Maternally administered oxytocin leads to changes in offspring social behavior and neurobiology.
Abstract
Oxytocin is used in approximately half of all births in the United States during labor induction and/or augmentation. However, the effects of maternal oxytocin administration on offspring development have not been fully characterized. Here, we used the socially monogamous prairie vole to examine the hypothesis that oxytocin exposure at birth can have long-term developmental consequences. Maternally administered oxytocin increased methylation of the oxytocin receptor (Oxtr) in the fetal brain. As adults, oxytocin-exposed voles were more gregarious, with increased alloparental caregiving toward pups and increased close social contact with other adults. Cross-fostering indicated that these effects were the result of direct action on the offspring, rather than indirect effects via postnatal changes in maternal behavior. Male oxytocin-exposed offspring had increased oxytocin receptor density and expression in the brain as adults. These results show that long-term effects of perinatal oxytocin may be mediated by an epigenetic mechanism.
INTRODUCTION
The hormone oxytocin (OXT) triggers uterine contractions at birth, facilitating expulsion of the fetus. In contemporary obstetric care, OXT is the most widely used uterotonic agent (1) and is used to both induce and augment labor. Since 1990, the rate of labor induction in the United States has increased from 9.5 to 23.8% of births in 2015 (2). When including labor augmentation, national surveys estimate that approximately 50% of women receive some form of exogenous OXT during labor (3, 4). Conversely, infants delivered by cesarean section have lower levels of OXT than those delivered vaginally (5). It has conventionally been thought that maternal OXT would neither reach the fetus nor produce long-lasting effects. However, this assumption has not been thoroughly tested.
What might be the consequences on offspring neurodevelopment as a result of exogenous OXT administration at birth? OXT serves a variety of functions in the neonate, all broadly related to helping achieve a transition from the uterine environment to the gradual process of establishing homeostatic independence (6). These functions in the fetus/neonate are analogous to how OXT triggers the onset of maternal behavior in the mother’s brain (7). At birth, OXT serves to protect the fetal brain from the hypoxic conditions of birth by triggering a transient switch in γ-aminobutyric acid signaling (8), along with several other related functions (9–12). The process by which exposure to a hormone in early life produces an enduring effect in the developing organism is known as “hormonal imprinting,” which has been observed following OXT exposure (13). The neonatal period appears to be a sensitive period in terms of OXT’s actions within the brain (6, 13, 14). This suggests that changes in perinatal OXT signaling can alter developmental trajectory. Following birth and across development, OXT regulates a variety of social behaviors typically associated with affiliation, caregiving, and bonding (15). We therefore hypothesized that OXT exposure at birth could influence subsequent social behavior.
In humans, labor induction is associated with changes in neonatal behavior (16) and delayed motor development (17) in the acute phase soon after birth. There has been little work done on the possible long-term consequences of OXT exposure beyond some inconsistent associations between maternal OXT administration (either induction or augmentation) and heightened risk for later development of autism in offspring (18–21). However, all of these associations have been correlational, and we do not know whether this association is due to the administration of exogenous OXT per se or the need for such administration is due to low endogenous OXT levels.
Here, we used the monogamous prairie vole to experimentally investigate the effects of maternally administered OXT in the immediate prepartum period on the brains and behavior of offspring. As a proof of concept, we tested whether maternal administration of a single IU of OXT (0.03 mg/kg) would produce acute physiological effects in the fetus (plasma OXT levels and c-fos immunoreactivity in the brain). Second, we sought to confirm fetal physiological sensitivity to maternal OXT without the confounding influence of induced uterine contractions and hence tested fetal heart rate following removal from the uterus while still maintaining the umbilical connection. Next, we tested how long-lasting consequences of OXT could be established in the fetal brain via changes to the epigenetic regulation of the OXT receptor (Oxtr) following a single administration of OXT (0.125 to 0.5 mg/kg, administered once on the expected day of delivery). Last, we used the intermediate dose of OXT (0.25 mg/kg) administered maternally on the day of delivery to explore the effects on offspring brain and behavior. The range of doses chosen for this study was based on previous work from our laboratory that used 0.4 mg/kg of OXT administered to voles daily (22) and 1 mg/kg administered once to pups on the first postnatal day (15, 23). Prairie voles have relatively high levels of plasma OXT (24), so the doses used in the present work are high by the standards of other species but still below threshold to induce labor in the vole.
RESULTS
The fetus is sensitive to maternally administered OXT
In experiments 1 and 2, we measured acute physiological responses of the fetus to maternal OXT administration to establish fetal sensitivity to maternal OXT. Maternally administered OXT (0.03 mg/kg) on the expected day of delivery resulted in increased fetal plasma OXT levels (experiment 1, P < 0.05; Fig. 1A) as well as an increase in neuronal activity in two OXT-sensitive regions, the paraventricular nucleus and supraoptic nucleus, both located within the hypothalamus (P < 0.05 for both comparisons; Fig. 1, B and C). Since OXT produces acute bradycardic effect in adults (25), we assessed the acute effect of OXT on heart rate in fetal voles in experiment 2. Similar to adults, direct administration of OXT (0.25 mg/kg) to vole fetuses produced an acute bradycardic response 5 min after injection (experiment 2, P < 0.05; Fig. 1D). Next, we tested whether maternally administered OXT would have a similar effect. To remove the confounding influence of uterine contractions restricting blood supply and thereby slowing fetal heart rate, we removed fetal pups from the uterus and excised them via laparotomy while still maintaining a connection to the maternal circulation via the umbilical cord (experiment 2b; Fig. 1E). Intraperitoneal administration of OXT to the dam produced a rapid decrease in fetal heart rate (Fig. 1F). The fetal heart rate decline continued over the course of the 5-min recording, an effect not seen in the group treated with saline vehicle (SAL) (P < 0.01; Fig. 1G). Pretreatment with the OXT antagonist L-368,899 (OTA) completely abolished this effect (P < 0.01, Fig. 1G). There were no effects of sex on fetal heart rate. OTA also led to a higher resting heart rate in the fetus, which suggests a tonically bradycardic role for OXT. Thus, we conclude that the fetal vole pup is acutely sensitive to maternally administered OXT.
Fig. 1. The fetus is acutely responsive to maternal OXT.
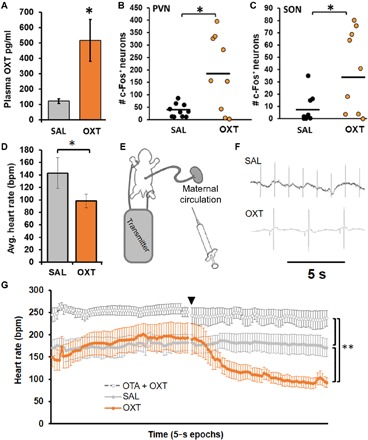
(A) Experiment 1a: Plasma OXT was higher in fetal vole pups after maternal treatment with OXT (0.03 mg/kg, n = 6) compared to saline vehicle (SAL, n = 7). Results from two OXT litters were discarded because of excessively high values that fell beyond the linear portion of the assay’s sensitivity curve. (B and C) Experiment 1b: In a separate set of animals, the number of c-fos + nuclei was higher in fetal vole pups after maternal treatment with OXT (0.03 mg/kg, n = 9) compared to SAL (n = 10) in both the paraventricular nucleus (PVN) (B) and supraoptic nucleus (SON) (C). (D) Experiment 2a: Heart rate in postnatal day 1 (PND 1) vole pups was lower after direct injection of OXT (0.25 mg/kg, n = 7) compared to SAL (n = 7). (E) Experiment 2b: Heart rate recording paradigm for term fetal vole pups while still connected to maternal blood supply via the umbilical cord. (F) Representative traces of fetal heart rate from experiment 2b. (G) Heart rate in fetal vole pups (approximately embryonic day 21) was lower after maternal OXT treatment (0.25 mg/kg, n = 6) compared to SAL (n = 7); this was prevented by pretreatment with an OXT antagonist (OTA, 2 mg/kg, n = 5) 10 min prior. Data are presented as 5 min of baseline with maternal treatment occurring at the time point denoted by the black triangle, followed by another 5 min of recording. Data shown are means ± SEM *P < 0.05; **P < 0.01.
Maternally administered OXT leads to regulatory changes in fetal Oxtr
In experiment 3, we measured Oxtr DNA methylation as a percentage of CpGs (cytosines prior to guanines) that were methylated across four sites conserved between prairie voles and humans (Fig. 2A) (26) at 90 min after maternal OXT administration (0.125 to 0.5 mg/kg). Variation in fetal weight served as a proxy for gestational development and revealed that expression and epigenetic regulation of Oxtr appear to change as parturition approaches. As predicted by the canonical relationship between methylation and transcription, Oxtr methylation negatively predicted Oxtr mRNA levels (Fig. 2C). Across all three brain regions, increasing fetal weight was negatively correlated with Oxtr methylation (Fig. 2D) and positively correlated with Oxtr mRNA expression (Fig. 2E). Analyses of Oxtr methylation revealed an effect of fetal weight [F(1,1071) = 11.16, P < 0.001] and a dose × fetal weight interaction [F(1,1071) = 6.01, P = 0.014], such that methylation was higher in the OXT-treated groups among heavier pups. Oxtr expression was evaluated as proportional to GAPDH mRNA, repeated throughout the same three brain regions, and analyses revealed effects of fetal weight [F(1,80) = 6.05, P = 0.016] and region [F(2,133) = 5.11, P = 0.007], as well as a dose × fetal weight × methylation × region interaction [F(2,132) = 11.05, P < 0.001], such that fetal weight led to increases in Oxtr expression, and methylation was generally associated with decreases in Oxtr expression.
Fig. 2. DNA methylation of Oxtr promoter within the fetal brain is reduced with advancing gestation but increased by maternal OXT.
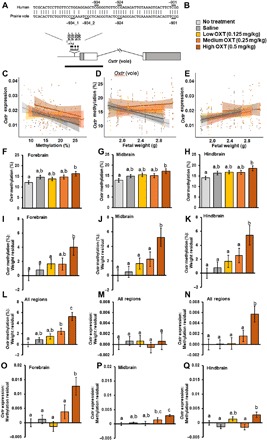
Experiment 3: The methylation and expression of Oxtr in fetal vole pup brains on the expected day of delivery and the acute effects of maternal OXT administration. (A) Sequence homology between human and prairie vole Oxtr genes with respect to four CpG sites assayed in this study. (B) Treatment key for the following graphs of data from approximately embryonic day 21 fetal vole pup brains (n = 15 to 22 per group). (C) Oxtr expression was significantly negatively predicted by Oxtr methylation in the saline and no-treatment conditions and not in the OXT treatment conditions. Oxtr expression was measured relative to GAPDH expression using the ΔCt method (2−ΔCt). (D) Oxtr DNA methylation was significantly negatively predicted by fetal weight in the saline and no-treatment conditions and not in the OXT treatment conditions. (E) Oxtr expression was significantly positively predicted by fetal weight in the saline and no-treatment conditions and not in the OXT treatment conditions. (F to H) The overall differences in Oxtr methylation between treatment conditions revealed higher methylation in the high-dose OXT condition compared to the no-treatment condition across all three brain regions. (I to K) Once fetal body weight was taken into account, there was a dose-dependent increase in Oxtr DNA methylation for a given fetal weight, with the high-dose OXT condition showing higher methylation levels than other conditions across all three brain regions. (L) Looking across the entire fetal brain, there was a dose-dependent increase in Oxtr DNA methylation for a given fetal weight following OXT treatment based on the expected relationship between fetal body weight and Oxtr methylation observed in the no-treatment condition. (M) Looking across the entire fetal brain, there were no treatment effects on the relationship between fetal weight and Oxtr expression level. (N) Looking across the entire fetal brain, the high-OXT treatment produced an increase in the level of Oxtr expression relative to the expected level of expression based on fetal body weight and Oxtr methylation observed in the no-treatment condition. This effect was most prominent in the forebrain (O), somewhat present in the midbrain, (P) and absent in the hindbrain (Q). Significant differences between groups are illustrated by different lowercase letters above each bar; groups sharing the same letter did not differ.
OXT treatment also affected the relationship between fetal weight and Oxtr regulation, especially in the high-OXT treatment group (0.5 mg/kg). We observed a dose-dependent increase in the slope of the weight-methylation relationship (Fig. 2D). For each of the relationships examined, i.e., (i) Oxtr methylation−fetal weight, (ii) Oxtr expression−fetal weight, and (iii) Oxtr expression−Oxtr methylation, there were significant correlations for the saline and no-treatment conditions (P < 0.05) but not for any of the OXT doses (Fig. 2, C to E). For a given weight, there was a dose-dependent increase in methylation across the three brain regions (Fig. 2, F and I to K). The relationship between methylation level and transcription remained the same but was shifted upward in the high-OXT group (Fig. 2C). For a given weight and degree of methylation, the high-OXT group responded with increased Oxtr expression (Fig. 2, N and O). There were no effects of either maternal OXT treatment or fetal body weight on expression levels of the vasopressin receptor V1aR, and no effect of sex on any measure, except V1aR expression, which was higher in males throughout the brain (P < 0.05).
Maternally administered OXT leads to changes in offspring social behavior throughout development
In experiment 4, we evaluated the impact of maternally administered OXT (0.25 mg/kg) at birth on offspring behavior throughout development. To account for the possibility of prenatal OXT affecting maternal behavior and thereby indirectly affecting offspring development, we implemented a cross-fostering paradigm (Fig. 3A). Because OXT has been shown to affect the production of ultrasonic vocalizations (USVs) in rodent pups (27), we chose to first examine this behavior. Overall, there were significant effects of age [F(22,167) = 5.397, P < 0.001], treatment [F(22,167) = 2.419, P = 0.001], and an interaction between treatment and sex [F(44,336) = 1.572, P = 0.015] such that male SAL pups vocalized for a longer duration than female SAL pups (P = 0.049). Across postnatal days 1 and 4, vole pups born to OXT-treated dams emitted more vocalizations (P = 0.013; Fig. 3B) and vocalized for longer (P = 0.002; Fig. 3C). There were no differences in pup weights at any age. In addition, we observed no differences in maternal time spent on the nest as a result of OXT administration (fig. S1).
Fig. 3. Maternal OXT at birth shapes offspring behavior throughout development.
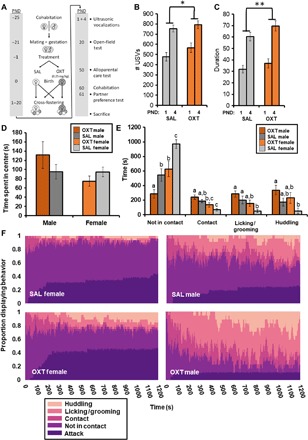
Experiment 4: The behavioral effects of maternal OXT administration across development. (A) Experimental design with cross-fostering to examine developmental consequences of maternal OXT administration (0.25 mg/kg) at birth. (B) Offspring delivered by OXT-treated dams emitted more vocalizations than offspring delivered by SAL-treated dams on postnatal days 1 and 4 (n = 11 to 20 per group). (C) Offspring (1 and 4 days old) delivered by OXT-treated dams vocalized for longer than offspring delivered by SAL-treated dams (n = 11 to 20 per group). (D) No effects of OXT treatment on behavior in the OFT at postnatal day 20. (E) In response to an unfamiliar, unrelated pup, male sex and OXT birth treatment were both associated with increases in alloparental caregiving at postnatal day 50 (n = 17 to 31 per group). (F) Alloparental caregiving behavior expressed as a proportion of animals within a group displaying each behavior, showing the progression from nonresponsive to alloparental over the 20-min time course and including subjects that responded aggressively (attacked). Data shown are means ± SEM. *P < 0.05; **P < 0.01. Significant differences between groups in (F) are illustrated by different lowercase letters above each bar; groups sharing the same letter did not differ.
At weaning age (postnatal day 20), offspring were tested for anxiety-like behavior in the open-field test (OFT) since OXT has been implicated in the regulation of anxiety (28). We observed no treatment effects nor sex differences in anxiety-like behavior (Fig. 3D). As subadults (postnatal days 50 to 55), offspring were tested for caregiving behavior in the alloparental care test (APC) since OXT has been implicated in the regulation of alloparenting (29, 30). As has been previously reported (31), we observed a significant effect of sex, such that male voles were more alloparental than females [F(5,55) = 6.208, P < 0.001]. In addition, there was a main effect of treatment [F(5,55) = 4.43, P = 0.002], such that offspring of OXT-treated dams displayed more alloparental caregiving (Fig. 3, E and F). The proportion of subjects attacking the pup did not vary between treatment conditions (SAL females: 12 of 31, OXT females: 14 of 28, SAL males: 5 of 21, and OXT males: 3 of 17). The USV, OFT, and APC findings were all replications from a preliminary study conducted without cross-fostering that showed a similar pattern of effects, albeit within the OXT-treated group, only females were more alloparental (fig. S2).
As adults (postnatal day 60), offspring were paired with an opposite sex conspecific and tested for partner preference formation in the partner preference test (PPT) since OXT has been implicated in social bonding and monogamy (32). Here, we observed that whereas females of both treatment conditions formed partner preferences, males did not (Fig. 4B). When time spent side-by-side huddling was collapsed across Partner and Stranger, offspring of OXT-treated dams spent more time side-by-side huddling compared to offspring of saline-treated dams [F(1,90) = 4.21, P = 0.015; Fig. 4C] and less time alone in the solitary cage [F(1,90) = 12.86, P = 0.001; Fig. 4D]. There was also a trend of offspring of OXT-treated dams spending more time in the Stranger’s cage (P = 0.056) and huddling with the Stranger (P = 0.056), along with a sex × treatment interaction indicating more time spent in the Partner’s cage [F(1,90) = 4.98, P = 0.028] among females of the OXT condition. In none of the behavioral measures tested did we observe an effect of rearing condition, that is, the treatment of the foster dam.
Fig. 4. The behavioral effects of maternal OXT administration on pair bonding in adulthood (experiment 4 continued).
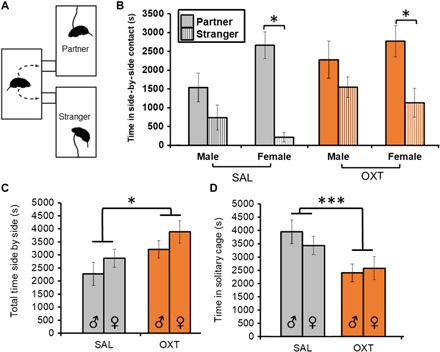
(A) The PPT paradigm, where a given subject is free to spend time with either of two conspecifics of the opposite sex, one unfamiliar (Stranger) and one with which the subject has cohabited for 24 hours (Partner), tested here in adulthood on postnatal day 60. (B) Partner preference formation, indexed by significantly more time spent in side-by-side contact with the Partner versus Stranger, was achieved in females of both treatment conditions (n = 16 to 29 per group). (C) When collapsed across both Partner and Stranger, OXT-treated animals showed increased total time in side-by-side contact (n = 16 to 29 per group). (D) OXT treatment was also associated with less time spent in the solitary cage (n = 16 to 29 per group). Data shown are means ± SEM *P < 0.05; ***P < 0.001.
Maternally administered OXT leads to changes in the endogenous OXT system within the brains of adult offspring
Following completion of this battery of behavioral tests, subjects were euthanized, and brain tissue was collected and immediately frozen. To examine the effects of OXT (0.25 mg/kg) exposure in early life on the state of endogenous OXT regulation in adulthood, we dissected brains for regional analysis of Oxtr at the level of DNA methylation, mRNA expression, and protein levels.
Autoradiographic analyses revealed sex- and region-specific changes in neuropeptide receptor densities in adulthood. The adult male offspring of OXT-treated dams displayed increased OXTR density across several brain regions (Fig. 5). We observed sex × treatment interaction effects on OXTR density in the central amygdala [F(1,67) = 4.3, P = 0.042; Fig. 5K], parietal cortex [F(1,68) = 5.03, P = 0.028; Fig. 5L], and insular cortex [F(1,53) = 11.46, P = 0.001; F(1,42) = 6.5, P = 0.014; and F(1,65) = 7.56, P = 0.008; Fig. 5M]. The insular cortex in particular, which was measured throughout the rostral-caudal axis of the brain at three points, showed a consistent pattern of increased OXTR density in males of the OXT birth treatment group. There was a trend of sex difference in the lateral septum [F(1,43) = 4.05, P = 0.051], with males showing increased OXTR density. No differences in OXTR were found in the bed nucleus of the stria terminalis, nucleus accumbens (NAcc) (both shell and core), and ventromedial hypothalamus. There were several trends for sex differences in V1aR, with males expressing V1aR at higher densities, and a sex × treatment effect in the ventral pallidum [F(1,58) = 4.31, P = 0.042], with OXT treatment decreasing V1aR density in males (fig. S6).
Fig. 5. Maternal OXT administration leads to increased OXTR density in the brains of male offspring in adulthood.
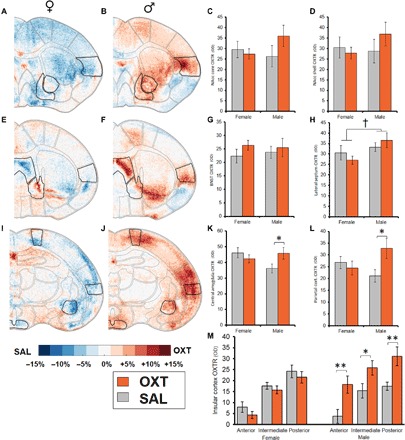
Experiment 4 (continued): Autoradiographic analyses of OXTR density throughout the brain expressed in terms of optical density (OD). Within-sex comparisons between adult offspring of OXT- and SAL-treated dams are shown as heat maps in which blue colors represent regions where SAL-treated animals displayed higher OXTR density and red colors represent regions where OXT-treated animals displayed higher OXTR density [n = 11 to 20 per group; females: (A), (E), and (I); males: (B), (F), and (J)]. Comparisons of specific regions of interest (outlined) are shown on the right. There were no effects observed in the NAcc core (C), shell (D), or BNST (G). (H) Male sex was associated with a trend toward increased OXTR density within the lateral septum. There were multiple sex × treatment interactions revealing that males exposed to OXT treatment condition showed increased OXTR density compared to SAL males in the central amygdala (K) and parietal cortex (L) and throughout the anterior-posterior extent of the insular cortex (M). Data shown are means ± SEM. †P = 0.051; *P < 0.05; **P < 0.01.
These findings were broadly consistent with molecular results from samples of three contralateral brain regions (central amygdala, insular cortex, and NAcc; fig. S7). We also observed a consistent pattern of females having greater methylation in the Oxtr promoter region than males in each of three regions examined (P = 0.003 to 0.025). Last, females that spent more time alongside the Partner than the Stranger showed consistently less Oxtr promoter methylation (P = 0.002 to 0.03).
DISCUSSION
Here, we have shown that the fetus is acutely sensitive to maternally administered OXT at birth and that such OXT exposure changes offspring development across a variety of OXT-sensitive brain regions and behaviors. This is the first study to show experimentally that there can be long-term changes in offspring brain and behavior following a single maternal OXT administration in the immediate prepartum. When we administered OXT to the pregnant female, fetal OXT levels increased, and neuronal activity in two OXT source nuclei increased. In addition, fetal heart rate declined after maternal OXT treatment, which was most likely not caused by uterine contractions, as the fetus had been removed from the uterus. Maternal OXT treatment led to dose-dependent increases in Oxtr methylation in the fetal brain and increased the Oxtr gene expression for a given level of methylation. Offspring exposed to prepartum OXT vocalized more following separation as pups and went on to display more alloparental care, more time in close proximity with adult conspecifics, and less time alone. Adult male offspring of OXT-treated dams showed increases in OXTR and a decrease in V1aR density in regions of the brain known to regulate social behavior and affect. When we used a cross-fostering paradigm to determine the contribution of possible OXT-induced changes in maternal behavior, we saw no effects of rearing condition on any behavior measured. We therefore conclude that the observed changes are via direct action of maternally administered OXT on the fetus, possibly by crossing the placenta, as has been shown previously (33).
Evidence continues to grow that OXT acts as a hormonal signal that prepares the fetal brain for the extrauterine environment (6). Thus, there is an adaptive reason why the fetal brain would be sensitive to maternal OXT levels, and the observed changes in offspring development may be due to changes in the transition toward homeostatic independence. This hypothesis is supported by the molecular results from fetal brains. Methylation and expression data suggested that the fetal brain becomes more sensitive to OXT as parturition approaches (indexed here via fetal weight), since Oxtr methylation levels decreased and mRNA levels correspondingly increased.
In the brains of adult male offspring of OXT-treated dams, we observed increases in OXTR density in the central amygdala, parietal cortex, and insular cortex and a decrease in V1aR density in the ventral pallidum. It is likely that these effects contributed to the behavioral differences that we observed in these animals, as these are brain regions well known to contribute to the expression of caregiving behavior (34). OXT signaling in the central amygdala (CeA) regulates maternal aggressive behavior in defense of pups (35), while OXT in the NAcc regulates pair bonding and alloparental caregiving (36). Of particular note were the robust increases in OXTR density along the rostral-caudal extent of the insular cortex, which is associated with caregiving responsiveness (34). In addition to the effects of maternal OXT treatment, pair-bond formation may have also led to changes in OXTR/V1aR densities.
Although there were no differences in overall levels of Oxtr DNA methylation within the brain in adulthood (fig. S7), the differences in OXTR density were somewhat paralleled by similar changes in molecular measures from the corresponding contralateral brain regions. For instance, increases in OXTR density were mirrored by increases in Oxtr expression in the amygdala and insular cortex. There was also a significant increase and a trend toward an increase in the ratio of expression to methylation level in the amygdala and insular cortex, respectively. This pattern in the brains of adult offspring resembles the changes in epigenetic regulation of Oxtr seen in the fetal brain samples and speaks to a possible mechanism by which perinatal OXT imparts long-lasting effects. At the acute 90-min time point in fetal brains, we observed effects of maternal OXT on fetal methylation and mRNA levels, especially at the high dose. Maternally administered exogenous OXT not only led to increases in methylation of the Oxtr promoter but also changed the relationship between methylation and mRNA expression. Because expression relatively increased for a given level of methylation, we hypothesize that this may indicate hydroxymethylation, which has previously been associated with increasing gene expression (37). Expression levels of the closely related V1aR were not affected, suggesting specificity of the effects of maternal OXT.
This work builds on previous studies that have shown young mammals to be sensitive to the organizational effects of OXT when it is administered directly to the pup on the first postnatal day (15, 23). Previous studies on the developmental consequences of early-life OXT exposure have relied on doses between 1 mg/kg (14, 38–40) and 5 mg/kg (13) delivered directly to the pup, rather than to pregnant females as in the present study. Previous work on the acute functions of maternal OXT on fetal preparedness for birth has used doses of 0.05 mg/kg in rats (8) and 1 mg/kg in guinea pigs (10). This is roughly in line with species differences in plasma OXT between rats and voles, where rat levels are approximately one-third of those in voles (24), which implies that higher doses would be needed to induce labor in voles. That said, the doses in the present study (0.03 to 0.5 mg/kg) were below the threshold to induce labor in this species. Thus, it is unlikely that the effects observed here were due to difficulties with the birth process. However, we cannot exclude indirect effects of OXT due to uterine contractions. Note also, that this study does not directly model the use of OXT in human labor. Humans typically receive much lower doses over much longer durations (41). Here, OXT was administered via a single bolus, in contrast to the use of a Pitocin drip, which typically involves regularly increasing administration rates up to a maximum of 40 mU/min (42). Future work should more closely model the dosing regimen used in humans.
The present findings’ relevance to human health should spur future studies to investigate the possible effects of maternally administered OXT on behavior in human neonates. Currently, approximately 50% of women are given OXT before the third stage of labor (3, 4). Translational animal research is needed to experimentally backfill in our understanding of the effects of this widespread practice in humans. Although rodent brains are far less developed than humans’ at birth, all mammals must navigate the transition from fetal life to the extrauterine environment via precise coordination between mother and offspring (43). OXT is one facet of this coordinated transition, and practices that affect maternal-fetal hormone signaling may therefore affect the fetus’ initiation of homeostatic independence.
Previous studies across human populations have found mixed results when examining the links between OXT administration at birth and subsequent autism diagnosis in offspring (18–21). The present findings suggest that OXT exposure can produce a gregarious phenotype in offspring, which would support the contention that a correlation between peripartum OXT administration and autism may arise because of a heightened need for OXT supplementation during labor resulting from low endogenous levels. However, the effects of neonatal OXT on subsequent social behavior vary by sex, species, and dose (13, 14, 40), so direct extrapolation of the present findings to humans is contentious. Even so, these data indicate the importance of consideration of OXT dose during labor in humans.
OXT administered to either induce or augment labor should be evaluated for potential effects on offspring social/neural development. The fact that it has not up to this point speaks to a need for more cross-talk between obstetrics and neuroscience, and a reevaluation of approaches effectively grandfathered into a widespread obstetric practice before we became aware of the diverse set of actions of OXT in the brain. We anticipate this process to be extremely challenging, as OXT has produced great reductions in fetal and maternal mortality. Any unintended side effects to social functioning resulting from prepartum OXT will need to be measured alongside the well-documented health benefits.
MATERIALS AND METHODS
Experimental design
Heterosexual pairs of prairie voles (Microtus ochrogaster) were used to generate the subjects for this study. Dams consisted of primiparous adult female voles at 60 to 90 days of age. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of either the University of Illinois at Chicago or Northeastern University. Prairie voles experience both induced estrus and induced ovulation; therefore, we created a timed mating paradigm to best predict the day of delivery (Fig. 3A). Untreated sires remained with the dam and litter from conception to weaning. OXT was always administered on the expected day of delivery, which was confirmed either by fetal body weight > 1.7 g or delivery within 24 hours in experiment 4, which focused on long-term consequences.
In experiment 1a, term pregnant female prairie voles were intraperitoneally injected with either OXT (0.03 mg/kg) or SAL and then rapidly decapitated 10 min later to measure peak plasma OXT levels. Fetal plasma was then pooled across each litter and assayed for OXT according to previously published methods (30). In experiment 1b, term pregnant females were treated with either OXT (0.03 mg/kg) or SAL and then anesthetized with isoflurane 90 min later to measure peak c-fos immunoreactivity. Fetal brains were then embedded in gelatin and processed for c-fos immunohistochemistry according to previously published methods (44).
In experiments 2a and 2b, pup/fetal heart rate was obtained by looping leads around opposite limbs (Fig. 1C) and applying electrode-conductive gel. In experiment 2a, the umbilical cord was clamped to prevent transfer, and the fetus was injected directly. In experiment 2b, the fetus was still connected to the maternal circulation via the umbilical cord and the pregnant female was injected. After 5 min of baseline recording, either the pup (experiment 2a, n = 7 per group) or pregnant female (experiment 2b, n = 6 to 7 per group) was randomly assigned to be injected with either SAL or OXT (0.25 mg/kg) intraperitoneally, and heart rate was recorded for another 5 min. In experiment 2b, a third treatment group received pretreatment with the OXT antagonist L-368,899 (Merck Pharmaceuticals, Kenilworth, NJ) at a dose of 2 mg/kg 10 min before treatment with OXT (n = 5).
In experiment 3, pregnant female prairie voles were randomly assigned to receive one of five treatments: low-OXT (0.125 mg/kg), medium-OXT (0.25 mg/kg), high-OXT (0.5 mg/kg), SAL, or no treatment on the expected day of delivery (n = 15 to 22 per group). One male and one female pup were included from each litter. Ninety minutes after treatment, subjects were anesthetized with isoflurane, and fetuses were removed and euthanized. Fetal brains were then extracted and sectioned into thirds, roughly corresponding to forebrain, midbrain, and hindbrain, before being frozen in dry ice and placed into −80°C storage. The resulting tissue was then analyzed for Oxtr mRNA expression and DNA methylation. Oxtr methylation was measured as a percentage of CpGs methylated across four sites conserved between prairie voles and humans (Fig. 2A) (26).
In experiment 4, pregnant female prairie voles were randomly assigned to be injected with either SAL or the medium dose of OXT (0.25 mg/kg) on the expected day of delivery. Only if a litter was delivered within 24 hours were offspring included in the study and cross-fostered to a different dam. After birth, individual pups were toe-clipped for identification. Dams each fostered litters comprising roughly 50% OXT and 50% SAL pups, balanced by sex as much as possible. What resulted was a 2 × 2 × 2 design: birth treatment (the treatment of the birth dam), rearing condition (the treatment of the foster dam) consisting of either SAL or OXT, and sex. Thus, there were eight groups (n = 7 to 18 per group, 101 subjects in total). Offspring were then followed throughout development and tested according to the paradigm illustrated in Fig. 3A. Specific procedural details can be found in the Supplementary Materials.
Statistical analyses
In experiments 1a and 1b, plasma OXT concentrations and regional c-fos intensity were compared between treatments by Student’s t test (Fig. 1, A to C). In experiments 2a and 2b, heart rate was compared between treatments by repeated-measures analysis of variance (ANOVA) and presented as either an average (experiment 2a; Fig. 1D) or time series (experiment 2b; Fig. 1G). In experiment 3, methylation and expression were compared between treatments as linear mixed-effects models that included fetal sex, fetal weight, litter size, and dose of OXT (and in the case of expression, methylation as well). Methylation data are also presented as the residuals of methylation as a function of fetal weight for a linear model based solely on the no-treatment control group (Fig. 2, F to K). Expression data are also presented as the residuals of expression as a function of fetal weight and methylation for a linear model based solely on the no-treatment control group (Fig. 2, L to N). In experiment 4, behaviors were compared between birth and rearing treatments as follows: for USVs, a repeated-measures ANOVA that included age, sex, birth treatment, and rearing treatment; for OFT, APC, and PPT behaviors, ANOVAs that included sex, birth treatment, and rearing treatment. For the molecular measures in adult brains, Oxtr methylation and mRNA expression were evaluated via an ANOVA that included sex, birth treatment, and brain region. For the autoradiographic analyses in adult brains, OXTR and V1aR were evaluated via a repeated-measures ANOVA (two repetitions per brain region) that included sex, birth treatment, and brain region. We did not observe any effects of litter size.
Supplementary Material
Acknowledgments
We acknowledge the contributions of J. Amacker, E. Grimsley, M. Barnes, N. Zingman-Daniels, D. Whalen, B. Yeung, A. I. Moore, and T. Rosenstein of Northeastern University, and the contributions of J.D. Seo and the Indiana Statistical Consulting Center at Indiana University. Funding: This work was funded by a grant from the NIH (P01HD075750). Author contributions: This study was originally formulated by W.M.K. and J.R.Y. with mentorship from C.S.C. and J.J.C. W.M.K., A.-M.P., and J.R.Y. carried out the experiments. Plasma OXT was assayed by W.M.K. and H.P.-N. DNA methylation and gene expression were carried out by T.S.L., E.F.F., K.L.W., and J.J.C. Heart rate, immunohistochemistry, vocalization analysis, behavior, and autoradiography were analyzed by W.M.K. Results and statistics were analyzed by W.M.K. C.S.C. and C.F.F. supervised the research. W.M.K. wrote the manuscript with input from all authors. All authors discussed and commented on the results and the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/5/eaav2244/DC1
Supplementary Methods
Fig. S1. Postnatal development and care.
Fig. S2. C-fos immunoreactivity.
Fig. S3. Behavioral results on the effects of maternal OXT administration (0.25 mg/kg, intraperitoneally) from an earlier study without cross-fostering.
Fig. S4. The workflow for consolidating autoradiographic images into group composites, which were then subtracted from one another to generate heat maps of group differentials.
Fig. S5. Autoradiographic analyses of OXTR density throughout the brain.
Fig. S6. Autoradiographic analyses of V1aR density throughout the brain.
Fig. S7. The effects of maternal OXT administration on offspring Oxtr expression and methylation in adulthood, experiment 4 (continued).
REFERENCES AND NOTES
- 1.Gallos I. D., Papadopoulou A., Man R., Athanasopoulos N., Tobias A., Price M. J., Williams M. J., Diaz V., Pasquale J., Chamillard M., Widmer M., Tunçalp Ö., Hofmeyr G. J., Althabe F., Gülmezoglu A. M., Vogel J. P., Oladapo O. T., Coomarasamy A., Uterotonic agents for preventing postpartum haemorrhage: A network meta-analysis. Cochrane Database Syst. Rev , Issue 12, CD011689 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin J. A., Hamilton B. E., Osterman M. J. K., Driscoll A. K., Mathews T. J., Births: Final data for 2015. Natl. Vital Stat. Rep. 66, 1 (2017). [PubMed] [Google Scholar]
- 3.Declercq E. R., Sakala C., Corry M. P., Applebaum S., Listening to mothers II: Report of the second national U.S. survey of women’s childbearing experiences: Conducted January–February 2006 for childbirth connection by Harris Interactive in partnership with Lamaze International. J. Perinat. Educ. 16, 9–14 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Declercq E. R., Sakala C., Corry M. P., Applebaum S., Herrlich A., Major survey findings of listening to MothersSM III: Pregnancy and birth: Report of the third national U.S. survey of women’s childbearing experiences. J. Perinat. Educ. 23, 9–16 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marchini G., Lagercrantz H., Winberg J., Uvnäs-Moberg K., Fetal and maternal plasma levels of gastrin, somatostatin and oxytocin after vaginal delivery and elective cesarean section. Early Hum. Dev. 18, 73–79 (1988). [DOI] [PubMed] [Google Scholar]
- 6.Kenkel W. M., Yee J. R., Carter C. S., Is oxytocin a maternal-foetal signalling molecule at birth? Implications for development. J. Neuroendocrinol. 26, 739–749 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Pedersen C. A., Prange A. J. Jr., Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc. Natl. Acad. Sci. U.S.A. 76, 6661–6665 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tyzio R., Cossart R., Khalilov I., Minlebaev M., Hübner C. A., Represa A., Ben-Ari Y., Khazipov R., Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science 314, 1788–1792 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Khazipov R., Tyzio R., Ben-Ari Y., Effects of oxytocin on GABA signalling in the foetal brain during delivery. Prog. Brain Res. 170, 243–257 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Nair P. K., Li T., Bhattacharjee R., Ye X., Folkesson H. G., Oxytocin-induced labor augments IL-1β-stimulated lung fluid absorption in fetal guinea pig lungs. Am. J. Physiol. Lung Cell. Mol. Physiol. 289, L1029–L1038 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Schaller F., Watrin F., Sturny R., Massacrier A., Szepetowski P., Muscatelli F., A single postnatal injection of oxytocin rescues the lethal feeding behaviour in mouse newborns deficient for the imprinted Magel2 gene. Hum. Mol. Genet. 19, 4895–4905 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Mazzuca M., Minlebaev M., Shakirzyanova A., Tyzio R., Taccola G., Janackova S., Gataullina S., Ben-Ari Y., Giniatullin R., Khazipov R., Newborn analgesia mediated by oxytocin during delivery. Front. Cell. Neurosci. 5, 3 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashemi F., Tekes K., Laufer R., Szegi P., Tóthfalusi L., Csaba G., Effect of a single neonatal oxytocin treatment (hormonal imprinting) on the biogenic amine level of the adult rat brain: Could oxytocin-induced labor cause pervasive developmental diseases? Reprod. Sci. 20, 1255–1263 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Bales K. L., van Westerhuyzen J. A., Lewis-Reese A. D., Grotte N. D., Lanter J. A., Carter C. S., Oxytocin has dose-dependent developmental effects on pair-bonding and alloparental care in female prairie voles. Horm. Behav. 52, 274–279 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller T. V., Caldwell H. K., Oxytocin during development: Possible organizational effects on behavior. Front. Endocrinol. 6, 76 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eishima K., The effects of obstetric conditions on neonatal behaviour in Japanese infants. Early Hum. Dev. 28, 253–263 (1992). [DOI] [PubMed] [Google Scholar]
- 17.González-Valenzuela M.-J., Lopez-Montiel D., González-Mesa E. S., Exposure to synthetic oxytocin during delivery and its effect on psychomotor development. Dev. Psychobiol. 57, 908–920 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Gregory S. G., Anthopolos R., Osgood C. E., Grotegut C. A., Miranda M. L., Association of autism with induced or augmented childbirth in North Carolina Birth Record (1990-1998) and Education Research (1997-2007) databases. JAMA Pediatr. 167, 959–966 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Wang C., Geng H., Liu W., Zhang G., Prenatal, perinatal, and postnatal factors associated with autism: A meta-analysis. Medicine 96, e6696 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weisman O., Agerbo E., Carter C. S., Harris J. C., Uldbjerg N., Henriksen T. B., Thygesen M., Mortensen P. B., Leckman J. F., Dalsgaard S., Oxytocin-augmented labor and risk for autism in males. Behav. Brain Res. 284, 207–212 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Guastella A. J., Cooper M. N., White C. R. H., White M. K., Pennell C. E., Whitehouse A. J. O., Does perinatal exposure to exogenous oxytocin influence child behavioural problems and autistic-like behaviours to 20 years of age? J. Child Psychol. Psychiatry 59, 1323–1332 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Grippo A. J., Trahanas D. M., Zimmerman R. R. II, Porges S. W., Carter C. S., Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology 34, 1542–1553 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carter C. S., Developmental consequences of oxytocin. Physiol. Behav. 79, 383–397 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Kramer K. M., Cushing B. S., Carter C. S., Wu J., Ottinger M. A., Sex and species differences in plasma oxytocin using an enzyme immunoassay. Can. J. Zool. 82, 1194–1200 (2004). [Google Scholar]
- 25.Hicks C., Ramos L., Reekie T., Misagh G. H., Narlawar R., Kassiou M., McGregor I. S., Body temperature and cardiac changes induced by peripherally administered oxytocin, vasopressin and the non-peptide oxytocin receptor agonist WAY 267,464: A biotelemetry study in rats. Br. J. Pharmacol. 171, 2868–2887 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perkeybile A. M., Carter C. S., Wroblewski K. L., Puglia M. H., Kenkel W. M., Lillard T. S., Karaoli T., Gregory S. G., Mohammadi N., Epstein L., Bales K. L., Connelly J. J., Early nurture epigenetically tunes the oxytocin receptor. Psychoneuroendocrinology 99, 128–136 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Insel T. R., Winslow J. T., Central administration of oxytocin modulates the infant rat’s response to social isolation. Eur. J. Pharmacol. 203, 149–152 (1991). [DOI] [PubMed] [Google Scholar]
- 28.Neumann I. D., Landgraf R., Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviors. Trends Neurosci. 35, 649–659 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Bales K. L., Kim A. J., Lewis-Reese A. D., Carter C. S., Both oxytocin and vasopressin may influence alloparental behavior in male prairie voles. Horm. Behav. 45, 354–361 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Kenkel W. M., Paredes J., Yee J. R., Pournajafi-Nazarloo H., Bales K. L., Carter C. S., Neuroendocrine and behavioural responses to exposure to an infant in male prairie voles. J. Neuroendocrinol. 24, 874–886 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Kenkel W. M., Perkeybile A. M., Carter C. S., The neurobiological causes and effects of alloparenting. Dev. Neurobiol. 77, 214–232 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carter C. S., Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology 23, 779–818 (1998). [DOI] [PubMed] [Google Scholar]
- 33.Malek A., Blann E., Mattison D. R., Human placental transport of oxytocin. J. Matern. Fetal Med. 5, 245–255 (1996). [DOI] [PubMed] [Google Scholar]
- 34.Riem M. M. E., Bakermans-Kranenburg M. J., Pieper S., Tops M., Boksem M. A. S., Vermeiren R. R. J. M., van IJzendoorn M. H., Rombouts S. A. R. B., Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: A randomized controlled trial. Biol. Psychiatry 70, 291–297 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Ferris C. F., Foote K. B., Meltser H. M., Plenby M. G., Smith K. L., Insel T. R., Oxytocin in the amygdala facilitates maternal aggression. Ann. N. Y. Acad. Sci. 652, 456–457 (1992). [DOI] [PubMed] [Google Scholar]
- 36.Ross H. E., Freeman S. M., Spiegel L. L., Ren X., Terwilliger E. F., Young L. J., Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J. Neurosci. 29, 1312–1318 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perera A., Eisen D., Wagner M., Laube S. K., Künzel A. F., Koch S., Steinbacher J., Schulze E., Splith V., Mittermeier N., Müller M., Biel M., Carell T., Michalakis S., TET3 is recruited by REST for context-specific hydroxymethylation and induction of gene expression. Cell Rep. 11, 283–294 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Eaton J. L., Roache L., Nguyen K. N., Cushing B. S., Troyer E., Papademetriou E., Raghanti M. A., Organizational effects of oxytocin on serotonin innervation. Dev. Psychobiol. 54, 92–97 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Jia R., Tai F., An S., Broders H., Sun R., Neonatal manipulation of oxytocin influences the partner preference in mandarin voles (Microtus mandarinus). Neuropeptides 42, 525–533 (2008). [DOI] [PubMed] [Google Scholar]
- 40.Mogi K., Ooyama R., Nagasawa M., Kikusui T., Effects of neonatal oxytocin manipulation on development of social behaviors in mice. Physiol. Behav. 133, 68–75 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Roloff K., Peng S., Sanchez-Ramos L., Valenzuela G. J., Cumulative oxytocin dose during induction of labor according to maternal body mass index. Int. J. Gynaecol. Obstet. 131, 54–58 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Smith J. G., Merrill D. C., Oxytocin for induction of labor. Clin. Obstet. Gynecol. 49, 594–608 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Lagercrantz H., Slotkin T. A., The "stress" of being born. Sci. Am. 254, 100–107 (1986). [DOI] [PubMed] [Google Scholar]
- 44.Cushing B. S., Yamamoto Y., Hoffman G. E., Carter C. S., Central expression of c-Fos in neonatal male and female prairie voles in response to treatment with oxytocin. Brain Res. Dev. Brain Res. 143, 129–136 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Kenkel W. M., Paredes J., Lewis G. F., Yee J. R., Pournajafi-Nazarloo H., Grippo A. J., Porges S. W., Carter C. S., Autonomic substrates of the response to pups in male prairie voles. PLOS ONE 8, e69965 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okhovat M., Chen I. C., Dehghani Z., Zheng D. J., Ikpatt J. E., Momoh H., Phelps S. M., Genetic variation in the developmental regulation of cortical avpr1a among prairie voles. Genes Brain Behav. 17, 36–48 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Kenkel W. M., Yee J. R., Porges S. W., Ferris C. F., Carter C. S., Cardioacceleration in alloparents in response to stimuli from prairie vole pups: The significance of thermoregulation. Behav. Brain Res. 286, 71–79 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kenkel W. M., Carter C. S., Voluntary exercise facilitates pair-bonding in male prairie voles. Behav. Brain Res. 296, 326–330 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kenkel W. M., Suboc G., Carter C. S., Autonomic, behavioral and neuroendocrine correlates of paternal behavior in male prairie voles. Physiol. Behav. 128, 252–259 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pérez-Escudero A., Vicente-Page J., Hinz R. C., Arganda S., de Polavieja G. G., idTracker: Tracking individuals in a group by automatic identification of unmarked animals. Nat. Methods 11, 743–748 (2014). [DOI] [PubMed] [Google Scholar]
- 51.Perkeybile A. M., Delaney-Busch N., Hartman S., Grimm K. J., Bales K. L., Intergenerational transmission of alloparental behavior and oxytocin and vasopressin receptor distribution in the prairie vole. Front. Behav. Neurosci. 9, 191 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yee J. R., Kenkel W. M., Kulkarni P., Moore K., Perkeybile A. M., Toddes S., Amacker J. A., Carter C. S., Ferris C. F., BOLD fMRI in awake prairie voles: A platform for translational social and affective neuroscience. Neuroimage 138, 221–232 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/5/eaav2244/DC1
Supplementary Methods
Fig. S1. Postnatal development and care.
Fig. S2. C-fos immunoreactivity.
Fig. S3. Behavioral results on the effects of maternal OXT administration (0.25 mg/kg, intraperitoneally) from an earlier study without cross-fostering.
Fig. S4. The workflow for consolidating autoradiographic images into group composites, which were then subtracted from one another to generate heat maps of group differentials.
Fig. S5. Autoradiographic analyses of OXTR density throughout the brain.
Fig. S6. Autoradiographic analyses of V1aR density throughout the brain.
Fig. S7. The effects of maternal OXT administration on offspring Oxtr expression and methylation in adulthood, experiment 4 (continued).


