Abstract
Objective:
Factor XI (FXI) contributes to thrombotic disease while playing a limited role in normal hemostasis. We generated a unique, humanized anti-FXI antibody, AB023, which blocks factor XIIa (FXIIa)-mediated FXI activation without inhibiting FXI activation by thrombin or the procoagulant function of FXIa. We sought to confirm the antithrombotic activity of AB023 in a baboon thrombosis model and to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics in healthy adult subjects.
Approach and Results:
In a primate model of acute vascular graft thrombosis, AB023 reduced platelet and fibrin accumulation within the grafts by >75%. To evaluate the safety of AB023, we performed a first-in-human study in healthy adult volunteers without any serious adverse events. Overall, 10 of 21 (48%) subjects experienced 20 treatment-emergent adverse events, with 7 of 16 (44%) subjects following active treatment and 3 of 5 (60%) subjects following placebo. AB023 did not increase bleeding or prothrombin times. Anticoagulation was verified by a saturable ~2-fold prolongation of the partial thromboplastin time for over one month after the highest dose.
Conclusions:
AB023, which inhibits contact activation-initiated blood coagulation in vitro and experimental thrombus formation in primates, produced a dose-dependent duration of limited anticoagulation without drug-related adverse effects in a phase 1 trial. When put in context with earlier observations suggesting that FXI contributes to venous thromboembolism and cardiovascular disease, while contributing minimally to hemostasis, our data further justify clinical evaluation of AB023 in conditions where contact-initiated FXI activation is suspected to have a pathogenic role.
Keywords: factor XI, thrombosis, anticoagulation, hemostasis
Graphical Abstract

Introduction
Cardiovascular disease (CVD) and venous thromboembolism (VTE) remain leading causes of death in the United States and other developed countries.1 Primary prevention, acute treatment and secondary preventative strategies for CVD and VTE such as anticoagulation and antiplatelet therapy are effective, but universally increase the risk of bleeding. With the widespread adoption of the direct oral anticoagulants (DOACs) for a large majority of VTE indications over the last several years, bleeding rates have been reduced, though not abolished; indeed, annual major bleeding rates with DOACs are reported to be 4%, with mortality rates reaching 25% after intracranial hemorrhage.2 In addition, breakthrough thrombosis remains problematic with DOACs as well as other forms of anticoagulation, and many clinical scenarios such as intravascular devices (e.g. hemodialysis, mechanical cardiac valves, ventricular assist devices or VAD) remain areas of critically unmet medical need. For example, device failure (16.2%) and stroke (29.7%) are common events with VADs despite anticoagulation with a vitamin K antagonist.3 In other settings such as sepsis, novel forms of anticoagulation, while initially promising, failed to show a convincing benefit in phase 3 trials.4, 5
Given the numerous clinical indications for anticoagulation, development of novel anticoagulants with the potential to outperform the current therapeutic standard has become a high research priority. Previously ignored by modern anticoagulation strategies, the contact activation system, including the intrinsic pathway of thrombin generation, has garnered increasing attention. Mounting pre-clinical and clinical evidence supports the role of contact factors XI (FXI) and XII (FXII) in promoting pathologic thrombosis and inflammation, and establishes these enzymes as promising targets for the development of drugs with both anticoagulant and anti-inflammatory properties, with the distinct potential benefit of leaving bleeding risk unaffected. Congenital FXII deficiency, for instance, appears to have no associated phenotypic bleeding diathesis; congenital FXI deficiency (hemophilia C) confers a mild bleeding risk that generally manifests only in the face of significant hemostatic challenge.6 Epidemiologic data suggest that individuals deficient in FXI are also significantly protected against venous and arterial thromboembolic diseases.7, 8 This association was later corroborated by the results of a prospective clinical trial using a FXI antisense oligonucleotide (ASO) which showed that reducing FXI levels provided superior prophylaxis against surgically-provoked VTE, as well as a lower incidence of bleeding, when compared to traditional anticoagulation.9
Our group developed a novel murine IgG2b monoclonal antibody (14E11) targeting the apple 2 (A2) domain of FXI. This antibody inhibits the activation of FXI by activated FXII (FXIIa) as well as inhibiting reciprocal FXII activation by FXIa, without inhibiting the activation of FIX by FXIa or hemostatic FXI activation by thrombin.10 The antithrombotic and anti-inflammatory effects of 14E11, administered intravenously or subcutaneously, were demonstrated in multiple in vivo models including device-associated thrombosis, arterial thrombosis, and polymicrobial or listeria sepsis.10–14 This work culminated in the development of AB023 (also referred to elsewhere as xisomab 3G3, or 3G3), a recombinant, humanized anti-FXI A2 domain antibody with ~70% homology to the 14E11 antibody, which we evaluated in preclinical safety and efficacy studies as well as the herein described phase 1 clinical trial. The aims of this first‐in‐human, dose‐escalation study were to describe the safety, tolerability, pharmacokinetics (PK), pharmacodynamics (PD) and immunogenicity of a single ascending, intravenous bolus dose of AB023 in healthy volunteers as compared to placebo.
Methods
Because of the sensitive nature of the data collected for this study, requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to the corresponding author at Aronora, Inc.
The AB023 phase 1 clinical study was preregistered prior to conducting research under the unique Clinicaltrials.gov identifier, NCT03097341.
Please see the Major Resources Table in the Supplemental Material.
AB023 humanization and manufacturing cell line development
The antibody AB023 was generated by CDR-grafting technology at Antitope, Ltd (Cambridge, UK). The variable region genes from the monoclonal mouse anti-human FXI antibody 14E11 were sequenced to design a series of humanized antibody variants. Humanized variable region genes were designed based on human germline sequences with the closest homology to the murine sequences and constructed by gene synthesis. These were then cloned into separate vectors containing human IgG4 genes. The humanized antibody was stably expressed and tested for binding to human FXI by competition ELISA against biotinylated 14E11. The lead candidate antibody was chosen based on the competition binding ELISA and activity in the in vitro activated partial thromboplastin (aPTT) assay, and a stable manufacturing cell line was generated at Antitope, Ltd (Cambridge, UK) using Composite CHO™ Technology.
AB023 manufacturing
The antibody AB023 was manufactured at Bayer Healthcare LLC, Berkeley, CA. Briefly, the cells used to inoculate production bioreactor originated from the AB023 master cell bank. Cells were cultured using a fed-batch process and harvested at 14 days. Purification consisted of three chromatography column steps, two specific viral clearance steps, followed by concentration and diafiltration. After final formulation in a buffer containing histidine, arginine-HCl, methionine, sucrose, and polysorbate 80, the AB023 was filtered into the drug substance containers and stored frozen until fill/finishing as a lyophilized drug product (15 mg/mL after reconstitution).
Binding affinity for FXI and FXIa
The antibody AB023 was biotinylated using the EZ-Link™ Sulfo-NHS-Biotinylation Kit (Thermo Scientific) as per instructions. FXI or FXIa (2 μg/mL, 100 μL/well) in 50 mM Na2CO3 pH 9.6 was incubated overnight at 4°C in Immulon 2HB microtiter plates (Thermo Scientific). Wells were blocked with 150 μL phosphate buffered saline (PBS) with 2% BSA for one hour (h) at RT. 100 μL biotinylated AB023 (0.7 pM to 6.7 μM) in 90 mM HEPES pH 7.2, 100 mM NaCl, 0.1% BSA, 0.1% Tween-20 (HBS) was added, and incubated for 90 min at room temperature (RT). After washing with PBS-0.1% Tween-20 (PBS-T), 100 μL streptavidin-HRP (Thermo Scientific, 1:8000 dilution in HBS) was added, with incubation at RT for 90 min. After washing, 100 μL substrate solution (12 mL 30 mM citric acid, 100 mM Na2HPO4 pH 5.0, 1 o-phenylenediamine dihydrochloride (OPD) tablet, 12 μL 30% H2O2) was added. Reactions were stopped after 10 min with 50 μL 2.5 M H2SO4. Absorbance at 495 nm was measured on a SpectroMax 340 microplate reader (Molecular Devices). The data was analyzed by non-linear regression analysis and the apparent Kd was calculated as the concentration of AB023 needed to achieve half-max binding at equilibrium using GraphPad Prism (v. 5.0).
Western blotting
Biotinylated AB023 (10 μg/mL) was the primary antibody in Western blots of recombinant FXI with individual apple domains replaced with corresponding domains from human prekallikrein (PK), or individual FXI apple domains attached to recombinant tissue plasminogen activator (tPA) as previously described.10 Detection was performed with streptavidin-HRP and chemiluminescence.
In vitro activation of FXI by FXIIa
FXI (30 nM) was incubated with 0.5 nM α-FXIIa and dextran sulfate (0.1 μg/mL) at 37°C in 25 mM HEPES, pH 7.4, 150 mM NaCl, and 0.1% BSA in the presence or absence of AB023 (0 nM to 300 nM). After 30 min of incubation, samples were removed and quenched with polybrene (6 μg/mL) to neutralize dextran sulfate and CTI (50 μg/mL) to inactivate FXIIa, after which the generation of FXIa was quantified by measuring rates of S-2366 hydrolysis at 405nm. Rates of S-2366 hydrolysis were converted to FXIa concentrations using a standard curve.
In vitro activation of FXI by thrombin
FXI (30 nM) was incubated with 2.5 nM α-thrombin and dextran sulfate (0.1 μg/mL) at 37°C in 25 mM HEPES, pH 7.4, 150 mM NaCl, and 0.1% BSA in the presence or absence of AB023 (300 nM). After 0, 5, 15, 30, or 60 minutes incubation, the samples were removed and quenched with polybrene (6 μg/mL) to neutralize dextran sulfate and hirudin (10 U/mL) to inactivate thrombin, after which the generation of FXIa was quantified by measuring rates of S-2366 hydrolysis at 405nm. Rates of S-2366 hydrolysis were converted to FXIa concentrations using a standard curve.
Coagulation assays
Activated partial thromboplastin time (aPTT) assay:
Pooled plasmas from human, baboon, rat or cynomolgus monkey (90 μL, from three individual subjects) anticoagulated with 0.38% sodium citrate were mixed with 10 μL of AB023 (0.183 to 1500 μg/mL) or control (PBS) and allowed to incubate at room temperature for 5 minutes. 40 μL of the plasma/antibody mixture was then incubated with 40 μL of aPTT reagent (SynthASil, #0020006800, Instrumentation Laboratory, Bedford, MA) for 3 min at 37°C. After incubation, 40 μL of CaCl2 was added and time to clot was determined on a KC4 ™Analyzer (Tcoag, Bray, Ireland). Each sample was assayed in duplicate. For each species tested, the aPTT data was expressed as fold change of baseline and plotted against the log10 concentration of AB023.
Prothrombin time (PT) assay:
PT of plasma samples was measured using Dade Innovin (Siemens Healthcare Diagnostics, Berlin, Germany), reconstituted according to the manufacturer’s recommendation prior to sample measurement. To measure PT, 40 μL of 0.32% sodium citrate anticoagulated plasma collected from baboons was incubated at 37°C for 3 min and 80 μL of PT reagent was added. Samples were measured in duplicate using a KC4 coagulometer.
Baboon vascular graft thrombosis model
All animal experiments were approved by the Institutional Animal Care and Use Committee of Oregon Health & Science University. A total of 8 non-terminal experiments were performed using 4 juvenile male baboons (Papio anubis) with a surgically placed, healed, chronic exteriorized arterio-venous (AV) shunt connecting the femoral artery and vein, as described previously.15 Briefly, a collagen-coated 20 mm long, 4 mm internal diameter vascular graft segment was deployed into the AV shunt of the baboon for the duration of 90 min-long acute thrombosis experiments. Only male animals were used in these studies in accordance with the NIH policy guidance NOT-OD-15–102 for single-sex studies when using acutely scarce resources, which applies to non-human primates due to animal safety concerns. To protect the chronically implanted shunt site, the animals wear protective suits which prevent them from accessing the shunt site at all times. Female baboons have significant swelling of their sex organs during their menstruation cycles, which prohibits the use of secure protective suits. Without these suits, a catastrophic failure is imminent as the animal is then able to physically remove the shunt. Every effort (including suit redesign, birth control to reduce swelling) has been made to attempt to alleviate this risk. Unfortunately, none are able to significantly protect the female animals and therefore, only male animals were used.
Thrombus formation in the graft during perfusion was assessed by dynamic quantitative gamma camera imaging of radio-labeled autologous platelet content in the graft segment, and further assessed by measurement of retained fibrin content within the graft after termination of each experiment, as previously described in detail.15 Briefly, platelet-associated 111In radioactivity within the grafts was determined at 5 min intervals using a GE-400A-61 gamma scintillation camera. Since graft thrombi formation propagate and extend distally (downstream) from the graft over time, platelet accumulation was also measured within a 10 cm-long region immediately distal to the graft. The real-time radio imaging of platelets thus monitors thrombus growth within the thrombogenic graft (thrombus “head”), as well as the thrombus that propagates distally to the collagen segment (thrombus “tail”). Homologous 125I-labeled baboon fibrinogen incorporation into the thrombus was assessed by measuring the radioactivity using a gamma counter (Wizard-3, PerkinElmer, Shelton, CT).
First in Human Trial Design
We performed a phase 1, randomized, double-blind, placebo-controlled, single ascending bolus dose study at a single site (Celerion, Tempe, AZ) in healthy adult volunteers to test the safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of AB023. This clinical trial (clinicaltrials.gov identifier: NCT03097341) was approved by the Chesapeake Institutional Review Board prior to initiation as required by and conducted in accordance with the Declaration of Helsinki and ICH Good Clinical Practice. Written informed consent was obtained from all participants prior to enrollment in the trial.
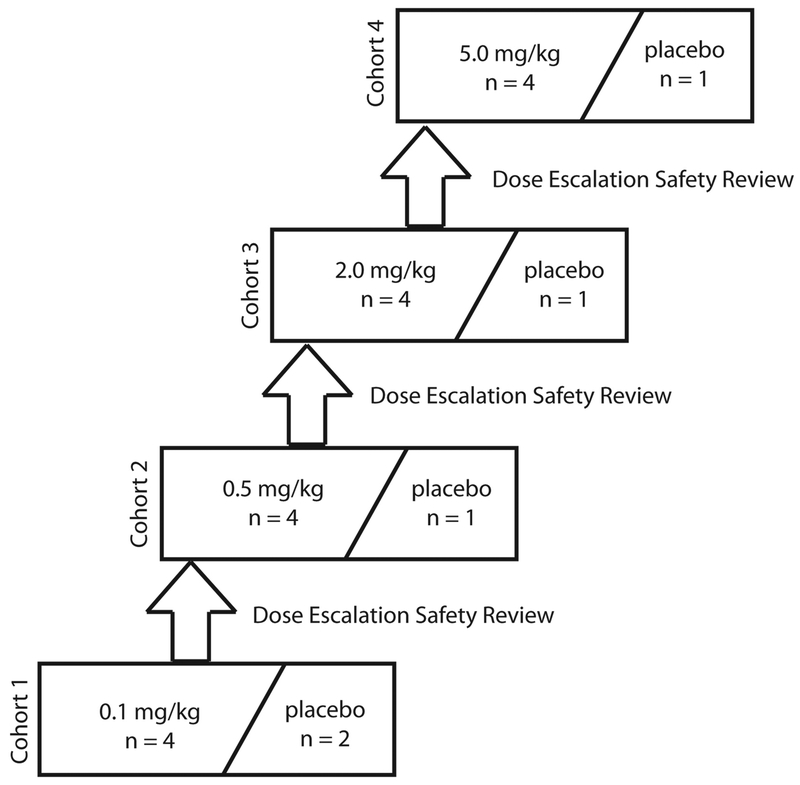
The study consisted of four ascending dose cohorts (0.1, 0.5, 2.0, 5.0 mg/kg). In each cohort, subjects were randomized to receive a single IV bolus injection of AB023 (n=4 per cohort) or placebo (n=1 per cohort, except for the 0.1 mg/kg dosing cohort in which n=2) (Figure 3). Both investigators and subjects were blinded to treatment. A data safety monitoring committee that was blinded to treatment provided oversight of this phase 1.
Figure 3: Dose escalation schedule for the AB023 first-in human phase 1 clinical trial.
Each cohort was dosed sequentially. Once subjects were screened and met eligibility criteria, they were randomized to receive either AB023 or placebo at a 4:1 ratio, except for cohort 1 where the AB023: placebo ratio was 4:2 to accommodate 2 sentinel subjects (1 active and 1 placebo). Before dose escalation to the next dose, all safety data was collected from each subject through Day 28 and reviewed by a safety review committee during a dose escalation safety review meeting.
Eligibility Criteria:
This study included healthy adults 18 to 48 years of age with a body mass index (BMI) ≥19 and ≤29.0 kg/m2 and weight between 50 and 125 kg with normal hepatic and renal function. Subjects were required to have aPTT, PT, the international normalized ratio (INR), and platelets within the limits of normal range at enrollment along with a bleeding time between 2 to 8 min. Only women of non-childbearing potential were eligible for the trial and all fertile males were required to use an approved form of birth control throughout the duration of the study.
Endpoints:
The primary endpoint of the study was the number and severity of treatment-emergent adverse events (TEAEs) following single doses of AB023 or placebo. The patients were observed for adverse events and blood samples were repeatedly drawn for laboratory analyses. Other safety parameters included PT, bleeding time (BT, Surgicutt®), clinical laboratory values, vital signs, and drug immunogenicity. Immunogenicity was evaluated from study plasma samples that were pretreated to remove endogenous FXI and an acid dissociation step to reduce interference. The samples were then assayed for anti-drug antibodies using a sandwich ELISA procedure using AB023 to capture anti-drug antibodies and biotinylated AB023 and streptavidin-HRP for detection. Secondary endpoints included drug PK and PD, the latter represented by aPTT values.
Pharmacokinetics and Pharmacodynamics:
For all subjects, blood samples for PK and PD assessment were collected at pre-dose, and at 0.083, 0.25, 0.5, 1, 3, 8, 24, 72, 120, 168, 216, 336, 504, and 672 h after the start of AB023 or placebo administration. Following the 672 h time point, sample collections for PK assessments continued every 7 days (±2 days) until aPTT values returned to the normal range or within ±10% of the baseline. Free plasma AB023 concentrations were determined using a validated ELISA method. PK endpoints included total exposure (AUC0-inf), exposure up to last measurable concentration (AUC0-t), the percent of the AUC0-inf that was extrapolated (AUC%extrap), elimination rate constant (Kel), maximum plasma concentration (Cmax), clearance (CL), half-life of elimination (T1/2), mean absorption time, and volume of distribution (Vss) at steady state. APTT was used as a PD biomarker.
Statistics:
All statistics were calculated using R (R foundation for statistical computing, Vienna, Austria, version 3.5.1) unless otherwise noted. For all tests reported here, statistical significance is defined as p<0.05. The assumptions underlying parametric tests were checked by confirming the normal distributions of model residuals using quantile-quantile plots. Equal variance assumptions were confirmed using Levene’s test.
Baboon vascular graft thrombosis model:
Numeric values are shown as mean and range in the text, while mean ± SEM are shown in the figures. Means of fibrin deposition were compared by Mann-Whitney U-test for treatment vs. control. Comparisons between AB023 and control treatment results of platelet deposition rates over time were performed by a linear mixed effects model with main effects calculated for treatment, time, and interaction, and random effects calculated for trial (package nlme version 3.1–137). Because the treatment variable had only two levels, post-hoc tests were not performed.
Phase 1 clinical trial:
The study sample size was chosen to enable appropriate safety and tolerability assessment while ensuring that a minimal number of healthy participants were exposed to the investigational drug. Descriptive statistics were used to evaluate safety endpoints. The appropriate noncompartmental PK parameters were calculated from the plasma AB023 concentration-time data using Phoenix® WinNonlin®, Version 7.0. Actual sample times were used in the calculations of the PK parameters. The calculation of the actual time for AB023 was in respect to the start of infusion time of AB023 on Day 1. The plasma concentrations of AB023 are shown as mean ± SEM concentration-time profiles are presented on a linear scale. PK concentrations and/or PK parameter descriptive statistics were generated using SAS, Version 9.3 (SAS Institute, Cary, NC, USA). Mean ± SEM aPTT and PT value-time profiles and change are presented on a linear scale. Bleeding time data were analyzed with a one-way ANOVA with treatment as a categorical variable while PT data were analyzed with a two-way repeated measures ANOVA against dosage and sample time. Bleeding time and PT data were analyzed with post-hoc Dunnett tests as appropriate.
Results:
The humanized anti-FXI antibody AB023 inhibits FXIIa activation of FXI
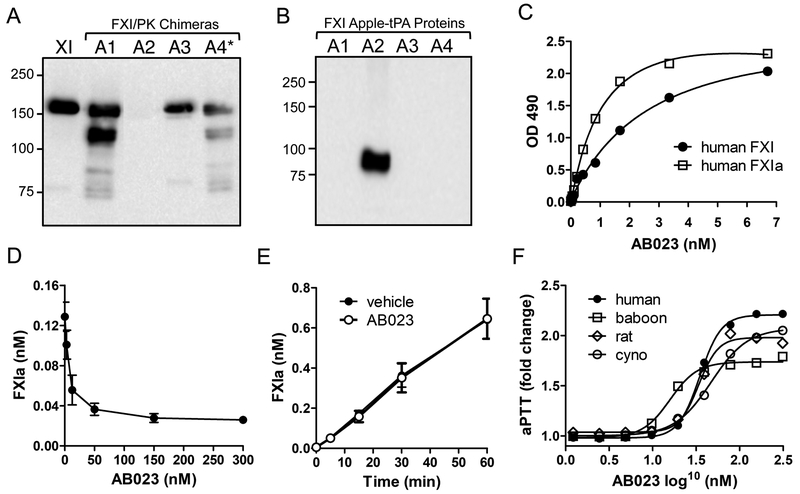
The recombinant humanized antibody, AB023, binds to the apple 2 (A2) domain of FXI and FXIa with high affinity (Kd=3.66 and 1.38 nM respectively16, Figure 1A–C) and inhibits activation of FXI by FXIIa in a concentration-dependent manner (Figure 1D) but does not inhibit thrombin-mediated activation of FXI (Figure 1E). AB023 prolonged the aPTT of plasma from normal rats, baboons, cynomolgus monkeys, and humans in a concentration-dependent manner (Figure 1F) by about 2-fold at and above saturating concentrations, which was similar to the anticoagulant effect of the murine antibody, 14E11.
Figure 1: Binding properties of AB023.
(A) Western blots of non-reducing 10% polyacrylamide gel of human FXI (lane 1) and human FXI in which the A1, A2, A3, or A4 domain has been replaced with the corresponding domain from prekallikrein (PK). (B) Western blots of non-reducing 10% polyacrylamide gel of individual human FXI apple domains (A1-A4) linked to recombinant tPA. AB023 recognizes the A2 domain of human FXI. (C) Binding of AB023 to human FXI (closed circles), human FXIa (open square). (D) AB023 concentration-dependently inhibits FXIIa activation of FXI. (E) AB023 does not prevent activation of FXI by thrombin. (F) AB023 prolongs the aPTT in a concentration dependent manner in plasma from human (black circles), baboons (open squares), cynomolgus monkeys (open circles) and rats (open diamonds). *The FXI/PKA4 chimeric protein (A4*) is a dimeric molecule created by replacing Cys326 with alanine in PK A4 domain.
AB023 is antithrombotic in a baboon model of thrombosis
The antithrombotic effects of AB023 were characterized in a well-established vascular thrombosis model performed as previously described.15, 17–19 A single 1.0 mg/kg intravenous injection of AB023 reduced platelet accumulation within collagen-coated vascular grafts (Figure 2A), and near complete inhibition of platelet accumulation was achieved in the region 10 cm immediately downstream of the graft (the “tail”) (Figure 2B) compared to vehicle control. Overall, total platelet accumulation (platelet accumulation within the thrombogenic graft plus the “tail”) was significantly reduced after pretreatment with 1.0 mg/kg AB023 (Figure 2C). Total fibrin deposition (graft + tail) was decreased after AB023 treatment (Figure 2D–F). These studies indicate that AB023, like its murine precursor, is antithrombotic in a primate acute vascular graft thrombosis model. APTT was prolonged following AB023 treatment at 30 and 60 min after injection (Table 1) and remained elevated beyond 24 h post-treatment (data not shown). No change in aPTT was observed in the control group. PT was also measured before AB023 treatment, as well as 60 min and 24 h after treatment; treatment with AB023 did not alter the PT compared to controls (Table 1).
Figure 2: AB023 reduces platelet-rich thrombus growth in a primate thrombosis model.
Effect of AB023 (1.0 mg/kg, IV) on platelet (A-C) deposition on (A) collagen-coated (4 mm diameter, 2 cm long) vascular grafts and (B) 10 cm downstream of the collagen-coated graft (“tail”). (C) Platelet deposition in both the graft + tail combined. (D-F) Fibrin deposition within the collagen graft (D), tail (E) and graft + tail combined (F) in control treated (black bar) and after AB023 treatment. Values are mean ± SEM, n = 4 experiments/ group in 4 animals. *p<0.05, **p<0.01, ***p<0.001 vs. control. Each animal underwent a control experiment followed by AB023 experiment.
Table 1:
aPTT and PT from baboon vascular thrombosis model
| aPTT (s) | |||
| Pre-study | 30 min | 60 min | |
| control | 33.2 ± 3.2 | 33.4 ± 3.2 | 32.7 ± 1.8 |
| AB023 | 78.4 ± 11.0 | 78.5 ± 12.4 | 78.7 ± 12.9 |
| PT (s) | |||
| Pre-study | 60 min | 24h | |
| control | 8.9 ± 0.1 | 8.8 ± 0.2 | - |
| AB023 | 9.2 ± 0.1 | 9.0 ± 0.4 | 9.1 ± 0.4 |
Data presented are mean ± standard deviation.
First-in-human (FIH) study
A total of 21 subjects entered the study, received the study medication, completed the study per protocol, and were included in the PD, PK and safety analyses. Patient demographics are listed in Table 2.
Table 2:
Subject disposition and basic characteristics in the AB023 phase 1 trial
| AB023 | Placebo | Total (%) | ||||
|---|---|---|---|---|---|---|
| 0.1 mg/kg | 0.5 mg/kg | 2.0 mg/kg | 5.0 mg/kg | |||
| No. of subjects included | 4 | 4 | 4 | 4 | 5 | 21 (100) |
| No. of subjects withdrawn | 0 | 0 | 0 | 0 | 0 | 0 |
| Sex (males) (females) |
1 3 |
2 2 |
3 1 |
3 1 |
3 2 |
12 (57) 9 (43) |
| Age (years) Mean (SD) |
42.8 (4.3) | 33.0 (9.8) | 32.0 (11.0) | 42.0 (2.2) | 32.2 (7.1) | 36.2 (8.4) |
| Weight (kg) Mean (SD) |
71.7 (17.6) | 68.3 (10.1) | 72.0 (13.4) | 75.7 (9.7) | 73.2 (11.6) | 72.2 (11.6) |
| BMI (kg/m2) Mean (SD) |
27.4 (2.5) | 24.2 (2.9) | 24.5 (2.8) | 25.6 (1.7) | 25.6 (2.6) | 25.5 (2.5) |
Abbreviations: BMI: body mass index; SD: tandard deviation
Safety
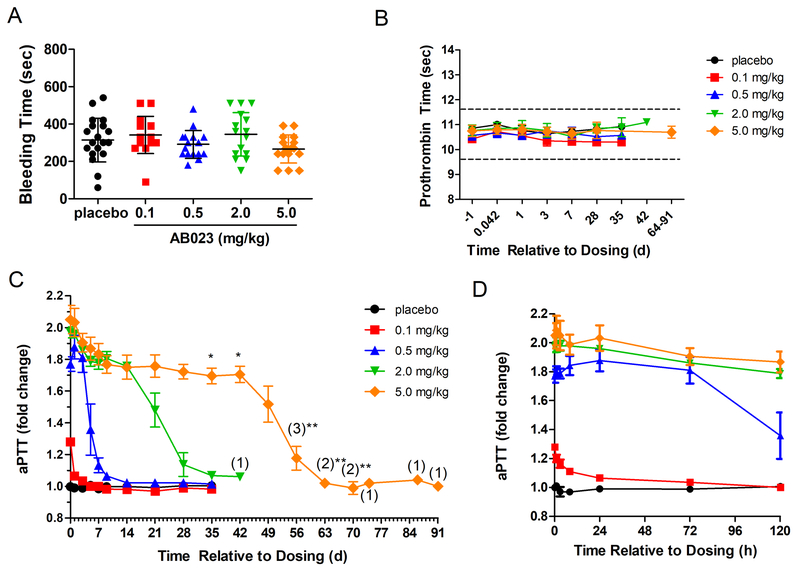
There were no serious adverse events (SAEs) experienced in this study. No subjects were removed from the study due to adverse events (AEs). Overall, 10 of 21 (48%) subjects experienced a total of 20 TEAEs in this study, with 7 of 16 (44%) subjects following active treatment and 3 of 5 (60%) subjects following placebo (Table 3). Three (19%) active-treatment subjects experienced 5 events that were suspected to be possibly treatment-related by the principal investigator (PI). The most common TEAE in this study was a productive cough, considered unrelated or unlikely treatment-related, experienced by two (13%) active-treatment subjects, with one subject receiving 0.5 mg/kg AB023 and one subject receiving 5.0 mg/kg AB023. One event of productive cough was mild (Grade 1) in severity (0.5 mg/kg AB023) and the other (5.0 mg/kg AB023) was moderate (Grade 2). Onset for these events was 35 days and 50 days post-dose, with resolution at 1 h and 9.5 days, respectively. All other TEAEs were experienced by one subject each. The majority of events (17) were mild (Grade 1) in severity and 3 were moderate (Grade 2). Of these AEs, the PI considered the following to be possibly treatment-related (treatment in parenthesis): increased diastolic BP (2.0 mg/kg AB023) which resolved spontaneously after 4.5 h, injection site ecchymosis on the inside of the left upper arm after several failed attempts to inject in the antecubital vein (0.5 mg/kg AB023), 2 events of injection site pain (0.5 mg/kg AB023), and generalized pruritus (0.5 mg/kg AB023). AB023 had no effect on mean BT or PT in any study subject in any of the examined cohorts (Figure 4A, B). Three subjects received acetaminophen during the study for resolution of events considered unrelated or unlikely treatment related. Two subjects received non-drug therapy for resolution of events considered unrelated or unlikely treatment-related. Finally, anti-drug antibodies were not detected in the plasma of any subjects. Overall, AB023 was found to be safe and well-tolerated in healthy human volunteers.
Table 3:
Summary of adverse events (AEs) following intravenous administration of AB023
| AB023 | Pooled Placebo | ||
|---|---|---|---|
| Treatment | Total | ||
| Trial Participants, n | 21 | 16 | 5 |
| Trial Participants Dosed, n (%) | 21 | 16 | 5 |
| Trial Participants with AEs, n (%) | 10 (48) | 7 (44) | 3 (60) |
| Total AEs, n | 20 | 15 | 5 |
| Mild | 17 | 12 | 5 |
| Moderate | 3 | 3 | 0 |
| Severe | 0 | 0 | 0 |
| Relationship to Study Drug, n | |||
| Unrelated | 5 | 3 | 2 |
| Unlikely | 10 | 7 | 3 |
| Possibly | 5 | 5 | 0 |
| Probably | 0 | 0 | 0 |
| Likely | 0 | 0 | 0 |
| Most Common AE, n | |||
| Productive cough | 2 | 2 | 0 |
| Possibly Treatment Related AEs, n | |||
| Diastolic blood pressure increased | 1 | 1 | 0 |
| Injection site ecchymosis | 1 | 1 | 0 |
| Injection site pain | 2 | 2 | 0 |
| Pruritus, generalized | 1 | 1 | 0 |
Figure 4: Coagulation parameters from all subjects from the AB023 phase 1, single ascending dose, randomized, placebo-controlled trial.
Four cohorts were administered a single dose of AB023 (cohort 1: 0.1 mg/kg, red squares, cohort 2: 0.5 mg/kg, blue triangles, cohort 3: 2.0 mg/kg, green inverted triangles, and cohort 4: 5.0 mg/kg, orange diamonds, n = 4 for each dose level) or placebo (all placebo-dosed subjects are grouped together (n = 5, black circles). (A) BT data from all subjects post AB023 or placebo administration. For each cohort, results from both left and right arms and 1h and 24h post-dose are combined. There was no statistical difference in BT found in any cohort compared to placebo. (B) PT time from all subjects pre- and post-dose. There was no statistical difference in PT found in any cohort compared to placebo. (C) aPTT of cohorts 1–4 for the duration of the phase 1 clinical trial in days (d) and (D) aPTT data from the first 120 hours (h) post-dose. Data represents mean ± SEM. Numbers in parentheses indicate the number of subjects at that time point. *blood sample taken within 24h of time point indicated. **blood sample taken within 48h of time point indicated.
Pharmacokinetics
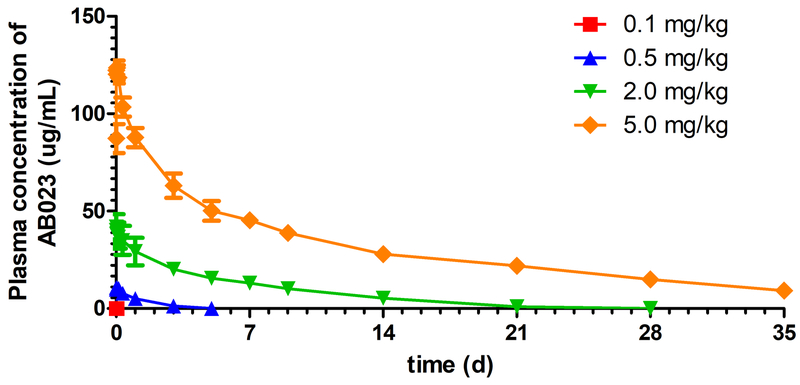
Plasma AB023 was detectable in all subjects at the time of first sampling (0.08 h post start of infusion). Plasma AB023 remained detectable in the majority of subjects up to 0.5 h and 120 h post-dose for the 0.1 and 0.5 mg/kg dose levels, respectively. Plasma AB023 remained detectable in all subjects throughout the entire sampling interval (672 h) for the 2.0 mg/kg and 5.0 mg/kg dose levels. Mean peak plasma concentrations of AB023 occurred at approximately 0.08 h for the 0.1 mg/kg and 2.0 mg/kg dose levels, 0.25 h for the 0.5 mg/kg dose level, and 1 h for the 5 mg/kg dose level. Ascending doses of AB023 increased the peak and overall AB023 exposure in a slightly more than a dose-proportional manner from the 0.5 mg/kg to 5.0 mg/kg dose levels (Figure 5).
Figure 5: Mean AB023 plasma concentrations after a single IV dose.
Plasma concentration of AB023 from all cohorts after a single injection of AB023 (cohort 1: 0.1 mg/kg, red squares, cohort 2: 0.5 mg/kg, blue triangles, cohort 3: 2.0 mg/kg, green inverted triangles, and cohort 4: 5.0 mg/kg, orange diamonds, n = 4 for each dose level. Data represents mean ± SEM.
Following administration of a single IV injection of AB023, plasma exposure (AUCs and Cmax) increased with increasing AB023 doses. Geometric mean AUC0-t, AUC0-inf, and Cmax values ranged from 57 to 28×106 ng*h/mL, 185 to 28×106 ng*h/mL, and 123 to 1.3×105 ng/mL, respectively, after administration of 0.1 mg/kg to 5.0 mg/kg dose. Median Tmax values varied between 0.08 h to 0.65 h post-dose. Arithmetic mean T1/2 values appeared to increase with increasing AB023 doses and ranged from approximately 1.3 h following the 0.1 mg/kg AB023 dose to approximately 121.5 h following the 5.0 mg/kg dose. Total plasma clearance (CL) was approximately 37.5 L/h and volume of distribution (Vss) was approximately 69.1 L for the 0.1 mg/kg dose (n=1 for both values), representing the underestimation of exposure at this dose level. Arithmetic mean CL appeared to decrease with increasing AB023 doses and ranged from approximately 0.094 L/h to 0.014 L/h following the 0.5 mg/kg to 5.0 mg/kg dose. Vss appeared to increase with increasing AB023 dose, ranging from approximately 2.5 L for the lowest to 4.3 L for the highest dose (Table 4).
Table 4:
Pharmacokinetic parameters following a single intravenous injection of AB023
| AUC0-t (ng*hr/mL) | 57.0 (46.5) [n= 3] | 361800 (21.9) [n=4] | 5540000 (23.9) [n=4] | 28120000 (11.8) [n=4] |
| AUC0-inf (ng*hr/mL) | 184.7 [n=1] | 363700 (21.8) [n=4] | 5550000 (24.0) [n=4] | 28140000 (11.8) [n=4] |
| AUC%extrap (%) | 57.3 [n=1] | 0.532 ± 0.176 [n=4] | 0.184 ± 0.072 [n=4] | 0.070 ± 0.036 [n=4] |
| Cmax (ng/mL) | 122.7 (23.3) [n=3] | 11210 (9.1) [n=4] | 42510 (12.3) [n=4] | 127200 (2.6) [n=4] |
| Tmax (hr) | 0.084 (0.08, 0.09) [n=3] | 0.649 (0.26, 3.02) [n=4] | 0.088 (0.08, 0.25) [n=4] | 0.387 (0.26, 3.00) [n=4] |
| Kel (1/hr) | 0.522 [n=1] | 0.042 ± 0.004 [n=4] | 0.011 ± 0.001 [n=4] | 0.006 ± 0.002 [n=4] |
| T1/2 (hr) | 1.33 [n=1] | 16.64 ± 1.56 [n=4] | 60.63 ± 4.45 [n=4] | 121.49 ± 40.69 [n=4] |
AUCs and Cmax values are presented as geometric mean and geometric CV%, when available.Tmax values are presented as median (minimum, maximum)Other parameters are presented as arithmetic mean (± SD), or just mean when SD is not available.
Abbreviations: AUC0-t :Area under the concentration-time curve, from time 0 to the last observed non-zero concentration (t); AUC0-inf: area under the concentration-time curve, from time 0 extrapolated to infinity; AUC%extrap: percent of AUC0-inf extrapolated; Cmax: maximum plasma concentration; CL: apparent total plasma clearance; Kel: apparent terminal elimination rate constant; T1/2: apparent terminal elimination half-life; Tmax: time to reach maximum observed concentration; Vss: total apparent volume of distribution following single IV dose
Pharmacodynamics
Individual pre-dose aPTT values ranged from 23 to 32 seconds in all subjects. Administration of AB023 produced an expected prolongation of aPTT in all subjects receiving the active compound (Figure 4C, D). Overall, the mean aPTT values reached a maximum aPTT prolongation at ≥0.5 mg/kg. This saturating effect was expected from AB023 as a result of binding all free FXI and reflects the understood mechanism of action of AB023. Mean aPTT values were highest or close to/within the error margins of maximum between 0.25 and 24 h post-dose at all concentrations tested (from 0.1 to 5.0 mg/kg). The aPTT values decreased with time whereby the lowest dose level (0.1 mg/kg) decreased faster than the highest dose level (5.0 mg/kg). The aPTT values returned to baseline (±10%) by 24 h and 336 h for all subjects at dose levels 0.1 mg/kg and 0.5 mg/kg, respectively. Most subjects in dose levels 2.0 mg/kg and all subjects in dose level 5.0 mg/kg returned to baseline past the last planned time point at 672 h (Figure 4C).
Discussion:
While currently available anticoagulants are effective at preventing thrombosis, all existing therapies can significantly increase bleeding risks, leading to substantial morbidity, elevated health care costs, and in some cases resulting in death. Consequently, safer anticoagulants that do not impair hemostasis are a critical unmet need. Recent clinical studies implicate the blood coagulation contact system as a promising target for safer anticoagulation.9 We have developed a novel, recombinant antibody, AB023, that binds the FXI A2 domain and selectively inhibits FXI activation by FXIIa, inhibits the activation of FXII by FXIa, as well as inhibits contact-associated FXI autoactivation in vitro.12 Importantly, our in vitro data also demonstrate that AB023, like its murine precursor 14E11, does not prevent FXI activation by thrombin.10 These observed substrate specific alterations conferred upon FXI/FXIa upon binding to AB023, and the lack of AB023’s effect on FXI activation by thrombin suggest a hypothetical mechanistic rationale for AB023 to not increase the iatrogenic bleeding risk that may be seen with more direct FXI/FXIa inhibition or antagonism.
While lowering FXI levels using ASOs was associated with less orthopedic surgery-related bleeding events than traditional heparin anticoagulation clinically,9 an abundance of epidemiologic data still suggests a mild yet variable bleeding phenotype associated with FXI deficiency.6 By preserving the proteolytic functions of the FXI active site, as well as the ability of thrombin to activate FXI, AB023 selectively halts the coagulation cascade when initiated by contact activation, essentially rendering AB023 as a functional FXIIa inhibitor within the classical cascade model of blood coagulation. Patients deficient in FXII, while rare, have not been described as having any abnormal bleeding phenotype, further supporting the notion that the pathway AB023 inhibits may be completely dispensable for hemostasis. In vivo, experimental thrombosis studies using the murine precursor 14E11 corroborate this hypothesis, with no unusual bleeding events nor changes in prothrombin or bleeding times detected.10, 11 Lastly, no bleeding side effects were observed in either of the pivotal GLP toxicity studies performed using supratherapeutic doses of AB023 in rats and cynomolgus monkeys (data not shown). These studies, along with the data presented here, demonstrate the potent antithrombotic efficacy and hemostatic safety of AB023 in experimental models and strengthen the hypothesis that FXIIa dependent FXI activation, while inconsequential for hemostasis, appears to be pathologic in certain settings.
Novel agents that completely abrogate FXI function, such as ASOs that inhibit the production of FXI or antibodies that inhibit the hemostatic functions of FXI/FXIa, are more likely to result in a bleeding phenotype similar to individuals with hemophilia C.9, 20 While bleeding in these cases is mild and typically only provoked by substantial hemostatic challenges medical support and anticoagulation reversal may be required in dire circumstances. As it pertains to hemostatic capacity, we propose that individuals who receive AB023 are likely to behave in a clinically similar fashion to individuals born deficient in FXII. Such individuals have no known bleeding or other pathologic phenotype and can undergo major surgery without any specific interventions.22 Therefore, bleeding that occurs in patients exposed to AB023 can likely be managed similarly to patients with an intact coagulation system.
The phase 1 trial described here is the first to evaluate the safety of AB023 in humans. Most notably, no high-grade adverse events occurred in patients who received AB023 at any of the escalating doses from 0.1 mg/kg to 5.0 mg/kg, suggesting the drug is safe and well tolerated. A single grade 1 injection site ecchymosis event was observed in an AB023 treated subject (0.5 mg/kg) after several failed attempts to inject in the antecubital vein resulted in an unconventional injection site on the upper arm. The most common AE (cough) was unlikely related to the drug. Finally, while AB023 prolonged aPTT in a dose-dependent manner, PT or BT were not detectably altered.
The pharmacokinetic parameters of AB023 obtained from this study revealed that escalating doses of AB023 predictably increased plasma drug exposure (AUCs and Cmax). Interestingly, T1/2 increased with increasing doses of the drug and, conversely, CL decreased with increasing doses. This phenomenon can likely be explained by both the use of an ELISA method that detected only free AB023, and target-mediated drug disposition, where a proportion of drug administered is bound with high affinity to plasma FXI, which is reflected in the PK properties of the drug.23, 24 These effects on apparent PK parameters such as T1/2, Vss, and CL are often dose-dependent, as was observed for AB023 in this study. Indeed, we found that the terminal elimination phase was better characterized at the highest AB023 doses, where plasma concentrations of antibody were far above target saturation. At these high antibody concentrations, relative to FXI, elimination is likely approaching a first-order process where the FcRn-mediated pathway is dominant and target-mediated drug disposition is less of a factor.23 Target-mediated drug disposition is also likely responsible for the Vss that was lower than the physiological blood volume. Vss was likely only characterized appropriately at the highest dose level where Vss approached the physiological blood volume. The use of an ELISA method that detects only free antibody is a clear limitation to the study and does not reveal the full scope of AB023 PK. In order to address this, an ELISA method to detect total AB023 is under development and banked plasma samples will be evaluated once this assay is validated and available.
We also found a predictable, dose-dependent response in aPTT after AB023 administration, with a 5.0 mg/kg dose measurably prolonging aPTT for over four weeks. This finding holds promise for patient populations that require anticoagulation for both subacute and chronic indications, including postoperative VTE prophylaxis (current guidelines mandate weeks of post-operative anticoagulation for certain scenarios25), mechanical heart valves (a population in which DOACs are inferior to alternatives),26 chronic indwelling catheters, hemodialysis patients with atrial fibrillation, and bacterial sepsis with disseminated intravascular coagulation. These considerations, along with the favorable results of this phase 1 trial, have led to our currently enrolling phase 2 trial evaluating the safety and preliminary efficacy of AB023 in end stage renal disease patients receiving chronic hemodialysis (ClinicalTrials.gov Identifier: NCT03612856) where the primary objective is to measure safety and tolerability in this patient population. Preliminary efficacy will also be evaluated as a secondary objective by visual assessment of thrombus accumulation within the dialyzer cartridge,27 protein accumulation within the dialyzer cartridge, and improved efficiency of hemodialysis.
While AB023 is a FXI antibody that essentially serves as a functional FXIIa inhibitor, it is distinct from antibodies in development targeting FXIIa itself.28 While these two strategies have not been compared in humans, and any potential differences in safety and efficacy are speculative, several possible distinctions are worth discussing. Besides the fact that the average concentrations of circulating plasma FXII and FXI differ to a substantial degree (375 nM vs. 30 nM respectively)29, 30 there is also a theoretical benefit to targeting the circulating zymogen form of FXI as opposed to the activated serine protease FXIIa; in the latter scenario, the antibody may compete with other substrates for the FXIIa catalytic domain. Lastly, AB023 may have fewer off-target effects by preserving the other in vivo functions of FXIIa, including activation of the kinin-kallikrein system.
There are limitations to the evaluation of AB023 in animals and humans that should be discussed. First, the ability of AB023 to inhibit growth of an already existing thrombus was not evaluated in our baboon thrombosis model and therefore it is unknown whether AB023 would be effective as an interventional treatment for thrombosis. In addition, while our studies show that AB023 reduced contact pathway-initiated experimental thrombosis, its effectiveness in preventing thrombosis initiated by tissue factor has not yet been evaluated. However, the parent antibody 14E11 was indeed able to limit lethal pulmonary embolism induced by tissue factor injection in a mouse model.10 Previous studies also show that total inhibition of FXI activity reduced tissue-factor induced experimental thrombus growth18, however, because this result could be due to FXI activation via thrombin31 it is unknown whether AB023 would be similarly effective.
Another significant limitation of the phase 1 study is the inability to accurately assess bleeding risk with existing techniques. While it is reassuring that no clinically significant bleeding occurred and that the PT and BT were not changed by any dose of AB023 in this study, these parameters have significant variability and are limited predictors of actual bleeding risk, e.g., in hemophilia, when the values are often within the normal range. Second, as with any phase 1 trial, small sample size limits the ability to detect rare side effects which are possible with any drug.
In conclusion, we have shown that targeting contact activation by selective antibody-mediated inhibition of FXIIa-dependent FXI activation is feasible with AB023. There were no major safety concerns in this first-in-human trial, and overall, AB023 was well tolerated in healthy volunteers. We have developed a strong preclinical rationale suggesting this drug could be efficacious in a variety of common settings that might benefit from inhibiting contact activation. Indeed, targeting the blood coagulation contact pathway with drugs like AB023 has the potential to uncouple bleeding from thrombosis, which has been inexorably linked with traditional anticoagulants, and alter the way we offer antithrombotic treatments going forward.
Supplementary Material
Highlights:
AB023 is a unique, humanized anti-FXI antibody which blocks FXIIa-mediated FXI activation without inhibiting FXI activation by thrombin or the procoagulant function of FXIa.
In an experimental baboon graft thrombosis model, AB023 reduced platelet and fibrin accumulation within the thrombogenic grafts by >75%.
AB023 was safe and well tolerated in healthy human volunteers in a phase 1, first-in-human clinical trial.
Acknowledgments
The authors would like to thank Jennifer Johnson for her support with the primate studies, and Marschelle Carris for her laboratory technical support.
Sources of Funding
This study was supported, in part, by the National Heart Lung and Blood Institute grants HL128016 to E. I. Tucker and A. Gruber, HL106919 to E.I. Tucker and C.U. Lorentz, and HL144113 and HL101972 to M. Hinds, A. Gruber and O. McCarty.
Non-standard Abbreviations
- A2
apple 2
- AE
adverse event
- APTT
activated partial thromboplastin time
- ASO
antisense oligonucleotide
- AUC
area under the concentration-time curve
- AUC%extrap
percent of AUC0-inf extrapolated
- AUC0-inf
area under the concentration-time curve, from time 0 extrapolated to infinity
- AUC0-t
Area under the concentration-time curve, from time 0 to the last observed non-zero concentration (t)
- AV
arteriovenous
- BMI
body mass index
- BP
blood pressure
- BSA
bovine serum albumin
- BT
bleeding time
- CDR
complementarity determining region
- CL
apparent total plasma clearance
- Cmax
maximum plasma concentration
- CTI
corn trypsin inhibitor
- CVD
cardiovascular disease
- DOAC
direct oral anticoagulant
- FcRn
neonatal FC receptor
- FXI
factor XI
- FXII
factor XII
- HRP
horse-radish peroxidase
- ICH
international council for harmonization
- INR
international normalized ratio
- IV
intravenous
- Kel
apparent terminal elimination rate constant
- PD
pharmacodynamics
- PI
principal investigator
- PK
pharmacokinetic
- PT
prothrombin time
- RT
room temperature
- SAE
severe adverse events
- TEAE
treatment-emergent adverse events
- T1/2
apparent terminal elimination half-life
- Tmax
time to reach maximum observed concentration
- tPA
tissue plasminogen activator
- VAD
ventricular assist device
- Vd
apparent volume of distribution
- Vss
total apparent volume of distribution following single IV dose
- VTE
venous thromboembolism
Footnotes
Disclosures
A. Gruber, C. U. Lorentz, E. I. Tucker, N. G. Verbout, and M. Wallisch are employees of Aronora, Inc., and they as well as OHSU may have a financial interest in the results of this study. J.J. Shatzel serves as a medical consultant for Aronora, Inc.
References:
- 1.Statistics NCfH. Health, united states, 2016: With chartbook on long-term trends in health. Hyattsville, MD: 2017. [PubMed] [Google Scholar]
- 2.Inohara T, Xian Y, Liang L, Matsouaka RA, Saver JL, Smith EE, Schwamm LH, Reeves MJ, Hernandez AF, Bhatt DL, Peterson ED, Fonarow GC. Association of intracerebral hemorrhage among patients taking non-vitamin k antagonist vs vitamin k antagonist oral anticoagulants with in-hospital mortality. JAMA. 2018;319:463–473. doi: 10.1001/jama.2017.21917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers JG, Pagani FD, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, Boyce SW, Najjar SS, Jeevanandam V, Anderson AS, Gregoric ID, Mallidi H, Leadley K, Aaronson KD, Frazier OH, Milano CA. Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med. 2017;376:451–460. doi: 10.1056/NEJMoa1602954 [DOI] [PubMed] [Google Scholar]
- 4.Jaimes F, De La Rosa G, Morales C, Fortich F, Arango C, Aguirre D, Munoz A. Unfractioned heparin for treatment of sepsis: A randomized clinical trial (the hetrase study). Crit Care Med. 2009;37:1185–1196. doi: 10.1097/CCM.0b013e31819c06bc [DOI] [PubMed] [Google Scholar]
- 5.Ranieri VM, Thompson BT, Barie PS, Dhainaut JF, Douglas IS, Finfer S, Gardlund B, Marshall JC, Rhodes A, Artigas A, Payen D, Tenhunen J, Al-Khalidi HR, Thompson V, Janes J, Macias WL, Vangerow B, Williams MD, Group P-SS. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055–2064. doi: 10.1056/NEJMoa1202290 [DOI] [PubMed] [Google Scholar]
- 6.Gailani AE, Neff AT. Rare coagulation factor deficiencies In: Hoffman H, Benze EJ, Shattil SJ, Furie B, Silberstein LE, McGlave P, Heslop H, eds. Hematology, basic principles and practice.: Churchill Livingstone-Elsevier; 2009. [Google Scholar]
- 7.Preis M, Hirsch J, Kotler A, Zoabi A, Stein N, Rennert G, Saliba W. Factor XI deficiency is associated with lower risk for cardiovascular and venous thromboembolism events. Blood. 2017;129:1210–1215. doi: 10.1182/blood-2016-09-742262 [DOI] [PubMed] [Google Scholar]
- 8.Salomon O, Steinberg DM, Zucker M, Varon D, Zivelin A, Seligsohn U. Patients with severe factor XI deficiency have a reduced incidence of deep-vein thrombosis. Thromb Haemost. 2011;105:269–273. doi: 10.1160/TH10-05-0307 [DOI] [PubMed] [Google Scholar]
- 9.Büller HR, Bethune C, Bhanot S, Gailani D, Monia BP, Raskob GE, Segers A, Verhamme P, Weitz JI, Investigators F-AT. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med. 2015;372:232–240. doi: 10.1056/NEJMoa1405760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng Q, Tucker EI, Pine MS, Sisler I, Matafonov A, Sun MF, White-Adams TC, Smith SA, Hanson SR, McCarty OJ, Renne T, Gruber A, Gailani D. A role for factor XIIa-mediated factor XI activation in thrombus formation in vivo. Blood. 2010;116:3981–3989. doi: 10.1182/blood-2010-02-270918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leung PY, Hurst S, Berny-Lang MA, Verbout NG, Gailani D, Tucker EI, Wang RK, McCarty OJ, Gruber A. Inhibition of factor XII-mediated activation of factor XI provides protection against experimental acute ischemic stroke in mice. Transl Stroke Res. 2012;3:381–389. doi: 10.1007/s12975-012-0186-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorentz CU, Verbout NG, Cao Z, Liu L, Hinds MT, McCarty OJT, Ivanov I, Tucker EI, Gailani D, Gruber A. Factor XI contributes to myocardial ischemia-reperfusion injury in mice. Blood Adv. 2018;2:85–88. doi: 10.1182/bloodadvances.2017004879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo D, Szaba FM, Kummer LW, Johnson LL, Tucker EI, Gruber A, Gailani D, Smiley ST. Factor XI-deficient mice display reduced inflammation, coagulopathy, and bacterial growth during listeriosis. Infect Immun. 2012;80:91–99. doi: 10.1128/IAI.05568-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tucker EI, Verbout NG, Leung PY, Hurst S, McCarty OJ, Gailani D, Gruber A. Inhibition of factor XI activation attenuates inflammation and coagulopathy while improving the survival of mouse polymicrobial sepsis. Blood. 2012;119:4762–4768. doi: 10.1182/blood-2011-10-386185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanson SR, Griffin JH, Harker LA, Kelly AB, Esmon CT, Gruber A. Antithrombotic effects of thrombin-induced activation of endogenous protein C in primates. J Clin Invest. 1993;92:2003–2012. doi: 10.1172/JCI116795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zilberman-Rudenko J, Reitsma SE, Puy C, Rigg RA, Smith SA, Tucker EI, Silasi R, Merkulova A, McCrae KR, Maas C, Urbanus RT, Gailani D, Morrissey JH, Gruber A, Lupu F, Schmaier AH, McCarty OJT. Factor XII activation promotes platelet consumption in the presence of bacterial-type long-chain polyphosphate in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2018;38:1748–1760. doi: 10.1161/ATVBAHA.118.311193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crosby JR, Marzec U, Revenko AS, Zhao C, Gao D, Matafonov A, Gailani D, MacLeod AR, Tucker EI, Gruber A, Hanson SR, Monia BP. Antithrombotic effect of antisense factor XI oligonucleotide treatment in primates. Arterioscler Thromb Vasc Biol. 2013;33:1670–1678. doi: 10.1161/ATVBAHA.113.301282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gruber A, Hanson SR. Factor XI-dependence of surface- and tissue factor-initiated thrombus propagation in primates. Blood. 2003;102:953–955. doi: 10.1182/blood-2003-01-0324 [DOI] [PubMed] [Google Scholar]
- 19.Tucker EI, Marzec UM, White TC, Hurst S, Rugonyi S, McCarty OJ, Gailani D, Gruber A, Hanson SR. Prevention of vascular graft occlusion and thrombus-associated thrombin generation by inhibition of factor XI. Blood. 2009;113:936–944. doi: 10.1182/blood-2008-06-163675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Horani RA, Desai UR. Factor xia inhibitors: A review of the patent literature. Expert Opin Ther Pat. 2016;26:323–345. doi: 10.1517/13543776.2016.1154045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gay ND, Azar S, Salomon O, Taylor JA. Management of severe factor XI deficiency in cardiac surgery: A case report and review of the literature. Haemophilia. 2017;23:e512–e514. doi: 10.1111/hae.13311 [DOI] [PubMed] [Google Scholar]
- 22.Sekine J, Tsuruda K, Matsunga S, Kamihira S, Ueno K, Inokuchi T. Surgical management in the patient with congenital factor XII deficiency. Report of a case. Oral Surg Oral Med Oral Pathol. 1994;77:13–15. doi: [DOI] [PubMed] [Google Scholar]
- 23.Liu L Pharmacokinetics of monoclonal antibodies and Fc-fusion proteins. Protein Cell. 2018;9:15–32. doi: 10.1007/s13238-017-0408-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mager DE. Target-mediated drug disposition and dynamics. Biochem Pharmacol. 2006;72:1–10. doi: 10.1016/j.bcp.2005.12.041 [DOI] [PubMed] [Google Scholar]
- 25.Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, Samama CM. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:e227S–e277S. doi: 10.1378/chest.11-2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, Blatchford J, Devenny K, Friedman J, Guiver K, Harper R, Khder Y, Lobmeyer MT, Maas H, Voigt JU, Simoons ML, Van de Werf F, Investigators R-A. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–1214. doi: 10.1056/NEJMoa1300615 [DOI] [PubMed] [Google Scholar]
- 27.Ronco C, Brendolan A, Nalesso F, Zanella M, De Cal M, Corradi V, Virzi GM, Ferrari F, Garzotto F, Lorenzin A, Karopadi AN, Sartori M, De Rosa S, Samoni S, Husain-Syed F, Spinelli A, Neri M, Villa G, Alghisi A. Prospective, randomized, multicenter, controlled trial (TRIATHRON 1) on a new antithrombogenic hydrophilic dialysis membrane. Int J Artif Organs. 2017;40:234–239. doi: 10.5301/ijao.5000608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao H, Biondo M, Lioe H, Busfield S, Rayzman V, Nieswandt B, Bork K, Harrison LC, Auyeung P, Farkas H, Csuka D, Pelzing M, Dower S, Wilson MJ, Nash A, Nolte MW, Panousis C. Antibody-mediated inhibition of FXIIa blocks downstream bradykinin generation. J Allergy Clin Immunol. 2018;142:1355–1358. doi: 10.1016/j.jaci.2018.06.014 [DOI] [PubMed] [Google Scholar]
- 29.Bach J, Endler G, Winkelmann BR, Boehm BO, Maerz W, Mannhalter C, Hellstern P. Coagulation factor XII (FXII) activity, activated FXII, distribution of FXII c46t gene polymorphism and coronary risk. J Thromb Haemost. 2008;6:291–296. doi: 10.1111/j.1538-7836.2007.02839.x [DOI] [PubMed] [Google Scholar]
- 30.Reddigari SR, Shibayama Y, Brunnee T, Kaplan AP. Human hageman factor (factor FXII) and high molecular weight kininogen compete for the same binding site on human umbilical vein endothelial cells. J Biol Chem. 1993;268:11982–11987. doi: [PubMed] [Google Scholar]
- 31.Kravtsov DV, Matafonov A, Tucker EI, Sun MF, Walsh PN, Gruber A, Gailani D. Factor XI contributes to thrombin generation in the absence of factor xii. Blood. 2009;114:452–458. doi: 10.1182/blood-2009-02-203604 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.