Abstract
Background
Bronchiectasis is being increasingly diagnosed and recognised as an important contributor to chronic lung disease in both adults and children in high‐ and low‐income countries. It is characterised by irreversible dilatation of airways and is generally associated with airway inflammation and chronic bacterial infection. Medical management largely aims to reduce morbidity by controlling the symptoms, reduce exacerbation frequency, improve quality of life and prevent the progression of bronchiectasis. This is an update of a review first published in 2000.
Objectives
To evaluate the efficacy and safety of inhaled corticosteroids (ICS) in children and adults with stable state bronchiectasis, specifically to assess whether the use of ICS: (1) reduces the severity and frequency of acute respiratory exacerbations; or (2) affects long‐term pulmonary function decline.
Search methods
We searched the Cochrane Register of Controlled Trials (CENTRAL), the Cochrane Airways Group Register of trials, MEDLINE and Embase databases. We ran the latest literature search in June 2017.
Selection criteria
All randomised controlled trials (RCTs) comparing ICS with a placebo or no medication. We included children and adults with clinical or radiographic evidence of bronchiectasis, but excluded people with cystic fibrosis.
Data collection and analysis
We reviewed search results against predetermined criteria for inclusion. In this update, two independent review authors assessed methodological quality and risk of bias in trials using established criteria and extracted data using standard pro forma. We analysed treatment as 'treatment received' and performed sensitivity analyses.
Main results
The review included seven studies, involving 380 adults. Of the 380 randomised participants, 348 completed the studies.
Due to differences in outcomes reported among the seven studies, we could only perform limited meta‐analysis for both the short‐term ICS use (6 months or less) and the longer‐term ICS use (> 6 months).
During stable state in the short‐term group (ICS for 6 months or less), based on the two studies from which data could be included, there were no significant differences from baseline values in the forced expiratory volume in the first second (FEV1) at the end of the study (mean difference (MD) ‐0.09, 95% confidence interval (CI) ‐0.26 to 0.09) and forced vital capacity (FVC) (MD 0.01 L, 95% CI ‐0.16 to 0.17) in adults on ICS (compared to no ICS). Similarly, we did not find any significant difference in the average exacerbation frequency (MD 0.09, 95% CI ‐0.61 to 0.79) or health‐related quality of life (HRQoL) total scores in adults on ICS when compared with no ICS, though data available were limited. Based on a single non‐placebo controlled study from which we could not extract clinical data, there was marginal, though statistically significant improvement in sputum volume and dyspnoea scores on ICS.
The single study on long‐term outcomes (over 6 months) that examined lung function and other clinical outcomes, showed no significant effect of ICS on any of the outcomes. We could not draw any conclusion on adverse effects due to limited available data.
Despite the authors of all seven studies stating they were double‐blind, we judged one study (in the short duration ICS) as having a high risk of bias based on blinding, attrition and reporting of outcomes. The GRADE quality of evidence was low for all outcomes (due to non‐placebo controlled trial, indirectness and imprecision with small numbers of participants and studies).
Authors' conclusions
This updated review indicates that there is insufficient evidence to support the routine use of ICS in adults with stable state bronchiectasis. Further, we cannot draw any conclusion for the use of ICS in adults during an acute exacerbation or in children (for any state), as there were no studies.
Plain language summary
Role of inhaled corticosteroids (ICS) in the management of bronchiectasis
Background
Bronchiectasis is a lung disease. People with bronchiectasis often experience long‐term symptoms such as productive or wet cough, repeated flare‐ups (exacerbations) and poor quality of life. People with bronchiectasis have airway inflammation and many have asthma‐like symptoms (such as cough and wheeze). Because of this, inhaled corticosteroids (ICS), commonly used in asthma, might also improve symptoms, reduce flare‐ups and/or reduce worsening of lung function for people with bronchiectasis. However, routine use of ICS may also cause unwanted side effects.
Review question
What are the benefits of using ICS regularly in the management of adults and children with bronchiectasis?
Study characteristics
We included studies that compared ICS with no ICS, or with a placebo (i.e. a medication made to look the same as ICS but with no active ingredients). We only included studies where it was decided at random who would receive ICS and who would not. The participants included in the seven studies were 380 adults who had bronchiectasis diagnosed by symptoms or from a detailed lung scan (computed tomography (CT)). We did not include studies that involved participants with cystic fibrosis, which can also cause bronchiectasis. Although we planned to include studies involving children with bronchiectasis, we did not find such studies.
What evidence did we find?
From available evidence up to June 2017, we found seven eligible studies involving adult participants that examined the role of ICS in bronchiectasis. The adults had stable bronchiectasis ‐ they were not having a flare‐up at the start of the study.
We were able to include results from two studies that gave ICS for less than six months to adults with stable bronchiectasis. ICS did not make a difference to lung function, number of exacerbations during the study or quality of life. In a different study, which also gave ICS for less than six months, we found a small reduction in sputum (phlegm) and improvement in breathlessness. However, as these results were from a study which did not use a placebo we cannot be certain about them.
The single study on long‐term use of ICS (i.e. for over 6 months) showed no meaningful benefit of ICS for any of the outcomes.
There were no studies conducted when the participants were having a flare‐up of their bronchiectasis. There were also no studies that involved children with bronchiectasis. Importantly, we do not know if ICS are linked to more unwanted side effects, because the studies did not provide much information about this.
Conclusion
The review found that there is not enough evidence for the routine use of ICS in adults with stable bronchiectasis. We can make no conclusions about the use of ICS for flare‐ups of bronchiectasis, or about their use in children, because we did not find any studies.
Quality of evidence
Overall, we judged the quality of evidence to be low. We were concerned because the largest study, which showed some benefits, did not use a placebo. This means that participants and staff in the study would have known who was getting ICS and who was not, which could affect the results. Also, our confidence was reduced because we only found a small number of studies to include in our review and some of the studies may have included people with other types of lung disease, in addition to bronchiectasis.
Summary of findings
Background
Description of the condition
Bronchiectasis, previously termed an 'orphan disease' is increasingly recognised as a major cause of respiratory morbidity, especially in low‐income countries (Karadag 2005; Karakoc 2001), and in some ethnic populations of affluent countries (Chang 2002; Edwards 2003; Singleton 2000). More recently, it has also been increasingly reported from some high‐income countries (Sanchez‐Munoz 2016). Bronchiectasis is the end result of a variety of airway insults and predisposing conditions that culminate in airway injury, recurrent or persistent airway infection and destruction (Chang 2010). The underlying aetiology of bronchiectasis varies from being a consequence of recurrent respiratory infections to rare immune deficiencies. Other causes include primary ciliary dyskinesia, allergic bronchopulmonary aspergillosis and mycobacterial infection (Olveira 2017; Shoemark 2007). A common feature of most patients is the impaired local and/or systemic host defences to infection.
The dominant symptoms and signs of bronchiectasis are productive or wet cough, dyspnoea on exertion and presence of other respiratory signs (clubbing, chest wall deformity, respiratory noises such as wheeze or crepitations on auscultation). In the long‐term, pulmonary function decline may occur (Keistinen 1997; Twiss 2006). In studies involving both children and adult cohorts, asthma‐like symptoms in people with bronchiectasis have been described and when present, are associated with accelerated pulmonary function decline when compared to those with bronchiectasis, but without asthma‐like symptoms (Field 1969; Keistinen 1997).
Like people with chronic obstructive pulmonary disease (COPD) and cystic fibrosis, children and adults with bronchiectasis also suffer from recurrent acute exacerbations, some of whom require hospitalised treatment. Pulmonary exacerbations present with a worsening of the baseline clinical state (Kapur 2012a), and are associated with increased morbidity (Kapur 2009), progressive deterioration in lung function (Ellerman 1997; Kapur 2010), and poor quality of life in both adults and children with bronchiectasis (Kapur 2012; Wilson 1997). Effective management regimes for bronchiectasis would reduce the frequency or severity of respiratory exacerbations, and/or the long‐term pulmonary function decline.
The hallmarks of bronchiectasis are stasis of infected airway secretions; reduced airway mucus clearance; and regional or diffuse airway wall dilatation, thickening and destruction with loss of airway structural integrity (Mandal 2013). Based on Cole's 'vicious circle hypothesis', microbial colonisation/infection is important in the pathophysiology of bronchiectasis, as it leads to bronchial obstruction and a normal or exaggerated inflammatory response (Cole 1986). As in COPD, chronic airway obstruction and bronchial hyper‐responsiveness is known to occur in bronchiectasis. A variety of proinflammatory airway cells such as neutrophils, lymphocytes, macrophages and eosinophils have been implicated in the pathogenesis of both bronchiectasis and COPD (Mandal 2013; Kim 2008).
Although inflammation is considered an important driver of bronchial hyper‐responsiveness and airway instability in airway diseases such as COPD and asthma (Berend 2008), evidence directly implicating it in bronchial hyper‐responsiveness in bronchiectasis is unclear. Since bronchiectasis involves similar airway inflammatory mediators, suppression of this inflammatory process could potentially slow the rate of airway damage.
Description of the intervention
The anti‐inflammatory effect of corticosteroids on the airways has been well documented in inflammatory airway diseases, such as asthma (Booth 1995), and COPD (Renkema 1996; Sobieraj 2008). Inhaled preparation of corticosteroids provide a non‐specific, local anti‐inflammatory effect with minimal systemic side effects. ICS are available as metered dose inhalers, dry powder devices or nebulised, and can be used for short or long durations. Short duration use of ICS likely has different effects compared to longer‐term ICS use for our primary outcome of interest (lung function), as well as for other adverse event outcomes, such as bone metabolism (Kerstjens 1994), and children's growth (Pruteanu 2014), further discussed in the last paragraph of the 'Data synthesis' section.
Inhaled corticosteroids (ICS) are beneficial in some (but not all) people with COPD (Ernst 2015). However, use of ICS is associated with adverse events in children and adults that range from mild (candidiasis) to serious (adrenal insufficiency (Holme 2008), osteoporosis, cataracts, pneumonia) events. Recent evidence indicates increased risk of pneumonia and lower respiratory tract infections with use of ICS in adults with COPD (Wang 2016). Since bronchiectasis involves similar disruption of the host airway defences, it is plausible that this cohort is also at higher risk of respiratory infections with the use of ICS.
How the intervention might work
Airway eosinophilia and bronchial hyper‐responsiveness has been reported in adults (Ip 1991;Tsikrika 2017), and children with bronchiectasis (Goyal 2016;Pizzutto 2013), hence ICS may be potentially beneficial for this group of patients, as ICS is usually efficacious in people with airway eosinophilia.
Why it is important to do this review
The role of ICS in bronchiectasis remains unclear. An update of the original systematic review on the efficacy of ICS in the management of children and adults with bronchiectasis (Kapur 2009) could help guide clinical practice.
Objectives
To evaluate the efficacy and safety of inhaled corticosteroids (ICS) in children and adults with stable state bronchiectasis, specifically to assess whether the use of ICS: (1) reduces the severity and frequency of acute respiratory exacerbations; or (2) affects long‐term pulmonary function decline.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) using inhaled corticosteroids (ICS) in patients with bronchiectasis, in comparison to placebo.
Types of participants
Children or adults with bronchiectasis (defined clinically or radiologically) not related to cystic fibrosis.
Types of interventions
All types of ICS.
Types of outcome measures
Primary outcomes
Change in objective measures of lung function.
Secondary outcomes
(A) for short‐term effectiveness (6 months or less)
Mean difference in bronchiectasis severity control (wheeze, dyspnoea, cough diary, etc).
Mean of respiratory exacerbations, or hospitalisations per participant, or both.
Sputum volume.
Mean difference in other objective indices (airway markers of inflammation, exhaled nitric oxide, etc).
Mean difference in quality of life indices.
Proportions experiencing adverse effects of the intervention, (e.g. pharyngeal candidiasis, voice change, pneumonia, etc).
(B) for medium‐ to long‐term outcomes (greater than 6 months)
Clinical indices of bronchiectasis severity control (quality of life, cough diary, Likert scale, visual analogue scale, level of interference of cough, etc).
Relevant airway markers of inflammation.
Proportions experiencing adverse effects of the intervention, (e.g. adrenal insufficiency, cataracts, linear growth, etc).
Frequency of exacerbation per subject.
Search methods for identification of studies
Electronic searches
This is an update of a previous Cochrane Review (Kapur 2009).
For this update, we identified studies from the following sources.
The Cochrane Airways Group Trials Register (updated June 2017).
The Cochrane Central Register of Controlled Trials (CENTRAL; Issue 5, 2016.
MEDLINE (1950 to June 2017).
Embase (1980 to June 2017).
The list of references in relevant publications.
Written communication with the study authors of the studies included in the review, when necessary.
For the full database topic search strategies, see Appendix 1. We searched all databases with no restriction on language of publication. We searched the CENTRAL database for handsearched conference abstracts and grey literature.
We also conducted searches of ClinicalTrials.gov (www.ClinicalTrials.gov), and the World Health Organization (WHO) trials portal (www.who.int/ictrp/en/), using the search strategy in Appendix 2. We searched all databases from their inception to 5 February 2018, and we imposed no restriction on language of publication.
Searching other resources
We wrote to the lead authors of all the studies included and three study authors replied; we incorporated any information provided.
Data collection and analysis
Selection of studies
From the title, abstract, or descriptors, two review authors (NK, AC) independently reviewed the literature searches to identify potentially relevant studies for full review. There was no disagreement between the review authors as to which studies should be included. We recorded the selection process in sufficient detail to complete the PRISMA (PRISMA 2009) flow diagram (Figure 1) and Characteristics of excluded studies table.
1.
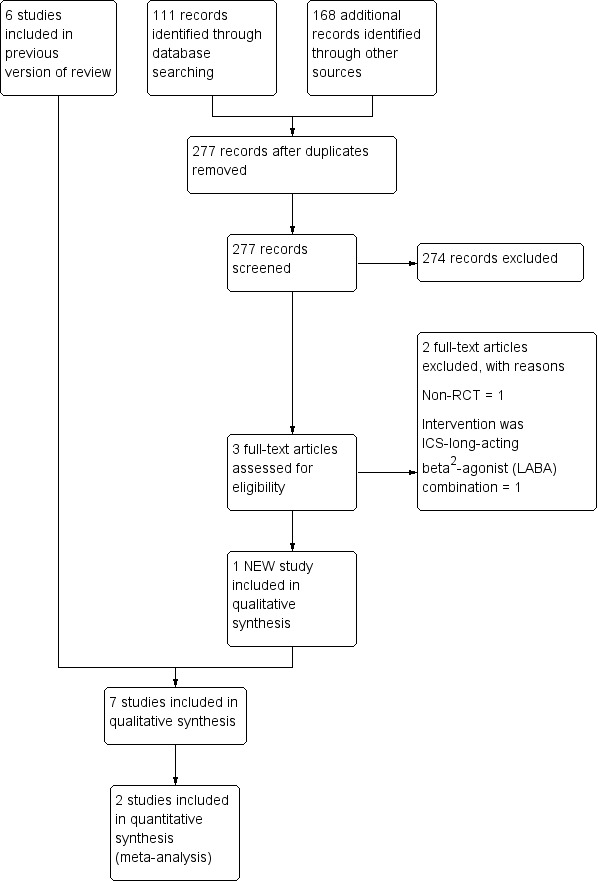
Study flow diagram.
Data extraction and management
We reviewed studies that satisfied the inclusion criteria and recorded the following information: study setting, year of study, source of funding, participant recruitment details (including number of eligible participants), inclusion and exclusion criteria, other symptoms, randomisation and allocation concealment method, numbers of participants randomised, blinding (masking) of participants, care providers and outcome assessors, dose and type of intervention, duration of therapy, cointerventions, numbers of participants not followed up, reasons for withdrawals from study protocol (clinical, side effects, refusal and other), details on side effects of therapy, and whether intention‐to‐treat analyses were possible. We extracted data independently on the outcomes described previously. We requested further information from the study authors, but only three responded (Hernando 2012; Joshi 2004; Martinez‐Garcia 2006); we incorporated the information received.
Assessment of risk of bias in included studies
We included all assessments in the 'Characteristics of included studies' table. We measured inter‐reviewer reliability for the identification of high quality studies for each component using the Kappa statistic. Agreement between the two review authors was excellent (weighted kappa score for quality assessment scores was 0.81).
Two review authors (NK, AC) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreement by discussion. We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low or unclear and provided a quote from the study report together with a justification in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. We carried out blinding separately for different key outcomes, where necessary. Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Measures of treatment effect
The statistical analysis proposed, included calculation of a pooled estimate of treatment effect for each dichotomous outcome across all studies (odds of outcome in participants allocated to receive treatment compared with odds of outcome in participants allocated to control group); and calculation of a pooled estimate of treatment effect for each continuous outcome across all studies in the form of a mean difference (MD). For continuous variables, we recorded mean absolute change from baseline for each group and standard deviation (SD) for each group.
Unit of analysis issues
We used the participant as the unit of analysis.
Dealing with missing data
We sought data on a number of participants with each outcome event listed above, by allocated treatment group in order for us to conduct an intention‐to‐treat (ITT) analysis. We also contacted the study investigators for clarification and further information where necessary.
Assessment of heterogeneity
We described and tested any heterogeneity between the study results to see if it reached statistical significance using a chi2 test. We estimated the 95% confidence interval (CI) using a random‐effects model whenever there were concerns about statistical heterogeneity.
Assessment of reporting biases
We checked all reports of the included studies to check that all the stated outcomes were reported and the results were presented in the 'Risk of bias table' in the 'Characteristics of included studies' table.
Data synthesis
We calculated odds ratio (OR) using a modified ITT analysis for the dichotomous outcome variables of each individual study. This analysis assumes that participants not available for outcome assessment have not improved (and probably represents a conservative estimate of effect). An initial qualitative comparison of all the individually analysed studies examined whether pooling of results (meta‐analysis) is reasonable. This took into account differences in study populations, inclusion/exclusion criteria, interventions, outcome assessment, and estimated effect size.
We included results from studies that met the inclusion criteria and reported any of the outcomes of interest in the subsequent meta‐analyses. We calculated the summary weighted risk ratio (RR) and 95% CI (fixed‐effect model) using Review Manager 5 (Review Manager 2014). For cross‐over studies, we calculated mean treatment differences from raw data. We extracted or imputed and entered the date as fixed‐effect generic inverse variance outcomes to provide summary weighted differences and 95% CIs. In cross‐over trials, we only included data from the first arm in meta‐analysis when the data were combined with parallel studies (Elbourne 2002). We calculated numbers needed to treat to benefit (NNTB) were calculated from the pooled OR and applied its 95% CI to a specified baseline risk using an online calculator (Cates 2008). If studies had reported outcomes using different measurement scales, we planned to use the standardised mean difference (SMD).
'Summary of findings' table
We created 'Summary of findings' tables using the following outcomes.
Short‐term ICS use (≤ 6 months)
Lung function indices forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) (in L, end of study minus baseline values)
Lung function indices diffusion capacity % predicted (end of study values)
Average number of exacerbations per participant
Pseudomonas aeruginosa (P aeruginosa) colonisation
There are various types of lung function abnormality in people with bronchiectasis (Guan 2014). As this was our primary outcome measure, we included the various indices that reflect these abnormalities.
Longer‐term ICS use (> 6 months)
Lung function indices FEV1 and FVC (in L, end of study minus baseline values)
Average number of exacerbations per participant
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contributed data to the meta‐analyses for the prespecified outcomes. We then used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using GRADEpro software (GRADEpro GDT 2015). We justified all decisions to downgrade the quality of studies using footnotes, and we made comments to aid the reader's understanding of the review where necessary.
We evaluated the outcomes described above based on short (6 months or less) and long duration (> 6 months) use of ICS, as done previously in our Cochrane Review (Kapur 2009). This arbitrary cut‐off is a commonly used time frame in studies (Daniel 2017; Kerstjens 1994; Lee 2012), when evaluating the effects of ICS, as duration of ICS use may impact on clinical and structural pulmonary outcomes (e.g. airway remodelling (Barnes 2010)), and adverse events (e.g. growth (Pruteanu 2014)), and bone metabolism (Kerstjens 1994). Lung function in people with bronchiectasis generally declines slowly over time and as this was our a priori defined primary outcome, the effect of short duration ICS theoretically could differ from longer duration ICS. Outcomes such as bronchial hyper‐responsiveness takes months of ICS therapy to plateau (Barnes 2010). An example with respect to adverse events: in the Cochrane Review on the effect of ICS on height in growing children with asthma, the authors found no difference between groups (ICS versus placebo) in the first six months of ICS use, but a significant difference between groups was present in ICS use at the 12 months time point (Pruteanu 2014).
Subgroup analysis and investigation of heterogeneity
We planned the following a priori subgroup analysis.
Children (aged 18 years or less) and adults (> 18 years).
Dose of ICS; low (< 400 µg), moderate (400 µg to 800 µg), high (> 800 µg) of beclomethasone equivalent.
Severity of bronchiectasis (based on lung function).
Sensitivity analysis
We also planned sensitivity analyses to assess the impact of the following potentially important factors on the overall outcomes.
Study quality.
Variation in the inclusion criteria.
Differences in the medications used in the intervention and comparison groups.
Analysis using random‐effects model.
Analysis by 'treatment received'.
Results
Description of studies
Results of the search
For the updated review in 2009 the Airways Group Register identified 341 potentially relevant titles. After assessing the abstracts, we obtained nine papers for consideration for inclusion in the review. We excluded three studies as inhaled corticosteroids (ICS) were not compared to placebo/no treatment or were non‐randomised studies or included participants with pneumonia (Ghosh 2002; Monton 1999; ONeil 2004; see Characteristics of excluded studies table).
For this 2018 update, we conducted searches from 2011 to 30 June 2017 and the Cochrane Airways Group's Register identified 111 new references; we identified an additional 277 titles through searches of ClinicalTrials.gov (www.ClinicalTrials.gov), and the WHO trials portal (www.who.int/ictrp/en/). After assessing the abstracts, we obtained three articles for consideration to be included in the review; we excluded two studies because ICS was compared to an ICS‐long‐acting beta2‐agonist (LABA) combination (Martinez‐Garcia 2012), or was a non‐randomised study (Guran 2008). We included one new study in this update (Hernando 2012) (Figure 1).
Included studies
See Characteristics of included studies and Table 3 'Summary of included studies characteristics'.
1. Summary of included studies characteristics.
| Study ID | Intervention | Control | Duration of intervention |
| Elborn 1992 | Beclomethasone dipropionate 750 µg twice daily by MDI | Placebo | 6 weeks |
| Hernando 2012 | Budesonide 400 µg twice daily | Placebo | 6 months |
| Joshi 2004 | Beclomethasone 800 µg per day over 2 doses | Placebo | 4 weeks |
| Martinez‐Garcia 2006 | Fluticasone 500 µg twice daily by MDI or 250 µg fluticasone twice daily | No treatment | 6 months |
| Tsang 1998 | Fluticasone 500 µg twice daily by accuhaler | Placebo | 4 weeks |
| Tsang 2004 | Fluticasone 500 µg twice daily by accuhaler | Placebo | 52 weeks |
| Tsang 2005 | Fluticasone 500 µg twice daily by accuhaler | Placebo | 52 weeks |
MDI: metered dose inhaler
We identified one new study for this review update (Hernando 2012), including 77 participants. A total of seven studies met the inclusion criteria for this review update (Elborn 1992; Hernando 2012; Joshi 2004; Martinez‐Garcia 2006; Tsang 1998; Tsang 2004; Tsang 2005). All included studies identified were published in English. We requested additional data from all study authors but only three authors responded (Hernando 2012; Joshi 2004; Martinez‐Garcia 2006), and provided additional data. We also requested data on outcomes at 24 weeks for the Tsang 2005 study as well as the lung function values in actual volumes (instead of percentage predicted) from the study authors.
Study design and population
All seven studies were single centre studies. The studies included participants with bronchiectasis diagnosed on bronchography (Elborn 1992), or high resolution computed tomography (HRCT) of the chest (Hernando 2012; Joshi 2004; Martinez‐Garcia 2006; Tsang 1998; Tsang 2004; Tsang 2005). All studies excluded participants with cystic fibrosis, with six studies excluding participants with bronchial asthma also (Hernando 2012; Joshi 2004; Martinez‐Garcia 2006; Tsang 1998; Tsang 2004; Tsang 2005). Participants with allergic bronchopulmonary aspergillosis were also excluded from the Elborn 1992 study and the Martinez‐Garcia 2006 study. None of the studies included any children.
The Joshi 2004 and Elborn 1992 studies were cross‐over studies and the others were parallel group studies. All were double‐blind placebo controlled trials except the Martinez‐Garcia 2006 study, which did not use placebo in the control group.
Participants with bronchiectasis were recruited during stable state, defined as "free from exacerbation for four weeks" (Hernando 2012; Joshi 2004; Martinez‐Garcia 2006), or "stable 24‐hour sputum volume, FEV1 and FVC" (Tsang 1998; Tsang 2004; Tsang 2005). No study was performed during an acute respiratory exacerbation.
Interventions
Moderate to high doses of ICS were used: budesonide 800 µg/day (Hernando 2012), beclomethasone 800 µg/day (Joshi 2004), beclomethasone 1500 µg/day in the Elborn 1992 study and fluticasone 1000 µg/day (2000 µg/day budesonide equivalent) (Martinez‐Garcia 2006; Tsang 1998;Tsang 2004;Tsang 2005). The Martinez‐Garcia 2006 study had a third arm with inhaled fluticasone 500 µg/day and we combined this data with the 1000 µg/day arm and compared them as a single group with the control group wherever possible.
The study duration ranged from a short duration of four to six weeks in the Elborn 1992, Joshi 2004 and Tsang 1998 studies to six months in the Martinez‐Garcia 2006 and Hernando 2012 studies. Two studies were of one year duration (Tsang 2004;Tsang 2005), with visits at four, 12, 24, 36 and 52 weeks.
Outcomes
Lung functions were available as an outcome variable in all studies except the Tsang 2004 study, which reported only fractional exhaled nitric oxide (FeNO) levels. Forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) (in litres or % predicted) were available from all studies. Peak expiratory flow rate (PEFR) was reported in the Elborn 1992, Joshi 2004 and Tsang 1998 studies, with the Martinez‐Garcia 2006 and Tsang 1998 studies giving details about total lung capacity, residual volume and diffusion capacity. We did not include spirometric parameters from the Joshi 2004 study in the final analysis since the 'pre‐treatment' values were reported for the whole 20 group together and these values were not available separately for the two groups at baseline. We also did not include data from the Tsang 1998 and Tsang 2004 studies in the final analysis, since geometric mean was used instead of arithmetic mean. We did not include any of the data from the Elborn 1992 study, since pre‐cross‐over data were not available separately.
Clinical parameters of cough, wheeze and dyspnoea were measured differently in different studies. The Elborn 1992 study used a visual analogue scale (VAS) to quantify these symptoms, the Martinez‐Garcia 2006 study defined significant cough as that persisting for > 50% of days. Dyspnoea was measured by using the transition dyspnoea index in the Martinez‐Garcia 2006 study. The Hernando 2012 study measured cough, sputum production and dyspnoea on a scale of 0 to 3 and combined these three as a total symptom score. Clinical parameters of cough, dyspnoea and wheezing, although reported by the Tsang 2005 study, were not defined properly and we did not include them in the analysis. Twenty‐four‐hour sputum volume was included as an outcome variable in the Elborn 1992, Martinez‐Garcia 2006, Tsang 1998 and Tsang 2005 studies.
Quality of life was included as an outcome parameter in the Martinez‐Garcia 2006 and Hernando 2012 studies, with both using the St. George Respiratory Questionnaire to calculate total scores as well as symptoms, activity and impact score.
The Martinez‐Garcia 2006, Tsang 1998 and Tsang 2005 studies defined exacerbation as persistent (> 24 hours) deterioration in at least three respiratory symptoms (including cough, dyspnoea, haemoptysis, increased sputum purulence or volume, and chest pain), with or without fever, radiographic deterioration, systemic disturbances, or deterioration in chest signs. The Hernando 2012 study defined exacerbation as worsening of more than 48 hours duration of at least three of the four symptoms of cough, sputum production, dyspnoea and fever.
The study characteristics are described in the 'Characteristics of included studies' table.
Excluded studies
We excluded five studies from the review. The reason for their exclusion is discussed in the 'Characteristics of excluded studies' table. The most common reason for exclusion was the study not being a RCT (two studies).
Risk of bias in included studies
Risk of bias judgements for included studies are summarised in Figure 2 and Figure 3.
2.
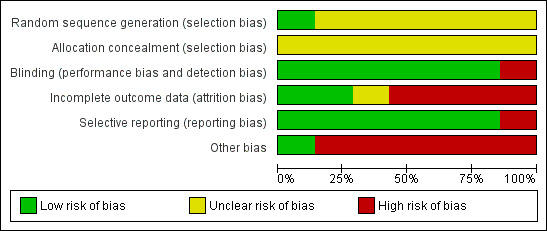
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
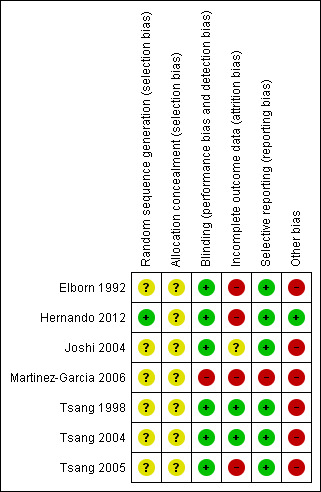
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The generation of randomisation sequence was not reported in six studies and so we judged risk of bias to be unclear (Elborn 1992; Joshi 2004; Martinez‐Garcia 2006; Tsang 1998; Tsang 2004; Tsang 2005). Only one study stated how the random sequence was generated and we judged this to have low risk of bias (Hernando 2012). Allocation concealment was unclear in all seven studies (Elborn 1992; Hernando 2012; Joshi 2004; Martinez‐Garcia 2006; Tsang 1998; Tsang 2004; Tsang 2005).
Blinding
We judged all studies as low risk as they were double‐blind, except Martinez‐Garcia 2006, which we judged as high risk as they did not have a placebo arm, and the blinding was done only for comparing two dosages of ICS.
Incomplete outcome data
Two studies reported the progress of all randomised participants in each group (Tsang 1998; Tsang 2004), resulting in low risk of bias, whereas in Joshi 2004, the bias was unclear as there was no mention of withdrawals or dropouts. We judged four studies as high risk of bias as they did not describe where their dropouts occurred (Elborn 1992; Hernando 2012; Martinez‐Garcia 2006; Tsang 2005). Furthermore, we could not extract pre‐cross‐over arm data from Elborn 1992 or include it in any of the meta‐analysis. Follow‐up was between 80% to 90% in Tsang 2005 and was unclear in Joshi 2004. The remaining studies reported outcomes in > 90% of the participants (Hernando 2012; Martinez‐Garcia 2006; Tsang 1998; Tsang 2004).
Selective reporting
We judged six studies to have low risk of bias (Elborn 1992; Hernando 2012; Joshi 2004; Tsang 1998; Tsang 2004; Tsang 2005), as they reported all data for outcomes. One study did not report all the outcome data for the 500 µg/day arm (Martinez‐Garcia 2006), therefore we judged it at high risk of bias.
Other potential sources of bias
We judged six studies as potentially having high risk of bias in other areas (Elborn 1992; Joshi 2004; Martinez‐Garcia 2006; Tsang 1998; Tsang 2004; Tsang 2005). The baseline values for lung functions, sputum amount and sputum inflammatory markers were significantly different clinically between the arms in three studies (Tsang 1998; Tsang 2004; Tsang 2005). We judged Elborn 1992 as high risk due to the cross‐over design with no washout period. Joshi 2004 included participants with significant post‐bronchodilator response which indicates ICS would be beneficial. Martinez‐Garcia 2006 did not complete ITT analysis.
Effects of interventions
Summary of findings for the main comparison. Inhaled corticosteroids compared to placebo for bronchiectasis (short‐term use of 6 months or less).
| Inhaled corticosteroids compared to placebo for bronchiectasis (< 6 months) | ||||||
| Patient or population: people with bronchiectasis Setting: university hospital Intervention: inhaled corticosteroids Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Inhaled corticosteroids | |||||
| Lung function (spirometry indices) ‐ FEV1 (in L, end study minus baseline values) | Mean change from baseline ranged from ‐0.038 to 0.805 | MD 0.09 lower (0.26 lower to 0.09 higher) | ‐ | 156 (2 studies) | ⊕⊕⊝⊝ Low1,2,3 |
|
| Lung function (spirometry indices) ‐ FVC (in L, end study minus baseline values) | Mean change from baseline ranged from ‐0.062 to 0.0218 | MD 0.01 higher (0.16 lower to 0.17 higher) | ‐ | 156 (2 studies) | ⊕⊕⊝⊝ Low1,2,3 |
|
| Lung function (other indices) ‐ diffusion capacity % predicted (end of study) | Mean end of study value 84.2 | MD 2.70 higher (2.49 lower to 7.89 higher) | ‐ | 57 (1 study) | ⊕⊕⊝⊝ Low1,2,3 |
|
| Lung function (other indices) ‐ RV % predicted (end of study values) | Mean end of study value 106 | MD 2.00 higher (9.41 lower to 13.41 higher) | ‐ | 57 (1 study) | ⊕⊕⊝⊝ Low1,2,3 |
|
| Lung function (other indices) ‐ TLC % predicted (end of study values) | Mean end of study value 86.4 | MD 3.20 higher (1.99 lower to 8.39 higher) | ‐ | 57 (1 study) | ⊕⊕⊝⊝ Low1,2,3 |
|
| Average number of exacerbations per participant | Average number of exacerbations per patient ranged from 0.97 to 1.31 | MD 0.17 lower (0.56 lower to 0.22 higher) | ‐ | 127 (2 studies) | ⊕⊕⊝⊝ Low1,2,3 |
|
| Pseudomonas aeruginosa (P aeruginosa) colonisation | 410 per 1000 | 395 per 1000 (238 to 576) | OR 0.94 (0.45 to 1.96) | 156 (2 studies) | ⊕⊕⊝⊝ Low1,2,3 |
|
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; MD: mean difference; OR: odds ratio; RV: residual volume; TLC: total lung capacity | ||||||
| GRADE Working Group grades of evidence High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate‐certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low‐certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low‐certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1The largest study was not a placebo controlled trial (Martinez‐Garcia 2006). 2Study participant numbers are small; outcome downgraded one point for imprecision. 3One study had an issue with directness (Joshi 2004), but did not contribute to this outcome.
Summary of findings 2. Inhaled corticosteroids compared to placebo for bronchiectasis (longer‐term use of > 6 months).
| Inhaled corticosteroids compared to placebo for bronchiectasis (medium‐ to long‐term outcomes) | ||||||
| Patient or population: people with bronchiectasis Setting: university hospital Intervention: inhaled corticosteroids Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Inhaled corticosteroids | |||||
| Lung function indices ‐ FEV1% predicted (end study minus baseline values) | Mean change from baseline 0 | MD 0.30 higher (17.43 lower to 18.03 higher) | ‐ | 86 (1 study) | ⊕⊕⊝⊝ Low1,2 |
|
| Lung function indices ‐ FVC % predicted (end study minus baseline values) | Mean change from baseline 0.9 | MD 0.90 lower (14.59 lower to 12.79 higher) | ‐ | 86 (1 study) | ⊕⊕⊝⊝ Low1,2 |
|
| Number of participants improved | 628 per 1000 | 490 per 1000 (288 to 693) | OR 0.57 (0.24 to 1.34) | 86 (1 study) | ⊕⊕⊝⊝ Low1,2 |
|
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; MD: mean difference; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate‐certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low‐certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect Very low‐certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect | ||||||
1Only a single study with small participant numbers; we downgraded outcome one point for imprecision. 2Single study with high/unclear risk of bias in several domains; we downgraded outcome one point for limitations.
The seven studies involved 380 participants, with 348 completing the studies (Elborn 1992; Hernando 2012; Joshi 2004; Martinez‐Garcia 2006; Tsang 1998; Tsang 2004; Tsang 2005). The data that we could include in the meta‐analysis were very limited, with just two studies contributing most data (Hernando 2012; Martinez‐Garcia 2006).
Stable state bronchiectasis: short‐term (≤ 6 months) outcomes
We included data from the Hernando 2012 and Martinez‐Garcia 2006 studies in the short‐term stable state analysis. See Table 1 for the main comparisons.
Primary outcome: lung function
We included date from two studies in this meta‐analysis (Hernando 2012; Martinez‐Garcia 2006). We used change from baseline data to remove bias in differences at baseline. For both FEV1 and FVC, we combined data of the two steroid dose arms for the Martinez‐Garcia 2006 study into a single intervention arm when comparing against the no steroid arm. For FEV1, the pooled data showed no difference between the groups (mean difference (MD) ‐0.09, 95% confidence interval (CI) ‐0.26 to 0.09; participants = 156; Analysis 1.1). A similar result was seen for FVC (MD 0.01, 95% CI ‐0.16 to 0.17; participants = 156; Analysis 1.1), with no significant heterogeneity between studies. The Elborn 1992 study also reported an improvement in the FEV1 in the ICS group compared to the placebo group (P = 0.03), but not in FVC; as the values were not reported, we were unable to pool this.
1.1. Analysis.
Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 1 Lung function (spirometry indices).
Based on a single study from which we could include data in the final analysis, ICS showed no difference in the following outcomes: diffusing capacity of the lungs for carbon monoxide (DLCO) (MD 2.70, 95% CI ‐2.49 to 7.89), residual volume (MD 2.00, 95% CI ‐9.41 to 13.41) and total lung capacity (MD 3.20, 95% CI ‐1.99 to 8.39) . We only used the 500 µg twice daily arm, since data on the lower dose ICS arm were not available (Martinez‐Garcia 2006).
Secondary outcome: clinical severity
We could only display data from a single non‐placebo study for these clinical parameters (Martinez‐Garcia 2006). We combined data from the two ICS dose arms for wheeze and sputum production, but not for dyspnoea, since lower dose steroid data were unavailable. The number of participants with sputum reduction as well as with improvement in dyspnoea was significantly higher in the ICS arm compared to the control arm (Analysis 1.3). There were no differences between groups for the clinical parameters of cough and wheeze. Although the Martinez‐Garcia 2006 study described a significant difference between groups for the number of participants experiencing reduced cough, we found no difference between groups when we performed ITT analyses. Also, as the methodology of subjective cough measures was not a validated method, we did not display this data as a forest plot. Although we did not include date from the Elborn 1992 study in the final analysis, the study reported that the ICS group had a significant improvement in cough (P = 0.02) but not wheeze and dyspnoea. Again, only the P value was reported. The data from the Hernando 2012 study showed no significant improvement in the total symptom score in the group on ICS compared to control (MD ‐3.22, 95% CI ‐8.15 to 1.71), but we could not include the data since the subdivision of the score was not available. None of the other studies reported these clinical outcomes.
1.3. Analysis.
Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 3 Clinical severity indices.
Secondary outcome: exacerbations
Data on average number of exacerbations per participant were available from two studies (Hernando 2012; Martinez‐Garcia 2006). The combined data showed no difference between the two groups (MD ‐0.17, 95% CI ‐0.56 to 0.22; participants = 127; Analysis 1.4). In the Tsang 1998 study, one participant in the fluticasone group experienced an exacerbation compared with three participants in the placebo group. In the Hernando 2012 study, the mean numbers of exacerbations in the ICS group was 0.68 compared to 0.97 in the placebo group, though this was not reported to be statistically significant. In the same study, a much higher proportion of participants (12%) needed hospital admission in the placebo group compared to the ICS group (2.7%), but this was not statistically significant. We only used the 500 µg twice daily arm for the Martinez‐Garcia 2006 study, since data on the lower dose ICS arm were not available for this parameter.
1.4. Analysis.
Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 4 Exacerbations.
Secondary outcome: sputum and biomarker characteristics
The Martinez‐Garcia 2006 study was the only study included in this analysis which showed a trend towards reduction in the sputum volume (MD ‐8.30, 95% CI ‐16.55 to ‐0.05; participants = 57; Analysis 1.5). We only used the 500 µg twice daily arm for the Martinez‐Garcia 2006 study, since data on the low dose ICS arm were not available. We did not include data on sputum volume and sputum inflammatory markers from the Tsang 1998 study in the final analysis due to clinically significant differences in the baseline value between the two groups. The Elborn 1992 study also described a significant improvement in the 24‐hour sputum volume in the ICS group (P = 0.003), but neither the size of the effect nor the CI was provided. The other studies did not include this as an outcome variable.
1.5. Analysis.
Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 5 Sputum and biomarkers characteristics.
Secondary outcome: fractional exhaled nitric oxide (FeNO)
The Tsang 2004 study showed no change in the FeNO between the two groups at 24 weeks. Since the data were skewed and geometric means were reported, we did not include this data in the analysis.
The proportion of eosinophils as a percentage of total cells were significantly less in the sputum at the end of ICS therapy compared to the end of placebo therapy in the Hernando 2012 study, although all other inflammatory sputum markers, including IL‐8, did not show any significant difference.
Secondary outcome: Pseudomonas colonisation
Data on proportion of participants colonised with P aeruginosa at the end of treatment were available from two studies (Hernando 2012; Martinez‐Garcia 2006), with pooled data showing no increased risk of P aeruginosa colonisation with ICS therapy. We used combined data for the two steroid dose arms for the Martinez‐Garcia 2006 study for this parameter. We included extra data provided by the Hernando 2012 study in this analysis.
The Tsang 1998 study explained that the density of P aeruginosa in the sputum was similar between groups at the end of treatment (median density of 1.0 x 10E7 cfu/mL (inter‐quartile range (IQR) 0.33 to 5.15) in the ICS group and 1.4 x 10E7 cfu/mL (IQR 0.7 to 4.67) in the placebo group.
However, the density of total bacteria and commensal bacteria in sputum increased after four weeks of therapy with ICS when compared to baseline (median baseline value was 2.6 x 10E7 cfu/mL (IQR 0.94 to 5.28) and median post‐treatment value was 11.6 x 10E7 cfu/mL (IQR 0.34 to 33.8) whilst the corresponding value in the placebo group was lower post‐treatment (median baseline value was 2.6 x 10E7 cfu/mL (IQR 0.34 to 4.3) and median post‐treatment value was 1.3 x 10E7 cfu/mL (IQR 0.55 to 2.75). The post‐treatment between‐group comparisons P value did not reach statistical significance (P = 0.06) (Tsang 1998).
Secondary outcome: quality of life
Data were available from two studies (Hernando 2012; Martinez‐Garcia 2006). When data of the two ICS dose arms were combined in the Martinez‐Garcia 2006 study, and clinical improvement in health‐related quality of life (HRQoL) analysed as a dichotomous variable, significantly more people in the ICS group experienced a clinically important improvement in their HRQoL score compared to the no treatment group (Analysis 1.3.4). The Hernando 2012 study reported worsening in total quality of life score in both the ICS and placebo group when compared to baseline, but the between‐group difference was not statistically significant (MD ‐3.22, 95% CI ‐8.15 to 1.71; participants = 70; Analysis 1.7). When we pooled the data from the two included studies, there was a statistically significant improvement in activity score but not for total scores or symptom score (Analysis 1.7). Only 500 µg twice daily ICS data were available for these parameters. Impact score data were only available from the Hernando 2012 study.
1.7. Analysis.
Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 7 St George HRQoL (end of study minus baseline).
Secondary outcome: adverse events
Only one study reported adverse events (Martinez‐Garcia 2006), and it was more frequent in the treatment group receiving 1000 µg versus 500 µg (19 participants versus 7, P = 0.04). The most common side effects were dry mouth, local irritation and transient dysphonia.
Stable state bronchiectasis: long‐term (> 6 months) outcomes
We included data from the Tsang 2004 study and Tsang 2005 study in the long‐term stable state analysis, although the Tsang 2004 study had only FeNO as the outcome variable. See Table 2 for the main comparisons.
Primary outcome: lung function
For FEV1 % predicted (end study minus baseline values), data from the single study, Tsang 2005, showed no difference between the two groups (MD 0.30, 95% CI ‐17.43 to 18.03; participants = 86; Analysis 2.1).
2.1. Analysis.
Comparison 2 Stable state (> 6 months), Outcome 1 Lung function indices.
For FVC % predicted (end study minus baseline values), data from the single study, Tsang 2005, showed no difference between the two groups (MD ‐0.90, 95% CI ‐14.59 to 12.79; participants = 86; Analysis 2.1).
Secondary outcome: clinical severity
We did not include any data for the clinical parameters from the two studies of more than six months duration (Tsang 2004; Tsang 2005).
Secondary outcome: exacerbations
The Tsang 2005 study showed a non‐significant improvement in exacerbation frequency in the ICS group compared to the control group (odds ratio (OR) 0.57, 95% CI 0.24 to 1.34; participants = 86; Analysis 2.2).
2.2. Analysis.
Comparison 2 Stable state (> 6 months), Outcome 2 Exacerbations.
Secondary outcome: sputum and biomarker characteristics
As an overall effect, ICS had no effect on the 24‐hour sputum volume when given for a period of 52 weeks, although we did not include the data for this outcome in the analysis because only median and interquartile ranges were available and the data were very skewed. As a subgroup analysis, the Tsang 2005 study reported a significant improvement in the amount of sputum volume/day in the subgroup of participants with sputum volume < 30 mL/ day, exacerbation frequency ≤ two/year, and sputum purulence score > 5 (data not available).
Sputum purulence was scored as 0, 1, 2, 3, 4, 5, 6, 7 or 8 (absence of, completely transparent, almost transparent, translucent but colourless, opaque, milky white grey, pale green, moderately green, and dark green sputum, respectively) in the Tsang 2005 study. After 52 weeks, there was no significant difference in the purulence scores between the ICS and placebo groups (MD 0.2, 95% CI ‐0.94 to 1.34; participants = 86; Analysis 2.3).
2.3. Analysis.
Comparison 2 Stable state (> 6 months), Outcome 3 Sputum and biomarker characteristics.
Secondary outcome: FeNO
The Tsang 2004 study showed no change in the FeNO between the two groups at 52 weeks, although we did not include the data due to its skewed nature.
Secondary outcome: adverse events
None of the studies reported adverse events.
Sensitivity analysis
Removing the study with a poor quality score (no placebo) made no difference to the results for FEV1 and FVC. The activity subscore of the St. George Respiratory Questionnaire (a measure of HRQoL) became non‐significant when we removed the non‐placebo controlled study (Martinez‐Garcia 2006).
For the clinical data, when we analysed separately the data from the fluticasone 1000 µg arm of the Martinez‐Garcia 2006 study and the 500 µg arm, in both arms in the meta‐analysis, those on ICS still had a higher proportion of participants with sputum volume reduction of > 50%. For the outcomes of wheeze and cough there was still no difference between the groups. For the outcome of dyspnoea, actual data were not provided for the 500 µg arm but the study authors mentioned that there was no difference between groups. Hence it is assumed that the significant effect present for the 1000 µg/day group (outcome 1.3.3) was no longer present for the 500 µg/day group.
Analysis using random‐effects did not alter the significance of any of the outcomes. None of the other planned sensitivity analysis were relevant.
Discussion
Summary of main results
We did not identify any studies involving children in our searches. Seven studies involving adult participants fulfilled the inclusion criteria. Of the 380 randomised, 348 participants completed the trials. In the short‐term group ( inhaled corticosteroids (ICS) for ≤ 6 months), use of high dose ICS in adults with bronchiectasis did not lead to any statistical or clinically significant change in the lung function indices of forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC). Further, there was no statistically significant improvement in the overall health‐related quality of life (HRQoL) indices except in the activity score. There was improvement in sputum production and dyspnoea scores in the ICS group from the single study with available data for this outcome (Martinez‐Garcia 2006), but the result should be interpreted with caution as the study was not placebo controlled. When we only included placebo controlled studies, there were no significant differences between groups in all outcomes examined (spirometry, clinical outcomes of exacerbation or sputum volume, etc.). The single study on long‐term outcomes showed no significant benefit of ICS in any of the outcomes.
Overall completeness and applicability of evidence
This Cochrane review is limited to seven studies involving adult participants with variable designs, variable doses and length of study. Also, data that could be combined for the meta‐analyses were limited to only two studies for the various outcomes examined. As there were no paediatric studies, data from this review cannot be extrapolated to children. Further, in children, the adverse effect of high dose, or long‐term ICS, or both, is likely to be more serious than in adults, in particular to the detrimental effect on linear growth (Philip 2014).
The meta‐analyses derived from two studies of the short‐term effect of ICS in bronchiectasis showed no benefit of ICS on lung function parameters of FEV1 and FVC (compared to controls), though the data included were limited (Hernando 2012; Martinez‐Garcia 2006). There was improvement in clinical parameters of dyspnoea and sputum production in the ICS group, based on data from the single study which was not placebo controlled. Similar improvement was also seen in the activity score of HRQoL in the ICS group, though the effect was marginal. We could not include data from Joshi 2004 and Tsang 1998 in the final analysis, but both studies had not reported any improvement in the lung function parameters between the steroid and placebo groups. On the contrary, data from the Elborn 1992 study (which we could not include in the meta‐analysis) showed a significant improvement in the FEV1 in the ICS group compared to the placebo group. Thus, it remains uncertain whether ICS confers any benefit in people with bronchiectasis.
Data on the short‐term effect of ICS on clinical parameters of cough, wheeze, dyspnoea and sputum volume were available from two studies (Hernando 2012; Martinez‐Garcia 2006). The Martinez‐Garcia 2006 study showed a significant improvement in dyspnoea in the 1000 µg/day fluticasone group and sputum volume for the combined 500 µg/day and 1000 µg/day group compared to the control group, although no such effect was seen in the total symptom score from the Hernando 2012 study. We did not include data on sputum volume from the Tsang 1998 study in the analysis in view of the clinically significant difference in the baseline values. The Martinez‐Garcia 2006 study showed a significant improvement in the quality of life score in the ICS group using the St. George Respiratory Questionnaire, though no such improvement was reported in the Hernando 2012 study. On pooling these data, the effect favoured ICS use largely due to the large effect of the non‐placebo controlled study (Martinez‐Garcia 2006).
Recurrent acute pulmonary exacerbations form part of the disease progression in patients with bronchiectasis and many of these exacerbations require hospital admission. Recurrent exacerbations are associated with progressive deterioration of lung functions (Kapur 2010), and are also one of the strong predictors of poor quality of life in bronchiectasis (Wilson 1997). In this review, based on limited data, short‐term use of ICS did not influence frequency of exacerbations. Prolonged ICS administration also did not influence exacerbation frequency (Tsang 2005). This becomes more relevant with the fact that ICS actually increased the bacterial density in the airways and most exacerbations in bronchiectasis are likely to be infective in origin (Tsang 1998).
Administration of ICS for a longer duration significantly increased the number of participants who had a more than 20% reduction in the 24‐hour sputum volume, but did not have any beneficial effect in the other clinical or spirometric parameters (Tsang 2004; Tsang 2005). This lack of effect again suggests that infection and not pure inflammation, is probably the more relevant underlying pathogenic mechanism of disease progression in bronchiectasis.
The ICS doses used in several studies were very high (up to 2000 µg/day budesonide equivalent). This limits applicability of the data to most patients in our current era where ICS doses used are generally lower, in light of the increased appreciation of adverse events related to prolonged use of high ICS doses, such as the effect on linear growth (Philip 2014). High dose ICS use is also associated with adverse events in children and adults that range from mild events (candidiasis) to serious events (pneumonia, adrenal insufficiency, osteoporosis, cataracts) (Sobieraj 2008). The Martinez‐Garcia 2006 study reported dry mouth, local irritation and transient dysphonia as the most common adverse effects. None of the other studies reported any adverse effects. While there was no significant difference in adverse events between groups, the total sample size was small, rendering a likely type‐1 error. The potential harm associated with prolonged ICS use, or high ICS doses, or both, need to be considered along with its potential benefit.
Bronchiectasis is a heterogenous condition, with a substantial overlap in people with chronic obstructive pulmonary disease (COPD). Not all the studies in this review excluded smokers and thus, whether data in our review can be universally applied is unknown. Nearly 50% of adults with COPD have bronchiectasis (Chang 2008). A Cochrane Review concluded that ICS administration reduces the rate of exacerbations as well as the rate of decline in quality of life in patients with stable state COPD (Yang 2012). As the Martinez‐Garcia 2006 study included participants who were smokers, there is a possibility that some of these participants had COPD and this may influence the positive findings in that study. It is possible that COPD‐associated bronchiectasis may respond differently to treatment modalities when compared to bronchiectasis associated with other causes.
Persistent inflammation plays a role in deterioration of lung function in bronchiectasis (Ip 1993). Studies in adults with cystic fibrosis suggest that ICS treatment improves bronchial hyper‐responsiveness and spirometric parameters (Van Haren 1995). Thus, it is theoretically possible that ICS may improve lung function, and with it clinical parameters in non‐cystic fibrosis bronchiectasis as well, although this review has shown that any benefit from ICS is inconsistent. However, given the increased presence of airway hyper‐responsiveness in patients with non‐cystic fibrosis bronchiectasis (Ip 1991), it is possible that ICS may have a role in this subgroup.
The bacteriology of sputum influences future morbidity and mortality; specifically, people with Pseudomonas aeruginosa (P aeruginosa) have more rapid lung function decline (Finch 2015). As the bacteriology in all the included studies were unclear, the applicability of this review in the different subsets of people with various bacteriology such as mycobacteria and P aeruginosa is also uncertain.
In the consideration of using ICS, especially long‐term ICS (> 6 months) in patients, clinicians should be cognisant of the possible effect of corticosteroids on cell immunity dysregulation (Sabroe 2013). Biologically, this effect could lead to an increase in infections that could worsen clinical outcomes, such as lung function and exacerbations (Sabroe 2013). Indeed, adults with COPD on ICS have an increased unadjusted risk of having pneumonia (Festic 2016). Although this adverse event is a clinically important issue in people with bronchiectasis, only one study specifically reported on adverse events and thus we could not assess this in this review. However, in the ICS group of shorter duration (6 months or less), two studies found no increased risk of P aeruginosa in the ICS arm compared to placebo (Analysis 1.6). One study found a non‐significant increase in sputum bacterial density in the ICS group (Tsang 1998), whilst there was a non‐decrease in the placebo group (between‐groups post‐treatment P = 0.06).
1.6. Analysis.
Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 6 Pseudomonas aeruginosa colonisation.
Quality of the evidence
The overall quality of evidence was low (Table 1; Table 2). The sample size and number of studies were small. The major contributor to the benefit of ICS found in the meta‐analyses was from a non‐placebo controlled study. The studies also did not exclude participants with airway hyper‐responsiveness or coexistent COPD, hence the potential of skewing the results in favour of ICS.
Potential biases in the review process
We conducted this review in accordance with a prespecified protocol. We have outlined changes from the protocol in the Differences between protocol and review section and we did not identify any major sources of bias in the review process.
Agreements and disagreements with other studies or reviews
Our findings are similar to that outlined in bronchiectasis guidelines of the British Thoracic Society (Pasteur 2010), and the Thoracic Society of Australia and New Zealand (Chang 2015). We found no other published systematic reviews on the use of ICS for people with bronchiectasis.
Whilst a separate disease, cystic fibrosis lung disease manifests with bronchiectasis. Studies in cystic fibrosis have also shown no benefits with ICS. A Cochrane Review of ICS in cystic fibrosis concluded that there is insufficient evidence to determine if they are beneficial or harmful (Balfour‐Lynn 2014). In a large prospective, multicentre study, withdrawal of ICS for six months was not associated with significant worsening of cystic fibrosis lung disease (Balfour‐Lynn 2006). Participants in whom ICS were discontinued did not have a change in lung function over time, an increased need for oral or intravenous antibiotics, or a shorter time to pulmonary exacerbation (Balfour‐Lynn 2006).
Authors' conclusions
Implications for practice.
In this review update, we identified one new study, adding a further 77 participants; however, these additional results did not change the original conclusions. The present review continues to indicate that there is insufficient evidence to suggest the routine use of inhaled corticosteroids (ICS) in adults with stable state bronchiectasis. The only study that showed a benefit was a non‐placebo controlled trial (Martinez‐Garcia 2006). Insufficient data precludes any definite conclusion on the use of ICS in adults during an acute exacerbation, or in children (for any state) as there were no studies.
Implications for research.
Further studies are required to examine the effect of ICS on short‐ and long‐term outcomes for children and adults with non‐cystic fibrosis bronchiectasis. Outcomes should include exacerbations (rate and hospital admission), symptoms, quality of life, lung function indices and inflammatory parameters and bacteriology. Studies are required in both stable state and during an acute exacerbation state. Studies involving adults should clearly differentiate coexistent COPD, as the presence of this may influence effectiveness of ICS. A priori analysis for those with bronchial hyper‐responsiveness should also be defined, since its presence may influence ICS response.
What's new
| Date | Event | Description |
|---|---|---|
| 30 June 2017 | New search has been performed | Literature search run. |
| 30 June 2017 | New citation required but conclusions have not changed | One new study included in the review. Addition of author. Review updated to reflect current Cochrane methods including addition of 'Summary of findings' table and PRISMA flow chart. See Differences between protocol and review. |
History
Protocol first published: Issue 2, 1997 Review first published: Issue 2, 1999
| Date | Event | Description |
|---|---|---|
| 15 August 2008 | New citation required and conclusions have changed | Aug 2008: new author team, protocol changed and previous included studies data amended, new studies added, conclusions changed. |
| 5 May 2008 | Amended | Converted to new review format. |
| 10 September 2007 | New search has been performed | New literature search performed. |
Acknowledgements
We thank Elizabeth Stovold for performing the search and Dr Chris Cates and the Cochrane Airways Group for their support.
Rebecca Normansell was the Editor for this review and commented critically on the review.
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
Appendices
Appendix 1. Database search strategies
| Database | Search | |
| Airways Register (searched through the Cochrane Register of Studies) | steroid* or corticosteroid* or glucocorticoid* or beclomet* or budesonide or fluticasone OR ciclesonide or triamcinolone or flunisolide | |
| CENTRAL and MEDLINE (Ovid) strategy (MEDLINE search combined with RCT search filter) |
#1 MeSH descriptor Bronchiectasis explode all trees #2 bronchiect* #3 (#1 OR #2) #4 MeSH descriptor Adrenal Cortex Hormones explode all trees #5 steroid* #6 corticosteroid* #7 glucocorticoid* #8 beclomet* #9 0fluticasone #11 ciclesonide #12 flunisolide #13 triamcinolone #14 (#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #12) #15 (#3 AND #14) | |
| Embase (Ovid) strategy (combined with RCT search filter) |
#1 Emtree descriptor Bronchiectasis explode all trees #2 bronchiect* #3 (#1 OR #2) #4 Emtree descriptor Corticosteroid explode all trees #5 steroid* #6 corticosteroid* #7 glucocorticoid* #8 beclomet* #9 budesonide #10 fluticasone #11 ciclesonide #12 flunisolide #13 triamcinolone #14 (#4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #12) #15 (#3 AND #14) |
Appendix 2. Search strategy to identify relevant trials from ClinicalTrials.gov and WHO trials portal
"inhaled corticosteroid" AND "bronchiectasis" AND "clinical trials"
Data and analyses
Comparison 1. Stable state bronchiectasis (6 months or less).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lung function (spirometry indices) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 FEV1 (in L, end study minus baseline values) | 2 | 156 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.26, 0.09] |
| 1.2 FVC (in L, end study minus baseline values) | 2 | 156 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.16, 0.17] |
| 2 Lung function (other indices) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Diffusion capacity % predicted (end of study) | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 2.70 [‐2.49, 7.89] |
| 2.2 RV % predicted (end of study values) | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐9.41, 13.41] |
| 2.3 TLC % predicted (end of study values) | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [‐1.99, 8.39] |
| 3 Clinical severity indices | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Number of participants with regular wheeze (combined) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Number of participants without sputum reduction of > 50% (combined) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Number of participants with no improvement in dyspnoea score > 1 (min important difference) (1000F) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Number of participants with no clinically significant improvement in HRQoL | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Exacerbations | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.56, 0.22] |
| 4.1 Average number of exacerbations per participant | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.56, 0.22] |
| 5 Sputum and biomarkers characteristics | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Sputum volume or weight (per day) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Pseudomonas aeruginosa colonisation | 2 | 156 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.45, 1.96] |
| 7 St George HRQoL (end of study minus baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Total score | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐3.54 [‐8.00, 0.92] |
| 7.2 Symptom score | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐4.75 [‐10.42, 0.92] |
| 7.3 Activity score | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐6.21 [‐12.40, ‐0.01] |
| 7.4 Impact score | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐3.63 [‐9.35, 2.09] |
1.2. Analysis.
Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 2 Lung function (other indices).
Comparison 2. Stable state (> 6 months).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Lung function indices | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 FEV1% predicted (end study minus baseline values) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 FVC % predicted (end study minus baseline values) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Exacerbations | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Number of participants improved | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Sputum and biomarker characteristics | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Sputum purulence score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Elborn 1992.
| Methods | A prospective, double‐blind, placebo controlled, randomised cross‐over design with study duration of 6 weeks. There were five participants who dropped out; we are unsure when these occurred. Two participants declined to take part in second limb of study. No washout period mentioned. |
|
| Participants | Twenty participants (12 females, mean age 50 years, range 30 to 65) were studied with bronchiectasis diagnosed by bronchogram in 18 and computed tomography scan in two. No participant received a course of antibiotics for at least 8 weeks prior to the study. Exclusion: participants with hypogammaglobulinaemia, cystic fibrosis, ABPA or primary ciliary dyskinesia, as well as those taking OCS or ICS. |
|
| Interventions | Inhaled beclomethasone dipropionate 750 µg twice daily by MDI or placebo for 6 weeks. | |
| Outcomes |
|
|
| Notes | Cross‐over design with no washout period. Separate results of first arm not available. Data not included in analysis. No funding source mentioned, though acknowledgement for ICS was given to Allen and Hanburys. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided within the published article about generation of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | No information about concealment was reported in the article. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double‐blinded" and "matched placebo". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were 5 patients who dropped out; we are unsure when these occurred. Two patients declined to take part in second arm of study. |
| Selective reporting (reporting bias) | Low risk | No suggestion that selective reporting may have been done. |
| Other bias | High risk | Cross‐over design with no washout period. Separate results of first arm not available. |
Hernando 2012.
| Methods | A prospective, double‐blind, parallel group placebo controlled trial. | |
| Participants | 77 participants included over a 3‐year period. Age range 40 to 85 years, mean age 68.06 years. Seven did not complete the study: three died from respiratory failure and four pulled out voluntarily. 37 analysed in the budesonide group and 33 in the placebo group. Mean age of the budesonide group was 68 years and that of the placebo group was 66 years. 19 (27.1%) participants in the budesonide group and 18 (25.7%) in the placebo group had Pseudomonas aeruginosa (P aeruginosa) cultured from their sputum. The mean (SD) baseline FEV1% predicted in the budesonide and the placebo group was 64.6% (25.1) and 64.7% (27), respectively. | |
| Interventions | 400 µg inhaled budesonide twice a day versus placebo for 6 months. | |
| Outcomes | Total symptom score which included I) cough and sputum production; and ii) dyspnoea.
|
|
| Notes | Additional information provided by the authors. The study was supported by the Catalan Foundation of Pneumology grant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Hospital pharmacy (third party) performed randomisation and dispensed inhalers. |
| Allocation concealment (selection bias) | Unclear risk | No mention how allocation was done. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote "parallel, placebo‐masked clinical trial". Probably done. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7 dropouts, 6 of whom were from the placebo group. Data of dropouts not included. Since 3 of these died of respiratory failure, all belonging to the placebo group, potential for bias on outcome if data not included. |
| Selective reporting (reporting bias) | Low risk | No suggestion that selective reporting may have been done. |
| Other bias | Low risk | No other risk of bias identified |
Joshi 2004.
| Methods | Randomised double‐blind, placebo controlled, cross‐over study with study duration of 4 weeks. Two‐week washout period between cross‐over. Details of dropouts not clear. |
|
| Participants | 20 participants (9 females) age range 15 to 60 years were prospectively enrolled. All participants treated with oral salbutamol 2 mg four times daily and oral theophylline 200 mg four times daily throughout the trial period. 14 participants had unilateral disease and six had bilateral disease. Inclusion: bronchiectasis confirmed by HRCT, chest in stable state (no exacerbation in previous 1 month) demonstrating significant post‐bronchodilator response (> 12% change) on spirometry Exclusion: atopy, bronchial asthma or smoking |
|
| Interventions | Inhaled beclomethasone 800 µg/day in two divided doses by MDI or placebo for 4 weeks. | |
| Outcomes |
|
|
| Notes | SD calculated from P value. Only first arm of the study before cross‐over used in analysis. Additional information provided by the authors. Spirometeric data not included in the analysis since baseline values not reported separately for the two groups and a common mean given for the group of 20. No mention of funding source. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided within the published article about generation of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | No mention how allocation was done. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "patients were randomised in a double blind manner". Comment: Probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of dropouts. |
| Selective reporting (reporting bias) | Low risk | No suggestion that selective reporting may have been done. |
| Other bias | High risk | Inclusion of participants who had a significant post‐bronchodilator response biased the study in favour of response to ICS since those with positive bronchodilator response are more likely to improve with ICS due to the asthma‐like reversibility in their airway. |
Martinez‐Garcia 2006.
| Methods | Randomised (stratified for prior smoking habit in pack year), double‐blind (only for dose of steroid), non‐placebo controlled prospective trial with study duration 6 months. | |
| Participants | Of the 132 participants initially included in the study, 39 were excluded prior to randomisation (23 had high probability of asthma, 4 did not give consent, 1 had Down's syndrome and 3 each had psychiatric disorder, systemic ICS and had disease of significant severity). 93 patients enrolled in the study. Seven dropouts during the study, three from the no steroid group and two each from the 500 µg and 1000 µg group. Study completed in 86 participants.
Inclusion: all participants with HRCT diagnosed bronchiectasis diagnosed between 1993 and June 2003 in Requena General Hospital. The participants were required to be free from acute exacerbation for at least 4 weeks. Exclusion: participants with asthma, cystic fibrosis and on whom ICS could not be stopped. |
|
| Interventions | Inhaled fluticasone 500 µg twice daily by MDI versus 250 µg fluticasone twice daily versus no treatment for 6 months | |
| Outcomes | Baseline data collection started 6 months prior to randomisation. During this period data prospectively collected on number of acute exacerbations, antibiotic use and hospital admissions.
During randomisation visit, information collected on dyspnoea score, daily sputum production (average of sputum produced over three days); cough and need for short‐acting bronchodilator in the 1‐month prior to randomisation, and HRQoL using the validated Spanish version of the SGRQ. After randomisation, TLC, RV and diffusion capacity analysed again after 6 months. HRQoL assessment at 3 and 6 months and all other tests at 1, 3 and 6 months. > 4‐point change in SGRQ considered significant. > 1‐point change is dyspnoea score considered significant. |
|
| Notes | Wherever available, data on the 250 µg twice daily group was combined with 500 µg twice daily group for comparison with no steroid group. Additional information provided by the authors. Study was supported by Grant Red Respira. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided within the published article about generation of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | No mention of how allocation was done. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "The study was conducted on a double blind basis regarding the effective inhalatory steroid dose administered (500 vs. 1000 µg/day), but not as relates to the administration or not of steroid treatment (i.e. 0 vs. 500 or 1000 µg/day)." Comment: No blinding for the two groups assessed by us (0 versus 1000 µg/day). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The patient characteristics and outcome data of those excluded or dropped out not described and not compared with those included for analysis. |
| Selective reporting (reporting bias) | High risk | Information on TLC, RV and DLCO gathered but not reported. Some outcomes that were reported in the 1000 µg group were not reported in the 500 µg group (such as sputum volume, TLC, RV and cough and wheeze clinical variables). |
| Other bias | High risk | Intention‐to‐treat analysis not done. Of the 39 participants excluded before randomisation, no details available for the reason for exclusion for 2 participants. |
Tsang 1998.
| Methods | Randomised double‐blind, placebo controlled prospective trial with study duration of 4 weeks. There were no dropouts. |
|
| Participants | 24 participants (mean age 51 years, 12 females) with HRCT proven bronchiectasis. Fluticasone group: n = 12, 6 females, age: mean 43 (SD 11). Placebo group: n = 12, 8 females, age: mean 56.8 (SD 11). Inclusion: daily sputum > 10 mL, absence of asthma or other unstable systemic disease; and "steady state" bronchiectasis (< 10% alteration of 24‐hour sputum volume, FEV1 and FVC). Exclusion: unreliable clinic attendance, known adverse reaction to fluticasone, regular use of ICS and asthma. |
|
| Interventions | Inhaled fluticasone 500 µg twice daily by accuhaler or placebo for 4 weeks. | |
| Outcomes |
Sputum leukocyte density, bacterial densities and concentrations of interleukin (IL)1B, IL 8, TNF alpha and leukotriene B4 were all measured at the time of randomisation and at 4 weeks. |
|
| Notes | Geometric mean was used instead of arithmetic mean. Hence, none of the lung function data were included in the final analysis. No mention of funding source. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided within the published article about generation of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | No mention of how allocation was done. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "performed a double blind, placebo controlled study". Comment: Probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts. |
| Selective reporting (reporting bias) | Low risk | No suggestion that selective reporting may have been done. |
| Other bias | High risk | The baseline values for lung functions, sputum amount and sputum inflammatory markers were significantly different, thus were subject to bias. |
Tsang 2004.
| Methods | Randomised double‐blind, prospective placebo controlled trial with study duration of 52 weeks. There were no dropouts. |
|
| Participants | 60 participants (mean age 56.4 years, 38 females) with HRCT proven bronchiectasis. Fluticasone group: n = 30, age: mean 56.1 (SD 14). Placebo group: n = 30, age: mean 56.7 (SD 11.3). 16 were P aeruginosa colonised and 44 were not. Inclusion: absence of asthma or other unstable systemic disease; and "steady state" bronchiectasis (< 20% alteration of 24‐hour sputum volume, FEV1 and FVC) and absence of deterioration in respiratory symptoms at baseline visit. Exclusion: unreliable clinic attendance, known adverse reaction to fluticasone and asthma. |
|
| Interventions | Inhaled fluticasone 500 µg twice daily by accuhaler or placebo for 52 weeks. | |
| Outcomes | The participants were followed up at ‐2, ‐1, 0, 4, 12, 24, 36, 48 and 52 weeks after commencement of therapy for measurement of FeNO. | |
| Notes | Data not included in the final analysis since medians and inter‐quartile range reported in this study. The study was supported by a CRCG Grant of the University of Hong Kong. The Accuhalers were donated by GlaxoWellcome, China. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided within the published article about generation of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | No mention on allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "performed a double blind, placebo controlled study". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no dropouts. |
| Selective reporting (reporting bias) | Low risk | No suggestion that selective reporting may have been done. |
| Other bias | High risk | Baseline value of the 2 groups were significantly different. |
Tsang 2005.
| Methods | Randomised double‐blind, prospective placebo controlled trial with study duration of 52 weeks. 5 dropouts in the placebo arm (1 at 4 weeks, 3 at 24 weeks and 1 at 52 weeks) and 8 dropouts in the fluticasone arm (2 at 4 weeks and 1 each at 6, 22, 32, 36, 50 and 52 weeks). |
|
| Participants | 89 patients recruited. Three participants withdrew. 86 participants (57 females, mean age 58.5 years) with HRCT proven bronchiectasis randomised between fluticasone and placebo. Fluticasone group: n = 43, 23 females, age: mean 57.7 (SD 14.4). Placebo group: n = 43, 34 females, age: mean 59.2 (SD 14.2). 23 were P aeruginosa colonised. Inclusion: absence of asthma or other unstable systemic disease; and "steady state" bronchiectasis (< 20% alteration of 24‐hour sputum volume, FEV1 and FVC) and absence of deterioration in respiratory symptoms at baseline visit. Exclusion: unreliable clinic attendance, known adverse reaction to fluticasone or quinolones and regular usage of ICS. |
|
| Interventions | Inhaled fluticasone 500 µg twice daily by accuhaler device or matched placebo for 52 weeks. | |
| Outcomes | The participants were followed up at ‐2, ‐1, 0, 4, 12, 24, 36, 48 and 52 weeks after commencement of therapy. Primary outcomes
Secondary outcomes
Improvement or deterioration was defined as > 20% change from baseline. |
|
| Notes | SD calculated from P value for sputum volume and exacerbation frequency and from confidence interval for the rest. The study was partially funded by GlaxoWelcome (Hong Kong). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomisation (block of 4)", however, no information about who generated the randomisation codes. |
| Allocation concealment (selection bias) | Unclear risk | No information about concealment was reported in the published article. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "double blind, placebo controlled study". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data of dropouts not compared to those included in analysis. |
| Selective reporting (reporting bias) | Low risk | No suggestion that selective reporting may have been done. |
| Other bias | High risk | Significant differences at the baseline on clinical features of "cough" and "dyspnoea" between the two groups to allow for post‐treatment comparison. |
ABPA: allergic bronchopulmonary aspergillosis DLCO: diffusing capacity of the lungs for carbon monoxide FeNO: fractional exhaled nitric oxide FEV1: forced expiratory volume (in 1 second) FVC: forced vital capacity HRCT: high resolution computed tomography HRQoL: health‐related quality of life ICS: inhaled corticosteroids MDI: metered dose inhaler OCS: oral corticosteroids PEFR: peak expiratory flow rate RV: residual volume SD: standard deviation SGRQ: St George's Respiratory Questionnaire TLC: total lung capacity TNF: tumour necrosis factor
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ghosh 2002 | Study compared effects of inhaled budesonide with inhaled ipratropium and not placebo |
| Guran 2008 | Not a RCT, withdrawal study with before‐and‐after outcomes |
| Martinez‐Garcia 2012 | Study compared budesonide with budesonide‐formoterol combination and not a placebo |
| Monton 1999 | Study included patients with pneumonia and not bronchiectasis. Used systemic steroids, not inhaled |
| ONeil 2004 | Study not a RCT. Studies subjective benefits of inhaler therapy including both ICS and bronchodilators |
ICS: inhaled corticosteroids RCT: randomised controlled trial
Differences between protocol and review
In the original protocol, we defined 'long‐term effect' as that measured at more than 12 months duration. We changed this to more than six months duration in the first review undertaken by the current group of review authors.
We included data from the Martinez‐Garcia 2006 study in the review, although the comparison between the untreated and the inhaled corticosteroids (ICS) groups were not blinded. Also, for clinical severity assessment in the Martinez‐Garcia 2006 study, we used outcome variables of sputum reduction > 50% and dyspnoea score improvement > 1 posthoc, since these were the ones available from the study.
We moved lung function to a primary outcome.
We included 'Summary of findings' tables in the review.
We added a study flow diagram.
We specified the methods in more detail following the MECIR standards.
We redrafted all sections under recommended headings.
Contributions of authors
The protocol was written by NK and AC based on previous protocols on cough in children. For the review update: NK and AC performed selection of articles from the search, data extraction, data analysis and writing of the review. HP prepared the manuscript. SB and JK reviewed the manuscript.
Sources of support
Internal sources
-
Royal Children's Hospital Foundation, Brisbane, Australia.
Salary support from the Foundation for the 2009 version of this review
External sources
-
National Health and Medical Research Council, Australia.
AC's Practitioner Fellowship and Project Grant
-
Asthma Australia, Australia.
HP is supported through an Early Career Fellowship
Declarations of interest
Nitin Kapur: none known. Helen Petsky: none known. Scott Bell: has received travel and accommodation support to attend investigator meetings (Vertex, Rempex), to participate in advisory boards and to speak at sponsored Symposia. Speakers fees and support to participate in preparation of educational materials and in advisory board have been paid to his Institution. John Kolbe: has received funds of approximately NZ $500 from Novartis for lecture to GPs as part of an educational symposium. John also received funds to attend investigator meetings from Aradigm, GSK, Insmed, and Corus. Anne Chang: grant provided by GSK is unrelated to this topic.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Elborn 1992 {published data only}
- Elborn JS, Johnston B, Allen F, Clarke J, McGarry J, Varghese G. Inhaled steroids in patients with bronchiectasis. Respiratory Medicine 1992;86(2):121‐4. [PUBMED: 1615177] [DOI] [PubMed] [Google Scholar]
Hernando 2012 {published data only}
- Hernando R, Drobnic ME, Cruz MJ, Ferrer A, Sune P, Montoro JB, et al. Budesonide efficacy and safety in patients with bronchiectasis not due to cystic fibrosis. International Journal of Clinical Pharmacy 2012;34:644‐50. [DOI] [PubMed] [Google Scholar]
Joshi 2004 {published data only}
- Joshi JM, Sundaram P. Role of inhaled steroids in stable bronchiectasis. Indian Practitioner 2004;57(4):243‐5. [Google Scholar]
Martinez‐Garcia 2006 {published and unpublished data}
- Martinez Garcia MA, Perpina‐Tordera M, Roman‐Sanchez P, Soler‐Cataluna JJ. Inhaled steroids improve quality of life in patients with steady state bronchiectasis. Respiratory Medicine 2006;100:1623‐32. [DOI] [PubMed] [Google Scholar]
Tsang 1998 {published data only}
- Tsang KW, Ho P, Lam W, Ip M, Chan K, Ho C, et al. Inhaled fluticasone reduces sputum inflammatory indices in severe bronchiectasis. American Journal of Respiratory and Critical Care Medicine 1998;158(3):723‐7. [PUBMED: 9730996] [DOI] [PubMed] [Google Scholar]
Tsang 2004 {published data only}
- Tsang KW, Tan KC, Ho PL, Ooi GC, Khong PL, Leung R, et al. Exhaled nitric oxide in bronchiectasis: the effects of inhaled corticosteroid therapy. International Journal of Tuberculosis and Lung Disease 2004;8(11):1301‐7. [PubMed] [Google Scholar]
Tsang 2005 {published data only}
- Tsang KW, Tan KC, Ho PL, Ooi GC, Ho JC, Mak J, et al. Inhaled fluticasone in bronchiectasis: a 12‐month study. Thorax 2005;60:239‐43. [PUBMED: 15741443] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ghosh 2002 {published data only}
- Ghosh CS, Joseraj R, Kumari A, Jayaprakash. A randomized controlled clinical trial comparing the effects of inhaled budesonide vs inhaled ipratropium in patients with bronchiectasis. Indian Journal of Allergy Asthma and Immunology 2002;16(1):55‐71. [Google Scholar]
Guran 2008 {published data only}
- Guran T, Ersu R, Karadag B, Karakoc F, Demirel Y, Hekim N, et al. Withdrawal of inhaled steroids in children with non‐cystic fibrosis bronchiectasis. Journal of Clinical Pharmacy and Therapeutics 2008;33:603‐11. [DOI] [PubMed] [Google Scholar]
Martinez‐Garcia 2012 {published data only}
- Martinez‐Garcia MA, Soler‐Cataluna JJ, Catalan‐Serra P, Roman‐Sanchez P, Tordera MP. Clinical efficacy and safety of budesonide‐formoterol in non‐cystic fibrosis bronchiectasis. Chest 2012;141(2):461‐8. [DOI] [PubMed] [Google Scholar]
Monton 1999 {published data only}
- Monton C, Ewig S, Torres A, El‐Ebiary M, Filella X, Rano A, et al. Role of glucocorticoids on inflammatory response in non‐immunosuppressed patients with pneumonia: a pilot study. European Respiratory Journal 1999;14:218‐20. [DOI] [PubMed] [Google Scholar]
ONeil 2004 {published data only}
- O'Neill B, Bradley JM, Macmohan J, Elborn S. Subjective benefit of inhaled therapies in patients with bronchiectasis: a questionnaire study. International Journal of Clinical Practice 2004;58(5):441‐3. [DOI] [PubMed] [Google Scholar]
Additional references
Balfour‐Lynn 2006
- Balfour‐Lynn IM, Lees B, Hall P, Phillips G, Khan M, Flather M, et al. Multicenter randomized controlled trial of withdrawal of inhaled corticosteroids in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2006;173(12):1356‐62. [DOI] [PubMed] [Google Scholar]
Balfour‐Lynn 2014
- Balfour‐Lynn I, Welch K. Inhaled corticosteroids for cystic fibrosis. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD001915] [DOI] [PubMed] [Google Scholar]
Barnes 2010
- Barnes PJ. Inhaled corticosteroids. Pharmaceuticals (Basel) 2010;3(3):514–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berend 2008
- Berend N, Salome CM, King GG. Mechanisms of airway hyperresponsiveness in asthma. Respirology 2008;13:624‐31. [DOI] [PubMed] [Google Scholar]
Booth 1995
- Booth H, Richmond I, Ward C, Gardiner PV, Harkawat R, Walters EH. Effect of high dose inhaled fluticasone propionate on airway inflammation in asthma. Americal Journal of Respiratory and Critical Care Medicine 1995;152(1):45‐52. [DOI] [PubMed] [Google Scholar]
Cates 2008 [Computer program]
- Cates C. Visual Rx. Online NNT Calculator. Dr Christopher Cates, 2008.
Chang 2002
- Chang AB, Grimwood K, Mulholland EK, Torzillo PJ. Bronchiectasis in Indigenous children in remote Australian communities. Medical Journal of Australia 2002;177(4):200‐4. [DOI] [PubMed] [Google Scholar]
Chang 2008
- Chang AB, Bilton D. Exacerbations in cystic fibrosis: 4‐non‐cystic fibrosis bronchiectasis. Thorax 2008;63(3):269‐76. [DOI] [PubMed] [Google Scholar]
Chang 2010
- Chang AB, Bell SC, Byrnes CA, Grimwood K, Holmes PW, King PT, et al. Chronic suppurative lung disease and bronchiectasis in children and adults in Australia and New Zealand. Medical Journal of Australia 2010;193:356. [DOI] [PubMed] [Google Scholar]
Chang 2015
- Chang AB, Bell SC, Torzillo PJ, King PT, Byrnes CA, Maguire GP, et al. Bronchiectasis and chronic suppurative lung disease (CSLD) in children and adults in Australia and New Zealand: Thoracic Society of Australia and New Zealand Guideline: an update. Medical Journal of Australia 2015;202:21‐3. [DOI] [PubMed] [Google Scholar]
Cole 1986
- Cole PJ. Inflammation: a two edged sword. The model of bronchiectasis. European Journal of Respiratory Disease. Supplement 1986;147:6‐15. [PubMed] [Google Scholar]
Daniel 2017
- Daniel S, Jose O. A study on HbA1c profile in children with asthma using inhaled corticosteroids. International Journal of Contemporary Pediatrics 2017;4(3):796‐800. [DOI: ] [Google Scholar]
Edwards 2003
- Edwards EA, Asher MI, Byrnes CA. Paediatric bronchiectasis in the twenty‐first century: experience of a tertiary children's hospital in New Zealand. Journal of Paediatrics and Child Health 2003;39(2):111‐7. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Ellerman 1997
- Ellerman A, Bisgaard H. Longitudinal study of lung function in a cohort of primary ciliary dyskinesia. European Respiratroy Journal 1997;10(10):2376‐9. [DOI] [PubMed] [Google Scholar]
Ernst 2015
- Ernst P, Saad N, Suissa S. Inhaled corticosteroids in COPD: the clinical evidence. European Respiratory Journal 2015;45(2):525‐37. [DOI: 10.1183/09031936.00128914] [DOI] [PubMed] [Google Scholar]
Festic 2016
- Festic E, Bansal V, Gupta E, Scanlon PD. Association of inhaled corticosteroids with incident pneumonia and mortality in COPD patients; systematic review and meta‐analysis. COPD 2016;13(3):312‐26. [DOI: 10.3109/15412555.2015.1081162] [DOI] [PMC free article] [PubMed] [Google Scholar]
Field 1969
- Field CE. Bronchiectasis. Third report on a follow‐up study of medical and surgical cases from childhood. Archives of Disease in Childhood 1969;44(237):551‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Finch 2015
- Finch S, McDonnell MJ, Abo‐Leyah H, Aliberti S, Chalmers JD. A comprehensive analysis of the impact of pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Annals of the American Thoracic Society 2015;12(11):1602‐11. [DOI] [PubMed] [Google Scholar]
Goyal 2016
- Goyal V, Grimwood K, Masters IB, Marchant JM, Chang AB. State of the art: pediatric bronchiectasis ‐ no longer an orphan disease. Pediatric Pulmonology 2016;51(5):450‐69. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed prior to 30 April 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guan 2014
- Guan WJ, Gao YH, Xu G, Lin ZY, Tang Y, Li HM, et al. Characterization of lung function impairment in adults with bronchiectasis. PLoS ONE 2014;9(11):e113373. [DOI: 10.1371/journal.pone.0113373] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holme 2008
- Holme J, Tomlinson JW, Stockley RA, Stewart PM, Barlow N, Sullivan AL. Adrenal suppression in bronchiectasis and impact of inhaled corticosteroids. European Respiratory Journal 2008;32:1047‐52. [DOI] [PubMed] [Google Scholar]
Ip 1991
- Ip M, Lam WK, Liong E, Chan CY, Tse KM. Analysis of factors associated with bronchial hyperreactivity to methacholine in bronchiectasis. Lung 1991;169(4):245. [DOI] [PubMed] [Google Scholar]
Ip 1993
- Ip M, Lauder IJ, Wong WY. Multivariate analysis of factors affecting pulmonary function in bronchiectasis. Respiration 1993;60:45‐50. [DOI] [PubMed] [Google Scholar]
Kapur 2009
- Kapur N, Masters IB, Chang AB. Exacerbations in noncystic fibrosis bronchiectasis: Clinical features and investigations. Respiratory Medicine 2009;103:1681. [DOI] [PubMed] [Google Scholar]
Kapur 2010
- Kapur N, Masters IB, Chang AB. Longitudinal growth and lung function in pediatric non‐CF bronchiectasis ‐ what influences lung function stability?. Chest 2010;138:158. [DOI] [PubMed] [Google Scholar]
Kapur 2012
- Kapur N, Masters IB, Newcombe P, Chang AB. The burden of disease in paediatric non‐cystic fibrosis bronchiectasis. Chest 2012;141:1018. [DOI] [PubMed] [Google Scholar]
Kapur 2012a
- Kapur N, Masters IB, Morris PS, Galligan J, Ware R, Chang AB. Defining pulmonary exacerbation in children with non‐cystic fibrosis bronchiectasis. Pediatric Pulmonology 2012;47(1):68. [DOI] [PubMed] [Google Scholar]
Karadag 2005
- Karadag B, Karakoc F, Ersu R, Kut A, Bakac S, Dagli E. Non‐cystic‐fibrosis bronchiectasis in children: a persisting problem in developing countries. Respiration 2005;72(3):233‐8. [DOI] [PubMed] [Google Scholar]
Karakoc 2001
- Karakoc GB, Yilmaz M, Altintas DU, Kendirli SG. Bronchiectasis: still a problem. Pediatric Pulmonology 2001;32(2):175‐8. [DOI] [PubMed] [Google Scholar]
Keistinen 1997
- Keistinen T, Saynajakangas O, Tuuponen T, Kivela SL. Bronchiectasis: an orphan disease with a poorly‐understood prognosis. European Respiratory Journal 1997;10(12):2784‐7. [DOI] [PubMed] [Google Scholar]
Kerstjens 1994
- Kerstjens HA, Postma DS, Doormaal JJ, Zanten AK, Brand PL, Dekhuijzen PN, et al. Effects of short‐term and long‐term treatment with inhaled corticosteroids on bone metabolism in patients with airways obstruction. Dutch CNSLD Study Group. Thorax 1994;49(7):652‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2008
- Kim V, Rogers TJ, Criner GJ. New concepts in the pathobiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008 May 1;5(4):478‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2012
- Lee C, Klaustermeyer WB. Effect of high dose inhaled corticosteroids on cell mediated immunity in patients with asthma. Allergologia et Immunopathologia (Madr) 2012;40(2):100‐3. [DOI] [PubMed] [Google Scholar]
Mandal 2013
- Mandal P, Hill AT. Bronchiectasis: breaking the cycle of inflammation and infection. Lancet Respiratory Medicine 2013;1:e5‐6. [DOI] [PubMed] [Google Scholar]
Olveira 2017
- Olveira C, Padilla A, Martínez‐García MÁ, Rosa D, Girón RM, Vendrell M, et al. Etiology of bronchiectasis in a cohort of 2047 patients. An analysis of the Spanish Historical Bronchiectasis Registry. Archivos Bronconeumologia 2017;53(7):366‐74. [DOI] [PubMed] [Google Scholar]
Pasteur 2010
- Pasteur MC, Bilton D, Hill AT. British Thoracic Society guideline for non‐CF bronchiectasis. Thorax 2010;65 (Suppl 1):i1‐i58. [DOI] [PubMed] [Google Scholar]
Philip 2014
- Philip J. The effects of inhaled corticosteroids on growth in children. Open Respiratory Medicine Journal 2014;8(Suppl 1:M3):66‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pizzutto 2013
- Pizzutto SJ, Grimwood K, Bauert P, Schultz KL, Yerkovich ST, Upham JW, et al. Flexible bronchoscopy contributes to the clinical management of Indigenous children with non‐cystic fibrosis bronchiectasis. Pediatric Pulmonology 2013;48:67‐73. [DOI] [PubMed] [Google Scholar]
PRISMA 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Annals of Internal Medicine 2009;151(4):264‐9. [DOI] [PubMed] [Google Scholar]
Pruteanu 2014
- Pruteanu AI, Chauhan BF, Zhang L, Prietsch SO, Ducharme FM. Inhaled corticosteroids in children with persistent asthma: dose‐response effects on growth. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD009878.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Renkema 1996
- Renkema TE, Schouten UP, Koeter GH, Postma DS. Effects of long‐term treatment with corticosteroids in COPD. Chest 1996;109(5):1156‐62. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sabroe 2013
- Sabroe I, Postma D, Heijink I, Dockrell DH. The yin and the yang of immunosuppression with inhaled corticosteroids. Thorax 2013;68(12):1085‐7. [DOI: 10.1136/thoraxjnl-2013-203773] [DOI] [PubMed] [Google Scholar]
Sanchez‐Munoz 2016
- Sánchez‐Muñoz G, López de Andrés A, Jiménez‐García R, Carrasco‐Garrido P, Hernández‐Barrera V, Pedraza‐Serrano F, et al. Time trends in hospital admissions for bronchiectasis: analysis of the Spanish national hospital discharge data (2004 to 2013). PLoS One 2016;11(9):e0162282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shoemark 2007
- Shoemark A, Ozerovitch L, Wilson R. Aetiology in adult patients with bronchiectasis. Respiratory Medicine 2007;101(6):1163‐70. [DOI] [PubMed] [Google Scholar]
Singleton 2000
- Singleton R, Morris A, Redding G, Poll J, Holck P, Martinez P, et al. Bronchiectasis in Alaskan Native children: causes and clinical courses. Pediatric Pulmonology 2000;29(3):182‐7. [DOI] [PubMed] [Google Scholar]
Sobieraj 2008
- Sobieraj DM, White CM, Coleman CI. Benefits and risks of adjunctive inhaled corticosteroids in chronic obstructive pulmonary disease: a meta‐analysis. Clinical Therapeutics 2008;30(8):1416‐25. [DOI] [PubMed] [Google Scholar]
Tsikrika 2017
- Tsikrika S, Dimakou K, Papaioannou AI, Hillas G, Thanos L, Kostikas K, et al. The role of non‐invasive modalities for assessing inflammation in patients with non‐cystic fibrosis bronchiectasis. Cytokine 2017;99:281‐6. [DOI] [PubMed] [Google Scholar]
Twiss 2006
- Twiss J, Stewart AW, Byrnes CA. Longitudinal pulmonary function of childhood bronchiectasis and comparison with cystic fibrosis. Thorax 2006;61(5):414‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Haren 1995
- Haren EH, Lammers JW, Festen J, Heijerman HG, Groot CA, Herwaarden CL. The effects of inhaled corticosteroid budesonide on lung function and bronchial hyper‐responsiveness in adult patients with cystic fibrosis. Respiratory Medicine 1995;89:209‐14. [DOI] [PubMed] [Google Scholar]
Wang 2016
- Wang CY, Lai CC, Yang WC, Lin CC, Chen L, Wang HC, et al. The association between inhaled corticosteroid and pneumonia in COPD patients: the improvement of patients' life quality with COPD in Taiwan (IMPACT) study. International Journal of Chronic Obstructive Pulmonary Disease 2016;11:2775‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilson 1997
- Wilson CB, Jones PW, O'Leary CJ, Cole PJ, Wilson R. Validation of the St. George's Respiratory Questionnaire in bronchiectasis. American Journal of Respiratory and Critical Care Medicine 1997;156:536‐41. [DOI] [PubMed] [Google Scholar]
Yang 2012
- Yang IA, Clark MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD002991.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Kapur 2009
- Kapur N, Bell S, Kolbe J, Chang AB. Inhaled steroids for bronchiectasis. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD000996.pub2] [DOI] [PubMed] [Google Scholar]
Kolbe 1998
- Kolbe J, Wells A. Inhaled steroids in the treatment of bronchiectasis. Cochrane Database of Systematic Reviews 1998, Issue 1. [DOI: 10.1002/14651858.CD000996] [DOI] [Google Scholar]
Kolbe 2000
- Kolbe J, Wells A, Ram FS. Inhaled steroids for bronchiectasis. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD000996] [DOI] [PubMed] [Google Scholar]


