Abstract
Background
In children, dental caries (tooth decay) is among the most prevalent chronic diseases worldwide. Pulp interventions are indicated for extensive tooth decay. Depending on the severity of the disease, three pulp treatment techniques are available: direct pulp capping, pulpotomy and pulpectomy. After treatment, the cavity is filled with a medicament. Materials commonly used include mineral trioxide aggregate (MTA), calcium hydroxide, formocresol or ferric sulphate.
This is an update of a Cochrane Review published in 2014 when insufficient evidence was found to clearly identify one superior pulpotomy medicament and technique.
Objectives
To assess the effects of different pulp treatment techniques and associated medicaments for the treatment of extensive decay in primary teeth.
Search methods
Cochrane Oral Health's Information Specialist searched the Cochrane Oral Health Group's Trials Register (to 10 August 2017), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2017, Issue 7), MEDLINE Ovid (1946 to 10 August 2017), Embase Ovid (1980 to 10 August 2017) and the Web of Science (1945 to 10 August 2017). OpenGrey was searched for grey literature. The US National Institutes of Health Trials Registry (ClinicalTrials.gov) and the World Health Organization International Clinical Trials Registry Platform were searched for ongoing trials. No restrictions were placed on the language or date of publication when searching the electronic databases.
Selection criteria
We included randomised controlled trials (RCTs) comparing interventions that combined a pulp treatment technique with a medicament or device in children with extensive decay in the dental pulp of their primary teeth.
Data collection and analysis
Two review authors independently extracted data and assessed 'Risk of bias'. We contacted authors of RCTs for additional information when necessary. The primary outcomes were clinical failure and radiological failure, as defined in trials, at six, 12 and 24 months. We performed data synthesis with pair‐wise meta‐analyses using fixed‐effect models. We assessed statistical heterogeneity by using I² coefficients.
Main results
We included 40 new trials bringing the total to 87 included trials (7140 randomised teeth) for this update. All were small, single‐centre trials (median number of randomised teeth = 68). All trials were assessed at unclear or high risk of bias.
The 87 trials examined 125 different comparisons: 75 comparisons of different medicaments or techniques for pulpotomy; 25 comparisons of different medicaments for pulpectomy; four comparisons of pulpotomy and pulpectomy; and 21 comparisons of different medicaments for direct pulp capping.
The proportion of clinical failures and radiological failures was low in all trials. In many trials, there were either no clinical failures or no radiographic failures in either study arm.
For pulpotomy, we assessed three comparisons as providing moderate‐quality evidence. Compared with formocresol, MTA reduced both clinical and radiological failures, with a statistically significant difference at 12 months for clinical failure and at six, 12 and 24 months for radiological failure (12 trials, 740 participants). Compared with calcium hydroxide, MTA reduced both clinical and radiological failures, with statistically significant differences for clinical failure at 12 and 24 months. MTA also appeared to reduce radiological failure at six, 12 and 24 months (four trials, 150 participants) (low‐quality evidence). When comparing calcium hydroxide with formocresol, there was a statistically significant difference in favour of formocresol for clinical failure at six and 12 months and radiological failure at six, 12 and 24 months (six trials (one with no failures), 332 participants).
Regarding pulpectomy, we found moderate‐quality evidence for two comparisons. The comparison between Metapex and zinc oxide and eugenol (ZOE) paste was inconclusive, with no clear evidence of a difference between the interventions for failure at 6 or 12 months (two trials, 62 participants). Similarly inconclusive, there was no clear evidence of a difference in failure between Endoflas and ZOE (outcomes measured at 6 months; two trials, 80 participants). There was low‐quality evidence of a difference in failure at 12 months that suggested ZOE paste may be better than Vitapex (calcium hydroxide/iodoform) paste (two trials, 161 participants).
Regarding direct pulp capping, the small number of studies undertaking the same comparison limits any interpretation. We assessed the quality of the evidence as low or very low for all comparisons. One trial appeared to favour formocresol over calcium hydroxide; however, there are safety concerns about formocresol.
Authors' conclusions
Pulp treatment for extensive decay in primary teeth is generally successful. Many included trials had no clinical or radiological failures in either trial arm, and the overall proportion of failures was low. Any future trials in this area would require a very large sample size and follow up of a minimum of one year.
The evidence suggests MTA may be the most efficacious medicament to heal the root pulp after pulpotomy of a deciduous tooth. As MTA is relatively expensive, future research could be undertaken to confirm if Biodentine, enamel matrix derivative, laser treatment or Ankaferd Blood Stopper are acceptable second choices, and whether, where none of these treatments can be used, application of sodium hypochlorite is the safest option. Formocresol, though effective, has known concerns about toxicity.
Regarding pulpectomy, there is no conclusive evidence that one medicament or technique is superior to another, and so the choice of medicament remains at the clinician's discretion. Research could be undertaken to confirm if ZOE paste is more effective than Vitapex and to evaluate other alternatives.
Regarding direct pulp capping, the small number of studies and low quality of the evidence limited interpretation. Formocresol may be more successful than calcium hydroxide; however, given its toxicity, any future research should focus on alternatives.
Plain language summary
Pulp treatment for extensive decay in primary teeth
Review question
How effective are different options for treating extensive tooth decay in children's primary (milk) teeth to resolve the child's symptoms (typically pain, swelling, abnormal movement) and tooth signs (as shown on an x‐ray)?
Background
In children, tooth decay is among the most common diseases. Tooth decay in the primary teeth tends to progress rapidly, often reaching the pulp ‐ the nerves, tiny blood vessels and connective tissue that make up the centre of a tooth. Dentists often have to perform one of three pulp treatment techniques: direct pulp capping (where a healing agent is placed directly over the exposed pulp), pulpotomy (removal of a portion of the pulp) or pulpectomy (removal of all of the pulp in the pulp chamber and root canal of a tooth).
The most common materials used for direct pulp capping are calcium hydroxide, the more recent but more expensive mineral trioxide aggregate, formocresol or an adhesive resin (placed directly over the tooth's nerve).
After a pulpotomy, one of four materials is generally used: ferric sulphate, formocresol, calcium hydroxide or mineral trioxide aggregate.
After a pulpectomy, a material is put into the space created by pulp removal. This material should not prevent the resorption of the primary tooth's root, to let the permanent tooth to grow in.
Study characteristics
Review authors working with Cochrane Oral Health carried out this review of randomised controlled trials. The evidence is current up to August 2017.
We included 87 trials that investigated the success of pulp treatment of milk teeth. The trials were published between 1989 and 2017 and provided 125 comparisons of different treatment options.
Key results
Pulp treatment for extensive decay in primary teeth is generally successful. The proportion of treatment failures was low, with many of the included trials having no failures with either of the treatments being compared.
After a pulpotomy, mineral trioxide aggregate (MTA) seems to be the best material (in terms of biocompatibility and efficacy) to put into contact with the remaining root dental nerve. The evidence showed it to be less likely to fail than either calcium hydroxide or formocresol.
After pulpectomy, it is not clear whether any medicament is superior to another. ZOE paste may give better results than Vitapex (calcium hydroxide/iodoform) paste, but more studies are needed to confirm this and to explore other treatment options.
Regarding direct pulp capping, the small number of studies undertaking the same comparison limits any interpretation. Formocresol may be superior to calcium hydroxide in terms of clinical and radiological failure, but because of toxic effects associated with formocresol, safer alternatives should be evaluated.
Quality of the evidence
We judged the quality of the evidence suggesting the superiority of MTA over calcium hydroxide or formocresol after pulpotomy to be moderate. For other comparisons, the quality of the evidence is low or very low, which means we cannot be certain about the findings. The low quality is due to shortcomings in the methods used within the individual trials, the small number of children included in the trials and the short‐term follow‐up after treatment.
Future trials to evaluate which healing agents are best for the three pulp treatments would require a very large sample size and should follow up the participants of a minimum of one year.
Summary of findings
Background
Description of the condition
Dental caries (tooth decay) is a bacterial infection that causes demineralisation and destruction of tooth tissues. The severity ranges from the early clinically visible changes in enamel caused by demineralisation to extensive cavitation. If the cavitation exposes dentine, then the caries has progressed to a 'distinct cavitation'. In more severe cases, there is obvious loss of tooth structure, the cavity is both deep and wide, and the dentine is clearly visible; a cavity that involves at least half of a tooth surface or possibly reaches the pulp is referred to as 'extensive' (ICDAS II 2011). In children, dental caries is among the most prevalent chronic diseases worldwide. Extensive tooth decay is the most common disease of primary teeth; 42% of children aged from two to 11 years have dental caries in their primary teeth, with a mean of 1.6 decayed teeth for each child (NHANES 2010; Selwitz 2007). Most dental caries in children are left untreated (CDC 2011). Decay in primary teeth is a risk factor for decay in permanent teeth (Al‐Shalan 1997; Finucane 2012; Kaste 1992).
Description of the intervention
Pulp interventions combine a pulp treatment technique and a medicament. The primary objective of pulp interventions is to maintain the integrity of the tooth and the health of its supporting tissues. Depending on the severity of the disease, three pulp treatment techniques are available: direct pulp capping, pulpotomy and pulpectomy (Guideline Pulp Therapy 2014; Guideline Pulp Therapy 2016). These treatments consist of the eviction of caries, followed by the eviction of a part of the pulp tissue and then setting in place medicaments. This treatment keeps the temporary tooth on the arch until it is replaced by the permanent tooth.
Direct pulp capping is usually indicated in a primary tooth with normal pulp (accidentally) exposed 1 mm or less. The exposed pulp is capped with a medicament before placing a restoration that seals the tooth. A pulpotomy is performed in a primary tooth with extensive caries but without evidence of radicular pathology. The coronal pulp is removed, and the remaining vital radicular pulp tissue is covered with a medicament. A pulpectomy is performed in a primary tooth with irreversible pulpitis. The radicular pulp is removed, and then a medicament is used to fill the canals. The tooth is restored with a restoration.
These treatments are combined with a variety of medicaments, to protect the pulp or the periradicular tissues, or to fill the substance loss, or both.
How the intervention might work
Pulp interventions involve the elimination of the infection and protection of the decontaminated tooth from future microbial invasion. Several medicaments are available for the obturation of the decontaminated surfaces or canals, the most frequently used are mineral trioxide aggregate (MTA), calcium hydroxide, formocresol or ferric sulphate.
Formocresol is a solution of cresol 35% and formaldehyde 19% in a vehicle of glycerine 15% and water (Buckley's formocresol). One part of this formula is normally mixed with three parts glycerine and one part water. This mixture prevents tissue autolysis by bonding to protein. Cresol is locally destructive to vital tissues but presents negligible potential for systemic distribution following the pulp treatment technique. However, formaldehyde is distributed systemically after pulp treatment technique and is classified by the International Agency for Research on Cancer (IARC) of the World Health Organization (WHO) as a known human carcinogen (IARC 2017). Although a 1:5 or 1:25 dilution of formocresol is generally advocated, many dentists use a more concentrated formula.
Ferric sulphate is a haemostatic compound that forms a metal‐protein clot at the surface of the pulp stumps, which seals blood capillaries and acts as a barrier to irritating components of the materials applied after. No concerns about toxic or harmful effects of ferric sulphate have been published in the dental or medical literature.
Calcium hydroxide was the first agent used in pulpotomies that demonstrated a capacity to induce dentine regeneration by becoming very alkaline when mixed with water. However, calcium hydroxide may possibly wound the primary tooth pulp to permit internal resorption or dystrophic calcification.
MTA is a recent mineral material that results ‐ when mixed with water ‐ in a hydrated calcium silicate gel containing calcium hydroxide. It is also very alkaline and promotes tissue regeneration when placed in contact with the pulp or periradicular tissues. It is biocompatible, non‐toxic and non‐resorbable and leads to minimal leakage around the margins.
Why it is important to do this review
Cochrane Oral Health undertook an extensive prioritisation exercise in 2014 to identify a core portfolio of titles that were the most clinically important to maintain on the Cochrane Library (Worthington 2015). Consequently, this review was identified as a priority title by the paediatric expert panel (Cochrane Oral Health priority review portfolio).
Because formocresol contains a known human carcinogen and is widely used for direct pulp capping and pulpotomy in children, finding a biocompatible and efficient alternative is a priority.
This is an update of a Cochrane Review first published in 2003 (Nadin 2003) and updated in October 2014 (Smaïl‐Faugeron 2014a). The 2003 version review included three randomised controlled trials (RCTs). We wrote a new protocol and searched for up‐to‐date evidence for an update in 2014. The 2014 update included 47 RCTs, on the basis of which the review authors concluded there was insufficient evidence supporting the superiority of one type of treatment over another. Since the 2014 version, results of new trials have been published and new medicaments have been introduced, and so we considered it important to synthesise new findings with existing evidence.
Objectives
To assess the effects of different pulp treatment techniques and associated medicaments for the treatment of extensive decay in primary teeth.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing different pulp interventions combining a pulp treatment technique and a medicament in primary teeth. We included trials that compared different medicaments for the same pulp treatment technique or different pulp treatment techniques with each other.
Types of participants
Children with extensive decay involving dental pulp in primary teeth.
Types of interventions
All pulp interventions combining a pulp treatment technique (pulpotomy, pulpectomy or direct pulp capping) and a medicament (any medication or device).
Types of outcome measures
Primary outcomes
We defined two primary outcomes: clinical failure and radiological failure as defined in primary studies, at six, 12 and 24 months.
Secondary outcomes
According to our classification of outcomes (Smaïl‐Faugeron 2013), we considered the following secondary outcomes to be relevant:
overall failure;
secondary clinical outcomes: pain, soft tissue pathology, pathological mobility, adjacent tissue inflammation, defective restoration (clinically), secondary caries at the margin (clinically), periodontal pocket formation, dental anxiety/phobia, premature tooth loss, signs of exfoliation, smell; and
secondary radiological outcomes: pathological radiolucency, pathological root resorption, pulp canal obliteration, dentin bridge formation, physiological root resorption, defective restoration (radiographically), secondary caries (radiographically), and filling material anomaly.
Search methods for identification of studies
Electronic searches
Cochrane Oral Health’s Information Specialist conducted systematic searches in the following databases for RCTs and controlled clinical trials. There were no language, publication year or publication status restrictions:
Cochrane Oral Health's Trials Register (to 10 August 2017) (Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 7) in the Cochrane Library (searched 10 August 2017) (Appendix 2);
MEDLINE Ovid (1946 to 10 August 2017) (Appendix 3);
Embase Ovid (1980 to 10 August 2017) (Appendix 4);
Web of Science (1945 to 10 August 2017) (Appendix 5); and
OpenGrey (to 10 August 2017) (Appendix 6).
There were no restrictions on the language or date of publication when searching the electronic databases. We identified and translated references in German, Serbian, Spanish, Japanese, Chinese, Danish, Italian, Arabic and Iranian.
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying RCTs and controlled clinical trials as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6 (Lefebvre 2011).
Searching other resources
Handsearching and identification of unpublished studies
The following databases were searched for ongoing trials, seeAppendix 7 for the search strategy:
US National Institutes of Health Ongoing Trials Register (clinicaltrials.gov; searched 10 August 2017); and
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 10 August 2017).
We handsearched the following journals:
Pediatric Dentistry (1995 to 2001);
European Journal of Paediatric Dentistry (2000 to 2002);
Journal of Clinical Pediatric Dentistry (1996 to 2002);
Journal of Endodontics (1996 to 2002); and
International Journal of Paediatric Dentistry (1991 to 2002).
Reference searching
We checked the references of all eligible trials for relevant studies. We scanned reference lists from review articles identified in the searches for further studies and consulted reference lists from paediatric dentistry textbooks.
We contacted experts in the field to help identify unpublished literature.
Data collection and analysis
Selection of studies
Two review authors independently scanned the titles of all records identified by the search to determine whether the studies were relevant. We resolved disagreements by discussion. Two review authors independently scanned selected abstracts to determine whether the study was relevant. If necessary, we obtained the full article. We resolved disagreements by discussion. We obtained the full report for all relevant articles. Two review authors independently scanned the full reports and completed the data extraction form to determine whether the article should be included or excluded. Disagreements were resolved by discussion. Finally, we included studies after checking for multiple publications of a given study (Characteristics of included studies). We recorded excluded studies, with reasons for exclusion (Characteristics of excluded studies).
Data extraction and management
Two review authors independently collected data using a specially designed data extraction form. Two review authors had pilot‐tested the data extraction form with 10 articles and modified it as required before use. We extracted data presented in graphs and figures whenever possible but included data only if both review authors independently had the same result or the authors could provide clarification of data. We resolved disagreements by discussion. We attempted to contact all study authors for clarification or missing information. We excluded data until further clarification was available, if we could not reach agreement. For each trial, we recorded the following data: year of publication and country of origin, inclusion/exclusion criteria specified, detailed description of interventions, sample size, mean age of participants, duration of follow‐up and outcome data. We tabulated all outcomes as reported in trials at six, 12, and 24 months.
Assessment of risk of bias in included studies
Two review authors independently graded all relevant articles in duplicate. This process followed the domain‐based evaluation described in the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (updated March 2011) (Higgins 2011). The two review authors compared evaluations and resolved any disagreements by discussion. The two review authors assessed the following domains in terms of 'low', 'unclear' or 'high' risk of bias: generation of sequence allocation, allocation concealment, blinding of participants and personnel, blinding of clinical outcome assessors, blinding of radiological outcome assessors and complete outcome data (both intention‐to‐treat and missing data). We tried to assess selective outcome reporting by looking for the trials in the clinicaltrials.gov register and comparing the 'Methods' and 'Results' sections of the publication.
Assessment of overall risk of bias considered the importance of different domains and studies and was classified as follows: low risk of bias (plausible bias unlikely to seriously alter the results) if all criteria were met; unclear risk of bias (plausible bias that raises some doubt about the results) if one or more criteria were assessed as unclear; or high risk of bias (plausible bias that seriously weakens confidence in the results) if one or more criteria were not met.
Measures of treatment effect
For dichotomous outcomes, we expressed the estimate of treatment effect as risk ratios together with 95% confidence intervals (CIs).
For continuous outcomes (such as mean participant satisfaction scores), where studies used the same scale to measure the outcome, we used the mean difference with 95% CIs. Where different scales were used, we expressed the treatment effect as a standardised mean difference and 95% CI.
Unit of analysis issues
The unit of analysis was the tooth, because teeth were randomly assigned to interventions. Some trials had a split‐mouth design, whereby one tooth was randomly allocated to the experimental treatment and another tooth in the same child was allocated to the control treatment. Pairing of data needed to be taken into account in the analysis. Split‐mouth trials that ignore the pairing show a unit‐of‐analysis error. Failure to account for correlation is likely to underestimate the precision of the trial (i.e. a CI that is too wide). We reported such errors, but could not re‐analyse data appropriately.
Dealing with missing data
To allow for an intention‐to‐treat analysis, we imputed missing outcome data as treatment success.
Assessment of heterogeneity
To investigate statistical heterogeneity, we examined forest plots, as well as Cochran's homogeneity tests, I² co‐efficients and between‐trial variances. We used the I² statistic with an approximate guide for interpretation as follows: 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100% represents considerable heterogeneity.
Assessment of reporting biases
We did not assess within‐study selective outcome reporting because we did not have access to study protocols. We planned to assess a possible between‐study reporting bias by producing a funnel plot of effect estimates against their standard errors if at least 10 trials were included in a meta‐analysis. If asymmetry of the funnel plot was found by inspection and confirmed by statistical tests, possible explanations were planned to be taken into account in the interpretation of the overall estimate of treatment effects.
Data synthesis
When two or more similar outcomes were reported in the same trial (e.g. spontaneous pain and pain on palpation), we considered only the most frequently reported outcome across all trials included in the meta‐analysis (Appendix 8). In addition, the different types of mineral trioxide aggregate (MTA) (unspecified MTA, grey MTA and white MTA) were combined, and if a trial compared two types of MTA, we included data for both arms.
We synthesised trials comparing different medicaments for the same pulp treatment technique (pulpotomy versus pulpotomy; pulpectomy versus pulpectomy; direct pulp capping versus direct pulp capping). The decision about whether to combine the results of individual studies depended on the assessment of heterogeneity. Combined estimates and associated 95% CIs were calculated by Mantel‐Haenszel fixed‐effect or random‐effects methods. In all cases, we considered the results from both fixed‐effect and random‐effects models. For random‐effects models, the estimate of the heterogeneity parameter is likely to be unreliable when the meta‐analysis is based on a small number of studies. Hence, when results from the trials were consistent, we preferred fixed‐effect analysis (Whitehead 2002). All P values were two‐sided and P value < 0.05 was deemed significant.
Subgroup analysis and investigation of heterogeneity
Where possible, subgroup analyses were to be undertaken to compare: results for teeth that were symptomatic versus symptom free preoperatively; effect of participant age at treatment, e.g. up to seven years and seven to 10 years; comparison of different types of final filling materials; and site of treatment ‐ primary versus secondary care sectors.
Sensitivity analysis
Sensitivity analyses were to be undertaken as follows:
excluding unpublished studies;
excluding studies of the lowest quality; and
excluding one or more large studies (if found) to assess how much they dominated the result.
Summarising findings and assessing the quality of the evidence
We created three 'Summary of findings' tables (one each for pulpotomy, pulpectomy and direct pulp capping) to present effect estimates for our main comparisons and primary outcomes. We also presented our assessment of the quality of the evidence, which we assessed as high, moderate, low or very low, according to GRADE critieria (Schünemann 2011).
Results
Description of studies
Results of the search
We provide summary details in the Characteristics of included studies, Characteristics of excluded studies and Characteristics of ongoing studies tables.
Searches from all sources identified 3330 references, 1709 of which remained after removing duplicates. After scanning the titles and abstracts (when available), we obtained the full reports of 157 records that looked potentially eligible and performed data extraction. After communication or attempted communication with 30 authors, and partial or complete translation of 20 papers, we listed 55 as excluded studies, with reasons for exclusion. We classified 14 registered trials as ongoing studies (see Characteristics of ongoing studies), none of which had reported results.
In total, 87 trials (91 references) satisfied the eligibility criteria and were included in the review (Figure 1).
1.
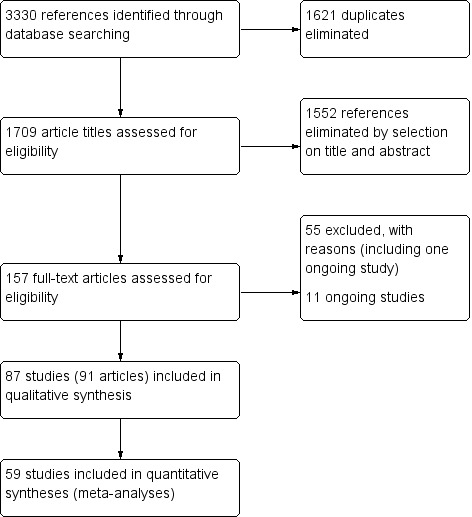
Study flow diagram
The 87 trials involved 7140 randomised teeth. Seventeen studies (20%) were split‐mouth design (without description of appropriate analysis); the remaining 70 studies (80%) were parallel‐arm design.
Included studies
Year of publication, setting and operators
The earliest trial was published in 1989 (Alaçam 1989), 34 trials (39%) were published between 2005 and 2012, and 38 trials (44%) were published between 2013 and 2017.
All included studies were single‐centre trials conducted primarily in paediatric dentistry departments of universities. Treatment settings and operators varied.
20 (23%) trials were conducted in India (Chandra 2014; Goyal 2014; Goyal 2016; Grewal 2016; Gupta 2015; Kalra 2017; Kusum 2015; Nadkarni 2000; Naik 2005; Niranjani 2015; Pinky 2011; Prabhakar 2008; Pramila 2016; Ramar 2010; Rewal 2014; Subramaniam 2009; Subramaniam 2011; Uloopi 2016; Yadav 2014);
16 (18%) in Turkey (Akcay 2014; Alaçam 1989; Alaçam 2009; Arikan 2016; Bezgin 2016; Cantekin 2014; Celik 2013; Demir 2007; Durmus 2014; Erdem 2011; Ozalp 2005; Ozmen 2017; Sonmez 2008; Tuna 2008; Ulusoy 2014a; Yildirim 2016);
12 (14%) in Iran (Aeinehchi 2007; Aminabadi 2010; Aminabadi 2016; Ansari 2010; Bahrololoomi 2008; Fallahinejad Ghajari 2013; Haghgoo 2009; Khorakian 2014; Malekafzali 2011; Mortazavi 2004; Noorollahian 2008; Shabzendedar 2013);
six (7%) in the USA (Dean 2002; Fei 1991; Fishman 1996; Vargas 2006; Zealand 2010; Zurn 2008);
six (7%) in Brazil (Coser 2008; Fernandes 2015; Lourenço 2015a; Moretti 2008; Oliveira 2013a; Sakai 2009);
four (5%) in Canada (Casas 2004; Doyle 2010; Nguyen 2017; Saltzman 2005);
three (3%) in Israel (Eidelman 2001; Fuks 1997; Holan 2005);
two in Egypt (Agamy 2004; Sabbarini 2008);
three in Saudi Arabia (El Meligy 2016; Farsi 2005; Shumayrikh 1999);
two in Thailand (Nakornchai 2010; Trairatvorakul 2008);
two in Spain (Cuadros‐Fernández 2016; Fernández 2013);
one each in Germany (Huth 2005), Kuwait (Ibricevic 2000), Mexico (Garrocho‐Rangel 2009), Serbia and Montenegro (Markovic 2005), Korea (Kang 2015), Nigeria (Olatosi 2015), Syria (Al‐Ostwani 2016) Belgium (Rajasekharan 2017), and the UK (Waterhouse 2000).
The study setting was not mentioned in 19 (22%) trials.
Operators were dentists in 38 (43%) trials, undergraduate dental students supervised by senior staff members of clinics in one trial (Alaçam 2009), postgraduate dental students supervised by one or two investigators in two trials (Cuadros‐Fernández 2016; Khorakian 2014), and professor, doctoral graduate, doctoral student, master graduate and master student in one trial (Rajasekharan 2017). Operators were not mentioned in 44 (50%) trials.
Participants
The weighted mean age of children in the 87 included studies was 6.3 years. Age‐related inclusion criteria varied among studies; children's ages ranged from two years to 13 years.
All included studies were small; the median number of enrolled children in each trial was 45.5 (interquartile range (IQR) 27 to 71; minimum to maximum 15 to 155). The median number of treated teeth for each trial was 70 (IQR 50 to 100; minimum to maximum 20 to 291).
Interventions
Number of arms
Overall, 17 (20%) were split‐mouth studies, 38 (44%) trials were two‐arm studies, 21 (24%) were three‐arm studies, 10 (11%) were four‐arm studies, and one trial described a five‐arm study (Demir 2007).
Treatments and medicaments
The 87 trials described 125 different combinations of pulp treatment (pulpotomy, pulpectomy or direct pulp capping) and medicament.
Pulpotomy
In total, 53 trials (61%) compared different medicaments/techniques for pulpotomy (75 comparisons):
-
Mineral trioxide aggregate (MTA) compared with formocresol in 19 trials (23%)
full strength formocresol (Aeinehchi 2007; Agamy 2004; Eidelman 2001; Farsi 2005; Haghgoo 2009; Holan 2005; Jayam 2014; Saltzman 2005; Yildirim 2016)
1:5 diluted formocresol (Ansari 2010; Erdem 2011; Fernández 2013; Moretti 2008; Naik 2005; Noorollahian 2008; Olatosi 2015; Sonmez 2008; Subramaniam 2009; Zealand 2010).
MTA compared with calcium hydroxide in six trials (Akcay 2014; Celik 2013; Liu 2011; Moretti 2008; Oliveira 2013a; Sonmez 2008);
MTA compared with ferric sulphate with or without eugenol, in five trials (Doyle 2010; Erdem 2011; Fernández 2013; Goyal 2016; Sonmez 2008) (two comparisons);
MTA compared with ferric sulphate + MTA (Doyle 2010);
MTA compared with Portland cement in three trials (Oliveira 2013a; Sakai 2009; Yildirim 2016);
MTA compared with calcium‐enriched mixture (CEM) (Malekafzali 2011);
MTA compared with sodium hypochlorite (NaOCl) (Fernández 2013);
MTA compared with calcium hydroxide + NaOCl (Akcay 2014);
MTA + NaOCl versus calcium hydroxide + NaOCl (Akcay 2014);
MTA compared with buffered glutaraldehyde (Goyal 2016);
MTA compared with zinc oxide eugenol (ZOE) (Erdem 2011);
MTA compared with diode laser (Niranjani 2015);
MTA + diode laser versus formocresol + ZOE (Saltzman 2005);
MTA compared with low‐level laser therapy (LLLT) (Uloopi 2016);
MTA compared with enamel matrix derivative (EMD) (Yildirim 2016);
MTA compared with Biodentine in four trials (Cuadros‐Fernández 2016; Kusum 2015; Niranjani 2015; Rajasekharan 2017);
MTA compared with propolis (Kusum 2015);
MTA compared with aloe vera (Aloe barbadensis Mill, family Liliaceae) (Kalra 2017);
MTA compared with Tempophore (iodoform‐based paste) (Rajasekharan 2017);
-
Comparisons between different types of MTA:
white MTA compared with grey MTA (Agamy 2004);
MTA versus MTA + NaOCl (Akcay 2014);
ProRoot MTA compared with OrthoMTA (Kang 2015);
ProRoot MTA compared with RetroMTA (Kang 2015);
OrthoMTA compared with RetroMTA (Kang 2015).
-
calcium hydroxide compared with formocresol in eight trials (9%):
full strength formocresol (Alaçam 2009; Markovic 2005);
1:5 diluted formocresol (Fernandes 2015; Huth 2005; Moretti 2008; Sonmez 2008; Waterhouse 2000; Zurn 2008).
calcium hydroxide compared with ferric sulphate in three trials (Huth 2005; Markovic 2005; Sonmez 2008);
calcium hydroxide compared with Portland cement (Oliveira 2013a);
calcium hydroxide compared with MTA + NaOCl (Akcay 2014);
calcium hydroxide compared with Er:YAG laser (Huth 2005);
calcium hydroxide compared with calcium hydroxide/iodoform (Alaçam 2009);
calcium hydroxide compared with low‐level laser therapy (LLLT) (Fernandes 2015);
calcium hydroxide compared with LLLT + calcium hydroxide (Fernandes 2015);
calcium hydroxide + LLLT compared with LLLT (Fernandes 2015);
calcium hydroxide compared with calcium hydroxide + NaOCl (Akcay 2014);
calcium hydroxide compared with Biodentine (Grewal 2016).
-
ferric sulphate compared with formocresol in 10 trials (11%):
full strength formocresol (Fei 1991; Ibricevic 2000; Markovic 2005);
1:5 diluted formocresol (Durmus 2014; Erdem 2011; Fernández 2013; Fuks 1997; Huth 2005; Ozmen 2017; Sonmez 2008).
ferric sulphate compared with NaOCl in two trials (Fernández 2013; Vargas 2006);
ferric sulphate compared with buffered glutaraldehyde (Goyal 2016);
ferric sulphate versus ZOE (Erdem 2011);
ferric sulphate compared with Er:YAG laser (Huth 2005);
ferric sulphate compared with diode laser in three trials (Durmus 2014; Gupta 2015; Yadav 2014);
ferric sulphate compared with electrosurgery in two trials (Gupta 2015; Yadav 2014);
ferric sulphate/MTA compared with ferric sulphate (with or without eugenol) (Doyle 2010) (two comparisons);
-
ferric sulphate compared with Ankaferd Blood Stopper in two trials (Cantekin 2014; Ozmen 2017):
full strength formocresol (Fei 1991; Ibricevic 2000; Markovic 2005);
1:5 diluted formocresol (Durmus 2014; Erdem 2011; Fernández 2013; Fuks 1997; Huth 2005; Ozmen 2017; Sonmez 2008).
Portland cement compared with full strength formocresol (Yildirim 2016);
Portland cement compared with EMD (Yildirim 2016);
Portland cement compared with Portland cement + radio‐opacifying agents (iodoform (CHI₃ or zirconium oxide (ZrO₂)) (Lourenço 2015a) (two comparisons);
Glutaraldehyde + calcium hydroxide compared with full strength formocresol (Alaçam 1989);
Glutaraldehyde + ZOE compared with full strength formocresol (Alaçam 1989);
Glutaraldehyde + calcium hydroxide compared with glutaraldehyde + ZOE in two trials (Alaçam 1989; Shumayrikh 1999);
Electrofulguration + calcium hydroxide compared with electrofulguration + ZOE (Fishman 1996).
-
Electrosurgery compared with formocresol in two trials:
full strength formocresol (Dean 2002);
1:5 diluted formocresol (Bahrololoomi 2008).
Electrosurgery compared with diode laser in two trials (Gupta 2015; Yadav 2014);
Electrosurgery compared with CEM (Khorakian 2014);
Biodentine compared with formocresol (El Meligy 2016)
Biodentine compared with diode laser (Niranjani 2015);
Biodentine compared with Tempophore (Rajasekharan 2017);
Biodentine compared with propolis (Kusum 2015).
1:5 diluted formocresol compared with NaOCl in two trials (Fernández 2013; Shabzendedar 2013);
Full strength formocresol compared with calcium hydroxide/iodoform (Alaçam 2009);
1:5 diluted formocresol compared with ZOE (Erdem 2011);
1:5 diluted formocresol compared with Er:YAG laser (Huth 2005);
1:5 diluted formocresol compared with diode laser (Durmus 2014);
1:5 diluted formocresol compared with LLLT (Fernandes 2015);
1:5 diluted formocresol compared with LLLT + calcium hydroxide (Fernandes 2015);
1:5 diluted formocresol compared with Ankaferd Blood Stopper (Ozmen 2017);
-
formocresol compared with EMD in two trials:
full strength formocresol (Yildirim 2016);
1:5 diluted formocresol (Sabbarini 2008);
Full strength formocresol compared with 1:5 diluted formocresol (Goyal 2014);
Full strength formocresol compared with 1:25 diluted formocresol (Goyal 2014);
1:5 diluted formocresol compared with 1:25 diluted formocresol (Goyal 2014).
Pulpectomy
In total, 15 trials (17%) compared different medicaments for pulpectomy (25 comparisons):
calcium hydroxide compared with ZOE in two trials (Nadkarni 2000; Ozalp 2005);
calcium hydroxide compared with Sealapex (composition: isobutyl salicylate resin, SiO₂, BiO₃, TiO₂, N‐ethyl toluene sulfenamide resin, ZnO, CaO, eugenol‐free calcium hydroxide) (Ozalp 2005);
calcium hydroxide compared with Vitapex (calcium hydroxide/ 50% iodoform) (Ozalp 2005);
Metapex (composition: calcium hydroxide < 36 w/w%, iodoform 30 to 37w/w%, polydimethylsiloxane < 26 w/w%) compared with ZOE in two trials (Al‐Ostwani 2016; Subramaniam 2011);
Metapex compared with ZOE + calcium hydroxide with iodoform (Endoflas) in two trials (Ramar 2010; Subramaniam 2011);
Metapex compared with ZOE with iodoform (RC Fill) (Ramar 2010);
Metapex compared with endoflas‐chlorophenol‐free (Endoflas‐CF) (Al‐Ostwani 2016);
Metapex compared with zinc oxide and propolis (ZOP) (Al‐Ostwani 2016);
Sealapex compared with ZOE (Ozalp 2005);
Sealapex compared with Vitapex (Ozalp 2005);
Vitapex compared with ZOE in five trials (Chen 2015; Mortazavi 2004; Ozalp 2005; Pramila 2016; Trairatvorakul 2008);
Vitapex compared with 3Mix (ciprofloxacin + metronidazole + minocycline) (Nakornchai 2010);
Vitapex compared with RC Fill (Pramila 2016);
Vitapex compared with a mixture of ZOE + calcium hydroxide + iodoform (unnamed product) (Chen 2015);
Endoflas compared with ZOE in two trials (Rewal 2014; Subramaniam 2011);
Endoflas compared with RC Fill (Ramar 2010);
Endoflas‐CF compared with ZOE (Al‐Ostwani 2016);
Endoflas‐CF compared with ZOP (Al‐Ostwani 2016);
ZOE compared with ozonated sesame oil‐ZO (Chandra 2014);
ZOE compared with RC Fill (Pramila 2016);
ZOE compared with ZOP (Al‐Ostwani 2016);
ZOE compared with ZOE + calcium hydroxide + iodoform (unnamed product) (Chen 2015);
ciprofloxacin + metronidazole + minocycline (3Mix) compared with ciprofloxacin + ornidazole + minocycline (Pinky 2011);
MTA compared with intermediate restorative material (IRM) (Arikan 2016);
MTA compared with gutta‐percha/AH‐Plus (Bezgin 2016).
Both pulpotomy and pulpectomy
Four trials compared pulpotomy and pulpectomy with different medicaments (four comparisons):
full strength formocresol pulpotomy compared with calcium hydroxide pulpectomy (Coser 2008).
ferric sulphate/ZOE pulpotomy compared with ZOE pulpectomy (Casas 2004);
ferric sulphate/MTA pulpotomy compared with ZOE (Sedanol) pulpectomy (Nguyen 2017).
3Mix (ciprofloxacin + metronidazole + minocycline) pulpotomy compared with 3Mix pulpectomy (Prabhakar 2008).
Direct pulp capping
Seven trials (8%) compared different medicaments for direct pulp capping (21 comparisons):
calcium hydroxide compared with formocresol (Aminabadi 2010);
calcium hydroxide compared with acetone‐based total‐etch adhesive (with or without non‐rinse conditioner or total etching with 36% phosphoric acid) (Demir 2007, four comparisons);
calcium hydroxide compared with EMD (Garrocho‐Rangel 2009);
calcium hydroxide compared with MTA (Tuna 2008);
Acetone‐based total‐etch adhesive compared with acetone‐based total‐etch adhesive + non‐rinse conditioner or total‐etching with 36% phosphoric acid or self etch adhesive system (Demir 2007) (3 comparisons);
Non‐rinse conditioner + acetone‐based total‐etch adhesive compared with acetone‐based total‐etch adhesive + total‐etching with 36% phosphoric acid or self‐etch adhesive system (Demir 2007, two comparisons);
Self etch adhesive system + acetone‐based total‐etch adhesive versus total‐etching with 36% phosphoric acid + acetone‐based total‐etch adhesive (Demir 2007);
MTA compared with CEM (Fallahinejad Ghajari 2013);
MTA compared with 3Mix (Aminabadi 2016);
MTA compared with 3Mixtatin (a combination of simvastatin and 3Mix antibiotic) (Aminabadi 2016);
MTA compared with simvastatin (Aminabadi 2016);
3Mix compared with 3Mixtatin (Aminabadi 2016);
3Mix compared with simvastatin (Aminabadi 2016);
3Mixtatin compared with simvastatin (Aminabadi 2016);
calcium hydroxide cement (Dycal) compared with a bone graft calcium sulphate hemihydrate material (DentoGen) (Ulusoy 2014a).
Duration of follow‐up
The duration of follow‐up was fixed in 78 (90%) trials. Data were assessed at six months in 70 (80%) trials, at 12 months in 59 (68%) trials and at 24 months in 24 (28%) trials.
Rubber dam
In 67 trials (77%), treatments were completed with rubber dam isolation. In four trials, either rubber dam or cotton rolls were used (Ozalp 2005; Waterhouse 2000; Zealand 2010; Zurn 2008); in four trials, cotton rolls were used (Alaçam 1989; Fallahinejad Ghajari 2013; Markovic 2005; Sonmez 2008); and in 12 trials there was insufficient information to determine if a rubber dam or cotton rolls were used (Arikan 2016; Cantekin 2014; Chen 2015; Demir 2007; Goyal 2014; Goyal 2016; Liu 2011; Mortazavi 2004; Nadkarni 2000; Naik 2005; Niranjani 2015; Pinky 2011).
Pulp access
Caries were removed prior to pulpal access in 68 (78%) trials. Pulp was accessed with a high‐speed bur in 37 (43%) trials, a slow‐speed bur in six trials (Aminabadi 2010; Aminabadi 2016; Markovic 2005; Moretti 2008; Ramar 2010; Shabzendedar 2013), a high‐speed followed by a slow‐speed bur in six trials (Casas 2004; Celik 2013; Cuadros‐Fernández 2016; Doyle 2010; Fernández 2013; Nguyen 2017), a combination of slow‐speed bur and excavator in one trial (Nadkarni 2000), a combination of high‐speed bur and round carbide bur in three trials (Fallahinejad Ghajari 2013; Kang 2015; Lourenço 2015a), a high‐speed followed by a combination of slow‐speed bur and round carbide bur in one trial (Ulusoy 2014a), a number 557 round bur in one trial (Kalra 2017), or a handpiece with a round bur (with no precision) followed by a high speed and round carbide bur in one trial (Oliveira 2013a).
Removal of coronal pulp involved an excavator in 35 (40%) trials, a combination of slow‐speed bur and excavator in 11 (13%) trials, a slow‐speed bur in 11 (13%) trials, a high‐speed bur in two trials (Ibricevic 2000; Markovic 2005), a high speed bur followed by excavator in one trial (Celik 2013), round burs numbers ½ and ¼ or excavator in one trial (Grewal 2016), or a number 6 carbide round bur in one trial (Shabzendedar 2013).
In the case of pulpectomy, complete extirpation of the pulp involved barbed broaches, K files or H files.
Haemostasis
Before application of the pulpotomy or direct pulp capping medicament, haemostasis of the pulp stumps was achieved with either dry or moistened (water or saline) cotton wool pellets in 53 trials (61%).
In two trials, haemostasis of the pulp stumps was achieved with techniques that differed according to the group: in Coser 2008, haemostasis was obtained with dry cotton wool pellets in the pulpotomy group and with moistened cotton pellets with saline in the pulpectomy group; whereas, in Doyle 2010, haemostasis was obtained with saline/water flush in the three ferric sulphate arms and with dry cotton pellets in the MTA arm.
In five trials, haemostasis was obtained using other techniques: a sterile cotton pellet soaked in 1.25% sodium hypochlorite solution and placed over the exposure site for 62 seconds without pressure (Demir 2007), a damp sterile cotton pellet (Farsi 2005), a dry sterile cotton pellet and electrofulguration (Fishman 1996), a cotton pellet moistened with 10% sodium hypochlorite maintained for one minute in one group (no haemostasis in the other group, Nakornchai 2010), a cotton pellet moistened with 3% hydrogen peroxide (Shumayrikh 1999), or diode laser (810 nm with the pulsed contact mode of application for two seconds delivered by optical fibre tip and 1.5 W power) (Niranjani 2015). The other trials involved no haemostasis technique or no details about haemostasis.
Irrigation
Irrigation was performed in 48 (55%) trials (in one group only in three trials (Nguyen 2017; Prabhakar 2008; Saltzman 2005). Irrigants used were:
saline in 33 (38%) trials;
0.9% saline solution (Shumayrikh 1999);
0.5% saline (Coser 2008);
5.25% sodium hypochlorite and distilled water (Al‐Ostwani 2016);
2.5% sodium hypochlorite in three trials (Chen 2015; Nakornchai 2010; Trairatvorakul 2008);
2.5% sodium hypochlorite and saline in four trials (Arikan 2016; Chandra 2014; Nadkarni 2000; Rewal 2014);
1% sodium hypochlorite and saline or water in three trials (Aminabadi 2016; Bezgin 2016; Subramaniam 2011);
5% sodium hypochlorite followed by a 0.5% metronidazole solution (Ozalp 2005);
a mixture of 2.25% sodium hypochlorite (1.5 mL) and 0.12% chlorhexidine gluconate (1.5 mL) (Ramar 2010);
alternating irrigations of sterile saline and a chlorhexidine solution (Garrocho‐Rangel 2009);
saline and finally with 2% chlorhexidine (Pramila 2016);
3% hydrogen peroxide and sterile saline (Alaçam 1989); and
water in three trials (Nguyen 2017; Vargas 2006; Yildirim 2016).
Number of visits
Only one intervention session for both groups was necessary for 62 (71%) trials. In six trials (7%), the number of visits was one in one treatment group and two (Akcay 2014; Ansari 2010; Bezgin 2016; Kang 2015; Noorollahian 2008; Sonmez 2008) or one or two (Ibricevic 2000; Nakornchai 2010) in the other groups.
The number of visits was:
one or two in both groups in two trials (Cantekin 2014; Ozalp 2005);
two in 12 (14%) trials (Chen 2015; Goyal 2014; Goyal 2016; Grewal 2016; Jayam 2014; Kusum 2015; Mortazavi 2004; Nadkarni 2000; Naik 2005; Shumayrikh 1999; Subramaniam 2009; Subramaniam 2011);
three in two trials (Arikan 2016; Pinky 2011); and
four in one trial (Coser 2008).
Description of medicaments used
Pulpotomy
The formocresol technique used in 31 trials (36%) involved application of a cotton wool pellet soaked with formocresol on the pulp stumps for five minutes after pulpotomy. Alaçam 2009 and Yildirim 2016 involved applying the cotton wool pellet soaked with formocresol on the pulp stumps for three to four minutes, and Subramaniam 2009 and Shabzendedar 2013 involved applying a cotton wool pellet soaked with formocresol on the pulp stumps for one minute after pulpotomy.
The MTA technique used after pulpotomy involved a 3:1 powder:saline ratio in 21 trials (24%), followed by placement of moistened cotton pellet over MTA for 15 minutes in one trial (Jayam 2014). The MTA technique used by Moretti 2008 after pulpotomy involved a 1:1 powder:saline ratio. Oliveira 2013a or Kalra 2017 did not define the powder:saline ratio involved (they tried to obtain a "homogeneous paste" or a "thick paste").
The calcium hydroxide technique used by Celik 2013 involved application of calcium hydroxide powder mixed with sterile water in a 3:1 ratio to produce a homogeneous paste. The calcium hydroxide technique used by Grewal 2016 involved application of calcium hydroxide paste with the help of disposable tip topped by light cured calcium hydroxide.
The ferric sulphate technique used by Casas 2004 and Nguyen 2017 involved application of a 16% or 15.5% aqueous ferric sulphate solution on the pulp stumps for 10 to 15 seconds after pulpotomy, followed by a water flush in the pulp chamber (with an air‐water syringe). The ferric sulphate technique used in nine (10%) trials involved application of 15.5% aqueous ferric sulphate or eugenol‐free ferric sulphate for 15 seconds (Doyle 2010; Durmus 2014; Erdem 2011; Fei 1991; Fuks 1997; Ibricevic 2000; Markovic 2005; Ozmen 2017; Sonmez 2008), or 10 to 15 seconds (Fuks 1997; Sonmez 2008), after pulpotomy. The technique used by Huth 2005 involved application of 15.5% ferric sulphate. The ferric sulphate technique used by Vargas 2006, Gupta 2015 and Cantekin 2014 was described as application of ferric sulphate for 15 seconds after pulpotomy (followed by irrigation of saline in Cantekin 2014). The ferric sulphate technique used by Goyal 2016 and Yadav 2014 involved application of a 15.5% ferric sulphate solution on the pulp stumps for 15 seconds after pulpotomy, followed by irrigation of normal saline.
The ferric sulphate‐MTA technique used by Doyle 2010 involved application of a 15.5% aqueous ferric sulphate solution, followed by MTA application for 15 seconds after pulpotomy.
The Portland cement technique, with or without CHI₃ or ZrO₂, used by Lourenço 2015a, involved application of cements prepared using an MTA kit spoon (1 g) of powder as the measure parameter with two drops (0.3 mL) of distilled water and mixed in sterilised glass to obtain a paste consistency; cements were applied with a spatula. The Portland cement technique used by Oliveira 2013a involved 0.1 g Portland cement (previously sterilised with ethylene oxide and then mixed with sterile water) mixed with sterile saline to produce a homogeneous paste. The Portland cement technique used by Yildirim 2016 was sterilised with ethylene oxide prior to use, and 0.16 g of the cement was mixed with distilled water until a homogeneous paste was obtained.
The 5% NaOCl technique used by Vargas 2006 and Fernández 2013 involved application of a cotton wool pellet soaked with 5% NaOCl on the pulp stumps for 30 seconds after pulpotomy. The 3% NaOCl technique used by Shabzendedar 2013 involved application of a cotton pellet saturated with 3% NaOCI on the pulp stumps for 30 seconds after pulpotomy.
The 2% unbuffered glutaraldehyde technique used by Alaçam 1989 involved application on the pulp stumps of 2% unbuffered glutaraldehyde for five minutes after pulpotomy, followed by ZOE in one group, and calcium hydroxide in the other group. Goyal 2016 used the same technique followed by ZOE. Shumayrikh 1999 used the same technique, except glutaraldehyde was applied for three minutes after pulpotomy, followed by eugenol + intermediate restorative material (IRM) (a reinforced ZOE) in one group and calcium hydroxide in the other group.
The CEM technique used by Khorakian 2014 involved application of a 2 mm layer of CEM cement directly over the radicular pulp (3:1 powder:liquid ratio).
The technique used by Niranjani 2015 involved a diode laser of 810 nm with the pulsed contact mode of application for two seconds delivered by an optical fibre tip with at 1.5 W. The diode laser technique used by Durmus 2014 involved a beam at a wavelength of 810 nm transmitted; the diode laser fibre tip was kept 1 mm to 2 mm away from the tissue; the pulp at canal orifices was treated for 10 seconds with a frequency of 30 Hz, at 50 mJ, and 1.5 W, under air‐cooling operation mode without water. In Gupta 2015, the pulp was ablated to the level of the canal orifice using a diode laser at 980 nm wavelength, 3 W power and the continuous pulse mode. The laser energy of 4.0 J/cm² was delivered through a 0.5 mm diameter optical fibre in contact with the pulp tissue for 2 minutes and 31 seconds. If additional ablation was required, subsequent multiple applications were administered. In Yadav 2014, the remaining coronal pulp tissue was exposed to laser energy through an optical fibre using a diode laser of 810 nm and 7 W set at a 3 W power in the continuous mode. The laser energy was delivered through a 400 μm diameter optical fibre in a non contact mode but close to the pulp tissue for not more than two to three seconds (PD = 2388.53, Fluence = 7165.60).
The erbium:yttrium‐aluminium garnet (Er:YAG) laser technique used by Huth 2005 involved an application of 2 Hz and 180 mJ laser in the pulse mode without water cooling, with a mean number (± standard deviation) of laser pulses for each tooth of 31.5 ± 5.9 equally distributed to each pulp stump.
The low‐level laser therapy (LLLT) technique used by Uloopi 2016 involved a diode laser wavelength 810 nm, under continuous mode; an energy of 2 J/cm² was applied over the radicular stumps for about 10 seconds. The InGaAlP laser radiation used by Fernandes 2015 was delivered through a 320 lm diameter optical fibre in contact with pulp tissue; the parameters were set at 660 nm wavelength, 10 mW power output, 2.5 J/cm² energy density, 50 to 60 Hz frequency, 0.04 cm² focus beam diameter and irradiation time of 10 seconds. The same author used the LLLT (as described before) followed by calcium hydroxide.
The electrosurgery technique used by Bahrololoomi 2008 and Dean 2002 involved a maximum of three applications of one second to each pulpal orifice, with cool‐down periods of five seconds (Dean 2002), or 10 to 15 seconds (Bahrololoomi 2008), between applications to limit heat build‐up, at 40% power. In Gupta 2015, an electrosurgery electrode tip (unit T4, fine wire; 50 W power; 110 V ± 5% 50/60 Hz 92 VA; work frequency 1.5 ˜ 1.7 MHz ± 5%) was used for the pulpotomy procedure. During the procedure, the electrode tip was positioned slightly above the pulp tissue but close enough for electrical arcing to occur (about 1 mm above the tissue). The current was applied for 1 to 2 seconds over each pulpal stump. This procedure was repeated up to three times on each pulpal orifice, until brown appearance was observed in the tissue. In Yadav 2014, the ART‐E1 electrosurgery unit was set to the COAG 1 mode to perform both electrofulguration and electrocoagulation.The handpiece with appropriate electrode tips, kept 1 to 2 mm away from the pulpal tissue, was used to deliver the electric arc. The duration of application was not more than two to three seconds followed by a cool‐down period of five seconds. If necessary, this procedure was repeated up to a maximum of three times. After each current application, a new large moist sterile cotton pellet was placed with pressure on the pulpal tissue near to orifice to absorb any blood or tissue fluids before the next current application (e.g. pellet‐electrode‐pellet‐electrode). When properly completed, the pulpal stumps appeared dry and completely blackened. The electrosurgery/electrofulguration (Hyfrecator) used by Fishman 1996 involved application of the active electrode tip about 1 mm above each pulpal stump tissue for one to two seconds; if additional fulguration was required, 10 seconds elapsed before subsequent current application. In Khorakian 2014, an electrosurgical ball‐shaped electrode was immediately used for tissue coagulation. The unit was set at 55 W, 3.69 MHz, 600 Ω, and COAG mode. The electrode was placed 1 to 2 mm above the pulp orifices and then electrical arc allowed to bridge for 1 second. This procedure was repeated up to three times on each pulpal orifice with 5 to 10 second cool‐down intervals, until a dark brown appearance was observed in the tissues; then copious irrigation.
The enamel matrix derivative (EMD) technique used by Sabbarini 2008 after pulpotomy involved application of a cotton pellet on the amputated pulpal stump; the tooth was then conditioned with polyacrylic acid gel; the cotton pellet was then removed, and the amputated pulpal stump was covered with protein EMD gel from a 0.3 mL syringe. The technique used by Yildirim 2016 involved 0.7 mL of EMD injected over the root pulp tissue.
The Biodentine technique used by Kusum 2015 involved mixing pre‐measured unit dose capsules for 30 seconds at 4200 rpm in a triturator to obtain a putty‐like consistency. It was then carried with an amalgam carrier and condensed lightly with a metal condenser on the pulp stumps, to a thickness of 2 to 3 mm. The Biodentine used by Grewal 2016 involved the following procedure: before the capsule was opened, it was tapped gently on a hard surface to diffuse the powder; five drops of liquid from the single‐dose dispenser were poured into the capsule, after which the capsule was placed in a triturator for 30 seconds; the material was then transferred with the aid of the manufacturer‐supplied spatula and placed inside the cavity with the aid of an amalgam carrier or spatula. A plugger or sterile cotton pellet was used to adjust the material against the walls without excessive compression.
The propolis technique used by Kusum 2015 involved 1.5 g 100% standardised propolis extract powder mixed with 1.75 mL of polyethylene glycol to form a thick consistency on a clean dry glass slab using a metal spatula. The paste was carried to the pulp stumps with a metal carrier and then condensed lightly to a thickness of 2 to 3 mm.
The aloe vera technique used by Kalra 2017 involved use of a healthy plant of pure aloe vera, approximately four years old, certified by the Indian Agricultural Research Institute, procured at regular intervals throughout the study period. A healthy leaf was selected from the plant and cut from its stem base, cleaned with 70% ethyl alcohol, and stored in distilled water for one hour to eliminate aloin. After one hour, the outer green rind portion was removed using a sterile Bard‐Parker blade, and the blade was introduced inside the inner mucilage layer. The mucilage or the inner clear jelly‑like substance (approximately 10 mm) was removed and washed again. The mucilage was cut in half and placed onto the pulp stumps of the tooth.
The Ankaferd Blood Stopper technique used by Cantekin 2014 and Ozmen 2017 involved application of solution to the pulp stumps with a dental syringe for 15 seconds, before the pulp stumps were rinsed with saline solution and pulp chamber dried with sterile cotton pellets.
The following techniques after pulpotomy were not described in sufficient detail: formocresol (Coser 2008), diode laser with MTA (Akcay 2014; Sakai 2009; Saltzman 2005), MTA (Kusum 2015; Liu 2011), ZOE (Erdem 2011), calcium hydroxide (Akcay 2014; Alaçam 2009; Aminabadi 2010; Coser 2008; Demir 2007; Fernandes 2015; Huth 2005; Liu 2011; Markovic 2005; Moretti 2008; Oliveira 2013a; Sonmez 2008; Waterhouse 2000; Zurn 2008), CEM (Malekafzali 2011), Portland cement (Sakai 2009) and Biodentine (Cuadros‐Fernández 2016; El Meligy 2016; Niranjani 2015).
Pulpectomy
The Vitapex paste technique used by Mortazavi 2004 after pulpectomy involved application of an formocresol‐moistened cotton pellet in the pulp chamber after pulpotomy, followed by a ZOE paste (zonalin) temporary restoration at the first visit. Vitapex was applied after pulpectomy during the second visit. The Vitapex technique used by (Pramila 2016) was available in preformed syringes, the syringe was inserted into the canal near the apex, the paste was extruded into the canal, and the syringe was then slowly withdrawn as it filled the entire canal.
For the ZOE technique, Mortazavi 2004 also applied an formocresol‐moistened cotton pellet in the pulp chamber after pulpotomy, followed by a ZOE paste (zonalin) temporary restoration at the first visit; a ZOE paste was applied after pulpectomy during the second visit. The ZOE technique used by Nadkarni 2000 and Chandra 2014 involved application of ZOE with a needle placed 1 or 2 mm short of the radiographic apex. The ZOE technique used by Casas 2004, Al‐Ostwani 2016 and Nguyen 2017 involved application of ZOE paste after pulpectomy to the root canal with a spiral paste filler inserted into the canal to a point just short of the apex. The ZOE technique used by Pramila 2016 involved application of ZOE in the root canal with an endodontic pressure syringe. The ZOE technique used by Rewal 2014 involved use of a Lentulo spiral mounted on a slow‐speed hand piece.
For the Metapex technique, Al‐Ostwani 2016 used performed syringe with disposable plastic needles to inject the paste into the root canal; after inserting the tape of the needle near the apex, and the paste was gently pressed into the canal pulling the tape back slowly until the canal was filled.
The calcium hydroxide technique used by Nadkarni 2000 involved application of calcium hydroxide with a needle placed 2 mm short of the radiographic apex.
The MTA technique used by Arikan 2016 involved application of approximately 3 mm of MTA on the pulpal floor, then a moistened cotton pellet in contact with the MTA was left in the cavity before application of the temporary filling material. The MTA technique used by Bezgin 2016 involved application of MTA (mixed according to the manufacturer’s recommendations) in the canal using the MTA Gun System and compacted using endodontic pluggers; the MTA was allowed to set completely by placing a cotton pellet moistened with sterile water inside the pulp chamber.
The RC Fill technique used by Pramila 2016 was available in powder and liquid form, mixed to the desired consistency according to the manufacturer’s instructions; a Lentulo spiral was used to place the RC Fill.
The gutta‐percha/AH‐Plus technique used by Bezgin 2016 involved application of gutta‐percha points filling root canals, using a size 30 master cone and size 25, 20 and 15 accessory cones, with finger spreaders sizes 25 and 20 and AH‐Plus Sealer using a cold lateral condensation technique.
The ozonated sesame oil‐ZO technique used by Chandra 2014 involved application of a mixture of ZO powder (0.2 g, arsenic free) and ozonated sesame oil filling root canals 1 mm short of the apex using Lentulo spirals.
The Endoflas technique used by Rewal 2014 involved a Lentulo spiral mounted on a slow‐speed hand piece.
The technique used by Al‐Ostwani 2016 involved application of Endoflas‐CF. The powder of Endoflas‐CF paste was synthesised by adding 56.5% zinc oxide, 40.6% iodoform, 1.63% barium sulphate and 1.07% calcium hydroxide, and mixed with eugenol without adding chlorophenol. Paste was inserted into the root canal using Lentulo spirals at low speed.
The 3Mix (ciprofloxacin + metronidazole + minocycline) and the ciprofloxacin + ornidazole + minocycline technique after pulpectomy involved application of ciprofloxacin, metronidazole and minocycline in the first group and ciprofloxacin, ornidazole and minocycline in the second group (Pinky 2011). After removal of the coating, the drugs were pulverised using a sterile porcelain mortar and pestle. The powdered drugs were mixed in two different combinations at a ratio of 1:3:3 and kept separately to prevent exposure to light and moisture. One increment of each powdered drug was mixed with propylene glycol to form an ointment just before use. Canal orifices were enlarged to receive the medication using a round bur, then cavities were cleaned and irrigated using saline and dried.
The pulpectomy techniques used by Prabhakar 2008 involved application of 3Mix after necrotic coronal pulp removal in one group and after removal of both necrotic coronal as well as all accessible radicular pulp tissue in the other group.
The pulpectomy technique used by Al‐Ostwani 2016 involved application of ZOP. The hydrolytic propolis of ZOP paste was extracted from raw propolis. ZOP paste was synthesised by mixing 50% zinc oxide powder with 50% hydrolytic propolis, to form a radiopaque paste with appropriate viscosity for filling the root canal. The paste was inserted into the root canal using Lentulo spirals at low speed.
The following techniques after pulpectomy were not described in sufficient detail: IRM (Arikan 2016), MTA (Celik 2013; Ozalp 2005; Subramaniam 2011), Vitapex technique (Nakornchai 2010; Ozalp 2005; Trairatvorakul 2008), 3Mix (Nakornchai 2010), Sealapex and calcium hydroxide (Ozalp 2005), RC Fill, Metapex, ZOE + Metapex techniques and Endoflas (Ramar 2010; Subramaniam 2011).
Direct pulp capping
The formocresol technique involved application of a cotton pellet soaked with formocresol on the pulp exposure for five minutes (Aminabadi 2010).
The MTA technique involved a 3:1 powder:saline ratio in Tuna 2008. In Aminabadi 2016, MTA was mixed with normal saline to form a creamy mixture delivered to the exposure site using a small amalgam carrier to reach a thickness of 1.5 to 2 mm and extending 2 mm beyond the margins of the exposure site. A wet cotton pellet was pressed slightly for better adaptation of capping material with pulp at the exposure site.
The etch‐and‐rinse adhesive technique involved application of 36% phosphoric acid gel on enamel margins for 15 seconds, followed by extending the gel application to the cavity for an additional 10 seconds with care not to contact the exposed pulp (Demir 2007).
The calcium sulphate hemihydrate technique involved application of calcium sulphate powder, mixed with three to four drops of regular‐set liquid until a putty‐like consistency was achieved, and applied with ball‐ended instruments at the exposure site (Ulusoy 2014a).
In Aminabadi 2016, 3Mix, 3Mixtatin and simvastatin were mixed with normal saline to form a creamy mixture and delivered to the exposure site using a small amalgam carrier to reach a thickness of 1.5 to 2 mm and extending 2 mm beyond the margins of the exposure site. A dry cotton pellet was pressed slightly for better adaptation of capping material with pulp at the exposure site.
The following techniques after direct pulp capping were not described in sufficient detail: EMD (Garrocho‐Rangel 2009), calcium hydroxide (Garrocho‐Rangel 2009; Tuna 2008), acetone‐based total‐etch adhesive, non‐rinse conditioner, self‐etch adhesive system (Demir 2007), calcium hydroxide cement (Dycal) (Ulusoy 2014a), CEM (Fallahinejad Ghajari 2013), Biodentine (Rajasekharan 2017), Tempophore (Rajasekharan 2017) and MTA (Fallahinejad Ghajari 2013; Rajasekharan 2017).
Intermediate restoration
Formocresol techniques were followed by placement of:
ZOE in 14 trials (16%);
ZOE and IRM in eight trials (Agamy 2004; Eidelman 2001; Farsi 2005; Fei 1991; Fernandes 2015; Fuks 1997; Holan 2005; Moretti 2008);
IRM in six trials (Ansari 2010; Dean 2002; El Meligy 2016; Huth 2005; Shabzendedar 2013; Zealand 2010);
two successive IRM temporary restorations (formocresol dressing changed after seven days, ZOE placement at third visit) (Coser 2008);
calcium hydroxide liner and glass‐ionomer cement (Markovic 2005);
Cavit (Sabbarini 2008);
ZOE and zinc phosphate cement (Sonmez 2008); and
ZOE and glass ionomer cement in two trials (Durmus 2014; Yildirim 2016).
The type of intermediate restoration after formocresol technique was not specified in two trials (Alaçam 2009; Goyal 2014).
MTA techniques were followed by placement of:
IRM in 15 trials (17%);
ZOE in nine trials (Erdem 2011; Goyal 2016; Jayam 2014; Naik 2005; Noorollahian 2008; Olatosi 2015; Sonmez 2008; Subramaniam 2009; Tuna 2008);
glass‐ionomer cement in six trials (Celik 2013; Kalra 2017; Liu 2011; Rajasekharan 2017; Uloopi 2016; Yildirim 2016);
metal‐reinforced glass ionomer cement (Arikan 2016);
reinforced glass ionomer cement (Bezgin 2016); and
ZOE and glass ionomer cement (Kusum 2015).
There was no intermediate restoration in three trials after MTA technique (Aeinehchi 2007; Fallahinejad Ghajari 2013; Saltzman 2005).
Calcium hydroxide techniques were followed by placement of:
ZOE in five trials (Aminabadi 2010; Nadkarni 2000; Niranjani 2015; Tuna 2008; Waterhouse 2000);
IRM in five trials (Coser 2008; Huth 2005; Moretti 2008; Oliveira 2013a);
ZOE and IRM (Fernandes 2015);
glass‐ionomer cement in four trials (Celik 2013; Grewal 2016; Liu 2011; Markovic 2005); and
dentine adhesive (Garrocho‐Rangel 2009).
The type of intermediate restoration after calcium hydroxide technique was not specified (Alaçam 2009), and there was no intermediate restoration in three trials (Demir 2007; Sonmez 2008; Zurn 2008).
Ferric sulphate techniques were followed by placement of:
IRM in three trials (Doyle 2010; Huth 2005; Vargas 2006);
IRM and glass ionomer cement (Cantekin 2014);
ZOE in six trials (Casas 2004; Erdem 2011; Goyal 2016; Gupta 2015; Ozmen 2017; Yadav 2014);
ZOE and glass‐ionomer cement (Durmus 2014); and
MTA followed by a layer of light‐cured glass ionomer (Nguyen 2017).
Eugenol‐free ferric sulphate was followed by placement of:
Cimpact S (Doyle 2010);
ZOE then IRM for five days over the ZOE paste for very uncooperative children (Ibricevic 2000);
calcium hydroxide and glass‐ionomer cement (Markovic 2005); and
ZOE and zinc phosphate cement (Sonmez 2008).
Ferric sulphate/MTA technique was followed by IRM (Doyle 2010).
Calcium hydroxide cement and calcium sulphate hemihydrate techniques were followed by glass‐ionomer cement restoration (Ulusoy 2014a).
Portland cement technique was followed by:
IRM in three trials (Lourenço 2015a; Oliveira 2013a; Sakai 2009); and
ZOE and glass ionomer cement (Yildirim 2016).
NaOCl techniques were followed by IRM in three trials (Fernández 2013; Shabzendedar 2013; Vargas 2006).
The techniques for 2% glutaraldehyde + eugenol + IRM and 2% glutaraldehyde + calcium hydroxide were followed by placement of compomer in Shumayrikh 1999. There was no intermediate restoration after 2% unbuffered glutaraldehyde + ZOE and 2% unbuffered glutaraldehyde + calcium hydroxide techniques in Alaçam 1989. Goyal 2016 reported use of 2% buffered glutaraldehyde followed by ZOE.
EMD technique was followed by ZOE and glass ionomer cement in Yildirim 2016. There was no intermediate restoration in Garrocho‐Rangel 2009 and Sabbarini 2008.
CEM technique was followed by IRM in Malekafzali 2011. There was no intermediate restoration after CEM in Fallahinejad Ghajari 2013 and Khorakian 2014.
Diode laser technique was followed by:
ZOE in three trials (Gupta 2015; Niranjani 2015; Yadav 2014); and
ZOE and glass ionomer cement (Durmus 2014).
Er:YAG laser technique was followed by IRM (Huth 2005).
Electrosurgery techniques were followed by placement of:
ZOE in five trials (Bahrololoomi 2008; Fishman 1996; Gupta 2015; Khorakian 2014; Yadav 2014);
IRM (Dean 2002); and
calcium hydroxide (Fishman 1996).
LLLT technique was followed by glass ionomer cement in Uloopi 2016 and by ZOE + IRM in Fernandes 2015.
Biodentine technique was followed by ZOE and glass ionomer cement in Kusum 2015, by IRM in Cuadros‐Fernández 2016, and by glass ionomer cement in Rajasekharan 2017. There was no intermediate restoration following Biodentine in three trials (El Meligy 2016; Grewal 2016; Niranjani 2015).
Propolis technique was followed by ZOE and glass ionomer cement in Kusum 2015.
Aloe vera technique was followed by a layer of collagen sponge and glass ionomer cement in Kalra 2017.
Ankaferd Blood Stopper technique was followed by IRM and glass ionomer cement in Cantekin 2014, and by ZOE in Ozmen 2017.
Tempophore was followed by glass ionomer cement in Rajasekharan 2017.
Vitapex technique was followed by IRM placement (Nakornchai 2010) and glass‐ionomer cement (Pramila 2016). There was no intermediate restoration in three trials (Mortazavi 2004; Ozalp 2005; Trairatvorakul 2008).
Metapex technique was followed by ZOE placement in Ramar 2010, ZOE and glass ionomer (Miracle mix) (Subramaniam 2011), and glass ionomer (Al‐Ostwani 2016).
ZOE + Metapex and RC Fill techniques were followed by placement of ZOE (Ramar 2010).
Endoflas technique was followed by placement of ZOE and glass ionomer (Miracle mix) (Subramaniam 2011), ZOE (Rewal 2014), and glass ionomer (Al‐Ostwani 2016).
ZOE technique was followed by glass ionomer in four trials (Al‐Ostwani 2016; Nguyen 2017; Pramila 2016; Subramaniam 2011), thick ZOE paste (Rewal 2014), and no intermediate restoration in six trials (Chandra 2014; Erdem 2011; Mortazavi 2004; Nadkarni 2000; Ozalp 2005; Trairatvorakul 2008).
IRM technique was followed by placement of metal‐reinforced glass ionomer cement (Arikan 2016).
The type of intermediate restoration after calcium hydroxide/iodoform techniques was not specified in Alaçam 2009.
3Mix and ciprofloxacin + ornidazole + minocycline techniques were followed by ZOE or IRM in three trials (Aminabadi 2016; Nakornchai 2010; Pinky 2011).
3Mixtatin and simvastatin were followed by IRM in Aminabadi 2016.
The gutta‐percha/AH‐Plus technique was followed by reinforced glass ionomer cement in Bezgin 2016.
There was no intermediate restoration following ozonated sesame oil‐ZO technique in Chandra 2014.
ZOP technique was followed by glass ionomer in Al‐Ostwani 2016.
There was no intermediate restoration after ZOE technique (Casas 2004), acetone‐based total‐etch adhesive (Demir 2007), Sealapex technique (Ozalp 2005), calcium hydroxide technique (Ozalp 2005), and antibacterial mix technique (Prabhakar 2008).
Final restoration
Final restorations after placement of formocresol were:
stainless‐steel crown in 18 (21%) trials;
amalgam in four trials (Bahrololoomi 2008; Erdem 2011; Markovic 2005; Sonmez 2008);
glass‐ionomer cement and stainless‐steel crown in three trials (Sabbarini 2008; Saltzman 2005; Subramaniam 2009);
amalgam or stainless‐steel crown in three trials (Ansari 2010; Ibricevic 2000; Ozmen 2017);
amalgam or glass‐ionomer cement (Aeinehchi 2007);
glass‐ionomer cement or stainless‐steel crown if the restoration was not satisfactory (Coser 2008);
composite or stainless‐steel crown (Holan 2005);
glass‐ionomer cement or composite or stainless‐steel crown (Huth 2005);
glass‐ionomer cement in two trials (Fernandes 2015; Moretti 2008);
stainless steel crown and/or glass ionomer restoration and silver amalgam (Jayam 2014);
glass‐ionomer cement or composite or amalgam, and stainless‐steel crown if indicated (Waterhouse 2000).
Final restoration after placement of formocresol was not mentioned in Alaçam 1989.
Final restorations after placement of MTA were:
stainless‐steel crown in 21 trials (24%);
amalgam in five trials (Aeinehchi 2007; Celik 2013; Fallahinejad Ghajari 2013; Sonmez 2008; Tuna 2008), followed by a light‐cured fissure sealant material in one trial (Celik 2013);
glass‐ionomer cement in two trials (Moretti 2008; Sakai 2009);
resin modified glass ionomer cement (Oliveira 2013a);
composite resin in two trials (Bezgin 2016; Liu 2011);
composite resin or amalgam or stainless (Holan 2005);
amalgam or stainless‐steel crown (Malekafzali 2011);
glass ionomer and amalgam (Aminabadi 2016);
stainless steel crown and/or glass ionomer restoration and silver amalgam (Jayam 2014);
glass‐ionomer cement and stainless‐steel crown (Subramaniam 2009).
Final restorations after placement of calcium hydroxide were:
stainless‐steel crown in four trials (Alaçam 2009; Aminabadi 2010; Garrocho‐Rangel 2009; Nadkarni 2000);
amalgam in four trials (Celik 2013; Demir 2007; Markovic 2005; Tuna 2008) followed by a light‐cured fissure sealant material (Celik 2013);
glass‐ionomer cement or stainless‐steel crown if the restoration was not satisfactory (Coser 2008);
glass‐ionomer cement or composite or stainless‐steel crown (Huth 2005);
glass‐ionomer cement in two trials (Fernandes 2015; Moretti 2008);
resin modified glass ionomer cement (Oliveira 2013a);
glass‐ionomer cement and amalgam (Sonmez 2008);
glass‐ionomer cement or composite or amalgam, and stainless‐steel crown if indicated (Waterhouse 2000);
glass‐ionomer cement and stainless‐steel crown (Zurn 2008)
composite resin (Liu 2011); and
nanohybrid composite resin (Grewal 2016).
Final restorations after placement of ferric sulphate were:
stainless‐steel crown in eight trials (Cantekin 2014; Doyle 2010; Durmus 2014; Fei 1991; Fuks 1997; Goyal 2016; Gupta 2015; Vargas 2006);
amalgam in three trials (Erdem 2011; Markovic 2005; Sonmez 2008);
amalgam or stainless‐steel crown in three trials (Casas 2004; Ibricevic 2000; Ozmen 2017);
glass‐ionomer cement (Yadav 2014);
acid etch resin (Nguyen 2017); and
glass‐ionomer cement or composite or stainless‐steel crown (Huth 2005).
Final restorations after placement of ZOE were amalgam in three trials (Erdem 2011; Mortazavi 2004; Ozalp 2005), stainless‐steel crown in four trials (Nadkarni 2000; Rewal 2014; Subramaniam 2011; Trairatvorakul 2008) and acid‐etch resin (Nguyen 2017).
Final restoration after placement of 2% glutaraldehyde + eugenol + IRM and 2% glutaraldehyde + calcium hydroxide techniques was stainless‐steel crown in one trial (Shumayrikh 1999). Final restoration after placement of 2% unbuffered glutaraldehyde + ZOE and 2% unbuffered glutaraldehyde + calcium hydroxide was not mentioned in one trial (Alaçam 1989). Final restoration after placement of 2% buffered glutaraldehyde was stainless steel crown in one trial (Goyal 2016).
Final restoration after diode laser was stainless steel crown in three trials (Durmus 2014; Gupta 2015; Niranjani 2015) and glass‐ionomer cement in one trial (Yadav 2014).
Final restorations after electrosurgery were stainless‐steel crowns in four trials (Dean 2002; Fishman 1996; Gupta 2015; Khorakian 2014), amalgam in one trial (Bahrololoomi 2008) and glass‐ionomer cement in one trial (Yadav 2014).
Final restoration after placement of acetone‐based total‐etch adhesive, acetone‐based total‐etch adhesive, total‐etching with 36% phosphoric acid and self etch adhesive system was composite in one trial (Demir 2007).
Final restorations after placement of EMD were stainless‐steel crowns in two trials (Garrocho‐Rangel 2009; Yildirim 2016), and glass‐ionomer cement and stainless‐steel crowns in one other trial (Sabbarini 2008).
Final restorations after Er:YAG laser were glass‐ionomer cement and composite or stainless‐steel crowns in one trial (Huth 2005).
Final restoration after LLLT was stainless‐steel crown in one trial (Uloopi 2016) and glass ionomer cement in one trial (Fernandes 2015).
Final restorations after placement of CEM were amalgam or stainless‐steel crowns in one trial (Malekafzali 2011), amalgam in one trial (Fallahinejad Ghajari 2013), and stainless steel crown in one trial (Khorakian 2014).
Final restoration after placement of Portland cement was:
glass‐ionomer cement in two trials (Lourenço 2015a; Sakai 2009);
resin‐modified glass‐ionomer cement in one trial (Oliveira 2013a); and
stainless steel crown in one trial (Yildirim 2016)
Final restoration after placement of calcium hydroxide cement and calcium sulphate hemihydrate was amalgam followed by a light‐cured fissure sealant material in one trial (Ulusoy 2014a).
Final restoration after placement of Biodentine was stainless steel crown in five trials (Cuadros‐Fernández 2016; El Meligy 2016; Kusum 2015; Niranjani 2015; Rajasekharan 2017) and nanohybrid composite resin in one trial (Grewal 2016).
Final restoration was stainless steel crown after placement of propolis (Kusum 2015), Tempophore (Rajasekharan 2017) and aloe vera (Kalra 2017).
Final restoration after Ankaferd Blood Stopper was stainless steel crown in one trial (Cantekin 2014), and amalgam or stainless steel crown in one trial (Ozmen 2017).
Final restorations after placement of Vitapex or Metapex were:
amalgams in two trials (Mortazavi 2004; Ozalp 2005);
glass‐ionomer cement and stainless‐steel crowns in one trial (Nakornchai 2010); and
stainless‐steel crowns in five trials (Al‐Ostwani 2016; Pramila 2016; Ramar 2010; Subramaniam 2011; Trairatvorakul 2008).
Final restorations after placement of 3Mix were glass‐ionomer cement and stainless‐steel crowns in two trials (Nakornchai 2010; Pinky 2011), and glass ionomer and amalgam in one trial (Aminabadi 2016).
Final restoration after placement of 3Mixtatin and simvastatin were glass ionomer and amalgam in one trial (Aminabadi 2016).
Final restoration after placement of Sealapex or calcium hydroxide was amalgam in one trial (Ozalp 2005).
Final restorations after placement of ciprofloxacin + metronidazole + minocycline and ciprofloxacin + ornidazole + minocycline were glass‐ionomer cement and stainless‐steel crowns in one trial (Pinky 2011).
Final restorations after antibacterial mix technique were made of glass‐ionomer cement and composite resin in one trial (Prabhakar 2008).
Final restoration after gutta‐percha/AH‐Plus technique was resin composite in one trial (Bezgin 2016).
Final restoration after placement of calcium hydroxide/iodoform (Alaçam 2009), ZOE (Al‐Ostwani 2016; Casas 2004; Chandra 2014; Pramila 2016), IRM (Arikan 2016), ozonated sesame oil‐ZO (Chandra 2014), ferric sulphate: MTA (Doyle 2010), RC Fill (Pramila 2016; Ramar 2010), ZOE + Metapex (Ramar 2010), Endoflas or Endoflas‐CF (Al‐Ostwani 2016; Rewal 2014; Subramaniam 2011), ZOP (Al‐Ostwani 2016) and 3% or 5% NaOCl (Fernández 2013; Shabzendedar 2013; Vargas 2006) was stainless‐steel crown in 10 trials.
Excluded studies
We excluded 55 studies: 30 were not RCTs, 14 had only an abstract, with insufficient information and no response from authors; in 2 articles biomaterials were not compared; 4 articles were reviews, 2 were case reports and 1 was a terminated trial; 1 was an in vitro study in dogs and humans, 1 focused on restorative dentistry, and 1 was a duplicate.
Risk of bias in included studies
Summary details are given in the Characteristics of included studies table and Figure 2.
2.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Random sequence generation
The risk of selection bias with regard to sequence generation was low in 37 trials (43%). The sequence was generated by random number tables or computerised random‐number generators in 24 trials (28%) and coin toss in 13 trials (15%).
One included trial described an alternate allocation (Ibricevic 2000), and was judged to be at high risk of bias.
There was insufficient information to make a clear judgement about risk of bias in 49 trials (56%).
Allocation concealment
Allocation concealment was applied for eight included studies (Celik 2013; Fishman 1996; Garrocho‐Rangel 2009; Huth 2005; Kalra 2017; Pramila 2016; Vargas 2006; Zealand 2010), which were assessed as being at low risk of bias for this domain. In the other 79 trials (91%), allocation concealment was unclear.
Blinding
Blinding of participants and personnel
Participants and personnel were blinded in five trials (Fallahinejad Ghajari 2013; Garrocho‐Rangel 2009; Khorakian 2014; Pramila 2016; Shumayrikh 1999). Blinding of participants and personnel was unclear in 82 included trials (94%).
Blinding of clinical outcomes assessment
The children were examined clinically by examiners blinded to the technique in 45 trials (50%). Children were examined clinically by examiners who were not blinded to the treatment in four trials (Eidelman 2001; Holan 2005; Ibricevic 2000; Saltzman 2005). There was insufficient information to make a clear judgement of blinding in 38 trials (44%).
Blinding of radiological outcomes assessment
The children were examined radiographically by examiners blinded to the technique in 51 trials (59%). Children were examined radiographically by examiners who were not blinded to the treatment in two trials (Bezgin 2016; Nguyen 2017). There was insufficient information to make a clear judgement on radiological blinding in 34 trials (39%).
Incomplete outcome data
The risk of bias for incomplete outcome data was low for 53 trials (61%): 32 trials (37%) had no missing data, the proportion of missing outcomes was lower than 10% of children or teeth randomly assigned for 18 trials (21%), and missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups in three trials (Aminabadi 2016; Cuadros‐Fernández 2016; Rajasekharan 2017).
The proportion of missing outcomes was higher than 10% of children or teeth randomly assigned for 31 trials (36%)
There was insufficient information pertaining to attrition/exclusion in three trials (Akcay 2014; Coser 2008; Waterhouse 2000).
Selective reporting
We assessed two trials as being at low risk of reporting bias (Cuadros‐Fernández 2016; Pramila 2016). We did not have access to 97% of trial protocols, so we judged these trials to be at unclear risk of bias for the selective outcome reporting. We judged Rajasekharan 2017 to be at high risk of bias.
Overall risk of bias
The overall risk of bias was high in 36 trials (41%) (Aeinehchi 2007; Agamy 2004; Ansari 2010; Bezgin 2016; Casas 2004; Doyle 2010; Eidelman 2001; Farsi 2005; Fei 1991; Fernández 2013; Goyal 2014; Goyal 2016; Grewal 2016; Holan 2005; Ibricevic 2000; Jayam 2014; Kalra 2017; Kang 2015; Khorakian 2014; Liu 2011; Malekafzali 2011; Mortazavi 2004; Nguyen 2017; Niranjani 2015; Noorollahian 2008; Oliveira 2013a; Pramila 2016; Rajasekharan 2017; Sakai 2009; Saltzman 2005; Shabzendedar 2013; Sonmez 2008; Tuna 2008; Vargas 2006; Zealand 2010; Zurn 2008).
For the other 51 trials (59%), the risk of bias was unclear, frequently due to lack of information about allocation concealment and blinding of participants and staff.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Pulpotomy compared with pulpotomy using alternative medicament/technique for extensive decay in primary teeth.
| Pulpotomy compared with pulpotomy using alternative medicament/technique for extensive decay in primary teeth | ||||||
|
Population: children with extensive decay in primary teeth Settings: primary care Intervention: pulpotomy with one type of medicament Comparison: pulpotomy using alternative medicament or different technique | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Experimental | |||||
| MTA versus formocresol | ||||||
|
Clinical failure (12 months) |
28 per 1000 | 8.6 per 1000 (2.8 per 1000 to 26.0 per 1000) | RR 0.31 (0.10 to 0.93) | 740 (12 studies) |
⊕⊕⊕⊝ moderate1 | Failure rate less than 3% across both the MTA and formocresol treatment groups. Seven of the 12 studies had no failures at 12 months. No evidence of a difference in clinical failure at 6 months or 24 months |
|
Radiological failure (12 months) |
50 per 1000 | 20.5 per 1000 (9.5 per 1000 to 44.5 per 1000) | RR 0.41 (0.19 to 0.89) | 740 (12 studies) | ⊕⊕⊕⊝ moderate1 | Failure rate 5% across formocresol treatment groups and 2.1% across MTA treatment groups. Five of the 12 studies had no failures at 12 months. Results similar at 6 and 24 months |
| MTA versus calcium hydroxide | ||||||
| Clinical failure (12 months) | 14 per 1000 | 2.2 per 1000 (0.02 per 1000 to 9.8 per 1000) | RR 0.16 (0.04 to 0.70) | 150 (4 studies) | ⊕⊕⊕⊝ moderate1 | Results similar at 24 months. No evidence of a difference in clinical failure at 6 months |
|
Radiological failure (12 months) |
351 per 1000 | 42.1 per 1000 (14 per 1000 to 126.4 per 1000) | RR 0.12 (0.04 to 0.36) | 150 (4 studies) | ⊕⊕⊝⊝ low2 | Results similar at 6 and 24 months |
| Calcium hydroxide versus formocresol | ||||||
| Clinical failure (12 months) | 115 per 1000 | 215 per 1000 (140.3 per 1000 to 332.4 per 1000) | RR 1.87 (1.22 to 2.89) | 332 (6 studies) | ⊕⊕⊕⊝ moderate1 | Results similar at 6 months No evidence of a difference in clinical failure at 24 months |
| Radiological failure (12 months) | 253 per 1000 | 470.6 per 1000 (359.3 per 1000 to 617.3 per 1000) | RR 1.86 (1.42 to 2.44) | 332 (6 studies) | ⊕⊕⊕⊝ moderate1 | Results similar at 6 and 24 months |
| Other comparisons assessed in more than one trial that had treatment failures | ||||||
| Clinical failure (at six, 12 and 24 months) | The quality of the evidence waslow for 4 comparisons3: laser versus ferric sulphate; Biodentine versus MTA; ferric sulphate versus formocresol; electrosurgery versus ferric sulphate; calcium hydroxide versus ferric sulphate. The quality of the evidence was very low for 5 comparisons: NaOCl versus ferric sulphate4; laser versus electrosurgery4; MTA versus ferric sulphate5; ABS versus ferric sulphate6; EMD versus formocresol7. |
|||||
| Radiological failure (at six, 12 and 24 months) | The quality of the evidence waslow for 8 comparisons: NaOCl versus ferric sulphate2; MTA versus ferric sulphate3; Biodentine versus MTA3; ferric sulphate versus formocresol3; laser versus ferric sulphate3; electrosurgery versus ferric sulphate3; ABS versus ferric sulphate3; laser versus electrosurgery3; calcium hydroxide versus ferric sulphate (favouring ferric sulphate)3. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. Downgraded 1 level due to high risk of bias 2. Downgraded 1 level due to high risk of bias and 1 level due to substantial inconsistency 3. Downgraded 1 level due to high risk of bias and 1 level due to imprecision 4. Downgraded 1 level due to high risk of bias and 2 levels due to imprecision 5. Downgraded 1 level due to high risk of bias, 1 level due to moderate inconsistency and 1 level due to imprecision 6. Downgraded 1 level due to high risk of bias and 2 levels due to very serious imprecision 7. Downgraded 1 level due to high risk of bias, 1 level due to substantial inconsistency and 1 level due to imprecision
Summary of findings 2. Pulpectomy compared with pulpectomy using alternative medicament for extensive decay in primary teeth.
| Pulpectomy compared with pulpectomy using alternative medicament for extensive decay in primary teeth | ||||||
|
Population: children with extensive decay in primary teeth Settings: primary care Intervention: pulpectomy with 1 type of medicament Comparison: pulpectomy using alternative medicament | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Experimental | |||||
| Endoflas versus ZOE | ||||||
| Clinical failure (6 months) | 128 per 1000 | 33.3 per 1000 (6.4 per 1000 to 192 per 1000) | RR 0.26 (0.05 to 1.50) | 80 (2 studies) | ⊕⊕⊕⊝ moderate1 | One trial assessed failure at 12 months: RR 1.00, 95% 0.07 to 14.55 |
| Radiological failure (6 months) | 128 per 1000 | 33.3 per 1000 (6.4 per 1000 to 192 per 1000) | RR 0.26 (0.05 to 1.50) | 80 (2 studies) | ⊕⊕⊕⊝ moderate1 | |
| Metapex versus ZOE | ||||||
| Clinical failure (12 months) | 97 per 1000 | 68.9 per 1000 (14.6 per 1000 to 323 per 1000) | RR 0.71 (0.15 to 3.33) | 62 (2 studies) | ⊕⊕⊕⊝ moderate1 | Results similar at 6 months |
| Radiological failure (12 months) | 129 per 1000 | 129 per 1000 (40 per 1000 to 421.8 per 1000) | RR 1.00 (0.31 to 3.27) | 62 (2 studies) | ⊕⊕⊕⊝ moderate1 | Results similar at 6 months |
| Other comparisons assessed in more than one trial that had treatment failures | ||||||
| Clinical failure | The quality of the evidence was rated as low for 1 comparison: Vitapex versus ZOE (favouring ZOE)2 | |||||
| Radiological failure | The quality of the evidence was rated as low for 2 comparisons: Vitapex versus ZOE2 (favouring ZOE); calcium hydroxide versus ZOE3 | |||||
1. Downgraded 1 level due to imprecision 2. Downgraded 2 levels due to very substantial inconsistency 3. Downgraded 1 level due to substantial inconsistency and 1 level due to imprecision
Summary of findings 3. Direct pulp capping compared with direct pulp capping using alternative medicament for extensive decay in primary teeth.
| Direct pulp capping compared with direct pulp capping using alternative medicament for extensive decay in primary teeth | ||||||
|
Population: children with extensive decay in primary teeth Settings: primary care Intervention: direct pulp capping with 1 type of medicament Comparison: direct pulp capping using alternative medicament | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Experimental | |||||
| Seven trials evaluated 22 comparisons of different medicaments for direct pulp capping. Each comparison was assessed by a single trial. There were no clinical or radiological failures in two comparisons: acetone‐based total‐etch adhesive versus calcium hydroxide; MTA versus calcium hydroxide. | ||||||
| Clinical failure (at six, 12 and 24 months) | The quality of the evidence was assessed as low for 5 comparisons1: calcium hydroxide versus formocresol (favouring formocrescol), MTA versus 3Mix and MTA versus simvastatin (favouring MTA), 3Mix versus 3Mixtatin and 3Mixtatin versus simvastatin (favouring 3Mixtatin). The quality of the evidence was rated as very low for all other comparisons.2 |
|||||
| Radiological failure (at six, 12 and 24 months) | The quality of the evidence was rated as low for 1 comparison: calcium hydroxide versus formocresol1 (favouring formocresol). The quality of the evidence was rated as very low for all other comparisons.2 |
|||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. Downgraded 1 level due to risk of bias and 1 level due to imprecision 2. Downgraded 1 level due to risk of bias and 2 levels due to severe imprecision
We identified 19 trials for the comparison of MTA and formocresol, six for the comparison of MTA and calcium hydroxide, five for the comparison of MTA and ferric sulphate, three for the comparison of MTA and Portland cement, four for the comparison of MTA and Biodentine, eight for the comparison of calcium hydroxide and formocresol, three for the comparison of calcium hydroxide and ferric sulphate, 10 for the comparison of ferric sulphate and formocresol, two for the comparison of NaOCl and ferric sulphate, three for the comparison of diode laser and ferric sulphate, two for the comparison of electrosurgery and ferric sulphate, two for the comparison of ferric sulphate and Ankaferd Blood Stopper, two for the comparison of glutaraldehyde + calcium hydroxide versus glutaraldehyde + ZOE, two for the comparison of diode laser and electrosurgery, two for the comparison of NaOCl and formocresol, two for the comparison of EMD and formocresol, two for the comparison of calcium hydroxide and ZOE, two for the comparison of Metapex and ZOE, two for the comparison of Metapex and Endoflas, five for the comparison of Vitapex and ZOE, and two for the comparison of Endoflas and ZOE. Two trials compared two different types of MTA (Agamy 2004; Celik 2013); we combined data for these two arms as prespecified. All other comparisons were addressed by only one trial each. Overall, only 59 of 87 trials (68%) were included in meta‐analyses.
Pulpotomy versus pulpotomy
We included 53 trials that compared pulpotomy using different types of medicaments. We assessed that 28 (32%) trials were at high risk of bias, and for 25 (29%) other trials, the risk of bias was unclear.
MTA versus full strength or 1:5 diluted formocresol
Clinical failure
At six months, data were extractable from 13 RCTs totaling 1048 teeth. In 10 of the 13 trials, there was no clinical failure in any of the participants regardless of the intervention. From the three remaining trials (N = 394 participants), the pooled results showed no statistically significant difference in clinical failure with MTA compared with formocresol. The pooled risk ratio (RR) was 0.37 (95% confidence interval (CI) 0.07 to 1.89). At 12 months, data were extractable from 12 RCTs totaling 740 teeth. In seven of the 12 trials, there was no clinical failure in any of the participants regardless of the intervention. From the five remaining trials, the results showed a statistically significant difference (RR 0.31, 95% CI 0.10 to 0.93) with no evidence of statistical heterogeneity among included trials (I² = 0%). At 24 months, data were extractable from nine RCTs totaling 548 teeth. In five of the nine trials, there was no clinical failure in any of the participants regardless of the intervention. From the four remaining trials, the results showed no statistically significant difference (RR 0.47, 95% CI 0.18 to 1.19; Analysis 1.1).
1.1. Analysis.
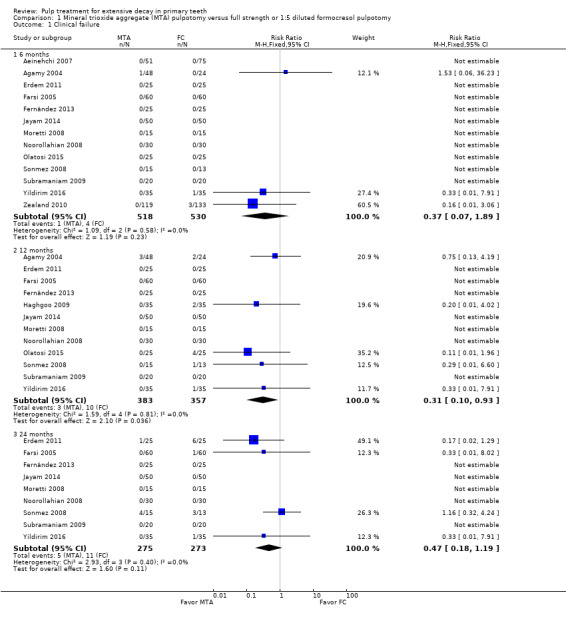
Comparison 1 Mineral trioxide aggregate (MTA) pulpotomy versus full strength or 1:5 diluted formocresol pulpotomy, Outcome 1 Clinical failure.
The results showed no statistically significant difference at any time point in clinical failure when full strength formocresol and 1:5 diluted formocresol results were not pooled (Analysis 2.1; Analysis 3.1).
2.1. Analysis.
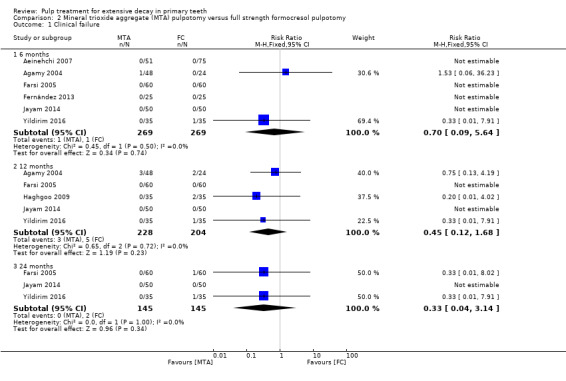
Comparison 2 Mineral trioxide aggregate (MTA) pulpotomy versus full strength formocresol pulpotomy, Outcome 1 Clinical failure.
3.1. Analysis.
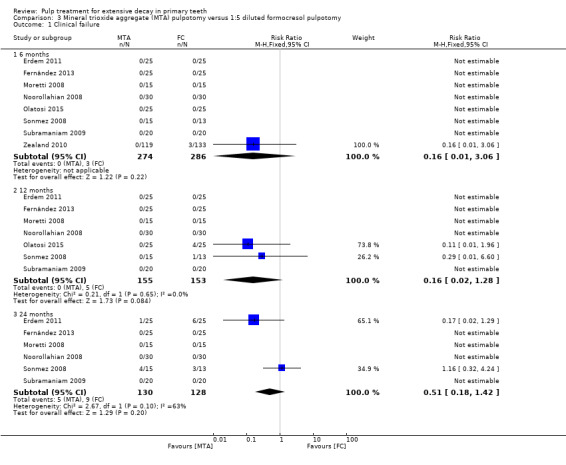
Comparison 3 Mineral trioxide aggregate (MTA) pulpotomy versus 1:5 diluted formocresol pulpotomy, Outcome 1 Clinical failure.
Radiological failure
At six months, data were extractable from 12 RCTs totaling 922 teeth. In eight of the 12 trials, there was no radiological failure in any participants regardless of the intervention. From the four remaining trials, the pooled results showed a statistically significant difference in radiological failure with MTA compared with formocresol. The pooled RR was 0.38 (95% CI 0.17 to 0.86) with no evidence of statistical heterogeneity among included trials (I² = 0%). At 12 months, results were similar with seven trials providing data for a pooled RR of 0.41 (95% CI 0.19 to 0.89) with no evidence of statistical heterogeneity among included trials (I² = 0%). At 24 months, data were extractable from nine RCTs totaling 548 teeth, with eight trials providing data. The results showed a statistically significant difference (RR 0.42, 95% CI 0.22 to 0.80) with no evidence of statistical heterogeneity among included trials (I² = 18%; Analysis 1.2).
1.2. Analysis.
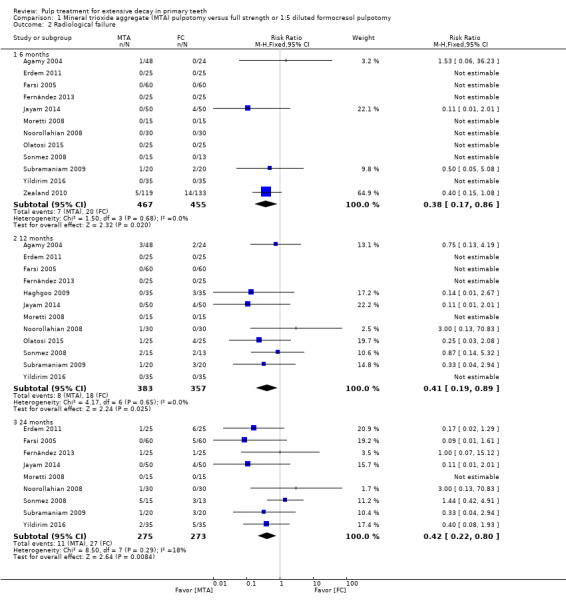
Comparison 1 Mineral trioxide aggregate (MTA) pulpotomy versus full strength or 1:5 diluted formocresol pulpotomy, Outcome 2 Radiological failure.
The results showed a statistically significant difference at 12 and 24 months in radiological failure for MTA compared with full strength formocresol (Analysis 2.2) (no statistically significant difference at 6 months); the results showed no statistically significant difference at any point in radiological failure for MTA compared with 1:5 diluted formocresol (Analysis 3.2).
2.2. Analysis.
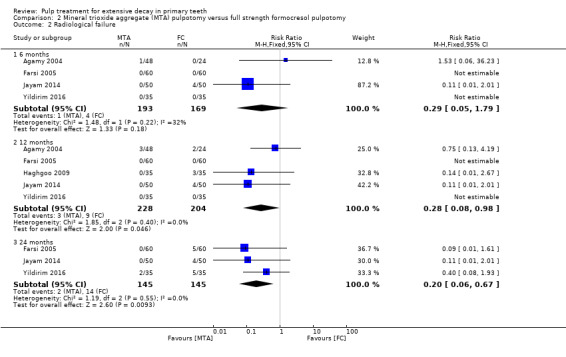
Comparison 2 Mineral trioxide aggregate (MTA) pulpotomy versus full strength formocresol pulpotomy, Outcome 2 Radiological failure.
3.2. Analysis.
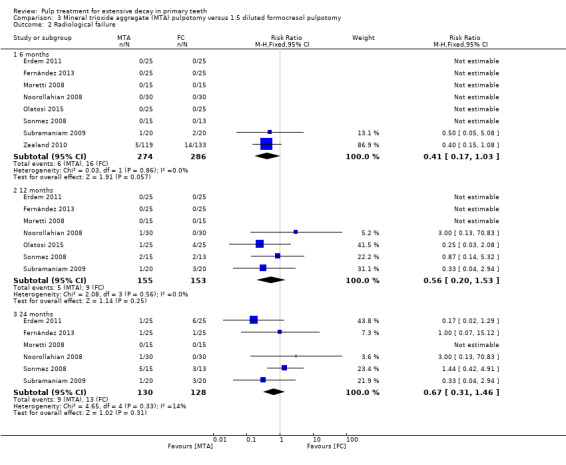
Comparison 3 Mineral trioxide aggregate (MTA) pulpotomy versus 1:5 diluted formocresol pulpotomy, Outcome 2 Radiological failure.
Overall failure
At six months, data were extractable from six RCTs totaling 328 teeth. In four of the six trials, there was no overall failure in any of the participants regardless of the intervention. From the two remaining trials, the results showed no statistically significant difference in overall failure with MTA compared with formocresol (RR 0.23, 95% CI 0.04 to 1.32). Results were similar at 12 months with four trials providing data for an overall pooled RR of 0.48 (96% CI 0.17 to 1.36). At 24 months, data were extractable from seven RCTs totaling 368 teeth, with all seven trials providing data for a pooled RR of 0.50 (95% CI 0.25 to 1.01; Analysis 1.3).
1.3. Analysis.
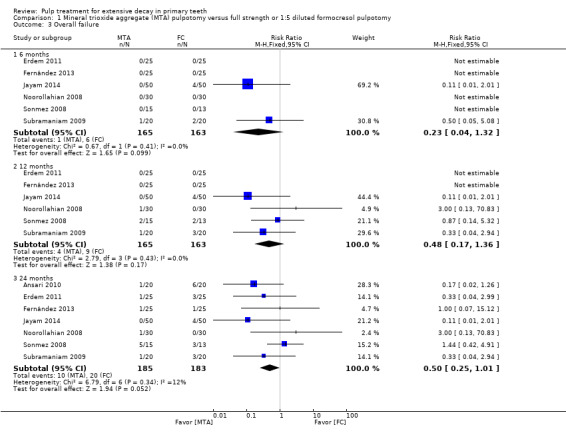
Comparison 1 Mineral trioxide aggregate (MTA) pulpotomy versus full strength or 1:5 diluted formocresol pulpotomy, Outcome 3 Overall failure.
Five of six included trials compared MTA with 1:5 diluted formocresol. One compared MTA with full strength formocresol with no statistically significant difference (Jayam 2014).
Two additional trials, which randomised 32 (Eidelman 2001) and 64 teeth (Holan 2005), did not assess overall failure at a fixed time point but at a mean (range) follow‐up of 13 (6 to 31) and 36 (4 to 74) months, respectively. The RRs were 0.30 (95% CI 0.01 to 6.77) for Eidelman 2001 and 0.19 (95% CI 0.02 to 1.52) for Holan 2005.
Pain
At six months, data were extractable from six RCTs totaling 390 teeth. In five trials, there was no pain in any of the participants regardless of the intervention. From the remaining trial, the results showed no statistically significant difference (RR 0.33, 95% CI 0.01 to 7.91). Results were similar at 12 months, with two trials providing data for a pooled RR of 0.25 (95% CI 0.03 to 2.18). At 24 months, data were extractable from four RCTs totaling 290 teeth. In one of the four trials, there was no pain in any of the participants regardless of the intervention. For the three remaining trials, the pooled results showed no statistically significant difference in pain with MTA compared with formocresol (RR 0.71, 95% CI 0.14 to 3.56; Analysis 1.4).
1.4. Analysis.
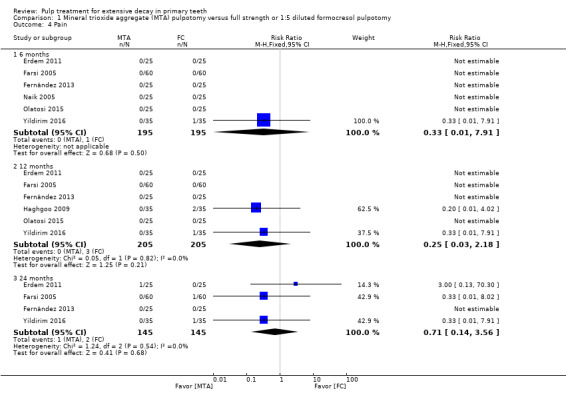
Comparison 1 Mineral trioxide aggregate (MTA) pulpotomy versus full strength or 1:5 diluted formocresol pulpotomy, Outcome 4 Pain.
The results showed no statistically significant difference at any point in pain for MTA compared with full strength formocresol or 1:5 diluted formocresol (Analysis 2.3; Analysis 3.3).
2.3. Analysis.
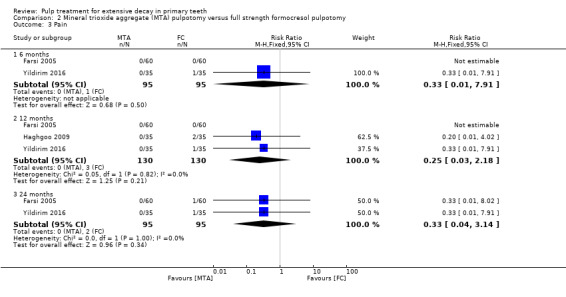
Comparison 2 Mineral trioxide aggregate (MTA) pulpotomy versus full strength formocresol pulpotomy, Outcome 3 Pain.
3.3. Analysis.
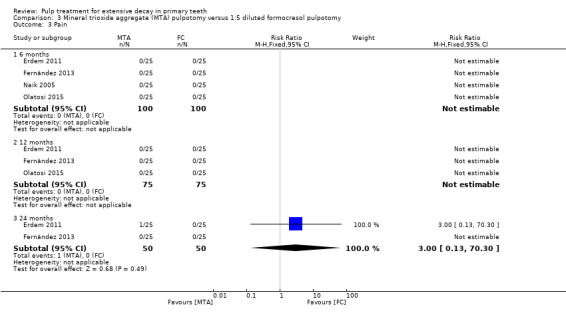
Comparison 3 Mineral trioxide aggregate (MTA) pulpotomy versus 1:5 diluted formocresol pulpotomy, Outcome 3 Pain.
One trial, which randomised 32 teeth, did not assess pain at a fixed time point but at a mean (range) follow‐up of 13 (6 to 31) (Eidelman 2001). There was no pain in any of the participants regardless of the intervention.
Soft tissue pathology
At six months, data were extractable from seven RCTs totaling 410 teeth. In six trials, there was no soft tissue pathology in any participants regardless of the intervention. For the remaining trial, the results showed no statistically significant difference (RR 0.33, 95% CI 0.01 to 7.91). At 12 months, in three trials, there was no soft tissue pathology in any participants regardless of the intervention. Of the remaining four trials, results showed no statistically significant difference (RR 0.22, 95% CI 0.05 to 1.01). At 24 months, data were extractable from five RCTs totaling 310 teeth, with two trials providing data for a pooled RR of 0.33 (95% CI 0.04 to 3.10; Analysis 1.5).
1.5. Analysis.
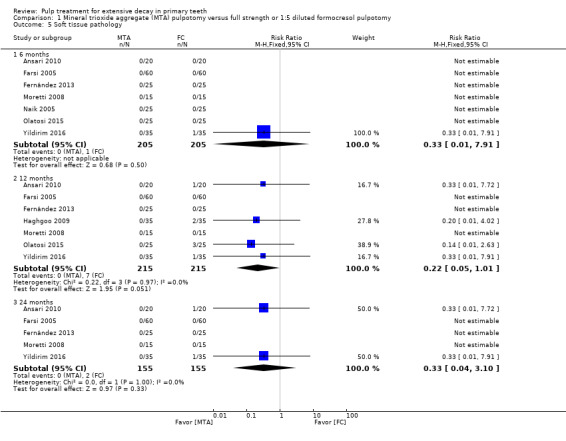
Comparison 1 Mineral trioxide aggregate (MTA) pulpotomy versus full strength or 1:5 diluted formocresol pulpotomy, Outcome 5 Soft tissue pathology.
The results showed no statistically significant difference at any time point in soft tissue pathology for MTA compared either with full strength formocresol or 1:5 diluted formocresol (Analysis 2.4; Analysis 3.4).
2.4. Analysis.
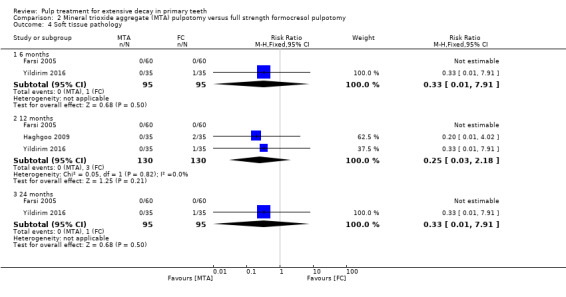
Comparison 2 Mineral trioxide aggregate (MTA) pulpotomy versus full strength formocresol pulpotomy, Outcome 4 Soft tissue pathology.
3.4. Analysis.
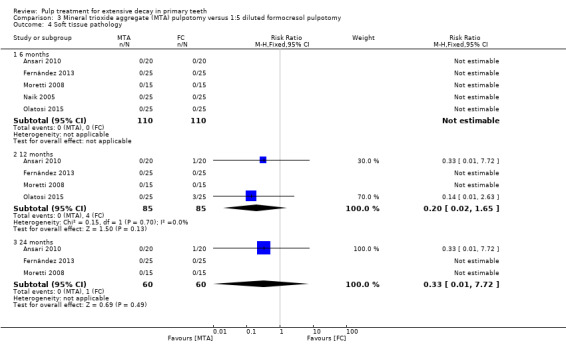
Comparison 3 Mineral trioxide aggregate (MTA) pulpotomy versus 1:5 diluted formocresol pulpotomy, Outcome 4 Soft tissue pathology.
In addition, two trials, which randomised 32 (Eidelman 2001) and 64 (Holan 2005) teeth, did not assess soft tissue pathology at a fixed time point but at a mean (range) follow‐up of 13 (6 to 31) for Eidelman 2001 and 38 (4 to 74) months for Holan 2005. There was no soft tissue pathology in any of the participants regardless of the intervention in Eidelman 2001, and for Holan 2005, the RR was 0.94 (95% CI 0.06 to 14.4).
Pathological mobility
At six months, data were extractable from five RCTs totaling 250 teeth. In both trials, there was no pathological mobility in any of the participants regardless of the intervention. At 12 months, data were extractable from four RCTs totaling 200 teeth. For three trials, there was no pathological mobility in any of the participants regardless of the intervention. Results from the remaining trial showed no statistically significant difference (RR 0.20, 95% CI 0.01 to 3.97). At 24 months, data were extractable from three RCTs totaling 150 teeth. In both trials, there was no pathological mobility in any of the participants regardless of the intervention (Analysis 1.6).
1.6. Analysis.
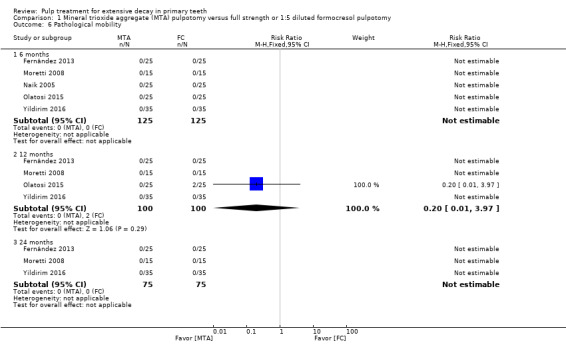
Comparison 1 Mineral trioxide aggregate (MTA) pulpotomy versus full strength or 1:5 diluted formocresol pulpotomy, Outcome 6 Pathological mobility.
The results showed no statistically significant difference at any time point in pathological mobility between MTA and either full strength formocresol or 1:5 diluted formocresol (Analysis 2.5; Analysis 3.5).
2.5. Analysis.
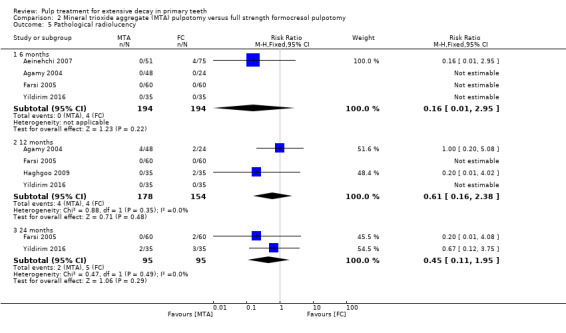
Comparison 2 Mineral trioxide aggregate (MTA) pulpotomy versus full strength formocresol pulpotomy, Outcome 5 Pathological radiolucency.
3.5. Analysis.
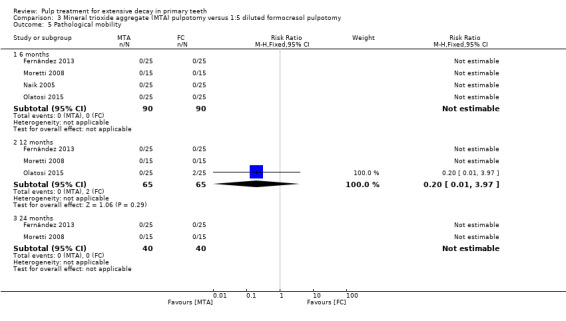
Comparison 3 Mineral trioxide aggregate (MTA) pulpotomy versus 1:5 diluted formocresol pulpotomy, Outcome 5 Pathological mobility.
Pathological radiolucency
At six months, data were extractable from 13 RCTs totaling 1010 teeth. In nine of the 13 trials, there was no pathological radiolucency in any of the participants regardless of the intervention. From the four remaining trials, the pooled results showed no evidence of a statistically significant difference in pathological radiolucency with MTA compared with formocresol (pooled RR 0.54, 95% CI 0.27 to 1.08). At 12 months, data were extractable from 11 RCTs totaling 652 teeth. In five of the 11 trials, there was no pathological radiolucency in any participants regardless of the intervention. From the six remaining trials, the pooled results showed evidence of a statistically significant difference in pathological radiolucency between MTA and formocresol (pooled RR 0.43, 95% CI 0.19 to 0.98) with no evidence of statistical heterogeneity among included trials (I² = 0%). At 24 months, data were extractable from eight RCTs totaling 460 teeth. In two of the eight trials, there was no pathological radiolucency in any participants regardless of the intervention. From the six remaining trials, the pooled results showed no evidence of a statistically significant difference in pathological radiolucency with MTA compared with formocresol (pooled RR 0.55, 95% CI 0.25 to 1.22; Analysis 1.7).
1.7. Analysis.
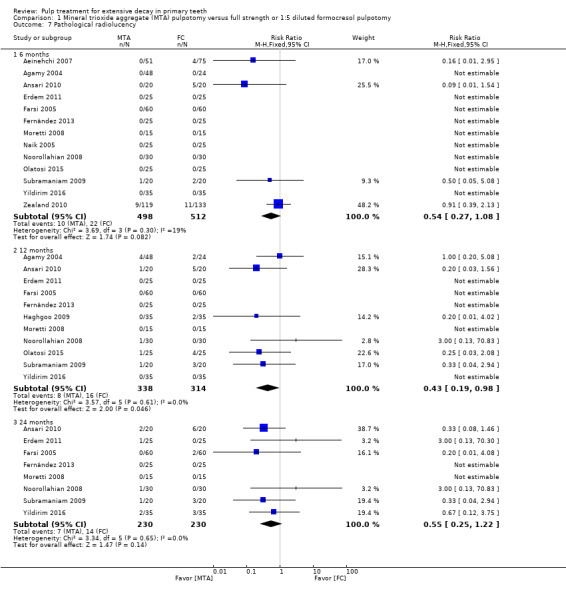
Comparison 1 Mineral trioxide aggregate (MTA) pulpotomy versus full strength or 1:5 diluted formocresol pulpotomy, Outcome 7 Pathological radiolucency.
The results showed no statistically significant difference at any time point in pathological radiolucency between MTA and either full strength formocresol or 1:5 diluted formocresol (Analysis 2.5; Analysis 3.6).
3.6. Analysis.
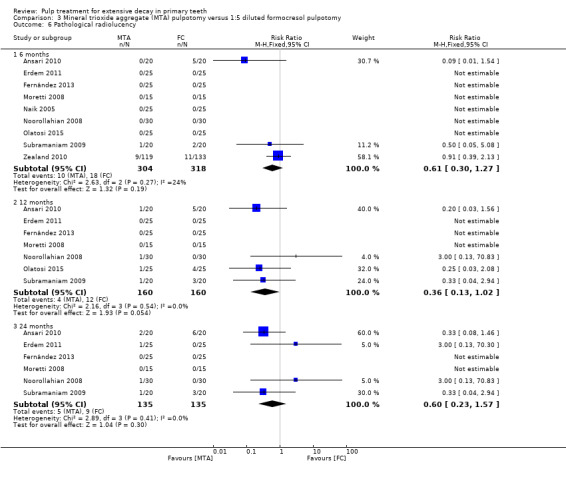
Comparison 3 Mineral trioxide aggregate (MTA) pulpotomy versus 1:5 diluted formocresol pulpotomy, Outcome 6 Pathological radiolucency.
In addition, two trials, which randomised 32 (Eidelman 2001) and 64 (Holan 2005) teeth, did not assess pathological radiolucency at a fixed time point but at a mean (range) follow‐up of 13 (6 to 31) for Eidelman 2001 and 36 (4 to 74) months for Holan 2005. For one trial, there was no pathological radiolucency in any of the participants regardless of the intervention, and for the other trial, the RR was 0.94 (95% CI 0.06 to 14.4).
Pathological root resorption
At six months, data were extractable from 11 RCTs totaling 866 teeth. In seven of the trials, there was no pathological root resorption in any participants regardless of the intervention. From the four remaining trials, the pooled results showed no evidence of a statistically significant difference in pathological root resorption with MTA compared with formocresol (pooled RR 0.47, 95% CI 0.18 to 1.21). At 12 months, data were extractable from nine RCTs totaling 508 teeth. In five of the trials, there was no pathological root resorption in any participants regardless of the intervention. From the four remaining trials, the pooled results showed no evidence of a statistically significant difference in pathological root resorption between MTA and formocresol (pooled RR 0.26, 95% CI 0.07 to 1.03). At 24 months, data were extractable from six RCTs totaling 338 teeth. In one of the five trials, there was no pathological root resorption in any of the participants regardless of the intervention. From the five remaining trials, the pooled results showed evidence of a statistically significant difference in pathological root resorption between MTA and formocresol (pooled RR 0.25, 95% CI 0.08 to 0.81), with no evidence of statistical heterogeneity among included trials (I² = 0%; Analysis 1.8).
1.8. Analysis.
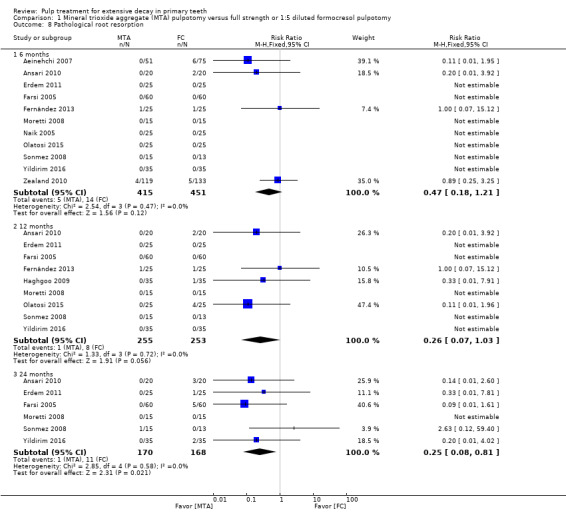
Comparison 1 Mineral trioxide aggregate (MTA) pulpotomy versus full strength or 1:5 diluted formocresol pulpotomy, Outcome 8 Pathological root resorption.
The results showed a statistically significant difference at 24 months in pathological root resorption between MTA and full strength formocresol; the results showed no statistically significant difference at 6 and 12 months between MTA and full strength formocresol, or at any point between MTA and 1:5 diluted formocresol (Analysis 2.6; Analysis 3.7).
2.6. Analysis.
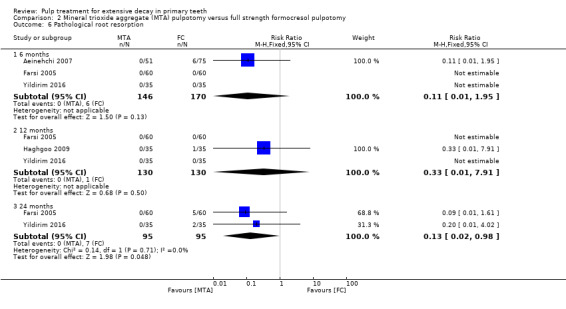
Comparison 2 Mineral trioxide aggregate (MTA) pulpotomy versus full strength formocresol pulpotomy, Outcome 6 Pathological root resorption.
3.7. Analysis.
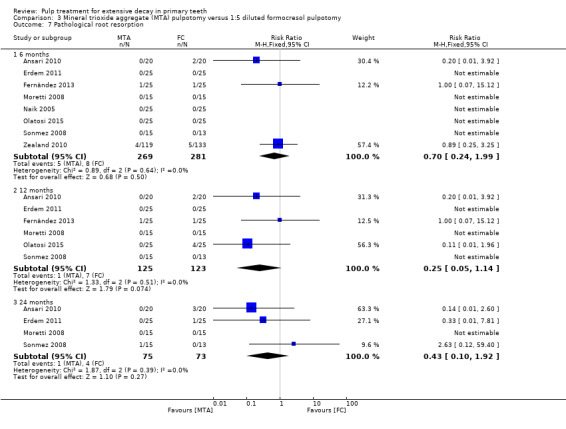
Comparison 3 Mineral trioxide aggregate (MTA) pulpotomy versus 1:5 diluted formocresol pulpotomy, Outcome 7 Pathological root resorption.
A further two trials, which randomised 32 (Eidelman 2001) and 64 (Holan 2005) teeth, did not assess pathological root resorption at a fixed time point but at a mean (range) follow‐up of 13 (6 to 31) months for Eidelman 2001 and 36 (4 to 74) months for Holan 2005. The RR was 0.06 (95% CI 0.00 to 0.92) for Eidelman 2001 and 0.30 (95% CI 0.01 to 6.77) for Holan 2005.
Pulp canal obliteration
At six months, data were extractable from nine RCTs totaling 712 teeth. In six of the trials, there was no pulp canal obliteration in any participants regardless of the intervention. From the three remaining trials, the pooled results showed no statistically significant difference in pulp canal obliteration between MTA and formocresol (pooled RR 1.52, 95% CI 1.00 to 2.30). Results were similar at 12 months, with five of seven trials providing data (RR 1.70, 95% CI 0.81 to 3.57). At 24 months, data were extractable from six RCTs totaling 338 teeth; the pooled results showed a larger risk of pulp canal obliteration with MTA compared with formocresol (RR 2.05, 95% CI 1.07 to 3.94), with no evidence of statistical heterogeneity among included trials (I² = 22%; Analysis 1.9).
1.9. Analysis.
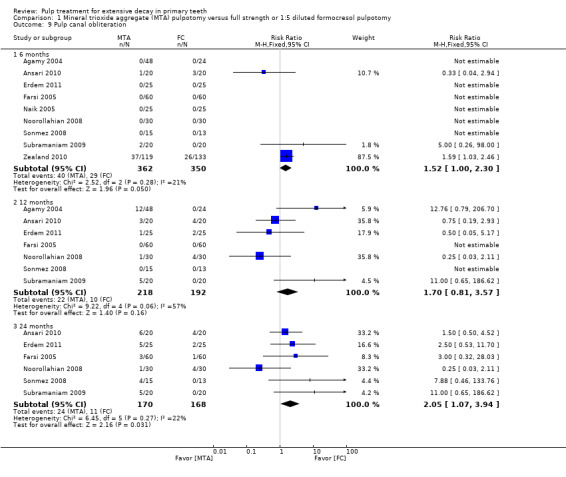
Comparison 1 Mineral trioxide aggregate (MTA) pulpotomy versus full strength or 1:5 diluted formocresol pulpotomy, Outcome 9 Pulp canal obliteration.
The results showed no statistically significant difference at any time point in pulp canal obliteration between MTA and either full strength or 1:5 diluted formocresol (Analysis 2.7; Analysis 3.8).
2.7. Analysis.
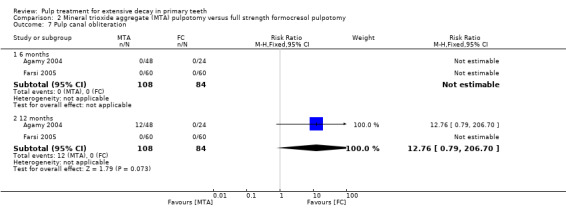
Comparison 2 Mineral trioxide aggregate (MTA) pulpotomy versus full strength formocresol pulpotomy, Outcome 7 Pulp canal obliteration.
3.8. Analysis.
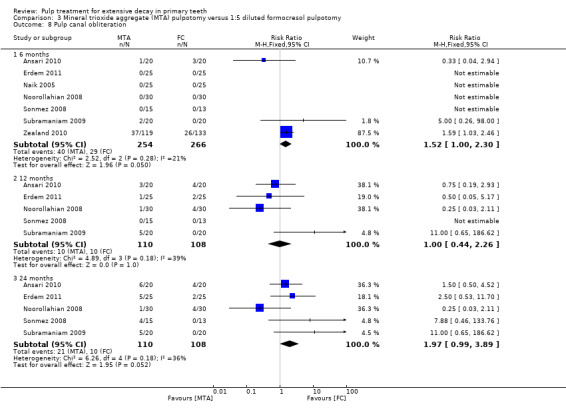
Comparison 3 Mineral trioxide aggregate (MTA) pulpotomy versus 1:5 diluted formocresol pulpotomy, Outcome 8 Pulp canal obliteration.
One additional trial, which randomised 64 teeth, did not assess pulp canal obliteration at a fixed time point but at a mean (range) follow‐up of 36 (4 to 74) months (Holan 2005). The RR was 1.19 (95% CI 0.75 to 1.90).
Dentin bridge formation
At six months, data were extractable from three RCTs totaling 322 teeth. The pooled results showed a greater chance of dentin bridge formation with MTA than with formocresol (RR 18.16, 95% CI 3.63 to 90.91), with evidence of a moderate statistical heterogeneity among included trials (I² = 34%). At 12 months, data were extractable from two RCTs totaling 70 teeth; the pooled results showed no statistically significant difference in dentin bridge formation with MTA compared with formocresol. The pooled RR was 6.00 (95% CI 0.76 to 47.22). Results were similar at 24 months (Analysis 1.10).
1.10. Analysis.
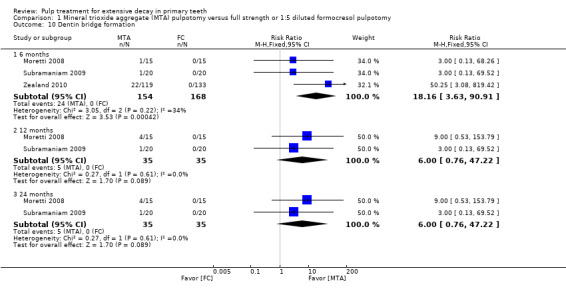
Comparison 1 Mineral trioxide aggregate (MTA) pulpotomy versus full strength or 1:5 diluted formocresol pulpotomy, Outcome 10 Dentin bridge formation.
All included trials compared MTA with 1:5 diluted formocresol.
In addition, the trial by Holan 2005, which randomised 64 teeth, did not assess dentin bridge formation at a fixed time point but at a mean (range) follow‐up of 36 (4 to 74) months. The RR was 2.82 (95% CI 0.12 to 66.82).
Physiological root resorption
At six months, data were extractable from two RCTs totaling 170 teeth. In both trials, there was no physiological root resorption in any of the participants regardless of the intervention. Results were similar at 12 months. At 24 months, one of the two trials showed no cases of physiological root resorption, regardless of the intervention. In the other trial, the results showed no statistically significant difference in physiological root resorption with MTA compared with formocresol (RR 0.33, 95% CI 0.01 to 7.83; Analysis 1.11).
1.11. Analysis.
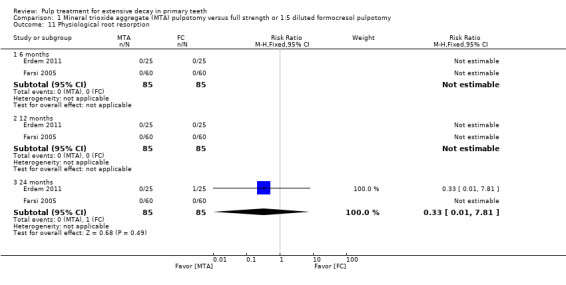
Comparison 1 Mineral trioxide aggregate (MTA) pulpotomy versus full strength or 1:5 diluted formocresol pulpotomy, Outcome 11 Physiological root resorption.
The trial providing data assessed 1:5 diluted formocresol.
MTA versus calcium hydroxide
Clinical failure
At six months, data were extractable from four RCTs totaling 150 teeth. In three trials, there was no clinical failure in any participants regardless of the intervention. For the remaining trial, the results showed no statistically significant difference (RR 0.20, 95% CI 0.01 to 3.85). At 12 months, with four trials providing data, the pooled results showed a statistically significant difference in clinical failure between MTA and calcium hydroxide (RR 0.16 (95% CI 0.04 to 0.70), with no evidence of statistical heterogeneity among the included trials (I² = 0%). At 24 months, data were extractable from five RCTs totaling 284 teeth. All trials provided data. The pooled results showed a statistically significant difference in favour of MTA (RR 0.25, 95% CI 0.12 to 0.52), with evidence of a moderate statistical heterogeneity among included trials (I² = 31%; Analysis 5.1).
5.1. Analysis.
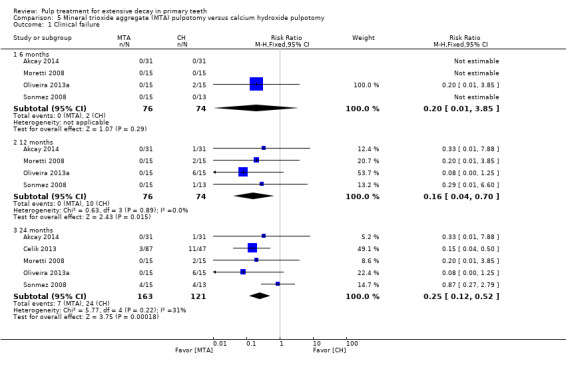
Comparison 5 Mineral trioxide aggregate (MTA) pulpotomy versus calcium hydroxide pulpotomy, Outcome 1 Clinical failure.
Radiological failure
At six months, data were extractable from four RCTs totaling 150 teeth. In one trial, there was no radiological failure in any participants regardless of the intervention. In the other trials, the results showed a statistically significant difference in radiological failure in favour of MTA compared to calcium hydroxide (RR 0.08, 95% CI 0.02 to 0.41), with no evidence of statistical heterogeneity among included trials (I² = 0%). Results were similar at 12 months (RR 0.12, 95% CI 0.04 to 0.36). At 24 months, data were extractable from five RCTs totaling 284 teeth. All trials provided data. The pooled results showed a statistically significant difference in favour of MTA (RR 0.14, 95% CI 0.08 to 0.26), with evidence of substantial statistical heterogeneity among included trials (I² = 68%; Analysis 5.2).
5.2. Analysis.
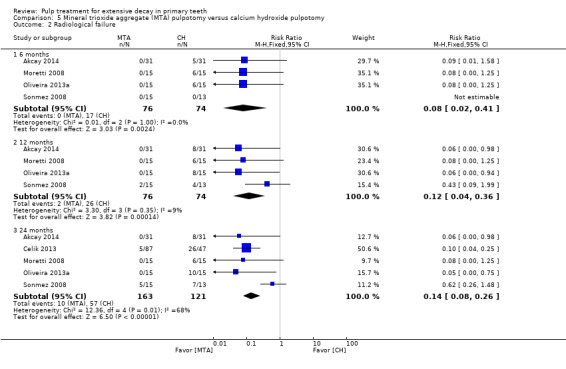
Comparison 5 Mineral trioxide aggregate (MTA) pulpotomy versus calcium hydroxide pulpotomy, Outcome 2 Radiological failure.
Overall failure
At six months, data were extractable from two RCTs totaling 68 teeth. In one trial, there was no overall failure in any of the participants regardless of the intervention. In the other trial, the results showed no statistically significant differences (RR 0.20, 95% CI 0.01 to 3.92). Results were similar at 12 months with all trials providing data (RR 0.34, 95% CI 0.10 to 1.19). At 24 months, the pooled results showed a statistically significant difference in favour of MTA (RR 0.42, 95% CI 0.18 to 0.95), with evidence of moderate statistical heterogeneity among included trials (I² = 36%; Analysis 5.3).
5.3. Analysis.
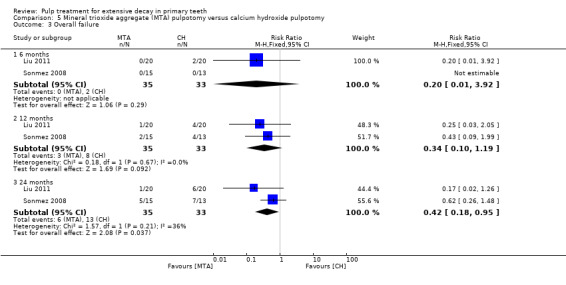
Comparison 5 Mineral trioxide aggregate (MTA) pulpotomy versus calcium hydroxide pulpotomy, Outcome 3 Overall failure.
Pain
At six and 12 months, one trial, which randomised 62 teeth, assessed spontaneous pain (Akcay 2014). There was no pain in any of the participants regardless of the intervention. At 24 months, data were extractable from two RCTs totaling 196 teeth. The results showed no statistically significant difference between MTA and calcium hydroxide (RR 0.41, 95% CI 0.09 to 1.73; Analysis 5.4).
5.4. Analysis.
Comparison 5 Mineral trioxide aggregate (MTA) pulpotomy versus calcium hydroxide pulpotomy, Outcome 4 Pain.
Defective restoration (clinically)
One trial, which randomised 139 teeth, assessed amalgam restorations with ditched margins (Celik 2013). At 24 months, there was no statistically significant difference between MTA and calcium hydroxide (RR 0.54, 95% CI 0.08 to 3.71).
Soft tissue pathology
At six months, data were extractable from three RCTs totaling 122 teeth. In one trial, there was no soft tissue pathology in any of the participants regardless of the intervention. In the other trials, results showed no statistically significant difference (RR 0.20, 95% CI 0.02 to 1.62). At 12 months, with all trials providing data, the results were statistically significant and in favour of MTA (RR 0.12, 95% CI 0.02 to 0.62), with no evidence of statistical heterogeneity among included trials (I² = 0%). At 24 months, data were extractable from four RCTs totaling 256 teeth. All trials provided data. The results were statistically significant (RR 0.17, 95% CI 0.06 to 0.47) with no evidence of statistical heterogeneity among included trials (I² = 0%; Analysis 5.5).
5.5. Analysis.
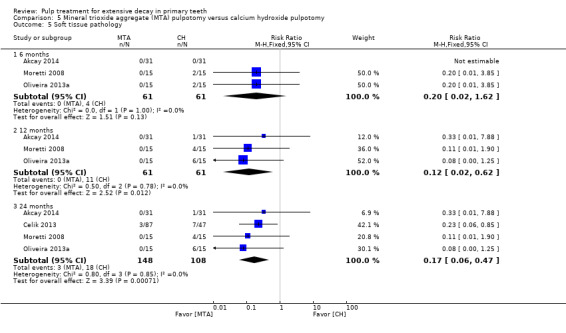
Comparison 5 Mineral trioxide aggregate (MTA) pulpotomy versus calcium hydroxide pulpotomy, Outcome 5 Soft tissue pathology.
Pathological mobility
At six and 12 months, data were extractable from three RCTs totaling 122 teeth. In one trial, there was no pathological mobility in any of the participants regardless of the intervention. In the other trials, results showed no statistically significant difference (RR 0.20, 95% CI 0.02 to 1.62). Results were statistically significant at 12 and 24 months, clearly favouring MTA (RR 0.09, 95% CI 0.01 to 0.66), with no evidence of statistical heterogeneity among included trials (I² = 0%). At 24 months, data were extractable from four RCTs totaling 256 teeth with all trials providing data (Analysis 5.6).
5.6. Analysis.
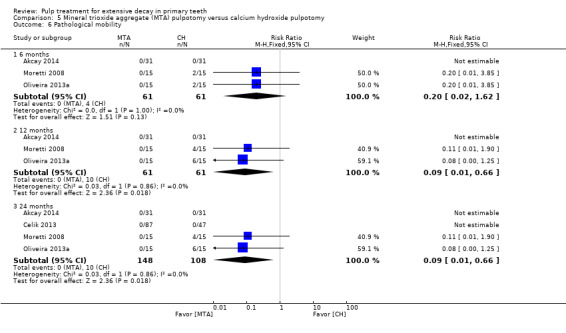
Comparison 5 Mineral trioxide aggregate (MTA) pulpotomy versus calcium hydroxide pulpotomy, Outcome 6 Pathological mobility.
Pathological radiolucency
At six months, data were extractable from four RCTs totaling 162 teeth. For one trial, there was no pathological radiolucency in any of the participants regardless of the intervention. For the remaining trials, results were statistically significant and in favour of MTA (RR 0.10, 95% CI 0.02 to 0.50), with no evidence of statistical heterogeneity among included trials (I² = 0%). Results were similar at 12 (RR 0.14, 95% CI 0.04 to 0.47) and 24 months (RR 0.08, 95% CI 0.03 to 0.22). At 24 months, data were extractable from five RCTs totaling 296 teeth with all trials providing data (Analysis 5.7).
5.7. Analysis.
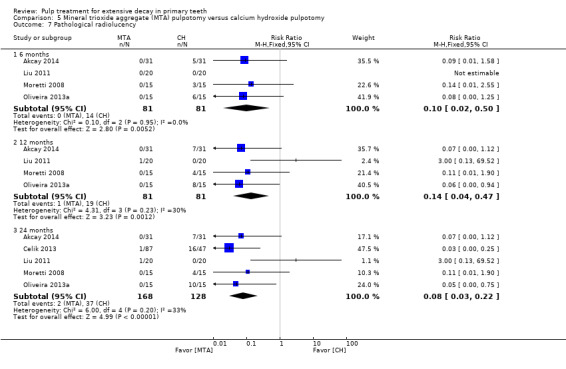
Comparison 5 Mineral trioxide aggregate (MTA) pulpotomy versus calcium hydroxide pulpotomy, Outcome 7 Pathological radiolucency.
Pathological root resorption
At six months, data were extractable from five RCTs totaling 190 teeth. In one of the trials, there was no pathological root resorption in any of the participants regardless of the intervention. From the other trials, the pooled results showed statistically significant difference in pathological root resorption between MTA and calcium hydroxide (RR 0.10, 95% CI 0.02 to 0.39) with no evidence of statistical heterogeneity among included trials (I² = 0%). Results were similar at 12 (RR 0.07, 95% CI 0.02 to 0.29) and 24 months (RR 0.08, 95% CI 0.03 to 0.18). At 24 months, data were extractable from five RCTs totaling 324 teeth with all trials providing data (Analysis 5.8).
5.8. Analysis.
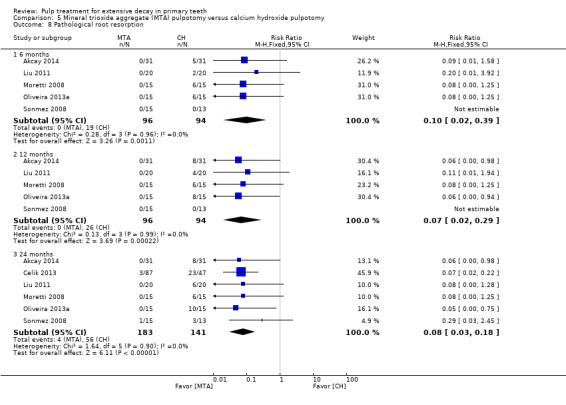
Comparison 5 Mineral trioxide aggregate (MTA) pulpotomy versus calcium hydroxide pulpotomy, Outcome 8 Pathological root resorption.
Pulp canal obliteration
At six months, data were extractable from three RCTs totaling 120 teeth. In one of the trials, there was no pulp canal obliteration in any of the participants regardless of the intervention. From the other trials, the results showed a statistically significant difference between MTA and calcium hydroxide (RR 7.77, 95% CI 1.56 to 38.69), showing a higher risk of pulp canal obliteration with MTA than with calcium hydroxide, with no evidence of statistical heterogeneity among included trials (I² = 0%). At 12 months, results were not statistically significantly different with all trials providing data (RR 2.01, 95% CI 0.97 to 4.17). At 24 months, data were extractable from four RCTs totaling 254 teeth, with all trials providing data. The results showed a statistically significantly higher risk of pulp canal obliteration with MTA than with calcium hydroxide (RR 2.05, 95% CI 1.01 to 4.19), with evidence of substantial statistical heterogeneity among included trials (I² = 69%; Analysis 5.9).
5.9. Analysis.
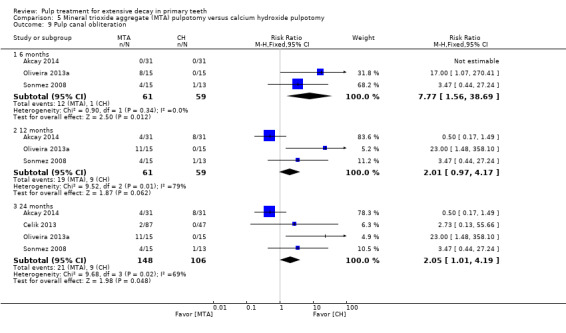
Comparison 5 Mineral trioxide aggregate (MTA) pulpotomy versus calcium hydroxide pulpotomy, Outcome 9 Pulp canal obliteration.
Dentin bridge formation
Data were extractable from two RCTs totaling 60 teeth. At six months, the results showed a statistically significant difference and a greater chance of dentin bridge formation when MTA is applied than when calcium hydroxide is applied (RR 0.20, 95% CI 0.05 to 0.84), with no evidence of statistical heterogeneity among included trials (I² = 0%). Results were not statistically significant at 12 and 24 months (RR 0.80, 95% CI 0.37 to 1.74; Analysis 5.10).
5.10. Analysis.
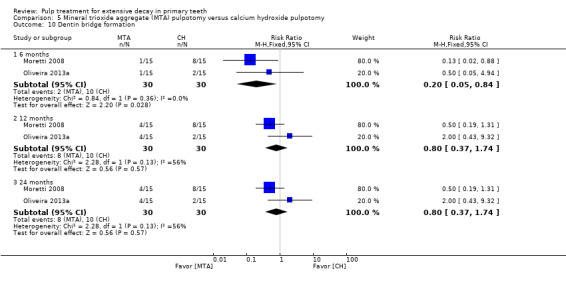
Comparison 5 Mineral trioxide aggregate (MTA) pulpotomy versus calcium hydroxide pulpotomy, Outcome 10 Dentin bridge formation.
MTA versus ferric sulphate
Clinical failure
At six months, data were extractable from four RCTs totaling 190 teeth. In three trials, there was no clinical failure in any of the participants regardless of the intervention. For the remaining trial, the results showed no statistically significant difference in clinical failure between MTA and ferric sulphate (RR 0.08, 95% CI 0.00 to 1.31). At 12 months, data were extractable from three RCTs totaling 130 teeth. In two trials, there was no clinical failure in any of the participants regardless of the intervention. For the remaining trial, the results showed no statistically significant difference in clinical failure for MTA compared with ferric sulphate (RR 0.20, 95% CI 0.01 to 3.97). Results were similar at 24 months, with all trials providing data (RR 0.52, 95% CI 0.20 to 1.39; Analysis 4.1).
4.1. Analysis.
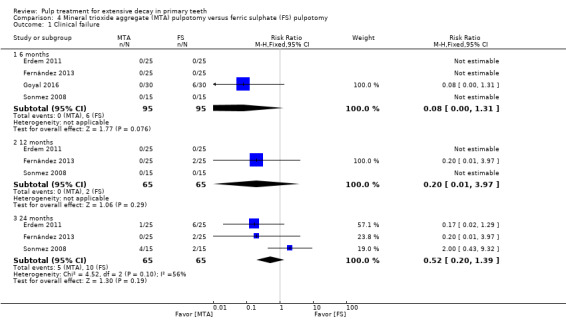
Comparison 4 Mineral trioxide aggregate (MTA) pulpotomy versus ferric sulphate (FS) pulpotomy, Outcome 1 Clinical failure.
Radiological failure
At six months, data were extractable from four RCTs totaling 190 teeth. In two trials, there was no clinical failure in any of the participants regardless of the intervention. For the two remaining trials, the pooled results showed a statistically significant difference (RR 0.06, 95% CI 0.01 to 0.40) with evidence of moderate statistical heterogeneity among included trials (I² = 30%). At 12 months, data were extractable from three RCTs totaling 130 teeth. In one trial, there was no clinical failure in any of the participants regardless of the intervention. For the remaining two trials, the pooled results showed no statistically significant difference (RR 0.71, 95% CI 0.15 to 3.44). Results were similar at 24 months, with all trials providing data (RR 0.58, 95% CI 0.25 to 1.36; Analysis 4.2).
4.2. Analysis.
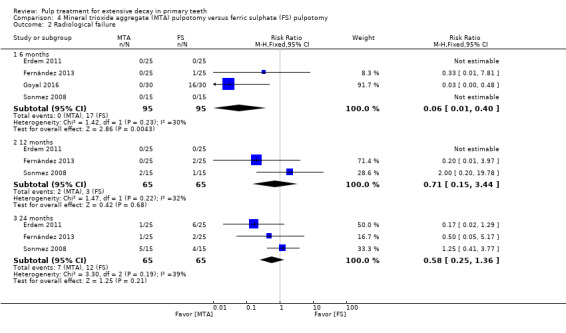
Comparison 4 Mineral trioxide aggregate (MTA) pulpotomy versus ferric sulphate (FS) pulpotomy, Outcome 2 Radiological failure.
Overall failure
At six months, data were extractable from four RCTs totaling 190 teeth. In two trials, there was no overall failure in any participants regardless of the intervention. For the two remaining trials, the pooled results showed a statistically significant difference favouring MTA over ferric sulphate (RR 0.06, 95% CI 0.01 to 0.40) with evidence of a moderate statistical heterogeneity among included trials (I² = 30%). At 12 months, data were extractable from three RCTs totaling 130 teeth. In one trial, there was no overall failure in any of the participants regardless of the intervention. For the remaining two trials, the pooled results showed no statistically significant difference (RR 0.71, 95% CI 0.15 to 3.44). Results were similar at 24 months, with all trials providing data (RR 0.78, 95% CI 0.32 to 1.89; Analysis 4.3).
4.3. Analysis.
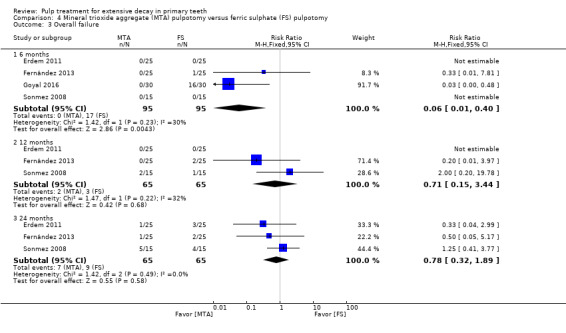
Comparison 4 Mineral trioxide aggregate (MTA) pulpotomy versus ferric sulphate (FS) pulpotomy, Outcome 3 Overall failure.
Pain
At six months, data were extractable from three RCTs totaling 160 teeth. In two trials, there was no pain in any of the participants regardless of the intervention. For the remaining trial, the results showed no statistically significant difference (RR 0.20, 95% CI 0.01 to 4.00). At 12 months, data were extractable from two RCTs totaling 100 teeth. In the two trials, there was no pain in any of the participants regardless of the intervention (Analysis 4.4).
4.4. Analysis.
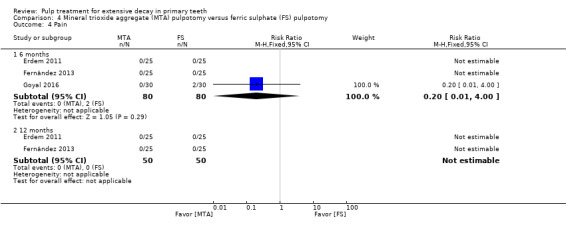
Comparison 4 Mineral trioxide aggregate (MTA) pulpotomy versus ferric sulphate (FS) pulpotomy, Outcome 4 Pain.
At 24 months, one trial, which randomised 50 teeth, assessed pain (Fernández 2013). There was no pain in any of the participants regardless of the intervention.
One additional trial, which randomised 111 teeth, did not assess pain at a fixed time point but at a mean (range) follow‐up of 22 (6 to 38) months (Doyle 2010). The RR was 0.36 (95% CI 0.02 to 8.75).
Soft tissue pathology
At six months, data were extractable from two RCTs totaling 110 teeth. In one trial, there was no soft tissue pathology in any of the participants regardless of the intervention. For the remaining trial, the results showed no statistically significant difference (RR 0.20, 95% CI 0.01 to 4.00; Analysis 4.5).
4.5. Analysis.
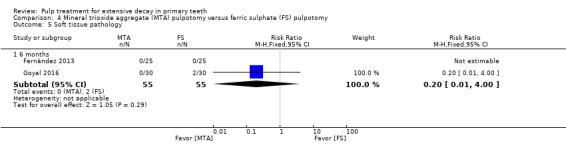
Comparison 4 Mineral trioxide aggregate (MTA) pulpotomy versus ferric sulphate (FS) pulpotomy, Outcome 5 Soft tissue pathology.
At 24 months, one trial, which randomised 50 teeth, assessed soft tissue pathology (Fernández 2013). There was no soft tissue pathology in any of the participants regardless of the intervention.
One additional trial assessed soft tissue pathology at a mean (range) follow‐up of 22 (6 to 38) months (Doyle 2010). There was no statistically significant difference between groups (RR 0.36, 95% CI 0.02 to 8.75).
Adjacent tissues inflammation
One trial, which randomised 50 teeth, assessed adjacent tissues inflammation (Fernández 2013). At six months, there was no adjacent tissue inflammation in any of the participants regardless of the intervention. At 12 and 24 months, the results showed no statistically significance between groups (RR 0.33, 95% CI 0.01 to 7.81; RR 0.20, 95% CI 0.01 to 3.97, respectively).
One additional trial, which randomised 111 teeth, assessed adjacent tissues inflammation at a mean (range) follow‐up of 22 (6 to 38) months (Doyle 2010). There was no statistically significant difference between groups (RR 0.36, 95% CI 0.02 to 8.75).
Pathological mobility
At six months, data were extractable from two RCTs totaling 110 teeth. In one trial, there was no pathological mobility in any of the participants regardless of the intervention. In the other trial, the results showed no statistically significant difference (RR 0.08, 95% CI 0.00 to 1.31; Analysis 4.6).
4.6. Analysis.
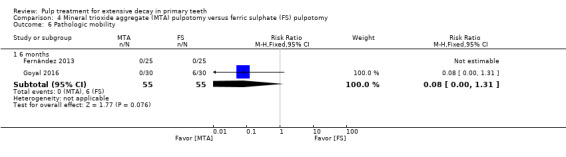
Comparison 4 Mineral trioxide aggregate (MTA) pulpotomy versus ferric sulphate (FS) pulpotomy, Outcome 6 Pathologic mobility.
At 12 and 24 months, one trial, which randomised 50 teeth, assessed pathological mobility (Fernández 2013). There was no pathological mobility in any of the participants regardless of the intervention.
One additional trial, which randomised 111 teeth, assessed pathological mobility at a mean (range) follow‐up of 22 (6 to 38) months (Doyle 2010). There was no statistically significant difference between groups (RR 0.22, 95% CI 0.01 to 4.45).
Pathological radiolucency
At six months, data were extractable from three RCTs totaling 160 teeth. In two trials, there was no pathological radiolucency in any of the participants regardless of the intervention. In the remaining trial, the results showed a statistically significant difference favouring MTA over ferric sulphate (RR 0.03, 95% CI 0.00 to 0.48). At 12 and 24 months, data were extractable from two RCTs totaling 100 teeth. In one trial, there was no pathological radiolucency in any of the participants regardless of the intervention. For the remaining trial, the results showed no statistically significant difference at 12 and 24 months (RR 0.33, 95% CI 0.01 to 7.81; RR 0.20, 95% CI 0.01 to 3.97; respectively; Analysis 4.7).
4.7. Analysis.
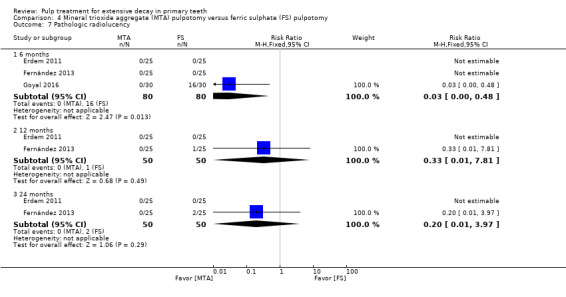
Comparison 4 Mineral trioxide aggregate (MTA) pulpotomy versus ferric sulphate (FS) pulpotomy, Outcome 7 Pathologic radiolucency.
One additional trial, which randomised 111 teeth, did not assess pathological radiolucency at a fixed time point but at a mean (range) follow‐up of 22 (6 to 38) months (Doyle 2010). The RR was 0.36 (95% CI 0.02 to 8.75).
Pathological root resorption
At six months, data were extractable from four RCTs totaling 190 teeth. In two trials, there was no pathological root resorption in any of the participants regardless of the intervention. For the two remaining trials, the pooled results showed a statistically significant difference favouring MTA over ferric sulphate (RR 0.07, 95% CI 0.01 to 0.53), with no evidence of statistical heterogeneity among included trials (I² = 8%). At 12 months, data were extractable from three RCTs totaling 130 teeth. In two trials, there was no pathological root resorption in any of the participants regardless of the intervention. For the remaining trial, the results showed no statistically significant difference (RR 0.20, 95% CI 0.01 to 3.97). Results were similar at 24 months, with all trials providing data (RR 0.56, 95% CI 0.12 to 2.51; Analysis 4.8).
4.8. Analysis.
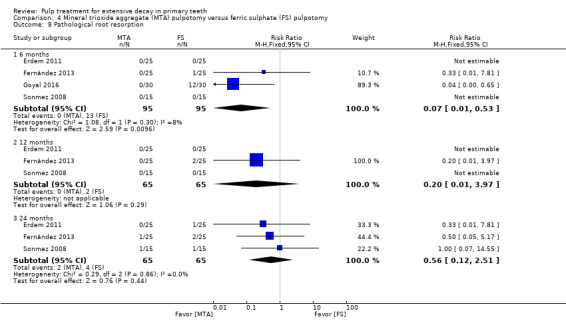
Comparison 4 Mineral trioxide aggregate (MTA) pulpotomy versus ferric sulphate (FS) pulpotomy, Outcome 8 Pathological root resorption.
One trial, which randomised 111 teeth, did not assess pathological root resorption at a fixed time point but at a mean (range) follow‐up of 22 (6 to 38) months (Doyle 2010). The RR was 0.22 (95% CI 0.07 to 0.71).
Pulp canal obliteration
At six months, data were extractable from three RCTs totaling 140 teeth. In the three trials, there was no pulp canal obliteration in any of the participants regardless of the intervention. At 12 months, data were extractable from two RCTs totaling 80 teeth. In one trial, there was no pulp canal obliteration in any of the participants regardless of the intervention. For the remaining trial, the results showed no statistically significant difference (RR 3.00, 95% CI 0.13 to 70.30). Results were similar at 24 months, with all trials providing data (RR 1.57, 95% CI 0.47 to 5.27; Analysis 4.9).
4.9. Analysis.
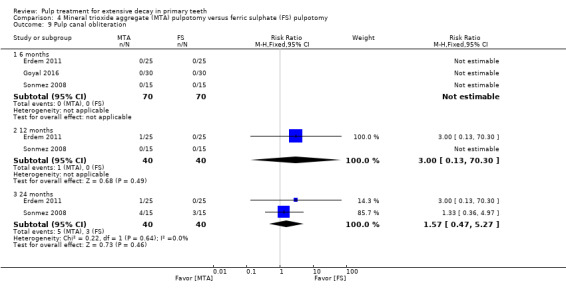
Comparison 4 Mineral trioxide aggregate (MTA) pulpotomy versus ferric sulphate (FS) pulpotomy, Outcome 9 Pulp canal obliteration.
One additional trial, which randomised 111 teeth, did not assess pulp canal obliteration at a fixed time point but at a mean (range) follow‐up of 22 (6 to 38) months (Doyle 2010). The RR was 1.68 (95% CI 0.93 to 3.04).
Physiological root resorption
One trial, which randomised 50 teeth, assessed physiological root resorption (Erdem 2011). At six and 12 months, there was no physiological root resorption in any of the participants regardless of the intervention. At 24 months, the RR was 0.14 (95% CI 0.01 to 2.63).
Finally, one registered trial assessed clinical and radiographic success at six, nine and 12 months, but no resulting information was published (NCT02783911).
Ferric sulphate + MTA versus MTA
One trial, which randomised 130 teeth, assessed ferric sulphate + MTA versus MTA based on pain, soft tissue pathology, pathological mobility, pathological radiolucency, pathological root resorption and pulp canal obliteration (Doyle 2010). This trial did not assess the outcomes at a fixed time point but at a mean (range) follow‐up of 22 (6 to 38) months. There were no statistically significant differences for any outcome (Table 4).
1. Pulpotomy (FS + MTA) versus pulpotomy (MTA).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Pain | mean 22 | 1 | Not estimable* |
| Soft tissue pathology | mean 22 | 1 | Not estimable* |
| Pathological mobility | mean 22 | 1 | Not estimable* |
| Pathological radiolucency | mean 22 | 1 | 3.46 (0.17 to 70.69) |
| Pathological root resorption | mean 22 | 1 | 2.75 (0.82 to 9.29) |
| Pulp canal obliteration | mean 22 | 1 | 0.83 (0.51 to 1.33) |
*due to lack of events
Abbreviations ‐ CI: confidence interval; FS: ferric sulphate; MTA: mineral trioxide aggregate
MTA versus Portland cement
Clinical failure
At six, 12 and 24 months, data were extractable from three RCTs totaling 130 teeth. In two trials, there was no clinical failure in any of the participants regardless of the intervention. For the remaining trial, the results showed no statistically significant difference (RR 0.20, 95% CI 0.01 to 4.02; Analysis 6.1).
6.1. Analysis.
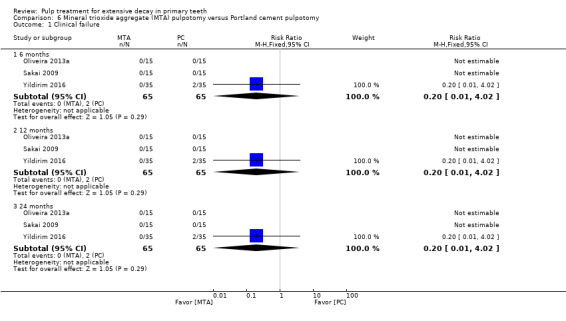
Comparison 6 Mineral trioxide aggregate (MTA) pulpotomy versus Portland cement pulpotomy, Outcome 1 Clinical failure.
Radiological failure
At six and 12 months, data were extractable from three RCTs totaling 130 teeth. In the three trials, there was no radiological failure in any of the participants regardless of the intervention. At 24 months, one trial provided data, and the results showed no statistically significant difference (RR 0.50, 95% CI 0.10 to 2.56; Analysis 6.2).
6.2. Analysis.
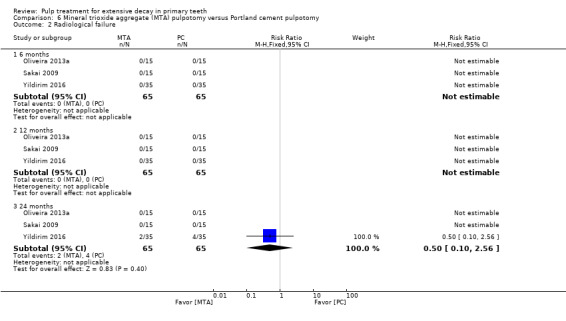
Comparison 6 Mineral trioxide aggregate (MTA) pulpotomy versus Portland cement pulpotomy, Outcome 2 Radiological failure.
Pain
At six, 12 and 24 months, data were extractable from three RCTs totaling 130 teeth. In two trials, there was no pain in any of the participants regardless of the intervention. For the remaining trial, the results showed no statistically significant difference (RR 0.33, 95% CI 0.01 to 7.91; Analysis 6.3).
6.3. Analysis.
Comparison 6 Mineral trioxide aggregate (MTA) pulpotomy versus Portland cement pulpotomy, Outcome 3 Pain.
Soft tissue pathology
At six, 12 and 24 months, data were extractable from three RCTs totaling 130 teeth. In two trials, there was no clinical failure in any of the participants regardless of the intervention. For the remaining trial, the results showed no statistically significant difference (RR 0.20, 95% CI 0.01 to 4.02; Analysis 6.4).
6.4. Analysis.
Comparison 6 Mineral trioxide aggregate (MTA) pulpotomy versus Portland cement pulpotomy, Outcome 4 Soft tissue pathology.
Pathological mobility
At six, 12 and 24 months, data were extractable from three RCTs totaling 130 teeth. In two trials, there was no pain in any of the participants regardless of the intervention. For the remaining trial, the results showed no statistically significant difference (RR 0.33, 95% CI 0.01 to 7.91; Analysis 6.5).
6.5. Analysis.
Comparison 6 Mineral trioxide aggregate (MTA) pulpotomy versus Portland cement pulpotomy, Outcome 5 Pathologic mobility.
Pathological radiolucency
At six and 12 months, data were extractable from three RCTs totaling 130 teeth. In the three trials, there was no radiological failure in any of the participants regardless of the intervention. At 24 months, one trial provided data, and the results showed no statistically significant difference (RR 0.67, 95% CI 0.12 to 3.75; Analysis 6.6).
6.6. Analysis.
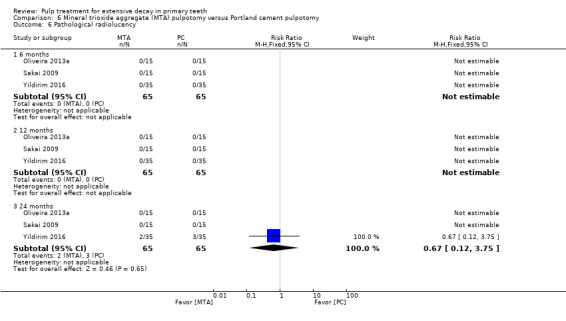
Comparison 6 Mineral trioxide aggregate (MTA) pulpotomy versus Portland cement pulpotomy, Outcome 6 Pathological radiolucency.
Pathological root resorption
At six and 12 months, data were extractable from three RCTs totaling 130 teeth. In the three trials, there was no radiological failure in any of the participants regardless of the intervention. At 24 months, one trial provided data, and the results showed no statistically significant difference (RR 0.33, 95% CI 0.01 to 7.91; Analysis 6.7).
6.7. Analysis.
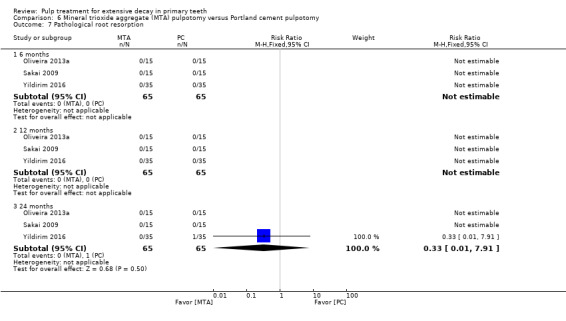
Comparison 6 Mineral trioxide aggregate (MTA) pulpotomy versus Portland cement pulpotomy, Outcome 7 Pathological root resorption.
Smell
One trial, which randomised 30 teeth, assessed smell at six, 12 and 24 months (Sakai 2009). There was no smell at any time point regardless of the intervention.
Pulp canal obliteration
Data were extractable from two RCTs totaling 60 teeth. All trials provided data. Results were not statistically significant at 6 (RR 0.73, 95% CI 0.49 to 1.08), 12 (RR 0.83, 95% CI 0.60 to 1.14) and 24 months (RR 0.96, 95% CI 0.71 to 1.29; Analysis 6.8).
6.8. Analysis.
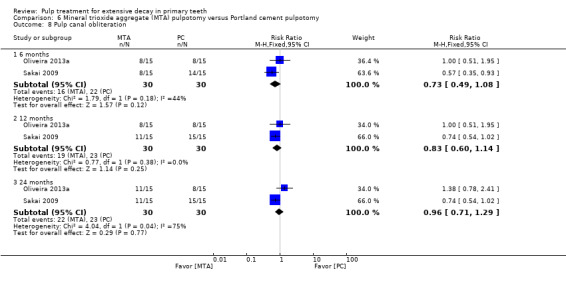
Comparison 6 Mineral trioxide aggregate (MTA) pulpotomy versus Portland cement pulpotomy, Outcome 8 Pulp canal obliteration.
Dentin bridge formation
Data were extractable from two RCTs totaling 60 teeth. Results were not statistically significant at 6 (RR 0.56, 95% CI 0.13 to 2.43), 12 and 24 months (RR 1.50, 95% CI 0.61 to 3.71; Analysis 6.9).
6.9. Analysis.
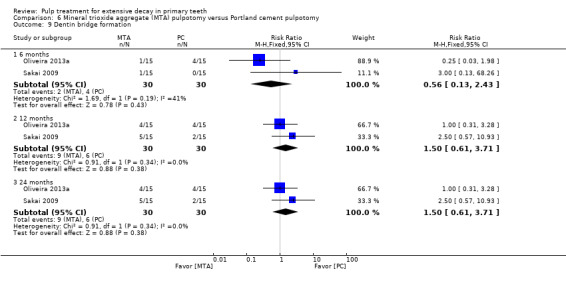
Comparison 6 Mineral trioxide aggregate (MTA) pulpotomy versus Portland cement pulpotomy, Outcome 9 Dentin bridge formation.
Calcium‐enriched mixture (CEM) cement versus MTA
One trial, which randomised 80 teeth, assessed CEM cement versus MTA based on clinical failure, radiological failure and pathological root resorption (Malekafzali 2011). There were no statistically significant differences for any outcome or time point (Table 5).
2. Pulpotomy (CEM cement) versus pulpotomy (MTA).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6, 12 and 18 | 1 | Not estimable* |
| Radiological failure | 6 | 1 | Not estimable* |
| 12 | 1 | 0.33 (0.04 to 2.94) | |
| 18 | 1 | 0.33 (0.04 to 2.94) | |
| Pathological root resorption | 6 | 1 | Not estimable* |
| 12 | 1 | 0.33 (0.04 to 2.94) | |
| 18 | 1 | 0.33 (0.04 to 2.94) |
*due to lack of events
Abbreviations ‐ CEM: calcium‐enriched mixture; CI: confidence interval; MTA: mineral trioxide aggregate
MTA versus sodium hypochlorite (NaOCl)
One trial, which randomised 50 teeth, assessed MTA versus NaOCl based on clinical failure, radiological failure, overall failure, pain, soft tissue pathology, pathological mobility, pathological radiolucency and pathological root resorption (Fernández 2013). There were no statistically significant differences for any outcome or time point (Table 6).
3. Pulpotomy (MTA) versus pulpotomy (NaOCl).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 and 12 | 1 | Not estimable* |
| 24 | 1 | 0.33 (0.01, 7.81) | |
| Radiological failure | 6 and 12 | 1 | 0.14 (0.01, 2.63) |
| 24 | 1 | 0.33 (0.04, 2.99) | |
| Overall failure | 24 | 1 | 0.33 (0.04, 2.99) |
| Pain | 6, 12 and 24 months | 1 | Not estimable* |
| Soft tissue pathology | 6 and 12 | 1 | Not estimable* |
| 24 | 1 | 0.33 (0.01, 7.81) | |
| Pathologic mobility | 6, 12 and 24 months | 1 | Not estimable* |
| Pathologic radiolucency | 6, 12 and 24 months | 1 | Not estimable* |
| Pathologicroot resorption | 6 and 12 | 1 | 0.14 (0.01, 2.63) |
| 24 | 1 | 0.33 (0.04, 2.99) |
*due to lack of events
Abbreviations ‐ CI: confidence interval; MTA: mineral trioxide aggregate; NaOCl: sodium hypochlorite
MTA versus calcium hydroxide + sodium hypochlorite (NaOCl)
One trial, which randomised 62 teeth, assessed MTA versus calcium hydroxide + NaOCl based on clinical failure, radiological failure, overall failure, pain, soft tissue pathology, pathological mobility, adjacent tissues pathology, pathological radiolucency, pathological root resorption and pulp canal obliteration (Akcay 2014). There were no statistically significant differences for any outcome or time point (Table 7).
4. Pulpotomy (MTA) versus pulpotomy (CH+NaOCl).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 and 12 | 1 | Not estimable* |
| Radiological failure | 6 | 1 | Not estimable* |
| 12 | 1 | 0.09 (0.01, 1.58) | |
| Soft tissue pathology | 6 and 12 | 1 | Not estimable* |
| Pathologic mobility | 6 and 12 | 1 | Not estimable* |
| Adjacent tissue inflammation | 6 and 12 | 1 | Not estimable* |
| Pathologic radiolucency | 6 | 1 | Not estimable* |
| 12 | 1 | 0.14 (0.01, 2.66) | |
| Pathologicroot resorption | 6 | 1 | Not estimable* |
| 12 | 1 | 0.14 (0.01, 2.66) | |
| Pulp canal obliteration | 6 | 1 | Not estimable* |
| 12 | 1 | 0.44 (0.15, 1.29) |
*due to lack of events
Abbreviations ‐ CI: confidence interval; MTA: mineral trioxide aggregate; CH: calcium hydroxyde; NaOCl; sodium hypochlorite
MTA + sodium hypochlorite (NaOCl) versus calcium hydroxide + NaOCl
One trial, which randomised 62 teeth, assessed MTA + NaOCl versus calcium hydroxide + NaOCl based on clinical failure, radiological failure, overall failure, pain, soft tissue pathology, pathological mobility, adjacent tissue pathology, pathological radiolucency, pathological root resorption and pulp canal obliteration (Akcay 2014). There were no statistically significant differences for any outcome or time point (Table 8).
5. Pulpotomy (MTA + NaOCl) versus pulpotomy (CH + NaOCl).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 and 12 | 1 | Not estimable* |
| Radiological failure | 6 | 1 | Not estimable* |
| 12 | 1 | 0.20 (0.02, 1.61) | |
| Soft tissue pathology | 6 and 12 | 1 | Not estimable* |
| Pathologic mobility | 6 and 12 | 1 | Not estimable* |
| Adjacent tissue inflammation | 6 and 12 | 1 | Not estimable* |
| Pathologic radiolucency | 6 | 1 | Not estimable* |
| 12 | 1 | 0.14 (0.01, 2.66) | |
| Pathologic root resorption | 6 | 1 | Not estimable* |
| 12 | 1 | 0.33 (0.04, 3.03) | |
| Pulp canal obliteration | 6 | 1 | Not estimable* |
| 12 | 1 | 0.67 (0.27, 1.65) |
*due to lack of events
Abbreviations ‐ CI: confidence interval; MTA: mineral trioxide aggregate; NaOCl; sodium hypochlorite; CH: calcium hydroxyde.
MTA versus buffered glutaraldehyde
One trial, which randomised 60 teeth, assessed MTA versus buffered glutaraldehyde based on pain, soft tissue pathology, pathological mobility, pathological radiolucency, pathological root resorption and pulp canal obliteration (Goyal 2016). The results showed a statistically significant difference for pain, pathological mobility, pathological radiolucency and pathological root resorption at six months, favouring MTA over buffered glutaraldehyde (RR 0.06, 95% CI 0.00 to 0.98; RR 0.06, 95% CI 0.00 to 0.98; RR 0.03, 95% CI 0.00 to 0.55; RR 0.05, 95% CI 0.00 to 0.78; respectively). There were no statistically significant differences for other outcomes at any time point (Table 9).
6. Pulpotomy (MTA) versus pulpotomy (2% buffered glutaraldehyde).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Pain | 6 | 1 | 0.06 (0.00, 0.98) |
| Soft tissue pathology | 6 | 1 | 0.08 (0.00, 1.31) |
| Pathologic mobility | 6 | 1 | 0.06 (0.00, 0.98) |
| Pathologic radiolucency | 6 | 1 | 0.03 (0.00, 0.55) |
| Pathologic root resorption | 6 | 1 | 0.05 (0.00, 0.78) |
| Pulp canal obliteration | 6 | 1 | 0.11 (0.01, 1.98) |
*due to lack of events
Abbreviations ‐ CI: confidence interval; MTA: mineral trioxide aggregate.
MTA versus zinc oxide and eugenol (ZOE)
One trial, which randomised 50 teeth, assessed MTA versus ZOE based on clinical failure, radiological failure, overall failure, pain, pathological radiolucency, pathological root resorption, pulp canal obliteration and physiological root resorption (Erdem 2011). There was a statistically significant difference in radiological failure at 24 months in favour of MTA (RR 0.10, 95% CI 0.01 to 0.72). There were no other statistically significant differences (Table 10).
7. Pulpotomy (MTA) versus pulpotomy (ZOE).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 | 1 | Not estimable* |
| 12 | 1 | Not estimable* | |
| 24 | 1 | 0.20 (0.03 to 1.59) | |
| Radiological failure | 6 | 1 | 0.33 (0.01 to 7.81) |
| 12 | 1 | 0.20 (0.01 to 3.97) | |
| 24 | 1 | 0.10 (0.01 to 0.72) | |
| Overall failure | 6 | 1 | 0.33 (0.01 to 7.81) |
| 12 | 1 | 0.20 (0.01 to 3.97) | |
| 24 | 1 | 0.13 (0.02 to 0.93) | |
| Pain | 6 | 1 | Not estimable* |
| 12 | 1 | Not estimable* | |
| 24 | 1 | 3.00 (0.13 to 70.30) | |
| Pathological radiolucency | 6 | 1 | Not estimable* |
| 12 | 1 | Not estimable* | |
| 24 | 1 | 3.00 (0.13 to 70.30) | |
| Pathological root resorption | 6 | 1 | 0.33 (0.01 to 7.81) |
| 12 | 1 | 0.33 (0.01 to 7.81) | |
| 24 | 1 | 0.08 (0.00 to 1.30) | |
| Pulp canal obliteration | 6 | 1 | Not estimable* |
| 12 | 1 | 3.00 (0.13 to 70.30) | |
| 24 | 1 | 11.0 (0.64 to 188.96) | |
| Physiological root resorption | 6 | 1 | Not estimable* |
| 12 | 1 | Not estimable* | |
| 24 | 1 | 0.20 (0.01 to 3.97) |
*due to lack of events
Abbreviations ‐ CI: confidence interval; MTA: mineral trioxide aggregate; ZOE: zinc oxide and eugenol
MTA versus diode laser
One trial, which randomised 40 teeth, assessed MTA versus diode laser based on pain, soft tissue pathology, pathological mobility, pathological radiolucency, pathological root resorption, and premature tooth loss (Niranjani 2015). There was no premature tooth loss at any time point regardless of the intervention.There were no statistically significant differences for the other outcomes at six months (RR 0.33, 95% CI 0.01 to 7.72).
Diode laser + MTA versus formocresol + zinc oxide and eugenol (ZOE)
One trial, which randomised 52 teeth, assessed diode laser + MTA versus formocresol + ZOE based on clinical failure, radiological failure, overall failure, pathological radiolucency and pathological root resorption (Saltzman 2005). This trial did not assess the outcomes at a fixed time point but at a mean (± standard deviation) follow‐up of 2.3 ± 2.1, 5.2 ± 1.9, 9.5 ± 2.3 and 15.7 ± 3 months. There was no clinical failure in any of the participants regardless of the delay. There was no statistically significant difference for radiological failure, overall failure, pathological radiolucency or pathological root resorption at any follow‐up session (Table 11).
8. Pulpotomy (diode laser + MTA) versus pulpotomy (FC + ZOE).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 15.7 | 1 | Not estimable* |
| Radiological failure | 15.7 | 1 | 4.00 (0.48 to 33.42) |
| Overall failure | 15.7 | 1 | 2.00 (0.40 to 9.99) |
| Pathological radiolucency | 15.7 | 1 | 3.00 (0.33 to 26.99) |
| Pathological root resorption | 15.7 | 1 | 1.50 (0.27 to 8.25) |
*due to lack of events
Abbreviations ‐ CI: confidence interval; FC: formocresol; MTA: mineral trioxide aggregate; ZOE: zinc oxide and eugenol
Low‐level diode laser versus MTA
One trial, which randomised 40 teeth, assessed low‐level diode laser versus MTA based on overall failure at six and 12 months (Uloopi 2016). There was no statistically significant difference at any time point (RR 3.00, 95% CI 0.34 to 26.45; RR 4.00, 95% CI 0.49 to 32.72, respectively).
MTA versus enamel matrix derivative (EMD)
One trial, which randomised 70 teeth, assessed MTA versus EMD based on clinical failure, radiological failure, overall failure, pain, soft tissue pathology, pathological mobility, pathological radiolucency and pathological root resorption (Yildirim 2016). There was no statistically significant difference for any outcome at any time point (Table 12).
9. Pulpotomy (MTA) versus pulpotomy (EMD).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6, 12 and 24 | 1 | 0.14 (0.01, 2.67) |
| Radiological failure | 24 | 1 | 0.29 (0.06, 1.28) |
| Overall failure | 6, 12 and 24 | 1 | 0.14 (0.01, 2.67) |
| Pain | 6, 12 and 24 | 1 | 0.33 (0.01, 7.91) |
| Soft tissue pathology | 6, 12 and 24 | 1 | 0.20 (0.01, 4.02) |
| Pathologic mobility | 6, 12 and 24 | 1 | 0.20 (0.01, 4.02) |
| Pathologic radiolucency | 24 | 1 | 0.40 (0.08, 1.93) |
| Pathologic root resorption | 24 | 1 | 0.20 (0.01, 4.02) |
Abbreviations ‐ CI: confidence interval; MTA: mineral trioxide aggregate; EMD: enamel matrix derivative
Biodentine versus MTA
Clinical failure
At six months, data were extractable from four RCTs totaling 234 teeth. In one trial, there was no clinical failure in any of the participants regardless of the intervention. For the remaining trials, the pooled results showed no statistically significant difference (RR 1.72, 95% CI 0.42 to 6.99). At 12 months, data were extractable from two RCTs totaling 144 teeth. The pooled results showed no statistically significant difference (RR 0.75, 95% CI 0.16 to 3.62; Analysis 7.1).
7.1. Analysis.
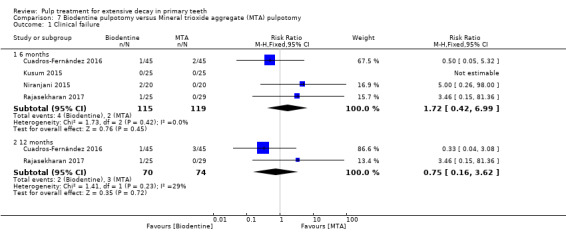
Comparison 7 Biodentine pulpotomy versus Mineral trioxide aggregate (MTA) pulpotomy, Outcome 1 Clinical failure.
Radiological failure
At six months, data were extractable from four RCTs totaling 234 teeth. In one trial, there was no clinical failure in any of the participants regardless of the intervention. For the remaining trials, the pooled results showed no statistically significant difference (RR 2.40, 95% CI 0.65 to 8.84). At 12 months, data were extractable from two RCTs totaling 144 teeth. The pooled results showed no statistically significant difference (RR 1.08, 95% CI 0.22 to 5.27; Analysis 7.2).
7.2. Analysis.
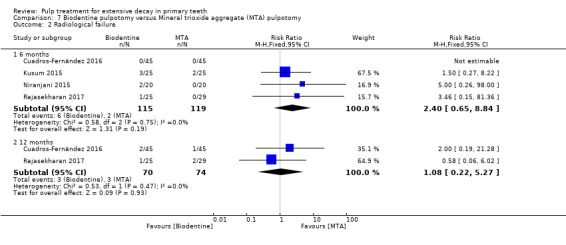
Comparison 7 Biodentine pulpotomy versus Mineral trioxide aggregate (MTA) pulpotomy, Outcome 2 Radiological failure.
Pain
At six months, data were extractable from three RCTs totaling 180 teeth. In two trials, there was no pain in any of the participants regardless of the intervention. For the remaining trial, the results showed no statistically significant difference (RR 5.00, 95% CI 0.26 to 98.00; Analysis 7.3). At 12 months, one trial, which randomised 90 teeth, assessed pain. There was no pain in any of the participants regardless of the intervention.
7.3. Analysis.
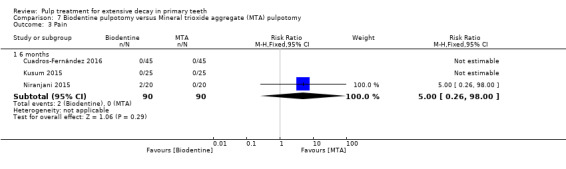
Comparison 7 Biodentine pulpotomy versus Mineral trioxide aggregate (MTA) pulpotomy, Outcome 3 Pain.
Soft tissue pathology
At six months, data were extractable from three RCTs totaling 180 teeth. In two trials, there was no soft tissue pathology in any of the participants regardless of the intervention. For the remaining trial, the results showed no statistically significant difference (RR 5.00, 95% CI 0.26 to 98.00; Analysis 7.4). At 12 months, one trial, which randomised 90 teeth, assessed soft tissue pathology. There was no soft tissue pathology in any of the participants regardless of the intervention.
7.4. Analysis.
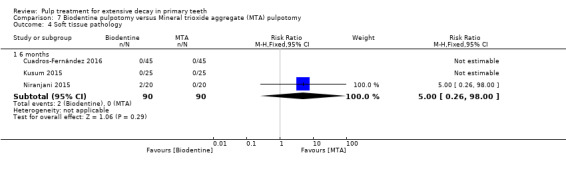
Comparison 7 Biodentine pulpotomy versus Mineral trioxide aggregate (MTA) pulpotomy, Outcome 4 Soft tissue pathology.
Adjacent tissue inflammation
One trial, which randomised 90 teeth, assessed adjacent tissue inflammation. There was no statistically significant difference at six (RR 0.50, 95% CI 0.05 to 5.32) and 12 months (R 0.33, 95% CI 0.04 to 3.08).
Pathological mobility
At six months, data were extractable from three RCTs totaling 180 teeth. In two trials, there was no pathological mobility in any of the participants regardless of the intervention. For the remaining trial, the results showed no statistically significant difference (RR 5.00, 95% CI 0.26 to 98.00; Analysis 7.5). At 12 months, one trial, which randomised 90 teeth, assessed pathological mobility. There was no pathological mobility in any of the participants regardless of the intervention.
7.5. Analysis.
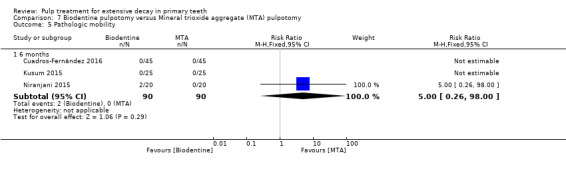
Comparison 7 Biodentine pulpotomy versus Mineral trioxide aggregate (MTA) pulpotomy, Outcome 5 Pathologic mobility.
Pathological radiolucency
Data were extractable from two RCTs totaling 144 teeth. At six months, in one trial, there was no pathological radiolucency in any of the participants regardless of the intervention. For the remaining trial, the results showed no statistically significant evidence (RR 3.46, 95% CI 0.15 to 81.36). At 12 months, the pooled results showed no statistically significant evidence (RR 1.09, 95% CI 0.19 to 6.27; Analysis 7.6).
7.6. Analysis.
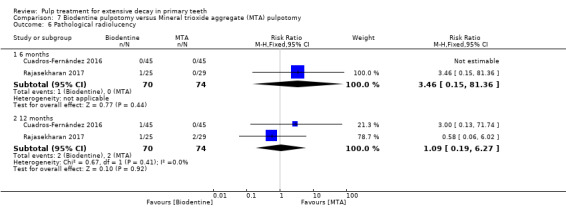
Comparison 7 Biodentine pulpotomy versus Mineral trioxide aggregate (MTA) pulpotomy, Outcome 6 Pathological radiolucency.
Pathological root resorption
Data were extractable from two RCTs totaling 144 teeth. At six months, in one trial, there was no pathological radiolucency in any of the participants regardless of the intervention. For the remaining trial, the results showed no statistically significant evidence (RR 2.32, 95% CI 0.22 to 24.09). At 12 months, the pooled results showed no statistically significant evidence (RR 1.12, 95% CI 0.30 to 4.19; Analysis 7.7).
7.7. Analysis.
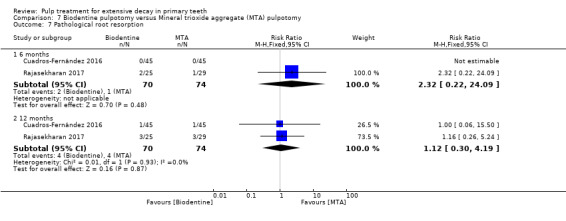
Comparison 7 Biodentine pulpotomy versus Mineral trioxide aggregate (MTA) pulpotomy, Outcome 7 Pathological root resorption.
Pulp canal obliteration
One trial, which randomised 54 teeth, assessed pulp canal obliteration (Rajasekharan 2017). There was a statistically significant difference at six (RR 5.22, 95% CI 1.24 to 21.94) and 12 months (RR 2.44, 95% CI 1.43 to 4.14) in favour of MTA.
Dentin bridge formation
One trial, which randomised 54 teeth, assessed pulp canal obliteration (Rajasekharan 2017). There was no statistically significant differences at six (RR 0.16, 95% CI 0.01 to 3.04) and 12 months (RR 1.28, 95% CI 0.65 to 2.49).
One additional registered trial assessed clinical success (no abscess or any swelling related to the tooth, no fistula or other pathology, no pathological mobility, no post‐operative pain, no pain on palpation or percussion), radiographic success (no root resorption (internal or external), no furcation involvement or periapical radiolucency, no loss of lamina dura, presence of normal appearance of periodontal ligament space) at 36 months (NCT02298504).
Propolis versus MTA
One trial, which randomised 50 teeth, assessed propolis versus MTA based on clinical failure and radiological failure (Kusum 2015). There was no statistically significant differences for any outcomes at six months (clinical failure: RR 3.00, 95% CI 0.13 to 70.30; radiological failure: RR 3.50, 95% CI 0.80 to 15.23).
Aloe vera versus MTA
One trial, which randomised 60 teeth, assessed Aloe vera versus MTA based on clinical failure, radiological failure and overall failure (Kalra 2017). For clinical and overall failures, there was a statistically significant difference at six (RR 51.00, 95% CI 3.25 to 801.15) and 12 months (RR 53.00, 95% CI 3.38 to 831.71) in favour of MTA. For radiological failure, there was a statistically significant difference at six and 12 months (RR 28.00, 95% CI 4.07 to 192.79) in favour of MTA.
Tempophore versus MTA
One trial which randomised 56 teeth, assessed Tempophore versus MTA based on clinical failure, radiological failure, pathological radiolucency, pathological root resorption, pulp canal obliteration and dentin bridge formation (Rajasekharan 2017). There were no statistically significant differences for all outcomes at any time points (Table 13).
10. Pulpotomy (Tempophore) versus pulpotomy (MTA).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 and 12 | 1 | 3.21 (0.14, 75.68) |
| Radiological failure | 6 | 1 | 9.64 (0.54, 171.09) |
| 12 | 1 | 2.69 (0.57, 12.70) | |
| Pathological radiolucency | 6 | 1 | 3.21 (0.14, 75.68) |
| 12 | 1 | 2.15 (0.43, 10.79) | |
| Pathological root resorption | 6 | 1 | 6.44 (0.83, 50.11) |
| 12 | 1 | 4.30 (1.00, 18.47) | |
| Pulp canal obliteration | 6 | 1 | 3.76 (0.85, 16.54) |
| 12 | 1 | 1.61 (0.78, 3.33) | |
| Dentine bridge formation | 6 | 1 | 0.15 (0.01, 2.83) |
| 12 | 1 | 0.07 (0.00, 1.19) |
Abbreviation: CI: confidence interval
MTA versus MTA + sodium hypochlorite (NaOCl)
One trial, which randomised 62 teeth, assessed MTA versus MTA+NaOCl based on clinical failure, radiological failure, overall failure, pain, soft tissue pathology, pathological mobility, adjacent tissue pathology, pathological radiolucency, pathological root resorption and pulp canal obliteration (Akcay 2014). There were no statistically significant differences for any outcome or time point (Table 14).
11. Pulpotomy (MTA) versus pulpotomy (MTA + NaOCl).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 and 12 | 1 | Not estimable* |
| Radiological failure | 6 and 12 | 1 | 0.33 (0.01, 7.88) |
| Soft tissue pathology | 6 and 12 | 1 | Not estimable* |
| Pathologic mobility | 6 and 12 | 1 | Not estimable* |
| Adjacent tissue inflammation | 6 and 12 | 1 | Not estimable* |
| Pathologic radiolucency | 6 and 12 | 1 | Not estimable* |
| Pathologic root resorption | 6 and 12 | 1 | 0.33 (0.01, 7.88) |
| Pulp canal obliteration | 6 | 1 | Not estimable* |
| 12 | 1 | 0.67 (0.21, 2.13) |
* due to lack of events
Abbreviations ‐ CI: confidence interval; MTA: mineral trioxide aggregate; NaOCl; sodium hypochlorite.
ProRoot MTA versus OrthoMTA
One trial, which randomised 94 teeth, assessed ProRoot MTA versus OrthoMTA based on clinical failure, radiological failure, pain, soft tissue pathology, pathological mobility, pathological radiolucency and pathological root resorption (Kang 2015). There were no statistically significant differences for any outcome or time point (Table 15).
12. Pulpotomy (ProRoot MTA) versus pulpotomy (OrthoMTA).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 | 1 | Not estimable* |
| 12 | 1 | 0.33 (0.01, 7.98) | |
| Radiological failure | 6 | 1 | 1.00 (0.06, 15.52) |
| 12 | 1 | 0.50 (0.05, 5.33) | |
| Pain | 6 and 12 | 1 | Not estimable* |
| Soft tissue pathology | 6 | 1 | Not estimable* |
| 12 | 1 | 0.33 (0.01, 7.98) | |
| Pathologic mobility | 6 and 12 | 1 | Not estimable* |
| Pathologic radiolucency | 6 | 1 | 0.33 (0.01, 7.98) |
| 12 | 1 | 0.20 (0.01, 4.06) | |
| Pathologic root resorption | 6 | 1 | 3.00 (0.13, 71.82) |
| 12 | 1 | 1.00 (0.06, 15.52) |
*due to lack of events
Abbreviation: CI: confidence interval.
ProRoot MTA versus RetroMTA
One trial, which randomised 96 teeth, assessed ProRoot MTA versus RetroMTA based on clinical failure, radiological failure, pain, soft tissue pathology, pathological mobility, pathological radiolucency and pathological root resorption (Kang 2015). There were no statistically significant differences for any outcome or time point (Table 16).
13. Pulpotomy (ProRoot MTA) versus pulpotomy (RetroMTA).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 and 12 | 1 | Not estimable* |
| Radiological failure | 6 and 12 | 1 | 0.35 (0.04, 3.22) |
| Pain | 6 and 12 | 1 | Not estimable* |
| Soft tissue pathology | 6 and 12 | 1 | Not estimable* |
| Pathologic mobility | 6 and 12 | 1 | Not estimable* |
| Pathologic radiolucency | 6 and 12 | 1 | 0.35 (0.01, 8.32) |
| Pathologic root resorption | 6 and 12 | 1 | 0.35 (0.04, 3.22) |
*due to lack of events
Abbreviation: CI: confidence interval.
OrthoMTA versus RetroMTA
One trial, which randomised 96 teeth, assessed OrthoMTA versus RetroMTA based on clinical failure, radiological failure, pain, soft tissue pathology, pathological mobility, pathological radiolucency and pathological root resorption (Kang 2015). There were no statistically significant differences for any outcome or time point (Table 17).
14. Pulpotomy (OrthoMTA) versus pulpotomy (RetroMTA).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 | 1 | Not estimable* |
| 12 | 1 | 3.13 (0.13, 74.85) | |
| Radiological failure | 6 | 1 | 0.35 (0.04, 3.22) |
| 12 | 1 | 0.70 (0.12, 3.98) | |
| Pain | 6 and 12 | 1 | Not estimable* |
| Soft tissue pathology | 6 | 1 | Not estimable* |
| 12 | 1 | 3.13 (0.13, 74.85) | |
| Pathologic mobility | 6 and 12 | 1 | Not estimable* |
| Pathologic radiolucency | 6 | 1 | 1.04 (0.07, 16.19) |
| 12 | 1 | 2.09 (0.20, 22.24) | |
| Pathologic root resorption | 6 | 1 | 0.15 (0.01, 2.81) |
| 12 | 1 | 0.35 (0.04, 3.22) |
*due to lack of events
Abbreviation: CI: confidence interval.
Calcium hydroxide versus formocresol
Clinical failure
At six months, data were extractable from six RCTs totaling 332 teeth. In two of the trials, there was no clinical failure in any of the participants regardless of the intervention. From the four remaining trials, the pooled results showed a larger risk of clinical failure with calcium hydroxide compared with formocresol. The pooled RR was 1.98 (95% CI 1.17 to 3.37). The statistical heterogeneity among included trials was substantial (I² = 57%). Results were similar at 12 months (RR 1.87, 95% CI 1.22 to 2.89). At 24 months, data were extractable from three RCTs totaling 150 teeth. The pooled results showed no statistically significant difference (RR 2.18, 95% CI 0.78 to 6.11; Analysis 8.1).
8.1. Analysis.
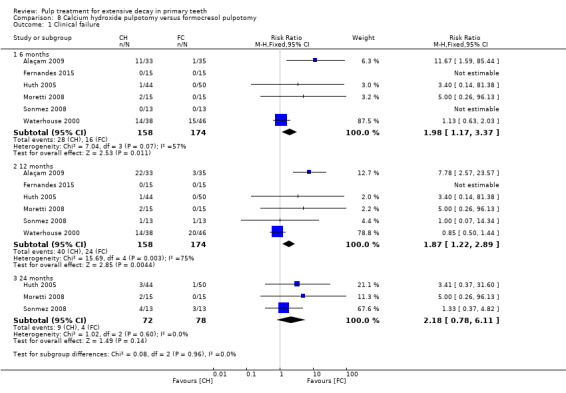
Comparison 8 Calcium hydroxide pulpotomy versus formocresol pulpotomy, Outcome 1 Clinical failure.
One additional trial, which randomised 76 teeth, did not assess clinical failure at a fixed time point but at an interval follow‐up of zero to six, seven to 12 and 13 to 24 months (Zurn 2008). Between zero and six months, the RR was 1.00 (95% CI 0.06 to 15.41). Results were similar between seven and 12 months and between 13 and 24 months.
Radiological failure
At six months, data were extractable from four RCTs totaling 154 teeth. In one of the trials, there was no radiological failure in any of the participants regardless of the intervention. From the three remaining trials, the pooled results showed a larger risk of radiological failure with calcium hydroxide compared with formocresol (RR 15.48, 95% CI 3.86 to 62.06) with no evidence of statistical heterogeneity among included trials (I² = 0%). Results at 12 months were RR 1.86 (95% CI 1.42 to 2.44) with six trials providing data (332 teeth randomised) and with substantial statistical heterogeneity (I² = 89%), and at 24 months the RR was 3.63 (95% CI 1.73 to 7.61) with three trials providing data (150 teeth randomised) and with no evidence of statistical heterogeneity (I² = 0%) (Analysis 8.2).
8.2. Analysis.
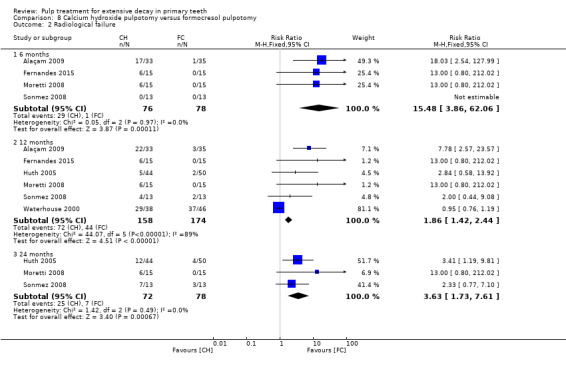
Comparison 8 Calcium hydroxide pulpotomy versus formocresol pulpotomy, Outcome 2 Radiological failure.
The trial by Zurn 2008 did not assess radiological failure at a fixed time point but at an interval follow‐up of zero to six, seven to 12 and 13 to 24 months. Between zero and six months, the RR was 1.00 (95% CI 0.06 to 15.41). Results were similar between seven and 12 months. Between 13 and 24 months, the RR was 9 (95% CI 1.20 to 67.60).
Overall failure
At 12 months, data were extractable from two RCTs totaling 120 teeth. The pooled results showed no statistically significant difference in overall failure with calcium hydroxide compared with formocresol. The pooled RR was 2.41 (95% CI 0.80 to 7.21). Results were similar at 24 months, although the difference was statistically significant and in favour of formocresol (RR 2.93, 95% CI 1.35 to 6.34), with no evidence of statistical heterogeneity (I² = 0%; Analysis 8.3).
8.3. Analysis.
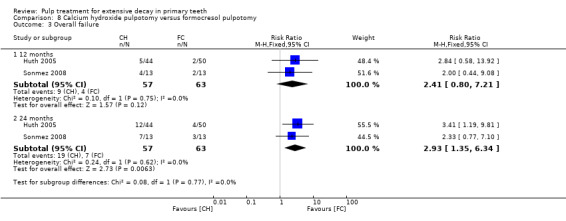
Comparison 8 Calcium hydroxide pulpotomy versus formocresol pulpotomy, Outcome 3 Overall failure.
One additional trial did not assess overall failure at a fixed time point but at an interval follow‐up of zero to six, seven to 12 and 13 to 24 months (Zurn 2008). Between zero and six months, the RR was 1.00 (95% CI 0.15 to 6.74). Between seven and 12 months, the RR was 4.50 (95% CI 1.04 to 19.47). Results were similar between 13 and 24 months.
Pain
At six months, data were extractable from four RCTs totaling 276 teeth. In three of the trials, there was no pain in any of the participants regardless of the intervention. The results for the remaining trial showed no statistically significant difference in pain with calcium hydroxide compared with formocresol (RR 3.18, 95% CI 0.35 to 29.08). At 12 months, the results were statistically significant (RR 6.30, 95% CI 1.15 to 34.40), in favour of formocresol with no evidence of statistical heterogeneity (I² = 0%; Analysis 8.4).
8.4. Analysis.
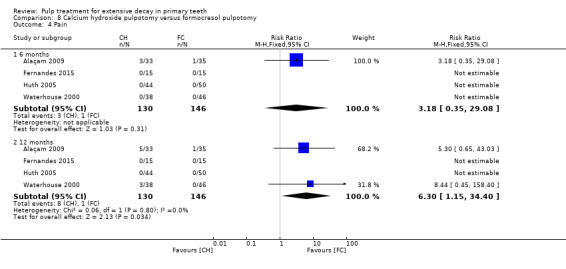
Comparison 8 Calcium hydroxide pulpotomy versus formocresol pulpotomy, Outcome 4 Pain.
Soft tissue pathology
At six months, data were extractable from five RCTs totaling 306 teeth. In three of the four trials, there was no soft tissue pathology in any of the participants regardless of the intervention. From the two remaining trials, the pooled results showed no statistical difference in soft tissue pathology with calcium hydroxide compared with formocresol. The pooled RR was 5.14 (95% CI 0.63 to 42.25). At 12 months, the results were statistically significant (RR 6.77, 95% CI 1.23 to 37.10) in favour of formocresol with three trials providing data and with no evidence of statistical heterogeneity (I² = 0%). However, this was not statistically significant at 24 months, with only two trials providing data (Analysis 8.5).
8.5. Analysis.
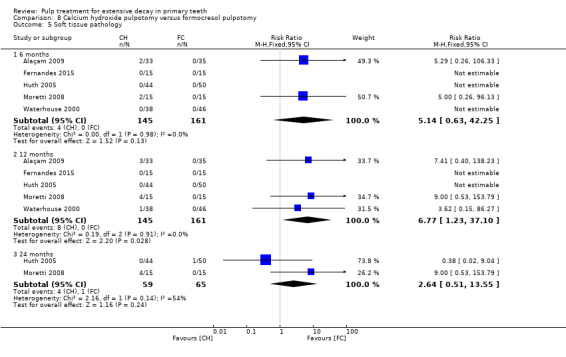
Comparison 8 Calcium hydroxide pulpotomy versus formocresol pulpotomy, Outcome 5 Soft tissue pathology.
One additional trial, which randomised 76 teeth, did not assess soft tissue pathology at a fixed time point but at an interval follow‐up of zero to six, seven to 12 and 13 to 24 months (Zurn 2008). Between zero and six months, the RR was 1.00 (95% CI 0.06 to 15.41). Results were similar between seven and 12 months and between 13 and 24 months.
Pathological mobility
At six months, data were extractable from four RCTs totaling 238 teeth. In three of the trials, there was no pathological mobility in any of the participants regardless of the intervention. From the remaining trial, the results showed no statistically significant difference in pathological mobility between calcium hydroxide and formocresol (RR 1.21, 95% CI 0.18 to 8.19). At 12 months, results were similar with two of the four trials providing data (RR 1.14, 95% CI 0.40 to 3.31). At 24 months, results were similar with one of two trials providing data (124 teeth randomised) (RR 9.00, 95% CI 0.53 to 153.79; Analysis 8.6).
8.6. Analysis.
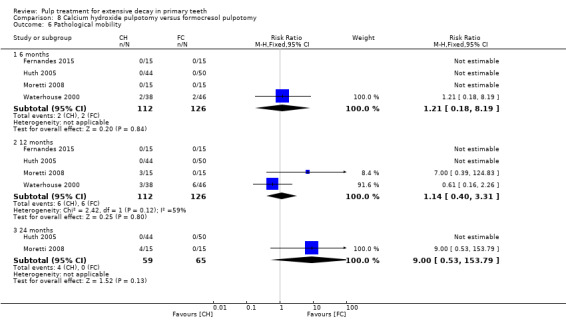
Comparison 8 Calcium hydroxide pulpotomy versus formocresol pulpotomy, Outcome 6 Pathological mobility.
Secondary caries at the margin (clinically)
One trial, which randomised 84 teeth, assessed secondary caries at the margin (Waterhouse 2000). At six months, the RR was 0.17 (95% CI 0.01 to 3.23). Results were similar at 12 months.
Defective restoration (clinically)
One trial, which randomised 84 teeth, assessed defective restoration (Waterhouse 2000). At six months, there was no statistically significant difference in the number of defective restorations between treatment groups (RR 0.40, 95% CI 0.02 to 9.59); at 12 months, the RR was 1.21 (95% CI 0.08 to 18.72).
Pathological radiolucency
At six months, data were extractable from three RCTs totaling 98 teeth. For one trial, there was no pathological radiolucency in any of the participants regardless of the intervention. For the remaining trials, the pooled results showed no statistically significant difference in pathological radiolucency for calcium hydroxide compared with formocresol (RR 3.78, 95% CI 0.64 to 22.17). Results were similar at 12 months with four trials providing data (276 teeth randomised) (RR 1.90, 95% CI 0.67 to 5.40), and at 24 months with two trials providing data (124 teeth randomised) (RR 3.24, 95% CI 0.79 to 13.28; Analysis 8.7).
8.7. Analysis.
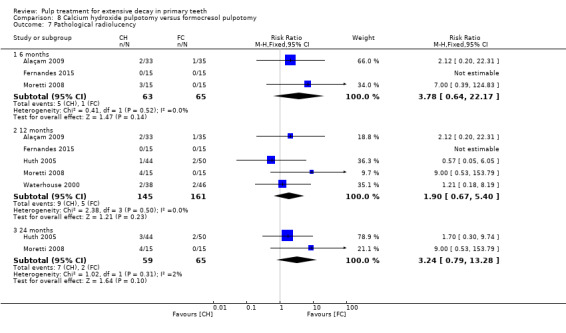
Comparison 8 Calcium hydroxide pulpotomy versus formocresol pulpotomy, Outcome 7 Pathological radiolucency.
One additional trial, which randomised 76 teeth, did not assess pathological radiolucency at a fixed time point but at an interval follow‐up of zero to six, seven to 12 and 13 to 24 months (Zurn 2008). Between zero and six months, the RR was 1.00 (95% CI 0.15 to 6.74). Between seven and 12 months, the RR was 4.50 (95% CI 1.04 to 19.47). Results were similar between 13 and 24 months.
Pathological root resorption
At six months, data were extractable from four RCTs totaling 154 teeth. In one of the trials, there was no pathological root resorption in any of the participants regardless of the intervention. From the remaining trials, the pooled results showed a larger risk of pathological root resorption with calcium hydroxide compared with formocresol (RR 11.87, 95% CI 2.33 to 60.40), with no evidence of statistical heterogeneity among trials (I² = 0%). Results were similar at 12 months, with five of six trials providing data (332 teeth randomised) (RR 6.25, 95% CI 2.04 to 19.14), and at 24 months, with three trials providing data (150 teeth randomised) (RR 4.59, 95% CI 1.33 to 15.81; Analysis 8.8).
8.8. Analysis.
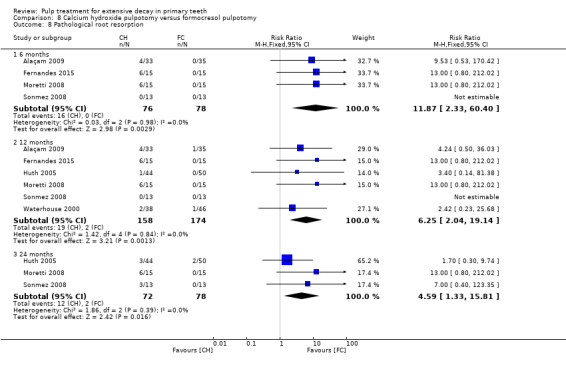
Comparison 8 Calcium hydroxide pulpotomy versus formocresol pulpotomy, Outcome 8 Pathological root resorption.
One additional trial, which randomised 76 teeth, did not assess pathological root resorption at a fixed time point but at an interval follow‐up of zero to six, seven to 12 and 13 to 24 months (Zurn 2008). Between zero and six months, the RR was 1.00 (95% CI 0.31 to 3.17). Results were similar between seven and 12 months and between 13 and 24 months.
Pulp canal obliteration
At six months, data were extractable from two trials totaling 56 teeth. There was no statistically significant difference (RR 4.00, 95% CI 0.47 to 33.75). At 12 months, data were extractable from three RCTs totaling 140 teeth. The pooled results showed no statistically significant difference in pulp canal obliteration with calcium hydroxide compared with formocresol (RR 2.68, 95% CI 0.91 to 7.95; Analysis 8.9).
8.9. Analysis.
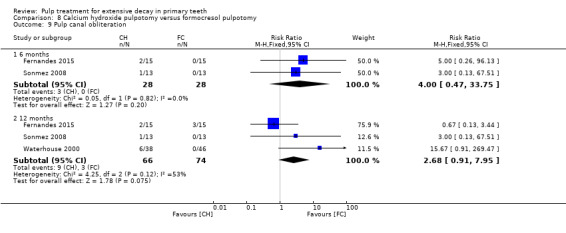
Comparison 8 Calcium hydroxide pulpotomy versus formocresol pulpotomy, Outcome 9 Pulp canal obliteration.
One additional trial, which randomised 76 teeth, did not assess pulp canal obliteration at a fixed time point but at an interval follow‐up of zero to six, seven to 12 and 13 to 24 months. Between zero and six months, the RR was 1.00 (95% CI 0.42 to 2.39) (Zurn 2008). Results were similar between seven and 12 months. Between 13 and 24 months, the RR was 1.88 (95% CI 1.29 to 2.72).
Dentin bridge formation
Data were extractable from two RCTs totaling 60 teeth. There was a statistically significant difference in favour of calcium hydroxide at six (RR 13.00, 95% CI 1.81 to 93.60) and 12 months (RR 14.00, 95% CI 1.95 to 100.26; Analysis 8.10).
8.10. Analysis.
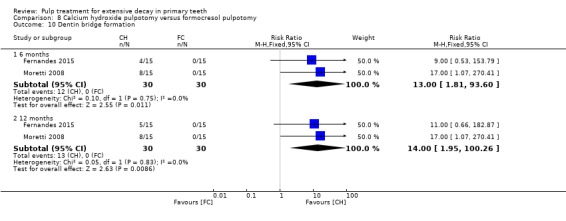
Comparison 8 Calcium hydroxide pulpotomy versus formocresol pulpotomy, Outcome 10 Dentin bridge formation.
Physiological root resorption
One trial, which randomised 84 teeth, assessed physiological root resorption at 12 months (Waterhouse 2000). There was no statistically significant difference between groups (RR 0.40, 95% CI 0.12 to 1.39).
Secondary caries (radiographically)
One trial, which randomised 84 teeth, assessed secondary caries at 12 months (Waterhouse 2000). There was no statistically significant difference between groups (RR 3.62, 95% CI 0.15 to 86.28).
Calcium hydroxide versus ferric sulphate
Clinical failure
At six, 12 and 24 months, data were extractable from two RCTs totaling 122 teeth. At six months, one trial showed no cases of clinical failure in either treatment group. For the second trial, the results showed no statistically difference in clinical failure with calcium hydroxide compared with ferric sulphate (RR 3.40, 95% CI 0.14 to 81.38). At 12 months, the pooled results showed no statistically significant difference in clinical failure with calcium hydroxide compared with ferric sulphate (RR 3.41, 95% CI 0.37 to 31.61). Results were similar at 24 months (RR 3.44, 95% CI 0.90 to 13.18; Analysis 9.1).
9.1. Analysis.
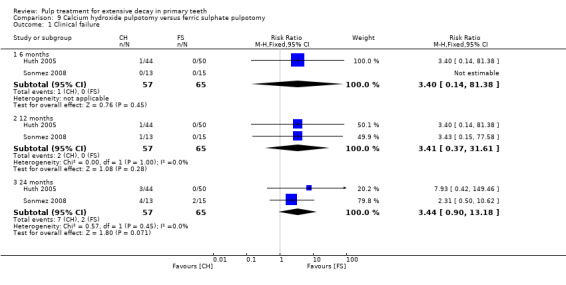
Comparison 9 Calcium hydroxide pulpotomy versus ferric sulphate pulpotomy, Outcome 1 Clinical failure.
Radiological failure
At 12 and 24 months, data were extractable from two RCTs totaling 122 teeth. At 12 months, the pooled results showed no statistically significant difference in radiological failure with calcium hydroxide compared with ferric sulphate. The pooled RR was 1.28 (95% CI 0.53 to 3.13). The direction of effect was the same at 24 months, although the difference was statistically significant (RR 1.97, 95% CI 1.04 to 3.75) in favour of ferric sulphate, and there was no evidence of statistical heterogeneity among included trials (I² = 0%; Analysis 9.2).
9.2. Analysis.
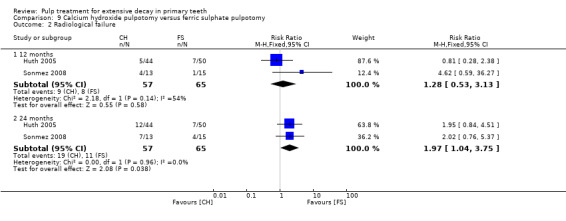
Comparison 9 Calcium hydroxide pulpotomy versus ferric sulphate pulpotomy, Outcome 2 Radiological failure.
Overall failure
At 12 and 24 months, data were extractable from two RCTs totaling 122 teeth. At 12 months, the pooled results showed no statistically significant difference in overall failure with calcium hydroxide compared with ferric sulphate (RR 1.28, 95% CI 0.53 to 3.13). The statistical heterogeneity among included trials was substantial (I² = 54%). At 24 months, the difference was statistically significant (RR 1.97, 95% CI 1.04 to 3.75) in favour of ferric sulphate, with no evidence of statistical heterogeneity among included trials (I² = 0%; Analysis 9.3).
9.3. Analysis.
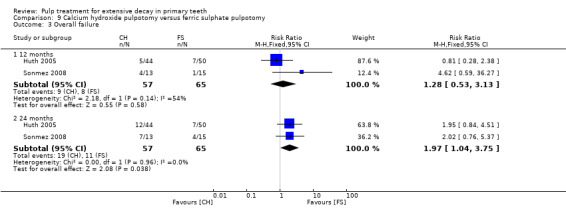
Comparison 9 Calcium hydroxide pulpotomy versus ferric sulphate pulpotomy, Outcome 3 Overall failure.
Pathological root resorption
At 12 and 24 months, data were extractable from two RCTs totaling 122 teeth. At 12 months, one of the two trials found no pathological root resorption in any of the participants regardless of the intervention. In the second trial, the results showed no statistically significant difference in pathological root resorption with calcium hydroxide compared with ferric sulphate (RR 0.57, 95% CI 0.05 to 6.05). Results were similar at 24 months, with the two trials providing data (RR 2.29, 95% CI 0.60 to 8.66; Analysis 9.4).
9.4. Analysis.
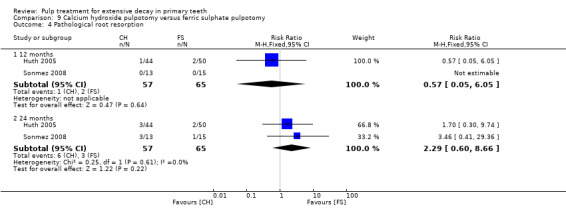
Comparison 9 Calcium hydroxide pulpotomy versus ferric sulphate pulpotomy, Outcome 4 Pathological root resorption.
Pulp canal obliteration
One trial, which randomised 28 teeth, assessed pulp canal obliteration (Sonmez 2008). At six and 12 months, there was no pulp canal obliteration in any of the participants regardless of the intervention. At 24 months, the RR was 0.38 (95% CI 0.05 to 3.26).
Calcium hydroxide versus Portland cement
One trial, which randomised 30 teeth, assessed calcium hydroxide versus Portland cement based on clinical failure, radiological failure, soft tissue pathology, pathological mobility, pathological radiolucency, pathological root resorption, and dentin bridge formation (Oliveira 2013a). There were statistically significant differences for radiological failure, pathological radiolucency and pathological root resorption at 12 (RR 17.00, 95% CI 1.07 to 270.41) and 24 months (RR 21.00, 95% CI 1.34 to 328.86). There were no statistically significant differences for other outcomes (Table 18).
15. Pulpotomy (CH) versus pulpotomy (PC).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 | 1 | 5.00 (0.26, 96.13) |
| 12 and 24 | 1 | 13.00 (0.80, 212.02) | |
| Radiological failure | 6 | 1 | 13.00 (0.80, 212.02) |
| 12 | 1 | 17.00 (1.07 to 270.41) | |
| 24 | 1 | 21.00 (1.34 to 328.86) | |
| Soft tissue pathology | 6 | 1 | 5.00 (0.26, 96.13) |
| 12 and 24 | 1 | 13.00 (0.80, 212.02) | |
| Pathologic mobility | 6 | 1 | 5.00 (0.26, 96.13) |
| 12 and 24 | 1 | 13.00 (0.80, 212.02) | |
| Adjacent tissue inflammation | 6, 12 and 24 | 1 | Not estimable * |
| Pathologic radiolucency | 6 | 1 | 13.00 (0.80, 212.02) |
| 12 | 1 | 17.00 (1.07 to 270.41) | |
| 24 | 1 | 21.00 (1.34 to 328.86) | |
| Pathologic root resorption | 6 | 1 | 13.00 (0.80, 212.02) |
| 12 | 1 | 17.00 (1.07 to 270.41) | |
| 24 | 1 | 21.00 (1.34 to 328.86) | |
| Dentine bridge formation | 6, 12 and 24 | 1 | 0.50 (0.11 to 2.33) |
*due to lack of events
Abbreviations ‐ CI: confidence interval; CH: calcium hydroxide; PC: Portland cement.
Calcium hydroxide versus MTA + sodium hypochlorite (NaOCl)
One trial, which randomised 62 teeth, assessed MTA+NaOCl versus calcium hydroxide + NaOCl based on clinical failure, radiological failure, overall failure, pain, soft tissue pathology, pathological mobility, adjacent tissue pathology, pathological radiolucency, pathological root resorption and pulp canal obliteration (Akcay 2014). The results showed statistically significant differences for radiological failure and pathological root resorption at 12 months (RR 8.00, 95% CI 1.06 to 60.21; RR 17.00, 95% CI 1.02 to 282.30, respectively). There were no statistically significant differences for other outcomes at any time point (Table 19).
16. Pulpotomy (CH) versus pulpotomy (MTA + NaOCl).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 | 1 | Not estimable* |
| 12 | 1 | 3.00 (0.13, 70.92) | |
| Radiological failure | 6 | 1 | 5.00 (0.62, 40.36) |
| 12 | 1 | 8.00 (1.06, 60.21) | |
| Soft tissue pathology | 6 | 1 | Not estimable* |
| 12 | 1 | 3.00 (0.13, 70.92) | |
| Pathologic mobility | 6 and 12 | 1 | Not estimable* |
| Adjacent tissue inflammation | 6 and 12 | 1 | Not estimable* |
| Pathologic radiolucency | 6 | 1 | Not estimable* |
| 12 | 1 | 15.00 (0.89, 251.77) | |
| Pathologic root resorption | 6 | 1 | Not estimable* |
| 12 | 17.00 (1.02, 282.30) | ||
| Pulp canal obliteration | 6 | 1 | Not estimable* |
| 12 | 1 | 1.33 (0.52, 3.39) |
*due to lack of events
Abbreviations ‐ CI: confidence interval; CH: calcium hydroxyde; MTA: mineral trioxide aggregate; NaOCl; sodium hypochlorite.
Erbium:yttrium‐aluminium garnet (Er:YAG) laser versus calcium hydroxide
One trial, which randomised 91 teeth, assessed Er:YAG laser versus calcium hydroxide based on clinical failure, radiological failure, overall failure, pain, soft tissue pathology, pathological mobility, pathological radiolucency and pathological root resorption (Huth 2005). Statistically significant differences were shown at 24 months with regard to radiological failure (RR 0.31 (95% CI 0.11 to 0.90) and overall failure (RR 0.31 (95% CI 0.11 to 0.90), in favour of Er:YAG laser. There were no statistically significant differences for any other outcome or time point (Table 20).
17. Pulpotomy (Er:YAG laser) versus pulpotomy (CH).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 | 1 | 0.31 (0.01 to 7.48) |
| 12 | 1 | 0.94 (0.60 to 14.52) | |
| 24 | 1 | 0.62 (0.11 to 3.56) | |
| Radiological failure | 12 | 1 | 0.56 (0.14 to 2.21) |
| 24 | 1 | 0.31 (0.11 to 0.90) | |
| Overall failure | 12 | 1 | 0.56 (0.14 to 2.21) |
| 24 | 1 | 0.31 (0.11 to 0.90) | |
| Pain | 6, 12 and 24 | 1 | Not estimable* |
| Soft tissue pathology | 6, 12 and 24 | 1 | Not estimable* |
| Pathological mobility | 6, 12 and 24 | 1 | Not estimable* |
| Pathological radiolucency | 12 | 1 | 0.31 (0.01 to 7.48) |
| 24 | 1 | 0.62 (0.11 to 3.56) | |
| Pathological root resorption | 12 | 1 | 0.94 (0.60 to 14.52) |
| 24 | 1 | 0.62 (0.11 to 3.56) |
*due to lack of events
Abbreviations ‐ CI: confidence interval; Er:YAG: erbium:yttrium‐aluminium garnet; CH: calcium hydroxide.
Calcium hydroxide/iodoform versus calcium hydroxide
One trial, which randomised 65 teeth, assessed calcium hydroxide/iodoform versus calcium hydroxide based on clinical failure, radiological failure, pain, soft tissue pathology, pathological radiolucency and pathological root resorption (Alaçam 2009). There were no statistically significant differences for any outcome or time point (Table 21).
18. Pulpotomy (CH/iodoform) versus pulpotomy (CH).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 | 1 | 1.41 (0.77 to 2.58) |
| 12 | 1 | 1.13 (0.82 to 1.54) | |
| Radiological failure | 6 | 1 | 1.33 (0.89 to 2.00) |
| 12 | 1 | 1.17 (0.87 to 1.59) | |
| Pain | 6 | 1 | 1.72 (0.45 to 6.61) |
| 12 | 1 | 1.03 (0.33 to 3.23) | |
| Soft tissue pathology | 6 | 1 | 1.55 (0.28 to 8.65) |
| 12 | 1 | 1.03 (0.22 to 4.74) | |
| Pathological radiolucency | 6 and 12 | 1 | 5.15 (0.26 to 103.31) |
| Pathological root resorption | 6 | 1 | 1.72 (0.45 to 6.61) |
| 12 | 1 | 1.29 (0.38 to 4.37) |
CI: confidence interval; CH: calcium hydroxide.
Low‐level laser therapy (LLLT) versus calcium hydroxide
One trial, which randomised 30 teeth, assessed LLLT versus calcium hydroxide based on clinical failure, radiological failure, pain, soft tissue pathology, adjacent tissue inflammation, pathologic mobility, pathologic radiolucency, pathologic root resorption, pulp canal obliteration, and dentin bridge formation at six and 12 months (Fernandes 2015). There was no clinical failure, pain, soft tissue pathology, adjacent tissue inflammation pathologic mobility and pathologic radiolucency in any of the participants regardless of the intervention. There were no statistically significant differences for radiological failure and pathologic root resorption at six and 12 months (RR 0.50, 95% CI 0.15 to 1.64), pulp canal obliteration at six (RR 0.50, 95% CI 0.05 to 4.94) and 12 months (RR 2.50, 95% CI 0.57 to 10.93), and dentin bridge formation at six (RR 0.11, 95% CI 0.01 to 1.90) and 12 months (RR 0.09, 95% CI 0.01 to 1.51).
Low‐level laser therapy (LLLT) + calcium hydroxide versus calcium hydroxide
One trial, which randomised 30 teeth, assessed LLLT + calcium hydroxide versus calcium hydroxide based on clinical failure, radiological failure, pain, soft tissue pathology, adjacent tissue inflammation, pathologic mobility, pathologic radiolucency, pathologic root resorption, pulp canal obliteration, and dentin bridge formation at six and 12 months (Fernandes 2015). There was no clinical failure, pain, soft tissue pathology, adjacent tissue inflammation pathologic mobility and pathologic radiolucency in any of the participants regardless of the intervention. There were no statistically significant differences for radiological failure and pathologic root resorption at six (RR 0.33, 95% CI 0.08 to 1.39) and 12 months (RR 0.50, 95% CI 0.15 to 1.64), pulp canal obliteration at six and 12 months (RR 0.50, 95% CI 0.05 to 4.94), and dentin bridge formation at six (RR 1.75, 95% CI 0.64 to 4.75) and 12 months (RR 1.40, 95% CI 0.57 to 3.43).
Low‐level laser therapy (LLLT) versus LLLT + calcium hydroxide
One trial, which randomised 30 teeth, assessed LLLT versus LLLT + calcium hydroxide based on clinical failure, radiological failure, pain, soft tissue pathology, adjacent tissue inflammation, pathologic mobility, pathologic radiolucency, pathologic root resorption, pulp canal obliteration, and dentin bridge formation at six and 12 months (Fernandes 2015). There was no clinical failure, pain, soft tissue pathology, adjacent tissue inflammation pathologic mobility and pathologic radiolucency in any of the participants regardless of the intervention. There were no statistically significant differences for radiological failure and pathologic root resorption at six (RR 1.50, 95% CI 0.29 to 7.73) and 12 months (RR 1.00, 95% CI 0.24 to 4.18), pulp canal obliteration at six (RR 1.00, 95% CI 0.07 to 14.55) and 12 months (RR 5.00, 95% CI 0.66 to 37.85), and dentin bridge formation at six and 12 months (RR 0.07, 95% CI 0.00 to 1.07).
Calcium hydroxide + sodium hypochlorite (NaOCl) versus calcium hydroxide
One trial, which randomised 62 teeth, assessed calcium hydroxide + NaOCl versus calcium hydroxide based on clinical failure, radiological failure, overall failure, pain, soft tissue pathology, pathological mobility, adjacent tissue pathology, pathological radiolucency, pathological root resorption and pulp canal obliteration (Akcay 2014). There were no statistically significant differences for any outcome or time point (Table 22).
19. Pulpotomy (CH + NaOCl) versus pulpotomy (CH).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 | 1 | Not estimable* |
| 12 | 1 | 0.33 (0.01, 7.88) | |
| Radiological failure | 6 | 1 | 0.09 (0.01, 1.58) |
| 12 | 1 | 0.63 (0.23, 1.70) | |
| Soft tissue pathology | 6 | 1 | Not estimable* |
| 12 | 1 | 0.33 (0.01, 7.88) | |
| Pathologic mobility | 6 and 12 | 1 | Not estimable* |
| Adjacent tissue inflammation | 6 and 12 | 1 | Not estimable* |
| Pathologic radiolucency | 6 | 1 | Not estimable* |
| 12 | 1 | 0.43 (0.12, 1.51) | |
| Pathologic root resorption | 6 | 1 | Not estimable* |
| 12 | 0.38 (0.11, 1.28) | ||
| Pulp canal obliteration | 6 | 1 | Not estimable* |
| 12 | 1 | 1.13 (0.50, 2.53) |
*due to lack of events
Abbreviations ‐ CI: confidence interval; CH: calcium hydroxide; NaOCl: sodium hypochlorite.
Biodentine versus calcium hydroxide
One trial, which randomised 62 teeth, assessed calcium hydroxide + NaOCl versus calcium hydroxide based on clinical failure, radiological failure, pain, soft tissue pathology, defective restoration (clinically), secondary caries at the margin (clinically), pathological radiolucency, and pathological root resorption (Grewal 2016). There were no radiological failures, no soft tissue pathology and no secondary caries at the margin at six and 12 months. There were no statistically significant differences for clinical failure and pain at six and 12 months (RR 0.33, 95% CI 0.01 to 7.72), defective restoration at 12 months (RR 1.00, 95% CI 0.39 to 2.58), and pathological root resorption at six (RR 0.33, 95% CI 0.01 to 7.72) and 12 months (RR 0.20, 95% CI 0.01 to 3.92).
Ferric sulphate versus formocresol
Clinical failure
At six months, data were extractable from six RCTs totaling 394 teeth. In five RCTs, there was no clinical failure in any of the participants regardless of the intervention. For the remaining trials, there was no statistically significant difference (RR 1.00, 95% CI 0.15 to 6.87). Results were similar at 12 months, with five trials providing data (RR 1.38, 95% CI 0.45 to 4.27). At 24 months, data were extractable from five RCTs totaling 258 teeth. The pooled results for the three trials showed no statistically significant difference in clinical failure (RR 0.83, 95% CI 0.40 to 1.70; Analysis 10.1).
10.1. Analysis.
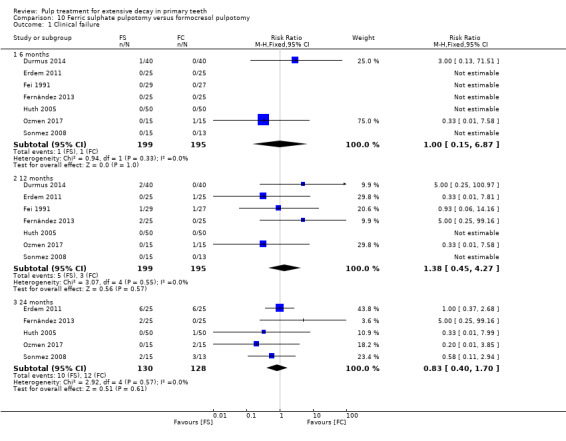
Comparison 10 Ferric sulphate pulpotomy versus formocresol pulpotomy, Outcome 1 Clinical failure.
One trial, which randomised 164 teeth, did not assess clinical failure at a fixed time point but at an interval follow‐up of three to 20 and 46 to 48 months (Ibricevic 2000). The RR was 1.00 (95% CI 0.02 to 49.04) at three to 20 months and RR 1.58 (95% CI 0.27 to 9.18) at 46 to 48 months.
Radiological failure
At six months, data were extractable from six RCTs totaling 294 teeth. In two trials, there was no radiological failure in any of the participants regardless of the intervention. From the remaining trials, the pooled results showed no statistically significant difference in clinical failure with ferric sulphate compared with formocresol (RR 0.79, 95% CI 0.32 to 1.92). Results were similar at 12 months, with six trials providing data (394 teeth randomised) (RR 1.33, 95% CI 0.73, 2.42), and at 24 months, with five trials providing data (258 teeth randomised) (RR 1.26, 95% CI 0.71 to 2.24; Analysis 10.2).
10.2. Analysis.
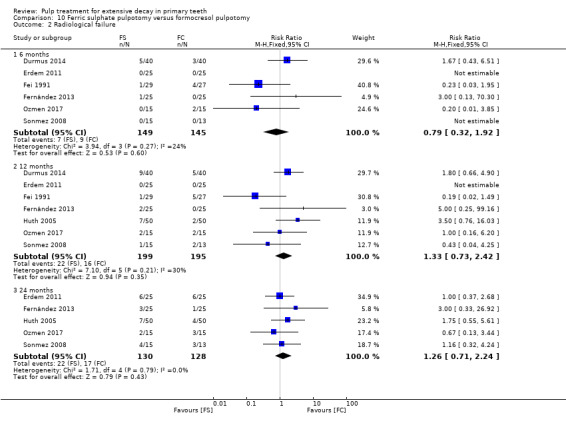
Comparison 10 Ferric sulphate pulpotomy versus formocresol pulpotomy, Outcome 2 Radiological failure.
One trial, which randomised 164 teeth, did not assess radiological failure at a fixed time point but at an interval follow‐up of three to 20 and 46 to 48 months (Ibricevic 2000). The RR was 1.00 (95% CI 0.42 to 2.36) at three to 20 months. Results were similar at 46 to 48 months.
Overall failure
At six months, data were extractable from four RCTs totaling 184 teeth. In two trials, there was no overall failure in any of the participants regardless of the intervention. From the two remaining trials, the pooled results showed no statistically significant difference in clinical failure with ferric sulphate compared with formocresol (RR 0.53, 95% CI 0.12 to 2.37). Results were similar at 12 months, with four of five trials providing data (284 teeth randomised) (RR 1.16, 95% CI 0.51, 2.64), and at 24 months, with four trials providing data (228 teeth randomised) (RR 1.49, 95% CI 0.74 to 3.01; Analysis 10.3).
10.3. Analysis.
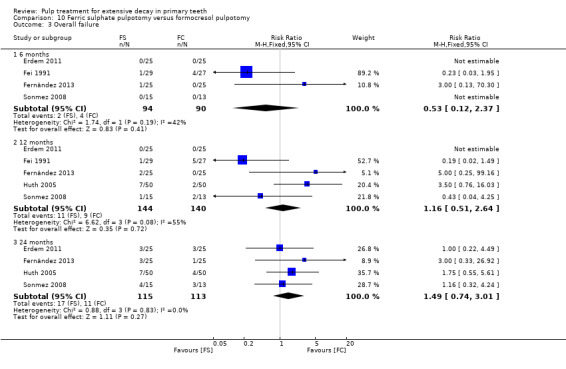
Comparison 10 Ferric sulphate pulpotomy versus formocresol pulpotomy, Outcome 3 Overall failure.
Two additional trials, which randomised 96 teeth (Fuks 1997) and 164 teeth (Ibricevic 2000), did not assess overall failure at a fixed time point but at an interval follow‐up of 24 to 35 months and 46 to 48 months, respectively. The RR was 0.44 (95% CI 0.13 to 1.45) for 24 to 35 months (Fuks 1997) and 1.33 (95% CI 0.44 to 4.03) for 46 to 48 months (Ibricevic 2000).
Pain
Data were extractable from four RCTs totaling 230 teeth. In three trials, no participant declared pain regardless of the intervention. For the remaining trial, there was no statistically significant difference at six, 12 (RR 0.33, 95% CI 0.01 to 7.58) and 24 months (RR 0.20, 95% CI 0.01 to 3.85; Analysis 10.4).
10.4. Analysis.
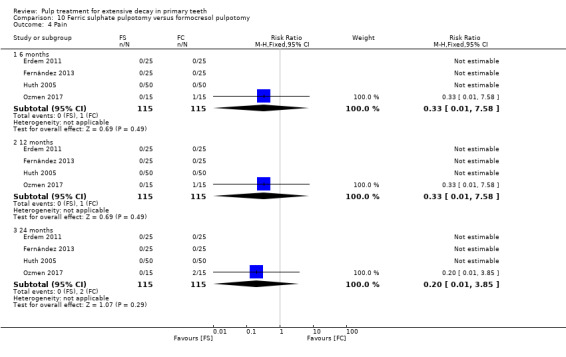
Comparison 10 Ferric sulphate pulpotomy versus formocresol pulpotomy, Outcome 4 Pain.
Soft tissue pathology
One trial, which randomised 30 teeth, assessed soft tissue pathology at six, 12 and 24 months (Ozmen 2017). There was no statistically significant evidence (RR 0.33, 95% CI 0.01 to 7.58).
Pathologic mobility
One trial, which randomised 30 teeth, assessed soft tissue pathology at six, 12 and 24 months (Ozmen 2017). There was no statistically significant evidence (RR 0.33, 95% CI 0.01 to 7.58).
Adjacent tissue inflammation
One trial, which randomised 50 teeth, assessed adjacent tissue inflammation (Fernández 2013). There was no adjacent tissue inflammation in any of the participants regardless of the intervention. At 12 and 24 months, there was no statistically significant difference (RR 5.00, 95% CI 0.25, 99.16).
Pathological radiolucency
At six months, two trials, which randomised 80 teeth, assessed pathological radiolucency (Fernández 2013, Ozmen 2017). There was no pathological radiolucency in any of the participants regardless of the intervention. At 12 months, data were extractable from four RCTs totaling 230 teeth. In two trials, there was no pathological radiolucency in any of the participants regardless of the intervention. From the remaining trials, the results showed no statistically significant difference in pathological radiolucency with ferric sulphate compared with formocresol (RR 1.80, 95% CI 0.40 to 8.17). Results were similar at 24 months (RR 2.20, 95% CI 0.51 to 9.50; Analysis 10.5).
10.5. Analysis.
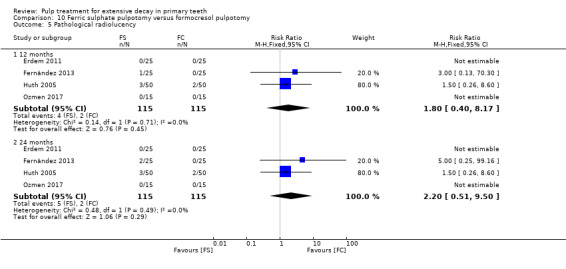
Comparison 10 Ferric sulphate pulpotomy versus formocresol pulpotomy, Outcome 5 Pathological radiolucency.
Two additional trials, which randomised 96 (Fuks 1997) and 164 (Ibricevic 2000) teeth, did not assess pathological radiolucency at a fixed time point but at an interval follow‐up of six to 11, 12 to 23 and 24 to 35 months for Fuks 1997 and three to 20 and 46 to 48 months for Ibricevic 2000. In the two trials, there was no pathological radiolucency in any of the participants regardless of the intervention at the first delays. At 24 to 35 months, the RR was 0.44 (95% CI 0.08 to 2.49) (Fuks 1997) and at 46 to 48 months RR 2.86 (95% CI 0.30 to 26.90) (Ibricevic 2000).
Pathological root resorption
At six months, data were extractable from five RCTs totaling 314 teeth. In three trials, there was no pathological root resorption in any of the participants regardless of the intervention. For the remaining trials, the pooled results showed no statistically significant evidence (RR 0.67, 95% CI 0.12 to 3.84). At 12 months, data were extractable from SIX RCTs totaling 314 teeth. In two trials, there was no pathological root resorption in any of the participants regardless of the intervention. From the remaining trials, the pooled results showed no statistically significant difference in pathological root resorption with ferric sulphate compared with formocresol (RR 1.64, 95% CI 0.53 to 5.08). Results were similar at 24 months with four trials providing data (258 teeth randomised) (RR 1.21, 95% CI 0.50 to 2.96; Analysis 10.6).
10.6. Analysis.
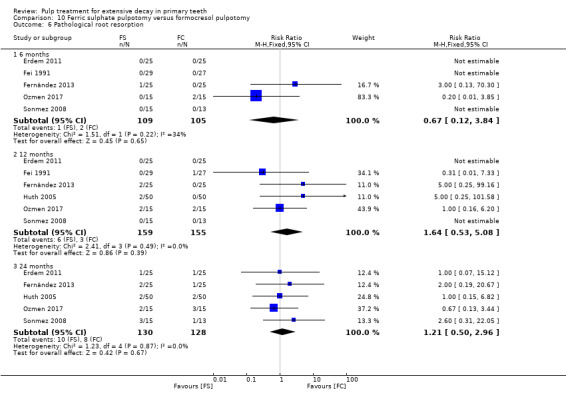
Comparison 10 Ferric sulphate pulpotomy versus formocresol pulpotomy, Outcome 6 Pathological root resorption.
Two additional trials did not assess pathological root resorption at a fixed time point but at an interval follow‐up of six to 11, 12 to 23 and 24 to 35 months for Fuks 1997 and three to 20 and 46 to 48 months for Ibricevic 2000. For Fuks 1997, there was no pathological root resorption in any of the participants regardless of the intervention at the first delays. At 24 to 35 months, the RR was 1.31 (95% CI 0.25 to 6.81). In Ibricevic 2000, the RR was 0.95 (95% CI 0.06 to 14.97) at three to 20 months and RR 0.63 (95% CI 0.11 to 3.70) at 46 to 48 months.
Pulp canal obliteration
At six months, data were extractable from three RCTs totaling 134 teeth. In all three trials, there was no pulp canal obliteration in any of the participants regardless of the intervention. At 12 months, data were extractable from three RCTs totaling 134 teeth. In one of the trials, there was no pulp canal obliteration in any of the participants regardless of the intervention. From the two remaining trials, the pooled results showed no statistically significant difference in pulp canal obliteration with ferric sulphate compared with formocresol (RR 0.94, 95% CI 0.54 to 1.64). At 24 months, data were extractable from two RCTs totaling 78 teeth. The pooled results showed no statistically significant difference in pulp canal obliteration with ferric sulphate compared with formocresol (RR 1.24, 95% CI 0.28 to 5.54; Analysis 10.7).
10.7. Analysis.
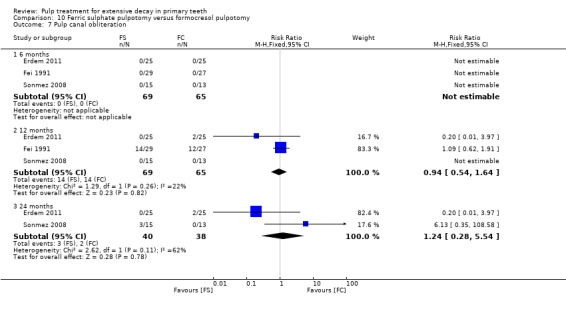
Comparison 10 Ferric sulphate pulpotomy versus formocresol pulpotomy, Outcome 7 Pulp canal obliteration.
One additional trial, which randomised 96 teeth, did not assess pulp canal obliteration at a fixed time point but at an interval follow‐up of six to 11, 12 to 23 and 24 to 35 months (Fuks 1997). There was no pulp canal obliteration in any of the participants regardless of the intervention at six to 11 or 12 to 23 months. At 24 to 35 months, the RR was 1.64 (95% CI 0.55 to 4.85).
Physiological root resorption
One trial, which randomised 50 teeth, assessed physiological root resorption (Erdem 2011). At six and 12 months, there was no physiological root resorption in any of the participants regardless of the intervention. At 24 months, the RR was 3.00 (95% CI 0.33 to 26.92).
Succedaneous tooth structural anomaly
One trial, which randomised 164 teeth, assessed succedaneous tooth structural anomaly (Ibricevic 2000). At three to 20 and 46 to 48 months, there was no succedaneous tooth structural anomaly in any of the participants regardless of the intervention.
Sodium hypochlorite (NaOCl) versus ferric sulphate
Clinical failure
At six months, data were extractable from two RCTs totaling 110 teeth. In the two trials, there was no clinical failure in any of the participants regardless of the intervention. At 12 months, data were extractable from two RCTs totaling 110 teeth. In one trial, there was no clinical failure in any of the participants regardless of the intervention. For the remaining trial, the results showed no statistically significant difference (RR 4.39, 95% CI 0.22 to 87.82; Analysis 11.1).
11.1. Analysis.
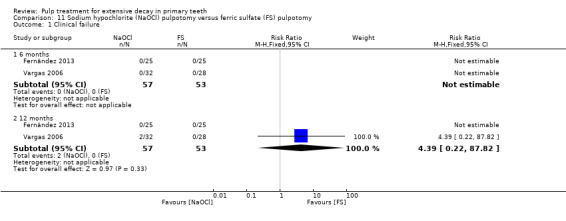
Comparison 11 Sodium hypochlorite (NaOCl) pulpotomy versus ferric sulfate (FS) pulpotomy, Outcome 1 Clinical failure.
At 24 months, one trial, which randomised 50 teeth, assessed clinical failure (Fernández 2013). The results showed no statistically significant difference (RR 3.00, 95% CI 0.13 to 70.30).
Radiological failure
At six months, data were extractable from two RCTs totaling 110 teeth. The results showed no statistically significant difference (RR 0.55, 95% CI 0.22 to 1.39). Results were similar at 12 months (RR 0.42, 95% CI 0.17 to 1.02; Analysis 11.2).
11.2. Analysis.
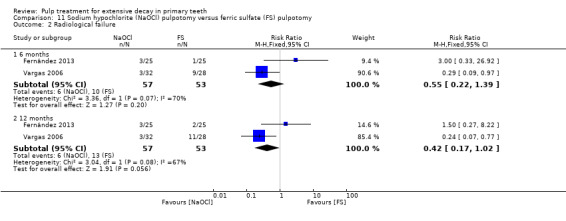
Comparison 11 Sodium hypochlorite (NaOCl) pulpotomy versus ferric sulfate (FS) pulpotomy, Outcome 2 Radiological failure.
At 24 months, one trial, which randomised 50 teeth, assessed radiological failure (Fernández 2013). The results showed no statistically significant difference (RR 1.50, 95% CI 0.27 to 8.22).
Overall failure
One trial, which randomised 50 teeth, assessed overall failure (Fernández 2013). The results showed no statistically significant difference at six months (RR 3.00, 95% CI 0.33 to 26.92), 12 and 24 months (RR 1.50, 95% CI 0.27 to 8.22).
Pain
At six and 12 months, data were extractable from two RCTs totaling 110 teeth. In the two trials, there was no pain in any of the participants regardless of the intervention (Analysis 11.3).
11.3. Analysis.
Comparison 11 Sodium hypochlorite (NaOCl) pulpotomy versus ferric sulfate (FS) pulpotomy, Outcome 3 Pain.
At 24 months, one trial, which randomised 50 teeth, assessed pain (Fernández 2013). There was no pain in any of the participants regardless of the intervention.
Soft tissue pathology
At six and 12 months, data were extractable from two RCTs totaling 110 teeth. In the two trials, there was no soft tissue pathology in any of the participants regardless of the intervention (Analysis 11.4).
11.4. Analysis.
Comparison 11 Sodium hypochlorite (NaOCl) pulpotomy versus ferric sulfate (FS) pulpotomy, Outcome 4 Soft tissue pathology.
At 24 months, one trial, which randomised 50 teeth, assessed soft tissue pathology (Fernández 2013). There was no soft tissue pathology in any of the participants regardless of the intervention.
Adjacent tissue inflammation
At six months, data were extractable from two RCTs totaling 110 teeth. In the two trials, there was no adjacent tissue inflammation in any of the participants regardless of the intervention. At 12 months, data were extractable from two RCTs totaling 110 teeth. The pooled results showed no statistically significant difference (RR 0.31, 95% CI 0.03 to 2.91; Analysis 11.5).
11.5. Analysis.
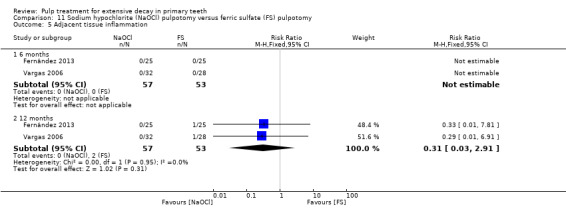
Comparison 11 Sodium hypochlorite (NaOCl) pulpotomy versus ferric sulfate (FS) pulpotomy, Outcome 5 Adjacent tissue inflammation.
At 24 months, one trial, which randomised 50 teeth, assessed clinical failure (Fernández 2013). The results showed no statistically significant difference (RR 0.50, 95% CI 0.05 to 5.17).
Pathological mobility
At six and 12 months, data were extractable from two RCTs totaling 110 teeth. In the two trials, there was no pathological mobility in any of the participants regardless of the intervention (Analysis 11.6).
11.6. Analysis.
Comparison 11 Sodium hypochlorite (NaOCl) pulpotomy versus ferric sulfate (FS) pulpotomy, Outcome 6 Pathologic mobility.
At 24 months, one trial, which randomised 50 teeth, assessed pathological mobility (Fernández 2013). There was no pathological mobility in any of the participants regardless of the intervention.
Pathological radiolucency
At six months, data were extractable from two RCTs totaling 110 teeth. In one trial, there was no pathological radiolucency in any of the participants regardless of the intervention. For the remaining trial, the results showed no statistically significant difference (RR 0.88, 95% CI 0.06 to 13.35). Results were similar at 12 months, with the two trials providing data (RR 0.56, 95% CI 0.07 to 4.17; Analysis 11.7).
11.7. Analysis.
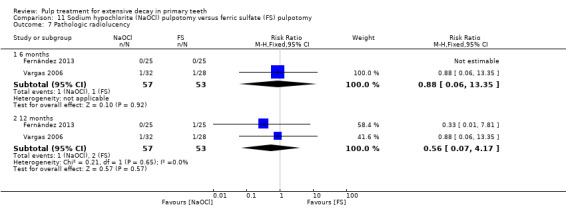
Comparison 11 Sodium hypochlorite (NaOCl) pulpotomy versus ferric sulfate (FS) pulpotomy, Outcome 7 Pathologic radiolucency.
At 24 months, one trial, which randomised 50 teeth, assessed clinical failure (Fernández 2013). The results showed no statistically significant difference (RR 0.20, 95% CI 0.01 to 3.97).
Pathological root resorption
At six months, data were extractable from two RCTs totaling 110 teeth. The pooled results showed no statistically significant difference (RR 0.51, 95% CI 0.18 to 1.42). Results were similar at 12 months (RR 0.38, 95% CI 0.15 to 1.01; Analysis 11.8).
11.8. Analysis.
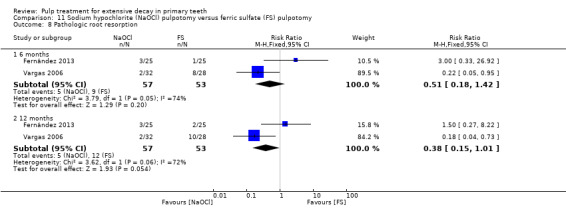
Comparison 11 Sodium hypochlorite (NaOCl) pulpotomy versus ferric sulfate (FS) pulpotomy, Outcome 8 Pathologic root resorption.
At 24 months, one trial, which randomised 50 teeth, assessed clinical failure (Fernández 2013). The results showed no statistically significant difference (RR 1.50, 95% CI 0.27 to 8.22).
Ferric sulphate versus buffered glutaraldehyde
One trial, which randomised 60 teeth, assessed ferric sulphate versus buffered glutaraldehyde based on pain, soft tissue pathology, pathological mobility, pathological radiolucency, pathological root resorption and pulp canal obliteration (Goyal 2016). There were no statistically significant differences for any outcome or time point (Table 23).
20. Pulpotomy (FS) versus pulpotomy (buffered glutaraldehyde).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Pain | 6 | 1 | 0.25 (0.06, 1.08) |
| Soft tissue pathology | 6 | 1 | 0.33 (0.07, 1.52) |
| Pathologic mobility | 6 | 1 | 0.75 (0.30, 1.90) |
| Pathologic radiolucency | 6 | 1 | 1.14 (0.69, 1.90) |
| Pathologic root resorption | 6 | 1 | 1.20 (0.61, 2.34) |
| Pulp canal obliteration | 6 | 1 | 0.11 (0.01, 1.98) |
*due to lack of events
Abbreviations ‐ CI: confidence interval; FS: ferric sulfate.
Ferric sulphate versus zinc oxide and eugenol (ZOE)
One trial, which randomised 50 teeth, assessed ferric sulphate versus ZOE based on clinical failure, radiological failure, overall failure, pain, pathological radiolucency, pathological root resorption, pulp canal obliteration and physiological root resorption (Erdem 2011). There were no statistically significant differences for any outcome or time point (Table 24).
21. Pulpotomy (FS) versus pulpotomy (ZOE).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 and 12 | 1 | Not estimable* |
| 24 | 1 | 1.20 (0.42 to 3.43) | |
| Radiological failure | 6 | 1 | 0.33 (0.01 to 7.81) |
| 12 | 1 | 0.20 (0.01 to 3.97) | |
| 24 | 1 | 0.60 (0.26 to 1.40) | |
| Overall failure | 6 | 1 | 0.33 (0.01 to 7.81) |
| 12 | 1 | 0.20 (0.01 to 3.97) | |
| 24 | 1 | 0.38 (0.11 to 1.25) | |
| Pain | 6, 12 and 24 | 1 | Not estimable* |
| Pathological radiolucency | 6, 12 and 24 | 1 | Not estimable* |
| Pathological root resorption | 6 | 1 | 0.33 (0.01 to 7.81) |
| 12 | 1 | 0.33 (0.01 to 7.81) | |
| 24 | 1 | 0.17 (0.02 to 1.29) | |
| Physiological root resorption | 6 and 12 | 1 | Not estimable* |
| 24 | 1 | 1.50 (0.27 to 8.22) | |
| Pulp canal obliteration | 6, 12 and 24 | 1 | Not estimable* |
*due to lack of events
Abbreviations ‐ CI: confidence interval; FS: ferric sulphate; ZOE: zinc oxide and eugenol
Erbium:yttrium‐aluminium garnet (Er:YAG) laser versus ferric sulphate
One trial, which randomised 97 teeth, assessed Er:YAG laser versus ferric sulphate by clinical failure, radiological failure, overall failure, pain, soft tissue pathology, pathological mobility, pathological radiolucency and pathological root resorption (Huth 2005). There were no statistically significant differences for any outcome or time point (Table 25).
22. Pulpotomy (Er:YAG laser) versus pulpotomy (FS).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 | 1 | Not estimable* |
| 12 | 1 | 3.19 (0.13 to 76.37) | |
| 24 | 1 | 5.31 (0.26 to 107.86) | |
| Radiological failure | 12 | 1 | 0.46 (0.13 to 1.66) |
| 24 | 1 | 0.61 (0.19 to 1.94) | |
| Overall failure | 12 | 1 | 0.46 (0.13 to 1.66) |
| 24 | 1 | 0.61 (0.19 to 1.94) | |
| Pain | 6, 12 and 24 | 1 | Not estimable* |
| Soft tissue pathology | 6, 12 and 24 | 1 | Not estimable* |
| Pathological mobility | 6, 12 and 24 | 1 | Not estimable* |
| Pathological radiolucency | 12 | 1 | 0.15 (0.01 to 2.86) |
| 24 | 1 | 0.71 (0.12 to 4.06) | |
| Pathological root resorption | 12 | 1 | 0.53 (0.05 to 5.67) |
| 24 | 1 | 1.06 (0.16 to 7.25) |
*due to lack of events
Abbreviations ‐ CI: confidence interval; Er:YAG: erbium:yttrium‐aluminium garnet; FS: ferric sulphate
Diode laser versus ferric sulphate
Clinical failure
At six months, data were extractable from three RCTs totaling 130 teeth. The pooled results showed no statistically significant difference (RR 0.23, 95% CI 0.04 to 1.30). Results were similar at 12 months, with two trials providing data (100 teeth randomised) (RR 0.20, 95% CI 0.02 to 1.62; Analysis 12.1).
12.1. Analysis.
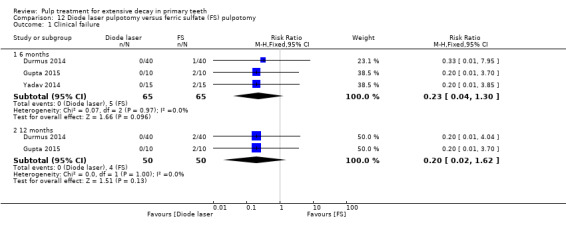
Comparison 12 Diode laser pulpotomy versus ferric sulfate (FS) pulpotomy, Outcome 1 Clinical failure.
Radiological failure
At six months, data were extractable from three RCTs totaling 130 teeth. The pooled results showed no statistically significant difference (RR 0.89, 95% CI 0.38 to 2.12). Results were similar at 12 months, with two trials providing data (100 teeth randomised) (RR 0.91, 95% CI 0.44 to 1.92; Analysis 12.2).
12.2. Analysis.
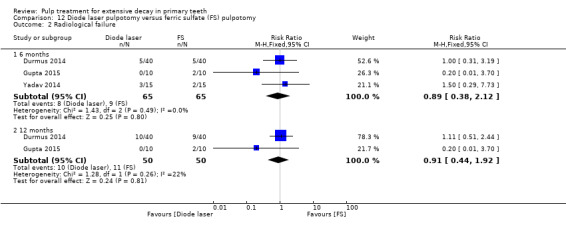
Comparison 12 Diode laser pulpotomy versus ferric sulfate (FS) pulpotomy, Outcome 2 Radiological failure.
Pain
At six months, data were extractable from two RCTs totaling 50 teeth. The pooled results showed no statistically significant difference (RR 0.20, 95% CI 0.03 to 1.60; Analysis 12.3).
12.3. Analysis.
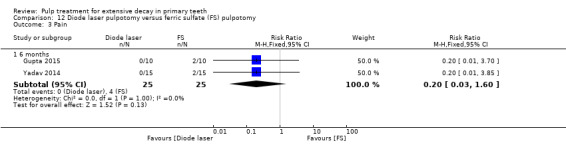
Comparison 12 Diode laser pulpotomy versus ferric sulfate (FS) pulpotomy, Outcome 3 Pain.
Pathological radiolucency
At six months, data were extractable from two RCTs totaling 50 teeth. The pooled results showed no statistically significant difference (RR 0.25, 95% CI 0.03 to 2.08; Analysis 12.4).
12.4. Analysis.
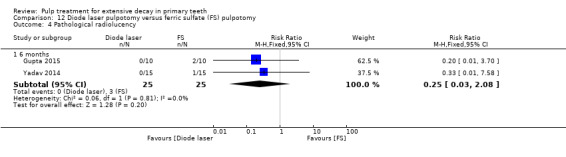
Comparison 12 Diode laser pulpotomy versus ferric sulfate (FS) pulpotomy, Outcome 4 Pathological radiolucency.
Pathological root resorption
At six months, data were extractable from twoRCTs totaling 50 teeth. In one trial, there was no pathological root resorption in any of the participants regardless of the intervention. For the remaining trial, the results showed no statistically significant difference (RR 1.50, 95% CI 0.29 to 7.73; Analysis 12.5).
12.5. Analysis.
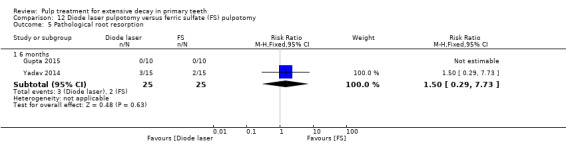
Comparison 12 Diode laser pulpotomy versus ferric sulfate (FS) pulpotomy, Outcome 5 Pathological root resorption.
Electrosurgery versus ferric sulphate
Clinical failure
At six months, data were extractable from two RCTs totaling 50 teeth. The pooled results showed no statistically significant difference (RR 0.56, 95% CI 0.13 to 2.34; Analysis 13.1).
13.1. Analysis.
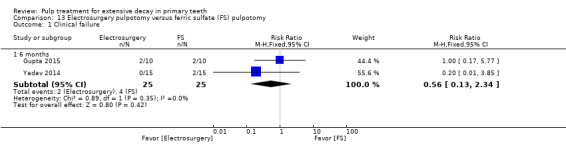
Comparison 13 Electrosurgery pulpotomy versus ferric sulfate (FS) pulpotomy, Outcome 1 Clinical failure.
At 12 months, one trial, which randomised 20 teeth, assessed clinical failure (Gupta 2015). The results showed no statistically significant difference (RR 1.00, 95% CI 0.17 to 5.77).
Radiological failure
At six months, data were extractable from two RCTs totaling 50 teeth. The pooled results showed no statistically significant difference (RR 1.25, 95% CI 0.38 to 4.12; Analysis 13.2).
13.2. Analysis.
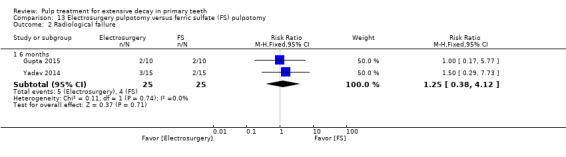
Comparison 13 Electrosurgery pulpotomy versus ferric sulfate (FS) pulpotomy, Outcome 2 Radiological failure.
At 12 months, one trial, which randomised 20 teeth, assessed radiological failure (Gupta 2015). The results showed no statistically significant difference (RR 1.00, 95% CI 0.17 to 5.77).
Pain
At six months, data were extractable from two RCTs totaling 50 teeth. The pooled results showed no statistically significant difference (RR 0.56, 95% CI 0.13 to 2.34) (Analysis 13.3).
13.3. Analysis.
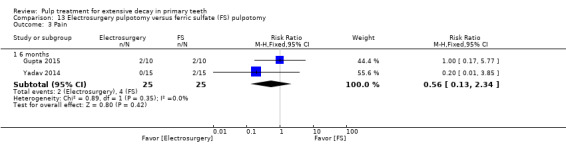
Comparison 13 Electrosurgery pulpotomy versus ferric sulfate (FS) pulpotomy, Outcome 3 Pain.
At 12 months, one trial, which randomised 20 teeth, assessed pain (Gupta 2015). The results showed no statistically significant difference (RR 1.00, 95% CI 0.17 to 5.77).
Pathological mobility
At six months, data were extractable from two RCTs totaling 50 teeth. In the two trials, there was no pathological mobility in any of the participants regardless of the intervention (Analysis 13.4).
13.4. Analysis.
Comparison 13 Electrosurgery pulpotomy versus ferric sulfate (FS) pulpotomy, Outcome 4 Pathological mobility.
At 12 months, one trial, which randomised 20 teeth, assessed pathological mobility (Gupta 2015). There was no pathological mobility in any of the participants regardless of the intervention.
Pathological radiolucency
At six months, data were extractable from two RCTs totaling 50 teeth. The pooled results showed no statistically significant difference (RR 0.25, 95% CI 0.03 to 2.08; Analysis 13.5).
13.5. Analysis.
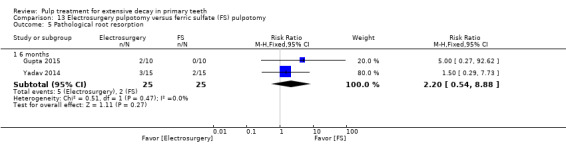
Comparison 13 Electrosurgery pulpotomy versus ferric sulfate (FS) pulpotomy, Outcome 5 Pathological root resorption.
At 12 months, one trial, which randomised 20 teeth, assessed pathological radiolucency (Gupta 2015). The results showed no statistically significant difference (RR 0.20, 95% CI 0.01 to 3.70).
Pathological root resorption
At six months, data were extractable from two RCTs totaling 50 teeth. The pooled results showed no statistically significant difference (RR 2.20, 95% CI 0.54 to 8.88; Analysis 13.5).
At 12 months, one trial, which randomised 20 teeth, assessed pathological root resorption (Gupta 2015). The results showed no statistically significant difference (RR 5.00, 95% CI 0.27 to 92.62).
Pulp canal obliteration
At six months, data were extractable from two RCTs totaling 50 teeth. In the two trials, there was no pulp canal obliteration in any of the participants regardless of the intervention (Analysis 13.6).
13.6. Analysis.
Comparison 13 Electrosurgery pulpotomy versus ferric sulfate (FS) pulpotomy, Outcome 6 Pulp canal obliteration.
At 12 months, one trial, which randomised 20 teeth, assessed pulp canal obliteration (Gupta 2015). There was no pulp canal obliteration in any of the participants regardless of the intervention.
Ferric sulphate + MTA versus ferric sulphate
One trial, which randomised 135 teeth, assessed ferric sulphate + MTA versus ferric sulphate based on pain, soft tissue pathology, pathological mobility, adjacent tissue inflammation, pathological radiolucency, pathological root resorption, and pulp canal obliteration (Doyle 2010). This trial did not assess the outcomes at a fixed time point but at a mean (range) follow‐up of 22 (6 to 38) months. There were no statistically significant differences for any outcome (Table 26).
23. Pulpotomy (FS + MTA) versus pulpotomy (FS).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Pain | mean 22 | 1 | 0.25 (0.01 to 6.08) |
| Soft tissue pathology | mean 22 | 1 | 0.25 (0.01 to 6.08) |
| Pathological mobility | mean 22 | 1 | 0.15 (0.01 to 3.09) |
| Adjacent tissue inflammation | mean 22 | 1 | 0.25 (0.01 to 6.08) |
| Pathological radiolucency | mean 22 | 1 | 0.29 (0.01 to 6.92) |
| Pathological root resorption | mean 22 | 1 | 0.60 (0.31 to 1.19) |
| Pulp canal obliteration | mean 22 | 1 | 1.39 (0.78 to 2.49) |
CI: confidence interval; FS: ferric sulphate; MTA: mineral trioxide aggregate
Ferric sulphate versus Ankaferd Blood Stopper
Clinical failure
Data were extractable from two trials totaling 100 teeth. At six months, there was no clinical failure in any of the participants regardless of the intervention. At 12 months, for one trial, there was no clinical failure in any of the participants regardless of the intervention. For the remaining trial, there was no statistically significant difference (RR 1.25, 95% CI 0.37 to 4.27; Analysis 14.1). At 24 months, one trial, which randomised 30 teeth, assessed clinical failure (Ozmen 2017). There were no statistically significant differences (RR 5.00, 95% CI 0.26 to 96.13).
14.1. Analysis.
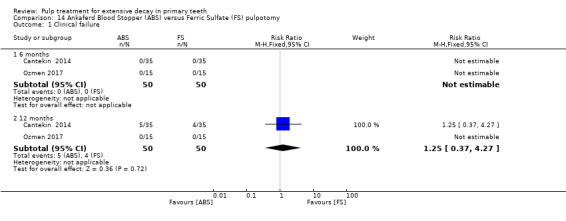
Comparison 14 Ankaferd Blood Stopper (ABS) versus Ferric Sulfate (FS) pulpotomy, Outcome 1 Clinical failure.
Radiological failure
Data were extractable from two trials totaling 100 teeth. At six months, for one trial, there was no radiological failure in any of the participants regardless of the intervention. For the remaining trial, there was no statistically significant difference (RR 1.00, 95% CI 0.16 to 6.20). At 12 months, the pooled results showed no statistically significant difference (RR 0.88, 95% CI 0.34 to 2.23; Analysis 14.2). At 24 months, one trial, which randomised 30 teeth, assessed radiological failure (Ozmen 2017). There was no statistically significant difference (RR 1.00, 95% CI 0.16 to 6.20).
14.2. Analysis.
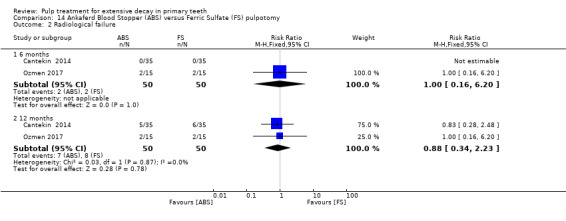
Comparison 14 Ankaferd Blood Stopper (ABS) versus Ferric Sulfate (FS) pulpotomy, Outcome 2 Radiological failure.
Pain
Data were extractable from two trials totaling 100 teeth. At six months, there was no pain in any of the participants regardless of the intervention. At 12 months, for one trial, there was no pain in any of the participants regardless of the intervention. For the remaining trial, there was no statistically significant differences (RR 1.00, 95% CI 0.15 to 6.71; Analysis 14.3). At 24 months, one trial, which randomised 30 teeth, assessed pain (Ozmen 2017). There was no pain in any of the participants regardless of the intervention.
14.3. Analysis.
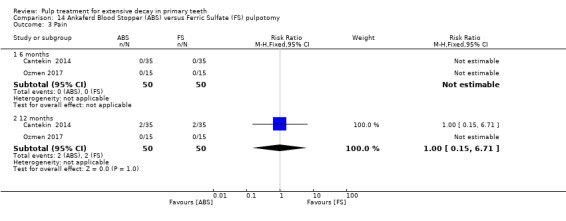
Comparison 14 Ankaferd Blood Stopper (ABS) versus Ferric Sulfate (FS) pulpotomy, Outcome 3 Pain.
Soft tissue pathology
Data were extractable from two trials totaling 100 teeth. At six months, there was no soft tissue pathology in any of the participants regardless of the intervention. At 12 months, for one trial, there was no soft tissue pathology in any of the participants regardless of the intervention. For the remaining trial, there was no statistically significant differences (RR 1.50, 95% CI 0.27 to 8.43; Analysis 14.4). At 24 months, one trial, which randomised 30 teeth, assessed soft tissue pathology (Ozmen 2017). There was no soft tissue pathology in any of the participants regardless of the intervention.
14.4. Analysis.
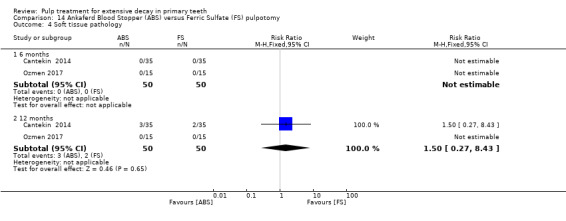
Comparison 14 Ankaferd Blood Stopper (ABS) versus Ferric Sulfate (FS) pulpotomy, Outcome 4 Soft tissue pathology.
Pathologic mobility
Data were extractable from two trials totaling 100 teeth. At six and 12 months, there was no pathologic mobility in any of the participants regardless of the intervention (Analysis 14.5). At 24 months, one trial, which randomised 30 teeth, assessed pathologic mobility (Ozmen 2017). There was no pathologic mobility in any of the participants regardless of the intervention.
14.5. Analysis.
Comparison 14 Ankaferd Blood Stopper (ABS) versus Ferric Sulfate (FS) pulpotomy, Outcome 5 Pathologic mobility.
Pathological radiolucency
Data were extractable from two trials totaling 100 teeth. At six months, there was no pathological radiolucency in any of the participants regardless of the intervention. At 12 months, for one trial, there was no pathological radiolucency in any of the participants regardless of the intervention. For the remaining trial, there was no statistically significant differences (RR 0.67, 95% CI 0.12 to 3.75; Analysis 14.6). At 24 months, one trial, which randomised 30 teeth, assessed pathological radiolucency (Ozmen 2017). There was no pathological radiolucency in any of the participants regardless of the intervention.
14.6. Analysis.
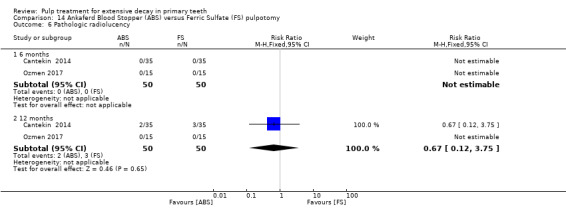
Comparison 14 Ankaferd Blood Stopper (ABS) versus Ferric Sulfate (FS) pulpotomy, Outcome 6 Pathologic radiolucency.
Pathological root resorption
Data were extractable from two trials totaling 100 teeth. At six months, for one trial, there was no pathological root resorption in any of the participants regardless of the intervention. For the remaining trial, there was no statistically significant difference (RR 5.00, 95% CI 0.26 to 96.13). At 12 months, the pooled results showed no statistically significant differences (RR 1.00, 95% CI 0.31 to 3.23; Analysis 14.7). At 24 months, one trial, which randomised 30 teeth, assessed pathological root resorption (Ozmen 2017). There was no statistically significant differences (RR 1.00, 95% CI 0.16 to 6.20).
14.7. Analysis.
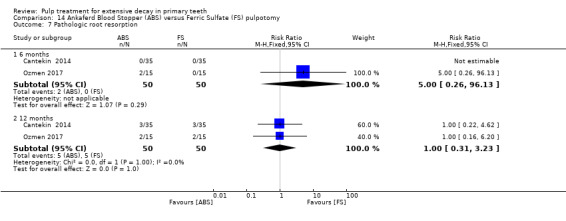
Comparison 14 Ankaferd Blood Stopper (ABS) versus Ferric Sulfate (FS) pulpotomy, Outcome 7 Pathologic root resorption.
Portland cement versus full strength formocresol
One trial, which randomised 70 teeth, assessed Portland cement versus formocresol based on clinical failure, radiological failure, overall failure, pain, soft tissue pathology, pathological mobility, pathological radiolucency and pathological root resorption (Yildirim 2016). There were no statistically significant differences for any outcomes at time point (Table 27).
24. Pulpotomy (PC) versus pulpotomy (FC).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6, 12 and 24 | 1 | 2.00 (0.19, 21.06) |
| Radiological failure | 24 | 1 | 0.80 (0.23, 2.73) |
| Overall failure | 6, 12 and 24 | 1 | 2.00 (0.19, 21.06) |
| Pain | 6, 12 and 24 | 1 | 1.00 (0.07, 15.36) |
| Soft tissue pathology | 6, 12 and 24 | 1 | 2.00 (0.19, 21.06) |
| Pathologic mobility | 6, 12 and 24 | 1 | 3.00 (0.13, 71.22) |
| Pathologic radiolucency | 24 | 1 | 1.00 (0.22, 4.62) |
| Pathologic root resorption | 24 | 1 | 0.50 (0.05, 5.27) |
Abbreviations ‐ CI: confidence interval; PC: Portland cement; FC: formocresol
Portland cement versus enamel matrix derivative (EMD)
One trial, which randomised 70 teeth, assessed MTA versus EMD based on clinical failure, radiological failure, overall failure, pain, soft tissue pathology, pathological mobility, pathological radiolucency and pathological root resorption (Yildirim 2016). There were no statistically significant differences for any outcomes at time point (Table 28).
25. Pulpotomy (PC) versus pulpotomy (EMD).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6, 12 and 24 | 1 | 0.67 (0.12, 3.75) |
| Radiological failure | 24 | 1 | 0.57 (0.18, 1.78) |
| Overall failure | 6, 12 and 24 | 1 | 0.67 (0.12, 3.75) |
| Pain | 6, 12 and 24 | 1 | 1.00 (0.07, 15.36) |
| Soft tissue pathology | 6, 12 and 24 | 1 | 1.00 (0.15, 6.71) |
| Pathologic mobility | 6, 12 and 24 | 1 | 0.50 (0.05, 5.27) |
| Pathologic radiolucency | 24 | 1 | 0.60 (0.16, 2.32) |
| Pathologic root resorption | 24 | 1 | 0.50 (0.05, 5.27) |
*due to lack of events
Abbreviations ‐ CI: confidence interval; PC: Portland cement; EMD: enamel matrix derivative
Portland cement versus Portland cement + iodoform (Portland cement + CHI₃) or Portland cement + zirconium oxide (Portland cement + ZrO₂)
One trial, which randomised 30 teeth, assessed Portland cement versus Portland cement + CHI₃ or Portland cement + ZrO₂ based on clinical failure, radiological failure, pathological root resorption, pathological radiolucency, soft tissue pathology, pathological mobility (Lourenço 2015a). There were no events in any of the participants regardless of the intervention.
Glutaraldehyde + calcium hydroxide versus full strength formocresol
One trial, which randomised 44 teeth, assessed glutaraldehyde + calcium hydroxide versus ZOE based on clinical failure and radiological failure (Alaçam 1989). There were no statistically significant differences for either outcome at three months (clinical failure: RR 1.10, 95% CI 0.17 to 7.10; radiological failure: RR 1.10, 95% CI 0.31 to 3.84). No longer‐term data were reported.
Glutaraldehyde + zinc oxide and eugenol (ZOE) versus full strength formocresol
One trial, which randomised 48 teeth, assessed glutaraldehyde + ZOE versus ZOE based on clinical failure and radiological failure (Alaçam 1989). There were no statistically significant differences for either outcome at three months (clinical failure: RR 0.46 95% CI 0.04 to 4.74; radiological failure: RR 0.46, 95% CI 0.09 to 2.28). No longer‐term data were reported.
Glutaraldehyde + calcium hydroxide versus glutaraldehyde + zinc oxide and eugenol (ZOE)
One trial, which randomised 46 teeth, assessed glutaraldehyde + calcium hydroxide versus glutaraldehyde + ZOE based on clinical failure and radiological failure (Alaçam 1989). There were no statistically significant differences for either outcome at three months (clinical failure: RR 2.38, 95% CI 0.23 to 24.46; radiological failure: RR 2.38, 95% CI 0.48 to 11.74). No longer‐term data were reported.
An additional trial, which randomised 61 teeth, assessed glutaraldehyde + calcium hydroxide versus glutaraldehyde + ZOE at 12 months based on clinical failure, radiological failure, pain, pathological radiolucency and pathological root resorption (Shumayrikh 1999). There were no statistically significant differences between treatment groups for any outcome (Table 29).
26. Pulpotomy (glutaraldehyde + CH) versus pulpotomy (glutaraldehyde + ZOE).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 12 | 1 | 2.90 (0.32 to 26.38) |
| Radiological failure | 12 | 1 | 1.11 (0.46 to 2.67) |
| Pain | 12 | 1 | Not estimable* |
| Pathological radiolucency | 12 | 1 | 0.97 (0.39 to 2.43) |
| Pathological root resorption | 12 | 1 | 0.97 (0.15 to 6.44) |
*due to lack of events
Abbreviations ‐ CH: calcium hydroxide; CI: confidence interval; ZOE: zinc oxide and eugenol
Electrofulguration + calcium hydroxide versus electrofulguration + zinc oxide eugenol (ZOE)
One trial, which randomised 47 teeth, assessed electrofulguration + calcium hydroxide versus electrofulguration + ZOE based on clinical failure, radiological failure, overall failure, pain, soft tissue pathology, pathological mobility, pathological radiolucency, pathological root resorption and pulp canal obliteration (Fishman 1996). There were no statistically significant differences for either outcome at six months (Table 30).
27. Pulpotomy (electrofulguration + CH) versus pulpotomy (electrofulguration + ZOE).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 | 1 | 0.83 (0.26, 2.73) |
| Radiological failure | 6 | 1 | 0.94 (0.47, 1.88) |
| Overall failure | 6 | 1 | 0.94 (0.47, 1.88) |
| Pain | 6 | 1 | Not estimable* |
| Soft tissue pathology | 6 | 1 | 0.83 (0.26, 2.73) |
| Pathologic mobility | 6 | 1 | Not estimable* |
| Pathologic radiolucency | 6 | 1 | 1.04 (0.43, 2.51) |
| Pathologic root resorption | 6 | 1 | 0.75 (0.28, 2.02) |
| Pulp canal obliteration | 6 | 1 | 1.04 (0.16, 6.80) |
*due to lack of events
Abbreviations ‐ CI: confidence interval; CH: calcium hydroxide; ZOE: zinc oxide eugenol
Electrosurgery versus formocresol
One trial, which randomised 70 teeth, assessed electrosurgery versus 1:5 diluted formocresol at fixed time points, reporting on clinical failure, radiological failure, pain, soft tissue pathology, pathological mobility, pathological radiolucency and pathological root resorption (Bahrololoomi 2008). There was no statistically significant difference at any time point for any outcome (Table 31).
28. Pulpotomy (electrosurgery) versus pulpotomy (FC).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 9 | 1 | 3.00 (0.13 to 71.22) |
| Radiological failure | 9 | 1 | 5.00 (0.62 to 40.64) |
| Pain | 6 | 1 | Not estimable* |
| Soft tissue pathology | 6 | 1 | 3.00 (0.13 to 71.22) |
| Pathological mobility | 6 | 1 | Not estimable* |
| Pathological radiolucency | 6 | 1 | 5.00 (0.25 to 100.54) |
| Pathological root resorption | 6 | 1 | 5.00 (0.25 to 100.54) |
*due to lack of events
Abbreviations ‐ CI: confidence interval; FC: formocresol
One additional trial, which randomised 50 teeth, did not assess electrosurgery versus full strength formocresol at a fixed time point but at a mean (range) follow‐up of 11.5 (5 to 25) months in one the formocresol group and 10.9 (6 to 31) in the other electrosurgery group (Dean 2002). There was no statistically significant difference for clinical failure (RR 3.00, 95% CI 0.13 to 70.30) or radiological failure (RR 2.00, 95% CI 0.40 to 9.95).
Diode laser versus electrosurgery
Clinical failure
At six months, data were extractable from two RCTs totaling 50 teeth. In one trial, there was no clinical failure in any of the participants regardless of the intervention. For the remaining trial, the results showed no statistically significant difference (RR 0.20, 95% CI 0.01 to 3.70; Analysis 15.1).
15.1. Analysis.
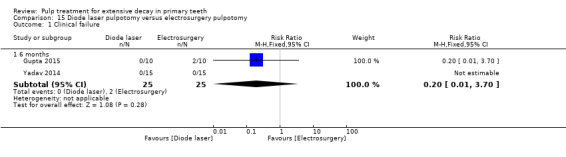
Comparison 15 Diode laser pulpotomy versus electrosurgery pulpotomy, Outcome 1 Clinical failure.
At 12 months, one trial, which randomised 20 teeth, assessed clinical failure (Gupta 2015). The results showed no statistically significant difference (RR 0.20, 95% CI 0.01 to 3.70).
Radiological failure
At six months, data were extractable from two RCTs totaling 50 teeth. The pooled results showed no statistically significant difference (RR 0.64, 95% CI 0.19 to 2.18; Analysis 15.2).
15.2. Analysis.
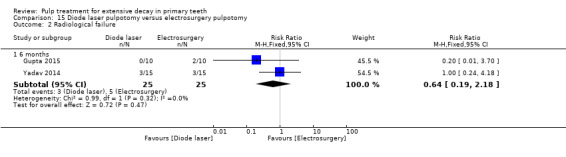
Comparison 15 Diode laser pulpotomy versus electrosurgery pulpotomy, Outcome 2 Radiological failure.
At 12 months, one trial, which randomised 20 teeth, assessed radiological failure (Gupta 2015). The results showed no statistically significant difference (RR 0.20, 95% CI 0.01 to 3.70).
Pain
At six months, data were extractable from two RCTs totaling 50 teeth. In one trial, there was no pain in any of the participants regardless of the intervention. For the remaining trial, the results showed no statistically significant difference (RR 0.20, 95% CI 0.01 to 3.70; Analysis 15.3).
15.3. Analysis.
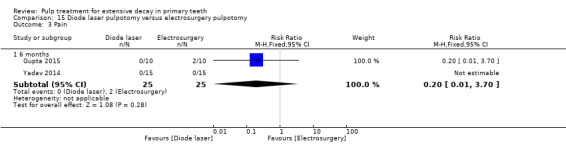
Comparison 15 Diode laser pulpotomy versus electrosurgery pulpotomy, Outcome 3 Pain.
At 12 months, one trial, which randomised 20 teeth, assessed pain (Gupta 2015). The results showed no statistically significant difference (RR 0.20, 95% CI 0.01 to 3.70).
Pathological mobility
At six months, data were extractable from two RCTs totaling 50 teeth. In the two trials, there was no pathological mobility in any of the participants regardless of the intervention (Analysis 15.4).
15.4. Analysis.
Comparison 15 Diode laser pulpotomy versus electrosurgery pulpotomy, Outcome 4 Pathological mobility.
At 12 months, one trial, which randomised 20 teeth, assessed pathological mobility (Gupta 2015). There was no pathological mobility in any of the participants regardless of the intervention.
Pathological radiolucency
At six months, data were extractable from two RCTs totaling 50 teeth. In the two trials, there was no pathological radiolucency in any of the participants regardless of the intervention (Analysis 15.5).
15.5. Analysis.
Comparison 15 Diode laser pulpotomy versus electrosurgery pulpotomy, Outcome 5 Pathological radiolucency.
At 12 months, one trial, which randomised 20 teeth, assessed pathological radiolucency (Gupta 2015). There was no pathological radiolucency in any of the participants regardless of the intervention.
Pathological root resorption
At six months, data were extractable from two RCTs totaling 50 teeth. The pooled results showed no statistically significant difference (RR 0.64, 95% CI 0.19 to 2.18; Analysis 15.6).
15.6. Analysis.
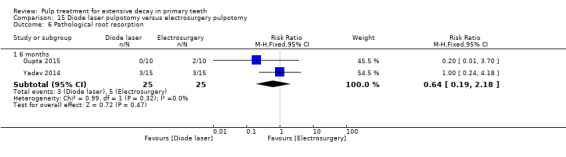
Comparison 15 Diode laser pulpotomy versus electrosurgery pulpotomy, Outcome 6 Pathological root resorption.
At 12 months, one trial, which randomised 20 teeth, assessed pathological root resorption (Gupta 2015). The results showed no statistically significant difference (RR 0.20, 95% CI 0.01 to 3.70).
Pulp canal obliteration
At six months, data were extractable from two RCTs totaling 50 teeth. In the two trials, there was no pulp canal obliteration in any of the participants regardless of the intervention (Analysis 15.7).
15.7. Analysis.
Comparison 15 Diode laser pulpotomy versus electrosurgery pulpotomy, Outcome 7 Pulp canal obliteration.
At 12 months, one trial, which randomised 20 teeth, assessed pulp canal obliteration (Gupta 2015). There was no pulp canal obliteration in any of the participants regardless of the intervention.
Electrosurgery versus calcium‐enriched mixture (CEM)
One trial, which randomised 102 teeth, assessed clinical failure, radiological failure, overall failure and pulp canal obliteration (Khorakian 2014). At six, 12 and 24 months, there was no clinical failure in any of the participants regardless of the intervention. For radiological/overall failure, there were no statistically significant differences at six (RR 0.20, 95% CI 0.01 to 4.07), 12 (RR 1.00, 95% CI 0.06 to 15.56) and 24 months (RR 0.50, 95% CI 0.10 to 2.61). For pulp canal obliteration, there were no statistically significant differences at 12 months (RR 1.22, 95% CI 0.88 to 1.70).
Biodentine versus formocresol
One trial, which randomised 112 teeth, assessed clinical failure, radiological failure, pain, soft tissue pathology, pathologic mobility, pathological radiolucency, pathological root resorption and pulp canal obliteration at six months (El Meligy 2016). There was no clinical failure, radiological failure, pain, soft tissue pathology, pathologic mobility, pathological radiolucency and pathological root resorption in any of the participants regardless of the intervention. For pulp canal obliteration, there was no statistically significant difference (RR 1.43, 95% CI 0.59 to 3.49).
Biodentine versus diode laser
One trial, which randomised 40 teeth, assessed Biodentine versus diode based on pain, soft tissue pathology, pathological mobility, pathological radiolucency, pathological root resorption, and premature tooth loss (Niranjani 2015). There was no premature tooth loss in any of the participants regardless of the intervention.There were no statistically significant differences for the other outcomes at six months (RR 2.00, 95% CI 0.20 to 20.33).
Biodentine versus Tempophore
One trial, which randomised 56 teeth, assessed Biodentine versus Tempophore based on clinical failure, radiological failure, pathological radiolucency, pathological root resorption, pulp canal obliteration and dentin bridge formation at six and 12 months (Rajasekharan 2017). There were no statistically significant differences for any outcomes at any time point (Table 32).
29. Pulpotomy (Biodentine) versus pulpotomy (Tempophore).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 and 12 | 1 | 1.08 (0.07, 16.36) |
| Radiological failure | 6 and 12 | 1 | 0.54 (0.11, 2.70) |
| Pathological radiolucency | 6 | 1 | 2.16 (0.21, 22.38) |
| 12 | 1 | 0.54 (0.11, 2.70) | |
| Pathological root resorption | 6 | 1 | 0.36 (0.08, 1.62) |
| 12 | 1 | 0.31 (0.07, 1.35) | |
| Pulp canal obliteration | 6 | 1 | 1.39 (0.61, 3.17) |
| 12 | 1 | 1.08 (0.60, 1.94) | |
| Dentine bridge formation | 6 | 1 | Not estimable* |
| 12 | 1 | 11.85 (0.69, 203.86) |
*due to lack of events
Abbreviations ‐ CI: confidence interval
Biodentine versus propolis
One trial, which randomised 50 teeth, assessed Biodentine versus propolis based on clinical failure and radiological failure (Kusum 2015). There were no statistically significant differences for any outcomes at six months (clinical failure: RR 0.33, 95% CI 0.01 to 7.81; radiological failure: RR 0.43, 95% CI 0.12 to 1.47).
Sodium hypochlorite (NaOCl) versus 1:5 diluted formocresol
Clinical failure
At six and 12 months, data were extractable from two RCTs totaling 150 teeth. In the two trials, there was no clinical failure in any of the participants regardless of the intervention (Analysis 16.1).
16.1. Analysis.
Comparison 16 Sodium hypochlorite (NaOCl) pulpotomy versus 1:5 diluted formocresol pulpotomy, Outcome 1 Clinical failure.
At 24 months, one trial, which randomised 50 teeth, assessed clinical failure (Fernández 2013). The results showed no statistically significant difference (RR 3.00, 95% CI 0.13 to 70.30).
Radiological failure
At six months, data were extractable from two RCTs totaling 150 teeth. The pooled results showed no statistically significant difference (RR 1.29, 95% CI 0.33 to 5.08). Results were similar at 12 months (RR 1.86, 95% CI 0.52 to 6.59; Analysis 16.2).
16.2. Analysis.
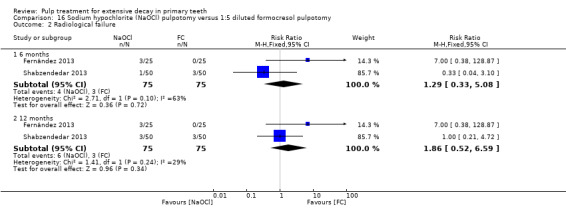
Comparison 16 Sodium hypochlorite (NaOCl) pulpotomy versus 1:5 diluted formocresol pulpotomy, Outcome 2 Radiological failure.
At 24 months, one trial, which randomised 50 teeth, assessed radiological failure (Fernández 2013). The results showed no statistically significant difference (RR 3.00, 95% CI 0.33 to 26.92).
Overall failure
At six, 12 and 24 months, one trial, which randomised 50 teeth, assessed radiological failure (Fernández 2013). The results showed no statistically significant difference (RR 7.00, 95% CI 0.38 to 128.87).
Pain
At six and 12 months, data were extractable from two RCTs totaling 150 teeth. In the two trials, there was no pain in any of the participants regardless of the intervention (Analysis 16.3).
16.3. Analysis.
Comparison 16 Sodium hypochlorite (NaOCl) pulpotomy versus 1:5 diluted formocresol pulpotomy, Outcome 3 Pain.
At 24 months, one trial, which randomised 20 teeth, assessed pain (Fernández 2013). There was no pain in any of the participants regardless of the intervention.
Soft tissue pathology
At six and 12 months, data were extractable from two RCTs totaling 150 teeth. In the two trials, there was no soft tissue pathology in any of the participants regardless of the intervention (Analysis 16.4).
16.4. Analysis.
Comparison 16 Sodium hypochlorite (NaOCl) pulpotomy versus 1:5 diluted formocresol pulpotomy, Outcome 4 Soft tissue pathology.
At 24 months, one trial, which randomised 20 teeth, assessed soft tissue pathology (Fernández 2013). There was no soft tissue pathology in any of the participants regardless of the intervention.
Adjacent tissue inflammation
One trial, which randomised 50 teeth, assessed adjacent tissue inflammation (Fernández 2013). At six and 12 months, there was no adjacent tissue inflammation in any of the participants regardless of the intervention. At 24 months, the results showed no statistically significant difference (RR 3.00, 95% CI 0.13 to 70.30).
Pathological mobility
At six and 12 months, data were extractable from two RCTs totaling 50 teeth. In the two trials, there was no pathological mobility in any of the participants regardless of the intervention (Analysis 16.5).
16.5. Analysis.
Comparison 16 Sodium hypochlorite (NaOCl) pulpotomy versus 1:5 diluted formocresol pulpotomy, Outcome 5 Pathologic mobility.
At 24 months, one trial, which randomised 20 teeth, assessed soft tissue pathology (Fernández 2013). There was no pathological mobility in any of the participants regardless of the intervention.
Pathological radiolucency
At six and 12 months, data were extractable from two RCTs totaling 50 teeth. In the two trials, there was no pathological radiolucency in any of the participants regardless of the intervention (Analysis 16.6).
16.6. Analysis.
Comparison 16 Sodium hypochlorite (NaOCl) pulpotomy versus 1:5 diluted formocresol pulpotomy, Outcome 6 Pathologic radiolucency.
At 24 months, one trial, which randomised 20 teeth, assessed pathological radiolucency (Fernández 2013). There was no pathological radiolucency in any of the participants regardless of the intervention.
Pathological root resorption
At six months, data were extractable from two RCTs totaling 50 teeth. The pooled results showed no statistically significant difference (RR 1.29, 95% CI 0.33 to 5.08; Analysis 16.7). Results were similar at 12 months (RR 1.86, 95% CI 0.52 to 6.59).
16.7. Analysis.
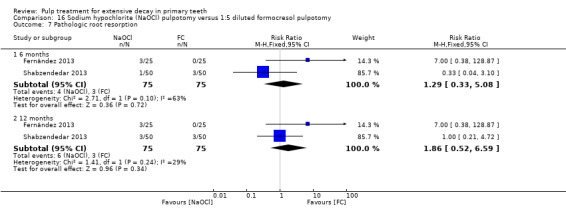
Comparison 16 Sodium hypochlorite (NaOCl) pulpotomy versus 1:5 diluted formocresol pulpotomy, Outcome 7 Pathologic root resorption.
At 24 months, one trial, which randomised 20 teeth, assessed pathological root resorption (Fernández 2013). The results showed no statistically significant difference (RR 3.00, 95% CI 0.33 to 26.92).
Calcium hydroxide/iodoform versus full strength formocresol
One trial, which randomised 67 teeth, assessed calcium hydroxide/iodoform versus formocresol, reporting on clinical failure, radiological failure, pain, soft tissue pathology, pathological radiolucency and pathological root resorption (Alaçam 2009). For clinical failure, the RR was 16.41 (95% CI 2.30 to 117.26) at six months and RR 9.11 (95% CI 3.04 to 27.31) at 12 months in favour of formocresol. For radiological failure, the RR was 24.06 (95% CI 3.44 to 168.43) at six months and RR 9.11 (95% CI 3.04 to 27.31) at 12 months. There was no statistically significant difference for any other outcome at either six or 12 months (Table 33).
30. Pulpotomy (CH/iodoform) versus pulpotomy (FC).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 | 1 | 16.41 (2.30 to 117.26) |
| 12 | 1 | 9.11 (3.04 to 27.31) | |
| Radiological failure | 6 | 1 | 24.06 (3.44 to 168.43) |
| 12 | 1 | 9.11 (3.04 to 27.31) | |
| Pain | 6 | 1 | 5.47 (0.67 to 44.34) |
| 12 | 1 | 5.47 (0.67 to 44.34) | |
| Soft tissue pathology | 6 | 1 | 7.64 (0.41 to 142.35) |
| 12 | 1 | 7.64 (0.41 to 142.35) | |
| Pathological radiolucency | 6 | 1 | 2.19 (0.21 to 22.99) |
| 12 | 1 | 2.19 (0.21 to 22.99) | |
| Pathological root resorption | 6 | 1 | 12.00 (0.69 to 208.77) |
| 12 | 1 | 5.47 (0.67 to 44.34) |
Abbreviations ‐ CH: calcium hydroxide; CI: confidence interval; FC: formocresol
Zinc oxide and eugenol (ZOE) versus formocresol
One trial, which randomised 50 teeth, assessed ZOE versus formocresol based on clinical failure, radiological failure, overall failure, pain, pathological radiolucency, pathological root resorption, pulp canal obliteration and physiological root resorption (Erdem 2011). At six and 12 months, there was no clinical failure in any of the participants regardless of the intervention. There was no statistically significant difference for any outcome at any time point (Table 34).
31. Pulpotomy (ZOE) versus pulpotomy (FC).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 | 1 | Not estimable* |
| 12 | 1 | Not estimable* | |
| 24 | 1 | 0.83 (0.29 to 2.38) | |
| Radiological failure | 6 | 1 | 3.00 (0.13 to 70.30) |
| 12 | 1 | 5.00 (0.25 to 99.17) | |
| 24 | 1 | 1.67 (0.71 to 3.89) | |
| Overall failure | 6 | 1 | 3.00 (0.13 to 70.30) |
| 12 | 1 | 5.00 (0.25 to 99.17) | |
| 24 | 1 | 2.67 (0.80 to 8.90) | |
| Pain | 6, 12 and 24 | 1 | Not estimable* |
| Pathological radiolucency | 6, 12 and 24 | 1 | Not estimable* |
| Pathological root resorption | 6 | 1 | 3.00 (0.13 to 70.30) |
| 12 | 1 | 3.00 (0.13 to 70.30) | |
| 24 | 1 | 6.00 (0.78 to 46.29) | |
| Pulp canal obliteration | 6 | 1 | Not estimable* |
| 12 | 1 | 0.20 (0.01 to 3.97) | |
| 24 | 1 | 0.20 (0.01 to 3.97) | |
| Physiological root resorption | 6 | 1 | Not estimable* |
| 12 | 1 | Not estimable* | |
| 24 | 1 | 2.00 (0.19 to 20.67) |
*due to lack of events
Abbreviations ‐ CI: confidence interval; FC: formocresol; ZOE: zinc oxide and eugenol
Erbium:yttrium‐aluminium garnet (Er:YAG) laser versus formocresol
One trial, which randomised 97 teeth, assessed Er:YAG laser versus formocresol based on clinical failure, radiological failure, overall failure, pain, soft tissue pathology, pathological mobility, pathological radiolucency and pathological root resorption (Huth 2005). There were no statistically significant differences for any outcome at six, 12 or 24 months (Table 35).
32. Pulpotomy (Er:YAG laser) versus pulpotomy (FC).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 | 1 | Not estimable* |
| 12 | 1 | 3.19 (0.13 to 76.37) | |
| 24 | 1 | 2.13 (0.20 to 22.70) | |
| Radiological failure | 6 | 1 | Not estimable* |
| 12 | 1 | 1.60 (0.28 to 9.13) | |
| 24 | 1 | 1.06 (0.28 to 4.01) | |
| Overall failure | 6 | 1 | Not estimable* |
| 12 | 1 | 1.60 (0.28 to 9.13) | |
| 24 | 1 | 1.06 (0.28 to 4.01) | |
| Pain | 6, 12 and 24 | 1 | Not estimable* |
| Soft tissue pathology | 6 | 1 | Not estimable* |
| 12 | 1 | Not estimable* | |
| 24 | 1 | 0.35 (0.01 to 8.49) | |
| Pathological mobility | 6, 12 and 24 | 1 | Not estimable* |
| Pathological radiolucency | 6 | 1 | Not estimable* |
| 12 | 1 | 0.21 (0.01 to 4.31) | |
| 24 | 1 | 1.06 (0.16 to 7.25) | |
| Pathological root resorption | 6 | 1 | Not estimable* |
| 12 | 1 | 3.19 (0.13 to 76.37) | |
| 24 | 1 | 1.06 (0.16 to 7.25) |
*due to lack of events
Abbreviations ‐ CI: confidence interval; Er:YAG: erbium:yttrium‐aluminium garnet; FC: formocresol
Diode laser versus formocresol
One trial, which randomised 80 teeth, assessed diode laser versus formocresol based on clinical failure and radiological failure (Durmus 2014). There were no statistically significant differences for any outcome at six and 12 months (Table 36).
33. Pulpotomy (diode laser) versus pulpotomy (FC).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 | 1 | Not estimable* |
| 12 | 1 | 0.33 (0.01, 7.95) | |
| Radiological failure | 6 | 1 | 1.67 (0.43, 6.51) |
| 12 | 1 | 2.00 (0.75, 5.33) |
*due to lack of events
Abbreviations ‐ CI: confidence interval; FC: formocresol.
Low‐level laser therapy (LLLT) versus formocresol
One trial, which randomised 30 teeth, assessed LLLT versus formocresol based on clinical failure, radiological failure, pain, soft tissue pathology, adjacent tissue inflammation, pathologic mobility, pathologic radiolucency, pathologic root resorption, pulp canal obliteration, and dentin bridge formation at six and 12 months (Fernandes 2015). There was no clinical failure, pain, soft tissue pathology, adjacent tissue inflammation pathologic mobility, pathologic radiolucency and dentin bridge formation in any of the participants regardless of the intervention. There were no statistically significant differences for radiological failure and pathologic root resorption at six and 12 months (RR 7.00, 95% CI 0.39 to 124.83), pulp canal obliteration at six (RR 3.00, 95% CI 0.13 to 68.26) and 12 months (RR 1.67, 95% CI 0.48 to 5.76).
Low‐level laser therapy (LLLT) + calcium hydroxide versus formocresol
One trial, which randomised 30 teeth, assessed LLLT + calcium hydroxide versus formocresol based on clinical failure, radiological failure, pain, soft tissue pathology, adjacent tissue inflammation, pathologic mobility, pathologic radiolucency, pathologic root resorption, pulp canal obliteration, and dentin bridge formation at six and 12 months (Fernandes 2015). There was no clinical failure, pain, soft tissue pathology, adjacent tissue inflammation pathologic mobility and pathologic radiolucency in any of the participants regardless of the intervention. There were no statistically significant differences for radiological failure and pathologic root resorption at six (RR 5.00, 95% CI 0.26 to 96.13) and 12 months (RR 7.00, 95% CI 0.39 to 124.83), pulp canal obliteration at six (RR 3.00, 95% CI 0.13 to 68.26) and 12 months (RR 0.33, 95% CI 0.04 to 2.85), and dentin bridge formation at six and 12 months (RR 15.00, 95% CI 0.93 to 241.20).
Ankaferd Blood Stopper versus formocresol
One trial, which randomised 30 teeth, assessed Ankaferd Blood Stopper versus formocresol based on clinical failure, radiological failure, pain, soft tissue pathology, pathological mobility, pathological radiolucency and pathologic root resorption (Ozmen 2017). There were no statistically significant differences for all outcomes at any time points (Table 37).
34. Pulpotomy (ABS) versus pulpotomy (FC).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 and 12 | 1 | 0.33 (0.01, 7.58) |
| 24 | 1 | 1.00 (0.16, 6.20) | |
| Radiological failure | 6 and 12 | 1 | 1.00 (0.16, 6.20) |
| 24 | 1 | 0.67 (0.13, 3.44) | |
| Pain | 6 and 12 | 1 | 0.33 (0.01, 7.58) |
| 24 | 1 | 1.00 (0.16, 6.20) | |
| Soft tissue pathology | 6, 12 and 24 months | 1 | 0.33 (0.01, 7.58) |
| Pathologic mobility | 6, 12 and 24 months | 1 | 0.33 (0.01, 7.58) |
| Pathological radiolucency | 6 and 12 | 1 | Not estimable* |
| Pathological root resorption | 6 and 12 | 1 | 1.00 (0.16, 6.20) |
| 24 | 1 | 0.67 (0.13, 3.44) |
*due to lack of events
Abbreviation ‐ CI: confidence interval
Enamel matrix derivative (EMD) versus formocresol
Two trials assessed EMD versus formocresol: one trial assessed full strength formocresol (Yildirim 2016), and the other trial assessed 1:5 diluted formocresol (Sabbarini 2008).
Clinical failure
At six months, data were extractable from two RCTs totaling 100 teeth. The results showed no statistically significant difference (RR 0.80, 95% CI 0.23 to 2.83; Analysis 17.1).
17.1. Analysis.
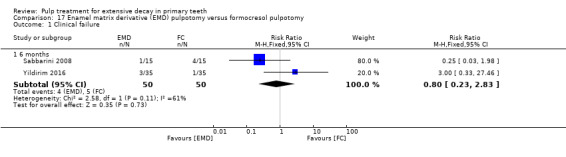
Comparison 17 Enamel matrix derivative (EMD) pulpotomy versus formocresol pulpotomy, Outcome 1 Clinical failure.
At 12 and 24 months, one trial, which randomised 70 teeth, assessed clinical failure (Yildirim 2016). There was no statistically significant difference (RR 3.00, 95% CI 0.33 to 27.46).
Radiological failure
At six months, one trial, which randomised 30 teeth, assessed radiological failure (Sabbarini 2008). There was a statistically significant difference in favour of EMD (RR 0.46, 95% CI 0.24 to 0.88).
At 24 months, one trial, which randomised 70 teeth, assessed radiological failure (Yildirim 2016). There was no statistically significant difference (RR 1.40, 95% CI 0.49 to 3.99).
Overall failure
At six, 12 and 24 months, one trial, which randomised 70 teeth, assessed overall failure (Yildirim 2016). There was no statistically significant difference (RR 3.00, 95% CI 0.33 to 27.46).
Pain
At six months, data were extractable from two RCTs totaling 100 teeth. The results showed no statistically significant difference (RR 0.40, 95% CI 0.08 to 1.92; Analysis 17.2).
17.2. Analysis.
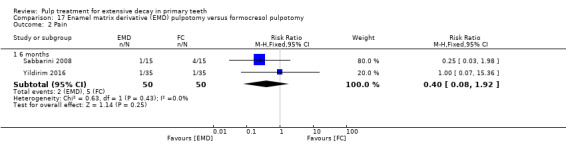
Comparison 17 Enamel matrix derivative (EMD) pulpotomy versus formocresol pulpotomy, Outcome 2 Pain.
At 12 and 24 months, one trial, which randomised 70 teeth, assessed pain (Yildirim 2016). There was no statistically significant difference (RR 1.00, 95% CI 0.07 to 15.36).
Soft tissue pathology
At six months, data were extractable from two RCTs totaling 100 teeth. In one trial, there was no soft tissue pathology in any of the participants regardless of the intervention. For the remaining trial, the results showed no statistically significant difference (RR 2.00, 95% CI 0.19 to 21.06; Analysis 17.3).
17.3. Analysis.
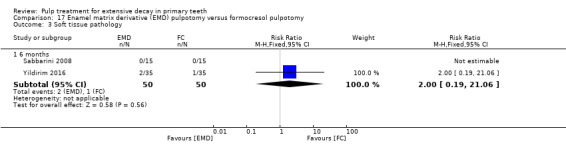
Comparison 17 Enamel matrix derivative (EMD) pulpotomy versus formocresol pulpotomy, Outcome 3 Soft tissue pathology.
At 12 and 24 months, one trial, which randomised 70 teeth, assessed clinical failure (Yildirim 2016). There was no statistically significant difference (RR 2.00, 95% CI 0.19 to 21.06).
Pathological mobility
At six months, data were extractable from two RCTs totaling 100 teeth. In one trial, there was no pathological mobility in any of the participants regardless of the intervention. For the remaining trial, the results showed no statistically significant difference (RR 5.00, 95% CI 0.26 to 96.13; Analysis 17.4).
17.4. Analysis.
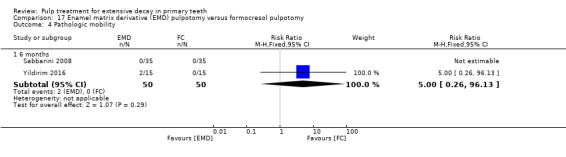
Comparison 17 Enamel matrix derivative (EMD) pulpotomy versus formocresol pulpotomy, Outcome 4 Pathologic mobility.
At 12 and 24 months, one trial, which randomised 70 teeth, assessed pathological mobility (Yildirim 2016). There was no statistically significant difference (RR 5.00, 95% CI 0.25 to 100.53).
Pathological radiolucency
At 24 months, one trial, which randomised 70 teeth, assessed pathological radiolucency (Yildirim 2016). There was no statistically significant difference (RR 1.67, 95% CI 0.43 to 6.45).
Pathological root resorption
At 24 months, one trial, which randomised 70 teeth, assessed pathological root resorption (Yildirim 2016). There was no statistically significant difference (RR 1.00, 95% CI 0.15 to 6.71).
Full strength formocresol compared with 1:5 diluted formocresol
One trial, which randomised 30 teeth, assessed clinical failure, radiological failure, pain, soft tissue pathology, pathological radiolucency and pathological root resorption at six months (Goyal 2014). There was no event in any of the participants regardless of the intervention.
Full strength formocresol compared with 1:25 diluted formocresol
One trial, which randomised 30 teeth, assessed clinical failure, radiological failure, pain, soft tissue pathology, pathological radiolucency and pathological root resorption at six months (Goyal 2014). There was no event in any of the participants regardless of the intervention.
1:5 diluted formocresol compared with 1:25 diluted formocresol
One trial, which randomised 30 teeth, assessed clinical failure, radiological failure, pain, soft tissue pathology, pathological radiolucency and pathological root resorption at six months (Goyal 2014). There was no event in any of the participants regardless of the intervention.
Pulpectomy versus pulpectomy
We assessed 15 trials that compared pulpectomy using different types of medicaments. Two trials were assessed at high risk of bias, and the risk of bias was unclear for 13 trials.
Calcium hydroxide versus zinc oxide and eugenol (ZOE)
Clinical failure
Ozalp 2005, which randomised 40 teeth, assessed calcium hydroxide versus ZOE based on clinical failure at six and 12 months; there was no statistically significant difference at either time point (six months: RR 3.00, 95% CI 0.13 to 69.52; 12 months: RR 9.00, 95% CI 0.52 to 156.92).
Nadkarni 2000, which randomised 70 teeth, assessed calcium hydroxide versus ZOE based on clinical failure at nine months. There was no statistically significant difference (RR 0.25, 95% CI 0.03 to 2.13).
Radiological failure
At six months, data were extractable from two RCTs totaling 110 teeth. The pooled results showed no statistically significant difference in radiological failure between calcium hydroxide and ZOE (RR 2.50, 95% CI 0.50 to 12.50; Analysis 18.1).
18.1. Analysis.
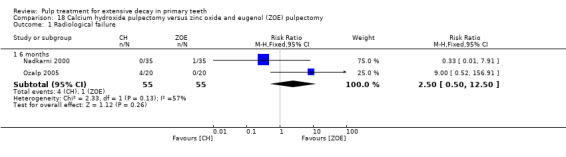
Comparison 18 Calcium hydroxide pulpectomy versus zinc oxide and eugenol (ZOE) pulpectomy, Outcome 1 Radiological failure.
Ozalp 2005 provided data on radiological failure at 12 months. There was no statistically significant difference (RR 9.00, 95% CI 0.52 to 156.92).
Overall failure
Nadkarni 2000, which randomised 70 teeth, provided data on overall failure. There was no statistically significant difference at nine months (RR 0.50, 95% CI 0.10 to 2.56). Data at other time points were unavailable.
Pain
At six months, data were extractable from two RCTs totaling 110 teeth. The pooled RR was 1.00 (95% CI 0.14 to 6.90; Analysis 18.2).
18.2. Analysis.
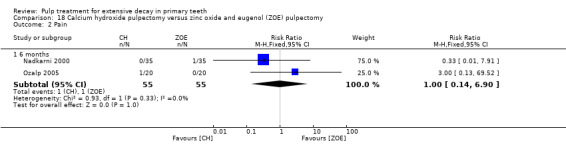
Comparison 18 Calcium hydroxide pulpectomy versus zinc oxide and eugenol (ZOE) pulpectomy, Outcome 2 Pain.
Ozalp 2005, which randomised 40 teeth, provided data on pain at 12 months. There was no statistically significant difference (RR 3.00, 95% CI 0.13 to 69.52).
Pathological mobility
At six months, data were extractable from two RCTs totaling 110 teeth. The pooled RR was 1.00 (95% CI 0.14 to 6.90; Analysis 18.3).
18.3. Analysis.
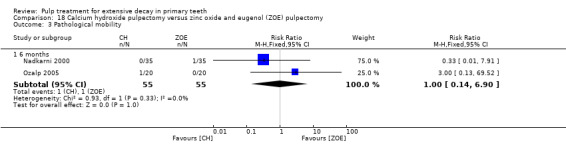
Comparison 18 Calcium hydroxide pulpectomy versus zinc oxide and eugenol (ZOE) pulpectomy, Outcome 3 Pathological mobility.
Ozalp 2005, which randomised 40 teeth, also provided data on pathological mobility at 12 months. There was no statistically significant difference (RR 3.00, 95% CI 0.13 to 69.52).
Pathological radiolucency
Two trials (110 teeth) provided data on pathological radiolucency at six months (Nadkarni 2000; Ozalp 2005). The pooled results showed no statistically significant difference between calcium hydroxide and ZOE (RR 1.50, 95% CI 0.26 to 8.72; Analysis 18.4).
18.4. Analysis.
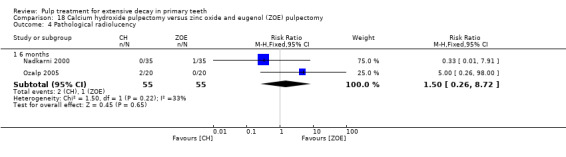
Comparison 18 Calcium hydroxide pulpectomy versus zinc oxide and eugenol (ZOE) pulpectomy, Outcome 4 Pathological radiolucency.
Ozalp 2005 provided data at 12 months. There was no statistically significant difference (RR 5.00, 95% CI 0.26 to 98.00).
Pathological root resorption
Ozalp 2005 provided data on pathological root resorption. There was no pathological root resorption in any of the participants regardless of the time of assessment.
Filling material anomaly
Ozalp 2005, which randomised 40 teeth, provided data on filling material anomaly. There was no filling material anomaly in any of the participants regardless of the time of assessment.
Sealapex versus calcium hydroxide
Ozalp 2005, which randomised 40 teeth, assessed Sealapex (eugenol‐free calcium hydroxide) versus calcium hydroxide based on clinical failure, radiological failure, pain, pathological mobility, pathological radiolucency, pathological root resorption and filling material anomaly. There was no statistically significant difference for any outcome at either six or 12 months (Table 38).
35. Pulpectomy (Sealapex) versus pulpectomy (CH).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 | 1 | 1.00 (0.07 to 14.90) |
| 12 | 1 | 0.50 (0.10 to 2.43) | |
| Radiological failure | 6 and 12 | 1 | 0.50 (0.10 to 2.43) |
| Pain | 6 and 12 | 1 | 1.00 (0.07 to 14.90) |
| Pathological mobility | 6 and 12 | 1 | 1.00 (0.07 to 14.90) |
| Pathological radiolucency | 6 and 12 | 1 | 0.20 (0.01 to 3.92) |
| Pathological root resorption | 6 and 12 | 1 | 5.00 (0.26 to 98.00) |
| Filling material anomaly | 6 and 12 | 1 | Not estimable* |
*due to lack of events
Abbreviations ‐ CI: confidence interval; Sealapex: eugenol‐free CH; CH: calcium hydroxide.
Vitapex versus calcium hydroxide
Ozalp 2005, which randomised 40 teeth, assessed Vitapex versus calcium hydroxide based on clinical failure, radiological failure, pain, pathological mobility, pathological radiolucency, pathological root resorption and filling material anomaly. There was no statistically significant difference for any outcome at either six or 12 months (Table 39).
36. Pulpectomy (Vitapex) versus pulpectomy (CH).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 | 1 | 0.33 (0.01 to 7.72) |
| 12 | 1 | 0.11 (0.01 to 1.94) | |
| Radiological failure | 6 and 12 | 1 | 0.11 (0.01 to 1.94) |
| Pain | 6 and 12 | 1 | 0.33 (0.01 to 7.72) |
| Pathological mobility | 6 and 12 | 1 | 0.33 (0.01 to 7.72) |
| Pathological radiolucency | 6 and 12 | 1 | 0.20 (0.01 to 3.92) |
| Pathological root resorption | 6 and 12 | 1 | Not estimable* |
| Filling material anomaly | 6 and 12 | 1 | 3.00 (0.13 to 69.52) |
*due to lack of events
Abbreviations ‐ CI: confidence interval; Vitapex: CH/iodoform; CH: calcium hydroxide.
Metapex versus zinc oxide and eugenol (ZOE)
Clinical failure
Data were extractable from two trials, totaling 62 teeth (Al‐Ostwani 2016; Subramaniam 2011). There were no statistically significant differences at six (RR 0.60, 95% CI 0.08 to 4.29) and 12 months (RR 0.71, 95% CI 0.15 to 3.33; Analysis 19.1).
19.1. Analysis.
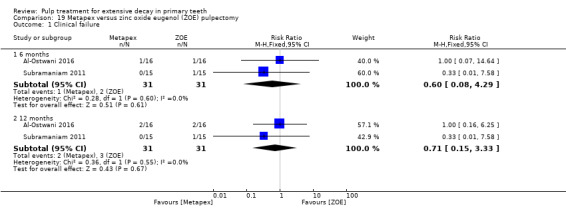
Comparison 19 Metapex versus zinc oxide eugenol (ZOE) pulpectomy, Outcome 1 Clinical failure.
Radiological failure
At six and 12 months, data were extractable from two trials, totaling 62 teeth. There was no statistically significant difference (RR 1.00, 95% CI 0.31 to 3.27; Analysis 19.2).
19.2. Analysis.
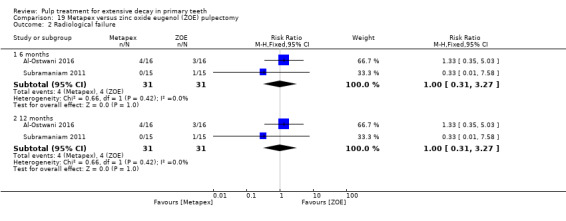
Comparison 19 Metapex versus zinc oxide eugenol (ZOE) pulpectomy, Outcome 2 Radiological failure.
Pain
At six and 12 months, one trial, which randomised 30 teeth, assessed pain (Subramaniam 2011). There was no statistically significant difference (RR 0.33, 95% CI 0.01 to 7.58).
Pathologic mobility
At six and 12 months, one trial, which randomised 30 teeth, assessed pathologic mobility (Subramaniam 2011). There was no statistically significant difference (RR 0.33, 95% CI 0.01 to 7.58).
Soft tissue pathology
At six and 12 months, one trial, which randomised 30 teeth, assessed soft tissue pathology (Subramaniam 2011). There was no soft tissue pathology in any of the participants regardless of the intervention.
Adjacent tissue inflammation
At six and 12 months, one trial, which randomised 30 teeth, assessed adjacent tissue inflammation (Subramaniam 2011). There were no adjacent tissue inflammation in any of the participants regardless of the intervention.
Pathologic radiolucency
At six and 12 months, data were extractable from two trials, totaling 62 teeth. There was no pathologic radiolucency in one trial; for the remaining trial, there was no statistically significant difference (RR 1.33, 95 CI 0.35 to 5.03) (Al‐Ostwani 2016).
Pathologic root resorption
At six and 12 months, one trial, which randomised 30 teeth, assessed adjacent tissue inflammation (Subramaniam 2011). There was no statistically significant difference (RR 0.33, 95% CI 0.01 to 7.58).
Metapex versus Endoflas
Clinical failure
At six months, data were extractable from two trials totaling 92 teeth. In one trial, there was no clinical failure in any of the participants regardless of the intervention. For the remaining trial, there was no statistically significant difference (RR 0.33, 95% CI 0.01 to 7.58; Analysis 20.1).
20.1. Analysis.
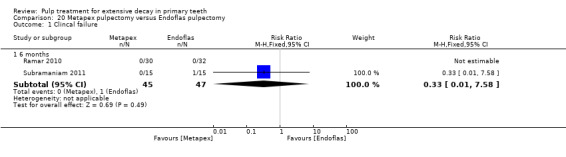
Comparison 20 Metapex pulpectomy versus Endoflas pulpectomy, Outcome 1 Clincal failure.
At 12 months, one trial, which randomised 30 teeth, assessed clinical failure (Subramaniam 2011). There was no statistically significant difference (RR 0.33, 95% CI 0.01 to 7.58).
Radiological failure
At six months, data were extractable from two trials totaling 92 teeth. There was no statistically significant difference (RR 2.02, 95% CI 0.79 to 5.15; Analysis 20.2).
20.2. Analysis.
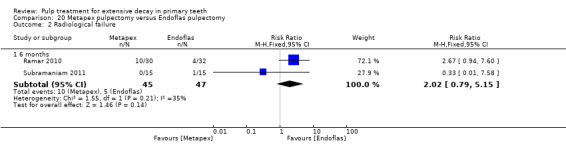
Comparison 20 Metapex pulpectomy versus Endoflas pulpectomy, Outcome 2 Radiological failure.
At 12 months, one trial, which randomised 30 teeth, assessed radiological failure (Subramaniam 2011). There was no statistically significant difference (RR 0.35, 95% CI 0.02 to 8.39).
Pain
At six months, data were extractable from two trials totaling 92 teeth. In one trial, there was no pain in any of the participants regardless of the intervention. For the remaining trial, there was no statistically significant difference (RR 0.33, 95% CI 0.01 to 7.58; Analysis 20.3).
20.3. Analysis.
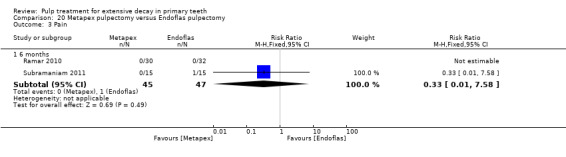
Comparison 20 Metapex pulpectomy versus Endoflas pulpectomy, Outcome 3 Pain.
At 12 months, one trial, which randomised 30 teeth, assessed pain (Subramaniam 2011). There was no statistically significant difference (RR 0.35, 95% CI 0.02 to 8.39).
Soft tissue pathology
At six months, data were extractable from two trials totaling 92 teeth. In one trial, there was no soft tissue pathology in any of the participants regardless of the intervention. For the remaining trial, there was no statistically significant difference (RR 0.33, 95% CI 0.01 to 7.58; Analysis 20.4).
20.4. Analysis.
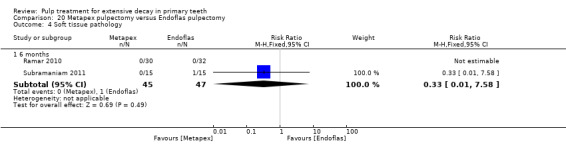
Comparison 20 Metapex pulpectomy versus Endoflas pulpectomy, Outcome 4 Soft tissue pathology.
At 12 months, one trial, which randomised 30 teeth, assessed soft tissue pathology (Subramaniam 2011). There was no statistically significant difference (RR 0.35, 95% CI 0.02 to 8.39).
Pathological mobility
At six months, data were extractable from two trials totaling 92 teeth. In the two trials, there was no pathological mobility in any of the participants regardless of the intervention (Analysis 20.5).
20.5. Analysis.
Comparison 20 Metapex pulpectomy versus Endoflas pulpectomy, Outcome 5 Pathologic mobility.
At 12 months, one trial, which randomised 30 teeth, assessed pathological mobility (Subramaniam 2011). There was no pathological mobility in any of the participants regardless of the intervention.
Pathological radiolucency
At six months, data were extractable from two trials totaling 92 teeth. There was no statistically significant difference (RR 2.02, 95% CI 0.79 to 5.15; Analysis 20.6).
20.6. Analysis.
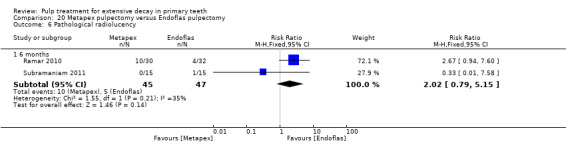
Comparison 20 Metapex pulpectomy versus Endoflas pulpectomy, Outcome 6 Pathological radiolucency.
At 12 months, one trial, which randomised 30 teeth, assessed pathological radiolucency (Subramaniam 2011). There was no statistically significant difference (RR 0.35, 95% CI 0.02 to 8.39).
Pathological root resorption
At six months, data were extractable from two trials totaling 92 teeth. In the two trials, there was no pathological root resorption in any of the participants regardless of the intervention (Analysis 20.7).
20.7. Analysis.
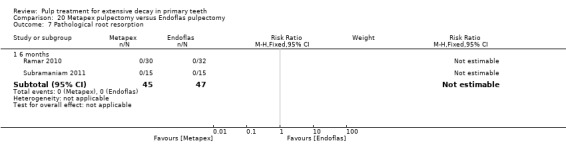
Comparison 20 Metapex pulpectomy versus Endoflas pulpectomy, Outcome 7 Pathological root resorption.
At 12 months, one trial, which randomised 30 teeth, assessed pathological root resorption (Subramaniam 2011). There was no pathological root resorption in any of the participants regardless of the intervention.
Succedaneous tooth anomaly (radiographically)
One trial, which randomised 30 teeth, assessed Metapex versus Endoflas based on unerupted succedaneous tooth anomaly (radiographically) (Subramaniam 2011). There was no cases in any of the participants regardless of the delay.
Metapex versus RC Fill
One trial, which randomised 64 teeth, assessed Metapex versus RC Fill based on pain, pathological radiolucency, pathological root resorption and filling material anomaly (Ramar 2010). There was no statistically significant difference for any outcome at six months (pain: RR 0.38, 95% CI 0.02 to 8.91; pathological radiolucency and filling material anomaly: RR 0.10, 95% CI 0.01 to 1.78; pathological root resorption: RR 2.27, 95% CI 0.87 to 5.89).
Metapex compared with Endoflas chlorophenol free (Endoflas‐CF)
One trial, which randomised 32 teeth, assessed Metapex versus Endoflas‐CF based on clinical failure and radiologic failure (pathologic radiolucency) (Al‐Ostwani 2016). There was no statistically significant differences for clinical failure at six (RR 3.00, 95% CI 0.13 to 68.57) and 12 months (RR 1.00, 95% CI 0.16 to 6.25), and for radiological failure (pathologic radiolucency) at six and 12 months (RR 1.33, 95% CI 0.35 to 5.03).
Metapex compared with zinc oxide and propolis (ZOP)
One trial, which randomised 32 teeth, assessed Metapex versus Endoflas‐CF based on clinical failure and radiologic failure (pathologic radiolucency) (Al‐Ostwani 2016). There were no statistically significant differences for clinical failure at six (RR 3.00, 95% CI 0.13 to 68.57) and 12 months (RR 2.00, 95% CI 0.20 to 19.91), and for radiological failure (pathologic radiolucency) at six (RR 1.33, 95% CI 0.35 to 5.03) and 12 months (RR 0.80, 95% CI 0.26 to 2.45).
Sealapex versus zinc oxide and eugenol (ZOE)
One trial, which randomised 40 teeth, assessed Sealapex (eugenol‐free calcium hydroxide) versus ZOE based on clinical failure, radiological failure, pain, pathological mobility, pathological radiolucency, pathological root resorption and filling material anomaly (Ozalp 2005). There was no statistically significant difference for any outcome at either six or 12 months. For clinical failure, the RR was 3.00 (95% CI 0.13 to 69.52) at six months and 5.00 (95% CI 0.26 to 98.00) at 12 months. For radiological failure and pathological root resorption, the RR was 5.00 (95% CI 0.26 to 98.00) at six and 12 months. For pain and pathological mobility, the RR was 3.00 (95% CI 0.13 to 69.52) at six and 12 months. There was no pathological radiolucency or filling material anomaly in any of the participants.
Vitapex versus Sealapex
One trial, which randomised 40 teeth, assessed Vitapex (calcium hydroxide/iodoform) versus Sealapex (eugenol‐free calcium hydroxide) based on clinical failure, radiological failure, pain, pathological mobility, pathological radiolucency, pathological root resorption and filling material anomaly (Ozalp 2005). There was no statistically significant difference for any outcome at either six or 12 months (Table 40).
37. Pulpectomy (Vitapex) versus pulpectomy (Sealapex).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 | 1 | 0.33 (0.01 to 7.72) |
| 12 | 1 | 0.20 (0.01 to 3.92) | |
| Radiological failure | 6 and 12 | 1 | 0.20 (0.01 to 3.92) |
| Pain | 6 and 12 | 1 | 0.33 (0.01 to 7.72) |
| Pathological mobility | 6 and 12 | 1 | 0.33 (0.01 to 7.72) |
| Pathological radiolucency | 6 and 12 | 1 | Not estimable* |
| Pathological root resorption | 6 and 12 | 1 | 0.20 (0.01 to 3.92) |
| Filling material anomaly | 6 and 12 | 1 | 3.00 (0.13 to 69.52) |
*due to lack of events
Abbreviations ‐ CI: confidence interval; Vitapex: CH/iodoform; CH: calcium hydroxide; Sealapex: eugenol‐free CH.
Vitapex versus zinc oxide and eugenol (ZOE)
Clinical failure
At six months, data were extractable from four RCTs totaling 287 teeth. In three of the trials, there was no clinical failure in any of the participants regardless of the intervention. From the remaining trial, the results showed no statistically significant difference in clinical failure with Vitapex compared with ZOE (RR 0.33, 95% CI 0.01 to 7.84). At 12 months, in two trials, there was no clinical failure in any of the participants regardless of the intervention. From the two remaining trials, the pooled results showed a statistically significant difference (RR 4.75, 95% CI 1.21 to 18.55) with evidence of statistical heterogeneity among included trials (I² = 78%; Analysis 21.1). Pramila 2016 (86 teeth randomised) assessed clinical failure at 30 months, and reported no failures.
21.1. Analysis.
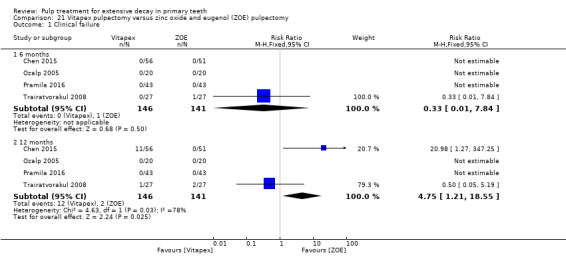
Comparison 21 Vitapex pulpectomy versus zinc oxide and eugenol (ZOE) pulpectomy, Outcome 1 Clinical failure.
Radiological failure
At six months, data were extractable from four RCTs totaling 287 teeth. In one of the trials, there was no radiological failure in any of the participants regardless of the intervention. From the three remaining trials, the pooled results showed no statistically significant difference in radiological failure with Vitapex compared with ZOE (RR 2.36, 95% CI 0.86 to 6.50). At 12 months, the pooled results showed a statistically significant difference (RR 6.56, 95% CI 2.58 to 16.67), with evidence of statistical heterogeneity among included trials (I² = 82%; Analysis 21.2). At 30 months, one trial, which randomised 86 teeth, assessed radiological failure, and there was a statistically significant difference (RR 19.00, 95% CI 1.14 to 316.52) (Pramila 2016).
21.2. Analysis.
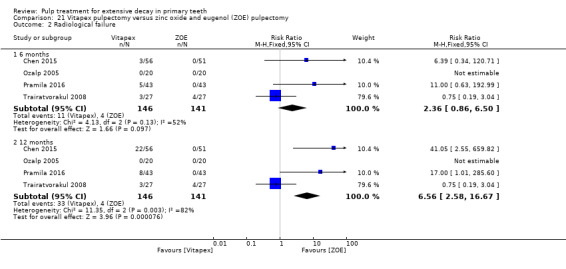
Comparison 21 Vitapex pulpectomy versus zinc oxide and eugenol (ZOE) pulpectomy, Outcome 2 Radiological failure.
Overall failure
Data were extractable from two trials totaling 140 teeth. There was no statistically significant difference at six (RR 1.89, 95% CI 0.63 to 5.66) and 12 months (RR 2.56, 95% CI 0.89 to 7.32; Analysis 21.3).
21.3. Analysis.
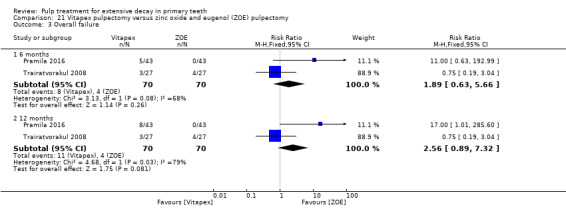
Comparison 21 Vitapex pulpectomy versus zinc oxide and eugenol (ZOE) pulpectomy, Outcome 3 Overall failure.
An additional trial, which randomised 58 teeth, did not assess the outcome at a fixed time point but at a mean (range) follow‐up of 12 (10 to 16) months (RR 1.01, 95% CI 0.63 to 1.64) (Mortazavi 2004).
Pain
At six and 12 months, data were extractable from three RCTs totaling 180 teeth. There was no pain in any of the participants in either trial or intervention (Analysis 21.4). At 30 months, one trial, which randomised 86 teeth, assessed pain, and there was no cases (Pramila 2016).
21.4. Analysis.
Comparison 21 Vitapex pulpectomy versus zinc oxide and eugenol (ZOE) pulpectomy, Outcome 4 Pain.
Mortazavi 2004, which randomised 58 teeth, did not assess the outcome at a fixed time point but at a mean (range) follow‐up of 12 (10 to 16) months. There was no pain in any of the participants regardless of the intervention.
Pathological mobility
At six and 12 months, data were extractable from three RCTs totaling 180 teeth. In two of the trials, there was no pathological mobility in any of the participants at either time point. For the remaining trial, the six‐ and 12‐month results showed no statistically significant difference in pathological mobility with Vitapex compared with ZOE (six months: RR 0.33, 95% CI 0.01 to 7.84; 12 months: RR 1.00, 95% CI 0.07 to 15.18; Analysis 21.5). At 30 months, one trial, which randomised 86 teeth, assessed pathologic mobility, and there was no cases (Pramila 2016).
21.5. Analysis.
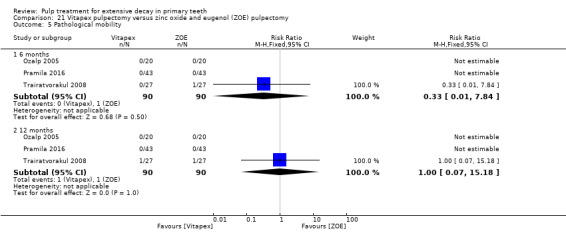
Comparison 21 Vitapex pulpectomy versus zinc oxide and eugenol (ZOE) pulpectomy, Outcome 5 Pathological mobility.
One additional trial, which randomised 58 teeth, did not assess the outcome at a fixed time point but at a mean (range) follow‐up of 12 (10 to 16) months (Mortazavi 2004). The RR was 0.14 (95% CI 0.01 to 2.41).
Soft tissue pathology
Three trials, randomising 155 teeth, assessed soft tissue pathology (Mortazavi 2004; Trairatvorakul 2008; Pramila 2016). There was no soft tissue pathology in any of the participants in either trial. At 30 months, one trial, which randomised 86 teeth, assessed soft tissue pathology, and there was no cases (Pramila 2016).
Pathological radiolucency
One trial, which randomised 40 teeth, assessed pathological radiolucency (Ozalp 2005). There was no pathological radiolucency in any of the participants regardless of the intervention, at any time point.
One additional trial, which randomised 58 teeth, did not assess the outcome at a fixed time point but at a mean (range) follow‐up of 12 (10 to 16) months (RR 0.14, 95% CI 0.01 to 2.41) (Mortazavi 2004).
Pathological root resorption
One trial provided data on pathological root radiolucency at 12 months (Ozalp 2005). There were no cases in any of the participants regardless of the intervention.
Filling material anomaly
One trial, which randomised 40 teeth, assessed filling material anomaly (Ozalp 2005). There was no statistically significant difference between groups at either six months (RR 3.00, 95% CI 0.13 to 69.52) or 12 months (RR 7.00, 95% CI 0.38 to 127.33).
Unerupted succedaneous tooth anomaly (radiographically)
One trial, which randomised 86 teeth, assessed unerupted succedaneous tooth anomaly (radiographically) at six, 12 and 30 months (Pramila 2016). There was no cases in any of the participants regardless of the intervention.
Vitapex (calcium hydroxide (calcium hydroxide)/iodoform) versus 3Mix (ciprofloxacin + metronidazole + minocycline)
One trial, which randomised 50 teeth, assessed Vitapex versus 3Mix (ciprofloxacin + metronidazole + minocycline) based on clinical failure, radiological failure, pain, soft tissue pathology, pathological mobility, pathological radiolucency, pathological root resorption and pulp canal obliteration (Nakornchai 2010). At 12 months, there was a statistically significant difference in favour of Vitapex for pathological radiolucency (RR 2.75, 95% CI 1.01 to 7.48). There was no other statistically significant difference for any other outcome at either six or 12 months (Table 41).
38. Pulpectomy (Vitapex) versus pulpectomy (3Mix).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 | 1 | Not estimable* |
| 12 | 1 | 1.0 (0.07 to 15.12) | |
| Radiological failure | 6 | 1 | 1.25 (0.38 to 4.12) |
| 12 | 1 | 1.83 (0.80 to 4.19) | |
| Pain | 6 | 1 | Not estimable* |
| 12 | 1 | 3.00 (0.13 to 70.30) | |
| Soft tissue pathology | 6 | 1 | Not estimable* |
| 12 | 1 | 1.0 (0.07 to 15.12) | |
| Pathological mobility | 6 and 12 | 1 | Not estimable* |
| Pathological radiolucency | 6 | 1 | 1.50 (0.27 to 8.22) |
| 12 | 1 | 2.75 (1.01 to 7.48) | |
| Pathological root resorption | 6 and 12 | 1 | 0.20 (0.01 to 3.97) |
| Pulp canal obliteration | 6 | 1 | Not estimable* |
| 12 | 1 | 0.20 (0.01 to 3.97) |
*due to lack of events
Abbreviations ‐ CI: confidence interval; Vitapex: CH/iodoform; CH: calcium hydroxide; 3Mix: ciprofloxacin + metronidazole + minocycline.
Vitapex versus RC Fill
One trial, which randomised 86 teeth, assessed Vitapex versus RC Fill based on clinical failure, radiological failure, overall failure, pain, pathologic mobility, soft tissue pathology, and unerupted succedaneous tooth anomaly (radiographically) (Pramila 2016). At six, 12 and 30 months, there was no cases of clinical failure, pain, pathologic mobility, soft tissue pathology, and unerupted succedaneous tooth anomaly (radiographically) in any of the participants regardless of the intervention. For radiological/overall failure, there were no statistically significant differences at six (RR 1.67, 95% CI 0.42 to 6.54), 12 (RR 2.00, 95% CI 0.65 to 6.15) and 30 months (RR 1.80, 95% CI 0.66 to 4.93).
Vitapex versus ZOE + calcium hydroxide + iodoform (unnamed product)
One trial, which randomised 109 teeth, assessed Vitapex versus ZOE+calcium hydroxide+iodoform (unnamed product) based on clinical failure and radiological failure (Chen 2015). At 12 months, there was a statistically significant difference in favour of ZOE+calcium hydroxide + iodoform (unnamed product) for clinical failure and radiological failure (clinical failure: RR 21.79, 95% CI 1.32 to 360.78; radiological failure: RR 42.63, 95% CI 2.65 to 685.54). There was no other statistically significant difference at six months (Table 42).
39. Pulpectomy (Vitapex) versus pulpectomy (MPRCF).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 | 1 | Not estimable* |
| 12 | 1 | 21.79 (1.32, 360.78) | |
| Radiological failure | 6 | 1 | 6.63 (0.35, 125.41) |
| 12 | 1 | 42.63 (2.65, 685.54) |
*due to lack of events
Abbreviations ‐ CI: confidence interval; Vitapex: CH/iodoform; MPRCF: ZOE (zinc oxide eugenol), calcium hydroxide, iodoform.
Endoflas versus zinc oxide and eugenol (ZOE)
Clinical failure
At six months, data were extractable from two trials, totaling 80 teeth. There was no statistically significant difference (RR 0.26, 95% CI 0.05 to 1.50; Analysis 22.1).
22.1. Analysis.
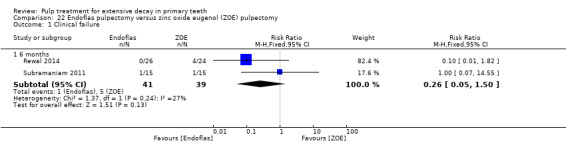
Comparison 22 Endoflas pulpectomy versus zinc oxide eugenol (ZOE) pulpectomy, Outcome 1 Clinical failure.
At 12 months, one trial, which randomised 30 teeth, assessed clinical failure (Subramaniam 2011). There was no statistically significant difference (RR 1.00, 95% CI 0.07 to 14.55).
Radiological failure
At six months, data were extractable from two trials, totaling 80 teeth. There was no statistically significant difference (RR 0.26, 95% CI 0.05 to 1.50; Analysis 22.2).
22.2. Analysis.
Comparison 22 Endoflas pulpectomy versus zinc oxide eugenol (ZOE) pulpectomy, Outcome 2 Radiological failure.
At 12 months, one trial, which randomised 30 teeth, assessed radiological failure (Subramaniam 2011). There was no statistically significant difference (RR 1.00, 95% CI 0.07 to 14.55).
Pain
At six months, data were extractable from two trials, totaling 80 teeth. There was no statistically significant differences (RR 0.26, 95% CI 0.05 to 1.50; Analysis 22.3).
22.3. Analysis.
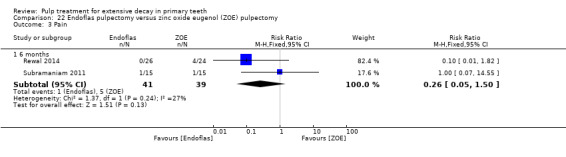
Comparison 22 Endoflas pulpectomy versus zinc oxide eugenol (ZOE) pulpectomy, Outcome 3 Pain.
At 12 months, one trial, which randomised 30 teeth, assessed pain (Subramaniam 2011). There was no statistically significant difference (RR 1.00, 95% CI 0.07 to 14.55).
Pathologic mobility
At six months, data were extractable from two trials, totaling 80 teeth. There was no statistically significant difference (RR 0.16, 95% CI 0.02 to 1.25; Analysis 22.4).
22.4. Analysis.
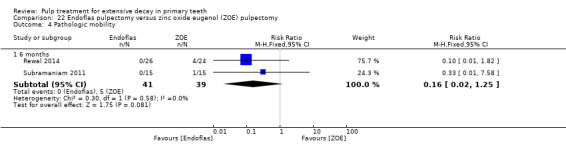
Comparison 22 Endoflas pulpectomy versus zinc oxide eugenol (ZOE) pulpectomy, Outcome 4 Pathologic mobility.
At 12 months, one trial, which randomised 30 teeth, assessed pathologic mobility (Subramaniam 2011). There was no statistically significant difference (RR 0.33, 95% CI 0.01 to 7.58).
Soft tissue pathology
At six months, data were extractable from two trials, totaling 80 teeth. There was no cases for one trial; for the remaining trial, there was no statistically significant difference (RR 3.00, 95% CI 0.13 to 68.26) (Subramaniam 2011).
At 12 months, one trial, which randomised 30 teeth, assessed soft tissue pathology (Subramaniam 2011). There was no statistically significant difference (RR 3.00, 95% CI 0.13 to 68.26).
Adjacent tissue inflammation
There were no adjacent tissue inflammation in any of the participants regardless of the intervention.
Pathologic radiolucency
At six months, data were extractable from two trials, totaling 80 teeth. There was no statistically significant difference (RR 0.64, 95% CI 0.11 to 3.63; Analysis 22.5).
22.5. Analysis.
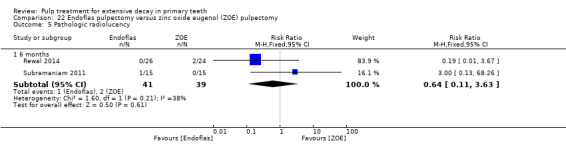
Comparison 22 Endoflas pulpectomy versus zinc oxide eugenol (ZOE) pulpectomy, Outcome 5 Pathologic radiolucency.
At 12 months, one trial, which randomised 30 teeth, assessed soft tissue pathology (Subramaniam 2011). There was no statistically significant difference (RR 3.00, 95% CI 0.13 to 68.26).
Pathologic root resorption
At six and 12 months, one trial, which randomised 30 teeth, assessed pathologic root resorption (Subramaniam 2011). There was no statistically significant difference (RR 0.33, 95% CI 0.01 to 7.58).
Filling material anomaly
One trial, which randomised 50 teeth, assessed filling material anomaly at six months (Rewal 2014). There was no statistically significant difference (RR 0.65, 95% CI 0.29 to 1.42).
Endoflas versus RC Fill
One trial, which randomised 66 teeth, assessed Endoflas versus RC Fill based on pain, pathological radiolucency, pathological root resorption and filling material anomaly (Ramar 2010). There was no statistically significant difference for any outcome at six months. For pain, the RR was 0.35 (95% CI 0.01 to 8.38); for pathological radiolucency and filling material anomaly, the RR was 0.10 (95% CI 0.01 to 1.68); for pathological root resorption, the RR was 0.85 (95% CI 0.25 to 2.88).
Endoflas‐CF versus ZOE
One trial, which randomised 32 teeth, assessed Endoflas‐CF versus ZOE based on clinical failure and radiologic failure (pathologic radiolucency) (Al‐Ostwani 2016). There were no statistically significant differences for clinical failure at six (RR 0.33, 95% CI 0.01 to 7.92) and 12 months (RR 1.00, 95% CI 0.16 to 6.25), and for radiological failure (pathologic radiolucency) at six and 12 months (RR 1.00, 95% CI 0.24 to 4.23).
Endoflas‐CF versus zinc oxide and propolis (ZOP)
One trial, which randomised 32 teeth, assessed Endoflas‐CF versus ZOP based on clinical failure and radiologic failure (pathologic radiolucency) (Al‐Ostwani 2016). There was no clinical failure at six months; there were no statistically significant differences for clinical failure at 12 months (RR 2.00, 95% CI 0.20 to 19.91), and for radiological failure (pathologic radiolucency) at six (RR 1.00, 95% CI 0.24 to 4.23) and 12 months (RR 0.60, 95% CI 0.17 to 2.10).
ZOE versus ozonated sesame oil‐zinc oxide (ZO)
One trial, which randomised 60 teeth, assessed ZOE versus ozonated sesame oil‐ZO based on clinical failure, radiological failure, overall failure, pathologic radiolucency at 12 months (Chandra 2014). For clinical failure, there was no statistically significant difference (RR 3.00, 95% CI 0.13 to 70.83). For radiological/overall failure (pathologic radiolucency), there was a statistically significant difference (RR 4.50, 95% CI 1.03 to 19.62).
ZOE versus RC Fill
One trial, which randomised 86 teeth, assessed ZOE versus RC Fill based on clinical failure, radiological failure, overall failure, pain, pathologic mobility, soft tissue pathology, and unerupted succedaneous tooth anomaly (radiographically) (Pramila 2016). At six, 12 and 30 months, there was no cases of clinical failure, pain, pathologic mobility, soft tissue pathology, and unerupted succedaneous tooth anomaly (radiographically) in any of the participants regardless of the intervention. For radiological/overall failure, there were no statistically significant differences at six (RR 0.14, 95% CI 0.01 to 2.68), 12 (RR 0.11, 95% CI 0.01 to 2.00) and 30 months (RR 0.09, 95% CI 0.01 to 1.59).
ZOE versus zinc oxide and propolis (ZOP)
One trial, which randomised 32 teeth, assessed ZOE versus ZOP based on clinical failure and radiologic failure (pathologic radiolucency) (Al‐Ostwani 2016). There were no statistically significant differences for clinical failure at six (RR 3.00, 95% CI 0.13 to 68.57) and 12 months (RR 2.00, 95% CI 0.20 to 19.91), and for radiological failure (pathologic radiolucency) at six (RR 1.00, 95% CI 0.24 to 4.23) and 12 months (RR 0.60, 95% CI 0.17 to 2.10).
ZOE versus ZOE + calcium hydroxide + iodoform (unnamed product)
One trial, which randomised 104 teeth, provided data on clinical failure, radiological failure and filling material anomaly at six and 12 months (Chen 2015). There were no clinical or radiological failures in any of the participants regardless of the intervention. For filling material anomaly, there was no statistically significant difference at six months (RR 1.73, 95% CI 0.95 to 3.17), and a statistically significant difference at 12 months (RR 3.12, 95% CI 1.35 to 7.22).
Ciprofloxacin + metronidazole + minocycline (3Mix) versus ciprofloxacin + ornidazole + minocycline
One trial, which randomised 40 teeth, assessed 3Mix versus ciprofloxacin+ornidazole+minocycline based on clinical failure, pain, pathological mobility, soft tissue pathology and pathological radiolucency (i.e. radiological failure) (Pinky 2011). There was no clinical failure, pain, pathological mobility, soft tissue pathology in any of the participants regardless of the time point. There was no pathological radiolucency at six months in any of the participants, and there was no statistically significant difference at 12 months (RR 5.00, 95% CI 0.26 to 98.00).
MTA versus IRM
One trial, which randomised 50 teeth, assessed MTA versus IRM based on clinical failure, radiological failure, overall failure, pain, pathological mobility, soft tissue pathology (Arikan 2016). At six and 12 months, there was no clinical failure, pain, pathological mobility and soft tissue pathology. For radiological/overall failure, there was no statistically significant difference at six (RR 0.20, 95% CI 0.01 to 3.97) or 12 months (RR 0.50, 95% CI 0.10 to 2.49).
MTA versus gutta‐percha/AH‐Plus
One trial, which randomised 20 teeth, assessed MTA versus gutta‐percha/AH‐Plus based on clinical failure, radiological failure, overall failure, pain, pathologic mobility, soft tissue pathology and pathologic radiolucency (Bezgin 2016). At six months, there was no failure. At 12 and 24 months, there were no cases of pain and pathologic mobility. At 12 months, there was no statistically significant difference for clinical failure/soft tissue pathology (RR 0.20, 95 CI 0.01 to 3.70), radiological failure/pathologic radiolucency and overall failure (RR 0.25, 95 CI 0.03 to 1.86). At 24 months, there was no statistically significant difference for clinical failure/soft tissue pathology (RR 0.14, 95 CI 0.01 to 2.45); at 24 months, there was a statistically significant difference for radiological failure/pathologic radiolucency and overall failure (RR 0.14, 95 CI 0.02 to 0.96).
Pulpotomy versus pulpectomy
Four trials compared pulpotomy with pulpectomy. Two trials were at high risk of bias, and, for two other trials, the risk of bias was unclear. We judged no trials comparing pulpotomy versus pulpectomy to be at low risk of bias.
Formocresol pulpotomy versus calcium hydroxide pulpectomy
One trial, which randomised 51 teeth, assessed formocresol pulpotomy versus calcium hydroxide pulpectomy (Coser 2008). There were no data provided for any outcomes.
Ferric sulphate/zinc oxide and eugenol (ZOE) pulpotomy versus Sedanol (ZOE) pulpectomy
One trial, which randomised 291 teeth, assessed ferric sulphate pulpotomy versus Sedanol pulpectomy based on radiological failure, pain, soft tissue pathology, pathological radiolucency, pathological root resorption and pulp canal obliteration (Casas 2004). There were statistically significant differences for pathological root resorption (RR 21.04, 95% CI 1.28 to 346.39) and pulp canal obliteration (RR 27.05, 95% CI 1.66 to 441.49) at 24 months. There was no statistically significant difference for any other outcome (Table 43).
40. Pulpotomy (FS) versus pulpectomy (Sedanol).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Pain | 24 | 1 | 1.80 (0.07 to 43.88) |
| Soft tissue pathology | 24 | 1 | 4.21 (0.22 to 80.70) |
| Pathological radiolucency | 24 | 1 | 0.60 (0.25 to 1.46) |
| Pathological root resorption | 24 | 1 | 21.04 (1.28 to 346.39) |
| Pulp canal obliteration | 24 | 1 | 27.05 (1.66 to 441.49) |
Abbreviations ‐ CI: confidence interval; FS: ferric sulphate; Sedanol=ZOE: zinc oxide and eugenol.
Ferric sulphate/MTA pulpotomy versus ZOE pulpectomy
One trial, which randomised 172 teeth, assessed ferric sulphate/MTA pulpotomy versus ZOE pulpectomy based on clinical failure and radiological failure (Nguyen 2017). There were no statistically significant differences for clinical failure at 12 (RR 3.61, 95% CI 0.18 to 74.16) and 18 months (RR 2.16, 95% CI 0.23 to 20.35), and for radiological failure at 12 (RR 0.36, 95% CI 0.09 to 1.39) and 18 months (RR 0.84, 95% CI 0.29 to 2.39).
3Mix (ciprofloxacin + metronidazole + minocycline) pulpotomy versus 3Mix pulpectomy
One trial, which randomised 60 teeth, assessed 3Mix pulpotomy versus 3Mix pulpectomy based on clinical failure, pain, pathological mobility, soft tissue pathology and pathological radiolucency (i.e. radiological failure) (Prabhakar 2008). There was a statistically significant difference at six months for pathological radiolucency (RR 23.00, 95% CI 1.42 to 373.46), that was not maintained at 12 months. There was no statistically significant difference for the other outcomes at any time point (Table 44).
41. Pulpotomy (3Mix) versus pulpectomy (3Mix).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 | 1 | 3.00 (0.13, 70.83) |
| 12 | 1 | 5.00 (0.25, 99.95) | |
| Pain | 6 | 1 | 3.00 (0.13, 70.83) |
| 12 | 1 | 5.00 (0.25, 99.95) | |
| Soft tissue pathology | 6 | 1 | Not estimable* |
| 12 | 1 | 3.00 (0.13, 70.83) | |
| Pathologic mobility | 6 and 12 | 1 | 3.00 (0.13, 70.83) |
| Pathologic radiolucency | 6 | 1 | 23.00 (1.42, 373.46) |
| 12 | 1 | 11.00 (0.64, 190.53) |
*due to lack events
Abbreviations ‐ CI: confidence interval; 3Mix: ciprofloxacin + metronidazole + minocycline.
Direct pulp capping versus direct pulp capping
Seven trials compared direct pulp capping using different medicaments. Six trials were at unclear risk of bias and one trial was at high risk of bias.
Calcium hydroxide versus formocresol
One trial, which randomised 120 teeth, assessed calcium hydroxide versus formocresol based on clinical failure, radiological failure, pain, soft tissue pathology, pathological radiolucency and pathological root resorption (Aminabadi 2010). There was a statistically significant difference for clinical failure (RR 3.83, 95% CI 1.68 to 8.74), radiological failure (RR 3.11, 95% CI 1.61 to 6.02) and pathological radiolucency (RR 5.00, 95% CI 1.14 to 21.86) at 24 months. There was no statistically significant difference for any other outcome or time point (Table 45).
42. Direct pulp capping (CH) versus direct pulp capping (FC).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 24 | 1 | 3.83 (1.68 to 8.74) |
| Radiological failure | 24 | 1 | 3.11 (1.61 to 6.02) |
| Pain | 6 | 1 | 7.00 (0.37 to 132.66) |
| 12 | 1 | 9.00 (0.50 to 163.59) | |
| 24 | 1 | 4.00 (0.89 to 18.06) | |
| Soft tissue pathology | 6 | 1 | 7.00 (0.37 to 132.66) |
| 12 | 1 | 2.5 (0.50 to 12.39) | |
| 24 | 1 | 1.8 (0.64 to 5.06) | |
| Pathological radiolucency | 6 | 1 | Not estimable* |
| 12 | 1 | 4.00 (0.46 to 34.75) | |
| 24 | 1 | 5.00 (1.14 to 21.86) | |
| Pathological root resorption | 6 | 1 | Not estimable* |
| 12 | 1 | 3.33 (0.96 to 11.51) | |
| 24 | 1 | 2.00 (0.87 to 4.60) |
*due to lack of events
Abbreviations ‐ CH: calcium hydroxide; CI: confidence interval; FC: formocresol.
Acetone‐based total‐etch adhesive versus calcium hydroxide
One trial, which randomised 40 teeth, assessed acetone‐based total‐etch adhesive versus calcium hydroxide based on clinical failure, radiological failure, pain, pathological radiolucency and pathological root resorption (Demir 2007). There was no clinical failure, radiological failure, pain, pathological radiolucency or pathological root resorption in any of the participants regardless of the time point.
Non‐rinse conditioner + acetone‐based total‐etch adhesive versus calcium hydroxide
One trial, which randomised 40 teeth, assessed non‐rinse conditioner + acetone‐based total‐etch adhesive versus calcium hydroxide based on clinical failure, radiological failure, pain, pathological radiolucency and pathological root resorption (Demir 2007). There was no clinical failure, pain or pathological root resorption in any of the participants regardless of the delay. There was no radiological failure or pathological radiolucency in any of the participants regardless of the intervention at six months. For radiological failure and pathological radiolucency at 12 and 24 months, the RR was 3.00 (95% CI 0.13 to 69.52).
Total‐etching with 36% phosphoric acid + acetone‐based total‐etch adhesive versus calcium hydroxide
One trial, which randomised 40 teeth, assessed total‐etching with 36% phosphoric acid + acetone‐based total‐etch adhesive versus calcium hydroxide based on clinical failure, radiological failure, pain, pathological radiolucency and pathological root resorption (Demir 2007). There was no statistically significant difference for any outcome at six, 12 or 24 months (Table 46).
43. Direct pulp capping (total‐etching with 36% phosphoric acid + acetone‐based total‐etch adhesive) versus direct pulp capping (CH).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 | 1 | Not estimable* |
| 12 and 24 | 1 | 3.00 (0.13 to 69.52) | |
| Radiological failure | 6 | 1 | Not estimable* |
| 12 | 1 | 3.00 (0.13 to 69.52) | |
| 24 | 1 | 7.00 (0.38 to 127.33) | |
| Pain | 6 | 1 | Not estimable* |
| 12 and 24 | 1 | 3.00 (0.13 to 69.52) | |
| Pathological radiolucency | 6 | 1 | Not estimable* |
| 12 | 1 | 3.00 (0.13 to 69.52) | |
| 24 | 1 | 7.00 (0.38 to 127.33) | |
| Pathological root resorption | 6, 12 and 24 | 1 | Not estimable* |
*due to lack of events
Abbreviations ‐ CH: calcium hydroxide; CI: confidence interval
Self etch adhesive system + acetone‐based total‐etch adhesive versus calcium hydroxide
One trial, which randomised 40 teeth, assessed self etch adhesive system + acetone‐based total‐etch adhesive versus calcium hydroxide based on clinical failure, radiological failure, pain, pathological radiolucency and pathological root resorption (Demir 2007). There was no statistically significant difference for any outcome at six, 12 or 24 months (Table 47).
44. Direct pulp capping (self etch adhesive system + acetone‐based total‐etch adhesive) versus direct pulp capping (CH).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6, 12 and 24 | 1 | 3.00 (0.13 to 69.52) |
| Radiological failure | 6 | 1 | Not estimable* |
| 12 and 24 | 1 | 3.00 (0.13 to 69.52) | |
| Pain | 6 | 1 | Not estimable* |
| 12 and 24 | 1 | 3.00 (0.13 to 69.52) | |
| Pathological radiolucency | 6 | 1 | Not estimable* |
| 12 and 24 | 1 | 3.00 (0.13 to 69.52) | |
| Pathological root resorption | 6, 12 and 24 | 1 | Not estimable* |
*due to lack of events
Abbreviations ‐ CH: calcium hydroxide; CI: confidence interval
Enamel matrix derivative (EMD) versus calcium hydroxide
One trial, which randomised 90 teeth, assessed EMD versus calcium hydroxide based on overall failure, pain, soft tissue pathology, pathological mobility and pathological root resorption (Garrocho‐Rangel 2009). For overall failure, the RR was 1.00 (95% CI 0.06 to 15.50) at six and 12 months. There was no pain, soft tissue pathology, pathological mobility or pathological root resorption in any of the participants regardless of the time point.
MTA versus calcium hydroxide
One trial, which randomised 50 teeth, assessed MTA versus calcium hydroxide based on clinical failure, radiological failure and pain (Tuna 2008). There were no clinical and radiological failures or pain in any of the participants regardless of the time point.
Non‐rinse conditioner + acetone‐based total‐etch adhesive versus acetone‐based total‐etch adhesive
One trial, which randomised 40 teeth, assessed non‐rinse conditioner + acetone‐based total‐etch adhesive versus acetone‐based total‐etch adhesive based on clinical failure, radiological failure, pain, pathological radiolucency and pathological root resorption (Demir 2007). There was no clinical failure, pain or pathological root resorption in any of the participants. There was no radiological failure or pathological radiolucency in any of the participants at six months. For radiological failure and pathological radiolucency, the RR was 3.00 (95% CI 0.13 to 69.52) at 12 and 24 months.
Total‐etching with 36% phosphoric acid + acetone‐based total‐etch adhesive versus acetone‐based total‐etch adhesive
One trial, which randomised 40 teeth, assessed total‐etching with 36% phosphoric acid + acetone‐based total‐etch adhesive versus acetone‐based total‐etch adhesive based on clinical failure, radiological failure, pain, pathological radiolucency and pathological root resorption (Demir 2007). There was no statistically significant difference for any outcome at six, 12 or 24 months (Table 48).
45. Direct pulp capping (total‐etching with 36% phosphoric acid + acetone‐based total‐etch adhesive) versus direct pulp capping (acetone‐based total‐etch adhesive).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 | 1 | Not estimable* |
| 12 and 24 | 1 | 3.00 (0.13 to 69.52) | |
| Radiological failure | 6 and 12 | 1 | 3.00 (0.13 to 69.52) |
| 24 | 1 | 7.00 (0.38 to 127.33) | |
| Pain | 6 | 1 | Not estimable* |
| 12 and 24 | 1 | 3.00 (0.13 to 69.52) | |
| Pathological radiolucency | 6 | 1 | Not estimable* |
| 12 and 24 | 1 | 3.00 (0.13 to 69.52) | |
| Pathological root resorption | 6, 12 and 24 | 1 | Not estimable* |
*due to lack of events
Abbreviation ‐ CI: confidence interval.
Self‐etch adhesive system + acetone‐based total‐etch adhesive versus acetone‐based total‐etch adhesive
One trial, which randomised 40 teeth, assessed self‐etch adhesive system + acetone‐based total‐etch adhesive versus acetone‐based total‐etch adhesive based on clinical failure, radiological failure, pain, pathological radiolucency and pathological root resorption (Demir 2007). There was no statistically significant difference for any outcome at six, 12 or 24 months (Table 49).
46. Direct pulp capping (self etch adhesive system + acetone‐based total‐etch adhesive) versus direct pulp capping (acetone‐based total‐etch adhesive).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6, 12 and 24 | 1 | 3.00 (0.13 to 69.52) |
| Radiological failure | 6 | 1 | Not estimable* |
| 12 and 24 | 1 | 3.00 (0.13 to 69.52) | |
| Pain | 6 | 1 | Not estimable* |
| 12 and 24 | 1 | 3.00 (0.13 to 69.52) | |
| Pathological radiolucency | 6 | 1 | Not estimable* |
| 12 and 24 | 1 | 3.00 (0.13 to 69.52) | |
| Pathological root resorption | 6, 12 and 24 | 1 | Not estimable* |
*due to lack of events
Abbreviation ‐ CI: confidence interval
Total‐etching with 36% phosphoric acid + acetone‐based total‐etch adhesive versus non‐rinse conditioner + acetone‐based total‐etch adhesive
One trial, which randomised 40 teeth, assessed total‐etching with 36% phosphoric acid + acetone‐based total‐etch adhesive versus non‐rinse conditioner + acetone‐based total‐etch adhesive based on clinical failure, radiological failure, pain, pathological radiolucency and pathological root resorption (Demir 2007). There was no statistically significant difference for any outcome at six, 12 or 24 months (Table 50).
47. Direct pulp capping (total‐etching with 36% phosphoric acid + acetone‐based total‐etch adhesive) versus direct pulp capping (non‐rinse conditioner + acetone‐based total‐etch adhesive).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 | 1 | Not estimable* |
| 12 and 24 | 1 | 3.00 (0.13 to 69.52) | |
| Radiological failure | 6 | 1 | Not estimable* |
| 12 | 1 | 3.00 (0.13 to 69.52) | |
| 24 | 1 | 7.00 (0.38 to 127.33) | |
| Pain | 6 | 1 | Not estimable* |
| 12 and 24 | 1 | 3.00 (0.13 to 69.52) | |
| Pathological radiolucency | 6 | 1 | Not estimable* |
| 12 | 1 | 3.00 (0.13 to 69.52) | |
| 24 | 1 | 7.00 (0.38 to 127.33) | |
| Pathological root resorption | 6, 12 and 24 | 1 | Not estimable* |
*due to lack of events
Abbreviations ‐ CI: confidence interval.
Self‐etch adhesive system + acetone‐based total‐etch adhesive versus non‐rinse conditioner + acetone‐based total‐etch adhesive
One trial, which randomised 40 teeth, assessed self‐etch adhesive system + acetone‐based total‐etch adhesive versus non‐rinse conditioner + acetone‐based total‐etch adhesive based on clinical failure, radiological failure, pain, pathological radiolucency and pathological root resorption (Demir 2007). There was no statistically significant difference for any outcome at six, 12 or 24 months (Table 51).
48. Direct pulp capping (self etch adhesive system + acetone‐based total‐etch adhesive) versus direct pulp capping (non‐rinse conditioner + acetone‐based total‐etch adhesive).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6, 12 and 24 | 1 | 3.00 (0.13 to 69.52) |
| Radiological failure | 6 | 1 | Not estimable* |
| 12 and 24 | 1 | 1.00 (0.07 to 14.90) | |
| Pain | 6 | 1 | Not estimable* |
| 12 and 24 | 1 | 3.00 (0.13 to 69.52) | |
| Pathological radiolucency | 6 | 1 | Not estimable* |
| 12 and 24 | 1 | 1.00 (0.07 to 14.90) | |
| Pathological root resorption | 6, 12 and 24 | 1 | Not estimable* |
*due to lack of events
Abbreviation ‐ CI: confidence interval.
Self‐etch adhesive system + acetone‐based total‐etch adhesive versus total‐etching with 36% phosphoric acid + acetone‐based total‐etch adhesive
One trial, which randomised 40 teeth, assessed self‐etch adhesive system + acetone‐based total‐etch adhesive versus total‐etching with 36% phosphoric acid + acetone‐based total‐etch adhesive based on clinical failure, radiological failure, pain, pathological radiolucency and pathological root resorption (Demir 2007). There was no statistically significant difference for any outcome at six, 12 or 24 months (Table 52).
49. Direct pulp capping (self etch adhesive system + acetone‐based total‐etch adhesive) versus direct pulp capping (total‐etching with 36% phosphoric acid + acetone‐based total‐etch adhesive).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 6 | 1 | 3.00 (0.13 to 69.52) |
| 12 and 24 | 1 | 1.00 (0.07 to 14.90) | |
| Radiological failure | 6 | 1 | 0.33 (0.01 to 7.72) |
| 12 | 1 | 1.00 (0.07 to 14.90) | |
| 24 | 1 | 0.33 (0.04 to 2.94) | |
| Pain | 6 | 1 | Not estimable* |
| 12 | 1 | 3.00 (0.13 to 69.52) | |
| 24 | 1 | 1.00 (0.07 to 14.90) | |
| Pathological radiolucency | 6 | 1 | Not estimable* |
| 12 | 1 | 1.00 (0.07 to 14.90) | |
| 24 | 1 | 0.33 (0.04 to 2.94) | |
| Pathological root resorption | 6, 12 and 24 | 1 | Not estimable* |
*due to lack of events
Abbreviation ‐ CI: confidence interval.
Calcium‐enriched mixture (CEM) cement versus MTA
One trial, which randomised 42 teeth, assessed CEM cement versus MTA based on clinical failure, radiological failure, pain, soft tissue pathology, pathological radiolucency and pathological root resorption at six months, and overall failure at 20 month (Fallahinejad Ghajari 2013). At six months, there was no pain, radiological failure, pathological radiolucency and pathological root resorption in any participants regardless of the intervention. There were no statistically significant differences for clinical failure and soft tissue pathology (RR 3.00, 95% CI 0.13 to 69.70). At 20 months, there was no statistically significant difference for overall failure (RR 1.00, 95% CI 0.07 to 14.95).
MTA versus 3Mix
One trial, which randomised 160 teeth, assessed MTA versus 3Mix based on clinical failure, radiological failure, overall failure, pain, soft tissue pathology, pathologic radiolucency, pathologic root resorption at 12 months (Aminabadi 2016). There was a statistically significant difference for clinical and overall failure (RR 0.17, 95% CI 0.04 to 0.70), pain and soft tissue pathology (RR 0.08, 95% CI 0.01 to 0.61). There was no radiological failure.
MTA versus 3Mixtatin
One trial, which randomised 160 teeth, assessed MTA versus 3Mixtatin based on clinical failure, radiological failure, overall failure, pain, soft tissue pathology, pathologic radiolucency, pathologic root resorption at 12 months (Aminabadi 2016). There were no statistically significant differences for clinical and overall failure (RR 0.67, 95% CI 0.12 to 3.78), pain (RR 0.33, 95% CI 0.04 to 3.07) and soft tissue pathology (RR 3.00, 95% CI 0.13 to 71.51). There was no radiological failure.
MTA versus simvastatin
One trial, which randomised 160 teeth, assessed MTA versus simvastatin based on clinical failure, radiological failure, overall failure, pain, soft tissue pathology, pathologic radiolucency, pathologic root resorption at 12 months (Aminabadi 2016). There was a statistically significant difference for clinical and overall failure (RR 0.17, 95% CI 0.04 to 0.70), pain and soft tissue pathology (RR 0.08, 95% CI 0.01 to 0.61). There was no radiological failure.
3Mix versus 3Mixtatin
One trial, which randomised 160 teeth, assessed 3Mix versus 3Mixtatin based on clinical failure, radiological failure, overall failure, pain, soft tissue pathology, pathologic radiolucency, pathologic root resorption at 12 months (Aminabadi 2016). There were statistically significant differences for clinical, overall failure, pain (RR 4.00, 95% CI 1.22 to 13.11), and soft tissue pathology (RR 25.00, 95% CI 1.53 to 408.39). There was no radiological failure.
3Mix versus simvastatin
One trial, which randomised 160 teeth, assessed 3Mix versus simvastatin based on clinical failure, radiological failure, overall failure, pain, soft tissue pathology, pathologic radiolucency, pathologic root resorption at 12 months (Aminabadi 2016). There were no statistically significant differences for clinical, overall failure, pain and soft tissue pathology (RR 1.00, 95% CI 0.51 to 1.95). There was no radiological failure.
3Mixtatin versus simvastatin
One trial, which randomised 160 teeth, assessed 3Mixtatin versus simvastatin based on clinical failure, radiological failure, overall failure, pain, soft tissue pathology, pathologic radiolucency, pathologic root resorption at 12 months (Aminabadi 2016). There was a statistically significant difference for clinical, overall failure and pain (RR 0.25, 95% CI 0.08 to 0.82), and soft tissue pathology (RR 0.04, 95% CI 0.00 to 0.65). There was no radiological failure.
Calcium hydroxide cement (Dycal) versus calcium sulphate hemihydrate (DentoGen)
One trial, which randomised 40 teeth, assessed Dycal versus DentoGen based on clinical failure, radiological failure, pain, soft tissue pathology, pathological mobility, pathological radiolucency and pathological root resorption (Ulusoy 2014a). There was no statistically significant difference for any outcome at six or 12 months (Table 53).
50. Direct pulp capping (Dycal) versus direct pulp capping (Dentogen).
| Outcome | Time point (months) | No. of studies | Risk ratio (95% CI) |
| Clinical failure | 1, 3, 6 | 1 | 5.00 (0.26, 98.00) |
| 9 | 1 | 7.00 (0.38, 127.32) | |
| 12 | 1 | 4.00 (0.49, 32.72) | |
| Radiological failure | 1, 3, 6 | 1 | 5.00 (0.26, 98.00) |
| 9 | 1 | 3.00 (0.34, 26.45) | |
| 12 | 1 | 1.67 (0.46, 6.06) | |
| Pain | 1, 3, 6, 9 | 1 | Not estimable* |
| 12 | 1 | 3.00 (0.13, 69.52) | |
| Soft tissue pathology | 1, 3, 6, 9 | 1 | 5.00 (0.26, 98.00) |
| 12 | 1 | 2.00 (0.20, 20.33) | |
| Pathologic mobility | 1, 3, 6, 9 | 1 | 5.00 (0.26, 98.00) |
| 12 | 1 | 2.00 (0.20, 20.33) | |
| Pathologic radiolucency | 1, 3, 6 | 1 | 5.00 (0.26, 98.00) |
| 9 | 1 | 3.00 (0.34, 26.45) | |
| 12 | 1 | 1.33 (0.34, 5.21) | |
| Pathologic root resorption | 1, 3, 6 | 1 | 5.00 (0.26, 98.00) |
| 9 | 1 | 2.00 (0.20, 20.33) | |
| 12 | 1.33 (0.34, 5.21) |
*due to lack of events
Abbreviation ‐ CI: confidence interval.
Discussion
Summary of main results
For this systematic review of pulp interventions for treatment of extensive decay in primary teeth of children, we included 87 randomised controlled trials (RCTs). These trials examined 125 different comparisons: 75 comparisons among different medicaments for pulpotomy (reported in 53 trials), 25 comparisons among different medicaments for pulpectomy (15 trials), four comparisons between pulpotomy and pulpectomy (4 trials), and 21 comparisons among different medicaments for direct pulp capping (7 trials). The risk of bias for all individual studies was assessed as either high or unclear.
Pulpotomy
The majority of the evidence with regards to pulpotomy versus pulpotomy came from trials of mineral trioxide aggregate (MTA) compared with formocresol (FC). MTA reduced both clinical and radiological failures with a statistically significant difference at 12 months for clinical failure and at six, 12 and 24 months for radiological failure (moderate‐quality evidence). MTA also showed favourable results for all secondary outcomes, especially pathological root resorption with around four times fewer cases at 24 months than when formocresol was used (effect size 0.25); pathological radiolucency was less than half as frequent with MTA than with formocresol at 12 months (effect size 0.43). MTA induced statistically significantly more pulp canal obliteration at 24 months and dentin bridge formation at six months.
MTA also showed favourable results compared with calcium hydroxide for all outcomes measured, with statistically significant differences at six, 12 and 24 months for radiological failure, pathological radiolucency and root resorption, at 12 and 24 months for clinical failure, soft tissue pathology and pathological mobility, and at 24 months for overall failure (moderate‐ to low‐quality evidence). The largest effect sizes concerned pathological radiolucency, pathological root resorption and pathological mobility with over 10 times fewer cases when MTA was used (effect sizes at 24 months respectively 0.08, 0.08 and 0.09). MTA induced statistically significantly more pulp canal obliteration at six and 24 months (about double), but also five times more dentin bridge formation at six months (the effect size was 0.2 at 6 months and 0.8 at 12 and 24 months, which seems to indicate that there is 25% more dentin bridge formation with MTA. Dentin bridge formation was faster with MTA).
Calcium hydroxide was compared with formocresol. There was a statistically significant difference in favour of formocresol for clinical failure at six and 12 months, radiological failure at six, 12 and 24 months, overall failure at 24 months, pain and soft tissue pathology at 12 months, and pathological root resorption at six, 12 and 24 months (moderate‐quality evidence). There was a statistically significant difference in favour of calcium hydroxide for dentin bridge formation at six and 12 months.
Calcium hydroxide was also compared with ferric sulphate. There was a statistically significant difference in favour of ferric sulphate for radiological and overall failure at 24 months (low‐quality evidence).
Compared with ferric sulphate, MTA had statistically significantly fewer radiological and overall failures and pathological radiolucency and root resorption at six months. (This difference was not significant at 12 and 24 months) (low‐ to very low‐quality evidence).
For all other comparisons of medicaments used during pulpotomies, despite the statistically significant difference for seven comparisons, each including only one trial (Kalra 2017; Sabbarini 2008Table 9; Table 10; Table 18; Table 19; Table 20; Table 33), the small number of studies and the inconsistency in results limits any interpretation.
Overall, MTA pulpotomy was superior to formocresol and ferric sulphate; all three treatments may be superior to calcium hydroxide pulpotomy. Although the evidence was weak for other medicaments, MTA seemed to stand out as the best treatment option for pulpotomy in primary teeth for the moment.
A challenge is that MTA is an expensive product with a relatively short setting time. The total treatment cost is incompatible with prices set by social welfare systems in many countries. However, MTA cost is reducing rapidly in Europe. Very few trials compared MTA with Biodentine, lasers, EMD or Ankaferd Blood Stopper for pulpotomy in primary teeth. Large, long‐term, well‐designed trials comparing these therapies in terms of efficacy and cost‐effectiveness would be useful. Research should also be encouraged to find a calcium silicate or inorganic material that is as effective as MTA for primary tooth pulpotomy, but with a shorter setting time.
Formocresol is a compound consisting of 48.5% formaldehyde, 48.5% cresol and 3% glycerine. Buckley's solution, introduced in 1904, is a diluted form of formocresol containing 19% formaldehyde, 35% cresol and 17.5% glycerine. Disinfecting agents used commonly for hospital floors and surfaces include sodium hypochlorite, chlorhexidine, ethanol, formaldehyde, glutaraldehyde, hydrogen peroxide, iodoform and calcium hydroxide (Ferreira 2007; Sassone 2003). However, formaldehyde is a carcinogen (of the second category according to modified 67/548/CEE) and mutagenic agent (of the third category according to modified 67/548/CEE) (Milnes 2006). Moreover, cresols are toxic if ingested or there is cutaneous contact and can cause severe skin burns and eye lesions. Formocresol should therefore not be applied without a dental dam. Researchers started to say that formocresol should not be used at the beginning of the 1980s (Lewis 2009); the American Association of Endodontists and the American Academy of Pediatric Dentistry advocate not using formocresol. We think the use of formocresol should be banned in children, and journals should not accept any publication of a trial including formocresol treatments in children. We think MTA should now be used as the 'gold standard' medicament in primary teeth pulpotomy trials.
Although the evidence is insufficient and a proper network meta‐analysis would be needed, calcium hydroxide and calcium hydroxide iodoform may be ineffective medicaments that should not be applied after pulpotomy of a primary molar. It seems that ZOE, electrosurgery, buffered glutaraldehyde and probably ferric sulphate may have medium to low efficacy. Ferric sulphate has proved to be less effective than MTA and may be less effective than formocresol (except for pain). Ferric sulphate may also be less effective than NaOCl. If MTA, laser, Biodentine or EMD cannot be applied to the pulp stumps, applying NaOCl may be the safest and cheapest option, although the efficacy of such a treatment clearly seems to be limited.
For the time being, the evidence supports applying MTA on the pulp stumps after pulpotomy in primary teeth. Where MTA is not accessible, research is needed to confirm if Biodentine, EMD, laser treatment or Ankaferd Blood Stopper are acceptable second choices, and if, where none of these treatments can be used, application of NaOCl would be the safest option.
Pulpectomy
For comparisons of medicaments used during pulpectomies, ZOE had statistically significantly fewer clinical and radiological failures at six and 12 months than calcium hydroxide iodoform (Vitapex) (low‐quality evidence). For all other comparisons of medicaments used during pulpectomies, despite the statistically significant difference for four comparisons, each including only one trial (Bezgin 2016; Chandra 2014; Table 41; Table 42), the small number of studies and the inconsistency in results limited interpretation. ZOE‐calcium hydroxide‐I (Endoflas) and 3Mix have been insufficiently evaluated and trials comparing ZOE, ZOE‐calcium hydroxide‐I, 3Mix (and maybe Metapex) should be conducted.
Thus, until further trials compare ZOE‐calcium hydroxide‐I and 3Mix, ZOE may be the best choice for filling the root canals after pulpectomy in primary teeth. ZOE seems to be an effective medicament; it is cheap and reasonably safe (Sarrami 2002) for use in children.
Pulpotomy versus pulpectomy
The evidence comparing pulpotomy and pulpectomy was limited as the four available trials each assessed a different combinations of medicament and pulpotomy/pulpectomy. Two of the trials found in favour of the pulpectomy arm for some secondary outcomes (Table 43; Table 44); however, we think this is due to the fact that the teeth included in both these trials presented root pulp irreversible inflammation or infection; actually, inclusion criteria in both these trials concerned teeth with furcal radiolucency, spontaneous pain, tenderness to percussion, abnormal mobility or an abscess or fistula. Such teeth should not be treated by pulpotomy. Pulpectomy and extraction are the two options in such cases. Researchers and clinicians should refer to the AAPD guideline on pulp therapy when conceiving a trial or choosing the appropriate pulp treatment (Guideline Pulp Therapy 2014; Guideline Pulp Therapy 2016).
Direct pulp capping
The evidence comparing different medicaments for use in direct pulp capping was limited as there was only one trial for each comparison. Demir 2007 compared different adhesive resins with each other and against calcium hydroxide, and did not find evidence that one medicament was superior to another. Aminabadi 2010 favoured formocresol over calcium hydroxide; MTA over 3Mix or simvastatin; and 3Mixtatin over 3Mix or simvastatin (low‐quality evidence). Calcium silicates such as MTA and Biodentine seem to be promising materials; however, no failure was identified in Tuna's trial comparing calcium hydroxide and MTA (Tuna 2008). As for pulpotomy, EMD may also be an alternative medicament for direct pulp capping in primary teeth; one trial compared EMD with calcium hydroxide and identified only two failures (one from each group) from 90 teeth evaluated over 12 months (Garrocho‐Rangel 2009).
Due to the toxicity of formocresol, conducting a large long‐term, well‐designed trial comparing MTA, Biodentine, EMD and DentoGen could help practitioners choose the right medicament to apply on a pulp exposure in a primary tooth.
Overall completeness and applicability of evidence
Overall completeness
Many trials have evaluated medicaments that can be used after pulpotomy of a deciduous molar. However, few trials have evaluated direct pulp capping medicaments. Several recent studies have added to the evidence comparing medicaments that can be applied to root canals after pulpectomy, but data are still scarce.
Some studies have compared the pulpotomy and pulpectomy treatments but no study has compared dental pulp capping and pulpotomy. However, AAPD recommendations already stipulate the conditions when direct pulp capping, pulpotomy or pulpectomy should be performed; moreover, these recommendations say direct pulp capping of a carious pulp exposure in a primary tooth is not recommended (Guideline Pulp Therapy 2014; Guideline Pulp Therapy 2016). It therefore seems there is no need for new trials comparing direct pulp capping and pulpotomy or pulpotomy and pulpectomy.
Applicability of evidence
Many countries ‐ rich or poor, north or south ‐ contributed data to our review, so results should be valid in all countries and regions.
In terms of context, all included trials were conducted in dental departments of hospitals. The context of a private practice is quite different and practice‐based studies, conducted in private practices, would enhance the external validity of data on pulp treatments in primary teeth. However, operators of included studies were of different types: either paediatric dentists, general practitioners, undergraduate or postgraduate students. Our results therefore do apply to all types of practitioners.
Quality of the evidence
The major limitation of our review is inherent to shortcomings across and within primary studies. The efficacy of pulp treatment techniques may be measured in various ways, commonly by both clinical and radiological dimensions. We found substantial diversity in reported outcomes, with 78 outcomes pertaining to primary teeth (39 clinical and 39 radiological outcomes). A success or failure composite outcome was often used but was defined by various component outcomes across trials. Consensus is lacking regarding the most relevant outcomes, especially for the definition of success or failure. Most investigators used their own criteria. In our review, the median number of component outcomes defining success or failure for each trial was nine (quartile 1 to quartile 3: 5 to 10, minimum to maximum 1 to 20). Moreover, all these outcomes were frequently assessed at different times within and across trials. Because of heterogeneous selection and measurement of outcomes across trials, performing meta‐analyses may be difficult, if not impossible. However, this variety did not always result in substantial statistical heterogeneity on meta‐analysis. The number of children included in most studies was very small. Power was thus a problem both at the study level and at the review level. Until further trials are conducted, a network meta‐analysis may help unravel the efficacy hierarchy that exists among pulpotomy materials.
The overall risk of bias of included studies was either high or unclear. Trial reporting often did not allow for assessment of all risk of bias domains because many methodological elements were not mentioned. Operative treatment cannot be blinded and the risk of bias could not be low. There is no solution to this problem. However, no included study would be at low risk if this risk of bias domain was not taken into account, which means the methodology of clinical trials evaluating pulp treatments in primary teeth can still be enhanced.
We assessed the quality of evidence regarding pulp treatments for extensive decay in primary teeth as moderate to very low (according to GRADE recommendations), depending on the comparison and main outcome (clinical or radiographic). The risk of bias was high or unclear in all studies, the small size of the studies often resulted in imprecision and methodological differences sometimes resulted in substantial inconsistency. However, some comparisons had a large (RR < 0.5 or > 2) to very large (RR < 0.2 or > 5) magnitude of effect, which may compensate for the methodological shortcomings or the heterogeneity. We could not assess the potential for publication bias.
In this review, the quality of the evidence seemed slightly better for radiographic outcomes: the risk of bias was rated as low, unclear and high in 51%, 44% and 5% of the included trials, respectively, for the clinical outcomes, compared to 59%, 39% and 2%, respectively, for the radiographic outcomes.
We assessed 32% of the pulpotomy trials to be at high risk of bias, compared with 13% of the pulpectomy trials and 14% of the direct pulp capping trials. The pulpotomy evidence may be in larger quantity but of lower quality than the pulpectomy and direct pulp capping evidence. However, it is difficult to be sure as all other trials were at unclear risk of bias.
We assessed 50% of the pulpotomy versus pulpectomy trials to be at high risk of bias, which is not surprising because the methodology of these trials was poor overall (in particular, inclusion criteria do not comply with AAPD guidelines).
The heterogeneity of the results was very often low (0% to 40%) and sometimes moderate to substantial (40% to 68%) in pulpotomy trials. It was low to very substantial (up to 82%) in pulpectomy trials. Direct pulp capping comparisons all involved only one trial so heterogeneity could not be assessed.
Imprecision was a common reason for downgrading the quality of the evidence because included trials were mostly small and yielded large confidence intervals if too few trials had been conducted.
Potential biases in the review process
Our search strategy was extensive and we contacted authors to query data. However, we cannot exclude the possibility of selective reporting of trials or outcomes. The small numbers of trials in comparisons preclude the assessment of small‐study biases through funnel plots and asymmetry tests. Regarding selective outcome reporting, we did not have access to most trial protocols. However, we found important differences between outcomes defined in the methods and results sections. In all 87 reports, at least one outcome defined in the methods section was not reported in the results section or vice versa. However, the large number of cases creates support for overall conclusions.
Agreements and disagreements with other studies or reviews
Pulpotomy
A few systematic reviews have compared pulpotomy medicaments.
One systematic review compared MTA, calcium hydroxide, ferric sulphate and electrosurgery; based on 30 trials, it concluded the superiority of MTA (Stringhini 2015a). One systematic review compared MTA, formocresol, ferric sulphate and calcium hydroxide; based on 18 RCTs and 10 clinical trials; results suggested that MTA was superior to formocresol, ferric sulphate and calcium hydroxide in all time periods up to exfoliation (Ng 2008). One systematic review compared MTA and ferric sulphate; based on four trials, the 24‐month data were in favour of MTA (Asgary 2014b). One systematic review compared MTA and calcium hydroxide; based on four trials, risk ratios were in favour of MTA after six, 12 and 24 months (Shirvani 2014b). The results of these four systematic reviews are in agreement with our results.
Four systematic reviews compared MTA and formocresol. One was based on five trials (377 teeth) and did not identify any significant difference in success between medicaments (Marghalani 2014a). One was based on six trials and its results showed a clinical and a radiographic risk ratio statistically significantly in favour of MTA (Peng 2006). One was based on eight trials and concluded that overall clinical and radiographic success rates were in favour of MTA (Fallahinejad 2008).One was based on 19 trials (1585 participants) and the resulting risk ratios were 0.26, 0.37 and 0.41 after six, 12 and 24 months, respectively, always in favour of MTA (Shirvani 2014c). Our results, based on 14 trials (1048 teeth), are very much in line with the latter as the risk ratios we observed were 0.23, 0.48 and 0.50, respectively. Overall, MTA is a more effective medicament than formocresol for the pulpotomy of primary molars.
One network meta‐analysis was published (Lin 2014). The results favoured MTA, ferric sulphate and formocresol over laser and calcium hydroxide. Considering the effect estimates and funnel plot of clinical and radiographic outcomes after 18 to 24 months, the medicaments followed the mentioned order in terms of success, with MTA being the most successful medicament and calcium hydroxide being the least successful. Concerning these two medicaments, our results are in agreement with this network meta‐analysis. However, our results are not completely in agreement with this publication for ferric sulphate, formocresol and laser. Our results were based on 53 trials while this network meta‐analysis was based on 37 studies (3 of which we excluded because they did not meet our inclusion criteria), 22 of which were included in the final network meta‐analysis. Thus, our effect estimates are much larger than those found in this review. Overall, our pair‐wise effect estimates favour the same medicament as in this review. A network meta‐analysis based on our results would certainly help in confirming the relative efficacy of medicaments such as ferric sulphate, laser or NaOCl.
Furthermore, one systematic review compared formocresol and ferric sulphate; based on four RCTs, four controlled clinical trials and three retrospective studies; its results did not show any statistically significant risk ratio between medicaments (Peng 2007). Another systematic review comparing formocresol and ferric sulphate found clinical data statistically significantly in favour of ferric sulphate and radiographic data that did not statistically significantly differ between medicaments (Loh 2004). As in our review, their clinical data seemed to be in favour of ferric sulphate while their radiographic data seemed to be in favour of formocresol.
Pulpectomy
We found no other systematic review comparing the different medicaments that can be used to fill the root canals of pulpectomised primary molars.
Direct pulp capping
We found no other systematic review comparing the different medicaments that can be applied on the pulp stumps for direct pulp capping of primary molars.
Comparison of pulp treatments
A recent systematic review supported indirect pulp therapy and pulpotomy (with either MTA or formocresol) over direct pulp capping as the latter showed similar failure rates but the quality of the evidence was lower (Coll 2015; Coll 2017). Our review did not evaluate indirect pulp capping. However, we found no randomised trial comparing pulpotomy and direct pulp capping. This recent systematic review was based on some of the trials included in this review, which compare medicaments for a given pulp treatment. We think the context and methodology varies too much across trials to conclude as to the superiority of pulpotomy or direct pulp capping: trials comparing these would be useful.
Authors' conclusions
Implications for practice.
Many of the included trials had no clinical or radiological failures in either arm, and the proportion of failures in other trials was small.
Mineral trioxide aggregate (MTA) may be the best medicament to apply on the pulp stumps after pulpotomy of a deciduous tooth. Formocresol is effective, but there are known concerns about toxicity. Where MTA is not accessible, Biodentine, enamel matrix derivatives (EMD), laser treatment or maybe Ankaferd Blood Stopper seem to be the second choices. Where none of these treatments can be used, application of sodium hypochlorite (NaOCl) could be the safest option.
Concerning pulpectomy of primary teeth, the evidence was inconclusive. Zinc‐oxide eugenol (ZOE) paste, which is cheap and considered relatively safe, may be more effective than Vitapex, but data were insufficient to draw conclusions about its relative efficacy compared to Endoflas and Metapex, or other alternatives.
For direct pulp capping in primary teeth, formocresol appeared to be more successful than calcium hydroxide, but should not be used in children due to its toxicity. Tricalcium silicates (MTA in particular), calcium sulphate hemihydrate (DentoGen) and EMD may be the best alternatives, but the quality of the evidence is low to very low and all comparisons were based on only one trial.
The overall quality of the evidence ranged from was moderate to very low and so it should be noted that future research could change our findings.
Implications for research.
Future trials in this area should take into consideration the high success rate, which means that very large trials would be needed and should follow up children for at least one year, ideally longer. Trials should use the criteria we proposed for the evaluation of pulp treatment techniques to facilitate future systematic reviews and meta‐analyses (Smaïl‐Faugeron 2013).
Concerning pulpotomy in primary teeth, well designed long‐term trials could compare MTA with Biodentine, laser therapy (diode or Er:Yag), EMD, Ankaferd Blood Stopper and maybe simple NaOCl application in terms of efficacy and cost‐effectiveness. Cost‐effectiveness trials from different countries could be useful to determine which medicament should be advocated in which economic setting. Laboratory research could also be encouraged to elaborate a calcium silicate or inorganic material as safe and effective as MTA for primary tooth pulpotomy, but with a shorter setting time. We suggest that researchers choose MTA as the reference treatment.
Concerning pulpectomy, well designed long‐term trials could compare ZOE paste with Endoflas, 3Mix and Metapex.
Concerning direct pulp capping of primary teeth, a trial (or trials) comparing MTA, EMD, calcium sulphate hemihydrate (DentoGen) and maybe other tricalcium silicates (such as Biodentine) or laser therapy could help in defining which is the most effective treatment.
What's new
| Date | Event | Description |
|---|---|---|
| 10 August 2017 | New search has been performed | Searches updated to August 2017. |
| 10 August 2017 | New citation required and conclusions have changed | Review update including 40 new studies bringing the total to 87 included studies. The methods have been updated and the risk of bias completed for all included studies. Conclusions changed. |
Acknowledgements
For this update, we thank Helen Worthington, Laura MacDonald, Anne Littlewood and Philip Riley of Cochrane Oral Health. We also thank the referees Suzanne Dunkley and Jaap Veerkamp, and copy editor Ann Jones.
For previous versions of the review, we thank Anne Littlewood (Cochrane Oral Health) for design and conduct of search strategies; Luisa Fernandez Mauleffinch and Philip Riley (Cochrane Oral Health) for their assistance; Laura Smales (BioMedEditing, Toronto, Canada) for editing the manuscript; and Gill Nadin for contribution as an author.
Appendices
Appendix 1. Cochrane Oral Health's Trials Register search strategy
From August 2017, searches of the Oral Health Group's Trials Register for this review were undertaken using the Cochrane Register of Studies and the search strategy below:
1 (("root canal" and (therap* or treat*)):ti,ab) AND (INREGISTER) 2 ((pulpectom* or pulpotom*):ti,ab) AND (INREGISTER) 3 ((pulp and cap*):ti,ab) AND (INREGISTER) 4 (#1 or #2 or #3) AND (INREGISTER) 5 ((primary or deciduous or milk or baby or temporary or natal):ti,ab) AND (INREGISTER) 6 ((child* or infant* or preschool or pre‐school):ti,ab) AND (INREGISTER) 7 (#5 or #6) AND (INREGISTER) 8 (#4 and #7) AND (INREGISTER) Previous searches of the Oral Health Group's Trials Register were undertaken using the Procite software and the search strategy below:
((DENTAL‐PULP‐CAPPING OR "pulp cap*" OR PULPECTOMY OR PULPOTOMY OR pulpectom* OR pulpotom* OR "ROOT‐CANAL‐THERAPY" OR ("root canal" AND (therapy or treatment)) OR ENDODONTICS OR endodontic*) AND (primary or deciduous OR milk OR baby OR temporary OR natal OR child* OR infant* OR child‐preschool))
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 DENTAL PULP CAPPING single term (MeSH) #2 PULPECTOMY single term (MeSH) #3 PULPOTOMY single term (MeSH) #4 ROOT CANAL THERAPY explode all trees (MeSH) #5 ENDODONTICS single term (MeSH) #6 (#1 or #2 or #3 or #4 or #5) #7 ((root next canal) and (therap* or treat*)) #8 pulpectom* #9 pulpotom* #10 (pulp near cap*) #11 (#7 or #8 or #9 or #10) #12 (primary or deciduous or milk or baby or temporary or natal) #13 CHILD explode (MeSH) #14 child* or infant* #15 #12 or #13 or #14 #16 #6 or #11 #17 (#15 AND #16)
Appendix 3. MEDLINE Ovid search strategy
1. Dental Pulp Capping/ 2. PULPECTOMY/ 3. PULPOTOMY/ 4. exp "Root Canal Therapy"/ 5. ENDODONTICS/ 6. or/1‐5 7. (root canal and (therap$ or treat$)).mp. [mp=title, original title, abstract, name of substance, mesh subject heading] 8. (pulpectom$ or pulpotom$).mp. [mp=title, original title, abstract, name of substance, mesh subject heading] 9. (pulp adj6 cap$).mp. [mp=title, original title, abstract, name of substance, mesh subject heading] 10. or/7‐9 11. (primary or deciduous or milk or baby or temporary or natal).mp. [mp=title, original title, abstract, name of substance, mesh subject heading] 12. exp Child/ 13. Infant/ 14. (child$ or infant$).mp. [mp=title, original title, abstract, name of substance, mesh subject heading] 15. or/11‐14 16. 6 or 10 17. 15 and 16
The above subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of theCochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 4. Embase Ovid search strategy
1. endodontics/ 2. (pulp adj6 cap$).mp. 3. (pulpectom$ or pulpotom$).mp. 4. ((root adj canal) and (therap$ or treat$)).mp. 5. or/1‐4 6. (primary or deciduous or milk or baby or temporary or natal).mp. 7. Child/ 8. Infant/ 9. (child$ or infant$).mp. 10. or/6‐9 11. 5 and 10
The above subject search was linked to adapted version of the Cochrane Embase Project filter for identifying RCTs in Embase Ovid (see http://www.cochranelibrary.com/help/central‐creation‐details.html for information):
1. Randomized controlled trial/ 2. Controlled clinical study/ 3. Random$.ti,ab. 4. randomization/ 5. intermethod comparison/ 6. placebo.ti,ab. 7. (compare or compared or comparison).ti. 8. ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 9. (open adj label).ti,ab. 10. ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab 11. double blind procedure/ 12. parallel group$1.ti,ab. 13. (crossover or cross over).ti,ab. 14. ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 15. (assigned or allocated).ti,ab. 16. (controlled adj7 (study or design or trial)).ti,ab. 17. (volunteer or volunteers).ti,ab. 18. trial.ti. 19. or/1‐18 20. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 21. 19 not 20
Appendix 5. Web of Science search strategy
TS=((pulp cap* OR pulpectom* OR pulpotom* OR endodontic* OR root canal therap* OR root canal treat*) AND (child* Or infant* OR primary OR deciduous OR milk Or baby OR temporary OR natal))
AND
TS=(random* or trial* or placebo* or group*)
Appendix 6. OpenGrey search strategy
A series of keyword searches was performed:
pulp and cap* and dental pulp and cap* and teeth pulp and cap* and tooth pulpectom* pulpotom* endodontic* and child* endodontic* and primary root canal and child* root canal and primary child* Or infant* OR primary OR deciduous OR milk Or baby OR temporary OR natal
Appendix 7. US National Institutes of Health Ongoing Trials Registry (ClinicalTrials.gov) and the World Health Organization International Clinical Trials Registry Platform search strategy
A series of keyword searches was performed in both trials registries:
pulp and capping and child pulpectomy and child pulpotomy and child endodontic and child root and canal and child
Appendix 8. Selected outcome in studies reporting two or more similar outcomes
Pain
Tenderness to percussion, to pressure, and spontaneous pain were reported component outcomes of pain.
Soft tissue pathology
Swelling, fistula, abscess, sinus tract were reported component outcomes of soft tissue pathology.
Adjacent tissue inflammation
Severe gingival inflammation, redness around the tooth‐crown, bleeding around the tooth or crown, erythema, inflammation in the adjacent tissues were reported component outcomes of adjacent tissue inflammation.
Pathological radiolucency
Pathological radiolucency, furcal radiolucency, periapical radiolucency, periodontal ligament widening, inter‐radicular bone destruction, periapical bone destruction, changes in the integrity of lamina dura, pathological radiolucency, periradicular radiolucency, bone radiolucency, periapical lesion and inter‐radicular radiolucency were reported component outcomes of pathological radiolucency.
Pathological root resorption
Pathological root resorption, internal root resorption and external root resorption were reported component outcomes of pathological root resorption.
Pulp canal obliteration
Pulp canal obliteration, Intracanal calcifications, calcific metamorphosis and calcific degeneration of the pulp were reported component outcomes of pulp canal obliteration.
Data and analyses
Comparison 1. Mineral trioxide aggregate (MTA) pulpotomy versus full strength or 1:5 diluted formocresol pulpotomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 months | 13 | 1048 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.07, 1.89] |
| 1.2 12 months | 12 | 740 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.10, 0.93] |
| 1.3 24 months | 9 | 548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.18, 1.19] |
| 2 Radiological failure | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 6 months | 12 | 922 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.17, 0.86] |
| 2.2 12 months | 12 | 740 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.19, 0.89] |
| 2.3 24 months | 9 | 548 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.22, 0.80] |
| 3 Overall failure | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 6 months | 6 | 328 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.04, 1.32] |
| 3.2 12 months | 6 | 328 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.17, 1.36] |
| 3.3 24 months | 7 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.25, 1.01] |
| 4 Pain | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 6 months | 6 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.91] |
| 4.2 12 months | 6 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.18] |
| 4.3 24 months | 4 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.14, 3.56] |
| 5 Soft tissue pathology | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 6 months | 7 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.91] |
| 5.2 12 months | 7 | 430 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 1.01] |
| 5.3 24 months | 5 | 310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.10] |
| 6 Pathological mobility | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 6 months | 5 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 12 months | 4 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.97] |
| 6.3 24 months | 3 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Pathological radiolucency | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 6 months | 13 | 1010 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.27, 1.08] |
| 7.2 12 months | 11 | 652 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.19, 0.98] |
| 7.3 24 months | 8 | 460 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.25, 1.22] |
| 8 Pathological root resorption | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 6 months | 11 | 866 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.18, 1.21] |
| 8.2 12 months | 9 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.07, 1.03] |
| 8.3 24 months | 6 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.08, 0.81] |
| 9 Pulp canal obliteration | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 6 months | 9 | 712 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.00, 2.30] |
| 9.2 12 months | 7 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.81, 3.57] |
| 9.3 24 months | 6 | 338 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [1.07, 3.94] |
| 10 Dentin bridge formation | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 6 months | 3 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 18.16 [3.63, 90.91] |
| 10.2 12 months | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.76, 47.22] |
| 10.3 24 months | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.76, 47.22] |
| 11 Physiological root resorption | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 6 months | 2 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.2 12 months | 2 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 24 months | 2 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.81] |
Comparison 2. Mineral trioxide aggregate (MTA) pulpotomy versus full strength formocresol pulpotomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 months | 6 | 538 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.09, 5.64] |
| 1.2 12 months | 5 | 432 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.12, 1.68] |
| 1.3 24 months | 3 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.14] |
| 2 Radiological failure | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 6 months | 4 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.05, 1.79] |
| 2.2 12 months | 5 | 432 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.08, 0.98] |
| 2.3 24 months | 3 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.06, 0.67] |
| 3 Pain | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 6 months | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.91] |
| 3.2 12 months | 3 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.18] |
| 3.3 24 months | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.14] |
| 4 Soft tissue pathology | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 6 months | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.91] |
| 4.2 12 months | 3 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.18] |
| 4.3 24 months | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.91] |
| 5 Pathological radiolucency | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 6 months | 4 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 2.95] |
| 5.2 12 months | 4 | 332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.16, 2.38] |
| 5.3 24 months | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.11, 1.95] |
| 6 Pathological root resorption | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 6 months | 3 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.95] |
| 6.2 12 months | 3 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.91] |
| 6.3 24 months | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 0.98] |
| 7 Pulp canal obliteration | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 6 months | 2 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 12 months | 2 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.76 [0.79, 206.70] |
Comparison 3. Mineral trioxide aggregate (MTA) pulpotomy versus 1:5 diluted formocresol pulpotomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 months | 8 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.06] |
| 1.2 12 months | 7 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.28] |
| 1.3 24 months | 6 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.18, 1.42] |
| 2 Radiological failure | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 6 months | 8 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.17, 1.03] |
| 2.2 12 months | 7 | 308 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.20, 1.53] |
| 2.3 24 months | 6 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.31, 1.46] |
| 3 Pain | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 6 months | 4 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 12 months | 3 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 24 months | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.30] |
| 4 Soft tissue pathology | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 6 months | 5 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 12 months | 4 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.02, 1.65] |
| 4.3 24 months | 3 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| 5 Pathological mobility | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 6 months | 4 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 12 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.97] |
| 5.3 24 months | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Pathological radiolucency | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 6 months | 9 | 622 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.30, 1.27] |
| 6.2 12 months | 7 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.13, 1.02] |
| 6.3 24 months | 6 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.23, 1.57] |
| 7 Pathological root resorption | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 6 months | 8 | 550 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.24, 1.99] |
| 7.2 12 months | 6 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.05, 1.14] |
| 7.3 24 months | 4 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.10, 1.92] |
| 8 Pulp canal obliteration | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 6 months | 7 | 520 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.00, 2.30] |
| 8.2 12 months | 5 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.44, 2.26] |
| 8.3 24 months | 5 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.99, 3.89] |
Comparison 4. Mineral trioxide aggregate (MTA) pulpotomy versus ferric sulphate (FS) pulpotomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 months | 4 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.31] |
| 1.2 12 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.97] |
| 1.3 24 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.20, 1.39] |
| 2 Radiological failure | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 6 months | 4 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.40] |
| 2.2 12 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.15, 3.44] |
| 2.3 24 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.25, 1.36] |
| 3 Overall failure | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 6 months | 4 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.40] |
| 3.2 12 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.15, 3.44] |
| 3.3 24 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.32, 1.89] |
| 4 Pain | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 6 months | 3 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.00] |
| 4.2 12 months | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Soft tissue pathology | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 6 months | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.00] |
| 6 Pathologic mobility | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 6 months | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.31] |
| 7 Pathologic radiolucency | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 6 months | 3 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.48] |
| 7.2 12 months | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.81] |
| 7.3 24 months | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.97] |
| 8 Pathological root resorption | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 6 months | 4 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.01, 0.53] |
| 8.2 12 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.97] |
| 8.3 24 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.12, 2.51] |
| 9 Pulp canal obliteration | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 6 months | 3 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 12 months | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.30] |
| 9.3 24 months | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.47, 5.27] |
Comparison 5. Mineral trioxide aggregate (MTA) pulpotomy versus calcium hydroxide pulpotomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 months | 4 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.85] |
| 1.2 12 months | 4 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.04, 0.70] |
| 1.3 24 months | 5 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.12, 0.52] |
| 2 Radiological failure | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 6 months | 4 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.02, 0.41] |
| 2.2 12 months | 4 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.04, 0.36] |
| 2.3 24 months | 5 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.08, 0.26] |
| 3 Overall failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 6 months | 2 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.92] |
| 3.2 12 months | 2 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.10, 1.19] |
| 3.3 24 months | 2 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.18, 0.95] |
| 4 Pain | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 24 months | 2 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.09, 1.73] |
| 5 Soft tissue pathology | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 6 months | 3 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.02, 1.62] |
| 5.2 12 months | 3 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.02, 0.62] |
| 5.3 24 months | 4 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.06, 0.47] |
| 6 Pathological mobility | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 6 months | 3 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.02, 1.62] |
| 6.2 12 months | 3 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.66] |
| 6.3 24 months | 4 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 0.66] |
| 7 Pathological radiolucency | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 6 months | 4 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.02, 0.50] |
| 7.2 12 months | 4 | 162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.04, 0.47] |
| 7.3 24 months | 5 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.03, 0.22] |
| 8 Pathological root resorption | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 6 months | 5 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.02, 0.39] |
| 8.2 12 months | 5 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.02, 0.29] |
| 8.3 24 months | 6 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.03, 0.18] |
| 9 Pulp canal obliteration | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 6 months | 3 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.77 [1.56, 38.69] |
| 9.2 12 months | 3 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.01 [0.97, 4.17] |
| 9.3 24 months | 4 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [1.01, 4.19] |
| 10 Dentin bridge formation | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 6 months | 2 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.05, 0.84] |
| 10.2 12 months | 2 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.37, 1.74] |
| 10.3 24 months | 2 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.37, 1.74] |
Comparison 6. Mineral trioxide aggregate (MTA) pulpotomy versus Portland cement pulpotomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.02] |
| 1.2 12 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.02] |
| 1.3 24 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.02] |
| 2 Radiological failure | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 6 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 12 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 24 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.10, 2.56] |
| 3 Pain | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 6 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.91] |
| 3.2 12 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.91] |
| 3.3 24 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.91] |
| 4 Soft tissue pathology | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 6 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.02] |
| 4.2 12 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.02] |
| 4.3 24 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.02] |
| 5 Pathologic mobility | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 6 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.91] |
| 5.2 12 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.91] |
| 5.3 24 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.91] |
| 6 Pathological radiolucency | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 6 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 12 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 24 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.75] |
| 7 Pathological root resorption | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 6 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 12 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 24 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.91] |
| 8 Pulp canal obliteration | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 6 months | 2 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.49, 1.08] |
| 8.2 12 months | 2 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.60, 1.14] |
| 8.3 24 months | 2 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.71, 1.29] |
| 9 Dentin bridge formation | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 6 months | 2 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.13, 2.43] |
| 9.2 12 months | 2 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.61, 3.71] |
| 9.3 24 months | 2 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.61, 3.71] |
Comparison 7. Biodentine pulpotomy versus Mineral trioxide aggregate (MTA) pulpotomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 months | 4 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.42, 6.99] |
| 1.2 12 months | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.16, 3.62] |
| 2 Radiological failure | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 6 months | 4 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [0.65, 8.84] |
| 2.2 12 months | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.22, 5.27] |
| 3 Pain | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 6 months | 3 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.26, 98.00] |
| 4 Soft tissue pathology | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 6 months | 3 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.26, 98.00] |
| 5 Pathologic mobility | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 6 months | 3 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.26, 98.00] |
| 6 Pathological radiolucency | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 6 months | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.46 [0.15, 81.36] |
| 6.2 12 months | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.19, 6.27] |
| 7 Pathological root resorption | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 6 months | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [0.22, 24.09] |
| 7.2 12 months | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.30, 4.19] |
Comparison 8. Calcium hydroxide pulpotomy versus formocresol pulpotomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 months | 6 | 332 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [1.17, 3.37] |
| 1.2 12 months | 6 | 332 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.22, 2.89] |
| 1.3 24 months | 3 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [0.78, 6.11] |
| 2 Radiological failure | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 6 months | 4 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.48 [3.86, 62.06] |
| 2.2 12 months | 6 | 332 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.42, 2.44] |
| 2.3 24 months | 3 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.63 [1.73, 7.61] |
| 3 Overall failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 12 months | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.41 [0.80, 7.21] |
| 3.2 24 months | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.93 [1.35, 6.34] |
| 4 Pain | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 6 months | 4 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.18 [0.35, 29.08] |
| 4.2 12 months | 4 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.30 [1.15, 34.40] |
| 5 Soft tissue pathology | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 6 months | 5 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.14 [0.63, 42.25] |
| 5.2 12 months | 5 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.77 [1.23, 37.10] |
| 5.3 24 months | 2 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.64 [0.51, 13.55] |
| 6 Pathological mobility | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 6 months | 4 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.18, 8.19] |
| 6.2 12 months | 4 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.40, 3.31] |
| 6.3 24 months | 2 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.53, 153.79] |
| 7 Pathological radiolucency | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 6 months | 3 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.78 [0.64, 22.17] |
| 7.2 12 months | 5 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.67, 5.40] |
| 7.3 24 months | 2 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.24 [0.79, 13.28] |
| 8 Pathological root resorption | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 6 months | 4 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.87 [2.33, 60.40] |
| 8.2 12 months | 6 | 332 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.25 [2.04, 19.14] |
| 8.3 24 months | 3 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.59 [1.33, 15.81] |
| 9 Pulp canal obliteration | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 6 months | 2 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.47, 33.75] |
| 9.2 12 months | 3 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.68 [0.91, 7.95] |
| 10 Dentin bridge formation | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 6 months | 2 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.0 [1.81, 93.60] |
| 10.2 12 months | 2 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 14.0 [1.95, 100.26] |
Comparison 9. Calcium hydroxide pulpotomy versus ferric sulphate pulpotomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 months | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.4 [0.14, 81.38] |
| 1.2 12 months | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.41 [0.37, 31.61] |
| 1.3 24 months | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.44 [0.90, 13.18] |
| 2 Radiological failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 12 months | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.53, 3.13] |
| 2.2 24 months | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [1.04, 3.75] |
| 3 Overall failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 12 months | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.53, 3.13] |
| 3.2 24 months | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [1.04, 3.75] |
| 4 Pathological root resorption | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 12 months | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.05, 6.05] |
| 4.2 24 months | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.29 [0.60, 8.66] |
Comparison 10. Ferric sulphate pulpotomy versus formocresol pulpotomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 months | 7 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.87] |
| 1.2 12 months | 7 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.45, 4.27] |
| 1.3 24 months | 5 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.40, 1.70] |
| 2 Radiological failure | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 6 months | 6 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.32, 1.92] |
| 2.2 12 months | 7 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.73, 2.42] |
| 2.3 24 months | 5 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.71, 2.24] |
| 3 Overall failure | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 6 months | 4 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.12, 2.37] |
| 3.2 12 months | 5 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.51, 2.64] |
| 3.3 24 months | 4 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.74, 3.01] |
| 4 Pain | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 6 months | 4 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.58] |
| 4.2 12 months | 4 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.58] |
| 4.3 24 months | 4 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.85] |
| 5 Pathological radiolucency | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 12 months | 4 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.8 [0.40, 8.17] |
| 5.2 24 months | 4 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.2 [0.51, 9.50] |
| 6 Pathological root resorption | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 6 months | 5 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.84] |
| 6.2 12 months | 6 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.53, 5.08] |
| 6.3 24 months | 5 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.50, 2.96] |
| 7 Pulp canal obliteration | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 6 months | 3 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.2 12 months | 3 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.54, 1.64] |
| 7.3 24 months | 2 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.28, 5.54] |
Comparison 11. Sodium hypochlorite (NaOCl) pulpotomy versus ferric sulfate (FS) pulpotomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 months | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 12 months | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.39 [0.22, 87.82] |
| 2 Radiological failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 6 months | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.22, 1.39] |
| 2.2 12 months | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.02] |
| 3 Pain | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 6 months | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 12 months | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Soft tissue pathology | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 6 months | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 12 months | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.91] |
| 5 Adjacent tissue inflammation | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 6 months | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 12 months | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.03, 2.91] |
| 6 Pathologic mobility | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 6 months | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 12 months | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Pathologic radiolucency | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 6 months | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.06, 13.35] |
| 7.2 12 months | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.07, 4.17] |
| 8 Pathologic root resorption | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 6 months | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.18, 1.42] |
| 8.2 12 months | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.15, 1.01] |
Comparison 12. Diode laser pulpotomy versus ferric sulfate (FS) pulpotomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.04, 1.30] |
| 1.2 12 months | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.02, 1.62] |
| 2 Radiological failure | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 6 months | 3 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.38, 2.12] |
| 2.2 12 months | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.44, 1.92] |
| 3 Pain | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 6 months | 2 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.03, 1.60] |
| 4 Pathological radiolucency | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 6 months | 2 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.08] |
| 5 Pathological root resorption | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 6 months | 2 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.29, 7.73] |
Comparison 13. Electrosurgery pulpotomy versus ferric sulfate (FS) pulpotomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 months | 2 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.13, 2.34] |
| 2 Radiological failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 6 months | 2 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.38, 4.12] |
| 3 Pain | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 6 months | 2 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.13, 2.34] |
| 4 Pathological mobility | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 6 months | 2 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Pathological root resorption | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 6 months | 2 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.2 [0.54, 8.88] |
| 6 Pulp canal obliteration | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 6 months | 2 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 14. Ankaferd Blood Stopper (ABS) versus Ferric Sulfate (FS) pulpotomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 months | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 12 months | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.37, 4.27] |
| 2 Radiological failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 6 months | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.16, 6.20] |
| 2.2 12 months | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.34, 2.23] |
| 3 Pain | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 6 months | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 12 months | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.71] |
| 4 Soft tissue pathology | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 6 months | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 12 months | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.27, 8.43] |
| 5 Pathologic mobility | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 6 months | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 12 months | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Pathologic radiolucency | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 6 months | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 12 months | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.75] |
| 7 Pathologic root resorption | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 6 months | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.26, 96.13] |
| 7.2 12 months | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.31, 3.23] |
Comparison 15. Diode laser pulpotomy versus electrosurgery pulpotomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 months | 2 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.70] |
| 2 Radiological failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 6 months | 2 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.19, 2.18] |
| 3 Pain | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 6 months | 2 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.70] |
| 4 Pathological mobility | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 6 months | 2 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Pathological radiolucency | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 6 months | 2 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Pathological root resorption | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 6 months | 2 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.19, 2.18] |
| 7 Pulp canal obliteration | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 6 months | 2 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 16. Sodium hypochlorite (NaOCl) pulpotomy versus 1:5 diluted formocresol pulpotomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 months | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 12 months | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Radiological failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 6 months | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.33, 5.08] |
| 2.2 12 months | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.52, 6.59] |
| 3 Pain | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 6 months | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 12 months | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Soft tissue pathology | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 6 months | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 12 months | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Pathologic mobility | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 6 months | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 12 months | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Pathologic radiolucency | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 6 months | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 12 months | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Pathologic root resorption | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 6 months | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.33, 5.08] |
| 7.2 12 months | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.52, 6.59] |
Comparison 17. Enamel matrix derivative (EMD) pulpotomy versus formocresol pulpotomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 months | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.83] |
| 2 Pain | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 6 months | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.08, 1.92] |
| 3 Soft tissue pathology | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 6 months | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.06] |
| 4 Pathologic mobility | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 6 months | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.26, 96.13] |
Comparison 18. Calcium hydroxide pulpectomy versus zinc oxide and eugenol (ZOE) pulpectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Radiological failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 months | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.50, 12.50] |
| 2 Pain | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 6 months | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.90] |
| 3 Pathological mobility | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 6 months | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.90] |
| 4 Pathological radiolucency | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 6 months | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.26, 8.72] |
| 5 Pathological radiolucency | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 6 months | 2 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.08] |
18.5. Analysis.
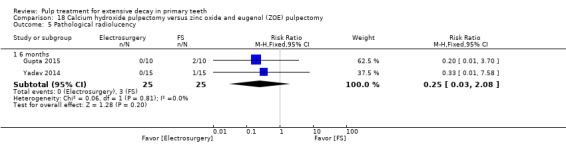
Comparison 18 Calcium hydroxide pulpectomy versus zinc oxide and eugenol (ZOE) pulpectomy, Outcome 5 Pathological radiolucency.
Comparison 19. Metapex versus zinc oxide eugenol (ZOE) pulpectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 months | 2 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.08, 4.29] |
| 1.2 12 months | 2 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.15, 3.33] |
| 2 Radiological failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 6 months | 2 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.31, 3.27] |
| 2.2 12 months | 2 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.31, 3.27] |
Comparison 20. Metapex pulpectomy versus Endoflas pulpectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clincal failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 months | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.58] |
| 2 Radiological failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 6 months | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.79, 5.15] |
| 3 Pain | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 6 months | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.58] |
| 4 Soft tissue pathology | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 6 months | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.58] |
| 5 Pathologic mobility | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 6 months | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Pathological radiolucency | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 6 months | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.79, 5.15] |
| 7 Pathological root resorption | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 6 months | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 21. Vitapex pulpectomy versus zinc oxide and eugenol (ZOE) pulpectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 months | 4 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.84] |
| 1.2 12 months | 4 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.75 [1.21, 18.55] |
| 2 Radiological failure | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 6 months | 4 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [0.86, 6.50] |
| 2.2 12 months | 4 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.56 [2.58, 16.67] |
| 3 Overall failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 6 months | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.63, 5.66] |
| 3.2 12 months | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.56 [0.89, 7.32] |
| 4 Pain | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 6 months | 3 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 12 months | 3 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Pathological mobility | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 6 months | 3 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.84] |
| 5.2 12 months | 3 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.18] |
Comparison 22. Endoflas pulpectomy versus zinc oxide eugenol (ZOE) pulpectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 6 months | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.05, 1.50] |
| 2 Radiological failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 6 months | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.05, 1.50] |
| 3 Pain | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 6 months | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.05, 1.50] |
| 4 Pathologic mobility | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 6 months | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.25] |
| 5 Pathologic radiolucency | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 6 months | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.11, 3.63] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aeinehchi 2007.
| Methods | RCT, parallel‐arm Children randomly assigned Conducted in the dental clinic of Azad University, Tehran, Iran. Operators were a dentist under the supervision of an endodontist |
|
| Participants | 126 children, 126 teeth, mean age 6.5 years, standard deviation age 1.16 years, age range 5 to 9 years | |
| Interventions |
Group 1: Pulpotomy (formocresol); n = 75 (1 visit) Rubber dam Caries removal prior to pulpal access: not mentioned Pulp access with high‐speed burr Pulpotomy amputation with excavator For haemostasis, moistened cotton pellet with saline Irrigation with saline Cotton wool pellet soaked with FC placed on pulp stumps for 5 minutes after pulpotomy, followed by zonalin dressings before being restored with amalgam or glass‐ionomer cement Group 2: Pulpotomy (MTA); n = 51 (1 visit) Rubber dam Caries removal prior to pulpal access: not mentioned Pulp access with high‐speed burr Pulpotomy amputation with excavator For haemostasis, moistened cotton pellet with saline Irrigation with saline MTA applied after pulpotomy, followed by amalgam |
|
| Outcomes | Clinical failure (spontaneous pain, swelling, pain on palpation or percussion and sinus tract formation, periodontal ligament widening, furcal radiolucency or apical radiolucency), pathological root resorption: evaluation at 3 and 6 months (at tooth level) Radiological failure (pathological root resorption, periodontal ligament widening and apical, lateral or furcal radiolucency): evaluation at 3 months (at tooth level) |
|
| Notes | Reasons for dropouts: quote: "18 in the FC group and 8 in the MTA group did not attend the 3‐month follow‐up" Comment: 55 participants excluded: not meeting inclusion (39 children), refused to participate (12 children), other reasons (4 children) Source of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number producing system |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% of children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Agamy 2004.
| Methods | RCT, split‐mouth Teeth randomly assigned Conducted in the Paediatric Dentistry Department, Alexandria University, Alexandria, Egypt. Operators not mentioned |
|
| Participants | 24 children, 72 teeth, mean age 6.1 years, age range 4‐8 years | |
| Interventions |
Group 1: Pulpotomy (grey MTA); n = 24 (1 visit) Rubber dam Caries removal prior to pulpal access not mentioned Pulp access with high‐speed bur Pulpotomy amputation with excavator For haemostasis, moistened cotton pellet with water No irrigation Grey MTA (3:1 powder:saline ratio) applied after pulpotomy, followed by IRM dressings before being restored with stainless‐steel crowns Group 2: Pulpotomy (white MTA); n = 24 (1 visit) Rubber dam Caries removal prior to pulpal access not mentioned Pulp access with high‐speed bur Pulpotomy amputation with excavator For haemostasis, moistened cotton pellet with water No irrigation White MTA (3:1 powder:saline ratio) applied after pulpotomy, followed by IRM dressings before being restored with stainless‐steel crowns Group 3: Pulpotomy (formocresol); n = 24 (1 visit) Rubber dam Caries removal prior to pulpal access not mentioned Pulp access with high‐speed bur Pulpotomy amputation with excavator For haemostasis, moistened cotton pellet with water No irrigation Cotton wool pellet soaked with FC placed on pulp stumps for 5 minutes after pulpotomy, followed by ZOE and IRM dressings before being restored with stainless‐steel crowns |
|
| Outcomes | Clinical success (no pain symptoms, or no tenderness to percussion, or no swelling, or no fistulation, or no pathological mobility), radiographic success (no radicular radiolucency, or internal or external resorption, or periodontal ligament space widening), radicular radiolucency, pulp canal obliteration: evaluation at 1, 3, 6 and 12 months (at tooth level) | |
| Notes | Reasons of dropouts: quote: "Four children with 12 pulpotimized molars, failed to return for evaluations and were excluded from the study" Comment: 17% of participants (8% of teeth) dropped out of the study. The reasons for failure to return were not reported Source of funding: quote "This study was supported by the Zawawi Pediatric Dentistry Fund of the Indiana University Foundation. [...] The authors also wish to thank Dentsply Tulsa Dental for donating the MTA materials used in this study" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "Two examiners, who were blinded to the treatment type, evaluated the teeth clinically" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "Two examiners, who were blinded to the treatment type, evaluated the teeth [...] and radiographically" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% of children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Akcay 2014.
| Methods | RCT, split‐mouth Teeth randomly assigned Conducted in the Pediatric Dentistry Department, Faculty of Dentistry, Ankara University. Operators not mentioned |
|
| Participants | 64 children, 128 teeth, mean age 8.2 years, age range 6‐10 years | |
| Interventions |
Group 1: Pulpotomy (CH NaCOl); n = 31 (1 visit) Rubber dam Caries removal prior to pulpal access not mentioned Pulp access not mentioned Pulpotomy amputation with excavator For haemostasis, dry cotton pellet Irrigation with 5% NaOCl for 30 seconds, then water CH, followed by IRM then stainless steel crown Group 2: Pulpotomy (CH); n = 31 (1 visit) Rubber dam Caries removal prior to pulpal access not mentioned Pulp access not mentioned Pulpotomy amputation with excavator For haemostasis, dry cotton pellet Irrigation with saline for 30 seconds then water CH, followed by IRM then stainless steel crown Group 3: Pulpotomy (MTA NaOCl); n = 31 (2 visits) Rubber dam Caries removal prior to pulpal access not mentioned Pulp access not mentioned Pulpotomy amputation with excavator For haemostasis, dry cotton pellet Irrigation with 5% NaOCl for 30 seconds then water MTA, followed by a moistened cotton pellet, followed by IRM. Second visit: IRM and the cotton pellets were removed after 24 hours, then stainless steel crown Group 4: Pulpotomy (MTA); n = 31 (2 visits) Rubber dam Caries removal prior to pulpal access not mentioned Pulp access not mentioned Pulpotomy amputation with excavator For haemostasis, dry cotton pellet Irrigation with saline for 30 seconds then water MTA, followed by a moistened cotton pellet, followed by IRM. Second visit: IRM and the cotton pellets were removed after 24 hours, then stainless steel crown |
|
| Outcomes | clinical success (absence of spontaneous pain, pathologic mobility, tenderness to percussion, swelling, fistula, or gingival inflammation), radiographic success (absence of internal/external root resorption and periapical/furcal radiolucency), calcific metamorphosis of the pulp: evaluation at 3, 6, 9 and 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | a toss of a coin |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | quote: "One examiner, who was blinded to treatment type, evaluated the teeth clinically " |
| Blinding of radiological outcomes assessment | Low risk | quote: "One examiner, who was blinded to treatment type, evaluated the teeth [...] radiographically" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | missing data balanced in numbers across intervention groups, with similar reasons for missing data across groups (2 in each group because of uncontrolled bleeding) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Al‐Ostwani 2016.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Department of Pedodontics and Preventive Dentistry at School of Dentistry, Damascus, Syria. Operators not mentioned |
|
| Participants | 39 children, 64 teeth, mean age 8.2 years, age range 3 to 9 years | |
| Interventions |
Group 1:Pulpectomy (zinc oxide and propolis); n = 16 (1 visit)
Group 2:Pulpectomy (endoflas‐chlorophenol‐free); n = 16 (1 visit)
Group 3:Pulpectomy (Metapex); n = 16 (2 visits)
Group 4:Pulpectomy (ZOE); n = 16 (2 visits)
|
|
| Outcomes | Clinical success (no abnormal mobility, pain, or sensitivity to percussion), radiographic success (decrease in the size of radiolucency and the presence of bone regeneration), at 6 and 12 months. Treatment failure was classified into two degrees as (a) the radiolucency slightly increased in size, but it was separated from succeeding bud with adequate bone and (b) the radiolucency threatening the succeeding buds, so the tooth was extracted. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "The treated molars were evaluated double‐blindly by three observers" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "The treated molars were evaluated double‐blindly by three observers" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Alaçam 1989.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in Turkey. Setting and operators not mentioned |
|
| Participants | 42 children, 69 teeth, age range 7 to 11 years | |
| Interventions |
Group 1:Pulpotomy (glutaraldehyde + ZOE); n = 25 (1 visit)
Group 2:Pulpotomy (glutaraldehyde + calcium hydroxide); n = 21 (1 visit)
Group 3:Pulpotomy (formocresol + ZOE); n = 23 (1 visit)
|
|
| Outcomes | Clinical success (pain symptoms, thermal sensitivity, tenderness to percussion, changes in the mucous membrane in the surrounding area, sensitivity to sour, sensitivity to sweet), radiological success (internal root resorption, changes in the integrity of lamina dura, abnormalities in the structure of trabecular bone): evaluation at 3 months (at tooth level) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Alaçam 2009.
| Methods | RCT, parallel‐arm Children randomly assigned Conducted in the University of Gazi Department of Pediatric Dentistry, Turkey. Operators were undergraduate dental students supervised by members of senior staff clinics |
|
| Participants | 105 children, 105 teeth, mean age 6.4 years, age range 4 to 8 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 35 (1 visit)
Group 2:Pulpotomy (calcium hydroxide); n = 35 (1 visit)
Group 3:Pulpotomy (calcium hydroxide/iodoform)n = 35 (1 visit)
|
|
| Outcomes | Clinical success (teeth remained asymptomatic, no tenderness to percussion, no sinus tract or no premature tooth loss), radiological success (no furcal or periapical radiolucencies or internal or external root resorption), tenderness to percussion, swelling, spontaneous pain, fistula, internal root resorption, external root resorption, periapical radiolucency, furcal radiolucency, widened periodontal ligament: evaluation at 1, 3, 6, 9 and 12 months (at tooth level) | |
| Notes | Quote: "9 children, with 9 pulpotomized molars, failed to return for evaluations and were excluded from the study" "5 bleeding cases were excluded from analysis" Group 1 ‐ received intervention, n = 35; no exclusions Group 2 ‐ received intervention, n = 33; excluded due to uncontrolled bleeding from paste placement n = 2 Group 3 ‐ received intervention, n = 32; excluded due to uncontrolled bleeding from paste placement n = 3 Source of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Low risk | Quote: "Radiographic outcome assessments were made by the primary investigator and 1 independent experienced clinician who was blind to the treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of missing outcomes < 10% of children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Aminabadi 2016.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Department of Pediatric Dentistry, Tabriz University of Medical Science, Iran. Operator was an expert paediatric dentist. |
|
| Participants | 83 children, 160 teeth, mean age 5.14 years, age range 3 to 6 years | |
| Interventions |
Group 1:direct pulp capping (3 Mix); n = 40 (1 visit)
Group 2:direct pulp capping (3 Mixtatin); n = 40 (1 visit)
Group 3:direct pulp capping (simvastatin); n = 40 (1 visit)
Group 4:direct pulp capping (White MTA); n = 40 (1 visit)
|
|
| Outcomes | Failure of treatment: pain, tenderness to palpation and percussion, sinus tract, and swelling; presence of internal or external root resorption, inter‐radicular radiolucency, and periapical lesion: evaluation at 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer random number generator |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The operator was not blinded to the treatment because of different manipulation techniques implemented for the study groups" |
| Blinding of clinical outcomes assessment | Low risk | Quote: "clinical and radiographic examinations were conducted at each appointment by two experienced paediatric dentists that were blinded to the techniques applied to each group" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "clinical and radiographic examinations were conducted at each appointment by two experienced paediatric dentists that were blinded to the techniques applied to each group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Aminabadi 2010.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Department of Pediatric Dentistry at Tabriz University of Medical Sciences School of Dentistry, Iran. Operators not mentioned |
|
| Participants | 84 children, 120 teeth, mean age 4.4 years, age range 4 to 5 years | |
| Interventions |
Group 1:Direct pulp capping (formocresol + ZOE); n = 60 (1 visit)
Group 2:Direct pulp capping (calcium hydroxide + ZOE); n = 60 (1 visit)
|
|
| Outcomes | Clinical success (spontaneous pain, or pain initiated by stimuli; signs of a defective restoration or recurrent caries; signs of mobility, sinus formation, tenderness to percussion, or soft tissue swelling; and signs of exfoliation, mobility or signs/symptoms of the successor tooth erupting), radiological success (defective restoration or recurrent caries; periradicular pathology such as periapical or furcal radiolucency; and pathological internal resorption, replacement resorption, intracanal calcifications, or physiological root resorption): evaluation at 24 months (at tooth level) Spontaneous pain, tenderness to percussion, fistula or parulis, periapical radiolucency or furcal radiolucency, internal resorption or external resorption: evaluation at 6, 12, 18 and 24 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin toss |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "objectivity was maximized by not having direct access during [...] clinical evaluation to records detailing which pulp therapy agent was used" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "objectivity was maximized by not having direct access during [...] radiological evaluation to records detailing which pulp therapy agent was used" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Ansari 2010.
| Methods | RCT, split‐mouth Teeth randomly assigned Conducted in the Paedodontic Department at Shahid Beheshti University, Dental School, Iran. Operator was an investigator |
|
| Participants | 17 children, 40 teeth, age range 4 to 9 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 20 (1 visit)
Group 2:Pulpotomy (MTA); n = 20 (2 visits)
|
|
| Outcomes | Signs of failure (internal resorption, radiographic signs of pathosis (periapical radiolucency), report of pain, presence of gingival swelling and sinus tract): evaluation at 24 months (at tooth level) Fistula, furcal radiolucency, periapical radiolucency, internal resorption, external resorption, periodontal ligament widening, pulp canal obliteration: evaluation at 6, 12 and 24 months (at tooth level) |
|
| Notes | Source of funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% of children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Arikan 2016.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in Turkey. Operator was a paediatric dentist |
|
| Participants | 50 children, 50 teeth, age range 4 to 9 years | |
| Interventions |
Group 1:Pulpectomy (IRM); n = 25 (3 visits)
Group 2:Pulpectomy (MTA); n = 25 (3 visits)
|
|
| Outcomes | Clinical failure (pain, pathological mobility, tenderness to percussion and palpation, and any soft tissue pathology and sinus tract) and radiographical failure (pathological root resorption, reduced size or healing of existing lesion, and absence of new lesions at the interradicular or periapical area): evaluation at 3, 6, 12 and 18 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "Examiners were blinded to the groups" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "Examiners were blinded to the groups" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Bahrololoomi 2008.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Pedodontics Department of Yazd Faculty of Dentistry, Iran. Operators were the principal investigator or co investigators |
|
| Participants | 46 children, 70 teeth, mean age 6.1 years, standard deviation age 1.4 years, age range 4 to 10 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 35 (1 visit)
Group 2:Pulpotomy (electrosurgery); n = 35 (2 visits)
In the experimental electrosurgical group, a series of large, sterile cotton pellets were placed in the chamber with pressure to obtain temporary haemostasis. The cotton pellets were then removed and the electrosurgery dental U‐shaped electrode (Whaledent perfect TCS, Colten Whaledent Inc., USA) was immediately placed 1 to 2 mm above the tissue. The electrosurgery unit power was set at 40%. The electrical arc was allowed to bridge the gap to the first pulpal stump for 1 second followed by a cool‐down period of 10 to 15 seconds. Heat was minimised by keeping the electrode as far away from the pulpal stumps and the tooth structure as possible while still allowing electrical arcing to occur. This procedure was repeated up to 3 times at each pulpal orifice. To avoid heat build‐up in any 1 area of the tooth, single applications of 1 second were performed to each orifice in a rotational sequence. After each current application, a new large sterile cotton pellet was placed with pressure on the next pulpal orifice to be electrosurgically treated to absorb any blood or tissue fluid before the next current application (i.e. pellet‐electrode‐pellet‐electrode). Pulpal stumps were dry and blackened, followed by ZOE dressings before being restored with amalgam |
|
| Outcomes | Clinical success (absence of pain, abscess, fistula or excessive mobility), radiological success (presence of a normal periodontal ligament space, absence of pathological root resorption or canal calcification, and no periradicular or furcal radiolucency): evaluation at 9 months (at tooth level) Pain symptoms, fistula, pathological mobility, abscess, furcal radiolucency, internal resorption, external resorption: evaluation at 3, 6 and 9 months |
|
| Notes | Source of funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "...examiner who was ...blind to the treatment" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "...examiner who was ...blind to the treatment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Bezgin 2016.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in Turkey. Operator was a paediatric dentist |
|
| Participants | 16 children, 20 teeth, age range 6 to 13 years, mean age 10.5 years | |
| Interventions |
Group 1:Pulpectomy (gutta‐percha/AH‐Plus); n = 10 (1 visit)
Group 2:Pulpectomy (MTA); n = 10 (2 visits)
|
|
| Outcomes | Clinical success (no symptoms of pain, tenderness to percussion, swelling, and presence of a fistula or pathological mobility), radiographic success (no evidence of periradicular or interradicular radiolucency or internal or external root resorption): evaluation at 6, 12, 18, 24 and 36 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | High risk | Quote: "examiners could not be blinded to the type of the root canal filling" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Cantekin 2014.
| Methods | RCT, split‐mouth Teeth randomly assigned Conducted in the clinic of the Department of Pediatric Dentistry, Erciyes University, Kayseri, Turkey. Operators were not mentioned |
|
| Participants | 35 children, 70 teeth, age range 4 to 6 years | |
| Interventions |
Group 1:Pulpotomy (Ankaferd Blood Stopper); n = 35 (1 or 2 visits)
Group 2:Pulpotomy (FS); n = 35 (1 or 2 visits)
|
|
| Outcomes | Clinical failure (pain, tenderness to percussion, gingival abscess, sinus/fistula, and pathological mobility), radiographic success (absence of abnormal root resorption, internal root resorption, furcation involvement, and periapical bone destruction), Calcification in pulpal tissue and pulp canal obliteration: evaluation at 3, 6, 9 and 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "All pre‐ and postoperative clinical and digital radiographic examinations were performed at followup by one experienced investigator who was blind to the group being studied" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "All pre‐ and postoperative clinical and digital radiographic examinations were performed at followup by one experienced investigator who was blind to the group being studied" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of missing outcomes < 10% of children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Casas 2004.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Hospital for Sick Children, Toronto, Canada. Operators were 3 paediatric dentists |
|
| Participants | 130 children, 291 teeth, mean age 4.4 years, standard deviation age 1.3 years | |
| Interventions |
Group 1:Pulpotomy (ferric sulphate); n = 182 (1 visit)
Group 2:Pulpectomy (ZOE); n = 109 (1 visit)
|
|
| Outcomes | Radiological success (N ‐ normal molar without evidence of radiographic change or H ‐ radiographic changes associated with normal physiological molar resorption): evaluation at 36 months (at tooth level) Furcal radiolucency, periapical radiolucency, internal root resorption, external resorption, periodontal ligament widening, pulp canal obliteration, N (score 4‐rx), H (score 4‐rx), Po (score 4‐rx), Px (score 4‐rx), pain symptoms, tenderness to percussion, (swelling or parulis), (fistula or swelling): evaluation at 24 and 36 months |
|
| Notes | Source of funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% of children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Celik 2013.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the paediatric dental clinic at the School of Dentistry, Hacettepe University, Ankara, Turkey. Operator was a paediatric dentist. |
|
| Participants | 75 children, 139 teeth, 3 to 9 years | |
| Interventions |
Group 1:Pulpotomy (ProRoot MTA); n = 46 (1 visit)
Group 2:Pulpotomy (MTA Angelus); n = 45 (1 visit)
Group 3:Pulpotomy (CH); n = 48 (1 visit)
|
|
| Outcomes | Clinical success (absence of spontaneous pain and/ or sensitivity to palpation/percussion; absence of fistula, swelling, and/or abnormal mobility), radiological success (absence of radiolucencies at the inter‐radicular and/or periapical regions, absence of pulp canal obliteration (fully obliterated canals); and absence of internal or external (pathologic) resorption), defective restoration (clinically): evaluation at 1, 3, 6, 12, 18, 24 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "...sequentially numbered opaque‐sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Operator blinding was not possible" |
| Blinding of clinical outcomes assessment | Low risk | Quote: "Two calibrated operators, blinded to group assignment and treatment, performed ...clinical ...recall examinations" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "Two calibrated operators, blinded to group assignment and treatment, performed ...radiographic recall examinations" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of missing outcomes < 10% of children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Chandra 2014.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in India. Operator not mentioned. |
|
| Participants | 52 children, 60 teeth, 3.8 to 7.6 years | |
| Interventions |
Group 1:Pulpectomy (ozonated oil‐ZO); n = 30 (1 visit)
Group 2:Pulpectomy (ZOE); n = 30 (1 visit)
|
|
| Outcomes | Clinical success (absence of pain, tenderness to percussion, absence or decrease in mobility and sinus opening), radiographic success (signs of resolution in the radiolucency, no new signs of post‐operative radiolucency and no signs of internal or external pathological root resorption), radiographic failure (increase in postoperative inter‐radicular radiolucency or development of new postoperative radiolucency): evaluation at 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "The teeth were evaluated for success or failure based on clinical and radiographic criteria by a blinded investigator" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "The teeth were evaluated for success or failure based on clinical and radiographic criteria by a blinded investigator" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Chen 2015.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Department of Pediatric Dentistry, First Dental Center, Peking University School and Hospital of Stomatology, Beijing, China. Operator was one investigator |
|
| Participants | 155 children, 160 teeth, average age: 5.88 ± 1.27 years | |
| Interventions |
Group 1:Pulpectomy (ZOE); n = 51 (2 visits)
Group 2:Pulpectomy (Vitapex); n = 56 (2 visits)
Group 3:Pulpectomy (MPRCF); n = 53 (2 visits)
|
|
| Outcomes | Clinical and radiologic success: evaluation at 6 and 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "...the clinical ...diagnoses were blindly assessed by other two investigators" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "...the ...radiogaphic diagnoses were blindly assessed by other two investigators" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Coser 2008.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the University Center Heminio Ometto, School of Dentistry, Araras, Sao Paulo, Brazil. Operators not mentioned |
|
| Participants | 29 children, 51 teeth, age range 4.5 to 6.5 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 28 (4 visits)
Group 2:Pulpectomy (calcium hydroxide); n = 23 (4 visits)
|
|
| Outcomes | No data provided | |
| Notes | Dropouts: no information provided Follow‐up for 48 months; reporting at baseline, 12, 24, 26, 48 months Source of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Cuadros‐Fernández 2016.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Department of Paediatric Dentistry at the Universitat Internacional de Catalunya, Spain. Operator was a postgraduate student |
|
| Participants | 68 children, 90 teeth, age range 4 to 9 years, mean 6.6 ± 1.3 years | |
| Interventions |
Group 1:Pulpotomy (MTA); n = 45 (1 visit)
Group 2:Pulpotomy (Biodentine); n = 45 (1 visit)
|
|
| Outcomes | Clinical success (no symptoms of pain, and there was no swelling or gingival inflammation, fistulation, or pathologic mobility), radiologic success (no evidence of internal or external resorption or periradicular radiolucency): evaluation at 6 and 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Protocol prospectively registered (NCT01591278). no discrepancies in outcomes between registered record and published RCT. |
Dean 2002.
| Methods | RCT, parallel‐arm Children randomly assigned Conducted in the dental clinics of the Indiana University Institutional Review Board, USA. Operators were investigators: "...standardization of the investigators in the experimental technique was attempted by using a clinician with over 20 years of experience in performing the electrosurgical…" |
|
| Participants | 50 children, 50 teeth mean age 5.3 years, age range 2.2 to 8.1 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 25 (1 visit)
Group 2:Pulpotomy (electrosurgery); n = 25 (1 visit)
|
|
| Outcomes | Clinical success (no pain, no abscess, no fistula or no excessive mobility), radiological success (normal periodontal ligament space, no pathological root resorption, no canal calcification and no periradicular radiolucency): mean evaluation at 11.5 (range 5‐25) months for Group 1 and 10.9 (6‐31) for Group 2 (at tooth level) | |
| Notes | Source of funding: quote: "This study was supported by Birtcher Medical Services, Inc" Comment: Birtcher Medical Systems, Inc. is a USA manufacturer of medical and surgical instruments | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...the patients were assigned randomly by the flip of a coin" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Demir 2007.
| Methods | RCT, parallel‐arm Teeth randomly assigned Setting not mentioned. Conducted in Turkey. Operators were investigators |
|
| Participants | 67 children, 100 teeth, age range 5 to 9 years | |
| Interventions |
Group 1:Direct pulp capping (calcium hydroxide); n = 20 (1 visit)
Group 2:Direct pulp capping (acetone‐based total‐etch adhesive); n = 20 (1 visit)
Group 3:Direct pulp capping (acetone‐based total‐etch adhesive ‐ non rinse conditioner); n = 20 (1 visit)
Group 4:Direct pulp capping (acetone‐based total‐etch adhesive ‐ total etching); n = 20 (1 visit)
Group 5:Direct pulp capping (acetone‐based total‐etch adhesive ‐ self‐etch); n = 20 (1 visit)
|
|
| Outcomes | Clinical success (no spontaneous pain or sensitivity (or both) to pressure/percussion, no fistula, oedema, abnormal mobility, or a combination), radiological success (no radiolucency at the inter‐radicular or periapical regions (or both), no internal or external (pathological) resorption that was not compatible with the expected resorption due to the exfoliation process), inter‐radicular radiolucency or periapical radiolucency, internal root resorption or external root resorption, pain symptoms or spontaneous pain: evaluation at 1, 3, 6, 9, 12, 18 and 24 months (at tooth level) | |
| Notes | Reasons for dropouts: 9 exfoliations (7 at 18 months, 2 at 24 months); 2 extractions (12 and 18 months), 1 extraction (6 months), 1 extraction (12 months) Source of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "...two calibrated operators, blinded to the treatments, performed the clinical ...recall examinations" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "...two calibrated operators, blinded to the treatments, performed the ...radiological recall examinations" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of missing outcomes < 10% of children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Doyle 2010.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Hospital for Sick Children, Toronto, Canada. Operators were 3 paediatric dentists |
|
| Participants | 112 children, 266 teeth, mean age 4.0 years, standard deviation age 1.1 years | |
| Interventions |
Group 1:Pulpotomy (FS + eugenol); n = 58 (1 visit)
Group 2:Pulpotomy (FS); n = 78 (1 visit)
Group 3:Pulpotomy (MTA); n = 53 (1 visit)
Group 4:Pulpotomy (MTA + FS + eugenol); n = 77 (1 visit)
|
|
| Outcomes | Tenderness to percussion, pathological mobility, erythema, parulis, pathological radiolucency, internal root resorption, external root resorption, periodontal ligament widening, pulp canal obliteration, N (score 5‐rx), Po (score 5‐rx), Px (score 5‐rx): mean evaluation at 22 (range 6 to 38) months (at tooth level) Quote: "Subjects were invited to return for clinical and radiographic assessments at 12, 24, and 36 months after treatment" |
|
| Notes | Source of funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Low risk | Quote: "2 blinded, disinterested raters classified each molar into 1 of 3 radiographic outcomes" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% of children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Durmus 2014.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the University of Marmara, Department of Paediatric Dentistry, in Istanbul. Operator was one paediatric dentist. |
|
| Participants | 58 children, 120 teeth, age range 5 to 9 years | |
| Interventions |
Group 1:Pulpotomy (diode laser); n = 40 (1 visit)
Group 2:Pulpotomy (formocresol); n = 40 (1 visit)
Group 3:Pulpotomy (ferric sulphate); n = 40 (1 visit)
|
|
| Outcomes | Clinical failure (spontaneous pain, percussion/palpation, abscess, swelling, fistula, or pathologic mobility), radiological failure (periapical radiolucency, widened periodontal ligament space (PDL), pathologic internal/external root resorption, or pathological changes of the alveolar bone in the furcation): evaluation at 1, 3, 6, 9, and 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "The outcome assessment and data analysis were blinded " |
| Blinding of radiological outcomes assessment | Unclear risk | Quote: "The outcome assessment and data analysis were blinded " BUT "Two blinded observers evaluated a set of radiographs separately" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Eidelman 2001.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the undergraduate and graduate Pediatric Dentistry Clinics of the Hebrew University‐Hadassah School of Dental Medicine, Israel. Operators were authors |
|
| Participants | 26 children, 45 teeth; 32 teeth from 18 children analysed, mean age 6.4 years, age range 5 to 12 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 17 teeth (1 visit)
Group 2:Pulpotomy (MTA); n = 15 teeth (1 visit)
|
|
| Outcomes | Signs of failure (internal root resorption, furcation radiolucency, periapical bone destruction, pain, swelling, or sinus tract), internal root resorption, furcation radiolucency, periapical bone destruction, pain, swelling, or sinus tract: evaluation at 13 (6 to 30) months (at tooth level) | |
| Notes | Reasons of dropouts: quotes: "a total of 45 primary molars were pulpotomized in 26 children. Of these 32 teeth in 18 children were available for follow‐up evaluation"; "4 children with 8 teeth had less than 6 months postoperative period at the time of data analysis. 3 children with 5 teeth were not available for follow‐up examination since they moved to another city" Source of funding: not reported, although the MTA material was provided by a colleague at another university in the USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin toss |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement of Yes or No |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement of Yes or No |
| Blinding of clinical outcomes assessment | High risk | Quote: "the children were examined clinically at follow‐up by one of the 3 authors who were not blind to which treatment group the subject belong" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "all 3 authors blindly evaluated the radiographs" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% of children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
El Meligy 2016.
| Methods | RCT, split‐mouth Teeth randomly assigned Conducted at the Pediatric Dental Clinics, Faculty of Dentistry, King Abdulaziz University (KAU), Jeddah. Operators not mentioned |
|
| Participants | 37 children, 56 pairs, 112 teeth; mean age 6 ± 0.75 years, age range 4 to 8 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 56 teeth (1 visit)
Group 2:Pulpotomy (Biodentine); n = 56 teeth (1 visit)
|
|
| Outcomes | Clinical success (absence of sensitivity, pain, or swelling, no tenderness to percussion, no abscess or fistulation, no tooth mobility), radiographic success (absence of furcation and periapical radiolucency, absence of internal or external root resorption), presence of a normal periodontal ligament space, presence of pulp canal obliteration: evaluation at 3 and 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement of Yes or No |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement of Yes or No |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement of Yes or No |
| Blinding of clinical outcomes assessment | Low risk | Quote: "Independently, two examiners who were blinded to treatment type evaluated the teeth clinically and radiographically." |
| Blinding of radiological outcomes assessment | Low risk | Quote: "Independently, two examiners who were blinded to treatment type evaluated the teeth clinically and radiographically." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Erdem 2011.
| Methods | RCT, parallel arm Teeth randomly assigned Setting not mentioned. Conducted in Turkey. Operators were 3 paediatric dentists |
|
| Participants | 32 children, 100 teeth, mean age 6.2 years, standard deviation age 0.7 years. age range 5 to 7 years | |
| Interventions |
Group 1:Pulpotomy (MTA); n = 25 (1 visit)
Group 2:Pulpotomy (ferric sulphate); n = 25 (1 visit)
Group 3:Pulpotomy (formocresol); n = 25 (1 visit)
Group 4:n = 25 (1 visit)
|
|
| Outcomes | Clinical failure (pain, swelling, mobility, percussion pain), radiological failure (internal root resorption, and furcation or periapical bone destruction (or both)), signs of failure (pain, swelling, mobility, percussion pain, internal root resorption, and furcation or periapical bone destruction (or both)), internal root resorption, pulp canal obliteration, tenderness to percussion, inter‐radicular bone destruction, physiological root resorption: evaluation at 6, 12 and 24 months (at tooth level) | |
| Notes | Source of funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "the children were examined clinically by 3 experienced pediatric dentists (not the operators) blinded to the technique" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "the children were examined radiographically by 3 experienced pediatric dentists (not the operators) blinded to the technique" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Fallahinejad Ghajari 2013.
| Methods | RCT, split‐mouth Teeth randomly assigned Setting not mentioned. Conducted in Iran. One operator (no detail) |
|
| Participants | 21 children, 42 teeth, mean age 6.9 ± 0.7, age range 5 to 8 years | |
| Interventions |
Group 1: Direct pulp capping (MTA); n = 21 (1 visit)
Group 2: Direct pulp capping (CEM); n = 21 (1 visit)
|
|
| Outcomes | Clinical failure (pain, swelling, tenderness to pressure, sinus tract, swelling and tenderness to percussion), radiological failure (internal and/or external root resorption, interradicular radiolucencies, and periapical lesions): evaluation at 6 and 20 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The single operator and children were blind to biomaterial/treatment |
| Blinding of clinical outcomes assessment | Low risk | Quote: "Treatment outcomes ...were evaluated at 20 months by a calibrated dentist, radiologist and a statistician who were also blind to the type of used biomaterial" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "Treatment outcomes ...were evaluated at 20 months by a calibrated dentist, radiologist and a statistician who were also blind to the type of used biomaterial" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of missing outcomes < 10% of children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Farsi 2005.
| Methods | RCT, parallel‐arm Children randomly assigned Setting not mentioned. Operators were paediatric dentists. Conducted in Saudi Arabia |
|
| Participants | 100 children, 120 teeth, mean age 6 years, standard deviation age 1.6 years, age range 3 to 8 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 60 (1 visit)
Group 2:Pulpotomy (MTA); n = 60 (1 visit)
|
|
| Outcomes | Clinical success (no pain, sinus tract or swelling), radiographic success (no evidence of furcation radiolucency, internal root resorption, or periapical bone destruction), physiological root resorption, furcation radiolucency, periapical radiolucency, internal root resorption, pulp canal obliteration, pain symptoms, sinus tract, swelling: evaluation at 6, 12, 18 and 24 months (at tooth level) | |
| Notes | Dropouts: "Out of 120 teeth, only 74 were assessed clinically and radiographically throughout the follow up period" Comment: only the results for these 74 teeth were reported Source of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin toss |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% of children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Fei 1991.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Pediatric Dental Clinic at the University of Southern California School of Dentistry. Operators not mentioned |
|
| Participants | 62 children, 83 teeth, mean age 6.7 years, age range 3.2 to 10.1 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = not stated (27 teeth analysed) (1 visit)
Group 2:Pulpotomy (ferric sulphate); n = not stated (29 teeth analysed) (1 visit)
|
|
| Outcomes | Clinical success (no symptoms of pain, tenderness to percussion, swelling, fistulation or pathological tooth mobility), radiographic success (normal periodontal ligament, absence of pathological internal root resorption, external root resorption, no intraradicular or no periapical radiolucency), signs of failure (symptoms of pain, tenderness to percussion, swelling, fistulation, pathological tooth mobility, abnormal periodontal ligament, pathological internal root resorption, external root resorption, intraradicular or periapical radiolucency), internal root resorption, pulp canal obliteration: evaluation at 3, 6 and 12 months (at tooth level) | |
| Notes | 56 teeth from 48 children were available for evaluation after 12 months (Group 1: 27; Group 2: 29) Source of funding: not reported, although FS material was provided by a manufacturer Quote: "The ferric sulphate tested in this study was provided by the Ultradent Company" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "Clinical evaluations of the teeth were conducted by two independent examiners who had no knowledge of the group to which the particular tooth was assigned" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "Radiological evaluations of the teeth were conducted by two independent examiners who had no knowledge of the group to which the particular tooth was assigned" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of randomised teeth unknown |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Fernandes 2015.
| Methods | RCT, parallel‐arm Children randomly assigned Setting not mentioned. Operators not mentioned. Conducted in Brazil |
|
| Participants | number of children not mentioned, 60 teeth, mean age 6.5 years, age range 5 to 9 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 15 (1 visit)
Group 2:Pulpotomy (CH); n = 15 (1 visit)
Group 3:Pulpotomy (LLLT); n = 15 (1 visit)
Group 4:Pulpotomy (CH+ LLLT); n = 15 (1 visit)
|
|
| Outcomes | Clinical success (absence of spontaneous pain, mobility, swelling, or fistula), Radiographic success (presence of hard tissue barrier formation and pulp calcifications, and absence of internal or external root resorption and furcation radiolucency) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "At each checkup, two blinded and calibrated investigators performed clinical and periapical radiographic examination of the pulpotomized teeth" |
| Blinding of radiological outcomes assessment | Low risk | quote: "At each checkup, two blinded and calibrated investigators performed clinical and periapical radiographic examination of the pulpotomized teeth" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of missing outcomes < 10% of children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Fernández 2013.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Department of Paediatric Dentistry at the Universitat Internacional de Catalunya. Operators were student and dentists. |
|
| Participants | 81 children, 100 teeth, mean age 6.7 ± 1.6 years, age range 3.2 to 10.1 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 25 (1 visit)
Group 2:Pulpotomy (MTA); n = 25 (1 visit)
Group 3:Pulpotomy (ferric sulphate); n = 25 (1 visit)
Group 4:Pulpotomy (sodium hypochlorite); n = 25 (1 visit)
|
|
| Outcomes | Clinical success (no pain, swelling, fistulation, or pathologic mobility), radiographic success (no evidence of internal or external resorption, or periradicular radiolucency), overall success: evaluation at 6, 12, 18, and 24 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Low risk | Quote: "the radiographs were ...re‐evaluated independently by two blinded observers" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% of children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Fishman 1996.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in a hospital‐based (Long Beach Memorial Medical Center) dental clinic in California, USA (noted as predominantly children from low‐income families). Operators not mentioned |
|
| Participants | 38 children, 47 teeth, mean age 5 years, age range 3.1 to 8.1 years | |
| Interventions |
Group 1:Pulpotomy (ZOE); n = 24 (1 visit)
Group 2:Pulpotomy (calcium hydroxide); n = 23 (1 visit)
|
|
| Outcomes | Clinical success (no excessive tooth mobility, no subjective symptoms of pain, no tenderness to percussion, and no fistula), radiographic success (normal periodontal ligament and absence of furcation or periapical radiolucency, internal or external resorption and calcific degeneration in the remaining pulp tissue), signs of failure (excessive tooth mobility, subjective symptoms of pain, tenderness to percussion, fistula, abnormal periodontal ligament, furcation or periapical radiolucency, internal or external resorption, and calcific degeneration in the remaining pulp tissue), periapical radiolucency, internal root resorption, external root resorption, periodontal ligament widening, pulp canal obliteration (parulis, fistula or swelling): evaluation at 1, 3 and 6 months (at tooth level) | |
| Notes | 47 teeth for treatment; 43 teeth from 35 children were available for evaluation after 6 months 1 month: 11 teeth in CH group and 10 teeth in ZOE group unavailable for recall; 3 months: 9 teeth in CH group and 8 teeth in ZOE group unavailable for recall; 6 months: 3 teeth in CH group and 1 tooth in ZOE group unavailable for recall Source of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Low risk | Numerical code which was available only to the operator |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "Clinical evaluation was determined by 2 examiners who had no knowledge if the experimental group of the tooth" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "radiologic evaluation was determined by 2 examiners who had no knowledge if the experimental group of the tooth" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Fuks 1997.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the paediatric dentistry undergraduate student's clinic of the Hebrew University‐Hadassah School of Dental Medicine, Israel Operators were the Israeli authors of this study |
|
| Participants | 72 children, 96 teeth, mean age 7.5 years, age range 4.5 to 10 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 38 (1 visit)
Group 2:Pulpotomy (ferric sulphate); n = 58 (1 visit)
|
|
| Outcomes | Radiographic success (internal root resorption, furcation radiolucency or periapical bone destruction), furcal radiolucency, periapical radiolucency, internal root resorption, pulp canal obliteration, faster root resorption compared with contralateral, slower root resorption compared with contralateral, similar root resorption compared with contralateral: evaluation at 20.5: (6 to 11), (12 to 23) and (24 to 35) months (at tooth level) Signs of failure (internal root resorption, furcation radiolucency, periapical bone destruction, pain, swelling, or sinus tract): evaluation at 20.5 (24 to 35) months (at tooth level) |
|
| Notes | Source of funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin toss |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of missing outcomes < 10% of children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Garrocho‐Rangel 2009.
| Methods | RCT, split‐mouth Teeth randomly assigned Pediatric Dentistry Postgraduate Program, Faculty of Dentistry of San Luis Potosi University, Mexico. A single operator performed all procedures |
|
| Participants | 45 children, 90 teeth, median age (± standard deviation) boys: 6.4 ± 1.16 years, girls: 5.7 ± 1.01 years | |
| Interventions |
Group 1:Direct pulp capping (EMD); n = 45 (1 visit)
Group 2:Direct pulp capping (calcium hydroxide); n = 45 (1 visit)
|
|
| Outcomes | Signs of failure (internal dentin resorption, spontaneous pain, gingival abscess (sinus tract), external root resorption or pathological mobility), internal dentin resorption, spontaneous pain, abscess, pathological root resorption, swelling or pathological mobility; evaluation at 1, 6 and 12 months (at tooth level) | |
| Notes | Source of funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random using number sequences generated by R 2.4.0 software |
| Allocation concealment (selection bias) | Low risk | Quote: "The operator was blind to the random number schemes until just before placing the materials" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The participants…were blind regarding capping material group assignation" |
| Blinding of clinical outcomes assessment | Low risk | Quote: "Assessing observer and analyst were blind regarding capping material group assignation" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "Assessing observer and analyst were blind regarding capping material group assignation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Goyal 2014.
| Methods | RCT, parallel arm Teeth randomly assigned Setting not mentioned. Conducted in India. Operator not mentioned |
|
| Participants | 41 children, 45 teeth, age range 4 to 8 years | |
| Interventions |
Group 1:Pulpotomy (full strength formocresol); n = 15 (2 visits)
Group 2:Pulpotomy (1:5 diluted formocresol); n = 15 (2 visits)
Group 3:Pulpotomy (1:25 diluted formocresol); n = 15 (2 visits) No details 1:25 diluted FC: 18 mL glycerine and 6 mL distilled water are thoroughly mixed and to which 1 part of FC is added. A number 1 foam pellet saturated with 1:25 diluted FC then twice squeezed to remove excess 1:25 diluted FC and placed for 5 minutes. Then teeth restored with stainless‐steel crown (second visit) |
|
| Outcomes | Clinical failure (pain, intra‐oral/extra‐oral swelling, tenderness on percussion, sinus/fistula), radiological failure (furcation radiolucency, periapical changes, internal/external resorption): evaluation at 1, 3, 6 and 9 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "the subjects were unaware of their group" Insufficient information to make a clear judgement for blinding of personnel |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% of children randomly assigned at 9 months |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Goyal 2016.
| Methods | RCT, parallel arm Teeth randomly assigned DAV Dental College Yamuna Nagar, Haryana. A single operator (investigator) performed all procedures |
|
| Participants | 42 children, 90 teeth, 4 to 8 years | |
| Interventions |
Group 1:Pulpotomy (15.5% ferric sulphate); n = 30 (2 visits)
Group 2:Pulpotomy (2% buffered glutaraldehyde); n = 30 (2 visits)
Group 3:Pulpotomy (MTA); n = 30 (2 visits)
|
|
| Outcomes | Clinical parameters (pain, sinus formation, swelling (intra oral), and mobility), radiological parameters (PDL widening, internal resorption, external resorption, periapical radiolucency, canal obliteration, and furcation radiolucency): evaluation at 24 h, 1 month, 3 months and 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% of children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Grewal 2016.
| Methods | RCT, split‐mouth Children randomly assigned Conducted at the Outpatient Department of Paediatric Dentistry, Punjab Government Dental College and Hospital, Amritsar, Punjab, India. Operators not mentioned. |
|
| Participants | 20 children, 40 teeth, mean age 7.35 years, age range 5 to 10 years | |
| Interventions |
Group 1:Pulpotomy (Biodentine); n = 20 (2 visits)
Group 2:Pulpotomy (CH); n = 20 (2 visits)
|
|
| Outcomes | Pain, swelling: evaluation at 3, 6 and 12 months; mean dentin thickness, internal root resorption: evaluation at 6 and 12 months; colour matching, marginal discolouration, secondary caries, anatomic form, surface texture, marginal integrity, pulp sensitivity: evaluation at 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random‐number |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "Blinded clinical and radiographic outcomes were observed" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "Blinded clinical and radiographic outcomes were observed" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% of children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Gupta 2015.
| Methods | RCT, parallel arm Teeth randomly assigned Outpatient Department of Pediatric Dentistry of Subharti Dental College, Meerut. A single operator (investigator) performed all procedures |
|
| Participants | 30 children, 30 teeth, 4 to 10 years | |
| Interventions |
Group 1: Pulpotomy (ferric sulphate); n = 10 (1 visit)
Group 2: Pulpotomy (electrosurgery); n = 10 (1 visit)
Group 3: Pulpotomy (diode laser); n = 10 (1 visit)
|
|
| Outcomes | Clinical success, radiological success, pain, furcal and periapical radiolucency, internal root resorption: evaluation at 3, 6, 9 and 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Haghgoo 2009.
| Methods | RCT, parallel‐arm Teeth randomly assigned |
|
| Participants | 70 teeth, age range 4 to 7 years | |
| Interventions |
Group 1: Pulpotomy (formocresol); n = 35 (1 visit) Group 2: Pulpotomy (Iranian Root MTA); n = 35 (1 visit) |
|
| Outcomes | Clinical failure, radiological failure, pain, soft tissue pathology, pathological radiolucency, pathological root resorption: evaluation at 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | The clinical ...follow up evaluations were performed at 6, 12 months by a blinded dentist |
| Blinding of radiological outcomes assessment | Low risk | The ...radiographic follow up evaluations were performed at 6, 12 months by a blinded dentist |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Holan 2005.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Pediatric Dentistry Clinic of the Hebrew University‐Hadassah School of Dental Medicine in Jerusalem, Israel. Operators were the authors of this study |
|
| Participants | 35 children, 64 teeth, mean age 6.5 years, age range 4.4 to 11 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 31 (1 visit)
Group 2:Pulpotomy (MTA); n = 33 (1 visit)
|
|
| Outcomes | Signs of failure (furcation radiolucency, periapical bone destruction, internal root resorption, swelling or sinus tract), abscess, pulp canal obliteration, dentine bridge formation, furcal radiolucency, periapical radiolucency, internal root resorption, external root resorption, calcific metamorphosis (periapical radiolucency or inter‐radicular radiolucency): evaluation at 36 (range 4 to 74) months (at tooth level) | |
| Notes | Reasons of dropouts: "Of the 64 pulpotomized teeth, 62 teeth in 33 children were available for analysis of success/failure rate. 2 molars in 2 patients, both of the FC group, were excluded from the study because the patients never returned for follow‐up examination" Comment: quotes: "when a patient did not respond or broke an appointment, further attempts were made to call the parents and a follow‐up examination was rescheduled"; "the follow‐up period was defined as the time elapsed between treatment and one of the following: 1/detection of pulpotomy failure; 2/naturally exfoliated tooth; 3/patient's last visit for recall examination. Teeth with less than 12 months follow‐up time were excluded from the study, unless a failure was detected during the first postoperative year" Source of funding: not reported, although the MTA material was provided by a colleague at another university in the USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin toss |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | High risk | Quote: "the children were then examined clinically by 1 of the 3 authors who were not blind to which treatment group the assessed tooth belonged" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "All 3 authors blindly evaluated the radiographs" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of missing outcomes < 10% of children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Huth 2005.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Pedodontic Section, Department of Restorative Dentistry and Periodontology, Ludwig‐Maximilians‐University, Munich, Germany. Operators were 2 paedodontists |
|
| Participants | 107 children, 191 teeth, mean age 4.8 years, standard deviation age 1.6 years, age range 2 to 8 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 50 (1 visit)
Group 2:Pulpotomy (Er:YAG); n = 47 (1 visit)
Group 3:Pulpotomy (calcium hydroxide); n = 44 (1 visit)
Group 4:Pulpotomy (ferric sulphate); n = 50 (1 visit)
|
|
| Outcomes | Clinical failure (spontaneous pain, tenderness to percussion, fistula, soft tissue swelling, and pathological tooth mobility), spontaneous pain, tenderness to percussion, swelling, fistula, pathological mobility: evaluation at 6, 12, 18 and 24 months Radiological failure (periapical or furcal radiolucency, pathological external or distinct internal root resorption, or widened periodontal ligament space), signs of failure (spontaneous pain, tenderness to percussion, fistula, soft tissue swelling, pathological tooth mobility, periapical or furcal radiolucency, pathological external or distinct internal root resorption, or widened periodontal ligament space), furcal radiolucency, periapical radiolucency, internal root resorption, external root resorption, periodontal ligament widening: evaluation at 12 and 24 months (at tooth level) |
|
| Notes | Reasons of dropouts: "103 patients (191 teeth followed up): 3 teeth from the laser group and 6 from the calcium hydroxide group were excluded from follow‐up and statistical analysis, due to uncontrollable bleeding during radiation or placement of calcium hydroxide, since a hyperemic, inflamed radicular pulp is considered a contraindication for vital pulpotomy"; "12 teeth had exfoliated physiologically" Comment: quotes: "4 patients moved away" Source of funding: quote: "The study was completely financed by Departmental funding". (Department of Restorative Dentistry & Periodontology, Dental School, Ludwig‐Maximilians‐University, Munich) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...by an assistant casting a concealed lot from a box containing 4 x 50 lots (block randomization)" |
| Allocation concealment (selection bias) | Low risk | Quote: "...all other contributors for the study were blinded to generation and implementation of the treatment assignment" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "clinical re‐evaluations …were performed independently by two experienced dentists (not the operators) blinded to the technique"; "the outcome assessment and data analysis were blinded, since the techniques were indistinguishable and coded" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "radiographic examinations were performed independently by two experienced dentists (not the operators) blinded to the technique"; "the outcome assessment and data analysis were blinded, since the techniques were indistinguishable and coded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of missing outcomes < 10% of children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Ibricevic 2000.
| Methods | RCT, parallel‐arm Children randomly assigned Setting not mentioned. Conducted in Kuwait. Operator was 1 senior paedodontist |
|
| Participants | 70 children, 164 teeth, mean age 4.3 years, age range 3 to 6 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 80 (1 visit)
Group 2:Pulpotomy (ferric sulphate); n = 84 (1 or 2 visits)
|
|
| Outcomes | Clinical success (absence of any fistula, abscess, swelling, spontaneous pain or pathological mobility), radiological failure (normal periodontal ligament space, no pathological internal or external root resorption and no intraradicular or periapical radiolucency), internal root resorption, periapical bone destruction, inter‐radicular bone destruction, succedaneous tooth structural anomaly: evaluation at 3 to 20 and 46 to 48 months (at tooth level) Signs of failure (internal root resorption, furcation radiolucency, periapical bone destruction, or a combination): evaluation at 46 to 48 months (at tooth level) |
|
| Notes | The first 70 teeth were all treated within 1 month. The pulpotomy therapy of a further 124 primary molars was performed on the same children, during the following 6 months. On the final recall after 42 to 48 months, only 60 children appeared within the 4 months' recall period Clinical follow‐up: every 3 months Radiographic follow‐up: 6, 20 and 42 to 48 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternate allocation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | High risk | Quote: "The clinical follow‐up by the same examiner who had performed all pulpotomies and was aware to which treatment groups the subjects belonged" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "Both authors, blindly, evaluated radiographs" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Jayam 2014.
| Methods | RCT, parallel‐arm Children randomly assigned Conducted at the outpatient department, Dr R Ahmed Dental College and Hospital, India. Operators not mentioned. |
|
| Participants | 66 children, 100 teeth, age range 3 to 7 years | |
| Interventions |
Group 1:Pulpotomy (MTA); n = 50 (2 visits)
Group 2:Pulpotomy (FC); n = 50 (2 visits)
|
|
| Outcomes | Clinical failure (history of pain, tenderness to palpation/percussion, pathological mobility, intra‐ or extra‐oral swelling, intra‐ or extra‐oral sinus, radiograph evaluation (integrity of lamina dura, presence or absence of radiolucencies in the apical or bifurcation areas of tooth, pathological internal or external root resorption, pulp canal obliteration), dentin bridge formation, overall success: evaluation at 1, 3, 6, 12 and 24 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% of children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Kalra 2017.
| Methods | RCT, parallel‐arm Children randomly assigned Conducted in India. Setting and operators not mentioned. |
|
| Participants | 48 children, 60 teeth, age range 4 to 10 years, mean 6.5 ± 1.2 years | |
| Interventions |
Group 1:Pulpotomy (MTA); n = 30 (1 visit)
Group 2:Pulpotomy (Aloe vera); n = 30 (1 visit)
|
|
| Outcomes | Clinical failure (ain, tenderness, mobility, swelling, sinus), radiographic failure (widening of periodontal ligament space, radiolucency, root resorption, pulp obliteration): evaluation at 1, 3, 6, 9, and 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "...the parent selecting a color‑coded stick out from an opaque bag mentioning the medicament" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% of children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Kang 2015.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Department of Pediatric Dentistry at the Yonsei University Dental Hospital. Operators were seven paediatric dentists |
|
| Participants | 102 children, 151 teeth, age range 3 to 10 years | |
| Interventions |
Group 1:Pulpotomy (RetroMTA); n = 49 (1 visit)
Group 2:Pulpotomy (OrthoMTA); n = 47 (2 visits)
Group 3:Pulpotomy (ProRoot MTA); n = 47 (2 visits)
|
|
| Outcomes | Clinical failure (spontaneous pain and/or sensitivity to palpation/percussion; fistula, gingival redness, and swelling and/or mobility), radiological failure (bone resorption at the periapical and/or interradicular regions; PDL space widening; and external/internal root resorption): evaluation at 3, 6 and 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Low risk | Quote: "...one dental radiologist (KT Kim) who were not involved in this study were blinded to the group assignment and treatment and performed all radiographic follow‐up examinations after the completion of the 12‐month study period." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Khorakian 2014.
| Methods | RCT, split‐mouth Teeth randomly assigned Conducted in the paediatric department of Mashhad Dental School, Iran. Operator was a postgraduate student of paediatric dentistry, who was supervised by two academic staff |
|
| Participants | 51 children, 102 teeth, age range 4 to 6 years | |
| Interventions |
Group 1:Pulpotomy (CEM); n = 51 (1 visit)
Group 2:Pulpotomy (ES/ZOE); n = 51 (1 visit)
|
|
| Outcomes | Clinical success (lack of pain, mobility, swelling, sinus tract, tenderness to percussion and bone swelling), radiographic success (PDL and periapical regions with normal width and trabeculation minimal internal resorption), pulp canal obliteration: evaluation at 6, 12 and 24 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random‐number |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "...both the patients and outcome assessors were blinded to the type of treatment" |
| Blinding of clinical outcomes assessment | Low risk | Quote: "...both the patients and outcome assessors were blinded to the type of treatment" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "...both the patients and outcome assessors were blinded to the type of treatment" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Kusum 2015.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Department of Pediatric and Preventive Dentistry, Faculty of Dental Sciences, King George’s Medical University, UP, Lucknow. Operator not mentioned. |
|
| Participants | 90 children, 90 teeth, mean age 6.8 years, age range 3 to 10 years | |
| Interventions |
Group 1:Pulpotomy (MTA); n = 25 (2 visits)
Group 2:Pulpotomy (Biodentine); n = 25 (2 visits)
Group 3:Pulpotomy (propolis); n = 25 (2 visits)
|
|
| Outcomes | Clinical and radiographic criteria for assessing teeth were explained along with a calibration process to the two observers on three initial cases. The criteria, based on Zurn and Seale has been used for scoring the clinical and radiographic findings. The scoring system was devised to represent severity of changes but not to define an individual tooth as a ‘success’ or ‘failure’, i.e. as the score gets larger, the pathologies get progressively more invasive and require more frequent follow‐up. Teeth scored as 1 or 2 were considered successful. Evaluation at 3, 6 and 9 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "The teeth were evaluated clinically and radiographically by two observers independently who were blinded to the treatment type" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "The teeth were evaluated clinically and radiographically by two observers independently who were blinded to the treatment type" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Liu 2011.
| Methods | RCT, split‐mouth Teeth randomly assigned Conducted at the Department of Pediatric dentistry, Pekin. Operator not mentioned. |
|
| Participants | 18 children, 40 teeth, age range 4 to 9 years | |
| Interventions |
Group 1:Pulpotomy (MTA); n = 25 (1 visit)
Group 2:Pulpotomy (CH); n = 25 (1 visit)
|
|
| Outcomes | Clinical success (no pain, swelling, fistula, tenderness to percussion, pathologic mobility), radiologic success (no periapical or interradicular radiolucency, pathological root resorption): evaluation at 10 to 56 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% of children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Lourenço 2015a.
| Methods | RCT, parallel arm Teeth randomly assigned Setting and operators not mentioned |
|
| Participants | 22 children, 30 teeth, mean age 6.6 years, standard deviation age 1.4 years. | |
| Interventions |
Group 1:Pulpotomy (Portland cement, PC); n = 10 (1 visit)
Group 2:Pulpotomy (PC + iodoform, PC + CHI₃); n = 10 (1 visit)
Group 3:Pulpotomy (PC + zirconium oxide, PC + ZrO₂); n = 10 (1 visit)
|
|
| Outcomes | Clinical success (no swelling, fistula, spontaneous pain, mobility), radiological success (no furcation radiolucency, internal root resorption, external root resorption): evaluation at 6, 12 and 24 months (at tooth level) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random‐number generator |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "...at each follow‐up examination two investigators who were blinded to the identification of the medicaments ... performed clinical... examination of the pulpotomized teeth" |
| Blinding of radiological outcomes assessment | Low risk | quote: "...at each follow‐up examination two investigators who were blinded to the identification of the medicaments ... performed... periapical examination of the pulpotomized teeth" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of missing outcomes < 10% of children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Malekafzali 2011.
| Methods | RCT, split‐mouth Teeth randomly assigned Conducted in the Paediatric Dental Clinic of Shahid Beheshti dental School, Tehran, Iran. Operators not mentioned |
|
| Participants | 40 children, 80 teeth, mean age 6 years, standard deviation age 0.8 years, age range 4 to 8 years | |
| Interventions |
Group 1:Pulpotomy (MTA); n = 40 (1 visit)
Group 2:Pulpotomy (calcium enriched mixture); n = 40 (1 visit)
|
|
| Outcomes | Clinical failure (swelling/abscess, sinus tract, spontaneous pain, pathological mobility, or a combination), radiological failure (furcation radiolucency, periapical bone destruction, internal root resorption and pathological external root resorption), external root resorption: evaluation at 6, 12 and 24 months (at tooth level) | |
| Notes | Source of funding: "This trial was supported by Iran National Science Foundation and Iranian Center for Endodontic Research, Shahid Beheshti Medical University, Tehran, Iran" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "The children were examined clinically by a blinded paediatric dentist" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "Radiographic evaluations were performed by a blinded oral radiologist and paedodontist" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% of children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Markovic 2005.
| Methods | RCT, parallel‐arm Children randomly assigned Conducted in the Clinic of Preventive and Pediatric Dentistry, School of Dentistry, University of Belgrade, Serbia and Montenegro. Operators were 3 paedodontists with a minimum of 5 years' clinical experience |
|
| Participants | 104 children, 104 teeth, mean age 6.4 years, standard deviation age 1.1 years, age range 4 to 9 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 33 (1 visit)
Group 2:Pulpotomy (calcium hydroxide); n = 34 (1 visit)
Group 3:Pulpotomy (ferric sulphate)n = 37 (1 visit)
|
|
| Outcomes | Radiographic success (pathological changes of the alveolar bone in the apical or furcation (or both) area, visible periapical or inter‐radicular radiolucency, integrity of lamina dura, pathological internal resorption, external root resorption), spontaneous pain, abnormal mobility, tenderness to percussion, changes in the integrity of lamina dura, pathological internal resorption, external root resorption, dentine bridge formation, abscess or fistula, apical and furcal destruction: reporting at 3, 6, 12 and 18 months (at tooth level) | |
| Notes | Source of funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Moretti 2008.
| Methods | RCT, parallel‐arm Teeth randomly assigned Setting not mentioned. Conducted in Brazil. Operators were 3 authors of the article |
|
| Participants | 23 children, 45 teeth, mean age 6 years, standard deviation age 0.4 years, age range 5 to 9 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 15 (1 visit)
Group 2:Pulpotomy (MTA); n = 15 (1 visit)
Group 3:Pulpotomy (calcium hydroxide); n = 15 (1 visit)
|
|
| Outcomes | Clinical success (no spontaneous pain, no mobility, no swelling, no fistula and no smell), radiographic success (no internal root resorption, no inter‐radicular bone destruction, no furcation radiolucency or dentine bridge formation), fistula, pathological mobility, inter‐radicular bone destruction, internal root resorption, dentine bridge formation: evaluation at 3, 6, 12, 18 and 24 months (at tooth level) | |
| Notes | Dropouts: "Two children… were lost to follow‐up because they moved to another city" Group 1: 6, 12, 18, 24 months: 1 exfoliation per month Group 2: 18 months: 1 exfoliation Group 3: 12 and 18 months: 1 exfoliation per month; 24 months: 3 exfoliations Exfoliations and extractions were excluded from analysis? No information Source of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number‐producing system |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "...each checkup involved a clinical examination of the pulpotomized teeth, which was performed by two blinded and previously calibrated investigators" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "...each checkup involved a periapical radiographic examination of the pulpotomized teeth, which was performed by two blinded and previously calibrated investigators" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of missing outcomes < 10% of children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Mortazavi 2004.
| Methods | RCT, parallel‐arm Teeth randomly assigned Setting and operators not mentioned. Conducted in Iran |
|
| Participants | 58 children, 58 teeth, mean age 5.7 years, standard deviation age 1.5 years, age range 3 to 13 years | |
| Interventions |
Group 1:Pulpectomy (ZOE); n = 32 (2 visits)
Group 2:Pulpectomy (calcium hydroxide + iodoform); n = 26 (2 visits)
|
|
| Outcomes | Signs of success (absence of pain, fistula, intraoral swelling, extraoral swelling, abnormal mobility, bone radiolucency or position and eruption pathway of the permanent successor tooth), pain symptoms, fistula, pathological mobility, extraoral swelling, intraoral swelling, bone radiolucency, position and eruption pathway of the permanent successor tooth: evaluation at 12 (range 10 to 16) months (at tooth level) | |
| Notes | Of the 58 original children selected and treated at the beginning of the study, 52 returned for follow‐up. Six children had either moved from the area, or changed addresses or phone numbers (or both), and contact was lost Source of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Nadkarni 2000.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Outpatient Department, Department of Pediatric Dentistry, Nair Hospital Dental College, Mumbai, India. Operators not mentioned |
|
| Participants | 60 children, 70 teeth, age range 4 to 8 years | |
| Interventions |
Group 1:Pulpectomy (calcium hydroxide); n = 35 (2 visits)
Group 2:Pulpectomy (ZOE); n = 35 (2 visits)
|
|
| Outcomes | Pain symptoms, tenderness to percussion, pathological mobility, pathological radiolucency: evaluation at 1, 3, 6 and 9 months (at tooth level) Radiographic success (the radiolucency did not increase and when it was the same as the preoperative status): evaluation at 3, 6 and 9 months (at tooth level) Clinical success (absence of pain, absence of tenderness to percussion and absence of, or decrease in mobility), signs of success (pain symptoms, tenderness to percussion, pathological mobility, pathological radiolucency): evaluation at 9 months (at tooth level) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Naik 2005.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Department of Pedodontics and Preventive Dentistry, Mangalore, India. Operators not mentioned |
|
| Participants | 38 children, 50 teeth | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 25 (2 visits)
Group 2:Pulpotomy (MTA); n = 25 (2 visits)
|
|
| Outcomes | Pain, mobility, swelling, sinus tract, internal root resorption, external root resorption (periapical or furcal radiolucency), root resorption in relation to contralateral tooth, pulp canal obliteration: evaluation at 1, 3 and 6 months (at tooth level) | |
| Notes | 3 teeth were not available for further follow‐up after 1 month Source of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of missing outcomes < 10% children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Nakornchai 2010.
| Methods | RCT, parallel‐arm Teeth randomly assigned Setting not mentioned. Conducted in Thailand. 1 operator not mentioned |
|
| Participants | 37 children, 50 teeth, age range 3 to 8 years | |
| Interventions |
Group 1:Pulpectomy (ciprofloxacin + metronidazole + minocycline); n = 25 (1 visit)
Group 2:Pulpectomy (calcium hydroxide + iodoform); n = 25 (1 or 2 visits)
|
|
| Outcomes | Clinical success (pain, gingival abscesses, fistula openings or abnormal mobility), radiological success (static or reduced size of bifurcation/periapical radiolucency, no progression of pathological external root resorption, no progression of internal root resorption and no newly formed radiographic lesions), spontaneous pain, tenderness to percussion, swelling, fistula, pathological mobility, abscess, furcal radiolucency, periapical radiolucency, internal root resorption, external root resorption, calcific metamorphosis: evaluation at 9 and 12 months (at tooth level) | |
| Notes | Source of funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin toss |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Blinded clinical evaluations were performed by the operator |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Nguyen 2017.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Hospital for Sick Children, Toronto, Canada. Operators were 5 paediatric dentists |
|
| Participants | 70 children, 172 teeth, mean age 2.5 years, standard deviation age 0.5 years, min‐max 1.5 to 4 years | |
| Interventions |
Group 1:Pulpotomy (ferric sulphate); n = 100 (1 visit)
Group 2:Pulpectomy (ZOE); n = 72 (1 visit)
|
|
| Outcomes | (1) N equals incisor without pathologic change; (2) P equals pathologic change present, follow‐up recommended; and (3) Px equals pathologic change present, extract. N or Pq were considered an acceptable outcome, while incisors rated as P were considered unacceptable. + presence or absence of periapical radiolucency, pathological external root resorption, widened PDL space, physiological root resorption, internal root resorption, PCO, and dentin bridge formation, and whether the restoration was intact or not + spontaneous pain, tenderness to percussion, fistula/sinus tract, soft tissue swelling, and/or pathological tooth mobility at 12 and 18 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random‐number generator |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement of Yes or No |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding of personnel providing treatment |
| Blinding of clinical outcomes assessment | Low risk | Quote: "A single investigator, who did not perform any pulp therapy or participate in radiographic evaluation, performed all clinical assessments." |
| Blinding of radiological outcomes assessment | High risk | No blinding of raters assessing radiographic outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Niranjani 2015.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Department of Pedodontics and Preventive dentistry, St. Joseph Dental College and Hospital, Eluru, Andhra Pradesh, India. Operator not mentioned |
|
| Participants | 60 children, 60 teeth, age range 5 to 9 years | |
| Interventions |
Group 1:Pulpotomy (Biodentine); n = 20 (1 visit)
Group 2:Pulpotomy (diode laser); n = 20 (1 visit)
Group 3:Pulpotomy (MTA); n = 20 (1 visit)
|
|
| Outcomes | Clinical criteria (pain, sinus tract, swelling and mobility), radiographic criteria (premature exfoliation, PDL widening, internal/external resorption and periapical/furcal radiolucency): evaluation at 3 and 6 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Noorollahian 2008.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Paediatric Dentistry Department, Faculty of Dentistry, Zahedan University of Medical Sciences, Iran. Operators not mentioned |
|
| Participants | 46 children, 60 teeth, mean age 6.1 years, age range 5 to 7 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 30 (1 visit)
Group 2:Pulpotomy (MTA); n = 30 (2 visits)
|
|
| Outcomes | Clinical success (no pain symptoms, no tenderness of percussion, no swelling, no fistulation or no pathological mobility), radiological success (no evidence of radicular radiolucency, no internal or external root resorption or no periodontal ligament space widening), signs of failure (internal root resorption, furcation radiolucency, periapical bone destruction, pain, swelling or sinus tract), furcation involvement, pulp canal obliteration: evaluation at 6, 12 and 24 months (at tooth level) | |
| Notes | Dropouts: at 24 months "12 out of 30 teeth in the two groups", no reasons stated Source of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "...the children were examined clinically by the author who was blind to which treatment group the subject belonged" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "...the children were examined radiographically by the author who was blind to which treatment group the subject belonged" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Olatosi 2015.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Pediatric Dentistry unit of the Department of Child Dental Health Lagos University Teaching Hospital. Operator was a paediatric dentist |
|
| Participants | 37 children, 50 teeth, mean age 6.1 years, age range 4 to 7 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 25 (1 visit)
Group 2:Pulpotomy (MTA); n = 25 (1 visit)
|
|
| Outcomes | Clinical success (no pain, no tenderness to percussion, no swelling or sinus tract, no pathologic tooth mobility), radiological success (normal periodontal ligament space, no furcation or periapical radiolucency, no active/progressing internal root resorption, no pathologic external root resorption): evaluation at 1, 3, 6, 9, and 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "...clinical assessment of the treated teeth which were only identified by code was carried out by two experienced dentists who were blinded to the treatment groups." |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of missing outcomes < 10% children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Oliveira 2013a.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Paediatric Dentistry Department, University of São Paulo, Brazil. Operators not mentioned |
|
| Participants | 45 children, 45 teeth, mean age not mentioned, age range 5 to 9 years | |
| Interventions |
Group 1:Pulpotomy (CH); n = 15 (1 visit)
Group 2:Pulpotomy (MTA); n = 15 (1 visit)
Group 3:Pulpotomy (PC); n = 15 (1 visit)
|
|
| Outcomes | Clinical success (no spontaneous pain, no swelling, no fistula or no mobility), radiological success (no evidence of furcation radiolucency, no internal root resorption, dentine bridge formation), intra‐canal obliteration, inter‐radicular bone destruction, intra‐canal obliteration: evaluation at 6, 12 and 24 months (at tooth level) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random‐number generator |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "...each check‐up involved a clinical... examination of the pulpotomized teeth, which was performed by two blinded and previously calibrated investigators" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "...each check‐up involved a... periapical radiographic examination of the pulpotomized teeth, which was performed by two blinded and previously calibrated investigators" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Ozalp 2005.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Department of Pediatric Dentistry, University of Ankara, Turkey. Operators were 2 experienced paediatric dentists |
|
| Participants | 76 children, 80 teeth, age range 4 to 9 years | |
| Interventions |
Group 1:Pulpectomy (ZOE); n = 20 (1 or 2 visits)
Group 2:Pulpectomy (calcium hydroxide eugenol free); n = 20 (1 or 2 visits)
Group 3:Pulpectomy (calcium hydroxide); n = 20 (1 or 2 visits)
Group 4:Pulpectomy (calcium hydroxide + iodoform); n = 20 (1 or 2 visits)
|
|
| Outcomes | Clinical success (no pain, no gingival swelling, no tenderness to percussion, no abnormal mobility, no fistula or no abscess), radiological success (no (increase in size of) furcation radiolucency, no (increase in size of) periapical radiolucency, no (increase in size of) discontinuity of lamina dura and no (increase in size of) pathological root resorption), tenderness to percussion, pathological mobility, periapical radiolucency, pathological root resorption, excess filling material and its resorption: evaluation at 2, 4, 6, 8, 10, 12 and 18 months (at tooth level) | |
| Notes | Source of funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "The clinical success were assessed by the clinicians blindly" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "The radiographic success were assessed by the clinicians blindly" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Ozmen 2017.
| Methods | RCT, parallel arm Teeth randomly assigned Conducted at the Department of Pediatric Dentistry, Turkey. Operator was an investigator |
|
| Participants | 26 children, 45 teeth, age range 6 to 9 years, mean age: 7.36 ± 0.96 years | |
| Interventions |
Group 1:Pulpotomy (FC); n = 15 (1 visit)
Group 2:Pulpotomy (FS); n = 15 (1 visit)
Group 3:Pulpotomy (Ankaferd Blood Stopper); n = 15 (1 visit)
|
|
| Outcomes | Clinical failure (spontaneous or severe pain, pathological mobility, swelling or sinus tract, tenderness to percussion or palpation), radiological failure (furcal or periapical radiolucency, didened periodontal ligament spaces, internal or external root resorption, loss of lamina dura), pulp canal obliteration: evaluation every 3 or 6 months (up to 24 months). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin tossing |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of missing outcomes < 10% children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Pinky 2011.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Outpatient Department of Pedodontics and Preventive Dentistry, College of Dental Sciences, Davangere, India. Operators not mentioned |
|
| Participants | 28 children, 40 teeth, age range 4 to 10 years | |
| Interventions |
Group 1:Pulpectomy (ciprofloxacin + metronidazole + minocycline); n = 20 (3 visits)
3Mix (ciprofloxacin + metronidazole + minocycline) after pulpectomy. Commercially available chemotherapeutic agents such as ciprofloxacin, metronidazole and minocycline, were used. After removal of enteric coating, these drugs were pulverised using sterile porcelain mortar and pestle. These powdered drugs were mixed into 2 different combinations in the ratio of 1:3:3, i.e. 1 group being 1 part of ciprofloxacin, 3 parts of metronidazole and 3 parts of minocycline, kept separately to prevent exposure to light and moisture. 1 increment of each powdered drug was mixed with propylene glycol to form an ointment just before use. Canal orifices were enlarged to receive medicament termed as "medication cavity". This was accomplished using a round bur, following which cavities were cleaned and irrigated with the help of saline and dried. The medication cavities were filled with 1 of the pastes and given a temporary dressing with ZOE. Children were recalled after 15 days for resolution of clinical signs and symptoms, following which permanent restoration was done with glass‐ionomer cement. At 30 days, following successful treatment, stainless‐steel crowns were placed and x‐rays taken Group 2:Pulpectomy (ciprofloxacin + ornidazole + minocycline); n = 20 (3 visits)
Ciprofloxacin + ornidazole + minocycline after pulpectomy. Commercially available chemotherapeutic agents such as ciprofloxacin, minocycline and ornidazole were used. After removal of enteric coating, these drugs were pulverised using sterile porcelain mortar and pestle. These powdered drugs were mixed into 2 different combinations in the ratio of 1:3:3, i.e. 1 group being 1 part of ciprofloxacin with 3 parts of ornidazole and 3 parts of minocycline, kept separately to prevent exposure to light and moisture. 1 increment of each powdered drug was mixed with propylene glycol to form an ointment just before use. Canal orifices were enlarged to receive medicament termed as "medication cavity". This was accomplished using a round bur, following which cavities were cleaned and irrigated with the help of saline and dried. The medication cavities were filled with 1 of the pastes and given a temporary dressing with ZOE. Children were recalled after 15 days for resolution of clinical signs and symptoms, following which permanent restoration was done with glass‐ionomer cement. At 30 days, following successful treatment, stainless‐steel crowns were placed and x‐rays taken |
|
| Outcomes | Clinical success (absence of spontaneous pain, tenderness to percussion, abnormal mobility and signs of pathology such as intraoral or extraoral abscess), pain symptoms, tenderness to percussion, abscess: evaluation at 3, 6 and 12 months (at tooth level) Radiological success (radiolucency decreased compared with preoperative status or remained same), furcal radiolucency: evaluation at 6 and 12 months (at tooth level) |
|
| Notes | Source of funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Prabhakar 2008.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the clinic of the department of Pedodontics and Preventive Dentistry, Bapuji Dental College and Hospital, Davangere, India. Operators not mentioned |
|
| Participants | 41 children, 60 teeth, age range 4 to 10 years | |
| Interventions |
Group 1:Pulpotomy (3Mix); n = 30 (1 visit)
Only the necrotic coronal pulp was removed for pulpotomy. The orifice of the canal was enlarged and was termed as "medication cavity" which was half‐filled with antibacterial mix, before being restored with glass‐ionomer cement and composite Group 2:Pulpectomy (3MIx); n = 30 (1 visit)
Both necrotic coronal as well as all accessible radicular pulp tissue was extirpated for pulpotomy. The orifice of the canal was enlarged and was termed as "medication cavity" which was half‐filled with antibacterial mix, before being restored with glass‐ionomer cement and composite |
|
| Outcomes | Pain symptoms, tenderness to percussion, pathological mobility, abscess: evaluation at 1, 6 and 12 months (at tooth level) Furcal radiolucency: evaluation at 6 and 12 months (at tooth level) |
|
| Notes | Source of funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Pramila 2016.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in Saveetha Dental College and Hospital, India. Operator was an investigator |
|
| Participants | 88 children, 129 teeth, age range 4 to 9 years | |
| Interventions |
Group 1:Pulpectomy (RC Fill); n = 43 (1 visit)
RC Fill available in powder and liquid form, mixed to the desired consistency according to the manufacturer’s instructions. A Lentulo spiral was used to place the RC Fill, followed by glass ionomer cement, before being restored with stainless steel crown. Group 2:Pulpectomy (Vitapex); n = 43 (1 visit)
Vitapex available in preformed syringes. The syringe was inserted into the canal near the apex. The paste was extruded into the canal, and the syringe was then slowly withdrawn as it filled the entire canal, followed by glass ionomer cement, before being restored with stainless steel crown. Group 3:Pulpectomy (ZOE); n = 43 (1 visit)
ZOE available in powder and liquid form, mixed to the desired consistency according to the manufacturer’s instructions. An Endodontic Pressure Syringe was used to place ZOE, followed by glass ionomer cement, before being restored with stainless steel crown. |
|
| Outcomes | Clinical failure (pain, tenderness to percussion, swelling/abscess, mobility and draining fistula), radiographic failure (furcation, radiolucency, periapical radiolucency, internal root resorption, external root resorption, lamina dura, deviation in the path of eruption, intraradicular resorption, resorption of extruded material), overall success: evaluation at 6, 12 and 30 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque and sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The participants and outcome assessors were blinded about the filling materials used" |
| Blinding of clinical outcomes assessment | Low risk | Quote: "The participants and outcome assessors were blinded about the filling materials used" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "The participants and outcome assessors were blinded about the filling materials used" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% of children randomly assigned |
| Selective reporting (reporting bias) | Low risk | Protocol prospectively registered (CTRI/2011/06/001776). no discrepancies in outcomes between registered record and published RCT. |
Rajasekharan 2017.
| Methods | RCT, parallel arm Teeth randomly assigned Conducted in the Department of Paediatric Dentistry and Special Care, University Hospital, Ghent University, Belgium. Operators were five (professor, doctoral graduate, doctoral student, master graduate and master student) |
|
| Participants | 58 children, 81 teeth, age range 3 to 8 years, mean age: 4.79 ± 1.23 | |
| Interventions |
Group 1:Pulpotomy (Biodentine); n = 25 (1 visit)
Group 2:Pulpotomy (MTA); n = 29 (1 visit)
Group 3:Pulpotomy (Tempophore); n = 27 (1 visit)
|
|
| Outcomes | Clinical and radiographic scoring criteria adapted from Zurn & Seale (2008): evaluation at 1, 6, 12 and 18 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "All teeth were followed up clinically and radiographically by two blinded calibrated investigators" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "All teeth were followed up clinically and radiographically by two blinded calibrated investigators" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups in two trials |
| Selective reporting (reporting bias) | High risk | Protocol retrospectively registered (NCT01733420). Registered primary outcomes were reported as secondary outcomes in the published article and new primary outcomes were introduced in the published article. |
Ramar 2010.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Department of Pedodontics and Preventive Dentistry, Ragas Dental College and Hospital, Chennai, India. Operators not mentioned |
|
| Participants | 77 children, 96 teeth, age range 4 to 7 years | |
| Interventions |
Group 1:Pulpectomy (ZOE); n = 34 (1 visit)
Group 2:Pulpectomy (calcium hydroxide + iodoform); n = 30 (1 visit)
Group 3:Pulpectomy (ZOE + calcium hydroxide); n = 32 (1 visit)
|
|
| Outcomes | Pain symptoms, furcal radiolucency, periapical radiolucency, excess filling material and its resorption, faster root resorption compared with contralateral, slower root resorption compared with contralateral, similar root resorption compared with contralateral: evaluation at 3, 6 and 9 months (at tooth level) | |
| Notes | Source of funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of missing outcomes < 10% children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Rewal 2014.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Department of Pedodontics and Preventive Dentistry, India. Operator was an investigator. |
|
| Participants | 50 children, 50 teeth, age range 4 to 9 years | |
| Interventions |
Group 1:Pulpectomy (ZOE); n = 24 (1 visit)
Group 2:Pulpectomy (Endoflas); n = 26 (1 visit)
|
|
| Outcomes | Clinical success (absence of pain, redness, swelling, tenderness on percussion, and sinus or fistula), radiographic success (reduction in the size of interradicular radiolucency or the size remaining the same): evaluation at 3, 6 and 9 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Sabbarini 2008.
| Methods | RCT, split‐mouth Teeth randomly assigned Conducted in the paediatric dental clinics, Department of Pediatric Dentistry, Alexandria University, Egypt. Operators not mentioned |
|
| Participants | 15 children, 30 teeth, mean age 5 years, standard deviation age 0.7 years, age range 4 to 7 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 15 (1 visit)
Group 2:Pulpotomy (EMD); n = 15 (1 visit)
A cotton pellet was placed to cover the amputated pulpal stumps, and the tooth was then conditioned with polyacrylic acid gel. The cotton pellet was then removed, and the amputated pulpal stumps were covered with protein EMD gel from a 0.3 mL syringe, before being restored with glass‐ionomer cement and stainless‐steel crown |
|
| Outcomes | Clinical success (absence of pain, pain on percussion, mobility, and abscess or fistula formation), radiological success (normal periodontal ligament space and had an absence of furcation and periapical radiolucency, pulp calcification, and internal resorption), pain symptoms, tenderness to percussion, pathological mobility, sinus tract, abscess: evaluation at 2, 4 and 6 months (at tooth level) | |
| Notes | Source of funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "2 examiners who were blinded to treatment type evaluated the teeth clinically" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "2 examiners who were blinded to treatment type evaluated the teeth radiographically" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Sakai 2009.
| Methods | RCT, parallel‐arm Teeth randomly assigned. Conducted in Brazil. Operators and setting not mentioned |
|
| Participants | 30 children, 30 teeth, mean age 6.8 years, age range 5 to 9 years | |
| Interventions |
Group 1:Pulpotomy (Portland cement); n = 15 (1 visit)
Group 2:Pulpotomy (MTA); n = 15 (1 visit)
|
|
| Outcomes | Clinical success (no spontaneous pain, no mobility, no swelling, no fistula or no smell), radiographic success (no internal root resorption and no furcation radiolucency, dentine bridge formation), swelling, pathological mobility, sinus tract, inflammation in the adjacent tissues, furcal radiolucency, internal resorption, pulp canal obliteration, dentine bridge formation: evaluation at 6, 12, 18 and 24 months (at tooth level) | |
| Notes | Reasons of dropouts: Group 1: 3 (of 24‐month follow‐up) + 3 (after 24 months) exfoliations Group 2: 1 (of 18‐month follow‐up) + 1 (of 24‐month follow‐up) + 3 (after 24 months) exfoliations Source of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The primary mandibular molars were randomly assigned to MTA or PC groups by the toss of a coin" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "Clinical examination… which was performed by two blinded and previously calibrated investigators" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "Periapical radiographic examination… which was performed by two blinded and previously calibrated investigators" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Saltzman 2005.
| Methods | RCT, split‐mouth Teeth randomly assigned Conducted in the University of Toronto Faculty of Dentistry Paediatric Clinic, Canada. Operators were 1 of 7 paediatric dental residents, including the primary investigator |
|
| Participants | 16 children, 52 teeth, mean age 5.1 years, age range 3 to 8 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 26 (1 visit)
Group 2:Pulpotomy (MTA); n = 26 (1 visit)
|
|
| Outcomes | Clinical success (teeth remained asymptomatic, absence of a sinus tract, premature tooth loss), radiographic success (absence of furcal radiolucencies, pathological root resorption, damage to succedaneous follicle, or a combination), signs of success (teeth remained asymptomatic, absence of a sinus tract, absence of furcal radiolucencies, pathological root resorption, damage to succedaneous follicle and premature tooth loss, or a combination), furcal radiolucency, periapical radiolucency, pathological root resorption, root resorption in relation to contralateral: evaluation at (mean ± standard deviation) 2.3 ± 2.1, 5.2 ± 1.9, 9.5 ± 2.3 and 15.7 ±3 months (at tooth level) | |
| Notes | 4 follow‐up visits (mean ± standard deviation): first: 2.3 ± 2.1, second: 5.2 ± 1.9, third: 9.5 ± 2.3, fourth: 15.7 ± 3.0 months Source of funding: quote: "The investigators wish to thank BioLitec and Lasers in Dentistry for the donation of the diode laser, and Dentsply for the donation of the MTA. Funding for this study was provided by the University of Toronto and Alpha Omega. The authors of this study do not have any financial interest in the commercial products used" Comment: Alpha Omega International Dental Fraternity is a Jewish philanthropic charity and presents no apparent conflict of interests |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin toss |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | High risk | Quote: "clinical outcome assessments were made by the primary investigator at each follow‐up visit" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "radiographic outcome assessments were made by the primary investigator and one independent experienced clinician who was blind to the treatment" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Shabzendedar 2013.
| Methods | RCT, parallel arm Teeth randomly assigned Conducted in the clinic of the Pediatric Dentistry Department of the university's School of Dentistry. Operators not mentioned |
|
| Participants | 100 children, 100 teeth, mean age 4.3 years, age range 3 to 6 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 50 (1 visit)
Group 2:Pulpotomy (3% NaOCl); n = 50 (1 visit)
|
|
| Outcomes | Clinical success (no pain symptoms, tenderness to percussion, swelling, fistula, and pathologic mobility), radiographic success (no evidence of inter‐radicular radiolucency, internal or external root resorption, and periapical radiolucency): evaluation at 6 and 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number generator |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "The dentists assessing the outcomes were blinded to group assignment" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "The dentists assessing the outcomes were blinded to group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Shumayrikh 1999.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the paediatric dentistry postgraduate clinics of King Saud University College of Dentistry, Saudi Arabia. Operators not mentioned |
|
| Participants | 19 children, 61 teeth, age range 5 to 10 years | |
| Interventions |
Group 1:Pulpotomy (glutaraldehyde + ZOE); n = 30 (2 visits)
Group 2:Pulpotomy (glutaraldehyde + calcium hydroxide); n = 31 (2 visits)
|
|
| Outcomes | Clinical success (no history of pain, no swelling or sinus tract, no history of thermal sensitivity and no tenderness to percussion), radiographic success (no loss of lamina dura, no loss of trabecular bone, no furcal or periapical radiolucency and no internal resorption), pain symptoms, thermal sensitivity, tenderness to percussion, internal root resorption, changes in the integrity of lamina dura, loss of trabecular bone, furcal or periapical radiolucency: evaluation at 12 months (at tooth level) | |
| Notes | Reasons of dropouts: 2 children with 4 treated teeth did not attend the follow‐up appointments Source of funding: quote: "This project was supported by a grant from the College of Dentistry Research Center (CDRC) at King Saud University" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "...the teeth treated by pulpotomy were only identified by code under the supervision of the assistant" |
| Blinding of clinical outcomes assessment | Low risk | Quote: "...clinical evaluations of the treated teeth were carried out by the investigator without knowing which tooth had been treated with" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "...radiographic evaluations of the treated teeth were carried out by the investigator without knowing which tooth had been treated with…" "the… radiographs… were also evaluated by the principal investigator and another pediatric dentist" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of missing outcomes < 10% children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Sonmez 2008.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Clinic of the Pediatric Dentistry Department at the Ankara University, Turkey. Operator was the same paedodontist |
|
| Participants | Treated: 16 children, 80 teeth; analysed: 11 children, 56 teeth, mean age 6.6 years, age range 4 to9 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 13 (analysed) (1 visit)
Group 2:Pulpotomy (ferric sulphate); n = 15 (analysed, not treated) (1 visit)
Group 3:Pulpotomy (calcium hydroxide); n = 13 (analysed, not treated) (1 visit)
Group 4:Pulpotomy (MTA); n = 15 (analysed, not treated) (2 visits)
|
|
| Outcomes | Clinical success (no symptoms of pain, no tenderness on percussion, no swelling, no fistulae or no pathological mobility), radiographic success (no periradicular or inter‐radicular radiolucency, no external or internal resorption or no periodontal ligament space widening), signs of success (no symptoms of pain, no tenderness of percussion, no swelling, no fistulae or no pathological mobility, no periradicular or inter‐radicular radiolucency, no external or internal resorption or no periodontal ligament space widening), internal resorption, external resorption, pulp canal obliteration: evaluation at 6, 12, 18 and 24 months (at tooth level) | |
| Notes | 5 excluded participants. Quote: "Four children did not come to follow‐up appointments 6 months after the first treatment and were excluded from the study. Follow‐up of one child with four pulpotomised primary molars had to be discontinued after 1 year because the family moved to another city." Source of funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Subramaniam 2009.
| Methods | RCT, split‐mouth Teeth randomly assigned Conducted in the Department of Pedodontics and Preventive Dentistry, The Oxford Dental College, Hospital and Research Center, Bangalore, India. Operators not mentioned |
|
| Participants | 19 children, 40 teeth, age range 6 to 8 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 20 (2 visits)
Group 2:Pulpotomy (MTA); n = 20 (2 visits)
|
|
| Outcomes | Clinical success (no history of pain, no tenderness to percussion, no gingival abscess, no sinus/fistula and no pathological mobility), radiographic success (no internal/external root resorption or no periapical/furcal radiolucency), signs of success (no history of pain, no tenderness to percussion, no gingival abscess, no sinus/fistula and no pathological mobility, no internal/external root resorption or no periapical/furcal radiolucency), furcal radiolucency, pulp canal obliteration, dentine bridge formation: evaluation at 1, 6, 12 and 24 months (at tooth level) | |
| Notes | Source of funding: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin toss |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Subramaniam 2011.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Department of Pedodontics and Preventive Dentistry, The Oxford Dental College, Hospital and Research Centre, Bangalore, India. Operators not mentioned |
|
| Participants | Number of enrolled children not mentioned, 45 teeth, age range 5 to 9 years | |
| Interventions |
Group 1: Pulpectomy (calcium hydroxide + iodoform); n = 15 (2 visits)
Group 2: Pulpectomy (calcium hydroxide + ZOE + iodoform); n = 15 (2 visits)
Group 3: Pulpectomy (ZOE); n = 15 (2 visits)
|
|
| Outcomes | Clinical success (no gingival swelling/inflammation/redness, no sinus opening in the oral mucosa or purulent exudate expressed from the gingival margin, no abnormal mobility other than mobility due to normal exfoliation, absence of pain on percussion/tenderness), radiographic success (no evidence of extensive pathological root resorption, reduction or no change in preoperative pathological inter‐radicular or periapical radiolucency (or both), no evidence of development of new postoperative pathological radiolucency involving the succedaneous tooth germ), pain symptoms, tenderness to percussion, swelling, pathological mobility, pathological root resorption, damage in succedaneous follicle: evaluation at 3, 6, 12 and 18 months (at tooth level) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Trairatvorakul 2008.
| Methods | RCT, parallel‐arm Teeth randomly assigned. Conducted in Thailand Setting not mentioned. Operator was 1 investigator (paediatric dentist) |
|
| Participants | 42 children, 54 teeth, mean age 5.6 years, standard deviation age 1.2 years, age range 3.3 to 7.8 years | |
| Interventions |
Group 1:Pulpectomy (ZOE); n = 27 (1 visit)
Group 2:Pulpectomy (calcium hydroxide + iodoform); n = 27 (1 visit)
|
|
| Outcomes | Clinical success (no pain, healthy soft tissue (defined as the absence of swelling, redness or sinus tract) and no abnormal mobility), radiographic success (radiographic continuity of the lamina dura, reduction in the size of any pathological inter‐radicular or periapical radiolucencies (or both) or evidence of bone regeneration), signs of success (absence of change or more discontinuity of lamina dura and absence of change in size of radiolucency area), pain symptoms, swelling, fistula, pathological mobility: evaluation at 6 and 12 months (at tooth level) | |
| Notes | Source of funding: "The authors wish to thank the Chulalongkorn University Postgraduate Research Fund for financial support" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "the clinical diagnoses were blindly assessed by another investigator" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "the radiographic diagnoses were blindly assessed by another investigator" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Tuna 2008.
| Methods | RCT, split‐mouth Teeth randomly assigned. Conducted in Turkey Setting and operators not mentioned |
|
| Participants | 25 children, 50 teeth, age range 5 to 8 years | |
| Interventions |
Group 1:Direct pulp capping (MTA + ZOE); n = 25 (1 visit)
Group 2: Direct pulp capping (CH + ZOE); n = 25 (1 visit)
|
|
| Outcomes | Clinical success (no spontaneous pain, no tenderness of percussion, no swelling, no fistulation or no pathological mobility), radiographic success (no furcation radiolucency, no periodontal ligament space widening or no internal or external root resorption), thermal sensitivity: evaluation at 3, 6, 12, 18 and 24 months (at tooth level) | |
| Notes | Reasons of dropouts: 1 child did not return for evaluation after 1 month, 1 after 9 months and 1 after 12 months because of the loss of restoration that had been placed on the pulp capping material, 1 tooth was excluded from the clinical study after 9 months and 1 tooth after 18 months, both from the CH group Lost to follow‐up: Group 1: failure to attend, n = 3; Group 2: failure to attend, n = 3; loss of restoration, n = 2 Analysed: Group 1: n = 22; Group 2: n = 20. No exclusions Source of funding: quote: "This study was supported financially by the Scientific Research Foundation of Gazi University, Ankara, Turkey (grant no. 03/2003‐15)" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "...two investigators, who attended a calibration session before the follow‐up examinations, blindly evaluated the teeth clinically" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "...two investigators, who attended a calibration session before the follow‐up examinations, blindly evaluated the teeth radiographically" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Uloopi 2016.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Department of Paediatric Dentistry, Vishnu Dental College, Bhimavaram. Operator not mentioned |
|
| Participants | 29 children, 40 teeth, mean 5.6, age range 4 to 7 years | |
| Interventions |
Group 1:Pulpotomy (MTA); n = 20 (1 visit)
Group 2:Pulpotomy (low‐level laser therapy);n = 20 (1 visit)
|
|
| Outcomes | Clinical success (no pain, tenderness to percussion, swelling, fistulation or pathologic mobility), radiologic success (no radicular radiolucency, internal or external root resorption or periodontal ligament space widening), overall success: evaluation at 3, 6 and 12 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of missing outcomes < 10% children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Ulusoy 2014a.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Department of Pedodontics, Faculty of Dentistry, Turkey. Operator was the principal investigator |
|
| Participants | 40 children, 40 teeth, mean age 7.3 years, age range 5 to 9 years | |
| Interventions |
Group 1:Pulpotomy (CH cement/Dycal); n = 20 (1 visit)
Group 2:Pulpotomy (calcium sulphate hemihydrate/Dentogen); n = 20 (1 visit)
|
|
| Outcomes | Clinical success (no pathologic mobility, fistula, spontaneous pain, sensitivity to percussion/palpation, oedema), radiographic success (no external root resorption, internal root resorption, inter‐radicular radiolucency, periapical radiolucency), inter‐radicular radiolucency, external root resorption, internal root resorption, fistula, pathologic mobility, spontaneous pain: evaluation at 1, 3, 6, 9 and 12 months (at tooth level). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "...all clinical... recall examinations were performed by the same clinician who was blinded to the treatment groups" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "...all... radiographic recall examinations were performed by the same clinician who was blinded to the treatment groups" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Vargas 2006.
| Methods | RCT, split‐mouth Teeth randomly assigned Conducted in the Department of Pediatric Dentistry, The University of Iowa, Iowa City, Iowa, USA. Operator was the principal investigator |
|
| Participants | 23 children, 60 teeth, mean age 5 years, age range 4 to 9 years | |
| Interventions |
Group 1:Pulpotomy (ferric sulphate); n = 28 (1 visit)
Group 2:Pulpotomy (sodium hypochlorite); n = 32 (1 visit)
|
|
| Outcomes | Clinical failure (mobility, swelling, fistula, history of spontaneous pain), radiographic success (no external root resorption, no internal root resorption, no inter‐radicular bone destruction), overall success ((% clinical success + % radiographic success)/2), pain palpation, swelling, fistula, pathological mobility, redness, bleeding, furcation involvement, internal resorption: evaluation at 6 and 12 months (at tooth level) | |
| Notes | No reason of dropouts, except "2 teeth exfoliated and were eliminated from further follow‐up" Source of funding: quote: "This research was supported by the Obermann Center for Advanced Studies Spelman Rockefeller Grant from The University of Iowa, Iowa City, Iowa" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random table |
| Allocation concealment (selection bias) | Low risk | Quote: "subject assignment was made at the consent appointment, but allocation of the tooth according to the allocation sequence was made the day of the treatment visit"; "this allocation followed the current guidelines for randomised clinical trials put forth by CONSORT [Consolidated Standards of Reporting Trials]" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Quote: "...the clinical examination was performed by the principal investigator without immediate knowledge of which treatment has been rendered on which tooth" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "...all radiographs were read… by 2 co‐investigators who were blinded to the technique used" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Waterhouse 2000.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the paediatric dental clinic within the Dental Hospital, Newcastle‐upon‐Tyne, UK. Operator not mentioned |
|
| Participants | 52 children, 84 teeth, mean age 5 years, age range 3.3 to 12.5 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 46 (1 visit)
Group 2:Pulpotomy (calcium hydroxide); n = 38 (1 visit)
|
|
| Outcomes | Clinical failure (symptoms from the treated tooth reported by the child or parent, spontaneous pain, pain initiated by stimuli, signs of defective restoration or recurrent caries, signs of mobility, sinus formation, tenderness to percussion, soft tissues swelling, signs of exfoliation, mobility or signs/symptoms of the successor tooth erupting), tenderness to percussion, swelling, pathological mobility, sinus tract, secondary caries, defective restoration: evaluation at 6 and 12 months (at tooth level) Radiographic success (defective restoration or recurrent caries, periradicular pathology such as periapical or furcal radiolucency, pathological internal resorption, replacement resorption, intracanal calcifications, physiological root resorption, position and eruption pathway of the permanent successor tooth), periradicular radiolucency, furcal radiolucency, periapical radiolucency, internal resorption, pulp canal obliteration, physiological root resorption, recurrent caries: evaluation at 12 months (at tooth level) |
|
| Notes | 5 teeth lost to follow‐up Clinical follow‐up: 22.5 months (range 6.1 to 38.5) Radiographic follow‐up: 18.9 months (range 1.3 to 36.9) Source of funding: "This study was supported by The Shirley Glasstone‐Hughes Memorial prize awarded to the authors by the British Dental Association, in September 1993" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin toss |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "Objectivity was maximized during clinical assessment, by not having direct access to records detailing which pulp therapy agent was used" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "Objectivity was maximized during radiographic assessment, by not having direct access to records detailing which pulp therapy agent was used" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Yadav 2014.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in Sudha Rustagi Dental College, Faridabad. Operator not mentioned |
|
| Participants | 37 children, 45 teeth, age range 4 to 7 years | |
| Interventions |
Group 1:Pulpotomy (15.5% ferric sulphate); n = 15 (1 visit)
Cotton pellet was first saturated with 15.5% ferric sulphate and later compressed between gauze to remove excess so it was just moistened with the solution. It was then placed for 15 seconds on amputated pulp stumps. After this the pulp stumps were observed for brownish to black discolouration of the fixed radicular pulp tissue. Excess ferric sulphate was flushed from the pulp chamber with copious amount of saline and clot remnants were removed from the chamber followed by placement of a thick mix of zinc oxide eugenol into the pulp chamber. Then teeth restored by glass ionomer cement Group 2:Pulpotomy (electrosurgery); n = 15 (1 visit)
The ART‐E1 electrosurgery unit was set at COAG 1 mode to perform both electrofulguration and electrocoagulation. The handpiece with appropriate electrode tips, kept 1 to 2 mm away from the pulpal tissue, was used to deliver the electric current. The duration of application was not more than 2 to 3 seconds followed by a cool down period of 5 seconds. If necessary, this procedure was repeated up to a maximum of three times. After each current application, a new large moist sterile cotton pellet was placed with pressure on the pulpal tissue near to orifice to absorb any blood or tissue fluids before the next current application (e.g. pellet‐electrode‐pellet‐electrode). When properly completed, the pulpal stumps appeared dry and completely blackened. This was followed by placement of a thick mix of zinc oxide eugenol into the pulp chamber. Then teeth restored by glass ionomer cement Group 3:Pulpotomy (diode laser); n = 15 (1 visit)
The remaining coronal pulp tissue was exposed to laser energy through an optical fibre using the diode laser (810 nm, output power: 7 W) set at 3 W of power in Continuous Wave. The laser energy was delivered through a 400 μm diameter optical fibre in a non contact mode with pulp tissue for not more than 2‐3 sec (PD = 2388.53, Fluence = 7165.60). Application of laser was administered until the pulp was ablated and complete haemostasis was achieved. All children and clinical staff wore appropriate eye protection during application of the laser. Applications were administered as per the requirement of each tooth followed by placement of a thick mix of zinc oxide eugenol into the pulp chamber. Then teeth restored by glass ionomer cement |
|
| Outcomes | Clinical success (absence of pain and tenderness, absence of abscess, absence of sinus or fistula, absence of mobility), radiographic success (absence of widened periodontal space, absence of inter‐radicular or periapical radiolucency, absence of sinus or fistula, absence of internal resorption, absence of abnormal canal calcification): evaluation at 1, 3, 6 and 9 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of missing outcomes < 10% children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Yildirim 2016.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Gülhane Military Medical Academy (GMMA) Pediatric Dentistry Clinic. One operator (investigator). |
|
| Participants | 65 children, 140 teeth, age range 5 to 9 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 35 (1 visit)
Group 2:Pulpotomy (MTA); n = 35 (1 visit)
Group 3:Pulpotomy (Portland cement); n = 35 (1 visit)
Group 4:Pulpotomy (EMD); n = 35 (1 visit)
|
|
| Outcomes | Clinical failure (spontaneous pain, swelling, fistula), radiological failure (radiolucency of the periapical or furcation, and pathological external root resorption), overall success, pulp canal obliteration, internal root resorption, marginal adaptation of the crown, crushing or deformities of the crown, changes in occlusion: evaluation at 3, 6, 12, 18, and 24 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of missing outcomes < 10% children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Zealand 2010.
| Methods | RCT, parallel‐arm Teeth randomly assigned Conducted in the Children's Clinic of the University of Michigan School of Dentistry, USA. Operator not mentioned |
|
| Participants | 152 children, 252 teeth, mean age 5.5 years, standard deviation age 1.5 years, age range 2.5 to 10 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 133 (1 visit)
Group 2:Pulpotomy (MTA); n = 119 (1 visit)
|
|
| Outcomes | Clinical success (not clearly defined), radiographic failure (internal root resorption, external root resorption, internal root resorption with perforated form, periradicular lesion), score 2‐1 (clinically), score 2‐2 (clinically), score 2‐3 (clinically), score 2‐4 (clinically), internal resorption, internal resorption with perforated form, external resorption, periodontal ligament widening, pulp canal obliteration, dentine bridge formation, score 7‐1 (rx), score 7‐2 (rx), score 7‐3 (rx), score 7‐4 (rx): evaluation at 6 months (at tooth level) | |
| Notes | 203/252 teeth were available for the 6‐month evaluation resulting in 19% lost at follow‐up Source of funding: "This study was supported by Michigan Institute for Clinical & Health Research and Delta Dental Foundation of Michigan. The authors declare no conflict of interest" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization of the medicament used was done by an envelope draw" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Low risk | Quote: "The blinded clinical examination was performed by 1 to 19 operators who were calibrated to the clinical scoring criteria" |
| Blinding of radiological outcomes assessment | Low risk | Quote: "All radiographs were viewed by 4 blinded, calibrated evaluators" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
Zurn 2008.
| Methods | RCT, split‐mouth Teeth randomly assigned Conducted in the Department of Pediatric Dentistry, Baylor College of Dentistry Texas, Health Science Center, Dallas, Texas, USA. Operator were 2 standardised operators |
|
| Participants | 23 children, 76 teeth, mean age 5.3 years, standard deviation age 1.7 years, age range 2.3 to 8.5 years | |
| Interventions |
Group 1:Pulpotomy (formocresol); n = 38 (1 visit)
Group 2:Pulpotomy (calcium hydroxide); n = 38 (1 visit)
|
|
| Outcomes | Clinical success (not clearly defined), radiographic failure (not clearly defined), overall success (the cumulative rate of failure due to clinical abscesses or osseous radiolucencies was calculated for each treatment, as was an overall cumulative rate of success. These calculations were based on the following equation: failure percentage = 100% x (previous failures + new failures)/(previous failures + currently examined teeth)), abscess, internal resorption, internal resorption with perforated form, external resorption, periodontal ligament widening, calcific metamorphosis, bone radiolucency: evaluation at 0 to 6, 7 to 12 and 13 to 24 months (at tooth level) | |
| Notes | 3 children were lost due to failure to return for follow‐up Analysed: 20 children, 68 teeth Source of funding: quote: "This research project won the Ralph E. MacDonald (sic) Award at the 2006 AAPD annual session for the most outstanding research presented by a graduate student" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin toss |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of clinical outcomes assessment | Unclear risk | Insufficient information to make a clear judgement |
| Blinding of radiological outcomes assessment | Low risk | Quote: "...all postoperative radiographs were digitally scanned and evaluated by 2 standardized and calibrated examiners. To blind the examiners to the treatment regimens, the coronal portions were blackened‐out" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of missing outcomes > 10% children randomly assigned |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to make a clear judgement |
CEM: calcium‐enriched mixture; CH: calcium hydroxide; clin: clinically; EMD: enamel matrix derivative; Er‐YAG: erbium:yttrium‐aluminium garnet; FC: formocresol; FS: ferric sulphate; IRM: intermediate restorative material (reinforced zinc oxide and eugenol); MTA: mineral trioxide aggregate; n: number of teeth; PC: Portland cement; RCT: randomised controlled trial; rx: radiographically; ZOE: zinc oxide and eugenol
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdel‐Aziz 1999 | Not an RCT |
| Aktoren 2000 | Abstract only. Insufficient information presented. Attempts to contact the authors were unsuccessful |
| Ansari 2009 | Abstract only. Insufficient information presented. Attempts to contact the authors were unsuccessful |
| Ayrton 1969 | Not an RCT |
| Badzian‐Kobos 1967 | Not an RCT |
| Barcelos 2011 | Biomaterials not compared |
| Beaver 1966 | Not an RCT |
| Berrebi 2009 | Abstract only. Insufficient information presented. Attempts to contact the authors were unsuccessful |
| Boggs 1969 | Not an RCT |
| Brannstrom 1979 | Human and dog permanent teeth. In vitro study |
| Casas 2003 | Abstract only. Insufficient information presented. Attempts to contact the authors were unsuccessful |
| Chien 2001 | Not an RCT |
| Cuisia 2001 | Abstract only. Insufficient information presented. Attempts to contact the authors were unsuccessful |
| Damle 1999 | Not an RCT |
| Droter 1967 | Review |
| Einwag 1991 | Not an RCT |
| Elomaa 1974 | Not an RCT |
| Fuks 2000 | Abstract only. Insufficient information presented. Attempts to obtain further information from the authors were unsuccessful |
| Grivu 1966 | Not an RCT |
| Hannah 1972 | Not an RCT |
| Hansen 1971 | Not an RCT |
| Hartsook 1966 | Review |
| Ibricevic 2001 | Abstract only. Insufficient information presented. Attempts to contact the authors were unsuccessful |
| Kalaskar 2004 | Not an RCT |
| Keszler 1987 | Not an RCT |
| Kouri 1969 | Not an RCT |
| Liu 2003 | Abstract only. Insufficient information presented. Attempts to contact the authors were unsuccessful |
| Liu 2006 | Not an RCT |
| Lourenço 2015 | Duplicate |
| Louwakul 2011 | Biomaterials not compared |
| Mani 1999 | Not an RCT |
| Massler 1968 | Not an RCT |
| Mejare 1979 | Restorative dentistry |
| NCT01622153 | Ongoing trial comparing pulpotomy formocresol with electrical pulpotomy, which was terminated because use of one of the materials was discontinued |
| Odabas 2007 | Not an RCT |
| Percinoto 2006 | Not an RCT |
| Punwani 1993 | Abstract only. Insufficient information presented. Attempts to contact the authors were unsuccessful |
| Ram 2001 | Abstract only. Insufficient information presented. Attempts to contact the authors were unsuccessful |
| Ravn 1968 | Not an RCT |
| Reddy 1996 | Abstract only. Insufficient information presented. Attempts to contact the authors were unsuccessful |
| Redig 1968 | Not an RCT |
| Riccioli 1971 | Case report |
| Ripa 1971 | Review |
| Ritwik 2003 | Abstract only. Insufficient information presented. Attempts to contact the authors were unsuccessful |
| Rivera 2003 | Not an RCT |
| Rocha 1999 | Abstract only. Insufficient information presented. Attempts to contact the authors were unsuccessful |
| Rule 1966 | Case report |
| Sargenti 1975 | Review |
| Sayegh 1967 | Not an RCT. Intact human teeth |
| Sogbe de Agell 1989 | Not an RCT |
| Szabo 1968 | Abstract only. Insufficient information presented. Attempts to contact the authors were unsuccessful |
| Tsai 1993 | Not an RCT |
| Velkova 1977 | Not an RCT |
| Yakushiji 1969 | Not an RCT |
| Yildiz 2014 | Not an RCT |
Abbreviation ‐ RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
CTRI/2011/06/001776.
| Trial name or title | Root Canal Treatment in Milk Teeth using Three Root Canal Filling Materials: a Double‐Blinded Randomized Controlled Trial |
| Methods | RCT with participant and outcome assessor blinding |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | RC fill Pulpdent root canal sealer Vitapex |
| Outcomes | Clinical evaluation: pain, redness, swelling/abscess, draining fistula and mobility
Radiographic evaluation: furcation radiolucency, periapical radiolucency, internal/external root resorption, deviated eruption of succedaneous teeth, excessive filling material and its resorption Clinical and radiographic evaluation of three root filling materials for a period of 3,6 and 12 months |
| Starting date | 10 June 2011 first recruitment |
| Contact information | Dr R Pramila (Postgraduate (Pedodontics))
Saveetha Dental College
162 Poonamallee Gigh Road
Velappanchavadi
Chennai
Tamil Nadu
600077
India dr.pramee@gmail.com |
| Notes |
NCT00802256.
| Trial name or title | Comparative Evaluation of Pulpotomized Primary Molars With Mineral Trioxide Aggregate and New Endodonthic Cement |
| Methods | "Forty patients are selected randomly. Each patient has at least 2 teeth which require pulpotomy treatment. After removing of carious teeth by a low speed round bur and pulp exposure, roof of pulp chamber is removed completely by a high speed 008 fissure bur. Life tissues of pulp are removed by sharp excavator and rinsing with normal saline. Hemostat is achieved and cavity will be cleaned by 0.5% hypo chlorate solution. MTA or NEC material is mixed according to manufacturer instruction and will be placed in pulp chamber and over pulpal canal orifices for at least 1 mm. The light cure glass ionomer is also mixed according to manufacturer instruction and is placed over the A or B material and cured for 40 minutes. The treated tooth will be restored with a stainless steel crown or amalgam filling material." |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental A: teeth that are treated with Mineral Trioxide Aggregate (MTA) material Experimental B: teeth that are treated with new Endodontic Cement (NEC) material |
| Outcomes | Primary outcome measures: clinical features and radiographic examination Time frame: six months and one year |
| Starting date | October 2008 |
| Contact information | Contact: Fatemeh Shekarchi, student |
| Notes |
NCT00972556.
| Trial name or title | Comparison of Mineral Trioxide Aggregate (MTA) and 20% formocresol in Pulpotomized Human Primary Molars |
| Methods |
|
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Primary: clinically and radiographically outcomes (time frame: six, 12, 18, and 24 months); Secondary: histological outcome (time frame: when the subjective tooth physically exfoliates from oral cavity) |
| Starting date | September 2009 |
| Contact information | Contact: Yuan‐Ling Lee, PhD, Contact: Hsiao‐Hua Chang, MS |
| Notes |
NCT01010451.
| Trial name or title | Antimicrobial Pulpotomy of Primary Molars |
| Methods | None |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | None |
| Starting date | August 2000 |
| Contact information | None |
| Notes |
NCT01591278.
| Trial name or title | MTA and Biodentine in Pulpotomized Primary Molars |
| Methods | Parallel RCT |
| Participants | Age 4 to 9 years Inclusion criteria
Exclusion criteria
|
| Interventions | Biodentine and MTA |
| Outcomes | Primary
Secondary
Time frame for all secondary outcomes: 6 and 12 months |
| Starting date | March 2012 |
| Contact information | Cristina Cuadros, International University of Catalonia |
| Notes |
NCT01733420.
| Trial name or title | Biodentine Versus White MTA Pulpotomy |
| Methods | Parallel RCT |
| Participants |
|
| Interventions | Experimental: Biodentine Active comparator: white mineral trioxide aggregate (MTA) Active comparator: Tempophore |
| Outcomes | Not provided |
| Starting date | October 2011 |
| Contact information | Luc Martens, Ghent University |
| Notes |
NCT02137967.
| Trial name or title | Sodium Hypochlorite Pulpotomies in Primary Molars: Comparison With Conventional 20% Formocresol Pulpotomies |
| Methods | Formocresol is the most universally taught and most widely used pulpotomy medicament in the primary teeth. However, concerns have been raised over the use of formocresol because of its toxicity and potential carcinogenicity. A substitute for formocresol has been investigated but evidence is lacking to conclude which is the most appropriate technique for pulpotomies in primary teeth. Sodium hypochlorite (NaOCl) has been used in root canal irrigant for more than 80 years, and it is at present the most popular irrigant in root canal treatment. Studies have showed that NaOCl is biological compatible and is a very good antimicrobial solution without being a pulpal irritant. Recent studies using sodium hypochlorite as pulpotomy medicament in primary molars showed promising results. In this project, the investigators propose a randomised clinical trial, which will be performed in Paediatric Dentistry Department of the National Taiwan University Hospital, to compare the treatment outcomes between NaOCl and formocresol in human primary molars needing pulpotomy treatment. The aim of this study is to determining whether NaOCl is a suitable replacement for formocresol in the pulpotomy of human primary molar teeth. To assess this aim, 200 healthy children aged from 2.5 to 9 years, who have at least one primary first or second molars diagnosed to receive pulpotomy treatment will be recruited. The involved teeth will be randomly assigned to the control group (dilute 20% formocresol (DFC)) or experimental group (2.5% NaOCl). At three, six, nine, 12, 15, 18, 21 and 24 months post‐treatment, the randomly assigned teeth will be clinically and radiographically evaluated by blinded independent evaluators to the treatment group. The differences will be statistically analysed using Chi² test, Fisher's exact test, and t‐test, using a statistical significance at P < 0.05. |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Primary: change of clinical findings (time frame: at three, six, nine, 12, 15, 18, 21 and 24 months post‐treatment). The outcome will be assessed first by clinical findings. We can discriminate the result are successful or not by scoring the clinical finding from 1 to 4. Criteria for clinical scoring asymptomatic, clinical score = 1 Slight discomfort: percussion sensitivity; mobility > 1 mm but < 2 mm, clinical score = 2 Minor discomfort: long‐lasting chewing sensitivity; gingival swelling; periodontal pocket formation without exudate; mobility> 2 mm but < 3 mm, clinical score = 3 major discomfort: Late pathological changes; spontaneous pain; periodontal pocket formation with exudate; sinus tract; mobility ≧ 3 mm; premature tooth loss due to pathology, clinical score = 4 Secondary: change of radiographic findings (time frame: at three, six, nine, 12, 15, 18, 21 and 24 months post‐treatment). The outcome will be assessed then by radiographic findings. We can discriminate the result are successful or not by scoring the radiographic findings from1 to 5. Criteria for radiographic scoring Dentinal bridge, clinical score = 1, regeneration tissue response No change, clinical score = 2, no pathological changes Pulp canal obliteration, clinical score = 3, slight pathological changes, no clinical significance Periodontal ligament widening, clinical score = 3, slight pathological changes, no clinical significance Internal root resorption (non‐perforated), clinical score = 4, minor pathological changes External root resorption, clinical score = 4, minor pathological changes Internal root resorption (perforated), clinical score = 4, minor pathological changes Peri‐radicular lesion, clinical score = 5, major pathological changes, treatment needed |
| Starting date | August 2011 |
| Contact information | Hsiao‐Hua Chang, PhD |
| Notes |
NCT02201498.
| Trial name or title | Randomized Clinical Trial for Primary Molar Pulpotomy, Biodentine versus Formocresol‐ZOE |
| Methods | None |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | September 2014 |
| Contact information | None |
| Notes |
NCT02286648.
| Trial name or title | Success Rate Evaluation of Miniature Pulpotomy With MTA in Primary Molars |
| Methods | None |
| Participants | 4 years to 7 years (child) Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Primary: successful outcome of treatment as indicated by clinical signs defined with observation and checklist (time frame: up to 12 months) success or failure of treatment defined with observation and checklist Secondary: successful outcome of treatment as indicated by radiographic signs defined with observation and checklist (time frame: up to 12 months) success or failure of treatment defined with observation and checklist |
| Starting date | February 2014 |
| Contact information | None |
| Notes |
NCT02298504.
| Trial name or title | Vital Pulp Treatment in Primary Teeth |
| Methods | Paediatric patients having deep decay in primary molars seen at UMMC, UMSOD, and University of Maryland Rehabilitation and Orthopaedic Institute, will be included in the sample. Teeth with deep caries, > 50% into dentin, will be randomly assigned using a table of random numbers to the three treatment groups: Group 1 pulpotomy with MTA, Group 2 pulpotomy with Biodentine, Group 3 indirect pulp treatment. Treatment will be performed by board certified paediatric dentists or they will directly supervise paediatric dental residents at each site as part of their regular protocol for treating deep caries. Radiographs will be taken as prescribed in the Guideline for taking Radiographs in Children by the American Academy of Pediatric Dentistry. Twice‐yearly clinical examinations will be performed by the treating dentists or paediatric dental residents to check for any soft tissue pathology such as abscess or mobility of treated tooth/teeth. If treatment success/failure consensus between the blinded dentists is not reached, a third dentist will be consulted. The success/failure data will be entered onto spreadsheets and examined statistically using statistical software. |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | November 2015 |
| Contact information | None |
| Notes |
NCT02393326.
| Trial name or title | Biodentine Partial Pulpotomy of Pulpally Exposed Primary Molars |
| Methods |
|
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Primary
Secondary
|
| Starting date | May 2015 |
| Contact information | Contact: Avia Fux‐Noy, DMD, Contact: Hadas Lemberg, PhD |
| Notes |
NCT02702505.
| Trial name or title | Success and Color Stability of MTA Pulpotomized Primary Molars: an RCT (MTA) |
| Methods | This randomised control, split‐mouth trial will use 50 paediatric participants selected from the patient population in the paediatric dental clinics at Baylor College of Dentistry and in select faculty private practices. The study will use a within‐subject control design whereby one tooth will be treated with a pulpotomy using the new formulation of MTA (NeoMTA Plus, Avalon Biomed Inc., Bradenton, FL, USA) and restored with a multi‐surface composite, and the other tooth with an MTA pulpotomy and restored with a SSC; thus, approximately 50 teeth will be treated for each treatment group. The restoration type will be randomised as to which side will receive the SSC or composite using sealed, opaque envelopes. Approximately 50 participants will be needed for the study in order to elicit any significant findings as demonstrated by a power analysis from a similar study. |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | November 2014 |
| Contact information | Carolyn A Kerins, DDS, PhD |
| Notes |
NCT02783911.
| Trial name or title | Comparison of mineral trioxide aggregate (MTA) and ferric sulfate pulpotomies |
| Methods | Comparison of clinical and radiographic success between MTA and ferric sulphate pulpotomies for primary molars. Recall appointments are completed six months, nine months and 12 months. Regular recall and follow‐up will be performed for patients who has MTA or ferric sulphate pulpotomies. Clinical and radiographic findings will be recorded. |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Comparison of the clinical and radiographic success between MTA and ferric sulphate pulpotomies in primary molars (time frame: 1 year). At recall visits six, nine and 12 months, blinded clinical examination will be completed by participating faculty members who are calibrated to clinical scaring criteria. Periapical radiographs will be taken and evaluated by 2 paediatric dentists and 1 endodontist, for presence of various pathologies. Scored based on the criteria established by Zurn and Seale 2008. Scores will be transferred to Microsoft Excel. The difference between the two materials will be analysed using the Mann‐Whitney U test, Chi² test and Fisher's exact test. Intra‐ and inter‐rater agreement will be measured for radiographic assessment using Cohen's kappa test |
| Starting date | April 2016 |
| Contact information | Jung‐Wei Chen, DDS, MS, PhD |
| Notes |
NCT02789423.
| Trial name or title | Clinical and radiographical evaluation of the effect of Dycal and Biodentine in DPC in primary teeth |
| Methods | "The aim of the present study is to compare Calcium Hydroxide cement (Dycal) and Calcium Silicate cement (Biodentine)TM as pulp capping agents in primary molars. The objective of this study include the evaluation of clinical and radiographic efficacy of Calcium Hydroxide cement (Dycal) and Calcium Silicate cement (Biodentine), and their response in direct pulp capping treatment on primary molars during a 6‐month follow‐up. After following the proper standardized procedure for direct pulp cap. In the current study direct pulp capping was performed using calcium hydroxide cement (Dycal) and Calcium Silicate cement (Biodentine) on 60 primary teeth of children equally divided between 2 study groups randomly of both sexes aged 4 to 9 years old. Complete case history was recorded in detail and intraoral periapical radiograph was also taken for teeth indicated for direct pulp capping. Written consent was obtained from the parents of participants before starting the procedure. Strict standardised procedure had been followed and the pulp capping agent (Dycal/Biodentine) were applied according to the manufacturer's instructions. Each participant was evaluated clinically and radiographically for any abnormal clinical signs and symptoms at one, three and six months postoperatively. Better results for the success of the study could be relatively enhanced by close attention to rigid criteria for case selection, standardisation of direct pulp capping procedure and meticulous performance of the procedure appear to be prerequisites for successful treatment." |
| Participants | Inclusion criteria
In addition, the teeth treated by direct pulp capping had only a pinpoint mechanical exposure (0.5 mm to 1 mm), for which haemorrhage control could be achieved within two minutes before proceeding with direct pulp capping. Radiographically, there was absence of internal resorption, external resorption, periapical or furcation radiolucencies and pathology of succedaneous permanent tooth follicle. Exclusion criteria
|
| Interventions |
|
| Outcomes | Evidence of effectiveness of Dycal and Biodentine as Direct Pulp Capping agent in primary molars confirmed by clinical and radiographic evaluation (time frame: 6 months) |
| Starting date | June 2014 |
| Contact information | Dr Komal IM Gandhi, BDS, Dr Mishthu Solanki, MDS |
| Notes |
Differences between protocol and review
None.
Contributions of authors
Conceiving the review ‐ Violaine Smaïl‐Faugeron (VSF), Hélène Fron Chabouis (HFC), Michele Muller‐Bolla (MMB), Anne‐Marie Glenny (AMG) Designing the review ‐ VSF, HFC, Pierre Durieux (PD), Frédéric Courson (FC), AMG Co‐ordinating the review ‐ VSF, HFC Developing the search strategy ‐ VSF, HFC, AMG, Anne Littlewood (AL) Undertaking searches ‐ VSF, HFC, AL Screening search results ‐ VSF, HFC Organising the retrieval of papers ‐ VSF Screening the retrieved papers against inclusion criteria ‐ VSF, HFC Appraising the quality of papers ‐ VSF, HFC Extracting data from papers ‐ VSF, HFC Writing to authors of papers for additional information ‐ VSF, HFC Data management for the review ‐ VSF Entering data into RevMan ‐ VSF Analysis of data ‐ VSF Interpretation of data ‐ VSF, HFC, FC Writing the review ‐ VSF, HFC, PD, FC
Sources of support
Internal sources
School of Dentistry, The University of Manchester, Manchester Academic Health Sciences Centre (MAHSC), the NIHR Manchester Biomedical Research Centre, UK.
School of Dentistry, The University of Manchester, UK.
External sources
-
Cochrane Oral Health Group Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships‐alliances). Contributors over the past year have been the American Association of Public Health Dentistry, USA; AS‐Akademie, Germany; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; NHS Education for Scotland, UK; and the Swiss Society for Endodontology, Switzerland.
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS or the Department of Health.
Declarations of interest
Violaine Smaïl‐Faugeron: none known Anne‐Marie Glenny: none known. I am Deputy Co‐ordinating Editor with Cochrane Oral Health. Frédéric Courson: none known Pierre Durieux: has received in the past three years research grants from the French Ministry of Health (PREQHOS, PHRC). He has been member of scientific committees of ANR (Agence Nationale de la Recherche), PREQHOS (Projets de Recherche en qualité hospitalière) and PREPS (Projets de Recherche en Performance de Soins) of the French Ministry of Health. He has received consultancies from Amgen and ONYX, and the Haute Autorité de Santé (HAS). The author has no financial relationships with any organisations that might have an interest in the review in the previous three years (except the fact that the author is employee of an acute care hospital). Michele Muller‐Bolla: none known Hélène Fron Chabouis: our lab (URB2i, Universite Paris Descartes) has developed industrial partnerships but these did not have any link with this review and did not influence my work.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Aeinehchi 2007 {published data only}
Agamy 2004 {published data only}
- Agamy HA, Bakry NS, Mounir MM, Avery DR. Comparison of mineral trioxide aggregate and formocresol as pulp‐capping agents in pulpotomized primary teeth. Pediatric Dentistry 2004; Vol. 26, issue 4:302‐9. [PubMed]
Akcay 2014 {published data only}
- Akcay M, Sari S. The effect of sodium hypochlorite application on the success of calcium hydroxide and mineral trioxide aggregate pulpotomies in primary teeth. Pediatric Dentistry 2014;36(4):316‐21. [PubMed] [Google Scholar]
Alaçam 1989 {published and unpublished data}
- Alaçam A. Long term effects of primary teeth pulpotomies with formocresol, glutaraldehyde‐calcium and glutaraldehyde‐zinc oxide eugenol on succudaneous teeth. Journal of Pedodontics 1989;13(4):307‐13. [PubMed] [Google Scholar]
- Alaçam A. Pulpal tissue changes following pulpotomies with formocresol, glutaraldehyde‐calcium hydroxide, glutaraldehyde‐zinc oxide eugenol pastes in primary teeth. Journal of Pedodontics 1989;13:123‐32. [PubMed] [Google Scholar]
Alaçam 2009 {published data only}
- Alaçam A, Odabaş ME, Tüzüner T, Sillelioğlu H, Baygin O. Clinical and radiographic outcomes of calcium hydroxide and formocresol pulpotomies performed by dental students. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics 2009;108(5):e127‐33. [DOI] [PubMed] [Google Scholar]
Al‐Ostwani 2016 {published data only}
- Al‐Ostwani AO, Al‐Monaqel BM, Al‐Tinawi MK. A clinical and radiographic study of four different root canal fillings in primary molars. Journal of the Indian Society of Pedodontics and Preventive Dentistry 2016;34:55‐9. [DOI] [PubMed] [Google Scholar]
Aminabadi 2010 {published data only}
- Aminabadi NA, Farahani RM, Oskouei SG. Formocresol versus calcium hydroxide direct pulp capping of human primary molars: two year follow‐up. Journal of Clinical Pediatric Dentistry 2010;34(4):317‐21. [PUBMED: 20831133] [DOI] [PubMed] [Google Scholar]
Aminabadi 2016 {published data only}
- Asl Aminabadi N, Satrab S, Najafpour E, Samiei M, Jamali Z, Shirazi S. A randomized trial of direct pulp capping in primary molars using MTA compared to 3Mixtatin: a novel pulp capping biomaterial. International Journal of Paediatric Dentistry 2016;26(4):281‐90. [DOI] [PubMed] [Google Scholar]
Ansari 2010 {published data only}
- Ansari G, Ranjpour M. Mineral trioxide aggregate and formocresol pulpotomy of primary teeth: a 2‐year follow‐up. International Endodontic Journal 2010;43(5):413‐8. [PUBMED: 20518934] [DOI] [PubMed] [Google Scholar]
Arikan 2016 {published data only}
- Arikan V, Sonmez H, Sari S. Comparison of two base materials regarding their effect on root canal treatment success in primary molars with furcation lesions. BioMed Research International 2016;2016:1429286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bahrololoomi 2008 {published data only}
Bezgin 2016 {published data only}
- Bezgin T, Ozgul BM, Arikan V, Sari S. Root canal filling in primary molars without successors: Mineral trioxide aggregate versus gutta‐percha/AH‐Plus. Australian Endodontic Journal 2016;42:73‐81. [DOI] [PubMed] [Google Scholar]
Cantekin 2014 {published data only}
- Cantekin K, Gümüş H. Success rates of ankaferd blood stopper and ferric sulfate as pulpotomy agents in primary molars. International Scholarly Research Notices 2014;2014:819605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Casas 2004 {published data only}
- Casas MJ, Kenny DJ, Johnston DH, Judd PL. Long‐term outcomes of primary molar ferric sulfate pulpotomy and root canal therapy. Pediatric Dentistry 2004;26(1):44‐8. [PubMed] [Google Scholar]
- Casas MJ, Layug MA, Kenny DJ, Johnston DH, Judd PL. Two‐year outcomes of primary molar ferric sulfate pulpotomy and root canal therapy. Pediatric Dentistry 2003;25(2):97‐102. [PUBMED: 12723832] [PubMed] [Google Scholar]
Celik 2013 {published data only}
- Celik B, Ataç AS, Cehreli ZC, Uysal S. A randomized trial of mineral trioxide aggregate cements in primary tooth pulpotomies. Journal of Dentistry for Children (Chicago, Ill.) 2013;80(3):126‐32. [PubMed] [Google Scholar]
Chandra 2014 {published data only}
- Chandra SP, Chandrasekhar R, Uloopi KS, Vinay C, Kumar NM. Success of root fillings with zinc oxide‐ozonated oil in primary molars: preliminary results. European Archives of Paediatric Dentistry 2014;15(3):191‐5. [DOI] [PubMed] [Google Scholar]
Chen 2015 {published data only}
- Chen XX, Lin BC, Zhong J, Ge LH. Degradation evaluation and success of pulpectomy with a modified primary root canal filling in primary molars. Beijing Da Xue Xue Bao Yi Xue Ban 2015;47(3):529‐35. [PubMed] [Google Scholar]
Coser 2008 {published data only}
- Coser RM, Gondim JO, Aparecida Giro EM. Evaluation of 2 endodontic techniques used to treat human primary molars with furcation radiolucency area: a 48‐month radiographic study. Quintessence International 2008;39(7):549‐57. [PubMed] [Google Scholar]
Cuadros‐Fernández 2016 {published data only}
- Cuadros‐Fernández C, Lorente Rodríguez AI, Sáez‐Martínez S, García‐Binimelis J, About I, Mercadé M. Short‐term treatment outcome of pulpotomies in primary molars using mineral trioxide aggregate and Biodentine: a randomized clinical trial. Clinical Oral Investigations 2016;20(7):1639‐45. [DOI] [PubMed] [Google Scholar]
Dean 2002 {published and unpublished data}
- Dean JA, Mack RB, Fulkerson BT, Sanders BJ. Comparison of electrosurgical and formocresol pulpotomy procedures on children. International Journal of Paediatric Dentistry 2002;12:177‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Demir 2007 {published data only}
- Demir T, Cehreli ZC. Clinical and radiographic evaluation of adhesive pulp capping in primary molars following hemostasis with 1.25% sodium hypochlorite: 2‐year results. American Journal of Dentistry 2007;20(3):182‐8. [PubMed] [Google Scholar]
Doyle 2010 {published data only}
- Doyle TL, Casas MJ, Kenny DJ, Judd PL. Mineral trioxide aggregate produces superior outcomes in vital primary molar pulpotomy. Pediatric Dentistry 2010;32(1):41‐7. [PUBMED: 20298652] [PubMed] [Google Scholar]
Durmus 2014 {published data only}
- Durmus B, Tanboga I. In vivo evaluation of the treatment outcome of pulpotomy in primary molars using diode laser, formocresol, and ferric sulphate. Photomedicine and Laser Surgery 2014;32(5):289‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eidelman 2001 {published data only}
- Eidelman E, Holan G, Fuks AB. Mineral trioxide aggregate vs. formocresol in pulpotomized primary molars: a preliminary report. Pediatric Dentistry 2001;23(1):15‐8. [MEDLINE: ] [PubMed] [Google Scholar]
El Meligy 2016 {published data only}
- Meligy O A, Allazzam S, Alamoudi N M. Comparison between biodentine and formocresol for pulpotomy of primary teeth: A randomized clinical trial. Quintessence International 2016;47(7):571‐80. [DOI] [PubMed] [Google Scholar]
Erdem 2011 {published data only}
- Erdem AP, Guven Y, Balli B, Ilhan B, Sepet E, Ulukapi I, et al. Success rates of mineral trioxide aggregate, ferric sulfate, and formocresol pulpotomies: a 24‐month study. Pediatric Dentistry 2011;33(2):165‐70. [PUBMED: 21703067] [PubMed] [Google Scholar]
Fallahinejad Ghajari 2013 {published data only}
- Fallahinejad Ghajari M, Asgharian Jeddi T, Iri S, Asgary S. Direct pulp‐capping with calcium enriched mixture in primary molar teeth: a randomized clinical trial. Iranian Endodontic Journal 2010;5(1):27‐30. [PMC free article] [PubMed] [Google Scholar]
- Fallahinejad Ghajari M, Asgharian Jeddi T, Iri S, Asgary S. Treatment outcomes of direct pulp‐capping after 20 months: a randomized controlled trial. Iranian Endodontic Journal 2013;8(4):149‐52. [PMC free article] [PubMed] [Google Scholar]
Farsi 2005 {published data only}
- Farsi N, Alamoudi N, Balto K, Mushayt A. Success of mineral trioxide aggregate in pulpotomized primary molars. Journal of Clinical Pediatric Dentistry 2005;29(4):307‐11. [DOI] [PubMed] [Google Scholar]
Fei 1991 {published and unpublished data}
- Fei AL, Udin RD, Johnson R. A clinical study of ferric sulfate as a pulpotomy agent in primary teeth. Pediatric Dentistry 1991;13(6):327‐32. [MEDLINE: ] [PubMed] [Google Scholar]
Fernandes 2015 {published data only}
- Fernandes A P, Lourenco Neto N, Teixeira Marques N C, Silveira Moretti A B, Sakai V T, Cruvinel Silva T, et al. Clinical and radiographic outcomes of the use of Low‐Level Laser Therapy in vital pulp of primary teeth. International Journal of Paediatric Dentistry 2015;25(2):144‐50. [DOI] [PubMed] [Google Scholar]
Fernández 2013 {published data only}
- Fernández CC, Martínez SS, Jimeno FG, Lorente Rodríguez AI, Mercadé M. Clinical and radiographic outcomes of the use of four dressing materials in pulpotomized primary molars: a randomized clinical trial with 2‐year follow‐up. International Journal of Paediatric Dentistry 2013;23(6):400‐7. [DOI] [PubMed] [Google Scholar]
Fishman 1996 {published data only}
- Fishman SA, Udin RD, Good DL, Rodef F. Success of electrofulguration pulpotomies covered by zinc oxide and eugenol or calcium hydroxide: a clinical study. Pediatric Dentistry 1996;18(5):385‐90. [MEDLINE: ] [PubMed] [Google Scholar]
Fuks 1997 {published and unpublished data}
- Fuks AB, Holan G, Davis JM, Eidelman E. Ferric sulfate versus dilute formocresol in pulpotomized primary molars: long‐term follow up. Pediatric Dentistry 1997;19(5):327‐30. [PubMed] [Google Scholar]
Garrocho‐Rangel 2009 {published data only}
- Garrocho‐Rangel A, Flores H, Silva‐Herzog D, Hernandez‐Sierra F, Mandeville P, Pozos‐Guillen AJ. Efficacy of EMD versus calcium hydroxide in direct pulp capping of primary molars: a randomized controlled clinical trial. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics 2009;107(5):733‐8. [DOI] [PubMed] [Google Scholar]
Goyal 2014 {published data only}
- Goyal S, Abuwala T, Joshi K, Mehta J, Indushekar KR, Hallikerimath S. The clinical, radiographic and histological evaluation of three different concentrations of formocresol as a pulpotomy agent. Journal of International Oral Health 2014;6(2):118‐25. [PMC free article] [PubMed] [Google Scholar]
Goyal 2016 {published data only}
- Goyal P, Pandit IK, Gugnani N, Gupta M, Goel R, Gambhir RS. Clinical and radiographic comparison of various medicaments used for pulpotomy in primary molars: A randomized clinical trial. European Journal of Dentistry 2016;10(3):315‐20. [PUBMED: 27403046] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grewal 2016 {published data only}
- Grewal N, Salhan R, Kaur N, Patel H B. Comparative evaluation of calcium silicate‐based dentin substitute (Biodentine(R)) and calcium hydroxide (pulpdent) in the formation of reactive dentin bridge in regenerative pulpotomy of vital primary teeth: Triple blind, randomized clinical trial. Contemporary Clinical Dentistry 2016;7(4):457‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2015 {published data only}
- Gupta G, Rana V, Srivastava N, Chandna P. Laser Pulpotomy‐An Effective Alternative to Conventional Techniques: A 12 Months Clinicoradiographic Study. Jaypees International Journal of Clinical Pediatric Dentistry 2015;8(1):18‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haghgoo 2009 {published data only}
- Haghgoo R, MollaAsadolla F, Abbasi F. Evaluation of clinical and radiographic success rates of root MTA and formocresol in pulpotomy of primary molars. Journal of Dental Medicine 2009;22(2):136‐41. [Google Scholar]
Holan 2005 {published data only}
- Holan G, Eidelman E, Fuks AB. Long‐term evaluation of pulpotomy in primary molars using mineral trioxide aggregate or formocresol. Pediatric Dentistry 2005; Vol. 27, issue 2:129‐36. [PubMed]
Huth 2005 {published data only}
- Huth KC, Paschos E, Hajek‐Al‐Khatar N, Hollweck R, Crispin A, Hickel R, et al. Effectiveness of 4 pulpotomy techniques ‐ randomized controlled trial. Journal of Dental Research 2005;84(12):1144‐8. [DOI] [PubMed] [Google Scholar]
Ibricevic 2000 {published and unpublished data}
- Ibricevic H, Al‐Jame Q. Ferric sulfate as pulpotomy agent in primary teeth: twenty month clinical follow‐up. Journal of Clinical Pediatric Dentistry 2000;24(4):269‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ibricevic H, Al‐Jame Q. Ferric sulphate and formocresol in pulpotomy of primary molars: long term follow‐up study. European Journal of Paediatric Dentistry 2003;4(1):28‐32. [PUBMED: 12870985] [PubMed] [Google Scholar]
Jayam 2014 {published data only}
- Jayam C, Mitra M, Mishra J, Bhattacharya B, Jana B. Evaluation and comparison of white mineral trioxide aggregate and formocresol medicaments in primary tooth pulpotomy: clinical and radiographic study. Journal of Indian Society of Pedodontics and Preventive Dentistry 2014;32(1):13‐8. [DOI] [PubMed] [Google Scholar]
Kalra 2017 {published data only}
- Kalra M, Garg N, Rallan M, Pathivada L, Yeluri R. Comparative evaluation of fresh aloe barbadensis plant extract and mineral trioxide aggregate as pulpotomy agents in primary molars: a 12‐month follow‐up study. Contemporary Clinical Dentistry 2017;8(1):106‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 2015 {published data only}
- Kang C‐M, Kim S‐H, Shin Y, Lee H‐S, Lee J‐H, Kim GT, et al. A randomized controlled trial of ProRoot MTA, Ortho MTA and Retro MTA for pulpotomy in primary molars. Oral Diseases 2015;21(6):785‐91. [DOI] [PubMed] [Google Scholar]
Khorakian 2014 {published data only}
- Khorakian F, Mazhari F, Asgary S, Sahebnasagh M, Alizadeh Kaseb A, Movahhed T, et al. Two‐year outcomes of electrosurgery and calcium‐enriched mixture pulpotomy in primary teeth: a randomised clinical trial. European Archives of Paediatric Dentistry 2014;15(4):223‐8. [DOI] [PubMed] [Google Scholar]
Kusum 2015 {published data only}
- Kusum B, Rakesh K, Richa K. Clinical and radiographical evaluation of mineral trioxide aggregate, biodentine and propolis as pulpotomy medicaments in primary teeth. Restorative Dentistry and Endodontics 2015;40(4):276‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2011 {published data only}
- Liu H, Zhou Q, Qin M. Mineral trioxide aggregate versus calcium hydroxide for pulpotomy in primary molars. Chinese Journal of Dental Research 2011;14(2):121‐5. [PubMed] [Google Scholar]
Lourenço 2015a {published data only}
- Lourenço Neto N, Marques NC, Fernandes AP, Hungaro Duarte MA, Abdo RC, Machado MA, et al. Clinical and radiographic evaluation of Portland cement added to radiopacifying agents in primary molar pulpotomies. European Archives of Paediatric Dentistry 2015;16(5):377‐82. [PUBMED: 25788172] [DOI] [PubMed] [Google Scholar]
Malekafzali 2011 {published data only}
- Malekafzali B, Shekarchi F, Asgary S. Treatment outcomes of pulpotomy in primary molars using two endodontic biomaterials. A 2‐year randomised clinical trial. European Journal of Paediatric Dentistry 2011;12(3):189‐93. [PUBMED: 22077689] [PubMed] [Google Scholar]
Markovic 2005 {published data only}
- Markovic D, Zivojinovic V, Vucetic M. Evaluation of three pulpotomy medicaments in primary teeth. European Journal of Paediatric Dentistry 2005; Vol. 6, issue 3:133‐8. [PubMed]
Moretti 2008 {published data only}
Mortazavi 2004 {published data only}
Nadkarni 2000 {published data only}
- Nadkarni U, Damle SG. Comparative evaluation of calcium hydroxide and zinc oxide eugenol as root canal filling materials for primary molars: a clinical and radiographic study. Journal of the Indian Society of Pedodontics and Preventive Dentistry 2000;18(1):1‐10. [MEDLINE: ] [PubMed] [Google Scholar]
Naik 2005 {published data only}
- Naik S, Hegde AH. Mineral trioxide aggregate as a pulpotomy agent in primary molars: an in vivo study. Journal of the Indian Society of Pedodontics and Preventive Dentistry 2005;23(1):13‐6. [PUBMED: 15858300] [DOI] [PubMed] [Google Scholar]
Nakornchai 2010 {published data only}
- Nakornchai S, Banditsing P, Visetratana N. Clinical evaluation of 3Mix and Vitapex as treatment options for pulpally involved primary molars. International Journal of Paediatric Dentistry 2010;20(3):214‐21. [PUBMED: 20409203] [DOI] [PubMed] [Google Scholar]
Nguyen 2017 {published data only}
- Nguyen T D, Judd P L, Barrett E J, Sidhu N, Casas M J. Comparison of ferric sulfate combined mineral trioxide aggregate pulpotomy and zinc oxide eugenol pulpectomy of primary maxillary incisors: an 18‐month randomized, controlled trial. Pediatric Dentistry 2017;39(1):34‐8. [PubMed] [Google Scholar]
Niranjani 2015 {published data only}
- Niranjani K, Prasad MG, Vasa AA, Divya G, Thakur MS, Saujanya K. Clinical evaluation of success of primary teeth pulpotomy using Mineral Trioxide Aggregate(®), laser and Biodentine(TM)‐ an in vivo study. Journal of Clinical and Diagnostic Research 2015;9(4):ZC35‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Noorollahian 2008 {published data only}
Olatosi 2015 {published data only}
- Olatosi OO, Sote EO, Orenuga OO. Effect of mineral trioxide aggregate and formocresol pulpotomy on vital primary teeth: a clinical and radiographic study. Nigerian Journal of Clinical Practice 2015;18(2):292‐6. [DOI] [PubMed] [Google Scholar]
Oliveira 2013a {published data only}
- Oliveira TM, Moretti AB, Sakai VT, Lourenço Neto N, Santos CF, Machado MA, et al. Clinical, radiographic and histologic analysis of the effects of pulp capping materials used in pulpotomies of human primary teeth. European Archives of Paediatric Dentistry 2013;14(2):65‐71. [PUBMED: 23549993] [DOI] [PubMed] [Google Scholar]
Ozalp 2005 {published data only}
- Ozalp N, Saroğlu I, Sönmez H. Evaluation of various root canal filling materials in primary molar pulpectomies: an in vivo study. American Journal of Dentistry 2005; Vol. 18, issue 6:347‐50. [PubMed]
Ozmen 2017 {published data only}
- Ozmen B, Bayrak S. Comparative evaluation of ankaferd blood stopper, ferric sulfate, and formocresol as pulpotomy agent in primary teeth: A clinical study. Nigerian Journal of Clinical Practice 2017;20(7):832‐8. [DOI] [PubMed] [Google Scholar]
Pinky 2011 {published data only}
- Pinky C, Shashibhushan KK, Subbareddy VV. Endodontic treatment of necrosed primary teeth using two different combinations of antibacterial drugs: an in vivo study. Journal of the Indian Society of Pedodontics and Preventive Dentistry 2011;29(2):121‐7. [PUBMED: 21911950] [DOI] [PubMed] [Google Scholar]
Prabhakar 2008 {published data only}
- Prabhakar AR, Sridevi E, Raju OS, Satish V. Endodontic treatment of primary teeth using combination of antibacterial drugs: an in vivo study. Journal of the Indian Society of Pedodontics and Preventive Dentistry 2008;26(5):5‐10. [PubMed] [Google Scholar]
Pramila 2016 {published data only}
- Pramila R, Muthu M S, Deepa G, Farzan J M, Rodrigues S J. Pulpectomies in primary mandibular molars: a comparison of outcomes using three root filling materials. International Endodontic Journal 2016;49(5):413‐21. [DOI] [PubMed] [Google Scholar]
Rajasekharan 2017 {published data only}
- Rajasekharan S, Martens L C, Vandenbulcke J, Jacquet W, Bottenberg P, Cauwels R G. Efficacy of three different pulpotomy agents in primary molars: a randomized control trial. International Endodontic Journal 2017;50(3):215‐28. [DOI] [PubMed] [Google Scholar]
Ramar 2010 {published data only}
- Ramar K, Mungara J. Clinical and radiographic evaluation of pulpectomies using three root canal filling materials: an in‐vivo study. Journal of the Indian Society of Pedodontics and Preventive Dentistry 2010;28(1):25‐9. [PUBMED: 20215668] [DOI] [PubMed] [Google Scholar]
Rewal 2014 {published data only}
- Rewal N, Thakur A S, Sachdev V, Mahajan N. Comparison of endoflas and zinc oxide eugenol as root canal filling materials in primary dentition. Journal of Indian Society of Pedodontics and Preventive Dentistry 2014;32(4):317‐21. [DOI] [PubMed] [Google Scholar]
Sabbarini 2008 {published data only}
Sakai 2009 {published data only}
- Sakai VT, Moretti ABS, Oliveira TM, Fornetti APC, Santos CF, Machado M, et al. Summary of pulpotomy of human primary molars with MTA and Portland cement: a randomised controlled trial. British Dental Journal 2009;207(3):128‐9. [0007‐0610] [DOI] [PubMed] [Google Scholar]
Saltzman 2005 {published data only}
- Saltzman B, Sigal M, Clokie C, Rukavina J, Titley K, Kulkarni GV. Assessment of a novel alternative to conventional formocresol‐zinc oxide eugenol pulpotomy for the treatment of pulpally involved human primary teeth: diode laser‐mineral trioxide aggregate pulpotomy. International Journal of Paediatric Dentistry 2005; Vol. 15, issue 6:437‐47. [DOI] [PubMed]
Shabzendedar 2013 {published data only}
- Shabzendedar M, Mazhari F, Alami M, Talebi M. Sodium hypochlorite vs formocresol as pulpotomy medicaments in primary molars: 1‐year follow‐up. Pediatric Dentistry 2013;35(4):329‐32. [PubMed] [Google Scholar]
Shumayrikh 1999 {published and unpublished data}
- Shumayrikh NM, Adenubi JO. Clinical evaluation of glutaraldehyde with calcium hydroxide and glutaraldehyde with zinc oxide eugenol in pulpotomy of primary molars. Endodontics and Dental Traumatology 1999;15(6):259‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sonmez 2008 {published data only}
- Sonmez D, Sari S, Cetinbaş T. A comparison of four pulpotomy techniques in primary molars: a long‐term follow‐up. Journal of Endodontics 2008;34(8):950‐5. [DOI] [PubMed] [Google Scholar]
Subramaniam 2009 {published data only}
Subramaniam 2011 {published data only}
- Subramaniam P, Gilhotra K. Endoflas, zinc oxide eugenol and Metapex as root canal filling materials in primary molars ‐ a comparative clinical study. Journal of Clinical Pediatric Dentistry 2011;35(4):365‐9. [PUBMED: 22046693] [DOI] [PubMed] [Google Scholar]
Trairatvorakul 2008 {published data only}
- Trairatvorakul C, Chunlasikaiwan S. Success of pulpectomy with zinc oxide‐eugenol vs calcium hydroxide/iodoform paste in primary molars: a clinical study. Pediatric Dentistry 2008; Vol. 30, issue 4:303‐8. [PubMed]
Tuna 2008 {published data only}
Uloopi 2016 {published data only}
- Uloopi KS, Vinay C, Ratnaditya A, Gopal AS, Mrudula KJ, Rao RC. Clinical evaluation of low level diode laser application for primary teeth pulpotomy. Journal of Clinical and Diagnostic Research 2016;10(1):ZC67‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ulusoy 2014a {published data only}
- Ulusoy AT, Bayrak S, Bodrumlu EH. Clinical and radiological evaluation of calcium sulfate as direct pulp capping material in primary teeth. European Journal of Paediatric Dentistry 2014;15(2):127‐31. [PUBMED: 25102461] [PubMed] [Google Scholar]
Vargas 2006 {published data only}
- Vargas KG, Packham B, Lowman D. Preliminary evaluation of sodium hypochlorite for pulpotomies in primary molars. Pediatric Dentistry 2006; Vol. 28, issue 6:511‐7. [PubMed]
Waterhouse 2000 {published and unpublished data}
- Waterhouse PJ, Nunn JH, Whitworth JM. An investigation of the relative efficacy of Buckley's formocresol and calcium hydroxide in primary molar vital pulp therapy. British Dental Journal 2000;188(1):32‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Waterhouse PJ, Nunn JH, Whitworth JM. Prostaglandin E2 and treatment outcome in pulp therapy of primary molars with carious exposures. International Journal of Paediatric Dentistry 2002;12(2):116‐23. [PUBMED: 11966889] [DOI] [PubMed] [Google Scholar]
Yadav 2014 {published data only}
- Yadav P, Indushekar K, Saraf B, Sheoran N, Sardana D. Comparative evaluation of Ferric Sulfate, Electrosurgical and Diode Laser on human primary molars pulpotomy: an "in‐vivo" study. Laser Therapy 2014;23(1):41‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yildirim 2016 {published data only}
- Yildirim C, Basak F, Akgun OM, Polat GG, Altun C. Clinical and radiographic evaluation of the effectiveness of formocresol, mineral trioxide aggregate, portland cement, and enamel matrix derivative in primary teeth pulpotomies: a two year follow‐Up. Journal of Clinical Pediatric Dentistry 2016;40(1):14‐20. [DOI] [PubMed] [Google Scholar]
Zealand 2010 {published data only}
- Zealand CM, Briskie DM, Botero TM, Boynton JR, Hu JC. Comparing gray mineral trioxide aggregate and diluted formocresol in pulpotomized human primary molars. Pediatric Dentistry 2010;32(5):393‐9. [PUBMED: 21070705] [PMC free article] [PubMed] [Google Scholar]
Zurn 2008 {published data only}
- Zurn D, Seale NS. Light‐cured calcium hydroxide vs formocresol in human primary molar pulpotomies: a randomized controlled trial. Pediatric Dentistry 2008; Vol. 30, issue 1:34‐41. [PubMed]
References to studies excluded from this review
Abdel‐Aziz 1999 {published data only}
- Abdel‐Aziz A, Abdella A. Clinical and histological evaluation of three antiseptics used in abscessed primary molars. Pediatric Dentistry 1999;21(5):115. [Google Scholar]
Aktoren 2000 {published and unpublished data}
- Aktoren O, Gencay K. A two year clinical‐radiographic follow‐up of the pulpotomies in primary molars. Journal of Dental Research 2000;79:543 (Abs No 3193). [Google Scholar]
Ansari 2009 {published and unpublished data}
- Ansari G, Vahid Golpaygani M, Chitsazan I, Fekrazad R. Clinical and radiographic evaluation of diode laser pulpotomy on human primary teeth: a preliminary study. Lasers in Medical Science Conference: World Federation for Laser Dentistry (WFLD). Hong Kong, 2009; Vol. 24(3):470‐1.
Ayrton 1969 {published data only}
- Ayrton Ode T, Benfatti SV, Andrioni JN. Clinical and morphological study of a therapeutic agent with formaldehyde base for the preservation of the deciduous teeth [Estudio clínico y morfológico de un medicamento con base de formaldehida para la conservación de los dientes temporales]. El Cooperador Dental; Cooperativismo, Información y Ciencia Odontológica 1969;35(1):16‐21. [PUBMED: 5255812] [PubMed] [Google Scholar]
Badzian‐Kobos 1967 {published data only}
- Badzian‐Kobos K, Walczak A. Clinical evaluation of the results of treatment of live dental pulp with Ledermix and Calxyl in some pulp diseases in children [Kliniczna ocena wynikow leczenia przyzyciowego niektorych stanow chorobowych miazgi zebow dzieciecych preparatami "Ledermix" i "Calxyl"]. Czasopismo Stomatologiczne 1967;20(11):1139‐43. [PUBMED: 4965461] [PubMed] [Google Scholar]
Barcelos 2011 {published data only}
- Barcelos R, Tannure PN, Rosario YMRV, Luiz RR, Gleiser R, Primo L. Permanent successors effects after smear layer removal in deciduous pulpectomies [abstract]. Proceedings of the 89th General Session of the International Association for Dental Research; 2011, Mar 16‐19; San Diego, California, United States. 2011:Abstract no: 3144.
Beaver 1966 {published data only}
- Beaver HA, Kopel HM, Sabes WR. The effect of zinc oxide ‐ eugenol cement on a formocresolized pulp. Journal of Dentistry for Children 1966;33:381‐96. [MEDLINE: ] [PubMed] [Google Scholar]
Berrebi 2009 {published and unpublished data}
- Berrebi J, Heysselaer D, Nyssen‐Behets C, Rocca JP, Mahler P, Limme M, et al. Clinical treatment of exposed pulp by direct capping/pulpotomy on primary and permanent immature teeth. Lasers in Medical Science Conference: World Federation for Laser Dentistry (WFLD). Hong Kong, 2009; Vol. 24(3):472.
Boggs 1969 {published data only}
- Boggs DC. Simple technique for treating non‐vital deciduous teeth ‐ a study. Northwest Dentistry 1969;48(2):102‐4. [PUBMED: 5253110] [PubMed] [Google Scholar]
Brannstrom 1979 {published data only}
- Brannstrom M, Nyborg H, Stromberg T. Experiments with pulp capping. Oral Surgery, Oral Medicine, and Oral Pathology 1979;48(4):347‐52. [PUBMED: 291861] [DOI] [PubMed] [Google Scholar]
Casas 2003 {published data only}
- Casas M, Kenny D, Johnston D, Judd P, Layug M. Three‐year prospective outcome study of ferric sulphate pulpotomies and root canal treatment in vital primary molars. International Journal of Paediatric Dentistry 2003;13 Suppl 1:9. [Google Scholar]
Chien 2001 {published data only}
- Chien MM, Setzer S, Cleaton‐Jones P. How does zinc oxide‐eugenol compare to ferric sulphate as a pulpotomy material?. Journal of the South African Dental Association 2001;56(3):130‐5. [CN‐00567178] [PubMed] [Google Scholar]
Cuisia 2001 {published data only}
- Cuisia ZE, Musselman R, Schneider P, Dummett C. A study of mineral trioxide aggregate pulpotomies in primary molars. Pediatric Dentistry 2001;23(2):168. [Google Scholar]
Damle 1999 {published data only}
- Damle SG, Nadkarni U. A comparative study of two root canal resorbable filling materials in primary molars. International Journal of Paediatric Dentistry 1999;9 Suppl 1:86 (Abs No 35.03). [Google Scholar]
Droter 1967 {published data only}
- Droter JA. Pulp therapy in primary teeth. Journal of Dentistry for Children 1967;34(6):507‐10. [PUBMED: 4864184] [PubMed] [Google Scholar]
Einwag 1991 {published data only}
- Einwag J. Endodontics in primary dentition [Endodontie im milchgebiss]. Zahnarztliche Mitteilungen 1991;81(9):878‐84. [PUBMED: 1906661] [PubMed] [Google Scholar]
Elomaa 1974 {published data only}
- Elomaa M, Rantanen L, Nystrom M. Use of chemotherapeutic‐corticoid combination in vital pulpotomy. Proceedings of the Finnish Dental Society. Suomen Hammaslaakariseuran Toimituksia 1974;70(1):1‐6. [PUBMED: 4821944] [PubMed] [Google Scholar]
Fuks 2000 {published data only}
- Fuks AB, Papagiannoulis L, Geki S, Koulatzidou M, Polychronopoulou A. One‐year comparative study in pulpotomized primary teeth using ferric sulfate and diluted formocresol. European Journal of Paediatric Dentistry 2000;1(3):128 (Abs No 98). [Google Scholar]
Grivu 1966 {published data only}
- Grivu O, Mecher E, Stoica E. Principles and present status of the therapy of pulp diseases in children [Principiile si stadiul actual al terapiei pulpopatiilor la copii]. Stomatologia 1966;13(3):233‐44. [PUBMED: 5224151] [PubMed] [Google Scholar]
Hannah 1972 {published data only}
- Hannah DR. Glutaraldehyde and calcium hydroxide. A pulp dressing material. British Dental Journal 1972;132(6):227‐31. [PUBMED: 4504039] [DOI] [PubMed] [Google Scholar]
Hansen 1971 {published data only}
- Hansen HP, Ravn JJ, Ulrich D. Vital pulpotomy in primary molars. A clinical and histologic investigation of the effect of zinc oxide‐eugenol cement and Ledermix. Scandinavian Journal of Dental Research 1971;79:13‐23. [PubMed] [Google Scholar]
Hartsook 1966 {published data only}
- Hartsook JT. Pulpal therapy in primary and young permanent teeth. Dental Clinics of North America 1966:377‐89. [PUBMED: 4222772] [PubMed]
Ibricevic 2001 {published and unpublished data}
- Ibricevic H, Al‐Jame G. Ferric sulfate as pulpotomy agent in primary teeth: clinical study. Journal of Dental Research 2001;80(4):1216 (Abs No 123). [Google Scholar]
Kalaskar 2004 {published data only}
- Kalaskar RR, Damle SG. Comparative evaluation of lyophilized freeze dried platelet derived preparation with calcium hydroxide as pulpotomy agents in primary molars. Journal of the Indian Society of Pedodontics and Preventive Dentistry 2004;22(1):24‐9. [PubMed] [Google Scholar]
Keszler 1987 {published data only}
- Keszler A, Dominguez FV. Current status of pulp treatment with formocresol [Estado actual del tratamiento pulpar con formocresol]. Revista Española de Endodoncia 1987;5(2):63‐70. [PUBMED: 3483227] [PubMed] [Google Scholar]
Kouri 1969 {published data only}
- Kouri EM, Matthews JL, Taylor PP. Epinephrine in pulpotomy. ASDC Journal of Dentistry for Children 1969;36(2):123‐8. [PUBMED: 4888301] [PubMed] [Google Scholar]
Liu 2003 {published and unpublished data}
- Liu JF. Nd:YAG laser pulpotomy of human primary teeth. Lasers in Dentistry, Proceedings: Revolution of Dental Treatment in the New Millennium 2003:251‐6.
Liu 2006 {published data only}
Lourenço 2015 {published data only}
- Lourenço Neto N, Marques NC, Fernandes AP, Hungaro Duarte MA, Abdo RC, Machado MA, et al. Clinical and radiographic evaluation of Portland cement added to radiopacifying agents in primary molar pulpotomies. European Archives of Paediatric Dentistry 2015;16(5):377‐82. [DOI] [PubMed] [Google Scholar]
Louwakul 2011 {published data only}
- Louwakul P, Prucksathamrongkul W. Effect of chlorhexidine on outcome of pulpectomy in primary molars [abstract]. Proceedings of the 45th Meeting of the Continental European Division of the International Association of Dental Research; 2011, Aug 31‐Sep 3; Budapest, Hungary. 2011:Abstract no: 497.
Mani 1999 {published data only}
- Mani SA, Chawla HS, Goel A. Zinc oxide‐eugenol or calcium hydroxide as root canal filling material in primary teeth. International Journal of Paediatric Dentistry 1999;9(Suppl 1):113. [Google Scholar]
Massler 1968 {published data only}
- Massler M. Preventive endodontics: vital pulp therapy. Journal of the Dental Association of South Africa 1968;23(1):208‐15. [PUBMED: 5243415] [PubMed] [Google Scholar]
Mejare 1979 {published data only}
- Mejare I. Pulpotomy of primary molars with coronal or total pulpitis using formocresol technique. Scandinavian Journal of Dental Research 1979;87(3):208‐16. [DOI] [PubMed] [Google Scholar]
NCT01622153 {published data only}
- NCT01622153. Electrical and formocresol pulpotomy in primary molars. Available from www.clinicaltrials.gov/ct2/show/record/NCT01622153 (accessed 22 November 2017).
Odabas 2007 {published data only}
Percinoto 2006 {published data only}
- Percinoto C, Castro AM, Pinto LM. Clinical and radiographic evaluation of pulpotomies employing calcium hydroxide and trioxide mineral aggregate. General Dentistry 2006;54(4):258‐61. [PUBMED: 16903198] [PubMed] [Google Scholar]
Punwani 1993 {published and unpublished data}
- Punwani I, Fadavi S. The efficacy of pulpfixÆ glutaraldehyde pulpotomy agent for the treatment of vital primary teeth with carious pulp exposure. Journal of Dental Research 1993;72:212 (IADR Abs No 869). [Google Scholar]
Ram 2001 {published and unpublished data}
- Ram D, Moskovitz M, Fuks AB. Ferric sulfate versus formocresol in pulpotomized primary molars: clinical and radiographic results. Journal of Dental Research 2001;80:1311 (Abs No 20). [Google Scholar]
Ravn 1968 {published data only}
- Ravn JJ, Svarrer M. A clinicoradiographic follow‐up study of coronal vital pulpotomy in 200 primary molars treated with zinc‐oxide‐eugenol paste [En klinisk‐radiologisk efterundersogelse af koronal vitalamputation i 200 primaere molarer, behandlet med zinkilte‐euge‐nol som amputationspasta]. Tandlaegebladet 1968;72(8):718‐26. [PUBMED: 5247171] [PubMed] [Google Scholar]
Reddy 1996 {published data only}
- Reddy VVS, Fernandes. Clinical and radiological evaluation of zinc oxide‐eugenol and Maisto's paste as obturating materials in infected primary teeth ‐ nine months study. Journal of the Indian Society of Pedodontics and Preventive Dentistry 1996;14(2):39‐44. [PubMed] [Google Scholar]
Redig 1968 {published data only}
- Redig DF. A comparison and evaluation of two formocresol pulpotomy technics utilizing "Buckley's" formocresol. Journal of Dentistry for Children 1968;35:22‐30. [MEDLINE: ] [PubMed] [Google Scholar]
Riccioli 1971 {published data only}
- Riccioli GA. Conservative therapy of the deciduous teeth [A proposito di terapie conservatrice dei decidui]. Mondo Odontostomatologico 1971;14(1):65‐7. [PUBMED: 5317205] [PubMed] [Google Scholar]
Ripa 1971 {published data only}
- Ripa LW. Pulp therapy in live deciduous teeth [Terapeutica pulpar para dientes temporales vivos]. Fauchard 1971;2(6):114‐7. [PUBMED: 5288029] [PubMed] [Google Scholar]
Ritwik 2003 {published data only}
- Ritwik P, Cuisia ZV, Dabir P, Musselman RJ. MTA pulpotomies in the primary molars of children: results after 3 years. International Journal of Paediatric Dentistry 2003;13(Suppl 1):11. [Google Scholar]
Rivera 2003 {published data only}
- Rivera N, Reyes E, Mazzaoui S, Moron A. Pulpal therapy for primary teeth: formocresol vs electrosurgery: a clinical study. Journal of Dentistry for Children 2003;70(1):71‐3. [PUBMED: 12762614] [PubMed] [Google Scholar]
Rocha 1999 {published data only}
- Rocha M, Baroni R, Santos L, Girardi K. Calcium hydroxide and MTA pulpotomies in primary teeth: one year results. International Journal of Paediatric Dentistry 1999;9(Suppl 1):102. [Google Scholar]
Rule 1966 {published data only}
- Rule DC, Winter GB. Root growth and apical repair subsequent to pulpal necrosis in children. British Dental Journal 1966;120(12):586‐90. [PUBMED: 5221182] [PubMed] [Google Scholar]
Sargenti 1975 {published data only}
- Sargenti A. Rational root therapy of deciduous teeth with N2 [Die rationelle Wurzelbehandlung der Milchzahne mit N2]. Zahnarztliche Praxis 1975;26(9):198‐200. [PUBMED: 1057341] [PubMed] [Google Scholar]
Sayegh 1967 {published data only}
- Sayegh FS, Reed AJ. Correlated clinical and histological evaluation of Hydrex in pulp therapy. Journal of Dentistry for Children 1967;34(6):471‐7. [PUBMED: 4864180] [PubMed] [Google Scholar]
Sogbe de Agell 1989 {published data only}
- Sogbe de Agell R. Clinical and radiographic evaluation of deciduous molars with necrotic pulp treated with two concentrations of formocresol. Acta Odontológica Venezolana 1989;27(1):3‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Szabo 1968 {published data only}
- Szabo H, Beczner S, Matavovszky M. Dental pulp treatment with "N2" material in deciduous molars [Tejmolarisok pulpakezelese "N2" anayaggal]. Fogorvosi Szemle 1968;61(12):268‐70. [PUBMED: 5251838] [PubMed] [Google Scholar]
Tsai 1993 {published and unpublished data}
- Tsai TP, Su HL, Tseng LH. Glutaraldehyde preparations and pulpotomy in primary molars. Oral Surgery, Oral Medicine, and Oral Pathology 1993;76(3):346‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Velkova 1977 {published data only}
- Velkova A. Treatment of pulpitis in deciduous molars [Prispevek k problematice leceni docasnych pulpitickych molaru]. Ceskoslovenska Stomatologie 1977;77(4):277‐81. [PUBMED: 269022] [PubMed] [Google Scholar]
Yakushiji 1969 {published data only}
- Yakushiji M, Sasamoto K, Imanishi T, Machida Y, Sekine N. Clinical study of vital pulpotomy with Calvital on deciduous teeth. Shikwa Gakuho 1969;69(2):271‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Yildiz 2014 {published data only}
- Yildiz E, Tosun G. Evaluation of formocresol, calcium hydroxide, ferric sulfate, and MTA primary molar pulpotomies. European Journal of Dentistry 2014;8(2):234‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
CTRI/2011/06/001776 {published data only}
- CTRI/2011/06/001776. Root canal treatment in milk teeth using three root canal filling materials: a double‐blinded randomized controlled trial. Available from www.ctri.nic.in (accessed 28 November 2017).
NCT00802256 {published data only}
- Comparative evaluation of pulpotomized primary molars with mineral trioxide aggregate and new endodontic cement. Available from www.clinicaltrials.gov/ct2/show/record/NCT00802256 (accessed 26 June 2017).
NCT00972556 {published data only}
- Comparison of mineral trioxide aggregate (MTA) and 20% formocresol (FC) in pulpotomized human primary molars. Available from www.clinicaltrials.gov/show/ (accessed 5 June 2014).
NCT01010451 {published data only}
- Antimicrobial pulpotomy of primary molars. Available from www.clinicaltrials.gov/ct2/show/record/NCT01010451 (accessed 26 June 2017).
NCT01591278 {published data only}
- NCT01591278. MTA and biodentine in pulpotomized primary molars. Available from www.clinicaltrials.gov/ct2/show/record/NCT01591278 (accessed 28 November 2017).
NCT01733420 {published data only}
- NCT01733420. Biodentine versus white MTA pulpotomy. Available from www.clinicaltrials.gov/ct2/show/record/NCT01733420 (accessed 28 November 2017).
NCT02137967 {published data only}
- Sodium hypochlorite pulpotomies in primary molars: comparison with conventional 20% formocresol pulpotomies. Available from www.clinicaltrials.gov/ct2/show/record/NCT02137967 (accessed 26 June 2017).
NCT02201498 {published data only}
- Randomized clinical trial for primary molar pulpotomy, biodentine vs formocresol‐ZOE. Available from www.clinicaltrials.gov/ct2/show/record/NCT02201498 (accessed 26 June 2017).
NCT02286648 {published data only}
- Success rate evaluation of miniature pulpotomy with MTA in primary molars. Available from www.clinicaltrials.gov/ct2/show/record/NCT02286648 (accessed 26 June 2017).
NCT02298504 {published data only}
- Vital pulp treatment in primary teeth. Available from www.clinicaltrials.gov/ct2/show/record/NCT02298504 (accessed 26 June 2017).
NCT02393326 {published data only}
- Biodentine partial pulpotomy of pulpally exposed primary molars. Available from www.clinicaltrials.gov/ct2/show/record/NCT02393326 (accessed 26 June 2017).
NCT02702505 {published data only}
- Success and color stability of MTA pulpotomized primary molars: an RCT (MTA). Available from www.clinicaltrials.gov/ct2/show/record/NCT02702505 (accessed 26 June 2017).
NCT02783911 {published data only}
- Comparison of mineral trioxide aggregate (MTA) & ferric sulfate (FS) pulpotomies. Available from www.clinicaltrials.gov/ct2/show/study/NCT02783911 (accessed 26 June 2017).
NCT02789423 {published data only}
- NCT02789423. Clinical & radiographical evaluation of the effect of dycal & biodentine in DPC in primary teeth. www.clinicaltrials.gov/ct2/show/record/NCT02789423 (first received 3 June 2016).
Additional references
Al‐Shalan 1997
- Al‐Shalan TA, Erickson PR, Hardie NA. Primary incisor decay before age 4 as a risk factor for future dental caries. Pediatric Dentistry 1997;19(1):37‐41. [PUBMED: 9048412] [PubMed] [Google Scholar]
Asgary 2014b
- Asgary S, Shirvani A, Fazlyab M. MTA and ferric sulfate in pulpotomy outcomes of primary molars: a systematic review and meta‐analysis. Journal of Clinical Pediatric Dentistry 2014;39(1):1‐8. [PUBMED: 25631717] [DOI] [PubMed] [Google Scholar]
CDC 2011
- USA National Center for Health Statistics, Centers for Disease Control and Prevention. CDC Data & Statistics. Feature: Untreated Dental Caries (Cavities) in Children Ages 2‐19, United States, 2011. Available from www.cdc.gov/Features/dsUntreatedCavitiesKids/ (accessed 5 June 2014).
Coll 2015
- Coll J, Seale NS, Vargas K, Chi DL, Marghalani AA, Graham L. Protocol for a systematic review and meta‐analysis of vital pulp therapy for children with deep caries in the primary dentition. Pediatric Dentistry 2015;37(5):418‐21. [PUBMED: 26531083] [PubMed] [Google Scholar]
Coll 2017
- Coll JA, Seale NS, Vargas K, Marghalani AA, Al Shamali S, Graham L. Primary tooth vital pulp therapy: a systematic review and meta‐analysis. Pediatric Dentistry 2017;39(1):16‐123. [PUBMED: 28292337] [PubMed] [Google Scholar]
Fallahinejad 2008
- Fallahinejad Ghajari M, Mirkarimi M, Vatanpour M, Kharrazi Fard MJ. Comparison of pulpotomy with formocresol and MTA in primary molars: a systematic review and meta‐analysis. Iranian Endodontic Journal 2008;3(3):45‐9. [PUBMED: 24146670] [PMC free article] [PubMed] [Google Scholar]
Ferreira 2007
- Ferreira FB, Torres SA, Rosa OP, Ferreira CM, Garcia RB, Marcucci MC, et al. Antimicrobial effect of propolis and other substances against selected endodontic pathogens. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2007;104(5):709‐16. [PUBMED: 17964476] [DOI] [PubMed] [Google Scholar]
Finucane 2012
- Finucane D. Rationale for restoration of carious primary teeth: a review. European Archives of Paediatric Dentistry 2012;13(6):281‐92. [PUBMED: 23235127] [DOI] [PubMed] [Google Scholar]
Guideline Pulp Therapy 2014
- Clinical Affairs Committee ‐ Pulp Therapy Subcommittee. Guideline on Pulp Therapy for Primary and Immature Permanent Teeth. American Academy of Pediatric Dentistry 2014:244‐52.
Guideline Pulp Therapy 2016
- Clinical Affairs Committee ‐ Pulp Therapy Subcommittee. Guideline on Pulp Therapy for Primary and Immature Permanent Teeth. Pediatric Dentistry 2016;38(6):280‐8. [PUBMED: 27931467] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from: www.cochrane‐handbook.org.
IARC 2017
- World Health Organization. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. monographs.iarc.fr/ENG/Classification/latest_classif.php (accessed 8 January 2018).
ICDAS II 2011
- International Caries Detection and Assessment System Coordinating Committee. Rationale and Evidence for the International Caries Detection and Assessment System (ICDAS II), 2011. Available from www.icdas.org/downloads (accessed 5 June 2014).
Kaste 1992
- Kaste LM, Marianos D, Chang R, Phipps KR. The assessment of nursing caries and its relationship to high caries in the permanent dentition. Journal of Public Health Dentistry 1992;52(2):64‐8. [PUBMED: 1564693] [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration.
Lewis 2009
- Lewis B. The obsolescence of formocresol. British Dental Journal 2009;207(11):525‐8. [PUBMED: 20010749] [DOI] [PubMed] [Google Scholar]
Lin 2014
- Lin PY, Chen HS, Wang YH, Tu YK. Primary molar pulpotomy: a systematic review and network meta‐analysis. Journal of Dentistry 2014;42(9):1060‐77. [PUBMED: 24513112] [DOI] [PubMed] [Google Scholar]
Loh 2004
- Loh A, O'Hoy P, Tran X, Charles R, Hughes A, Kubo K, et al. Evidence‐based assessment: evaluation of the formocresol versus ferric sulfate primary molar pulpotomy. Pediatric Dentistry 2004;26(5):401‐9. [PUBMED: 15460294] [PubMed] [Google Scholar]
Marghalani 2014a
- Marghalani AA, Omar S, Chen JW. Clinical and radiographic success of mineral trioxide aggregate compared with formocresol as a pulpotomy treatment in primary molars: a systematic review and meta‐analysis. Journal of the American Dental Association (1939) 2014;145(7):714‐21. [PUBMED: 24982277] [DOI] [PubMed] [Google Scholar]
Milnes 2006
- Milnes AR. Persuasive evidence that formocresol use in pediatric dentistry is safe. Journal of the Canadian Dental Association 2006;72(3):247‐8. [PUBMED: 16696891] [PubMed] [Google Scholar]
Ng 2008
- Ng FK, Messer LB. Mineral trioxide aggregate as a pulpotomy medicament: an evidence‐based assessment. European Archives of Paediatric Dentistry 2008;9(2):58‐73. [PUBMED: 18534173] [DOI] [PubMed] [Google Scholar]
NHANES 2010
- National Health and Nutrition Examination Survey (NHANES). National Health and Nutrition Examination Survey Data (1999‐2004). Available from www.cdc.gov/nchs/data/datalinkage/nh99+_mort_file_layout_public_2010.pdf (accessed 5 June 2014).
Peng 2006
- Peng L, Ye L, Tan H, Zhou X. Evaluation of the formocresol versus mineral trioxide aggregate primary molar pulpotomy: a meta‐analysis. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2006;102(6):e40‐4. [PUBMED: 17138165] [DOI] [PubMed] [Google Scholar]
Peng 2007
- Peng L, Ye L, Guo X, Tan H, Zhou X, Wang C, et al. Evaluation of formocresol versus ferric sulphate primary molar pulpotomy: a systematic review and meta‐analysis. International Endodontic Journal 2007;40(10):751‐7. [PUBMED: 17714467] [DOI] [PubMed] [Google Scholar]
Sarrami 2002
- Sarrami N, Pemberton MN, Thornhill MH, Theaker ED. Adverse reactions associated with the use of eugenol in dentistry. British Dental Journal 2002;193(5):257‐9. [PUBMED: 12353045] [DOI] [PubMed] [Google Scholar]
Sassone 2003
- Sassone LM, Fidel RA, Fidel SR, Dias M, Hirata RJ. Antimicrobial activity of different concentrations of NaOCl and chlorhexidine using a contact test. Brazilian Dental Journal 2003;14(2):99‐102. [PUBMED: 12964652] [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. [Google Scholar]
Selwitz 2007
- Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet 2007;369(9555):51‐9. [DOI] [PubMed] [Google Scholar]
Shirvani 2014b
- Shirvani A, Hassanizadeh R, Asgary S. Mineral trioxide aggregate vs. calcium hydroxide in primary molar pulpotomy: a systematic review. Iranian Endodontic Journal 2014;9(2):83‐8. [PUBMED: 24688575] [PMC free article] [PubMed] [Google Scholar]
Shirvani 2014c
- Shirvani A, Asgary S. Mineral trioxide aggregate versus formocresol pulpotomy: a systematic review and meta‐analysis of randomized clinical trials. Clinical Oral Investigations 2014;18(4):1023‐30. [PUBMED: 24452827] [DOI] [PubMed] [Google Scholar]
Smaïl‐Faugeron 2013
- Smaïl‐Faugeron V, Fron Chabouis H, Durieux P, Attal J P, Muller‐Bolla M, Courson F. Development of a core set of outcomes for randomized controlled trials with multiple outcomes ‐ example of pulp treatments of primary teeth for extensive decay in children. PLoS One 2013;8(1):e51908. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stringhini 2015a
- Stringhini Junior E, Vitcel ME, Oliveira LB. Evidence of pulpotomy in primary teeth comparing MTA, calcium hydroxide, ferric sulphate, and electrosurgery with formocresol. European Archives of Paediatric Dentistry 2015;16(4):303‐12. [PUBMED: 25833280] [DOI] [PubMed] [Google Scholar]
Whitehead 2002
- Whitehead A. Meta‐Analysis of Controlled Clinical Trials. Chichester: Wiley, 2002. [Google Scholar]
Worthington 2015
- Worthington H, Clarkson J, Weldon J. Priority oral health research identification for clinical decision‐making. Evidence‐based Dentistry 2015;16(3):69‐71. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Nadin 2003
- Nadin G, Goel BR, Yeung CA, Glenny AM. Pulp treatment for extensive decay in primary teeth. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD003220] [DOI] [PubMed] [Google Scholar]
Smaïl‐Faugeron 2014a
- Smaïl‐Faugeron V, Courson F, Durieux P, Muller‐Bolla M, Glenny AM, Fron Chabouis H. Pulp treatment for extensive decay in primary teeth. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD003220.pub2] [DOI] [PubMed] [Google Scholar]


