Abstract
Histamine is a neurotransmitter with actions throughout the nervous system of vertebrates and invertebrates. Nevertheless, the actions of only a few identified histamine-containing neurons have been characterized. Here, we present the actions of a histaminergic projection neuron on the rhythmically active pyloric and gastric mill circuits within the stomatogastric ganglion (STG) of the crab Cancer borealis. An antiserum generated against histamine labeled profiles throughout the C. borealis stomatogastric nervous system. Labeling occurred in several somata and neuropil within the paired commissural ganglia as well as in neuropil within the STG and at the junction of the superior oesophageal and stomatogastric nerves. The source of all histamine-like immunolabeling in the STG neuropil was one pair of neuronal somata, the previously identified inferior ventricular (IV) neurons, located in the supraoesophageal ganglion. These neurons also exhibited FLRFamide-like immunoreactivity. Activation of the IV neurons in the crab inhibited some pyloric and gastric mill neurons and, with inputs from the commissural ganglia eliminated, terminated both rhythms. Focal application of histamine had comparable effects. The actions of both applied histamine and IV neuron stimulation were blocked, reversibly, by the histamine type-2 receptor antagonist cimetidine. With the commissural ganglia connected to the STG, IV neuron stimulation elicited a longer-latency activation of commissural projection neurons which in turn modified the pyloric rhythm and activated the gastric mill rhythm. These results support the hypothesis that the histaminergic/peptidergic IV neurons are projection neurons with direct and indirect actions on the STG circuits of the crab C. borealis.
Keywords: crustacea, neuromodulation, stomatogastric ganglion, cotransmission, histamine, FLRFamide-like peptides
Neuronal circuits that control behavior are modulated by a host of neuroactive substances (Christie et al., 1995a; Marder and Calabrese, 1996; Marder and Bucher, 2001; Nusbaum et al., 2001; Skiebe, 2001). These substances are often colocalized and coreleased from neurons that modulate neuronal circuits. Cotransmitters commonly include at least one small molecule transmitter and one neuropeptide. The actions of coreleased transmitters are often not a simple summation of their individual effects on each target neuron (Nusbaum et al., 2001; Nusbaum, 2002). Thus, a complete understanding of neural circuit modulation requires the identification of the neurotransmitters used to alter circuit activity, including their mode of delivery (e.g., hormonal vs. local release), an accurate description of their patterns of colocalization in inputs to that circuit, and their specific actions on target neurons and circuits.
The crustacean stomatogastric nervous system (STNS) is particularly amenable to such studies. The STNS is an extension of the crustacean central nervous system that contains the unpaired stomatogastric (STG) and oesophageal (OG) ganglia plus the paired commissural ganglia (CoGs). Neural circuits contained within these ganglia control the rhythmic processing of food through the foregut. Overlapping sets of STG neurons comprise the gastric mill and pyloric circuits, which generate the gastric mill (chewing) and pyloric (filtering of chewed food) rhythms (Harris-Warrick et al., 1992; Nusbaum and Beenhakker, 2002). There are 25–30 neurons in the STG, depending on the species (Maynard, 1971; King, 1976; Kilman and Marder, 1996), and their properties and connectivity are well-characterized (Harris-Warrick et al., 1992; Weimann and Marder, 1994; Marder and Calabrese, 1996; Nusbaum and Beenhakker, 2002).
A variety of neuromodulators evoke different forms of the pyloric and gastric mill rhythms (Marder and Hooper, 1985; Harris-Warrick et al., 1992; Marder and Weimann, 1992; Nusbaum et al., 2001). These modulators include numerous peptides, small molecule transmitters, and the gas nitric oxide (Nusbaum et al., 2001; Scholz et al., 2001; Skiebe, 2001). Projection neurons whose somata are located in the OG and CoGs provide most of the modulatory innervation to the STG circuits (Coleman et al., 1992). In the crab Cancer borealis, there are likely to be fewer than 20 distinct neuron types that project to the STG from these ganglia (Coleman et al., 1992). Five of these projection neurons are well characterized (Norris et al., 1994, Norris et al., 1996; Nusbaum et al., 2001; Wood and Nusbaum, 2002).
In this report, we aimed to expand our characterization of projection neurons in C. borealis by studying a pair of projection neurons whose somata are located in the supraoesophageal ganglion, commonly referred to as the brain. These neurons are the inferior ventricular (IV) neurons (Dando and Selverston, 1972). Although not previously studied in C. borealis, the IV neurons have been identified and characterized in several different crustaceans. In lobsters, the IV neurons exhibit a histaminergic/ peptidergic phenotype (Claiborne and Selverston, 1984a; Le Feuvre et al., 2001; Pulver et al., 2003) and they evoke complex fast and slow synaptic potentials in STG neurons (Russell and Hartline, 1981; Sigvardt and Mulloney, 1982a; Claiborne and Selverston, 1984a; Marder and Eisen, 1984; Cazalets et al., 1990b; Dickinson et al., 1990, 2001). Some of the IV neuron actions are distinct in different lobster species (Dickinson et al., 1990, 1993, 2001; Meyrand et al., 1991, 1994).
Histamine (HA) is used as a neurotransmitter at several invertebrate synapses, where it commonly plays an inhibitory role (Claiborne and Selverston, 1984a; Weiss et al., 1986b; Nassel et al., 1988; McClintock and Ache, 1989; Skiebe et al., 1990; Battelle et al., 1991, 1999; Homberg and Hildebrand, 1991; Hashemzadeh-Gargari and Freschi, 1992; Buchner et al., 1993; Schmid and Duncker, 1993; el Manira and Clarac, 1994; Horner et al., 1996; Callaway and Stuart, 1999; Evans et al., 1999; Nassel, 1999). However, there are few previous studies of how identified histaminergic neurons influence rhythmically active neural circuits (Weiss et al., 1986b; Evans et al., 1999).
In this study, we show that the IV neurons in C. borealis exhibit both HA-like and FLRFamide-like immunoreactivity and that the direct actions of the IV neurons on the STG circuits are largely inhibitory and histaminergic. In contrast, we show that the IV neurons have indirect excitatory actions on these circuits as a consequence of activating CoG projection neurons. We discuss the possibility that these latter actions result from the FLRFamide-like cotransmitter. We also discuss the similarities and differences between the IV neuron actions on the STG circuits and projection neurons in C. borealis with those previously documented in lobsters. Some of this work appeared previously in abstract form (Christie et al., 2000).
MATERIALS AND METHODS
Animals
Jonah crabs, Cancer borealis, were obtained from Commercial Lobster and Seafood Company (Boston, MA) and the Marine Biological Laboratory (Woods Hole, MA). Animals were maintained in recirculating artificial seawater aquaria at 10–12°C and fed fresh squid approximately twice per week. Animals were anesthetized by packing in ice for 30–60 minutes immediately before dissection. Dissection of the STNS was done in chilled (~4°C) physiological saline (composition in mM: NaCl, 440; MgCl2, 26; CaCl2, 13; KCl, 11; Trizma base, 10; maleic acid, 5; pH 7.4–7.6).
Antibodies
Histamine immunoprocessing was accomplished by using a rabbit polyclonal antiserum generated against HA (Panula et al., 1988) at a final dilution of 1:500 with an incubation time of approximately 72 hours. This antibody was obtained commercially (Accurate Chemical and Scientific Corp., Westbury, NY; Immunostar, Hudson, WI). Allatostatin-like immunoreactivity was examined by using a rabbit polyclonal antiserum generated against allatostatin 5 and was a kind gift of Dr. René Feyereisen (University of Arizona, Tucson, AZ). This antibody was used at a final dilution of 1:500 with an incubation time of 24–48 hours. Cholecystokinin (CCK) -like immunolabeling used a mouse monoclonal antibody (anti-CCKC36-9H) generated against mammalian CCK8 (Christie et al., 1995b). This antibody was a kind gift from Dr. Anthony Stretton (University of Wisconsin, Madison, WI) and was used at a final dilution of 1:300 and an incubation time of 12–24 hours. FLRFamide-like immunoprocessing used either of several rabbit polyclonal antisera, including anti-FMRFamide231 (O’Donohue et al., 1984), anti-FMRFamide671 (Marder et al., 1987), and anti-FMRFamideImmunostar (Blitz et al., 1999). Because the native C. borealis peptides are themselves N-terminally extended FLRFamides, specifically SDRNFLRFamide, TNRNFLRFamide, and NRNFLRFamide (Weimann et al., 1993; Li et al., 2003), immunoreactivity resulting from the use of these reagents will be indicated as FLRFamide-related immunolabeling. Anti-FMRFamide231 was a kind gift from Dr. Winsor Watson (University of New Hampshire). Anti-FMRFImmunostar was purchased commercially (Immunostar). All anti-FMRFamide antibodies were used at a final dilution of 1:300 with incubation times of 12–24 hours. The anti-gamma-amino butyric acid (anti-GABA) antibody was a rabbit polyclonal antiserum obtained from Sigma (St. Louis, MO) and was used at a final dilution of 1:300 with an incubation time of 3 to 7 days (Swensen et al., 2000). We used two different anti-proctolin antisera that were kind gifts from Dr. Hans Agricola (Friedrich-Schiller-Universitat, Jena, Germany) and Dr. Dick R. Nassel (Department of Zoology, Stockholm University, Stockholm, Sweden). Both were rabbit polyclonal antisera used with incubation times of 12–24 hours. The former antiserum was used at a final dilution of 1:1500, whereas the latter antiserum was used at a final dilution of 1:5,000 (Blitz et al., 1999). The anti-red pigment concentrating hormone antibody was a rabbit polyclonal antiserum that was a kind gift from Dr. Robert Elde (College of Biological Sciences, University of Minnesota, Minneapolis, MN). This antiserum was used at a final dilution of 1:200, with an incubation time of 24–48 hours (Nusbaum and Marder, 1988). All secondary antibodies were purchased from Calbiochem (San Diego, CA) and were used at final dilutions of 1:25–1:300.
Histamine conjugate production
To help determine the specificity of HA-like immunoreactivity, a synthetic antigen identical to that used for the production of the anti-HA antiserum was produced (Panula et al., 1984). In brief, a solution of histamine dihydrochloride (20 mg; Sigma) and succinylated keyhole limpet hemocyanin (KLH;10 mg; Sigma) in distilled water (3.0 ml) was prepared, to which was added a solution of 100 mg/ml 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDAC; Sigma) in distilled water (0.2 ml). The pH of this solution was adjusted to 5.0–6.0, and the solution was maintained overnight at room temperature (approximately 20°C). The resulting conjugate was then dialyzed against distilled water and subsequently lyophilized for storage. Dried conjugate was reconstituted immediately before being used (see below).
Anatomy
Whole-mount immunocytochemistry.
Whole-mount immunocytochemistry was performed as described previously (Christie et al., 1995b; Blitz et al., 1999). Briefly, the isolated STNS was fixed for 2–24 hours in either freshly made 4% EDAC in 0.1 M sodium phosphate buffer (P, pH 7.4) or 4% paraformaldehyde in P (pH 7.4). Fixed tissue was rinsed five times over approximately 5 hours in a solution of 0.1 M sodium phosphate (pH 7.2) containing 0.3% Triton X-100 (P-Triton). EDAC fixative was used for all preparations processed with the anti-HA antibody (Panula et al., 1988; Mulloney and Hall, 1991). Preparations involving other antibodies were fixed with the paraformaldehyde solution. Fixations for anti-GABA immunolabeling were 2–3 hours (Swensen et al., 2000). All other fixations were 12–24 hours (Christie et al., 1995b; Blitz et al., 1999). Incubation in primary antibody was done in P-Triton, with 10% goat normal serum (GNS) added to diminish nonspecific binding. After incubation in primary antibody, tissues were again rinsed five times over approximately 5 hours in P-Triton and then incubated for 18–24 hours in either goat anti-rabbit or goat anti-mouse IgG conjugated with either fluorescein isothiocyanate (FITC) or rhodamine (secondary antibody determined by the host species in which the primary antibody was raised). As with the primary antibody, secondary antibody incubation was done in P-Triton with 10% GNS. After secondary antibody incubation, each preparation was rinsed five times over approximately 5 hours in P and then mounted between a glass microscope slide and coverslip by using a solution of 80% glycerin, 20% 20 mM sodium carbonate, pH ~9.0.
Preadsorption controls.
To strengthen the likelihood that the HA-like and FLRFamide-like immunoreactivity seen in the crab STNS were due to the presence of HA and a N-terminally extended FLRFamide, respectively, we conducted a series of preadsorption controls. The polyclonal anti-HA antibody used in our study was generated in rabbit against an HA-KLH conjugate. Thus, in our preadsorption controls, we used either HA-KLH conjugate, HA, unconjugated KLH, or TNRNFLRFamide as blocking agents. In each blocking experiment, the anti-HA antibody was incubated with one of these blocking agents for 2 hours at room temperature before applying the solution to the STNS. Immunoprocessing was then performed as described above. A similar set of preadsorption controls were done for the anti-FLRFamide antibody supplied by Immunostar. Here, TNRNFLRFamide and HA-KLH conjugates were used as the blocking agents.
Nerve backfills.
To determine the projection pattern and cotransmitter complement of the IV neurons, Lucifer yellow-CH (LY; Sigma) backfills of several different nerves were made by using standard techniques (Coleman et al., 1992; Blitz et al., 1999). In these experiments, a Vaseline well was built around the nerve of interest and the saline within the well was replaced with distilled water. After several minutes, the distilled water was removed and replaced with a solution of 10–20% LY in distilled water, after which the nerve was transected within the well. The preparation was then incubated at 4°C for 18–72 hours, after which the dye was removed from the well and the preparation either fixed and immunoprocessed as described above or fixed and mounted for viewing as described below.
Laser scanning confocal microscopy.
After processing as described above, whole-mounts of the STNS were viewed and images were collected by using either a BioRad MRC 600 laser scanning confocal microscope (Li et al., 2002) or a Leica (Nussloch, Germany) TCS_NT laser scanning confocal microscope. Most work was performed with the Leica system. This system was equipped with a Leica DMRBE upright microscope as well as argon and krypton/argon lasers. Leica-supplied FITC (RSP500, BP530/30), tetrarhodamine isothiocyanate (TRITC; DD488/568, LP590), and FITC-TRITC (DD488/568, RSP580, Bp530/30, LP590) filter sets and Leica TCS_NT software were used to collect images on this system. Confocal photomicrographs were produced using Photoshop software (version 3.0.1 for Silicon Graphics; Adobe Systems, Inc., San Jose, CA).
Electrophysiology
Electrophysiological experiments were performed by using standard techniques (Bartos and Nusbaum, 1997; Blitz et al., 1999). Briefly, the entire C. borealis STNS was pinned down in a silicone polymer (Sylgard 184; Dow-Corning) -lined Petri dish. The STNS consists of four ganglia plus their connecting and peripheral nerves (Fig. 1). These ganglia include the STG (26 neurons), OG (14 neurons), and the paired CoGs (approximately 500 neurons each). In some electrophysiology experiments, the STG was isolated from the CoGs by transection of the paired superior oesophageal (sons) and inferior oesophageal (ions) nerves.
Fig. 1.
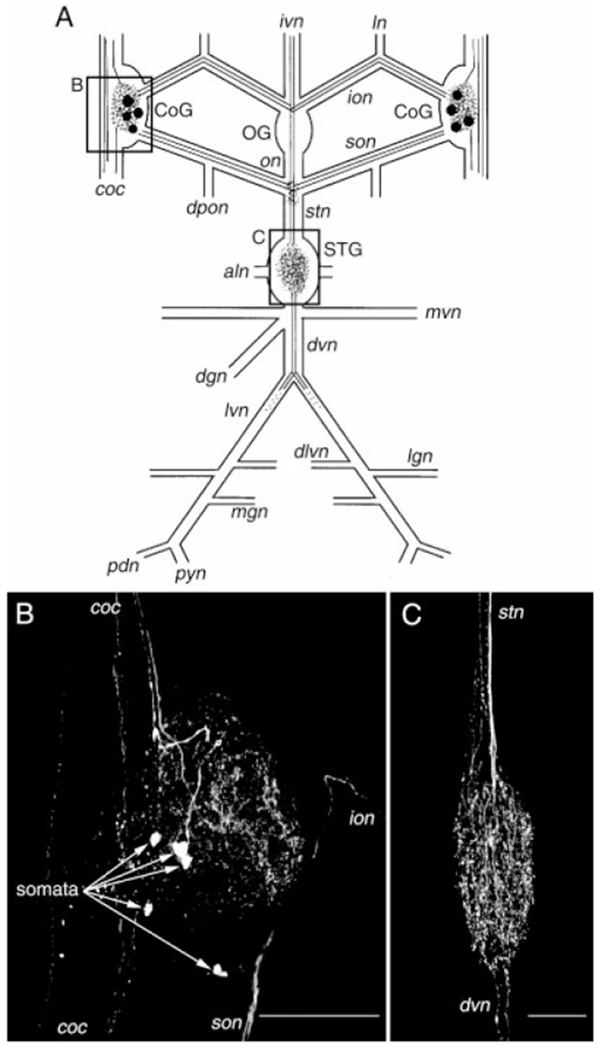
Distribution of histamine-like (HA-like) immunolabeling in the stomatogastric nervous system (STNS) of Cancer borealis. A: Schematic representation of the STNS showing the distribution of HA-like labeling. Labeled somata are indicated by filled circles, axons by solid or dotted lines, and neuropil by stippling. The boxed areas of the schematic show the relative positions of the photomicrographs shown in B and C. B: Confocal photomicrograph showing an HA-like immunolabeled commissural ganglion (CoG). In this image, five somata are present (arrows) as well as neuropil and axons in several of the nerves that connect with this ganglion. C: Confocal photomicrograph showing the distribution of HA-like immunolabeling in the stomatogastric ganglion (STG). HA-like staining within the STG is restricted to the neuropil. None of the approximately 25 neurons present in this ganglion exhibit HA-like immunoreactivity. B and C are composite images of 41 and 28 optical sections, respectively, taken at approximately 2μm intervals. Ganglia: OG, oesophageal ganglion. Nerves: aln, anterior lateral nerve; coc, circumoesophageal connective; dgn, dorsal gastric nerve; dlvn, dorsal lateral ventricular nerve; dpon, dorsal posterior oesophageal nerve; dvn dorsal ventricular nerve; ion, inferior oesophageal nerve; ivn, inferior ventricular nerve; lgn, lateral gastric nerve; ln, labral nerve; lvn, lateral ventricular nerve; mgn, medial gastric nerve; mvn, medial ventricular nerve; on, oesophageal nerve; pdn, pyloric dilator nerve; pyn, pyloric nerve; stn, stomatogastric nerve; son, superior oesophageal nerve. Scale bars = 250 μm in B, 100 μm in C.
Histamine was applied to the STG neuropil by focal pressure ejection. Other substances were applied to the STG by either superfusion or focal pressure ejection. Superfusion rates were 7–10 ml/min with a bath volume of approximately 10 ml. Bath temperature was maintained at 10–12°C. Pressure ejection (2–5 seconds duration, 6 PSI) was accomplished by using a Picospritzer II pressure unit (General Valve Company, Fairfield, NJ) with micropipettes ranging from 1 to 10 MΩ resistance. The STG was desheathed to facilitate both access of applied substances to the STG neuropil and intracellular recordings of STG neurons. For intracellular recording, the STG was viewed with darkfield illumination (Nikon, Tokyo, Japan). Intracellular recordings of STG somata were made using glass microelectrodes (15–25 MΩ) filled with 4 M potassium acetate plus 20 mM KCl. Extracellular recordings of the peripheral nerves were accomplished by placing a Vaseline well around a section of each nerve of interest and recording the activity in that nerve relative to ground by using one stainless steel wire electrode in the well and a second wire electrode in the common bath. Intracellular current injection was performed by using Axoclamp 2 amplifiers (Axon Instruments, Foster City, CA) in single-electrode discontinuous current-clamp (DCC) mode. Sample rates during DCC were 2–3 kHz.
The IV neurons were stimulated extracellularly by means of the inferior ventricular nerve (ivn: 15–40 Hz) by using a Grass S88 stimulator and Grass SIU5 stimulus isolation unit (Astro-Med/Grass Instruments, Warwick, RI). Extracellular IV neuron stimulation has been performed routinely in P. interruptus (Sigvardt and Mulloney, 1982a; Dickinson et al., 2001). STG neurons were identified on the basis of their axonal projection patterns, their activity patterns, and their interactions with other STG neurons (Weimann et al., 1991; Norris et al., 1996; Bartos and Nusbaum, 1997).
Data were collected on a MT95000 chart recorder (Astro-Med/Grass Instruments) and videotape (Wintron Technologies, Howard, PA). Figures were prepared by scanning sequences of recordings by using an HP Scanjet IIc (Hewlett Packard) with Deskscan II (version 2.0) software. Final figures were prepared with CorelDraw (version 3.0 for Windows) and Photo-Paint (version 3.0, Corel Corp., Ottawa, Ontario, Canada) software. Statistics were generated using Sigma Plot 4.0 and Sigma Stat 2.03 (SPSS, Inc., Chicago, IL). Data are presented as means ± standard deviation.
RESULTS
Histamine-like immunolabeling in the stomatogastric nervous system
To examine the distribution of histamine-like immunoreactivity in the STNS (Fig. 1A), we carried out whole-mount immunocytochemistry followed by confocal microscopy. Whole-mount immunolabeling in the commissural ganglia (n = 23 crabs, 45 ganglia) revealed HA-like immunoreactivity in as many as six neuronal somata per CoG (mean, 3.5 ± 1.7 somata; range, 0–6 somata) as well as numerous neuropilar processes (Fig. 1B). One of these somata projected an axon through the CoG neuropil, arborized therein and then continued toward the brain through the circumoesophageal connective (coc). The coc links the brain with the thoracic ganglionic ring. The remaining immunopositive somata were often less intensely labeled by the anti-HA antibody. Therefore, it was not possible to determine the projection pattern of these neurons. In addition to the axon originating from the brightly labeled CoG soma, several additional fibers were present in the coc. Several of these fibers projected from the coc into the CoG neuropil and may well have contributed to the HA-like-immunoreactive profiles seen in this neuropil. HA-like-immunopositive axons were also present in both the superior and inferior oesophageal nerves. Within each son (n = 46 sons), two axons were always labeled by the HA antibody. Two HA-like-immunopositive axons were also always seen in each ion (n = 46 ions).
None of the intrinsic OG somata exhibited HA-like immunolabeling, but several immunopositive fibers projected through this ganglion (n = 23 preparations; data not shown). Specifically, in 22 of 23 preparations, a pair of HA-like-immunoreactive axons projected through the OG from the ivn, which connects the OG with the brain. Within the OG, the HA-like-immunopositive ivn axons branched and projected one axon each into the oesophageal nerve (on) and into each ion. The on-projecting branch of these axons branched again at the junction of the on, son, and stomatogastric nerve (stn) (Fig. 1A). At this junction, one branch projected through each son to each CoG, and one branch projected through the stn to the STG. No other HA-like-immunoreactive axons were present in the on, ions, sons, or stn.
The OG contains no neuropil region, but an extra-ganglionic neuropil is located at the junction of the son, on, and stn. This may function as the neuropilar plexus for the OG. There was considerable HA-like immunolabeling in this plexus (n = 23 of 23 preparations), all of which appeared to originate from the HA-immunolabeled axon branches that projected through the stn (data not shown).
HA-like immunolabeling in the stomatogastric ganglion was confined to the neuropil (n = 23 preparations; Figs. 1C, 2A). No STG somata exhibited HA-like immunolabeling in any of these preparations. The immunolabeling in the STG neuropil appeared to derive entirely from the stn axons that projected from the ivn. Within the stn, at the entrance to the STG, we consistently found HA-like immunolabeling in two relatively large diameter axons (~5 μm) and a set of smaller diameter ones (<2 μm; Fig. 2B). The larger diameter axons were the continuation of the HA-like-immunolabeled axons that projected from the ivn, while the smaller processes appeared to be ascending branches of the primary axons that entered the STG from the stn. In all preparations, these fine processes projected no further than the middle of the stn, where they often terminated in large varicose swellings. These were the only HA-like-immunolabeled axons present in the stn (Fig. 2D). Two HA-like-immunopositive axons were also occasionally seen in the dorsal ventricular nerve (dvn: 5 of 23 preparations; Fig. 2C,E). When present, these axons bifurcated at the junction of the dvn and lateral ventricular nerves (lvns), projecting one axon through each lvn. Within a short distance of entering the lvns, this HA-like immunolabeling became bead-like in nature and subsequently terminated. In all likelihood, these axons too were derived from the paired HA-like-immunolabeled axons in the stn.
Fig. 2.
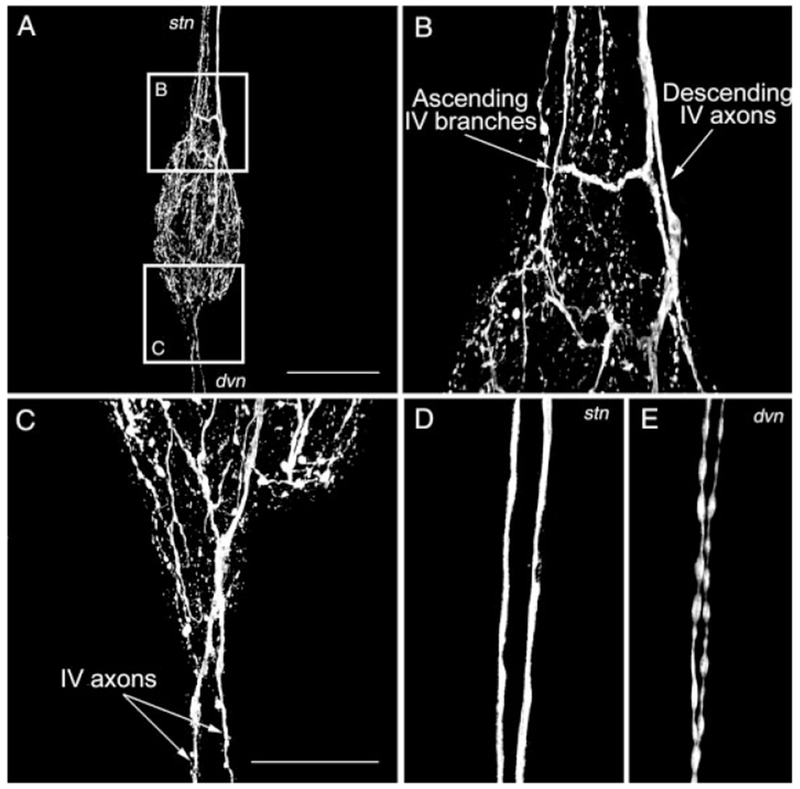
Branching pattern of the histaminergic projections in the stomatogastric ganglion. A: Composite image of HA-like immunolabeling in the STG. Note the presence of multiple fibers where the stomatogastric nerve (stn) and the dorsal ventricular nerve (dvn) connect with the STG. The boxed areas represented magnified regions shown in B and C. B: The HA-like immunolabeling of fibers at the junction of the stn and STG represents both relatively small diameter ascending fibers that extend no further than halfway along the stn and two larger diameter descending fibers that project from the IV neurons in the brain to the STG neuropil. C: In some STGs, HA-like immunolabeling projects past the STG in the dvn. D,E: The descending stn (D) and dvn (E) HA-like immunolabeled fibers exhibit different morphology. The descending stn fibers have a relatively large diameter and appear to be fibers of passage. The dvn fibers have a smaller diameter and a beads on a string morphology, comparable to that of the fibers ascending from the STG. The descending stn fibers could be traced directly from the immunolabeled fibers in the ivn. The image in D was collected in the anterior region of the stn, at a point anterior to the region containing the smaller immunolabeled fibers that ascend from the STG. The images in A-E are composites of 34, 26, 23, 19, and 12 optical sections, respectively. The optical sections were collected at 2-μm intervals in A and 1-μm intervals in B–E. Scale bars = 250 μm in A, 100 μm in C (applies to B–E).
IV neurons are the source of histamine-like immunolabeling in the STG
In the spiny lobster Panulirus interruptus, there are a pair of neurons located in the brain that project through the ivn to the CoGs and STG and contain histamine (Claiborne and Selverston, 1984a,b). The species-equivalent neurons in the American lobster Homarus americanus, the European lobster Homarus gammarus, and the crayfish Pacifastacus leniusculus also exhibit HA-like immunolabeling (Mulloney and Hall, 1991; Le Feuvre et al., 2001; Pulver et al., 2003). To determine whether the axons that provide the HA-like immunolabeling in the C. borealis STG are projections from the species-equivalent IV neurons, we conducted a series of immunocytochemical experiments in which the brain remained connected to the STNS by means of the ivn (n = 14 preparations). We were able to consistently trace the HA-like-immunoreactive axons in the ivn to somata located in the dorsal posterior portion of the brain, close to the insertion point of the ivn (Fig. 3A,B). In 12 of these brain-attached preparations, we followed the axons from their somata in the brain to the STG neuropil (Fig. 3C1). These preparations showed that the two HA-like-immunopositive stn axons were indeed the axons of the two IV neurons. In two preparations, the IV neuron somata were located in the ivn (Fig. 3C2), rather than at the base of the brain, as also occurs in some other decapod crustaceans (Mulloney and Hall, 1991; Le Feuvre et al., 2001; Pulver et al., 2003).
Fig. 3.
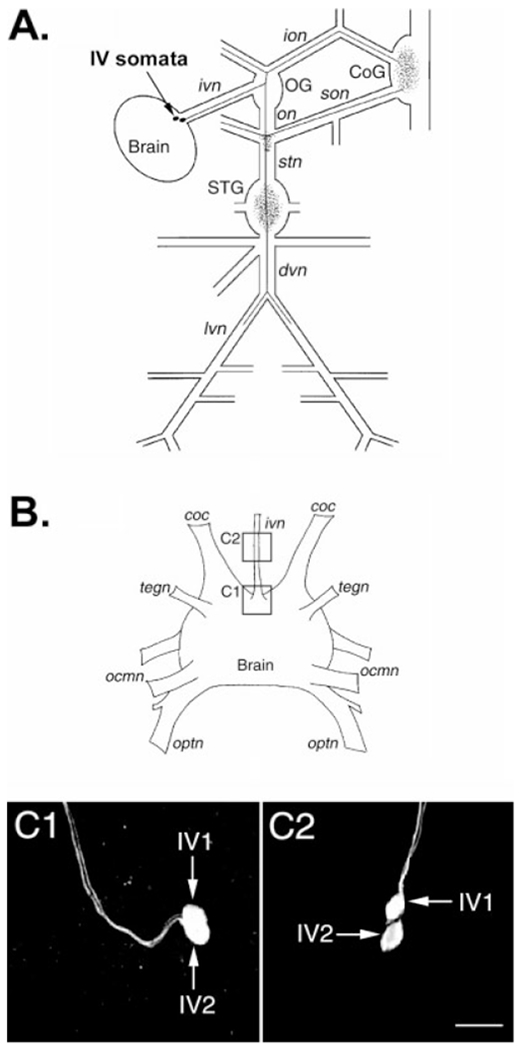
Soma location and projection pattern of the inferior ventricular (IV) neurons in Cancer borealis. A: Schematic representation of the stomatogastric nervous system connected with the supraoesophageal ganglion (Brain), showing the projection pattern of one of the paired IV neurons. These two neurons have identical projection patterns. The IV neuron somata are indicated by filled circles, and the axonal projection pattern is represented by solid lines. The neuropilar areas of the IV neurons are indicated by stippling. B: Schematic representation of the brain of C. borealis with some of its major nerves. The boxed regions in this schematic show the location of the confocal photomicrographs of HA-immunolabeled preparations shown in C1 and C2. C: C1 shows the IV neurons (IV1, IV2) in their more common location, within the brain at the insertion point of the inferior ventricular nerve (ivn). C2 shows the IV neurons within the ivn, where it occurred in fewer than 10% of the preparations examined. C1 and C2, which were taken from different preparations, are composite images of 25 and 16 optical sections, respectively. Images for both panels were collected at approximately 2-μm intervals and are shown at the same scale. ocmn, oculomotor nerve; optn, optic nerve; tegn, tegumentary nerve. For other abbreviations, see Figure 1. Scale bar = 50 μm in C2 (applies to C1,C2).
IV neurons contain a FLRFamide-like peptide cotransmitter
Many of the modulatory neurons that arborize within the STG neuropil contain more than one neuroactive substance (Katz et al., 1989; Skiebe and Schneider, 1994; Blitz et al., 1999; Meyrand et al., 2000; Nusbaum et al., 2001; Skiebe, 2001; Thirumalai and Marder, 2002). To determine whether the IV neurons contained cotransmitters, we immunoprocessed brains with ivns attached by using different anti-transmitter antibodies known to label STG neuropilar processes. Immunoprocessing with antibodies generated against GABA, allatostatin, cholecystokinin, proctolin, and red pigment concentrating hormone revealed no labeled somata in this location (n = 3 preparations for each antibody). In contrast, immunoprocessing with several different antibodies generated against FLRFamide peptides did stain neurons in the region commonly occupied by the IV neurons (n = 13 preparations). It should be noted, however, that not all FLRFamide antisera that stain the STG neuropil immunolabeled these neurons. Specifically, anti-FLRF671, which stains a set of profiles in the STNS apparently identical to those labeled by anti-FLRF231 and anti-FLRFImmunostar (A.E. Christie, unpublished observations), did not label somata in the region of the brain where the IV neurons were located.
To confirm the identity of these FLRFamide-like-immunoreactive neurons as the IV neurons, LY backfills of the ivn were paired with FLRFamide immunoprocessing (Fig. 4). The IV neurons were the only somata at the junction of the brain and the ivn that filled with dye when the ivn was backfilled. As is shown in Figure 4, when dye-filled IV neurons were processed with anti-FLRFamide antibody, they exhibited FLRFamide-like immunoreactivity in their somata and/or axons (n = 8).
Fig. 4.
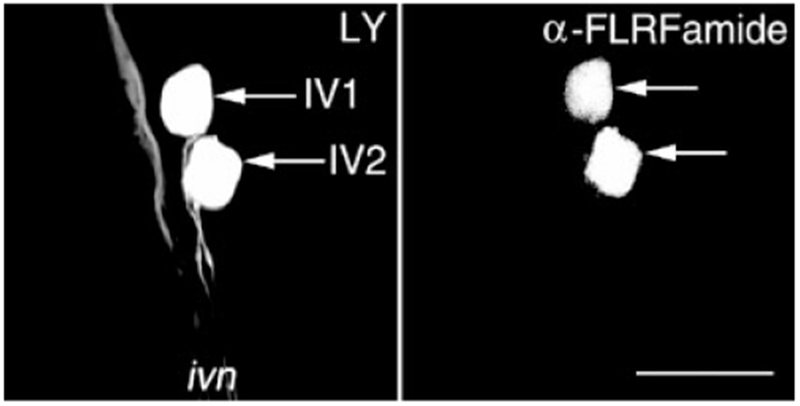
The IV neurons of Cancer borealis contain a FLRFamide-like peptide cotransmitter. Lucifer yellow (LY) nerve backfills of the inferior ventricular nerve (ivn) were paired with anti-FLRFamide immunoprocessing to show that the IV neurons exhibit FLRFamide-like immunoreactivity. Left: LY backfill of the ivn resulted in LY labeling in the brain, near the insertion point of the ivn, of only the two IV neuron somata (arrows). Right: The distribution of FLRFamide-like immunolabeling, in the same region of the brain from the same preparation as the LY backfill panel, visualized by means of a rhodamine-conjugated secondary antibody. The two IV neurons (arrows) exhibit FLRFamide-like immunolabeling. Each panel is a composite of 31 optical sections taken at approximately 1-μm intervals that were collected simultaneously. Scale bar = 100 μm.
Specificity of histamine-like and FLRFamide-like immunolabeling
A previous study performed in several crustacean species showed that the anti-HA antibody used in our study is specific for HA in crustacean nervous tissue (Mulloney and Hall, 1991). However, the fixation regimen used in our study for the anti-HA antibody differed slightly from those used previously, and, because the antibody is a polyclonal antiserum, we conducted experiments to confirm that the staining we report here was also suppressed specifically by preadsorption of the antiserum with histamine (see Materials and Methods section for details). Incubation of the HA antiserum with a HA-KLH conjugate (10−6 M) completely abolished HA-like immunolabeling in the STNS (n = 4 preparations). The same concentration of HA alone did not alter the immunolabeling (n = 4 preparations), but incubation of the antiserum with a higher concentration of HA (10−3 M) did block this immunolabeling (n = 3 preparations). Staining in the STNS after the antiserum was preincubated with either KLH or TNRNFLRFamide (10−3 M; n = 3/3 preparations for each substance) was no different from that after antiserum preincubations at room temperature with no blocking agent present.
To test whether the FLRFamide-like immunolabeling in the IV neurons was due to cross-reactivity to histamine, we conducted preadsorption experiments in which the anti-FLRFamide antibody was incubated with HA-KLH conjugate for 2 hours at room temperature before it was used in immunoprocessing. Incubation of the antiserum with 10−3 M HA-KLH conjugate did not alter FLRFamide-like immunolabeling in the IV neurons (n = 3). To test whether this neuropeptide-like immunolabeling in the IV neurons resulted from binding of the FMRFamide antibody within the polyclonal antiserum, we preincubated this antiserum with TNRNFLRFamide (10−6 M; n = 3). All immunolabeling in the STNS was eliminated in all three of these preincubation experiments.
Comparison of histamine application and IV neuron stimulation
We characterized and compared the actions of histamine and IV neuron stimulation on the pyloric and gastric mill rhythms. To study the actions of HA, we focally applied it to the STG neuropil by means of pressure ejection. In these experiments, the STG was isolated from the CoGs by transecting the sons and ions. When HA was focally applied (10−4 M pipette concentration; 2 seconds duration; 6 PSI), the ongoing pyloric rhythm was consistently turned off (n = 23/23 preparations) (Fig. 5A). This HA-mediated termination of the ongoing pyloric rhythm occurred 1.23 ± 0.3 seconds (n = 10) after the start of the HA application. It is likely that much of the delay resulted from the time required for the pressure ejected HA to reach and penetrate the STG neuropil. Comparable applications of saline did not alter the pyloric rhythm. The pyloric rhythm did not resume until 11.62 ± 8.3 seconds (n = 10) after an HA application (Fig. 5A). Note in Figure 5A that all impulse activity ceased in the pyloric dilator (PD) neurons. The PD neurons are members of the pyloric pacemaker group. Activity also routinely ceased in the inferior cardiac (IC) neuron (Fig. 5A) and, when it was active before HA application, in the ventricular dilator (VD) neuron (data not shown). In contrast, the pyloric (PY) neurons switched from rhythmic to tonic activity, while activity in the lateral pyloric (LP) neuron initially terminated and then often resumed as intermittent tonic activity. In the lobster P. interruptus, resumption of the pyloric rhythm after IV neuron-mediated rhythm termination is associated with an initially stronger and faster rhythm (Russell and Hartline, 1981; Marder and Eisen, 1984). A comparable event occurred in only 1 of these 23 experiments with C. borealis.
Fig. 5.
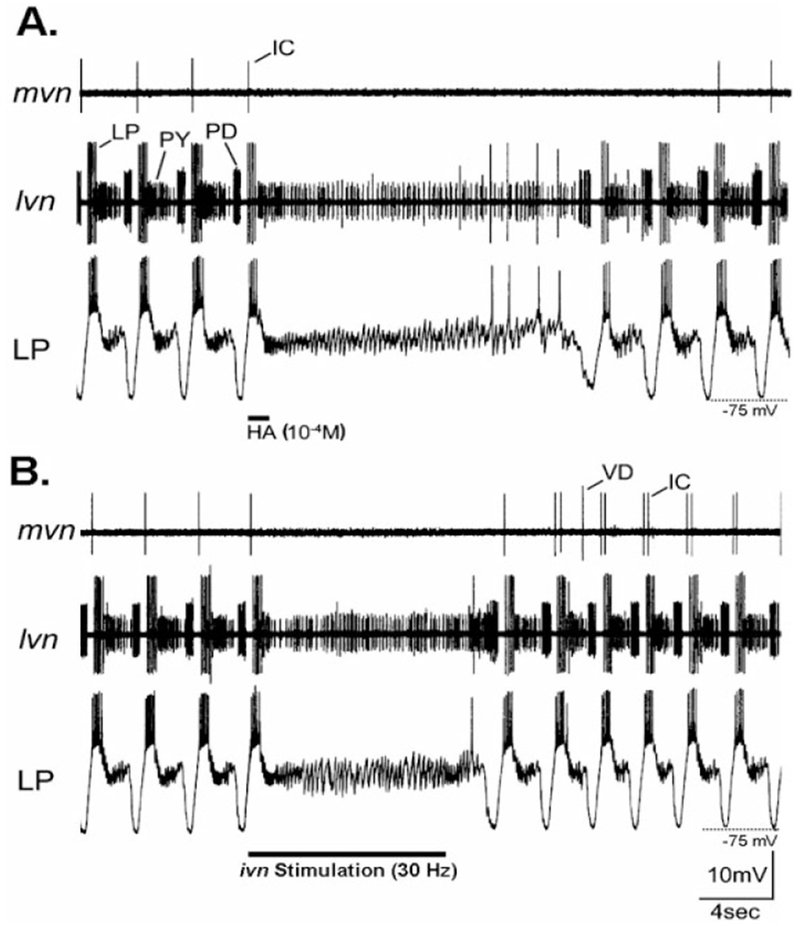
Inhibition of the pyloric rhythm by HA application and IV neuron stimulation in preparations where the STG was isolated from the commissural ganglia. A: Focal application of HA (bar) onto the STG neuropil caused a long-lasting but reversible suppression of the pyloric rhythm. Note the loss of rhythmic neuronal activity in both extracellular recordings (mvn, lvn) and in the intracellular recording of the lateral pyloric (LP) neuron. During this time, only PY neuron activity persisted (lvn), although the LP neuron fired a few action potentials just before the pyloric rhythm resumed. The inhibitory postsynaptic potentials (IPSPs) that occurred in the LP neuron while the pyloric rhythm was turned off represent input from the PY neurons (Selverston and Moulins, 1987). In this preparation, there was no ongoing VD neuron activity after the STG was isolated. B: Stimulation of the ivn (30 Hz) reversibly terminated the ongoing pyloric rhythm. This rhythm resumed approximately 2 seconds after the stimulation was stopped. Comparable to the pyloric rhythm response to HA application, the persisting tonic activity in the lvn recording during ivn stimulation was from the PY neurons. As in A, the PY neurons were responsible for the persisting IPSPs that occurred in the LP neuron during nerve stimulation. A and B are from the same preparation. For abbreviations, see list. Scale bars are the same for both panels.
To assess the influence of the histamine-containing IV neurons on the pyloric rhythm, we activated the IV neurons by means of extracellular stimulation of the ivn (15–40 Hz). This procedure for selective IV neuron activation has been used previously in the lobster STNS (Dando and Selverston, 1972; Sigvardt and Mulloney, 1982a; Claiborne and Selverston, 1984a,b; Hooper and Moulins, 1990; Dickinson et al., 2001). As with HA application, these experiments were conducted using preparations in which the influence of CoG projection neurons was removed by means of transection of the sons and ions. In 23 of the 25 preparations tested, extracellular stimulation of the ivn weakened or suppressed the ongoing pyloric rhythm (Fig. 5B). This rhythm modification persisted for the duration of the stimulation (5–10 seconds) and outlasted it by 2.1 ± 0.9 second (n = 10). When ivn stimulation suppressed the ongoing pyloric rhythm, this suppression was nearly identical to that seen with focal application of HA (Fig. 5). This included suppression of impulse activity in the PD, IC, and VD neurons and a switch from rhythmic to tonic activity in the PY neurons. Also similar to HA applications, the LP neuron either stopped firing or switched from rhythmic to tonic activity. In the two preparations in which ivn stimulation did not inhibit the pyloric rhythm, this rhythm instead sped up during the stimulation.
The pyloric rhythm response to both HA application and ivn stimulation appeared to result primarily from a HA-mediated inhibition of the pyloric pacemaker neurons. In C. borealis, the pyloric pacemaker group includes the single anterior burster (AB), paired PD, and paired lateral posterior gastric (LPG) neurons. Among this set of pacemaker neurons, at least the PD neurons maintained a hyperpolarized membrane potential in response to both HA application (Fig. 6A) and IV neuron stimulation (Fig. 6B) (n = 13). For example, within two seconds of an HA application, the PD neuron membrane potential stopped oscillating and rested at −66 ± 4.2 mV (n = 5). This membrane potential was generally near or more hyperpolarized than the trough of the slow wave oscillations occurring in this neuron during the ongoing pyloric rhythm (Fig. 6A,B). In contrast to the hyperpolarized membrane potential in the PD neuron in response to either HA application or IV neuron stimulation, the PY and LP neurons remained relatively depolarized (HA application response: PY, −52 ± 0.5 mV, n = 3; LP, −59 ± 2.4 mV, n = 4). These more depolarized potentials enabled the persistent activity in the PY neurons and occasional firing in the LP neuron.
Fig. 6.
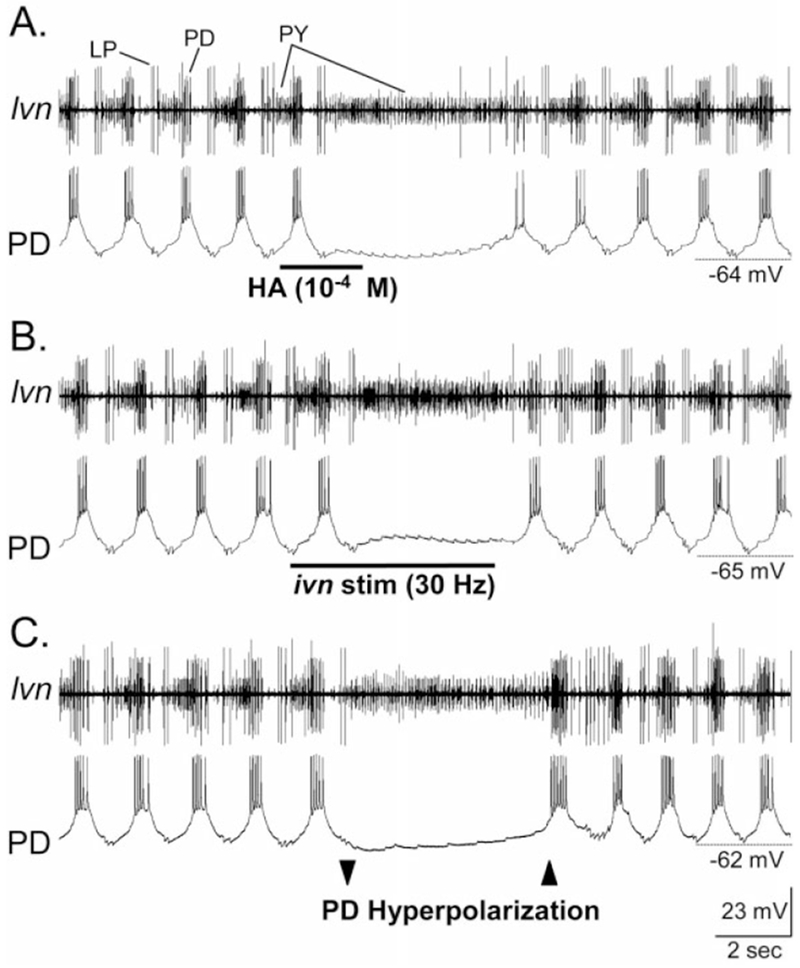
The pyloric circuit response to HA application and ivn stimulation is similar to that resulting from direct hyperpolarization of the pyloric pacemaker neurons. A: Focal application of HA onto the STG neuropil caused a long-lasting but reversible suppression of the pyloric rhythm (lvn). Tonic PY neuron activity (lvn) was all that persisted during pyloric rhythm suppression. Note that the PD neuron membrane potential remained hyperpolarized in response to the HA application. There are two PD neurons, both of which are members of the electrically coupled, pyloric pacemaker ensemble. B: IV neuron stimulation terminated the ongoing pyloric rhythm. As occurred in response to HA application, only PY neuron activity persisted and the PD neuron remained at a hyperpolarized membrane potential. C: Intracellular hyperpolarizing current injection into a PD neuron terminated the ongoing pyloric rhythm (lvn). As occurred in response to HA application and ivn stimulation, only the PY neurons remained active during the PD neuron hyperpolarization. In this experiment, one PD neuron was injected with hyperpolarizing current (not shown), while the second PD neuron was simultaneously recorded. All three panels are from the same preparation, in which the STG was isolated from the CoGs. For abbreviations, see list. Scale bars are the same for all panels.
We tested the hypothesis that inhibition of the pacemaker neurons was the primary means by which ivn stimulation and HA application inhibited the pyloric rhythm. To this end, we hyperpolarized one or both of the PD neurons, thereby terminating the pyloric rhythm (Fig. 6C). During this time, the PY neurons became tonically active, and there was occasional activity in the LP neuron. Both neurons maintained membrane potentials comparable to those occurring during HA application and ivn stimulation (n = 6; Fig. 6). Also comparable to the latter two conditions, impulse activity in IC and VD was terminated during PD neuron hyperpolarization (n = 6). We also tested whether there was any further influence of either HA application or ivn stimulation on the pyloric circuit when the pacemaker neurons were hyperpolarized by current injection into the PD neurons. During this time, neither HA application (n = 4) nor ivn stimulation (n = 5) further altered the activity of the other pyloric neurons (data not shown).
The gastric mill rhythm was also inhibited by both focal application of histamine and ivn stimulation. Unlike the pyloric rhythm, the gastric mill rhythm is not spontaneously active in this system when the CoGs are removed. However, with the CoGs removed the gastric mill rhythm is readily activated by selective stimulation of an identified CoG projection neuron called modulatory commissural neuron 1 (MCN1), by means of extracellular stimulation of one or both ions (Bartos and Nusbaum, 1997). The gastric mill rhythm persists for the duration of MCN1 stimulation. These MCN1-elicited gastric mill rhythms were consistently terminated by HA application (n = 10/11 preparations, Fig. 7A). They generally resumed 16.5 ± 6.6 seconds (n = 7) after HA application. This ability of HA to inhibit the ongoing gastric mill rhythm suggested that the gastric mill circuit would also be sensitive to IV neuron stimulation. We tested this hypothesis by stimulating the ivn (15–40 Hz) during a MCN1-elicited gastric mill rhythm. These rhythms indeed were terminated by ivn stimulation (Fig. 7B, n = 10/11) and resumed 5.2 ± 2.0 second (n = 8) after the end of the ivn stimulation. It is also noteworthy that both HA application and ivn stimulation were able to terminate the gastric mill rhythm, as well as the pyloric rhythm (Fig. 7), during these MCN1 stimulations, despite the fact that, during this time, MCN1 was providing continuous modulatory drive to these circuits (Bartos and Nusbaum, 1997; Bartos et al., 1999).
Fig. 7.
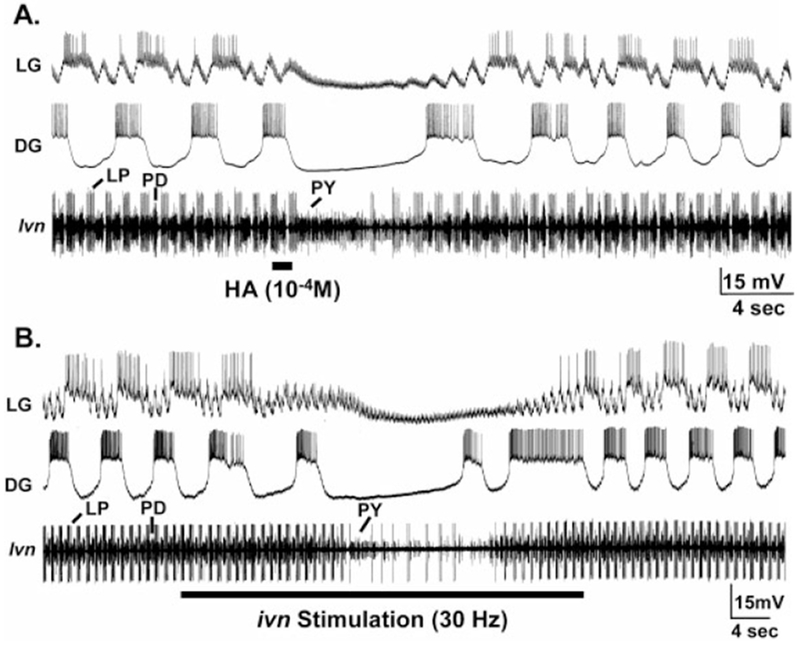
Inhibition of the gastric mill rhythm by histamine (HA) application and IV neuron stimulation in preparations where the STG was isolated from the commissural ganglia. A: Focal application of HA onto the STG neuropil reversibly terminated the gastric mill (LG, DG) and pyloric rhythms (lvn). The gastric mill rhythm was driven, and the pyloric rhythm modulated, by continuous stimulation (15 Hz each) of both MCN1 projection neurons for the duration of the recording. The persisting excitatory postsynaptic potentials (EPSPs) in the LG neuron during ivn stimulation represent electrical EPSPs from MCN1 (Coleman et al., 1995). Most hyperpolarized Vm: DG, −68 mV; LG, −76 mV. B: Extracellular stimulation of the ivn (30 Hz) reversibly terminated ongoing gastric mill (LG, DG) and pyloric rhythms (lvn). As in A, both MCN1s were stimulated tonically (15 Hz each) for the duration of the recording, and the electrical EPSPs from MCN1 in the LG neuron persisted during the gastric mill response to HA application. Most hyperpolarized Vm: DG, −68mV; LG, −74 mV. A and B are from the same preparation. For abbreviations, see list.
The activity of one gastric mill neuron, the dorsal gastric (DG) neuron, was consistently more effectively inhibited by HA application than ivn stimulation. For example, when HA was applied during an MCN1-elicited gastric mill rhythm, DG neuron activity stopped when the gastric mill rhythm stopped, and its activity did not resume until the gastric mill rhythm resumed (Fig. 7A). When ivn stimulation occurred during an MCN1-elicited gastric mill rhythm, the DG neuron often generated a burst after the gastric mill rhythm was terminated and it then resumed its bursting activity before the end of ivn stimulation (Fig. 7B). In contrast, the gastric mill rhythm did not resume until after ivn stimulation (Fig. 7B). These results suggested that the DG neuron was either less affected by ivn activity than by HA application or else was excited by a cotransmitter of the IV neurons, such as the FLRFamide-like peptide (see above). A further hint for such an excitatory action of the IV neurons came from experiments in which we stimulated the ivn without concomitant MCN1 stimulation. In some experiments, ivn stimulations activated the DG neuron without activating any other gastric mill or pyloric neurons (n = 6 preparations, data not shown). Furthermore, focal application of TNRNFLRFamide (10−4 M in the pipette), a FLRFamide peptide family member found in C. borealis (Weimann et al., 1993; Li et al., 2003), excited the DG neuron (n = 10/10, data not shown).
Cimetidine suppresses the actions of histamine and IV neuron stimulation on the pyloric rhythm
Previous studies on the crustacean nervous system showed that the histamine H2 receptor antagonist cimetidine effectively blocks the actions of exogenously applied histamine (Hashemzadeh-Gargari and Freschi, 1992; el Manira and Clarac, 1994). Therefore, to further support the hypothesis that the IV neurons use HA as a transmitter in the STG, and to determine whether the actions of the IV neurons on the pyloric rhythm are due solely to HA, we investigated whether cimetidine could eliminate all of the actions of HA application and ivn stimulation on the pyloric rhythm. Superfusion of cimetidine (5 × 10−3 M) by itself did not alter the ongoing pyloric rhythm in most preparations. In a few preparations, it caused a modest increase in the speed and strength of this rhythm. For example, the pyloric cycle frequency was not significantly different, in the absence of HA application and ivn stimulation, during superfusion with saline alone or with cimetidine (saline, 0.77 ± 0.2 Hz; cimetidine, 0.82 ± 0.2 Hz; saline wash, 0.76 ± 0.2 Hz; one-way analysis of variance [ANOVA]; P > 0.5; n = 11). There was also no difference between these two conditions in the number of LP neuron spikes/burst (saline, 4.1 ± 2.0; cimetidine, 4.8 ± 1.9; saline wash, 4.3 ± 2.2; one-way ANOVA; P > 0.5; n = 11).
In eight of eight preparations, cimetidine (5 × 10−3 M) eliminated the pyloric circuit response to focal application of HA (10−4 M). For example, the pyloric cycle frequency was unchanged when HA was coapplied with cimetidine (cimetidine, 0.87 ± 0.2 Hz; cimetidine + HA, 0.86 ± 0.2 Hz; P > 0.5, t test; n = 8). The same was true for the number of LP neuron spikes/burst (cimetidine, 6.1 ± 3.1; cimetidine + HA, 6.0 ± 2.9; P > 0.5, t test; n = 8).
The inhibitory actions of ivn stimulation on the pyloric rhythm were also eliminated in cimetidine (5 × 10−3 M). For example, in 8 of the 10 preparations examined in the presence of cimetidine, there was no effect of ivn stimulation on either the mean pyloric cycle frequency (cimetidine, 0.80 ± 0.1 Hz; cimetidine plus ivn stimulation, 0.86 ± 0.1 Hz; P > 0.3, t test; n = 8), or the mean activity of the LP neuron (cimetidine, 5.1 ± 2.6 spikes/burst; cimetidine plus ivn stimulation, 4.6 ± 2.2; P > 0.5, t test; n = 8). In the other two preparations, the inhibitory action of ivn stimulation on the pyloric rhythm during saline superfusion became an excitatory action during cimetidine superfusion, resulting in an increase in the pyloric cycle frequency. This effect again reversed to inhibition during the subsequent saline wash.
IV neuron stimulation excites the pyloric and gastric mill rhythms by means of actions in the commissural ganglion
As we described earlier, the IV neurons project to the commissural ganglia, by means of both the ions and sons, as well as to the STG (Fig. 3). Therefore, we asked whether these neurons also influenced the STG circuits by means of actions on CoG projection neurons. To this end, we stimulated the ivn in preparations in which the CoGs remained connected to the STG. In some crustaceans, the IV neurons fire action potential bursts in a rhythmic repeating pattern that is time-locked to the cardiac sac rhythm (Dickinson and Marder, 1989; Hooper and Moulins, 1989; Hooper et al., 1990). Therefore, we used a standardized ivn stimulation protocol in this set of experiments that approximated such a rhythmic activity pattern. Specifically, the ivn was stimulated in 10 rhythmic bursts (burst duration, 6 seconds; intraburst frequency, 15 Hz) at a frequency of 0.06 Hz. In these preparations, ivn stimulation initially elicited a brief period of pyloric rhythm inhibition, which was presumably a consequence of the direct actions of the IV neurons on the pyloric circuit. This inhibition was followed by a prolonged enhancement of the pyloric rhythm and, in 17 of 30 preparations, activation of the gastric mill rhythm (Fig. 8). The enhanced pyloric rhythm that followed ivn stimulation included an increase in pyloric cycle frequency and more intense activity in several pyloric neurons. The pyloric cycle frequency increased from 1.20 ± 0.2 Hz before ivn stimulation to 1.44 ± 0.2 Hz during ivn stimulation (P < 0.01, t test; n = 14). Similarly, there were changes in the activity level of the IC and VD neurons. IC neuron activity increased from 1.3 ± 1.2 spikes/burst to 3.4 ± 1.6 spikes/ burst (P < 0.001, t test; n = 13), while VD neuron activity increased from 1.6 ± 1.1 to 5.1 ± 1.4 spikes/burst (P < 0.001, t test; n = 14). In contrast, the number of spikes/ burst in the pyloric LP neuron was unchanged (7.4 ± 2.1 prestimulation, 8.7 ± 1.7 poststimulation; P > 0.05, t test; n = 13).
Fig. 8.
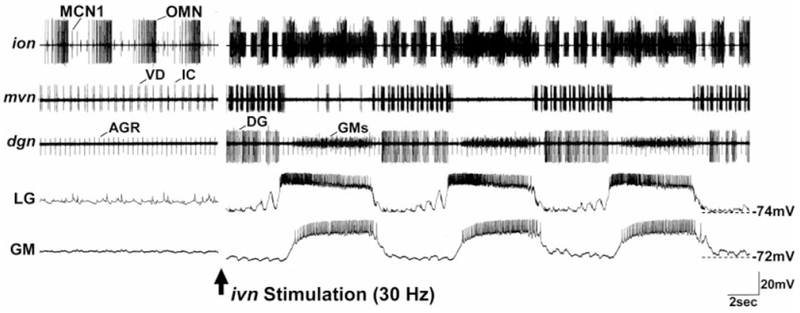
IV neuron stimulation activates the gastric mill rhythm, strengthens the pyloric and oesophageal rhythms, and excites CoG projection neurons when the STG remains connected with the CoGs. Left: Before ivn stimulation, there was no gastric mill rhythm (GM, LG, dgn, mvn), relatively weak pyloric (mvn) and oesophageal (ion) rhythms, and little spontaneous activity in the MCN1 projection neuron (ion). The oesophageal rhythm is monitored by rhythmic bursting of an oesophageal motor neuron (OMN: ion). The tonically active unit in the dgn represents the activity of the anterior gastric receptor (AGR) sensory neuron, which is commonly tonically active in the isolated stomatogastric nervous system. Right: After ivn stimulation (30 Hz), the gastric mill rhythm was elicited and the pyloric and oesophageal rhythms were strengthened. Note that there was gastric mill-timed inhibition of the pyloric-timed activity in the IC and VD neurons (mvn), as commonly occurs during some versions of the gastric mill rhythm (Blitz and Nusbaum, 1997). Similarly, MCN1 (ion) exhibited both a gastric mill-timed (during the LG/GM neuron burst) and pyloric-timed (during the DG neuron burst) activity pattern. For other abbreviations, see list.
The ivn-elicited gastric mill rhythm was characterized by rhythmic alternating bursting of the gastric mill neurons, including the lateral gastric (LG), gastric mill (GM), and DG neurons (Fig. 8). This also included gastric mill-timed inhibition of the IC and VD neurons, during each LG neuron burst, as routinely occurs in at least one version of the gastric mill rhythm (Blitz and Nusbaum, 1997). These actions of the IV neurons tended to outlast the period of stimulation by 235.0 ± 151.8 seconds (n = 12). In every preparation in which ivn stimulation activated the gastric mill rhythm, subsequent transection of the sons and ions eliminated this action.
The version of the gastric mill rhythm elicited by ivn stimulation was similar to that resulting from activation of two previously identified CoG projection neurons, including MCN1 and commissural projection neuron 2 (CPN2; Blitz and Nusbaum, 1997). Therefore, during these ivn stimulations, we monitored MCN1 activity by means of extracellular recordings of the ion (Coleman and Nusbaum, 1994). These recordings indicated that ivn activation of the gastric mill rhythm was always accompanied by excitation of MCN1 (n = 17) (Fig. 8). The MCN1 response always persisted for at least the duration of the ivn-elicited gastric mill rhythm. Typically, we observed strong bursting activity in the MCN1 from both CoGs. In preparations wherein ivn stimulation did not elicit a gastric mill rhythm, MCN1 was often activated but its activity was considerably weaker than when a gastric mill rhythm was elicited. We did not record CPN2 activity during these experiments.
IV neuron excitation of the oesophageal rhythm
In addition to its actions on CoG projection neurons that influence the STG circuits, ivn stimulation influenced the oesophageal rhythm in the CoGs. The oesophageal rhythm is generated by a neuronal circuit occurring in each CoG (Spirito, 1975). Like the pyloric rhythm, the oesophageal rhythm is usually spontaneously active in the isolated stomatogastric nervous system. This finding is evident from the ion recording in Figure 8, wherein the oesophageal rhythm was monitored by means of the largest unit in this recording. This unit represents the activity of an oesophageal motor neuron (OMN) that projects through the ion and OG to innervate oesophageal muscles in the region of the OG (D.M. Blitz and M.P. Nusbaum, unpublished observations). IV neuron stimulation increased the cycle frequency of this motor pattern (pre-ivn stimulation, 0.25 ± 0.06 Hz; post-ivn stimulation, 0.38 ± 0.1 Hz; P < 0.001, t test; n = 11). Additionally, ivn stimulation routinely reduced the OMN burst duration (prestimulation, 1.9 ± 0.5 seconds; poststimulation, 1.32 ± 0.3 seconds; P < 0.005; n = 10) (see also Fig. 8).
Parallel IV neuron projections to each commissural ganglion
The IV neurons project through both the ions and sons to reach each CoG (Fig. 3). We aimed to determine whether both or only one of these pathways is used to activate MCN1. To this end, we transected either the ion or son on a given side of a preparation, after determining that ivn stimulation did indeed activate MCN1 (Fig. 9). Transection of the son eliminated the ivn-mediated activation of MCN1 in most preparations (n = 7/8; Fig. 9C). In one of these preparations a weakened excitation of MCN1 by ivn stimulation persisted after son transection. In all preparations, the gastric mill rhythm disappeared simultaneously with the removal of MCN1 activity. In contrast, in most preparations, the transection of the ion did not prevent MCN1 from being activated in response to ivn stimulation (n = 5/6; Fig. 9C). In one preparation, the activation of MCN1 by ivn stimulation was weaker than before ion transection. In preparations where the ion was transected, ivn stimulation did not elicit a gastric mill rhythm, because, although MCN1 activity was enhanced, this activity did not reach the STG.
Fig. 9.
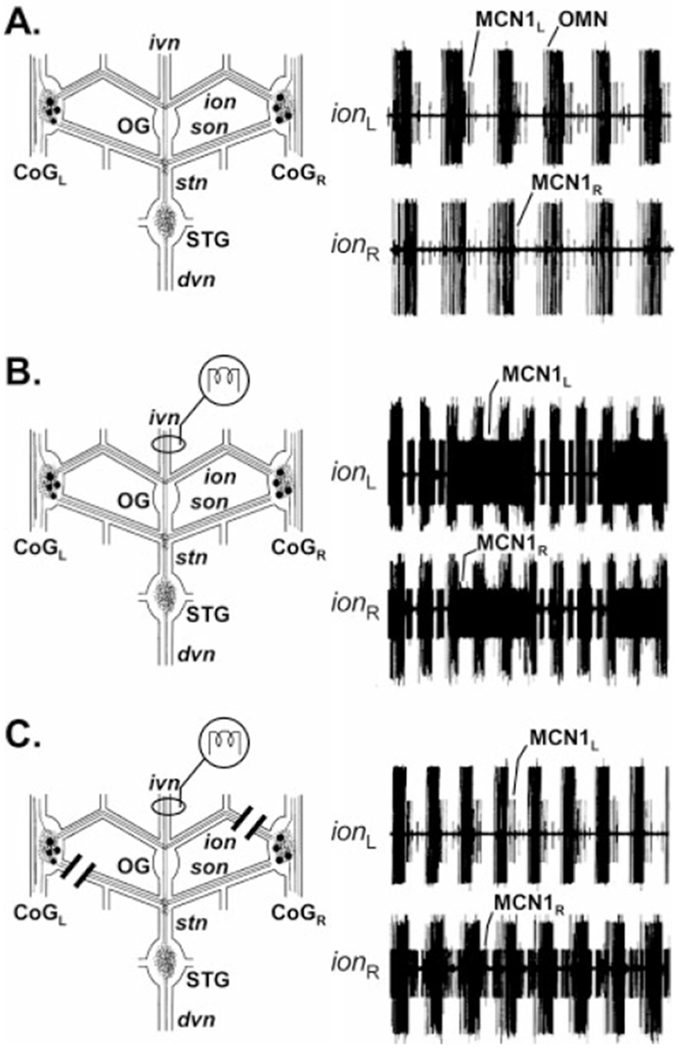
Only one of the two IV neuron projections to each CoG excites the MCN1 projection neuron. A: Simultaneous extracellular recordings of the left (ionL) and right (ionR) ions before ivn stimulation showed an ongoing oesophageal rhythm (OMN) and weak oesophageal-timed activity from both MCN1s. B: After ivn stimulation, both MCN1s exhibited strengthened activity that was now time-locked with both the pyloric rhythm (short duration bursts) and the gastric mill rhythm (long duration bursts) (see Fig. 8). C: After transection of the sonL and ionR, ivn stimulation no longer excited MCN1L but it still excited MCN1R. This result indicated that IV neuron excitation of MCN1 occurs only by means of the son axon of the IV neuron. Note that the ionR was transected on the STG side of the ionR recording electrode. This method enabled continued recording of MCN1 activity projecting from the CoG, but it prevented this MCN1 activity from reaching the STG. No gastric mill rhythm was activated, because MCN1L was not excited and MCN1R activity did not reach the STG. The recordings in all three panels are from the same preparation.
DISCUSSION
We have documented the actions of a pair of identified histaminergic/peptidergic projection neurons, the IV neurons, which influence at least three motor circuits in the STNS of the crab C. borealis. In this species, the primary actions of the IV neurons in the STG are direct inhibition and indirect excitation of the gastric mill and pyloric circuits (Fig. 10). The direct inhibitory actions were relatively short-lasting and were mimicked by focal application of HA. Moreover, the H2 receptor antagonist cimetidine suppressed both the IV neuron- and HA-evoked inhibition in the STG. Cimetidine-sensitive histamine receptors also occur in other arthropods, where it has been determined that these receptors are pharmacologically distinct from the classic mammalian H2 type of HA receptors (Hashemzadeh-Gargari and Freschi, 1992; Gisselmann et al., 2001).
Fig. 10.
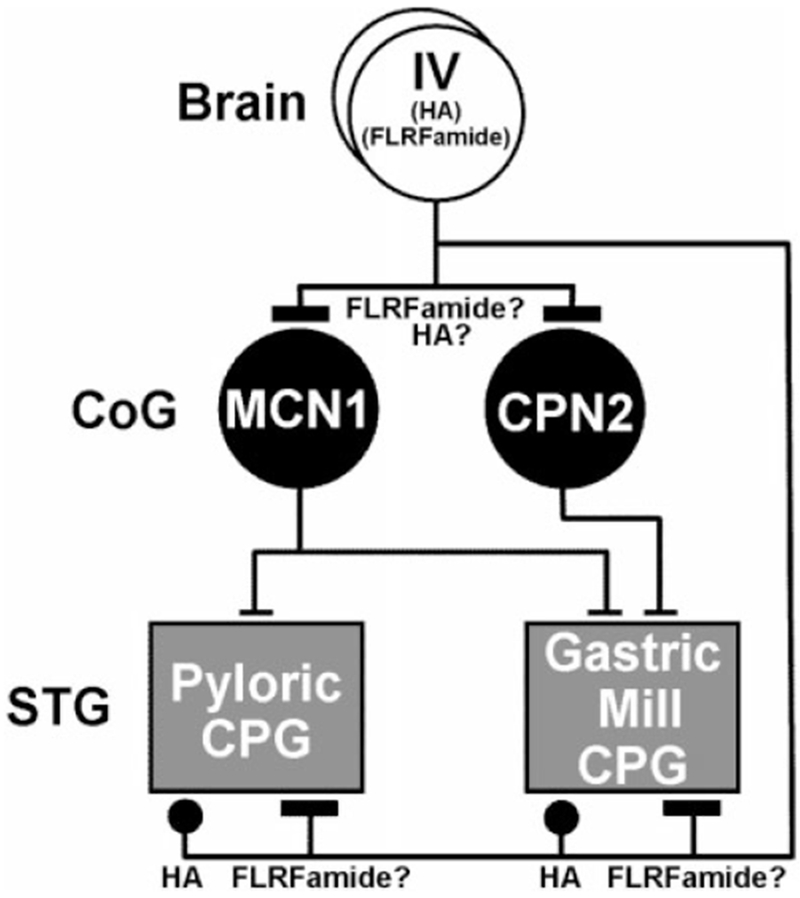
Schematic of the direct and indirect actions of the IV neurons on the gastric mill and pyloric central pattern generating circuits (CPGs) in the STG of the crab Cancer borealis. The IV neurons use the neurotransmitter histamine (HA) to elicit a direct, relatively short-lasting inhibition of both of these CPGs. It remains to be determined whether there is also a peptidergic (FLRFamide-like) component to the IV neuron actions in the STG. By means of their excitation of CoG projection neurons, including MCN1 and CPN2, the IV neurons also elicit an indirect, relatively long-lasting excitation of the gastric mill and pyloric CPGs. The IV neuron excitation of these projection neurons may result from the actions of its peptide cotransmitter. For other abbreviations, see list. Symbols: T-bars, excitation; Filled circles, inhibition.
The indirect excitatory actions of the IV neurons on the STG circuits were relatively long-lasting and resulted at least partly from their activation of projection neurons in the CoGs, including the previously identified projection neuron MCN1 (Fig. 10; Coleman and Nusbaum, 1994; Blitz et al., 1999). The IV neurons also consistently increased the speed of the oesophageal rhythm in the CoGs. There are two other known histaminergic neurons that influence motor pattern generation. Both of these are sensory neurons in the Aplysia californica nervous system, where they influence the neural circuit underlying feeding (Weiss et al., 1986a,b; Evans et al., 1999). The IV neurons are not sensory neurons, but do receive strong excitatory drive from sensory structures in the crustacean foregut (Sigvardt and Mulloney, 1982b; Hooper et al., 1990; Meyrand et al., 1994).
The IV neurons have been studied previously in several other crustacean species, including the spiny lobsters P. interruptus and P. vulgaris, the European lobster Homarus gammarus, the American lobster H. americanus as well as several crayfish species (Dando and Selverston, 1972; Russell and Hartline, 1981; Sigvardt and Mulloney, 1982a,b; Claiborne and Selverston, 1984a,b; Dickinson and Marder, 1989; Cazalets et al., 1990a,b; Dickinson et al., 1990, 1993; Hooper and Moulins, 1990; Meyrand et al., 1991, 1994; Pulver et al., 2003; Skiebe, 2003). In all of these species, this neuronal pair retains its soma location, its dual transmitter phenotype, and exhibits the same axonal projection pattern. A second neuropeptide, orcokinin, has also recently been localized to the IV neuron in several crayfish and lobsters (Li et al., 2002; Skiebe, 2003). Whether orcokinin is also present in the IV neurons of other species awaits further study. There is also a conserved physiological action of this projection neuron across species in terms of its ability to inhibit the pyloric rhythm by means of HA-mediated synaptic inhibition. Many, but not all, of the same pyloric neurons are inhibited by this pathway in the different species.
Despite this degree of retained phenotype, there are also differences in some of the physiological actions of these neurons across species. For example, in P. interruptus and H. americanus, there is an additional, excitatory component to the IV neuron influence on pyloric and gastric mill neurons (Russell and Hartline, 1981; Claiborne and Selverston, 1984a; Marder and Eisen, 1984; Cazalets et al., 1990a,b; Dickinson et al., 1993). This excitation includes both unitary excitatory postsynaptic potentials (EPSPs) and an enhancement of the bursting ability of some neurons. No such EPSPs were observed in any pyloric neurons, and an excitation of the pyloric rhythm occurred in only a few preparations in C. borealis. In P. interruptus, these excitatory actions of the IV neurons were blocked by the type-1 HA receptor antagonist pyrilamine, suggesting a histaminergic source for these effects (Claiborne and Selverston, 1984a). In H. gammarus, where the IV neurons are called the pyloric suppressor (PS) neurons, short-term activation of these neurons has similar actions on the pyloric and oesophageal rhythms to those in C. borealis. However, the inhibition of the pyloric rhythm persists for considerably longer in H. gammarus (Cazalets et al., 1990a; Meyrand et al., 1994). In contrast, longer-lasting activation of these neurons in H. gammarus has a distinct influence on the stomatogastric circuits from that in C. borealis. Instead of separate actions on the aforementioned three rhythms as occurred in C. borealis, PS neuron activation dismantles the pyloric, gastric mill, and oesophageal circuits and recombines subsets of neurons from each circuit to produce a separate rhythmic motor pattern (Meyrand et al., 1991, 1994). Regardless of the IV neuron stimulation frequency and duration, these latter influences were never observed in C. borealis. The result of prolonged ivn stimulation in P. interruptus is distinct from that in both C. borealis and H. gammarus. In P. interruptus, such stimulation modifies the pyloric rhythm in a manner comparable to that occurring during activation of the cardiac sac rhythm (Dickinson et al., 1993).
The ability of the species-equivalent neuron to evoke different responses from the same neuronal circuits in different species was documented previously in this system for another projection neuron (Meyrand et al., 2000). There are also some examples of differences in the cotransmitter complement of species-equivalent neurons in this system, although their physiological consequences are not yet documented (Skiebe, 2001, 2003). The extent to which these differences in IV/PS neuron actions across species result from pre- vs. postsynaptic changes was not investigated. Nevertheless, these examples of differences in what is apparently the same neuron in different species provides a cautionary note for comparable studies in more complex systems where the access to detailed cellular-level information known for the stomatogastric system is not as readily available.
The direct IV neuron actions on the pyloric and gastric mill circuits are dominated by HA (Fig. 10). For example, in the presence of cimetidine, there was usually no change in the pyloric or gastric mill rhythms during ivn stimulation. The presence of an occasional excitatory influence of ivn stimulation within the STG suggests that the IV neurons either have a second, cimetidine-insensitive HA action or a peptidergic action on the pyloric circuit. Perhaps this excitatory action is more strongly expressed when the system is in another state, such as during generation of the cardiac sac rhythm, which was not present in our experiments. The IV neurons participate in the cardiac sac rhythm (Dickinson and Marder, 1989; Dickinson et al., 1990; Hooper et al., 1990). Previous work showed that the FLRFamide peptides native to C. borealis effectively modulate the pyloric and gastric mill rhythms (Weimann et al., 1993). However, there are additional projection neurons that innervate the STG and exhibit FLRFamide immunoreactivity (Coleman et al., 1992). One of these other projection neurons may be responsible for these previously characterized physiological actions. Although we did not investigate the contributions of the IV neuron cotransmitters to its long-lasting excitatory actions on CoG projection neurons, these actions may be peptidergic, because application of a native FLRFamide peptide family member to the CoGs activates projection neurons that, in turn, initiate the gastric mill rhythm in the STG (Weimann et al., 1993; D.M. Blitz, M.P. Nusbaum, unpublished observations).
The IV neuron-mediated long-lasting activation of the gastric mill rhythm and strengthening of the pyloric rhythm resulted at least partly from IV neuron activation of CoG projection neurons, including MCN1 (Fig. 10). MCN1 is instrumental for activating the gastric mill rhythm, and it excites the pyloric rhythm (Bartos and Nusbaum, 1997; Blitz et al., 1999; Bartos et al., 1999). The ivn stimulus used in our experiments is likely to coactivate MCN1 and CPN2 (Fig. 10), based on the similarity of the expressed gastric mill rhythm to the one resulting from the coactivation of these two projection neurons (Blitz and Nusbaum, 1997). The extent to which the IV/PS neurons influence the STG circuits by means of actions on CoG projection neurons in other crustaceans is not known, although the PS neurons do make an excitatory synapse on at least one CoG projection neuron in H. gammarus (Meyrand et al., 1994).
There are several identified projection neurons whose influence on the STG circuits has been characterized (Nagy and Dickinson, 1983; Meyrand et al., 1994; Nagy and Cardi, 1994; Combes et al., 1999; Nusbaum et al., 2001; Nusbaum, 2002). Thus far, the IV/PS neurons are the only ones that terminate the activity of these circuits. However, there are likely to be additional inhibitory projection neurons in this system, given the presence of several other transmitters whose exogenous application inhibits pyloric and/or gastric mill activity (Cazalets et al., 1987; Skiebe and Schneider, 1994; Swensen et al., 2000; Skiebe, 2003). Although most identified projection neurons that influence rhythmic motor activity are excitatory, there are several examples of projection neurons in other systems that terminate such activity (Brodfuehrer and Thorogood, 2001; Perrins et al., 2002).
The distinct direct and indirect actions of the IV/PS neurons on the STG circuits provide a clear example for why caution should always be exercised when extrapolating results from the reduced to the more intact preparation. It remains to be determined why the IV neurons have the apparently contradictory effect of direct, brief inhibitory actions and indirect, long-lasting excitatory actions on the pyloric and gastric mill circuits. One possibility is that there are situations during which its synaptic actions in either the STG or the CoGs are selectively inhibited, enabling it to act as a either a pure activator or suppressor of STG motor output. Previous work in the spiny lobster has shown that there are clearly ganglion-specific influences on the IV neuron (Dickinson et al., 1993).
There remain too few examples of cotransmission in the context of neuronal circuit activity to discern general principles. Thus far, however, within the stomatogastric system, there does not appear to be a consistent pattern to the use of cotransmitters. For example, in contrast to the dominance of the small molecule cotransmitter in the direct IV neuron actions on the STG circuits, previous studies have documented the extensive influence of neuropeptides on the pyloric and gastric mill circuits (Marder et al., 1997; Skiebe, 2001; Nusbaum, 2002; Thirumalai and Marder, 2002). With respect to specific projection neurons in C. borealis, there are functionally important roles for the peptide cotransmitters of MCN1 and the modulatory proctolin neuron (MPN) on the STG circuits (Nusbaum et al., 2001). In fact, in the case of MPN, its direct actions on the pyloric circuit are largely peptidergic, while its actions on CoG projection neurons are entirely mediated by its small molecule cotransmitter (Blitz and Nusbaum, 1999). Thus, it will be instructive to examine the relative contributions of the IV neuron cotransmitters on the CoG projection neurons in C. borealis and compare those contributions with the ones made by these same cotransmitters onto the STG circuit neurons. One concept that is developing from this and related studies in this system is that the direct alteration of neuronal circuit activity by a multitransmitter projection neuron is often dominated by a subset of the cotransmitters released from that projection neuron.
ACKNOWLEDGMENTS
We thank Shari Hertzberg for her assistance with data analysis, and Lynn Spruce for her advice and help in the production of synthetic antigen.
Grant sponsor: National Institute of Neurological Disorders and Stroke Grant number: NS29936 (M.P.N.); Grant number: NS42813 (E.M.); Grant number: NS17813; Grant sponsor: Individual National Research Service Award; Grant number: F32-NS09718 (A.E.C); Grant number: F31-NS41894 (M.P.B.).
Abbreviations
- AB
anterior burster neuron
- CCK
cholecystokinin
- coc
circumoesophageal connective
- CoG
commissural ganglion
- CPN2
commissural projection neuron 2
- DCC
discontinuous current clamp
- DG
dorsal gastric neuron
- dgn
dorsal gastric nerve
- dvn
dorsal ventricular nerve
- EDAC
1-ethyl-3-(3-dimethylaminopropyl)carbodiimide
- GABA
gamma-amino butyric acid
- GM
gastric mill neuron
- GNS
goat normal serum
- H2
histamine type-2 receptor
- HA
histamine
- IC
inferior cardiac neuron
- ion
inferior oesophageal nerve
- IV
inferior ventricular neuron
- ivn
inferior ventricular nerve
- KLH
keyhole limpet hemocyanin
- LG
lateral gastric neuron
- LP
lateral pyloric neuron
- LPG
lateral posterior gastric neuron
- lvn
lateral ventricular nerve
- LY
Lucifer yellow
- MCN1
modulatory commissural neuron 1
- MPN
modulatory proctolin neuron
- mvn
median ventricular nerve
- OG
oesophageal ganglion
- OMN
oesophageal motor neuron
- on
oesophageal nerve
- P
phosphate buffer
- PD
pyloric dilator neuron
- PS
pyloric suppressor neuron
- P-Triton
phosphate buffer with Triton X-100
- PY
pyloric neuron
- son
superior oesophageal nerve
- stn
stomatogastric nerve
- STG
stomatogastric ganglion
- STNS
stomatogastric nervous system
- VD
ventricular dilator neuron
LITERATURE CITED
- Bartos M, Nusbaum MP. 1997. Intercircuit control of motor pattern modulation by presynaptic inhibition. J Neurosci 17:2247–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Manor Y, Nadim F, Marder E, Nusbaum MP. 1999. Coordination of fast and slow rhythmic neuronal circuits. J Neurosci 19:6650–6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battelle BA, Calman BG, Andrews AW, Grieco FD, Mleziva MB, Callaway JC, Stuart AE. 1991. Histamine: a putative afferent neurotransmitter in Limulus eyes. J Comp Neurol 305:527–542. [DOI] [PubMed] [Google Scholar]
- Battelle BA, Calman BG, Hart MK. 1999. Cellular distributions and functions of histamine, octopamine, and serotonin in the peripheral visual system, brain, and circumoesophageal ring of the horseshoe crab Limulus polyphemus. Microsc Res Tech 44:70–80. [DOI] [PubMed] [Google Scholar]
- Blitz DM, Nusbaum MP. 1997. Motor pattern selection via inhibition of parallel pathways. J Neurosci 17:4965–4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Nusbaum MP. 1999. Distinct functions for cotransmitters mediating motor pattern selection. J Neurosci 19:6774–6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz DM, Christie AE, Coleman MJ, Norris BJ, Marder E, Nusbaum MP. 1999. Different proctolin neurons elicit distinct motor patterns from a multifunctional neuronal network. J Neurosci 19:5449–5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodfuehrer PD, Thorogood MS. 2001. Identified neurons and leech swimming behavior. Prog Neurobiol 63:371–381. [DOI] [PubMed] [Google Scholar]
- Buchner E, Buchner S, Burg MG, Hofbauer A, Pak WL, Pollack I. 1993. Histamine is a major mechanosensory neurotransmitter candidate in Drosophila melanogaster. Cell Tissue Res 273:119–125. [DOI] [PubMed] [Google Scholar]
- Callaway JC, Stuart AE. 1999. The distribution of histamine and serotonin in the barnacle’s nervous system. Microsc Res Tech 44:94–104. [DOI] [PubMed] [Google Scholar]
- Cazalets JR, Cournil I, Geffard M, Moulins M. 1987. Suppression of oscillatory activity in crustacean pyloric neurons: implication of GABAergic inputs. J Neurosci 7:2884–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalets JR, Nagy F, Moulins M. 1990a. Suppressive control of the crustacean pyloric network by a pair of identified interneurons. I. Modulation of the motor pattern. J Neurosci 10:448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalets JR, Nagy F, Moulins M. 1990b. Suppressive control of the crustacean pyloric network by a pair of identified interneurons. II. Modulation of neuronal properties. J Neurosci 10:458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie A, Skiebe P, Marder E. 1995a. Matrix of neuromodulators in neurosecretory structures of the crab, Cancer borealis. J Exp Biol 198:2431–2439. [DOI] [PubMed] [Google Scholar]
- Christie AE, Baldwin D, Turrigiano G, Graubard K, Marder E. 1995b. Immunocytochemical localization of multiple cholecystokinin-like peptides in the stomatogastric nervous system of the crab, Cancer borealis. J Exp Biol 198:263–271. [DOI] [PubMed] [Google Scholar]
- Christie AE, Stein W, Quinlan JE, Nusbaum MP. 2000. Histaminergic innervation of the crab stomatogastric system. Soc Neurosci Abstr 26:449. [Google Scholar]
- Claiborne BJ, Selverston AI. 1984a. Histamine as a neurotransmitter in the stomatogastric nervous system of the spiny lobster. J Neurosci 4:708–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claiborne BJ, Selverston AI. 1984b. Localization of stomatogastric IV neuron cell bodies in lobster brain. J Comp Physiol A 154:27–32. [Google Scholar]
- Coleman MJ, Nusbaum MP. 1994. Functional consequences of compartmentalization of synaptic input. J Neurosci 14:6544–6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MJ, Nusbaum MP, Cournil I, Claiborne BJ. 1992. Distribution of modulatory inputs to the stomatogastric ganglion of the crab, Cancer borealis. J Comp Neurol 325:581–594. [DOI] [PubMed] [Google Scholar]
- Coleman MJ, Meyrand P, Nusbaum MP. 1995. A switch between two modes of synaptic transmission mediated by presynaptic inhibition. Nature 378:502–505. [DOI] [PubMed] [Google Scholar]
- Combes D, Meyrand P, Simmers J. 1999. Motor pattern specification by dual descending pathways to a lobster rhythm-generating network. J Neurosci 19:3610–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dando MR, Selverston AI. 1972. Command fibres from the supraooesophageal ganglion to the stomatogastric ganglion in Panulirus argus. J Comp Physiol 78:138–175. [Google Scholar]
- Dickinson PS, Marder E. 1989. Peptidergic modulation of a multioscillator system in the lobster. I. Activation of the cardiac sac motor pattern by the neuropeptides proctolin and red pigment-concentrating hormone. J Neurophysiol 61:833–844. [DOI] [PubMed] [Google Scholar]
- Dickinson PS, Mecsas C, Marder E. 1990. Neuropeptide fusion of two motor-pattern generator circuits. Nature 344:155–158. [DOI] [PubMed] [Google Scholar]
- Dickinson PS, Mecsas C, Hetling J, Terio K. 1993. The neuropeptide red pigment concentrating hormone affects rhythmic pattern generation at multiple sites. J Neurophysiol 69:1475–1483. [DOI] [PubMed] [Google Scholar]
- Dickinson PS, Hauptman J, Hetling J, Mahadevan A. 2001. RCPH modulation of a multi-oscillator network: effects on the pyloric network of the spiny lobster. J Neurophysiol 85:1424–1435. [DOI] [PubMed] [Google Scholar]
- el Manira A, Clarac F 1994. Presynaptic inhibition is mediated by histamine and GABA in the crustacean escape reaction. J Neurophysiol 71:1088–1095. [DOI] [PubMed] [Google Scholar]
- Evans CG, Alexeeva V, Rybak J, Karhunen T, Weiss KR, Cropper EC. 1999. A pair of reciprocally inhibitory histaminergic sensory neurons are activated within the same phase of ingestive motor programs in Aplysia. J Neurosci 19:845–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisselmann G, Pusch H, Hovemann BT, Hatt H. 2001. Two cDNAs coding for histamine-gated ion channels in D. melanogaster. Nat Neurosci 5:11–12. [DOI] [PubMed] [Google Scholar]
- Harris-Warrick RM, Marder E, Selverston AI, Moulins M. 1992. Dynamic biological networks: the stomatogastric nervous system. Cambridge, MA: MIT Press. [Google Scholar]
- Hashemzadeh-Gargari H, Freschi JE. 1992. Histamine activates chloride conductance in motor neurons of the lobster cardiac ganglion. J Neurophysiol 68:9–15. [DOI] [PubMed] [Google Scholar]
- Homberg U, Hildebrand JG. 1991. Histamine-immunoreactive neurons in the midbrain and subooesophageal ganglion of sphinx moth Manduca sexta. J Comp Neurol 307:647–657. [DOI] [PubMed] [Google Scholar]
- Hooper SL, Moulins M. 1989. Switching of a neuron from one network to another by sensory-induced changes in membrane properties. Science 244:1587–1589. [DOI] [PubMed] [Google Scholar]
- Hooper SL, Moulins M. 1990. Cellular and synaptic mechanisms responsible for a long-lasting restructuring of the lobster pyloric network. J Neurophysiol 64:1574–1589. [DOI] [PubMed] [Google Scholar]
- Hooper SL, Moulins M, Nonnotte L. 1990. Sensory input induces long-lasting changes in the output of the lobster pyloric network. J Neurophysiol 64:1555–1573. [DOI] [PubMed] [Google Scholar]
- Horner M, Helle J, Schurmann FW. 1996. The distribution of histamine-immunoreactive neurons in the ventral nerve cord of the cricket, Gryllus bimaculatus. Cell Tissue Res 286:393–405. [DOI] [PubMed] [Google Scholar]
- Katz PS, Eigg MH, Harris-Warrick RM. 1989. Serotonergic/cholinergic muscle receptor cells in the crab stomatogastric nervous system. I. Identification and characterization of the gastropyloric receptor cells. J Neurophysiol 62:558–570. [DOI] [PubMed] [Google Scholar]
- Kilman VL, Marder E. 1996. Ultrastructure of the stomatogastric ganglion neuropil of the crab, Cancer borealis. J Comp Neurol 374:362–375. [DOI] [PubMed] [Google Scholar]
- King DG. 1976. Organization of crustacean neuropil. I. Patterns of synaptic connections in lobster stomatogastric ganglion. J Neurocytol 5:207–237. [DOI] [PubMed] [Google Scholar]
- Le Feuvre Y, Fenelon VS, Meyrand P. 2001. Ontogeny of modulatory inputs to motor networks: early established projection and progressive neurotransmitter acquisition. J Neurosci 21:1313–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Pulver SR, Kelley WP, Thirumalai V, Sweedler JV, Marder E. 2002. Orcokinin peptides in developing and adult crustacean stomatogastric nervous systems and pericardial organs. J Comp Neurol 444:227–244. [DOI] [PubMed] [Google Scholar]
- Li L, Kelley WP, Billimoria C, Christie AE, Pulver SR, Sweedler JV, Marder E. 2003. Mass spectrometric investigation of the neuropeptide complement and release in the pericardial organs of the crab, Cancer borealis. J Neurochem 87:642–656. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D. 2001. Central pattern generators and the control of rhythmic movements. Curr Biol 11:R986–R996. [DOI] [PubMed] [Google Scholar]
- Marder E, Calabrese RL 1996. Principles of rhythmic motor pattern generation. Physiol Rev 76:687–717. [DOI] [PubMed] [Google Scholar]
- Marder E, Eisen JS. 1984. Electrically coupled pacemaker neurons respond differently to same physiological inputs and neurotransmitters. J Neurophysiol 51:1362–1374. [DOI] [PubMed] [Google Scholar]
- Marder E, Hooper SL. 1985. Neurotransmitter modulation of the stomatogastric ganglion of decapod crustaceans In: Selverston AI, editor. Model neural networks and behavior. New York: Plenum Press; p 319–337. [Google Scholar]
- Marder E, Weimann JM. 1992. Modulatory control of multiple task processing in the stomatogastric nervous system In: Kien J, McCrohan C, Winlow B, editors. Neurobiology of motor programme selection. New York: Pergamon Press; p 3–19. [Google Scholar]
- Marder E, Calabrese RL, Nusbaum MP, Trimmer B. 1987. Distribution and partial characterization of FMRFamide-like peptides in the stomatogastric nervous systems of the rock crab, Cancer borealis, and the spiny lobster, Panulirus interruptus. J Comp Neurol 259:150–163. [DOI] [PubMed] [Google Scholar]
- Marder E, Jorge-Rivera JC, Kilman V, Weimann JM. 1997. Peptidergic modulation of synaptic transmission in a rhythmic motor system In: Festoff BW, Hantai D, Citron BA, editors. The synapse: in development, health, and disease. Greenwich, CT: JAI Press Inc; p 213–233. [Google Scholar]
- Maynard EA. 1971. Electron microscopy of stomatogastric ganglion in the lobster, Homarus americanus. Tissue Cell 3:137–149. [DOI] [PubMed] [Google Scholar]
- McClintock TS, Ache BW. 1989. Histamine directly gates a chloride channel in lobster olfactory receptor neurons. Proc Natl Acad Sci USA 86:8137–8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyrand P, Simmers J, Moulins M 1991. Construction of a pattern-generating circuit with neurons of different networks. Nature 351:60–63. [DOI] [PubMed] [Google Scholar]
- Meyrand P, Simmers J, Moulins M. 1994. Dynamic construction of a neural network from multiple pattern generators in the lobster stomatogastric nervous system. J Neurosci 14:630–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyrand P, Faumont S, Simmers J, Christie AE, Nusbaum MP. 2000. Species-specific modulation of pattern-generating circuits. Eur J Neurosci 12:2585–2596. [DOI] [PubMed] [Google Scholar]
- Mulloney B, Hall WM. 1991. Neurons with histaminelike immunoreactivity in the segmental and stomatogastric nervous systems of the crayfish Pacifastacus leniusculus and the lobster Homarus americanus. Cell Tissue Res 266:197–207. [DOI] [PubMed] [Google Scholar]
- Nagy F, Cardi P. 1994. A rhythmic modulatory gating system in the stomatogastric nervous system of Homarus gammarus. II. Modulatory control of the pyloric CPG. J Neurophysiol 71:2490–2502. [DOI] [PubMed] [Google Scholar]
- Nagy F, Dickinson PS. 1983. Control of a central pattern generator by an identified modulatory interneurone in crustacea. I. Modulation of the pyloric motor output. J Exp Biol 105:33–58. [DOI] [PubMed] [Google Scholar]
- Nassel DR. 1999. Histamine in the brain of insects: a review. Microsc Res Tech 44:121–136. [DOI] [PubMed] [Google Scholar]
- Nassel DR, Holmqvist MH, Hardie RC, Hakanson R, Sundler F. 1988. Histamine-like immunoreactivity in photoreceptors of the compound eyes and ocelli of the flies Calliphora erythrocephala and Musca domestica. Cell Tissue Res 253:639–646. [DOI] [PubMed] [Google Scholar]
- Norris BJ, Coleman MJ, Nusbaum MP. 1994. Recruitment of a projection neuron determines gastric mill motor pattern selection in the stomatogastric nervous system of the crab, Cancer borealis. J Neurophysiol 72:1451–1463. [DOI] [PubMed] [Google Scholar]
- Norris BJ, Coleman MJ, Nusbaum MP. 1996. Pyloric motor pattern modification by a newly identified projection neuron in the crab stomatogastric nervous system. J Neurophysiol 75:97–108. [DOI] [PubMed] [Google Scholar]
- Nusbaum MP. 2002. Regulating peptidergic modulation of rhythmically active neural circuits. Brain Behav Evol 60:378–387. [DOI] [PubMed] [Google Scholar]
- Nusbaum MP, Beenhakker MP. 2002. A small-systems approach to motor pattern generation. Nature 417:343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum MP, Marder E. 1988. A neuronal role for a crustacean red pigment concentrating hormone-like peptide: neuromodulation of the pyloric rhythm in the crab, Cancer borealis. J Exp Biol 135:165–181. [Google Scholar]
- Nusbaum MP, Blitz DM, Swensen AM, Wood D, Marder E. 2001. The roles of co-transmission in neural network modulation. Trends Neurosci 24:146–154. [DOI] [PubMed] [Google Scholar]
- O’Donohue TL, Bishop JF, Chronwall BM, Groome J, Watson WH. 1984. Characterization and distribution of FMRFamide immunoreactivity in the rat central nervous system. Peptides 5:563–568. [DOI] [PubMed] [Google Scholar]
- Panula P, Yang HY, Costa E. 1984. Histamine-containing neurons in the rat hypothalamus. Proc Natl Acad Sci USA 81:2572–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panula P, Happola O, Airaksinen MS, Auvinen S, Virkamaki A. 1988. Carbodiimide as a tissue fixative in histamine immunohistochemistry and its application in developmental neurobiology. J Histochem Cytochem 36:259–269. [DOI] [PubMed] [Google Scholar]
- Perrins R, Walford A, Roberts A. 2002. Sensory activation and role of inhibitory reticulospinal neurons that stop swimming in hatchling frog tadpoles. J Neurosci 22:4229–4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulver SR, Thirumalai V, Richards KS, Marder E. 2003. Dopamine and histamine in the developing stomatogastric system of the lobster Homarus americanus. J Comp Neurol 462:400–414. [DOI] [PubMed] [Google Scholar]
- Russell DF, Hartline DK. 1981. Amultiaction synapse evoking both EPSPs and enhancement of endogenous bursting. Brain Res 223:19–38. [DOI] [PubMed] [Google Scholar]
- Schmid A, Duncker M. 1993. On the function of histamine in the central nervous system of arthropods. Acta Biol Hung 44:67–75. [PubMed] [Google Scholar]
- Scholz NL, de Vente J, Truman JW, Graubard K. 2001. Neural network partitioning by NO and cGMP. J Neurosci 21:1610–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selverston AI, Moulins M. 1987. The crustacean stomatogastric system: a model for the study of central nervous systems. Berlin: Springer-Verlag. [Google Scholar]
- Sigvardt KA, Mulloney B. 1982a. Properties of synapses made by IVN command-interneurones in the stomatogastric ganglion of the spiny lobster Panulirus interruptus. J Exp Biol 97:153–168. [DOI] [PubMed] [Google Scholar]
- Sigvardt KA, Mulloney B. 1982b. Sensory alteration of motor patterns in the stomatogastric nervous system of the spiny lobster Panulirus interruptus. J Exp Biol 97:137–152. [DOI] [PubMed] [Google Scholar]
- Skiebe P 2001. Neuropeptides are ubiquitous chemical mediators: using the stomatogastric nervous system as a model system. J Exp Biol 204:2035–2048. [DOI] [PubMed] [Google Scholar]
- Skiebe P 2003. Neuropeptides in the crayfish stomatogastric nervous system. Microsc Res Tech 60:302–312. [DOI] [PubMed] [Google Scholar]
- Skiebe P, Schneider H. 1994. Allatostatin peptides in the crab stomatogastric nervous system: inhibition of the pyloric motor pattern and distribution of allatostatin-like immunoreactivity. J Exp Biol 194:195–208. [DOI] [PubMed] [Google Scholar]
- Skiebe P, Corrette BJ, Wiese K. 1990. Evidence that histamine is the inhibitory transmitter of the auditory interneuron ON1 of crickets. Neurosci Lett 116:361–366. [DOI] [PubMed] [Google Scholar]
- Spirito CP. 1975. Organization of the crayfish ooesophageal nervous system. J Comp Physiol 102:237–249. [Google Scholar]
- Swensen AM, Golowasch J, Christie AE, Coleman MJ, Nusbaum MP, Marder E. 2000. GABA and responses to GABA in the stomatogastric ganglion of the crab Cancer borealis. J Exp Biol 203:2075–2092. [DOI] [PubMed] [Google Scholar]
- Thirumalai V, Marder E. 2002. Colocalized neuropeptides activate a central pattern generator by acting on different circuit targets. J Neurosci 22:1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimann JM, Marder E. 1994. Switching neurons are integral members of multiple oscillatory networks. Curr Biol 4:896–902. [DOI] [PubMed] [Google Scholar]
- Weimann JM, Meyrand P, Marder E. 1991. Neurons that form multiple pattern generators: identification and multiple activity patterns of gastric/pyloric neurons in the crab stomatogastric system. J Neurophysiol 65:111–122. [DOI] [PubMed] [Google Scholar]
- Weimann JM, Marder E, Evans B, Calabrese RL. 1993. The effects of SDRNFLRFamide and TNRNFLRFamide on the motor patterns of the stomatogastric ganglion of the crab Cancer borealis. J Exp Biol 181:1–26. [DOI] [PubMed] [Google Scholar]
- Weiss KR, Chiel HJ, Kuperfermann I. 1986a. Sensory function and gating of histaminergic neuron C2 in Aplysia. J Neurosci 6:2416–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss KR, Shapiro E, Kupfermann I. 1986b. Modulatory synaptic actions of an identified histaminergic neuron on the serotonergic metacerebral cell of Aplysia. J Neurosci 6:2393–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood DE, Nusbaum MP. 2002. Extracellular peptidase activity tunes motor pattern modulation. J Neurosci 22:4185–4195. [DOI] [PMC free article] [PubMed] [Google Scholar]


