analysis 1.2 and Figure 5. One of the studies in this analysis reported very small standard deviations (SDs), which varied markedly from those reported in other papers (Haydardedeoglu 2017). We are attempting to contact the trial authors and whilst awaiting response have assumed the SD to be in fact standard error (SE); we recalculated this accordingly.
1.2. Analysis.

Comparison 1 Follicular flushing, Outcome 2 Oocyte yield per woman randomised (normally distributed data).
5.

Forest plot of comparison: 1 Follicular flushing, outcome: 1.2 Oocyte yield per woman randomised (normally distributed data).
None of the studies providing data that could not be included in the meta‐analysis provided any evidence of a difference in oocyte yield between the two groups (Haines 1989; Kingsland 1991; Mok‐Lin 2013; Tan 1992) . See Analysis 1.3.
1.3. Analysis.
Comparison 1 Follicular flushing, Outcome 3 Oocyte yield per woman randomised (non‐normally distributed data).
| Oocyte yield per woman randomised (non‐normally distributed data) | |||
|---|---|---|---|
| Study | Aspiration/flush | Aspiration only | p value |
| Haines 1989 | Mean oocyte yield: 5.6 (range 2‐15) | Mean oocyte yield: 6.8 (range 2‐14) | p = 0.22 (NS) |
| Kingsland 1991 | Median oocyte yield: 7 | Median oocyte retrieved: 8.5 | NS |
| Mok‐Lin 2013 | Median oocyte yield: 3 (IQR 2‐5) | Median oocyte yield: 4 (IQR 2‐6) | p = 0.41 |
| Tan 1992 | Median oocyte yield: 9 (range 1‐22) | Median oocyte yield 11 (range: 1‐24) | NS |
1.4. Duration of oocyte retrieval
The duration of oocyte retrieval was markedly longer in the aspiration/flush group than in the aspiration only group (MD 166,01 seconds, 95% CI 141.96 to 190.06; six RCTs; n = 714; I2 = 88%; very low‐quality evidence). See Analysis 1.4 and Figure 6. One of the studies in this analysis reported very small SDs, which varied markedly compared with those reported in other papers (Haydardedeoglu 2017). We are attempting to contact the study authors and whilst awaiting response have assumed the SD to be in fact SE; we have recalculated this accordingly.
1.4. Analysis.

Comparison 1 Follicular flushing, Outcome 4 Duration of oocyte retrieval (normally distributed data; seconds).
6.

Forest plot of comparison: 1 Follicular flushing, outcome: 1.4 Duration of oocyte retrieval (normally distributed data; seconds).
Sensitivity analysis with removal of the study reporting markedly different SD showed similar results (Haydardedeoglu 2017) (MD 192.70 seconds, 95% CI 165.85 to 219.56; five RCTs; n = 634; five RCTs; I2= 82%; very low‐quality evidence).
Both studies providing data that could not be included in the meta‐analysis yielded evidence of aspiration/flush lasting longer than aspiration alone (Kingsland 1991; Tan 1992). See Analysis 1.5.
1.5. Analysis.
Comparison 1 Follicular flushing, Outcome 5 Time taken for procedure (non‐normally distributed data).
| Time taken for procedure (non‐normally distributed data) | |||
|---|---|---|---|
| Study | Aspiration/flush | Aspiration only | P value |
| Kingsland 1991 | Median time taken for procedure: 35 minutes | Median time taken for procedure: 20 minutes | P<0.001 |
| Tan 1992 | Median time taken: 30 minutes (range 15 to 70 minutes) | Median time taken: 15 minutes (range 4 to 30 minutes) | P<0.00001 |
1.5. Total number of embryos
We found no evidence of a difference in the total number of embryos per woman randomised (MD ‐0.10 embryos, 95% CI ‐0.34 to 0.15; two RCTs; n = 160; I2 = 58%; low‐quality evidence). See Analysis 1.6.
1.6. Analysis.

Comparison 1 Follicular flushing, Outcome 6 Total number of embryos.
1.6. Number of cryopreserved embryos
Two studies reported on the number of cryopreserved embryos per woman randomised. However, meta‐analysis was not possible, as the mean number in the aspiration/flush group in Mok‐Lin 2013 was 0. See Analysis 1.7. The other study in this analysis reported very small SDs, which varied markedly from those reported in other papers (Haydardedeoglu 2017). We are attempting to contact study authors and whilst awaiting response have assumed the SD to be in fact SE; we have recalculated this accordingly.
1.7. Analysis.

Comparison 1 Follicular flushing, Outcome 7 Number of embryos cryopreserved per woman randomised.
1.7. Clinical pregnancy rate
We found no evidence of a difference in clinical pregnancy rate per woman randomised (OR 1.07, 95% CI 0.78 to 1.46; five RCTs; n = 704; I2 = 49%; moderate‐quality evidence). See Analysis 1.8 and Figure 7.
1.8. Analysis.

Comparison 1 Follicular flushing, Outcome 8 Clinical pregnancy rate per woman randomised.
7.

Forest plot of comparison: 1 Follicular flushing, outcome: 1.8 Clinical pregnancy rate per woman randomised.
1.8. Ongoing pregnancy rate
We found no evidence of a difference in ongoing pregnancy rate per woman randomised (OR 1.21, 95% CI 0.73 to 2.02; four RCTs; n = 344; I2 = 0%; moderate‐quality evidence). See Analysis 1.9 and Figure 8.
1.9. Analysis.
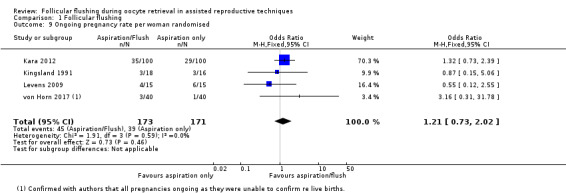
Comparison 1 Follicular flushing, Outcome 9 Ongoing pregnancy rate per woman randomised.
8.

Forest plot of comparison: 1 Follicular flushing, outcome: 1.9 Ongoing pregnancy rate per woman randomised.
1.9. Adverse events
von Horn 2017 reported no evidence of a difference on the Depression Anxiety and Stress Scale (DASS)‐21 in depression (MD 0.60 points, 95% CI ‐0.66 to 1.86; one RCT; n = 80), anxiety (MD 0.00 points, 95% CI ‐0.60 to 0.60; one RCT, n = 80), or stress (MD 1.10 points, 95% CI ‐0.42 to 2.62; n = 80; moderate‐quality evidence). See Analysis 1.10 and Figure 9.
1.10. Analysis.

Comparison 1 Follicular flushing, Outcome 10 Adverse events (continuous data).
9.

Forest plot of comparison: 1 Follicular flushing, outcome: 1.10 Adverse events (continuous data).
Tan 1992 reported on three adverse events: blockage of the needle (OR 7.44, 95% CI 0.37 to 147.92; one RCT; n = 100), vomiting (OR 5.21, 95% CI 0.24 to 111.24; one RCT; n = 100), and hypotension (OR 5.21, 95% CI 0.24 to 111.24; one RCT; n = 100). We found no evidence of a difference between aspiration/flush compared with aspiration alone for any of these outcomes (Analysis 1.11; Figure 10). Tan 1992 reported that significantly less analgesia was required with the aspiration alone procedure compared with added flushing (median 50 mg, range 50 to 100 mg for aspiration alone; median 100 mg, range 50 to 100 mg for aspiration/flushing). It should be noted that event rates are low and were derived from a single study, hence caution is advised in interpreting these data.
1.11. Analysis.
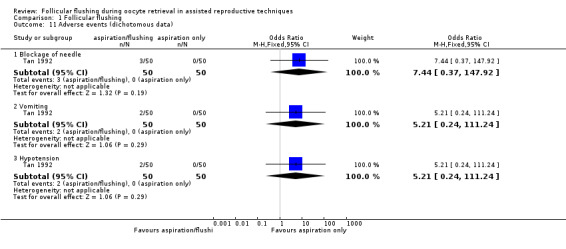
Comparison 1 Follicular flushing, Outcome 11 Adverse events (dichotomous data).
10.

Forest plot of comparison: 1 Follicular flushing, outcome: 1.11 Adverse events (dichotomous data).
No study provided data on safety.
Discussion
Summary of main results
This Cochrane review aimed to evaluate the effectiveness of follicular flushing (aspiration/flush) compared with aspiration alone in women undergoing in vitro fertilisation (IVF) or intracytoplasmic sperm injection (ICSI). Moderate‐quality evidence shows probably little or no difference in the primary outcome of live birth with follicular flushing. No studies reported on the primary outcome of miscarriage. Moderate‐quality evidence shows probably little or no difference in oocyte yield with follicular flushing compared with aspiration alone. The oocyte retrieval process was noted to last significantly longer with follicular flushing than with aspiration alone, although this evidence is of very low quality. No differences were noted in total number of embryos, number of embryos cryopreserved, or clinical or ongoing pregnancy rates (Table 1). Evidence was insufficient to allow firm conclusions with respect to adverse events or safety.
Overall completeness and applicability of evidence
Only three of the included studies reported on live birth rate, which is likely to be the most important outcome for women embarking on fertility treatment. Furthermore, none of the studies reported a miscarriage rate. No studies reported on this review's subgroup of maternal age. Additionally, only one study focussed on women with poor ovarian reserve (Haydardedeoglu 2017). However all participants in this study also had a poor response to ovarian stimulation; we decided to include these patients in this subgroup as it represents the more clinically relevant subgroup. Two studies looked at poor response to ovarian stimulation and noted no difference in the live birth rate (Haydardedeoglu 2017; Mok‐Lin 2013); we deemed this to be high‐quality evidence.
Most of the included papers focussed on oocyte yield and four studies incorporated data that could not be used for meta‐analysis (Haines 1989; Kingsland 1991; Mok‐Lin 2013; Tan 1992). Nevertheless, these studies reported no change in oocyte yield with follicular flushing. In addition, two studies incorporated data on duration of oocyte retrieval that could not be incorporated into the meta‐analysis (Kingsland 1991; Tan 1992). These data mirrored data presented in the meta‐analysis, suggesting increased procedure length with follicular flushing. It appears that follicular flushing is associated with a significantly longer procedure compared with aspiration alone. Nevertheless, these data should be interpreted with caution, as we found a high degree of heterogeneity, mainly attributable to one study (Haydardedeoglu 2017).
Although the included studies provided few data on adverse events, von Horn 2017 reported no differences in depression, anxiety, or stress in either group, and Tan 1992 reported no differences in needle blockage, vomiting, or hypotension. Data on adverse events should be interpreted with caution, as individual studies are relatively small and event rates low.
A study carried out in the early 2000s reported that more than 50% of fertility units routinely perform follicular flushing (Knight 2001). Although changes in clinical practice are usually slow to be implemented, this Cochrane review provides evidence suggesting no benefit for follicular flushing and hence would encourage a shift away from it.
Quality of the evidence
For this review, we identified and included only published data originating from ten RCTs and incorporating 928 women. We have summarised in Figure 2 and Figure 3 risk of bias for individual studies.
We rated the quality of evidence on the basis of GRADE criteria. The quality of the evidence ranged from very low to moderate, with issues arising as the result of lack of blinding, imprecision, and inconsistency. Although lack of blinding was a feature of several included studies, and blinding of the operator was not possible, we suggest that this was not essential, as study outcomes were objective. See Table 1.
Potential biases in the review process
Review authors aimed to reduce the risk of publication bias by conducting systematic searches of multiple databases and trial registries to identify ongoing studies. We contacted trial authors to request further information when applicable, and unfortunately we did not receive a response in all cases. Subgroup analysis was not possible for subgroups of age and poor ovarian reserve owing to lack of data. As prespecified, we performed sensitivity analysis for the primary outcome of live birth. We were unable to construct a funnel plot owing to the small number of included studies.
Agreements and disagreements with other studies or reviews
Older studies, which were not randomised controlled trials (RCTs), have suggested that oocyte yield increases with follicular flushing. For example, Bagtharia 2005 found 40% of oocytes in primary aspiration without flushing of the follicle and retrieved up to 82% of oocytes with two flushes and up to 97% with four flushes. Mendez Lozano 2008 observed a 46.8% oocyte recovery rate with aspiration only compared with 84.6% with additional follicular flushing in 165 infertile women with low ovarian reserve who were undergoing 271 consecutive minimal stimulation IVF cycles.
However, data from this systematic review contradict these findings and show no increase in oocyte yield nor in the more clinically relevant outcome of live birth. In addition, the two most recent systematic reviews on this topic, which date to 2012 (Levy 2012; Roque 2012), reported findings that are similar to those presented in this systematic review. Both of these systematic reviews incorporated studies that we have included here.
Authors' conclusions
Implications for practice.
This review suggests that follicular flushing probably has little or no effect on live birth rates compared with aspiration alone. None of the included trials reported on effects of follicular aspiration and flushing on the miscarriage rate. Data suggest little or no difference between follicular flushing and aspiration alone with respect to oocyte yield, total embryo number, or number of cryopreserved embryos. In addition, follicular flushing probably makes little or no difference in the clinical pregnancy rate. Evidence was insufficient to allow any firm conclusions with respect to adverse events or safety.
Implications for research.
Although the body of evidence against use of follicular flushing is growing, further research predominantly centred on population selection and outcomes is required. Study design could be improved by blinding participants, the embryologist, and those assessing outcomes.
Population
Most research so far has not focussed on specific populations that may benefit from follicular flushing. Future directions could involve focussing on populations such as older women, women with poor ovarian reserve, and women with a poor response to ovarian stimulation.
Outcomes
Although more recent studies have focused on the outcome of live birth rate, this remains an underreported outcome. Further research should make this the primary outcome. In addition, data on the miscarriage rate associated with follicular flushing are required.
What's new
| Date | Event | Description |
|---|---|---|
| 20 March 2018 | New citation required but conclusions have not changed | The addition of 6 new studies has not led to a change in the conclusions of this review. |
| 20 March 2018 | New search has been performed | New searches have identified 6 studies at this update (Haines 1989;Haydardedeoglu 2011; Haydardedeoglu 2017; Kara 2012; Mok‐Lin 2013; von Horn 2017). |
History
Protocol first published: Issue 1, 2004 Review first published: Issue 9, 2010
| Date | Event | Description |
|---|---|---|
| 31 March 2010 | New search has been performed | This review has had a search run. One new study was identified for the update, and formatting has been amended to include all subheadings for RevMan 5. Amendments to the original protocol have been made, and some outcomes and objectives have been removed. |
| 19 January 2010 | New search has been performed | Review completed, no changes to protocol |
| 2 April 2008 | Amended | Converted to new review format |
| 11 November 2003 | New citation required and major changes | Substantive amendments |
Acknowledgements
We wish to thank the CGFG editorial group for patience and kind support provided. In particular, we would like to thank Helen Nagels for assistance with all of our queries, and Marian Showell for help in conducting these searches.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Group (CGFG) specialised register search strategy
Searched 18 July 2017
Procite platform
Keywords CONTAINS "follicular flushing" or "follicular rinsing" or "Flushing" or "flushing media" or "flushing outcome" or "tubal flushing" or "follicle aspiration" or "follicular aspiration" or "Flushing‐Outcome" or "flushing media" or "Flushing" or Title CONTAINS "follicular flushing" or "follicular rinsing" or "Flushing" or "flushing media" or "flushing outcome" or "tubal flushing" or "follicle aspiration" or "follicular aspiration" or "Flushing‐Outcome" or "flushing media" or "Flushing" (314 hits)
Appendix 2. CENTRAL Register of Studies Online (CRSO) search strategy
Searched 18 July 2017
Web platform
#1 (follic* adj15 flush*):TI,AB,KY (27)
#2 (follic* adj15 wash*):TI,AB,KY (6)
#3 ((flush* or wash*) adj15 oocyte*):TI,AB,KY (22)
#4 (ovar* adj15 flush*):TI,AB,KY (13)
#5 (ovar* adj15 wash*):TI,AB,KY (1)
#6 #1 OR #2 OR #3 OR #4 OR #5 (54)
Appendix 3. MEDLINE search strategy
Searched from 1946 to 18 July 2017
Ovid platform 1 (follic$ adj15 flush$).tw. (139) 2 (follic$ adj15 wash$).tw. (118) 3 ((flush$ or wash$) adj15 oocyte$).tw. (400) 4 (ovar$ adj15 flush$).tw. (225) 5 (ovar$ adj15 wash$).tw. (308) 6 or/1‐5 (1044) 7 randomized controlled trial.pt. (469461) 8 controlled clinical trial.pt. (94440) 9 randomized.ab. (411777) 10 randomised.ab. (80733) 11 placebo.tw. (196669) 12 clinical trials as topic.sh. (187425) 13 randomly.ab. (285289) 14 trial.ti. (184823) 15 (crossover or cross‐over or cross over).tw. (76205) 16 or/7‐15 (1207988) 17 exp animals/ not humans.sh. (4440485) 18 16 not 17 (1114171) 19 6 and 18 (58)
Appendix 4. Embase search strategy
Searched from 1980 to 18 July 2017
Ovid platform
1 (follic$ adj15 flush$).tw. (168) 2 (follic$ adj15 wash$).tw. (161) 3 ((flush$ or wash$) adj15 oocyte$).tw. (535) 4 (ovar$ adj15 flush$).tw. (250) 5 (ovar$ adj15 wash$).tw. (425) 6 or/1‐5 (1339) 7 Clinical Trial/ (930972) 8 Randomized Controlled Trial/ (459267) 9 exp randomization/ (74757) 10 Single Blind Procedure/ (28357) 11 Double Blind Procedure/ (138107) 12 Crossover Procedure/ (52384) 13 Placebo/ (296350) 14 Randomi?ed controlled trial$.tw. (163117) 15 Rct.tw. (24914) 16 random allocation.tw. (1660) 17 randomly allocated.tw. (27801) 18 allocated randomly.tw. (2252) 19 (allocated adj2 random).tw. (781) 20 Single blind$.tw. (19437) 21 Double blind$.tw. (173262) 22 ((treble or triple) adj blind$).tw. (695) 23 placebo$.tw. (252265) 24 prospective study/ (390466) 25 or/7‐24 (1766082) 26 case study/ (48502) 27 case report.tw. (333445) 28 abstract report/ or letter/ (1002945) 29 or/26‐28 (1376884) 30 25 not 29 (1720844) 31 6 and 30 (138)
Appendix 5. PsycINFO search strategy
Searched from 1806 to 18 July 2017
Ovid platform
1 (follic$ adj15 flush$).tw. (2) 2 (follic$ adj15 wash$).tw. (0) 3 ((flush$ or wash$) adj15 oocyte$).tw. (1) 4 (ovar$ adj15 flush$).tw. (7) 5 (ovar$ adj15 wash$).tw. (2) 6 or/1‐5 (11) 7 random.tw. (50386) 8 control.tw. (389567) 9 double‐blind.tw. (20779) 10 clinical trials/ (10459) 11 placebo/ (4898) 12 exp Treatment/ (689504) 13 or/7‐12 (1067763) 14 6 and 13 (3)
Appendix 6. CINAHL search strategy
Searched from 1961 to 18 July 2017
Ebsco Platform
| # | Query | Results |
| S19 | S6 AND S18 | 14 |
| S18 | S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 | 1,147,142 |
| S17 | TX allocat* random* | 7,005 |
| S16 | (MH "Quantitative Studies") | 16,053 |
| S15 | (MH "Placebos") | 10,235 |
| S14 | TX placebo* | 46,640 |
| S13 | TX random* allocat* | 7,005 |
| S12 | (MH "Random Assignment") | 43,441 |
| S11 | TX randomi* control* trial* | 127,953 |
| S10 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 897,233 |
| S9 | TX clinic* n1 trial* | 208,458 |
| S8 | PT Clinical trial | 80,031 |
| S7 | (MH "Clinical Trials+") | 217,256 |
| S6 | S1 OR S2 OR S3 OR S4 OR S5 | 60 |
| S5 | TX ovar* N15 wash* | 24 |
| S4 | TX ovar* N15 flush* | 19 |
| S3 | TX (flush* or wash*) N15 oocyte* | 7 |
| S2 | TX follic* N15 wash* | 3 |
| S1 | TX follic* N15 flush* | 13 |
Appendix 7. Inclusion criteria
| Date | |
| Assessor | EG PM |
| First author | |
| Publication year | |
| Journal | |
| Language | |
| Retrieval | Electronic search Handsearched |
| Study design | |
| Q1: Is the study a randomised controlled trial? | Yes No Unclear |
| If 'no', trial excluded. If yes, then proceed to Q2. | |
| Participants | |
| Q2: Are the participants undergoing assisted conception treatment by IVF or ICSI? | Yes No Unclear |
| Q3: Did study participants use their own gametes? | Yes No Unclear |
| If 'no' to either Q2 or Q3, trial excluded. If yes, then proceed to Q4. | |
| Intervention | |
| Q4: Was the intervention follicular aspiration and flushing versus follicular aspiration alone? |
Yes No Unclear |
| Final decision | |
| Study included if 'yes' to Q1, Q2, Q3, and Q4 | Include Exclude |
| Reasoning for exclusion | |
| If 'unclear', action taken | |
| Both assessors in agreement? | Yes No |
| If no, outcome of discussion and/or arbitration. |
Appendix 8. Data extraction form
| Date | ||
| Assessor | EG PM |
|
| First author | ||
| Publication year | ||
| Published | Yes No |
|
| Language | ||
| Retrieval | Electronic search Handsearched |
|
| Study design | ||
| Randomised controlled trial? | Yes No |
|
| What type of randomised controlled trial? | Parallel (intervention vs control) Cross‐over (participants used as intervention and control groups) |
|
| Participant recruitment | Prospective Retrospective Unclear |
|
| Participants | ||
| Country | ||
| Site (single or multiple centres, location) | ||
| Age | Mean+SD/Median+Range Intervention group Control group |
|
| Inclusion criteria | ||
| Exclusion criteria | ||
| Power calculation was performed and followed | Yes No Unclear |
|
| Study size | ||
| Number recruited | ||
| Number randomised | ||
| Number excluded | ||
| Number analysed | ||
| Number lost to follow‐up | ||
| Interventions | ||
| To include description of the ovarian stimulation protocol (when appropriate), as well as details of follicular aspiration and flushing procedures |
||
| Primary outcomes | ||
| Live birth rate | Occurrence of outcome | Non‐occurrence of outcome |
| Intervention group | ||
| Control group | ||
| Total (by event) | ||
| Miscarriage rate | Occurrence of outcome | Non‐occurrence of outcome |
| Intervention group | ||
| Control group | ||
| Total (by event) | ||
| Secondary outcomes | ||
| Oocyte yield | Occurrence of outcome | Non‐occurrence of outcome |
| Intervention group | ||
| Control group | ||
| Total (by event) | ||
| Duration of oocyte retrieval | Occurrence of outcome | Non‐occurrence of outcome |
| Intervention group | ||
| Control group | ||
| Total (by event) | ||
| Total number of embryos | Occurrence of outcome | Non‐occurrence of outcome |
| Intervention group | ||
| Control group | ||
| Total (by event) | ||
| Number of embryos cryopreserved | Occurrence of outcome | Non‐occurrence of outcome |
| Intervention group | ||
| Control group | ||
| Total (by event) | ||
| Ongoing pregnancy rate | Occurrence of outcome | Non‐occurrence of outcome |
| Intervention group | ||
| Control group | ||
| Total (by event) | ||
| Adverse event: | Occurrence of outcome | Non‐occurrence of outcome |
| Intervention group | ||
| Control group | ||
| Total (by event) | ||
| Adverse event: | Occurrence of outcome | Non‐occurrence of outcome |
| Intervention group | ||
| Control group | ||
| Total (by event) | ||
| Adverse event: | Occurrence of outcome | Non‐occurrence of outcome |
| Intervention group | ||
| Control group | ||
| Total (by event) | ||
|
Subgroups: Age Poor ovarian reserve Poor response to ovarian stimulation |
||
| Live birth rate | Occurrence of outcome | Non‐occurrence of outcome |
| Intervention group | ||
| Control group | ||
| Total (by event) | ||
| Miscarriage rate | Occurrence of outcome | Non‐occurrence of outcome |
| Intervention group | ||
| Control group | ||
| Total (by event) | ||
| Risk of bias assessment | ||
| Selection bias | Was the allocation sequence adequately generated? (adequate: computerised random number generator; random numbers table) |
Yes No Unclear |
| Was participant allocation concealment adequate? (adequate: central computer randomisation; sequentially numbered, sealed opaque envelopes) |
Yes No Unclear |
|
| Performance bias | Were participants blinded? | Yes No Unclear |
| Were personnel (embryologist) blinded? | Yes No Unclear |
|
| Detection bias | Were those assessing outcomes blinded? | Yes No Unclear |
| Attrition bias (incomplete outcome data) |
Was loss to follow‐up accounted for? | Yes No Unclear |
| Was an intention‐to‐treat analysis performed? | Yes No Unclear |
|
| Selective outcome reporting | Are reports of the study free of the suggestion of selective outcome reporting? |
Yes No Unclear |
| Other sources of bias (high risk of bias: commercial funding source, early stopping, baseline imbalances, poor choice of design) |
||
Data and analyses
Comparison 1. Follicular flushing.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth rate | 3 | 303 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.58, 1.56] |
| 1.1 Poor response to ovarian stimulation | 2 | 130 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.25, 1.47] |
| 1.2 Normal response to ovarian stimulation | 1 | 173 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.64, 2.16] |
| 2 Oocyte yield per woman randomised (normally distributed data) | 6 | 708 | Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐0.64, 0.09] |
| 3 Oocyte yield per woman randomised (non‐normally distributed data) | Other data | No numeric data | ||
| 4 Duration of oocyte retrieval (normally distributed data; seconds) | 6 | 714 | Mean Difference (IV, Fixed, 95% CI) | 166.01 [141.96, 190.06] |
| 5 Time taken for procedure (non‐normally distributed data) | Other data | No numeric data | ||
| 6 Total number of embryos | 2 | 160 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.34, 0.15] |
| 7 Number of embryos cryopreserved per woman randomised | 2 | 324 | Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐0.94, 0.06] |
| 8 Clinical pregnancy rate per woman randomised | 5 | 704 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.78, 1.46] |
| 9 Ongoing pregnancy rate per woman randomised | 4 | 344 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.73, 2.02] |
| 10 Adverse events (continuous data) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Depression | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.66, 1.86] |
| 10.2 Anxiety | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.60, 0.60] |
| 10.3 Stress | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 1.10 [‐0.42, 2.62] |
| 11 Adverse events (dichotomous data) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Blockage of needle | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.44 [0.37, 147.92] |
| 11.2 Vomiting | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.21 [0.24, 111.24] |
| 11.3 Hypotension | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.21 [0.24, 111.24] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Haines 1989.
| Methods | Parallel randomised trial | |
| Participants | Country: Australia Site: Flinders University, Flinders Medical Centre, Adelaide Patients: 36 patients undergoing IVF treatment Mean age + SD: not specified Inclusion: not specified Exclusion: not specified |
|
| Interventions | Ovarian hyperstimulation was achieved with clomiphene citrate (Clomid, Merrell Dow), 50 mg twice daily on days 5 to 9 of the cycle, and human menopausal gonadotropin (Humegon, Organon), 2 ampoules daily from day 6 and continued according to response. Human chorionic gonadotropin (Profasi, Serono), 5000 IU, was administered when the dominant follicle reached 18 mm in the presence of appropriate oestradiol levels. Oocyte pickup was performed with the patient under intravenous analgesia via a single‐lumen (W.A. Cook, Australia; 17 G, 23.5 cm, K‐OPS‐1023‐RWH) or double‐lumen (W.A. Cook, Australia; 17 G, 25 cm; K‐OPSD‐1725) needle. Flushing was performed up to 5 times. The single‐lumen oocyte pickup represented the control group (n = 18), and the double‐lumen oocyte pickup represented the intervention group (n = 18). |
|
| Outcomes | Number of follicles aspirated (mean + range) Fertilisation rate (%) |
|
| Notes | No statement regarding competing interests. No declaration of funding source(s), if any | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women were analysed. |
| Selective reporting (reporting bias) | Unclear risk | No details |
| Other bias | Unclear risk | No details |
Haydardedeoglu 2011.
| Methods | Prospective parallel randomised trial | |
| Participants | Country: Turkey Site: Department of Obstetrics and Gynaecology, Baskent University Adana Patients: 274 women undergoing ICSI treatment Mean age ± SD: 30.58 ± 4.66 in the single‐lumen needle group, 30.75 ± 4.96 in the double‐lumen needle group Inclusion: not specified Exclusion: patients with a poor response (< 6 follicles over 12 mm on the day of hCG), patients undergoing a microdose flare protocol, patients with a high response (polycystic ovarian syndrome and polycystic ovaries) |
|
| Interventions | Patients underwent luteal down‐regulation with 1.0 mg leuprolide acetate (Lucrin; Abbott, Istanbul, Turkey) for at least 10 days until day 2 to 3 of menses, at which point baseline ultrasonography and blood tests were carried out. If there were no cysts ≥2 cm and estradiol (E2) levels were <50 pg/mL, gonadotrophin stimulation was performed with 150 to 225 IU gonadotropin (Puregon; Organon, Turkey). E2 monitoring began on the morning of stimulation day 5. Other patients underwent a GnRH antagonist cycle with baseline ultrasonography and blood tests. If there were no cysts ≥2 cm and the progesterone level was <1 ng/mL, gonadotropin stimulation was performed with 150 to 225 IU gonadotropin. E2 monitoring began on the morning of stimulation day 5. GnRH antagonist (Orgalutran; Organon) was added on day 6. Ultrasound and E2 monitoring continued until hCG administration criteria were met: at least 3 follicles with maximum diameter > 17 mm. In the single‐lumen needle group (n = 125), a 17‐gauge needle (Cook Ireland Ltd., Limerick, Ireland) was used to aspirate the follicles. A 17‐gauge needle was used in the double‐lumen needle group (n = 149); 2 mL flush medium was injected and was aspirated once for each punctured follicle. Oocyte‐corona complexes were denuded and intracytoplasmic sperm injection performed after 2 hours of incubation. Embryos were transferred on day 3 with individualised transfer protocols for poor‐grade embryos. All participants had luteal support with 90 mg progesterone (8% gel, Crinon; Serono, Istanbul, Turkey) administered vaginally each day after embryo transfer. |
|
| Outcomes | Number of retrieved oocytes (mean ± SD) Number of metaphase II oocytes (mean ± SD) Number of germinal vesicles (mean ± SD) Duration of oocyte retrieval (min, mean ± SD) Fertilisation rate (%, mean ± SD) Number of transferred embryos (mean ± SD) Biochemical pregnancy rate (mean ± SD) Clinical pregnancy rate (mean ± SD) Live birth rate (mean ± SD) Rate of patients hospitalised with ovarian hyperstimulation syndrome (%) Cancellation rate (%) |
|
| Notes | Using a baseline live birth rate for normal‐responding participants with ICSI of 35% with detectable difference between groups at 5%, a sample size of 1471 participants in each group was required to achieve 0.80 power. Recruitment was terminated after 13 months, when it became evident it would not be possible to recruit this number of participants at a single centre. No statement regarding competing interests. No declaration of funding source(s), if any |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "allocation sequence generated from a random numbers table" |
| Allocation concealment (selection bias) | Low risk | Quote: "use of consecutively numbered opaque, sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "open‐label, randomized controlled trial" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "open‐label, randomized controlled trial" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women were analysed. |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes were reported. |
| Other bias | Low risk | No differences in basal participant characteristics. No statement regarding conflicts of interest |
Haydardedeoglu 2017.
| Methods | Prospective parallel randomised trial | |
| Participants | Country: Turkey Site: Division of Reproductive Endocrinology and IVF Unit, Department of Obstetrics and Gynaecology, Baskent University Adana Patients: 80 women undergoing ICSI treatment Mean age ± SD: 34.3 ± 5.4 in the single‐lumen needle group, 36.2 ± 3.9 in the double‐lumen needle group Inclusion: women aged 20 to 43 years with poor ovarian response defined as 5 or fewer follicles ≥ 13 mm in size, serum progesterone level < 1.5 ng/mL on the day of hCG administration, and known poor functional ovarian reserve to predict a poor ovarian response to gonadotropin stimulation diagnosed by an antral follicle count (AFC) < 6 in both ovaries together with an anti‐Müllerian hormone (AMH) level < 0.8 ng/mL Exclusion: monofollicular ovarian response, natural in vitro fertilisation (IVF) cycle programme, and presence of ovarian endometrioma |
|
| Interventions | Approximately half of poor responders took part in a low‐dose luteal GnRH agonist programme consisting of a luteal dose of 0.5 mg leuprorelin acetate (Lucrin; Abbott, Paris, France) until day 2 or 3 following menses. After ovarian suppression was achieved, the dose was reduced to 0.25 mg until the date of 10,000 IU hCG (Pregnyl ampoule; MSD) administration. If there were no cysts ≥2 cm and the estradiol (E2) level was < 50 pg/mL, then gonadotropin stimulation with 300 IU (Puregon; MSD, Oss, the Netherlands) was performed. Ultrasound and blood E2 monitoring continued until administration of 10,000 IU hCG when at least 2 follicles had reached maximum diameter > 17 mm. The GnRH antagonist protocol involved administration of letrozole (Femara; Novartis, Basel, Switzerland) and rFSH (Puregon; MSD). On day 2 or 3 of menses, letrozole 5 mg/d was started and continued for 5 days. Administration of gonadotropins was started on the same day with 150 IU rFSH plus 150 IU pure human menopausal gonadotropin (hMG) (Menopur; Ferring Pharmaceuticals, Lozan Saint‐Prex, Switzerland). A GnRH antagonist (Orgalutran; MSD) was added to this regimen when the leading follicle had reached 14 mm. Ultrasound and blood E2 monitoring continued until the hCG administration criterion was met, with at least 2 follicles having a maximum diameter of > 17 mm. Four participants were placed on the FSH + pure hMG/GnRH antagonist protocol, which administered gonadotropins and started on day 2 or 3 of menses with 150 IU rFSH plus 150 IU pure hMG. Orgalutran was added to this regimen when the leading follicle reached 14 mm. After the leading follicle reached > 17 mm, 10,000 IU of hCG and 0.2 mg/mL triptorelin were injected. Allocation sequence was done using a random numbers table to assign participants to single‐lumen (direct aspiration) or double‐lumen (follicular flushing) needle groups. Consecutively numbered opaque, sealed envelopes were used on the day of oocyte retrieval. All participants were blinded to randomisation for the duration of the study. Clinicians performing the oocyte retrieval procedure were notified of treatment allocation on the day of retrieval to record duration of the procedure and were given anaesthetic drug amounts. Transvaginal ultrasound‐guided oocyte retrieval was performed 36 hours after trigger under sedation with 1% propofol (Fresenius Kabi, Homburg, Germany). For the single‐lumen needle group (n = 40), a 17‐gauge needle (Cook Ireland, Limerick, Ireland) was used to aspirate follicles. A 17‐gauge needle (Cook Ireland) was also used in the double‐lumen needle group (n = 40), and 2 mL was injected into each follicle via a manually pressed syringe containing 10 mL of culture medium warmed to 37 °C and re‐aspirated and re‐injected 3 times for each punctured follicle. The pressure at which we aspirated the follicles was strictly maintained at 80 mmHg. The oocyte–corona complexes were denuded, and intracytoplasmic sperm injection was performed after a 2‐hour incubation. Embryos were transferred on day 3. All participants received luteal support with daily intravaginal 90 mg progesterone (Crinone 8% gel; Merck Serono, Darmstadt, Germmany) and 0.1 mg/ml triptorelin on the third day after embryo transfer. |
|
| Outcomes | Number of metaphase II oocytes retrieved (mean ± SD) Number of punctured follicles (n) Number of retrieved oocytes (n) Fertilisation rate (%, mean ± SD) Implantation rate (%, mean ± SD) Duration of procedure (seconds, mean ± SD) Total use of anaesthetic (mean ± SD) Clinical pregnancy rate (%) Live birth date (%) |
|
| Notes | No competing interests. Funding by Baskent University Faculty of Medicine | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a random numbers table, the 80 eligible patients were assigned randomly" |
| Allocation concealment (selection bias) | Low risk | Quote: "using consecutively numbered opaque, sealed envelopes on the day of oocyte retrieval" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "All patients were blinded to the randomisation for the duration of the study" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No statement regarding blinding of personnel assessing outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women were analysed. |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes were reported. |
| Other bias | Low risk | No differences in basal patient characteristics. No competing interests declared |
Kara 2012.
| Methods | Prospective parallel randomised trial | |
| Participants | Country: Turkey Site: Department of Obstetrics and Gynecology, Bozok University Medical Faculty, Yozgat, Turkey Patients: 200 patients undergoing ICSI treatment Mean age ± SD: 28.1 ± 5.5 in the aspiration group, 30.1 ± 5.3 in the aspiration group Inclusion: not specified Exclusion: not specified |
|
| Interventions | In all participants, the pituitary was down‐regulated with 0.5 mg leuprolide acetate (Lucrin, Abbott, USA), starting on the 21st day of the previous cycle. The dose was reduced to 0.25 mg and was continued until the day of hCG injection. Controlled ovarian stimulation was performed with FSH on cycle day 3. The starting FSH dose was 300 IU, and this was individually adjusted on the basis of previous treatment cycles, BMI, and age. Follicular development was monitored with E2 levels and ultrasonographic measurements. When 1 or 2 follicles reached 17 mm, hCG (Pregnyl, Schering‐Plough, USA) was administered. Transvaginal utrasound‐guided needle aspiration of follicular fluid was carried out 35 to 36 hours after hCG administration. For the aspiration only group (group 1), a single‐lumen transvaginal oocyte retrieval needle (Otrieva Tapered Ovum Aspiration Needle, K‐TIVM‐172035‐US, Cook Medical, Spencer, IN, USA) was used. In the flushing group (group 2), a double‐lumen transvaginal oocyte retrieval needle (Echo Tip Double Lumen Aspiration Needle, K‐OPSD‐1635‐A‐L, Cook Medical, Spencer, IN, USA) was used. Flushing was with 2 mL flush medium. Women were anaesthetised with Propofol 1000 mg/mL (Abbott, USA) during the oocyte pickup procedure. All women underwent intracytoplasmic sperm injection. Up to 4 embryos were transferred on day 2, 3, or 5 after oocyte retrieval using Rocket THin wall Transfer set (Rocket Medical, Hingham, MA, USA). Luteal support was provided by vaginal progesterone administration (Crinon 8% vaginal gel, Merck‐Serono, Switzerland). Progesterone administration was initiated on the oocyte pickup day and continued for 12 days until the day of pregnancy testing. In cases of pregnancy, progesterone was continued until the 12th gestational week. All women were initially randomly numbered. Then, computer‐assisted randomisation was utilised according to the instructions at www.randomization.com. |
|
| Outcomes | Number of retrieved oocytes (likely mean, unclear if ± SD) Number of metaphase II oocytes (likely mean, unclear if ± SD) Number of metaphase I oocytes (likely mean, unclear if ± SD) Fertilisation rate (%) Clinical pregnancy rate (%) Ongoing pregnancy rate (%) Cancellation rate (%) Duration of procedure (min, likely mean, unclear if ± SD) |
|
| Notes | Trial authors contacted to clarify whether data consist of mean ± SD (reply awaited). No competing interests. No declaration of funding source(s), if any |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer assisted randomization was utilized" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women were analysed. |
| Selective reporting (reporting bias) | Unclear risk | No a priori statement regarding outcomes |
| Other bias | Low risk | No differences in basal participant characteristics. No competing interests declared |
Kingsland 1991.
| Methods | Prospective parallel randomised trial | |
| Participants | Country: UK Site: 34 women undergoing IVF Median age: 31 years for group 1, 30.5 years for group 2 Inclusion: aged 35 years or younger with tubal damage as the sole cause of infertility Exclusion: no details |
|
| Interventions | Down‐regulation with long‐luteal regimen using buserelin. Ovarian stimulation with human menopausal gonadotropin, hCG administered when at least 3 follicles > 18 mm diameter Transvaginal ultrasound‐guided retrieval via JP6L double‐channeled needle Pain relief: 1 mg lorazepam given orally on the evening before oocyte retrieval and repeated on the morning of aspiration. A single dose of 150 mg pethidine was administered IM 20 minutes before aspiration. No participants required additional anaesthesia. Group 1 had aspiration only vs Group 2 had follicles emptied, then flushed with 10 mL Earle's balanced salt solution (EBSS, Gibco, Paisley, UK) supplemented with pyruvate and bicarbonate and buffered with HEPES, if the oocyte was not retrieved in the aspiration. A maximum of 2 mL of fluid was instilled into each follicle at each flush (maximum of 5 flushes per follicle). All oocyte retrievals were done by the same operator. Oocytes were washed once in flushing medium, incubated at 37 °C in 5% CO2 in air, pre‐equilibrated 1 mL drops of EBSS supplemented with 0.11 mg/mL sodium pyruvate, 1% sodium bicarbonate, 0.02 mg gentamicin, and 10% IMS. |
|
| Outcomes | Number of oocytes obtained (median) Time taken for oocyte retrieval (min, median) Fertilization rate (%) Ongoing pregnancy rate (n) |
|
| Notes | No statement regarding competing interests. No declaration of funding source(s), if any | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women were analysed. |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes were reported. |
| Other bias | Unclear risk | No differences in baseline participant characteristics. No statement regarding conflicts of interest |
Levens 2009.
| Methods | Prospective randomised study | |
| Participants | Patients: 30 poor responders undergoing ART Site: Walter Reed Army Medical Center ART Program, USA Mean age 37.1 ± 3.2 and 36.2 ± 3.4 years for single‐ and double‐lumen groups, respectively (P = 0.48) Inclusion criteria: low responders with a cumulative follicle count of 4 to 8 follicles > 12 mm (with at least 2 follicles achieving > 16 mm) |
|
| Interventions | Pretreatment with OCPs during the cycle preceding ovarian stimulation. A combination of rFSH (Gonal‐F) and hMG (Repronex, Ferring) was given twice daily. Adequate follicular development was assessed by serial serum E2 ultrasound. hCG 10,000 IU was given, followed by transvaginal oocyte retrieval 34 to 36 hours later. Assignment to single‐ or double‐lumen group was done immediately before oocyte retrieval. Computerised randomisation in blocks of 10 to 20 was used to ensure balanced group size. Concealment was achieved by using sequentially numbered, opaque envelopes that were opened in the OR after anaesthesia was administered. The length and diameter of retrieval needles were standardised (35 cm, 16G) to control flow dynamics within the needle that may affect oocyte recovery. Cook Echotip single‐lumen (K‐J‐ANC‐16R‐35) and double‐lumen (K‐OPSD‐1635‐B‐S) transvaginal oocyte retrieval needles were used. Suction pressure of 150 to 200 mmHg (provided by Pioneer Pro‐pump, GenX International, CT) was used under direct transvaginal ultrasound guidance (Acuson Sequoia 512 with an 8‐MHz probe). Those in the single‐lumen needle group did not undergo saline follicular flushing (direct aspiration), whereas those in the double‐lumen group had each aspirated follicle flushed once with 2 mL sterile PBS and subsequently re‐aspirated. | |
| Outcomes | Number of oocytes obtained (mean ± SD) Total oocytes mature, maturity (%) Fertilization rate (%) Implantation rate (%) Ongoing pregnancy rate (%) Retrieval times (sec, mean ± SD) |
|
| Notes | No statement regarding competing interests. No declaration of funding source(s), if any | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned" Quote: "computerised randomization in blocks of 10 and 20 to ensure balanced group size" |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation was performed by the Walter Reed Army Medical Center Department of Clinical Investigation and concealed by using sequentially numbered, opaque envelopes that were opened in the operating theater after anesthesia was administered" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the embryologist identifying and collecting the oocytes remained blinded to the group assignments" Quote: "The providers performing the oocyte retrieval remained blinded to the number of oocytes retrieved until the completion of the procedure" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women were analysed. |
| Selective reporting (reporting bias) | Low risk | A priori outcomes were reported. |
| Other bias | Unclear risk | No differences in basal participant characteristics. No statement regarding competing interests |
Mok‐Lin 2013.
| Methods | Prospective parallel randomised study | |
| Participants | Country: USA Site: Ronald O. Perelman and Claudia Cohen Center for Reproductive Medicine of Weill Cornell Medical College Patients: 50 women undergoing IVF Mean age ± SD: 39.5 ± 3.2 in the direct aspiration group, 38.2 ± 4.3 in the flushing group Inclusion: poor responders with 4 or fewer follicles ≥ 12 mm Exclusion: patients without a planned fresh embryo transfer, patients undergoing natural IVF, patients whose cycles were cancelled before hCG administration, patients offered enrolment or randomised in a previous cycle |
|
| Interventions | Most poor responders utilised a GnRH antagonist protocol with luteal oestrogen priming. Participants were placed on a 0.1‐mg oestradiol patch every other day beginning 8 to 10 days after an LH surge, followed by controlled ovarian hyperstimulation (COH) on day 2 of menses. Other protocols included use of a GnRH antagonist without priming, 5 days of clomiphene citrate or letrozole, and daily subcutaneous 40 micrograms leuprolide with COH. COH was performed with rFSH and human menopausal gonadotropins. hCG 10,000 IU was administered intramuscularly when 1 to 2 follicles ≥ 17 mm were present. Participants were randomised on the day of hCG administration to direct aspiration or flushing. Treatment allocation was performed via a computer‐generated randomisation sequence. Allocation was concealed by sequentially numbered, opaque envelopes. All participants were blinded to randomisation. Embryologists were blinded to the allocation scheme. In the direct aspiration group, a 16‐gauge single‐lumen oocyte retrieval needle (Echotipwovum aspiration needle; Cook Medical; Bloomington, IN, USA) was used to aspirate follicles with transvaginal ultrasound guidance. In the flushing group, each aspirated follicle was flushed up to 4 times via a manually pressed syringe with 5 mL of culture media warmed to 37 °C and was re‐aspirated with a 16‐gauge double‐lumen needle (Echotip Double Lumen Aspiration Needle; Cook Medical). All embryos were transferred on day 3, and transferring physicians were blinded to the assigned intervention. |
|
| Outcomes | Number of oocytes retrieved (mean ± SD) Anaesthesia time (min, mean ± SD) Procedure time (min, mean ± SD) Number of mature oocytes (mean ± SD) Number of embryos transferred (mean ± SD) Implantation rate (%) Clinical pregnancy rate (%) Live birth rate (%) |
|
| Notes | No competing interests. No external funding for the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Treatment allocation was performed using a computer‐generated randomization sequence" |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation was performed by the research team and concealed using sequentially numbered, opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All patients were blinded to the randomization for the duration of the study" Quote: "Determination of need for ICSI and selection of embryos for transfer were performed by embryologists blinded to the allocation scheme" Quote: "All embryos were transferred on Day 3 and transferring physicians were blinded to the assigned intervention" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No statement regarding blinding of personnel assessing outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women were analysed. |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes were reported. |
| Other bias | Low risk | No differences in basal participant characteristics. No competing interests declared |
Scott 1989.
| Methods | Prospective parallel randomised study | |
| Participants | Country: USA Patients: 44 women undergoing IVF Median ages of both groups not given No inclusion or exclusion criteria given |
|
| Interventions | All participants underwent gonadotropin stimulation via previously described protocols (in textbook, no details given in the paper). Retrieval with single‐lumen needle (SLN) (n = 22) was done with a Swe‐Med needle (outer diameter 1.5 mm, inner diameter 1 mm). The follicle was aspirated with a hand‐held 20‐mL syringe, and the needle was removed from the participant; this was followed by aspiration of an additional 2 mL of heparinised Dulbecco's solution through the system to wash fluid in the dead space back into the syringe. The double‐lumen needle (DLN; Swe‐Med Lab, Frolunda, Sweden) had an inner diameter of the aspiration lumen of 1 mm and an outer diameter of 1.6 mm. The follicle was aspirated, then 1 to 3 mL of heparinised Dulbecco's solution was injected into the follicle through the second port. This volume was then aspirated back into the syringe. Lavage was performed 1 more time until the oocyte was recovered, or until the follicle was not re‐expanding well, before proceeding to the next follicle. Pain relief: method not mentioned |
|
| Outcomes | Number of follicles aspirated and number of oocytes retrieved (mean ± SE) Incidence of fractured zona in both groups (%) |
|
| Notes | No statement regarding competing interests. No declaration of funding source(s), if any | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women were analysed. |
| Selective reporting (reporting bias) | Unclear risk | Fertilisation rate and clinical pregnancy rate were not described. |
| Other bias | Unclear risk | No details |
Tan 1992.
| Methods | Prospective parallel randomised study | |
| Participants | Country: UK Site: 100 women undergoing IVF treatment at an assisted conception unit Median age for group 1 was 32 (25 to 42 years), and for group 2 32.5 (23 to 43 years). Inclusion: not specified Exclusion: women who had developed > 25 or < 4 follicles wider than 14 mm diameter on the day of hCG administration |
|
| Interventions | Long follicular protocol, starting buserelin acetate (Suprefact; Hoechst, Hounslow, UK) was administered intranasally (200 μg 4‐hourly) on day 1 or 2 of the menstrual cycle. When serum oestradiol concentration was < 200 pmol/L, human menopausal gonadotropin (Pergonal; Serono, Welwyn Garden City, UK) was started at 2 to 6 ampoules daily. hCG (Profasi; Serano) 10,000 IU was administered when there were at least 4 follicles > 14 mm in diameter and mean diameter of the largest follicle was > 20 mm. Transvaginal ultrasound‐guided follicle aspiration was performed 33 to 38 hours post hCG as an outpatient procedure. Pain relief was achieved with IV pethidine 50 to 100 mg in bolus doses of 25 mg as required. Aspiration via JP6L double‐channel needle (Casmed, Cheam, UK). Maximum aspiration pressure of 100 mmHg was used in both groups. Group 1 (aspiration only; n = 50): inner channel of needle removed to convert it to a single‐channel needle. Each follicle was aspirated until empty. The probe was moved around until all follicular fluid was aspirated as evidenced by some blood‐stained fluid in the tubing. The same procedure was repeated until all follicles > 10 mm had been aspirated from the first ovary. After dead space in the needle was cleared, the procedure was repeated in the second ovary. Group 2 (aspiration and flushing, n = 50): double‐channel needle used and the follicle aspirated through the inner channel. This initial aspirate was termed A1. Once the follicle had been emptied, the collecting tube was changed and, with the valve open, flushing medium was injected until 1.5 mL of fluid had been collected. This was termed A2. A1 and A2 were examined separately, and if no oocyte was observed, the follicle was flushed up to a maximum of 6 times. One to three embryos were transferred 48 to 72 hours after oocyte recovery. |
|
| Outcomes | Number of follicles aspirated and number of oocytes obtained (median + range) Time taken for oocyte aspiration (min, median + range) Dose of pethidine required (mg, median + range) Fertilization rate (%, range) Number of embryos transferred (median + range) Clinical pregnancy rate (%, range) |
|
| Notes | No statement regarding competing interests. No declaration of funding source(s), if any | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear as to how randomisation was performed |
| Allocation concealment (selection bias) | Low risk | Randomised by drawing serially number sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for all women were reported. |
| Selective reporting (reporting bias) | Low risk | A priori outcomes were reported. |
| Other bias | Unclear risk | No differences in basal participant characteristics. No statement on competing interests |
von Horn 2017.
| Methods | Prospective parallel randomised study | |
| Participants | Country: Germany Site: Department of Reproductive Endocrinology and Reproductive Medicine, University Hospital of Schleswig‐ Holstein, Lübeck Patients: 80 women undergoing ICSI treatment Mean age ± SD: 38.7 ± 5.0 in the no flushing group, 37.5 ± 4.3 in the flushing group Inclusion: BMI >18 kg/m2 and <35 kg/m2, between the age of 18 and 45, presenting with a total ≤ five follicles >10 mm in both ovaries combined at the end of the follicular phase of the treatment cycle Exclusion: 1 ovary absent (e.g. after ovarectomy) or 1/both ovaries foreseeably difficult to puncture (e.g. heterotopic site because of adhesions) |
|
| Interventions | IVF protocol used is not described in detail. Final oocyte maturation was induced by 5000 IU urinary hCG as soon as the leading follicle reached a mean diameter of 18 mm or the day thereafter, and oocyte pickup was scheduled 34–38 h thereafter. On the day of the decision to trigger final oocyte maturation, participants were randomised to either study group (Steiner‐Tan Needle) or control group (Gynetics). The Steiner‐Tan needles and the flushing system were provided for free by the manufacturer. Randomisation was performed by one of the doctors who performed sonographic monitoring by opening a sealed, opaque, and sequentially numbered envelope containing allocation of the participant. The random sequence was software‐generated and was produced by one of the trial authors. Blocks of 4 were used. In the study group (n = 40), all visible follicles were aspirated with suction pressure of 180 mmHg, then were flushed 3 times under ultrasound. In the control group (n = 40), all visible follicles were aspirated with suction pressure of 180 mmHg. Intracytoplasmic sperm injection was performed as per standard operating procedure. |
|
| Outcomes | Number of cumulus‐oocyte complexes (mean ± SD) and oocyte retrieval rate Number of metaphase II oocytes (mean ± SD) Number of fertilised oocytes (mean ± SD) Proportion of participants undergoing embryo transfer (%) Ongoing pregnancy rate (n) Duration of procedure (min, mean ± SD) Depression Anxiety and Stress Scale score (DASS‐21) Pain assessment by visual analogue scale 2 hours post procedure |
|
| Notes | Steiner‐Tan needles were provided for free by the manufacturer. Personal fees received by study authors were declared. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random sequence was software‐generated and was produced by one of the authors (G.G.). Blocks of four were used" |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed, opaque and sequentially numbered envelope containing the allocation of the patient" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "randomized, controlled, open, superiority trial" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "randomized, controlled, open, superiority trial" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised women were analysed. |
| Selective reporting (reporting bias) | Low risk | All a priori outcomes were reported. |
| Other bias | Low risk | No differences in basal participant characteristics. Competing interests declared |
AFC: antral follicle count.
AMH: anti‐Müllerian hormone.
ART: assisted reproductive technology.
BMI: body mass index.
DASS‐21: Depression Anxiety and Stress Scale‐21.
DLN: double‐lumen needle.
E2: estradiol.
EBSS: Earle's balanced salt solution.
FSH: follicle‐stimulating hormone.
GnRH: gonadotropin‐releasing hormone.
hCG: human chorionic gonadotropin.
HEPES: 4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid.
hMG: human menopausal gonadotropin.
ICSI: intracytoplasmic sperm injection.
IM: intramuscular.
IMS: inactivated maternal serum.
IVF: in vitro fertilisation.
OCPs: oral contraceptive pills.
PBS: phosphate‐buffered saline.
rFSH: recombinant follicle‐stimulating hormone.
SD: standard deviation.
SE: standard error.
SLN: single‐lumen needle.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Avila 2013 | 148 participants divided into 2 groups: 75 allocated to follicular flushing (case), 73 allocated to aspiration only (control). Total oocytes retrieved were 11.80 ± 1.3 in the flushing group vs 9.59 ± 6.1 (P = 0.691) in the control group. Further, no differences were reported in positive pregnancy test (43% and 32%, respectively) or fertilisation rate (55.5% and 55.85%, respectively). Reason for exclusion: retrospective, descriptive study |
| Aydin 2017 | 45 poor responders who all underwent aspiration followed by flushing up to 3 times. Trial authors reported an increase in the number of oocytes retrieved with sequential flushing. Reason for exclusion: prospective cohort study, not a randomised study; no aspiration only group |
| Bagtharia 2005 | All participants had repeated flushing of the follicles. Study compared number of oocytes obtained with each flushing after primary aspiration of the follicle. Study authors concluded that 40% of oocytes were retrieved with primary aspiration without flushing of the follicle, and up to 82% of oocytes were retrieved with 2 flushes and up to 97% with 4 flushes. Study was excluded as it was not an RCT and no control (aspiration only) group was present. |
| Biljan 1997 | 35 participants were randomised to have the left or right ovary flushed with heparinised normal saline or heparinised culture medium. Oocytes obtained from each side were cultured separately and were assessed for fertilisation 18 to 21 hours after insemination. From the side flushed with saline, 185 oocytes were collected from 237 follicles, which was not significantly different from 181 oocytes collected from 244 follicles on the side flushed with culture medium (OR 1.23, 95% CI 0.79 to 1.92). No significant difference in fertilization rates was observed between oocytes obtained after saline (median 71.4%) and culture medium flush (median 75%) (OR 1.08, 95% CI 0.68 to 1.72). Reason for excluding from meta‐analysis: no aspiration only group |
| Dean 1997 | This was an abstract of the same study as Biljan MM et al (1997) ‐ published in Fertility and Sterility 1997;68:1132‐4. Reason for exclusion: duplicate study (under different first trial author) |
| el Hussein 1992 | Study evaluated 100 consecutive patients undergoing 100 cycles of IVF. Four patients were excluded because their embryos were electively cryopreserved. Study reported an overall oocyte recovery rate of 87.8%. Of 1046 oocytes collected, 40.3% were from initial aspiration (A1), 41.3% from dead space in the collecting system (A2), 13.7% from the first 2‐mL flush (F1), and 4.7% from the second 2‐mL flush (F2). Comparable numbers of viable and fertilised oocytes and cleaved, transferred, and frozen embryos in tubes A1 and A2, but all these parameters were significantly lower in tubes F1 and F2 (P < 0.0001). All these parameters were also significantly higher in F1 compared with F2 (P < 0.001), except for numbers of embryos frozen, which showed no difference. Overall pregnancy rate/cycle was 28.1% and pregnancy rate per ET was 31%. No pregnancy was reported in any of the cycles in which embryos originating from F2 were transferred, nor was pregnancy found in cycles in which only embryos from F1 were transferred. Study authors concluded that follicular aspiration together with one 2‐mL flush maximises the recovery of oocytes that will result in pregnancies. Reason for exclusion: not a randomised study. Aspiration done in all cases |
| Faller 2010 | Randomised study comparing aspiration alone (39 participants) vs aspiration and flushing (40 participants) in poor responders. In the flushing group, 123 oocytes were collected, whereas 106 were obtained from the aspiration alone group (P = 0.0594). No difference was found in fertilisation or pregnancy rates. Reason for exclusion: study authors contacted owing to similarity with another conference abstract (Pirrello 2011) with differing participant numbers. Study authors clarified that issues were identified with study ethics and inclusion criteria. |
| Ghosh 2002 | This is a comparative evaluation comparing aspiration alone (group A; 156 participants) vs repeated follicular flushing (group B; 172 participants) in women with tubal block. Study authors reported oocyte recovery of 5.2 ± 1.1 in group A and 6.2 ± 1.3 in group B. Pregnancy rate was 34.6% in group A and 34.9% in group B; miscarriage rates were 9.2% and 21.6, respectively. Reason for exclusion: not a randomised study |
| Gordon 2002 | A randomised study comparing 2 flushing media (Medicult flushing medium 25 cases, SynVitroFlush 22 cases) for follicle irrigation of women undergoing IVF/ICSI treatment. Study authors observed no differences in numbers of oocytes retrieved, fertilisation rates, numbers of embryos replaced, and clinical pregnancy per oocyte collection (2/25 or 8% vs 6/22 or 27.2% for Medicult and SynVitroFlush medium, respectively; P = 0.052). Reason for exclusion: no aspiration‐only group for comparison |
| Khalifa 1999 | Study included 40 IVF cycles in cases with > 10 follicles. Each case was randomised to the first half of follicles (> 14 mm) flushed with non‐heparinised EBSS and the second half with non‐heparinised normal saline, or vice versa. 185 oocytes out of 276 follicles (67%) were retrieved when EBSS was used (group I), and 187 out of 284 follicles (65.8%) when normal saline was used (group II). Data show no significant differences in fertilisation (150/185, 81% vs 153/187, 82%; NS), cleavage rates (136/150, 90.6% vs 141/153, 92%; NS), or grade I embryos at 48 hours (74% vs 76%) and 72 hours (68% vs 67%) in groups I and II, respectively. Reason for exclusion: no aspiration‐only group for comparison |
| Knight 2001 | A retrospective study involving 1139 cycles of oocyte aspiration only and 1139 cycles of aspiration plus flushing at City West IVF during 1991‐1993. 23 women had failed collections in each group and were excluded (leaving only 1139 in each group). (Total number of participants in the abstract (2378) did not match that in the text (1139+1139+23+23=2324).) Reason for exclusion: historical comparison of aspiration alone and aspiration with additional flushing of each follicle. Not a randomised trial |
| Lenz 1987 | Oocyte collection was done in 53 cases by ultrasonically guided abdominal puncture under local or epidural anaesthesia. After follicle aspiration, 2 to 6 flushes with culture medium were performed with a syringe. A total of 196 oocytes were collected, 84 of which (42.9%) were found in the flushes. Mechanical damage was observed in 5.1% of oocytes. Cleavage rates in mature oocytes (157) after 48 hours in culture were similar in the aspirate group (56.5%) and the flush group (54.2%). 10 clinical pregnancies were reported, corresponding to a pregnancy rate of 18.9%. Reason for exclusion: TAS not TVS approach, only 1 group of aspiration with flushing |
| Mehri 2014 | One of the aims of this study was to determine whether oocytes retrieved with or without follicular flushing have different developmental competence. 49 cycles were studied; if an oocyte was not collected with aspiration alone, flushing would be conducted twice. Data show no difference in oocyte maturity between flushed and not flushed groups and no differences in fertilisation and cleavage rates. Reason for exclusion: observational study |
| Mendez Lozano 2008 | Study prospectively included 165 infertile women with low ovarian reserve, 20 to 37 years of age, undergoing 271 consecutive minimal stimulation IVF cycles from January 2005 to December 2006. Oocyte retrieval was performed 34 hours after hCG administration, rather than after 36 hours, to avoid risk of possible follicular rupture before aspiration. Follicular fluid was aspirated with a single‐channel 16‐gauge needle attached to a 10‐mL syringe. The aspiration needle was kept steady inside the follicle until the oocyte was found and isolated (follicular aspiration group; FA group). In case of negative oocyte recovery, sequential flushings were performed via 10‐mL syringes filled with 3 mL of Tyrode's salt solution. These oocytes were entered into the follicular flushing group (FF). Data show 46.8% oocyte recovery with aspiration only, compared with 84.6% with additional follicular flushings. In addition, oocytes retrieved by follicular flushing demonstrated better morphological quality (top‐quality embryos 43/75 or 59.7% vs 40/98 or 41.2%; P = 0.01) and implantation outcomes (implantation rate 34.8% vs 20.4%; P = 0.04) for the corresponding embryo compared with those already present in follicular fluid. Reason for exclusion: Recruited women underwent > 1 cycle of treatment. Not an RCT, as aspiration was followed by flushing only when no oocyte was obtained |
| Neyens 2016 | 138 patients undergoing IVF underwent aspiration (A) and up to 3 flushes (F1, F2, F3) and were inspected for the presence of oocyte‐cumular complexes (OCCs). 91% of OCCs were obtained with aspiration only (A) after 1 flush (F1); significantly more mature oocytes were collected with aspiration only (P = 0.03). Fertilisation rates were similar in all groups. Clinical pregnancy rate and live birth rate were not affected by the first 2 flushes. Reason for exclusion: observational study |
| Pirrello 2011 | A randomised study comparing aspiration alone (36 participants) with aspiration and flushing (38 participants) among poor responders. An equivalent number of oocytes was collected in both groups (P = 0.0594). Reason for exclusion: study authors contacted regarding similarity with another conference abstract (Faller 2010) but with differing participant numbers. Study authors clarified that these data are duplicated in Faller 2010, and that differences in numbers were due to issues related to study ethics and inclusion criteria. |
| Waterstone 1992 | All 50 participants had follicle aspiration with flushing. The origin of each oocyte was established ‐ whether it had been obtained in the initial part of the aspirate, in the dead space aspirate, in the first to third flushes, or in the fourth to sixth flushes. Trialists concluded that 20% more oocytes were obtained than with aspiration alone. Reason for exclusion: not an RCT; only 1 group included |
| Ziebe 2000 | In 107 IVF/ICSI cycles, Medicult and SynVitro flushing media were prospectively randomised for use in follicle flushing. No adverse effects were noted during oocyte recovery in either of the 2 groups. Average numbers of oocytes collected (8.2 ± 2.8 vs 8.3 ± 2.9), recovery rates (86.8 ± 14.6 vs 82.8 ± 15), cleavage rates (60.7 ± 30.3 vs 61.1 ± 28.2), implantation rates (21.1% vs 18.3%), and ongoing pregnancy rates per completed cycle (27.7% vs 27.5%) were similar with SynVitro and Medicult flushing media, respectively. Reason for exclusion: no aspiration‐only group for comparison |
A: aspiration.
CI: confidence interval.
EBSS: Earle's balanced salt solution.
ET: embryo transfer.
F: flushing.
FA: follicular aspiration.
FF: follicular flushing.
hCG: human chorionic gonadotropin.
ICSI: intracytoplasmic sperm injection.
IVF: in vitro fertilisation.
NS: not significant.
OCC: oocyte‐cumulus complex.
OR: odds ratio.
RCT: randomised controlled trial.
TAS: transabdominal sonography.
TVS: transvaginal sonography.
Characteristics of ongoing studies [ordered by study ID]
NCT01329302.
| Trial name or title | Benefit of follicular flushing during oocyte retrieval for poor responder patient in an assisted reproductive technology programme |
| Methods | Prospective single‐blind randomised controlled trial |
| Participants | ‐ Patients undergoing IVF or ICSI treatment ‐ Long agonist, antagonist, or short stimulation protocol ‐ Fewer than 5 follicles of 14 mm or more per day of hCG ‐ 18 to 43 years old ‐ Patients within a married couple or who could prove a married life of over 2 years |
| Interventions | Direct aspiration with single‐lumen needle Echo tip Cook, or follicular flushing with double‐lumen needle Echo tip Cook for double‐lumen aspiration |
| Outcomes | Primary outcome measures: ‐ Number of mature oocytes collected ‐ Quality of embryos obtained Secondary outcome measures: ‐ Number of embryos obtained ‐ Number of transferable embryos (transferred and frozen) ‐ Number of pregnancies obtained |
| Starting date | March 2011 |
| Contact information | Dr. Olivier Pirrello Centre d'Assistance Médicale à la Procréation, CMCO‐SIHCUS Schiltigheim France, 67300 |
| Notes | Advised by study authors that the study is due to be submitted for peer review shortly |
NCT02277210.
| Trial name or title | Follicular flushing in patients with suboptimal responses |
| Methods | Prospective single‐blind randomised controlled trial |
| Participants | Age of women: < 43 years Normal uterine cavity on saline sonogram Endometrial thickness ≥ 8mm on the day of hCG |
| Interventions | Non‐flushing group ‐ Aspiration alone for all follicles larger than 10 mm on both sides. Follicular fluid and flushing will be examined by embryologists to identify any oocytes. Active comparator: flushing group ‐ Aspiration of follicles larger than 10 mm on both sides followed by follicular flushing up to 4 times. Follicular fluid and flushing will be examined by embryologists to identify any oocytes. |
| Outcomes | Primary outcome measures: ‐ Ongoing pregnancy rate Secondary outcome measures: ‐ Number of oocytes retrieved ‐ Number of embryos available for transfer ‐ Clinical pregnancy rate ‐ Operation time for retrieval ‐ Pain score for retrieval |
| Starting date | October 2014 |
| Contact information | Professor Ernest Hung‐Yu Ng Department of Obstetrics and Gynaecology The University of Hong Kong Hong Kong PR China |
| Notes | Estimated study completion date: September 2020 |
NCT02641808.
| Trial name or title | Does follicular flushing improve the outcomes in monofollicular IVF therapy? |
| Methods | Prospective open‐label randomised controlled trial |
| Participants | ‐ Indication for in vitro fertilisation ‐ Desire for natural cycle IVF and ICSI fertilisation ‐ Regular menstrual cycle; both ovaries can be reached for follicle aspiration ‐ 18 to 42 years ‐ Size of follicle ≥16 mm ‐ Max 2 previous embryo transfers |
| Interventions | Experimental: follicular flushing group ‐ Monofollicular IVF therapy with follicular flushing up to 5 times after aspiration of the follicle to the time of oocyte pickup Active comparator: aspiration group ‐ Monofollicular IVF therapy with aspiration only at the time of oocyte pickup |
| Outcomes | Primary outcome measures: ‐ Proportion of mature oocytes retrieved Secondary outcome measures: ‐ Number of flushings necessary to retrieve the oocyte (intervention arm only) ‐ Fertilisation rate ‐ Embryo quality (BLEFCO and ASEBIR scores) on day 2 after fertilisation ‐ Transfer rate ‐ Pregnancy rate ‐ Pain during intervention measured on a VAS scale ‐ Time taken for intervention |
| Starting date | August 2016 |
| Contact information | Dr. Alexandra S. Kohl Schwartz University Hospital Inselspital University of Bern Berne Switzerland |
| Notes | Estimated primary completion date: July 2018 (final data collection date for primary outcome measure) |
ASEBIR: classical EQC of the Spanish Society for the Study of Reproductive Biology.
BLEFCO: Biologistes des Laboratoires d'Etude de la Fécondation et de la Conservation de l'oeuf scale.
hCG: human chorionic gonadotropin.
ICSI: intracytoplasmic sperm injection.
IVF: in vitro fertilisation.
VAS: visual analogue scale.
Differences between protocol and review
For the 2010 review
The methods indicated that we planned to compare single versus multiple flushes, and different volumes for flushing, in terms of live births and ongoing pregnancies in women undergoing IVF and ICSI. However, as aspiration and aspiration with flushing do not yield any differences in clinical and ongoing pregnancies, nor in the number of oocytes obtained, this analysis becomes both irrelevant and unnecessary, and we removed the secondary objective from the final review.
Clinical pregnancy has been moved to be a primary outcome, with ongoing pregnancy.
We have removed several secondary outcomes from the protocol, as they did not contribute to the overall aim of the review, or they were potentially biased, as data not could not be analysed per woman randomised. These outcomes include fertilisation rate, rate of embryo cleavage, rates of congenital and chromosomal abnormalities, amount of anaesthetic required, and cost per oocyte retrieval procedure performed.
We have added the outcome of adverse events as a primary outcome.
For the 2018 update
We have updated primary and secondary outcomes. We have updated search methods and data collection and analysis in keeping with the most recent Cochrane Gynaecology and Fertility Group guidelines.
Contributions of authors
E. Georgiou was involved in preparing all sections of the review.
P. Melo was involved in data extraction for the review.
J. Brown made substantial editorial amendments to the review.
I. Granne was involved in preparing all sections of the protocol and made substantial editorial amendments to the review.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Haines 1989 {published data only}
- Haines CJ, Emes AL, O'Shea RT, Weiss TJ. Choice of needle for ovum pickup. Journal of In Vitro Fertilisation and Embryo Transfer 1989;6(2):111‐2. [DOI] [PubMed] [Google Scholar]
Haydardedeoglu 2011 {published data only}
- Haydardedeoglu B, Cok T, Kilicdag EB, Parlakgumus AH, Simsek E, Bagis T. In vitro fertilization‐intracytoplasmic sperm injection outcomes in single‐ versus double‐lumen oocyte retrieval needles in normally responding patients: a randomized trial. Fertility and Sterility 2011;95(2):812‐4. [DOI] [PubMed] [Google Scholar]
Haydardedeoglu 2017 {published data only}
- Haydardedeoglu B, Gjemalaj F, Aytac PC, Kilicdag EB. Direct aspiration versus follicular flushing in poor responders undergoing intracytoplasmic sperm injection: a randomised controlled trial. British Journal of Obstetrics and Gynaecology 2017;124(8):1190‐6. [DOI] [PubMed] [Google Scholar]
Kara 2012 {published data only}
- Kara M, Aydin T, Turktekin N. Is follicular flushing really effective? A clinical study. Archives of Gynecology and Obstetrics 2012;286(4):1061‐4. [DOI] [PubMed] [Google Scholar]
Kingsland 1991 {published data only}
- Kingsland CR, Taylor CT, Aziz N, Bickerton N. Is follicular flushing necessary for oocyte retrieval? A randomized trial. Human Reproduction 1991;6(3):382‐3. [DOI] [PubMed] [Google Scholar]
Levens 2009 {published data only}
- Levens ED, Whitcomb BW, Payson MD, Larsen FW. Ovarian follicular flushing among low‐responding patients undergoing assisted reproductive technology. Fertility and Sterility 2009;91 Suppl(4):1381‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mok‐Lin 2013 {published data only}
- Mok‐Lin E, Brauer AA, Schattman G, Zaninovic N, Rosenwaks Z, Spandorfer S. Follicular flushing and in vitro fertilization outcomes in the poorest responders: a randomized controlled trial. Human Reproduction 2013;28(11):2990‐5. [DOI: 10.1093/humrep/det350] [DOI] [PubMed] [Google Scholar]
Scott 1989 {published data only}
- Scott RT, Hofmann GE, Muasher SJ, Acosta AA, Kreiner DK, Rosenwaks Z. A prospective randomized comparison of single‐ and double‐lumen needles for transvaginal follicular aspiration. Journal of In Vitro Fertilization and Embryo Transfer 1989;6(2):98‐100. [DOI] [PubMed] [Google Scholar]
Tan 1992 {published data only}
- Tan SL, Waterstone J, Wren M, Parsons J. A prospective randomized study comparing aspiration only with aspiration and flushing for transvaginal ultrasound‐directed oocyte recovery. Fertility and Sterility 1992;58(2):356‐60. [DOI] [PubMed] [Google Scholar]
von Horn 2017 {published and unpublished data}
- Horn K, Depenbusch M, Schultze‐Mosgau A, Griesinger G. Randomized, open trial comparing a modified double‐lumen needle follicular flushing system with a single‐lumen aspiration needle in IVF patients with poor ovarian response. Human Reproduction 2017;32(4):832‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Avila 2013 {published data only}
- Avila A, Diaz‐Spndola P, Santos‐Haliscak R, Obeso‐Montoya I, Davila‐Garza A, Garcia‐Villafaña G. Follicle flushing does not improve reproductive outcomes in art program. Fertility and Sterility 2013;100(3 Suppl):S499–S500. [Google Scholar]
Aydin 2017 {published data only}
- Aydin A, Taplamaciogly F, Gokce F. What is the effect of follicle flushing on oocyte yield and quality in ART cycles of poor responders?. Human Reproduction Abstract Book of the 33rd Annual Meeting of the ESHRE, Switzerland 2017;32:i246‐7. [Google Scholar]
Bagtharia 2005 {published data only}
- Bagtharia S, Haloob ARK. Is there a benefit from routine follicular flushing for oocyte retrieval?. Journal of Obstetrics and Gynaecology 2005;25(4):374‐6. [DOI] [PubMed] [Google Scholar]
Biljan 1997 {published data only}
- Biljan MM, Dean N, Hemmings R, Bissonnette F, Tan SL. Prospective randomized trial of the effect of two flushing media on oocyte collection and fertilization rates after in vitro fertilization. Fertility and Sterility 1997;68(6):1132‐4. [DOI] [PubMed] [Google Scholar]
Dean 1997 {published data only}
- Dean N, Biljan MM, Hemmings R, Bissonette F, Tan SL. Prospective randomized trial on effects of different flushing media on collection and fertilization rates in IVF procedures. Journal of Reproduction and Fertility 1992;19:22 (Abstract No. 44). [Google Scholar]
el Hussein 1992 {published data only}
- Hussein E, Balen AH, Tan SL. A prospective study comparing the outcome of oocytes retrieved in the aspirate with those retrieved in the flush during transvaginal ultrasound directed oocyte recovery for in‐vitro fertilization. British Journal of Obstetrics and Gynaecology 1992;99(10):841‐4. [DOI] [PubMed] [Google Scholar]
Faller 2010 {published and unpublished data}
- Faller E, Pirrello O, Wittemer C, Ohl J. Follicular flushing with double‐lumen needle among low responder patients: preliminary study of 79 cases. Human Reproduction, Abstract Book of the 26th Annual Meeting of the ESHRE, Rome 2010;25 Suppl 1:i153. [Google Scholar]
Ghosh 2002 {published data only}
- Ghosh S, Chatterjee R, Goswami S, Chakraborty BN. Follicular flushing at OCR ‐ should we do away with it?. Human Reproduction Abstract Book of the 18th Annual Meeting of the ESHRE, Vienna, July 1‐3, 2002. 2002;17 Suppl 1(Abstract O‐145):51. [Google Scholar]
Gordon 2002 {published data only}
- Gordon AC, Gadd SC, Kendrew H, Spearman P, Williams C, Walker D, et al. Prospective randomized comparison of two commercially prepared flushing media for IVF/ICSI. Human Reproduction, Abstract Book of the 18th Annual Meeting of the ESHRE, Vienna, July 1‐3, 2002. 2002;17 Suppl 1(Abstract P‐4810):163. [Google Scholar]
Khalifa 1999 {published data only}
- Khalifa EAM, Buraidah KFSH. Routine use of normal saline as flushing media has no impact on fertilization, embryo development and pregnancy rates in assisted reproductive technologies. Fertility and Sterility 1999;72 Suppl 1:193‐4. [Google Scholar]
Knight 2001 {published data only}
- Knight DC, Tyler JP, Driscoll GL. Follicular flushing at oocyte retrieval: a reappraisal. Australian & New Zealand Journal of Obstetrics & Gynaecology 2001;41(2):210‐3. [DOI] [PubMed] [Google Scholar]
Lenz 1987 {published data only}
- Lenz S, Lindenberg S, Fehilly C, Petersen K. Are ultrasonic‐guided follicular aspiration and flushing safe for the oocyte?. Journal of In Vitro Fertilization and Embryo Transfer 1987;4(3):159‐61. [DOI] [PubMed] [Google Scholar]
Mehri 2014 {published data only}
- Mehri S, Levi Setti PE, Greco K, Sakkas D, Martinez G, Patrizio P. Correlation between follicular diameters and flushing versus no flushing on oocyte maturity, fertilization rate and embryo quality. Journal of Assisted Reproduction and Genetics 2014;31(1):73‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mendez Lozano 2008 {published data only}
- Mendez Lozano DH, Scheffer JB, Frydman N, Fay S, Fanchin R, Frydman R. Optimal reproductive competence of oocytes retrieved through follicular flushing in minimal stimulation IVF. RBM Online 2008;16(1):119‐23. [DOI] [PubMed] [Google Scholar]
Neyens 2016 {published data only}
- Neyens S, Neubourg D, Peeraer K, Jaegher N, Spiessens C, Debrock S, et al. Is there a correlation between the number of follicular flushings, oocyte/embryo quality and pregnancy rate in assisted reproductive technology cycles? Results from a prospective study. Gynecologic and Obstetric Investigation 2016;81(1):34‐40. [DOI] [PubMed] [Google Scholar]
Pirrello 2011 {published data only}
- Pirrello O, Faller E, Catherine R, Jeanine O, Laurence M, Brigitte F, et al. Follicular flushing with double lumen needle among low responder patients: a preliminary study. Human Fertility (Abstract of the 7th Biennial Conference of the UK Fertility Societies: The Association of Clinical Embryologists, British Fertility Society and the Society for Reproduction and Fertility. Also Participating: Irish Clinical Embryologists Association, ICE and the Irish Fertility Society 2011;14(2):143. [Google Scholar]
Waterstone 1992 {published data only}
- Waterstone JJ, Parsons JH. A prospective study to investigate the value of flushing follicles during transvaginal ultrasound‐directed follicle aspiration. Fertility and Sterility 1992;57(1):221‐3. [DOI] [PubMed] [Google Scholar]
Ziebe 2000 {published data only}
- Ziebe S, Sunde A, Erb K. Evaluation of a new fully synthetic flushing medium in a prospective randomised multi‐centre study. Human Reproduction 2000;15:74 (Abstract of the 16th ESHRE, Bologna, 25‐28 June 2000; O‐0185). [Google Scholar]
References to ongoing studies
NCT01329302 {unpublished data only}
- NCT01329302. Benefit of follicular flushing during oocyte retrieval for poor responder patient in an assisted reproductive technology program. Available from https://clinicaltrials.gov/ct2/show/NCT01329302. First posted 5 April 2011.
NCT02277210 {unpublished data only}
- NCT02277210. Follicular flushing in patients with suboptimal responses. Available from https://clinicaltrials.gov/ct2/show/NCT02277210. First posted 28 October 2014.
NCT02641808 {unpublished data only}
- NCT02641808. Does follicular flushing improve the outcome in monofollicular IVF therapy? (Flushing). Available from https://clinicaltrials.gov/ct2/show/NCT02641808. First posted 29 December 2015.
Additional references
GRADEpro GDT 2015 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 23 October 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Janssenwillen 1997
- Janssenswillen, C. Christiaens, F. Camu, F. Van Steirteghem, A. The effect of propofol on parthenogenetic activation, in vitro fertilization and early development of mouse oocytes. Fertility and Sterility 1997;67:769‐74. [DOI] [PubMed] [Google Scholar]
Levy 2012
- Levy G, Hill MJ, Ramirez CI, Correa L, Ryan ME, DeCherney AH, et al. The use of follicle flushing during oocyte retrieval in assisted reproductive technologies: a systematic review and meta‐analysis. Human Reproduction 2012;27(8):2373‐9. [DOI: 10.1093/humrep/des174] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roque 2012
- Roque M, Sampaio M, Geber S. Follicular flushing during oocyte retrieval: a systematic review and meta‐analysis. Journal of Assisted Reproduction and Genetics 2012;29(11):1249‐54. [DOI: 10.1007/s10815-012-9869-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tatone 1998
- Tatone C, Francion A, Marinangeli F, Lottan M, Varrassi G, Colonna R. An evaluation of propofol toxicity on mouse oocytes and preimplantation embryos. Human Reproduction 1998;13:430‐5. [DOI] [PubMed] [Google Scholar]
Wood 2000
- Wood C, Trounson AO. Historical perspectives of IVF. In: Trounson AO, Gardner DK editor(s). Handbook of In Vitro Fertilization. Second Edition. New York: CRC Press, 2000:4. [Google Scholar]
Zegers‐Hochschild 2017
- Zegers‐Hochschild F, Adamson GD, Dyer S, Racowsky C, Mouzon J, Sokol R, et al. The International Glossary on Infertility and Fertility Care, 2017. Fertility and Sterility 2017;108(3):393‐406. [DOI: 10.1016/j.fertnstert.2017.06.005] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Wongtra‐ngan 2004
- Wongtra‐ngan S, Edi‐Osagie ECO, Vutyavanich T. Follicular flushing during oocyte retrieval in assisted reproductive techniques. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD004634] [DOI] [PubMed] [Google Scholar]
Wongtra‐Ngan 2010
- Wongtra‐Ngan S, Vutyavanich T, Brown J. Follicular flushing during oocyte retrieval in assisted reproductive techniques. Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD004634.pub2] [DOI] [PubMed] [Google Scholar]


