Abstract
Background
Postpartum haemorrhage (PPH) is the leading cause of maternal mortality worldwide. Prophylactic uterotonic drugs can prevent PPH, and are routinely recommended. There are several uterotonic drugs for preventing PPH but it is still debatable which drug is best.
Objectives
To identify the most effective uterotonic drug(s) to prevent PPH, and generate a ranking according to their effectiveness and side‐effect profile.
Search methods
We searched Cochrane Pregnancy and Childbirth’s Trials Register (1 June 2015), ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) for unpublished trial reports (30 June 2015) and reference lists of retrieved studies.
Selection criteria
All randomised controlled comparisons or cluster trials of effectiveness or side‐effects of uterotonic drugs for preventing PPH.
Quasi‐randomised trials and cross‐over trials are not eligible for inclusion in this review.
Data collection and analysis
At least three review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. We estimated the relative effects and rankings for preventing PPH ≥ 500 mL and PPH ≥ 1000 mL as primary outcomes. We performed pairwise meta‐analyses and network meta‐analysis to determine the relative effects and rankings of all available drugs. We stratified our primary outcomes according to mode of birth, prior risk of PPH, healthcare setting, dosage, regimen and route of drug administration, to detect subgroup effects.The absolute risks in the oxytocin are based on meta‐analyses of proportions from the studies included in this review and the risks in the intervention groups were based on the assumed risk in the oxytocin group and the relative effects of the interventions.
Main results
This network meta‐analysis included 140 randomised trials with data from 88,947 women. There are two large ongoing studies. The trials were mostly carried out in hospital settings and recruited women who were predominantly more than 37 weeks of gestation having a vaginal birth. The majority of trials were assessed to have uncertain risk of bias due to poor reporting of study design. This primarily impacted on our confidence in comparisons involving carbetocin trials more than other uterotonics.
The three most effective drugs for prevention of PPH ≥ 500 mL were ergometrine plus oxytocin combination, carbetocin, and misoprostol plus oxytocin combination. These three options were more effective at preventing PPH ≥ 500 mL compared with oxytocin, the drug currently recommended by the WHO (ergometrine plus oxytocin risk ratio (RR) 0.69 (95% confidence interval (CI) 0.57 to 0.83), moderate‐quality evidence; carbetocin RR 0.72 (95% CI 0.52 to 1.00), very low‐quality evidence; misoprostol plus oxytocin RR 0.73 (95% CI 0.60 to 0.90), moderate‐quality evidence). Based on these results, about 10.5% women given oxytocin would experience a PPH of ≥ 500 mL compared with 7.2% given ergometrine plus oxytocin combination, 7.6% given carbetocin, and 7.7% given misoprostol plus oxytocin. Oxytocin was ranked fourth with close to 0% cumulative probability of being ranked in the top three for PPH ≥ 500 mL.
The outcomes and rankings for the outcome of PPH ≥ 1000 mL were similar to those of PPH ≥ 500 mL. with the evidence for ergometrine plus oxytocin combination being more effective than oxytocin (RR 0.77 (95% CI 0.61 to 0.95), high‐quality evidence) being more certain than that for carbetocin (RR 0.70 (95% CI 0.38 to 1.28), low‐quality evidence), or misoprostol plus oxytocin combination (RR 0.90 (95% CI 0.72 to 1.14), moderate‐quality evidence)
There were no meaningful differences between all drugs for maternal deaths or severe morbidity as these outcomes were so rare in the included randomised trials.
Two combination regimens had the poorest rankings for side‐effects. Specifically, the ergometrine plus oxytocin combination had the higher risk for vomiting (RR 3.10 (95% CI 2.11 to 4.56), high‐quality evidence; 1.9% versus 0.6%) and hypertension [RR 1.77 (95% CI 0.55 to 5.66), low‐quality evidence; 1.2% versus 0.7%), while the misoprostol plus oxytocin combination had the higher risk for fever (RR 3.18 (95% CI 2.22 to 4.55), moderate‐quality evidence; 11.4% versus 3.6%) when compared with oxytocin. Carbetocin had similar risk for side‐effects compared with oxytocin although the quality evidence was very low for vomiting and for fever, and was low for hypertension.
Authors' conclusions
Ergometrine plus oxytocin combination, carbetocin, and misoprostol plus oxytocin combination were more effective for preventing PPH ≥ 500 mL than the current standard oxytocin. Ergometrine plus oxytocin combination was more effective for preventing PPH ≥ 1000 mL than oxytocin. Misoprostol plus oxytocin combination evidence is less consistent and may relate to different routes and doses of misoprostol used in the studies. Carbetocin had the most favourable side‐effect profile amongst the top three options; however, most carbetocin trials were small and at high risk of bias.
Amongst the 11 ongoing studies listed in this review there are two key studies that will inform a future update of this review. The first is a WHO‐led multi‐centre study comparing the effectiveness of a room temperature stable carbetocin versus oxytocin (administered intramuscularly) for preventing PPH in women having a vaginal birth. The trial includes around 30,000 women from 10 countries. The other is a UK‐based trial recruiting more than 6000 women to a three‐arm trial comparing carbetocin, oxytocin and ergometrine plus oxytocin combination. Both trials are expected to report in 2018.
Consultation with our consumer group demonstrated the need for more research into PPH outcomes identified as priorities for women and their families, such as women’s views regarding the drugs used, clinical signs of excessive blood loss, neonatal unit admissions and breastfeeding at discharge. To date, trials have rarely investigated these outcomes. Consumers also considered the side‐effects of uterotonic drugs to be important but these were often not reported. A forthcoming set of core outcomes relating to PPH will identify outcomes to prioritise in trial reporting and will inform futures updates of this review. We urge all trialists to consider measuring these outcomes for each drug in all future randomised trials. Lastly, future evidence synthesis research could compare the effects of different dosages and routes of administration for the most effective drugs.
Keywords: Female; Humans; Network Meta‐Analysis; Drug Therapy, Combination; Drug Therapy, Combination/adverse effects; Drug Therapy, Combination/methods; Ergonovine; Ergonovine/adverse effects; Ergonovine/therapeutic use; Fever; Fever/chemically induced; Hypertension; Hypertension/chemically induced; Misoprostol; Misoprostol/therapeutic use; Oxytocics; Oxytocics/therapeutic use; Oxytocin; Oxytocin/adverse effects; Oxytocin/analogs & derivatives; Oxytocin/therapeutic use; Postpartum Hemorrhage; Postpartum Hemorrhage/prevention & control; Vomiting; Vomiting/chemically induced
Which drug is best for reducing excessive blood loss after birth?
What is the issue?
The aim of this Cochrane review was to find out which drug is most effective in preventing excessive blood loss at childbirth and has the least side‐effects. We collected and analysed all the relevant studies to answer this question.
Why is this important?
Bleeding after birth is the most common reason why mothers die in childbirth worldwide. Although most healthy women can cope well with some bleeding at childbirth, others do not, and this can pose a serious risk to their health and even life. To reduce excessive bleeding at childbirth, the routine administration of a drug to contract the uterus (uterotonic) has become standard practice across the world. The aim of this research was to identify which drug is most effective in preventing excessive bleeding after childbirth with the least side‐effects.
Different drugs given routinely at childbirth have been used for preventing excessive bleeding. They include oxytocin, misoprostol, ergometrine, carbetocin, and combinations of these drugs, each with different effectiveness and side‐effects. Some of the side‐effects identified include: vomiting, high blood pressure and fever. We analysed all the available evidence to compare all of these drugs and calculated a ranking among them, providing robust effectiveness and side‐effect profiles for each drug.
What evidence did we find?
We searched for evidence in June 2015 and found 140 studies involving a total of 88,947 women. The results suggest that an ergometrine plus oxytocin combination, carbetocin, and a misoprostol plus oxytocin combination are the most effective drugs for preventing excessive bleeding after childbirth and are more effective than the drug oxytocin currently recommended by the World Health Organization (WHO). However, ergometrine plus oxytocin and misoprostol plus oxytocin were the worst drugs for side‐effects, with carbetocin having the most favourable side‐effect profile (less vomiting, high blood pressure and fever). More effective drugs could probably prevent one out of three women from bleeding excessively after childbirth compared to oxytocin. However, existing carbetocin studies were small and of poor quality.
What does this mean?
We found that ergometrine plus oxytocin, misoprostol plus oxytocin, and carbetocin were more effective drugs for reducing excessive bleeding at childbirth than oxytocin which is the current standard drug used to prevent this condition. Carbetocin has the least side‐effects among the top three drug options, but to date studies of carbetocin were small and of poor quality.
There are some ongoing studies that are not yet complete, including two key studies. One is a large study (involving around 30,000 women across 10 different countries) comparing the effectiveness of carbetocin versus oxytocin for preventing PPH among women having a vaginal birth. The other is a UK‐based trial (involving more than 6000 women) comparing carbetocin, oxytocin and ergometrine plus oxytocin combination. Both trials are expected to report in 2018 and these results will be incorporated when this review is updated.
Consultation with our consumer group has demonstrated a need for more research into PPH outcomes identified as priorities for women and their families, such as women’s views regarding the drugs used, clinical signs of excessive blood loss, neonatal unit admissions and breastfeeding at discharge. Trials to date have rarely investigated these outcomes. Consumers also considered the side‐effects of uterotonic drugs to be important and these were often not reported. A set of standardised PPH outcomes are being developed and will be incorporated in future updates of this review. We would hope that future trials would also consider adopting those outcomes. Finally, future systematic reviews could compare the effects of different doses and ways of administering the most effective drugs.
Summary of findings
Summary of findings for the main comparison.
| Effects of uterotonic drugs for preventing postpartum haemorrhage: a network meta‐analysis | |||||
|
Patient or population: Women giving birth and at the third stage of labour Settings: Hospital setting Intervention: Ergometrine plus oxytocin, Carbetocin, Misoprostol plus oxytocin Comparison: Oxytocin | |||||
| Outcomes | Effects and 95% confidence intervals in the effects. Main comparator is oxytocin. | Comments | |||
| Risk with ergometrine plus oxytocin* | Risk with carbetocin* | Risk with misoprostol plus oxytocin* | Risk with oxytocin** | ||
| PPH ≥500 mL |
7.2% (6 to 8.7) for vaginal births 51.7% (42.7 to 62.2) for caesareans |
7.6% (5.5 to 10.5) for vaginal births 53.9% (38.9 to 74.9) for caesareans |
7.7% (6.3 to 9.5) for vaginal births 54.7% (44.9 to 67.4) for caesareans |
10.5% (9.8 to 11.3) for vaginal births 74.9% (65.7 to 85.4) for caesareans |
There was evidence of global inconsistency in this analysis ( P = 0.046). However, the comparisons in this table were consistent except for the comparison of ergometrine versus no treatment not included in this table‐based on a single study. |
| RR 0.69 (0.57 to 0.83) (NMA) RR 0.72 (0.56 to 0.92) (Pairwise) |
RR 0.72 (0.52 to 1.00) (NMA) RR 0.69 (0.45 to 1.07) (Pairwise) |
RR 0.73 (0.60 to 0.90) (NMA) RR 0.74 (0.62 to 0.88) (Pairwise) |
1 | ||
| ⊕⊕⊕⊝ moderate confidence in estimate due to inconsistency based on 10 studies (13,138 women, I2=57.4%) | ⊕⊝⊝⊝ very low confidence in estimate due to risk of bias, imprecision and inconsistency based on 8 studies (917 women, I2 = 49.9%) | ⊕⊕⊕⊝ moderate confidence in estimate due to inconsistency based on 12 studies (9651 women, I2 = 60.5%) | |||
| PPH ≥1000 mL |
2.8% (2.2 to 3.4) for vaginal births 10.7% (8.5 to 13.2) for caesareans |
2.5% (1.4 to 4.6) for vaginal births 9.7% (5.3 to 17.8) for caesareans |
3.2% (2.6 to 4.1) for vaginal births 12.5% (10 to 15.8) for caesareans |
3.6% (3.4 to 3.9) for vaginal births 13.9% (11.7 to 16.6) for caesareans |
There was no evidence of global inconsistency (P = 0.345) in this analysis. |
| RR 0.77 (0.61 to 0.95) (NMA) RR 0.73 (0.57 to 0.93) (Pairwise) |
RR 0.70 (0.38 to 1.28) (NMA) RR 0.71 (0.38 to 1.35) (Pairwise) |
RR 0.90 (0.72 to 1.14) (NMA) RR 0.89 (0.71 to 1.12) (Pairwise) |
1 | ||
| ⊕⊕⊕⊕ high confidence in estimate based on 9 studies (13,038 women, I2 = 0%) | ⊕⊕⊝⊝ low confidence in estimate due to risk of bias and imprecision based on 7 studies (1026 women, I2 = 0%) | ⊕⊕⊕⊝ moderate confidence in estimate due to imprecision based on 14 studies (9897 women, I2 = 0%) | |||
| Vomiting |
1.9% (1.3 to 2.7) for vaginal births 16.1% (11 to 23.7) for caesareans |
0.5% (0.3 to 0.9) for vaginal births 4.6% (2.9 to 7.4) for caesareans |
1.3% (0.8 to 2) for vaginal births 11.2% (7.1 to 17.6) for caesareans |
0.6% (0.5 to 0.6) for vaginal births 5.2% (4.9 to 5.5) for caesareans |
There was no evidence of global inconsistency (P = 0.06) in this analysis. |
| RR 3.10 (2.11 to 4.56) (NMA) RR 3.15 (1.72 to 5.78) (Pairwise) |
RR 0.89 (0.55 to 1.42) (NMA) RR 0.88 (0.39 to 1.99) (Pairwise) |
RR 2.16 (1.37 to 3.39) (NMA) RR 2.25 (1.45 to 3.48) (Pairwise) |
1 | ||
| ⊕⊕⊕⊕ high confidence in estimate based on 8 studies (9811 women, I2 = 48.1%) | ⊕⊝⊝⊝ very low confidence in estimate due to risk of bias, inconsistency and imprecision based on 10 studies (1939 women, I2 = 59.2%) | ⊕⊕⊕⊕ high confidence in estimate due to imprecision based on 9 studies (5015 women, I2 = 30.1%) | |||
| Hypertension |
1.2% (0.4 to 4) for vaginal births 29.6% ( to ) for caesareans |
0.6% (0.1 to 3.3) for vaginal births 14.2% (2.5 to 79.7) for caesareans |
Risks not available as no studies report this outcome |
0.7% (0.7 to 0.8) for vaginal births 16.7% (11.2 to 24.9) for caesareans |
There was no evidence of global inconsistency (P = 0.481) in this analysis. |
| RR 1.77 (0.55 to 5.66) (NMA) RR 0.95 (0.10 to 8.38) (Pairwise) |
RR 0.85 (0.15 to 4.77) (NMA) | RR not available as no studies reported this outcome | 1 | ||
| ⊕⊕⊝⊝ low confidence in estimate due to inconsistency and imprecision based on 2 studies (1039 women, I2 = 73.2%) | ⊕⊕⊝⊝ low confidence in estimate due to imprecision and based only on indirect evidence | Quality of the evidence cannot be assessed as no studies report this outcome | |||
| Fever |
3% (1.5 to 6) for vaginal births 11.7% (6.5 to 23.2) for caesareans |
3.1% (0.8 to 12.1) for vaginal births 12% (3.1 to 46.6) for caesareans |
11.4% (8 to 16.4) for vaginal births 44.2% (30.9 to 63.2) for caesareans |
3.6% (3.4 to 3.9) for vaginal births 13.9% (11.7 to 16.6) for caesareans |
There was no evidence of global inconsistency (P = 0.352) in this analysis. |
| RR 0.84 (0.42 to 1.67) (NMA) RR 1.07 (0.47 to 2.43) (Pairwise) |
RR 0.86 (0.22 to 3.35) (NMA) RR 2.11 (0.18 to 24.40) (Pairwise) |
RR 3.18 (2.22 to 4.55) (NMA) RR 2.96 (1.95 to 4.51) (Pairwise) |
1 | ||
| ⊕⊕⊕⊝ moderate confidence in estimate due to imprecision based on 2 studies (1591 women, I2 = 0%) | ⊕⊝⊝⊝ very low confidence in estimate due to risk of bias, inconsistency and imprecision based on 3 studies (292 women, I2 = 40.9%) | ⊕⊕⊕⊝ moderate confidence in estimate due to inconsistency based on 15 studies (8209 women, I2 = 77.8%) | |||
| *The risks in the ergometrine plus oxytocin, carbetocin, misoprostol plus oxytocin groups (and their 95% confidence interval) are based on the assumed risk in the oxytocin group and the relative effects of the interventions (and its 95% CI). **The risk in the oxytocin group (and its 95% confidence interval) is based on a meta‐analysis of proportions from the studies included in this review for this group. RR: Risk ratio | |||||
|
GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
Background
Description of the condition
An estimated 303,000 women died during childbirth in 2015 (Alkema 2016). Postpartum haemorrhage (PPH) accounted for up to a third of all these maternal deaths (Say 2014). Almost all deaths occurred in low‐ or middle‐income countries. Even when death from PPH is avoided, the need for blood transfusion, hysterectomy and additional care place a huge burden on health services (Penney 2007; Souza 2013).
The third stage of labour, defined as the period of time from birth until the delivery of the placenta, and the immediate postpartum period are the most hazardous periods of childbirth due to the risk of PPH. The World Health Organization (WHO) defines PPH as blood loss after birth exceeds 500 mL in the first 24 hours (WHO 2012). Even though healthy women can easily cope with this amount of blood loss, for those who may be malnourished and/or anaemic it can cause considerable morbidity and mortality. The most common cause of PPH is uterine atony (failure of the uterus to contract after delivery), which accounts for 75% of cases (Weekes 1956). Even though risk factors for adverse maternal outcomes from severe haemorrhage have been identified (Souza 2013), often PPH is unpredictable as it occurs in the absence of identifiable clinical or historical risk factors (Combs 1991). Therefore, effective prevention of PPH is advocated for all women during childbirth (WHO 2012). The administration of uterotonic drugs routinely in the third stage of labour is the key intervention that prevents PPH, although there is uncertainty about which drug may be the most effective.
Description of the intervention
The administration of uterotonic drugs to prevent PPH is part of the active management of the third stage of labour, which can prevent two out of three events of PPH (Begley 2015). The active management of the third stage of labour refers to the administration of a uterotonic drug, early cord clamping, and controlled cord traction until delivery of the placenta. The WHO guideline development group recently revisited the evidence underpinning each component of active management of third stage of labour and considered the use of uterotonics as the main intervention within this package (WHO 2012). Uterotonics are also essential for the treatment of PPH, but this is not considered in this review.
How the intervention might work
Several different uterotonic drugs have been used for preventing PPH. These drugs include ergometrine, misoprostol, carbetocin, oxytocin, and the combinations of misoprostol plus oxytocin and ergometrine plus oxytocin.
Oxytocin
Oxytocin (Syntocinon®) is the most widely used uterotonic drug. At low doses, it produces rhythmic uterine contractions that are indistinguishable in frequency, force and duration from those observed during spontaneous labour, but at higher dosages, it causes sustained uterine contractions (MEDICINES.ORG.UK). It has a short half‐life, approximately three to five minutes, and can be used as an infusion to maintain uterine contraction. When used intramuscularly, the latent phase lasts two to five minutes, but the uterine activity can last two to three hours (MEDICINES.ORG.UK). However, oxytocin cannot be used orally. It is unstable in ambient temperatures and it requires a cold chain through storage and transport. It should also not be given intravenously as a large bolus, because it can cause severe hypotension (Thomas 2007). Because of its anti‐diuretic effect, water intoxication can occur with prolonged infusion of oxytocin (MEDICINES.ORG.UK). Oxytocin has a favourable side‐effect profile and it is not significantly worse than placebo for common side‐effects such as nausea and vomiting, but the evidence is scarce (Westhoff 2013).
Ergometrine
Ergometrine and methylergometrine are ergot alkaloids that increase the uterine muscle tone by causing sustained uterine contractions. They have a latent phase of two to five minutes after intramuscular injection and the plasma half‐life is 30 to 120 minutes (de Groot 1998). However, ergometrine and methylergometrine are unstable in heat with an unpredictable bioavailability, which precludes oral use (de Groot 1996a). They are vasoconstrictive and increase the risk of hypertension postpartum (Liabsuetrakul 2007). Other side‐effects with ergot alkaloids are pain after birth, nausea and vomiting (Liabsuetrakul 2007).
Misoprostol
Misoprostol is a prostaglandin E1 analogue, which is licensed for the prevention and treatment of gastric ulcers. It is well known for its off‐label use as a uterotonic agent (Tuncalp 2012). It is water‐soluble and heat stable (Davies 2001). It is absorbed after nine to 15 minutes after sublingual, oral, vaginal, and rectal use. The half‐life is about 20 to 40 minutes. Oral and sublingual routes have the advantage of rapid onset of action, while the vaginal and rectal routes result in prolonged activity and greater bioavailability (Schaff 2005). However, it is associated with side‐effects such as diarrhoea, abdominal pain, nausea and vomiting, shivering and pyrexia (Tuncalp 2012).
Carbetocin
Carbetocin is a newer long‐acting synthetic analogue of oxytocin with agonist properties. After intravenous injection, it produces sustained uterine contractions within two minutes, lasting for approximately six minutes followed by rhythmic contractions for 60 minutes (Hunter 1992). When carbetocin is administered by an intramuscular injection, the sustained uterine contractions last for approximately 11 minutes and the rhythmic contractions for 120 minutes (Hunter 1992). Carbetocin is heat stable and the side‐effect profile appears to be similar to oxytocin (Su 2012).
Combination drugs
The use of combinations of uterotonic drugs is also popular and the most commonly used preparation is ergometrine plus oxytocin (Syntometrine®). This combination is associated with a statistically significant reduction of PPH ≥ 500 mL when compared with oxytocin alone, attributable to the additive ergometrine effect (odds ratio (OR) 0.82, 95% confidence interval (CI) 0.71 to 0.95) (McDonald 2004). Another combination is misoprostol plus oxytocin that is also found to be associated with a small reduction in PPH ≥ 500 mL (risk ratio (RR) 0.71, 95% CI 0.53 to 0.95) (Tuncalp 2012). However, both these combinations are associated with significant side‐effects and despite the difference in PPH ≥ 500 mL, there was no difference found for more severe PPH when compared to oxytocin defined as PPH ≥ 1000 mL. Hence, the WHO guideline recommends oxytocin over these combinations (WHO 2012).
The WHO recommends that all women giving birth should be offered uterotonics during the third stage of labour for the prevention of PPH; oxytocin (intramuscular/intravenous, 10 international units (IU)) is the uterotonic drug of choice (WHO 2012). Other injectable uterotonics and misoprostol are recommended as alternatives for the prevention of PPH in settings where oxytocin is not available. Carbetocin is found to reduce the need for additional uterotonics (RR 0.62, 95% CI 0.44 to 0.88), but it is more expensive and not better than oxytocin for preventing PPH ≥ 1000 mL (WHO 2012).
Why it is important to do this review
Cochrane reviews have compared individual uterotonic agents against another uterotonic agent, placebo or no treatment (Begley 2015; Liabsuetrakul 2007; McDonald 2004; Su 2012; Tuncalp 2012; Westhoff 2013). Such pairwise meta‐analyses can only compare two agents that have been compared directly in head‐to‐head trials (direct evidence). In the absence of a single randomised controlled trial comparing all available uterotonic agents, uncertainty remains over their relative effectiveness and ranking. We conducted a network meta‐analysis synthesizing all direct and indirect trial evidence of relative treatment effects in a single coherent analysis for all the competing agents. Indirect evidence is obtained when the relative effectiveness of two competing drugs is inferred through a common comparator, even though this pair may not have been compared directly (Caldwell 2005; Lumley 2002). Our network meta‐analysis provides effectiveness and side‐effect profiles, along with the ranking for each uterotonic agent.
Objectives
Primary
To identify the most effective uterotonic drug(s) to prevent postpartum haemorrhage (PPH) with a favourable side‐effect profile, and to generate a clinically useful ranking of all available uterotonics.
Secondary
To provide the relative effectiveness and side‐effect profile of each drug for our primary outcomes within: a) population subgroups (prior risk of PPH, mode of birth and healthcare setting) and b) treatment subgroups (different dosages, routes or regimens of administration of each uterotonic drug).
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled comparisons or cluster trials of effectiveness or side‐effects of uterotonic drugs for preventing postpartum haemorrhage (PPH) were included. Quasi‐randomised trials and cross‐over trials were excluded.
Types of participants
The review included studies of pregnant women following a vaginal or caesarean birth in hospital or community settings.
Types of interventions
Trials were eligible if they administered uterotonic agents of any dosage, route or regimen systemically at birth for preventing PPH, and compared them against other uterotonic agents, placebo or no treatment. Trials evaluating uterotonic drugs administered locally or not immediately after birth, or exclusively comparing different dosages, routes or regimens of the same uterotonic agent were excluded. We included trials in which non‐pharmacologic co‐interventions such as controlled cord traction, cord clamping, or uterine massage was performed as a randomised intervention in all arms of the trial and the effects of such co‐interventions were tested through a sensitivity analysis.
We classified drugs into oxytocin, carbetocin, misoprostol, ergometrine (included also ergonovine, methylergonovine), ergometrine plus oxytocin (Syntometrine, oxytocin combined with ergometrine, ergonovine, or methylergonovine), and misoprostol plus oxytocin. We excluded synthetic prostaglandin analogues of PGF2α (carboprost), and PGE2 (prostin, sulprostone), because these drugs are usually used for treating (and not preventing) PPH, and are not currently recommended by the WHO as alternatives (WHO 2012).
For this review, we assumed that any woman who meets the inclusion criteria is, in principle, equally likely to be randomised to any of the eligible uterotonic drugs.
Types of outcome measures
We estimated the relative effects and rankings of the competing interventions according to the following outcomes.
Primary outcomes
The primary outcomes of the review were:
PPH ≥ 500 mL; and
PPH ≥ 1000 mL.
Secondary outcomes
The secondary outcomes of the review were:
maternal deaths;
maternal deaths or severe morbidity events adapted from WHO “near miss” criteria (WHO 2011) to include major surgery (laparotomy, uterine artery ligation, internal iliac artery ligation, B‐Lynch suture, hysterectomy, extensive vaginal repair, admission to the intensive care unit, or vital organ failure (temporary or permanent);
additional uterotonics requirement;
transfusion requirement;
manual removal of the placenta;
mean volumes of blood loss (mL);
mean durations of the third stage of labour (minutes);
change in haemoglobin measurements before and after birth (g/L);
clinical signs of excessive blood loss (as defined by the trialists);
neonatal unit admission requirement;
breastfeeding at discharge; and
side‐effects such as nausea, vomiting, hypertension, headache, tachycardia, hypotension, abdominal pain, fever and shivering in the first 24 hours postpartum.
Search methods for identification of studies
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (1 June 2015). We updated this search on 27 October 2017 and added the results to Studies awaiting classification to be assessed and incorporated at the next update.
The Register is a database containing over 23,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings; and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, the Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports using the terms given in Appendix 1 (30 June 2015).
Searching other resources
We retrieved additional relevant references cited in papers identified through the above search strategy and we did search for the full texts of trials initially identified as abstracts. We sought information from primary authors to investigate whether these studies met our eligibility criteria, and to obtain outcome and study data. Trials that compared at least two of the drugs were eligible and we searched for all possible comparisons formed by the drugs of interest. We did not apply any language or date restrictions.
Data collection and analysis
Selection of studies
Three review authors retrieved and independently assessed for inclusion all the potential studies we identified (IDG, AM, HW). We resolved any disagreements through discussion or, if required, in consultation with a third person (AC). We created a study flow diagram to map out the number of records identified, included and excluded (Figure 1).
Figure 1.
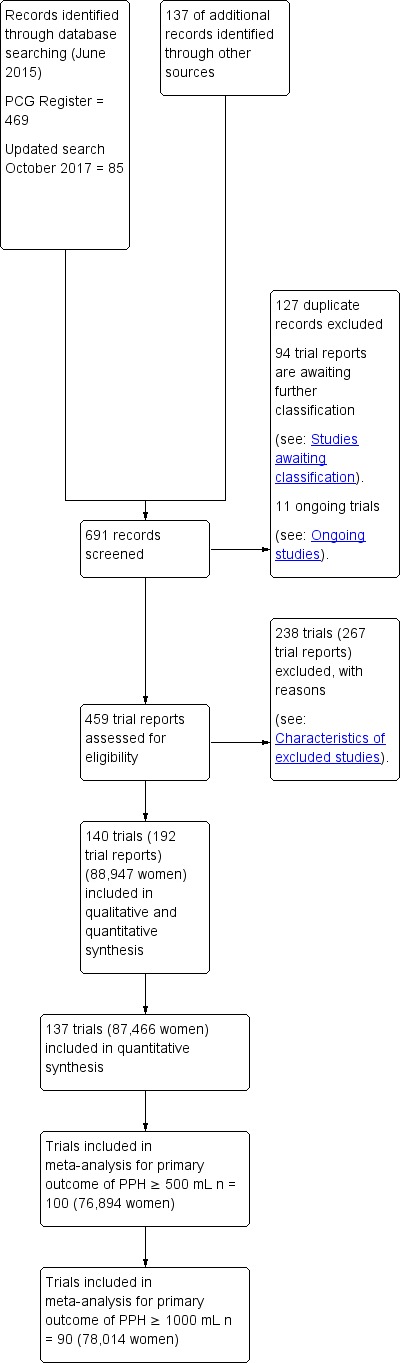
Study flow diagram.
Data extraction and management
We designed an electronic form on ©Microsoft Access to extract data. For eligible studies, at least three review authors independently extracted the data using a blank electronic form (IDG, HW, AM, DL, HG, OT). We resolved discrepancies through discussion or, if required, we consulted another person (AC). We entered data into STATA and Review Manager software (RevMan 2014) and checked for accuracy. When information was unclear, we attempted to contact authors of the original reports to provide further details. The following data were extracted.
Outcome data
From each included study we extracted: the number of participants, the gestational age and the parity of participants, and any exclusion criteria. We also extracted: the interventions being compared, and their respective primary and secondary outcomes. All relevant arm level data were extracted (e.g. number of events and number of patients for binary outcomes).
Data on potential effect modifiers
From each included study we extracted the following study, intervention and population characteristics that may act as effect modifiers:
mode of delivery (vaginal or caesarean birth);
prior risk of PPH (as defined by trialists and categorised as low, high, mixed or not stated);
dosage, regimen, and route of drug administration (sublingual, subcutaneous, intramuscular, rectal, oral, intravenous bolus and/or infusion); and
setting of the study (community or hospital).
Other data
From each included study we extracted the following additional information:
country or countries in which the study was performed;
date of publication;
type of publication (full‐text publication, abstract publication, unpublished data); and
trial registration reference.
Assessment of risk of bias in included studies
At least three (IDG, HW, AM, DL, HG, OT) review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreements were resolved by discussion or by involving another assessor (AC).
(1) Random sequence generation (checking for possible selection bias)
Studies were excluded if found to be at high risk for bias for random sequence generation (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number). We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the methods as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator); or
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); or
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to have affected the results.
We assessed the methods as:
low, high or unclear risk of bias for participants; and
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or supplied by the trial authors, we re‐included missing data in the analyses. We assessed methods to handle incomplete outcome data as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups and less than 10% of missing outcome data);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation or more than 10% of missing outcome data); or
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns about other possible sources of bias, such as the source of funding and potential conflicts of interest.
We assessed these interests as:
low risk of other bias (public funding or no funding and no significant conflicts of interest identified);
high risk of other bias (industry funding or significant conflicts of interest identified); or
unclear risk of other bias.
Another source of bias was generated by the method of measuring blood loss. We assessed the method described in each study and classified it as at:
low risk of other bias (objective measurements such as weighing sponges, measurements in drapes, volumetric assessment, tagged red cells, etc);
high risk of other bias (subjective measurement such as clinical or visual estimates); or
unclear risk of other bias (unspecified methods of measurement).
(7) Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to have impacted on the findings. For our primary outcomes, we combined quality items and judged trials as “low risk of bias” if they were double‐blinded, had allocation concealment and with little loss to follow‐up (less than 10%). Trials were judged as “intermediate risk of bias” if they demonstrated adequate allocation concealment, with assessor blinding and little loss to follow‐up (less than 10%). Alternatively, trials were considered to be at “high risk of bias”. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis for information about how the risk of bias was incorporated in the sensitivity analysis.
Summary of findings
A “Summary of findings” table is presented as described by Puhan et al (Puhan 2014). This table shows the overall quality of the body of evidence for the primary review outcomes and important side‐effects, using GRADE criteria. GRADE ratings were determined on the basis of risk of bias, inconsistency, indirectness and imprecision. The risks of bias was assessed conventionally for each included trial. A judgement was made to downgrade the quality of the evidence if the majority of the trials for each outcome or each direct comparison were at high risk of bias. The evidence was also downgraded in quality if we found inconsistency between estimates produced by the network meta‐analysis and direct estimates obtained from pairwise comparisons. Heterogeneity across studies for each pairwise meta‐analysis was assessed using I2. The evidence was downgraded for indirectness if the included trials for specific direct comparisons were considered to be more restrictive or different than the overall review question. Lastly, evidence was downgraded if there was imprecision. Imprecision relates to the overall level of confidence that may be placed in the estimated treatment effects. Each quality element considered to have ‘serious’ or ‘very serious’ limitations was rated down one or two levels respectively. GRADE assessments were made for the most effective drugs (ergometrine plus oxytocin, carbetocin, and misoprostol plus oxytocin) in comparison with the most frequently used and recommended drug (oxytocin) as a comparison for the primary outcomes and important side‐effects. The risk calculated in the comparison group (oxytocin) (and its 95% confidence interval (CI)) was based on a meta‐analysis of proportions from the studies included in this review. The risks (and their 95% CIs) calculated in the intervention groups were based on the assumed risk in the comparison group and the relative effects of the interventions (and their 95% CIs). The risks differed significantly by the mode of birth subgroup and they are presented separately for vaginal births and caesareans. Assessments were carried out by IDG and checked by AC.
Measures of treatment effect
Relative treatment effects
We summarised relative treatment effects for dichotomous outcomes as risk ratios (RR) and for continuous outcomes as mean difference (MD) with 95% CIs (Dias 2013).
Relative treatment ranking
We estimated the cumulative probabilities for each treatment being at each possible rank and obtained a treatment hierarchy using the surface under the cumulative ranking curve (SUCRA); the larger the SUCRA the higher its rank among all available drug options (Salanti 2011). The probabilities to rank the treatments are estimated under a Bayesian model with flat priors, assuming that the posterior distribution of the parameter estimates is approximated by a normal distribution with mean and variance equal to the frequentist estimates and variance–covariance matrix (White 2015).
Unit of analysis issues
Cluster‐randomised trials
For the only cluster‐randomised trial included in this review (Stanton 2013), we used the unadjusted standard errors as the clusters and the Intracluster Correlation Co‐efficient (ICC) was small (ICC = 0.012). We considered it reasonable to combine the results from the cluster‐randomised and the individually‐randomised trials as there was little heterogeneity between the study designs and any interaction between the relative effects of agents and the choice of randomisation unit was considered to be unlikely. The effect of the unit of randomisation was also assessed in sensitivity analysis (Higgins 2011).
Cross‐over trials
This type of trial was not deemed appropriate for this intervention.
Multi‐arm trials
Multi‐arm trials were included and we accounted for the correlation between the effect estimates in the network meta‐analysis. We treated multi‐arm studies as multiple independent comparisons in pairwise meta‐analyses and these were not combined in any analysis.
Dealing with missing data
For included studies, we noted the levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis. For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we included all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. We used the number randomised minus any participants whose outcomes were known to be missing as the denominator for each outcome in each trial.
Assessment of clinical and methodological heterogeneity within treatment comparisons
To evaluate the presence of clinical heterogeneity, we described the study population characteristics across all included trials. We assessed the presence of clinical heterogeneity by comparing these characteristics.
Assessment of transitivity across treatment comparisons
In this context we expect that the transitivity assumption holds assuming the following: 1) the common treatment used to compare different uterotonics indirectly is similar when it appears in different trials (e.g. oxytocin is administered in a similar way in oxytocin versus misoprostol trials and in oxytocin versus oxytocin plus ergometrine trials); 2) all pairwise comparisons do not differ with respect to the distribution of effect modifiers (e.g. the design and study characteristics of oxytocin versus misoprostol trials are similar to oxytocin versus oxytocin plus ergometrine trials). The assumption of transitivity was evaluated epidemiologically by comparing the clinical and methodological characteristics of sets of studies from the various treatment comparisons.
Assessment of reporting biases
We assessed potential reporting bias for the primary outcomes by assessing the sensitivity of results to exclusion of studies with fewer than 400 participants.
Data synthesis
Methods for direct treatment comparisons
Initially, we performed pairwise meta‐analyses using a random‐effects model in Stata for every treatment comparison with at least two studies (DerSimonian 1986).
Methods for indirect and mixed comparisons
We performed the network meta‐analysis within a frequentist framework using multivariate meta‐analysis estimated by restricted maximum likelihood. All analyses were done using Stata statistical software, release 14 (StataCorp, College Station, TX). We used the network suite of Stata commands designed from this purpose (White 2012; White 2015).
Assessment of statistical heterogeneity
Assumptions when estimating the heterogeneity
In pairwise meta‐analyses we estimated the heterogeneity for each comparison. In network meta‐analysis we assumed a common estimate for the heterogeneity variance across all of the different comparisons.
Measures and tests for heterogeneity
We assessed statistically the presence of heterogeneity within each pairwise comparison for the primary outcomes using the I2 statistic that measures the percentage of variability that cannot be attributed to random error (Higgins 2002). The assessment of statistical heterogeneity in the entire network was based on the magnitude of the heterogeneity variance parameter estimated from the multivariate meta‐analysis.
Assessment of statistical inconsistency
To check the assumption of consistency in the entire network we used the “design‐by‐ treatment” interaction model as described by Higgins (Higgins 2012). This method accounts for a different source of inconsistency that can occur when studies with different designs (two‐arm trials versus three‐arm trials) give different results as well as disagreement between direct and indirect evidence. Using this approach we inferred about the presence of inconsistency from any source in the entire network based on a Chi2 test.
Investigation of heterogeneity and inconsistency
Where we found important heterogeneity and/or inconsistency, we explored the possible sources for primary outcomes. Where sufficient studies were available, we performed multivariate meta‐analyses or subgroup analyses by using the following potential effect modifiers as possible sources of inconsistency and/or heterogeneity.
Population: prior risk of PPH (high versus low), mode of delivery (vaginal versus caesarean birth), setting (hospital versus community).
Intervention: dose of misoprostol ( ≥ 600 mcg versus < 600 mcg), and regimen of oxytocin (bolus versus bolus plus infusion versus infusion only).
Risk of bias of the studies: studies are ranked as “low risk of bias” if they are double‐blinded, and have allocation concealment with little loss to follow‐up (less than 10%). The concealed studies with assessor blinding and little loss to follow‐up (less than 10%) are ranked as “intermediate risk of bias” and the rest as “high risk of bias”. We considered that assessor blinding was likely to be very important, in order to eliminate any risk of bias in subjective measurements or estimates of blood loss (not all studies measure this outcome objectively). We considered protocol publication in advance of the results to be an unsuitable criterion for sensitivity analyses, because protocol publication only became widespread in recent years.
Funding source (high versus low risk of bias).
Whether an objective method of outcome assessment was employed (objective versus subjective). Objective methods of blood loss measurement were considered to be all methods that employed a measurement of the blood loss. This is in contrast to subjective methods where a healthcare professional is estimating the blood loss, usually visually.
Trial size (excluding small studies, in recognition of the greater likelihood for small studies than large or multi‐centre studies to suffer publication bias). In terms of trial size, there is evidence that smaller studies can exaggerate estimated benefits (Nüesch 2010). However, the cut‐off for deciding the definition of a small study can vary between research topics. For this topic, it appears that trials with more than 400 participants are more likely to be of higher quality, prospectively registered and overall at low risk of bias.
Randomisation unit (cluster versus individual).
Subgroup analysis
For the primary outcomes we carried out the following subgroup analyses.
Population: prior risk of PPH (high versus low), mode of delivery (vaginal versus caesarean birth), setting (hospital versus community).
Intervention: dose of misoprostol (≥ 600 mcg versus < 600 mcg), and regimen of oxytocin (bolus versus bolus plus infusion versus infusion only).
We assessed subgroup differences by firstly comparing the network diagram for each subgroup. Next, we performed a network meta‐analysis for each subgroup and we compared their relative treatment effects and their relative treatment ranking..
Sensitivity analysis
For the primary outcomes we performed sensitivity analysis for the following.
Risk of bias of the studies as described previously.
Funding source as described previously.
Whether an objective method of outcome assessment was employed (objective versus subjective).
Trial size as described previously.
Trials that also randomised participants to co‐interventions such as uterine massage or controlled cord traction.
Trials with more than 10% missing data.
Trials published before 1990.
Randomisation unit (cluster versus individual).
Choice of relative effect measure (RR versus OR).
Use of fixed‐effect versus random‐effects model.
Differences were assessed by evaluating the relative effects and assessment of model fit.
Results
Description of studies
Results of the search
The results of the search strategy are summarised in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow diagram (Figure 1).
The search of Cochrane Pregnancy and Childbirth's (CPC) Trials Register in June 2015 retrieved 469 trial reports. A further 137 records were retrieved from additional author searches and manual searching of reference lists. In October 2017, an updated search of the CPC Register retrieved an additional 85 trial reports. After exclusion of duplicates, we assessed for eligibility 378 trials by full‐text evaluation. We have included in this systematic review 140 randomised trials (192 trial reports) involving 88,947 women, From these, 137 trials involving 87,466 women, comparing six active drugs contributed data to the network meta‐analysis.
We have contacted the authors from 95 primary randomised trials for additional data or clarifications and were able to add in this review data not reported in the published reports for 40 randomised trials.
We excluded 238 trials (267 trial reports) and 11 trials are ongoing (Ongoing studies). We also have 94 trial reports that are awaiting further classification and we plan to assess these at the next update (see: Studies awaiting classification).
Included studies
Most studies were reported in English;nine translations were obtained (four Spanish, two French, two Turkish and one Chinese). The studies were conducted in various countries and often involved more than one country. The UK was the country where most studies were conducted (11 studies). A number of multi‐arm trials were identified: two five‐arm trials, six four‐arm trials and 15 three‐arm trials. The median size of the trials was around 248 participants (interquartile (IQR) 136 to 622).
Most trials (96.4%, 135/140) were performed in a hospital setting with only four community trials (2.9%) and one (0.7%) in a mixed setting. The majority of the trials included women undergoing a vaginal birth (74.3%, 104/140), and 36 trials (25.7%) involved women undergoing elective or emergency caesareans. Women included in the trials were judged to be at high risk for postpartum haemorrhage (PPH) in 43 of 140 trials (30.7%), low risk in 42 trials (30%) and 50 trials (35.7%) included women both at high or low risk for PPH. The risk for PPH was not specified in five trials (3.6%).
The gestational age of women included in the trials was not specified in 70 of 140 trials (50%). Thirty‐two trials (22.9%) included women with term pregnancies and the remaining 38 trials (27.1%) included women with both pre‐term or term pregnancies. Eighty‐two trials (58.6%) included women with a singleton pregnancy, 23 trials (16.4%) included women with either singleton or multiple pregnancies and 35 trials (25%) did not specify this criterion. Four trials (2.9%) included only nulliparous or primigravida women, one trial included only multiparous women (0.7%), 35 trials (25%) included women of all parities and 100 trials (71.4%) did not specify the parity of the women included in the trials. Exclusion criteria varied significantly and usually encompassed women with significant medical comorbidities. See Characteristics of included studies for details.
Excluded studies
We excluded 238 randomised trials (for details seeCharacteristics of excluded studies).
Risk of bias in included studies
We present summaries of the methodological quality of the included studies for each of the domains we assessed across all studies (Figure 2) and for each included study (Figure 3).
Figure 2.
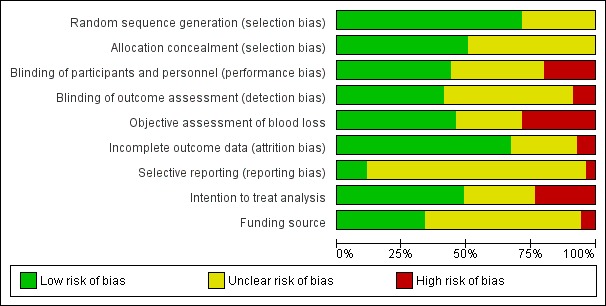
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Trials with evidence of inadequate random sequence generation were excluded from this review. As a result 100 of 140 included trials (71.4%) were found to have used an adequate method generating the random sequence and were at low risk of bias. However, 40 trials (28.6%) did not report the method used in sufficient detail and the risk of bias was judged to be unclear. Seventy‐one of 140 trials (50.7%) reported adequate methods for allocation concealment and were judged to be at low risk of bias. Sixty‐nine trials (49.3%), did not provide enough information to assess allocation concealment and the risk of bias was judged to be unclear.
Blinding
In total, 61 of 140 trials (43.6%) reported adequate methods for blinding both participants and personnel to treatment allocation. Twenty‐eight trials (20.0%) were judged to be at high risk of bias for blinding of participants and personnel. Fifty‐one trials (36.4%) did not provide enough information to assess the blinding of participants and personnel and the risk of bias was judged to be unclear. Fifty‐eight of 140 trials (41.47%) reported adequate methods for blinding the assessment of the primary outcomes. Twelve trials (8.6%) were judged to be at high risk of bias for blinding the assessment of the primary outcomes. Seventy trials (50.0%) did not provide enough information for blinding the assessment of the primary outcomes and the risk of bias was judged to be unclear.
Incomplete outcome data
Ninety‐four of 140 trials (67.1%) were judged to be at a low risk of bias. In these trials, missing outcome data were less than 10% and balanced in numbers across intervention groups with similar reasons for missing data across groups. In 10 trials (7.1%), more than 10% of patients dropped out or were not analysed as per the “intention‐to‐treat” principles following randomisation, indicating a high risk of bias. Thirty‐six trials (25.7%) did not provide enough information to assess so that it was uncertain whether or not the handling of incomplete data was appropriate and the risk of bias was judged to be unclear in these trials.
Selective reporting
Only 16 of 140 trials (11.4%) pre‐specified all outcomes in publicly available study protocols and were judged to be at low risk of bias. Five trials (3.6%) did not report all pre‐specified outcomes as reported in their published protocols or methodology within the main report and were judged to be at high risk of bias for selective reporting. For most trials (119 trials; 85.0%), we were unable to trace a published protocol and the risk of bias was judged to be unclear.
Other potential sources of bias
We found that 47 of 140 trials (33.6%) were either conducted with public or no funding and did not declare potential conflicts of interest. Eight trials (5.7%) were judged to be at high risk of bias as they were funded directly by the pharmaceutical industry. Eighty‐five trials (60.7%) did not provide enough information to assess the source of funding or potential conflicts of interest and the risk of bias was judged to be unclear.
Among all the studies, 64 of 140 trials (45.7%) reported relatively objective methods for measuring blood loss such as weighing sponges, measurements in drapes or volumetric assessment and were judged to be at low risk of bias. Forty trials (28.6%) were judged to be at high risk of bias for measuring blood loss as they used subjective measurement such as clinical or visual estimates. Thirty‐six trials (25.7%) did not measure blood loss or did not provide enough information to assess the method for measuring blood loss, and the risk of bias was judged to be unclear. Three included studies did not report useable blood loss data and were not included in the network meta‐analysis (Fawole 2011, Kikutani 2006,Ramirez 2001).
For the purpose of sensitivity analysis we analysed how many trials were judged to be at low, intermediate or high overall risk of bias. For PPH ≥ 500 mL, 29 of 100 trials (29%) were found to be at low overall risk of bias. Seventy‐one of 100 trials (71%) were judged to be at high risk of bias as they were judged to be either at high risk or unclear risk of bias for at least one of the domains mentioned above. There were no trials judged as intermediate risk of bias ‐ see Sensitivity analysis for information about how this risk of bias has impacted the results.
Effects of interventions
See: Table 1
Primary outcomes
Postpartum haemorrhage (PPH) ≥ 500 mL
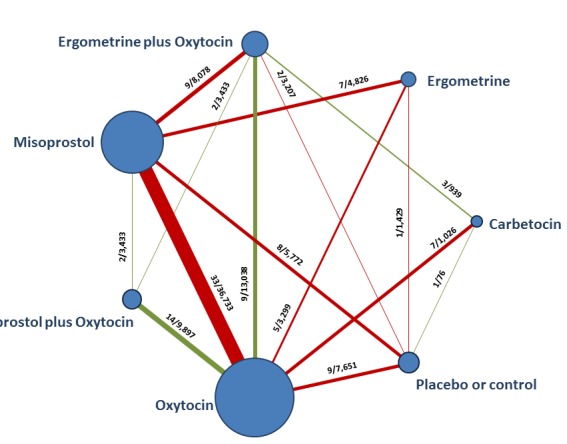
The network diagram for PPH ≥ 500 mL is presented in Figure 4. Oxytocin was the most frequently investigated uterotonic agent (82%, 82 of 100 trials) (Figure 4).
Figure 4.
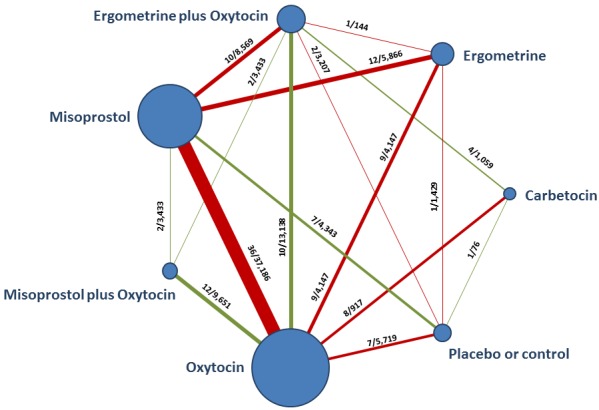
Network diagram for PPH ≥ 500 mL. The nodes represent an intervention and their size is proportional to the number of trials comparing this intervention to any other in the network. The lines connecting each pair of interventions represent a direct comparison and are drawn proportional to the number of trials making each direct comparison. Numbers on the lines represent the number of trials and participants for each comparison. The colour of the line is green when more than 50% of the trials involved in the specific direct comparison are judged to be at “low risk of bias” if they were double‐blinded, and had allocation concealment with little loss to follow‐up (less than 10%). The colour is red when less than 50% of the trials are at “low risk of bias”. Multi‐arm trials contribute to more than one comparison.
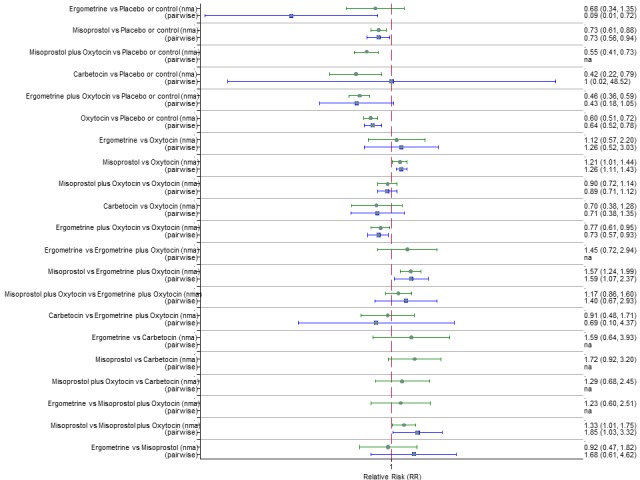
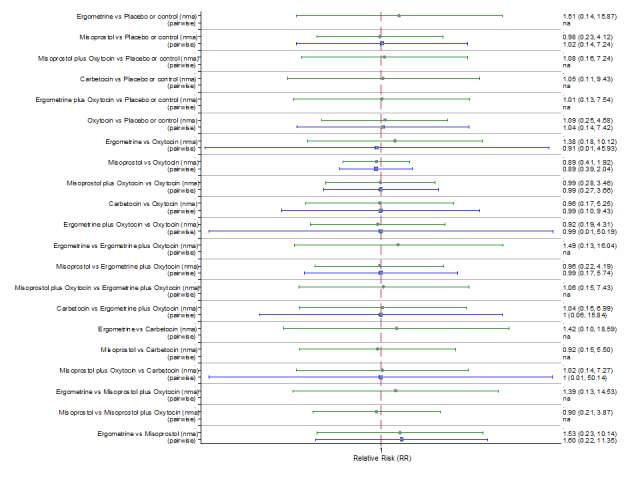
Pooled effect sizes from the network meta‐analysis of 100 trials suggested that all drugs were effective for preventing PPH ≥ 500 mL when compared with placebo or no treatment (Figure 5). The three most effective options for prevention of PPH ≥ 500 mL were ergometrine plus oxytocin combination, carbetocin, and misoprostol plus oxytocin combination. All three drugs more effectively reduced the risk of PPH ≥ 500 mL than oxytocin (ergometrine plus oxytocin risk ratio (RR) 0.69 (95% confidence interval (CI) 0.57 to 0.83); carbetocin RR 0.72 (95% CI 0.52 to 1.00); misoprostol plus oxytocin RR 0.73 95% CI (0.60 to 0.90), (Figure 5). Ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin were also found to be more effective when compared with misoprostol and ergometrine when used alone. There was evidence of global inconsistency in this analysis, where the direct and network (combining direct and indirect) randomised evidence were not in agreement (P = 0.046). The inconsistency was driven by a single unblinded study of ergometrine versus no treatment (Begley 1990).
Figure 5.
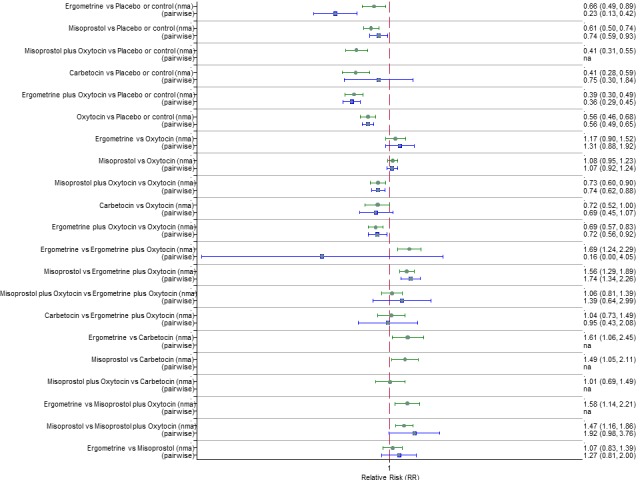
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL.
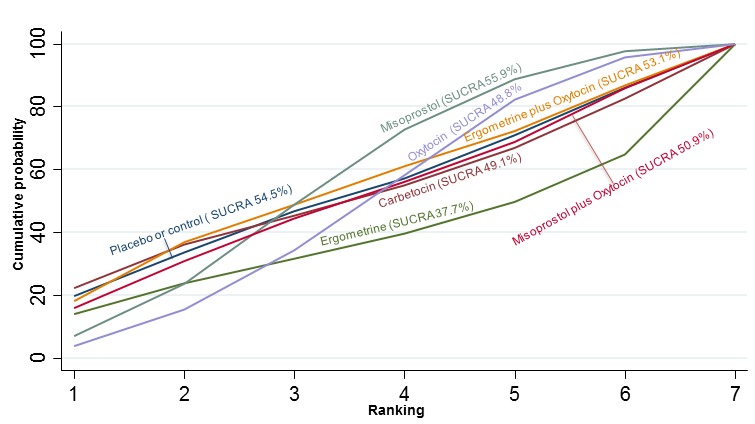
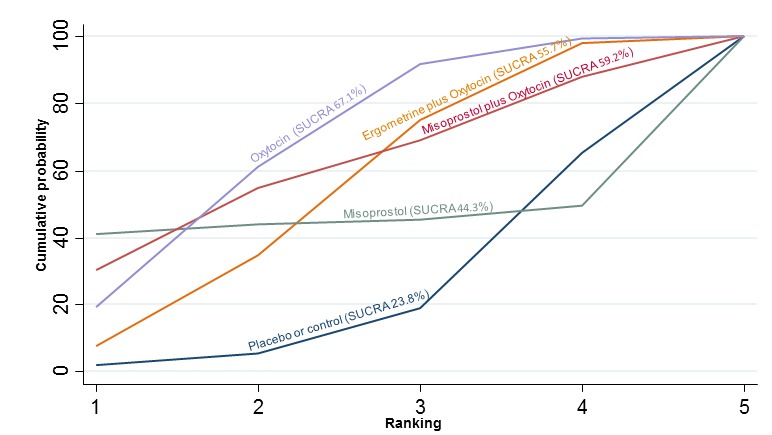
The cumulative probabilities for each agent being at each possible rank for preventing PPH ≥ 500 mL are shown in Figure 6. Ranking indicates the cumulative probability of being the best drug, the second best, the third best, etc. The highest ranked agents were ergometrine plus oxytocin combination, carbetocin, and misoprostol plus oxytocin combination with an almost 100% probability of these three agents being ranked first, second or third best. Oxytocin was ranked fourth and its probability of being ranked in the top three agents was close to 0%.
Figure 6.
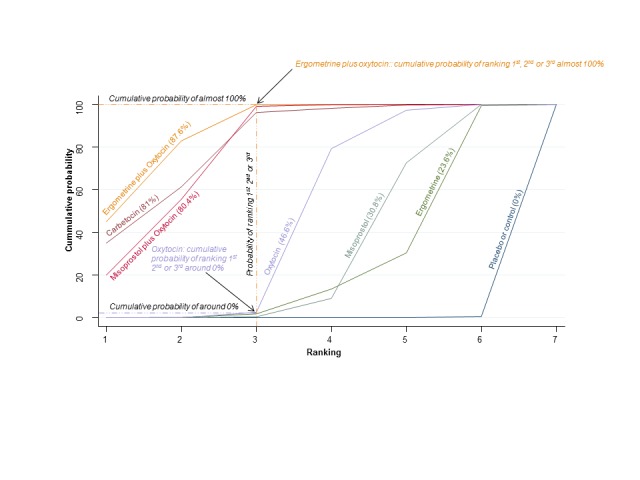
Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL. Ranking indicates the cumulative probability of being the best drug, the second best, the third best, etc. The x‐axis shows the relative ranking and the y‐axis the cumulative probability of each ranking. We estimate the SUrface underneath this Cumulative RAnking line (SUCRA); the larger the SUCRA the higher its rank among all available drug options.
According to GRADE, the quality of evidence was rated as moderate due to inconsistency for the comparisons of ergometrine plus oxytocin versus oxytocin and misoprostol plus oxytocin versus oxytocin (Table 1). However, the quality of evidence was ranked very low for the comparison of carbetocin versus oxytocin due to the risk of bias in the studies comparing the two uterotonics, inconsistency and imprecision (Table 1).
Postpartum haemorrhage (PPH) ≥ 1000 mL
The network diagram for PPH ≥ 1000 mL is presented in Figure 7. Oxytocin was the most frequently investigated uterotonic agent (85.6%, 77 of 90 trials) (Figure 7).
Figure 7.
Network diagram for PPH ≥ 1000 mL.
Pooled effect estimates from the network meta‐analysis of 90 trials suggested that all agents except ergometrine were effective for preventing PPH ≥ 1000 mL when compared with placebo or no treatment (Figure 8). Ergometrine plus oxytocin combination was the only agent found to be more effective when compared with the standard agent oxytocin (RR 0.77, 95% CI 0.61 to 0.95) although carbetocin (RR 0.70, 95% CI 0.38 to 1.28) and misoprostol plus oxytocin combination (RR 0.90, 95% CI 0.72 to 1.14) demonstrated a trend towards reduction in this outcome (Figure 8). There was no evidence of global inconsistency (P = 0.345).
Figure 8.
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 1000 mL.
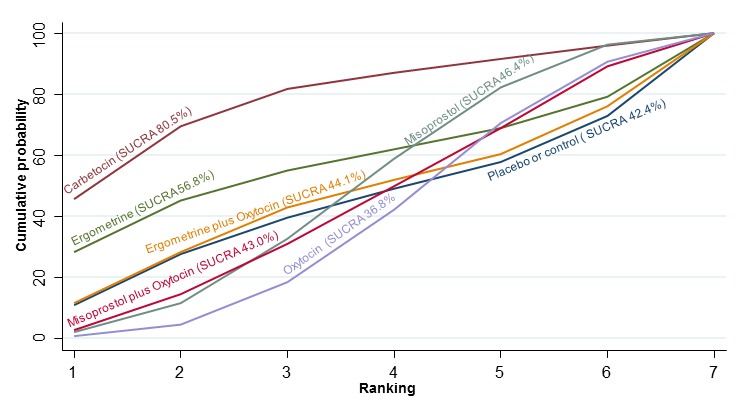
The cumulative probabilities for each agent being at each possible rank for PPH ≥ 1000 mL are shown in Figure 9. The highest ranked agents were ergometrine plus oxytocin combination, carbetocin, and misoprostol plus oxytocin combination. Oxytocin was still ranked fourth and its probability of being ranked in the top three agents was approximately 20%.
Figure 9.
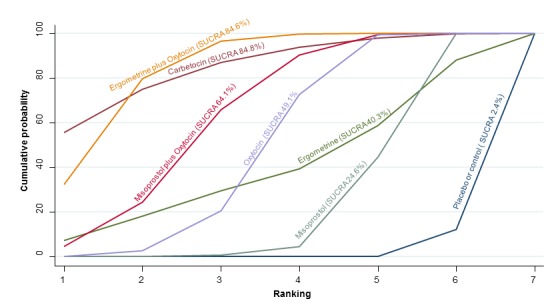
Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 1000 mL.
According to GRADE, the quality of evidence was rated as high for the comparison of ergometrine plus oxytocin combination versus oxytocin (Table 1). However, the quality of evidence was ranked moderate for the comparison of misoprostol plus oxytocin combination versus oxytocin due to imprecision in the confidence intervals. The quality of evidence for carbetocin versus oxytocin was ranked as low due to the risk of bias in the studies comparing the two uterotonics and the imprecision (Table 1).
Secondary outcomes
Maternal death
The network diagram for maternal death is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 50 trials suggested that there were no meaningful differences between all uterotonic agents for maternal deaths as this outcome was so rare (Figure 10). There was no evidence of global inconsistency in this analysis (P = 0.999). Figure 11 shows the cumulative probabilities for each agent being at each possible rank for maternal death. No reliable ranking could be derived for this outcome.
Figure 10.
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for maternal death.
Figure 11.
Cumulative rankograms comparing each of the uterotonic drugs for prevention of maternal death.
Maternal deaths or severe morbidity
The network diagram for maternal death or severe morbidity is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 37 trials suggested that there were no detectable differences between all agents for maternal deaths or severe morbidity as this outcome was still so rare (Figure 12). There was no evidence of global inconsistency in this analysis (P = 0.884). Figure 13 shows the cumulative probabilities for each agent being at each possible rank for maternal death or severe morbidity. No sensible ranking could be derived for this outcome due to limited data.
Figure 12.

Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for maternal death or severe morbidity.
Figure 13.
Cumulative rankograms comparing each of the uterotonic drugs for prevention of maternal deaths or severe morbidity events.
Additional uterotonics
The network diagram for the requirement of additional uterotonics is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 107 trials suggested that all agents were effective at reducing the requirement of additional uterotonics when compared with placebo or no treatment (Figure 14). Ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin were found to be more effective when compared with the standard agent oxytocin (Figure 14). Ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin were also found to be more effective when compared with misoprostol and ergometrine when used alone. There was no evidence of global inconsistency in this analysis (P = 0.275). Figure 15 shows the cumulative probabilities for each agent being at each possible rank for the requirement of additional uterotonics. The highest ranked agents were carbetocin, misoprostol plus oxytocin and ergometrine plus oxytocinwith an almost 100% probability of these three agents being ranked in the top three. Oxytocin was ranked fourth and its probability in being ranked in the top three agents was close to 0%. The lowest ranked agents were misoprostol, ergometrine and placebo or no treatment.
Figure 14.
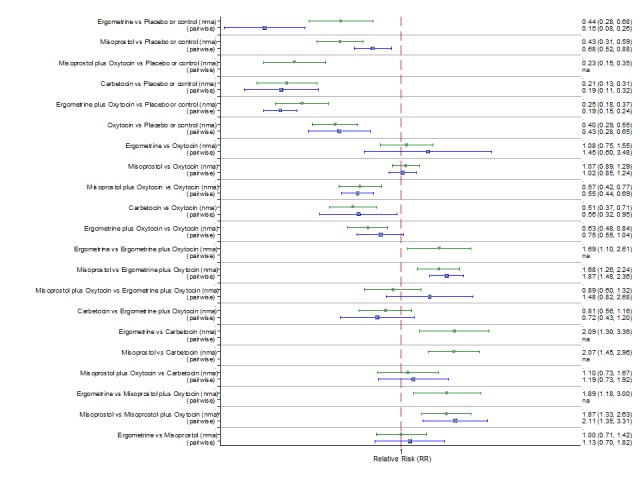
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for the requirement of additional uterotonics.
Figure 15.
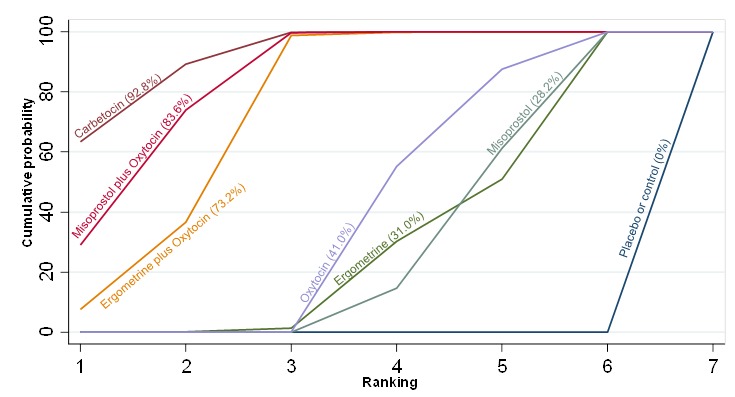
Cumulative rankograms comparing each of the uterotonic drugs for the requirement of additional uterotonics.
Transfusion
The network diagram for blood transfusion is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 92 trials suggested that all agents except ergometrine were effective for preventing blood transfusion when compared with placebo or no treatment (Figure 16). Misoprostol plus oxytocin was the only agent found to be more effective when compared with the standard agent oxytocin. Carbetocin and ergometrine plus oxytocin demonstrated a trend towards reduction of this outcome (Figure 16). There was no evidence of global inconsistency in this analysis (P = 0.061). Figure 17 shows the cumulative probabilities for each agent being at each possible rank for preventing blood transfusion. The highest ranked agents were misoprostol plus oxytocin, carbetocin and ergometrine plus oxytocin. Oxytocin was ranked fifth behind misoprostol and its probability of being ranked in the top three agents was less than 10%.
Figure 16.
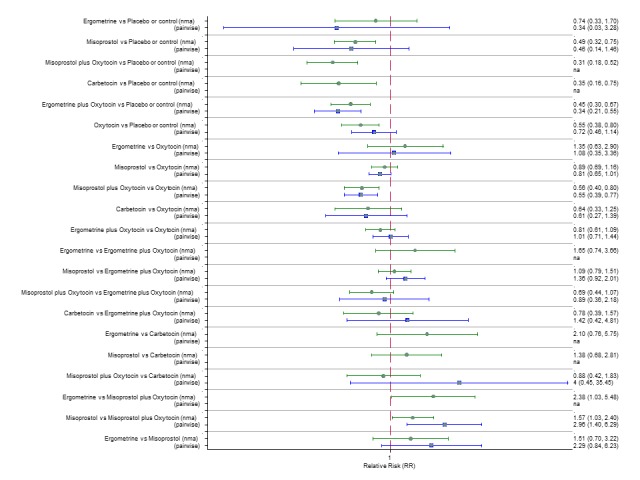
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for the requirement of blood transfusion.
Figure 17.
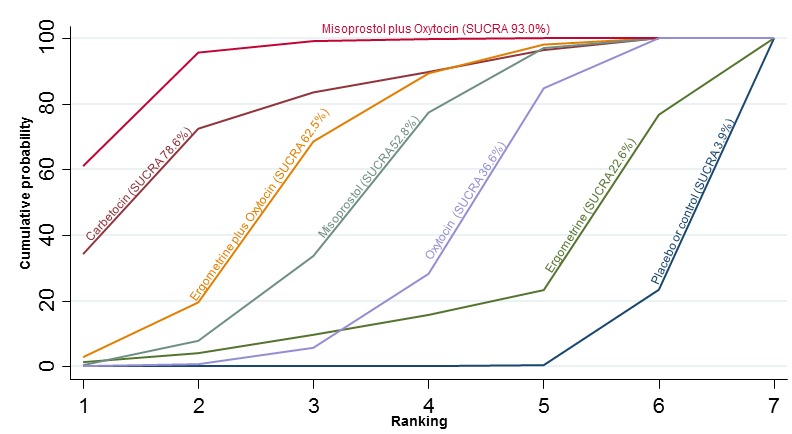
Cumulative rankograms comparing each of the uterotonic drugs for the requirement of blood transfusion.
Manual removal of the placenta
The network diagram for the requirement of manual removal of placenta is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 67 trials suggested that there are no clear differences between all agents for this outcome (Figure 18). There was evidence of global inconsistency in this analysis (P = 0.025). However, we note that the CIs for both the network meta‐analysis and direct evidence were overlapping across all comparisons suggesting locally‐consistent results except for ergometrine versus placebo or no treatment and carbetocin versus oxytocin based on single studies. Figure 19 shows the cumulative probabilities for each agent being at each possible rank for prevention of the manual removal of placenta. No clear ranking could be derived for this outcome with all agents being comparable, except for carbetocin that appeared to have the highest probability in being the top ranked agent with a probability close to 80%.
Figure 18.
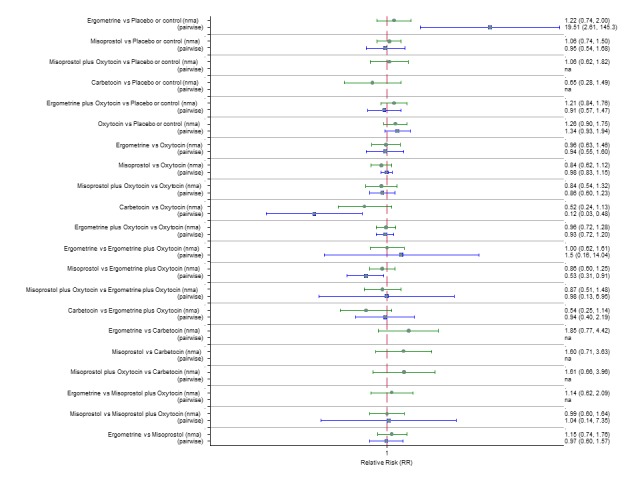
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for the requirement of manual removal of placenta.
Figure 19.
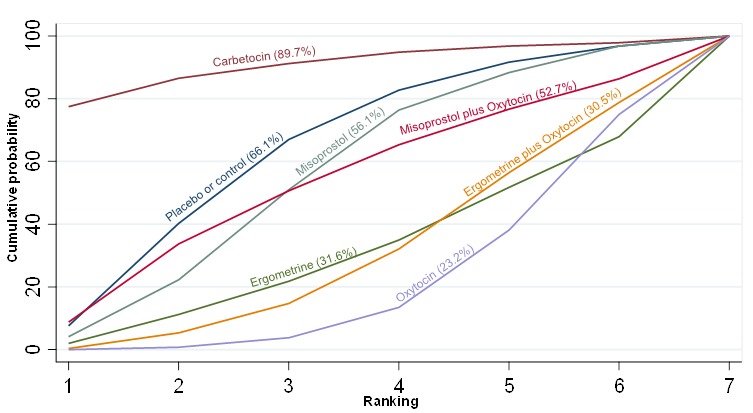
Cumulative rankograms comparing each of the uterotonic drugs for the requirement of manual removal of placenta.
Mean volumes of blood loss
The network diagram for blood loss (mL) as a continuous outcome is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 102 trials suggested that all agents are effective for reducing blood loss as a continuous outcome when compared with placebo or no treatment (Figure 20). Carbetocin and misoprostol plus oxytocin were found to be more effective when compared with the standard agent oxytocin. Ergometrine plus oxytocin also demonstrated a trend towards reduction of this outcome (Figure 20). Carbetocin and misoprostol plus oxytocin were more effective than ergometrine plus oxytocin in reducing blood loss. Carbetocin and misoprostol plus oxytocin were also found to be more effective when compared with misoprostol and ergometrine when used alone. There was no evidence of global inconsistency in this analysis (P = 0.111). Figure 21 shows the cumulative probabilities for each agent being at each possible rank for preventing blood loss (mL) as a continuous outcome. The highest ranked agents were carbetocin, misoprostol plus oxytocin and ergometrine plus oxytocin. Oxytocin was ranked fourth and its probability in being ranked in the top three agents was less than 10%. The lowest ranked agents were misoprostol, ergometrine and placebo or no treatment.
Figure 20.
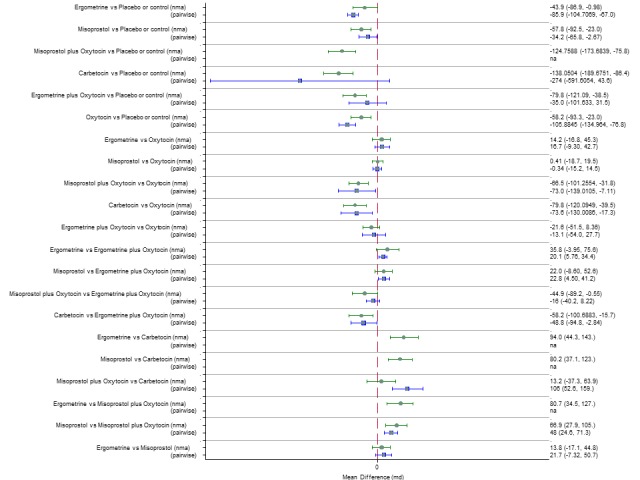
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for blood loss (mL).
Figure 21.
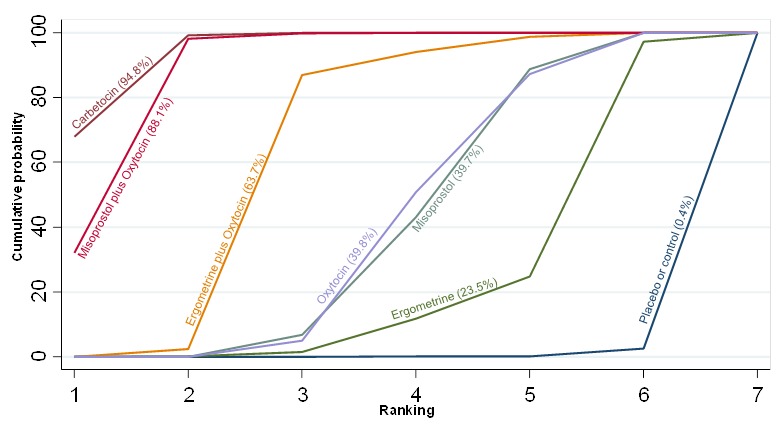
Cumulative rankograms comparing each of the uterotonic drugs for blood loss (mL).
Mean durations of the third stage of labour
The network diagram for the duration of the third stage (minutes) as a continuous outcome is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 58 trials suggested that all agents are effective for reducing the duration of the third stage as a continuous outcome when compared with placebo or no treatment except for carbetocin and misoprostol plus oxytocin that demonstrated a similar trend towards reduction of this outcome (Figure 22). There were no significant differences between all active agents for this outcome (Figure 22). There was evidence of global inconsistency in this analysis (P = 0.011) and these results need to be interpreted with caution. Figure 23 shows the cumulative probabilities for each agent being at each possible rank for the reduction of the duration of the third stage. No sensible ranking could be derived for this outcome with all agents being comparable. The exception was ergometrine plus oxytocin that appeared to have the highest probability in being the top ranked agent with a probability close to 60% and the placebo or no treatment that appeared to have the lowest ranking.
Figure 22.
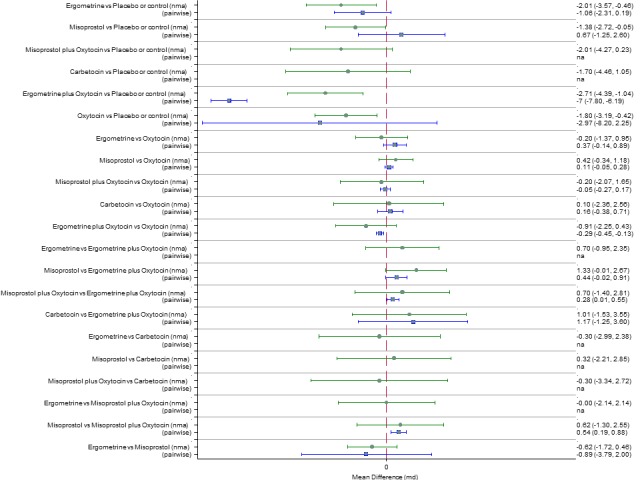
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for duration of third stage (minutes).
Figure 23.
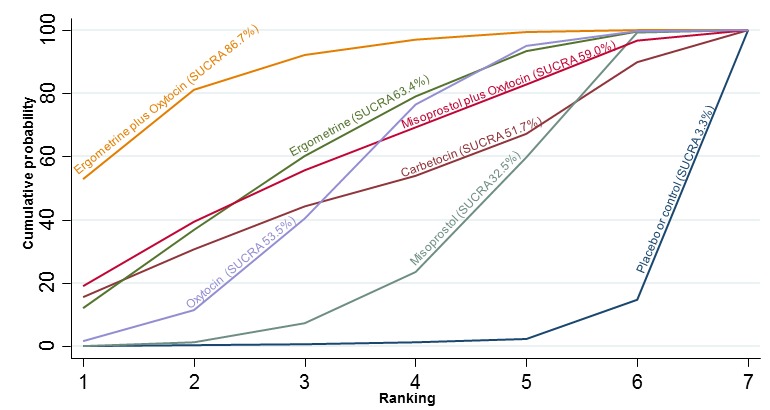
Cumulative rankograms comparing each of the uterotonic drugs for duration of third stage (minutes).
Change in haemoglobin
The network diagram for the change in haemoglobin measurements before and after birth (g/L) is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 74 trials suggested that misoprostol plus oxytocin and carbetocin are effective for reducing the change in haemoglobin measurements when compared with placebo or no treatment (Figure 24). Misoprostol plus oxytocin was the only agent found to be more effective when compared with the standard agent oxytocin (Figure 24). The combination of misoprostol plus oxytocin was also more effective than misoprostol and ergometrine when used alone. Carbetocin was more effective than ergometrine when used alone. However, there was evidence of substantial global inconsistency in this analysis (P = 0.001). Figure 25 shows the cumulative probabilities for each agent being at each possible rank for change in haemoglobin measurements before and after birth (g/L). The highest ranked agents were misoprostol plus oxytocin, carbetocin and ergometrine plus oxytocin. Oxytocin was ranked fourth and its probability in being ranked in the top three agents was just over 20%. The lowest ranked agents were misoprostol, ergometrine and placebo or no treatment.
Figure 24.
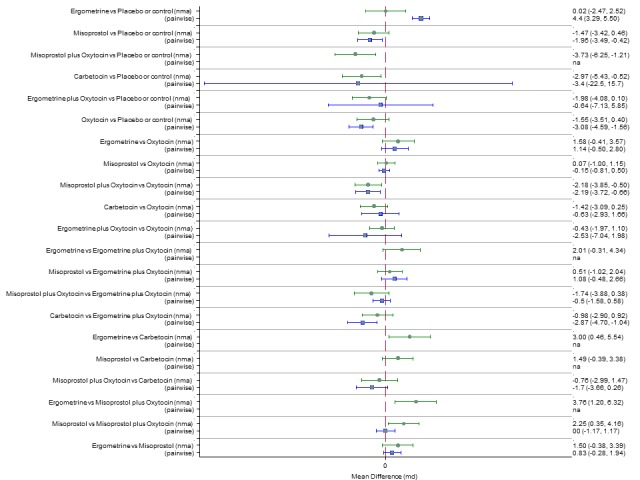
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for change in haemoglobin measurements before and after birth (g/L).
Figure 25.
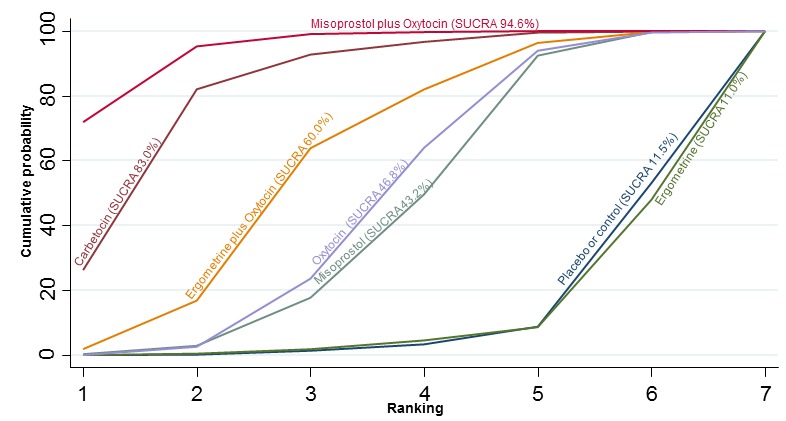
Cumulative rankograms comparing each of the uterotonic drugs for change in haemoglobin measurements before and after birth (g/L).
Clinical signs of blood loss
There were no trials reporting clinical signs of acute blood loss.
Neonatal unit admission
The network diagram for neonatal unit admissions is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of only six trials did not point towards any meaningful differences between all agents for this outcome (Figure 26). There was no evidence of global inconsistency in this analysis (P = 0.989). Figure 27 shows the cumulative probabilities for each agent being at each possible rank for neonatal unit admissions. No sensible ranking could be derived for this outcome because of too few studies reporting this outcome.
Figure 26.
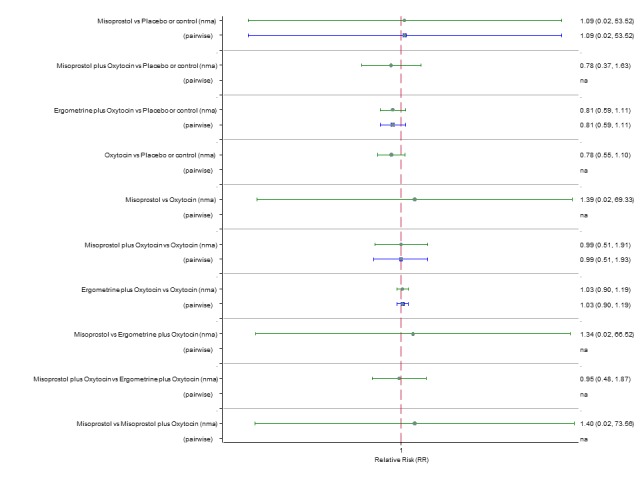
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for neonatal unit admissions.
Figure 27.
Cumulative rankograms comparing each of the uterotonic drugs for neonatal unit admissions.
Breastfeeding at discharge
The network diagram for breastfeeding at discharge is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of only five trials did not point towards any meaningful differences between agents for this outcome (Figure 28). There was no evidence of global inconsistency in this analysis (P = 0.167). Figure 29 shows the cumulative probabilities for each agent being at each possible rank for breastfeeding at discharge. No clear ranking could be derived for this outcome with all agents being comparable again because of too few studies.
Figure 28.
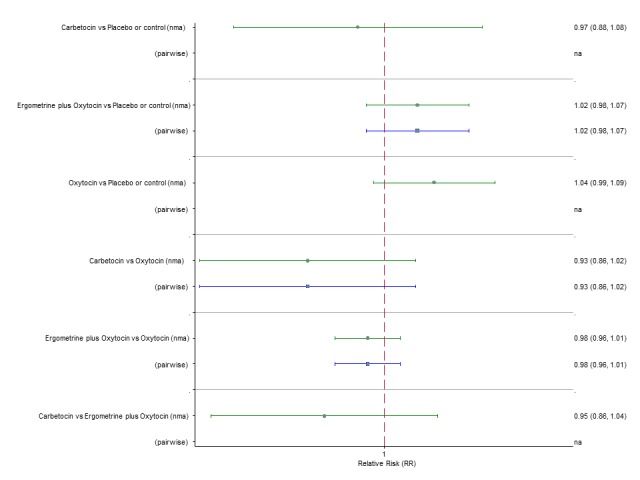
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for breastfeeding at discharge.
Figure 29.
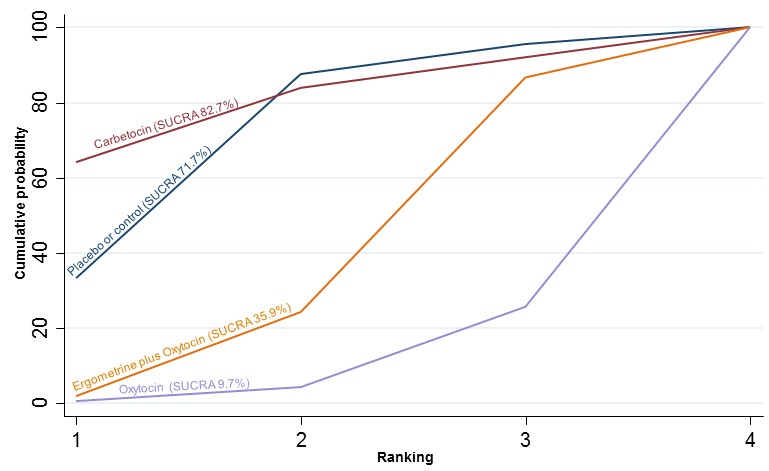
Cumulative rankograms comparing each of the uterotonic drugs for breastfeeding at discharge.
Side‐effects
Nausea
The network diagram for nausea is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 74 trials suggested that ergometrine and ergometrine plus oxytocin are worse than placebo or no treatment in causing nausea (Figure 30). Ergometrine, ergometrine plus oxytocin, misoprostol and misoprostol plus oxytocin were found to be worse in causing nausea when compared with the standard agent oxytocin (Figure 30). Ergometrine, ergometrine plus oxytocin and misoprostol plus oxytocin were significantly worse in causing nausea than carbetocin. There was evidence of global inconsistency in this analysis (P = 0.005). However, we note that the CIs for both the network meta‐analysis and direct evidence were overlapping across all comparisons suggesting locally‐consistent results except for ergometrine versus placebo or no treatment based on a single study. Figure 31 shows the cumulative probabilities for each agent being at each possible rank for causing nausea. The highest ranked and the agents with the least risk of nausea were carbetocin, oxytocin and placebo or no treatment. The lowest ranked and most likely agents to cause nausea were ergometrine plus oxytocin and ergometrine.
Figure 30.
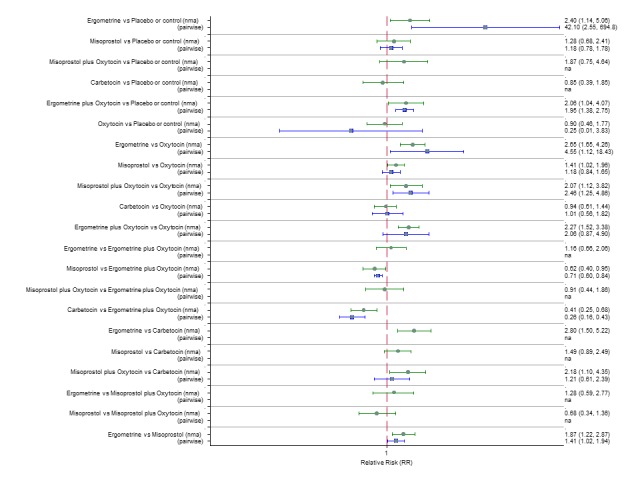
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for nausea.
Figure 31.
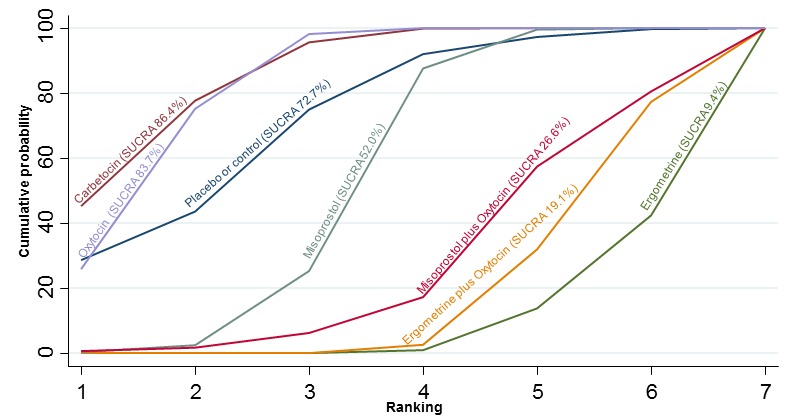
Cumulative rankograms comparing each of the uterotonic drugs for nausea.
Vomiting
The network diagram for vomiting is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 83 trials suggested that ergometrine and ergometrine plus oxytocin are worse than placebo or no treatment in causing vomiting (Figure 32). Ergometrine, ergometrine plus oxytocin, misoprostol and misoprostol plus ocytocin were found to be worse in causing vomiting when compared with the standard agent oxytocin (Figure 32). Ergometrine, ergometrine plus oxytocin, misoprostol and misoprostol plus oxytocin were significantly worse in causing vomiting than carbetocin. There was no evidence of global inconsistency in this analysis (P = 0.06). Figure 33 shows the cumulative probabilities for each agent being at each possible rank for causing vomiting. The highest ranked agents were carbetocin, oxytocin and placebo or no treatment with an almost 100% probability of these three agents being ranked in the top three. The lowest ranked agents were ergometrine plus oxytocin and ergometrine.
Figure 32.
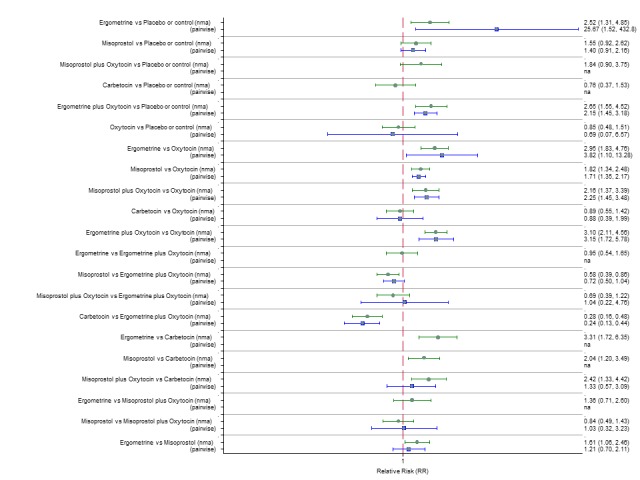
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for vomiting.
Figure 33.
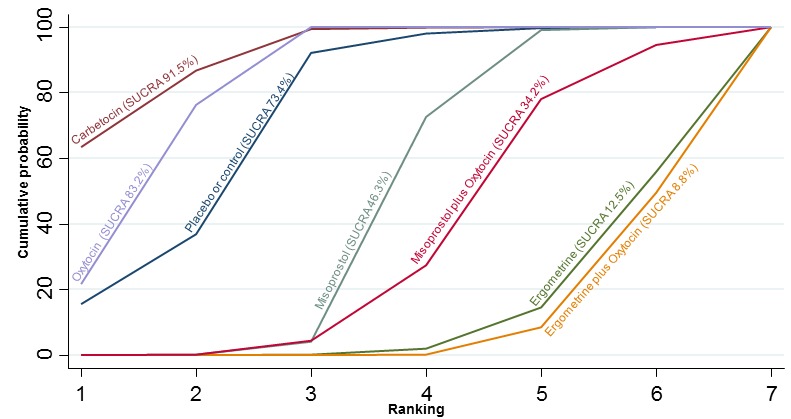
Cumulative rankograms comparing each of the uterotonic drugs for vomiting.
Hypertension
The network diagram for hypertension is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 15 trials suggested that ergometrine is worse than placebo or no treatment in causing hypertension (Figure 34). Ergometrine was found to be worse in causing hypertension when compared with the standard agent oxytocin (Figure 34). Ergometrine was also significantly worse in causing hypertension than carbetocin and misoprostol. There was no evidence of global inconsistency in this analysis (P = 0.481). Figure 35 shows the cumulative probabilities for each agent being at each possible rank for causing hypertension. The lowest ranked agents were ergometrine and ergometrine plus oxytocin. However, not all agents could be ranked because of too few studies in this analysis.
Figure 34.
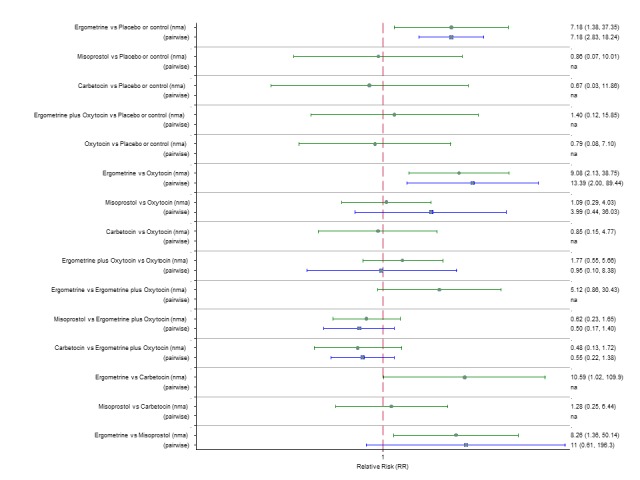
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for hypertension.
Figure 35.
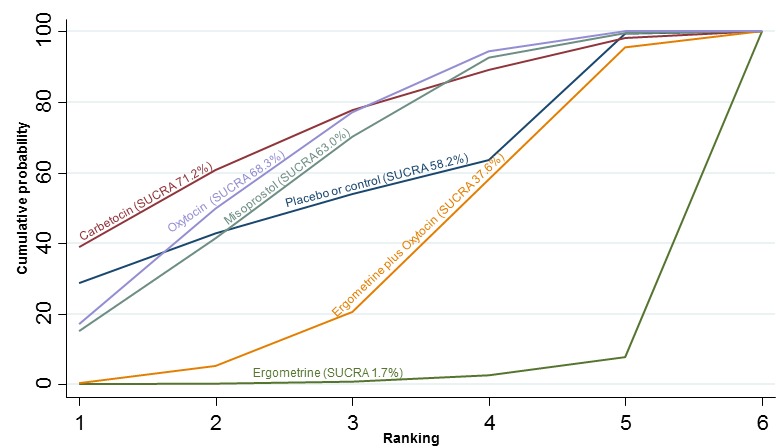
Cumulative rankograms comparing each of the uterotonic drugs for hypertension.
Headache
The network diagram for headache is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 45 trials suggested that ergometrine is worse than placebo or no treatment in causing headache (Figure 36). Ergometrine was found to be worse in causing headache when compared with the standard agent oxytocin (Figure 36). Ergometrine was also significantly worse in causing headache than carbetocin and misoprostol. There was no evidence of global inconsistency in this analysis (P = 0.826). Figure 37 shows the cumulative probabilities for each agent being at each possible rank for causing headache. The lowest ranked agents were ergometrine, misoprostol plus oxytocin and ergometrine plus oxytocin. The highest ranked agents were placebo or no treatment, carbetocin and oxytocin.
Figure 36.
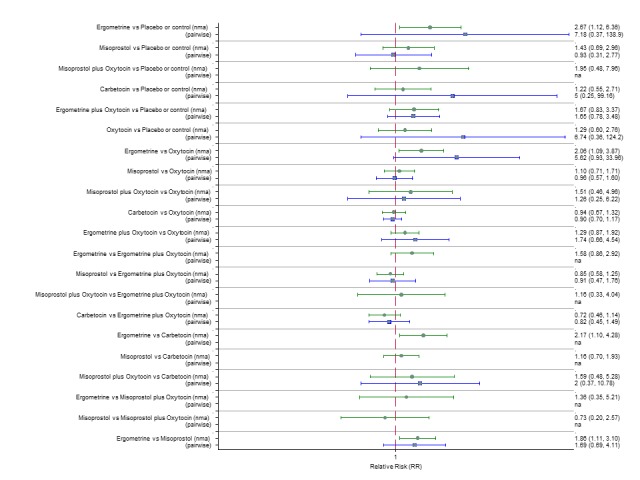
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for headache.
Figure 37.
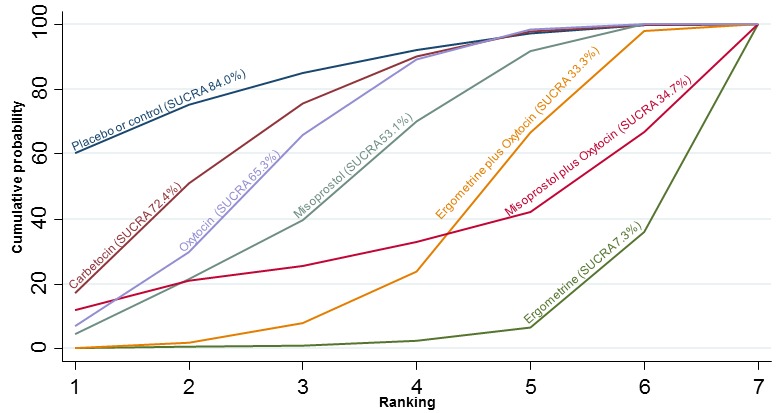
Cumulative rankograms comparing each of the uterotonic drugs for headache.
Fever
The network diagram for fever is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 64 trials suggested that misoprostol and misoprostol plus oxytocin are worse than placebo or no treatment in causing fever (Figure 38). Misoprostol and misoprostol plus oxytocin were found to be worse in causing fever when compared with the standard agent oxytocin (Figure 38). Misoprostol and misoprostol plus oxytocin were also significantly worse in causing fever than carbetocin, ergometrine and ergometrine plus oxytocin with the exception of the comparison carbetocin versus misoprostol plus oxytocin which fell just short of being statistically significant. There was no evidence of global inconsistency in this analysis (P = 0.352). Figure 39 shows the cumulative probabilities for each agent being at each possible rank for causing fever. The highest ranked agents were carbetocin, oxytocin and placebo or no treatment. The lowest ranked agents were misoprostol and misoprostol plus oxytocin. The rest of the agents were similar in ranking to the placebo or no treatment group.
Figure 38.
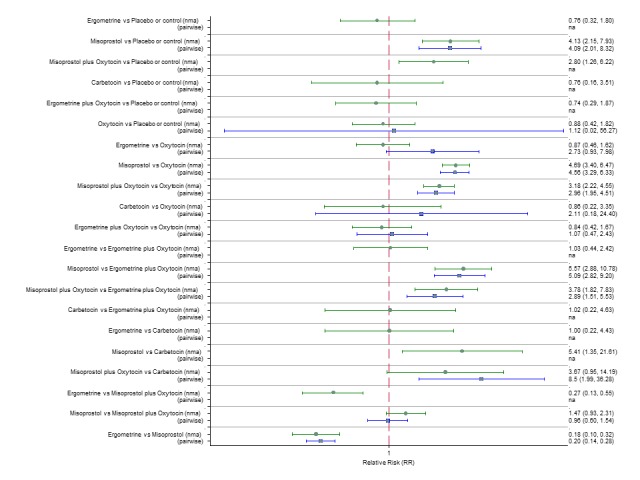
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for fever.
Figure 39.
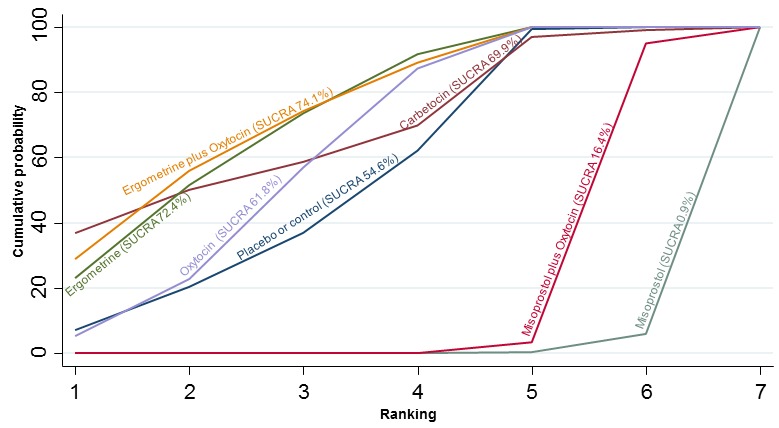
Cumulative rankograms comparing each of the uterotonic drugs for fever.
Shivering
The network diagram for shivering is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 87 trials suggested that misoprostol and misoprostol plus oxytocin are worse than placebo or no treatment in causing shivering (Figure 40). Misoprostol and misoprostol plus oxytocin were found to be worse in causing shivering when compared with the standard agent oxytocin (Figure 40). Misoprostol and misoprostol plus oxytocin were also significantly worse in causing shivering than carbetocin, ergometrine and ergometrine plus oxytocin. There was no evidence of global inconsistency in this analysis (P = 0.923). Figure 41 shows the cumulative probabilities for each agent being at each possible rank for causing shivering. The highest ranked agents were carbetocin and oxytocin. The lowest ranked agents were misoprostol and misoprostol plus oxytocin. Ergometrine and ergometrine plus oxytocin were similar in ranking to the placebo or no treatment group.
Figure 40.
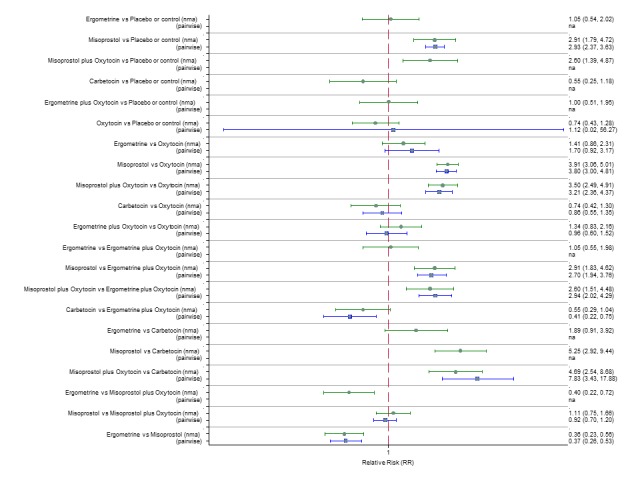
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for shivering.
Figure 41.
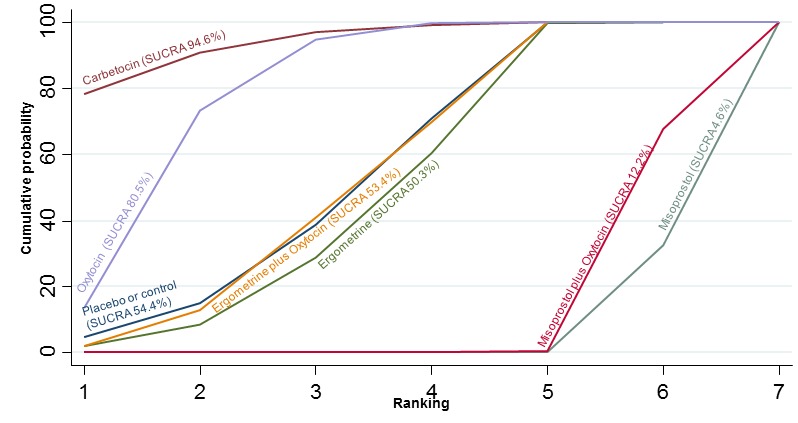
Cumulative rankograms comparing each of the uterotonic drugs for shivering.
Tachycardia
The network diagram for tachycardia is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of seven trials suggested only that carbetocin is worse than oxytocin and ergometrine plus oxytocin in causing tachycardia, but most of the comparisons were based on single studies (Figure 42). There was no evidence of global inconsistency in this analysis (P = 0.361). Figure 43 shows the cumulative probabilities for each agent being at each possible rank for causing tachycardia. No clear ranking emerged and not all agents could be ranked because of the lack of studies in this analysis.
Figure 42.
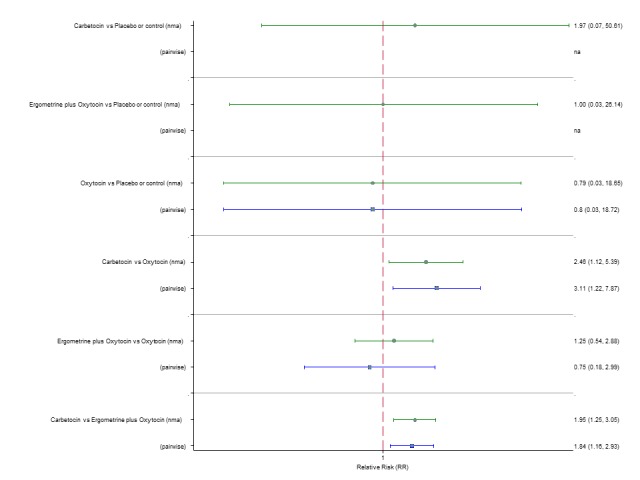
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for tachycardia.
Figure 43.
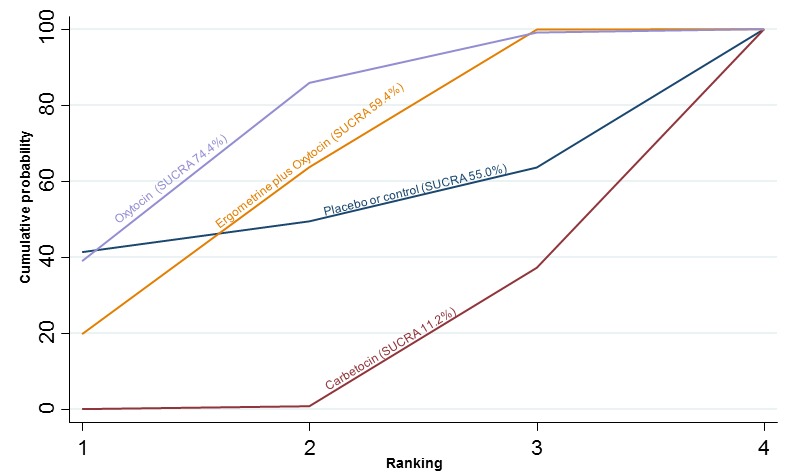
Cumulative rankograms comparing each of the uterotonic drugs for tachycardia.
Hypotension
The network diagram for hypotension is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 8 trials suggested a lack of evidence that any agent is worse or better than any other as most of the comparisons were based on single studies (Figure 44). There was no evidence of global inconsistency in this analysis (P = 0.304). Figure 45 shows the cumulative probabilities for each agent being at each possible rank for causing hypotension. The highest ranked agents were misoprostol and placebo or no treatment. For the rest of the agents no clear ranking emerged and not all agents could be ranked because of the lack of studies in this analysis.
Figure 44.
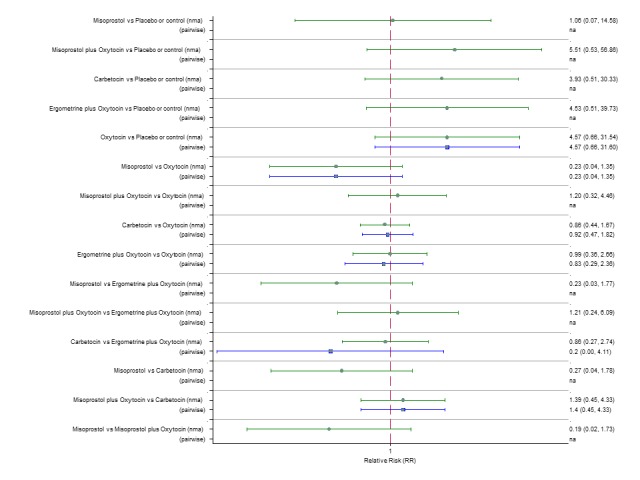
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for hypotension.
Figure 45.
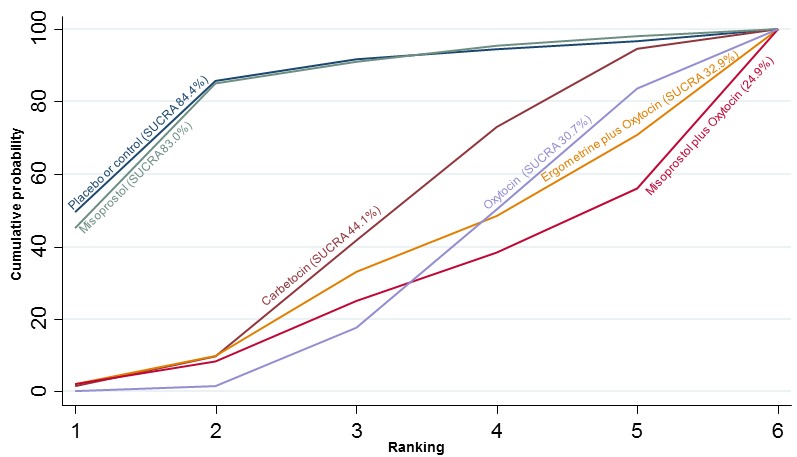
Cumulative rankograms comparing each of the uterotonic drugs for hypotension.
Abdominal pain
The network diagram for abdominal pain is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 25 trials suggested that misoprostol plus oxytocin is worse than placebo or no treatment in causing abdominal pain (Figure 46). No active agent was found to be worse or better than any other. There was evidence of global inconsistency in this analysis (P = 0.035). However, we note that the CIs for both the network meta‐analysis and direct evidence were overlapping across all comparisons suggesting locally fairly consistent results. Figure 47 shows the cumulative probabilities for each agent being at each possible rank for causing abdominal pain. The highest ranked agent was placebo or no treatment. For the rest of the agents no clear ranking emerged because of the lack of studies in this analysis.
Figure 46.
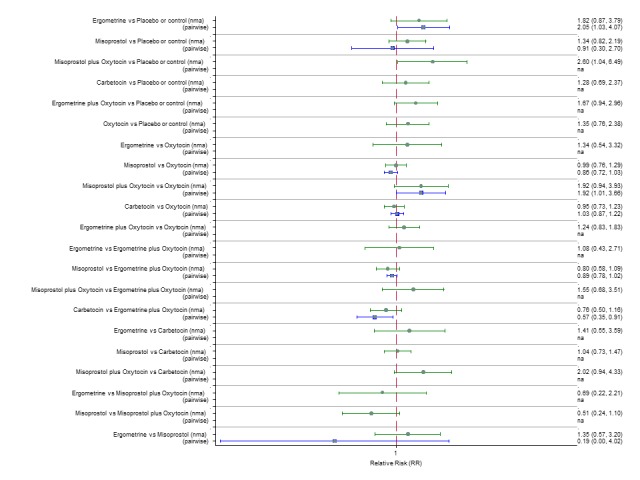
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for abdominal pain.
Figure 47.
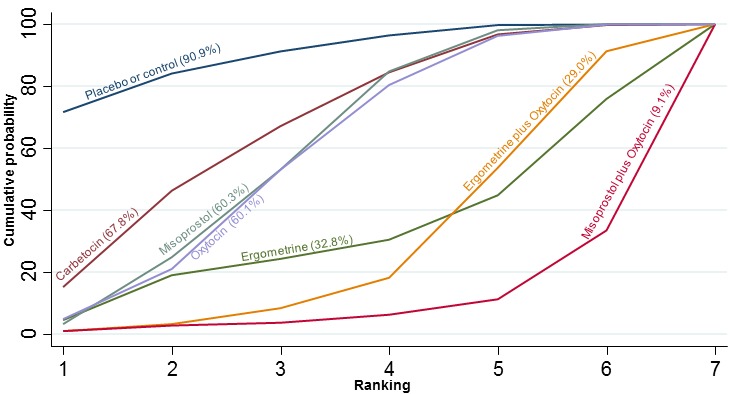
Cumulative rankograms comparing each of the uterotonic drugs for abdominal pain.
Subgroup analyses
Mode of birth
Vaginal birth
PPH ≥ 500 mL
The network diagram for PPH ≥ 500 mL for the subgroup including only vaginal births is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 85 trials suggested that all agents are effective for preventing PPH ≥ 500 mL when compared with placebo or no treatment (Figure 48). Ergometrine plus oxytocin, and misoprostol plus oxytocin were found to be more effective when compared with the standard agent oxytocin. Carbetocin also demonstrated a trend towards reduction of this outcome (Figure 48). Ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin were also found to be more effective when compared with misoprostol and ergometrine when used alone. There was no evidence of global inconsistency in this analysis (P = 0.06). Figure 49 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 500 mL for the subgroup including only vaginal births. The highest ranked agents were ergometrine plus oxytocin, carbetocin, and misoprostol plus oxytocin with an almost 100% probability of these three agents being ranked first, second or third. Oxytocin was ranked fourth and its probability of being ranked in the top three agents was close to 0%.
Figure 48.
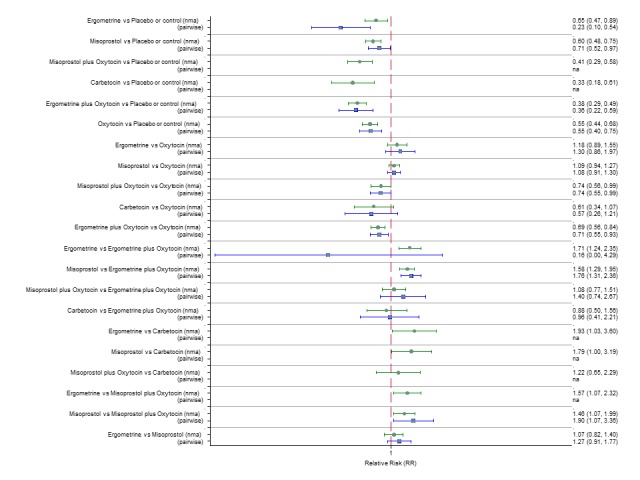
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL by mode of birth (vaginal birth).
Figure 49.
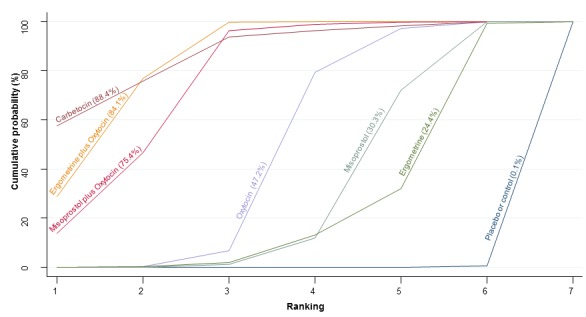
Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL by mode of birth (vaginal birth).
PPH ≥ 1000 mL
Pooled effect estimates from the network meta‐analysis of 71 trials suggested that all agents except carbetocin and ergometrine are effective for preventing PPH ≥ 1000 mL when compared with placebo or no treatment (Appendix 3). Ergometrine plus oxytocin was the only agent found to be more effective when compared with the standard agent oxytocin. Carbetocin and misoprostol plus oxytocin demonstrated a trend towards reduction of this outcome (Appendix 3). There was no evidence of global inconsistency in this analysis (P = 0.206). Appendix 3 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 1000 mL for the subgroup including only vaginal births. The highest ranked agents were carbetocin, ergometrine plus oxytocin, and misoprostol plus oxytocin. Oxytocin was ranked fourth and its probability in being ranked in the top two agents was close to 0%.
Caesarean section
PPH ≥ 500 mL
Pooled effect estimates from the network meta‐analysis of 15 trials suggested that only misoprostol plus oxytocin is better than oxytocin alone in preventing PPH ≥ 500 mL for women undergoing caesareans, but most of the comparisons were based on single studies (Figure 50). There was no evidence of global inconsistency in this analysis (P = 0.249). Figure 51 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 500 mL for the subgroup including only caesareans. The highest ranked agents were misoprostol plus oxytocin and carbetocin. Oxytocin was ranked third and its probability in being ranked in the top two agents was close to 5%. Ergometrine and ergometrine plus oxytocin could not be ranked as there were no studies found comparing those with any other agents in the network.
Figure 50.
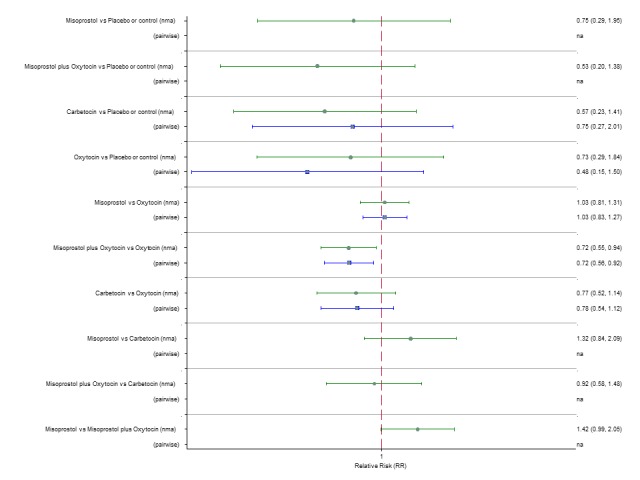
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL by mode of birth (caesarean).
Figure 51.
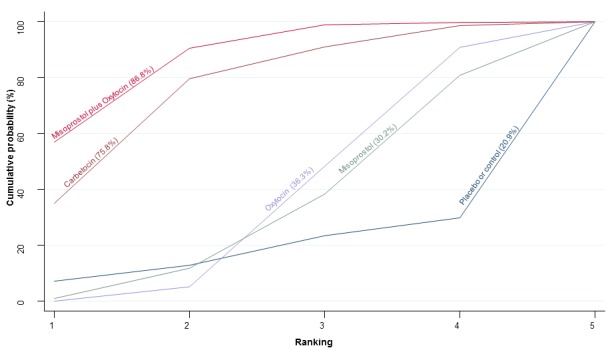
Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL by mode of birth (caesarean).
PPH ≥ 1000 mL
Pooled effect estimates from the network meta‐analysis of 19 trials suggested a lack of evidence that any agent is worse or better than any other in preventing PPH ≥ 1000 mL in women undergoing caesareans, but many of the comparisons were based on single studies (Appendix 3). There was no evidence of global inconsistency in this analysis (P = 0.86). Appendix 3 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 1000 mL for the subgroup including only caesareans. No clear ranking emerged in this analysis. Ergometrine and ergometrine plus oxytocin could not be ranked as there were no studies found comparing those with any other agents in the network.
Prior risk of PPH
Low risk for PPH
PPH ≥ 500 mL
Pooled effect estimates from the network meta‐analysis of 35 trials suggested that only ergometrine plus oxytocin and misoprostol are better than placebo or no treatment in preventing PPH ≥ 500 mL in women at low risk for PPH, but most of the comparisons were based on single studies (Figure 52). There was no evidence of global inconsistency in this analysis (P = 0.236). Figure 53 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 500 mL for the subgroup including only trials with women at low risk for PPH. The highest ranked agents were ergometrine plus oxytocin and carbetocin. Oxytocin was ranked fourth behind misoprostol and its probability in being ranked in the top two agents was close to 10%. Misoprostol plus oxytocin could not be ranked as there were no studies found comparing this agent with any other agents in the network.
Figure 52.
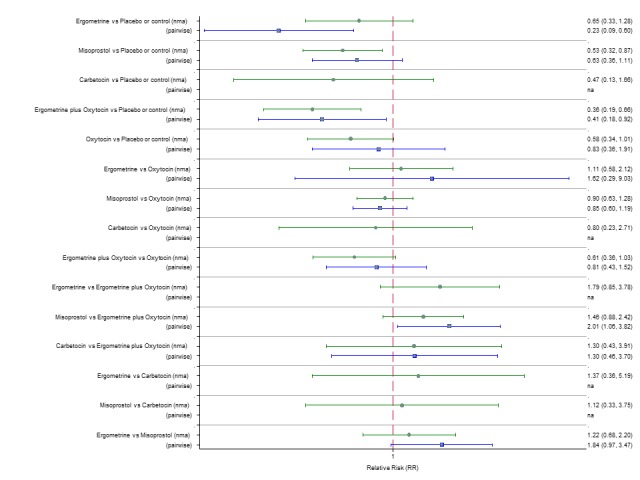
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL by prior risk for PPH (low risk).
Figure 53.
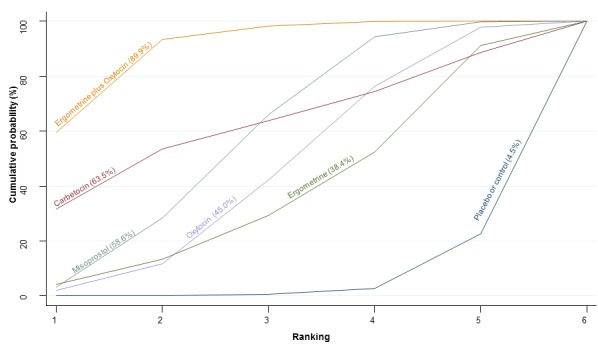
Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL by prior risk for PPH (low risk).
PPH ≥ 1000 mL
Pooled effect estimates from the network meta‐analysis of 32 trials suggested that ergometrine plus oxytocin, oxytocin, ergometrine and misoprostol are better than placebo or no treatment in preventing PPH ≥ 1000 mL in women at low risk for PPH (Appendix 3). The comparisons between active agents appeared to be underpowered to detect differences between them. There was no evidence of global inconsistency in this analysis (P = 0.477). Appendix 3 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 1000 mL for the subgroup including only trials with women at low risk for PPH. No clear ranking emerged in this analysis. Ergometrine could not be ranked as there were no studies found comparing those with any other agents in the network.
High risk for PPH
PPH ≥ 500 mL
Pooled effect estimates from the network meta‐analysis of 21 trials suggested that only isoprostol plus oxytocin is better than oxytocin in preventing PPH ≥ 500 mL and carbetocin showed a similar trend towards prevention of this outcome for women at high risk for PPH, but most of the comparisons were based on single studies (Figure 54). There was no evidence of global inconsistency in this analysis (P = 0.211). Figure 55 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 500 mL for the subgroup including only trials with women at high risk for PPH. The highest ranked agents were misoprostol plus oxytocin and carbetocin. Oxytocin was ranked third closely followed by misoprostol and its probability in being ranked in the top two agents was close to 0%.
Figure 54.
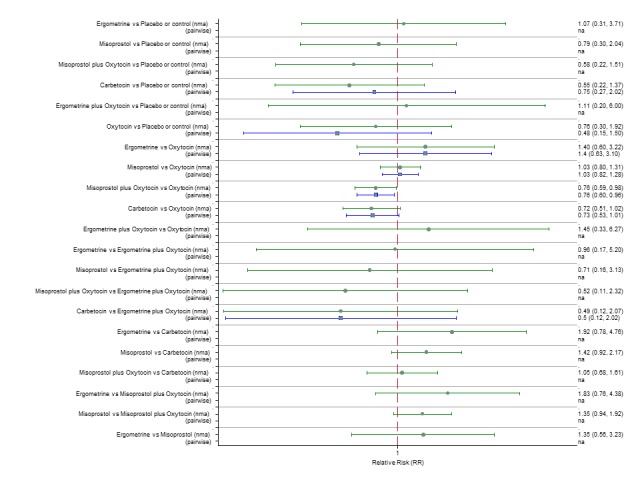
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL by prior risk for PPH (high risk).
Figure 55.
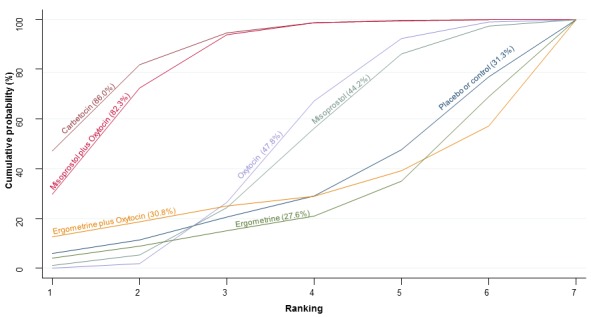
Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL by prior risk for PPH (high risk).
PPH ≥ 1000 mL
Pooled effect estimates from the network meta‐analysis of 22 trials suggested a lack of evidence that any agent is worse or better than any other in preventing PPH ≥ 1000 mL in women at high risk for PPH; many of the comparisons were based on single studies (Appendix 3). There was no evidence of global inconsistency in this analysis (P = 0.851). Appendix 3 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 1000 mL for the subgroup including only trials with women at high risk for PPH. No clear ranking emerged in this analysis. Ergometrine and ergometrine plus oxytocin could not be ranked as there were no studies found comparing those with any other agents in the network.
Healthcare setting
Hospital setting
PPH ≥ 500 mL
The network diagram for PPH ≥ 500 mL for the subgroup including trials carried out in the hospital setting is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 95 trials suggested that all agents are effective for preventing PPH ≥ 500 mL when compared with placebo or no treatment (Figure 56). Ergometrine plus oxytocin, and misoprostol plus oxytocin were found to be more effective when compared with the standard agent oxytocin. Carbetocin also demonstrated a trend towards reduction of this outcome (Figure 56). Ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin were also found to be more effective when compared with misoprostol and ergometrine when used alone. There was evidence of global inconsistency in this analysis (P = 0.0448). However, we note that the CIs for both the network and direct evidence were overlapping across all comparisons suggesting locally‐consistent results except for ergometrine versus placebo or no treatment based on a single study. Figure 57 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 500 mL for the subgroup including only trials carried out in the hospital setting. The highest ranked agents were ergometrine plus oxytocin, carbetocin, and misoprostol plus oxytocin with an almost 100% probability of these three agents being ranked first, second or third. Oxytocin was ranked fourth and its probability in being ranked in the top three agents was close to 0%.
Figure 56.
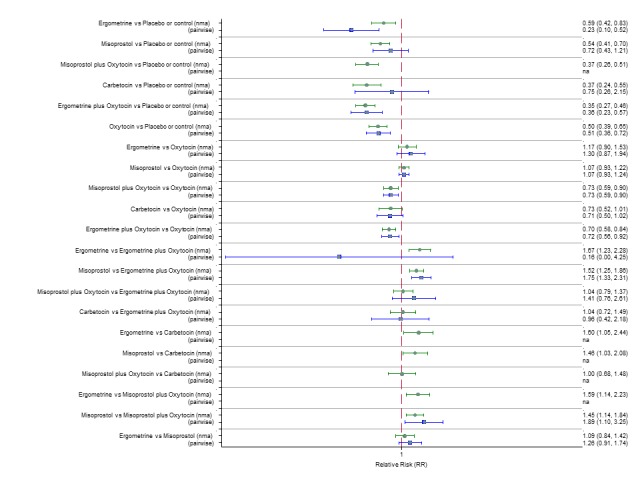
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL by healthcare setting (hospital setting).
Figure 57.
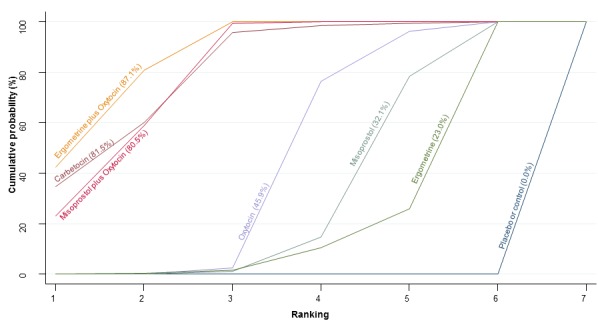
Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL by healthcare setting (hospital setting).
PPH ≥ 1000 mL
Pooled effect estimates from the network meta‐analysis of 85 trials suggested that all agents except ergometrine are effective for preventing PPH ≥ 1000 mL when compared with placebo or no treatment for the subgroup including only trials carried out in the hospital setting (Appendix 3). Ergometrine plus oxytocin was the only agent found to be more effective when compared with the standard agent oxytocin. Carbetocin and misoprostol plus oxytocin demonstrated a trend towards reduction of this outcome (Appendix 3). There was no evidence of global inconsistency in this analysis (P = 0.389). Appendix 3 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 1000 mL for the subgroup including trials carried out in the hospital setting. The highest ranked agents were carbetocin, ergometrine plus oxytocin and misoprostol plus oxytocin. Oxytocin was still ranked fourth and its probability in being ranked in the top three agents was close to 20%.
Community setting
PPH ≥ 500 mL
Pooled effect estimates from the network meta‐analysis of four trials suggested that only oxytocin and misoprostol are effective for preventing PPH ≥ 500 mL when compared with placebo or no treatment for the subgroup including only trials carried out in the community setting (Figure 58). There was evidence of global inconsistency in this analysis (P = 0.03), but most of the comparisons were based on a small number of studies. Figure 59 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 500 mL for the subgroup including trials carried out in the community setting. No clear ranking emerged in this analysis. Carbetocin, misoprostol plus oxytocin, ergometrine and ergometrine plus oxytocin could not be ranked as there were no studies found comparing those with any other agents in the network.
Figure 58.
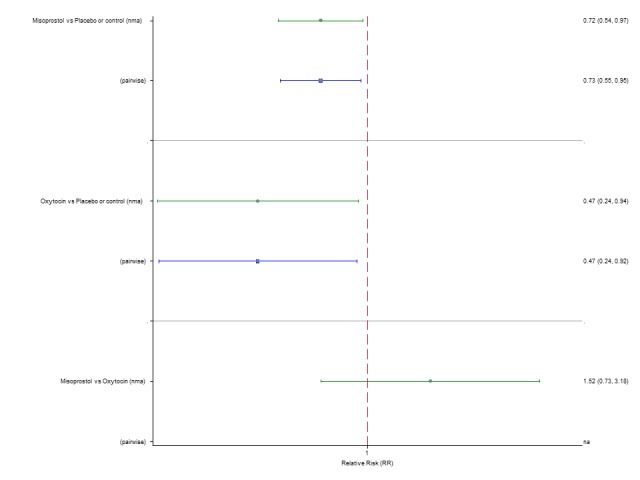
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL by healthcare setting (community setting).
Figure 59.
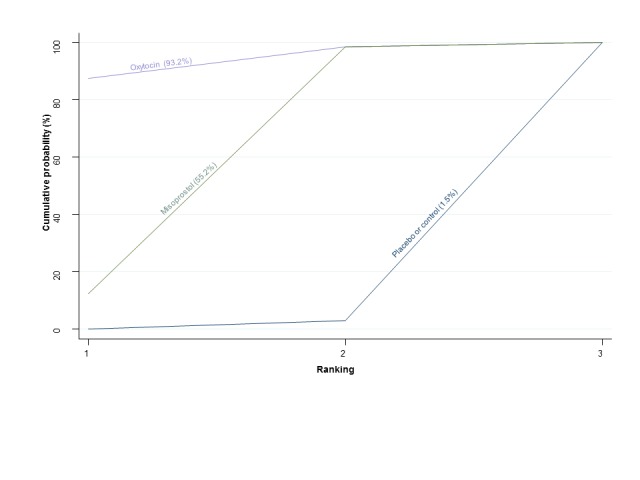
Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL by healthcare setting (community setting).
PPH ≥ 1000 mL
Pooled effect estimates from the network meta‐analysis of four trials suggested that only misoprostol is more effective for preventing PPH ≥ 1000 mL when compared with placebo or no treatment. Oxytocin also demonstrated a trend towards reduction of this outcome for the subgroup including trials carried out in the community setting (Appendix 3). There was evidence of global inconsistency in this analysis (P = 0.004), but most of the comparisons were based on single studies. Appendix 3 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 1000 mL for the subgroup including trials carried out in the community setting. No clear ranking emerged in this analysis. Carbetocin, misoprostol plus oxytocin, ergometrine and ergometrine plus oxytocin could not be ranked as there were no studies found comparing those with any other agents in the network.
Intervention: dose, regimen or route
Low‐dose misoprostol
PPH ≥ 500 mL
The network diagram for PPH ≥ 500 mL for the subgroup including only misoprostol studies that used a low dose (< 600 mcg) is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 72 trials suggested that all agents are effective for preventing PPH ≥ 500 mL when compared with placebo or no treatment (Figure 60). Ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin were found to be more effective when compared with the standard agent oxytocin (Figure 60). Ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin were also found to be more effective when compared with misoprostol and ergometrine when used alone. There was evidence of global inconsistency in this analysis (P = 0.016). However, we note that the CIs for both the network and direct evidence were overlapping across all comparisons suggesting locally‐consistent results except for ergometrine versus control or no treatment based on a single study. Figure 61 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 500 mL for the subgroup restricted to misoprostol trials that used a low dose. The highest ranked agents were ergometrine plus oxytocin, carbetocin, and misoprostol plus oxytocin with almost 100% probability of these three agents being ranked first, second or third. Oxytocin was ranked fourth and its probability in being ranked in the top three agents was close to 0%.
Figure 60.
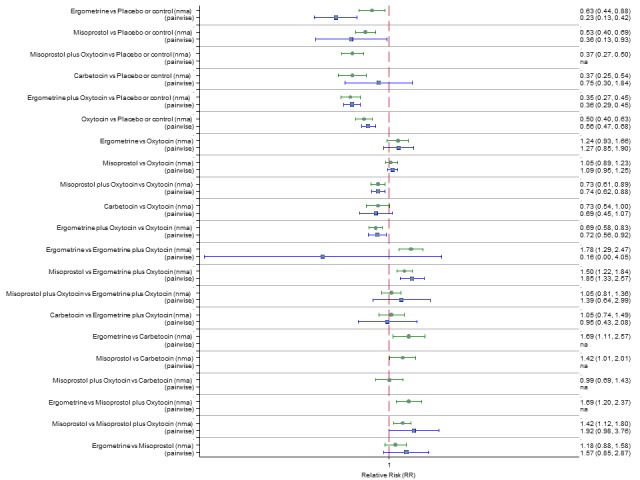
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL restricted to misoprostol studies that use a low dose (less or equal to 500 mcg).
Figure 61.
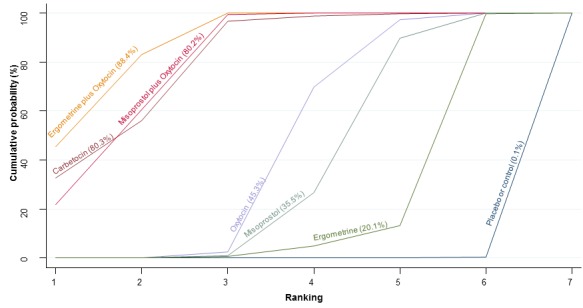
Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL restricted to misoprostol studies that use a low dose (less or equal to 500 mcg).
PPH ≥ 1000 mL
Pooled effect estimates from the network meta‐analysis of 69 trials suggested that all agents except ergometrine are effective for preventing PPH ≥ 1000 mL when compared with placebo or no treatment for the subgroup including only misoprostol trials that used a low dose (Appendix 3). Ergometrine plus oxytocin was the only agent found to be more effective when compared with the standard agent oxytocin. Carbetocin also demonstrated a trend towards reduction of this outcome (Appendix 3). There was no evidence of global inconsistency in this analysis (P = 0.401). Appendix 3 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 1000 mL for the subgroup restricted to misoprostol trials that used a low dose. The highest ranked agents were carbetocin, ergometrine plus oxytocin and misoprostol plus oxytocin. Oxytocin was still ranked fourth and its probability in being ranked in the top three agents was close to 20%.
High‐dose misoprostol
PPH ≥ 500 mL
The network diagram for PPH ≥ 500 mL for the subgroup including only misoprostol studies that used a high dose (≥ 600 mcg) is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 83 trials suggested that all agents are effective for preventing PPH ≥ 500 mL when compared with placebo or no treatment for the subgroup including only misoprostol trials that used a high dose (Figure 62). Ergometrine plus oxytocin and misoprostol plus oxytocin were found to be more effective when compared with the standard agent oxytocin. Carbetocin also showed a trend towards reduction of this outcome (Figure 62). Ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin were also found to be more effective than misoprostol when used alone. There was no evidence of global inconsistency in this analysis (P = 0.322). Figure 63 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 500 mL for the subgroup including only misoprostol trials that used a high dose. The highest ranked agents were ergometrine plus oxytocin, carbetocin, and misoprostol plus oxytocin with more than 80% probability of these three agents being ranked first, second or third. Oxytocin was ranked fifth behind ergometrine and its probability in being ranked in the top three agents was close to 0%.
Figure 62.
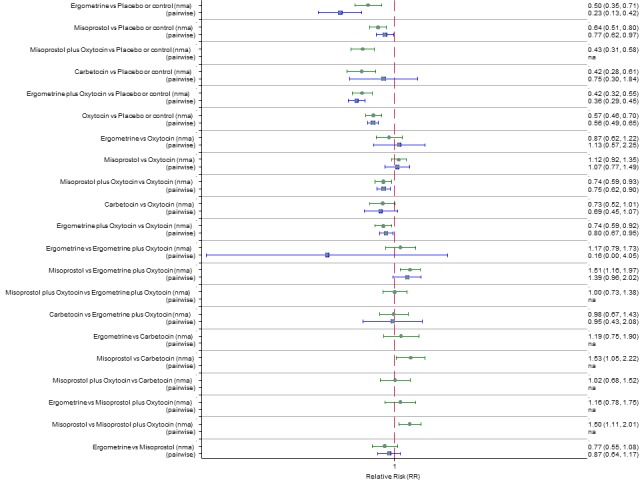
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL restricted to misoprostol studies that use a high dose (600 mcg or more).
Figure 63.
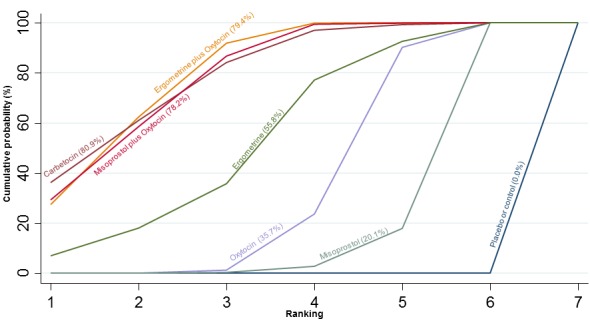
Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 1000 mL restricted to misoprostol studies that use a high dose (600 mcg or more).
PPH ≥ 1000 mL
Pooled effect estimates from the network meta‐analysis of 62 trials suggested that all agents except ergometrine are effective for preventing PPH ≥ 1000 mL when compared with placebo or no treatment for the subgroup including only misoprostol trials that used a high dose (Appendix 3). Ergometrine plus oxytocin was the only agent found to be more effective when compared with the standard agent oxytocin. Carbetocin also demonstrated a trend towards reduction of this outcome (Appendix 3). Ergometrine plus oxytocin, carbetocin, misoprostol plus oxytocin and oxytocin when used alone were found to be more effective than misoprostol despite misoprostol being used at a high dose. There was no evidence of global inconsistency in this analysis (P = 0.625). Appendix 3 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 1000 mL for the subgroup restricted to misoprostol trials that used a high dose. The highest ranked agents were carbetocin, ergometrine plus oxytocin, ergometrine and misoprostol plus oxytocin. Oxytocin was still ranked fifth and its probability in being ranked in the top three agents was less than 20%.
Oxytocin bolus only
PPH ≥ 500 mL
The network diagram for PPH ≥ 500 mL for the subgroup is presented in Appendix 2. This subgroup includes all trials, but for trials including oxytocin as an arm, this analysis is restricted to oxytocin studies that used an intravenous or intramuscular bolus of any dose and excluded studies that used a bolus plus infusion or infusion only of oxytocin. Pooled effect estimates from the network meta‐analysis of 84 trials suggested that all agents are effective for preventing PPH ≥ 500 mL when compared with placebo or no treatment for the subgroup including trials that only used an intramuscular or intravenous bolus of oxytocin at any dose (Figure 64). Ergometrine plus oxytocin was the only agent found to be more effective when compared with the standard agent oxytocin. Carbetocin and misoprostol plus oxytocin also demonstrated a trend towards reduction of this outcome (Figure 64). Ergometrine plus oxytocin was also found to be more effective than misoprostol when used alone. There was no evidence of global inconsistency in this analysis (P = 0.134). Figure 65 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 500 mL for the subgroup including trials that used an intramuscular or intravenous bolus of oxytocin at any dose. The highest ranked agents were ergometrine plus oxytocin, misoprostol plus oxytocin and carbetocin with more than 80% probability of these three agents being ranked first, second or third. Oxytocin was still ranked fourth and its probability in being ranked in the top three agents was less than 20%.
Figure 64.
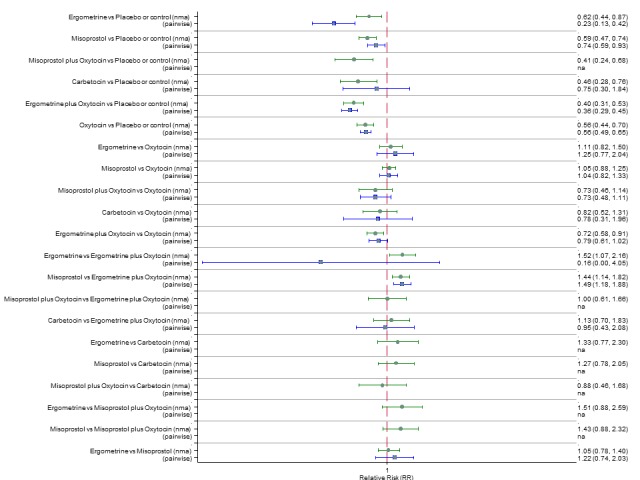
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL restricted to oxytocin studies that used an intramuscular or intravenous bolus of any dose.
Figure 65.
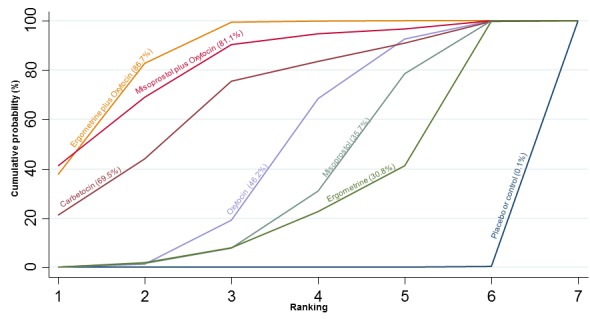
Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL restricted to oxytocin studies that used an intramuscular or intravenous bolus of any dose.
PPH ≥ 1000 mL
The network diagram for PPH ≥ 1000 mL for the subgroup including all trials, but restricted to trials that used an intravenous or intramuscular bolus of oxytocin at any dose is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 68 trials suggested that all agents except carbetocin and ergometrine are effective for preventing PPH ≥ 500 mL when compared with placebo or no treatment for the subgroup including only trials that used an intramuscular or intravenous bolus of oxytocin at any dose (Appendix 3). None of the agents was found to be more effective when compared with the standard agent oxytocin (Appendix 3). Ergometrine plus oxytocin and oxytocin when used alone were found to be more effective than misoprostol. There was no evidence of global inconsistency in this analysis (P = 0.468). Appendix 3 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 1000 mL for the subgroup restricted to oxytocin studies that used an intramuscular or intravenous bolus of any dose. The highest ranked agent was ergometrine plus oxytocin with less clear ranking amongst the other agents.
Oxytocin bolus plus infusion
PPH ≥ 500 mL
The network diagram for PPH ≥ 500 mL for this subgroup is presented in Appendix 2. This subgroup includes all trials, but when oxytocin was used as an arm in the trial this analysis is restricted only to studies that used an intravenous bolus with an intravenous infusion of oxytocin at any dose and excluded studies that used an intravenous or intramuscular bolus or an intravenous infusion only of oxytocin. Pooled effect estimates from the network meta‐analysis of 31 trials suggested that all agents except oxytocin and misoprostol plus oxytocin are effective for preventing PPH ≥ 500 mL when compared with placebo or no treatment for the subgroup including oxytocin trials that used an intravenous bolus plus an infusion of any dose (Figure 66). The active agents were comparable between them, but most of the comparisons were underpowered to detect a difference. There was no evidence of global inconsistency in this analysis (P = 0.081). Figure 67 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 500 mL for the subgroup including only trials of oxytocin that used an intravenous bolus plus an infusion of any dose. No clear ranking emerged in this analysis.
Figure 66.
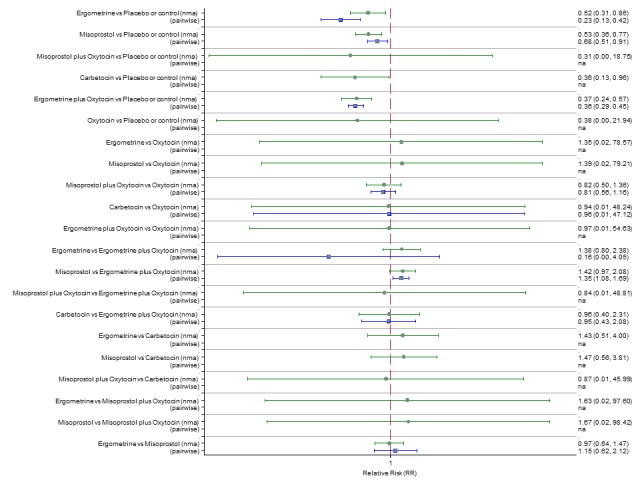
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL restricted to oxytocin studies that used an intravenous bolus plus an infusion of any dose.
Figure 67.
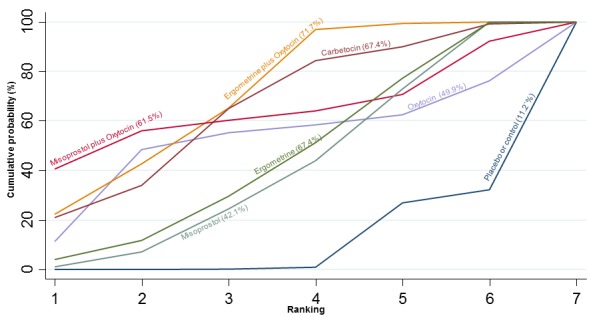
Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL restricted to oxytocin studies that used an intravenous bolus plus an infusion of any dose.
PPH ≥ 1000 mL
The network diagram for PPH ≥ 1000 mL for this subgroup is presented in Appendix 2. This subgroup includes all trials, but it is restricted to studies that used an intravenous bolus with an intravenous infusion of oxytocin at any dose. Pooled effect estimates from the network meta‐analysis of 29 trials suggested that all agents demonstrated a similar trend for reducing occurrence of this outcome, but only ergometrine, misoprostol and ergometrine plus oxytocin reached statistical significance when compared with placebo or no treatment for this subgroup (Appendix 3). The active agents were comparable between them, but most of the comparisons were underpowered to detect a difference. There was no evidence of global inconsistency in this analysis (P = 0.315). Appendix 3 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 1000 mL for the subgroup including oxytocin studies that used an intravenous bolus plus an infusion of any dose. No clear ranking emerged in this analysis.
Oxytocin infusion only
PPH ≥ 500 mL
The network diagram for PPH ≥ 500 mL for this subgroup is presented in Appendix 2. This subgroup includes all trials, but when oxytocin was used as an arm in the trial this analysis is restricted to studies that used an intravenous infusion only of oxytocin at any dose and excluded studies that used an intravenous or intramuscular bolus or an intravenous bolus plus an intravenous infusion of oxytocin. Pooled effect estimates from the network meta‐analysis of 48 trials suggested that all agents are effective for preventing PPH ≥ 500 mL when compared with placebo or no treatment for the subgroup including oxytocin trials that used an intravenous infusion only of any dose (Figure 68). The active agents were comparable between them, but most of the comparisons were underpowered to detect a difference. There was no evidence of global inconsistency in this analysis (P = 0.135). Figure 69 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 500 mL for the subgroup including oxytocin trials that used an intravenous infusion only of any dose. The highest ranked agents were carbetocin, ergometrine plus oxytocin and misoprostol plus oxytocin with almost 100% probability of these three agents being ranked first, second or third. Oxytocin was ranked fourth and its probability in being ranked in the top three agents was almost 0%.
Figure 68.
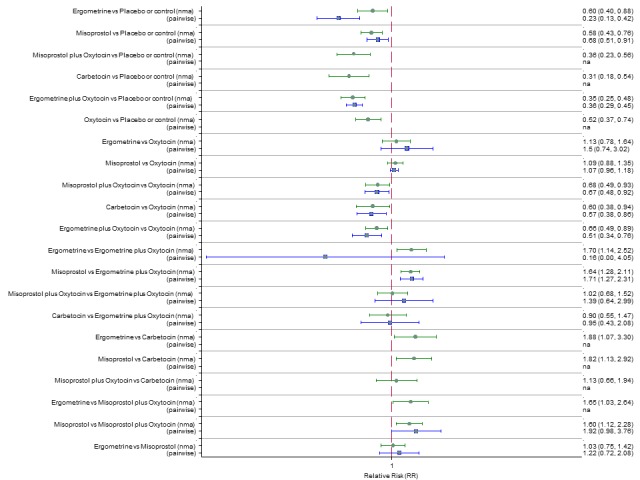
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL restricted to oxytocin studies that used an intravenous infusion only of any dose.
Figure 69.
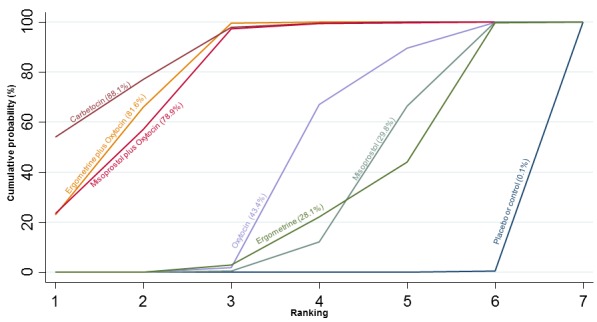
Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL restricted to oxytocin studies that used an intravenous infusion only of any dose.
PPH ≥ 1000 mL
The network diagram for PPH ≥ 1000 mL for the subgroup including all trials, but restricted to oxytocin trials that used an intravenous infusion only of any dose is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 41 trials suggested that all agents except oxytocin and ergometrine are effective for preventing PPH ≥ 500 mL when compared with placebo or no treatment for the subgroup including only oxytocin trials that used an intravenous infusion only of any dose (Appendix 3). Ergometrine plus oxytocin and carbetocin were found to be more effective when compared with the standard agent oxytocin (Appendix 3). Ergometrine plus oxytocin and carbetocin were also found to be more effective than misoprostol. There was no evidence of global inconsistency in this analysis (P = 0.232). Appendix 3 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 1000 mL for the subgroup including oxytocin studies that used an intravenous infusion only of any dose. The highest ranked agent was carbetocin. There is less clear ranking for the rest of the agents but on this analysis oxytocin was ranked sixth lower than ergometrine and misoprostol with 0% probability of being ranked in the top three.
Sensitivity analyses
Low risk of bias studies
PPH ≥ 500 mL
The network diagram for PPH ≥ 500 mL for low risk of bias trials (double‐blinded, adequately concealed with less than 10% loss to follow‐up) is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 29 low risk of bias trials suggested that all agents except carbetocin are effective for preventing PPH ≥ 500 mL when compared with placebo or no treatment. Carbetocin demonstrated a similar trend towards reduction of this outcome (Figure 70). Ergometrine plus oxytocin, and ergometrine were found to be more effective when compared with the standard agent oxytocin (Figure 70). Ergometrine plus oxytocin and ergometrine were also found to be more effective when compared with misoprostol when used alone. There was no evidence of global inconsistency in this analysis (P = 0.844). Figure 71 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 500 mL for the low risk of bias trials. The highest ranked agents were ergometrine, ergometrine plus oxytocin and misoprostol plus oxytocin. Oxytocin was ranked fourth and its probability in being ranked in the top three agents was less than 10%. Carbetocin dropped its ranking from second in the global analysis for PPH ≥ 500 mL to fifth behind oxytocin in this analysis including only low risk of bias trials. The ranking of ergometrine is an extreme outlier in this analysis and is based on a single study.
Figure 70.
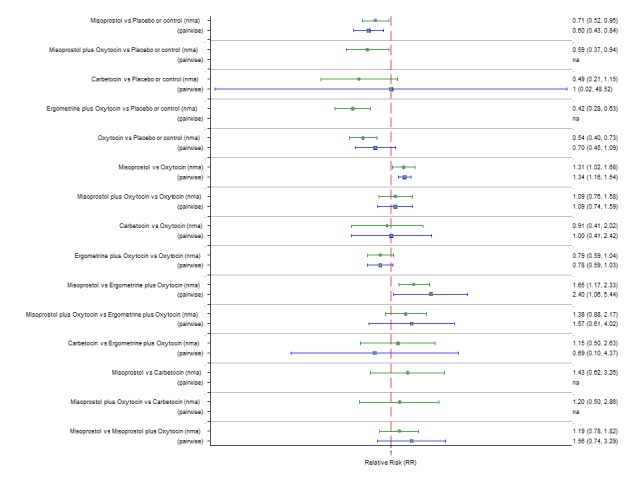
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL restricted to low risk of bias studies only.
Figure 71.
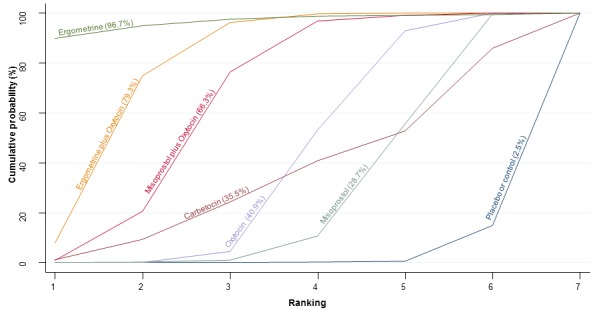
Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL restricted to low risk of bias studies only.
PPH ≥ 1000 mL
The network diagram for PPH ≥ 1000 mL for low risk of bias trials is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 30 high‐quality trials suggested that all agents except carbetocin are effective for preventing PPH ≥ 1000 mL when compared with placebo or no treatment. Carbetocin demonstrated a similar trend towards reduction of this outcome (Appendix 3). Oxytocin was found to be better than misoprostol when used alone (Appendix 3). Ergometrine plus oxytocin was also found to be more effective when compared with misoprostol when used alone. There was no evidence of global inconsistency in this analysis (P = 0.802). Appendix 3 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 1000 mL for the low risk of bias trials. The highest ranked agent was ergometrine plus oxytocin. The ranking for carbetocin, oxytocin and misoprostol plus oxytocin was very close without a clear hierarchy.
Studies with funding source at low risk of bias (public or no funding)
PPH ≥ 500 mL
The network diagram for PPH ≥ 500 mL for studies with public or no funding is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 32 trials suggested that all agents except carbetocin and ergometrine are effective for preventing PPH ≥ 500 mL when compared with placebo or no treatment. All other agents demonstrated a similar trend towards reduction of this outcome (Figure 72). There were no significant differences between the active agents. There was evidence of global inconsistency in this analysis (P = 0.0003). However, we note that the CIs for both the network and direct evidence were overlapping across all comparisons suggesting locally‐consistent results except for ergometrine versus misoprostol based on a single study. Figure 73 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 500 mL for trials with public or no funding. The highest ranked agent was ergometrine plus oxytocin. The ranking for carbetocin, oxytocin and misoprostol plus oxytocin was very close without a clear hierarchy.
Figure 72.
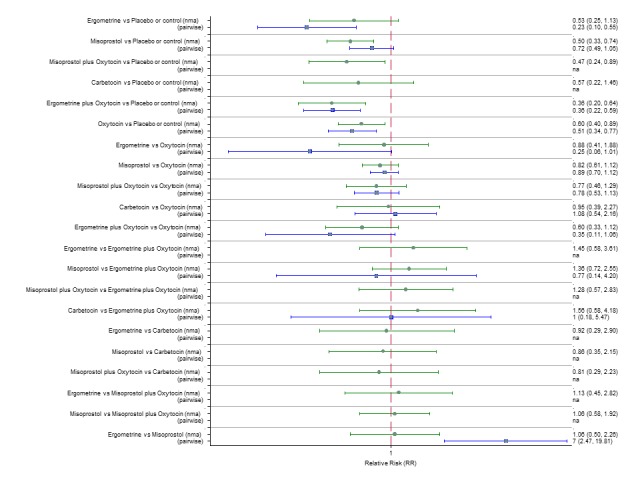
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL restricted to studies with funding source at low risk of bias (public or no funding).
Figure 73.
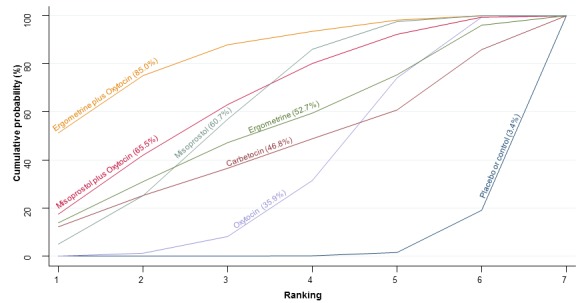
Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL restricted to studies with funding source at low risk of bias (public or no funding).
PPH ≥ 1000 mL
The network diagram for PPH ≥ 1000 mL for trials with public or no funding is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 35 trials suggested that all agents except carbetocin are effective for preventing PPH ≥ 1000 mL when compared with placebo or no treatment. Carbetocin demonstrated a similar trend towards reduction of this outcome (Appendix 3). No agent was found to be significantly better or worse than oxytocin (Appendix 3). Ergometrine was found to be more effective when compared with misoprostol. There was no evidence of global inconsistency in this analysis (P = 0.739). Appendix 3 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 1000 mL for the trials with public or no funding. The highest ranked agents were ergometrine and ergometrine plus oxytocin. The ranking for carbetocin, oxytocin and misoprostol plus oxytocin was very close without a clear hierarchy. The ranking of ergometrine was an outlier in this analysis and was based on a single study.
Studies with an objective method of measuring blood loss
PPH ≥ 500 mL
The network diagram for PPH ≥ 500 mL for the trials that used an objective method for measuring blood loss is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 56 trials suggested that all agents except ergometrine are effective for preventing PPH ≥ 500 mL when compared with placebo or no treatment (Figure 74). Ergometrine plus oxytocin and misoprostol plus oxytocin were found to be more effective when compared with the standard agent oxytocin with carbetocin also demonstrating a similar trend (Figure 74). Ergometrine plus oxytocin and misoprostol plus oxytocin were also found to be more effective than misoprostol and ergometrine when used alone. There was no evidence of global inconsistency in this analysis (P = 0.455). Figure 75 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 500 mL for trials that used an objective method of measuring blood loss. The highest ranked agents were ergometrine plus oxytocin and misoprostol plus oxytocin followed closely by carbetocin with almost 100% probability of these three agents being ranked first, second or third. Oxytocin was ranked fourth and its probability in being ranked in the top three agents was less than 0%.
Figure 74.
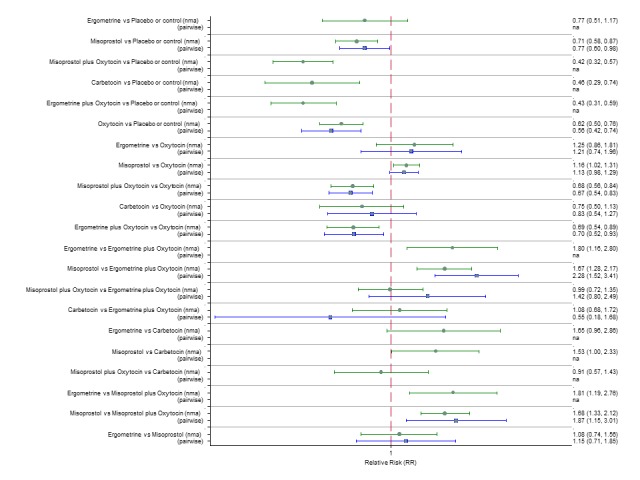
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL restricted to studies with an objective method of measuring blood loss.
Figure 75.
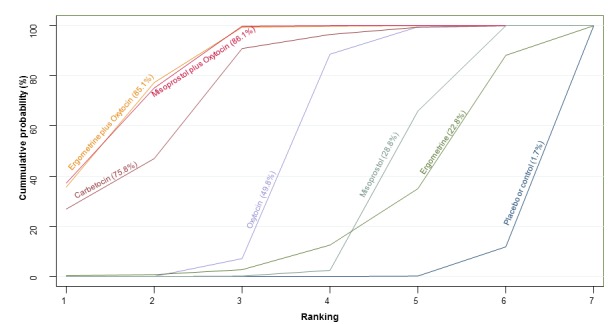
Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL restricted to studies with an objective method of measuring blood loss.
PPH ≥ 1000 mL
The network diagram for PPH ≥ 1000 mL for studies that used an objective method of measuring blood loss is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 49 trials suggested that all agents except carbetocin and ergometrine are effective for preventing PPH ≥ 1000 mL when compared with placebo or no treatment. Carbetocin demonstrated a similar trend towards reduction of this outcome (Appendix 3). Ergometrine plus oxytocin was found to be more effective when compared with the standard agent oxytocin. Ergometrine plus oxytocin was also found to be more effective than misoprostol and ergometrine when used alone. There was no evidence of global inconsistency in this analysis (P = 0.606). Appendix 3 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 1000 mL for the studies that used an objective method of measuring blood loss. The highest ranked agent was ergometrine plus oxytocin. The ranking for carbetocin, oxytocin and misoprostol plus oxytocin was very close without a clear hierarchy.
Large studies only (> 400 participants)
PPH ≥ 500 mL
The network diagram for PPH ≥ 500 mL restricted to large trials with more than 400 participants is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 46 trials suggested that all agents except ergometrine are effective for preventing PPH ≥ 500 mL when compared with placebo or no treatment (Figure 76). Ergometrine plus oxytocin and misoprostol plus oxytocin were found to be more effective when compared with the standard agent oxytocin with carbetocin not being included in this analysis as there were no large studies comparing carbetocin to any of the other agents (Figure 76). Ergometrine plus oxytocin and misoprostol plus oxytocin were also found to be more effective than misoprostol and ergometrine when used alone. There was evidence of global inconsistency in this analysis (P = 0.011). However, we note that the CIs for both the network and direct evidence were overlapping across all comparisons suggesting locally‐consistent results except for ergometrine versus placebo or no treatment based on a single study. Figure 77 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 500 mL for large trials. The highest ranked agents were ergometrine plus oxytocin and misoprostol plus oxytocin. Oxytocin was ranked third and its probability in being ranked in the top two agents was close to 0%. Carbetocin could not be ranked as there were no studies found comparing it with any other agents in the network.
Figure 76.
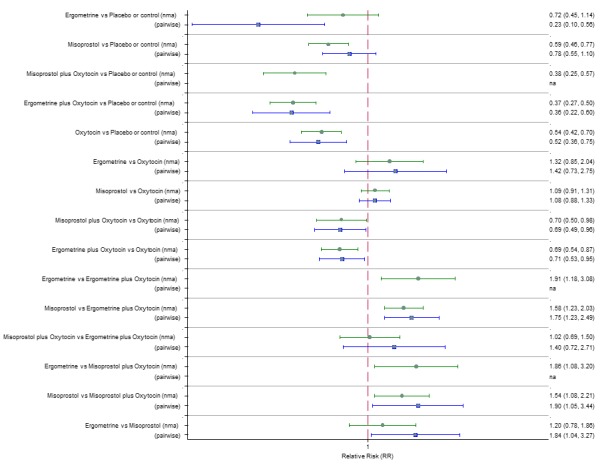
Forest plot with relative risk ratios and 95% CIs from network meta‐analysis and pairwise analyses for prevention of PPH ≥ 500 mL restricted to large studies (> 400 participants).
Figure 77.
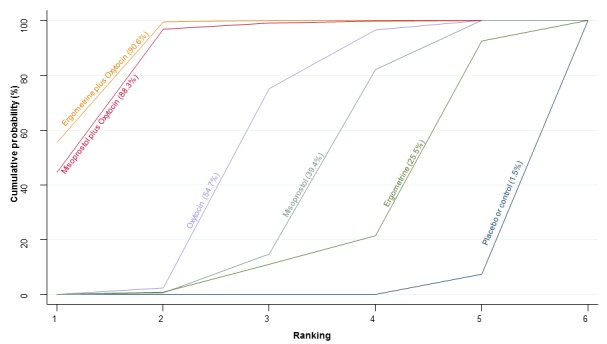
Cumulative rankograms comparing each of the uterotonic drugs for prevention of PPH ≥ 500 mL restricted to large studies (> 400 participants).
PPH ≥ 1000 mL
The network diagram for PPH ≥ 1000 mL restricted to large trials with more than 400 participants is presented in Appendix 2. Pooled effect estimates from the network meta‐analysis of 46 trials suggested that all agents except ergometrine are effective for preventing PPH ≥ 500 mL when compared with placebo or no treatment (Appendix 3). Ergometrine plus oxytocin was found to be more effective when compared with the standard agent oxytocin with carbetocin not being included in this analysis as there were no large studies comparing carbetocin to any of the other agents (Appendix 3). Ergometrine plus oxytocin and oxytocin alone were also found to be more effective than misoprostol when used alone. There was no evidence of global inconsistency in this analysis (P = 0.122). Appendix 3 shows the cumulative probabilities for each agent being at each possible rank for PPH ≥ 1000 mL for the large trials. The highest ranked agents were ergometrine plus oxytocin and misoprostol plus oxytocin. Oxytocin was ranked third and its probability in being ranked in the top two agents was close to 10%. Carbetocin could not be ranked as there were no studies found comparing it with any other agents in the network.
Further sensitivity analyses
Further sensitivity analyses for the primary outcomes were performed by removing trials published earlier than 1990 (three trials), a cluster trial (one trial), removing trials with high level of missing data (10 trials) and removing trials where participants were also randomised to co‐agents such as uterine massage and/or early controlled cord traction (three trials). Sensitivity analyses were also performed according to the choice of relative effect measure (RR versus OR) and the statistical model (fixed‐effect versus random‐effects model). We found that the overall ranking did not vary and the confidence intervals of the relative effects did not substantially change. Of note is that the global inconsistency was substantially reduced when the trials randomising to co‐interventions were removed (P = 0.218).
Discussion
Summary of main results
This network meta‐analysis found that an ergometrine plus oxytocin combination, carbetocin, and a misoprostol plus oxytocin combination were more effective uterotonic drugs for preventing postpartum haemorrhage (PPH) ≥ 500 mL than the standard drug recommendation of oxytocin. An ergometrine plus oxytocin combination was also more effective in preventing PPH ≥ 1000 mL while a trend was noted for carbetocin and a misoprostol plus oxytocin combination. However, there was a higher risk of significant side‐effects with the two combination regimens. Carbetocin had a favourable side‐effect profile similar to oxytocin. However, carbetocin trials were small and at high risk of bias, and when we restricted the analysis including only trials at low risk of bias, carbetocin lost its top ranking, although there was significant uncertainty around the effect estimates.
Overall completeness and applicability of evidence
This network meta‐analysis provides the relative effectiveness of all drugs used for the prevention of PPH in a coherent and methodologically robust way across important clinical outcomes by combining both direct and indirect evidence, thus increasing the statistical power and confidence in the results. We found that most of the included trials reported our primary outcomes and most of the secondary outcomes. This increased the power across most of our analyses and contributed to the consistency in the ranking across all blood loss outcomes. We were thorough in our evaluation of the important potential treatment effect modifiers (mode of birth, prior risk of PPH, healthcare setting, dose, route and regimen of the drugs). We did not encounter important differences in the distribution of the effect modifiers between the different comparisons. In addition, the ranking of the drugs in each of the subgroups was comparable with the overall ranking. The results of the network meta‐analyses were mostly consistent and where there was significant inconsistency this was likely due to unstable estimates from single studies. Through our sensitivity analyses we were able to identify that research underpinning the carbetocin effectiveness is based on small studies at high risk of bias.
Many trials excluded women with significant comorbidities and at very high risk for PPH. Women recruited to the included studies were predominantly delivered at more than 37 weeks of gestation. Most of the trials were carried out in hospital settings and with women having a vaginal birth. For women having a vaginal birth, uterotonic drug administration used to be a component of the active management of the third stage of labour, alongside controlled cord traction and early cord clamping. The most up‐to‐date guidelines from the WHO (WHO 2012), place emphasis on the administration of a uterotonic drug as the main agent within this package for prevention of PPH. These guidelines state that early cord clamping is generally not advised, whilst controlled cord traction is optional where skilled birth attendants are present (WHO 2012). Rankings of the available agents were similar in subgroups including only trials of women having a vaginal birth or undergoing a caesarean section, but there were no trials that used ergometrine plus oxytocin or ergometrine alone for prevention of PPH at caesarean section births. For women undergoing caesarean deliveries, the risk of PPH ≥500 mL was reduced from 75% with oxytocin down to almost 50% with more effective agents. Evidently, uterine tone plays a major role in PPH at caesarean section, with a relative reduction of PPH ≥500 mL similar to the one of women undergoing vaginal births when more effective agents are used. The ranking is relevant to women at either high or low risk for PPH in hospital settings. There were not enough trials to be able to recommend a ranking in community settings, even though a similar ranking in terms of effectiveness can be expected.
The dosages, regimens and routes of drug administration for the most effective drugs varied. Carbetocin in most of the studies was administered as a single intravenous bolus of 100 mcg (15 studies) or intramuscularly (seven studies). The combination of ergometrine plus oxytocin was usually administered intramuscularly combining 500 mcg of ergometrine plus 5 IU (international units) of oxytocin (21 studies). Misoprostol plus oxytocin combinations varied greatly, with some studies administering an intravenous infusion of 20 IU of oxytocin and 400 mcg of misoprostol sublingually (three studies), or 200 mcg of misoprostol sublingually (two studies), others administering an intravenous bolus of oxytocin of 10 IU plus 400 mcg misoprostol sublingually (two studies) while others administered an intravenous infusion of 10 IU of oxytocin and 400 mcg of misoprostol rectally (two studies). There were another 12 ways of administering the oxytocin plus misoprostol combination described and each was employed in only one included study (see Characteristics of included studies).
Quality of the evidence
The majority of included trials were at uncertain risk of bias, with more than half of the quality domains not reported. We did not downgrade the estimates from the network meta‐analysis involving combinations of either ergometrine or misoprostol plus oxytocin due to risk of bias (See Table 1). However, we regarded risk of bias from studies contributing to carbetocin comparisons to warrant downgrading for PPH ≥ 500 mL and PPH ≥ 1000 mL. We considered serious imprecision to warrant downgrading the quality of evidence for PPH ≥ 500 mL for carbetocin and for PPH ≥1000 mL for the combination of misoprostol plus oxytocin, and for carbetocin. We also downgraded the quality of the evidence for PPH ≥ 500 mL for ergometrine plus oxytocin, carbetocin and misoprostol plus oxytocin because of inconsistency in the comparisons with oxytocin.
Potential biases in the review process
The earliest included trial was conducted in 1976 (Moodie 1976), and in the decades since then, the clinical care and the clinical response to PPH may have improved. These temporal changes could have contributed to heterogeneity and increased the uncertainty of findings. However, we carried out a sensitivity analysis by removing trials published before 1990 and this did not vary the ranking of the drugs. As objective methods of measuring blood loss became increasingly available this could perhaps have also led to apparent changes in reported blood loss. The trials included in the review recruited women with varied clinical characteristics, and it is important to consider this when interpreting results. The inclusion criteria were not always reported in detail and, when they were, these varied across trials. Further heterogeneity may also be present in the overall analysis related to the dose, route or regimen of the drugs. Even though we did not observe subgroup effects when we examined the dose of misoprostol or regimen of oxytocin administration, we were not able to perform subgroup analyses for every single increment in dosage or route of drug administration. Lastly, not all trials reported data on side‐effects hence these analyses were often underpowered.
Agreements and disagreements with other studies or reviews
Our results agree with existing Cochrane reviews (Begley 2015; Liabsuetrakul 2007; McDonald 2004; Su 2012; Tuncalp 2012; Westhoff 2013) that focus on the comparison of a uterotonic drug versus another (direct comparisons). However, this network meta‐analysis has several more studies than included in the previous reviews because of its nature of comparing all available uterotonic drugs in one single analysis and because it is the most up‐to‐date including recently published trials. Hence, some estimates differ slightly, as expected.
Authors' conclusions
The current WHO recommendation for preventing PPH is 10 IU of intramuscular or intravenous oxytocin (WHO 2012). Oxytocin should be kept refrigerated (2 °C to 8 °C) or stored at room temperature (25 °C or lower). Several studies have demonstrated that oxytocin loses potency if stored at room temperature for too long or at higher temperatures, making its use difficult in low‐resource countries (Hogerzeil 1993; WHO 1993). Since we have shown that oxytocin is ranked fourth in effectiveness, and and ergometrine plus oxytocin combination, carbetocin, and misoprostol plus oxytocin combination are more effective, our results could have important implications for clinical practice. Ergometrine plus oxytocin combination is the only agent that significantly reduces PPH ≥ 500 mL and PPH ≥ 1000 mL compared with oxytocin on both network and pairwise estimates. Misoprostol plus oxytocin combination evidence is less consistent and this may be related to the different routes and doses of misoprostol used in the studies. Carbetocin is more effective compared with oxytocin with a similar side‐effect profile to oxytocin. However, when we restricted our analysis to trials with low risk of bias, the ranking of carbetocin changed and it did not appear to be more effective than oxytocin. The manufacturer of carbetocin (Ferring Pharmaceuticals) has recently developed a room temperature stable (RTS) formulation, which makes it an attractive option for countries where maintaining the cold chain is problematic. Therefore, we conclude that there is an urgent need for a high‐quality large trial comparing the current standard of oxytocin with carbetocin, and especially RTS carbetocin, to confirm or reject the findings of small and at high risk of bias trials that have involved carbetocin to date.
There are two key studies that will inform a future update of this review. The first one is a WHO‐led multi‐centre phase III clinical study comparing the effectiveness of RTS carbetocin versus oxytocin (administered intramuscularly) for the prevention of PPH among women having a vaginal birth (Widmer 2016). This trial is led by several of the study authors (MW, MG, JH and AC) and includes approximately 30,000 women from 10 countries: Argentina, Egypt, India, Kenya, Nigeria, Singapore, South Africa, Thailand, Uganda, and the UK. Results are expected before the end of 2018. If the results of the study support carbetocin, the aim is to provide access to RTS carbetocin to public sector providers in low‐income countries with a high burden of maternal mortality, at an affordable and sustainable price comparable to oxytocin. Another trial based in the UK is recruiting more than 6000 women to a three‐arm trial comparing carbetocin, oxytocin and ergometrine plus oxytocin combination (Draycott 2014). This trial is also expected to report in 2018. These trials will provide the high‐quality evidence needed to update our network meta‐analysis.
Consultation with our consumer group has demonstrated a need for more research into PPH outcomes identified as priorities for women and their families, such as women’s views regarding the drugs used, clinical signs of excessive blood loss, neonatal unit admissions and breastfeeding at discharge. Trials to date have rarely investigated these outcomes. Consumers also considered the side‐effects of uterotonic drugs to be important and these were often not reported. The Postpartum Haemorrhage Core Outcome Sets Project (http://www.comet‐initiative.org/studies/details/706) will elucidate outcomes to prioritise in trial reporting and will inform futures updates of this review. We would urge all trialists to consider reporting these outcomes for each drug in all future randomised trials. Lastly, future evidence synthesis research could compare the effects of different dosages and routes of administration for the most effective drugs.
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of Cochrane Pregnancy and Childbirth's international panel of consumers and the Group's Statistical Adviser.
This review is supported by Ammalife (www.ammalife.org), and is undertaken as part of independent research funded by the UK National Institute for Health Research (Health Technology Assessment Project 14/139/17 "Uterotonic drugs for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis"). The views expressed in this publication are those of the review authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health.
The World Health Organization (WHO) and Ioannis D Gallos, Helen M Williams, Malcolm J Price, Abi Merriel, Harold Gee, David Lissauer, Vidhya Moorthy, Aurelio Tobias, Jonathan J Deeks, G Justus Hofmeyr and Arri Coomarasamy retain copyright and all other rights in their respective contributions to the manuscript of this review as submitted for publication.
We are grateful for the advice by the investigators of the Postpartum Haemorrhage Core Outcome Sets Project and particularly to Shireen Meher, Anna Cuthbert, Zarko Alfirevic, Jamie Kirkham and Paula Williamson.
Appendices
Appendix 1. Search terms
ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP)
Third stage AND labo(u)r AND oxytocin
Third stage AND labo(u)r AND misoprostol
Third stage AND labo(u)r AND carbetocin
Third stage AND labo(u)r AND ergometrine
uterotonic* AND oxytocin
uterotonic* AND misoprostol
uterotonic* AND carbetocin
uterotonic* AND ergometrine
uterotonic* AND labo(u)r
uterotonic* AND h(a)emorrhage
h(a)emorrhage AND postpartum AND ergometrine
h(a)emorrhage AND postpartum AND oxytocin
h(a)emorrhage AND postpartum AND carbetocin
h(a)emorrhage AND postpartum AND misoprostol
Appendix 2. Network diagrams for secondary outcomes and subgroup analyses
Please see NIHR HTA report for all diagrams (link).
Appendix 3. Subgroup and sensitivity analyses for PPH ≥ 1000 mL
Please see NIHR HTA report for all diagrams (https://www.journalslibrary.nihr.ac.uk/programmes/hta/1413917/#/)
Differences between protocol and review
There are some differences between the published protocol for this review (Gallos 2015) and the full review, these are listed below.
Objectives
We have clarified the objectives of this review.
In our protocol the stated objectives were: We aim to assess the clinical effectiveness and side‐effect profile of uterotonic drugs to prevent PPH, and to generate a clinically useful ranking of available uterotonics according to their effectiveness and side‐effects. We will explore the effects according to various key prognostic and treatment factors. The population of interest is women following a vaginal birth or a caesarean section in the hospital or the community setting. All uterotonic drugs considered by the WHO are eligible and the outcomes include blood loss‐related outcomes and side‐effects.
In the review, our objectives are listed as:
Primary
To identify the most effective uterotonic drug(s) to prevent postpartum haemorrhage (PPH) with a favourable side‐effect profile, and to generate a clinically useful ranking of all available uterotonics.
Secondary
To provide the relative effectiveness and side‐effect profile of each drug for our primary outcomes within: a) Population subgroups (prior risk of PPH, mode of birth and healthcare setting) and b) Treatment subgroups (different dosages, routes or regimens of administration of each uterotonic drug).
Methods/types of agents
The text in this section has been edited to add sensitivity analyses that became necessary during the review and explain how we grouped the agents for analysis.
In the protocol, this section stated:
We will consider trials of uterotonics described by WHO (WHO 2012) (oxytocin, ergometrine, misoprostol, carbetocin, or combinations of uterotonics) administered prophylactically by healthcare professionals for preventing PPH via any systemic route (sublingual, subcutaneous, intramuscular, rectal, oral, intravenous bolus and/or infusion) compared with another uterotonic or with placebo or no treatment. If we identify in the included studies interventions that we are not aware of, we will consider them as eligible and include them in the network after assessing their comparability with those named above. We will include trials in which non‐pharmacologic co‐interventions such as controlled cord traction, cord clamping, or uterine massage was performed as a randomised intervention in all arms of the trial. We will stratify all drugs according to mode of birth, prior risk of PPH, healthcare setting, specific dosage, regimen and route, to detect inequalities in subgroups that could affect comparative effectiveness.
Figure 1 (in the published protocol) shows the overall network of eligible comparisons in the review at a drug level.
Multi‐arm trials that compare different dosages, regimens or routes of one uterotonic drug, but also compare those versus another uterotonic drug, will be included. Intervention arms of different dosages, regimens or routes of the same uterotonic drug will be merged together for the global analysis of all outcomes and treated as separate independent comparisons only for the relevant subgroup analysis according to dosage, regimen and route of drug administration, while taking into account the correlation between the comparisons. We will exclude trials comparing exclusively different dosages, regimens or routes of administration of the same uterotonic drug. The review will be restricted to studies evaluating uterotonic drugs administered systemically at the birth of the baby for preventing PPH. Studies considering non‐uterotonic drugs, uterotonic drugs administered locally (for example, via intraumbilical or intrauterine routes) or at a later stage of delivery (for example, for the treatment of PPH or for retained placenta) will be excluded.
In our review this section now states:
Trials were eligible if they administered uterotonic agents of any dosage, route or regimen systemically following birth for preventing PPH, and compared them against other uterotonic agents, placebo or no treatment. Trials evaluating uterotonic drugs administered locally or not immediately after birth, or exclusively comparing different dosages, routes or regimens of the same uterotonic agent were excluded. We included trials in which non‐pharmacologic co‐interventions such as controlled cord traction, cord clamping, or uterine massage was performed as a randomised intervention in all arms of the trial and the effects of such co‐interventions were tested through a sensitivity analysis.
We classified drugs into oxytocin, carbetocin, misoprostol, ergometrine (included also ergonovine, methylergonovine), oxytocin plus ergometrine (Syntometrine, oxytocin combined with ergometrine, ergonovine, or methylergonovine), and oxytocin plus misoprostol. We excluded synthetic prostaglandin analogues of PGF2α (carboprost), and PGE2 (prostin, sulprostone), because these drugs are usually used for treating (and not preventing) PPH, and are not currently recommended by the World Health Organization (WHO) as alternatives (WHO 2012).
Methods/search methods
The search methods have been updated in line with the current standard search methods text of Cochrane Pregnancy and Childbirth.
Methods/types of outcomes/secondary outcomes
We have edited the outcome 'clinical signs of blood loss' to 'clinical signs of excessive blood loss (as defined by the trialists).'
Methods/investigation of heterogeneity and inconsistency and also subgroup analysis
We have edited the intervention subgroups for exploring heterogeneity and inconsistency and also subgroup analysis. In the protocol, these sections stated:
Intervention: dose, regimen or route.
In our review these sections now state:
Intervention: Dose of misoprostol (≥ 600 mcg versus < 600 mcg), and regimen of oxytocin (bolus versus bolus plus infusion versus infusion only).
Methods/investigation of heterogeneity and inconsistency and also subgroup analysis
We have carried out additional sensitivity analyses that became necessary during the conduct of the review. These are listed below:
Trials that also randomised participants to co‐interventions such as uterine massage or controlled cord traction.
Trials with more than 10% missing data.
Trials published before 1990.
Analysis
Since publication of the protocol for this review, further methods became available to perform the analysis with a frequentist approach in STATA. We changed our analysis for this reason to STATA rather than WinBUGS and a Bayesian environment.
'Summary of findings' table
We have added a GRADE Working Group approach for rating the quality of treatment effect estimates from network meta‐analysis, which was not planned during the protocol stage as the methods for this only became available recently for network meta‐analyses.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | 3‐arm controlled randomised trial. | |
| Participants | Between September 2006 and February 2009, 1964 parturients were randomised in a hospital setting in Egypt and South Africa. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with medical complications such as hypertension and diabetes, previous caesarean section, or an abdominal wall that was not thin enough to allow easy palpation of the uterus after delivery. | |
| Interventions | 10 IU of oxytocin administered intramuscularly (n = 1302) versus placebo or control (n = 662). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta. death. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated to 1 of 3 groups by selecting the next number in a computer‐generated random number sequence. |
| Allocation concealment (selection bias) | Low risk | The allocated group was noted inside opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | In Assiut, investigators evaluated blood loss by collection with a calibrated plastic drape placed under the mother within 30 minutes of delivery. At the East London Hospital Complex, investigators evaluated blood loss by collection with a low profile plastic “fracture” bedpan placed under the mother. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Investigators were unable to collect outcome data from 14 randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was registered retrospectively (ACTRN: 12609000372280). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the institution of the authors, or conducted without external funding. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 60 parturients were randomised in a hospital setting in the UK. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria were not specified. | |
| Interventions | 10 IU of oxytocin administered by an intravenous bolus (n = 30) versus 400 mcg of misoprostol administered orally (n = 30). | |
| Outcomes | The study recorded the following outcomes: PPH at 1000, additional uterotonics, transfusion, blood loss (mL), change in Hb level, vomiting, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed using sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated intra‐operative blood loss by the estimation of attending physicians, and by measurement of preoperative and postoperative Hb concentration and haematocrit. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between 1st July 2010 and 31st March 2011, 218 parturients were randomised in a hospital setting in Nigeria. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients with altered serum electrolytes, peritonitis, sepsis, previous bowel surgery, thyroid disease, inflammatory bowel disease, or chronic constipation. | |
| Interventions | 25 IU of oxytocin administered by an intravenous bolus plus infusion (n = 109) versus 600 mcg plus 5 IU of misoprostol plus oxytocin administered rectally plus by an intravenous bolus (n = 109). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, blood loss (mL), nausea, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The treatment allocation sequence was developed by 1 researcher (O.O.) using a computer‐generated table of random numbers with varied permutated blocks. |
| Allocation concealment (selection bias) | Low risk | Investigators used sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The same researcher administered the drugs intra‐operation and set up the infusions in the operating room; he was the only person who was not blind to the drug allocation and he did not take any further part in the active running of the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 200 parturients were randomised in a hospital setting in Nigeria. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing induction of labour or caesarean section, or those with haematocrit of less than 30%, pre‐eclampsia/eclampsia, grand multiparity (5 or more), multiple pregnancy, coagulopathy, or medical disorders. | |
| Interventions | 10 IU of oxytocin administered intramuscularly (n = 100) versus 400 mcg of misoprostol administered orally (n = 100). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb, third‐stage duration (min), nausea, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised into 2 groups, A and B, by blocked (restrictive) double‐blind randomisation using random table generated numbers. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss at delivery by collection with a large kidney dish, for measurement in a graduated measuring jar. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 80 parturients were randomised in a hospital setting in Egypt. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean. Exclusion criteria comprised parturients with risk factors for excessive blood loss e.g. those with placenta praevia or placental abruption. | |
| Interventions | 100 mcg of carbetocin administered by an intravenous bolus (n = 40) versus 10 IU of oxytocin administered by an intravenous bolus (n = 40). | |
| Outcomes | The study recorded the following outcomes: blood loss (mL). | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study was "single‐blind" but the identity of those blinded and the method of blinding were not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 3‐arm controlled randomised trial. | |
| Participants | Between October 2009 and February 2011, 120 parturients were randomised in a hospital setting in Egypt. The population comprised women of parity 4 or less, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing induction of labour or instrumental delivery, or those with previous caesarean section, extensive perineal, vaginal or cervical lacerations, bleeding disorders, Hb less than 100 g/L, uterine malformations, grand multiparity, multiple pregnancy, polyhydramnios, intrauterine fetal death, medical problems such as pre‐eclampsia, diabetes, cardiopulmonary problems, bowel disease, or allergy to prostaglandins. | |
| Interventions | Placebo or control (n = 40) versus 200 mcg of misoprostol administered sublingually (n = 40) versus 5 IU of oxytocin administered intramuscularly (n = 40). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, blood loss (mL), change in Hb level. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Investigators used closed envelopes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Objective assessment of blood loss | Low risk | Assessor blinding was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Investigators evaluated blood loss by collection with sterile packs weighed beforehand and afterwards. |
| Selective reporting (reporting bias) | Unclear risk | "Following randomisation, 16 study participants were excluded from our analysis. Of these, 14 patients received intrapartum oxytocin, 1 patient experienced extensive vaginal laceration and another experienced a cervical laceration." |
| Intention to treat analysis | High risk | The protocol of the study was unavailable for verification. |
| Funding source | Unclear risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between 1st December 1997 and 20th April 1998, 213 parturients were randomised in a hospital setting in Belgium. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with hypertensive disorders, gestational age less than 32 weeks, intrauterine fetal death, uterine malformations, inflammatory bowel disease, obliterative vascular or coronary disease, sepsis, allergy to prostaglandins or alkaloids. | |
| Interventions | 600 mcg of misoprostol administered orally (n = 105) versus 200 mcg of ergometrine administered by an intravenous bolus (n = 108). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonic, transfusion, manual removal of placenta, nausea, vomiting, headache, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was allocated by a computer‐generated list and randomisation in blocks. |
| Allocation concealment (selection bias) | Low risk | The study box contained either 2 capsules of misoprostol and an ampoule containing placebo, or 2 capsules with placebo and an ampoule containing methylergometrine. The study boxes and capsules were indistinguishable in the 2 groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "213 women were enrolled in the study, but the data for 13 were excluded because a caesarean section was performed after randomisation (n = 3), or because no predelivery (n = 3) or postpartum (n = 7, short hospital stay) blood sample was taken." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between May 2011 and May 2012, 200 parturients were randomised in a hospital setting in Pakistan. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with traumatic PPH, bleeding disorders, prolonged labour, placenta praevia, placental abruption, multiple pregnancy, BMI more than 30, or previous PPH. | |
| Interventions | 5 IU of oxytocin administered by an intravenous bolus (n = 100) versus 800 mcg of misoprostol administered rectally (n = 100). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, morbidity, manual removal of placenta, death, blood loss (mL), third‐stage duration (min), vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with special drapes placed under the mother until 1‐hour postpartum, and weighed beforehand and afterwards. Blood was also collected in graduated plastic bags. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between May 2009 and December 2009, 240 parturients were randomised in a hospital setting in Kuwait. The population comprised women of parity 5 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients less than 18 years old and those with known or suspected coagulopathy, grand multiparity (5 or more), uterine fibroids, polyhydramnios, multiple pregnancy, fetal macrosomia, severe anaemia, cervical tears or who required prophylactic oxytocin infusion. The presence of contraindications for the use of either Syntometrine or carbetocin that include pre‐existing hypertension, pre‐eclampsia, asthma, cardiac, renal or liver diseases, epilepsy, or history of hypersensitivity to Syntometrine or carbetocin. | |
| Interventions | 100 mcg of carbetocin administered intramuscularly (n = 120) versus 5 IU and 500 mcg of ergometrine plus oxytocin administered intramuscularly (n = 120). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, blood loss (mL), change in Hb level, nausea, vomiting, hypertension, headache, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was allocated by a computer‐generated code prepared before the recruitment. |
| Allocation concealment (selection bias) | Low risk | Investigators used sealed, consecutively‐numbered, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with a new plastic sheet placed under the mother following delivery of the placenta, and weighed (together with any gauzes, tampons and pads applied during the delivery) beforehand and 2 hours afterwards. A digital scale was used for weight measurement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between November 2006 and July 2007, 377 parturients were randomised in a hospital setting in the UK. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean. Exclusion criteria comprised parturients undergoing caesarean section with general anaesthesia, gestational age less than 37 weeks performed for fetal or maternal distress where, due to time constraints, it was not possible to recruit or randomise, or those with multiple pregnancy, placenta praevia or placental abruption. | |
| Interventions | 100 mcg of carbetocin administered by an intravenous bolus (n = 188) versus 5 IU of oxytocin administered by an intravenous bolus (n = 189). | |
| Outcomes | The study recorded the following outcomes: PPH at 1000, morbidity,. additional uterotonics, transfusion, death, blood loss (mL), change in Hb level, nausea, vomiting, headache, tachycardia, hypotension, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation sequence (1:1 ratio—blocks of 10, no stratification) was generated by computer. |
| Allocation concealment (selection bias) | Low risk | The preparation of the ampoules was undertaken by DHP Ltd. (Powys, UK) which provided sequentially numbered and labelled boxes each containing a 1 mL ampoule of the study drug. All boxes and ampoules were identically labelled, with the study number being the only differentiating feature between different drug packs. The random allocation sequence was not known to the investigators until the study had finished and the analysis was started. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Blood loss was estimated by the attending surgeon "in the usual way (visual estimation, number of used swabs and amount of aspirated blood)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered prospectively (EudraCT 2005‐002812‐94). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | Ferring Pharmaceuticals funded the cost of preparation of blinded medication ampoules. No other external funding was required for the study. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between 23rd September 2012 and 9th September 2013, 1140 parturients were randomised in a hospital setting in Uganda. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing induction or augmentation of labour or elective caesarean section, or those with intrauterine fetal death, heart disease, severe malaria or acute bacterial infection, multiple pregnancy, antepartum haemorrhage, altered cognitive status or reported hypersensitivity to prostaglandins. | |
| Interventions | 10 IU of oxytocin administered intramuscularly (n = 570) versus 600 mcg of misoprostol administered sublingually (n = 570). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb level.. third‐stage duration (min), nausea, vomiting, headache, fever, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A study biostatistician generated a randomisation list with a block size of 10. |
| Allocation concealment (selection bias) | Low risk | The study clinical pharmacist prepared the study drugs and placebos. The midwife research assistants received opaque envelopes with affixed study codes, containing both an injection (1 mL of oxytocin 10 IU or its placebo) and 3 pills (misoprostol 600 mg or its placebo). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "To achieve blinding of the participants and assessors, both inactive agents were manufactured and packaged to resemble actual study medicines in terms of shape, size, and colour." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with a clean plastic sheet placed under the mother during and after the third stage of labour. The sheet was specifically designed and piloted for the purpose. Blood was then drained into a calibrated container to improve accuracy in blood loss measurement. Furthermore, "mothers were given pre‐weighed standard sanitary pads to place in the perineum at all times. These pads were changed and weighed hourly for the first 6 hours, and then every 6 hours until 24 hours postpartum. Blood loss was estimated as 1 mL per g of weight of the pad after subtracting the dry pad weight." Investigators added the estimated blood loss in pads, to the volume of blood already collected with the plastic sheet. To improve consistency in the estimation of blood loss, standardised electronic scales were used to weigh soiled sanitary pads. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (ClinicalTrials.gov NCT01866241). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by scholarship funding from the Father Bash Foundation (public funding). |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between 1st April 2009 and 31st December 2009, 264 parturients were randomised in a hospital setting in Nigeria. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients in the second or third stage of labour, or those with cervical lacerations or coagulopathy. | |
| Interventions | 30 IU of oxytocin administered by an intravenous bolus plus infusion (n = 132) versus 20 IU plus 600 mcg of misoprostol plus oxytocin administered rectally plus by an intravenous infusion (n = 132). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, death, blood loss (mL), vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation code produced by an independent statistician using a computer‐generated random number sequence. |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially numbered sealed packets made of identical opaque brown‐paper envelopes prepared by the hospital pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with a BRASS‐V calibrated drape "which is a sterile intrapartum blood collection mat with a calibrated receptacle" placed under the mother after the delivery of the baby and immediate clamping of the umbilical cord. The drape included ribbons tied around the abdomen of the mother to optimise blood collection. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "6 women from the misoprostol group and 3 from the oxytocin group were excluded from statistical analysis. 5 of these women in the misoprostol group and all 3 in the oxytocin group were excluded because of the occurrence of cervical lacerations in them. The sixth woman excluded in the misoprostol group had developed features of disseminated intravascular coagulopathy (DIC). Analysis was thus based on 255 parturients (126 in the misoprostol group and 129 in the oxytocin group)." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Low risk | The study was conducted without external funding. |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between 12th June 2005 and 18th December 2006, 48 parturients were randomised in a hospital setting in Canada. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by emergency caesarean section. Exclusion criteria comprised parturients requiring general anaesthesia, or those with cardiac disease, hypertension or any condition predisposing to uterine atony and PPH, such as placenta praevia, multiple pregnancy, pre‐eclampsia, macrosomia, polyhydramnios, uterine fibroids, bleeding disorders, chorioamnionitis, previous uterine atony, previous PPH or allergy/hypersensitivity to oxytocin or ergot derivatives. | |
| Interventions | 250 mcg plus 20 IU of ergometrine plus oxytocin administered by an intravenous bolus (n = 24) versus 20 IU of oxytocin administered by an intravenous bolus plus infusion (n = 24). | |
| Outcomes | The study recorded the following outcomes: additional uterotonics, transfusion, blood loss (mL), nausea, vomiting, hypertension, tachycardia. hypotension. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated list of numbers. |
| Allocation concealment (selection bias) | Low risk | Investigators used consecutively‐numbered opaque sealed packets or envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by measurement of haematocrit preoperatively and 48 hours postoperatively. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the institution of the authors. |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between dates unspecified, 550 parturients were randomised in a hospital setting in South Africa. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 400 mcg of misoprostol administered rectally (n = 275) versus placebo or control (n = 275). | |
| Outcomes | The study recorded the following outcomes: PPH at 1000, additional uterotonics, manual removal of placenta, third‐stage duration (min), vomiting, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was by means of sealed, opaque containers containing 400 mg misoprostol or placebo tablets. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The placebo tablets were similar in size and colour but were not identical in shape to the misoprostol tablets. Blinding of the midwife administering the tablets was therefore not possible." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with an absorbent plastic‐backed linen saver and a low‐profile plastic “fracture” bedpan placed under the mother. Blood collection in the plastic bedpan continued until 1 hour after delivery of the baby. At 1 hour after delivery, all the blood on the linen saver was scooped into the bedpan with the blood already collected there, and "the total blood was carefully measured." All the used linen savers and vaginal pads were weighed, and the known dry weights of these materials were subtracted from the measured total weight. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Records of 4 of the 550 allocations (all from the placebo group) could not be traced." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 491 parturients were randomised in a hospital setting in South Africa. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 400 mcg of misoprostol administered rectally (n = 241) versus 500 mcg and 5 IU respectively of ergometrine plus oxytocin administered intramuscularly (n = 250). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, additional uterotonics, transfusion, manual removal of placenta, blood loss (mL), change in Hb level third‐stage duration (min). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was by means of sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "About halfway through enrolment it was discovered that a small number of women had been excluded from the Syntometrine [ergometrine plus oxytocin] group because of hypertension detected after enrolment (thus contraindicating the use of Syntometrine [ergometrine plus oxytocin]. The Syntometrine envelopes had been reallocated to subsequent participants, and it was not possible to trace the women originally allocated." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the South African Medical Research Council (public funding). |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between dates unspecified, 119 parturients were randomised in a hospital setting in the USA. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria were not specified. | |
| Interventions | 100 mcg of carbetocin administered by an intravenous bolus (n = 62) versus placebo or control (n = 57). | |
| Outcomes | The study recorded the following outcome: additional uterotonics. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between October 2000 and February 2004, 622 parturients were randomised in a hospital setting in Canada. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with placenta praevia, placental abruption, coagulopathy or unstable asthma. | |
| Interventions | 5 IU of oxytocin administered by an intravenous bolus (n = 311) versus 400 mcg of misoprostol administered orally (n = 311). | |
| Outcomes | The study recorded the following outcomes: PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, death, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation cards were produced. |
| Allocation concealment (selection bias) | Low risk | Investigators used sealed, opaque, sequentially numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The packages were prepared by the hospital pharmacy and their active drug unknown to the physicians and nurses." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by a combination of the visual estimation of attending physicians and measurement of blood volume in a kidney dish placed under the mother during the third stage of labour. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Nova Scotia Health Research Foundation (public funding). |
| Methods | 2‐arm controlled randomised trial. | |
| Participants | Between 1st October 1987 and 31st October 1988, 1429 parturients were randomised in a hospital setting in Ireland. The population comprised women of parity 5 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, vaginal breech or instrumental delivery, or those with hypertension, epidural anaesthesia, antepartum haemorrhage, placenta praevia, placental abruption, first stage of labour more than 15 hours, "quick" delivery or needing resuscitation. | |
| Interventions | 500 mcg of ergometrine administered by an intravenous bolus (n = 705) versus placebo or control (n = 724). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, blood loss (mL), change in Hb level. third‐stage duration (min), nausea, vomiting, hypertension, headache, aAbdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables were used. The first number was selected from the table and the numbers were then allocated in blocks of 100, following in sequence. |
| Allocation concealment (selection bias) | Low risk | Investigators used numbered, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss "as accurately as possible, with full realisation of the well‐documented problems of clinical measuring and estimation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses but dropouts for change in Hb |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by public funding, or conducted without external funding. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between dates unspecified, 652 parturients were randomised in a hospital setting in India. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section or instrumental delivery, or those with medical disorders, in active labour with more than 4 cm dilatation or stillbirths. | |
| Interventions | 400 mcg of misoprostol administered sublingually (n = 321) versus 10 IU of oxytocin administered intramuscularly (n = 331). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), third‐stage duration (min), nausea, vomiting, fever, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to treatment with a 1:1 ratio using computer‐generated simple randomisation. |
| Allocation concealment (selection bias) | Low risk | The study medications and placebos were packaged in appropriately coded envelopes by administrative staff from the department of clinical pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with a BRASS‐V calibrated drape placed under the mother before delivery of the baby. "The calibrated blood collection receptacle was opened after delivery and drainage of amniotic fluid. The blood collected in the drape was transferred to a measuring jar with 10 mL calibrations for accuracy. Blood‐soaked swabs were weighed in g, and the known dry weight of the swabs was subtracted; this volume was added to the measured blood volume from the drape (assuming an equivalence of 1 g and 1 mL)." Blood loss was measured at 1 and 2 hours after delivery of the baby. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was registered retrospectively (ClinicalTrials.gov NCT01373359). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from Jawaharlal Nehru Medical College (the institution of the authors). Study medications were donated by Cipla (misoprostol) and AstraZeneca (oxytocin). |
| Methods | 3‐arm controlled randomised trial. | |
| Participants | Between November 1999 and June 2000, 602 parturients were randomised in a hospital setting in France. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with gestational age less than 32 weeks, previous PPH, intrauterine fetal death, previous uterine scar, multiple pregnancy or pre‐eclampsia. | |
| Interventions | Placebo or control (n = 220) versus 2.5 IU of oxytocin (n = 196) administered intramuscularly versus 600 mcg of misoprostol administered orally (n = 186). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, blood loss (mL), change in Hb level, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Slips with the words “control,” “Syntocinon,” and “Cytotec” were placed into envelopes which were then drawn at random upon admission into the delivery room to determine to which group the woman would belong. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by weighing (methods of collecting blood were not reported). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between October 2000 and December 2002, 756 parturients were randomised in a hospital setting in the USA. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with a bleeding disorder. | |
| Interventions | 200 mcg plus 20 IU of misoprostol plus oxytocin administered sublingually plus by an intravenous infusion (n = 377) versus 20 IU of oxytocin administered by an intravenous infusion (n = 379). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb level, third‐stage duration (min), vomiting, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Agent vials were coded with a number, which had been assigned using a random number table. |
| Allocation concealment (selection bias) | Low risk | Investigators used opaque vials containing either a 200 mcg misoprostol tablet or placebo. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The placebo tablets were similar in size and colour, but not identical in shape to the misoprostol tablet." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "The placebo tablets were similar in size and colour, but not identical in shape to the misoprostol tablet." |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between 1st September 2007 and 5th January 2008, 104 parturients were randomised in hospital settings in France and Italy. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean. Exclusion criteria comprised parturients with toxaemia, eclampsia or epilepsy. | |
| Interventions | 100 mcg of carbetocin administered by an intravenous bolus (n = 52) versus 10 IU of oxytocin administered by an intravenous infusion (n = 52). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, additional uterotonics, blood loss (mL), vomiting, headache, hypotension, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The patients were divided in 2 groups with blinding to the study medication." Blinding of caregivers was unconfirmed. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by "a sensitive colorimetric method." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | High risk | The authors "do not have a financial relationship with the organisation that sponsored the research." No other source(s) of funding for the study were reported. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between dates unspecified, 60 parturients were randomised in a hospital setting in Canada. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients with heart disease or cardiac arrhythmia, hypertension or liver/renal/endocrine disease. | |
| Interventions | 100 mcg of carbetocin administered by an intravenous bolus (n = 29) versus 32.5 IU of oxytocin administered by an intravenous bolus plus infusion (n = 28). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, death, blood loss (mL), nausea, vomiting, headache, fever, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by a sensitive colorimetric measurement of the Hb concentration of blood loss collected "by means of aspiration from the operative field [that] began immediately after administration of the study drug and ceased at the time of skin closure. All gauzes used during this timeframe were placed in 15% Lyse solution. All aspirated blood, gauzes, and the reference blood sample were sent to the laboratory for quantification of total blood volume. Blood on gauzes was extracted with Lyse solution, and Hb content was determined with a sensitive colorimetric method adapted to the Cobas FARA analyser. Haemoglobin concentration is proportional to the absorbance of a hydrogen peroxide‐activated aminophenazone‐phenol mixture measured at a wavelength of 500 nm. The inter‐assay coefficient of variation averaged 3.3%, and the limit of detection of the assay was 14 mg/dL. The amount of blood collected in gauzes was calculated with the following formula: blood loss in dL = amount of Hb in surgical gauzes in mg /Hb concentration in mg/dL before caesarean section. Total blood loss was calculated by means of summing the volumes of blood aspirated and collected with gauzes." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "3 patients who received general instead of epidural anesthesia were excluded from the study and did not receive the study medication" but the study report did not specify whether these exclusions occurred before or after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | High risk | The study was supported by funding from Ferring Pharmaceuticals. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between dates unspecified, 164 parturients were randomised in a hospital setting in Canada. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients younger than 18 years old, or those without known PPH risk, known or suspected coagulopathy, heart disease or cardiac arrhythmia, chronic liver/renal/endocrine disease or hypersensitivity to study drugs. | |
| Interventions | 100 mcg of carbetocin administered intramuscularly (n = 84) versus 10 IU of oxytocin administered by an intravenous infusion (n = 80). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, additional uterotonics, blood loss (mL), change in Hb level, nausea, vomiting, headache, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Investigators used computer‐generated randomisation codes with a block size of 4. |
| Allocation concealment (selection bias) | Unclear risk | Investigators used consecutively‐numbered sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was "double‐blind": "for each study subject, kits containing both the study medication and a placebo were prepared in the hospital pharmacy according to the randomisation schedule, to assure blinding of the clinical staff." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "4 women did not receive study medication and were therefore not included in the analysis (3 were excluded as a result of caesarean births). Had these 4 women completed the study and received the medication, 1 would have received carbetocin and 3 would have received oxytocin. This factor contributed to the lower reported number of women receiving oxytocin." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | High risk | The study was supported by funding from Ferring Pharmaceuticals. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between dates unspecified, 700 parturients were randomised in a hospital setting in Mozambique. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing induction or augmentation of labour. | |
| Interventions | 400 mcg of misoprostol administered rectally (n = 350) versus 10 IU of oxytocin administered intramuscularly (n = 350). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, blood loss (mL), third‐stage duration (min), vomiting, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Neither the investigators nor the nurses participating in the study had access to the codes until the completion of the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss with a metallic collector placed under the mother, from immediately after delivery of the baby until the mother was removed from the delivery room. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "A few subjects were excluded after randomisation for emergency caesarean section or incomplete data collection." |
| Selective reporting (reporting bias) | High risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of retained placenta were omitted). |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Low risk | This study was financed by the Maputo Central Hospital (the institution of the authors) and the Special Program on Research and Research Training in Human Reproduction of the WHO (public funding). |
| Methods | 5‐arm placebo‐controlled randomised trial. | |
| Participants | Between July 2008 and April 2009, 75 parturients were randomised in a hospital setting in the USA. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients with active labour, ruptured membranes, drug allergy, multiple pregnancy, significant obstetric disease, risk factors for PPH (abnormal placentation, fibroids, previous PPH, previous classical uterine incision), coagulopathy or thrombocytopenia. | |
| Interventions | Placebo or control (n = 15) versus 5, 3, 1, or 0.5 IU of oxytocin administered by an intravenous bolus (n = 60). | |
| Outcomes | The study recorded the following outcomes: additional uterotonics, transfusion, blood loss (mL), nausea, vomiting, tachycardia, hypotension. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using Microsoft Excel‐generated random number allocations. |
| Allocation concealment (selection bias) | Unclear risk | Investigators used opaque envelopes containing group assignments. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The obstetrician and anaesthetist involved in each case were blinded to the oxytocin dose assignments." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss "by estimating blood collected by suction and by calculating the weight of blood on surgical swabs." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "75 patients were enrolled, and 74 patients completed the study; 1 patient was excluded due to protocol violation (obstetrician request for supplemental oxytocin despite adequate uterine tone)." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Low risk | The study was supported by funding from the Department of Anaesthesia of the Stanford University School of Medicine (the institution of the authors). |
| Methods | 4‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between 1st January 2000 and 1st October 2000,1633 parturients were randomised in a hospital setting in Turkey. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with gestational age less than 32 weeks or hypersensitivity to prostaglandins. | |
| Interventions | 400 mcg plus 10 IU of misoprostol plus oxytocin administered rectally plus by an intravenous infusion (n = 407) versus 400 mcg of misoprostol administered rectally (n = 405) versus 10 IU of oxytocin administered by an intravenous infusion (n = 412) versus 200 mcg plus 10 IU of ergometrine plus oxytocin administered intramuscularly plus by an intravenous infusion (n = 409). | |
| Outcomes | The study recorded the following outcomes: PPH at 500. PPH at 1000, additional uterotonics, transfusion, change in Hb level, third‐stage duration (min), vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was based on a table of computer‐generated blocks of random numbers. |
| Allocation concealment (selection bias) | Low risk | Investigators used sealed consecutively‐numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All medications were applied by midwives, but residents who treat[ed] the birth and the third stage of labour were blinded to the identity of medication. Only the midwife who applied the medication opened the envelope once to read the code and then transferred the randomisation code into another identical envelope. The identities of the placebo and active medication were also concealed from caregivers and residents who followed the patient for the next 24 hours. The randomisation code was not broken until study completion." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All medications were applied by midwives, but residents who treat[ed] the birth and the third stage of labour were blinded to the identity of medication. Only the midwife who applied the medication opened the envelope once to read the code and then transferred the randomisation code into another identical envelope. The identities of the placebo and active medication were also concealed from caregivers and residents who followed the patient for the next 24 hours. The randomisation code was not broken until study completion." |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with a sterile steel bedpan and plastic bed linen. Gauzes and pads were also collected and weighed until 1 hour after delivery of the placenta. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "The study enrolled 1633 women, but the data for 27 women were excluded because of lack of predelivery (n = 13) or postpartum (n = 14, short hospital stay) haemoglobin concentrations." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 4‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between January 2000 and October 2000, 1800 parturients were randomised in a hospital setting in Turkey. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with gestational age less than 32 weeks or hypersensitivity to prostaglandins. | |
| Interventions | 400 mcg plus 10 IU of misoprostol plus oxytocin administered orally plus by an intravenous infusion (n = 450) versus 400 mcg of misoprostol administered orally (n = 450) versus 10 IU of oxytocin administered by an intravenous infusion (n = 450) versus 200 mcg plus 10 IU of ergometrine plus oxytocin administered intramuscularly plus by an intravenous infusion (n = 450). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, blood loss (mL), change in Hb level, third‐stage duration (min), vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was computer‐generated without any blocking or stratification. |
| Allocation concealment (selection bias) | Low risk | Investigators used sealed, consecutively‐numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The placebo tablets were similar in size and colour but were not identical in shape to the misoprostol tablets. To minimise this limitation, the preparation and administration of the medication were carried out by a midwife who had not been involved in the management of the patient except for drug administration. The identity of medication was concealed from the resident physicians who managed the delivery and the third stage of labor. The caregivers and residents who followed up on the patient for the next 24 hours were also blind as to which patients received placebo and which received active medication. The randomisation code was not broken until the completion of the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The placebo tablets were similar in size and colour but were not identical in shape to the misoprostol tablets. To minimise this limitation, the preparation and administration of the medication were carried out by a midwife who had not been involved in the management of the patient except for drug administration. The identity of medication was concealed from the resident physicians who managed the delivery and the third stage of labor. The caregivers and residents who followed up on the patient for the next 24 hours were also blind as to which patients received placebo and which received active medication. The randomisation code was not broken until the completion of the study." |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with a sterile steel bedpan and plastic bed linen from immediately after delivery. Gauzes and pads were also collected 1 hour after delivery of the placenta and weighed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "The data for 226 patients were excluded because of caesarean deliveries performed after randomisation (n = 206) and the lack of predelivery (n = 6) or postpartum (n = 14, short hospital stay) haemoglobin concentrations." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between April 2007 and October 2008, 1410 parturients were randomised in a hospital setting in Spain. The population comprised women of parity 4 or less, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section or instrumental delivery, or those with gestational age less than 32 weeks, coagulopathy, Hb less than 80 g/L, liver or kidney disorder, grand multiparity (5 or more), hypersensitivity or any contraindication for use of prostaglandins. | |
| Interventions | 400 mcg and 200 mcg plus 10 IU of misoprostol plus oxytocin administered sublingually and rectally plus intramuscularly (n = 702) versus 10 IU of oxytocin administered intramuscularly (n = 698). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb level, third‐stage duration (min), NNU admissions, nausea, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignments were generated by computer. |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially‐numbered, opaque, sealed envelopes prepared by people not related to the study. This process was supervised by an analyst. Every morning a secretary received the sealed envelopes for distribution and this process was monitored by someone working on the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | After delivery of the baby, investigators evaluated blood loss by collection with a sterile waterproof cloth placed under the mother, to channel blood into a bottle with capacity of 2 L: the volume reading was collected once beyond the third stage of labour. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "3 y 7 de las mujeres aleatorizadas a los grupos I y II, respectivamente, no recibieron ningún tratamiento por incumplimiento del protocolo y, como la información correspondiente a ellas no fue registrada en ningún momento, no forman parte de este informe": 3 women in the misoprostol plus active management group, and 7 women in the active management group, were excluded from the analysis due to protocol deviations and non‐availability of the information. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Low risk | The study was supported by the Science and Ethics Committee of the Hospital Eusebio Hernandez in Habana, Cuba in conjunction with the Clinica Mediterranea Medica in Valencia, Spain (the institutions of the authors). |
| Methods | 4‐arm controlled randomised trial. | |
| Participants | Between January 2005 and November 2008, 160 parturients were randomised in a hospital setting in Turkey. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean. Exclusion criteria comprised parturients with thyroid disorder, inflammatory bowel disease or other bowel diseases, previous bariatric surgery or hypersensitivity to prostaglandins. | |
| Interventions | 200 mcg, 400 mcg, or 600 mcg plus 10 IU of misoprostol plus oxytocin administered rectally plus by an intravenous infusion (n = 120) versus 10 IU of oxytocin administered by an intravenous infusion (n = 40). | |
| Outcomes | The study recorded the following outcomes: fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between 1st December 2007 and 31st May 2009, 200 parturients were randomised in a hospital setting in India. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean. Exclusion criteria comprised parturients undergoing caesarean section for cord prolapse or bradycardia, or those with cardiovascular, respiratory, liver or haematological disorders or known hypersensitivity to prostaglandins. | |
| Interventions | 800 mcg of misoprostol administered rectally (n = 100) versus 40 IU of oxytocin administered by an intravenous infusion (n = 100). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, death, blood loss (mL), change in Hb level, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using computer‐generated random numbers in a 1:1 ratio. |
| Allocation concealment (selection bias) | Low risk | The packets containing the 2 drugs were sealed and opaque, and could not be identified by the surgeons and anaesthetists. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The packets containing the 2 types of drug were sealed and opaque, and could not be identified by the surgeons and anaesthetist." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated intraoperative blood loss by collection with a suction bottle for volumetric measurement, combined with linen savers and mops weighed before and after delivery. They added the approximate volume of the contents of the suction bottle (a) to the difference in weight between dry (b) and soaked (c) linen savers and mops (1 g equivalent to 1 mL). Amniotic fluid volume (d) was calculated by multiplying amniotic fluid index by 30 mL. Finally, intraoperative blood loss was determined by subtracting amniotic fluid volume from approximate blood loss ((a plus (c ‐ b)) ‐ d). Furthermore, investigators evaluated postoperative bleeding over the next 8 hours by weighing soaked pads and subtracting the dry weight. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "4 women in group 1 [misoprostol] and 6 women in group 2 [oxytocin] were excluded from the analysis: 4 women required conversion to general anaesthesia, 5 women had traumatic intraoperative bleeding (extension of lower segment incision or ligament hematoma), and 1 woman had placenta accreta resulting in hysterectomy." |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (CTRI 2009/091/000075). |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between 1st September 2009 and 31st August 2010, 530 parturients were randomised in a hospital setting in India. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing augmentation of labour, caesarean section or instrumental delivery, or those with risk factors for PPH, including BMI more than 30, grand multiparity (5 or more), polyhydramnios, fetal macrosomia, antepartum haemorrhage, prolonged labour, previous PPH, Hb less than 80 g/L, severe pre‐eclampsia, asthma or coagulopathy. | |
| Interventions | 400 mcg of misoprostol administered sublingually (n = 265) versus 10 IU of oxytocin administered intramuscularly (n = 265). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated random number sequence. |
| Allocation concealment (selection bias) | Low risk | Investigators used pre‐prepared sealed and opaque packet. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The misoprostol and placebo tablets were similar in size, shape, and colour. The ampoules of oxytocin and placebo were also similar. Selection, enrolment, and randomisation were done by the resident doctors, whereas preparation of packets and confidential record maintenance was done by the labour room nursing staff in charge." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with specially designed, pre‐weighed absorbent thick cotton pads with plastic lining, placed under the mother. Blood clots, if any, were expressed from the vagina into a polythene bag. Any episiotomy wound was repaired immediately, and the swabs used for the purpose of episiotomy were not included in blood loss assessment. If necessary, pads were replaced during the observational hour after delivery. Then the soaked pad(s) and the blood clots were weighed. "The specific gravity of blood being 1.08, the amount of blood lost in mL was approximately equal to the weight in g." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "2 women in the study group and 1 woman in the control group refused sublingual administration of the drug." |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (CTRI 2009/091/000672). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between 1st October 2012 and 31st December 2013, 396 parturients were randomised in a hospital setting in India. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by emergency caesarean section. Exclusion criteria comprised parturients requiring conversion to general anaesthesia, or those with cardiovascular, hepatic, or haematological disorders or any contraindication for the use of misoprostol or oxytocin. | |
| Interventions | 400 mcg plus 20 IU of misoprostol plus oxytocin administered sublingually plus by an intravenous bolus and infusion (n = 198) versus 20 IU of oxytocin administered by an intravenous bolus plus infusion (n = 198). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, death, blood loss (mL), change in Hb level, nausea, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a computer‐generated random number sequence and blocks of size 8. |
| Allocation concealment (selection bias) | Low risk | Assignments were contained in sealed, opaque and sequentially‐numbered packets. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Randomisation and confidential record maintenance were performed by residents who were not involved in the trial, and the operation theatre midwife prepared the sealed packets and allocated and administered the drugs. Thus, clinicians, investigators, data analysts, and participants were masked to the treatment allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated intraoperative blood loss from after delivery of the placenta. Blood was collected with a suction bottle, linen savers and mops: the dry weights of these materials were subtracted from the soaked weights, and the total volume of intraoperative blood loss calculated on the basis that 1 g is equivalent to 1 mL. Investigators also evaluated postoperative blood loss by weighing soaked pads. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (CTRI 2013/05/003645). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 3‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 300 parturients were randomised in a hospital setting in India. The population comprised women of parity 5 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing augmentation of labour, caesarean section or instrumental delivery, or those with grand multiparity (more than 5), multiple pregnancy, pregnancy‐induced hypertension, antepartum haemorrhage, previous caesarean, Hb less than 80 g/L, other obstetric problems or known hypersensitivity to prostaglandins. | |
| Interventions | 100 mcg or 200 mcg of misoprostol administered sublingually (n = 200) versus 200 mcg of ergometrine administered by an intravenous bolus (n = 100). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, death, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, headache, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using random number tables. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Investigators evaluated blood loss by "measuring blood and blood clots collected in sponges." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 991 parturients were randomised in a hospital setting in Hong Kong. The population comprised women of parity 3 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with medical conditions that precluded the use of ergometrine, such as pre‐eclampsia, cardiac disease or conditions that required prophylactic oxytocin infusion after delivery such as grand multiparity (4 or more) or presence of uterine fibroids. | |
| Interventions | 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 500) versus 10 IU of oxytocin administered by an intravenous bolus (n = 491). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, blood loss (mL), change in Hb level, nausea, vomiting, hypertension, headache. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Investigators used sealed consecutively‐numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The preparation and administration of the medication was carried out by a second midwife who was not involved in the management of the patient except for the drug administration. The medical attendant who delivered the baby was not informed of the type of oxytocics used." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss "by measuring the amount of blood clots and weighing the towels and swabs used." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Low risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 3‐arm active‐controlled randomised trial. | |
| Participants | Between December 1997 and December 1998, 930 parturients were randomised in a hospital setting in Australia, Papua and China. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing elective caesarean section, or those with coagulopathy, asthma, heart disease, severe renal disease, epilepsy or hypertension. | |
| Interventions | 400 mcg of misoprostol administered orally (n = 455) versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 310) versus 10 IU of oxytocin administered intramuscularly (n = 129). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, blood loss (mL), change in Hb level, third‐stage duration (min). | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved by a random number list in blocks of 20, with separate randomisation for each centre. |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially‐numbered sealed security (opaque) envelopes containing the appropriate drug label for each centre. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Investigators evaluated blood loss by combining "estimated" and "measured" values according to the standard clinical practice of each study centre. The "estimated" blood loss was judged by the attending senior midwives and/or clinicians. The "measured" blood loss was calculated as the actual volume of blood collected in a calibrated measuring jug, combined with the difference in weight between dry and blood‐stained undersheets and sanitary pads. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were not collected completely from 67 study participants: "the main reasons for exclusion prior to randomisation, and following randomisation but before treatment, were the need for caesarean section and development of hypertension, either before or during labour. Two women (1 in each group) were not included in the analysis as no record was made of the primary outcome of blood loss." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between February 1992 and December 1994, 694 parturients were randomised in a hospital setting in Canada. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients undergoing general anaesthesia or requiring a classical uterine incision, or those with heart disease, chronic hypertension requiring treatment, liver, renal, or endocrine disorders, coagulopathy, placenta praevia or placental abruption. | |
| Interventions | 100 mcg of carbetocin administered by an intravenous bolus (n = 348) versus 25 IU of oxytocin administered by an intravenous bolus plus infusion (n = 346). | |
| Outcomes | The study recorded the following outcomes: additional uterotonics, transfusion, change in Hb level, nausea, vomiting, headache, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was allocated by a computer‐generated randomisation code, stratified by centre and with use of random blocks of 2. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "All physicians and nurses involved, all investigators and their staff, and all sponsor representatives were kept blinded to the treatment codes at all times." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Informed consent was obtained from 694 patients. 35 patients were withdrawn from the study before they received study drug, leaving a total of 659 patients who received [the] study drug and were included in the safety analysis." Major protocol violations. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | High risk | The study was supported by funding from Ferring Pharmaceuticals. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 196 parturients were randomised in a hospital setting in Indonesia. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at unspecified for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 600 mcg of misoprostol administered orally (n = 98) versus 10 IU of oxytocin administered intramuscularly (n = 98). | |
| Outcomes | The study recorded the following outcomes: blood loss (mL), third‐stage duration (min), shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 3‐arm placebo‐controlled randomised trial. | |
| Participants | Between July 1993 and July 1994, 371 parturients were randomised in a hospital and community setting in the Netherlands. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing induction or augmentation of labour or instrumental delivery, requiring tocolysis or those who refuse to take part or with cardiac disease, multiple pregnancy, non‐cephalic presentation, polyhydramnios, coagulopathy, stillbirth, antepartum haemorrhage, Hb less than 4.8 mmol/L or previous complication in third stage. | |
| Interventions | Placebo or control (n = 143) versus 5 IU of oxytocin administered intramuscularly (n = 78). There were 4 exclusions post randomisation but it was unclear from which group. The oral ergometrine group was merged with the control group for analysis. | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated random list. |
| Allocation concealment (selection bias) | Low risk | Investigators used identical study boxes. Care was taken that no difference could be seen or heard between the packages of the ergometrine/placebo tablets and the oxytocin ampoules. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was "double‐blind" with placebo to match ergometrine treatment, but "to allow comparison with a standard prophylactic regimen a third group receiving the standard intramuscular oxytocin was added, but for obvious reasons this could not be conducted in a blind manner." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with a "fresh" perineal pad placed under the mother from immediately after birth until 1 hour after the delivery of the placenta. The difference in the weight of the pad before and after delivery was calculated on the basis that 1 g is equivalent to 1 mL of blood. "During delivery some blood was usually spattered on the drapes and gowns of the attendants, although attempts were made to minimise such losses. This gave a constant error of approximately 10%. In addition, the placental interstices contain maternal blood (about 9% of placental weight). As systematic overestimations (amniotic fluid) and underestimations (blood loss) are likely to be equally distributed among the groups, no corrections have been made for them." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "4 women with exclusion criteria were entered erroneously (3 forcipal extractions, 1 augmentation). They are considered as non‐participants." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between September 2002 and December 2005, 1620 parturients were randomised in a community setting in India. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients at high risk and inappropriate for home or community births according to India’s ministry of health guidelines including those undergoing elective caesarean section or breech vaginal delivery, or those previous caesarean section, Hb less than 80 g/L, antepartum haemorrhage, hypertension, multiple pregnancy, history of previous antepartum or PPH, retained placenta, uterine inversion, diabetes, heart disease, seizures, placenta praevia, asthma or contraindications to misoprostol. | |
| Interventions | 600 mcg of misoprostol administered orally (n = 812) versus placebo or control (n = 808). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), nausea, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a randomisation list with a random block size generated by the data co‐ordinating centre and stratified by the midwife. |
| Allocation concealment (selection bias) | Low risk | The envelopes were numbered and each envelope had a 5‐digit code number assigned to it. The first 2 digits were the auxiliary nurse midwife number, followed by a sequence number beginning with 001 and ending with 100, assigned to the individual participant. Non‐distinguishable envelopes in batches of 100 were distributed to each of the midwives affiliated with the 4 selected primary‐health centres. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The identical placebo was specifically manufactured for the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with a polyurethane blood collection drape placed under the mother from immediately after birth until 1 hour after delivery of the baby. The blood collection drape included a calibrated receptacle specifically developed for the study. In the event of persistent bleeding beyond 1 hour, the drape was removed at 1 hour, blood loss measured, and a new drape used with a second measurement made at 2 hours. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (ClinicalTrials.gov NCT00097123). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the National Institute of Child Health and Human Development (public funding) and the Bill and Melinda Gates Foundation (public funding). |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between December 2011 and May 2013, 100 parturients were randomised in a hospital setting in India. The population comprised women of parity 4 or less, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with grand multiparity (not defined), rhesus negative blood group, cardiac disease, diabetes, bleeding disorder, precipitated labour, overdistended uterus, traumatic PPH, PROM/chorioamnionitis, intrauterine death, previous caesarean section/scar on uterus or inability to obtain the informed consent. | |
| Interventions | 10 IU of oxytocin administered intramuscularly (n = 50) versus 200 mcg of ergometrine administered intramuscularly (n = 50). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, additional uterotonics, transfusion, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, headache. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Investigators used a systematic random sampling method. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with drapes that were weighed together with mops and clots, and by measurement of Hb concentration and haematocrit of a sample of venous blood before delivery and 24 hours after birth. A sample of venous blood before delivery and 24 hours after the birth was also collected, for Hb and haematocrit measurement "as an objective index of blood loss." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 50 parturients were randomised in a hospital setting in the UK. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at unspecified for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 10 IU of oxytocin administered intramuscularly (n = 25) versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 25). | |
| Outcomes | The study recorded the following outcome: Blood loss (mL). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between the beginning of August 2007 and the end of December 2007, 100 parturients were randomised in a hospital setting in Iran. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients with multiple pregnancy, prolonged labour more than 12 h, 2 or more previous caesarean sections, previous uterine rupture, Hb less than 80 g/L, who had a history of heart, renal or liver disorders or had a coagulopathy. | |
| Interventions | 400 mcg of misoprostol administered sublingually (n = 50) versus 20 IU of oxytocin administered by an intravenous infusion (n = 50). | |
| Outcomes | The study recorded the following outcomes: additional uterotonics, blood loss (mL), change in Hb level. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | By a simple randomisation method, patients were allocated into 2 equal groups. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection in a suction bottle, and with drapes and pads beneath the mother. Amniotic fluid was suctioned and measured, and then subtracted from the total volume of the suction bottle. Meanwhile the known dry weight(s) of drapes and pads were subtracted from the soaked weights of these materials. Measurements of blood collected in the suction bottle and on drapes and pads were added together. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | High risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of transfusion were omitted). |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between 1st January 2013 and 31st June 2014,180 parturients were randomised in a hospital setting in Egypt. The population comprised nulliparous women with a singleton pregnancy, at high risk for PPH, who delivered by emergency caesarean section. Exclusion criteria comprised parturients undergoing elective caesarean section, vaginal delivery or general anaesthesia, or those who were multigravida, or with malpresentation, fetal anomalies, placenta praevia, diabetes, hypertension, pre‐eclampsia or cardiac disease. | |
| Interventions | 100 mcg of carbetocin administered by an intravenous bolus (n = 90) versus 20 IU of oxytocin administered by an intravenous infusion (n = 90). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, death, blood loss (mL), change in Hb level, nausea, headache, fever. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Investigators used sealed, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was "double‐blinded": "a double dummy system for administration was used." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss "in the usual way (visual estimation, number of used swabs and amount of aspirated blood)." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "100 cases were excluded (4 had congenital fetal anomalies, 7 cases had placenta praevia, 5 cases were diabetic, 8 had hypertension, 9 had pre‐eclampsia, 3 cases were cardiac, 28 cases [required] general anaesthesia, 17 cases delivered vaginally and 19 delivered by elective caesarean section)." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between dates unspecified, 382 parturients were randomised in a hospital setting in Egypt. The population comprised women of parity 3 or less, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients with asthma, anaemia, bleeding disorders, cardiac disease, inflammatory disease, bowel disease, multiple pregnancy, pre‐eclampsia, placenta praevia, placental abruption, previous APH, previous PPH, grand multiparity (not defined), fibroids, growth restriction, fetal malformations or allergy to prostaglandins. | |
| Interventions | 400 mcg plus 10 IU of misoprostol plus oxytocin administered sublingually plus by an intravenous bolus (n = 191) versus 10 IU of oxytocin administered by an intravenous infusion (n = 191). | |
| Outcomes | The study recorded the following outcomes: morbidity, additional uterotonics, transfusion, death, blood loss (mL), vomiting, fever, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation code was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially‐numbered sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo and misoprostol tables "looked identical in size, colour, and packing." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated intraoperative blood loss by collection in a suction bottle minus sonographically estimated amniotic fluid volume, together with visual estimates of the volume of blood on the floor and the weight differences between dry and used towels, linens, and swabs. Visual estimates were performed by obstetricians blinded to treatment allocation. Towels, linen and swabs were weighed with an electronic scale. Weights were added to volumetric values on the basis that 1 g is equivalent to 1 mL. Investigators evaluated postoperative blood loss by weighing bed linen, gowns and perineal pads. Furthermore, blinded investigators estimated blood loss by multiplying maternal blood volume in mL by the difference between preoperative and postoperative haematocrit measurements, all divided by preoperative haematocrit measurements. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "4 patients in the placebo group and 12 patients in the misoprostol group were excluded from the study due to loss to follow‐up or missed preoperative hematocrit data." |
| Selective reporting (reporting bias) | Unclear risk | The study report matches the study protocol that was registered retrospectively (ClinicalTrials.gov NCT01466530). |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Low risk | The study was supported by funding from Mansoura University (the institution of the authors). |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between April 1996 and March 1998, 1000 parturients were randomised in a hospital setting in the UK. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section or water birth, or those with severe asthma. | |
| Interventions | 500 mcg of misoprostol administered orally (n = 501) versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 499). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta. death, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, headache, fever, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Statistician using computer‐generated block randomisation with varying block size. |
| Allocation concealment (selection bias) | Low risk | Investigators used opaque, sequentially‐numbered sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between February 2010 and October 2012, 380 parturients were randomised in a hospital setting in Egypt. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients undergoing general anaesthesia, or those with coagulopathy, coronary artery disease, hypertension, PPH due to causes other than uterine atony or hypersensitivity to carbetocin. | |
| Interventions | 400 mcg plus 20 IU of misoprostol plus oxytocin administered sublingually plus by an intravenous infusion (n = 190) versus 100 mcg of carbetocin administered by an intravenous bolus (n = 190). | |
| Outcomes | The study recorded the following outcomes: morbidity, additional uterotonics, transfusion, death, blood loss (mL), change in Hb level, nausea, vomiting, headache, hypotension, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated random number sequence. |
| Allocation concealment (selection bias) | Low risk | Drugs were in pre‐prepared sealed and opaque packets. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Randomisation was done by the resident doctors immediately before transfer to theatre, whereas preparation of packets and confidential record maintenance was done by the labour room nursing staff... Caesarean delivery was performed by 4 senior obstetricians who were blinded to the allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss "in the usual way (visual estimation, number of used swabs and amount of aspirated blood)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between 1st January 2008 and 1st January 2009, 400 parturients were randomised in a hospital setting in Egypt. The population comprised women of parity 4 or less, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients undergoing their first elective caesarean section, or those unsure of gestation or with hypertension, diabetes, oligohydramnios, abnormal placenta or abnormal laboratory investigations. | |
| Interventions | 400 mcg plus 10 IU of misoprostol plus oxytocin administered rectally plus by an intravenous infusion (n = 200) versus 10 IU of oxytocin administered by an intravenous infusion (n = 200). | |
| Outcomes | The study recorded the following outcomes: PPH at 1000, additional uterotonics, transfusion, blood loss (mL), change in Hb level, NNU admissions, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using computer‐generated tables. |
| Allocation concealment (selection bias) | Unclear risk | Allocation was placed in sealed envelopes until the time of operation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Attending obstetricians and other caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss from after uterine incision, by collection in 2 separate suction sets administered by a nurse, and by weighing surgical towels before and after each operation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was registered retrospectively (ACTRN 12611000638932). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the institution of the authors, or conducted without external funding. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between 4th January 2004 and 30th January 2005, 864 parturients were randomised in a hospital setting in Nigeria. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with pre‐eclampsia, hypertension, cardiac disease, severe anaemia, asthma, renal/hepatic disorders, grand multiparity (not defined), multiple pregnancy, polyhydramnios, previous PPH, fibroids or contraindications to misoprostol or ergometrine. | |
| Interventions | 400 mcg of misoprostol administered orally (n = 432) versus 500 mcg of ergometrine administered intramuscularly (n = 432). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, manual removal of placenta, death, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, headache, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was by simple random selection. An independent statistician generated sets of 4 random letters, which were in boxes, and each box contained 4 separate random allocations which was equivalent to an opaque sealed envelope stratified in a block of 4. |
| Allocation concealment (selection bias) | Low risk | Investigators used opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study was "single‐blinded." The identity of those blinded was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by a combination of careful collection in a receptacle after the delivery of the baby, by visual estimation of blood loss, and by extrapolation of blood loss using the weight difference of the total perineal pad used up to 24 hours postpartum. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of transfusion, chest pain and abdominal pain were omitted). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the National Postgraduate Medical College and Faculty of Obstetrics and Gynaecology of the University College Hospital in Ibadan, Nigeria (the institution of the authors). |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between 1st September 2011 and 31st May 2012, 300 parturients were randomised in a hospital setting in Nigeria. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with premature labour (less than 28 weeks), multiple pregnancy, antepartum haemorrhage, hypertension in pregnancy, severe anaemia or haemoglobinopathy. | |
| Interventions | 10 IU of oxytocin administered intramuscularly (n = 151) versus 500 mcg of ergometrine administered intramuscularly (n = 149). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, additional uterotonics, transfusion, manual removal of placenta, blood loss (mL), third‐stage duration (min), nausea, vomiting, hypertension, headache. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using computer‐generated random tables. |
| Allocation concealment (selection bias) | Low risk | A person uninvolved with the study prepared the study drugs. The labels on the ampoules (which were similar in size and colour) were removed and the ampoules were placed in opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "A person uninvolved with the study prepared the study drugs… The labels on the ampoules (which were similar in size and colour) were removed and the ampoules were placed in opaque sealed envelopes, such that only the computer‐generated randomisation numbers on the envelopes were available to identify the study drug during unblinding." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with "a fresh large perineal pad with plastic backing." They placed all the gauzes and perineal pads used to absorb the blood into a polythene bag, and subtracted the dry weight from the wet weight. Volume of blood loss was calculated on the basis that 1 g is equivalent to 1 mL. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Low risk | The study protocol was registered (PACTR 201105000292708). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the institution of the authors. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between 1st January 2002 and 30th June 2002, 97 parturients were randomised in a hospital setting in Turkey. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing elective caesarean section or instrumental delivery, or those with premature labour (less than 37 weeks), postmaturity (more than 43 weeks), grand multiparity (more than 4), twin pregnancy, growth restriction, macrosomia, Hb less than 100 g/L, systemic disorder, prolonged third stage, manual removal of placenta or additional lacerations due to episiotomy or where it took longer than 30 min to repair lacerations after episiotomy. | |
| Interventions | 400 mcg of misoprostol administered rectally (n = 49) versus 200 mcg plus 10 IU of ergometrine plus oxytocin administered intramuscularly (n = 48). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, blood loss (mL), change in Hb level. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was achieved using block randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with scale vessels, and by subtraction of the dry weight(s) of cloths and pads from the soaked weight(s) of these items. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm placebo‐controlled randomised trial | |
| Participants | 1345 parturients were randomised in a hospital setting in Nigeria. The population comprised multiparous women, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered vaginally. Exclusion criteria comprised severe allergic conditions or asthma, age below 18 years, pyrexia above 38°C, or abortion of the pregnancy. | |
| Interventions | 400 mcg of misoprostol administered sublingually plus 10 IU of oxytocin or 250 mcg to 500 mcg of ergometrine administered intramuscularly or by an intravenous bolus (n = 658) or intravenous bolus versus 10 IU of Oxytocin or 250 mcg to 500 mcg of ergometrine administered intramuscularly or intravenously (n = 660). | |
| Outcomes | Could not include in the analysis as could not separate out the patients that received oxytocin from those who received ergometrine. | |
| Notes | Contact with study authors for additional information: Yes. Additional data from authors: Yes, but data not provided separate for each drug used and could not be included in the meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was allocated in blocks of 6–8 women by the research nurse, who used a computer‐generated randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | The trial drugs were concealed in sealed, sequentially numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was identical in shape, colour, size, and design. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded. |
| Objective assessment of blood loss | Low risk | Blood collection was initiated as soon as possible after administration of the trial medication. A low‐profile plastic fracture bedpan was placed below the woman's perineum to collect all subsequent blood loss for a period of 1 hour. Blood collected in the bedpan and all blood soaked small gauze swabs were emptied into a plastic measuring jar and the volume was measured. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses stated by authors but 27 women randomised were not included in the analysis for the primary outcome. |
| Selective reporting (reporting bias) | Unclear risk | No available protocol. |
| Intention to treat analysis | Unclear risk | 27 women randomised were not included in the analysis for the primary outcome. |
| Funding source | Low risk | The trial was funded by theMedical Research Council of South Africa. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified in 2009, 100 parturients were randomised in a hospital setting in Iran. The population comprised women of parity 3 or less, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients with twin pregnancy, fetal distress, pregnancy‐induced hypertension, oligohydramnios, polyhydramnios, macrosomia, grand multiparity (4 or more), HELLP syndrome, coagulopathy, asthma, heart/lung/liver disease, previous more than 1 caesarean section, previous myomectomy, previous other abdominal operations, febrile diseases or sensitivity to prostaglandins. | |
| Interventions | 400 mcg of misoprostol administered rectally (n = 50) versus 10 IU of oxytocin administered by an intravenous infusion (n = 50). | |
| Outcomes | The study recorded the following outcomes: transfusion, blood loss (mL), nausea, vomiting, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Investigators used a table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated intraoperative blood loss by collection with an isolated suction. The volume of blood collected in suction was combined with the volume of blood collected in gauzes and gowns: every small gauze soaked with blood was considered to contain 20 mL, and every large gauze soaked with blood 50 mL, and every g increase in the weight of a gown was considered as equivalent to 1 mL of blood. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Kashan University of Medical Sciences (the institution of the authors). |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between 1st March 2007 and 1st June 2007, 250 parturients were randomised in a hospital setting in Tunisia. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean. Exclusion criteria comprised parturients undergoing caesarean section with general anaesthesia, or those with placenta praevia, retroplacental clot, multiple pregnancy, premature labour (less than 32 weeks), intra‐uterine death, Hb less than 80 g/L, coagulopathy, HELLP syndrome, antepartum haemorrhage, ruptured uterus, previous more than 2 caesareans or other uterine scar, prolonged labour (more than 12 hours) or pyrexia. | |
| Interventions | 200 mcg plus 20 IU of misoprostol plus oxytocin administered sublingually plus by an intravenous bolus and infusion (n = 125) versus 20 IU of oxytocin administered by an intravenous bolus plus infusion (n = 125). | |
| Outcomes | The study recorded the following outcomes: PPH at 1000, transfusion, blood loss (mL), change in Hb level, nausea, vomiting, headache, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | A slip of paper was placed inside an opaque, sealed envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated perioperative blood loss as a combination of the volume of liquid in the suction collection jar, and the weight of swabs and pads. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between May 2011 and August 2011, 75 parturients were randomised in a hospital setting in the Philippines. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with pre‐existing hypertension, pre‐eclampsia, diabetes, asthma, cardiac/renal diseases, coagulopathy, abnormal laboratory tests or allergy to the study medication. | |
| Interventions | 100 mcg of carbetocin administered by an intravenous bolus (n = 39) versus 10 IU of oxytocin administered by an intravenous infusion (n = 36). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics. transfusion, blood loss (mL,. change in Hb level, nausea, vomiting, headache, tachycardia, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Investigators used sealed, consecutively‐numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The patient and the principal investigator attending the delivery were blinded to the type of medication administered" [additional information from the authors]. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "In addition to these, the following were recorded by assigned personnel not blinded in this study: any adverse events or experiences from the 2 groups (carbetocin versus oxytocin); vital signs before and after drug infusion, the need for an additional uterotonic in each group, the need for a uterine massage and the intensity of the uterine contraction after infusion of the assigned drug." |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by visual estimation, not including blood loss considered to result from repair of lacerations. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "9 women in the carbetocin group and 6 women in the oxytocin group failed to have a paired haemoglobin test to measure the change in haemoglobin 24 hours after delivery because they refused further blood extraction. These 15 women were excluded." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm controlled randomised trial. | |
| Participants | Between October 2002 and April 2003, 156 parturients were randomised in a hospital setting in China. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 400 mcg of misoprostol administered orally (n = 80) versus placebo or control (n = 76). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, blood loss (mL). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss in the 2 hours after delivery and after all amniotic fluids had been drained, by collection in a small tray and absorption into disposable, sterile, water‐resistant gauze. The contents were weighed and volume was determined on the basis that 1.05 g is equivalent to 1 mL of blood. A measuring cup was used to estimate the blood in the tray; blood that soaked into the gauze was measured on the basis that material measuring 10 cm by 10 cm holds 10 mL of blood. These 3 measurements were combined to ascertain total blood loss. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between 2002 and 2003, 200 parturients were randomised in a hospital setting in India. The population comprised women of primigravidas, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 600 mcg of misoprostol administered orally (n = 100) versus 200 mcg of ergometrine administered by an intravenous bolus (n = 100). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, additional uterotonics, manual removal of placenta, third‐stage duration (min), nausea, vomiting, headache, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised in 1:1 ratio by random number sequence. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 100 parturients were randomised in a hospital setting in Ecuador. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients with Hb less than 80 g/L, multiple pregnancy, polyhydramnios, previous uterine rupture, bleeding disorders, intrauterine death or hyperthermia (more than 38.5°C). | |
| Interventions | 400 mcg of misoprostol administered sublingually (n = 50) versus 10 IU of oxytocin administered by an intravenous infusion (n = 50). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, blood loss (mL), nausea, vomiting, headache, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated postoperative blood loss by collection with "suction apparatus and sterile drapes before irrigation" and by weighing the blood collected in abdominal swabs and gauzes with a calibrated scale (Zhongshan Camry Electronic Co Ltd, model EK 4052‐E, Guangdong, China). Investigators estimated the volume of blood loss "by subtraction of amniotic fluid at 30 cc per each centimetre reported by amniotic fluid index." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between dates unspecified, 400 parturients were randomised in a hospital setting in the USA. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with multiple pregnancy, coagulopathy, Hb less than 70 g/L, indication for caesarean section or contraindication to prostaglandin or oxytocin use. | |
| Interventions | 400 mcg of misoprostol administered rectally (n = 201) versus 20 IU of oxytocin administered by an intravenous infusion (n = 199). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, nausea, vomiting, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by an uninvolved party and determined by a random number sequence. |
| Allocation concealment (selection bias) | Low risk | The random number sequence was prepared by a third party and was concealed until the patient was enrolled. Packets were prepared in advance of randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The random number sequence was "concealed until the patient was enrolled" and "packets were prepared in advance of randomisation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss (a) by collection with drapes placed under the mother. Each drape included a plastic pouch and measured volume in mL. Meanwhile the dry weights of delivery linen and sponges were subtracted from bloodied weights to determine the volume of blood collected with these materials, on the basis that 1 g is equivalent to 1 mL. The volumes of blood in drapes and linen were added together. Furthermore "if amniotic fluid loss [after placement of the drape] was significant... the approximate percentage was recorded on the data sheet and blood loss was adjusted accordingly." Investigators evaluated blood loss (b) by estimation of the delivery attendant(s). Investigators evaluated blood loss (c) by measurement of Hb and haematocrit values were obtained on admission and on postpartum day 1. The differences between these 2 values were recorded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Of the 75 women who were excluded from analysis, 73 underwent caesarean deliveries, 1 woman was discharged to home before delivery, and 1 had an initial Hb of 6.8 mg/dL." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between April 1998 and November 1999, 18530 parturients were randomised in a hospital setting in Argentina, China, Egypt, Ireland, Nigeria, South Africa, Switzerland, Thailand, and Vietnam. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing elective or emergency caesarean section after randomisation, or those with asthma, severe chronic allergic conditions, abortion, pyrexia (more than 38°C) or inability to give consent. | |
| Interventions | 600 mcg of misoprostol administered orally (n = 9264) versus 10 IU of oxytocin administered intramuscularly or by an intravenous bolus (n = 9266). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), third‐stage duration (min, nausea, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random allocation schedule was generated centrally at WHO, Geneva, Switzerland, by computer‐generated random numbers and was stratified by country. Within the strata, women were individually randomised into 1 of 2 intervention groups with randomly varying block sizes of 4–6 women. |
| Allocation concealment (selection bias) | Low risk | The treatment packs were sealed, numbered sequentially, and could only be taken from the dispenser consecutively. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The treatment packs and their contents were identical in shape, colour, weight, and feel… Double‐blinding, including double placebos, ensured that ascertainment bias in the measurement of blood loss and use of additional uterotonics was unlikely. However, unblinding could have occurred because of the higher rate of shivering associated with misoprostol." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss from the time of delivery of the baby until the third stage of the labour was completed, when the mother was transferred to postnatal care (usually up to 1 hour postpartum). Immediately after the cord was clamped and cut, they passed a flat bedpan or an unsoiled receiver under the mother. The collected blood was poured into a standard measuring jar provided by WHO for volumetric measurement. "To simplify the procedure... small gauze swabs soaked with blood were put into the measuring jar and included in the measurement together with the blood and clots." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Investigators excluded "37 and 34 women with emergency caesarean section, and 13 and 4 women lost to follow‐up in misoprostol and oxytocin groups, respectively, for blood loss at least 1000 mL, and 2 and 4 women without information on the need for additional uterotonics." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Low risk | The study was supported by funding from the UNDP/UNFPA/WHO/World Bank Special Programme of Research, Development and Research Training. Searle (Skokie, IL, USA) and Novartis (Basel, Switzerland) donated the active and placebo medications used in the trial. |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between dates unspecified, 200 parturients were randomised in a hospital setting in India. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 600 mcg of misoprostol administered rectally (n = 100) versus 10 IU of oxytocin administered intramuscularly (n = 100). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, blood loss (mL). change in Hb level, third‐stage duration (min), nausea, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using computer‐generated random tables. |
| Allocation concealment (selection bias) | Unclear risk | A sealed envelope with a code number was opened when vaginal delivery was imminent. The code was not broken till the end of the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was "double‐blind." "Each envelope contained either 3 tablets of 200 mcg misoprostol and an ampoule of normal saline or 3 identical looking placebo tablets and an ampoule of 10 IU oxytocin." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with a BRASS‐V calibrated drape placed under the mother. Pre‐weighed gauzes were used to clean any perineal tears or episiotomy. After 1 hour the dry weight of the sponges was subtracted from the soiled weight, and added to the volume of blood collected in the drape on the basis that 1 g is equivalent to 1 mL. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between August 2000 and May 2004, 352 parturients were randomised in a hospital setting in the USA. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean. Exclusion criteria were not specified. | |
| Interventions | 200 mcg plus 20 IU of misoprostol plus oxytocin administered sublingually plus by an intravenous infusion (n = 173) versus 20 IU of oxytocin administered by an intravenous infusion (n = 179). | |
| Outcomes | The study recorded the following outcomes: PPH at 1000, additional uterotonics, transfusion, blood loss (mL), change in Hb level. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Low risk | The group assignments were available only to the pharmacy. The nurse selected an opaque vial from the drug cabinet that contained either a 200 mg misoprostol tablet or placebo. The vial number (which had been assigned in the pharmacy) and patient identification were sent to the pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were unclear. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Over 6 months between dates unspecified, 140 parturients were randomised in a hospital setting in West Indies. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with previous PPH, hypertension, previous caesarean, intrauterine death, sepsis/pyrexia (more than 38°C), antepartum haemorrhage or Hb less than 80 g/L. | |
| Interventions | 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 70) versus 400 mcg of misoprostol administered rectally (n = 70). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, hypertension, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation was used to randomly assign participants. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Both the patient and the midwife conducting the delivery were aware of the drug administered." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with a modified plastic drape placed under the mother from the commencement of the third stage of labour, until 1 hour after delivery. The collection drape measured 168 cm by 84 cm, and contained folded over side‐wings (to act as a chute) and a 34‐cm collection pouch made by folding the distal end of the drape. Standard sterile drapes were placed above the blood collection drape. Every effort was made to avoid soiling the sterile drapes before delivery of the baby, because they were not weighed. After delivery, overlying sterile drapes were removed to facilitate the use of the collection drape. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Mona Campus and Research Publication Committee of the University of the West Indies (the institution of the authors). |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between dates unspecified, 500 parturients were randomised in a hospital setting in South Africa. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing augmentation of labour, or those with hypertension, diabetes or previous caesarean. | |
| Interventions | 400 mcg of misoprostol administered orally (n = 250) versus placebo or control (n = 250). | |
| Outcomes | The study recorded the following outcomes: PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated random sequence, in balanced blocks of 8. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The tablets were either misoprostol 2 x 200 mcg or 2 placebo tablets similar in size and colour but not shape. Efforts to obtain identical placebo tablets were unsuccessful. This method of blinding proved to be effective. In only 1 case did the attending midwife inadvertently catch sight of the tablets." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Within a minute of delivery, investigators removed any linen soiled with amniotic fluid, and placed a fresh, disposable absorbent linen‐saver sheet with plastic backing, and a low wedge‐shaped plastic "fracture" bedpan under the mother. "This was found to be a comfortable and efficient way of collecting the great majority of blood lost after delivery, and could be left in place without discomfort even during perineal suturing. When active bleeding had stopped, any blood clots were expressed from the uterus, the bedpan was removed and a sanitary towel was applied. The [volume of] blood in the bedpan was measured in a measuring jug. An hour after delivery, any bloodstained linen‐savers and sanitary towels were placed in a plastic bag and weighed in g." After subtracting the known dry weights of these materials, the bloodstained weights were added to the volume of blood collected in the bedpan to ascertain the total blood loss in the first hour after delivery. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the South African Medical Research Council (public funding). |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between dates unspecified, 600 parturients were randomised in a hospital setting in South Africa. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at unspecified for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 600 mcg of misoprostol administered orally (n = 300) versus placebo or control (n = 300). | |
| Outcomes | The study recorded the following outcomes: PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, nausea, vomiting, fever, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignments were generated by computer in blocks of 18. |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially‐numbered, opaque test tubes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Misoprostol and placebo were similar in size and colour but not shape |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Within a minute of delivery, investigators removed any linen soiled with amniotic fluid, and placed a fresh, disposable absorbent linen‐saver sheet with plastic backing, and a low wedge‐shaped plastic "fracture" bedpan under the mother. "This was found to be a comfortable and efficient way of collecting the great majority of blood lost after delivery, and could be left in place without discomfort even during perineal suturing. When active bleeding had stopped, any blood clots were expressed from the uterus, the bedpan was removed and a sanitary towel was applied. The [volume of] blood in the bedpan was measured in a measuring jug. An hour after delivery, any bloodstained linen‐savers and sanitary towels were placed in a plastic bag and weighed in g." After subtracting the known dry weights of these materials, the bloodstained weights were added to the volume of blood collected in the bedpan to ascertain the total blood loss in the first hour after delivery". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "There were no withdrawals after randomisation and all outcomes were analysed in the allocated group." However the primary outcome data of 1 study participant in the placebo group were unavailable. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the South African Medical Research Council (public funding) and University of the Witwatersrand (the institution of the authors). |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between 6th March 2006 and 13th August 2007, 1103 parturients were randomised in a hospital setting in South Africa, Uganda, and Nigeria. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section or instrumental delivery, or those who declined participation or were unable to consent, were too ill or distressed to participate or with a not viable pregnancy. | |
| Interventions | 400 mcg plus 10 IU of misoprostol plus oxytocin administered sublingually plus intramuscularly (n = 547) versus 10 IU of oxytocin administered intramuscularly (n = 556). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, manual removal of placenta, death, blood loss (mL), fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers were stratified by country in blocks of 6–8. |
| Allocation concealment (selection bias) | Low risk | The trial medication was provided, and the study drug packs were prepared, by Gynuity Health Projects. When a participant enrolled, the researcher took the next study drug pack from the dispenser and immediately wrote the woman's name both on the pack and in the participant number list, which was kept separate from the case record forms. Enrolment took place when the pack was removed from the pack dispenser. The pack could not be used for another woman or returned to the dispenser. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was "double‐blind." "The packs were identical in shape, colour, weight, and feel, and contained either 2 tablets of 200 mcg of misoprostol (HRA Pharma, Paris, France) or 2 matching placebo tablets." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Similarly to the study team of Gulmezoglu 2001, investigators evaluated blood loss by collection with a fresh non‐absorbent sheet and low plastic “fracture” bedpan placed under the mother from as soon as possible after delivery until 1 hour postpartum. Investigators considered that "longer‐term blood loss measurement is more difficult to standardise." They transferred the blood collected in the sheet and the bedpan (together with any soaked small gauze swabs) to a measuring jar to ascertain the volume. Alternatively, they collected blood with a plastic sheet placed under the mother immediately after delivery. If bleeding continued beyond 1 hour, investigators restarted collection and measurement until bleeding subsided. Attempts were made to minimise any losses on the drapes and gowns of delivery attendants. In addition, "the placental interstices also contain maternal blood (about 9% of placental weight). Because overestimations (amniotic fluid) and underestimations (blood loss) were likely to be distributed equally between the 2 study groups, and most would have occurred before the onset of measurement, the data were not corrected. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Data for the primary outcome were not available for 4 of the 1103 women." |
| Selective reporting (reporting bias) | High risk | The prospectively registered protocol of the study (ClinicalTrials.gov NCT 00124540) lists some secondary outcomes different to those included the study report (at least 1000 mL within the first hour only, transfusion, Hb less than 8 g/dL 24 hours after delivery). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from Gynuity Health Projects through a grant from the Bill and Melinda Gates Foundation (public funding). |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between March 2003 and August 2004, 661 parturients were randomised in a community setting in Guinea‐Bissau. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 600 mcg of misoprostol administered sublingually (n = 330) versus placebo or control (n = 331). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, manual removal of placenta, death, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a list of random numbers. |
| Allocation concealment (selection bias) | Low risk | Investigators used opaque envelopes that were consecutively‐numbered and filled with the study drugs. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Misoprostol and placebo tablets of identical form, size, colour, and packing were produced." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | After delivery of the baby and drainage of the amniotic fluid, investigators placed a clean plastic‐lined absorbent drape under the mother. They changed the drape as many times as needed. The mother stayed on the drape or was asked to wear a pad over the next 60 minutes. All drapes and pads were weighed with an electronic scale and the known dry weights were subtracted in order to ascertain the volume of blood loss on the basis that 1 g is equivalent to 1 mL. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Danish Society of Obstetrics and Gynaecology, the Illum Foundation, and the Danish International Development Agency (public funding). |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between dates unspecified, 214 parturients were randomised in a hospital setting in Korea. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at high risk for PPH, who delivered by caesarean (unspecified whether elective or emergency). Exclusion criteria were not specified. | |
| Interventions | 20 IU of oxytocin administered by an intravenous infusion (n = 118) versus 400 mcg plus 20 IU of misoprostol plus oxytocin administered rectally plus by an intravenous infusion (n = 96). | |
| Outcomes | The study recorded the following outcomes: additional uterotonics, transfusion, change in Hb level, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blinding was not reported, but the use of placebo impeded knowledge of treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 200 parturients were randomised in a hospital setting in India. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 400 mcg of misoprostol administered rectally (n = 100) versus unspecified of ergometrine administered intramuscularly (n = 100). | |
| Outcomes | The study recorded the following outcomes: third‐stage duration (min), nausea, vomiting, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between January 2001 and December 2002, 510 parturients were randomised in a hospital setting in Nigeria. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing induction or augmentation of labour or instrumental delivery, or those requiring epidural analgesia or with hypertension in pregnancy, existing hypertension, chronic renal disease, diabetes, vascular diseases, cardiac disease, anticoagulation therapy or allergy to ergometrine or oxytocin. | |
| Interventions | 500 mcg of ergometrine administered intramuscularly (n = 254) versus 10 IU of oxytocin administered by an intravenous bolus (n = 256). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, blood loss (mL), hypertension. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated list of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Investigators used numbers that were labelled on envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm controlled randomised trial. | |
| Participants | Between November 2006 and April 2008, 1802 parturients were randomised in a hospital setting in Sweden. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing elective caesarean section, or those who were non‐Swedish speaking or with previous PPH, pre‐eclampsia, grand multiparity (more than 4) or intrauterine death. | |
| Interventions | 10 IU of oxytocin administered by an intravenous bolus (n = 903) versus of placebo or control (n = 899). | |
| Outcomes | The study recorded the following outcomes: PPH at 1000, transfusion, manual removal of placenta, blood loss (mL), change in Hb level, third‐stage duration (min). | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Investigators used sealed envelopes containing the randomisation group prepared in consecutive order and kept in another unit. At randomisation, midwives phoned the staff at the other unit who opened the envelopes and disclosed the assigned intervention and trial number. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Because of the nature of the study, blinding was not possible for the midwives, but the parturients were not informed of which management was to be used for them." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by removing pads soaked with amniotic fluid and placing a dry sanitary pad under the mother, immediately after the birth of the baby. They weighed all sanitary towels and pads before and after use. Blood loss was recorded (a) between the birth of the baby and the expulsion of the placenta, and (b) from expulsion of the placenta up to 2 hours postpartum. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 171 randomised women were not included in the study analysis. Among those randomised to receive oxytocin, 4 withdrew consent, 75 had caesareans, and 14 were lost to follow‐up. In the control group, 2 withdrew consent, 56 had caesareans, and 20 were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | The authors excluded 131 randomised study participants from the analysis because they experienced caesarean deliveries. |
| Funding source | Low risk | The study was supported by funding from the Research and Development Board in Göteborg and Bohuslän, Baby Bag and the SU Foundation in Sweden (public funding). |
| Methods | 2‐arm controlled randomised trial. | |
| Participants | Between February 2005 and March 2005, 130 parturients were randomised in a hospital setting in Tunisia. The population comprised women of parity 5 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with placenta praevia, antepartum haemorrhage, non‐cephalic presentation, intrauterine death, grand multiparity, (more than 5), fibroids, anticoagulation therapy, previous PPH or previous caesarean. | |
| Interventions | 5 IU of oxytocin administered by an intravenous bolus (n = 65) versus placebo or control (n = 65). | |
| Outcomes | The study recorded the following outcomes: PPH at 1000, transfusion, manual removal of placenta, death, change in Hb level, third‐stage duration (min). | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were unclear. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between 1st June 1998 and 31st December 1998,140 parturients were randomised in a hospital setting in Thailand. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at unspecified for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 600 mcg of misoprostol administered orally (n = 70) versus 200 mcg of ergometrine administered intramuscularly (n = 70). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, blood loss (mL). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 238 parturients were randomised in a hospital setting in Canada. The population comprised women of parity 5 or less, unspecified whether singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with coagulopathy, anticoagulation therapy, previous PPH or previous caesarean. | |
| Interventions | 400 mcg of misoprostol administered rectally (n = 100) versus 5 IU of oxytocin administered by an intravenous bolus or intramuscularly (n = 113). There were 15 exclusions post randomisation but it was unclear from which group. | |
| Outcomes | The study recorded the following outcomes: additional uterotonics, transfusion, manual removal of placenta, change in Hb level. third‐stage duration (min), nausea, vomiting, headache, fever, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A statistician developed blocked randomisation tables for each centre. |
| Allocation concealment (selection bias) | Low risk | Pharmacy assembled consecutively‐numbered opaque, sealed packets that contained the group allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "13 women randomised subsequently delivered by caesarean and were excluded from analysis. 2 women were lost to follow‐up early in the trial when their packets were opened but the manoeuvre was not completed and no data were recorded." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Low risk | The study was supported by funding from the physicians of Ontario, through the Physician Services Incorporated Foundation (public funding). |
| Methods | 3‐arm controlled randomised trial. | |
| Participants | Between dates unspecified, 140 parturients were randomised in a hospital setting in Hungary. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at unspecified for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 200 mcg of ergometrine administered by an intravenous bolus (n = 50) versus placebo or control (n = 43) versus 1 mg dinoprost administered intramuscularly (n = 47). The dinoprost arm was not included in the analysis. | |
| Outcomes | The study recorded the following outcome: third‐stage duration (min). | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Investigators evaluated blood loss by collection in a container placed under the mother during the third stage of labour until 2 hours postpartum. The contents of the container were transferred to a measuring cylinder. However, blood loss data were not reported in a format that could be extracted for the purpose of this review. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between 1st January 1991 and 30th June 1991, 2040 parturients were randomised in a hospital setting in United Arab Emirates. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing induction or augmentation of labour, caesarean section or instrumental delivery, or requiring general anaesthesia, epidural or diazepam, or those with antenatal hypertension (160/100 mmHg or more), hypertension on antihypertensive drugs, multiple pregnancy, cardiac disease or Hb of 90 g/L or less. | |
| Interventions | 10 IU of oxytocin administered intramuscularly (n = 1017) versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 1023). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, transfusion, manual removal of placenta, vomiting, headache. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Treatment was allocated as per a number code generated by the hospital pharmacist who alone was aware of the content of the ampoules. |
| Allocation concealment (selection bias) | Low risk | Participants were assigned an opaque sealed envelope. Each envelope carried the instruction to use a numbered vial of the study drug. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss "in the standard way" by measurement of blood and clots in a graduated jug, and by weighing swabs and linen. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "12 patients had to be excluded from the trial (oxytocin 5; syntometrine [ergometrine plus oxytocin] 7) after randomisation because they no longer fulfilled the inclusion criteria... [including] 2 who required caesarean section (1 in each group) and 10 who were delivered by forceps or ventouse (oxytocin 4; syntometrine [ergometrine plus oxytocin] 6)." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 4‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 136 parturients were randomised in a hospital setting in Japan. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who were scheduled for caesarean with ASA I or II. Exclusion criteria comprised parturients that were affected by cardiovascular conditions, were scheduled for autologous blood transfusion, who had tocolytics administered or premature rupture of membranes. | |
| Interventions | 10 IU up to 20 IU of oxytocin administered by an intravenous bolus (n = 102) versus 200 mcg ergometrine administered by an intravenous bolus (n = 34). | |
| Outcomes | The study recorded the following outcome: blood loss (mL). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no and data cannot be extracted for meta‐analysis. Manuscript translated from Japanese in full. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Methods of blinding were not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | Not reported. |
| Funding source | Unclear risk | Not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between August 2003 and March 2004, 55 parturients were randomised in a hospital setting in Turkey. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean. Exclusion criteria comprised parturients with multiple pregnancy, hypertension or vascular diseases. | |
| Interventions | 10 IU of oxytocin administered by an intravenous bolus plus infusion (n = 35) versus 200 mcg plus 10 IU of ergometrine plus oxytocin administered by an intravenous bolus plus by intravenous bolus plus infusion (n = 20). | |
| Outcomes | The study recorded the following outcome: blood loss (mL). | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated intraoperative blood loss by weighing compresses and rolls before and after the birth of the baby, and calculating the difference between these measurements. Pre‐weighted pads were distributed in advance to each mother, and collected at intervals of 3‐6 hours hour intervals after the aspiration of amniotic fluid. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between October 1999 and February 2000, 500 parturients were randomised in a hospital setting in Zimbabwe. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing instrumental delivery, or those with previous PPH, antepartum haemorrhage, coagulopathy, multiple pregnancy, asthma or allergies to prostaglandins or oxytocin. | |
| Interventions | 400 mcg of misoprostol administered orally (n = 243) versus 10 IU of oxytocin administered intramuscularly (n = 256). There was 1 exclusion post randomisation but it was unclear as to which group it was randomised to. | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity,additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), third‐stage duration (min), nausea, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | The participant was asked to randomly pick a numbered sealed opaque envelope from the study cooler‐box. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Identical placebo tablets could not be obtained from the manufacturers. The tablets were similar in size and colour but not in shape. However, most reviewed trials on misoprostol had this similar problem although this method of blinding proved to be effective." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "The data sheet was completed by the midwife supervising the delivery and collected and checked by the research assistant." |
| Objective assessment of blood loss | Low risk | After delivery, investigators evaluated blood loss by removing linen soiled with amniotic fluid, and then placing a fresh disposable incontinence pad with a plastic backing under the mother. Blood expressed from the uterus was measured with a calibrated measuring jug. The volume of blood soiling linen savers and sanitary pads was determined as the difference between dry weights and soiled weights: these measurements were added to the volume recorded by the calibrated jug. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Data for 1 woman were excluded because she delivered undiagnosed twins after randomisation." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 60 parturients were randomised in a hospital setting in China (Hong Kong SAR). The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing induction or augmentation of labour, or those with antepartum haemorrhage, anaemia, 2 or more surgical terminations, previous manual removal of placenta, previous PPH or previous third stage complications. | |
| Interventions | 500 mcg plus 5 IU of ergometrine plus oxytocin administered by an intravenous bolus (n = 30) versus 600 mcg of misoprostol administered sublingually (n = 30). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, manual removal of placenta, death, fever. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was allocated using a random number‐generated table. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss during the third stage by visual estimation, and by objective measurement on the basis of a method previously described by Newton et al. Whilst any blood clots were collected and measured with a jug, white linen was placed under the mother during delivery and subsequently processed for 15 minutes with sodium hydroxide solution in an automatic stomacher (laboratory blender), to achieve the formation of alkaline hematin. "The optical density at 550 nm of the alkaline hematin was measured by spectrophotometry and compared with that of a known volume of a sample of the patient’s venous blood" to calculate the volume of blood loss. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | It was unclear from the study report whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between January 1999 and February 2002, 56 parturients were randomised in a hospital setting in Switzerland. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients undergoing emergency caesarean section, or those with fetal distress, fetal malformations, pre‐eclampsia, HELLP syndrome, coagulopathy, severe systemic disorders, an American Society of Anaesthesiologists physical status of 3 or greater, severe asthma, previous myomectomy, pyrexia (more than 38.5°C) or hypersensitivity to prostaglandins. | |
| Interventions | 25 IU of oxytocin administered by an intravenous bolus plus infusion (n = 28) versus 800 mcg plus 5 IU of misoprostol plus oxytocin administered orally plus by an intravenous bolus (n = 28). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, death, blood loss (mL), nausea, headache, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The hospital pharmacy performed the 1:1 computer‐generated randomisation that assigned the participants to their group. |
| Allocation concealment (selection bias) | Low risk | Investigators used identical study boxes from pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was "double‐blind": "the study drugs and placebos [were provided by the pharmacy] in unidentifiable form." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | When the membranes ruptured before delivery, investigators evaluated intraoperative and postoperative blood loss by determining the difference in weight of cloths and pads used to absorb blood during surgery and in the intermediate care unit. When membranes did not rupture preoperatively, investigators evaluated blood loss by collection in suction bottles and subtracting estimated amniotic fluid volume. Investigators considered that 1 g is equivalent to 1 mL of blood or amniotic fluid. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "3 patients in the oxytocin group were excluded from statistical analysis because of errors in drug administration." Moreover calculated blood loss data were unavailable in 13 cases and for these women the primary outcome was estimated clinically. |
| Selective reporting (reporting bias) | High risk | The study protocol that was registered retrospectively (ClinicalTrials.gov) lists PPH as the primary outcome of the study, but the study report lists the primary outcomes as intraoperative and postoperative blood loss and drug‐related adverse effects (these items are listed only as secondary outcomes in the registration file). The study does not report the incidence of PPH at 500 mL, nor PPH at 1000 mL. |
| Intention to treat analysis | High risk | The authors excluded 3 study participants in the oxytocin group from the analysis because they incurred errors in drug administration. |
| Funding source | Low risk | The study was supported by funding from the Scientific Pool of Basel University Hospital (the institution of the authors). |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between July 2004 and March 2005, 329 parturients were randomised in a hospital setting in Hong Kong. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients requiring prophylactic oxytocin infusion, or those with pre‐existing hypertension, pre‐eclampsia, asthma, cardiac/renal/liver diseases, grand multiparity or fibroids. | |
| Interventions | 100 mcg of carbetocin administered intramuscularly (n = 165) versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 164). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, hypertension, headache, tachycardia, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated code before recruitment. |
| Allocation concealment (selection bias) | Low risk | This was performed by opening a sealed, consecutively‐numbered, opaque envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by visual estimation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "15 women in the carbetocin group and 14 women in the syntometrine [ergometrine plus oxytocin] group failed to have a paired haemoglobin test to measure the change in haemoglobin 48 hours after delivery either because they had requested early home discharge or refused." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of fever were omitted). |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | High risk | The study was supported by funding from Ferring Pharmaceuticals. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 40 parturients were randomised in a hospital setting in the UK. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean. Exclusion criteria comprised parturients with 2 or more previous caesarean sections or previous uterine rupture. | |
| Interventions | 10 IU of oxytocin administered by an intravenous bolus (n = 20) versus 500 mcg of misoprostol administered orally (n = 20). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, blood loss (mL), change in Hb level, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was undertaken by means of computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Investigators used sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The obstetrician, surgical assistant, scrub nurse and recovery midwife were blinded to the treatment. The anaesthetist and the anaesthetic assistant were not blinded as it was important for patient safety that a record was kept of all drugs administered." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated intraoperative and postoperative (up to 1 hour) blood loss by visual estimation "in a standard manner (volume of blood in suction bottle plus soiling of swabs and bed sheets)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by "assistance" from the Department of Anaesthesia at University College London Hospitals NHS Trust (the institution of the authors). |
| Methods | 3‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between dates unspecified, 597 parturients were randomised in a hospital setting in South Africa and Thailand. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing elective caesarean section or abortion, or those with asthma, other severe chronic allergic conditions a contraindication to use of misoprostol or if they were not willing or able to give informed consent. | |
| Interventions | 600 mcg or 400 mcg of misoprostol administered orally (n = 397) versus 10 IU of oxytocin administered intramuscularly (n = 200). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), nausea,. vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random allocation sequence was generated centrally. |
| Allocation concealment (selection bias) | Low risk | The treatment packs were consecutively‐numbered and sealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The packs were identical in shape, colour, weight and feel. Each woman received an injection and 3 tablets. Thus, the trial was double‐blinded using double placebos." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss from the delivery of the baby until the mother was transferred to postnatal care. The collected blood was poured into a standard measuring jar provided by WHO for the purpose of volumetric measurement. Linen was not weighed but clots and small gauze swabs soaked with blood were included in the measurement. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion after randomisation: “8 women did not comply with treatment in the oxytocin group (6 because of emergency caesarean section, 1 was HIV positive (mistakenly excluded despite HIV sero‐positivity not being an exclusion criterion), and in another case the ampoule could not be located in the treatment pack. There was 1 woman in the misoprostol 600 mcg group who did not comply with the treatment, because the tablets could not be located after the treatment pack was opened. Finally, in the misoprostol 400 mcg group, 1 woman had an emergency caesarean section after the treatment pack was opened and could not receive the allocated treatment." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the WHO (public funding). Active and placebo medications, syringes and swabs were donated by Searle, Novartis Pharma AG and Becton Dickinson International. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between May 2013 and December 2014, 200 parturients were randomised in a hospital setting in Egypt. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with placenta praevia, coagulopathy, pre‐eclampsia, cardiac/renal/liver disorders, epilepsy or known hypersensitivity to oxytocin or carbetocin. | |
| Interventions | 100 mcg of carbetocin administered intramuscularly (n = 100) versus 5 IU of oxytocin administered intramuscularly (n = 100). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, headache, tachycardia, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were equally randomised using an automated web‐based randomisation system. |
| Allocation concealment (selection bias) | Unclear risk | The study report states that investigators ensured allocation concealment, but gives no further details. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by weighing swabs and using pictorial charts. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between 19th February 1990 and 20th October 1991, 3497 parturients were randomised in a hospital setting in Australia. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing emergency or elective caesarean section, or requiring general anaesthetic for instrumental delivery, or those with hypertension in labour (more than 150/100 mmHg), antenatal hypertension, maternal distress, advanced stage in labour, language barrier, fetal abnormality, intrauterine death or medical disorder. | |
| Interventions | 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 1730) versus 10 IU of oxytocin administered intramuscularly (n = 1753). There were 14 exclusions post randomisation but it was unclear from which group. | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, NNU admissions, breastfeeding, nausea, vomiting. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The ampoules were numbered by Sandoz using simple randomisation. There was no blocking or prognostic stratification. |
| Allocation concealment (selection bias) | Low risk | The ampoules were numbered by third party (Sandoz). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Delivery attendants were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending obstetricians and midwives. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All women allocated to receive a drug were included in that group, excluding only the 14 women for whom drug allocation was not recorded." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | High risk | The study was supported by funding from Sandoz. |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between dates unspecified in 1984, 461 parturients were randomised in a hospital setting in UK. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing elective caesarean section, or those with significant hypertension or cardiac disease. | |
| Interventions | 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 230) versus 5 IU of oxytocin administered intramuscularly (n = 231). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, manual removal of placenta, blood loss (mL), third‐stage duration (min). | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear sequence: described as without any blocking or stratification. |
| Allocation concealment (selection bias) | Low risk | Investigators used identical study boxes prepared by third party (Sandoz). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss "in the standard way by graduated jug measurement plus an allowance for spillage." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Perinatal Trials Service (public funding), for the Department of Health for England and Wales, and for Birthright (the charitable arm of the RCOG). Coded medication ampoules were provided by Sandoz. |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between June 2006 and June 2008, 1119 parturients were randomised in a community setting in Pakistan. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with hypertension, non‐cephalic presentation, polyhydramnios, previous caesarean, multiple pregnancy, intrauterine death, antepartum haemorrhage or Hb less than 80 g/L. | |
| Interventions | 600 mcg of misoprostol administered orally (n = 534) versus placebo or control (n = 585). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, manual removal of placenta, death, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, headache, fever. shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated random code in blocks of 6 was maintained by Gynuity Health Projects in New York and not revealed until data collection and cleaning were completed. |
| Allocation concealment (selection bias) | Low risk | Study medication was packed in numbered colour‐coded boxes by Gynuity Health Projects in New York. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Both women and TBAs were blinded to study assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | To evaluate postpartum blood loss, blood was collected with a perineal sheet and bedpan placed under the mother for a minimum of 1 hour or until active bleeding stopped (whichever occurred last). "Blood collected in the bedpan was transferred to a measuring jar, which was then closed, and the perineal sheet and cotton roll were placed in a sealed plastic bag. The closed measuring jar and sealed plastic bag were then placed inside a plastic cooler which was tightly closed and stored in a secure place in the woman’s home until the local health visitor or community health nurse arrived for weighing, 1–2 days after delivery." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Invalid blood loss measures, which mainly occurred when monitoring visits were not possible because of poor weather conditions, were excluded from our analysis." |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (ClinicalTrials.gov NCT00120237). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Bill and Melinda Gates Foundation (public funding). |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between January 2008 and July 2008, 84 parturients were randomised in a hospital setting in Austria. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients requiring general anaesthesia, or those with placenta praevia, placental abruption, multiple pregnancy, pre‐eclampsia, gestational diabetes, pre‐existing insulin‐dependent diabetes, cardiovascular/renal disorders, hypo‐/hyperthyroidism or women on cardiovascular system medications. | |
| Interventions | 100 mcg of carbetocin administered by an intravenous bolus (n = 28) versus 5 IU of oxytocin administered by an intravenous bolus (n = 28). There were 28 exclusions post randomisation but it was unclear from which group. | |
| Outcomes | The study recorded the following outcomes: additional uterotonics. change in Hb level, nausea, headache. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by a computer‐generated randomisation sequence in a 1:1 ratio with blocks of 10 and no stratification. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Study medication was double‐blinded to the clinical staff (obstetricians as well as anaesthesiologists) and the technicians performing the measurements." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Investigators did not evaluate blood loss. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | After randomisation, investigators excluded 28 women from analysis for technical problems (n = 15), change to general anaesthesia (n = 9), recording artefacts (n = 3) and patient withdrawal (n = 1). |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (EudraCT 2007‐005498‐78). |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Low risk | CNSystems Medizintechnik AG in Graz, Austria provided the Task Force® Monitor 3040i system used to measure haemodynamic parameters. No other external funding was required for the study. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 88 parturients were randomised in a hospital setting in the UK. The population comprised women of primigravidas, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 500 mcg of ergometrine administered by an intravenous bolus (n = 44) versus 10 IU of oxytocin administered by an intravenous bolus (n = 44). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, blood loss (mL), nausea. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by "the haemoglobin extraction‐dilution technique, which is acceptably accurate (Roe, Gardiner and Dudley, 1962; Thornton et al, 1963) and particularly suited to obstetric use (Moir and Wallace, 1967; Wallace, 1967). The pedometer apparatus was used and all blood and blood‐stained linen were collected." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 148 parturients were randomised in a hospital setting in the UK. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 500 mcg of ergometrine administered by an intravenous bolus (n = 78) versus 5 IU of oxytocin administered by an intravenous bolus (n = 70). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, blood loss (mL), nausea. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with the placenta bowl and soiled linen and swabs. "The principles of the haemoglobin extraction‐dilution technique employed have been discussed by Roe, Gardiner and Dudley (1962) and Thornton and colleagues (1963)." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were 148 study participants but blood loss data were available in only 80 cases. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between May 2010 and October 2011, 200 parturients were randomised in a hospital setting in India. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing emergency or elective caesarean section, or those with eclampsia, asthma, epilepsy, cardiac/kidney disorder or coagulopathy. | |
| Interventions | 600 mcg of misoprostol administered orally (n = 100) versus 10 IU of oxytocin administered intramuscularly (n = 100). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, additional uterotonics, nausea, vomiting, fever, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly divided into 2 equal groups. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Investigators evaluated blood loss in mL, by collection with a calibrated plastic drape, after the drainage of amniotic fluid and delivery of the baby until the third stage of labour was completed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between 1st January 2013 and 30th June 2013, 235 parturients were randomised in a hospital setting in Nigeria. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing planned instrumental, or those who received oxytocin and/or misoprostol other than in the third stage of labour, or those with grand multiparity (more than 4), multiple pregnancy, fibroids, polyhydramnios, pre‐eclampsia, eclampsia, hypertension, cardiac disorder, asthma, antepartum haemorrhage previous PPH, prolonged rupture of membranes or Hb less than 100 g/L). | |
| Interventions | 600 mcg of misoprostol administered orally (n = 121) versus 10 IU of oxytocin administered intramuscularly (n = 114). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, morbidity, additional uterotonics, manual removal of placenta, death, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation was blocked (restrictive), using computer‐generated random numbers prepared by an independent statistician. |
| Allocation concealment (selection bias) | Unclear risk | Investigators used opaque envelopes but no other details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Participants, caregivers, and outcome assessors (researchers or research assistants) were masked to group allocation. Investigators were not masked for data analysis." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Participants, caregivers, and outcome assessors (researchers or research assistants) were masked to group allocation. Investigators were not masked for data analysis." |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by "the gravimetric method" (Ambardekar 2009) until 1 hour after delivery. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 235 study participants were randomised but only 200 were analysed due to protocol deviations and missing data. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was registered retrospectively (PACTR 201407000825227). |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Low risk | The study was supported by funding from the University of Ilorin Teaching Hospital (the institution of the authors). |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between dates unspecified, 514 parturients were randomised in a hospital setting in Egypt. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with antepartum haemorrhage, coagulopathy, hypertension in pregnancy or the need for anticoagulants. | |
| Interventions | 800 mcg of misoprostol administered rectally (n = 257) versus 5 IU of oxytocin administered by an intravenous infusion (n = 257). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, third‐stage duration (min), nausea, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was allocated by a computer‐generated random allocation system created at the Statistics Unit of Assiut University Hospital. |
| Allocation concealment (selection bias) | Low risk | Allocation codes were placed in sealed, opaque, consecutively‐numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was "double‐blind": active treatments and placebo treatments were "identical‐looking." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were unclear. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between June 1998 and February 1999, 2058 parturients were randomised in a hospital setting in Hong Kong. The population comprised women of parity 3 or less, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients requiring oxytocin infusion in the third stage, or those with pre‐eclampsia, cardiac disorder, asthma, grand multiparity (more than 3), fibroids or contraindications for the use of either misoprostol or syntometrine. | |
| Interventions | 600 mcg of misoprostol administered orally (n = 1026) versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 1032). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb level, nausea, vomiting, hypertension, headache, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was based on a table of computer‐generated blocks of random numbers. |
| Allocation concealment (selection bias) | Low risk | Allocation was placed in consecutively‐numbered opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "This was not a double‐blinded study." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between April 2000 and January 2001, 360 parturients were randomised in a hospital setting in Hong Kong. The population comprised women of parity 3 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients requiring oxytocin infusion in the third stage, or those with pre‐eclampsia, cardiac disorder, asthma, grand multiparity (more than 3), fibroids or contraindications for the use of either misoprostol or syntometrine. | |
| Interventions | 400 mcg of misoprostol administered orally (n = 178) versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 177). There were 5 exclusions post randomisation but it was unclear from which group. | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb level, nausea, vomiting, hypertension, headache, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was based on a table of computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Investigators used consecutively‐numbered and sealed opaque packages. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The placebo was identical in size and colour but had a different shape to the misoprostol tablet. All women were asked to swallow the tablets directly from the opaque cup without looking at them. The identity of the active medication and placebo were concealed from the caregivers and the parturient." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "5 women were excluded from the analysis because of missing post‐delivery haemoglobin level." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of tachycardia and dizziness were omitted). |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 120 parturients were randomised in a hospital setting in Malaysia. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients younger than 18 years old, or those with cardiac disorder, hypertension requiring treatment, liver/renal/vascular/endocrine disorder (excluding gestational diabetes) or hypersensitivity to oxytocin or carbetocin. | |
| Interventions | 100 mcg of carbetocin administered intramuscularly (n = 60) versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 60). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb level, nausea, vomiting, hypertension, headache, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Investigators used sealed, sequentially‐numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The preparation and administration of the medication was carried out by midwives who were not involved in the management of the patient except for the drug administration." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by "the gravimetric method" from immediately after drug administration. They used a digital scale (Soehnle, Venezia) for weight measurement. In order to minimise confounding by fluid absorbed into drapes, they collected blood with a new plastic sheet placed under the mother after delivery of the baby. They also weighed any gauzes, tampons and pads used in the first hour after delivery of the placenta, and subtracted the dry weights of these materials to calculate blood loss on the basis that 1 g is equivalent to 1 mL. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between 16th December 1993 and 6th October 1994, 1000 parturients were randomised in a hospital setting in Sweden. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 10 IU of oxytocin administered by an intravenous bolus (n = 513) versus placebo or control (n = 487). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, blood loss (mL), third‐stage duration (min). | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Ampoules were prepared at the hospital pharmacy and consecutively‐numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The content of the ampullas was unknown to mothers, midwives and doctors until the study was completed." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss "by measuring collected blood and adding what was estimated to have been absorbed by surgical cloths and tissues." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the County Council and County Health Authority Research and Development Foundation in the County of Jämtland, Sweden (public funding). |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between August 2000 and July 2001, 496 parturients were randomised in a hospital setting in Nigeria. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing induction or augmentation of labour, or those with previous caesarean, Hb less than 80 g/L, previous PPH, grand multiparity (not defined), multiple pregnancy, polyhydramnios, fibroids or precipitate labour. | |
| Interventions | 10 IU of oxytocin administered intramuscularly (n = 249) versus 600 mcg of misoprostol administered orally (n = 247). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using random tables. |
| Allocation concealment (selection bias) | Low risk | Pharmacy prepared opaque sealed sequentially‐numbered packets. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The identity of the active medication and placebo were concealed from the caregivers and parturients." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending obstetricians. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 3‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 144 parturients were randomised in a hospital setting in Nigeria. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing instrumental delivery, or those with previous PPH, multiple pregnancy, polyhydramnios or vaginal lacerations. | |
| Interventions | 200 mcg or 500 mcg of ergometrine administered intramuscularly (n = 96) versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 48). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, manual removal of placenta, blood loss (mL). | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Investigators performed restricted random allocation. |
| Allocation concealment (selection bias) | Unclear risk | Investigators used sealed sequentially‐numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The identity of the various drugs was not known to the investigators until after completion of the trial." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by collection in a dish pressed against the vulva for 3 minutes: the contents were carefully measured. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | High risk | The study was supported by funding from Sandoz. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between January 2006 and September 2007, 600 parturients were randomised in a hospital setting in Nigeria. The population comprised women of parity 6 or less, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with hypertension in pregnancy, packed cell volume less than 30%, previous PPH, haemoglobinopathy or cardiac disorder. | |
| Interventions | 10 IU of oxytocin administered by an intravenous bolus (n = 297) versus 250 mcg of ergometrine administered by an intravenous bolus (n = 303). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, manual removal of placenta, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, hypertension, headache. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation was done by sealed sequentially‐numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Investigators evaluated blood loss by "using a pre‐weighed gauze that was weighed again after delivery." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of transfusion and PPH at least 1000 mL were omitted). |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 3‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 156 parturients were randomised in a hospital setting in Spain. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients with comorbidities, refractory hypotension due to neuraxial blockage, vasoactive drugs needed to control haemodynamic issues or multiple pregnancy. | |
| Interventions | 100 mcg of carbetocin administered by an intravenous bolus (n = 52) versus 61 IU of oxytocin administered by an intravenous bolus plus infusion (n = 104). | |
| Outcomes | The study recorded the following outcomes: additional uterotonics, nausea, vomiting, headache, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using a computer‐generated sequence. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Investigators evaluated blood loss by the estimation of delivery attendants, but blood loss data were not reported in a format way that could be extracted for the purpose of this review. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between June 2006 and April 2007, 100 parturients were randomised in a hospital setting in Nigeria. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean. Exclusion criteria comprised parturients requiring general anaesthesia, or those with multiple pregnancy, placenta praevia, antepartum haemorrhage, cardiac/renal/liver disorders, coagulopathy, asthma, glaucoma, pre‐eclampsia, eclampsia, prolonged labour or contraindications to administration of prostaglandins. | |
| Interventions | 20 IU of oxytocin administered by an intravenous infusion (n = 50) versus 400 mcg of misoprostol administered sublingually (n = 50). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, blood loss (mL), change in Hb level, nausea, vomiting, headache, hypotension, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The treatment allocation sequence was developed by a statistician who was not otherwise involved with the study using computer‐generated table of random numbers and varied permutated blocks. |
| Allocation concealment (selection bias) | Low risk | Investigators used sealed, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The anaesthetist was blind to the allocation until he opened each participant’s envelope at surgery… The obstetricians were unaware of what oxytocic was given as the faces of the patients were screened off during the surgery." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "The obstetricians were unaware of what oxytocic was given as the faces of the patients were screened off during the surgery." |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection in a suction bottle, and by weighing delivery drapes and gauzes on the basis that 1 g is equivalent to 1 mL of blood. "Both the surgeon and anaesthetist estimated blood loss independently... The scrub nurse weighed the drapes and gauze before and after the operation, noted the amount of blood in the suction bottle, and recorded these... The postoperative care nurse also recorded the blood loss during the first 4 hours after surgery." Finally a research assistant (not part of the medical team) calculated the mean estimated blood loss from all these values. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between April 2002 and October 2002, 450 parturients were randomised in a hospital setting in Ghana. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with asthma, epilepsy or contraindications to prostaglandins. | |
| Interventions | 10 IU of oxytocin administered intramuscularly (n = 225) versus 800 mcg of misoprostol administered orally (n = 225). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, hypertension, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The treatment allocation was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially‐numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "We acknowledge that unblinding for some participants was possible because the envelopes for women who were initially randomised but who subsequently underwent caesarean section were returned and used for the next women enrolled." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending physicians and midwives. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from Matercare International and the Society of Obstetricians and Gynaecologists of Canada (public funding). |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between April 2002 and December 2002, 450 parturients were randomised in a hospital setting in Ghana. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with asthma, epilepsy or contraindications to prostaglandins. | |
| Interventions | 10 IU of oxytocin administered intramuscularly (n = 226) versus 800 mcg of misoprostol administered rectally (n = 224). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, hypertension, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially‐numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Unblinding for some participants was possible because the envelopes for women who were initially randomised but who subsequently underwent caesarean section were returned and used for the next women enrolled." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending physicians and midwives. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Estimated blood loss data were unavailable in 9 cases (misoprostol 7; oxytocin 2) and Hb measurements (misoprostol 4; oxytocin 6) were unavailable in 10 cases. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from Matercare International and the Society of Obstetricians and Gynaecologists of Canada (public funding). |
| Methods | 3‐arm active‐controlled randomised trial. | |
| Participants | Between 29th October 2000 and 6th November 2000, 78 parturients were randomised in a hospital setting in Colombia. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with asthma, multiple pregnancy, intrauterine death, coagulopathy, cervical tear or water in the blood collector. | |
| Interventions | 50 mcg of misoprostol administered sublingually (n = 25) versus 16 mLU/min of oxytocin administered by an intravenous infusion (n = 25) versus 200 mcg of ergometrine administered intramuscularly (n = 25). There were 3 exclusions post randomisation but it was unclear from which group. | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, blood loss (mL), third‐stage duration (min), vomiting, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Investigators evaluated blood loss from cord clamping until 1 hour after delivery. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 women were excluded from the analysis after entering the study: "se excluyeron 3 pacientes por obito fetal, desgarro de cervix severo, vertimiento de agua en el recipiente recolector de sangre." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm controlled randomised trial. | |
| Participants | Between 1st January 1986 and 31st January 1987, 1695 parturients were randomised in a hospital setting in the UK. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with cardiac disorder, antepartum haemorrhage, non‐cephalic presentation, multiple pregnancy, intrauterine death but after change in the protocol multiple other exclusion criteria were introduced. | |
| Interventions | 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 846) versus of placebo or control (n = 849). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, change in Hb level, NNU admissions, breastfeeding, vomiting, headache. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially‐numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the South Western Regional Health Authority of the United Kingdom (public funding). |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between dates unspecified, 400 parturients were randomised in a hospital setting in Iran. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with placenta praevia, placental abruption, coagulopathy, previous caesarean, macrosomia (more than 4 kg), polyhydramnios or uncontrolled asthma. | |
| Interventions | 20 IU of oxytocin administered by an intravenous infusion (n = 200) versus 400 mcg of misoprostol administered orally (n = 200). | |
| Outcomes | The study recorded the following outcomes: additional uterotonics, transfusion, blood loss (mL), change in Hb level, hypotension, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was allocated using simple randomisation with computer‐generated numbers in a 1:1 ratio. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was "double‐blind": "for blinding the study, identical‐appearing solutions and tablets corresponding to the 2 pharmacological groups were prepared by the pharmacy and kept in the fridge until required." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Investigators evaluated blood loss during the first hour after delivery, by collection with pads weighed before and after absorbance of blood. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | The study protocol was registered (ClinicalTrials.gov NCT01863706) but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of diarrhoea, nausea and vomiting were not completely reported). Moreover, the study publication reports outcomes (hypotension, nausea, transfusion) not listed in the registered protocol. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Hormozgan University of Medical Sciences (the institution of the authors). |
| Methods | 3‐arm controlled randomised trial. | |
| Participants | Between dates unspecified, an unspecified number of parturients were randomised in an unspecified setting and country. The population comprised primiparous women, with singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients that were multiparous, severely anaemic, and hypertensive conditions during pregnancy. | |
| Interventions | 5 IU of oxytocin administered by an intravenous bolus versus 200 mcg ergometrine administered by an intravenous bolus vs placebo or control. | |
| Outcomes | The study recorded the following outcome: Change in Hb level. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. Abstract only available and data provided could not be used for meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Methods of blinding were not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | Not reported. |
| Funding source | Unclear risk | Not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between January 2003 and December 2003, 686 parturients were randomised in a hospital setting in Saudi Arabia. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section or requiring oxytocin infusion in the third stage, or those with pre‐eclampsia, cardiac disorder, hypertension on treatment, antepartum haemorrhage, pre‐term labour (less than 37 weeks), post maturity (more than 42 weeks) or Hb less or equal to 90 g/L. | |
| Interventions | 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 340) versus 10 IU of oxytocin administered by an intravenous infusion (n = 346). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, blood loss (mL), third‐stage duration (min), nausea, vomiting headache. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using computer‐generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Investigators used sequentially‐numbered, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss "clinically in a standard way" by collection with a plastic sheet that was subsequently drained (with clots) into a graduated measuring jug, and by weighing swabs and towels. "Any delayed haemorrhage within 24 hours after delivery was calculated." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of requirement for additional syntometrine [ergometrine plus oxytocin] were omitted). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 200 parturients were randomised in a hospital setting in India. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing elective caesarean section, or those with pre‐term labour (less than 32 weeks), prolonged labour, antepartum haemorrhage, pre‐eclampsia, intrauterine death, multiple pregnancy, epilepsy, asthma, cardiac/kidney disorder, coagulopathy or anaemia. | |
| Interventions | 400 mcg of misoprostol administered orally (n = 100) versus an unspecified dose of ergometrine administered by an unspecified injectable method (n = 100). | |
| Outcomes | The study recorded the following outcomes: additional uterotonics, transfusion, manual removal of placenta, hypertension. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Investigators evaluated blood loss in the first 2 hours after delivery of the placenta, by "clinical estimation." However, blood loss data were not reported in a format that could be extracted for the purpose of this review. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between August 2008 and August 2009, 144 parturients were randomised in a hospital setting in Panama. The population comprised women of parity 5 or more, a singleton pregnancy, at high risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing emergency caesarean section, or those with coagulopathy, unknown parity or known allergy to carbetocin. | |
| Interventions | 100 mcg of carbetocin administered by an intravenous bolus (n = 45) versus 20 IU of oxytocin administered by an intravenous infusion (n = 90). There were 9 exclusions post randomisation but it was unclear from which group. | |
| Outcomes | The study recorded the following outcomes: additional uterotonics, transfusion, manual removal of placenta, breastfeeding, nausea, vomiting. Headache. Shivering. Abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Se pudieron reclutar 144 pacientes, en quienes se dio la aleatorización en dos grupos (carbetocina:oxitocina) en una proporción 1:2. Cualquier causal de falla en el seguimiento del protocolo establecido obligaba a la exclusión de la paciente del estudio. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of PPH were omitted). |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Unclear risk | Ferring Pharmaceuticals donated carbetocin. No other external funding was required for the study. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between July 2010 and September 2010, 57 parturients were randomised in a hospital setting in Panama. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by both caesarean and vaginal delivery. Exclusion criteria comprised parturients with HELLP syndrome, blood dyscrasia or multiple pregnancy. | |
| Interventions | 100 mcg of carbetocin administered by an intravenous bolus (n = 26) versus 10 IU of oxytocin administered by an intravenous infusion (n = 29). There were 2 exclusions post randomisation but it was unclear from which group. | |
| Outcomes | The study recorded the following outcomes: additional uterotonics, transfusion, change in Hb level, third‐stage duration (min), breastfeeding. Vomiting. Headache. Fever. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The randomisation was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Investigators used opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study was "double‐blind": "because the 2 drugs are administered differently, a double dummy system for administration was used." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 women were excluded from the study analysis after randomisation ("1 given drug before expulsion of placenta; 1 ampoule of the drug broken before use"). |
| Selective reporting (reporting bias) | High risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Those who withdrew from the study after randomisation were not included in the analysis. |
| Funding source | Low risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm controlled randomised trial. | |
| Participants | Between June 1993 and December 1995, 1512 parturients were randomised in a hospital setting in the UK. The population comprised women of parity 5 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing augmentation of labour or instrumental delivery or requiring epidural analgesia, or those with placenta praevia, previous PPH, antepartum haemorrhage, Hb less than 100 g/L or mean corpuscular volume less than 75 fL, non‐cephalic presentation, multiple pregnancy, intrauterine death, grand multiparity (more than 5), fibroids, anticoagulation therapy, pre‐term labour (less than 32 weeks) or contraindications to any of the drugs. | |
| Interventions | An unspecified dose of ergometrine plus oxytocin administered by an unspecified route (n = 748) versus placebo or control (n = 764). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000.,Aaditional uterotonics, transfusion, manual removal of placenta, blood loss (mL), change in Hb level, third‐stage duration (min), NNU admissions, breastfeeding, nausea, vomiting, headache. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation schedule used variably sized balanced blocks, and the randomisation envelopes were prepared in advance in the National Perinatal Epidemiology Unit (NEPU). |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially‐numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending midwives. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Blood loss data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Public Health and Operational Research Committee of the Anglia and Oxford Regional Health Authority, UK (public funding). |
| Methods | 3‐arm placebo‐controlled randomised trial. | |
| Participants | Between November 2009 and September 2011, 76 parturients were randomised in a hospital setting in Norway. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by elective caesarean section. Exclusion criteria comprised parturients with pre‐eclampsia, placenta praevia, placenta accreta, von Willebrand disease or other bleeding disorder or preoperative systolic arterial pressure less than 90 mmHg. | |
| Interventions | 5 IU of oxytocin administered by an intravenous bolus (n = 26) versus 100 mcg of carbetocin administered by an intravenous bolus (n = 25) versus placebo or control (n = 25). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, blood loss (mL), change in Hb level, headache. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was allocated by a computer‐generated list of random numbers. The block size varied between 6 and 9, with stratification into 2 strata: BMI less than 30 and BMI of 30 or more. |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially‐numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was "double‐blinded": "to maintain blinding of the participants and investigators, the test medicine was delivered to the Department of Anaesthesiology in 10 mL syringes containing 5 mL of solution marked only with trial identification and randomisation numbers. The 10 mL syringes with the test medicines were prepared by a staff anaesthesiologist, who was otherwise uninvolved in the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss with the following formula: (0.75 x height in inches x 50) plus (weight in pounds x 50) x ((predelivery haematocrit measurement ‐ postdelivery haematocrit measurement)/predelivery haematocrit measurement). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (ClinicalTrials.gov NCT00977769). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | High risk | The study was supported by funding from Ferring Pharmaceuticals. |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between dates unspecified, 1721 parturients were randomised in a hospital setting in France. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing emergency caesarean section, or those with known hypersensitivity to prostaglandins. | |
| Interventions | 400 mcg plus 10 IU of misoprostol plus oxytocin administered orally plus by an intravenous bolus (n = 863) versus 10 IU of oxytocin administered by an intravenous bolus (n = 857). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, death, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "The study excluded 57 women from the misoprostol group and 61 from the placebo group because they had caesareans." The study report did not specify whether exclusions occurred before or after randomisation. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors excluded 119 study participants from the analysis because they experienced caesarean deliveries: it was unclear from the study report whether these exclusions occurred before or after randomisation. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between November 2009 and September 2011, 1865 parturients were randomised in a hospital setting in Nigeria. The population comprised women of parity 6 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing instrumental delivery, or those with diabetes, non‐cephalic presentation, anaemia, antepartum haemorrhage, multiple pregnancy, grand multiparity (more than 6) or known allergy. | |
| Interventions | 10 IU of oxytocin administered by an intravenous bolus (n = 900) versus 600 mcg of misoprostol administered orally (n = 900). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, blood loss (mL), change in Hb level. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignments were generated by dice‐box. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss at delivery by collection with pre‐calibrated kidney dishes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "46 of the administered questionnaires were invalidated leaving a total of 1819 valid questionnaires (912 for oxytocin and 907 for misoprostol). The data were further reduced through a process of computer randomisation so as to have [an] equal study population in the 2 medication groups: oxytocin group (900 subjects) and misoprostol group (900 subjects)”. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Low risk | The study was supported by funding from the University of Maiduguri Teaching Hospital. Study medications were donated by Emzor Pharmaceutical Industries. |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between March 2011 and June 2011, 216 parturients were randomised in a hospital setting in Iran. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with hypertension, pre‐eclampsia, uterine rupture, cervical tear, asthma, cardiovascular/renal/liver disorders, grand multiparity (not defined), fibroids or previous PPH. | |
| Interventions | 100 mcg of carbetocin administered intramuscularly (n = 109) versus 200 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 107). | |
| Outcomes | The study recorded the following outcomes: morbidity, additional uterotonics, death,.change in Hb level, nausea, vomiting, tachycardia, hypotension, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a random number table. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients and medical personnel were blinded to the type of drug." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 24 hours postpartum, blood samples could not be collected from 16 women (9 in the carbetocin group and 7 in the ergometrine plus oxytocin group). |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (Iranian registry of clinical trials number 138810212854N2). |
| Intention to treat analysis | High risk | The authors excluded 16 study participants from the analysis because postpartum Hb measurements were not available. |
| Funding source | Unclear risk | The study was supported by funding from the Kashan University of Medical Sciences (the institution of the authors). |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between 1st September 2009 and 28th February 2010, 200 parturients were randomised in a hospital setting in Nepal. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with polyhydramnios, chorioamnionitis, preterm labour, previous caesarean, asthma, cardiac disorder or contraindication/hypersensitivity to the use of prostaglandin and uterotonics. | |
| Interventions | 1000 mcg of misoprostol administered rectally (n = 100) versus 10 IU of oxytocin administered intramuscularly (n = 100). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, morbidity, death, blood loss (mL), change in Hb level, third‐stage duration (min), fever, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was randomly allocated as per the lottery technique. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss in the 48 hours postpartum, by collection with pre‐weighed sterile pads and a calibrated bucket. All the soaked drapes and pads were weighed and the dry weights of these materials were subtracted to calculate blood loss on the basis that 1 g is equivalent to 1 mL. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 4‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between dates unspecified, 300 parturients were randomised in a hospital setting in India. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing augmentation of labour, or those with intrauterine death, antepartum haemorrhage, multiple pregnancy, malpresentation, cardiac disorder, rhesus‐negative mother, hypertension, Hb less than 70 g/L or hypersensitivity/contraindication to prostaglandins. | |
| Interventions | 400 mcg or 600 mcg of misoprostol administered sublingually (n = 150) versus 5 IU of oxytocin administered by an intravenous bolus (n = 75) versus 200 mcg of ergometrine administered by an intravenous bolus (n = 75). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, additional uterotonics, transfusion, manual removal of placenta, blood loss (mL), third‐stage duration (min), fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drug packets were sealed and coded by the same individual using a computer‐generated random number chart. |
| Allocation concealment (selection bias) | Unclear risk | Investigators used sealed drug packets. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was "double‐blind": active treatments and placebo treatments were "identical" and investigators were "thus blinded." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators removed any linen soiled with amniotic fluid, and placed a disposable and absorbent pre‐weighed linen saver sheet with a pre‐weighed polythene bag under the mother to collect blood from the uterine cavity. Any blood clots were expressed from the vagina into the polythene bag, which was then removed and weighed. A fresh pre‐weighed sanitary napkin was applied. Separate swabs were not included in the final calculation (addition of the various gravimetric measurements), that was performed 1 hour after delivery. "The specific gravity of blood being 1.08, the amount of blood lost in mL was equal to the weight in grams." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (changes in Hb measurements were unspecified beyond textual summary that "all groups showed a slight decrease in mean haemoglobin concentration 24 hours postpartum [maximum decrease of 0.6 g/dL]; however, the difference was not significant [ANOVA, P > 0.05]"). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 4‐arm active‐controlled randomised trial. | |
| Participants | Between April 2002 and February 2003, 1200 parturients were randomised in a hospital setting in Egypt. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with traumatic PPH, blood disorders, chorioamnionitis, placenta praevia or placental abruption. | |
| Interventions | 200 mcg of ergometrine administered intramuscularly (n = 300) versus 600 mcg to 1000 mcg of misoprostol administered sublingually (n = 900). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb level, third‐stage duration (min), vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Investigators used opaque, closed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by collection with a graduated plastic bag, and by weighing towels, linen and gauzes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "144 women were excluded from analysis because they were exposed to trauma to the perineum, vagina or cervix during labour and had traumatic excessive bleeding." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between June 2003 and July 2005, 174 parturients were randomised in a hospital setting in India. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean. Exclusion criteria were not specified. | |
| Interventions | 400 mcg plus 20 IU of misoprostol plus oxytocin administered sublingually plus by an intravenous infusion (n = 90) versus 20 IU of oxytocin administered by an intravenous infusion (n = 84). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, blood loss (mL), change in Hb level, nausea, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved by computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially‐numbered, opaque, sealed envelopes made at pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study participants and caregivers were blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated intraoperative blood loss by collection with suction apparatus and sterile drapes before irrigation, and by evaluating the blood in abdominal swabs and gauzes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm cluster controlled randomised trial. | |
| Participants | Between 21st April 2011 and 30th November 2012, 1586 parturients were randomised in a community setting in Ghana. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 10 IU of oxytocin administered intramuscularly (n = 689) versus placebo or control (n = 897). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, death. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The 52 CHOs were randomly allocated equally to either the intervention or the control group; this allocation was stratified by both district and distance (at least 10 km, or less than 10 km) to emergency obstetric care. The randomisation sequence was determined using Stata (version 12). |
| Allocation concealment (selection bias) | Unclear risk | .Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The random allocation was not masked." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors were not blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated postpartum blood loss by collection with a BRASS‐V calibrated plastic drape placed under the mother, who was asked to remain recumbent for 1 hour following delivery of the baby, or for 2 hours if active bleeding persisted. "Fluids, urine, and faeces were excluded from the blood loss measure by sweeping them to the side and into a receptacle." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "7 and 9 enrolled women in the oxytocin and control arms, respectively, lacked a blood‐loss measure." |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered (ClinicalTrials.gov NCT01108289). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Bill and Melinda Gates Foundation (public funding). |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between January 2005 and April 2008, 370 parturients were randomised in a hospital setting in Singapore. The population comprised women of unspecified parity, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing elective caesarean section, or those with multiple pregnancy, previous PPH, coagulopathy, coronary artery disease, hypertension or hypersensitivity/contraindications for the use of Syntometrine or carbetocin. | |
| Interventions | 100 mcg of carbetocin administered intramuscularly (n = 185) versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 185). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, blood loss (mL), third‐stage duration (min), nausea, vomiting, headache, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was blocked and stratified by parity. The randomisation list with the allocation of the mode of intervention was forwarded from the Biostatistics Unit to the Department of Pharmacy at National University Hospital, where the purchased medications were kept. |
| Allocation concealment (selection bias) | Low risk | Investigators used opaque packages made at pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The identities of the medications were not known to the midwives, obstetricians and the participants. The medication codes were only broken following completion of the trial." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the visual estimation of attending obstetricians and midwives. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Low risk | The study protocol was registered 2 years after beginning recruitment (ClinicalTrial.gov NCT00499005). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the National Healthcare Group of Singapore (public funding). |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between January 2003 and December 2003, 400 parturients were randomised in a hospital setting in Bangladesh. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with previous caesarean. | |
| Interventions | 400 mcg of misoprostol administered orally (n = 210) versus 10 IU of oxytocin administered intramuscularly (n = 190). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending physicians after collection in a plastic bowl. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm placebo‐controlled randomised trial. | |
| Participants | Between May 1997 and April 1998, 65 parturients were randomised in a Hospital setting in Switzerland. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with multiple pregnancy, pre‐eclampsia, previous PPH or antepartum haemorrhage. | |
| Interventions | 600 mcg of misoprostol administered orally (n = 31) versus placebo or control (n = 34). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, additional uterotonics, blood loss (mL), change in Hb level, third‐stage duration (min), NNU admissions, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using random tables. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed by the pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was "double‐masked": "for proper masking, the study drugs were prepared by the hospital pharmacy as 3 identical gelatine capsules." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between March 2010 and February 2011, 100 parturients were randomised in a hospital setting in India. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with grand multiparity (more than 4), anaemia, malpresentation, polyhydramnios, antepartum haemorrhage, liver/renal disorder, previous caesarean, previous PPH, uterine anomaly, traumatic PPH or contraindications to use misoprostol or oxytocin. | |
| Interventions | 10 IU of oxytocin administered by an intravenous infusion (n = 50) versus 600 mcg of misoprostol administered sublingually (n = 50). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, death, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting. fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | .Randomisation was achieved using a computer‐generated random number sequence. |
| Allocation concealment (selection bias) | Unclear risk | Investigators used sequentially‐numbered, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Due to [the] nature of administration of the drugs, [the] patient or clinical care team could not be blinded. However, [the] statistician was unaware of the group allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators removed any linen soiled with amniotic fluid, and placed a calibrated plastic bag under the mother to collect blood from the uterine cavity. After delivery of the placenta, a pre‐weighed pad was placed high up in vagina until 1 hour afterwards. In cases of episiotomy, a separate pad was applied to the episiotomy site, and the fluid collected by this pad was not included in blood loss measurements. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm controlled randomised trial. | |
| Participants | Between January 1988 and February 1990, 193 parturients were randomised in a hospital setting in the UK. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing induction or augmentation of labour or instrumental delivery, or those with grand multiparity (not defined), malpresentation, multiple pregnancy, previous caesarean, previous PPH, antepartum haemorrhage, hypertension in pregnancy, intrauterine death, preterm rupture of membranes, cervical lacerations or third degree perineal tears. | |
| Interventions | Placebo or control (n = 90) versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 103). | |
| Outcomes | The study recorded the following outcomes: additional uterotonics, transfusion, manual removal of placenta, blood loss (mL), change in Hb level, third‐stage duration (min). | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was randomly allocated using standard randomisation tables. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | The study was conducted without external funding. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between 21st April 2011 and 31st March 2012, 120 parturients were randomised in a hospital setting in Nigeria. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean. Exclusion criteria comprised parturients requiring general anaesthesia, or those with multiple pregnancy, placenta praevia, pre‐eclampsia, eclampsia, undiagnosed vaginal bleeding, prolonged labour, prolonged obstructed labour, cardiac/renal/liver disorders or fever. | |
| Interventions | 400 mcg plus 20 IU of misoprostol plus oxytocin administered sublingually plus by an intravenous infusion (n = 60) versus 20 IU of oxytocin administered by an intravenous infusion (n = 60). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, death, blood loss (mL), change in Hb level, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment allocations were generated by random tables. |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially‐numbered, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "There were no look‐alike placebo tablets for women who had oxytocin alone... The obstetricians and the scrub nurses were unaware of which oxytocic was given to each patient, as they were screened off during the surgery... No member of the obstetric team had knowledge of which agent the patient received”. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "There were no look‐alike placebo tablets for women who had oxytocin alone... The obstetricians and the scrub nurses were unaware of which oxytocic was given to each patient, as they were screened off during the surgery... No member of the obstetric team had knowledge of which agent the patient received”. |
| Objective assessment of blood loss | Low risk | Investigators evaluated intraoperative and postoperative blood loss by collection in a suction bottle. Furthermore, soiled drapes, abdominal packs and pieces of gauze were weighed and the known dry weights subtracted. Finally, vulva pads applied during the 4 hours post‐operation, were also weighed and the known dry weights subtracted. Measurements obtained by these 3 methods were added together. Weight measurements were performed with a weighing scale made in China, of total weighing capacity of 5 kg and graduations of 0.25 g. Investigators considered that 1 g is equivalent to 1 mL of blood. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification, but not all of the outcomes projected by methodological descriptions were reported as results in the study report (cases of nausea, vomiting, diarrhoea, headaches, fatigue, dizziness, chills, flatulence and abdominal pain were omitted). |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between 1st January 2012 and 30th June 2012, 100 parturients were randomised in a hospital setting in India. The population comprised women of parity 2 to 4, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with previous PPH, multiple pregnancy, previous caesarean, macrosomia, pre‐eclampsia, diabetes, cardiac/lung/bleeding/clotting disorders or taking anticoagulants. | |
| Interventions | 10 IU of oxytocin administered by an intravenous bolus (n = 50) versus 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 50). | |
| Outcomes | The study recorded the following outcome: PPH at 500. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study participants (patients) were divided by a lottery system in the 2 groups, each group comprising 50 patients. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss after the delivery of baby "by squeezing the soaked pads and quantifying the amount of blood clots in a kidney tray of standard size to be equal to 500 mL." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 5‐arm controlled randomised trial. | |
| Participants | Between dates unspecified, 248 parturients were randomised in a hospital setting in Turkey. The population comprised women of parity 5 or less, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with placenta praevia, previous PPH, antepartum haemorrhage, non‐cephalic presentation, multiple pregnancy, intrauterine death, grand multiparity (more than 5), fibroids, pre‐eclampsia or anticoagulation therapy. | |
| Interventions | Placebo or control (n = 49) versus 400 mcg to 800 mcg of misoprostol administered orally, vaginally or rectally (n = 199). | |
| Outcomes | The study recorded the following outcomes: additional uterotonics, transfusion, third‐stage duration (min), shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was generated by random tables. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between dates unspecified, 200 parturients were randomised in a hospital setting in India. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with cardiac disorder in pregnancy, uterine tumour in pregnancy, secondary PPH, grand multiparity (not defined), multiple pregnancy, polyhydramnios, anaemia, coagulopathy, antepartum haemorrhage, previous PPH, prolonged labour, precipitate labour or known allergic or hypersensitivity reaction to prostaglandins. | |
| Interventions | 10 IU of oxytocin administered by an intravenous infusion (n = 100) versus 800 mcg of misoprostol administered rectally (n = 100). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, blood loss (mL), nausea, fever, shivering. | |
| Notes | Contact with study authors for additional information: no. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Investigators used simple random sampling. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Unclear risk | Methods of evaluating blood loss were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 3‐arm active‐controlled randomised trial. | |
| Participants | Over 10 months between dates unspecified, 200 parturients were randomised in a hospital setting in India. The population comprised women of parity 4 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients with grand multiparity (more than 4), multiple pregnancy, preterm labour (less than 32 weeks), HELLP syndrome, polyhydramnios, coagulopathy, asthma, cardiac/renal disorder, epilepsy, hypertension, Hb less than 80 g/L or known drug allergy. | |
| Interventions | 400 mcg of misoprostol administered sublingually (n = 66) versus 200 mcg of ergometrine administered intramuscularly (n = 67). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, Additional uterotonics, transfusion, manual removal of placenta, nausea, vomiting, fever, shivering, abdominal pain. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was allocated by a computer‐generated random number. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | After the drainage of amniotic fluid, investigators evaluated blood loss by collection with a sterile calibrated BRASS‐V drape placed under the mother. The drape remained in place for 1 hour. Furthermore, "blood loss in gauze pieces was calculated by subtracting the weight of dry gauze from the weight of blood‐soaked gauze pieces." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between 2005 and 2006, 200 parturients were randomised in a hospital setting in India. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria were not specified. | |
| Interventions | 400 mcg of misoprostol administered sublingually (n = 100) versus 200 mcg of ergometrine administered intramuscularly (n = 100). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, additional uterotonics, manual removal of placenta, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was "double‐blind": active treatments and placebo treatments were "identical‐looking." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss "accurately with a specially designed calibrated blood collection drape (BRASS‐V drape)." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | It was unclear from the study report whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were unclear. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between October 2002 and January 2003, 120 parturients were randomised in a hospital setting in India. The population comprised women of parity 5 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing induction or augmentation of labour or caesarean section, or those with preterm labour (less than 37 weeks), grand multiparity (more than 5), multiple pregnancy, hypertension in pregnancy, Hb less than 80 g/L or known hypersensitivity to prostaglandins. | |
| Interventions | 400 mcg of misoprostol administered sublingually (n = 60) versus 200 mcg of ergometrine administered by an intravenous bolus (n = 60). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, headache, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment was allocated by random tables. |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially‐numbered, opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Treatments were administered via different routes and the authors did not report any double dummy. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss by the estimation of attending nurses and obstetricians. After delivery of the baby, amniotic fluid was allowed to drain away, and amniotic fluid‐soaked bed linens were covered with dry disposable ‘linen‐savers’. A wedge‐shaped plastic bedpan was placed under the mother for 1 hour. Blood and clots from the bedpan were decanted into a measuring cylinder and measured. Blood‐soaked swabs and linen‐savers were weighed; the known dry weights were subtracted, for the weight of blood contained within them to be added to the value indicated by the measuring cylinder. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 2‐arm active‐controlled randomised trial. | |
| Participants | Between August 2004 and April 2005, 100 parturients were randomised in a hospital setting in India. The population comprised women of unspecified parity, a singleton pregnancy, at high risk for PPH, who delivered by either elective or emergency caesarean. Exclusion criteria comprised parturients with multiple pregnancy, antepartum haemorrhage, polyhydramnios, prolonged labour (more than 12 hours), previous more than 1 caesarean, previous uterine rupture, cardiac/liver/renal disorder, coagulopathy or Hb less than 80 g/L. | |
| Interventions | 400 mcg of misoprostol administered sublingually (n = 50) versus 20 IU of oxytocin administered by an intravenous infusion (n = 50). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, blood loss (mL), change in Hb level, vomiting, headache,fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Investigators used opaque, sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study participants and caregivers were not blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | Investigators evaluated blood loss intraoperatively and in the first hour postoperatively "in a standard manner." They measured the volume of blood in the suction bottle, and weighed blood‐soaked sponges and linen savers. Then they added the difference between dry and blood‐soaked weights of sponges and linen savers, to the volume measured in the suction bottle. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were collected completely from all randomised study participants. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from the Division of Reproductive Health and Nutrition, Indian Council of Medical Research (public funding). |
| Methods | 2‐arm active‐controlled double‐dummy randomised trial. | |
| Participants | Between 15th June 1998 and 15th May 1999, 401 parturients were randomised in a hospital setting in Ghana. The population comprised women of parity 5 or less, a singleton pregnancy, at low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing induction or augmentation of labour or caesarean section, or those with grand multiparity (more than 5), multiple pregnancy, preterm labour (less than 32 weeks), hypertension in pregnancy, HELLP syndrome, polyhydramnios, previous PPH, coagulopathy, precipitate labour, chorioamnionitis, Hb less than 80 g/L or a known hypersensitivity to prostaglandins. | |
| Interventions | 400 mcg of misoprostol administered orally (n = 203) versus 10 IU of oxytocin administered intramuscularly (n = 198). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), Change in Hb level, third‐stage duration (min), nausea, vomiting, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Investigators used sequentially‐numbered, opaque packets made by administrative staff. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The identity of the placebo and active medications were concealed from caregivers and participants." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | High risk | Investigators evaluated blood loss by the estimation of attending physicians. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of those women randomised, blood loss measurements were unavailable in 3 cases, and postpartum Hb samples were unavailable in 9 cases. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Low risk | All those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from MaterCare International and the Canadian International Development Agency (public funding). |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between dates unspecified, 58 parturients were randomised in a hospital setting in Australia. The population comprised women of unspecified parity, either singleton or multiple pregnancy, at high risk for PPH, who delivered by emergency caesarean section. Exclusion criteria comprised parturients undergoing elective caesarean section or requiring general anaesthesia, or those with vascular/liver/renal disorders, preterm labour (less than 37 weeks), placenta praevia, placental abruption, previous more than 2 caesareans or an adverse reaction to carbetocin/oxytocin. | |
| Interventions | 100 mcg of carbetocin administered by an intravenous bolus (n = 30) versus 5 IU of oxytocin administered by an intravenous bolus (n = 28). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, blood loss (mL), change in Hb level. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Investigators used computer‐generated randomisation at pharmacy level, and none of the operating or anaesthetic doctors had access to this. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed by the pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was "double‐blinded": women received "a blinded bolus of carbetocin" or "a blinded oxytocin bolus." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Low risk | Investigators evaluated intra‐operative blood loss by the estimation of attending physicians. Excess blood was collected in measuring container by suction, and weighed together with any swabs soaked in blood. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Low risk | The study report matches the study protocol that was registered prospectively (ACTRN 12612000466842). |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Low risk | The study was supported by funding from Frankston Hospital (the institution of the authors). |
| Methods | 2‐arm active‐controlled double‐blinded randomised trial. | |
| Participants | Between February 1993 and March 1993, 1000 parturients were randomised in a hospital setting in Hong Kong. The population comprised women of unspecified parity, a singleton pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients requiring oxytocin infusion in the third stage, or those with pre‐eclampsia or cardiac disorder. | |
| Interventions | 500 mcg plus 5 IU of ergometrine plus oxytocin administered intramuscularly (n = 496) versus 10 IU of oxytocin administered intramuscularly (n = 495). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, morbidity, additional uterotonics, transfusion, manual removal of placenta, death, change in Hb level, nausea, vomiting, headache. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: no. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using computer‐generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Investigators used sequentially‐numbered, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "When a patient entered the study, a nursing officer who was not involved in the management of the patient drew up the indicated medication and handed this to the patient’s attendants." Study participants and caregivers were thus blinded to treatment allocations. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to treatment allocations. |
| Objective assessment of blood loss | Unclear risk | Investigators evaluated blood loss during delivery "by measuring the amount of blood clots and weighing the towels used." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "9 [randomised participants] were excluded: 3 had a twin pregnancy, 1 had blood transfusion during labour, and the other 5 had unavailable records." |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | High risk | Not all study participants were included in the analysis. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
| Methods | 3‐arm active‐controlled randomised trial. | |
| Participants | Over 8 months between dates unspecified, 2023 parturients were randomised in a hospital setting in India. The population comprised women of unspecified parity, unspecified whether singleton or multiple pregnancy, at both high and low risk for PPH, who delivered by vaginal delivery. Exclusion criteria comprised parturients undergoing caesarean section, or those with asthma, cardiac disorder, rhesus factor incompatibility or hypertension. | |
| Interventions | 400 mcg of misoprostol administered orally (n = 730) versus 10 IU of oxytocin administered intramuscularly (n = 617) versus 200 mcg of ergometrine administered by an intravenous bolus (n = 676). | |
| Outcomes | The study recorded the following outcomes: PPH at 500, PPH at 1000, additional uterotonics, transfusion, manual removal of placenta, death, blood loss (mL), change in Hb level, third‐stage duration (min), nausea, vomiting, headache, fever, shivering. | |
| Notes | Contact with study authors for additional information: yes. Additional data from authors: yes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved using computer‐generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding (of study participants and caregivers) was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding was not reported. |
| Objective assessment of blood loss | Low risk | After the drainage of amniotic fluid, investigators evaluated blood loss by collection with a large sterile plastic bag placed under the mother until she was transferred to the postnatal department. The blood collected in the plastic bag was then transferred to a measuring jar. Mops were not used in the labour room, and gauze pieces were counted. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study authors did not mention any incomplete outcome data. |
| Selective reporting (reporting bias) | Unclear risk | The protocol of the study was unavailable for verification. |
| Intention to treat analysis | Unclear risk | The authors did not specify whether all those who were enrolled and randomly allocated to treatment were included in the analysis, in the groups to which they were randomised. |
| Funding source | Unclear risk | Source(s) of funding for the study were not reported. |
ACTRN, Australian Clinical Trials Registration Number; ANOVA, one‐way Analysis Of Variance; ASA I or II, ASA Physical Status Classification System: ASA I represents a normal healthy patient, ASA II represents a patient with mild systemic disease; BMI, Body Mass Index; cc, cubic centimetres; CHOs, community health officers; cm, centimetres; CTRI, Clinical Trials Registry of India; DIC, Disseminated Intravascular Coagulopathy; dL, decilitres; EudraCT, European Clinical Trials database; fL, femtolitres (measurement of mean corpuscular volume); g, grams; Hb, Haemoglobin; HELLP syndrome, Hemolysis (destruction of red blood cells), Elevated Liver enzymes (which indicate liver damage), and Low Platelet count; HIV, Human Immunodeficiency Virus; Hong Kong SAR, Hong Kong Special Adminstrative Region; IU, International Units; kg, kilograms; km, kilometres; L, litres; mcg, micrograms; mg, milligrams; min, minutes; mL, millilitres; mmHG, millimetres of mercury (unit of pressure); mmol, millimoles; NCT, National Clinical Trial (number); NEPU, National Perinatal Epidemiology Unit; NHS, National Health Service; nm, nanometres; NNU, Neonatal Unit; PACTR, Pan African Clinical Trials Registry; PPH, Postpartum Haemorrhage; PROM, Premature Rupture Of Membranes; RCOG, Royal College of Obstetricians and Gynaecologists; UK, United Kingdom; UNDP/UNFPA, United Nations Development Programme/United Nations Population Fund; USA, United States of America; WHO, World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdel‐Aleem 1993 | Not eligible intervention |
| Abdel‐Aleem 1997 | Not eligible intervention |
| Abdel‐Aleem 2013 | Not eligible intervention |
| Abdollahy 2000 | Not eligible intervention |
| Al‐Harazi 2009 | Same drug intervention both arms and only different route of misoprostol administration |
| Anandakrishnan 2013 | Same drug intervention both arms and only different dose of carbetocin administration |
| Anjaneyulu 1988 | Not eligible intervention |
| Anvaripour 2013 | Intervention given after the third stage of labour |
| Athavale 1991 | Not eligible intervention |
| Ayedi 2011a | Same drug intervention both arms and only different dose of oxytocin administration |
| Ayedi 2011b | Not eligible intervention |
| Aziz 2014 | Quasi‐randomised |
| Bader 2000 | Not eligible intervention |
| Badhwar 1991 | Not eligible intervention |
| Bai 2014 | Not eligible uterotonic |
| Balki 2006 | Same drug intervention both arms and only different dose of oxytocin administration |
| Banovska 2013 | Not eligible intervention |
| Barbaro 1961 | Not eligible intervention |
| Baumgarten 1983 | Not eligible uterotonic |
| Bhattacharya 1988 | Not eligible uterotonic |
| Bhavana 2013 | Not eligible intervention |
| Bider 1991 | Not eligible intervention |
| Bider 1992 | Not eligible intervention |
| Bisri 2011 | Same drug intervention both arms and only different route of oxytocin administration |
| Biswas 2007 | Not eligible uterotonic |
| Bivins 1993 | Not eligible uterotonic |
| Blum 2010 | Intervention for treatment of PPH |
| Bonham 1963 | Quasi‐randomised |
| Bonis 2012 | Quasi‐randomised |
| Cappiello 2006 | Not eligible intervention |
| Carvalho 2004 | Same drug intervention both arms and only different dose of oxytocin administration |
| Catanzarite 1990 | Not eligible intervention |
| Chaplin 2009 | Not eligible intervention |
| Chaudhuri 2014 | Inappropriate population (excluded women who had PPH) |
| Chestnut 1987 | Not eligible intervention |
| Chou 1994 | Not eligible intervention |
| Chua 1995 | Not eligible intervention |
| Chukudebelu 1963 | Quasi‐randomised |
| Cooper 2004 | Same drug intervention both arms and only different dose of oxytocin administration |
| Cordovani 2012 | Same drug intervention both arms and only different dose of carbetocin administration |
| Dagdeviren 2014 | Same drug intervention both arms and only different route of oxytocin administration |
| Dahiya 1995 | Not eligible intervention |
| Daley 1951 | Quasi‐randomised |
| Daly 1999 | Inappropriate population |
| Dao 2009 | Intervention for treatment of PPH |
| Davies 2005 | Same drug intervention both arms and only different route of oxytocin administration |
| De bonis 2012 | Quasi‐randomised |
| Dennehy 1998 | Same drug intervention both arms and only different route of oxytocin administration |
| Devi 1988 | Not eligible intervention |
| Diab 1999 | Quasi‐randomised |
| Dickinson 2009 | Not eligible population (terminations 2nd trimester) |
| Dommisse 1980 | Not randomised |
| Dong 2011 | Not eligible intervention |
| Durocher 2012 | Quasi‐randomised |
| Dutta 2000 | Quasi‐randomised |
| Dweck 2000 | Not eligible intervention |
| Dzuba 2012 | Same drug intervention both arms and only different route of oxytocin administration |
| Elati 2011 | Same drug intervention both arms and only different route of misoprostol administration |
| Erkkola 1984 | Not eligible intervention |
| Farber 2013 | Not eligible intervention |
| Farber 2015 | Not eligible intervention |
| Fatemeh 2011 | Same drug intervention both arms and only different route of oxytocin administration |
| Fawzy 2012 | Treatment (not prevention) of PPH. |
| Forster 1957 | Quasi‐randomised |
| Francis 1965 | Quasi‐randomised |
| Friedman 1957 | Quasi‐randomised |
| Fugo 1958 | Quasi‐randomised |
| Gai 2004 | Not eligible intervention |
| George 2010 | Same drug intervention both arms and only different route of oxytocin administration |
| Ghulmiyyah 2007 | Not eligible intervention |
| Gobbur 2011 | Not eligible intervention |
| Gohel 2007 | Not eligible intervention |
| Goswami 2013 | Not eligible intervention |
| Groeber 1960 | Not eligible intervention |
| Gungorduk 2010a | Same drug intervention both arms and only different route of oxytocin administration |
| Gungorduk 2010b | Not eligible intervention |
| Gungorduk 2011 | Not eligible intervention |
| Gungorduk 2013 | Not eligible intervention |
| Gupta 2014 | Not eligible intervention |
| Habek 2007 | Not eligible intervention |
| Hacker 1979 | Not randomised |
| Halder 2013 | Not eligible intervention |
| Hoffman 2006 | Not appropriate intervention (comparing timing of oxytocin) |
| Hofmeyr 2004 | Intervention for treating PPH |
| Howard 1964 | Not eligible intervention |
| Huh 2004 | Same drug intervention both arms and only different route of oxytocin administration |
| Hunt 2013 | Not eligible intervention |
| Häivä 1994 | Quasi‐randomised |
| Ilancheran 1990 | Not randomised |
| Irons 1994 | Inappropriate population (excluded women who had PPH) |
| Jackson 2001 | Not appropriate intervention (comparing timing of oxytocin) |
| Jiang 2001 | Same drug intervention both arms and only different route of oxytocin administration |
| Jin 2000 | Not eligible intervention |
| Jolivet 1978 | Not eligible intervention (given oral ergometrine for 6 days) |
| Jonsson 2010 | Same drug intervention both arms and only different route of oxytocin administration |
| Kashanian 2010 | Ineligible population (excluded women with PPH) |
| Kemp 1963 | Quasi‐randomised |
| Khan 1997 | Not eligible intervention |
| Khan 2003 | Same drug intervention both arms and only different route of misoprostol administration |
| Khan 2012 | Same drug intervention both arms and only different route of oxytocin administration |
| Khanun 2011 | Same drug intervention both arms and only different route of misoprostol administration |
| Khurshid 2010 | Not eligible intervention |
| Kikutani 2003a | Innapropriate population |
| Kikutani 2003b | Innapropriate population |
| King 2010 | Same drug intervention both arms and only different route of oxytocin administration |
| Kintu 2012 | Same drug intervention both arms and only different dose of oxytocin administration |
| Kiran 2012 | Same drug intervention both arms and only different dose of oxytocin administration |
| Kore 2000 | Not eligible intervention |
| Kovacheva 2015 | Same drug intervention both arms and only different route of oxytocin administration |
| Kovavisarach 1998 | Not eligible intervention |
| Kumar 2011 | Not eligible intervention (carboprost) |
| Kushtagi 2006 | Not eligible intervention (carboprost) |
| Lamont 2001 | Not eligible intervention (carboprost) |
| Le 2000 | Not eligible intervention |
| Leader 2002 | Not eligible population (2nd trimester) |
| Li 2002 | Not eligible intervention |
| Li 2003 | Not eligible intervention |
| Li 2011 | Not eligible intervention |
| Lin 2009 | Not eligible intervention |
| Liu 1997 | Not eligible intervention |
| Liu 2002 | Not eligible intervention |
| Luamprapas 1994 | Not eligible intervention |
| Mangla 2012 | Not eligible intervention |
| Mankuta 2006 | Not eligible intervention |
| Mansouri 2011 | Same drug intervention both arms and only different route of misoprostol administration |
| Martinez 2006 | Not eligible intervention |
| McGinty 1956 | Quasi‐randomised |
| Miller 2009 | Not eligible intervention |
| Mirghafourvand 2015 | Not eligible intervention |
| Mollitt 2009 | Same drug intervention both arms and only different route of oxytocin administration |
| Moore 1956 | Same drug intervention both arms and only different type of the same drug |
| Movafegh 2011 | Not eligible intervention |
| Muller 1996 | Not randomised |
| Munishankarappa 2009 | Same drug intervention both arms and only different route of oxytocin administration |
| Munn 2001 | Same drug intervention both arms and only different route of oxytocin administration |
| Murphy 2009 | Same drug intervention both arms and only different route of oxytocin administration |
| Nankali 2013 | Not eligible intervention |
| NCT01710566 2012 | Study withdrawn |
| Nellore 2006 | Not eligible intervention |
| Nelson 1983 | Not eligible intervention |
| Newton 1961 | Quasi‐randomised |
| Nguyen‐Lu 2013 | Same drug intervention both arms and only different dose of carbetocin administration |
| Nieminen 1964 | Not eligible intervention |
| Norchi 1988 | Not eligible intervention |
| Oberbaum 2005 | Not eligible intervention |
| Oguz 2014 | Same drug intervention both arms and only different route and timing of oxytocin administration |
| Ozalp 2010 | Not eligible intervention |
| Ozcan 1996 | Not eligible intervention |
| Ozkaya 2005 | Inappropriate population (excluded women who had PPH) |
| Padhy 2006 | Not eligible intervention |
| Palacio 2011 | Same drug intervention both arms and only different dose of oxytocin administration |
| Paull 1977 | Same drug intervention both arms and only different doses of drug administration |
| Pei 1996 | Not randomised |
| Perdiou 2009 | Not eligible intervention |
| Phromboot 2010 | Not eligible intervention |
| Pierre 1992 | Quasi‐randomised |
| Pinder 2002 | Same drug intervention both arms and only different doses of drug administration |
| Pisani 2012 | Quasi‐randomised |
| Poeschmann 1991 | Quasi‐randomised |
| Porter 1991 | Not eligible intervention |
| Priya 2015 | Inappropriate population (measured blood loss after the delivery of the placenta) |
| Puri 2012 | Not eligible intervention |
| Qiu 1999 | Not eligible population (2nd stage) |
| Quiroga 2009 | Not eligible intervention |
| Rajwani 2000 | Not eligible intervention |
| Reddy 1989 | Not eligible intervention |
| Reddy 2001 | Not eligible intervention |
| Rooney 1985 | Quasi‐randomised |
| Rosales‐Ortiz 2013 | Quasi‐randomised |
| Rouse 2011 | Same drug intervention both arms and only different doses of drug administration |
| Sadeghipour 2013 | Not eligible intervention |
| Saito 2007 | Qausi‐randomised |
| Samuels 2005 | Not eligible intervention |
| Sariganont 1999 | Not randomised |
| Sarna 1997 | Same drug intervention both arms and only different doses of drug administration |
| Sartain 2008 | Same drug intervention both arms and only different doses of drug administration |
| Schaefer 2004 | Same drug intervention both arms and only different timings of drug administration |
| Schemmer 2001 | Same drug intervention both arms and only different timings of drug administration |
| Sekhavat 2009 | Not eligible intervention |
| Sentilhes 2014 | Not eligible intervention |
| Senturk 2013 | Not eligible intervention |
| Shahid 2013 | Not eligible intervention |
| Sharma 2014 | Not randomised |
| Sheehan 2011 | Same drug intervention both arms and only different doses of drug administration |
| Shirazi 2013 | Not eligible intervention |
| Shrestha 2007 | Not eligible intervention |
| Singh 2005 | Not eligible intervention |
| Siriwarakul 1991 | Not eligible intervention |
| Soiva 1964 | Quasi‐randomised |
| Sorbe 1978 | Quasi‐randomised |
| Soriano 1995 | Quasi‐randomised |
| Stearn 1963 | Quasi‐randomised |
| Svanstrom 2008 | Innapropriate population |
| Symes 1984 | Innapropriate population |
| Taj 2014 | Not eligible intervention |
| Takagi 1976 | Not eligible intervention |
| Tanir 2009 | Not eligible intervention |
| Tarabrin 2012 | Not eligible intervention |
| Tariq 2015b | Administered for treatment of PPH |
| Tehseen 2008 | Not eligible intervention |
| Terry 1970 | Not eligible intervention |
| Tessier 2000 | Same drug intervention both arms and only different doses of drug administration |
| Tharakan 2008 | Same drug intervention both arms and only different doses of drug administration |
| Thomas 2007 | Same drug intervention both arms and only different doses of drug administration |
| Thornton 1988 | Quasi‐randomised |
| Tita 2012 | Same drug intervention both arms and only different doses of drug administration |
| Tripti 2006 | Not eligible intervention |
| Tripti 2009 | Not randomised |
| Tudor 2006 | Not eligible intervention |
| Van den Enden 2009 | Same drug intervention both arms and only different doses of drug administration |
| Van Selm 1995 | Not eligible uterotonic |
| Vasegh 2005 | Quasi‐randomised |
| Vaughan 1974 | Innapropriate population |
| Ventoskovskiy 1990 | Not eligible intervention |
| Verghese 2008 | Not eligible intervention |
| Vogel 2004 | Not eligible outcomes |
| Wallace 2008 | Same drug intervention both arms and only different regimen of oxytocin administration |
| Walraven 2005 | Not eligible uterotonic (oral ergometrine) |
| Wang 2000 | Not eligible intervention |
| Weeks 2013 | Self‐administered drug |
| Weihong 1998 | Not eligible intervention |
| Weiss 1975 | Not eligible outcomes |
| Wetta 2013 | Same drug intervention both arms and only different doses of drug administration |
| Winikoff 2012 | Same drug intervention both arms and only different doses of drug administration |
| Wong 2006 | Same drug intervention both arms and only different doses of drug administration |
| Wright 2006 | Not eligible intervention |
| Wu 2007 | Not eligible intervention |
| Xu 2003 | Not eligible intervention |
| Xu 2013 | Not eligible intervention |
| Yamaguchi 2011 | Same drug intervention both arms and only different doses of drug administration |
| Yan 2000 | Not eligible intervention |
| Yang 2001 | Not eligible intervention |
| Young 1988 | Not eligible intervention |
| Zamora 1999 | Not eligible intervention |
| Zaporozhan 2013 | Not eligible intervention |
| Zhao 1998 | Not eligible intervention |
| Zhao 2003 | Not eligible intervention |
| Zhou 1994 | Same drug intervention both arms and only different doses of drug administration |
PPH: postpartum haemorrhage
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | Randomised trial. |
| Participants | 100 women with singleton term pregnancy undergoing caesarean with spinal anaesthesia |
| Interventions | 20 IU of oxytocin administered by an intravenous infusion or 400 mcg of misoprostol administered sublingually |
| Outcomes | Additional uterotonics, blood loss (mL), change in Hb level, fever,.shivering. |
| Notes | Method of randomisation not clear and 'Risk of bias' assessment is uncertain. Abstract only. Unable to contact authors. |
| Methods | Randomised trial. |
| Participants | 542 nulliparous pregnant women |
| Interventions | 20 IU of oxytocin administered intravenously or 400 mcg of misoprostol administered sublingually |
| Outcomes | PPH (not defined), third‐stage duration (min), headache, shivering. |
| Notes | Method of randomisation not clear and 'Risk of bias' assessment is uncertain. Written in Persian and awaiting translation. |
| Methods | Randomised trial. |
| Participants | 120 women |
| Interventions | 10 IU of oxytocin administered intramuscularly or 400 mcg of misoprostol administered sublingually |
| Outcomes | PPH at 500. Blood loss (mL). |
| Notes | Method of randomisation not clear and 'Risk of bias' assessment is uncertain. Cannot obtain full text. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | Cluster‐randomised trial. |
| Participants | 1200 women from 30 health centres |
| Interventions | 600 mcg of Methylergometrine administered intramuscularly or 600 mcg of misoprostol administered orally |
| Outcomes | PPH at 500, third‐stage duration (min).,additional uterotonics,transfusion, blood loss (mL). |
| Notes | Method of randomisation not clear and 'Risk of bias' assessment is uncertain. Require intracluster correlation coefficient. |
| Methods | Randomised trial. |
| Participants | 200 women |
| Interventions | Not known dose of ergometrine administered intravenously or not known dose of misoprostol administered orally |
| Outcomes | Additional uterotonics, PPH (not defined), third‐stage duration (min), transfusion. |
| Notes | Method of randomisation not clear and 'Risk of bias' assessment is uncertain. Abstract only. Unable to contact authors. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | Randomised trial. |
| Participants | 152 women with no clear inclusion criteria |
| Interventions | Not known dose of carbetocin of unknown route or not known dose of oxytocin of unknown route |
| Outcomes | PPH (not defined). |
| Notes | Method of randomisation not clear and 'Risk of bias' assessment is uncertain. Abstract only. Unable to contact authors. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | Randomised trial. |
| Participants | 1750 women |
| Interventions | 5 IU of oxytocin administered intramuscularly then increased to 10 IU or ergometrine 500 mcg plus 5 IU of oxytocin administered intramuscularly |
| Outcomes | PPH at 500, blood loss (mL). |
| Notes | Method of randomisation not clear and 'Risk of bias' assessment is uncertain. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | Open‐label randomised trial. |
| Participants | 143 women with term singleton pregnancies |
| Interventions | Not known dose of oxytocin of unknown route or Not known dose of oxytocin of unknown route plus 600 mcg of misoprostol administered rectally. |
| Outcomes | Change in Hb level. |
| Notes | Method of randomisation not clear and 'Risk of bias' assessment is uncertain. Abstract only. Unable to contact authors. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | Double‐blinded randomised trial. |
| Participants | Not known how many women randomised |
| Interventions | Not known dose of oxytocin administered intravenously or 400 mcg of misoprostol administered orally. |
| Outcomes | PPH at 500, PPH at 1000, additional uterotonics, transfusion, change in Hb level, blood loss (mL), fever, shivering. |
| Notes | Method of randomisation not clear and 'Risk of bias' assessment is uncertain. Abstract only. Unable to contact authors. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | Randomised trial. |
| Participants | Not known how many women randomised |
| Interventions | 200 mcg of Methylergometrine of unknown route or 400 mcg of misoprostol administered sublingually. |
| Outcomes | PPH (not defined), additional uterotonics, change in HB level, third‐stage duration (min), blood loss (mL). |
| Notes | Method of randomisation not clear and 'Risk of bias' assessment is uncertain. Abstract only. Unable to contact authors. |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Hb, haemoglobin; IU, international unit;mcg, microgram; mL,: milliltre; PPH, postpartum haemorrhage
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Buccal misoprostol during cesarean section for preventing postpartum hemorrhage |
| Methods | Placebo‐controlled randomised trial. |
| Participants | 120 women undergoing an elective or emergency caesarean birth at 24 weeks of gestation or later with risk factors for PPH. |
| Interventions | 400 mcg of misoprostol administered buccally or placebo |
| Outcomes | PPH at 1000, additional uterotonics, transfusion. |
| Starting date | February 2008, Updated 2012 |
| Contact information | Dr. Jose E. Gonzalez |
| Notes | This study is shown as currently recruiting participants. |
| Trial name or title | Comparing misoprostol and oxytocin in UnijectTM for Postpartum Hemorrhage (PPH) Prevention in Mali |
| Methods | Double‐blinded randomised trial. |
| Participants | 140 women with a pregnancy over 34 weeks and a risk factor for PPH. |
| Interventions | 100 mcg of carbetocin administered intravenously or 10 IU of oxytocin administered intravenously. |
| Outcomes | Change in Hb level, nausea, vomiting, fever, shivering. |
| Starting date | Start date not known. |
| Contact information | Milton Cesar Gomez Gomez |
| Notes | This study is shown as not yet recruiting. |
| Trial name or title | Comparing misoprostol and oxytocin in Uniject for postpartum hemorrhage (PPH) prevention in Senegal |
| Methods | Open‐label cluster‐randomised trial. |
| Participants | 1365 women giving birth in community health centres with a trained study provider. |
| Interventions | 600 mcg of misoprostol administered orally or 10 IU of oxytocin administered intramuscularly. |
| Outcomes | Change in Hb level, nausea, vomiting, fever, shivering. |
| Starting date | June 2012 |
| Contact information | Gynuity health projects |
| Notes | This study is shown as completed. |
| Trial name or title | Intramuscular oxytocics: a comparison study of intramuscular carbetocin, syntocinon and syntometrine for the third stage of labour following vaginal birth (IMox) |
| Methods | Randomised trial |
| Participants | Women delivering vaginally, singleton pregnancy |
| Interventions | One dose of 100 mcg intramuscular Carbetocin given for active management of the third stage of labour, immediately after the birth of the baby. One dose of 10 IU intramuscular Syntocinon given for active management of the third stage of labour, immediately after the birth of the baby. One dose of 500 mcg/5 IU intramuscular Syntometrine given for active management of the third stage of labour, immediately after the birth of the baby. |
| Outcomes | Requirement for additional uterotonic drugs |
| Starting date | February 2015 |
| Contact information | Tim Draycott, North Bristol NHS Trust/University of Bristol |
| Notes | Study Chair: |
| Trial name or title | Efficiency of carbetocin in the prevention of the postpartum haemorrhage: a clinical double‐blinded randomised study |
| Methods | Open‐label randomised trial. |
| Participants | Women undergoing a vaginal birth at home with a trained study provider. |
| Interventions | 600 mcg of misoprostol administered orally or 10 IU of oxytocin administered intramuscularly. |
| Outcomes | PPH at 1000, additional uterotonics, transfusion, nausea, headache, abdominal pain. |
| Starting date | 15/07/2010 |
| Contact information | Milton Cesar Gomez Gomez |
| Notes | This study is shown as not yet recruiting. |
| Trial name or title | Comparison of the effect of rectal misoprostol and syntometrin in prevention of postpartum hemorrhage |
| Methods | Double‐blinded randomised trial. |
| Participants | 200 women with a singleton pregnancy undergoing a vaginal birth. |
| Interventions | 500 mcg of ergometrine plus 5 IU of oxytocin administered intramuscularly or 600 mcg of misoprostol administered rectally. |
| Outcomes | Additional uterotonics, change in Hb level. |
| Starting date | 21/4/2010 |
| Contact information | Dr. Mansoureh Samimi |
| Notes | This study is shown as recruitment complete. |
| Trial name or title | Comparison effect of carbetocine and syntometrin in prevention of postpartum hemorrhage |
| Methods | Double‐blinded randomised trial. |
| Participants | 200 women with a singleton pregnancy undergoing a vaginal birth. |
| Interventions | 500 mcg of ergometrine plus 5 IU of oxytocin administered intramuscularly or 100 mcg of carbetocin administered intramuscularly. |
| Outcomes | Additional uterotonics, change in Hb level. |
| Starting date | 21/1/2010 |
| Contact information | Dr. Mansoureh Samimi |
| Notes | This study is shown as recruitment complete. |
| Trial name or title | Comparison of misoprostol and oxytocin in reduction of postpartum hemorrhage |
| Methods | Randomised trial. |
| Participants | 300 women with singleton, term pregnancies. |
| Interventions | 10 IU of oxytocin administered intravenously or 400 mcg of misoprostol administered orally. |
| Outcomes | Change in haemoglobin. |
| Starting date | 22/12/2009 |
| Contact information | Simindokht Moradi |
| Notes | This study is shown as recruitment complete. |
| Trial name or title | Misoprostol versus oxytocin for prevention of post partum hemorrhage |
| Methods | Double‐dummy randomised trial. |
| Participants | 400 women undergoing vaginal birth with a singleton pregnancy. |
| Interventions | 400 mcg misoprostol administered orally or 20 IU of oxytocin administered through an intravenous infusion. |
| Outcomes | Change in Hb level, vomiting, fever, shivering. |
| Starting date | May 2013 |
| Contact information | Dr Minoo Rajaei |
| Notes | This study is shown as ongoing, but not recruiting participants. |
| Trial name or title | Comparison between rectal & sublingual misoprostol before caesarian section to reduce intra & post‐operative blood loss |
| Methods | Placebo‐controlled randomised trial. |
| Participants | 635 women undergoing elective caesarean with a singleton term pregnancy and only 1 previous caesarean. |
| Interventions | 400 mcg of misoprostol administered rectally or 400 mcg of misoprostol administered sublingually or placebo. |
| Outcomes | Change in Hb level, blood loss. |
| Starting date | February 2013 |
| Contact information | Mohamed S Sweed, |
| Notes | This study is shown as completed. |
| Trial name or title | Room temperature stable carbetocin for the prevention of postpartum haemorrhage during the third stage of labour in women delivering vaginally. |
| Methods | Randomized, non‐inferiority trial at 22 centres in 10 countries. |
| Participants | Women delivering vaginally, cervical dilatation equal to or less than 6cm, singleton pregnancy. |
| Interventions | Carbetocin RTS 100 micrograms solution for intramuscular (IM) injection to be administered once during the third stage of labour. Oxytocin 10 IU solution for intramuscular (IM) injection to be administered once during the third stage of labour. |
| Outcomes | The proportion of women with blood loss of 500 mL or more or the use of additional uterotonics at one hour and up to two hours for women who continue to bleed after one hour. (composite primary outcome). The blood loss will be measured with a plastic drape placed under the woman's buttocks. |
| Starting date | July 2015 |
| Contact information | Mariana Widmer (widmerm@who.int) |
| Notes | ACTRN12614000870651 |
Hb, haemoglobin; IU, international unit;mcg, microgram; PPH, postpartum haemorrhage; RTS, room temperature stable
Contributions of authors
Ioannis D Gallos (IDG) and Arri Coomarasamy (AC) conceived the idea for this study. IDG, Helen M Williams (HMW), Malcolm J Price (MP), Abi Merriel (AM), Harold Gee (HG), David Lissauer (DL), Vidhya Moorthy (VM), Özge Tunçalp (OT), A Metin Gülmezoglu (AMG), Jonathan J Deeks (JJD), G Justus Hofmeyr (GJH) and AC designed the meta‐analysis. IDG designed all electronic data collection forms. IDG, HMW, AM, HG, DL, VM and OT screened trials and extracted data. MP and Aurelio Tobias (AT) performed the statistical analysis. MP, AT and JJD provided statistical advice and input. IDG drafted the protocol and all versions of the review. HMW, MP, AM, HG, DL, OT, MW, AMG, AT, JJD, GJH and AC edited and revised the review.
Sources of support
Internal sources
-
University of Birmingham, UK.
The authors of this review are employed by the institutions indicated by their respective affiliations except where otherwise stated.
-
World Health Organization, Switzerland.
The authors of this review are employed by the institutions indicated by their respective affiliations except where otherwise stated.
-
Sandwell and West Birmingham NHS Trust, UK.
The authors of this review are employed by the institutions indicated by their respective affiliations except where otherwise stated.
-
University of the Witwatersrand, South Africa.
The authors of this review are employed by the institutions indicated by their respective affiliations except where otherwise stated.
External sources
-
National Institute for Health Research, UK.
This review is undertaken as part of independent research funded by the UK National Institute for Health Research (Health Technology Assessment Project 14/139/17 Uterotonic drugs for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis).
-
Birmingham Women’s NHS Foundation Trust, UK.
Additional financial support to meet the employment costs of Helen M Willliams (HMW) and Abi Merriel (AM) is provided by Birmingham Women’s NHS Foundation Trust.
-
Medical Research Council, UK.
Additional financial support to meet the employment costs of Malcolm J Price (MP) is provided by the Medical Research Council (Project MR/J013595/1).
-
Ammalife, UK.
Additional financial support to meet the employment costs of Abi Merriel (AM) is provided by Ammalife (UK Registered Charity 1120236).
Declarations of interest
Ioannis D Gallos (IDG): is a co‐applicant to the UK National Institute for Health Research HTA Project Award 14/139/17 entitled “Uterotonic drugs for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis”. He has been involved in one or more previous or ongoing trials related to the use of uterotonics for the prevention of PPH that could be eligible for inclusion in this review. He will not participate in any decisions regarding these trials (i.e. assessment for inclusion/exclusion, trial quality, data extraction) for the purposes of this review or future updates – these tasks will be carried out by other members of the team who are not directly involved in the trials. Ferring Pharmaceuticals and Novartis have supplied carbetocin and oxytocin for studies and an ongoing study is supported by WHO/Merck for Mothers. IDG has been supported by the MSD for mothers initiative for travel to a meeting for the study.
Helen M Williams (HMW): is part‐funded by the Birmingham Women’s NHS Foundation Trust, and a co‐applicant to the UK National Institute for Health Research HTA Project Award 14/139/17 entitled “Uterotonic drugs for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis”. Her salary is part‐funded by Tommy's. She is a member of the Executive Board of Ammalife (UK registered charity 1120236). She has also assisted the administration of activities at a single study site in contribution to a multinational randomised controlled trial of carbetocin versus oxytocin,that could potentially be eligible for inclusion in this review. The trial is sponsored by the World Health Organization and supported by Merck for Mothers. She will not participate in decisions regarding the inclusion of this trial in the review or any tasks related to it such as data extraction or quality assessment.
Malcolm J Price (MP) is funded as a research fellow by the UK Medical Research Council (MRC) Project Award MR/J013595/1, and a co‐applicant to the UK National Institute for Health Research HTA Project Award 14/139/17 entitled “Uterotonic drugs for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis”.
Aurelio Tobias: none known.
Abi Merriel (AM): was part‐funded by Ammalife (UK Registered Charity 1120236) and the Birmingham Women’s NHS Foundation Trust.
Harold Gee (HG): is a Trustee of Ammalife (UK Registered Charity 1120236).
David Lissauer (DL): was previously a Trustee of Ammalife (UK Registered Charity 1120236).
Vidhya Moorthy: none known.
Mariana Widmer (MW): is involved in an ongoing trial related to the use of uterotonics for the prevention of PPH that could be eligible for inclusion in this review. Ferring Pharmaceuticals and Novartis have supplied carbetocin and oxytocin for the trial and the study is supported by WHO/Merck for Mothers. MW will not participate in any decisions regarding this trial (i.e. assessment for inclusion/exclusion, trial quality, data extraction)for the purposes of this review or future updates – these tasks will be carried out by other members of the team who are not directly involved in the trial.
Özge Tunçalp (OT): is a co‐applicant to the UK National Institute for Health Research HTA Project Award 14/139/17 entitled “Uterotonic drugs for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis”.
A Metin Gulmezoglu (AMG): is a co‐applicant to the UK National Institute for Health Research HTA Project Award 14/139/17 entitled “Uterotonic drugs for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis”. AMG was involved in the large multicentre trial (as part of the central coordination unit) which may be included in the review. AMG is involved in an ongoing trial related to the use of uterotonics for the prevention of PPH that could be eligible for inclusion in this review. Ferring Pharmaceuticals and Novartis have supplied carbetocin and oxytocin for the trial and the study is supported by WHO/Merck for Mothers. AMG will not participate in any decisions regarding this or previous trials (i.e. assessment for inclusion/exclusion, trial quality, data extraction)for the purposes of this review or future updates – these tasks will be carried out by other members of the team who are not directly involved in the trial.
Jonathan J Deeks (JJD): is a co‐applicant to the UK National Institute for Health Research HTA Project Award 14/139/17 entitled “Uterotonic drugs for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis”.
G Justus Hofmeyr (GJH) has been and continues to be involved in a number of studies that may be eligible for inclusion in this review, but will not participate in data extraction or quality assessment of the studies in which he was involved. He is a co‐investigator on the UK National Institute for Health Research HTA Project Award 14/139/17 entitled "Uterotonic drugs for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis". Neither he nor his institution receives funding from this grant.
Arri Coomarasamy (AC): is the Chief Investigator of UK National Institute for Health Research HTA Project Award 14/139/17 entitled "Uterotonic drugs for preventing postpartum haemorrhage: a network meta‐analysis and cost‐effectiveness analysis". He has been involved in one or more previous or ongoing trials related to the use of uterotonics for the prevention of PPH that could be eligible for inclusion in this review. Ferring Pharmaceuticals and Novartis have supplied carbetocin and oxytocin for studies and an ongoing study is supported by WHO/Merck for Mothers. AC will not participate in any decisions regarding these trials (i.e. assessment for inclusion/exclusion, trial quality, data extraction)for the purposes of this review or future updates – these tasks will be carried out by other members of the team who are not directly involved in the trials. AC is a member of the Executive Board of Ammalife (UK registered charity 1120236). He does not receive any payment for this relationship.
New
References
References to studies included in this review
- Abdel‐Aleem H, Singata M, Abdel‐Aleem M, Mshweshwe N, Williams X, Hofmeyr GJ. Uterine massage to reduce postpartum hemorrhage after vaginal delivery. International Journal of Gynecology & Obstetrics 2010;111(1):32‐6. [DOI] [PubMed] [Google Scholar]
- Acharya G, Al‐Sammarai MT, Patel N, Al‐Habib A, Kiserud T. A randomized, controlled trial comparing effect of oral misoprostol and intravenous syntocinon on intra‐operative blood loss during cesarean section. Acta Obstetricia et Gynecologica Scandinavica 2001;80(3):245‐50. [DOI] [PubMed] [Google Scholar]
- Adanikin AI, Orji EO, Adanikin PO, Olaniyan O. Comparative study of rectal misoprostol to oxytocin infusion in preventing postpartum haemorrhage post‐caesarean section. International Journal of Gynecology & Obstetrics 2012;119(Suppl 3):S825. [Google Scholar]; Adanikin AI, Orji EO, Fasubaa OB, Onwudiegwu U, Ijarotimi OA, Olaniyan O. The effect of post‐cesarean rectal misoprostol on intestinal motility. International Journal of Gynecology and Obstetrics 2012;119(2):159‐62. [DOI] [PubMed] [Google Scholar]; Orji EO, Adanikin AI. Prospective randomised double blind study on the effect of post‐caesarean rectal misoprostol on intestinal motility. International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S446‐S447. [DOI] [PubMed] [Google Scholar]; Orji EO, Adanikin AO. The effect of post‐caesarean rectal misoprostol on intestinal motility. BJOG: an international journal of obstetrics and gynaecology 2013;120(Suppl s1):21. [DOI] [PubMed] [Google Scholar]
- Afolabi EO, Kuti O, Orji EO, Ogunniyi SO. Oral misoprostol versus intramuscular oxytocin in the active management of the third stage of labour. Singapore Medical Journal 2010;51(3):207‐11. [PubMed] [Google Scholar]
- Ahmed WAS, Ibrahim ZM, Mostafa I, Kishk EA, Elbahie MA. Safety and efficacy of carbetocin in hypertensive pregnant women undergoing cesarean delivery. Journal of Maternal‐Fetal & Neonatal Medicine 2014;27(Suppl 1):49. [Google Scholar]
- Al‐Sawaf A, El‐Mazny A, Shohayeb A. A randomised controlled trial of sublingual misoprostol and intramuscular oxytocin for prevention of postpartum haemorrhage. Journal of Obstetrics and Gynaecology 2013;33(3):277‐9. [DOI] [PubMed] [Google Scholar]
- Amant F, Spitz B, Timmerman D, Corresmans A, Assche FA. Misoprostol compared with methylergometrine for the prevention of postpartum haemorrhage: a double‐blind randomised trial. British Journal of Obstetrics and Gynaecology 1999;106:1066‐70. [DOI] [PubMed] [Google Scholar]
- Amin N. Prophylactic use of misoprostol in management of third stage of labour and prevention of atonic uterus. Journal of Postgraduate Medical Institute 2014;28(2):196‐200. [Google Scholar]
- Askar AA, Ismail MT, El‐Ezz AA, Rabie NH. Carbetocin versus syntometrine in the management of third stage of labor following vaginal delivery. Archives of Gynecology and Obstetrics 2011;284(6):1359‐65. [DOI] [PubMed] [Google Scholar]
- Attilakos G, Psaroudakis D, Ash J, Buchanan R, Winter C, Donald F, et al. Can a new oxytocin analogue reduce the need for additional oxytocics after caesarean section? The results of a double‐blind randomised trial. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(Suppl 1):Fa51. [Google Scholar]; Attilakos G, Psaroudakis D, Ash J, Buchanan R, Winter C, Donald F, et al. Carbetocin versus oxytocin for the prevention of postpartum haemorrhage following caesarean section: the results of a double‐blind randomised trial. BJOG: an international journal of obstetrics and gynaecology 2010;117(8):929‐36. [DOI] [PubMed] [Google Scholar]; Attilakos G, Psaroudakis D, Ash J, Buchanan R, Winter C, Donald F, et al. The haemodynamic effects of oxytocin and carbetocin following caesarean section: the results of a double‐blind randomised study. BJOG: an international journal of obstetrics and gynaecology 2008;115(s1):140‐1. [DOI] [PubMed] [Google Scholar]; Attilakos G, Psaroudakis D, Ash J, Buchanen R, Winter C, Draycott T. Low recruitment rate for a drug trial in obstetrics: an effect of the publicity following the TGN1412 clinical trial at the PAREXEL Research Unit in Northwick Park Hospital? [abstract]. 31st British International Congress of Obstetrics and Gynaecology; 2007 July 4‐6; London, UK. 2007:110. [Google Scholar]
- Atukunda EC, Siedner MJ, Obua C, Mugyenyi GR, Twagirumukiza M, Agaba AG. Sublingual misoprostol versus intramuscular oxytocin for prevention of post‐partum haemorrhage in Uganda: a randomised, controlled, non‐inferiority trial. Lancet 2014;384(Suppl 1):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Atukunda EC, Siedner MJ, Obua C, Mugyenyi GR, Twagirumukiza M, Agaba AG. Sublingual misoprostol versus intramuscular oxytocin for prevention of postpartum hemorrhage in Uganda: a double‐blind randomized non‐inferiority trial. PLoS Medicine 2014;11(11):e1001752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badejoko OO, Ijarotimi AO, Awowole IO, Loto OM, Badejoko BO, Olaiya DS, et al. Adjunctive rectal misoprostol versus oxytocin infusion for prevention of postpartum hemorrhage in women at risk: a randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2012;38(11):1294‐301. [DOI] [PubMed] [Google Scholar]
- Balki M, Dhumne S, Kasodekar S, Kingdom J, Windrim R, Carvalho JC. Oxytocin‐ergometrine co‐administration does not reduce blood loss at caesarean delivery for labour arrest. BJOG: an international journal of obstetrics and gynaecology 2008;115(5):579‐84. [DOI] [PubMed] [Google Scholar]
- Bamigboye AA, Hofmeyr GJ, Merrell DA. Rectal misoprostol in the prevention of postpartum haemorrhage: a placebo controlled trial. Proceedings of the 17th Conference on Priorities in Perinatal Care; 1998; South Africa. 1998:49‐52. [DOI] [PubMed] [Google Scholar]; Bamigboye AA, Hofmeyr GJ, Merrell DA. Rectal misoprostol in the prevention of postpartum hemorrhage: a placebo‐controlled trial. American Journal of Obstetrics and Gynecology 1998;179(4):1043‐6. [DOI] [PubMed] [Google Scholar]
- Bamigboye AA, Merrell DA, Hofmeyr GJ, Mitchell R. Randomized comparison of rectal misoprostol with syntometrine for management of third stage of labor. Acta Obstetricia et Gynecologica Scandinavica 1998;77:178‐81. [PubMed] [Google Scholar]
- Barton SR, Jackson A. The safety and efficiency of carbetocin to control uterine bleeding following caesarean section. Prenatal and Neonatal Medicine 1996;1(Suppl 1):185. [Google Scholar]
- Baskett TF, Persad V, Clough H, Young D. Prophylactic use of misoprostol in the third stage of labor [abstract]. Obstetrics & Gynecology 2005;105(4 Suppl):39S. [Google Scholar]; Baskett TF, Persad VL, Clough HJ, Young DC. Misoprostol versus oxytocin for the reduction of postpartum blood loss. International Journal of Gynecology & Obstetrics 2007;97(1):2‐5. [DOI] [PubMed] [Google Scholar]; Chandra S, Persad V, Young D, Baskett T. A preliminary study of cutaneous blood flow associated with postpartum use of oral misoprostol. Journal of Obstetrics & Gynaecology Canada: JOGC 2004;26(12):1073‐6. [DOI] [PubMed] [Google Scholar]
- Begley CM. Comparative studies in the third stage of labour [MSc thesis]. Dublin: Trinity College, University of Dublin, 1990. [Google Scholar]; Begley CM. A comparison of 'active' and 'physiological' management of the third stage of labour. Midwifery 1990;6:3‐17. [DOI] [PubMed] [Google Scholar]; Begley CM. The effect of ergometrine on breast feeding. Midwifery 1990;6:60‐72. [DOI] [PubMed] [Google Scholar]
- Bellad M, Ganachari TDM, Mallapur M. Sublingual (SL) powdered misoprostol (400 mcg) vs IM oxytocin (10 IU) for prevention of postpartum blood loss ‐ a randomized controlled trial. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S124‐5. [Google Scholar]; Bellad MB, Tara D, Ganachari MS, Mallapur MD, Goudar SS, Kodkany BS, et al. Prevention of postpartum haemorrhage with sublingual misoprostol or oxytocin: a double‐blind randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2012;119(8):975‐86. [DOI] [PubMed] [Google Scholar]
- Benchimol M, Gondry J, Mention JE, Gagneur O, Boulanger JC. Role of misoprostol in the delivery outcome. [French] [Place du misoprostol dans la direction de la delivrance.]. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 2001;30(6):576‐83. [PubMed] [Google Scholar]
- Bhullar A, Carlan SJ, Hamm J, Lamberty N, White L, Richichi K. Buccal misoprostol to decrease blood loss after vaginal delivery: a randomized trial. Obstetrics & Gynecology 2004;104(6):1282‐8. [DOI] [PubMed] [Google Scholar]
- Borruto F, Treisser A, Comparetto C. Utilization of carbetocin for prevention of postpartum hemorrhage after cesarean section: a randomized clinical trial. Archives of Gynecology and Obstetrics 2009;280(5):707‐12. [DOI] [PubMed] [Google Scholar]
- Boucher M, Horbay GL, Griffin P, Deschamps Y, Desjardins C, Schulz M, et al. Double‐blind, randomized comparison of the effect of carbetocin and oxytocin on intraoperative blood loss and uterine tone of patients undergoing cesarean section. Journal of Perinatology 1998;18(3):202‐7. [PubMed] [Google Scholar]
- Boucher M, Nimrod C, Tawagi G. Carbetocin IM injection vs oxytocin IV infusion for prevention of postpartum hemorrhage in women at risk following vaginal delivery. American Journal of Obstetrics and Gynecology 2001;185(6 Pt 2):Abstract no: 494. [Google Scholar]; Boucher M, Nimrod CA, Tawagi GF, Meeker TA, Rennicks White RE, Varin J. Comparison of carbetocin and oxytocin for the prevention of postpartum hemorrhage following vaginal delivery:a double‐blind randomized trial. Journal of Obstetrics & Gynaecology Canada: JOGC 2004;26(5):481‐8. [DOI] [PubMed] [Google Scholar]
- Bugalho A, Daniel A, Foundes A, Cunha M. Misoprostol for the prevention of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2001;73:1‐6. [DOI] [PubMed] [Google Scholar]
- Butwick AJ, Coleman L, Cohen SE, Riley ET, Carvalho B. A study of the minimum effective dose of oxytocin in patients undergoing elective cesarean delivery. American Society of Anaesthesiologists Annual Meeting; 2009 Oct 17‐21; New Orleans, USA. 2009. [Google Scholar]; Butwick AJ, Coleman L, Cohen SE, Riley ET, Carvalho B. Minimum effective bolus dose of oxytocin during elective caesarean delivery. British Journal of Anaesthesia 2010;104(3):338‐43. [DOI] [PubMed] [Google Scholar]
- Caliskan E, Meydanli M, Dilbaz B, Aykan B, Sonmezer M, Haberal A. Is rectal misoprostol really effective in the treatment of third stage of labor? A randomized controlled trial. American Journal of Obstetrics and Gynecology 2002;187:1038‐45. [DOI] [PubMed] [Google Scholar]
- Caliskan E, Dilbaz B, Meydanli MM, Ozturk N, Narin MA, Haberal A. Oral misoprostol for the third stage of labor: a randomized controlled trial. Obstetrics & Gynecology 2003;101(5 Pt 1):921‐8. [DOI] [PubMed] [Google Scholar]
- Carbonell i Esteve JL, Hernandez JMR, Piloto M, Setien SA, Texido CS, Tomasi G, et al. Active management of the third phase of labour plus 400 mug of sublingual misoprostol and 200 mug of rectal misoprostol versus active management only in the prevention of post‐partum haemorrhage. A randomised clinical trial [Manejo activo de la tercera fase del parto mas 400 mug de misoprostol sublingual y 200 mug de misoprostol rectal frente a manejo activo solo en la prevencion de la hemorragia posparto. Ensayo clinico aleatorizado]. Progresos de Obstetricia y Ginecologia 2009;52(10):543‐51. [Google Scholar]
- Cayan F, Doruk A, Sungur MA, Dilek S. Comparison of the different dosages of rectal misoprostol on I=intestinal motility and pain score in high risk cesarean delivery. Turkiye Klinikleri Journal of Medical Sciences 2010;30(4):1154‐9. [Google Scholar]
- Chaudhuri P, Banerjee GB, Mandal A. Rectally administered misoprostol versus intravenous oxytocin infusion during cesarean delivery to reduce intraoperative and postoperative blood loss. International Journal of Gynecology & Obstetrics 2010;109(1):25‐9. [DOI] [PubMed] [Google Scholar]
- Chaudhuri P, Biswas J, Mandal A. Sublingual misoprostol versus intramuscular oxytocin for prevention of postpartum hemorrhage in low‐risk women. International Journal of Gynecology and Obstetrics 2012;116(2):138‐42. [DOI] [PubMed] [Google Scholar]
- Chaudhuri P, Majumdar A. Sublingual misoprostol as an adjunct to oxytocin during cesarean delivery in women at risk of postpartum hemorrhage. International Journal of Gynaecology & Obstetrics 2015;128:48‐52. [DOI] [PubMed] [Google Scholar]
- Chhabra S, Tickoo C. Low‐dose sublingual misoprostol versus methylergometrine for active management of the third stage of labor. Journal of Obstetrics and Gynaecology Research 2008;34(5):820‐3. [DOI] [PubMed] [Google Scholar]
- Choy CMY, Lau WC, Tam WH, Yuen PM. A randomised controlled trial of intramuscular syntometrine and intravenous oxytocin in the management of the third stage of labour. BJOG: an international journal of obstetrics and gynaecology 2002;109:173‐7. [DOI] [PubMed] [Google Scholar]
- Cook CM, Spurrett B, Murray H. A randomized clinical trial comparing oral misoprostol with synthetic oxytocin or syntometrine in the third stage of labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 1999;39(4):414‐9. [DOI] [PubMed] [Google Scholar]
- Dansereau J. Comparison of carbetocin vs. oxytocin in prevention of uterine atony post cesarean section. Prenatal and Neonatal Medicine 1996;1(Suppl 1):80. [Google Scholar]; Dansereau J, Gambling D, Joshi A, Helewa M, Doran T, Lange I, et al. Double‐blind comparison of carbetocin vs oxytocin in preventing uterine atony post Cesarean section. International Journal of Gynecology & Obstetrics 1994;46 Suppl:77. [Google Scholar]; Dansereau J, Joshi AK, Helewa ME, Doran TA, Lange IR, Luther ER, et al. Double blind comparison of carbetocin versus oxytocin in prevention of uterine atony after cesarean section. American Journal of Obstetrics and Gynecology 1999;18(3 Pt 1):670‐6. [DOI] [PubMed] [Google Scholar]; Dansereau J, Joshi AK, Helewa ME, Doran TA, Lange IR, Luther ER, et al. Double‐blind comparison of carbetocin vs oxytocin in preventing uterine atony post Cesarean section. European Journal of Obstetrics & Gynecology and Reproductive Biology 1996;69:37. [DOI] [PubMed] [Google Scholar]; Gambling D, Dansereau J, Schulz M, Horbay GLA, Waasenaar W. Double‐blind, randomized comparison of a single dose of carbetocin vs 8 hours oxytocin infusion after cesarean delivery: safety data. A Canadian multi‐center trial [abstract]. International Journal of Obstetric Anesthesia 1994;3:113‐4. [Google Scholar]; Gambling DR, Dansereau J, Wassenaar W, Schulz M, Horbay GLA. Double‐blind randomized comparison of a single dose of carbetocin versus 8 hours oxytocin infusion after cesarean delivery: safety data. Anesthesia & Analgesia 1994;78 Suppl:S127. [Google Scholar]
- Dasuki D, Emilia O, Harini S. Randomized clinical trial: the effectiveness of oral misoprostol versus oxytocin in prevention of postpartum hemorrhage [abstract]. Journal of Obstetrics and Gynaecology Research 2002;28(1):46. [Google Scholar]
- Groot ANJA, Roosmalen J, Dongen PWJ, Borm GF. A placebo‐controlled trial of oral ergometrine to reduce postpartum hemorrhage. Acta Obstetricia et Gynecologica Scandinavica 1996;75:464‐8. [DOI] [PubMed] [Google Scholar]
- Derman RJ, Kodkany BS, Goudar SS, Geller SE, Naik VA, Bellad MB, et al. Oral misoprostol in preventing postpartum haemorrhage in resource‐poor communities: a randomised controlled trial. Lancet 2006;368(9543):1248‐53. [DOI] [PubMed] [Google Scholar]; Geller SE, Goudar SS, Adams MG, Naik VA, Patel A, Bellad MB, et al. Factors associated with acute postpartum hemorrhage in low‐risk women delivering in rural India. International Journal of Gynecology & Obstetrics 2008;101(1):94‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]; Geller SE, Patel A, Niak VA, Goudar SS, Edlavitch SA, Kodkany BS, et al. Conducting international collaborative research in developing nations. International Journal of Gynecology & Obstetrics 2004;87(3):267‐71. [DOI] [PubMed] [Google Scholar]; Goudar SS, Chakraborty H, Edlavitch SA, Naik VA, Bellad MB, Patted SS, et al. Variation in the postpartum hemorrhage rate in a clinical trial of oral misoprostol. Journal of Maternal‐Fetal & Neonatal Medicine 2008;21(8):559‐64. [DOI] [PubMed] [Google Scholar]; Kodkany BS, Derman RJ, Goudar SS, Geller SE, Edlavitch SA, Naik VA, et al. Initiating a novel therapy in preventing postpartum hemorrhage in rural India: a joint collaboration between the United States and India. International Journal of Fertility & Womens Medicine 2004;49(2):91‐6. [PubMed] [Google Scholar]; Kodkany BS, Goudar SS, Derman RJ. The efficacy of oral misoprostol in preventing postpartum hemorrhage in a community setting: a randomized double‐blind placebo‐controlled trial. International Journal of Gynecology & Obstetrics 2006;94(Suppl 2):S141‐S142. [DOI] [PubMed] [Google Scholar]; NCT00097123. RCT of misoprostol for postpartum hemorrhage in India. clinicaltrials.gov/ct2/show/NCT00097123 (first received 18 November 2004). ; Patted SS, Goudar SS, Naik VA, Bellad MB, Edlavitch SA, Kodkany BS, et al. Side effects of oral misoprostol for the prevention of postpartum hemorrhage: results of a community‐based randomised controlled trial in rural India. Journal of Maternal‐Fetal & Neonatal Medicine 2009;22(1):24‐8. [DOI] [PubMed] [Google Scholar]
- Dhananjaya BS, Charishma S. Comparative study of efficacy and safety of intramuscular oxytocin with intramuscular methylergometrine in the active management of third stage of labour. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2014;5(3):734‐9. [Google Scholar]
- Docherty PW, Hooper M. Choice of an oxytocic agent for routine use at delivery. Journal of Obstetrics and Gynaecology 1981;2:60. [Google Scholar]
- Eftekhari N, Doroodian M, Lashkarizadeh R. The effect of sublingual misoprostol versus intravenous oxytocin in reducing bleeding after caesarean section. Journal of Obstetrics and Gynaecology 2009;29(7):633‐6. [DOI] [PubMed] [Google Scholar]
- Behery MM, Sayed GA, Hameed AA, Soliman BS, Abdelsalam WA, Bahaa A. Carbetocin versus oxytocin for prevention of postpartum hemorrhage in obese nulliparous women undergoing emergency cesarean delivery. Journal of Maternal‐Fetal & Neonatal Medicine2015:1‐4. [DOI] [PubMed]
- Elgafor El Sharkwy IA. Carbetocin versus sublingual misoprostol plus oxytocin infusion for prevention of postpartum hemorrhage at cesarean section in patients with risk factors: a randomized, open trail study. Archives of Gynecology and Obstetrics 2013;288(6):1231‐6. [DOI] [PubMed] [Google Scholar]
- El‐Refaey H, Nooh R, O'Brien P, Abdalla M, Geary M, Walder J, et al. The misoprostol third stage of labour study: a randomised controlled comparison between orally administered misoprostol and standard management. BJOG: an international journal of obstetrics and gynaecology 2000;107:1104‐10. [DOI] [PubMed] [Google Scholar]
- Elsedeek MS. Impact of preoperative rectal misoprostol on blood loss during and after elective cesarean delivery. International Journal of Gynecology & Obstetrics 2012;118(2):149‐52. [DOI] [PubMed] [Google Scholar]
- Tahan MR, Warda OM, Rashad A, Yasseen AM, Ramzy EA, Ahmady MS, et al. Effects of preoperative sublingual misoprostol on uterine tone during isoflurane anesthesia for cesarean section. Revista Brasileira de Anestesiologia 2012;62(5):625‐35. [DOI] [PubMed] [Google Scholar]
- Enakpene CA, Morhason‐Bello IO, Enakpene EO, Arowojolu AO, Omigbodun AO. Oral misoprostol for the prevention of primary post‐partum hemorrhage during third stage of labor. Journal of Obstetrics and Gynaecology Research 2007;33(6):810‐7. [DOI] [PubMed] [Google Scholar]
- Ezeama CO, Eleje GU, Ezeama NN, Igwegbe AO, Ikechebelu JI, Ugboaja JO, et al. A comparison of prophylactic intramuscular ergometrine and oxytocin for women in the third stage of labor. International Journal of Gynecology & Obstetrics 2014;124(1):67‐71. [DOI] [PubMed] [Google Scholar]
- Fararjeh C, Gezer A, Cepni I, Benian A, Ocal P, Kosebay D. The efficacy of misoprostol in preventing postpartum bleeding [Postpartum Kanama Profilaksisinde Rektal Mizoprostol Kulanımının Etkinliği.]. Jinekoloji ve Obstetrik Dergisi 2003;17(4):218‐23. [Google Scholar]
- Fawole AO, Sotiloye OS, Hunyinbo KI, Umezulike AC, Okunlola MA, Adekanle DA, et al. A double‐blind, randomized, placebo‐controlled trial of misoprostol and routine uterotonics for the prevention of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2011;112(2):107‐11. [DOI] [PubMed] [Google Scholar]
- Fazel MR, Mansoure‐Samimi, Esmaeil‐Fakharian. A comparison of rectal misoprostol and intravenous oxytocin on hemorrhage and homeostatic changes during cesarean section. Middle East Journal of Anesthesiology 2013;22(1):41‐6. [PubMed] [Google Scholar]
- Fekih M, Jnifene A, Fathallah K, Ben Regaya L, Memmi A, Bouguizene S, et al. Benefit of misoprostol for prevention of postpartum hemorrhage in cesarean section: a randomized controlled trial. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 2009;38(7):588‐93. [DOI] [PubMed] [Google Scholar]
- Fenix AM. Double‐blind randomized controlled trial comparing the effect of carbetocin with oxytocin for the prevention of postpartum hemorrhage among high risk women following vaginal delivery. International Journal of Gynaecology & Obstetrics 2012;119(Suppl 3):S347‐S348. [Google Scholar]
- Fu YX, Ran KQ, Wang M. Prevention of early postpartum hemorrhage by way of oral misoprostol. Journal of Nursing Science 2003;18(12):910‐1. [Google Scholar]
- Garg P, Batra S, Gandhi G. Oral misoprostol versus injectable methylergometrine in management of the third stage of labor. International Journal of Gynecology & Obstetrics 2005;91(2):160‐1. [DOI] [PubMed] [Google Scholar]
- Gavilanes P, Morales MF, Velasco S, Teran E. Sublingual misoprostol is as effective as intravenous oxytocin to reduce intra‐operative blood loss during cesarean delivery in women living at high altitude. Journal of Maternal‐Fetal & Neonatal Medicine 2016;29(4):559‐61. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld TS, Wing DA. Rectal misoprostol versus intravenous oxytocin for the prevention of postpartum hemorrhage after vaginal delivery. American Journal of Obstetrics and Gynecology 2001;185:878‐82. [DOI] [PubMed] [Google Scholar]
- Gulmezoglu AM, Villar J, Ngoc NTN, Piaggio G, Carroli G, Adetoro L, et al. WHO multicentre randomised trial of misoprostol in the management of the third stage of labour. Lancet 2001;358:689‐95. [DOI] [PubMed] [Google Scholar]; Lumbiganon P, Hofmeyr J, Gulmezoglu AM, Pinol A, Villar J. Misoprostol dose‐related shivering and pyrexia in the third stage of labour Who collaborative trial of misoprostol in the management of the third stage of labour. British Journal of Obstetrics and Gynaecology 1999;106(4):304‐8. [DOI] [PubMed] [Google Scholar]; Lumbiganon P, Villar J, Piaggio G, Gulmezoglu AM, Adetoro L, Carroli G. Side effects of oral misoprostol during the first 24 hours after administration in the third stage of labour. BJOG: an international journal of obstetrics and gynaecology 2002;109:1222‐6. [DOI] [PubMed] [Google Scholar]
- Gupta B, Jain V, Aggarwal N. Rectal misoprostol versus oxytocin in the prevention of postpartum hemorrhage ‐ a pilot study. International Journal of Gynecology & Obstetrics 2006;94(Suppl 2):S139‐S140. [DOI] [PubMed] [Google Scholar]
- Hamm J, Russell Z, Botha T, Carlan SJ, Richichi K. Buccal misoprostol to prevent hemorrhage at cesarean delivery: a randomized study. American Journal of Obstetrics and Gynecology 2005;192:1404‐6. [DOI] [PubMed] [Google Scholar]
- Harriott J, Christie L, Wynter S, DaCosta V, Fletcher H, Reid M. A randomized comparison of rectal misoprostol with syntometrine on blood loss in the third stage of labour. West Indian Medical Journal 2009;58(3):201‐6. [PubMed] [Google Scholar]
- Hofmeyr GJ, Nikodem C, Jager M, Drakely A, Gilbart B. Oral misoprostol for labour third stage management: randomised assessment of side effects. Proceedings of the 17th Conference on Priorities in Perinatal Care; 1998; South Africa. 1998:53‐4. [Google Scholar]; Hofmeyr GJ, Nikodem VC, Jager M, Gelbart BR. A randomised placebo controlled trial of oral misoprostol in the third stage of labour. British Journal of Obstetrics and Gynaecology 1998;105(9):971‐5. [DOI] [PubMed] [Google Scholar]; Hofmeyr GJ, Jager M, Rose L, Nikodem VC, Lawrie T. Misoprostol for third stage of labour management: a double blind, placebo controlled clinical trial. Proceedings of the 16th Conference on Priorities in Perinatal Care; 1997; South Africa. 1997:29‐31. [Google Scholar]
- Hofmeyr GJ, Nikodem VC, Jager M, Drakely AJ. Side effects of oral misoprostol in the third stage of labour: a random allocation placebo controlled trial. Journal of Obstetrics & Gynaecology 2000;20(Suppl 1):S40‐1. [Google Scholar]; Hofmeyr GJ, Nikodem VC, Jager M, Drakely A. Side‐effects of oral misoprostol in the third stage of labour‐‐a randomised placebo‐controlled trial. South African Medical Journal 2001;91(5):432‐5. [PubMed] [Google Scholar]
- Hofmeyr GJ, Fawole B, Mugerwa K, Godi NP, Blignaut Q, Mangesi L, et al. Administration of 400mug of misoprostol to augment routine active management of the third stage of labor. International Journal of Gynecology and Obstetrics 2011;112(2):98‐102. [DOI] [PubMed] [Google Scholar]; NCT00124540. Misoprostol for preventing postpartum hemorrhage. clinicaltrials.gov/ct2/show/NCT00124540 Date first received: 26 July 2005.
- Hoj L, Cardoso P, Nielsen BB, Hvidman L, Nielsen J, Aaby P. Effect of sublingual misoprostol on severe postpartum haemorrhage in a primary health centre in Guinea‐Bissau: randomised double blind clinical trial. BMJ 2005;331:723‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nielsen BB, Hoj L, Hvidman LE, Nielsen J, Cardoso P, Aaby P. Reduced post‐partum bleeding after treatment with sublingual misoprostol: a randomized double‐blind clinical study in a developing country ‐ secondary publication [Reduceret post partum‐blodning efter sublingval misoprostol: et randomiseret dobbeltblindt klinisk studie i et udviklingsland ‐ sekundaerpublikation]. Ugeskrift for Laeger 2006;168(13):1341‐3. [PubMed] [Google Scholar]
- Hong SC, Kim JW, Park HT, Seol HJ, Kim HJ, Kim SH, et al. Additional rectal misoprostol plus intravenous oxytocin versus intravenous oxytocin for the prevention of postpartum hemorrhage after cesarean section. American Journal of Obstetrics and Gynecology 2007;197(6 Suppl 1):S99, Abstract no: 321. [Google Scholar]
- Is S, Gr V, Keranahalli S. Comparison of intramuscular ergometrine and per rectal misoprostol for prophylaxis against atonic post patrum haemorrhage. International Journal of Gynecology & Obstetrics 2012;119(Suppl 3):S797‐S798. [Google Scholar]
- Jago AA, Ezechi OC, Achinge GI, Okunlola MA. Effect of oxytocics on the blood pressure of normotensive Nigerian parturients. Journal of Maternal‐Fetal & Neonatal Medicine 2007;20(9):703‐5. [DOI] [PubMed] [Google Scholar]
- Jangsten E, Mattsson L, Lyckestam I, Hellstro¨m A, Berg M. A comparison of active management and expectant management of the third stage of labour: a Swedish randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2011;118:362–9. [DOI] [PubMed] [Google Scholar]
- Jerbi M, Hidar S, Elmoueddeb, Chaieb A, Khairi H. Oxytocin in the third stage of labor. International Journal of Gynecology & Obstetrics 2007;96(3):198‐9. [DOI] [PubMed] [Google Scholar]
- Jirakulsawas J, Khooarmompattana S. Comparison of oral misoprostol and intramuscular methylergonovine for prevention of postpartum hemorrhage. Thai Journal of Obstetrics and Gynaecology 2000;12(4):332. [Google Scholar]
- Karkanis SG, Caloia D, Salenieks ME, Kingdom J, Walker M, Meffe F, et al. Randomized controlled trial of rectal misoprostol versus oxytocin in third stage management. Journal of Obstetrics and Gynaecology Canada: JOGC 2002;24(2):149‐54. [DOI] [PubMed] [Google Scholar]
- Kerekes L, Domokos N. The effect of prostaglandin F2alpha on third stage labour. Prostaglandins 1979;18:161‐6. [DOI] [PubMed] [Google Scholar]
- Khan GQ, John IS, Chan T, Wani S, Hughes AO, Stirrat GM. Abu Dhabi third stage trial: oxytocin vs syntometrine in the active management of the third stage of labour. European Journal of Obstetrics & Gynecology and Reproductive Biology 1995;58:147‐51. [DOI] [PubMed] [Google Scholar]; Khan GQ, Susheela J I, Chan T, Wani S, Hughes AO, Stirrat GM. Abu Dhabi third stage trial: oxytocin versus syntometrine in the active management of the third stage of labour. 27th British Congress of Obstetrics and Gynaecology; 1995 July 4‐7; Dublin, Ireland. 1995:Abstract no: 212. [DOI] [PubMed] [Google Scholar]
- Kikutani T, Kikutani M, Oshima M, Sugimoto K, Shimada Y. Effects of methylergometrine and oxytocin on blood loss and uterine contraction during cesarean section. Masui ‐ Japanese Journal of Anesthesiology 2006;55(5):590‐4. [PubMed] [Google Scholar]
- Kumru S, Gurates B, Parmaksiz C. Investigation of the usefulness of methyl ergonovine application in cesarean section cases [Sezaryen olgularinda metil ergonovin uygulamasinin yararliliginin arastirilmasi]. Journal of the Turkish German Gynecology Association Artemis 2005;6(1):42‐5. [Google Scholar]
- Kundodyiwa TW, Majoko F, Rusakaniko S. Misoprostol versus oxytocin in the third stage of labor. International Journal of Gynecology & Obstetrics 2001;75:235‐41. [DOI] [PubMed] [Google Scholar]
- Lam H, Tang OS, Lee CP, Ho PC. A pilot‐randomized comparison of sublingual misoprostol with syntometrine on the blood loss in third stage of labor. Acta Obstetricia et Gynecologica Scandinavica 2004;83:647‐50. [DOI] [PubMed] [Google Scholar]
- Lapaire O, Schneider MC, Stotz M, Surbek DV, Holzgreve W, Hoesli IM. Oral misoprostol vs. intravenous oxytocin in reducing blood loss after emergency cesarean delivery. International Journal of Gynecology & Obstetrics 2006;95(1):2‐7. [DOI] [PubMed] [Google Scholar]
- Leung SW, Ng PS, Wong WY, Cheung TH. A randomised trial of carbetocin versus syntometrine in the management of the third stage of labour. BJOG: an international journal of obstetrics and gynaecology 2006;113:1459‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokugamage AU, Moodley J, Sullivan K, Rodeck CH, Niculescu L, Tigere P. The Durban primary postpartum haemorrhage study. Women's Health ‐ into the new millennium. Proceedings of the 4th International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1999 October 3‐6; Cape Town South Africa. RCOG, 1999:77‐8. [Google Scholar]; Lokugamage AU, Paine M, Bassau‐Balroop H, El‐Refaey K, Sullivan K, Rodek C. Active management of the third stage at caesarean section: misoprostol vs syntocinon. XVI FIGO World Congress of Obstetrics & Gynecology; 2000 Sept 3‐8; Washington DC, USA. 2000; Vol. Book 2:54. [Google Scholar]; Lokugamage AU, Paine M, Bassaw‐Balroop K, Sullivan KR, El‐Refaey H, Rodeck CH. Active management of the third stage at caesarean section: a randomised controlled trial of misoprostol versus syntocinon. Australian & New Zealand Journal of Obstetrics & Gynaecology 2001;41(4):411‐4. [DOI] [PubMed] [Google Scholar]
- Lumbiganon P, Hofmeyr J, Gülmezoglu AM, Pinol A, Villar J. Misoprostol dose‐related shivering and pyrexia inthe third stage of labour. British Journal of Obstetrics and Gynaecology 1999;106:304‐8. [DOI] [PubMed] [Google Scholar]
- Maged AM, Hassan AM, Shehata NA. Carbetocin versus oxytocin for prevention of postpartum hemorrhage after vaginal delivery in high risk women. Journal of Maternal‐Fetal & Neonatal Medicine 2016;29(4):532‐6. [DOI] [PubMed] [Google Scholar]
- McDonald S. The Perth third stage oxytocic trial. Proceedings of National Conference on Research in Midwifery; 1992; Reading, UK. 1992. [Google Scholar]; McDonald S, Prendiville WJ. A randomized controlled trial of syntocinon vs syntometrine as part of the active management of the third stage of labour. Journal of Perinatal Medicine 1992;20(Suppl 1):97. [Google Scholar]; McDonald S, Prendiville WJ, Blair E. A randomised controlled trial of syntocinon vs syntometrine as part of the active management of the third stage of labour. Proceedings of 26th British Congress of Obstetrics and Gynaecology; 1992 July 7‐10; Manchester, UK. 1992:87. [Google Scholar]; McDonald SJ. Management in the third stage of labour [thesis]. University of Western Australia, 1996. [Google Scholar]; McDonald SJ, Prendiville WJ, Blair E. Randomised controlled trial of oxytocin alone vs oxytocin and ergometrine in active management of third stage of labour. BMJ 1993;307:1167‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GG, Elbourne DR. The Salford third stage trial: oxytocin plus ergometrine vs oxytocin alone in the active management of the third stage of labor. Online Journal of Current Clinical Trials 1993;2:Doc 83. [PubMed] [Google Scholar]
- Mobeen N, Durocher J, Zuberi NF, Jahan N, Blum J, Wasim S, et al. Administration of misoprostol by trained traditional birth attendants to prevent postpartum haemorrhage in homebirths in Pakistan: a randomised placebo‐controlled trial. BJOG: an international journal of obstetrics and gynaecology 2011;118:353‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mobeen N, Durocher J, Zuberi NF, Jahan N, Blum J, Wasim S, et al. Use of misoprostol by trained traditional birth attendants to prevent postpartum haemorrhage during home deliveries in Pakistan: a randomised placebo‐controlled trial. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S92. [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT00120237. Misoprostol for the prevention of postpartum hemorrhage in rural Pakistan. clinicaltrials.gov/ct2/show/NCT00120237 Date first received: 7 July 2005.
- Moertl M, Kraschl J, Friedrich S, Pickel K, Ulrich D, Eder M, et al. Hemodynamic changes of carbetocin and oxytocin in women undergoing cesarean section. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S112. [Google Scholar]; Moertl MG, Friedrich S, Kraschl J, Wadsack C, Lang U, Schlembach D. Haemodynamic effects of carbetocin and oxytocin given as intravenous bolus on women undergoing caesarean delivery: a randomised trial. BJOG: an international journal of obstetrics and gynaecology 2011;118(11):1349‐56. [DOI] [PubMed] [Google Scholar]; Mortl M, Pickel K, Friedrich S, Ulrich D, Lang U, Schlembach D. Hemodynamic changes of carbetocin and oxytocin given as i.v. bolus on women undergoing cesarean section. Geburtshilfe und Frauenheilkunde 2008;68:S46. [Google Scholar]
- Moir DD, Amoa AB. Ergometrine or oxytocin? Blood loss and side‐effects at spontaneous vertex delivery. British Journal of Anaesthesia 1979;51(2):113‐7. [DOI] [PubMed] [Google Scholar]
- Moodie JE, Moir DD. Ergometrine, oxytocin and extradural analgesia. British Journal of Anaesthesia 1976;48:571‐4. [DOI] [PubMed] [Google Scholar]
- Mukta M, Sahay PB. Role of misoprostol 600 mcg oral in active management of third stage of labor: a comparative study with oxytocin 10 IU i.m. Journal of Obstetrics and Gynecology of India 2013;6:325‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa AO, Ijaiya MA, Saidu R, Aboyeji AP, Jimoh AA, Adesina KT, et al. Double‐blind randomized controlled trial comparing misoprostol and oxytocin for management of the third stage of labor in a Nigerian hospital. International Journal of Gynecology & Obstetrics2015; Vol. 129, issue 3:227‐30. [DOI] [PubMed]
- Nasr A, Shahin AY, Elsamman AM, Zakherah MS, Shaaban OM. Rectal misoprostol versus intravenous oxytocin for prevention of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2009;105(3):244‐7. [DOI] [PubMed] [Google Scholar]
- Ng PS, Chan ASM, Sin WK, Tang LCH, Cheung KB, Yuen PM. A multicentre randomized controlled trial of oral misoprostol and im syntometrine in the management of the third stage of labour. Human Reproduction 2001;16(1):31‐5. [DOI] [PubMed] [Google Scholar]; Ng PS, Chan ASM, Sin WK, Tang LCH, Cheung KB, Yuen PM. Comparison of oral misoprostol and intramuscular syntometrine in the management of the third stage of labor ‐ a multicenter randomised controlled trial. XVI FIGO World Congress of Obstetrics & Gynecology. Book 4; 2000 Sept 3‐8; Washington DC, USA. 2000:29. [Google Scholar]
- Ng PS, Lai CY, Sahota DS, Yuen PM. A double‐blind randomized controlled trial of oral misoprostol and intramuscular syntometrine in the management of the third stage of labor. Gynecologic and Obstetric Investigation 2007;63(1):55‐60. [DOI] [PubMed] [Google Scholar]; Yuen PM, Ng PS, Sahota DS. A double‐blind randomised controlled trial of oral misoprostol in addition to intra‐muscular syntometrine in the management of the third stage of labour. 30th British Congress of Obstetrics and Gynaecology; 2004 July 7‐9; Glasgow, UK. 2004:62. [Google Scholar]
- Nirmala K, Zainuddin AA, Ghani NA, Zulkifli S, Jamil MA. Carbetocin versus syntometrine in prevention of post‐partum hemorrhage following vaginal delivery. Journal of Obstetrics and Gynaecology Research 2009;35(1):48‐54. [DOI] [PubMed] [Google Scholar]
- Nordstrom L, Fogelstam K, Fridman G, Larsson A, Rydhstroem H. Routine oxytocin in the third stage of labour: a placebo controlled randomised trial. British Journal of Obstetrics and Gynaecology 1997;104(7):781‐6. [DOI] [PubMed] [Google Scholar]
- Oboro VO, Tabowei TO. A randomised controlled trial of misoprostol versus oxytocin in the active management of the third stage of labour. Journal of Obstetrics & Gynaecology 2003;23(1):13‐6. [DOI] [PubMed] [Google Scholar]
- Ogunbode O, Obisesan K, Ayeni O. Methergin in the management of the third stage of labor: a comparative clinical trial with syntometrine and ergometrine. Current Therapeutic Research, Clinical and Experimental 1979;26:460‐5. [Google Scholar]
- Orji E, Agwu F, Loto O, Olaleye O. A randomized comparative study of prophylactic oxytocin versus ergometrine in the third stage of labor. International Journal of Gynecology & Obstetrics 2008;101(2):129‐32. [DOI] [PubMed] [Google Scholar]
- Ortiz‐Gomez JR, Morillas‐Ramirez F, Fornet‐Ruiz I, Palacio‐Abizanda FJ, Bermejo‐Albares L. [Clinical and pharmacological study of the efficacy of carbetocin in elective caesareans compared to low and usual doses of oxytocin]. [Spanish]. Revista Espanola de Anestesiologia y Reanimacion 2013;60(1):7‐15. [DOI] [PubMed] [Google Scholar]
- Owonikoko KM, Arowojolu AO, Okunlola MA. Effect of sublingual misoprostol versus intravenous oxytocin on reducing blood loss at cesarean section in Nigeria: A randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2011;37(7):715‐21. [DOI] [PubMed] [Google Scholar]
- Parsons SM, Walley RL, Crane JM, Matthews K, Hutchens D. Oral misoprostol versus oxytocin in the management of the third stage of labour. Journal of Obstetrics and Gynaecology Canada 2006;28(1):20‐6. [DOI] [PubMed] [Google Scholar]
- Parsons S, Ntumy YM, Walley RL, Wilson JB, Crane JMG, Matthews K, et al. Rectal misoprostol vs intramuscular oxytocin in the routine management of the third stage of labour. 30th British Congress of Obstetrics and Gynaecology; 2004 July 7‐9; Glasgow, UK. 2004:18. [Google Scholar]; Parsons SM, Walley RL, Crane JM, Matthews K, Hutchens D. Rectal misoprostol versus oxytocin in the management of the third stage of labour. Journal of Obstetrics and Gynaecology Canada 2007;29(9):711‐8. [DOI] [PubMed] [Google Scholar]
- Penaranda WA, Arrieta OB, Yances BR. Active management of childbirth with sublingual misoprostol: a controlled clinical trial in the Hospital de Maternidad Rafael Calvo [Manejo activo del alumbramiento con misoprostol sublingual: un estudio clinico controlado en al hospital de maternidad rafael calvo de cartagena]. Revista Colombiana de Obstetricia y Ginecologia 2002;53(1):87‐92. [Google Scholar]
- Prendiville WJ, Harding JE, Elbourne DR, Stirrat GM. The Bristol third stage trial: active versus physiological management of third stage of labour. BMJ 1988;297(6659):1295‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaei M, Karimi S, Shahboodaghi Z, Mahboobi H, Khorgoei T, Rajaei F. Safety and efficacy of misoprostol versus oxytocin for the prevention of postpartum hemorrhage. Journal of Pregnancy 2014;2014:713879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez O, Benito V, Jimenez R, Valido C, Hernandez C, Garcia JA. Third stage of labour: active or expectant management? preliminary results [abstract]. Journal of Perinatal Medicine 2001;Suppl 1(Pt 2):364. [Google Scholar]
- Rashid M, Clark A, Rashid MH. A randomised controlled trial comparing the efficacy of intramuscular syntometrine and intravenous syntocinon, in preventing postpartum haemorrhage. Journal of Obstetrics and Gynaecology 2009;29(5):396‐401. [DOI] [PubMed] [Google Scholar]
- Ray A, Mukherjee P, Basu G, Chatterjee A. Misoprostol and third stage of labour. Journal of Obstetrics and Gynecology of India 2001;51(6):53‐4. [Google Scholar]
- Reyes OA, Gonzalez GM. Carbetocin versus oxytocin for prevention of postpartum hemorrhage in patients with severe preeclampsia: a double‐blind randomized controlled trial. Journal of Obstetrics and Gynaecology Canada: JOGC 2011;33(11):1099‐104. [DOI] [PubMed] [Google Scholar]
- Reyes OA. Carbetocin vs. oxytocin for the prevention of postpartum hemorrhage grand multipara patients: randomized controlled trial [Carbetocina vs. oxitocina para la prevención de hemorragia posparto en pacientes grandes multíparas: estudio aleatorizado controlado]. Clinica e Investigacion en Ginecologia y Obstetricia 2011;38:2‐7. [Google Scholar]
- Rogers J, Wood J, McCandlish R, Ayers S, Truesdale A, Elbourne D. Active versus expectant management of third stage of labour: the Hinchingbrooke randomised controlled trial. Lancet 1998;351(9104):693‐9. [DOI] [PubMed] [Google Scholar]
- Gawecka E, Rosseland LA. A secondary analysis of a randomized placebo‐controlled trial comparing the analgesic effects of oxytocin with carbetocin: postcesarean delivery morphine equivalents. Anesthesia and Analgesia 2014;119(4):1004. [DOI] [PubMed] [Google Scholar]; Rosseland LA, Hauge TH, Grindheim G, Stubhaug A, Langesaeter E. Changes in blood pressure and cardiac output during cesarean delivery: the effects of oxytocin and carbetocin compared with placebo. Anesthesiology 2013;119(3):541‐51. [DOI] [PubMed] [Google Scholar]
- Rozenberg P, Quibel T, Ghout I, Salomon L, Bussiere L, Goffinet F. Active management of the third stage of labor with routine oxytocin and misoprostol for the prevention of postpartum hemorrhage: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2015;212(1 Suppl 1):S18. [DOI] [PubMed] [Google Scholar]
- Sadiq UG, Kwanashie O, Mairiga G, Gamaniel S, Isa H, Abdu A, et al. A randomised clinical trial comparing the efficacy of oxytocin injection and oral misoprostol tablet in the prevention of postpartum haemorrhage in Maiduguri Nigeria. International Research Journal of Pharmacy 2011;2(8):76‐81. [Google Scholar]
- Samimi M, Imani‐Harsini A, Abedzadeh‐Kalahroudi M. Carbetocin vs. syntometrine in prevention of postpartum hemorrhage: a double blind randomized control trial. Iranian Red Crescent Medical Journal 2013;15(9):817‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha A, Dongol A, Chawla CD, Adhikari RK. Rectal misoprostol versus intramuscular oxytocin for prevention of post partum hemorrhage. Kathmandu University Medical Journal 2011;33(1):8‐12. [DOI] [PubMed] [Google Scholar]
- Singh G, Radhakrishnan G, Guleria K. Comparison of sublingual misoprostol, intravenous oxytocin, and intravenous methylergometrine in active management of the third stage of labor. International Journal of Gynecology & Obstetrics 2009;107(2):130‐4. [DOI] [PubMed] [Google Scholar]
- Soltan MH, El‐Gendi E, Imam HH, Fathi O. Different doses of sublingual misoprostol versus methylergometrine for the prevention of atonic postpartum haemorrhage. International Journal of Health Sciences 2007;1(2):229‐36. [PMC free article] [PubMed] [Google Scholar]
- Sood AK, Singh S. Sublingual misoprostol to reduce blood loss at cesarean delivery. Journal of Obstetrics and Gynaecology of India 2012;62(2):162‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01108302. Effectiveness, safety and feasibility of auxiliary nurse midwives' (ANM) use of oxytocin in uniject™ to prevent postpartum hemorrhage in India. clinicaltrials.gov/ct2/show/NCT01108302 Date first received: 2 April 2010. ; Stanton CK, Newton S, Mullany LC, Cofie P, Agyemang CT, Adiibokah E, et al. Impact on postpartum hemorrhage of prophylactic administration of oxytocin 10 IU via Uniject by peripheral health care providers at home births: Design of a community‐based cluster‐randomized trial. BMC Pregnancy and Childbirth 2012;12:42. [DOI] [PMC free article] [PubMed] [Google Scholar]; Stanton CK, Newton S, Mullany LC, Cofie P, Tawiah Agyemang C, Adiibokah E, et al. Effect on postpartum hemorrhage of prophylactic oxytocin (10 IU) by injection by community health officers in Ghana: a community‐based, cluster‐randomized trial. PLoS Medicine. NCT01108289 2013; Vol. 10, issue 10:e1001524. [DOI] [PMC free article] [PubMed]
- Su LL, Rauff M, Chan YH, Mohamad Suphan N, Lau TP, Biswas A, et al. Carbetocin versus syntometrine for the third stage of labour following vaginal delivery‐‐a double‐blind randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2009;116(11):1461‐6. [DOI] [PubMed] [Google Scholar]
- Sultana N, Khatun M. Misoprostol versus oxytocin in the active management of the third stage of labour. Journal of Bangladesh College of Physicians and Surgeons 2007;25(2):73‐6. [Google Scholar]
- Surbek DV, Fehr PM, Hoesli I, Holzgreve W. Misoprostol for prevention of postpartum hemorrhage: a randomized controlled trial [abstract]. XVI FIGO World Congress of Obstetrics & Gynecology; 2000 Sept 3‐8; Washington DC, USA 2000;Book 1:33. [Google Scholar]; Surbek DV, Fehr PM, Hoesli I, Holzgreve W. Oral misoprostol vs placebo for third stage of labour [Orales misoprostol reduziert den postpartalen blutverlust]. Gynakologisch Geburtshilfliche Rundschau 1999;39:144. [Google Scholar]
- Tewatia R, Rani S, Srivastav U, Makhija B. Sublingual misoprostol versus intravenous oxytocin in prevention of post‐partum hemorrhage. Archives of Gynecology and Obstetrics 2014;289:739‐42. [DOI] [PubMed] [Google Scholar]
- Thilaganathan B, Cutner A, Latimer J, Beard R. Management of the third stage of labour in women at low risk of postpartum haemorrhage. European Journal of Obstetrics & Gynecology and Reproductive Biology 1993;48:19‐22. [DOI] [PubMed] [Google Scholar]
- Ugwu IA, Enabor OO, Adeyemi AB, Lawal OO, Oladokun A, Olayemi O. Sublingual misoprostol to decrease blood loss after caesarean delivery: a randomised controlled trial. Journal of Obstetrics and Gynaecology 2014;34(5):407‐11. [DOI] [PubMed] [Google Scholar]
- Uncu Y, Karahasan M, Uyaniklar O, Uncu G. Prophylactic misoprostol for the prevention of postpartum hemorrhage: a randomized controlled trial. European Review for Medical and Pharmacological Sciences 2015;19(1):15‐22. [PubMed] [Google Scholar]
- Un Nisa S, Usmani SY. Role of intravenous syntocinon in prevention of primary postpartum haemorrhage. Pakistan Journal of Medical and Health Sciences 2012;6(4):1020‐4. [Google Scholar]
- Vagge DS, Mamatha KR, Rohatgi V. A comparative study to assess the efficacy and tolerability of per rectal misoprostol versus intravenous oxytocin in prevention of primary postpartum haemorrhage in a tertiary care hospital. Indian Journal of Pharmacology 2013;45 Suppl:S45. [Google Scholar]; Vagge DS, Mamatha KR, Shivamurthy G, Rohatgi V. A comparative study to assess the efficacy and tolerability of per rectal misoprostol and intravenous oxytocin in prevention of primary postpartum haemorrhage in a tertiary care hospital. Journal of Chemical and Pharmaceutical Research 2014;6(3):1134‐40. [Google Scholar]
- Vaid A, Dadhwal V, Mittal S, Deka D, Misra R, Sharma JB, et al. A randomized controlled trial of prophylactic sublingual misoprostol versus intramuscular methyl‐ergometrine versus intramuscular 15‐methyl PGF2alpha in active management of third stage of labor. Archives of Gynecology and Obstetrics 2009;280(6):893‐7. [DOI] [PubMed] [Google Scholar]
- Verma P, Aggarwal N, Jain V, Suri V. A double‐blind randomized controlled trial to compare sublingual misoprostol with methylergometrine for prevention of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2006;94(Suppl 2):S137‐S138. [DOI] [PubMed] [Google Scholar]
- Vimala N, Mittal S, Kumar S, Dadhwal V, Mehta S. Sublingual misoprostol versus methylergometrine for active management of third stage of labor. International Journal of Gynecology & Obstetrics 2004;87:1‐5. [DOI] [PubMed] [Google Scholar]
- Vimala N, Mittal S, Kumar S. Sublingual misoprostol versus oxytocin infusion to reduce blood loss at cesarean section. International Journal of Gynecology & Obstetrics 2006;92(2):106‐10. [DOI] [PubMed] [Google Scholar]
- Walley RL, Wilson JB, Crane JM, Matthews K, Sawyer E, Hutchens D. A double‐blind placebo controlled randomised trial of misoprostol and oxytocin in the management of the third stage of labour. BJOG: an international journal of obstetrics and gynaecology 2000;107(9):1111‐5. [DOI] [PubMed] [Google Scholar]
- Whigham CA, Gorelik A, Loughnan T, Trivedi A. Carbetocin versus oxytocin in active labour. BJOG: An International Journal of Obstetrics and Gynaecology 2014;121(Suppl 2):88. [Google Scholar]
- Yuen PM, Chan NST, Yim SF, Chang AMZ. A randomised double blind comparison of syntometrine and syntocinon in the management of the third stage of labour. British Journal of Obstetrics and Gynaecology 1995;102:377‐80. [DOI] [PubMed] [Google Scholar]
- Zachariah ES, Naidu M, Seshadri L. Oral misoprostol in the third stage of labor. International Journal of Gynecology & Obstetrics 2006;92(1):23‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Abdel‐Aleem H, Abol‐Oyoun EM, Moustafa SAM, Kamel HS, Abdel‐Wahab HA. Carboprost trometamol in the management of the third stage of labor. International Journal of Gynecology & Obstetrics 1993;42:247‐50. [DOI] [PubMed] [Google Scholar]
- Abdel‐Aleem H. Management of the third stage of labour with carboprost trometamol in high risk patients for postpartum haemorrhage. Personal communication1997. ; Abdel‐Aleem H, Mostafa SAM, Makarem MH, Abol‐Oyoun EM, Makhlouf A, Shoukry M. Management of the third stage of labour with carboprost trometamol in high risk patients for postpartum hemorrhage. Research activities on reproductive health: annual report of Assiut University Department of Obstetrics and Gynecology November 1997. Assiut University, Faculty of Medicine, 1997:75. [Google Scholar]
- Abdel‐Aleem H, Alhusaini TK, Abdel‐Aleem MA, Menoufy M, Gulmezoglu AM. Effectiveness of tranexamic acid on blood loss in patients undergoing elective cesarean section: randomized clinical trial. Journal of Maternal‐Fetal & Neonatal Medicine 2013;26(17):1705‐9. [DOI] [PubMed] [Google Scholar]
- Abdollahy F. Comparison effect of oxytocin and normal salin injection intra umbelical venuse [abstract]. Gynecological Endocrinology 2000;14(Suppl 2):49. [Google Scholar]
- Al‐Harazi AH, Frass KA. Sublingual misoprostol for the prevention of postpartum hemorrhage. Saudi Medical Journal 2009;30(7):912‐6. [PubMed] [Google Scholar]
- Anandakrishnan S, Balki M, Farine D, Seaward G, Carvalho JC. Carbetocin at elective cesarean delivery: a randomized controlled trial to determine the effective dose, part 2. Canadian Journal of Anaesthesia 2013;60(11):1054‐60. [DOI] [PubMed] [Google Scholar]
- Anjaneyulu R, Devi PK, Kanthamani CR, Vijaya R, Raghavan KS. Prophylactic use of 15(S)15 methyl PGF2alpha by intramuscular route ‐ a controlled clinical trial. Acta Obstetricia et Gynecologica Scandinavica Supplementa 1988;145:9‐11. [DOI] [PubMed] [Google Scholar]
- Anvaripour A, Shahryari H, Ahmadi S, Ghasemi S, Mirzaei K. Comparison the effects of oxytocin and methylergonovine in elective caesarean section under spinal anesthesia. Archives of Gynecology and Obstetrics 2013;287(5):979‐83. [DOI] [PubMed] [Google Scholar]
- Athavale RD, Nerurkar NM, Dalvi SA, Bhattacharya MS. Umbilical vein oxytocin in the management of third stage of labour. Journal of Postgraduate Medicine 1991;37(4):219‐20. [PubMed] [Google Scholar]
- Ayedi M, Zouche I, Smaoui L, Bouaziz I, Smaoui M, Kolsi K. Comparison of 2 versus 5 units of oxytocin in caesarean section. European Journal of Anaesthesiology 2011;28 Suppl:159‐60. [Google Scholar]
- Ayedi M, Jarraya A, Smaoui M, Zouari J, Smaoui L, Kolsi K. Effect of tranexamic acid on post partum hemorrhage by uterine atony: a preliminary result of a randomized, placebo controlled trial. European Journal of Anaesthesiology 2011;28 Suppl 1:165. [Google Scholar]
- Aziz S, Kazi S, Haq G, Soomro N. Oral misoprostol versus oxytocin in the management of third stage of labour. JPMA ‐ Journal of the Pakistan Medical Association 2014;64(4):428‐32. [PubMed] [Google Scholar]
- Bader W, Ast S, Hatzmann W. The significance of acupuncture in the third stage of labour [Dit Bedeutung der Akupunktur in der Plazentarperiode]. Deutsche Zeitschrift fur Akupunktur 2000;43:264‐8. [Google Scholar]; Bader W, Ast S, Reinehr J, Hackmann J, Hatzmann W. Oxytocin versus acupuncture in the third stage of labour ‐ a prospective randomized study [Oxytocin versus Akupunktur in der Plazentarperiode ‐ eine prospektiv randomisierte Studie]. Geburtshilfe und Frauenheilkunde 2000;60 Suppl 1:S73. [Google Scholar]
- Badhwar L, Singh K, Sethi N, Gupta I, Aggarwal N. The value of nipple stimulation in the management of third stage of labour. International Journal of Gynecology & Obstetrics 1991;36 Suppl:16. [Google Scholar]
- Bai J, Sun Q, Zhai H. A comparison of oxytocin and carboprost tromethamine in the prevention of postpartum hemorrhage in high‐risk patients undergoing cesarean delivery. Experimental and Therapeutic Medicine 2014;7(1):46‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balki M, Ronayne M, Davies S, Fallah S, Kingdom J, Windrim R, et al. Minimum oxytocin dose requirement after cesarean delivery for labor arrest. Obstetrics & Gynecology 2006;107(1):45‐50. [DOI] [PubMed] [Google Scholar]; Balki M, Ronayne M, Davies S, Kingdom J, Windrim R, Carvalho J. Oxytocin requirements at cesarean section for failure to progress in labor: a dose‐finding study [abstract]. Anesthesiology 2005;102(Suppl 1):10. [Google Scholar]
- Banovska J, Goffard P, Suball M, Origer P, Delatte P, Kapessidou P. Efficiency of temporary balloon occlusion of iliac arteries in patients at high hemorrhagic risk undergoing cesarean section. European Journal of Anaesthesiology 2013;30:176. [Google Scholar]
- Barbaro CA, Smith GO. Clinical trial of SE505 ‐ a new oxytocic mixture. Australian and New Zealand Journal of Obstetrics and Gynaecology 1961;1:147‐50. [Google Scholar]
- Baumgarten K, Schmidt J, Horvat A, Neumann M, Cerwenka R, Gruber W, et al. Uterine motility after post‐partum application of sulprostone and other oxytocics. European Journal of Obstetrics & Gynecology and Reproductive Biology 1983;16:181‐92. [DOI] [PubMed] [Google Scholar]
- Bhattacharya P, Devi PK, Jain S, Kanthamani CR, Raghavan KS. Prophylactic use of 15(S)15 methyl PGF2alpha by intramuscular route for control of postpartum bleeding ‐ a comparative trial with methylergometrine. Acta Obstetricia et Gynecologica Scandinavica Supplement 1988;145:13‐5. [DOI] [PubMed] [Google Scholar]
- Bhavana G, Mittal S. Evaluation of efficacy of prophylactic injection tranexamic acid in decreasing blood loss before and after caesarean section. BJOG: an international journal of obstetrics and gynaecology 2013;120(Suppl s1):32. 23107369 [Google Scholar]
- Bider D, Menashe Y, Dulitzky M, Mashiach S, Ben‐Rafael Z. Oxytocin or saline injected intra‐umbilically did not influence the third stage of labor. Acta Obstetricia et Gynecologica Scandinavica 1991;70:321‐3. [DOI] [PubMed] [Google Scholar]
- Bider D, Ben‐Rafael Z, Dulitzky M, Menashe Y, Mashiach S, Barkai G. Effect of intraumbilical prostaglandin F2alpha injection on the third stage of labor. Journal of Reproductive Medicine 1992;37(4):317‐9. [PubMed] [Google Scholar]
- Bisri Y, Redjeki IS, Himendra A. The comparative of effect of bolus‐infusion oxytocine with infusion oxytocine on blood pressure, heart rate, and uterine contraction of women undergoing elective caesarean section with general anesthesia N2O‐sevoflurane. European Journal of Anaesthesiology 2011;28 Suppl:159. [Google Scholar]
- Biswas A, Bal R, Kundu MK, Kyal A, Halder M. A study of prophylactic use of 15‐methyl prostaglandin f2alpha in the active management of third stage of labour. Journal of the Indian Medical Association 2007;105(9):506‐9. [PubMed] [Google Scholar]
- Bivins HA, Cope DA, Newman RB, Eller DP. Randomized trial of intraumbilical vein oxytocin. American Journal of Obstetrics and Gynecology 1993;168:435. [DOI] [PubMed] [Google Scholar]; Bivins HAJ, Cope DA, Newman RB, Eller DP. Randomized trial of intraumbilical vein oxytocin in midtrimester pregnancy losses. American Journal of Obstetrics and Gynecology 1993;169(4):1070‐3. [DOI] [PubMed] [Google Scholar]
- Blum J, Winikoff B, Raghavan S, Dabash R, Ramadan MC, Dilbaz B, et al. Treatment of post‐partum haemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double‐blind, randomised, non‐inferiority trial. Lancet 2010;375(9710):217‐23. [DOI] [PubMed] [Google Scholar]
- Bonham DG. Intramuscular oxytocics and cord traction in third stage of labour. BMJ 1963;2:1620‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonis M, Torricelli M, Leoni L, Berti P, Ciani V, Puzzutiello R, et al. Carbetocin versus oxytocin after caesarean section: similar efficacy but reduced pain perception in women with high risk of postpartum haemorrhage. Journal of Maternal‐Fetal & Neonatal Medicine 2012;25(6):732‐5. [DOI] [PubMed] [Google Scholar]
- Cappiello E, Lugo L, Kodali B, Hepner D, Harnett M, Tsen LC. A double‐blinded, randomized, placebo‐controlled trial of calcium chloride for the augmentation of uterine tone following cesarean delivery [abstract]. Anesthesiology 2006;104(Suppl 1):32. [Google Scholar]
- Carvalho JCA, Balki M, Kingdom J, Windrim R. Oxytocin requirements at elective cesarean delivery: A dose finding study. Obstetrics & Gynecology 2004;104:1005‐10. [DOI] [PubMed] [Google Scholar]
- Catanzarite VA. Prophylactic intramyometrial carboprost tromethamine does not substantially reduce blood loss relative to intramyometrial oxytocin at routine cesarean section. American Journal of Perinatology 1990;7:39‐42. [DOI] [PubMed] [Google Scholar]
- Chaplin AC, George RB, McKeen D, McLeod LC. Up‐down determination of the ED90 of oxytocin infusions for the prevention of postpartum uterine atony in parturients undergoing an elective caesarean delivery. Canadian Journal of Anaesthesia 2009;56(Suppl 1):S62. [DOI] [PubMed] [Google Scholar]
- Chaudhuri P, Mandi S, Mazumdar A. Rectally administrated misoprostol as an alternative to intravenous oxytocin infusion for preventing post‐partum hemorrhage after cesarean delivery. Journal of Obstetrics and Gynaecology Research 2014;40(9):2023‐30. [DOI] [PubMed] [Google Scholar]
- Chestnut DH, Wilcox LL. Influence of umbilical vein administration of oxytocin on the third stage of labor. Proceedings of 19th Annual Meeting of Society for Obstetric Anesthesia and Perinatology; 1987 May 20‐23; Halifax, Nova Scotia, Canada. 1987:49. [Google Scholar]; Chestnut DH, Wilcox LL. Influence of umbilical vein administration of oxytocin on the third stage of labor: a randomized, double‐blind, placebo‐controlled study. American Journal of Obstetrics and Gynecology 1987;157:160‐2. [DOI] [PubMed] [Google Scholar]
- Chou MM, MacKenzie IZ. A prospective, double‐blind, randomized comparison of prophylactic intramyometrial 15‐methyl prostaglandin F2, 125 micrograms, and intravenous oxytocin, 20 units, for the control of blood loss at elective cesarean section. American Journal of Obstetrics and Gynecology 1994;171:1356‐60. [DOI] [PubMed] [Google Scholar]
- Chua S, Chew SL, Yeoh CL, Roy AC, Ho LM, Selamat N, et al. A randomized controlled study of prostaglandin 15‐Methyl F2 alpha compared with syntometrine for prophylactic use in the third stage of labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 1995;35:413‐6. [DOI] [PubMed] [Google Scholar]
- Chukudebelu WO, Marshall AT, Chalmers JA. Use of 'syntometrine' in the third stage of labour. BMJ 1963;1:1390‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN07452238. A study to determine the cardiovascular effects of different methods of administering the oxytocic drug syntocinon. isrctn.com/ISRCTN07452238. ISRCTN07452238 Date first received: 12 September 2003.
- Cordovani D, Balki M, Farine D, Seaward G, Carvalho JCA. Carbetocin at elective cesarean delivery: a randomized controlled trial to determine the effective dose. Canadian Journal of Anesthesia 2012;59(8):751‐7. [DOI] [PubMed] [Google Scholar]
- NCT02080104. Intramuscular versus intravenous prophylactic oxytocin for hemorrhage after vaginal delivery (oxytocin). clinicaltrials.gov/ct2/show/NCT02080104 Date first received: 1 March 2014.
- Dahiya P, Puri M, Rathee S. Influence of intraumbilical oxytocin on the third stage of labour. Indian Journal of Medical Science 1995;49:23‐7. [PubMed] [Google Scholar]
- Daley D. The use of intramuscular ergometrine at the end of the second stage of normal labour. Journal of Obstetrics and Gynaecology of the British Empire 1951;57:388‐97. [DOI] [PubMed] [Google Scholar]
- Daly S, Andolina K, Tolosa JE, Roberts N, Wapner R. A randomized controlled trial of misoprostol versus oxytocin in preventing postpartum blood loss. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S68. [Google Scholar]
- Dao B, Blum J, Barrera G, Cherine Ramadan M, Dabash R, Darwish E, et al. Side effect profiles for misoprostol and oxytocin in the treatment of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S150. [Google Scholar]
- Davies GAL, Tessier JL, Woodman MC, Lipson A, Hahn PM. Maternal hemodynamics after oxytocin bolus compared with infusion in the third stage of labor: a randomized controlled trial. Obstetrics & Gynecology 2005;105:294‐9. [DOI] [PubMed] [Google Scholar]
- Bonis M, Torricelli M, Leoni L, Berti P, Ciani V, Puzzutiello R, et al. Carbetocin versus oxytocin after caesarean section: similar efficacy but reduced pain perception in women with high risk of postpartum haemorrhage. Journal of Maternal‐Fetal & Neonatal Medicine 2012;25(6):732‐5. [DOI] [PubMed] [Google Scholar]
- Dennehy KC, Rosaeg OP, Cicutti NJ, Krepski B, Sylvain JP. Oxytocin injection after caesarean delivery: intravenous or intramyometrial?. Canadian Journal of Anaesthesia 1998;45(7):635‐9. [DOI] [PubMed] [Google Scholar]
- Devi PK, Sutaria UD, Raghavan KS. Prophylactic use of 15(S)15 methyl PGF2alpha for control of postpartum bleeding. Acta Obstetricia et Gynecologica Scandinavica Supplementa 1988;145:7‐8. [DOI] [PubMed] [Google Scholar]
- Diab KM, Ramy AR, Yehia MA. The use of rectal misoprostol as active pharmacological management of the third stage of labor. Journal of Obstetrics and Gynaecology Research 1999;25(5):327‐32. [DOI] [PubMed] [Google Scholar]
- Dickinson JE, Doherty DA. Optimization of third‐stage management after second‐trimester medical pregnancy termination. American Journal of Obstetrics & Gynecology 2009;201(3):303.e1‐303.e7. [DOI] [PubMed] [Google Scholar]
- Dommisse J. The routine use of oxytocic drugs in the third stage of labour [letter]. South African Medical Journal 1980;46:549. [PubMed] [Google Scholar]
- Dong Y. Effects of carboprost on prevention of hemorrhage after induced labor with scarred uterus. Journal of Shanghai Jiaotong University (Medical Science) 2011;31(8):1212‐5. [Google Scholar]
- Durocher J, Blum J, Sheldon WR, Trussell J, Winikoff B. Does the effect of oxytocin prophylaxis on post‐partum blood loss depend on route of administration?. International Journal of Gynecology & Obstetrics 2012;119(Suppl 3):S332. [Google Scholar]
- Dutta DK, Saha KK. Comparative study on role of syntometrine and prostaglandin in the prevention of PPH. XVI FIGO World Congress of Obstetrics & Gynecology; 2000 Sept 3‐8; Washington DC, USA. 2000; Vol. Book 4:29. [Google Scholar]
- Dweck MF, Lynch CM, Spellacy WN. Use of methergine for the prevention of postoperative endometritis in non‐elective cesarean section patients. Infectious Diseases in Obstetrics & Gynecology 2000;8:151‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzuba I, Durocher J, Dilbaz B, Gelisen O, Ngoc NTN, Montesinos R, et al. Route of administration of oxytocin in prevention of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2012;119(Suppl 3):S333. [Google Scholar]
- Elati A, Elmahaishi MS, Elmahaishi MO, Elsraiti OA, Weeks AD. The effect of misoprostol on postpartum contractions: a randomised comparison of three sublingual doses. BJOG: an international journal of obstetrics and gynaecology 2011;118(4):466‐73. [DOI] [PubMed] [Google Scholar]
- Erkkola R, Kero P, Kanto J, Korvenranta H, Nanto V, Peltonen T. Delayed cord clamping in cesarean section with general anesthesia. American Journal of Perinatology 1984;1(2):165‐9. [DOI] [PubMed] [Google Scholar]
- NCT02026297. Tranexamic acid and thromboelastography during cesarean delivery (TA TEG). clinicaltrials.gov/ct2/show/NCT02026297 Date first received: 22 December 2013.
- Farber MK, Schultz R, Lugo L, Liu X, Huang C, Tsen LC. The effect of co‐administration of intravenous calcium chloride and oxytocin on maternal hemodynamics and uterine tone following cesarean delivery: a double‐blinded, randomized, placebo‐controlled trial. International Journal of Obstetric Anesthesia 2015;24(3):217‐24. [DOI] [PubMed] [Google Scholar]
- Fatemeh F, Zohreh S, Abbas MG, Layla H. Maternal haemodynamic effects of oxytocin bolus or infusion in the third stage of labour. Pakistan Journal of Medical Sciences 2011;27(3):656‐9. [Google Scholar]
- Fawzy AEMA, Swelem M, Abdelrehim AI, Titeli S, Elghazal ZS, El‐Gahwagi MM, et al. Active management of third stage of labor by intravenous ergometrine and rectal versus sublingual misoprostol (a double‐center study). Alexandria Journal of Medicine 2012;48(4):381‐5. [Google Scholar]
- Forster FMC. A comparative study of ergometrine and 'methergin' used in the management of the third stage of labour. Medical Journal of Australia 1957;2:155‐6. [Google Scholar]
- Francis HH, Miller JM, Porteous CR. Clinical trial of an oxytocin‐ergometrine mixture (first of two trials). Australian and New Zealand Journal of Obstetrics and Gynaecology 1965;5:47‐51. [DOI] [PubMed] [Google Scholar]
- Friedman EA. Comparative clinical evaluation of postpartum oxytocics. American Journal of Obstetrics and Gynecology 1957;73:1306‐13. [DOI] [PubMed] [Google Scholar]
- Fugo NW, Dieckmann WJ. A comparison of oxytocic drugs in the management of the placental stage. American Journal of Obstetrics and Gynecology 1958;76:141‐6. [DOI] [PubMed] [Google Scholar]
- Gai MY, Wu LF, Su QF, Tatsumoto K. Clinical observation of blood loss reduced by tranexamic acid during and after caesarian section: a multi‐center, randomized trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 2004;112:154‐7. [DOI] [PubMed] [Google Scholar]
- George RB, McKeen D, Chaplin AC, McLeod L. Up‐down determination of the ED(90) of oxytocin infusions for the prevention of postpartum uterine atony in parturients undergoing Cesarean delivery. Canadian Journal of Anaesthesia 2010;57(6):578‐82. [DOI] [PubMed] [Google Scholar]
- Ghulmiyyah LM, Wehbe SA, Saltzman SL, Ehleban C, Sibai BM. Effects of intraumbilical vein injection of saline versus oxytocin plus saline on duration of the third stage of labor: a randomized double‐blind placebo trial [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S18. [Google Scholar]; Ghulmiyyah LM, Wehbe SA, Saltzman SL, Ehleben C, Sibai BM. Intraumbilical vein injection of oxytocin and the third stage of labor: randomized double‐blind placebo trial. American Journal of Perinatology 2007;24(6):347‐52. [DOI] [PubMed] [Google Scholar]
- Gobbur VR, Reddy SV, Bijapur UJ. Efficacy of tranexamic acid in reducing blood loss during lower segment caesarean section. 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:92. [Google Scholar]
- Gohel M, Patel P, Gupta A, Desai P. Efficacy of tranexamic acid in decreasing blood loss during and after cesarean section: a randomized case controlled prospective study. Journal of Obstetrics and Gynaecology of India 2007;57(3):228‐30. [Google Scholar]
- Goswami U, Sarangi S, Gupta S, Babbar S. Comparative evaluation of two doses of tranexamic acid used prophylactically in anemic parturients for lower segment cesarean section: a double‐blind randomized case control prospective trial. Saudi Journal of Anaesthesia 2013;7(4):427‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeber WR, Bishop EH. Methergine and ergonovine in the third stage of labor. Obstetrics & Gynecology 1960;15:85‐8. [PubMed] [Google Scholar]
- Gungorduk K, Asicioglu O, Celikkol O, Olgac Y, Ark C. Use of additional oxytocin to reduce blood loss at elective caesarean section: a randomised control trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2010;50(1):36‐9. [DOI] [PubMed] [Google Scholar]
- Gungorduk K, Asicioglu O, Besimoglu B, Gungorduk OC, Yildirm G, Ark C, et al. Using intraumbilical vein injection of oxytocin in routine practice with active management of the third stage of labor: a randomized controlled trial. Obstetrics & Gynecology 2010;116(3):619‐24. [DOI] [PubMed] [Google Scholar]
- Gungorduk K, Yildirim G, Asicioglu O, Gungorduk OC, Sudolmus S, Ark C. Efficacy of intravenous tranexamic acid in reducing blood loss after elective cesarean section: a prospective, randomized, double‐blind, placebo‐controlled study. American Journal of Perinatology 2011;28(3):233‐40. [DOI] [PubMed] [Google Scholar]
- Gungorduk K, Asicioglu O, Yildirim G, Ark C, Tekirdag AI, Besimoglu B. Can intravenous injection of tranexamic acid be used in routine practice with active management of the third stage of labor in vaginal delivery? A randomized controlled study. American Journal of Perinatology 2013;30(5):407‐13. [DOI] [PubMed] [Google Scholar]
- Gupta M, Bhosale U. Comparative study of methylergometrine and low dose carboprost (PGF2‐x) in active management of 3rd stage labor. BJOG: an international journal of obstetrics and gynaecology 2014;121(Suppl 2):139. [Google Scholar]
- Habek D, Franicevic D. Intraumbilical injection of uterotonics for retained placenta. International Journal of Gynecology & Obstetrics 2007;99(2):105‐9. [DOI] [PubMed] [Google Scholar]
- Hacker NF, Biggs JSG. Blood pressure changes when uterine stimulants are used after normal delivery. British Journal of Obstetrics and Gynaecology 1979;86:633‐6. [DOI] [PubMed] [Google Scholar]
- Häivä L, Hartikainen A. Pharmacological management of third stage of labor in primiparae and multiparae [Kohtua supistavan lääkkeen valinta ensi‐ ja uudelleensynnyttäjille]. Suomen Lääkärilehti 1994;49(33):3442‐4. [Google Scholar]
- Halder S, Samanta B, Sardar R, Chattopadhyay S. Tranexamic acid used before caesarean section reduces blood loss based on pre‐ and postoperative hemoglobin level: A case‐control study. Journal of the Indian Medical Association 2013;111(3):184‐6. [PubMed] [Google Scholar]
- Hoffman M, Castagnola D, Naqvi F. A randomized trial of active versus expectant management of the third stage of labor [abstract]. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S107. [Google Scholar]; Hoffman M, Naqvi F, Sciscione A. A randomized trial of active versus expectant management of the third stage of labor [abstract]. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S82. [Google Scholar]
- Hofmeyr GJ, Ferreira S, Nikodem VC, Mangesi L, Singata M, Jafta Z, et al. Misoprostol for treating postpartum haemorrhage: a randomized controlled trial. BMC Pregnancy and Childbirth 2004;4(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard WF, McFadden PR, Keettel WC. Oxytocic drugs in fourth stage of labor. Journal of the American Medical Association 1964;189:411‐3. [DOI] [PubMed] [Google Scholar]
- Huh W, Chelmow D, Malone FD. A randomized, double‐blinded, placebo controlled trial of oxytocin at the beginning versus the end of the third stage of labor for prevention of postpartum hemorrhage. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S130. [DOI] [PubMed] [Google Scholar]; Huh WK, Chelmow D, Malone FD. A double‐blinded, randomized, controlled trial of oxytocin at the beginning versus the end of the third stage of labour for prevention of postpartum hemorrhage. Gynecologic and Obstetric Investigation 2004;58(2):72‐6. [DOI] [PubMed] [Google Scholar]
- Hunt BJ. Tranexamic acid for the treatment of postpartum haemorrhage‐preliminary results of the woman trial. Transfusion Medicine 2013;23(Suppl 1):7. [Google Scholar]
- Ilancheran A, Ratnam SS. Effect of oxytocics on prostaglandin levels in the third stage of labour. Gynecologic and Obstetric Investigation 1990;29:177‐80. [DOI] [PubMed] [Google Scholar]
- Irons DW, Sriskandabalan P, Bullough CHW. A simple alternative to parenteral oxytocics for the third stage of labor. International Journal of Gynecology & Obstetrics 1994;46:15‐8. [DOI] [PubMed] [Google Scholar]
- Jackson KWJ, Allbert JR, Schemmer GK, Elliot M, Humphrey A, Taylor J. A randomized controlled trial comparing oxytocin administration before and after placental delivery in the prevention of postpartum hemorrhage. American Journal of Obstetrics and Gynecology 2001;185:873‐7. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Wang P, Cao W. Effect on different doses of misoprostol to prevent postpartum hemorrhage. Chinese Nursing Research 2001;15(6):313‐4. [Google Scholar]
- Jin LJ, Zhou L. Application of anus misoprostol to decrease the volume of post partum hemorrhage. Journal of Practical Nursing 2000;16(2):9‐10. [Google Scholar]
- Jolivet A, Robyn C, Huraux‐Rendu C, Gautray JP. Effect of ergot alkaloid derivatives on milk secretion in the immediate postpartum period. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1978;7:129‐34. [PubMed] [Google Scholar]
- Jonsson M, Hanson U, Lidell C, Norden‐Lindeberg S. ST depression at caesarean section and the relation to oxytocin dose. A randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2010;117(1):76‐83. [DOI] [PubMed] [Google Scholar]; Jonsson M, Norden Lindeberg S, Hanson U. ST depression at caesarean section and the relation to oxytocin dose. A randomised controlled trial. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S214. [DOI] [PubMed] [Google Scholar]
- Kashanian M, Fekrat M, Masoomi Z, Sheikh Ansari N. Comparison of active and expectant management on the duration of the third stage of labour and the amount of blood loss during the third and fourth stages of labour:a randomised controlled trial. Midwifery 2010;26:241‐5. [DOI] [PubMed] [Google Scholar]
- Kemp J. Clinical trial of "syntometrine" in the third stage of labour. British Medical Journal 1963;1(5342):1391‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan GQ, John IS, Wani S, Doherty T, Sibai BM. Controlled cord traction versus minimal intervention techniques in delivery of the placenta: a randomized controlled trial. American Journal of Obstetrics and Gynecology 1997;177(4):770‐4. [DOI] [PubMed] [Google Scholar]
- Khan RU, El‐Refaey H. Pharmacokinetics and adverse‐effect profile of rectally administered misoprostol in the third stage of labor. Obstetrics & Gynecology 2003;101(5 Pt 1):968‐74. [DOI] [PubMed] [Google Scholar]
- Khan MS, Sinha SK, Sultana T, Singhal S. Comparison of two oxytocin infusions in patients undergoing emergency cesarean sections: A double blind study. International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S389. [Google Scholar]
- Khanun A, Khanum S. Oral versus rectal misoprostol in the prevention of primary postpartum hemorrage. Pakistan Journal of Medical and Health Sciences 2011;5(3):587‐8. [Google Scholar]
- Khurshid R, Fatima K, Parveen S, Ul Shamas I, Salman R. A comparison between intramuscular PGF2 a125 mG and intravenous methyl ergometrine 0.2 Mg in the active management of third stage labor. Internet Journal of Gynecology and Obstetrics2010; Vol. 14 (Number 1).
- Kikutani T, Shimada Y. Effects of methylergometrine and oxytocin on thoracic epidural pressure during cesarean section. Journal of Obstetrics and Gynaecology Research 2003;29(3):180‐5. [DOI] [PubMed] [Google Scholar]
- Kikutani T, Oshima M, Sugimoto K, Shimada Y. Effects of intravenous infusion rate of oxytocin on thoracic epidural pressure in parturients undergoing elective cesarean section. Journal of Nippon Medical School = Nihon Ika Daigahu Zasshi 2003;70(6):475‐9. [DOI] [PubMed] [Google Scholar]
- King KJ, Douglas J, Unger W, Wong AB. A randomized double‐blind comparison of a 5 unit intravenous oxytocin bolus versus placebo as a strategy to prevent uterine atony at cesarean section in women who are at increased risk of post‐partum hemorrhage [abstract]. Anesthesiology 2006;104(Suppl 1):41. [Google Scholar]; King KJ, Douglas J, Unger W, Wong AB, Espinosa V, King RA. 5U bolus oxytocin at cesarean section in women at risk of atony [abstract]. Anesthesiology 2007;106(Suppl 1):14. [Google Scholar]; King KJ, Douglas MJ, Unger W, Wong A, King RAR. Five unit bolus oxytocin at cesarean delivery in women at risk of atony: a randomized, double‐blind, controlled trial. Anesthesia & Analgesia 2010;111(6):1460‐6. [DOI] [PubMed] [Google Scholar]
- Kintu A, Nakubulwa S, Mijumbi C, Kwizera A, Tindimwebwa J. Uterotonic efficacy of oxytocin 2.5 versus 10 units during caesarean section at mulago hospital: a double blinded placebo controlled randomised clinical trial. British Journal of Anaesthesia 2012;108:ii197‐8. [Google Scholar]
- Kiran S, Anand A, Singh T, Gupta N. Effective dose of oxytocin in caesarean delivery. British Journal of Anaesthesia 2012;108:ii195. [Google Scholar]
- Kore S, Srikrishna S, Hegde A, Ambiye VR, Vaidya PR. Active management of third stage of labour with intraumbilical oxytocin injection. Journal of Obstetrics and Gynecology of India 2000;50(3):54‐5. [Google Scholar]
- Kovacheva VP, Soens MA, Tsen LC. A randomized, double‐blinded trial of a "rule of threes" algorithm versus continuous infusion of oxytocin during elective cesarean delivery. Anesthesiology2015. [DOI] [PubMed]
- Kovavisarach E, Rojsangruang S. Effect of umbilical vein oxytocin injection on the third stage of labor : a randomized controlled study. 9th Congress of the Federation of the Asia and Oceania Perinatal Societies; 1996 November 10‐14; Singapore. 1996:59. [PubMed] [Google Scholar]; Kovavisarach E, Rojsangruang S. Effect of umbilical vein oxytocin injection on the third stage of labor: a randomized controlled study. Journal of the Medical Association of Thailand 1998;81:693‐7. [PubMed] [Google Scholar]
- Kumar S. A study to determine the efficacy of 600 mcg misoprostol in prevention of post partum haemorrhage. 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:305. [Google Scholar]
- Kushtagi P, Verghese LM. Evaluation of two uterotonic medications for the management of the third stage of labor. International Journal of Gynecology & Obstetrics 2006;94(1):47‐8. [DOI] [PubMed] [Google Scholar]
- Lamont RF, Morgan DJ, Logue M, Gordon H. A prospective randomised trial to compare the efficacy and safety of hemabate and syntometrine for the prevention of primary postpartum haemorrhage. Prostaglandins & Other Lipid Mediators 2001;66(3):203‐10. [DOI] [PubMed] [Google Scholar]
- Le J. Prevention of postpartum hemorrhage by carboprost and oxytocin in 90 cases analysis. Acta Medicinae Sinica 2000;13(2):140‐1. [Google Scholar]
- Leader J, Bujnovsky M, Carlan SJ, Triana T, Richichi K. Effect of oral misoprostol after second‐trimester delivery: a randomized, blinded study. Obstetrics & Gynecology 2002;100(4):689‐94. [DOI] [PubMed] [Google Scholar]
- Li X, Wang H, Wang J, Cao X L, Ma Y. Prophylactic and therapeutic effect of misoprofil plus oxytocin on postpartum hemorrhage in patients with pregnancy‐induced hypertension syndrome. Journal of Postgraduates of Medicine 2002;25(7):34‐5. [Google Scholar]
- Li DP, Bei HZ. Clinical study on reduction of postpartum bleeding in the risk factors by misoprostol. Hainan Medical Journal 2003;14(11):11‐2. [Google Scholar]
- Li H, Afzal A, Lian Q, Kramer GC, Svenson C, Prough D. Restricted fluid therapy decreases surgical blood loss ‐ a clinical study of two fluid regimens during cesarean section under spinal anesthesia. Anesthesia & Analgesia 2011;112:S‐291. [Google Scholar]; Li H, Simon M, Lian Q, Afzal A, Christer Svenson C, Prough D. Restricted fluid therapy decreases surgical blood loss ‐ A clinical study of two fluid regimens during cesarean section under spinal anesthesia. American Society of Anesthesiologists Annual Meeting; 2011, October 15‐19; Chicago, Illinois. 2011. [Google Scholar]
- Lin JH, Lin QD, Liu XH, Yan JY, He J, Li L, et al. [Multi‐center study of motherwort injection to prevent postpartum hemorrhage after caesarian section]. [Chinese]. Chung‐Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics & Gynecology] 2009;44(3):175‐8. [PubMed] [Google Scholar]
- Liu C, Wang D, Li X. Clinical study on reduction of postpartum bleeding by methyl carprost suppository. Chung‐Hua Fu Chan Ko Tsa Chih/Chinese Journal of Obstetrics and Gynaecology 1997;32(1):22‐4. [PubMed] [Google Scholar]
- Liu DY, Fan L, Huang XH. Clinical observation on treatment of postpartum hemorrhage by xuesaitong soft capsule. Chinese Journal of Integrated Traditional and Western Medicine 2002;22(3):182‐4. [PubMed] [Google Scholar]
- Luamprapas A. A study of umbilical vein administration of oxytocin to shorten the third stage of labor. Chon Buri Hospital Journal 1994;19(2):14‐25. [Google Scholar]
- Mangla D, Goel JK, Goel R. Prophylactic intramyometrial oxytocin before placenta delivery during cesarean section prevents postpartum hemorrhage: a prospective randomized study of 150 women. Journal of South Asian Federation of Obstetrics and Gynaecology 2012;4(2):93‐6. [Google Scholar]
- NCT00405626. Double blind placebo controlled bellis perenis and arnica montana as a drug for PPH. clinicaltrials.gov/ct2/show/NCT00405626 Date first received: 29 November 2006.
- Mansouri HA, Alsahly N. Rectal versus oral misoprostol for active management of third stage of labor: a randomized controlled trial. Archives of Gynecology and Obstetrics 2011;283(5):935‐9. [DOI] [PubMed] [Google Scholar]
- Martinez MM, Lopez Farfan JA, Ramos Alvarez G, Lopez Colombo A. Oxitocin trough umbilical vein to shorten the third stage of labor [Oxitocina transvena umbilical para acortar el tercer periodo de trabajo de parto]. Ginecologia y Obstetricia de Mexico 2006;74(2):89‐94. [PubMed] [Google Scholar]
- McGinty LB. A study of the vasopressor effects of oxytocics when used intravenously in the third stage of labour. Western Journal of Surgery 1956;64:22‐8. [PubMed] [Google Scholar]
- Miller S, Tudor C, Thorsten V, Nyima, Kalyang, Sonam, et al. Randomized double masked trial of Zhi Byed 11, a Tibetan traditional medicine, versus misoprostol to prevent postpartum hemorrhage in Lhasa, Tibet. Journal of Midwifery & Women's Health 2009;54(2):133‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirghafourvand M, Alizadeh SM, Abasalizadeh F, Shirdel M. The effect of intravenous tranexamic acid on hemoglobin and hematocrit levels after vaginal delivery: a randomized controlled trial. Iranian Journal of Obstetrics, Gynecology and Infertility 2013;16(60):1‐8. [Google Scholar]; Mirghafourvand M, Mohammad‐Alizadeh S, Abbasalizadeh F, Shirdel M. The effect of prophylactic intravenous tranexamic acid on blood loss after vaginal delivery in women at low risk of postpartum haemorrhage: a double‐blind randomised controlled trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2015;55(1):53‐8. [DOI] [PubMed] [Google Scholar]
- Mollitt C, Ssenoga A, Grassman C, Barclay PM. Randomised controlled trial comparing the effects of oxytocin i.v. bolus vs. oxytocin i.v. infusion on cardiac output during caesarean section. International Journal of Obstetric Anesthesia 2009;18(Suppl 1):S11. [Google Scholar]
- Moore JH. Is methylergonovine tartrate superior to ergonovine maleate. American Journal of Obstetrics and Gynecology 1956;71:908‐11. [DOI] [PubMed] [Google Scholar]
- Movafegh A, Eslamian L, Dorabadi A. Effect of intravenous tranexamic acid administration on blood loss during and after cesarean delivery. International Journal of Gynecology & Obstetrics 2011;115(3):224‐6. [DOI] [PubMed] [Google Scholar]
- Muller R, Beck G. Active management of the third stage of labour. 19th Swiss Congress of the Swiss Society of Gynecology and Obstetrics; 1996 June; Interlaken, Switzerland. 1996. [Google Scholar]
- Munishankarappa B, McLeod GA, MacGregor H, Murphy D. Maternal haemodynamic at elective caesarean section following oxytocin 5‐unit bolus and placebo infusion compared to oxytocin 5‐unit bolus and 30‐unit infusion. International Journal of Obstetric Anesthesia 2009;18(Suppl 1):S48. [DOI] [PubMed] [Google Scholar]
- Munn MB, Owen J, Hauth J. Oxytocin regimens for the prevention of uterine atony at cesarean delivery [abstract]. American Journal of Obstetrics and Gynecology 2001;184(1):S14. [DOI] [PubMed] [Google Scholar]; Munn MB, Owen J, Vincent R, Wakefield M, Chestnut DH, Hauth JC. Comparison of two oxytocin regimens to prevent uterine atony at cesarean delivery: a randomized controlled trial. Obstetrics & Gynecology 2001;98(3):386‐90. [DOI] [PubMed] [Google Scholar]
- ISRCTN17813715. A randomised controlled trial of oxytocin bolus versus oxytocin bolus and infusion for the control of blood loss at elective caesarean section. isrctn.com/ISRCTN17813715 Date first received: 25 March 2008. ; Murphy DJ, Carey M, Montgomery AA, Sheehan SR, the ECSSITSG. Study protocol. ECSSIT ‐ Elective Caesarean Section Syntocinon Infusion Trial. A multi‐centre randomised controlled trial of oxytocin (Syntocinon) 5 IU bolus and placebo infusion versus oxytocin 5 IU bolus and 40 IU infusion for the control of blood loss at elective caesarean section. BMC Pregnancy and Childbirth 2009;9:36. [DOI] [PMC free article] [PubMed] [Google Scholar]; Murphy DJ, MacGregor H, Munishankar B, McLeod G. A randomised controlled trial of oxytocin 5IU and placebo infusion versus oxytocin 5IU and 30IU infusion for the control of blood loss at elective caesarean section‐‐pilot study. European Journal of Obstetrics & Gynecology and Reproductive Biology 2009;142(1):30‐3. [DOI] [PubMed] [Google Scholar]
- Nankali A, Keshavarzi F, Fakheri T, Zare S, Rezaei M, Daeichin S. Effect of intraumbilical vein oxytocin injection on third stage of labor. Taiwanese Journal of Obstetrics & Gynecology 2013;52(1):57‐60. [DOI] [PubMed] [Google Scholar]
- NCT01710566. Misoprostol and oxytocin in Uniject® for postpartum hemorrhage prevention in communities. clinicaltrials.gov/ct2/show/NCT01710566 Date first received: 8 May 2012.
- Nellore V, Mittal S, Dadhwal V. Rectal misoprostol vs. 15‐methyl prostaglandin F2alpha for the prevention of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2006;94(1):45‐6. [DOI] [PubMed] [Google Scholar]
- Nelson GH. Use of 15‐methyl prostaglandin F2alpha postpartum to contract the uterus in normal pregnant women. Journal of the Medical Association of Georgia 1983;72:703‐6. [PubMed] [Google Scholar]
- Newton M, Mosey LM, Egli GE, Gifford WB, Hull CT. Blood loss during and immediately after delivery. Obstetrics & Gynecology 1961;17:9‐18. [PubMed] [Google Scholar]
- Nguyen‐Lu N, Carvalho J, Farine D, Seaward G, Downey K, Balki M. Carbetocin at cesarean delivery for labor arrest: A randomised controlled trial to determine ED90. Canadian Journal of Anesthesia 2013;60(Suppl 1):S112. [Google Scholar]
- Nieminen U, Jaervinen PA. A comparative study of different medical treatments of the third stage of labour. Annales Chirurgiae et Gynaecologiae Fenniae 1964;53:424‐9. [PubMed] [Google Scholar]
- Norchi S, Beretta E, Zanini A, Bottino S. Prevention of primary post‐partum haemorrhage (PPH). Controlled clinical trial: Sulprostone vs Metilergometrina. 12th FIGO World Congress of Gynecology and Obstetrics; 1988 October 23‐28; Brazil. 1988. [Google Scholar]
- NCT01156194. Effect of a homeopathic remedy on the third stage of delivery: a prospective, randomized, double‐blind study. clinicaltrials.gov/show/NCT01156194 Date first received: 1 July 2010. ; Oberbaum M, Galoyan N, Lerner‐Geva L, Singer SR, Grisaru S, Shashar D, et al. The effect of the homeopathic remedies arnica montana and bellis perennis on mild postpartum bleeding‐‐a randomized, double‐blind, placebo‐controlled study‐‐preliminary results. Complementary Therapies in Medicine 2005;13(2):87‐90. [DOI] [PubMed] [Google Scholar]
- Oguz Orhan E, Dilbaz B, Aksakal SE, Altinbas S, Erkaya S. Prospective randomized trial of oxytocin administration for active management of the third stage of labor. International Journal of Gynecology & Obstetrics 2014;127(2):175‐9. [DOI] [PubMed] [Google Scholar]
- Ozalp E, Tanir HM, Sener T. Dinoprostone vaginal insert versus intravenous oxytocin to reduce postpartum blood loss following vaginal or cesarean delivery. Clinical and Experimental Obstetrics and Gynecology 2010;37(1):53‐5. [PubMed] [Google Scholar]
- Ozcan T, Sahin G, Senoz S. The effect of intraumbilical oxytocin on the third stage of labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 1996;36:9‐11. [DOI] [PubMed] [Google Scholar]
- Ozkaya O, Sezik M, Kaya H, Desdicioglu R, Dittrich R. Placebo‐controlled randomized comparison of vaginal with rectal misoprostol in the prevention of postpartum hemorrhage. Journal of Obstetrics & Gynaecology Research 2005;31(5):389‐93. [DOI] [PubMed] [Google Scholar]
- Padhy AK, Panigrahi R, Mohapatra KR. Alternative method of active management of 3rd stage of labour with 10 units of intraumbilical oxytocin injection [abstract]. 49th All India Congress of Obstetrics and Gynaecology; 2006 January 6‐9; Cochin, Kerala State, India. 2006:76. [Google Scholar]
- Palacio FJ, Morillas F, Ortiz‐Gomez JR, Fornet I, Bermejo L, Cantalejo F. [Efficacy of low‐dose oxytocin during elective cesarean section]. [Spanish]. Revista Espanola de Anestesiologia y Reanimacion 2011;58(1):6‐10. [DOI] [PubMed] [Google Scholar]
- Paull JD, Ratten GJ. Ergometrine and third stage blood loss. Medical Journal of Australia 1977;1:178‐9. [Google Scholar]
- Pei JL, Zhao DF. Study of the effects of using uterine stimulants on milk secretion during delivery. Zhonghua Hu Li Za Zhi 1996;31(7):384‐5. [PubMed] [Google Scholar]
- Perdiou A. The effect of 3rd generation colloids on primary haemostasis in pregnant women. European Hematology Association 14th Annual Congress; 2009 June 4‐7; Berlin, Germany. 2009. [Google Scholar]; Perdiou A, Kousoulakou A, Papadopoulou G, Leveta G, Trigka A, Andromida M, et al. The effect of 3rd generation colloids on primary haemostasis in pregnant women. Haematologica 2009;94(s2):527, Abstract no. 1334. [Google Scholar]
- Phromboot T. Efficacy of intraumbilical vein methylergonovine maleate on duration of third stage of labor. Thai Journal of Obstetrics and Gynaecology 2010;20:29‐33. [Google Scholar]
- Pierre F, Mesnard L, Body G. For a systematic policy of iv oxytocin inducted placenta deliveries in a unit where a fairly active management of third stage of labour is yet applied: results of a controlled trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 1992;43:131‐5. [DOI] [PubMed] [Google Scholar]
- Pinder AJ, Dresner M, Calow C, ORiordan J, Johnson R. Haemodynamic changes caused by oxytocin during caesarean section under spinal anaesthesia. International Journal of Obstetric Anesthesia 2002;11:156‐9. [DOI] [PubMed] [Google Scholar]
- Pisani I, Tiralongo GM, Gagliardi G, Scala RL, Todde C, Frigo MG, et al. The maternal cardiovascular effect of carbetocin compared to oxytocin in women undergoing caesarean section. Pregnancy Hypertension 2012;2(2):139‐42. [DOI] [PubMed] [Google Scholar]
- Poeschmann RP, Doesburg WH, Eskes TKAB. A randomized comparison of oxytocin, sulprostone and placebo in the management of the third stage of labour. British Journal of Obstetrics and Gynaecology 1991;98:528‐30. [DOI] [PubMed] [Google Scholar]; Poeschmann RP, Eskes TKAB, Doesburg WH. Oxytocin and sulprostone reduce post partum blood loss and shorten the third stage in low risk term women. International Journal of Gynecology & Obstetrics 1991;36 Suppl:312. [Google Scholar]; Poeschmann RP, Eskes TKAB, Doesburg WH, Lemmens WAJG, Benneker JCLH. Oxytocin and sulprostone reduce postpartum blood loss in low risk term women compared to saline. Proceedings of 1st European Congress on Prostaglandins in Reproduction; 1988 July 6‐9; Vienna, Austria. 1988:176. [Google Scholar]
- Porter KB, O'Brien WF, Bruskivage L, Collins MK, Knuppel RA, Givens P. Prospective randomized study on the effects of umbilical vein oxytocin on puerperal blood loss, length of the third stage of labor and on alpha‐fetoprotein levels. American Journal of Obstetrics and Gynecology 1991;164:326. [Google Scholar]; Porter KB, O'Brien WF, Collins MK, Givens P, Knuppel R, Bruskivage L. A randomized comparison of umbilical vein and intravenous oxytocin during the puerperium. Obstetrics & Gynecology 1991;78:254‐6. [PubMed] [Google Scholar]
- Priya GP, Veena P, Chaturvedula L, Subitha L. A randomized controlled trial of sublingual misoprostol and intramuscular oxytocin for prevention of postpartum hemorrhage. Archives of Gynecology and Obstetrics2015. [DOI] [PubMed]
- Puri M, Taneja P, Gami N, Rehan HS. Effects of different doses of intraumbilical oxytocin on the third stage of labor. International Journal of Gynecology & Obstetrics 2012;118(3):210‐2. [DOI] [PubMed] [Google Scholar]
- Qiu H, Zhu H, Ouyang W. Clinical study on chanlibao in accelerating second stage of labor [Chinese]. Chung‐Kuo Chung Hsi i Chieh Ho Tsa Chih 1998;18:214‐6. [PubMed] [Google Scholar]; Qiu H, Zhu H, Ouyang W, Wang Z, Sun H. Clinical effects and mechanism of chanlibao in accelerating second stage of labor. Journal of Tongji Medical University 1999;19(2):141‐4. [DOI] [PubMed] [Google Scholar]
- Quiroga Diaz R, Cantu Mata R, Tello Gutierrez HE, Puente Villalobos M, Montemayor Garza R, Martinez Mendoza A. Intrauterine misoprostol for the prevention of bleeding cesarean. Ginecologia y Obstetricia de Mexico 2009;77(10):469‐74. [PubMed] [Google Scholar]
- Rajwani J, Survana K. Active management of third stage of labor ‐ a comparative study [abstract]. XVI FIGO World Congress of Obstetrics & Gynecology (Book 3); 2000 Sept 3‐8; Washington DC, USA. 2000:54. [Google Scholar]
- Reddy VV, Carey JC. Effect of umbilical vein oxytocin on puerperal blood loss and length of the third stage of labor. American Journal of Obstetrics and Gynecology 1989;160(1):206‐8. [DOI] [PubMed] [Google Scholar]
- Reddy R, Shenoy JV. Active management of third stage of labour. A comparative study in high risk patients for atonic postpartum haemorrhage. Journal of Obstetrics and Gynecology of India 2001;51(2):44‐7. [Google Scholar]
- Rooney I, Hughes P, Calder AA. Is routine administration of syntometrine still justified in the management of the third stage of labour?. Health Bulletin 1985;43:99‐101. [PubMed] [Google Scholar]
- Rosales‐Ortiz S. Prophylaxis of obstetric haemorrhage: Experience using carbetocin vs. Oxytocin in patients with risk factors for postpartum haemorrhage. Journal of Perinatal Medicine 2013;41(Suppl 1):140. [Google Scholar]
- Rouse D, Abramovici A, Szychowski J, Seals S, Andrews W, Hauth J, et al. Oxytocin dose‐regimens to prevent uterine atony after vaginal delivery: does treatment efficacy vary by risk status?. American Journal of Obstetrics and Gynecology 2011;204(1 Suppl):S50‐1. [Google Scholar]
- IRCT2013052613473N1. The role of tranexamic acid in management of uterine atony during delivery. en.search.irct.ir/view/13710 Date first received: 14 July 2013.
- Saito K, Haruki A, Ishikawa H, Takahashi T, Nagase H, Koyama M, et al. Prospective study of intramuscular ergometrine compared with intramuscular oxytocin for prevention of postpartum hemorrhage. Journal of Obstetrics and Gynaecology Research 2007;33(3):254‐8. [DOI] [PubMed] [Google Scholar]
- Samuels N, Oberbaum M. The effect of the homoeopathic remedies arnica montana and bellis perennis on postpartum bleeding ‐ a randomised, double‐blind, placebo‐controlled study [abstract]. Focus on Alternative and Complementary Therapies 2005;10 Suppl 1:47. [DOI] [PubMed] [Google Scholar]
- Sariganont J. Comparative study between syntocinon and methergin in prevention of postpartum hemorrhage. Thai Journal of Obstetrics and Gynaecology 1999;11(4):248. [Google Scholar]
- Sarna MC, Soni AK, Gomez M, Oriol NE. Intravenous oxytocin in patients undergoing elective cesarean section [see comments]. Anesthesia & Analgesia 1997;84(4):753‐6. [DOI] [PubMed] [Google Scholar]
- Sartain JB, Barry JJ, Howat PW, McCormack DI, Bryant M. Intravenous oxytocin bolus of 2 units is superior to 5 units during elective caesarean section. British Journal of Anaesthesia 2008;101(6):822‐6. [DOI] [PubMed] [Google Scholar]
- Schaefer A, Klein L, Wolfe P, Heindricks G, Downs L, Guinn D. Double blind rct of early versus traditional oxytocin management in the third stage to prevent blood loss [abstract]. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S69. [Google Scholar]
- Schemmer G. A randomized controlled trial comparing prophylactic administration of oxytocin before and after placental delivery in the prevention of postpartum hemorrhage [abstract]. American Journal of Obstetrics and Gynecology 2001;184(1):S20. [DOI] [PubMed] [Google Scholar]
- Sekhavat L, Tabatabaii A, Dalili M, Farajkhoda T, Tafti AD. Efficacy of tranexamic acid in reducing blood loss after cesarean section. Journal of Maternal‐Fetal & Neonatal Medicine 2009;22(1):72‐5. [DOI] [PubMed] [Google Scholar]
- NCT02302456. Tranexamic acid for preventing postpartum haemorrhage following a vaginal delivery (TRAAP). clinicaltrials.gov/ct2/show/NCT02302456 Date first received: 17 November 2014.
- Senturk MB, Cakmak Y, Yildiz G, Yildiz P. Tranexamic acid for cesarean section: a double‐blind, placebo‐controlled, randomized clinical trial. Archives of Gynecology and Obstetrics 2013;287(4):641‐5. [DOI] [PubMed] [Google Scholar]
- Shahid A, Khan A. Tranexamic acid in decreasing blood loss during and after caesarean section. Journal of the College of Physicians and Surgeons‐‐Pakistan : JCPSP2013; Vol. 23, issue 7:459‐62. [PubMed]
- Sharma M, Kaur P, Kaur K, Kaur A, Kaur PK, Kaur MM. A comparative study of oxytocin/misoprostol/methylergometrine for active management of the third stage of labor. Journal of Obstetrics and Gynecology of India 2014;64(3):175‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan S, Carey M, Murphy D. A cohort study of 500 patients recruited to ECSSIT ‐ Elective Caesarean Section Syntocinon Infusion Trial. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S492. [Google Scholar]; Sheehan S, Montgomery AA, Carey M, McAuliffe F, Eogan M, Gleeson R, et al. Ecssit‐elective caesarean section Syntocinon infusion trial a multicentre randomized controlled trial of oxytocin (Syntocinon) 5 IU bolus and placebo infusion versus oxytocin 5 IU bolus and 40 IU infusion for the control of blood loss at elective caesarean section. Irish Journal of Medical Science 2011;180(Suppl 4):S119. [Google Scholar]; Sheehan SR, Montgomery AA, Carey M, McAuliffe FM, Eogan M, Gleeson R, et al. Oxytocin bolus versus oxytocin bolus and infusion for control of blood loss at elective caesarean section: Double blind, placebo controlled, randomised trial. BMJ 2011;343(7819):d4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRCT201204079399N1. A placebo‐controlled clinical trial to assess efficacy of tranexamic acid in reducing hemorrhage after vaginal delivery. en.search.irct.ir/view/9264 Date first received: 15 November 2012.
- Shrestha P, Babu CS. Influence of umbilical vein oxytocin on blood loss and length of third stage of labour. Nepal Medical College Journal 2007;9(3):176‐8. [PubMed] [Google Scholar]
- Singh N, Singh U. Methylergometrine and carboprost tromethamine prophylaxis for postpartum hemorrhage. Journal of Obstetrics and Gynaecology of India 2005;55(4):325‐8. [Google Scholar]
- Siriwarakul W. A study of umbilical vein administration of oxytocin to shorten the third stage of labor. Chon Buri Hospital Journal 1991;16(1):40‐51. [Google Scholar]
- Soiva K, Koistinen O. Clinical experience with simultaneous intramuscular injection of oxytocin and methylergometrine. Annales Chirurgiae et Gynaecologiae Fenniae 1964;53:173‐8. [PubMed] [Google Scholar]
- Sorbe B. Active pharmacologic management of the third stage of labor. A comparison of oxytocin and ergometrine. Obstetrics & Gynecology 1978;52:694‐7. [PubMed] [Google Scholar]
- Soriano D, Dulitzki M, Schiff E, Barkai G, Seidman DS. A randomized prospective trial of oxytocin plus ergometrin versus oxytocin alone for prevention of postpartum hemorrhage. American Journal of Obstetrics and Gynecology 1995;172(1 pt 2):361. [Google Scholar]
- Stearn RH. Syntometrine in the management of the third stage of labour. Journal of Obstetrics and Gynaecology of the British Commonwealth 1963;70:593‐6. [DOI] [PubMed] [Google Scholar]
- Svanstrom MC, Biber B, Hanes M, Johansson G, Naslund U, Balfors EM. Signs of myocardial ischaemia after injection of oxytocin: a randomized double‐blind comparison of oxytocin and methylergometrine during caesarean section. British Journal of Anaesthesia 2008;100(5):683‐9. [DOI] [PubMed] [Google Scholar]
- Symes JB. A study on the effect of ergometrine on serum prolactin levels following delivery. Journal of Obstetrics and Gynaecology 1984;5:36‐8. [Google Scholar]
- Taj N, Firdous A, Akhtar N, Chaudhary MH, Sarah, Bajwa Z, et al. Efficacy of tranexamic acid in reducing blood loss during and after cesarean section. Rawal Medical Journal 2014;39(3):311‐3. [Google Scholar]
- Takagi S, Yoshida T, Togo Y, Tochigi H, Abe M, Sakata H, et al. The effects of intramyometrial injection of prostaglandin F2alpha on severe post‐partum hemorrhage. Prostaglandins 1976;12(4):565‐79. [DOI] [PubMed] [Google Scholar]
- Tanir H, Sener T, Ozalp E. Dinoprostone vaginal insert versus intravenous oxytocin to reduce the postpartum blood loss following vaginal or cesarean delivery. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S506‐7. [PubMed] [Google Scholar]
- Tarabrin O, Kaminskiy V, Galich S, Tkachenko R, Gulyaev A, Shcherbakov S, et al. Efficacy of tranexamic acid in decreasing blood loss during cesarean section. Critical Care 2012;16 Suppl 1:S157. [Google Scholar]
- Tariq N, Khakwani M, Parveen R. Effectiveness of misoprostol in the prevention of postpartum hemorrhage. Pakistan Journal of Medical and Health Sciences 2015;9(1):268‐70. [Google Scholar]
- Tehseen F, Anwar A, Arfat Y. Intraumbilical veinous injection oxytocin in the active management of third stage of labour. Journal of the College of Physicians and Surgeons‐‐Pakistan 2008;18(9):551‐4. [PubMed] [Google Scholar]
- Terry MF. A management of the third stage to reduce feto‐maternal transfusion. Journal of Obstetrics and Gynaecology of the British Commonwealth 1970;77:129‐32. [DOI] [PubMed] [Google Scholar]
- Tessier JL, Davies GAL, Woodman MC, Lipson A. Maternal hemodynamics after oxytocin bolus versus infusion in the third stage of labor. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S128. [DOI] [PubMed] [Google Scholar]
- Tharakan T, Jha J. Randomized double blind prospective trial of active management of the third stage of labor. Archives of Medical Science 2008;4(1):79‐82. [Google Scholar]; Tharakan T, Jha J. Randomized double‐blind prospective trial of active management of the third stage of labor [abstract]. Obstetrics & Gynecology 2007;109(4 Suppl):1S. 17403920 [Google Scholar]
- Thomas JS, Koh SH, Cooper GM. Haemodynamic effects of intravenous bolus or infusion of oxytocin in women undergoing caesarean section [abstract]. International Journal of Obstetric Anesthesia 2006;15 Suppl 1:S13. [Google Scholar]; Thomas JS, Koh SH, Cooper GM. Haemodynamic effects of oxytocin given as i.v. bolus or infusion on women undergoing caesarean section. British Journal of Anaesthesia 2007;98(1):116‐9. [DOI] [PubMed] [Google Scholar]
- Thornton S, Davison JM, Baylis PH. Plasma oxytocin during third stage of labour: comparison of natural and active management. BMJ 1988;297:167‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]; Thornton S, Davison JM, Baylis PH. Plasma oxytocin in the third stage of human labour with and without syntometrine [abstract]. Clinical Science 1987;73 Suppl 17:2P. [Google Scholar]
- Tita ATN, Szychowski JM, Rouse DJ, Bean CM, Chapman V, Nothern A, et al. Higher‐dose oxytocin and hemorrhage after vaginal delivery: A randomized controlled trial. Obstetrics and Gynecology 2012;119(2 Pt 1):293‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripti N, Manju E. Intramuscular PGF2 alpha 125 microg versus intravenous methyl ergometrine 0.2 mg in the active management of third stage of labor. Journal of Obstetrics and Gynecology of India 2006;56(5):396‐8. [Google Scholar]
- Tripti N, Balram S. 400 ug oral misoprostol versus 0.2mg intravenous methyl ergometrine for the active management of third stage of labor. Journal of Obstetrics and Gynecology of India 2009;59(3):228‐34. [Google Scholar]
- Tudor C, Miller S, Nyima, Sonam, Droyoung, Varner M. Preliminary progress report: randomized double‐blind trial of Zhi Byed 11, a Tibetan traditional medicine, versus misoprostol to prevent postpartum hemorrhage in Lhasa, Tibet. International Journal of Gynecology & Obstetrics 2006;94(Suppl 2):S145‐6. [DOI] [PubMed] [Google Scholar]
- Enden E, Lahousse J, Devlieger R, Vandermeersch E, Velde M. Haemodynamic effects of a bolus or infusion of oxytocin: a randomised double‐blind trial. International Journal of Obstetric Anesthesia 2009;18(Suppl 1):S45. [DOI] [PubMed] [Google Scholar]
- Selm M, Kanhai HHH, Keirse MJNC. Preventing the recurrence of atonic postpartum hemorrhage: a double‐blind trial. Acta Obstetricia et Gynecologica Scandinavica 1995;74:270‐4. [DOI] [PubMed] [Google Scholar]
- Vasegh FR, Bahiraie A, Mahmoudi M, Salehi L. Comparison of active and physiologic management of third stage of labor. HAYAT: The Journal of Tehran Faculty of Nursing & Midwifery 2005;10(23):102. [Google Scholar]
- Vaughan Williams CA, Johnson A, Ledward R. A comparison of central venous pressure changes in the third stage of labour following oxytocic drugs and diazepam. Journal of Obstetrics and Gynaecology of the British Commonwealth 1974;81:596‐9. [DOI] [PubMed] [Google Scholar]
- Ventoskovskiy BM, Popov AV. Homoeopathy as a practical alternative to traditional obstetrics methods. British Homoeopathic Journal 1990;79:201‐5. [Google Scholar]
- Verghese L, Kushtagi P. Evaluation of carboprost as a prophylactic oxytocic in the management of third stage of labour. BJOG: an international journal of obstetrics and gynaecology 2008;115(s1):74. [Google Scholar]
- Vogel D, Burkhardt T, Rentsch K, Schweer H, Watzer B, Zimmerman R, et al. Misoprostol versus methylergometrine: pharmacokinetics in human milk. American Journal of Obstetrics and Gynecology 2004;191:2168‐73. [DOI] [PubMed] [Google Scholar]
- Wallace EM. A double‐blind randomised controlled trial of oxytocin bolus plus placebo infusion versus oxytocin bolus plus oxytocin infusion at elective caesarean section. Australian New Zealand Clinical Trials Registry (www.anzctr.org.au) (accessed 19 February 2008). ACTRN12607000631404 2008.
- Walraven G, Blum J, Dampha Y, Sowe M, Morison L, Winikoff B, et al. Misoprostol in the management of the third stage of labour in the home delivery setting in rural Gambia: a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2005;112:1277‐83. [DOI] [PubMed] [Google Scholar]
- Wang BI, Du JM. Clinical study on reduction of postpartum bleeding using carprost suppository. Henan Medical Research 2000;9(2):155‐6. [Google Scholar]
- Weeks A, Ditai J, Ononge S, Faragher B, Mirembe F, Byamugisha J, et al. Self‐administered misoprostol to prevent bleeding after homebirths in Uganda: a placebo‐controlled randomised trial. BJOG: an international journal of obstetrics and gynaecology 2013;120:76. [Google Scholar]
- Weihong H, Hanrong C, Hong L, Linan C. Preventing of postpartum haemorrhage by carboprost methylate suppository administered through vagina or sublingually. Acta Academiae Medicinae Shanghai 1998;25:137‐9. [Google Scholar]
- Weiss G, Klein S, Shenkman L, Kataoka K, Hollander CS. Effect of methylergonovine on puerperal prolactin secretion. Obstetrics & Gynecology 1975;46:209‐10. [PubMed] [Google Scholar]
- Wetta L, Szychowski J, Seals S, Mancuso M, Hauth J, Tita A. Risk factors for uterine atony at vaginal delivery: a comprehensive evaluation. American Journal of Obstetrics and Gynecology 2011;204(1 Suppl):S71‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wetta LA, Szychowski JM, Seals S, Mancuso MS, Biggio JR, Tita ATN. Risk factors for uterine atony/postpartum hemorrhage requiring treatment after vaginal delivery. American Journal of Obstetrics and Gynecology 2013;209(1):51e1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01608958. Intravenous and intramuscular administration of oxytocin in the third stage of labor for prevention of postpartum hemorrhage. clinicaltrials.gov/ct2/show/NCT01608958 Date first received: 29 May 2012.
- NCT00257803. Does the rapid intravenous administration of oxytocin after delivery of the baby decrease the bleeding during cesarean section in women at risk of bleeding during cesarean section?. clinicaltrials.gov/ct2/show/NCT00257803 Date first received: 21 November 2005.
- Wright L. Preventing postpartum hemorrhage using a Tibetan traditional medicine. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 21 March 2006)2006.
- Wu LF, Liu Y, Ruan Y. Clinical study on prevention of postpartum hemorrhage of cesarean section using hemabat in high risk pregnant women. Chinese Journal of Obstetrics & Gynecology 2007;42(9):577‐81. [PubMed] [Google Scholar]
- Xu H. Misoprostol on preventing postpartum bleeding in cesarean. Hebei Medicine 2003;9(9):806‐7. [Google Scholar]
- Xu J, Gao W, Ju Y. Tranexamic acid for the prevention of postpartum hemorrhage after cesarean section: a double‐blind randomization trial. Archives of Gynecology and Obstetrics 2013;287(3):463‐8. [DOI] [PubMed] [Google Scholar]
- Yamaguchi ET, Cardoso MM, Torres ML, Nascimento RC, Ribeiro MC, Frerichs E, et al. Serum oxytocin concentrations in elective caesarean delivery: a randomized comparison of three infusion regimens. International Journal of Obstetric Anesthesia 2011;20(3):224‐8. [DOI] [PubMed] [Google Scholar]
- Yan WG, Ling MX, Mao HY. Clinical study on reduction of postpartum bleeding in cesarean operation by misoprostol. Journal of Zhenjiang Medical College 2000;10(3):440‐1. [Google Scholar]
- Yang H, Zheng S, Shi C. [Clinical study on the efficacy of tranexamic acid in reducing postpartum blood lose: a randomized comparative, multicenter trial] [Chinese]. Chung‐Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics and Gynaecology] 2001;36(10):590‐2. [PubMed] [Google Scholar]
- Young SB, Martelly PD, Greb L, Considine G, Coustan DR. The effect of intraumbilical oxytocin on the third stage of labor. Obstetrics & Gynecology 1988;71:736‐8. [PubMed] [Google Scholar]
- Zamora LAL. A randomized controlled trial of oxytocin administered at the end of the second stage of labor versus oxytocin administered at the end of the third stage of labor in the prevention of postpartum hemorrhage. Philippine Journal of Obstetrics and Gynecology 1999;23(4):125‐33. [PubMed] [Google Scholar]
- Zaporozhan V, Tarabrin O, Gavrychenko D, Mazurenko G, Saleh O, Lyoshenko I. Effcacy of tranexamic acid in decreasing blood loss during cesarean section. Critical Care 2013;17(Suppl 2):S135‐6. [Google Scholar]
- Zhao Y, Li X, Peng Y. Clinical study on reduction of postpartum bleeding in cesarean section by misoprostol. Chung‐Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics & Gynecology] 1998;33:403‐5. [PubMed] [Google Scholar]
- Zhao SF, Sun XF. Clinical study on preventing and curing postpartum hemorrhage in the third stage of labor. Journal of Practical Obstetrics and Gynecology 2003;19(5):278‐80. [Google Scholar]
- Zhou HL, Zhang L. Study on the effect of third stage of labor through different channels of injection of oxytocin. Chinese Journal of Nursing 1994;29(8):453‐5. [Google Scholar]
References to studies awaiting assessment
- Adanikin AI, Orji E, Adanikin PO, Olaniyan O. Comparative study of rectal misoprostol to oxytocin infusion in preventing postpartum haemorrhage after caesarean section. Nepal Journal of Obstetrics and Gynaecology 2013;8(2):34‐7. [Google Scholar]
- Adhikari S, Rana A, Bista KD. Active management of third stage of labour: comparison between prophylactic intramuscular methylergometrine and intramuscular oxytocin. Nepal Journal of Obstetrics and Gynaecology 2007;2(2):24‐8. [Google Scholar]
- Ahmed MR, Sayed Ahmed WA, Madny EH, Arafa AM, Said MM. Efficacy of tranexamic acid in decreasing blood loss in elective caesarean delivery. Journal of Maternal‐Fetal & Neonatal Medicine 2015;28(9):1014‐8. [DOI] [PubMed] [Google Scholar]
- Akinaga C, Uchizaki S, Kurita T, Taniguchi M, Makino H, Suzuki A, et al. Randomized double‐blind comparison of the effects of intramyometrial and intravenous oxytocin during elective cesarean section. Journal of Obstetrics and Gynaecology Research 2016;42(4):404‐9. [DOI] [PubMed] [Google Scholar]
- Ali R, Hina F. Postpartum hemorrhage; comparison of efficacy of ergometrine with misoprostol in prophylaxis in cesarean section. Professional Medical Journal 2012;19(3):360‐4. [Google Scholar]
- Alli QO. Comparing effectiveness of sublingual misoprostol with oxytocin infusion to reduce blood loss at caesarean section: double blind, randomised study. BJOG: an international journal of obstetrics and gynaecology 2013;120:77‐8. 23679822 [Google Scholar]
- Alwani M, Singh S, Thakur R, Mishra S. A randomized study comparing rectally administered misoprostol after spinal anesthesia versus intramuscular oxytocin for prevention of postpartum hemorrhage in caesarean section. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2014;3(3):512‐5. [Google Scholar]
- Ashwal E, Hiersch L, Wertheimer A, Krispin E, Aviram A, Dayan DB, et al. The effect of post‐partum oxytocin regimen on hemoglobin decline–a randomized controlled trial. American Journal of Obstetrics and Gynecology 2016;214(1 Suppl):S197‐S198, Abstract no: 351. [Google Scholar]
- Asmat R, Ashraf T, Asmat F, Asmat S, Asmat N. Effectiveness of per rectal misoprostol versus intramuscular oxytocin for prevention of primary postpartum haemorrhage. Journal of the College of Physicians and Surgeons Pakistan 2017;27(1):13‐7. [PubMed] [Google Scholar]
- Ayedi M, NCT01599468. Effects of tranexamic acid on post partum hemorrhage by uterine atony after cesarean section delivery: a randomized, placebo controlled trial. clinicaltrials.gov/ct2/show/NCT01599468 (first received 14 May 2012).
- Baig FS, Shahzad N, Khurshid HN, Malik A. Postpartum haemorrhage; comparison of intra umbilical and intra venous injection of oxytocin on blood loss in third stage of labour. Professional Medical Journal 2015;22(6):793‐7. [Google Scholar]
- Begum T, Yeasmin S, Chakma S. Sublingual misoprostol versus oxitocin infusion to reduce blood loss in caesarean section. BJOG: an international journal of obstetrics and gynaecology 2015;122(Suppl S1):258. [Google Scholar]
- Beigi A, Tabarestani H, Moini A, Zarrinkoub F, Kazempour M, Hadian Amree A. [Sublingual misoprostol versus intravenous oxytocin in the management of postpartum hemorrhage]. Tehran University Medical Journal 2009;67(8):556‐61. [Google Scholar]
- Bhatti K, Mahar T, Hafeez R, Shoaib‐u‐Nisa. A randomized controlled trial on prevention of postpartum haemorrhage with sublingual misoprostol or oxytocin. Medical Forum Monthly 2014;25(1):10‐2. [Google Scholar]
- Boopathi A, Nayak SR, Rao A, Rao B. Oxytocin versus methylergometrine in the active management of third stage of labour. Open Journal of Obstetrics and Gynecology 2014;4:666‐71. [Google Scholar]
- Carrillo‐Gaucin S, Torres‐Gomez LG. Carbetocin and oxytocin: prevention of postpartum hemorrhage in patients with risk factors for uterine atony. Revista Medica Del Instituto Mexicano Del Seguro Social 2016;54 Suppl 3:S284‐90. [PubMed] [Google Scholar]
- Chalermpolprapa V. Efficacy of sublingual misoprostol in prevention of postpartum hemorrhage in cesarean section: A randomized double‐blinded, placebo‐controlled trial. Region 4‐5 Medical Journal 2010;29(3):325‐35. [Google Scholar]
- Chandhiok N, Dhillon BS, Datey S, Mathur A, Saxena NC. Oral misoprostol for prevention of postpartum hemorrhage by paramedical workers in India. International Journal of Gynecology & Obstetrics 2006;92(2):170‐5. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. Misoprostol and the 3rd stage. XVI FIGO World Congress of Obstetrics & Gynecology; 2000 Sept 3‐8; Washington DC, USA. 2000; Vol. Book 4:29. [Google Scholar]
- Chatterjee S, Sarkar A, Rao KD. Using misoprostol for primary versus secondary prevention of postpartum haemorrhage ‐ Do costs matter?. Plos One 2016;11(10):e0164718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri P, Majumdar A. A randomized trial of sublingual misoprostol to augment routine third‐stage management among women at risk of postpartum hemorrhage. International Journal of Gynecology and Obstetrics 2016;132:191‐5. [DOI] [PubMed] [Google Scholar]
- Chou LT, Da AW, Murizah MZ1, Rushdan M, Rashid Z. A randomised controlled trial on low dose versus high dose oxytocin infusion in prevention of uterine atony at caesarean delivery. Journal of Obstetrics and Gynaecology Research 2015;41(Suppl S1):44‐5, Abstract no: FC 8.11. 25163390 [Google Scholar]
- Cordovani D, Farine D, Balki M, Seaward G, Carvalho JC. Carbetocin at elective cesarean delivery: A dose‐finding study. Canadian Journal of Anesthesia 2011;58(Suppl 1):S90. [Google Scholar]
- Dabbaghi Gale T, Elmizadeh KH, Moradi SD, Rashvand Melli E. Comparison of intravenous oxytocin and oral misoprostol in reduction of postpartum hemorrhage. Journal of Zanjan University of Medical Sciences and Health Services 2012;20(81):1‐8. [Google Scholar]
- Dagdeviren H, Cengiz H, Heydarova U, Caypinar SS, Kanawati A, Guven E, et al. Intramuscular versus intravenous prophylactic oxytocin for postpartum hemorrhage after vaginal delivery: a randomized controlled study. Archives of Gynecology and Obstetrics 2016;294(5):911‐6. [DOI] [PubMed] [Google Scholar]
- Angel‐Garcia G, Garcia‐Contreras F, Constantino‐Casas P, Nevarez‐Sida A, Lopez‐Gonzalez N, Garcia‐Constantino M, et al. Economic evaluation of carbetocin for the prevention of uterine atony in patients with risk factors in Mexico. Value in Health 2006;9(6):A254. [Google Scholar]
- Dell‐Kuster S, Hoesli I, Lapaire O, Seeberger E, Steiner LA, Bucher HC, et al. Efficacy and safety of carbetocin applied as an intravenous bolus compared to as a short‐infusion for caesarean section: study protocol for a randomised controlled trial. Trials 2016;17(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell‐Kuster S, Hoesli I, Lapaire O, Seeberger E, Steiner LA, Bucher HC, et al. Efficacy and safety of intravenous carbetocin as a bolus compared to a short infusion for caesarean section. Journal of Obstetric Anesthesia 2016;26(Suppl 1):S7. [Google Scholar]
- Dell‐Kuster S, Hoesli I, Lapaire O, Seeberger E, Steiner LA, Bucher HC, et al. Efficacy and safety of carbetocin given as an intravenous bolus compared with short infusion for caesarean section ‐ double‐blind, double‐dummy, randomized controlled non‐inferiority trial. British Journal of Anaesthesia 2017;118(5):772‐80. [DOI] [PubMed] [Google Scholar]
- Deshpande HG, Madkar CS, Patel KK. Comparative study between intravenous and intraumbilical oxytocin as active management of third stage in elective and emergency caeserean section. Indian Journal of Obstetrics and Gynaecology Research 2016;3(1):55‐8. [Google Scholar]
- Diop A, Daff B, Sow M, Blum J, Diagne M, Sloan NL, et al. Oxytocin via Uniject (a prefilled single‐use injection) versus oral misoprostol for prevention of postpartum haemorrhage at the community level: a cluster‐randomised controlled trial. Lancet. Global Health 2016;4(1):e37‐44. [DOI] [PubMed] [Google Scholar]; NCT01487278. Comparing misoprostol and oxytocin in unijectTM for postpartum hemorrhage (PPH) prevention in Mali. clinicaltrials.gov/ct2/show/NCT01487278 (first received: 5 December 2011).
- Dumoulin JG. A reappraisal of the use of ergometrine. Journal of Obstetrics and Gynaecology 1981;1:178‐81. [Google Scholar]
- Dutta BK, Gupta KR. A comparative study on rectal misoprostol versus intramuscular oxytocin to prevent postpartum haemorrhage. New Indian Journal of OBGYN 2016;2(2):98‐103. [Google Scholar]
- Elbohoty AEH, Mohammed WE, Sweed M, Eldin AMB, Nabhan A, Abd‐El‐Maeboud KHI. Randomized controlled trial comparing carbetocin, misoprostol, and oxytocin for the prevention of postpartum hemorrhage following an elective cesarean delivery. International Journal of Gynaecology and Obstetrics 2016;134(3):324‐8. [DOI] [PubMed] [Google Scholar]
- Fahmy AA, Fawzy M. Oxytocin infusion after oxytocin bolus and carbetocin bolus to reduce blood loss during and after cesarean section ‐ a randomized clinical trial. Medical Journal of Cairo University 2015;83(1):79‐83. [Google Scholar]
- Fahmy NG, Yousef HM, Zaki HV. Comparative study between effect of carbetocin and oxytocin on isoflurane‐induced uterine hypotonia in twin pregnancy patients undergoing cesarean section. Egyptian Journal of Anaesthesia 2016;32(1):117‐21. [Google Scholar]
- Fakour F, Mirzayi M, Reza Naghipour M, Ebrahimi H, Mahdavi M. Comparison between sublingual misoprostol and intravenous oxytocin in management of third stage of labor. Iranian Journal of Obstetrics, Gynecology and Infertility 2013;15(34):7‐14. [Google Scholar]
- Frye L, Durocher J, Weeks A, Ditai J, Ononge S, Faragher B, et al. On the trail of misoprostol in the community: A secondary analysis of self‐administered misoprostol for the prevention of postpartum hemorrhage in Uganda. International Journal of Gynaecology and Obstetrics 2015;131(Suppl 5):E354‐5. [Google Scholar]
- Fuks AM, Khanna P, Yusaf T, Aslian A, Kowalska D, Salafia CM. Use of prophylactic misoprostol in reduction of blood loss at vaginal delivery. Obstetrics and Gynecology 2014;123(5 Suppl):144S‐5S. [Google Scholar]
- Ghulmiyyah LM, Usta IM, Ghazeeri G, Taher N, Abu‐Ghannam G, Nassar AH, et al. Intravenous oxytocin use to decrease blood loss during scheduled cesarean delivery: a randomized double‐blinded controlled trial (oxytrial). American Journal of Perinatology 2017;34(4):379‐87. [DOI] [PubMed] [Google Scholar]
- Gulmezoglu M. The WHO Champion Trial. International Journal of Gynecology and Obstetrics 2015;131(Suppl 5):E29‐30. [Google Scholar]
- Hernandez‐Castro F, Lopez‐Serna N, Trevino‐Salinas EM, Soria‐Lopez JA, Sordia‐Hernandez LH, Cardenas‐Estrada E. Randomized double‐blind placebo‐controlled trial of buccal misoprostol to reduce the need for additional uterotonic drugs during cesarean delivery. International Journal of Gynaecology and Obstetrics 2016;132(2):184‐7. [DOI] [PubMed] [Google Scholar]
- Islam A, Siraj A, Arif N. Post partum hemorrhage prophylaxis; comparison of the efficacy of misoprostol and ergometrine in cesarean delivery. Professional Medical Journal 2008;15(3):323‐7. [Google Scholar]
- Jagielska I, Kazdepka‐Zieminska A, Kaczorowska A, Madej A, Kolossa T, Grabiec M. [Evaluation of carbetocin and oxytocin efficacy in prevention of postpartum hemorrhage in women after cesarean section]. [Polish]. Ginekologia Polska 2015;86(9):689‐93. [PubMed] [Google Scholar]
- Jans S, Herschderfer KC, Diem MT, Aitink M, Rijnders M, Pal‐Bruin K, et al. LENTE study: effectiveness of prophylactic intramuscular oxytocin during third stage of labour amongst low risk women.. A randomized controlled trial. 31st International Confederation of Midwives Triennial Congress. Midwives ‐ Making a Difference in the World; 2017 June 18‐22; Toronto, Canada. 2017:Abstract no: P1.063. [Google Scholar]
- Javadi EHS, Sadeghipour Z, Barikani A, Javadi M. Tranexamic acid in the control of uterine atony during labor. Biotechnology and Health Sciences 2015;2(2):e26898. [Google Scholar]
- Kabir N, Akter D, Daisy TA, Jesmin S, Razzak M, Tasnim S, et al. Efficacy and safety of carbetocin in comparison to oxytocin in the active management of third stage of labour following vaginal delivery: an open label randomized control trial. Bangladesh Journal of Obstetrics and Gynecology 2015;30(1):3‐9. [Google Scholar]
- Khan M, Balki M, Ahmed I, Farine D, Searward G, Carvalho JCA. Carbetocin at elective cesarean delivery: a randomized controlled trial to determine the effective dose, part 3 final. Society for Obstetric Anesthesia and Perinatology (SOAP) 45th Annual Meeting; 2013 April 24‐28; San Juan, Puerto Rico. 2013:Abstract no: GM 3. [Google Scholar]
- Koen S, Snyman LC, Pattinson RC, Makin JA. A randomised controlled trial comparing oxytocin and oxytocin + ergometrine for prevention of postpartum haemorrhage at caesarean section. South African Medical Journal = Suid‐Afrikaanse Tydskrif Vir Geneeskunde 2016;106(4):399‐402. [DOI] [PubMed] [Google Scholar]
- Liu Y, Chen HX, Kang DL, Kuang XH, Liu WX, Ni J. Influence of dexmedetomidine on incidence of adverse reactions introduced by hemabate in postpartum hemorrhage during cesarean section. International Journal of Clinical and Experimental Medicine 2015;8(8):13776‐82. [PMC free article] [PubMed] [Google Scholar]
- Liu W, Ma S, Pan W, Tan W. Combination of motherwort injection and oxytocin for the prevention of postpartum hemorrhage after cesarean section. Journal of Maternal‐Fetal & Neonatal Medicine 2016;29(51):2489‐92. [DOI] [PubMed] [Google Scholar]
- Maged AM, Helal OM, Elsherbini MM, Eid MM, Elkomy RO, Dahab S, et al. A randomized placebo‐controlled trial of preoperative tranexamic acid among women undergoing elective cesarean delivery. International Journal of Gynecology and Obstetrics 2015;131:265‐8. [DOI] [PubMed] [Google Scholar]
- Maged AM, Ragab AS, Elnassery N, Al Mostafa W, Dahab S, Kotb A. Carbetocin versus syntometrine for prevention of postpartum hemorrhage after cesarean section. Journal of Maternal‐Fetal & Neonatal Medicine 2017;30(8):962‐6. [DOI] [PubMed] [Google Scholar]
- Makvandi S, Shoushtari SZ, Hosseini VZ. Management of third stage of labour: A comparison of intraumbilical oxytocin and placental cord drainage. Shiraz E Medical Journal 2013;14(2):83‐90. [Google Scholar]
- Mirteimouri M, Tara F, Teimouri B, Sakhavar N, Vaezi A. Efficacy of rectal misoprostol for prevention of postpartum hemorrhage. Iranian Journal of Pharmaceutical Research2013; Vol. 12, issue 2:469‐74. [PMC free article] [PubMed]
- Mockler JC, Malkoutzis V, Davis‐Tuck M, Wallace EM. Oxytocin infusion at elective caesarean section: a double blind, randomised controlled trial. Journal of Paediatrics and Child Health 2015;51(Suppl 1):54. 25586845 [Google Scholar]
- Modi V, Goel JK, Kashyap A, Arya SB, Kar J, Goel R. Active management of third stage of labor: A comparison of various uterotonic. Journal of South Asian Federation of Obstetrics and Gynaecology ‐ SAFOG 2014;6(3):151‐5. [Google Scholar]
- Mohamadian S, Shorab NJ, Mirzakhani K. The effect of the timing of intramuscular oxytocin injection on maternal bleeding during the third stage of labour. Journal of Midwifery & Reproductive Health 2013;1(2):66‐70. [Google Scholar]
- Mohamed HF, Mustafa GF, Ibrahim MA, Stefanos GE. Comparative study between intravenous bolus dose of carbetocin versus oxytocin during cesarean delivery in healthy parturients on blood loss: a randomized control trial. Medical Journal of Cairo University 2015;83(2):167‐72. [Google Scholar]
- Murphy D, ISRCTN14718882. A study to compare the effectiveness of intravenous oxytocin with intramuscular oxytocin given at the third stage of labour at preventing bleeding at vaginal birth. isrctn.com/ISRCTN14718882 (first received 14 December 2015).
- Nankaly A, Jalilian N, Eshghiali S, Rezaei M. The effects of sublingual misoprostol and intravenous oxytocin in reducing bleeding among cesarean deliveries. Acta Medica Mediterranea 2016;32:953‐7. [Google Scholar]
- Narenji F. Comparison the effect of intramuscular injection of oxytocin and nipple stimulation on the third stage of delivery length and bleeding. IRCT Iranian Registry of Clinical Trials (www.irct.ir) [accessed 16 July 2015]. IRCT201206099981N1 2012.
- Neri‐Mejia M, Pedraza‐Aviles AG. Active management of the third stage of labor: three schemes of oxytocin: randomised clinical trial. Ginecologia y Obstetricia De Mexico 2016;84(5):306‐13. [PubMed] [Google Scholar]
- Ng PS, Yuen PM, Sahota DS. Comparison of oral misoprostol and intravascular syntocinon in the management of the third stage of labour ‐ a double‐blind randomised controlled trial. 30th British Congress of Obstetrics and Gynaecology; 2004 July 7‐9; Glasgow, UK. 2004:69. [Google Scholar]
- Nguyen‐Lu N, Carvalho JC, Farine D, Seaward G, Ye XY, Balki M. Carbetocin at cesarean delivery for labour arrest: a sequential allocation trial to determine the effective dose. Canadian Journal of Anaesthesia//Journal Canadien D'anesthesie 2015;62(8):866‐74. [DOI] [PubMed] [Google Scholar]
- Ononge S, Campbell OMR, Kaharuza F, Lewis JJ, Fielding K, Mirembe F. Effectiveness and safety of misoprostol distributed to antenatal women to prevent postpartum haemorrhage after child‐births: a stepped‐wedge cluster‐randomized trial. BMC Pregnancy and Childbirth 2015;15:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othman ER, Fayez MF, Aal DEMA, El‐Dine Mohamed HS, Abbas AM, Ali MK. Sublingual misoprostol versus intravenous oxytocin in reducing bleeding during and after cesarean delivery: a randomized clinical trial. Taiwanese Journal of Obstetrics and Gynecology 2016;55(6):791‐5. [DOI] [PubMed] [Google Scholar]
- Pakniat H, Khezri MB. The effect of combined oxytocin‐misoprostol versus oxytocin and misoprostol alone in reducing blood loss at cesarean delivery: a prospective randomized double‐blind study. Journal of Obstetrics and Gynaecology of India 2015;65(6):376‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil NB, Patted SS. A randomised controlled trial of oral misoprostol vs injection methylergometrine for prevention of post partum hemorrhage. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 2013;2(3):296‐303. [Google Scholar]
- Quibel T, Ghout I, Goffinet F, Salomon LJ, Fort J, Javoise S, et al. Active management of the third stage of labor with a combination of oxytocin and misoprostol to prevent postpartum hemorrhage: a randomized controlled trial. Obstetrics and Gynecology 2016;128(4):805‐11. [DOI] [PubMed] [Google Scholar]
- Rabow S, Jonsson E, Jonsson H, Olofsson P. Cardiovascular effects of oxytocin and carbetocin at caesarean section, a prospective double‐blind randomised study using non‐invasive pulse wave analysis. Acta Anaesthesiologica Scandinavica2017; Vol. 61, issue 8:1053. [DOI] [PubMed]
- Ragab A, Barakat R, Alsammani MA. A randomized clinical trial of preoperative versus postoperative misoprostol in elective cesarean delivery. International Journal of Gynaecology and Obstetrics 2016;132(1):82‐4. [DOI] [PubMed] [Google Scholar]
- Raghavan S, Geller S, Miller S, Goudar SS, Anger H, Yadavannavar MC, et al. Misoprostol for primary versus secondary prevention of postpartum haemorrhage: a cluster‐randomised non‐inferiority community trial. BJOG : aan international journal of obstetrics and gynaecology 2016;123:120‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray D, Ghosh S, Bhattacharya S, Mandal RD, Basak A. Oxytocin administration during caesarian delivery: comparison between bolus versus infusion. Society for Obstetric Anesthesia and Perinatology (SOAP) 44th Annual Meeting; 2015 May 2‐5; Monterey, USA. 2012:T‐26. [Google Scholar]
- Razali N, Md Latar IL, Chan YK, Omar SZ, Tan PC. Carbetocin compared to oxytocin in emergency cesarean section: a randomized trial. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2016;198:35‐9. [DOI] [PubMed] [Google Scholar]
- Reyes OA. Carbetocin vs oxytocin for the prevention of postpartum hemorrhage in grand multiparous patients: A randomized controlled trial. [Spanish]. Clinica e investigacion en ginecologia y obstetricia2011; Vol. 38, issue 1:2‐7.
- Rosales‐Ortiz S, Aguado RP, Hernandez RS, Castorena M, Cristobal FL, Gonzalez MC, et al. Carbetocin versus oxytocin for prevention of postpartum haemorrhage: A randomised controlled trial. Lancet 2014;383:S51. [DOI] [PubMed] [Google Scholar]
- Sangkhomkhamhang U, ACTRN12612000624886. A randomised controlled trial of intravenous versus intramuscular oxytocin in the management of third stage of labor. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12612000624886 (first received 12 June 2012).
- Sentilhes L, Daniel V, Darsonval A, Deruelle P, Vardon D, Perrotin F, et al. Study protocol. TRAAP ‐ TRAnexamic Acid for Preventing postpartum hemorrhage after vaginal delivery: a multicenter randomized, double‐blind, placebo‐controlled trial. BMC Pregnancy and Childbirth 2015;15:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senturk S, Kagitci M, Balik G, Arslan H, Kir Sahin F. The effect of the combined use of methylergonovine and oxytocin during caesarean section in the prevention of post‐partum haemorrhage. Basic & Clinical Pharmacology & Toxicology 2016;118(5):338‐43. [DOI] [PubMed] [Google Scholar]
- Shrestha A, Urala MS, Upreti D, Niraula S. Comparison of intramyometrial and intramuscular 15 methyl PGF2x against traditional prophylactic intramuscular methergin for the active management of third stage of labor. Nepal Journal of Obstetrics and Gynaecology 2008;3(2):35‐9. [Google Scholar]
- Shrivasatava DD, Khamsara D. Critical evaluation of sublingual misoprostol and methyl ergometrine in active management of third stage of labour. International Journal of Gynecology and Obstetrics 2012;119(Suppl 3):S484. [Google Scholar]
- Soleimani Z, Naini AA. The effectiveness of sublingual misoprostol in prevention of bleeding during cesarean delivery. [Persian]. Iranian Journal of Obstetrics, Gynecology and Infertility2014; Vol. 17, issue 125:1‐7.
- Sunil Kumar KS, Shyam S, Batakurki P. Carboprost versus oxytocin for active management of third stage of labor: a prospective randomized control study. Journal of Obstetrics and Gynaecology of India 2016;66(Suppl 1):S229‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheripanah R, Shoman A, Ali Karimzadeh M, Zamaniyan M, Malih N. Efficacy of oxytocin vs. Carbetocin in prevention of postpartum hemorrhage after cesarean section under general anesthesia: a prospective randomized clinical trial. Journal of Maternal‐Fetal & Neonatal Medicine2017 [epub ahead of print]. [DOI] [PubMed]
- Tali K, Ignacio Alensuela A. The effect of prophylactic intravenous tranexamic acid in reducing blood loss after vaginal delivery in women at low risk of postpartum haemorrhage: A prospective, randomised, double‐blind, placebo‐controlled study. Australian and New Zealand Journal of Obstetrics and Gynaecology 2016;56(Suppl 1):61. [Google Scholar]
- Ugwu IA, Oluwasola TA, Enabor OO, Anayochukwu‐Ugwu NN, Adeyemi AB, Olayemi OO. Randomized controlled trial comparing 200mug and 400mug sublingual misoprostol for prevention of primary postpartum hemorrhage. International Journal of Gynaecology and Obstetrics 2016;133:173‐7. [DOI] [PubMed] [Google Scholar]
- Un Nisa S, Usmani SY. Role of intravenous Syntocinon in prevention of primary postpartum haemorrhage. Pakistan Journal of Medical and Health Sciences2012; Vol. 6, issue 4:1020‐3.
- Vlassoff M, Diallo A, Philbin J, Kost K, Bankole A. Cost‐effectiveness of two interventions for the prevention of postpartum hemorrhage in Senegal. International Journal of Gynecology and Obstetrics 2016;133(3):307‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voltolini C, Bonis M, Vellucci F, Regini C, Orlandini C, Vannuccini S, et al. Carbetocin versus oxytocin after caesarean section: Similar efficacy but reduced pain perception in women with high risk of postpartum haemorrhage. International Journal of Gynecology and Obstetrics 2012;119:S806‐7. [DOI] [PubMed] [Google Scholar]
- Weeks AD, Ditai J, Ononge S, Faragher B, Frye LJ, Durocher J, et al. The MamaMiso study of self‐administered misoprostol to prevent bleeding after childbirth in rural Uganda: a community‐based, placebo‐controlled randomised trial. BMC Pregnancy and Childbirth 2015;15(1):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whigham CA, Gorelik A, Loughnan TE, Trivedi A. Carbetocin versus oxytocin to reduce additional uterotonic use at non‐elective caesarean section: a double‐blind, randomised trial. Journal of Maternal‐Fetal & Neonatal Medicine 2016;29(23):3866‐9. [DOI] [PubMed] [Google Scholar]
- Winikoff B, Dzuba I, Carroli G, NCT02954068. Intravenous versus intramuscular administration of oxytocin and its relationship with postpartum bleeding and other clinical signs: a randomized placebo‐controlled study. clinicaltrials.gov/show/NCT02954068 (first received 28 October 2016).
References to ongoing studies
- NCT01733329. Buccal misoprostol during cesarean section for preventing postpartum hemorrhage in women with risk factors for uterine atony. clinicaltrials.gov/ct2/show/NCT01733329 16 November 2012.
- NCT01487278. Comparing misoprostol and oxytocin in UnijectTM postpartum hemorrhage (PPH) prevention in Mali. clinicaltrials.gov/ct2/show/NCT01487278 Date first received: 5 December 2011.
- NCT01713153. Comparing misoprostol and oxytocin in uniject for postpartum hemorrhage (PPH) prevention in Senegal. clinicaltrials.gov/ct2/show/NCT01713153 Date first received: 22 October 2012.
- Draycott T, Nelson HA. Intramuscular oxytocics: a comparison study of intramuscular carbetocin, syntocinon and syntometrine for the third stage of labour following vaginal birth (IMox). clinicaltrials.gov/ct2/show/NCT02216383 (first received 15 August 2014).
- Gomez MCG. Comparison of the effectiveness of carbetocin vs oxytocin in managing the third stage of labor in a group of women with risk factors for postpartum hemorrhage. Australian New Zealand Clinical Trials Registry (www.anzctr.org.au) (accessed 16 February 2011). ACTRN12610000550000 2011.
- IRCT138810212854N2. Comparison effect of carbetocine and syntometrin in prevention of post partum hemorrhage. en.search.irct.ir/view/2059 Date first received: 15 March 2010.
- IRCT201008212854N5. Comparison of the effect of rectal misoprostol and syntometrin in prevention of post partum hemorrhage. en.search.irct.ir/view/3932 Date first received: 9 September 2010.
- IRCT138812223548N1. Comparison of misoprostol and oxytocin in reduction of postpartum hemorrhage. en.search.irct.ir/view/2616 Date first received: 29 May 2010.
- NCT01863706. Misoprostol versus oxytocin for prevention of post partum hemorrhage. clinicaltrials.gov/ct2/show/NCT01863706 Date first received: 23 May 2013.
- NCT02083107. Comparison between rectal and sublingual misoprostol before caesarian section to reduce intra & post‐operative blood loss. clinicaltrials.gov/ct2/show/NCT02083107 Date first received: 7 March 2014.
- Widmer M, Piaggio G, Abdel‐Aleem H, Carroli G, Chong Y, Coomarasamy A, et al. Room temperature stable carbetocin for the prevention of postpartum haemorrhage during the third stage of labour in women delivering vaginally: study protocol for a randomized controlled trial. Trials 2016;17:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
- Alkema L, Chou D, Hogan D, Zhang S, Moller AB, Gemmill A, et al. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario‐based projections to 2030: a systematic analysis by the UN Maternal Mortality Estimation Inter‐Agency Group. Lancet 2016;387:462‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley CM, Gyte GML, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD007412.pub4] [DOI] [PubMed] [Google Scholar]
- Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331:897‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs CA, Murphy EL, Laros RK Jr. Factors associated with postpartum hemorrhage with vaginal birth. Obstetrics and Gynecology 1991;77:69‐76. [PubMed] [Google Scholar]
- Davies NM, Longstreth J, Jamali F. Misoprostol therapeutics revisited. Pharmacotherapy 2001;21:60‐73. [DOI] [PubMed] [Google Scholar]
- Groot AN. The role of oral (methyl) ergometrine in the prevention of postpartum haemorrhage. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1996;69:31‐6. [DOI] [PubMed] [Google Scholar]
- Groot AN, Dongen PW, Vree TB, Hekster YA, Roosmalen J. Ergot alkaloids. Current status and review of clinical pharmacology and therapeutic use compared with other oxytocics in obstetrics and gynaecology. Drugs 1998;56:523‐35. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
- Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta‐analysis of randomized controlled trials. Medical Decision Making2013; Vol. 33, issue 5:607‐17. [DOI] [PMC free article] [PubMed]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency innetwork meta‐analysis: Concepts and models for multi‐arm studies. Res Synth Methods 2012;3(2):98‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogerzeil HV, Walker GJA, Goeje MJ. Stability of Injectable Oxytocics in Tropical Climates. Results of Field Surveys and Simulation Studies on Ergometrine, Methylergometrine and Oxytocin. Geneva: World Health Organization (WHO), 1993. [Google Scholar]
- Hunter DJ, Schulz P, Wassenaar W. Effect of carbetocin, a long‐acting oxytocin analog on the postpartum uterus. Clinical Pharmacology and Therapeutics 1992;52:60‐7. [DOI] [PubMed] [Google Scholar]
- Liabsuetrakul T, Choobun T, Peeyananjarassri K, Islam QM. Prophylactic use of ergot alkaloids in the third stage of labour. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD005456.pub2] [DOI] [PubMed] [Google Scholar]
- Lumley T. Network meta‐analysis for indirect treatment comparisons. Statistics in Medicine 2002;21(16):2313‐24. [DOI] [PubMed] [Google Scholar]
- McDonald SJ, Abbott JM, Higgins SP. Prophylactic ergometrine‐oxytocin versus oxytocin for the third stage of labour. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD000201.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicines.org.uk. Syntocinon Ampoules 5 IU/ml ‐ Summary of Product Characteristics (SPC) ‐ (eMC). Available from: http://www.medicines.org.uk/emc/medicine/16423/SPC2014; Vol. [cited 10 October 2014].
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta‐analyses of osteoarthritis trials: meta‐epidemiological study. BMJ 2010;341:c3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penney G, Brace V. Near miss audit in obstetrics. Current Opinion in Obstetrics & Gynecology 2007;19:145‐50. [DOI] [PubMed] [Google Scholar]
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello‐Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta‐analysis. BMJ 2014;349:g5630. [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: An overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163‐71. [DOI] [PubMed] [Google Scholar]
- Say L, Chou D, Gemmill A, Tunçalp O, Moller A, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Global Health 2014;2(6):e323‐e333. [DOI] [PubMed] [Google Scholar]
- Schaff EA, DiCenzo R, Fielding SL. Comparison of misoprostol plasma concentrations following buccal and sublingual administration. Contraception 2005;71(1):22‐5. [DOI] [PubMed] [Google Scholar]
- Souza JP, Gülmezoglu AM, Vogel J, Carroli G, Lumbiganon P, Qureshi Z, et al. Moving beyond essential interventions for reduction of maternal mortality (the WHO Multicountry Survey on Maternal and Newborn Health): a cross‐sectional study. Lancet 2013;381:1747‐55. [DOI] [PubMed] [Google Scholar]
- Su LL, Chong YS, Samuel M. Carbetocin for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD005457.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuncalp O, Hofmeyr GJ, Gulmezoglu AM. Prostaglandins for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD000494.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weekes LR, O'Toole DM. Postpartum hemorrhage; a five‐year study at Queen of Angels Hospital. American Journal of Obstetrics and Gynecology 1956;71:45‐50. [PubMed] [Google Scholar]
- Westhoff G, Cotter AM, Tolosa JE. Prophylactic oxytocin for the third stage of labour to prevent postpartum haemorrhage. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD001808.pub2] [DOI] [PubMed] [Google Scholar]
- White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network metaanalysis:Model estimation using multivariate meta‐regression. Research Synthesis Methods 2012;3(2):111‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White IR. Network meta‐analysis. Stata Journal 2015;15(4):951‐85. [Google Scholar]
- WHO. Stability of Injectable Oxytocics in Tropical Climates: Results of Field Surveys and Simulation Studies on Ergometrine, Methylergometrine, and Oxytocin. Geneva: World Health Organization, 1993. [Google Scholar]
- WHO. Evaluating the Quality of Care for Severe Pregnancy Complications: the WHO Near‐Miss Approach for Maternal Health. Geneva: World Health Organization, 2011. [Google Scholar]
- WHO. WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage. Geneva: Department of Reproductive Health, World Health Organization, 2012. [PubMed] [Google Scholar]
References to other published versions of this review
- Gallos ID, Williams HM, Price MJ, Merriel A, Gee H, Lissauer D, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta‐analysis. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD011689] [DOI] [PMC free article] [PubMed] [Google Scholar]


