Abstract
Background
Vestibular migraine is a common cause of episodic vertigo. Many preventive treatments have been proposed for this condition, including calcium antagonists, beta‐blockers, antidepressants, anticonvulsants, selective 5‐HT1 agonists, serotonin antagonists and non‐steroidal anti‐inflammatory drugs (NSAIDs).
Objectives
To assess the effects of pharmacological agents for the prevention of vestibular migraine.
Search methods
The Cochrane Ear, Nose and Throat Disorders Group (CENTDG) Trials Search Co‐ordinator searched the CENTDG Trials Register; Central Register of Controlled Trials (CENTRAL 2015, Issue 5); PubMed; EMBASE; CINAHL; Web of Science; Clinicaltrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 5 June 2015.
Selection criteria
Randomised controlled trials (RCTs) in adults (over 18 years) with a diagnosis of vestibular migraine orprobable vestibular migraine according to the Bárány Society/International Headache Society (IHS) criteria, treated in any setting, comparing pharmacological treatments used in the prevention of vestibular migraine, including beta‐blockers, calcium antagonists, anticonvulsants, antidepressants, serotonin antagonists and non‐steroidal anti‐inflammatory drugs (NSAIDs) against placebo or no treatment.
Data collection and analysis
We used the standard methodological procedures expected by The Cochrane Collaboration.
Main results
Our literature search identified 558 reports, however only 11 were sufficiently relevant for further assessment. We excluded two studies because they did not use the IHS diagnostic criteria for vestibular migraine. We excluded a further eight studies for various reasons related to their design (e.g. lack of placebo or no treatment comparator), aim (e.g. treatment of vestibular migraine rather than prevention) or conduct (e.g. early termination). We identified one ongoing study comparing metoprolol to placebo. The results of this study are awaited; recruitment of the last patient is expected by the end of 2016.
Authors' conclusions
We found no evidence from RCTs to answer the question set out in the review objectives. This review has identified the need for well‐designed randomised controlled trials to answer questions about the efficacy of current and new treatments.
Plain language summary
Medicines to prevent vestibular migraine
Review question
Which medicines are effective for preventing vestibular migraine?
Background
Vestibular migraine is a form of migraine in which the main symptoms relate to dizziness and the balance system. It is a common problem, known to affect 1% of the general population and possibly more because it is thought to be under diagnosed. There is still no agreement on which is the best treatment available to prevent attacks of vestibular migraine.
Study characteristics
We found only one ongoing study on this topic. This study is investigating a beta‐blocker (metoprolol) compared with a placebo (sham treatment).
Key results
We identified no completed randomised controlled trials that met the review inclusion criteria. Results of the ongoing study are awaited. Recruitment of the last patient is expected by the end of 2016.
The evidence in this review is current to June 2015.
Background
Description of the condition
Vestibular migraine is a form of migraine in which the patient experiences dizziness.
Migraine is a primary episodic headache disorder with internationally accepted diagnostic criteria (International Headache Society (IHS) Migraine Classification: ICHD 2013; Silberstein 2004). It can be classified as migraine with or without aura, depending on the presence or absence of other features. Focal neurological symptoms can accompany the headache. Migraine involves dysfunction of brain‐stem pathways that normally modulate sensory input. The key pathways for the pain are the trigeminovascular input from the meningeal vessels, which passes through the trigeminal ganglion and synapses on second order neurons in the trigeminocervical complex. These neurons, in turn, project through the quintothalamic tract and, after decussating in the brain stem, form synapses with neurons in the thalamus (crossing over from left to right and vice versa). There is a reflex connection between neurons in the superior salivatory nucleus in the pons, which results in a cranial parasympathetic outflow that is mediated through the pterygopalatine, otic and carotid ganglia (Goadsby 2002).
In migraine the headache typically takes place in recurrent attacks lasting four to 72 hours. It is often unilateral and pulsating, and of a moderate to severe (not mild) intensity. The criteria defined by the International Headache Society (IHS) in the International Classification of Headache Disorders (ICHD 2013) are described in Appendix 1. Aura symptoms are reversible and last less than 60 minutes. They may be present even when the headache does not take place. Appendix 2 describes the IHS criteria for the symptoms of aura.
All forms of dizziness are more prevalent in patients with migraine than in the general population (Cha 2007). This includes rotational vertigo, positional vertigo, imbalance, dizziness and Ménière's disease. Vertigo is present in 26.5% of patients with migraine, compared with 7.8% of patients with tension headache. Some studies have found vertigo in more than 40% of patients with classical migraine, and vertigo is more severe in patients suffering from migraine than in those suffering from tension headache (Cummings 2010). Migraine and vertigo do not always coincide in time. Up to 50% of patients with migraine and vertigo have been found to suffer vertigo in headache‐free periods (Brantberg 2005). Basilar type migraine is a special form of migraine with brainstem symptoms, or affecting both hemispheres simultaneously but without motor symptoms (the IHS criteria for basilar type migraine may be found in Appendix 3). In basilar type migraine, the dizziness symptoms have the characteristics of an aura phenomenon. However, basilar type migraine is responsible for just a small percentage of patients with migraine and vertigo. Benign paroxysmal vertigo of childhood is one the periodic syndromes that are commonly precursors of migraine, the other two being cyclical vomiting and abdominal migraine. For the IHS criteria of benign paroxysmal vertigo of childhood see Appendix 4.
There has been a lack of agreement upon the nomenclature and definition of migraine‐related dizziness. Terms like migraine‐associated dizziness, migraine‐related vestibulopathy, migrainous vertigo and vestibular migraine have been proposed. To our knowledge, Dieterich and Brandt were the first to use the term 'vestibular migraine' (Dieterich 1999).
Diagnostic criteria for definite and probable migrainous vertigo have been proposed by Neuhauser (Neuhauser 2001). The diagnosis is dependent on a description of the symptoms experienced and the exclusion of other potential causes by appropriate examination and investigation. There is no objective diagnostic test or biomarker for vestibular migraine. Based on the diagnostic criteria proposed by Neuhauser, Furman has established a flowchart for the diagnosis of definite migrainous vertigo and probable migrainous vertigo (Furman 2003). A recent joint position paper by the Bárány Society and the International Headache Society has updated these criteria (ICHD 2013; Lempert 2012) (Appendix 5). In this review we will use the updated set of criteria for the diagnosis of vestibular migraine.
Description of the intervention
There are two main groups of pharmacological treatments for vestibular migraine (Birsdorf 2011; Oas 2008): treatments for relief of individual attacks and prophylactic treatments to reduce the frequency and severity of attacks.
Drugs used in individual attacks include:
triptans (selective 5‐HT1 agonists);
calcium antagonists (verapamil, diltiazem, nifedipine, amlodipine, nitrendipine, nicardipine, isradipine, felodipine, nisoldipine); and
non‐steroidal anti‐inflammatory drugs (NSAIDs).
Pharmacological agents that have been used for the prevention of migraine include (Carod‐Artal 2014; Silberstein 2004):
beta‐blockers (alprenolol, nadolol, oxprenolol, penbutolol, pindolol, propanolol, sotalol, timolol, carteolol, acebutolol, atenolol, betaxolol, bisoprolol, celiprolol, esmolol, metoprolol, nebivolol, butoxamine, alpha‐methyl propanolol, carvedilol, labetalol, bucindolol and nebivolol);
calcium antagonists (verapamil, diltiazem, nifedipine, amlodipine, nitrendipine, nicardipine, isradipine, felodipine, nisoldipine);
anticonvulsants (valproic acid, carbamazepine, ethosuximide, phenytoin, phenobarbital, primidone, felbamate, gabapentin, oxcarbazepine, lamotrigine, levetiracetam, pregabalin, tiagabine, topiramate, vigabatrin and zonisamide);
antidepressants (amitriptyline, fluoxetine);
selective 5‐HT1 agonists (triptans) (i.e. sumatriptan, zolmitriptan, naratriptan, almotriptan, rizatriptan, eletriptan, frovatriptan);
serotonin antagonists (cyproheptadine, methysergide, pizotifen);
NSAIDs (aspirin, ibuprofen, tolfenamic acid, naproxen, acetaminophen);
riboflavin, coenzyme Q10, magnesium; and
angiotensin‐converting‐enzyme (ACE) inhibitors and related drugs (candesartan, lisinopril).
Adverse effects of these drugs used for migraine treatment include drowsiness, weight gain or loss, tremor, haematologic or liver abnormalities, hair loss, depression, anxiety, insomnia, parkinsonism, leg cramps or swelling, retroperitoneal fibrosis, constipation, heart conduction abnormalities and paraesthesias (Goadsby 2002).
The main focus of this review will be preventive drugs. Other treatments used to prevent vestibular migraine are diet manipulation, avoidance of triggering factors and sleep hygiene. Although biofeedback techniques have been used to treat other types of vertigo, to our knowledge they have not been used for vestibular migraine.
How the intervention might work
Different drugs might affect vestibular migraine in diverse ways according to their pharmacological characteristics. The use of blood pressure treatments appears to reduce the overall prevalence of headache in general (Bajwa 2012). It is not clear how beta‐blockers may work as a migraine preventive agent. Noradrenergic blocking, 5‐HT1 antagonism and antiplatelet effects have been suggested (Pazos 2008). Calcium antagonists seem to work by reducing vascular reactivity and interfering with trigeminal mediators. Triptans have three possible mechanisms of action: cranial vasoconstriction, peripheral neuronal inhibition and inhibition of transmission through second‐order neurons of the trigeminal complex (Goadsby 2002). Serotonin antagonists block 5HT‐2 receptors in the brain and act on the central phase of the migraine crisis.
Why it is important to do this review
Finding out which is the most effective treatment for vestibular migraine is one of the top 10 research priorities defined by a Priority Setting Partnership organised by the James Lind Alliance: Ear, Nose and Throat ‐ Aspects of Balance (JLA 2011). No systematic review or meta‐analysis has been prepared on this topic. The latest narrative review states the need for evidence on prophylactic treatment in vestibular migraine (Birsdorf 2011). Important conclusions may be drawn from a systematic review.
Objectives
To assess the effects of pharmacological agents for the prevention of vestibular migraine.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials, including cluster‐randomised trials. We did not plan to include quasi‐randomised studies or historically controlled trials.
Types of participants
Adults (over 18 years) with a diagnosis of vestibular migraine orprobable vestibular migraine according to the Bárány Society/IHS criteria, treated in any setting.
Definition of the disease
When trying to define patients with vestibular migraine we face a dilemma. If we strictly apply the criteria for the diagnosis of vestibular migraine or probable vestibular migraine as defined by the Bárány Society and the IHS (Lempert 2012), the population of trial participants (patients) will be tightly defined. However, we risk not finding any trials that have applied these criteria as they have only recently been codified. If we include trials with patients with migraine and any vestibular symptom we will undoubtedly have a broader range of patients, but their disease status will be ill‐defined and the population more heterogeneous.
We decided only to include trials where the participants fulfil the 2012 Bárány Society/IHS criteria for vestibular migraine and probablevestibular migraine for two reasons: firstly because of the robustness of the diagnosis made using those criteria and secondly because using these internationally accepted criteria (which have been issued by two international societies, one neurological, the other neuro‐otologic) will encourage the use of standardised criteria in future studies.
Since we anticipated a small number of trials, we decided to merge definite andprobable vestibular migraine patients into a single group, but we planned to investigate this decision using subgroup analyses.
Types of interventions
Active interventions
Pharmacological agents used in the prevention of vestibular migraine, including beta‐blockers, calcium antagonists, anticonvulsants, antidepressants, serotonin antagonists and NSAIDs.
Control
Placebo or no treatment.
These interventions had to be used in the context of the prevention of vestibular migraine episodes, rather than as treatment for an attack.
Types of outcome measures
We included all outcomes that we consider to be relevant to the general public, primary health care providers, vertigo specialists and policy decision‐makers. These include quality of life parameters, number of new episodes of vertigo, duration of the episodes, secondary effects and economic parameters.
We planned to analyse the following outcomes in the review, but did not plan to use them as a basis for including or excluding studies.
Primary outcomes
Overall improvement in symptoms
Secondary outcomes
Duration of episodes
Withdrawal from study
Adverse effects, specifically tiredness, drowsiness, weight gain or loss, tremor, haematologic or liver abnormalities, hair loss, depression, anxiety, insomnia, parkinsonism, leg cramps or swelling, retroperitoneal fibrosis, constipation, heart conduction abnormalities and paraesthesias (Goadsby 2002)
Intensity of the crisis
Quality of life
Economic factors (information that will help in modelling cost‐effectiveness)
Search methods for identification of studies
The Cochrane Ear, Nose and Throat Disorders Group (CENTDG) Trials Search Co‐ordinator (TSC) conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. The date of the search was 5 June 2015.
Electronic searches
The TSC searched:
the CENTDG Trials Register (searched 5 June 2015);
the Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 5);
Ovid MEDLINE (1946 to 5 June 2015)
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations) (searched 5 June 2015);
PubMed (as a top up to searches in Ovid MEDLINE) (searched 5 June 2015);
Ovid EMBASE (1974 to 2015 week 22);
Ovid CAB Abstracts (1910 to 2015 week 22);
EBSCO CINAHL (1982 to 5 June 2015);
LILACS (searched 5 June 2015);
KoreaMed (searched 5 June 2015);
IndMed (searched 5 June 2015);
PakMediNet (searched 5 June 2015);
Web of Knowledge, Web of Science (1945 to 5 June 2015);
ClinicalTrials.gov, www.clinicaltrials.gov (searched via the Cochrane Register of Studies 5 June 2015);
ICTRP (searched 5 June 2015);
ISRCTN, www.isrctn.com (searched 5 June 2015);
Google Scholar (searched 5 June 2015);
Google (searched 5 June 2015).
The TSC modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by The Cochrane Collaboration for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011). Search strategies for major databases including CENTRAL are provided in Appendix 6.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, the TSC searched PubMed, TRIPdatabase, The Cochrane Library and Google to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials.
We wrote to internationally renowned authors in vertigo to ask them if they knew of any unpublished data that could be included in this review.
We also searched the references of the main textbooks on vertigo to find further trials.
Data collection and analysis
We analysed single studies (not reports), meaning that we linked together multiple reports of a given study. Likewise, if a report included several studies, we considered the studies independently (Egger 2007).
In order to detect duplicate or multiple publication, we evaluated the following:
author names (most duplicate reports have authors in common);
location and setting of the studies (institutions, such as hospitals);
specific details of the interventions, such as dose or frequency;
numbers of participants and baseline data;
date and duration of the study (which can also clarify whether different sample sizes are due to different periods of recruitment).
If doubts remained, we contacted authors to clarify whether there was multiple publication of a single trial.
We used the Review Manager 5 software to record and analyse the data (RevMan 2014). We also followed the PRISMA guidelines to strengthen the quality of the systematic review (Moher 2009).
We studied adverse effects with a narrow scope, focusing on the detection of well‐known adverse effects of the drugs studied, rather than trying to detect a wide spectrum of effects, known or unknown. The reason for this strategy is that we can focus on important side effects and may draw more solid conclusions than with a wide focus. We were aware that a wide approach would detect more effects, but this is highly resource‐consuming and retrieves little useful information in comparison with the narrow focus approach (Handbook 2011). Furthermore, unknown adverse effects are better detected by primary surveillance rather than with a systematic review.
Selection of studies
Miguel Maldonado Fernández (MMF), Louisa Murdin (LM) and Jasminder Birdi (JB) independently reviewed the studies obtained, selecting double‐blinded, randomised controlled trials. Any disagreement was settled by discussion among the team of review authors.
Data extraction and management
Miguel Maldonado Fernandez, Ilkka Kivekäs, Michael Strupp and Greg Irving extracted the data independently.
We used a data collection form for each study (Appendix 7), based on a Cochrane template, to record the criteria for the eligibility of trials, to keep track of all the decisions regarding the trial and to save the relevant data that would be used in the meta‐analysis.
We used an electronic database (Excel) to record the trials. The data collection form had a Microsoft Word format, so that open‐ended data could be recorded. This electronic format also enabled the review authors to share and compare their work over the internet. Two review authors independently performed a pilot test of the database and the data collection form, to try to detect flaws that needed correction. We highlighted and corrected any errors in data entry and kept track of them in the data extraction form.
We used the RevMan 5 software to analyse the data.
Assessment of risk of bias in included studies
MMF and JB would have undertaken the assessment of the risk of bias of the included trials independently, using the Cochrane 'Risk of bias' tool in RevMan 5 (RevMan 2014), which involves describing each of seven domains as reported in the trial and then assigning a judgement about the adequacy of each entry: 'low', 'high' or 'unclear' risk of bias. The following domains were to be taken into consideration, as guided by theCochrane Handbook for Systematic Reviews of Interventions (Handbook 2011).
Sequence generation.
Allocation concealment.
Blinding of participants and personnel (double‐blinding).
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting. There are no well‐developed statistical methods to detect within‐study reporting biases, therefore we planned to use the following methods. If there was access to the protocol for the trial, we would compare the objectives in the protocol with the actual results reported in the trial. If the protocol was not available, we would compare the objectives mentioned in the methods section with the actual data reported in the results section. If there were discrepancies, we would report these and contact the authors to clarify them. They would be asked to provide the protocol and the full report of the results. We would measure the possible impact of selective outcome reporting using sensitivity analysis.
Other sources of bias.
Measures of treatment effect
MMF, JB and GI were to enter, analyse and interpret the data.
In measuring the treatment effect compared to the control (placebo or other interventions) we aimed to answer the following questions:
What is the direction of effect?
What is the size of effect?
Is the effect consistent across studies?
What is the strength of evidence for the effect?
In our review we originally expected to find several treatments for migraine‐related vestibular symptoms. We planned to analyse the different treatments independently in order to achieve meaningful comparisons (that is, we would avoid merging studies that analysed different active drugs). We intended to group studies according to the active treatment studied.
We considered that studies would be totally comparable when they used the same drug, dose and the same route of administration, and when the control group received the same alternative treatment (no treatment/placebo). For studies that used different routes, or different doses, we would include the trials in the group but carry out subgroup analysis to investigate whether these factors affect the effects observed.
We planned to perform a meta‐analysis within each homogeneous group that we encountered (defined by the treatment studied).
We foresaw several types of data to be obtained:
dichotomous data (some studies might classify participants according to having suffered vertigo symptoms or not, although a time‐to‐event design would be more appropriate; existence of secondary effects or not);
continuous data (number of crises, duration of symptoms);
ordinal scales to classify severity of symptoms;
counts and rates (number of events that each individual experiences); and
censored time‐to‐event data (i.e. time to a vertigo crisis).
For binary (dichotomous) data we expected to use the OR (odds ratio), RR (relative risk or risk ratio), RD (risk difference, also called absolute risk reduction) and NNT (number of participants needed to treat to avoid a case of the disease).
For the effect measures for continuous data we anticipated the use of the difference in means (MD) between the groups, if we found that the different studies used the same measuring scale, and SMD (standardised mean difference or, properly, the difference in standardised means) if they used different scales to measure the variable. The SMD assumes that all variability among studies comes from differences in the scale of measurement, which may not be the case, for example if pragmatic trials are included in the comparison. If the SMD was measured, we planned to take care to ensure that the direction of all scales was the same (e.g. that all scales increase with disease severity). If not, we would have multiplied the group of mean values from scales that decrease with disease severity by ‐1 and record this step in the data extraction form.
For ordinal data studies, we would have checked the reference to the ordinal scale used, first to see if the scale had been validated (and therefore measures what it claims to measure) and, secondly, to be sure that the authors of the study have not used a version of the scale adapted by themselves. Although special methods for proportional odds ratios exist for analysing ordinal outcome data, they are not available in RevMan 5. As suggested in the Cochrane Handbook for Systematic Reviews of Interventions, we would analyse small ordinal scales as dichotomous and large ordinal scales as continuous (Handbook 2011).
We had planned to analyse counts and rates (number of events, such as crisis of vertigo, which each individual experiences) with rate ratios (RR), in the case of rare events (with a Poisson distribution). If they were common events, we would treat them like continuous outcome data.
We would have analysed time‐to‐event data using survival analysis and expressed intervention effects as hazard ratios, defined as how many times more (or less) likely a participant is to suffer the event at a particular point in time if they receive the experimental rather than the control intervention. We would make the proportional hazard assumption (the hazard ratio is considered constant across the follow‐up period, even though hazards themselves may vary continuously).
Unit of analysis issues
Cluster‐randomised trials
Cluster‐randomised trials allocate groups instead of individuals. The participants in each group may be related in some way, therefore this needs to be taken into account in the analysis, otherwise we would incur a unit of analysis error (the allocation unit being different from the analysis unit), which would produce an artificially small P value and a risk of false positive results.
If cluster‐randomised trials were found, we would have sought statistical advice to determine whether the study had used an appropriate statistical method. If an appropriate method was used, we would have entered the reported effect estimates and standard errors into RevMan 5 using the generic inverse variance method.
If studies had not taken into account the clustering effect, we would have used the effective sample size (as detailed in Chapter 16.3.4 of the Cochrane Handbook for Systematic Reviews of Interventions) as an estimate for the clustering effects.
Cross‐over trials
Cross‐over trials of pharmacological treatments for vestibular migraine are not expected to have a strong carry‐over effect. In addition, outcomes are not irreversible and the nature of the disease does not change significantly over time, as in the case of a patient with a degenerative condition like Alzheimer's disease.
Multi‐arm studies
If we had found studies with more than two groups (several active treatments being tested, or several placebos being used), we would have established which of the comparisons are relevant to the systematic review, and relevant to each of the meta‐analyses that we might have implemented. We would have had to combine groups and create a single pair‐wise comparison. We would have tried to avoid selecting one pair of comparisons and discarding the rest, because this would mean losing information.
Repeated observations on participants
In long studies, we expected that results may have been harvested from several periods (e.g. three‐month, six‐month, one‐year follow‐up). In order to avoid unit of analysis error when combining these results in a single meta‐analysis (and therefore double‐counting participants), we would either have used data from time‐to‐event analysis if reported, or only extracted and analysed data for the most relevant time point(s) and ensured that data from participants were only included in an analysis once.
Dealing with missing data
In the case of missing data from trials, we would have contacted the authors for clarification. If no useful information could be obtained, we would have included all available data reported by the studies in the meta‐analysis (the available case analysis approach) and we would not have undertaken any imputations, except for calculations to obtain standard deviations using the methods suggested by the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011).
We would have performed sensitivity analysis in these cases, to assess the impact of missing data on the overall result. In any case, we would have addressed the fact that missing data may affect the results in the Discussion section of the review.
Assessment of heterogeneity
We expected that the trials included in the systematic review would have been performed according to different protocols, therefore a certain degree of heterogeneity was anticipated, due to differences in the participants, clinical settings or ways used to deliver the treatment. The presence of considerable heterogeneity would have not excluded the studies from subsequent meta‐analysis.
A rule of thumb for checking if the results in the trials are homogeneous is to compare the mean outcomes in the trials and see if there is consistency in the results. Another way is to see if there is overlap in the confidence intervals of the results in the trials.
A statistical way to look for heterogeneity is to use the Chi2 test. There are two main problems with this method. One is that the power of the test is low when the number of trials is small. For that reason, a non‐significant result cannot be taken as proof of homogeneity. A low number of trials was the expected situation in this systematic review and therefore we agreed to measure heterogeneity using the I2 statistic (Higgins 2003). I2 ranges from 0% to 100%, with 0% meaning complete lack of heterogeneity and bigger values meaning increasing heterogeneity.
We planned to interpret heterogeneity according to the I2 statistic results as follows (Handbook 2011):
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
We would have used funnel plots (scatter plot of the treatment effect estimates from individual studies against the standard error of the effect in each study) to detect reporting biases. We would have tried to spot void areas in the scatter plot that might correspond to studies which, for some reason, may have not been published. We would have used RevMan 5 for this purpose. However, we are aware that funnel plot asymmetry detects small sample effects, which may be due to publication bias but also to other reasons, such as poor methodological quality due to a small sample. Heterogeneity is another possible source of funnel plot asymmetry (severe patients, who may respond more significantly to the treatment, are prone to be included in the early smaller studies). Sampling variation and chance may be other explanations for plot asymmetry.
We would have plotted ratio measures of intervention effect (such as odds ratios and risk ratios) on a logarithmic scale so that effects of the same size but opposite directions (i.e. OR of 0.5 and 2) are equidistant to 1.
We would have used tests for funnel plot asymmetry only if at least 10 studies were included in the meta‐analysis (for fewer studies the test would not distinguish between chance and real asymmetry). We would have interpreted the results of the test according to the visual information in the funnel plot.
Data synthesis
Choosing between fixed‐effect or random‐effects models
If there had been substantial clinical or methodological heterogeneity in the methodology in the different studies within a comparison, or the statistical heterogeneity was substantial, we would have chosen a random‐effects model (Handbook 2011).
Meta‐analysis
We would have carried out meta‐analysis with a double‐step procedure:
We would have calculated a summary statistic describing the observed intervention effect for each study.
We would have calculated a pooled intervention effect as a weighted mean of the summaries of each study.
We would have performed meta‐analysis of continuous data with the inverse‐variance method. For meta‐analysis of dichotomous data, we would have used the Mantel‐Haenszel method for the fixed‐effect model and the DerSimonian and Laird method for the random‐effects model. In each case we would have used RevMan 5.
Subgroup analysis and investigation of heterogeneity
In order to detect variations of effect related to characteristics of the population or the intervention, including the dose of the drugs or route of administration (i.e. intravenous, intramuscular, subcutaneous, oral, intranasal), we might have performed subgroup analysis. However, we would have regarded subgroup analysis with extreme caution due to the risk of finding spurious associations because repeated comparisons were made. We would have used the RevMan 5 tool if subgroup analyses were carried out.
Sensitivity analysis
To measure the robustness of the results, we would have performed sensitivity analysis to see if the conclusion obtained by the review was affected by the estimation of uncertain data. If a problem with the data was detected using sensitivity analysis, we would have presented the final results in the form of a summary table, instead of individual forest plots.
Results
Description of studies
Results of the search
A flowchart with the search and selection results can be seen in Figure 1. We identified a total of 898 references, which reduced to 558 after duplicates were removed. In addition, we wrote to internationally renowned authors on vertigo to ask them if they knew of any unpublished data that could be included in this review. One did not answer; the rest replied that they did not know of any unpublished information. We searched the references of the main textbooks on vertigo and the reference lists of identified study reports to find other trials, but we identified no further studies.
1.
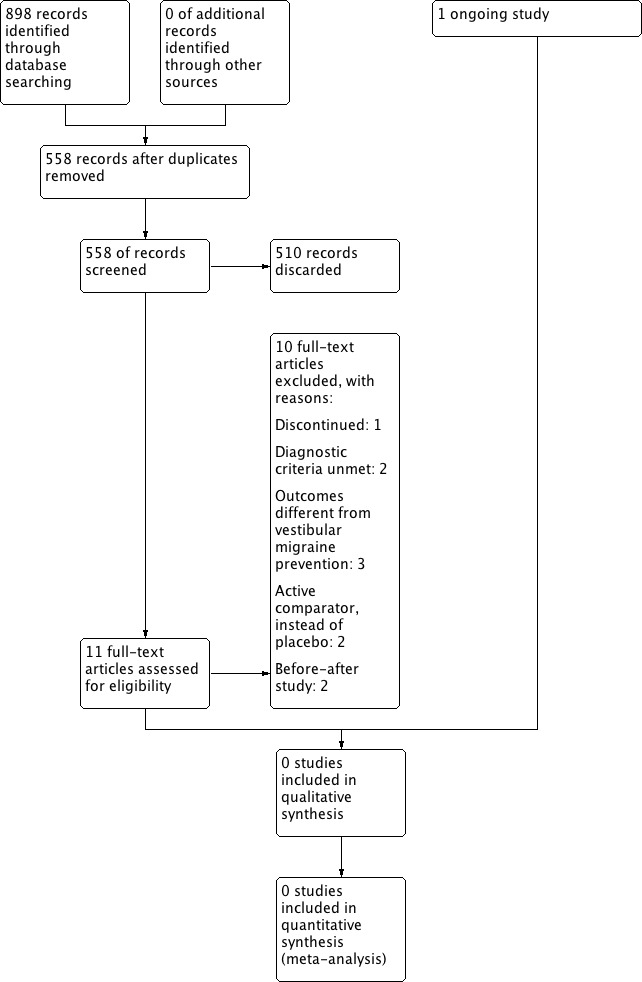
Process for sifting search results and selecting studies for inclusion
We compared the 558 articles retrieved by the search against the inclusion criteria. We initially selected 11 of these articles for further review. After further assessment we excluded 10 studies (Baloh 2014; Gode 2010; Lepcha 2013; Liu 2013; Lustig 2008; Mikulec 2014; Neuhauser 2003; Salviz 2014; Staab 2012; Wu 2007). One study is ongoing (Strupp 2008).
Included studies
None of the 11 articles initially selected were finally included in the review.
Excluded studies
See Characteristics of excluded studies.
Of the 11 selected articles, six were clinical trial registration documents (Strupp 2008, investigating metoprolol; Staab 2012, investigating verapamil; Lustig 2008, investigating topiramate; Mikulec 2014, investigating topiramate; Baloh 2014, investigating rizatriptan; Salviz 2014, investigating venlafaxine).
We excluded two trials because they did not use the IHS diagnostic criteria for vestibular migraine (Lepcha 2013; Mikulec 2014).
Baloh 2014 is a registered protocol for an ongoing double‐blind, placebo‐controlled trial comparing rizatriptan against placebo. We excluded this trial because it is investigating treatment of vestibular migraine instead of prevention and therefore falls outside the scope of this review.
Salviz 2014 is an open‐label, randomised, parallel‐group trial that is investigating venlafaxine compared to propanolol. We excluded this ongoing trial because the comparator is an active intervention, not placebo.
Staab 2012 was a trial registration document registered in ClinicalTrials.gov in August 2012. Although at first this seems to be a parallel clinical trial to investigate the efficacy of verapamil for vestibular migraine and sertraline for another condition called 'chronic subjective dizziness' (CSD), a thorough analysis revealed that the aim of this trial was to distinguish one disease from the other on the grounds of their response to verapamil (which, the authors of the study hypothesise, preferentially improves the symptoms of vestibular migraine) and the response to sertraline (which is hypothesised by the authors to improve CSD more than vestibular migraine). Accordingly, the design of the trial is not appropriate to measure the efficacy of verapamil in vestibular migraine because of circular reasoning (they define vestibular migraine symptoms if they respond to verapamil instead of sertraline).
Mikulec 2014 was a trial protocol registered in June 2014. The inclusion criteria for this trial are patients of both sexes, aged 18 to 70 years, who fall into three groups:
patients with Neuhauser definitive vestibular migraine;
patients with probable vestibular migraine; and
patients with dizziness that falls outside the Neuhauser criteria (non‐Neuhauser vestibular migraine). This would include those patients who did not fit the criteria for definitive vestibular migraine and probable vestibular migraine but were felt by the investigator to have underlying migraine as a possible cause of their dizziness, i.e. patients with a remote history of migraines, those with visual auras without headache, those with recurring self described "sinus pain" and those with significant motion intolerance, either to their own head motion or motion in their surroundings.
We excluded this study because it did not fit the inclusion criteria established in our protocol (Bárány Society/International Headache Society diagnostic criteria for vestibular migraine, which are different from the original Neuhauser criteria).
Lustig 2008 intended to study topiramate but it was withdrawn prior to enrolment (https://clinicaltrials.gov/ct2/show/results/NCT00732108).
We excluded Gode 2010 because, even though it seemed at first to be a true controlled trial on the prevention of vestibular migraine with topiramate, its main outcome was the comparison of the frequency and severity of vertigo and headache before and after topiramate treatment.
Neuhauser 2003 was a pilot study for a placebo‐controlled trial on the efficacy of vestibular migraine with zolmitriptan. We excluded it because it focused on acute relief (treatment) rather than prevention.
Lepcha 2013 was a randomised controlled trial (allocating clusters of four) on the prevention of vestibular migraine, which compared two arms of treatment, one with 10 mg flunarizine once daily at bedtime, and the other without it (and without placebo for the flunarizine). Both arms received treatment with betahistine 16 mg three times a day for 48 hours, paracetamol 1 g for acute attacks and vestibular exercises. The reason for excluding this trial was because, according to our protocol, we would only include trials that used the 2012 Bárány Society/IHS criteria for vestibular migraine and probable vestibular migraine. Here the Neuhauser diagnostic criteria were used instead.
Liu 2013 was written in Chinese and only the abstract was available in English. It was not possible to contact the main author to request information from the study. Two Chinese speaking scholars (Aaron Lai and Thomas Ming) kindly clarified the details from the main text. The author claims that this is a randomised trial, although the method of randomisation is not stated. No information on the method of allocation concealment is available. A total of 176 patients were studied. It is not mentioned how many patients were allocated to each group. The control group received (quote) "conventional western medicine care treatment". It was explained in the main text that the treatment was cinnarizine 5 mg twice daily. The active intervention group received (quote) "Chinese dialectical therapy on the basis of conventional western medicine care treatment", which seems to mean adding Chinese dialectical therapy to conventional western medical care. If this is the case, it is not mentioned whether a placebo intervention was used as a substitute for the Chinese dialectical therapy. We excluded this study because the IHS/Barany diagnostic criteria were not used.
Wu 2007 was written in Chinese and only the abstract was available in English. The authors stated that the objective of the trial was to "observe the effects of betahistine mesylate as a treatment to benign positional paroxysmal vertigo (BPPV), posterior circulation ischemia (PCI), migrainous vertigo (MV) and teenager benign paroxysmal vertigo and to study the causal relationships of dosages". The authors formed three groups (BPPV, posterior circulation ischaemia and migrainous vertigo) and each group (n = 30) was split into two subgroups, one receiving betahistine mesylate 6 mg three times a day and the other receiving betahistine mesylate 12 mg three times a day. They studied a group of 25 patients with teenager benign paroxysmal vertigo, which received 6 mg three times a day for a duration of one month, with no control group. It is not mentioned in the abstract if the study was randomised. In addition, no information is given about allocation concealment. The reason for excluding this trial is that outcome variables (high stimulating rate of auditory brainstem response and Dizziness Handicap Inventory) were (quote) "compared before and after administration of betahistine mesylate", and therefore this is not a true clinical trial comparing an active intervention with a control intervention, but a but a trial comparing a group before and after an intervention.
Ongoing studies
One ongoing trial exists, investigating metoprolol (Strupp 2008). No results are yet available. We contacted the author to ask about the status of the trial. Recruitment of the last patient is expected by the end of 2016.
Risk of bias in included studies
We identified no studies that met the review inclusion criteria.
Effects of interventions
We identified no studies that met the review inclusion criteria.
Discussion
Summary of main results
Vestibular migraine is the most common central cause of episodic vertigo and the second most frequent overall cause. Finding out which is the most effective treatment for vestibular migraine is one of the top 10 research priorities defined by a Priority Setting Partnership organised by the James Lind Alliance: Ear, Nose and Throat ‐ Aspects of Balance. However, this systematic review did not identify any completed clinical trials on pharmacological treatment for the prevention of vestibular migraine. We identified one ongoing trial in a preliminary phase, from which no results are yet available (Strupp 2008, investigating metoprolol). Recruitment of the last patient is expected by the end of 2016.
Overall completeness and applicability of evidence
Although vestibular migraine is the most common cause of central vertigo, we unfortunately did not find any completed trials from which to draw evidence. We expect to have relevant information when the results of the one ongoing trial are finally published (Strupp 2008, investigating metoprolol). December 2016 is the anticipated date of recruitment of the last patient.
According to Yaffe 2012, reviews may be empty because:
the area of review is new and no trials are available;
the question they seek to answer is very restricted and specific;
the inclusion criteria for the studies to be included are very strict and difficult to meet. This problem is analogous to the question of explanatory and pragmatic clinical trials (Treweek 2009), where explanatory trials have very restrictive inclusion criteria, making them methodologically more robust, but making extrapolation to the average patient more difficult. On the other hand, pragmatic trials have more flexible inclusion criteria to make the results more easily extrapolated.
Empty systematic reviews may lead us to conclude that there is a need for a new clinical trial to answer the research question. Although this is totally advisable in many instances, it may not be the case if there is clear indirect evidence of severe adverse effects of an experimental drug (thus making new trials both unadvisable and unethical) (Hammerstrøm 2011).
Based on this, empty systematic reviews are, when appraised correctly, a powerful tool to detect unexploited areas of research.
Quality of the evidence
After a thorough search we did not find any studies to include in this review.
Potential biases in the review process
This systematic review aimed to include all existing information on pharmacological treatment for the prevention of vestibular migraine. We tried to gather published and grey literature using a thorough search strategy. We also reviewed cross‐references from published books and contacted internationally renowned authors to ask for any unpublished data of which they might be aware. Therefore, we hope that we have limited the risk of introducing bias during the selection of the studies.
One of the authors of this review (Michael Strupp) is the main investigator of the ongoing trial (Strupp 2008). This trial has no available data as yet, so currently is not included in the review. This study was evaluated by other review authors (Miguel Maldonado, Jasminder Birdi) to avoid bias.
We excluded two trials, Lepcha 2013 and Mikulec 2014, because they did not use the International Headache Society (IHS) diagnostic criteria for vestibular migraine, which we had agreed to use for the sake of consistency and clarity. In the near future these criteria will probably be more widely used by researchers.
Agreements and disagreements with other studies or reviews
We ran a search in June 2015 to identify any new reviews on this subject. This is, to our knowledge, the only systematic review on pharmacological treatment for the prevention of vestibular migraine.
Authors' conclusions
Implications for practice.
This systematic review did not find any trials on which to base practical recommendations for the pharmacological prevention of vestibular migraine. This is, to our knowledge, the only systematic review on this subject. One ongoing trials exists, investigating metoprolol, but no results are yet available. Several drugs are widely used for the treatment of this condition and all of them have well‐documented side effects. As there is still no evidence to support these drugs, the balance between harm and benefit should be carefully evaluated in each case.
Implications for research.
Vestibular migraine is a frequent and disabling cause of episodic vertigo, but there are as yet no trials on which to base recommendations for its pharmacological prevention. Future randomised controlled trials are required. Participants should be adults over 18 years of age with a diagnosis of vestibular migraine according to international diagnostic criteria (Bárány Society and the International Headache Society) (Lempert 2012). When selecting the intervention drug, one strategy would be to seek the best evidence for migraine treatment and choose the drug that has been proven to have the highest efficacy. Another option would be to try emerging drugs for migraine treatment, although their efficacy for vestibular migraine itself might not yet have been tested in some cases. The comparator should be placebo or no treatment. Primary outcomes should be the number, duration and severity of vertigo spells. Secondary outcomes should be quality of life scores and adverse effects.
What's new
| Date | Event | Description |
|---|---|---|
| 10 April 2018 | Amended | Contact author Declarations of interest statement updated with dates. |
Acknowledgements
The authors would like to thank:
Samantha Faulkner, Trial Search Co‐ordinator and Assistant Managing Editor of the Cochrane Ear, Nose & Throat Disorders Group, for her invaluable work with the search strategy for trials.
Jenny Bellorini, Managing Editor of the Cochrane Ear, Nose & Throat Disorders Group, for her constant support.
Kamal Mahtani and David Nunan for their kind suggestions.
Aaron Lai and Thomas Ming for their kind help in the translation from Chinese.
Ángel Mones for his library resources.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to the Cochrane ENT Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Migraine diagnostic criteria (The International Classification of Headache Disorders, 3rd edition beta version)
A. At least five attacks1 fulfilling criteria B–D.
B. Headache attacks lasting 4 to 72 hours (untreated or unsuccessfully treated).2,3
C. Headache has at least two of the following four characteristics: 1. unilateral location; 2. pulsating quality; 3. moderate or severe pain intensity; 4. aggravation by or causing avoidance of routine physical activity (e.g. walking or climbing stairs).
D. During headache at least one of the following: 1. nausea and/or vomiting; 2. photophobia and phonophobia.
E. Not better accounted for by another ICHD‐3 diagnosis.
Notes: 1. One or a few migraine attacks may be difficult to distinguish from symptomatic migraine‐like attacks. Furthermore, the nature of a single or a few attacks may be difficult to understand. Therefore, at least five attacks are required. Individuals who otherwise meet the criteria for 1.1 'Migraine without aura' but have had fewer than five attacks, should be coded 1.5.1 'Probable migraine without aura'. 2. When the patient falls asleep during a migraine attack and wakes up without it, duration of the attack is reckoned until the time of awakening. 3. In children and adolescents (aged under 18 years), attacks may last 2 to 72 hours (the evidence for untreated durations of less than two hours in children has not been substantiated).
Appendix 2. International Headache Society diagnostic criteria for aura symptoms
At least two attacks fulfilling criteria 2 to 4.
-
Aura consisting of at least one of the following, but no motor weakness:
fully reversible visual symptoms including positive features (e.g. flickering lights, spots or lines) and/or negative features (i.e. loss of vision);
fully reversible sensory symptoms including positive features (i.e. pins and needles) and/or negative features (i.e. numbness);
fully reversible dysphasic speech disturbance.
-
At least two of the following:
homonymous visual symptoms and/or unilateral sensory symptoms;
at least one aura symptom develops gradually over ≥ 5 minutes and/or different aura symptoms occur in succession over ≥ 5 minutes;
each symptom lasts ≥ 5 and ≤ 60 minutes.
Headache fulfilling criteria 2 to 4 in Appendix 1 (migraine diagnostic criteria) begins during the aura or follows aura within 60 minutes.
Not attributed to another disorder.
Appendix 3. IHS diagnostic criteria for basilar migraine
At least two attacks fulfilling criteria 2 to 4.
-
Aura consisting of at least two of the following fully reversible symptoms, but no motor weakness:
dysarthria;
vertigo;
tinnitus;
hypacusia;
diplopia;
visual symptoms simultaneously in both temporal and nasal fields of both eyes;
ataxia;
decreased level of consciousness;
simultaneously bilateral paraesthesias.
-
At least one of the following:
at least one aura symptom develops gradually over ≥ 5 minutes and/or different aura symptoms occur in succession over ≥ 5 minutes;
each aura symptom lasts ≥ 5 and ≤ 60 minutes.
Headache fulfilling criteria 2 to 4 in Appendix 1 (migraine diagnostic criteria) begins during the aura or follows aura within 60 minutes.
Not attributed to another disorder.
Appendix 4. IHS criteria for benign paroxysmal vertigo of childhood
At least five attacks fulfilling criterion 2.
Multiple episodes of severe vertigo1, occurring without warning and resolving spontaneously after minutes to hours.
Normal neurological examination; audiometric and vestibular functions between attacks.
Normal electroencephalogram.
1Often associated with nystagmus or vomiting; unilateral throbbing headache may occur in some attacks.
Appendix 5. Diagnostic criteria for vestibular migraine and probable vestibular migraine according to the joint paper by the Bárány Society and the International Headache Society (2012)
1. Vestibular migraine
A. At least five episodes with vestibular symptoms of moderate or severe intensity, lasting five minutes to 72 hours.
B. Current or previous history of migraine with or without aura according to the International Classification of Headache Disorders (ICHD).
C. One or more migraine features with at least 50% of the vestibular episodes:
headache with at least two of the following characteristics: one‐sided location, pulsating quality, moderate or severe pain intensity, aggravation by routine physical activity;
photophobia and phonophobia;
visual aura.
D. Not better accounted for by another vestibular or ICHD diagnosis
2. Probable vestibular migraine
A. At least five episodes with vestibular symptoms of moderate or severe intensity, lasting five minutes to 72 hours.
B. Only one of the criteria B and C for vestibular migraine is fulfilled (migraine history or migraine features during the episode).
C. Not better accounted for by another vestibular or ICHD diagnosis.
Appendix 6. Search strategies
| CENTRAL and Cochrane Ear, Nose and Throat Disorders Group Trials Register | Ovid MEDLINE | EMBASE and CAB Abstracts (Ovid) |
| #1 MeSH descriptor: [Migraine Disorders] explode all trees #2 MeSH descriptor: [Vestibular Diseases] explode all tree #3 MeSH descriptor: [Vestibule, Labyrinth] explode all trees and with qualifiers: [Physiopathology ‐ PP] #4 MeSH descriptor: [Vestibular Nerve] explode all trees and with qualifiers: [Physiopathology ‐ PP] #5 MeSH descriptor: [Dizziness] explode all trees #6 #2 or #3 or #4 or #5 #7 #1 and #6 #8 migrain* near (vertig* or dizz* or vestibul* or spinning) #9 #7 or #8 |
1 exp Migraine Disorders/ 2 exp Vestibular Diseases/ 3 exp Vestibule, Labyrinth/pp [Physiopathology] 4 exp Vestibular Nerve/pp [Physiopathology] 5 exp Dizziness/ 6 2 or 3 or 4 or 5 7 1 and 6 8 (migrain* adj6 (vertig* or dizz* or vestibul* or spinning)).tw. 9 7 or 8 |
1 (migrain* and (vertig* or dizz* or vestibul* or spinning or lightheaded*)).tw. |
| CINAHL | Web of Science | Trial Registries |
| S1 (MH "Migraine") S2 (MH "Vestibular Diseases+") S3 (MH "Vestibule, Labyrinth+/PP") S4 (MH "Vestibular Nerve/PP") S5 (MH "Dizziness") S6 S2 OR S3 OR S4 OR S5 S7 S1 AND S6 S8 TX migrain* N6 (vertig* or dizz* or vestibul* or spinning) S9 S7 OR S8 |
TS=(migrain* and (vertig* or dizz* or vestibul* or spinning or lightheaded*)) |
Clinicaltrials.gov (migraine OR migrainous) AND (vertigo OR vertiginous OR dizzy OR dizziness OR vestibular OR spinning) ICTRP migraine AND vertigo OR migraine AND vestibular OR migraine AND dizzy OR migraine AND dizziness OR migraine AND spinning |
Appendix 7. Data collection form
| Review title or ID |
| Study ID(surname of first author and year first full report of study was published, e.g. Smith 2001) |
| Report IDs of other reports of this study(e.g. duplicate publications, follow‐up studies) |
|
Notes: |
1. General information
| Date form completed(dd/mm/yyyy) | |
| Name/ID of person extracting data | |
|
Report title (title of paper/abstract/report that data are extracted from) |
|
|
Report ID (ID for this paper/abstract/report) |
|
|
Reference details |
|
| Report author contact details | |
|
Publication type (e.g. full report, abstract, letter) |
|
|
Study funding sources (including role of funders) |
|
|
Possible conflicts of interest (for study authors) |
|
|
Notes: | |
2. Study eligibility
| Study characteristics |
Eligibility criteria (Insert eligibility criteria for each characteristic as defined in the protocol) |
Yes | No | Unclear |
Location in text (page & ¶/fig/table) |
|
| Type of study | Randomised controlled trial | |||||
| Controlled clinical trial (quasi‐randomised trial) |
||||||
|
Participants |
|
|||||
| Types of intervention | |
|||||
| Types of outcome measures | |
|||||
| INCLUDE | EXCLUDE | |||||
|
Reason for exclusion |
||||||
|
Notes: | ||||||
DO NOT PROCEED IF STUDY EXCLUDED FROM REVIEW
3. Population and setting
|
Description Include comparative information for each group (i.e. intervention and controls) if available |
Location in text (page & ¶/fig/table) |
||
|
Population description (from which study participants are drawn) |
|||
|
Setting (including location and social context) |
|||
| Inclusion criteria | |||
| Exclusion criteria | |||
| Method/s of recruitment of participants | |||
|
Informed consent obtained |
Yes No Unclear |
||
|
Notes: | |||
4. Methods
|
Descriptions as stated in report/paper |
Location in text (page & ¶/fig/table) |
||
|
Aim of study |
|||
| Design(e.g. parallel, cross‐over, cluster) | |||
|
Unit of allocation (by individuals, cluster/groups or body parts) |
|||
|
Start date |
|
||
|
End date |
|
||
|
Total study duration |
|||
| Ethical approval needed/obtained for study | Yes No Unclear |
||
|
Notes: | |||
5. 'Risk of bias' assessment
See Chapter 8 of the Cochrane Handbook
| Domain |
Risk of bias |
Support for judgement |
Location in text (page & ¶/fig/table) |
||
| Low risk | High risk | Unclear | |||
|
Random sequence generation (selection bias) |
|||||
|
Allocation concealment (selection bias) |
|||||
|
Blinding of participants and personnel (performance bias) |
Outcome group: All |
||||
| (if required) |
Outcome group: |
||||
|
Blinding of outcome assessment (detection bias) |
Outcome group: All |
||||
| (if required) |
Outcome group: |
||||
|
Incomplete outcome data (attrition bias) |
|||||
|
Selective outcome reporting? (reporting bias) |
|||||
|
Other bias |
|||||
|
Notes: | |||||
6. Participants
Provide overall data and, if available, comparative data for each intervention or comparison group.
|
Description as stated in report/paper |
Location in text (page & ¶/fig/table) |
|
|
Total no. randomised (or total population at start of study for non‐RCTs) |
||
|
Clusters (if applicable, no., type, no. people per cluster) |
||
| Baseline imbalances | ||
|
Withdrawals and exclusions (if not provided below by outcome) |
||
| Age | ||
| Sex | ||
| Race/ethnicity | ||
| Severity of illness | ||
|
Co‐morbidities |
||
| Other treatment received(additional to study intervention) | ||
|
Other relevant socio‐demographics |
||
|
Subgroups measured |
||
|
Subgroups reported |
||
|
Notes: | ||
7. Intervention groups
Copy and paste table for each intervention and comparison group
Intervention group 1
|
Description as stated in report/paper |
Location in text (page & ¶/fig/table) |
|
|
Group name |
||
|
No. randomised to group (specify whether no. people or clusters) |
||
|
Theoretical basis(include key references) |
||
| Description(include sufficient detail for replication, e.g. content, dose, components) | ||
| Duration of treatment period | ||
| Timing(e.g. frequency, duration of each episode) | ||
| Delivery(e.g. mechanism, medium, intensity, fidelity) | ||
|
Providers (e.g. no., profession, training, ethnicity etc. if relevant) |
||
|
Co‐interventions |
||
| Economic variables (i.e. intervention cost, changes in other costs as result of intervention) | ||
|
Resource requirements to replicate intervention (e.g. staff numbers, cold chain, equipment) |
||
|
Notes: | ||
8. Outcomes
Copy and paste table for each outcome.
Outcome 1
|
Description as stated in report/paper |
Location in text (page & ¶/fig/table) |
||
|
Outcome name |
|||
| Time points measured | |||
| Time points reported | |||
| Outcome definition(with diagnostic criteria if relevant) | |||
| Person measuring/reporting | |||
|
Unit of measurement (if relevant) |
|||
| Scales: upper and lower limits(indicate whether high or low score is good) | |||
| Is outcome/tool validated? | Yes No Unclear |
||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | |||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
|||
| Power | |||
|
Notes: | |||
9. Results
Copy and paste the appropriate table for each outcome, including additional tables for each time point and subgroup as required.
Dichotomous outcome
|
Description as stated in report/paper |
Location in text (page & ¶/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Time point (specify whether from start or end of intervention) | ||||||
| Results | Intervention | Comparison | ||||
| No. events | No. participants | No. events | No. participants | |||
| No. missing participants and reasons | ||||||
| No. participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
|
Unit of analysis(by individuals, cluster/groups or body parts) |
||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||
| Reanalysis possible? | Yes No Unclear |
|||||
| Reanalysed results | ||||||
|
Notes: | ||||||
Continuous outcome
|
Description as stated in report/paper |
Location in text (page & ¶/fig/table) |
|||||||||
| Comparison | ||||||||||
| Outcome | ||||||||||
| Subgroup | ||||||||||
| Time point (specify whether from start or end of intervention) | ||||||||||
| Post‐intervention or change from baseline? | ||||||||||
| Results | Intervention | Comparison | ||||||||
| Mean | SD (or other variance) | No. participants | Mean | SD (or other variance) | No. participants | |||||
| No. missing participants and reasons | ||||||||||
| No. participants moved from other group and reasons | ||||||||||
|
Any other results reported |
||||||||||
|
Unit of analysis (individuals, cluster/groups or body parts) |
||||||||||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||||||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||||||
| Reanalysis possible? | Yes No Unclear |
|||||||||
| Reanalysed results | ||||||||||
|
Notes: |
||||||||||
Other outcome
|
Description as stated in report/paper |
Location in text (page & ¶/fig/table) |
|||||
| Comparison | ||||||
| Outcome | ||||||
| Subgroup | ||||||
| Time point (specify whether from start or end of intervention) | ||||||
| Results | Intervention result | SD (or other variance) | Control result | SD (or other variance) | ||
| Overall results | SE (or other variance) | |||||
| No. participants | Intervention | Control | ||||
| No. missing participants and reasons | ||||||
| No. participants moved from other group and reasons | ||||||
| Any other results reported | ||||||
| Unit of analysis(by individuals, cluster/groups or body parts) | ||||||
| Statistical methods used and appropriateness of these methods | ||||||
| Reanalysis required?(specify) | Yes No Unclear |
|||||
| Reanalysis possible? | Yes No Unclear |
|||||
| Reanalysed results | ||||||
|
Notes: | ||||||
10. Applicability
| Have important populations been excluded from the study?(consider disadvantaged populations, and possible differences in the intervention effect) | Yes No Unclear |
|
| Is the intervention likely to be aimed at disadvantaged groups?(e.g. lower socioeconomic groups) | Yes No Unclear |
|
|
Does the study directly address the review question? (any issues of partial or indirect applicability) |
Yes No Unclear |
|
|
Notes: | ||
11. Other information
|
Description as stated in report/paper |
Location in text (page & ¶/fig/table) |
|
|
Key conclusions of study authors |
||
|
References to other relevant studies |
||
| Correspondence required for further study information(from whom, what and when) | ||
|
Notes: | ||
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baloh 2014 | This ongoing clinical trial focuses on treatment, not prevention and was excluded for this reason |
| Gode 2010 | This study compared 2 doses of topiramate (50 mg/day or 100 mg/day); there was no placebo or no treatment group |
| Lepcha 2013 | This study used the Neuhauser diagnostic criteria; according to the protocol for this review, we only included studies using the Bárány Society/International Headache Society diagnostic criteria |
| Liu 2013 | This study did not use standard criteria for the diagnosis of vestibular migraine; according to the protocol for this review, we only included studies using the Bárány Society/International Headache Society diagnostic criteria |
| Lustig 2008 | This trial was excluded because it was withdrawn before the enrolment of any patients |
| Mikulec 2014 | This was a cross‐over trial with 2 active comparators (nortriptyline and topiramate) and no placebo or no treatment arm. Patients could switch to the other intervention within the first treatment period if they were intolerant. In addition, it does not meet the diagnostic inclusion criteria; according to the protocol for this review, we only included studies using the Bárány Society/International Headache Society diagnostic criteria |
| Neuhauser 2003 | This study was focused on the treatment, not the prevention, of vestibular migraine |
| Salviz 2014 | This ongoing clinical trial is comparing the efficacy of venlafaxine to propanolol. It was excluded because the comparator is an active intervention, not placebo |
| Staab 2012 | This study did not focus on the effectiveness of preventive treatment of vestibular migraine, but on clarifying the diagnostic criteria for vestibular migraine and chronic subjective dizziness |
| Wu 2007 | This study was not a true clinical trial comparing the effectiveness of an intervention with a control; it was a trial comparing a group before and after an intervention |
Characteristics of ongoing studies [ordered by study ID]
Strupp 2008.
| Trial name or title | Prophylactic treatment of vestibular migraine with metoprolol |
| Methods | Multicentre, national, randomised, double‐masked, placebo‐controlled, 2‐arm, parallel‐group efficacy of treatment study |
| Participants | Inclusion criteria: Diagnosis of definite vestibular migraine according to the criteria of Neuhauser et al 2001: 1.1. Episodic vestibular symptoms of at least moderate severity (rotational vertigo, other illusory self or object motion, positional vertigo, head motion intolerance, i.e. sensation of imbalance or illusory self or object motion that is provoked by head motion) 1.2. Migraine according to the International Headache Society (IHS) criteria 1.3. At least 1 of the following migrainous symptoms during at least 2 vertiginous attacks: migrainous headache, photophobia, phonophobia, visual or other auras 1.4. Other causes ruled out by appropriate investigations 2. At least 2 attacks per month for at least 3 subsequent months 3. Aged 18 to 80 years, either sex 4. Written informed consent, signed and dated by the patient (or patient's authorised representative) and by the person obtaining the consent, indicating agreement to comply with all protocol‐specified procedures Exclusion criteria: 1. Other vestibular disorders such as Ménière's disease, phobic postural vertigo, benign paroxysmal positioning vertigo, vestibular paroxysmia, central disorders such as paroxysmal brainstem attacks, transient ischaemic attacks (TIAs) 2. Contraindications for treatment metoprolol such as: 2.1. Known allergic reaction to one of the trial drugs 2.2. Pregnancy or breast‐feeding 2.3. Sinoatrial (SA)‐block, atrioventricular (AV)‐block, sick sinus syndrome, bradycardia less than 50 bpm at rest, systolic blood pressure less than 100 mmHg, end‐grade peripheral arterial disease and bronchial asthma 2.4. Pheochromocytoma 2.5. Poorly controlled diabetes mellitus 2.6. Porphyria 2.7. Psoriasis 2.8. Disorders of haemostasis 2.9. Concurrent medications, such as monoamine oxidase (MAO)‐inhibitor, sympathomimetic drugs 2.10. Known severe coronary heart disease or heart failure 2.11. Persistent hypertension with systolic blood pressure greater than 180 mmHg or diastolic blood pressure greater than 110 mmHg (mean of 3 consecutive arm‐cuff readings over 20 to 30 minutes), which cannot be controlled by antihypertensive therapy 2.12. Life expectancy of less than 12 months 3. Other serious illness, e.g. severe hepatic, cardiac or renal failure, acute myocardial infarction, neoplasm or a complex disease that may confound treatment assessment 4. Participation in another study with an investigational drug or device within the last 30 days, prior participation in the current study or planned participation in another trial |
| Interventions | Active intervention: metoprolol (95 mg per day) Control: placebo The total treatment time will be 6 months with a 3‐month follow‐up |
| Outcomes | Primary outcome:
The number of vertigo attacks and number of migraine attacks in the 2 treatment groups during the last 3 months of the 6‐month treatment period Secondary outcomes: 1. Number of vertigo attacks during the last 3 months of the total follow‐up period of 9 months 2. Median duration and severity of vertigo attacks during the last 3 months of the 6‐month treatment period and the last 3 months of the total follow‐up period 3. Number of headache days per month during the last 3 months of the 6‐month treatment period and the last 3 months of the total follow‐up period 4. Change of peripheral vestibular function and handicap/impairment due to vertigo between baseline, 6‐month visit and 9‐month visit |
| Starting date | 1 January 2010 |
| Contact information | Contact name Prof Michael Strupp Address Marchioninistr. 15 City/town Munich Zip/Postcode 81377 Country Germany |
| Notes | Prof. Michael Strupp is one of the authors of this systematic review. He kindly asked to enter the team when we contacted him as the main author of the trial, and as a renowned international expert on vertigo. Dr. Strupp foresees the recruitment of the last patient of the trial at the end of 2016. 105 participants had been recruited by the end of 2014. This study uses the IHS/Bárány criteria and will therefore be eligible for the review when it is finished |
Differences between protocol and review
In the Types of studies section, we stated in our protocol that (quote) "In order to diminish bias, only double‐blind trials will be included in the review". Blinding is a risk of bias issue, however, therefore non‐blinded studies would normally be included and dealt with in the 'Risk of bias' assessment. Such studies would be considered high risk, affecting data analysis/strength of evidence judgements. This was corrected in the final manuscript. However, it did not affect the trials selected because no trial was rejected from this systematic review for this reason.
Two authors (Ilkka Kivekäs, Michael Strupp) joined the review as it developed. Both asked to be co‐review authors when they were contacted for information about unpublished data.
Contributions of authors
All the members of the team were involved in writing the manuscript under the co‐ordination of Miguel Maldonado Fernandez.
Miguel Maldonado Fernandez, Louisa Murdin and Jasminder Birdi independently reviewed the studies obtained.
Miguel Maldonado Fernandez, Ilkka Kivekäs, Michael Strupp and Greg Irving extracted the data.
Miguel Maldonado Fernandez and Ilkka Kivekäs assessed the potential bias of the studies.
Sources of support
Internal sources
-
University of Oxford, UK.
Miguel Maldonado, Jasminder Birdi and Greg Irving are enrolled in the MSc in Evidence Based Healthcare at the University of Oxford and therefore have access to a bibliographic engine (SOLO System) from this institution.
University College London, UK.
University of Liverpool, UK.
-
Servicio de Salud del Principado de Asturias (SESPA), Spain.
Bibliographic support.
External sources
-
National Institute for Health Research, UK.
Infrastructure funding for the Cochrane ENT Group
Declarations of interest
Miguel Maldonado Fernandez has previously received honoraria from Menarini (2011) and Schering‐Plough‐MSD (2009).
Jasminder Birdi has no interest to declare.
Greg Irving has no interests to declare.
Louisa Murdin has no interests to declare.
Ilkka Kivekäs has no interests to declare.
Michael Strupp is Joint Chief Editor of the Journal of Neurology, Editor in Chief of Frontiers of Neuro‐otology and Section Editor of F1000. He has received speakers' honoraria from Abbott, Actelion, UCB, GSK, TEVA, Biogen Idec, Pierre‐Fabre, Eisai and Hennig Pharma. He is the main investigator of one of the ongoing trials. His study was evaluated by other review authors (Miguel Maldonado, Jasminder Birdi) to avoid bias.
Edited (no change to conclusions)
References
References to studies excluded from this review
Baloh 2014 {published data only}
- Baloh RW. A phase II/III trial on rizatriptan for vestibular migraine. https://clinicaltrials.gov/ct2/show/study/NCT02447991 (accessed 9 June 2015). [NCT02447991]
Gode 2010 {published data only}
- Gode S, Celebisoy N, Kirazli T, Akyuz A, Bilgen C, Karapolat H, et al. Clinical assessment of topiramate therapy in patients with migrainous vertigo. Headache 2010;1:77‐84. [DOI: 10.1111/j.1526-4610.2009.01496.x] [DOI] [PubMed] [Google Scholar]
Lepcha 2013 {published and unpublished data}
Liu 2013 {published data only}
- Liu Y. Observation on the comprehensive efficacy of Chinese dialectical method in the treatment of migrainous vertigo. Clinical Medicine & Engineering 2013;4:462‐3. [Google Scholar]
Lustig 2008 {published and unpublished data}
- Hwang HS, Eisele DW, Lustig LR. Efficacy of topiramate in patients with migraine‐associated dizziness. Resident research application form, University of California, San Francisco (https://accelerate.ucsf.edu/files/CTST‐Hwang‐resident‐research‐app.doc) 2008.
Mikulec 2014 {published data only}
- Mikulec A. A prospective randomized cross‐over trial of nortriptyline and topiramate in the initial treatment of vestibular migraine. http://clinicaltrials.gov/show/NCT02169830 2014.
Neuhauser 2003 {published data only}
- Neuhauser H, Radtke A, Brevern M, Lempert T. Zolmitriptan for treatment of migrainous vertigo: a pilot randomized placebo‐controlled trial. Neurology 2003;60(5):882‐3. [PUBMED: 12629256] [DOI] [PubMed] [Google Scholar]
Salviz 2014 {published data only}
- Salviz M. Effectivity of propranolol and venlafaxine in treatment of vestibular migraine: a randomized controlled clinical trial. https://clinicaltrials.gov/ct2/show/study/NCT02350985 (accessed 9 June 2015). [NCT02350985]
Staab 2012 {published and unpublished data}
- Staab JP. Verapamil vs. sertraline for vestibular migraine & chronic subjective dizziness. http://clinicaltrials.gov/show/NCT01669304.
Wu 2007 {published data only}
- Wu Z, Zhang S, Liu X, Chen A, Ji F, Yang W, et al. The effect of betahistine mesylate as a treatment to vertigo induced by inner ear ischemia. Chinese Scientific Journal of Hearing and Speech Rehabilitation 2007;5:26‐9. [Google Scholar]
References to ongoing studies
Strupp 2008 {published data only}
- Prophylactic treatment of vestibular migraine with metoprolol: a double‐blind, placebo‐controlled trial. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=2009‐013701‐34 2009.
- Strupp M. Prophylactic treatment of vestibular migraine with metoprolol: a double‐blind, placebo‐controlled trial. http://www.controlled‐trials.com/ISRCTN72824329 2009. [DOI] [PMC free article] [PubMed]
Additional references
Bajwa 2012
- Bajwa ZH, Sabahat A. Preventive treatment of migraine in adults. In: Basow DS editor(s). UpToDate. Waltham, MA: UpToDate, 2012. [Google Scholar]
Birsdorf 2011
- Birsdorf AR. Management of vestibular migraine. Therapeutic Advances in Neurological Disorders 2011;4(3):183‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brantberg 2005
- Brantberg K, Trees N, Baloh RW. Migraine‐associated vertigo. Acta Oto‐Laryngologica 2005;125:276‐9. [DOI] [PubMed] [Google Scholar]
Carod‐Artal 2014
- Carod‐Artal FJ. Tackling chronic migraine: current perspectives. Journal of Pain Research 2014;7:185‐94. [DOI: 10.2147/JPR.S61819] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cha 2007
- Cha YH, Baloh RW. Migraine associated vertigo. Journal of Clinical Neurology 2007;3(3):121‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cummings 2010
- Crane BT, Eggers SDZ, Zee DS, Baloh RW. Central vestibular disorders. Cummings Otolaryngology: Head and Neck Surgery. 5th Edition. Mosby, 2010:Chapter 166. [Google Scholar]
Dieterich 1999
- Dieterich M, Brandt T. Episodic vertigo related to migraine (90 cases): vestibular migraine?. Journal of Neurology 1999;246(10):883‐92. [DOI] [PubMed] [Google Scholar]
Egger 2007
- Egger M, Davey Smith G, Altman DG. Systematic Reviews. 1st Edition. London: BMJ Publishing Group, 2007. [Google Scholar]
Furman 2003
- Furman JM, Marcus DA, Babalan CD. Migrainous vertigo: development of a pathogenetic model and structured diagnostic interview. Current Opinion in Neurology 2003;16(1):5‐13. [DOI] [PubMed] [Google Scholar]
Goadsby 2002
- Goadsby PJ, Lipton RB, Ferrari MD. Migraine ‐ current understanding and treatment. New England Journal of Medicine 2002;346(4):257‐70. [DOI] [PubMed] [Google Scholar]
Hammerstrøm 2011
- Hammerstrøm KT, Bjørndal A. If there are no randomised controlled trials, do we always need more research?. Cochrane Database of Systematic Reviews 2011;8:ED000024. [DOI: 10.1002/14651858.ED000024] [DOI] [PMC free article] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557–60. [DOI: 10.1136/bmj.327.7414.557] [DOI] [PMC free article] [PubMed] [Google Scholar]
ICHD 2013
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33(9):629‐808. [DOI: 10.1177/0333102413485658] [DOI] [PubMed] [Google Scholar]
JLA 2011
- Burton M, Firkins L. James Lind Alliance. Ear, Nose and Throat ‐ Aspects of Balance Priority Setting Partnership. http://www.lindalliance.org/top‐tens.asp 2011 (accessed 25 March 2013).
Lempert 2012
- Lempert T, Olesenb J, Furman J, Waterston J, Seemungal B, Carey J, et al. Vestibular migraine: diagnostic criteria. Consensus document of the Bárány Society and the International Headache Society. Journal of Vestibular Research 2012;22:167‐72. [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. BMJ 2009;339:b2535. [DOI: 10.1136/bmj.b2535] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neuhauser 2001
- Neuhauser H, Leopold M, Brevern M, Arnold G, Lempert T. The interrelations of migraine, vertigo and migrainous vertigo. Neurology 2001;56:436‐41. [DOI] [PubMed] [Google Scholar]
Oas 2008
- Oas JG. Migraine‐induced vestibulopathy. In: Weber PC editor(s). Vertigo and Disequilibrium. A Practical Guide to Diagnosis and Management. New York: Thieme, 2008:125‐9. [Google Scholar]
Pazos 2008
- Pazos A. Cell mediators I. Histamine and 5 hydroxytryptamine. Pharmacology of migraine [Mediadores celulares I. Histamina y 5‐Hidroxitriptamina. Farmacología de la Migraña]. In: Florez J editor(s). Farmacología Humana. 5th Edition. Madrid: Elsevier, 2008:367‐90. [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Silberstein 2004
- Silberstein SD. Migraine. Lancet 2004;363(9406):381‐91. [DOI] [PubMed] [Google Scholar]
Treweek 2009
- Treweek S, Zwarenstein M. Making trials matter: pragmatic and explanatory trials and the problem of applicability. Trials 2009;10:37. [DOI: 10.1186/1745-6215-10-37] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yaffe 2012
- Yaffe J, Montgomery P, Hopewell S, Shepard LD. Empty reviews: a description and consideration of Cochrane systematic reviews with no included studies. PloS One 2012;7(5):e36626. [DOI] [PMC free article] [PubMed] [Google Scholar]


