Abstract
Background
Beyond term, the risks of stillbirth or neonatal death increase. It is unclear whether a policy of labour induction can reduce these risks. This Cochrane review is an update of a review that was originally published in 2006 and subsequently updated in 2012
Objectives
To assess the effects of a policy of labour induction at or beyond term compared with a policy of awaiting spontaneous labour or until an indication for birth induction of labour is identified) on pregnancy outcomes for infant and mother.
Search methods
We searched Cochrane Pregnancy and Childbirth’s Trials Register, ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) (9 October 2017), and reference lists of retrieved studies.
Selection criteria
Randomised controlled trials (RCTs) conducted in pregnant women at or beyond term, comparing a policy of labour induction with a policy of awaiting spontaneous onset of labour (expectant management). We also included trials published in abstract form only. Cluster‐RCTs, quasi‐RCTs and trials using a cross‐over design are not eligible for inclusion in this review.
We included pregnant women at or beyond term. Since a risk factor at this stage of pregnancy would normally require an intervention, only trials including women at low risk for complications were eligible. We accepted the trialists' definition of 'low risk'. The trials of induction of labour in women with prelabour rupture of membranes at or beyond term were not considered in this review but are considered in a separate Cochrane review.
Data collection and analysis
Two reviewers independently assessed trials for inclusion, assessed risk of bias and extracted data. Data were checked for accuracy. We assessed the quality of evidence using the GRADE approach.
Main results
In this updated review, we included 30 RCTs (reporting on 12,479 women). The trials took place in Norway, China, Thailand, the USA, Austria, Turkey, Canada, UK, India, Tunisia, Finland, Spain, Sweden and the Netherlands. They were generally at a moderate risk of bias.
Compared with a policy of expectant management, a policy of labour induction was associated with fewer (all‐cause) perinatal deaths (risk ratio (RR) 0.33, 95% confidence interval (CI) 0.14 to 0.78; 20 trials, 9960 infants; moderate‐quality evidence). There were two perinatal deaths in the labour induction policy group compared with 16 perinatal deaths in the expectant management group. The number needed to treat to for an additional beneficial outcome (NNTB) with induction of labour in order to prevent one perinatal death was 426 (95% CI 338 to 1337). There were fewer stillbirths in the induction group (RR 0.33, 95% CI 0.11 to 0.96; 20 trials, 9960 infants; moderate‐quality evidence); there was one stillbirth in the induction policy arm and 10 in the expectant management group.
For women in the policy of induction arms of trials, there were fewer caesarean sections compared with expectant management (RR 0.92, 95% CI 0.85 to 0.99; 27 trials, 11,738 women; moderate‐quality evidence); and a corresponding marginal increase in operative vaginal births with induction (RR 1.07, 95% CI 0.99 to 1.16; 18 trials, 9281 women; moderate‐quality evidence). There was no evidence of a difference between groups for perineal trauma (RR 1.09, 95% CI 0.65 to 1.83; 4 trials; 3028 women; low‐quality evidence), postpartum haemorrhage (RR 1.09 95% CI 0.92 to 1.30, 5 trials; 3315 women; low‐quality evidence), or length of maternal hospital stay (average mean difference (MD) ‐0.34 days, 95% CI ‐1.00 to 0.33; 5 trials; 1146 women; Tau² = 0.49; I² 95%; very low‐quality evidence).
Rates of neonatal intensive care unit (NICU) admission were lower (RR 0.88, 95% CI 0.77 to 1.01; 13 trials, 8531 infants; moderate‐quality evidence) and fewer babies had Apgar scores less than seven at five minutes in the induction groups compared with expectant management (RR 0.70, 95% CI 0.50 to 0.98; 16 trials, 9047 infants; moderate‐quality evidence).
There was no evidence of a difference for neonatal trauma (RR 1.18, 95% CI 0.68 to 2.05; 3 trials, 4255 infants; low‐quality evidence), for induction compared with expectant management.
Neonatal encephalopathy, neurodevelopment at childhood follow‐up, breastfeeding at discharge and postnatal depression were not reported by any trials.
In subgroup analyses, no clear differences between timing of induction (< 41 weeks versus ≥ 41 weeks' gestation) or by state of cervix were seen for perinatal death, stillbirth, NICU admission, caesarean section, or perineal trauma. However, operative vaginal birth was more common in the inductions at < 41 weeks' gestation subgroup compared with inductions at later gestational ages. The majority of trials (about 75% of participants) adopted a policy of induction at ≥ 41 weeks (> 287 days) gestation for the intervention arm.
Authors' conclusions
A policy of labour induction at or beyond term compared with expectant management is associated with fewer perinatal deaths and fewer caesarean sections; but more operative vaginal births. NICU admissions were lower and fewer babies had low Apgar scores with induction. No important differences were seen for most of the other maternal and infant outcomes.
Most of the important outcomes assessed using GRADE had a rating of moderate or low‐quality evidence ‐ with downgrading decisions generally due to study limitations such as lack of blinding (a condition inherent in comparisons between a policy of acting and of waiting), or imprecise effect estimates. One outcome (length of maternal stay) was downgraded further to very low‐quality evidence due to inconsistency.
Although the absolute risk of perinatal death is small, it may be helpful to offer women appropriate counselling to help choose between scheduled induction for a post‐term pregnancy or monitoring without (or later) induction).
The optimal timing of offering induction of labour to women at or beyond term warrants further investigation, as does further exploration of risk profiles of women and their values and preferences. Individual participant meta‐analysis is likely to help elucidate the role of factors, such as parity, in influencing outcomes of induction compared with expectant management.
Plain language summary
Induction of labour in women with normal pregnancies at or beyond term
What is the issue?
A normal pregnancy lasts about 40 weeks from the start of the woman's last menstrual period, but anything from 37 to 42 weeks is considered as being at term (within the normal range). If a pregnancy goes too long, a woman and her clinician may wish to intervene to bring the birth on, for example, by induction.
Why is this important?
Births after 42 weeks' gestation may slightly increase risks for babies, including a greater risk of death (before or shortly after birth). However induction of labour may also have risks for mothers and their babies, especially if women are not ready to labour. No tests can predict if babies would be better to stay inside their mother or if labour should be induced to make the birth happen sooner. Many hospitals therefore have policies for how long pregnancies should continue. This update (originally published in 2006 and subsequently updated in 2012) looks to see if inducing labour at a set time at or beyond term, could reduce risks for the babies.
What evidence did we find?
We searched for evidence up 9 October 2017 and identified 30 trials with over 12,000 women. The trials took place in Norway, China, Thailand, the USA, Austria, Turkey, Canada, UK, India, Tunisia, Finland, Spain, Sweden and the Netherlands. The evidence was mostly of moderate quality. The trials compared a policy to induce labour at or later than term (usually after 41 completed weeks of gestation (> 287 days)) with waiting for labour to start and/or waiting for a period before inducing labour.
We found that there were fewer deaths of babies in hospitals with a policy to induce when a pregnancy was continuing beyond term (moderate‐quality evidence). Fewer caesarean births were required with induction compared with waiting, but more assisted vaginal births were required with induction. There were fewer admissions to the intensive care nursery and fewer low Apgar scores at five minutes after birth (a simple test to test babies' health) in the induction groups compared with waiting (moderate‐quality evidence). We found that there were no clear differences between a policy to induce at or later than term or waiting in the risks of mothers having trauma to their perineum or bleeding after birth (both low‐quality evidence), in the length of their hospital stay (very‐low quality evidence), or in their babies having trauma (low‐quality evidence), None of the trials provided information on breastfeeding at discharge from hospital, postnatal depression, or whether the babies had encephalopathy (early abnormal neurological function), or child development.
What does this mean?
A policy of labour induction compared with expectant management is associated with fewer deaths of babies and fewer caesarean sections; but more assisted vaginal births. Although the chances of babies dying are small, it may help to offer women appropriate counselling to make an informed choice between induction of labour for pregnancies at, or later than, term ‐ or waiting for labour to start and/or waiting before inducing labour.
The best time to offer induction of labour to women at or beyond term is not yet clear and warrants further investigation. The risk profiles of women as well as their values and preferences could also be considered.
Summary of findings
Background
Description of the condition
A pregnant women is 'at term' when her pregnancy duration reaches 37 weeks. Up to 10% of pregnancies continue beyond 294 days (420 weeks) and are described as being 'post‐term' or 'postdate' (Olesen 2003; Roos 2010; Zeitlin 2007). In 2015 in the USA, 6.5% of pregnancies progressed to 41 weeks and 0.4% continued to 42 weeks or later (Martin 2017).
While the aetiology of post‐term birth is not well elucidated (Mandruzzato 2010), risk factors such as obesity, nulliparity and maternal age greater than 30 years have been associated with an increased risk of post‐term birth (Arrowsmith 2011; Caughey 2009; Heslehurst 2017; Roos 2010). Placental senescence may play a role in the pathophysiology of post‐term birth (Mandruzzato 2010), and genetic/epigenetic factors have also been implicated (Schierding 2014).
Both the mother and the infant are at increased risk of adverse events when the pregnancy continues beyond term (Hilder 1998). In a study from the Norwegian Birth Registry (Heimstad 2008), the perinatal death rate was 0.018% at day 287 (41 weeks) and 0.51% at day 302+ (> 43 weeks). These findings are important in that, even in a setting where early booking allows accurate assessment of gestational age and antenatal services are accessible for most women, post‐term pregnancy constitutes a high‐risk situation, especially for the baby. In another Norwegian study of nearly two million births from 1967 to 2006, the risk of post‐term infant death was strongly associated with growth restriction (Morken 2014).
The obstetric problems associated with post‐term pregnancy include induction of labour with an unfavourable cervix, caesarean section, prolonged labour, postpartum haemorrhage and traumatic birth. It is likely that some of these unwanted outcomes result from intervening when the uterus and cervix are not ready for labour (Caughey 2004).
Description of the intervention
Induction of labour is widely practised to try and prevent problems or outcomes such as caesarean section, prolonged labour, postpartum haemorrhage and traumatic birth (Caughey 2004), and to improve health outcomes for women and their infants. In the USA, nearly one in four births is induced (23.8% in 2015 ‐ Martin 2017). For post‐term pregnancies, this may be one in every two births (e.g. 52% induction rate for gestations ≥ 41 weeks ‐ Wolff 2016).
Variation in rates of post‐term births suggests that different policies and practices for managing post‐term pregnancies (especially timing of inductions) are used in Europe (Zeitlin 2007), and elsewhere. There is concern about the high and increasing induction rate in many countries, and increasing caesarean rates despite increasing induction rates (Keirse 2010).
Earlier versions of this review included interventions involving monitoring, such as early pregnancy ultrasound, that may have an effect on the outcomes of pregnancies for women at or beyond term. This topic is addressed in the Cochrane review 'Ultrasound for fetal assessment in early pregnancy' (Whitworth 2015). In this update, we evaluate the effects of timing of labour induction at or beyond term compared with expectant management (which may include various intensities and forms of monitoring).
How the intervention might work
When the cervix is favourable (usually a Bishop score of six or more), induction is often carried out by oxytocin and artificial rupture of amniotic membranes. If the cervix is not favourable then usually a prostaglandin gel or tablet is placed in the vagina or cervix to ripen the cervix and initiate the uterine contractions and labour. Many protocols are used with varying repeat intervals and transition to oxytocin and amniotomy depending on the onset of uterine contractions and progress of cervical dilatation.
Why it is important to do this review
Determining the threshold for induction of post‐term pregnancies has been described as 'the 41 week to 42 week dilemma' (Kortekaas 2014), with many hospitals now adopting a policy of induction at 41 weeks rather than a policy of waiting to induce at 42 weeks if spontaneous labour has not occurred. This 41‐week policy may substantially increase numbers of inductions ‐ in the Netherlands this policy would mean that 18% of all pregnant women would be induced compared with 1.5% if a 42‐week policy was adopted (Kortekaas 2014). It is important to assess whether improved outcomes such as reduced perinatal death and fewer caesarean sections can be achieved with earlier inductions and to determine the optimal gestational threshold for induction.
Objectives
To assess the effects of a policy of labour induction at or beyond term compared with a policy of awaiting spontaneous labour indefinitely (until a later gestational age or until a maternal or fetal indication for induction of labour is identified) on pregnancy outcomes for the infant and the mother.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials. We included trials presented only as abstracts as well as trials published in full‐text manuscript format.
Cluster‐randomised trials, quasi‐randomised trials and cross‐over trials are not eligible for inclusion in this review.
Types of participants
We included pregnant women at or beyond term. Since a risk factor at this stage of pregnancy would normally require an intervention, only trials including women at low risk for complications were eligible. We accepted the trialists' definition of 'low risk'. The trials of induction of labour in women with prelabour rupture of membranes at or beyond term were not considered in this review (and are considered in the Cochrane review 'Planned early birth versus expectant management (waiting) for prelabour rupture of membranes at term (37 weeks or more)' (Middleton 2017)), although some women participating in the eligible trials in this review may have had ruptured membranes.
Types of interventions
The intervention evaluated in this review is a policy of labour induction at a predetermined gestational age at or beyond term. This policy is compared with 'expectant management' until an indication for birth arises. The trial protocols differ according to:
gestational age used in the policy;
actual method of labour induction (prostaglandins, misoprostol, +/‐ oxytocin), protocol used (dosage of any drugs, timing, frequency of use and mode of administration);
expectant management protocols (intensity of fetal well‐being assessment and fetal monitoring techniques used).
Types of outcome measures
Primary outcomes
The primary outcome of this review was perinatal death, defined as intrauterine deaths plus neonatal deaths in the first week of life.
Secondary outcomes
For the infant/child
Stillbirth
Neonatal death within first week
Birth asphyxia (as defined by trialists)
Admission to neonatal intensive care unit
Neonatal convulsions
Neonatal encephalopathy
Use of anticonvulsants
Meconium aspiration syndrome
Pneumonia
Apgar score less than seven at five minutes
Birthweight
Birthweight > 4000 g
Neonatal trauma
Neurodevelopment at childhood follow‐up
For the mother
Mode of birth (caesarean section)
Operative vaginal birth (forceps or ventouse)
Analgesia used
Perineal trauma
Prolonged labour (cut‐off used by the trialists was used)
Postpartum haemorrhage (cut‐off used by the trialists was used)
Anxiety before birth
Other measures of satisfaction with the approach
Breastfeeding at discharge
Postnatal depression
Health services use
Length of maternal postnatal stay
Length of neonatal postnatal stay
Length of labour
Cost‐related analyses are described in the Discussion.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (9 October 2017).
The Register is a database containing over 24,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (9 October 2017) (see: Appendix 1 for search methods used).
Searching other resources
We searched the reference lists of retrieved studies. We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeGülmezoglu 2012.
For this update, the following methods were used for assessing the 15 reports that were identified as a result of the updated search. Where required, information pertaining to the previously included studies was updated according to methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014), and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving the third review author.
(1) Allocation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blinded outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blinded outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to have impacted on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
For this update we used the GRADE approach as outlined in the GRADE handbook, in order to assess the quality of the body of evidence relating to the following outcomes.
For the infant/child
Perinatal death, defined as intrauterine deaths plus neonatal deaths in the first week of life
Stillbirth
Admission to neonatal intensive care unit
Neonatal encephalopathy
Apgar score less than seven at five minutes
Neonatal trauma
Neurodevelopment at childhood follow‐up
For the mother
Mode of birth (caesarean section)
Operative vaginal birth (forceps or ventouse)
Perineal trauma
Postpartum haemorrhage (cut‐off reported by the trialists was used)
Breastfeeding at discharge
Postnatal depression
Length of maternal postnatal stay
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014), in order to create ’Summary of findings’ tables, comparing a policy of labour induction versus expectant management. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
Cluster‐randomised trials were not eligible for inclusion.
Cross‐over trials
Cross‐over trials were not eligible for inclusion.
Multiple pregnancies
We did not identify any eligible studies that reported multiple pregnancies separately. If studies with multiple pregnancies are reported separately in trials included in future updates of this review, we will adjust for clustering in the analyses wherever possible, and use the inverse variance method for adjusted analyses, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and in Yelland 2011.
Multi‐armed trials
Where we included studies with multiple arms, we created single pair‐wise comparisons, by including only the groups relevant to this review, or by combining groups. In Gelisen 2005, we combined the three induction arms for the relevant analyses.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if more eligible studies are included, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analyses.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. Where we identified substantial heterogeneity (above 30%), we aimed to explore it using pre‐specified subgroup analyses.
Assessment of reporting biases
Where there were 10 or more studies in the meta‐analyses, we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we planned to perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where studies were examining the same intervention, and the studies' populations and methods were judged sufficiently similar.
Where there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or where substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary has been treated as the average of the range of possible treatment effects and we have discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we decided that we would not combine trials. Where we used random‐effects analyses, the results have been presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
Where we identified substantial heterogeneity, we planned to investigate it using subgroup and sensitivity analyses. We planned to consider whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce it.
We carried out the following subgroup analyses.
Gestational age by week of gestation when induction was intended in the intervention arm.
In this update we have presented the main groups as close to this as study reporting would allow ‐ gestational ages ≤ 41 weeks, and > 41 completed weeks (> 287 days). In Brane 2014, the gestational age at induction in the intervention spanned 37 to 42 weeks.
State of the cervix (favourable versus unfavourable).
We were unable to conduct subgroup analyses by method of induction, due to wide variation in reporting of dosage, timing, frequency and mode of administration.
Where possible, we used the following outcomes in subgroup analyses.
For the infant/child
Perinatal death, defined as intrauterine deaths plus neonatal deaths in the first week of life
Stillbirth
Admission to neonatal intensive care unit
For the mother
Mode of birth (caesarean section)
Operative vaginal birth (forceps or ventouse)
Perineal trauma
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We carried out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result. Poor quality was defined as high risk of bias. We used the following outcomes in our sensitivity analyses.
For the infant/child
Perinatal death, defined as intrauterine deaths plus neonatal deaths in the first week of life
Stillbirth
Admission to neonatal intensive care unit
For the mother
Mode of birth (caesarean section)
Operative vaginal birth (forceps or ventouse)
Perineal trauma
Results
Description of studies
Results of the search
See Figure 1.
1.
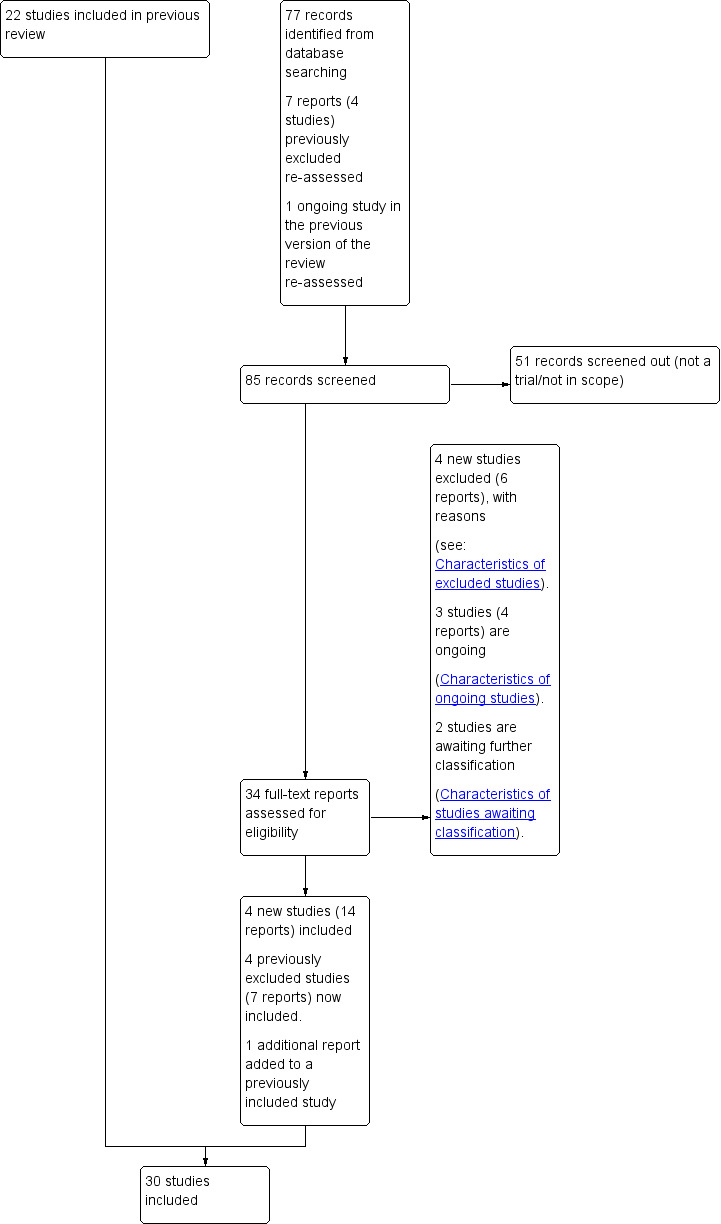
Study flow diagram.
For this update, we assessed 26 new trial reports along with four studies (seven reports) which were previously excluded and one previously ongoing study. We have included eight additional trials (21 reports) (Brane 2014; Cohn 1992; Kortekaas 2014; Martin 1978; Miller 2015; Sande 1983; Tylleskar 1979; Walker 2016), excluded four studies (six reports) (Frass 2011; Gregson 2015; Neri 2014; Rijnders 2011), and added three ongoing studies (four reports) (Elden 2016; Othman 2017; Reddy 2013). Two studies are awaiting classification, one pending a translation (Benito Reyes 2010), and another awaiting further detail (Harrington 2003). We also added an additional earlier report to an already included study (Heimstad 2007a).
This updated review is now comprised of 30 included trials which randomised 12,479 women and their babies (seeCharacteristics of included studies), 64 excluded studies (seeCharacteristics of excluded studies) and three ongoing studies (seeCharacteristics of ongoing studies).
Included studies
Settings
Of the 30 included trials:
six were conducted in the USA (Dyson 1987; Martin 1989; Miller 2015; NICHHD 1994; Nielsen 2005; Witter 1987);
four in the UK (Scotland; England; Ireland) (Cole 1975; Henry 1969; Martin 1978; Walker 2016);
three in China (Bergsjo 1989; Cohn 1992; Roach 1997);
three in Norway (Augensen 1987; Heimstad 2007a; Sande 1983);
two in India (Chakravarti 2000; James 2001);
two in Thailand (Chanrachkul 2003; Herabutya 1992);
two in Sweden (Brane 2014; Tylleskar 1979);
one in Tunisia (Sahraoui 2005);
one in Turkey (Gelisen 2005);
one in Canada (Hannah 1992);
one in France (Breart 1982);
one in Austria (Egarter 1989);
one in Spain (Ocon 1997);
one in the Netherlands (Kortekaas 2014); and
one in Finland (Suikkari 1983).
Cervix status
Fifteen trials did not mention or specify cervix status as an inclusion criterion (Augensen 1987; Bergsjo 1989; Brane 2014; Breart 1982; Chakravarti 2000; Cohn 1992; Cole 1975; Heimstad 2007a; Henry 1969; James 2001; Martin 1978; Roach 1997; Suikkari 1983; Walker 2016; Witter 1987). Nine trials included women with unfavourable cervix (Dyson 1987; Gelisen 2005; Hannah 1992; Herabutya 1992; Martin 1989; Miller 2015; NICHHD 1994; Ocon 1997; Sahraoui 2005), and six with favourable cervical status (Chanrachkul 2003; Egarter 1989; Kortekaas 2014; Nielsen 2005; Sande 1983; Tylleskar 1979).
Interventions
All trials were conducted in hospitals with various intensities of fetal monitoring both in the induction and expectant management groups (seeCharacteristics of included studies).
Timing of induction ‐ induction group
The information on timing of induction in each trial's induction arm is summarised below.
37 to 39 weeks: one trial (Breart 1982), induced women at 37 to 39 weeks' gestation.
39 weeks: one trial (Martin 1978), induced women at 39 weeks' gestation.
39 to 40 weeks: four trials (Cole 1975; Miller 2015; Nielsen 2005; Walker 2016), induced women at 39 to 40 weeks' gestation.
40 weeks: two trials (Egarter 1989; Tylleskar 1979), induced women at their expected due date.
37 to 42 weeks: one trial (Brane 2014), induced women at 370 to 416 weeks' gestation.
40 to 41 weeks: one trial (Sande 1983), induced women between 40 and 41 weeks' gestation.
< 41 weeks: one trial (Chakravarti 2000), induced women at less than 41 weeks' gestation.
41 completed weeks: five trials reported that they induced women at 41 completed weeks (410 or 287 days (Gelisen 2005; James 2001; Kortekaas 2014; Martin 1989); or 413 or 290 days (Chanrachkul 2003)).
> 41 weeks: in the remaining 15 trials (Augensen 1987; Bergsjo 1989; Cohn 1992; Dyson 1987; Hannah 1992; Heimstad 2007a; Henry 1969; Herabutya 1992; Kortekaas 2014; NICHHD 1994; Ocon 1997; Roach 1997; Sahraoui 2005; Suikkari 1983; Witter 1987), women were generally induced after 41 completed weeks (after 287 days) up to 42 completed weeks (294 days), with Kortekaas 2014 spanning induction across 41‐2+2 weeks and the NICHHD 1994 trial extending from 41 to 43 completed weeks (430 or 301 days).
In this update of the review, we have collapsed these categories into:
induced at < 41 weeks: 10 trials (Breart 1982; Chakravarti 2000; Cole 1975; Egarter 1989; Martin 1978; Miller 2015; Nielsen 2005; Sande 1983; Tylleskar 1979; Walker 2016);
induced at ≥ 41 weeks: 19 trials (Augensen 1987; Bergsjo 1989; Chanrachkul 2003; Cohn 1992; Dyson 1987; Gelisen 2005; Hannah 1992; Heimstad 2007a; Henry 1969; Herabutya 1992; James 2001; Kortekaas 2014; Martin 1989; NICHHD 1994; Ocon 1997; Roach 1997; Sahraoui 2005; Suikkari 1983; Witter 1987);
inductions spanning 37 to 42 weeks: one trial (Brane 2014).
(In the previous version of this review, we grouped studies into the following three categories: 39 to 40 weeks; 41 weeks; and > 41 weeks.)
In some trials, the actual gestational age at induction in the induction groups may have been slightly later than the gestational threshold specified at trial entry (e.g. Hannah 1992).
See Characteristics of included studies table for further details.
Method of induction ‐ induction group
Labour induction was by oxytocin with or without artificial rupture of membranes in most trials. In trials recruiting women with an unfavourable cervix, priming with prostaglandins or laminaria was often undertaken before induction.
Of the 30 included trials:
two trials did not report the method used (Chakravarti 2000; Cohn 1992);
23 trials used oxytocin infusion in some or all women in their intervention group (Augensen 1987; Bergsjo 1989; Brane 2014; Breart 1982; Chanrachkul 2003; Cole 1975; Dyson 1987; Gelisen 2005; Hannah 1992; Heimstad 2007a; Henry 1969; Herabutya 1992; James 2001; Kortekaas 2014; Martin 1989; Miller 2015; NICHHD 1994; Nielsen 2005; Sande 1983; Suikkari 1983; Tylleskar 1979; Walker 2016; Witter 1987). Of those trials, only one used oxytocin as the sole method of induction (Augensen 1987). Seventeen trials used artificial rupture of membranes (AROM), as well as oxytocin infusion (when possible) (Bergsjo 1989; Brane 2014; Breart 1982; Chanrachkul 2003; Cole 1975; Heimstad 2007a; Henry 1969; Herabutya 1992; James 2001; Kortekaas 2014; Miller 2015; Nielsen 2005; Sande 1983; Suikkari 1983; Tylleskar 1979; Walker 2016; Witter 1987);
none of the included trials used AROM as the sole method of induction;
10 trials used intravaginal prostaglandin E2 for some or all women in the intervention group (in either gel or pessary form) (Brane 2014; Dyson 1987; Egarter 1989; Hannah 1992; Herabutya 1992; NICHHD 1994; Ocon 1997; Roach 1997; Sahraoui 2005; Walker 2016). Four trials used prostaglandin E2 as the sole method of induction (Egarter 1989; Ocon 1997; Roach 1997; Sahraoui 2005) and six trials used a combination of prostaglandin and oxytocin +/‐ AROM (Brane 2014; Dyson 1987; Hannah 1992; Herabutya 1992; NICHHD 1994; Walker 2016);
three trials used vaginal misoprostol in some or all women in the intervention group (Gelisen 2005; Heimstad 2007a; Miller 2015);
two trials had more than one intervention group (Gelisen 2005; NICHHD 1994), although the placebo priming and oxytocin arm in NICHHD 1994 was not included in this review. The Gelisen 2005 trial had three labour induction arms with misoprostol, oxytocin and Foley catheter.
Expectant management group protocols
For the majority of trials, expectant management protocols included various combinations of fetal heart rate monitoring, ultrasound for amniotic fluid measurements and, in earlier studies, biochemical tests.
No gestational age limit for induction was imposed or reported in nine of the trials (Brane 2014; Cohn 1992; Dyson 1987; Henry 1969; James 2001; Ocon 1997; Roach 1997; Suikkari 1983; Witter 1987). In the remaining 21 trials, women were induced at the following times (unless they went into spontaneous labour earlier) in the expectant management groups.
41 weeks (Cole 1975).
41 to 42 weeks (Walker 2016).
42 weeks (Breart 1982; Chakravarti 2000; Egarter 1989; Gelisen 2005; Kortekaas 2014; Martin 1978; Miller 2015; Nielsen 2005; Sahraoui 2005; Sande 1983; Tylleskar 1979).
42 to 43 weeks (Augensen 1987; Heimstad 2007a).
43 weeks (Bergsjo 1989; Martin 1989).
44 weeks (Chanrachkul 2003; Hannah 1992; Herabutya 1992; NICHHD 1994).
See Characteristics of included studies for further details.
Outcomes
The primary outcome of perinatal death was reported in 20 of the 30 included trials. Caesarean section was reported in 27 trials. Other outcomes, such as many of the adverse pregnancy and neonatal outcomes, were reported in less than half of the included trials. Only two trials reported on maternal satisfaction and no trials have yet reported on maternal anxiety or depression, or breastfeeding.
Funding
Nine of the 30 included trials reported their funding sources as follows: Karolinska Institute Foundations and Funds (Brane 2014); Ramathibodi Hospital Research Grants (Chanrachkul 2003; Herabutya 1992); Community Service Program of Kaiser Foundation Hospitals (Dyson 1987); Medical Research Council of Canada (Hannah 1992); and Upjohn Company of Canada supplied the prostaglandin gel for this study; ZonMW (The Netherlands Organisation for Health Research and Development) (Kortekaas 2014); Vicksburg Hospital Medical Foundation (Martin 1989); National Institute of Child Health and Human Development, NIH, USA (NICHHD 1994); and one grant from the Research for Patient Benefit Programme of the National Institute for Health Research (Walker 2016).
Twenty‐one of the 30 included trials did not report their funding sources.
Declarations of interest
Walker 2016 declared relevant interests on the part of one author, Dr Smith: "Dr. Smith reports receiving fees for serving on an advisory board from Roche Diagnostics, consulting fees from GlaxoSmithKline, equipment loans from Roche Diagnostics and General Electric, travel support from Roche Diagnostics and Chiesi, and grant support from GlaxoSmithKline and Action Medical Research, and being named as an inventor on a pending patent (PCT/EP2014/062602) filed by GlaxoSmithKline related to retosiban as a preventive treatment for preterm labor in women with increased uterine stretch". No other potential conflict of interest was reported by the authors of this study.
Kortekaas 2014 declared that they had no conflicts of interest, and Miller 2015 declared that they had no financial conflicts of interest.
Of the remaining 27 studies, none included any declarations of interest.
Excluded studies
Most of the excluded trials were comparisons of different labour induction (17 trials: Ascher‐Walsh 2000; de Aquino 2003; Evans 1983; Kipikasa 2005; Lee 1997; Lemancewicz 1999; Magann 1999; Mancuso 1998; Meydanli 2003; Misra 1994; Müller 1995; Papageorgiou 1992; Rijnders 2011; Satin 1991; Stenlund 1999; Su 1996; Surbek 1997) or cervical ripening protocols (28 trials: Bell 1993; Berghella 1996; Boulvain 1998; Buttino 1990; Damania 1992; Dare 2002; Doany 1997; Elliott 1984; El‐Torkey 1992; Giacalone 1998; Hage 1993; Ingemarsson 1987; Jenssen 1977; Kadar 1990; Klopper 1969; Lien 1998; Lyons 2001; Magann 1998; Newman 1997; Rayburn 1988; Rayburn 1999; Roberts 1986; Sawai 1991; Sawai 1994; Williams 1990; Wing 2000; Wong 2002; Ziaei 2003). Nine studies were not randomised trials (Amano 1999; Cardozo 1986; Garry 2000; Heden 1991; Hernandez‐Castro 2008; Iqbal 2004; Katz 1983; Knox 1979; Ohel 1996) and there were variety of reasons for excluding the remaining nine studies (Alcalay 1996; Conway 2000; Dunn 1989; Frass 2011; Gregson 2015; Imsuwan 1999; Neri 2014; Nicholson 2008; Paul 1988). More details are provided in the Characteristics of excluded studies tables.
Risk of bias in included studies
Three trials (Chakravarti 2000; Cohn 1992; Suikkari 1983), were available only as abstracts and despite extensive searches we could not locate full publications of the studies, which limited our assessment of their risk of bias. Another trial (Kortekaas 2014), is available only in abstract form, with full publication planned.
We judged the majority of included trials to be at moderate risk of bias (Figure 2; Figure 3), largely due to a lack of reporting.
2.
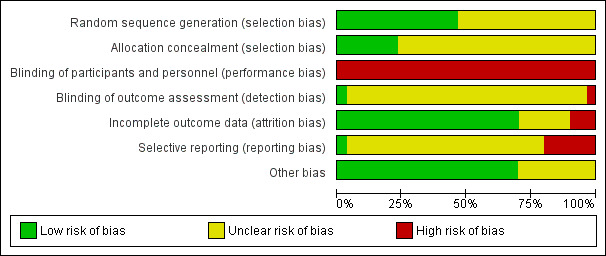
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged 14 trials (Augensen 1987; Bergsjo 1989; Chanrachkul 2003; Dyson 1987; Heimstad 2007a; James 2001; Kortekaas 2014; Martin 1978; Miller 2015; NICHHD 1994; Nielsen 2005; Sahraoui 2005; Walker 2016; Witter 1987), to be at low risk of selection bias, reporting some form of adequate random sequencing such as a computer‐generated sequence or a list of random numbers. We judged the remaining 16 trials to be at unclear risk of selection bias, as they did not report how a random sequence was generated (Brane 2014; Breart 1982; Chakravarti 2000; Cohn 1992; Cole 1975; Egarter 1989; Gelisen 2005; Hannah 1992; Henry 1969; Herabutya 1992; Martin 1989; Ocon 1997; Roach 1997; Sande 1983; Suikkari 1983; Tylleskar 1979).
Of the 29 included trials, only seven reported a method of allocation concealment likely to have a low risk of bias ‐ either central randomisation or sequentially numbered sealed opaque envelopes (Hannah 1992; Heimstad 2007a; Kortekaas 2014; Miller 2015; NICHHD 1994; Nielsen 2005; Walker 2016). Eight trials reported that they used an envelope system with an unclear risk of bias (Brane 2014; Breart 1982; Dyson 1987; Gelisen 2005; James 2001; Martin 1989; Roach 1997; Witter 1987), one trial reported a partial third party system also with unclear risk of bias (Augensen 1987), and 14 trials did not report a method for concealing allocation and were rated as being at unclear risk of bias (Bergsjo 1989; Chakravarti 2000; Chanrachkul 2003; Cohn 1992; Cole 1975; Egarter 1989; Henry 1969; Herabutya 1992; Martin 1978; Ocon 1997; Sahraoui 2005; Sande 1983; Suikkari 1983; Tylleskar 1979).
Blinding
Performance bias
Given the nature of the intervention (induction of labour) and comparison (expectant management), it was not possible for women or clinicians to be blinded to the treatment group in any of the 29 trials, and thus risk of performance bias was judged to be high. For the more objective outcomes such as perinatal death, this lack of blinding is unlikely to be a major source of bias.
Detection bias
It would have been possible for outcome assessment to have been undertaken by someone blinded to allocation groups. However, only two studies reported whether or not outcome assessment was blinded. One study indicated partial blinding of outcome assessment (Hannah 1992), with an adjudication of abnormal neonatal outcomes undertaken by a neonatologist who was unaware of the mothers' group assignments (rated unclear risk of bias). A further trial (Martin 1978) reported blinded outcome assessment (rated as low risk of bias). The remaining 27 trials did not detail whether outcome assessment was to be blinded, and thus we judged risk of detection bias to be unclear.
Measurement of outcomes such as perinatal death should not be biased by lack of blinding.
Incomplete outcome data
We considered the majority of trials (21) (Augensen 1987; Bergsjo 1989; Breart 1982; Chanrachkul 2003; Dyson 1987; Gelisen 2005; Hannah 1992; Heimstad 2007a; Henry 1969; Herabutya 1992; James 2001; Kortekaas 2014; Martin 1989; Miller 2015; NICHHD 1994; Nielsen 2005; Ocon 1997; Roach 1997; Sahraoui 2005; Walker 2016; Witter 1987) to be at low risk of attrition bias, with minimal/no losses to follow‐up or exclusions. We judged six trials to be at unclear risk of attrition bias (Brane 2014; Chakravarti 2000; Cohn 1992; Cole 1975; Egarter 1989; Suikkari 1983), commonly due to some post‐randomisation exclusions and/or missing data, or due to insufficient information to determine losses or exclusions (due to publication in abstract form only).
We judged three trials to be at high risk of attrition bias. In both Martin 1978 and Tylleskar 1979, between 25% and 30% of the women randomised were excluded post‐randomisation due to going into labour prior to their planned date of induction (for women in the induction group), due to obstetric abnormalities or failure to go into spontaneous labour before 42 weeks (women in the expectant management group of Martin 1978), going into labour prior to their expected delivery date (women in the expectant management group of Tylleskar 1979). In Sande 1983, a per protocol analysis was performed, whereby women were not analysed in the group to which they were randomised, rather according to whether they had their labour induced, or delivered spontaneously.
Selective reporting
Only one trial (Walker 2016) was judged to be at low risk of reporting bias, with outcomes reported as pre‐specified in the published protocol. We judged 23 trials (Brane 2014; Breart 1982; Chakravarti 2000; Chanrachkul 2003; Cohn 1992; Cole 1975; Dyson 1987; Gelisen 2005; Hannah 1992; Heimstad 2007a; Henry 1969; Herabutya 1992; James 2001; Kortekaas 2014; Martin 1978; Miller 2015; NICHHD 1994; Nielsen 2005; Ocon 1997; Roach 1997; Sahraoui 2005; Suikkari 1983; Witter 1987) to be at unclear risk of reporting bias, largely due to insufficient information to assess selective reporting (i.e. no access to trial protocols and limited detail reported in manuscript methods). We considered six trials (Augensen 1987; Bergsjo 1989; Egarter 1989; Martin 1989; Sande 1983; Tylleskar 1979), to be at high risk of reporting bias, predominately due to the incomplete reporting of outcomes data (such as in text or figures only, with statements such as "no significant difference between groups" made) such that outcome data could not be included in review meta‐analyses.
Other potential sources of bias
Most of the trials (21/30) (Augensen 1987; Bergsjo 1989; Brane 2014; Breart 1982; Chanrachkul 2003; Cole 1975; Dyson 1987; Gelisen 2005; Hannah 1992; Heimstad 2007a; Henry 1969; Herabutya 1992; James 2001; Martin 1989; Miller 2015; NICHHD 1994; Ocon 1997; Roach 1997; Sahraoui 2005; Walker 2016; Witter 1987), appeared to be free of other potential sources of bias. We judged the other nine trials to be at unclear risk of bias, six trials due to limited reporting (abstract only or limited methodological detail provided) (Chakravarti 2000; Cohn 1992; Kortekaas 2014; Martin 1978; Suikkari 1983; Tylleskar 1979); and one trial each for imbalance in the numbers of women randomised to each group (Egarter 1989), baseline characteristic imbalance (Nielsen 2005), or lack of reporting of baseline characteristics (Sande 1983).
Effects of interventions
Summary of findings for the main comparison. Labour induction versus expectant management (infant/child outcomes).
| Induction of labour for improving birth outcomes for women at or beyond term | ||||||
| Population: pregnant women at or beyond term Setting: Norway, China, Thailand, the USA, Austria, Turkey, Canada, UK, India, Tunisia, Finland, Spain, Sweden and the Netherlands Intervention: labour induction Comparison: expectant management | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with expectant management | Risk with labour induction | |||||
| Perinatal death | Study population | RR 0.33 (0.14 to 0.78) | 9960 (20 RCTs) | ⊕⊕⊕⊝ MODERATE1 | ||
| 3 per 1000 | 1 per 1000 (0 to 3) | |||||
| Stillbirth | Study population | RR 0.33 (0.11 to 0.96) | 9960 (20 RCTs) | ⊕⊕⊕⊝ MODERATE1 | ||
| 2 per 1000 | 1 per 1000 (0 to 2) | |||||
| Admission to neonatal intensive care unit | Study population | RR 0.88 (0.77 to 1.01) | 8531 (13 RCTs) | ⊕⊕⊕⊝ MODERATE1 | ||
| 85 per 1000 | 75 per 1000 (60 to 86) | |||||
| Neonatal encephalopathy | Study population | ‐ | (0 RCTs) | ‐ | No RCTs reported data for this outcome. | |
| see comment | see comment | |||||
| Apgar score less than 7 at 5 minutes | Study population | RR 0.70 (0.50 to 0.98) | 9047 (16 RCTs) | ⊕⊕⊕⊝ MODERATE1 | ||
| 17 per 1000 | 12 per 1000 (7 to 17) | |||||
| Neonatal trauma | Study population | RR 1.18 (0.68 to 2.05) | 4255 (3 RCTs) | ⊕⊕⊝⊝ LOW1,2 | ||
| 10 per 1000 | 12 per 1000 (7 to 21) | |||||
| Neurodevelopment at childhood follow‐up | Study population | ‐ | (0 RCTs) | ‐ | No RCTs reported data for this outcome. | |
| see comment | see comment | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Studies contributing data had some design limitations. (‐1) 2Wide confidence intervals crossing the line of no effect. (‐1)
Summary of findings 2. Labour induction versus expectant management (maternal outcomes).
| Induction of labour for improving birth outcomes for women at or beyond term | ||||||
| Population: women at or beyond term Setting: Norway, China, Thailand, the USA, Austria, Turkey, Canada, UK, India, Tunisia, Finland, Spain, Sweden, France and the Netherlands Intervention: labour induction Comparison: expectant management | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with expectant management | Risk with labour induction | |||||
| Caesarean section | Study population | RR 0.92 (0.85 to 0.99) | 11,738 (27 RCTs) | ⊕⊕⊕⊝ MODERATE1 | ||
| 184 per 1000 | 169 per 1000 (157 to 182) | |||||
| Operative vaginal birth (forceps or ventouse) | Study population | RR 1.07 (0.99 to 1.16) | 9281 (18 RCTs) | ⊕⊕⊕⊝ MODERATE1 | ||
| 193 per 1000 | 206 per 1000 (191 to 223) | |||||
| Perineal trauma | Study population | RR 1.09 (0.65 to 1.83) | 3028 (4 RCTs) | ⊕⊕⊝⊝ LOW1,2 | ||
| 17 per 1000 | 18 per 1000 (11 to 31) | |||||
| Postpartum haemorrhage | Study population | RR 1.09 (0.92 to 1.30) | 3315 (5 RCTs) | ⊕⊕⊝⊝ LOW1,2 | ||
| 122 per 1000 | 133 per 1000 (112 to 159 | |||||
| Breastfeeding at discharge | Study population | ‐ | (0 RCTs) | ‐ | No RCTs reported data for this outcome. | |
| see comment | see comment | |||||
| Postnatal depression | Study population | ‐ | (0 RCTs) | ‐ | No RCTs reported data for this outcome. | |
| see comment | see comment | |||||
| Length of maternal hospital stay (days) | ‐ | ‐ | Average MD 0.34 days shorter for women who were induced (1 day shorter to 0.33 days longer) | 1146 (5 RCTs) | ⊕⊝⊝⊝ VERY LOW1,2,3 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Studies contributing data had some design limitations. (‐1) 2Wide confidence intervals crossing the line of no effect. (‐1) 3Statistical heterogeneity (I² = 95%). Variation in size and direction of effect. (‐2)
Labour induction versus expectant management (all trials)
Primary outcome
Perinatal death
Fewer perinatal deaths occurred in the labour induction groups than in the expectant management groups: two perinatal deaths occurred in the induction group compared with 16 in the expectant group (risk ratio (RR) 0.33, 95% confidence interval (CI) 0.14 to 0.78; 20 trials; 9960 infants; moderate‐quality evidence; Analysis 1.1).
1.1. Analysis.
Comparison 1 Labour induction versus expectant management (all trials), Outcome 1 Perinatal death.
Interaction tests failed to demonstrate significant differences between the timing of induction subgroups for perinatal deaths (Chi² = 0.00, P = 0.99, I² = 0%; Analysis 2.1) or for subgroups according to state of cervix (Chi² = 0.08, P = 0.96, I² = 0%; Analysis 3.1).
2.1. Analysis.
Comparison 2 Labour induction versus expectant management (gestational age at induction), Outcome 1 Perinatal death.
3.1. Analysis.
Comparison 3 Labour induction versus expectant management (status of cervix), Outcome 1 Perinatal death.
Some trials (e.g. Hannah 1992), excluded perinatal deaths due to congenital anomalies while other trials included these. If the three deaths reported to be due to congenital anomalies are excluded, there was then one death in the labour induction group and 14 in the expectant management group. This made little difference to the overall result (RR 0.30, 95% CI 0.11 to 0.76).
Table 3 details, where known, the respective causes of death (stillbirths and neonatal deaths) for the 15 babies, including the stillbirth reported in Martin 1978 (where it was not clear if there were any neonatal deaths).
1. Causes of death (stillbirths and livebirth deaths).
| Study | Cause of death | |
| Intervention Group | Control Group | |
| Augensen 1987 | No deaths | No deaths |
| Bergsjo 1989 | 1. Severe malformations (Livebirth) GA at birth and timing of death after birth not reported |
1. Malformation (Livebirth) GA at birth and timing of death after birth not reported 2. Pneumonia (Livebirth) GA at birth and timing of death after birth not reported |
| Chanrachkul 2003 | No deaths | No deaths |
| Cole 1975 | No deaths | 1. Congenital heart condition (Stillbirth) GA at detection of death not reported |
| Dyson 1987 | No deaths | 1. Meconium aspiration and persistent fetal circulation (Livebirth) GA at birth was 43 + 4 and the timing of death after birth was not reported |
| Egarter 1989 | No deaths | 1. Cord complication (Stillbirth) GA at detection of fetal death was 40 + 3 weeks |
| Gelisen 2005 | No deaths | 1. Intrauterine fetal death (Stillbirth) GA at death 41 + 5 weeks |
| Hannah 1992 | No deaths | 1. Hypoxic ischaemic encephalopathy (Stillbirth) GA at detection of death not reported 2. Massive aspiration of meconium (Stillbirth) GA at detection of death not reported |
| Heimstad 2007a | No deaths | 1. Birth asphyxia secondary to a true knot in the umbilical cord (Livebirth) Birth at 294 days GA; death at 2 days of age |
| Henry 1969 | No deaths | 1. Stillbirth in a patient with an abnormal glucose tolerance test (Stillbirth) GA at detection of death not reported 2. Neonatal death from meconium inhalation in a woman with a positive amnioscopy who refused surgical induction of labour (Livebirth) GA at detection of death not reported |
| Herabutya 1992 | No deaths | 1. Congenital abnormality (Livebirth) Birth at 43 weeks; death at 3 days of age |
| James 2001 | No deaths | No deaths |
| Kortekaas 2014 | One fetal death (no further details reported) | 2 fetal deaths (no further details reported) |
| Martin 1978 | No deaths reported | 1. Stillbirth (Stillbirth) Stillbirth after induction of labour at 42 weeks for postmaturity and meconium |
| Martin 1989 | No deaths | No deaths |
| NICHHD 1994 | No deaths | No deaths |
| Sahraoui 2005 | No deaths | 1. Intrauterine fetal death (Stillbirth) Death detected at 42 weeks' GA |
| Sande 1983 | No deaths | No deaths |
| Suikkari 1983 | No deaths | No deaths |
| Walker 2016 | No deaths | No deaths |
GA: gestational age
The number needed to treat for an additional beneficial outcome (NNTB) with a policy of induction of labour in order to prevent one perinatal death was 464 (95% CI 361 to 1412).
Ten trials (Brane 2014; Breart 1982; Chakravarti 2000; Cohn 1992; Miller 2015; Nielsen 2005; Ocon 1997; Roach 1997; Tylleskar 1979; Witter 1987), did not report on perinatal deaths.
Sensitivity analyses
Only seven of the 30 trials were judged to be of higher quality, defined as adequate allocation concealment and low attrition (Hannah 1992; Heimstad 2007a; Kortekaas 2014; Miller 2015; NICHHD 1994; Nielsen 2005; Walker 2016). We have presented each of the sensitivity analyses under each of the relevant prespecified outcomes (perinatal death, stillbirth).
Perinatal death sensitivity analysis: RR 0.38, 95% 0.10 to 1.41; five trials, 6698 infants. There was one perinatal death in the induction group and four perinatal deaths in the expectant management group. On sensitivity analysis, conventional statistical significance was lost, although the point estimate of 62% relative risk reduction was similar to the overall analysis (RR 0.33 95% CI 0.14 to 0.78).
Secondary outcomes for the infant/child
Stillbirth
Eleven of the 16 perinatal deaths were stillbirths. One stillbirth occurred in the induction group and 10 stillbirths occurred in the expectant management groups (RR 0.33, 95% CI 0.11 to 0.96; 20 trials; 9960 infants; moderate‐quality evidence; Analysis 1.2). Interaction tests failed to demonstrate significant differences between the timing of induction subgroups (Chi² = 0, P = 0.98, I² = 0%; Analysis 2.2) or for subgroups according to state of cervix (Chi² = 0.01, P = 0.95, I² = 0%; Analysis 3.2) for the outcome of stillbirth.
1.2. Analysis.
Comparison 1 Labour induction versus expectant management (all trials), Outcome 2 Stillbirth.
2.2. Analysis.
Comparison 2 Labour induction versus expectant management (gestational age at induction), Outcome 2 Stillbirth.
3.2. Analysis.
Comparison 3 Labour induction versus expectant management (status of cervix), Outcome 2 Stillbirth.
Stillbirth sensitivity analysis: RR 0.34, 95% CI 0.05 to 2.12; five trials, 6698 infants. There was one stillbirth in the induction group and four stillbirths in the expectant management group. On sensitivity analysis, conventional statistical significance was lost, although the point estimate of a relative risk reduction of 66% was similar to the overall analysis (RR 0.30 95% CI 0.11 to 0.96).
Neonatal death
There were seven live birth deaths (all occurring before seven days of life). One of these was in the induction group and six were in the expectant management groups (RR 0.37, 95% CI 0.10 to 1.38; 19 trials; 9776 infants; Analysis 1.3).
1.3. Analysis.
Comparison 1 Labour induction versus expectant management (all trials), Outcome 3 Neonatal death.
Birth asphyxia
Rates of birth asphyxia were not clearly different between the induction and expectant management groups (RR 1.66, 95% CI 0.61 to 4.55: four trials; 1456 infants; Analysis 1.4).
1.4. Analysis.
Comparison 1 Labour induction versus expectant management (all trials), Outcome 4 Birth asphyxia.
Admission to neonatal intensive care unit (NICU)
Rates of NICU admissions were lower when labour induction was compared with expectant management (RR 0.88, 95% CI 0.77 to 1.01; 13 trials; 8531 infants; moderate‐quality evidence; Analysis 1.5). Interaction tests failed to demonstrate significant differences between the timing of induction subgroups (Chi² = 0.45, P = 0.80, I² = 0%; Analysis 2.3) or for subgroups according to state of cervix (Chi² = 0.86, P = 0.65, I² = 0%; Analysis 3.3) for the outcome of NICU admission.
1.5. Analysis.
Comparison 1 Labour induction versus expectant management (all trials), Outcome 5 Admission to neonatal intensive care unit.
2.3. Analysis.
Comparison 2 Labour induction versus expectant management (gestational age at induction), Outcome 3 Admission to neonatal intensive care unit.
3.3. Analysis.
Comparison 3 Labour induction versus expectant management (status of cervix), Outcome 3 Admission to neonatal intensive care unit.
Admission to the NICU sensitivity analysis: RR 0.87, 95% CI 0.77 to 1.02; seven trials, 6702 infants. On sensitivity analysis, results were very similar to the overall analysis (RR 0.88 95% CI 0.77 to 1.01).
Neonatal convulsions
There were no clear differences in instances of neonatal convulsions when labour induction was compared with expectant management (RR 0.54, 95% CI 0.15 to 1.97; three trials, 4365 infants; Analysis 1.6).
1.6. Analysis.
Comparison 1 Labour induction versus expectant management (all trials), Outcome 6 Neonatal convulsions.
Use of anticonvulsants
No clear differences between induction and expectant groups were evident for use of anticonvulsants in a single trial (RR 0.34, 95% CI 0.01 to 8.17; 349 infants; Analysis 1.7).
1.7. Analysis.
Comparison 1 Labour induction versus expectant management (all trials), Outcome 7 Use of anticonvulsants.
Meconium aspiration syndrome
There was a 23% relative reduction in the risk of meconium aspiration syndrome in the induction groups compared with the expectant management groups (RR 0.77 95% CI 0.62 to 0.96; 11 trials; 7781 infants; Analysis 1.8).
1.8. Analysis.
Comparison 1 Labour induction versus expectant management (all trials), Outcome 8 Meconium aspiration syndrome.
Apgar score less than seven at five minutes
Fewer babies had Apgar scores less than seven at five minutes in the induction groups compared with the expectant management groups (RR 0.70, 95% CI 0.50 to 0.98; 16 trials; 9047 infants; moderate‐quality evidence; Analysis 1.9).
1.9. Analysis.
Comparison 1 Labour induction versus expectant management (all trials), Outcome 9 Apgar score less than 7 at 5 minutes.
Birthweight (g)
On average, infants born to mothers in the induction group had lower birthweights than those born to mothers in the expectant management group (mean difference (MD) ‐69.43 g, 95% CI ‐96.83 to ‐42.02; 14 trials; 3799 infants; Analysis 1.10).
1.10. Analysis.
Comparison 1 Labour induction versus expectant management (all trials), Outcome 10 Birthweight (g).
Birthweight greater than 4000 g
There was a 28% relative reduction in the rate of macrosomia (greater than 4000 g) in the labour induction groups (average RR 0.72, 95% CI 0.54 to 0.96; eight trials; 5593 infants; Tau² = 0.09; Chi² = 20.84, P = 0.004; I² = 66%; Analysis 1.11). (Hannah 1992 used a cutoff‐of 4500 g rather than 4000 g for this outcome.)
1.11. Analysis.
Comparison 1 Labour induction versus expectant management (all trials), Outcome 11 Birthweight > 4000 g.
Neonatal trauma
On meta‐analysis of data from three trials no clear difference in rates of birth trauma in newborns was seen between labour induction and expectant management (RR 1.18, 95% CI 0.68 to 2.05; 4255 infants; low‐quality evidence; Analysis 1.12).
1.12. Analysis.
Comparison 1 Labour induction versus expectant management (all trials), Outcome 12 Neonatal trauma.
Unreported outcomes
No trials reported on neonatal encephalopathy, pneumonia, or neurodevelopment at childhood follow‐up (although Bergsjo 1989 reported no signs of neurological impairment in children at two years of age).
Secondary outcomes for the mother
Caesarean section
There were fewer caesarean sections (a relative reduction of 8%) in the induction groups compared with the expectant management groups on meta‐analysis of data from 27 trials (RR 0.92, 95% CI 0.85 to 0.99; 11,738 women; moderate‐quality evidence; Analysis 1.13). Subgroup interaction tests did not show clear differences according to timing of induction (Chi² = 4.10, P = 0.13, I² = 51.2%; Analysis 2.4) or by state of cervix (Chi² = 1.06, P = 0.59, I² = 0%; Analysis 3.4) for this outcome.
1.13. Analysis.
Comparison 1 Labour induction versus expectant management (all trials), Outcome 13 Caesarean section.
2.4. Analysis.
Comparison 2 Labour induction versus expectant management (gestational age at induction), Outcome 4 Caesarean section.
3.4. Analysis.
Comparison 3 Labour induction versus expectant management (status of cervix), Outcome 4 Caesarean section.
Caesarean section sensitivity analysis: RR 0.94, 95% CI 0.85 to 1.03; seven trials, 7080 women. On sensitivity analysis, results were very similar to the overall analysis (RR 0.92 95% CI 0.85 to 0.99), although conventional statistical significance was lost.
Operative vaginal birth (forceps or ventouse)
On meta‐analysis of data from the 18 trials that reported this outcome, the rate of operative vaginal birth was higher in the policy of labour induction groups compared with expectant management (RR 1.07, 95% CI 0.99 to 1.16; 9281 women; moderate‐quality evidence; Analysis 1.14). Subgroup interaction tests according to timing of induction indicated that the excess of operative vaginal births occurred in the induction at < 41 weeks' gestation group (Chi² = 7.87, P = 0.02, I² = 74.6%; Analysis 2.5). No clear differences were seen in the subgroup analyses by state of cervix (Chi² = 0.45, P = 0.80, I² = 0%; Analysis 3.5) for this outcome.
1.14. Analysis.
Comparison 1 Labour induction versus expectant management (all trials), Outcome 14 Operative vaginal birth (forceps or ventouse).
2.5. Analysis.
Comparison 2 Labour induction versus expectant management (gestational age at induction), Outcome 5 Operative vaginal birth (forceps or ventouse).
3.5. Analysis.
Comparison 3 Labour induction versus expectant management (status of cervix), Outcome 5 Operative vaginal birth (forceps or ventouse).
Operative vaginal birth sensitivity analysis: RR 1.03, 95% CI 0.94 to 1.12; five trials, 6570 women. On sensitivity analysis, results were very similar to the overall analysis (RR 1.07 95% CI 0.99 to 1.16).
Analgesia used
In nine trials with 3724 women, there was substantial variation in type of analgesia/anaesthesia used and so data were not pooled. In general, there were few differences seen in need for analgesia between the induction and expectant management groups (Analysis 1.15).
1.15. Analysis.
Comparison 1 Labour induction versus expectant management (all trials), Outcome 15 Analgesia used.
Perineal trauma
On meta‐analysis of data from four trials, no clear differences in perineal trauma were seen between induction and expectant management (RR 1.09, 95% 0.65 to 1.83; 3028 women; low‐quality evidence; Analysis 1.16). Interaction tests failed to detect any differences for subgroups by timing of induction (Chi² = 3.49, P = 0.17, I² = 42.7%; Analysis 2.6) or by state of cervix (tests for subgroup differences: not applicable; Analysis 3.6) for this outcome.
1.16. Analysis.
Comparison 1 Labour induction versus expectant management (all trials), Outcome 16 Perineal trauma.
2.6. Analysis.
Comparison 2 Labour induction versus expectant management (gestational age at induction), Outcome 6 Perineal trauma.
3.6. Analysis.
Comparison 3 Labour induction versus expectant management (status of cervix), Outcome 6 Perineal trauma.
Perineal trauma sensitivity analysis: RR 1.31, 95% CI 0.74 to 2.31; three trials, 2937 women. On sensitivity analysis, results were similar to the overall analysis (RR 1.09 95% CI 0.65 to 1.83).
Prolonged labour
The outcome of prolonged labour was reported in several different ways by three trials with 869 women, with none of the four comparisons showing clear differences between the induction and expectant management groups (Analysis 1.17).
1.17. Analysis.
Comparison 1 Labour induction versus expectant management (all trials), Outcome 17 Prolonged labour.
Postpartum haemorrhage
No clear difference in rates of postpartum haemorrhage was seen between induction and expectant management groups (RR 1.09 95% CI 0.92 to 1.30; five trials, 3315 women; low‐quality evidence; Analysis 1.18).
1.18. Analysis.
Comparison 1 Labour induction versus expectant management (all trials), Outcome 18 Postpartum haemorrhage.
Other measures of satisfaction with the approach
In one trial of 496 women, more women in the induction group said that they would choose the same arm in a future trial compared with women in the expectant management group (RR 1.93, 95% CI 1.62 to 2.30), but in another trial of 184 women, similar numbers of women indicated that they preferred the group they had been allocated to (RR 0.90, 95% CI 0.72 to 1.13; Analysis 1.19). Due to the high heterogeneity (I² = 96%) we did not pool the results of these two trials.
1.19. Analysis.
Comparison 1 Labour induction versus expectant management (all trials), Outcome 19 Maternal satisfaction.
Unreported outcomes
No trials reported on anxiety before birth, breastfeeding at discharge, or postnatal depression.
Secondary outcomes relating to health service use
Length of maternal hospital stay (days)
No clear overall differences between induction and expectant management were observed for duration of maternal hospital stay (average MD ‐0.34 days 95% CI ‐1.00 to 0.33; five trials; 1146 women; very low‐quality evidence; Analysis 1.20). There was, however, very substantial heterogeneity (Tau² = 0.49; Chi² = 77.02, P < 0.00001; I² = 95%) between the trials for this outcome.
1.20. Analysis.
Comparison 1 Labour induction versus expectant management (all trials), Outcome 20 Length of maternal hospital stay (days).
Length of neonatal hospital stay (days)
In one trial of 302 babies, there was a slightly shorter mean hospital stay for the induction group compared with the expectant management group (MD ‐0.30 day, 95% CI ‐0.61 to 0.01; Analysis 1.21).
1.21. Analysis.
Comparison 1 Labour induction versus expectant management (all trials), Outcome 21 Length of neonatal hospital stay (days).
Length of labour (hours)
Overall, length of labour was slightly shorter for women undergoing induction compared with expectant management (average MD ‐1.01 hours, 95% CI ‐1.72 to ‐0.31; nine trials; 1980 women; Tau² = 0.97; Chi² = 34.04, P < 0.0002; I² = 71%; Analysis 1.22).
1.22. Analysis.
Comparison 1 Labour induction versus expectant management (all trials), Outcome 22 Length of labour (hours).
Funnel plots
We assessed funnel plots for the outcomes: perinatal death (Figure 4), stillbirth (Figure 5), neonatal death (Figure 6), admission to NICU (Figure 7), meconium aspiration syndrome (Figure 8), Apgar score less than seven at five minutes (Figure 9), birthweight (Figure 10), caesarean section (Figure 11), operative vaginal birth (Figure 12). Typical visual asymmetry was not evident in any of the forest plots although perinatal death (Figure 4), stillbirth (Figure 5), neonatal death (Figure 6) and birthweight (Figure 10) showed patterns of asymmetry that were difficult to interpret.
4.

Funnel plot of comparison: 1 Labour induction versus expectant management (all trials), outcome: 1.1 Perinatal death.
5.
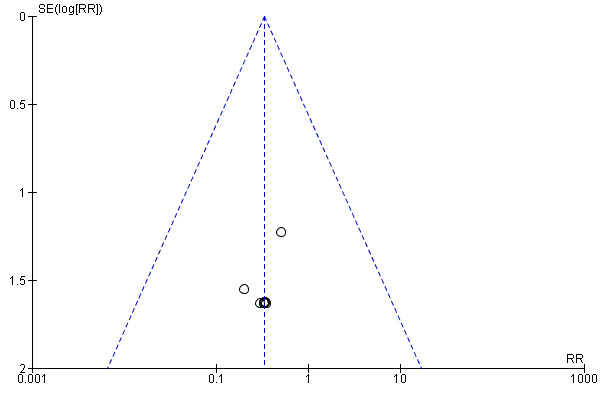
Funnel plot of comparison: 1 Labour induction versus expectant management (all trials), outcome: 1.2 Stillbirth.
6.

Funnel plot of comparison: 1 Labour induction versus expectant management (all trials), outcome: 1.3 Neonatal death.
7.

Funnel plot of comparison: 1 Labour induction versus expectant management (all trials), outcome: 1.5 Admission to neonatal intensive care unit.
8.

Funnel plot of comparison: 1 Labour induction versus expectant management (all trials), outcome: 1.8 Meconium aspiration syndrome.
9.

Funnel plot of comparison: 1 Labour induction versus expectant management (all trials), outcome: 1.9 Apgar score less than 7 at 5 minutes.
10.
Funnel plot of comparison: 1 Labour induction versus expectant management (all trials), outcome: 1.10 Birthweight (g).
11.

Funnel plot of comparison: 1 Labour induction versus expectant management (all trials), outcome: 1.13 Caesarean section.
12.
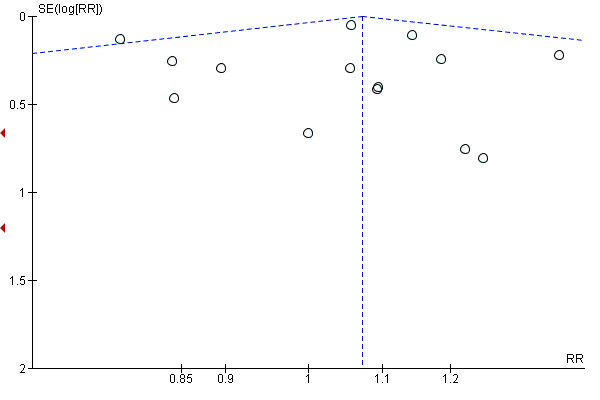
Funnel plot of comparison: 1 Labour induction versus expectant management (all trials), outcome: 1.14 Operative vaginal birth (forceps or ventouse).
Discussion
Summary of main results
In this updated review, we included 30 randomised controlled trials (reporting on 12,479 women).
We included pregnant women at or beyond term. Since a risk factor at this stage of pregnancy would normally require an intervention, only trials including women at low risk for complications were eligible. We accepted the trialists' definition of 'low risk'. The trials of induction of labour in women with prelabour rupture of membranes at or beyond term were not considered in this review. A policy of labour induction was associated with fewer perinatal deaths (with two perinatal deaths in the labour induction policy group compared with 16 perinatal deaths in the expectant management group) (moderate‐quality evidence). When restricted to a policy of induction at later gestational ages (> 41 weeks), there were two and 13 perinatal deaths, respectively. Although some trials excluded deaths from congenital anomalies, other trials did not exclude these deaths. When the three deaths reported to be due to congenital anomalies were excluded, the overall findings remained very similar. There were also fewer stillbirths in the induction group (one versus 10 in the expectant management group (moderate‐quality evidence)).
We found that there were fewer caesarean sections with a policy of induction compared with expectant management (moderate‐quality evidence). There was also a concomitant marginal increase in the rate of operative vaginal births in the induction group (moderate‐quality evidence). Rates of neonatal intensive care unit (NICU) admission were lower (moderate‐quality evidence) and fewer babies had Apgar scores less than seven at five minutes (moderate‐quality evidence) in the induction groups compared with expectant management. Other important outcomes did not show clear differences between induction and expectant management. These included neonatal trauma, perineal trauma and postpartum haemorrhage (low‐quality evidence), and length of maternal hospital stay (very low‐quality evidence). Neonatal encephalopathy, neurodevelopment at childhood follow‐up, breastfeeding and postnatal depression were not reported by any of the included trials.
Subgroup interaction tests according to timing of induction indicated more operative vaginal births occurred with earlier induction (< 41 weeks' gestation) compared with later induction (≥ 41 weeks' gestation). No clear differences between timing of induction and cervical status (favourable; unfavourable; mixed) subgroups were apparent for the other main outcomes of the review.
For the sensitivity analyses, all six prespecified outcomes demonstrated results in the same direction as the main analyses and there was little material difference in the overall results, although some outcomes lost conventional statistical significance when analyses were restricted to the combined smaller sample sizes of the higher‐quality trials.
This review evaluates trials where a policy of induction has been compared with a policy of waiting. However, women scheduled to be induced may not have ending up being induced; and women allocated to wait may have ended up being induced. For example, about one‐third of the women randomised to the induction policy group in the Hannah trial were not induced; and about one‐third of the women randomised to waiting or expectant management were induced (Hannah 1992; Keirse 2010).
Overall completeness and applicability of evidence
The body of evidence for this review is now quite extensive, including 30 trials and over 12,000 women. This has been sufficient to detect a difference in perinatal death between the induction and expectant management groups. The decrease seen in caesarean section with a policy of induction has been questioned by some authors (Keirse 2010; Mandruzzato 2010). They have pointed out that the women in the large Hannah 1992 trial who were induced in the policy of induction group (66% of this group), may have had a more effective cervical ripening regimen (prostaglandin) than the women who were induced in the expectant management group (33% of this group), and that more women in the expectant management group had a caesarean section for fetal distress (8.3% versus 5.7% in the induction group) (Keirse 2010;Mandruzzato 2010). Since review results for caesarean section were similar when the Hannah 1992 trial was omitted, this is not likely to have been a major issue.
Compared with expectant management, induction of labour at 41 weeks in nulliparous women has been shown to be cost‐effective; ranging from US$2932 to $21,612 per quality‐adjusted life years (QALY) gained (Kaimal 2011). Walker 2017 has reported that induction of labour at 39 weeks for nulliparous women aged 35 years and over was associated with a mean cost saving of £263 and a small additional gain in QALYs, without considering QALY gains from stillbirth prevention. Probabilistic sensitivity analyses have shown that induction of labour in nulliparous women at 41 weeks would be a cost‐effective intervention 96% of the time, if society was willing to bear the cost of $50,000 per QALY (Kaimal 2011). In the Hannah 1992 trial, the mean cost of a woman undergoing induction for a post‐term pregnancy was $193 lower than for a woman managed through monitoring, due mainly to the costs of additional monitoring and the significantly higher rates of caesarean section among these women.
As noted above, randomised trials completed to date have provided very little information about longer‐term outcomes for children and cohort studies have shown inconsistent results as to whether post‐term birth has a negative, positive or null impact on childhood development. A large cohort study from Denmark has suggested that more children born at 41 weeks' gestation or more achieved developmental milestones compared with children born at earlier term gestations (39 to 40 weeks) (Olesen 2015).
The trials included in this review employed a wide range of methods and combinations of induction techniques (see Characteristics of included studies), and so it was not possible to assess differences in outcomes by method of induction through conducting subgroup analyses.
There is mixed evidence that induction at term in first pregnancies increases the risk of caesarean section (Davey 2016; Mishanina 2014). While we could not elucidate this further in our review, an individual participant data meta‐analysis by parity may be able to answer this question.
Women with a post‐term pregnancy have described this period of unexpected waiting as being in a state of limbo, with increasingly negative feelings as the pregnancy continues, which could be addressed with more information and support from their healthcare professionals (Wessberg 2017).
Quality of the evidence
Included trials were generally at moderate risk of bias.
Most of the important outcomes assessed using GRADE had a rating of moderate‐ or low‐quality evidence ‐ with downgrading decisions generally due to study limitations such as lack of blinding (a condition inherent in comparisons between a policy of acting and of waiting), or imprecise effect estimates. However, for the majority of outcomes assessed using GRADE, statistical heterogeneity was mostly low, countering claims (e.g. Davey 2016), that some of the trials had unreliable results due to being outdated or flawed. Only one outcome (length of maternal stay) was downgraded further to very low‐quality evidence due to inconsistency.
Potential biases in the review process
Due to the rigorous methods used (comprehensive searching, double screening and data extraction, and careful appraisal and analysis), biases are likely to be low.
As mentioned above, there have been several criticisms of trials and reviews on this topic. Wood 2014 and colleagues point out that the decision to perform a caesarean section is often subjective and anxiety from medical staff about the dangers of a prolonged pregnancy may be a factor in determining when to carry out a caesarean, as could factors such as fetal distress or fetal size. They also make the observation whatever the reason(s) may be, this does not change that the fact that induction has shown reduced risk of caesarean section in clinical trials of induction of labour versus expectant management in women with intact membranes.
Agreements and disagreements with other studies or reviews
Both observational studies and systematic reviews of randomised controlled trials of timing of induction have shown mixed findings for outcomes such as perinatal death and caesarean section rates (Davey 2016).
Wood 2014 is the most comparable systematic review in terms of included studies. However, the authors only report the outcome of caesarean section, finding a similar reduction with a policy of induction as found in our review. The Mishanina 2014 systematic review of induction versus expectant management at any gestational age also found a reduction in caesarean birth for inductions from 37 weeks onwards (but not if induction was at less than 37 weeks); and an overall reduction in fetal death with a policy of induction.
Many of the current relevant guidelines recommend offering women induction of labour after 41 completed weeks of gestation (ACOG 2014; NICE 2008; SOGC 2008; WHO 2011). A postpartum survey of women who participated in the Heimstad 2007a trial indicated that most women would choose induction at 41 to 42 weeks in a subsequent pregnancy (Heimstad 2007b).
Authors' conclusions
Implications for practice.
The message from this review is that a policy of induction of labour at or beyond term is associated with fewer perinatal deaths, specifically stillbirths, (although the absolute risk is small). There is also a reduced risk of caesarean section with a possible increase in operative vaginal birth. Healthcare professionals may consider offering women the option of labour induction, probably at 41 to 42 completed weeks, with information about the absolute and relative risks of perinatal death at different gestational age time points and for different groups such as nulliparous or obese women, recognising that their assessments, values and preferences may differ. If a woman chooses to wait for spontaneous labour onset, it may be prudent to have regular fetal monitoring as longitudinal epidemiological studies suggest increased risk of perinatal death by increasing gestational age.
Implications for research.
The optimal timing of offering induction of labour to women at or beyond term warrants further investigation, as does further exploration of risk profiles of women and their values and preferences. Individual participant meta‐analysis is likely to help elucidate the role of factors, such as parity, in influencing outcomes of induction compared with expectant management.
Feedback
Marowitz, 14 April 2011
Summary
Both my students and myself are unable to understand the following sentence in text for ‘Effects of the intervention':
"Women induced at 37 to 40 completed weeks were more likely to have a caesarean section with expectant management than those in the labour induction group (RR 0.58; 95% CI 0.34 to 0.99)."
Are there errors in the wording of this sentence?
[Comment submitted by Amy Marowitz, April 2011]
Reply
Thank you for your feedback. We have corrected the error.
Contributors
A Metin Gülmezoglu
What's new
| Date | Event | Description |
|---|---|---|
| 28 December 2017 | New search has been performed | Search updated and eight additional trials included (Brane 2014; Cohn 1992; Kortekaas 2014; Martin 1978; Miller 2015; Sande 1983; Tylleskar 1979; Walker 2016). We have updated the methods in line with the standard methods used by Cochrane Pregnancy and Childbirth and we now use GRADE to assess the quality of the body of evidence. For this update, the overall conclusions have not changed. However, there is moderate certainty evidence to suggest that induction was associated with fewer stillbirths and fewer babies with low Apgar scores. |
| 9 October 2017 | New citation required but conclusions have not changed | Conclusions not changed. |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 31 March 2012 | New search has been performed | Search updated ‐ no new trials identified. Trial reports that were previously awaiting classification have now been incorporated into the review. We have added three new included trials (Heimstad 2007a; Nielsen 2005; Sahraoui 2005), three new excluded trials (Hernandez‐Castro 2008; Imsuwan 1999; Nicholson 2008) and one ongoing trial (Rijnders 2007). This updated review is now comprised of 22 included studies (reporting on 9383 women); 64 excluded studies and one ongoing study. Results are now presented as 37‐39 weeks; 39‐40 weeks; < 41 weeks, 41 weeks and > 41 weeks. A new author joined the team to help prepare this update. |
| 31 March 2012 | New citation required and conclusions have changed | Whilst the overall conclusions have not changed, there is now evidence to show that induction of labour at or beyond term is associated with a lower rate of caesarean section. |
| 6 July 2011 | Feedback has been incorporated | Feedback from Amy Marowitz added. |
| 6 July 2011 | Amended | Error corrected in response to feedback from Amy Marowitz (Feedback). |
| 14 July 2009 | Amended | Search updated. Eight reports of five trials added to Studies awaiting classification (Heimstad 2007a; Hernandez‐Castro 2008a; Imsuwan 1999a; Nicholson 2008a; Rijnders 2007a). |
| 3 September 2008 | Amended | Converted to new review format. |
| 28 February 2007 | Amended | The Implications for research section has been amended to include the uncertainty about timing of labour induction beyond term, which was unintentionally left out during the revision process. |
| 21 August 2006 | New citation required but conclusions have not changed | This version has been re‐written, including a new protocol which now limits the scope to labour induction |
| 30 June 2006 | New search has been performed | The previous version of this review included studies up to 1997 and included 21 labour induction trials (Gülmezoglu 2006). This version has been re‐written, including a new protocol which now limits the scope to labour induction, and includes 19 trials. Thirteen of the 21 trials included in the previous version are included in this version. The remaining eight trials were excluded because of alternate allocation (Cardozo 1986; Heden 1991; Katz 1983), a high proportion of postrandomization exclusion (greater than 30% in Martin 1978a and greater than 24% in Tylleskar 1979a), cervical ripening with breast stimulation (Elliott 1984; Kadar 1990), and analysis by intervention received (i.e. groups switched, Sande 1983a). Six trials published since the publication of the previous version have been included in this update (Chakravarti 2000; Chanrachkul 2003; Gelisen 2005; James 2001; Ocon 1997; Roach 1997). |
Acknowledgements
We acknowledge the support from Cochrane Pregnancy and Childbirth editorial team in Liverpool and the Australian and New Zealand Satellite of Cochrane Pregnancy and Childbirth (funded by NHMRC).
We thank Therese Dowswell from Cochrane Pregnancy and Childbirth who provided support for this update. Therese Dowswell assisted with data extraction, and the production of 'Summary of findings' tables.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane programme grant funding, and Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
We would like to acknowledge the contribution of Machiko Suganuma who assisted with data extraction for this update.
We would like to acknowledge the contribution of A Metin Gulmezoglu who initiated, led and assisted in the review preparation until 2016 and Emer Van Ryswyk for her contribution to the previous version of this review (Gülmezoglu 2012).
As part of the pre‐publication editorial process, this review has been commented on by four peers (an editor and three referees who are external to the editorial team), a member of Cochrane Pregnancy and Childbirth's international panel of consumers and the Group's Statistical Adviser.
Appendices
Appendix 1. Search methods used for ICTRP and ClinicalTrials.gov
ICTRP
Each line was run separately
induction AND expectant
induction AND wait(ing)
post‐term
postterm
postdate(s)
post‐date(s)
term AND pregnancy and expectant
ClinicalTrials.gov
Advanced search ‐ Intervention studies
pregnancy, prolonged AND (expectant OR wait OR waiting OR monitor)
post‐term pregnancy AND (expectant OR wait OR waiting OR monitor)
expectant AND labor
postterm OR post‐term
postdates OR post‐dates
Data and analyses
Comparison 1. Labour induction versus expectant management (all trials).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perinatal death | 20 | 9960 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.14, 0.78] |
| 2 Stillbirth | 20 | 9960 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.11, 0.96] |
| 3 Neonatal death | 19 | 9776 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.10, 1.38] |
| 4 Birth asphyxia | 4 | 1456 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.61, 4.55] |
| 5 Admission to neonatal intensive care unit | 13 | 8531 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.77, 1.01] |
| 6 Neonatal convulsions | 3 | 4365 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.15, 1.97] |
| 7 Use of anticonvulsants | 1 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.17] |
| 8 Meconium aspiration syndrome | 11 | 7781 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.62, 0.96] |
| 9 Apgar score less than 7 at 5 minutes | 16 | 9047 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.50, 0.98] |
| 10 Birthweight (g) | 14 | 3799 | Mean Difference (IV, Fixed, 95% CI) | ‐69.43 [‐96.83, ‐42.02] |
| 11 Birthweight > 4000 g | 8 | 5593 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.54, 0.96] |
| 12 Neonatal trauma | 3 | 4255 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.68, 2.05] |
| 13 Caesarean section | 27 | 11738 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.85, 0.99] |
| 14 Operative vaginal birth (forceps or ventouse) | 18 | 9281 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.99, 1.16] |
| 15 Analgesia used | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16 Perineal trauma | 4 | 3028 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.65, 1.83] |
| 17 Prolonged labour | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 First stage | 1 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.20, 1.45] |
| 17.2 Second stage | 1 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.36, 1.22] |
| 17.3 Third stage | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.02 [0.12, 73.52] |
| 17.4 No definition | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.30] |
| 18 Postpartum haemorrhage | 5 | 3315 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.92, 1.30] |
| 19 Maternal satisfaction | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 19.1 Hoping to be randomised to the same trial arm as they had been in this study | 1 | 496 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.62, 2.30] |
| 19.2 Preferred their allocation | 1 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.72, 1.13] |
| 20 Length of maternal hospital stay (days) | 5 | 1146 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐1.00, 0.33] |
| 21 Length of neonatal hospital stay (days) | 1 | 302 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐0.61, 0.01] |
| 22 Length of labour (hours) | 9 | 1980 | Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.72, ‐0.31] |
Comparison 2. Labour induction versus expectant management (gestational age at induction).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perinatal death | 20 | 9960 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.14, 0.78] |
| 1.1 < 41 weeks | 5 | 1552 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.05, 2.06] |
| 1.2 ≥ 41 weeks | 15 | 8408 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.13, 0.87] |
| 2 Stillbirth | 20 | 9960 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.11, 0.96] |
| 2.1 < 41 weeks | 5 | 1552 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.05, 2.06] |
| 2.2 ≥ 41 weeks | 15 | 8408 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.09, 1.24] |
| 3 Admission to neonatal intensive care unit | 13 | 8531 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.77, 1.01] |
| 3.1 < 41 weeks | 3 | 1005 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.41, 2.05] |
| 3.2 ≥ 41 weeks | 9 | 7397 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.76, 1.01] |
| 3.3 37 ‐ 42 weeks | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.18, 21.18] |
| 4 Caesarean section | 27 | 11738 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.85, 0.99] |
| 4.1 < 41 weeks | 9 | 2806 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.87, 1.24] |
| 4.2 ≥ 41 weeks | 17 | 8803 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.83, 0.98] |
| 4.3 37 ‐ 42 weeks | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.36, 1.06] |
| 5 Operative vaginal birth (forceps or ventouse) | 18 | 9281 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.99, 1.16] |
| 5.1 < 41 weeks | 7 | 2401 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.08, 1.48] |
| 5.2 ≥ 41 weeks | 10 | 6751 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.91, 1.10] |
| 5.3 37 ‐ 42 weeks | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.79, 4.93] |
| 6 Perineal trauma | 4 | 3028 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.65, 1.83] |
| 6.1 < 41 weeks | 1 | 618 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.58 [0.50, 13.21] |
| 6.2 ≥ 41 weeks | 2 | 2319 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.63, 2.15] |
| 6.3 37 ‐ 42 weeks | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.06, 1.53] |
Comparison 3. Labour induction versus expectant management (status of cervix).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perinatal death | 20 | 9960 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.14, 0.78] |
| 1.1 Favourable cervix | 3 | 771 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 7.27] |
| 1.2 Unfavourable cervix | 7 | 4938 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.07, 1.17] |
| 1.3 Unknown/mixed state of cervix | 10 | 4251 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.12, 1.15] |
| 2 Stillbirth | 20 | 9960 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.11, 0.96] |
| 2.1 Favourable cervix | 3 | 771 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 7.27] |
| 2.2 Unfavourable cervix | 7 | 4938 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.05, 1.66] |
| 2.3 Unknown/mixed state of cervix | 10 | 4251 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.09, 1.68] |
| 3 Admission to neonatal intensive care unit | 13 | 8531 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.77, 1.01] |
| 3.1 Favourable cervix | 2 | 475 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.02 [0.12, 73.52] |
| 3.2 Unfavourable cervix | 5 | 4380 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.77, 1.05] |
| 3.3 Unknown/mixed state of cervix | 6 | 3676 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.59, 1.12] |
| 4 Caesarean section | 27 | 11738 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.85, 0.99] |
| 4.1 Favourable cervix | 4 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.76, 1.64] |
| 4.2 Unfavourable cervix | 9 | 5212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.82, 1.00] |
| 4.3 Unknown/mixed state of cervix | 14 | 5609 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.81, 1.04] |
| 5 Operative vaginal birth (forceps or ventouse) | 18 | 9281 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.99, 1.16] |
| 5.1 Favourable cervix | 3 | 655 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.42, 1.82] |
| 5.2 Unfavourable cervix | 4 | 3650 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.95, 1.18] |
| 5.3 Unknown/mixed state of cervix | 11 | 4976 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.97, 1.24] |
| 6 Perineal trauma | 4 | 3028 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.65, 1.83] |
| 6.1 Unknown/mixed state of cervix | 4 | 3028 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.65, 1.83] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Augensen 1987.
| Methods | RCT | |
| Participants | Number of women randomised: 409
Setting: Bergen, Norway Inclusion criteria
Exclusion criteria
State of cervix: mixed (about 35% in each group had unripe cervix) |
|
| Interventions |
Induction group (n = 214): immediate induction with oxytocin (5 IU increased in a stepwise manner). GA at intervention 41+ weeks (290‐297 days) versus Expectant management group (n = 195): NST every 3‐4 days, IOL after 7 days |
|
| Outcomes |
Mother: caesarean section; assisted vaginal birth; length of labour; length of hospital stay Baby: perinatal death; birthweight; neonatal jaundice; meconium‐stained amniotic fluid; NICU admission |
|
| Notes |
Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | List of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was unclear given that it was not undertaken by a staff member or team clearly uninvolved in the trial. It was reported that the midwife undertook allocation using a random number list, and this list was inaccessible to the participating physicians. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/214 in the IOL group went into labour before IOL but data for these women have been included in the IOL group for analyses. No apparent losses to follow‐up or exclusions. |
| Selective reporting (reporting bias) | High risk | No outcomes were pre‐specified in the methods; some outcomes reported incompletely in text, e.g. "There was no significant difference between the groups in the use of analgesia, sedatives, and epidural anaesthesia." |
| Other bias | Low risk | Appears to be free of other bias |
Bergsjo 1989.
| Methods | RCT | |
| Participants | Number of women randomised: 188 Setting: Wuhan, Hubei province, China Inclusion criteria
Exclusion criteria
State of cervix: not mentioned |
|
| Interventions |
Induction group (n = 94): stripping of membranes followed by oxytocin infusion and AROM if cervix sufficiently dilated. GA for intervention: 42 completed weeks (294 days) versus Expectant management group (n = 94): no intervention for 1 week, IOL at 43 weeks. |
|
| Outcomes |
Mother:operative vaginal birth; duration of labour; caesarean section; breastfeeding (timing of recording of this outcome in relation to birth or discharge time was not specified) Baby: perinatal death; meconium aspiration syndrome |
|
| Notes |
Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | List of random numbers |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Appears that blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8/94 in IOL group went into labour before IOL but were kept in the allocated group. No apparent losses to follow‐up or exclusions. |
| Selective reporting (reporting bias) | High risk | Most pre‐specified outcomes were reported; however, limited information was provided for some outcomes (e.g. combined maternal complications) and neonatal outcomes, e.g. "Maternal complications, including protracted labor, cervical edema, cervical laceration, post‐partum hemorrhage and unspecified post‐partum morbidity accounted to about 15% in both groups, with no significant differences;" and "About 90 mothers in each group were breastfeeding." |
| Other bias | Low risk | Appears to be free of other bias. |
Brane 2014.
| Methods | RCT | |
| Participants | Number of women randomised: 138
Setting: Stockholm, Sweden Inclusion criteria
Exclusion criteria
State of cervix: Bishop score at presentation ranged from 1‐8 in induction group and 2‐9 in expectant management group. |
|
| Interventions |
Induction group (n = 71): 5 hours after medication to promote 'therapeutic rest' IOL performed, with method dependent on state of cervix: intravaginal PGE2 or transcervical catheter +/‐ AROM if cervical dilation permitted; followed by IV oxytocin (augmented every 20‐30 min) if no progress after AROM. versus Expectant management group (n = 67): spontaneous labour awaited as long as possible; IOL if women wanted, or if the obstetrician/midwife considered suitable. |
|
| Outcomes |
Mother: mode of birth; experience of birth; duration of labour; labour analgesia; oxytocin for augmentation; birth presentation; postpartum haemorrhage; sphincter tears. Baby: Apgar score < 7 at 5 min; cord artery metabolic acidosis; birthweight; head circumference; admission to NICU. |
|
| Notes | Women in both groups given medication to promote 'therapeutic rest' (1 g paracetamol, 10 mg zolpidem, 10 mg morphine). During active phase of labour (cervical dilation ≥ 4 cm or ROM) women monitored according to local protocol; slow progress (arrest of dilation for 203 hours) was treated with AROM or oxytocin. Funding: Karolinska Institute Foundations and Funds Declaration of interests: the authors report no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The randomization was performed in blocks of 5–10 in each group." Method of sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | "A sealed envelope containing coded protocols for the respective groups… was opened by the midwife." No further detail provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Some attrition and missing data particularly for the women’s views questionnaire. Most of the sample were included in the analyses for the primary outcome (65/71, and 64/67 in main analysis for mode of birth). The reasons for missing data for some clinical outcomes were not reported. |
| Selective reporting (reporting bias) | Unclear risk | No access to trial protocol to confidently assess selective reporting. Perinatal death not reported. |
| Other bias | Low risk | Appears to be free of other bias. |
Breart 1982.
| Methods | RCT (1:2 randomisation) | |
| Participants | Number of women randomised: 716
Setting: Paris, France Inclusion criteria: GA: 37‐39 weeks Exclusion criteria: high risk, contraindication for IOL State of cervix: not mentioned |
|
| Interventions |
Induction group (n = 235): oxytocin and AROM at GA 37‐39 weeks. versus Expectant management group (n = 481): FHR checking and amnioscopy every 2‐3 days. |
|
| Outcomes | Mother: duration of labour; mode of birth Baby: morbidity (Apgar scores, resuscitation) | |
| Notes |
Funding: not reported Declaration of interests: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | It was reported that a closed envelope system was used for allocation concealment, although no further detail was available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 202/235 in the induction group and 173/481 in the expectant group followed the trial protocol; trial results were reported for all 716 women and their babies. |
| Selective reporting (reporting bias) | Unclear risk | No access to trial protocol to confidently assess selective reporting. Perinatal death was not reported. |
| Other bias | Low risk | Appears to be free of other bias. |
Chakravarti 2000.
| Methods | RCT | |
| Participants | Number of women randomised: 231
Setting: Calcutta, India Inclusion criteria
State of cervix: not mentioned |
|
| Interventions |
Induction group (n = 117): IOL, no details of the method are available.
versus Expectant management group (n = 114 randomised): daily fetal movement counts, biophysical profile and ultrasound; IOL after 1 week. |
|
| Outcomes | Only caesarean section rates were adequately reported in the abstract. | |
| Notes | Reported as conference abstract. Only data for caesarean included in meta‐analysis. Funding: not reported Declaration of interests: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Appears that blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to determine. |
| Selective reporting (reporting bias) | Unclear risk | No outcomes were pre‐specified in the methods (conference abstract). Insufficient information to determine. |
| Other bias | Unclear risk | Unable to identify other bias based on the abstract. |
Chanrachkul 2003.
| Methods | RCT | |
| Participants | Number of women randomised: 249
Setting: Bangkok, Thailand Inclusion criteria
Exclusion criteria
State of cervix: favourable (Bishop score 6 or more) |
|
| Interventions |
Induction group (n = 124): AROM + oxytocin (if uterine contractions inadequate after 2 hours); versus Expectant management group (n = 125): spontaneous labour awaited unless 1) non‐reactive NST or 2) amniotic fluid index < 5 cm or 3) medical or obstetric indication for birth or 4) reaching 44 completed weeks. |
|
| Outcomes | Mother: prolonged labour; modes of birth and their indications; death; postpartum haemorrhage. Baby: perinatal death, birthweight; birth asphyxia, NICU admission, birthweight > 4000 g; Apgar < 7 at 5 mins. | |
| Notes |
Funding: Ramathibodi Hospital Research Grant 2/2542 Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out using computer‐generated numbers. |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 woman (in IOL group) excluded after randomisation because of misclassification (breech presentation). No apparent losses to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | While pre‐specified outcomes (in the methods) were reported, no access to trial protocol to further assess selective reporting. |
| Other bias | Low risk | Appears to be free of other bias. |
Cohn 1992.
| Methods | RCT | |
| Participants | Number of women randomised: 248
Setting: Hong Kong, China Inclusion criteria
Exclusion criteria
State of cervix: not reported |
|
| Interventions |
Induction group (n = not reported): induction versus Expectant management group (n = not reported): women were seen twice weekly. |
|
| Outcomes |
Mother: length of stay in hospital; analgesia; caesarean birth; meconium‐stained liquor Baby: neonatal admission for meconium aspiration; birthweight; Apgar scores; umbilical cord vein pH |
|
| Notes | Abstract only. No data included in meta‐analyses Funding: not reported Declaration of interests: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | " A prospective randomised study". The method of sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on losses to follow‐up or exclusions. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only. |
| Other bias | Unclear risk | Abstract only. Baseline characteristics were not described. |
Cole 1975.
| Methods | Randomly allocated, no further details available. | |
| Participants | Number of women randomised: 228
Setting: Glasgow, Scotland Inclusion criteria
State of cervix: not reported |
|
| Interventions |
Induction group (n = 111): IOL with AROM + oxytocin
versus Expectant management group (n = 117): no intervention until 41 weeks, thereafter IOL. |
|
| Outcomes |
Mother: length of labour; mode of birth (including operative versus non operative); analgesia requirements; postpartum blood loss Baby: perinatal deaths; meconium staining; Apgar scores; birthweight; neonatal jaundice |
|
| Notes |
Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7/118 and 2/119 in the intervention and control groups excluded after randomisation because of misclassification as low risk. |
| Selective reporting (reporting bias) | Unclear risk | No outcomes were pre‐specified in the methods; no access to trial protocol to further assess selective reporting. |
| Other bias | Low risk | Appears to be free of other bias. |
Dyson 1987.
| Methods | RCT | |
| Participants | Number of women randomised: 302
Setting: Kaiser Permanente Medical Care Hospital in California, USA Inclusion criteria
Exclusion criteria
State of cervix: unfavourable (Bishop score < 6) |
|
| Interventions |
Induction group (n = 152): PE2 gel (initially 3 mg but later reduced to 0.5 mg). If no labour in 24 hours, repeat PE2 and oxytocin if needed versus Expectant management group (n = 150): NST twice weekly, pelvic examination and amniotic fluid determination weekly between 41‐42 weeks and twice weekly afterwards. |
|
| Outcomes |
Mother: length of hospital stay; caesarean section; length of labour Baby: perinatal death; 1 min Apgar score < 7; 5 min Apgar score < 7; meconium‐stained amniotic fluid; meconium aspiration syndrome; post‐maturity syndrome; fetal distress; birthweight; birthweight > 4000 g; infant hospital stay length |
|
| Notes |
Funding: Community Service Program of Kaiser Foundation Hospitals Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A table of random numbers was used. |
| Allocation concealment (selection bias) | Unclear risk | The authors reported " using a series of consecutively numbered, sealed envelopes..." for allocation concealment, but no mention was made of envelope opaqueness. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses to follow‐up or exclusions. |
| Selective reporting (reporting bias) | Unclear risk | No outcomes were pre‐specified in the methods; no access to trial protocol to further assess selective reporting. |
| Other bias | Low risk | Appears to be free of other bias. |
Egarter 1989.
| Methods | RCT | |
| Participants | 345 women randomised
Setting: Vienna, Austria Inclusion criteria
Exclusion criteria
State of cervix: favourable (Modified Bishop score > 4) |
|
| Interventions |
Induction group (n = 180): vaginal PE2 (3 mg) tablets repeated 6 and 24 hours later if no active labour versus Expectant management group (n = 165): spontaneous labour awaited until 42 weeks. NST monitoring every 2‐3 days. |
|
| Outcomes |
Mother: birth interval (onset of contractions to birth in hours); rate and indication for operative birth; length of labour; analgesia requirements; caesarean section Baby: birthweight; length of baby at birth; incidence of meconium‐stained amniotic fluid; Apgar scores; results of umbilical cord pH determination; perinatal death |
|
| Notes |
Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 8/180 women in the induction group refused to be induced; and 3/165 women in the expectant group requested induction; and these 11 women were excluded from analysis post‐randomisation. |
| Selective reporting (reporting bias) | High risk | No outcomes were pre‐specified in the methods, limited information was provided for some outcomes, e.g. "The incidence of prolonged labor was not different in both groups... both groups required analgetic treatment in 35%... Birthweight and length, the incidence of meconium‐stained amniotic fluid, of low Apgar scores, and the results of pH determination were not different between the two groups." |
| Other bias | Unclear risk | Some imbalance in the numbers randomised to each group (180 versus 165). |
Gelisen 2005.
| Methods | RCT | |
| Participants | Number of women randomised: 600
Setting: teaching hospital in Ankara, Turkey; recruitment dates not reported Inclusion criteria
Exclusion criteria
State of cervix: unfavourable ‐ Bishop score < 5 Just under half the women were nulliparous. |
|
| Interventions |
Induction group: labour induction (3 methods*) (1) vaginal administration of 50 mg misoprostol (n = 100) (2) oxytocin induction (n = 100), and (3) transcervical insertion of a Foley balloon (n = 100) versus Expectant management group: spontaneous follow‐up with twice‐weekly nonstress testing and amniotic fluid measurement and once‐weekly biophysical scoring (n = 300); 24% of women were induced after 42 completed weeks. *the 3 induction arms were combined for analyses |
|
| Outcomes | Mother: oligohydramnios; pre‐eclampsia; tachysystole; hyperstimulation; vaginal birth; caesarean (emergent abdominal birth for worrying FHR); failed IOL Baby: perinatal death; shoulder dystocia; meconium stained amniotic fluid; meconium aspiration syndrome; fetal anomaly; low Apgar scores (< 7 at 5 mins); umbilical artery pH < 7.16; NICU admission; fetal macrosomia; birthweight; birthweight > 4000 g; length of hospital stay | |
| Notes |
Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was by sealed, opaque envelopes but there is no mention of numbering and sequential opening of the envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding: "Staff members in charge of labor were not blinded to the type of medication used for induction". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses to follow‐up or exclusions. |
| Selective reporting (reporting bias) | Unclear risk | While pre‐specified outcomes (in the methods) were reported, no access to trial protocol to further assess selective reporting. |
| Other bias | Low risk | Appears to be free of other bias. |
Hannah 1992.
| Methods | RCT | |
| Participants | Number of women randomised: 3418 enrolled (data available for 3407 women only)
Setting: 22 hospitals across Canada Inclusion criteria
Exclusion criteria
State of cervix: unfavourable at trial entry (first ripening and then IOL in the intervention group) |
|
| Interventions |
Induction group (n = 1701): up to 3 x 0.5 mg doses of PGE2 gel administered intracervically (if NST was normal and cervix unfavourable at time of induction = 77% of women), followed by either AROM or IV oxytocin infusion, or both. versus Expectant management group (n = 1706): daily fetal movement counting, NST and amniotic fluid measurement 2‐3 times per week. If either the NST or amniotic fluid volume assessment was abnormal, or other complications developed, labour was induced (28% of women induced in the expectant group received some form of PE2 (not gel)). |
|
| Outcomes | Mother: caesarean section; operative vaginal birth Baby: perinatal death (stillbirth or neonatal death before discharge excluding deaths caused by lethal congenital abnormalities); birthweight > 4000 g; Apgar score < 7 at 5 min; asphyxial encephalopathy (seizures, alterations in levels of consciousness or tone, or a need for tube feeding during the first 48 hours of life), respiratory distress (oxygen requirement > 40% and respiratory rate > 60 breaths/min, both within 12 hours after birth and persisting for more than 24 hours, or assisted ventilation for more than 24 hours): meconium aspiration syndrome; neonatal trauma; NICU admission | |
| Notes | Most women (89%) were enrolled at 410 to 416 weeks' gestation (3% before 41 weeks and 8% at or beyond 42 weeks), of whom 86.2% in the induced group and 63.6% in the expectant group gave birth before 42 weeks' gestation. In the induction group, 31% of women were not induced and in the expectant management group, 34% of women were induced. Funding: Medical Research Council of Canada: MA‐8472; Upjohn Company of Canada supplied the prostaglandin gel. Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation was not reported. |
| Allocation concealment (selection bias) | Low risk | Randomisation was carried out at a site separate from the trial ("centrally controlled at McMaster University"). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study was partially blinded; an adjudication of abnormal neonatal outcomes was undertaken by a neonatologist who was unaware of the mothers' group assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3418 women enrolled (data available for 3407 women); 7 women whose babies had lethal congenital anomalies were excluded after randomisation from the analysis of perinatal and neonatal outcomes ‐ induction group (1 woman) and expectant management group (6 women). |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified outcomes (from the methods) were reported; some results reported incompletely in text, e.g. "The frequency of postpartum maternal morbidity (hemorrhage, sepsis, endometritis) did not differ between the two groups (data not shown)." No access to trial protocol to further assess selective reporting. |
| Other bias | Low risk | Appears to be free of other bias; although methods of induction differed between the induction group and the women requiring induction in the expectant management group. |
Heimstad 2007a.
| Methods | RCT | |
| Participants | Number of women randomised: 508
Setting: St. Olavs University Hospital, Trondheim, Norway Inclusion criteria
State of cervix: all stages included |
|
| Interventions |
Induction group (n = 254): if cervix favourable (Bishop score ≥ 6) AROM + oxytocin, if not (Bishop score < 6) 50 µg misoprostol vaginally versus Expectant management group (n = 254): twice‐weekly ultrasound and CTG, labour induction after 300 days of pregnancy. |
|
| Outcomes | Mother: prolonged labour; mode of birth; perineal trauma; maternal satisfaction; postpartum haemorrhage Baby: perinatal death; neonatal morbidity, for which a score was tallied (by evaluating the degree of deviation from the potential of a perfect outcome for each newborn as defined by the authors); neonatal trauma; birthweight; birthweight > 4000 g, NICU admission, birth asphyxia, meconium aspiration syndrome, Apgar < 7 at 5 mins | |
| Notes |
Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation using blocks of 16 with no stratification. |
| Allocation concealment (selection bias) | Low risk | Central allocation ‐ clinical trials office. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No women were lost to follow‐up for clinical outcomes; 8 women did not complete the inclusion questionnaire; for the post‐birth telephone survey, 12 women were lost to follow‐up (4 in induction group and 8 in expectant management group). |
| Selective reporting (reporting bias) | Unclear risk | While all pre‐specified outcomes (in the methods) were reported, with no access to trial protocol it is not possible to further assess selective reporting. |
| Other bias | Low risk | Appears to be free of other bias. |
Henry 1969.
| Methods | RCT with inadequately reported randomisation methods. | |
| Participants | Number of women randomised: 112
Setting: Birmingham, UK Inclusion criteria (not well specified)
Exclusion criteria
State of cervix: not mentioned as a criterion |
|
| Interventions |
Induction group (n = 55): AROM and oxytocin ("surgical" group)
versus Expectant management group (n = 57): weekly amnioscopy. |
|
| Outcomes |
Mother: number of days past term; prolonged labour; mode of birth Baby: perinatal death; birthweight |
|
| Notes | 4 women in expectant group and 1 in induction group were randomised before 41 weeks Funding: not reported Declaration of interests: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses to follow‐up or exclusions. |
| Selective reporting (reporting bias) | Unclear risk | No outcomes were pre‐specified in the methods; no access to trial protocol to further assess selective reporting. |
| Other bias | Low risk | Appears to be free of other bias. |
Herabutya 1992.
| Methods | RCT | |
| Participants | Number of women randomised: 108
Setting: Bangkok, Thailand Inclusion criteria
Exclusion criteria
State of cervix: unfavourable cervix (Bishop score 6 or less) |
|
| Interventions |
Induction group (n = 57): PGE2 intracervical, repeated after 6 hours, AROM and oxytocin on day 2 according to contractions versus Expectant management group (n = 51): a) NST between 42 and 43 completed weeks. 2) NST between 43 and 44 completed weeks; women underwent IOL if there were abnormalities in antepartum fetal testing as non‐reactive NST, or variable decelerations on NST or if Bishop score > 6 on reaching 44 completed weeks' gestation. |
|
| Outcomes |
Mother: length of first stage of labour; mode of birth; cephalopelvic disproportion; fetal distress Baby: birthweight; meconium staining; Apgar score < 7 at 1 min; Apgar score < 7 at 5 min; intubation required; admission to special care baby unit; perinatal death |
|
| Notes |
Funding: Ramathibodi Hospital Research Fund Grant 1988 Declaration of interests: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses to follow‐up or exclusions. |
| Selective reporting (reporting bias) | Unclear risk | No outcomes were pre‐specified in the methods; no access to trial protocol to further assess selective reporting. |
| Other bias | Low risk | Appears to be free of other bias. |
James 2001.
| Methods | RCT | |
| Participants | Number of women randomised: 74 Setting: Vellore, India Inclusion criteria
Exclusion criteria
State of cervix: not mentioned as a criterion |
|
| Interventions |
Induction group (n = 37): Bishop < 5: cervical ripening with extra‐amniotically placed 16F Foley catheter with 20 mL of saline Bishop > 5: stripping of membranes Then, 12 hours later, IOL by AROM and oxytocin infusion versus Expectant management group (n = 37): daily fetal movement counts; biophysical profile every second day |
|
| Outcomes |
Mother: mode of birth and indications; duration of labour; mean hospital stay Baby: meconium staining of amniotic fluid; meconium aspiration; Apgar scores < 7 (at 1 and 5 min); need for neonatal intubation; birthweight; birthweight > 4000 g; signs of post maturity; perinatal deaths; abnormal electronic fetal trace monitoring |
|
| Notes |
Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A table of random numbers was used. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was unclear since "... a series of consecutively numbered, sealed envelopes..." was used but no mention was made of opaqueness of the envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up or post‐randomisation exclusion. |
| Selective reporting (reporting bias) | Unclear risk | All of the outcomes mentioned in the methods section were reported on in the results section; mean duration of labour was reported with no measure of variance. No access to trial protocol to further assess selective reporting. |
| Other bias | Low risk | Appears to be free of other bias. |
Kortekaas 2014.
| Methods | Multi‐centre RCT (non‐inferiority trial) | |
| Participants | Number of women randomised: 1811 (August 2012 to March 2016) Setting: Dutch Obstetric Consortium in cooperation with the Midwifery Research Network of the Netherlands including 200 centres (university hospitals, teaching hospitals, non‐teaching hospitals and midwifery practices). Inclusion criteria: low‐risk women > 18 years with a singleton pregnancy in stable cephalic position and a certain gestational age of 41‐2/+2 weeks. Exclusion criteria: age < 18 years, uncertain gestational age, obstetrical indications for secondary care (e.g. hypertension, proteinuria, pre‐existing maternal heart or kidney diseases, gestational diabetes, previous caesarean section, multiple pregnancy, intrauterine growth restriction, non‐reassuring fetal status (no fetal movements, abnormal fetal heart rate, known fetal abnormalities which could influence perinatal outcome (including abnormal karyotype), ruptured membranes at time of randomisation and a non‐reassuring fetal status at time of randomisation). |
|
| Interventions |
Induction* of labour at 410‐2 weeks (n = 902) versus Expectant management until 42 weeks (n = 909) *Women with a cervix that is judged to be ripe at vaginal examination (Bishop Score of 6 or more), will have labour induced with amniotomy followed by intravenous oxytocin according to local protocol. In case rupturing of membranes is not possible, cervical ripening will be accomplished in accordance with national guidelines. |
|
| Outcomes | Primary outcome (of study): composite of perinatal death and neonatal morbidity (adverse perinatal outcomes are defined as a composite of perinatal death, a 5‐minute Apgar‐score < 7 and/or an arterial pH < 7.05, meconium aspiration syndrome, plexus brachialis injury, intracranial haemorrhage and/or NICU admission). Secondary outcomes (of study): individual components of the composite; maternal outcomes (instrumental vaginal birth, Caesarean section), analgesia (epidural, spinal, opiates), postpartum haemorrhage ≥ 1000 mL, severe perineal injury (third‐ or fourth‐degree perineal tear). |
|
| Notes |
Funding: ZonMW grant number 17120200 Declarations of interest: the authors declare that they have no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated code |
| Allocation concealment (selection bias) | Low risk | Computer‐based randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and staff were aware of assignments |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Staff making treatment decisions and recording outcomes were aware of assignments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Results available from 96% of 1811 women (but abstract does not report the 109 losses by group) |
| Selective reporting (reporting bias) | Unclear risk | Trial protocol published, results not fully published yet |
| Other bias | Unclear risk | Abstract only published to date |
Martin 1978.
| Methods | RCT | |
| Participants | Number of women randomised: 264 "admitted to this trial"
Setting: Royal Maternity Hospital, Northern Ireland, UK Trial recruitment: timing not reported Inclusion criteria
Exclusion criteria
State of cervix: not clearly reported; assumed to be mixed (Bishop score recorded). |
|
| Interventions |
Induction group (n = 131 admitted; 92 analysed): IOL at 39 weeks' GA. Women were admitted for fasting at 8:30 am; their forewaters were punctured soon after and Bishop score and cervical dilatation recorded. IV oxytocin commenced at 2.5 mU/min and doubled every 30 min until satisfactory uterine response achieved; dose varied to maintain adequate contractions. All women continuously monitored with internal tocography and fetal scalp electrode versus Expectant management group (n = 133 admitted; 92 analysed): await spontaneous labour until 42 weeks, unless IOL required earlier for medical reasons. Women had, if necessary, augmentation of labour by puncture of the forewaters or IV oxytocin; when possible, they were also monitored. |
|
| Outcomes |
Mother: mode of birth (assisted birth; caesarean birth); Induction to birth interval; Unexplained postpartum pyrexia; Analgesia demand; Type 1 and 2 diabetes; Meconium staining of the amniotic fluid; duration of gestation; attitudes towards management Baby: Apgar scores at 1 and 5 min; Dubowitz scores < 45; stillbirth; hyperbilirubinaemia |
|
| Notes |
Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A prospective randomized controlled trial"; "allocated using random number tables." |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “All monitor records were examined blind at completion of the trial.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 184/264 women followed up (30% loss to follow‐up). 264 women eligible, 34 excluded: induction group: obstetric abnormality (8 women); refusal (10 women); defaulter (6 women); spontaneous labour before attending clinic (1 woman). Expectant management group: obstetric reasons (9); leaving 230 women in the trial (106 in planned birth, 124 in expectant management group) Of the 106 women in the induction group, 13 went into spontaneous labour before the date of admission and 1 was excluded due to medical reasons – therefore 92 women were induced and analysed. Of the 124 women in the expectant management group, a further 32 were excluded due to obstetric abnormalities or failure to go into spontaneous labour before 42 weeks – therefore only 92 were analysed |
| Selective reporting (reporting bias) | Unclear risk | Some results reported incompletely, e.g.: “There was no difference with respect to the distribution of Apgar scores…at five minutes.” |
| Other bias | Unclear risk | Groups appeared comparable at baseline however analyses excluded 30% of women admitted to the trial. Limited methodological detail provided to further assess other bias. |
Martin 1989.
| Methods | RCT | |
| Participants | Number of women randomised: 22
Setting: Jackson, USA Inclusion criteria
Exclusion criteria
State of cervix: unripe (Bishop score 5 or less) included |
|
| Interventions |
Induction group (n =12): laminaria tents followed by oxytocin versus Expectant management group (n = 10): weekly ultrasound for amniotic fluid assessment and NST. |
|
| Outcomes |
Mother: mode of birth; length of labour; type of analgesia; length of hospital stay; labour‐associated morbidity Baby: birthweight; Apgar score; perinatal deaths; neonatal course; meconium staining |
|
| Notes |
Funding: Vicksburg Hospital Medical Foundation Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation in sealed envelopes but no mention of opaqueness, numbering and sequential opening of envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses to follow‐up or exclusions. |
| Selective reporting (reporting bias) | High risk | No outcomes were pre‐specified in the methods; a number of outcomes reported without measures of variance (e.g. birthweight, length of labour, hospital stay), and thus these outcomes could not be used in the meta‐analyses. No access to protocol to further assess selective reporting. |
| Other bias | Low risk | Appears to be free of other bias. |
Miller 2015.
| Methods | RCT | |
| Participants | Number of women randomised: 162
Setting: Military tertiary care medical centre, USA Inclusion criteria
Exclusion criteria
State of cervix: modified Bishop score ≤ 5 |
|
| Interventions |
Induction group (n = 82): IOL ≤ 1 weeks of randomisation; not before 39 weeks' GA. Where possible IOL by Foley catheter (single balloon, 60 mL water), taped in place until expulsion or 12 hours. After Foley catheter, oxytocin (2 per min, increasing every 20 min to 36 mIU per min max) until adequate contractions. If Foley catheter placement not possible, IOL by misoprostol 25 µg vaginally, repeated every 4 hours; AROM after 3 cm dilation; oxytocin administered after last if adequate contractions not observed with misoprostol versus Expectant management group (n = 80): scheduled for routine appointments and birthed for obstetric indications no later than 42 weeks' GA. |
|
| Outcomes |
Mother: caesarean birth; mode of delivery; number of visits after randomisation; unscheduled clinic or triage visits; number of antepartum fetal testing appointments; GA at admission; Bishop score at admission; admission diagnosis; indication for operative birth; use of regional anaesthesia; chorioamnionitis; estimated blood loss; blood transfusion; endomyometritis; labour and birth length of stay; postpartum stay Baby: meconium‐stained amniotic fluid; NICU admission; birthweight; SGA; LGA; Apgar score < 5 at 5 min |
|
| Notes |
Funding: not reported Declarations of interest: the authors declared that they had no financial conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers in permuted blocks of 4. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed by the use of opaque, sealed, sequentially numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding: "blinding of health care providers to the indication for delivery was deemed impractical." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Little loss to follow‐up following randomisation (82/82 and 79/80 women included in the analyses). |
| Selective reporting (reporting bias) | Unclear risk | No access to trial protocol to assess selective reporting. Perinatal death not reported. |
| Other bias | Low risk | Appears to be free of other bias. |
NICHHD 1994.
| Methods | RCT | |
| Participants | Number of women randomised: 440
Setting: University hospitals in the USA Inclusion criteria
Exclusion criteria
State of cervix: unfavourable (Bishop score 6 or less) |
|
| Interventions |
Induction group (n = 174): cervical priming with PGE2 gel followed 12 hours later with oxytocin versus Expectant management group (n = 175): weekly cervix assessments, twice weekly NST and amniotic fluid volume assessment. A total of 265 women were randomised to the intervention arm; however, 91 of these women were randomised to placebo gel with oxytocin 12 hours later and these women have not been included in this review. |
|
| Outcomes | Mother: time to birth from randomisation; maternal infection; need for transfusion; uterine hyperactivity; mode of birth; maternal death Baby: mechanical ventilation; nerve injury; seizures; babies with ≽1 adverse outcome; perinatal death; birthweight; Apgar score < 4 at 5 min; late decelerations in labour; meconium in amniotic fluid; meconium in aspiration pneumonia | |
| Notes | The initial sample size intended was 2800. However, after 18 months and 440 participants, the study was stopped, since the incidence of adverse outcome was only 1.1% and therefore a sample size of 5600 would be required to adequately test the hypothesis proposed. Funding: National Institute of Child Health and Human Development, NIH, USA Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation sequence generation was performed using a computer‐generated randomisation scheme stratified by site and GA. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed by using central allocation by a data co‐ordinating centre. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses to follow‐up or exclusions. |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes (in methods) were reported; no access to trial protocol to further assess selective reporting. |
| Other bias | Low risk | Appears to be free of other bias. |
Nielsen 2005.
| Methods | RCT | |
| Participants | Number of women randomised: 226
Setting: Army Medical Center, Tacoma, Washington, USA Inclusion criteria
Exclusion criteria
State of cervix: favourable (≥ 5 for nulliparous and ≥ 4 for multiparous women) |
|
| Interventions |
Induction group (n = 116): AROM, oxytocin or both versus Expectant management group (n = 110): weekly follow‐up until 42 weeks. Labour induced after 42 weeks. Weekly monitoring with CTG and ultrasound, increased to twice a week after 41 weeks. |
|
| Outcomes |
Mother: randomisation to birth interval; admission to birth interval; Indication for admission; epidural analgesia; mode of birth; EBL; length of labour; chorioamnionitis; postpartum days Baby: birthweight; admission to NICU; Apgar score < 7 at 5 mins |
|
| Notes | The study was discontinued after recruitment of 226 women (target of 600) due to slow recruitment and no observed difference in the 2 groups Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation sequence was generated using a computer‐generated list. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was achieved using sequentially numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses to follow‐ up or exclusions. 23/116 (19.8%) in induction group went into spontaneous labour, 10/110 (9.1%) in the expectant management group required labour induction and results for these women were analysed according to which group they were randomised. |
| Selective reporting (reporting bias) | Unclear risk | No access to trial protocol for confidently assess selective reporting. Perinatal death not reported. |
| Other bias | Unclear risk | Baseline imbalance for Bishop score "The only significant difference noted was EM patients had a more favorable Bishop score on admission than IND patients (7.2+2.1 versus 8.6+2.0, p<0.0001)." |
Ocon 1997.
| Methods | RCT (partially translated). | |
| Participants | Number of women randomised: 113
Setting: Gran Canaria, Spain Inclusion criteria
Exclusion criteria
State of cervix: unfavourable (Bishop score < 5) |
|
| Interventions |
Induction group (n = 57): intracervical PGE2 gel (0.5 mg); unclear whether further intervention occurred (full translation not available) versus Expectant management group (n = 56): monitoring by NST, biophysical profile and amnioscopy. |
|
| Outcomes | Mother: time to birth; mode of birth Baby: meconium staining; NICU admission; birthweight > 4000 g; Apgar score < 7 at 5 mins (other outcomes may have been present, but were not reported in the translation) | |
| Notes |
Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation was not reported according to the translation. |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was not reported according to the translation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses to follow‐up or exclusions. |
| Selective reporting (reporting bias) | Unclear risk | No access to trial protocol for confidently assess selective reporting. Perinatal deaths appear not to have been reported according to the translation, although this has not been verified by a second translation. |
| Other bias | Low risk | Appears to be free of other bias. |
Roach 1997.
| Methods | RCT | |
| Participants | Number of women randomised: 201
Setting: Hong Kong, China Inclusion criteria
Exclusion criteria
State of cervix: not mentioned as a criterion |
|
| Interventions |
Induction group (n = 96): PGE2 pessaries 6‐hourly if necessary versus Expectant management group (n = 105): serial monitoring with NST (x2) and amniotic fluid index measurements (x1) weekly. |
|
| Outcomes |
Mother: spontaneous labour; caesarean section; fetal distress in labour Baby: birthweight; Apgar score < 7 (1 min/5 min); cord blood pH; admission to NICU; meconium below the vocal cords |
|
| Notes |
Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation in a series of identical envelopes but no mention of sealed envelopes, opaqueness and sequential numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses to follow‐up or exclusions. 17/96 (18%) in the induction group went into spontaneous labour and 12/105 (11%) in the expectant management group were induced and the results for these women were included in the analyses. |
| Selective reporting (reporting bias) | Unclear risk | Very few outcomes reported; "We did not address perinatal mortality in this study." No access to trial protocol to further assess selective reporting. |
| Other bias | Low risk | Appears to be free of other bias. |
Sahraoui 2005.
| Methods | RCT | |
| Participants | Number of women randomised: 150
Setting: Sousse, Tunisie (Tunisia) Inclusion criteria
Exclusion criteria
State of cervix: cervix unripe (Bishop score < 4) |
|
| Interventions |
Induction group (n = 75): PGE2 gel intracervically (daily cervical ripening by PGE2 gel, maximum 3 gels) versus Expectant management group (n = 75): CTG every second day until 42 completed weeks. After that, PGE2 gel if no spontaneous labour. |
|
| Outcomes |
Mother: duration of labour; mode of birth; GA at birth; duration of mother’s hospital stay (hours); need for augmentation of labour using synthetic oxytocin (Recours aux ocytociques); effect of Bishop score on admission on duration of labour (Effet du score de Bishop à l’admission sur la durée (duration) du travail (labour); progress in labour; time between final dose of PE2 gel and birth Baby: duration of infant’s hospital stay (hours); total cost of care; admission to neonatal unit; stained amniotic fluid; Apgar score at 1 min; perinatal death; stillbirth; neonatal death; macrosomia; signs of post‐maturity; need for resuscitation at birth; number of doses of gel administered |
|
| Notes | This article is in French. Funding: not reported Declaration of interests: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer. |
| Allocation concealment (selection bias) | Unclear risk | Article in French. Appears not to have been reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses to follow‐up or exclusions. |
| Selective reporting (reporting bias) | Unclear risk | No access to trial protocol to confidently assess selective reporting. |
| Other bias | Low risk | Appears to be free of other bias. |
Sande 1983.
| Methods | RCT | |
| Participants | Number of women randomised: 166
Setting: Ulleval Hospital, Norway Inclusion criteria
Exclusion criteria
State of cervix: Bishop score ≥ 5 |
|
| Interventions |
Induction group (n = 76): IOL on the following morning after randomisation; IV oxytocin (10 units in 1 L 5% glucose) immediately following AROM. Women were monitored by CTG versus Expectant management group (n = 90): waited for spontaneous labour to occur, following the normal procedure for the department (labour was induced after 42 weeks). |
|
| Outcomes |
Mother: duration of first and second stages of labour; pain relief; mode of birth (caesarean birth; vacuum; forceps; spontaneous birth); postpartum bleeding; induction to birth interval Baby: liveborn; neonatal death; birthweight; Apgar scores at 1 and 5 min; morbidity (transfer to NICU; paediatric examination of 1st and 5th day) |
|
| Notes | Only data for perinatal death, stillbirth and neonatal death included in meta‐analyses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generated was not reported. |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Per protocol analysis performed. Of the 76 women randomised to the induction group, 23 birthed spontaneously before induction In the expectant management group, 15 of the 90 women passed 42 weeks and had their labour induced Therefore, there were a total of 68 women who had their labour induced, and 98 birthed spontaneously (results analysed as such, not as per randomisation). |
| Selective reporting (reporting bias) | High risk | A number of outcomes such as birthweight, postpartum bleeding, and neonatal morbidity are reported incompletely in the text, e.g. “no differences” and “ns.” |
| Other bias | Unclear risk | No information by randomisation group. |
Suikkari 1983.
| Methods | Randomised trial, no further details. | |
| Participants | Number of women randomised: 119
Setting: Lappenranta, Finland Inclusion criteria
Exclusion criteria
State of cervix: not used as a criterion |
|
| Interventions |
Induction group (n = 66): oxytocin alone or with AROM depending on the cervix versus Expectant management group (n = 53): obstetric examination, NST, biochemical tests and amniotic fluid determination every 3 days. |
|
| Outcomes | Mother: mode of birth (reported only as operative); duration of labour; mean blood loss during labour; maternal death Baby: mean birthweight; Apgar scores; perinatal death; stillbirth; neonatal death | |
| Notes | The study is available as an abstract only. Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generated was not reported. |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to determine losses to follow‐up or exclusions. |
| Selective reporting (reporting bias) | Unclear risk | No outcomes were pre‐specified (abstract). |
| Other bias | Unclear risk | Unable to identify other bias based on the abstract; some degree of imbalance in numbers randomised to each group (66 and 53). |
Tylleskar 1979.
| Methods | RCT | |
| Participants | Number of women randomised: 112
Setting: Linköping and Motala, Sweden Inclusion criteria
Exclusion criteria
State of cervix: primipara with pelvic score of at least 5 points and engaged head; multipara with pelvic score at least 4 points |
|
| Interventions |
Induction group (n = 57): after AROM, an open‐ended saline‐filled catheter was inserted for measurement of intraamniotic pressure and a scalp electrode applied for continuous recording of FHR. IV oxytocin (using Cardiff Infusion System Mark II) was started 15 min later at 1 mU/min, increased continuously until the intensity of contractions was at least 33 mm Hg, with a frequency of at least 1 contraction every 150 seconds; the infusion rate doubled every 12.5 min versus Expectant management group (n = 55): women were asked to come to the delivery ward as soon as labour started; external CTG recordings were made until definite labour activity was demonstrated. AROM was performed, a catheter and scalp electrode applied when for primipara the cervix was at least 50% effaced and dilated more than 2 cm, and for multipara when the cervix was dilated to 3 cm. If the pregnancy lasted more than 14 days beyond the estimated time of birth labour was induced using IV oxytocin. |
|
| Outcomes |
Mother: duration of labour; uterine activity at 6 cm cervical dilatation; total amount of oxytocin used; fetal heart rate patterns (early decelerations; late decelerations; bradycardia); amount of bleeding during the third stage of labour; mode of birth (vacuum extraction); placental retention; maternal pH at birth; analgesia (pudendal block, nitrous oxide, pethidine/atarax); women's experiences of the birth Baby: birthweight; birth asphyxia; Apgar score at 1 and 5 min; baby pH; lowest weight in first week; haemoglobin and haematocrit in umbilical vein and day 2; Bilirubin levels day 1‐3; 4 dimensions of the Brazelton Scale |
|
| Notes |
Funding: not reported Declaration of interests: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generated was not reported. |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 112 women were randomised. 13/57 in the induction group and 12/55 in the expectant management group went into labour before the expected due date and were excluded. 3 further women were excluded – 1 in the expectant management group birthed rapidly and data collection was not possible; 1 woman in each group had a caesarean due to feto‐pelvic disproportion. Thus 43/57 in the induction group and 41/55 in the expectant management group were analysed; overall 84/112 (75%). |
| Selective reporting (reporting bias) | High risk | Results for a number of outcomes are reported incompletely, e.g. "Nor were there any differences in maternal pH at delivery"; "Analgesia in the form of pudendal block or nitrous oxide was given in the same frequency in the two groups;" and "An analysis of the questionnaire with respect to the patients experiences of the delivery indicate a positive attitude... No statistical differences between groups were found." |
| Other bias | Unclear risk | Limited methodological detail provided. Only baseline characteristics reported were age, pelvic score and number of previous pregnancies, and only for women analysed. |
Walker 2016.
| Methods | RCT | |
| Participants | Number of women randomised: 619
Setting: 39 National Health Service Hospitals, UK Inclusion criteria
Exclusion criteria
State of cervix: not clearly reported (mixed) |
|
| Interventions |
Induction group (n = 305): IOL between 390 and 396 weeks, with method dependent on local protocol and cervical ripeness (most participating units used prostaglandin ripening followed, if necessary, by AROM and oxytocin). Prostaglandin tablet regimen (n = 49), prostaglandin gel regimen (n = 63), prostaglandin slow release pessary (N = 158), AROM (n = 129), oxytocin (n = 137) (women could have > 1 intervention) versus Expectant management group (n = 314): waiting for spontaneous onset of labour, unless a situation developed necessitating birth by IOL or caesarean. Women underwent IOL between 41‐42 weeks (7‐14 days after due date) depending on preference and physician's usual practice; if a woman declined induction at 42 weeks, she could undergo a scan to determine fetal growth and amniotic fluid volume daily or every other day, CTG and monitoring according to usual practice. |
|
| Outcomes |
Mother: caesarean; method of birth; onset of labour; indication for induction; method of induction; indication for caesarean; intrapartum and postpartum complications (e.g. systemic infection, need for blood transfusion); mother’s expectations and experience of childbirth; analgesia; perineal trauma; postpartum haemorrhage Baby: live or stillbirth; birthweight; admission to NICU; birth trauma; 2 composite outcomes for serious neonatal complications (direct trauma and hypoxia); Apgar < 7 at 5 mins |
|
| Notes | Staff were encouraged to use the same methods of IOL in the induction group and the expectant management group who were subsequently induced. Funding: Grant (PB‐PG‐0610‐22275) from the Research for Patient Benefit Programme of the National Institute for Health Research Declarations of interest: Dr. Smith reports receiving fees for serving on an advisory board from Roche Diagnostics, consulting fees from GlaxoSmithKline, equipment loans from Roche Diagnostics and General Electric, travel support from Roche Diagnostics and Chiesi, and grant support from GlaxoSmithKline and Action Medical Research, and being named as an inventor on a pending patent (PCT/EP2014/062602) filed by GlaxoSmithKline related to retosiban as a preventive treatment for preterm labor in women with increased uterine stretch. No other potential conflict of interest relevant to this article was reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1 ratio using a computer‐generated code with the use of permuted blocks of randomly varying size generated by a clinical trials unit. Stratified by trial centre and maternal age. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed by using central allocation by the Clinical Trials Unit. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis included all but 1/619 women; 83% completed childbirth experience questionnaires. |
| Selective reporting (reporting bias) | Low risk | Trial protocol published. |
| Other bias | Low risk | Appears to be free of other bias. |
Witter 1987.
| Methods | RCT | |
| Participants | Number of women randomised: 200
Setting: Baltimore, USA Inclusion criteria
Exclusion criteria No additional criteria State of cervix: not mentioned |
|
| Interventions |
Induction group (n = 103): oxytocin infusion with AROM when possible
versus Expectant management group (n = 97): Estriol measurements 2‐3/week. In both groups women initiated fetal movement counting. If reduced fetal movements, FHR and estriol testing were undertaken at 41 completed weeks. |
|
| Outcomes | Mother: GA at birth; length of hospital stay; urinary estriol/creatinine ratio; maternal complications; endometritis; pre‐eclampsia; PROM; caesarean section + indications Baby: birthweight; biparietal diameter; placental weight; Dubowitz score (assesses infant GA); SGA/AGA/LGA; fetal distress; meconium staining; infant complications; Apgar scores (< 7 at 5 mins); fetal anomalies; post‐mature infants; meconium aspiration | |
| Notes |
Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation sequence was generated using a computer‐generated random number table. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was achieved using sequentially labelled sealed envelopes, but there was no mention of opaqueness. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Appears that blinding was not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinded outcome assessment was not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/103 women and 2/97 women in the induction and expectant management groups dropped out of the study; 35/103 women and 39/97 in the induction and expectant management groups birthing prior to 42 completed weeks (and were included); all were included in the analyses. |
| Selective reporting (reporting bias) | Unclear risk | No detailed outcomes were pre‐specified in the methods; perinatal death was not reported. No access to trial protocol to further assess selective reporting. |
| Other bias | Low risk | Appears to be free of other bias. |
AGA: appropriate for gestational age AROM: artificial rupture of membranes/amniotomy CTG: cardiotocography EBL: estimated blood loss EM: expectant management FHR: fetal heart rate GA: gestational age IOL: induction of labour IND: induction IU: international units IUFD: intrauterine fetal death IV: intravenous IVF: in‐vitro fertilisation LGA: large‐for‐gestational age LMP: last menstrual period min: minutes mIU: milli‐international units mU: milli‐units NICU: neonatal intensive care unit NST: nonstress test PGE2 (and PE2): prostaglandin E2 PROM: premature rupture of membranes RCT: randomised controlled trial SGA: small‐for‐gestational age
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alcalay 1996 | PROM at term. |
| Amano 1999 | Alternate allocation trial. |
| Ascher‐Walsh 2000 | Compares 2 forms of IOL. |
| Bell 1993 | Trial of cervical ripening not IOL. |
| Berghella 1996 | Membrane stripping to decrease the need for formal IOL. |
| Boulvain 1998 | Membrane stripping to decrease the need for formal IOL. |
| Buttino 1990 | Trial of cervical ripening not IOL. |
| Cardozo 1986 | Alternate allocation trial. |
| Conway 2000 | Trial of active versus expectant management in women with oligohydramnios. |
| Damania 1992 | Trial of cervical ripening (2 methods) not IOL. |
| Dare 2002 | Trial of cervical ripening not IOL. |
| de Aquino 2003 | Compares 2 forms of IOL. |
| Doany 1997 | Trial of cervical ripening not IOL. |
| Dunn 1989 | Intervention not a policy to induce labour compared with expectant management. |
| El‐Torkey 1992 | Trial of cervical ripening not IOL. |
| Elliott 1984 | Trial of nipple stimulation as a method of cervical ripening. No commitment to delivery within a given time or protocol. |
| Evans 1983 | Compares 2 forms of IOL. |
| Frass 2011 | Trial where all women were judged to be at risk (severe pre‐eclampsia). |
| Garry 2000 | Alternate allocation trial. |
| Giacalone 1998 | Trial of cervical ripening not IOL. |
| Gregson 2015 | Assessing effectiveness of acupuncture for IOL (role of acupuncture not established). |
| Hage 1993 | Trial of cervical ripening not IOL. |
| Heden 1991 | Alternate allocation trial. |
| Hernandez‐Castro 2008 | Not a RCT. |
| Imsuwan 1999 | This is a RCT evaluating the effectiveness of weekly membrane sweeping in labour initiation for women at 41 completed weeks. It is not evaluating a policy of stopping the pregnancy at 41 weeks. |
| Ingemarsson 1987 | Trial of cervical ripening not IOL. |
| Iqbal 2004 | Alternate allocation trial. |
| Jenssen 1977 | Trial of cervical ripening not IOL. |
| Kadar 1990 | Trial of nipple stimulation as a method of cervical ripening. No commitment to delivery within a given time or protocol. |
| Katz 1983 | Alternate allocation trial. |
| Kipikasa 2005 | Comparing alternate methods for IOL. |
| Klopper 1969 | Trial of cervical ripening not IOL. |
| Knox 1979 | Quasi‐randomised (last digit of hospital number). |
| Lee 1997 | Compares 2 forms of IOL. |
| Lemancewicz 1999 | Compares 2 forms of IOL. |
| Lien 1998 | Trial of cervical ripening not IOL. |
| Lyons 2001 | Trial of cervical ripening not IOL. |
| Magann 1998 | Trial of cervical ripening not IOL. |
| Magann 1999 | Compares 2 forms of IOL. |
| Mancuso 1998 | Compares 2 forms of IOL. |
| Meydanli 2003 | Compares 2 forms of IOL. |
| Misra 1994 | Compares 2 forms of IOL. |
| Müller 1995 | Compares 2 forms of IOL. |
| Neri 2014 | Assessing effectiveness of acupressure for IOL (role of acupuressure not established). |
| Newman 1997 | Trial of cervical ripening not IOL. |
| Nicholson 2008 | Trial where all women were judged to be at risk. |
| Ohel 1996 | Alternate allocation. |
| Papageorgiou 1992 | Compares 2 forms of IOL. |
| Paul 1988 | Protocol for RCT only ‐ no results. |
| Rayburn 1988 | Trial of cervical ripening not IOL. |
| Rayburn 1999 | Trial of cervical ripening not IOL. |
| Rijnders 2011 | Compares 2 alternative management strategies for IOL. |
| Roberts 1986 | Trial of cervical ripening not IOL. |
| Satin 1991 | Compares 2 forms of IOL. |
| Sawai 1991 | Trial of cervical ripening not IOL. |
| Sawai 1994 | Trial of cervical ripening not IOL. |
| Stenlund 1999 | Mifepristone versus placebo for IOL, but all women given PGE2 if necessary after 48 hours. |
| Su 1996 | Both groups induced within 2 days with alternative methods. |
| Surbek 1997 | Compares 2 forms of IOL. |
| Suzuki 1999 | Immediate IOL versus expectant management in twin pregnancies. |
| Williams 1990 | Trial of cervical ripening not IOL. |
| Wing 2000 | Trial of cervical ripening not IOL. |
| Wong 2002 | Trial of cervical ripening not IOL. |
| Ziaei 2003 | Trial of cervical ripening not IOL. |
IOL: induction of labour PGE2: prostaglandin E2 PROM: premature rupture of membranes RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Benito Reyes 2010.
| Methods | Quote: "prospective study" |
| Participants | 200 |
| Interventions | Elective induction versus expectant management |
| Outcomes | Caesarean section |
| Notes |
Harrington 2003.
| Methods | RCT (ISRCTN74323479) |
| Participants | 100 pregnant primiparous women who have not laboured at or beyond 41 weeks' gestation with cervical length > 3 cm |
| Interventions | Mifepristone versus standard care |
| Outcomes | Length of time from induction to onset of labour |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
Elden 2016.
| Trial name or title | SWEdish Post‐term Induction Study (SWEPIS) (ISRCTN26113652) |
| Methods | Multi‐centre RCT |
| Participants | 10,038 healthy women ≥18 years old with a normal live singleton pregnancy in cephalic presentation at 41+0 GW |
| Interventions | Labour induction at 41 weeks' gestation (early induction) or expectant management and induction at 42 weeks' gestation (late induction). |
| Outcomes | Primary outcome: composite of stillbirth, neonatal death and neonatal morbidity (defined as at least 1 of the following variables: Apgar score < 7 at 5 min, metabolic acidosis defined as pH < 7.05 and base deficit > 12 mmol/L in umbilical artery or pH < 7.00 in umbilical artery, HIE I‐III, intracranial haemorrhage, neonatal convulsions, meconium aspiration syndrome (MAS), mechanical ventilation, obstetric brachial plexus injury). |
| Starting date | 01/09/2015 |
| Contact information | Prof Helen Elden, Perinatal Centre, Department of Obstetrics and Gynecology, Institute of Clinical Sciences, Sahlgrenska Academy, East Hospital, Gothenburg, 416 85, Sweden |
| Notes |
Othman 2017.
| Trial name or title | Induction of labour at 39 weeks or beyond in multiparous women with a favourable cervix (ISRCTN15646866) |
| Methods | RCT |
| Participants | 160 pregnant women ≥ 39 weeks' gestation, with at least 1 previous vaginal birth |
| Interventions | 1) induction of labour at 39 weeks 2) expectant management with recommendation to induce at 41 weeks if woman has not given birth |
| Outcomes | Time of giving birth; maternal satisfaction |
| Starting date | 30 September 2016 |
| Contact information | Dr Aida Othman, Dept Obstetrics & Gynaecology, University Malaya Medical Centre, Kuala Lumpur, 59100, Malaysia |
| Notes |
Reddy 2013.
| Trial name or title | A randomized trial of induction versus expectant management (ARRIVE) |
| Methods | RCT |
| Participants | 6000 nulliparous women at 38 weeks 0 days to 38 weeks 6 days gestation |
| Interventions | 1) elective induction of labour between 39 weeks 0 days and 39 weeks 4 days 2) expectant management until at least 40 weeks 5 days (unless a medical indication arises |
| Outcomes | Primary outcome: composite of severe neonatal morbidity and perinatal mortality (any 1 of antepartum, intrapartum or neonatal death; intubation, CPAP, or high‐flow nasal cannula for ventilation or cardiopulmonary resuscitation within the first 72 hours, Apgar ≤ 3 at 5 minutes, neonatal encephalopathy, seizures, sepsis, pneumonia, meconium aspiration syndrome, birth trauma, intracranial haemorrhage (including IVH), hypotension requiring pressor support |
| Starting date | March 2014 |
| Contact information | Uma Reddy, Eunice Kennedy Shriver National Institute of Child Health and Human Development, USA |
| Notes | Final data collection for primary outcome completed November 2017 |
CPAP: continuous positive airway pressure GW: gestational weeks HIE: Hypoxic Ischemic Encephalopathy IVH: intraventricular haemorrhage RCT: randomised controlled trial
Differences between protocol and review
2018 update of the review
We have updated the methods in line with those in the standard template used by Cochrane Pregnancy and Childbirth.
We have omitted the outcome of vaginal birth as it is the obverse of caesarean section.
We have used the GRADE approach to assess the quality of the body of evidence and we have included ’Summary of findings’ tables.
We have added three new infant secondary outcomes (birthweight; birthweight > 4000 g; neonatal trauma), which were reported as non pre‐specified, but important, outcomes in the previous version of this review, in our main outcomes list.
The secondary infant outcome 'Perinatal death (stillbirth, newborn deaths within first week)' (which is the same as the primary outcome) has been changed to two separate outcomes, 'Stillbirth' and 'Neonatal death within the first week'.
The subgroup analyses by gestational age are now reported by induction at < 41 weeks; and at ≥ 41 weeks.
We have added in an additional search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP).
In the previous (Gülmezoglu 2012) update of this review:
The subgroup analyses by gestational age were reported by induction at 39‐40 weeks; at 41 weeks; and at > 41 weeks;
Tthe methods were updated to reflect the latest Cochrane Handbook for Systematic Reviews of Interventions version (Higgins 2011).
Contributions of authors
For this update review, PM and Emily Shepherd (ES) applied the selection criteria, extracted data for included studies and assessed risk of bias. All three authors (PM, ES and Caroline Crowther) contributed to drafting and editing of this update.
Sources of support
Internal sources
Australian Research Centre for Health of Women and Babies (ARCH), Robinson Research Institute, The University of Adelaide, Adelaide, Australia.
Healthy Mothers, Babies and Children, South Australian Health and Medical Research Institute (SAHMRI), Adelaide, Australia.
Liggins Institute, The University of Auckland, Auckland, New Zealand.
External sources
-
NHMRC: National Health and Medical Research Council, Australia.
Funding for the Cochrane Pregnancy and Childbirth Australian and New Zealand Satellite
-
NIHR: National Institute for Health Research, UK.
NIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines
Declarations of interest
Philippa Middleton: none known.
Caroline A Crowther: none known.
Emily Shepherd: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Augensen 1987 {published data only}
- Augensen K, Bergsjo P, Eikeland T, Ashvik K, Carlsen J. Randomized comparison of early versus late induction of labour in post‐term pregnancy. BMJ 1987;294:1192‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augensen K, Bergsjo P, Eikeland T, Askvik K, Carlsen J. Induction of labour in prolonged pregnancy. A prospective randomized study. 10th European Congress of Perinatal Medicine; 1986 Aug 12‐16; Leipzig, Germany. 1986.
Bergsjo 1989 {published data only}
- Bergsjo P, Huang GD, Yu SQ, Gao Z, Bakketeig LS. Comparison of induced vs non‐induced labor in post‐term pregnancy. Acta Obstetricia et Gynecologica Scandinavica 1989;68:683‐7. [DOI] [PubMed] [Google Scholar]
Brane 2014 {published data only}
- Brane E, Olsson A, Andolf E. A randomized controlled trial on early induction compared to expectant management of nulliparous women with prolonged latent phases. Acta Obstetricia Et Gynecologica Scandinavica 2014;93:1042‐9. [DOI] [PubMed] [Google Scholar]
Breart 1982 {published data only}
- Breart G, Goujard J, Maillard F, Chavigny C, Rumeau‐Rouquette C, Sureau C. Comparison of two obstetrical policies with regard to artificial induction of labour at term. A randomised trial. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1982;11:107‐12. [PubMed] [Google Scholar]
Chakravarti 2000 {published data only}
- Chakravarti S, Goenka B. Conservative policy of induction of labor in uncomplicated postdated pregnancies. XVI FIGO World Congress of Obstetrics & Gynecology; 2000 Sept 3‐8; Washington DC, USA (Book 3). 2000:62.
Chanrachkul 2003 {published data only}
- Chanrachkul B, Herabutya Y. Postterm with favorable cervix: is induction necessary?. European Journal of Obstetrics & Gynecology and Reproductive Biology 2003;106:154‐7. [DOI] [PubMed] [Google Scholar]
Cohn 1992 {published data only}
- Cohn M, Rogers M. Post maturity; a randomised study in a Hong Kong population. 26th British Congress of Obstetrics and Gynaecology; 1992 July 7‐10; Manchester, UK. 1992:306.
Cole 1975 {published data only}
- Cole RA, Howie PW, MacNaughton MC. Elective induction of labour. A randomised prospective trial. Lancet 1975;1:767‐70. [DOI] [PubMed] [Google Scholar]
- Engleman SR, Hilland MA, Howie PW, McIlwaine GM, McNay MB. An analysis of the economic implications of elective induction of labour at term. Community Medicine 1979;1:191‐8. [DOI] [PubMed] [Google Scholar]
Dyson 1987 {published data only}
- Dyson D, Miller PD, Armstrong MA. Management of prolonged pregnancy: induction of labour versus antepartum testing. American Journal of Obstetrics and Gynecology 1987;156:928‐34. [DOI] [PubMed] [Google Scholar]
- Dyson DC, Miller P, Miller M. Management of prolonged pregnancy ‐ induction versus antepartum fetal testing. 6th Annual Meeting of the Society of Perinatal Obstetricians; 1986 Jan 30‐Feb 1; San Antonio, Texas, USA. 1986:205.
Egarter 1989 {published data only}
- Egarter CH, Kofler E, Fitz R, Husslein P. Is induction of labour indicated in prolonged pregnancy? Results of a prospective randomised trial. Gynecologic and Obstetric Investigation 1989;27:6‐9. [DOI] [PubMed] [Google Scholar]
- Husslein P, Egarter C, Sevelda P, Genger H, Salzer H, Kofler E. Induction of labour with Prostaglandin E2 vaginal tablets ‐ a revival of elective induction? Results of a prospective randomised trial [Geburtseinleitung mit 3mg Prostaglandin E2‐vaginaltabletten: eine renaissance der programmierten geburt?]. Geburtshilfe und Frauenheilkunde 1986;46:83‐7. [DOI] [PubMed] [Google Scholar]
Gelisen 2005 {published data only}
- Gelisen O, Caliskan E, Dilbaz S, Ozdas E, Dilbaz B, Ozdas E, et al. Induction of labor with three different techniques at 41 weeks of gestation or spontaneous follow‐up until 42 weeks in women with definitely unfavorable cervical scores. European Journal of Obstetrics & Gynecology and Reproductive Biology 2005;120(2):164‐9. [DOI] [PubMed] [Google Scholar]
Hannah 1992 {published data only}
- Farquharson D, Hannah ME, Hannah WJ, Hewson SA, Willan A, Young D, et al. The Canadian multicentre postterm pregnancy trial (CMPPT): outcome in the induction group based on method of induction. 49th Annual Clinical Meeting of the Society of Obstetricians and Gynaecologists of Canada; 1993 June 22‐26; Ottawa, Ontario, Canada. 1993:15.
- Goeree R, Hannah M, Hewson S, for the Canadian Postterm Pregnancy Trial Group. Cost‐effectiveness of induction of labour versus serial antenatal monitoring in the Canadian multicentre postterm pregnancy trial. Canadian Medical Association Journal 1995;152:1445‐50. [PMC free article] [PubMed] [Google Scholar]
- Hannah M, Canadian MG. The Canadian Multicentre Postterm Pregnancy Trial. International Journal of Gynecology & Obstetrics 1994;46:31. [Google Scholar]
- Hannah ME, Hannah WJ, Hellman J, Hewson S, Milner R, Willan A. Induction of labour as compared with serial antenatal monitoring in post‐term pregnancy. A randomized controlled trial. New England Journal of Medicine 1992;326:1587‐92. [DOI] [PubMed] [Google Scholar]
- Hannah ME, Huh C, Hewson SA, Hannah WJ. Postterm pregnancy: putting the merits of a policy of induction into perspective. Birth 1996;23(1):13‐9. [DOI] [PubMed] [Google Scholar]
Heimstad 2007a {published data only}
- Heimstad R, Romundstad PR, Hyett J, Mattsson LA, Salvesen KA. Women's experiences and attitudes towards expectant management and induction of labor for post‐term pregnancy. Acta Obstetricia et Gynecologica Scandinavica 2007;86(8):950‐6. [DOI] [PubMed] [Google Scholar]
- Heimstad R, Skogvoli E, Mattsson L, Johansen OJ, Eik‐Nes SH, Romundstad PR, et al. Induction of labour or serial antenatal fetal monitoring in post‐term pregnancy. A randomised controlled trial. 36th Nordic Congress of Obstetrics and Gynecology; 2008 June 14‐17; Reykjavik, Iceland. 2008:84. [DOI] [PubMed]
- Heimstad R, Skogvoll E, Mattsson LA, Johansen OJ, Eik‐Nes SH, Salvesen KA. Induction of labor or serial antenatal fetal monitoring in postterm pregnancy: a randomized controlled trial. Obstetrics & Gynecology 2007;109(3):609‐17. [DOI] [PubMed] [Google Scholar]
- Heimstad U. Post term pregnancy ‐ induction of labor or monitoring of pregnancy. clinicaltrials.gov/show/NCT00385229 (first received 9 October 2006).
Henry 1969 {published data only}
- Henry GR. A controlled trial of surgical induction of labour and amnioscopy in the management of prolonged pregnancy. Journal of Obstetrics and Gynaecology of the British Commonwealth 1969;76:795‐8. [DOI] [PubMed] [Google Scholar]
Herabutya 1992 {published data only}
- Herabutya Y, Prasertsawat PO, Tongyai T, Isarangura N, Ayudthya N. Prolonged pregnancy: the management dilemma. International Journal of Gynecology & Obstetrics 1992;37:253‐8. [DOI] [PubMed] [Google Scholar]
James 2001 {published data only}
- James C, George SS, Gaunekar N, Seshadri L. Management of prolonged pregnancy: a randomized trial of induction of labour and antepartum foetal monitoring. National Medical Journal of India 2001;14:270‐3. [PubMed] [Google Scholar]
Kortekaas 2014 {published data only}
- Bruinsma A, Keulen J, Bakker J, Post J, Miranda E, Kortekaas J, et al. Induction of labor at 41 weeks or expectant management until 42 weeks‐preliminary results of the INDEX trial. American Journal of Obstetrics and Gynecology 2017;216(1 Suppl):S27‐S28, Abstract no: 40. [Google Scholar]
- Kortekaas JC, Bruinsma A, Keulen JKJ, Dillen J, Oudijk MA, Zwart JJ, et al. Effects of induction of labour versus expectant management in women with impending post‐term pregnancies: the 41 week ‐ 42 week dilemma. BMC Pregnancy and Childbirth 2014;14(1):350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol B. Costs and effects of induction of labour at 41 weeks compared to waiting for spontaneous labour until 42 weeks. trialregister.nl/trialreg/admin/rctview.asp?TC=3431 (first received 12 May 2012).
Martin 1978 {published data only}
- Martin DH, Thompson W, Pinkerton JHM, Watson JD. A randomised controlled trial of selective planned delivery. British Journal of Obstetrics and Gynaecology 1978;85:109‐13. [DOI] [PubMed] [Google Scholar]
Martin 1989 {published data only}
- Martin JN, Sessums JK, Howard P, Martin RW, Morrison JC. Alternative approaches to the management of gravidas with prolonged post‐term postdate pregnancies. Journal of the Mississippi State Association 1989;30:105‐11. [PubMed] [Google Scholar]
Miller 2015 {published data only}
- Miller N. Elective induction of nulliparous labor. clinicaltrials.gov/ct2/show/NCT01076062 (first received 25 February 2010).
- Miller NR, Cypher RL, Foglia LM, Pates JA, Nielsen PE. Elective induction of labor compared with expectant management of nulliparous women at 39 weeks of gestation: a randomized controlled trial. Obstetrics and Gynecology 2015;126(6):1258‐64. [DOI] [PubMed] [Google Scholar]
- Miller NR, Cypher RL, Foglia LM, Pates JA, Nielsen PE. Elective induction of nulliparous labor at 39 weeks of gestation: a randomized clinical trial. Obstetrics and Gynecology 2014;123(5 Suppl):72S. [DOI] [PubMed] [Google Scholar]
NICHHD 1994 {published data only}
- Medearis AL. Postterm pregnancy: active labor induction (PGE2 gel) not associated with improved outcomes compared to expectant management. A preliminary report. 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 January 23‐27; Houston, Texas, USA. 1990:17.
- National Institute of Child Health and Human Development Network of Maternal‐Fetal Medicine Units. A clinical trial of induction of labor versus expectant management in postterm pregnancy. American Journal of Obstetrics and Gynecology 1994;170:716‐23. [PubMed] [Google Scholar]
Nielsen 2005 {published data only}
- Nielsen PE, Howard BC, Hill CC, Larson PL, Holland RH, Smith PN. Comparison of elective induction of labor with favorable Bishop scores versus expectant management: a randomized clinical trial. Journal of Maternal‐Fetal & Neonatal Medicine 2005;18(1):59‐64. [DOI] [PubMed] [Google Scholar]
Ocon 1997 {published data only}
- Ocon L, Hurtado R, Coteron JJ, Zubiria A, Ramirez O, Garcia JA. Prolonged pregnancy: procedure guidelines [Gestacion prolongada: pautas de actuacion]. Progresos de Obstetricia y Ginecologia 1997;40:101‐6. [Google Scholar]
Roach 1997 {published data only}
- Roach VJ, Rogers MS. Pregnancy outcome beyond 41 weeks gestation. International Journal of Gynecology & Obstetrics 1997;59:19‐24. [DOI] [PubMed] [Google Scholar]
Sahraoui 2005 {published data only}
- Sahraoui W, Hajji S, Bibi M, Nouira M, Essaidi H, Khair H. Management of pregnancies beyond forty‐one week's gestation with an unfavorable cervix [Prise en charge obstetricale des grossesses prolongees au‐dela de 41 semaines d'amenorrhee avec un score de Bishop defavorable]. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 2005;34(5):454‐62. [DOI] [PubMed] [Google Scholar]
Sande 1983 {published data only}
- Sande HA, Tuveng J, Fonstelien T. A prospective randomized study of induction of labor. International Journal of Gynecology & Obstetrics 1983;21:333‐6. [DOI] [PubMed] [Google Scholar]
Suikkari 1983 {published data only}
- Suikkari AM, Jalkanen M, Heiskala H, Koskela O. Prolonged pregnancy: induction or observation. Acta Obstetricia et Gynecologica Scandinavica Supplement 1983;116:58. [Google Scholar]
Tylleskar 1979 {published data only}
- Leijon I, Finnstrom O, Hedenskog S, Ryden G, Tylleskar J. Spontaneous labor and elective induction ‐ a prospective randomized study. II Bilirubin levels in the neonatal period. Acta Obstetricia et Gynecologica Scandinavica 1980;59:103‐6. [DOI] [PubMed] [Google Scholar]
- Leijon I, Finnstrom O, Hedenskog S, Ryden G, Tylleskar J. Spontaneous labour and elective induction ‐ a prospective randomised study. Behavioural assessment and neurological examination in the newborn period. Acta Paediatrica Scandinavica 1979;68:553‐60. [DOI] [PubMed] [Google Scholar]
- Tylleskar J, Finnstrom O, Hedenskog S, Leijon I, Ryden G. Spontaneous delivery‐elective induction for convenience, a comparative study. 6th European Congress of Perinatal Medicine; 1978 Aug 29‐Sept 1; Vienna, Austria. 1978:345.
- Tylleskar J, Finnstrom O, Leijon I, Hedenskog S, Ryden G. Spontaneous labor and elective induction ‐ a prospective randomized study. Effects on mother and fetus. Acta Obstetricia et Gynecologica Scandinavica 1979;58:513‐8. [DOI] [PubMed] [Google Scholar]
Walker 2016 {published data only}
- Walker F, Bugg J, Macpherson M, McCormick C, Grace N, Wildsmith C, et al. Randomized trial of labor induction in women 35 years of age or older. New England Journal of Medicine 2016;374(9):813‐22. [DOI] [PubMed] [Google Scholar]
- Walker K. Induction of labour versus expectant management for women over 35 years. isrctn.com/ISRCTN11517275 (first received 23 August 2012).
- Walker K, Bugg G, Macpherson M, McCormick C, Wildsmith C, Smith G, et al. The 35/39 trial: A multi‐centre prospective randomised controlled trial of induction of labour versus expectant management for nulliparous women over 35 years of age. International Journal of Gynecology and Obstetrics 2015;131(Suppl 5):E221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KF, Bugg G, Macpherson M, McCormick C, Wildsmith C, Smith G, et al. Induction of labour versus expectant management for nulliparous women over 35 years of age: a multi‐centre prospective, randomised controlled trial. BMC Pregnancy and Childbirth 2012;12:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KF, Bugg G, Macpherson M, McCormick C, Wildsmith C, Smith G, et al. Induction of labour versus expectant management for nulliparous women over 35 years of age: protocol for a multicentre prospective, randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2013;120(Suppl s1):495. [Google Scholar]
- Walker KF, Bugg GJ, Macpherson M, McCormick C, Wildsmith C, Smith GC, et al. The 35/39 trial: a multicentre prospective randomised controlled trial of induction of labour versus expectant management for nulliparous women over 35 years of age. BJOG: an international journal of obstetrics and gynaecology 2015;122(Suppl S2):5. [Google Scholar]
- Walker KF, Dritsaki M, Bugg G, Macpherson M, McCormick C, Grace N, et al. Labour induction near term for women aged 35 or over: an economic evaluation. BJOG: an international journal of obstetrics and gynaecology 2017;124(6):929‐34. [DOI] [PubMed] [Google Scholar]
Witter 1987 {published data only}
- Witter FR, Weitz CM. A randomised trial of induction at 42 weeks of gestation vs expectant management for postdates pregnancies. American Journal of Perinatology 1987;4:206‐11. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alcalay 1996 {published data only}
- Alcalay M, Hourvitz A, Reichman B, Luski A, Quint J, Barkai G, et al. Prelabour rupture of membranes at term: early induction of labour vs expectant management. European Journal of Obstetrics & Gynecology and Reproductive Biology 1996;70:129‐33. [DOI] [PubMed] [Google Scholar]
Amano 1999 {published data only}
- Amano K, Saito K, Shoda T, Tani A, Yoshihara H, Nishijima M. Elective induction of labour at 39 weeks of gestation: a prospective randomized trial. Journal of Obstetrics and Gynaecology Research 1999;25:33‐7. [DOI] [PubMed] [Google Scholar]
Ascher‐Walsh 2000 {published data only}
- Ascher‐Walsh C, Burke B, Baxi L. Outpatient management of prolonged pregnancy with misoprostol (MP): a randomized, double‐blind placebo controlled study, prelim. data. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S20. [Google Scholar]
Bell 1993 {published data only}
- Bell RJ, Permezel M, MacLennan, Hughes C, Healy D, Brennecke S. A randomized, double‐blind controlled trial of the safety of vaginal recombinant human relaxin for cervical ripening. Obstetrics & Gynecology 1993;82:328‐33. [PubMed] [Google Scholar]
Berghella 1996 {published data only}
- Berghella V, Mickens R. Stripping of membranes as a safe method to reduce prolonged pregnancies. XIV World Congress of Gynecology and Obstetrics (FIGO);1994 Sept 26‐30; Montreal, Canada. 1994:PO34.16.
- Berghella V, Rogers RA, Lescale K. Stripping of membranes as a safe method to reduce prolonged pregnancies. Obstetrics & Gynecology 1996;87:927‐31. [DOI] [PubMed] [Google Scholar]
Boulvain 1998 {published data only}
- Boulvain M, Fraser WD, Marcoux S, Fontaine J‐Y, Bazin S, Pinault J‐J, et al. Does sweeping of the membranes reduce the need for formal induction of labour? A randomised controlled trial. British Journal of Obstetrics and Gynaecology 1998;105:34‐40. [DOI] [PubMed] [Google Scholar]
Buttino 1990 {published data only}
- Buttino L, Garite T. Intracervical prostaglandin in postdate pregnancy. Journal of Reproductive Medicine 1990;35(2):155‐8. [PubMed] [Google Scholar]
Cardozo 1986 {published data only}
- Cardozo L, Fysh J, Pearce JM. Prolonged pregnancy: the management debate. BMJ 1986;293:1059‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardozo L, Pearce JM, Fysh J. Conservative management of post‐maturity. Journal of Obstetrics and Gynaecology of the British Commonwealth 1983;4:69‐72. [Google Scholar]
- Pearce JM, Cardozo L. Prolonged pregnancy: results of supplemental analysis. BMJ 1988;297:715‐7. [Google Scholar]
Conway 2000 {published data only}
- Conway DL, Groth S, Adkins WB, Langer O. Management of isolated oligohydramnios in the term pregnancy: a randomized clinical trial. American Journal of Obstetrics and Gynecology 2000;182:S21. [Google Scholar]
Damania 1992 {published data only}
- Damania KK, Natu U, Mhatre PN, Mataliya M, Mehta AC, Daftary SN. Evaluation of two methods employed for cervical ripening. Journal of Postgraduate Medicine 1992;38(2):58‐9. [PubMed] [Google Scholar]
Dare 2002 {published data only}
- Dare FO, Oboro VO. The role of membrane stripping in prevention of post‐term pregnancy: a randomised clinical trial in Ile‐Ife, Nigeria. Journal of Obstetrics and Gynaecology 2002;22(3):283‐6. [DOI] [PubMed] [Google Scholar]
de Aquino 2003 {published data only}
- Aquino MMA, Cecatti JG. Misoprostol versus oxytocin for labour induction in term and post‐term pregnancy: randomized controlled trial. Sao Paulo Medical Journal 2003;121:102‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doany 1997 {published data only}
- Doany W. Outpatient management of postdate pregnancy with intravaginal prostaglandin E2 and membrane stripping. American Journal of Obstetrics and Gynecology 1997;174(1 Pt 2):351. [DOI] [PubMed] [Google Scholar]
- Doany W, McCarty J. Outpatient management of the uncomplicated postdate pregnancy with intravaginal prostaglandin E2 gel and membrane stripping. Journal of Maternal‐Fetal Medicine 1997;6:71‐8. [DOI] [PubMed] [Google Scholar]
Dunn 1989 {published data only}
- Dunn PA, Rogers D, Halford K. Transcutaneous electrical nerve stimulation at acupuncture points in the induction of uterine contractions. Obstetrics & Gynecology 1989;73:286‐90. [PubMed] [Google Scholar]
Elliott 1984 {published data only}
- Elliott JP, Flaherty JF. The use of breast stimulation to prevent postdate pregnancy. American Journal of Obstetrics and Gynecology 1984;149:628‐32. [DOI] [PubMed] [Google Scholar]
El‐Torkey 1992 {published data only}
- El‐Torkey M, Grant JM. Sweeping of the membranes is an effective method of induction of labour in prolonged pregnancy: a report of a randomized trial. British Journal of Obstetrics and Gynaecology 1992;99:455‐8. [DOI] [PubMed] [Google Scholar]
Evans 1983 {published data only}
- Evans MI, Dougan MB, Moawad AH, Evans WJ, Bryant‐Greenwood GD, Greenwood FC. Ripening of the human cervix with porcine ovarian relaxin. American Journal of Obstetrics and Gynecology 1983;147:410‐4. [DOI] [PubMed] [Google Scholar]
Frass 2011 {published data only}
- Frass KA, Shuaib AA, Al‐Harazi AH. Misoprostol for induction of labor in women with severe preeclampsia at or near term. Saudi Medical Journal 2011;32(7):679‐84. [PubMed] [Google Scholar]
Garry 2000 {published data only}
- Garry D, Figueroa R, Guillaume J, Cucco V. Use of castor oil in pregnancies at term. Alternative Therapies 2000;6(1):77‐9. [PubMed] [Google Scholar]
Giacalone 1998 {published data only}
- Giacalone PL, Targosz V, Laffargue F, Boog G, Faure JM. Cervical ripening with mifepristone before labor induction. Obstetrics & Gynecology 1998;92(4 Pt 1):487‐92. [DOI] [PubMed] [Google Scholar]
Gregson 2015 {published data only}
- Gregson S, Tiran D, Absalom J, Older L, Bassett P. Acupressure for inducing labour for nulliparous women with post‐dates pregnancy. Complementary Therapies in Clinical Practice 2015;21(4):257‐61. [DOI] [PubMed] [Google Scholar]
Hage 1993 {published data only}
- Hage P, Shawi J, Zarou D, Fleisher J. Double blind randomized trial to evaluate the role of outpatient use of PGE2 in cervical ripening. American Journal of Obstetrics and Gynecology 1993;168:430. [Google Scholar]
Heden 1991 {published data only}
- Heden L, Ingemarsson I, Ahlstrom H, Solum T. Induction of labor versus conservative management in prolonged pregnancy: controlled study. International Journal of Feto‐Maternal Medicine 1991;4(4):148‐52. [Google Scholar]
Hernandez‐Castro 2008 {published data only}
- Hernandez‐Castro F, Alvarez‐Chavez LD, Martinez‐Gaytan V, Cortes‐Flores R. Ambulatory treatment of prolonged pregnancy with prostaglandin E2 gel [Embarazo de 41 semanas o mayor. Manejo ambulatorio con gel de prostaglandina E2]. Revista Medica del Instituto Mexicano del Seguro Social 2008;46(2):191‐4. [PubMed] [Google Scholar]
Imsuwan 1999 {published data only}
- Imsuwan Y, Tanapat Y. Reduction of pregnancy with gestational age more than 41 weeks by membrane stripping to induce labor: a randomized controlled clinical trial. Thai Journal of Obstetrics and Gynaecology 1999;11(4):267. [Google Scholar]
Ingemarsson 1987 {published data only}
- Ingemarsson I, Heden L, Montan S, Sjoberg NO. Effect of intracervical prostaglandin gel in postterm women. Personal communication 1987.
Iqbal 2004 {published data only}
- Iqbal S. Management of prolonged pregnancy. JCPSP, Journal of the College of Physicians & Surgeons, Pakistan 2004;14(5):274‐7. [PubMed] [Google Scholar]
Jenssen 1977 {published data only}
- Jenssen H, Wright PB. The effect of dexamethasone therapy in prolonged pregnancy. Acta Obstetricia et Gynecologica Scandinavica 1977;56:467‐73. [DOI] [PubMed] [Google Scholar]
Kadar 1990 {published data only}
- Kadar N, Tapp A, Wong A. The influence of nipple stimulation at term on the duration of pregnancy. Journal of Perinatology 1990;10(2):164‐6. [PubMed] [Google Scholar]
Katz 1983 {published data only}
- Katz Z, Yemini M, Lancet M, Mogilner BM, Ben‐Hur H, Caspi B. Non‐aggressive management of post‐date pregnancies. European Journal of Obstetrics & Gynecology and Reproductive Biology 1983;15:71‐9. [DOI] [PubMed] [Google Scholar]
Kipikasa 2005 {published data only}
- Kipikasa JH, Adair CD, Williamson J, Breen JM, Medford LK, Sanchez‐Ramos L. Use of misoprostol on an outpatient basis for postdate pregnancy. International Journal of Gynecology & Obstetrics 2005;88:108‐11. [DOI] [PubMed] [Google Scholar]
Klopper 1969 {published data only}
- Klopper AI, Dennis KJ, Farr V. Effect of intra‐amniotic oestriol sulphate on uterine contractions. BMJ 1969;786(2):786‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knox 1979 {published data only}
- Knox GE, Huddleston JF, Flowers CE. Management of prolonged pregnancy: results of a prospective randomized trial. American Journal of Obstetrics and Gynecology 1979;134:376‐84. [DOI] [PubMed] [Google Scholar]
Lee 1997 {published data only}
- Lee HY. A randomised double‐blind study of vaginal misoprostol vs dinoprostone for cervical ripening and labour induction in prolonged pregnancy. Singapore Medical Journal 1997;38(7):292‐4. [PubMed] [Google Scholar]
Lemancewicz 1999 {published data only}
- Lemancewicz A, Urban R, Skotnicki MZ, Karpiuk A, Urban J. Uterine and fetal Doppler flow changes after misoprostol and oxytocin therapy for induction of labor in post‐term pregnancies. International Journal of Gynecology & Obstetrics 1999;67:139‐45. [DOI] [PubMed] [Google Scholar]
Lien 1998 {published data only}
- Lien JM, Morgan MA, Garite TJ, Kennedy KA, Sassoon DA, Freeman RK. Antepartum cervical ripening: applying prostaglandin E2 gel in conjunction with scheduled nonstress tests in postdate pregnancies. American Journal of Obstetrics and Gynecology 1998;179:453‐8. [DOI] [PubMed] [Google Scholar]
Lyons 2001 {published data only}
- Lyons C, Rumney P, Huang W, Morrison E, Thomas S, Nageotte M, et al. Outpatient cervical ripening with oral misoprostol post‐term: induction rates decreased. American Journal of Obstetrics and Gynecology 2001;184(1):S116. [Google Scholar]
Magann 1998 {published data only}
- Magann EF, Chauhan SP, Nevils BG, McNamara MF, Kinsella MJ, Morrison JC. Management of pregnancies beyond forty‐one weeks' gestation with an unfavorable cervix. American Journal of Obstetrics and Gynecology 1998;178:1279‐87. [DOI] [PubMed] [Google Scholar]
Magann 1999 {published data only}
- Magann EF, Chauhan SP, McNamara MF, Bass JD, Estes CM, Morrison JC. Membrane sweeping versus dinoprostone vaginal insert in the management of pregnancies beyond 41 weeks with an unfavorable cervix. Journal of Perinatology 1999;19(2):88‐91. [DOI] [PubMed] [Google Scholar]
Mancuso 1998 {published data only}
- Mancuso S, Ferrazzani S, Carolis S, Carducci B, Santis L, Caruso A. Term and postterm low‐risk pregnancies: management schemes for the reduction of high rates of cesarean section. Minerva Ginecologica 1998;48:95‐8. [PubMed] [Google Scholar]
Meydanli 2003 {published data only}
- Meydanli MM, Caliskan E, Burak F, Narin MA, Atmaca R. Labor induction post‐term with 25 micrograms vs. 50 micrograms of intravaginal misoprostol. International Journal of Gynecology & Obstetrics 2003;81:249‐55. [DOI] [PubMed] [Google Scholar]
Misra 1994 {published data only}
- Misra M, Vavre S. Labour induction with intracervical prostaglandin E2 gel and intravenous oxytocin in women with a very unfavourable cervix. Australia and New Zealand Journal of Obstetrics and Gynaecology 1994;34(5):511‐5. [Google Scholar]
Müller 1995 {published data only}
- Müller T, Rempen A. Comparison of 0,5 mg PG‐E2‐Intracervical‐gel versus 3 mg PG‐E2‐vaginal tablet for the induction of labour [Geburtseinleitung mit Prostaglandinen: 0,5 mg PG‐E2‐Intrazervikalgel versus 3 mg PG‐E2‐vaginaltablette]. Zeitschrift fur Geburtshilfe und Neonatologie 1995;199:30‐4. [PubMed] [Google Scholar]
Neri 2014 {published data only}
- Neri I, Monari F, Salvioli C, Facchinetti F. Acupuncture in post‐date pregnancy: a pilot study. Journal of Maternal‐Fetal & Neonatal Medicine 2014;27(9):874‐8. [DOI] [PubMed] [Google Scholar]
Newman 1997 {published data only}
- Newman M, Newman R. Multiple‐dose PGE2 cervical ripening on an outpatient basis: safety and efficacy. American Journal of Obstetrics and Gynecology 1997;176:S112. [Google Scholar]
Nicholson 2008 {published data only}
- Nicholson J, Caughey A, Parry S, Rosen S, Evans A, Macones G. Prospective randomized trial of the active management of risk in pregnancy at term: improved birth outcomes from prostaglandin‐assisted preventive labor induction. American Journal of Obstetrics and Gynecology 2007;197(6 Suppl 1):S37, Abstract no: 84. [Google Scholar]
- Nicholson JM, Parry S, Caughey AB, Rosen S, Keen A, Macones GA. The impact of the active management of risk in pregnancy at term on birth outcomes: a randomized clinical trial. American Journal of Obstetrics & Gynecology 2008;198(5):511.e1‐511.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pri‐Paz SM. Active management of risk in pregnancy at term to reduce rate of cesarean deliveries (AMOR IPAT). ClinicalTrials.gov (http://clinicaltrials.gov) (accessed 19 February 2008).
Ohel 1996 {published data only}
- Ohel G, Rahav D, Rothbart H, Ruach M. Randomised trial of outpatient induction of labor with vaginal PGE2 at 40‐41 weeks of gestation versus expectant management. Archives of Gynecology and Obstetrics 1996;258:109‐12. [DOI] [PubMed] [Google Scholar]
Papageorgiou 1992 {published data only}
- Papageorgiou I, Tsionou C, Minaretzis D, Michalas S, Aravantinos D. Labor characteristics of uncomplicated prolonged pregnancies after induction with intracervical prostaglandin E2 gel versus intravenous oxytocin. Gynecologic and Obstetric Investigation 1992;34:92‐6. [DOI] [PubMed] [Google Scholar]
Paul 1988 {published data only}
- Paul R, Romero R. Clinical trial of induction versus expectant management in postterm pregnancy. Personal communication 1988.
Rayburn 1988 {published data only}
- Rayburn W, Gosen R, Ramadei C, Woods R, Scott J. Outpatient cervical ripening with prostaglandin E2 gel in uncomplicated postdate pregnancies. American Journal of Obstetrics and Gynecology 1988;158:1417‐23. [DOI] [PubMed] [Google Scholar]
Rayburn 1999 {published data only}
- Rayburn WF, Gittens LN, Lucas MJ, Gall SA, Martin ME. Weekly administration of prostaglandin E2 gel compared with expectant management in women with previous cesareans. Obstetrics & Gynecology 1999;94:250‐4. [DOI] [PubMed] [Google Scholar]
Rijnders 2011 {published data only}
- ISRCTN47736435. Costs and effects of amniotomy at home for induction of post term pregnancy. isrctn.com/ISRCTN47736435 (first received: 9 January 2006).
- Rijnders MEB. A randomised controlled trial of amniotomy at home for induction between 292 and 294 days gestation. Interventions in Midwife led care in the Netherlands to Achieve Optimal Birth Outcomes: Effects and Women's Experiences. Amsterdam: University of Amsterdam, 2011. [ISRCTN47736435] [Google Scholar]
- Rijnders MEB. Costs and effects of amniotomy at home for induction of post term pregnancy. trialregister.nl/trialreg/admin/rctview.asp?TC=504 (first received 13 December 2005).
Roberts 1986 {published data only}
- Roberts WE, North DH, Speed JE, Martin JN, Palmer SM, Morrison JC. Comparative study of prostaglandin, laminaria, and minidose oxytocin for ripening of the unfavorable cervix prior to induction of labor. Journal of Perinatology 1986;6:16‐9. [Google Scholar]
Satin 1991 {published data only}
- Satin AJ, Hankins GDV, Yeomans ER. A prospective study of two dosing regimens of oxytocin for the induction of labor in patients with unfavorable cervices. American Journal of Obstetrics and Gynecology 1991;165:980‐4. [DOI] [PubMed] [Google Scholar]
- Satin AJ, Hankins GDV, Yeomans ER. A randomized study of two dosing regimens of oxytocin for the induction of patients with an unfavorable cervix. American Journal of Obstetrics and Gynecology 1991;164:307. [DOI] [PubMed] [Google Scholar]
Sawai 1991 {published data only}
- Sawai SK, Williams MC, O'Brien WF, Angel JL, Mastrogiannis DS, Johnson L. Sequential outpatient application of intravaginal prostaglandin E2 gel in the management of postdates pregnancies. Obstetrics & Gynecology 1991;78:19‐23. [PubMed] [Google Scholar]
Sawai 1994 {published data only}
- Sawai SK, O'Brien WF, Mastrogiannis DS, Krammer J, Mastry MG, Porter GW. Patient‐administered outpatient intravaginal prostaglandin E2 suppositories in post‐date pregnancies: a double‐blind, randomized, placebo‐controlled study. Obstetrics & Gynecology 1994;84(5):807‐10. [PubMed] [Google Scholar]
- Sawai SK, O'Brien WF, Mastrogiannis MS, Mastry MG, Porter GW, Johnson L. Outpatient prostaglandin E2 suppositories in postdates pregnancies. American Journal of Obstetrics and Gynecology 1992;166(1 Pt 2):400. [Google Scholar]
Stenlund 1999 {published data only}
- Stenlund PM, Bygdeman M, Ekman G. Induction of labor with mifepristone (RU 486). A randomized double‐blind study in post‐term pregnant women with unripe cervices. Acta Obstetricia et Gynecologica Scandinavica Supplement 1994;73(161):FP50. [PubMed] [Google Scholar]
- Stenlund PM, Ekman G, Aedo AR, Bygdeman M. Induction of labor with mifepristone: a randomized, double‐blind study versus placebo. Acta Obstetrica et Gynecologica Scandinavica 1999;78:793‐8. [PubMed] [Google Scholar]
Su 1996 {published data only}
- Su H, Li E, Weng L. Clinical observation on mifepristone for induction of term labor. Chinese Journal of Obstetrics & Gynecology 1996;31:676‐80. [PubMed] [Google Scholar]
Surbek 1997 {published data only}
- Surbek DV, Boesiger H, Hoesli L, Pavu N, Holzgreve W. Cervical priming and labor induction with intravaginal misoprostol versus PGE2: a double‐blind randomized trial. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S112. [DOI] [PubMed] [Google Scholar]
Suzuki 1999 {published data only}
- Suzuki S, Otsubo Y, Sawa R, Yoneyama Y, Araki T. Clinical trial of induction of labor versus expectant management in twin pregnancy. Gynecologic and Obstetric Investigation 1999;49:24‐7. [DOI] [PubMed] [Google Scholar]
Williams 1990 {published data only}
- Williams MG, O'Brien WF, Sawai SK, Knuppel RA. Outpatient cervical ripening in the postdates pregnancy. 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 January 23‐27; Houston, Texas, USA. 1990:533.
Wing 2000 {published data only}
- Wing DA, Fassett MJ, Mishell DR. Mifepristone for preinduction cervical ripening beyond 41 weeks' gestation: a randomized controlled trial. Obstetrics & Gynecology 2000;96(4):543‐8. [DOI] [PubMed] [Google Scholar]
Wong 2002 {published data only}
- Wong SF, Hui SK, Choi H, Ho LC. Does sweeping of membranes beyond 40 weeks reduce the need for formal induction of labour?. BJOG: an international journal of obstetrics and gynaecology 2002;109:632‐6. [DOI] [PubMed] [Google Scholar]
Ziaei 2003 {published data only}
- Ziaei S, Rosebehani N, Kazeminejad A, Zafarghandi S. The effects of intramuscular administration of corticosteroids on the induction of parturition. Journal of Perinatal Medicine 2003;31:134‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Benito Reyes 2010 {published data only}
- Benito Reyes V, Hurtado Mendoza R, Rodriguez Rodriguez F, Reyes Suarez D, Alvarez Leon EE, Garcia Hernandez JA. Elective termination versus expectant management in prolonged pregnancy: A prospective study of 200 pregnant women [Finalizacion electiva versus manejo expectante en el control de la gestacion prolongada: estudio prospectivo de 200 gestantes]. Progresos de Obstetricia y Ginecologia 2010;53(11):446‐53. [Google Scholar]
Harrington 2003 {published data only}
- Harrington K, ISRCTN74323479. What shall we do with the postdates woman who is not in labour?. isrctn.com/ISRCTN74323479 (first received 12 September 2003).
References to ongoing studies
Elden 2016 {published data only}
- Elden H. Induction of labor at 41 completed gestational weeks versus expectant management and induction at 42 completed gestational weeks. isrctn.com/ISRCTN26113652 (first received 21 March 2015).
- Elden H, Hagberg H, Wessberg A, Sengpiel V, Herbst A, Bullarbo M, et al. Study protocol of SWEPIS a Swedish multicentre register based randomised controlled trial to compare induction of labour at 41 completed gestational weeks versus expectant management and induction at 42 completed gestational weeks. BMC Pregnancy and Childbirth 2016;16:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Othman 2017 {published data only}
- Othman A. Induction of labour at 39 weeks or beyond in multiparous women with a favourable cervix. isrctn.com/ISRCTN15646866 (first received 27 January 2017).
Reddy 2013 {published data only}
- Reddy U. A randomized trial of induction versus expectant management (ARRIVE). clinicaltrials.gov/ct2/show/record/NCT01990612 (first received 21 November 2013).
Additional references
ACOG 2014
- ACOG. Management of late‐term and postterm pregnancies practice bulletin 146. Obstetrics and Gynecology 2014;124(2 Pt 1):390‐6. [DOI] [PubMed] [Google Scholar]
Arrowsmith 2011
- Arrowsmith S, Wray S, Quenby S. Maternal obesity and labour complications following induction of labour in prolonged pregnancy. BJOG: an international journal of obstetrics and gynaecology 2011;118(5):578‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caughey 2004
- Caughey AB, Musci TJ. Complications of term pregnancy beyond 37 weeks of gestation. Obstetrics and Gynecology 2004;103(1):57‐62. [DOI: 10.1097/01AOG.0000109216.24211] [DOI] [PubMed] [Google Scholar]
Caughey 2009
- Caughey AB, Sundaram V, Kaimal AJ, Gienger A, Cheng YW, McDonald KM, et al. Systematic review: elective induction of labor versus expectant management of pregnancy. Annals of Internal Medicine 2009;151:252‐63. [DOI] [PubMed] [Google Scholar]
Davey 2016
- Davey MA, King J. Caesarean section following induction of labour in uncomplicated first births‐ a population‐based cross‐sectional analysis of 42,950 births. BMC Pregnancy and Childbirth 2016;16:92. [DOI: 10.1186/s12884-016-0869-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heimstad 2007b
- Heimstad R, Romundstad PR, Hyett J, Mattsson LA, Salvesen KA. Women's experiences and attitudes towards expectant management and induction of labor for post‐term pregnancy. Acta Obstetricia et Gynecologica Scandinavica 2007;86:950‐6. [DOI] [PubMed] [Google Scholar]
Heimstad 2008
- Heimstad R, Romundstad PR, Salvesen KA. Induction of labour for post‐term pregnancy and risk estimates for intrauterine and perinatal death. Acta Obstetricia et Gynecologica Scandinavica 2008;87:247‐9. [DOI] [PubMed] [Google Scholar]
Heslehurst 2017
- Heslehurst N, Vieira R, Hayes L, Crowe L, Jones D, Robalino S, et al. Maternal body mass index and post‐term birth: a systematic review and meta‐analysis. Obesity Reviews 2017;18(3):293‐308. [DOI: 10.111/obr.12489] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hilder 1998
- Hilder L, Costeloe K, Thilaganathan B. Prolonged pregnancy: evaluating gestation‐specific risks of fetal and infant mortality. British Journal of Obstetrics and Gynaecology 1998;105:169‐73. [DOI] [PubMed] [Google Scholar]
Kaimal 2011
- Kaimal AJ, Little SE, Odibo AO, Stamilio DM, Grobman WA, Long EF, et al. Cost‐effectiveness of elective induction of labor at 41 weeks in nulliparous women. American Journal of Obstetrics and Gynecology 2011;204:137.e1‐9. [DOI] [PubMed] [Google Scholar]
Keirse 2010
- Keirse MJNC. Elective induction, selective deduction,and cesarean section. Birth 2010;37(3):252‐6. [DOI] [PubMed] [Google Scholar]
Mandruzzato 2010
- Mandruzzato G, Alfirevic Z, Chervenak F, Gruenebaum A, Heimstad R, Heinonen S, et al. Guidelines for the management of postterm pregnancy. Journal of Perinatal Medicine 2010;38:111‐9. [DOI] [PubMed] [Google Scholar]
Martin 2017
- Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Mathews TJ. Births: final data for 2015. National Vital Statistics Report 2017;66(1):1. [PubMed] [Google Scholar]
Middleton 2017
- Middleton P, Shepherd E, Flenady V, McBain RD, Crowther CA. Planned early birth versus expectant management (waiting) for prelabour rupture of membranes at term (37 weeks or more). Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD005302.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mishanina 2014
- Mishanina E, Rogozinska E, Thatthi T, Uddin‐Khan R, Khan K, Meads C. Use of labour induction and risk of cesarean delivery: a systematic review and meta‐analysis. Canadian Medical Association Journal 2014;186(9):665‐73. [DOI: 10.1503/cmaj.130925] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morken 2014
- Morken NH, Klungsøyr K, Skjaerven R. Perinatal mortality by gestational week and size at birth in singleton pregnancies at and beyond term: a nationwide population‐based cohort study. BMC Pregnancy and Childbirth 2014;14:172. [DOI: 10.1186/1471-2393-14-172] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2008
- National Institute for Health and Clinical Excellence. Induction of Labour Clinical Guideline. http://guidance.nice.org.uk/CG70/Guidance/pdf/English (last accessed 27 June 2017) 2008.
Olesen 2003
- Olesen AW, Westergaard JG, Olsen J. Perinatal and maternal complications related to postterm delivery: a national register‐based study, 1978‐1993. American Journal of Obstetrics and Gynecology 2003;189:222‐7. [DOI] [PubMed] [Google Scholar]
Olesen 2015
- Olesen AW, Olsen J, Zhu JL. Developmental milestones in children born post‐term in the Danish National Birth Cohort: a main research article. BJOG: an international journal of obstetrics and gynaecology 2015;122(10):1331‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2014.
Roos 2010
- Roos N, Sahlin L, Ekman‐Ordeberg G, Kilere H, Stephansson O. Maternal risk factors for postterm pregnancy and cesarean delivery following labour induction. Acta Obstetricia et Gynecologica Scandinavica 2010;89:1003‐10. [DOI] [PubMed] [Google Scholar]
Schierding 2014
- Schierding W, O'Sullivan JM, Derraik JGB, Cutfield WS. Genes and post‐term birth: late for delivery. BMC Research Notes 2014;7:720. [DOI: 10.1186/1756-0500-7-720] [DOI] [PMC free article] [PubMed] [Google Scholar]
SOGC 2008
- The Society of Obstetricians and Gynaecologists of Canada. Guidelines for the Management of Pregnancy at 41+0 to 42+0 Weeks. http://www.sogc.org/guidelines/index_e.asp#Obstetrics (accessed 3 March 2011).
Walker 2017
- Walker KF, Dritsaki M, Bugg G, Macpherson M, McCormick C, Grace N, et al. Labour induction near term for women aged 35 or over: an economic evaluation. BJOG: an International Journal of Obstetrics and Gynaecology 2017;124(6):929‐34. [DOI] [PubMed] [Google Scholar]
Wessberg 2017
- Wessberg A, Lundgren I, Elden H. Being in limbo: Women's lived experiences of pregnancy at 41 weeks of gestation and beyond ‐ A phenomenological study. BMC Pregnancy and Childbirth 2017;17(1):162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whitworth 2015
- Whitworth M, Bricker L, Mullan C. Ultrasound for fetal assessment in early pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD007058.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2011
- WHO. WHO recommendations for induction of labour. http://www.who.int/reproductivehealth/publications/maternal_perinatal_health/9789241501156/en/ [last accessed 27 june 2017] 2011.
Wolff 2016
- Wolff SL, Lorentzen I, Kaltoft AP, Schmidt H, Jeppesen MM, Maimburg RD. Has perinatal outcome improved after introduction of a guideline in favour of routine induction and increased surveillance prior to 42 weeks of gestation?: A cross‐sectional population‐based registry study. Sexual and Reproductive Healthcare 2016;10:19‐24. [DOI: 10.1016/j.srhc.2016.03.002] [DOI] [PubMed] [Google Scholar]
Wood 2014
- Wood S, Cooper S, Ross S. Does induction of labour increase the risk of caesarean section? A systematic review and meta‐analysis of trials in women with intact membranes. BJOG: an international journal of obstetrics and gynaecology 2014;121(6):674‐85. [DOI: 10.1111/1471-0528] [DOI] [PubMed] [Google Scholar]
Zeitlin 2007
- Zeitlin J, Blondel B, Alexander S, Bréart G and the PERISTAT Group. Variation in rates of postterm birth in Europe: reality or artefact?. BJOG: an international journal of obstetrics and gynaecology 2007;114:1097‐103. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gülmezoglu 2004
- Gülmezoglu AM, Crowther CA. Induction of labour for improving birth outcomes for women at or beyond term. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004945] [DOI] [PubMed] [Google Scholar]
Gülmezoglu 2006
- Gülmezoglu AM, Crowther CA, Middleton P. Interventions for preventing or improving the outcome of delivery at or beyond term. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD004945.pub2] [DOI] [Google Scholar]
Gülmezoglu 2012
- Gülmezoglu AM, Crowther CA, Middleton P, Heatley E. Induction of labour for improving birth outcomes for women at or beyond term. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD004945.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


