Abstract
Background
Occupational irritant hand dermatitis (OIHD) causes significant functional impairment, disruption of work, and discomfort in the working population. Different preventive measures such as protective gloves, barrier creams and moisturisers can be used, but it is not clear how effective these are. This is an update of a Cochrane review which was previously published in 2010.
Objectives
To assess the effects of primary preventive interventions and strategies (physical and behavioural) for preventing OIHD in healthy people (who have no hand dermatitis) who work in occupations where the skin is at risk of damage due to contact with water, detergents, chemicals or other irritants, or from wearing gloves.
Search methods
We updated our searches of the following databases to January 2018: the Cochrane Skin Specialised Register, CENTRAL, MEDLlNE, and Embase. We also searched five trials registers and checked the bibliographies of included studies for further references to relevant trials. We handsearched two sets of conference proceedings.
Selection criteria
We included parallel and cross‐over randomised controlled trials (RCTs) which examined the effectiveness of barrier creams, moisturisers, gloves, or educational interventions compared to no intervention for the primary prevention of OIHD under field conditions.
Data collection and analysis
We used the standard methodological procedures expected by Cochrane. The primary outcomes were signs and symptoms of OIHD developed during the trials, and the frequency of treatment discontinuation due to adverse effects.
Main results
We included nine RCTs involving 2888 participants without occupational irritant hand dermatitis (OIHD) at baseline. Six studies, including 1533 participants, investigated the effects of barrier creams, moisturisers, or both. Three studies, including 1355 participants, assessed the effectiveness of skin protection education on the prevention of OIHD. No studies were eligible that investigated the effects of protective gloves. Among each type of intervention, there was heterogeneity concerning the criteria for assessing signs and symptoms of OIHD, the products, and the occupations. Selection bias, performance bias, and reporting bias were generally unclear across all studies. The risk of detection bias was low in five studies and high in one study. The risk of other biases was low in four studies and high in two studies.
The eligible trials involved a variety of participants, including: metal workers exposed to cutting fluids, dye and print factory workers, gut cleaners in swine slaughterhouses, cleaners and kitchen workers, nurse apprentices, hospital employees handling irritants, and hairdressing apprentices. All studies were undertaken at the respective work places. Study duration ranged from four weeks to three years. The participants' ages ranged from 16 to 67 years.
Meta‐analyses for barrier creams, moisturisers, a combination of both barrier creams and moisturisers, or skin protection education showed imprecise effects favouring the intervention. Twenty‐nine per cent of participants who applied barrier creams developed signs of OIHD, compared to 33% of the controls, so the risk may be slightly reduced with this measure (risk ratio (RR) 0.87, 95% confidence interval (CI) 0.72 to 1.06; 999 participants; 4 studies; low‐quality evidence). However, this risk reduction may not be clinically important. There may be a clinically important protective effect with the use of moisturisers: in the intervention groups, 13% of participants developed symptoms of OIHD compared to 19% of the controls (RR 0.71, 95% CI 0.46 to 1.09; 507 participants; 3 studies; low‐quality evidence). Likewise, there may be a clinically important protective effect from using a combination of barrier creams and moisturisers: 8% of participants in the intervention group developed signs of OIHD, compared to 13% of the controls (RR 0.68, 95% CI 0.33 to 1.42; 474 participants; 2 studies; low‐quality evidence). We are uncertain whether skin protection education reduces the risk of developing signs of OIHD (RR 0.76, 95% CI 0.54 to 1.08; 1355 participants; 3 studies; very low‐quality evidence). Twenty‐one per cent of participants who received skin protection education developed signs of OIHD, compared to 28% of the controls.
None of the studies addressed the frequency of treatment discontinuation due to adverse effects of the products directly. However, in three studies of barrier creams, the reasons for withdrawal from the studies were unrelated to adverse effects. Likewise, in one study of moisturisers plus barrier creams, and in one study of skin protection education, reasons for dropout were unrelated to adverse effects. The remaining studies (one to two in each comparison) reported dropouts without stating how many of them may have been due to adverse reactions to the interventions. We judged the quality of this evidence as moderate, due to the indirectness of the results. The investigated interventions to prevent OIHD probably cause few or no serious adverse effects.
Authors' conclusions
Moisturisers used alone or in combination with barrier creams may result in a clinically important protective effect, either in the long‐ or short‐term, for the primary prevention of OIHD. Barrier creams alone may have slight protective effect, but this does not appear to be clinically important. The results for all of these comparisons were imprecise, and the low quality of the evidence means that our confidence in the effect estimates is limited. For skin protection education, the results varied substantially across the trials, the effect was imprecise, and the pooled risk reduction was not large enough to be clinically important. The very low quality of the evidence means that we are unsure as to whether skin protection education reduces the risk of developing OIHD. The interventions probably cause few or no serious adverse effects.
We conclude that at present there is insufficient evidence to confidently assess the effectiveness of interventions used in the primary prevention of OIHD. This does not necessarily mean that current measures are ineffective. Even though the update of this review included larger studies of reasonable quality, there is still a need for trials which apply standardised measures for the detection of OIHD in order to determine the effectiveness of the different prevention strategies.
Plain language summary
Treatments to prevent hand skin irritation in the workplace
Review question
In this review, we set out to assess the available evidence on the effect of barrier creams, moisturisers, gloves, and educational programmes for employees who are at risk of developing irritation of the skin on the hands. We found nine studies. None of them investigated protective gloves. The evidence in this review is current to 17 January 2018.
Background
Occupational irritant hand dermatitis (OIHD) is a skin disease that occurs on the hands of employees in certain jobs. The first signs are red and scaly patches in the finger webs and on the knuckle area of the hands. Itchy blisters, painful cracks, and possibly infection are common, and eventually the skin becomes thickened.
Hand skin irritation can affect employees who regularly come into contact with water, detergents, chemicals, and other irritants, or who wear gloves during their working day. People at particular risk include hairdressers, nurses, cleaners, builders, and people who work in the dye, printing, metal, and food industries. The condition is relatively common and affects about 5 to 20 out of 10,000 full‐time workers per year. Preventing OIHD from developing is important because it is difficult to clear once it starts.
Study characteristics
We included nine studies in this review, involving 2888 male and female workers aged between 16 and 67. The studies included several types of workers: metal workers, dye and print factory workers, gut cleaners in swine slaughterhouses, cleaners and kitchen workers, hospital employees, and hairdressing apprentices. We were unable to find out whether or not the preventive measures were equally effective in all these professions because there were too few trials. The studies lasted from four weeks up to three years.
Key results
Some of the preventive measures may reduce the risk of hand skin irritations. However, there were too few studies to be sure of this. The studies were too different from each other to combine in a meaningful way, and the results were too imprecise. Our results are therefore still debatable.
Various barrier creams, moisturisers, and skin protection education programs were investigated. It is possible that barrier creams may slightly reduce the risk of developing OIHD. This result was based on four studies. In these studies, 29% of people who applied barrier creams developed hand skin irritations. In the control group, who did not apply barrier creams, 33% developed hand skin irritations. The results of three studies showed that moisturisers may reduce the risk of developing OIHD by a useful amount. Thirteen per cent of the people who used moisturisers developed hand skin irritations, compared to 19% of those who did not use moisturisers. Two studies showed that using a combination of barrier creams and moisturisers may reduce the risk of developing OIHD by a useful amount. Eight per cent of the people who used moisturisers and barrier creams developed hand skin irritations, compared to 13% of the control group. Based on three studies, we are uncertain whether skin protection education reduces the risk of developing OIHD. In these studies, 21% of the people who received skin protection education developed hand skin irritations, compared to 28% of the people in the control group.
The safety and tolerability of these measures were not systematically addressed in these studies. However, no serious reactions to the treatments were reported. Mild reactions like itching or reddening of the skin were reported for only few people who applied the barrier creams or moisturisers. The measures to prevent hand skin irritations probably cause only few or no serious adverse effects.
Quality of the evidence
For barrier creams, moisturisers, or a combination of both, the quality of the evidence was low concerning the prevention of OIHD. There was not enough information and hand dermatitis was assessed differently across the studies.
For educational programmes, the quality of the evidence was very low concerning the prevention of hand skin irritation. There was not enough information, hand dermatitis was assessed differently across the studies, and the studies were poorly conducted in some important respects.
For the other key outcome, safety and tolerability of the treatments, the quality of the evidence was moderate because only indirect results were available.
Summary of findings
Background
Description of the condition
Definition and epidemiology
Occupational hand dermatitis is the most frequent work‐related skin disease in many Western countries (Diepgen 2003). The two major subgroups are occupational irritant hand dermatitis (OIHD) and occupational allergic hand dermatitis (OAHD) (Johansen 2011). This review focuses on OIHD and especially on the primary prevention of OIHD in healthy individuals, because preventing the development of dermatitis may help to reduce the development of severe and chronic dermatitis, and possibly related outcomes such as loss of employment (Brans 2016; Wulfhorst 2011). Studies of interventions which treat existing OIHD with the aim of preventing worsening of symptoms or repeat episodes of OIHD or those studies which focus on improving existing symptoms do exist, but are outside the scope of this review.
Occupational irritant hand dermatitis (OIHD) is an inflammatory response of the skin on the hands after contact with various irritant factors, such as water, detergents, soaps, solvents, gloves, food, and oils which cause direct damage to the skin (Lodi 2000; Skoet 2004). Clinically, OIHD shows a wide range of symptoms from acute, to subacute and chronic. Morphologically the clinical features in acute cases range from redness, oedema, and vesiculation, to thickening of the skin, hyperkeratosis, desquamation, and fissuring in chronic cases. Itching, burning sensations, and cracks are the most common complaints, sometimes leading to pain and infection ( Johansen 2011; McFadden 2001). Mild dermatitis typically starts in the finger webs and the knuckle areas of the hands. In moderate cases the area enlarges to the back of the hands and the fingers. In severe cases the entire hands and the wrists can be affected, and there may also be pain or infection.
Occupational allergic hand dermatitis (OAHD) is caused by sensitisation to contact allergens, e.g. metals, fragrance and fragrance‐related allergens, rubber ingredients, and preservatives. Skin lesions usually appear between 24 and 48 hours after direct skin contact with the allergens, at the contact point. Signs and symptoms resemble that of OIHD. In chronic cases, this can lead to diagnostic difficulties. Diagnostic patch testing with a standard series of allergens can help rule out a contact allergy as a contributing factor (Johansen 2011).
Epidemiological data on the incidence of occupational hand dermatitis in Europe and the USA are available from occupational skin disease registers from Ministries of Labour and insurance organisations. Other sources are case series and cross‐sectional studies in occupations that are at high risk of occupational hand dermatitis. Despite differences in definitions and ways of registration, the pattern of occupational skin diseases is similar in Europe and the USA. In most Western countries occupational hand dermatitis has been the most frequent, or at least the second most frequent, occupational disease in recent years, accounting for approximately 30% of the total occupational disease burden (Burnett 1998; Cherry 2000; Diepgen 2003; DGUV 2008; Karjalainen 1998). On the basis of different data sources, Diepgen and Coenraads calculated an incidence rate of registered occupational hand dermatitis of about 5 to 20 cases per 10,000 full‐time workers per year (Diepgen 1999). In reality the figures are probably considerably higher than this due to the well known phenomenon of under‐diagnosis and under‐reporting of occupational diseases for fear of job loss (Diepgen 2002; Meding 1987; Smit 1993).
Causes
Occupational irritant hand dermatitis (OIHD) occurs mainly in employees who perform a high amount of 'wet work' in their occupational life, e.g. hairdressers, health professionals, food industry workers, metal workers, and brick layers who have to frequently expose their hands to wet working conditions as part of their job. Additionally, in outdoor occupations winter weather might negatively influence the skin condition. OIHD results from continued, unprotected, low‐grade exposure to mild irritants such as detergents, soaps, solvents, water, food ingredients, and cutting oils or fluids but also from the frequent wearing of gloves, and develops when the regenerative capacities of the skin are exhausted and contact with the irritants continues (Diepgen 1996; Johansen 2011; Malten 1981).
In addition to external factors, other endogenous factors have been identified as risk factors for the development of OIHD. Patients with a proven tendency for atopic dermatitis were shown to be at higher risk of developing OIHD of the hands when working in occupations where the skin is at risk of damage (Bauer 1997; Bauer 1998; Bauer 2001; Coenraads 1998; Dickel 2003; Smit 1994; Uter 1998a; Uter 1999). The role of other attributes, such as age, sex, genetics, and ethnic differences, in predisposing people to OIHD are still unclear (Diepgen 1999; Kezic 2009).
Impact
Occupational irritant hand dermatitis (OIHD) is not a life‐threatening disease and mild forms do not usually interfere with daily life to a large extent, but in more severe cases the impact of OIHD on all aspects of an individual's quality of life can be considerable (Jowett 1985; Boehm 2012). It may cause long‐term illness with uncertain prognosis, social isolation, and eventually unemployment or change of occupation (Cvetkovski 2005; Lerbaek 2008; Meding 2005). This can be devastating in times of high unemployment and limited government social support (Meding 1990). The costs of OIHD for the individual and social security systems are likely to be significant (Diepgen 2013; Mathias 1985; Politiek 2016; Saetterstrøm 2014).
Assessment
Signs, symptoms, and severity of OIHD can vary from redness and dryness of the skin to chronic dermatitis with thickening and fissuring. Assessments of the severity of OIHD can be reported in a number of ways using different scores, which include qualitative and quantitative measures of signs and symptoms (erythema, oedema, vesiculation, dryness, scaling, hyperkeratosis, fissuring, itching, burning) and the area of hands involved. Recently several validated scoring systems for assessing the severity of hand dermatitis have been established, such as the hand eczema severity index (HECSI) by Held 2005, and the Osnabrück Hand Eczema Severity Index (OHSI) and Manuscore by Dulon 2009. The impact of the condition on employees is also reflected in the numbers staying or leaving the occupation due to OIHD.
Bioengineering methods can measure changes in the skin's barrier function or hydration even before visible changes appear. One such bioengineering method is tewametry, a technique that measures the amount of water that is lost through the outside layer of the skin (known as TEWL, transepidermal water loss). TEWL values are reported as g/m²h (amount of water lost from skin measured in gram per square metre per hour). Very often, inflamed skin does not hold water very well, and as a result the water in the body is lost more easily through the disrupted outer layer of the skin. An increase in TEWL has been demonstrated in cases of diseased or damaged skin, reflecting the impairment of the barrier function (Pinnagoda 1989). TEWL is typically used as an objective measure in clinical evaluation (Pinnagoda 1990).
Corneometry, another bioengineering method, is a tool used to measure the levels of skin hydration in healthy and diseased skin, i.e. how much water the skin holds. It is widely used to assess the efficacy of skin care and protection ointments in hydrating the stratum corneum. The stratum corneum, which is made of dead skin cells, is the outer layer of the skin and has an important barrier function (Fischer 1998; Leveque 1983).
Description of the intervention
The principles and methods of prevention strategies in occupations at high risk of OIHD are well defined. First line prevention strategies are based on technical‐organisational hazard control, e.g. automation of processes, replacing the need of workers to expose their skin to irritants, the replacement of dangerous substances by less toxic, less irritative, or less allergenic ones. Examples of additional strategies include changing the environment by substitution of wet work and encouraging changes in worker behaviour such as frequency of hand washing. Since these first options are more fundamental than personal protection, they are usually given priority over the other measures, but if these strategies cannot be put into action, individual protective measures, e.g. protective gloves, barrier creams, and moisturisers, are recommended.
Barrier creams/skin protection creams
A barrier cream, also called skin protection cream, is a topical preparation that is applied to the skin to provide a barrier, helping to reduce the effect of skin contact with contaminants. Barrier creams are used to protect employees against work‐related skin hazards. Ideally they are specially designed for and adapted to the profile of the workplace. Barrier creams are recommended for use before work, and two to three times during work time when necessary.
Under experimental conditions there is evidence that barrier creams show protective effects against the acute irritation caused by solvents (Mahmoud 1984; Mahmoud 1985). Different skin protection products have been shown to prevent or significantly reduce detergent‐induced irritation (Frosch 1994; Patterson 1999; Schliemann 2014; Zhai 1996). The effects of barrier creams on improvement of OIHD in hairdressers has been reported (Bock 2001). However, an international survey revealed that the majority of international experts are sceptical about the specific properties of barrier creams (Hogan 1990).
Moisturisers/emollients/skin care creams
Moisturisers, also called emollients or skin care creams, are used for regenerative skin care during and after work, and should be applied regularly during work time after hand washing, and after work at home to support the regenerative capacities of the skin (Halkier‐Sørensen 1993; Mathias 1990; Wigger‐Alberti 1997).
Protective gloves
Protective gloves are meant to be used when contact with toxic or irritant substances, allergens, or infectious material should be avoided. Although it is widely accepted that gloves protect against irritants, allergens, and microbial agents, there are concerns that occlusive gloves themselves are a substantial factor in the promotion of OIHD and OAHD if not used properly (Ramsing 1996; Rose 2009; Wrangsjö 1994).
Complex interventions using barrier creams, moisturisers, and protective gloves
Barrier creams and gloves combined with adequate moisturisers are widely recommended as the most important means of personal protection in professions where the skin is at risk. Various in vivo and in vitro methods have investigated their efficacy (Boman 1989; Fluhr 2007; Frosch 1994; Gabard 1995; Henry 1994; Mellström 1994; Treffel 1994; Wahlberg 1996).
Skin protection education
Most studies reveal a considerable lack of knowledge of exposed workers regarding the essential aspects of skin protection (Wulfhorst 2011). Skin protection education may address varying aspects of preventing OIHD. This includes advice on how to apply barrier creams, moisturiser, and gloves. Also, workers can be advised to reduce the extent of hand washing and wet work or avoid wearing jewellery at work. Practical training can be included as part of educational interventions for exposed workers. Behavioural interventions supported by health psychological approaches are used to promote the dissemination of knowledge concerning skin protection. Apart from practical training, these may include role models, working groups, and reminders. The programmes will be described individually for the included studies.
How the intervention might work
Barrier creams/skin protection creams
Barrier creams are meant to provide a thin layer on the skin and thereby help to reduce contact to irritants. The layer is thought to facilitate the removal of contaminants, thereby reducing the irritations of intensive hand washing (Kütting 2008; Mathias 1990). Barrier creams can also contain active ingredients which may trap or transform irritants (Frosch 1994; Lachapelle 1996; Zhai 2006). It is however controversial if there exists an essential difference between barrier creams and moisturisers or if this is only a matter of timing (before versus after exposure).
Moisturisers/emollients/skin care creams
Moisturisers are topical preparations that use a variety of agents designed to increase the hydration of the outer layers of the skin by reducing water loss from the skin. Moisturisers have been shown under experimental and real‐life conditions to have significant preventive and therapeutic effects. They prevent irritant skin reactions induced by detergents and have been shown to accelerate the regeneration of a disrupted barrier in irritated skin (De Paepe 2000; Loden 1997; Ramsing 1997; Williams 2010; Zhai 1998).
Protective gloves
Gloves are worn in order to reduce contact to irritants.
Skin protection education
Providing knowledge about skin protection can help workers at risk of OIHD to adopt an adequate preventive behaviour. The potential benefit of skin protection education is indirect because it also depends on the effectiveness of the advised measures. As knowledge alone does not guarantee the uptake of preventive behaviour it may be reasonable to include behavioural and psychological elements which aim to overcome impediments and promote the workers' motivation to protect their skin.
Why it is important to do this review
Occupational irritant hand dermatitis (OIHD) may cause serious problems for the individuals affected and their families. Even with social security systems in place, long‐term illness, unemployment or the necessity of occupational change can affect families to a large extent, especially in times of high unemployment, and uncertain prospects for future employment, even after retraining.
There are many indications that protective measures may be effective in the prevention of OIHD. However, the actual benefit of each measure, when used singly or in combination, under real‐world conditions in the work place is still unclear. In particular, it is important to establish whether individual protection measures are really beneficial, or whether they are potentially hazardous for employees under certain circumstances (Hogan 1990; Wigger‐Alberti 1998).
This review is an update of 'Interventions for preventing occupational irritant hand dermatitis' (Bauer 2010).
Objectives
To assess the effects of primary preventive interventions and strategies (physical and behavioural) for preventing occupational irritant hand dermatitis (OIHD) in healthy people (who have no hand dermatitis) who work in occupations where the skin is at risk of damage due to contact with water, detergents, chemicals or other irritants, or from wearing gloves.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials (RCTs) for inclusion, which investigated the efficacy of interventions in the primary prevention of occupational irritant hand dermatitis (OIHD), and were conducted under normal working conditions in the work place. We included parallel, split‐body, or cross‐over trials. We did not include controlled clinical trials (CCTs) because they provide a lower level of evidence.
Types of participants
We considered any employee in 'wet work' occupations for inclusion, where there is a risk of developing OIHD (incident cases), e.g. nurses, hairdressers, employees in the food processing industry, cleaners, metal workers, printers, bricklayers, etc. We included only primary prevention studies and not studies where participants had existing hand dermatitis, unless the participant population was mixed and disaggregated data were available for those participants who were healthy with no hand dermatitis at the start of the study. Whether or not hand dermatitis was present at baseline was decided based on the baseline data reported by the study investigators. The diagnostic criteria were not evaluated in this regard.
Types of interventions
We included studies of interventions for the primary prevention of OIHD, in working populations. This did not include experimental studies.
Examples of primary prevention interventions include:
barrier creams;
moisturisers;
protective gloves;
complex interventions using combinations of interventions e.g. barrier creams, moisturisers, and protective gloves;
skin protection education.
We included studies in which interventions were compared with another intervention or compared with no intervention.
Types of outcome measures
We included studies that measured the following outcomes, at any follow‐up time.
Primary outcomes
The proportion of participants developing any signs and symptoms of OIHD (incident cases) measured by clinical scores (IGA) and/or hand dermatitis scores (e.g. HECSI, Manuscore) as rated by the investigator (physician/nurse) or the participant.
Frequency of treatment discontinuation due to adverse effects. We did not perform a separate search for adverse effects of the target intervention. However, we did examine data on adverse effects from the included studies we identified.
Secondary outcomes
Severity of clinical signs and symptoms in incident cases of OIHD (measured by clinical scores (IGA) or hand dermatitis scores, e.g. HECSI, Manuscore), or both, as rated by the investigator (physician/nurse) or the participant.
Proportion of participants with significant changes (difference in average score or difference from baseline, or both) in barrier function or hydration, measured using TEWL (skin barrier), and corneometry (skin hydration).
Change of occupation because of OIHD versus staying in the occupation.
Proportion of participants satisfied with the products given (cosmetic, preventive, therapeutic properties of the products).
Other adverse outcomes: those that are not severe enough to warrant participants to leave the study (e.g. mild irritation or other complaints about products applied in the studies).
Search methods for identification of studies
We aimed to identify all relevant RCTs regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
For this update, we revised all the search strategies in line with current Cochrane Skin practices. Details of the previous search strategies are available in Bauer 2010.
We searched the following databases up to 17 January 2018:
the Cochrane Skin Specialised Register, using the search strategy in Appendix 1;
the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 12) in the Cochrane Library, using the strategy in Appendix 2;
MEDLINE via Ovid (from 1946), using the strategy in Appendix 3; and
Embase via Ovid (from 1974), using the strategy in Appendix 4.
Trials registers
We searched the following trials databases up to 22nd January 2018:
the ISRCTN registry (www.isrctn.com), using the search terms: (Occupational OR contact OR irritant OR prevention) AND ("hand dermatitis" OR "hand eczema") OR OIHD;
ClinicalTrials.gov (www.clinicaltrials.gov), using the search terms: (Occupational OR contact OR irritant OR prevention) AND ("hand dermatitis" OR "hand eczema") OR OIHD;
the Australian New Zealand Clinical Trials Registry (www.anzctr.org.au), using the search terms: (Occupational OR contact OR irritant OR prevention) AND (hand dermatitis OR hand eczema) OR OIHD;
the World Health Organization International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/), using the search terms: occupational AND hand dermatitis OR contact AND hand dermatitis OR irritant AND hand dermatitis OR occupational AND hand eczema OR contact AND hand eczema OR irritant AND hand eczema OR OIHD OR hand eczema AND primary prevention; and
the EU Clinical Trials Register (www.clinicaltrialsregister.eu), using the search terms: hand eczema OR hand dermatitis.
Searching other resources
Searching reference lists
We checked the bibliographies of included studies for further references to relevant trials.
Unpublished literature
One review author (AB) searched the following dermatology conference proceeding abstracts from 1999 up to January 2018:
Arbeitsgemeinschaft Berufs und Umweltdermatologie; and
American Contact Dermatitis Society.
Data collection and analysis
Selection of studies
For the first published review version (Bauer 2010), one review author (AB) checked titles and abstracts identified from the searches. Two review authors, AB and Jochen Schmitt (co‐author of the first published review version), independently assessed the full‐text versions of all possibly relevant studies. Two review authors (AB, JSch) decided which studies met the inclusion criteria, and recorded their methodological quality. The review authors (AB, JSch) resolved any disagreement by discussion. One review author (AB) attempted to obtain missing information from the trial reports by contacting the study investigators.
For the update, four review authors (HR, PE, AB, HCW) checked titles and abstracts identified in the updated searches. Two review authors (HR, PE) independently assessed the full‐text versions of all possibly relevant studies and three review authors (AB, PE, HR) decided which studies met the inclusion criteria. Any disagreement was resolved in discussion. One review author (HR) attempted to obtain missing information from the trial reports by contacting the study investigators.
Data extraction and management
For the first published review version, two review authors (AB, JSch) performed the data extraction and a third (HCW) resolved discrepancies (Bauer 2010). We slightly modified and then pilot‐tested the Cochrane Skin data collection form for intervention reviews (Version 3, April 2014) for the extractions.
Two review authors (HR, JL) independently extracted data from studies which were added during the update and reviewed by three authors (DD, MLS, AB). We resolved all discrepancies through discussion (HR, AB, JL, DD, MLS) and a consensus was reached.
We entered data into Cochrane Review Manager 5.3 software for data management and analysis (RevMan 2014).
Assessment of risk of bias in included studies
In the quality assessment we evaluated the components listed below for each included study, since there is some evidence that these are associated with biased estimates of treatment effect (Juni 2001). Two authors (AB, JSchm) independently assessed the risk of bias in the four studies included in the first published version of the review, according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions version 5.0.0 (Higgins 2008). For this update, one author (HR) re‐assessed these as described in the Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (chapter 8) (Higgins 2011). Two review authors (HR, DD) independently assessed the four newly included studies. One newly included trial was independently assessed by two authors (HR, AB) (Brüning 2008). We used the criteria listed below and categorised the studies' risk of bias as 'low', 'high', or 'unclear' for each domain.
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias): lack of blinding possibly influences the proportion of OIHD or the other outcomes, but there is insufficient information to judge whether this is likely. The bias risk of studies which did not blind participants or key personnel was therefore judged as 'unclear' for all outcomes.
-
Blinding of outcome assessment (detection bias): lack of blinding is considered to introduce:
a low risk of detection bias for the objectively measured secondary outcome 2 (TEWL and/or corneometry)
a high risk of detection bias for all other outcomes, which were subjectively assessed.
-
Incomplete outcome data (attrition bias) was assessed separately for the following outcome groups. (When attrition was low or reasons for loss to follow‐up were unlikely to be related to the outcome, we judged the risk as 'low'; when attrition was considerable and reasons for loss to follow‐up were likely to be related to the outcome, we judged the risk as 'high'; when in doubt whether or not reasons for missing were likely to be related to the outcome, we judged the risk as 'unclear'.)
Outcomes related to signs and symptoms of hand eczema (primary outcome 1 and 2, secondary outcomes 1, 2, 3, and 5)
Secondary outcome 4 (proportion of participants satisfied with the products given (cosmetic, preventive, therapeutic properties of the products))
Selective reporting (reporting bias): we judged the risk as 'low' only if a protocol or other convincing text was available.
We resolved all discrepancies through discussion (HR, DD, MLS, AB; with advice from CB) and reached consensus.
Measures of treatment effect
For measuring of treatment effect we used risk ratios (RRs) and corresponding 95% confidence intervals (CIs). Where possible we calculated RR (95% CI) from the information given in the trial papers. The risk ratio can easily be interpreted as the risk of developing OIHD in the intervention group compared to the control group. For continuous outcomes, we planned to calculate mean difference (MD and 95% CI). Where we were unable to perform meta‐analyses, we reported the results from the individual trials.
Unit of analysis issues
When no correction for cluster randomisation was performed by the study investigators, we tried to retrieve the necessary data and calculated the intraclass correlation coefficients (ICCs) and corresponding design effects. We divided numbers from such trials (number of participants, number of events) by the design effect before including them in the meta‐analysis. When study investigators neither performed corrections for cluster randomisation nor provide the requested data, we searched the literature for appropriate ICC estimates and performed a Sensitivity analysis.
Studies with split‐body or cross‐over designs may only report their data as if they were derived from a parallel design (Higgins 2011 section 16.4.5 and 9.3.8). This can introduce unit‐of‐analysis issues because confidence intervals for such trials are likely to be too wide and the studies receive too little weight in meta‐analyses. It is controversial how serious these issues are (Higgins 2011 section 16.4.5). When the data required to include a paired analysis in a meta‐analysis were not given, we included them as if they were not paired and conducted a Sensitivity analysis that excluded these trials.
Dealing with missing data
We did not perform any intention‐to‐treat (ITT) calculations and missing data were dealt with descriptively (see Characteristics of included studies: attrition bias).
Concerning studies that did not report primary intervention data or other crucial information, two review authors (AB, HR) obtained missing data from the study investigators where possible.
Assessment of heterogeneity
We assessed statistical heterogeneity in the studies' results concerning OIHD using the I² statistic (Higgins 2011).
We commented on clinical and methodological diversity — including diagnostic criteria for OIHD (degree of certainty for the diagnosis of OIHD), scoring system for the severity of OIHD, and quality of bioengineering methods — in the appropriate sections (Included studies; Effects of interventions; Discussion; Characteristics of included studies) (Pinnagoda 1990).
Assessment of reporting biases
The Cochrane Handbook for Systematic Reviews of Interventions states that 'reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results' (Higgins 2011). We tried to minimise reporting bias by extensive searching of online databases, etc. (see Search methods for identification of studies).
We could not use funnel plots due to the small number of the included studies, varying interventions and varying methods to determine the main review outcome OIHD across studies (Higgins 2011 chapter 10.4.3.1). Funnel plot assessment of reporting bias will only be used in future if the number of included studies increases at subsequent updates of this review.
Data synthesis
We always used outcome data from the last follow‐up time point. When meta‐analysis was possible, we assessed risk ratio (RR) and corresponding 95% confidence interval (95% CI) applying the Mantel‐Haenszel method in a random‐effects model for dichotomous outcomes. For continuous outcomes we had planned to calculate the standardised mean difference.
Due to the small number of studies, we pooled trials without accounting for their risk of bias; the risk was addressed in the risk of bias tables (Risk of bias in included studies) and in the results section (Characteristics of included studies).
In studies with more than two arms, we used the control groups for several comparisons but never double‐counted within one comparison of interventions.
Whenever we had identified insufficient comparable trials to perform meta‐analyses, we described these outcomes by a narrative approach.
Subgroup analysis and investigation of heterogeneity
We were not able to conduct the subgroup analyses that we had planned (subgrouping according to less than, and greater than 30 years of age; sex; atopy; and occupation). See Differences between protocol and review for more details.
Sensitivity analysis
We excluded each trial with potential Unit of analysis issues (uncorrected cluster design, no paired analysis of data from cross‐over or split‐body designs) for the sensitivity analyses in order to assess whether or not the findings were robust to these issues. For cases where an uncorrected cluster‐randomised trial had an impact on whether or not significance was reached, we planned to calculate a critical design effect and corresponding ICC, above which overall significance would be reached. Correcting for cluster‐randomisation reduces a study's effective sample size in a meta‐analysis (Higgins 2011 chapter 16.3.4). The assessment of a critical ICC is based on the assumption that with rising ICC (and therefore reduced sample size), a study's impact on the meta‐analysis will drop. The relation is to a minor degree subject to rounding errors.
Another issue was the measurement of the first primary outcome (proportion of participants developing any signs and symptoms of OIHD). We excluded all trials that reported signs of hand eczema instead of manifest hand eczema in the sensitivity analyses in order to evaluate whether this was influential on the overall results.
'Summary of findings' tables
We chose two key outcomes (signs and symptoms of OIHD, treatment discontinuation due to adverse effects) as important for decision making, and presented them in our 'Summary of findings' tables. We assessed the quality of the evidence for these outcomes using GRADEproGDT software (GRADEpro GDT 2015). In the GRADE system, evidence derived from RCTs, as in this review, receives a high quality of evidence rating, but the quality can be downgraded due to weaknesses in the following domains: risk of bias, indirectness of evidence, inconsistency of evidence, imprecision of the estimated effect, or publication bias (Schünemann 2013). We described the rationale for downgrading in the footnotes of the respective tables.
Results
Description of studies
Results of the search
We combined the results of the searches for this update with those from the searches for the last published version of this review.
We identified a total of 1845 records through the database searches (after removing duplicates). We identified six additional records through other sources (including reference lists), giving a total of 1851 results. We excluded 1798 records based on titles and abstracts.
We assessed 53 records in full text. Of these, we excluded 34 (see Characteristics of excluded studies). We categorised two further studies (reported in three references) as ongoing (see Characteristics of ongoing studies) and one study is awaiting classification (reported in one reference) (see Characteristics of studies awaiting classification).
Nine studies (described in 15 records), met the review inclusion criteria and were included (see Characteristics of included studies). Five of these were new to this update. We included a total of seven studies in the meta‐analyses.
For a full description of the screening process see our study flow diagram (Figure 1).
1.

Study flow diagram including all previous searches.
Included studies
Our searches of electronic databases identified nine randomised controlled trials (RCTs), with a total of 2888 initially healthy participants, which met the inclusion criteria (Brüning 2008; Duca 1994; Flyvholm 2005; Goh 1994; Halkier‐Sørensen 1993; Kütting 2010; Löffler 2006; Meer 2015; Perrenoud 2001a). Please see Characteristics of included studies for more details of the trial conditions and 'Risk of bias' assessments for each study. Five studies were funded by official funding sources: German metal cooperative union (Vereinigung der Metall‐Berufsgenossenschaften VMBG); Danish Ministry of Health; Danish Insurance Association, Copenhagen, L. P. Hansen's fund, Odense, and Danfoss A/S, Nordborg, Denmark; German Statutory Accident Insurance (DGUV) and the Franz‐Koelsch‐Stiftung e.V.; Netherlands Organization for Health Research and Development (ZONMW). One study was funded by industry (Asche Chiesi GmbH, Hamburg, Germany) and three studies did not report any funding sources.
Design
All of the nine included studies were randomised controlled trials. With the exception of two cross‐over studies (Halkier‐Sørensen 1993; Perrenoud 2001a), the studies had a parallel design. Four parallel studies were cluster‐randomised (Flyvholm 2005; Kütting 2010; Löffler 2006; Meer 2015). All of these were analysed by the study investigators on the individual level without accounting for the clustering and without reporting intraclass correlation coefficients (ICCs). This introduces over‐precise results with standard errors and P values which are too small (Higgins 2011). The comparability of such studies to individually randomised studies is compromised (Brüning 2008; Duca 1994; Goh 1994; Halkier‐Sørensen 1993; Perrenoud 2001a). For the data from Meer 2015, we calculated an ICC of 0.005 and a corresponding design effect of 1.0989, based on data provided by the study investigators.
Additionally to the individual randomisation to two parallel study groups, one study randomised the participants' hands to the interventions in a second step, thus creating four study arms (Brüning 2008). This design introduces a minor unit of analysis issue and possibly also contamination effects.
Most of the included studies had two arms, while one was a three‐armed trial (Goh 1994), and two had four arms (Brüning 2008; Kütting 2010).
We could not include the two cross‐over studies, Perrenoud 2001a and Halkier‐Sørensen 1993, in the meta‐analyses of the first primary outcome because they did not report evaluable quantitative data. One reported only scores and no dichotomised data (Perrenoud 2001a), while the other reported the required data only for the no‐treatment period (Halkier‐Sørensen 1993).
Sample sizes
A total of 2888 participants were evaluable for this review (healthy at the beginning of the study, not lost to follow‐up). Most studies reported some attrition and some studies also recruited participants with existing occupational irritant hand dermatitis (OIHD). The numbers of participants who were excluded or lost to follow‐up are given in the Characteristics of included studies tables.The sample sizes varied from 16 to 893 participants in the individual trials.
The sample sizes reported throughout the review text do not necessarily refer to the actual number of participants that were eligible for evaluation. In order to correct for cluster design in the meta‐analyses, a reduced 'effective sample size' (Higgins 2011, sections 16.3.4 and 16.3.5) was estimated for the respective studies. The sample sizes of natural participants are reported in the Characteristics of included studies tables.
Participants and setting
Exclusively healthy participants were recruited for five studies (Brüning 2008; Goh 1994; Halkier‐Sørensen 1993; Kütting 2010; Perrenoud 2001a). Four studies (Duca 1994; Flyvholm 2005; Löffler 2006; Meer 2015) also recruited workers who were suffering from OIHD at the beginning of the study, but data for initially healthy participants was available.
Three studies (Goh 1994; Löffler 2006; Perrenoud 2001a) included only apprentices or newly employed workers. The mean age of participants in these trials ranged from 18 to 22 years. In the remaining studies (Brüning 2008; Duca 1994; Flyvholm 2005; Halkier‐Sørensen 1993; Kütting 2010; Meer 2015) the mean age ranged from 32 to 41 years.
Two trials included exclusively male workers (Brüning 2008; Kütting 2010). In three trials the majority of participants was male (65% to 92%) (Duca 1994; Flyvholm 2005; Goh 1994), and in the remaining trials the majority was female (78% to 99%) (Halkier‐Sørensen 1993; Löffler 2006; Meer 2015; Perrenoud 2001a).
All nine included studies were field studies in occupations prone to OIHD. The studies dealt with different occupations and different stages of experience:
metal workers (Brüning 2008; Goh 1994: newly employed; Kütting 2010);
dye and print industry workers (Duca 1994);
gut cleaners in swine slaughterhouses (Flyvholm 2005);
cleaners and kitchen assistants (Halkier‐Sørensen 1993);
hospital employees (Löffler 2006: 1st year nurse apprentices; Meer 2015);
apprentice hairdressers (Perrenoud 2001a).
Eight trials were performed in the following European countries: Denmark (Halkier‐Sørensen 1993; Flyvholm 2005); Germany (Brüning 2008; Kütting 2010; Löffler 2006); Italy (Duca 1994); Netherlands (Meer 2015); Switzerland (Perrenoud 2001a). One trial was performed in Singapore (Goh 1994).
Interventions
The duration of the interventions was between four weeks and three years. The participants of five studies received barrier creams, also called skin protection creams (Brüning 2008; Duca 1994; Goh 1994; Kütting 2010; Perrenoud 2001a).
In Duca 1994, the barrier creams were provided by the employer, were applied twice per day for 12 months, and fell into two main groups: silicone or hydrocarbon containing barrier creams.
In Brüning 2008, the participants received skin protection (Travabon or Stoko Protect), skin care (Estolan), both, or no product for 12 months. The products were applied to one hand while using a glove for the other hand. Further requirements for the application were not described.
In Goh 1994, 54 healthy, newly employed metal workers exposed to cutting fluids were randomised to apply a barrier cream, to apply a moisturiser, or to the control group for six months. The barrier cream (Arretil) was used on the hands before work and after each meal break. The moisturiser (Keri Lotion) was used daily as an after‐work emollient.
In Kütting 2010, the volunteers received skin protection, skin care, both, or no recommendation for 12 months. All participants used the skin care and protection products that were provided by the employer. Barrier creams were used before or during working hours. Skin care products were applied solely after work.
In Perrenoud 2001a, the participants started either with a barrier cream (Excipial protect) or with its vehicle. Excipial protect contains aluminium hydroxychloride and glycerine; the vehicle was designed specifically for skin care for occupational users. The first cream was applied five days per week for two weeks with a washout period of two days followed by another two‐week treatment period with the second cream and vice versa.
In four studies, the participants received moisturiser, also called skin care creams or emollients. Three of them are described above because they also investigated the effects of barrier creams (Brüning 2008; Goh 1994; Kütting 2010).
In Halkier‐Sørensen 1993, the participants were randomly allocated to two cross‐over groups for two lots of two weeks. One group started with a moisturiser (Locobase); the other group started with no treatment. Application requirements were not described.
Three studies implemented skin protection education programmes (Flyvholm 2005; Löffler 2006; Meer 2015), which could include providing products (Löffler 2006; Meer 2015). All three educational programmes advised the participants to substitute hand washing with alcohol‐based hand disinfection when there is no visible contamination, to wear gloves appropriately, and to apply skin care creams.
In Flyvholm 2005, a prevention programme (skin protection education) was evaluated for 12 months. The prevention strategy consisted of a two‐part concept, with an evidence‐based prevention programme giving recommendations for prevention of work‐related skin problems in wet work occupations, and a documented method for implementation. The recommendations were aimed at the management and at the employees. The local project group included two to five gut cleaners who acted as role models.
In Löffler 2006, the intervention group received skin protection education (educational lecture with practical parts), and skin care cream (Asche Basis Creme). The cream was also given to participants in of the control group. The lectures took place three times in the first year, and twice in the second and third year.
In Meer 2015, a multifaceted implementation strategy (skin protection education) was evaluated for 12 months. The intervention included participatory working groups, role modes, an educational programme including reminders, and a leaflet, while the comparison group received only the leaflet.
Comparisons
The included trials fell in four categories of interventions:
barrier creams versus no intervention (Brüning 2008; Duca 1994; Goh 1994; Kütting 2010);
moisturisers versus no intervention (Brüning 2008; Goh 1994; Kütting 2010);
combination of barrier creams and moisturisers versus no intervention (Brüning 2008; Kütting 2010);
skin protection education versus no or minimal intervention (Flyvholm 2005; Löffler 2006; Meer 2015).
We identified no trials which used the remaining predefined types of interventions (protective gloves; complex interventions using barrier creams, moisturisers, and protective gloves).
Outcomes
This section describes how the review outcomes were reported in the included studies. For results, see Effects of interventions.
Primary outcome 1: the proportion of participants developing any signs and symptoms of OIHD (incident cases) measured by clinical scores (IGA) and/or hand dermatitis scores (e.g. HECSI, Manuscore) as rated by the investigator (physician/nurse) or the participant
None of the included studies used the term OIHD. We decided that the following outcomes were eligible as primary review outcome 1:
hand eczema (Flyvholm 2005; Halkier‐Sørensen 1993; Kütting 2010; Meer 2015);
abnormal morphology (Brüning 2008: 'klinischer Hautbefund'; Duca 1994: 'esame obiettivo positivo per uno o più dei segni'; Löffler 2006: 'Morphologie auffällig');
cutting fluid dermatitis (Goh 1994).
One study applied scores for measuring skin damage and did not dichotomise their data (Perrenoud 2001a), so that no proportion could be extracted.
The outcome was assessed by the study personnel except for three studies (Flyvholm 2005; Kütting 2010; Meer 2015), in which the participants reported hand eczema in standardised interviews.
The proportion was reported as point prevalence at last follow‐up (Brüning 2008; Halkier‐Sørensen 1993), period prevalence of the last three or six months (Flyvholm 2005; Kütting 2010; Meer 2015), or as proportion of participants with signs of OIHD at either follow‐up (Duca 1994; Goh 1994; Löffler 2006).
Primary outcome 2: frequency of treatment discontinuation due to adverse effects
In one cross‐over study (Halkier‐Sørensen 1993), this outcome was addressed to some extent, while in some others it was evident from the dropout analyses that no participant was lost to follow‐up because of adverse effects (Brüning 2008; Goh 1994; Löffler 2006).
Secondary outcome 1: severity of clinical signs and symptoms in incident cases of OIHD (measured by clinical scores (IGA) or hand dermatitis scores (e.g. HECSI, Manuscore), or both, as rated by the investigator (physician/nurse) or the participant
Some studies applied scores (Halkier‐Sørensen 1993; Kütting 2010; Meer 2015; Perrenoud 2001a), but they were not reported separately for incident cases of OIHD.
Secondary outcome 2: proportion of participants with significant changes (difference in average score or difference from baseline, or both) in barrier function or hydration, measured using transepidermal water loss (TEWL), and corneometry (skin hydration)
TEWL was reported in three studies (Brüning 2008; Goh 1994; Halkier‐Sørensen 1993), but in Brüning 2008, quartiles were given instead of mean and standard deviation. Only figures were provided in Halkier‐Sørensen 1993.
Skin hydration was assessed in two studies (Brüning 2008; Halkier‐Sørensen 1993). One study assessed TEWL, corneometry, and chromometry (measurement of colour), but did not report their results (Perrenoud 2001a).
Secondary outcome 4: proportion of participants satisfied with the products given (cosmetic, preventive, therapeutic properties of the products)
Two studies addressed the participants' opinion on the products (Halkier‐Sørensen 1993; Perrenoud 2001a).
Secondary outcome 5: other adverse outcomes: those that are not severe enough to warrant participants to leave the study (e.g. mild irritation or other complaints about products applied in the studies)
Two studies described this outcome (Halkier‐Sørensen 1993; Perrenoud 2001a).
Excluded studies
We excluded 20 studies that may have been expected to be included. Of these, 11 were RCTs, eight were controlled clinical trials (CCTs), and one was a qualitative study.
Reasons for excluding the RCTs are as follows.
We excluded three studies because they violated the inclusion criteria by exclusively including workers suffering from OIHD and therefore dealing with secondary prevention not with primary prevention of OIHD (Arbogast 2004; Berndt 2000; McCormick 2000).
We excluded Held 2002, because some workers who already had hand dermatitis at baseline ('skin problems': 25% to 30%) participated. The study investigators responded that providing the required data for initially healthy participants would be too difficult because the study was conducted almost 15 years ago. The study investigators of another trial, Winker 2009, replied that they would not provide the requested data because they feared the power would be too low if participants with OIHD at baseline were removed from the sample size. We were unable to contact the study investigators of Frosch 2003, which did not provide sufficient information about hand dermatitis at baseline. Furthermore, the design of this trial showed weaknesses: only five laboratories were randomised to four products; and it used only a partial cross‐over‐design (two out of four products were tested in the same laboratory). In this update we were able to include two studies which had previously been excluded, after the study investigators provided the requested data (Flyvholm 2005; Löffler 2006).
We excluded Perrenoud 2001b, which investigated protective cream versus no intervention, because only preliminary data (no quantitative data) were available on OIHD and it was unclear if participants with existing OIHD were included. One study did not address the prevention of OIHD and did not report data on OIHD or any other review outcome (Mody 2003). We excluded another study, Winnefeld 2000, because its interventions (non‐medicated soap versus an alcohol‐based hand rinse) were not defined as interventions to prevent OIHD according to this review. Furthermore, the trial only took eight days and the incidence of OIHD was not assessed. We excluded another study, Dobson 1979, because it examined the effect of industrial hand cleansers, an intervention that was not considered in this review, and there was no data on OIHD. Their only outcome was TEWL. The only study concerning protective glove use was excluded because it was performed in an experimental setting (Davis 2005).
We excluded seven controlled clinical trials because they were not randomised (Amphoux 1975; Bauer 2002; Bolam 1971; Bregnhøj 2012; Held 2001; Schwanitz 2003; Sell 2005). We also excluded Glantz 1976, because it was probably not randomised and no relevant outcome data was assessed.
We excluded one study, Brown 2007, because for the most part it was a qualitative study (intervention implementation research) and it did not provide sufficient data on OIHD for the different interventions.
Risk of bias in included studies
A summary of the 'Risk of bias' assessments, which we carried out for each included study, can be seen in Figure 2 and Figure 3. Details can be found in the Characteristics of included studies tables.
2.
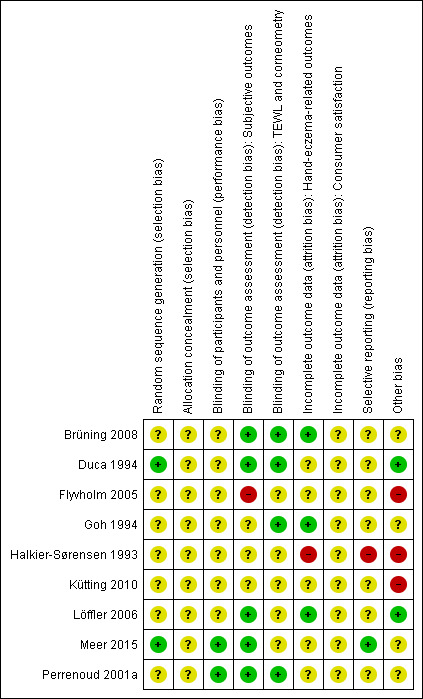
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
3.
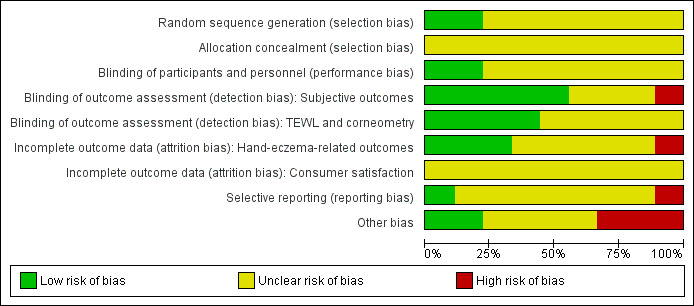
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
Allocation
Most studies did not provide any information on allocation except that it was at random, and we judged the risk of bias as unclear in these cases. Allocation concealment was not mentioned in any study; we therefore judged the risk of bias for this domain as unclear, except for in Kütting 2010. The risk of bias in this trial was high because potential confounders were significantly unbalanced at baseline.
Blinding
We judged the risk of performance bias as unclear for all four studies which did not blind the workers, and for four studies with unclear blinding. Only two studies reported blinding their participants (Meer 2015; Perrenoud 2001a).
In five studies, the outcome assessors were blinded, so we judged them as having a low risk of detection bias (Brüning 2008; Duca 1994; Löffler 2006; Meer 2015; Perrenoud 2001a). For one study, we judged the risk of bias as high because the outcome assessors were not blinded (Flyvholm 2005). Three studies probably did not blind the outcome assessors and so we judged them as having an unclear risk of bias for this domain (Goh 1994; Halkier‐Sørensen 1993; Kütting 2010).
Incomplete outcome data
Three trials had a low risk of attrition bias (Brüning 2008; Goh 1994; Löffler 2006). The risk of attrition bias was high in one study (Halkier‐Sørensen 1993). For five studies, the risk of bias was unclear because there was there was no dropout analysis or the analysis did not provide the information required for the risk of bias assessment (Duca 1994; Flyvholm 2005; Kütting 2010; Meer 2015; Perrenoud 2001a).
Selective reporting
We assessed most trials as having an unclear risk of reporting bias, because the study protocols were not available. One study, Meer 2015, reported the predefined outcomes so we assessed this study as having a low risk of reporting bias. Another study, Halkier‐Sørensen 1993, did not report their results according to the original randomisation, but according to the ability of the participants to complete the study period. Thus, the results were not reported in a way appropriate for an RCT, so we assigned a judgement of high risk of bias. They did not provide any data on OIHD that may have been used in the meta‐analysis.
Other potential sources of bias
In three of the four cluster‐randomised trials, no correction for the design effect was applied (Flyvholm 2005; Kütting 2010; Löffler 2006). They were analysed on the individual level without accounting for the clustering. This does not lead to a biased estimate of effect but it introduces over‐precise results with too small standard errors and P values (Higgins 2011). The comparability to individually randomised studies is therefore compromised. Data from one trial, Kütting 2010, was used in the meta‐analyses of the effect of barrier creams or moisturisers (or both) on OIHD (comparisons 1, 2, 3). All three studies included in the meta‐analysis of educational programmes (comparison 4) were cluster‐randomised trials.
Baseline imbalances introduced a high risk of bias to two studies (Flyvholm 2005; Halkier‐Sørensen 1993). They introduced an unclear risk of bias in three studies (Duca 1994: insufficient information; Goh 1994; Perrenoud 2001a: insufficient information). One study reported a possible, unclear bias introduced through differential diagnostic activity across study groups (Meer 2015). Apart from these exceptions, there was a low risk of bias concerning design, baseline imbalances, funding sources, and blocked randomisation. For three remaining studies, all these possible risks of bias were low (Brüning 2008; Kütting 2010; Löffler 2006).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Barrier creams compared to no treatment for preventing occupational irritant hand dermatitis.
| Barrier creams compared to no treatment for preventing occupational irritant hand dermatitis | ||||||
| Patient or population: workers at risk of occupational irritant hand dermatitis Setting: metal or dye/print factories Intervention: barrier creams Comparison: no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no treatment | Risk with barrier creams | |||||
| The proportion of participants developing any signs and symptoms of OIHD (incident cases) measured by clinical scores (IGA) or hand dermatitis scores (e.g. HECSI, Manuscore), or both, as rated by the investigator (physician/nurse) or the participant (proportion of OIHD) Follow up: range 6 months to 12 months |
Study population | RR 0.87 (0.72 to 1.06) | 999 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 | ||
| 334 per 1000 | 291 per 1000 (241 to 354) | |||||
| Frequency of treatment discontinuation due to adverse effects Follow up: range 2 weeks to 12 months |
All dropout reasons were unrelated to the treatment: the numbers of participants who dropped out of the individual trials ranged from 0% to 24%. | 111 (3 RCTs) |
⊕⊕⊕⊝ MODERATE2 | Information only available from dropout analyses, which were not designed to detect adverse effects. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The risk in the comparison group is based on mean proportion observed in the comparison groups. CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; OIHD: occupational irritant hand dermatitis | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded by two levels. Downgraded one level for imprecision because the confidence intervals were wide and included 1 as well as a clinically significant relative risk (0.75 or less). Downgraded one level for inconsistency because criteria for the diagnosis of OIHD varied across the included studies; signs and symptoms of OIHD were assessed by dermatologists, by study personnel, or by the participants.
2 Downgraded by one level due to the indirectness of the results. None of the studies reported directly on treatment discontinuation due to adverse effects. Instead, the extracted results are based on dropout analyses, which did not focus on adverse effects. For the remaining two studies in this comparison the dropout analyses were not detailed enough to extract whether or not adverse effects were among the reasons. It cannot be fully excluded that some of the participants who completed these studies may have stopped applying the products without the researchers' knowledge.
Summary of findings 2. Moisturisers compared to no treatment for preventing occupational irritant hand dermatitis.
| Moisturisers compared to no treatment for preventing occupational irritant hand dermatitis | ||||||
| Patient or population: workers at risk of occupational irritant hand dermatitis Setting: metal factories Intervention: moisturisers Comparison: no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no treatment | Risk with moisturisers | |||||
| The proportion of participants developing any signs and symptoms of OIHD (incident cases) measured by clinical scores (IGA) or hand dermatitis scores (e.g. HECSI, Manuscore), or both, as rated by the investigator (physician/nurse) or the participant (proportion of OIHD) Follow up: range 6 months to 12 months |
Study population | RR 0.71 (0.46 to 1.09) | 507 (3 RCTs) | ⊕⊕⊝⊝ LOW 1 | ||
| 187 per 1000 | 133 per 1000 (86 to 204) | |||||
| Frequency of treatment discontinuation due to adverse effects Follow up: range 2 weeks to 12 months |
All dropout reasons were unrelated to the treatment: the numbers of participants who dropped out of the individual trials ranged from 0% to 37% | 133 (2 RCTs) |
⊕⊕⊕⊝ MODERATE2 | Information only available from dropout analyses, which were not designed to detect adverse effects. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The risk in the comparison group is based on mean proportion observed in the comparison groups. CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; OIHD: occupational irritant hand dermatitis | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded by two levels. Downgraded one level for imprecision because the confidence intervals were wide and included 1 as well as a clinically significant relative risk (0.75 or less). Downgraded one level for inconsistency because criteria for the diagnosis of OIHD varied across the included studies; signs and symptoms of OIHD were assessed by dermatologists, by study personnel, or by the participants.
2 Downgraded by one level due to the indirectness of the results. None of the studies reported directly on treatment discontinuation due to adverse effects. Instead, the extracted results are based on dropout analyses, which did not focus on adverse effects. For the remaining two studies in this comparison the dropout analyses were not detailed enough to extract how often adverse effects were a reason. It cannot be fully excluded that some of the participants who completed these studies may have stopped applying the products without the researchers' knowledge.
Summary of findings 3. Barrier creams and moisturisers compared to no treatment for preventing occupational irritant hand dermatitis.
| Barrier creams and moisturisers compared to no treatment for preventing occupational irritant hand dermatitis | ||||||
| Patient or population: workers at risk of occupational irritant hand dermatitis Setting: metal factories Intervention: barrier creams and moisturisers Comparison: no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no treatment | Risk with barrier creams and moisturisers | |||||
| The proportion of participants developing any signs and symptoms of OIHD (incident cases) measured by clinical scores (IGA) or hand dermatitis scores (e.g. HECSI, Manuscore), or both, as rated by the investigator (physician/nurse) or the participant (proportion of OIHD) Follow up: median 12 months |
Study population | RR 0.68 (0.33 to 1.42) | 474 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 | ||
| 126 per 1000 | 85 per 1000 (41 to 178) | |||||
| Frequency of treatment discontinuation due to adverse effects Follow up: range 2 weeks to 12 months |
All dropout reasons were unrelated to the treatment: the numbers of participants who dropped out of the trial ranged from 8% to 24% | 100 (1 RCT) |
⊕⊕⊕⊝ MODERATE2 | Information only available from dropout analyses, which were not designed to detect adverse effects. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The risk in the comparison group is based on mean proportion observed in the comparison groups. CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; OIHD: occupational irritant hand dermatitis | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded by two levels. Downgraded one level for imprecision because the confidence intervals were wide and included 1 as well as a clinically significant relative risk (0.75 or less). Downgraded one level for inconsistency because criteria for the diagnosis of OIHD varied across the included studies; signs and symptoms of OIHD were assessed by dermatologists, or by the participants.
2 Downgraded by one level due to the indirectness of the results. None of the studies reported directly on treatment discontinuation due to adverse effects. Instead, the extracted results are based on dropout analyses, which did not focus on adverse effects. For the remaining study in this comparison no dropout analysis was performed. It cannot be fully excluded that some of the participants who completed these studies may have stopped applying the products without the researchers' knowledge.
Summary of findings 4. Skin protection education compared to no or minimal intervention for preventing occupational irritant hand dermatitis.
| Skin protection education compared to no or minimal intervention for preventing occupational irritant hand dermatitis | ||||||
| Patient or population: workers at risk of occupational irritant hand dermatitis Setting: slaughterhouses, nursing schools, and hospitals Intervention: skin protection education Comparison: no or minimal intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no or minimal intervention | Risk with skin protection education | |||||
| The proportion of participants developing any signs and symptoms of OIHD (incident cases) measured by clinical scores (IGA) or hand dermatitis scores (e.g. HECSI, Manuscore), or both, as rated by the investigator (physician/nurse) or the participant (proportion of OIHD) Follow up: range 1 years to 3 years |
Study population | RR 0.76 (0.54 to 1.08) | 13551 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 2 | ||
| 275 per 1000 | 209 per 1000 (148 to 297) | |||||
| Frequency of treatment discontinuation due to adverse effects Follow up: range 2 weeks to 12 months |
All dropout reasons were unrelated to the treatment: the numbers of participants who dropped out of the trial ranged from 35% to 38% | 250 (1 RCT) |
⊕⊕⊕⊝ MODERATE3 | Information only available from dropout analyses, which were not designed to detect adverse effects. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The risk in the comparison group is based on mean proportion observed in the comparison groups. CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; OIHD: occupational irritant hand dermatitis | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 'effective sample size' after correcting for cluster design in one study; number of natural participants: N = 1443
2 Downgraded by three levels. Downgraded one level for risk of bias as the risk ratios may have been overestimated (due to detection bias and baseline imbalance) or underestimated (due to differential diagnostic criteria). Downgraded one level for imprecision because the confidence intervals were wide and included 1 as well as a clinically significant relative risk (0.75 or less). Downgraded one level for inconsistency because criteria for the diagnosis of OIHD varied across the included studies; signs and symptoms of OIHD were assessed by a physician, or by the participants.
3 Downgraded by one level due to the indirectness of the results. None of the studies reported directly on treatment discontinuation due to adverse effects. Instead, the extracted results are based on dropout analyses, which did not focus on adverse effects. For the remaining two studies in this comparison no dropout reasons were reported. It cannot be fully excluded that some of the participants who completed these studies may have stopped applying the products without the researchers' knowledge.
We performed four meta‐analyses for the main outcome, proportion of participants developing signs or symptoms of occupational irritant hand dermatitis (OIHD), with up to four trials for each type of intervention.
For details on clinical and methodological diversity of the pooled studies, see Characteristics of included studies.
Studies relevant to this review fall into four comparisons. In total there were nine relevant randomised studies.
Comparison 1: barrier creams versus no treatment or vehicle
Four trials compared barrier creams against no treatment (Brüning 2008; Duca 1994; Goh 1994; Kütting 2010), while one study compared a protection cream against its vehicle (Perrenoud 2001a).
1.1 Primary outcome 1: the proportion of participants developing any signs and symptoms of OIHD (incident cases) measured by clinical scores (IGA) or hand dermatitis scores (e.g. HECSI, Manuscore), or both, as rated by the investigator (physician/nurse) or the participant
We identified four studies relevant to this outcome (total number of participants (N) = 999). The trials investigated metal workers exposed to cutting fluids (almost exclusively male) or dye/print factory workers. The duration was between six and 12 months. Except for Brüning 2008, all trials showed a slightly reduced risk of developing OIHD when applying barrier creams. In the intervention groups, 29% of participants developed symptoms of OIHD, compared to 33% in the control groups. However, when these data were pooled, the wide confidence intervals were consistent with either a reduced risk or no effect (risk ratio (RR) 0.87, 95% confidence interval (CI) 0.72 to 1.06; P = 0.18), and the quality of the evidence was low (Table 1). Across the individual studies, the RR ranged from 0.51 to 1.29, but the I2 measure showed no substantial heterogeneity (I2 = 9%) (Analysis 1.1).
1.1. Analysis.
Comparison 1 Barrier creams versus no treatment, Outcome 1 Proportion of OIHD.
Sensitivity analysis
The results of the sensitivity analyses are shown in Table 5. When we removed Kütting 2010, a cluster‐randomised trial, from the meta‐analysis, the confidence intervals were still wide and included no effect. We deduced that correcting for cluster randomisation in this study would still yield no significant results.
1. Sensitivity analysis for comparison 1.
| Excluded trials | Rationale for exclusion | Number of included trials |
Number of included participants |
RR (95% CI) | P | I2 |
| ‐ | ‐ | 4 | 999 | 0.87 (0.72 ‐ 1.06) | 0.18 | 9% |
| Kütting 2010 | cluster design without correction |
3 | 629 | 0.89 (0.75 ‐ 1.06) | 0.18 | 0% |
| Brüning 2008 | split‐body design without paired analysis |
3 | 907 | 0.84 (0.70 ‐ 1.00) | 0.05 | 0% |
|
Brüning 2008 Duca 1994 |
PO1 was not manifest hand dermatitis |
2 | 410 | 0.75 (0.43 ‐ 1.29) | 0.30 | 35% |
There was another unit‐of‐analysis issue since one study, Brüning 2008, had a partial split‐body design. The hands that were randomised to the barrier cream belonged to the same individuals as the hands randomised to the control arm. When we excluded this study from the analysis, the relative risk was marginally lower, and the confidence intervals only just included no effect. Apart from the unit‐of‐analysis issue, contamination between the two study arms may have obliterated the preventive effect in this study.
Including only studies with the outcome hand eczema or hand dermatitis showed a greater effect, but the confidence intervals were still wide and included no effect.
Overall the sensitivity analyses revealed that the findings were quite robust concerning unit‐of‐analysis and measures‐of‐effect issues.
Other data
One study could not be pooled with other trials (Perrenoud 2001a). It found that the barrier cream (Excipial protect) and its vehicle, used for five days a week for two weeks, were similarly protective. Most participants developed no or mild symptoms. We could not estimate RR or score values because no quantitative data were reported by the study investigators.
1.2 Primary outcome 2: frequency of treatment discontinuation due to adverse effects
In two studies, it was evident from the dropout analyses that no participant was lost to follow‐up because of adverse effects from the barrier creams (Brüning 2008; Goh 1994). In one further study, dropout reasons were unrelated to the trial (Perrenoud 2001a). However, we cannot fully exclude the possibility that some of the participants who completed these studies may have stopped applying the products without the researchers' knowledge. In two studies, dropout analyses were not performed or not detailed enough to extract whether or not adverse effects were among the reasons for dropout (Kütting 2010; Duca 1994). The numbers of participants who dropped out of the individual trials ranged from 0% to 24% and are given in the Characteristics of included studies tables.
1.3 Secondary outcome 1: severity of clinical signs and symptoms in incident cases of OIHD (measured by clinical scores (IGA) or hand dermatitis scores (e.g. HECSI, Manuscore), or both) as rated by the investigator (physician/nurse) or the participant
Two studies applied scores (Kütting 2010; Perrenoud 2001a), but they were not reported separately for incident cases of OIHD. One study reported the follow‐up proportions of OIHD in participants with OIHD at baseline, but they did not report any scores (Duca 1994). Two studies did not address this outcome (Brüning 2008; Goh 1994).
1.4 Secondary outcome 2: proportion of participants with significant changes (difference in average score or difference from baseline, or both) in barrier function or hydration, measured using trans epidermal water loss (TEWL) (skin barrier) and corneometry (skin hydration)
TEWL
Tewl, a measure of skin barrier function, was reported in three studies (Brüning 2008; Goh 1994; Perrenoud 2001a), but one study gave quartiles instead of mean and standard deviation (Brüning 2008), and another only gave figures and P values (Perrenoud 2001a). One study, Goh 1994, reported TEWL only for one time point but did not clarify for which. We were therefore unable to pool the data. The TEWL differences in the barrier cream groups compared to their respective controls were neither clinically important nor statistically significant in any of the three trials.
In Brüning 2008, the metal workers' median TEWL after one year was lower than at baseline in all study arms, including the control, but the changes were not significant (Table 6). TEWL in the barrier cream group was somewhat lower at the last follow‐up compared to control (not significant).
2. Median skin hydration and TEWL in Brüning 2008.
| Study arm | N |
Skin hydration1 baseline |
Skin hydration1 last follow‐up |
TEWL2 baseline |
TEWL2 last follow‐up |
| Barrier cream + moisturiser | 50 | 24.73 | 27.18 | 17.65 | 14.9 |
| Moisturiser | 50 | 24.42 | 26.43 | 16.93 | 14.4 |
| Barrier cream | 46 | 25.15 | 28.5 | 16 | 13.68 |
| Control3 | 46 | 25.73 | 27.35 | 16 | 14.5 |
1 Skin hydration measured by corneometry
2 Skin barrier function measured as TEWL (transepidermal water loss, g/m2/h)
3 Differences of interventions compared to the control were not significant at baseline or last follow‐up (no P values reported)
In Goh 1994, it remains unclear for which time point basal TEWL was reported. The values showed no significant differences between the groups. Results for TEWL analysis were as follows: in the control group the mean basal TEWL was 16.7 g/m²h while in the barrier cream group it was 15.5 g/m²h; the mean TEWL difference between groups was 1.2 g/m²h (not clinically important nor statistically significant). Concerning the three trial groups (barrier cream, moisturiser, control), the trial investigators found that changes of the metal workers' TEWL, 'throughout the six‐month study period, were almost identical'.
In Perrenoud 2001a, no important changes in TEWL values were observed. TEWL increased during barrier cream treatment without reaching statistical significance (Table 7).
3. Median skin hydration and TEWL in Perrenoud 2001.
| Study arm |
Skin hydration1 after 11 days control |
Skin hydration1 after 11 days barrier cream |
TEWL2 after 11 days control |
TEWL2 after 11 days barrier cream |
| Control before barrier cream |
67 | 60 | 10 | 14 |
| Barrier cream before control |
74 | 64 | 13 | 12 |
| Mean of both study arms3 | 70.5 | 62 | 11.5 | 13 |
1 Skin hydration measured by corneometry (units), figures extracted from diagram
2 Skin barrier function measured as TEWL (transepidermal water loss, g/m2/h), figures extracted from diagram
3 Differences of barrier cream compared to control group were significant for skin hydration (P < 0.01) and not significant for TEWL (no P values reported)
Skin hydration
This outcome was assessed in two studies (Brüning 2008; Perrenoud 2001a), but figures and P values were only provided in Perrenoud 2001a.
In Brüning 2008, median skin hydration was higher in the barrier cream group at last follow‐up compared to baseline (P = 0.0491, Table 6). Skin hydration at baseline and after one year in the intervention group was almost equal to the controls.
In Perrenoud 2001a, skin hydration of apprentice hairdressers measured by corneometry was significantly higher during the two weeks of vehicle use (P < 0.01, Table 7) than for the barrier cream. However, skin hydration was already visibly lower at the beginning of the verum period compared to the vehicle.
The details of these bioengineering methods are given in the Characteristics of included studies tables.
1.5 Secondary outcome 3: change of occupation because of OIHD versus staying in the occupation
None of the trials reported this outcome.
1.6 Secondary outcome 4: proportion of participants satisfied with the products given (cosmetic, preventive, therapeutic properties of the products)
Only one study addressed this outcome (Perrenoud 2001a). The satisfaction with the products was generally high. No difference was noted between the creams except with regard to texture. The vehicle was regarded as too oily by 11 out of 16 participants, while four considered the barrier cream to be too oily. Five participants did not plan to use the vehicle, two would not use the barrier cream, and one would not use either again.
1.7 Secondary outcome 5: other adverse outcomes: those that are not severe enough to warrant participants to leave the study (e.g. mild irritation or other complaints about products applied in the studies)
Only one study addressed this outcome. Mild adverse events like transient reddening and itching (no values reported) after use of Excipial protect or its vehicle were reported in Perrenoud 2001a.
Comparison 2: moisturisers versus no treatment
Four trials compared moisturisers against no treatment (Brüning 2008; Goh 1994; Halkier‐Sørensen 1993; Kütting 2010).
2.1 Primary outcome 1: the proportion of participants developing any signs and symptoms of OIHD (incident cases) measured by clinical scores (IGA) or hand dermatitis scores (e.g. HECSI, Manuscore), or both, as rated by the investigator (physician/nurse) or the participant
We identified three studies relevant to this outcome (N = 507). The trials investigated metal workers (almost exclusively male) exposed to cutting fluids. The duration was between six and 12 months. All trials showed a reduced risk of developing OIHD when applying moisturisers. In two individual trials as well as in the pooled analysis, the risk reduction was clinically important (0.75 or less). In the intervention groups, 13% of participants developed symptoms of OIHD compared to 19% in the control groups. However, when these data were pooled, the wide confidence intervals were consistent with either an important effect or no effect (RR 0.71, 95% CI 0.46 to 1.09; P = 0.11) and the quality of evidence was low (Table 2). Across the individual studies, RR ranged from 0.63 to 0.99. The heterogeneity was not substantial ( I2 = 10%) (Analysis 2.1).
2.1. Analysis.
Comparison 2 Moisturisers versus no treatment, Outcome 1 Proportion of OIHD.
Sensitivity analysis
The results of the sensitivity analysis are shown in Table 8. Despite low heterogeneity, data from the remaining two studies showed a reduced risk of developing OIHD when moisturisers were applied after removing Brüning 2008. In this comparison, the study by Brüning and colleagues was the only study which did not define hand eczema or hand dermatitis as their outcome (it used abnormal morphology instead). Another limitation of this study was the partial split‐body design, which may have introduced contamination.
4. Sensitivity analysis for comparison 2.
| Excluded trials | Rationale for exclusion |
Number of included trials |
Number of included participants |
RR (95% CI) | P | I2 |
| ‐ | ‐ | 3 | 507 | 0.71 (0.46 ‐ 1.09) | 0.11 | 10% |
| Kütting 2010 | cluster design without correction |
2 | 133 | 0.71 (0.36 ‐ 1.43) | 0.34 | 53% |
| Brüning 2008 | PO1 was not manifest hand dermatitis; split‐body design |
2 | 411 | 0.55 (0.31 ‐ 0.94) | 0.03 | 0% |
When removing Kütting 2010, the confidence intervals were still very wide and included no effect. We deduced that correcting for cluster randomisation in this study would still not yield significant results.
The sensitivity analysis revealed that the findings were robust concerning uncorrected cluster design (Kütting 2010), but the preventive effect of moisturisers appeared much clearer without data from Brüning 2008.
Other data
We could not pool one cross‐over study with other trials because no dichotomised data were available for the treatment period (Halkier‐Sørensen 1993). The study found that cleaners and kitchen workers often developed signs of OIHD during the two weeks of the control period: nineteen participants (20.4%) developed dry eczema, another 15 (16.1%) developed dryness and scaling, and 38 (40.9%) developed dryness only (N = 93 workers who completed both periods (group one), or ceased from the control period prematurely (group two)). For the two‐week treatment period with a moisturiser (Locobase), no such data were reported. However, a score which summarised severity ratings for several symptoms of OIHD was significantly reduced after the treatment period compared to the control period (Wilcoxon test, P < 0.001).
2.2 Primary outcome 2: frequency of treatment discontinuation due to adverse effects
In two studies it was evident from the dropout analyses that no participant was lost to follow‐up because of adverse effects from the moisturisers (Brüning 2008; Goh 1994). However, we cannot fully exclude the possibility that some of the participants who completed these studies may have stopped applying the products without the researchers' knowledge.
In one cross‐over study (Halkier‐Sørensen 1993), 12 participants violated the protocol or declined to continue the study because they developed or feared to develop hand dermatitis during the no‐treatment period. Six participants turned up only once, did not find Locobase acceptable, or went on vacation. It remains unclear if some of these discontinued the treatment phase due to adverse effects. Severe dryness of the skin caused 23 participants (20.7%) to drop out during the no‐treatment phase.
No dropout analyses were performed in Kütting 2010.
The numbers of participants who dropped out of the individual trials ranged from 0% to 37% and are given in the Characteristics of included studies tables.
2.3 Secondary outcome 1: severity of clinical signs and symptoms in incident cases of OIHD (measured by clinical scores (IGA) or hand dermatitis scores (e.g. HECSI, Manuscore), or both) as rated by the investigator (physician/nurse) or the participant
None of the included studies addressed this outcome. Some studies applied scores (Halkier‐Sørensen 1993; Kütting 2010), but they were not reported separately for incident cases of OIHD.
2.4 Secondary outcome 2: proportion of participants with significant changes (difference in average score or difference from baseline, or both) in barrier function or hydration, measured using TEWL (skin barrier) and corneometry (skin hydration)
TEWL
TEWL was reported in three studies (Brüning 2008; Goh 1994; Halkier‐Sørensen 1993), but one study gave quartiles instead of mean and standard deviation (Brüning 2008), and another only gave figures and P values (Halkier‐Sørensen 1993). One study reported TEWL only for one time point but did not clarify for which (Goh 1994). The TEWL differences in the barrier cream groups compared to their respective controls were neither clinically relevant nor statistically significant in any of the three trials.
In Brüning 2008, the metal workers' median TEWL after one year was lower than at baseline in all study arms, including the control, but the authors reported that the changes were not significant (Table 6). There was no important difference between moisturiser and control at both points in time. For metal workers exposed to cutting fluids, the application of a moisturiser over one year had no significant effects on TEWL.
In Goh 1994, it remains unclear for which time point basal TEWL was reported. The values showed no significant differences between the groups. Results for TEWL analysis were as follows: In the control group, the mean basal TEWL was 16.7 g/m²h. In the moisturiser group, the mean basal TEWL was 15.4 g/m²h (not statistically significant). Concerning the three trial groups (barrier cream, moisturiser, control), the study investigators found that changes of the metal workers' TEWL, 'throughout the six‐month study period, were almost identical'.
In Halkier‐Sørensen 1993, TEWL after the moisturisers period in was marginally higher compared to the control period (not significant, Table 9).
5. Mean skin hydration and TEWL in Halkier‐Sørensen 1993.
| Study arm | N |
Skin hydration1 after 2 weeks control |
Skin hydration1 after 2 weeks moisturiser |
TEWL2 after 2 weeks control |
TEWL2 after 2 weeks moisturiser |
| Control before moisturiser group I3 | 70 | 72 | 80 | 39 | 42 |
| Moisturiser before control group I | 70 | 72 | 83 | 40 | 41 |
| Control before moisturiser group II4 | 0 | ‐ | ‐ | ‐ | ‐ |
| Moisturiser before control group II | 23 | 66 | 86 | 41 | 37 |
| Mean of both study arms and both groups5 | ‐ | 71.2 | 82.1 | 39.7 | 40.9 |
1 Skin hydration measured by corneometry (capacitance), figures extracted from diagram
2 Skin barrier function measured as TEWL (transepidermal water loss, g/m2/h), figures extracted from diagram
3 Group 1: participants who completed both periods
4 Group 2: participants who completed the moisturiser period but dropped out of period C after an average of 6 days (1‐10 days) because they developed severe dryness of the skin
5 Differences of moisturiser compared to control were not significant for skin hydration or TEWL (no P values reported)
Skin hydration
This outcome was assessed in two studies (Brüning 2008; Halkier‐Sørensen 1993), but only one of these gave figures and P values (Halkier‐Sørensen 1993).
In both moisturiser group and controls of Brüning 2008, median skin hydration was higher at last follow‐up compared to baseline (not significant, Table 6). Skin hydration was marginally lower in the moisturisers group at baseline and after one year compared to controls (not significant).
In Halkier‐Sørensen 1993, after the no‐treatment period, the cleaners' and kitchen workers' skin hydration was considerably lower compared to the period when they used a moisturiser (P < 0.001, Table 9).
The details of these bioengineering methods are given in the Characteristics of included studies tables.
2.5 Secondary outcome 3: change of occupation because of OIHD versus staying in the occupation
None of the trials reported this outcome.
2.6 Secondary outcome 4: proportion of participants satisfied with the products given (cosmetic, preventive, therapeutic properties of the products)
Only one study addressed this outcome. In Halkier‐Sørensen 1993, 80% of the participants rated the quality of the moisturiser as good or very good. Thirteen per cent stated that it had no effect. Two participants did not find Locobase cosmetically acceptable. No data on adherence were given in that study.
2.7 Secondary outcome 5: other adverse outcomes: those that are not severe enough to warrant participants to leave the study (e.g. mild irritation or other complaints about products applied in the studies)
Only one study addressed this outcome. Itching, stinging, and dry skin after application of Locobase were reported in 7% of the participants of the study of Halkier‐Sørensen 1993.
Comparison 3: barrier creams and moisturisers versus no treatment
Four trials compared a combination of barrier creams and moisturisers against no treatment (Brüning 2008; Goh 1994; Halkier‐Sørensen 1993; Kütting 2010).
3.1 Primary outcome 1: the proportion of participants developing any signs and symptoms of OIHD (incident cases) measured by clinical scores (IGA) or hand dermatitis scores (e.g. HECSI, Manuscore), or both, as rated by the investigator (physician/nurse) or the participant
We identified two studies relevant to this outcome (N = 474). Two studies of male German metal workers were pooled for the meta‐analysis of barrier creams and moisturisers versus no intervention. The duration was 12 months. Both trials showed a reduced risk of developing OIHD when applying barrier creams and moisturisers. The risk reduction was clinically important (less than 0.75) in one individual trial and in the pooled analysis. In the intervention groups, 8% of participants developed symptoms of OIHD compared to 13% in the control groups, but when the data were pooled the very wide confidence intervals were consistent with either an important effect or no effect (RR 0.68, 95% CI 0.33 to 1.42; P = 0.30) and the quality of evidence was low (Table 3). RR ranged from 0.43 to 0.92. This outcome had moderate levels of heterogeneity (Chi2 = 1.84; df = 1.0; P = 0.17; I2 = 46%) (Analysis 3.1).
3.1. Analysis.
Comparison 3 Barrier creams and moisturisers vs no treatment, Outcome 1 Proportion of OIHD.
Sensitivity analysis
The results of the sensitivity analysis are shown in Table 10. When we excluded the trial Brüning 2008, which addressed abnormal morphology instead of hand eczema and had a partial split‐body design, the remaining study showed a considerable effect but this did not reach significance.
6. Sensitivity analysis for comparison 3.
| Excluded trials | Rationale for exclusion |
Number of included trials |
Number of included participants |
RR (95% CI) | P | I2 |
| ‐ | ‐ | 2 | 474 | 0.68 (0.33 ‐ 1.42) | 0.30 | 46% |
| Kütting 2010 | cluster design without correction |
1 | 96 | 0.92 (0.49 ‐ 1.72) | 0.79 | ‐ |
| Brüning 2008 | PO1 was not manifest hand dermatitis; split‐body design |
1 | 378 | 0.43 (0.17 ‐ 1.07) | 0.07 | ‐ |
When we removed Kütting 2010, the effect estimate was still very imprecise. We deduced that correcting for cluster randomisation in this study would still yield no significant results.
Because only two trials with moderate statistical heterogeneity were included in this comparison, the sensitivity analysis showed a considerable change in RR but not in statistical significance when either study was removed.
3.2 Primary outcome 2: frequency of treatment discontinuation due to adverse effects
In one study it was evident from the dropout analyses that no participant was lost to follow‐up because of adverse effects from the moisturisers (Brüning 2008). However, we cannot fully exclude the possibility that some of the participants who completed this study may have stopped applying the products without the researchers' knowledge.
No dropout analyses were performed in Kütting 2010.
The numbers of participants who dropped out of the individual trials ranged from 8% to 24% and are given in the Characteristics of included studies tables.
3.3 Secondary outcome 1: severity of clinical signs and symptoms in incident cases of OIHD (measured by clinical scores (IGA) or hand dermatitis scores (e.g. HECSI, Manuscore), or both) as rated by the investigator (physician/nurse) or the participant
None of the included studies addressed this outcome. One study applied scores (Kütting 2010), but they were not reported separately for incident cases of OIHD.
3.4 Secondary outcome 2: proportion of participants with significant changes (difference in average score and/or difference from baseline) in barrier function or hydration, measured using TEWL (skin barrier) and corneometry (skin hydration)
TEWL
TEWL was reported in one study (Brüning 2008). The study investigators gave quartiles instead of mean and standard deviation. The metal workers' median TEWL after one year was lower than at baseline in all study arms, including the control, but the changes were small and not significant (Table 6). There was no clinically important difference between barrier creams plus moisturiser and control at the last follow‐up.
Skin hydration
This outcome was assessed in one study (Brüning 2008). The study investigators gave quartiles instead of mean and standard deviation. For barrier creams plus moisturiser, median skin hydration was somewhat higher at last follow‐up compared to baseline (P = 0.0402). Without reaching significance or clinical importance, skin hydration was lower in this group at baseline and after one year.
The details of these bioengineering methods are given in the Characteristics of included studies tables.
3.5 Secondary outcome 3: change of occupation because of OIHD versus staying in the occupation
None of the trials reported this outcome.
3.6 Secondary outcome 4: proportion of participants satisfied with the products given (cosmetic, preventive, therapeutic properties of the products)
None of the trials reported this outcome.
3.7 Secondary outcome 5: other adverse outcomes: those that are not severe enough to warrant participants to leave the study (e.g. mild irritation or other complaints about products applied in the studies)
None of the trials reported this outcome.
Comparison 4: skin protection education versus no or minimal intervention
We identified three studies for this comparison.
4.1 Primary outcome 1: the proportion of participants developing any signs and symptoms of OIHD (incident cases) measured by clinical scores (IGA) or hand dermatitis scores (e.g. HECSI, Manuscore), or both, as rated by the investigator (physician/nurse) or the participant
For this outcome we found three relevant studies. A total number of 1443 gut cleaners, nurse apprentices, or hospital employees were included. In order to account for the cluster design in Meer 2015, the effective sample size needed to be corrected to a total of 1355 participants. The duration was between one and three years. With a skin protection education, the risk of developing OIHD was reduced in two studies (Flyvholm 2005; Löffler 2006). In these two studies, the risk reduction was clinically important (less than 0.75). One study showed an non‐significant but clinically important increased risk compared to the minimal implementation group (leaflet only) (Meer 2015). When we pooled these data, the wide confidence intervals were consistent with either a reduced risk or no effect (RR 0.76, 95% CI 0.54 to 1.08; P = 0.12) and the GRADE quality of evidence was very low (Table 4). In the intervention groups 21% of participants developed symptoms of OIHD, compared to 28% in the control groups. RR ranged from 0.69 to 1.26. We observed high levels of statistical heterogeneity (Chi2 = 4.44; df = 2.0; P = 0.11; I2 = 55%) (Analysis 4.1).
4.1. Analysis.
Comparison 4 Skin protection education versus no or minimal intervention, Outcome 1 Proportion of OIHD.
Sensitivity analysis
The results of the sensitivity analysis are shown in Table 11. All three trials were cluster‐randomised and only the authors of one study provided the data required for the correction of the design effect (Meer 2015). After removing Flyvholm 2005, the effect was still very imprecise and the CI included 1. The same was true for removing Löffler 2006, or both trials, from the meta‐analysis. We deduced that correcting for cluster randomisation in these two studies would still yield no significant results. Only one study in this comparison did not measure hand eczema or hand dermatitis, but instead measured morphological changes (Löffler 2006).
7. Sensitivity analysis for comparison 4.
| Excluded trials | Rationale for exclusion |
Number of included trials |
Number of included participants |
RR (95% CI) | P | I2 |
| ‐ | ‐ | 3 | 1355 | 0.76 (0.54 ‐ 1.08) | 0.12 | 55% |
| Flyvholm 2005 | cluster design without correction |
2 | 1143 | 0.86 (0.43 ‐ 1.70) | 0.66 | 78% |
| Löffler 2006 | cluster design without correction; PO1 was not manifest hand dermatitis |
2 | 1105 | 0.90 (0.51 ‐ 1.61) | 0.73 | 54% |
|
Flyvholm 2005 Löffler 2006 |
cluster design without correction |
1 | 893 | 1.26 (0.67 ‐ 2.35) | 0.47 | ‐ |
The risk reduction appeared considerably smaller when either or both studies with uncorrected cluster design were removed. One partial reason for this is that uncorrected cluster designs often show an artificially greater effect. Another reason is that the only study without unit‐of‐analysis issues showed a negative effect of skin protection education. The study authors speculate that this was the case because the participants of their intervention group were more aware of hand dermatitis due to the education and may have reported it more often only due to this awareness.
4.2 Primary outcome 2: frequency of treatment discontinuation due to adverse effects
In one study it was evident from the dropout analyses that no participant was lost to follow‐up because of adverse effects (Löffler 2006). However, we cannot fully exclude the possibility that some of the participants who completed the study may have stopped applying the products without the researchers' knowledge.
Two studies did not report any dropout reasons (Flyvholm 2005; Meer 2015).
The numbers of participants who dropped out of the individual trials ranged from 35% to 38% and are given in the Characteristics of included studies tables.
4.3 Secondary outcome 1: severity of clinical signs and symptoms in incident cases of OIHD (measured by clinical scores (IGA) or hand dermatitis scores (e.g. HECSI, Manuscore), or both) as rated by the investigator (physician/nurse) or the participant
None of the included studies addressed this outcome. One study applied scores (Meer 2015), but they were not reported separately for incident cases of OIHD
4.4 Secondary outcome 2: proportion of participants with significant changes (difference in average score or difference from baseline, or both) in barrier function or hydration, measured using TEWL (skin barrier) and corneometry (skin hydration)
None of the trials in this comparison reported these outcomes.
4.5 Secondary outcome 3: change of occupation because of OIHD versus staying in the occupation
None of the trials in this comparison reported these outcomes.
4.6 Secondary outcome 4: proportion of participants satisfied with the products given (cosmetic, preventive, therapeutic properties of the products)
None of the trials in this comparison reported these outcomes.
4.7 Secondary outcome 5: other adverse outcomes: those that are not severe enough to warrant participants to leave the study (e.g. mild irritation or other complaints about products applied in the studies)
None of the trials in this comparison reported these outcomes.
Discussion
Summary of main results
We included nine randomised controlled trials (RCTs) involving 2888 initially randomised participants without occupational irritant hand dermatitis (OIHD) from different occupations. The primary outcomes were signs and symptoms of OIHD developed during the trials, and the frequency of treatment discontinuation due to adverse effects. Six studies including 1533 participants investigated the effects of barrier creams, moisturisers, or both, compared to no intervention. Three studies including 1355 participants assessed the effectiveness of skin protection education on the prevention of OIHD compared to no intervention. Among these four types of intervention, the comparability of the studies was limited since the criteria for assessing signs and symptoms of OIHD, the products, and the occupations varied.
The trials involved metal workers exposed to cutting fluids, dye and print factory workers, gut cleaners in swine slaughterhouses, cleaners and kitchen workers, nurse apprentices, hospital employees handling irritants, and hairdressing apprentices. All studies were undertaken at the respective work places. Study duration ranged from four weeks up to three years. The participants' ages ranged from 16 to 67 years.
We performed four meta‐analyses, each comprising a maximum of four trials, for the primary outcome of signs and symptoms of OIHD. The meta‐analyses for barrier creams, moisturisers, a combination of both barrier creams and moisturisers, or skin protection education showed imprecise effects favouring the intervention. Barrier creams alone were investigated for metal workers, print and dye industry workers and may have a limited protective effect in these occupations compared to no intervention (Analysis 1.1; low‐quality evidence according to GRADE criteria). Moisturisers alone were investigated for metal workers and may have a clinically important protective effect in these occupations compared to no intervention (Analysis 2.1; low‐quality evidence). Likewise, there may be a clinically important reduced risk of developing OIHD in participants using a combination of barrier creams and moisturisers (Analysis 3.1; low‐quality evidence). We are uncertain whether skin protection education has a protective effect in gut cleaners and nurse apprentices (Analysis 4.1; very‐low quality evidence).
Sensitivity analyses showed that the findings concerning barrier creams were robust (Table 5), but the findings concerning the other interventions were not, as the magnitude of the protective effects varied depending on which trials were included (Table 8; Table 10; Table 11).
When we excluded Brüning 2008, the meta‐analyses revealed slightly stronger preventive effects of barrier creams (Table 5), and considerably stronger effects of moisturisers (Table 8), or both (Table 10). In this study there might have been some contamination due to the partial split‐body design.
No major harmful or other adverse effects were identified. None of the studies addressed the frequency of treatment discontinuation due to adverse effects of the products directly. However, in four studies the dropout reasons were unrelated to adverse effects. The investigated interventions to prevent OIHD probably cause few or no serious adverse effects. The quality of evidence was moderate, according to GRADE criteria.
Although the findings of this review were generally positive, the results were imprecise and the GRADE quality of evidence concerning effectiveness was rated as low or very low. We concluded that there is insufficient evidence, at present, for the effectiveness of most of the interventions identified for preventing new cases of OIHD in the workplace.
Overall completeness and applicability of evidence
There are a number of potential limitations to the applicability of the findings of this review. We identified only a limited number of randomised controlled trials investigating the effectiveness of measures to prevent OIHD under field study settings in different occupations. The lack of studies may be due to the high costs and effort involved in conducting field studies rather than ineffectiveness of primary prevention measures. Also, such studies are not required in order to sell barrier creams and moisturisers as they are classed as cosmetics.
In occupational settings, chronic OIHD is caused by a summation of subclinical exposures. Two of the nine studies we identified were short‐term studies (28 and 29 days) providing only limited information on long‐term effectiveness. Occupational irritant hand dermatitis is most prevalent in workers who have been exposed to high cumulative irritant damage (Malten 1981). Quite a long observation period is necessary when the effect of protective measures in a field study setting should be evaluated (Bauer 1997; Bauer 1998; Smit 1994; Uter 1998a). Moreover, interindividual differences in susceptibility and regenerative capacities play a major role, indicating an individual threshold for irritation (Agner 1991; Fartasch 1995; Pinnagoda 1989; Rietschel 1997; Tupker 1989a; Tupker 1989b; Kezic 2009).
No RCTs on the use of gloves for preventing OIHD were identified. We speculate that this is mainly because in many occupations it would be unethical to assign participants to a control group without glove use. In practice, guidelines and recommendations on glove use are mainly based on rules and regulations as well as on expert opinion (Mellström 1994).
Apart from OIHD, the review outcomes (including adverse effects) were usually addressed in very few studies, allowing only a narrative description of the findings.
Despite the limited body of evidence, our results do provide information on the effectiveness of barrier creams and moisturisers in primary prevention. However, there is insufficient evidence to make recommendations about their use. The data suggest that protection creams, moisturisers may reduce the risk of developing signs of OIHD to some extent. From the observed confidence intervals, we consider it unlikely for any intervention to reduce the risk to 50% or less. Policy makers, providers and users of primary prevention strategies for OIHD may find this information useful.
Quality of the evidence
For barrier creams, moisturisers, or both, we assessed the quality of evidence (according to GRADE criteria) as low concerning outcome 1 (the proportion of participants developing OIHD). We downgraded our quality assessments for these comparisons by two levels because the results were imprecise and included inconsistent outcomes, as described below (Table 1; Table 2; Table 3). For skin protection education, we assessed the quality of evidence as very low, according to GRADE criteria (Table 4). We downgraded our quality assessment for this comparison by three levels because the results were imprecise, included inconsistent outcomes, and were subject to a high risk of bias (the risk ratio may have been underestimated or overestimated). Since only few studies could be included in each comparison and the upper limits of the confidence intervals only barely included 1 (except for barrier creams and moisturisers combined), it is very likely that future research will have an important impact on deciding whether or not the investigated interventions do reduce the risk of developing signs and symptoms of OIHD.
We assessed the quality of evidence for primary outcome 2 (safety) as moderate for all comparisons because the only available data were indirect.
We did not apply the GRADE criteria to the secondary review outcomes because they are not among our key outcomes. Very few data were reported for these outcomes and meta‐analyses were not possible.
A variety of scores were assessed and are described in the Characteristics of included studies tables (Brüning 2008; Halkier‐Sørensen 1993; Kütting 2010; Meer 2015; Perrenoud 2001a). They either consisted of three categories or were quantitative. In most cases, the scores were apparently developed by the study investigators and not validated. The two exceptions were Kütting 2010, with good to excellent inter‐ and intra‐observer reliability, and Meer 2015, which applied a participant‐reported rating which belongs to a validated questionnaire (NOSQ 2002).
TEWL or corneometry (or both) were measured in four studies (Brüning 2008; Goh 1994; Halkier‐Sørensen 1993; Perrenoud 2001a). The methods were generally well‐described and adequate, but it was not always ensured that the measurements were taken from the exact same skin areas (Brüning 2008; Perrenoud 2001a).
Skin signs and symptoms of OIHD occur after repetitive contact with various irritant factors (known as consecutive subclinical irritation). OIHD develops over an extended period of time (weeks up to months). Therefore extended study periods are necessary. The study period was adequate in the majority of studies: three years (Löffler 2006), one year (Duca 1994; Brüning 2008; Flyvholm 2005; Kütting 2010; Meer 2015), and six months (Goh 1994). However the study period was too short to investigate a long‐term protective effect in two studies: 28 days in Halkier‐Sørensen 1993, and 29 days in Perrenoud 2001a.
Study limitations (design and risk of bias)
In each of the three comparisons of barrier creams and/or moisturisers, one trial was cluster‐randomised without correction (Kütting 2010). For educational programmes two out of three trials lacked appropriate correction for the design effect. We did not downgrade our GRADE quality assessments in any of these cases because sensitivity analyses showed that correcting for any value of design effect would not change the conclusion that the preventive effects were not significant.
Across all domains and trials the risk of bias was predominantly unclear. We decided not to downgrade our GRADE quality assessments for the comparisons of barrier creams and/or moisturisers. Biases generally result in more positive effect estimates whereas the observed effects were often small and mostly not significant in the included trials. There was no evidence to lower our confidence in the estimated RRs concerning risk of bias.
For skin protection education, the risk of bias was mainly unclear but was high for some domains. The relative risk may be higher than estimated due to lack of blinding of outcome assessors and baseline imbalances in Flyvholm 2005. On the other hand, RR may be lower than estimated due to an overestimation of OIHD in the intervention group in Meer 2015. We downgraded the quality of evidence of the primary review outcome 1 by one level for this comparison.
Inconsistency of results
Concerning methodological consistency, the diagnostic criteria for the diagnosis of OIHD varied across the included studies. Signs and symptoms of OIHD were assessed by dermatologists (Brüning 2008), by study personnel (Duca 1994; Goh 1994; Halkier‐Sørensen 1993; Löffler 2006; Perrenoud 2001a), or by the participants (with a standardised interview, as in Kütting 2010, or with the validated questionnaire NOSQ 2002, as in Flyvholm 2005; Meer 2015). None of the trials was designed to exclude endogenous/atopic hand eczema or allergic contact dermatitis of the hands. Non‐occupational hand eczema could not be excluded, either.
The limited number of eligible studies and the even smaller number of studies reporting manifest hand eczema made it necessary to define the key review outcome very broadly and investigate any signs and symptoms of OIHD. Accordingly, the majority of the studies showed methodological weaknesses. A variety of non‐standardised semi‐quantitative methods were used to assess the key review outcome signs and symptoms of OIHD. In two studies the criteria were not described; four studies reported any irritant skin changes; and three studies had the presence of OIHD assessed by the participant.
Therefore, we downgraded our GRADE assessments of the primary review outcome 1 by one level for all comparisons. This limitation will be an issue until further studies, with more consistently defined outcomes concerning OIHD, are available for subsequent updates of the review.
Another methodological inconsistency concerns the study populations. Occupations varied across trials evaluating barrier creams, and skin protection education, respectively. Moreover, the workers were employed in their respective occupations for varying and often unreported durations. While it is hard to tell whether this introduced a bias, it does lower our confidence in the results. Still, since we already downgraded for methodological inconsistency, we judged that no further downgrade was necessary.
Concerning statistical consistency of the results, two studies showed less positive or even negative effects of their respective interventions, possibly due to their study designs (Brüning 2008; Meer 2015). However, we decided not to downgrade our GRADE ratings of these comparisons further because the inconsistencies were not significant and 95% confidence intervals did overlap. Also, the main problem introduced by statistical inconsistency was the wide confidence intervals. This issue was already considered when we downgraded by one level due to imprecision.
Indirectness of evidence
For signs and symptoms of OIHD, no comparison was downgraded for indirectness of the evidence because the populations, interventions, comparisons, and outcome measures were reported as predefined for the review.
We downgraded the primary review outcome 2 by one level due to the indirectness of the results throughout all comparisons. None of the studies reported directly on treatment discontinuation due to adverse effects. Instead, the extracted results are based on dropout analyses, which did not focus on adverse effects of the interventions. No study reported whether or not treatment was discontinued by participants who did not drop out. For several studies, dropout analyses were not performed or not detailed enough to extract whether or not adverse effects were among the reasons.
Imprecision
None of the comparisons yielded significant results. However, the confidence intervals included 1 as well as clinically relevant RRs of 0.75 or less and were therefore too wide to be certain that the interventions had no impact.
For all comparisons, we downgraded our quality assessments of the evidence for primary review outcome 1 by one level due to imprecision.
Publication bias
An assessment of the reporting bias was not possible (Assessment of reporting biases). We did not downgrade any comparison due to publication bias.
Potential biases in the review process
We did not impose any language restriction and searched the grey literature, proceedings of relevant conferences, and contacted experts of the field. However, we might have missed trials on primary prevention of OIHD, especially those not published because of null or negative findings (publication bias). Therefore potential biases in the review process cannot be excluded.
In this review, we interpreted prevention as meaning interventions applied to healthy people. We chose to investigate the effectiveness of primary preventive measures applied to people who were assessed by the study investigators as not having any signs or symptoms of OIHD. In reality there will be some participants with some level of disease especially when it is very prevalent like dermatitis, or there will be those who have not (yet) asked for medical help because they are not aware that they have a medical problem. We acknowledge that exclusion of studies that were not primary prevention studies, i.e. where some participants had existing OIHD (unless the participant population was mixed and disaggregated data was available for those participants who were healthy with no OIHD at the start of the study), may not be directly applicable to field conditions. Interventions to prevent worsening or improve existing OIHD are available, but analysis of their effectiveness was outside the scope of this review and should be addressed in a separate systematic review.
Most included studies assessed the proportion of hand eczema. However, other dichotomised data were chosen for the primary outcome 1. Also, the time point varied across studies as we always used data from the last follow‐up. The weighting of trials in the meta‐analyses led to an over‐representation of studies that focused on minor irritant skin changes. The alternative would have been to weight all studies equally. We discarded this option because the primary outcome was any signs and symptoms of OIHD, and because only five of the nine trials assessed hand eczema or hand dermatitis. A related problem was that the confidence intervals of the risk ratios depend on the numbers of cases. This means that confidence intervals tended to be broader in studies which observed OIHD and narrower in studies which observed any irritant skin changes. The results of the meta‐analyses must be considered as preliminary.
Subjectivity occurred while judging the risk of performance bias and conducting GRADE quality assessments. One could judge that not blinding the participants introduces only a low risk of performance bias concerning the outcome OIHD (instead of an unclear bias). The risk of attrition bias might as well be judged as high in some cases, but we decided to judge the risk as unclear when the dropout reasons were not distinctly connected to the outcome OIHD. Other researchers might have further downgraded the quality of evidence based on the unclear risk of bias in most trials and domains.
Agreements and disagreements with other studies or reviews
We compared our findings to other reviews in this field of investigation. Individual studies were outside the scope of this overview.
The principles and measures of technical‐organisational hazard control and individual protective measures in workplaces where the skin is at risk are well‐defined (Halkier‐Sørensen 1993; Mathias 1990; Wigger‐Alberti 1997). In the available literature, barrier creams and gloves combined with adequate moisturisers are recommended widely as the most important means of personal protective equipment in professions with skin hazards.
In their 2003 review of skin protection creams (barrier creams) for the prevention of occupational dermatitis, Kütting and Drexler identify most of the studies included in our review which were available by that time (Duca 1994; Goh 1994; Perrenoud 2001a, but not Halkier‐Sørensen 1993) (Kütting 2003). Moreover, they discuss experimental trials, primary prevention trials, and trials which were not randomised. They argue that it is unclear whether experimental settings are suitable to simulate real workplace conditions. Most clinical trials they identify were too short, did not have a non‐intervention control, or were only suited to evaluate therapeutic effects. Similar to our findings, they conclude that they could not answer the question whether or not barrier creams and gloves can prevent or provoke contact dermatitis based on the available data. Concerning the protection of barrier creams against resorption of dangerous substances at the workplace, they conclude that the data was controversial. This question was beyond the scope of our review. Kütting and Drexler summarised that further studies under workplace conditions were needed in order to give evidence‐based recommendations about skin protection measures.
Saary and colleagues systematically reviewed the literature on the treatment and prevention of contact dermatitis (Saary 2005). Their focus is on occupational dermatitis, but experiments and studies involving other populations are included. They found good‐quality evidence that certain barrier creams can prevent irritant contact dermatitis. There is also good‐quality evidence for the preventive effects of moisturisers, especially with high lipid content. Saary and colleagues could not identify any good‐quality studies on educational programmes and found little evidence of their preventive effects. One fair‐quality study shows evidence that the use of cotton glove liners prevents from signs of irritant contact dermatitis that originates from wearing occlusive gloves.
Kampf and colleagues reviewed the prevention of irritant dermatitis among health care workers concerning hand hygiene practices (Kampf 2007). They report that there exists category IA (CDC/HICPAC guidelines) evidence concerning the recommendations of hand wash with soap and hand disinfection. The review concludes that healthcare workers should wash their hands with soap and water only as a rare exception, when there is visible soiling. Instead, they recommend to apply alcohol‐based hand rubs routinely when the hands have been contaminated. While this question was beyond the predefined comparisons of our review (except as part of an educational programme), the recommendations are highly relevant for the prevention of OIHD in health care workers.
Kütting and Drexler reviewed the efficacy of the three‐step programme of skin protection for primary prevention as opposed to secondary prevention (Kütting 2008). While the review focuses on occupational dermatitis, trials involving other populations are not excluded. Kütting and Drexler encourage the avoidance or substitution of hand cleansing, but the evidence they present is very scarce. Based on three studies, Perrenoud 2001a, Berndt 2000, and McCormick 2000, they report no significant benefit of barrier creams compared to vehicles or an oil‐containing lotion. They found very weak but undisputed evidence that barrier creams may help in removing contaminations more gently (Mathias 1990). Morevover, they point to adverse effects of barrier creams (increased susceptibility to irritants or allergens), which are reported in four studies. Three studies, which were excluded from our review, also report an increased susceptibility after the use of skin care creams (moisturisers). However, Kütting and Drexler argue that skin care can be used after work without the risk of an increased resorption of working substances. They report that after‐work skin care can prevent OIHD in subclinically damaged skin, based on five trials which were not included in our review. They also present four studies which show benefits of educational programmes (excluded from our review). Nevertheless, they conclude that a primary prevention benefit of barrier creams and skin care creams is not yet based on evidence.
With reference to Saary 2005, and some individual studies, Nicholson reports mixed evidence for the protective effects of pre‐work (barrier) creams (Nicholson 2010). This paper concludes that some creams may have protective effects, but that pre‐work creams are not generally effective in the prevention of OIHD; only scarce information on protective gloves is presented. They found evidence that educational programmes help to reduce the incidence of occupational hand dermatitis. Only one of the three referenced studies matched our inclusion criteria (Flyvholm 2005). Referring to Saary 2005, and the trial Arbogast 2004, Nicholson et al report that regular application of emollients helps to prevent occupational contact dermatitis.
One systematic review looks at RCTs and controlled clinical trials (CCTs) on primary and secondary prevention strategies of patients who are at risk of hand dermatitis or have signs and symptoms, i.e. their review is not limited to occupational irritant dermatitis (Van Gils 2011). They include only two of the trials that are also included in our review. Their results are hard to compare to those of our review because the outcomes were categorised as 'occurrence' of hand dermatitis, 'adherence to preventive measures', 'clinical outcomes and skin condition' (including signs of hand dermatitis), and 'self‐reported outcomes' (including self‐reported hand dermatitis). Moreover, the varying preventive strategies were not presented separately. Van Gils et al conclude that there is evidence that preventive strategies are effective for workers at risk for or with hand dermatitis. Our meta‐analyses provide only limited support for this assessment.
In Holness 2013, the evidence on occupational dermatitis prevention is briefly reviewed. She identifies systematic reviews and summarises that their results found moderate evidence for the effectiveness of prevention programmes (Saary 2005; Bauer 2010; Nicholson 2010; Van Gils 2011). She also argues that protective creams, while shown to be effective in experimental studies, may be applied in too small amounts in real workplace environments.
Based on the findings of Saary 2005, one paper reports that barrier creams show varying effects in interventional trials (Hines 2017). Referring to Kütting 2008, and Zhai 2006, they judge that recent research does demonstrate the effectiveness of barrier creams. Kütting and Drexler however indicate that only minimal evidence is available for the effectiveness of barrier creams whereas Zhai et al report that there is good evidence from experimental studies. Hines and colleagues also point to the significant benefit reported by Kütting 2010, referring to the outcome 'skin score', whereas the efficacy of barrier creams in this study is substantial but not significant for our primary outcome. Concerning skin care creams (moisturisers), Hines and colleagues consider four studies of primary research. Half of these studies are also included in our review (Goh 1994; Kütting 2010), while the other two are excluded because they are not primary prevention trials (Arbogast 2004; Winker 2009). Hines and colleagues adopt the study investigators' conclusions that skin care creams are beneficial, but they do not report or discuss how the effectiveness is measured.
Authors' conclusions
Implications for practice.
Low‐quality evidence suggests that moisturisers alone or combined with barrier creams may achieve a clinically important protective effect, either in the long‐ or short‐term, for primarily preventing occupational hand dermatitis (OIHD).
Low‐quality evidence indicates that barrier creams alone may have a slight protective effect on OIHD, but this does not appear to be clinically important. We are unsure whether the result for educational interventions can be generalised for practical application because of the very low quality of the evidence.
The interventions probably cause few or no serious adverse effects.
There is a complete absence of evidence to support or refute the use of protective gloves in the prevention of OIHD. However, protective gloves are indispensable for personal protection against chemical, biologic and physical hazards in many occupations.
We conclude that at present there is insufficient evidence to confidently assess the effectiveness of interventions used in the primary prevention of OIHD in the workplace. This does not necessarily mean that current measures are ineffective. There is still a need for trials which apply standardised measures for the detection of OIHD in order to determine the effectiveness of the different prevention strategies.
Implications for research.
The absence of substantial evidence of efficacy to support specific or complex interventions that are in use for the primary prevention of OIHD does not necessarily imply that they are not effective. Our results indicate that further large randomised controlled trials (RCTs) over extended time periods (six months up to one year) are needed to determine whether complex or single interventions for preventing OIHD in skin risk occupations are working. There is not enough evidence available at present to comment on whether moisturisers, barrier creams, gloves, and other skin protection measures work in real‐world wet work settings. Further trials, which are pragmatic (in real‐life conditions, to determine if an intervention works or not) and explanatory (in experimental conditions, to determine why and how an intervention might work), could explore the effectiveness of gloves, barrier creams, and moisturisers in different occupational settings with different irritant profiles. Such studies need to include new cases and examine primary prevention strategies in different work places.
In particular, we noticed a lack of RCTs concerning the effectiveness of gloves. While it may often be unethical to prohibit control group participants from using gloves, future studies can evaluate the effectiveness of different gloves, the use of cotton liners, or compare the promoted use of gloves to usual practice.
The agreement on diagnostic criteria of established OIHD are quite clear, but research should be directed to further develop and validate hand dermatitis scoring systems for a better and standardised discrimination of irritant skin changes from irritant contact dermatitis. A standard core outcome set is necessary in order to allow more direct comparisons across studies.
Researchers conducting cluster‐randomised trials need to apply a correction for the design effect to their evaluations as well as to their sample size calculations. Published intraclass correlation coefficients (ICCs) are lacking in the context of OIHD. Reporting ICCs would not only improve an individual study's quality, but also provide comparable estimates for planning and evaluating other studies. Even publishing ICCs for trials which are already concluded will be a valuable contribution. Currently, the only available ICC estimate in this context is low (Meer 2015; ICC = 0.005) but may be considerably higher in other studies. Sample sizes for future trials must be inflated accordingly.
Future researchers should be aware that having OIHD assessed by the participants has its strengths and weaknesses. On the one hand, it may be more convenient and it makes it possible to detect OIHD not only at a specific time point, but over a larger range (e.g. three or six months), which produces more cases and therefore yields higher power. On the other hand, participants are harder to blind in comparison to study personnel. Also, participants of the intervention groups may be more sensitised to spotting OIHD compared to the controls.
In order to facilitate subgroup analyses, researchers should additionally present their data stratified by sex, age (more or less than 30 years), history of atopy and hand dermatitis.
Various in vivo and in vitro methods are in place to investigate the efficacy of skin protective measures under experimental conditions (Boman 1989; Frosch 1994; Gabard 1995; Henry 1994; Mellström 1994; Treffel 1994; Wahlberg 1996; Zimmerli 1996). The results of those experimental studies are not sufficient to forecast the effectiveness of the products under real‐life conditions. In skin risk occupations, the controlled conditions of an experimental setting never exist and a variety of irritants and allergens as well as mechanical factors may repetitively impair the skin at the same time or follow each other. Field studies therefore seem to be the best way to ensure that recommendations given are substantiated by evidence. However, field studies incur specific problems like the possible contamination of the groups after randomisation, difficulties with blinding, the necessity of large sample sizes, and changes in compliance because of the presence of the investigator (Coenraads 2003).
In addition, it is challenging to conduct RCTs in the workplace environment. While high‐quality evidence is still lacking, it may be helpful to conduct another systematic review with broader inclusion criteria (e.g. experimental trials, non‐randomised controlled trials) in order to provide a complete overview of the available evidence. However, data from experimental settings is hardly meaningful to forecast efficacy at workplaces, and the inclusion of controlled clinical trials would lead to an even lower evidence level.
What's new
| Date | Event | Description |
|---|---|---|
| 17 January 2018 | New search has been performed | We included five new studies and were able to perform meta‐analyses of four different prevention strategies (up to four studies per intervention). We identified three ongoing studies. We reassessed risk of bias for the four studies included in the first review version, in accordance with the methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions. For one study (Goh 1994), we selected a different study outcome as matching the primary review outcome. We chose to use risk ratio, instead of odds ratio, as the measure of treatment effect. |
| 17 January 2018 | New citation required and conclusions have changed | We included five new studies and performed meta‐analyses of two additional prevention strategies. We reassessed risk of bias for the four studies included in the first review version. We chose to use risk ratio, instead of odds ratio, as the measure of treatment effect. We used GRADE methodology to assess evidence quality and draw conclusions about our certainty in the review findings. |
History
Protocol first published: Issue 4, 2003 Review first published: Issue 6, 2010
| Date | Event | Description |
|---|---|---|
| 7 October 2008 | Amended | Converted to new review format. |
Acknowledgements
The review authors would like to thank Laura Prescott, Emma Mead and Helen Scott of the Cochrane Skin Group for their supervision and assistance in this review, and Finola Delamere and Elizabeth Doney for their substantial help with searching for trials. We also thank Helen Wakeford and Liz Bickerdike of the Cochrane Editorial Unit for co‐ordinating the Fast‐Track Service editorial process. We thank Claudia Herzog, who checked if the review was relevant and comprehensible for consumers. We are also very grateful for the comments and suggestions from the consumer referees Esther Martin and Sean Chua, which helped us improve the wording of the abstract and plain language summary. We thank the peer referees D Linn Holness, Tove Agner and Ira Madan for their thoughtful and most helpful remarks. Moreover we would like to thank Jan Bong who contributed to the compilation of the protocol on which this review is based, and Giada Meinel for helping to extract study data which was only available in Italian. We would also like to thank Jochen Schmitt and John English for their valuable contribution to the first published review version.
We thank the following researchers for providing additional data and information to their studies: Cécile Boot, Hans Drexler, Mari‐Ann Flyvholm, Harald Löffler, and Dirk Taeger.
Appendices
Appendix 1. Cochrane Skin Group Specialised Register search strategy
(((dermat* or eczema or dermatos*) and (occupation* or irritant* or contact) and (hand* or finger* or palm*))) AND (INREGISTER) [REFERENCE] [STANDARD]
Appendix 2. CENTRAL (Cochrane Library) search strategy
#1 ((dermat* or eczema) and (occupation* or irritant* or contact) and (hand* or finger* or palm*)):ti,ab,kw #2 MeSH descriptor: [Hand Dermatoses] this term only #3 MeSH descriptor: [Dermatitis, Occupational] this term only #4 MeSH descriptor: [Dermatitis, Allergic Contact] this term only #5 MeSH descriptor: [Dermatitis, Contact] this term only #6 MeSH descriptor: [Dermatitis, Irritant] this term only #7 {or #3‐#6} #8 (hand* or finger* or palm*):ti,ab,kw #9 MeSH descriptor: [Hand] explode all trees #10 #8 or #9 #11 #7 and #10 #12 #1 or #2 or #11
Appendix 3. MEDLINE (Ovid) search strategy
1. ((dermat$ or eczema) and (occupation$ or irritant$ or contact) and (hand$ or finger$ or palm$)).ti,ab. 2. Hand Dermatoses/ 3. Dermatitis, Occupational/ 4. Dermatitis, Allergic Contact/ 5. Dermatitis, Contact/ 6. Dermatitis, Irritant/ 7. or/3‐6 8. (hand$ or finger$ or palm$).mp. 9. exp Hand/ 10. 8 or 9 11. 7 and 10 12. 1 or 2 or 11 13. randomised controlled trial.pt. 14. controlled clinical trial.pt. 15. randomized.ab. 16. placebo.ab. 17. clinical trials as topic.sh. 18. randomly.ab. 19. trial.ti. 20. 13 or 14 or 15 or 16 or 17 or 18 or 19 21. exp animals/ not humans.sh. 22. 20 not 21 23. 12 and 22
[Lines 13‐22: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision)]
Appendix 4. Embase (Ovid) search strategy
1. ((dermat$ or eczema) and (occupation$ or irritant$ or contact) and (hand$ or finger$ or palm$)).ti,ab. 2. hand eczema/ 3. occupational eczema/ 4. contact dermatitis/ 5. exp occupational skin disease/ 6. skin allergy/ 7. irritant dermatitis/ 8. or/3‐7 9. exp hand/ 10. (hand$ or finger$ or palm$).mp. 11. 9 or 10 12. 8 and 11 13. 1 or 2 or 12 14. crossover procedure.sh. 15. double‐blind procedure.sh. 16. single‐blind procedure.sh. 17. (crossover$ or cross over$).tw. 18. placebo$.tw. 19. (doubl$ adj blind$).tw. 20. allocat$.tw. 21. trial.ti. 22. randomised controlled trial.sh. 23. random$.tw. 24. or/14‐23 25. exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 26. human/ or normal human/ 27. 25 and 26 28. 25 not 27 29. 24 not 28 30. 13 and 29
Data and analyses
Comparison 1. Barrier creams versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of OIHD | 4 | 999 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.72, 1.06] |
Comparison 2. Moisturisers versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of OIHD | 3 | 507 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.46, 1.09] |
Comparison 3. Barrier creams and moisturisers vs no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of OIHD | 2 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.33, 1.42] |
Comparison 4. Skin protection education versus no or minimal intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of OIHD | 3 | 1355 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.54, 1.08] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brüning 2008.
| Methods | Individually randomised controlled trial Combination of parallel and split‐body design, 2x2 arms Duration: 12 months 3 follow‐ups at 3, 6, and 12 months |
|
| Participants |
Final number evaluable after 12 months: N = 96 healthy metal workers exposed to cutting fluids
Number of participants randomised: N = 100 (group 1: N = 50; group 2: N = 50) Lost to follow‐up: 4 in group 1 Mean age in years (participants evaluable after 12 months): group 1: 35.5 (range 17 to 59); group 2: 41.5 (range 17 to 59) Sex: male Inclusion criteria
Exclusion criteria
Setting: one medium‐sized German factory |
|
| Interventions | Comparison of the effectiveness of skin protection, skin care, or both, versus no intervention (4 study arms, all relevant to the review). The metal workers' hands were randomised to:
No details on application described, but participants received a flyer and the product. One‐sided application using a glove for the other hand. Participants were randomised to use product on either left or right hand. |
|
| Outcomes |
All outcomes were measured at baseline, after 3, 6, and 12 months. For the visits at 3 and 6 months, abnormal morphology was not reported while the other outcomes were reported in diagrams of the medians only. Diagnostic criteria for OIHD (degree of certainty for the diagnosis of OIHD) Strengths
Limitations
Comparability
Scoring system for the severity of OIHD: the study investigators applied a score which consisted of 3 categories: 'unauffällige klinische Befundung' (inconspicuous findings), 'geringfügig auffällige klinische Befundung' (minor conspicuous findings) and 'auffällige klinische Befundung' (conspicuous findings). Strengths
Limitations
Quality of bioengineering methods (Pinnagoda 1990): the measurements were performed on four defined skin areas (back of the left and right hand, distal part of the volar site of the lower left and right arm) after a 20‐minute adjustment phase at a standardised temperature (20°C) and relative humidity (50%). The participants were asked not to take a shower, eat or drink anything and to refrain from smoking within the hour before the measurement. TEWL was measured according to international recommendations (Pinnagoda 1990, Rogiers 2001). For the corneometry, electrical measurements of conductibility (κ), impedance (Ω) and capacity (F) were performed. Strengths
Limitations
|
|
| Notes | The left/right hand design may have led to failures when applying the products. Study funding sources: Vereinigung der Metall‐Berufsgenossenschaften (VMBG) (financial, content‐related and organisational support) Possible conflicts of interest not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described. Quote: 'Während der Erstuntersuchung wurden die Probanden zufällig in zwei Gruppen aufgeteilt. Danach erfolgte in jeder Probandengruppe die zufällige Aufteilung der Probanden in jeweils zwei Interventionsarme (jeweils einer pro Hand).' (p. 7) [At baseline, probands were randomly assigned to two groups. Subsequently in each group of probands, the probands were randomly assigned to two intervention arms each (one for each hand).] |
| Allocation concealment (selection bias) | Unclear risk | Block randomisation was performed, no details given. Unclear selection bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding of participants, participants could have influenced performance by intentionally applying products to the wrong hand. Possible influence on proportion of OIHD. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Physician was blinded. |
| Blinding of outcome assessment (detection bias) TEWL and corneometry | Low risk | No influence of blinding is expected for these objective measurements. |
| Incomplete outcome data (attrition bias) Hand‐eczema‐related outcomes | Low risk | Only 4 withdrawals, due to personal reasons, in group 1 (control/skin protection). |
| Incomplete outcome data (attrition bias) Consumer satisfaction | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. All outcomes mentioned in the report were reported at least for first and last examination. |
| Other bias | Unclear risk |
Design: more than one intervention arm. This was accounted for in meta‐analysis. The control group was not double‐counted within one meta‐analysis. The split‐body design may have introduced contamination, especially concerning the control arm. (unclear risk) Baseline imbalances
Blocked randomisation in unblinded trials: block randomisation was performed, no details given. Unclear selection bias. Differential diagnostic activity: no different diagnostic activities across study arms. |
Duca 1994.
| Methods | Individually randomised controlled trial
Parallel groups, 2 arms Duration: 1 year 3 follow‐ups at 4, 8, and 12 months |
|
| Participants |
Final number evaluable after 12 months: N = 497 initially healthy dye and print factory workers (intervention: N = 248; control: N = 249) Number of participants randomised: N = 868 (intervention: N = 428; control: N = 440) Lost to follow‐up: N = 211 (intervention: N = 102; control: N = 109) Excluded from review due to OIHD at beginning of study: N = 160 (intervention: N = 78; control: N = 82) Mean age in years (all included participants): intervention: 32.6; control group: 32.1 Sex: 91% male, balanced between groups Mean duration of employment (all 868 participants): barrier cream and control group, 7.9 years Inclusion criteria: dye and print industry workers Exclusion criteria: other dermatological disease on the hands Setting: field study in 13 dye and print factories, North Italy |
|
| Interventions | Comparison of the effectiveness of:
The workers were randomised to receive either the barrier creams (silicone or hydrocarbon containing barrier creams), which they used twice a day for 1 year, or no intervention. |
|
| Outcomes |
Assessments took place after 4, 8, and 12 months. Diagnostic criteria for OIHD (degree of certainty for the diagnosis of OIHD): objective skin lesions assessed by study personnel (erythema, edema, exudation, vesicles, blisters, desquamation, hyperkeratosis, rhagades, dryness, atrophy, lichenisation) Strengths
Limitations
Broad outcome definition ‐> comparatively many cases expected Time frame: combined incidence at 4, 8, or 12 months Scoring system for the severity of OIHD: no score assessed Quality of bioengineering methods (Pinnagoda 1990): not relevant |
|
| Notes |
Study funding sources: Not described. Possible conflicts of interest: Not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: 'La procedura di randomizzazione.....base di una sequenza di numeri casuali' (p. 233) [The randomisation procedure ... based on a sequence of random numbers] Matched pairs were used in order to achieve balanced groups. |
| Allocation concealment (selection bias) | Unclear risk | Inadequate concealment of the allocation sequence may have resulted from pairwise assigning the first person of the pair to group A if the random number was even, to group B if odd. The second person of the pair was assigned to the alternative group. Efforts to conceal the allocation were not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants remains unclear. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: 'Controllo clinici periodici ....all`oscuro del gruppo' [Periodical clinical examination ... blinded to group] ‐ Outcome assessors were blinded. |
| Blinding of outcome assessment (detection bias) TEWL and corneometry | Low risk | TEWL and corneometry were not measured. |
| Incomplete outcome data (attrition bias) Hand‐eczema‐related outcomes | Unclear risk | 12 months follow‐up: 102 missing from the barrier cream group, 109 missing from the control group due to dropout and unavailability at the examination time points. The study investigators did mention ITT analysis but this merely meant that compliance was ignored. Missing values were not estimated. Dropout was due to the long duration of recruitment and due to workers leaving the factories, but there was no detailed analysis. |
| Incomplete outcome data (attrition bias) Consumer satisfaction | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | Low risk |
Design: no sources of bias were identified. Baseline imbalances: demographic data of participants were only available for the entire study population (N = 868). It remains unclear whether the demographic characteristics of the initially healthy workers from intervention and control group (N = 708) differed significantly in this study (low risk) Blocked randomisation in unblinded trials: not enough information was given to decide whether or not allocation concealment was broken by the pairwise allocation. (unclear allocation concealment bias) Differential diagnostic activity: no different diagnostic activities across study arms. |
Flyvholm 2005.
| Methods | Cluster‐randomised controlled trial; 18 clusters (departments) Parallel design, 2 arms Duration: 1 year 1 follow‐up at 1 year |
|
| Participants |
Final number evaluable after 12 months: N = 212 initially healthy Danish gut cleaners (intervention: N = 59; control: N = 153) Number of participants randomised: N = 644 baseline responders (intervention: N = 205; control: N = 439) Lost to follow‐up: N = 228 were lost to follow‐up or stopped working as gut cleaners (intervention: N = 69; control: N = 159) Excluded from review due to OIHD at beginning of study: N = 204 (intervention: N = 77; control: N = 127) Mean age in years (all follow‐up respondents including those with OIHD at baseline and those not available at baseline): intervention: 36.1 (range 17‐62 years); control: 37.8 (range 17‐66) Sex: 66.3% male (intervention), 64.1% male (control) Inclusion criteria: not described Exclusion criteria: not described Setting: field study in 18 swine slaughterhouses in different Danish cities, all departments belonging to one company |
|
| Interventions | Comparison of the effectiveness of a:
The prevention strategy consisted of a two part concept, with an evidence based prevention programme giving recommendations for prevention of work related skin problems in wet work occupations, and a documented method for implementation. The recommendations were aimed at the management and at the employees.
The local project group included 2‐5 gut cleaners who acted as role models. |
|
| Outcomes |
Diagnostic criteria for OIHD (degree of certainty for the diagnosis of OIHD) Strengths
Limitations
Comparability
Scoring system for the severity of OIHD: no score assessed Quality of bioengineering methods (Pinnagoda 1990): not relevant |
|
| Notes | 79 participants had stopped working as gut cleaners and were analysed as a separate group in the report regardless of the intervention. For this review, they were not considered and treated as dropouts. Demographic data were only available for participants who were not lost to follow‐up, including those with OIHD at baseline. There was a mistake in the paper concerning primary outcome 1, which MA Flyvholm corrected in an email (05/052015): 'For the intervention group (corrected: comparison group), 67% of those with eczema at baseline reported eczema at follow up and 33% no eczema; 37% with no eczema at baseline reported eczema at follow up.' (p. 646) Study funding sources: 'The project was financially supported by an appropriation for prevention of asthma and allergy, administered by the Danish Ministry of Health.' (p. 648) Possible conflicts of interest: none declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described ('randomly allocated' p. 643). |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding, possible influence on proportion of OIHD. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No blinding likely to influence reporting of OIHD. |
| Blinding of outcome assessment (detection bias) TEWL and corneometry | Unclear risk | TEWL and corneometry were not measured. |
| Incomplete outcome data (attrition bias) Hand‐eczema‐related outcomes | Unclear risk | 149 participants (23,1%) lost to follow‐up, an unspecified number of which (max. 51) dropped out without stating reasons. |
| Incomplete outcome data (attrition bias) Consumer satisfaction | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information. |
| Other bias | High risk |
Design: no correction for cluster randomisation ‐> no bias, but over‐precise results and limited comparability. Baseline imbalances
Blocked randomisation in unblinded trials: no block randomisation reported. Differential diagnostic activity: no different diagnostic activities across study arms. |
Goh 1994.
| Methods | Individually randomised controlled trial
Parallel groups, 3 arms Duration: 6 months (also reported as 30 weeks) for each participant, 3 years altogether 3‐weekly follow‐ups |
|
| Participants |
Final number evaluable after 6 months: N = 54 initially healthy, newly employed metal workers exposed to cutting fluids (barrier cream: N = 17; moisturiser: N = 14; control: N = 23) Number of participants randomised: N = 54 Lost to follow‐up: N = 0 Mean age in years: barrier cream group: 22 (range 16 to 37); moisturiser group: 22 (range 16 to 36); control: 22 (range 17 to 35) Sex: 50 out of 54 male Exclusion criteria: 'Only machinists who had not handled cutting fluids previously were included in the study. Machinists who had already worked for more than I week were excluded.' (p. 177) Setting: field study in the grinding and turning sections of one large ball‐bearing manufacturing factory, Singapore |
|
| Interventions | Comparison of the effectiveness of:
The participants were randomised to receive either a barrier cream (60 g tube every 6 weeks used on the hands before work and after each meal break), or a moisturiser used daily as an after work emollient, or no intervention. Arretil is a water‐soluble, non‐oily, silicone‐free barrier cream. Keri lotion is a liquid paraffin lotion (16%) and contains lanolin oil. The participants were followed up every 3 weeks for 6 months. |
|
| Outcomes |
Diagnostic criteria for OIHD (degree of certainty for the diagnosis of OIHD): classification of dermatitis severity: mild = less than 25% of the total surface area of either hand involved (up to wrist line), moderate = more than 25% of the total surface area of either hand involved (up to wrist line). Strengths
Limitations
Comparability
Scoring system for the severity of OIHD: no score assessed Quality of bioengineering methods (Pinnagoda 1990): 'Baseline TEWL measurement was made on the skin overlying the dorsal 3rd metarcarpophalangeal (MCP) joint of both hands with an Evaporimeter (Servomed, Vallingby, Sweden). We chose the 3rd MCP joint because of the ease of identifying the exact spot for repeat measurements. We conducted a pilot measurement on mid‐dorsal hand, 1st, 3rd, 5th dorsal MCP joints, mid‐ventral forearms and mid‐dorsal forearms on the 1st 30 volunteers. All showed fairly consistent recordings. We found the 3rd dorsal MCP joints to have the highest TEWL recordings and that these were fairly consistently reproducible. The method of TEWL measurement was as follows. The machinist rested in the examination room for 10 to 15 min. The TEWL on the dorsal 3rd MCP joint of each hand was then measured. TEWL values on the left hand followed by those on the right hand were recorded. The procedure was repeated once. 2 recordings of TEWL values for each hand were thus obtained; the average was recorded.' (p. 177) Strengths
|
|
| Notes | Groups were comparable at baseline. No dropouts Study funding sources not described. ('A research grant was obtained.' p. 176) Possible conflicts of interest not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: 'Machinists were randomly assigned.' (p. 177). No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not blinded to the intervention because original samples of the products were supplied. Possible influence on proportion of OIHD. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No information provided on blinding of outcome assessors. |
| Blinding of outcome assessment (detection bias) TEWL and corneometry | Low risk | No influence of blinding is expected for the objective measurement of TEWL. Corneometry was not measured. |
| Incomplete outcome data (attrition bias) Hand‐eczema‐related outcomes | Low risk | No dropouts reported in either group. |
| Incomplete outcome data (attrition bias) Consumer satisfaction | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported. No protocol available. |
| Other bias | Unclear risk |
Design: participants were recruited consecutively (when they started work) over a period of 3 years, in which the work conditions and exposures might have been changed ‐> unclear bias More than one intervention group. This was accounted for in meta‐analysis. The control group was not double‐counted within one meta‐analysis. Baseline imbalances: baseline imbalance regarding turning vs grinding section: controls ‐ 14 vs 9; barrier cream ‐ 8 vs 9; emollient cream ‐ 4 vs 10 ‐> unclear bias Blocked randomisation in unblinded trials: no block randomisation reported Differential diagnostic activity: no different diagnostic activities across study arms |
Halkier‐Sørensen 1993.
| Methods | Individually randomised controlled trial
Cross‐over design, 2 arms Duration: 2x2 weeks 2 follow‐ups (at 2 and 4 weeks or at time of dropout) |
|
| Participants |
Final number completing the 2x2 weeks: N = 70 initially healthy cleaners and kitchen workers Number of participants randomised: N = 111 (N = 56 started with moisturiser; N = 55 started with no treatment) Lost to follow‐up: N = 41 Mean age in years (groups I, II, III): 41 (range 19 to 67) Mean duration of employment (groups I, II, III): 10 (range 1 to 35) years Sex: 110 out of 111 included participants were female. Setting: field study, Denmark |
|
| Interventions | Comparison of the effectiveness of:
in a 2x2 weeks cross‐over design. Application requirements were not described. Locobase, manufactured by Ferndale Laboratories, USA, is a wound and skin emulsion formulation intended for topical application. No details were given about the composition of the cream. |
|
| Outcomes | The study investigators reported the results after grouping all participants in 4 groups according to their ability to complete the 4 week study. Finally, only results of groups I and II were reported. Comparisons of intervention vs no treatment were only reported for corneometry, TEWL, and sum score in groups I and II
Diagnostic criteria for OIHD (degree of certainty for the diagnosis of OIHD): the degree of certainty could not be assessed because no diagnostic criteria for OIHD were described. Scoring system for the severity of OIHD
Strengths
Limitations
Quality of bioengineering methods (Pinnagoda 1990): 'Skin surface temperature (digital thermometer, Ellab type TRD, probe diameter 12 mm), transepidermal water loss (TEWL) (Evaporimeter EPI, Stockholm, Servomed, Sweden), and electrical capacitance (Corneometer CM420, Schwarzhaupt, W. Germany) were measured on both the right and left side and on the volar and dorsal aspect of the distal part of the 3rd finger, middle of the hands, and on the forearms (12 measurement points). The results are presented as overall mean values (the mean values for all measurements on all sites). All probes were hand‐held. The measurements were performed in a separate room with minor draught and protection shields. The mean room temperature was 23°C (20‐26°C) and the mean relative humidity (RH) 34% (25‐42%).' (p. 267). Strengths
|
|
| Notes | The study investigators did not report the results according to the groups randomised, but regrouped all participants in 4 groups according their ability to complete the 4 week study period. Therefore not included in meta‐analysis. Study funding sources: 'The study was supported by the Danish Insurance Association, Copenhagen, L. P. Hansen's fund, Odense, and Danfoss A/S, Nordborg, Denmark.' (p. 270) Possible conflicts of interest not described. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: 'They were randomised into 2 groups' (p. 267). No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not blinded due to study design. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Blinding of outcome assessors was probably not done. Quote: 'Clinical examination and skin physiological measurements were performed on entry...' (p. 267). |
| Blinding of outcome assessment (detection bias) TEWL and corneometry | Unclear risk | No influence of blinding is expected for these objective measurements. |
| Incomplete outcome data (attrition bias) Hand‐eczema‐related outcomes | High risk | N = 23 dropped out in period C after developing severe dryness of the skin, N = 12 violated the protocol or declined to continue the study because they developed or feared to develop hand dermatitis, N = 6 turned up only once, did not find Locobase acceptable, or went on vacation. |
| Incomplete outcome data (attrition bias) Consumer satisfaction | Unclear risk | Dropout analysis did not address this outcome conclusively. |
| Selective reporting (reporting bias) | High risk | Results were not reported according to the original randomisation, but according to the ability of the participants to complete the study period. This was an attempt to deal with the high risk of attrition bias. Consequently, the results were not reported in a way appropriate for an RCT. |
| Other bias | High risk | In‐study use of moisturiser was twice as high in participants who dropped out in the no‐treatment period (N = 23) compared to the participants who completed both parts of the study (2.3 g/person/day versus 1.2 g/person/day). Design: cross‐over ‐> unclear bias Baseline imbalances: pre‐study use of moisturiser differed significantly between the groups despite randomisation (96% versus 82%, P < 0.01). ‐> high risk Blocked randomisation in unblinded trials: no block randomisation reported. Differential diagnostic activity: not applicable because study arms were not evaluated separately. |
Kütting 2010.
| Methods | Cluster‐randomised controlled trial; 18 clusters (enterprises) Parallel design, 4 arms Duration: 12 months 2 follow‐ups at 6 and 12 months |
|
| Participants |
Final number evaluable after 12 months: N = 800 initially healthy metal workers exposed to cutting fluids (group 1: N = 217 in 6 enterprises; group 2: N = 209 in 5 enterprises; group 3: N = 213 in 4 enterprises; control: N = 161 in 3 enterprises) Number of participants randomised: N = 1020 (group 1: N = 263; group 2: N = 253; group 3: N = 258; control: N = 246) Lost to follow‐up: N = 220 (21.6%) withdrawal/exclusion at 2nd follow‐up (group 1: N = 46; group 2: N = 44; group 3: N = 45; control: N = 85) Median age (all included participants): group 1: 40; group 2: 40; group 3: 44,5; control: 42 Sex: male Inclusion criteria
Exclusion criteria:
Setting: German factories mainly of small or medium‐size |
|
| Interventions | Comparison of the effectiveness of skin protection and/or skin care creams vs no recommendation in a 4‐armed trial
Pragmatic approach: 'All participants used the skin care and protection products provided by the employer.' (p. 363). |
|
| Outcomes |
Diagnostic criteria for OIHD (degree of certainty for the diagnosis of OIHD) Strengths
Limitations
Comparability
Scoring system for the severity of OIHD: percentage change of skin score as primary outcome and relative change from baseline as secondary outcome: 'A quantitative skin score was used. In brief, the score comprised all morphological criteria and physiological abnormalities (e.g. dryness) characteristic of hand eczema. Extent, intensity and anatomical site of each type of skin lesion were also recorded.' (pp. 363‐4). 'All three physicians underwent standardized training before using the score. Moreover, during the study period digital photographs of the hands of randomly chosen participants were regularly evaluated and results discussed in order to maintain similarity of visual assessments between observers.' (p. 366). Strengths
Quality of bioengineering methods (Pinnagoda 1990): not relevant |
|
| Notes | Primary intervention: no inclusion of workers with manifest hand eczema (despite high baseline values on their very sensitive score). This was confirmed through correspondence with H Drexler. Study funding sources: 'The study was funded by the German Statutory Accident Insurance (DGUV) and the Franz‐Koelsch‐Stiftung e.V.' (p. 369). Possible conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: 'Randomization was performed on the basis of the 19 included enterprises, assigning each enterprise randomly to one of four study arms.' (p. 363) Apparently, an undescribed method was applied to ensure approximately equal size of the groups despite varying numbers of workers per enterprise. |
| Allocation concealment (selection bias) | Unclear risk | It cannot be excluded that allocation was biased by the results of the baseline screening. Potential confounders differed significantly at baseline. Even though the study investigators do not report significant associations with their outcomes, they did not test the association with review outcome 'proportion of OIHD'. Baseline skin condition was better in the control group, thus allowing less improvement after 1 year compared to other groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding reported; possible to influence proportion of OIHD. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Blinding of participants (= outcome assessors) was probably not done. This would be likely to influence reporting of OIHD, but clear information on blinding is lacking. |
| Blinding of outcome assessment (detection bias) TEWL and corneometry | Unclear risk | No influence of blinding is expected for these objective measurements. |
| Incomplete outcome data (attrition bias) Hand‐eczema‐related outcomes | Unclear risk | 78.4% of recruited participants were available at last follow‐up (1 year), no analysis of dropouts. Attrition in control was 2x as high as in other groups. 'High risk' judgement was therefore considered but discarded because dropout reasons are unknown. |
| Incomplete outcome data (attrition bias) Consumer satisfaction | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Skin score was presented in various ways: percentage change of skin score as primary outcome, categorized percentage change as secondary outcome; further outcomes including absolute change of skin score were reported. This is questionable, but at least the study investigators did not omit their less significant calculations. No protocol available |
| Other bias | High risk |
Design: no correction for cluster randomisation ‐> no bias, but over‐precise results and limited comparability. More than one intervention group. This was accounted for in meta‐analysis. The control group was not double‐counted within one meta‐analysis. Baseline imbalances: significant differences of potential confounders and dyshidrotic eczema, but not of other dermatological disorders. It remains unclear whether this was due to lack of allocation concealment or happened by chance and was worsened by the cluster design ‐> high risk of bias Blocked randomisation in unblinded trials: no block randomisation reported. Differential diagnostic activity: no different diagnostic activities across study arms. |
Löffler 2006.
| Methods | Cluster‐randomised controlled trial; 14 clusters (nursing schools) Parallel groups, 2 arms Duration: 3 years 2 follow‐ups at 1.5 and 3 years |
|
| Participants |
Final number evaluable after 3 years: N = 250 initially healthy 1st‐year nurse apprentices (intervention: N = 121; control N = 129) Number of participants randomised: N = 521 (numbers of participants allocated to groups were not reported) Lost to follow‐up: N = 196 (37.6%; dropout per group was not reported) Excluded from review due to OIHD at beginning of study: only the number of healthy participants who were evaluable were provided (N = 250). This included participants who had OIHD at least at 1 follow‐up before they dropped out. Mean age in years (all included participants): intervention: 20.9 (SD 4.9); control: 23.6 (SD 7.7) Sex: 13% male (intervention), 12% male (control) Mean duration of employment: 0 years, baseline examination was before beginning of the nurse training Inclusion criteria: not described Exclusion criteria: not described Setting: field study in 14 nursing schools (general nurses, paediatric and geriatric nurses, midwifes), Central Germany |
|
| Interventions | Comparison of the effectiveness of a:
The intervention group received regular training (educational lecture with practical parts), and skin care cream (Asche Basis Creme). The lectures took place 3 times in the first year, 2 times in the second and third year. The nurse apprentices were encouraged to use alcoholic hand disinfection instead of hand washing or scrubbing. The effect of applying skin care cream was assessed by the use of a fluorescence technique with the Dermalux‐system as part of the training. Participants in the control group also received the skin care cream. |
|
| Outcomes |
Diagnostic criteria for OIHD (degree of certainty for the diagnosis of OIHD): Irritant Skin changes were recorded using the operational definitions of Uter 1998b. Strengths
Limitations
Comparability
Scoring system for the severity of OIHD: no scores assessed Quality of bioengineering methods (Pinnagoda 1990): not relevant |
|
| Notes | Demographic data and dropout rates were only available for the entire study population of 521 nurse trainees. Study funding sources: 'This work was generously supported by a grant from Asche Chiesi GmbH, Hamburg, Germany.' (p. 207) Possible conflicts of interest: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: 'Randomly divided' (p. 203); details not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants unclear, possible influence on proportion of OIHD. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Evaluation of skin changes was performed by 2 blinded physicians. |
| Blinding of outcome assessment (detection bias) TEWL and corneometry | Unclear risk | No influence of blinding is expected for these objective measurements. |
| Incomplete outcome data (attrition bias) Hand‐eczema‐related outcomes | Low risk | Quote: 'The dropout rate was 37.6% which was not due to skin problems, but due to their absence because of illness at the date of evaluation.' (p. 204) Quote: 'For follow up of dropouts, we performed a telephone interview with the trainee or (if he could not be reached) with his teacher. The aim of this interview was to evaluate, whether or not skin changes were responsible for the dropout.' (p. 204) Low bias because the study investigators convincingly explain that they ensured no dropout was related to hand eczema |
| Incomplete outcome data (attrition bias) Consumer satisfaction | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk |
Design: no correction for cluster randomisation ‐> no bias, but over‐precise results and limited comparability. Baseline imbalances: no imbalances Blocked randomisation in unblinded trials: no block randomisation reported Differential diagnostic activity: no different diagnostic activities across study arms |
Meer 2015.
| Methods | Cluster‐randomised controlled trial; 48 clusters (hospital departments) Parallel design with different recruitment dates, 2 arms Duration: 12 months 4 follow‐ups at 3, 6, 9, and 12 months |
|
| Participants |
Final number evaluable after 12 months: N = 981 initially healthy hospital employees handling irritants during work (intervention: N = 559; control: N = 422) After correction for ICC = 0.005: N = 893 initially healthy hospital employees handling irritants during work (intervention: N = 509; control: N = 384) Number of participants randomised: N = 1649 (intervention: N = 876; control: N = 773) Excluded from review due to OIHD at beginning of study: N = 144 (intervention: N = 64; control: N = 80) Lost to follow‐up: N = 524 (intervention: N = 253; control: N = 271) out of the 1505 initially healthy participants Mean age in years (all included participants): intervention: 40.07 years, SD 11.5; controls: 40.8 years, SD 11.3 Sex: 78.4% female (intervention), 78.3% female (control) Inclusion criteria
Exclusion criteria: not handling irritants during work Setting: field study in 48 hospital departments (different cities in the Netherlands) |
|
| Interventions | Comparison of the effectiveness of:
The multifaceted implementation strategy included participatory working groups, role modes, educational programme including reminders, and a leaflet. The main recommendations were: '1. When there is no visible contamination of the hands, use an alcohol‐based hand disinfectant instead of water and soap to disinfect the hands* 2. Wear gloves when performing wet work 3. Wear cotton undergloves when you wear gloves for longer than 10 min 4. Use a moisturizer on a daily basis to nurse the skin and do not use a body lotion 5. Do not wear jewellery at work 6. Perform as little wet work as possible * This means that the use of disinfectant should be increased and the use of water and soap should be decreased.' (p. 3) All workers who were present at the educational session received a bag with one moisturiser, a pair of cotton undergloves, and two disinfectants (no product names or details reported). The intervention went on for 4 months. The comparison group received only the leaflet. |
|
| Outcomes |
Study investigators did not evaluate healthy skin score separately for participants in incident cases (as defined as review outcome). Diagnostic criteria for OIHD (degree of certainty for the diagnosis of OIHD) Strengths
Limitations
Comparability
Scoring system for the severity of OIHD: 'Workers assessed the health of the skin on their hands by means of the following question: ‘How would you judge the health of your hands/forearms at the moment on a scale from 0 to 10?’ (0, unhealthy skin; 10, healthy skin). This question was based on question D12 of the NOSQ‐2002.' (p. 4) Strengths
Limitations
Quality of bioengineering methods (Pinnagoda 1990): not relevant |
|
| Notes | Demographic data and only reported for the study population including those with hand eczema at baseline. Contingency table and dropouts were calculated based on the raw data received from the study investigators. Study funding sources: 'This study was supported by a grant from the Netherlands Organization for Health Research and Development (ZONMW).' Possible conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: 'Based on the sequence of inclusion, randomisation was performed in strata of two by an independent researcher.' (Meer 2011, p. 3) |
| Allocation concealment (selection bias) | Unclear risk | Not described. Randomisation took place before baseline measurements by an independent researcher and was stratified by cluster‐based criteria. The study investigators excluded 17 out of 1666 baseline responders after randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: 'Workers were not informed about the design of the study and outcome of randomisation.' (pp. 2‐3) No blinding of personnel and department managers is expected to introduce only a low risk. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | OIHD was self‐reported and participants were blinded. |
| Blinding of outcome assessment (detection bias) TEWL and corneometry | Unclear risk | No influence of blinding is expected for these objective measurements. |
| Incomplete outcome data (attrition bias) Hand‐eczema‐related outcomes | Unclear risk | Only superficial analysis of dropouts, reasons for dropout unknown. Quote: ‘Another limitation was that there was a non‐response rate of >30% at the final follow‐up measurement. However, the differences between the baseline values of the non‐responders and the baseline values of the total population were minimal.’ (p. 11) |
| Incomplete outcome data (attrition bias) Consumer satisfaction | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Low risk | Primary study outcome predefined in strategy and reported; secondary study outcomes predefined in strategy. |
| Other bias | Unclear risk |
Design: no correction for cluster randomisation ‐> no bias, but over‐precise results and limited comparability. Baseline imbalances (whole study population including participants with HE at baseline)
Blocked randomisation in unblinded trials: no block randomisation reported Differential diagnostic activity: study investigators speculate that participants of the intervention group are more aware of HE and were therefore more likely to report them: 'The educational session given during the intervention period might have increased awareness among the participants in the intervention group concerning their hand eczema symptoms. They may have evaluated their symptoms — which might have already been present at baseline — differently after the educational session. This might have led to an increase in hand eczema reports at follow‐up as compared with the control group that may be independent of a change in clinical status.' (p. 10) |
Perrenoud 2001a.
| Methods | Individually randomised controlled trial
Cross‐over design, 2 arms Duration: 2 x 2 weeks Follow‐ups: day 12, day 15, day 26, day 29 |
|
| Participants |
Final number evaluable after 2x2 weeks: N = 16 initially healthy 2nd year apprentice hairdressers (numbers allocated to groups not reported) Number of participants randomised: N = 21 (numbers allocated to groups not reported) Lost to follow‐up: N = 5 (numbers allocated to groups not reported) Sex: 20 female out of 21 included participants Median age (all included participants): 18 (range 16 to 30) Atopy: 1 had a probable and 3 had a possible atopic disposition Inclusion criteria: at least 5 times shampooing per day without gloves Exclusion criteria: hand dermatitis Setting: field study with apprentices from the main regional occupational training centre, Geneva, Switzerland |
|
| Interventions | Comparison of the effectiveness of:
The participants were randomised to start either with barrier cream (Excipial protect) or with its vehicle. Excipial protect contains aluminium hydroxychloride and glycerine. Glycerine promotes water retention in the skin and the aluminium salt reduces excess sweating. The vehicle was designed specifically for skin care for occupational users. The first cream was applied 5 days a week for 2 weeks with a washout period of 2 days followed by another 2‐week treatment period with the second cream. |
|
| Outcomes |
Participants were assessed at the beginning at day 12, day 15, day 26, and day 29. The primary outcome measures were the proportion of irritant skin changes (score defined by study investigators). The secondary outcome measures were the amount of skin barrier impairment (TEWL), skin hydration (corneometry), and skin colour (chromometry) in the groups. A further secondary outcome measure was the subjective opinion of the participants concerning different features of the creams (ease of use, consistency, oiliness, protective effect, tolerance, general aspects). Diagnostic criteria for OIHD (degree of certainty for the diagnosis of OIHD): not relevant Scoring system for the severity of OIHD: three scores were assessed: Dryness, redness, breaks in the skin. Scale of 0 to 3 (0 = none, 1 = mild, 2 = strong, 3 = maximum). Strengths
Limitations
Quality of bioengineering methods (Pinnagoda 1990) 'The biometric measurements were taken on the back of the dominant hand. The following instruments
Strengths
Limitations
|
|
| Notes | Not included in meta‐analysis because no quantitative data reported. Study funding sources: not described Possible conflicts of interest: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: 'The subjects were randomly assigned.' No further information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The participants were blinded: They received 3x 50 g tubes with identical markings at the beginning of the study and another 3 tubes after 2 weeks. Comment: Probably done |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote: 'Double‐blind' (p. 135). Participants, and outcome assessors were blinded. Comment: Probably done |
| Blinding of outcome assessment (detection bias) TEWL and corneometry | Low risk | No influence of blinding is expected for these objective measurements. |
| Incomplete outcome data (attrition bias) Hand‐eczema‐related outcomes | Unclear risk | 5 out of 21 apprentices dropped out during only 4 weeks. The study investigators stated that the dropouts were 'for reasons not related to the study. Their withdrawal did not effect the balance between the 2 groups to which they had been assigned' (p. 134). No further details provided. Dropout reasons unknown although it is doubtful they were truly unrelated to the study or outcome. |
| Incomplete outcome data (attrition bias) Consumer satisfaction | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Results concerning primary outcome measure was only summarised briefly. Quote: 'Clinical scores were generally very low... under either cream' (p. 136), but not reported separately for verum and vehicle. |
| Other bias | Unclear risk | Application frequency of barrier cream and vehicle unclear. Design: cross‐over ‐> no impact because no quantitative data for proportion of HE provided Baseline imbalances: comparability of groups at baseline unclear, no details given. Blocked randomisation in unblinded trials: no block randomisation reported Differential diagnostic activity: no different diagnostic activities across study arms |
g: gram HE: hand eczema ITT: intention‐to‐treat N: number OIHD: occupational irritant hand dermatitis RCT: randomised controlled trial SD: standard deviation TEWL: transepidermal water loss
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amphoux 1975 | Double‐blind CCT: inclusion of workers with hand dermatitis (intervention group: 21.0%, control group 21.4%), no separate data for healthy workers available. No inclusion of dropouts and withdrawals in the analysis. |
| Arbogast 2004 | RCT: only workers with compromised skin were included. Outcome: secondary prevention of OIHD. |
| Bauer 2002 | CCT: quality criteria for quasi‐RCT not fulfilled. No blinding of participants, clinicians and outcome assessors. |
| Berndt 2000 | RCT: only workers with compromised skin were included. Outcome: secondary prevention of OIHD. |
| Bolam 1971 | Not randomised, but groups were similar. Housewives. |
| Bregnhøj 2012 | Not randomised. No primary intervention (8 out of 397 apprentices showed HD at inclusion). |
| Brown 2007 | Qualitative study, intervention implementation research, no quantitative data available. |
| Davis 2005 | RCT: no field study, laboratory experiment. |
| Dobson 1979 | RCT: effect of industrial hand cleansers on TEWL was evaluated. This type of intervention was not considered in this review. No data on OIHD, only outcome is TEWL. |
| Frosch 2003 | RCT: no separate data for healthy dental technicians available. Only 5 laboratories were randomised to 4 products; only partial cross‐over‐design (2 out of 4 products were tested in the same laboratory). |
| Glantz 1976 | Probably not randomised. Under field conditions it was only investigated whether or not the protective ointment had a negative influence on the handling of dental instruments. The protective effects were only measured in a laboratory experiment. |
| Held 2001 | CCT: inclusion of apprentices with hand dermatitis (intervention group 25%, control group 20%), no separate data for healthy apprentices available. No blinding of participants and clinicians, blinding of outcome assessors unclear. |
| Held 2002 | RCT: inclusion of workers with hand dermatitis (intervention group 25%, control group 30% with two or more of the following symptoms: redness, vesicles, papules, itching, scaling, dryness, fissuring, rough and thickened, or suppurate skin changes), no separate data for healthy workers available. |
| McCormick 2000 | RCT: only workers with compromised skin were included. Outcome: secondary prevention of OIHD. |
| Mody 2003 | RCT: the objectives were to investigate the impact of an alcohol‐based hand rub on knowledge, compliance, and hand colonisation of healthcare workers. The prevention of OIHD was not addressed. |
| Perrenoud 2001b | RCT in an intensive care unit: unclear if only healthy participants were included, only preliminary data available. No quantitative data on OIHD available. |
| Schwanitz 2003 | CCT: inclusion of apprentices with hand dermatitis (intervention group 13.7%, control group 27.8%), no separate data for healthy apprentices available. No blinding of participants, clinicians, and outcome assessors. No information on loss to follow‐up. |
| Sell 2005 | CCT: inclusion of workers with hand dermatitis (12%), data on healthy workers were provided, but study could not be included because of violation of quality criteria (no blinding of participants, clinicians, and outcome assessors). |
| Winker 2009 | RCT: Primary and secondary intervention mixed. Winker answered to our emails, but did not provide the requested data for initially healthy participants (primary prevention). He stated that the number of cases (HE) would be too small to detect significant differences between groups. This is probably true, but may introduce reporting bias to our review. Their study included 456 participants without and 27 with HE at baseline. At last follow‐up, 24 participants had eczema. |
| Winnefeld 2000 | RCT: Comparison of a non‐medicated soap vs an alcohol‐based hand rinse: This type of intervention was not considered in this review. Primary outcome proportion of OIHD was not assessed. Only 8 days of duration. |
CCT: controlled clinical trial HE: hand eczema RCT: randomised controlled trial OIHD: occupational irritant hand dermatitis TEWL: transepidermal water loss
Characteristics of studies awaiting assessment [ordered by study ID]
Visscher 2014.
| Methods | Randomised controlled trial Parallel design, 2 arms Duration: 4 weeks 3 follow‐ups at 1, 2, and 4 weeks |
| Participants |
Number of participants randomised: N = 63 intensive care HCWs (over 60% with knuckle dryness and erythema scores of 2 at baseline) Setting: hospital |
| Interventions | 'The objective was to determine the effects on hand skin condition of
'Application was 33 daily.' |
| Outcomes | 'The primary outcome was skin condition measured as
after 1, 2, and 4 weeks.' |
| Notes | Conference publication, only abstract available. 'There was significant irritant dermatitis at baseline as over 60% of HCWs had knuckle dryness and erythema scores of 2.' This trial will only be eligible if the data on OIHD is available in a dichotomised form and separately provided for HCWs without OIHD at baseline. |
Characteristics of ongoing studies [ordered by study ID]
Madan 2016.
| Trial name or title | A behavioural change package to prevent hand dermatitis in nurses working in the national health service (the SCIN trial) |
| Methods | Cluster‐randomised controlled trial (35 sites) Parallel design, 4 arms Duration: 12 months |
| Participants | Nurses working in the National Health Service (NHS) who are particularly at risk Focus on two groups of staff:
Sample size: 'Field workers will be encouraged to recruit as many eligible student nurses and ICU nurses as possible, with the aim of recruiting at least 40 student nurses and 40 nurses from the ICUs at each site.' Setting: '12 NHS acute hospital trusts/health boards which provide OH care to both student and ICU nurses, 18 NHS trusts which provide OH care to ICU nurses and 5 university OH departments which provide OH care to student nurses' |
| Interventions | Comparison of the effectiveness of:
|
| Outcomes |
|
| Starting date | 'At the time of submission, the main trial has been underway since September 2014, with final recruitment planned for March 2016.' |
| Contact information | ira.madan@kcl.ac.uk |
| Notes | Inclusion and exclusion criteria might be refined. It is unclear whether or not nurses with hand dermatitis present at baseline will be included. Study funding sources: The SCIN Trial is funded by the National Institute of Health Research, Health Technology Assessment (HTA) programme grant: NIHR grant number 11/94/01. The trial is sponsored by the National Institute for Health Research (NIHR) Biomedical Research Centre based as Guy's and St Thomas' NHS Foundation Trust and the UKCRC‐registered King's Clinical Trials Unit at King's College London. Possible conflicts of interest: 'There are no competing interests to declare by any of the authors.' |
Soltanipoor 2016.
| Trial name or title | The healthy hands project: Effectiveness of a skincare programme for the prevention of contact dermatitis in healthcare workers |
| Methods | Cluster‐randomised controlled trial Parallel design, 2 arms Duration: 18 months Questionnaire every 6 months |
| Participants |
Number of participants randomised: healthcare workers (N unknown, 34 wards) Setting: University Medical Center |
| Interventions | 'The experimental intervention will comprise provision of hand creams in dispensers at the wards, with regular training and feedback, including reports of the electronically monitored consumption. Both the experimental and control groups will receive basic education on skin protection (as care as usual).' |
| Outcomes | 'The primary outcome is the change in Hand Eczema Severity Index from baseline to 12 months. The secondary outcomes are the natural moisturizing factor levels in the stratum corneum as a biomarker of skin barrier damage, and the total consumption of creams per ward.' |
| Starting date | Probably 2016 |
| Contact information | Not known |
| Notes | Conference publication, only abstract available Unknown whether purely primary prevention |
Differences between protocol and review
The protocol was completed in 2003, therefore predating the Cochrane 'Risk of bias' tool. This update was conducted applying the current version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), which required some substantial amendments and modifications. The applied methods were described more clearly in order to comply with theHandbook (Higgins 2011):
Assessment of risk of bias in included studies was done according to Higgins 2011. The protocol referred to Juni 2001.
Unit of analysis issues were addressed more clearly according to Higgins 2011. They were not mentioned in the protocol.
We clarified how heterogeneity was assessed in the Assessment of heterogeneity section of the review. This was not mentioned in the protocol.
We clarified in the Assessment of reporting biases section that we planned to create funnel plots but that they could not be applied due to the small number and methodological heterogeneity of included trials. Reporting bias was not addressed in the protocol.
Data synthesis: the protocol did not define how to deal with multiple outcome data. When this became apparent during data extraction we decided to extract data from the last available time point of each study. Apart from timing, multiple outcome data were not an issue in the included studies.
We have defined our key outcomes (primary outcomes 1 and 2) and presented them in one 'Summary of findings' table for each preventive intervention. The protocol did not address key outcomes.
GRADE methods and 'Summary of findings' tables were applied in this update. These tools were not available when the protocol was completed.
Several changes from the protocol were predefined in the proposal (the title registration form) of this update and were adopted in the update:
Terminology: in the protocol different terms for OIHD were used, which was leading to confusion. In the proposal of the update only OIHD was used, except when referring to the terminology applied in study reports.
We clarified the inclusion criteria and have stated more clearly throughout the review that we include only primary prevention studies and not studies where participants had existing OIHD, unless the participant population was mixed and disaggregated data was presented for those participants who were healthy with no OIHD at the start of the study.
The protocol allowed for the inclusion of quasi‐RCTs. We only considered RCTs for this update.
The protocol listed five categories of interventions. Before starting the update, the comparison of behavioural and psychological interventions versus no intervention was added to the types of interventions to be reviewed. However, these are elements of the existing category 'skin protection education', not a separate category. Accordingly, the Types of interventions section of this review still lists only the five categories that were specified in the protocol.
We defined the outcomes under investigation more clearly and numerated them differently (compare Primary outcomes; Secondary outcomes).
The protocol had only one primary outcome: 'Proportion of participants developing any signs and symptoms of hand dermatitis (incident cases) as rated by the investigators (physician/nurse) or the participants.' We amended the definition.
We added a second primary outcome (concerning adverse effects).
We added a more precise definition of the secondary outcome concerning clinical course of signs and symptoms of hand dermatitis.
We modified the secondary outcome concerning adverse effects, to cover only less severe adverse effects that are not addressed as a primary outcome.
The following changes from the protocol were also necessary.
We did not identify any eligible trials in the predefined comparison of complex interventions using barrier creams, moisturisers, and protective gloves versus no intervention. Instead, we analysed the effects of the combination of barrier creams and moisturisers versus no intervention.
The protocol did not clarify which meta‐analyses were planned. In this update, meta‐analyses were only undertaken for the main outcome, OIHD, because there were not sufficient comparable data for the meta‐analysis of other outcome
The protocol predefined sensitivity analyses for investigating the impact of lower‐quality studies and the potential impact of missing data. In order to avoid excessive subjective judgment, we clarified that we would only investigate the following methodological weaknesses in sensitivity analyses: cluster design without correction, split‐body or cross‐over design, and reporting only minor signs and symptoms of OIHD (as opposed to manifest OIHD). All but one study had missing data due to attrition. Dropout rates were sometimes considerably higher than cases of OIHD so that applying worst‐ or best‐case scenarios would have introduced a large bias. Data needed for other types of ITT assumptions were not available. We therefore did not perform any ITT calculations and missing data was dealt with descriptively.
No standardised mean difference could be calculated for continuous outcomes because the studies did not report sufficient data. These results were reported descriptively in the review.
We planned to do subgroup analyses (at less than, and greater than 30 years of age; sex; atopy; occupation). In this review, we did not analyse subgroups of trials because we could not perform meta‐analysis for most outcomes, or too few trials were included per comparison. We did not analyse subgroups of participants either, because such data were not available. Subgroups were assessed descriptively only.
Search methods: we did not correspond with authors and pharmaceutical companies to identify unpublished or ongoing trials, or grey literature. Instead we expanded the number of trials registers we searched in line with current Cochrane Skin methods.
We stated in the protocol that we would calculate odds ratios (ORs) as the measure of the treatment effect. However, we chose to report relative risk (RR) instead for the meta‐analyses in this and future review versions because odds ratios are more difficult to understand and often misinterpreted as risk ratios (Higgins 2011). This decision was made without considering any results which would have occurred by using odds ratios.
Split‐body designs were eligible for this update.
For this update, other review authors than predefined in the protocol performed the study selection and extraction.
Contributions of authors
AB was the contact person with the editorial base. AB and HR co‐ordinated contributions from the authors. HR worked on the methods section. AB and HR obtained data on ongoing and unpublished studies. HR, PE, AB, and HCW independently screened papers against eligibility criteria. HR, JL, DD, MLS, and AB extracted data for the review. HR, DD, AB, and MLS appraised the risk of bias of the included trials. HR sought additional information about papers, entered data into Review Manager, and analysed and interpreted the data. SMJ assessed the diagnostic criteria for the outcomes of the included studies and worked on the final draft of the review. AB, HR, and HCW judged the quality of the evidence. AB and HR wrote the final draft of the review.
CB worked with AB on the previous published version of the review, provided data extraction templates, advised on the update, and provided critical appraisal of the draft review.
HR responded to the comments of the referees. HCW gave advice concerning the revisions and the final draft of the review. AB is the guarantor of the update.
Disclaimer
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
Sources of support
Internal sources
-
TU Dresden, Germany.
The TU Dresden provided computer workplaces and access to literature.
-
Cochrane Skin Group, UK.
The CSG provided financial support and accompanied the entire review process.
External sources
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
Andrea Bauer: "I have received lecture fees from manufacturers of skin care and protection products (Spirig, Astellas, GSK, and La Roche‐Posay). I was the chief investigator (lead author) of an excluded study (Bauer 2002)."
Henriette Rönsch: none known.
Peter Elsner: "I was the co‐author of an excluded study (Bauer 2002)."
Daan Dittmar: none known.
Cathy Bennett: "I am the proprietor of Systematic Research Ltd., and received a consultancy fee for my work on this review."
Marie‐Louise A Schuttelaar: none known.
Judit Lukács: none known.
Swen Malte John: "I have received lecture fees from Galderma, Admirall, and Biogen‐Idec."
Hywel C Williams: "I have helped a team led by Dr Ira Madan in London to design and deliver a national clinical trial to prevent occupational irritant hand dermatitis in nurses, which is due to start recruiting in late 2014. The study will probably report in 2017 or 2018, and at some point will be eligible for inclusion in this systematic review future update. The trial tests the behavioural package alongside supply of emollients at work. I was the co‐author of an excluded study (Bauer 2002)."
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Brüning 2008 {published data only}
- Brüning T, Goldscheid N, Haufs M, Merget R, Pesch B, Schöneweis S, et al. Intervention study 'Skin Protection'. Comparative investigation to test the effectiveness of skin protection preparations in line with the three‐step skin protection program ‐ hazard analysis and protective measures [Interventionsstudie „Hautschutz“. Vergleichende Untersuchung zur Überprüfung der Wirksamkeit von Hautschutzpräparaten im Rahmen des dreistufigen Hautschutzplanes ‐ Gefährdungsanalyse und Schutzmaßnahme; Erratum 04.05.2016]. In: BGFA – Forschungsinstitut für Arbeitsmedizin der Deutschen Gesetzlichen Unfallversicherung editor(s). BGFA‐Report 1. Bochum: Institut der Ruhr‐Universität Bochum, 2008 and Erratum 2016. [Google Scholar]
- Dickel H, Pesch B, Leiste A, Schoeneweis S, Haufs M, Taeger D, et al. Occupational study of the effectiveness of barrier creams and moisturizers. Contact Dermatitis 2006;55(Suppl 1):39. [CENTRAL: CN‐00616076] [Google Scholar]
- Leiste A, Dickel H, Pesch B, Taeger D, Goldscheid N, Haufs M, et al. Efficacy of barrier creams and skin care ointments in employees exposed to cooling agents. A randomised controlled intervention study [Die Wirksamkeit von Hautschutz ‐ und Hautpflegemitteln unter Kühlschmierstoffexposition ‐ Eine randomisierte kontrollierte Interventionsstudie]. Journal der Deutschen Dermatologischen Gesellschaft (JDDG) 2007;5(Suppl 2):S186‐7. [Google Scholar]
Duca 1994 {published data only}
- Duca PG, Pelfini G, Ferguglia G, Settimi L, Peverelli C, Sevosi I, et al. Efficacy of barrier creams in preventing skin complaints in workers of a fabric dyeing and printing factory. Results of a random experiment. [Efficia dell'uso di creme‐barriera nel prevenire affezioni dermatologiche in lavoratori delle tintostamperie: Risultati di una sperimentatione randomizzata]. La Medicina del Lavoro 1994;85(3):231‐8. [CENTRAL: CN‐00169944] [PubMed] [Google Scholar]
Flyvholm 2005 {published data only (unpublished sought but not used)}
- Flyvholm MA, Mygind K, Sell L, Jensen A, Jepsen KF. A randomised controlled intervention study on prevention of work related skin problems among gut cleaners in swine slaughterhouses. Occupational and Environmental Medicine 2005;62(9):642‐9. [CENTRAL: CN‐00523951] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mygind K, Borg V, Flyvholm MA, Sell L, Frydendall Jepsen K. A study of the implementation process of an intervention to prevent work‐related skin problems in wet‐work occupations. International Archives of Occupational and Environmental Health 2006;79(1):66‐74. [CENTRAL: CN‐00561164] [DOI] [PubMed] [Google Scholar]
Goh 1994 {published data only}
- Goh CL, Gan SL. Efficacies of a barrier cream and an after work emollient cream against cutting fluid dermatitis in metalworkers: A prospective study. Contact Dermatitis 1994;31(3):176‐80. [PUBMED: 7821012] [DOI] [PubMed] [Google Scholar]
Halkier‐Sørensen 1993 {published data only}
- Halkier‐Sørensen L, Thesterup‐Pedersen K. The efficacy of a moisturizer (Locobase) among cleaners and kitchen assistants during everyday exposure to water and detergents. Contact Dermatitis 1993;29(5):266‐71. [CENTRAL: CN‐00099282] [DOI] [PubMed] [Google Scholar]
Kütting 2010 {published data only}
- Kütting B, Baumeister T, Weistenhöfer W, Pfahlberg A, Uter W, Drexler H. Effectiveness of skin protection measures in prevention of occupational hand eczema: Results of a prospective randomized controlled trial over a follow‐up period of 1 year. British Journal of Dermatology 2010;162(2):362‐70. [CENTRAL: CN‐00752880] [DOI] [PubMed] [Google Scholar]
Löffler 2006 {published and unpublished data}
- Löffler H, Bruckner T, Diepgen T, Effendy I. Primary prevention in health care employees: A prospective intervention study with a 3‐year training period. Contact Dermatitis 2006;54(4):202‐9. [CENTRAL: CN‐00564898] [DOI] [PubMed] [Google Scholar]
Meer 2015 {published and unpublished data}
- Meer EW, Boot CR, Jungbauer FH, Klink JJ, Rustemeyer T, Coenraads PJ, et al. Hands4U: a multifaceted strategy to implement guideline‐based recommendations to prevent hand eczema in health care workers: design of a randomised controlled trial and (cost) effectiveness evaluation. BMC Public Health 2011;11(669):1‐11. [CENTRAL: CN‐00806279] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meer EW, Boot CR, Twisk JW, Coenraads PJ, Jungbauer FH, Gulden JW, et al. Hands4U: The effectiveness of a multifaceted implementation strategy on behaviour related to the prevention of hand eczema ‐ A randomised controlled trial among healthcare workers. Occupational and Environmental Medicine 2014;71(7):492‐9. [CENTRAL: CN‐00996155] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meer EW, Boot CR, Gulden JW, Knol DL, Jungbauer FH, Coenraads PJ, et al. Hands4U: the effects of a multifaceted implementation strategy on hand eczema prevalence in a healthcare setting. Results of a randomized controlled trial. Contact Dermatitis 2015;72(5):312‐24. [CENTRAL: CN‐01254728] [DOI] [PubMed] [Google Scholar]
- Meer EW, Dongen JM, Boot CR, Gulden JW, Bosmans JE, Anema JR. Economic evaluation of a multifaceted implementation strategy for the prevention of hand eczema among healthcare workers in comparison with a control group: the Hands4U Study. Acta Dermato‐Venereologica 2016;96(4):499‐504. [CENTRAL: CN‐01178190] [DOI] [PubMed] [Google Scholar]
Perrenoud 2001a {published data only}
- Perrenoud D, Gallezot D, Melle G. The efficacy of a protective cream in a real‐world apprentice hairdresser environment. Contact Dermatitis 2001;45(3):134‐8. [CENTRAL: CN‐00770005] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Amphoux 1975 {published data only}
- Amphoux M, Robin J. Double blind test with a protective ointment (Ivosin) on the hands of cement workers [Doppelblindversuch mit einer Schutzsalbe (Ivosin) an den Händen von Zementarbeitern]. Berufs‐Dermatosen 1975;23(6):214‐26. [CENTRAL: CN‐00705625] [PubMed] [Google Scholar]
Arbogast 2004 {published data only}
- Arbogast JW, Fendler EJ, Hammond BS, Cartner TJ, Dolan MD, Ali Y, et al. Effectiveness of a hand care regimen with a moisturizer in manufacturing facilities where the workers are prone to occupational irritant dermatitis. Dermatitis 2004;15(1):10‐7. [PUBMED: 15573643] [DOI] [PubMed] [Google Scholar]
Bauer 2002 {published data only}
- Bauer A, Kelterer D, Bartsch R, Schlegel A, Pearson J, Stadeler M, et al. Prevention of hand dermatitis in bakers' apprentices: different efficacy of skin protection measures and UVB hardening. International Archives of Occupational and Environmental Health 2002;75(7):491‐9. [PUBMED: 12172896] [DOI] [PubMed] [Google Scholar]
Berndt 2000 {published data only}
- Berndt U, Wigger‐Alberti W, Gabard B, Elsner P. Efficacy of a barrier cream and its vehicle as protective measures against occupational irritant contact dermatitis. Contact Dermatitis 2000;42(2):77‐80. [CENTRAL: CN‐00275795] [DOI] [PubMed] [Google Scholar]
Bolam 1971 {published data only}
- Bolam RM, Hepworth R, Bowerman LT. In‐use evaluation of safety to skin of enzyme‐containing washing products. British Medical Journal 1971;2(5760):499‐501. [CENTRAL: CN‐00005787] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bregnhøj 2012 {published data only}
- Bregnhøj A, Menné T, Johansen JD, Søsted H. Prevention of hand eczema among Danish hairdressing apprentices: an intervention study. Occupational and Environmental Medicine 2012;69(5):310‐6. [PUBMED: 22267449] [DOI] [PubMed] [Google Scholar]
Brown 2007 {published data only}
- Brown TP, Rushton L, Williams HC, English JS. Intervention implementation research: An explorative study of reduction strategies for occupational contact dermatitis in the printing industry. Contact Dermatitis 2007;56:16‐20. [DOI] [PubMed] [Google Scholar]
Davis 2005 {published data only}
- Davis DD, Harper RA. Using gloves coated with a dermal therapy formula to improve skin condition. Association of periOperative Registered Nurses (AORN) Journal 2005;81:157‐66. [DOI] [PubMed] [Google Scholar]
Dobson 1979 {published data only}
- Dobson RL. Evaluation of hand cleansers. Contact Dermatitis 1979;5(5):305‐7. [CENTRAL: CN‐00568793] [DOI] [PubMed] [Google Scholar]
Frosch 2003 {published data only}
- Frosch PJ, Peiler D, Grunert V, Grunenberg B. Efficacy of barrier creams in comparison to skin care products in dental laboratory technicians ‐ A controlled trial. Journal der Deutschen Dermatologischen Gesellschaft [Journal of the German Society of Dermatology] 2003;1(7):547‐57. [CENTRAL: CN‐00474825] [DOI] [PubMed] [Google Scholar]
Glantz 1976 {published data only}
- Glantz PO, Larsson K, Nyquist G. A new skin protecting ointment against acrylic resins. Odontologisk Revy 1976;27(4):265‐72. [PUBMED: 138105] [PubMed] [Google Scholar]
Held 2001 {published data only}
- Held E, Wolff C, Gyntelberg F, Agner T. Prevention of work‐related skin problems in student auxiliary nurses. Contact Dermatitis 2001;44(5):297‐303. [CENTRAL: CN‐00347186] [DOI] [PubMed] [Google Scholar]
Held 2002 {published data only}
- Held E, Mygind K, Wolff C, Gyntelberg F, Agner T. Prevention of work related skin problems: An intervention study in wet work employees. Occupational and Environmental Medicine 2002;59(8):556‐61. [PUBMED: 12151613] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCormick 2000 {published data only}
- McCormick RD, Buchman TL, Maki DG. Double‐blind, randomized trial of a scheduled use of a novel barrier cream and an oil‐containing lotion for protecting hands of health care workers. American Journal of Infection Control 2000;28(4):302‐10. [CENTRAL: CN‐00298736] [DOI] [PubMed] [Google Scholar]
Mody 2003 {published data only}
- Mody L, McNeil SA, Sun R, Bradley SF, Kauffman C. Introduction of a waterless alcohol‐based hand rub in a long‐term‐care facility. Infection control and hospital epidemiology 2003;24(3):165‐71. [CENTRAL: CN‐00436468] [DOI] [PubMed] [Google Scholar]
Perrenoud 2001b {published data only}
- Perrenoud D, Gallezot D, Lübbe J, Akapko C, Ruffieux C, Melle G, et al. Effect of a protective cream against skin irritation associated with hand hygiene practices: preliminary report. Occupational and Environmental Medicine 2001;49(1 A):91‐4. [CENTRAL: CN‐00424757] [Google Scholar]
Schwanitz 2003 {published data only}
- Schwanitz HJ, Riehl U, Schlesinger T, Bock M, Skudlik C, Wulfhorst B. Skin care management: Educational aspects. International Archives of Occupational and Environmental Health 2003;76(5):374‐81. [PUBMED: 12719982 ] [DOI] [PubMed] [Google Scholar]
Sell 2005 {published data only}
- Sell L, Flyvholm MA, Lindhard G, Mygind K. Implementation of an occupational skin disease prevention programme in Danish cheese dairies. Contact Dermatitis 2005;53(3):155‐61. [PUBMED: 16128755] [DOI] [PubMed] [Google Scholar]
Winker 2009 {published data only}
- Winker R, Salameh B, Stolkovich S, Nikl M, Barth A, Ponocny E, et al. Effectiveness of skin protection creams in the prevention of occupational dermatitis: Results of a randomized, controlled trial. International Archives of Occupational & Environmental Health 2009;82(5):653‐62. [CENTRAL: CN‐00705763] [DOI] [PubMed] [Google Scholar]
Winnefeld 2000 {published data only}
- Winnefeld M, Richard MA, Drancourt M, Grob JJ. Skin tolerance and effectiveness of two hand decontamination procedures in everyday hospital use. British Journal of Dermatology 2000;143(3):546‐50. [CENTRAL: CN‐00332205] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Visscher 2014 {published data only}
- Visscher MO, DiMuzio A, Jones J, Adams L, Billhimer W. Effect of pseudoceramide cream on irritant hand dermatitis in health care workers. Journal of the American Academy of Dermatology 2014;70(5 Suppl 1):AB68. [EMBASE: 71390362] [Google Scholar]
References to ongoing studies
Madan 2016 {published data only}
- Madan I, Parsons V, Cookson B, English J, Lavender T, McCrone P, et al. A behavioural change package to prevent hand dermatitis in nurses working in the national health service (the SCIN trial): Study protocol for a cluster randomised controlled trial. Trials 2016;17(1):145. [PUBMED: 26987818] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan I, Parsons V, Cookson B, English J, Lavender T, McCrone P, et al. The SCIN (Skin Care Intervention in Nurses) trial: A cluster randomized trial. Contact Dermatitis 2016;75:101‐2. [EMBASE: 612248209] [Google Scholar]
Soltanipoor 2016 {published data only}
- Soltanipoor M, Hines J, Sluiter J, Kezic S, Rustemeyer T. The Healthy Hands Project: Effectiveness of a skincare programme for the prevention of contact dermatitis in healthcare workers. Contact Dermatitis 2016;75:100. [EMBASE: 612248185] [Google Scholar]
Additional references
Agner 1991
- Agner T. Basal transepidermal water loss, skin thickness, skin blood flow and skin colour in relation to sodium‐lauryl‐sulfate‐induced irritation in normal skin. Contact Dermatitis 1991;25(2):108‐14. [PUBMED: 1935039] [DOI] [PubMed] [Google Scholar]
Bauer 1997
- Bauer A, Seidel A, Bartsch R, Wollina U, Gebhardt M, Diepgen TL. Development of skin damage during vocational training in occupations with skin hazards [Entwicklung von Hautproblemen bei Berufsanfängern in Hautrisikoberufen]. Allergologie 1997;20(4):179‐83. [EMBASE: 1997155320] [Google Scholar]
Bauer 1998
- Bauer A, Bartsch R, Stadeler M, Schneider W, Grieshaber R, Wollina U, et al. Development of occupational skin diseases during vocational training in baker and confectioner apprentices: A follow up study. Contact Dermatitis 1998;39(6):307‐11. [PUBMED: 9874022] [DOI] [PubMed] [Google Scholar]
Bauer 2001
- Bauer A, Bartsch R, Hersmann C, Stadeler M, Kelterer K, Schneider W, et al. Occupational hand dermatitis in food industry apprentices: Results of a three year follow up cohort study. International Archives of Occupational and Environmental Health 2001;74(6):437‐42. [PUBMED: 11563607] [DOI] [PubMed] [Google Scholar]
Bock 2001
- Bock M, Wulfhorst B, Gabard B, Schwanitz HJ. Efficacy of skin protection creams for the treatment of irritant dermatitis in hairdresser apprentices. Occupational and Environmental Medicine 2001;49(1A):73‐6. [EMBASE: 2001126305] [Google Scholar]
Boehm 2012
- Boehm D, Schmid‐Ott G, Finkeldey F, John SM, Dwinger C, Werfel T, et al. Anxiety, depression and impaired health‐related quality of life in patients with occupational hand eczema. Contact Dermatitis 2012;67(4):184‐92. [PUBMED: 22564098] [DOI] [PubMed] [Google Scholar]
Boman 1989
- Boman A, Mellström GA. Percutaneous absorption of 3 organic solvents in the guinea pig. (III). Effect of barrier creams. Contact Dermatitis 1989;21(3):134‐40. [PUBMED: 2791538] [DOI] [PubMed] [Google Scholar]
Brans 2016
- Brans R, Skudlik C, Weisshaar E, Scheidt R, Ofenloch R, Elsner P, et al. Multicentre cohort study 'Rehabilitation of Occupational Skin Diseases ‐ Optimization and Quality Assurance of Inpatient Management (ROQ)': results from a 3‐year follow‐up. Contact Dermatitis 2016;75(4):205‐12. [PUBMED: 27356809] [DOI] [PubMed] [Google Scholar]
Burnett 1998
- Burnett CA, Lushniak BD, McCarthy W, Kaufman J. Occupational dermatitis causing days away from work in U.S. private industry, 1993. American Journal of Industrial Medicine 1998;34(6):568‐73. [PUBMED: 9816414] [DOI] [PubMed] [Google Scholar]
Cherry 2000
- Cherry N, Meyer JD, Adisesh A, Brooke R, Owen‐Smith V, Swales C, et al. Surveillance of occupational skin disease: EPIDERM and OPRA. British Journal of Dermatology 2000;142(6):1128‐34. [PUBMED: 10848735] [DOI] [PubMed] [Google Scholar]
Coenraads 1998
- Coenraads PJ, Diepgren TL. Risk for hand eczema for employees with past or present atopic dermatitis. International Archives of Occupational and Environmental Health 1998;71(1):7‐13. [PUBMED: 9523243] [DOI] [PubMed] [Google Scholar]
Coenraads 2003
- Coenraads PJ, Diepgen TL. Problems with trials and intervention studies on barrier creams and emollients at the workplace. International Archives of Occupational and Environmental Health 2003;76(5):362‐6. [PUBMED: 12768427] [DOI] [PubMed] [Google Scholar]
Cvetkovski 2005
- Cvetkovski RS, Rothman KJ, Olsen J, Mathiesen B, Iversen L, Johansen JD, et al. Relation between diagnoses on severity, sick leave and loss of job among patients with occupational hand eczema. British Journal of Dermatology 2005;152(1):93‐8. [PUBMED: 15656807] [DOI] [PubMed] [Google Scholar]
De Paepe 2000
- Paepe K, Derde MP, Roseeuw D, Rogier V. Claim substantiation and efficacy of hydrating body lotions and protective creams. Contact Dermatitis 2000;42(4):227‐34. [PUBMED: 10750855] [DOI] [PubMed] [Google Scholar]
DGUV 2008
- DGUV (Deutsche gesetzliche Unfallversicherung). Business report of the German Statutory Accident Insurances 2008. In: Deutsche Gesetzliche Unfallversicherung editor(s). Geschäfts‐ und Rechnungsergebnisse der gewerblichen Berufsgenossenschaften und Versicherungsträger der öffentlichen Hand 2008. Paderborn: Bonifatius GmbH, 2009. [Google Scholar]
Dickel 2003
- Dickel H, Bruckner TM, Schmidt A, Diepgen TL. Impact of atopic skin diathesis on occupational skin disease incidence in a working population. Journal of Investigative Dermatology 2003;121(1):37‐40. [PUBMED: 12839561] [DOI] [PubMed] [Google Scholar]
Diepgen 1996
- Diepgen TL, Schmidt A, Berg A, Plinske W. Occupational retraining in employees suffering from occupational skin disease [Berufliche Rehabilitation von hautkranken Beschäftigten]. Deutsches Arzteblatt 1996;93:31‐40. [Google Scholar]
Diepgen 1999
- Diepgen TL, Coenraads PJ. The epidemiology of occupational contact dermatitis. International Archives of Occupational and Environmental Health 1999;72(8):496‐506. [PUBMED: 10592001] [DOI] [PubMed] [Google Scholar]
Diepgen 2002
- Diepgen TL, Schmidt A. Are incidence and prevalence of occupational dermatoses underestimated? [Werden Inzidenz und Prävalenz berufsbedingter Hauterkrankungen unterschätzt?]. Arbeitsmedizin, Sozialmedizin, Praventivmedizin 2002;37:477‐80. [Google Scholar]
Diepgen 2003
- Diepgen TL. Occupational skin‐disease data in Europe. International Archives of Occupational and Environmental Health 2003;76(5):331‐8. [PUBMED: 12690490] [DOI] [PubMed] [Google Scholar]
Diepgen 2013
- Diepgen TL, Scheidt R, Weisshaar E, John SM, Hieke K. Cost of illness from occupational hand eczema in Germany. Contact Dermatitis 2013;69(2):99‐106. [PUBMED: 23869729] [DOI] [PubMed] [Google Scholar]
Dulon 2009
- Dulon M, Skudlik C, Nübling M, John SM, Nienhaus A. Validity and responsiveness of the Osnabrück Hand Eczema Severity Index (OHSI): A methodological study. British Journal of Dermatology 2009;160(1):137‐42. [PUBMED: 19016701] [DOI] [PubMed] [Google Scholar]
Fartasch 1995
- Fartasch M. Human barrier formation and reaction to irritation. Current Problems in Dermatology 1995;23:95‐103. [PUBMED: 9035933] [DOI] [PubMed] [Google Scholar]
Fischer 1998
- Fischer T, Greif C, Wigger‐Alberti W, Elsner P. Instrumental methods for the evaluation of effectiveness and safety of cosmetic products [Instrumentelle methoden zur bewertung der sicherheit und wirksamkeit von kosmetika]. Aktuelle Dermatologie 1998;24(8‐9):243‐50. [EMBASE: 1998322618] [Google Scholar]
Fluhr 2007
- Fluhr JW, Miteva M, Elsner P. Efficacy and safety testing. The clinical perspective. Current Problems in Dermatology 2007;34:33‐46. [PUBMED: 17312355] [DOI] [PubMed] [Google Scholar]
Frosch 1994
- Frosch PJ, Kurte A. Efficacy of barrier creams (IV). The repetitive irritation test (RIT) with a set of 4 standard irritants. Contact Dermatitis 1994;31(3):161‐8. [PUBMED: 7821009] [DOI] [PubMed] [Google Scholar]
Gabard 1995
- Gabard B, Treffel P, Charton‐Picard F, Eloy R. Irritant reactions on hairless micropig skin: A model for testing barrier creams. Current Problems in Dermatology 1995;23:275‐87. [PUBMED: 9035922] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro Guideline Development Tool. McMaster University (developed by Evidence Prime, Inc.), 2015.
Held 2005
- Held E, Skoet R, Johansen JD, Agner T. The hand eczema severity index (HECSI): A scoring system for clinical assessment of hand eczema. A study of inter‐ and intraobserver reliability. British Journal of Dermatology 2005;152(2):302‐7. [PUBMED: 15727643] [DOI] [PubMed] [Google Scholar]
Henry 1994
- Henry NW. Protective gloves for occupational use ‐ US rules, regulations, and standards. In: Mehlström GA, Wahlberg JE, Maibach HI editor(s). Protective gloves for occupational use. Boca Raton, Florida: CRC Press, 1994:45‐9. [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. [Google Scholar]
Hines 2017
- Hines J, Wilkinson SM, John SM, Diepgen TL, English J, Rustemeyer T, et al. The three moments of skin cream application: an evidence‐based proposal for use of skin creams in the prevention of irritant contact dermatitis in the workplace. Journal of the European Academy of Dermatology and Venereology : JEADV 2017;31(1):53‐64. [PUBMED: 27545662] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hogan 1990
- Hogan DJ, Dannaker CJ, Lal S, Maibach HI. An international survey on the prognosis of occupational contact dermatitis of the hands. Dermatosen in Beruf und Umwelt. Occupation and Environment 1990;38(5):143‐7. [PUBMED: 2149550] [PubMed] [Google Scholar]
Holness 2013
- Holness DL. Recent advances in occupational dermatitis. Current Opinion in Allergy and Clinical Immunology 2013;13(2):145‐50. [PUBMED: 23324811] [DOI] [PubMed] [Google Scholar]
Johansen 2011
- Johansen JD, Frosch PJ, Lepoittevin J‐P (Eds.). Contact Dermatitis. 5th Edition. Springer, 2011. [Google Scholar]
Jowett 1985
- Jowett S, Ryan T. Skin disease and handicap: An analysis of the impact of skin conditions. Social Science & Medicine 1985;20(4):425‐9. [PUBMED: 3158080] [DOI] [PubMed] [Google Scholar]
Juni 2001
- Juni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [PUBMED: 11440947] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kampf 2007
- Kampf G, Löffler H. Prevention of irritant contact dermatitis among health care workers by using evidence‐based hand hygiene practices: a review. Industrial Health 2007;45(5):645‐52. [PUBMED: 18057807] [DOI] [PubMed] [Google Scholar]
Karjalainen 1998
- Karjalainen A, Aalto L, Jolanski R, Keskinen H, Savela A. Occupational skin disease in Finland in 1996. Helsinki: Finnish Institute of Occupational Health (FIOH), 1998. [Google Scholar]
Kezic 2009
- Kezic S, Visser MJ, Verberk MM. Individual susceptibility to occupational contact dermatitis. Industrial Health 2009;47(5):469‐78. [PUBMED: 19834255] [DOI] [PubMed] [Google Scholar]
Kütting 2003
- Kütting B, Drexler H. Effectiveness of skin protection creams as a preventive measure in occupational Dermatitis: a critical update according to criteria of evidence‐based medicine. International Archives of Occupational and Environmental Health 2003;76(4):253‐9. [PUBMED: 12684811 ] [DOI] [PubMed] [Google Scholar]
Kütting 2008
- Kütting B, Drexler H. The three‐step programme of skin protection. A useful instrument of primary prevention or more effective in secondary prevention?. Deutsche Medizinische Wochenschrift (1946) 2008;133(5):201‐5. [PUBMED: 18213554] [DOI] [PubMed] [Google Scholar]
Lachapelle 1996
- Lachapelle JM. Efficacy of protective creams and/or gels. Current Problems in Dermatology 1996;25:182‐92. [PUBMED: 9026410] [DOI] [PubMed] [Google Scholar]
Lerbaek 2008
- Lerbaek A, Kyvik KO, Ravn H, Menne T, Agner T. Clinical characteristics and consequences of hand eczema ‐ An 8‐year follow‐up study of a population‐based twin cohort. Contact Dermatitis 2008;58(4):210‐6. [PUBMED: 18353028] [DOI] [PubMed] [Google Scholar]
Leveque 1983
- Leveque JL, Rigal J. Impedance methods for studying skin moisturization. Journal of the Society of Cosmetic Chemists 1983;34:419‐28. [Google Scholar]
Loden 1997
- Loden M. Barrier recovery and influence of irritant stimuli in skin treated with a moisturizing cream. Contact Dermatitis 1997;36(5):256‐60. [PUBMED: 9197961] [DOI] [PubMed] [Google Scholar]
Lodi 2000
- Lodi A, Mancini LL, Ambonati M, Coassini A, Ravanelli G, Crosti C. Epidemiology of occupational contact dermatitis in a North Italian population. European Journal of Dermatology 2000;10(2):128‐32. [PUBMED: 10694312] [PubMed] [Google Scholar]
Mahmoud 1984
- Mahmoud G, Lachapelle JM, Neste D. Histological assessment of skin damage by irritants: Its possible use in the evaluation of a 'barrier cream'. Contact Dermatitis 1984;11(3):179‐85. [PUBMED: 6499417] [DOI] [PubMed] [Google Scholar]
Mahmoud 1985
- Mahmoud G, Lachapelle JM. Evaluation of the protective value of an antisorbent gel by Laser Doppler flowmetry and histology. Contact Dermatitis 1985;13(1):14‐9. [PUBMED: 2931238] [DOI] [PubMed] [Google Scholar]
Malten 1981
- Malten KE. Thoughts on irritant contact dermatitis. Contact Dermatits 1981;7(5):238‐47. [PUBMED: 7030611] [DOI] [PubMed] [Google Scholar]
Mathias 1985
- Mathias CG. The cost of occupational skin disease. Archives of Dermatology 1985;121(3):332‐4. [PUBMED: 3156562] [PubMed] [Google Scholar]
Mathias 1990
- Mathias CG. Prevention of occupational contact dermatitis. Journal of the American Academy of Dermatology 1990;23(4 pt 1):742‐8. [PUBMED: 2146291] [DOI] [PubMed] [Google Scholar]
McFadden 2001
- McFadden JP. Hand eczema. In: Rycroft RJG, Menne T, Frosch PJ, Lepoittevin JP editor(s). Textbook of Contact Dermatitis. Berlin: Springer, 2001:403‐411. [Google Scholar]
Meding 1987
- Meding B, Swanbeck G. Prevalence of hand eczema in an industrial city. British Journal of Dermatology 1987;116(5):627‐34. [PUBMED: 2954578] [DOI] [PubMed] [Google Scholar]
Meding 1990
- Meding B, Swanbeck G. Consequences of having hand eczema. Contact Dermatitis 1990;23(1):6‐14. [PUBMED: 2144814] [DOI] [PubMed] [Google Scholar]
Meding 2005
- Meding B, Wrangsjö K, Järvholm B. Fifteen‐year follow‐up of hand eczema: Persistence and consequences. British Journal of Dermatology 2005;152(5):975‐80. [PUBMED: 15888155] [DOI] [PubMed] [Google Scholar]
Mellström 1994
- Mellström GA, Carlsson B. European standards on protective gloves. In: Mellström GA, Wahlberg JE, Maibach HI editor(s). Protective gloves for occupational use. Boca Raton, Florida: CRC Press, 1994:39‐43. [Google Scholar]
Nicholson 2010
- Nicholson PJ. Evidence‐based guidelines: occupational contact dermatitis and urticaria. Occupational Medicine 2010;60(7):502‐4. [PUBMED: 20871017] [DOI] [PubMed] [Google Scholar]
NOSQ 2002
- Flyvholm M‐A, Susitaival P, Meding B, Kanerva L, Lindberg M, Svensson Å, Ólafsson J H. Nordic Occupational Skin Questionnaire – NOSQ–2002. Nordic questionnaire for surveying work‐related skin diseases on hands and forearms and relevant exposures. TemaNord Copenhagen, Nordic Council of Ministers. Vol. 518, Copenhagen: Nordic Council of Ministers, 2002:1‐186. [Google Scholar]
Patterson 1999
- Patterson SE, Williams JV, Marks JG Jr. Prevention of sodium lauryl sulfate irritant contact dermatitis by Pro‐Q aerosol foam skin protectant. Journal of the American Academy of Dermatology 1999;40(5 Pt 1):783‐5. [PUBMED: 10321615] [DOI] [PubMed] [Google Scholar]
Pinnagoda 1989
- Pinnagoda J, Tupker RA, Coenraads PJ, Nater JP. Comparability and reproducibility of the results of water loss measurement: A study of 4 evaporimeters. Contact Dermatitis 1989;20(4):241‐6. [PUBMED: 2752735] [DOI] [PubMed] [Google Scholar]
Pinnagoda 1990
- Pinnagoda J, Tupker RA, Agner T, Serup J. Guidelines for transepidermal water loss (TEWL) measurement. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermatitis 1990;22(3):164‐78. [PUBMED: 2335090] [DOI] [PubMed] [Google Scholar]
Politiek 2016
- Politiek K, Oosterhaven JA, Vermeulen KM, Schuttelaar ML. Systematic review of cost‐of‐illness studies in hand eczema. Contact Dermatitis 2016;75(2):67‐76. [PUBMED: 27218305] [DOI] [PubMed] [Google Scholar]
Ramsing 1996
- Ramsing DW, Agner T. Effect of glove occlusion on human skin (II). Long term experimental exposure. Contact Dermatitis 1996;34(4):258‐62. [PUBMED: 8730163] [DOI] [PubMed] [Google Scholar]
Ramsing 1997
- Ramsing DW, Agner T. Preventive and therapeutic effects of a moisturizer. An experimental study in human skin. Acta Dermato‐Venereologica 1997;77(5):335‐7. [PUBMED: 9298122] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rietschel 1997
- Rietschel RL. Mechanisms in irritant contact dermatitis. Clinics in Dermatology 1997;15(4):557‐9. [PUBMED: 9255462] [DOI] [PubMed] [Google Scholar]
Rogiers 2001
- Rogiers V, EEMCO Group. EEMCO guidance for the assessment of transepidermal water loss in cosmetic sciences. Skin Pharmacology and Applied Skin Physiology 2001;14(2):117‐28. [PUBMED: 11316970] [DOI] [PubMed] [Google Scholar]
Rose 2009
- Rose RF, Lyons P, Horne H, Wilkinson MS. A review of the materials and allergens in protective gloves. Contact Dermatitis 2009;61(3):129‐37. [PUBMED: 19780770] [DOI] [PubMed] [Google Scholar]
Saary 2005
- Saary J, Qureshi R, Palda V, DeKoven J, Pratt M, Skotnicki‐Grant S, et al. A systematic review of contact dermatitis treatment and prevention. Journal of the American Academy of Dermatology 2005;53(5):845. [PUBMED: 16243136] [DOI] [PubMed] [Google Scholar]
Saetterstrøm 2014
- Saetterstrøm B, Olsen J, Johansen JD. Cost‐of‐illness of patients with contact dermatitis in Denmark. Contact Dermatitis 2014;71(3):154‐61. [PUBMED: 24750019] [DOI] [PubMed] [Google Scholar]
Schliemann 2014
- Schliemann S, Petri M, Elsner P. Preventing irritant contact dermatitis with protective creams: influence of the application dose. Contact Dermatitis 2014;70(1):19‐26. [PUBMED: 23844826] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. The GRADE Working Group, 2013. [Google Scholar]
Skoet 2004
- Skoet R, Olsen J, Mathiesen B, Iversen L, Johansen JD, Agner T. A survey of occupational hand eczema in Denmark. Contact Dermatitis 2004;51(4):159‐66. [PUBMED: 15500664] [DOI] [PubMed] [Google Scholar]
Smit 1993
- Smit HA, Burdorf A, Coenraads PJ. The prevalence of hand dermatitis in different occupations. International Journal of Epidemiology 1993;22(2):288‐93. [PUBMED: 8505186] [DOI] [PubMed] [Google Scholar]
Smit 1994
- Smit HA, Rijssen A, Vandenbroucke JP, Coenraads PJ. Susceptibility to and incidence of hand dermatitis in a cohort of apprentice hairdressers and nurses. Scandinavian Journal of Work, Environment & Health 1994;20(2):113‐21. [PUBMED: 8079132] [DOI] [PubMed] [Google Scholar]
Treffel 1994
- Treffel P, Gabard B, Juch R. Evaluation of barrier creams: An in vitro technique on human skin. Acta Dermato‐Venereologica 1994;74(1):7‐11. [PUBMED: 7511868] [DOI] [PubMed] [Google Scholar]
Tupker 1989a
- Tupker RA, Pinnagoda J, Coenraads PJ, Nater JP. The influence of the repeated exposure of surfactants on the human skin as determined by transepidermal water loss and visual scoring. Contact Dermatitis 1989;20(2):108‐14. [PUBMED: 2706957] [DOI] [PubMed] [Google Scholar]
Tupker 1989b
- Tupker RA, Pinnagoda J, Coenraads PJ, Nater JP. Baseline transepidermal water loss (TEWL) as a prediction of susceptibility to sodium lauryl sulfate. Contact Dermatitis 1989;20(4):265‐9. [PUBMED: 2752737] [DOI] [PubMed] [Google Scholar]
Uter 1998a
- Uter W, Pfahlberg A, Gefeller O, Schwanitz HJ. Prevalence and incidence of hand dermatitis in hairdressing apprentices: Results of the POSH study. Prevention of occupational skin disease in hairdressers. International Archives of Occupational and Environmental Health 1998;71(7):487‐92. [PUBMED: 9826082] [DOI] [PubMed] [Google Scholar]
Uter 1998b
- Uter W, Pfahlberg A, Gefeller O, Schwanitz H J. Hand eczema in a prospectively‐followed cohort of office‐workers. Contact Dermatitis 1998;38(2):83–9. [PUBMED: 9506220] [PubMed] [Google Scholar]
Uter 1999
- Uter W, Pfahlberg A, Gefeller O, Schwanitz HJ. Hand dermatitis in a prospectively followed cohort of hairdressing apprentices: Final results of the POSH study. Contact Dermatitis 1999;41(5):280‐6. [PUBMED: 10554064] [DOI] [PubMed] [Google Scholar]
Van Gils 2011
- Gils RF, Boot CR, Gils PF, Bruynzeel D, Coenraads PJ, Mechelen W, et al. Effectiveness of prevention programmes for hand dermatitis: a systematic review of the literature. Contact Dermatitis 2011;64(2):63‐72. [PUBMED: 21210820] [DOI] [PubMed] [Google Scholar]
Wahlberg 1996
- Wahlberg JE, Boman AS. Prevention of contact dermatitis from solvents. Current Problems in Dermatology 1996;25:57‐66. [PUBMED: 8787589] [DOI] [PubMed] [Google Scholar]
Wigger‐Alberti 1997
- Wigger‐Alberti W, Elsner P. Preventive measures in contact dermatitis. Clinics in Dermatology 1997;15(4):661‐5. [PUBMED: 9255478] [DOI] [PubMed] [Google Scholar]
Wigger‐Alberti 1998
- Wigger‐Alberti W, Elsner P. Do barrier creams and gloves prevent or provoke contact dermatitis?. American Journal of Contact Dermatitis 1998;9(2):100‐6. [PUBMED: 9601897] [PubMed] [Google Scholar]
Williams 2010
- Williams C, Wilkinson SM, McShane P, Lewis J, Pennington D, Pierce S, et al. A double‐blind, randomized study to assess the effectiveness of different moisturizers in preventing dermatitis induced by hand washing to simulate healthcare use. British Journal of Dermatology 2010;162(5):1088‐92. [PUBMED: 20199550] [DOI] [PubMed] [Google Scholar]
Wrangsjö 1994
- Wrangsjö K, Osterman K, Hage‐Hamsten M. Glove‐related skin symptoms among operation theatre and dental care unit personnel. (I) Interview investigation. Contact Dermatitis 1994;30(2):102‐7. [PUBMED: 8187484] [DOI] [PubMed] [Google Scholar]
Wulfhorst 2011
- Wulfhorst B, Bock M, Skudlik C, Wigger‐Alberti W, John SM. Prevention of hand eczema – gloves, barrier creams and workers’ education. In: Duus Johansen J, Frosch PJ, Lepoittevin JP editor(s). Contact Dermatitis. 5th Edition. Berlin: Springer, 2001:305‐45. [Google Scholar]
Zhai 1996
- Zhai H, Maibach HI. Effect of barrier creams: Human skin in vivo. Contact Dermatitis 1996;35(2):92‐6. [PUBMED: 8917826] [DOI] [PubMed] [Google Scholar]
Zhai 1998
- Zhai H, Maibach HI. Moisturizers in preventing irritant contact dermatitis: An overview. Contact Dermatitis 1998;38(5):241‐4. [PUBMED: 9667439] [PubMed] [Google Scholar]
Zhai 2006
- Zhai H, Maibach HI. Barrier creams. In: Chew A‐L, Maibach HI editor(s). Irritant Dermatitis. Berlin: Springer, 2006. [Google Scholar]
Zimmerli 1996
- Zimmerli T. European standards on protective gloves. Current Problems in Dermatology 1996;25(177):P6. [PUBMED: 8787602] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bauer 2010
- Bauer A, Schmitt J, Bennett C, Coenraads PJ, Elsner P, English J, et al. Interventions for preventing occupational irritant hand dermatitis. Cochrane Database of Systematic Reviews 2010, Issue 6. [DOI: 10.1002/14651858.CD004414.pub2] [DOI] [PubMed] [Google Scholar]


