Abstract
The transcription factor RUNX1 is required in the embryo for formation of the adult hematopoietic system. Here we describe the seminal findings that led to the discovery of RUNX1 and of its critical role in blood cell formation in the embryo from hemogenic endothelium. We also present RNA-Seq data demonstrating that hemogenic endothelial cells in different anatomic sites, which produce hematopoietic progenitors with dissimilar differentiation potentials, are molecularly distinct. Hemogenic and non-hemogenic endothelial cells in the yolk sac are more closely related to each other than either are to hemogenic or non-hemogenic endothelial cells in the major arteries. Thus, a major driver of the different lineage potentials of the committed erythro-myeloid progenitors that emerge in the yolk sac, versus hematopoietic stem cells that originate in the major arteries, is likely to be the distinct molecular properties of the hemogenic endothelial cells from which they are derived. We use bioinformatics analyses to predict signaling pathways active in arterial hemogenic endothelium, which include the functionally validated pathways Notch, Wnt, and Hedgehog. We also use a novel bioinformatics approach to assemble transcriptional regulatory networks and predict transcription factors that may be specifically involved in hematopoietic cell formation from arterial hemogenic endothelium, which is the origin of the adult hematopoietic system.
History
The discovery of RUNX1
An account of the developmental origin of blood cannot be told without the major protagonist of the story, RUNX1. RUNX was first discovered in the fly, in the classic screen conducted by Nusslein-Volhard and Wieschaus to identify mutations that affect development [1]. The mutation runt was named for the fact that it resulted in runted embryos - a defect caused by pre-segmentation patterning defects. The runt gene was cloned by Gergen and colleagues, and the protein it encoded shown to be nuclear, but its identity as a transcription factor was not discovered at that time [2].
The human RUNX1 gene was the next to be cloned by Ohki and colleagues, as one of a pair of genes disrupted by the (8;21)(q22;q22) translocation in acute myeloid leukemia (AML) [3]. Homology to the Drosophila runt gene was noted [4], but since the biochemical function of the Drosophila runt protein was not known, the function of the human homologue remained a mystery. Not long after, however, three labs using two different approaches uncovered RUNX1’s function. Our group (Speck) purified RUNX1 as a sequence-specific DNA binding protein that regulated the disease specificity of a mouse retrovirus [5]. The team of Ito and Shigesada purified RUNX1’s homologue RUNX2 based on its role in murine polyomavirus replication and transcription [6]. Both groups showed that the purified transcription factors consisted of two subunits, one that bound DNA directly (RUNX1 or RUNX2), and a common non-DNA binding subunit called core binding factor β (CBFβ) that increased the affinity of RUNX1 and RUNX2 for DNA [6–8]. The name “core binding factor” (CBF) derives from the DNA motif in the polyomavirus and retrovirus enhancers to which the RUNX proteins bound, which had previously been named “Core” [9]. At around the same time RUNX1 and CBFβ were purified and cloned, the Hiebert lab used a “selection and amplification binding” technique to determine whether the human RUNX1 protein bound DNA and, if so, which DNA sequence it recognized [10]. The Hiebert lab identified the same DNA sequence that was used by our lab and the Ito/Shigesada labs to purify the proteins. Closing the circle, Liu et al. showed that the inv(16)(p13.1;q22) associated with AML created a chimeric protein that fused the non DNA binding CBFβ subunit to the coiled-coil tail region of a smooth muscle myosin heavy chain [11]. Hence multiple lines of investigation converged, linking RUNX1 to CBFβ, the t(8;21) to the inv(16), and human leukemia to mouse leukemia. These discoveries are a great example of the major contribution the study of viruses and model organisms made to our understanding of human disease.
A role for RUNX1 in the embryonic origin of blood
Of all of the paths that led to the discovery of RUNX1 and CBFβ, only the chromosomal translocations hinted at an essential role at the earliest stages of blood cell formation. As background, hematopoiesis in the embryo unfolds in three waves, and both RUNX1 and CBFβ are required in the last two waves. The first, primitive wave produces primitive erythrocytes, diploid megakaryocytes, and primitive macrophages, all of which differentiate from mesoderm in the yolk sac blood islands beginning at embryonic day (E) 7.25 in the mouse [12–14]. Wave 2 consists of the first “definitive” progenitors, which include erythro-myeloid progenitors (EMPs) that emerge in the yolk sac beginning at E8.25 [12, 15], and lymphoid progenitors that appear at E9.5 in the yolk sac, and in the caudal part of the embryo in the dorsal aorta, vitelline and umbilical arteries [16–21]. Wave 3, the final wave of blood formation, includes pre-hematopoietic stem cells (pre-HSCs) that are unable to engraft adult mice directly, but colonize the fetal liver where they mature into adult-repopulating HSCs [22–27]. Wave 3 also includes a small number of adult-repopulating HSCs in the dorsal aorta, vitelline and umbilical arteries, and in the placenta, which presumably have matured in situ from pre-HSCs [24, 26, 28–34].
Knockouts of RUNX1 and CBFβ resulted in the absence of all wave 2 and 3 progenitors, including adult-repopulating HSCs [35–40]. To gain insight into the nature of this hematopoietic block, our lab introduced a lacZ reporter gene into the Runx1 locus to learn where RUNX1 was expressed in the blood lineage [41]. We found RUNX1 only in the relatively rare wave 2 and 3 progenitors in the embryo, which are far outnumbered by primitive erythrocytes that are initially RUNX1+ but quickly lose RUNX1 expression. This restriction of RUNX1 expression to wave 2 and 3 progenitors (and also to wave 1 macrophages) was actually a key feature that enabled us to pinpoint their origin. Some RUNX1+ wave 2 and 3 progenitors could be found in the circulation, and in the fetal liver, but we also found clusters of round RUNX1+ hematopoietic cells attached to RUNX1+ endothelial cells in arteries, specifically in anatomic sites where the first functional wave 2 and 3 progenitors, and histological evidence of blood cell formation had been described [17, 24, 32, 42–51]. Most importantly, we showed that RUNX1 loss blocked the formation of wave 2 and 3 progenitors from this rare population of “hemogenic” endothelial cells.
The concept that blood formed from hemogenic endothelium had been proposed in the early 20th century [44, 52, 53], and just prior to our work several groups showed that blood could differentiate from endothelial cells [54, 55]. Despite these compelling data, the notion that hemogenic endothelium was the immediate precursor of blood was not widely accepted at the time of our discovery. Our identification of RUNX1 as the first specific marker of hemogenic endothelium, and demonstration that RUNX1 was required for blood cell formation from hemogenic endothelium [41, 56, 57], lent considerable support to the notion that hemogenic endothelial cells were the immediate precursor of the adult hematopoietic organ. Later, live imaging studies performed by multiple laboratories showed blood cells forming from endothelial cells in real time, leading to the consensus that endothelial cells are the immediate precursor of blood cells [58–61].
Heterogeneity of hemogenic endothelium
An interesting question is how different populations of hemogenic endothelial (HE) cells produce embryonic blood progenitors with distinct properties. For example, HE cells in the yolk sac primarily give rise to EMPs, whilst in the major arteries HE cells give rise to pre-HSCs and HSCs. We hypothesize that differences in the lineage potential of hematopoietic stem and progenitor cells (HSPCs) produced in the yolk sac and major arteries likely originates, at least in part, from differences in the intrinsic properties of the HE cells from which they arise. Several lines of evidence support this hypothesis. For instance, HE cells that give rise to EMPs are both arterial and venous in origin, whereas pre-HSCs differentiate from arterial HE [62–64]. Second, different signaling pathways are involved in EMP and pre-HSC formation. For example, EMP formation from HE in the yolk sac vascular plexus does not require Notch signaling, while HSPC production from arterial endothelium is strictly dependent on Notch [65–68]. Similarly, inflammatory signaling regulates the numbers of HSPCs produced in the major arteries, but appears to have no influence on EMP numbers in the yolk sac [69–72].
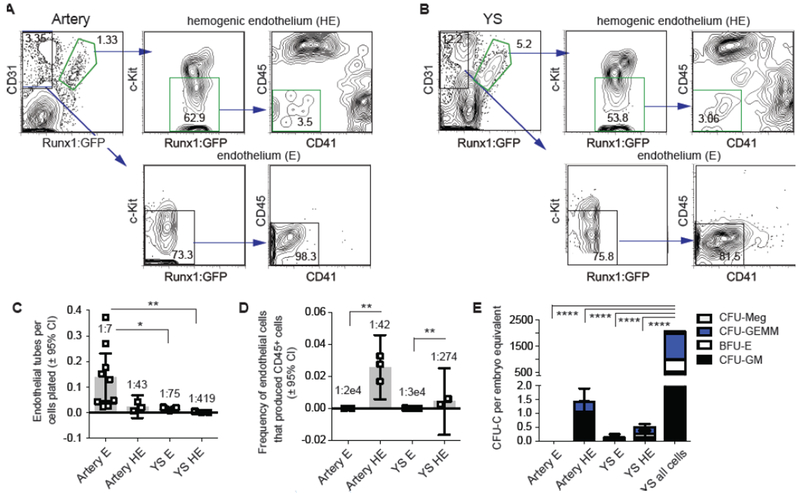
To determine whether HE cells in different anatomic sites are indeed molecularly distinct, we purified HE cells and non-hemogenic endothelial (E) cells from the yolk sac and major arteries at two different embryonic stages, embryonic day 9.5 (E9.5) and E10.5, and determined their transcriptomes by RNA-Seq. We isolated major arteries (dorsal aorta, umbilical, vitelline) by dissecting the caudal region of the mouse embryo from which the head, heart, pulmonary regions, liver, gut tube, tail and limb buds were removed. We used green fluorescent protein (GFP) expression from a Runx1:GFP knockin allele [73] as a tool to separate HE from E. We purified HE from the arteries and yolk sac as Runx1:GFP+ CD31+ Kitlo/- CD45− CD41− cells, and E as Runx1:GFP− CD31+ Kit− CD45− CD41− cells (Fig. 1A,B). We confirmed the functional purity of the cells in ex vivo assays. Both HE and E cells could form endothelial tubes in culture (Fig. 1C). On the other hand, endothelial cells with the ability to form CD45+ blood cells in culture were highly enriched in the purified Runx1:GFP+ HE population (the frequency was 500x higher in HE versus E from the arteries, and 100X higher in HE versus E from the yolk sac, Fig. 1D). Purified HE and E cells produced very few hematopoietic colonies in methylcellulose cultures (0.5–1.5 colony forming units per embryo equivalent) (Fig. 1E), indicating that the HE cells were not significantly contaminated by HSPCs.
Figure 1. Isolation and functional characterization of hemogenic endothelial (HE) and endothelial (E) cells from the arteries and yolk sac.
A) FACS gating strategy to purify HE and E cells from the arteries. Shown are profiles from E10.5 embryos.
B) Purification of HE and E from yolk sacs (YS), E10.5.
C) Frequency of cells that produced endothelial tubes on OP9 cells cultured with vascular endothelial growth factor (VEGF). Data from E10.5 HE and E are shown. Average frequencies are indicated above the bars. Significance determined by ANOVA and Tukey’s multiple comparisons test (mean ± 95% CI, *P < 0.05, **P < 0.01, n=3 experiments).
D) Frequency of HE and E cells that produced CD45+ hematopoietic cells following 8–10 days of culture on OP9 stromal cells in the presence of stem cell factor (SCF), interleukin 3 (IL-3), FLT3 ligand (Flt3L) and IL-7. Data from E10.5 HE and E are shown. Significance determined by ANOVA and Tukey’s multiple comparison tests (mean ± 95% CI, **P < 0.01, n=3 experiments).
E) Methylcellulose assays to enumerate colony forming units - culture (CFU-C) in sorted populations of endothelial cells, and unfractionated yolk sac cells as a control, represented as the number of CFU-Cs per embryo equivalent. Data from E10.5 samples shown. Significance determined by ANOVA and Tukey’s multiple comparison tests (mean ± SD, P < 0.0001, n = 4–8 samples, data combined from 3 experiments).
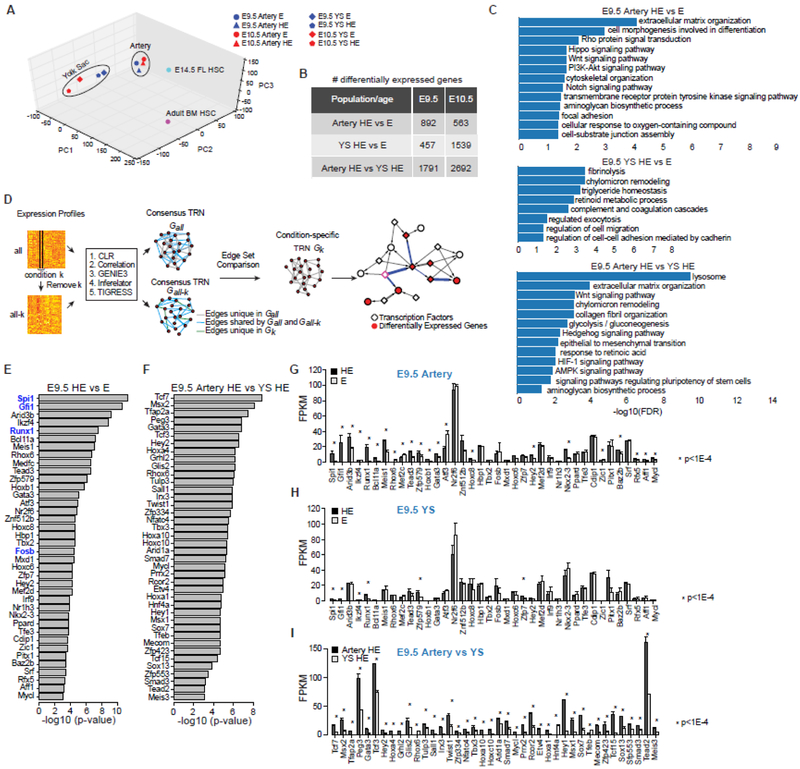
We bulk sequenced approximately 20,000 cells, obtaining ~74.7 million uniquely mapped reads per sample (Accession: GSE103813). The RNA-seq data is highly reproducible, with an average transcriptome-wide correlation of biological replicates of 0.95 (not shown). Principle component analysis (PCA) of the HE and E RNA-seq data, along with data from E14.5 fetal liver (FL) HSCs (Lin−Sca1+Kit+CD48−CD150+) and adult bone marrow (BM) HSCs (Lin−Sca1+Kit+CD34−CD48−CD150+) clustered HE and E far away from FL and BM HSCs (Figure 2A). The tissue of origin was the strongest driver of clustering, with HE and E cells from yolk sacs clustered closely with each other, and less closely with their equivalent populations in the arteries. Similarly, HE and E from the major arteries clustered more closely to each other than to the corresponding cells in the yolk sac. Therefore, HE and E from the major arteries are molecularly more closely related to each other than either is to HE or E from the yolk sac. We used EBSeq [74] to identify differentially expressed genes between pairs of endothelial populations. Consistent with the result of the PCA analysis, there were more differentially expressed genes between HE in the yolk sac and arteries, than between HE and E from a single anatomic site (Fig. 2B, Supplemental Table 1). The close relationship of HE and E cells from the major arteries was also reported by Baron et al. in their recently published single cell RNA-seq study [75].
Figure 2. Global comparison of the transcriptomes of HE and E.
A) Principle component analysis of the transcriptome data. Also shown are fetal liver (FL) HSCs and adult bone marrow (BM) HSCs for comparison. The t-distributed stochastic neighbor embedding (t-SNE) algorithm [98] yielded the same result (not shown).
B) Number of differentially expressed genes based on pairwise comparisons (false discovery rate of 0.05 and fold change of 1.5).
C) Enriched pathways among differentially expressed genes from each pairwise comparison of E9.5 HE and E (false discovery rate of 0.05).
D) A novel computational framework for condition-specific transcriptional regulatory network (TRN) construction and identification of key transcription factors. Nodes in the TRN represent genes, and edges represent regulatory relationship between TFs and target genes. The framework first constructs a consensus TRN for each cell type by using five methods, CLR (the context likelihood of relatedness) [99], a method based on Pearson correlation [99], GENIE3 (gene network inference with ensemble of trees) [100], Inferelator [101], and TIGRESS (trustful inference of gene regulation using stability selection) [102]. Next, the framework prioritizes key TFs based on their potentials of regulating the entire set of differentially expressed genes between the two TRNs compared. Details are provided in the Supplemental Methods.
E) Prioritized key TFs for E9.5 HE (artery HE plus YS HE) versus E (artery plus YS E). Shown in blue are the four TFs used by Sandler et al. and Lis et al. to convert endothelial cells into blood cells [82, 83]. Analysis of comparable E10.5 samples also prioritized Pitx1, Spi1, Runx1, Tead3, Rhox6, Tbx2, Hox8, Hbp1, Mxd1, Znf512B, and Hoxc8 (not shown).
F) Prioritized key TFs for E9.5 artery versus YS HE. TFs prioritized in both E9.5 and E10.5 artery versus YS HE include Tead2, Zfp423, Mecom, Sox7, Msx1, Etv4, Rcor2, Mycl, Tbx3, Twist1, Rhox6, Hoxa4, Tcf3, and Tcf7 (not shown).
G–I) Expression levels of prioritized TFs in corresponding comparisons of E9.5 HE and E. FPKM, Fragments Per Kilobase of transcript per Million mapped reads.
We performed pathway enrichment analysis among differentially expressed genes for each comparison of E9.5 endothelial cell populations. For simplicity sake we focused on the earlier time point, E9.5, which is the peak of EMP formation in the yolk sac, and is the first time RUNX1+ HE cells are abundant in the arteries [12, 63] (Fig. 2C). The Notch pathway was significantly enriched in the arterial HE versus E comparison but not in yolk sac HE versus E. Additional signaling pathways known to be involved in HSPC formation from the arteries such as Wnt, hedgehog, retinoid acid, and hypoxia [76–80] were predicted to be more active in arterial HE than in yolk sac HE.
Cell-type-specific transcriptional regulatory networks and key transcriptional regulators
To identify novel transcription factors (TFs) regulating HE formation, we developed a computational method for constructing condition-specific transcriptional regulatory networks (TRNs) and identifying key TFs using the constructed TRNs. By comparing a pair of cell-type-specific TRNs, we could then predict key TFs that mediate differential expression between the two TRNs (Fig. 2D). We identified 38 TFs in the comparison of E9.5 HE to E (combining YS and artery samples for each cell type), and 40 TFs in the artery HE versus yolk sac HE comparison (Fig. 2E–I, Supplemental Methods). GFI1 and RUNX1, both of which are essential for HSPC formation from HE [41, 81], are among the top ranked TFs in the HE versus E comparison, supporting our bioinformatics approach (Fig. 2E). SPI1 and FOSB, which together with RUNX1 and GFI1 were shown to be sufficient to reprogram endothelial cells into hematopoietic cells [82, 83] were also highly ranked. Other significantly ranked TFs in the HE to E comparison include the TEAD factors, known to regulate hematopoietic specification via the Hippo pathway [84]. TFs with known roles in hematopoiesis, but as of yet no reported function in embryonic HSPC formation, including Arid3b, Ikzf4, and Bcl11a [85–87] were also highly ranked. A similar comparison of E10.5 HE to E resulted in a list of 34 TFs, 10 of which overlapped with TFs predicted to be active at E9.5 (see Fig. 2E, legend). Several top-ranked TFs in the E9.5 artery versus yolk sac HE comparison (Fig. 2F), including Tcf7, Gata3, Hey1, and Hey2, are direct Notch targets [88–90], consistent with the important role for Notch signaling in arterial specification and hematopoiesis. The E9.5 artery HE versus yolk sac HE comparison also predicts that multiple Hox family genes (Hoxa1, Hoxa4, Hoxa10 and Hoxc10) may be in arterial HE. Meis1, shown to play an important role in embryonic HSPC formation [91, 92], was also highly ranked in the comparison of arterial versus yolk sac HE. Meis1 was recently shown to be critical for the transition of HE into progenitor cells in human induced pluripotent stem cell cultures [93].
In conclusion, the study of RUNX1 has provided critical insights into the embryonic origins of blood, and continues to be an extensively used marker of HE. Reporters based on RUNX1 expression have been useful for isolating and studying the generation of HE, and for analyzing the differentiation of HSPCs from HE [41, 73, 94–97]. Here we used expression of GFP from the endogenous Runx1 locus to isolate and compare HE from different anatomic sites. We provide molecular data confirming that HE cells from different sites of HSPC formation are molecularly distinct. These data will be a useful resource for investigators aiming to generate specific subsets of HE in culture, including arterial HE that is the source of pre-HSCs and HSCs.
Supplementary Material
Highlights.
Discovery of RUNX1 and its role in hemogenic endothelium.
Hemogenic endothelium from different anatomic sites is molecularly distinct.
Novel bioinformatics approach predicts transcription factors in hemogenic endothelium
Acknowledgements
This research was supported by NIH grants R01HL091724 (NAS), R01 HG006130 (KT), R01 GM108716 (KT), and R01 HL136024 (KT and NAS). NAS is grateful for her outstanding scientists and colleagues in the field of hematology, and indebted to all of the students and postdoctoral fellows she has had the extraordinary privilege to work with over the course of her career.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability
HE and E RNA-Seq data generated in this study has been deposited in the Gene Expression Omnibus (GEO) database under the accession number GSE103813. FL and BM HSC RNA-seq data will be published elsewhere (Gao et al., in preparation)
References
- [1].Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. [DOI] [PubMed] [Google Scholar]
- [2].Kania MA, Bonner AS, Duffy JB, Gergen JP. The Drosophila segmentation gene runt encodes a novel nuclear regulatory protein that is also expressed in the developing nervous system. Genes Dev. 1990;4:1701–1713. [DOI] [PubMed] [Google Scholar]
- [3].Miyoshi H, Shimizu K, Kozu T, Maseki N, Kaneko Y, Ohki M. t(8;21) breakpoints on chromosome 21 in acute myeloid leukemia are clustered within a limited region of a single gene, AML1. Proc Natl Acad Sci USA. 1991;88:10431–10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Daga A, Tighe JE, Calabi F. Leukaemia/Drosophila homology. Nature (London). 1992;356:484. [DOI] [PubMed] [Google Scholar]
- [5].Wang S, Speck NA. Purification of core-binding factor, a protein that binds the conserved core site in murine leukemia virus enhancers. Mol Cell Biol. 1992;12:89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kamachi Y, Ogawa E, Asano M, et al. Purification of a mouse nuclear factor that binds to both the A and B cores of the polyomavirus enhancer. J Virol. 1990;64:4808–4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ogawa E, Inuzuka M, Maruyama M, et al. Molecular cloning and characterization of PEBP2b, the heterodimeric partner of a novel Drosophila runt-related DNA binding protein PEBP2a. Virology. 1993;194:314–331. [DOI] [PubMed] [Google Scholar]
- [8].Wang S, Wang Q, Crute BE, Melnikova IN, Keller SR, Speck NA. Cloning and characterization of subunits of the T-cell receptor and murine leukemia virus enhancer corebinding factor. Mol Cell Biol. 1993;13:3324–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Weiher H, Zonig M, Gruss P. Multiple point mutations affecting the simian virus 40 enhancer. Science. 1983;219:626–631. [DOI] [PubMed] [Google Scholar]
- [10].Meyers S, Downing JR, Hiebert SW. Identification of AML-1 and the (8;21) translocation protein (aMl-1/ETO) as sequence-specific DNA-binding proteins: the runt homology domain is required for DNA binding and protein-protein interactions. Mol Cell Biol. 1993;13:6336–6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liu P, Tarle SA, Hajra A, et al. Fusion between transcription factor CBFb/PEBP2b and a myosin heavy chain in acute myeloid leukemia. Science. 1993;261:1041–1044. [DOI] [PubMed] [Google Scholar]
- [12].Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. [DOI] [PubMed] [Google Scholar]
- [13].Tober J, Koniski A, McGrath KE, et al. The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis. Blood. 2007;109:1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bertrand JY, Jalil A, Klaine M, Jung S, Cumano A, Godin I. Three pathways to mature macrophages in the early mouse yolk sac. Blood. 2005;106:3004–3011. [DOI] [PubMed] [Google Scholar]
- [15].McGrath KE, Frame JM, Fegan KH, et al. Distinct Sources of Hematopoietic Progenitors Emerge before HSCs and Provide Functional Blood Cells in the Mammalian Embryo. Cell reports. 2015;11:1892–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yoshimoto M, Montecino-Rodriguez E, Ferkowicz MJ, et al. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc Natl Acad Sci U S A. 2011;108:1468–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Godin IE, Garcia-Porrero JA, Coutinho A, Dieterlen-Lièvre F, Marcos MAR. Para-aortic splanchnopleura from early mouse embryos contain B1a cell progenitors. Nature. 1993;364:67–70. [DOI] [PubMed] [Google Scholar]
- [18].Yoshimoto M, Porayette P, Glosson NL, et al. Autonomous murine T-cell progenitor production in the extra-embryonic yolk sac before HSC emergence. Blood. 2012;119:5706–5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Boiers C, Carrelha J, Lutteropp M, et al. Lymphomyeloid contribution of an immune-restricted progenitor emerging prior to definitive hematopoietic stem cells. Cell Stem Cell. 2013;13:535–548. [DOI] [PubMed] [Google Scholar]
- [20].Lin Y, Yoder MC, Yoshimoto M. Lymphoid progenitor emergence in the murine embryo and yolk sac precedes stem cell detection. Stem Cells Dev. 2014;23:1168–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yokota T, Huang J, Tavian M, et al. Tracing the first waves of lymphopoiesis in mice. Development. 2006;133:2041–2051. [DOI] [PubMed] [Google Scholar]
- [22].Rybtsov S, Sobiesiak M, Taoudi S, et al. Hierarchical organization and early hematopoietic specification of the developing HSC lineage in the AGM region. J Exp Med. 2011;208:1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Taoudi S, Gonneau C, Moore K, et al. Extensive hematopoietic stem cell generation in the AGM region via maturation of VE-cadherin+CD45+ pre-definitive HSCs. Cell Stem Cell. 2008;3:99–108. [DOI] [PubMed] [Google Scholar]
- [24].Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. [DOI] [PubMed] [Google Scholar]
- [25].Rybtsov S, Batsivari A, Bilotkach K, et al. Tracing the origin of the HSC hierarchy reveals an SCF-dependent, IL-3-independent CD43(−) embryonic precursor. Stem cell reports. 2014;3:489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gordon-Keylock S, Sobiesiak M, Rybtsov S, Moore K, Medvinsky A. Mouse extraembryonic arterial vessels harbor precursors capable of maturing into definitive HSCs. Blood. 2013;122:2338–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kieusseian A, Brunet de la Grange P, Burlen-Defranoux O, Godin I, Cumano A.Immature hematopoietic stem cells undergo maturation in the fetal liver. Development. 2012;139:3521–3530. [DOI] [PubMed] [Google Scholar]
- [28].de Bruijn MF, Speck NA, Peeters MC, Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J. 2000;19:2465–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ottersbach K, Dzierzak E. The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Developmental cell. 2005;8:377–387. [DOI] [PubMed] [Google Scholar]
- [30].Gekas C, Dieterlen-Lievre F, Orkin SH, Mikkola HK. The placenta is a niche for hematopoietic stem cells. Developmental cell. 2005;8:365–375. [DOI] [PubMed] [Google Scholar]
- [31].Li Z, Lan Y, He W, et al. Mouse embryonic head as a site for hematopoietic stem cell development. Cell Stem Cell. 2012;11:663–675. [DOI] [PubMed] [Google Scholar]
- [32].Müller AM, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1:291–301. [DOI] [PubMed] [Google Scholar]
- [33].Ivanovs A, Rybtsov S, Welch L, Anderson Ra, Turner Ml, Medvinsky A. Highly potent human hematopoietic stem cells first emerge in the intraembryonic aorta-gonad-mesonephros region. J Exp Med. 2011;208:2417–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yoder MC. Inducing definitive hematopoiesis in a dish. Nat Biotechnol. 2014;32:539–541. [DOI] [PubMed] [Google Scholar]
- [35].Wang Q, Stacy T, Binder M, Marín-Padilla M, Sharpe AH, Speck NA. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc Natl Acad Sci USA. 1996;93:3444–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wang Q, Stacy T, Miller JD, et al. The CBFb subunit is essential for CBFa2 (AML1) function in vivo. Cell. 1996;87:697–708. [DOI] [PubMed] [Google Scholar]
- [37].Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84:321–330. [DOI] [PubMed] [Google Scholar]
- [38].Cai Z, de Bruijn MFTR, Ma X, et al. Haploinsufficiency of AML1/CBFA2 affects the embryonic generation of mouse hematopoietic stem cells. Immunity. 2000;13:423–431. [DOI] [PubMed] [Google Scholar]
- [39].Sasaki K, Yagi H, Bronson RT, et al. Absence of fetal liver hematopoiesis in mice deficient in transcriptional coactivator core binding factor beta. Proc Natl Acad Sci USA. 1996;93:12359–12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Niki M, Okada H, Takano H, et al. Hematopoiesis in the fetal liver is impaired by targeted mutagenesis of a gene encoding a non-DNA binding subunit of the transcription factor, polyomavirus enhancer binding protein 2/core binding factor. Proc Natl Acad Sci USA. 1997;94:5697–5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].North TE, Gu T-L, Stacy T, et al. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development. 1999;126:2563–2575. [DOI] [PubMed] [Google Scholar]
- [42].Dantschakoff V Uber das erste aufreten der blut-elemente in hühnerembryo. Folia Haematol. 1907;4:159–166. [Google Scholar]
- [43].Dieterlen-Lièvre F On the origin of haematopoietic stem cells in avian embryos: an experimental approach. J Embryol Exp Morphol. 1975;33:609–619. [PubMed] [Google Scholar]
- [44].Emmel VE. The cell clusters in the dorsal aorta of mammalian embryos. Am J Anat. 1916;19:401–421. [Google Scholar]
- [45].Garcia-Porrero JA, Godin IE, Dieterlen-Lièvre F. Potential intraembryonic hemogenic sites at pre-liver stages in the mouse. Anat Embryol. 1995;192:425–435. [DOI] [PubMed] [Google Scholar]
- [46].Jordon HE. Aortic cell clusters in vertebrate embryos. Proc Natl Acad Sci USA. 1917;3:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Jordon HE. A study of a 7mm human embryo: with special reference to its peculiar spirally twisted form, and its large aortic cell-clusters. Anat Rec. 1918;14:479–492. [Google Scholar]
- [48].Maximov AA. Untersuchengen über blut und bindegewebe. I. Die frühesten entwicklingsstadien der blut und bindegewebzellen beim säugetier-embryo, bis zum anfang der blutbildung in der leber. Archiv für mikroskopische anatomie. 1909;73:444–450. [Google Scholar]
- [49].Smith RA, Glomski CA. “Hemogenic endothelium” of the embryonic aorta: does it exist? Dev Comp Immunol. 1982;6:359–368. [DOI] [PubMed] [Google Scholar]
- [50].Wood HB, May G, Healy L, Enver T, Morriss-Kay GM. CD34 expression patterns during early mouse development are related to modes of blood vessel formation and reveal additional sites of hematopoiesis. Blood. 1997;90:2300–2311. [PubMed] [Google Scholar]
- [51].Godin I, Dieterlen-Lièvre F, Cumano A. Emergence of multipotent hematopoietic cells in the yolk sac and paraaortic splanchnopleura of 8.5 dpc mouse embryos. Proc Natl Acad Sci USA. 1995;92:773–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Jordon HE. Evidence of hemogenic capacity of endothelium. Anat Rec. 1916;10:417–420. [Google Scholar]
- [53].Sabin FR. Studies on the origin of blood vessels and of red corpuscles as seen in the living blastoderm of the chick during the second day of incubation. Contributions to Embryology. 1920;9:213–262. [Google Scholar]
- [54].Jaffredo T, Gautier R, Eichmann A, Dieterlen-Lièvre F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development. 1998;125:4575–4583. [DOI] [PubMed] [Google Scholar]
- [55].Nishikawa S-I, Nishikawa S, Kawamoto H, et al. In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity. 1998;8:761–769. [DOI] [PubMed] [Google Scholar]
- [56].Li Z, Chen MJ, Stacy T, Speck NA. Runx1 function in hematopoiesis is required in cells that express Tek. Blood. 2006;107:106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457:889–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kissa K, Herbomel P. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature. 2010;464:112–115. [DOI] [PubMed] [Google Scholar]
- [59].Eilken HM, Nishikawa S, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457:896–900. [DOI] [PubMed] [Google Scholar]
- [60].Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DY, Traver D. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature. 2010;464:108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature. 2010;464:116–120. [DOI] [PubMed] [Google Scholar]
- [62].Frame JM, Fegan KH, Conway SJ, McGrath KE, Palis J. Definitive Hematopoiesis in the Yolk Sac Emerges from Wnt-Responsive Hemogenic Endothelium Independently of Circulation and Arterial Identity. Stem cells. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ad Yzaguirre, Speck NA. Insights into blood cell formation from hemogenic endothelium in lesser-known anatomic sites. Dev Dyn. 2016;245:1011–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Chen MJ, Li Y, De Obaldia ME, et al. Erythroid/Myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell. 2011; 9:541–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hadland BK, Huppert SS, Kanungo J, et al. A requirement for Notch1 distinguishes 2 phases of definitive hematopoiesis during development. Blood. 2004;104:3097–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Robert-Moreno A, Espinosa L, de la Pompa JL, Bigas A. RBPjkappa-dependent Notch function regulates Gata2 and is essential for the formation of intra-embryonic hematopoietic cells. Development. 2005;132:1117–1126. [DOI] [PubMed] [Google Scholar]
- [67].Kumano K, Chiba S, Kunisato A, et al. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity. 2003;18:699–711. [DOI] [PubMed] [Google Scholar]
- [68].Burns CE, Traver D, Mayhall E, Shepard JL, Zon LI. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes Dev. 2005;19:2331–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Li Y, Esain V, Teng L, et al. Inflammatory signaling regulates embryonic hematopoietic stem and progenitor cell production. Genes Dev. 2014;28:2597–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Espin-Palazon R, Stachura DL, Campbell CA, et al. Proinflammatory signaling regulates hematopoietic stem cell emergence. Cell. 2014;159:1070–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Sawamiphak S, Kontarakis Z, Stainier DY. Interferon gamma signaling positively regulates hematopoietic stem cell emergence. Developmental cell. 2014;31:640–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].He Q, Zhang C, Wang L, et al. Inflammatory signaling regulates hematopoietic stem and progenitor cell emergence in vertebrates. Blood. 2015;125:1098–1106. [DOI] [PubMed] [Google Scholar]
- [73].Lorsbach RB, Moore J, Ang SO, Sun W, Lenny N, Downing JR. Role of Runx1 in adult hematopoiesis: analysis of Runx1-IRES-GFP knock-in mice reveals differential lineage expression. Blood. 2004;103:2522–2529. [DOI] [PubMed] [Google Scholar]
- [74].Leng N, Dawson JA, Thomson JA, et al. EBSeq: an empirical Bayes hierarchical model for inference in RNA-seq experiments. Bioinformatics. 2013;29:1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Baron CS, Kester L, Klaus A, et al. Single-cell transcriptomics reveal the dynamic of haematopoietic stem cell production in the aorta. Nature communications. 2018;9:2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Chanda B, Ditadi A, Iscove NN, Keller G. Retinoic acid signaling is essential for embryonic hematopoietic stem cell development. Cell. 2013;155:215–227. [DOI] [PubMed] [Google Scholar]
- [77].Goessling W, North TE, Loewer S, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136:1136–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Gering M, Patient R. Hedgehog signaling is required for adult blood stem cell formation in zebrafish embryos. Developmental cell. 2005;8:389–400. [DOI] [PubMed] [Google Scholar]
- [79].Imanirad P, Solaimani Kartalaei P, Crisan M, et al. HIF1alpha is a regulator of hematopoietic progenitor and stem cell development in hypoxic sites of the mouse embryo.Stem Cell Res. 2014;12:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Harris JM, Esain V, Frechette GM, et al. Glucose metabolism impacts the spatiotemporal onset and magnitude of HSC induction in vivo. Blood. 2013;121:2483–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Lancrin C, Mazan M, Stefanska M, et al. GFI1 and GFI1B control the loss of endothelial identity of hemogenic endothelium during hematopoietic commitment. Blood. 2012;120:314–322. [DOI] [PubMed] [Google Scholar]
- [82].Lis R, Karrasch CC, Poulos MG, et al. Conversion of adult endothelium to immunocompetent haematopoietic stem cells. Nature. 2017;545:439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Sandler VM, Lis R, Liu Y, et al. Reprogramming human endothelial cells to haematopoietic cells requires vascular induction. Nature. 2014;511:312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Goode DK, Obier N, Vijayabaskar MS, et al. Dynamic Gene Regulatory Networks Drive Hematopoietic Specification and Differentiation. Developmental cell. 2016;36:572–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kurkewich JL, Klopfenstein N, Hallas WM, et al. Arid3b Is Critical for B Lymphocyte Development. PLoS One. 2016;11:e0161468. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [86].Pan F, Yu H, Dang EV, et al. Eos mediates Foxp3-dependent gene silencing in CD4+ regulatory T cells. Science. 2009;325:1142–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Liu P, Keller JR, Ortiz M, et al. Bcl11a is essential for normal lymphoid development. Nat Immunol. 2003;4:525–532. [DOI] [PubMed] [Google Scholar]
- [88].Weber BN, Chi AW, Chavez A, et al. A critical role for TCF-1 in T-lineage specification and differentiation. Nature. 2011;476:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Fang TC, Yashiro-Ohtani Y, Del Bianco C, Knoblock DM, Blacklow SC, Pear WS. Notch directly regulates Gata3 expression during T helper 2 cell differentiation. Immunity. 2007;27:100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Maier MM, Gessler M. Comparative analysis of the human and mouse Hey1 promoter: Hey genes are new Notch target genes. Biochem Biophys Res Commun. 2000;275:652–660. [DOI] [PubMed] [Google Scholar]
- [91].Azcoitia V, Aracil M, Martinez AC, Torres M. The homeodomain protein Meis1 is essential for definitive hematopoiesis and vascular patterning in the mouse embryo. Dev Biol. 2005;280:307–320. [DOI] [PubMed] [Google Scholar]
- [92].Hisa T, Spence SE, Rachel RA, et al. Hematopoietic, angiogenic and eye defects in Meis1 mutant animals. Embo J. 2004;23:450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Wang H, Liu C, Liu X, et al. MEIS1 Regulates Hemogenic Endothelial Generation, Megakaryopoiesis, and Thrombopoiesis in Human Pluripotent Stem Cells by Targeting TAL1 and FLI1. Stem cell reports. 2018;10:447–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Nottingham WT, Jarratt A, Burgess M, et al. Runx1-mediated hematopoietic stem-cell emergence is controlled by a Gata/Ets/SCL-regulated enhancer. Blood. 2007;110:4188–4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].North TE, de Bruijn MF, Stacy T, et al. Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity. 2002;16:661–672. [DOI] [PubMed] [Google Scholar]
- [96].Ditadi A, Sturgeon CM, Tober J, et al. Human definitive haemogenic endothelium and arterial vascular endothelium represent distinct lineages. Nat Cell Biol. 2015;17:580–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Swiers G, Baumann C, O’Rourke J, et al. Early dynamic fate changes in haemogenic endothelium characterized at the single-cell level. Nature communications. 2013;4:2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].van der Maaten LJP, and Hinton GE Visualizing high-dimensional data using t-SNE. Journal of machine learning research. 2008;9:2579–2605. [Google Scholar]
- [99].Faith JJ, Hayete B, Thaden JT, et al. Large-scale mapping and validation of Escherichia coli transcriptional regulation from a compendium of expression profiles. PLoS Biol. 2007;5:e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Huynh-Thu VA, Irrthum A, Wehenkel L, Geurts P. Inferring regulatory networks from expression data using tree-based methods. PLoS One. 2010;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Bonneau R, Reiss DJ, Shannon P, et al. The Inferelator: an algorithm for learning parsimonious regulatory networks from systems-biology data sets de novo. Genome Biol. 2006;7:R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Haury AC, Mordelet F, Vera-Licona P, Vert JP. TIGRESS: Trustful Inference of Gene REgulation using Stability Selection. BMC Syst Biol. 2012;6:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.