Abstract
How neuronal networks enable animals, humans included, to make coordinated movements is a continuing goal of neuroscience research. The stomatogastric nervous system of decapod crustaceans, which contains a set of distinct but interacting motor circuits, has contributed significantly to the general principles guiding our present understanding of how rhythmic motor circuits operate at the cellular level. This results from a detailed documentation of the circuit dynamics underlying motor pattern generation in this system as well as its modulation by individual transmitters and neurons.
Graphical Abstract

In the quest to understand how the human nervous system enables us to interact with our environment, neuroscientists use a diverse collection of model systems. Investigators working with the mammalian central nervous system (CNS) have made significant progress over the past ten years with respect to the physiological and synaptic properties of individual neurons. However, the large number of neurons and associated complexity of the neuronal networks in these systems have limited progress in understanding how they work. So far, the systems that have provided many of the concepts that guide our understanding of neural circuit operation come from a small number of invertebrate preparations. Among these are neuronal circuits, called central pattern generators (CPGs), that control the generation of rhythmic motor behaviours.
How CPGs generate the patterned neural output underlying rhythmic movements has been studied for nearly 100 years1. Rhythmic behaviours include all motor acts that at their core involve a rhythmic, repeating set of movements, such as locomotion, respiration and mastication. All CPGs, in both invertebrates and vertebrates, operate on the same general principles2–4. One such principle is that these networks remain functional in the completely isolated nervous system, in the absence of all rhythmic neuronal input, including the rhythmic feedback from the sensory systems that normally monitor the elicited movements. This property facilitates circuit analysis at the level of the synaptic interactions between the component neurons (that is, cellular-level studies). It should not be forgotten, however, that the ability of sensory and higher-order systems to influence CPG activity is pivotal to ensuring that the resulting behaviour is appropriate for the situation at hand2,3,5. CPGs that are studied in the isolated nervous system include those controlling heartbeat in leeches, feeding in molluscs and crustaceans, locomotion in leeches, molluscs, crustaceans and various vertebrates, and respiration in mammals2–4.
Although the details differ in each circuit, all CPGs use the same set of cellular-level mechanisms for circuit construction2–4. This includes the prevalence of synaptic inhibition and a set of voltage-dependent firing patterns. Another, more recently appreciated similarity among CPGs is their state dependence. CPG circuits are not dedicated to producing a single neuronal activity pattern. Instead, a CPG is a flexible construct whose output is altered by neuromodulatory transmitters and hormones, enabling them to generate either variants of a single motor pattern (for example, different types of chewing) or, in some cases, distinct activity patterns2,3. This flexibility results largely from the ability of different neuromodulators to change the cellular and synaptic properties of individual circuit neurons. When the properties of circuit components are changed, the output of the circuit itself is modified. These and related aspects of CPG operation have revealed principles that are often shared by non-CPG circuits, enabling CPGs to serve as a model system for understanding neuronal circuit operation in general6.
The isolated stomatogastric nervous system
One model system that has contributed importantly to the general understanding of neural circuit operation is the stomatogastric nervous system of decapod crustaceans (lobsters, crabs, crayfish and shrimp). The value of this system has resulted from its accessibility, the use of several innovative techniques and the combined research effort of around 15 laboratories over the past ~30 years2,7,8. Here we highlight the insights gained from studying CPG operation at the cellular level by describing some of the contributions that have come from studying this preparation; a complete list of publications on the stomatogastric system is available at http//stg.rutgers.edu.
The stomatogastric nervous system is an extension of the crustacean CNS which contains four ganglia plus their connecting and peripheral nerves (Fig. 1a). These ganglia include the paired commissural ganglia (~550 neurons each) and the unpaired oesophageal ganglion (12–15 neurons) and stomatogastric ganglion (STG; 25–30 neurons). Within these ganglia are a set of distinct but interacting CPGs that generate the motor rhythms underlying different aspects of feeding in the oesophagus and multi-compartment stomach, including swallowing (oesophagus), food storage (cardiac sac), chewing (gastric mill) and the filtering of chewed food (pylorus)7. The two best characterized of these CPGs are the gastric mill and pyloric circuits, both of which are located in the STG. The gastric mill and pyloric motor rhythms have been studied in the intact animal (Fig. 1a), in semi-intact conditions, and in the isolated stomatogastric nervous system2,7,9–11. Although most research in this system has used the isolated nervous system, comparisons with semi-intact and in vivo preparations indicate that comparable events occur in all of these conditions9,10,12.
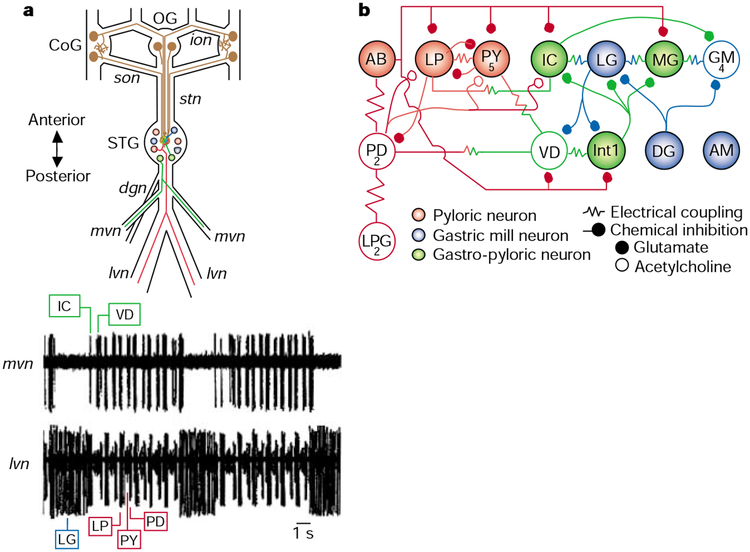
Figure 1.
The gastric mill and pyloric circuits in the stomatogastric nervous system. a, Schematic of the stomatogastric nervous system in the crab Cancer borealis (top panel). The neurons with somata in the commissural (CoG) and oesophageal (OG) ganglia represent modulatory projection neurons that influence the gastric mill (blue), pyloric (red) and gastro-pyloric (green) neurons in the stomatogastric ganglion (STG). Gastro-pyloric neurons are active with both the gastric mill and the pyloric rhythms. Most STG neurons are motor neurons that project axons posteriorly through the lateral (lvn) or medial (mvn) ventricular nerves. Illustrated are two STG neurons that project through these nerves. The bottom panel shows that the gastric mill and pyloric rhythms are readily recorded extracellularly via these two nerves in situ (recordings modified from ref. 12). Comparable recordings are routinely made in vitro (in completely isolated stomatogastric nervous system). The gastric mill rhythm is represented by the relatively long duration, rhythmic action potential bursts in the lateral gastric (LG) neuron and the concomitant elimination of activity in the inferior cardiac (IC) and ventricular dilator (VD) neurons. The faster pyloric rhythm is evident from the sequentially repeating action potential bursts in the lateral pyloric (LP), pyloric (PY), pyloric dilator (PD), IC and VD neurons. The activity in the LP, PY and PD neurons is obscured during each LG neuron burst. Additional abbreviations: dgn, dorsal gastric nerve; ion, inferior oesophageal nerve; son, superior oesophageal nerve; stn, stomatogastric nerve. b, Schematic of the STG neural network the crab Cancer borealis, showing gastric mill and pyloric circuits. The pyloric neurons (red) are shown in their normal sequence of pyloric-timed activity, with time progressing from left to right. The neurons that show gastric mill-timed activity are represented so that the neurons active during protraction of the teeth are on top, and those active during retraction of the teeth are below. Neurons labelled as ‘gastro-pyloric neurons’ exhibit both gastric mill- and pyloric-timed activity. Abbreviations: AB, anterior burster neuron; MG, medial gastric neuron; GM, gastric mill neuron; Int1, Interneuron 1; DG, dorsal gastric neuron. Adapted from ref. 13.
Physiological identification of each gastric mill and pyloric neuron is straightforward because nearly all of the STG neurons are members of one or both of these circuits13. All STG neurons are readily recorded and identified in extracellular recordings, enabling a continuous monitor of the activity of all circuit components (Fig. 1a). Additionally, all of these neurons have large-diameter somata (range, 25–120 μm), and each one occurs as either a unique individual or a small group of functionally equivalent neurons. These features enable prolonged (of the order of hours), simultaneous intracellular recordings from several STG neurons, which has greatly facilitated characterization of their synapses and membrane properties, as well as determining the role of each neuron in circuit dynamics. In fact, all synapses among the STG neurons have been identified and characterized, as have the transmitters used by these neurons8,14,15 (Fig. 1b). Many of their membrane properties also have been characterized15,16, including endogenous oscillatory capability, plateau potential generation, post-inhibitory rebound and escape from inhibition (Fig. 2).
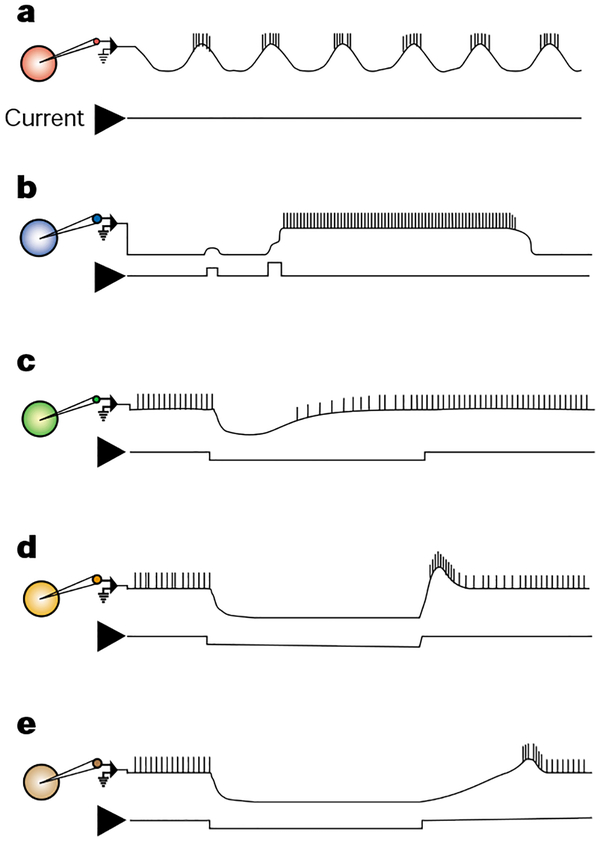
Figure 2.
Illustrations of some of the membrane properties occurring in intracellularly recorded neurons (coloured circles) of central pattern generating circuits, including those in the STG. Each current trace is a monitor of depolarizing (up) and/or hyperpolarizing (down) current injections into the recorded neuron. Comparable events can be elicited by excitatory and inhibitory synaptic input. a, An endogenous oscillator undergoes rhythmic membrane potential oscillations without requiring any rhythmic input. b, The plateau potential is a persistent depolarizing response that generates persistent action potential activity and outlasts the triggering stimulus. It has a membrane potential threshold for its activation and it eventually self-terminates. c, Escape from inhibition is an excitation that is triggered by a sufficient amplitude and duration of hyperpolarization. It enables the neuron to depolarize, often to the point of firing action potentials, despite the continued presence of a hyperpolarizing input. d, Post-inhibitory rebound (PIR) is also triggered by hyperpolarization, but it represents an overshooting of the resting potential with an associated burst of action potentials after termination of the hyperpolarization. e, PIR delay shows a slower rebound depolarization, compared with PIR, after a period of hyperpolarization.
Two techniques originally developed with the STG system — photoinactivation to selectively delete neurons in a physiological preparation14,15,17,18 and the use of the dynamic clamp to selectively reintroduce specific ionic or synaptic currents19–21 — were key to obtaining a cellular-level understanding of STG circuit operation. The use of these techniques has also extended well beyond the stomatogastric system22–27.
The gastric mill circuit generates a two-phase rhythm that underlies chewing (protraction and retraction of the teeth) within the gastric mill compartment of the stomach9. The rhythmic output of this circuit is an emergent property of the synaptic connectivity and membrane properties of the circuit neurons20,28. The circuit is activated by extrinsic inputs that are usually not spontaneously active15,29,30. In contrast, the pyloric rhythm is ‘pacemaker-driven’, as the circuit includes an endogenous oscillator neuron (a ‘pacemaker neuron’) whose intrinsically generated rhythmicity and synaptic outputs enable it to drive this rhythm14,17,18, an ability that is conditional on the presence of one or more neuromodulators. The complete activity pattern generated by the pyloric circuit, however, results from the connectivity and membrane properties of all circuit components14. As long as the STG continues to receive spontaneously active (non-rhythmic) modulatory input from the commissural ganglia, the pyloric rhythm persists. Pacemaker neurons are also found in other rhythmic systems, including the CPGs underlying mammalian respiration31 and locomotion32. This type of neuron seems to be key to the rhythmic activity of the brainstem respiratory circuit31,33, but its role in pattern generation during locomotion remains to be determined.
The conditional membrane and synaptic properties that characterize the STG neurons are regulated by the actions of more than 15 neuromodulatory transmitters contained in the STG terminals of the sensory and projection neurons that innervate this ganglion8,34,35 (Fig. 3a). There are several documented cases of co-localized transmitters within these neurons8,11,34 (Fig. 3b), and many of these same modulatory substances, plus some additional ones, also influence the STG as circulating hormones8,35. Application of particular modulators often accelerates and strengthens the pyloric rhythm, with each one eliciting a different version2,35 (Fig. 3a), although a few modulators instead inhibit the rhythmic activity8,15. Different modulatory projection neurons also elicit different versions of the pyloric and gastric mill rhythms11,30,36,37 (Fig. 3b).
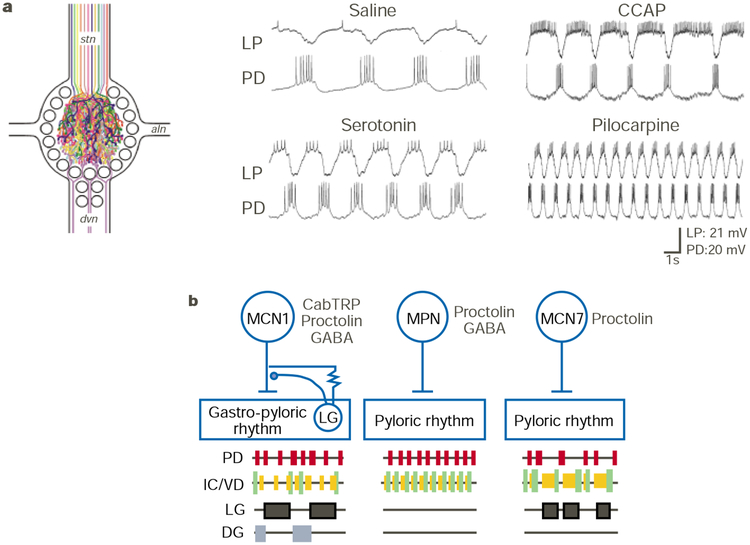
Figure 3.
Modulation of the pyloric and gastric mill rhythms in the STG. a, Schematic of the modulatory innervation of the STG (left panel; adapted from ref. 4). Each colour represents a distinct neuromodulatory transmitter or complement of transmitters localized to the axon and STG terminals of projection neurons that innervate the STG via the stomatogastric nerve (stn) and of sensory neurons that innervate the STG via the dorsal ventricular nerve (dvn). Individual bath application of different modulators to the isolated STG in the crab Cancer borealis elicits different versions of the pyloric rhythm (right panel; S. R. Hertzberg, M.P.B. & M.P.N., unpublished data). The pyloric rhythm is monitored by intracellular recordings of two pyloric circuit neurons (PD and LP). Modulator concentrations: serotonin (10−5 M), crustacean cardioactive peptide (CCAP; 10−6 M), and the muscarinic agonist pilocarpine (10−5 M). Each application was followed by a saline wash, during which the rhythm returned to control conditions (saline). All recordings are from the same preparation. b, Selective activation of distinct modulatory projection neurons which have a peptide co-transmitter in common (proctolin) have different actions on the pyloric and gastric mill circuits in the STG. Note that the inhibitory and electrical synapses from the LG neuron to MCN1 occur within the STG neuropil29,71. Abbreviations: CabTRP, Cancer borealis tachykinin-related peptide; MCN1/7, modulatory commissural neuron 1/7; MPN, modulatory proctolin neuron. Modified from ref. 11.
Neural output from the STG circuits is not always followed faithfully by the target muscles. The striated muscles of the stomatogastric system are ‘slow’ non-twitch muscles that generally respond in a graded fashion to their excitatory input. Although some muscle types do faithfully replicate the pattern of their neural input, others have a sufficiently slow contractile response that they exhibit a tonic contraction despite receiving a rhythmic neural input38. Furthermore, although single motor neurons innervate several different muscles in this system, the individual muscles can generate distinct contraction patterns despite their common innervation38. An active reinterpretation of the neural input at the muscle level has been noted in other systems39, and there is also plasticity at the periphery, increasing even further the potential outputs generated by these motor systems40–42.
Insight into neuronal circuit operation has also come from studying the stomatogastric system in several different crustacean species43,44. One conclusion drawn from these studies is that the functionally equivalent neuron in different species can differ in transmitter phenotype8,45 and/or physiological actions15,45,46. This work has implications for work in the vertebrate CNS where the same neurons are studied in different species without the availability of an extended cellular characterization in each case.
Basic circuit operation
Synaptic transmission and membrane properties
In the 1960s and early 1970s, it was believed that the neuronal basis of behaviour could be explained once all of the neurons in a circuit were identified and their synaptic connectivity determined. However, as CPG circuit analyses progressed, it became evident that there was more to motor pattern generation than the presence of excitation and inhibition. Specifically, neuronal membrane properties were found to be equally important for circuit operation14,16. Furthermore, we learned that neuromodulation changed these membrane properties and synaptic strengths, enabling neural circuits to generate multiple activity patterns14,15,47. It is now clear that these influences are key to the operation of all neural circuits.
One feature shared by many CPG circuits is the prevalence of inhibitory synapses2,3. Within the STG circuits, all transmitter-mediated actions are inhibitory16 (Fig. 1b). During the gastric mill and pyloric rhythms, these inhibitory synapses are responsible for the inactive phase of most STG neurons, whereas their active phase results from the activation of voltage-dependent membrane properties that are primed by the preceding inhibition2,14,16. For example, the rate of post-inhibitory rebound in different pyloric neurons contributes importantly to the timing of their activity within the pyloric rhythm48, whereas plateau potential generation strengthens the intensity of the action potential bursts in many pyloric and gastric mill neurons15,16. The interplay between synapses and membrane properties is further exemplified by the fact that the rhythmic synaptic inhibition in the pyloric circuit restricts the burst duration of at least some pyloric neurons to their period of most intense activity49.
Another functional consequence of the particular cellular properties associated with STG circuit neurons is that the pyloric rhythm retains its essential features, such as the relative timing of action potential bursts (activity phase within the rhythm) in each neuron, across a broad range of frequencies (~0.2–2.0 cycles per second)50. Voltage-dependent membrane properties such as those in STG neurons are common features of all CPG neurons, although in many cases their specific roles within these circuits are yet to be elucidated2,3.
Some non-CPG circuits also combine synaptic inhibition and membrane properties to generate patterned neuronal output. One well-characterized example is the circuit underlying sleep-related rhythms in the thalamus6. In this system, the basic circuit includes a spatially iterated set of reciprocally connected inhibitory (perigeniculate/reticular) neurons and excitatory (thalamocortical) neurons. The activity pattern generated by these neurons relies on the effective activation of such membrane properties as post-inhibitory rebound, escape from inhibition and plateau-like potentials. The switch from the sleep to the waking state in this system seems to result largely from modulatory inputs that depolarize the circuit neurons sufficiently to prevent the same inhibitory synapses within the circuit from activating these membrane properties6.
Each of the membrane properties in STG neurons is generally associated with a particular set of ionic currents, although in many cases more than one set of currents can mediate the same event51. Molecular approaches have provided a more detailed description of some of the channels and currents underlying these properties, including the genes that encode them and their specific location(s) within individual neurons52,53. This work has also been extended to the functional level using multiphoton imaging and voltage-clamp analysis of Ca2+ currents in an identified STG neuron54.
These studies provide the basis for a more accurate understanding of the physiological properties of individual circuit neurons, as well as their response to synaptic and modulatory inputs. It has become evident that mammalian dendrites also exhibit regions with distinct physiological properties, as a result of clustering of distinct channels55. Additionally, the molecular characterization of the ion channels and currents present in STG neurons is facilitating a more accurate determination of the identity or distinctiveness of similar neurons. The use of molecular identity should be particularly valuable in more complex systems where there exist populations of apparently equivalent neurons.
At most of the STG circuit synapses, graded transmitter release is predominant16. This occurs independently of action potentials and, once past a threshold level, increases in a graded fashion with increasing depolarization. The mechanisms underlying this release, as well as its modulation, have been studied extensively in the STG16,56, where important principles for comparable signalling events in other systems have been generated. Although graded release is pivotal within the STG circuit, these same circuit neurons use action potentials for exporting the STG circuit output to the muscles and to more central ganglia.
Modelling of circuit activity has become a valuable tool for elucidating neural circuit operation, even in small systems such as the STG, because of the complexity of neuronal circuits relative to available experimental techniques. This approach, combined with experimental tests of the predictions from each model, has been instrumental in determining the function of a number of specific cellular and synaptic properties of STG circuit neurons57–60. For example, efforts have led to several non-intuitive insights, including the presence of distinct types of endogenous oscillatory properties61, the use of membrane properties to transduce temporal patterns into a neural code58, and the presence of intercircuit coordination20,62.
Electrical coupling
Gap junction-mediated electrical coupling is prevalent throughout both vertebrate and invertebrate nervous systems. It links functionally equivalent neurons into co-active groups, provides fast and reliable synaptic communication and can enable coincidence detection63–66. Electrical coupling also provides additional degrees of freedom within neuronal circuits67,68. For example, within the pyloric circuit there are several neuronal pairs that are connected synaptically via both chemical and electrical synapses (Fig. 1b). Under steady-state conditions, inhibition is the dominant action at these dual synapses and the net result is that the coupled neurons fire in alternation instead of synchronously69,70. However, in certain modulatory environments the relative strength of these two types of synapses changes, causing a switch in the net response from inhibition to excitation70.
Another unusual use of electrical synapses occurs in the gastric mill circuit, where there is a voltage-dependent electrical synapse between a gastric mill neuron (the lateral gastric neuron) and the STG terminals of a modulatory projection neuron called MCN1 (ref. 71; and Fig. 3b). Because of the voltage-dependence of the electrical synapse and the particular circuit configuration, the projection neuron excites the gastric mill circuit via its released co-transmitters during one half of each cycle and via electrical coupling during the other half of each cycle71.
Even when neuronal ensembles are tightly electrically coupled, as occurs among the pyloric pacemaker group, the different components can have different functional roles17,18. In the spiny lobster, the pyloric pacemaker group includes a single oscillator neuron (called the anterior burster neuron), plus paired pyloric dilator neurons. Their electrical coupling enables the slow oscillations of their membrane potentials to occur synchronously, and both neuron types make inhibitory synapses on nearly all other pyloric neurons. However, they do so using different neurotransmitters67 (Fig. 1b). The glutamatergic inhibition from the anterior burster neuron has a relatively fast onset and offset, whereas the cholinergic pyloric dilator neuron evokes a slower onset and longer lasting inhibition in the same target neurons. One way by which modulatory inputs elicit different pyloric rhythms is by having differential actions on these two pacemaker neurons17,18,56,67. For example, dopamine application enhances the activity of the anterior burster neuron while inhibiting the pyloric dilator neuron18,67. In this case, the faster synaptic actions of the anterior burster neuron are dominant, contributing to a change in the timing of activity in the other pyloric neurons. These actions of dopamine work in concert with dopamine modulation of a specific potassium current (IA) in the other pyloric neurons to bring about the altered timing of their activity56. The extent to which different subsets of a population of electrically coupled neurons have different roles in circuit activity remains to be determined in most systems.
Another functional distinction between the electrically coupled anterior burster and pyloric dilator neurons was discovered from determining how their individual responses to neuromodulators influences the pyloric cycle frequency17,18. For example, the neuropeptide proctolin increases the speed of weakly cycling pyloric rhythms, but it evokes a maximal pyloric cycle frequency of 1 cycle s−1 despite the ability of this rhythm to cycle faster under other conditions. By studying the actions of this peptide separately on each pyloric circuit neuron, Hooper and Marder17 found that proctolin increased the rhythmic cycling of the isolated anterior burster neuron to frequencies of 2–3 cycles s−1, but it had no effect on the isolated pyloric dilator neurons. By sequentially photoinactivating each pyloric dilator neuron in the otherwise intact circuit, they showed that the lack of responsiveness of these neurons to proctolin slows the cycling speed of the anterior burster neuron, via their electrical coupling. This was a clear indication that the non-responsiveness of a neuron to a neuromodulator can have an impact on the circuit response to that modulator, and highlights the importance of knowing the function(s) of all circuit components for fully understanding circuit dynamics.
Short-term synaptic dynamics
Short-term synaptic dynamics include processes, such as short-term depression and facilitation, that have been studied extensively at the level of individual synapses in many systems72. The contribution of this form of synaptic plasticity to circuit dynamics has been intimated from studies of cortical neurons72, but a direct role for these processes within an operating circuit has only recently been documented. For example, modulation of the spinal locomotor CPG in lamprey alters the impact of synaptic depression and facilitation on the CPG output73. In the stomatogastric system, regulation of synaptic depression seems to be a determining factor for whether the pyloric cycle frequency is controlled by the endogenous oscillatory property of the pyloric pacemaker neuron or the inhibitory synaptic input that this neuron receives from the lateral pyloric neuron72,74 (Fig. 1b). Under steady-state conditions, the latter synapse is effectively depressed and the intrinsic properties of the pacemaker neuron control pyloric cycle speed. However, it seems likely that under some modulatory conditions the lateral pyloric synapse onto the pacemakers is strengthened such that it effectively regulates the speed of this rhythm. In another study, Combes et al.30 showed that short-term facilitation is used to switch the version of the gastric mill rhythm that is elicited by activation of an identified sensory pathway. When the sensory neuron fires at a moderate level, it preferentially activates one particular projection neuron that drives the gastric mill rhythm. However, higher-frequency firing of the sensory neuron produces facilitating excitatory postsynaptic potentials in another projection neuron whose activation changes the gastric mill rhythm.
Activity-dependent plasticity
Considerable attention has focused on homeostatic regulation of neural network activity75,76. Some of the pioneering studies of this phenomenon, performed with lobster STG neurons77, documented the ability of neurons to compensate for long-lasting changes in their activity by altering their cellular properties, with the consequence that their activity level ultimately returned to its previous steady state. These compensatory changes in the activity of pyloric neurons are mediated by long-term changes in the balance of active ionic currents57,78. One remarkable conclusion from this work has been that the same neuronal activity pattern can be achieved in the same neuron by different balances of ionic currents. Previous studies involving modulation of individual ion currents focused on the ability of this modulation to evoke short-term changes in the activity pattern of the neuron. Clearly, this same event can preserve the original activity pattern of the neuron over the long term. Additionally, because the STG work was performed in a network context, we learned that not only is the individual neuronal activity conserved, but also the circuit output.
Intercircuit coordination
Many behaviours involve the coordination of different sets of movements, such as the coordination of breathing with strenuous activities like running or swimming. There are few existing cellular-level biological models for how the distinct neuronal circuits underlying these movements coordinate their activity patterns. This type of coordination is being elucidated using the gastric mill and pyloric circuits. These two circuits operate in distinct time domains, with the pyloric rhythm ranging from ~0.5–2.0 cycles s−1 and the gastric mill rhythm ranging from ~0.05–0.2 cycles s−1 (Fig. 1a), and they can operate independently20,79. There are, however, identified synapses that enable each circuit to regulate the output of the other20,79. The gastric mill circuit regulates the pyloric rhythm through its ability to synaptically inhibit the STG terminals of the modulatory projection neuron MCN1, whose activity drives the gastric mill rhythm and enhances the pyloric rhythm29,71,79 (Fig. 3b). Thus, during one half of each gastric mill cycle, the lateral gastric neuron inhibits MCN1 and thereby eliminates the MCN1 excitation of the pyloric circuit. During the other half of each cycle the lateral gastric neuron is silent, enabling MCN1 to release its co-transmitters and excite the pyloric circuit.
Insight into the pyloric circuit regulation of the gastric mill rhythm developed from a computational model of the gastric mill rhythm elicited by MCN1 stimulation62. By manipulating the biological circuits, Bartos et al.20 verified the predictions of the model that the pyloric circuit regulates the speed of the gastric mill rhythm via an identified synapse, and that this same synapse links the start of each gastric mill cycle to the start of a pyloric cycle, thereby coordinating their activity patterns. There are comparable examples of coordination between distinct motor patterns in numerous systems, but the cellular-level mechanisms underlying this coordination are yet to be determined80,81.
Neuromodulation in the stomatogastric system
Early work on neural circuit modulation in the isolated STG showed that bath-applied neuromodulators reproducibly elicit stable, distinct pyloric and gastric mill rhythms, enabling a detailed analysis of these events47. The discovery that CPGs are functionally flexible marked a paradigm shift that was subsequently extended to all types of neuronal circuits. This ability to modify circuit output is largely a consequence of the relatively slow time course of modulatory actions and the fact that their primary targets are voltage- and time-dependent currents82. These currents, by definition, are each active in only a limited range of membrane potentials outside of which they have no influence. Comparable application of transmitters with ionotropic actions instead drives all target neurons towards the reversal potential of the current(s) opened by the transmitter and thereby disrupts any ongoing rhythmic activity82.
The degree of flexibility afforded to neuronal networks by modulatory inputs is extensive. For example, the same STG circuit neuron can be active with the pyloric rhythm, gastric mill rhythm or both rhythms simultaneously13. The same circuit neuron can also be switched to participate in different rhythms46,83,84. The gastric mill and pyloric circuits can even be dismantled, with some components participating in a different motor pattern and other components remaining silent46,83 (Fig. 4). It has not yet been possible to prove that comparable events occur in the vertebrate CNS, but the available evidence is strongly suggestive85–87.
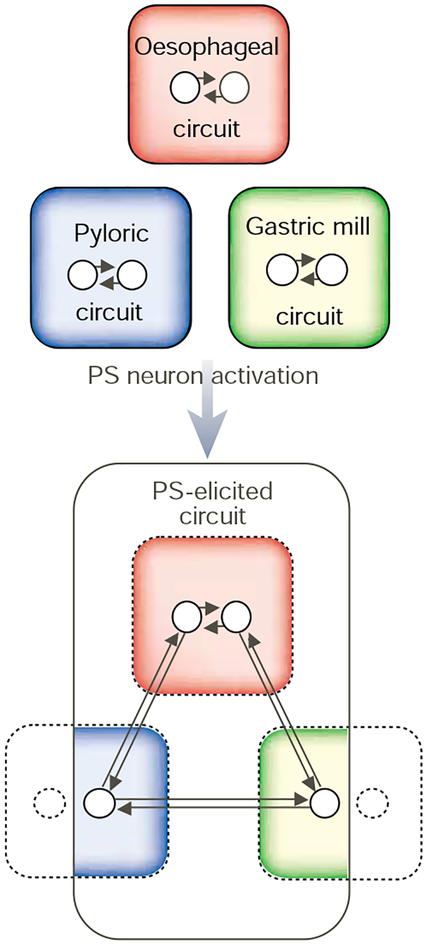
Figure 4.
Modulatory input can dismantle and reconfigure the STG circuits. Generally, the gastric mill, pyloric and oesophageal circuits generate distinct motor rhythms. However, activation of some modulatory inputs, such as the pyloric suppressor (PS) neuron, can eliminate these rhythms and replace them with a single, conjoint motor pattern. The PS-elicited motor circuit includes a subset of the neurons (circles within coloured regions) that comprise the three circuits. The PS neuron also inhibits the activity of the gastric mill and pyloric circuit neurons (circles with dashed outlines located outside coloured regions) that do not participate in this conjoint motor pattern. Adapted from ref. 83; see also ref. 46.
One realization that developed from these studies of neuromodulation is that the activity phenotype, membrane properties and synaptic actions of individual neurons are state dependent. For example, the same neuron under different conditions can be an endogenous oscillator, generate plateau potentials and/or post-inhibitory rebound, or express none of these properties2. Similarly, the strength, sign or even presence of synaptic actions can come and go. Changes such as these have significant consequences for circuit output2,56,88.
Modulation of CPG activity in the STG involves many different small-molecule, peptide and gaseous neurotransmitters8,35,89. The second-messenger pathways involved in these modulatory actions are not well described, but it is clear from imaging studies in physiological preparations that cyclic AMP levels within the STG are upregulated in unique ways by different neuromodulators90.
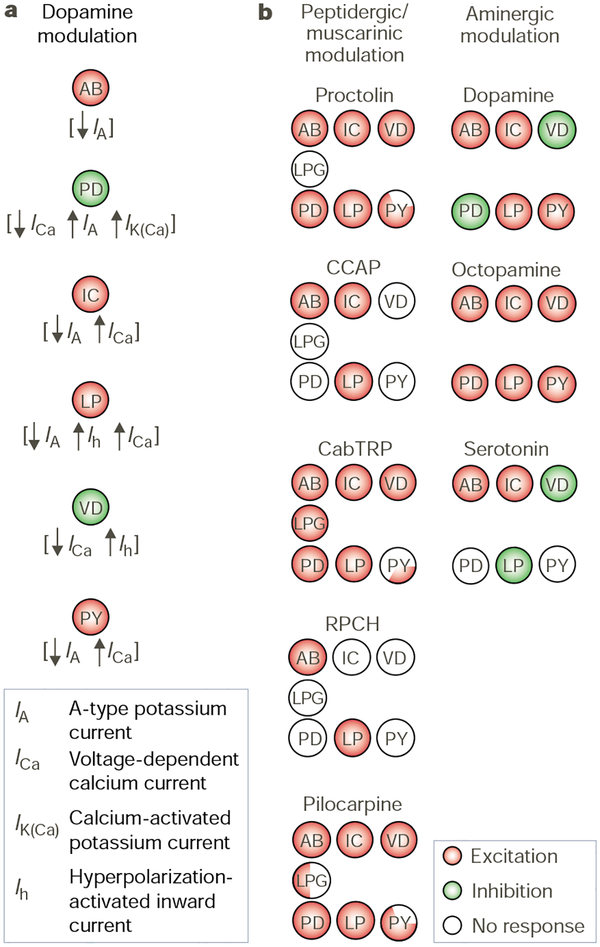
The presence of so many different neuromodulators in this system has provided an opportunity to determine the extent to which there is convergence and divergence of action. The actions produced by muscarinic acetylcholine, biogenic amines and neuropeptides in the STG provide examples of both21,56,91. For example, dopamine has a different effect on each pyloric neuron owing to its differential actions on multiple ion currents56 (Fig. 5a). In contrast, at least four different neuropeptides plus muscarinic agonists have convergent actions on a single, voltage-dependent ion current in the pyloric neurons56,91 (Fig. 5b). Despite this convergence at the current level, each of the modulators belonging to this latter group elicits different pyloric rhythms47,92 (Fig. 3a). At least part of this apparent discrepancy is because each modulator has direct actions on a distinct but overlapping subset of pyloric neurons91 (Fig. 5b). The same is true for the biogenic amines dopamine, serotonin and octopamine15 (Fig. 5b). In fact, the pyloric rhythm elicited by crustacean cardioactive peptide can be transformed into the pyloric rhythm elicited by the peptide proctolin by injecting the dynamic clamp version of the peptide-activated current into two pyloric neurons that respond only to proctolin21. Clearly, there is not a single underlying principle to describe the means by which different modulators elicit distinct outputs from the same network.
Figure 5.
Convergence and divergence of transmitter actions. a, Dopamine influences a distinct but overlapping set of ionic currents in each pyloric circuit neuron. Moreover, it can have opposite effects on the same current in different neurons. For example, dopamine has opposite effects on IA in the electrically coupled AB and PD neurons. Adapted from ref. 56. b, Different neuromodulators influence overlapping but distinct subsets of pyloric circuit neurons. All of the peptidergic and muscarinic actions are excitatory, as are the actions of octopamine. Dopamine and serotonin excite some pyloric neurons and inhibit others. RPCH, red pigment concentrating hormone; LPG, lateral posterior gastric neuron. Peptides and muscarinic actions adapted from ref. 91; amines adapted from ref. 15.
Co-transmission
In most systems, modulation of neural circuit activity is studied by direct application of a neuromodulator, usually because it is difficult to identify and selectively activate the modulatory neurons that release these substances. However, CPGs can generate distinct motor patterns not only when different modulators are applied directly to the isolated nervous system (Fig. 3a), but also when different modulatory neurons are activated11,15,36,93 (Fig. 3b).
Some early reports from work in the stomatogastric system supported the hypothesis that bath application of neuromodulators is equivalent to activating the relevant modulatory neuron11,15,34. However, modulatory neurons in this and other systems also utilize mechanisms that cannot be replicated using bath-application techniques11,46,83,94. For example, neurons commonly contain more than one neurotransmitter: often at least one neuropeptide and a small-molecule transmitter11,35. In addition to modulatory actions, the small-molecule transmitter usually has ionotropic actions, and ionotropic actions on rhythmically active circuits are not effectively mimicked by bath application.
Several modulatory neurons with identified co-transmitters occur in the stomatogastric system, including projection neurons11 (Fig. 3b) and sensory neurons8,34. Work with these neurons has illustrated that their co-transmitters exhibit both convergence and divergence of action. In some cases, these projection neurons affect subsets of their targets via only some of their co-transmitters11,95. Additionally, different projection neurons can elicit distinct STG rhythms despite having a neuropeptide (proctolin) transmitter in common11 (Fig. 3b). This results in part from the presence of distinct co-transmitters in these neurons, but also from a difference in the strength of the proctolin action onto the same pyloric neurons when this peptide is released from different projection neurons11,96. This latter result is at least partly a consequence of a differential regulation of proctolin, released from each neuron, by extracellular peptidase activity96.
Development of circuit modulation
Neural circuit development has been studied extensively in the vertebrate CNS, although little is known regarding the physiological development of neural circuits at the cellular level. However, recent developmental studies in the stomatogastric system are providing information that resonates with and extends those findings in the vertebrate systems97,98. For example, prior to the onset of feeding behaviour in the embryonic lobster, the STG rhythms are slower and less regular than in the adult, a situation paralleled in other developing networks99,100. Furthermore, the STG circuits are rhythmically active before the complete maturation of their modulatory inputs. This results in distinct activity patterns at different developmental times. Gradual maturation of the modulatory projection neurons to the STG through development seems to result primarily from the distinct developmental time at which different neuromodulators, including co-localized ones, first become expressed.
Closing the gaps
So far, most attention in the STG and comparable systems has focused on relating cellular and synaptic properties to circuit dynamics under steady-state and modulatory conditions. The stage is now set for expanding the scope of this endeavour to focus more intensively at how events at both the molecular and systems levels contribute to the control of neuronal circuit output. Additionally, to better bridge the gap between in vitro studies and normal activity in the intact animal, the next step is likely to involve working with the stomatogastric system while it remains connected with the rest of the CNS, isolated from the rest of the animal.
Previously, the complexity within invertebrate neural systems was thought to be a specialized adaptation of these animals that enabled them to use a relatively small number of neurons to perform a rich repertoire of behaviours. Because of their significantly larger numbers, neurons in the vertebrate nervous system were not expected to have the same range of computational complexity. It was therefore not certain whether the same basic principles guided neural circuit operation in these two groups of animals. It is now evident that vertebrate neurons and neural circuits are as multifunctional as their invertebrate counterparts2,3,6,87. Because of the similarities underlying neural circuit activity in both groups of animals, invertebrate models such as the crustacean stomatogastric nervous system continue to provide considerable insight into how the comparable neural circuits operate in the numerically larger and less accessible vertebrate CNS.
Acknowledgements
We thank E. Marder for helpful discussions and R. Harris-Warrick for feedback on an earlier version of the manuscript. Research support in the our laboratory is from National Institute of Neurological Disorders and Stroke grants.
References
- 1.Brown TG On the nature of the fundamental activity of the nervous centres; together with an analysis of the conditioning of rhythmic activity in progression, and a theory of the evolution of function in the nervous system. J. Physiol 48, 18–46 (1914). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marder E & Calabrese RL Principles of rhythmic motor pattern generation. Physiol. Rev 76, 687–717 (1996). [DOI] [PubMed] [Google Scholar]
- 3.Stein PSG, Grillner S, Selverston AI & Stuart DG (eds) Neurons, Networks, and Motor Behavior (The MIT Press, Cambridge, MA, 1997). [Google Scholar]
- 4.Marder E & Bucher D Central pattern generators and the control of rhythmic movements. Curr. Biol 11, R986–R996 (2001). [DOI] [PubMed] [Google Scholar]
- 5.Pearson KG Neural adaptation in the generation of rhythmic behavior. Annu. Rev. Physiol 62, 723–753 (2000). [DOI] [PubMed] [Google Scholar]
- 6.McCormick DA & Bal T Sleep and arousal: thalamocortical mechanisms. Annu. Rev. Neurosci 20, 185–215 (1997). [DOI] [PubMed] [Google Scholar]
- 7.Harris-Warrick RM, Marder E, Selverston AI & Moulins M (eds) Dynamic Biological Networks. The Stomatogastric Nervous System (MIT Press, Cambridge, MA, 1992). [Google Scholar]
- 8.Skiebe P Neuropeptides are ubiquitous chemical mediators: using the stomatogastric nervous system as a model system. J. Exp. Biol 204, 2035–2048 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Turrigiano GG & Heinzel H-G in Dynamic Biological Networks: The Stomatogastric Nervous System (eds Harris-Warrick RM, Marder E, Selverston AI & Moulins M) 197–220 (MIT Press, Cambridge, MA, 1992). [Google Scholar]
- 10.Clemens S, Combes D, Meyrand P & Simmers J Long-term expression of two interacting motor pattern-generating networks in the stomatogastric system of freely behaving lobster. J. Neurophysiol 79, 1396–1408 (1998). [DOI] [PubMed] [Google Scholar]
- 11.Nusbaum MP, Blitz DM, Swensen AM, Wood D & Marder E The roles of co-transmission in neural network modulation. Trends Neurosci 24, 146–154 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Heinzel HG, Weimann JM & Marder E The behavioral repertoire of the gastric mill in the crab, Cancer pagurus: an in situ endoscopic and electrophysiological examination. J. Neurosci 13, 1793–1803 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weimann JM & Marder E Switching neurons are integral members of multiple oscillatory networks. Curr. Biol 4, 896–902 (1994). [DOI] [PubMed] [Google Scholar]
- 14.Miller JP in The Crustacean Stomatogastric System (eds Selverston AI & Moulins M) 109–136 (Springer, Berlin, 1987). [Google Scholar]
- 15.Harris-Warrick RM, Nagy F & Nusbaum MP in Dynamic Biological Networks: The Stomatogastric Nervous System (eds Harris-Warrick RM, Marder E, Selverston AI & Moulins M) 87–138 (MIT Press, Cambridge, MA, 1992). [Google Scholar]
- 16.Hartline DK & Graubard K in Dynamic Biological Networks: The Stomatogastric Nervous System (eds Harris-Warrick RM, Marder E, Selverston AI & Moulins M) 31–86 (MIT Press, Cambridge, MA, 1992). [Google Scholar]
- 17.Hooper SL & Marder E Modulation of the lobster pyloric rhythm by the peptide proctolin. J. Neurosci 7, 2097–2112 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayali A & Harris-Warrick RM Monoamine control of the pacemaker kernel and cycle frequency in the lobster pyloric network. J. Neurosci 19, 6712–6722 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharp AA, O’Neil MB, Abbott LF & Marder E The dynamic clamp: artificial conductances in biological neurons. Trends Neurosci 16, 389–394 (1993). [DOI] [PubMed] [Google Scholar]
- 20.Bartos M, Manor Y, Nadim F, Marder E & Nusbaum MP Coordination of fast and slow rhythmic neuronal circuits. J. Neurosci 19, 6650–6660 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swensen AM & Marder E Modulators with convergent cellular actions elicit distinct circuit outputs. J. Neurosci 21, 4050–4058 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selverston AI, Kleindienst HU & Huber F Synaptic connectivity between cricket auditory interneurons as studied by selective photoinactivation. J. Neurosci 5, 1283–1292 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warzecha AK, Egelhaaf M & Borst A Neural circuit tuning fly visual interneurons to motion of small objects. I. Dissection of the circuit by pharmacological and photoinactivation techniques. J. Neurophysiol 69, 329–339 (1993). [DOI] [PubMed] [Google Scholar]
- 24.Kemenes G & Elliott CJ Analysis of the feeding motor pattern in the pond snail, Lymnaea stagnalis: photoinactivation of axonally stained pattern-generating interneurons. J. Neurosci 14, 153–166 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulrich D & Huguenard JR GABAA-receptor-mediated rebound burst firing and burst shunting in thalamus. J. Neurophysiol 78, 1748–1751 (1997). [DOI] [PubMed] [Google Scholar]
- 26.Jaeger D & Bower J Synaptic control of spiking in cerebellar Purkinje cells: dynamic current clamp based on model conductances. J. Neurosci 19, 6090–6101 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiehn O, Kjaerulff O, Tresch MC & Harris-Warrick R Contributions of intrinsic motor neuron properties to the production of rhythmic motor output in the mammalian spinal cord. Brain Res 53, 649–659 (2000). [DOI] [PubMed] [Google Scholar]
- 28.Elson RC & Selverston AI Mechanisms of gastric rhythm generation in the isolated stomatogastric ganglion of spiny lobsters: bursting pacemaker potentials, synaptic interactions, and muscarinic modulation. J. Neurophysiol 68, 890–907 (1992). [DOI] [PubMed] [Google Scholar]
- 29.Coleman MJ & Nusbaum MP Functional consequences of compartmentalization of synaptic input. J. Neurosci 14, 6544–6552 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Combes D, Meyrand P & Simmers J Dynamic restructuring of a rhythmic motor program by a single mechanoreceptor neuron in lobster. J. Neurosci 19, 3620–3628 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koshiya N & Smith JC Neuronal pacemaker for breathing visualized in vitro. Nature 400, 360–363 (1999). [DOI] [PubMed] [Google Scholar]
- 32.Parker D & Grillner S Neuronal mechanisms of synaptic and network plasticity in the lamprey spinal cord. Prog. Brain Res 125, 381–398 (2000). [DOI] [PubMed] [Google Scholar]
- 33.Thoby-Brisson M & Ramirez JM Role of inspiratory pacemaker neurons in mediating the hypoxic response of the respiratory network in vitro. J. Neurosci 20, 5858–5866 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katz PS & Harris-Warrick RM Neuromodulation of the crab pyloric central pattern generator by serotonergic/cholinergic proprioceptive afferents. J. Neurosci 10, 1495–1512 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marder E, Christie AE & Kilman VL Functional organization of cotransmission systems: lessons from small nervous systems. Invert. Neurosci 1, 105–112 (1995). [DOI] [PubMed] [Google Scholar]
- 36.Norris BJ, Coleman MJ & Nusbaum MP Pyloric motor pattern modification by a newly identified projection neuron in the crab stomatogastric nervous system. J. Neurophysiol 75, 97–108 (1996). [DOI] [PubMed] [Google Scholar]
- 37.Combes D, Meyrand P & Simmers J Motor pattern specification by dual descending pathways to a lobster rhythm-generating network. J. Neurosci 19, 3610–3619 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morris LG, Thuma JB & Hooper SL Muscles express motor patterns of non-innervating neural networks by filtering broad-band input. Nature Neurosci 3, 245–250 (2000). [DOI] [PubMed] [Google Scholar]
- 39.Hooper SL & Weaver AL Motor neuron activity is often insufficient to predict motor response. Curr. Opin. Neurobiol 10, 676–682 (2000). [DOI] [PubMed] [Google Scholar]
- 40.Katz PS, Kirk MD & Govind CK Facilitation and depression at different branches of the same motor axon: evidence for presynaptic differences in release. J. Neurosci 13, 3075–3089 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jorge-Rivera JC, Sen K, Birmingham JT, Abbott LF & Marder E Temporal dynamics of convergent modulation at a crustacean neuromuscular junction. J. Neurophysiol 80, 2559–2570 (1998). [DOI] [PubMed] [Google Scholar]
- 42.Gutovitz S, Birmingham JT, Luther JA, Simon DJ & Marder E GABA enhances transmission at an excitatory glutamatergic synapse. J. Neurosci 21, 5935–5943 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katz PS & Tazaki K in Dynamic Biological Networks: The Stomatogastric Nervous System (eds Harris-Warrick RM, Marder E, Selverston AI & Moulins M) 221–262 (MIT Press, Cambridge, MA, 1992). [Google Scholar]
- 44.Katz PS & Harris-Warrick RM The evolution of neuronal circuits underlying species-specific behavior. Curr. Opin. Neurobiol 9, 628–633 (1999). [DOI] [PubMed] [Google Scholar]
- 45.Meyrand P, Faumont S, Simmers J, Christie AE & Nusbaum MP Species-specific modulation of pattern-generating circuits. Eur. J. Neurosci 12, 2585–2596 (2000). [DOI] [PubMed] [Google Scholar]
- 46.Meyrand P, Simmers J & Moulins M Dynamic construction of a neural network from multiple pattern generators in the lobster stomatogastric nervous system. J. Neurosci 14, 630–644 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marder E & Hooper SL in Model Neural Networks and Behavior (ed. Selverston AI) 319–337 (Plenum, New York, 1985). [Google Scholar]
- 48.Tierney AJ & Harris-Warrick RM Physiological role of the transient potassium current in the pyloric circuit of the lobster stomatogastric ganglion. J. Neurophysiol 67, 599–609 (1992). [DOI] [PubMed] [Google Scholar]
- 49.Elson RC, Huerta R, Abarbanel HD, Rabinovich MI & Selverston AI Dynamic control of irregular bursting in an identified neuron of an oscillatory circuit. J. Neurophysiol 82, 115–122 (1999). [DOI] [PubMed] [Google Scholar]
- 50.Hooper SL Phase maintenance in the pyloric pattern of the lobster (Panulirus interruptus) stomatogastric ganglion. J. Comput. Neurosci 4, 191–205 (1997). [DOI] [PubMed] [Google Scholar]
- 51.Harris-Warrick RM & Flamm RE Multiple mechanisms of bursting in a conditional bursting neuron. J. Neurosci 7, 2113–2128 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baro DJ & Harris-Warrick RM Differential expression and targeting of K+ channel genes in the lobster pyloric central pattern generator. Ann. NY Acad. Sci 860, 281–295 (1998). [DOI] [PubMed] [Google Scholar]
- 53.Baro DJ et al. Molecular underpinnings of motor pattern generation: differential targeting of shal and shaker in the pyloric motor system. J. Neurosci 20, 6619–6630 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kloppenburg P, Zipfel WR, Webb WW & Harris-Warrick RM Highly localized Ca2+ accumulation revealed by multiphoton microscopy in an identified motoneuron and its modulation by dopamine. J. Neurosci 20, 2523–2533 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reyes A Influence of dendritic conductances on the input-output properties of neurons. Annu. Rev. Neurosci 24, 653–675 (2001). [DOI] [PubMed] [Google Scholar]
- 56.Harris-Warrick RM et al. Distributed effects of dopamine modulation in the crustacean pyloric network. Ann. NY Acad. Sci 860, 155–167 (1998). [DOI] [PubMed] [Google Scholar]
- 57.Goldman MS, Golowasch J, Marder E & Abbott LF Global structure, robustness, and modulation of neuronal models. J. Neurosci 21, 5229–5238 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hooper SL Transduction of temporal patterns by single neurons. Nature Neurosci 1, 720–726 (1998). [DOI] [PubMed] [Google Scholar]
- 59.Soto-Treviño C, Thoroughman KA, Marder E & Abbott LF Activity-dependent modification of inhibitory synapses in models of rhythmic neural networks. Nature Neurosci 4, 297–303 (2000). [DOI] [PubMed] [Google Scholar]
- 60.Selverston AI et al. Reliable circuits from irregular neurons: a dynamical approach to understanding central pattern generators. J. Physiol. Paris 94, 357–374 (2000). [DOI] [PubMed] [Google Scholar]
- 61.Marder E et al. Physiological insights from cellular and network models of the stomatogastric nervous systems of lobsters and crabs. Am. Zool 33, 29–39 (1993). [Google Scholar]
- 62.Nadim F, Manor Y, Nusbaum MP & Marder E Frequency regulation of a slow rhythm by a fast periodic input. J. Neurosci 18, 5053–5067 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Edwards DH, Yeh SR & Krasne FB Neuronal coincidence detection by voltage-sensitive electrical synapses. Proc. Natl Acad. Sci. USA 95, 7145–7150 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perez-Velazquez JL & Carlen PL Gap junctions, synchrony and seizures. Trends Neurosci 23, 68–74 (2000). [DOI] [PubMed] [Google Scholar]
- 65.Galarreta M & Hestrin S Electrical synapses between GABA-releasing interneurons. Nature Rev. Neurosci 2, 425–433 (2001). [DOI] [PubMed] [Google Scholar]
- 66.Schmitz D et al. Axo-axonal coupling: a novel mechanism for ultrafast neuronal communication. Neuron 31, 831–840 (2001). [DOI] [PubMed] [Google Scholar]
- 67.Marder E Roles for electrical coupling in neural circuits as revealed by selective neuronal deletions. J. Exp. Biol 112, 147–167 (1984). [DOI] [PubMed] [Google Scholar]
- 68.Kiehn O & Tresch MC Gap junctions and motor behavior. Trends Neurosci 25, 108–115 (2002). [DOI] [PubMed] [Google Scholar]
- 69.Graubard K & Hartline DK Full-wave rectification from a mixed electrical-chemical synapse. Science 237, 535–537 (1987). [DOI] [PubMed] [Google Scholar]
- 70.Johnson BR, Peck JH & Harris-Warrick RM Differential modulation of chemical and electrical components of mixed synapses in the lobster stomatogastric ganglion. J. Comp. Physiol. A 175, 233–249 (1994). [DOI] [PubMed] [Google Scholar]
- 71.Coleman MJ, Meyrand P & Nusbaum MP A switch between two modes of synaptic transmission mediated by presynaptic inhibition. Nature 378, 502–505 (1995). [DOI] [PubMed] [Google Scholar]
- 72.Nadim F & Manor Y The role of short-term synaptic dynamics in motor control. Curr. Opin. Neurobiol 10, 683–690 (2000). [DOI] [PubMed] [Google Scholar]
- 73.Parker D & Grillner S Activity-dependent metaplasticity of inhibitory and excitatory synaptic transmission in the lamprey spinal cord locomotor network. J. Neurosci 19, 1647–1656 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Manor Y & Nadim F Synaptic depression mediates bistability in neuronal networks with recurrent inhibitory connectivity. J. Neurosci 21, 9460–9470 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Turrigiano G Homeostatic plasticity in neuronal networks: the more things change, the more they stay the same. Trends Neurosci 22, 221–217 (1999). [DOI] [PubMed] [Google Scholar]
- 76.Turrigiano GG & Nelson SB Hebb and homeostasis in neuronal plasticity. Curr. Opin. Neurobiol 10, 358–364 (2000). [DOI] [PubMed] [Google Scholar]
- 77.Turrigiano GG, LeMasson G & Marder E Selective regulation of current densities underlies spontaneous changes in the activity of cultured neurons. J. Neurosci 15, 3640–3652 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mizrahi A et al. Long-term maintenance of channel distribution in a central pattern generator neuron by neuromodulatory inputs revealed by decentralization in organ culture. J. Neurosci 21, 7331–7339 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bartos M & Nusbaum MP Intercircuit control of motor pattern modulation by presynaptic inhibition. J. Neurosci 17, 2247–2256 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kawahara K, Kumagai S, Nakazono Y & Myamoto Y Coupling between respiratory and stepping rhythms during locomotion in decerebrate cats. J. Appl. Physiol 67, 110–115 (1989). [DOI] [PubMed] [Google Scholar]
- 81.Larson CR, Yajima Y & Ko P Modification of activity of medullary respiratory-related neurons for vocalizing and swallowing. J. Neurophysiol 71, 2294–2304 (1994). [DOI] [PubMed] [Google Scholar]
- 82.Marder E & Meyrand M in Neuronal and Cellular Oscillators (ed. Jacklet J) 317–338 (Dekker, New York, 1989). [Google Scholar]
- 83.Meyrand P, Simmers J & Moulins M Construction of a pattern-generating circuit with neurons of different networks. Nature 351, 60–63 (1991). [DOI] [PubMed] [Google Scholar]
- 84.Hooper SL & Moulins M Cellular and synaptic mechanisms responsible for a long-lasting restructuring of the lobster pyloric network. J. Neurophysiol 64, 1574–1589 (1990). [DOI] [PubMed] [Google Scholar]
- 85.Soffe SR Two distinct rhythmic motor patterns are driven by common premotor and motor neurons in a simple vertebrate spinal cord. J. Neurosci 13, 4456–4469 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Earhart GM & Stein PSG Step, swim and scratch motor patterns in the turtle. J. Neurophysiol 84, 2181–2190 (2000). [DOI] [PubMed] [Google Scholar]
- 87.Lieske SP, Thoby-Brisson M, Telgkamp P & Ramirez JM Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps. Nature Neurosci 3, 600–607 (2000). [DOI] [PubMed] [Google Scholar]
- 88.Dickinson PS, Mecsas C & Marder E Neuropeptide fusion of two motor-pattern generator circuits. Nature 344, 155–158 (1990). [DOI] [PubMed] [Google Scholar]
- 89.Scholz NL, de Vente J, Truman JW & Graubard K Neural network partitioning by NO and cGMP. J. Neurosci 21, 1610–1618 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hemple CM, Vincent P, Adams SR, Tsien RY & Selverston AI Spatio-temporal dynamics of cyclic AMP signals in an intact neural circuit. Nature 384, 166–169 (1996). [DOI] [PubMed] [Google Scholar]
- 91.Swensen AM & Marder E Multiple peptides converge to activate the same voltage-dependent current in a central pattern-generating circuit. J. Neurosci 20, 6752–6759 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marder E & Calabrese RL Principles of rhythmic motor pattern generation. Physiol. Rev 76, 687–717 (1996). [DOI] [PubMed] [Google Scholar]
- 93.Nagy F & Cardi P A rhythmic modulatory gating system in the stomatogastric nervous system of Homarus gammarus. II. Modulatory control of the pyloric CPG. J. Neurophysiol 71, 2490–2502 (1994). [DOI] [PubMed] [Google Scholar]
- 94.Perrins R & Weiss KR Compartmentalization of information processing in an aplysia feeding circuit interneuron through membrane properties and synaptic interactions. J. Neurosci 18, 3977–3989 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wood DE, Stein W & Nusbaum MP Projection neurons with shared cotransmitters elicit different motor patterns from the same neural circuit. J. Neurosci 20, 8943–8953 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wood DE & Nusbaum MP Extracellular peptidase activity tunes motor pattern modulation. J. Neurosci 22, 4185–4195 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Richards KS & Marder E The actions of crustacean cardioactive peptide on adult and developing stomatogastric ganglion motor patterns. J. Neurobiol 44, 31–44 (2000). [PubMed] [Google Scholar]
- 98.Le Feuvre Y, Fenelon VS & Meyrand P Ontogeny of modulatory inputs to motor networks: early established projection and progressive neurotransmitter acquisition. J. Neurosci 21, 1313–1326 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.O’Donovan MJ The origin of spontaneous activity in developing networks of the vertebrate nervous system. Curr. Opin. Neurobiol 9, 94–104 (1999). [DOI] [PubMed] [Google Scholar]
- 100.Wong ROL Retinal waves and visual system development. Annu. Rev. Neurosci 22, 29–47 (1999). [DOI] [PubMed] [Google Scholar]