Abstract
Background
Crohn's disease (CD) is a chronic, relapsing and remitting disease of the gastrointestinal tract that can cause significant morbidity and disability. Current treatment guidelines recommend early intervention with immunosuppressant or biological therapy in high‐risk patients with a severe disease phenotype at presentation. The feasibility of therapeutic de‐escalation once remission is achieved is a commonly encountered question in clinical practice, driven by patient and clinician concerns regarding safety, adverse events, cost and national regulations. Withdrawal of immunosuppressant and biologic drugs in patients with quiescent CD may limit adverse events and reduce healthcare costs. Alternatively, stopping these drug therapies may result in negative outcomes such as disease relapse, drug desensitization, bowel damage and need for surgery.
Objectives
To assess the feasibility and safety of discontinuing immunosuppressant or biologic drugs, administered alone or in combination, in patients with quiescent CD.
Search methods
We searched CENTRAL, MEDLINE, Embase and the Cochrane IBD Group Specialized Register from inception to 19 December 2017. We also searched the reference lists of potentially relevant manuscripts and conference proceedings to identify additional studies.
Selection criteria
Randomized controlled trials (RCTs) and prospective cohort studies that followed patients for a minimum duration of six months after drug discontinuation were considered for inclusion. The patient population of interest was adults (> 18 years) with CD (as defined by conventional clinical, endoscopic or histologic criteria) who had achieved remission while receiving immunosuppressant or biologic drugs administered alone or in combination. Patients then discontinued the drug regimen following a period of maintenance therapy of at least six months. The comparison was usual care (i.e. continuation of the drug regimen).
Data collection and analysis
The primary outcome measure was the proportion of patients who relapsed following discontinuation of immunosuppressant or biologic drugs, administered alone or in combination. Secondary outcomes included: the proportion of patients who responded to the reintroduction of immunosuppressant or biologic drugs, given as monotherapy or combination therapy; the proportion of patients who required surgery following relapse; the proportion of patients who required hospitalization for CD following relapse; the proportion of patients who developed new CD‐related complications (e.g. fistula, abscesses, strictures) following relapse; the proportion of patients with elevated biomarkers of inflammation (CRP, fecal calprotectin) in those who stop and those who continue therapy; the proportion of patients with anti‐drug antibodies and low serum trough drug levels; time to relapse; and the proportion of patients with adverse events, serious adverse events and withdrawal due to adverse events. For dichotomous outcomes, we calculated the risk ratio (RR) and 95% confidence interval (95% CI). Data were analyzed on an intention‐to‐treat basis where patients with missing outcome data were assumed to have relapsed. The overall quality of the evidence supporting the primary and secondary outcomes was assessed using the GRADE criteria.
Main results
A total of six RCTs (326 patients) evaluating therapeutic discontinuation in patients with quiescent CD were eligible for inclusion. In four RCTs azathioprine monotherapy was discontinued, and in two RCTs azathioprine was discontinued from a combination therapy regimen consisting of azathioprine with infliximab. No studies of biologic monotherapy withdrawal were eligible for inclusion. The majority of studies received unclear or low risk of bias ratings, with the exception of three open‐label RCTs, which were rated as high risk of bias for blinding. Four RCTs (215 participants) compared discontinuation to continuation of azathioprine monotherapy, while two studies (125 participants) compared discontinuation of azathioprine from a combination regimen to continuation of combination therapy. Continuation of azathioprine monotherapy was shown to be superior to withdrawal for risk of clinical relapse. Thirty‐two per cent (36/111) of azathioprine withdrawal participants relapsed compared to 14% (14/104) of participants who continued with azathioprine therapy (RR 0.42, 95% CI 0.24 to 0.72, GRADE low quality evidence). However, it is uncertain if there are any between‐group differences in new CD‐related complications (RR 0.34, 95% CI 0.06 to 2.08, GRADE low quality evidence), adverse events (RR 0.88, 95% CI 0.67 to 1.17, GRADE low quality evidence), serious adverse events (RR 3.29, 95% CI 0.35 to 30.80, GRADE low quality evidence) or withdrawal due to adverse events (RR 2.59, 95% CI 0.35 to 19.04, GRADE low quality evidence). Common adverse events included infections, mild leukopenia, abdominal symptoms, arthralgias, headache and elevated liver enzymes. No differences between azathioprine withdrawal from combination therapy versus continuation of combination therapy were observed for clinical relapse. Among patients who continued combination therapy with azathioprine and infliximab, 48% (27/56) had a clinical relapse compared to 49% (27/55) of patients discontinued azathioprine but remained on infliximab (RR 1.02, 95% CI 0.68 to 1.52, P = 0.32; GRADE low quality evidence). The effects on adverse events (RR 1.11, 95% CI 0.44 to 2.81, GRADE low quality of evidence) or serious adverse events are uncertain (RR 1.00, 95% CI 0.21 to 4.66; GRADE very low quality of evidence). Common adverse events in the combination therapy studies included infections, liver test elevations, arthralgias and infusion reactions.
Authors' conclusions
The effects of withdrawal of immunosuppressant therapy in people with quiescent Crohn's disease are uncertain. Low quality evidence suggests that continuing azathioprine monotherapy may be superior to withdrawal for avoiding clinical relapse, while very low quality evidence suggests that there may be no difference in clinical relapse rates between discontinuing azathioprine from a combination therapy regimen, compared to continuing combination therapy. It is unclear whether withdrawal of azathioprine, initially administered alone or in combination, impacts on the development of CD‐related complications, adverse events, serious adverse events or withdrawal due to adverse events. Further high‐quality research is needed in this area, particularly double‐blind RCTs in which biologic therapy or an immunosuppressant other than azathioprine is withdrawn.
Plain language summary
Is withdrawal of drug therapy feasible in patients with CD who have achieved remission?
Background
Crohn's disease is a serious, chronic, inflammatory disease of the small and large intestine. Symptoms include abdominal pain, diarrhea, bleeding and weight loss. When people with Crohn's disease are experiencing symptoms the disease is 'active'. When the symptoms stop, it is called 'remission'. When people in remission experience symptoms it is called a 'relapse'. Immunosuppressant drugs (e.g. azathioprine, 6‐mercaptopurine and methotrexate) and biologic medications (e.g. infliximab, adalimumab, vedolizumab and ustekinumab) are commonly used alone or in combination to treat Crohn's disease. While effective for initially controlling disease (i.e. inducing remission), there are safety and cost concerns regarding the long‐term use of these drugs for the prevention of relapse in people with Crohn's disease in remission.
Study characteristics
We performed a comprehensive literature review and identified six randomized controlled trials (an experiment in which participants are randomly assigned to receive two or more interventions and the results are compared) that involved a total of 326 participants. Four of the six studies assigned patients who had been receiving azathioprine alone to either continue or discontinue therapy (215 participants). Two of the six studies assigned patients who had been receiving azathioprine in addition to infliximab to continue therapy or discontinue azathioprine (111 participants).
Key results
Clinical relapse occurred in 13% (14/104) of patients who continued azathioprine monotherapy compared to 32% (36/111) of patients who discontinued azathioprine monotherapy. No differences were observed for Crohn's disease‐related complications, side effects, serious side effects and withdrawal due to side effects. Common side effects included infections, mild decrease in the number of white blood cells, abdominal symptoms, joint pain, headache and elevated liver enzymes. Among patients who continued combination therapy with azathioprine and infliximab, 48% (27/56) had a clinical relapse compared to 49% (27/55) of patients discontinued azathioprine but remained on infliximab. No differences in side effects, serious side effects or withdrawal due to side effects were observed. Common side effects reported in the combination therapy studies included infections, liver test elevations, joint pain and infusion reactions (a hypersensitivity reaction to the biologic medication).
Quality of evidence
Overall, the quality of evidence for each outcome was low due to a high risk of study bias and small numbers of patients evaluated.
Conclusions
The effects of withdrawal of immunosuppressant therapy in people with Crohn's disease in remission are uncertain. Low quality evidence suggests that continuing azathioprine monotherapy may be superior to withdrawal of azathioprine for avoiding clinical relapse in people with Crohn's disease in remission. Low quality evidence suggests that stopping the immunosuppressive after combination therapy does not seem to impact on the risk of relapsing. It is unclear whether the withdrawal of azathioprine, initially administered alone or in combination, impacts on the development of Crohn's disease‐related complications, side effects, serious side effects, or withdrawal from the studies due to side effects. Additional research is needed in this area to better inform clinical practice, particularly high‐quality randomized controlled trials examining outcomes when biologic therapy is withdrawn.
Summary of findings
Background
Description of the condition
Crohn's disease (CD) is a chronic, relapsing and remitting, immune‐mediated disease of the gastrointestinal tract which can result in significant morbidity and disability through progressive bowel damage. Typical symptoms include abdominal pain, chronic diarrhoea, weight loss, fatigue and impaired quality of life. The exact etiology of CD remains unknown, however a complex interplay between environmental factors, genetic susceptibility and intestinal microbiota likely cause the abnormal immune response and compromised epithelial barrier function that characterize the condition (Haag 2015).
Young adults are primarily affected, with the highest incidence of diagnosis reported during the second or third decades of life (Molodecky 2012). Epidemiological studies show a steady increase in the worldwide incidence of CD (Molodecky 2012). The highest incidence rates are reported in Australia (29.3 per 100,000 person‐years; Wilson 2010), Canada (20 per 100,000 person‐years; Bernstein 2006), New Zealand (16 per 100,000 person‐years; Gearry 2006) and Northern Europe (10 per 100,000 person‐years; Thompson 1998).
CD most commonly affects the terminal ileum and colon, yet any segment of the gastrointestinal tract may be involved. Extra‐intestinal manifestations may occur in up to half of all patients (Harbord 2016). Inflammatory disease is the most frequent phenotype at diagnosis, however approximately 50% of patients eventually develop stricturing or penetrating disease which often necessitates surgery (Peyrin‐Biroulet 2012). Over 90% of CD patients develop endoscopic disease recurrence within 15 years of the first bowel resection (Buisson 2012).
Description of the intervention
Current treatment guidelines recommend a sequential 'step‐up' approach, with initial use of first‐line drugs such as oral corticosteroids and subsequent escalation to immunosuppressant and biologic agents as monotherapy or in combination if necessary (Colombel 2010a). Conversely, the 'step‐down' approach involves initially treating high‐risk patients and those with severe disease at diagnosis with immunosuppressant and biologic medications. Evidence in support of the step‐down strategy has accumulated over the last decade (Colombel 2010b; D'Haens 2010). For example, in the SONIC trial (The Study of Biologic and Immunomodulator Naive Patients in Crohn’s Disease), 56.8% of patients treated with combination therapy achieved steroid‐free remission at week 26, as compared with 44.4% of patients receiving biologic alone, or with 30% of patients receiving azathioprine alone (Colombel 2010b). Findings from D'Haens 2008 also suggest that early combination therapy may be more effective than conventional management for induction of remission in CD. At week 26, 60% of patients receiving combination infliximab and azathioprine achieved clinical remission compared to 23% of those who received corticosteroids followed in sequence by azathioprine and infliximab (D'Haens 2008). Consequently, in this era of early intervention, there is an increasing number of patients being exposed to immunosuppressants and biologics early in their disease course and for a longer time horizon.
The feasibility of de‐escalation of therapy once remission is achieved is a common question encountered in clinical practice, driven by patient and clinician concerns around safety, adverse events, cost, convenience and national regulations. Long‐term exposure to thiopurines has been associated with the development of lymphoproliferative disorders (Beaugerie 2009), and cancer of the skin and urinary tract (Bourrier 2016; Peyrin‐Biroulet 2011). Tumor necrosis factor‐alpha (TNF‐α) antagonist therapies may increase the risk of infections (Billioud 2013), and conflicting evidence about a potential increase in the risk of melanoma exists (Andersen 2014; Long 2012). Patients receiving combination therapy may have an increased risk of developing hepatosplenic T cell lymphoma (Subramaniam 2014), non‐Hodgkin’s lymphoma (Siegel 2009), and infections. The potential long‐term consequences of exposure to biologic agents remains unknown.
Biologic medications are relatively expensive. It is estimated that TNF‐α antagonists account for 64% of the direct costs of CD treatment (van der Valk 2014), although costs are likely to decrease with the approval of biosimilar drugs. Questions have been raised as to whether discontinuing immunosuppressant and biologic therapies in patients with quiescent CD may limit adverse events and reduce the high healthcare costs associated with these therapies while maintaining remission.
How the intervention might work
Withdrawal of immunosuppressant and biologic drugs in patients with quiescent CD may reduce the risk of adverse events and reduce healthcare costs without any impact upon the disease state. Alternatively, stopping these drug therapies may result in negative outcomes such as disease relapse, drug desensitization, bowel damage and need for surgery.
Why it is important to do this review
A systematic appraisal of the literature was required to highlight the potential benefits and risks of withdrawing immunosuppressant and biologic drug therapy in CD patients who have achieved remission.
Objectives
The primary objective of this review was to assess the feasibility and safety of discontinuing immunosuppressant or biologic drugs, administered alone or in combination, in patients with quiescent CD.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs), controlled clinical trials, and prospective cohort studies with a control group were considered for inclusion. Included studies followed patients for a minimum duration of six months after drug discontinuation.
Types of participants
Adults (> 18 years) with CD (as defined by conventional clinical, endoscopic or histologic criteria) who achieved remission (as defined by the original study) while receiving immunosuppressant or biologic drugs administered alone or in combination were considered for inclusion.
Types of interventions
The intervention of interest was the discontinuation of immunosuppressant or biologic drugs, administered alone or in combination, following a period of maintenance therapy of at least six months. The comparison group was usual care.
Types of outcome measures
Primary outcomes
The primary outcome was the proportion of patients who relapsed (as defined by the included studies) following discontinuation of immunosuppressant or biologic drugs, administered alone or in combination.
Secondary outcomes
Secondary outcomes (where available) included: 1) the proportion of patients who responded to the reintroduction of immunosuppressant or biologic drugs, given as monotherapy or combination therapy; 2) the proportion of patients who required surgery following relapse; 3) the proportion of patients who required hospitalization for CD following relapse; 4) the proportion of patients who developed new CD‐related complications (e.g. fistula, abscesses, strictures) following relapse; 5) the proportion of patients with elevated biomarkers of inflammation (CRP, fecal calprotectin) in those who stop and those who continue therapy; 6) the proportion of patients with anti‐drug antibodies and low serum trough drug levels; 7) time to relapse and 8) the proportion of patients with adverse events, serious adverse events and withdrawal due to adverse events.
Search methods for identification of studies
Electronic searches
The following electronic databases were searched:
1. MEDLINE (Ovid, 1946 to 19 December 2017);
2. EMBASE (Ovid, 1984 to 19 December 2017);
3. CENTRAL; and
4. The Cochrane IBD Group Specialized Register.
The search strategies are listed in Appendix 1. The search was not limited by language, year of publication or type of publication.
Searching other resources
We also searched the reference lists of potentially relevant manuscripts to identify additional studies. Conference proceedings from Digestive Disease Week, the European Crohn's and Colitis Organisation annual meeting, and United European Gastroenterology Week published during the last five years were also searched to identify studies reported in abstract form only. ClinicalTrials.gov was searched to identify ongoing studies.
Data collection and analysis
Selection of studies
Two authors (JT and RB) independently screened titles and abstracts identified by the literature search to determine eligibility based on the inclusion criteria described above. Any disagreements between authors regarding study inclusion were resolved by discussion.
Data extraction and management
A data extraction form was used to collect information from the relevant studies. Two authors (JT and RB) independently extracted data. Consensus regarding data extraction was reached after discussion between authors. The following data were retrieved from the included studies:
1) general information: title, journal, year, publication type;
2) study information: study type, study design, setting, inclusion/exclusion criteria, methods of randomization, concealment of allocation and blinding, study duration, and definitions of remission and relapse;
3) population characteristics: number of participants recruited, total number of patients screened and randomized, total number of participants followed, baseline characteristics (e.g. age, sex, race, disease phenotype, disease duration, concurrent medications; prior medications);
4) intervention characteristics: type and dose of medication discontinued, duration of remission prior to discontinuation, duration of therapy prior to discontinuation;
5) follow‐up (length of follow‐up, withdrawals, number of patients lost to follow‐up); and
5) outcomes: primary and secondary outcomes, as described above.
Assessment of risk of bias in included studies
The methodological quality of each included RCT was independently assessed by two authors (JT and RB) using the Cochrane risk of bias tool (Higgins 2011a). Consensus was reached after discussion between authors on all studies. Factors assessed included:
1) sequence generation (i.e. was the allocation sequence adequately generated?);
2) allocation sequence concealment (i.e. was allocation adequately concealed?);
3) blinding (i.e. was knowledge of the allocated intervention adequately prevented during the study?);
4) incomplete outcome data (i.e. were incomplete outcome data adequately addressed?);
5) selective outcome reporting (i.e. are reports of the study free of suggestion of selective outcome reporting?); and
6) other potential sources of bias (i.e. was the study apparently free of other problems that could put it at high risk of bias?).
Studies will be assigned a low risk of bias, high risk of bias, or unclear risk of bias for each category.
We planned to assess the quality of non‐randomized, cohort studies was assessed using the Newcastle‐Ottawa scale (NOS) (Wells 2017). Factors assessed for cohort studies were:
1) Selection:
a) representativeness of the exposed cohort;
b) selection of the non‐exposed cohort; and
c) ascertainment of exposure.
2) Comparability:
a) comparability of cohorts on the basis of the design or analysis.
3) Outcome:
a) assessment of outcome;
b) appropriate length of follow‐up for outcomes to occur; and
c) adequacy of follow‐up of cohorts.
The quality of the total body of evidence for the primary and secondary outcomes of interest was assessed using the GRADE criteria (Schünemann 2011). Randomized trials are considered high quality and were downgraded due to:
1) risk of bias;
2) indirect evidence;
3) inconsistency (unexplained heterogeneity);
4) imprecision; and
5) publication bias.
The overall quality of evidence for each outcome was classified as high quality (i.e. further research is very unlikely to change our confidence in the estimate of effect); moderate quality (i.e. further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate); low quality (i.e. further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate); or very low quality (i.e. we are very uncertain about the estimate).
Measures of treatment effect
Review Manager (RevMan 5.3.5) was used to analyze data. All data were analyzed on an intention‐to‐treat (ITT) basis. The risk ratio (RR) and corresponding 95% confidence interval (CI) were calculated for dichotomous outcomes. We planned to calculate the mean difference (MD) and corresponding 95% CI for continuous outcomes and the hazard ratio (HR) and corresponding 95% CI using the generic inverse‐variance method for studies reporting the log hazard ratio and standard error for time‐to‐event outcomes.
Unit of analysis issues
When studies reported multiple observations for the same outcome, the outcomes were combined for fixed intervals of follow‐up (e.g. clinical remission at eight weeks). Cross‐over trials were included if data was available from the first phase of the study (i.e. before any cross‐over). Where studies allocated participants to more than one treatment arm, these arms were to be pooled for the primary analysis. Subgroup analyses were to be performed to compare efficacy and safety among different doses of drugs. The primary analysis considered the proportion of patients who experienced at least one efficacy or safety event.
Dealing with missing data
The original study authors were contacted in the case of unclear or missing data. Data that remained missing were considered to be a treatment failure, in accordance with the ITT principle. Where appropriate, sensitivity analyses were conducted to assess the impact of including unclear data on the effect estimate.
Assessment of heterogeneity
Heterogeneity was assessed using the Chi2 test (a P value of 0.10 was considered statistically significant) and the I2 statistic. An I2 value of 25% indicates low heterogeneity, 50% indicates moderate heterogeneity and 75% indicates high heterogeneity (Higgins 2003). We used sensitivity analyses to explore potential explanations for heterogeneity. If the I2 statistic showed a moderate to high degree of heterogeneity and the Chi2 test was statistically significant, forest plots were visually inspected for obvious outliers and sensitivity analysis were performed excluding the outlier to see if this explains the heterogeneity.
Assessment of reporting biases
Potential reporting bias was evaluated by comparing outcomes listed in protocols to published manuscripts. When protocols were not available, we compared outcomes listed in the methods section of published manuscripts to those described in the results section. If a sufficient number of studies are included (i.e. > 10) in the pooled analyses, we planned to investigate potential publication bias using funnel plots (Egger 1997).
Data synthesis
Data from individual trials were combined for meta‐analysis when the interventions, patient groups and outcomes were sufficiently similar (as determined by consensus). The pooled RR and 95% CI were calculated for dichotomous outcomes. For continuous outcomes, we planned to calculate the pooled MD and corresponding 95% CI. The standardized mean difference (SMD) and 95% CI were planned to be calculated when difference scales were used to measure the same outcome. Where studies reported estimates of log hazard ratios and standard errors obtained from Cox proportional hazards regression models, we planned to pool study results using the generic inverse‐variance method to obtain the pooled HR and corresponding 95% CI. Where a mixture of log‐rank and Cox model estimates are obtained from the studies, we planned to convert the log‐rank estimates into log hazard ratios and standard errors (Higgins 2011b). A fixed‐effect model was used to pool data unless heterogeneity exists between the studies. A random‐effects model was employed if heterogeneity existed (I2 50 to 75%). We did not pool data for meta‐analysis if a high degree of heterogeneity (I2 ≥ 75%) was detected.
Subgroup analysis and investigation of heterogeneity
Planned subgroup analyses included: different drug doses and routes of administration and different study designs (RCTs versus observational studies).
Sensitivity analysis
Sensitivity analyses were planned to examine the impact of the following variables on the pooled effect: random‐effects versus fixed‐effect modelling; low risk of bias only versus unclear or high risk of bias; and relevant loss to follow‐up (> 10% versus < 10%).
Results
Description of studies
Results of the search
A literature search conducted on 19 December 2017 identified 10,902 records. After excluding duplicate records, two authors (RB and JT) independently examined the abstracts of 6860 citations to identify eligible studies. Once the non‐applicable studies were excluded, 77 full‐text articles were assessed (Figure 1). A total of 67 of these records were excluded with reasons (see Characteristics of excluded studies). Eight reports of six studies that enrolled a total of 326 CD patients were determined to be eligible for inclusion in the review (Lémann 2005; O'Donoghue 1978; Roblin 2017; Van Assche 2008; Vilien 2004; Wenzl 2015; See Characteristics of included studies). Two ongoing studies were also identified (NCT01817426; NCT02177071). Four of the six included studies were RCTs in which immunosuppressant monotherapy (i.e. azathioprine) was discontinued (Lémann 2005; O'Donoghue 1978; Vilien 2004; Wenzl 2015). Two RCTs evaluated azathioprine withdrawal in patients who had achieved remission with azathioprine and infliximab combination therapy (Roblin 2017; Van Assche 2008). No studies of biologic monotherapy withdrawal were eligible for inclusion.
1.

Study flow diagram.
Included studies
Roblin 2017 was an open‐label randomized trial that followed three cohorts of patients with inflammatory bowel disease (N = 81; 45 patients with CD) who had been treated with azathioprine and infliximab combination therapy for at least one year. These patients were in deep remission for at least six months. In cohort A patients continued to receive a stable dose of combination therapy; in cohort B, the initial azathioprine dose was reduced by half; and in cohort C, azathioprine therapy was completely withdrawn while the initial infliximab dose remained stable. The primary outcome was clinical relapse as defined by a Crohn's Disease Activity Index (CDAI) > 220 with an increase in CDAI of greater than 70 points from the previous assessment or need to change the original therapeutic regimen because of adverse events or drug intolerance.at 12 months. Patients with CD from cohorts A and C (n = 31) were included in this review.
Lémann 2005 was a non‐inferiority placebo‐controlled double‐blind RCT in which 83 patients with quiescent CD who had received azathioprine for at least 42 months were allocated to either continue azathioprine (n = 40) or switch to placebo (n = 43). The primary outcome was clinical relapse (as defined by a CDAI score between 150 and 250 for 3 consecutive weeks with an increase of at least 75 points above baseline value, or the need for CD‐related surgery except for limited perianal surgery) at 18 months.
O'Donoghue 1978 was a placebo‐controlled double‐blind trial in which 51 CD patients who achieved clinical remission after receiving at least 6 months of azathioprine monotherapy were randomized to continue azathioprine (n = 27) or switch to placebo (n = 24). The primary outcome was clinical relapse (defined as a significant deterioration in clinical state requiring a change in treatment as judged by two blinded physicians) at 12 months.
In Van Assche 2008, 80 patients who were in clinical remission and had received combination (azathioprine and infliximab) therapy for more than 6 months were randomized to either discontinue (n = 40) or continue (n = 40) azathioprine. The primary outcome was the proportion of patients who required a change in the infliximab dosing interval or stopped infliximab therapy. Patients were followed for 104 weeks.
Vilien 2004 was an open‐label RCT in which 29 CD patients were randomized to continue (n = 14) or stop (n = 15) azathioprine monotherapy. The patients had received azathioprine for at least two years and were in clinical remission at the time of withdrawal. The primary outcome was clinical relapse (as defined as a CDAI rise of greater or equal to 75 points and a total CDAI of at least 150, or, disease activity necessitating intervention) at month 12.
Wenzl 2015 was a placebo‐controlled double‐blind trial in which 52 CD patients in stable remission for at least 42 months and a minimum four‐year treatment history with azathioprine were randomized to continue azathioprine therapy (n = 26) or switch to placebo (n = 26). The primary outcome was clinical relapse at week 24, defined as either: a) a CDAI score greater than 150 with an increase of at least 60 points above the baseline CDAI score in the absence of infectious diarrhea; b) the development of at least one new fistula in patients who were fistula‐free prior to trial enrolment; c) an increase of four or more Perianal Disease Activity Index points compared to baseline; d) disease activity necessitating therapy with oral corticosteroids or anti‐TNF‐α drugs; e) the need for CD‐related abdominal or perianal surgery).
Excluded studies
Sixty‐seven reports were excluded during the screening process (See Characteristics of excluded studies). One study was excluded because it was a case report (Begun 2016). Helwig 2017 was excluded because it was a review article. Two letters were excluded (Benitez 2015; Chaparro 2015). Two of the reports described studies in which an immunosuppressant or biologic drug, or combination therapy, was reduced in dose rather than being completely withdrawn (Amiot 2016; Paul 2015). In three studies patients did not receive maintenance therapy for the minimum six‐month duration prior to therapy withdrawal (Feagan 2015a; Feagan 2015b; Rismo 2013). A total of nine reports exclusively enrolled pediatric patients (Crombe 2010a; Crombe 2010b; Crombe 2011; Grossi 2015b; Kang 2016; Kierkus 2015; Nuti 2010a; Nuti 2010b; Wynands 2008), and in one study CD specific data were not available (de Lima 2016). Sixteen studies were of a retrospective design and therefore did not met the inclusion criteria (Bouhnik 1996; Chauvin 2014; Domenech 2005; Fischer 2014; Gallego 2015; Hlavaty 2016b; Iborra 2016a; Iborra 2016b; Iimuro 2011; Kennedy 2016; Kim 1999; Monterubbianesi 2015; Monterubbianesi 2016a; Monterubbianesi 2016b; Waugh 2010a; Waugh 2010b). The most common reason for exclusion was the lack of a control group (i.e. a group of patients who continued to receive drug therapy), with 32 studies falling into this category (Bodini 2015; Bortlik 2015a; Bortlik 2015b; Bortlik 2016; Bots 2016a; Bots 2016b; Brooks 2017; Chen 2015a; Chen 2015b; Cortes 2016; Dai 2014; De Suray 2012a; De Suray 2012b; Duricová 2015; Echarri 2013; Farkas 2014; Grossi 2015a; Helwig 2016; Louis 2012; Molander 2014; Molander 2015; Molander 2016; Molnar 2013; Qiu 2015a; Qiu 2015b; Schnitzler 2009; Seirafi 2011a; Seirafi 2011b; Squires 2015; Thomsen 2015; Treton 2009; Zelinkova 2013).
Risk of bias in included studies
The Cochrane risk of bias analysis is summarized in Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Four of the included RCTs were rated as low risk of bias (Lémann 2005; Roblin 2017; Vilien 2004; Wenzl 2015), and two were rated as unclear risk of bias with respect to random sequence generation (O'Donoghue 1978; Van Assche 2008). O'Donoghue 1978 reported that the treatment groups were randomly divided, but it is unclear how this was performed. Similarly, Van Assche 2008 does not clearly describe the randomization process.
Four of the included RCTs were rated as low risk of bias (Roblin 2017; Van Assche 2008; Vilien 2004; Wenzl 2015), and two were rated as unclear risk of bias (Lémann 2005; O'Donoghue 1978) with respect to allocation concealment (Lémann 2005; O'Donoghue 1978). Both Lémann 2005 and O'Donoghue 1978 fail to report sufficient detail regarding the method used to ensure that the individual performing the randomization was unaware of the next treatment assignment.
Blinding
Three of the included RCTs were rated as low risk of bias (Lémann 2005; O'Donoghue 1978; Wenzl 2015), and the remaining three studies were rated as high risk of bias with respect to blinding of participants and personnel (Roblin 2017; Van Assche 2008; Vilien 2004). Due to the open‐label design utilized in three studies no placebo was used and as such participants and personnel were aware of treatment assignment (Roblin 2017; Van Assche 2008; Vilien 2004).
With respect to blinding of outcome assessors, two of the RCTs were rated as unclear risk of bias (O'Donoghue 1978; Wenzl 2015), three were rated as high risk of bias (Roblin 2017; Van Assche 2008; Vilien 2004), and one was rated as low risk of bias (Lémann 2005). In Lémann 2005, biological tests results were reviewed by co‐investigators who were blinded to clinical information and the endoscopist was responsible for independently calculating the Crohn's Disease Endoscopic Index of Severity score. Blinded outcome assessment was not performed in three studies (Roblin 2017; Van Assche 2008; Vilien 2004).
Incomplete outcome data
All six RCTs were rated as low risk of bias for incomplete outcome data (Lémann 2005; O'Donoghue 1978; Roblin 2017; Van Assche 2008; Vilien 2004; Wenzl 2015).
Selective reporting
All six RCTs were rated as low risk of bias for selective reporting (Lémann 2005; O'Donoghue 1978; Roblin 2017; Van Assche 2008; Vilien 2004; Wenzl 2015).
Other potential sources of bias
Four of the five RCTs were rated as low risk of bias for other potential sources of bias (Lémann 2005; O'Donoghue 1978; Van Assche 2008; Vilien 2004). Wenzl 2015 and Roblin 2017 received an unclear rating for this factor since the trials were prematurely stopped due to slow enrolment.
Effects of interventions
Summary of findings for the main comparison. Usual care compared to immunosuppressive withdrawal after monotherapy for patients with quiescent Crohn's disease.
| Usual care compared to immunosuppressive withdrawal after monotherapy for patients with quiescent Crohn's disease | ||||||
| Patient or population: Patients with quiescent Crohn's disease Setting: Outpatient Intervention: Usual care Comparison: Immunosuppressive withdrawal after monotherapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
|
Risk with immunosuppressive withdrawal after monotherapy |
Risk with usual care | |||||
| Relapse at 12, 18 or 24 months | Study population | RR 0.42 (0.24 to 0.72) | 215 (4 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Sparse data (50 events) | |
| 324 per 1,000 | 136 per 1,000 (78 to 234) | |||||
| New CD‐related complications | Study population | RR 0.34 (0.06 to 2.08) | 135 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 3 4 | Very sparse data (5 events) | |
| 58 per 1,000 | 20 per 1,000 (3 to 121) | |||||
| Adverse events | Study population | RR 0.88 (0.67 to 1.17) | 186 (3 RCTs) | ⊕⊕⊝⊝ LOW 2 5 | Sparse data (45 events) | |
| 240 per 1,000 | 211 per 1,000 (161 to 280) | |||||
| Serious adverse events | Study population | RR 3.29 (0.35 to 30.80) | 134 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 3 4 | Very sparse data (2 events) | |
| 0 per 1,000 | 0 per 1,000 (0 to 0) | |||||
| Withdrawal due to adverse events | Study population | RR 2.59 (0.35 to 19.04) | 135 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 3 4 | Very sparse data (5 events) | |
| 14 per 1,000 | 38 per 1,000 (5 to 276) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level due to high risk of bias for blinding in one study and unclear risk of bias in three studies in the pooled analysis
2 Downgraded one level due to sparse data
3 Downgraded one level due to unclear risk of bias in the two studies in the pooled analysis
4 Downgraded two levels due to very sparse data
5 Downgraded one level due to unclear risk of bias in the three studies in the pooled analysis
Summary of findings 2. Usual care compared to immunosuppressive withdrawal after combination therapy for patients with quiescent Crohn's disease.
| Usual care compared to immunosuppressive withdrawal after combination therapy for patients with quiescent Crohn's disease | ||||||
| Patient or population: Patients with quiescent Crohn's disease Setting: Outpatient Intervention: Usual care Comparison: Immunosuppressive withdrawal after combination therapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
|
Risk with immunosuppressive withdrawal after combination therapy |
Risk with usual care | |||||
| Relapse at 12 or 24 months | Study population | RR 1.02 (0.68 to 1.52) | 111 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Sparse data (54 events) | |
| 491 per 1,000 | 501 per 1,000 (334 to 746) | |||||
| Adverse events | Study population | RR 1.11 (0.44 to 2.81) | 111 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | Sparse data (51 events) | |
| 455 per 1,000 | 505 per 1,000 (200 to 1,000) | |||||
| Serious adverse events | Study population | RR 1.00 (0.21 to 4.66) | 80 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 3 | Very sparse data (6 events) | |
| 75 per 1,000 | 75 per 1,000 (16 to 350) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level due to high risk of bias for blinding
2 Downgraded one level due to sparse data
3 Downgraded two levels due to very sparse data
Withdrawal of immunosuppressant monotherapy
The proportion of patients who relapse following discontinuation
Four RCTs with a total of 215 participants provided data on the rate of clinical relapse following the discontinuation of azathioprine monotherapy (Lémann 2005; O'Donoghue 1978; Vilien 2004; Wenzl 2015). The follow‐up period was 12 months for two studies (O'Donoghue 1978, Vilien 2004), 18 months for one study (Lémann 2005) and 24 months for one study (Wenzl 2015). A total of 32.4% (36/111) participants assigned to azathioprine withdrawal experienced clinical relapse compared to 13.5% (14/104) patients assigned to therapeutic continuation. The pooled RR for the primary outcome was 0.42 (95% CI 0.24 to 0.72, P = 0.002, I² = 0%), indicating a statistically significant benefit in favour of continuing azathioprine monotherapy (Analysis 1.1). The GRADE analysis indicated that the overall quality of evidence for the primary outcome (clinical relapse) for placebo‐controlled studies was low due to high risk of bias for blinding in one study (Vilien 2004), unclear risk of bias in three studies (Lémann 2005; O'Donoghue 1978; Wenzl 2015) and sparse data (see Table 1).
1.1. Analysis.

Comparison 1 Usual care versus immunosuppressive withdrawal after monotherapy, Outcome 1 Relapse at 12, 18 or 24 months.
The proportion of patients with new CD‐related complications (e.g. fistula, abscesses, strictures) following discontinuation
Two RCTs with a total of 135 participants provided data on the proportion of patients with new CD‐related complications following the discontinuation of azathioprine monotherapy (Lémann 2005; Wenzl 2015). A total of 5.8% (4/69) patients in the monotherapy withdrawal group experienced a new CD‐related complication compared to 1.5% (1/66) participants in the monotherapy continuation group. The pooled RR demonstrated no statistically significant difference between the two groups (RR 0.34, 95% CI 0.06 to 2.08, P = 0.24; I² = 0%; Analysis 1.2). The GRADE analysis indicated that the overall quality of evidence for this outcome was very low due to unclear risk of bias and very sparse data (see Table 1).
1.2. Analysis.
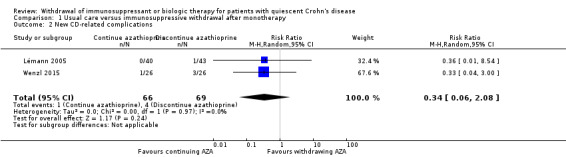
Comparison 1 Usual care versus immunosuppressive withdrawal after monotherapy, Outcome 2 New CD‐related complications.
The proportion of patients with adverse events following discontinuation
Three trials with a total of 186 participants provided data on the proportion of patients who experienced an adverse event following discontinuation of azathioprine monotherapy (Lémann 2005; O'Donoghue 1978; Wenzl 2015). A total of 24.0% (23/96) of participants randomized to discontinue therapy versus 24.4% (22/90) participants randomized to continue therapy experienced an adverse event during the observation period. The pooled RR was 0.88 (95% CI 0.67 to 1.17, P = 0.39; I² = 0%) showing no statistically significant difference between the withdrawal and continuation groups (Analysis 1.3).The GRADE analysis indicated that the overall quality of evidence for this outcome was low due to sparse data and unclear risk of bias (see Table 1). Common adverse events included infections, mild leukopenia, abdominal symptoms, arthralgias, headache and elevated liver enzymes.
1.3. Analysis.
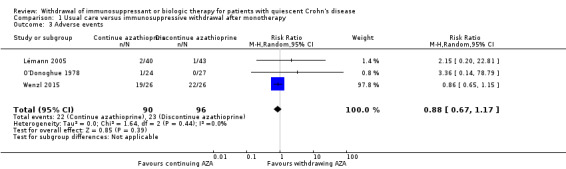
Comparison 1 Usual care versus immunosuppressive withdrawal after monotherapy, Outcome 3 Adverse events.
The proportion of patients with serious adverse events following discontinuation
Two trials (N = 134) provided data on the proportion of patients with serious adverse events following discontinuation of azathioprine monotherapy (Lémann 2005; O'Donoghue 1978 ). A total of 0% (0/70) of participants who discontinued therapy versus 3.1% (2/64) of participants who continued therapy experienced a serious adverse event during the observation period. The pooled RR was 3.29 (95% CI 0.35 to 30.80, P = 0.30; I² = 0%) and demonstrated no statistically significant difference between treatment groups (Analysis 1.4). The GRADE analysis indicated that the quality of evidence for this outcome was very low due to unclear risk of bias and very sparse data (see Table 1). The two serious adverse events included myelodysplastic syndrome (Lémann 2005), and pancytopenia (O'Donoghue 1978).
1.4. Analysis.

Comparison 1 Usual care versus immunosuppressive withdrawal after monotherapy, Outcome 4 Serious adverse events.
The proportion of patients who withdraw due to adverse events following discontinuation
Two trials with a total of 135 participants provided data on the proportion of patients who withdrew due to adverse events following discontinuation of azathioprine monotherapy (Lémann 2005; Wenzl 2015). A total of 1.4% (1/69) of participants randomized to therapeutic discontinuation versus 6.0% (4/66) of participants randomized to therapeutic continuation withdrew from the study due to an adverse event. The pooled RR of 2.59 (95% CI 0.35 to 19.04, P = 0.35; I² = 0%) failed to demonstrate a statistically significant difference between azathioprine monotherapy discontinuation compared to continuation (Analysis 1.5). The GRADE analysis indicated that the quality of evidence for this outcome was very low due to unclear risk of bias and very sparse data (see Table 1).
1.5. Analysis.

Comparison 1 Usual care versus immunosuppressive withdrawal after monotherapy, Outcome 5 Withdrawal due to adverse events.
Other outcomes
There were no data available to assess between‐group differences in: time to relapse; the proportion of patients who responded to the reintroduction of immunosuppressant monotherapy; the proportion of patients with elevated biomarkers of inflammation; the proportion of patients with anti‐drug antibodies and low serum trough drug levels; the proportion of patients who required surgery following relapse; or the proportion of patients who required hospitalization following relapse.
Withdrawal of anti‐TNF‐α monotherapy or other biologics
None of the included studies examined withdrawal of anti‐TNF‐α monotherapy, or withdrawal of other biologics used in Crohn's disease.
Withdrawal of immunosuppressant from combination therapy
Proportion of patients who relapse following discontinuation of immunosuppressant from combination therapy
Two studies (Roblin 2017; Van Assche 2008), reported on the proportion of patients who relapsed following discontinuation of an immunosuppressant from combination therapy (N = 111). The follow‐up period was 12 months for one study (Roblin 2017), and 24 months for one study (Van Assche 2008). When data from these studies were combined, a total of 49% (27/55) of patients who were withdrawn from azathioprine (but continued to receive infliximab) experienced clinical relapse compared to 48% (27/56) of patients who continued combination therapy with both azathioprine and infliximab. The pooled RR revealed no statistically significant difference between groups (RR 1.02, 95% CI 0.68 to 1.52, P = 0.92, I2 = 4%; (Analysis 2.1). The GRADE analysis indicated that the quality of evidence for this outcome was low due to high risk of bias for blinding and sparse data (see Table 2).
2.1. Analysis.

Comparison 2 Usual care versus immunosuppressive withdrawal after combination therapy, Outcome 1 Relapse at 12 or 24 months.
The proportion of patients with adverse events following discontinuation of immunosuppressant from combination therapy
Two RCTs (Roblin 2017; Van Assche 2008), reported on the proportion of patients who experienced an adverse event following discontinuation of an immunosuppressant from combination therapy (N = 111). When data were pooled, 45% (25/55) of patients who were withdrawn from azathioprine (but continued to receive infliximab) experienced an adverse event compared to 46% (26/56) of patients who continued combination therapy with both azathioprine and infliximab. The pooled RR revealed no statistically significant difference between groups (RR 1.11, 95% CI 0.44 to 2.81, P = 0.83, I2 = 16%; Analysis 2.2). Likewise, the RR in each type of study neither revealed no differences between groups (RR 1.09, 95% CI 0.75 to 1.59, in the RCT (Van Assche 2008); and RR of 0.56, 95% CI 0.16 to 1.96 in the observational study (Roblin 2017)). The GRADE analysis indicated that the quality of evidence for this outcome was low due to high risk of bias for blinding and sparse data (see Table 2). Common adverse events included infections, liver test elevations, arthralgias and infusion reactions.
2.2. Analysis.

Comparison 2 Usual care versus immunosuppressive withdrawal after combination therapy, Outcome 2 Adverse events.
The proportion of patients with serious adverse events following discontinuation of immunosuppressant from combination therapy
One study (Van Assche 2008) reported on the proportion of patients who experienced a serious adverse event following discontinuation of an immunosuppressant from combination therapy (N = 80). In both the azathioprine discontinuation and continuation groups, 0.07% (3/40) of patients experienced a serious adverse event. Thus, there was no statistically significant between‐group difference (RR 1.00, 95% CI 0.21 to 4.66; Analysis 2.3). The GRADE analysis indicated that the quality of evidence for this outcome was very low due to high risk of bias for blinding and very sparse data (see Table 2). Serious adverse events in the azathioprine discontinuation group include appendectomy (n = 1), skin carcinoma (n = 1), and ureterolithiasis (n = 1). Serious adverse events in group that continued combination therapy include pregnancy (n = 1), pneumonia (n = 1) and partial colectomy (n =1).
2.3. Analysis.

Comparison 2 Usual care versus immunosuppressive withdrawal after combination therapy, Outcome 3 Serious adverse events.
Other outcomes
There were no data available to assess between‐group differences in: time to relapse; the proportion of patients who experienced a CD‐related complication; the proportion of patients who responded to the reintroduction of immunosuppressant monotherapy; the proportion of patients with elevated biomarkers of inflammation; the proportion of patients with anti‐drug antibodies and low serum trough drug levels; the proportion of patients who required surgery following relapse; the proportion of patients who required hospitalization following relapse; or the proportion of patients who withdrew from the study due to adverse events.
Withdrawal of an anti‐TNF‐αagent from combination therapy
None of the included studies examined withdrawal of an anti‐TNF‐α agent from a combination therapy regimen.
Due to the small number of included studies and limited data, pre‐specified sensitivity analyses to assess the impact of risk of bias were not performed. Sensitivity analyses to examine the impact of using random effects versus fixed effect modelling were performed; the results are presented in Table 3. The application of fixed effect modelling had no or minimal change on the point estimates for all comparisons.
1. Sensitivity analysis: random effects vs fixed effect modelling.
| Comparison 1: Usual care versus immunosuppressive withdrawal after monotherapy | |||
| Outcome | Random Effects RR (95% CI) | Fixed Effect RR (95% CI) | Impact |
| 1.1 Relapse at 12, 18 or 24 months | 0.42 (0.24‐0.72) | 0.42 (0.24‐0.72) | No change |
| 1.2 New CD‐related complications | 0.34 (0.06‐2.08) | 0.34 (0.06‐2.08) | No change |
| 1.3 Adverse events | 0.88 (0.67‐1.17) | 0.97 (0.71‐1.32) | Minimal |
| 1.4 Serious adverse events | 3.29 (0.35‐30.80) | 3.29 (0.35‐30.80) | No change |
| 1.5 Withdrawal due to adverse events | 2.59 (0.35‐19.04) | 3.10 (0.49‐19.41) | Minimal |
| Comparison 2: Usual care versus Immunosuppressive withdrawal after combination therapy | |||
| Outcome | Random Effects RR (95% CI) | Fixed Effect RR (95% CI) | |
| 2.1 Relapse at 12 or 24 months | 1.02 (0.68‐1.52) | 0.99 (0.69‐1.43) | Minimal |
| 2.2 Adverse events | 1.11 (0.44‐2.81) | 1.04 (0.73‐1.47) | Minimal |
| 2.3 Serious adverse events | No pooling | No pooling | No pooling |
Discussion
Summary of main results
Six RCTs enrolling a total of 326 patients were identified by the literature search and met the criteria for inclusion for this review (Lémann 2005; O'Donoghue 1978; Roblin 2017; Van Assche 2008; Vilien 2004; Wenzl 2015).
Immunosuppressant monotherapy withdrawal
Our primary results suggest that patients with quiescent CD who continue azathioprine monotherapy may be less likely to experience clinical relapse compared to those who discontinue azathioprine therapy (RR 0.42, 95% CI 0.24 to 0.72, P = 0.002). However, these results should be interpreted with caution, since GRADE analysis revealed that the quality of evidence was low due to high risk of bias for blinding and sparse data. Furthermore, it is important to note that none of the studies were performed specifically for patients in 'deep remission' (clinical, biochemical and endoscopic remission).
No statistically significant between‐group differences were observed regarding the secondary outcomes. However, the quality of evidence supporting these findings ranges from very low to low , indicating the further research may impact on our confidence in the estimate of effect, and may ultimately change the estimate.
Immunosuppressant withdrawal from combination therapy
Two RCTs evaluated the withdrawal of azathioprine from a combination therapy regimen in patients with inactive CD (Roblin 2017; Van Assche 2008). These studies reported on the proportion of patients who relapsed and the proportion of patients who experienced an adverse event. Pooled and separate analyses were conducted, revealing with no statistical significant differences between the discontinuation and continuation groups. The overall quality of evidence was low, indicating that further research may impact on the confidence in the estimate of effect, and may ultimately change the estimate.
Van Assche 2008 was the only study to both evaluate the withdrawal of azathioprine from a combination therapy regimen and report on the proportion of patients with serious adverse events. No statistically significant difference was observed between treatment groups. The overall quality of evidence supporting this outcome was rated as very low. Thus, our confidence in the estimate of effect is limited. It should be noted that Van Assche 2008 was designed as a superiority study, with continued azathioprine therapy assumed to be superior to discontinued azathioprine therapy. This study was not adequately powered to detect non‐inferiority between the withdrawal and control groups.
Overall completeness and applicability of evidence
An exhaustive literature search was performed to identify potential included studies, and handsearching was performed to ensure that no eligible reports were missed. Unfortunately, several of our pre‐specified secondary outcomes of interest were not addressed in the included studies. There were no data on time to relapse; the proportion of patients who responded to immunosuppressant reintroduction; the proportion of patients with elevated biomarkers of inflammation; the proportion of patients with anti‐drug antibodies and low serum trough levels; the proportion of patients requiring surgery due to relapse; and the proportion of patients requiring hospitalization due to relapse in any of the included studies. Furthermore, the literature search failed to identify any controlled data regarding the withdrawal of anti‐TNF‐α therapy (as part of either a monotherapy or combination therapy regimen) in CD patients with inactive disease.
All of the included studies evaluated azathioprine monotherapy, or a combination therapy administered using azathioprine and infliximab, which may limit the applicability of the results of this systematic review to these specific medications. The findings of this systematic review are confined to patients in remission at the time of drug withdrawal. It is worth emphasizing that there were variations between included studies in the definition of remission and length of remission prior to drug withdrawal (summarized in Table 4).
2. Definition of remission by study.
| Study | Length of remission prior to drug withdrawal | Definition of remission prior to drug withdrawal |
| Roblin 2017 | Minimum 6 months | CDAI ≤ 150 and fecal calprotectin levels < 250 μg/g |
| Lémann 2005 | Mean 62 months (standard deviation 26 months); Minimum 42 months | Clinical remission (CDAI ≤ 150) and no need for medical/surgical therapy in the previous 42 months |
| O'Donoghue 1978 | Minimum 6 months | Clinical remission not otherwise specified |
| Van Assche 2008 | Minimum 6 months | Clinical response to infliximab and disease control |
| Vilien 2004 | Not specified | Clinical remission: physician's global assessment |
| Wenzl 2015 | Minimum 12 months | Clinical remission, no need for new medical therapy in the previous 12 months |
Both studies evaluating the withdrawal of azathioprine from a combination therapy regimen (Roblin 2017; Van Assche 2008) are likely to have had patient groups comprised primarily of those who had previously failed immunosuppressant monotherapy. It could be argued that in this cohort, the subsequent withdrawal of this agent would not be expected to have a significant effect (compared to, for example, a patient cohort in which patients initially commenced combination therapy and then had their immunosuppressant withdrawn).
The literature search did not identify any controlled studies of anti‐TNF‐α withdrawal, however it should be noted that the STORI trial, a relatively large, uncontrolled, prospective cohort study (N = 115) examined infliximab withdrawal from a combination therapy regimen in patients with quiescent CD (Louis 2012). Details pertaining to this study are listed in the Characteristics of excluded studies.
Quality of the evidence
The Cochrane risk of bias tool was used to assess the risk of bias of the included RCTs. Three of the RCTs received an unclear risk of bias rating for items including random sequence generation (O'Donoghue 1978), allocation concealment (Lémann 2005; O'Donoghue 1978), blinding of outcome assessors (O'Donoghue 1978; Wenzl 2015), and other bias (Wenzl 2015). Three of the RCTs were open‐label trials, and therefore received a high risk of bias rating for blinding of study participants and personnel (Roblin 2017; Van Assche 2008; Vilien 2004), while the remaining three RCTs received a low risk of bias rating for this factor. With regard to blinding of outcome assessors, three RCTs received a high risk of bias rating (Roblin 2017; Van Assche 2008; Vilien 2004), and one RCT received a low risk of bias rating (Lémann 2005). All six RCTs received low risk of bias ratings for incomplete outcome data and selective reporting (see Figure 2).
The overall quality of evidence supporting each outcome was assessed using the GRADE approach. As described in the Summary of findings table 2, the GRADE assessments for immunosuppressive monotherapy withdrawal compared to continuation ranged from very low (i.e. we are very uncertain about the estimate) to low (i.e. the true effect may be substantially different from the estimate of effect).
The GRADE assessments for immunosuppressive withdrawal from combination therapy compared to uninterrupted combination therapy are described in the Summary of findings table 2. Quality assessments ranged from very low (i.e. we are very uncertain about the estimate) to low (i.e. the true effect may be substantially different from the estimate of effect).
Potential biases in the review process
The methods and reporting structure employed for this systematic review were based on recommendations provided by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). A comprehensive literature search was developed and conducted, and the protocol was published in advance to facilitate transparency. The literature screening and data extraction were independently performed by two authors, and the quality of included studies and evidence was rigorously assessed. However, there are some potential limitations of the current systematic review. First, few additional data were obtained from unpublished sources. While we planned to investigate the possibility of publication bias using funnel plots, this was prevented by the insufficient number of included studies (i.e. less than 10 studies). Second, definitions of clinical relapse varied across studies; however, it should be noted that high statistical heterogeneity was not detected. Third, during the review execution there were some deviations from the published protocol. These derivations are transparently reported, and we feel they are unlikely to change our conclusions (see Differences between protocol and review).
Agreements and disagreements with other studies or reviews
The available evidence on cessation of immunosuppressant and anti‐TNF‐α drug therapy in patients with inactive CD has been systematically reviewed in six other reviews (Doherty 2018; French 2011; Gisbert 2016; Kennedy 2016; Pariente 2014; Torres 2015).
Doherty 2018 is a topical review, on the withdrawal of treatment in inflammatory bowel disease. This review summarizes expert opinions and was published by the European Crohn's and Colitis Organisation. It is stated that a systematic review of the literature was conducted, however the search date and search strategy were not reported. No risk of bias or quality assessments were performed, nor were the data meta‐analyzed. The authors conclude that a) there is a strong rationale for ceasing or de‐escalating immunosuppressant and anti‐TNF‐α therapy, particularly when used together in a combination regimen; b) CD patients who are withdrawn from drug therapy should be in clinical, biochemical and endoscopic remission and at low risk for relapse; and c) the decision to stop therapy should be based on the individual patient. It is also noted that further evidence from RCTs on the withdrawal of drug therapy is needed.
French 2011 is a systematic review and meta‐analysis that evaluated the impact of azathioprine withdrawal on the rate of relapse in CD patients with inactive disease. The literature search was conducted in September 2010, and a total of five studies, three RCTs (Lémann 2005; O'Donoghue 1978; Vilien 2004), and two retrospective cohort studies were included (Kim 1999; Sokol 2010). The authors conclude that immunosuppressant (i.e. azathioprine and 6‐mercaptopurine) continuation is more effective than withdrawal for remission of Crohn's disease. The current study differs from French 2011 in that three additional RCTs (Roblin 2017; Van Assche 2008; Wenzl 2015), were identified and included, and no retrospective data were included (Kim 1999; Sokol 2010).
Torres 2015 is a systematic review focused on the withdrawal of immunosuppressants or biologics in patients with quiescent CD or ulcerative colitis. A total of 7 RCTs, 16 prospective cohort studies and 43 retrospective cohort studies were deemed eligible for inclusion. Two of the RCTs, and 15 of the prospective cohort studies and all retrospective cohort studies were not included in the current review because the study population was not of interest (i.e. ulcerative colitis, pregnant and pediatric patients) or there was no control group. No meta‐analyses were performed. The authors conclude that disease activity, prognostic factors and complicated disease were associated with future relapse, and that approximately 50% of inflammatory bowel disease patients who discontinue therapy relapse. They also state that the cessation of drug therapy is a decision that should be made based on the individual patient.
Pariente 2014 performed a systematic literature review to identify studies evaluating the cessation of immunosuppressants or anti‐TNF‐α agents in inflammatory bowel disease patients. Studies that employed a retrospective design, lacked a control group, and exclusively enrolled ulcerative colitis patients met the eligibility criteria. A total of 11 relevant studies were identified, including one of the five RCTs included in the current systematic review (Van Assche 2008). No risk of bias assessments or meta‐analyses were performed. The authors conclude that therapeutic de‐escalation should be initiated on a case‐by‐case basis.
Gisbert 2016 performed a systematic literature review and meta‐analysis of studies evaluating the risk of relapse after discontinuation of anti‐TNF‐α therapy in clinically quiescent CD or ulcerative colitis. In contrast to the current review, studies that employed a retrospective design or lacked a control group, and those enrolling patients with ulcerative colitis met the eligibility criteria. The authors conclude that approximately one‐third of patients with inflammatory bowel disease relapsed after one year of discontinuing anti‐TNF‐α therapy, and this proportion increased to about one‐half in the longer term.
Kennedy 2016 performed a systematic literature review and meta‐analysis of studies assessing outcomes following anti‐TNF‐α withdrawal in patients with inflammatory bowel disease in clinical remission. This review included studies that employed a retrospective design or lacked a control group. The authors conclude that approximately one‐third of patients with IBD flare within one year of withdrawal of anti‐TNF‐α therapy.
Authors' conclusions
Implications for practice.
Immunosuppressant and anti‐TNF‐α agents are frequently used as induction and maintenance therapy in patients with moderate to severe CD. A common clinical dilemma is the feasibility of treatment withdrawal in patients who have achieved a sustained period of remission due to concerns regarding safety and cost. The effects of withdrawal of immunosuppressant therapy in quiescent Crohn's disease are uncertain. Low quality evidence suggests that continuing azathioprine monotherapy may be superior to withdrawal for avoiding clinical relapse. In terms of discontinuing azathioprine from a combination therapy regimen compared to continuing azathioprine and infliximab combination therapy, our analysis could not detect any difference between the two strategies although it is important to note that the quality of the evidence was very low. With respect to the development of CD‐related complications, adverse events, serious adverse events and withdrawal due to adverse events, no differences were observed between continuation compared to withdrawal for azathioprine monotherapy or combination therapy with azathioprine and infliximab. It is important to note that none of the included studies focused on the withdrawal of an anti‐TNF‐α agent from a monotherapy or combination therapy regimen, and azathioprine and infliximab were the only immunosuppressive and anti‐TNF‐α drugs studied, respectively. Furthermore, these studies were relatively limited in size with respect to participants and events, and as such our results should be interpreted with caution.
Implications for research.
There is a lack of controlled data on the withdrawal of drug therapy in patients with quiescent CD, particularly in the clinically relevant scenario of anti‐TNF‐α withdrawal from a combination therapy regimen. There is also a paucity of controlled data on withdrawal from anti‐TNF‐α monotherapy, and from other biologics used in CD such as vedolizumab and ustekinumab. Further research is necessary to confirm the possible risks and benefits associated with therapeutic cessation. We are aware of two ongoing RCTs in this area (NCT02177071; NCT01817426), and plan to include available data from these trials in future updates of this systematic review.
Acknowledgements
Funding for the Cochrane IBD Group (May 1, 2017 ‐ April 30, 2022) has been provided by Crohn's and Colitis Canada (CCC).
Appendices
Appendix 1. search strategies
EMBASE (1984 to present)
1. Exp inflammatory bowel diseases/ 2. Inflammatory bowel disease.mp. 3. Ulcerative colitis.mp. 4. Crohn.mp. 5. Exp Ulcerative colitis/ 6. Exp Crohn/ 7. IBD.mp. 8. Down titrat*.mp. 9. Dose titrat*.mp. 10. Dose reduc*.mp. 11. Dose de‐escalation.mp. 12. Withdraw*.mp. 13. Discontinu*.mp. 14. Dose taper*.mp. 15. Cessation.mp. 16. Stop*.mp. 17. Exp Tumor necrosis factor‐alpha/ 18. Exp Antibodies, Monoclonal/ 19. Exp Antimetabolites/ 20. Exp Immunosuppressive agents/ 21. Tumor necrosis factor.mp. 22. TNF‐alpha.mp. 23. TNFalpha.mp. 24. Anti‐TNF.mp. 25. AntiTNF.mp. 26. Anti‐tumor necrosis.mp. 27. Antitumor necrosis.mp. 28. Monoclonal antibod*mp. 29. Infliximab.mp. 30. Remicade.mp. 31. Certolizumab.mp. 32. Cimzina.mp. 33. Adalimumab.mp. 34. Humira.mp. 35. Antimetabolites.mp. 36. Anti‐metabolites.mp. 37. Immunomodulator*.mp. 38. Thiopurine.mp. 39. Mercaptopurine.mp. 40. 6‐MP.mp. 41. Azathioprine.mp. 42. Methotrexate.mp. 43. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 44. Or/1‐7 45. Or/8‐16 46. Or/17‐42 47. 43 and 44 and 45 and 46
MEDLINE (1946 to present)
1. Exp inflammatory bowel diseases/ 2. Inflammatory bowel disease.mp. 3. Ulcerative colitis.mp. 4. Crohn.mp. 5. Exp Ulcerative colitis/ 6. Exp Crohn/ 7. IBD.mp. 8. Down titrat*.mp. 9. Dose titrat*.mp. 10. Dose reduc*.mp. 11. Dose de‐escalation.mp. 12. Withdraw*.mp. 13. Discontinu*.mp. 14. Dose taper*.mp. 15. Cessation.mp. 16. Stop*.mp. 17. Exp Tumor necrosis factor‐alpha/ 18. Exp Antibodies, Monoclonal/ 19. Exp Antimetabolites/ 20. Exp Immunosuppressive agents/ 21. Tumor necrosis factor.mp. 22. TNF‐alpha.mp. 23. TNFalpha.mp. 24. Anti‐TNF.mp. 25. AntiTNF.mp. 26. Anti‐tumor necrosis.mp. 27. Antitumor necrosis.mp. 28. Monoclonal antibod*mp. 29. Infliximab.mp. 30. Remicade.mp. 31. Certolizumab.mp. 32. Cimzina.mp. 33. Adalimumab.mp. 34. Humira.mp. 35. Antimetabolites.mp. 36. Anti‐metabolites.mp. 37. Immunomodulator*.mp. 38. Thiopurine.mp. 39. Mercaptopurine.mp. 40. 6‐MP.mp. 41. Azathioprine.mp. 42. Methotrexate.mp. 43. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 44. Or/1‐7 45. Or/8‐16 46. Or/17‐42 47. 43 and 44 and 45 and 46
CENTRAL (inception to present)
#1 MeSH descriptor: [Inflammatory bowel diseases] explode all trees #2 MeSH descriptor: [Crohn Disease] explode all trees #3 MeSH descriptor: [Colitis, Ulcerative] explode all trees #4 Crohn #5 Ulcerative colitis #6 “Down titrat*” OR “dose titrat*” OR “dose reduc*” OR “dose de‐escalation” OR “withdraw*” OR “discontinu*” OR “dose taper” OR “cessation” OR “stop*” #7 MeSH descriptor: [Tumor necrosis factor‐alpha] explode all trees #8 MeSH descriptor: [Antibodies, Monoclonal/] explode all trees #9 MeSH descriptor: [Antimetabolites/] explode all trees #10 MeSH descriptor: [Immunosuppressive agents/] explode all trees #11 “TNF‐alpha” OR “TNFalpha” OR “Anti‐TNF” OR “AntiTNF” OR “Anti‐Tumor” OR “monoclonal antibod*” OR “infliximab” OR “Remicade” OR “certolizumab” OR “Cimzia” OR “adalimumab” OR “Humira” OR “antimetabolite*” OR “anti‐metabolite*” OR “immunomodulator*” OR “thiopurine*” OR “mercaptopurine” OR “6‐MP” OR “azathioprine” OR “methotrexate” #12 #1 and #2 and #3 and #4 and #5 #13 #7 and #8 and #9 and #10 and #11 #14 #6 and #12 and #13
The Cochrane IBD Group Specialized Register (inception to present)
1. crohn OR colitis OR inflammatory bowel disease or IBD
2. “Down titrat” OR “dose titrat” OR “dose reduc” OR “dose de‐escalation” OR “withdraw” OR “discontinu*” OR “dose taper” OR “cessation” OR “stop”
3. "necrosis" OR "TNF" OR "Anti‐TNF" OR "AntiTNF" OR "monoclonal" OR "immunosuppress" or "immunomodulat" OR "infliximab" OR "remicade" OR "certolizumab" OR "Cimzia" OR "adalimumab" OR "humira" OR "antimetabolite" OR "anti‐metab" OR "thiopurine" OR "mercaptopurine" OR "6‐MP" OR "azathoprine" OR "methotrexate"
4. 1 AND 2 AND 3
Data and analyses
Comparison 1. Usual care versus immunosuppressive withdrawal after monotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse at 12, 18 or 24 months | 4 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.24, 0.72] |
| 2 New CD‐related complications | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.06, 2.08] |
| 3 Adverse events | 3 | 186 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.67, 1.17] |
| 4 Serious adverse events | 2 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 3.29 [0.35, 30.80] |
| 5 Withdrawal due to adverse events | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 2.59 [0.35, 19.04] |
Comparison 2. Usual care versus immunosuppressive withdrawal after combination therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse at 12 or 24 months | 2 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.68, 1.52] |
| 2 Adverse events | 2 | 111 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.44, 2.81] |
| 3 Serious adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Lémann 2005.
| Methods | Randomized, double‐blind, multi‐centre, placebo controlled withdrawal trial (N = 83) 11 sites in France, 1 site in Belgium |
|
| Participants | Adults (> 18 years) with CD as diagnosed by established clinical, endoscopic, radiological and histological criteria who had received continuous azathioprine therapy for > 42 months Patients were ineligible if they had: a) experienced a flare‐up while receiving azathioprine; b) had active disease at entry (CDAI score > 150); c) had isolated perianal disease; or d) were treated with azathioprine for the prevention of post‐operative recurrence |
|
| Interventions | 1:1 randomization ratio Group 1: oral azathioprine once daily at the dose taken prior to study enrolment (n = 40) Group 2: placebo (n = 43) Follow‐up duration: 18 months |
|
| Outcomes | Primary outcome: proportion of patients with relapse (defined as CDAI score > 250, a CDAI score 150‐250 on 3 consecutive weeks with an increase of > 75 points from baseline, or the need for surgery) over the 18 month study period | |
| Notes | The definition of relapse was chosen to eliminate small/transient increases of the CDAI score, which could be attributable to a cause other than relapse such as irritable bowel syndrome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomization using permutation tables of 2 or 4 |
| Allocation concealment (selection bias) | Unclear risk | Not adequately described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The placebo and azathioprine were identical in appearance and taste |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Patients filled out diary cards Biological test results were reviewed by co‐investigators who had no patient contact and recorded results in a separate case report form The endoscopists calculated the CDEIS (it is unclear, but assumed that they were blinded to clinical information) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs were balanced across treatment groups 37/40 in the azathioprine group completed the study compared to 40/43 in the placebo group |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | The study appears to be free from other sources of bias |
O'Donoghue 1978.
| Methods | Randmized, double‐blind, placebo‐controlled withdrawal trial of azathioprine in CD (N = 51) | |
| Participants | Adults (>18 years) with CD as diagnosed by established criteria who were on azathioprine for > 6 months and had been in clinical remission for > 6 months | |
| Interventions | Group 1: oral azathioprine at the dose taken prior to study enrolment (n = 27) Group 2: placebo (n = 24) Duration of follow‐up: 12 months |
|
| Outcomes | Primary outcome: proportion of patients with relapse (defined as a significant deterioration in clinical state requiring change in treatment as judged by two doctors unaware of the patient's treatment) over the 12 month study period | |
| Notes | This represents the first withdrawal trial of azathioprine monotherapy in CD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study only states the groups were randomly divided, no information on how this was performed |
| Allocation concealment (selection bias) | Unclear risk | Not adequately described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind trial with control tablets utilized |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not adequately described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs were balanced across treatment groups 21/24 in the azathioprine group completed the study compared to 25/27 in the placebo group |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | This study appears to be free from other sources of bias |
Roblin 2017.
| Methods | Randomized open‐label trial (N = 81: UC N = 36; CD N = 45) | |
| Participants | Patients with IBD (UC and CD) in deep remission for at least 6 months who were treated with combination therapy (infliximab and azathioprine) for at least one year | |
| Interventions | Cohort A: azathioprine and infliximab continued (usual care) (UC n = 12; CD n = 16) Cohort B: azathioprine dose halved (UC n = 13; CD n = 14) Cohort C: azathioprine stopped (infliximab continued as monotherapy) (UC = 11; CD n = 15) |
|
| Outcomes | Primary: clinical relapse (CDAI score > 220 with a delta CDAI > 70 from the previous assessment and/or need to change the original therapeutic regimen because of adverse events or drug intolerance Secondary: infliximab trough levels and anti‐drug antibodies |
|
| Notes | Authors were contacted, and they provided information regarding CD‐specific results Patients with CD from cohorts A and C (n = 31) were included in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomised into three parallel groups with randomisation balanced by blocks" |
| Allocation concealment (selection bias) | Low risk | "The randomisation was not stratified and was centrally performed by an interactive web response system (IWRS)" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs were balanced across treatment groups 13/16 in the combination therapy group completed the study compared to 10/15 in the group where azathioprine was stopped |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes reported CD and UC data not reported separately, however authors supplied this information upon request |
| Other bias | Unclear risk | Premature stopping of the trial due to slow enrolment |
Van Assche 2008.
| Methods | Randmized, open‐label controlled trial (N = 80) | |
| Participants | Patients (> 16 years) with luminal CD on a combination regimen consisting of infliximab 5 mg/kg IV and an immunosuppressant (azathioprine/6‐mercaptopurine or methotrexate) for at least 6 months Patients must have had full disease control at entry |
|
| Interventions | 1:1 randomization ratio Group 1: azathioprine and infliximab continued (n = 40) Group 2: placebo with only infliximab continued (n = 40) Duration of follow‐up: 104 weeks |
|
| Outcomes | Primary outcome: the proportion of patients who required a change in the dosing interval or completely stopped infliximab therapy due to disease flare (i.e. clinical relapse) Secondary outcomes: infliximab trough levels, adverse events and mucosal healing |
|
| Notes | This study was not powered as a non‐inferiority trial; continuation therapy was assumed to be superior. To study non‐inferiority, > 250 patients per treatment arm would be required. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not adequately described (no explicit statement about the method used) |
| Allocation concealment (selection bias) | Low risk | Centralized randomization was performed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the open‐label design no placebo was given |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessment was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs were balanced across treatment groups 29/40 in the combination therapy group completed the study compared to 31/40 in the group where azathioprine was stopped |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | This study appears to be free from other sources of bias |
Vilien 2004.
| Methods | Randomized, open‐label, controlled trial (N = 29) | |
| Participants | Patients with quiescent CD who had received a continuous dose of azathioprine for at least 2 years | |
| Interventions | Group 1: azathioprine withdrawal (n = 15) Group 2: continued azathioprine treatment at an unchanged dose (n = 14) |
|
| Outcomes | Primary outcome: clinical relapse (defined as a rise in CDAI > 75 with a total CDAI > 150; or, any increased disease activity requiring new medical or surgical treatment) Follow‐up duration was 12 months |
|
| Notes | Randomization took place after stratification by azathioprine dose
High azathioprine dose was defined as > 1.60 mg/kg/day Low azathioprine dose was defined as < 1.60 mg/kg/day |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated 1:1 randomization |
| Allocation concealment (selection bias) | Low risk | Randomization was performed centrally |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial; participants and personnel not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was only one drop‐out (in the azathioprine group) |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | This study appears to be free from other sources of bias |
Wenzl 2015.
| Methods | Randmized, double‐blind, placebo‐controlled withdrawal trial (N = 52) | |
| Participants | CD patients in stable clinical remission (CDAI < 150) on azathioprine for > 4 years | |
| Interventions | 1:1 randomization ratio Group 1: placebo (n = 26) Group 2: azathioprine at dose prior to study entry (n = 26) Follow‐up duration: 24 months |
|
| Outcomes | Primary outcome: Time interval between first intake of the study drug and disease relapse Secondary outcomes: Disease activity (CDAI), quality of life, and laboratory parameters associated with active disease (CRP, serum hemoglobin, serum albumin, and platelet count) |
|
| Notes | Slow recruitment led to premature cessation of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization tables were used according to a 1:1 ratio |
| Allocation concealment (selection bias) | Low risk | Centralized randomization was performed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind: medicine was provided in identical‐appearing tablets |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not adequately described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs were balanced across treatment groups 19/26 in the azathioprine group completed the study compared to 23/26 in the placebo group |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Unclear risk | Premature stopping of the trial due to slow enrolment. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amiot 2016 | Reduced dose (not withdrawal) of therapy |
| Begun 2016 | Case report |
| Benitez 2015 | Letter (not study) |
| Bodini 2015 | No control (usual care) |
| Bortlik 2015a | No control (usual care) |
| Bortlik 2015b | No control (usual care) |
| Bortlik 2016 | No control (usual care) |
| Bots 2016a | No control (usual care) |
| Bots 2016b | No control (usual care) |
| Bouhnik 1996 | Retrospective study |
| Brooks 2017 | No control (usual care) |
| Chaparro 2015 | Letter (not study) |
| Chauvin 2014 | Retrospective study |
| Chen 2015a | No control (usual care) |
| Chen 2015b | No control (usual care) |
| Cortes 2016 | No control (usual care) |
| Crombe 2010a | Pediatric study |
| Crombe 2010b | Pediatric study |
| Crombe 2011 | Pediatric study |
| Dai 2014 | No control (usual care) |
| de Lima 2016 | CD specific data unavailable |
| De Suray 2012a | No control (usual care) |
| De Suray 2012b | No control (usual care) |
| Domenech 2005 | Retrospective study |
| Duricová 2015 | No control (usual care) |
| Echarri 2013 | No control (usual care) |
| Farkas 2014 | No control (usual care) |
| Feagan 2015a | < 6 months of treatment prior to withdrawal |
| Feagan 2015b | < 6 months of treatment prior to withdrawal |
| Fischer 2014 | Retrospective study |
| Gallego 2015 | Retrospective study |
| Grossi 2015a | No control (usual care) |
| Grossi 2015b | Pediatric study |
| Helwig 2016 | No control (usual care) |
| Helwig 2017 | Review article |
| Hlavaty 2016b | Retrospective study |
| Iborra 2016a | Retrospective study |
| Iborra 2016b | Retrospective study |
| Iimuro 2011 | Retrospective study |
| Kang 2016 | Pediatric study |
| Kennedy 2016 | Retrospective study |
| Kierkus 2015 | Pediatric study |
| Kim 1999 | Retrospective study |
| Louis 2012 | No control (usual care) In the STORI trial (N =115), infliximab was withdrawn from patients with quiescent CD who were receiving combination therapy (infliximab and an immunosuppressant). After 2 years of follow up, approximately 50% of patients relapsed. |
| Molander 2014 | No control (usual care) |
| Molander 2015 | No control (usual care) |
| Molander 2016 | No control (usual care) |
| Molnar 2013 | No control (usual care) |
| Monterubbianesi 2015 | Retrospective study |
| Monterubbianesi 2016a | Retrospective study |
| Monterubbianesi 2016b | Retrospective study |
| Nuti 2010a | Pediatric study |
| Nuti 2010b | Pediatric study |
| Paul 2015 | Reduced dose (not withdrawal) of therapy |
| Qiu 2015a | No control (usual care) |
| Qiu 2015b | No control (usual care) |
| Rismo 2013 | < 6 months of treatment prior to withdrawal |
| Schnitzler 2009 | No control (usual care) |
| Seirafi 2011a | No control (usual care) |
| Seirafi 2011b | No control (usual care) |
| Squires 2015 | No control (usual care) |
| Thomsen 2015 | No control (usual care) |
| Treton 2009 | No control (usual care) |
| Waugh 2010a | Retrospective study |
| Waugh 2010b | Retrospective study |
| Wynands 2008 | Pediatric study |
| Zelinkova 2013 | No control (usual care) |
Characteristics of ongoing studies [ordered by study ID]
NCT01817426.
| Trial name or title | Discontinuation of infliximab therapy in patients With CD during sustained complete remission (STOP IT) |
| Methods | Prospective, double‐blind, 2‐arm RCT Intervention Model: Parallel Assignment Masking: Triple (Participant, Care Provider, Investigator) Primary Purpose: Treatment |
| Participants | Patients with luminal Crohn's disease in sustained complete remission on infliximab |
| Interventions | Arm 1: infliximab at an unchanged dose Arm 2: placebo |
| Outcomes | Primary outcome: proportion of patients who maintain remission (CDAI < 150) |
| Starting date | Start date: November 2012 Estimated end date: November 2016 |
| Contact information | Sine Schnoor Buhl MD, sine_buhl@hotmail.com Mark Ainsworth MD PhD DMSc, Marain01@heh.regionh.dk |
| Notes |
NCT01817426 The recruitment status of this study is unknown. The completion date has passed and the status has not been verified in more than two years. |
NCT02177071.
| Trial name or title | A prospective randomized controlled trial comparing infliximab‐antimetabolites combination therapy to antimetabolites monotherapy and infliximab monotherapy in Crohn's disease patients in sustained steroid‐free remission on combination therapy (SPARE) |
| Methods | Prospective, open‐label, three‐arm RCT |
| Participants | Patients with luminal CD in steroid‐free remission for at least 6 months who have received combination therapy with infliximab and anti‐metabolites for at least 1 year N = 300 (100 per arm) |
| Interventions | Study treatment: Infliximab; 6‐mercaptopurine, azathioprine or methotrexate Arm 1: combination therapy with infliximab and anti‐metabolite continued Arm 2: discontinue infliximab, continue anti‐metabolite Arm 3: discontinue anti‐metabolite, continue infliximab |
| Outcomes | Co‐primary outcome: clinical relapse rate at 2 years; mean remission duration within 2 years |
| Starting date | Study duration: 2 + 2 years Enrollment: 2 years + 1 year Follow‐up: 2 years Start date: October 2015 Estimated end date: January 2020 |
| Contact information | Edouard Louis PhD, edouard.louis@ulg.ac.be |
| Notes | NCT02177071 |
Differences between protocol and review
The title of this review in its original protocol: "Withdrawal of drug therapy for patients with quiescent Crohn's disease", was changed to:"Withdrawal of immunosuppressant or biologic therapy for patients with quiescent Crohn's disease". A description of the factors assessed by the NOS was added to the methods section. We also decided to perform subgroup analyses based on study design (RCTs versus observational studies) as detailed in the most current version of the methods section. The subgroup analysis for pediatric studies was removed because the study participants included were defined as adults (> 18 years). Finally, a random‐effects model and not a fixed‐effect model was used for analyzing the data.
Contributions of authors
Ray Boyapati: study conception and design, acquisition of data, analysis and interpretation of data, drafting and critical revision of the manuscript.
Joana Torres: study conception and design, acquisition of data, analysis and interpretation of data, drafting and critical revision of the manuscript.
Carolina Palmela: acquisition of data.
Claire E Parker: study conception and design, acquisition of data, analysis and interpretation of data, drafting and critical revision of the manuscript.
Orli M Silverberg: acquisition of data.
Sonam D Upadhyaya: acquisition of data.
Tran M. Nguyen: designed search strategies, ran searches, acquisition of data.
Jean‐Frédéric Colombel: study conception and design, analysis and interpretation of data and critical revision of the manuscript.
Declarations of interest
Ray Boyapati: has no known conflicts of interest.
Joana Torres has served as a consultant for Takeda, and Abbvie; and has received travel support from Ferring and Abbvie. All of these activities are outside the submitted work.
Carolina Palmelahas received consultancy fees from Laboratorios Vitoria and travel grant from Abbvie and MSD. All of these activities are outside the submitted work.
Claire E Parker: has no known conflicts of interest.
Orli M Silverberg: has no known conflicts of interest.
Sonam D Upadhyaya: has no known conflicts of interest.
Tran M. Nguyen: has no known conflicts of interest.
Jean‐Frédéric Colombel has received consulting fees from AbbVie, Amgen, Boehringer‐Ingelheim, Arena Pharmaceuticals, Celgene Corporation, Celltrion, Enterome, Eli Lilly, Ferring Pharmaceuticals, Genentech, Janssen and Janssen, Medimmune, Merck & Co., Nextbiotix, Novartis Pharmaceuticals Corporation, Otsuka Pharmaceutical Development & Commercialization, Inc., Pfizer, Protagonist, Second Genome, Gilead, Seres Therapeutics, Shire, Takeda, Theradiag; Grants/Grants pending from Abbvie, Takeda, Janssen Pharmaceuticals; Payments for lectures from AbbVie, Ferring, Takeda, Celgene Corporation; stocks and stock options from Intestinal Biotech Development and Genfit. All of these activities are outside the submitted work.
New
References
References to studies included in this review
Lémann 2005 {published data only}
- Lémann M, Mary JY, Colombel JF, Duclos B, Soule JC, Lerebours E, et al. A randomized, double‐blind, controlled withdrawal trial in Crohn’s disease patients in long‐term remission on azathioprine. Gastroenterology 2005;128(7):1812‐8. [DOI] [PubMed] [Google Scholar]
O'Donoghue 1978 {published data only}
- O’Donoghue DP, Dawson AM, Powell‐Tuck J. Double blind withdrawal trial of azathioprine as maintenance treatment for Crohn’s disease. Lancet 1978;2(8097):955–7. [DOI] [PubMed] [Google Scholar]
Roblin 2017 {published data only}
- Tedesco E, Paul S, Marotte H, Jarlot C, Williet N, Phelip JM, et al. Azathioprine dose reduction in inflammatory bowel disease patients on combination therapy: A prospective study. Gastroenterology 2016;150(4 Suppl 1):S143‐4. [Google Scholar]
- Tedesco E, Paul S, Marotte H, Jarlot C, Williet N, Phelip JM, et al. Azathioprine dose reduction in patients with inflammatory bowel disease on combination therapy: A prospective study. Journal of Crohn's and Colitis 2016;10:S7‐8. [Google Scholar]
- Roblin X, Boschetti G, Williet N, Nancey S, Marotte H, Berger A, et al. Azathioprine dose reduction in inflammatory bowel disease patients on combination therapy: an open‐label, prospective and randomised clinical trial. Alimentary Pharmacology and Therapeutics 2017;46(2):142‐9. [DOI] [PubMed] [Google Scholar]
Van Assche 2008 {published data only}
- Assche G, Magdelaine‐Beuzelin C, D’Haens G, Baert F, Noman M, Vermeire S, et al. Withdrawal of immunosuppression in Crohn’s disease treated with scheduled infliximab maintenance: A randomized trial. Gastroenterology 2008;134:1861‐8. [DOI] [PubMed] [Google Scholar]
Vilien 2004 {published data only}
- Vilien M, Dahlerup JF, Munck LK, Nørregaard P, Grønbaek K, Fallingborg J. Randomized controlled azathioprine withdrawal after more than two years treatment in Crohn’s disease: Increased relapse rate the following year. Alimentary Pharmacology and Therapeutics 2004;19(11):1147‐52. [DOI] [PubMed] [Google Scholar]
Wenzl 2015 {published data only}
- Wenzl HH, Primas C, Novacek G, Teml A, Öfferlbauer‐Ernst A, Högenauer C, et al. Withdrawal of long‐term maintenance treatment with azathioprine tends to increase relapse risk in patients with Crohn’s disease. Digestive Diseases and Science 2015;60(5):1414‐23. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Amiot 2016 {published data only}
- Amiot A, Hulin A, Belhassan M, Andre C, Gagniere C, Baleur Y, et al. Therapeutic drug monitoring is predictive of loss of response after de‐escalation of infliximab therapy in patients with inflammatory bowel disease in clinical remission. Clinics and Research in Hepatology and Gastroenterology 2016;40(1):90‐8. [DOI] [PubMed] [Google Scholar]
Begun 2016 {published data only}
- Begun J. Inflammatory bowel disease in the clinic: Escalation and de‐escalation of therapy: A longitudinal case‐based discussion. Journal of Gastroenterology and Hepatology (Australia) 2016;31:12‐3. [DOI] [PubMed] [Google Scholar]
Benitez 2015 {published data only}
- Benitez JM, Garcia‐Sanchez V, Gisbert JP. Letter: Thiopurine withdrawal during sustained clinical remission in inflammatory bowel disease. Alimentary Pharmacology & Therapeutics 2015;41(5):494‐5. [DOI] [PubMed] [Google Scholar]
Bodini 2015 {published data only}
- Bodini G, Savarino V, Dulbecco P, Baldissarro I, Savarino E. IBD recurrence after stopping anti‐TNF‐alpha therapy: A prospective randomized controlled study comparing mesalamine and azathioprine‐preliminary results. Digestive and Liver Disease 2015;47:e137‐8. [Google Scholar]
Bortlik 2015a {published data only}
- Bortlik M, Machkova N, Hruba V, Mitrova K, Romanko I, Bina V, et al. Deep remission in Crohn's disease does not prevent disease relapse after withdrawal of anti‐TNF alpha therapy. Gastroenterology 2015;148(4 Suppl 1):S61. [Google Scholar]
Bortlik 2015b {published data only}
- Bortlik M, Duricova D, Machkova N, Hruba V, Mitrova K, Romanko I, et al. Deep remission in Crohn's disease does not prevent disease relapse after withdrawal of anti‐TNFa therapy. Journal of Crohn's and Colitis 2015;9:S4. [Google Scholar]
Bortlik 2016 {published data only}
- Bortlik M, Duricova D, Machkova N, Hruba V, Lukas M, Mitrova K, et al. Discontinuation of anti‐tumor necrosis factor therapy in inflammatory bowel disease patients: A prospective observation. Scandinavian Journal of Gastroenterology 2016;51(2):196‐202. [DOI] [PubMed] [Google Scholar]
Bots 2016a {published data only}
- Bots S, Kuin S, Lowenberg M, Ponsioen C, Brink G, D'Haens G. Relapse risk and predictors for relapse in a real‐life cohort of IBD patients after discontinuation of anti‐TNF therapy. Gastroenterology 2016;1):S814. [DOI] [PubMed] [Google Scholar]
Bots 2016b {published data only}
- Bots S, Kuin S, Lowenberg M, Ponsioen C, Brink G, D'Haens G. Relapse risk and predictors for relapse in a real‐life cohort of inflammatory bowel disease patients after discontinuation of anti‐tumour necrosis factor therapy. Journal of Crohn's and Colitis 2016;10:S397. [Google Scholar]
Bouhnik 1996 {published data only}
- Bouhnik Y, Lemann M, Mary JY, Scemama G, Tai R, Matuchansky C, et al. Long‐term follow‐up of patients with Crohn's disease treated with azathioprine or 6‐mercaptopurine. Lancet 1996;347(8996):215‐9. [DOI] [PubMed] [Google Scholar]
Brooks 2017 {published data only}
- Brooks AJ, Sebastian S, Cross SS, Robinson K, Warren L, Wright A, et al. Outcome of elective withdrawal of anti‐tumour necrosis factor‐alpha therapy in patients with Crohn's disease in established remission. Journal of Crohn's and Colitis 2017;11(12):1456‐62. [DOI] [PubMed] [Google Scholar]
Chaparro 2015 {published data only}
- Chaparro M, Gisbert JP. Letter: Infliximab de‐escalation based on trough levels in patients with inflammatory bowel disease. Alimentary Pharmacology and Therapeutics 2015;42(7):940‐1. [DOI] [PubMed] [Google Scholar]
Chauvin 2014 {published data only}
- Chauvin A, Thuaut A, Belhassan M, Baleur Y, Mesli F, Bastuji‐Garin S, et al. Infliximab as a bridge to remission maintained by antimetabolite therapy in Crohn's disease: A retrospective study. Digestive and Liver Disease 2014;46(8):695‐700. [DOI] [PubMed] [Google Scholar]
Chen 2015a {published data only}
- Chen Z, Xiang C, Hu HQ, Jiang B, Qiu C, Wang GZ, et al. Discontinuation of infliximab therapy in patients with Crohn's disease: Outcome and risk factors. Journal of Digestive Diseases 2015;16:11. [Google Scholar]
Chen 2015b {published data only}
- Chen Z, Xiang C, Hu H, Jiang B, Qiu C, Wang G, et al. Discontinuation of infliximab therapy in patients with Crohn's disease: Outcome and risk factors. Journal of Gastroenterology and Hepatology (Australia) 2015;30:154. [Google Scholar]
Cortes 2016 {published data only}
- Cortes X, Moles JR, Fernandez S, Clofent J, Moreno M, Rodriguez J, et al. Persistence of remission amongst patients with inflammatory bowel disease after adalimumab therapy is stopped: Economic and clinical implications. Journal of Crohn's and Colitis 2016;10:S355‐6. [Google Scholar]
Crombe 2010a {published data only}
- Crombe V, Salleron J, Savoye G, Dupas JL, Vernier‐Massouille G, Lerebours E, et al. Long‐term outcome of treatment with infliximab in paediatric Crohn's disease: A population‐based study. Gastroenterology 2010;138(5 Suppl 1):S300. [DOI] [PubMed] [Google Scholar]
Crombe 2010b {published data only}
- Crombe V, Salleron J, Savoye G, Dupas J, Vernier‐Massouille G, Lerebours E, et al. Long‐term outcome of treatment with infliximab (IFX) in pediatric Crohn's disease (CD): A population‐based study. Journal of Pediatric Gastroenterology and Nutrition 2010;50:E114‐5. [Google Scholar]
Crombe 2011 {published data only}
- Crombe V, Salleron J, Savoye G, Dupas JL, Vernier‐Massouille G, Lerebours E, et al. Long‐term outcome of treatment with infliximab in pediatric‐onset Crohn's disease: A population‐based study. Inflammatory Bowel Diseases 2011;17(10):2144‐52. [DOI] [PubMed] [Google Scholar]
Dai 2014 {published data only}
- Dai C, Liu WX, Jiang M, Sun MJ. Mucosal healing did not predict sustained clinical remission in patients with IBD after discontinuation of one‐year infliximab therapy. PLoS ONE 2014;9(10):e110797. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Lima 2016 {published data only}
- Lima A, Zelinkova Z, Ent C, Steegers EA, Woude CJ. Tailored anti‐TNF therapy during pregnancy in patients with IBD: maternal and fetal safety. Gut 2016;65(8):1261‐8. [DOI] [PubMed] [Google Scholar]
De Suray 2012a {published data only}
- Suray N, Salleron J, Vernier‐Massouille G, Grimaud JC, Bouhnik Y, Laharie D, et al. Close monitoring of CRP and fecal calprotectin levels to predict relapse in Crohn's disease patients: A sub‐analysis of the STORI study. Journal of Crohn's and Colitis 2012;6:S118‐9. [Google Scholar]
De Suray 2012b {published data only}
- Suray N, Salleron J, Vernier‐Massouille G, Grimaud J C, Bouhnik Y, Laharie D, et al. Close monitoring of CRP and fecal calprotectin is able to predict clinical relapse in patients with Crohn's disease in remission after infliximab withdrawal. A sub‐analysis of the STORI study. Gastroenterology 2012;142(5 Suppl 1):S149. [Google Scholar]
Domenech 2005 {published data only}
- Domenech E, Hinojosa J, Nos P, Garcia‐Planella E, Cabre E, Bernal I, et al. Clinical evolution of luminal and perianal Crohn's disease after inducing remission with infliximab: How long should patients be treated?. Alimentary Pharmacology and Therapeutics 2005;22(11‐12):1107‐13. [DOI] [PubMed] [Google Scholar]
Duricová 2015 {published data only}
- Duricová D, Bortlik M, Machkova N, Hruba V, Lukas M, Mitrova K, et al. Deep remission in Crohn’s disease does not prevent disease relapse after withdrawal of anti‐TNFa therapy. Gastroenterology 2015;148(4 Suppl 1):S‐61. [Google Scholar]
Echarri 2013 {published data only}
- Echarri A, Ollero V, Rodriguez JA, Gallego JC, Castro J. Predictors of relapse after discontinuing anti‐TNF therapy in Crohn's disease patients on deep remission. Journal of Crohn's and Colitis 2013;7:S171. [Google Scholar]
Farkas 2014 {published data only}
- Farkas K, Lakatos PL, Szucs M, Pallagi‐Kunstar E, Balint A, Nagy F, et al. Frequency and prognostic role of mucosal healing in patients with Crohn's disease and ulcerative colitis after one‐year of biological therapy. World Journal of Gastroenterology 2014;20(11):2995‐3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feagan 2015a {published data only}
- Feagan BG, Siegel CA, Melmed GY, Isaacs K, Lasch K, Rosario M, et al. Efficacy of vedolizumab with and without continued immunosuppressant use in GEMINI 1 and GEMINI 2. United European Gastroenterology Journal 2015;1:A17‐8. [Google Scholar]
Feagan 2015b {published data only}
- Feagan B, Siegel CA, Melmed G, Isaacs K, Lasch K, Rosario M, et al. Efficacy of vedolizumab maintenance therapy with and without continued immunosuppressant use in GEMINI 1 and GEMINI 2. American Journal of Gastroenterology 2015;110:S791. [Google Scholar]
Fischer 2014 {published data only}
- Fischer M, Campbell SC, Johnson CS, Helper DJ, Chiorean MV. Risk factors for rescue therapy in Crohn’s patients on combination therapy after discontinuation of the immunomodulator. Gastroenterology 2014;146(5 Suppl 1):S‐450. [Google Scholar]
Gallego 2015 {published data only}
- Gallego JC, Echarri A, Porta A, Bencheikh SE, Ollero V, Castro J. Ileal Crohn's disease: Magnetic resonance enterography as a predictor of relapse after antiTNF discontinuation. United European Gastroenterology Journal 2015;1:A237‐8. [Google Scholar]
Grossi 2015a {published data only}
- Grossi V, Lerer T, Griffiths A, LeLeiko N, Cabrera J, Otley A, et al. Concomitant use of immunomodulators affects the durability of infliximab therapy in children With Crohn's disease. Clinical Gastroenterology and Hepatology 2015;13(10):1748‐56. [DOI] [PubMed] [Google Scholar]
Grossi 2015b {published data only}
- Grossi L, Pagliaro M, Tullio A M, Tavani R, Cocciolillo S, Berardino M, et al. Maintenance of remission after prolonged therapy with Infliximab: A "real‐life" experience from a single Italian centre in patients affected by Crohn's Disease and Ulcerative Colitis. Journal of Crohn's and Colitis 2015;9:S211. [Google Scholar]
Helwig 2016 {published data only}
- Helwig U, Lutter F, Koppka N, Schreiber S. Proposal for an anti‐tumour necrosis factor‐exit strategy based on trough serum level. Journal of Crohn's and Colitis 2016;10:S252‐3. [Google Scholar]
Helwig 2017 {published data only}
- Helwig, U. Relapse after withdrawal from anti‐TNF therapy for inflammatory bowel disease. Zeitschrift fur Gastroenterologie 2017;55(1):85. [DOI] [PubMed] [Google Scholar]
Hlavaty 2016b {published data only}
- Hlavaty T, Krajcovicova A, Letkovsky J, Sturdik I, Koller T, Toth J, et al. Relapse rates of inflammatory bowel disease patients in deep and clinical remission after discontinuing anti‐tumour necrosis factor alpha therapy: A prospective single‐centre open label study. Journal of Crohn's and Colitis 2016;10:S337. [DOI] [PubMed] [Google Scholar]
Iborra 2016a {published data only}
- Iborra M, Navarro B, Cortes X, Bosca M, Blazquez T, Herreras J, et al. Risk of relapse after azathioprine discontinuation in inflammatory bowel disease patients in maintained remission. Journal of Crohn's and Colitis 2016;10:S333‐4. [Google Scholar]
Iborra 2016b {published data only}
- Iborra M, Herreras J, Bosca‐Watts MM, Cortes X, Navarro B, Martinez MTB, et al. Risk of relapse after azathioprine (AZA) discontinuation in inflammatory bowel disease (IBD) patients in maintained remission. Gastroenterology 2016;150(4 Suppl 1):S442. [Google Scholar]
Iimuro 2011 {published data only}
- Iimuro M, Nakamura S, Sato T, Ogawa T, Mikio K, Nogami K, et al. Long term outcome of top‐down therapy in Crohn’s disease: a single center experience. Inflammatory Bowel Diseases 2011;17:S49‐50. [Google Scholar]
Kang 2016 {published data only}
- Kang B, Choe YH, Lee K, Choi SY, Kim K. Factors associated with relapse after infliximab cessation in pediatric Crohn's disease patients treated with combined immunosuppression. Journal of Pediatric Gastroenterology and Nutrition 2016;63:S350‐1. [Google Scholar]
Kennedy 2016 {published data only}
- Kennedy NA, Warner B, Johnston EL, Flanders L, Hendy P, Ding NS, et al. Relapse after withdrawal from anti‐TNF therapy for inflammatory bowel disease: An observational study, plus systematic review and meta‐analysis. Alimentary Pharmacology and Therapeutics 2016;43(8):910‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kierkus 2015 {published data only}
- Kierkus J, Iwanczak B, Wegner A, Dadalski M, Grzybowska‐Chlebowczyk U, Lazowska I, et al. Monotherapy with infliximab versus combination therapy in the maintenance of clinical remission in children with moderate to severe Crohn disease. Journal of Pediatric Gastroenterology & Nutrition 2015;60(5):580‐5. [DOI] [PubMed] [Google Scholar]
Kim 1999 {published data only}
- Kim PS, Zlatanic J, Korelitz BI, Gleim GW. Optimum duration of treatment with 6‐mercaptopurine for Crohn's disease. American Journal of Gastroenterology 1999;94(11):3254‐7. [DOI] [PubMed] [Google Scholar]
Louis 2012 {published data only}
- Louis E, Mary JY, Verniermassouille G, Grimaud JC, Bouhnik Y, Laharie D, et al. Maintenance of remission among patients with Crohn's disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology 2012;142(1):63‐70. [DOI] [PubMed] [Google Scholar]
Molander 2014 {published data only}
- Molander P, Farkkila M, Salminen K, Kemppainen H, Blomster T, Koskela R, et al. Outcome after discontinuation of TNF alpha‐blocking therapy in patients with inflammatory bowel disease in deep remission. Inflammatory Bowel Diseases 2014;20(6):1021‐8. [DOI] [PubMed] [Google Scholar]
Molander 2015 {published data only}
- Molander P, Farkkila M, Ristimaki A, Salminen K, Kemppainen H, Blomster T, et al. Does fecal calprotectin predict short‐term relapse after stopping TNFalpha‐blocking agents in inflammatory bowel disease patients in deep remission?. Journal of Crohn's and Colitis 2015;9(1):33‐40. [DOI] [PubMed] [Google Scholar]
Molander 2016 {published data only}
- Molander P, Farkkila M, Kemppainen H, Blomster T, Jussila A, Mustonen H, et al. Long‐term outcome of inflammatory bowel disease patients with deep remission after discontinuation of TNFalpha ‐blocking agents. Journal of Crohn's and Colitis 2016;10:S47. [DOI] [PubMed] [Google Scholar]
Molnar 2013 {published data only}
- Molnar T, Lakatos PL, Farkas K, Nagy F, Szepes Z, Miheller P, et al. Predictors of relapse in patients with Crohn's disease in remission after 1 year of biological therapy. Alimentary Pharmacology and Therapeutics 2013;37(2):225‐33. [DOI] [PubMed] [Google Scholar]
Monterubbianesi 2015 {published data only}
- Monterubbianesi R, Papi C, Kohn A. Maintenance of clinical remission in Crohn's disease patients after discontinuation of long term treatment with infliximab: Results of a single centre cohort. Digestive and Liver Disease 2015;47:e100. [Google Scholar]
Monterubbianesi 2016a {published data only}
- Monterubbianesi R, Furfaro F, Costantino G, Bezzio C, Giannarelli D, Fries W, et al. Maintenance of clinical remission in IBD patients after discontinuation of anti‐TNF agents, an Italian experience. Digestive and Liver Disease 2016;48:e158. [Google Scholar]
Monterubbianesi 2016b {published data only}
- Monterubbianesi R, Furfaro F, Costantino G, Bezzio C, Giannarelli D, Fries W, et al. Maintenance of clinical remission in inflammatory bowel disease patients after discontinuation of anti‐TNF agents, an Italian experience. Journal of Crohn's and Colitis 2016;10:S386‐7. [Google Scholar]
Nuti 2010a {published data only}
- Nuti F, Conte F, Cavallari N, Civitelli F, Aloi M, Giudice E, et al. Long term efficacy of infliximab in inflammatory bowel disease at a single tertiary center. Journal of Pediatric Gastroenterology and Nutrition 2010;50:E108. [Google Scholar]
Nuti 2010b {published data only}
- Nuti F, Conte F, Cavallari N, Civitelli F, Aloi M, Alessandri C, et al. Long term efficacy of infliximab in inflammatory bowel disease at a single tertiary center. Digestive and Liver Disease 2010;42:S326‐7. [Google Scholar]
Paul 2015 {published data only}
- Paul S, Roblin X, Peyrin Biroulet L. Infliximab de‐escalation based on trough levels in inflammatory bowel disease patients: A proof‐of concept study. United European Gastroenterology Journal 2015;1:A617‐8. [Google Scholar]
Qiu 2015a {published data only}
- Qiu Y, Mao R, Chen BL, Yao H, Zeng ZR, Chen M. Predictors of disease relapse of patients with Crohn's disease in deep remission: Who and when can withdraw thiopurine maintenance therapy?. United European Gastroenterology Journal 2015;1:A434‐5. [Google Scholar]
Qiu 2015b {published data only}
- Qiu Y, Mao R, Chen BL, He Y, Zeng ZR, Chen MH. Predictors of disease relapse of patients with Crohn's disease in deep remission: Who and when can withdraw thiopurine maintenance therapy?. Journal of Crohn's and Colitis 2015;9:S61. [Google Scholar]
Rismo 2013 {published data only}
- Rismo R, Olsen T, Cui G, Paulssen EJ, Christiansen I, Johnsen K, et al. Normalization of mucosal cytokine gene expression levels predicts long‐term remission after discontinuation of anti‐TNF therapy in Crohn's disease. Scandinavian Journal of Gastroenterology 2013;48(3):311‐9. [DOI] [PubMed] [Google Scholar]
Schnitzler 2009 {published data only}
- Schnitzler F, Fidder H, Ferrante M, Noman M, Arijs I, Assche G, et al. Long‐term outcome of treatment with infliximab in 614 patients with Crohn's disease: Results from a single‐centre cohort. Gut 2009;58(4):492‐500. [DOI] [PubMed] [Google Scholar]
Seirafi 2011a {published data only}
- Seirafi M, Treton X, Vroey B, Cosnes J, Roblin X, Allez M, et al. Anti‐TNF therapy and pregnancy in inflammatory bowel disease: A prospective cohort study from the GETAID. Journal of Crohn's and Colitis 2011;5 (1):S70. [Google Scholar]
Seirafi 2011b {published data only}
- Seirafi M, Treton X, Vroey B, Cosnes J, Roblin X, Allez M, et al. Anti‐TNF therapy and pregnancy in inflammatory bowel disease: A prospective cohort study from the GETAID. Gastroenterology 2011;140(5 Suppl 1):S175. [Google Scholar]
Squires 2015 {published data only}
- Squires S, Boal A, Hamid R, Heydtmann M, Naismith G. Prospective anti‐TNF withdrawal in quiescent Crohn's disease‐12 month clinical outcomes. Gut 2015;64:A437. [Google Scholar]
Thomsen 2015 {published data only}
- Thomsen SB, Theede K, Nielsen AM, Kiszka‐Kanowitz M. Optimization of thiopurine therapy improves remission rates after cessation of anti‐TNFa in Crohn's disease. United European Gastroenterology Journal 2015;1:A32. [Google Scholar]
Treton 2009 {published data only}
- Treton X, Bouhnik Y, Mary J, Colombel J, Duclos B, Soule J, et al. Azathioprine withdrawal in patients With Crohn's disease maintained on prolonged remission: A high risk of relapse. Clinical Gastroenterology and Hepatology 2009;7(1):80‐5. [DOI] [PubMed] [Google Scholar]
Waugh 2010a {published data only}
- Waugh AW, Wong K, Fedorak RN. Maintenance of clinical benefit in Crohn's disease patients after discontinuation of infliximab: Long term follow‐up of a single center cohort. Gastroenterology 2010;138(5 Suppl 1):S685. [DOI] [PubMed] [Google Scholar]
Waugh 2010b {published data only}
- Waugh AW, Garg S, Matic K, Gramlich L, Wong C, Sadowski DC, et al. Maintenance of clinical benefit in Crohn's disease patients after discontinuation of infliximab: long‐term follow‐up of a single centre cohort. Alimentary Pharmacology & Therapeutics 2010;32(9):1129‐34. [DOI] [PubMed] [Google Scholar]
Wynands 2008 {published data only}
- Wynands J, Belbouab R, Candon S, Talbotec C, Mougenot JF, Chatenoud L, et al. 12‐month follow‐up after successful infliximab therapy in pediatric Crohn's disease. Journal of Pediatric Gastroenterology and Nutrition 2008;46(3):293‐8. [DOI] [PubMed] [Google Scholar]
Zelinkova 2013 {published data only}
- Zelinkova Z, Ent C, Bruin KF, Baalen O, Vermeulen HG, Smalbraak HJ, et al. Effects of discontinuing anti‐tumor necrosis factor therapy during pregnancy on the course of inflammatory bowel disease and neonatal exposure. Clinical Gastroenterology & Hepatology 2013;11(3):318‐21. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01817426 {published data only}
- NCT01817426. Discontinuation of infliximab therapy in patients with CD during sustained complete remission (STOP IT). clinicaltrials.gov/ct2/show/NCT01817426 (accessed 1 February 2018).
NCT02177071 {published data only}
- NCT02177071. A prospective randomized controlled trial comparing infliximab‐antimetabolites combination therapy to antimetabolites monotherapy and infliximab monotherapy in Crohn's disease patients in sustained steroid‐free remission on combination therapy (SPARE). clinicaltrials.gov/ct2/show/NCT02177071 (accessed 1 February 2018).
Additional references
Andersen 2014
- Andersen N, Pasternak B, Basit S, Andersson M, Svanström H, Caspersen S, et al. Association between tumor necrosis factor‐α antagonists and risk of cancer in patients with inflammatory bowel disease. JAMA 2014;311(23):2406‐13. [DOI] [PubMed] [Google Scholar]
Beaugerie 2009
- Beaugerie L, Brousse N, Bouvier AM, Colombel JF, Lémann M, Cosnes J, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet 2009;374(9701):1617‐25. [DOI] [PubMed] [Google Scholar]
Bernstein 2006
- Bernstein CN, Wajda A, Svenson LW, MacKenzie A, Koehoorn M, Jackson M, et al. The epidemiology of inflammatory bowel disease in Canada: a population‐based study. American Journal of Gastroenterology 2006;101(7):1559‐68. [DOI] [PubMed] [Google Scholar]
Billioud 2013
- Billioud V, Ford AC, Tedesco ED, Colombel JF, Roblin X, Peyrin‐Biroulet L. Preoperative use of anti‐TNF therapy and postoperative complications in inflammatory bowel diseases: a meta‐analysis. Journal of Crohn's and Colitis 2013;7(11):853‐67. [DOI] [PubMed] [Google Scholar]
Bourrier 2016
- Bourrier A, Carrat F, Colombel JF, Bouvier AM, Abitbol V, Marteau P, et al. Excess risk of urinary tract cancers in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Alimentary Pharmacology and Therapeutics 2016;43(2):252‐61. [DOI] [PubMed] [Google Scholar]
Buisson 2012
- Buisson A, Chevaux JB, Allen PB, Bommelaer G, Peyrin‐Biroulet L. Review article: the natural history of postoperative Crohn's disease recurrence. Alimentary Pharmacology and Therapeutics 2012;35(6):625‐33. [DOI] [PubMed] [Google Scholar]
Colombel 2010a
- Colombel JF. Understanding combination therapy with biologics and immunosuppressives for the treatment of Crohn's disease. Gastroenterology and Hepatology 2010;6(8):486‐90. [PMC free article] [PubMed] [Google Scholar]
Colombel 2010b
- Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. New England Journal of Medicine 2010;362(15):1383‐95. [DOI] [PubMed] [Google Scholar]
D'Haens 2008
- D’Haens G, Baert F, Assche G, Caenepeel P, Vergauwe P, Tuynman H, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet 2008;371:660‐7. [DOI] [PubMed] [Google Scholar]
D'Haens 2010
- D'Haens, GR. Top‐down therapy for IBD: rationale and requisite evidence. Nature Reviews Gastroenterology and Hepatology 2010;7(2):86‐92. [DOI] [PubMed] [Google Scholar]
Doherty 2018
- Doherty G, Katsanos KH, Burisch J, Allez M, Papamichael K, Stallmach A, et al. European Crohn’s and Colitis Organisation topical review on treatment withdrawal (‘exit strategies’) in inflammatory bowel disease. Journal of Crohn's and Colitis 2018;12(1):17‐31. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
French 2011
- French H, Mark Dalzell A, Srinivasan R, El‐Matary W. Relapse rate following azathioprine withdrawal in maintaining remission for Crohn's disease: a meta‐analysis. Digestive Diseases and Sciences 2011;56(7):1929‐36. [DOI] [PubMed] [Google Scholar]
Gearry 2006
- Gearry RB, Richardson A, Frampton CM, Collett JA, Burt MJ, Chapman BA, et al. High incidence of Crohn's disease in Canterbury, New Zealand: results of an epidemiologic study. Inflammatory Bowel Diseases 2006;12(10):936‐43. [DOI] [PubMed] [Google Scholar]
Gisbert 2016
- Gisbert JP, Marín AC, Chaparro M. The risk of relapse after anti‐TNF discontinuation in inflammatory bowel disease: systematic review and meta‐analysis. American Journal of Gastroenterology 2016;111(5):632‐47. [DOI] [PubMed] [Google Scholar]
Haag 2015
- Haag LM, Siegmund B. Intestinal microbiota and the innate immune system ‐ a crosstalk in Crohn's disease pathogenesis. Frontiers in Immunology 2015;6:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harbord 2016
- Harbord M, Annese V, Vavricka SR, Allez M, Barreiro‐de Acosta M, Boberg KM. The first European evidence‐based consensus on extra‐intestinal manifestations of inflammatory bowel disease. Journal of Crohn's and Colitis 2016;10(3):239‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Higgins 2011b
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Higgins 2011c
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Long 2012
- Long MD, Martin CF, Pipkin CA, Herfarth HH, Sandler RS, Kappelman MD. Risk of melanoma and non melanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology 2012;143(2):390‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Molodecky 2012
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012;142(1):46‐54. [DOI] [PubMed] [Google Scholar]
Pariente 2014
- Pariente B, Laharie D. Review article: why, when and how to de‐escalate therapy in inflammatory bowel diseases. Alimentary Pharmacology and Therapeutics 2014;40(4):338‐53. [DOI] [PubMed] [Google Scholar]
Peyrin‐Biroulet 2011
- Peyrin‐Biroulet L, Khosrotehrani K, Carrat F, Bouvier AM, Chevaux JB, Simon T, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology 2011;141(5):1621‐8. [DOI] [PubMed] [Google Scholar]
Peyrin‐Biroulet 2012
- Peyrin‐Biroulet L, Harmsen SW, Tremaine WJ, Zinsmeister AR, Sandborn WJ, Loftus EV. Surgery in a population‐based cohort of Crohn’s disease from Olmsted County, Minnesota (1970 – 2004). American Joural of Gastroenterology 2012;107:1693–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editors(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration. Available from www.cochrane‐handbook.org, 2011. [Google Scholar]
Siegel 2009
- Siegel CA, Marden SM, Persing SM, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti‐tumor necrosis factor and immunomodulator therapy for the treatment of Crohn's disease: a meta‐analysis. Clinical Gastroenterology and Hepatology 2009;7(8):874‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sokol 2010
- Sokol H, Seksik P, Nion‐Larmurier I, Vienne A, Beaugerie L, Cosnes J. Current smoking, not duration of remission, delays Crohn’s disease relapse following azathioprine withdrawal. Inflammatory Bowel Diseases 2010;16(3):362–3. [DOI] [PubMed] [Google Scholar]
Subramaniam 2014
- Subramaniam K, Yeung D, Grimpen F, Joseph J, Fay K, Buckland M, et al. Hepatosplenic T‐cell lymphoma, immunosuppressive agents and biologicals: what are the risks?. Internal Medicine Journal 2014;44(3):287‐90. [DOI] [PubMed] [Google Scholar]
Thompson 1998
- Thompson NP, Fleming DM, Charlton J, Pounder RE, Wakefield AJ. Patients consulting with Crohn's disease in primary care in England and Wales. European Journal of Gastroenterology Hepatology 1998;10(12):1007‐12. [DOI] [PubMed] [Google Scholar]
Torres 2015
- Torres J, Boyapati RK, Kennedy NA, Louis E, Colombel JF, Satsangi J. Systematic Review of Effects of Withdrawal of Immunomodulators or Biologic Agents From Patients With Inflammatory Bowel Disease. Gastroenterology 2015;149(7):1716‐30. [DOI] [PubMed] [Google Scholar]
van der Valk 2014
- Valk ME, Mangen MJ, Leenders M, Dijkstra G, Bodegraven AA, Fidder HH, et al. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti‐TNFα therapy: results from the COIN study. Gut 2014;63(1):72‐9. [DOI] [PubMed] [Google Scholar]
Wells 2017
- Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‐Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta‐analyses. www.ohri.ca/programs/clinical_epidemiology/oxford.asp. (accessed 30 January 2017).
Wilson 2010
- Wilson J, Hair C, Knight R, Catto‐Smith A, Bell S, Kamm M, et al. High incidence of inflammatory bowel disease in Australia: a prospective population‐based Australian incidence study. Inflammatory Bowel Diseases 2010;16(9):1550‐6. [DOI] [PubMed] [Google Scholar]


